Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
163 results about "Sustained delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
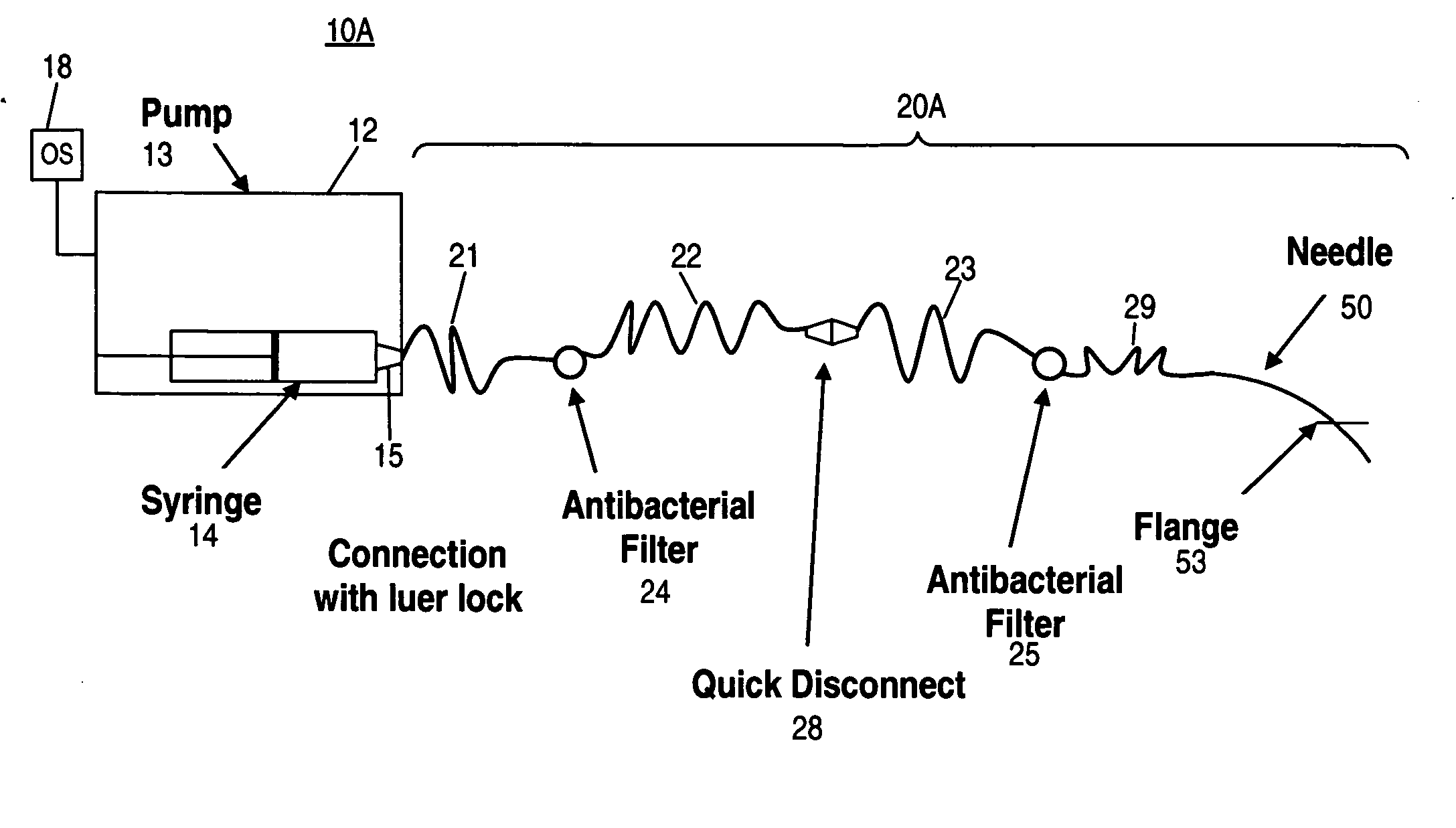
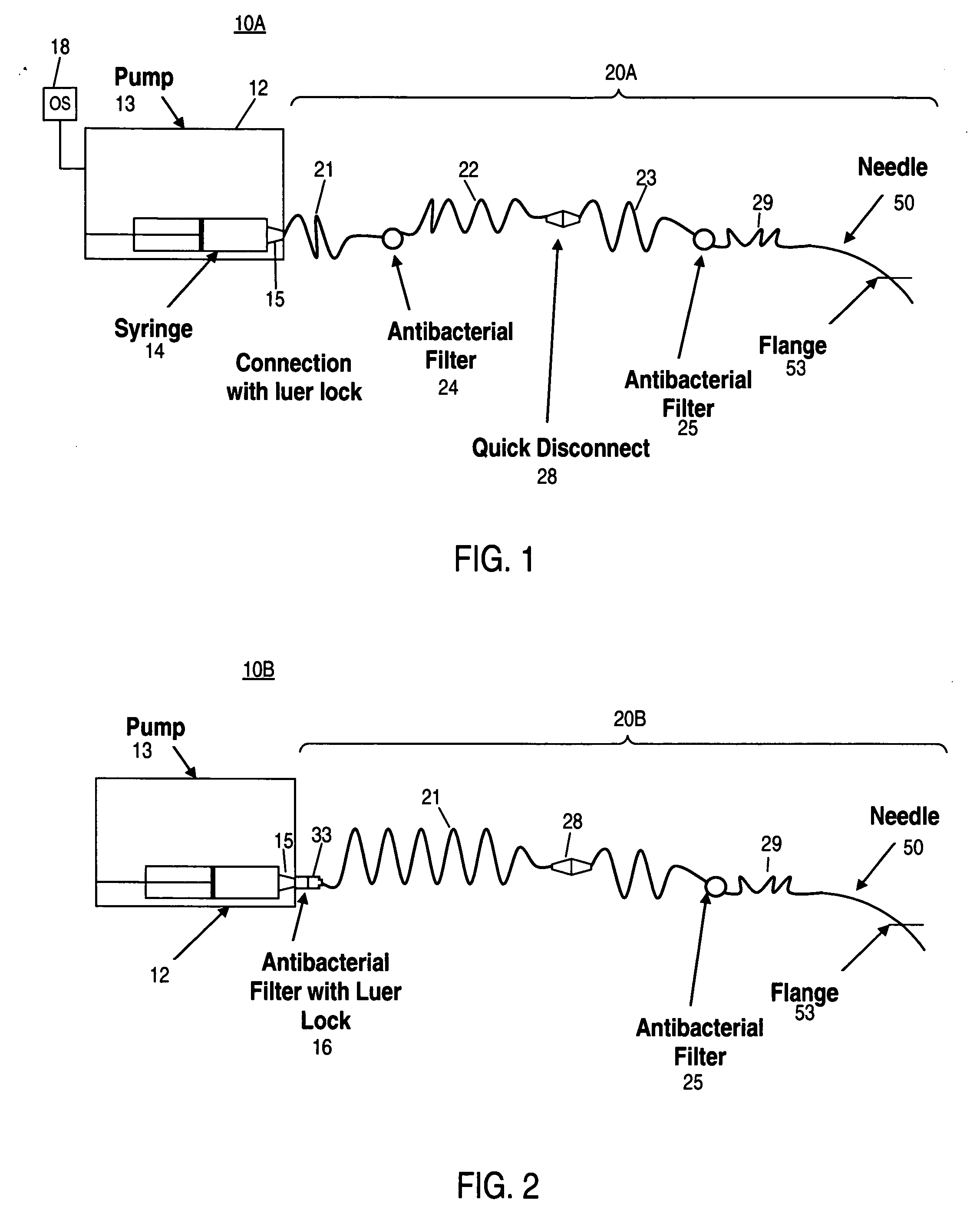
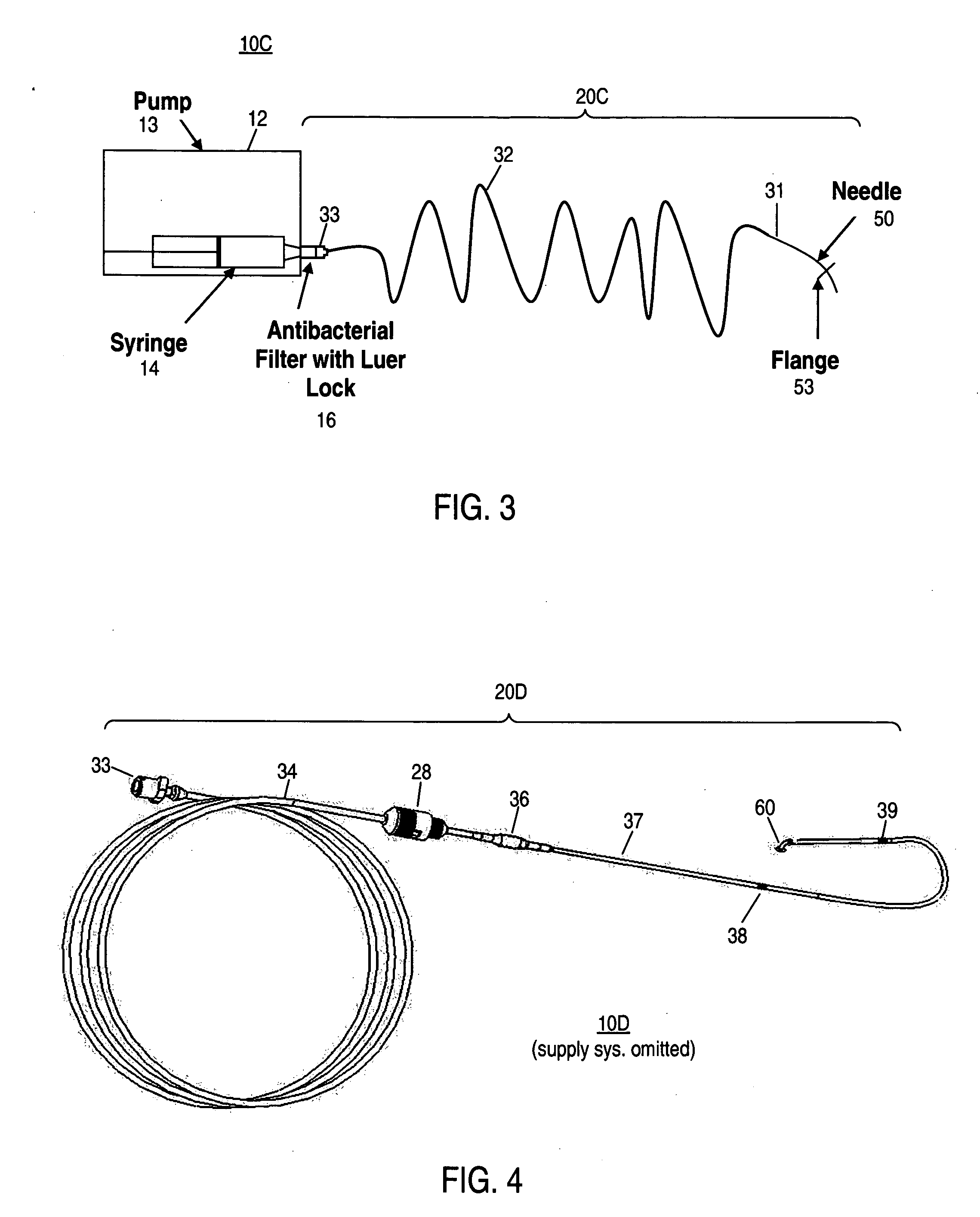
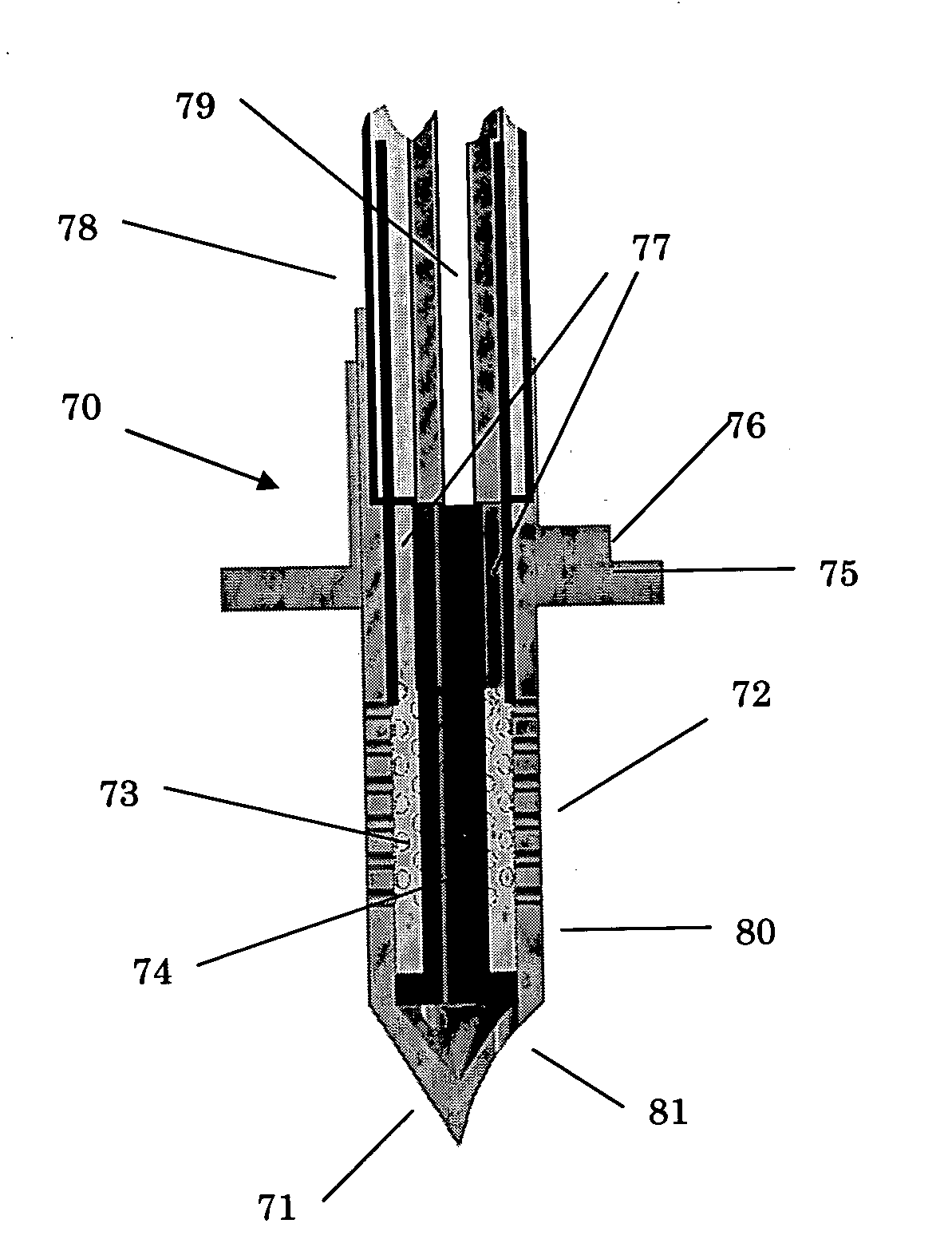
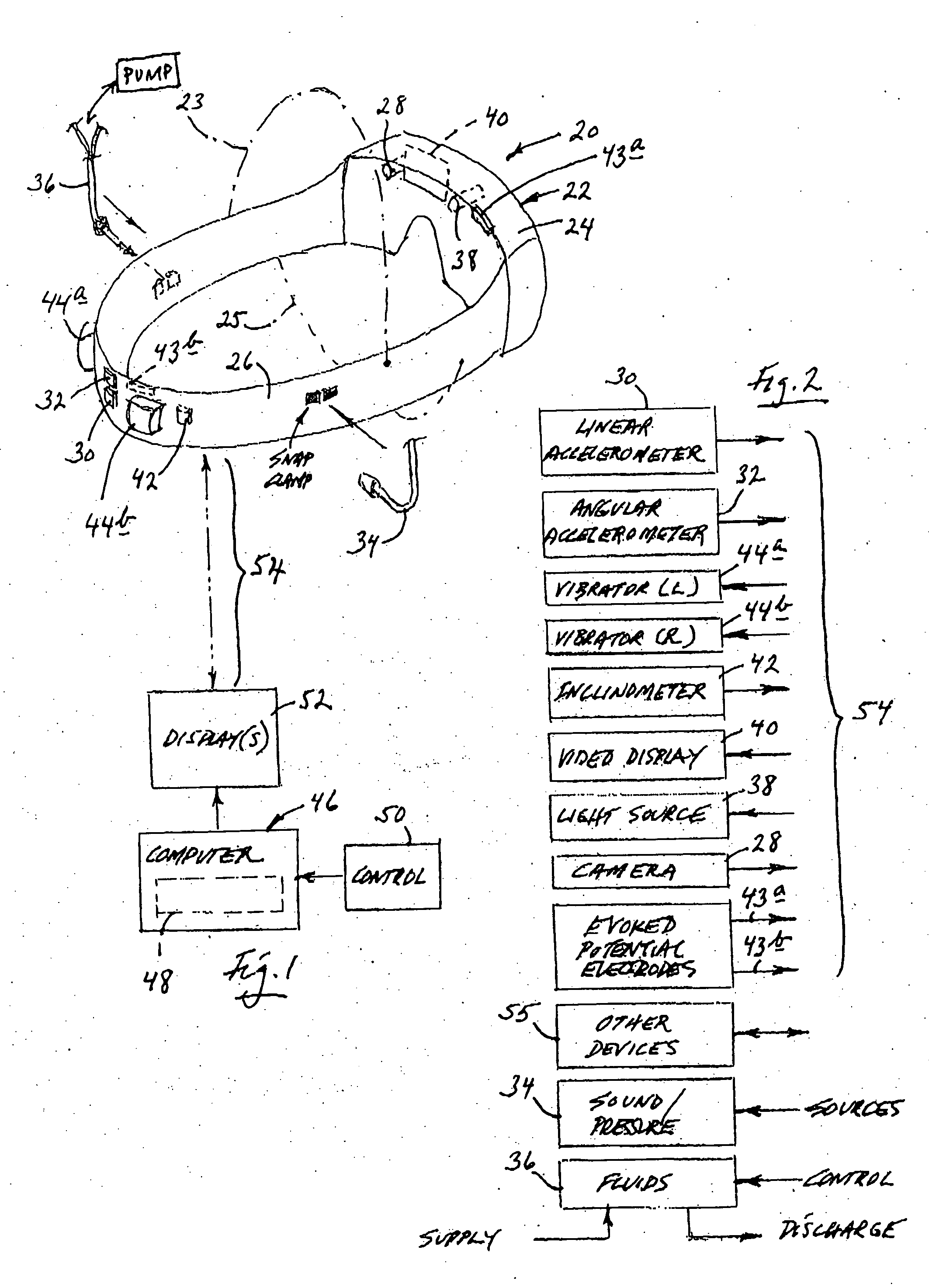
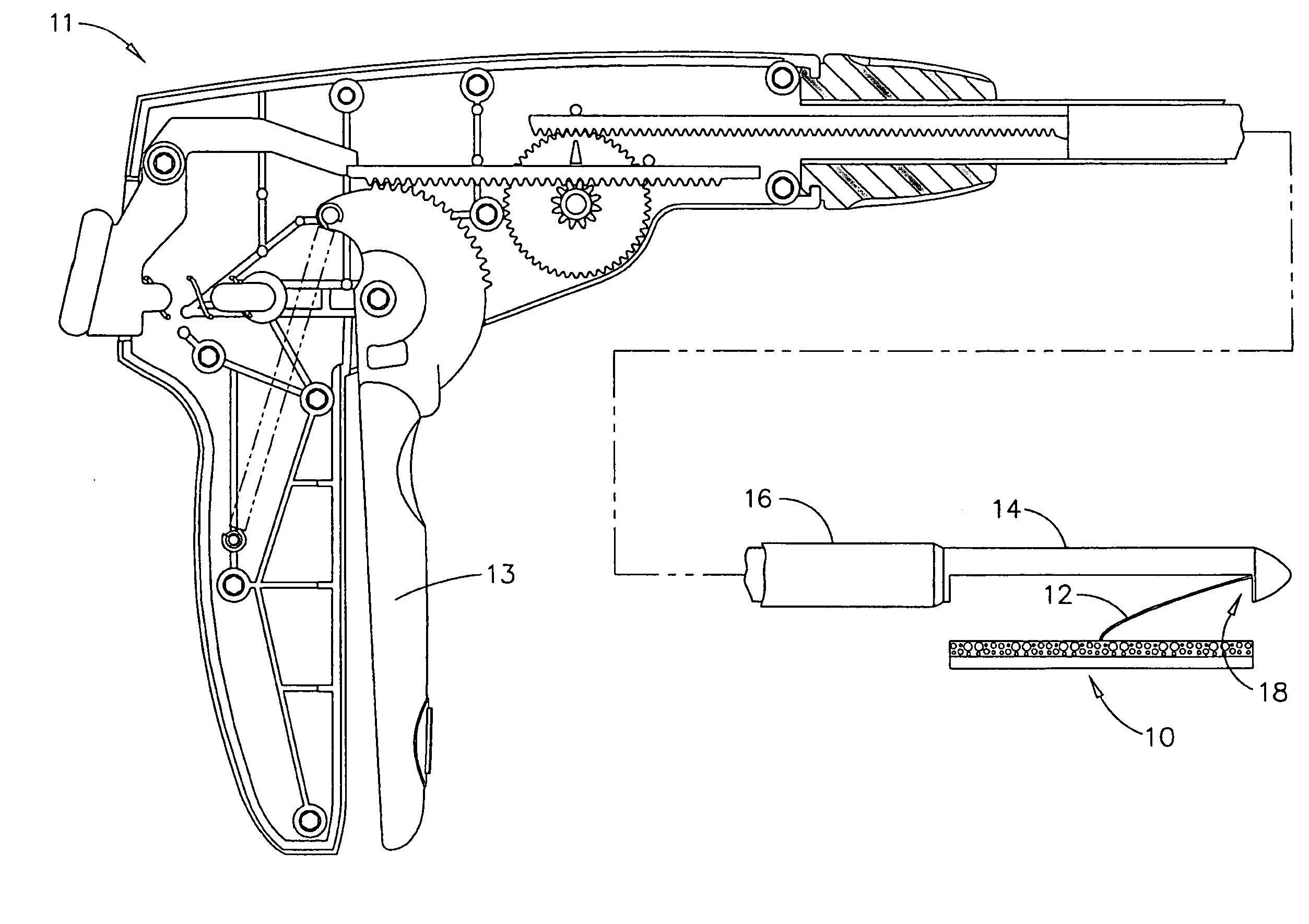
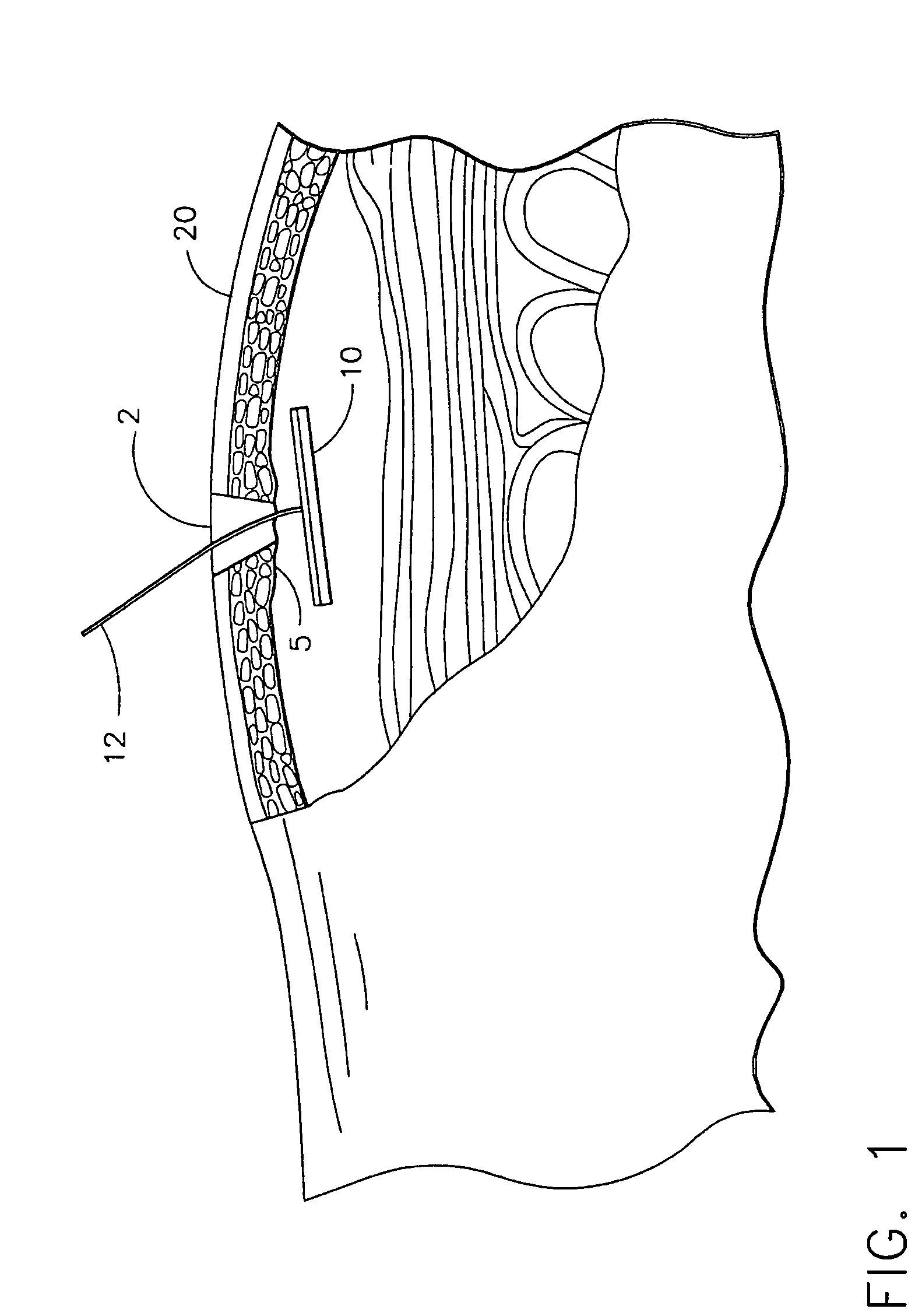
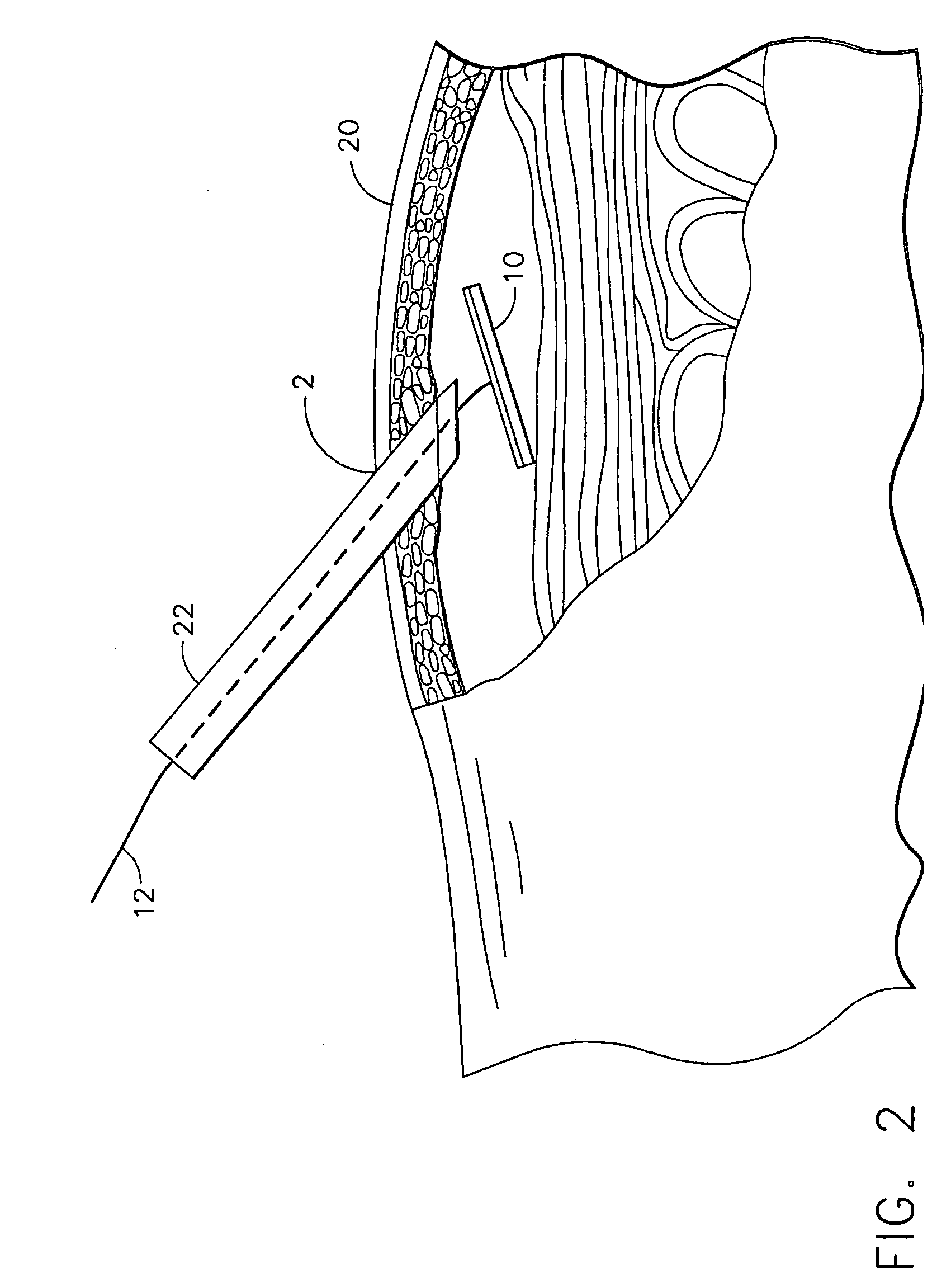
Apparatus and method for delivering therapeutic and/or other agents to the inner ear and to other tissues
An apparatus may include a needle for sustained delivery of drugs and other agents to the inner ear or other tissues of a human or an animal. The needle can include an insertion stop, and can be placed through the round window membrane or through a surgically-prepared hole in a bone. The needle can be in fluid communication with a port and / or with a micro-infusion or osmotic pump. A cochlear implant electrode can be used instead of a needle.
Owner:NEUROSYSTEC CORP
Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases
This invention provides a polymeric drug delivery system including a hydrogel containing one or more drugs for the treatment of a posterior segment disease. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
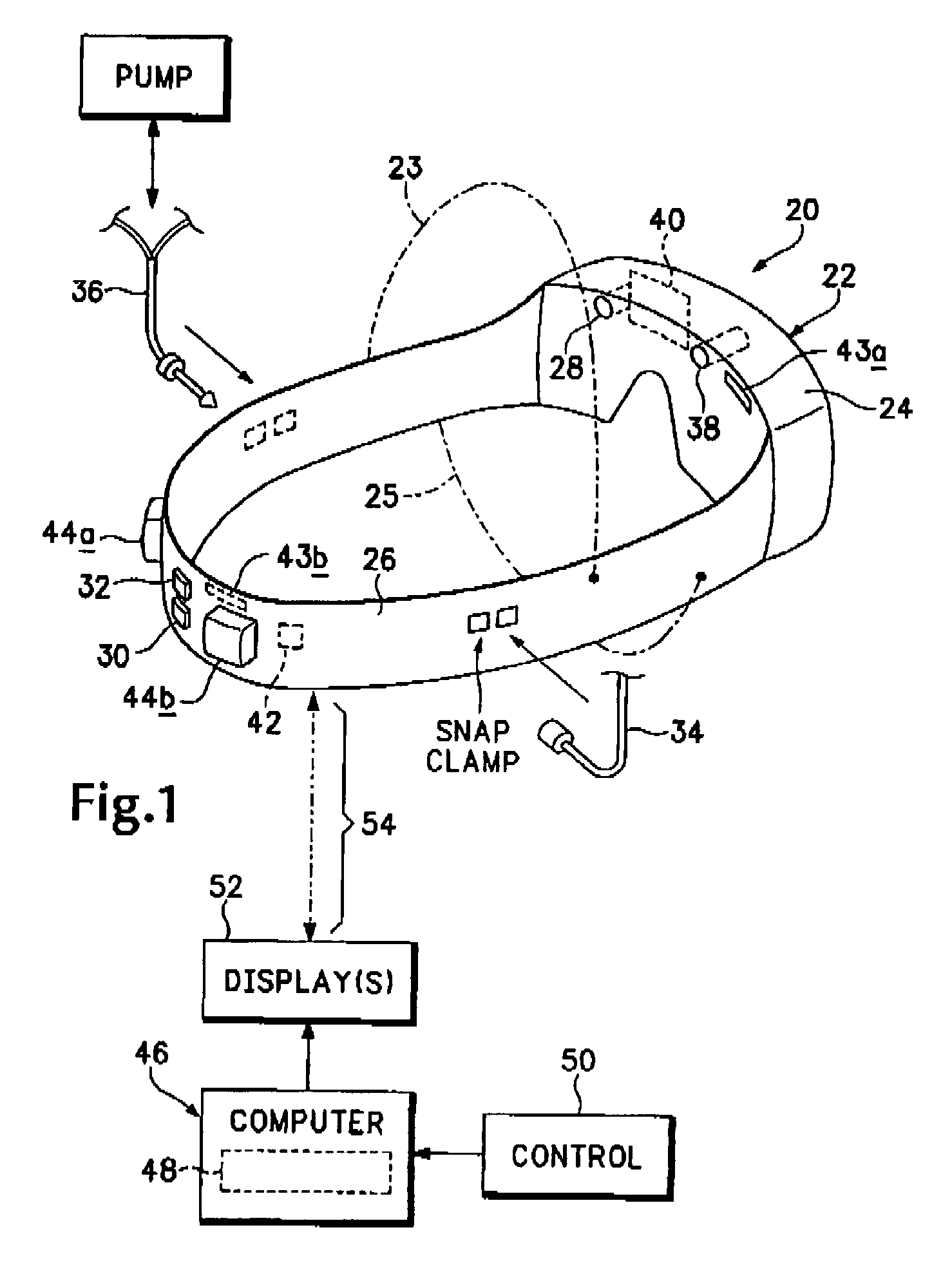
Minimally invasive, sustained, intra-tympanic drug delivery system
InactiveUS20050182385A1Easy to set upMinimization of systemic side effectElectrotherapyEar treatmentEngineeringCatheter
A convenient and preferably wearable system and method for implementing the controlled and sustained delivery of a medical liquid through the tympanic membrane and into the middle ear including port structure that produces a minimal opening in the membrane, a wearable and fixated fluid-conduit structure coupleable to the port structure, a reservoir adapted to contain delivery fluid, and an operationally controllable pump system applied to the fluid conduit structure, and / or an iontophoretic electrode system applied within the fluid conduit structure and to the subject's body, and operable to effect the delivery of reservoir-held fluid or medically active ions through the fluid conduit and port structures to the middle ear.
Owner:EPLEY JOHN M
Reservoir device for intraocular drug delivery
ActiveUS7883717B2Eliminate riskMinimally invasiveEye surgeryPharmaceutical delivery mechanismLimited accessSurgery
A delivery device that allows for the sustained release of an agent, particularly useful for the sustained release of a therapeutic agent to limited access regions, such as the posterior chamber of the eye and inner ear. The delivery device is minimally invasive, refillable and may be easily fixed to the treatment area. The delivery device includes a hollow body with an inlet port at its proximal end for insertion of the agent, a reservoir for holding the agent and a delivery mechanism for the sustained delivery of the agent from the reservoir to the patient.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods and articles for the delivery of medicaments to the eye for the treatment of posterior segment diseases
InactiveUS20050255144A1Pharmaceutical delivery mechanismEye treatmentHigh concentrationDelivery system
This invention provides articles and methods for drug delivery including a hydrogel containing one or more drugs for the treatment of a posterior segment disease and / or dry eye conditions. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20050009910A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaEster prodrug
The present invention relates to method of sustained-delivery of an active drug to a posterior part of an eye of a mammal to treat or prevent a disease or condition affecting said mammal, wherein said disease or condition can be treated or prevented by the action of said active drug upon said posterior part of the eye, comprising administering an effective amount of an ester prodrug of the active drug subconjunctivally or periocularly. Preferably, the active drug is more than about 10 times as active as the prodrug. Other aspects of this invention deal with the treatment of certain diseases by the periocular or subconjunctival delivery of an ester prodrug, and certain pharmaceutical products containing ester prodrugs for periocular or subconjunctival administration.
Owner:ALLERGAN INC
Method and device for minimally invasive implantation of biomaterial
A minimally invasive method of placing a delivery device substantially adjacent to vascular tissue and a device for use with such a method are disclosed. The delivery device may be a flexible biological construct with a flexible tethering means. The delivery device may be percutaneously inserted near vascular tissue such as, for example, peritoneal tissue. When the delivery device has been inserted, the tether may be used to pull the delivery device toward the vascular tissue and secure the device thereto. Contact between the front surface of the delivery device and the vascular tissue may be maintained by making and keeping the tether substantially taut. The delivery device may serve accomplish sustained delivery of active agents.
Owner:ETHICON ENDO SURGERY INC
Formulation for sustained delivery
Disclosed is an extended or controlled release dosage form of citalopram or its related forms and other newer antidepressants for oral administration to treat chronic patients suffering from depression and to minimize the side effects associated with the current drug treatment.
Owner:CHALLAPALLI PRASAD V N +2
Polysaccharide gel compositions and methods for sustained delivery of drugs
InactiveUS20090143348A1Enhanced release propertiesGood sustained releaseCosmetic preparationsBiocideDiseaseMedicine
Methods of producing a biocompatible polysaccharide gel composition having sustained release properties are disclosed. Also disclosed is a biocompatible polysaccharide gel composition having sustained release properties, a method of treating a disease or condition using the present biocompatible polysaccharide gel composition, and a method of controlling rate of release of at least one target solute from the biocompatible polysaccharide gel composition. Pharmaceutical compositions which include the present biocompatible polysaccharide gel composition also are disclosed.
Owner:ALLERGAN INC
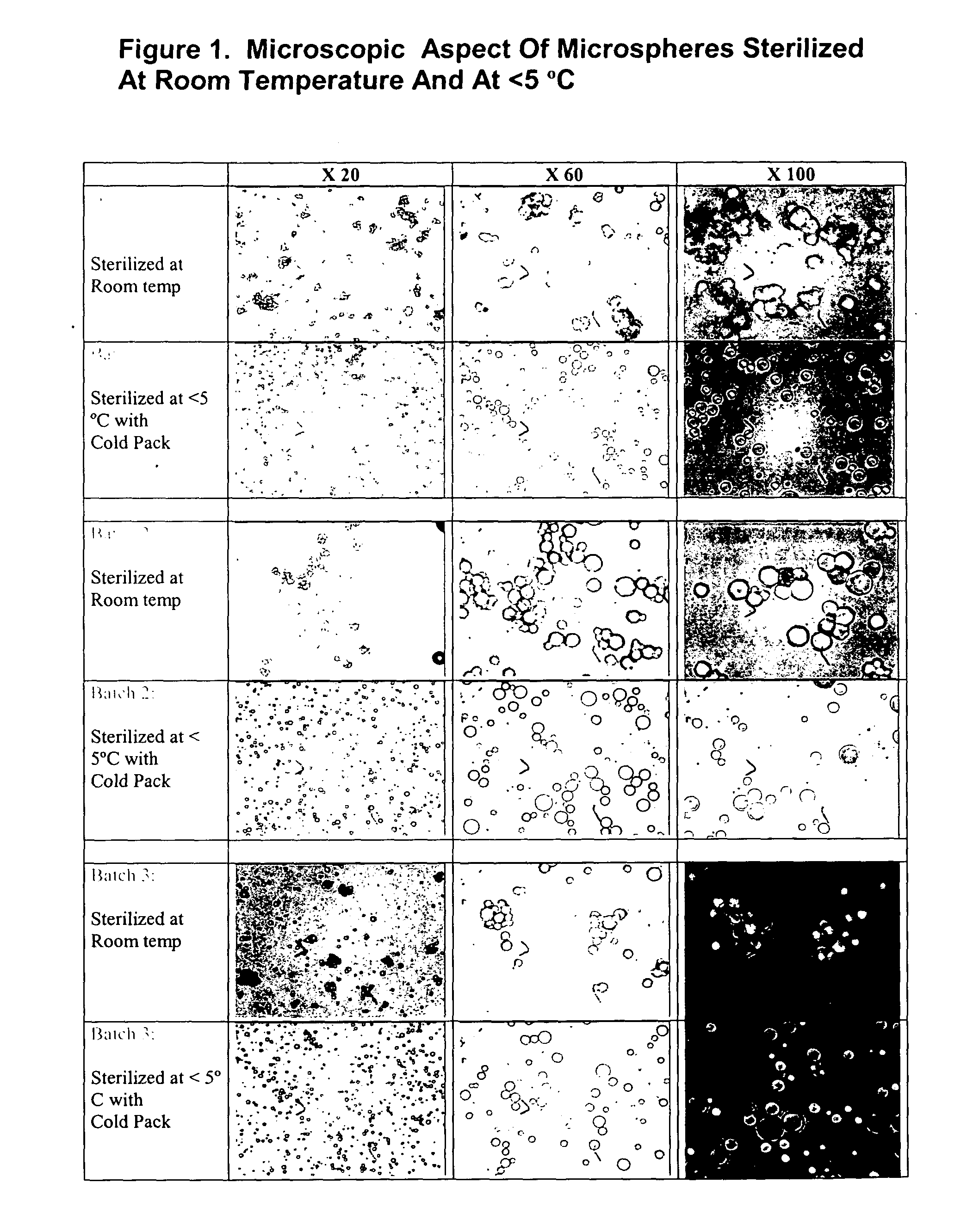
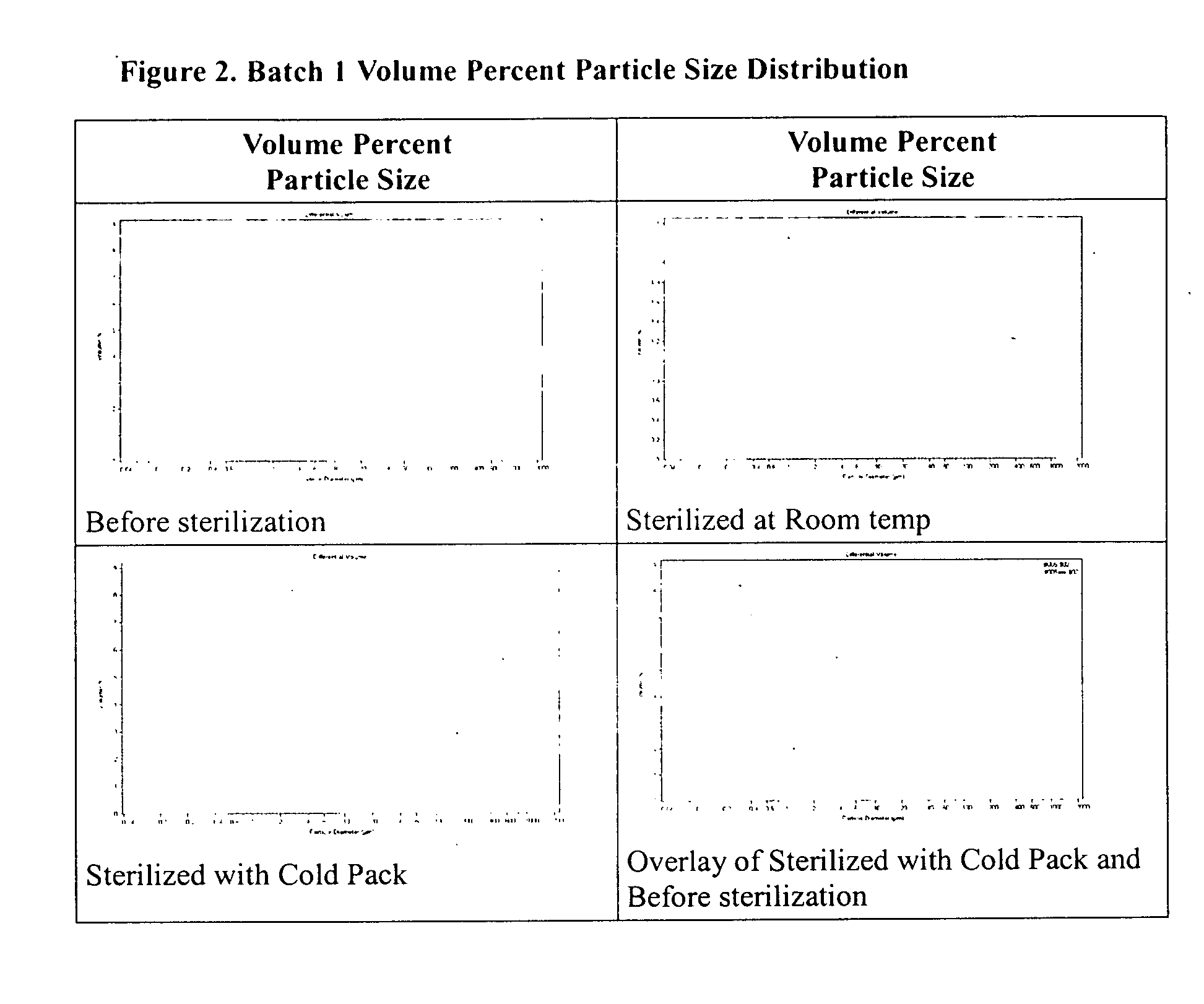
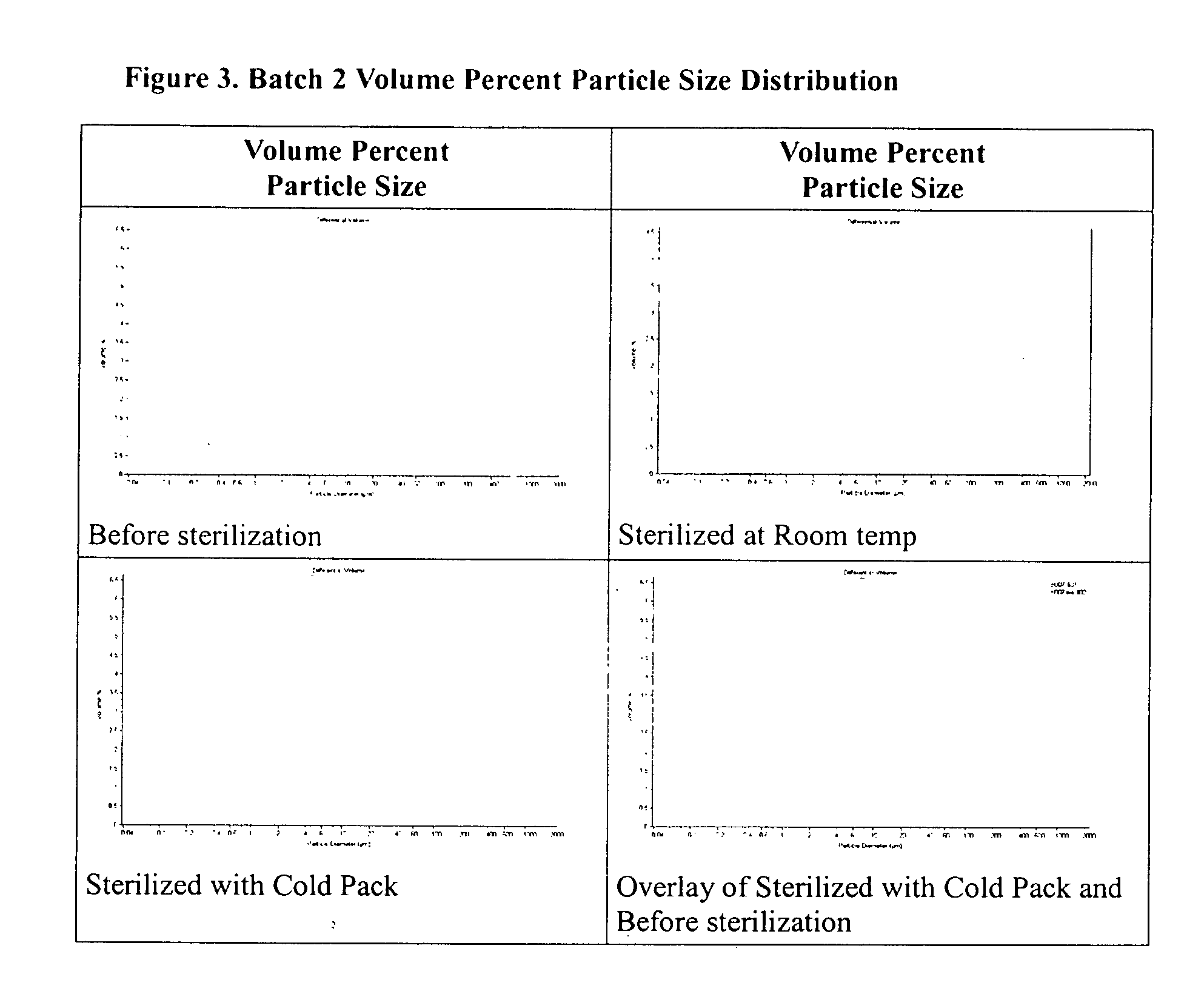
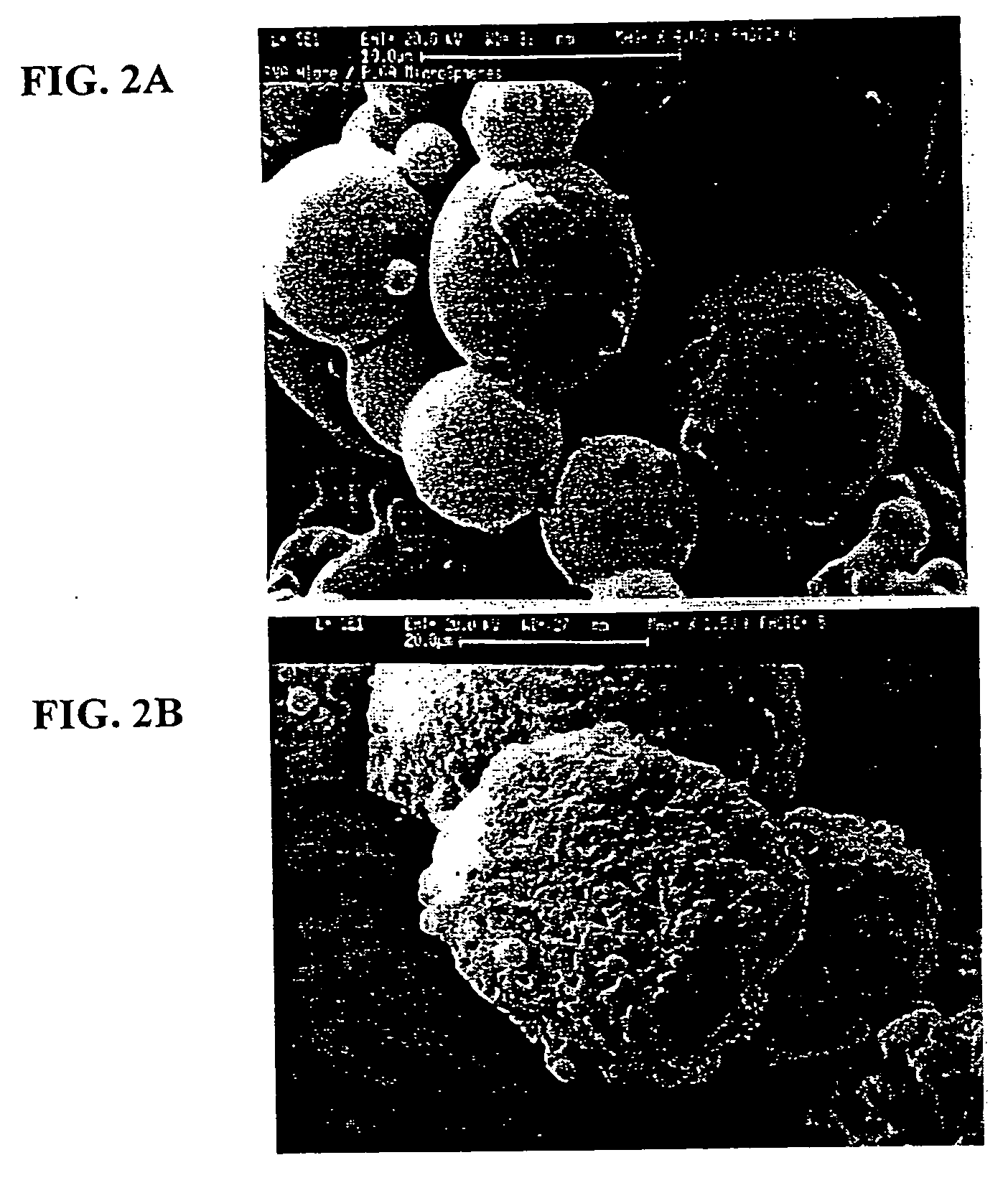
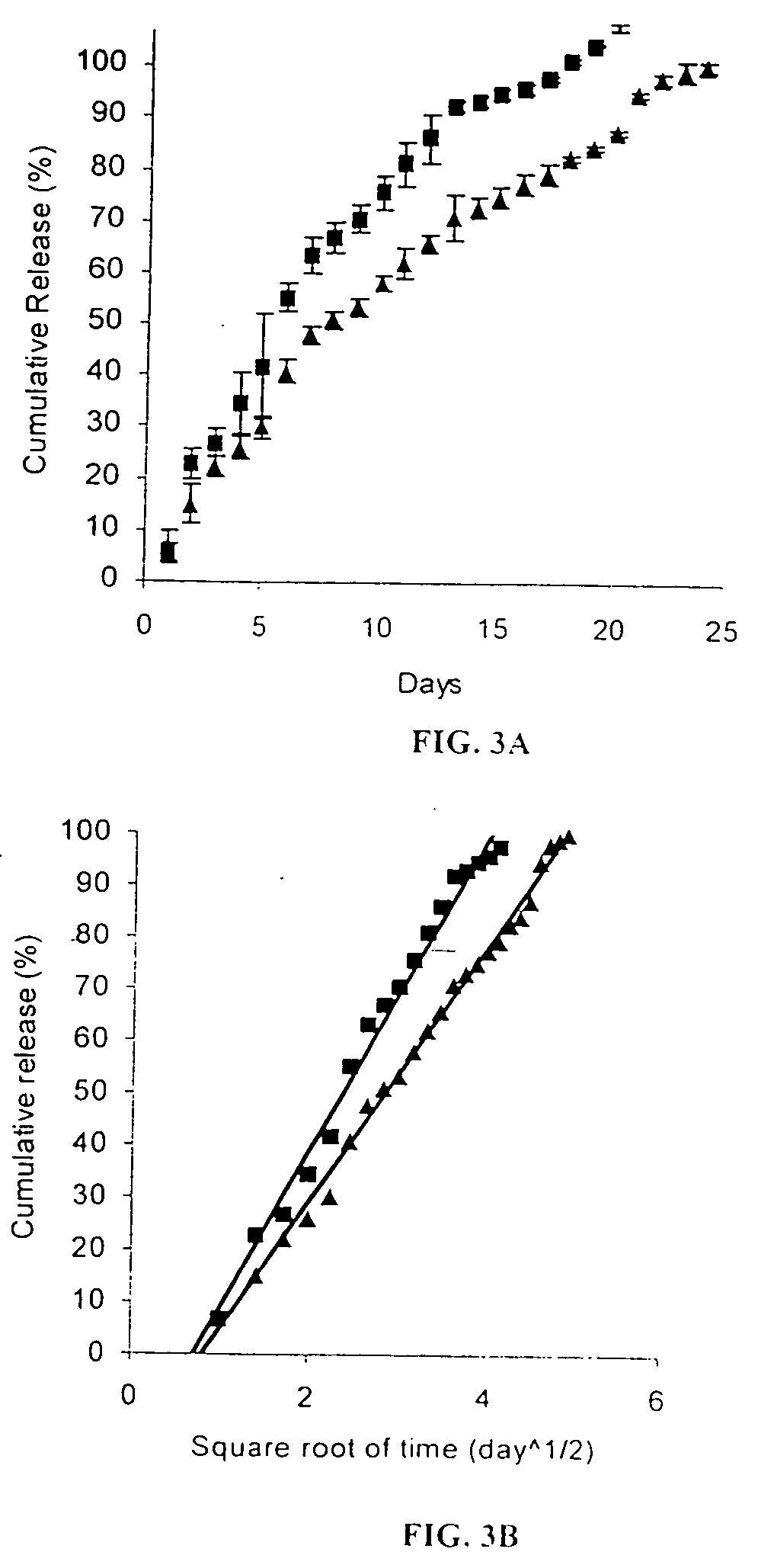
Method of sterilization of polymeric microparticles
InactiveUS20050003007A1Increased use of materialReduce executionOrganic active ingredientsPowder deliveryActive agentMicroparticle
This invention relates to the sterilization of polymeric material by irradiation for use in a body of a mammal. We have surprisingly discovered that the sterilization of polymeric materials for use in a mammal by irradiation is improved by reducing the temperature at which the irradiation is carried out. One aspect of this invention relates to a sterilized polymeric material for use in a body of a mammal wherein said polymeric material is sterilized by irradiation at a reduced temperature Another aspect of this invention relates to methods of sustained delivery of a therapeutically active agent to a mammal by using a polymeric material that has been sterilized at a reduced temperature by irradiation.
Owner:ALLERGAN INC
Drug delivery systems and use thereof
InactiveUS20050175708A1Reduced inhibitory activityTreat and inhibit diseasePowder deliveryBiocideVascular endothelial growth factorDelivery system
The invention provides a microsphere formulation for the sustained delivery of an aptamer, for example, an anti-Vascular Endothelial Growth Factor aptamer, to a preselected locus in a mammal, such as the eye. In addition, the invention provides methods for making such formulations, and methods of using such formulations to deliver an aptamer to a preselected locus in a mammal. In particular, the invention provides a method for delivering the aptamer to an eye for the treatment of an ocular disorder, for example, age-related macular degeneration.
Owner:MASSACHUSETTS EYE & EAR INFARY
Injectable, oral, or topical sustained release pharmaceutical formulations
Pharmaceutical formulations and methods are provided for the sustained delivery of a pharmaceutical agent to a patient by injection, by oral administration or by topical administration. The injectable formulation includes porous microparticles which comprise a pharmaceutical agent and a matrix material, wherein upon injection of the formulation a therapeutically or prophylactically effective amount of the pharmaceutical agent is released from the microparticles for at least 24 hours. The oral formulation includes porous microparticles which comprise a pharmaceutical agent and a matrix material, wherein a therapeutically or prophylactically effective amount of the pharmaceutical agent is released from the microparticles for at least 2 hours following oral administration. The topical formulation includes porous microparticles which comprise a pharmaceutical agent and a matrix material, wherein a therapeutically or prophylactically effective amount of the pharmaceutical agent is released from the microparticles for at least 2 hours following topical administration.
Owner:ACUSPHERE INC
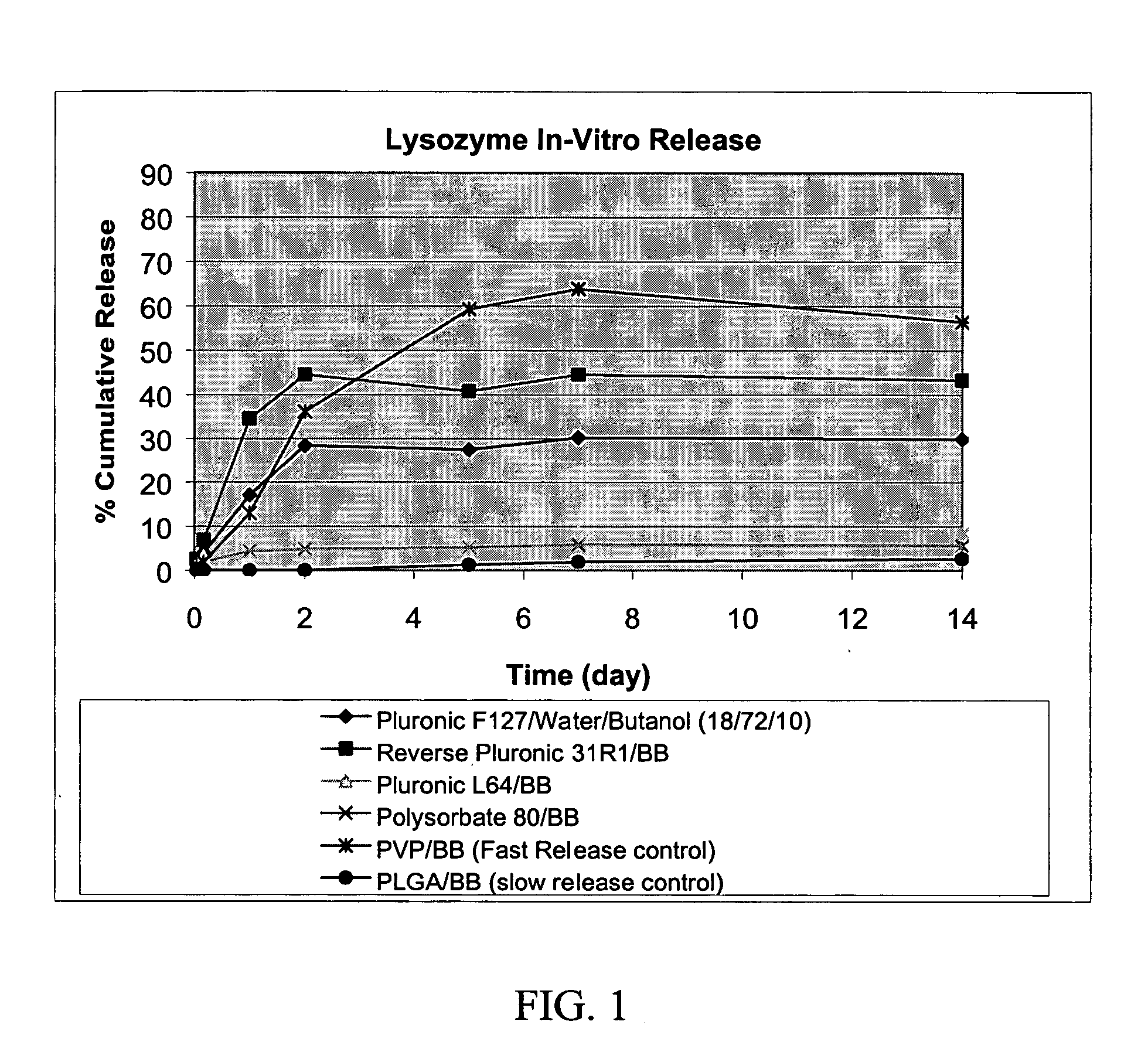
Polyol/oil suspensions for the sustained release of proteins
The present invention relates to the preparation of polyol / thickened oil suspensions containing a biologically active agent, for the sustained delivery of the biologically active agent. The described protein / glycerol / oil suspensions show sustained release of protein, e.g., G-CSF, of up to at least one week.
Owner:AMGEN INC
Sustained delivery of PDGF using self-assembling peptide nanofibers
InactiveUS20060148703A1Improve membrane permeabilityReduce deathPeptide/protein ingredientsMuscular disorderMedicineSelf-assembling peptide
The present invention is directed to a therapeutic composition in which human PDGF is bound directly to peptides that self assemble into a biologically compatible gel. When implanted in a patient's body, the composition provides for the slow, sustained release of PDGF. The composition will be especially useful in treating patients who have undergone a myocardial infarction.
Owner:THE BRIGHAM & WOMENS HOSPITAL INC
Ophthalmic devices for sustained delivery of active compounds
ActiveUS20060251696A1Sufficient amountPharmaceutical delivery mechanismOptical articlesCompound (substance)Time control
The invention relates to an ophthalmic product which has a capability of delivering a guest material (e.g., a lubricant or a drug) in a time-controlled-releasing manner. The invention also provides a process for making an ophthalmic product of the invention. In addition, the invention provides a method for time-controlled delivery of a drug or a lubricant.
Owner:ALCON INC
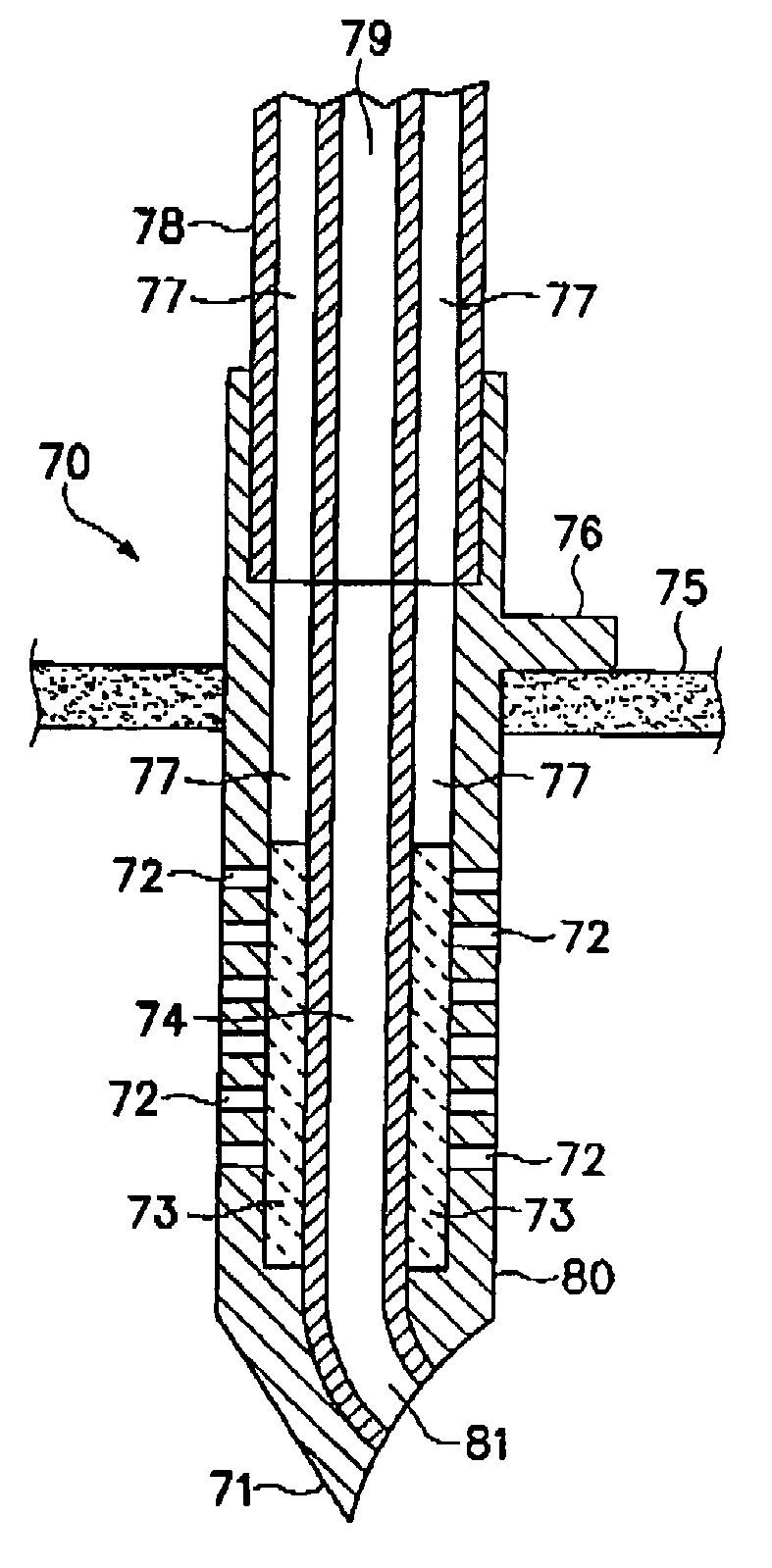
Cardiac drug delivery system
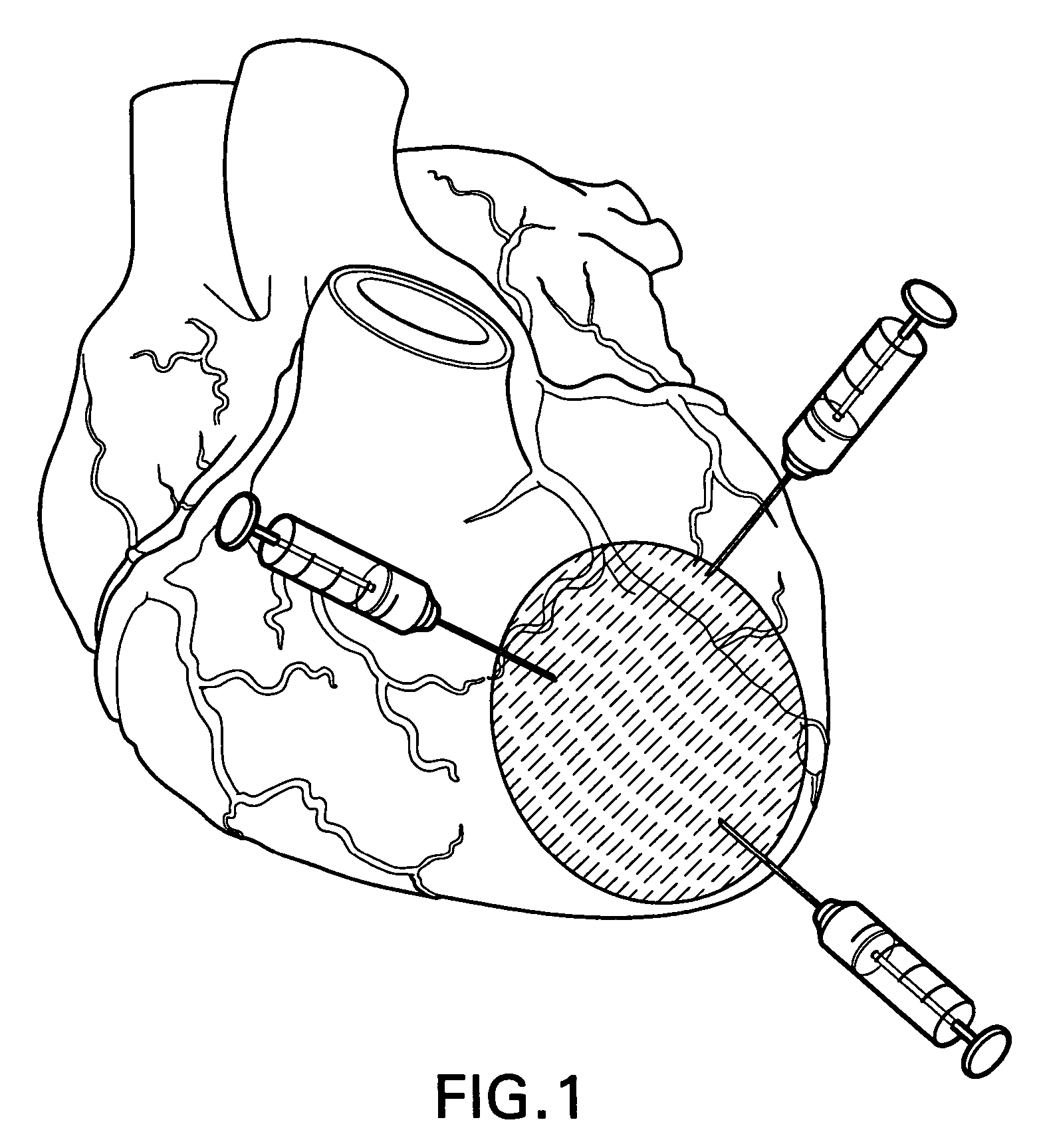
A system is disclosed, for administering a therapeutic agent locally and to a depth within cardiac tissue. An elongate, flexible catheter contains a flexible electric conductor and supports at its distal end an implantable electrode incorporating a penetrating element, typically a fixation helix or a linear needle that penetrates cardiac tissue as the electrode is implanted. A therapeutic agent is delivered through the electrode, to the cardiac tissue surrounding the penetrating element. The electrode can act as a sensor, to monitor an electrical condition of the surrounding cardiac tissue, and to control delivery of the agent responsive to the sensed electrical condition. Several embodiments feature a distal reservoir adjacent the electrode for effecting transient deliveries of the therapeutic agent in minute quantities. Other embodiments are disclosed for providing sustained deliveries of the agents.
Owner:BIOCARDIA
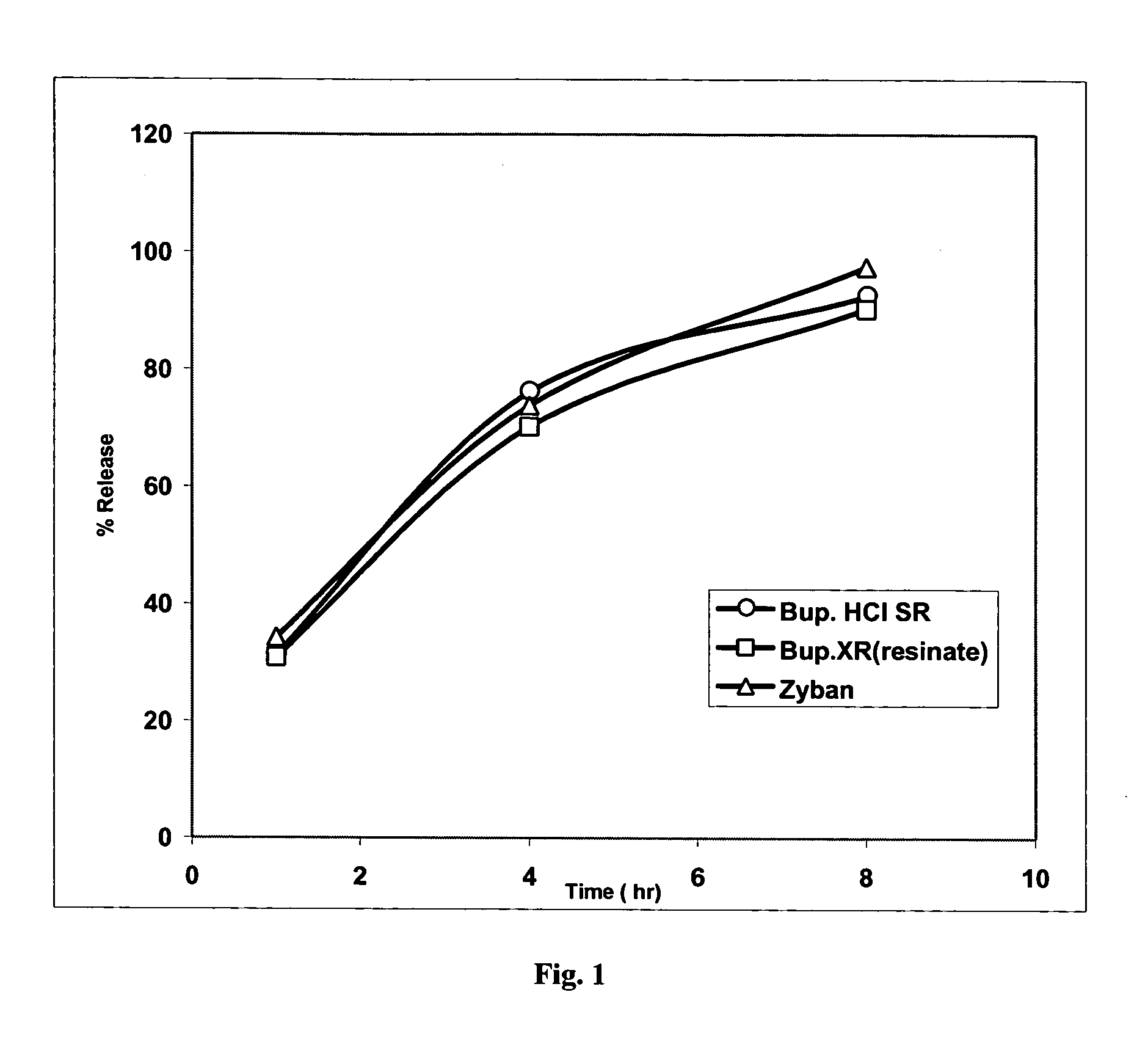
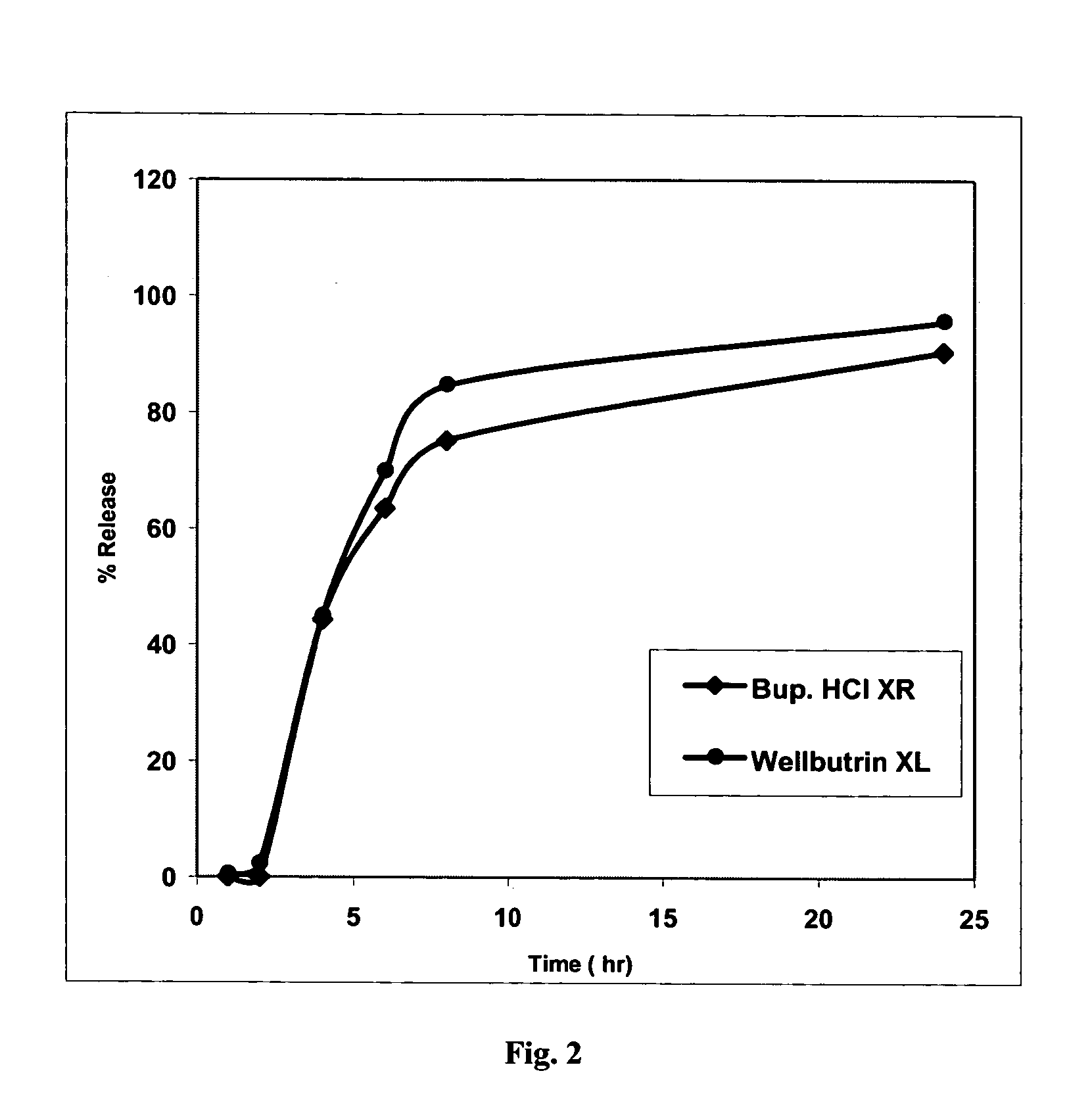
Bupropion formulation for sustained delivery
InactiveUS20050112198A1Improve stabilityProvide controlPharmaceutical non-active ingredientsPill deliveryAdditive ingredientPharmaceutical formulation
Disclosed is a pharmaceutical formulation for the stabilization and sustained delivery of an active pharmaceutical ingredient, such as the antidepressant, bupropion.
Owner:MURTY PHARMA
Minimally invasive, sustained, intra-tympanic drug delivery system
InactiveUS7351246B2Minimization of systemic side effectControlled absorptionElectrotherapyEar treatmentEngineeringTympanic Membranes
A convenient and preferably wearable system and method for implementing the controlled and sustained delivery of a medical liquid through the tympanic membrane and into the middle ear including port structure that produces a minimal opening in the membrane, a wearable and fixated fluid-conduit structure coupleable to the port structure, a reservoir adapted to contain delivery fluid, and an operationally controllable pump system applied to the fluid conduit structure, and / or an iontophoretic electrode system applied within the fluid conduit structure and to the subject's body, and operable to effect the delivery of reservoir-held fluid or medically active ions through the fluid conduit and port structures to the middle ear.
Owner:EPLEY JOHN M
Devices for intraocular drug delivery
InactiveUS20050059956A1Increase surface areaControl lengthSenses disorderEye surgeryLimited accessTherapeutic Area
An therapeutic agent delivery device that can allows is particularly suitable for delivery of a therapeutic agent to limited access regions, such as the posterior chamber of the eye and inner ear. Preferred devices of the invention are minimally invasive, refillable and may be easily fixed to the treatment area. Preferred delivery devices of the invention also include those that comprise a non-linear shaped body member body housing one or more substances and a delivery mechanism for the sustained delivery of the one or more substances from the non-linear shaped body member to the patient.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Composition for sustained delivery of hydrophobic drugs and process for the preparation thereof
InactiveUS7153520B2Enhance pharmacological effectsPowder deliverySolution deliveryPolythylene glycolPharmaceutical Substances
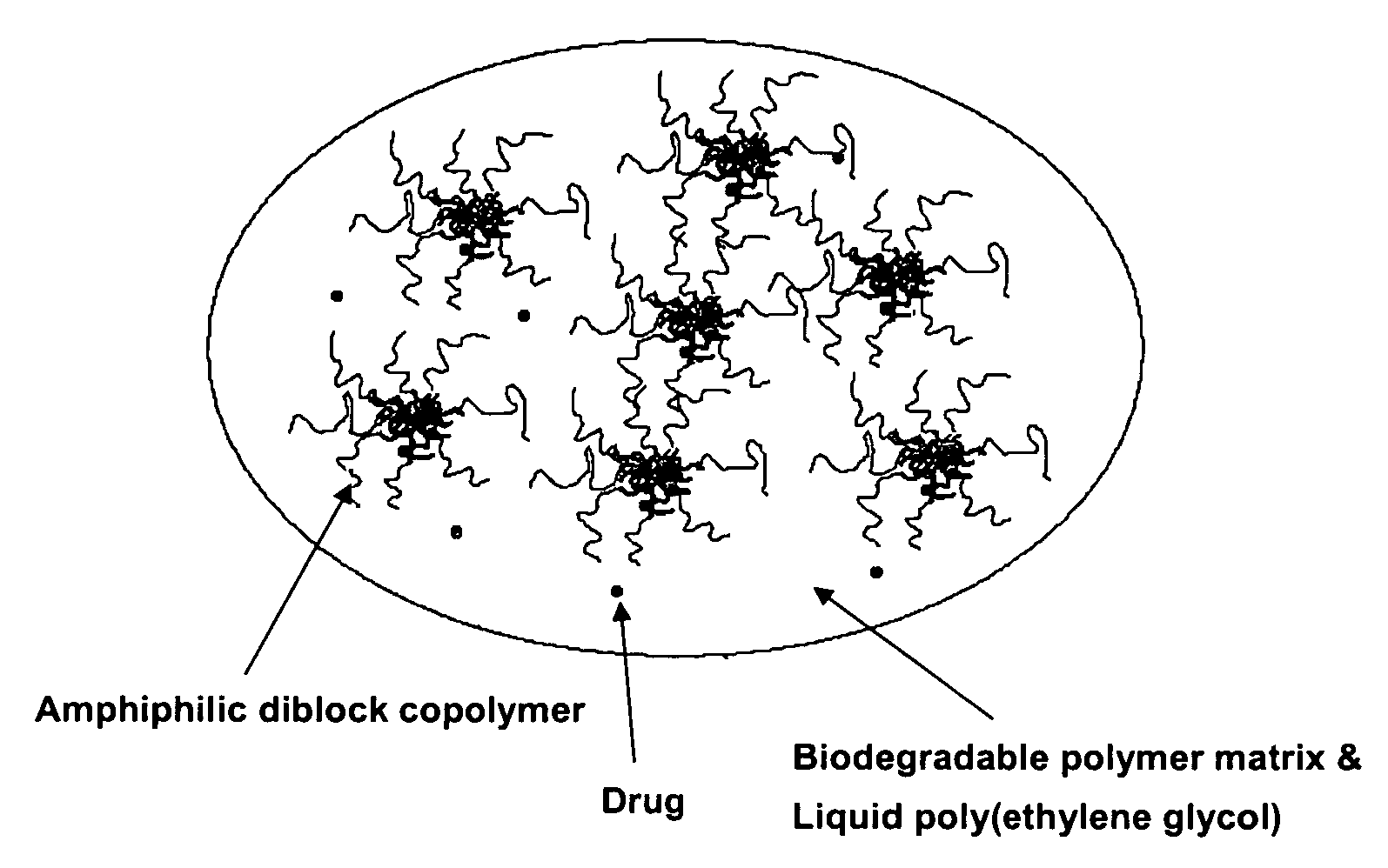
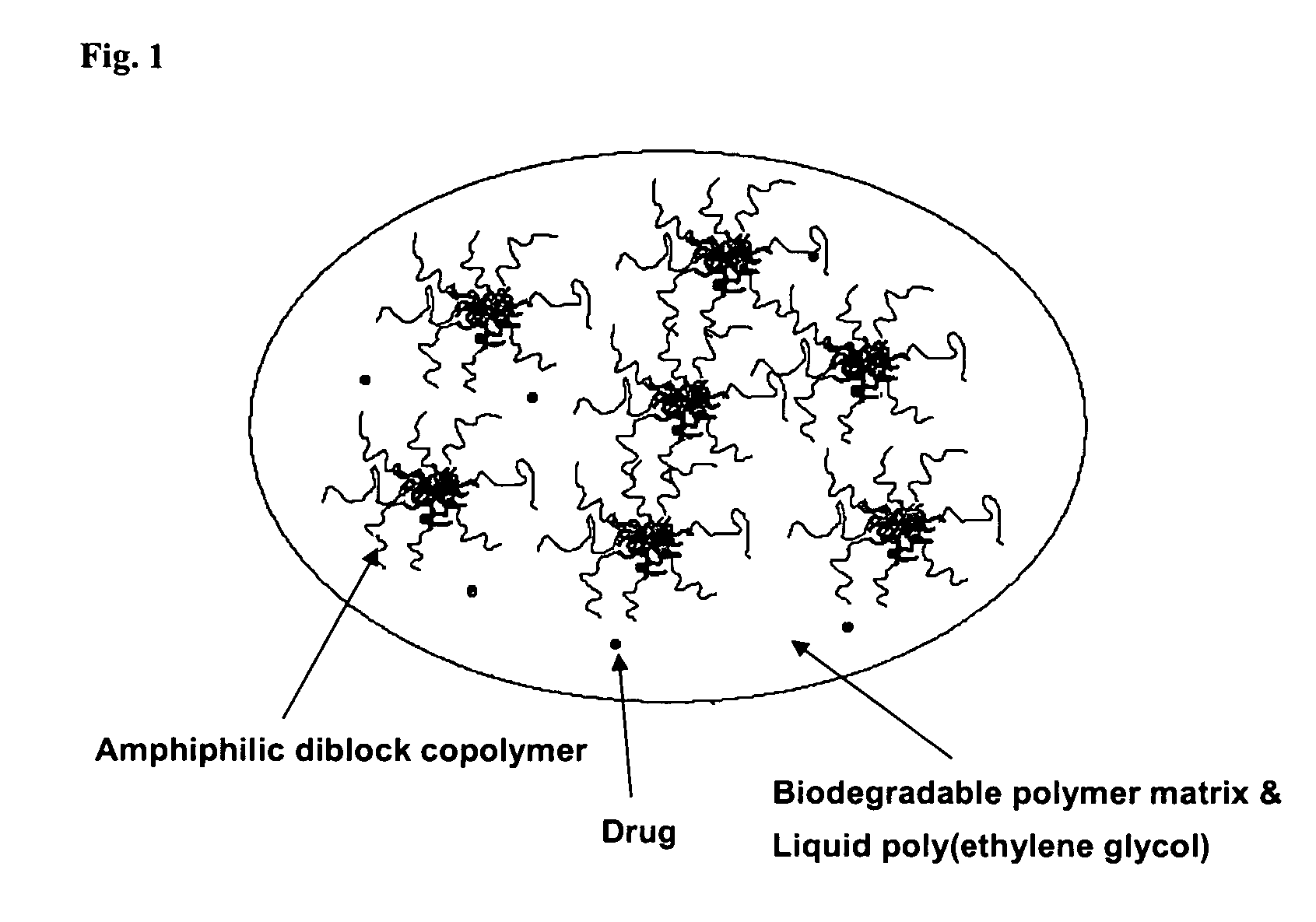
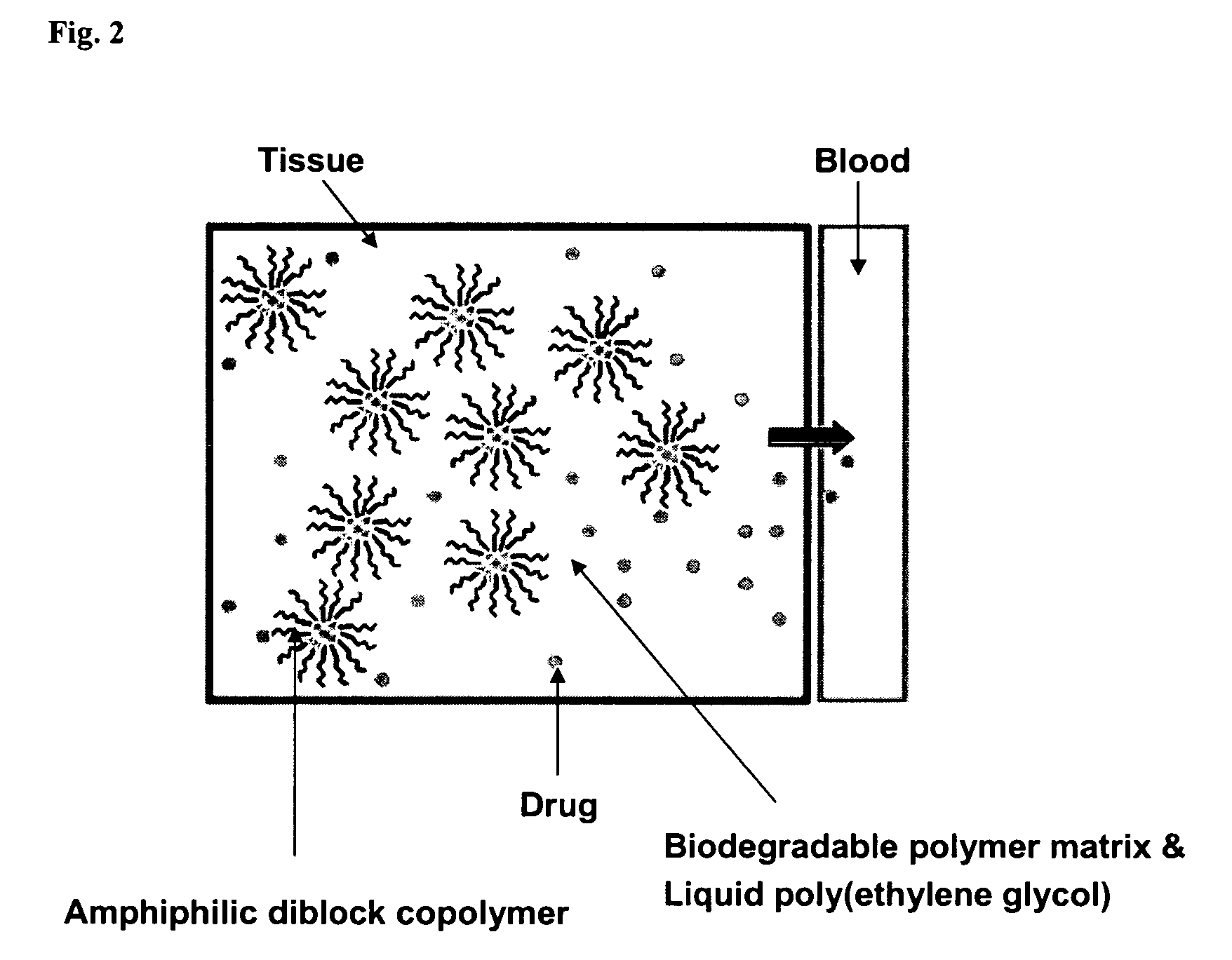
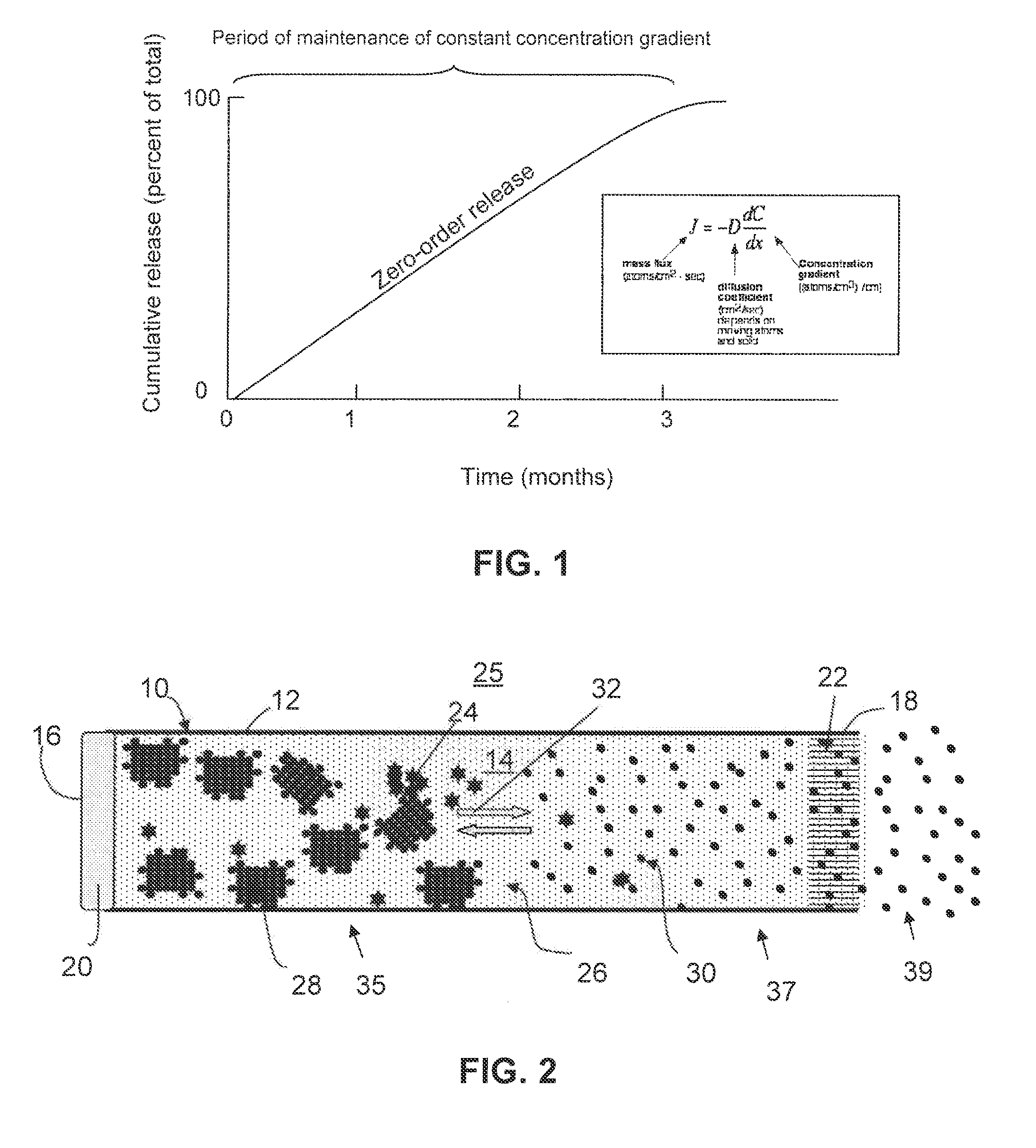
A composition for the sustained delivery of a drug comprising an amphiphilic diblock copolymer; a poorly water-soluble drug; a biodegradable polymer; and liquid poly(ethylene glycol) or functional derivatives thereof and a process for preparing the composition are disclosed. When administered into a particular body site, the composition forms an implant containing the drug and drug containing polymeric micelles, which are slowly released from the implant to maintain a constant drug concentration for an extended period of time.
Owner:SAMYANG HLDG CORP
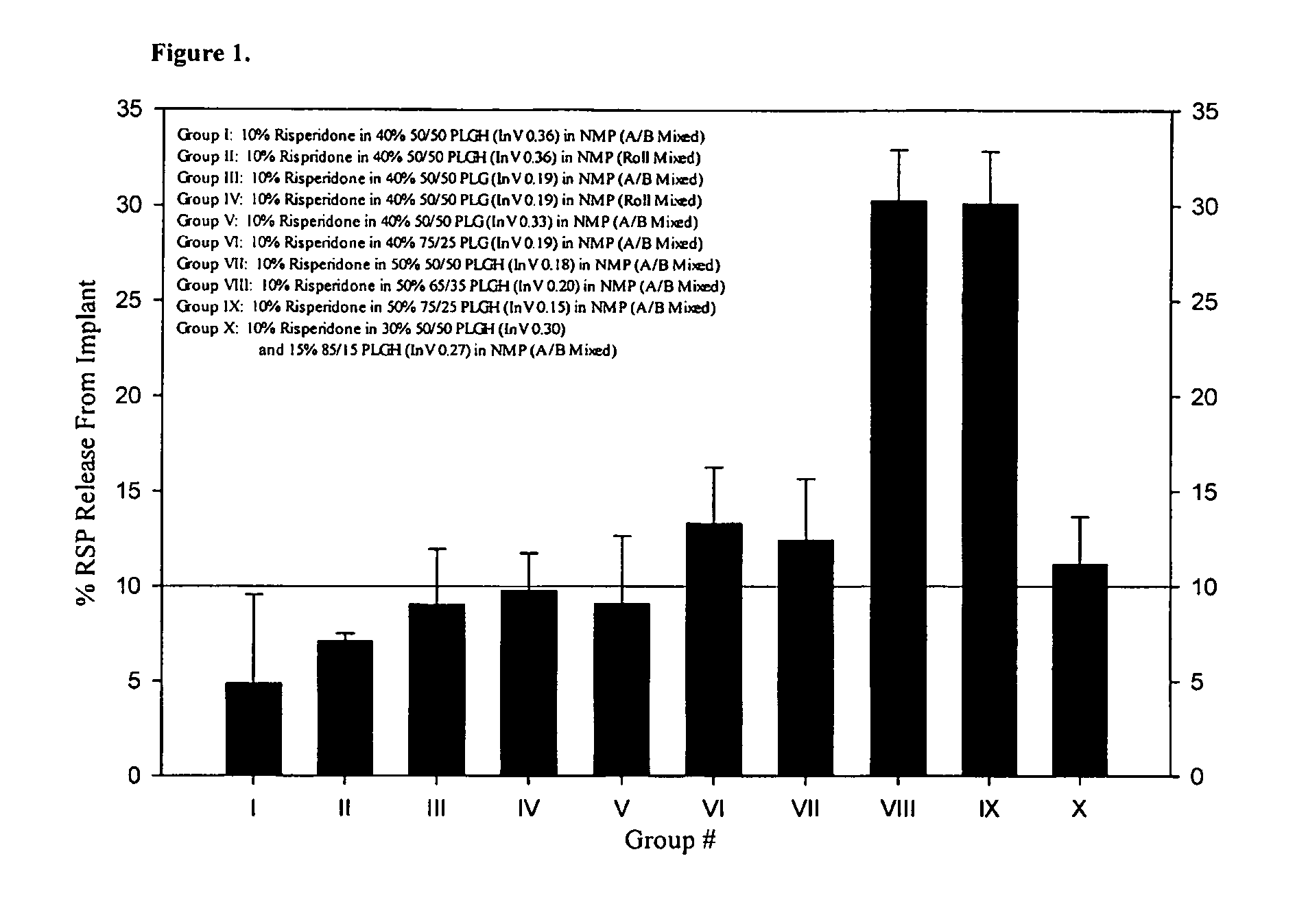
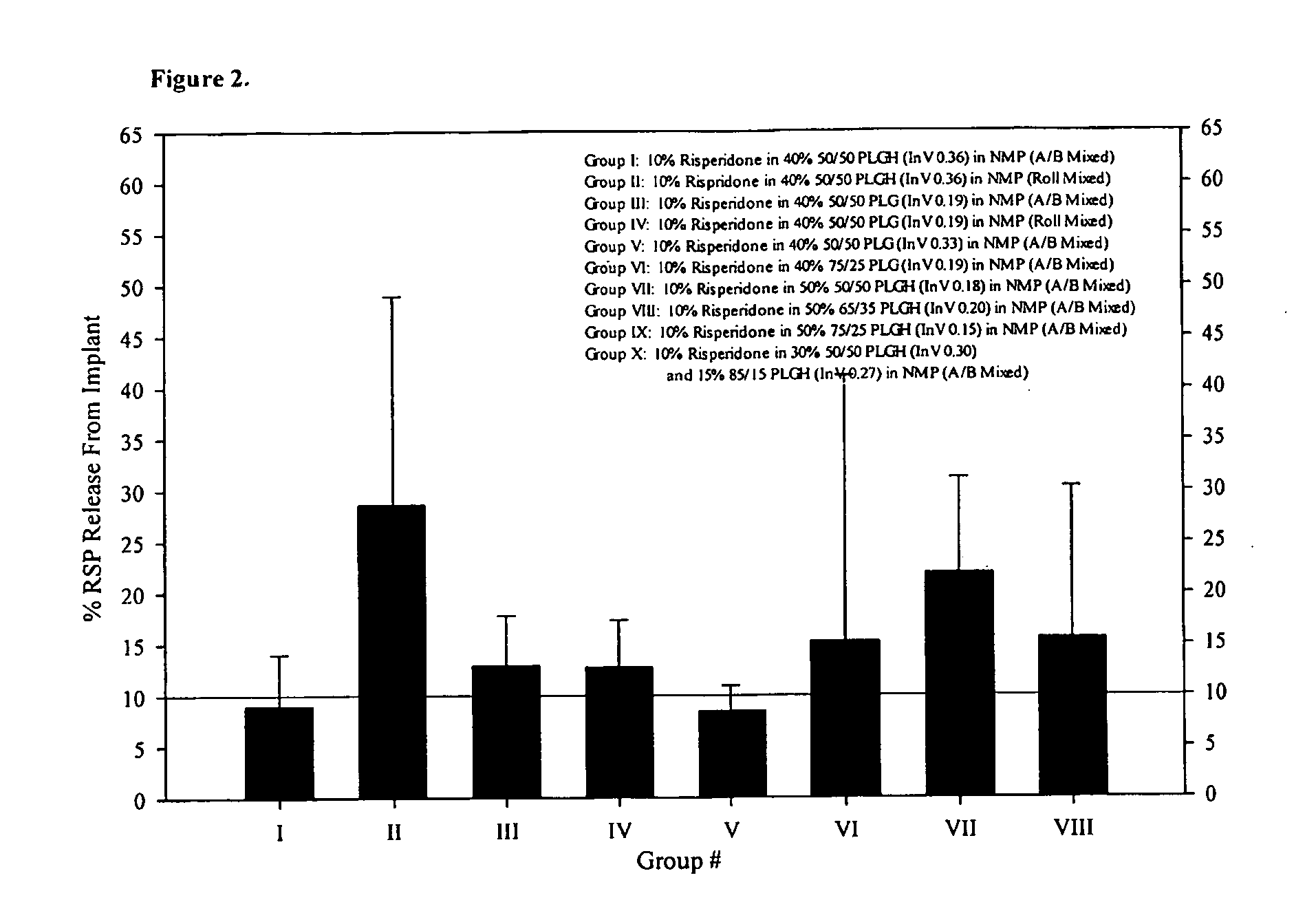
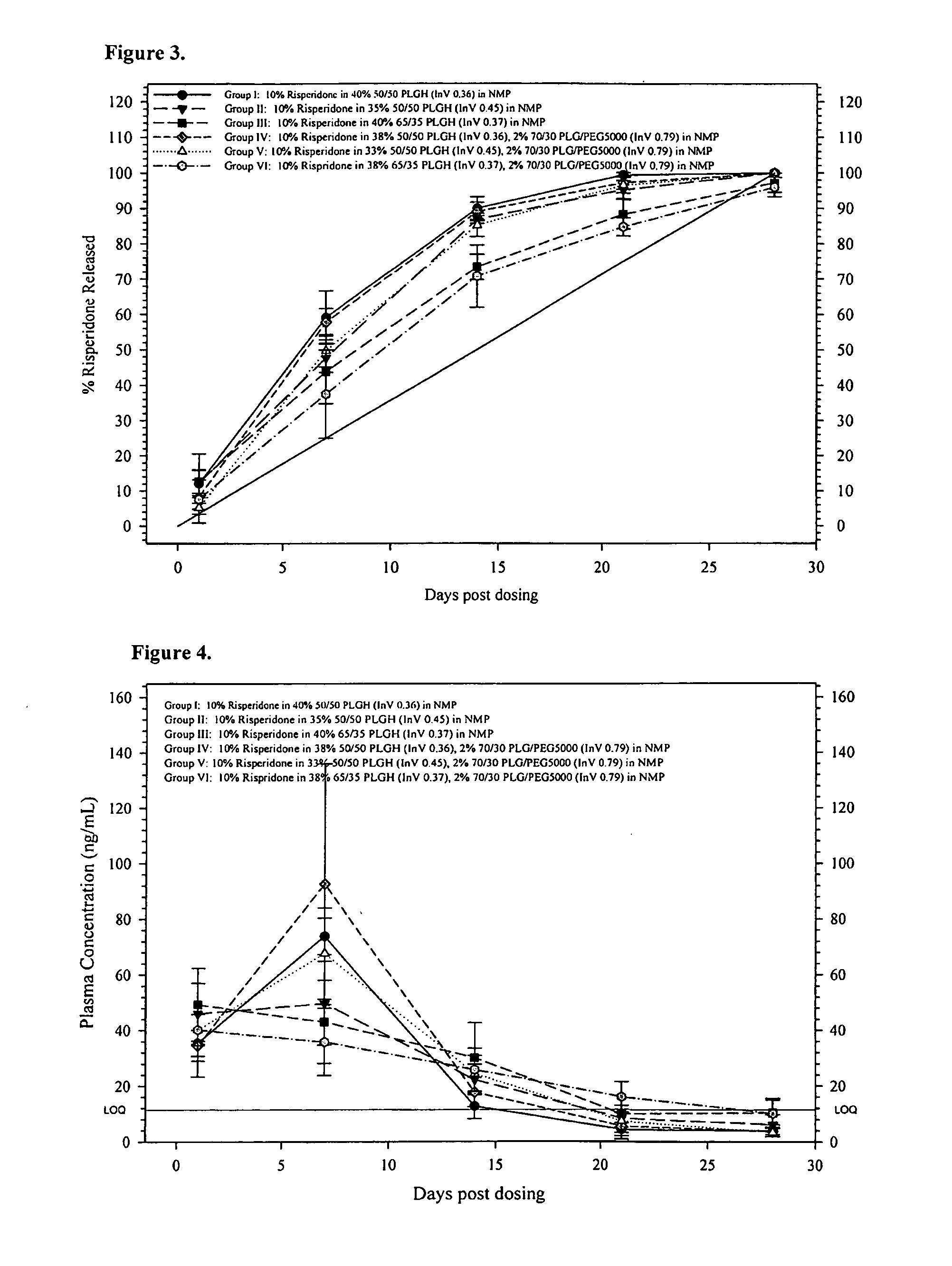
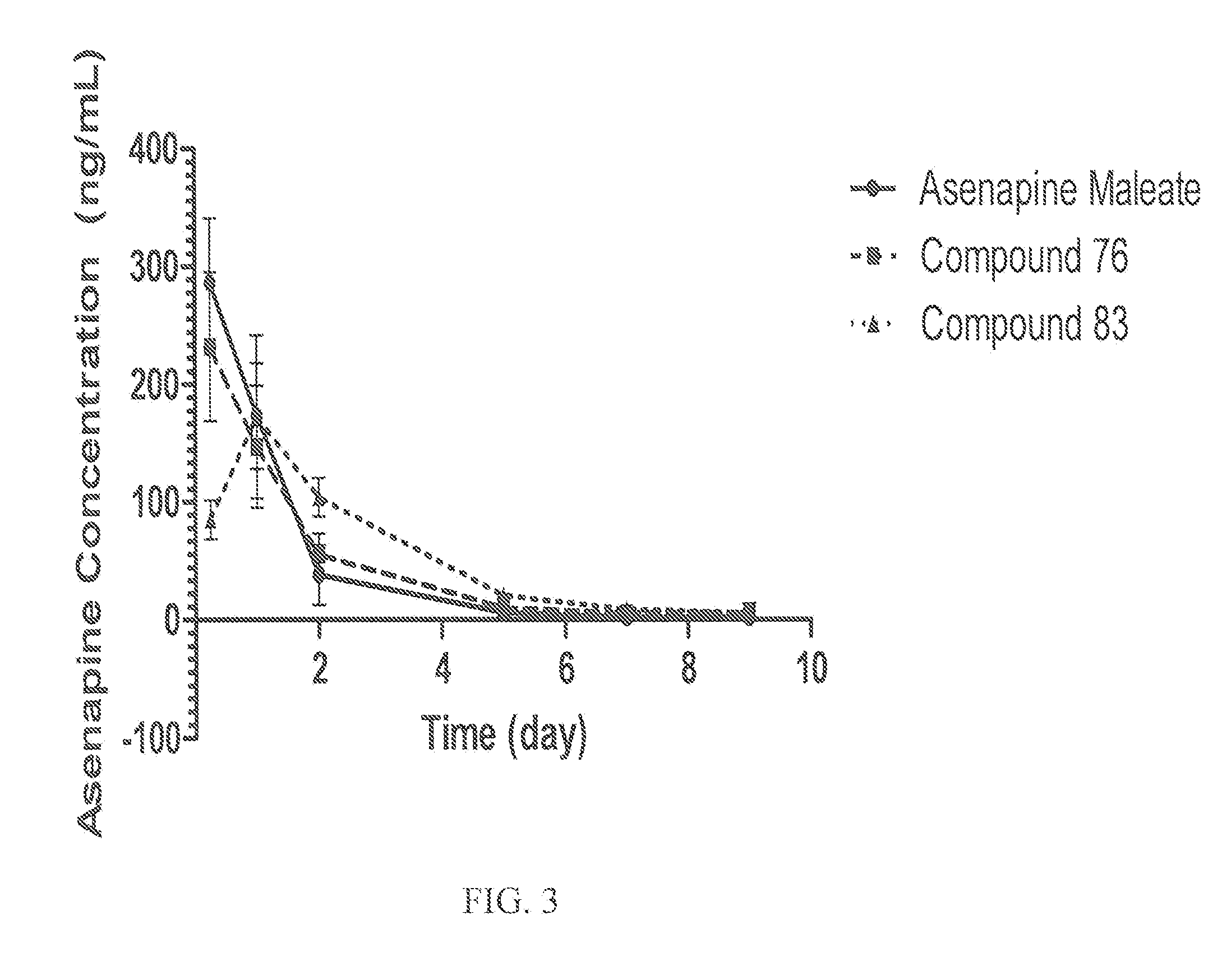
Sustained delivery formulations of risperidone compounds
ActiveUS20100266655A1Improve bioavailabilityLeast riskBiocideOrganic active ingredientsMetaboliteOrganic fluid
The present invention relates to a risperidone sustained release delivery system for treatment of medical conditions relating delusional psychosis, schizophrenia, bipolar disorder, psychotic depression, obsessive-compulsion disorder, Tourette syndrome, and autistic spectrum disorders. The sustained release delivery system includes a flowable composition containing risperidone, a metabolite, or a prodrug thereof and an implant containing risperidone, a metabolite, or a prodrug thereof. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid, and risperidone, a metabolite, or a prodrug thereof.
Owner:INDIVIOR UK
Methods and compositions for sustained delivery of drugs
The present disclosure relates to methods and compositions for the topical sustained delivery of therapeutic agents. Topical application of compositions containing a muscle fasciculating agent result in the sustained release of any therapeutic agent contained within the composition. More particularly, topical application of such compositions to the outer surface of the eyelid of a patient results in increased absorption and sustained release of the therapeutic agent into the eyes or systemically.
Owner:INTRATUS
Surfactant-based gel as an injectable, sustained drug delivery vehicle
The present invention provides methods and compositions for the sustained delivery of beneficial agents. In certain embodiments, the invention provides compositions comprising a surfactant, a solvent, and a beneficial agent, wherein upon exposure to a hydrophilic environment, the surfactant and solvent form a viscous gel and the beneficial agent is dispersed or dissolved in the gel. In other embodiments, the invention provides compositions comprising a surfactant, a solvent, a hydrophilic media, and a beneficial agent, wherein the surfactant, solvent, and hydrophilic media form a viscous gel and the beneficial agent is dispersed or dissolved in the gel.
Owner:DURECT CORP
Implantable device for long-term delivery of drugs
A device for sustained delivery of a poorly water soluble drug is described. A drug reservoir within the device, when in operation, contains an aqueous suspension of the drug mixed with a suspension of an excipient that, in one embodiment, generates acidic groups for a sustained period of time to maintain a desired pH in the aqueous suspension that in turn provides a constant concentration of a soluble form of the drug.
Owner:DELPOR
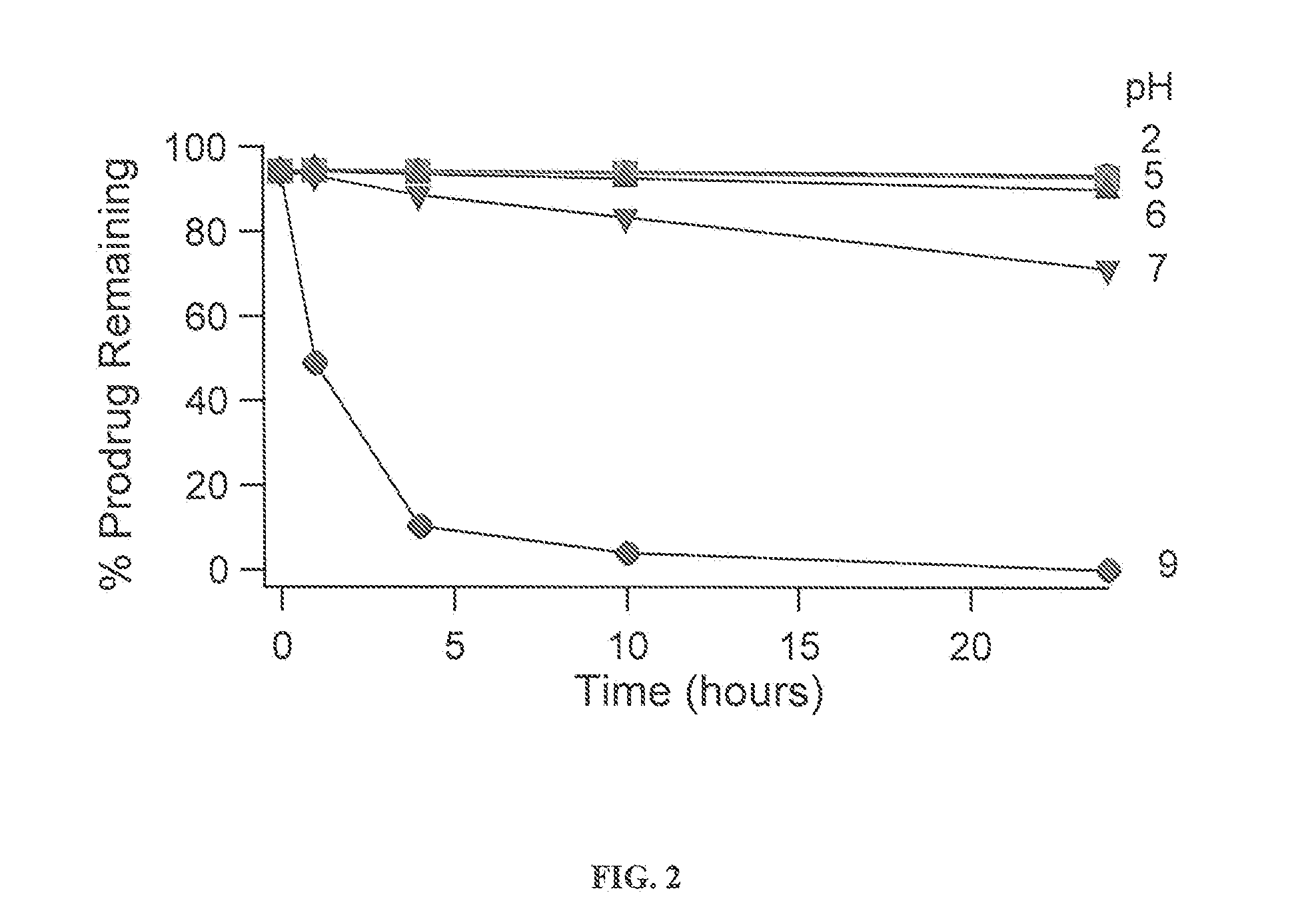
Prodrugs of nh-acidic compounds: ester, carbonate, carbamate and phosphonate derivatives
ActiveUS20110319422A1Long duration of actionReduce polarityOrganic active ingredientsNervous disorderImideCarbamate
The invention provides a method of sustained delivery of a lactam, imide, amide, sulfonamide, carbamate or urea containing parent drug by administering to a patient an effective amount of a prodrug compound of the invention wherein upon administration to the patient, release of the parent drug from the prodrug is sustained release. Prodrug compounds suitable for use in the methods of the invention are labile conjugates of parent drugs that are derivatized through carbonyl linked prodrug moieties. The prodrug compounds of the invention can be used to treat any condition for which the lactam, imide, amide, sulfonamide, carbamate or urea containing parent drug is useful as a treatment.
Owner:ALKERMES PHARMA IRELAND LTD
Quaternary Ammonium Salt Prodrugs
ActiveUS20110178068A1Long duration of actionReduce solubilityBiocideNervous disorderPharmaceutical drugPerylene derivatives
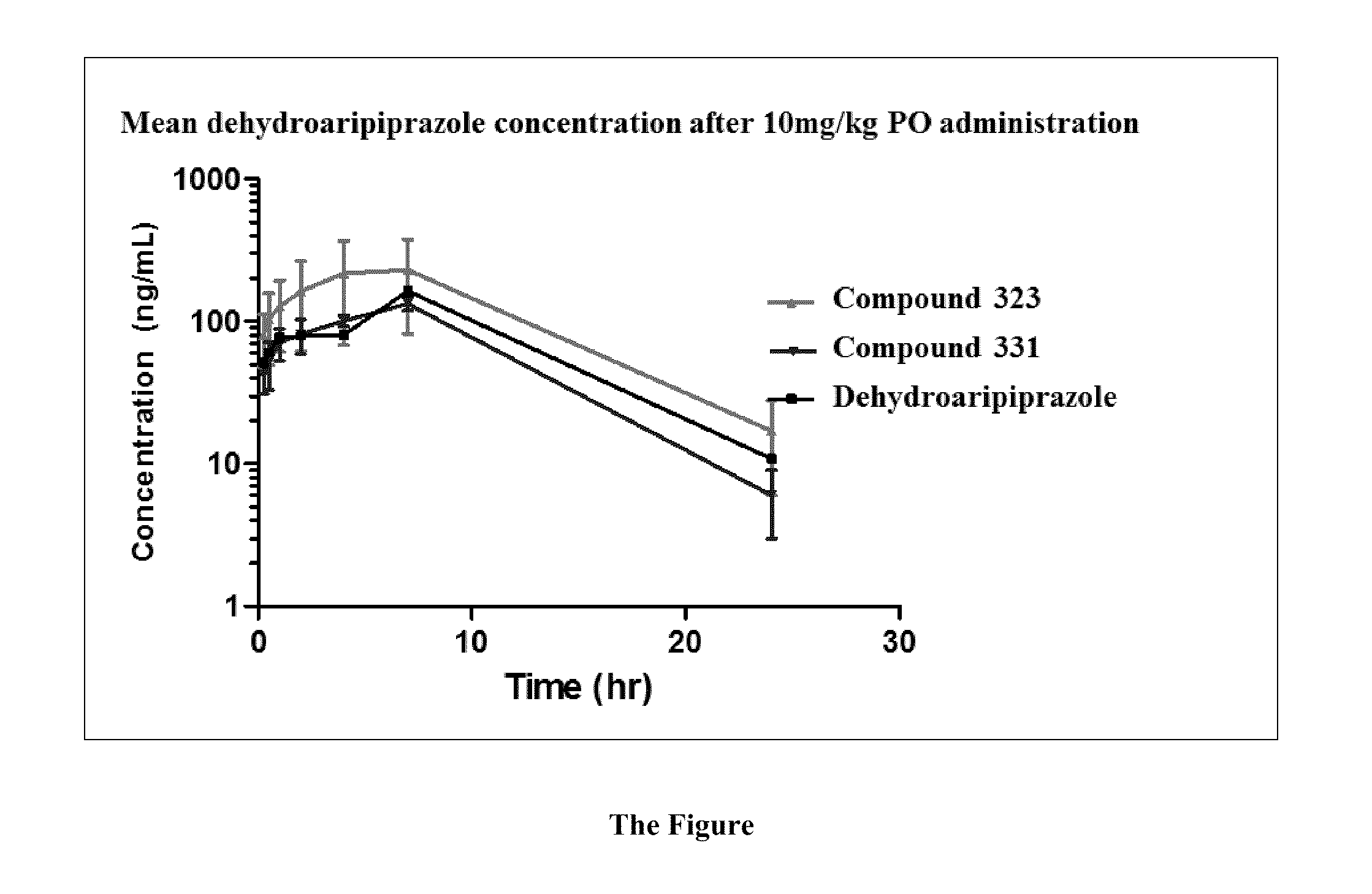
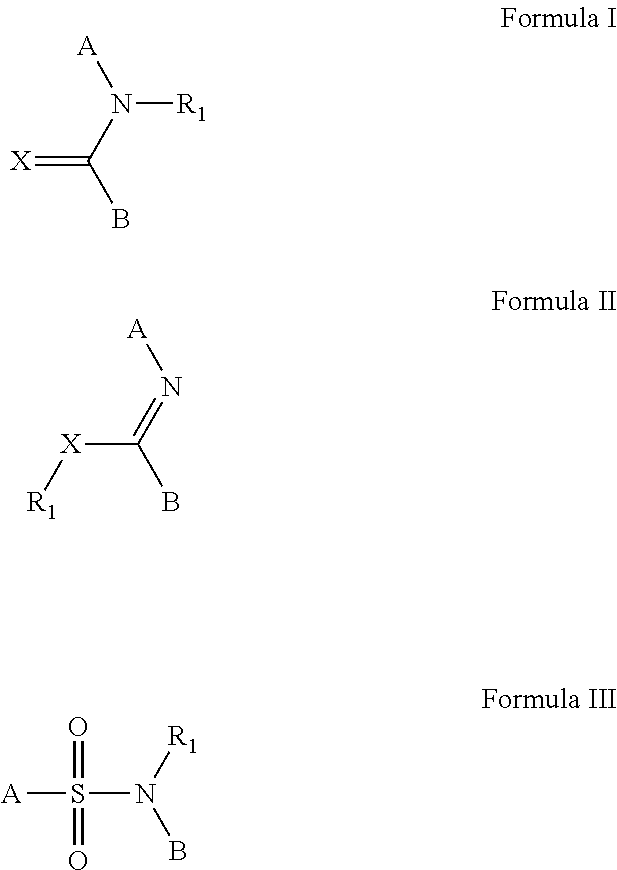
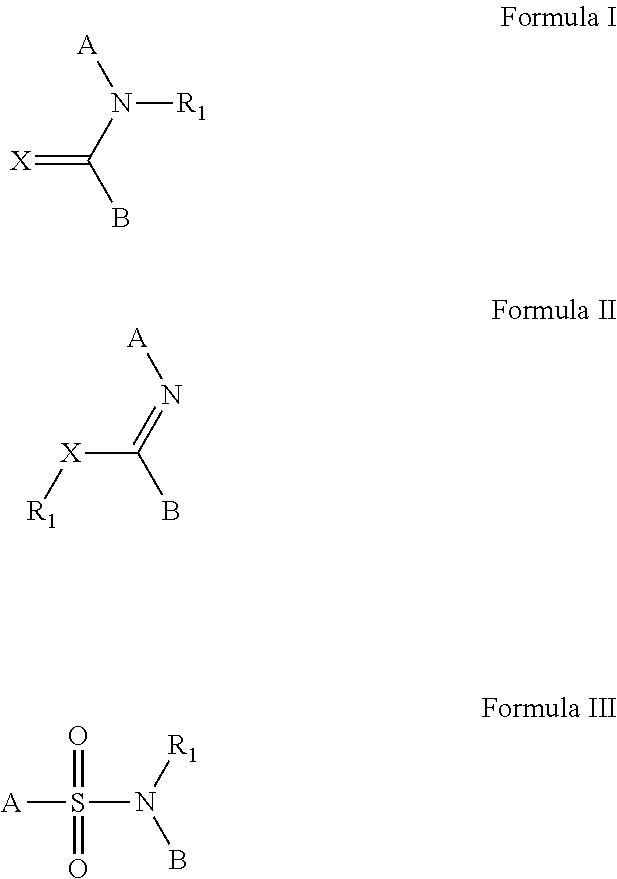
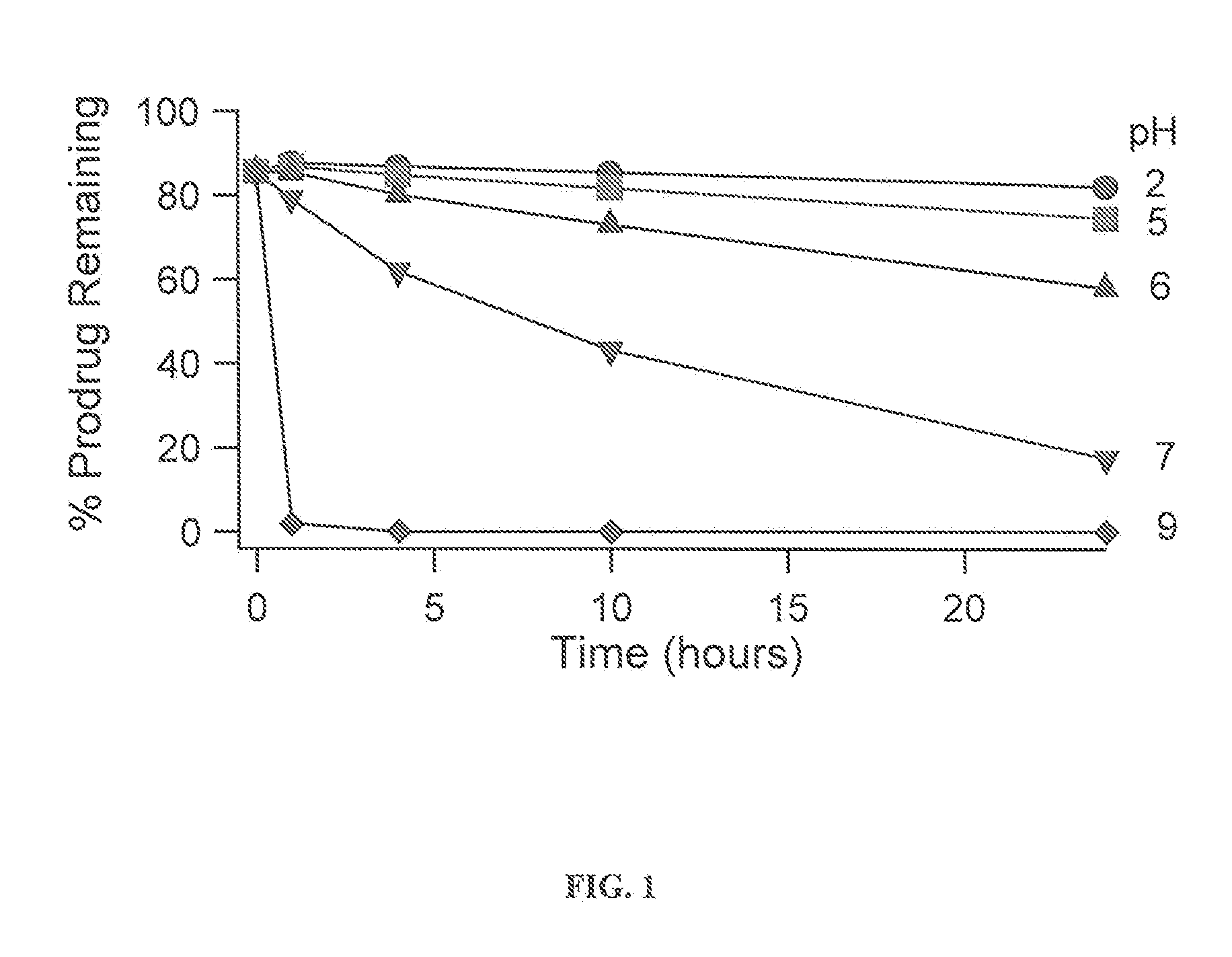
The invention provides a method of sustained delivery of a tertiary amine-containing parent drug comprising administering to a patient an effective amount of a prodrug compound of the invention wherein upon administration to the patient, release of the parent drug from the prodrug is sustained release. Prodrug compounds suitable for use in the methods of the invention are labile quaternary ammonium salts of tertiary amine-containing parent drugs (or tertiary imine-containing parent drugs) that are derivatized through aldehyde-linked prodrug moieties that reduce the solubility of the prodrug compound at a reference pH as compared to the parent drug. The physical, chemical and solubility properties of these derivatives can be further modulated by the choice of counterion X−. In one embodiment, the present invention provides a prodrug compound of Formula I:where R1-R5 are defined in the written description of the invention. The prodrug compounds of the invention can be used to treat any condition for which the tertiary amine-containing parent drug or tertiary imine-containing parent drug is useful as a treatment.
Owner:ALKERMES PHARMA IRELAND LTD
Prostate hypertrophy treatment composition and method
InactiveUS20050271597A1Improve permeabilityHigh permeation rate prevents undesirable modifications of the hormone within the skinOrganic active ingredientsPowder deliveryPhysiologyProgesterones
The present invention provides a method and composition for treatment of benign prostate hyperplasia (BPH) in men via a transscrotal delivery system. The composition of the present invention includes the steroid hormone progesterone containing permeation enhancers that greatly facilitate permeation through the skin, thus preventing modification of the constituents therein and providing continuous and sustained delivery of progesterone for several hours that mimics the circadian rhythm of endogenous progesterone. The progesterone composition preferably is capable of delivering an effective dosage amount of about 65-100 mg of progesterone per ml when applied directly onto the surface of scrotum.
Owner:KEITH ALEC D
Sustained delivery of an active agent using an implantable system
InactiveUS7655257B2Improve sealingPowder deliveryPeptide/protein ingredientsActive agentBiomedical engineering
The invention is directed to a device for delivering an active agent formulation for a predetermined administration period. An impermeable reservoir is divided into a water-swellable agent chamber and an active agent formulation chamber. Fluid from the environment is imbibed through a semipermeable plug into the water-swellable agent chamber and the active agent formulation is released through a back-diffusion regulating outlet. Delivery periods of up to 2 years are achieved.
Owner:INTARCIA THERAPEUTICS INC
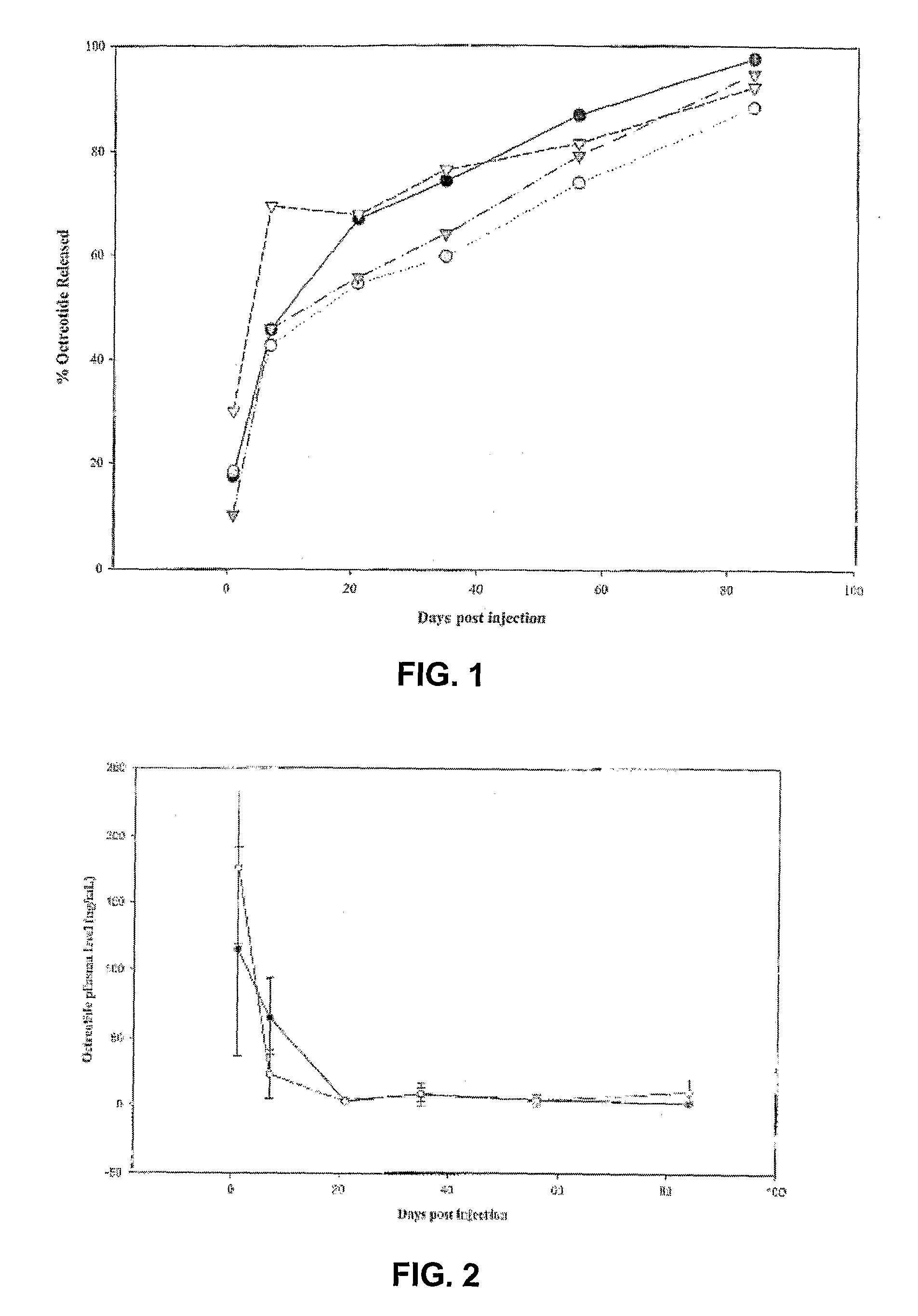
Sustained Delivery Formulations of Octreotide Compounds
InactiveUS20090092650A1Improve bioavailabilityLeast riskSenses disorderPeptide/protein ingredientsMedicineOrganic liquids
The present invention relates to an octreotide sustained release delivery system for treatment of diseases relating to somatotropin and / or somatostatin. The sustained release delivery system of the invention includes a flowable composition containing an octreotide compound, and an implant containing the octreotide compound. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid and an octreotide compound.
Owner:QLT USA INC
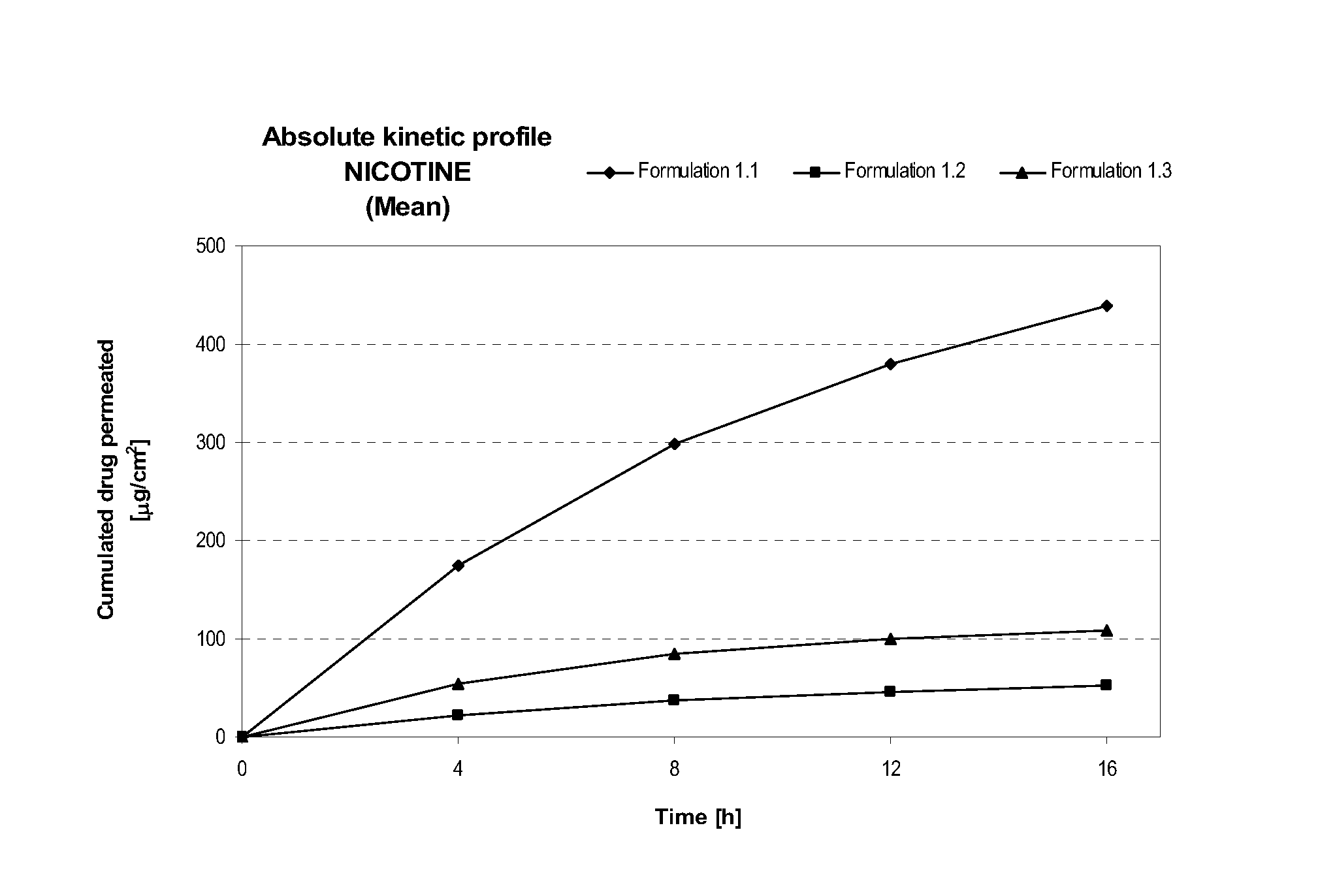
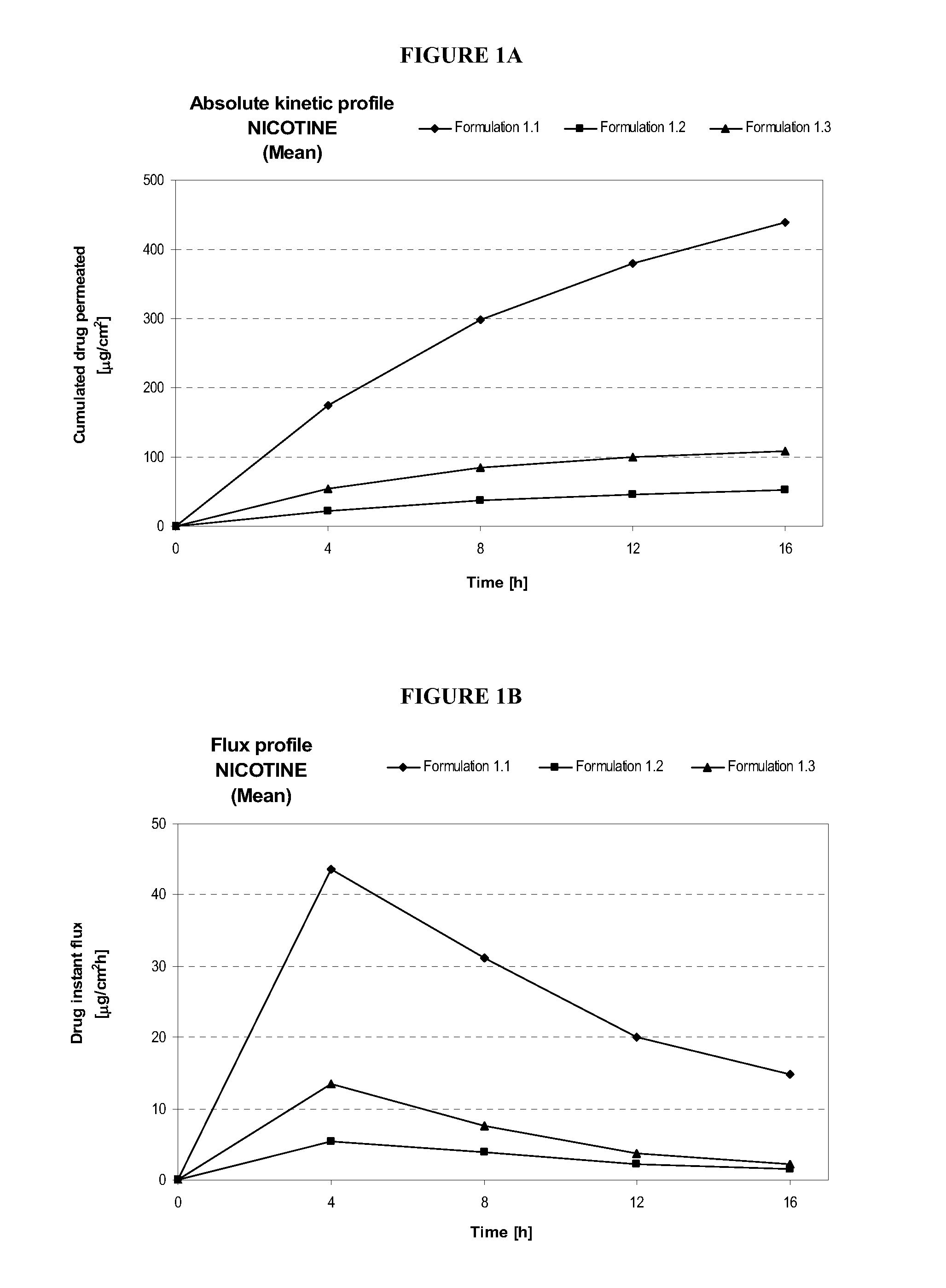
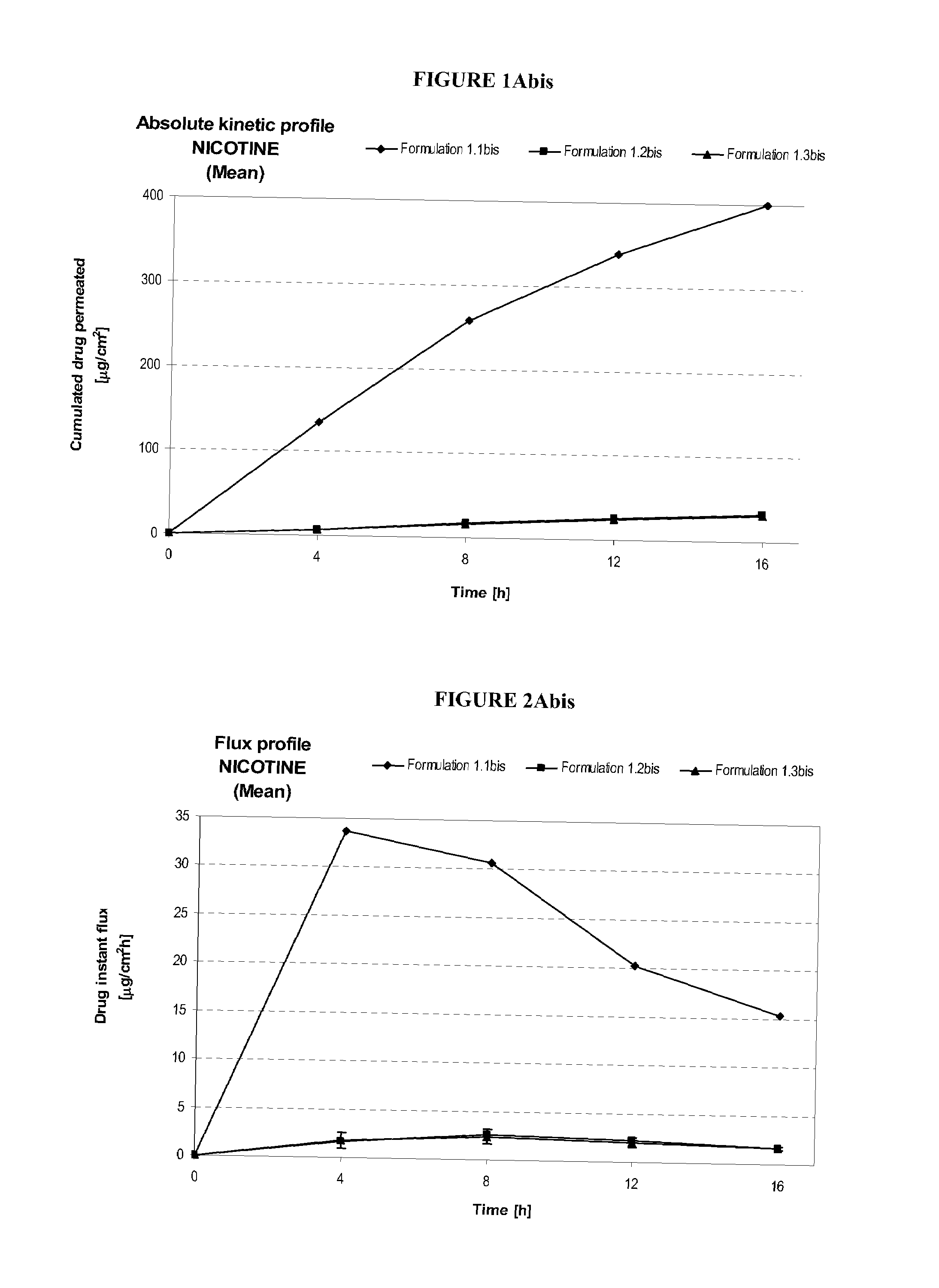
Pharmaceutical compositions of nicotine and methods of use thereof
The present invention comprises non occlusive compositions for transdermal delivery of nicotine, and more particularly pharmaceutically acceptable salts thereof, and methods of making same. The composition may, for example, be a gel suitable for transdermal or transmucosal applications. The compositions of the present invention typically comprise a mixture of water and alcohol, and a solvent system having a mono alkyl ether of diethylene glycol and a glycol present in specified ratios and in specific amounts, wherein the pH of the gel is usually between a pH of 5.5 and 7. The compositions may include further components, for example, the hydroalcoholic vehicle may further comprise additional penetration enhancer(s), buffering agent(s), antioxidant(s), stabilizer(s) and / or gelling agent(s). The invention also relates to a method for the sustained delivery of nicotine pharmaceutically acceptable salts to treat a variety of conditions and disorders.
Owner:ANTARES PHARMA IPL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com