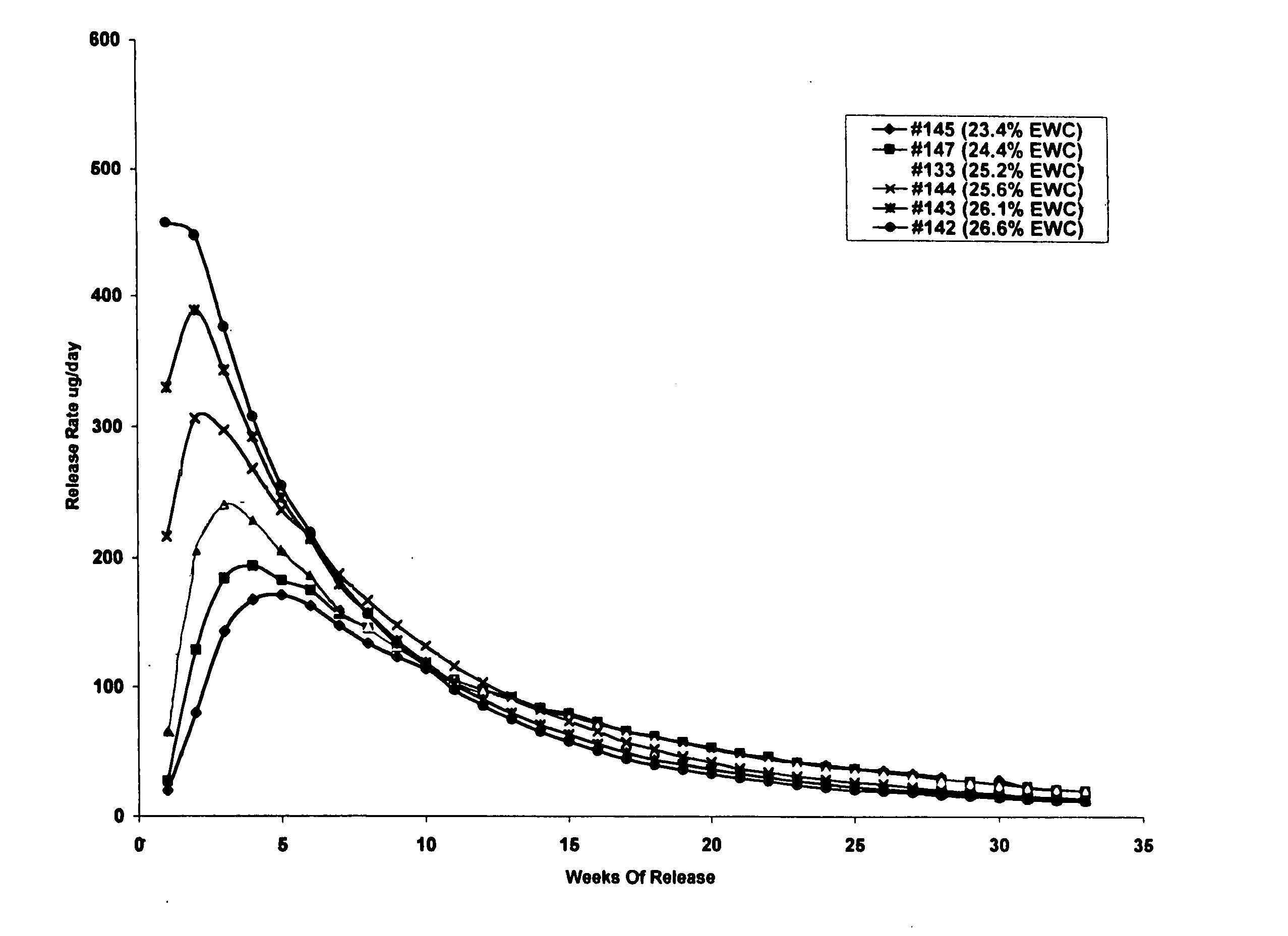
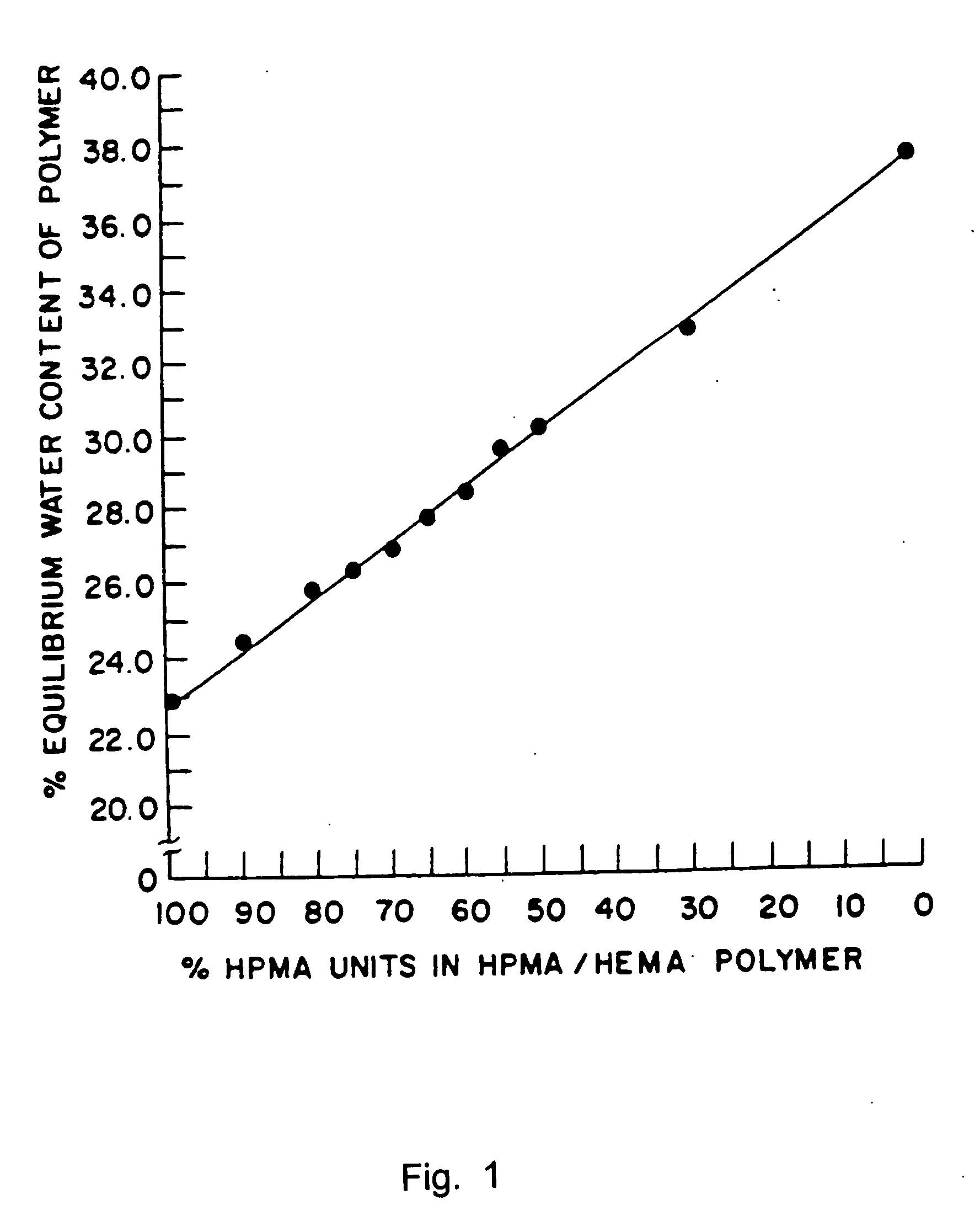
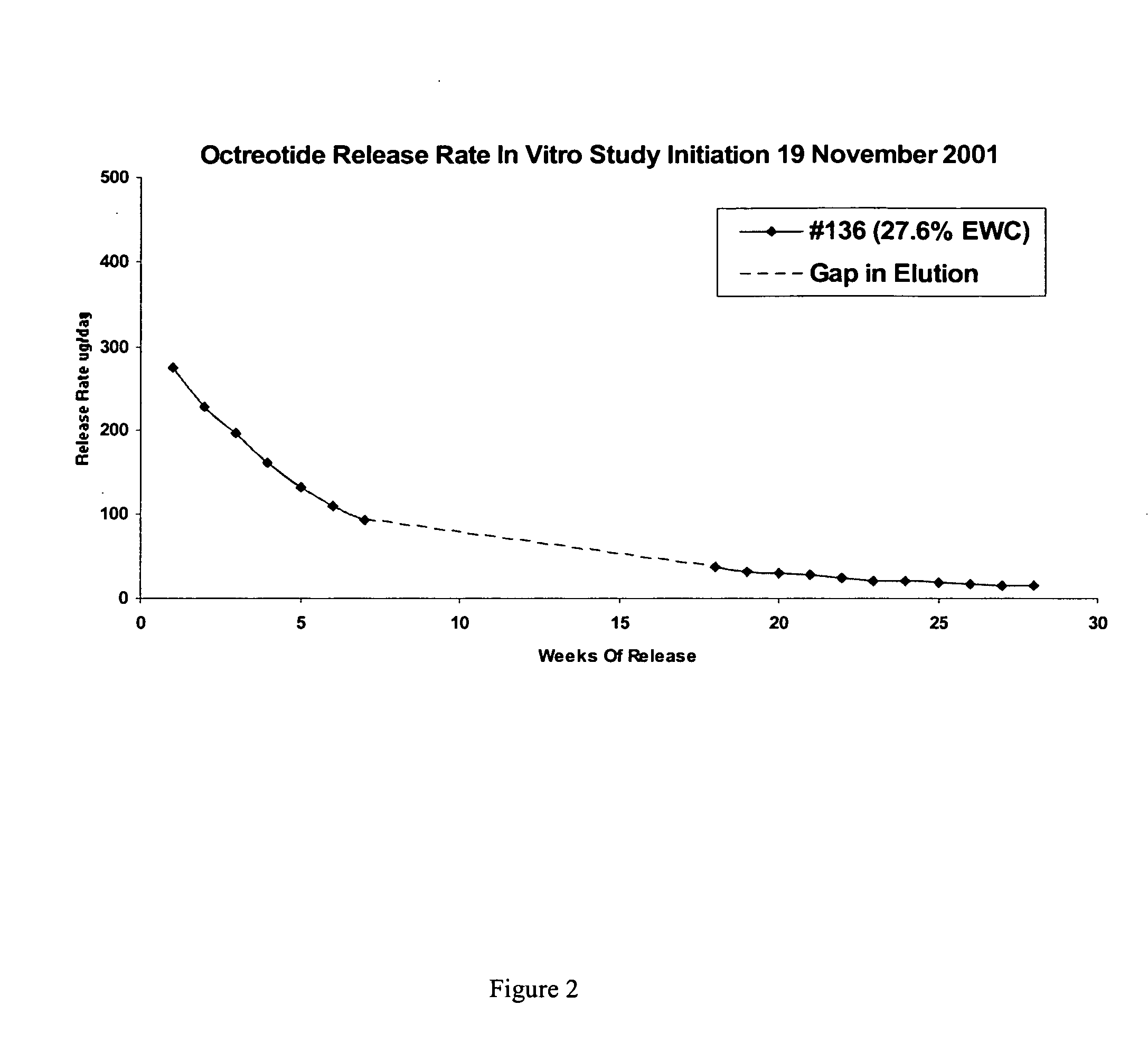
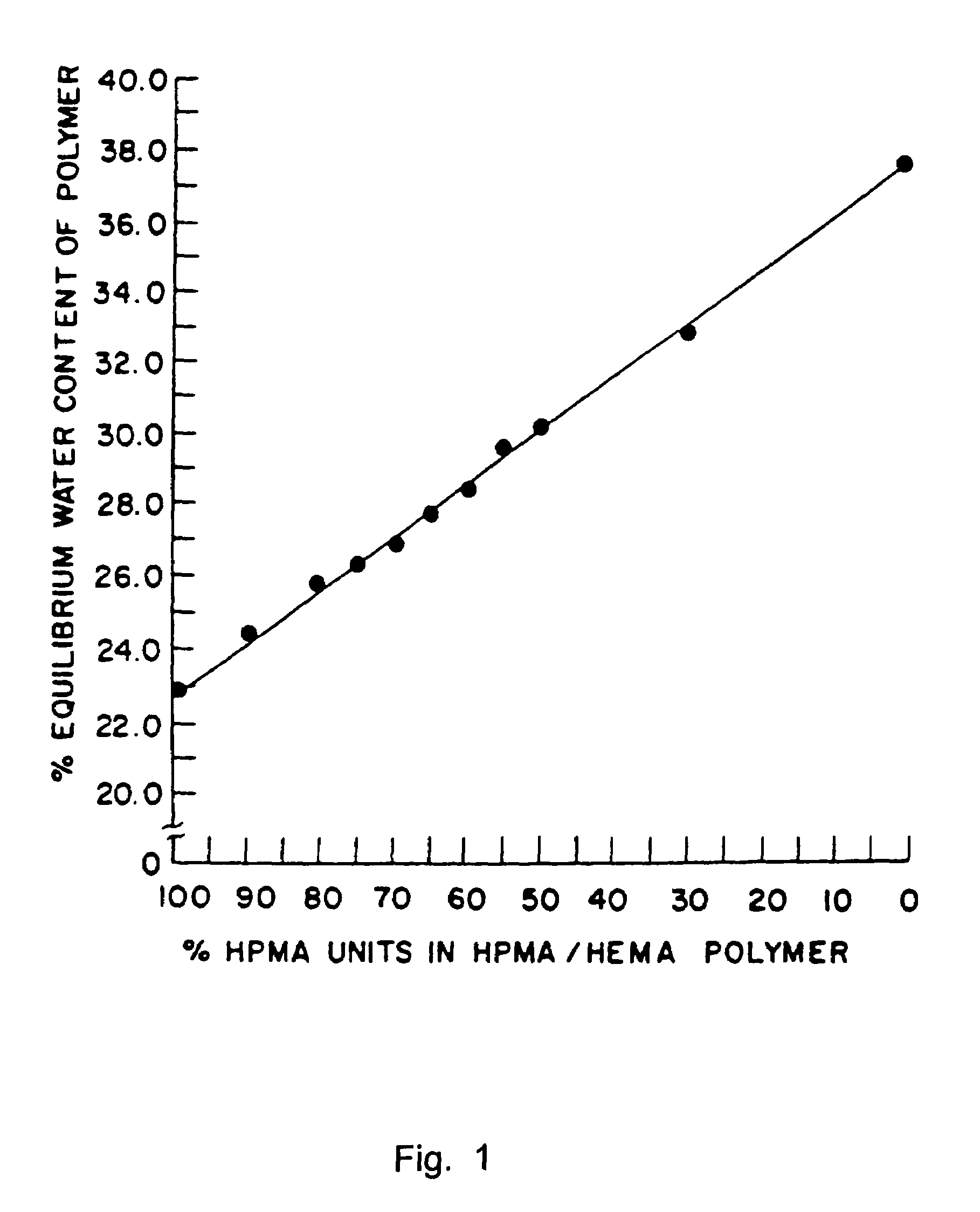
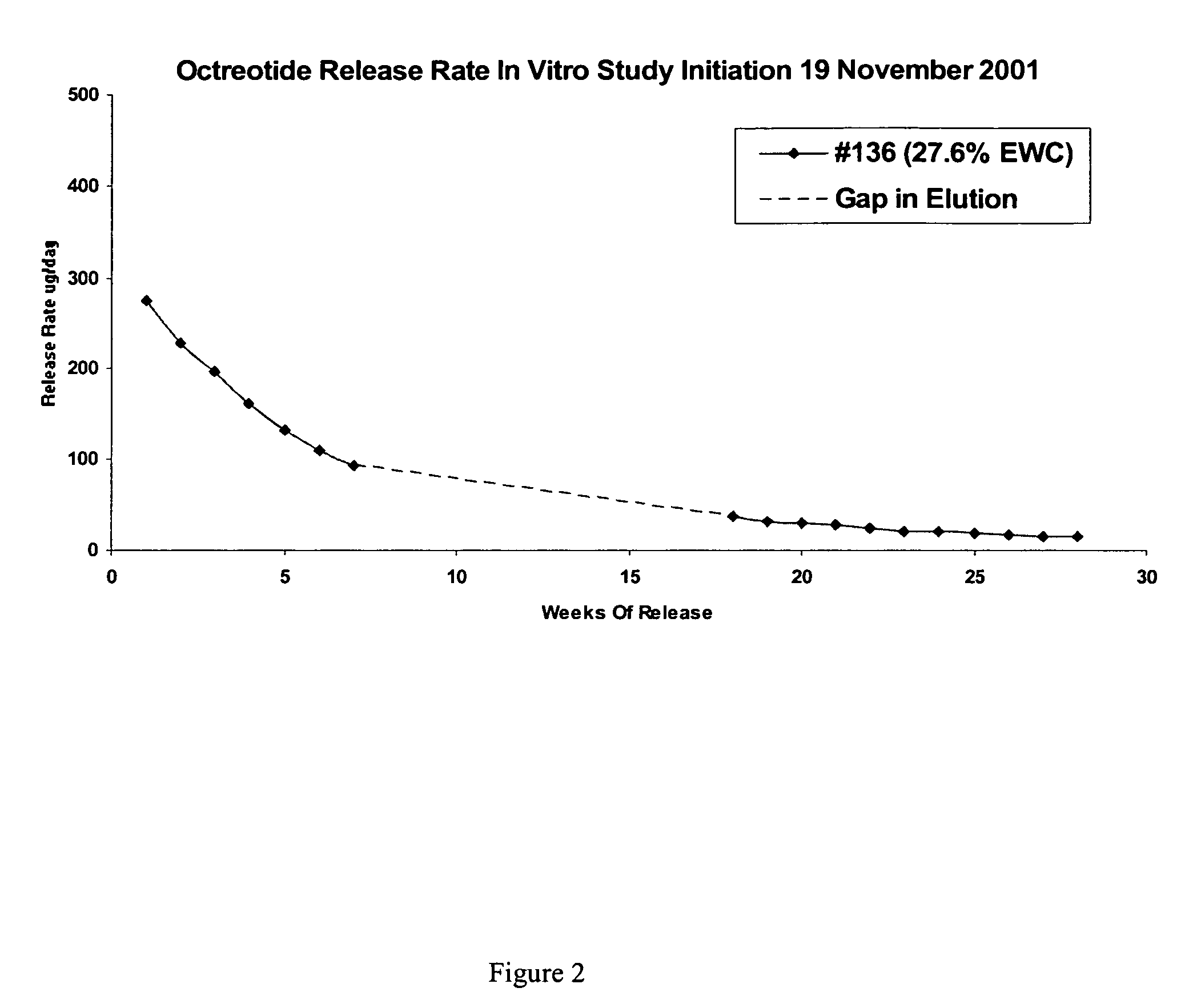
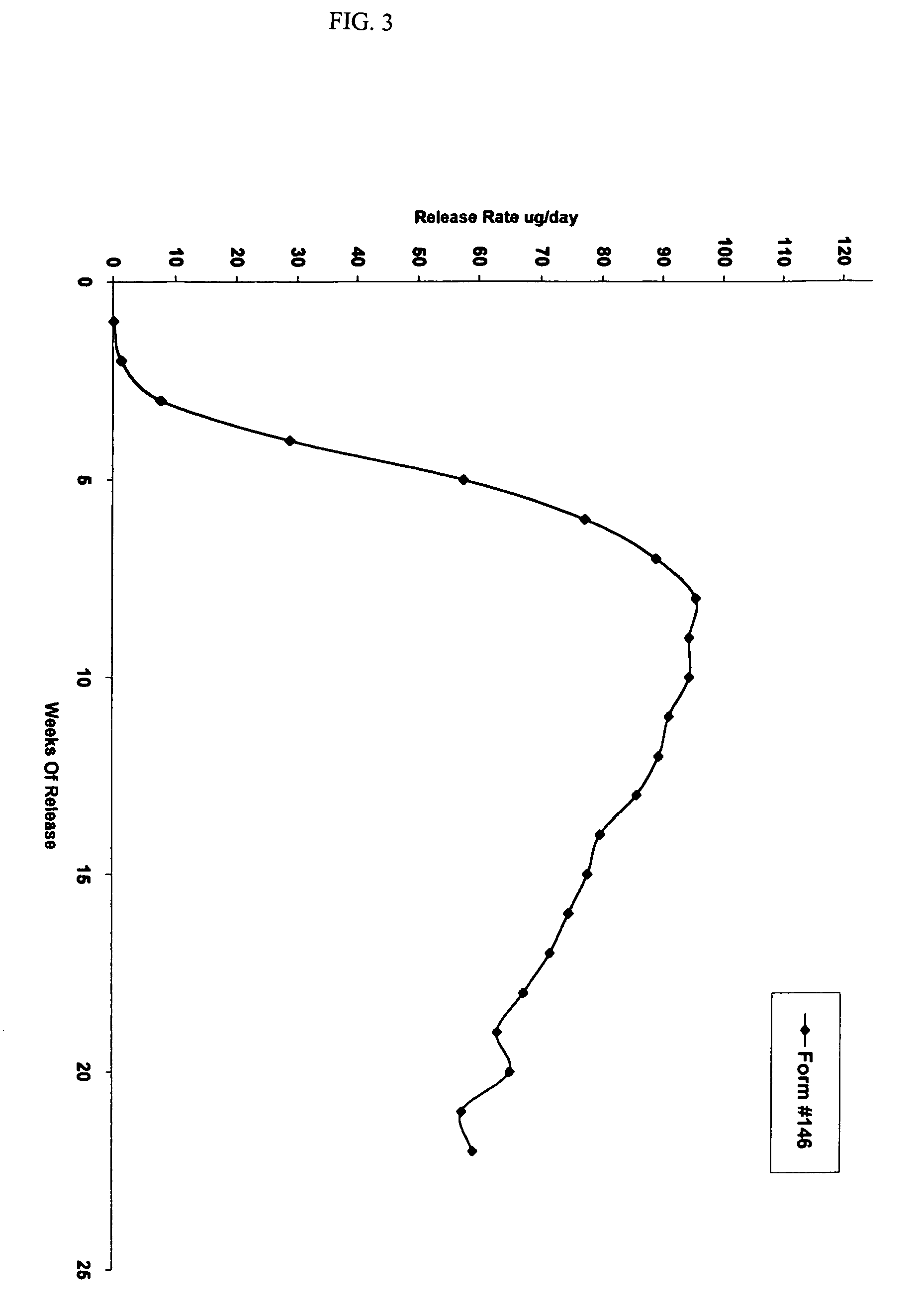
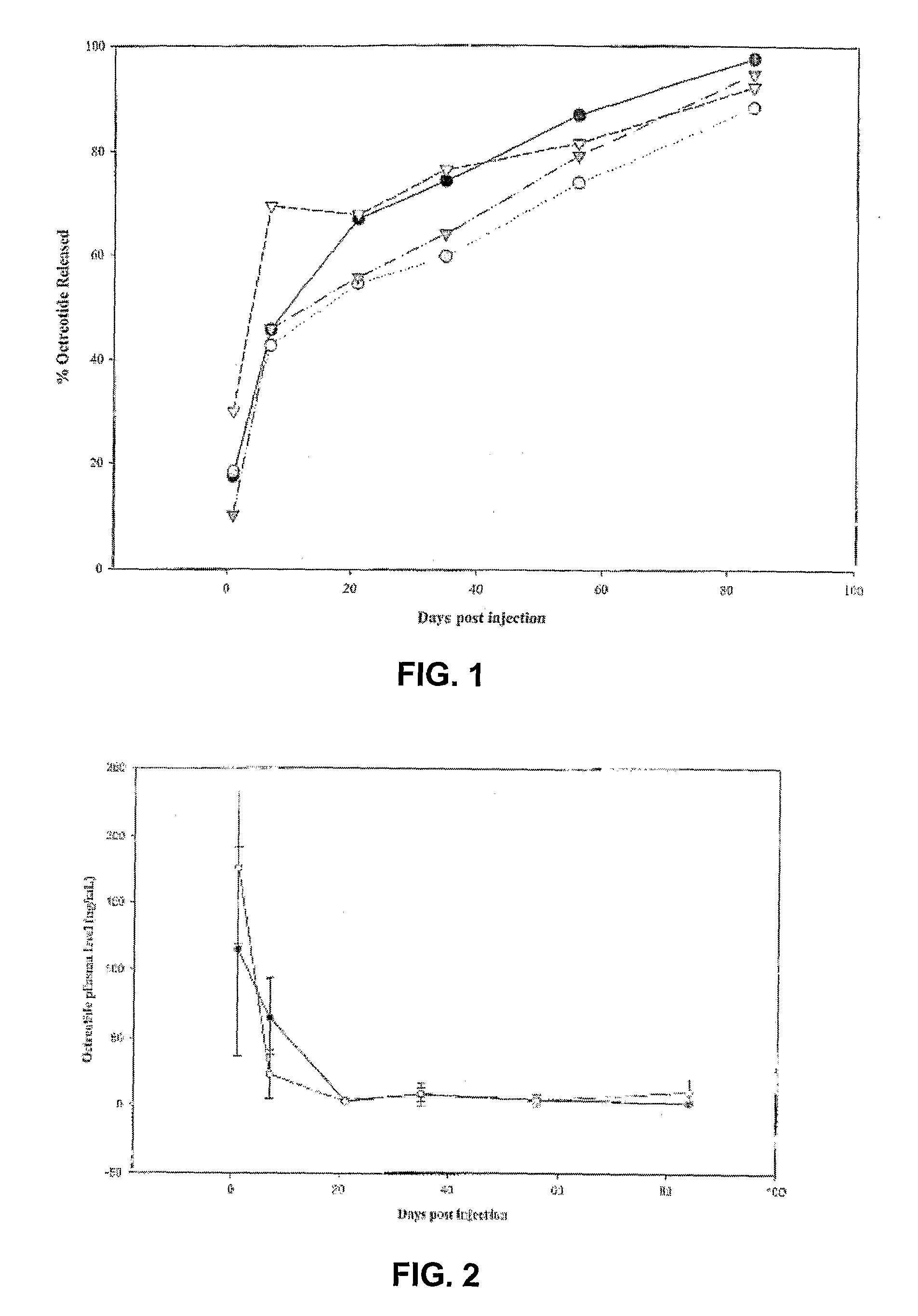
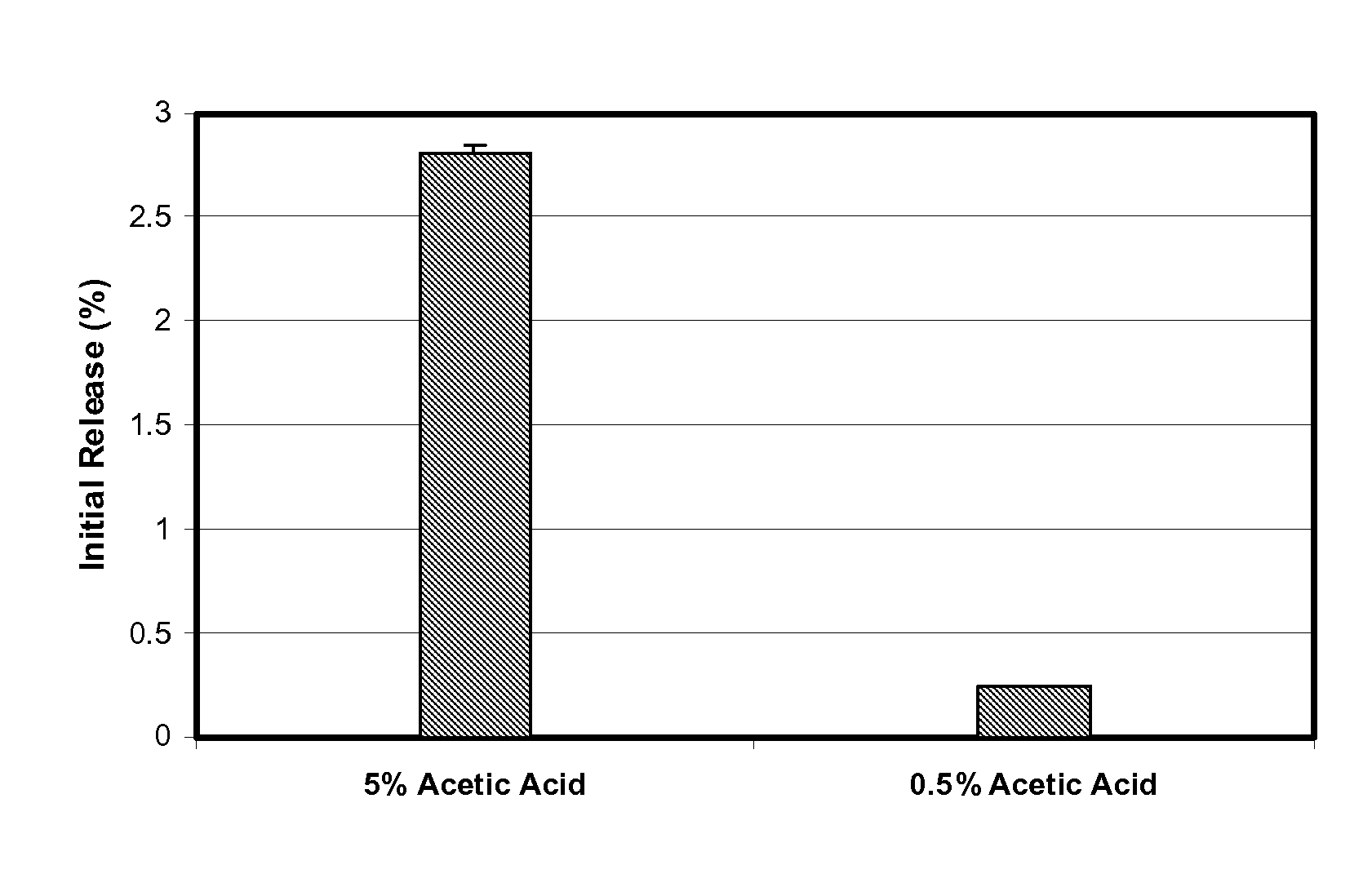
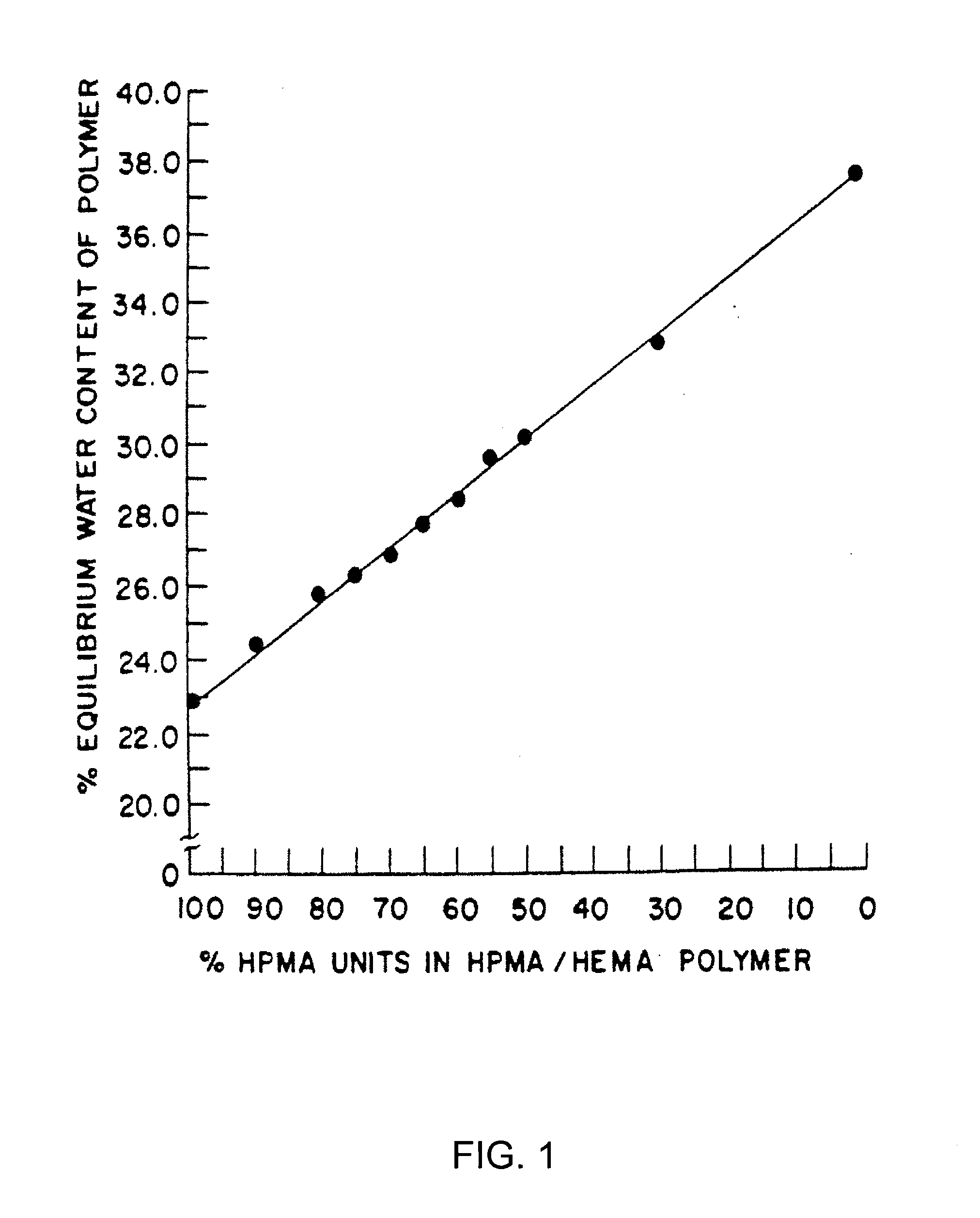
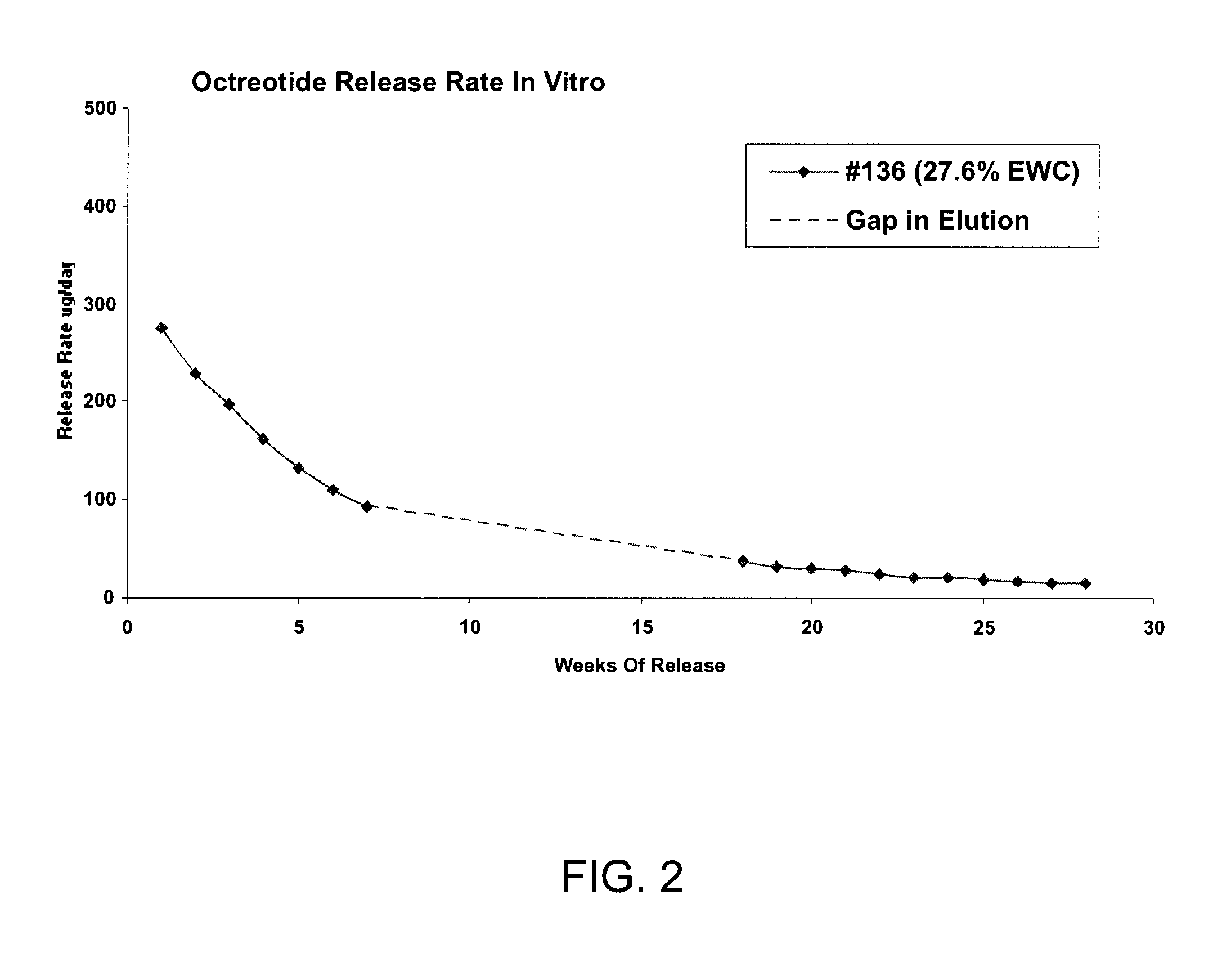
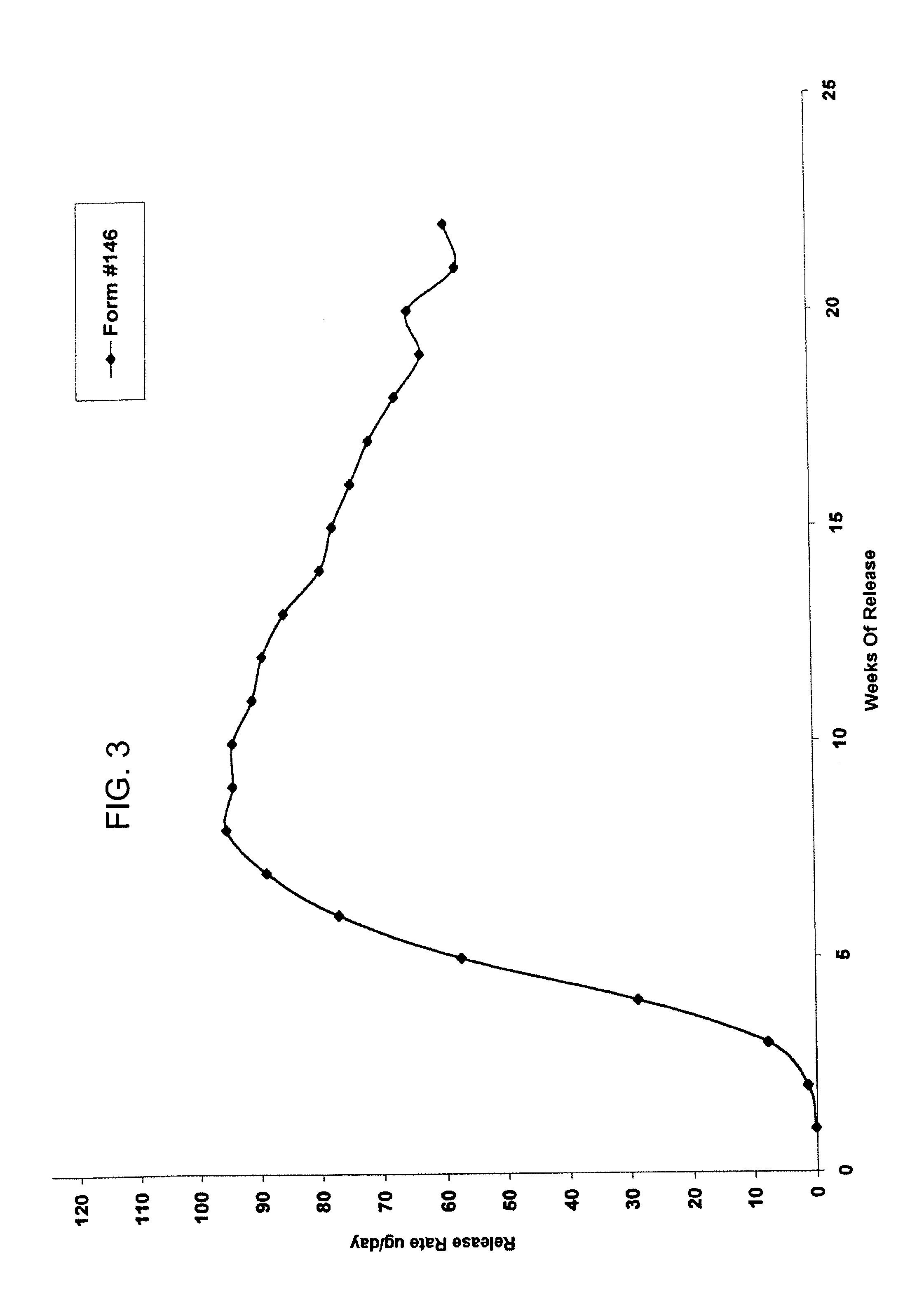
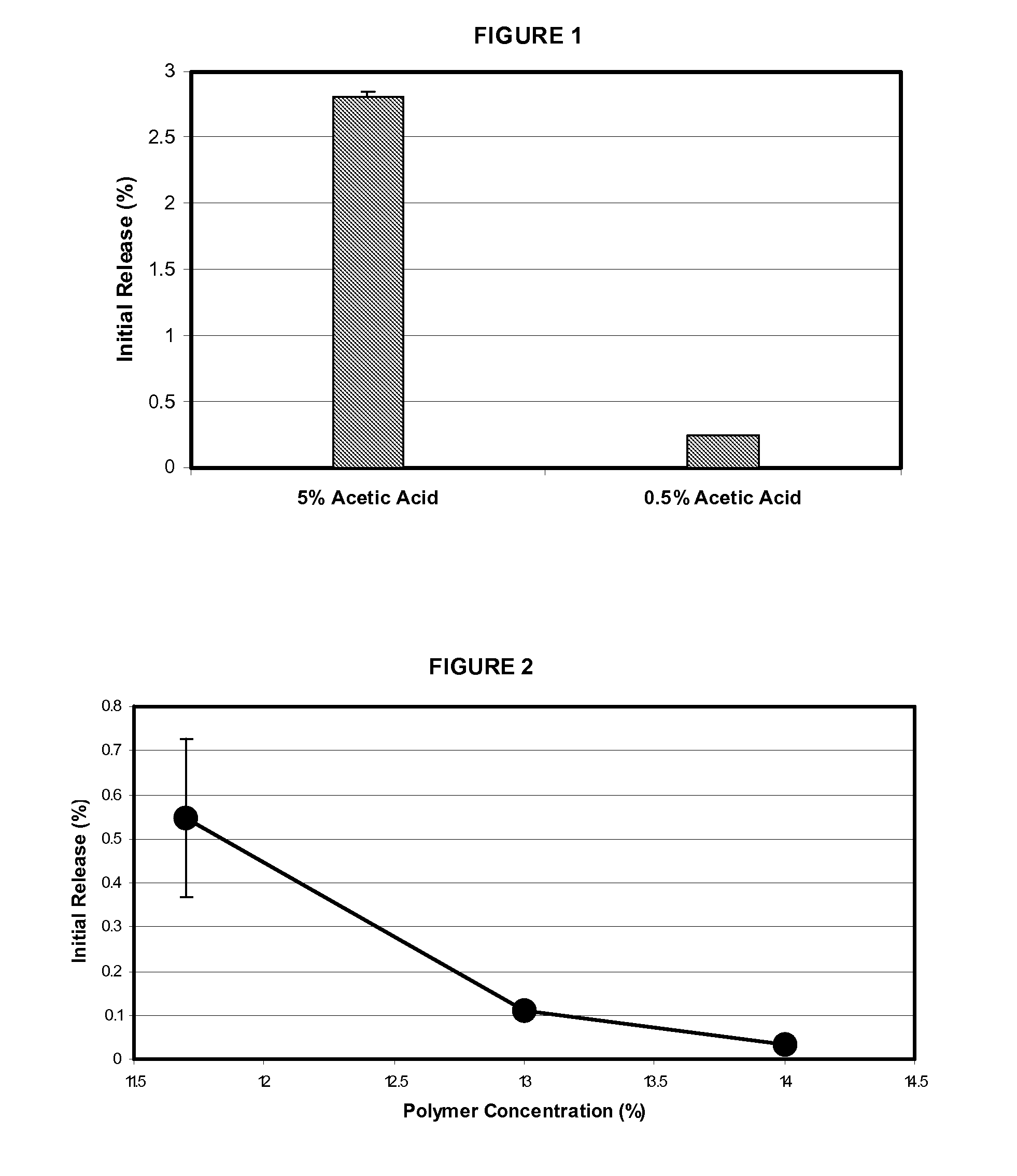
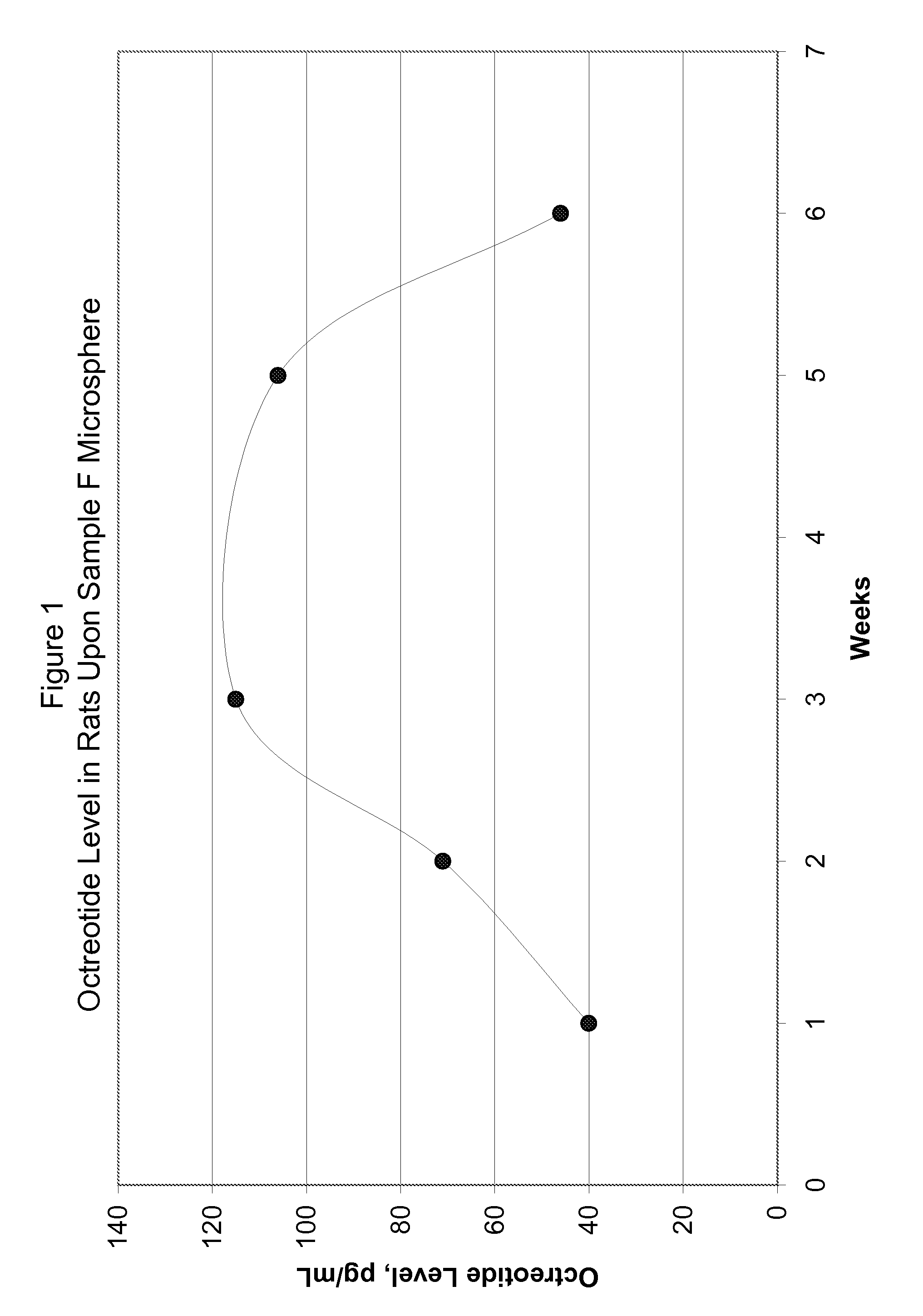
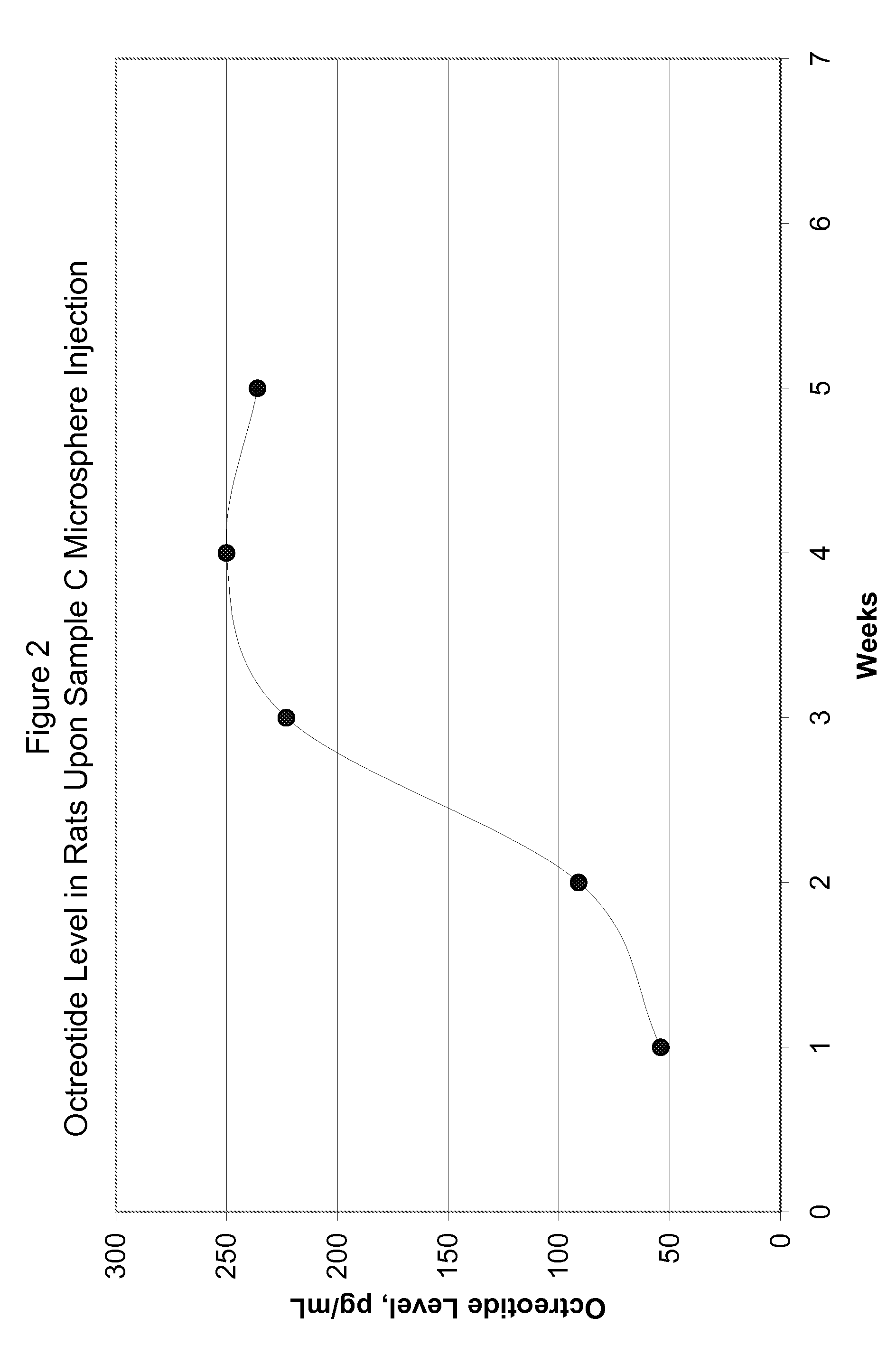
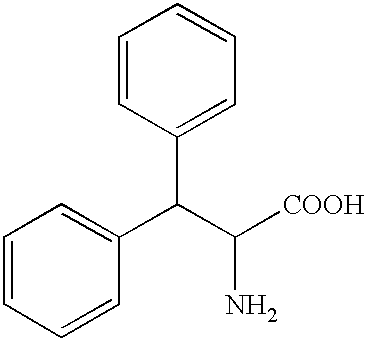
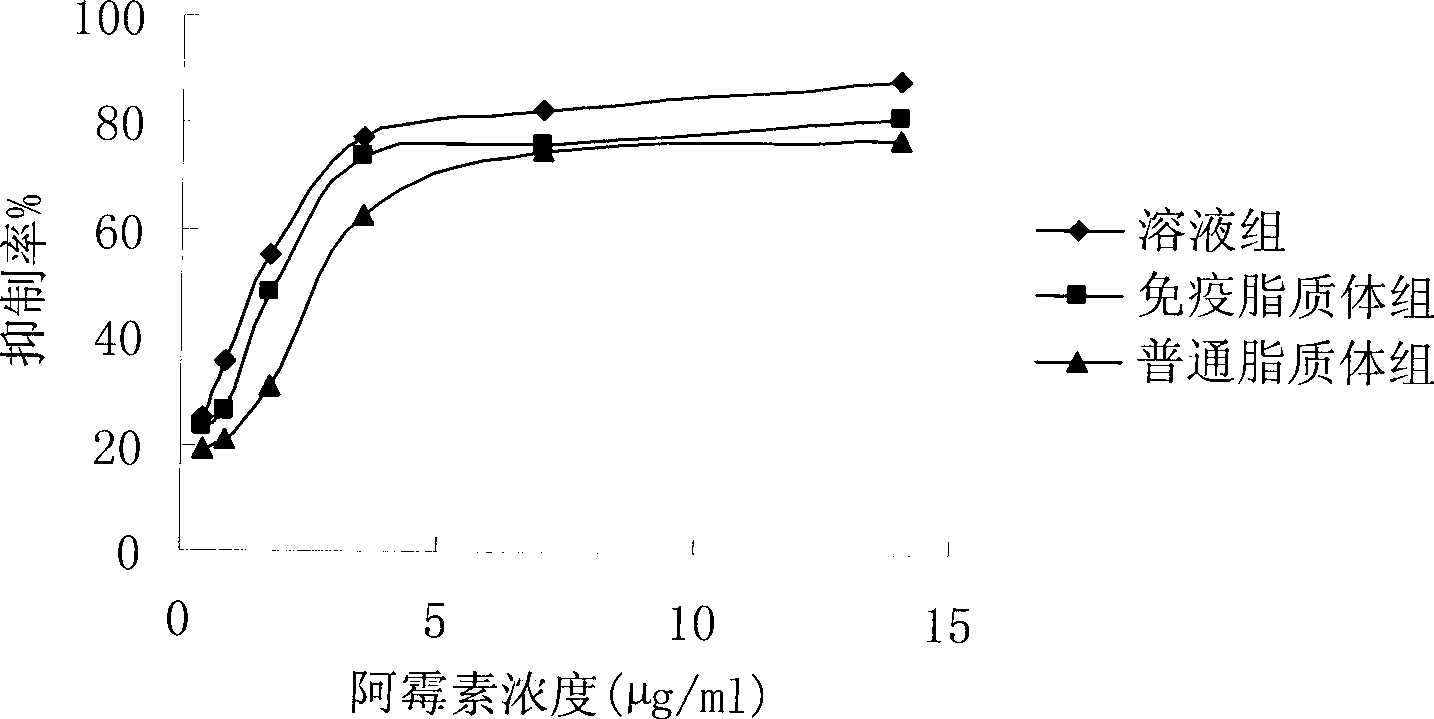
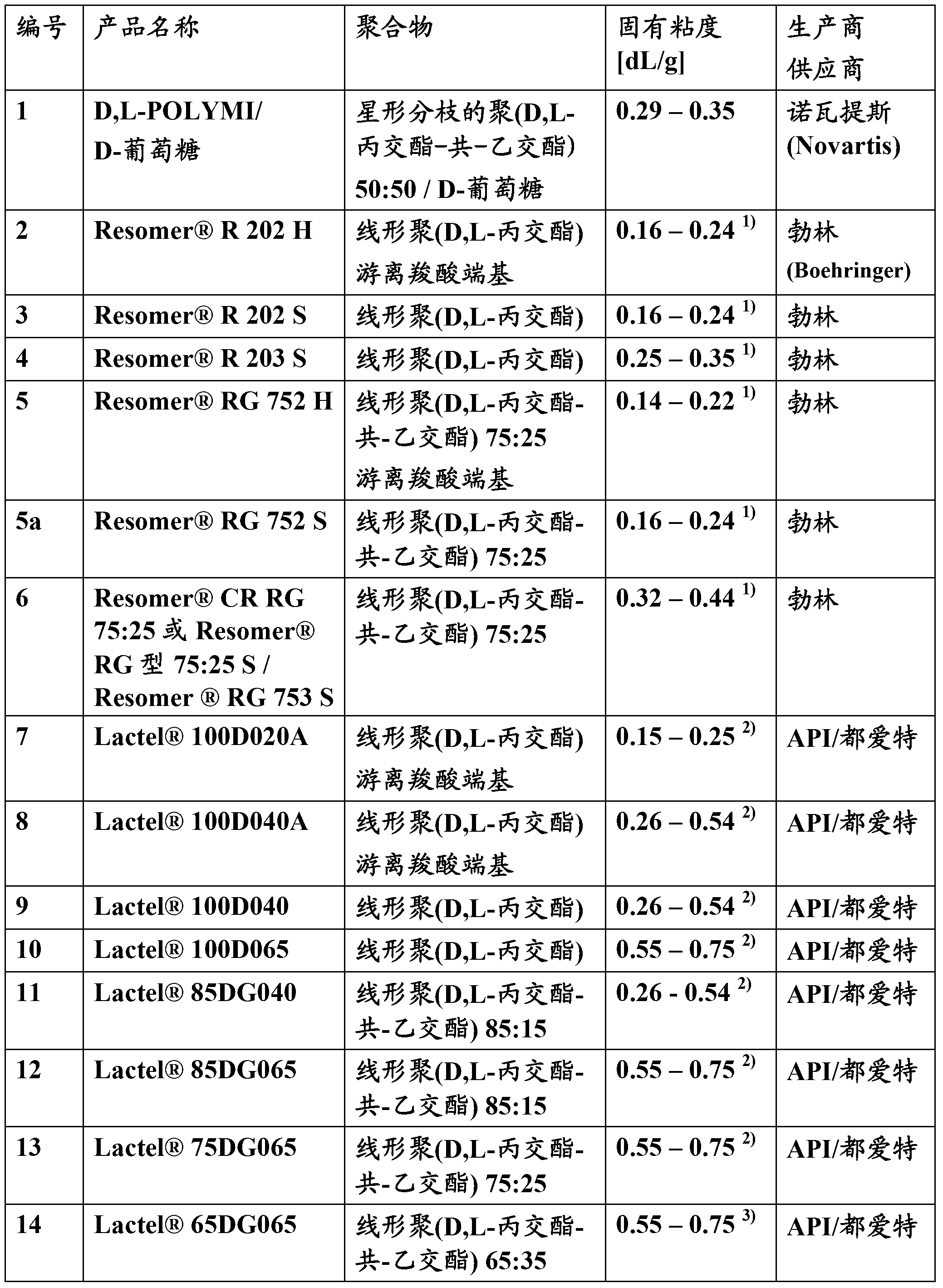
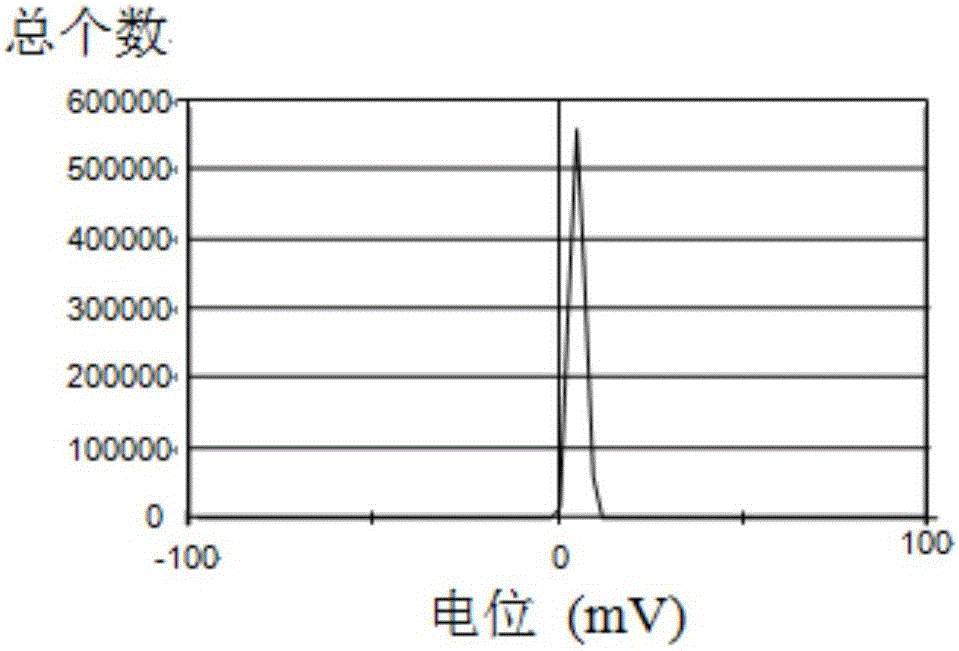
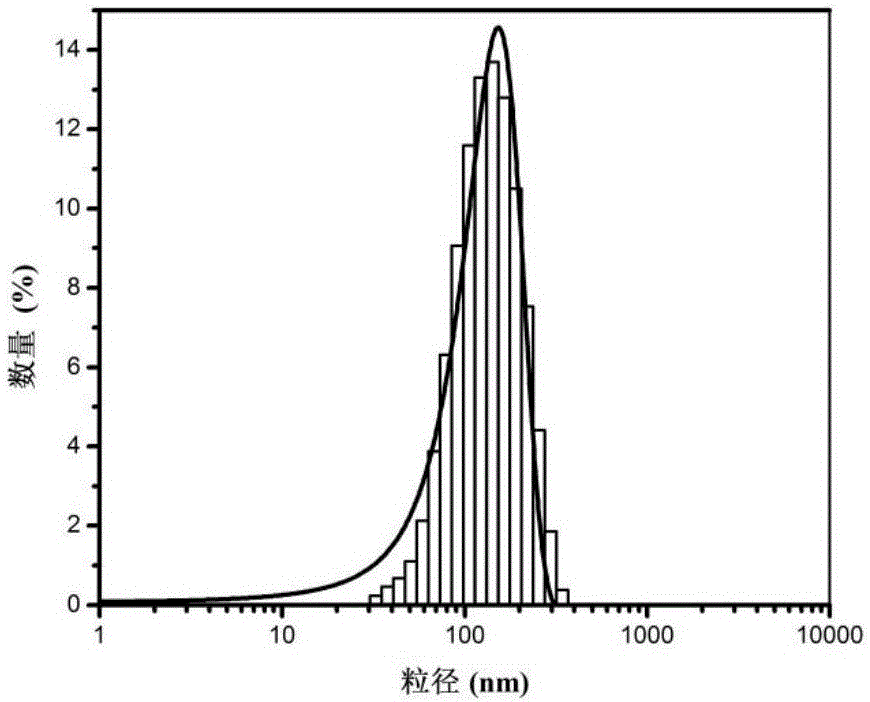
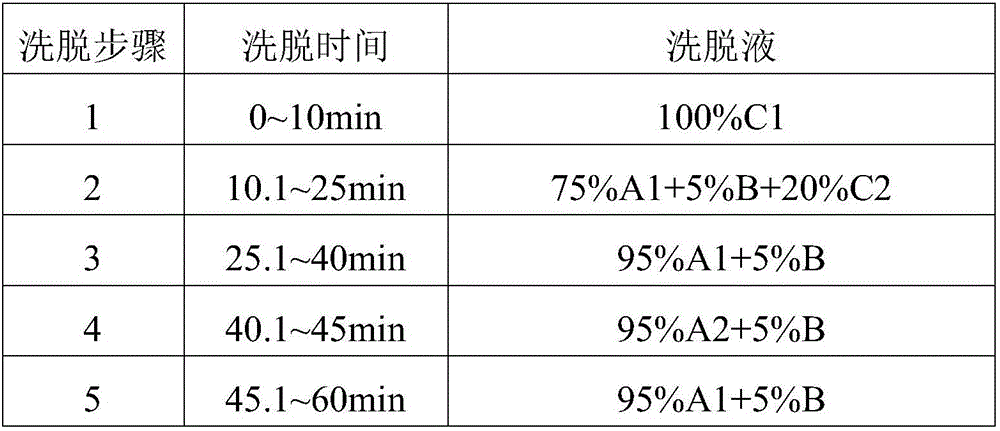
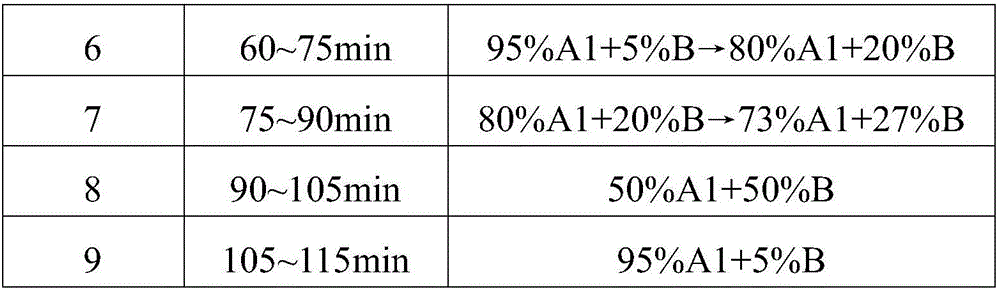
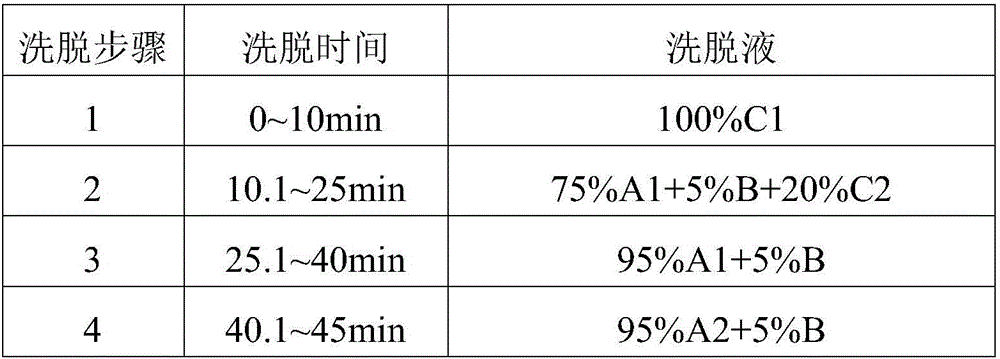
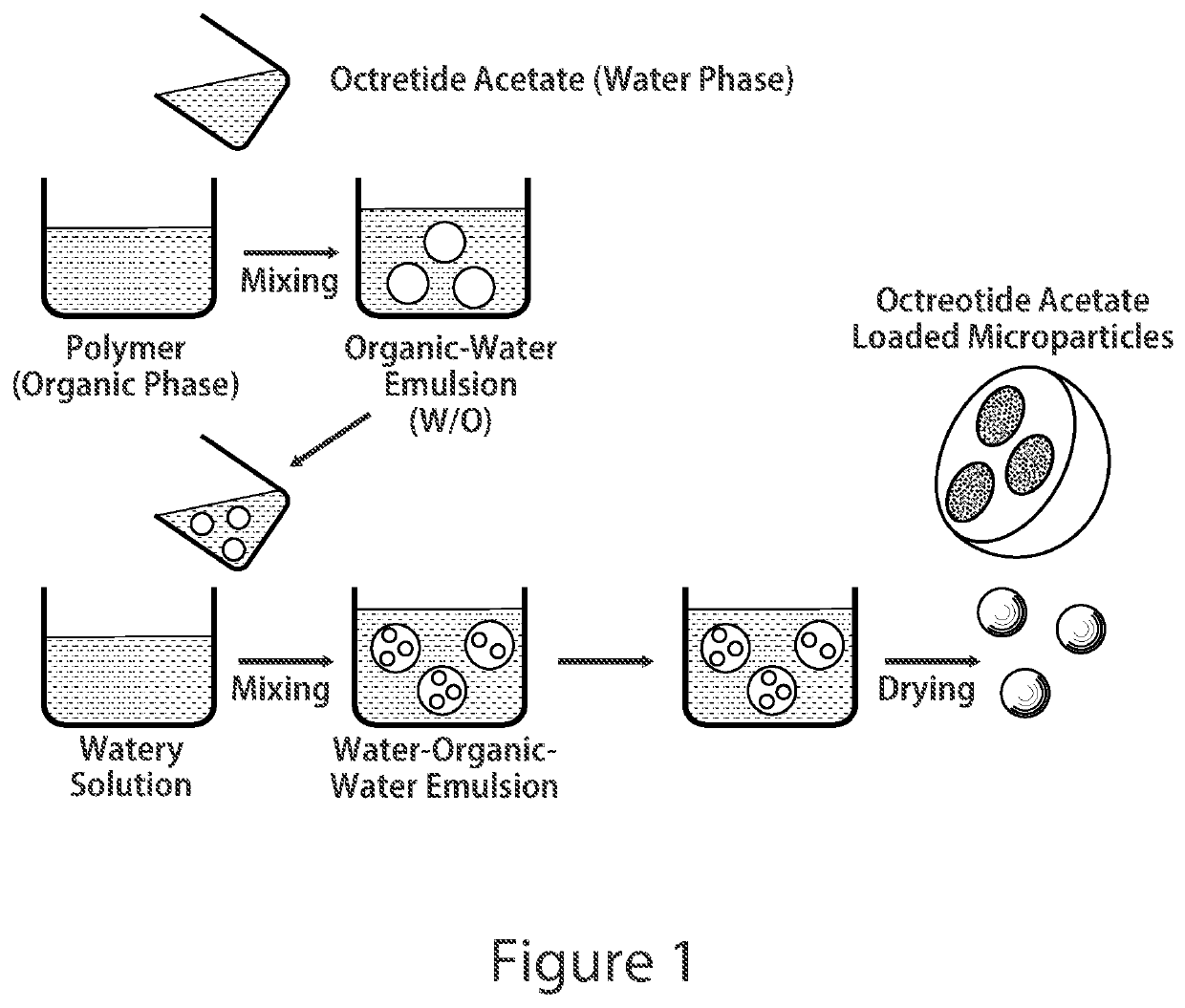
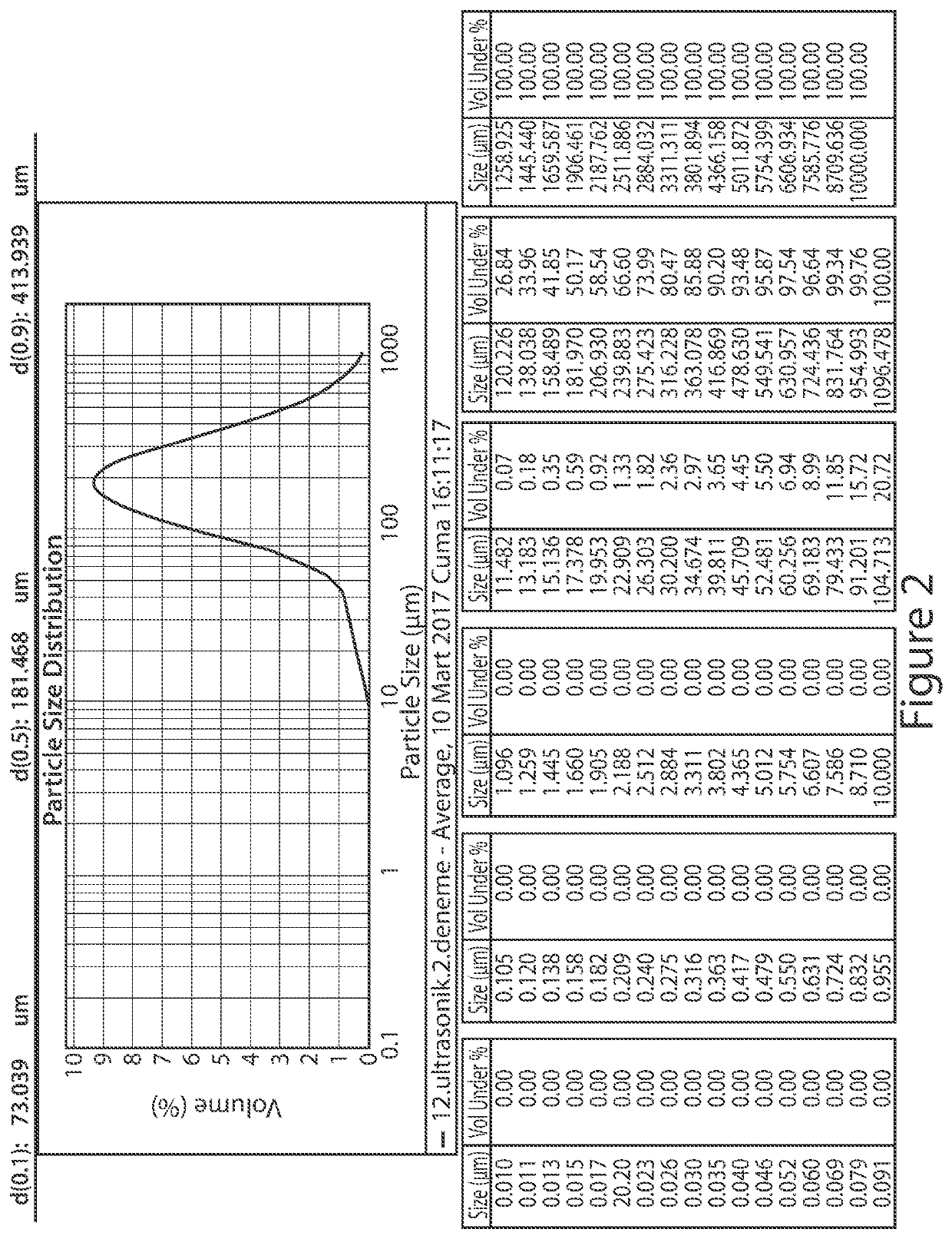
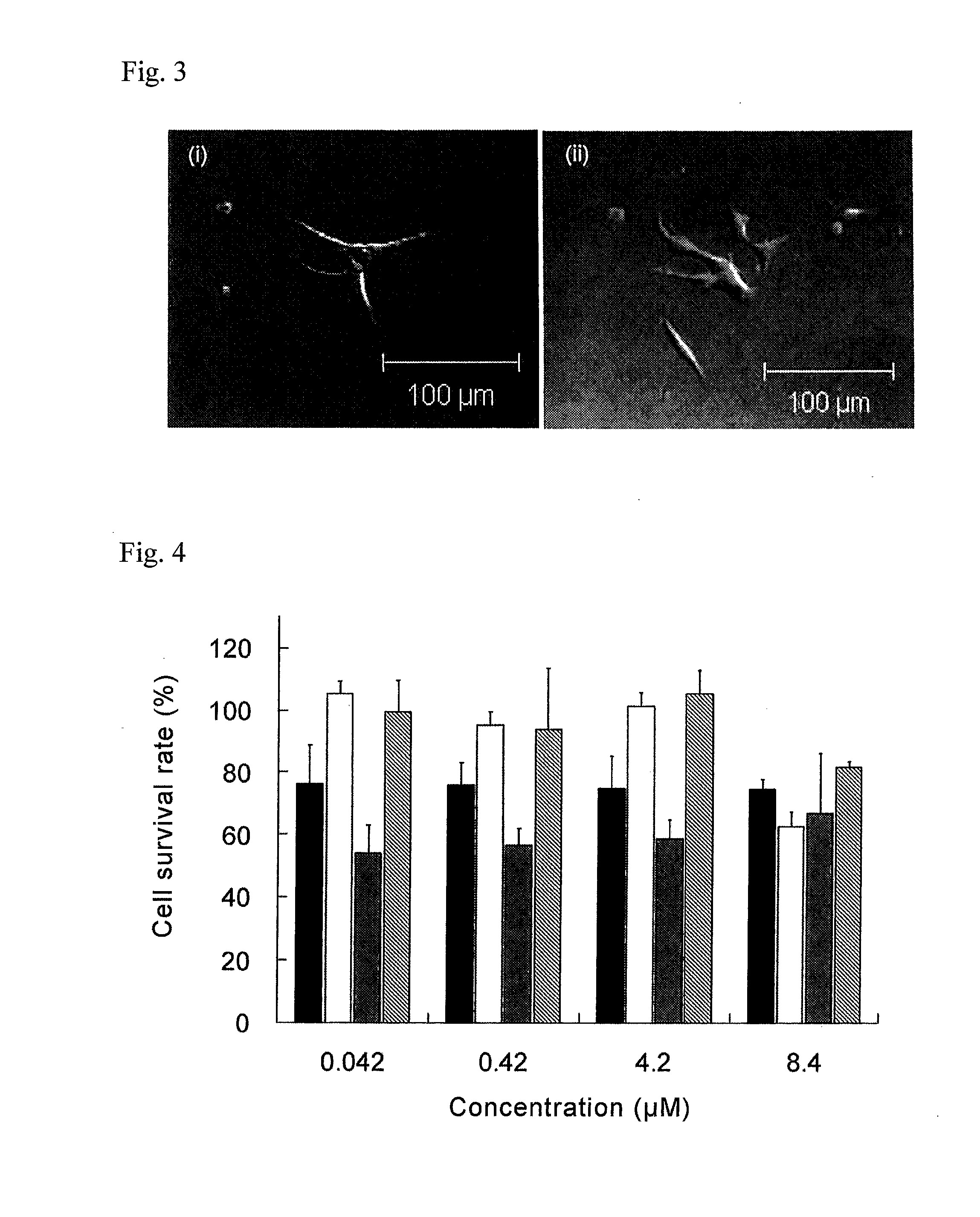
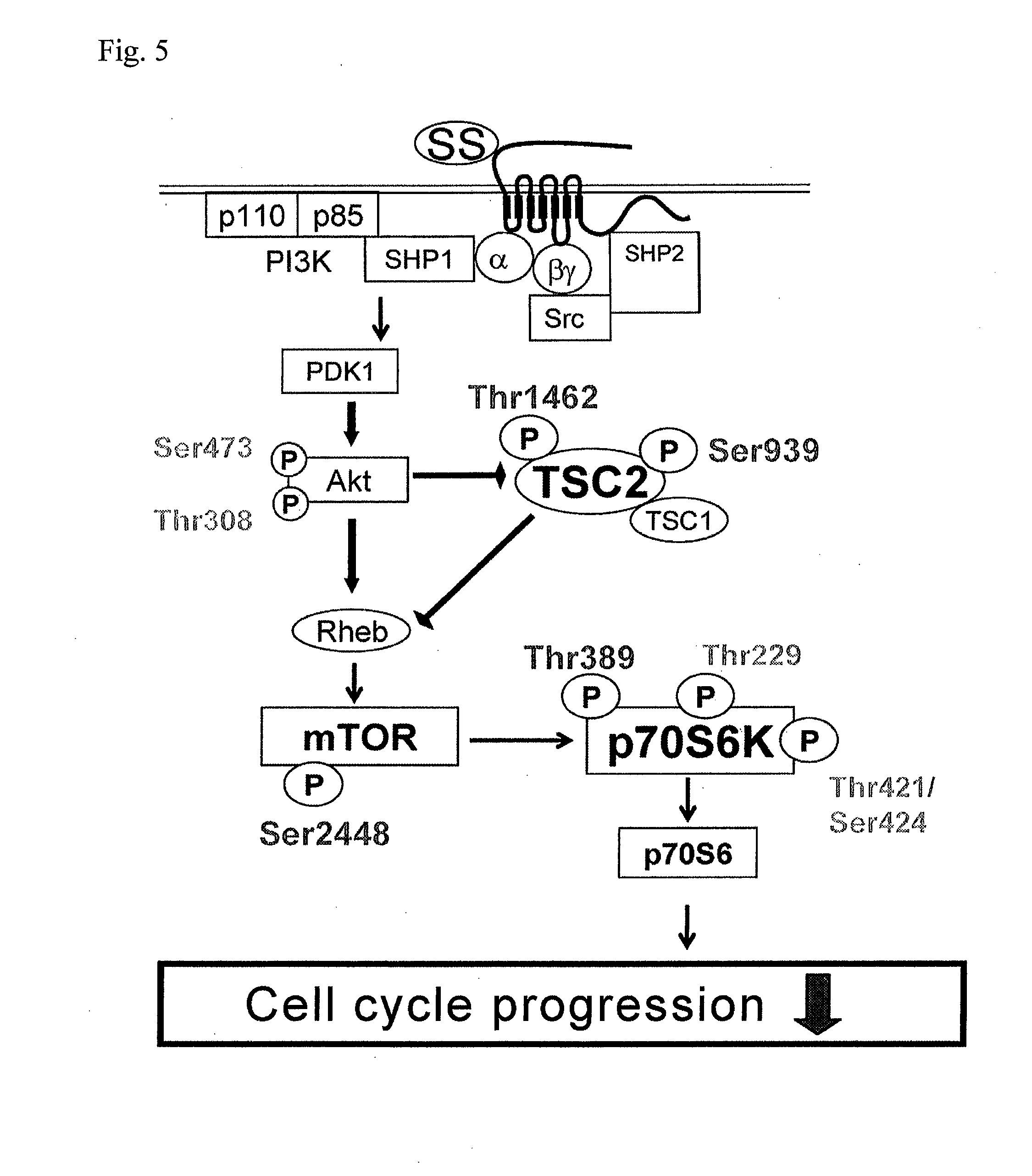
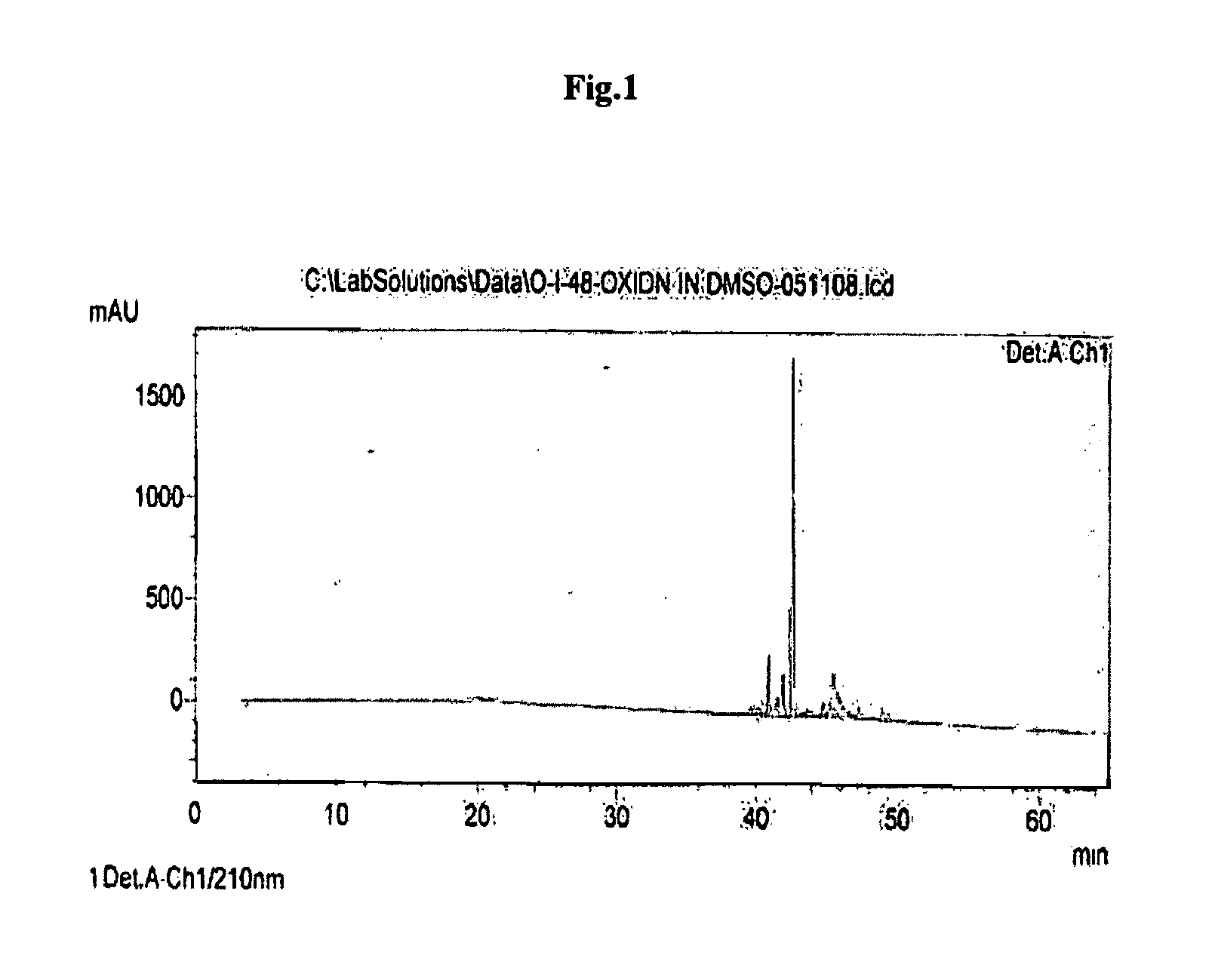
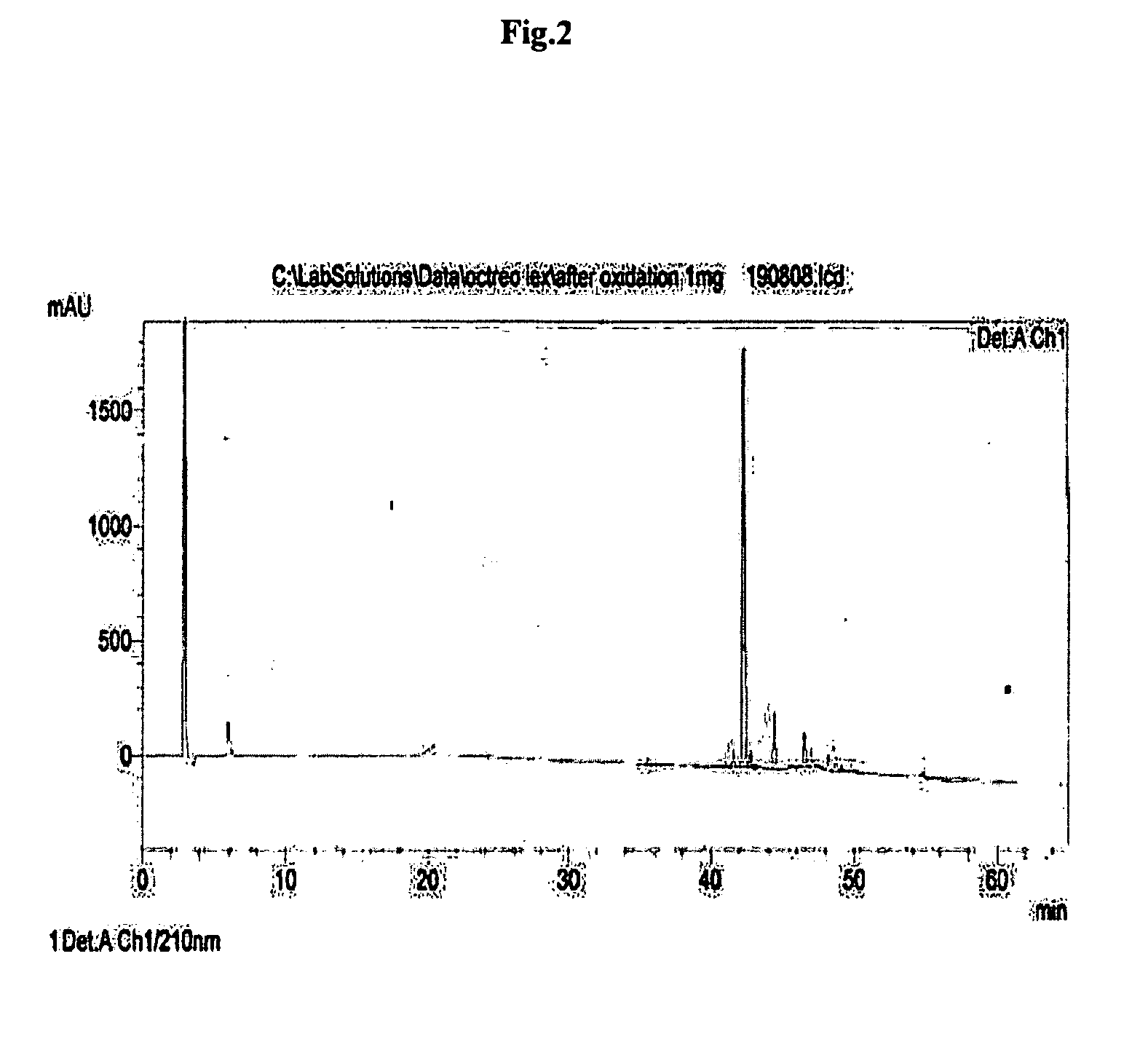
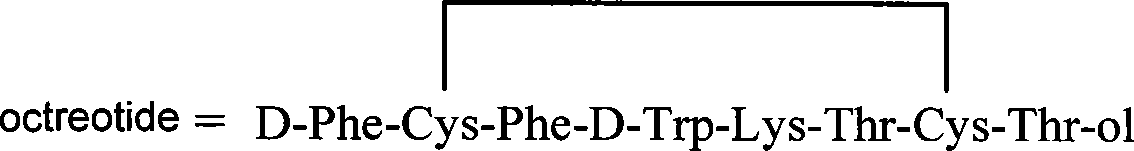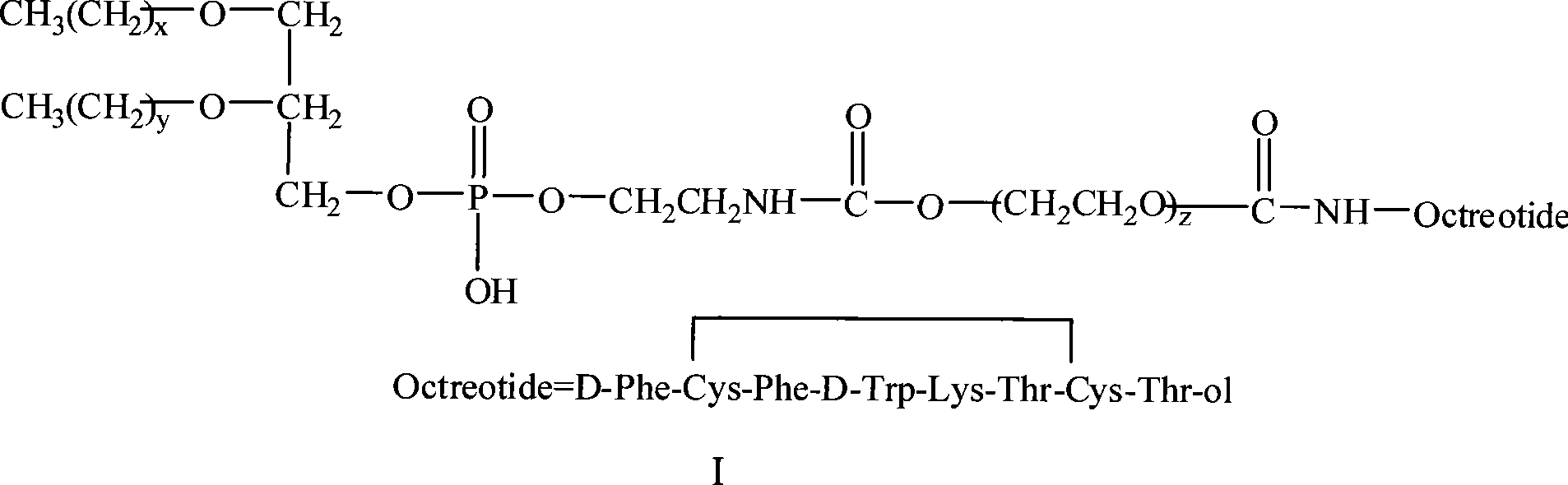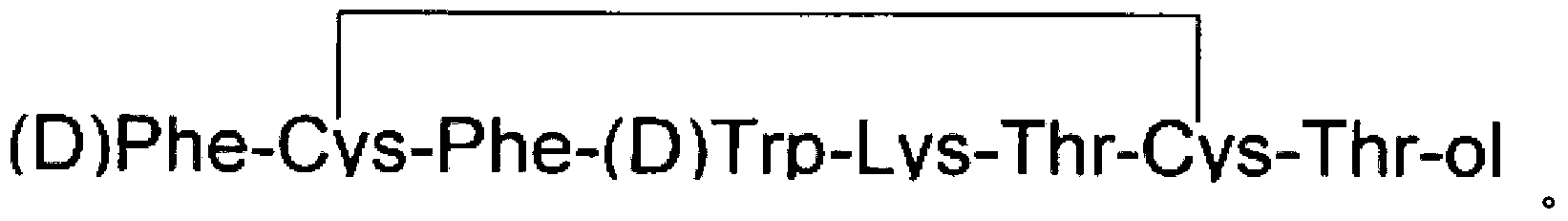Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
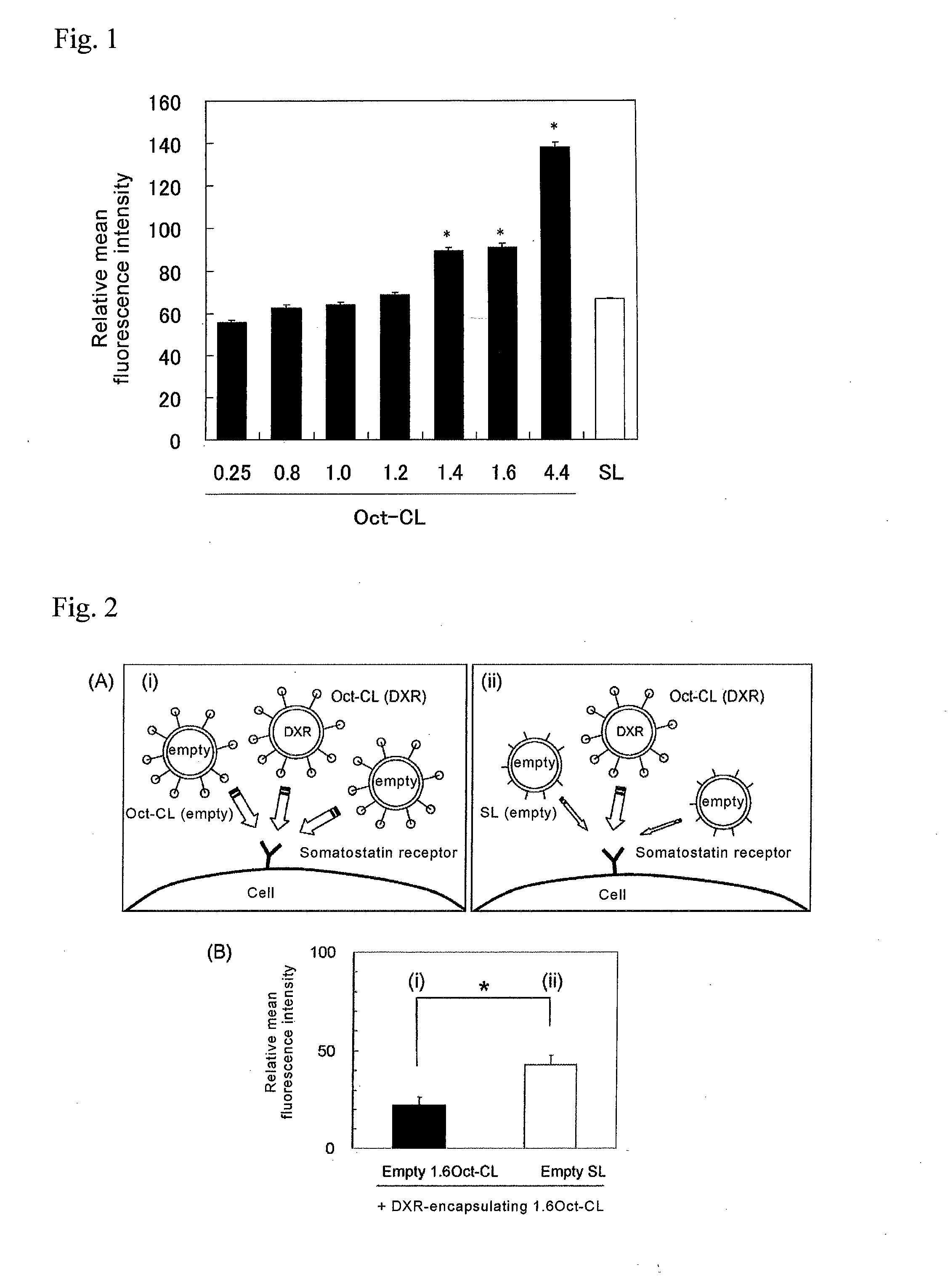
45 results about "Octreotide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Octreotide is used to treat severe watery diarrhea and sudden reddening of the face and neck caused by certain types of tumors (e.g., carcinoid tumors, vasoactive intestinal peptide tumors) that are found usually in the intestines and pancreas.
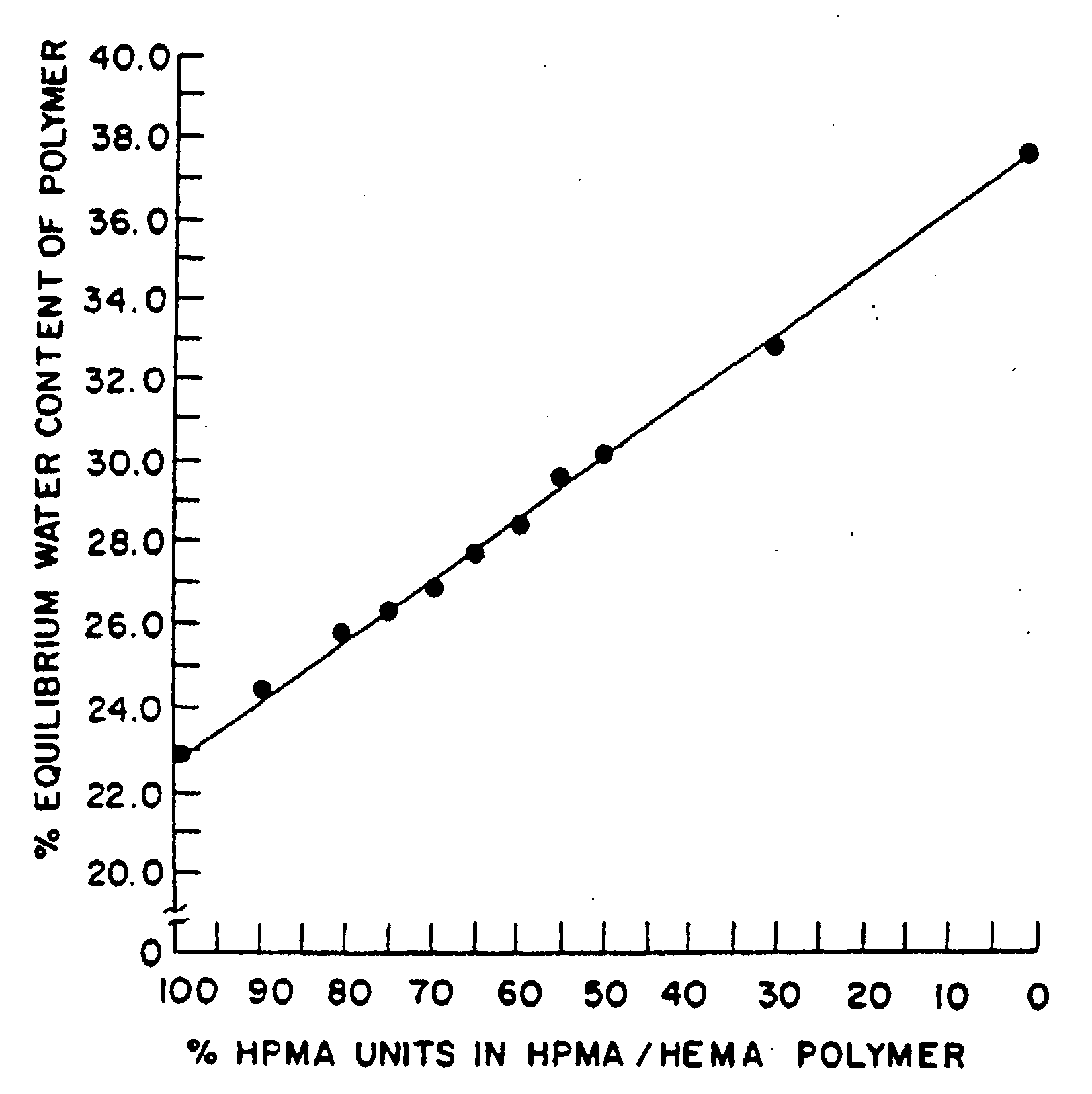
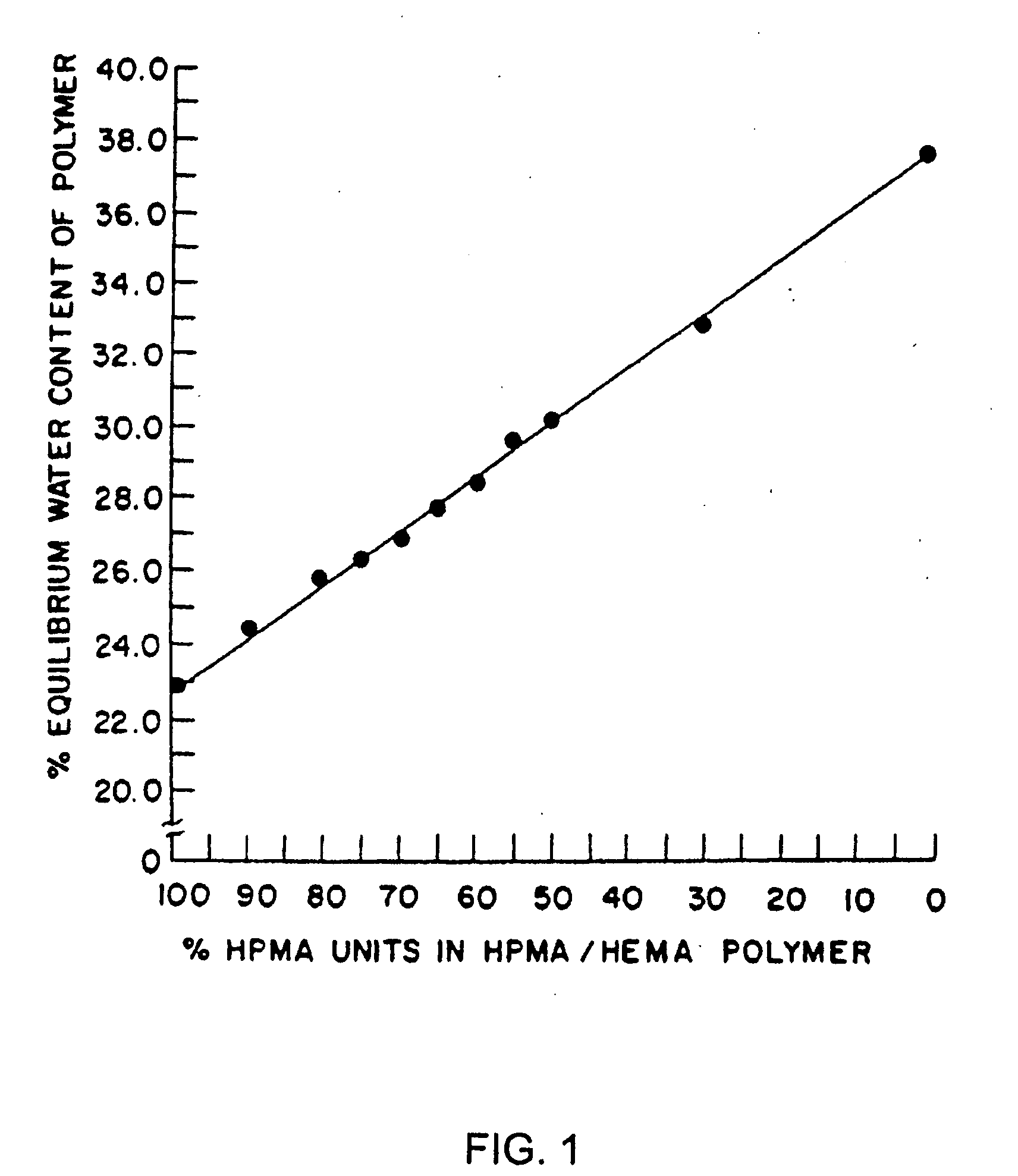
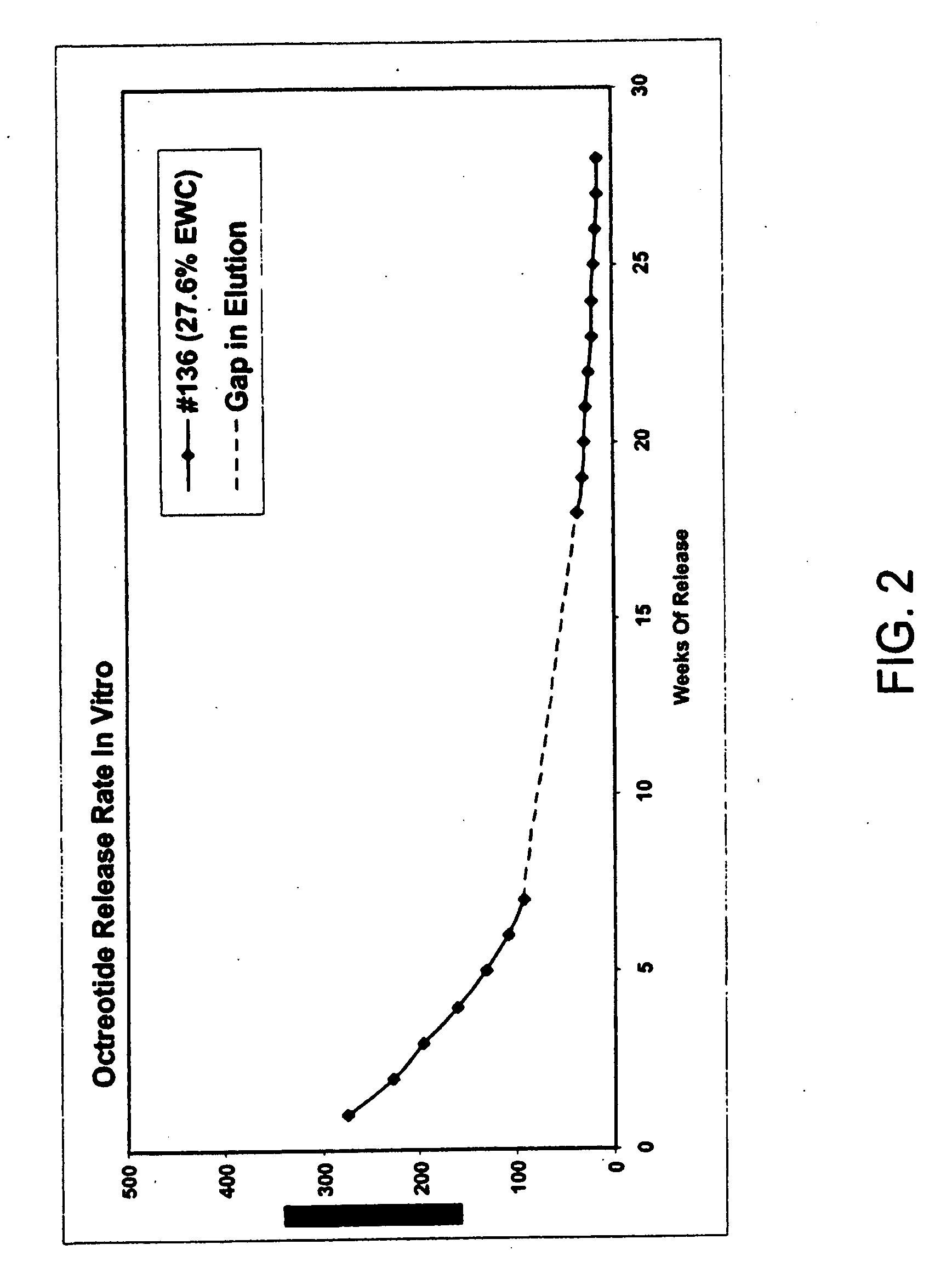
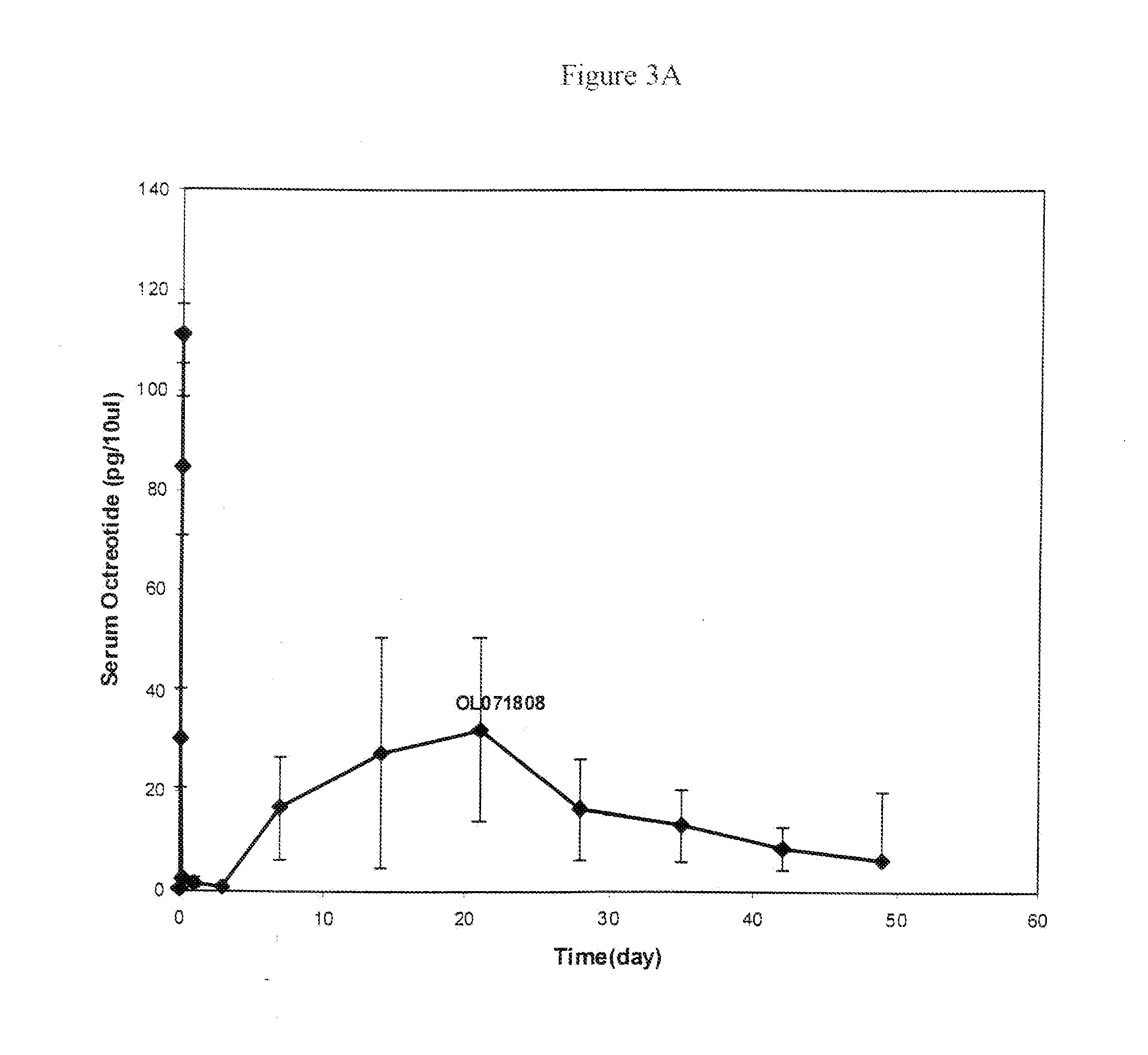
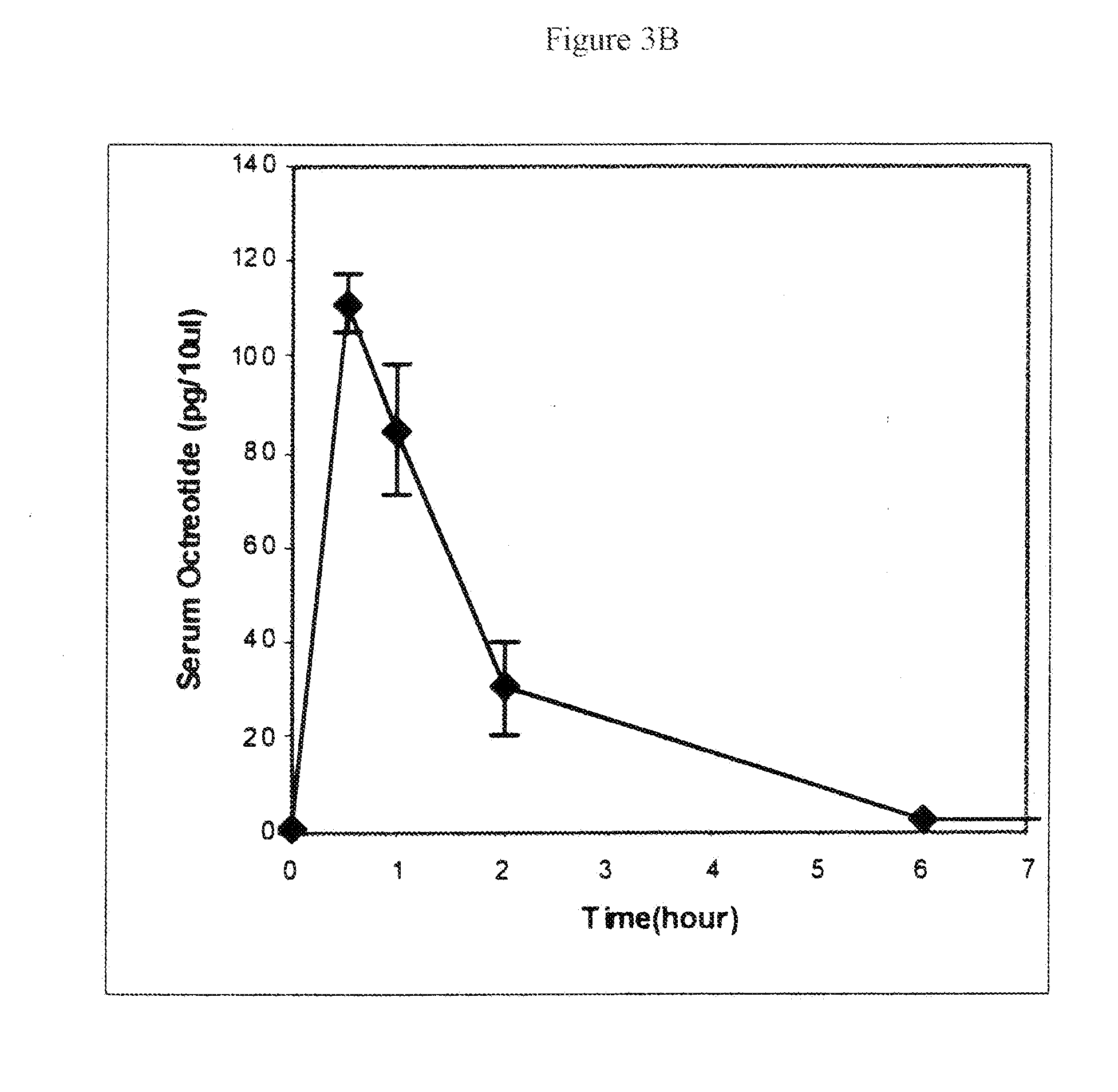
Controlled release formulations of octreotide
ActiveUS20060204540A1Avoid large peakImprove the level ofPeptide/protein ingredientsMetabolism disorderAcromegalyMalignant carcinoid tumors
A formulation of octreotide or pharmaceutically acceptable salts thereof, which provides controlled release of a therapeutically effective amount of octreotide for a period of at least about two months. Methods of treating acromegaly, decreasing growth hormone, decreasing IGF-1, and treating conditions associated with carcinoid tumors and VIPomas by administering a controlled release formulation of octreotide are provided herein.
Owner:ENDO PHARMA SOLUTIONS
Controlled release formulations of octreotide
ActiveUS7452868B2Reduce needLower Level RequirementsPeptide/protein ingredientsMetabolism disorderAcromegalyMalignant carcinoid tumors
Owner:ENDO PHARMA SOLUTIONS
Sustained Delivery Formulations of Octreotide Compounds
InactiveUS20090092650A1Improve bioavailabilityLeast riskSenses disorderPeptide/protein ingredientsMedicineOrganic liquids
The present invention relates to an octreotide sustained release delivery system for treatment of diseases relating to somatotropin and / or somatostatin. The sustained release delivery system of the invention includes a flowable composition containing an octreotide compound, and an implant containing the octreotide compound. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid and an octreotide compound.
Owner:QLT USA INC
Microspheres for the sustained release of octreotide with a low initial burst
InactiveUS20100086597A1Initial burst can be loweredAvoid painPowder deliveryPeptide/protein ingredientsMicrosphereL lactide
This disclosure features microspheres and a method of making them. The microspheres are for sustained release of an octreotide compound with a low initial burst, comprising a poly(D,L-lactide-co-glycolide) polymer matrix and an octreotide compound dispersed in the polymer matrix. The microspheres release less than 1% of a total amount of the octreotide compound within 1 hour at 37° C. and pH 7.4.
Owner:OAKWOOD LAB LLC
Preparation method for octreotide
The invention relates to the field of preparation of compounds, especially to a preparation method for octreotide. The preparation method comprises the following steps: subjecting dipeptide and a solid-phase synthetic hexapeptide resin to condensation so as to prepare linear octapeptide; dissolving linear octapeptide obtained after cracking in an aqueous solution of N,N-dimethyl formamide; and adding an oxidizing agent for cyclization and purification so as to prepare octreotide. The preparation method enables yield and purity of octreotide to be improved.
Owner:HANGZHOU HUADI GRP CO LTD
Preparation method of octreotide
InactiveCN101863961AHigh yieldSuitable for mass productionPeptide preparation methodsOctreotideSolid phases
The invention relates to a preparation method of octreotide, which comprises the steps of preparing crude linear peptide by an Fmoc / tBu solid-phase peptide synthesis method and preparing octreotide from the crude linear peptide through oxidizing reaction, wherein the oxidizing reaction is carried out under the pH of 7.0-7.5 and in the presence of hydrogen peroxide, and the charging molar ratio of the hydrogen peroxide and the crude linear peptide is 7-9 / 1. The invention adopts the hydrogen peroxide to oxidize the crude linear peptide so as to obtain the octreotide, the oxidizing reaction can be finished only within 1-3h, compared with the traditional air oxidizing method, the reaction time is greatly shortened, the octreotide product yield is tremendously improved, and the method is suitable for mass production of the octreotide and salts thereof.
Owner:苏州中科天马肽工程中心有限公司
Delivery of dry formulations of octreotide
Methods and devices are described for delivering octreotide to a patient, comprising implanting a controlled release composition for delivering octreotide, wherein the composition does not require hydration prior to implantation, and wherein the composition optionally comprises a release agent.
Owner:ENDO PHARMA SOLUTIONS
Delivery of dry formulations of octreotide
Methods and devices are described for delivering octreotide to a patient, comprising implanting a controlled release composition for delivering octreotide, wherein the composition does not require hydration prior to implantation, and wherein the composition optionally comprises a release agent.
Owner:ENDO PHARMA SOLUTIONS
Microspheres for the sustained release of octreotide with a low initial burst
InactiveUS20120021018A1Initial burst can be loweredAvoid painPowder deliveryCyclic peptide ingredientsMicrosphereL lactide
This disclosure features microspheres and a method of making them. The microspheres are for sustained release of an octreotide compound with a low initial burst, comprising a poly(D,L-lactide-co-glycolide) polymer matrix and an octreotide compound dispersed in the polymer matrix. The microspheres release less than 1% of a total amount of the octreotide compound within 1 hour at 37° C. and pH 7.4.
Owner:OAKWOOD LAB LLC
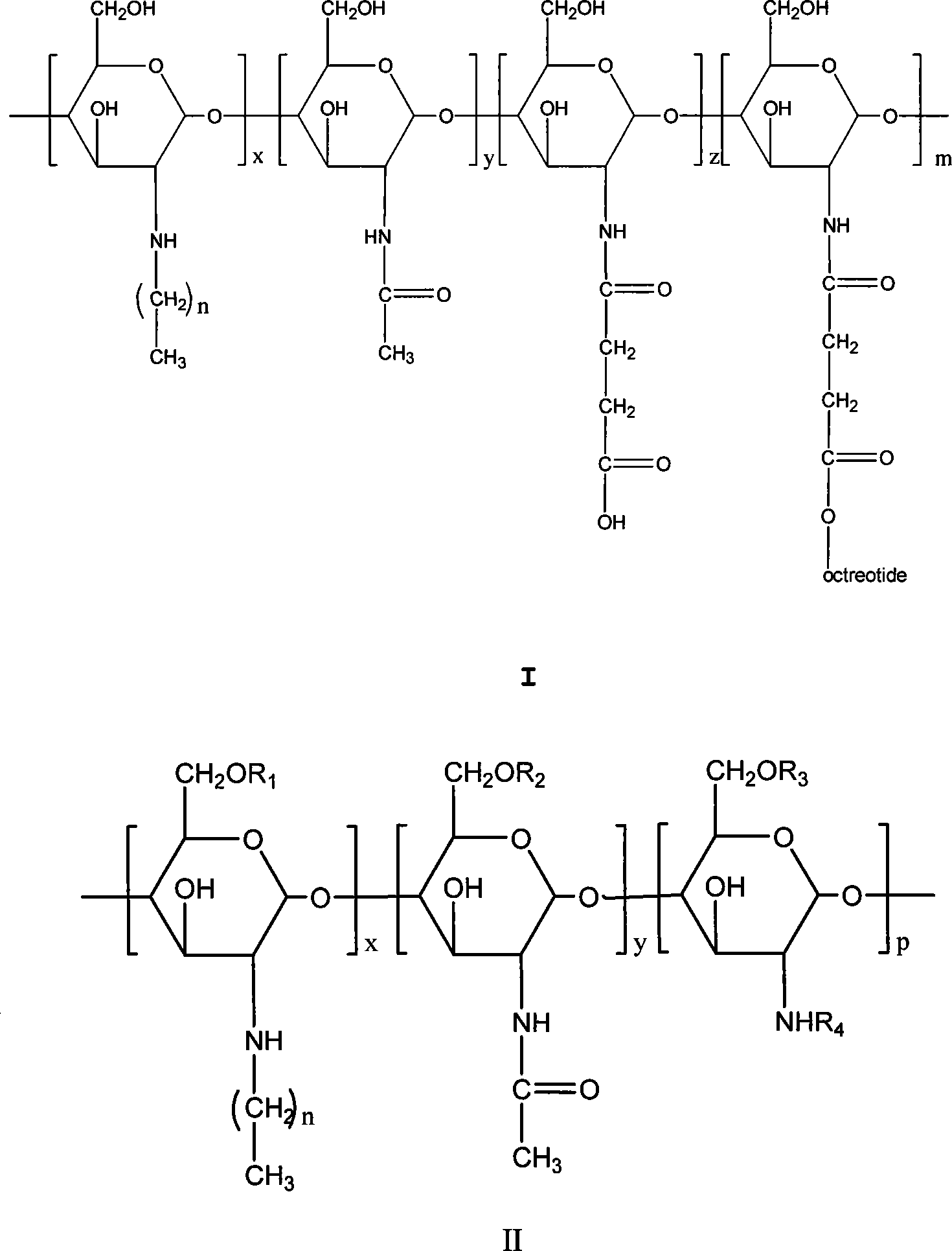
Chitose derivates using octreotide as target ligand and use thereof in medicament
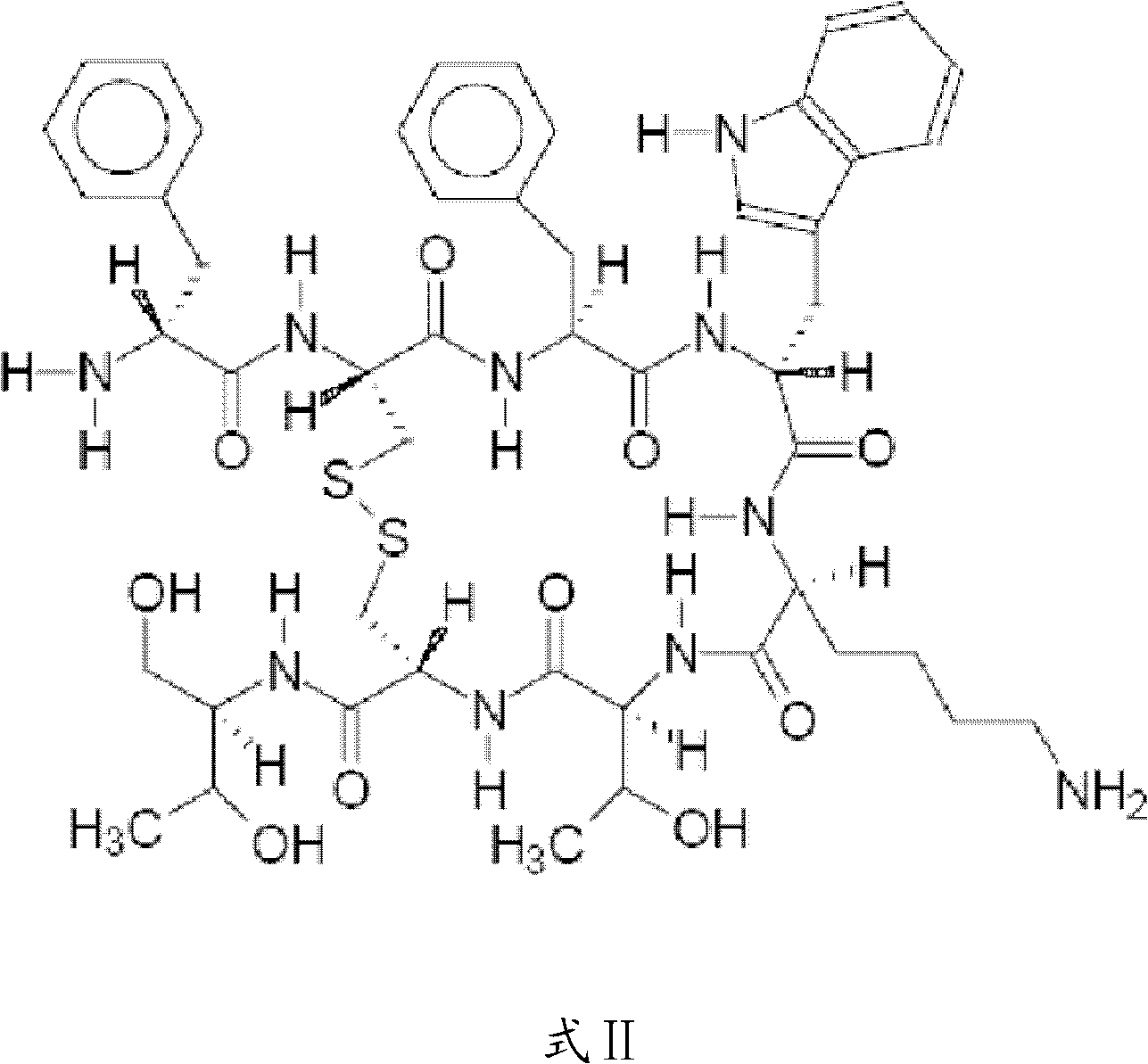
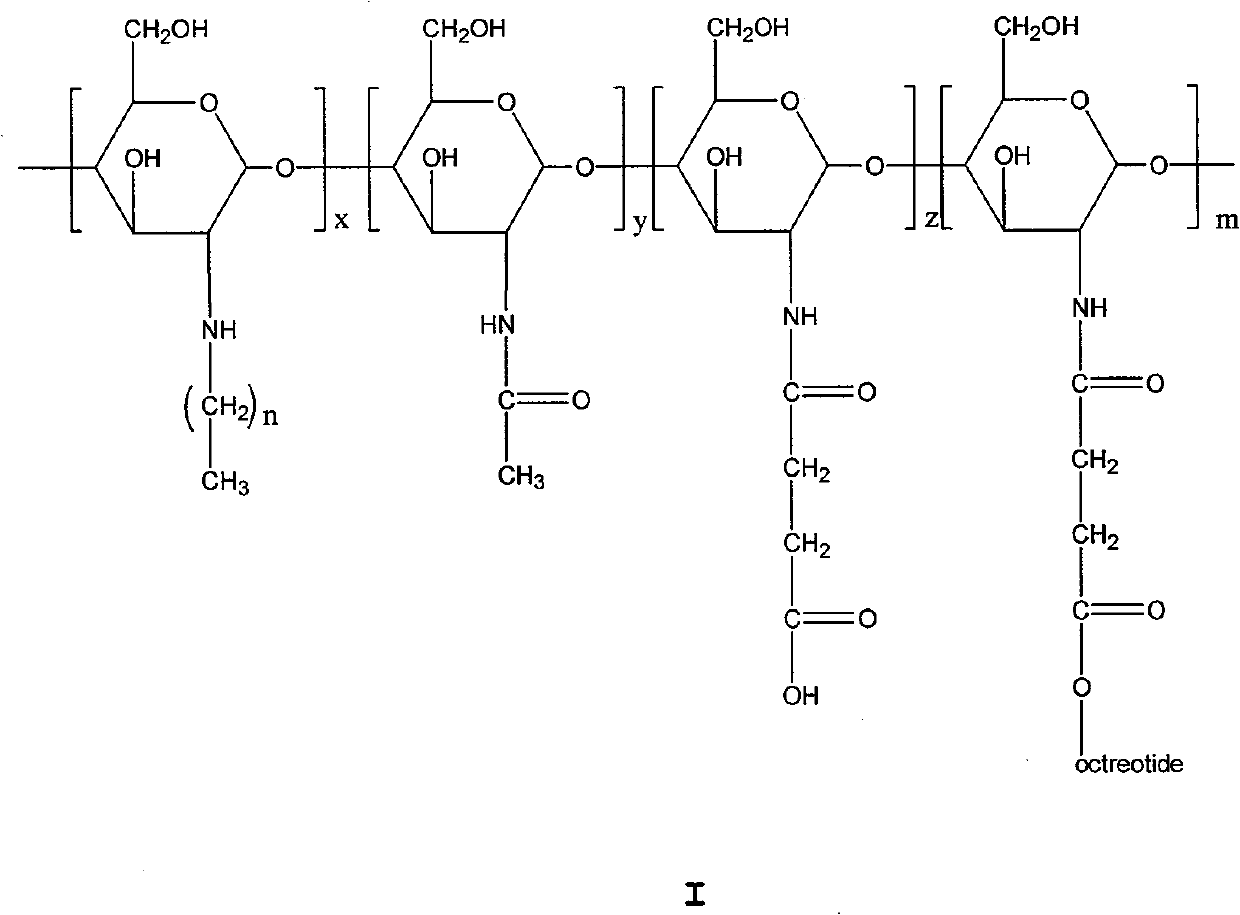
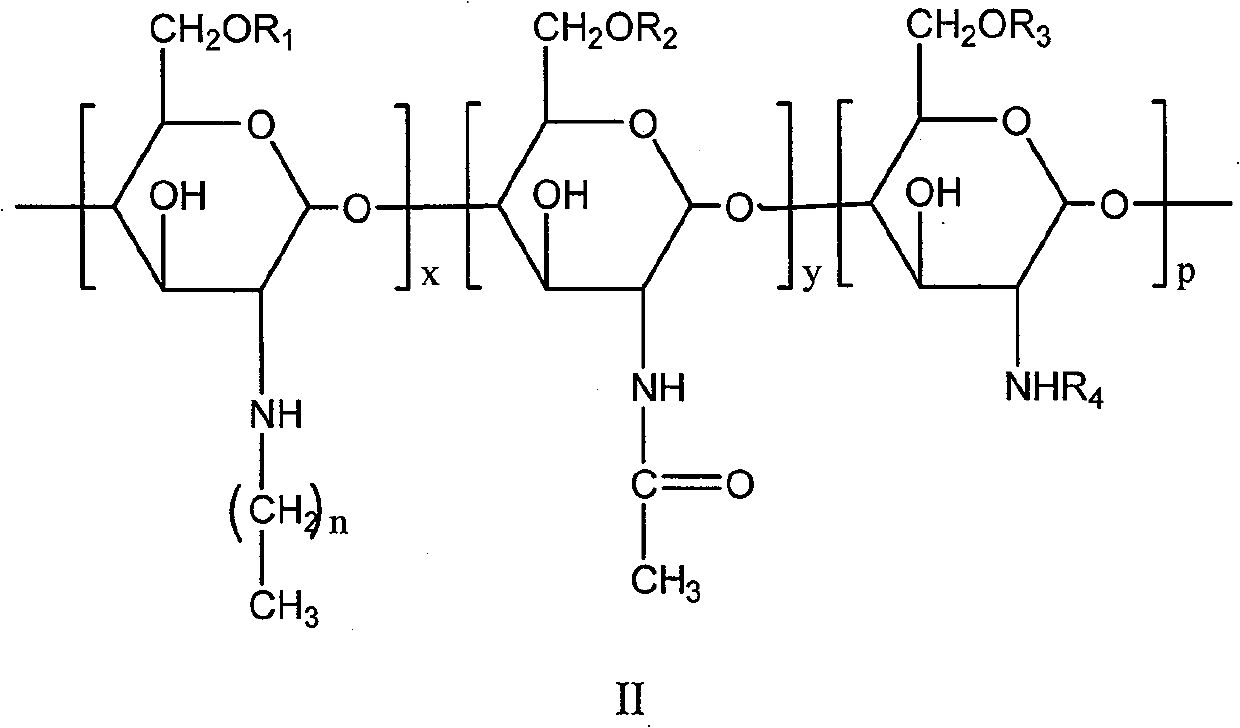
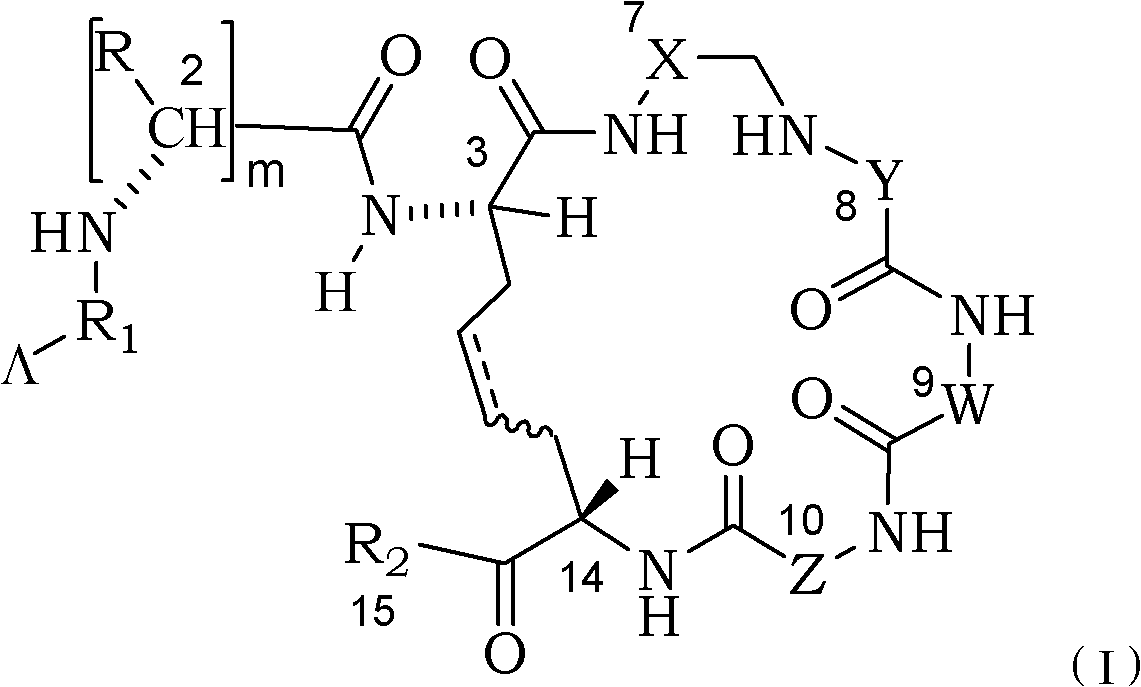
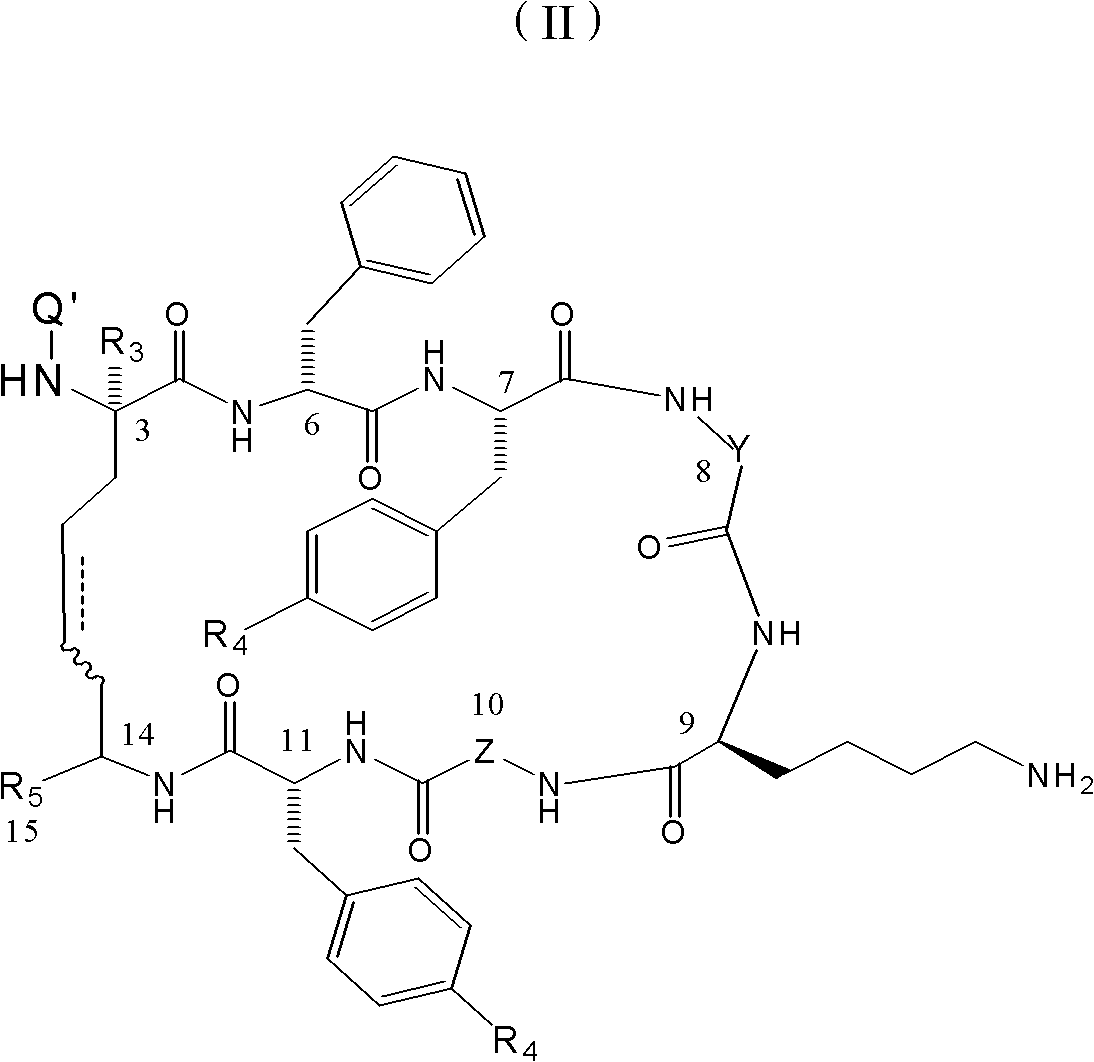
The invention relates to the field of polymer chemistry and the field of pharmaceutical excipients. The invention particularly relates to targeted chitosan derivatives (I) or (II) containing octreotide, in particular to the N-succinyl-N-alkylate chitosan derivative and the N-alkylate carboxymethyl chitosan and a preparation method thereof, the invention further relates to an effect thereof as a solubilizer of an insoluble drug, a modification effect on micelles, liposomes and other vectors and a pharmaceutical composition which leads the derivatives to have the target function and contains the derivatives.
Owner:CHINA PHARM UNIV
Delivery of dry formulations of octreotide
Methods and devices are described for delivering octreotide to a patient, comprising implanting a controlled release composition for delivering octreotide, wherein the composition does not require hydration prior to implantation, and wherein the composition optionally comprises a release agent.
Owner:ENDO PHARMA SOLUTIONS
Synthesis method for industrial production of octreotide
ActiveCN106543269AAvoid harsh reaction conditionsMild reaction conditionsPeptide preparation methodsWater bathsSynthesis methods
The invention relates to synthesis method for industrial production of octreotide. The method is characterized by comprising the following steps of: 1. taking aminomethyl resin as the starting resin, utilizing condensation reagents HOBT and DIC to couple the raw material Fmoc-Thr-x to the resin to generate Fmoc-Thr-x AM Resin; then coupling amino acids Cys, Thr, Lys, Trp, Phe, Cys and Phe to Fmoc-Thr-x AM Resin one by one in order, thus obtaining octreotide peptide resin; 2. adding a lysate solution, stirring the substances evenly and performing cracking for 2-3h; 3. dissolving octreotide crude peptide into a TFA / water mixed solution, then putting the mixture into 35DEG C-38DEG C water bath to perform heating for 2-3h to make the linear peptide of octreotide have single peak pattern in HP LC chromatogram; and 4. adding DMSO, then performing oxidation for 1-1.5h, conducting HPLC detection of a cyclization end point, and performing separation and purification to obtain a refined product of octreotide. In short, the synthesis method provided by the invention has simple technique, and is suitable for industrial production. After post-treatment, the HPLC peak pattern of octreotide crude peptide is simple, the cyclization method is easy to operate, cyclization is complete, the yield is high and the purity is 98% or more.
Owner:苏州天马医药集团天吉生物制药有限公司
Microspheres for releasing an octreotide compound without an initial time lag
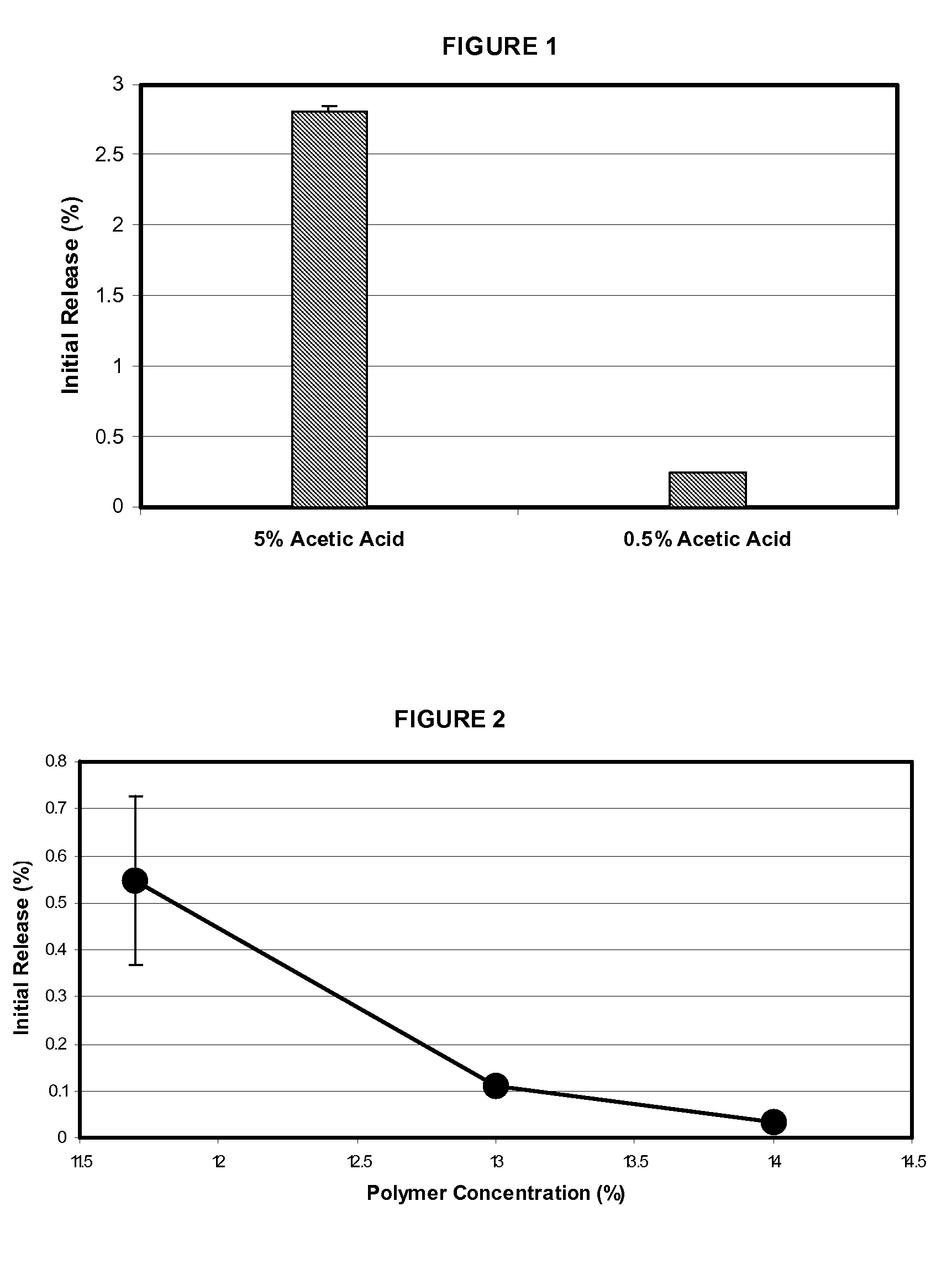
InactiveUS20100086596A1Fast preparationFaster drug clearingPowder deliveryPeptide/protein ingredientsAcetic acidTime lag
Microspheres for releasing an octreotide compound without an initial time lag include a poly(D,L-lactide-co-glycolide) polymer (PLGA polymer) matrix having a ratio of lactide to glycolide ranging from 80:20 to 90:10 mol %. The polymer has a molecular weight ranging from about 6000 to 16000. The octreotide compound is dispersed in the polymer matrix. The microspheres can be made by forming a dispersed phase by combining the above polymer, dichloromethane, the octreotide compound, methanol and acetic acid. A target loading of the octreotide compound in the dispersed phase ranges from 7 to 12% by weight. Polyvinyl alcohol is dissolved in water to form a continuous phase. The dispersed phase is mixed in the continuous phase to form a microsphere suspension. The dichloromethane, acetic acid, methanol and polyvinyl alcohol are removed from the microsphere suspension. Residual dichloromethane and methanol are removed from the microspheres by washing.
Owner:OAKWOOD LAB LLC
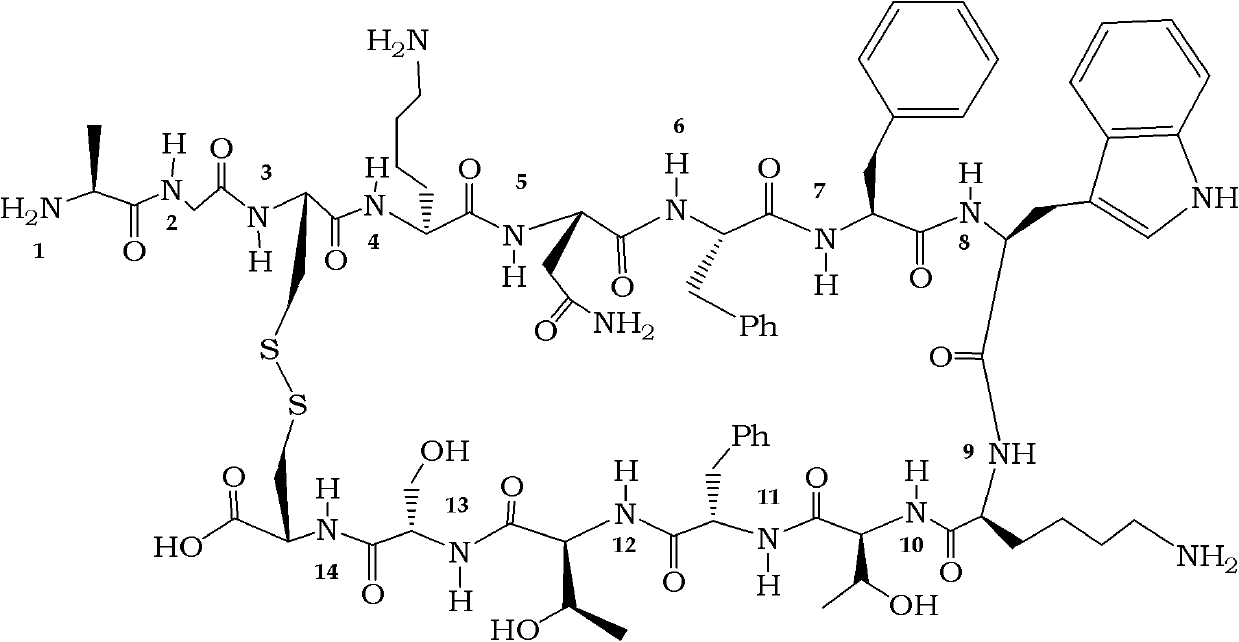
Penta-or tetrapeptide binding to somatostatin receptors and the use of the same
InactiveUS20030114362A1Reduce inflammationReduce decompositionIn-vivo radioactive preparationsSomatostatinsApoptosisCarboxylic acid
The subject matter of the present invention is a cyclic or linear tetra- or pentapeptide binding to somatostatin receptors. The compounds of the invention are characterised in that they contain the radical of an amino carboxylic acid bearing a five-membered ring in the peptide backbone which may optionally contain O, S, Se, N, or P. These compounds are easy to prepare and display increased stability against peptidases. The compounds of the present invention induce apoptosis of tumour cells and the use of said compounds for cancer therapy is described. In particular, the compounds are characterised in that they are active even against tumour cells displaying resistance against other somatostatin derivatives such as octreotide. In addition, the use of the compounds of the invention for tumour diagnosis by means of positron-emission tomography is described, as well as their use as agents against neurogenic inflammation.
Owner:NOVASPIN BIOTECH
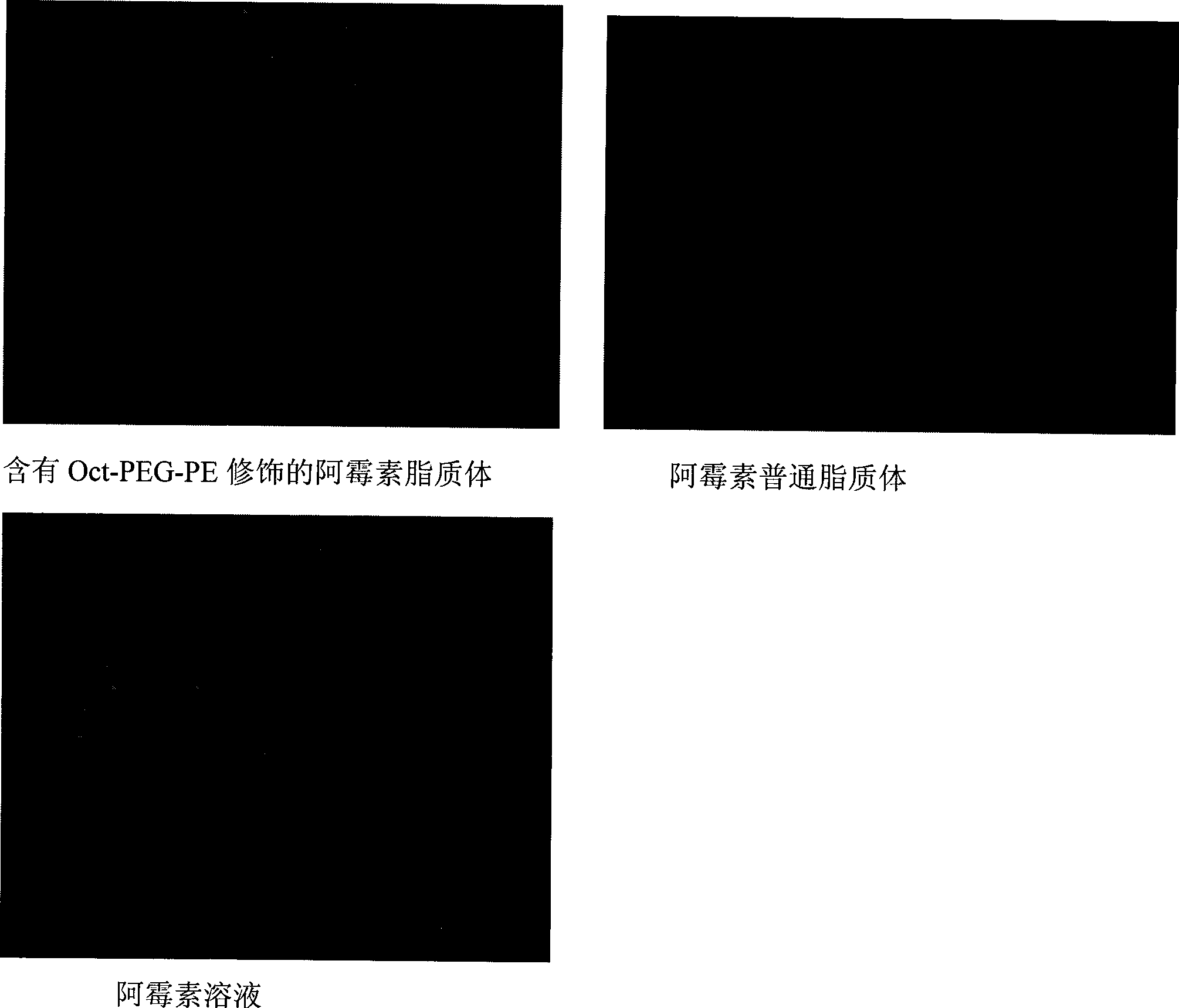
PEG-decorated phospholipid derivative using octreotide as target ligand and production method thereof
InactiveCN101455845AMacromolecular non-active ingredientsAntineoplastic agentsPolyethylene glycolCell membrane
The invention relates to the field of medical polymer auxiliary materials and medicines, in particular to polyethylene glycol modified phospholipids derivatives (I) which take octreotide as a targeting ligand. In the polyethylene glycol modified phospholipids derivatives (I), a polyethylene glycol long chain has the function of spatial stabilization and makes modified liposome realize long circulation; simultaneously, the octreotide is connected to the outside of a PEG hydrophilic chain, exposed to the outer layer of the liposome and not influenced by the PEG chain; and the conjugation between active targeting tumor cells and a cell membrane SSTR receptor is maintained after the liposome is effluent out of a tumor novel blood vessel, so as to improve uptake of liposome-containing medicines of tumor cells.
Owner:CHINA PHARM UNIV
Somatostatin precursor compound and somatostatin ligand compound of octreotide and preparation and application thereof
InactiveCN109824760ALittle impact on targetingImprove stabilityRadioactive preparation carriersPeptide preparation methodsRadioactive drugCurative effect
The invention provides a somatostatin precursor compound of octreotide, and belongs to the technical field of radioactive drugs and nuclear medicine.The precursor compound is NOTA-TATE, and the structure is shown in the following formula I (the formula is shown in the description). The invention also provides a somatostatin ligand compound of the octreotide, wherein the precursor compound NOTA-TATE is labeled by using radionuclide A to obtain A-NOTA-TATE.The invention also provides a method for preparing the somatostatin precursor compound and the somatostatin ligand compound of the octreotide.The ligand compound obtained by nuclide labeled NOTA-TATE is a colorless transparent liquid injection, has the advantages of simple preparation process, short time-consuming, high labeling rate, stable labeling, good affinity with somatostatin receptors, good targeting for tumor and good imaging effect,can be used for differential diagnosis, staging, accurate localization of focus, treatment andcurative effect monitoring of neuroendocrine tumors, and has good application prospects.
Owner:THE AFFILIATED HOSPITAL OF SOUTHWEST MEDICAL UNIV
Sustained release formulation comprising octreotide and two or more polylactide-co-glycolide polymers
The invention relates to a sustained release formulation comprising octreotide and two or more polylactide-co-glycolide polymers. In other words, the present invention relates to sustained release formulations comprising as active ingredient octreotide or a pharmaceutically-acceptable salt thereof and two or more different polylactide-co-glycolide polymers (PLGAs).
Owner:NOVARTIS AG
Drug for neuroendocrine tumors and process thereof
PendingCN109550060AReduce leakageSmall toxicityOrganic active ingredientsEmulsion deliverySide effectSomatostatin receptor
The invention discloses a drug for neuroendocrine tumors and a process thereof, and specifically relates to the field of biopharmaceuticals, wherein the drug includes the following raw materials: cysteamine hydrochloride, Boc2O, TPE-NHS, Boc-SS-NH2, HOOC-PEG5k-NH2, BLG-NCA, NHS, octreotide, 3-aminophenylboric acid and ETO. With phenylborate bonds contained in the drug, micelles can maintain structural stability in blood circulation, thereby protecting an encapsulated drug, reducing drug leakage, and reducing systemic toxic and side effects. When the drug-loaded micelles are gathered in tumor tissues through the EPR effect, octreotide on the surface of the micelles can specifically bind to the overexpressed somatostatin acceptors of neuroendocrine tumor cells, so as to enhance the targetingability of the micelles.
Owner:南京苏睿医药科技有限公司
Fe3O4/octreotide modified nano-liposome and preparing method thereof
InactiveCN107029246AGood lookingWith magneto-thermal conversion propertiesOrganic active ingredientsEnergy modified materialsTreatment effectSide effect
A Fe3O4 / octreotide modified nano-liposome is an octreotide modified drug-loading nano-liposome with a particle size of 80-200 nm and with magnetic Fe3O4 nano particles on the surface. According to a preparing method of the Fe3O4 / octreotide modified nano-liposome, a drug-loading liposome is modified with L-glutamic acid, so that the surface of the drug-loading liposome is negatively charged, and the surface of the nano-liposome is then modified with octreotide through electrostatic interaction; and Fe3O4 nano particles solution is mixed with the nano-liposome modified with octreotide based on a certain volume, and the Fe3O4 / octreotide modified dual-target nano-liposome is prepared through interaction between PEG on the surface of Fe3O4 and the hydroxyl group on the surface of the lipidosome. The method is simple, operation is easy, the preparing condition is mild, the cost is low, adopted raw materials have no toxic or side effect on the human body, and the cancer treatment effect is improved by means of magnetic targeting, precise drug release and the specific binding capacity of octreotide to tumor cells.
Owner:YANSHAN UNIV
Injection preparation before pancreas islet extraction by piglet pancreas
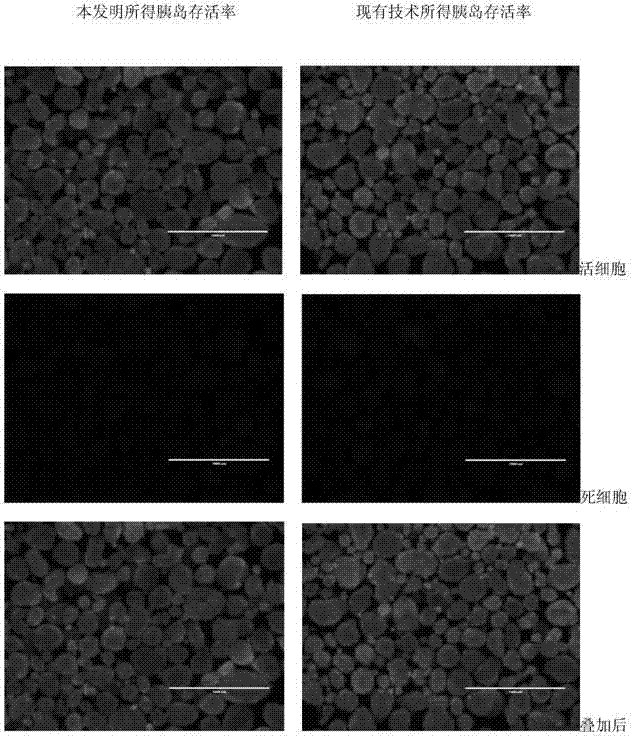
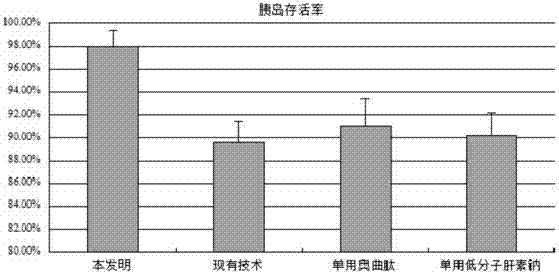
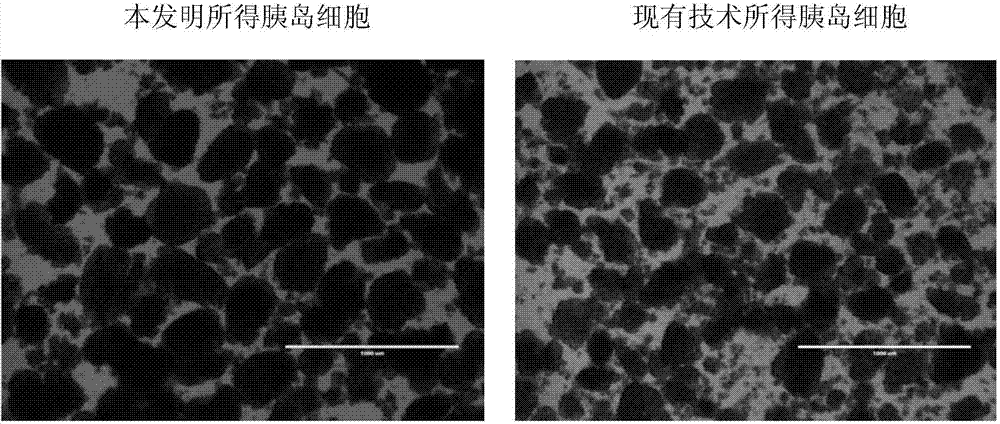
ActiveCN104324392AImprove Separation ResultsInhibition of secretionIn-vivo testing preparationsSecretionSecreted substance
The invention discloses an injection preparation before pancreas islet extraction by piglet pancreas. The injection preparation comprises octreotide and low molecular heparin sodium, the injection amount of octreotide is 1-3mum / kg; and the injection amount of low molecular heparin sodium is 5-15IU / kg. The operation is simple and easy to perform, pancreatin secretion can be greatly reduced, pancreas ischemic time is shortened, ischemic damage is reduced, pancreas islet loss is finally reduced, islet yield can be effectively increased, activity can be protected, the injection preparation provides available resource for preparing and transplanting provide, and has wide clinical application prospect.
Owner:HUNAN XENO LIFE SCI
All-solid-phase method for synthesizing octreotide
ActiveCN107778351AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionTrifluoroacetic acidHydrogen bromide
The invention relates to the field of medicinal synthesis and discloses a method for synthesizing octreotide. According to the method disclosed by the invention, special Boc-Thr(Bzl)-OL-Merrifield serves as an initial resin, an iodine oxidation method, a trifluoroacetic acid solution acidolysis resin of hydrogen bromide, protecting groups and other manners are matched to realize all-solid-phase preparation of the octreotide. Finally, the prepared octreotide has high purity and total yield. Meanwhile, the preparation process is greatly simplified, the preparation time is shortened, and the production cost is reduced.
Owner:CHENGDU SHENGNUO BIOPHARM
Octreotide-modified gold spherical shell nano liposome and preparation method thereof
InactiveCN106491535AMild experimental conditionsSimple and fast operationOrganic active ingredientsEnergy modified materialsTherapeutic effectLiposome
The invention relates to an octreotide-modified gold spherical shell nano liposome which is formed by forming a compact nano gold spherical shell on the surface of an oleanolic acid nano liposome and coating an octreotide ligand on the gold spherical shell surface. The particle size of the octreotide-modified gold spherical shell nano liposome is 50-200nm, and the surface plasma resonance absorption wavelength is 650-950nm. The preparation method mainly comprises the following steps: modifying the oleanolic acid liposome with glutathione so that the oleanolic acid liposome surface is provided with mercapto groups; carrying out crystal seed growth to prepare the gold spherical shell nano liposome; and finally, under mild conditions, coupling octreotide onto the gold nanoparticle surface through surface amino and hydroxy groups under the condition that the octreotide solution / gold spherical shell nano liposome volume ratio is (0.3-1):1, and carrying out co-incubation to obtain the octreotide-modified gold spherical shell nano liposome. The method is simple to operate, easy to control and mild in conditions. The treatment effect on cancer is enhanced by utilizing the photothermal effect of nano gold spherical shells, controlled release of drugs and specific targeting property of octreotide to tumor cells.
Owner:YANSHAN UNIV
Polyethylene glycol modified octreotide and preparation method thereof
InactiveCN110563809AGood slow and controlled release effectPeptide/protein ingredientsDigestive systemHalf-lifeTrifluoroacetic acid
The invention relates to the technical field of polypeptide drug preparation, and provides polyethylene glycol modified octreotide and a preparation method thereof. The method is characterized in thatthe method adopts a solid-phase synthesis method to couple octreotide main chains onto resin from a C end to an N end in sequence, an N end protecting group Fmoc is removed and then coupled with oneend of adipic acid through an amido bond, the other end of the adipic acid is coupled with polyethylene glycol, and finally, trifluoroacetic acid is used for deprotection and then liquid-phase cyclization is carried out. The compound can keep the original activity of the octreotide, overcomes the problem of short half-life period of the octreotide, greatly improves the clinical application compliance and has better application value.
Owner:应连心
Preparation method for octreotide
ActiveCN106749528APrevent elutionSuitable for continuous productionPeptide preparation methodsDesalinationReversed-Phase Liquid Chromatography
The invention discloses a preparation method for octreotide. The preparation method comprises the following step: successively subjecting a solution of a crude octreotide precursor product to reversed-phase cyclization, reversed-phase purification and reversed-phase desalination by adopting a highly-efficient liquid-phase reversed-phase chromatography process, wherein a filling material for the highly-efficient liquid-phase reversed-phase chromatography process is a styrene-divinyl benzene copolymer; and the crude octreotide precursor product contains two free sulfhydryl groups. The preparation method provided by the invention prepares a pure polypeptide product by adopting reversed-phase adsorption for cyclization, purification and desalination through a one-step method, optimizes production process, and is applicable to continuous industrial production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Octreotide Microsphere-Based Arterial Embolization for Treating Obesity
PendingUS20220088150A1Reduce in quantitySurgical adhesivesPeptide/protein ingredientsGastric arteryBiology
This invention provides a method for causing weight loss in a subject comprising introducing biodegradable microspheres into one or more of the subject's gastric arteries, wherein the microspheres (i) have a d90 value from 40 μm to 500 μm; (ii) comprise polylactic acid (PLA) and / or polylactic co-glycolic acid (PLGA); (iii) carry a therapeutically effective amount of pharmaceutical octreotide; (iv) embolize gastric arterial vessels supplied by the one or more arteries into which they are introduced; and (v) release octreotide during embolization.
Owner:BIOVENA SCI LLC
Chitose derivates using octreotide as target ligand and use thereof in medicament
Owner:CHINA PHARM UNIV
Octreotide sustained-release microspheres with high encapsulation rate and preparation method thereof
ActiveCN102940609AHigh encapsulation efficiencyHigh activityPeptide/protein ingredientsPharmaceutical non-active ingredientsMicrospherePolyvinyl alcohol
The invention discloses octreotide sustained-release microspheres with high encapsulation rate and a preparation method thereof. The octreotide sustained-release microspheres are characterized in that the weight ratio of octreotide to polylactic-co-glycolic acid (PLGA) is 1:10-1:5; trehalose is used as a dispersion phase stabilizer, and the weight ratio of the trehalose to the octreotide is 1:10-1:1; polyvinyl alcohol and polysorbate 80 are continuous phase emulsifiers, the concentrations of the polyvinyl alcohol and the polysorbate 80 are respectively 5-30mg / ml and 0.1-0.6mg / ml, and the ratio of the polyvinyl alcohol to the polysorbate 80 is 50:1; and the pH value of a continuous phase is 7.0-9.0. The obtained octreotide microspheres are round, uniform in granularity distribution and high in encapsulation rate, the in-vitro medicament release performance accords with the characteristics of long-acting preparations, the medicament release percentage in 24 hours is less than 20 percent, and the accumulated release in 30 days reach over 80 percent.
Owner:SHANGHAI SOHO YIMING PHARMA
Dicabba-analogues of octreotide
Owner:ADVANCED ACCELERATOR APPLICATIONS SA
Octreotide-modified nanomedicine for cancer treatment or cancer palliative care
ActiveUS20130171243A1Organic active ingredientsCyclic peptide ingredientsCancer preventionCancer therapy
The present invention relates to a medical composition for treatment, palliative care or prevention of cancer, such as medullary thyroid carcinoma. Specifically, the invention relates to a medical composition for treatment, palliative care or prevention of medullary thyroid carcinoma, which comprises liposomes modified with octreotide.
Owner:NANOSION
Process for synthesis of cyclic octapeptide
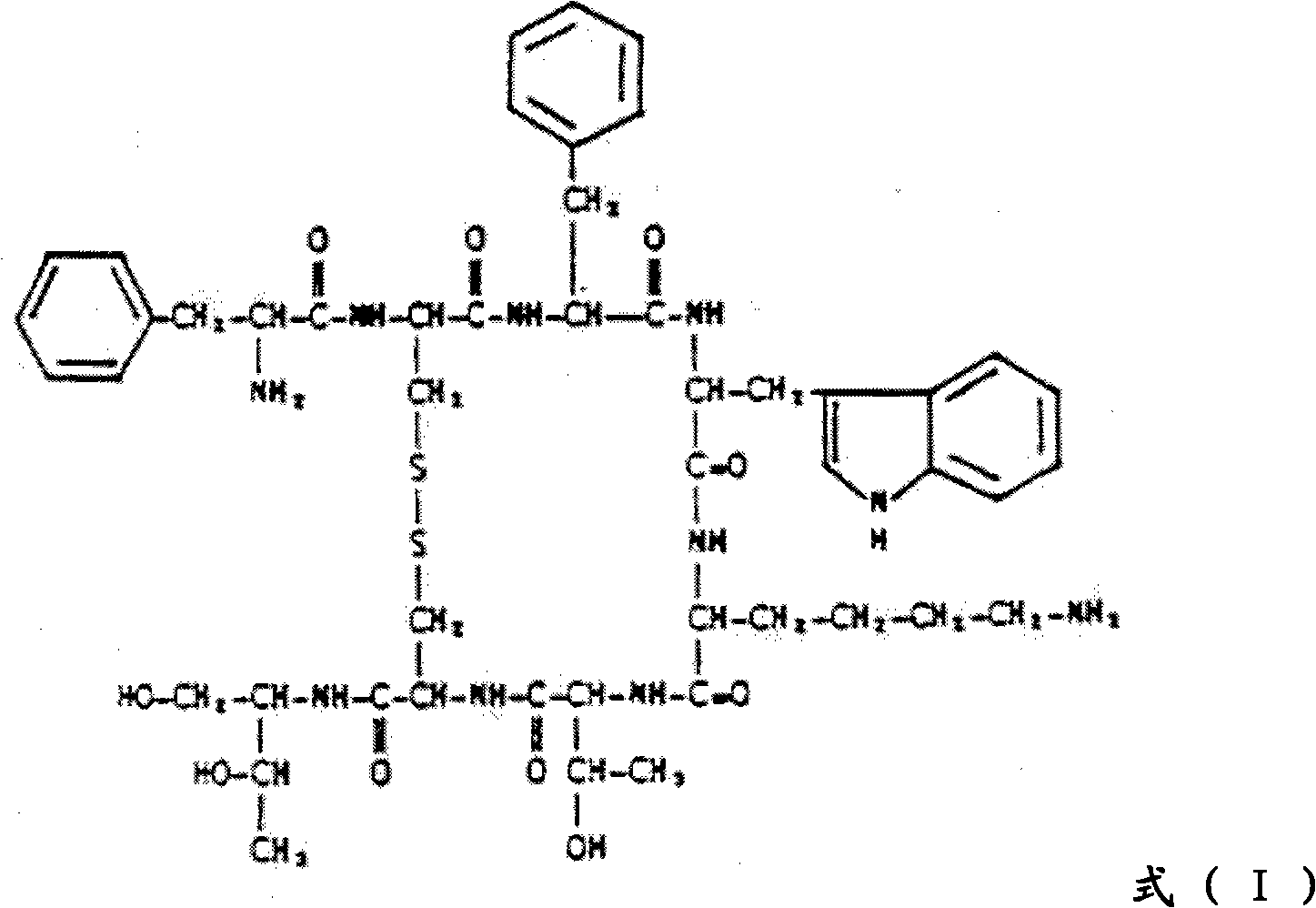
This invention relates a process for preparing octreotide and derivatives thereof. The starting material, Cys(Trt)-2-Chlorotrityl resin is coupled with various amino acids to obtain a protected heptapeptide of formula (2): Boc-D-Phe-Cys(Trt)-Phe-D-Trp-Lys(Boc)-Thr(OBut)-Cys(Trt)-2-Chlorotrityl resin. The linear protected peptide of formula (2) is cleaved from the support using TFA5TIS and water to yield linear protected peptide of formula (3) Boc-D-Phe-Cys(Trt)-Phe-D-Trp-Lys(Boc)-Thr(OBut)-Cys(Trt)-OH Linear protected heptapeptide of formula (3) is deprotected to yield heptapeptide of formula (6): D-Phe-Cys-Phe-D-Tip-Lys-Thr-Cys-OH; which is cyclized using hydrogen peroxide and to the cyclic peptide of formula (7) D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-OH; threoninol is coupled at C terminal to yield octreotide. Alternatively threoninol is coupled to the heptapeptide of formula (3) to yield protected octapeptide of formula (4) Boc-D-Phe-Cys(Trt)-Phe-D-Trp-Lys(Boc)-Thr(OBut)-Cys(Trt)-Thr-OL which is subsequently deprotected to yield linear octapeptide of formula (5) D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-OL and cyclized with hydrogen peroxide to yield cyclic octreotide with a yield of >95%.
Owner:USV LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com