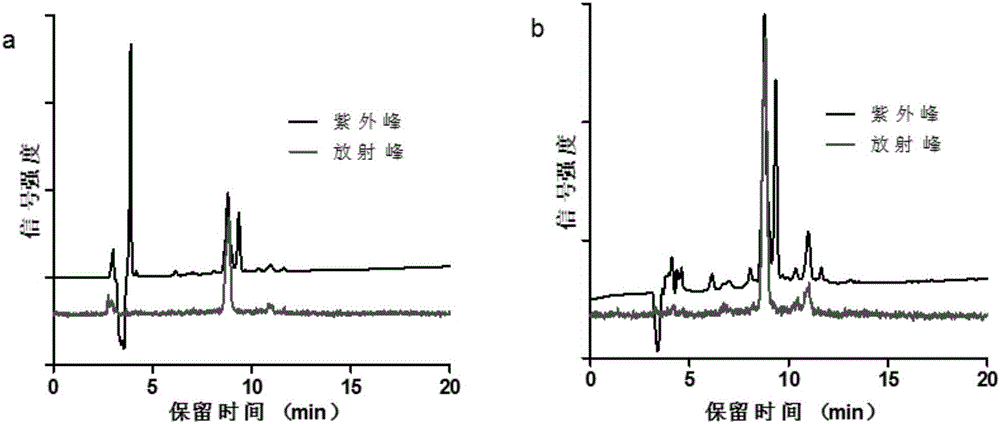
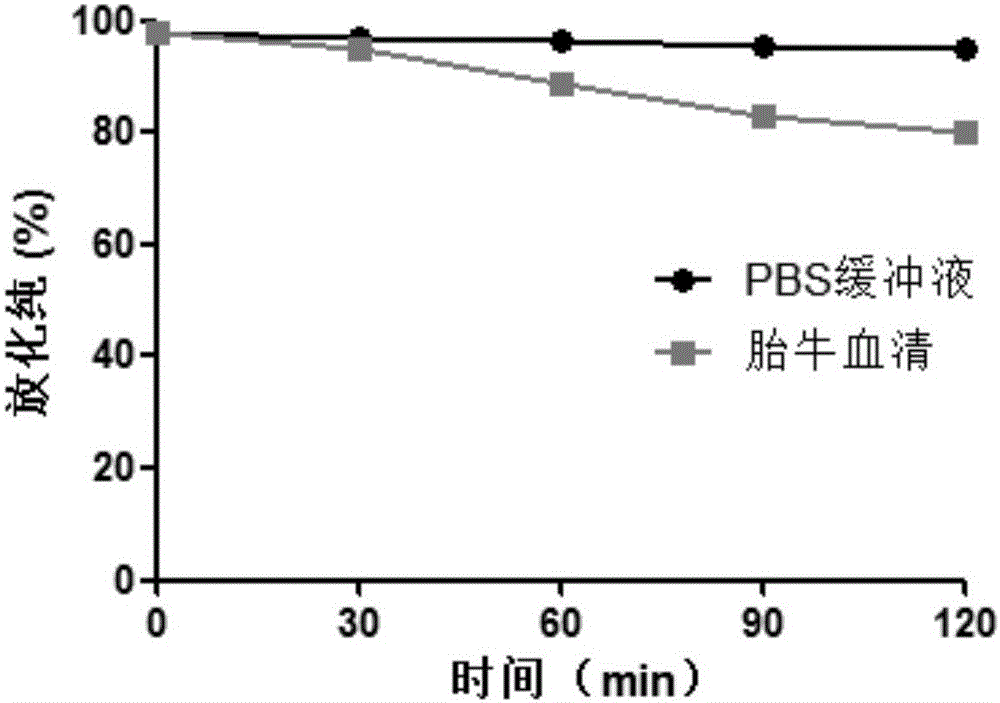
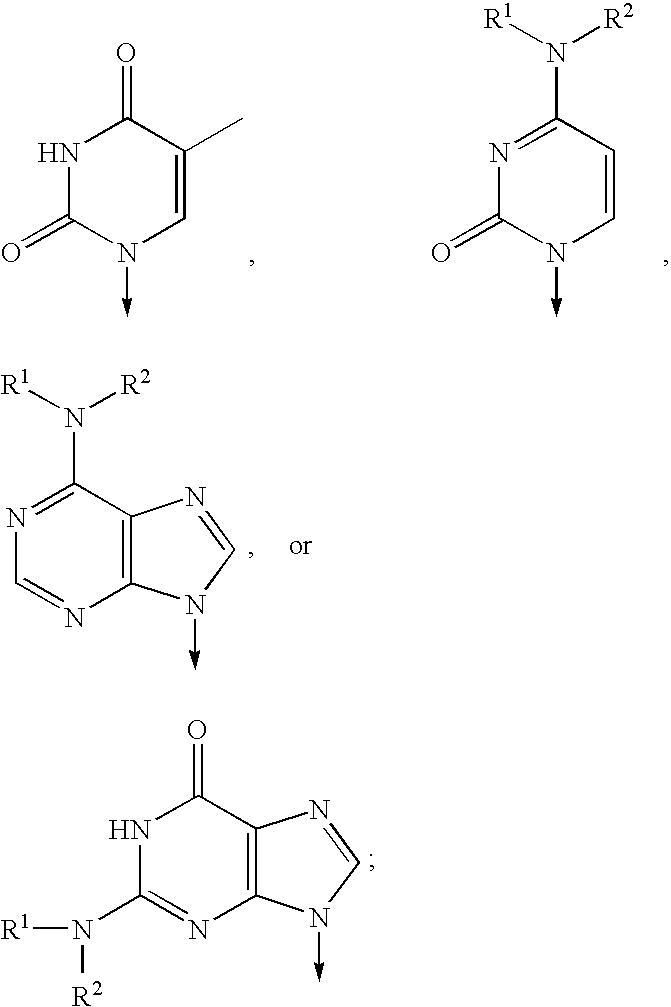
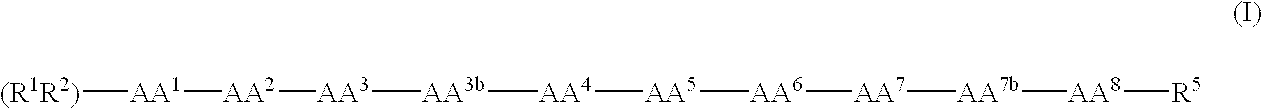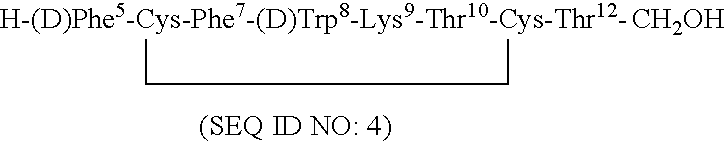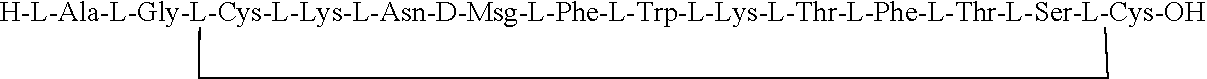Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Somatostatin receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Somatostatin receptors are receptors for the ligand somatostatin, a small neuropeptide associated with neural signaling, particularly in the post-synaptic response to NMDA receptor co-stimulation/activation. Somatostatin is encoded by a CRE and is very susceptible to gene promoter region activation by transcription factor CREB.
Compound having agonistic activity on somatostatin receptor, and use thereof for medical purposes
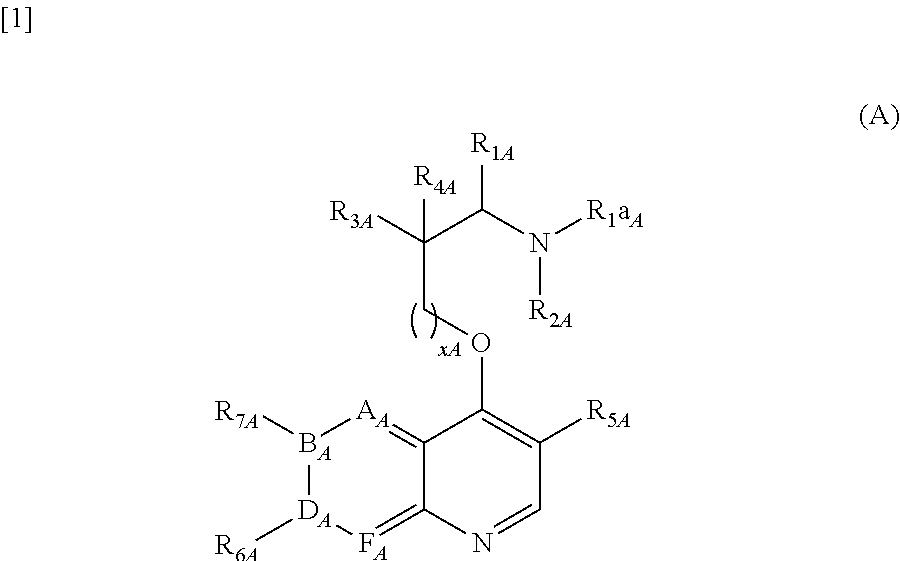
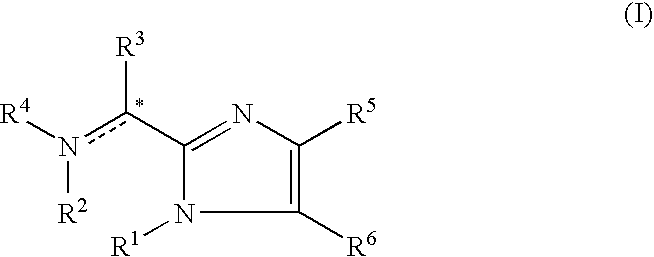
Provision of orally-available and low-toxic somatostatin receptor subtype 2 agonist. Since the compound represented by the general formula (I):[wherein all symbols represent the same meanings as those described in the description] a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof is non-peptidic low-molecular compound which has strong somatostatin receptor subtype 2 agonist activity, the compound is orally-available. Additionally, since the compound is low-toxic, the compound is useful for the prevention and / or treatment of the somatostatin related diseases such as acromegaly or gastrointestinal obstruction.
Owner:ONO PHARMA CO LTD
Anticancer therapy
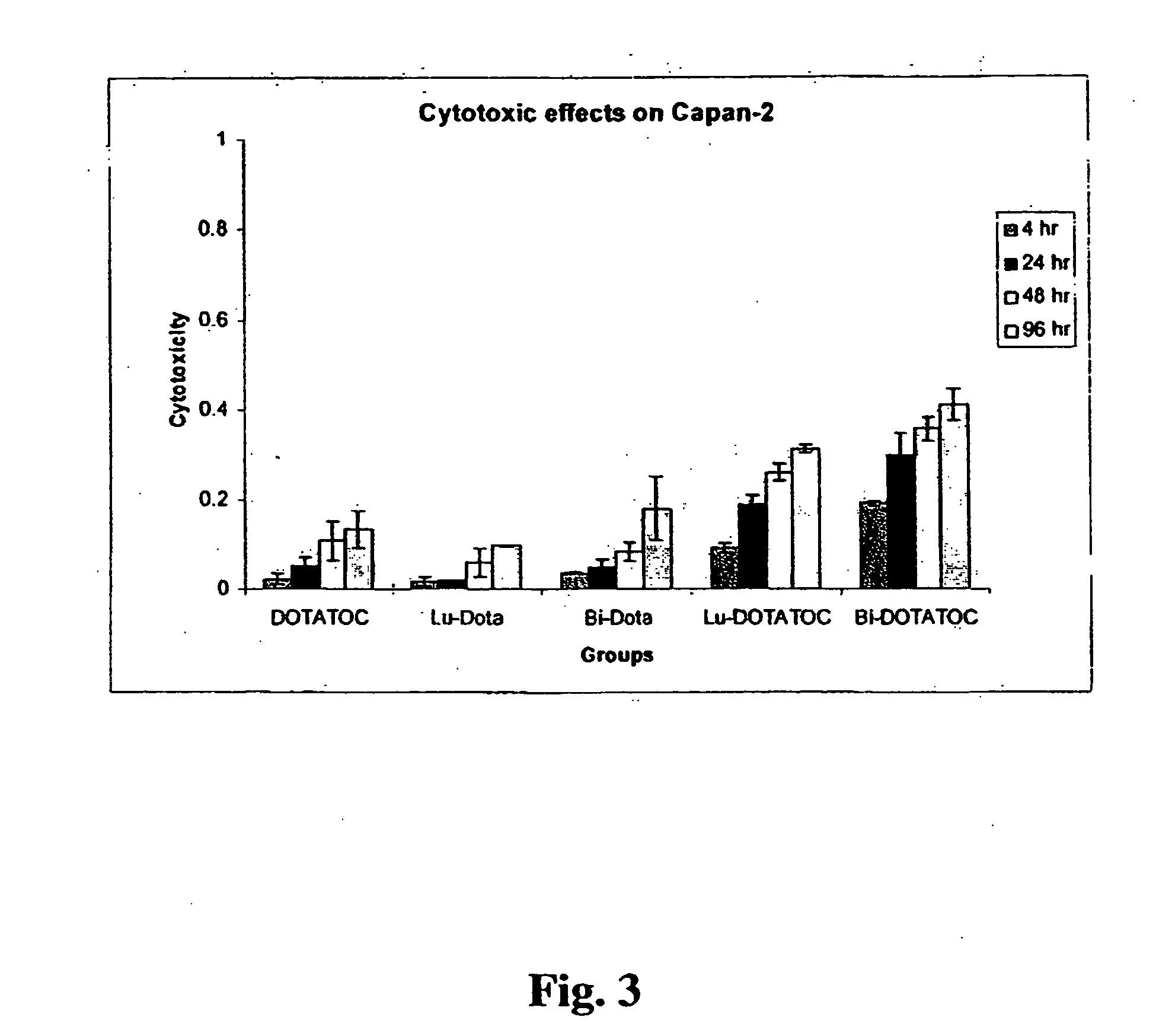
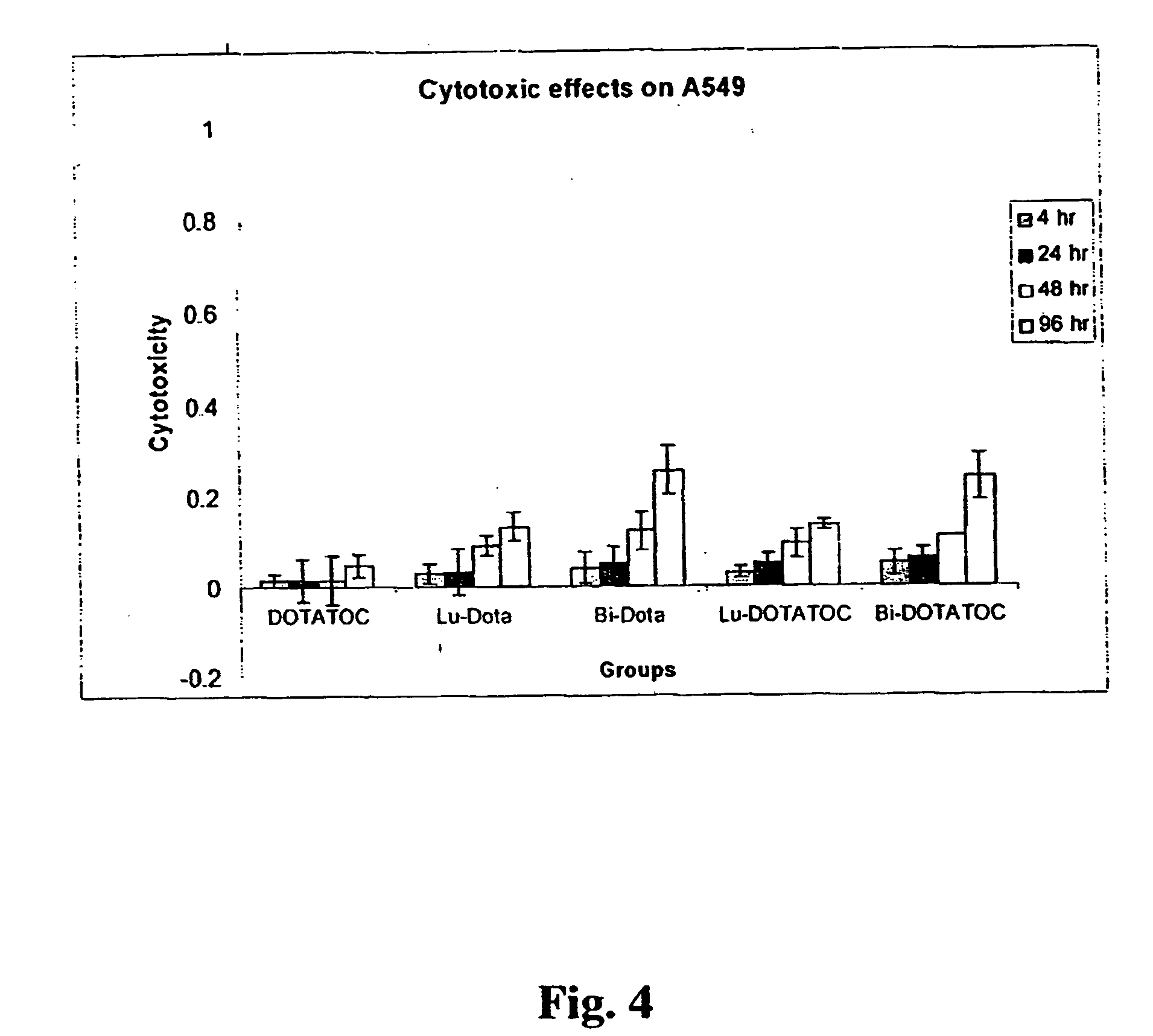
InactiveUS20070025910A1High expressionReduce the possibilityOrganic active ingredientsPeptide/protein ingredientsSomatostatin analogCytotoxicity
A subject afflicted with a cancer or precancerous condition is treated by administering an agent that increases expression of somatostatin receptors, and a cytotoxic recognition ligand. In an alternative embodiment, somatostatin analogs, which are radiolabeled are used to treat cancer or precancerous conditions.
Owner:STC UNM
Al18F-NOTA-PEG6-TATE of targeted somatostatin receptor and preparation method and application of Al18F-NOTA-PEG6-TATE
ActiveCN106084005AEasy to markMarking method saves timeRadioactive preparation carriersPeptide preparation methodsAcetic acid18f labeling
The invention discloses Al18F-NOTA-PEG6-TATE of a targeted somatostatin receptor and a preparation method and application of Al18F-NOTA-PEG6-TATE. The preparation method of the label includes the steps that a somatostatin derivative TATE and a difunctional chelating agent NOTA are connected through PEG to generate a radiolabelled precursor, then positron nuclide 18F labeling is carried out through a one-pot method, and the 18F labeled somatostatin derivative Al18F-NOTA-PEG6-TATE can be obtained. The method can be carried out in an acetate buffer solution system or an acetonitrile reaction system, and the method has the advantages of being easy and convenient to operate and high in labeling rate and product purity. The prepared positron label of the targeted somatostatin receptor can be applied to preparation of a PET / CT molecular probe or tumor therapy medicine, and provides a novel thought for synthesis of the clinical PET / CT molecular probe.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Anticancer therapy
ActiveUS20150196673A1High expressionReduce the possibilityOrganic active ingredientsPeptide/protein ingredientsSomatostatin AnalogueSomatostatin receptor
A subject afflicted with a cancer or precancerous condition is treated by administering an agent that increases expression of somatostatin receptors, and a cytotoxic recognition ligand. In an alternative embodiment, somatostatin analogs, which are radiolabeled are used to treat cancer or precancerous conditions.
Owner:STC UNM
Neuromedin b and somatostatin receptor agonists
Owner:IPSEN PHARMA SAS
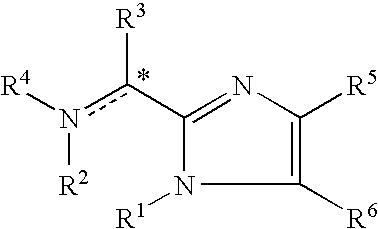
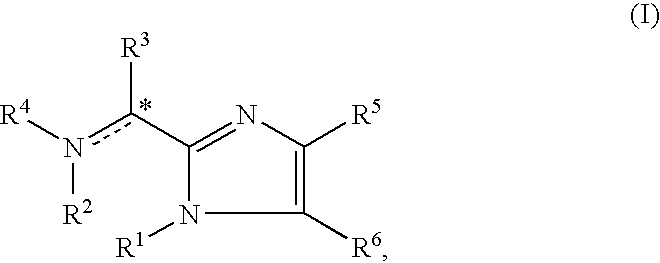
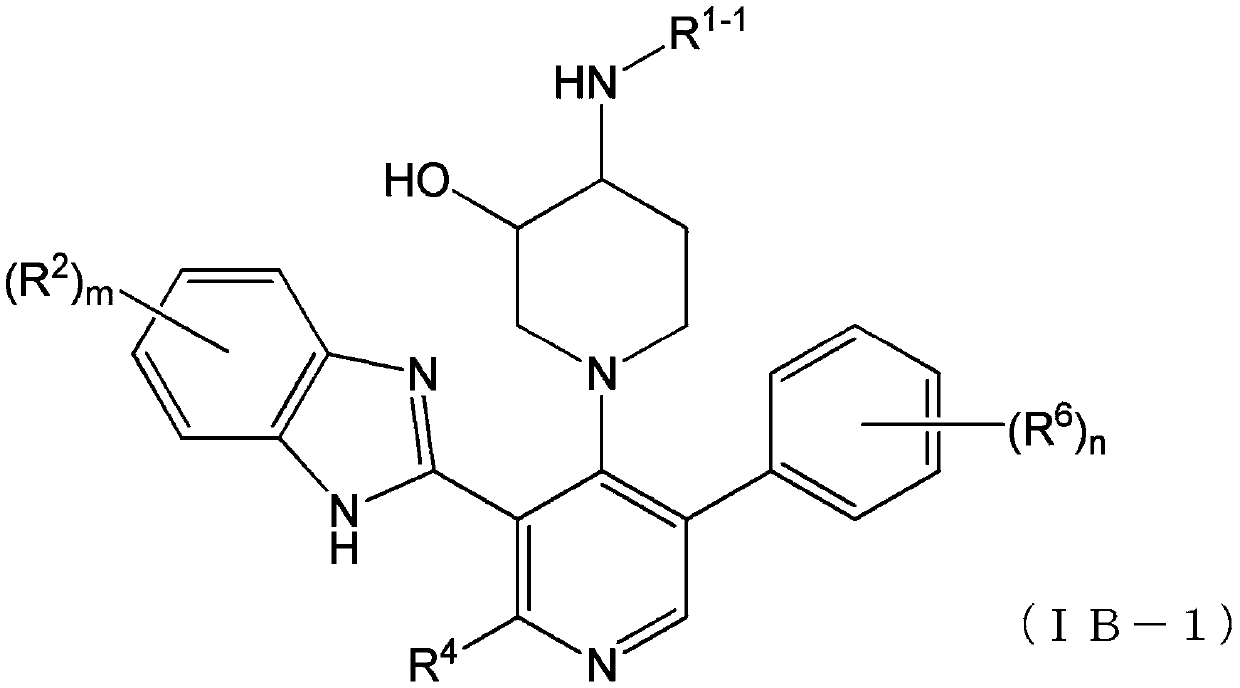
Imidazolyl derivatives
The present invention is directed to imidazolyl derivatives of the formula: where the substituents are defined in the specification, or a pharmaceutically acceptable salt thereof. The derivatives bind selectively to the somatostatin subtype receptors and elicit either an agonist or antagonist effect from the somatostatin subtype receptors. The derivatives are useful for treating a variety of diseases including acromegaly, restenosis, Crohn's disease, systemic sclerosis, external and internal pancreatic pseudocysts and ascites, VIPoma, nesidoblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, diarrhea, AIDS related diarrhea, chemotherapy related diarrhea, scleroderma, Irritable Bowel Syndrome, pancreatitis, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Cushing's Syndrome, gonadotropinoma, hyperparathyroidism, Graves' Disease, diabetic neuropathy, Paget's disease, polycystic ovary disease, cancer, cancer cachexia, hypotension, postprandial hypotension, panic attacks, GH secreting adenomas or TSH secreting adenomas.
Owner:IPSEN PHARMA SAS
Conformationally constrained backbone cyclized somatostatin analogs
InactiveUS6930088B2Improve stabilityHigh selectivityNervous disorderPeptide/protein ingredientsSomatostatin analogReceptor subtype
Novel peptides which are conformationally constrained backbone cyclized somatostatin analogs, having somatostatin receptor subtype selectivity are disclosed. These patterns or receptor subtype selectivity provide compounds having improved therapeutic utility. Methods for synthesizing the somatostatin analogs and for screening of the somatostatin analogs are also disclosed. Furthermore, pharmaceutical compositions comprising somatostatin analogs, and methods of using such compositions are disclosed.
Owner:CORTENDO
Imidazolyl derivatives
The present invention is directed to imidazolyl derivatives or pharmaceutically acceptable salts thereof which are useful as agonists or antagonists of somatostatin receptors, having the following formula (I),wherein the substituents are defined in the specification.
Owner:IPSEN PHARMA SAS
Somatostatin Receptor Antagonists and Glucose Control or Hypoglycemia
ActiveUS20110064742A1Reduce threatImprove responseOrganic active ingredientsMetabolism disorderGlycemicHypoglycemia
The present disclosure provides methods and uses for controlling tight blood glucose levels in a subject comprising administering an effective amount of a somatostatin inhibitor. The present disclosure provides methods and uses for treating or preventing hypoglycemia in a subject comprising administering an effective amount of a somatostatin inhibitor.
Owner:THE GOVERNING COUNCIL OF THE UNIV OF TORONTO
Photo-active backbone cyclized somatostatin analogs for photodynamic therapy and imaging
InactiveUS7700717B2Unique and superior chemicalUnique and superior and metabolicPeptide/protein ingredientsPhotodynamic therapySomatostatin analogReceptor subtype
Novel photo-active labeled diagnostic and therapeutic peptides which are conformationally constrained backbone cyclized somatostatin analogs, having improved somatostatin receptor subtype affinity and selectivity are disclosed. The backbone cyclized peptide analogs disclosed possess unique and superior properties over other analogs, such as chemical and metabolic stability, selectivity, increased bioavailability and improved pharmacokinetics. Furthermore, the unique patterns of receptor subtype selectivity provide compounds having improved diagnostic and therapeutic utilities. Pharmaceutical compositions comprising the photo-active backbone cyclized somatostatin analogs, reagents for synthesizing same, and methods of using such compositions for diagnostic and therapeutic purposes including optical imaging and photodynamic therapy are also disclosed.
Owner:DEVELOGEN ISRAEL
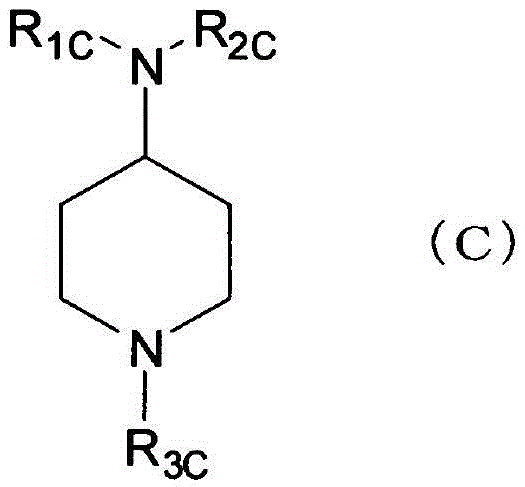
Substituted piperidinamines as somatostatin receptor subtype 5 (SSTR5) antagonists
Owner:F HOFFMANN LA ROCHE & CO AG
Compound having agonistic activity to somatostatin receptor and medicinal use thereof
InactiveCN105593221APossess strong agonistic activityRelieve painOrganic active ingredientsOrganic chemistryDiseaseAcromegaly
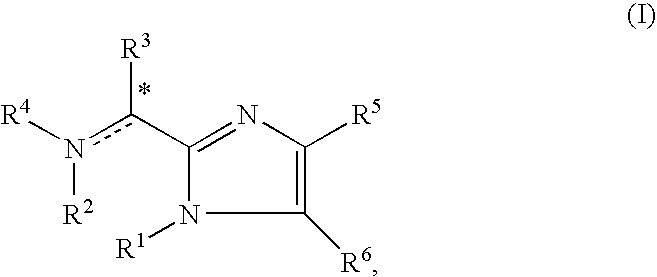
Provided is a somatostatin receptor subtype-2 agonist. A compound represented by general formula (I) [wherein each symbol has the same meaning as defined in the description], a salt thereof, an N-oxide thereof or a solvate thereof, or prodrugs of the same, which are low-molecular compounds having potent agonistic activity to somatostatin receptor subtype-2 and, therefore, can be administered in a simpler manner and have high safety and low toxicity, are useful in preventing and / or treating somatostatin-related diseases such as acromegaly and gastrointestinal obstruction.
Owner:ONO PHARMA CO LTD
Somatostatin analogs and IGF-I inhibition for breast cancer prevention
InactiveUS20090325863A1Decreased cell divisionInhibit apoptosisCompound screeningApoptosis detectionSomatostatin analogSSTR5 receptor
The present invention relates generally to the use and application of compounds or agents, including somatostatin analogs, with effect on, affinity for, or specificity to SSTR3 and / or SSTR5 somatostatin receptors, particularly in the breast, for the treatment of breast hyperplasia, pre-neoplastic lesions and breast carcinoma and / or prevention or reduction of risk for breast cancer or treatment of breast cancer, including DCIS. The invention also relates to use of somatostatin analog SOM230 in treatment of breast hyperplasia and / or prevention or treatment of breast cancer. The invention includes assays and methods for screening and identifying breast hyperplasia with elevated SSTR3 and / or SSTR5 receptors and for chemotherapy and identifying compounds of use in the invention which are specific for, modulate via, or bind to SSTR3 and / or SSTR5 receptors.
Owner:NEW YORK UNIV SCHOOL OF MEDICINE
Selective treatment of endothelial somatostatin receptors
InactiveUS20060089299A1Inhibit angiogenesisSenses disorderPeptide/protein ingredientsHuman useAgonist
The invention provides for the use of somatostatin receptor selective ligands (selective for SSTR1 or SSTR4) to treat human endothelial cells and to formulate medicaments for human use. The medicaments may for example be used to treat an angiogenic disease. In various embodiments, the angiogenic disease may for example be macular degeneration or a solid tumor. The SSTR1 or SSTR4 selective agonists may include the SSTR1 agonist (des-AA1,2,5[DTrp8,IAamp9]SS).
Owner:HSIANG YORK +3
Method of Treatment of Neuroendocrine Tumors That Over-Express Somatostatatin Receptors
InactiveUS20180185524A1Reducing tumorigenicityPrevent proliferationRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsImmunooncologySomatostatin receptor
The present invention relates to methods of treating cancers that over-express somatostatin receptors. More specifically, the invention provides a combined therapy in which a combination of Peptide Receptor Radionuclide Therapy (PRRT) and Immuno Oncology therapy (I-O therapy) are administered for the treatment of neuroendocrine tumors.
Owner:ADVANCED ACCELERATOR APPLICATIONS SA
Labelled somatostatin analogs backbone cyclized through metal complexation
InactiveUS20050226813A1Unique and superior chemicalUnique and superior and metabolicNervous disorderPeptide/protein ingredientsSomatostatin analogMetabolic stability
Novel diagnostic and therapeutic peptides disclosed herein are somatostatin analogs backbone cyclized through metal complexation, and having improved somatostatin receptor subtype affinity and selectivity. These backbone cyclized peptide analogs possess unique and superior properties over other analogs, including chemical and metabolic stability, selectivity, increased bioavailability and improved pharmacokinetics. Pharmaceutical compositions that include these backbone cyclized somatostatin analogs, radiolabelled analogs, reagents for synthesizing same, and methods of using such compositions for diagnostic and therapeutic purposes are also disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
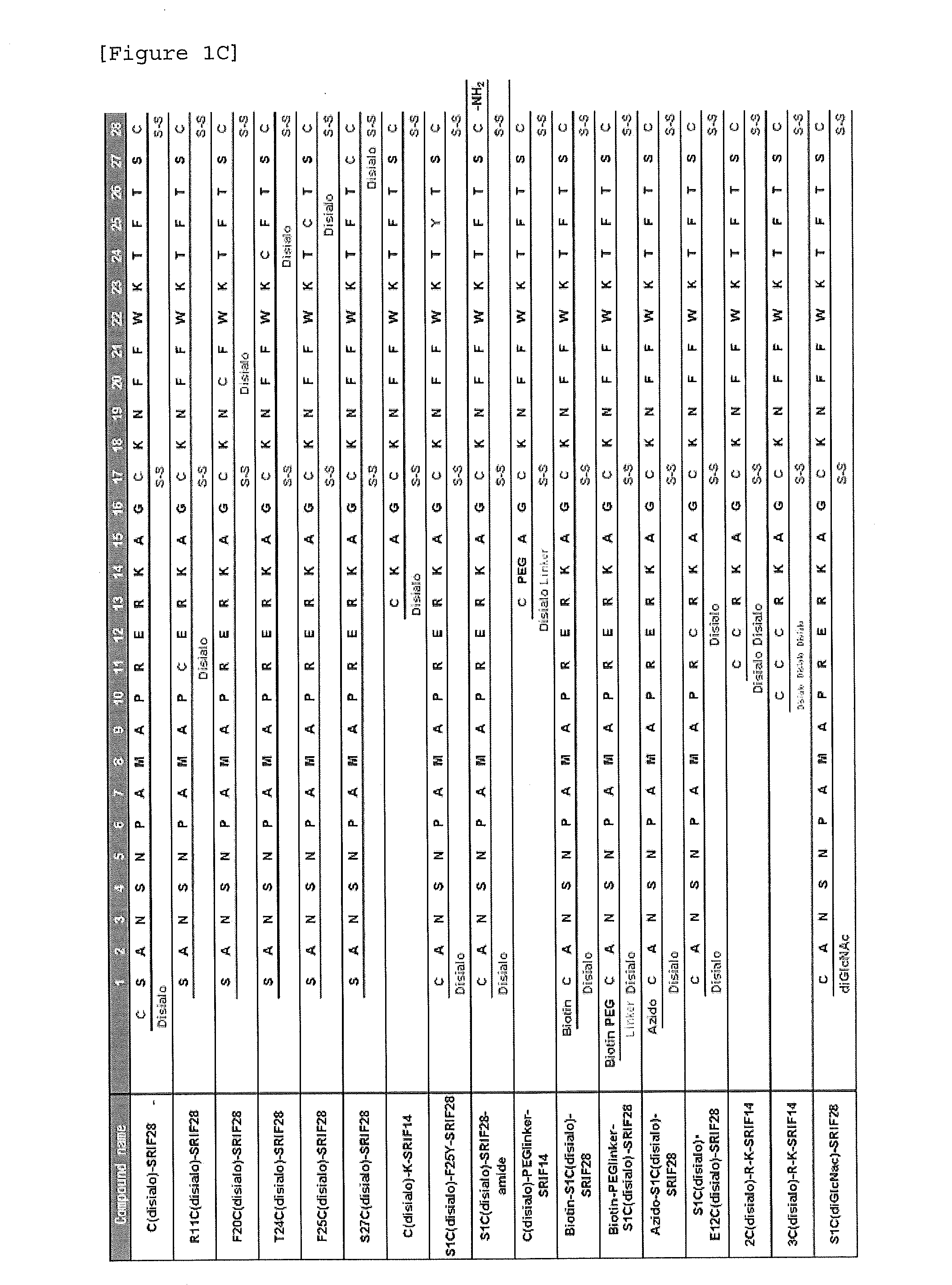
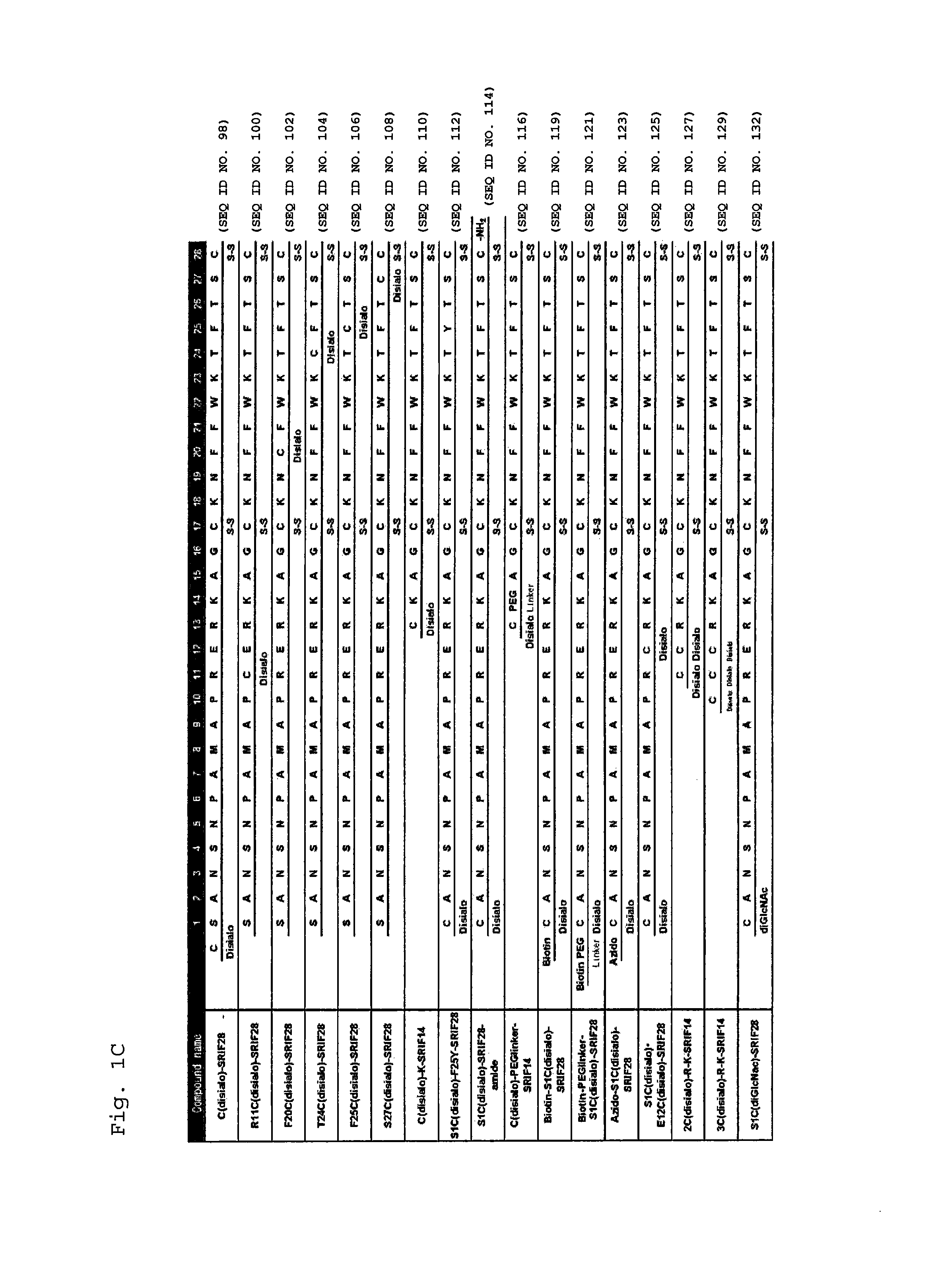
Glycosylated Polypeptide and Drug Composition Containing Said Polypeptide
ActiveUS20140315800A1Improve stabilityEasy to degradeSenses disorderNervous disorderPharmaceutical drugSomatostatin receptor
[Problem] To provide a glycosylated polypeptide having affinity to somatostatin receptors and, compared to somatostatins, having improved in-blood stability. [Solution] The glycosylated polypeptide is characterized by at least two amino acids in a somatostatin or an analogue thereof being replaced by glycosylated amino acids.
Owner:GLYTECH
Somatostatin receptor 1 and/or 4 selective agonists and antagonists
The invention relates to (hetero)arylsulfonylamino based peptidomimetics of formula (I), wherein R1, R2, R3, A, B, D, Q, k and n are defined as disclosed, or a pharmaceutically acceptable salt or ester thereof. Compounds of formula (I) possess high affinity and selectivity for the somatostatin receptor subtypes SSTR1 and / or SSTR4 and can be used for the treatment or diagnosis of diseases or conditions wherein an interaction with SSTR1 and / or SSTR4 is indicated to be useful.
Owner:WURSTER SIEGFRIED
Compound having somatostatin receptor agonistic activity and pharmaceutical use thereof
InactiveCN110300749AStrong agonistic activityRelieve painOrganic chemistryPeptide/protein ingredientsPhospholipinSide effect
Provided is a somatostatin receptor subtype 2 agonist. A compound disclosed in the present invention that is represented by general formula (I) (wherein each symbol has the same meaning as defined inthe description) or a salt thereof is a low molecular compound having a strong agonistic activity to somatostatin receptor subtype 2. The compound according to the present invention can be orally administered and, therefore, can be easily taken and contribute to the reduction of patient's pain associated with a therapeutic treatment. Moreover, the compound according to the present invention has sufficiently weak hERG inhibitory activity and / or phospholipidosis effect, compared with the SSTR2 agonistic activity thereof, and, therefore, can inhibit or suppress side effects caused by the aforesaid activity and / or effect.
Owner:ONO PHARMA CO LTD
Delivery carrier for targeting to cells expressed with somatostatin receptors
The present invention relates to a delivery carrier including liposomes or nanoparticles for targeting the cells expressed with somatostatin receptor, consisting of a plurality of liposomes that have one phospholipid bilayer coating, one hydrophilic core and a bioactive substance. The bioactive substance is packaged in the hydrophilic core, or embedded in the phospholipid bilayer, or electrically bound with liposomes as a complex, wherein the phospholipid bilayer coating is conjugated with a plurality of molecules in the outer surface. The molecules recognize the somatostatin receptor in the surface of the target cells and induce receptor-mediated endocytosis.
Owner:IND TECH RES INST
Peptide ligands of somatostatin receptors
Owner:BCN PEPTIDES SA
Compound having agonistic activity on somatostatin receptor, and use thereof for medical purposes
Provision of orally-available and low-toxic somatostatin receptor subtype 2 agonist. Since the compound represented by the general formula (I):[wherein all symbols represent the same meanings as those described in the description] a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof is non-peptidic low-molecular compound which has strong somatostatin receptor subtype 2 agonist activity, the compound is orally-available. Additionally, since the compound is low-toxic, the compound is useful for the prevention and / or treatment of the somatostatin related diseases such as acromegaly or gastrointestinal obstruction.
Owner:ONO PHARMA CO LTD
Glycosylated polypeptide and drug composition containing said polypeptide
ActiveUS9441024B2Improve stabilityEasy to degradeSenses disorderNervous disorderSomatostatin receptorAmino acid change
[Problem] To provide a glycosylated polypeptide having affinity to somatostatin receptors and, compared to somatostatins, having improved in-blood stability. [Solution] The glycosylated polypeptide is characterized by at least two amino acids in a somatostatin or an analogue thereof being replaced by glycosylated amino acids.
Owner:GLYTECH
Imidazolyl derivatives
The present invention is directed to imidazolyl derivatives of formula (I) where the substituents are defined in the specification, which are useful as agonists or antagonists of somatostatin receptors.
Owner:SOC DE CONSEILS DE RECH & DAPPLICATIONS SCI SAS +1
Glycosylated Polypeptide and Drug Composition Containing Said Polypeptide
ActiveUS20140336116A1Improve stabilityEasy to degradeSenses disorderNervous disorderPharmaceutical drugSomatostatin receptor
[Problem] To provide a glycosylated polypeptide having an affinity to somatostatin receptors, and, compared to somatostatins, having improved in-blood stability. [Solution] The glycosylated polypeptide is characterized by at least one amino acid in a somatostatin or an analog thereof being replaced with a glycosylated amino acid.
Owner:GLYTECH
Glycosylated polypeptide and drug composition containing said polypeptide
ActiveUS9422357B2Improve stabilityEasy to degradeSenses disorderNervous disorderSomatostatin receptorAmino acid
[Problem] To provide a glycosylated polypeptide having an affinity to somatostatin receptors, and, compared to somatostatins, having improved in-blood stability. [Solution] The glycosylated polypeptide is characterized by at least one amino acid in a somatostatin or an analog thereof being replaced with a glycosylated amino acid.
Owner:GLYTECH
Compound having somatostatin receptor agonistic activity and pharmaceutical use thereof
InactiveUS20200000816A1Excellent agonistic activityEasy to manageOrganic chemistryPeptide/protein ingredientsPharmaceutical drugAgonist
A somatostatin receptor subtype 2 agonist represented by the general formula (I):wherein all symbols have the same meanings as described in the specification, or a salt thereof, which can be easily administered and can alleviate pain accompanied by the therapy of patients.
Owner:ONO PHARMA CO LTD
Somatostatin precursor compound and somatostatin ligand compound of octreotide and preparation and application thereof
InactiveCN109824760ALittle impact on targetingImprove stabilityRadioactive preparation carriersPeptide preparation methodsRadioactive drugCurative effect
The invention provides a somatostatin precursor compound of octreotide, and belongs to the technical field of radioactive drugs and nuclear medicine.The precursor compound is NOTA-TATE, and the structure is shown in the following formula I (the formula is shown in the description). The invention also provides a somatostatin ligand compound of the octreotide, wherein the precursor compound NOTA-TATE is labeled by using radionuclide A to obtain A-NOTA-TATE.The invention also provides a method for preparing the somatostatin precursor compound and the somatostatin ligand compound of the octreotide.The ligand compound obtained by nuclide labeled NOTA-TATE is a colorless transparent liquid injection, has the advantages of simple preparation process, short time-consuming, high labeling rate, stable labeling, good affinity with somatostatin receptors, good targeting for tumor and good imaging effect,can be used for differential diagnosis, staging, accurate localization of focus, treatment andcurative effect monitoring of neuroendocrine tumors, and has good application prospects.
Owner:THE AFFILIATED HOSPITAL OF SOUTHWEST MEDICAL UNIV
Drug for neuroendocrine tumors and process thereof
PendingCN109550060AReduce leakageSmall toxicityOrganic active ingredientsEmulsion deliverySide effectSomatostatin receptor
The invention discloses a drug for neuroendocrine tumors and a process thereof, and specifically relates to the field of biopharmaceuticals, wherein the drug includes the following raw materials: cysteamine hydrochloride, Boc2O, TPE-NHS, Boc-SS-NH2, HOOC-PEG5k-NH2, BLG-NCA, NHS, octreotide, 3-aminophenylboric acid and ETO. With phenylborate bonds contained in the drug, micelles can maintain structural stability in blood circulation, thereby protecting an encapsulated drug, reducing drug leakage, and reducing systemic toxic and side effects. When the drug-loaded micelles are gathered in tumor tissues through the EPR effect, octreotide on the surface of the micelles can specifically bind to the overexpressed somatostatin acceptors of neuroendocrine tumor cells, so as to enhance the targetingability of the micelles.
Owner:南京苏睿医药科技有限公司
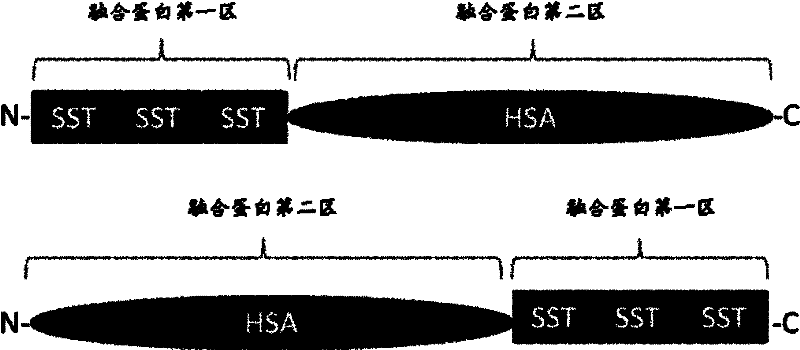
Fusion protein of human somatostatin tetradecapeptide and human serum albumin, and coding gene and preparation method thereof
InactiveCN102391376AHas clinical medicinal valueExtended stayFungiBacteriaSomatotropic hormoneSomatostatin receptor
The invention discloses a fusion protein of human somatostatin tetradecapeptide and human serum albumin, comprising a first region homologous with at least 85% sequence of the human somatostatin tetradecapeptide and a second region homologous with at least 85% sequence of the human serum albumin or a second region with partial amino acid sequence of the human serum albumin, wherein the first region homologous with the human somatostatin tetradecapeptide is positioned at the N terminal of the fusion protein, the second region homologous with the human serum albumin is positioned at the C terminal of the fusion protein, and no connecting peptide is added between the first and second regions; or the second region homologous with at least 85% sequence of the human serum albumin is positioned at the N terminal of the fusion protein, the first region homologous with at least 85% sequence of the human somatostatin tetradecapeptide is positioned at the C terminal of the fusion protein, and no connecting peptide is added between the first and second regions. The fusion protein disclosed by the invention prolongs the retention time of the human somatostatin tetradecapeptide molecules in the blood circulation system, and can be used for treating various diseases caused by surplus secretion of growth hormone, for treating diseases caused by endocrine disorder of gastrointestinal tract, for inhibiting growth of tumors expressing somatostatin receptors (SSTRs) and not expressing SSTRs, and for reducing the death rate caused by radiation injury of gastrointestinal tract.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com