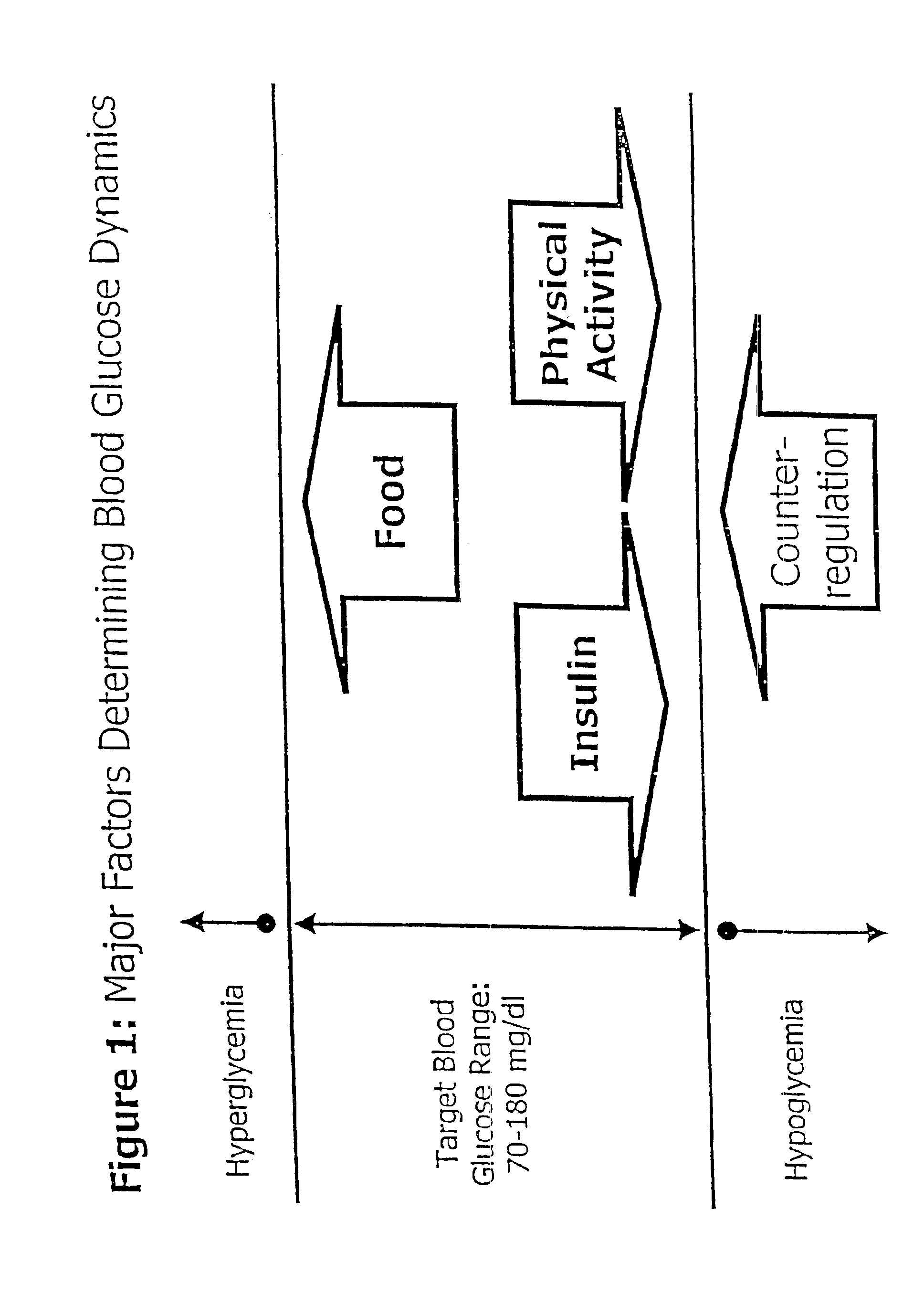
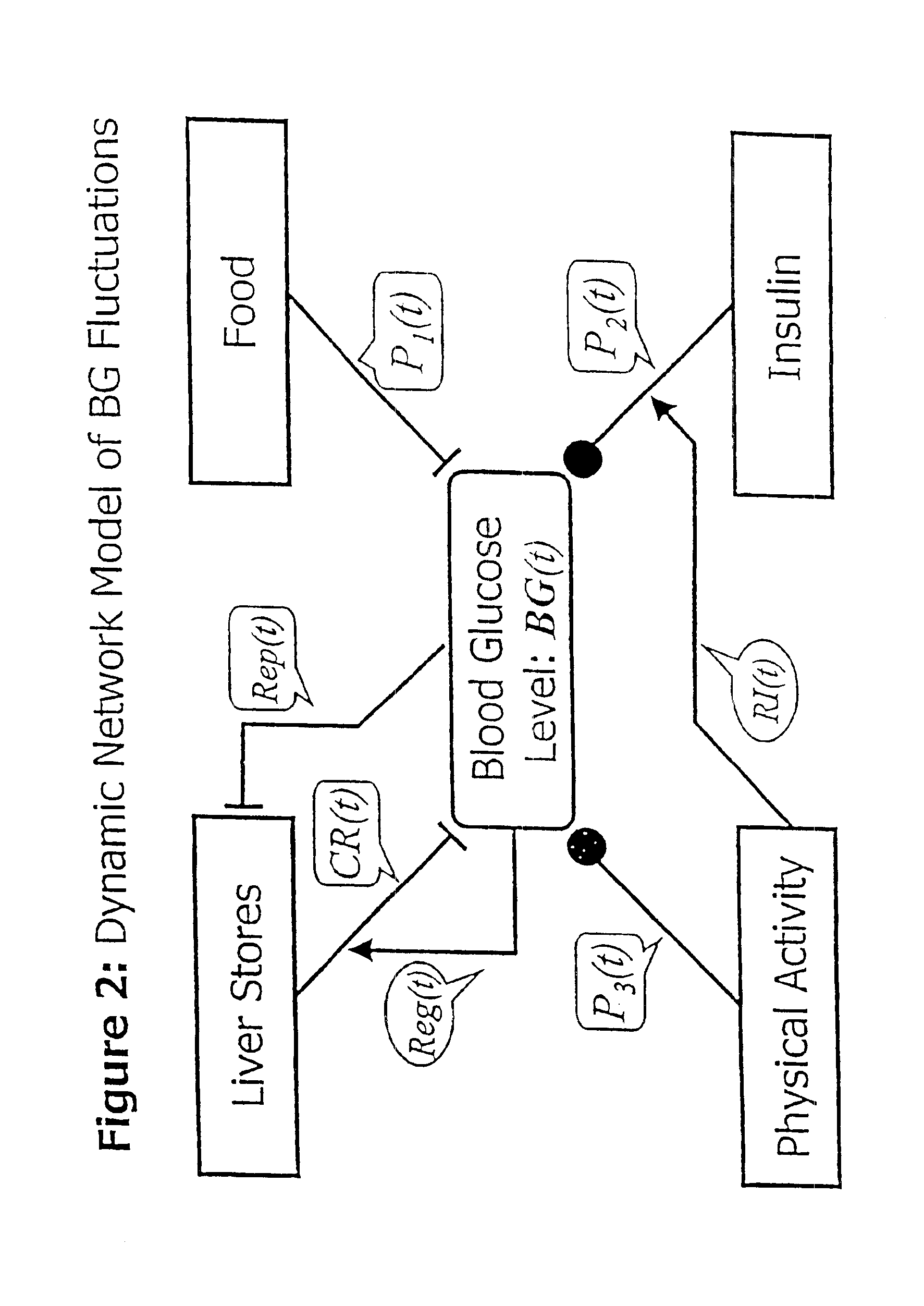
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
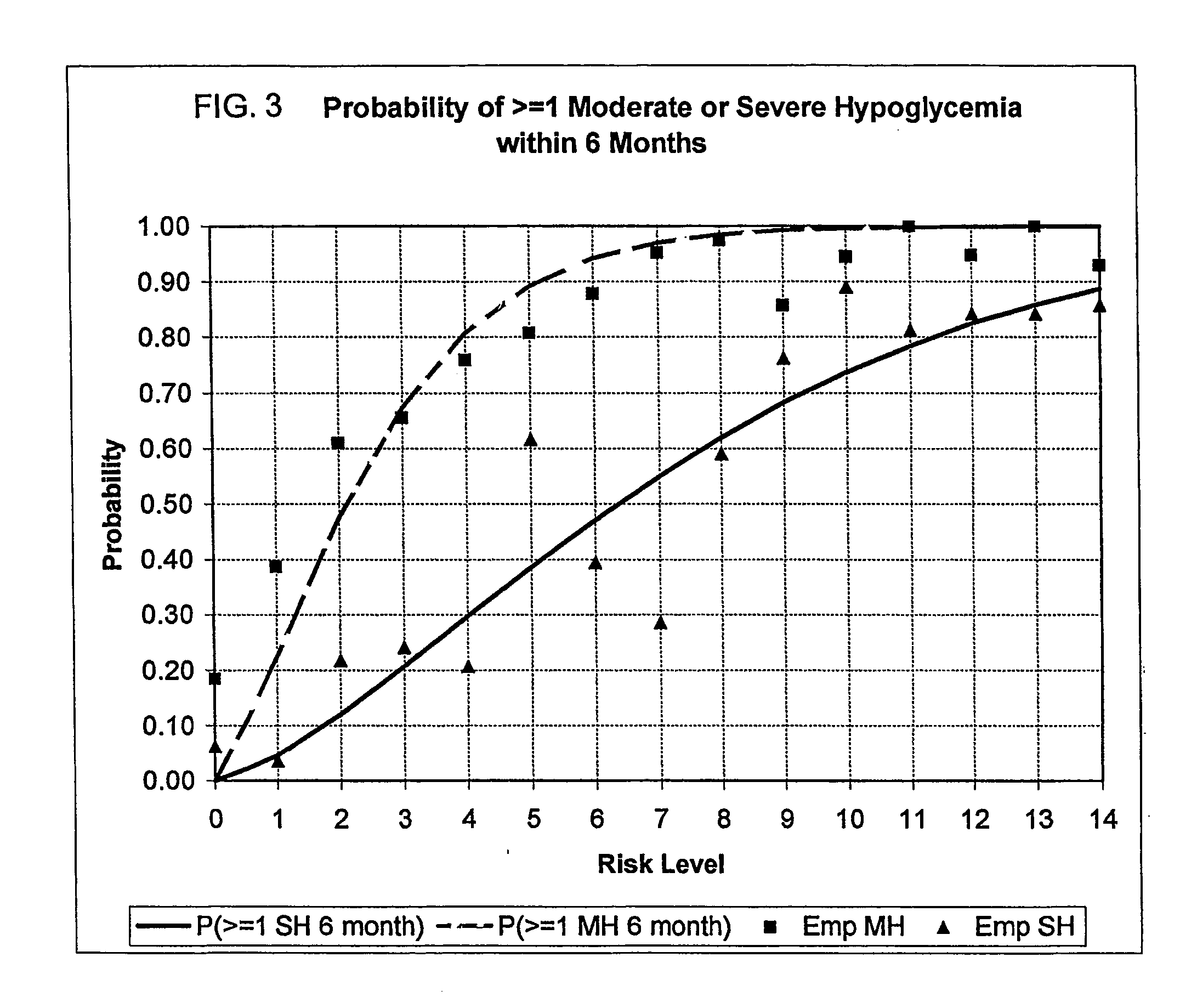
491 results about "Hypoglycemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition resulting when the blood glucose levels drop below the specified limits (4 mmol/L or 72mg/dL).
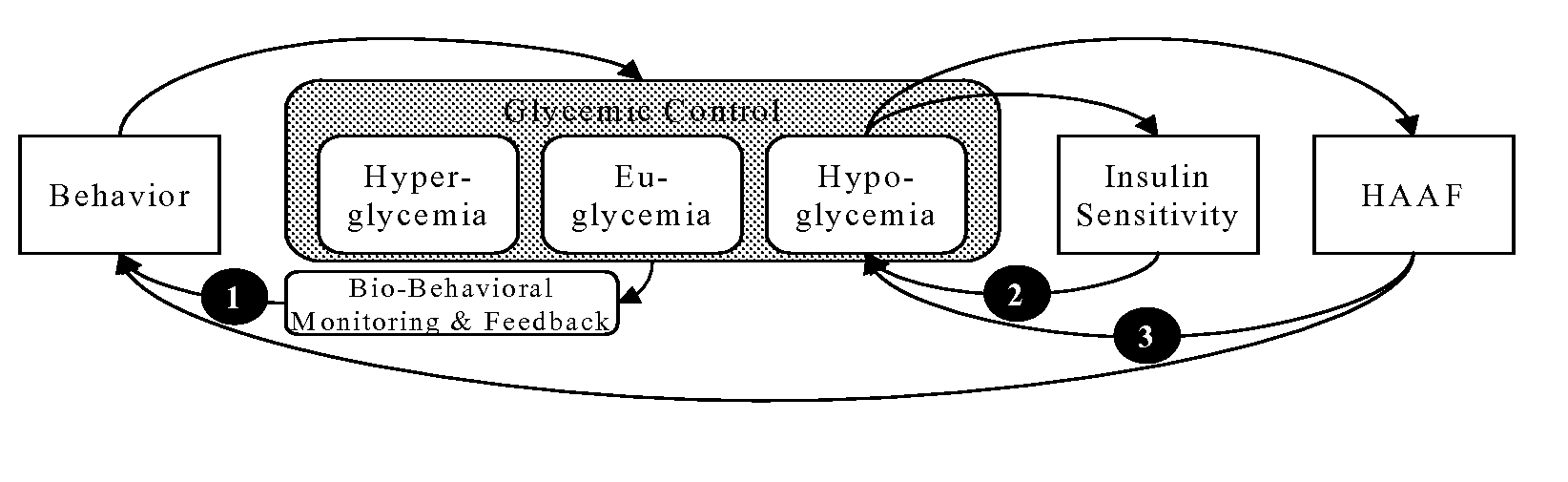
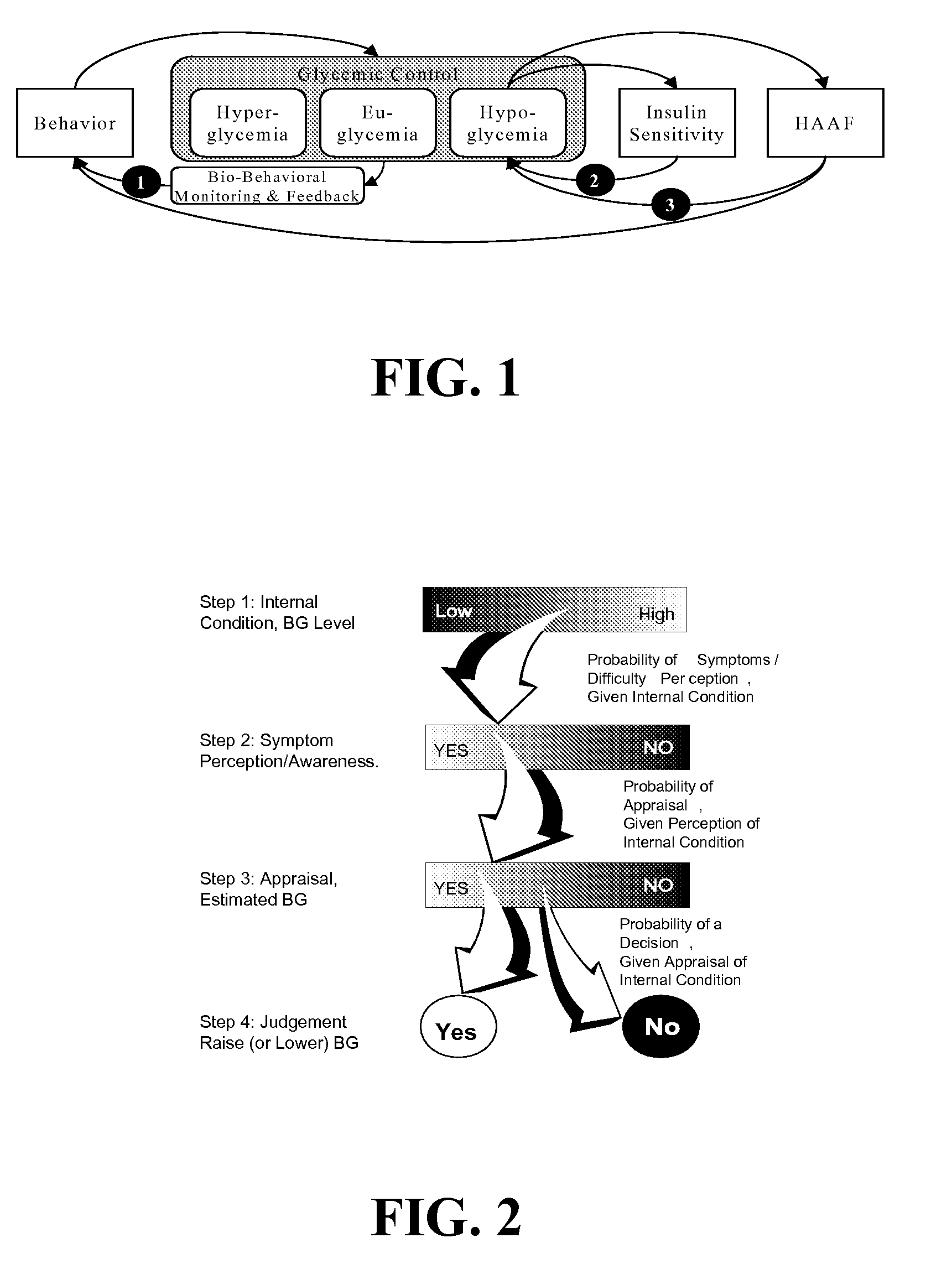
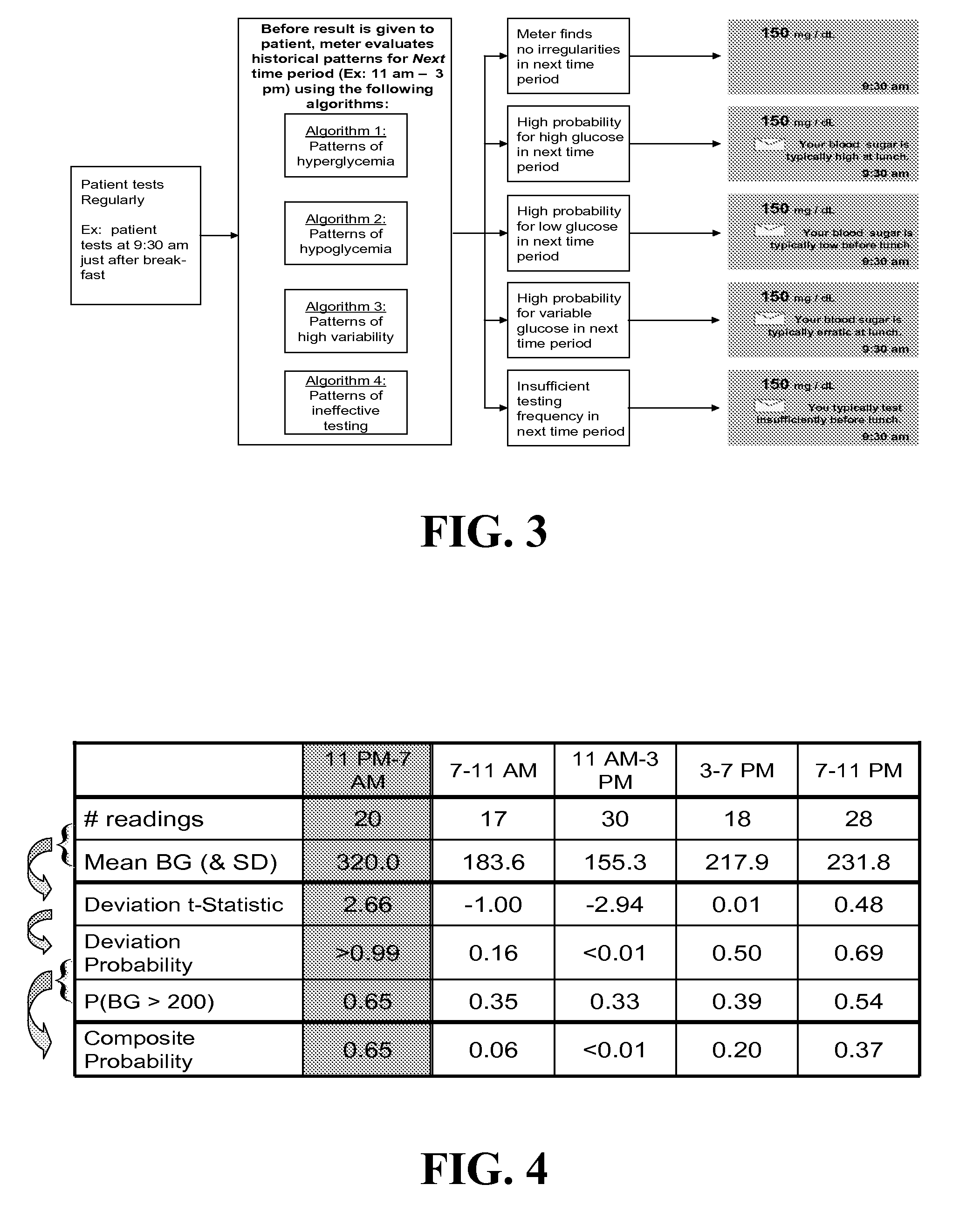
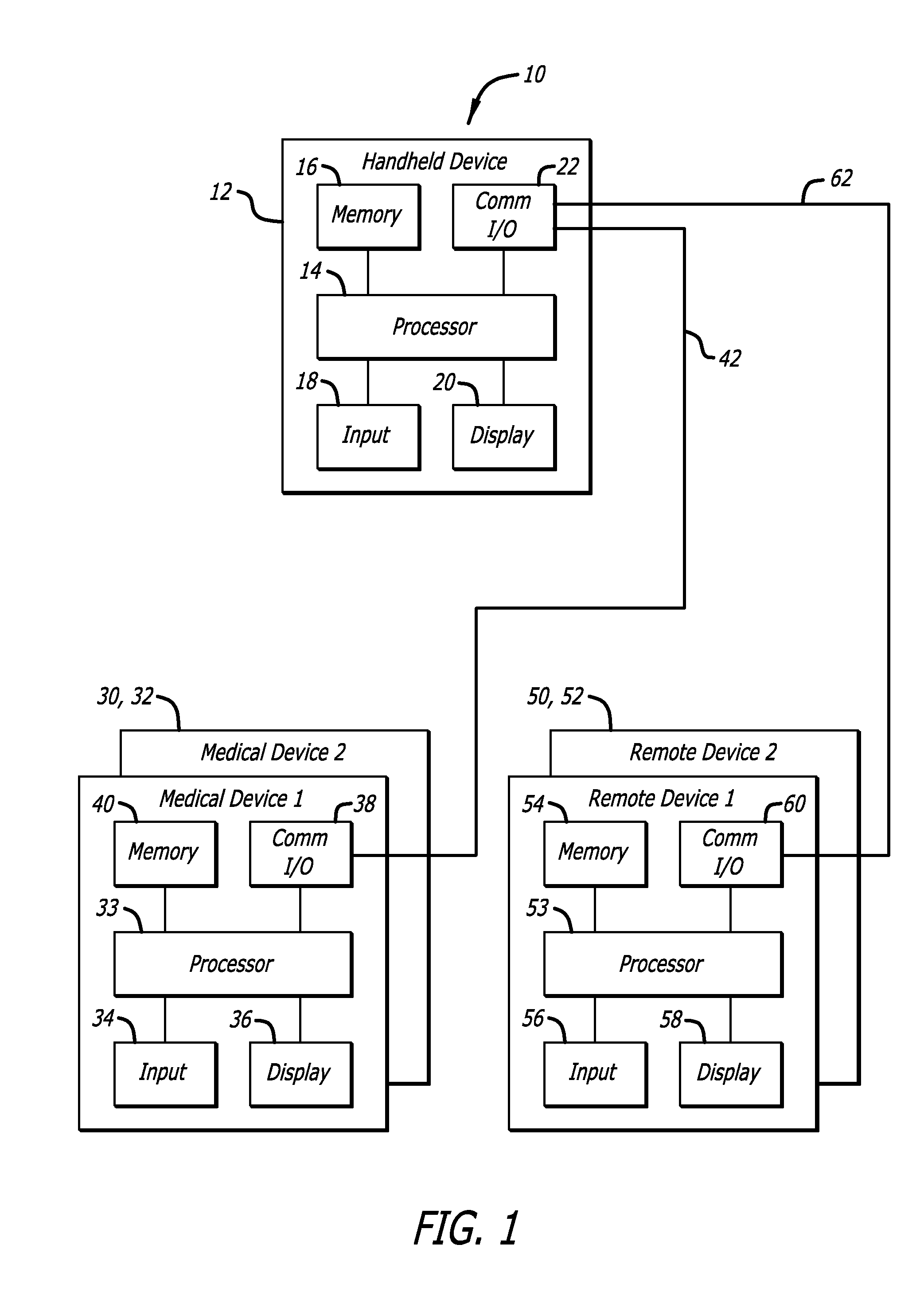
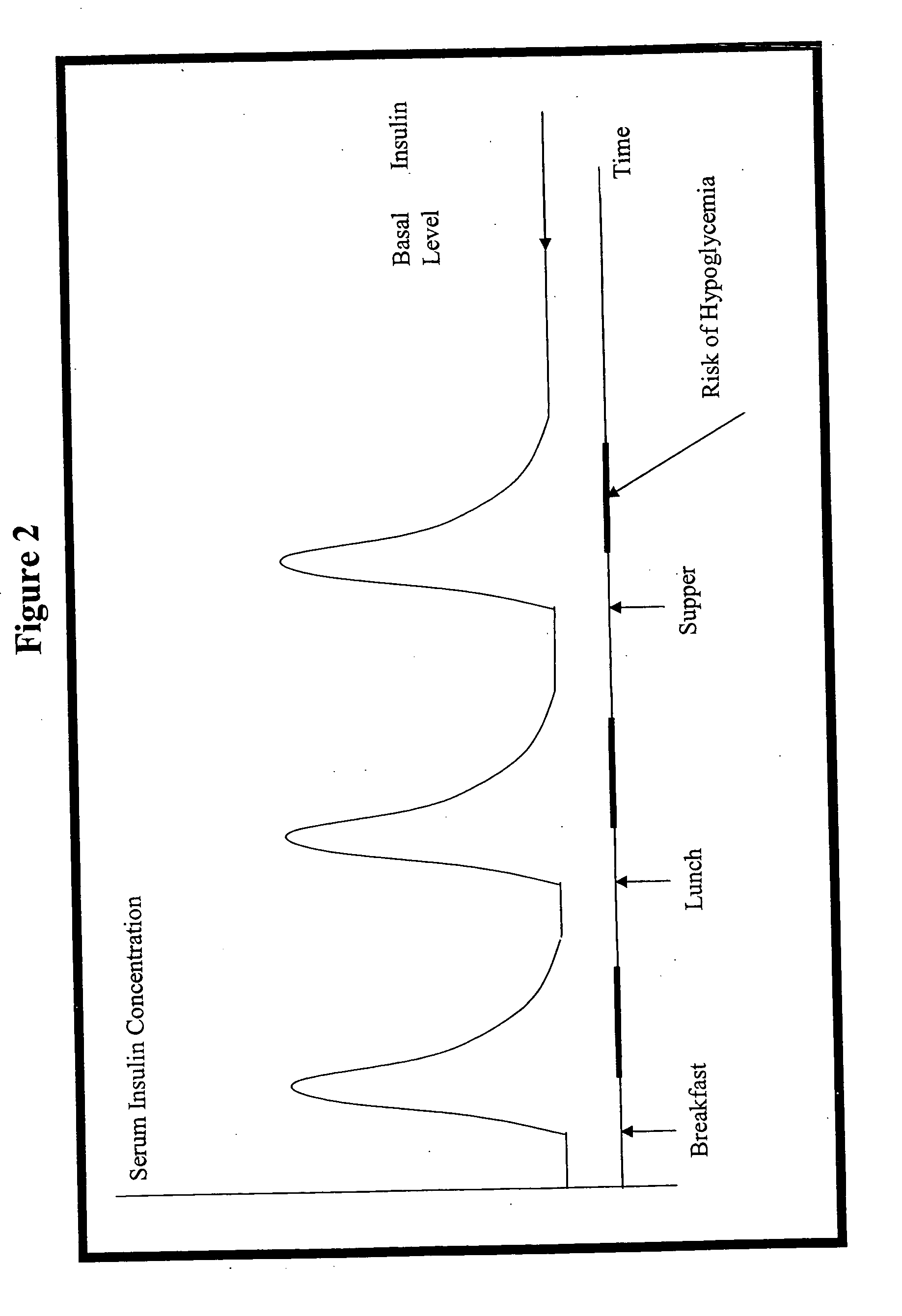
Systems, Methods and Computer Program Codes for Recognition of Patterns of Hyperglycemia and Hypoglycemia, Increased Glucose Variability, and Ineffective Self-Monitoring in Diabetes
InactiveUS20080154513A1Evaluate effectivenessEnhance existing SMBG devicesDrug and medicationsDigital computer detailsAcute hyperglycaemiaOptimal control
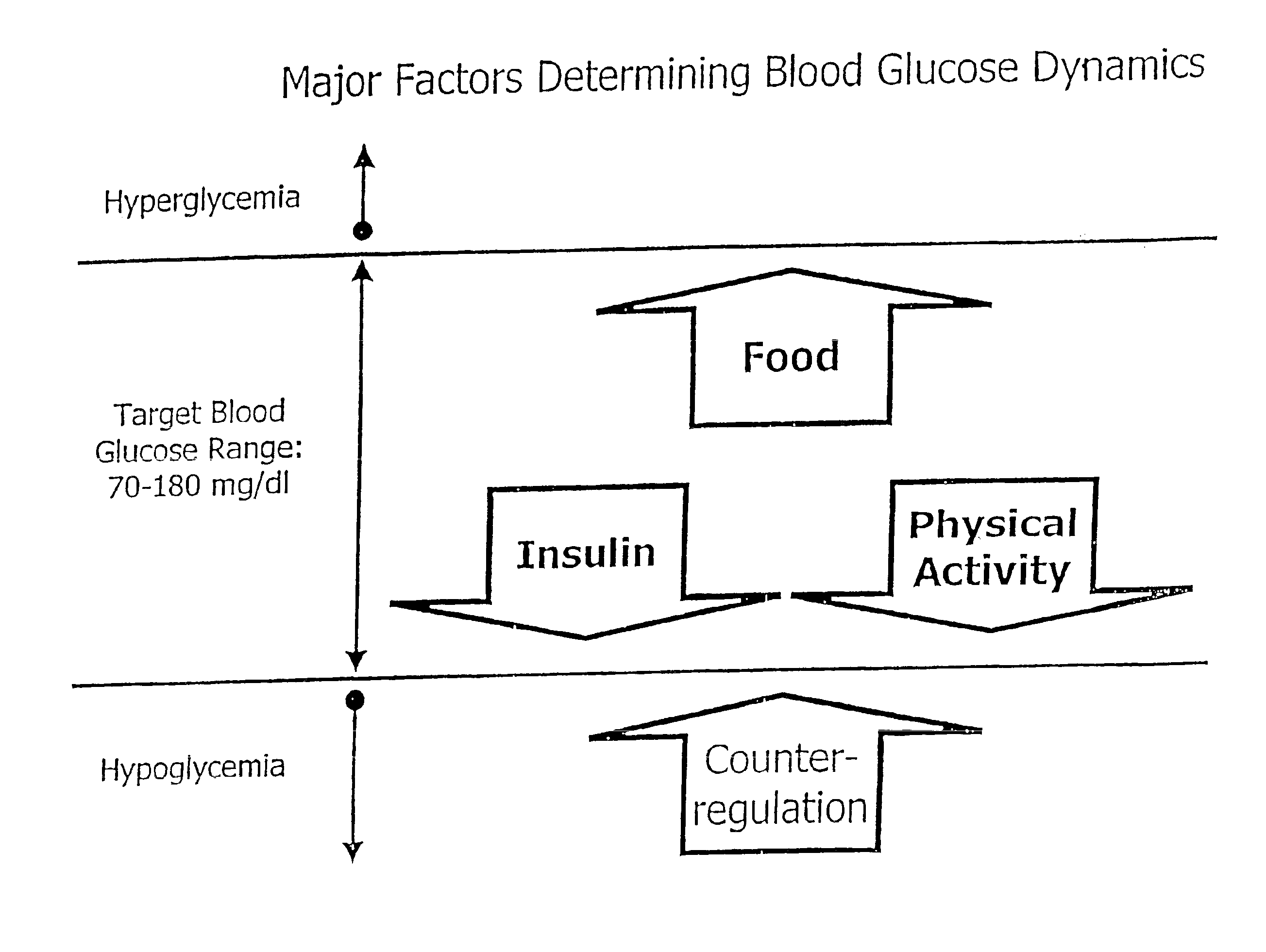
A method, system, and computer program product related to the maintenance of optimal control of diabetes, and is directed to predicting patterns of hypoglycemia, hyperglycemia, increased glucose variability, and insufficient or excessive testing for the upcoming period of time, based on blood glucose readings collected by a self-monitoring blood glucose device. The method, system, and computer program product pertain directly to the enhancement of existing home blood glucose monitoring devices, by introducing an intelligent data interpretation component capable of predicting and alerting the user to periods of increased risk for hyperglycemia, hypoglycemia, increased glucose variability, and ineffective testing, and to the enhancement of emerging self-monitoring blood glucose devices by the same features. With these predictions the diabetic can take steps to prevent the adverse consequences associated with hyperglycemia, hypoglycemia, and increased glucose variability.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
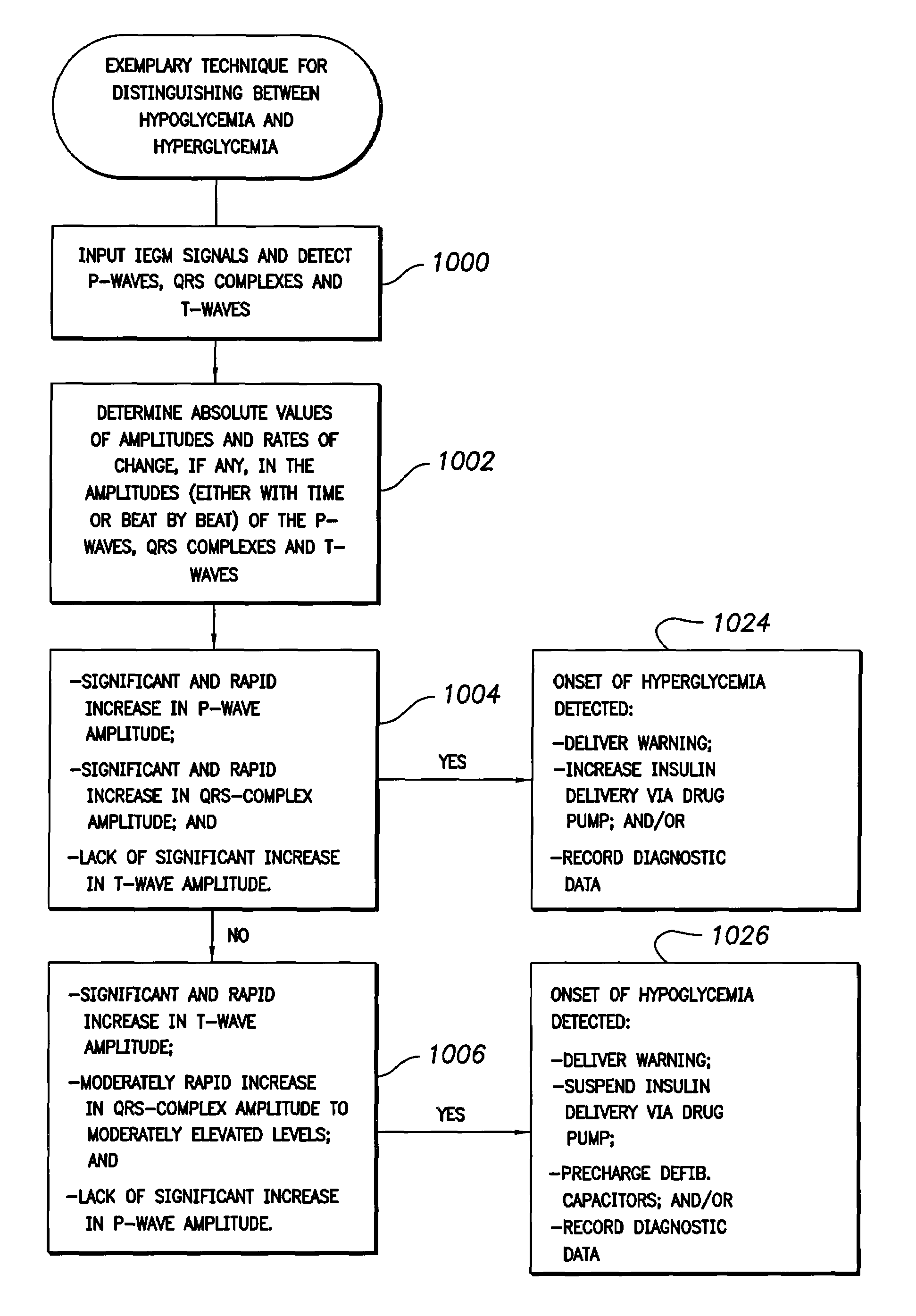
Method and apparatus for predicting the risk of hypoglycemia
InactiveUS6923763B1Reduced responseOptimal glucose controlMedical simulationHealth-index calculationInsulin infusionMedicine
The invention relates to a method which utilizes blood glucose (“BG”) sampling, insulin infusion / injection records, heart rate (“HR”) information and heart rate varability (“HRV”) information to estimate BG in the near future and to estimate of the risk of the onset of hypoglycemia. The invention also relates to an apparatus for predicting BG levels and for assessing the risk of the onset of hypoglycemia in the near future. The invention is based on two predetermined bio-mathematical routines: a network model of BG fluctuations and a BG profile for assessment of the risk of hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Method, system, and computer program product for the processing of self-monitoring blood glucose(smbg)data to enhance diabetic self-management
ActiveUS20050214892A1Easy to monitorContinuous informationMedical simulationMicrobiological testing/measurementAcute hyperglycaemiaOptimal control
A method, system, and computer program product related to the maintenance of optimal control of diabetes, and is directed to predicting the long-term exposure to hyperglycemia, and the long-term and short-term risks of severe or moderate hypoglycemia in diabetics, based on blood blucose readings collected by a self-monitoring blood glucose device. The method, system, and computer program product pertain directly to the enhancement of existing home blood glucose monitoring devices, by introducing an intelligent data interpretation component capable of predicting both HbA1c and periods of increased risk of hypoglycemia, and to the enhancement of emerging continuous monitoring devices by the same features. With these predictions the diabetic can take steps to prevent the adverse consequences associated with hyperglycemia and hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Methods of monitoring glucose levels in a subject and uses thereof
Methods of frequently monitoring glucose amounts and / or concentrations in a subject who is at risk for hypoglycemia, hyperglycemia, and / or glucose level fluctuations that put the subject at risk are provided. Also provided are methods of monitoring the effects of one or more pharmaceutical compositions on the levels of glucose in a subject.
Owner:ANIMAS TECH +1
Safety features for integrated insulin delivery system
ActiveUS20100298765A1Preventing hypoglycemiaLimited hyperglycemiaDrug and medicationsMedical devicesGlucose sensorsHypoglycemia
Safety features are applied to an integrated insulin delivery system to enhance safety while accounting for glucose sensor bias and calibration errors. One safety feature includes comparisons of calibrations of the sensor to nominal sensitivity and taking action, such as limiting insulin delivery or taking a further calibration of the sensor. In another feature, an automatic resumption of a basal delivery rate is programmed into the delivery device to avoid the possibility of complete loss of delivery of insulin in the event that communication with the delivery device is disrupted. Other features include steps taken to avoid hypoglycemia in the event that the sensor is negatively biased.
Owner:ABBOTT DIABETES CARE INC
Method and device for utilizing analyte levels to assist in the treatment of diabetes
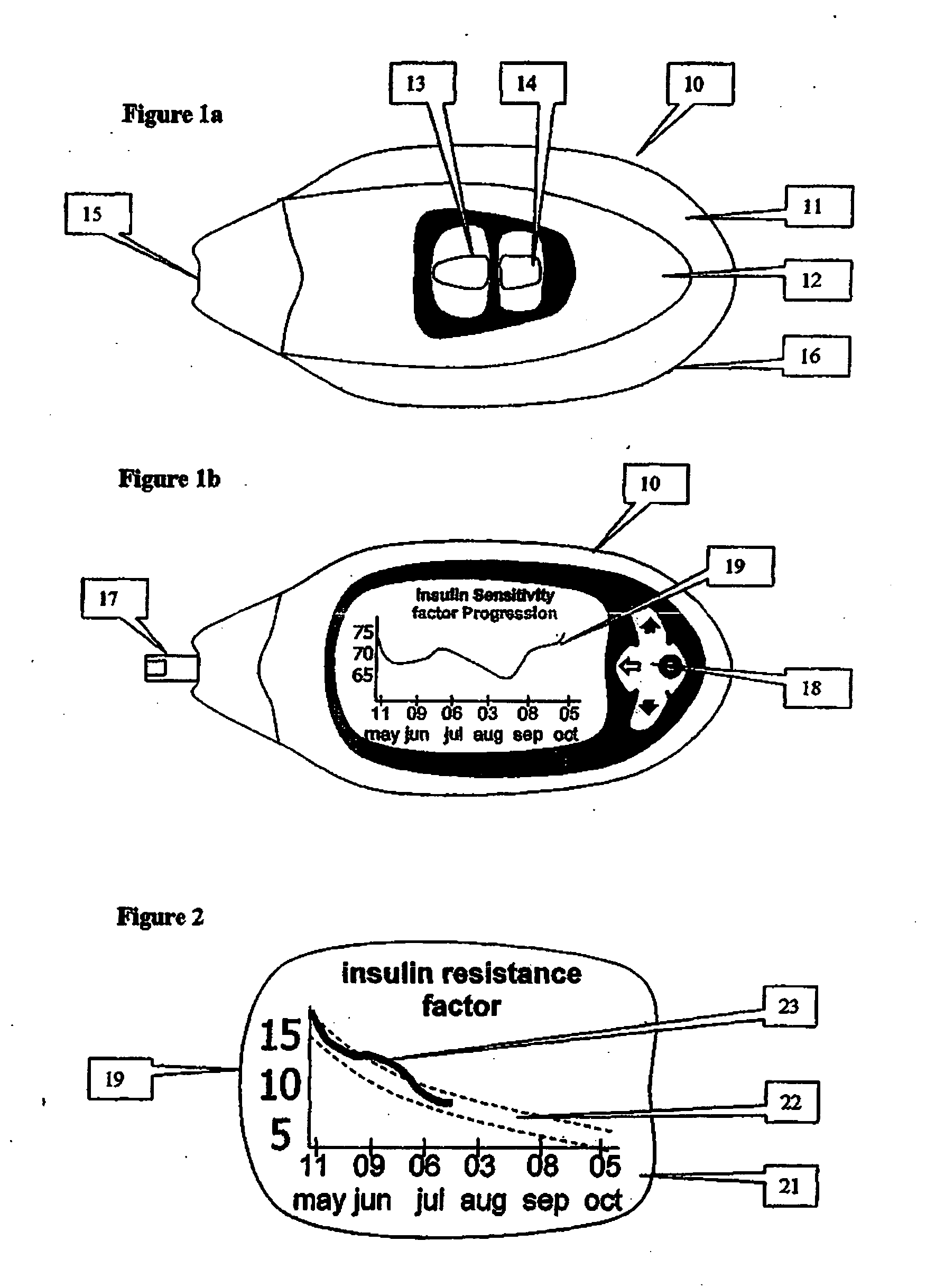
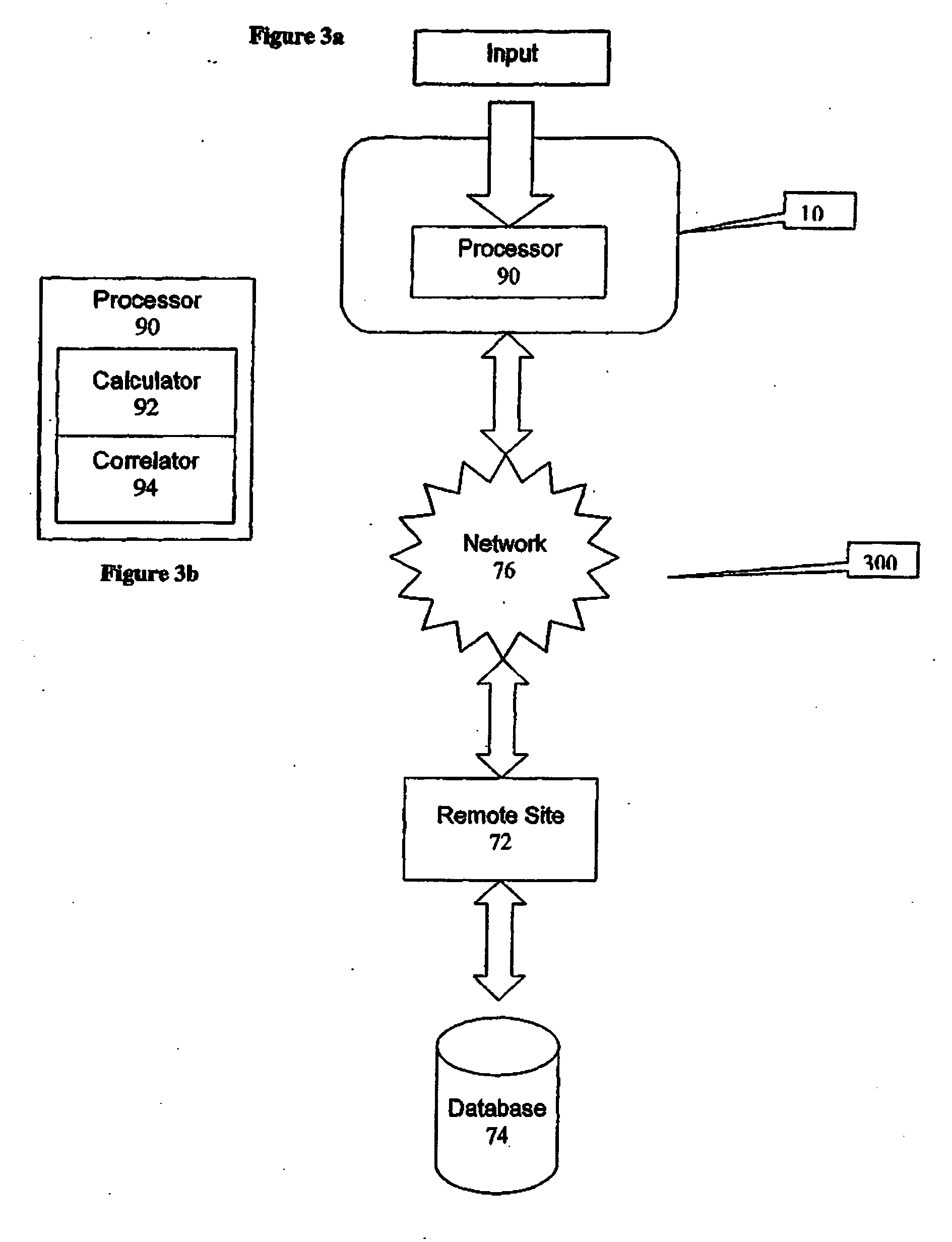
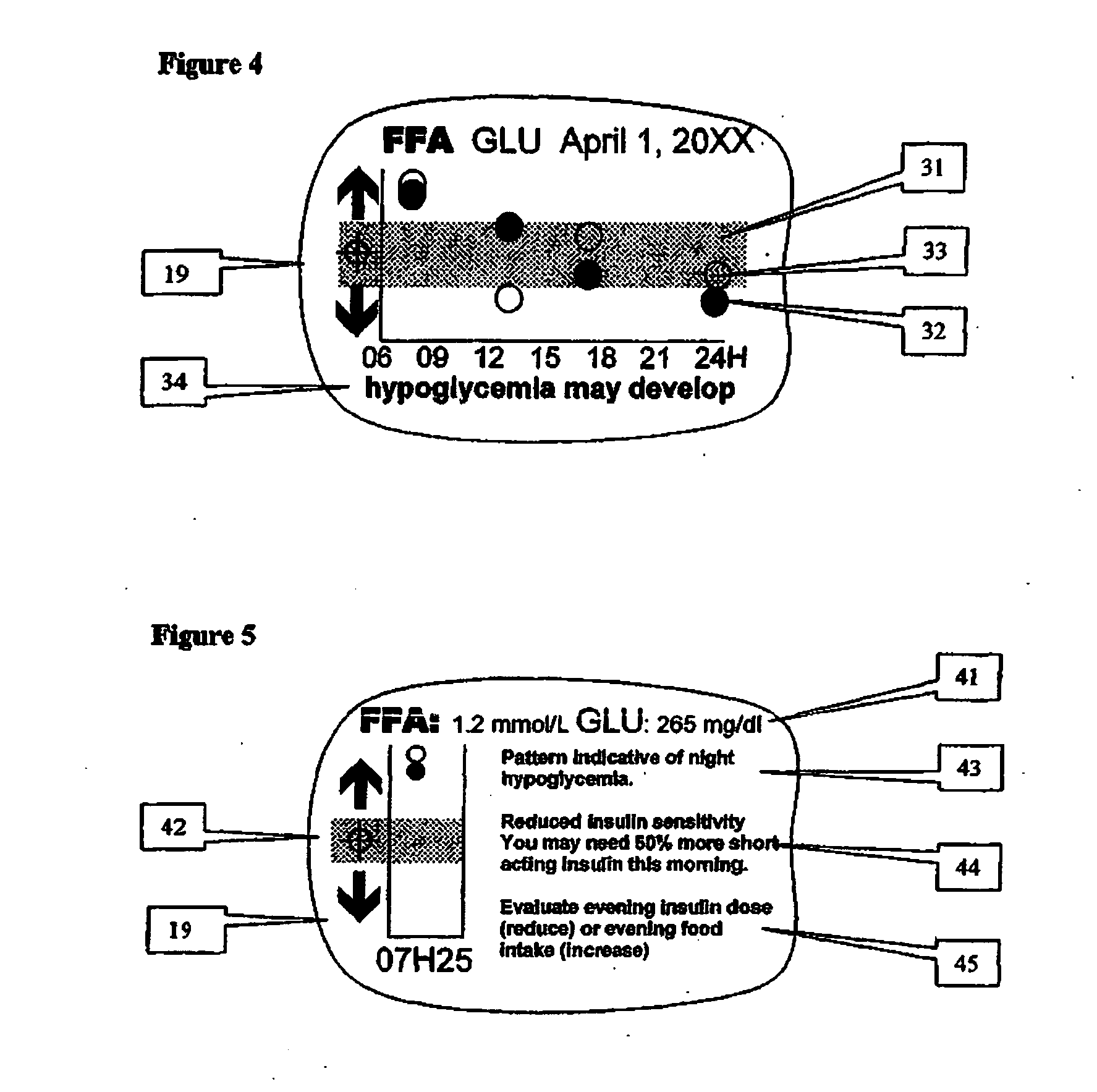
A health-monitoring device assesses the health of a user based on levels of two analytes in a biological fluid. A first analyte that is utilized to assess a user's health is a fat metabolism analyte, such as ketones, free fatty acids and glycerol, which is indicative of fat metabolism. A second analyte that is utilized is a glucose metabolism analyte, such as glucose. The levels of the two analytes may be used to assess insulin sensitivity, to detect both recent hypoglycemia and the cause of high glucose levels, and / or to guide therapeutic intervention. The dual analyte model may calculate a discrepancy between an actual insulin activity level and a theoretical insulin activity level. The dual analyte model of the present invention may be used to identify individuals at risk for metabolic syndrome, insulin resistance and non-insulin dependent diabetes, and allows monitoring of the progression of those disease states, as well as progress made by therapeutic interventions.
Owner:ABBOTT DIABETES CARE INC
Methods for reducing false hypoglycemia alarm occurrence
Owner:ABBOTT DIABETES CARE INC +1
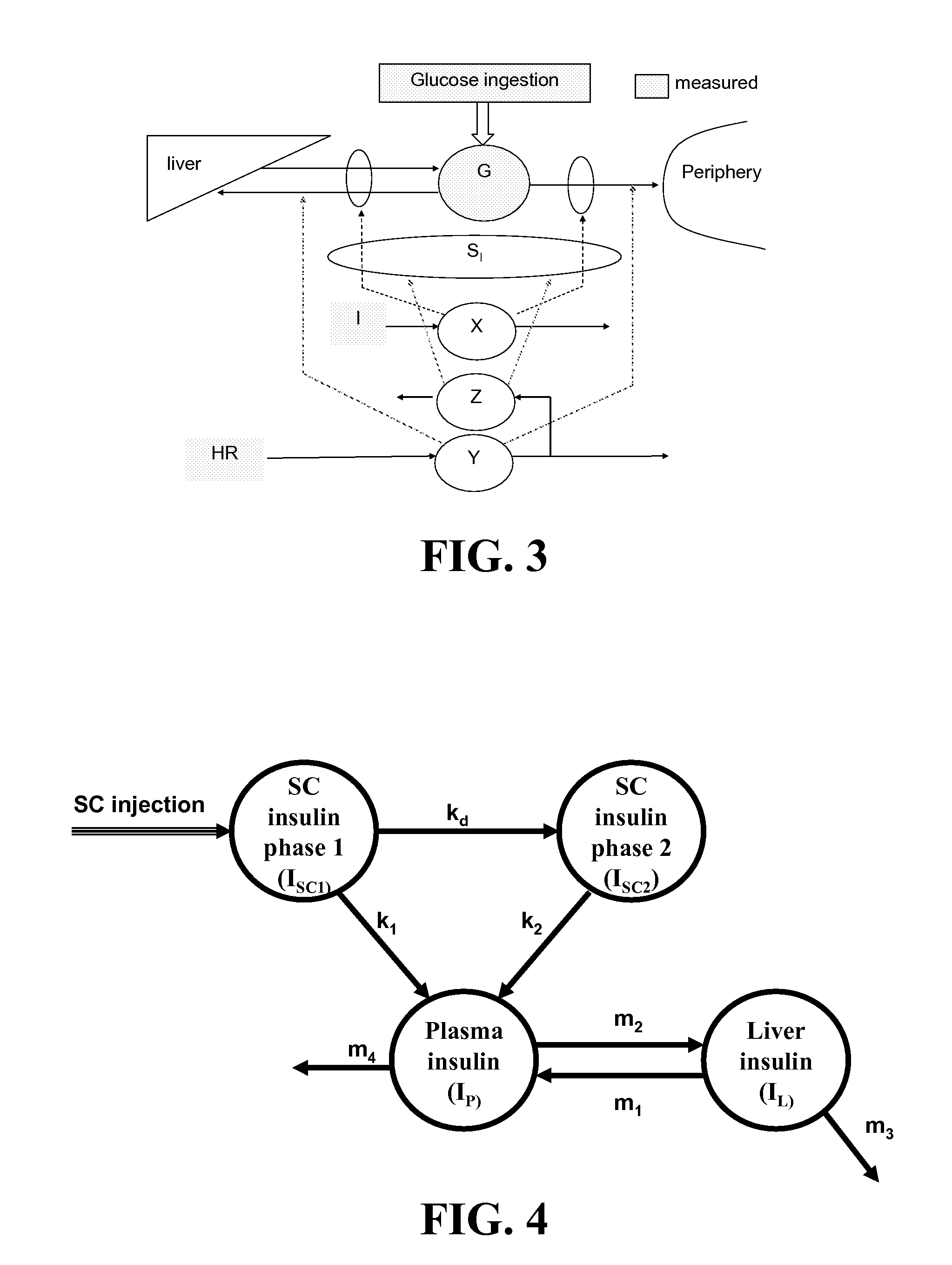
Method, System, and Computer Program Product for the Detection of Physical Activity by Changes in Heart Rate, Assessment of Fast Changing Metabolic States, and Applications of Closed and Open Control Loop in Diabetes
ActiveUS20100057043A1Minimize frequencyAvoid the needMedical simulationDrug and medicationsClosed loopHypoglycemia
A method, system, and computer program product related to the detection of physical activity using changes in heart rate. The method, system, and computer program product evaluates short term glucose demand and long term insulin action due to physical activity. The method, system, and computer program product is further related to the improvement of open and closed loop control of diabetes by accounting for the metabolic changes due to physical activity. The method, system, and computer program product is directed to detecting in real time the short and long term effects of physical activity on insulin action via heart rate analysis, and recommending changes in insulin dosing to compensate for the effects of physical activity. With these recommendations, the open and closed loop control of diabetes can be improved and steps can be taken to prevent hypoglycemia that may result from increased insulin sensitivity due to physical activity.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
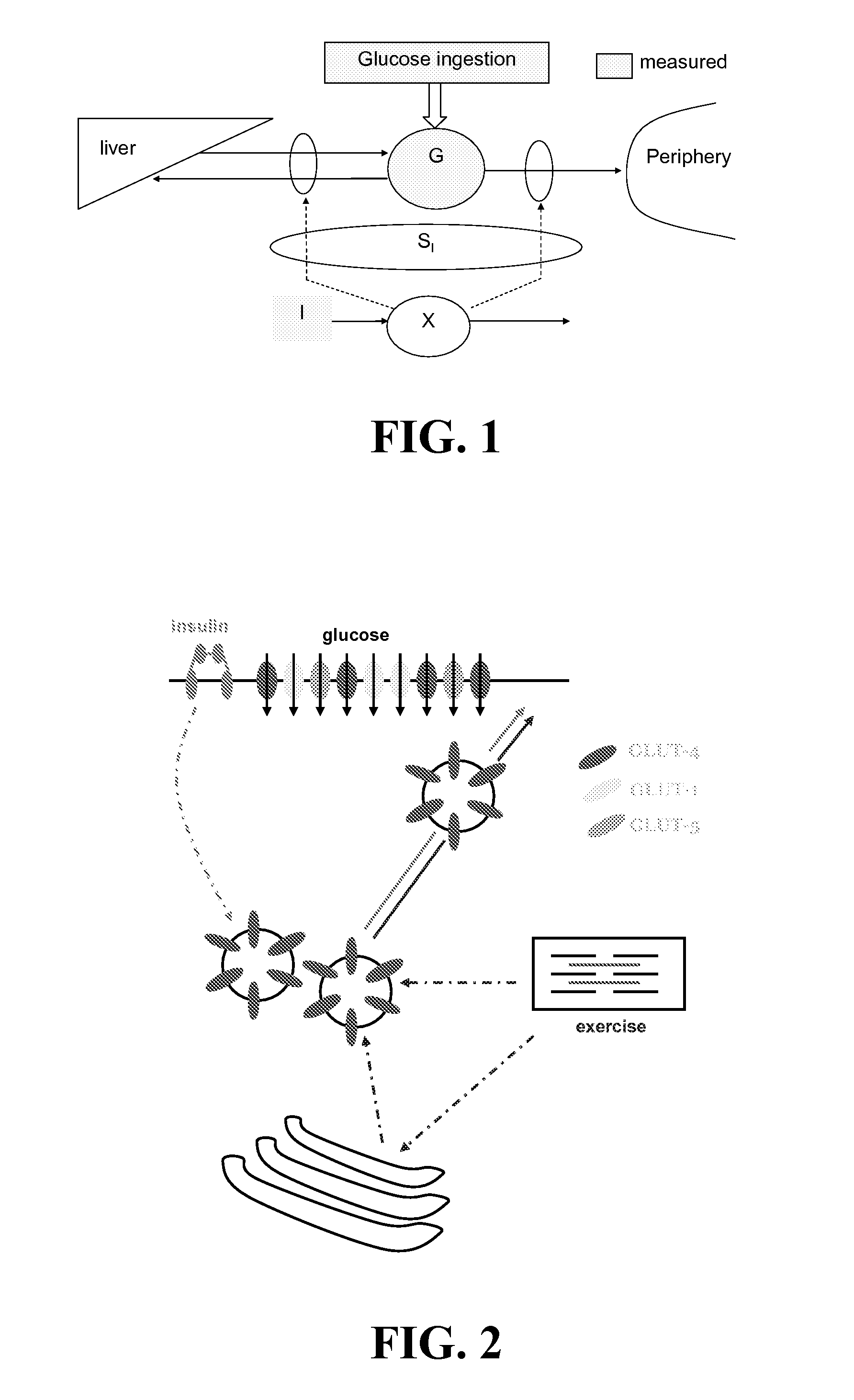
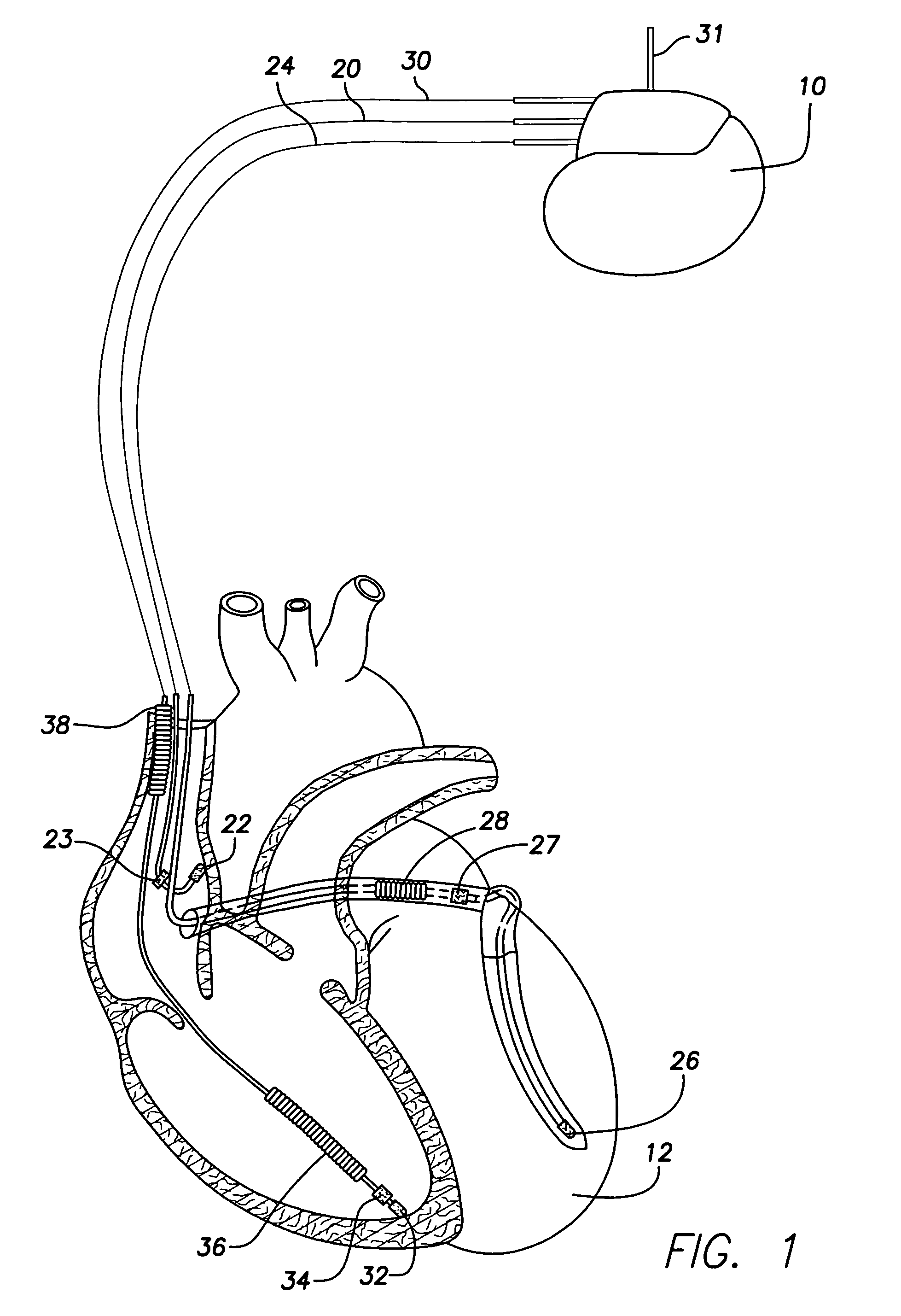
System and method for distinguishing between hypoglycemia and hyperglycemia using an implantable medical device
ActiveUS7524287B2Improve blood sugar controlReduce deliveryElectrotherapyElectrocardiographyAcute hyperglycaemiaT wave
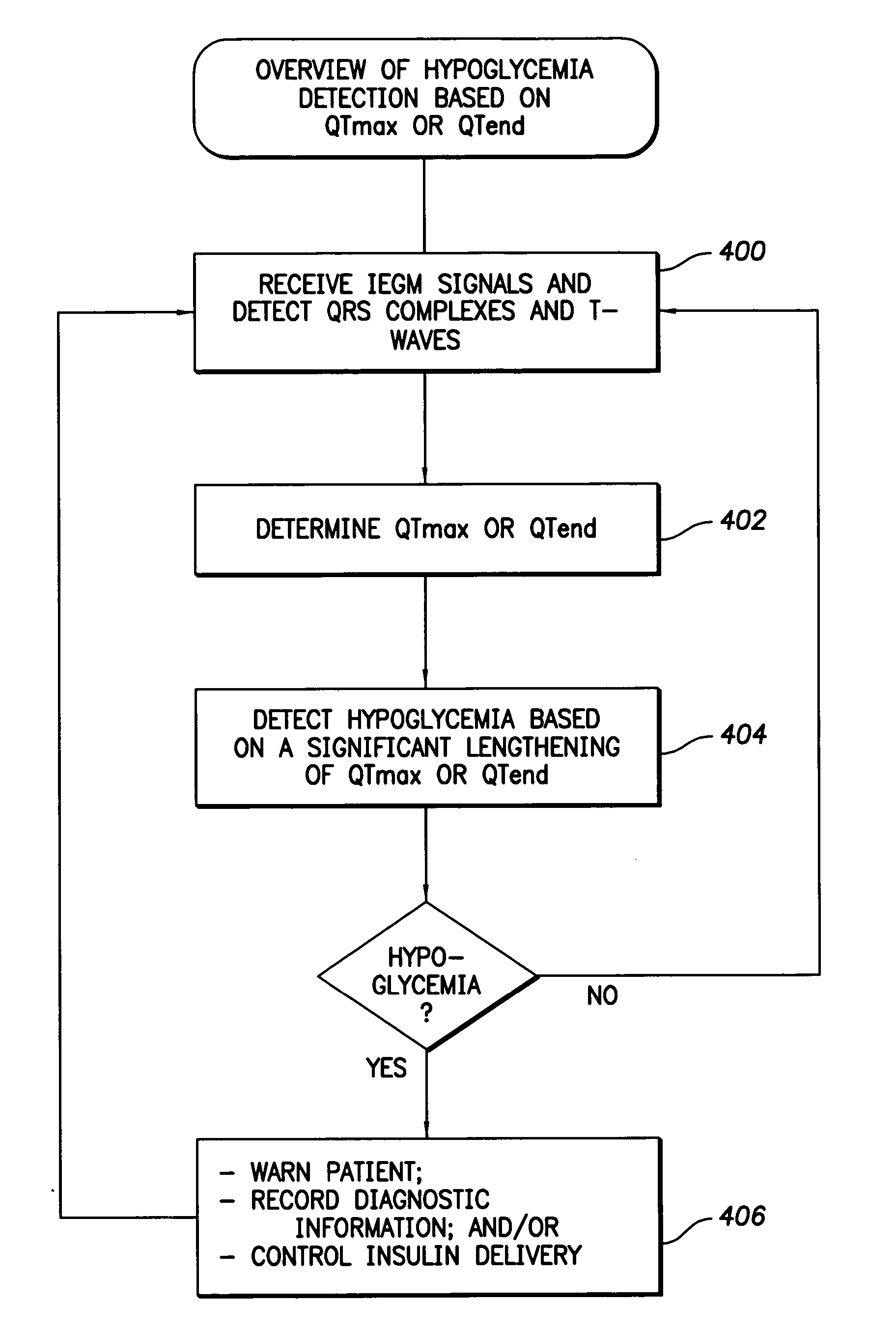
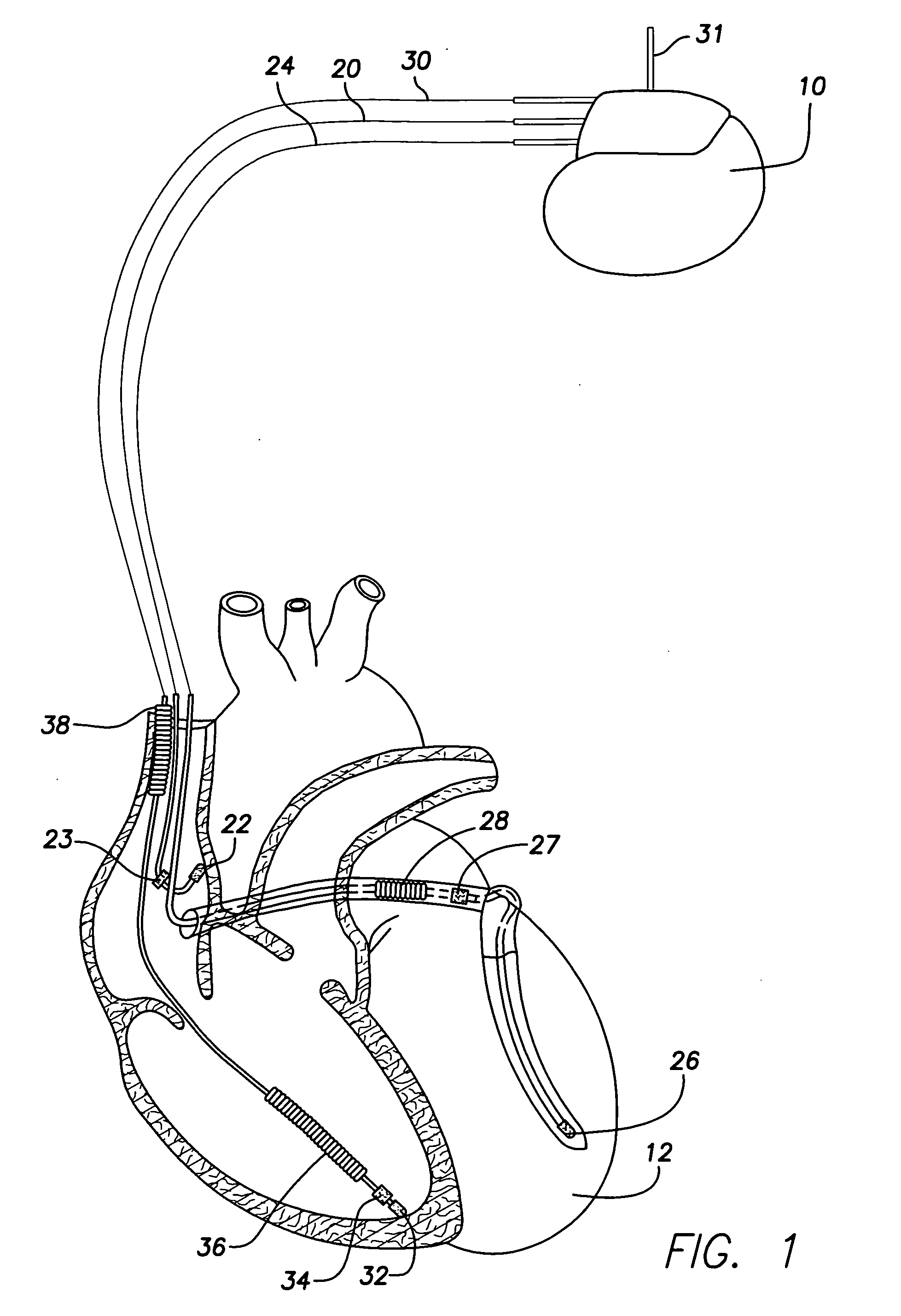
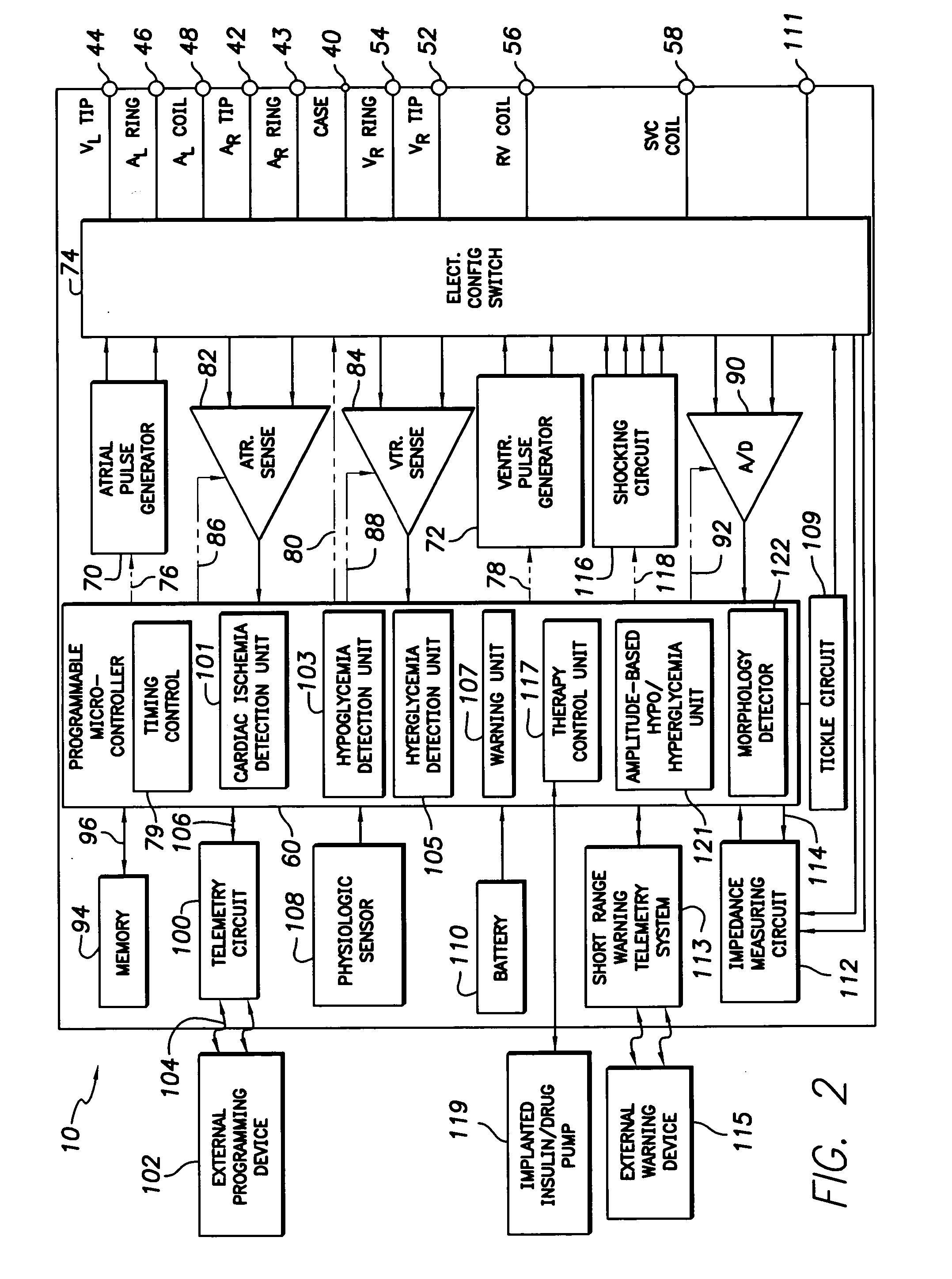
Techniques are described for detecting and distinguishing among ischemia, hypoglycemia or hyperglycemia based on intracardiac electrogram (IEGM) signals. In one technique, these conditions are detected and distinguished based on an analysis of: the interval between the QRS complex and the peak of a T-wave (QTmax), the interval between the QRS complex and the end of a T-wave (QTend), alone or in combination with a change in ST segment elevation. By exploiting QTmax and QTend in combination with ST segment elevation, changes in ST segment elevation caused by hypo / hyperglycemia can be properly distinguished from changes caused by cardiac ischemia. In another technique, hyperglycemia and hypoglycemia are predicted, detected and / or distinguished from one another based on an analysis of the amplitudes of P-waves, QRS-complexes and T-waves within the IEGM. Appropriate warning signals are delivered and therapy is automatically adjusted.
Owner:PACESETTER INC
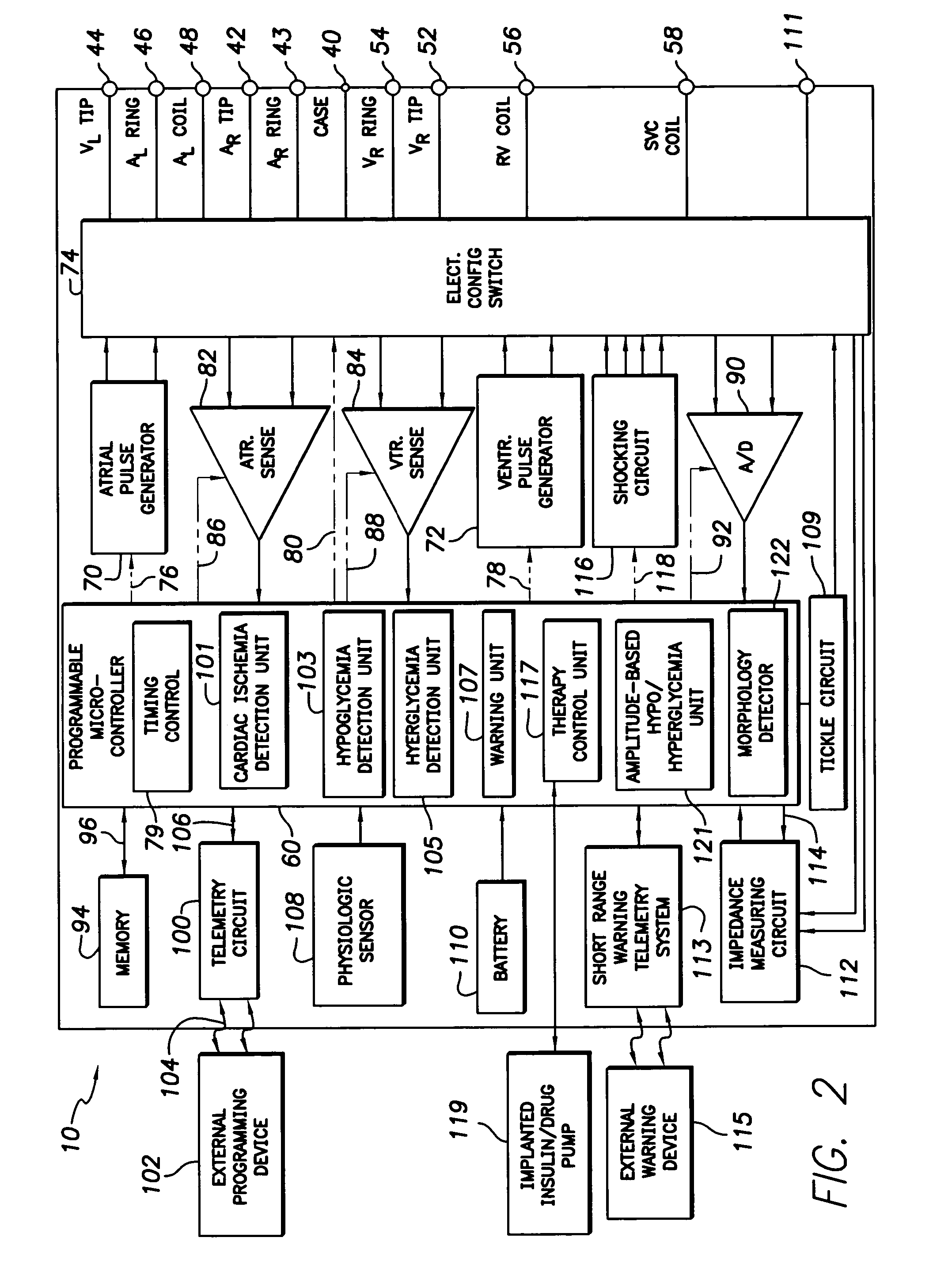
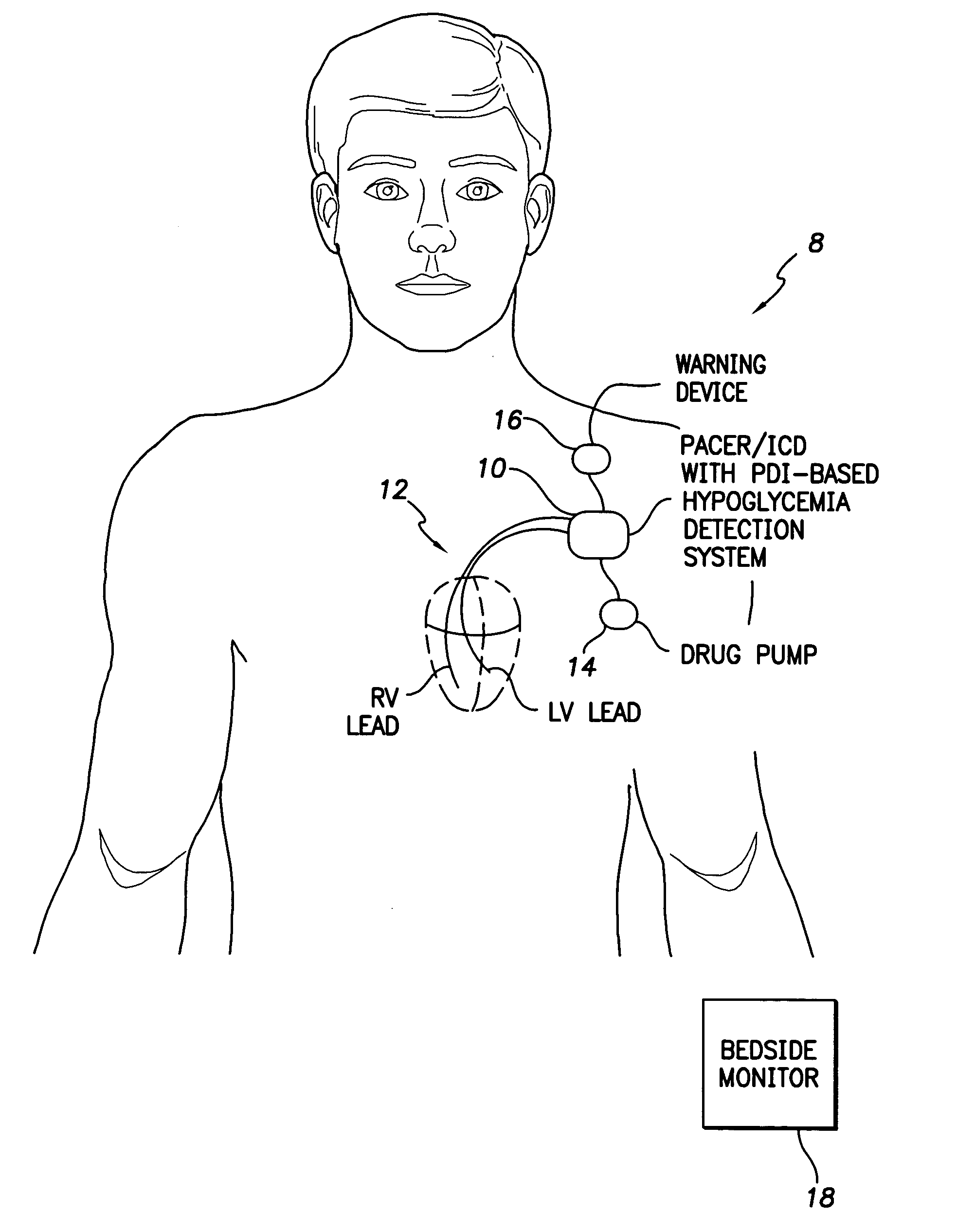
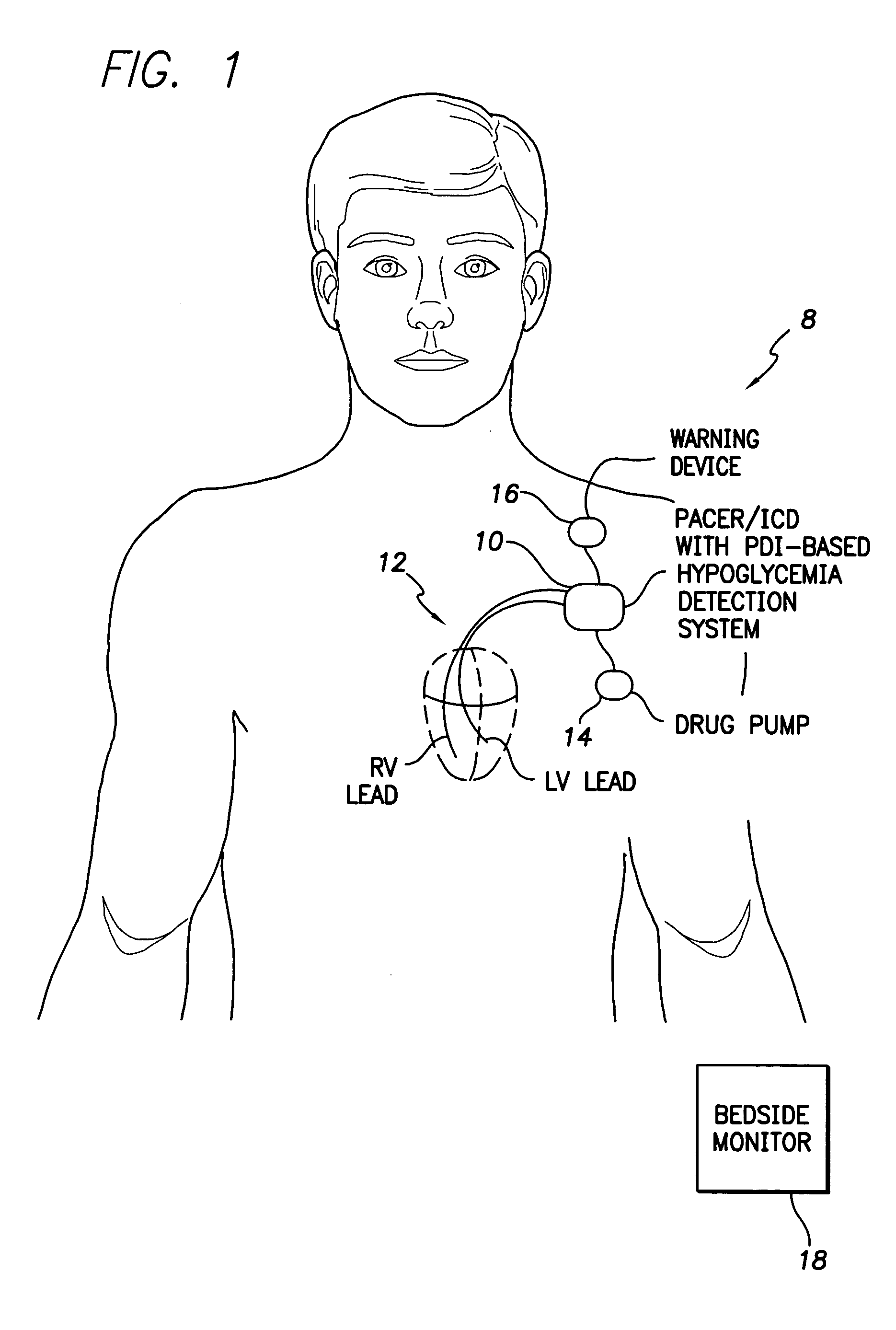
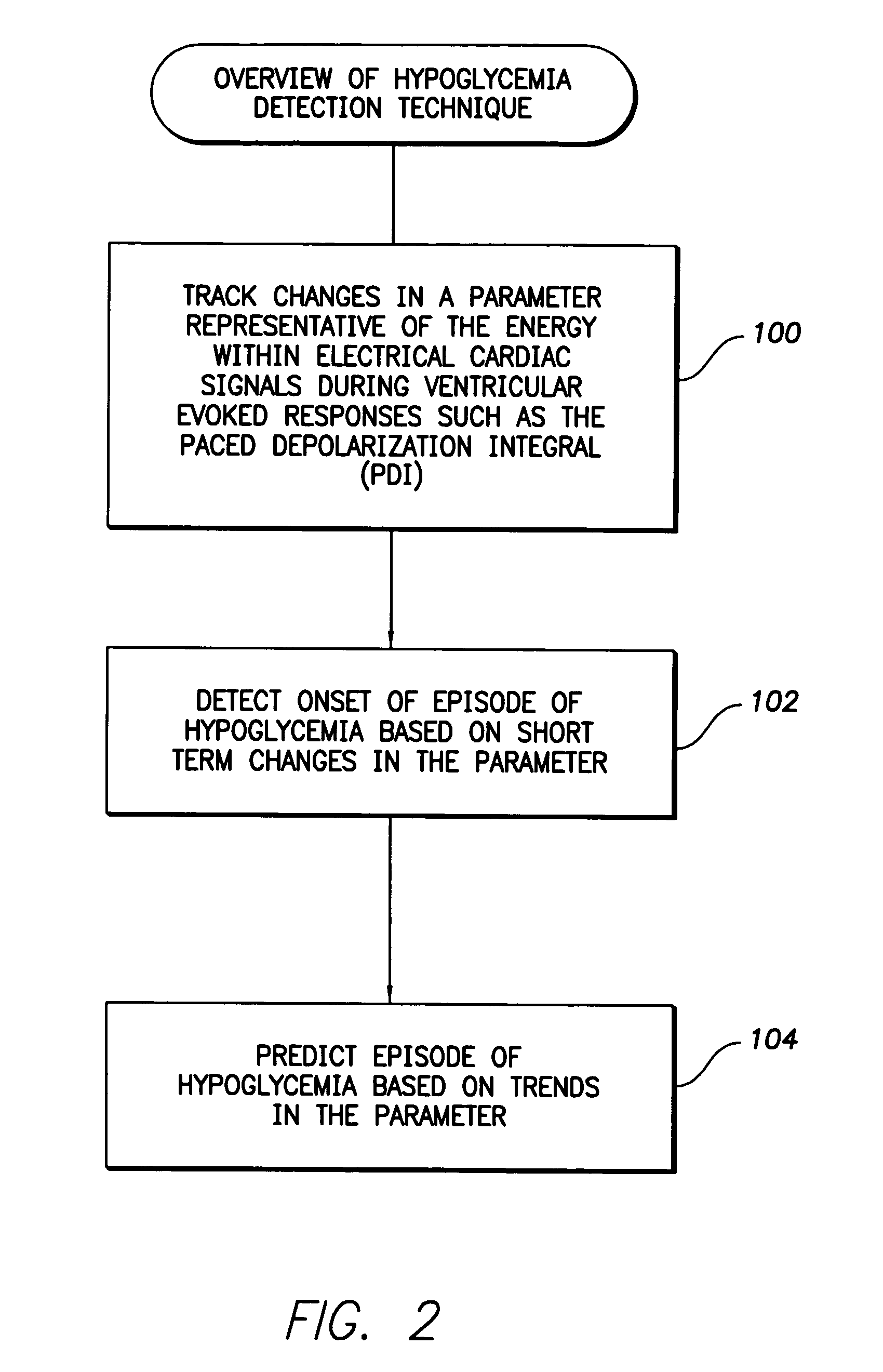
System and method for detecting hypoglycemia based on a paced depolarization integral using an implantable medical device
ActiveUS20060247685A1Improve blood sugar controlReduce deliveryElectrocardiographyMedical devicesCardiac pacemaker electrodeInsulin dependent
Techniques are provided for use with an implantable medical device such as a pacemaker or implantable cardioverter / defibrillator (ICD) for predicting and detecting hypoglycemia. In one example, the device tracks changes in a paced depolarization integral (PDI). A significant increase in PDI over a relatively short period of time indicates the onset of hypoglycemia (this can also be confirmed with QT changes). Upon detection of hypoglycemia, appropriate warning signals are generated to alert the patient. Certain therapies automatically provided by the implantable device may also be controlled in response to hypoglycemia. For example, if the patient is an insulin-dependent diabetic and the implantable device is equipped with an insulin pump capable of delivering insulin directly into the bloodstream, insulin delivery is automatically suspended until blood glucose levels return to acceptable levels. If the device is an ICD, it may be controlled to begin charging defibrillation capacitors upon detection of hypoglycemia so as to permit prompt delivery of a defibrillation shock, which may be needed if hypoglycemia triggers ventricular fibrillation. The detection techniques may be used in conjunction with other hypoglycemia detection techniques to improve detection specificity.
Owner:PACESETTER INC
System and method for distinguishing between hypoglycemia and hyperglycemia using an implantable medical device
ActiveUS20060167365A1Improve blood sugar controlReduce deliveryElectrotherapyElectrocardiographyAcute hyperglycaemiaT wave
Techniques are described for detecting and distinguishing among ischemia, hypoglycemia or hyperglycemia based on intracardiac electrogram (IEGM) signals. In one technique, these conditions are detected and distinguished based on an analysis of: the interval between the QRS complex and the peak of a T-wave (QTmax), the interval between the QRS complex and the end of a T-wave (QTend), alone or in combination with a change in ST segment elevation. By exploiting QTmax and QTend in combination with ST segment elevation, changes in ST segment elevation caused by hypo / hyperglycemia can be properly distinguished from changes caused by cardiac ischemia. In another technique, hyperglycemia and hypoglycemia are predicted, detected and / or distinguished from one another based on an analysis of the amplitudes of P-waves, QRS-complexes and T-waves within the IEGM. Appropriate warning signals are delivered and therapy is automatically adjusted.
Owner:PACESETTER INC
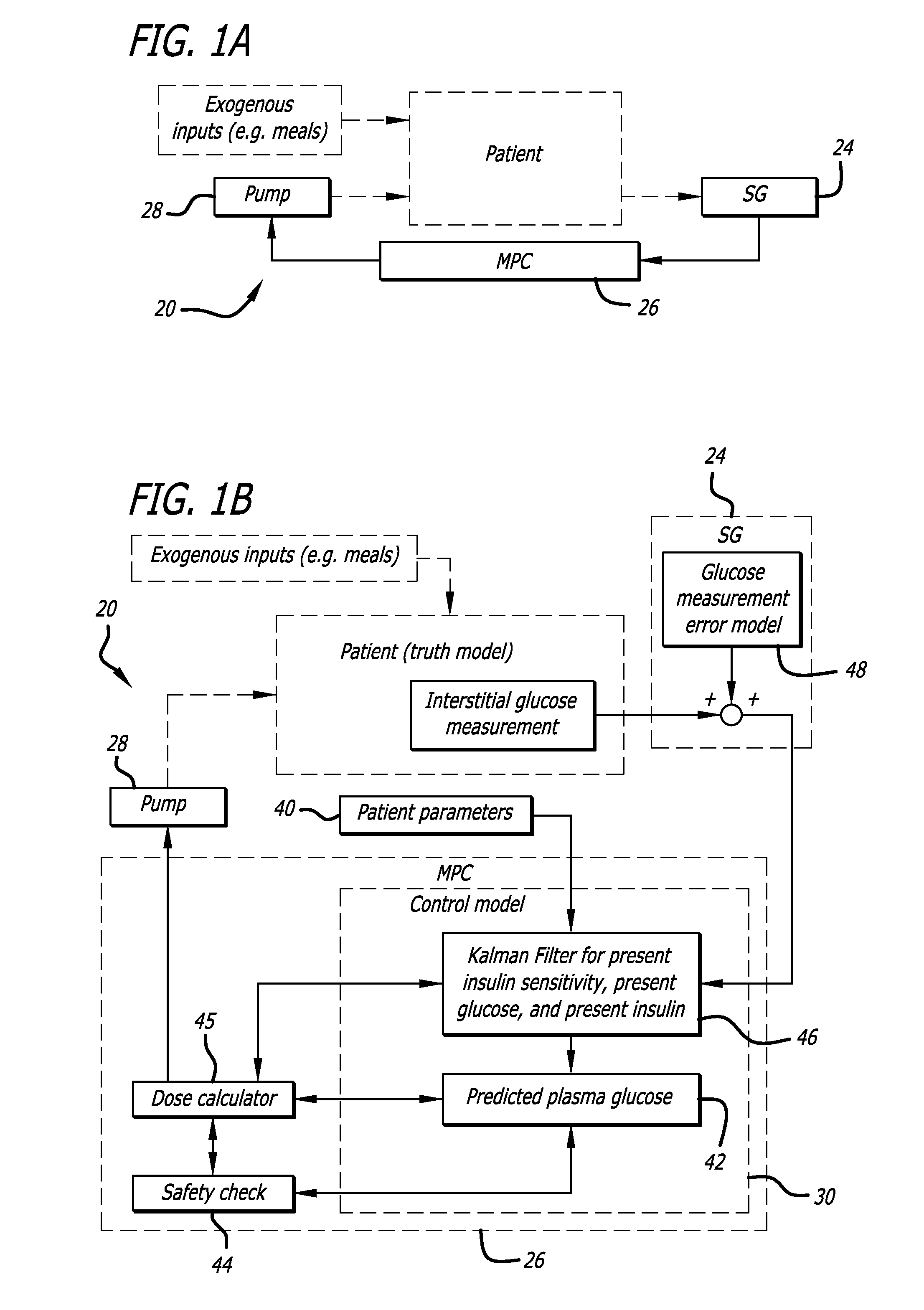
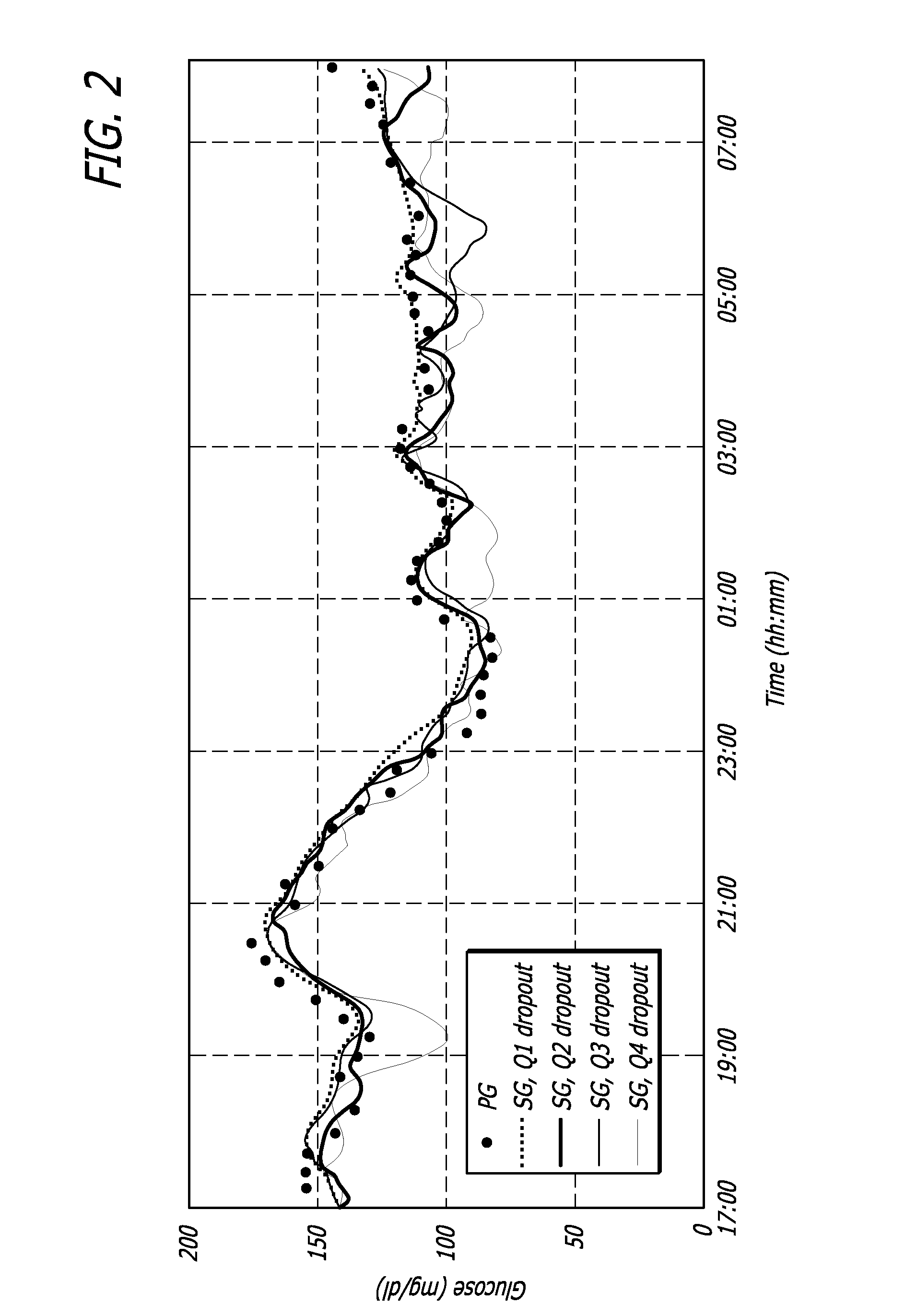
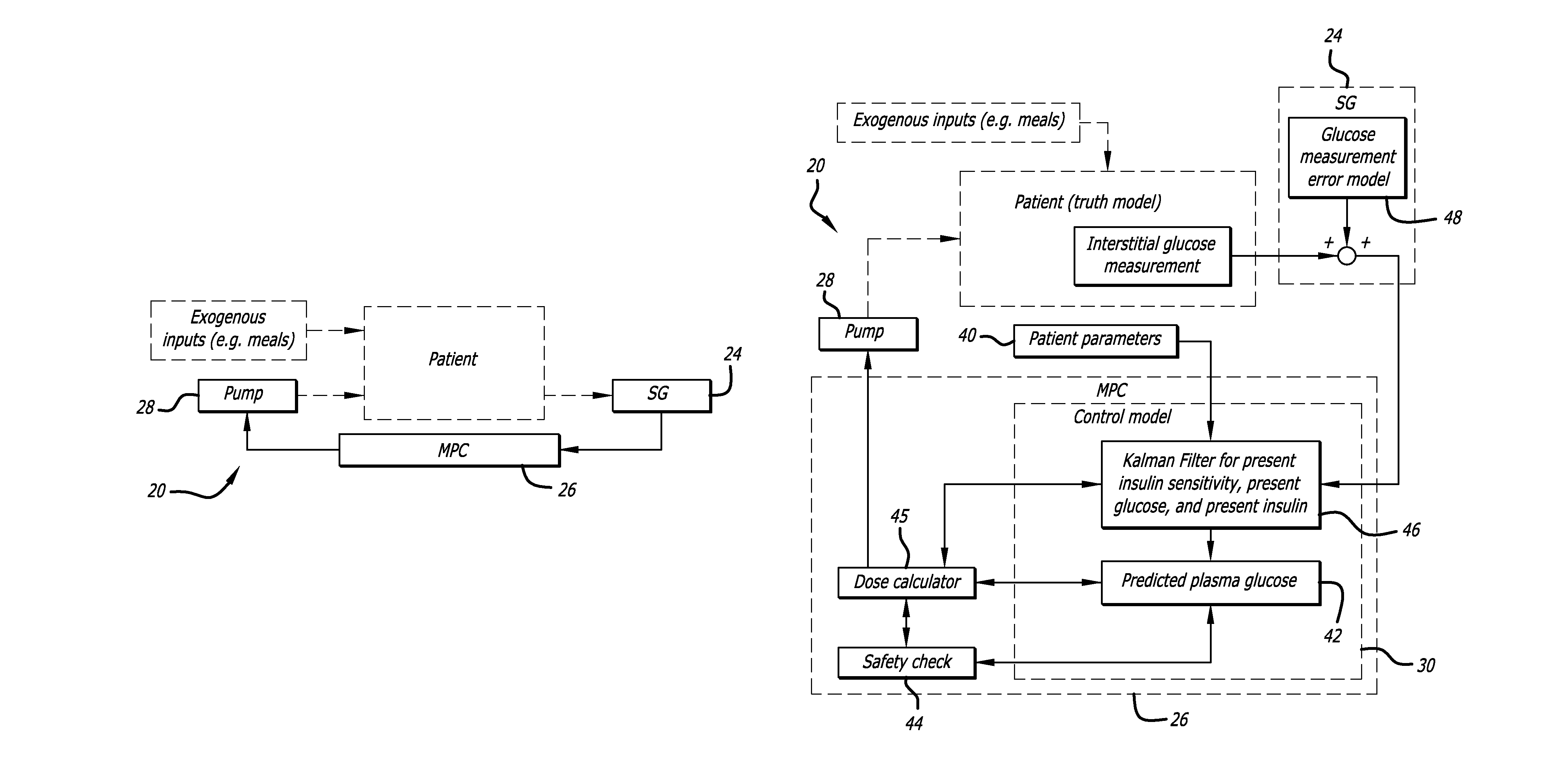
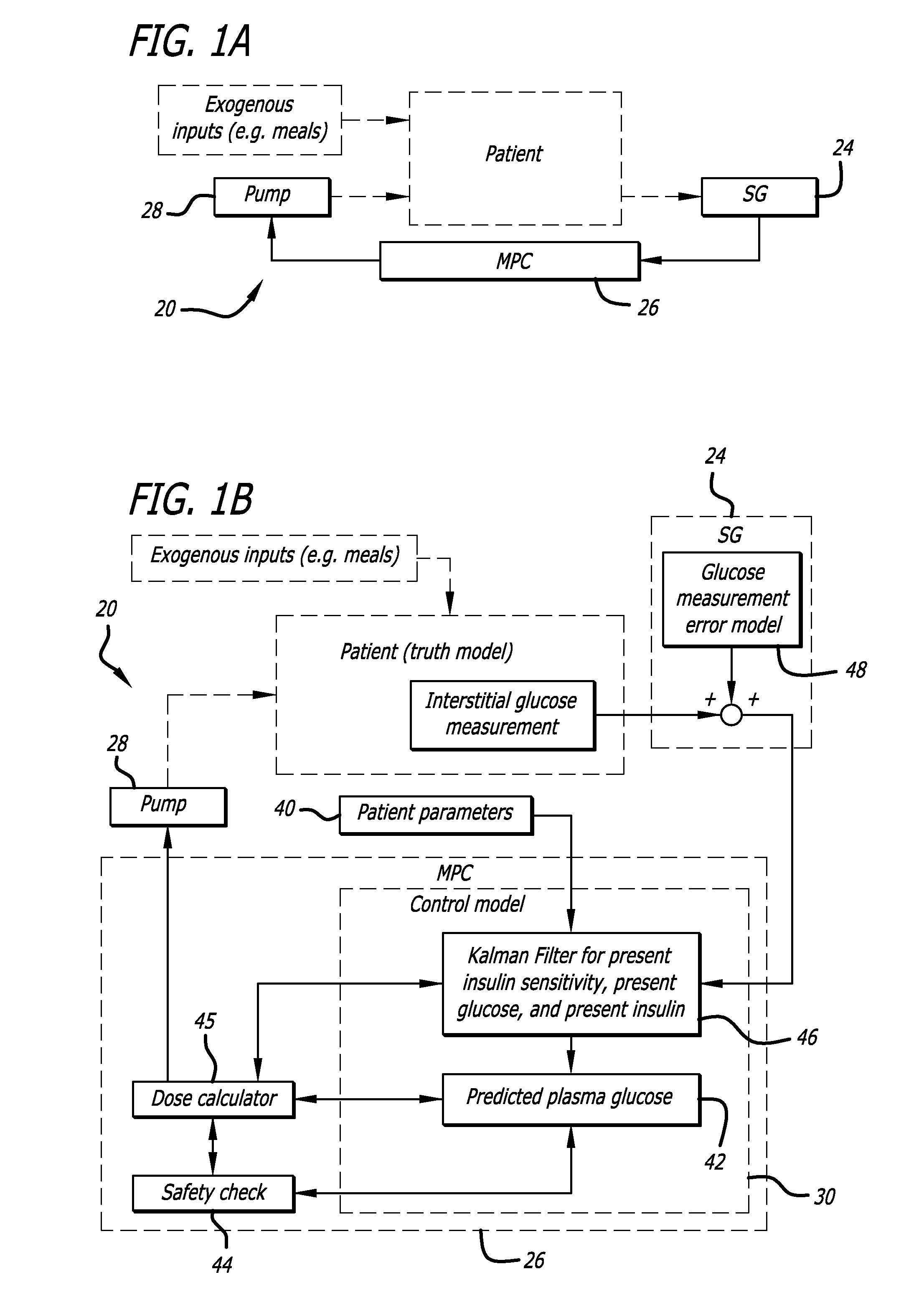
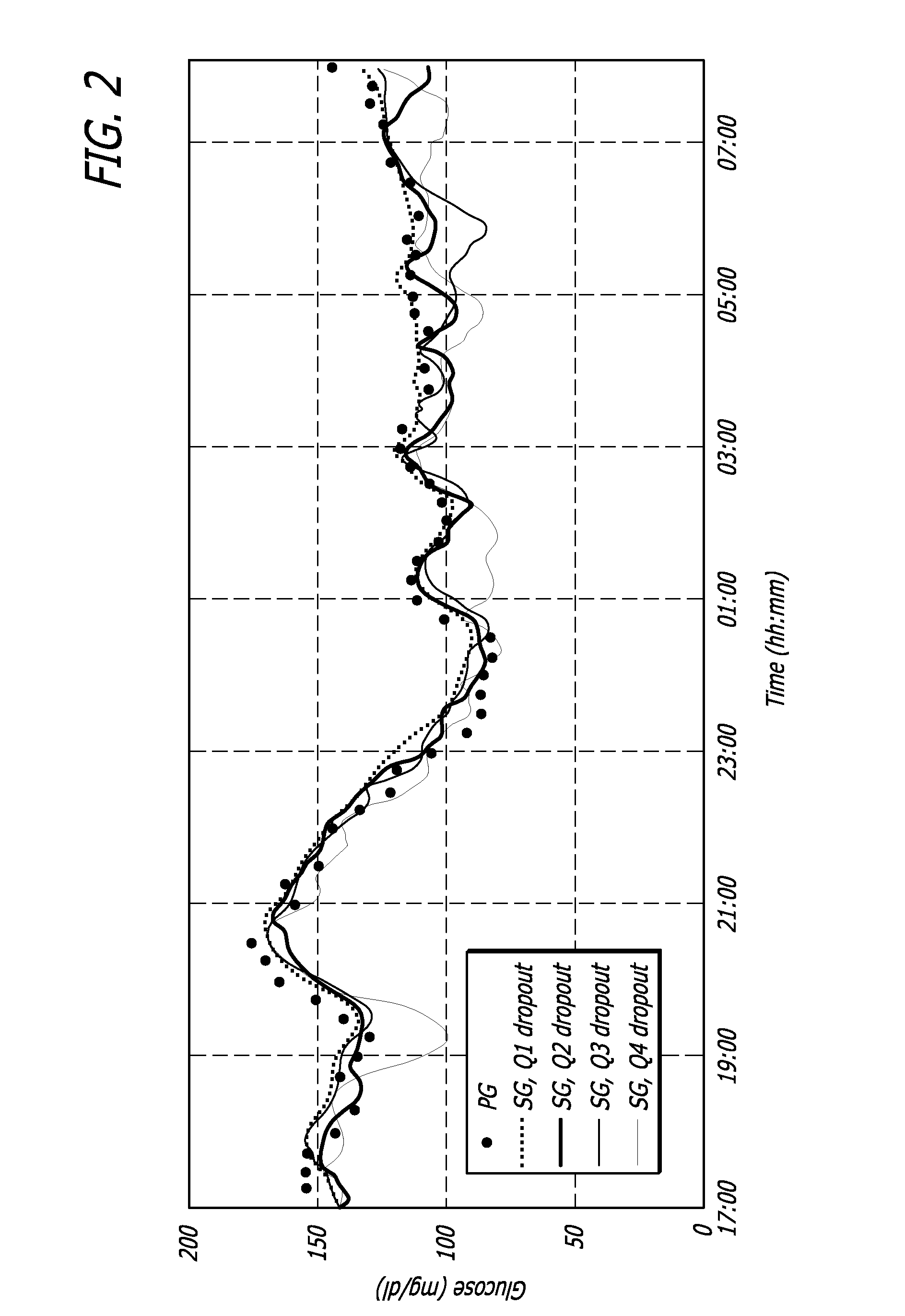
Overnight closed-loop insulin delivery with model predictive control and glucose measurement error model
ActiveUS20100280441A1Reducing insulin deliveryReduce deliveryDrug and medicationsMedical devicesGlucose sensorsClosed loop
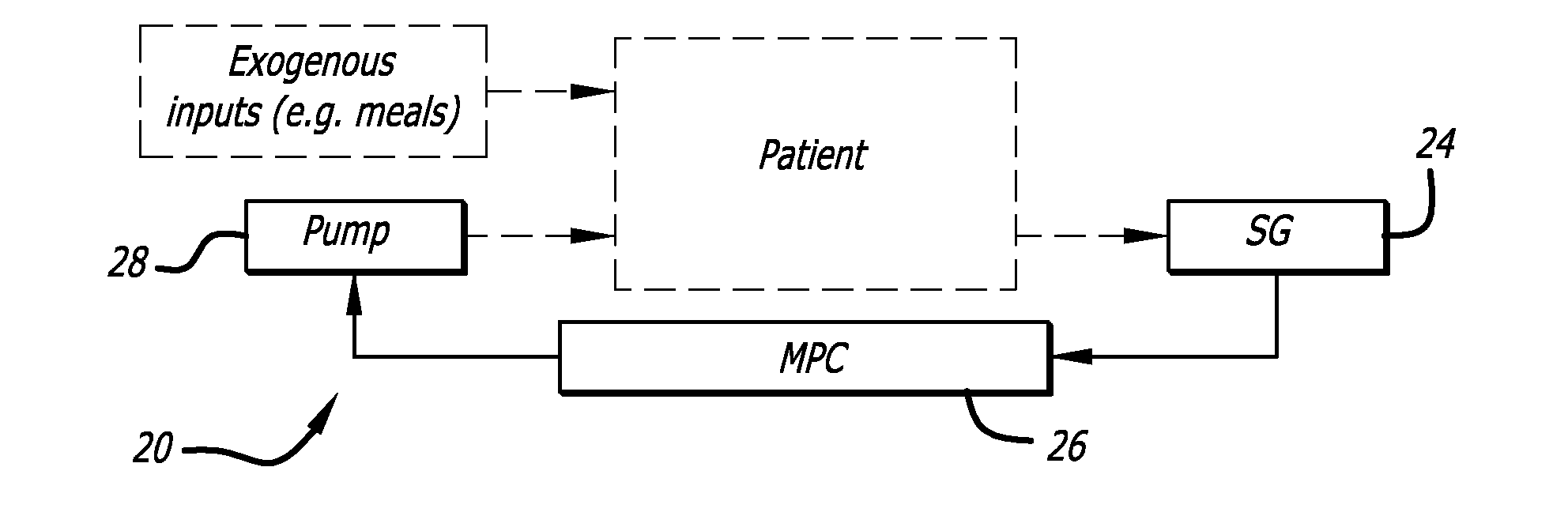
A closed-loop system for insulin infusion overnight uses a model predictive control algorithm (“MPC”). Used with the MPC is a glucose measurement error model which was derived from actual glucose sensor error data. That sensor error data included both a sensor artifacts component, including dropouts, and a persistent error component, including calibration error, all of which was obtained experimentally from living subjects. The MPC algorithm advised on insulin infusion every fifteen minutes. Sensor glucose input to the MPC was obtained by combining model-calculated, noise-free interstitial glucose with experimentally-derived transient and persistent sensor artifacts associated with the FreeStyle Navigator® Continuous Glucose Monitor System (“FSN”). The incidence of severe and significant hypoglycemia reduced 2300- and 200-fold, respectively, during simulated overnight closed-loop control with the MPC algorithm using the glucose measurement error model suggesting that the continuous glucose monitoring technologies facilitate safe closed-loop insulin delivery.
Owner:CAMBRIDGE ENTERPRISE LTD +1
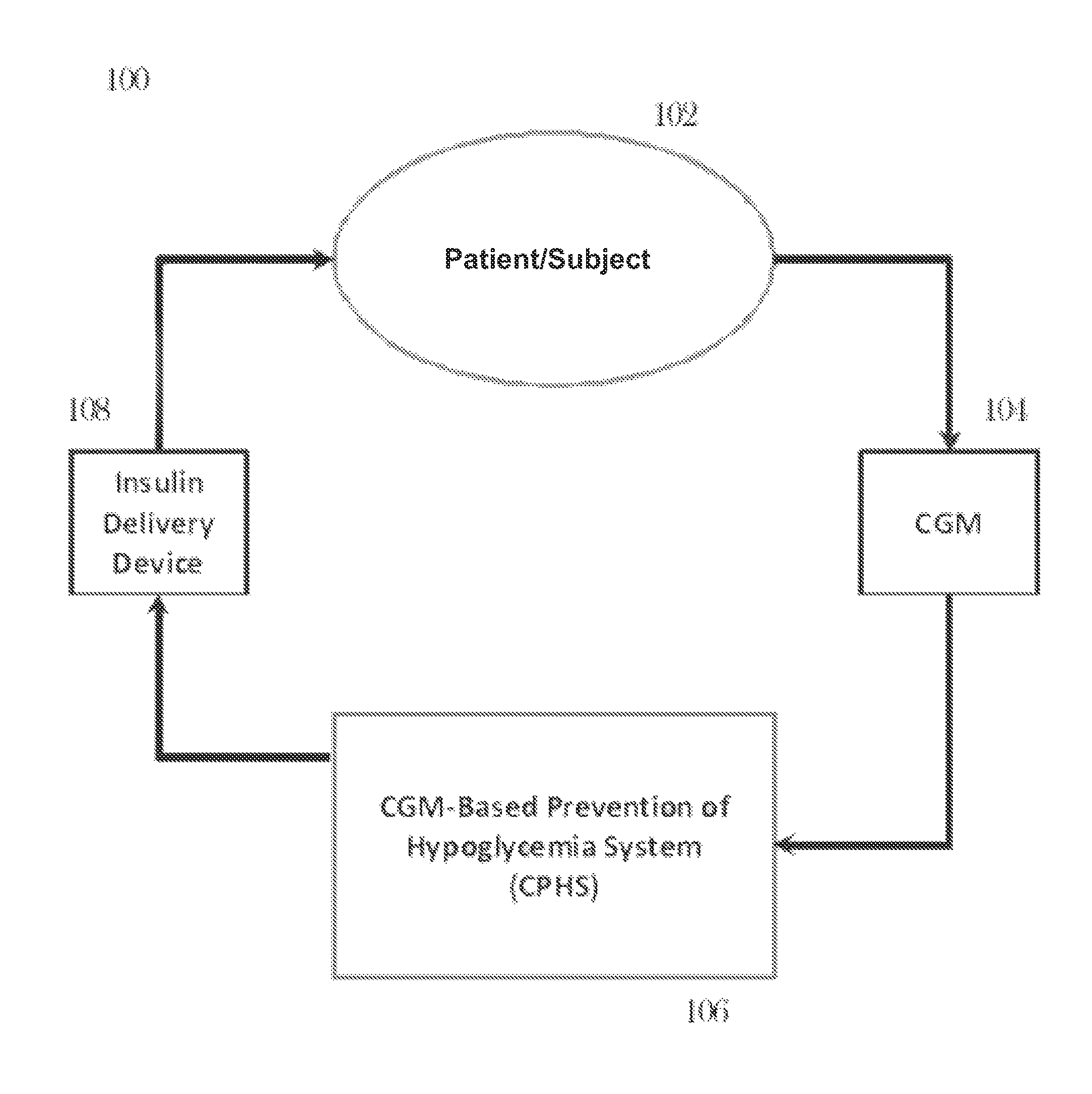
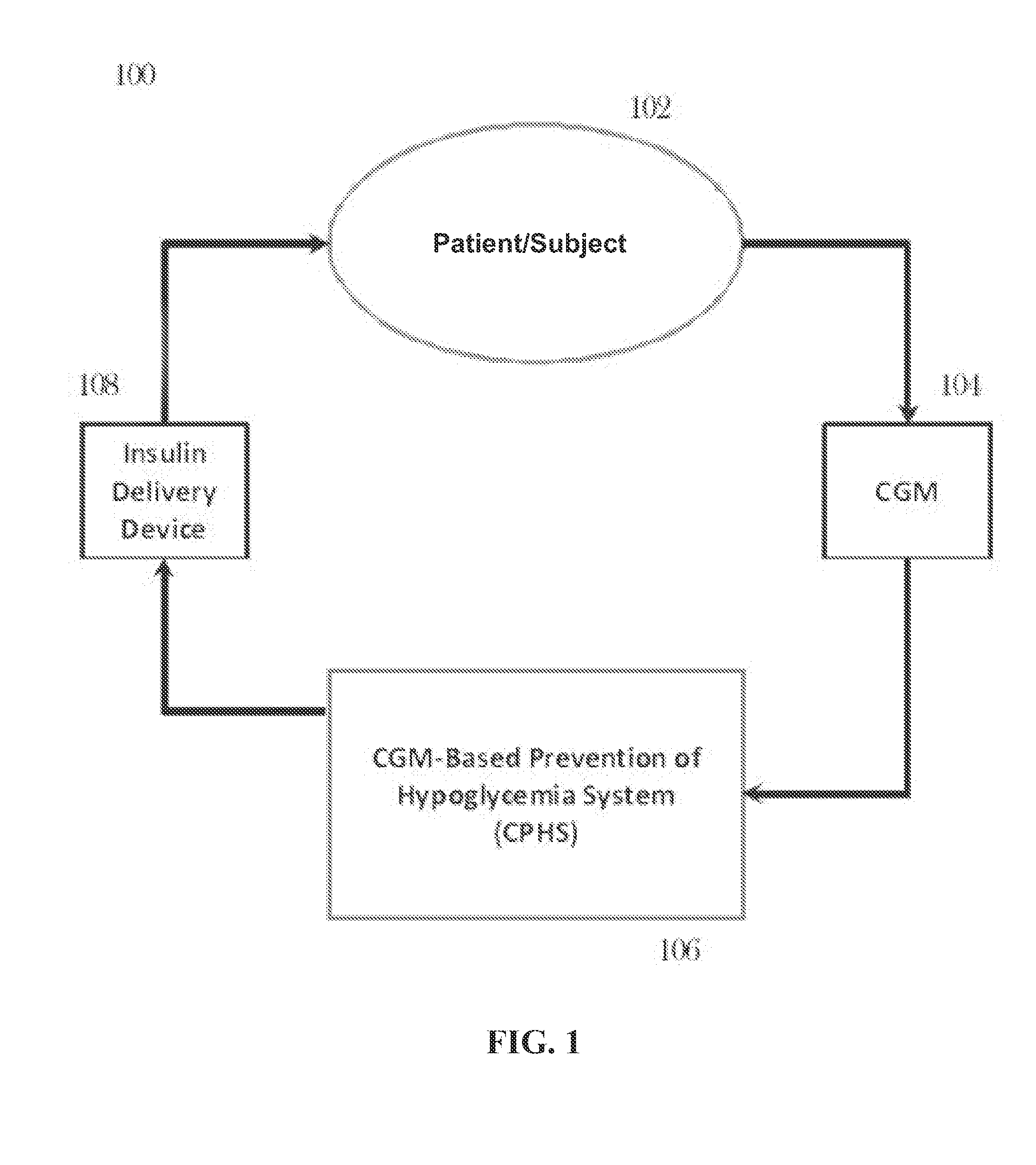
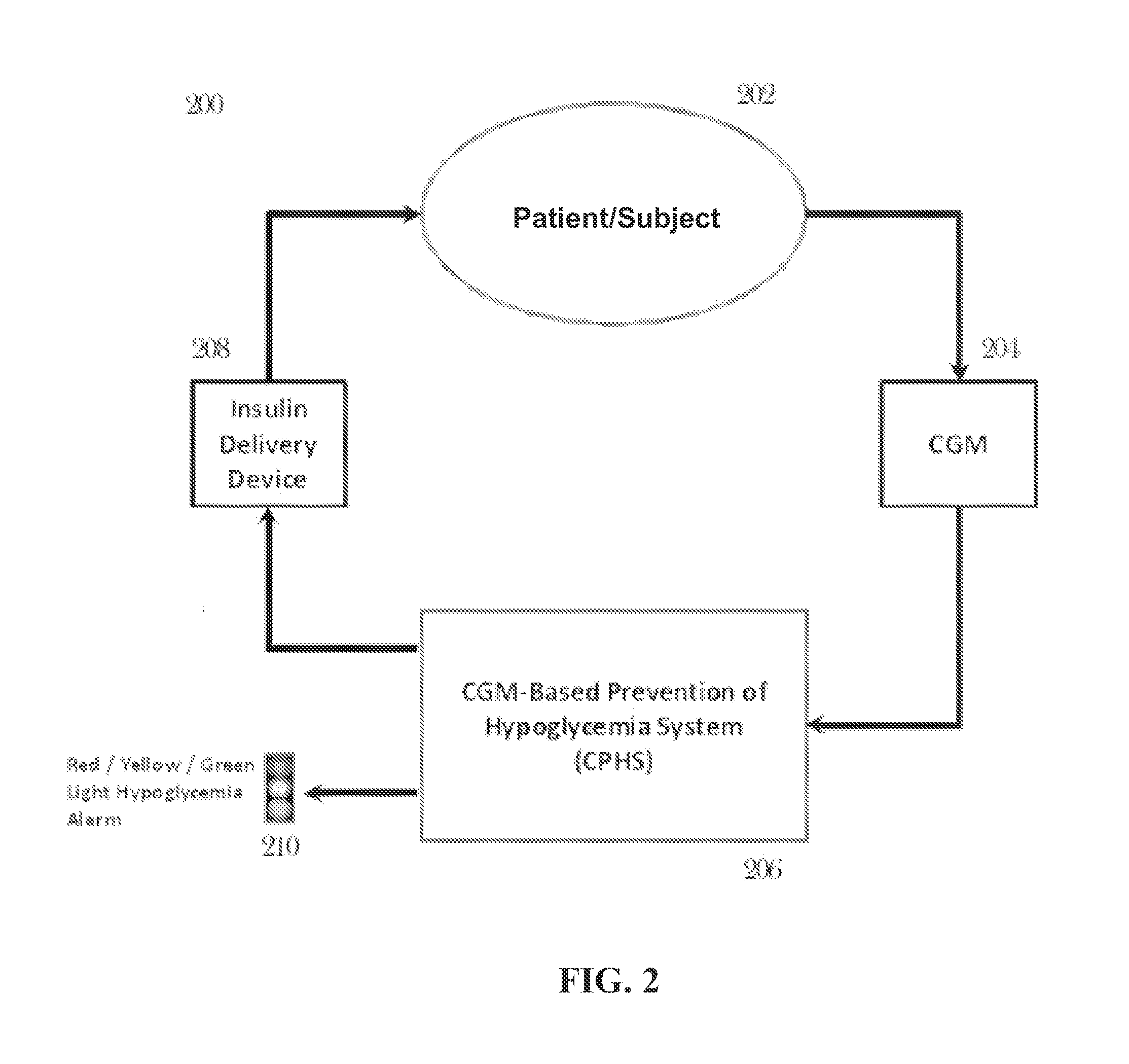
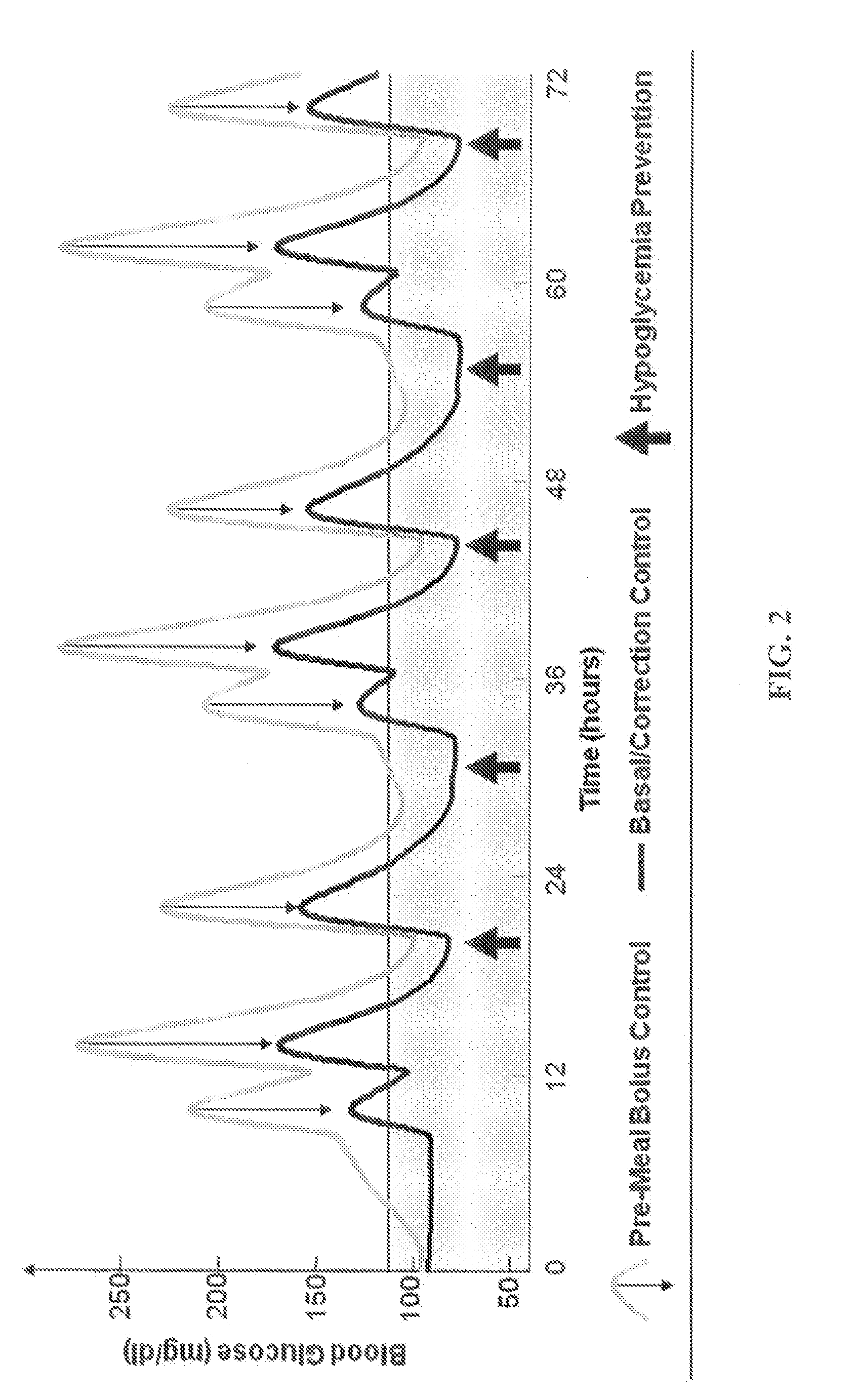
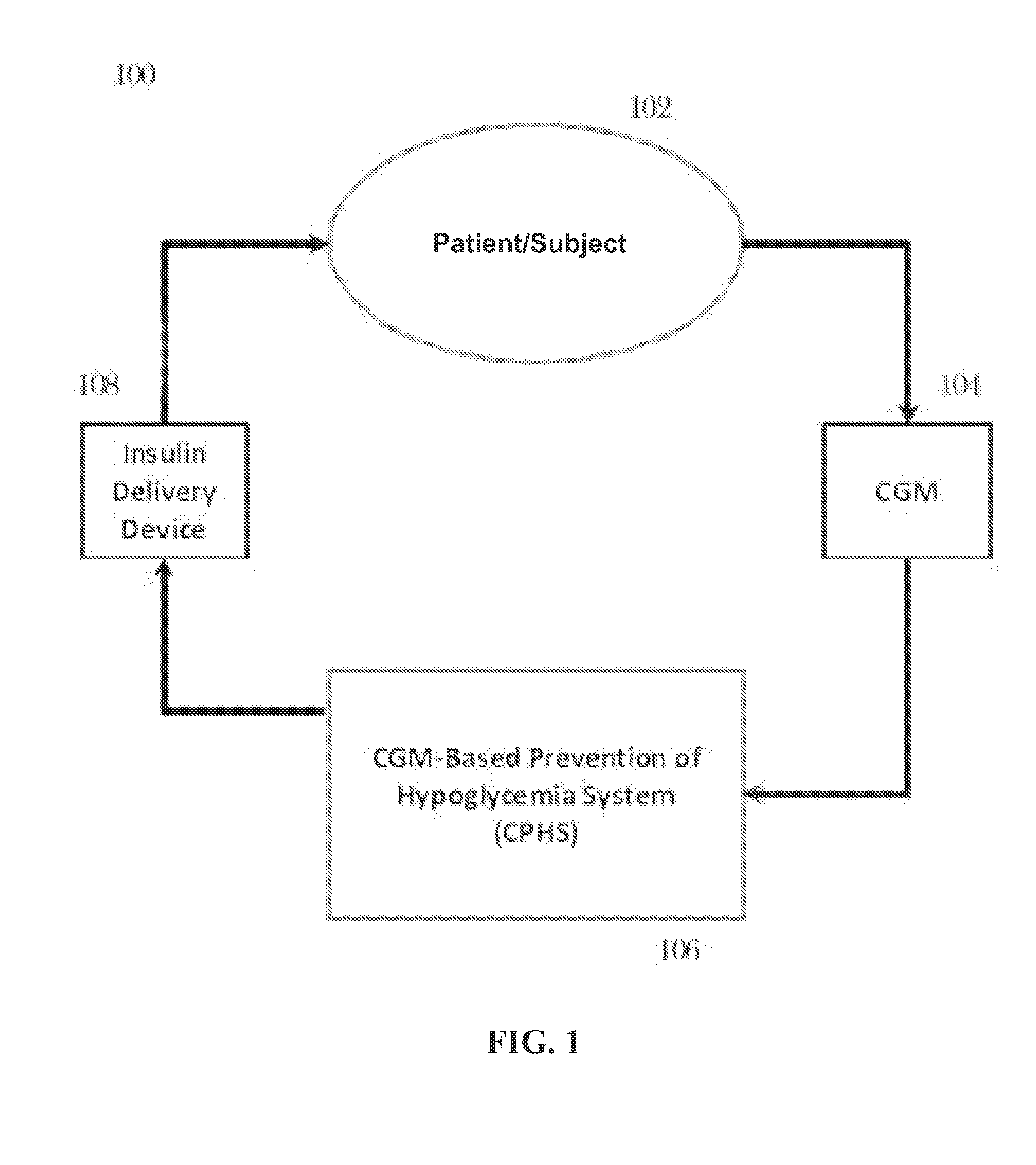
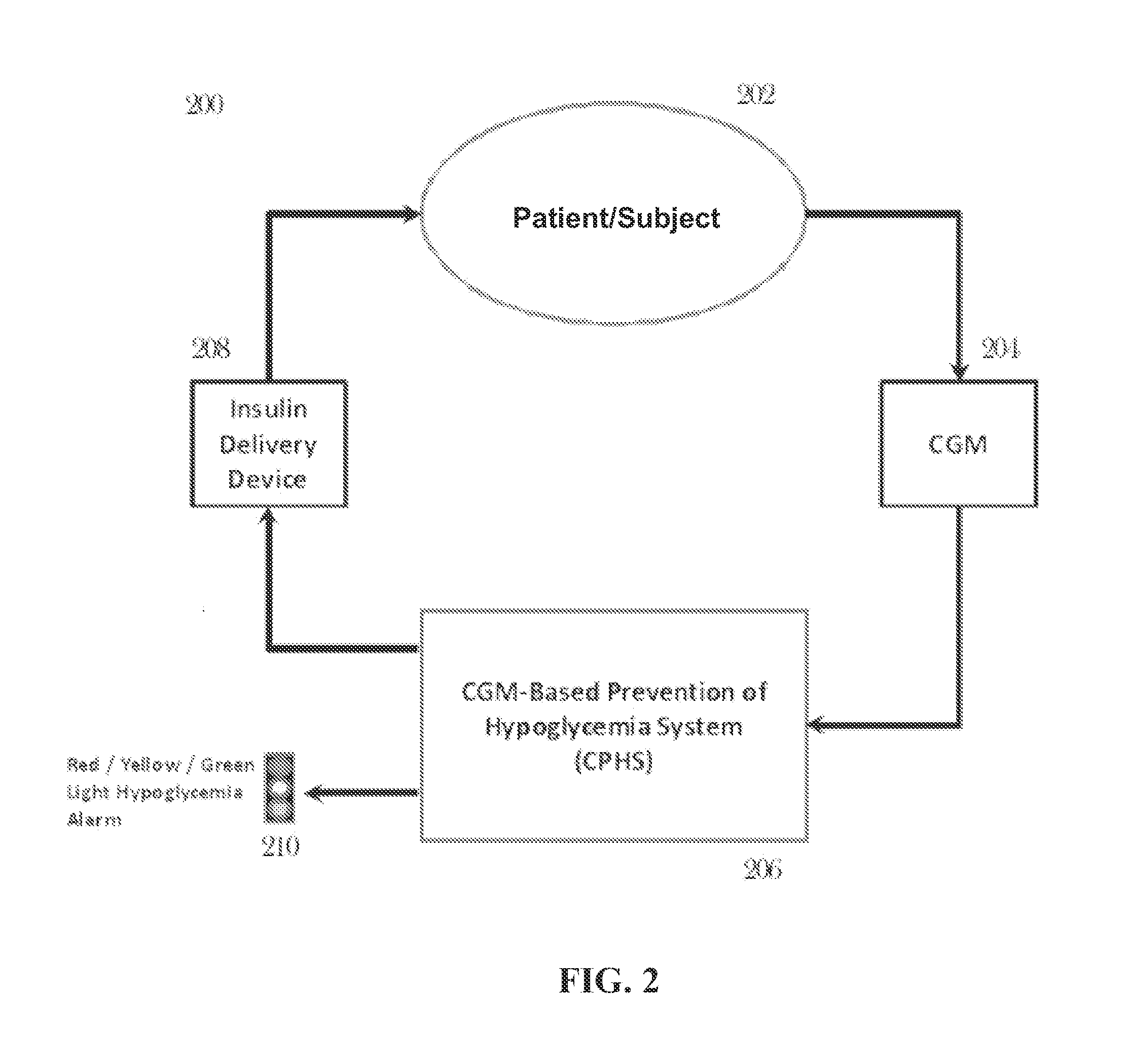
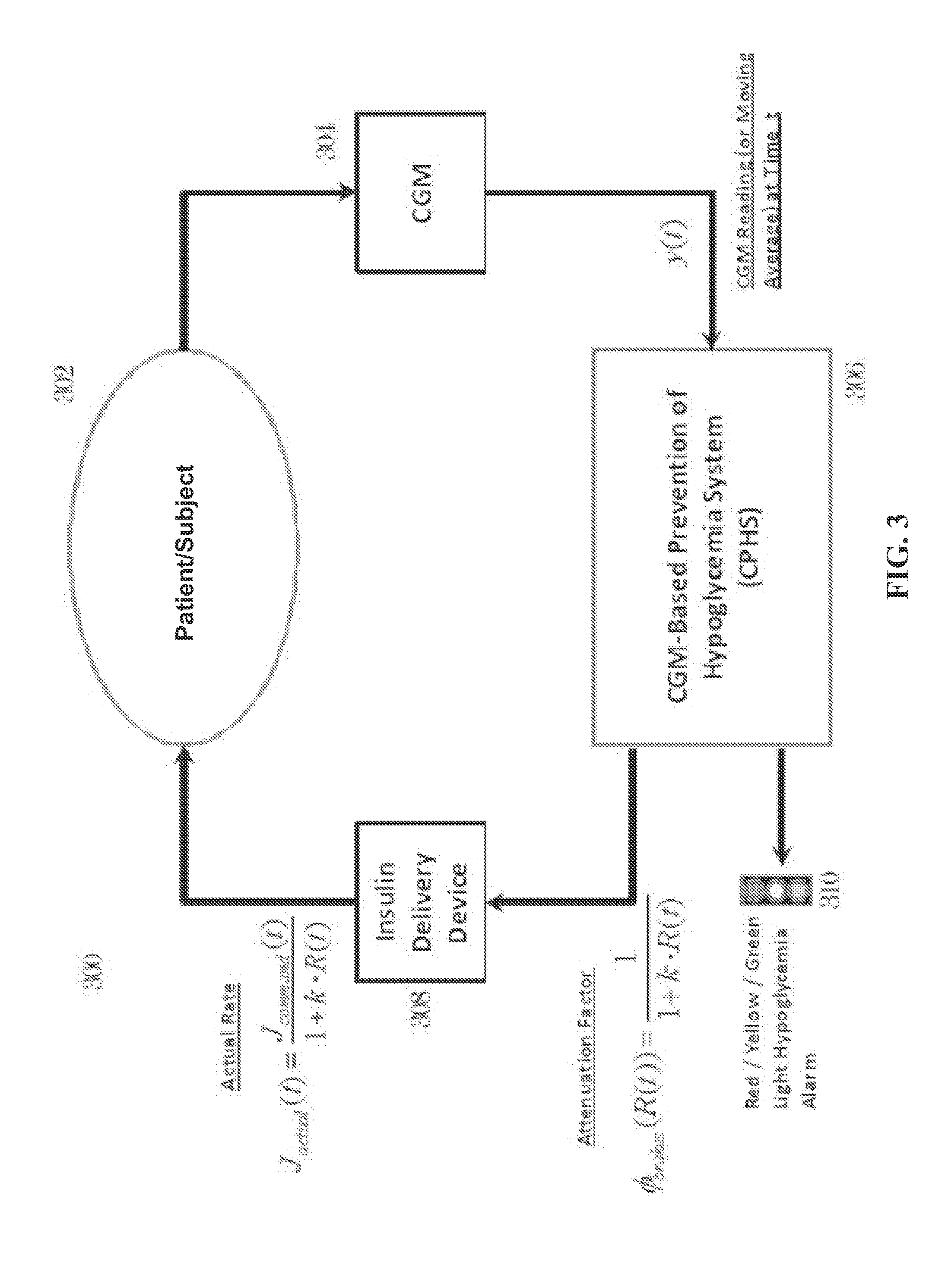
CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery
ActiveUS8562587B2Avoiding under-insulinizationReduce riskMedical simulationMetabolism disorderMedicineClosed loop
An aspect of an embodiment or partial embodiment of the present invention (or combinations of various embodiments in whole or in part of the present invention) comprises, but not limited thereto, a method and system (and related computer program product) for continually assessing the risk of hypoglycemia for a patient and then determining what action to take based on that risk assessment. A further embodiment results in two outputs: (1) an attenuation factor to be applied to the insulin rate command sent to the pump (either via conventional therapy or via open or closed loop control) and / or (2) a red / yellow / green light hypoglycemia alarm providing to the patient an indication of the risk of hypoglycemia. The two outputs of the CPHS can be used in combination or individually.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Safety features for integrated insulin delivery system
ActiveUS8257300B2Limited hyperglycemiaPreventing hypoglycemiaDrug and medicationsMedical devicesGlucose sensorsEngineering
Safety features are applied to an integrated insulin delivery system to enhance safety while accounting for glucose sensor bias and calibration errors. One safety feature includes comparisons of calibrations of the sensor to nominal sensitivity and taking action, such as limiting insulin delivery or taking a further calibration of the sensor. In another feature, an automatic resumption of a basal delivery rate is programmed into the delivery device to avoid the possibility of complete loss of delivery of insulin in the event that communication with the delivery device is disrupted. Other features include steps taken to avoid hypoglycemia in the event that the sensor is negatively biased.
Owner:ABBOTT DIABETES CARE INC
Method and composition for enhanced parenteral nutrition
A composition and method for improved parenteral administration of nutrients is provided. The method comprises co-administration of nutrients, especially carbohydrates, and an insulinotropic peptide, or its derivatives, analogs, and fragments. The method and composition provide high carbohydrate nutrition while avoiding hyper- and hypoglycemia and their attendant deleterious effects.
Owner:AMYLIN PHARMA INC +1
Analyte testing method and device for calculating basal insulin therapy
Described herein are various methods to ensure safety and the compliance of therapeutic diabetes protocols. The method can be achieved by performing safeguards against hypoglycemia of the user prior to any change in basal insulin dosage based on the plurality of data.
Owner:LIFESCAN IP HLDG LLC
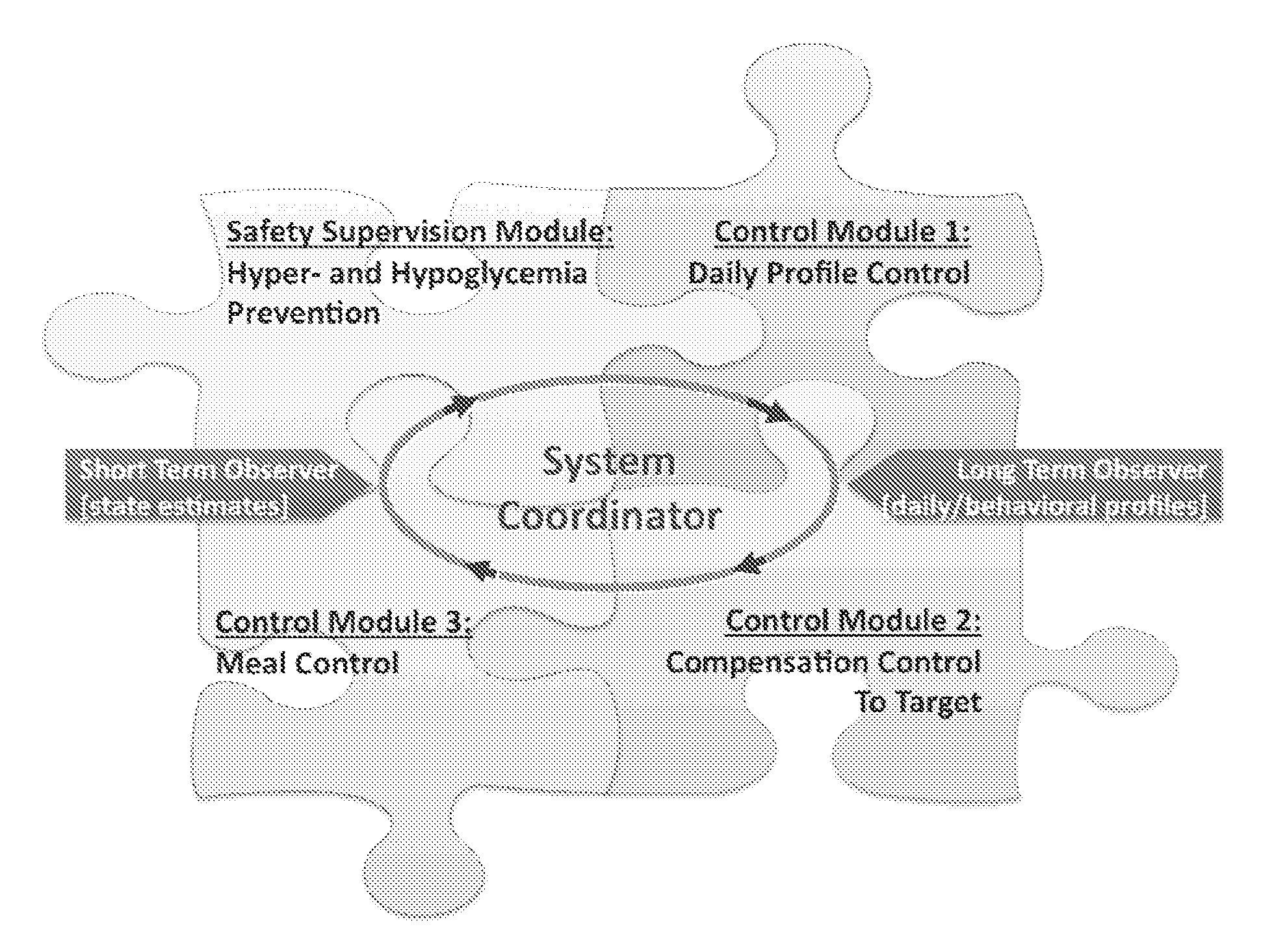
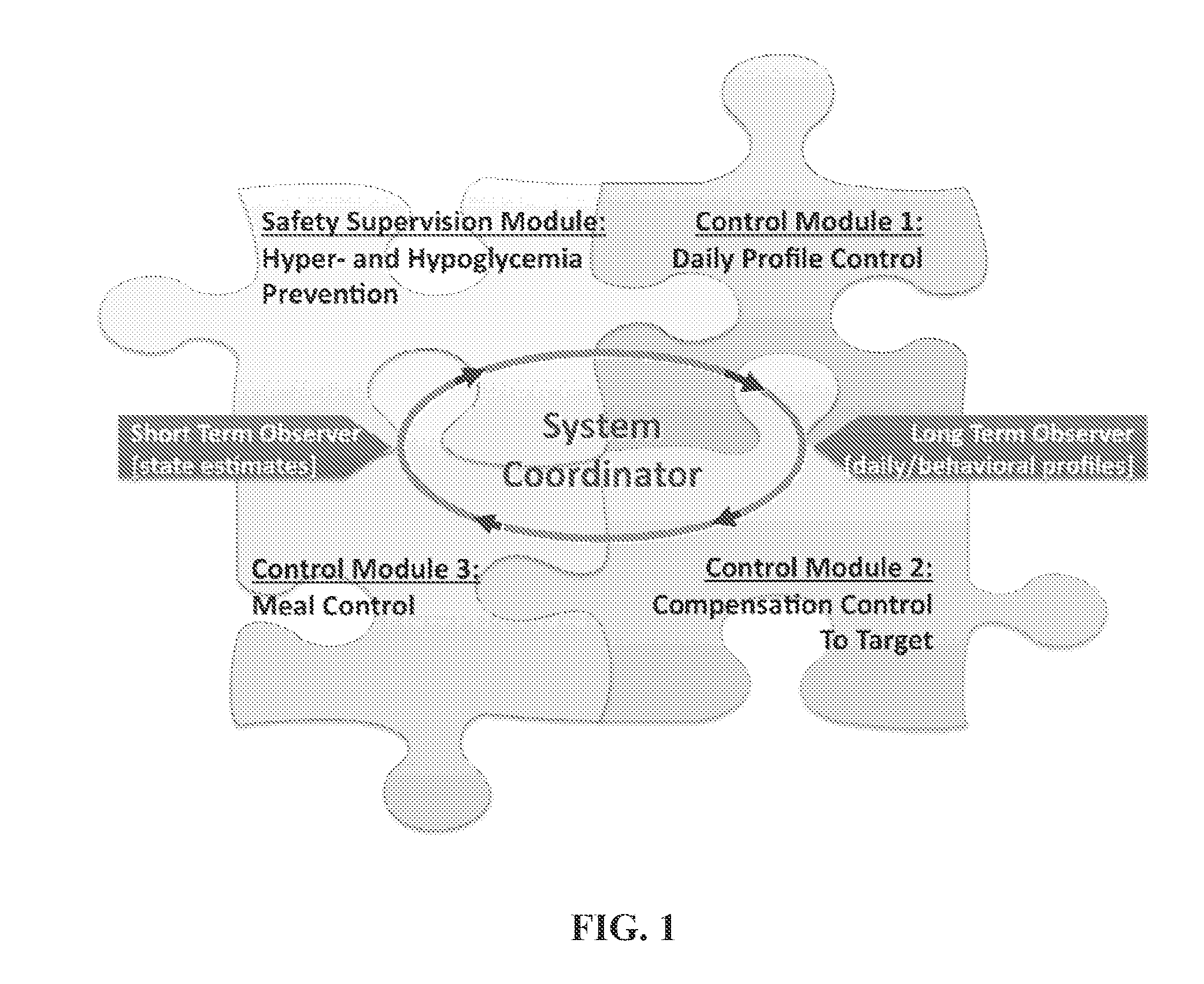
System Coordinator and Modular Architecture for Open-Loop and Closed-Loop Control of Diabetes
A structure, method, and computer program product for a diabetes control system provides, but is not limited thereto, the following: open-loop or closed-loop control of diabetes that adapts to individual physiologic characteristics and to the behavioral profile of each person. An exemplary aspect to this adaptation is biosystem (patient or subject) observation and modular control. Consequently, established is the fundamental architecture and the principal components for a modular system, which may include algorithmic observers of patients' behavior and metabolic state, as well as interacting control modules responsible for basal rate, insulin boluses, and hypoglycemia prevention.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
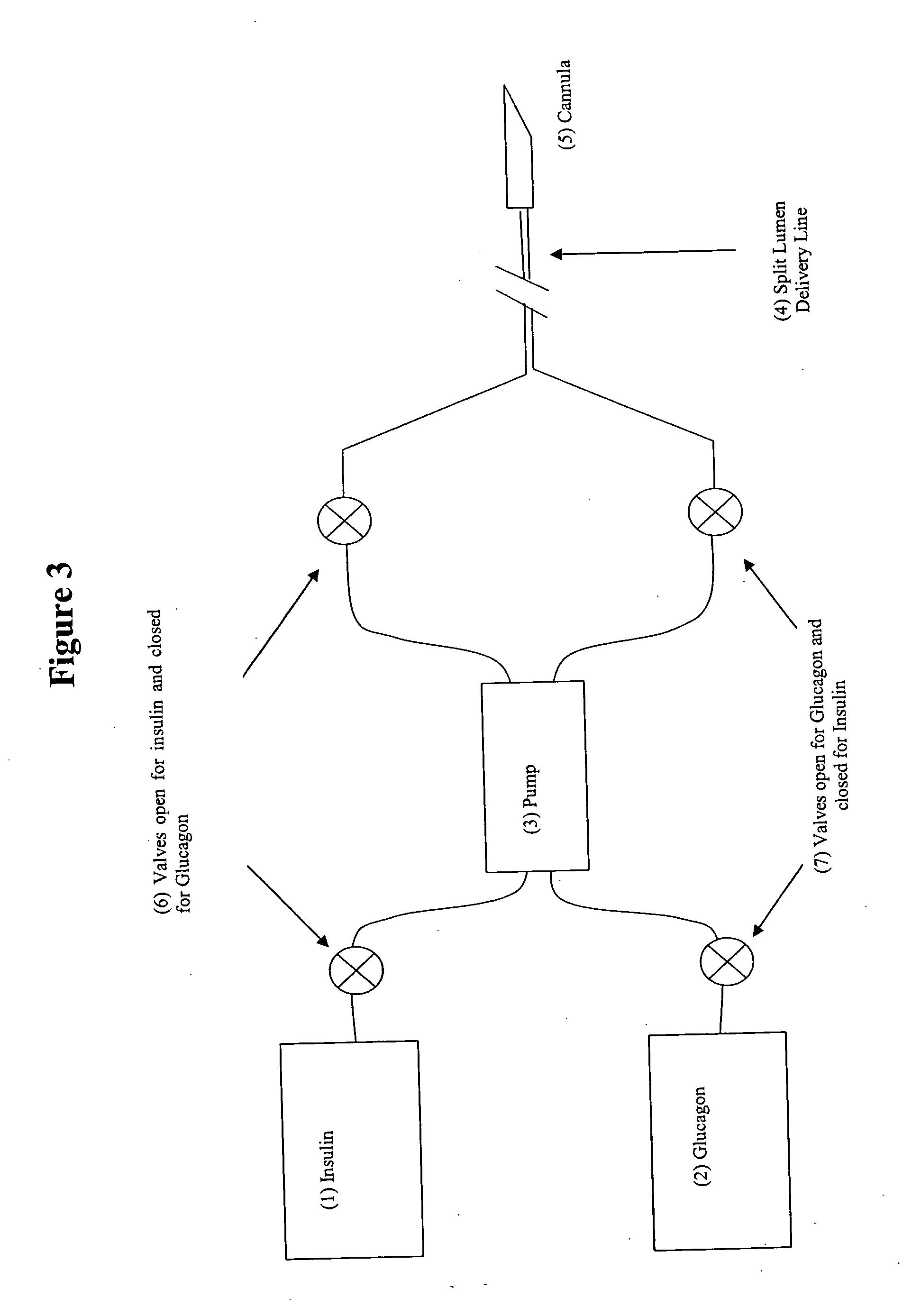
Compositions and methods for the prevention and control of insulin-induced hypoglycemia
InactiveUS20060014670A1Preventing hypoglycemiaPrevents a hypoglycemic eventIn-vivo radioactive preparationsPeptide/protein ingredientsMedicineInsulin induced hypoglycemia
Pharmaceutical compositions comprising both insulin and glucagon can be administered to control and treat diabetes while reducing or eliminating the risk of insulin-induced hypoglycemia.
Owner:ENJECT
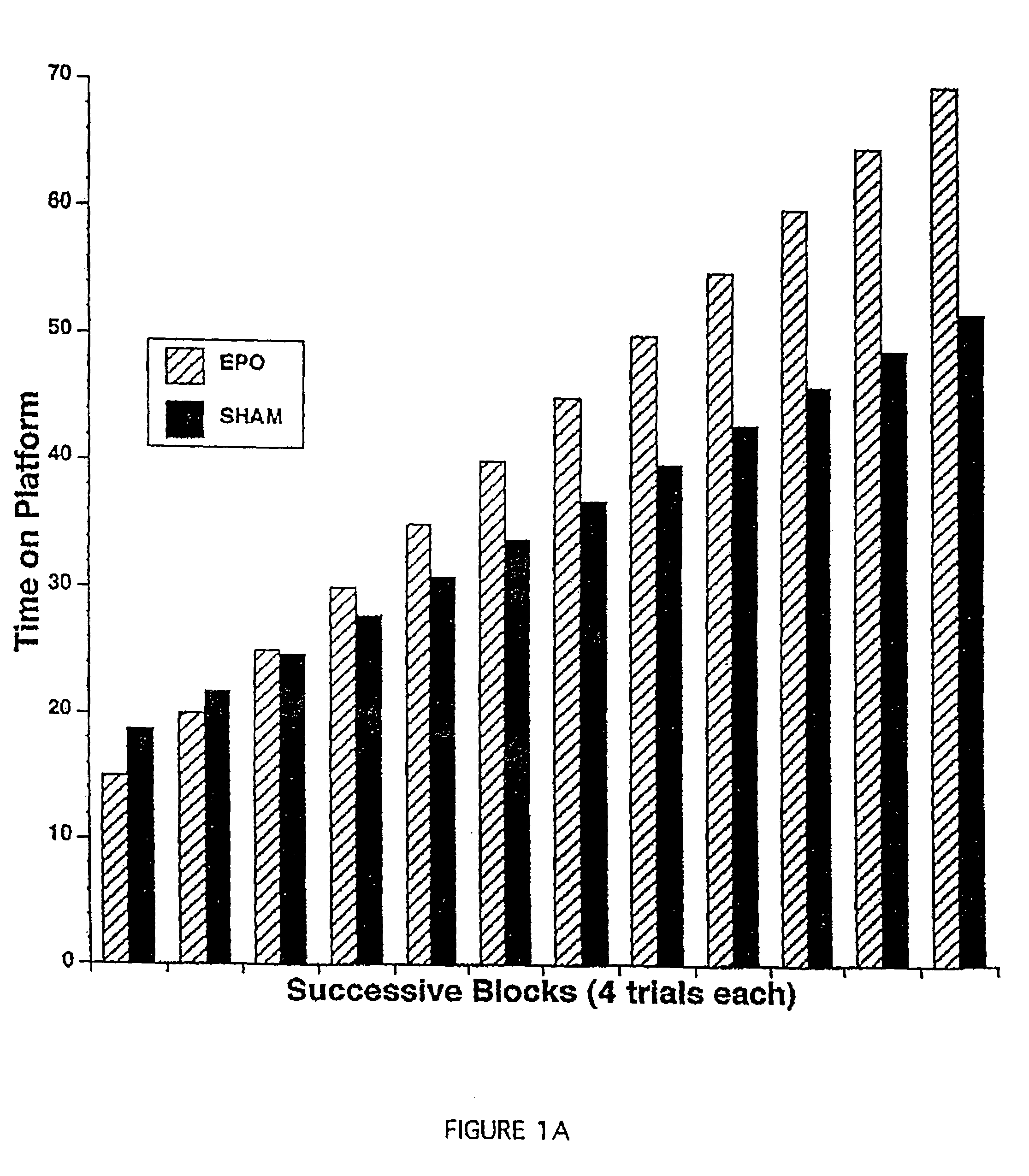
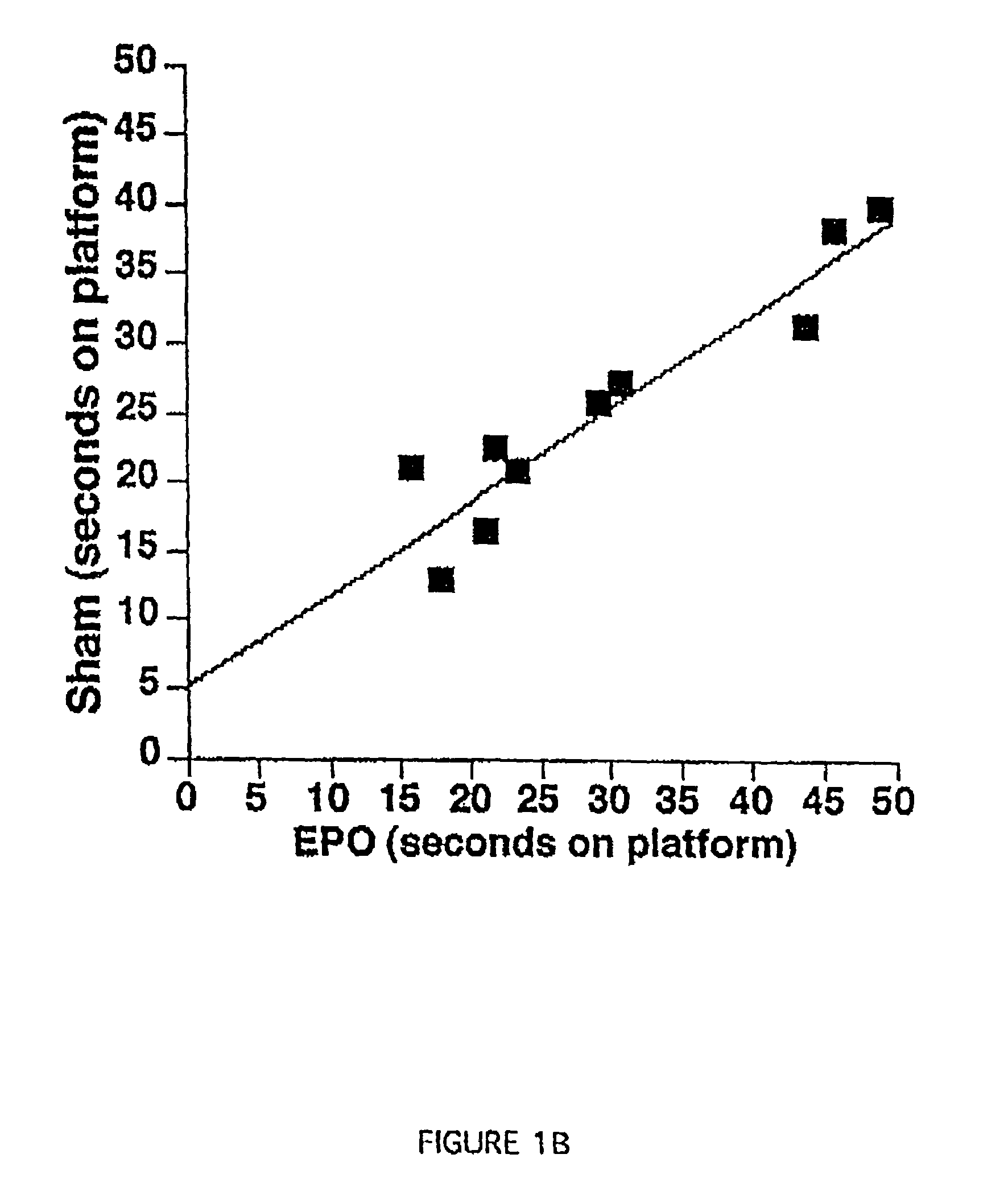
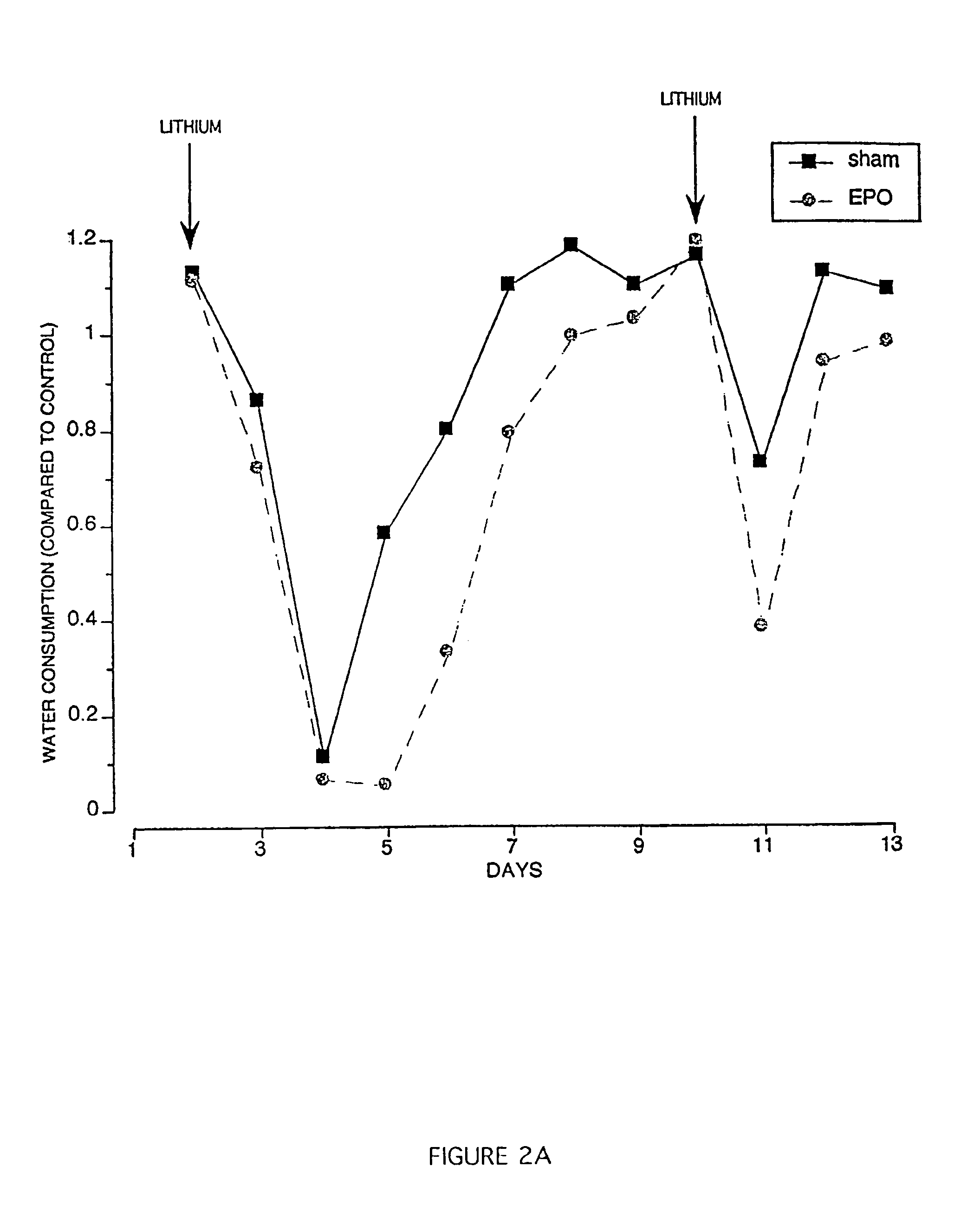
Methods for treatment and prevention of neuromuscular and muscular conditions by peripherally administered erythropoietin
InactiveUS7309687B1Improve cognitive functionAvoid damageNervous disorderPeptide/protein ingredientsDiseaseCentral neuron
Methods and compositions are provided for protecting or enhancing excitable tissue function in mammals by systemic administration of an erythropoietin receptor activity modulator, such as erythropoietin, which signals via an EPO-activated receptor to modulate the function of excitable tissue. Excitable tissues include central neuronal tissues, such as the brain, peripheral neuronal tissues, retina, and heart tissue. Protection of excitable tissues provides treatment of hypoxia, seizure disorders, neurodegenerative diseases, hypoglycemia, and neurotoxin poisoning. Enhancement of function is useful in learning and memory. The invention is also directed to compositions and methods for facilitating the transport of molecules across endothelial cell tight junction barriers, such as the blood-brain barrier, by association of molecules with an erythropoietin receptor activity modulator, such as an erythropoietin.
Owner:THE KENNETH S WARREN INST
Overnight closed-loop insulin delivery with model predictive control and glucose measurement error model
A closed-loop system for insulin infusion overnight uses a model predictive control algorithm (“MPC”). Used with the MPC is a glucose measurement error model which was derived from actual glucose sensor error data. That sensor error data included both a sensor artifacts component, including dropouts, and a persistent error component, including calibration error, all of which was obtained experimentally from living subjects. The MPC algorithm advised on insulin infusion every fifteen minutes. Sensor glucose input to the MPC was obtained by combining model-calculated, noise-free interstitial glucose with experimentally-derived transient and persistent sensor artifacts associated with the FreeStyle Navigator® Continuous Glucose Monitor System (“FSN”). The incidence of severe and significant hypoglycemia reduced 2300- and 200-fold, respectively, during simulated overnight closed-loop control with the MPC algorithm using the glucose measurement error model suggesting that the continuous glucose monitoring technologies facilitate safe closed-loop insulin delivery.
Owner:CAMBRIDGE ENTERPRISE LTD +1
Methods of monitoring glucose levels in a subject and uses thereof
Methods of frequently monitoring glucose amounts and / or concentrations in a subject who is at risk for hypoglycemia, hyperglycemia, and / or glucose level fluctuations that put the subject at risk are provided. Also provided are methods of monitoring the effects of one or more pharmaceutical compositions on the levels of glucose in a subject.
Owner:ANIMAS TECH
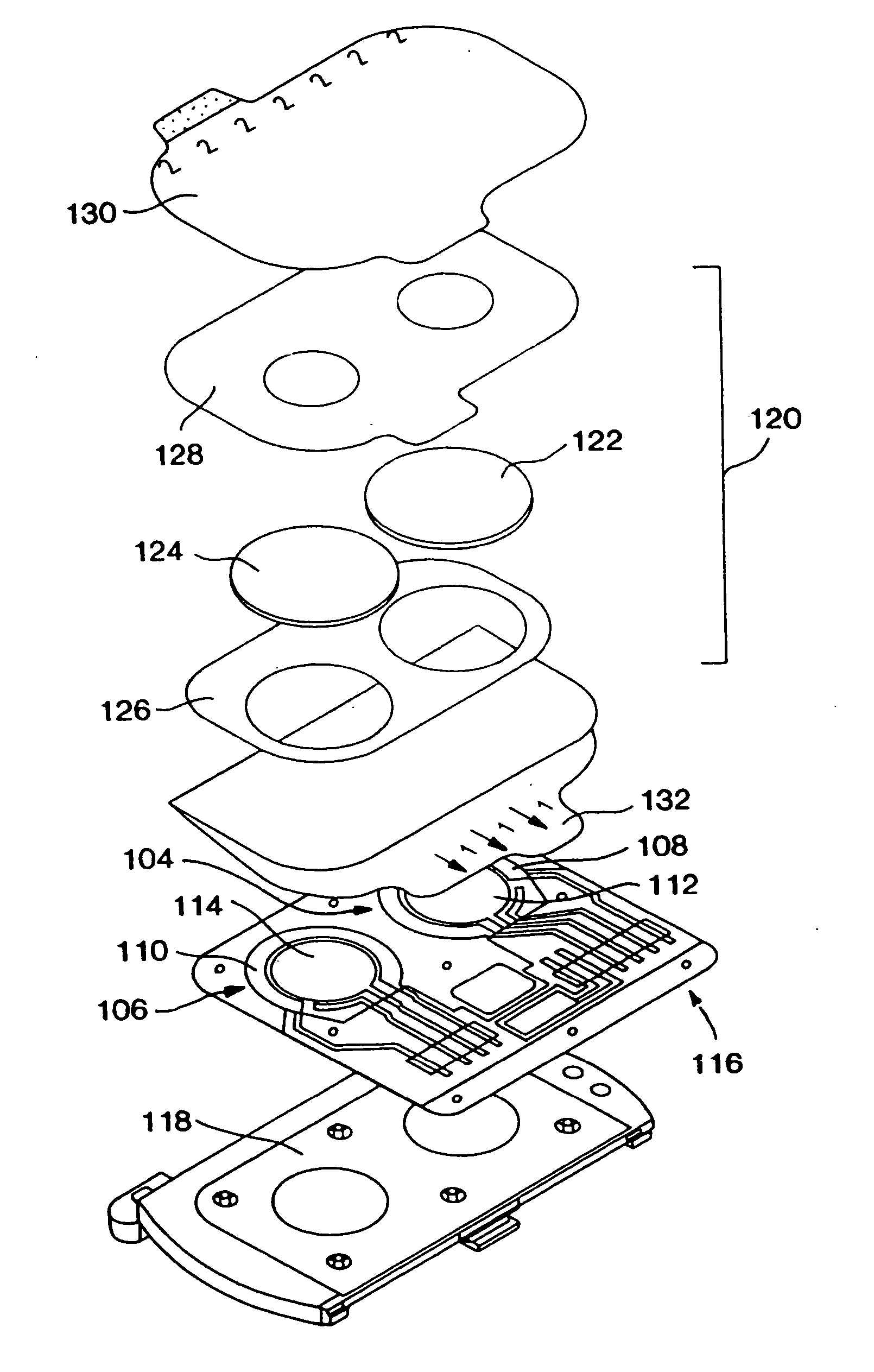
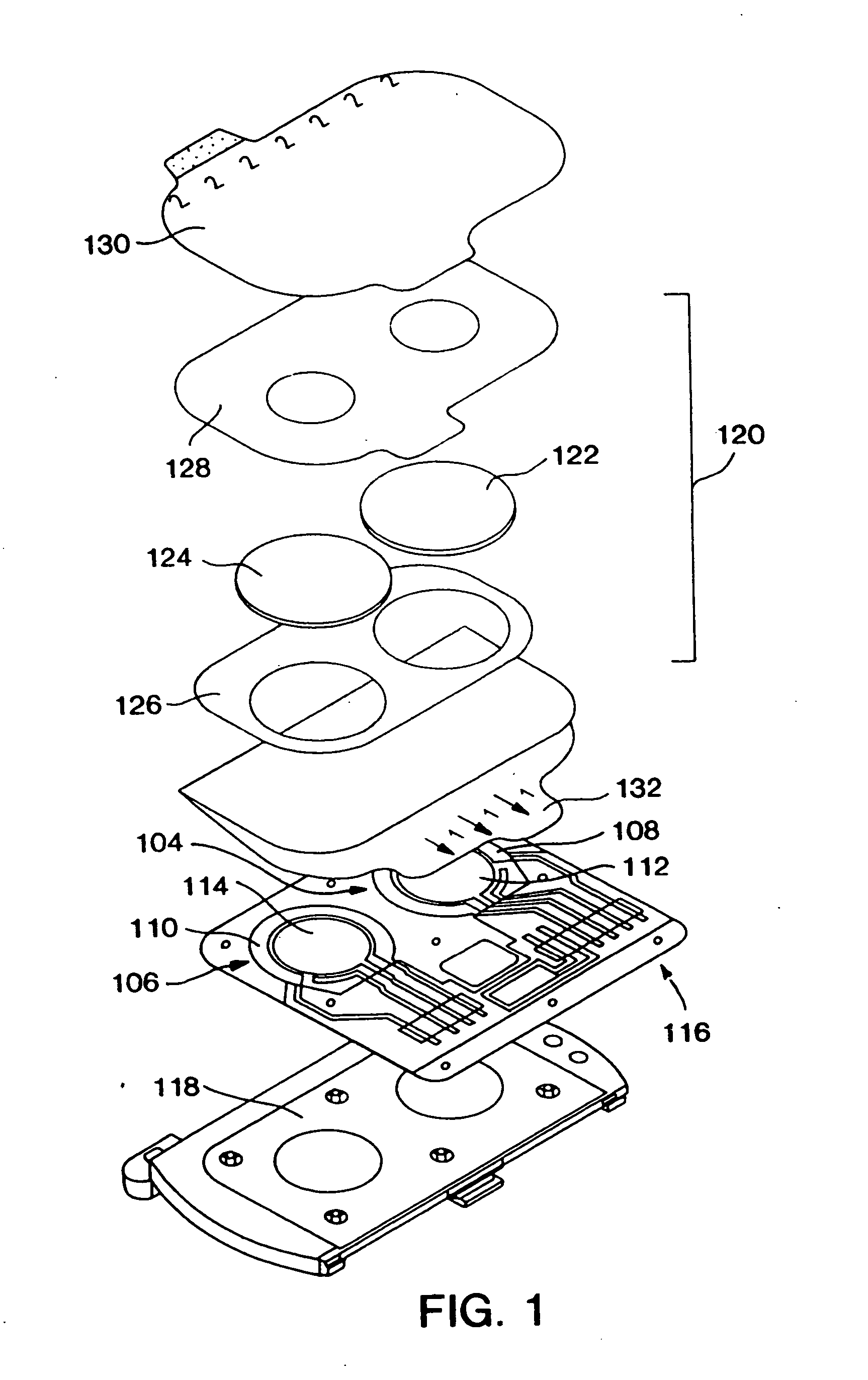
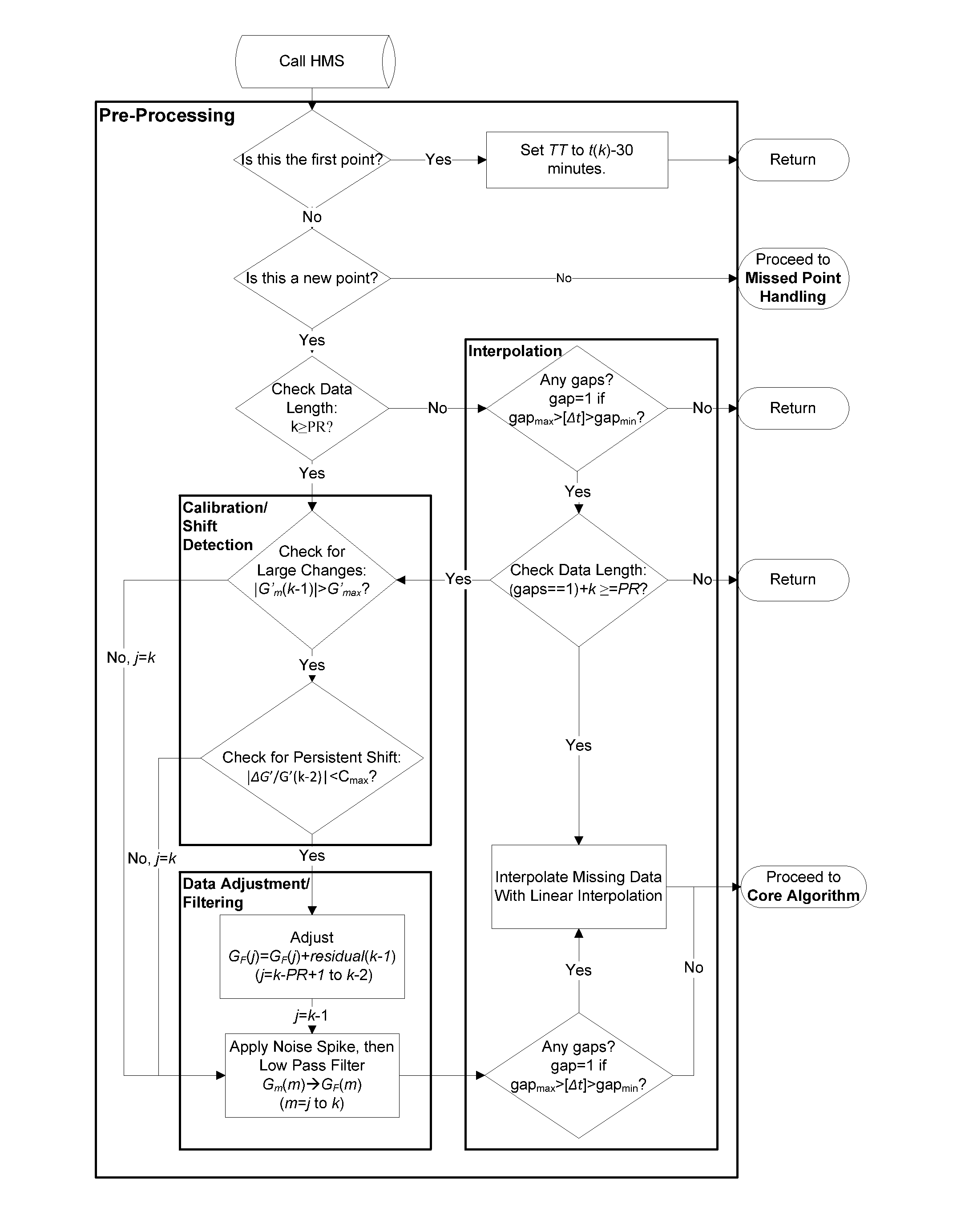
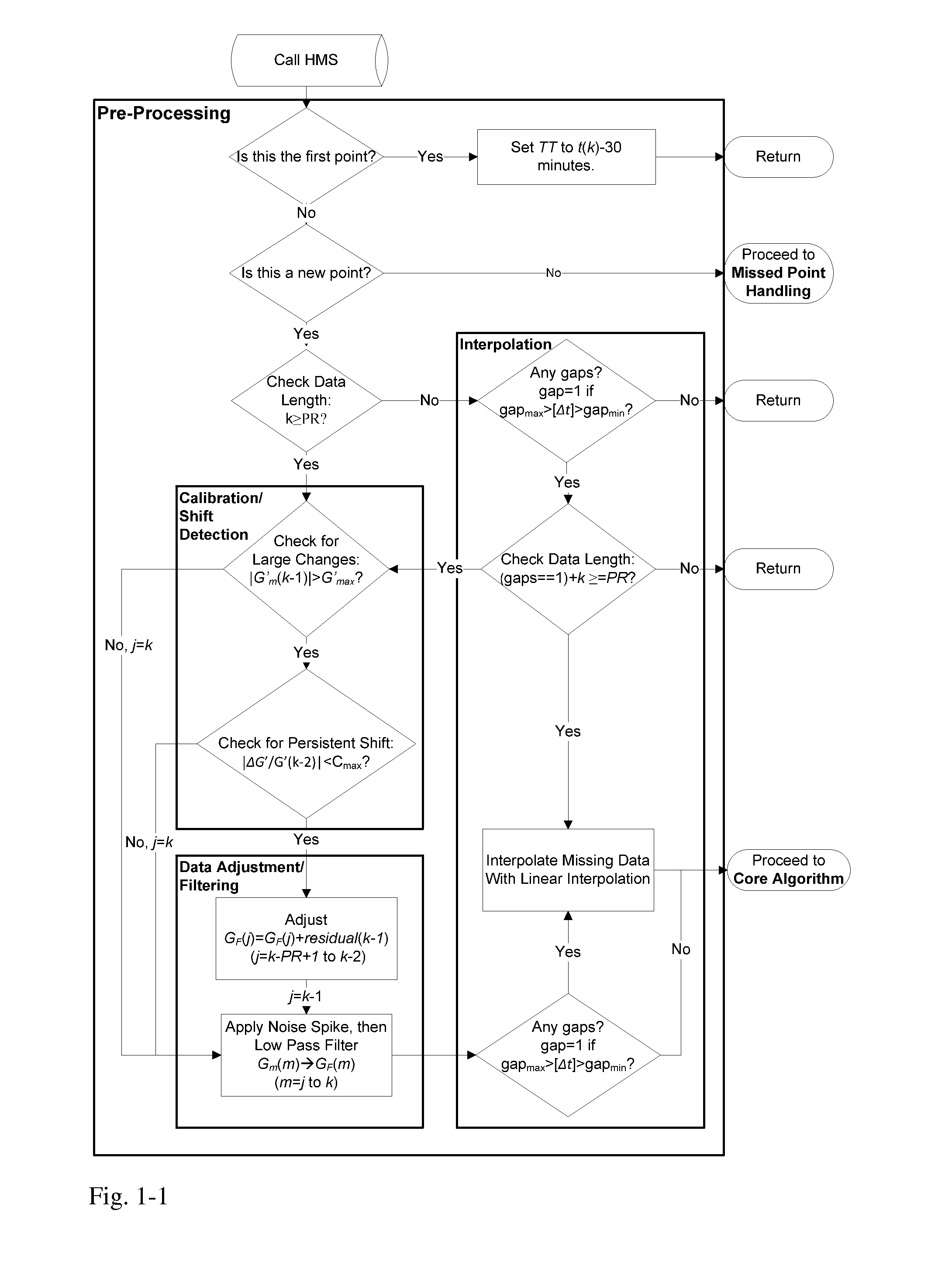
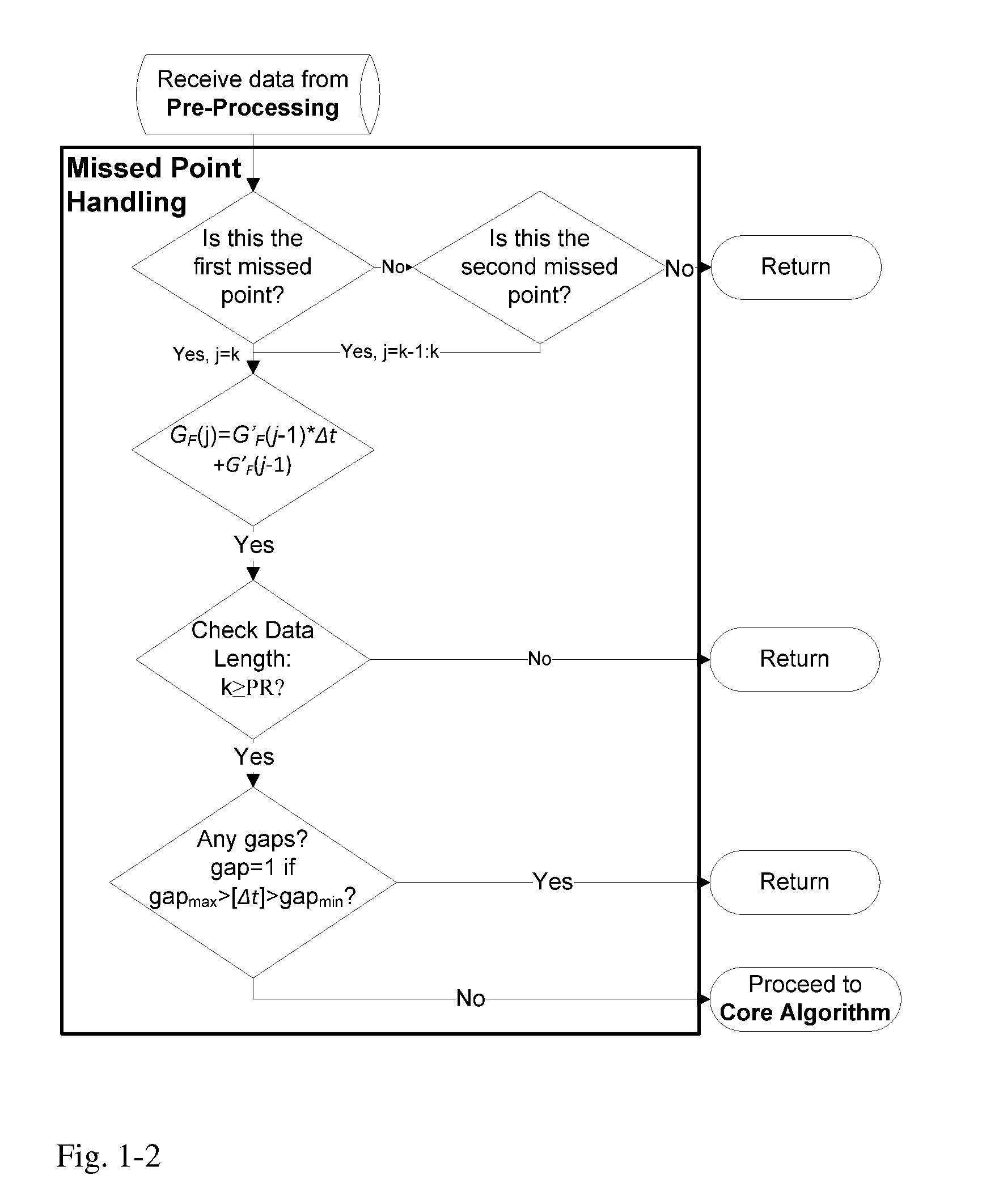
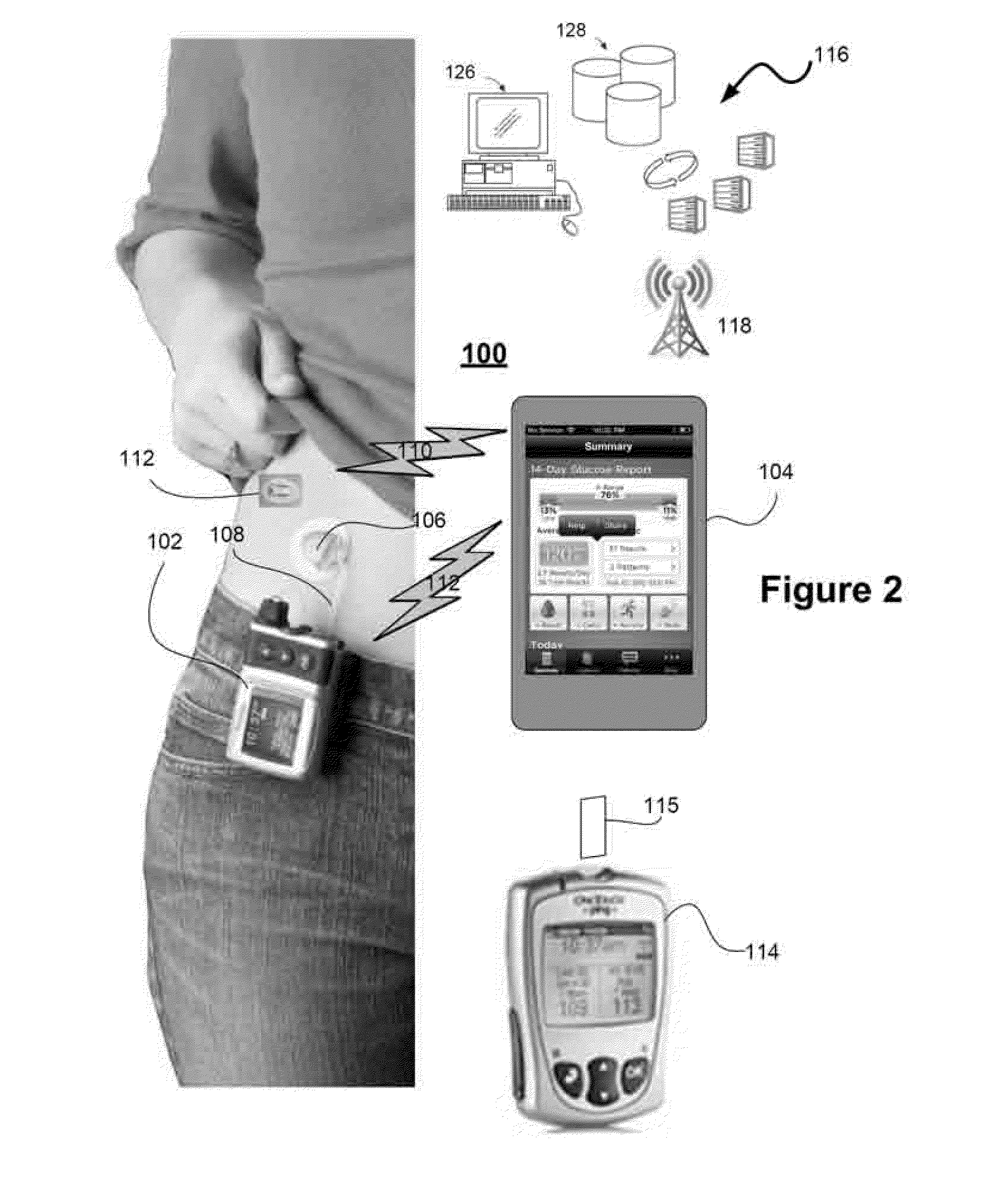
Health Monitoring System
InactiveUS20110313680A1Reduce noisePoint was missedTelemedicineNutrition controlNegative feedbackMissing data
A machine for processing continuous glucose monitoring data and issuing an alert if hypoglycemia is imminent has three modules: (a) a pre-processing module that receives and modulates continuous glucose monitoring data by reducing noise and adjusting for missed data points and shifts due to calibration; (b) a core algorithm module that receives data from the pre-processing module and calculates a rate of change to make a hypoglycemia prediction, and determine if hypoglycemia is imminent; and (c) an alarm mode module that receives data from the core algorithm and issues an audio or visual alert or warning message or a negative feedback signal to an insulin delivery device if hypoglycemia is imminent.
Owner:RGT UNIV OF CALIFORNIA
Amylin formulations
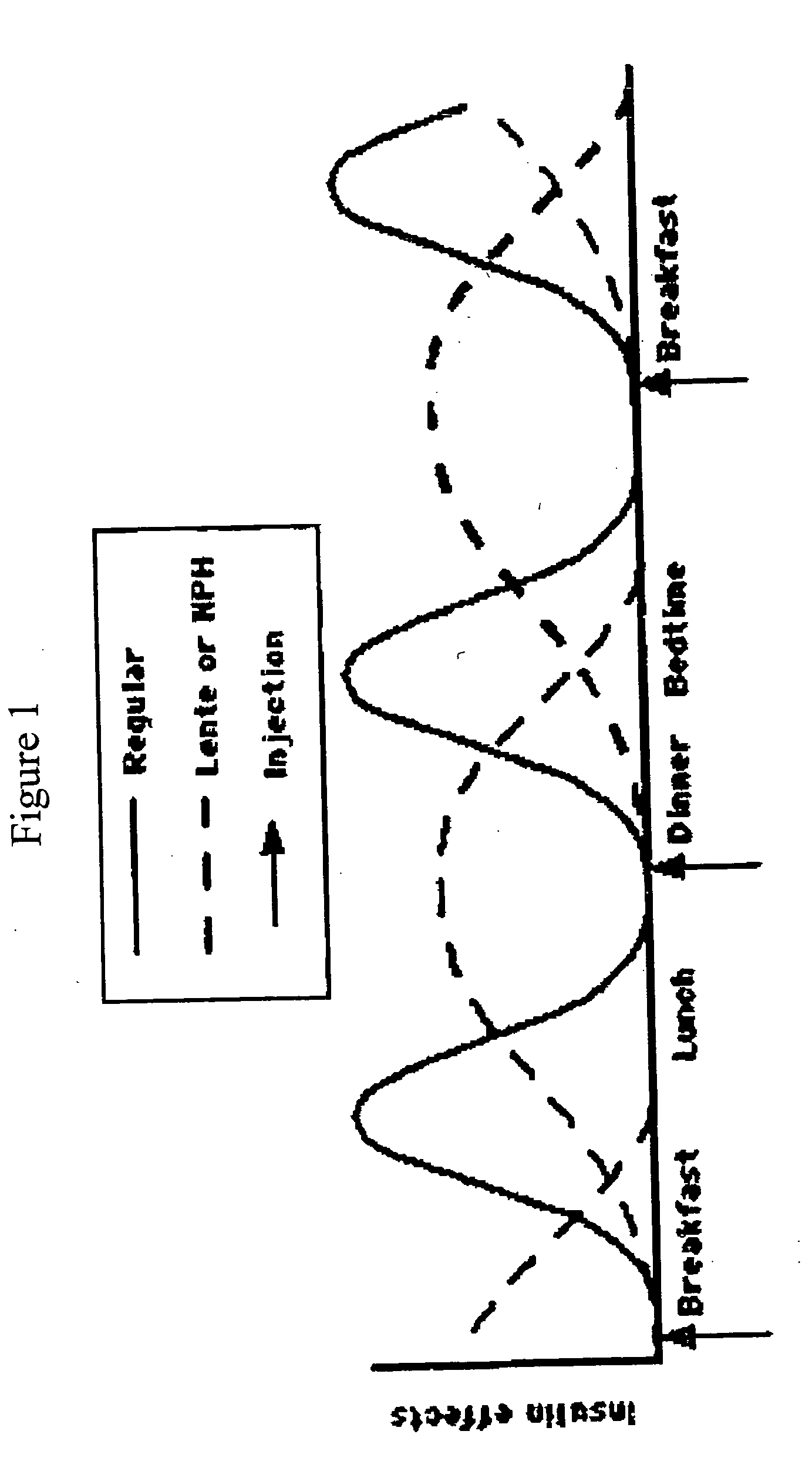
InactiveUS20080248999A1Reduce in quantityAct quicklyPeptide/protein ingredientsMetabolism disorderGLP-1 MimeticsBefore Breakfast
A combined insulin and amylin and / or GLP-1 mimetic formulation has been developed wherein the pH of the insulin is decreased so that the amylin and / or GLP-1 remains soluble when mixed together with the insulin. In the preferred embodiment, a bolus insulin is administered by injection before breakfast, providing adequate bolus insulin levels to cover the meal, without producing hypoglycemia after the meal. This can be combined with an adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of the insulin and amylin and / or GLP-1 mimetic combination. A GLP-1 mimetic may be combined with either rapid acting or basal insulin formulations. As a result, a patient using the combination formulation therapy may only need to inject half as many times per day.
Owner:BIODEL INC
CGM-Based Prevention of Hypoglycemia Via Hypoglycemia Risk Assessment and Smooth Reduction of Insulin Delivery
ActiveUS20120059353A1Reduced level of insulinAvoiding under-insulinizationDrug and medicationsMedical devicesMedicineClosed loop
An aspect of an embodiment or partial embodiment of the present invention (or combinations of various embodiments in whole or in part of the present invention) comprises, but not limited thereto, a method and system (and related computer program product) for continually assessing the risk of hypoglycemia for a patient and then determining what action to take based on that risk assessment. A further embodiment results in two outputs: (1) an attenuation factor to be applied to the insulin rate command sent to the pump (either via conventional therapy or via open or closed loop control) and / or (2) a red / yellow / green light hypoglycemia alarm providing to the patient an indication of the risk of hypoglycemia. The two outputs of the CPHS can be used in combination or individually.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
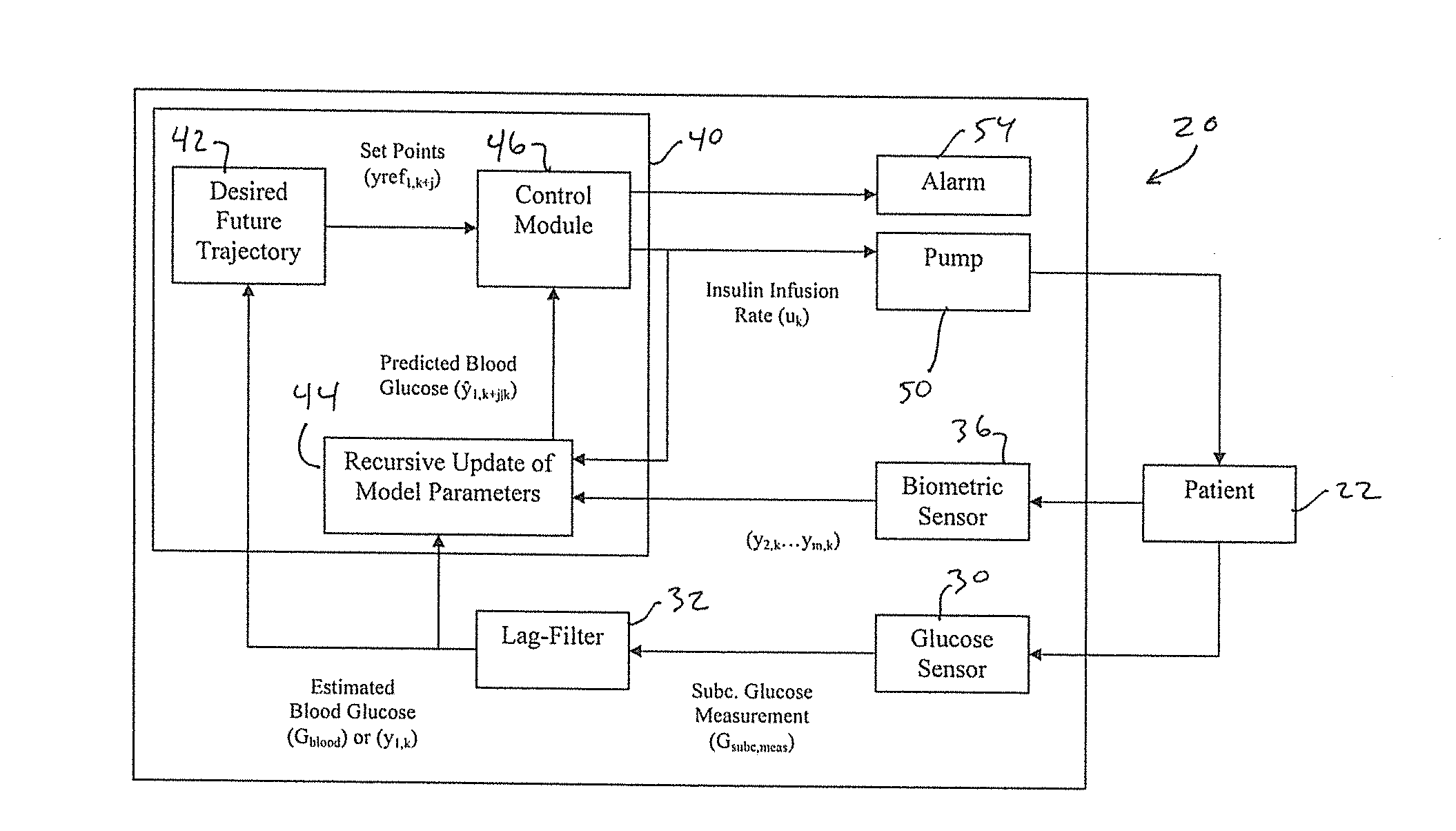
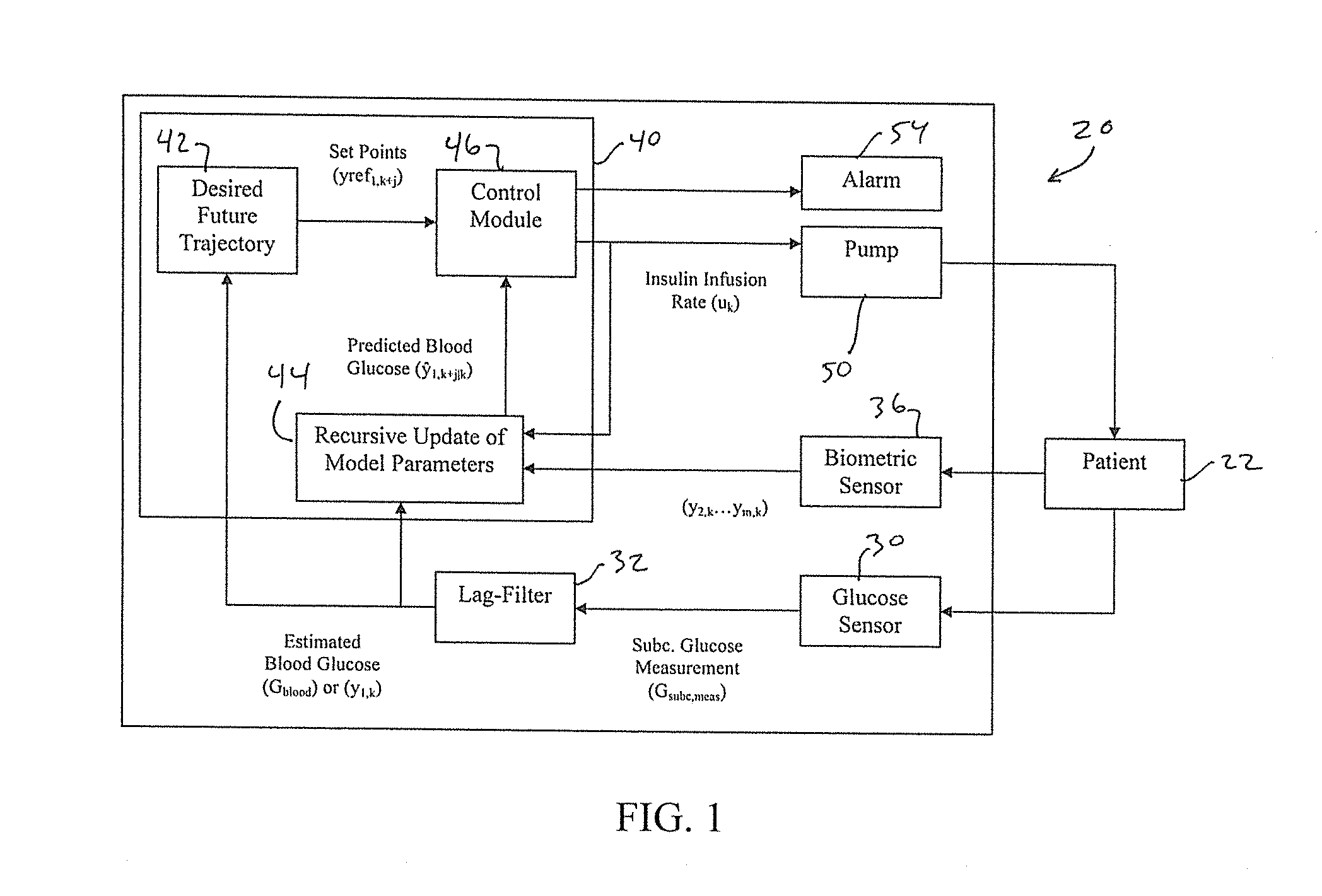
Multivariable artificial pancreas method and system
ActiveUS20160354543A1Easy to controlIncrease heart rateLocal control/monitoringMedical devicesAcute hyperglycaemiaHypoglycemia
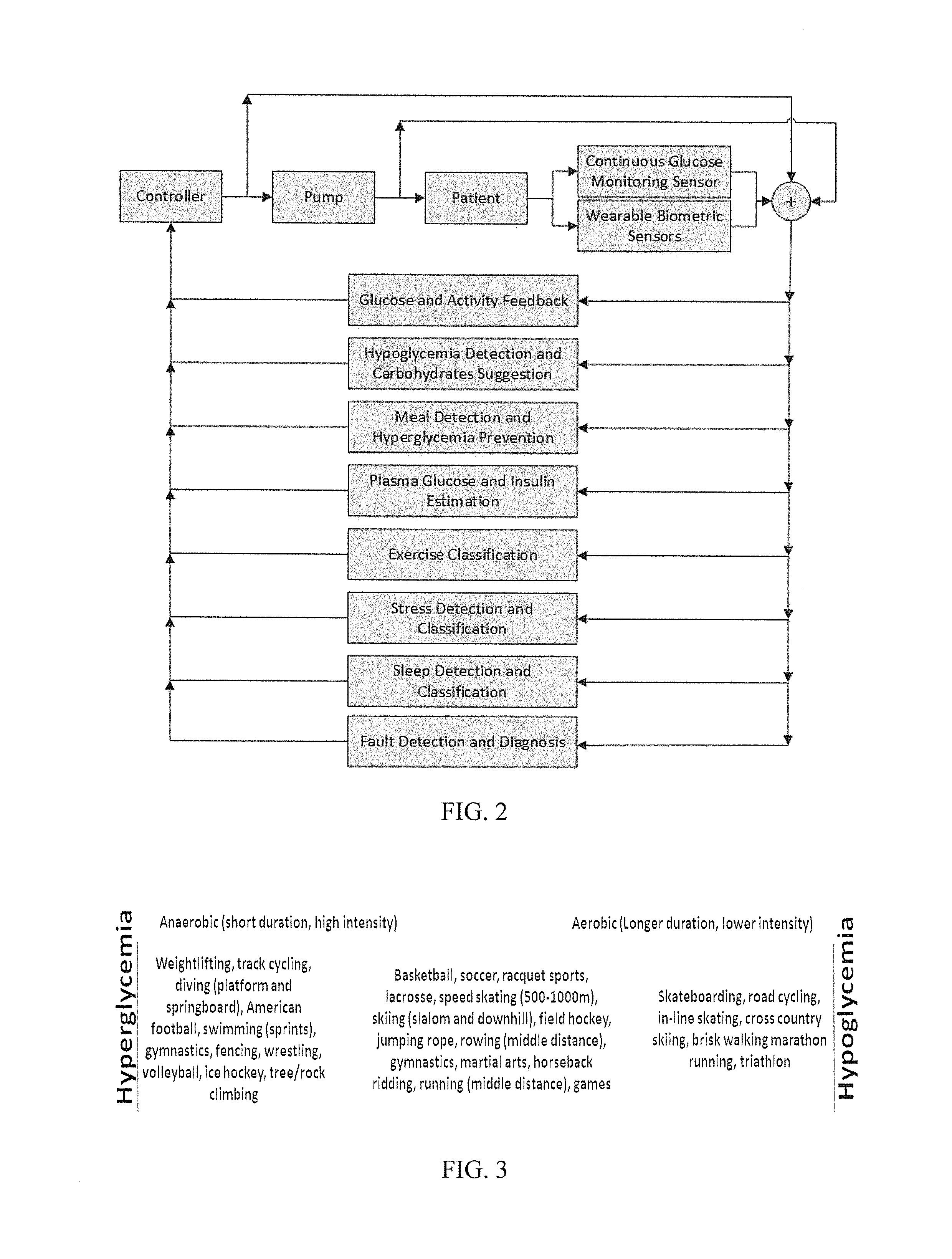
Methods and modules for using physiological (biometric) variables to advance the state of the artificial pancreas. The method and system includes one or more modules for recursive model identification, hypoglycemia early alert and alarm, adaptive control, hyperglycemia early alert and alarm, plasma insulin concentration estimation, assessment of physical activity (e.g., presence, type, duration, expected effects on insulin sensitivity and GC), detection of acute stress and assessment of its impact on insulin sensitivity, detection of sleep and its stages and assessment of sleep stages on GC, sensor fault detection and diagnosis, software and controller performance evaluation and adjustment and / or pump fault detection and diagnosis.
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
Method, System and Computer Readable Medium for Adaptive and Advisory Control of Diabetes
ActiveUS20150190098A1Reducing basal rateMedical simulationPhysical therapies and activitiesPhysiologyInsulin pump
An Adaptive Advisory Control (AA Control) interactive process involving algorithm-based assessment and communication of physiologic and behavioral parameters and patterns assists patients with diabetes with the optimization of their glycemic control. The method and system may uses all available sources of information about the patient; (i) EO Data (e.g. self-monitoring of blood glucose (SMBG) and CMG), (ii) Insulin Data (e.g. insulin pump log files or patient treatment records), and (iii) Patient Self Reporting Data (e.g. self treatment behaviors, meals, and exercise) to: retroactively assess the risk of hypoglycemia, retroactively assess risk-based reduction of insulin delivery, and then report to the patient how a risk-based insulin reduction system would have acted consistently to prevent hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
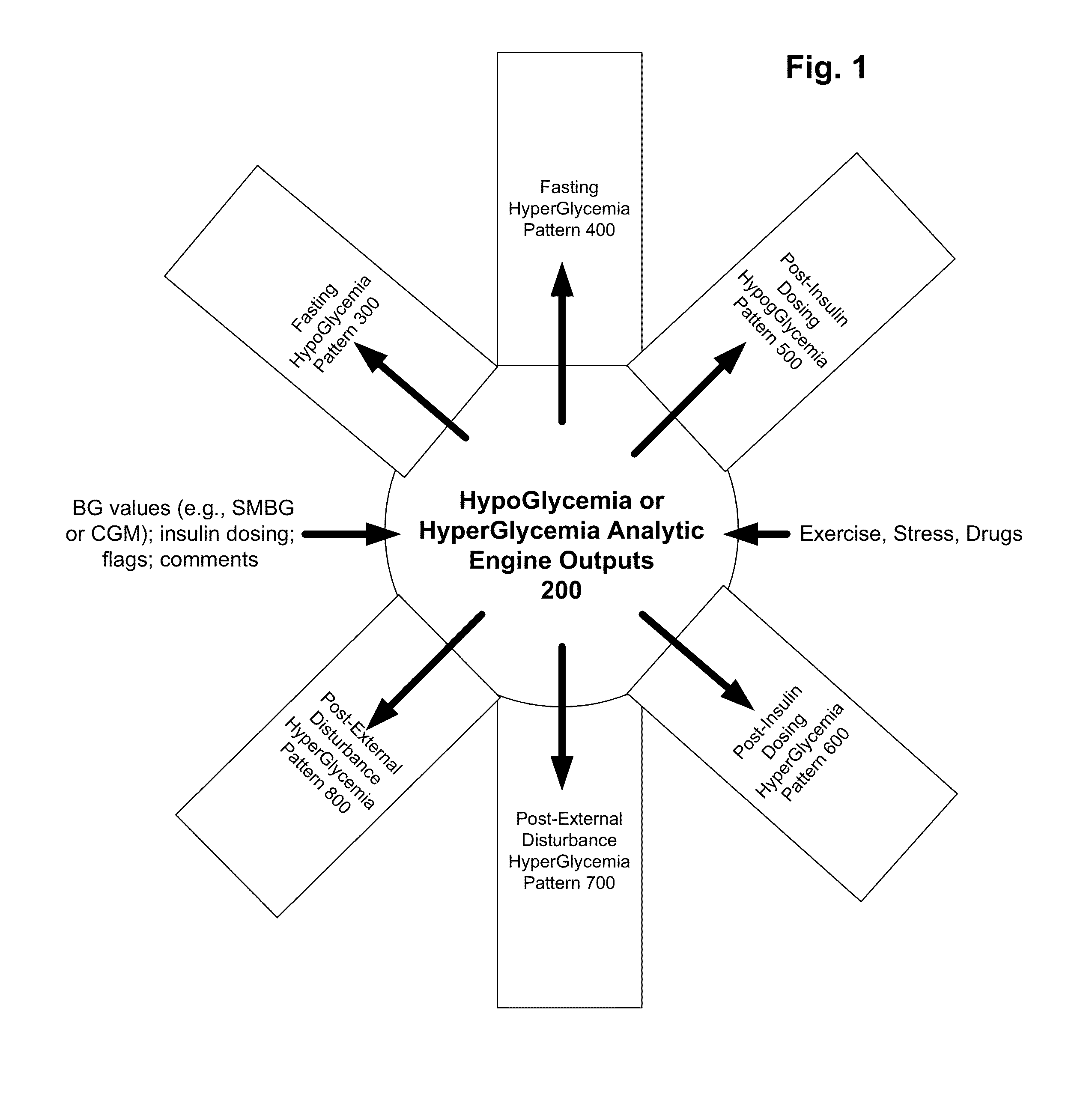
Method and system to indicate hyperglycemia or hypoglycemia for people with diabetes
Various methods and systems to manage diabetes of a subject using data relating to patterns to provide insight into how a patient's daily activities impact glycemic control of the subject. These patterns help to identify very specific areas of glycemic excursions, enable patients and HCPs to more easily identify patterns of hypoglycemia and hyperglycemia in order to take steps to improve glycemic control of the person with diabetes.
Owner:LIFESCAN INC
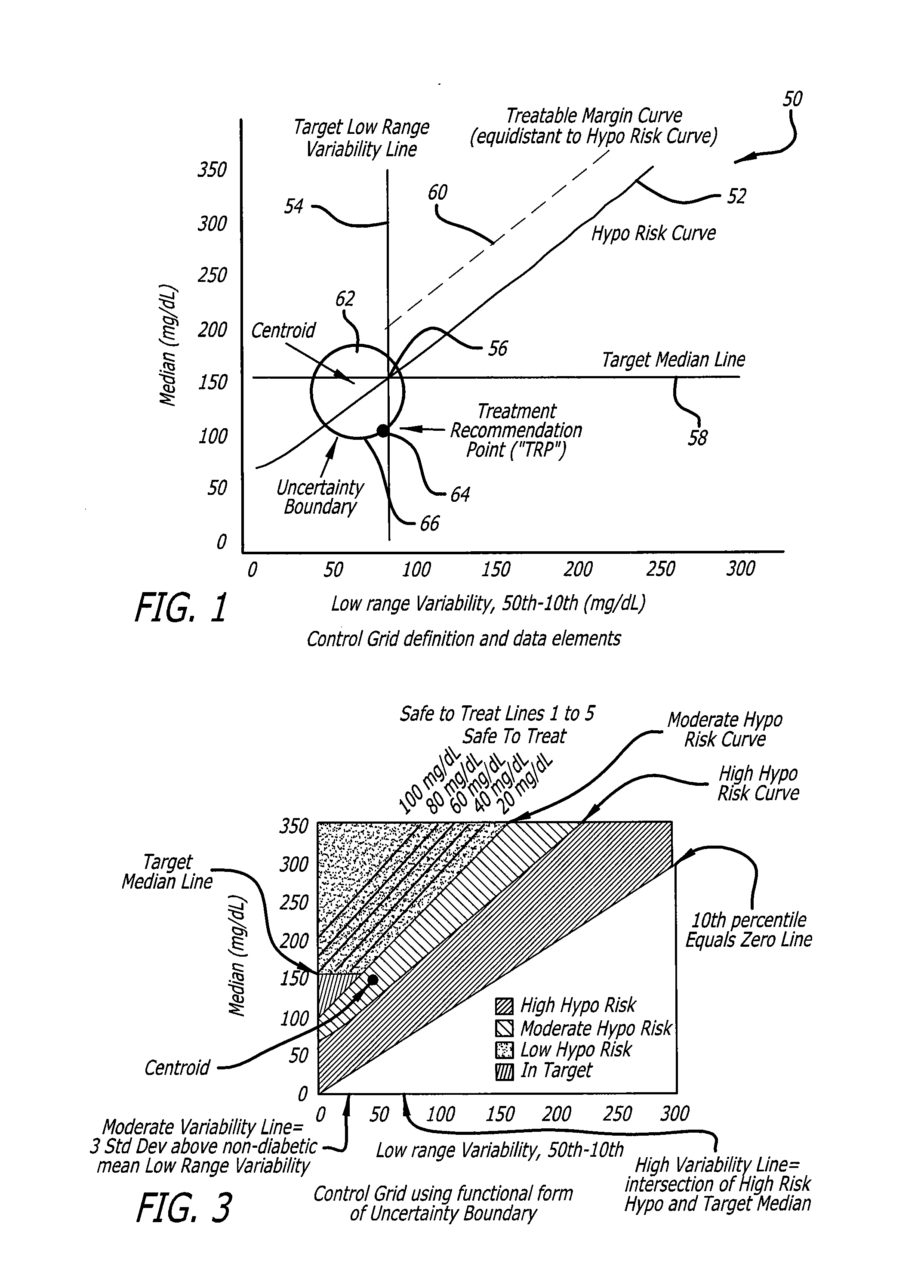
Analysis of glucose median, variability, and hypoglycemia risk for therapy guidance
ActiveUS20140188400A1Improve accuracyHealth-index calculationMedical automated diagnosisLow glucoseDiabetes Therapy
A system and method to provide guidance for diabetes therapy includes determining glycemic risks based on an analysis of glucose data. The analysis includes visualization of a glucose median, the variability of glucose in a patient, and the risk of hypoglycemia. An Advanced Daily Patterns report includes a visualization of an ambulatory glucose profile and a glucose control measure. The glucose control measure provides a highly visible and understandable display of the glucose condition of a patient visually expressed in the categories of low glucose, median glucose, and glucose variability.
Owner:ABBOTT DIABETES CARE INC
Chronic access system for extracorporeal treatment of blood including a continously wearable hemodialyzer
ActiveUS8109893B2More convenientEliminates the need for time-consuming, sedentary, hospital or clinic dialysisSemi-permeable membranesSolvent extractionIntravenous cannulaHaemodialysis machine
Owner:LANDE ARNOLD J
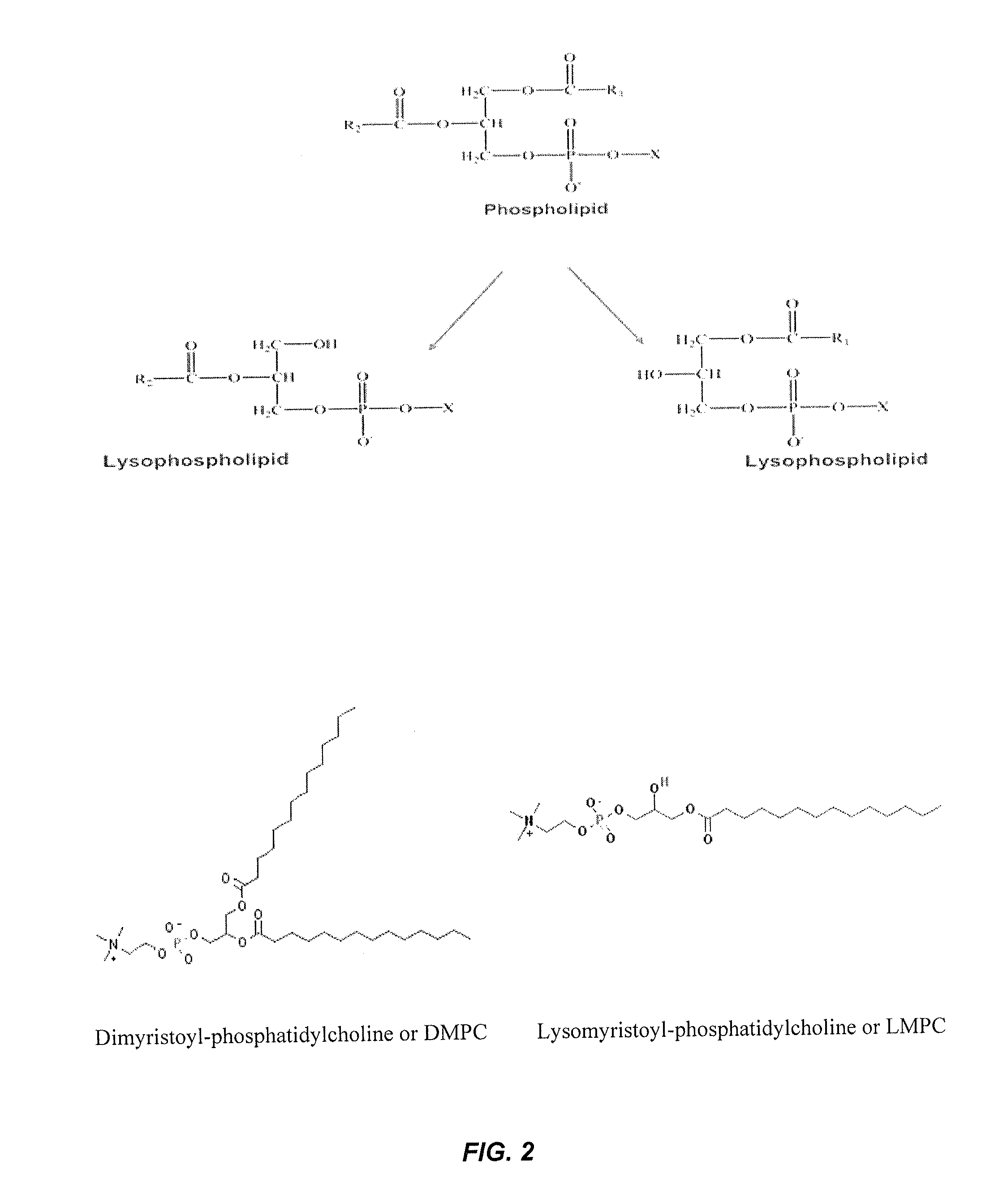
Stabilized glucagon nanoemulsions
InactiveUS20140378381A1Reduce wastePeptide/protein ingredientsMetabolism disorderMedicineHypoglycemia
The present invention provides an oil-in-water nanoemulsion containing glucagon, an oily phase, and an aqueous phase, wherein the glucagon is physically and chemically stable and the nanoemulsion is suitable for administration by manual injection or by a pump to treat hypoglycemia.
Owner:LATITUDE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com