Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
161 results about "Insulin delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
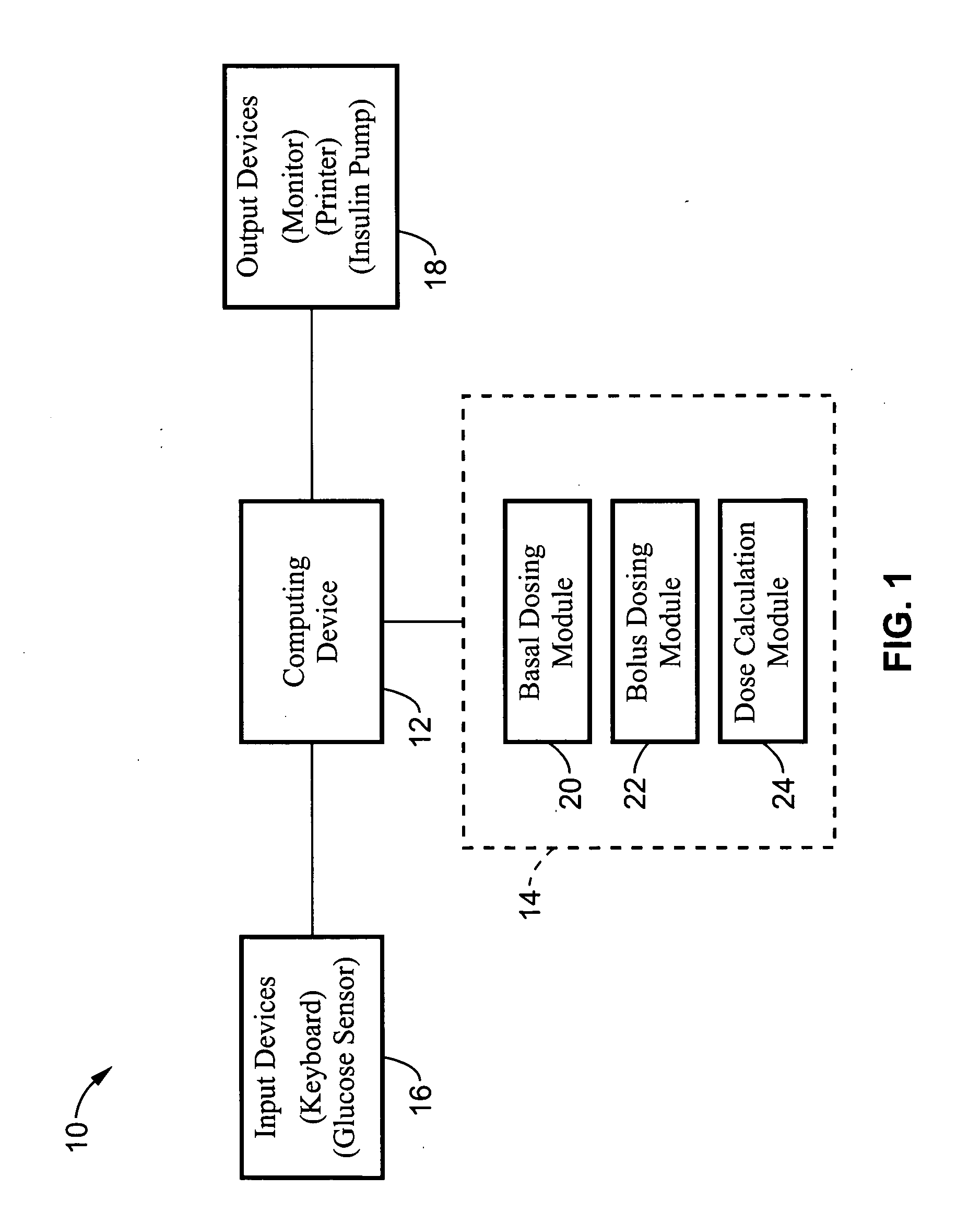
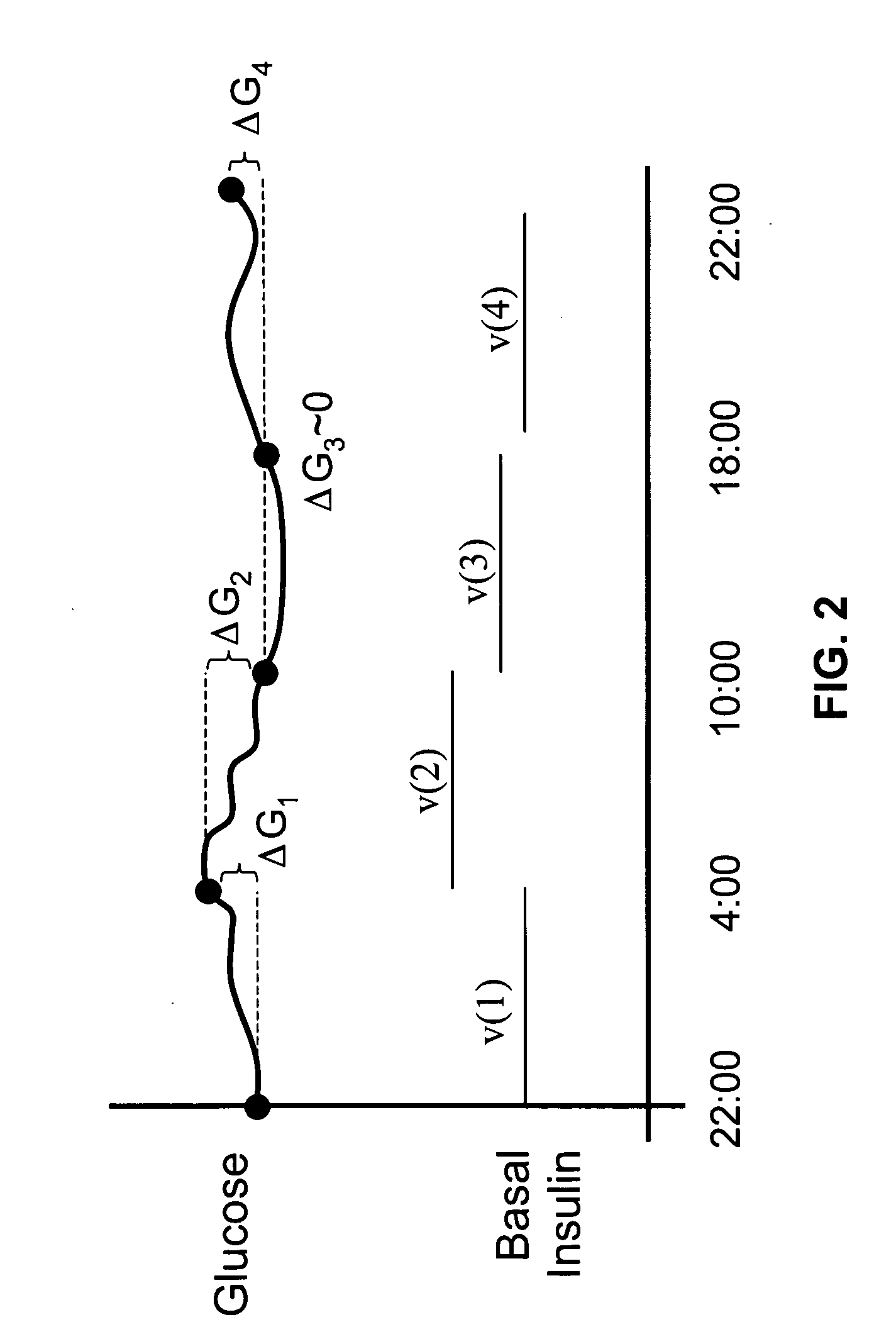
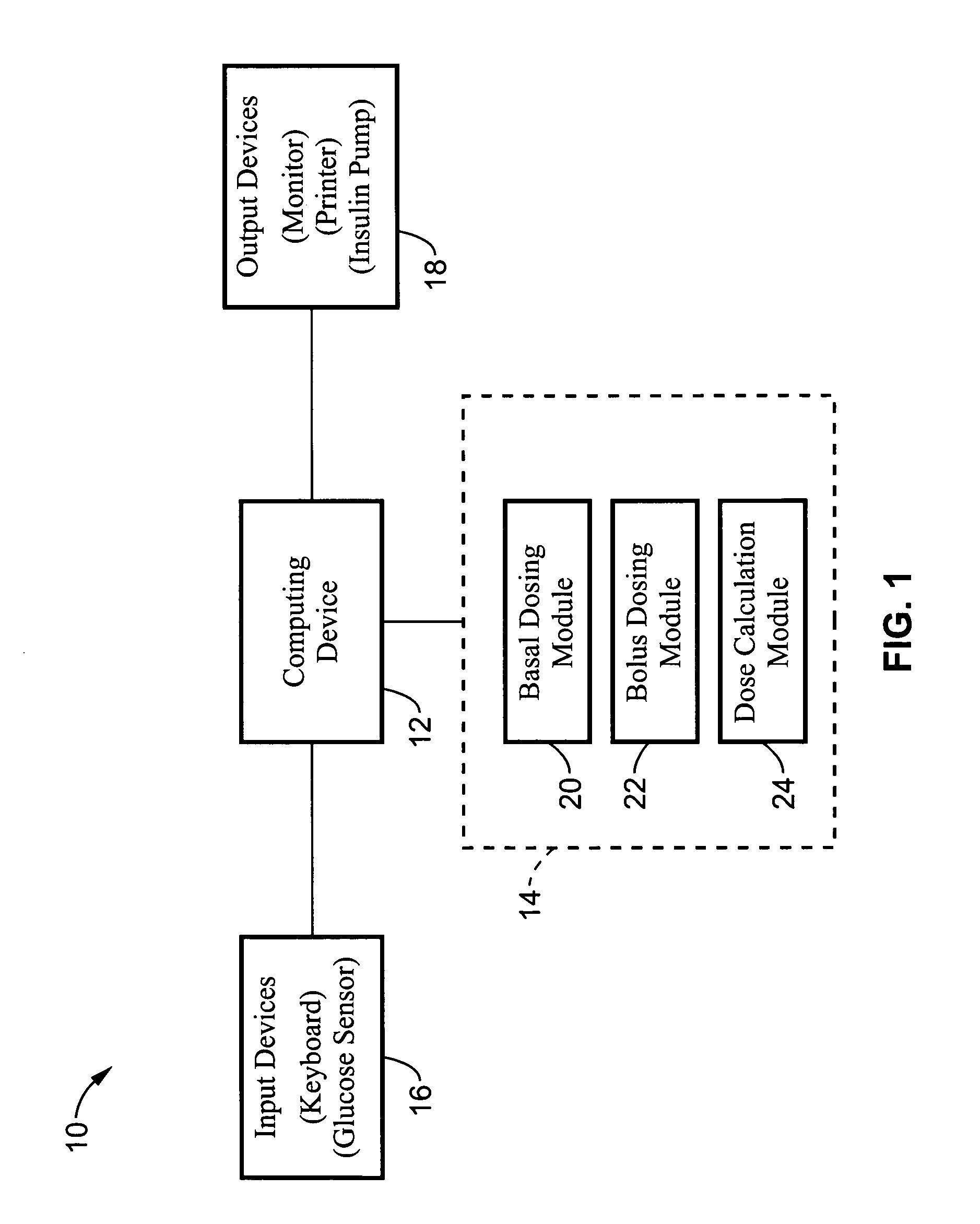
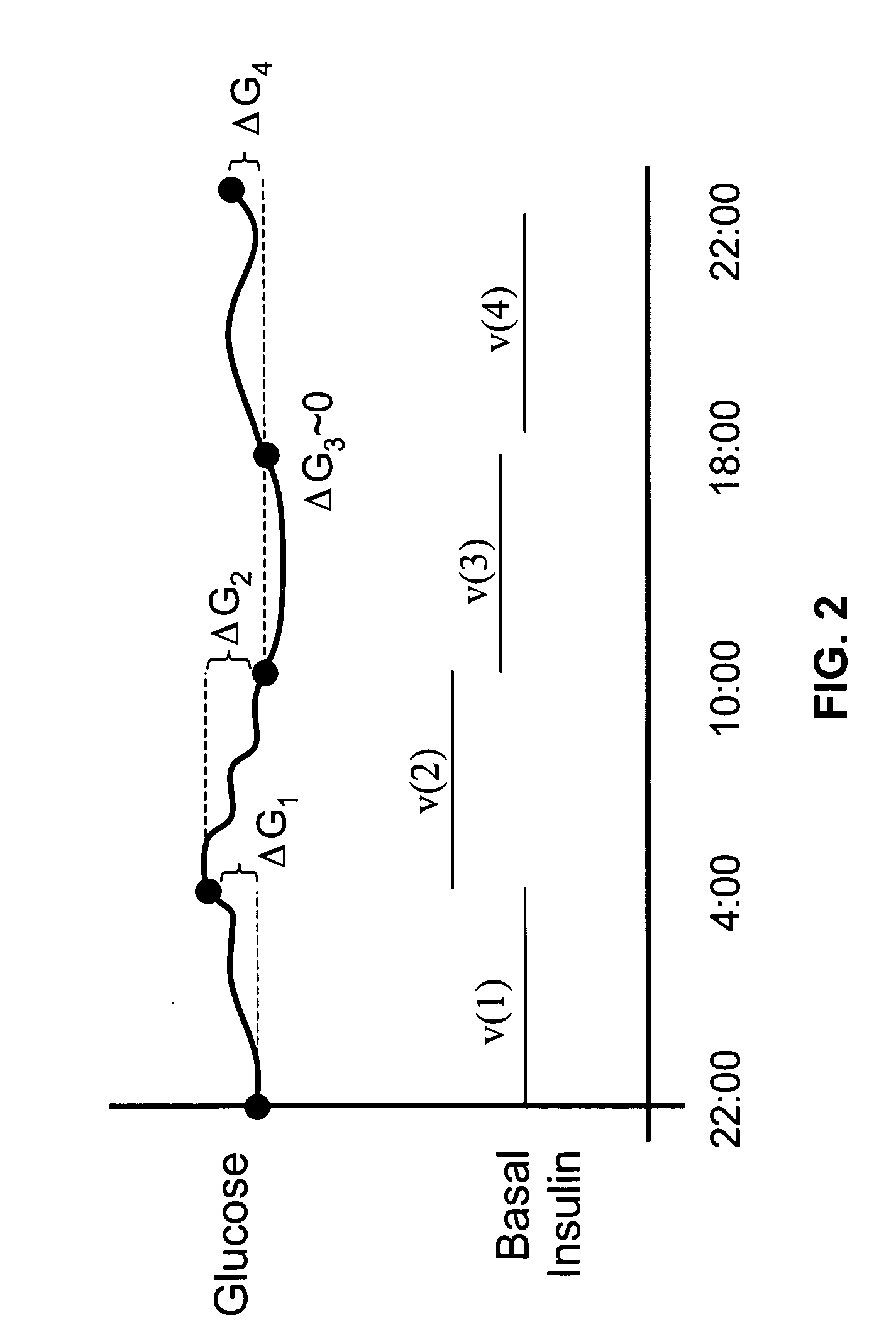
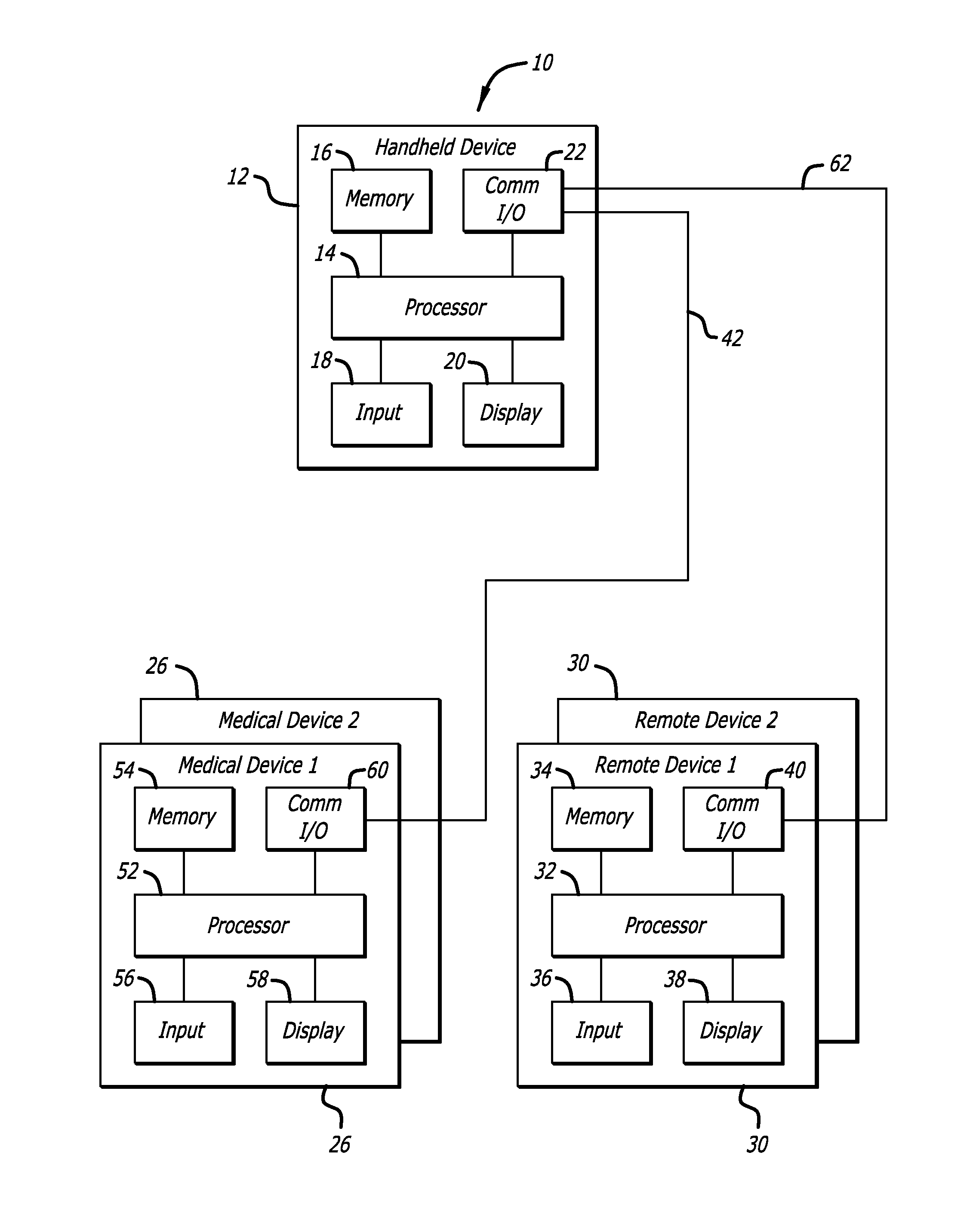
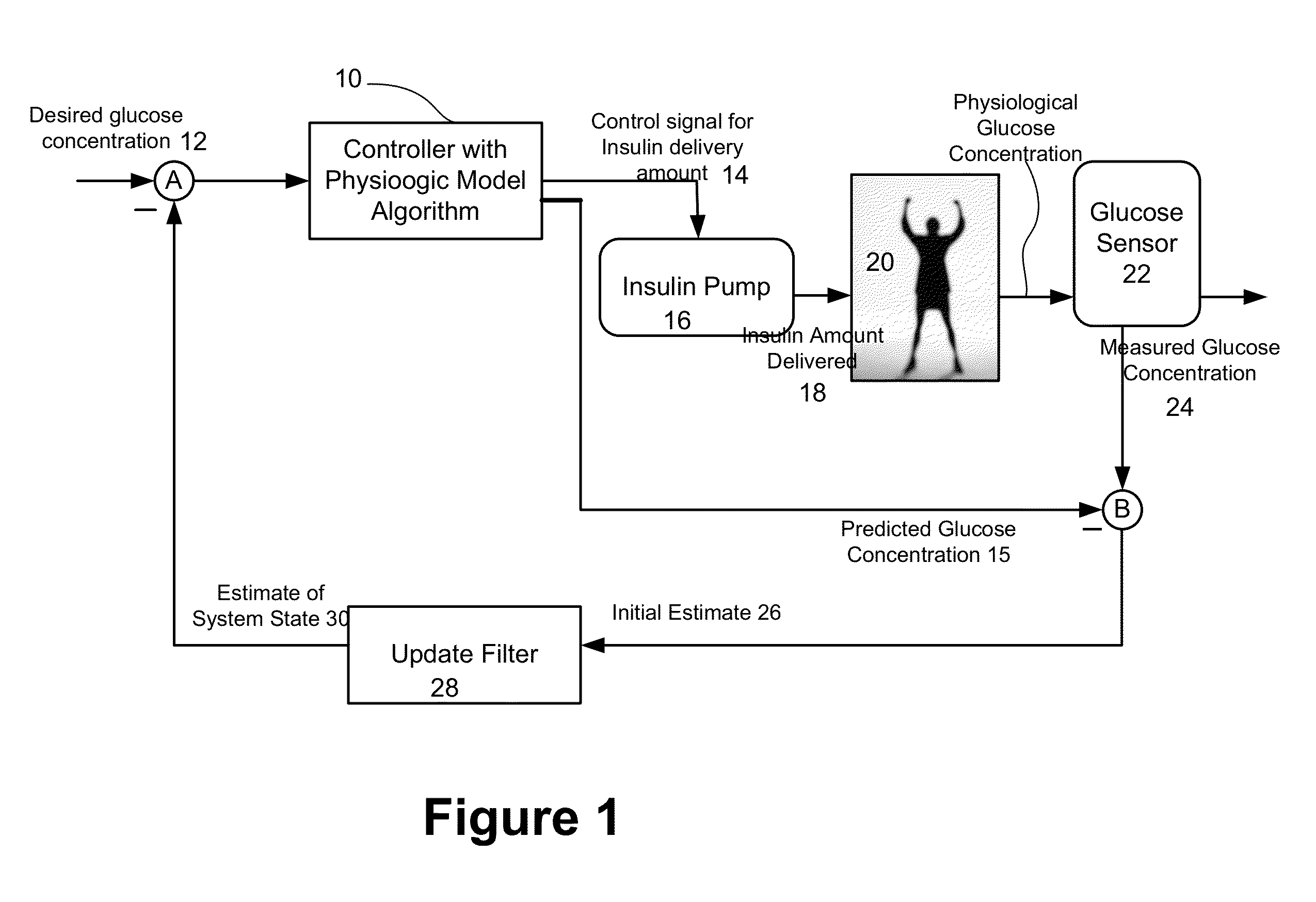
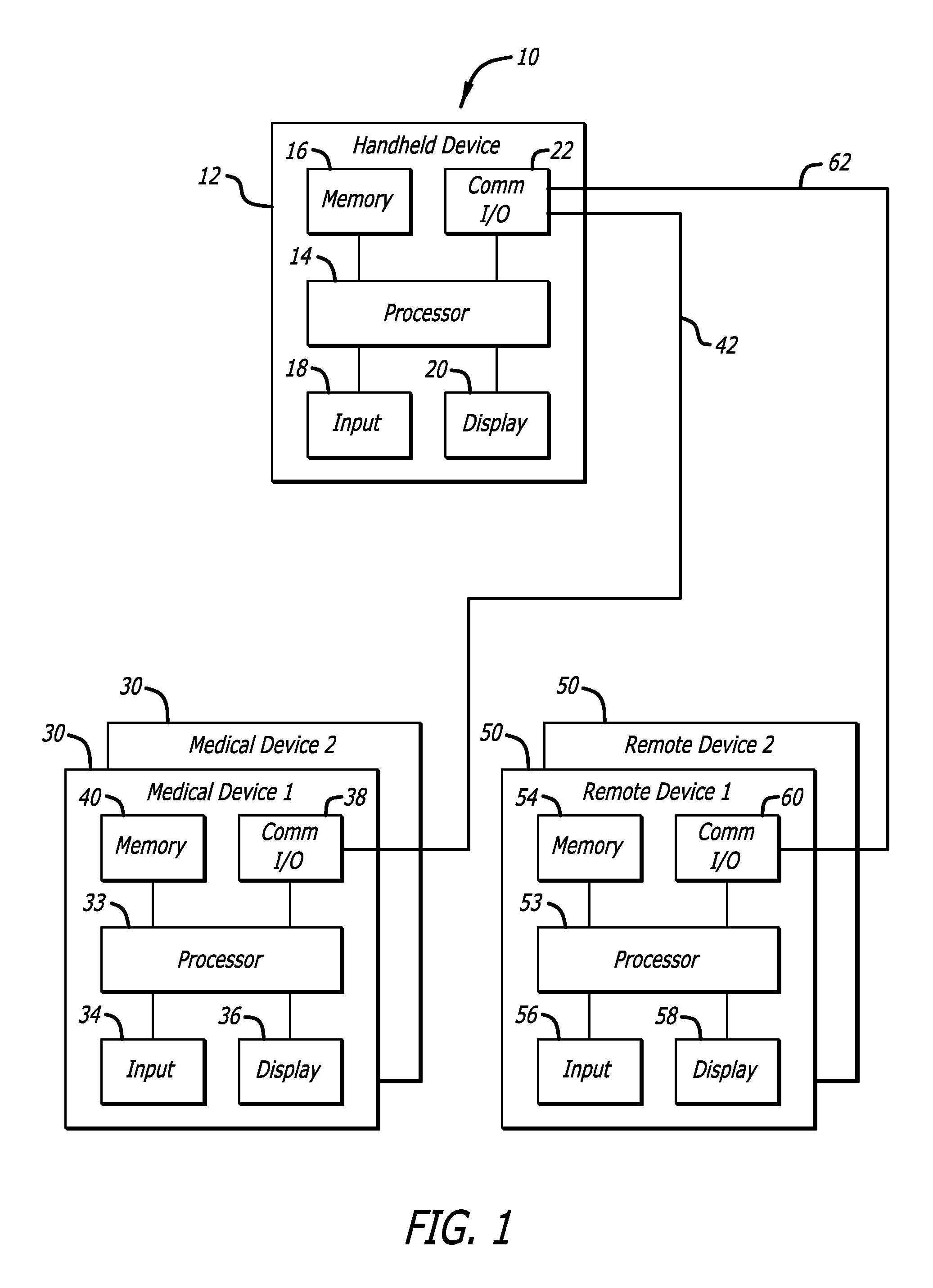
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS20050272640A1Ensure robustnessAccurately predicting insulin bolus dosagesPeptide/protein ingredientsDrug and medicationsPhysiologyMonitors blood glucose
A computer implemented method and associated apparatus for the combined control of insulin bolus dosing and basal delivery for the goal of achieving normal glycemic response to meals, exercise, stressors, and other perturbations to blood glucose levels. A run-to-run algorithm is used to monitor blood glucose levels and adjust insulin delivery as conditions are varied.
Owner:RGT UNIV OF CALIFORNIA
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS7651845B2Accurately predicting insulin bolus dosagesProcess controlPeptide/protein ingredientsDrug and medicationsMonitors blood glucoseGlycemic
Owner:RGT UNIV OF CALIFORNIA
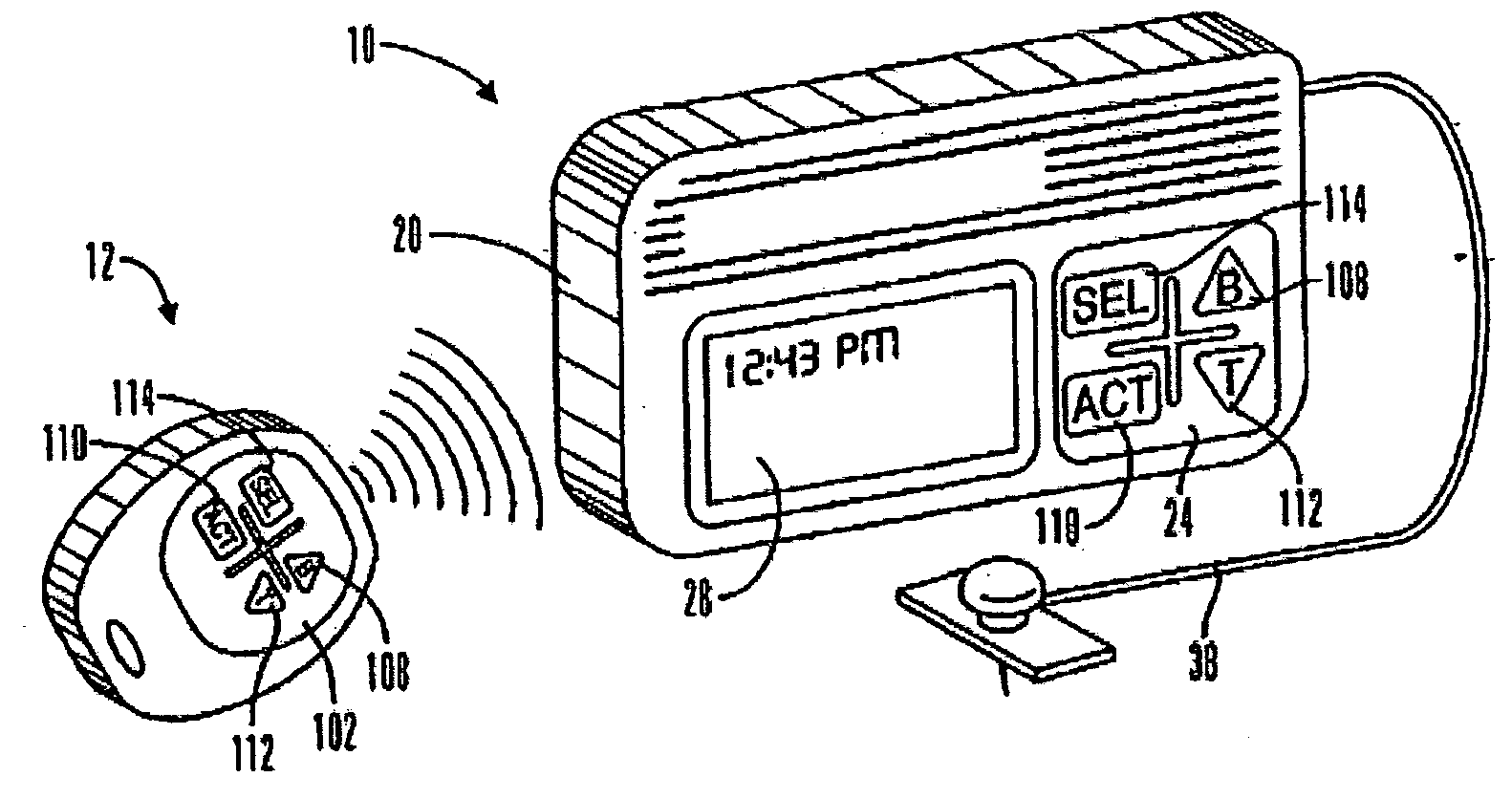
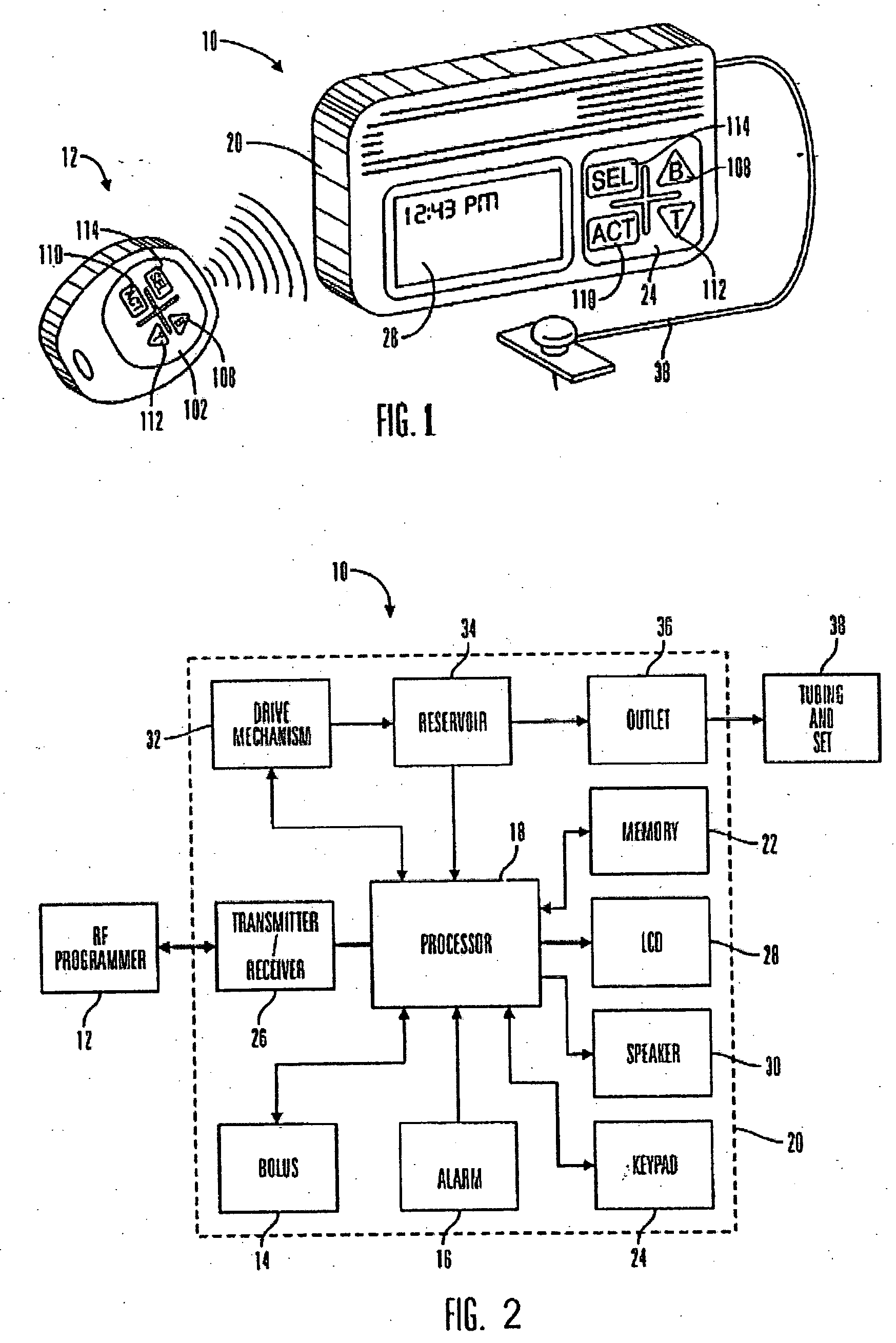
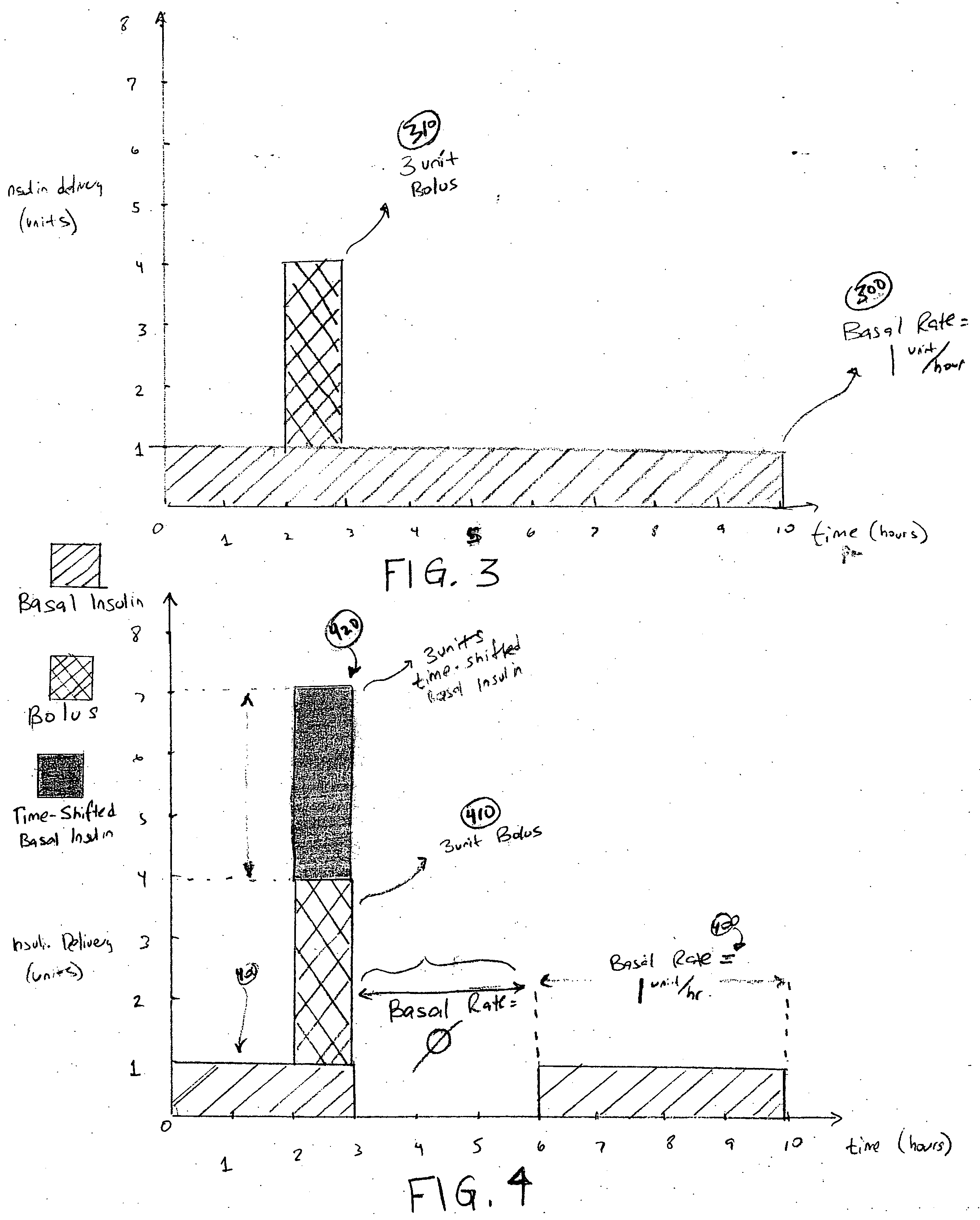
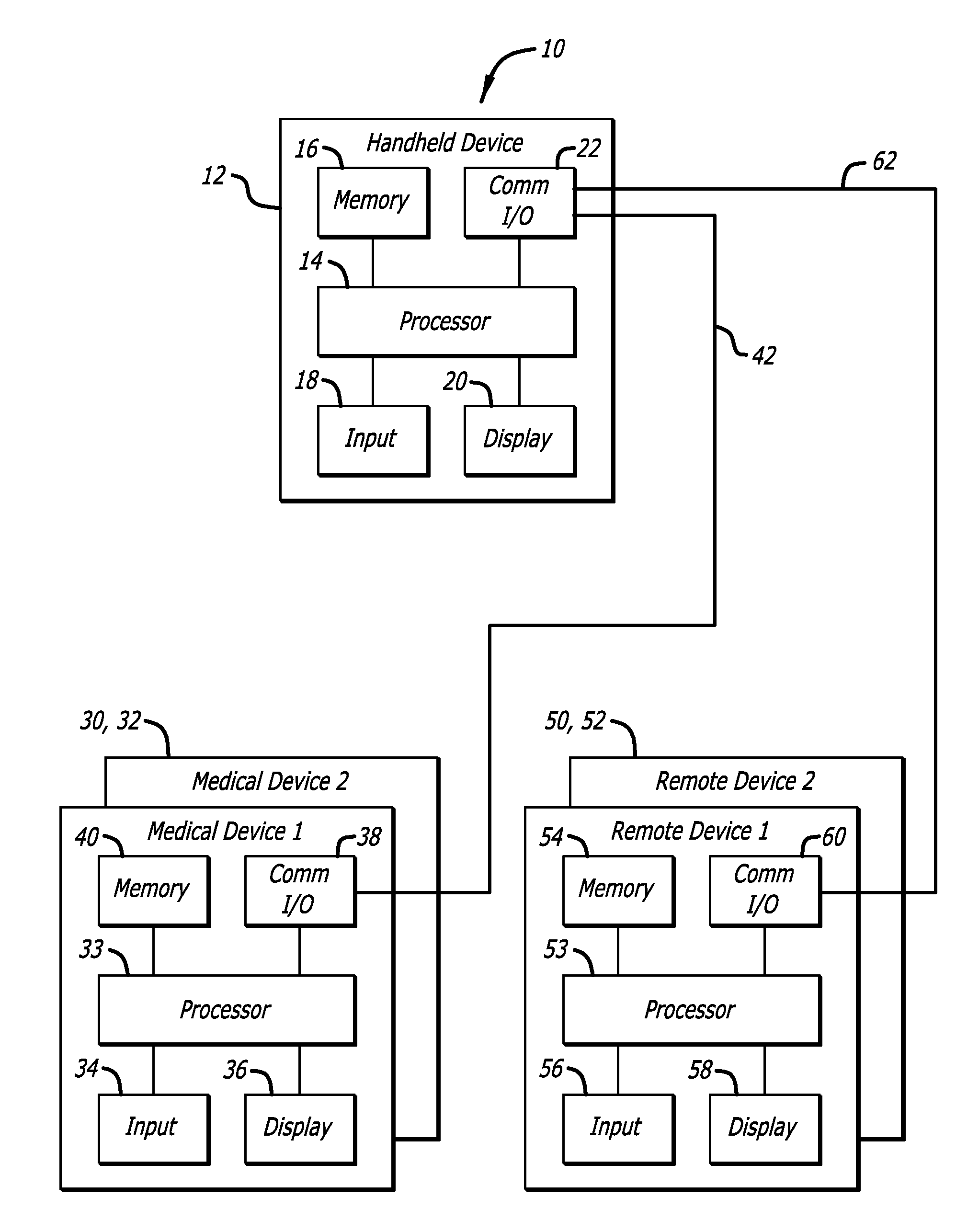
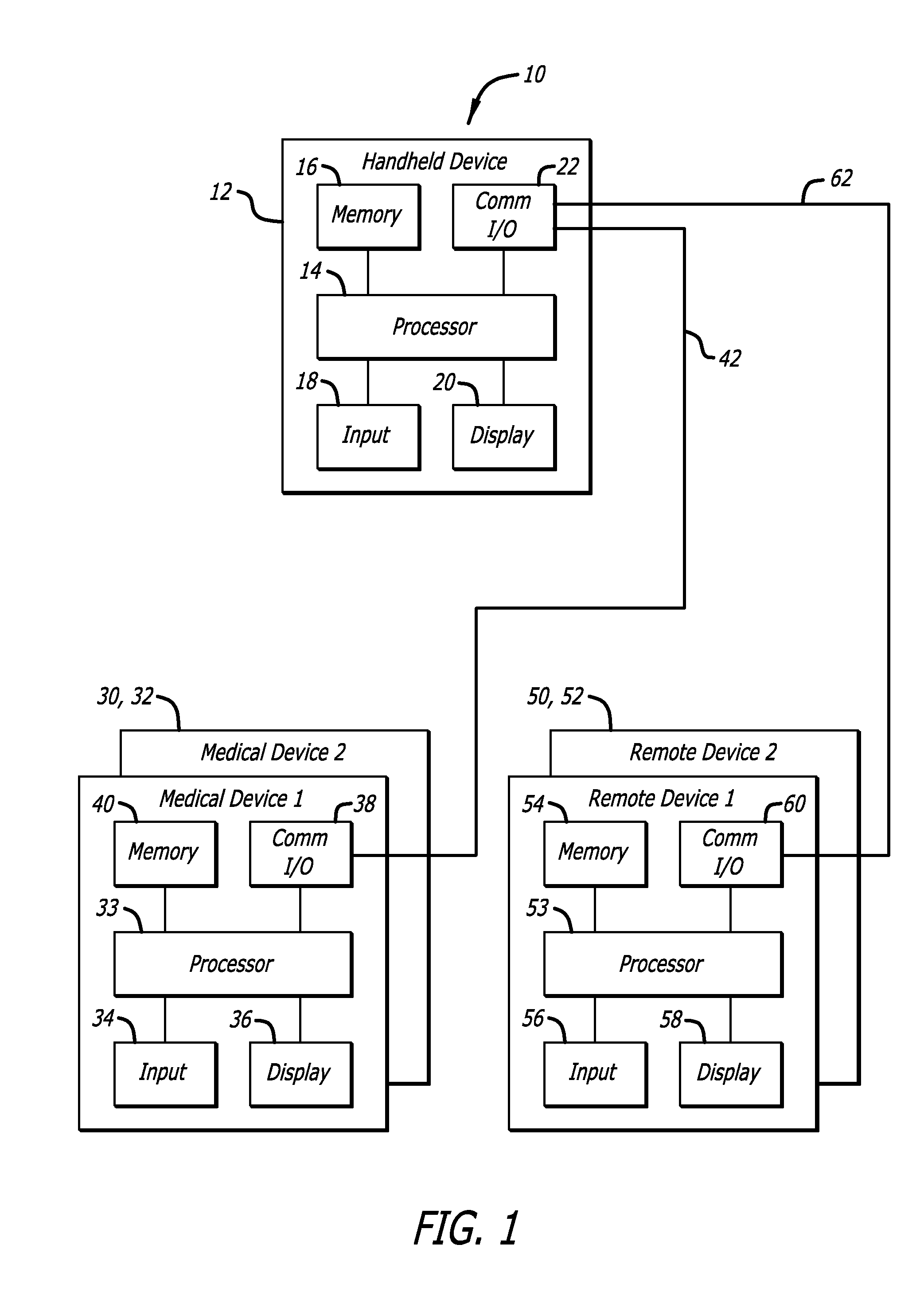
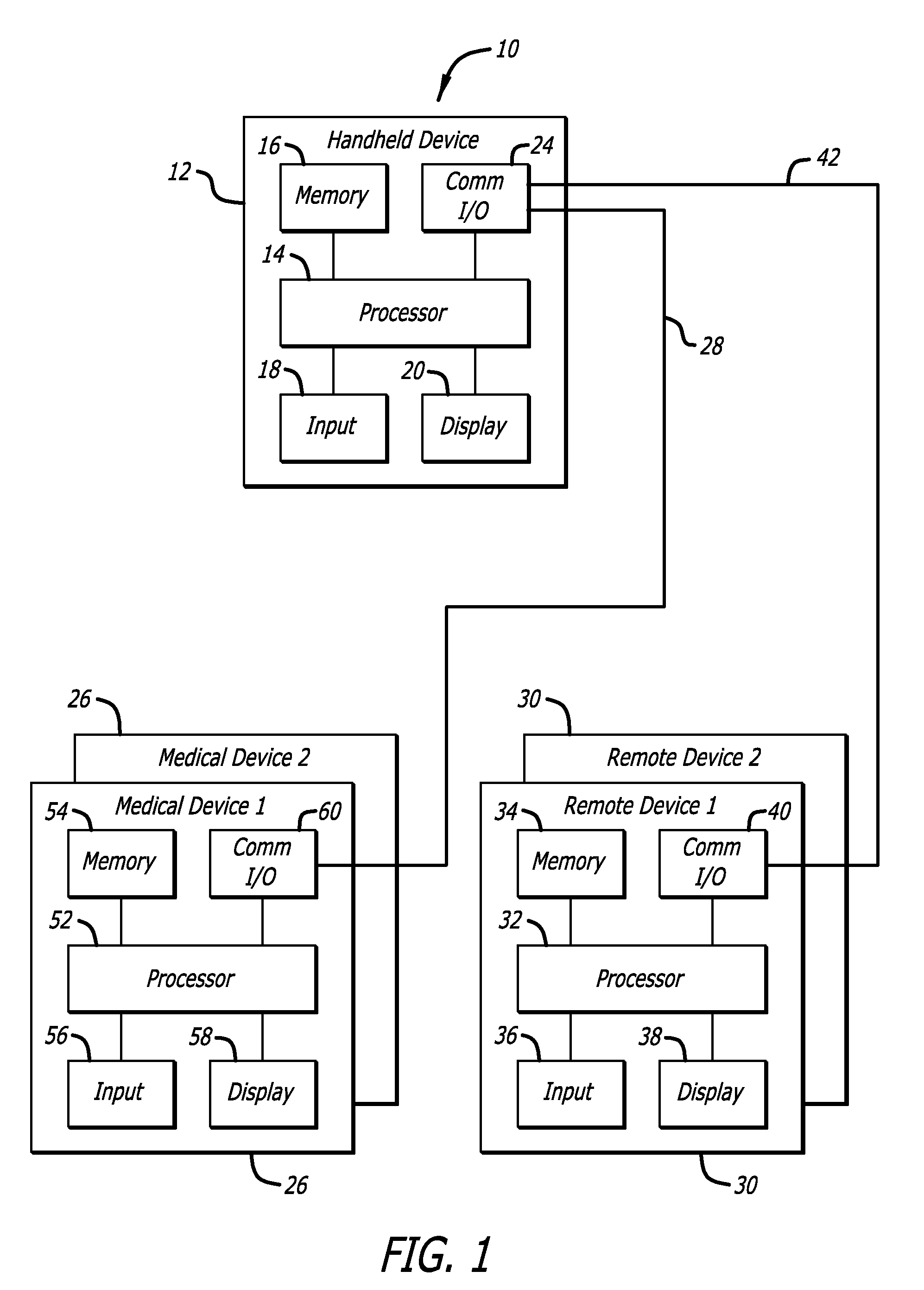
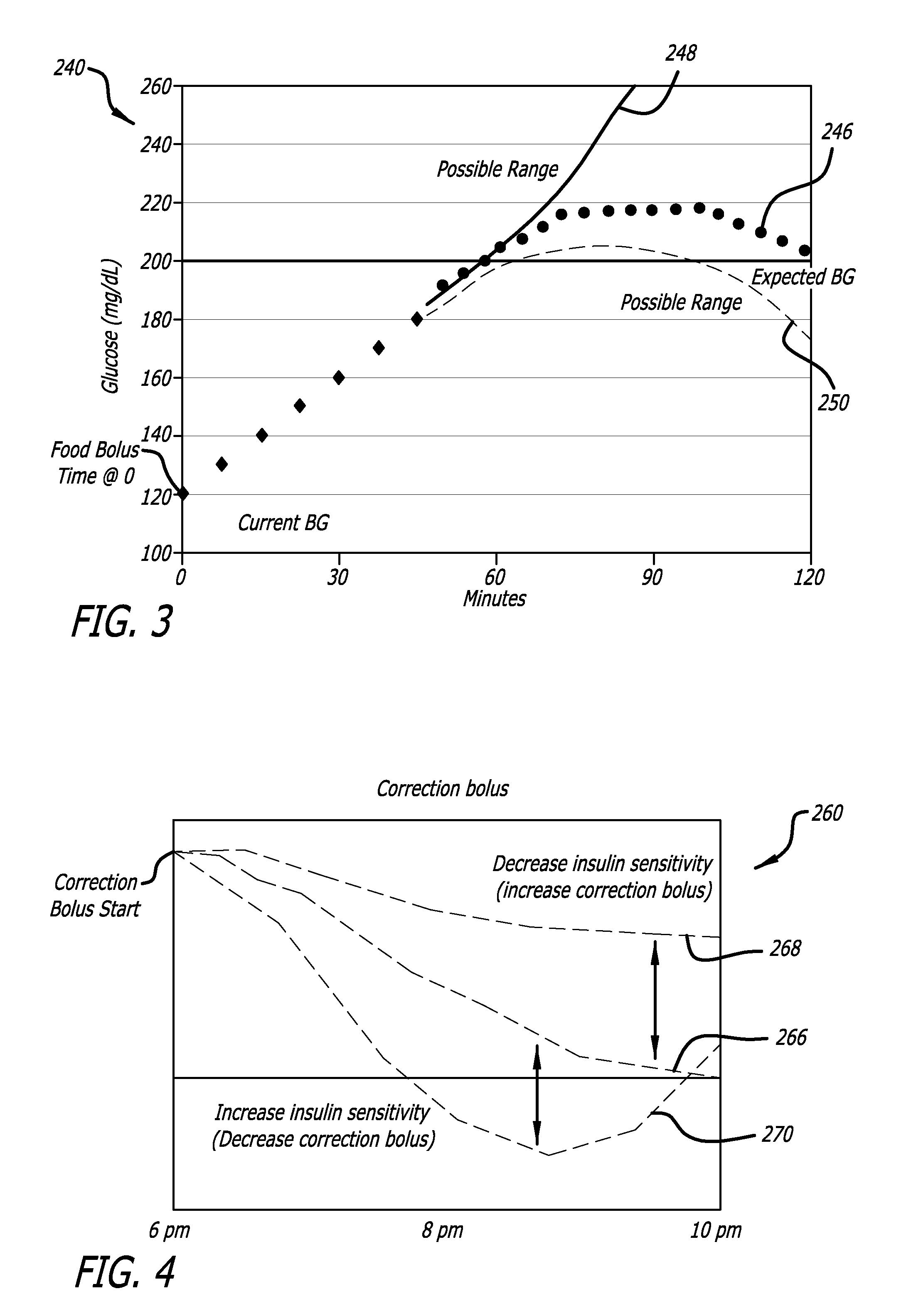
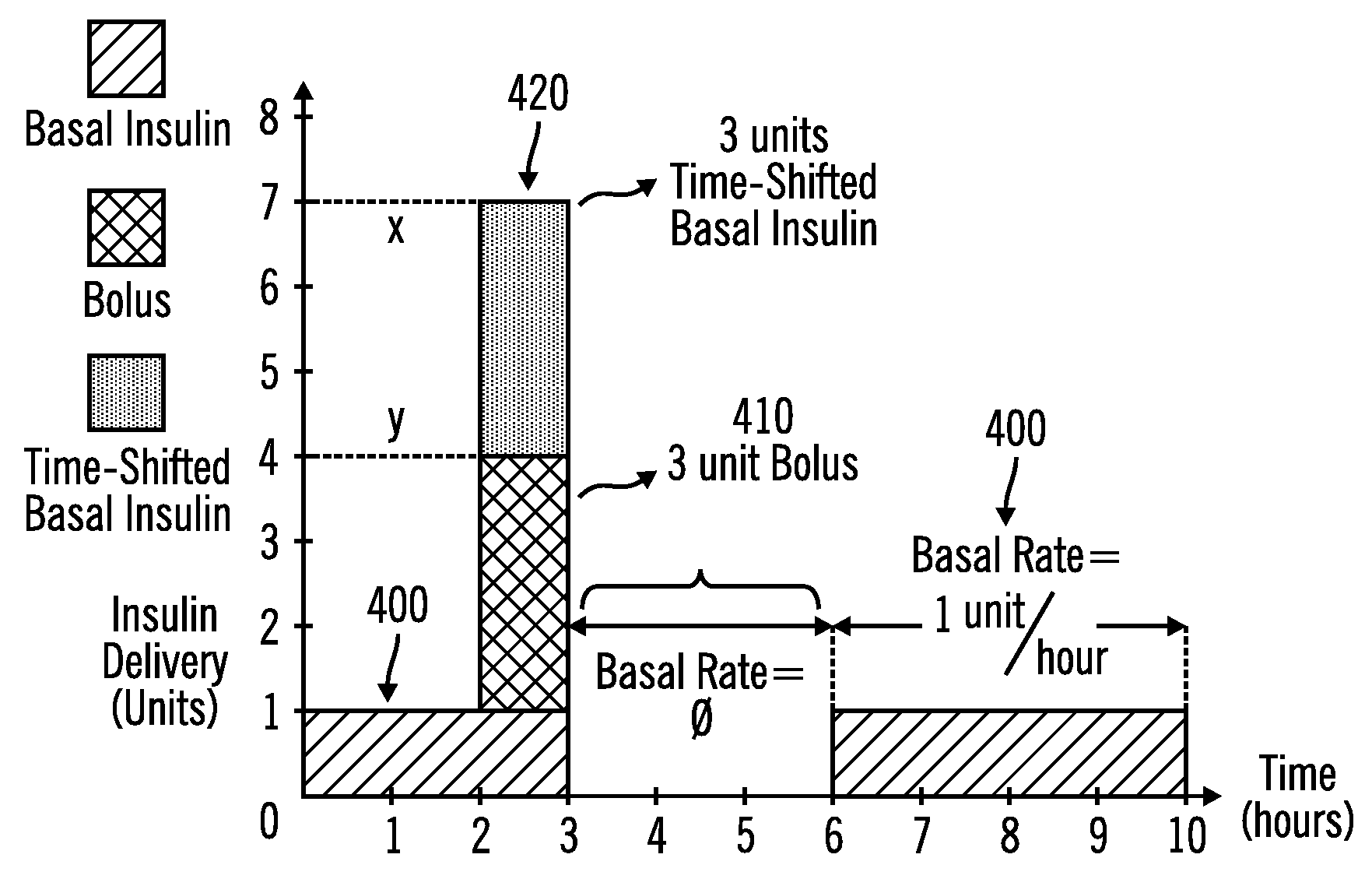
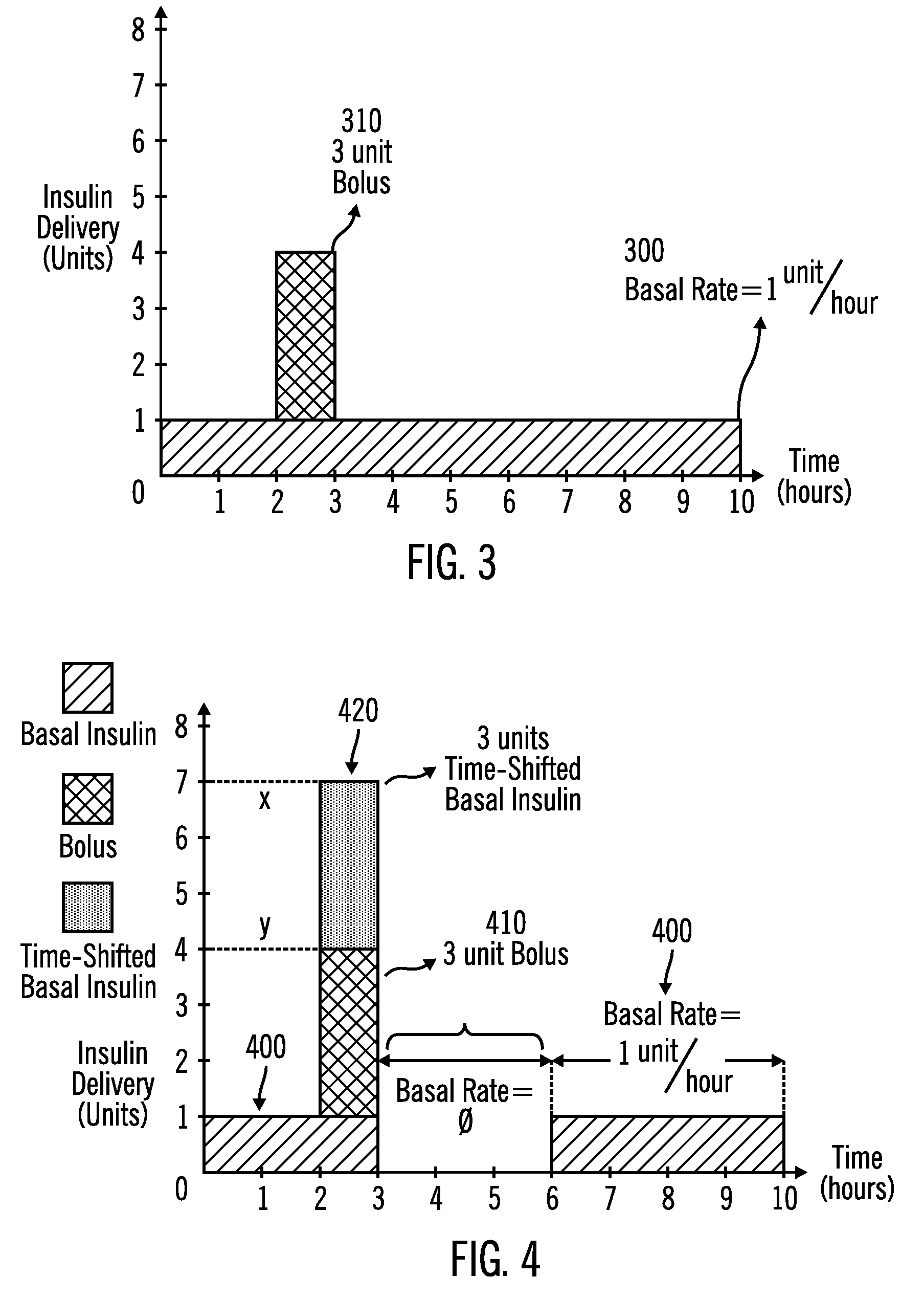
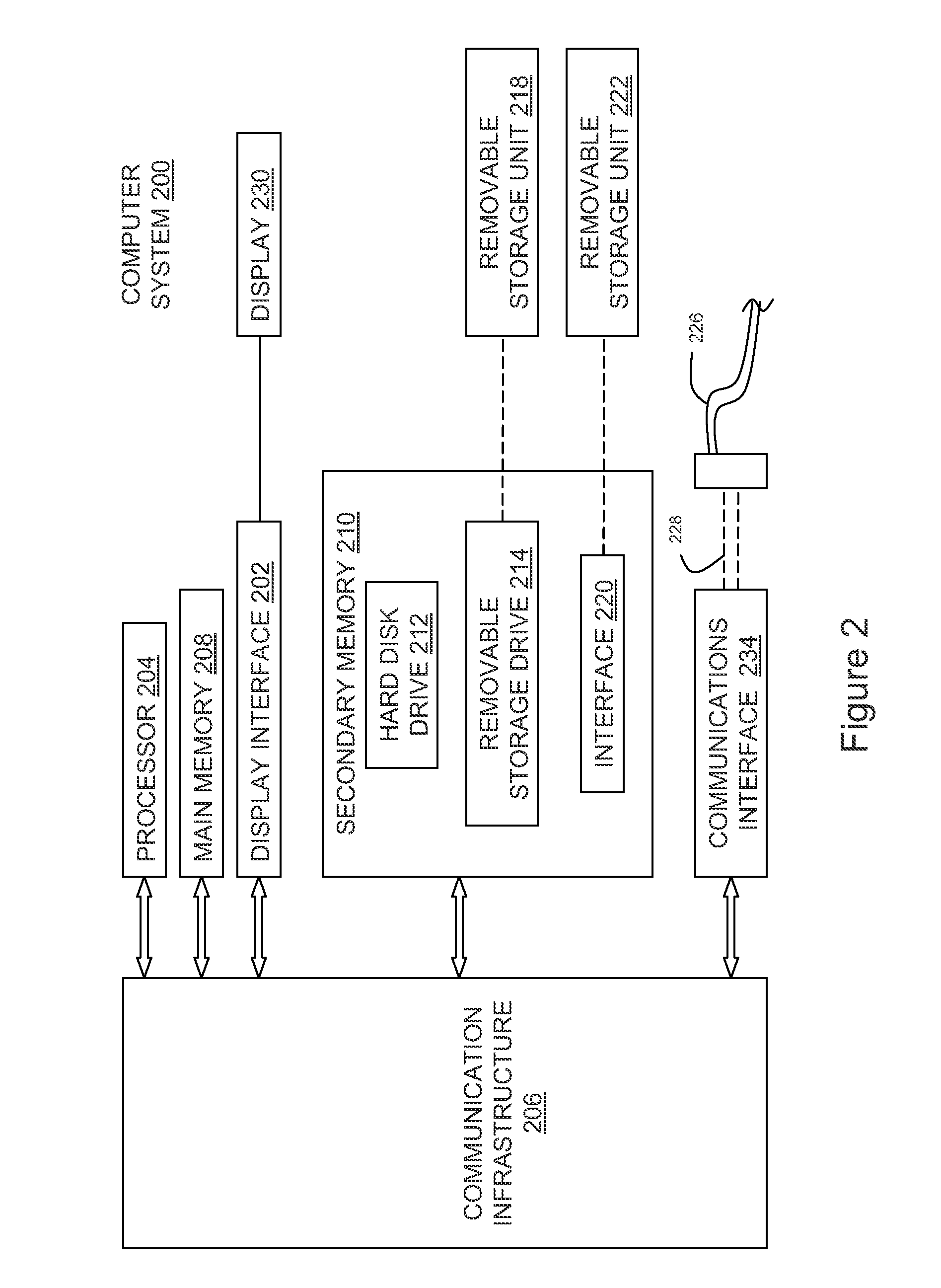
External infusion device with programmable capabilities to time-shift basal insulin and method of using the same
An external infusion device for delivering insulin from a reservoir into a body of a user includes the capability to deliver time-shifted basal insulin. The external infusion device includes at least one drive mechanism operatively couplable to the reservoir to deliver insulin into the body of the user, at least one processor to control the external infusion device, at least one power supply, at least one display device operatively coupled to the processor to provide visual information to the user, at least one input device operatively coupled to the processor to allow the user to command the processor, and a housing. Time-shifting of basal insulin occurs when a portion of basal insulin is added to a bolus, to a current basal rate, or to a bolus and a current basal rate.
Owner:MEDTRONIC MIMIMED INC
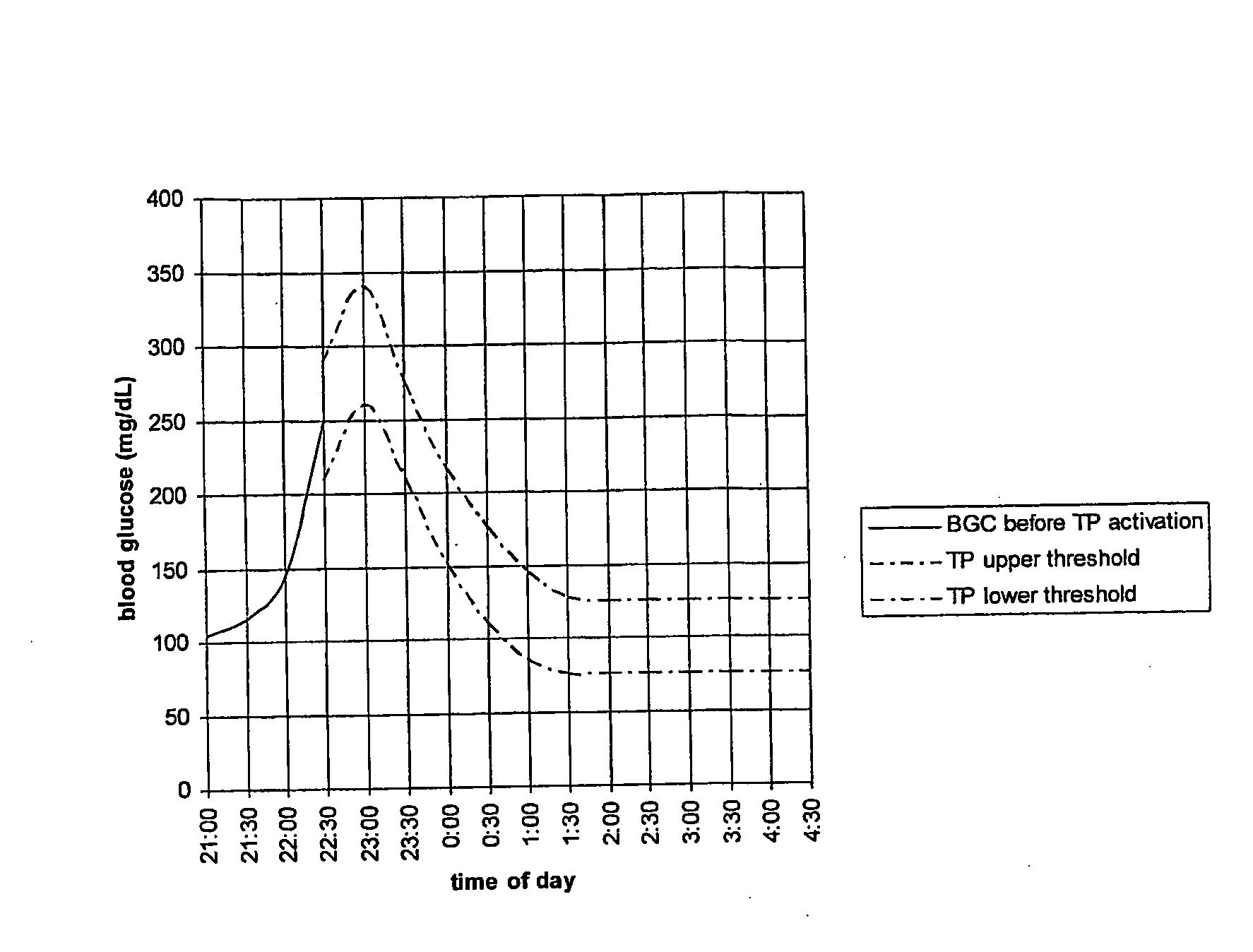
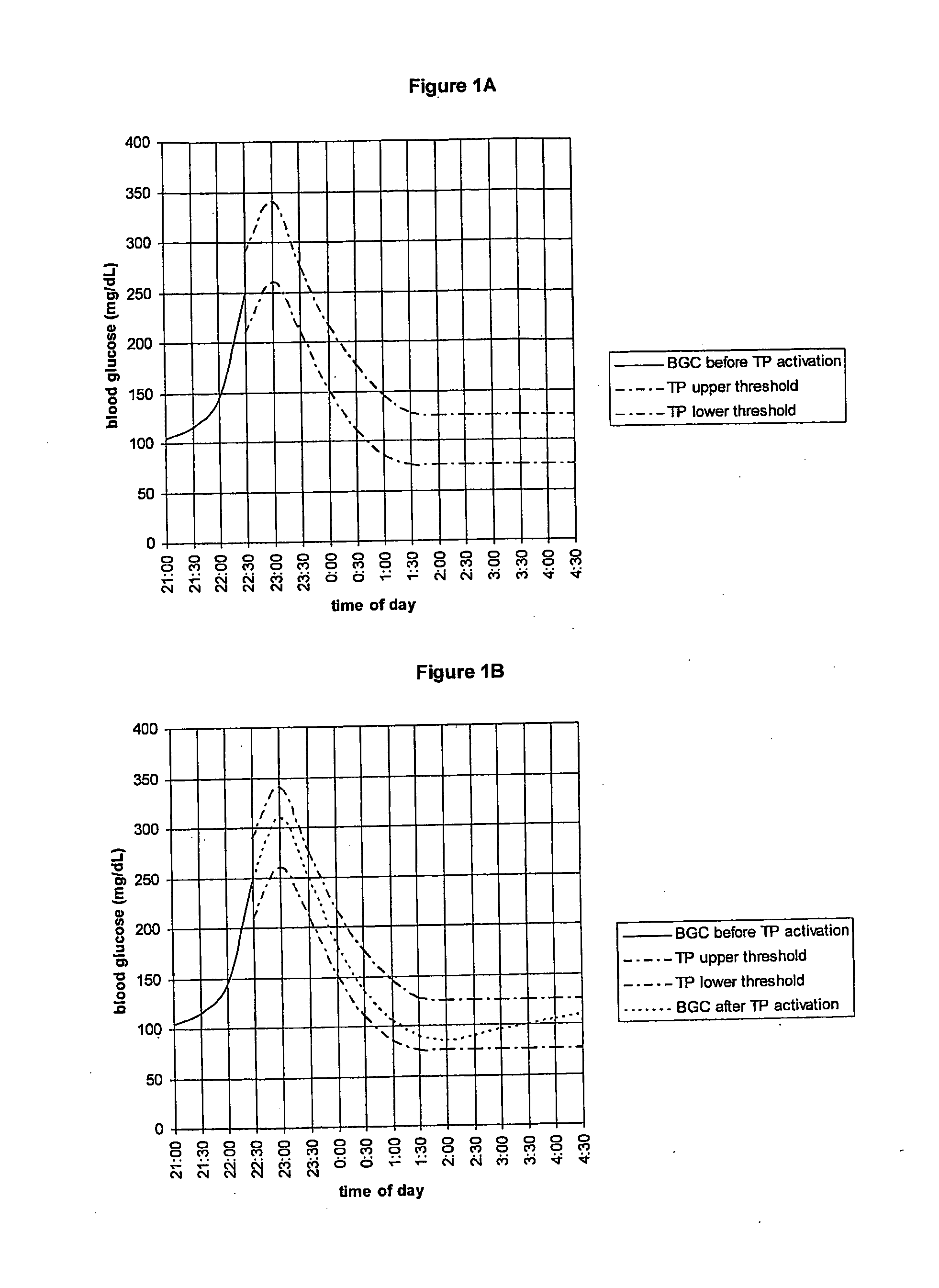
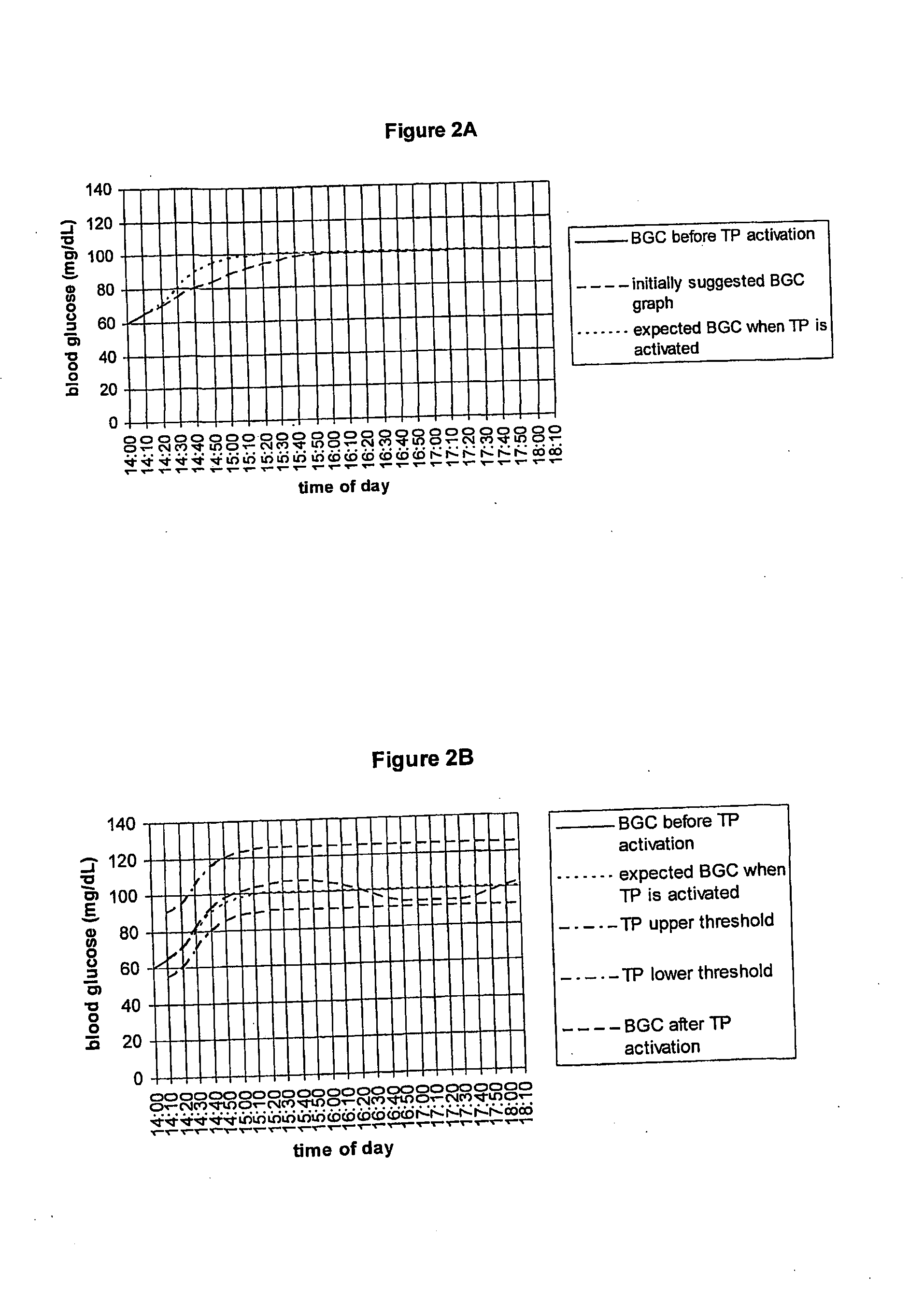
Fluctuating Blood Glucose Notification Threshold Profiles and Methods of Use
ActiveUS20080228055A1Improve visualizationHighly visualDiagnostic recording/measuringSensorsContinuous glucose monitoringGlucose polymers
Embodiments of the present invention provide a new system and methods for monitoring blood glucose concentration. A user of a continuous glucose monitor may program upper and lower blood glucose notification thresholds to fluctuate over time in order to facilitate management of the short-term effects of food consumption, insulin delivery aberrations, physical activity, emotions, and unforeseen circumstances.
Owner:SHER PHILIP M
Methods for modeling insulin therapy requirements
InactiveUS20110098548A1Improve fitShorten the timeMedical simulationDrug and medicationsGlycemicGlucose control
Various methods for improving the use of model based prediction of future blood glucose control in a patient having diabetes are described. A system for processing diabetes related information, including glucose information, for accurately predicting future glucose levels as a function of glucose data, carbohydrate intake, insulin delivery history and exercise history and then providing recommendations related to the predicted future glucose levels, is also described.
Owner:ABBOTT DIABETES CARE INC
Safety features for integrated insulin delivery system
ActiveUS20100298765A1Preventing hypoglycemiaLimited hyperglycemiaDrug and medicationsMedical devicesGlucose sensorsHypoglycemia
Safety features are applied to an integrated insulin delivery system to enhance safety while accounting for glucose sensor bias and calibration errors. One safety feature includes comparisons of calibrations of the sensor to nominal sensitivity and taking action, such as limiting insulin delivery or taking a further calibration of the sensor. In another feature, an automatic resumption of a basal delivery rate is programmed into the delivery device to avoid the possibility of complete loss of delivery of insulin in the event that communication with the delivery device is disrupted. Other features include steps taken to avoid hypoglycemia in the event that the sensor is negatively biased.
Owner:ABBOTT DIABETES CARE INC
Usability features for integrated insulin delivery system
ActiveUS20100295686A1Easy to useEnhancing alarm avoidanceDrug and medicationsMedical devicesUsabilityIntensive care medicine
Various systems and methods for improving the usability of continuous glucose monitors and drug delivery pumps are described.
Owner:ABBOTT DIABETES CARE INC
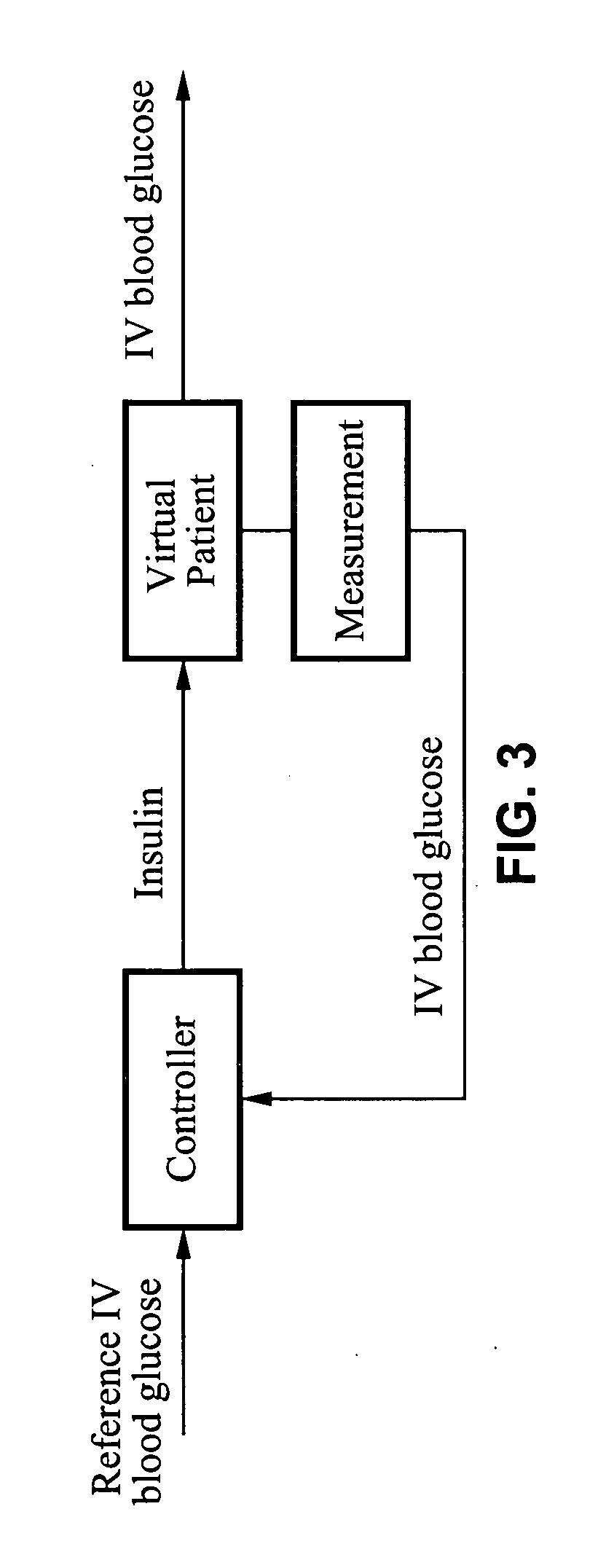
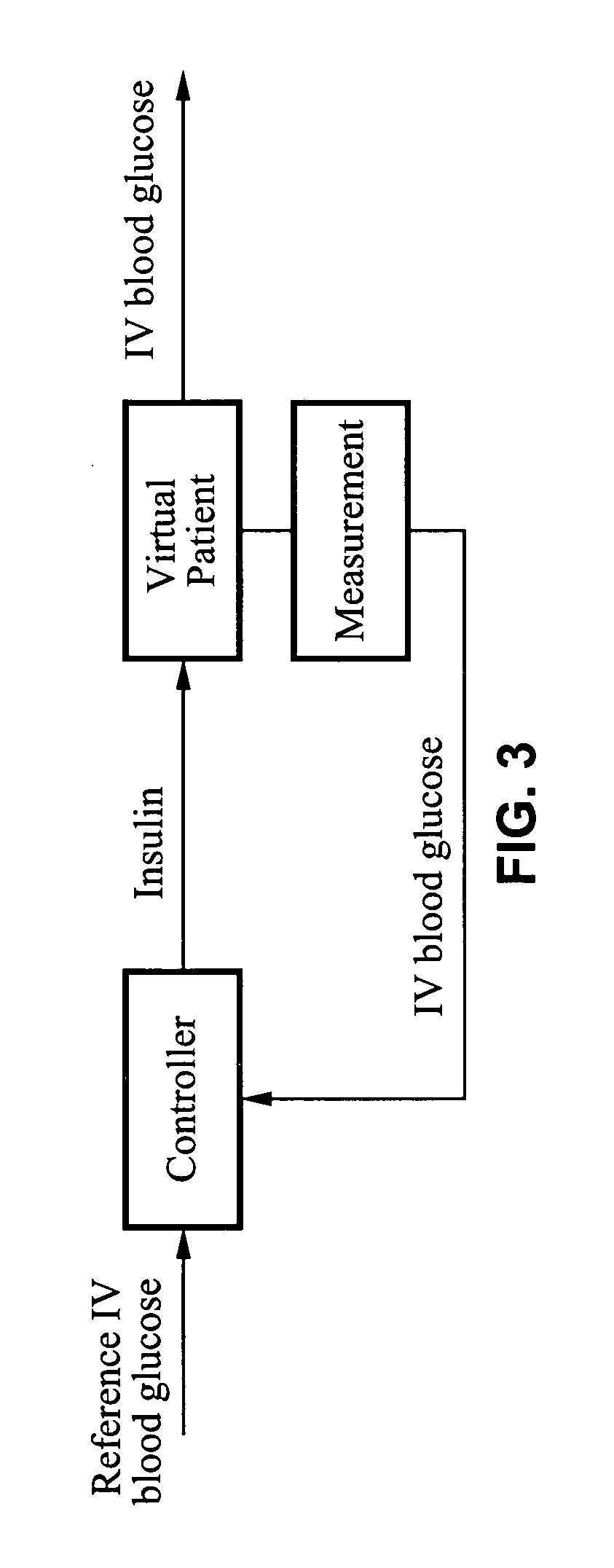
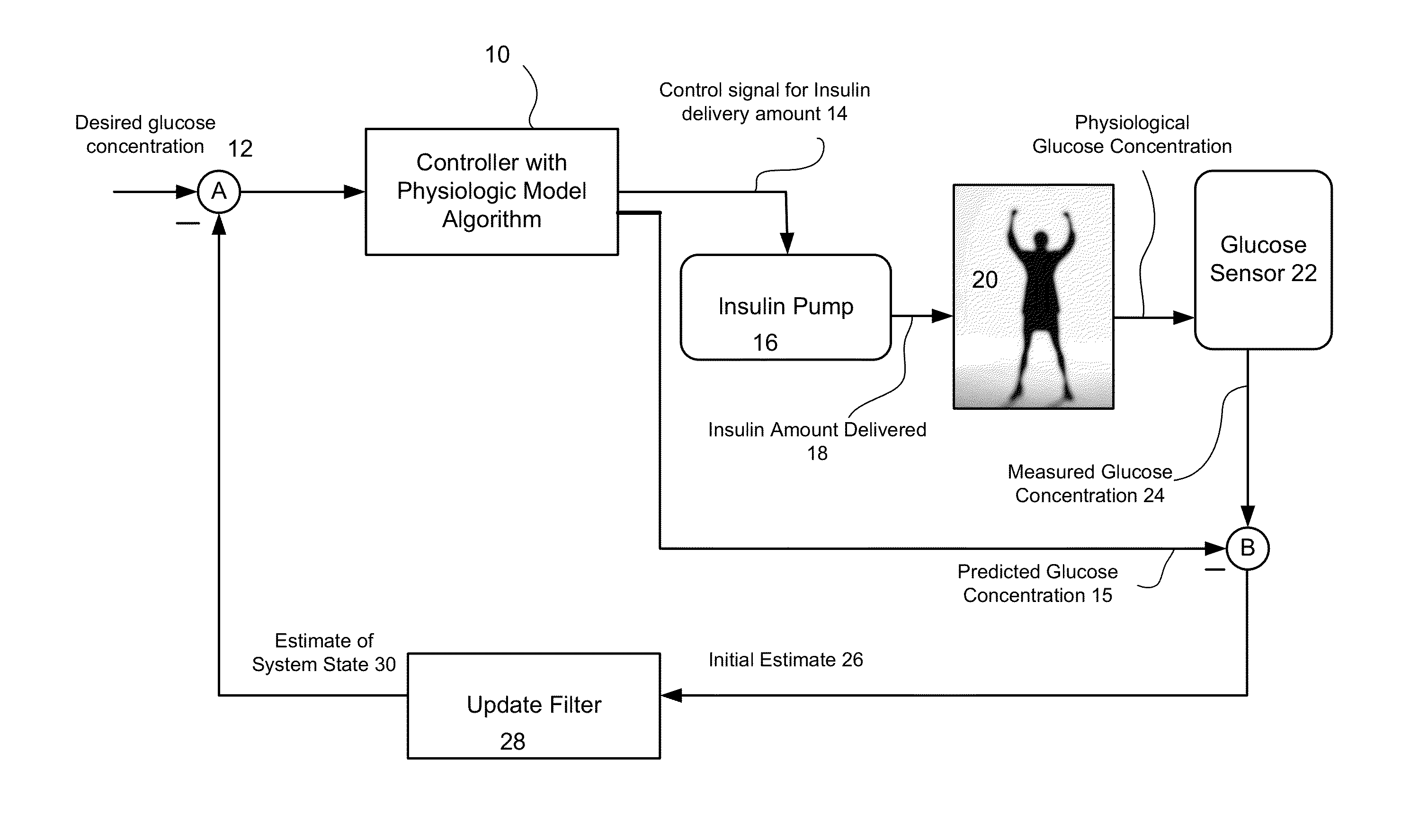
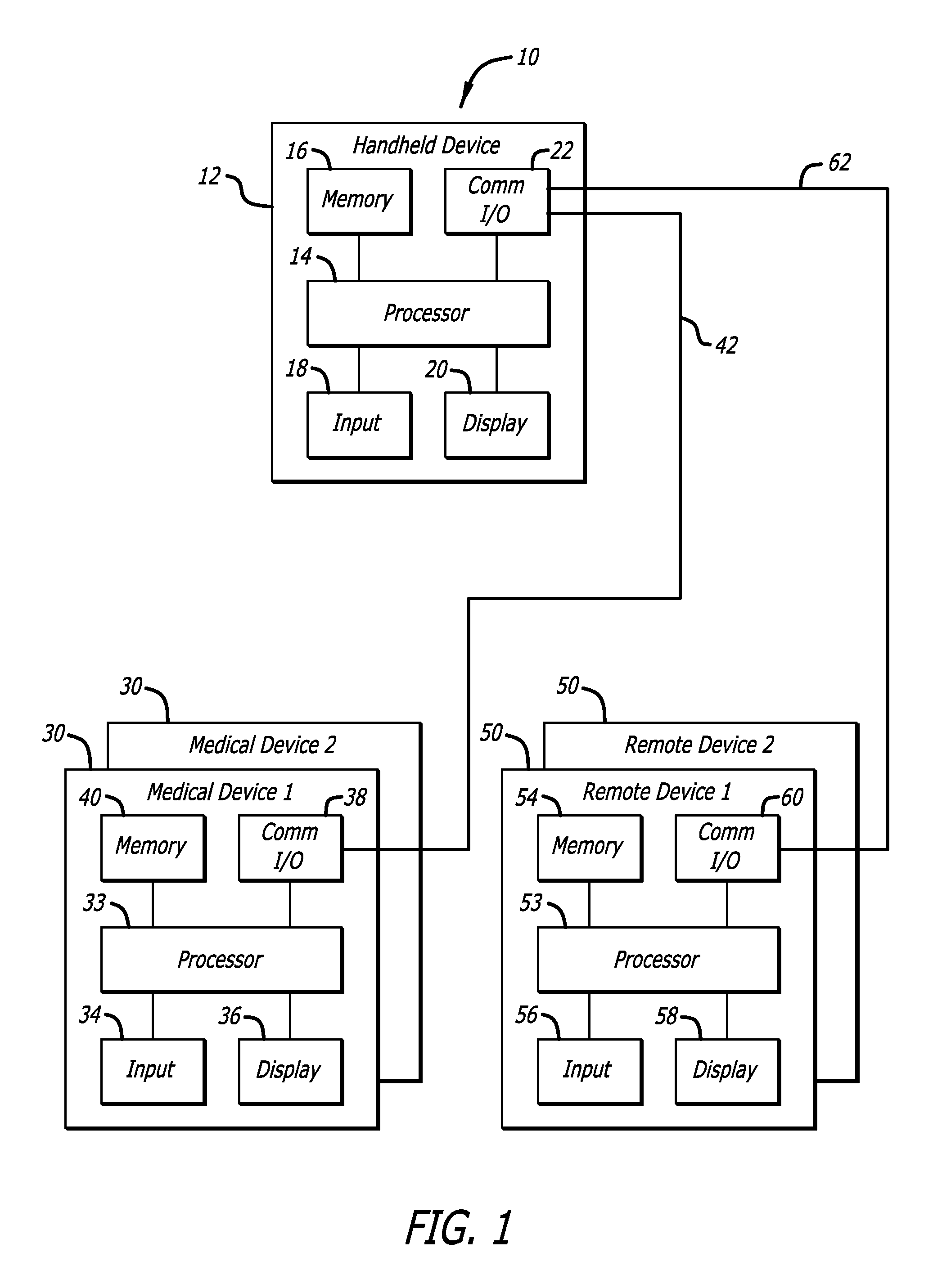
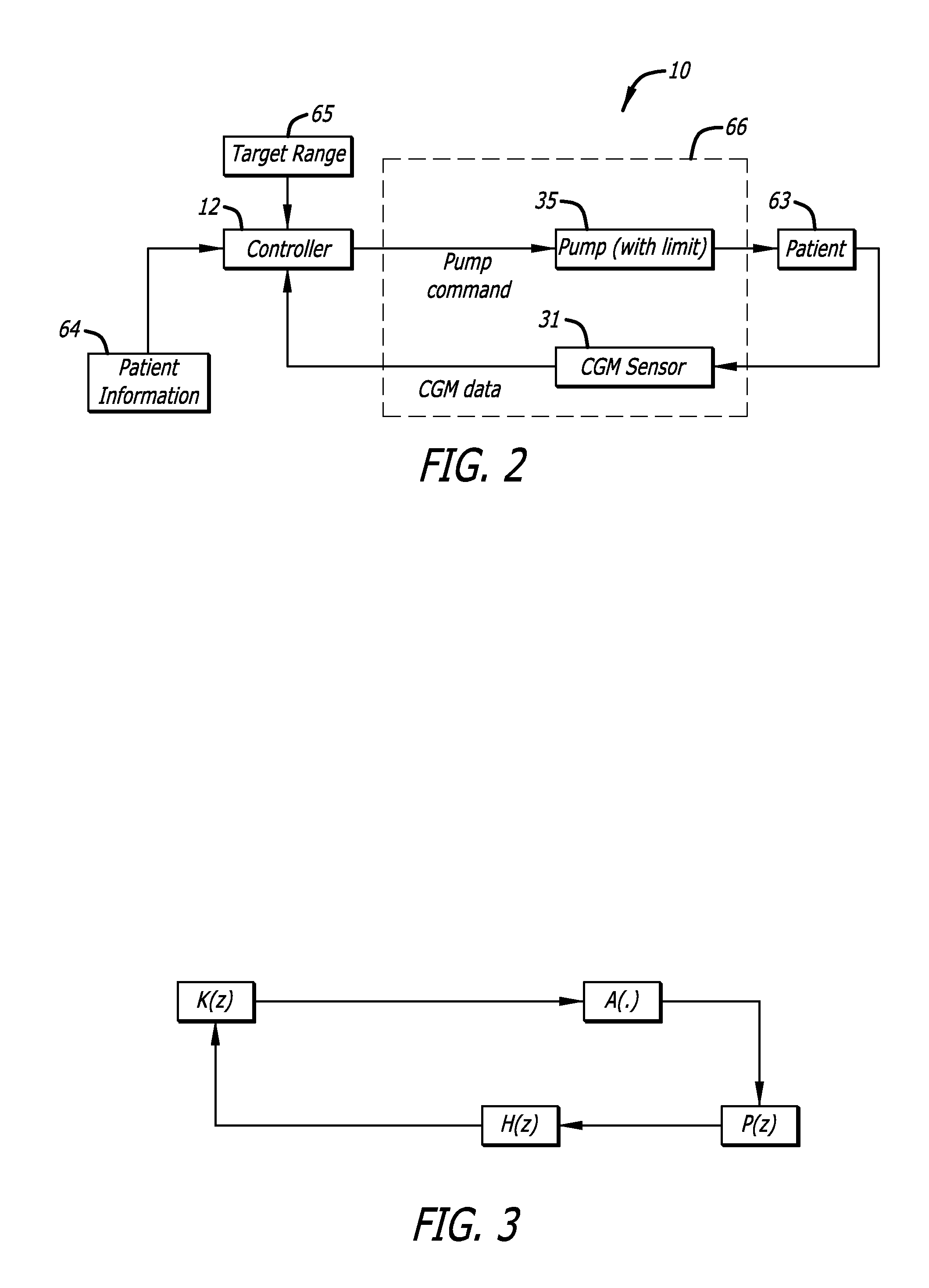
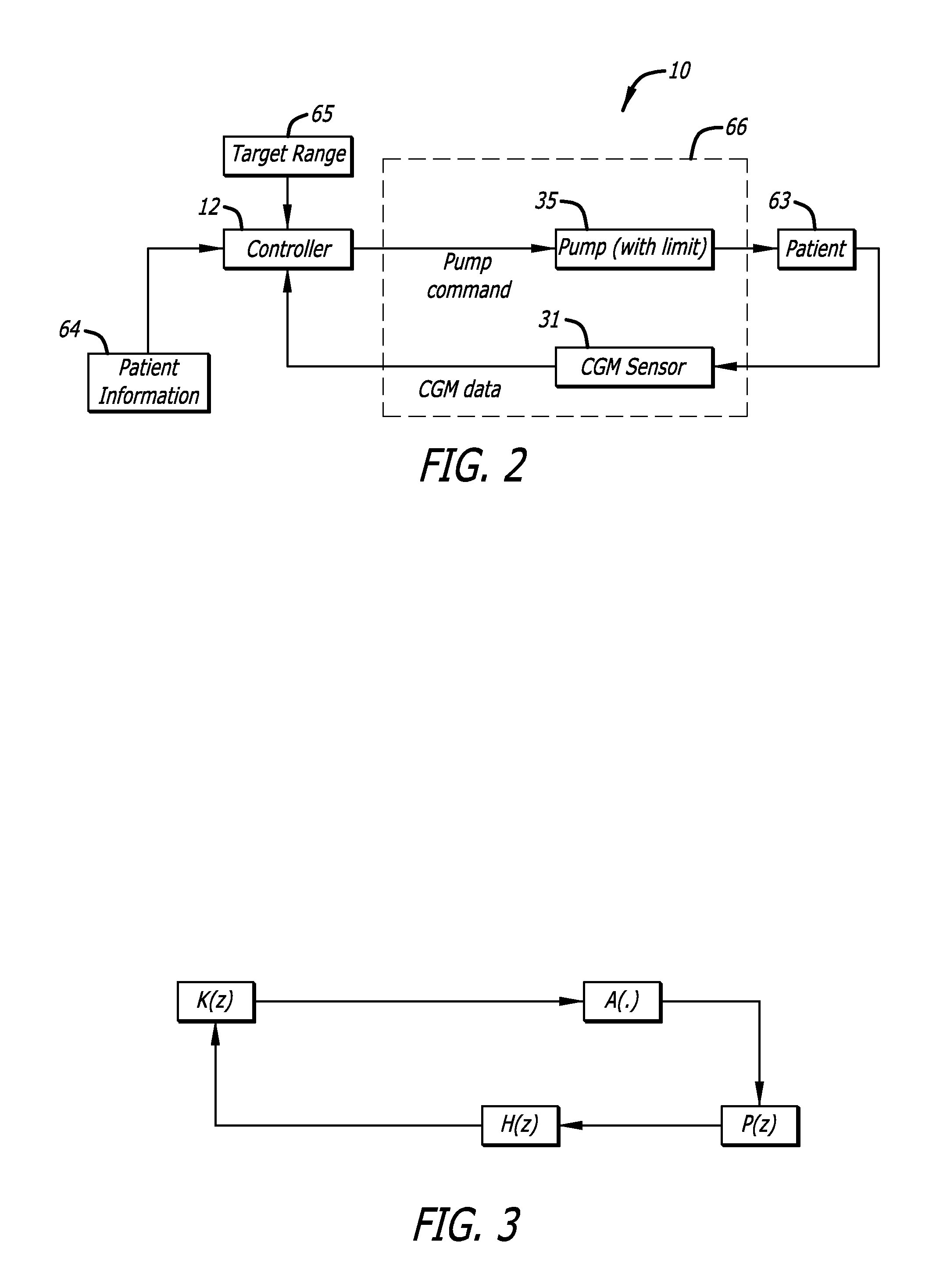
Predictive control based system and method for control of insulin delivery in diabetes using glucose sensing
A system and method for providing optimal insulin injections to a subject, using a controller, a continuous glucose monitor, and an insulin delivery unit is disclosed. The controller possesses a discrete-time, linear model predictive control law, means for sending information to the insulin delivery unit, and means for receiving information from the CGM. The control law implemented is derived from a discrete-time model of glucose insulin dynamics and an aggressiveness parameter. The result is that using only glucose measurements obtained from sensor readings and, prior values of external insulin infusion and meal and exercise announcement the optimal insulin injection necessary to safely regulate blood glucose can be calculated.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Real time management of data relating to physiological control of glucose levels
ActiveUS20110021898A1Physical therapies and activitiesMedical simulationInsulin on boardPatient specific
Continuous glucose monitoring (CGM) data and insulin delivery data are used to generate more reliable projected alarms related to a projected glucose levels. A memory stores endogenous data related to measurements of glucose level in a patient, and also stores exogenous data, such as insulin on board, both of which are used by a processor to create projected alarms. Profiles of CGM data are created for use in tuning patient-specific insulin data, such at basal rate, carb ratio, and insulin sensitivity. A processor searches for patterns in the data profiles and if found, recommended changes to patient-specific insulin data are provided to permit more accurate control over a patient's glucose levels.
Owner:ABBOTT DIABETES CARE INC
External infusion device with programmable capabilities to time-shift basal insulin and method of using the same
An external infusion device for delivering insulin from a reservoir into a body of a user includes the capability to deliver time-shifted basal insulin. The external infusion device includes at least one drive mechanism operatively couplable to the reservoir to deliver insulin into the body of the user, at least one processor to control the external infusion device, at least one power supply, at least one display device operatively coupled to the processor to provide visual information to the user, at least one input device operatively coupled to the processor to allow the user to command the processor, and a housing. Time-shifting of basal insulin occurs when a portion of basal insulin is added to a bolus, to a current basal rate, or to a bolus and a current basal rate.
Owner:MEDTRONIC MIMIMED INC
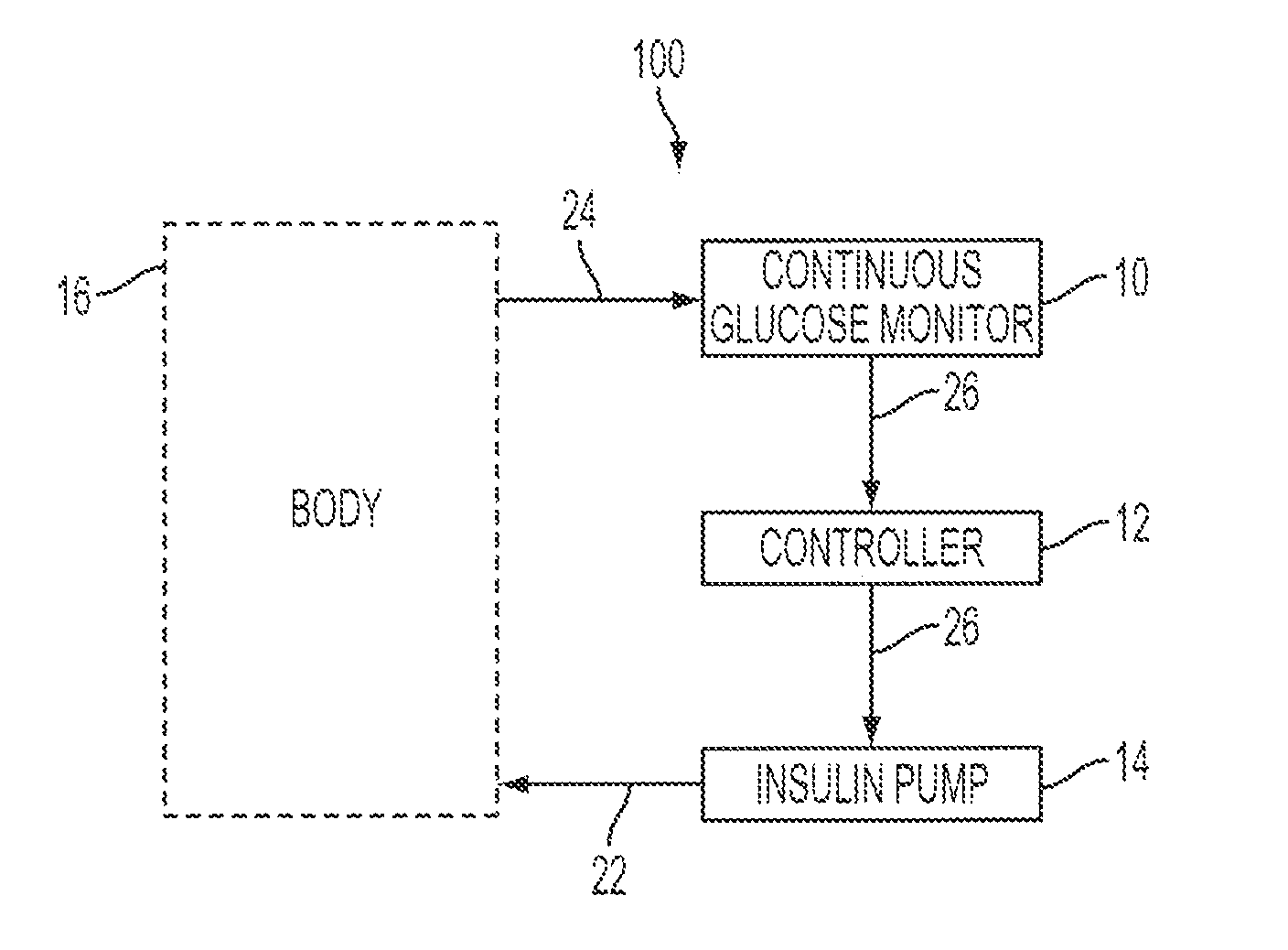
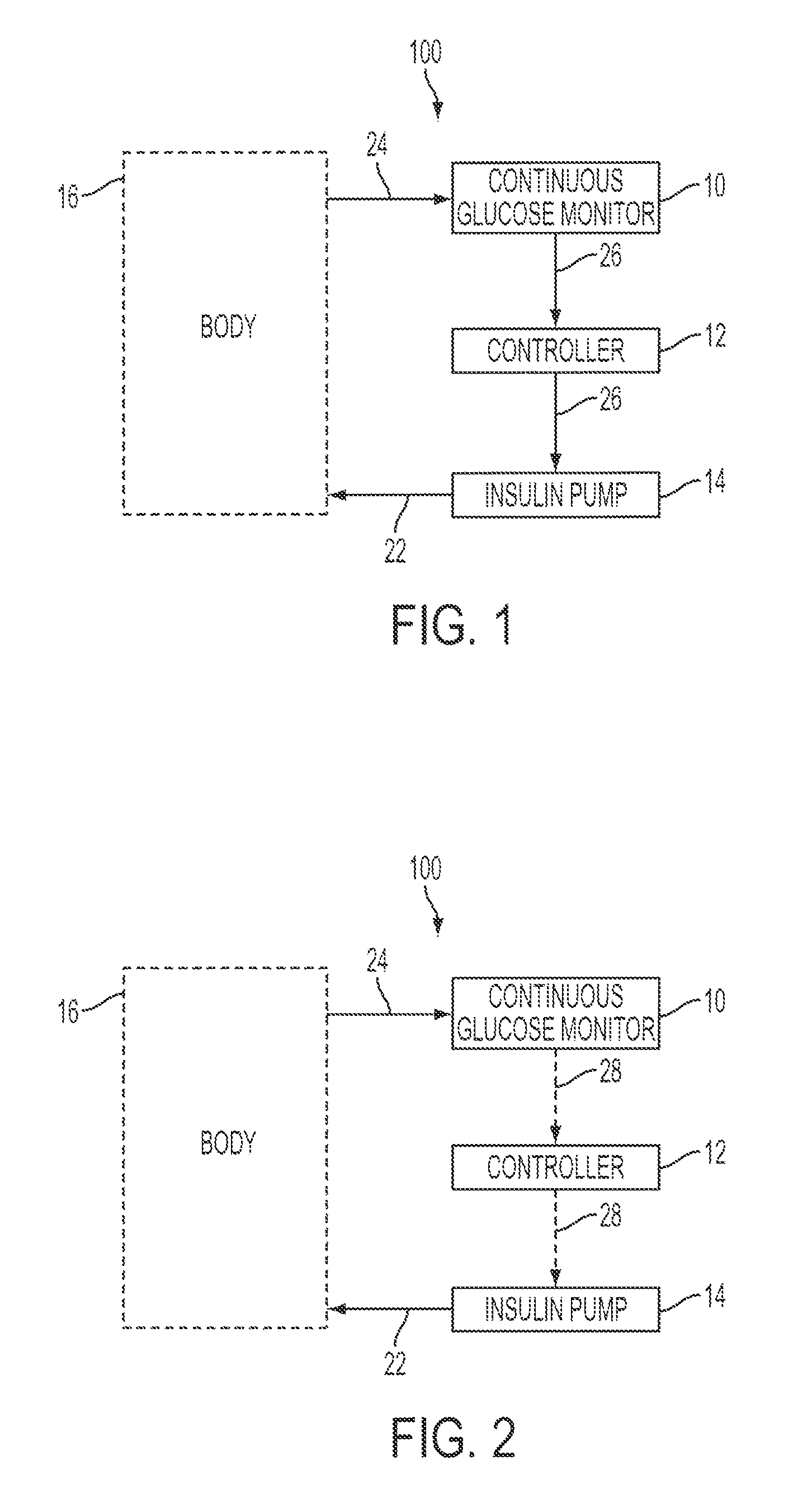
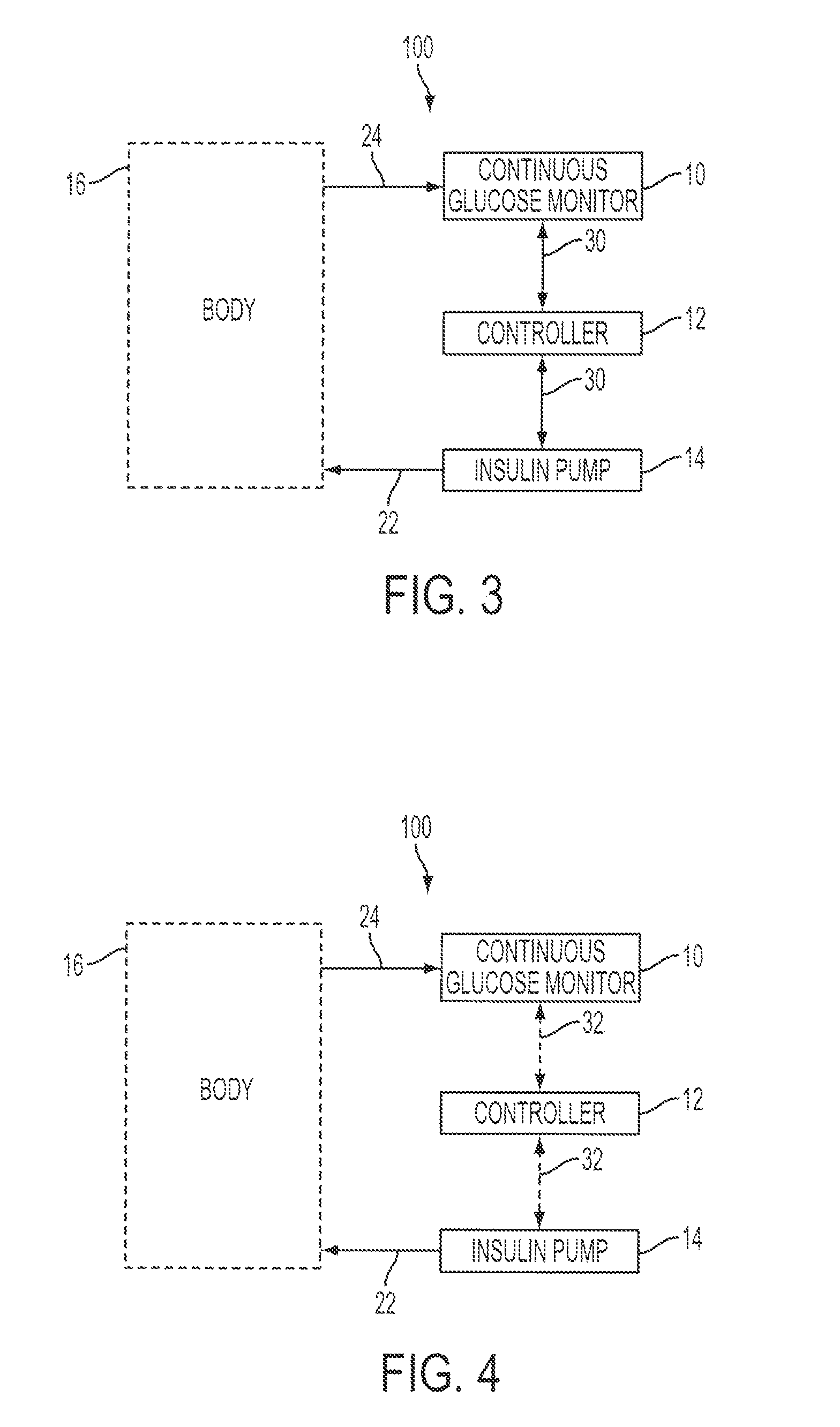
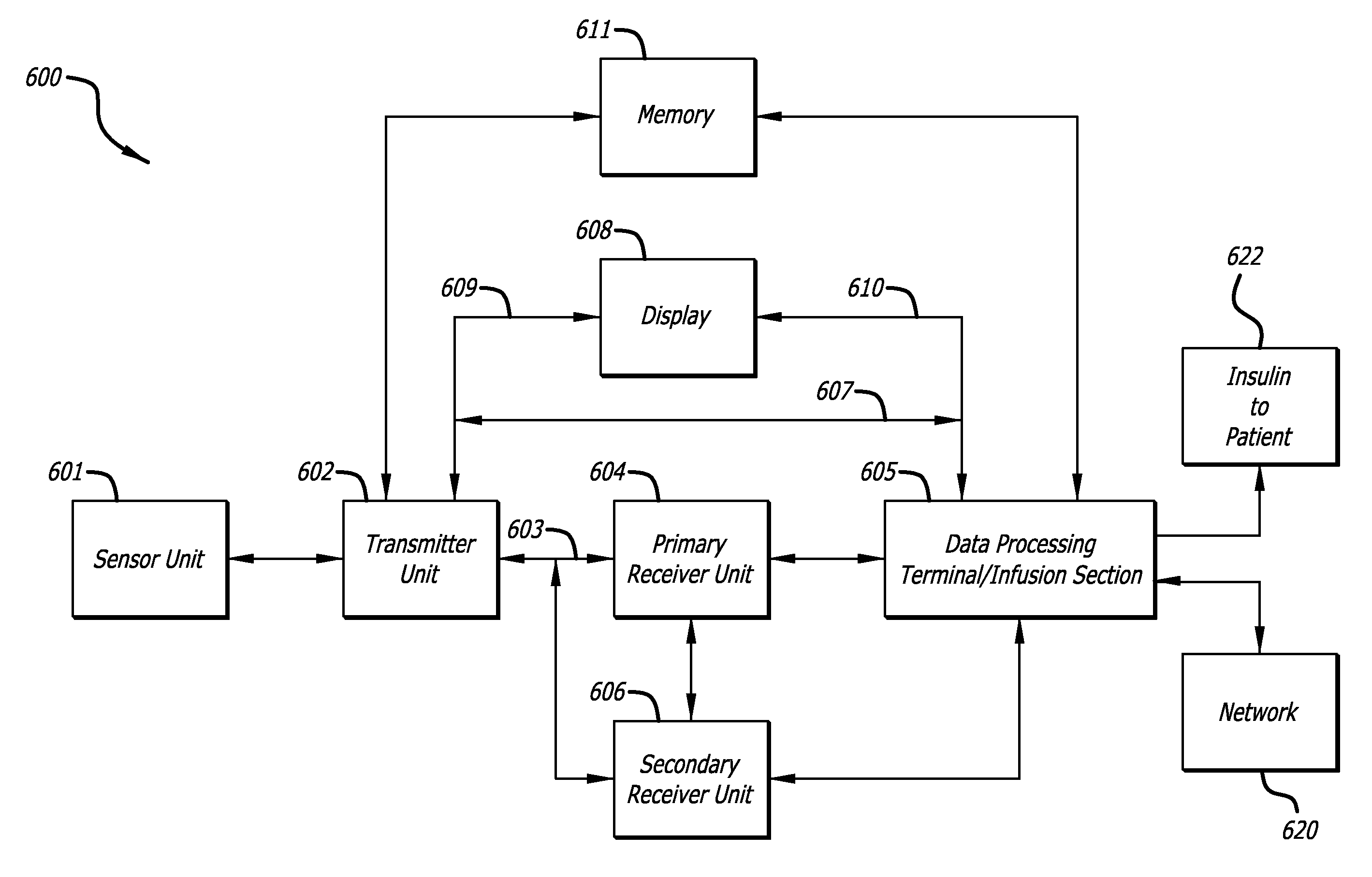
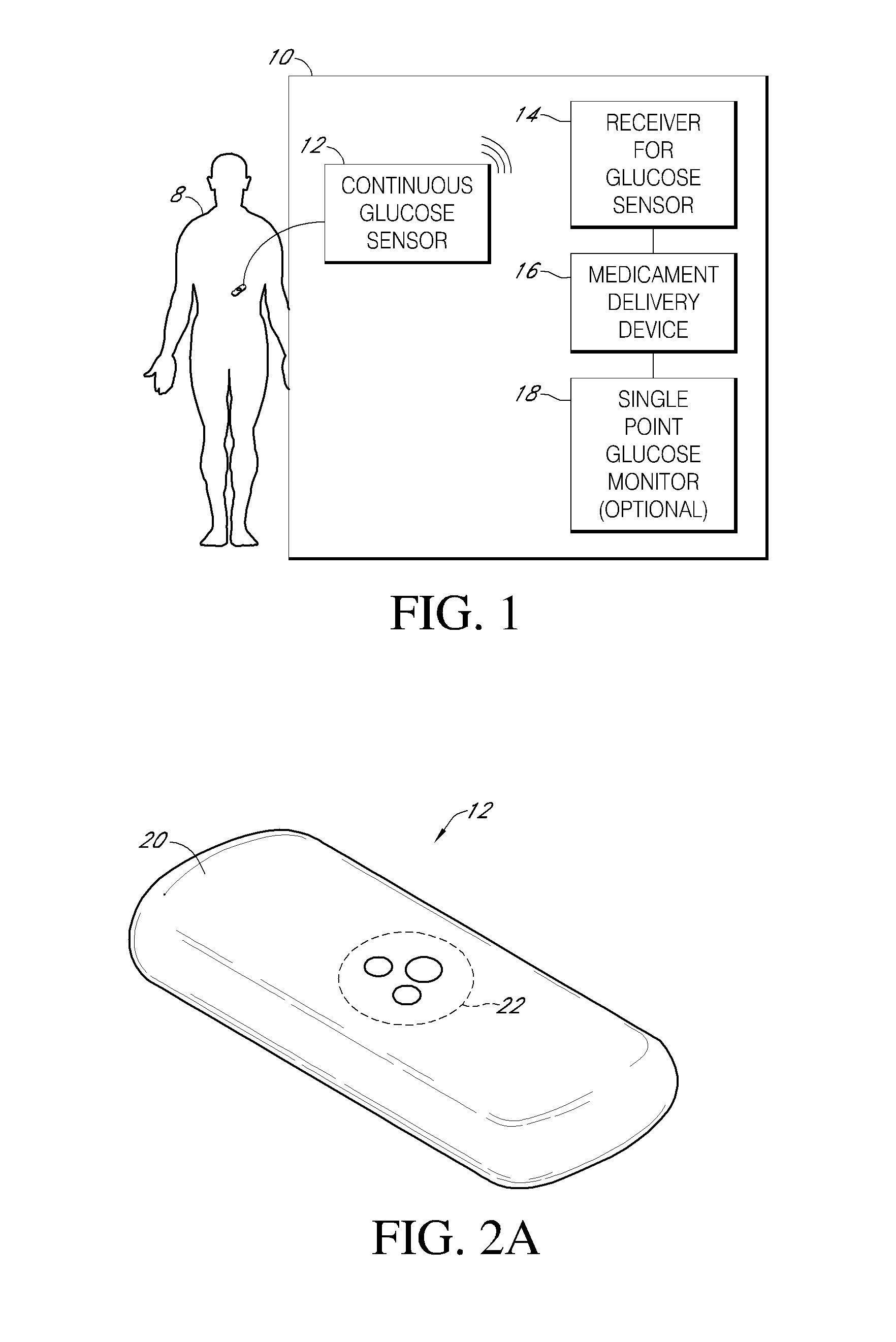
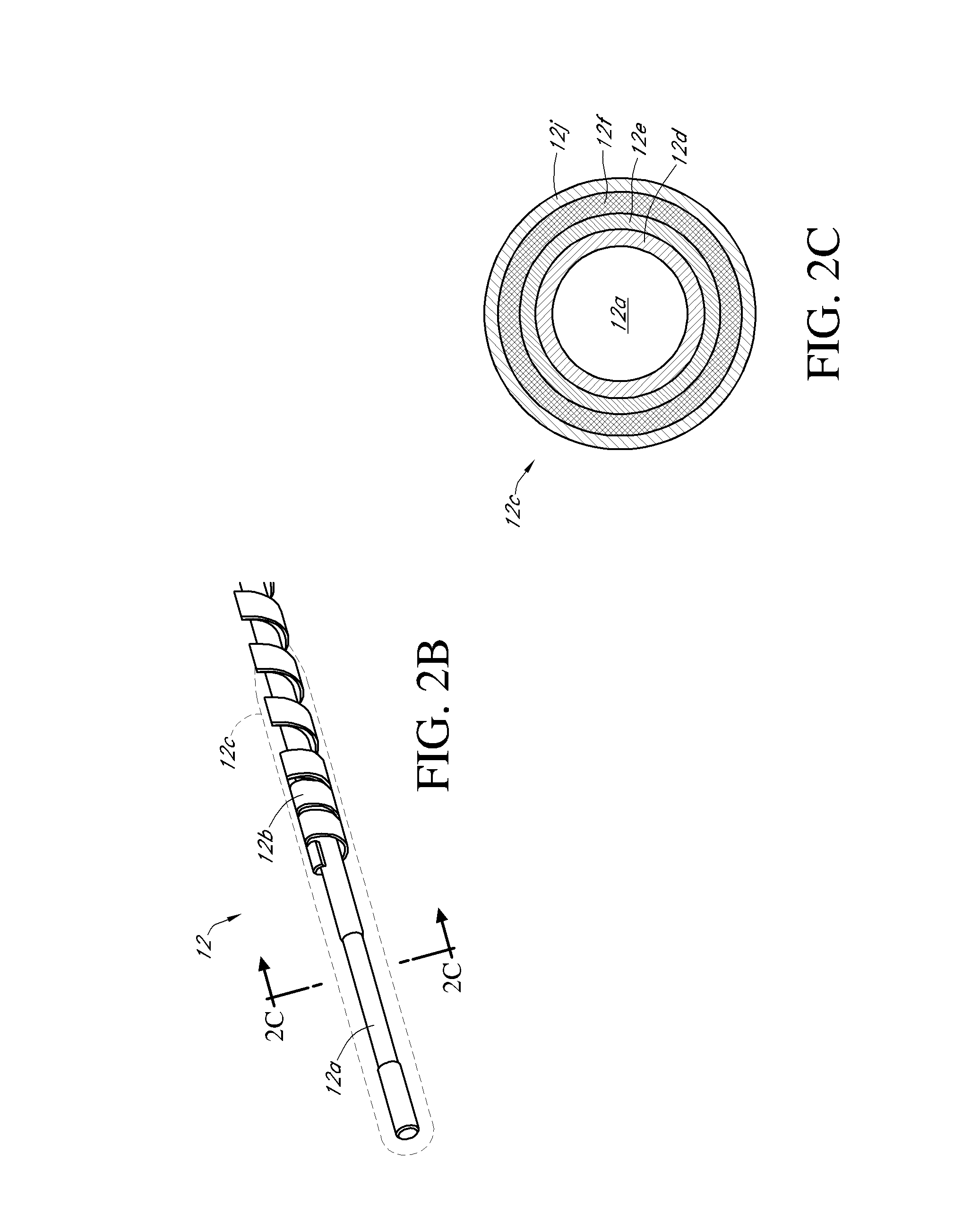
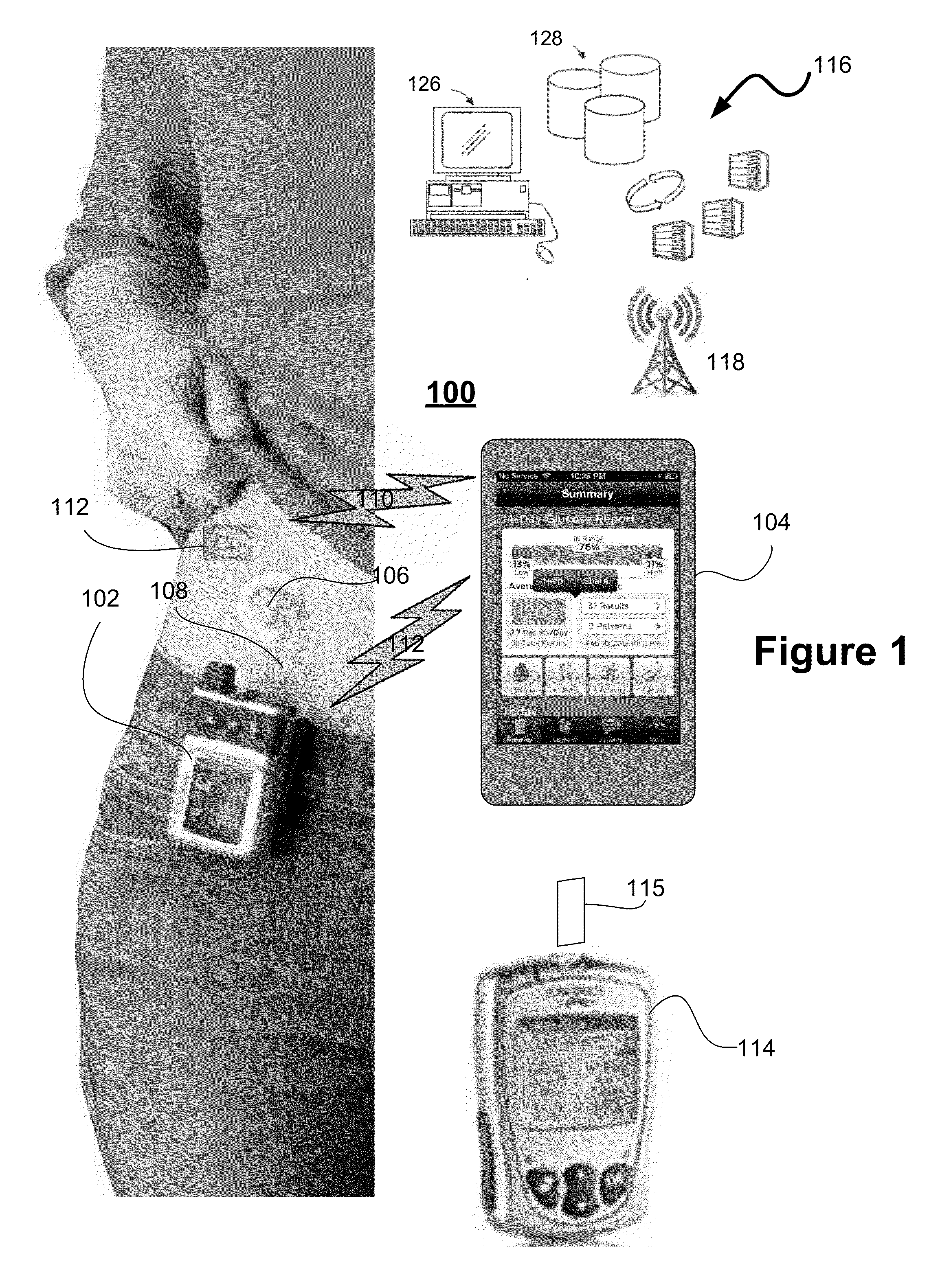
Integrated insulin delivery system with continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor 12, including a receiver 14, a medicament delivery device 16, a controller module, and optionally a single point glucose monitor 18 are provided. Integration may be manual, semi-automated and / or fully automated.
Owner:DEXCOM
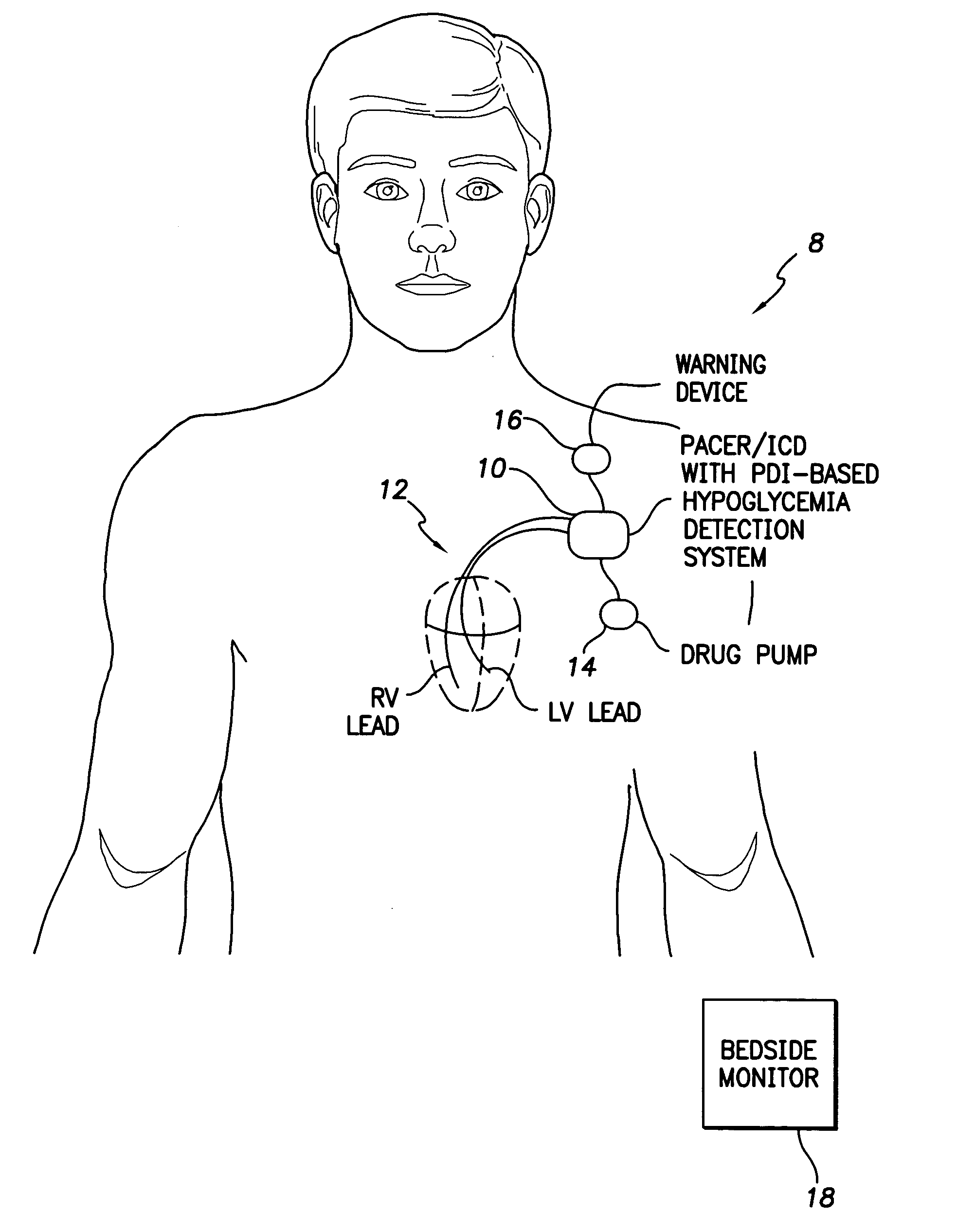
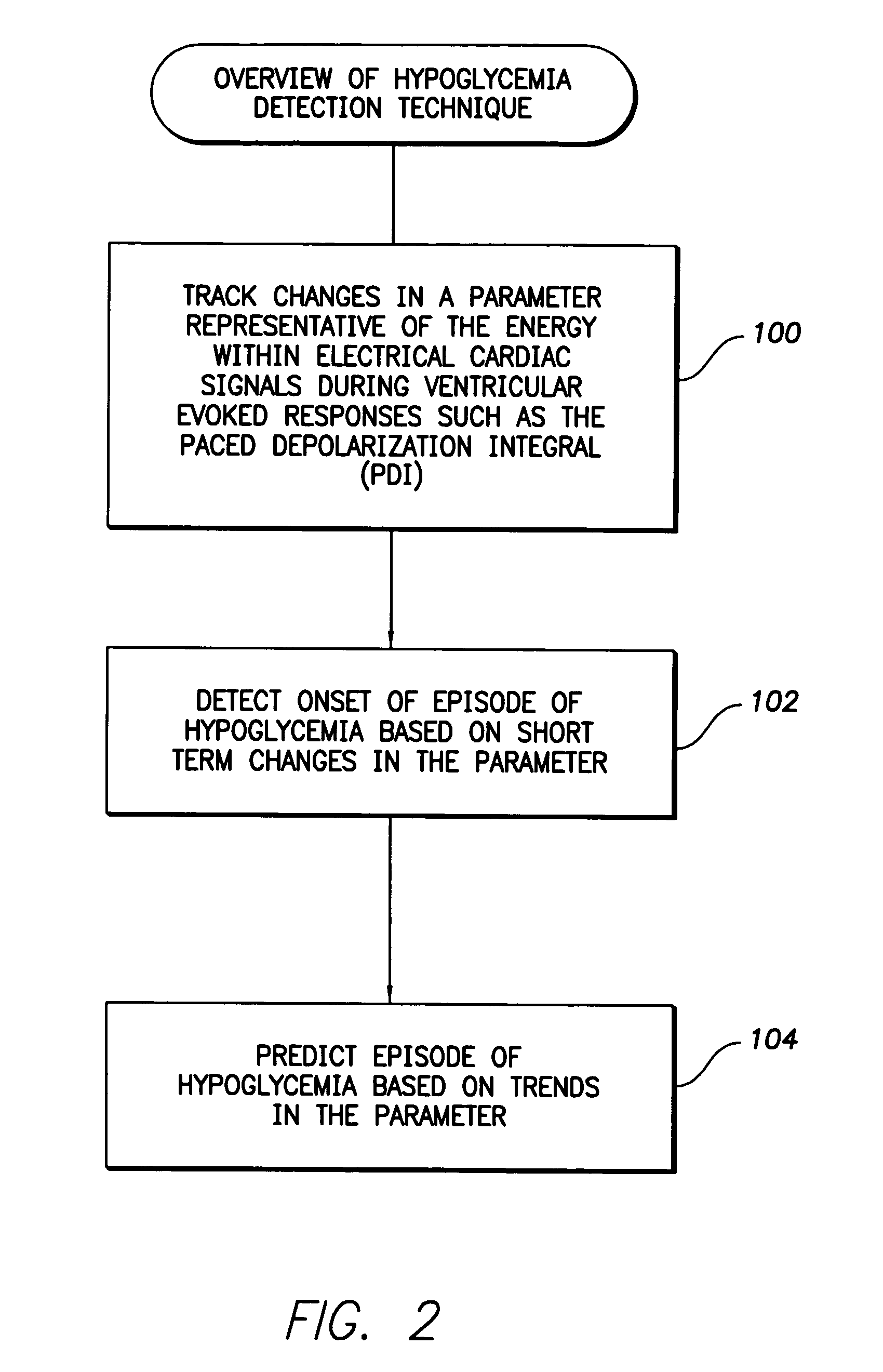
System and method for detecting hypoglycemia based on a paced depolarization integral using an implantable medical device
ActiveUS20060247685A1Improve blood sugar controlReduce deliveryElectrocardiographyMedical devicesCardiac pacemaker electrodeInsulin dependent
Techniques are provided for use with an implantable medical device such as a pacemaker or implantable cardioverter / defibrillator (ICD) for predicting and detecting hypoglycemia. In one example, the device tracks changes in a paced depolarization integral (PDI). A significant increase in PDI over a relatively short period of time indicates the onset of hypoglycemia (this can also be confirmed with QT changes). Upon detection of hypoglycemia, appropriate warning signals are generated to alert the patient. Certain therapies automatically provided by the implantable device may also be controlled in response to hypoglycemia. For example, if the patient is an insulin-dependent diabetic and the implantable device is equipped with an insulin pump capable of delivering insulin directly into the bloodstream, insulin delivery is automatically suspended until blood glucose levels return to acceptable levels. If the device is an ICD, it may be controlled to begin charging defibrillation capacitors upon detection of hypoglycemia so as to permit prompt delivery of a defibrillation shock, which may be needed if hypoglycemia triggers ventricular fibrillation. The detection techniques may be used in conjunction with other hypoglycemia detection techniques to improve detection specificity.
Owner:PACESETTER INC
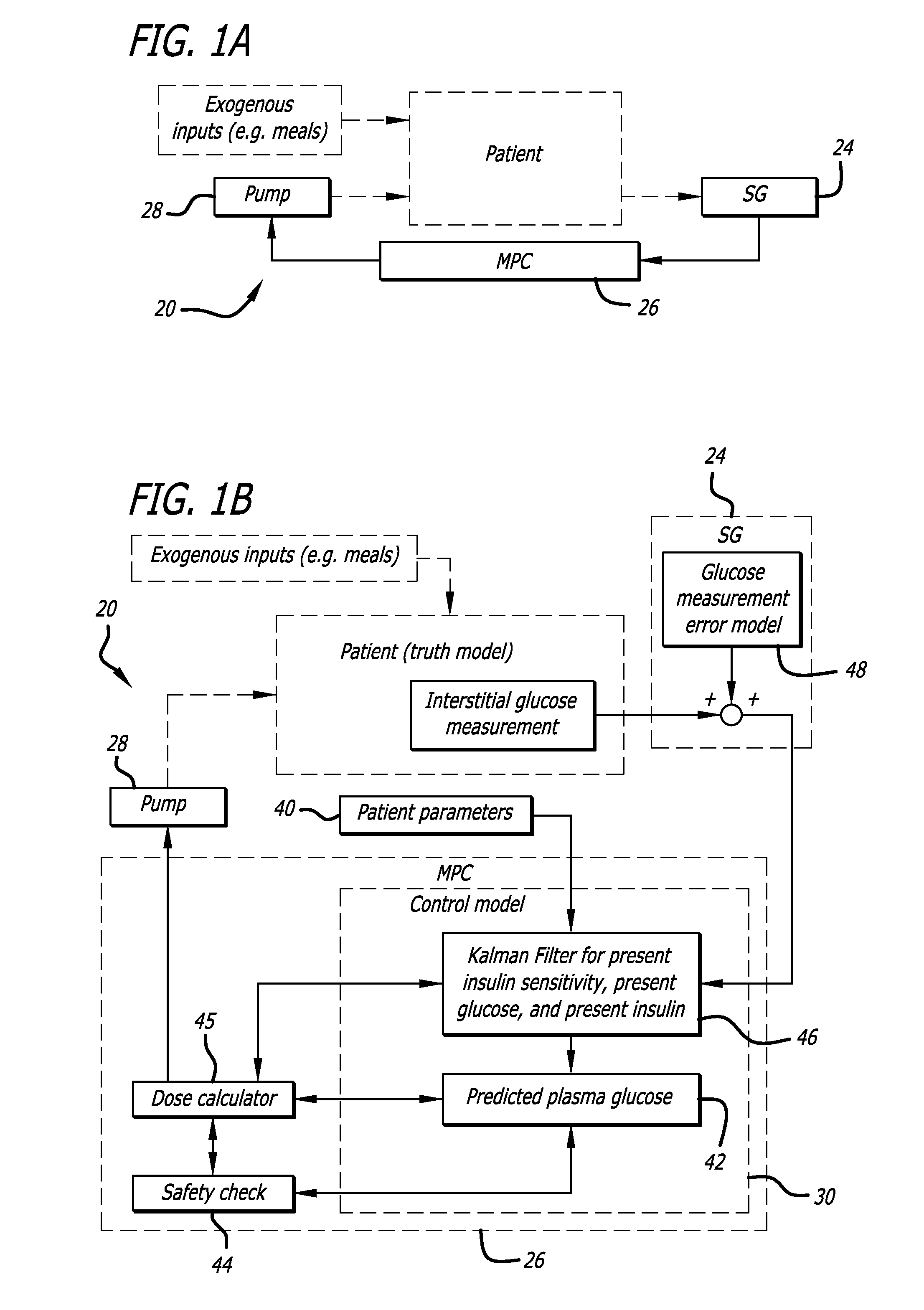
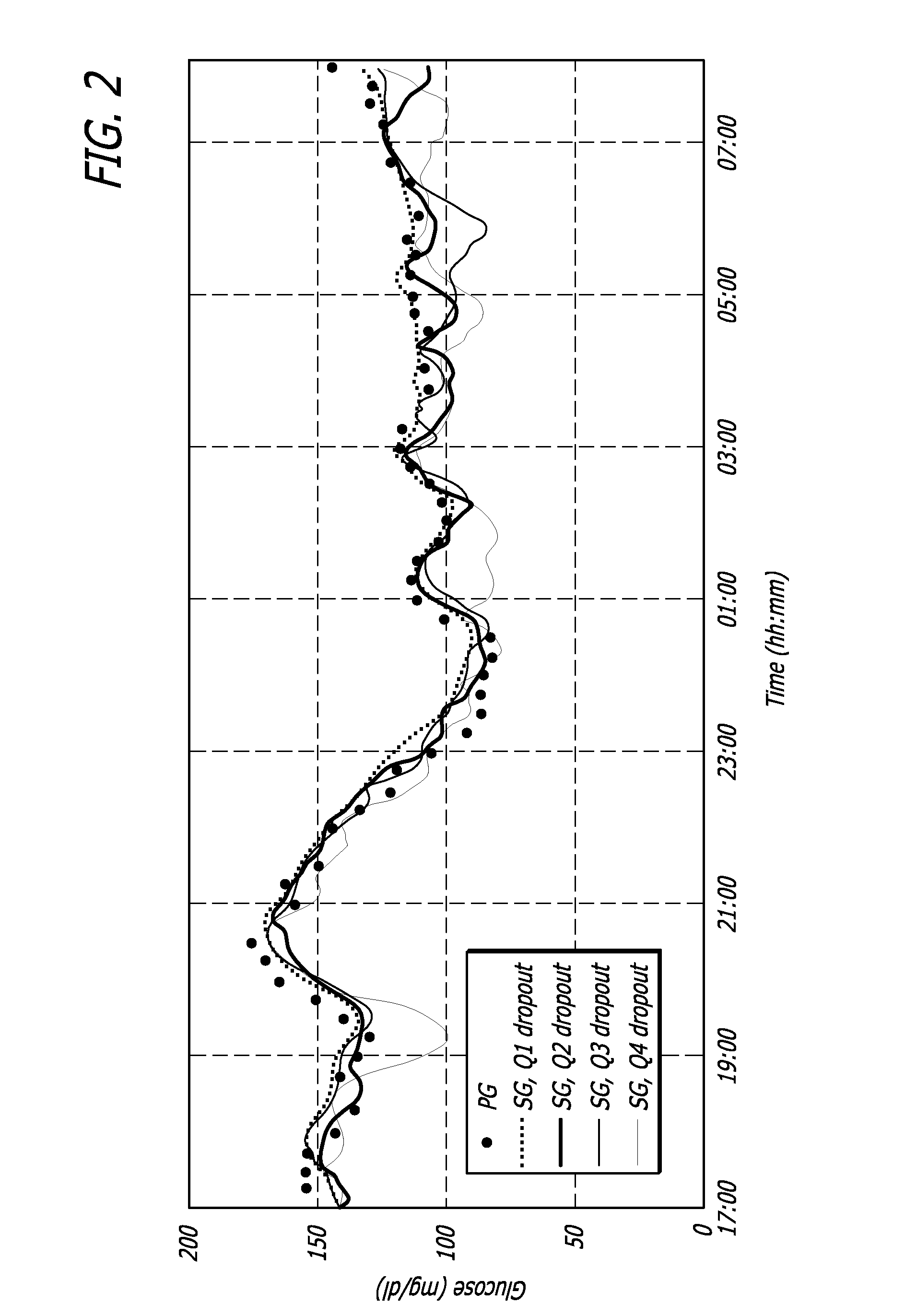
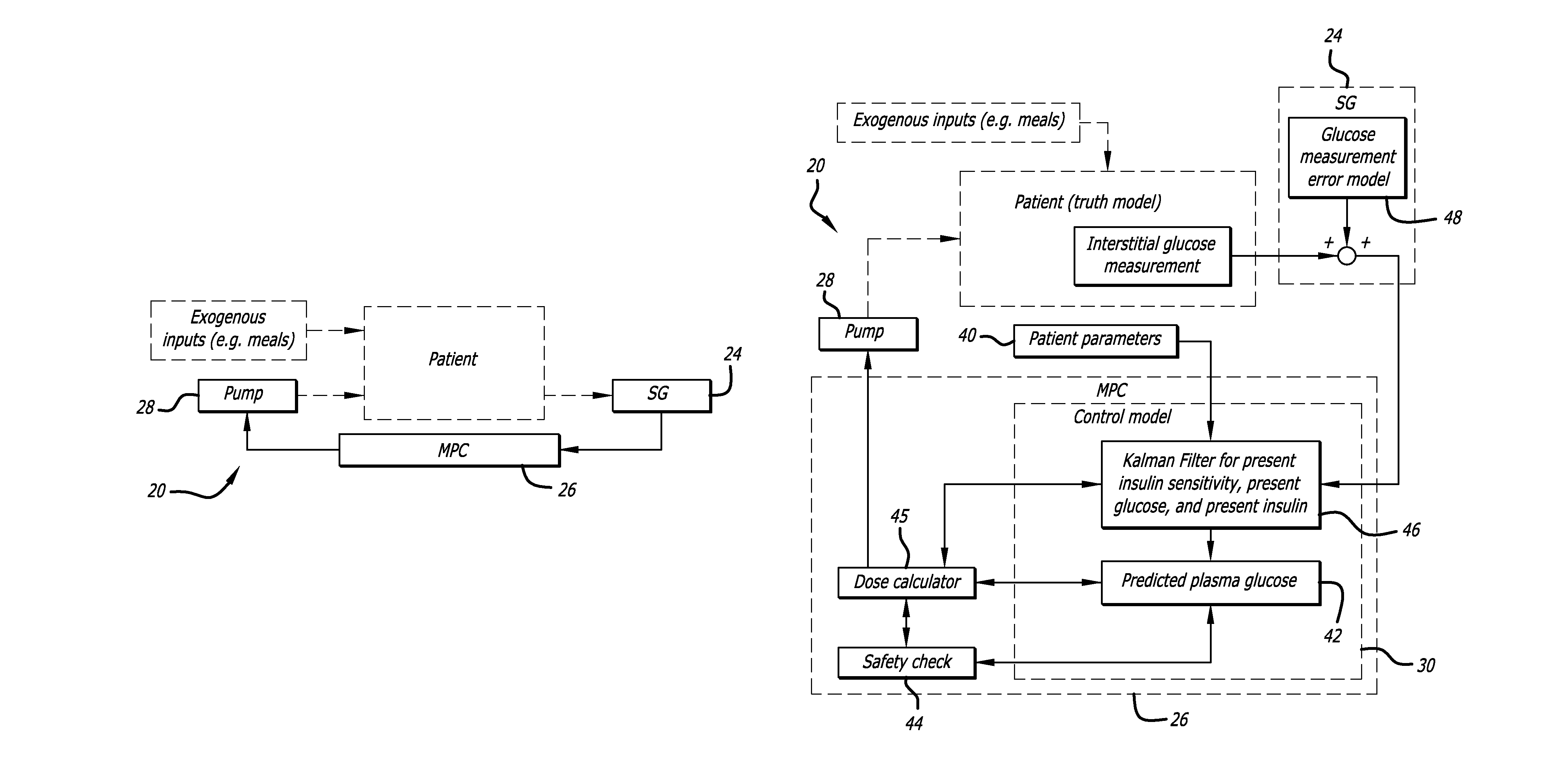
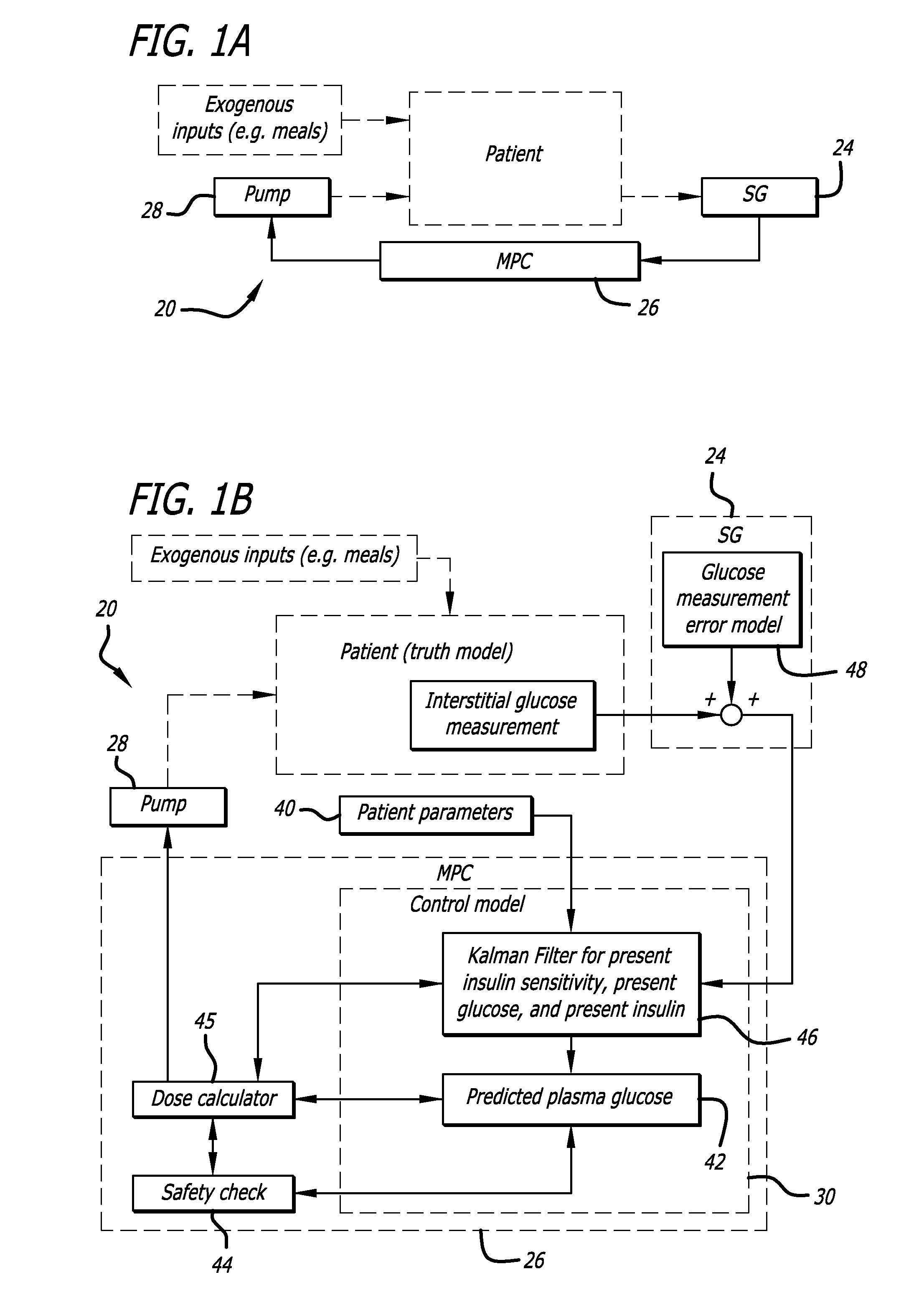
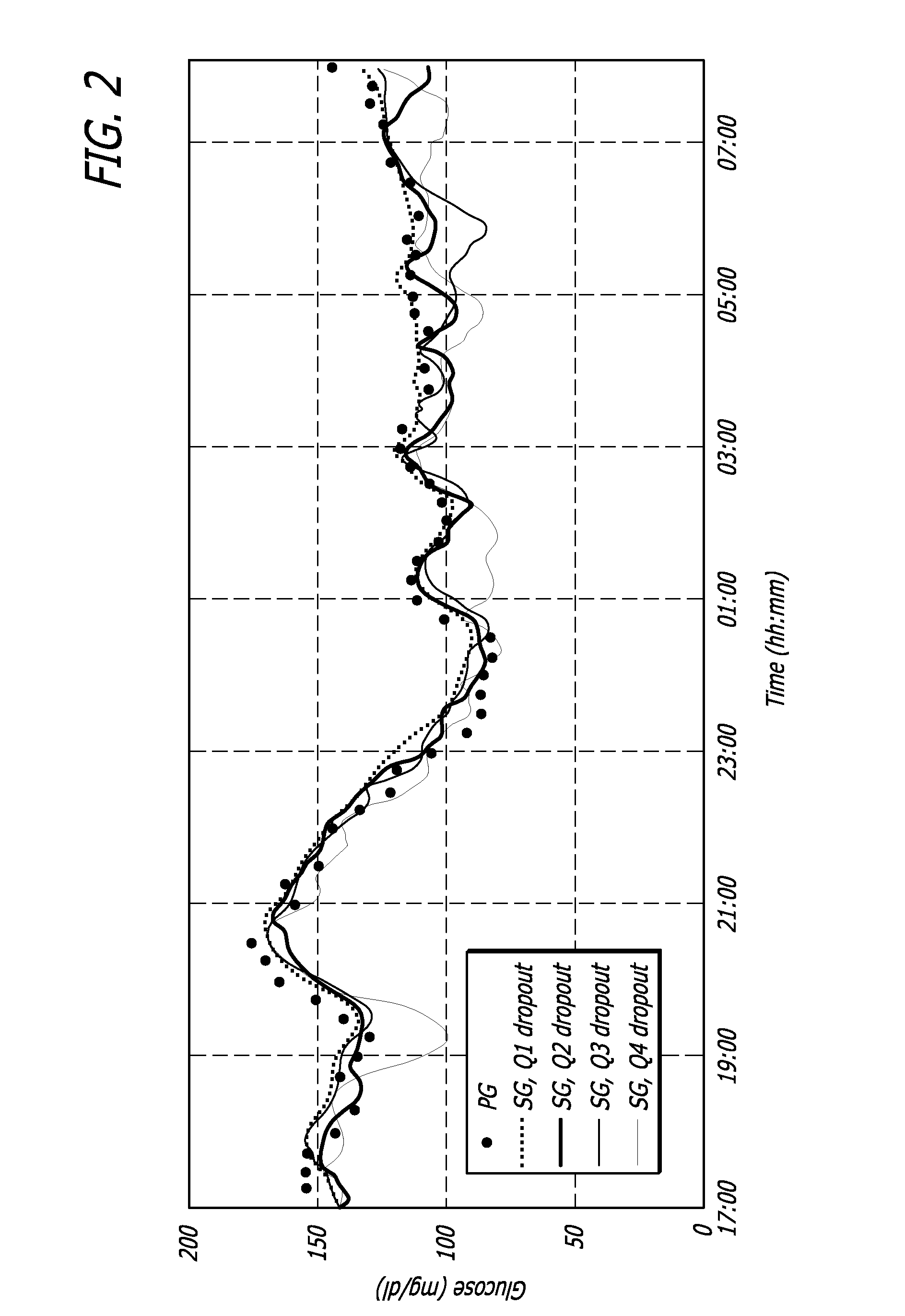
Overnight closed-loop insulin delivery with model predictive control and glucose measurement error model
ActiveUS20100280441A1Reducing insulin deliveryReduce deliveryDrug and medicationsMedical devicesGlucose sensorsClosed loop
A closed-loop system for insulin infusion overnight uses a model predictive control algorithm (“MPC”). Used with the MPC is a glucose measurement error model which was derived from actual glucose sensor error data. That sensor error data included both a sensor artifacts component, including dropouts, and a persistent error component, including calibration error, all of which was obtained experimentally from living subjects. The MPC algorithm advised on insulin infusion every fifteen minutes. Sensor glucose input to the MPC was obtained by combining model-calculated, noise-free interstitial glucose with experimentally-derived transient and persistent sensor artifacts associated with the FreeStyle Navigator® Continuous Glucose Monitor System (“FSN”). The incidence of severe and significant hypoglycemia reduced 2300- and 200-fold, respectively, during simulated overnight closed-loop control with the MPC algorithm using the glucose measurement error model suggesting that the continuous glucose monitoring technologies facilitate safe closed-loop insulin delivery.
Owner:CAMBRIDGE ENTERPRISE LTD +1
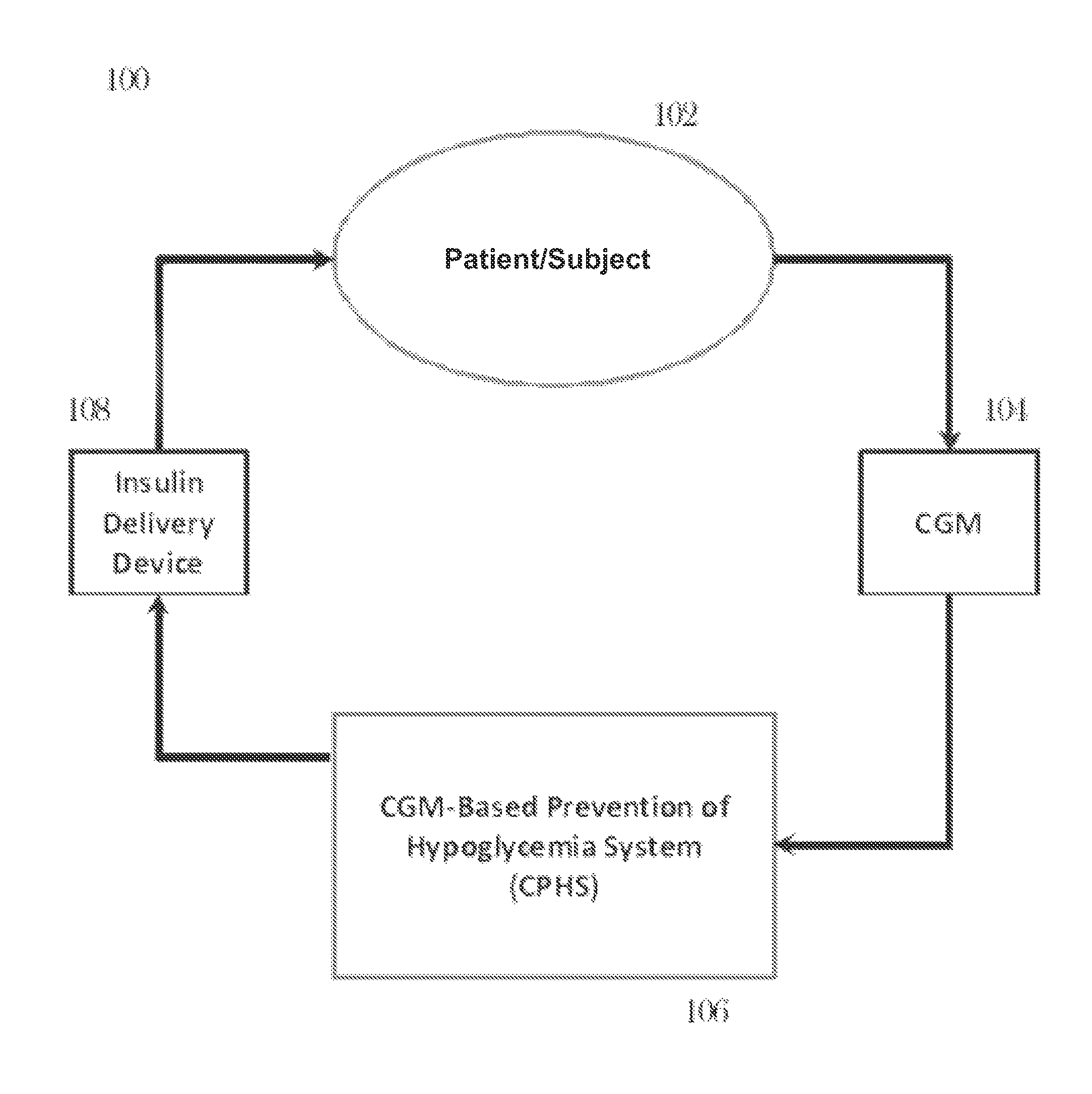
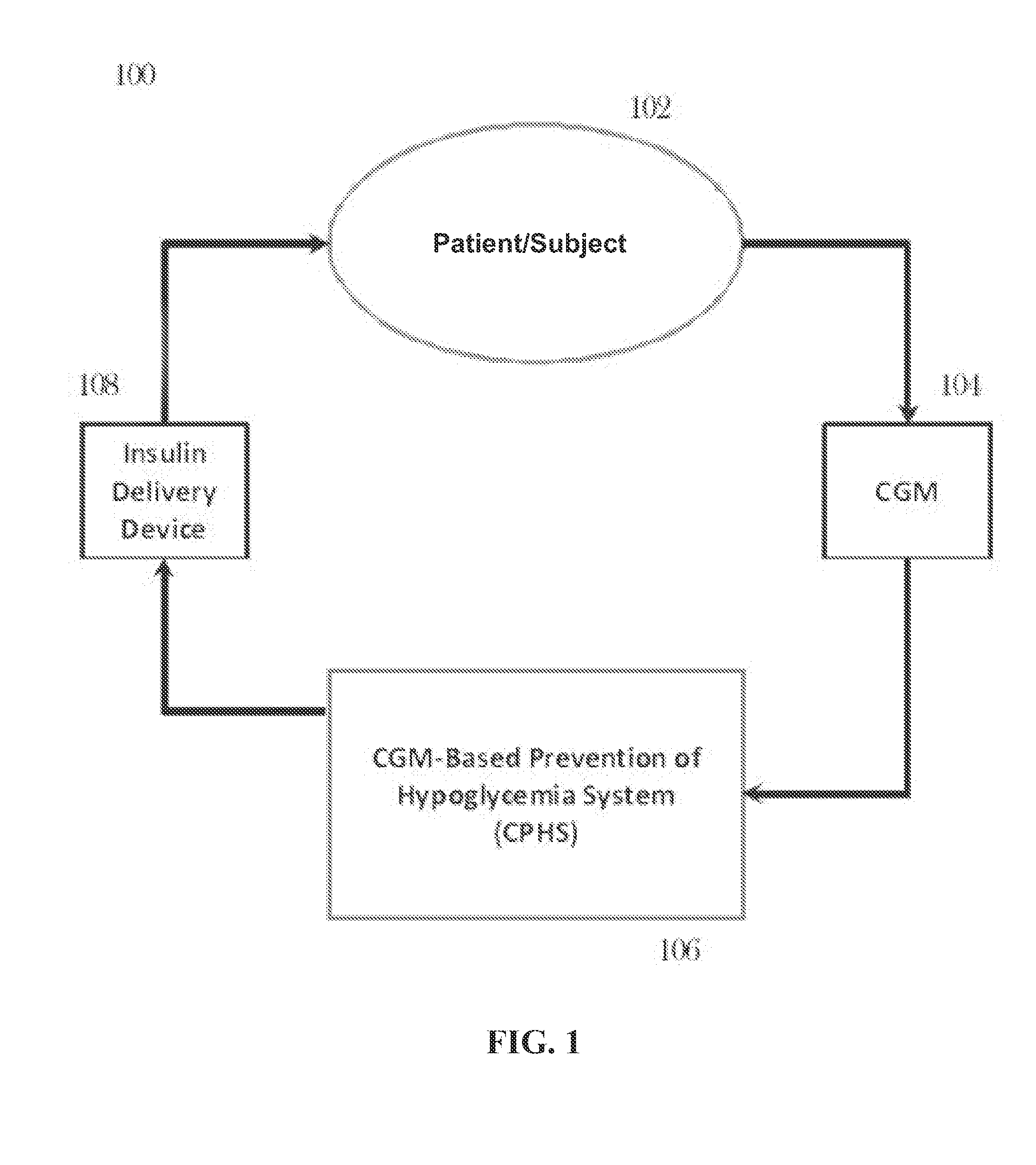
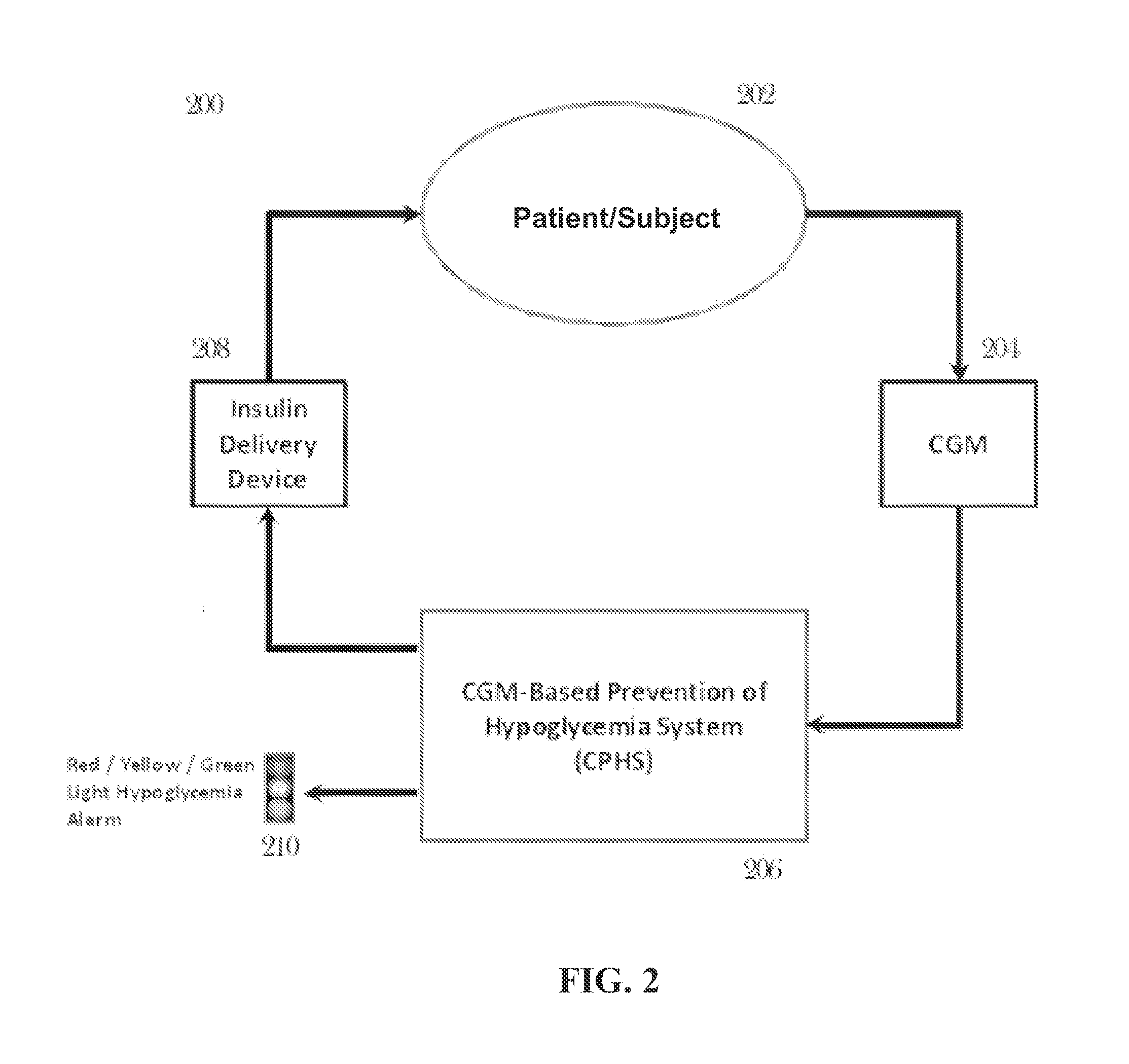
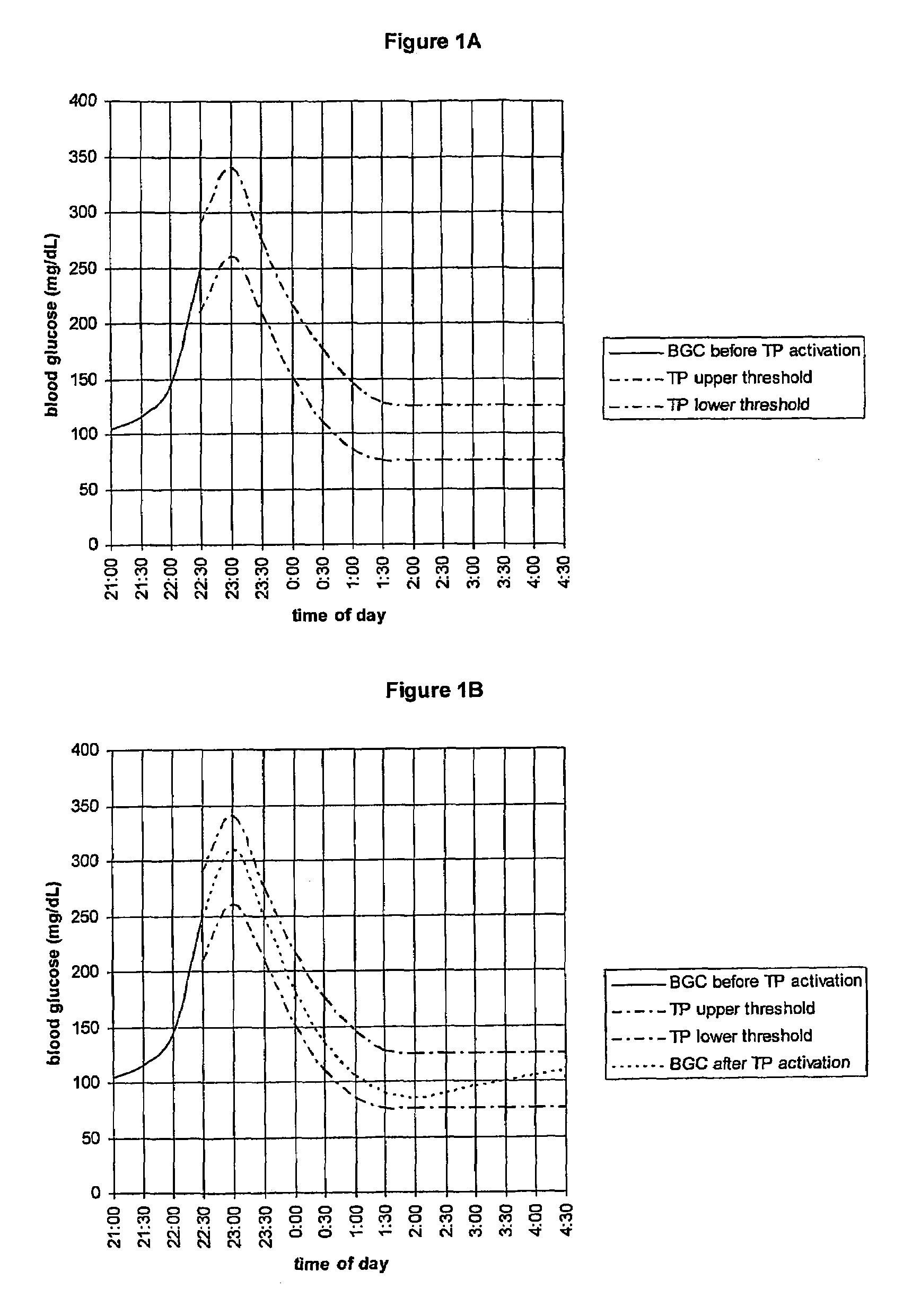
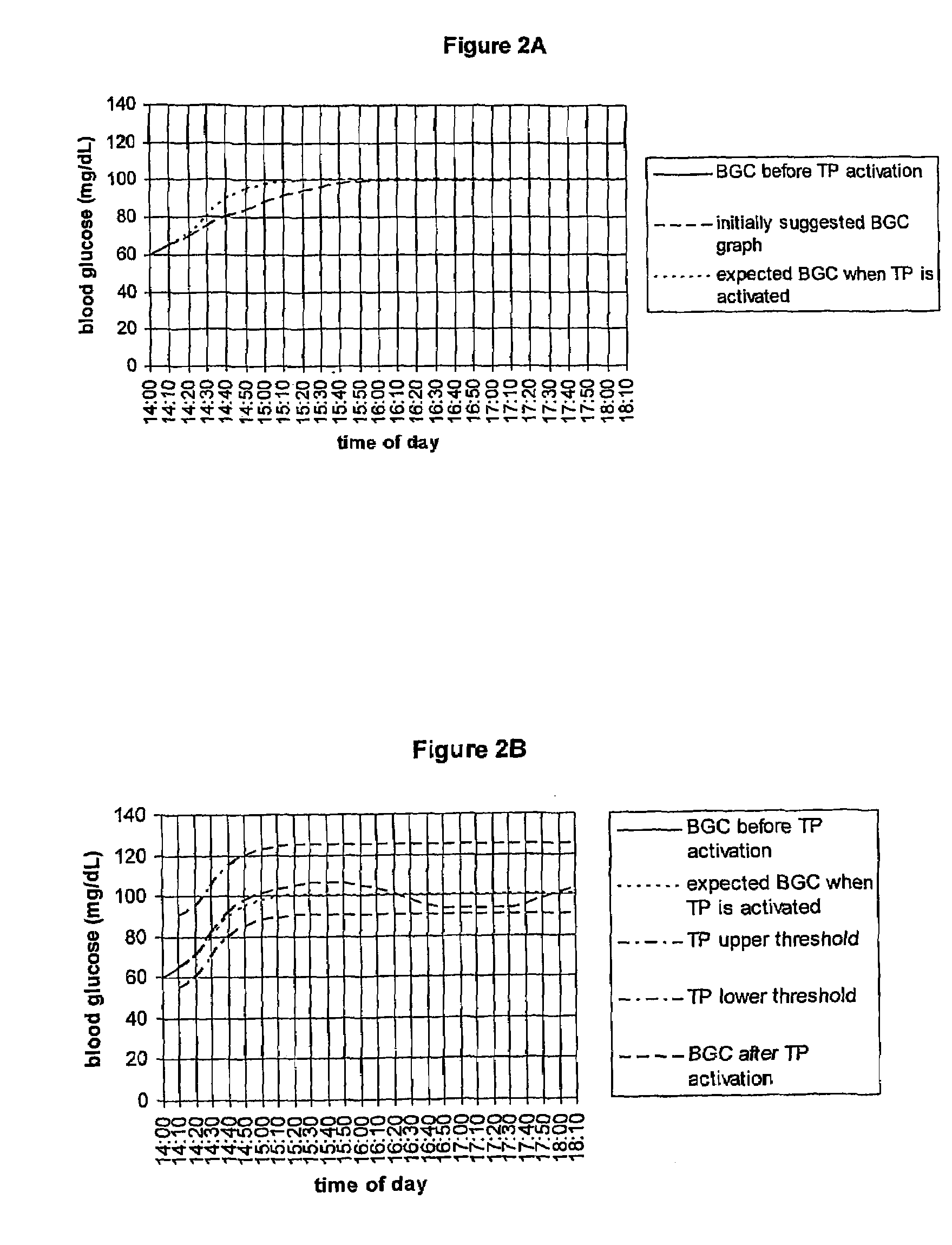
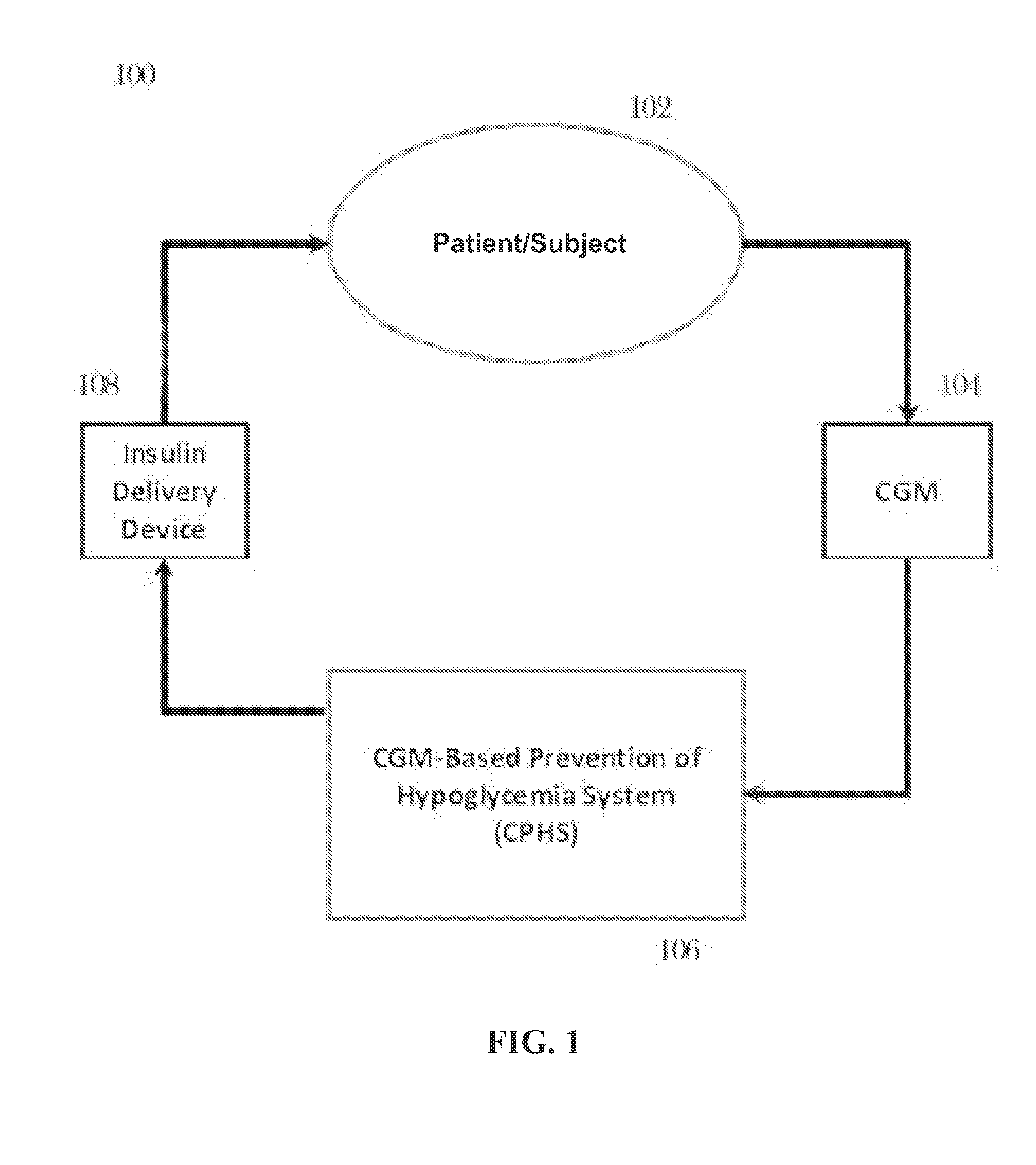
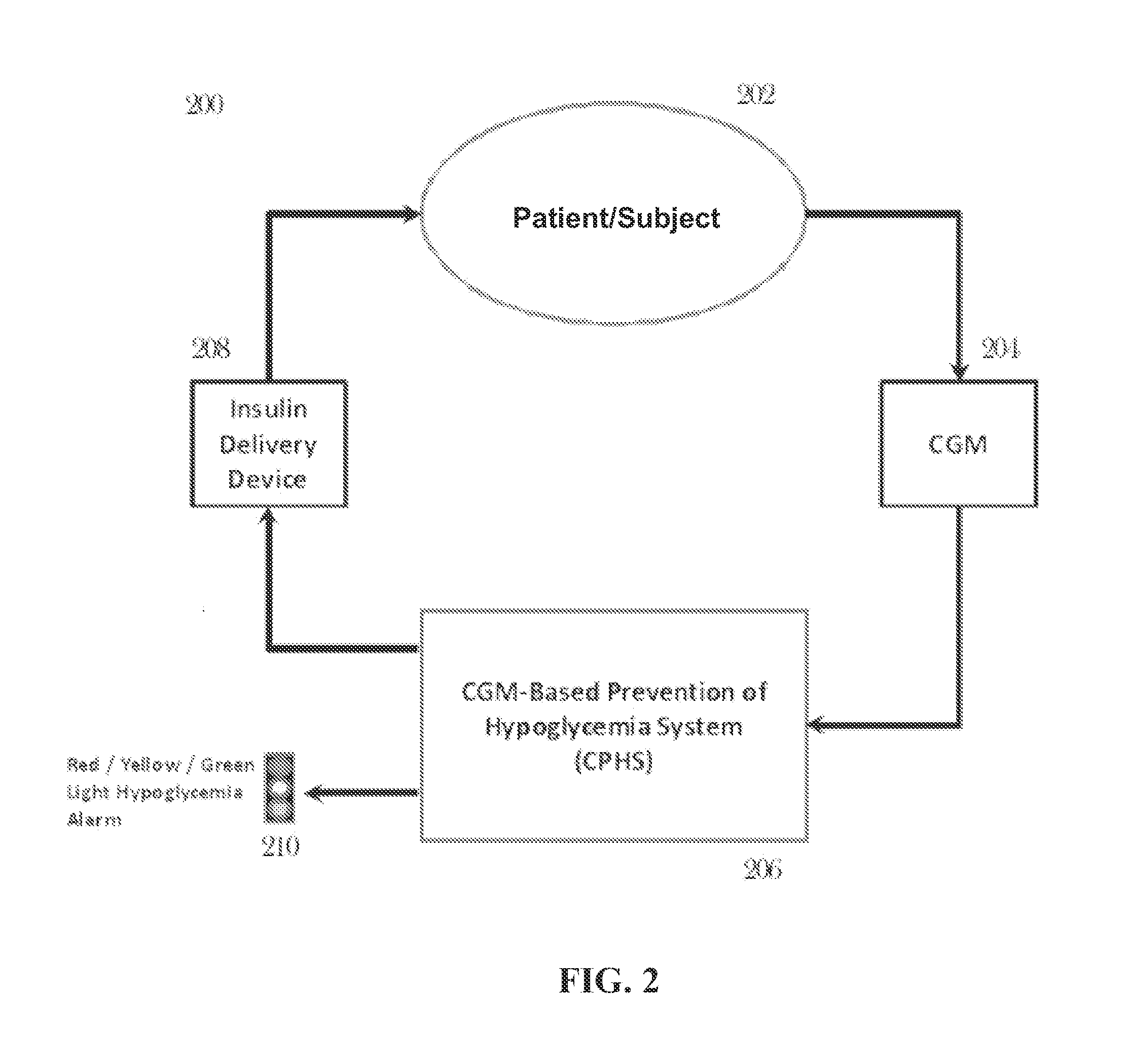
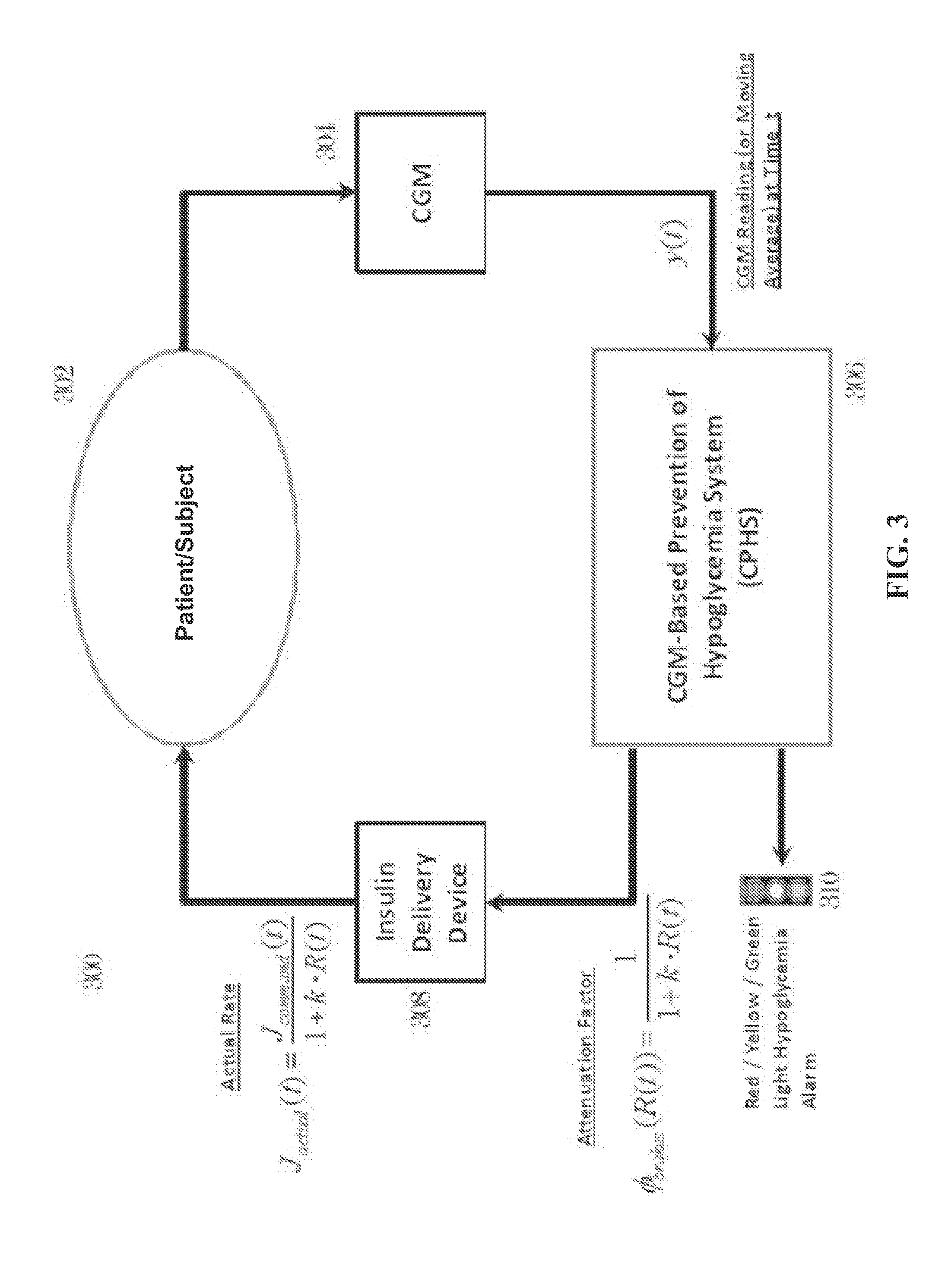
CGM-based prevention of hypoglycemia via hypoglycemia risk assessment and smooth reduction of insulin delivery
ActiveUS8562587B2Avoiding under-insulinizationReduce riskMedical simulationMetabolism disorderMedicineClosed loop
An aspect of an embodiment or partial embodiment of the present invention (or combinations of various embodiments in whole or in part of the present invention) comprises, but not limited thereto, a method and system (and related computer program product) for continually assessing the risk of hypoglycemia for a patient and then determining what action to take based on that risk assessment. A further embodiment results in two outputs: (1) an attenuation factor to be applied to the insulin rate command sent to the pump (either via conventional therapy or via open or closed loop control) and / or (2) a red / yellow / green light hypoglycemia alarm providing to the patient an indication of the risk of hypoglycemia. The two outputs of the CPHS can be used in combination or individually.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Safety features for integrated insulin delivery system
ActiveUS8257300B2Limited hyperglycemiaPreventing hypoglycemiaDrug and medicationsMedical devicesGlucose sensorsEngineering
Safety features are applied to an integrated insulin delivery system to enhance safety while accounting for glucose sensor bias and calibration errors. One safety feature includes comparisons of calibrations of the sensor to nominal sensitivity and taking action, such as limiting insulin delivery or taking a further calibration of the sensor. In another feature, an automatic resumption of a basal delivery rate is programmed into the delivery device to avoid the possibility of complete loss of delivery of insulin in the event that communication with the delivery device is disrupted. Other features include steps taken to avoid hypoglycemia in the event that the sensor is negatively biased.
Owner:ABBOTT DIABETES CARE INC
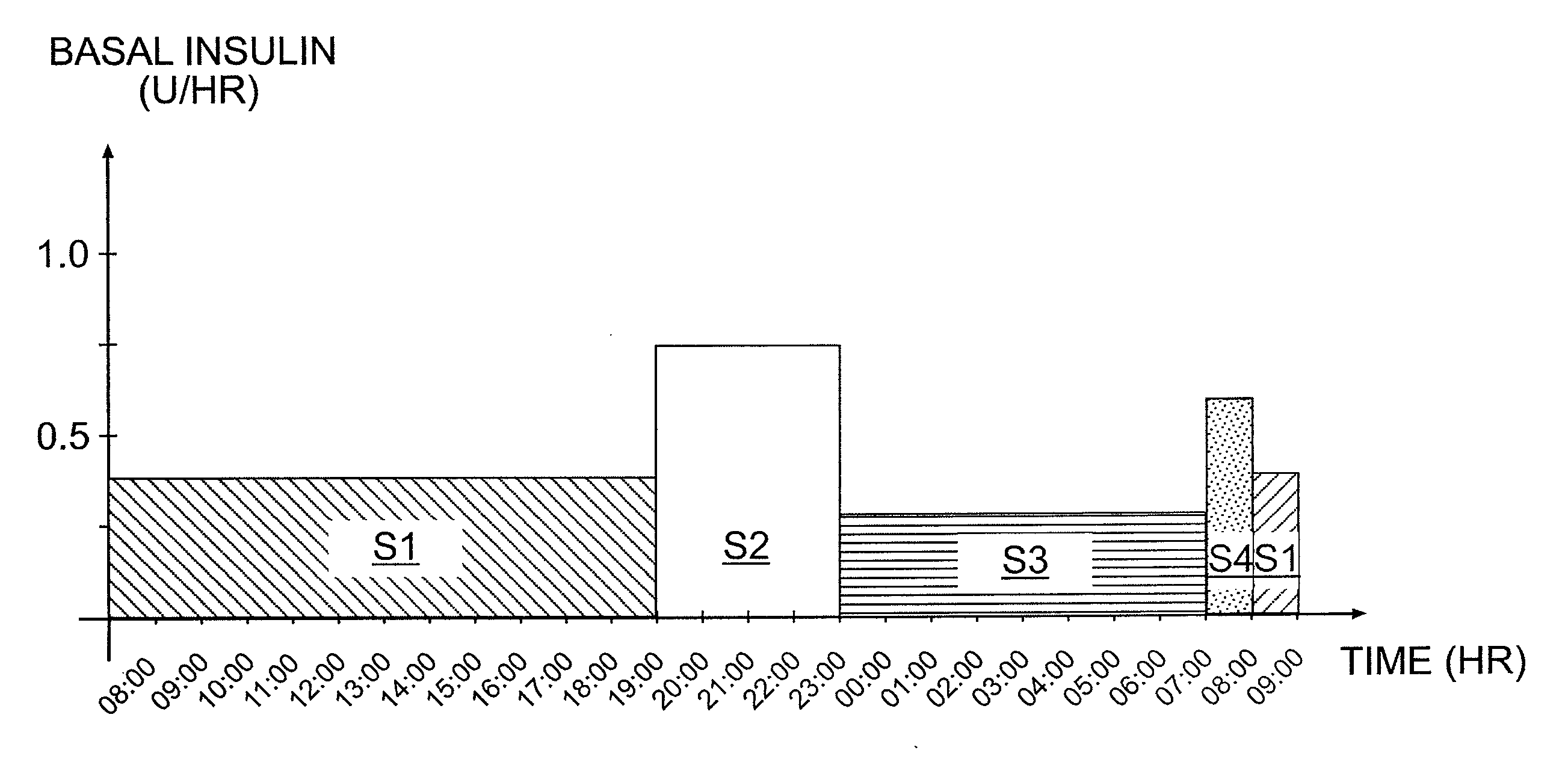
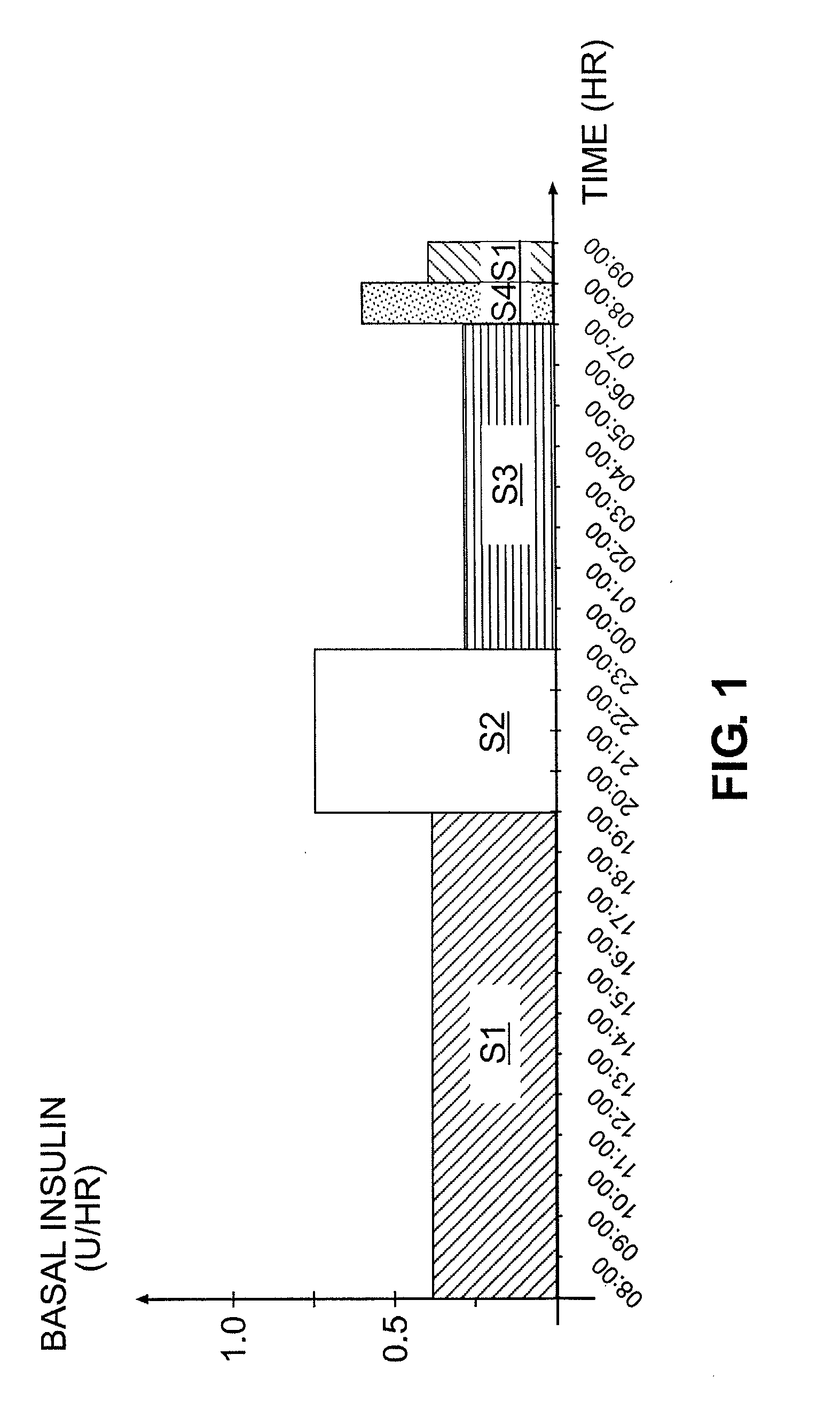
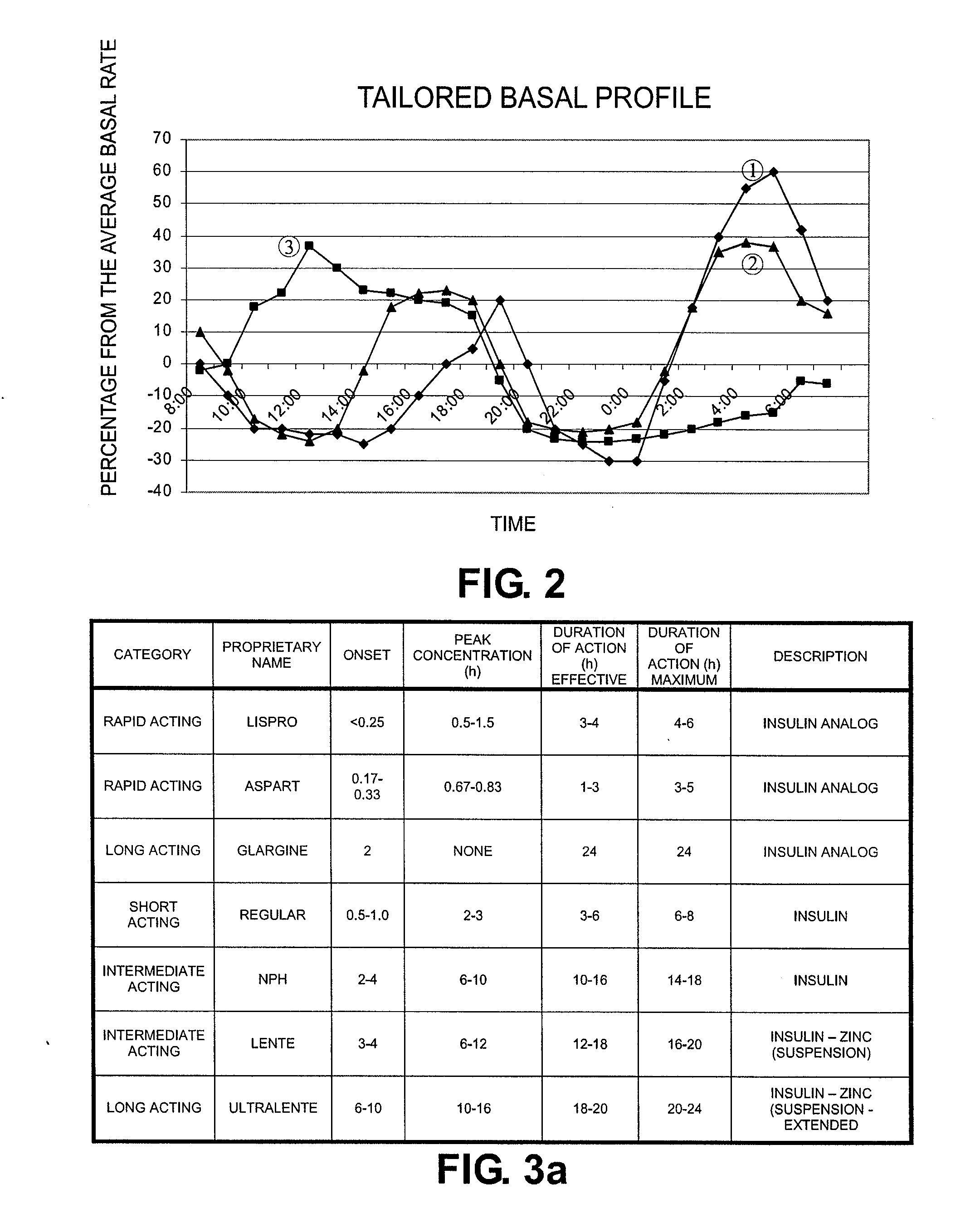
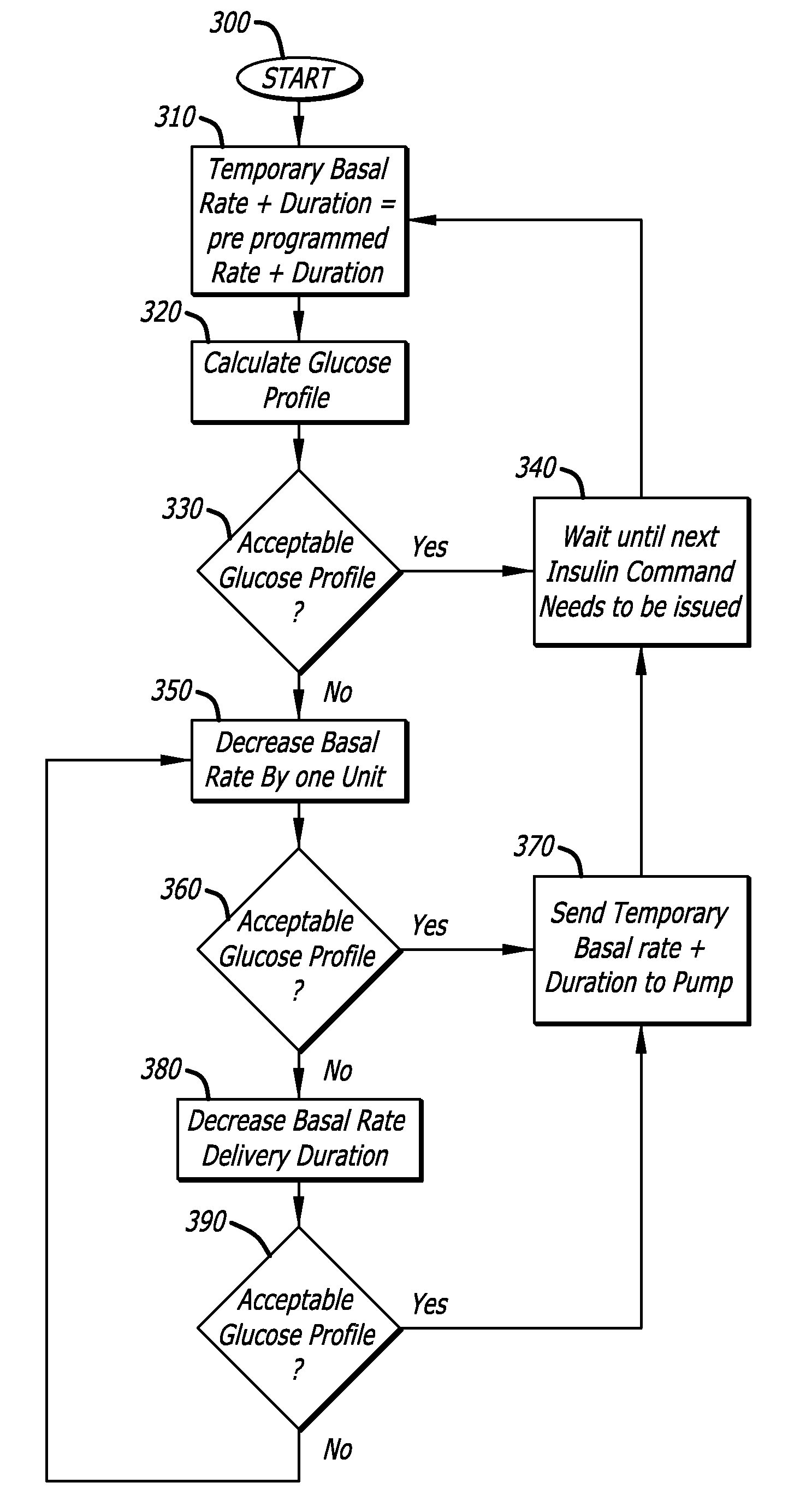
Tailored Basal Insulin Delivery System and Method
ActiveUS20080319384A1Monitor body glucose levelEconomical and cost-effectiveMetabolism disorderHealth-index calculationDelivery systemControlled delivery
Disclosed is a system to deliver insulin to a user's body. The system includes a dispensing unit to deliver the insulin to the body, and a control mechanism to control delivery of basal insulin according to a predetermined basal infusion pattern, the basal infusion pattern selected from a plurality of predetermined basal infusion patterns. In some embodiments, selection of the basal infusion pattern may be based on one or more personal characteristics of the user.
Owner:ROCHE DIABETES CARE INC
Method and system to handle manual boluses or meal events for closed-loop controllers
ActiveUS20140005633A1Negates portionReduce the impactDrug and medicationsMedical devicesGlucose sensorsDiabetes management
Described and illustrated is a diabetes management system that includes an infusion pump, glucose sensor and controller with a method programmed into the controller. The infusion pump is configured to deliver insulin to a subject. The glucose sensor is configured to sense glucose levels in the subject and provide output signals representative of the glucose levels in the subject. The controller is programmed to receive signals from at least one of the glucose sensor and the pump and configured to issue signals to the pump to deliver an amount of insulin determined by a feedback controller that utilizes a model predictive control and also configured to deliver at least the basal amount of insulin whenever the subject has initiated a manual bolus of insulin and a sensed or measured glucose level is at least a first threshold within a first duration of time.
Owner:JDRF INT +1
Adaptive insulin delivery system
A proactive system and method in which levels of glucose are monitored after a meal signal and compared to a safe range. If a monitored glucose level is outside the safe range, a post-prandial vertex of the glucose level is identified and an action is provided to more rapidly return the glucose level to a target level within the safe range than if no action was provided. In another aspect a control parameter in an IDM system is adjusted by determining a performance metric of the system as a function of the levels of glucose and a medication administration signal over a first window of time; and, if the performance metric is outside an expected range, adjusting the control parameter to adjust an amount of medication and to bring the performance metric inside the expected range.
Owner:ABBOTT DIABETES CARE INC
Overnight closed-loop insulin delivery with model predictive control and glucose measurement error model
A closed-loop system for insulin infusion overnight uses a model predictive control algorithm (“MPC”). Used with the MPC is a glucose measurement error model which was derived from actual glucose sensor error data. That sensor error data included both a sensor artifacts component, including dropouts, and a persistent error component, including calibration error, all of which was obtained experimentally from living subjects. The MPC algorithm advised on insulin infusion every fifteen minutes. Sensor glucose input to the MPC was obtained by combining model-calculated, noise-free interstitial glucose with experimentally-derived transient and persistent sensor artifacts associated with the FreeStyle Navigator® Continuous Glucose Monitor System (“FSN”). The incidence of severe and significant hypoglycemia reduced 2300- and 200-fold, respectively, during simulated overnight closed-loop control with the MPC algorithm using the glucose measurement error model suggesting that the continuous glucose monitoring technologies facilitate safe closed-loop insulin delivery.
Owner:CAMBRIDGE ENTERPRISE LTD +1
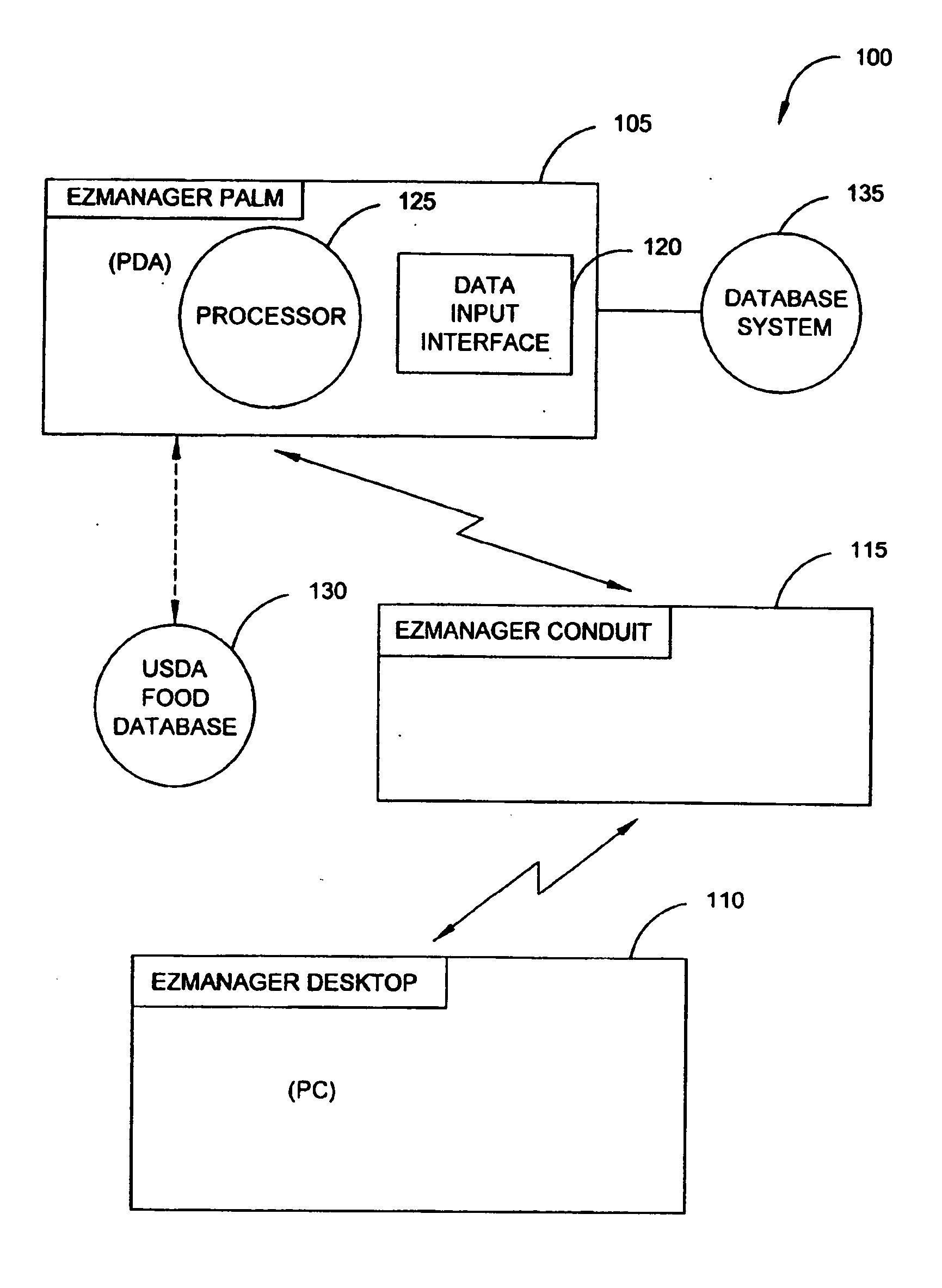
System and method for managing diabetes
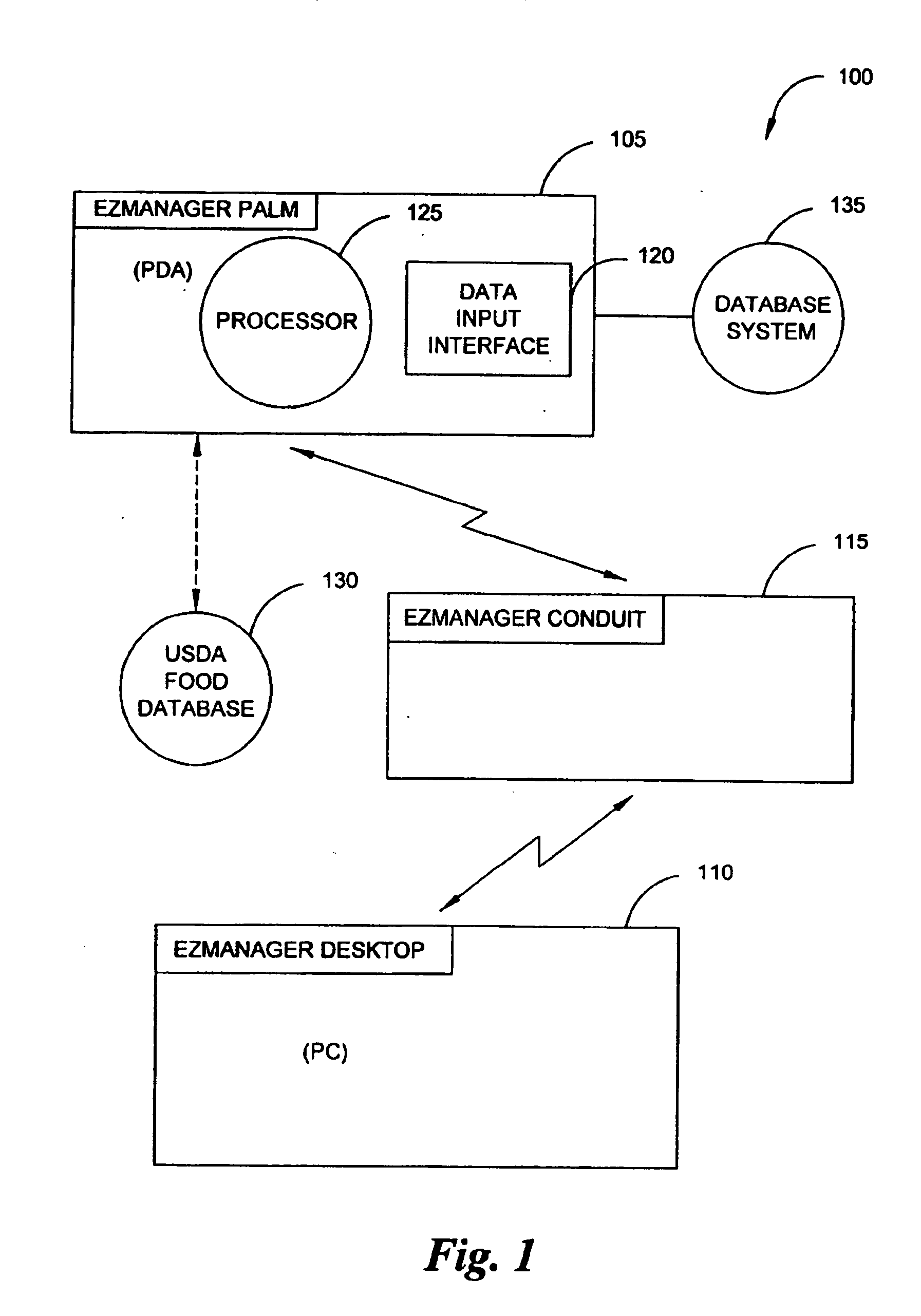
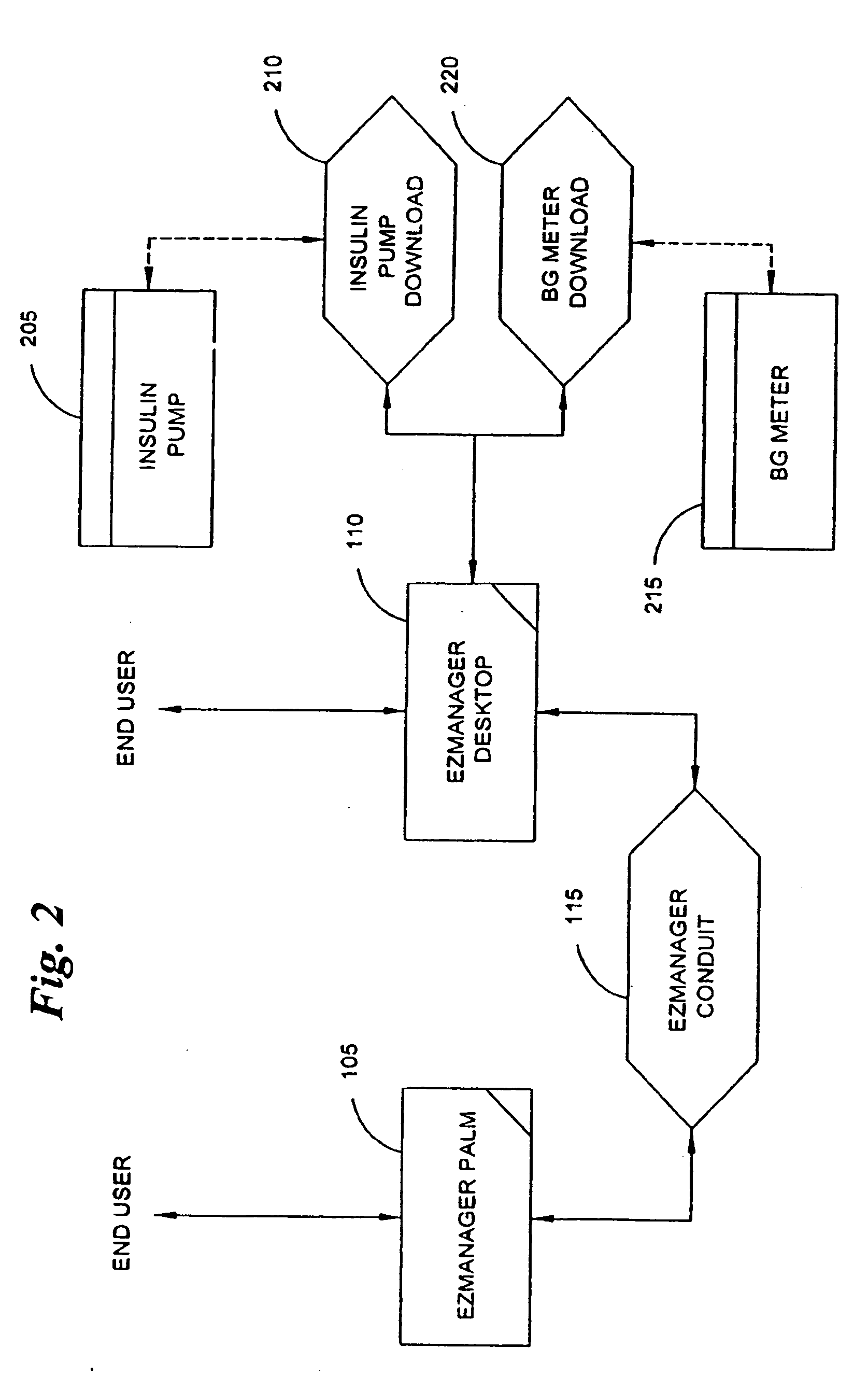
A diabetes management system, an infusion pump, and methods for managing a blood glucose level of a diabetes patient. The infusion pump includes a processor for monitoring an amount of insulin delivered to a patient and an internal database in communication with the processor. The internal database is for storing food information. The diabetes management system includes a blood glucose monitor, a food database, and an infusion pump for delivering insulin to a patient. The food database comprises one or more records of information on various foods. The infusion pump of the diabetes management system includes (i) a processor for monitoring an amount of insulin delivered to the patient and (ii) a second database. The second database is for receiving at least a portion of the information from the food database.
Owner:CROTHALL KATHERINE D +2
Method and system for a hybrid control-to-target and control-to-range model predictive control of an artificial pancreas
InactiveUS20140180240A1Medical simulationDrug and medicationsGlucose sensorsModel predictive control
Described and illustrated is a system for management of diabetes that includes an infusion pump, glucose sensor and controller with a method programmed into the controller. The infusion pump is configured to deliver insulin to a subject. The glucose sensor is configured to sense glucose levels in the subject and provide output signals representative of the glucose levels in the subject. The controller is programmed to switchover from one mode of MPC control based on a predetermined range of blood glucose values to another MPC mode based on a predetermined target.
Owner:JDRF INT +1
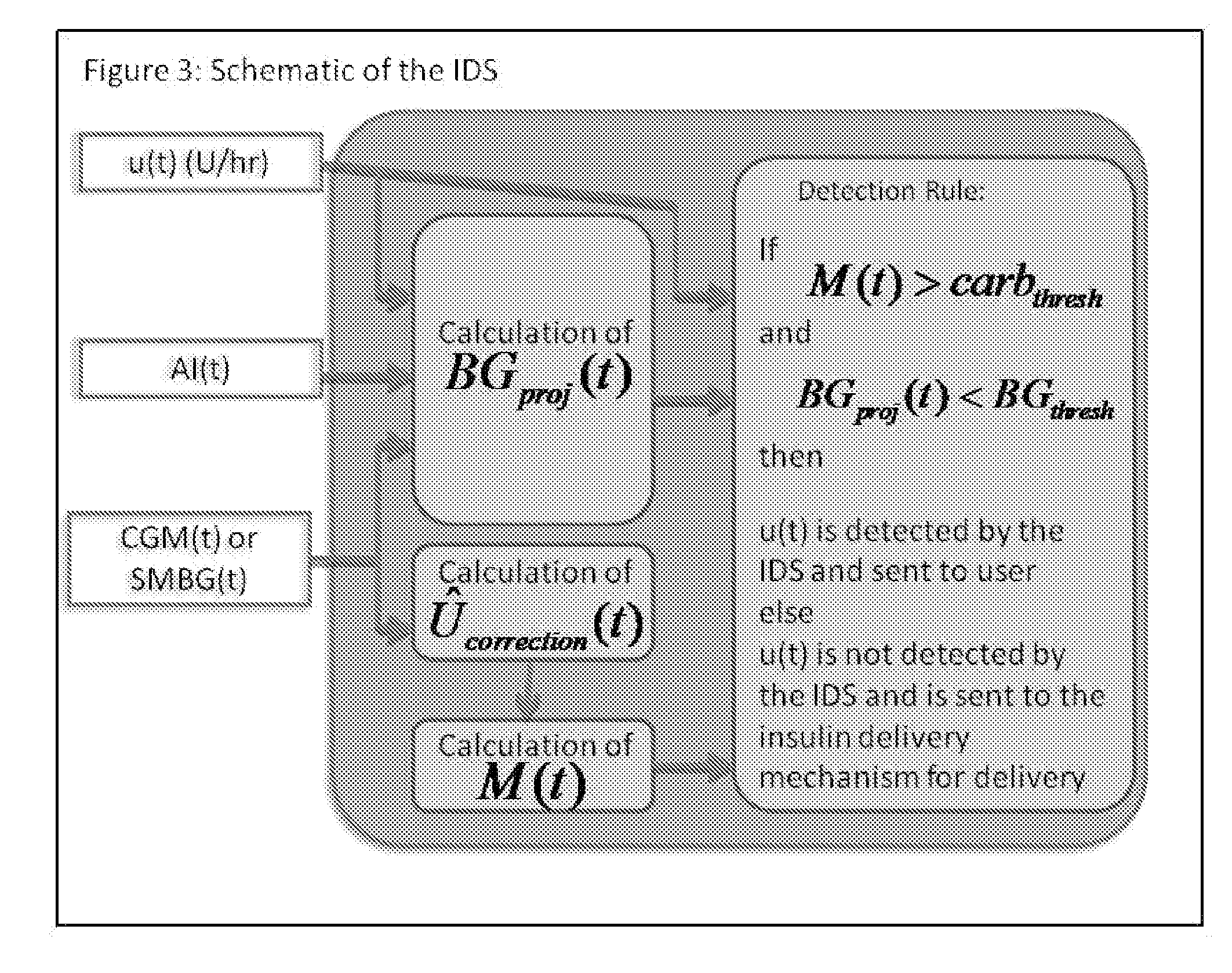
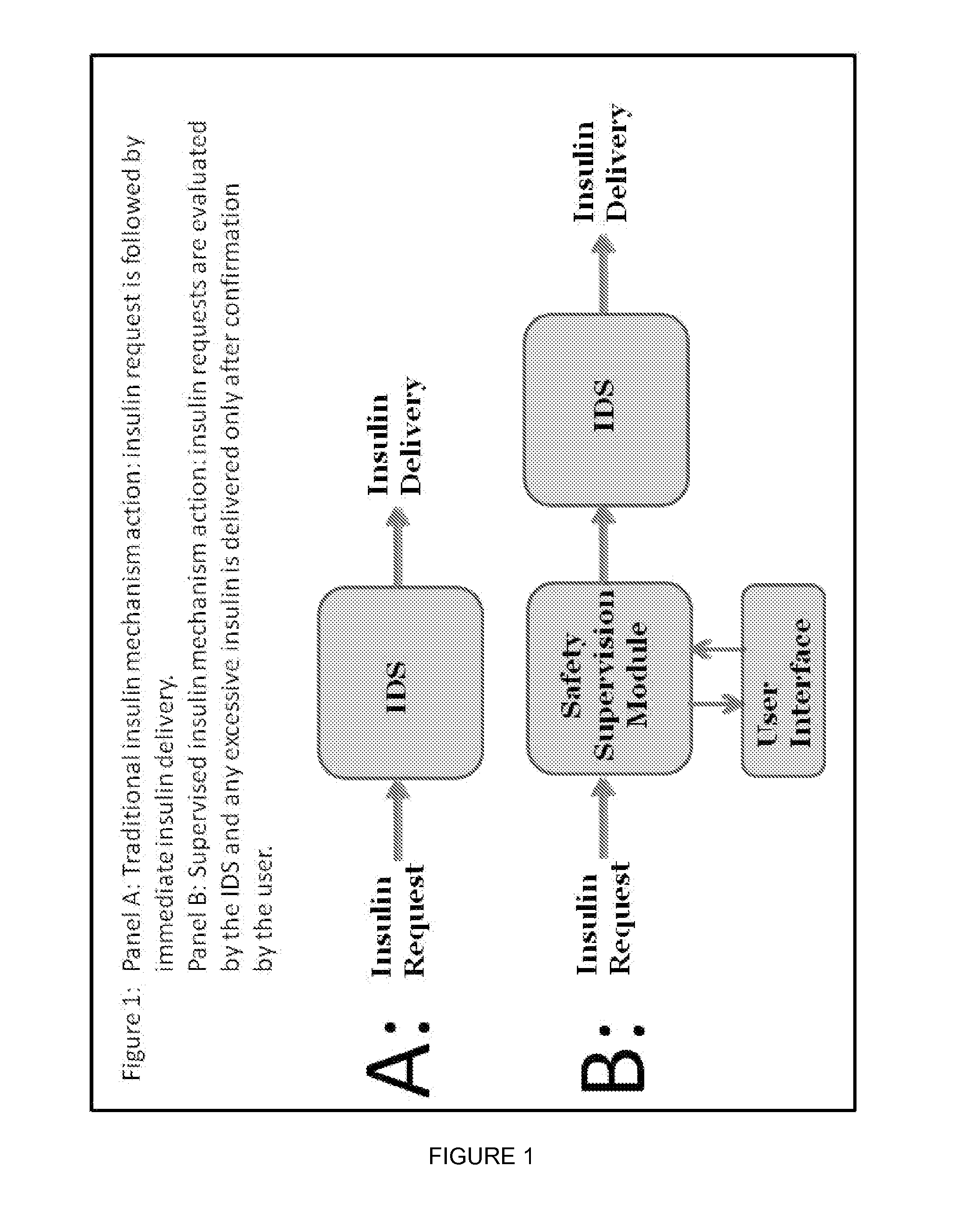
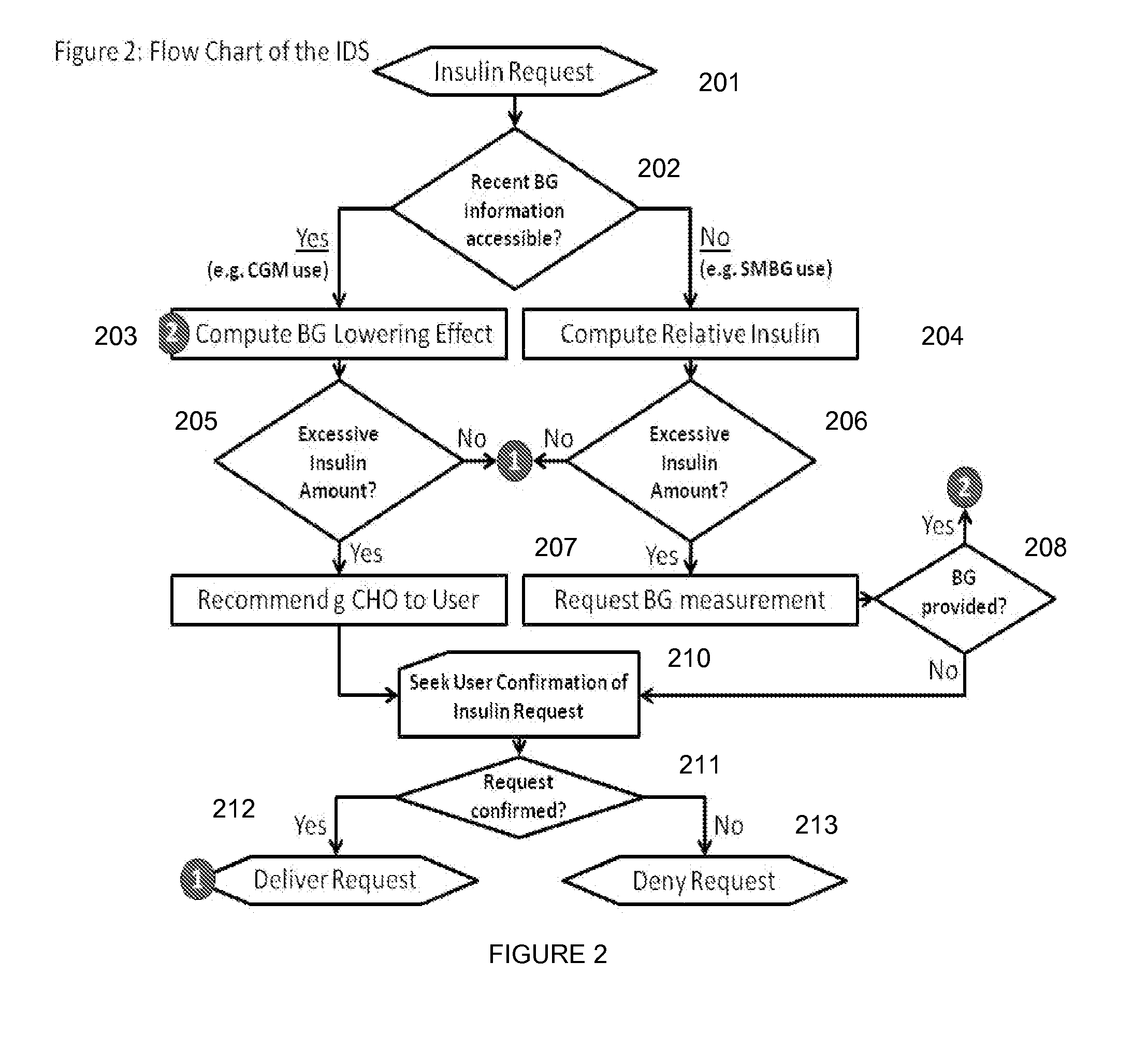
Method and System For The Safety, Analysis and Supervision of Insulin Pump Action and Other Modes of Insulin Delivery in Diabetes
ActiveUS20130116649A1Improve securityHealth-index calculationDrug and medicationsInsulin pumpGlycemic
An insulin delivery supervisor (IDS) with a safety analysis and supervision function that can reside between the insulin request and the insulin delivery and can intercept any excessive insulin requests before the insulin was delivered. The IDS can be implemented in any system based on insulin pump or pen and will work with either SMBG or CGM modes of blood glucose monitoring.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Monitoring device for management of insulin delivery
ActiveUS20120246106A1Minimize changesDrug and medicationsMedical devicesInsulin activityTreatment management
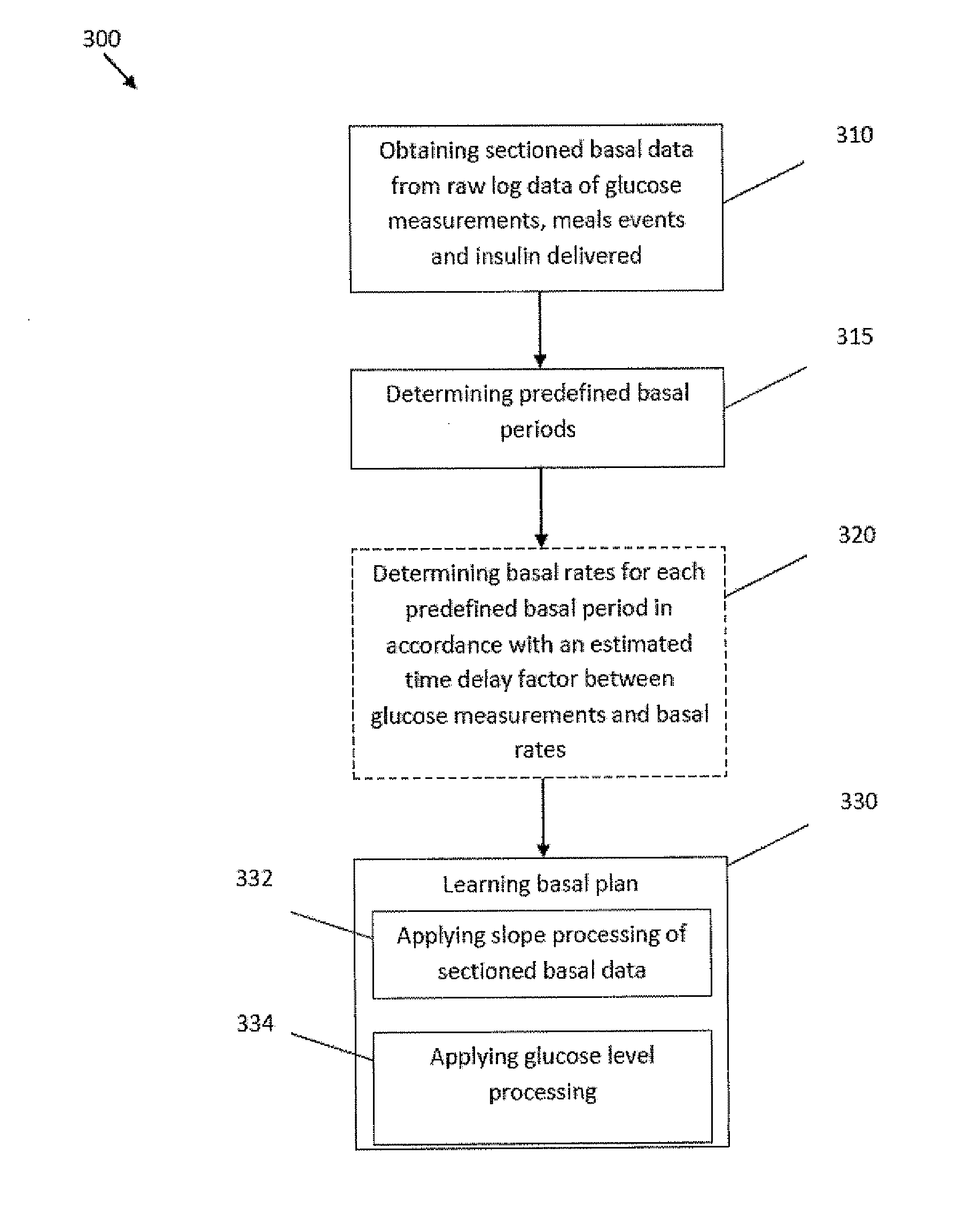
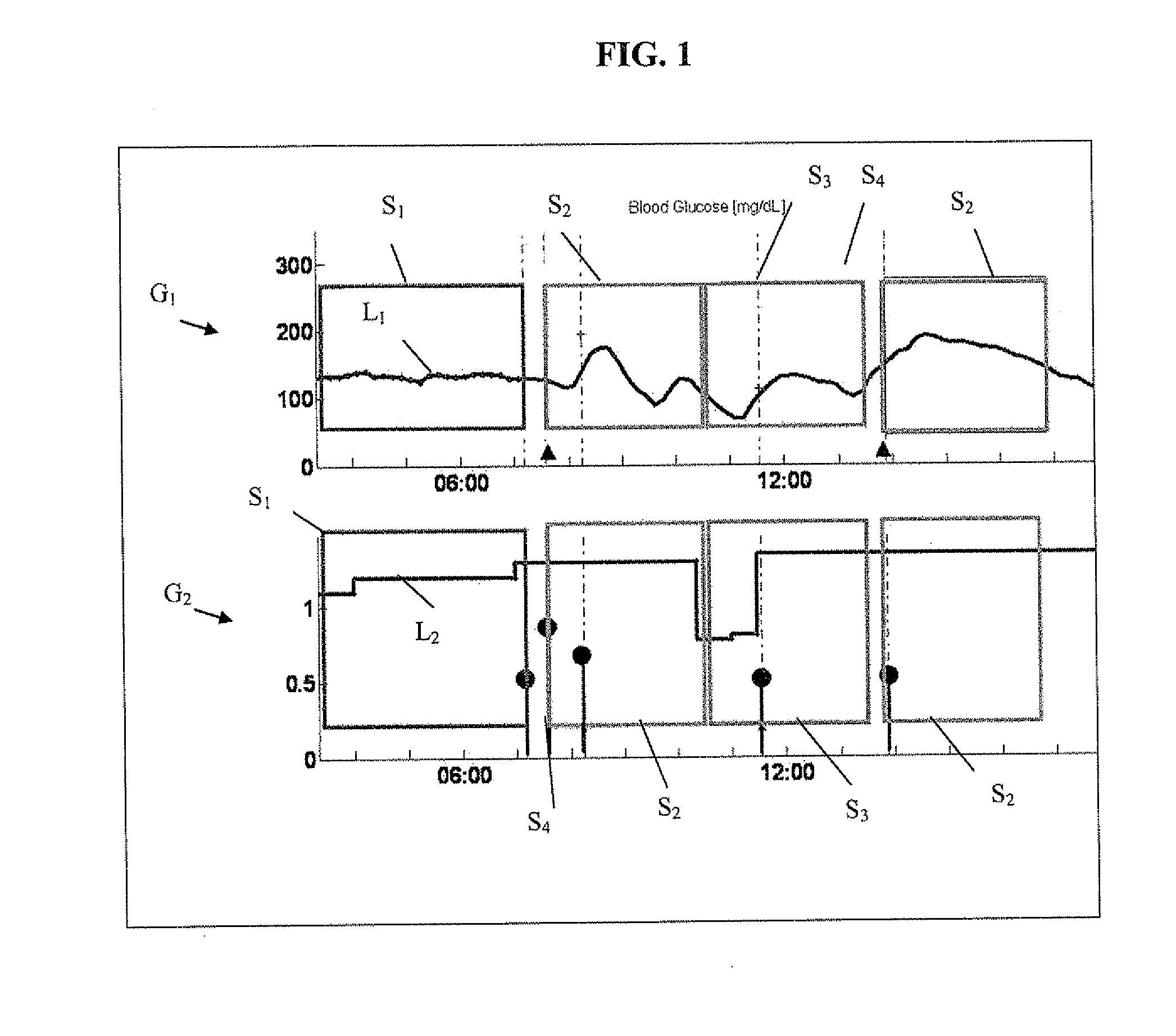
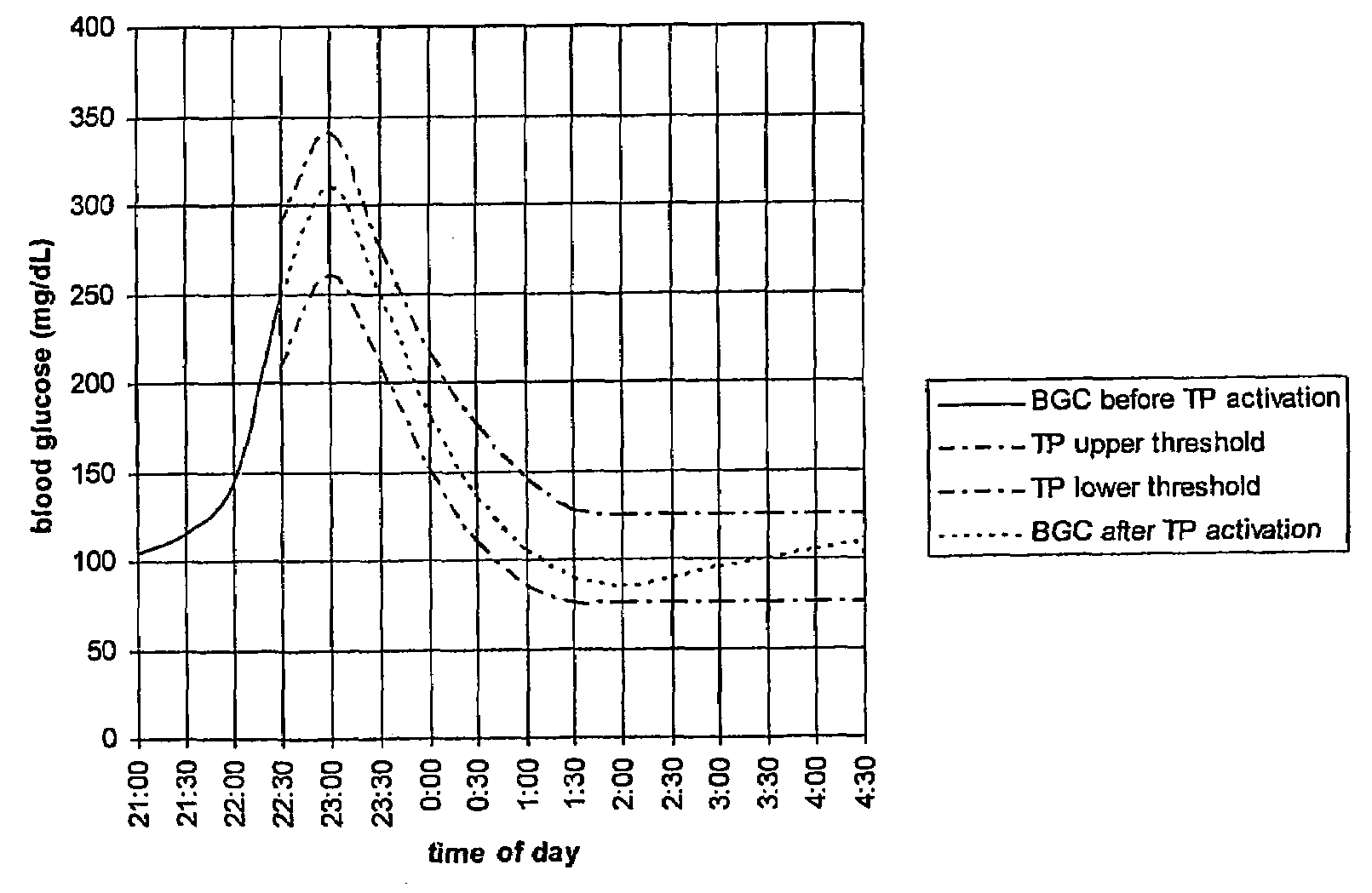
Monitoring system and method for use with diabetic treatment management. The system includes: a communication interface configured to permit access to stored raw log data, obtained over a certain time, being indicative of glucose measurements, meals consumed and insulin delivery; and a control unit including an unsupervised learning controller configured to receive and process said raw log data and determine at least one global insulin pump setting of basal rate, correction factor, carbohydrate ratio and insulin activity curve parameters. The system may include a processing unit including a first processor for processing measured data indicative of blood glucose level and generating first processed data, a second processor including at least one fuzzy logic module which receives input parameters corresponding to the measured data, the first processed data and a reference data, and processes the data to produce a qualitative output parameter to determine whether any treatment parameter should be modified.
Owner:DREAMED DIABETES
Fluctuating blood glucose notification threshold profiles and methods of use
Embodiments of the present invention provide a new system and methods for monitoring blood glucose concentration. A user of a continuous glucose monitor may program upper and lower blood glucose notification thresholds to fluctuate over time in order to facilitate management of the short-term effects of food consumption, insulin delivery aberrations, physical activity, emotions, and unforeseen circumstances.
Owner:SHER PHILIP M
CGM-Based Prevention of Hypoglycemia Via Hypoglycemia Risk Assessment and Smooth Reduction of Insulin Delivery
ActiveUS20120059353A1Reduced level of insulinAvoiding under-insulinizationDrug and medicationsMedical devicesMedicineClosed loop
An aspect of an embodiment or partial embodiment of the present invention (or combinations of various embodiments in whole or in part of the present invention) comprises, but not limited thereto, a method and system (and related computer program product) for continually assessing the risk of hypoglycemia for a patient and then determining what action to take based on that risk assessment. A further embodiment results in two outputs: (1) an attenuation factor to be applied to the insulin rate command sent to the pump (either via conventional therapy or via open or closed loop control) and / or (2) a red / yellow / green light hypoglycemia alarm providing to the patient an indication of the risk of hypoglycemia. The two outputs of the CPHS can be used in combination or individually.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Usability features for integrated insulin delivery system
ActiveUS8597274B2Easy to useEnhancing alarm avoidanceDrug and medicationsMedical devicesUsabilityIntensive care medicine
Owner:ABBOTT DIABETES CARE INC
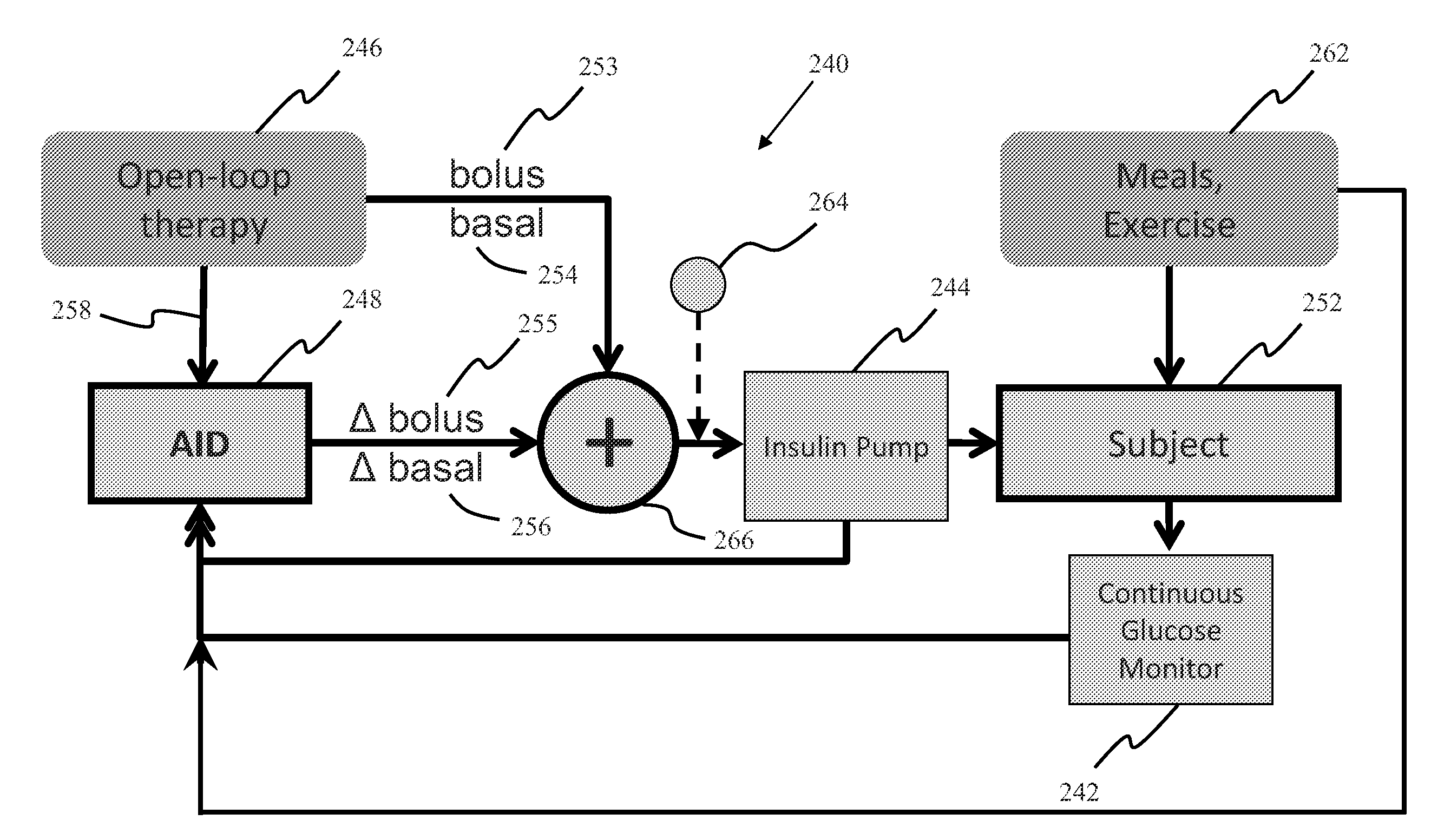
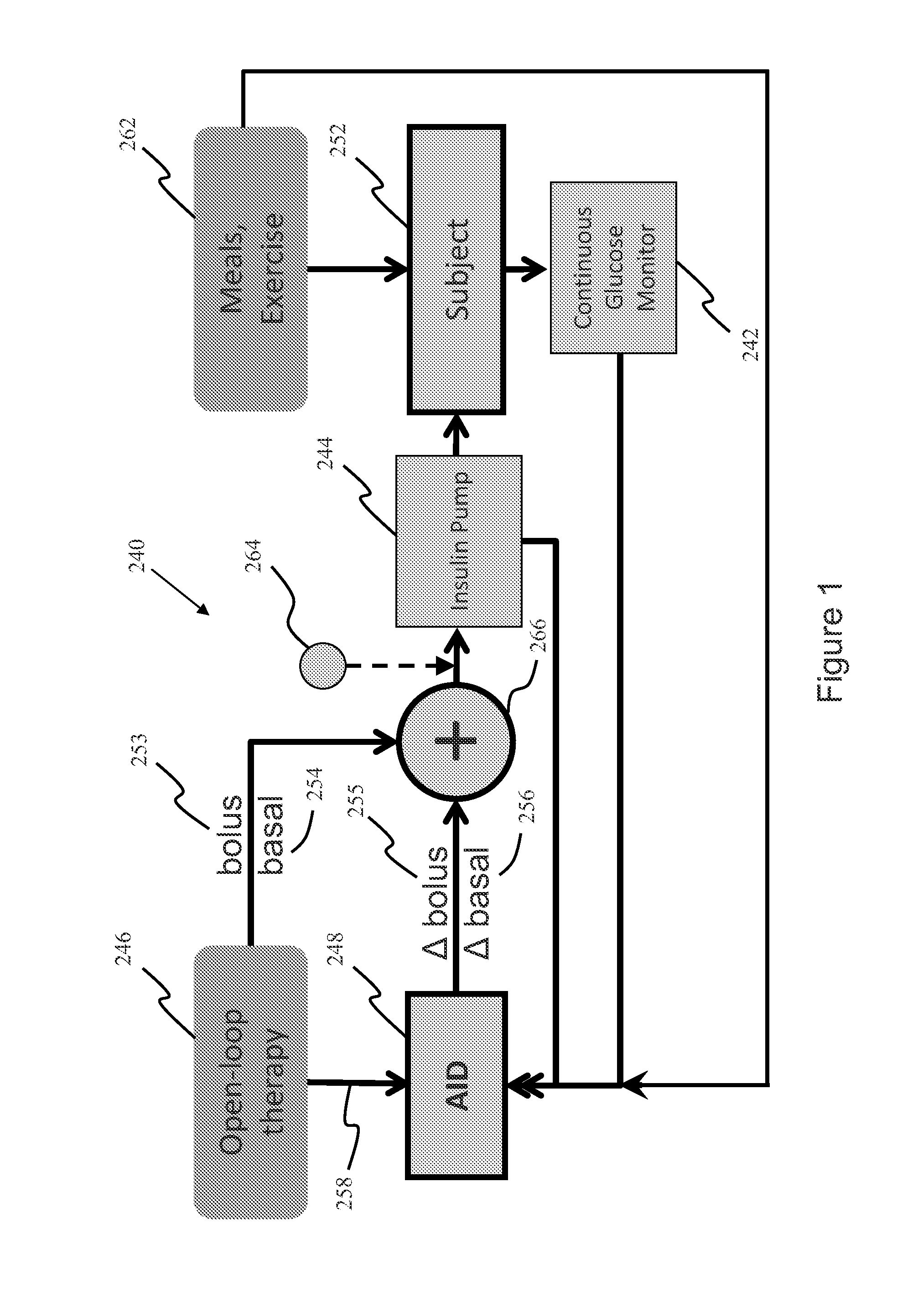
System, Method and Computer Program Product For Adjustment of Insulin Delivery in Diabetes Using Nominal Open-Loop Profiles
InactiveUS20120245556A1Medical simulationPhysical therapies and activitiesGlycemicBlood glucose status
A method, system and computer program product for correcting a nominal treatment strategy of a subject with diabetes. The method, system and computer program product may be configured for providing input whereby the input may include: open-loop therapy settings for the subject, data about glycemic state of the subject; and (optionally) data about meals and / or exercise of the subject. The method, system and computer program product may be configured for providing output, whereby the out-put may include an adjustment (correction) to the open-loop therapy settings for the subject for insulin delivery to the subject.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
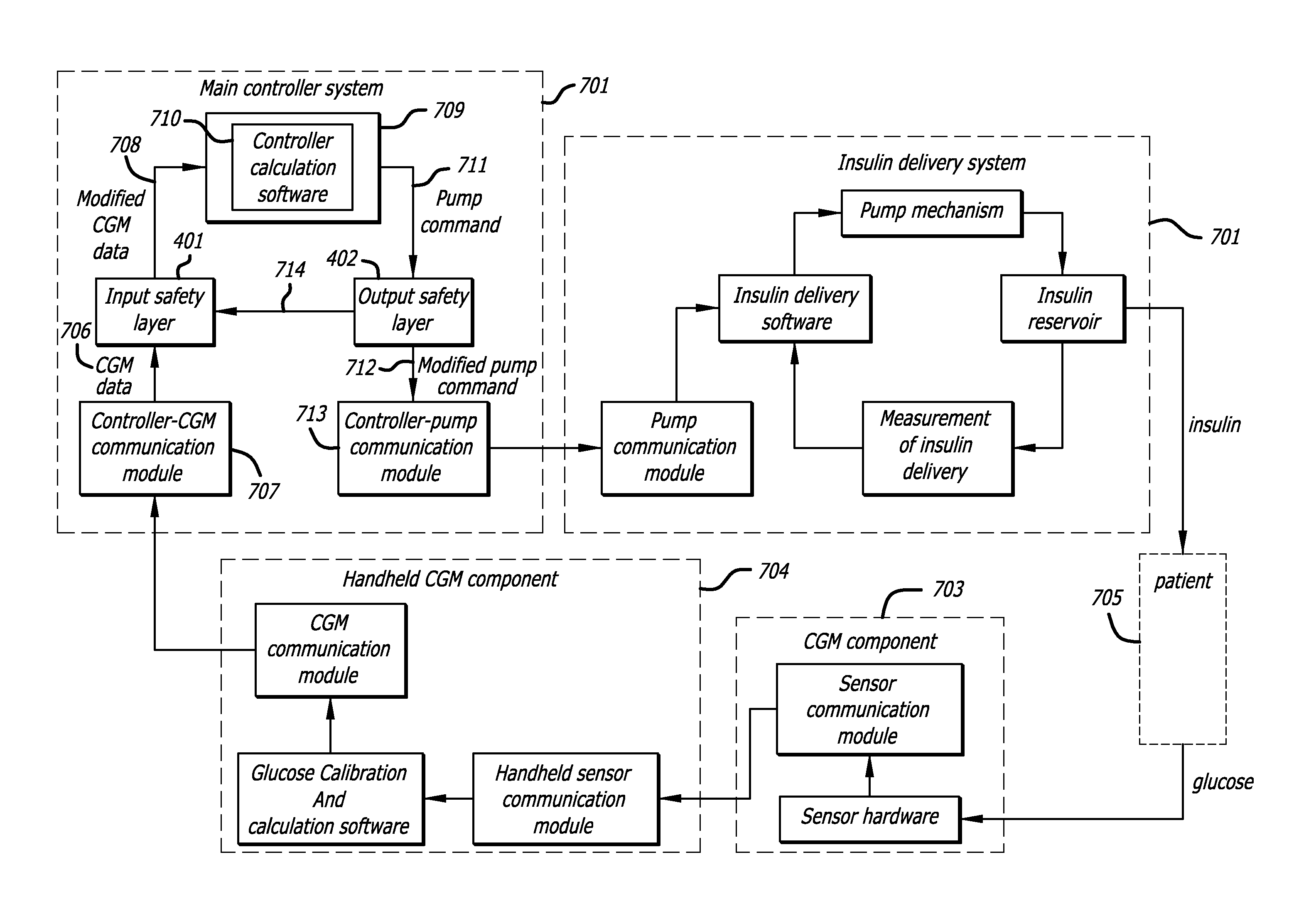
Safety layer for integrated insulin delivery system
An integrated diabetes management (IDM) system includes a safety layer which, in one configuration has two components, one located between a glucose sensor and a controller and a second component located between a controller and a pump, to monitor various aspects of signals and modify those signals for compatibility and safety purposes. In one application, the safety layer receives output control signals from a controller and modifies those control signals as a function of an actual amount of insulin delivered to the user. The safety layer allows for an increased level of safety in the IDM system and permits development of separate hardware and software upgrades with the safety layer assuring that compatibility between components will continue.
Owner:ABBOTT DIABETES CARE INC
Method, System and Computer Readable Medium for Adaptive and Advisory Control of Diabetes
ActiveUS20150190098A1Reducing basal rateMedical simulationPhysical therapies and activitiesPhysiologyInsulin pump
An Adaptive Advisory Control (AA Control) interactive process involving algorithm-based assessment and communication of physiologic and behavioral parameters and patterns assists patients with diabetes with the optimization of their glycemic control. The method and system may uses all available sources of information about the patient; (i) EO Data (e.g. self-monitoring of blood glucose (SMBG) and CMG), (ii) Insulin Data (e.g. insulin pump log files or patient treatment records), and (iii) Patient Self Reporting Data (e.g. self treatment behaviors, meals, and exercise) to: retroactively assess the risk of hypoglycemia, retroactively assess risk-based reduction of insulin delivery, and then report to the patient how a risk-based insulin reduction system would have acted consistently to prevent hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Safety layer for integrated insulin delivery system
An integrated diabetes management (IDM) system includes a safety layer which, in one configuration has two components, one located between a glucose sensor and a controller and a second component located between a controller and a pump, to monitor various aspects of signals and modify those signals for compatibility and safety purposes. In one application, the safety layer receives output control signals from a controller and modifies those control signals as a function of an actual amount of insulin delivered to the user. The safety layer allows for an increased level of safety in the IDM system and permits development of separate hardware and software upgrades with the safety layer assuring that compatibility between components will continue.
Owner:ABBOTT DIABETES CARE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com