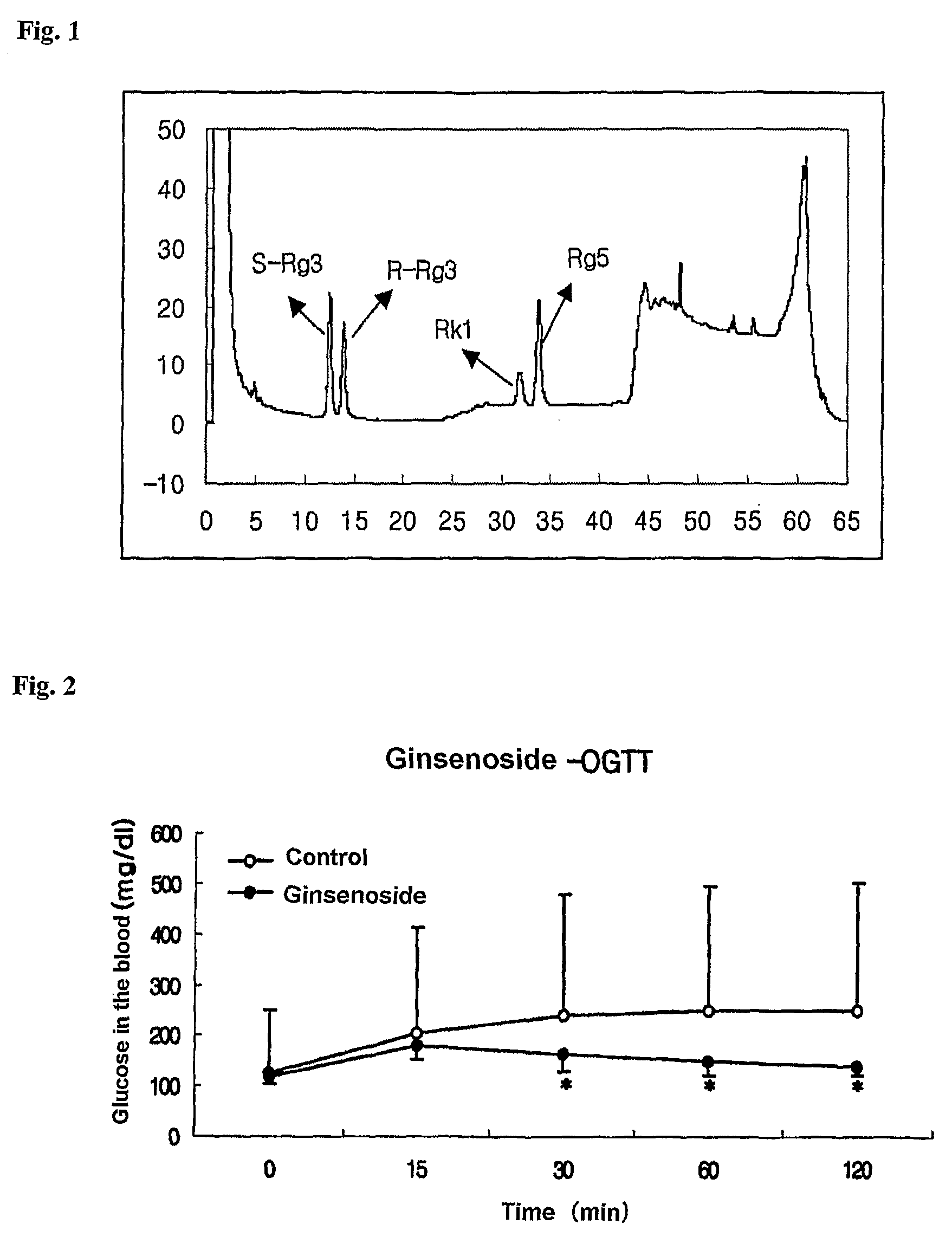
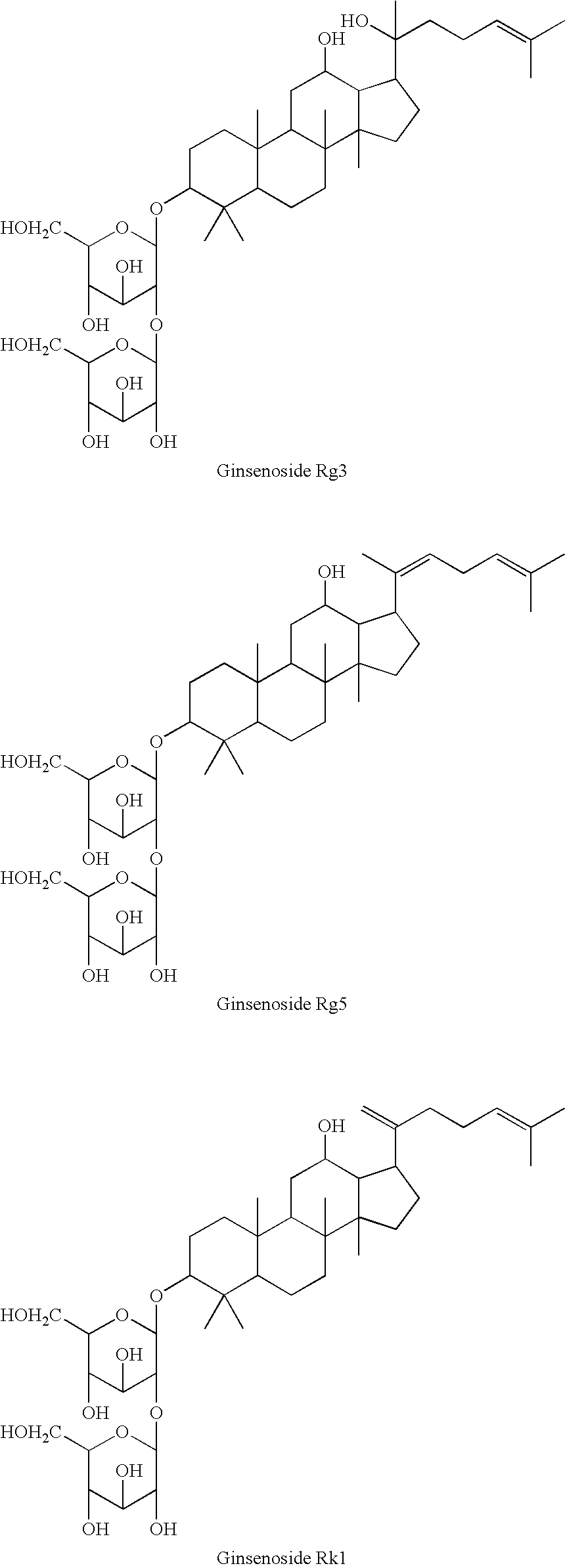
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
204 results about "Glucose control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
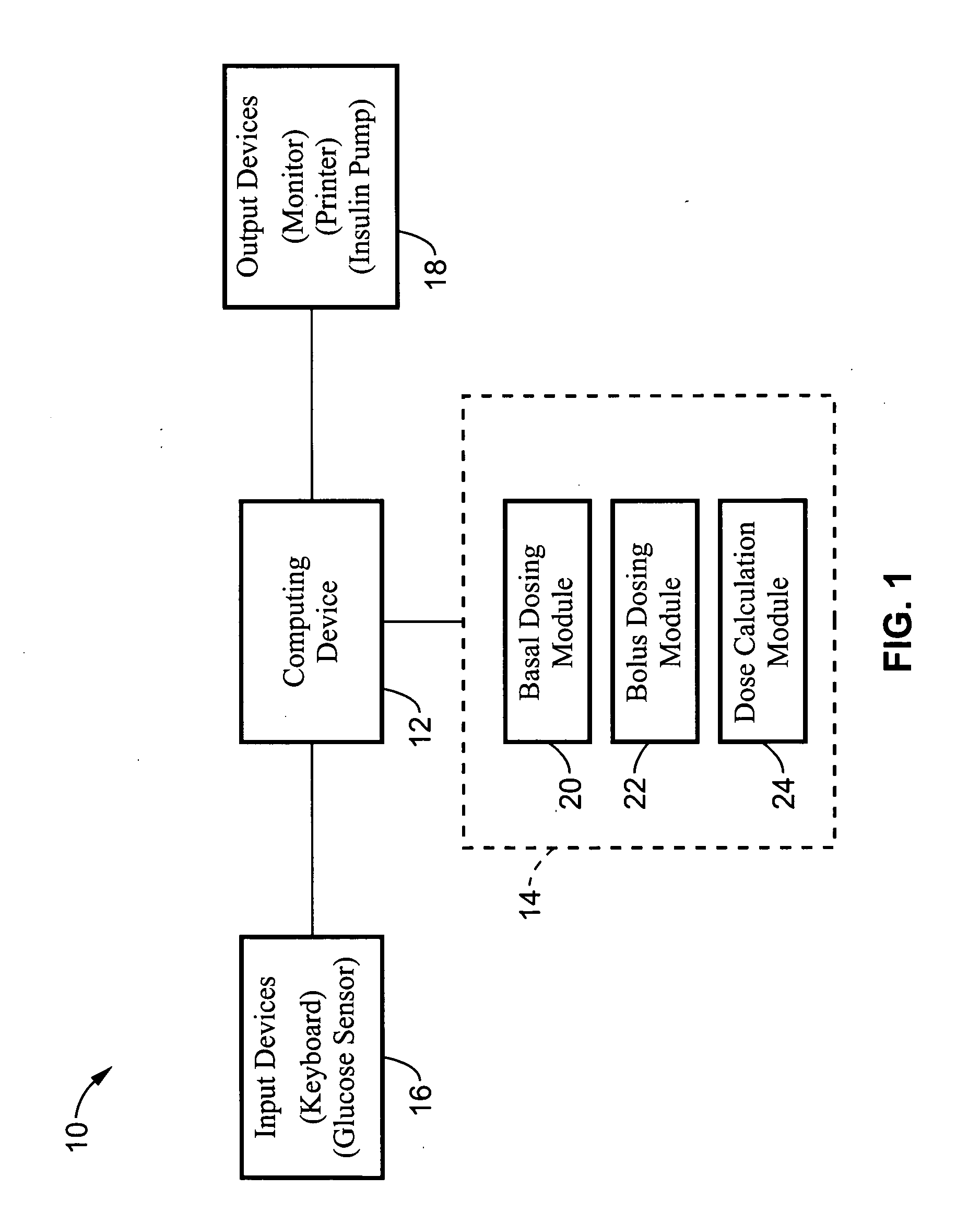
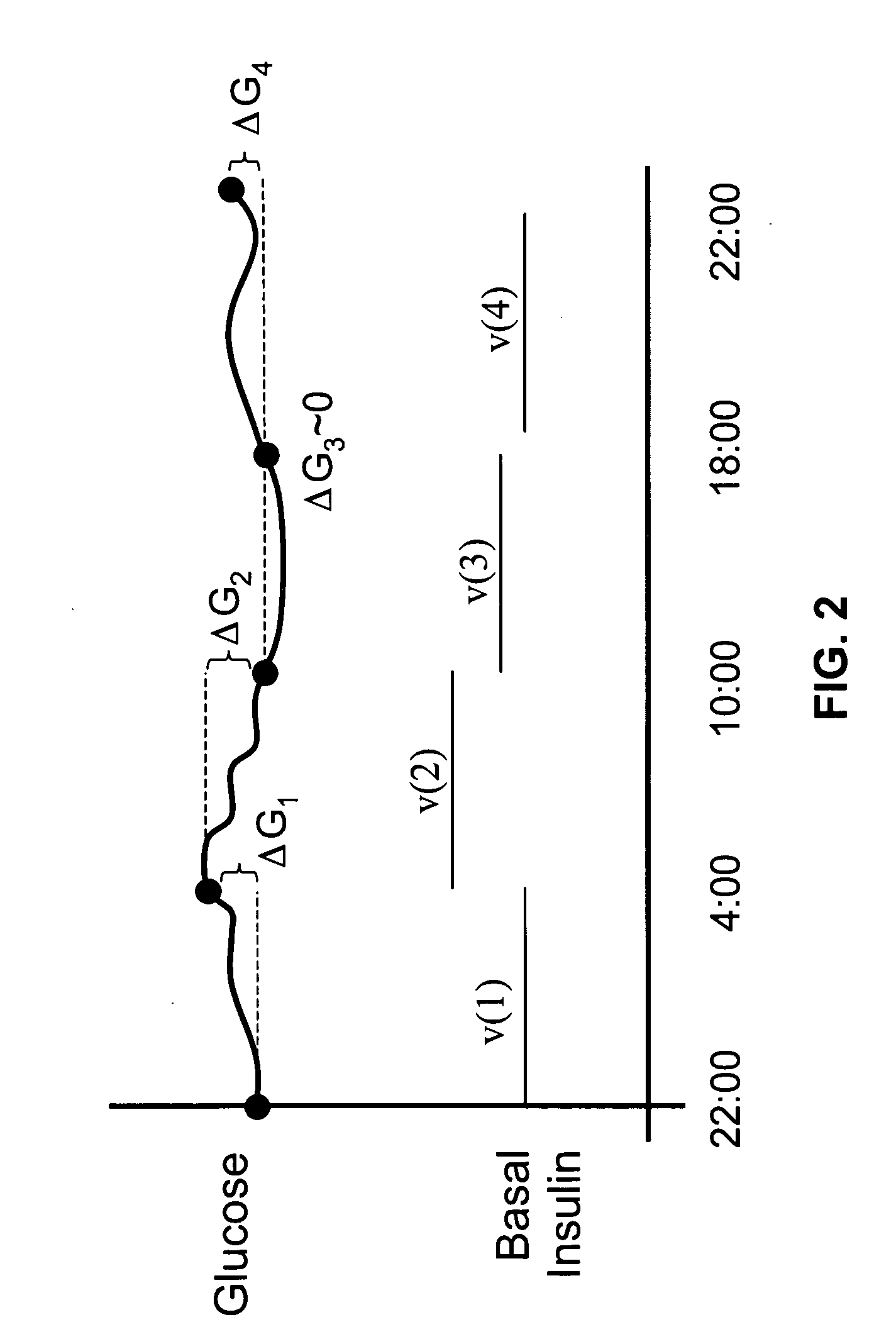
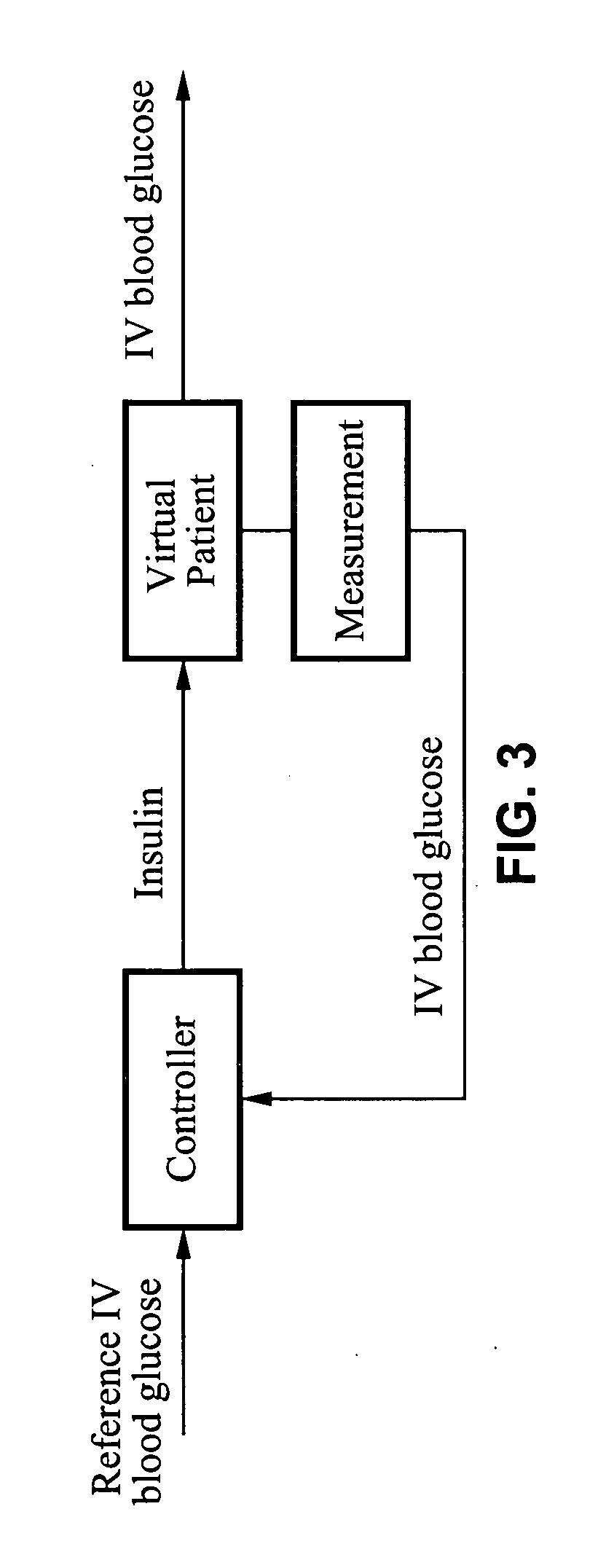
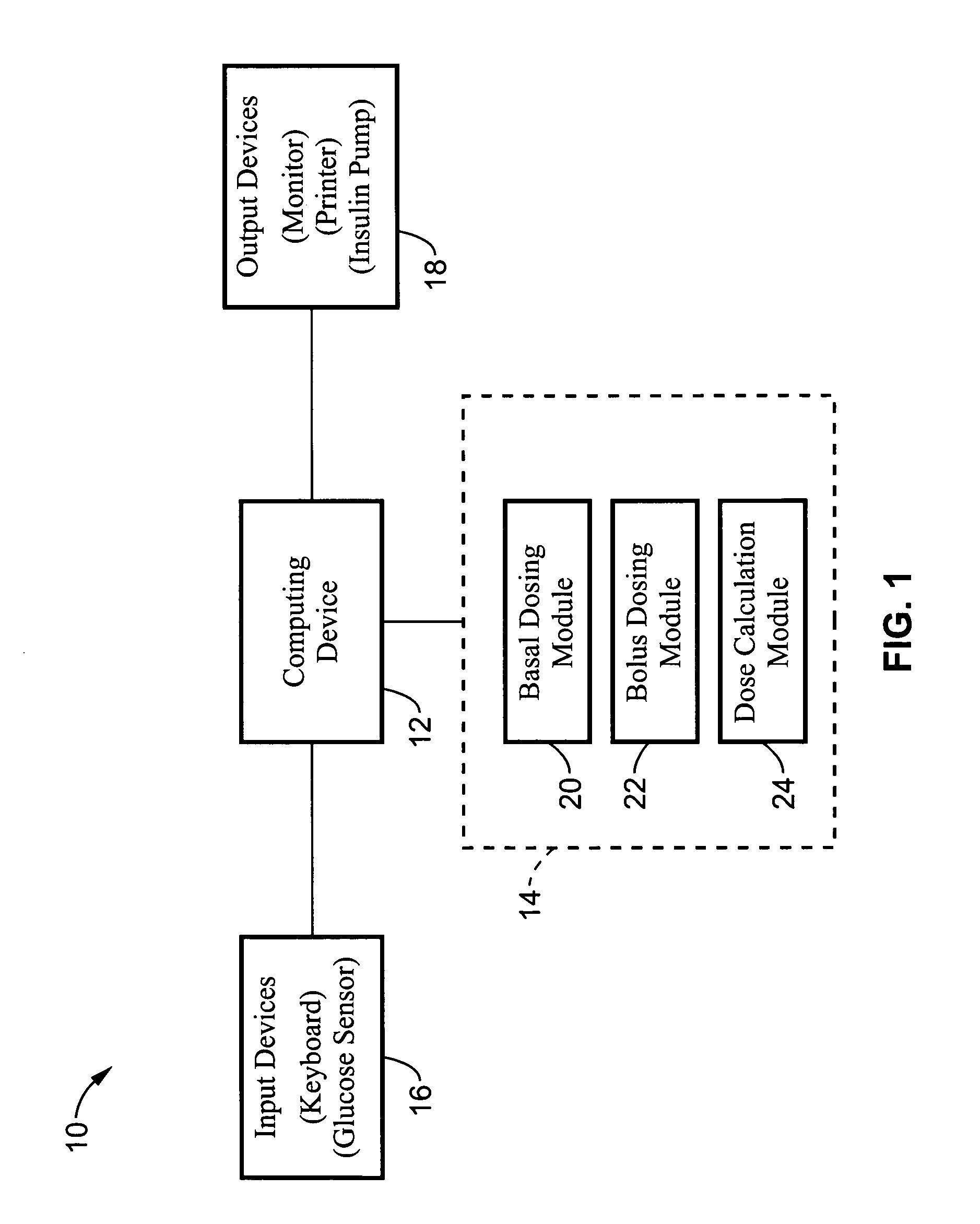
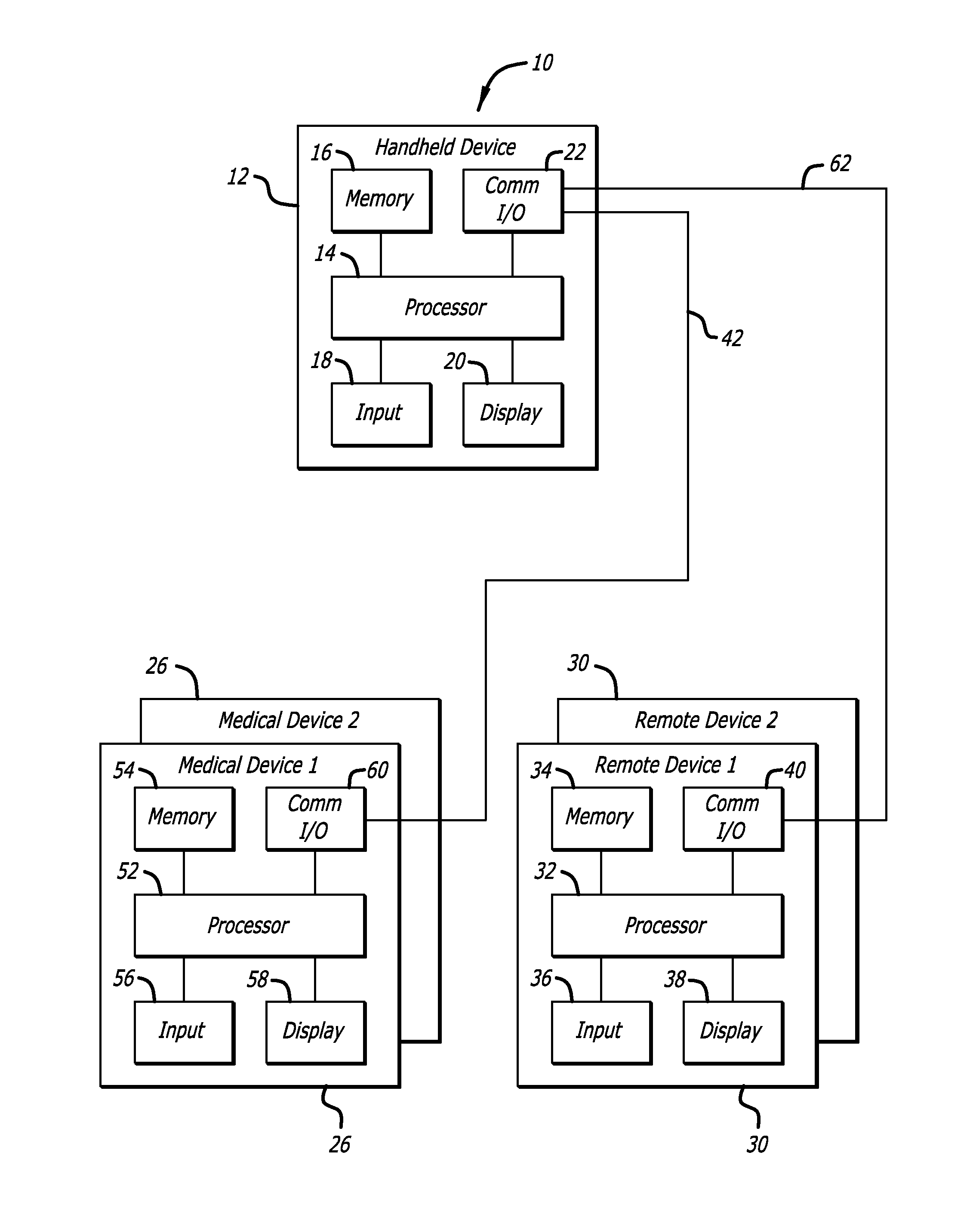
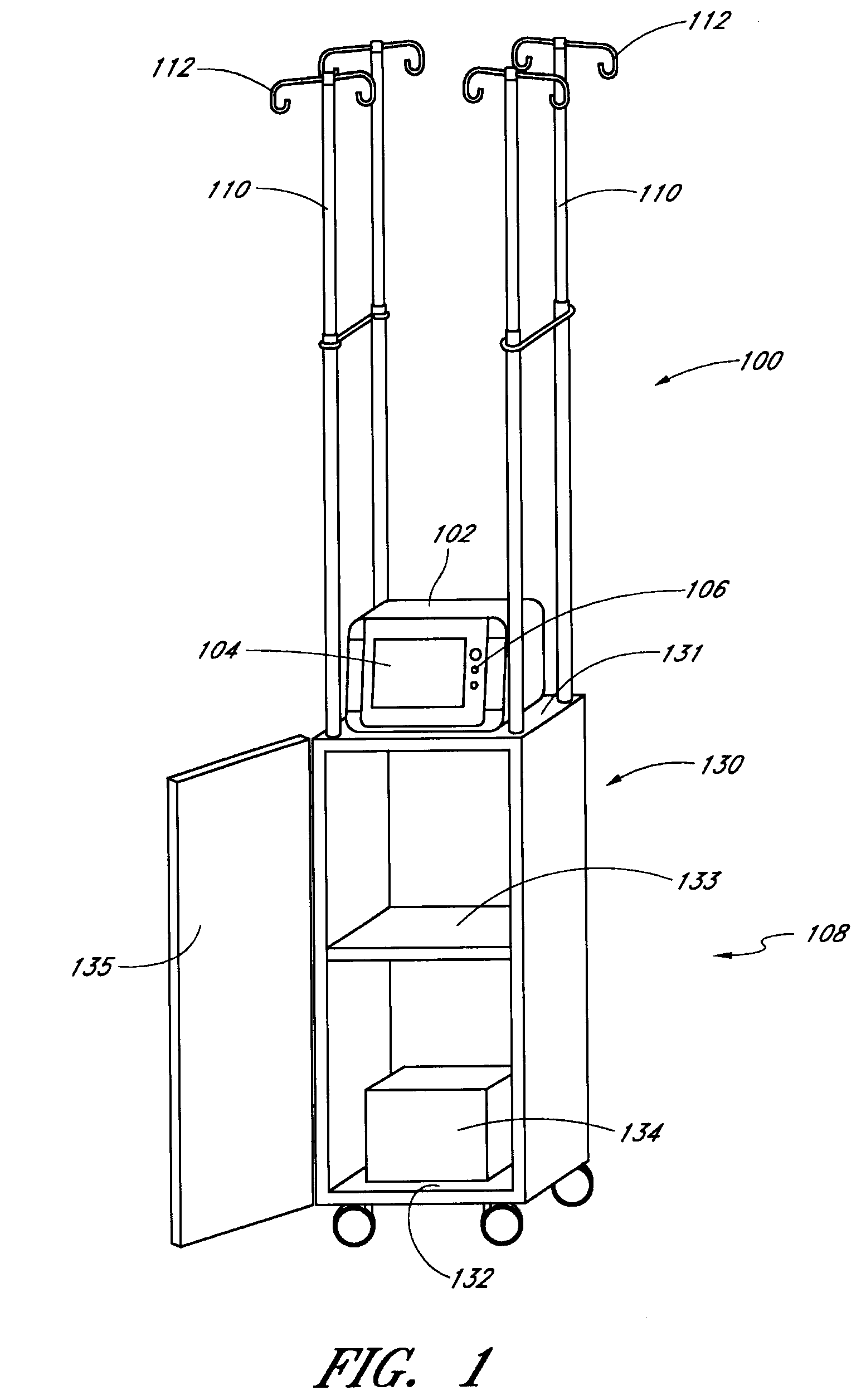
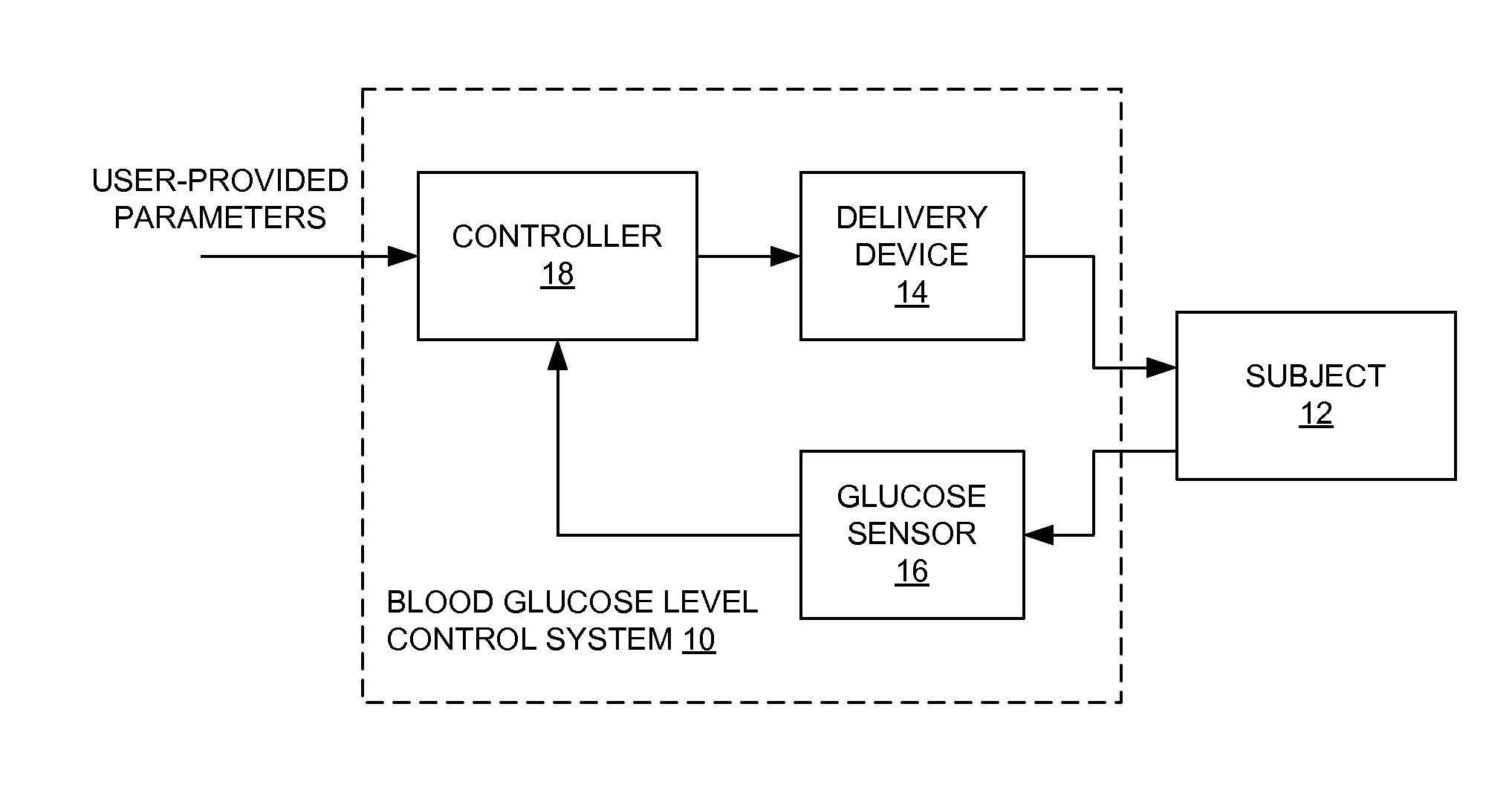
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS20050272640A1Ensure robustnessAccurately predicting insulin bolus dosagesPeptide/protein ingredientsDrug and medicationsPhysiologyMonitors blood glucose
A computer implemented method and associated apparatus for the combined control of insulin bolus dosing and basal delivery for the goal of achieving normal glycemic response to meals, exercise, stressors, and other perturbations to blood glucose levels. A run-to-run algorithm is used to monitor blood glucose levels and adjust insulin delivery as conditions are varied.
Owner:RGT UNIV OF CALIFORNIA
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS7651845B2Accurately predicting insulin bolus dosagesProcess controlPeptide/protein ingredientsDrug and medicationsMonitors blood glucoseGlycemic
Owner:RGT UNIV OF CALIFORNIA
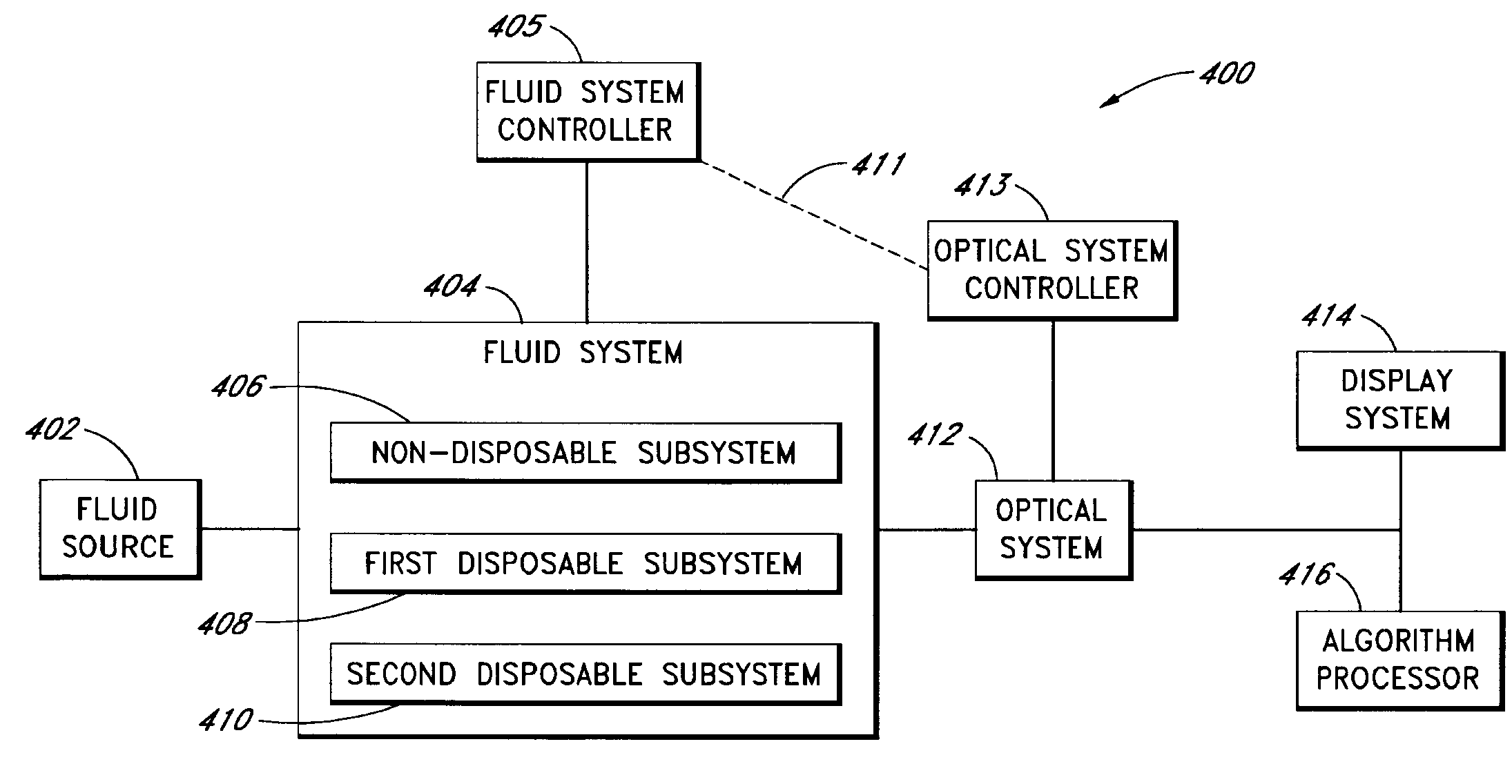
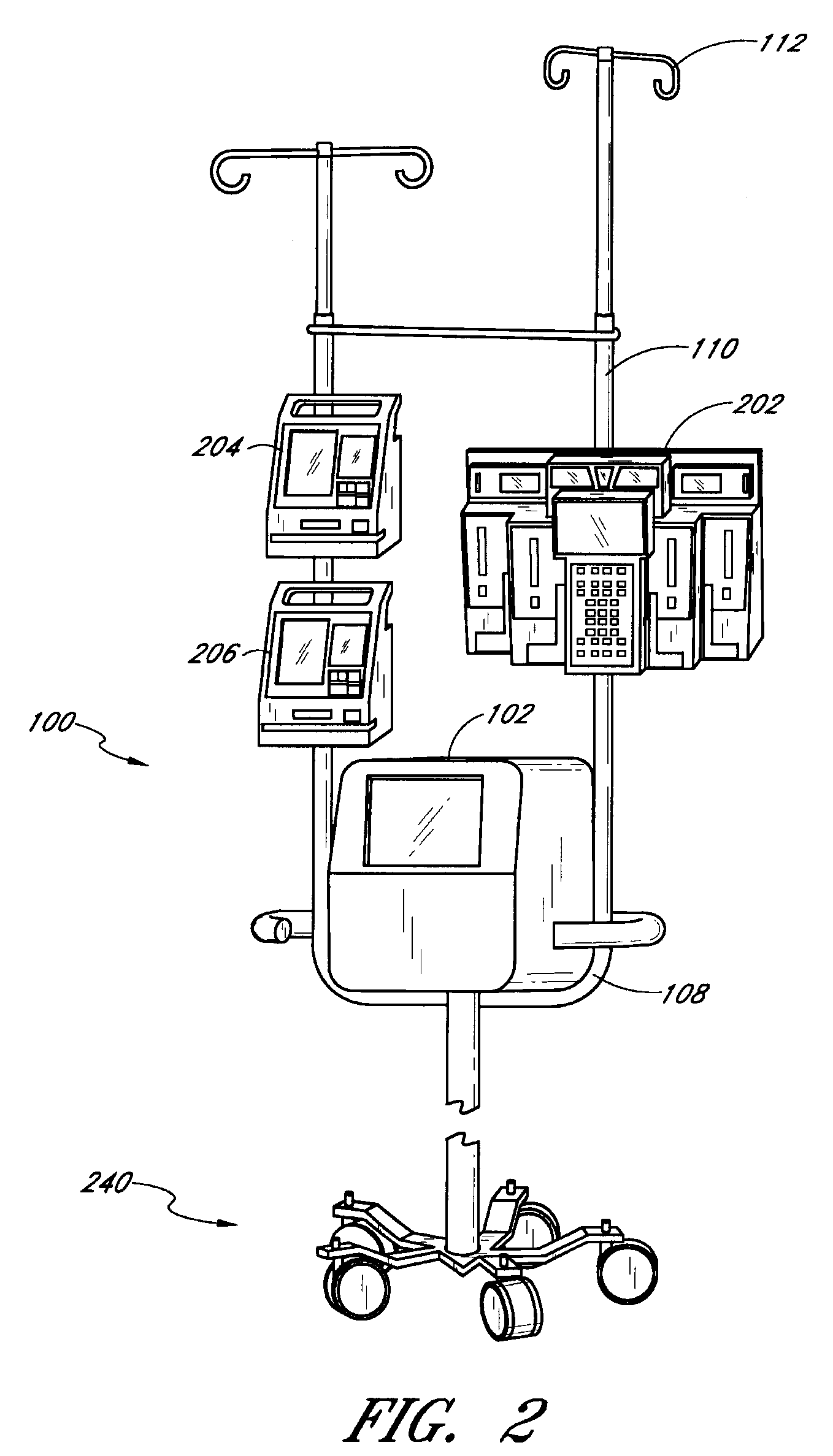
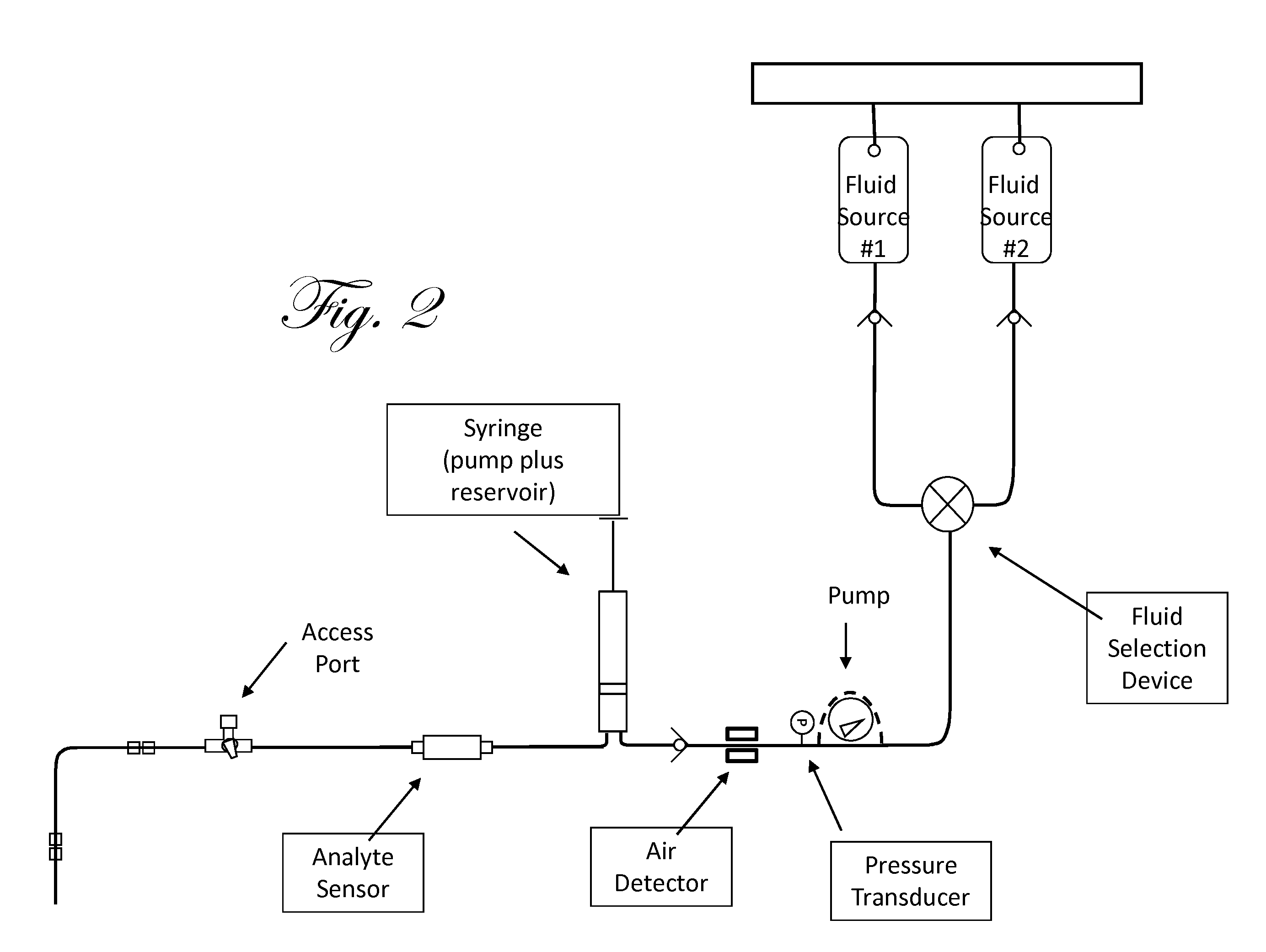
Fluid component analysis system and method for glucose monitoring and control
Owner:INSULET CORP +1
Method to determine the degree and stability of blood glucose control in patients with diabetes mellitus via the creation and continuous update of new statistical indicators in blood glucose monitors or free standing computers
InactiveUS20070010950A1Increase contributionShorten the timeDiagnostic recording/measuringSensorsInstabilityGlucose control
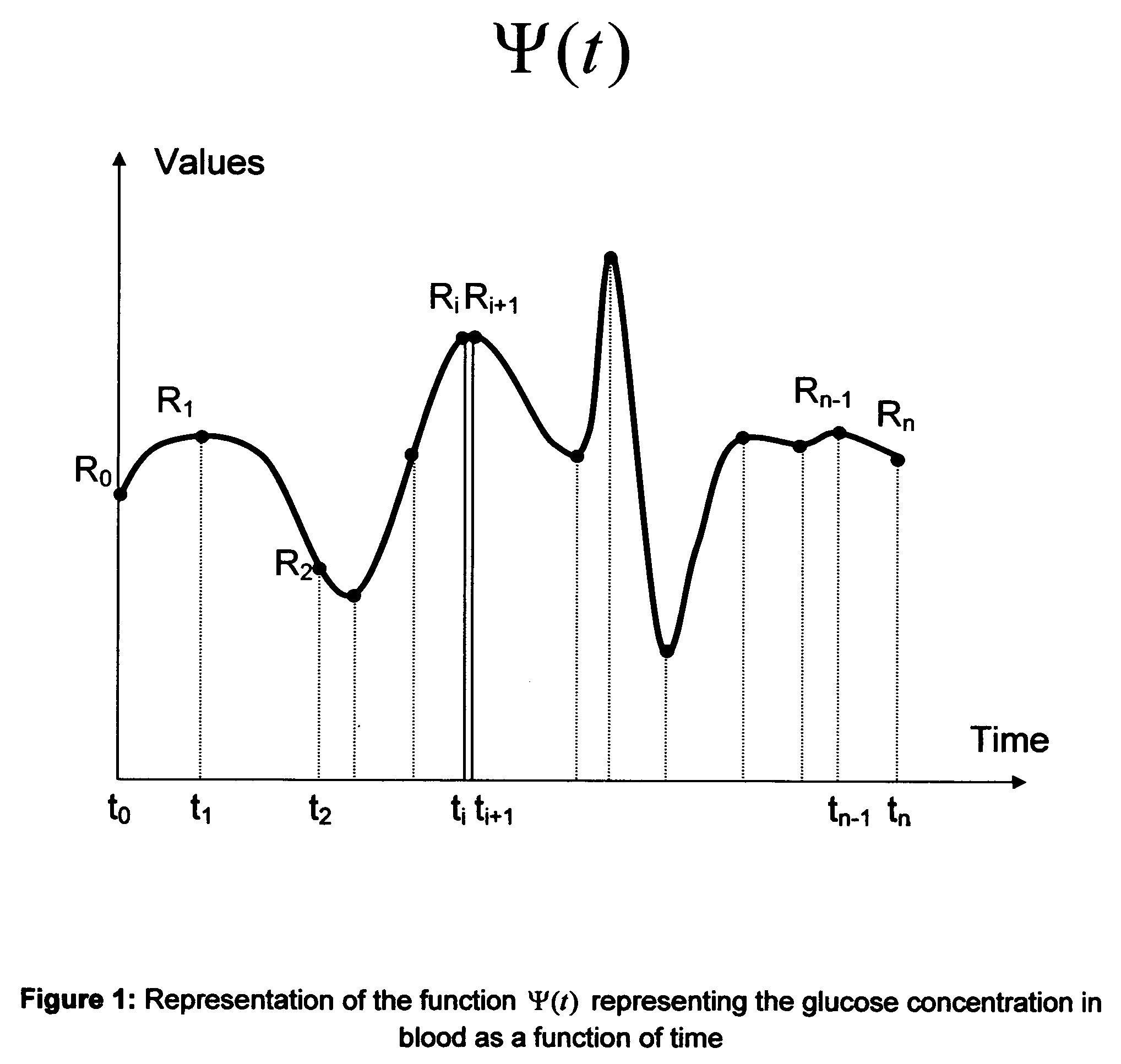
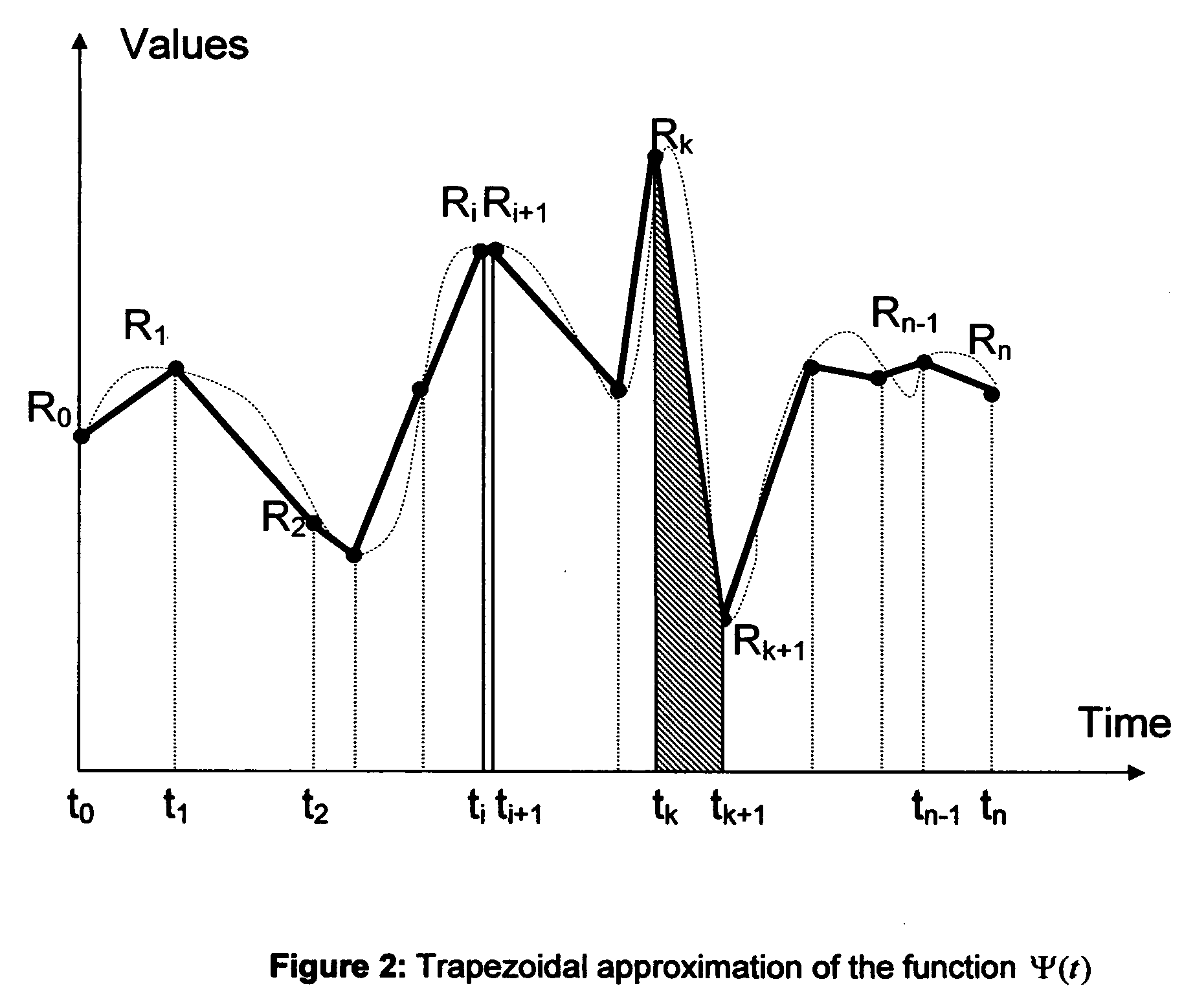
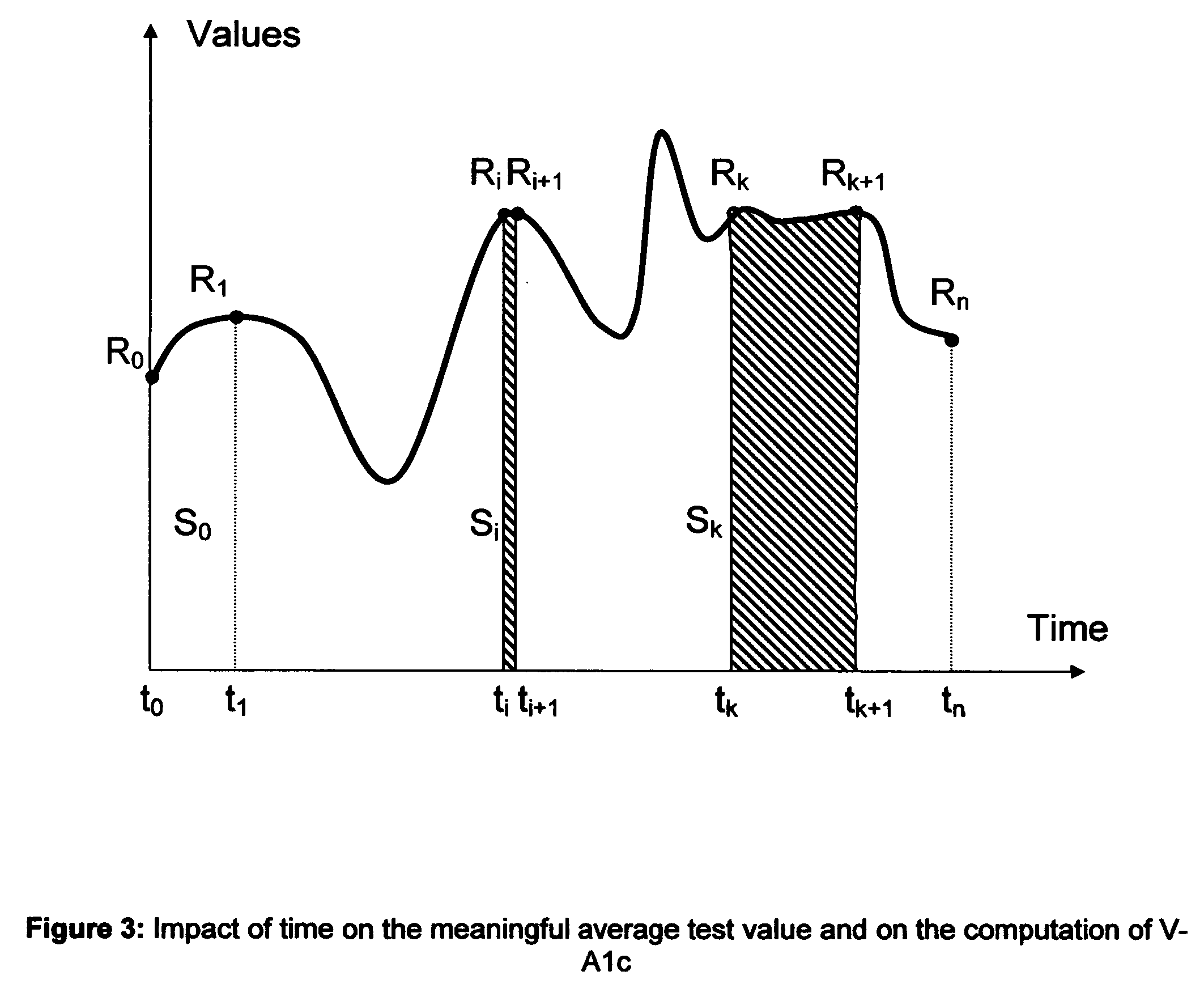
Microvascular complications of diabetes mellitus are closely related to blood glucose levels and fluctuations. The Glycostator statistical package was created to allow patients and health care providers simple access to “glycemic indicators” which permit a “snapshot view” of the effectiveness of the patient's diabetes management program. Glycostator functions provide a simple way of enhancing the information already provided by home blood glucose monitoring devices. To this end, a set of new indices, including one called the Virtual A1c, are computed in a recursive fashion from blood glucose test results to provide a more meaningful day-to-day assessment of glycemic control. All indices can be made available at the meter user interface on request. The displayed indices allow patients to improve glycemic control by identifying problems with blood glucose control and lability that are less easily recognized in traditional blood glucose meter statistical packages. Virtual A1c emulates hemoglobin A1c continuously and provides better day-to-day assessment of long term glycemic control than does the traditional average blood glucose report. The method for computing each of these indices, including the Virtual A1c, allows for their implementation in commercial blood glucose monitors.
Owner:ROCHE DIABETES CARE INC
Methods for modeling insulin therapy requirements
InactiveUS20110098548A1Improve fitShorten the timeMedical simulationDrug and medicationsGlycemicGlucose control
Various methods for improving the use of model based prediction of future blood glucose control in a patient having diabetes are described. A system for processing diabetes related information, including glucose information, for accurately predicting future glucose levels as a function of glucose data, carbohydrate intake, insulin delivery history and exercise history and then providing recommendations related to the predicted future glucose levels, is also described.
Owner:ABBOTT DIABETES CARE INC
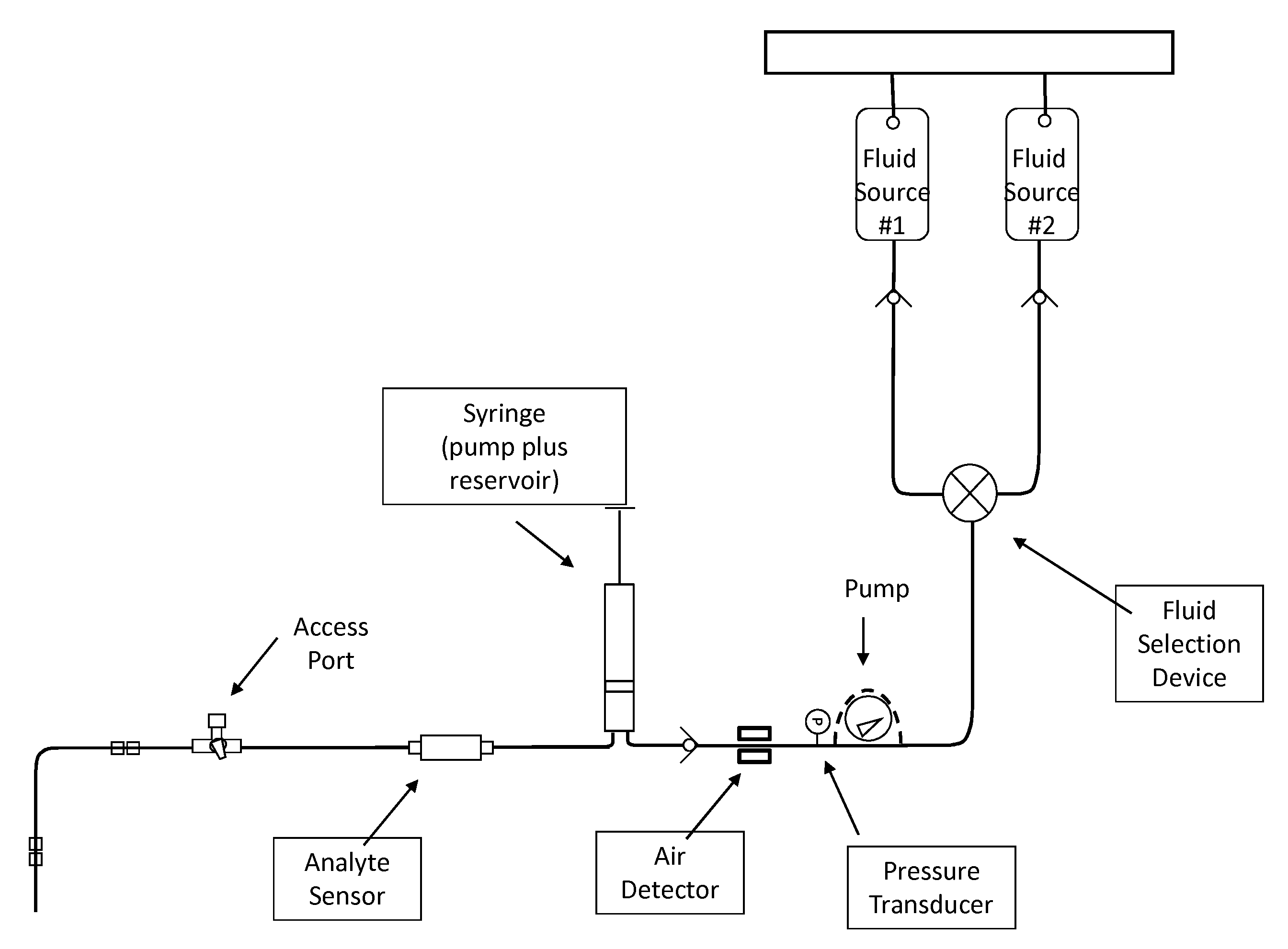
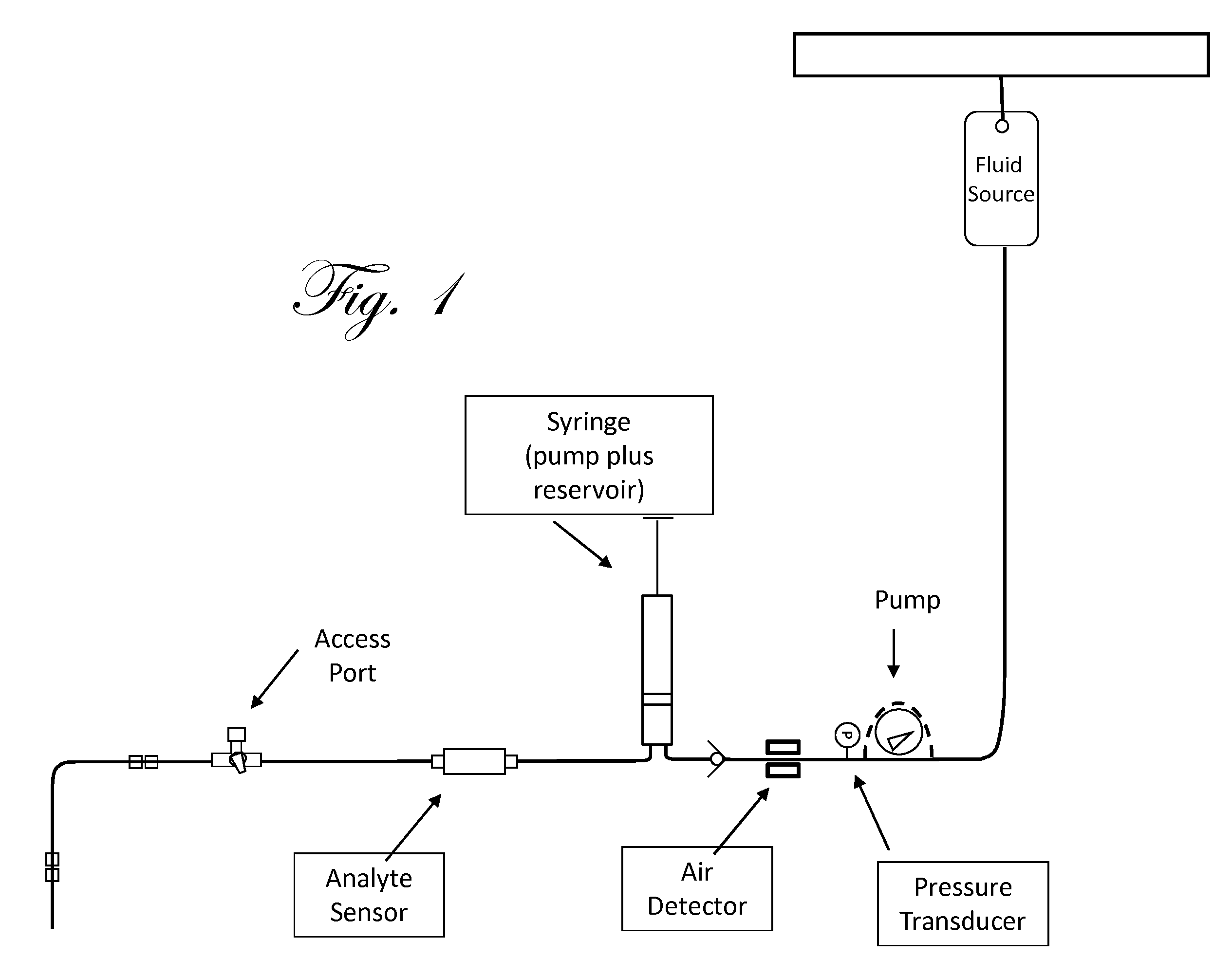
Fluid component analysis system and method for glucose monitoring and control
Disclosed are methods and apparatus for determining analyte concentration in a sample such as bodily fluid. Systems and methods disclosed herein can also include a treatment dosing system to infuse or inject a treatment drug (e.g. insulin or glucose) and provide glycemic control. The dose of the treatment drug may be based on the concentration of the analyte or the average value for the concentration of the analyte and / or the rate of change of the value of the concentration of the analyte.
Owner:INSULET CORP +1
Methods and apparatuses related to blood analyte measurement system
InactiveUS20100168535A1Accurate measurementEfficient implementationMedical devicesCatheterOperational systemPotassium
The present invention relates to a blood analyte measurement system for the procurement of blood samples for measurement of blood properties such as analyte concentration or analyte presence. A blood access system can be coupled with a measurement system such as an electrochemical sensor, and can also be used with other measurement modalities. Embodiments of the present invention can facilitate accurate measurement of blood glucose by the clinician in a sterile manner. Embodiments of the present invention can also enable the calibration of the sensor at one or more calibration points. One desired analyte of measurement is glucose for the effective implementation of glycemic control protocols. Embodiments of the present invention can also be used for the measurement of other analytes such as arterial blood gases, lactate, hemoglobin, potassium and urea. Additionally, embodiments of the present invention can function effectively on a variety of blood access points and specifically enables glucose monitoring in an existing arterial line that is already in place for hemodynamic monitoring. The present invention does not consume a significant amount of blood. Some embodiments of the present invention can re-infuse the blood into the patient, which can facilitate operation of the system in a sterile manner.
Owner:ROBINSON MARK RIES +4
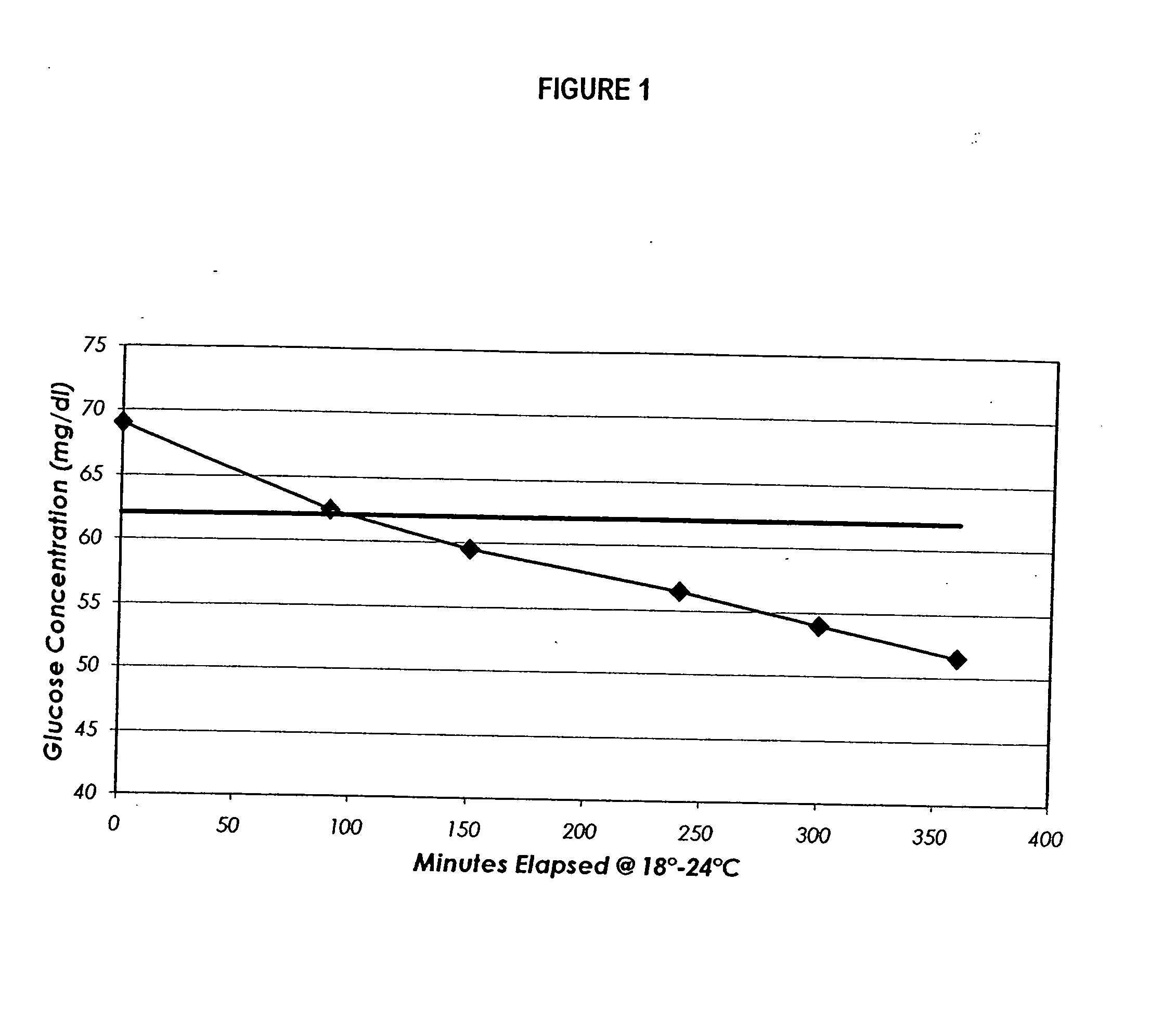
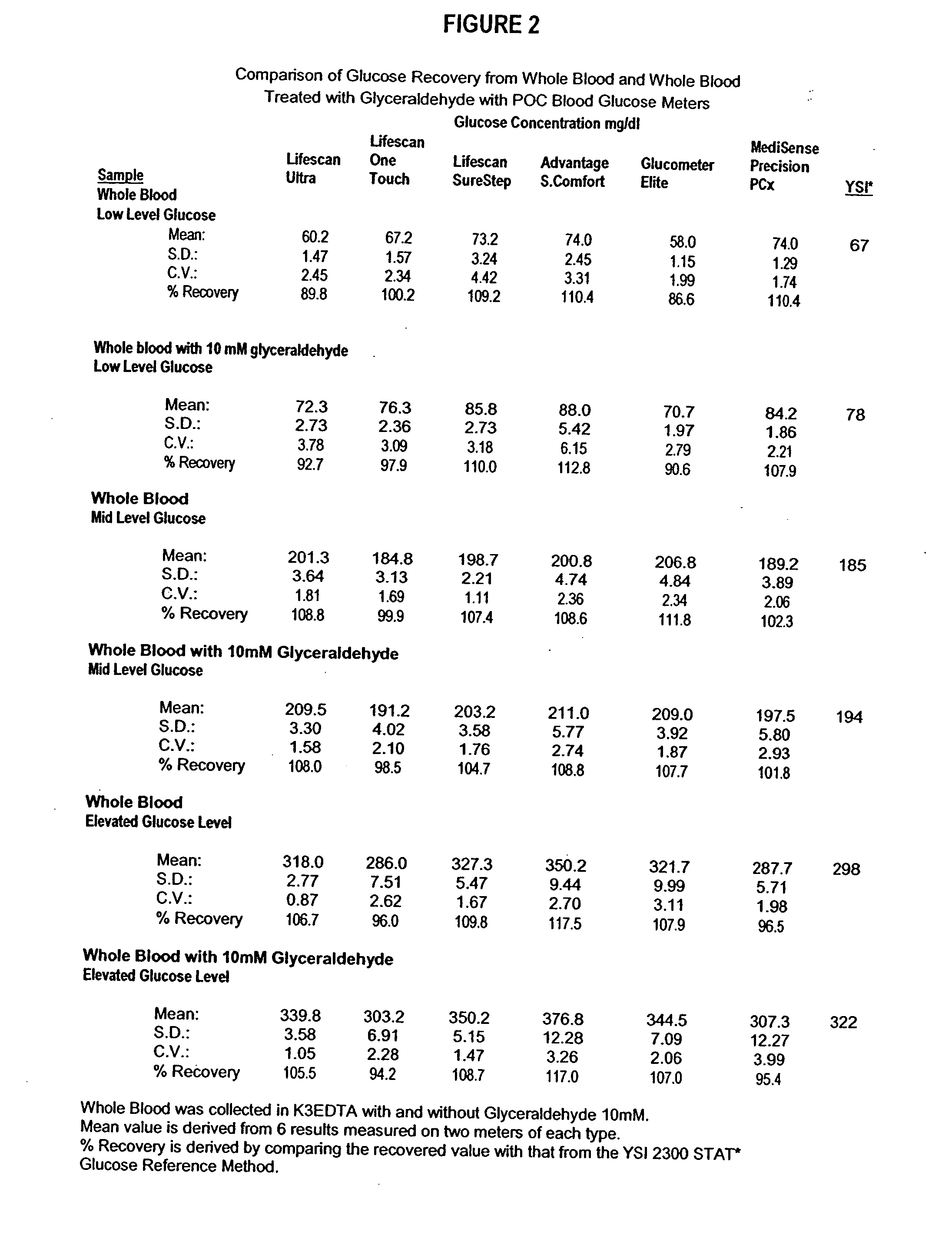
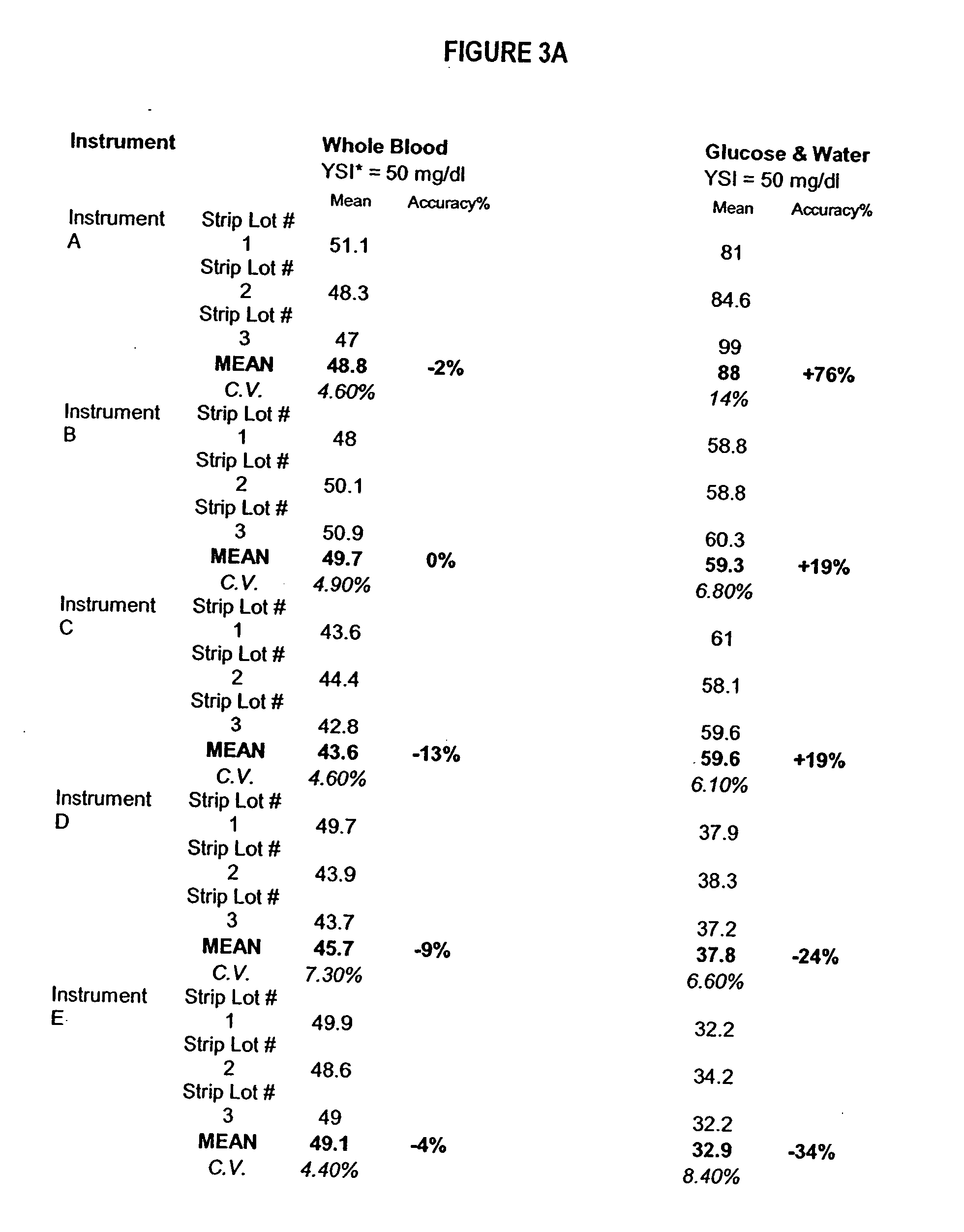
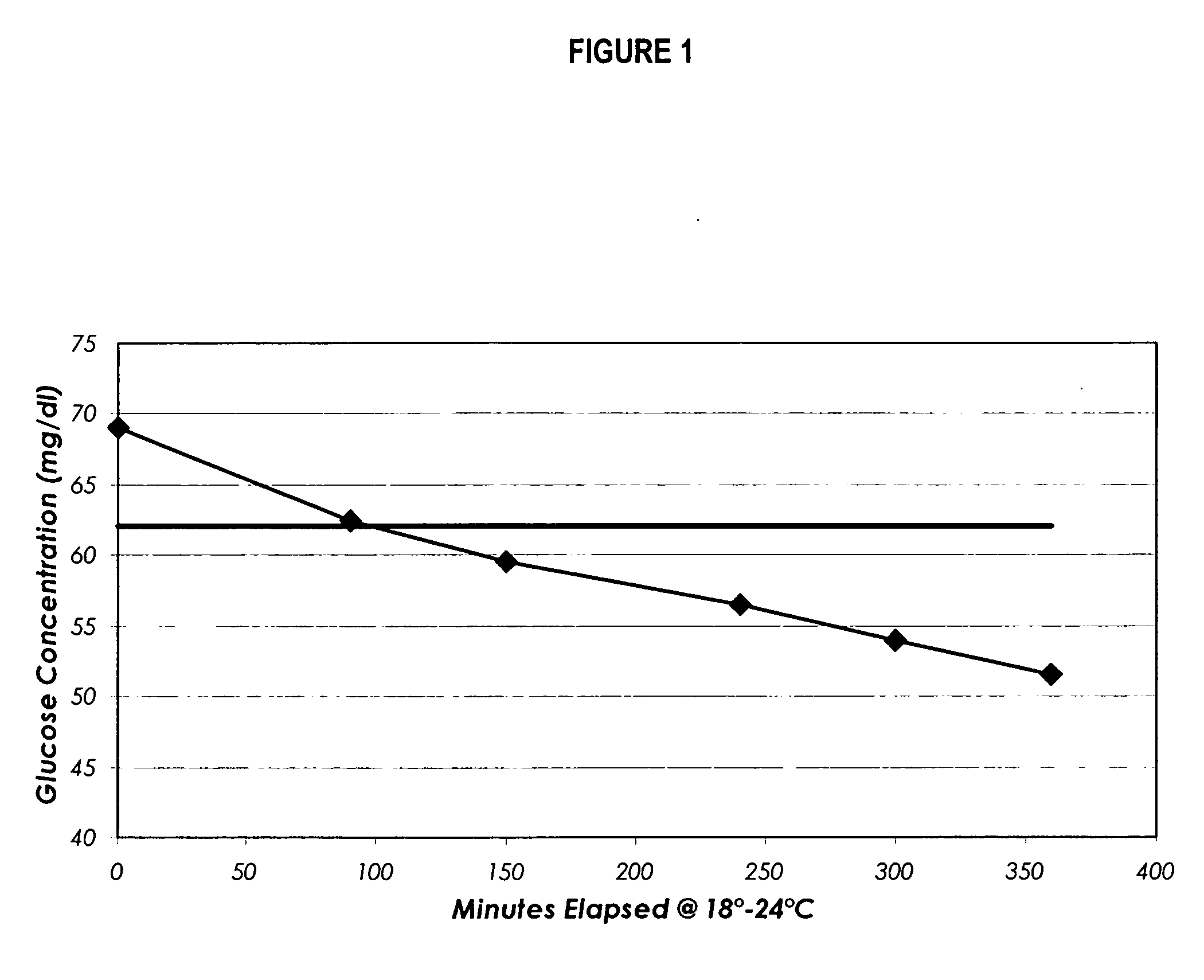
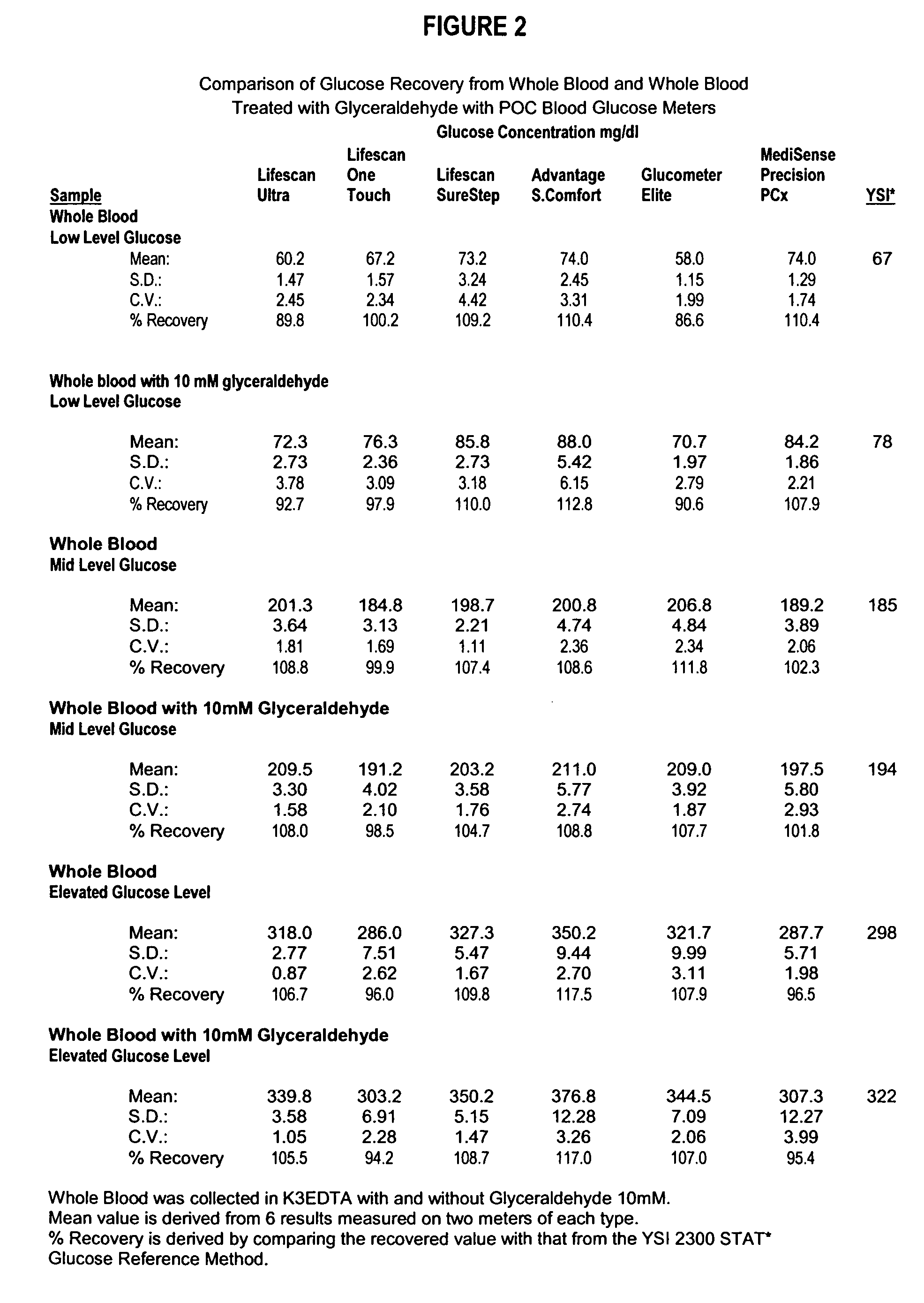
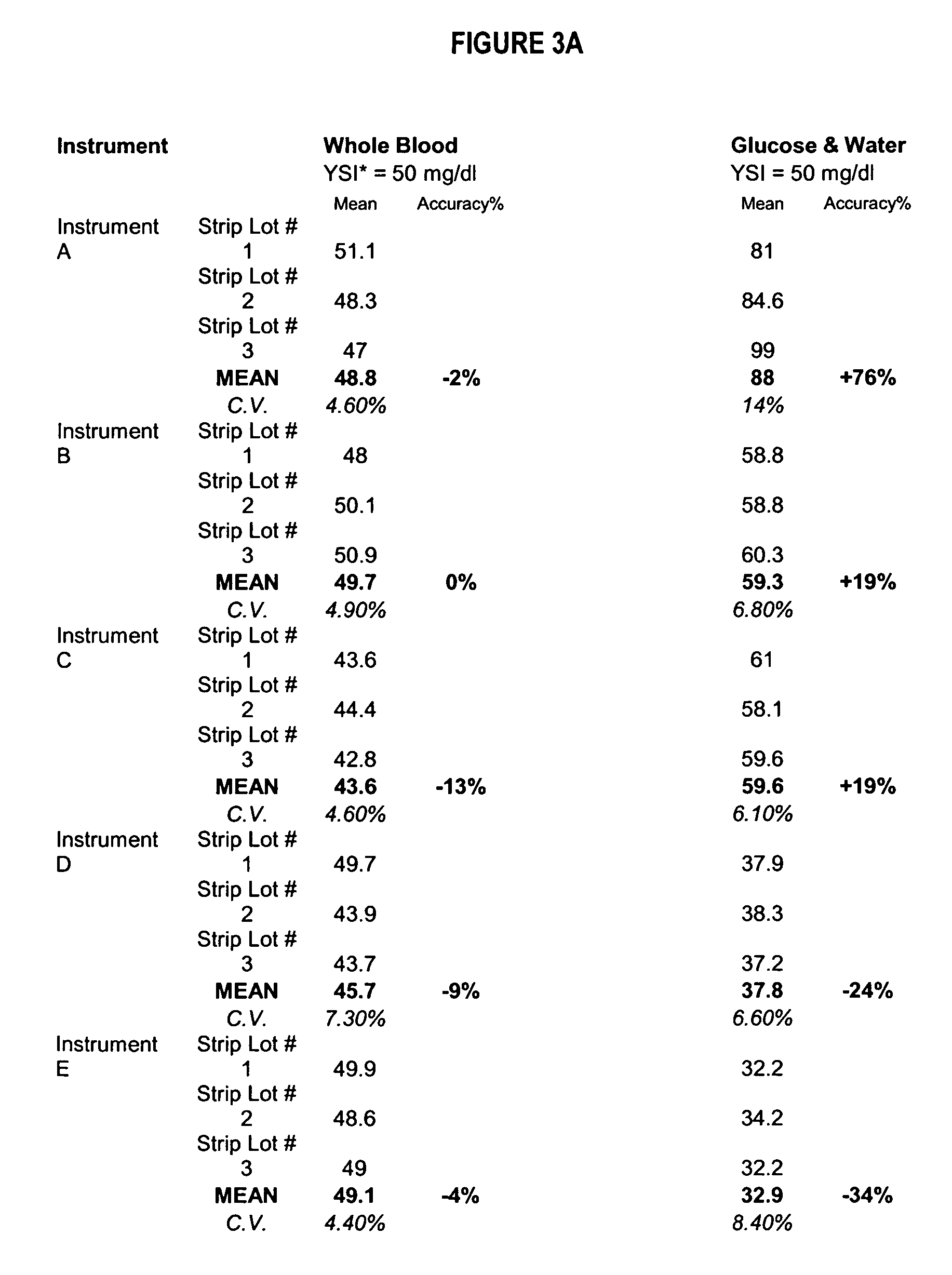
Process, composition and kit for providing a stable whole blood calibrator/control
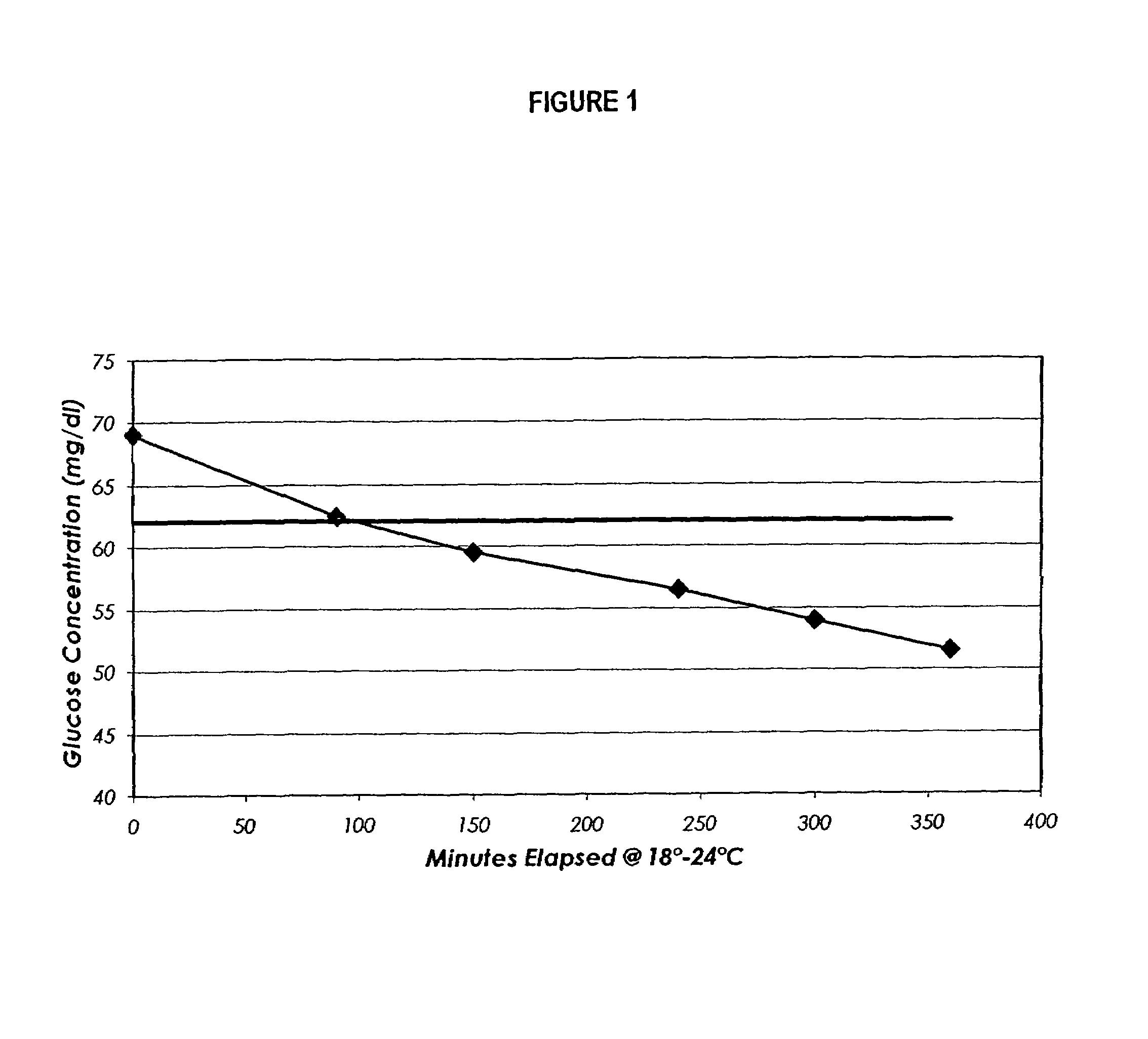
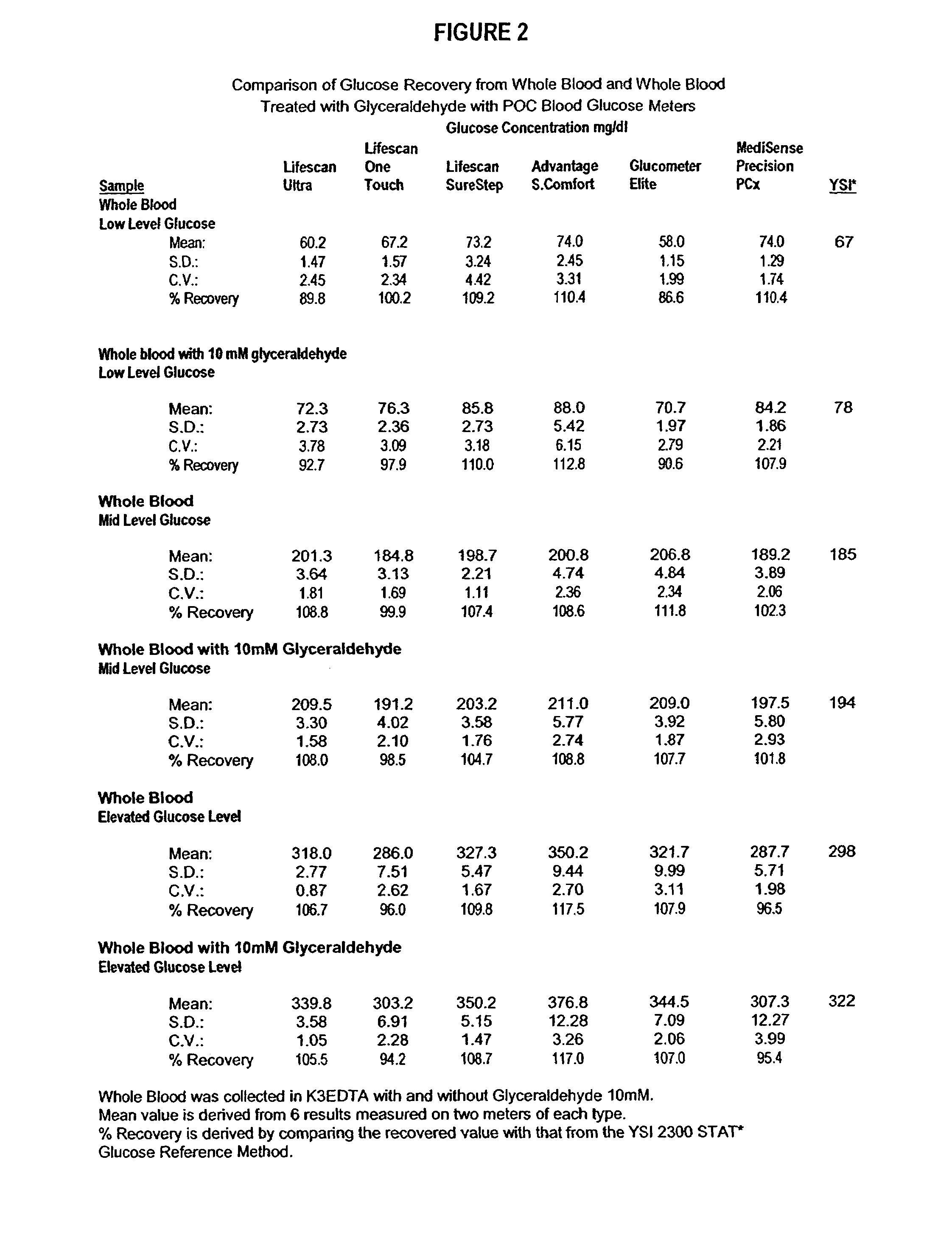
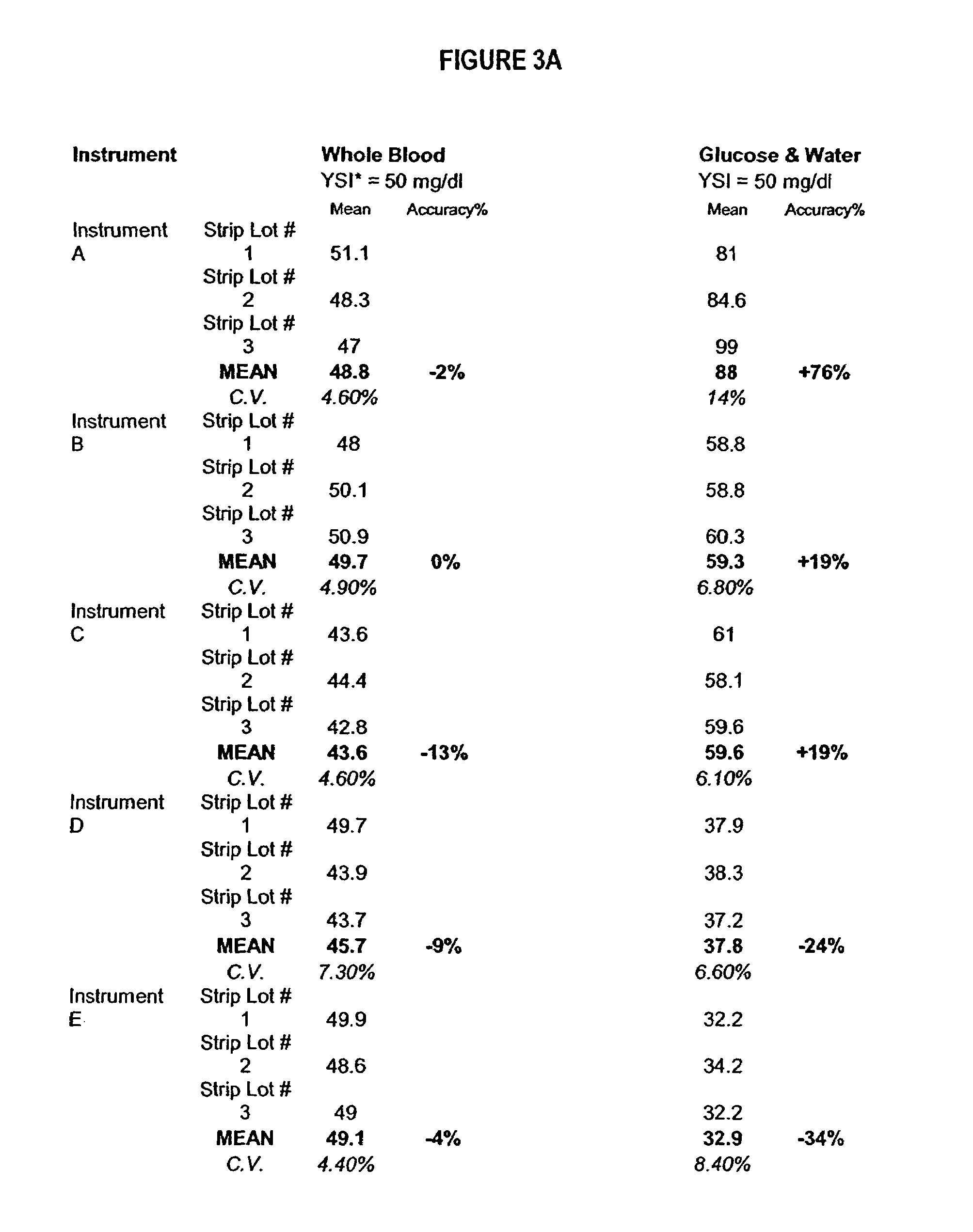
InactiveUS20060211072A1Quality improvementSimple and elegantSamplingMicrobiological testing/measurementMedicineConcentrations glucose
The present invention is directed toward a stable calibrator and / or control, kit and process for using in a glucose monitoring instrumentation. Principally, the instant invention teaches a glycolyzed red blood cell component which has been treated with a glycolysis stabilizing effective amount of at least one non-crosslinking aldehyde compound which may be added to fresh plasma along with an amount of glucose to form a simulated whole blood glucose control product, effective for maintaining a particular and essentially stable glucose concentration over a period of time sufficient for accurate measurement and calibration of a glucose measuring instrument.
Owner:STRECK INC
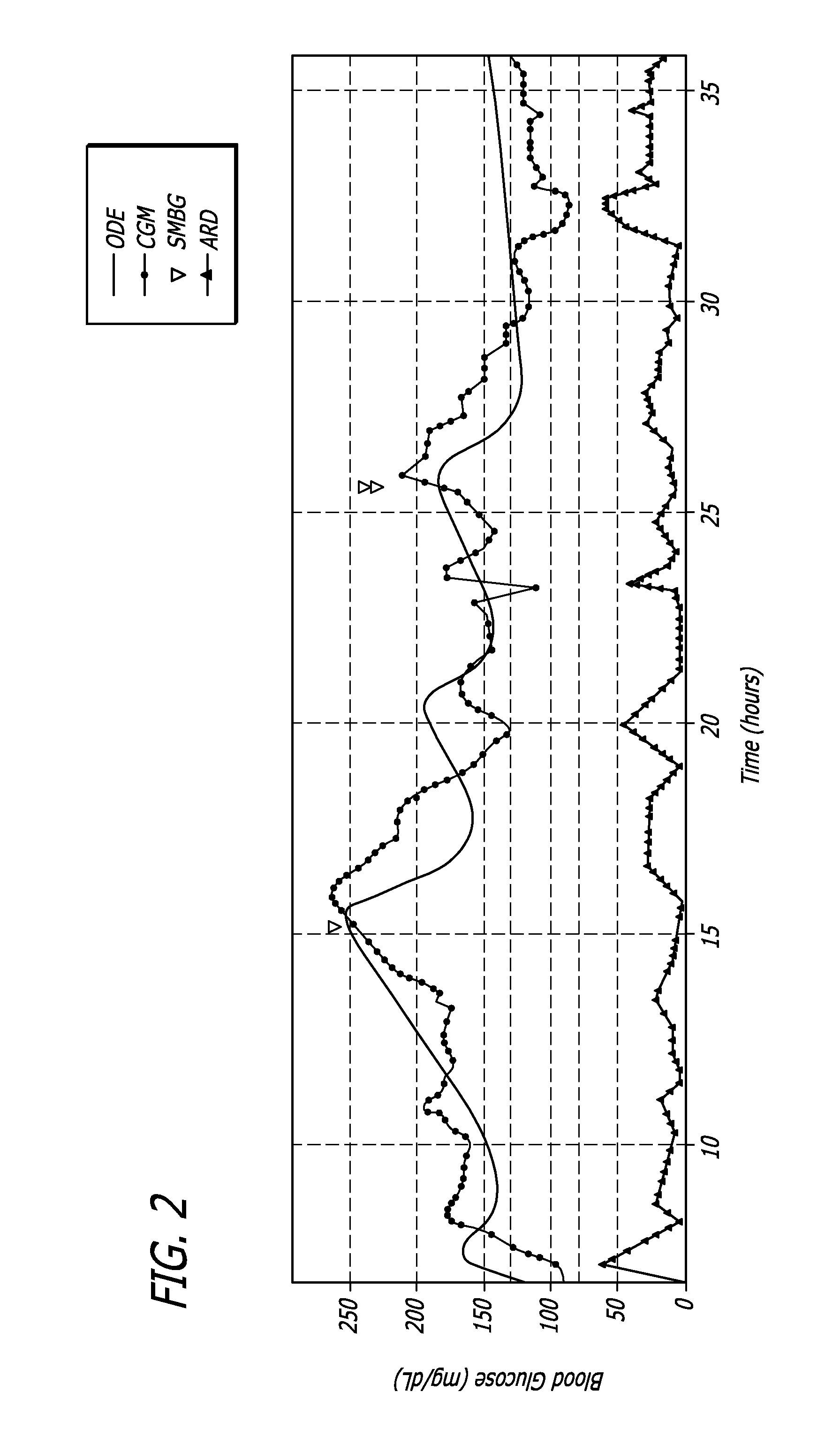
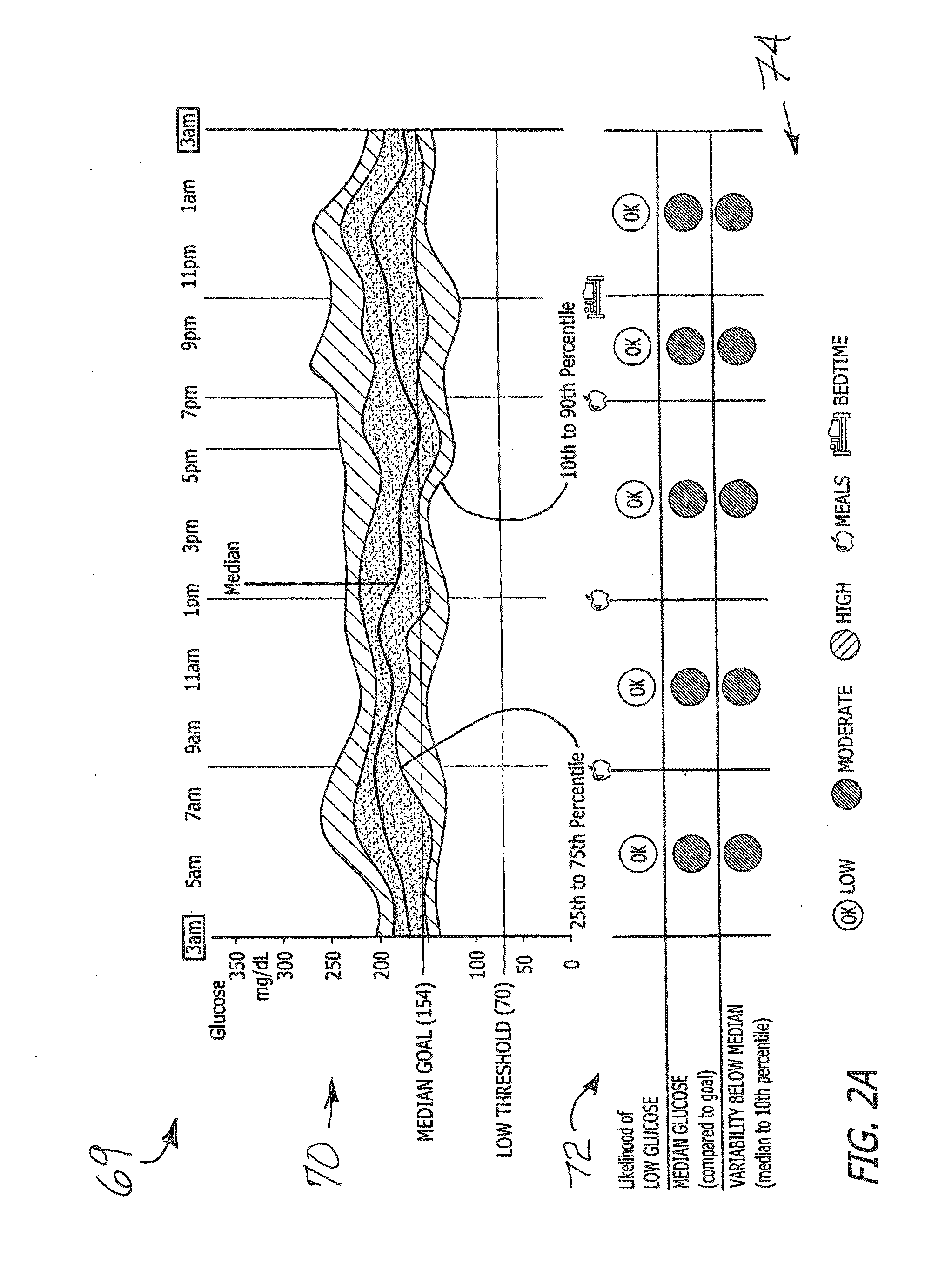
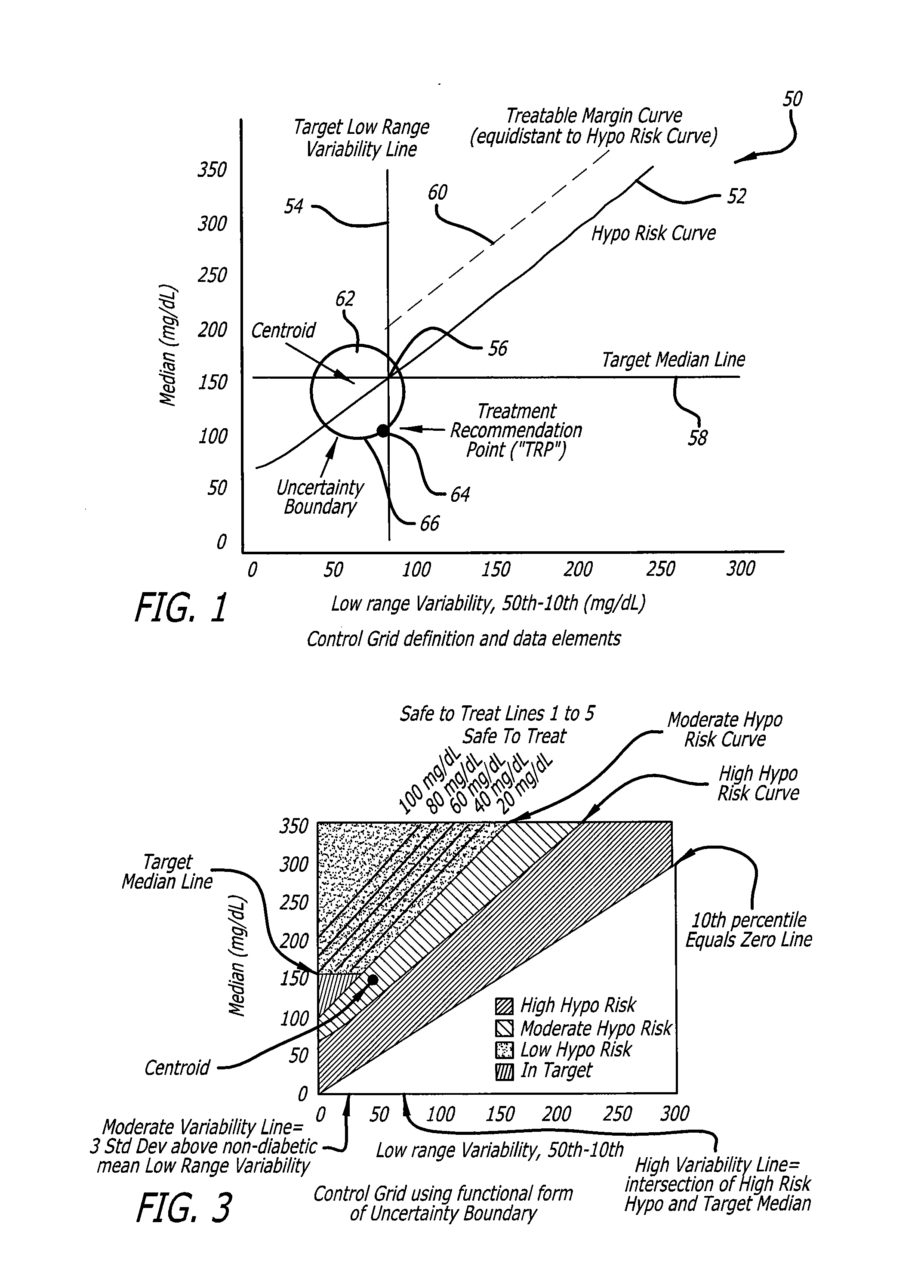
System and method to manage diabetes based on glucose median, glucose variability, and hypoglycemic risk
A system and method provides a glucose report for determining glycemic risk based on an ambulatory glucose profile of glucose data over a time period, a glucose control assessment based on median and variability of glucose, and indicators of high glucose variability. Time of day periods are shown at which glucose levels can be seen. A median glucose goal and a low glucose line provide coupled with glucose variability provide a view into effects that raising or lowering the median goal would have. Likelihood of low glucose, median glucose compared to goal, and variability of glucose below median provide probabilities based on glucose data. Patterns can be seen and provide guidance for treatment.
Owner:ABBOTT DIABETES CARE INC
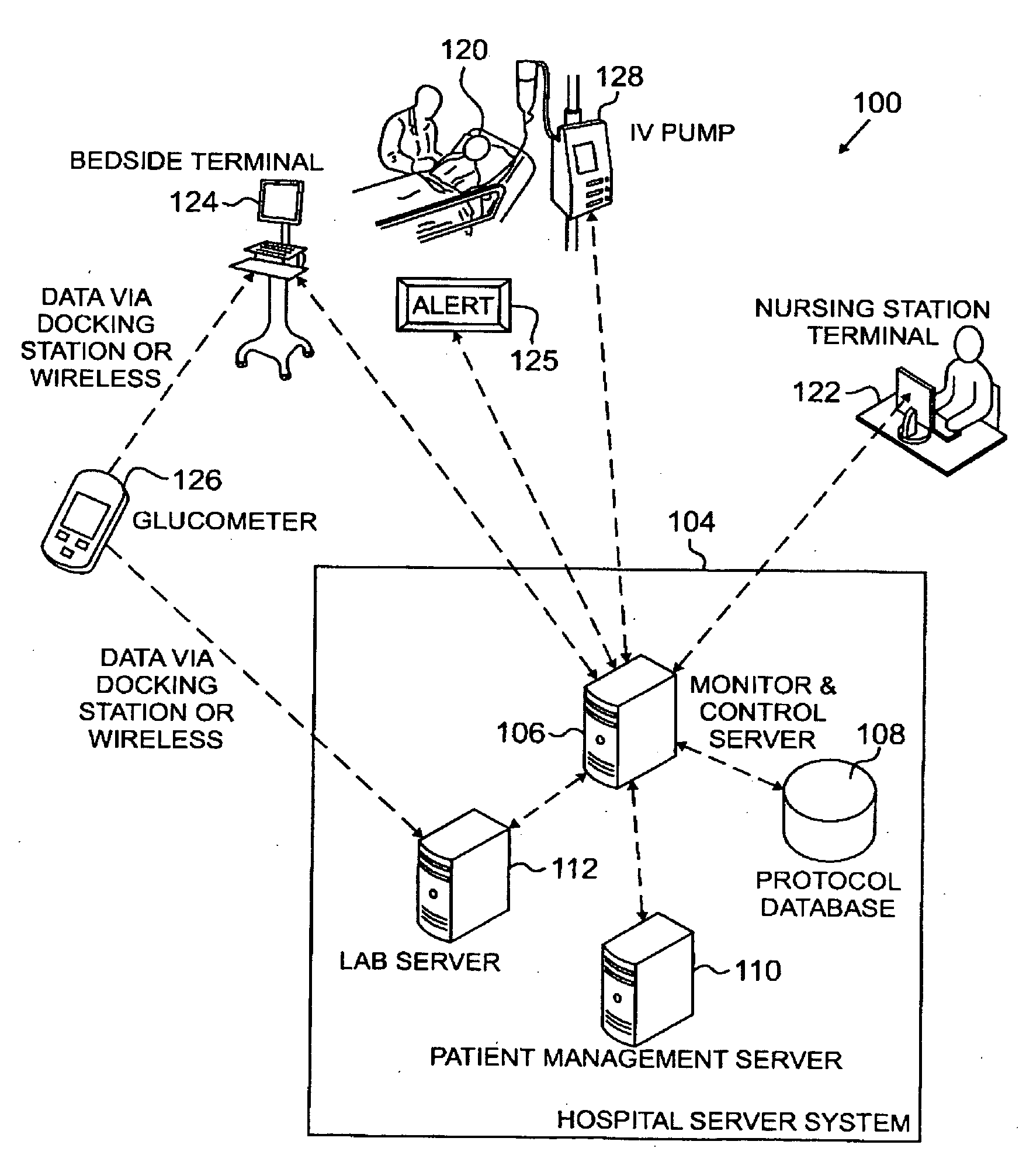
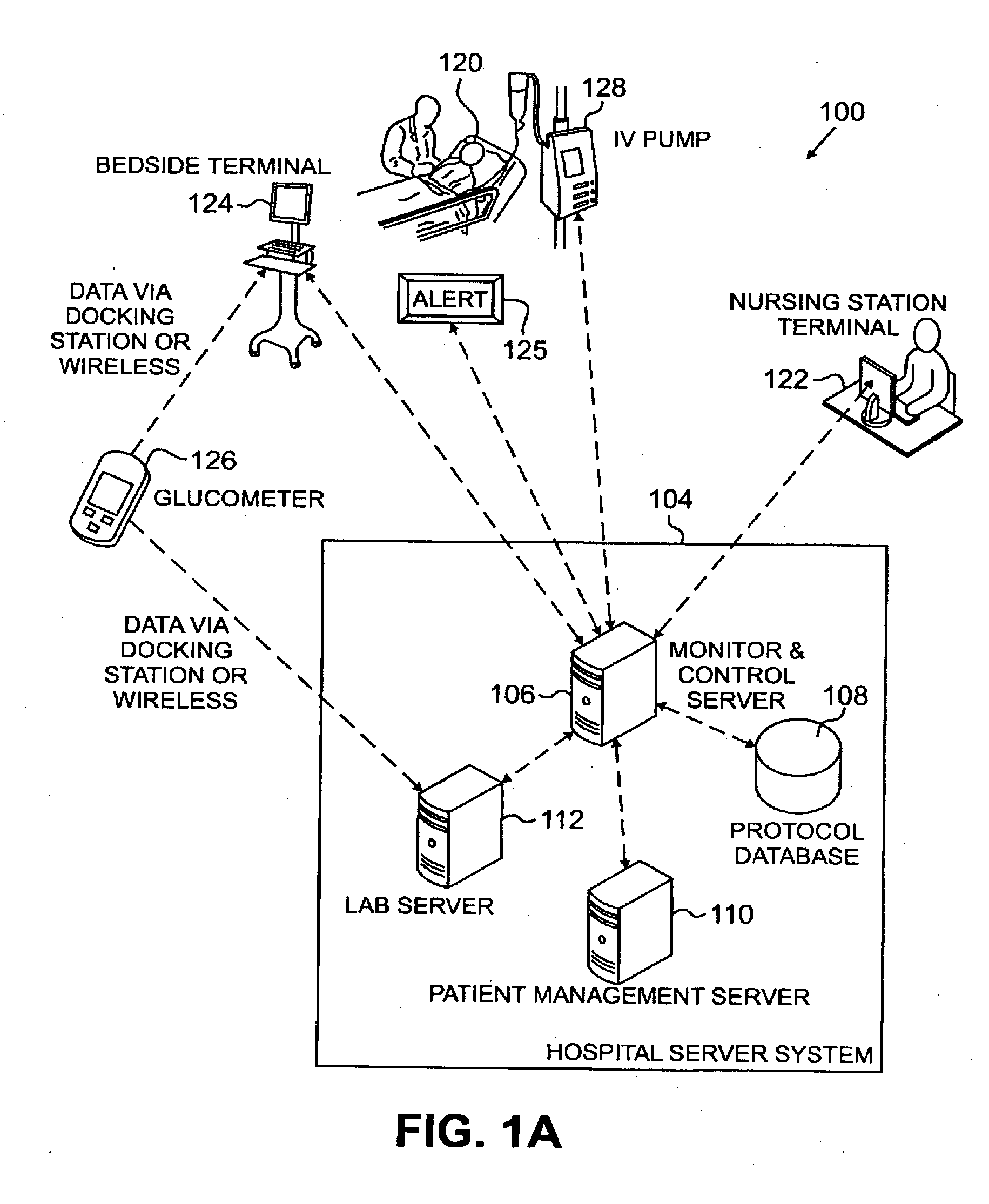
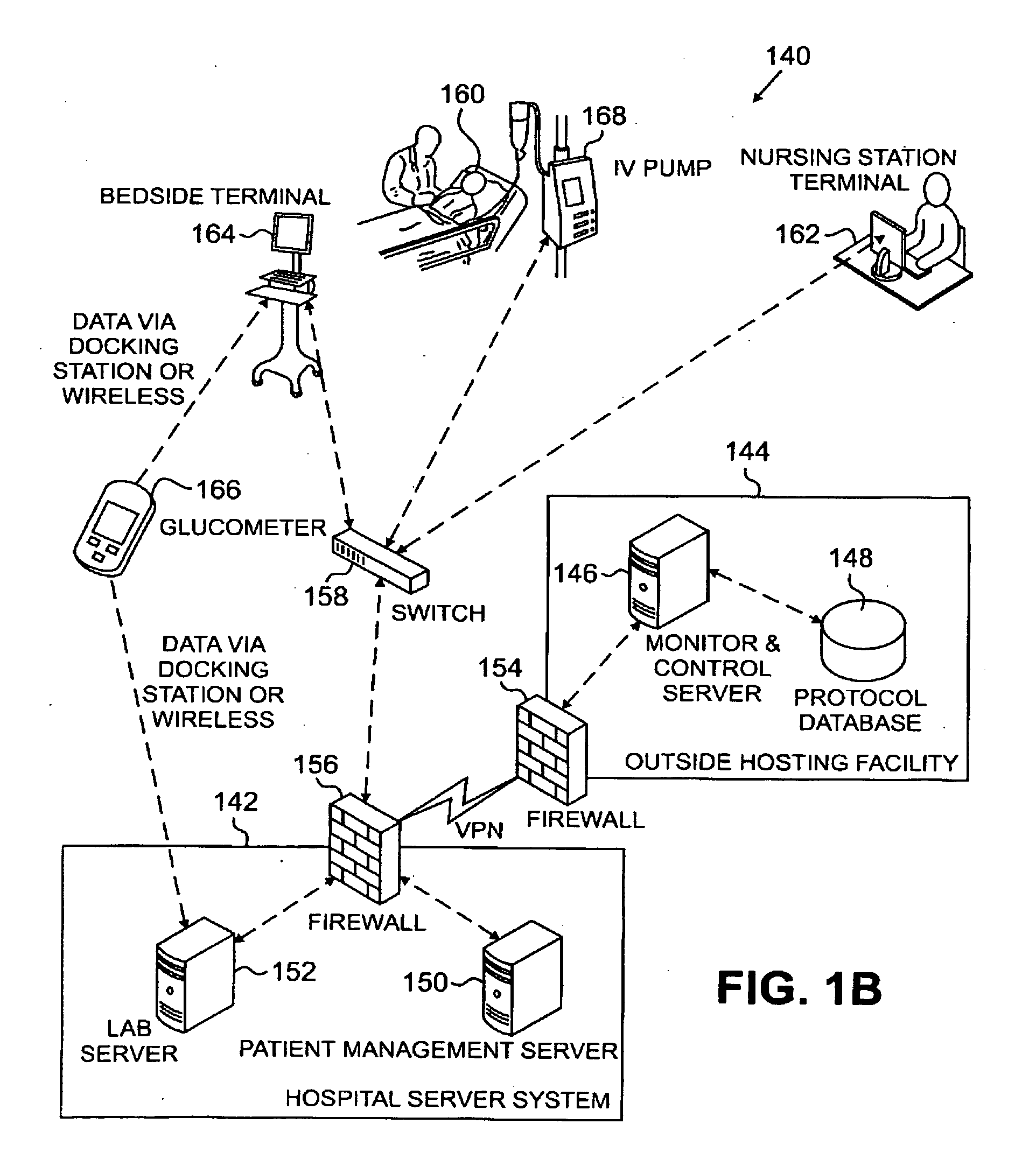
Systems, Methods, and Computer Program Product for Improved Management of Medical Procedures for Patients on Medical Protocols
Techniques are described for improved monitoring and control of a patient's physiologic status, such as blood glucose control according to a protocol for use in a patient care unit, such as an intensive care unit. Instructions to medical staff are adapted to factor in variations in responses to protocol recommendations. For example, a patient's physiological data as a result of a blood analysis is submitted as input to a program. An instruction for a medical procedure for the patient in response to the physiological data is automatically provided by the program. A confirmation response that the medical procedure has been accomplished and results of following the medical procedure are requested by the program. The time when the medical procedure was accomplished and the results of following the medical procedure are evaluated by the program to determine whether to adapt a subsequent instruction.
Owner:PRONIA MEDICAL SYST
Methods for improving blood glucose control
InactiveUS20120295985A1Increase insulin sensitivityReduce insulin resistanceBiocideOrganic chemistryQuinoneGlycemic
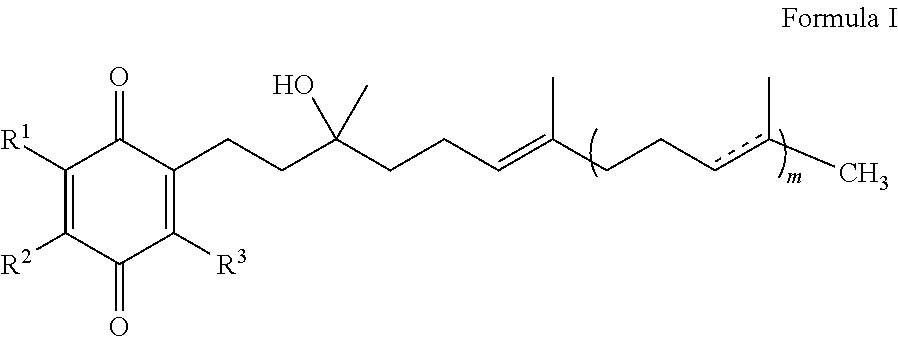
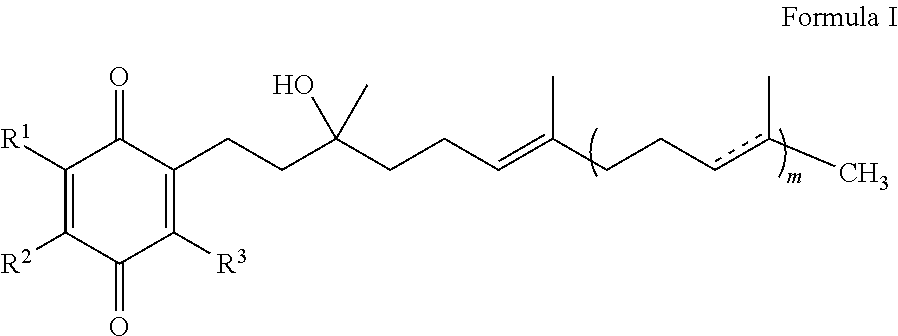
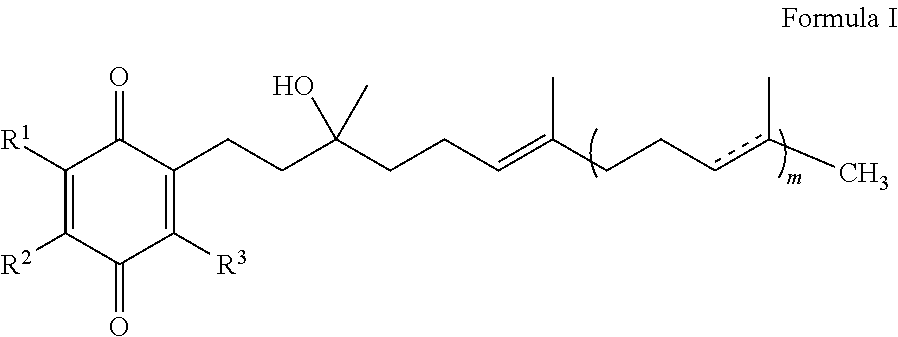
The present invention is concerned with a method for improving blood glucose control or for preventing or treating a condition requiring increasing insulin sensitivity or reducing insulin resistance in a subject in need of such control which comprises administering to the subject a composition comprising a therapeutically effective amount of one or more quinones of Formula I.
Owner:EDISON PHARMA
Formulation of a mixture of Free-B-ring flavonoids and flavans as a therapeutic agent
The present invention provides a novel composition of matter comprised of a mixture of two specific classes of compounds—Free-B-ring flavonoids and flavans—for use in the prevention and treatment of diseases and conditions mediated by the COX-2 and 5-LO pathways. The present invention further provides a novel method for simultaneously inhibiting the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) enzymes, and reducing cox-2 mRNA production. Finally, the present invention includes a method for weight loss and blood glucose control. The methods of this invention are comprised of administering to a host in need thereof an effective amount of the composition of this invention together with a pharmaceutically acceptable carrier. This invention relates generally to the prevention and treatment of diseases and conditions mediated by the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) pathways, including but not limited to the relief joint discomfort and pain associated with conditions such as osteoarthritis, rheumatoid arthritis, and other injuries that result from overuse.
Owner:UNIGEN
Dietary supplement for supressing appetite, enhancing and extending satiety, improving glycemic control, and stimulant free
This invention relates to a nutritional intervention composition for enhancing satiety prior to a meal and extending satiety after a meal. The nutritional intervention composition decreases food intake producing weight loss over time. The composition consists of Niacin, Vitamin B6, Calcium, Phosphorous, Magnesium, Chromium, Chitosan, Fenugreek, Ginseng, White willow bark, Garcinia cambogia, Aloe Vera gel powder, Momordica charantia, Griffonia simplicifolia, Lagerstroemia speciosa and Vanadyl sulfate. The invention does not require stimulants or anabolic ingredients. There are three phases of activity within the composition. One, enhanced satiety through elevated serotonin. Two, improved carbohydrate metabolism, reduced blood glucose and slowed gastric emptying. Three, enhanced fiber binding of lipids and excess bile acids.
Owner:NEEDLEMAN ALVIN +1
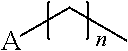
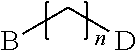
Polypeptide production in animal cell culture
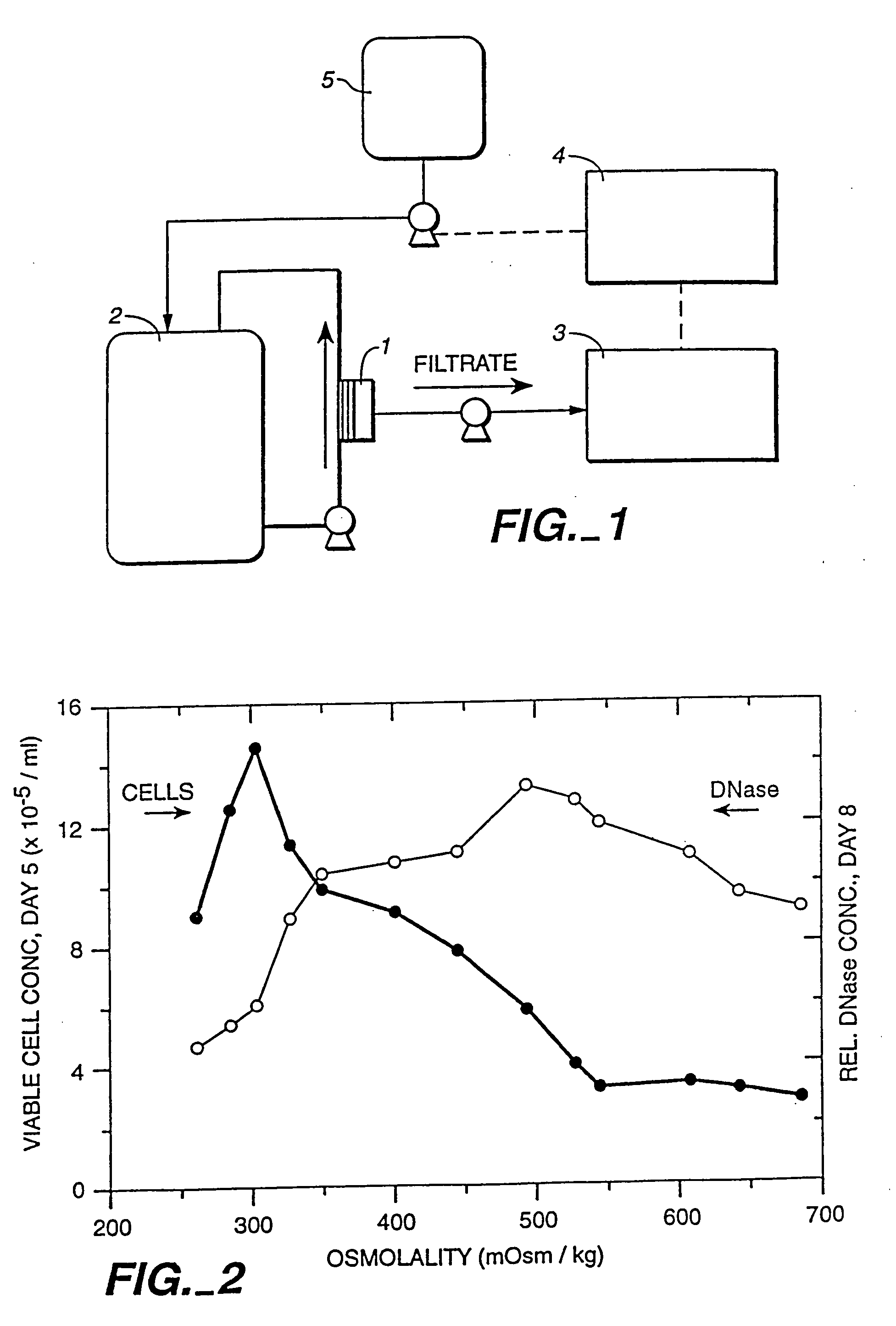
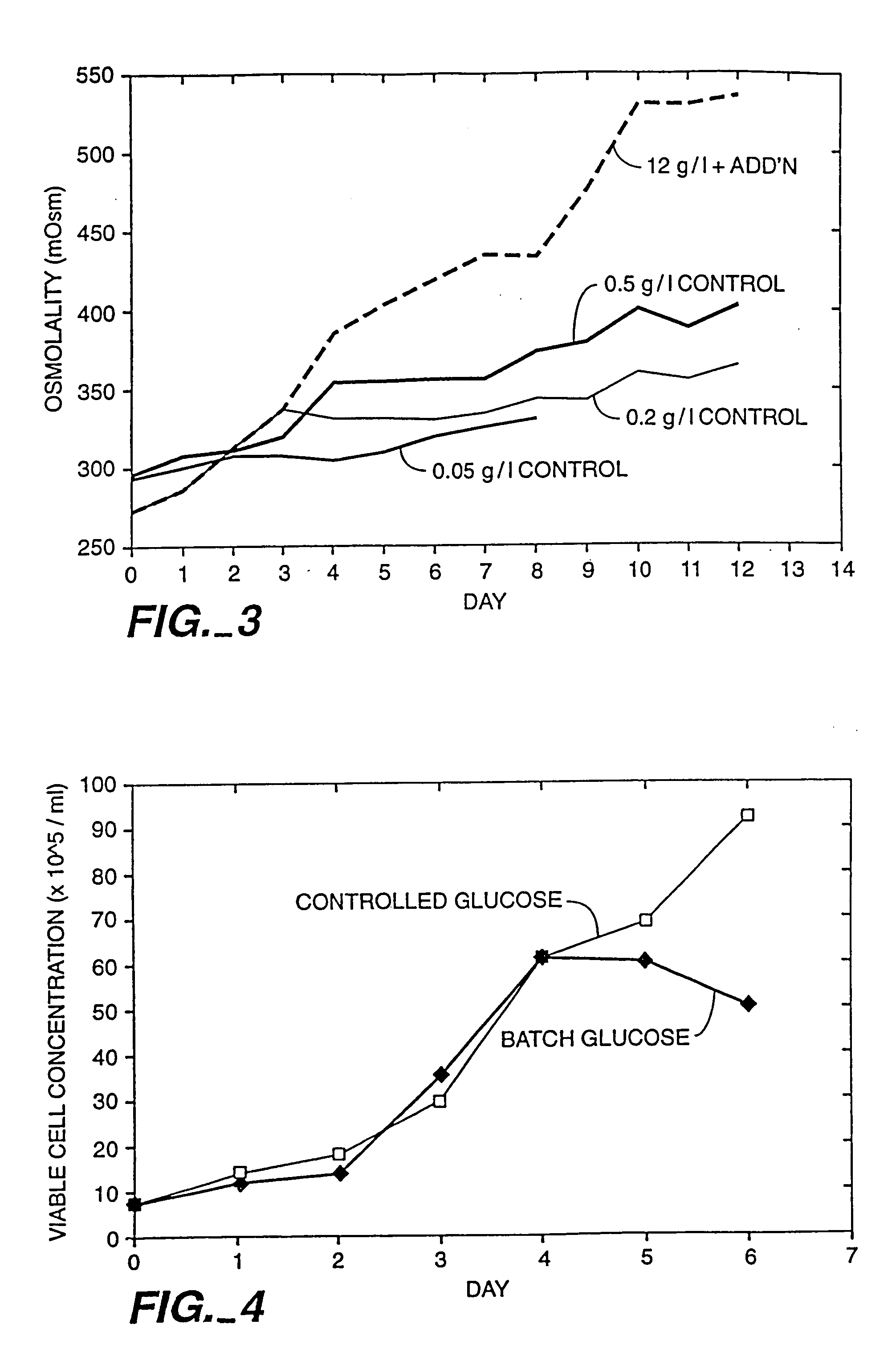
InactiveUS20050272124A1Promote cell growthHigh protein yieldGenetically modified cellsCulture processBiotechnologyGrowth phase
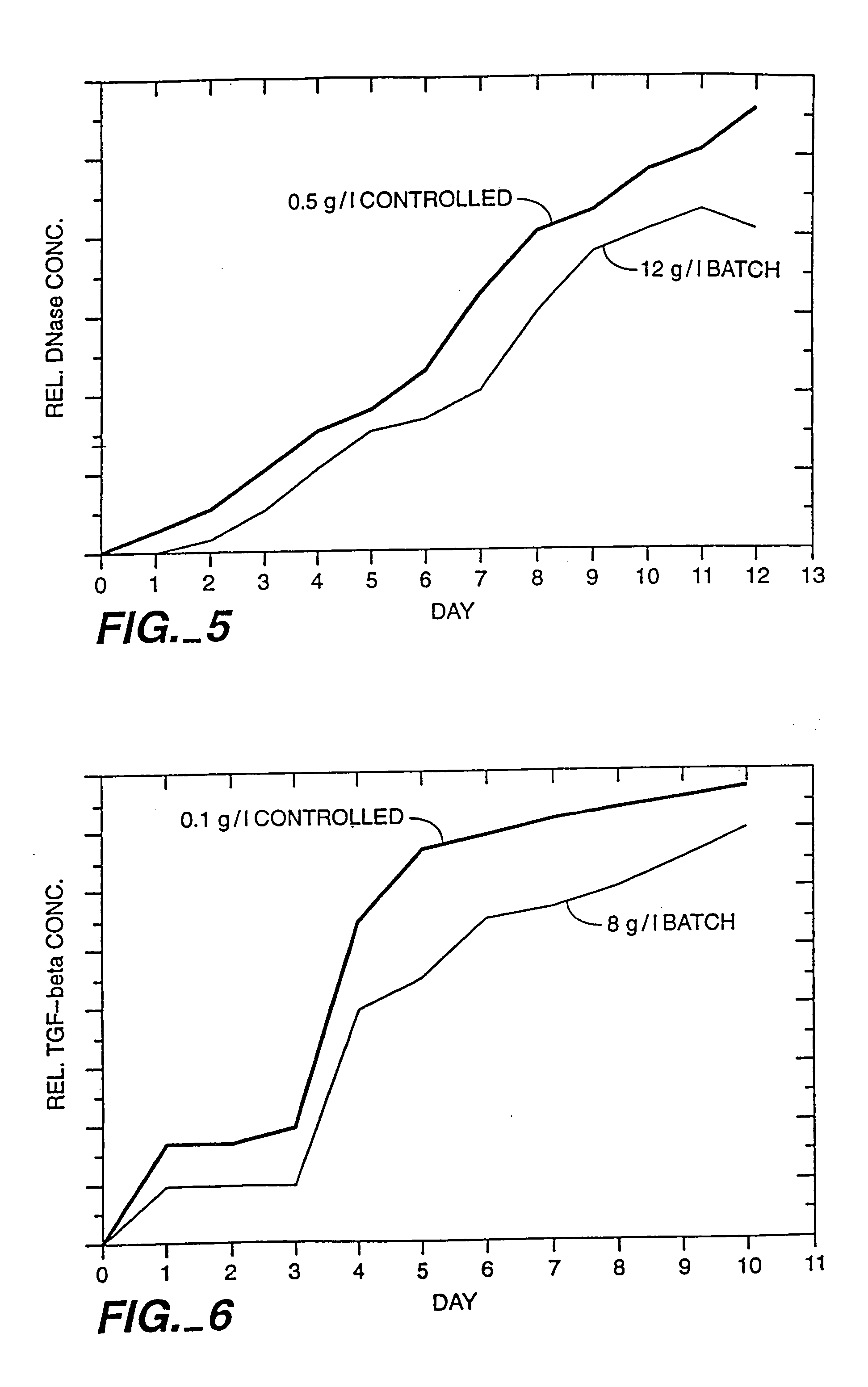
A method of producing a polypeptide in fed batch cell culture is provided which involves an initial cell growth phase and a distinct production phase. In the initial growth stage, animal cells having nucleic acid encoding the polypeptide are cultured at a starting osmolality of about 280-330 mOsm in the presence of a concentration of glucose controlled throughout the culturing to be within a range between about 0.01 and 1 g / L. This is followed by a production phase, where the cultured animal cells of the growth phase are inoculated at a cell seed density of at least 1.0×106 cells / mL and the cells are cultured at a starting osmolarity of about 400-600 mOsm in the presence of a concentration of glucose controlled throughout the culturing to be within a range between about 0.01 and 1 g / L. Preferably, the glutamine concentration in the cell culture medium is simultaneously controlled in order to curtail production of lactic acid and ammonia which result from unnecessarily high glutamine concentrations. During the growth phase, production of potentially detrimental metabolic waste products, such as lactic acid, is controlled thereby curtailing the increase of osmolality due to accumulation and neutralization of waste products. Thus, the cell growth can be improved. In the production phase, the cell culture conditions are modified in order to arrest or reduce cell growth and thereby direct nutrient utilization toward production, as opposed to cell growth. Overall, it is intended that the method results in an improvement in specific productivity, reduction in production run times and / or an increase in final product concentration.
Owner:GENENTECH INC
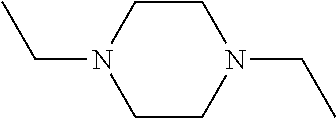
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Methods and compositions for weight management and for improving glycemic control
InactiveUS20120052151A1Reduce the amount requiredIncrease satietyFood ingredient as viscosity modification agentDough treatmentMedicineBody weight
The present invention provides methods, compositions and modified foods and foodstuffs useful for weight management and glycemic control.
Owner:GELESIS
Method, System and Computer Readable Medium for Adaptive and Advisory Control of Diabetes
ActiveUS20150190098A1Reducing basal rateMedical simulationPhysical therapies and activitiesPhysiologyInsulin pump
An Adaptive Advisory Control (AA Control) interactive process involving algorithm-based assessment and communication of physiologic and behavioral parameters and patterns assists patients with diabetes with the optimization of their glycemic control. The method and system may uses all available sources of information about the patient; (i) EO Data (e.g. self-monitoring of blood glucose (SMBG) and CMG), (ii) Insulin Data (e.g. insulin pump log files or patient treatment records), and (iii) Patient Self Reporting Data (e.g. self treatment behaviors, meals, and exercise) to: retroactively assess the risk of hypoglycemia, retroactively assess risk-based reduction of insulin delivery, and then report to the patient how a risk-based insulin reduction system would have acted consistently to prevent hypoglycemia.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Analysis of glucose median, variability, and hypoglycemia risk for therapy guidance
ActiveUS20140188400A1Improve accuracyHealth-index calculationMedical automated diagnosisLow glucoseDiabetes Therapy
A system and method to provide guidance for diabetes therapy includes determining glycemic risks based on an analysis of glucose data. The analysis includes visualization of a glucose median, the variability of glucose in a patient, and the risk of hypoglycemia. An Advanced Daily Patterns report includes a visualization of an ambulatory glucose profile and a glucose control measure. The glucose control measure provides a highly visible and understandable display of the glucose condition of a patient visually expressed in the categories of low glucose, median glucose, and glucose variability.
Owner:ABBOTT DIABETES CARE INC
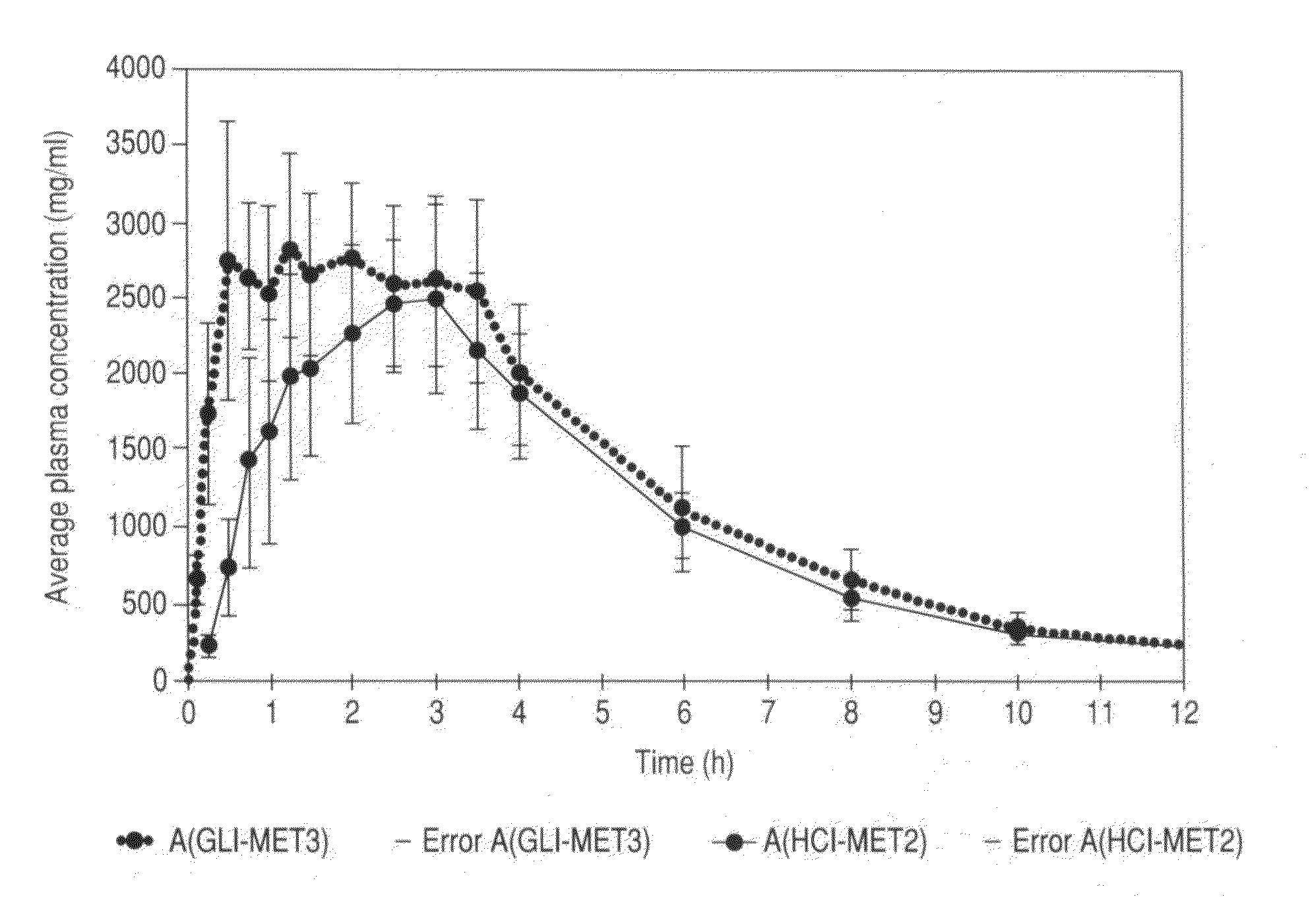
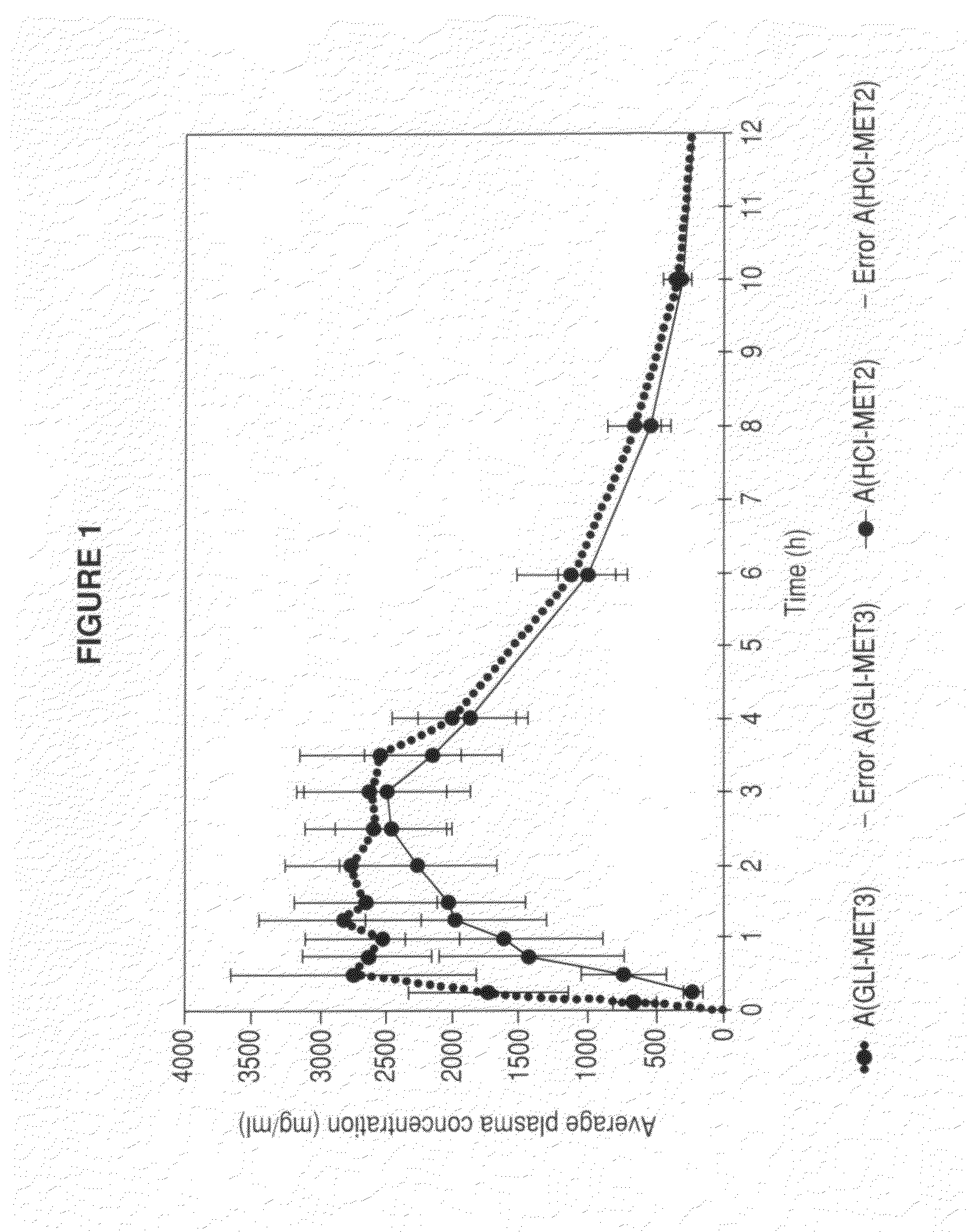
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
Composition for controlling increase in blood glucose
The present application describes a composition for blood glucose control containing green tea extracts (GTE) with gallated catechins (GC) and macromolecule to prevent the intestinal absorption of GC.
Owner:IND ACADEMIC COOPERATION FOUND KEIMYUNG UNIV
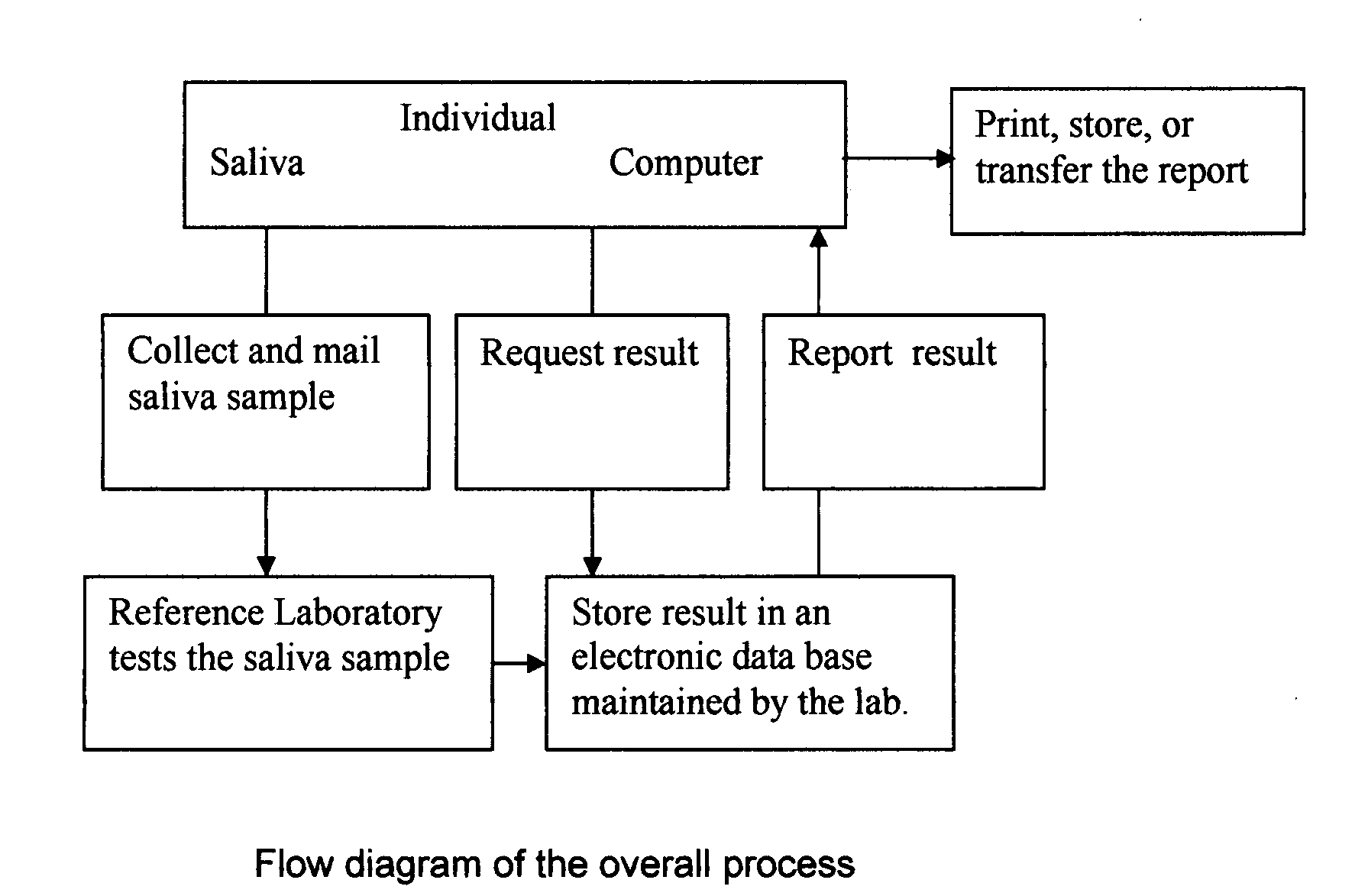
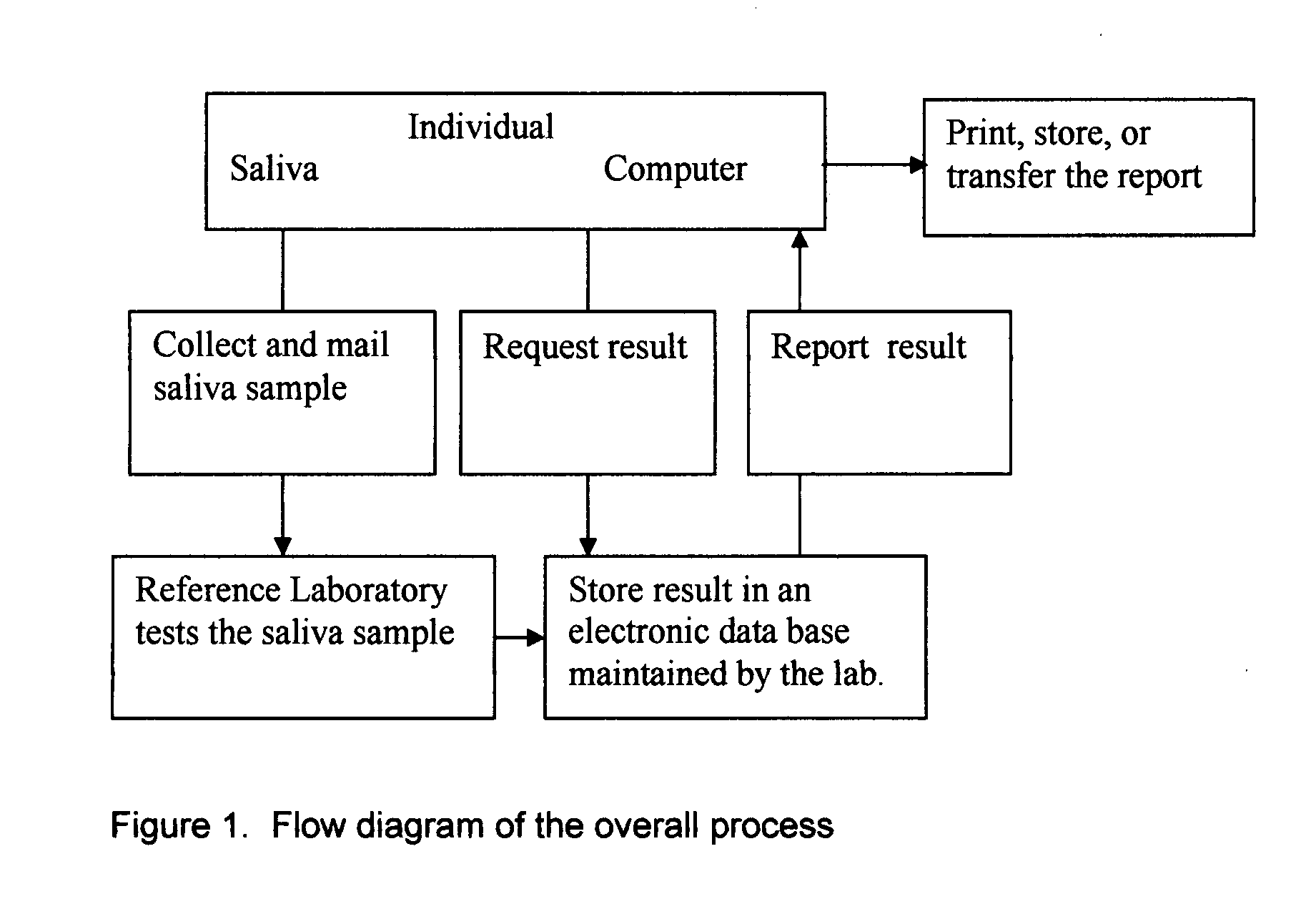
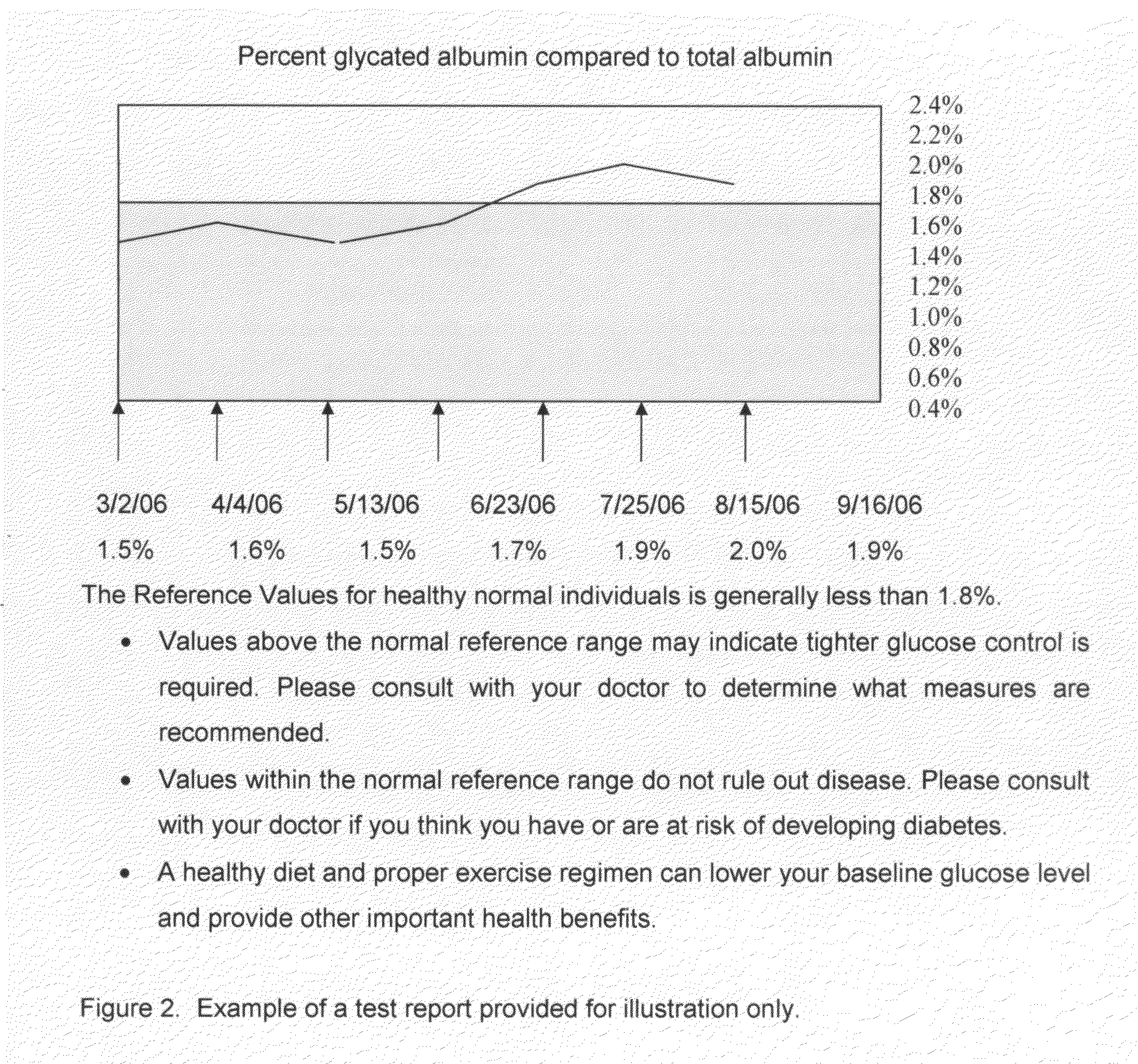
Home test for glycated albumin in saliva
InactiveUS20080227210A1Withdrawing sample devicesVaccination/ovulation diagnosticsSaliva sampleSaliva collection
A home test for measuring glycated albumin levels in saliva. The saliva sample is collected at home using a standardized saliva collection kit and mailed to a testing laboratory that performs the test and reports the result directly back to the customer via the internet. The home test can be used to monitor glucose control in diabetics and in healthy individuals. It may also be used as a diagnostic aid in identifying individuals with diabetes, or who are at risk of developing diabetes.
Owner:SMITH HENRY
Process, composition and kit for providing a stable whole blood calibrator/control
InactiveUS20060188995A1Quality improvementSimple and elegantMaterial analysis by optical meansBiological testingMedicineMeasuring instrument
The present invention is directed toward a stable calibrator and / or control, kit and process for using in a glucose monitoring instrumentation. Principally, the instant invention teaches a glycolyzed red blood cell component, which has been treated with a glycolysis stabilizing effective amount of at least one non-crosslinking aldehyde compound. The glycolyzed red blood cell component may be added to fresh plasma along with an amount of glucose to form a simulated whole blood glucose control product, effective for maintaining a particular and essentially stable glucose concentration over a period of time sufficient for accurate measurement and calibration of a glucose measuring instrument.
Owner:STRECK INC
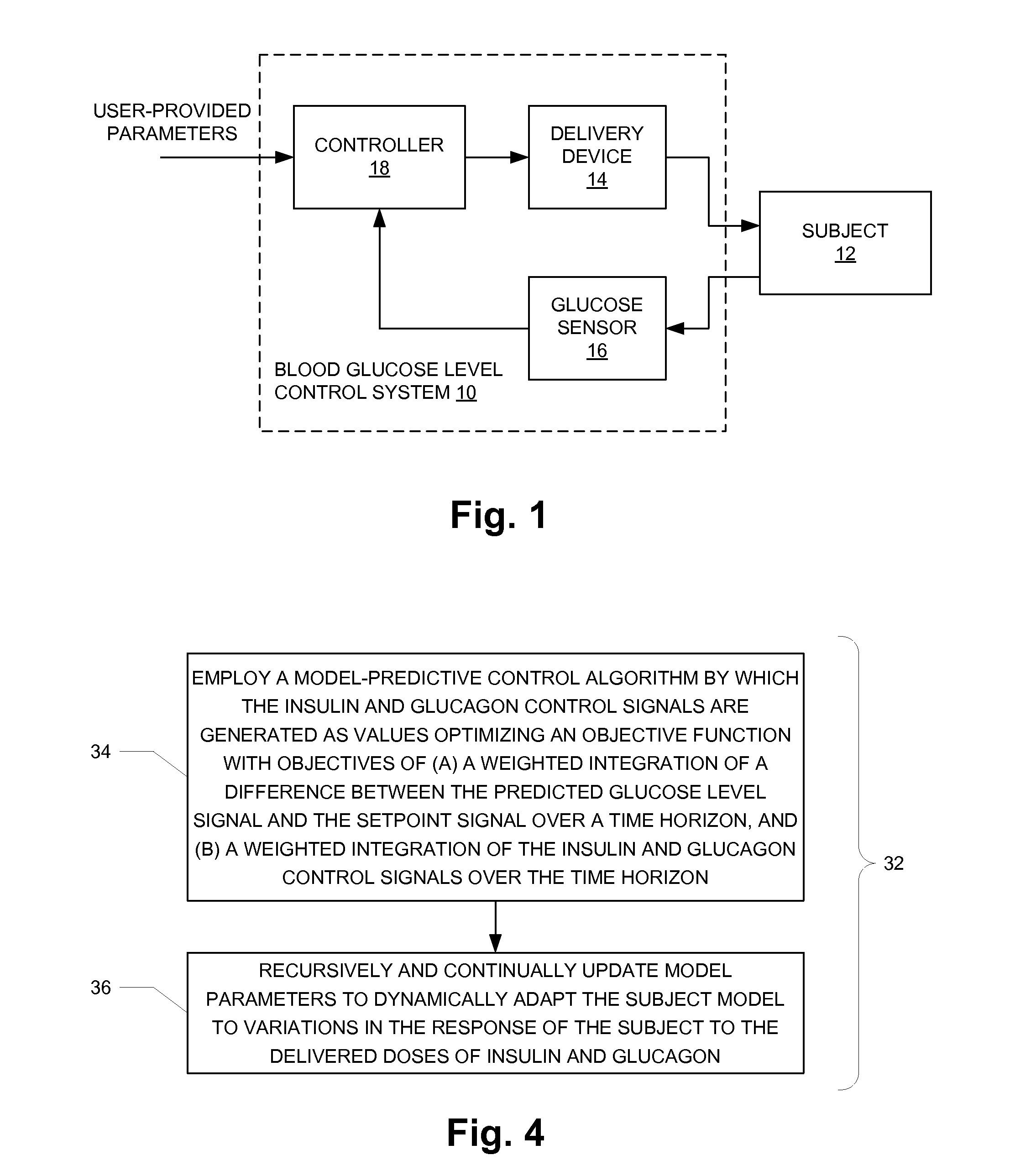
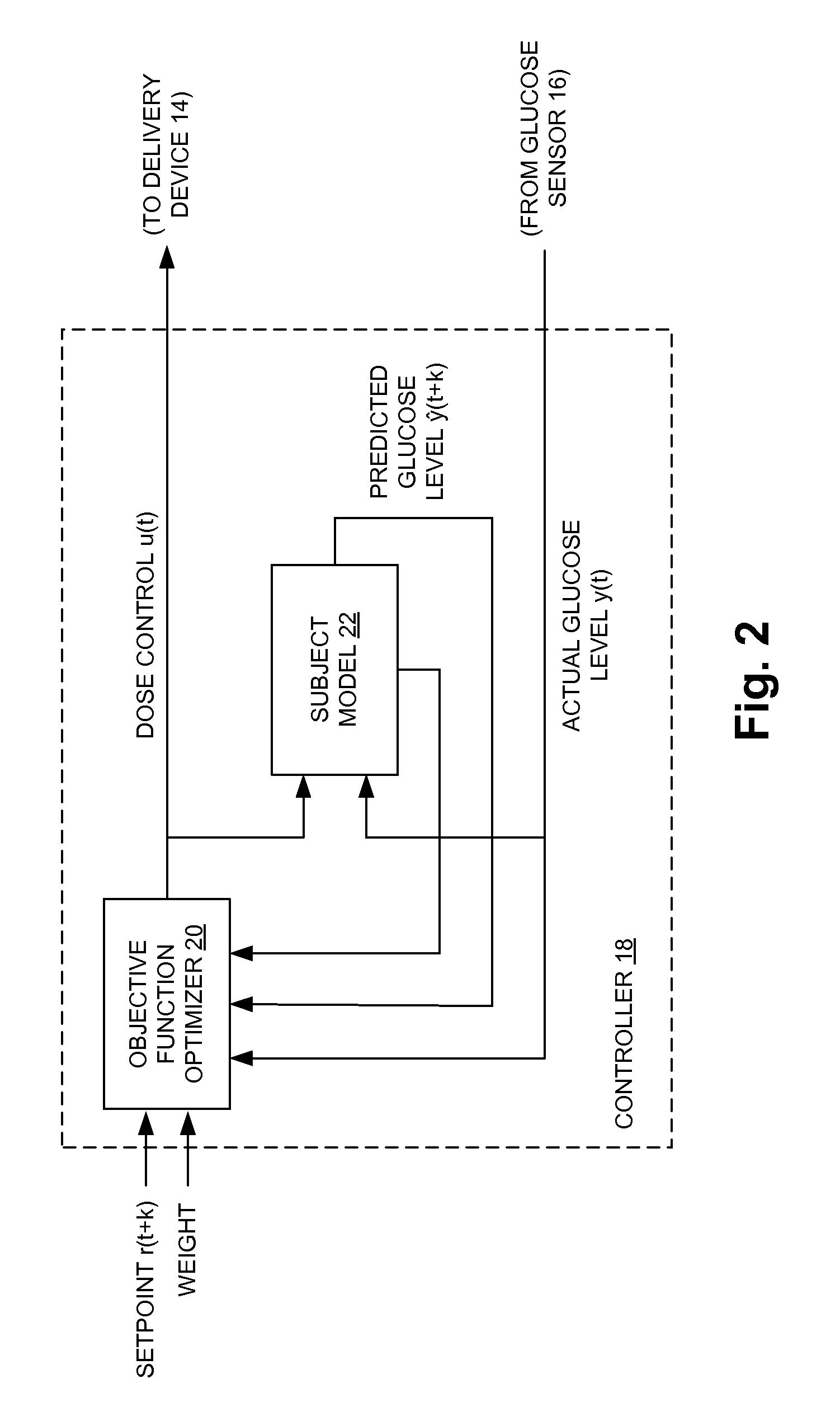
Fully automated control system for type 1 diabetes
ActiveUS8273052B2Reduce the burden onRegulate blood glucose levelMedical simulationMetabolism disorderControl signalPeak value
An augmented, adaptive algorithm utilizing model predictive control (MPC) is developed for closed-loop glucose control in type 1 diabetes. A linear empirical input-output subject model is used with an MPC algorithm to regulate blood glucose online, where the subject model is recursively adapted, and the control signal for delivery of insulin and a counter-regulatory agent such as glucagon is based solely on online glucose concentration measurements. The MPC signal is synthesized by optimizing an augmented objective function that minimizes local insulin accumulation in the subcutaneous depot and control signal aggressiveness, while simultaneously regulating glucose concentration to a preset reference set point. The mathematical formulation governing the subcutaneous accumulation of administered insulin is derived based on nominal temporal values pertaining to the pharmacokinetics (time-course of activity) of insulin in human, in terms of its absorption rate, peak absorption time, and overall time of action. The MPC algorithm is also formulated to provide control action with an integral effect, and in essence minimizes overall drug consumption. When employed as a modulator in an automated integrated glucose-control system for type 1 diabetes, the control algorithm provides the system with self-learning capability that enables it to operate under unrestricted activity of the subject.
Owner:TRUSTEES OF BOSTON UNIV
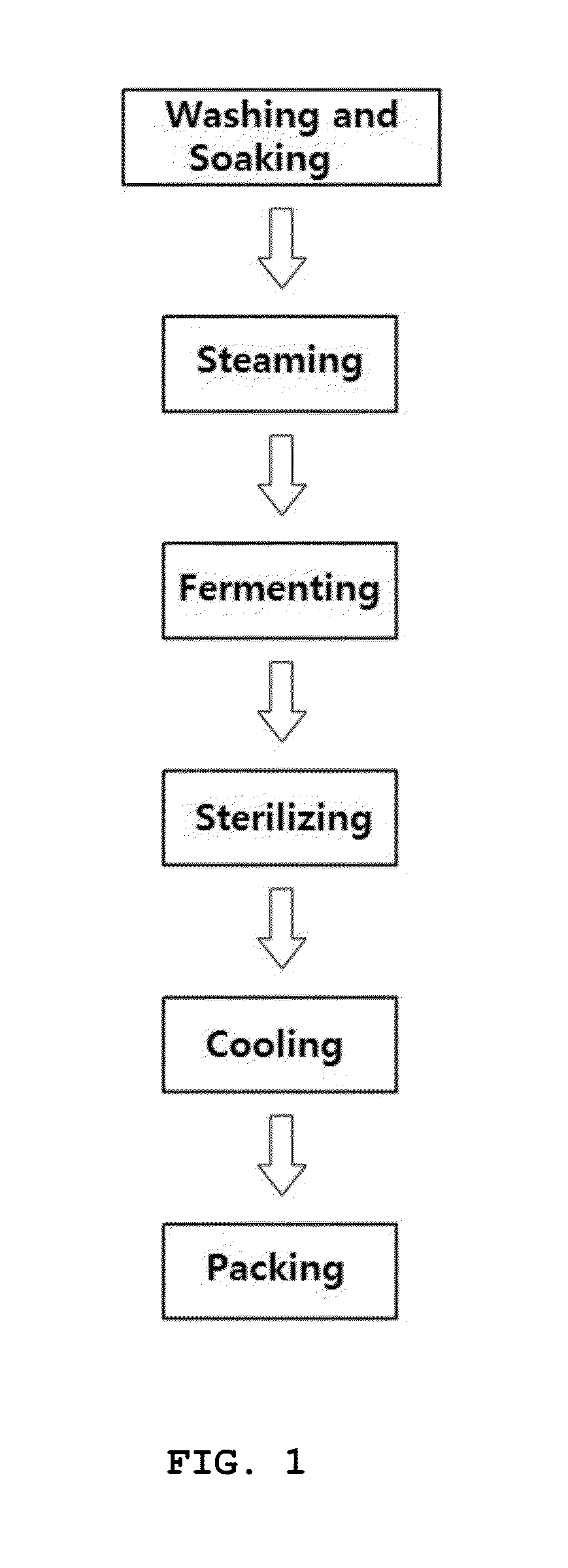
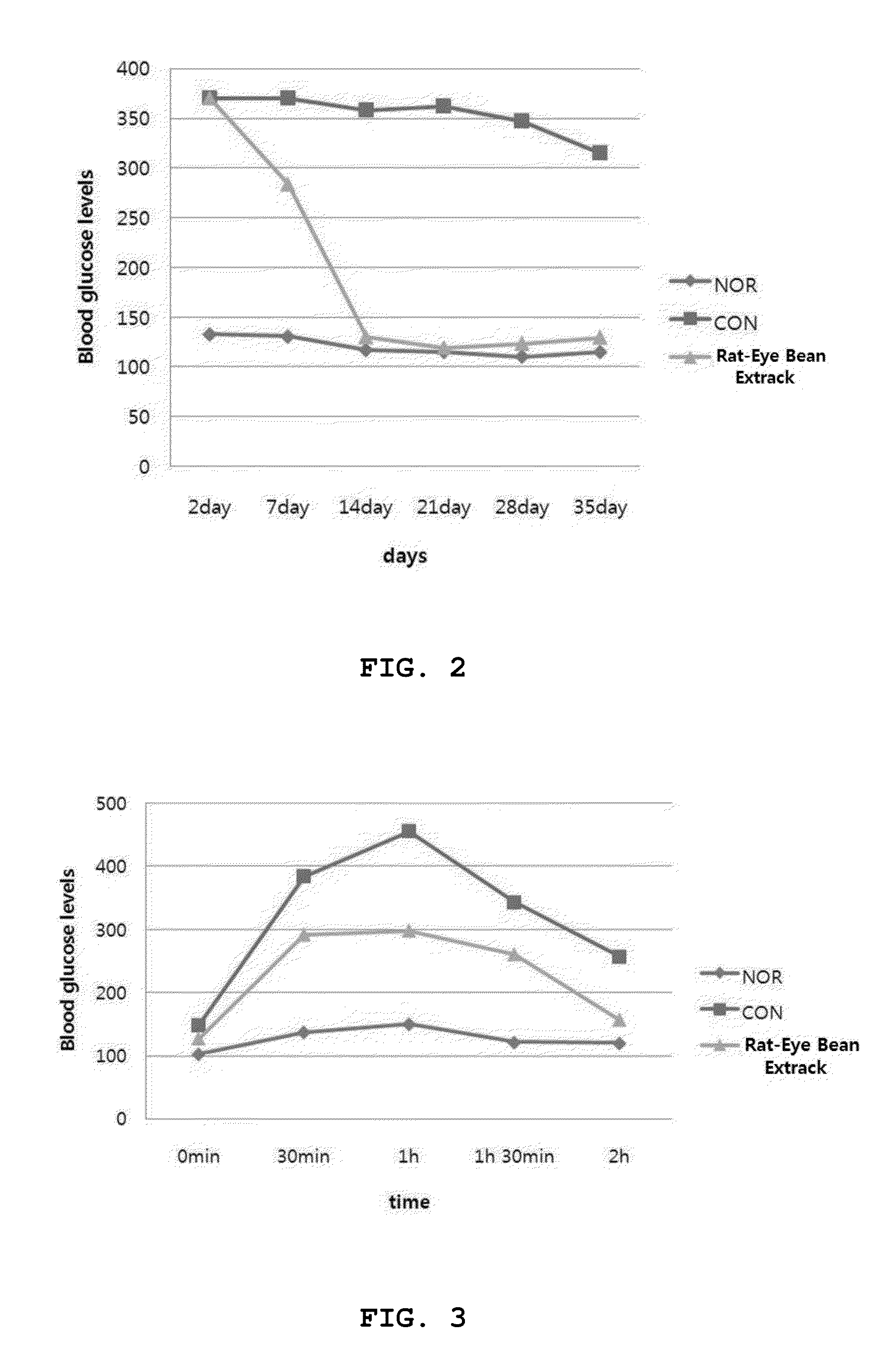
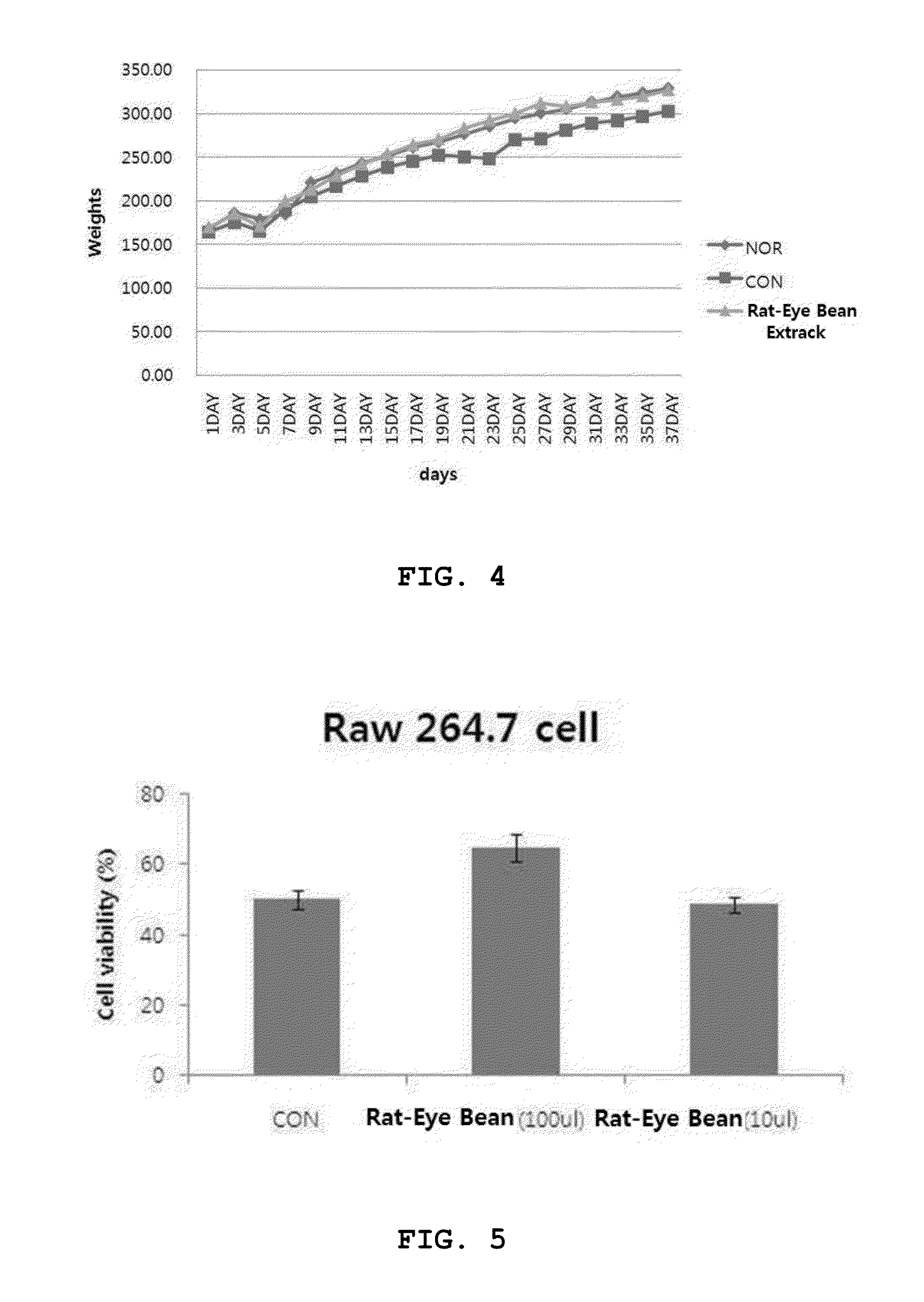
Rat-eye bean extract improving blood glucose control and bioactivity and method of producing the same
InactiveUS20140004211A1Improving bioactivityImproving blood glucose controlBiocideAnimal repellantsGlycemicBean extract
Provided are a rat-eye bean extract improving blood glucose control and bioactivity, which is produced from rat-eye beans as health food so as to be able to improving storability, portability and potability, and a method of producing the same. The rat-eye bean extract is produced by putting rat-eye beans, which are cleanly washed without foreign materials and are soaked in water for five hours, and water into a steam pot at a volume ratio of 3:1 along with rice straw, fermenting water, which is boiled to steam the rat-eye beans in weak flames for five hours, in a hot-floored room for two to three days, putting the fermented water and a grapefruit juice into the steam pot at a weight ratio of 99:1, and heating a mixture of the fermented water and the grapefruit juice in a closed state at a temperature of 110 to 120° C. for four to five hours.
Owner:CHOI BYEONGCHEOL +2
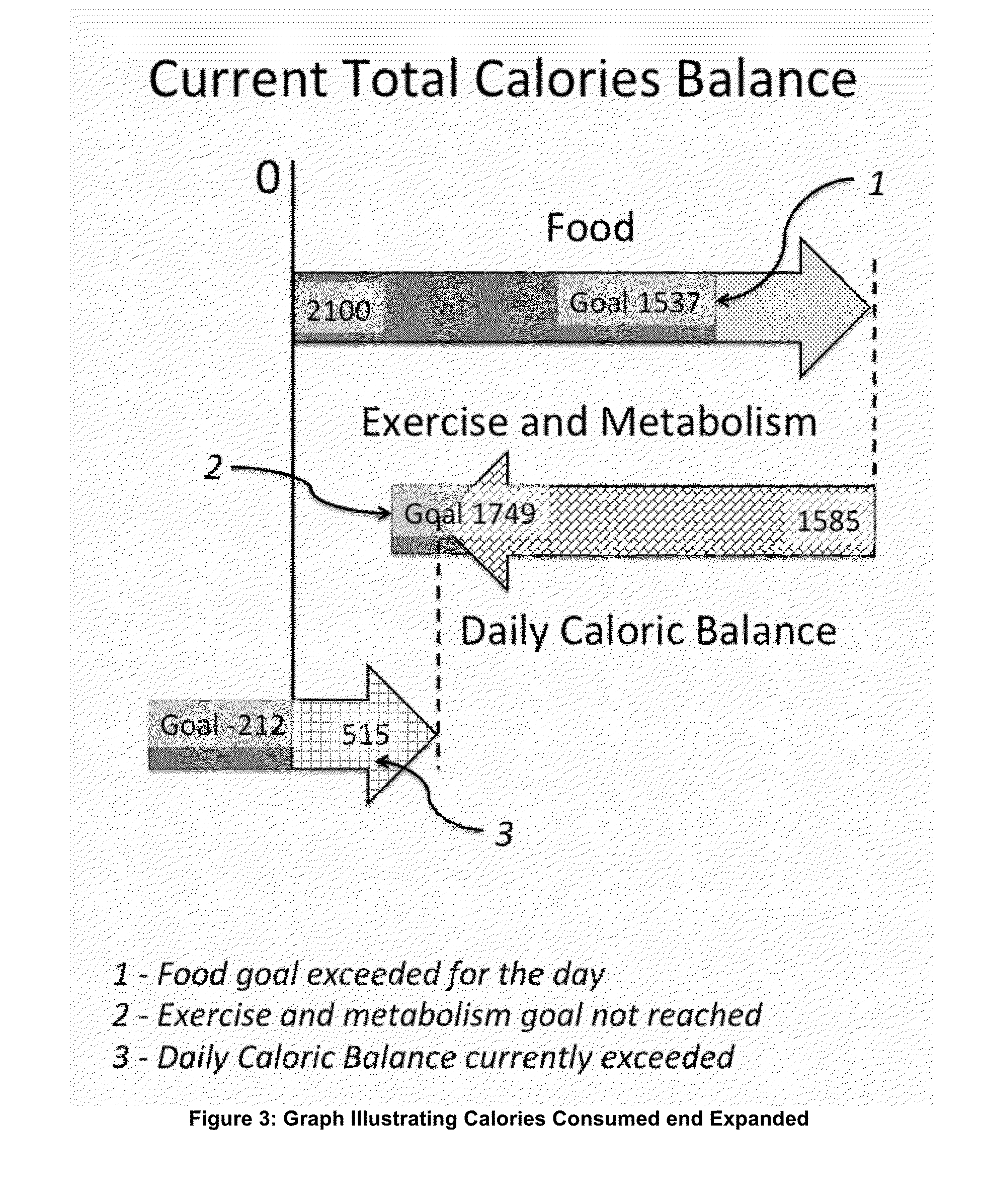
Energy and Food Consumption Tracking for Weight and Blood Glucose Control
InactiveUS20160166195A1Good estimateMinimize effortPhysical therapies and activitiesPerson identificationPedometerGlycemic
The present invention relates to the area of lifestyle devices and applications, particularly to daily trackers of activities, such as pedometers used for exercise monitoring, and working in collaboration with tools analyzing and planning the food intake and amount of exercises to keep or obtain the desired weight. This invention aims at better controlling: fitness level, food consumption, blood glucose level and weight of a person throughout the day. For daily and weekly tracking of the weight, the ingested and expended calories are reported. Calories meal goals are adjusting daily reflecting the over the goal exercise levels as well as mismatch in food consumed and meal goals. The method for better blood glucose control predicts the effects of the food ingested on raising a post-meal blood glucose level, and suggests a suitable timing and the duration of the exercise following the food intake to minimize a post-meal blood glucose peak.
Owner:RADECKA KATARZYNA +1
Glucagon analogues
InactiveUS20130316941A1Avoid weight gainGood for weight lossPeptide/protein ingredientsAntipyreticWeight gain preventionGlycemic
The invention provides glucagon analogue peptides and their use for promoting weight loss or preventing weight gain, and the treatment of obesity or excess body weight and associated conditions. The compounds may also be used to improve glycemic control and / or for the treatment of diabetes. The compounds may mediate their effect, inter alia, by having increased selectivity for the GLP-1 receptor as compared to human glucagon.
Owner:BOEHRINGER INGELHEIM INT GMBH +1
Fully automatic intelligent infusion method and device based on model predictive control for large doses of insulin
The invention provides an intelligent method based on model predictive control for automatic infusion of large doses of insulin, which is characterized in that: a continuous glucose monitoring system (CGMS) and an insulin pump are used as the basis of hardware; online detection of food is carried out based on a strong tracking filter; when the food is detected, large doses of insulin is transfused immediately, and the amount of insulin depends on historical food; whether the dosage is needed to be increased is judged every 30 minutes based on the model predictive control, and the dosage is designed; when the blood glucose concentration decreases, whether the basic amount of insulin is needed to be maintained is determined according to the predicted blood glucose value; and finally the blood glucose concentration is controlled to be within the safe range. Compared with the existing corresponding technology, the invention has the advantages of high degree of intelligence and fully automatic operation, and the blood glucose control effects can be improved significantly in case that self-management cannot be carried out by patients.
Owner:BEIJING UNIV OF CHEM TECH
Process, composition and kit for providing a stable whole blood calibrator/control
InactiveUS7390663B2Quality improvementSimple and elegantSamplingMicrobiological testing/measurementMedicineConcentrations glucose
The present invention is directed toward a stable calibrator and / or control, kit and process for using in a glucose monitoring instrumentation. Principally, the instant invention teaches a glycolyzed red blood cell component which has been treated with a glycolysis stabilizing effective amount of at least one non-crosslinking aldehyde compound which may be added to fresh plasma along with an amount of glucose to form a simulated whole blood glucose control product, effective for maintaining a particular and essentially stable glucose concentration over a period of time sufficient for accurate measurement and calibration of a glucose measuring instrument.
Owner:STRECK INC
Oral insulin therapies and protocol
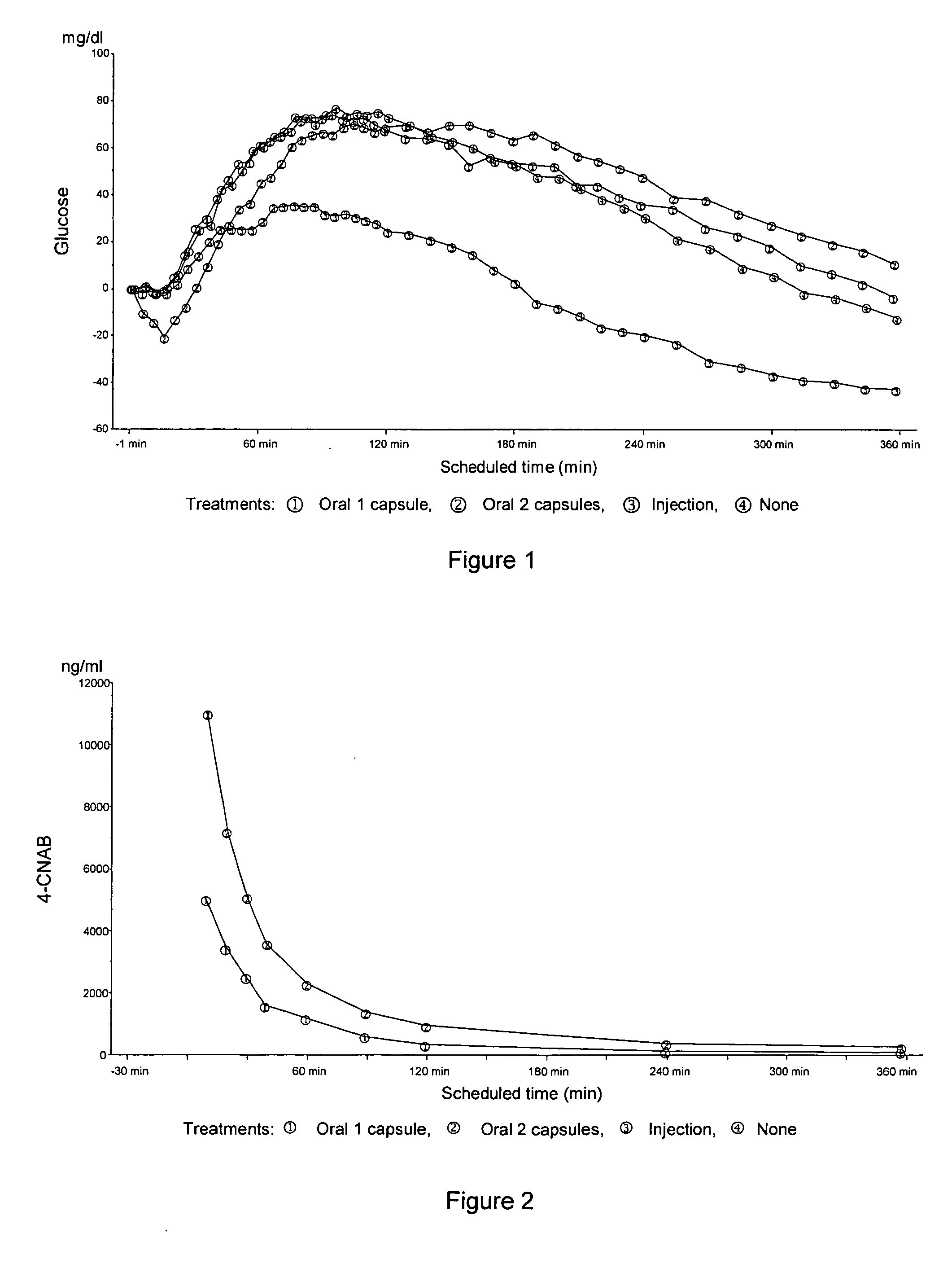
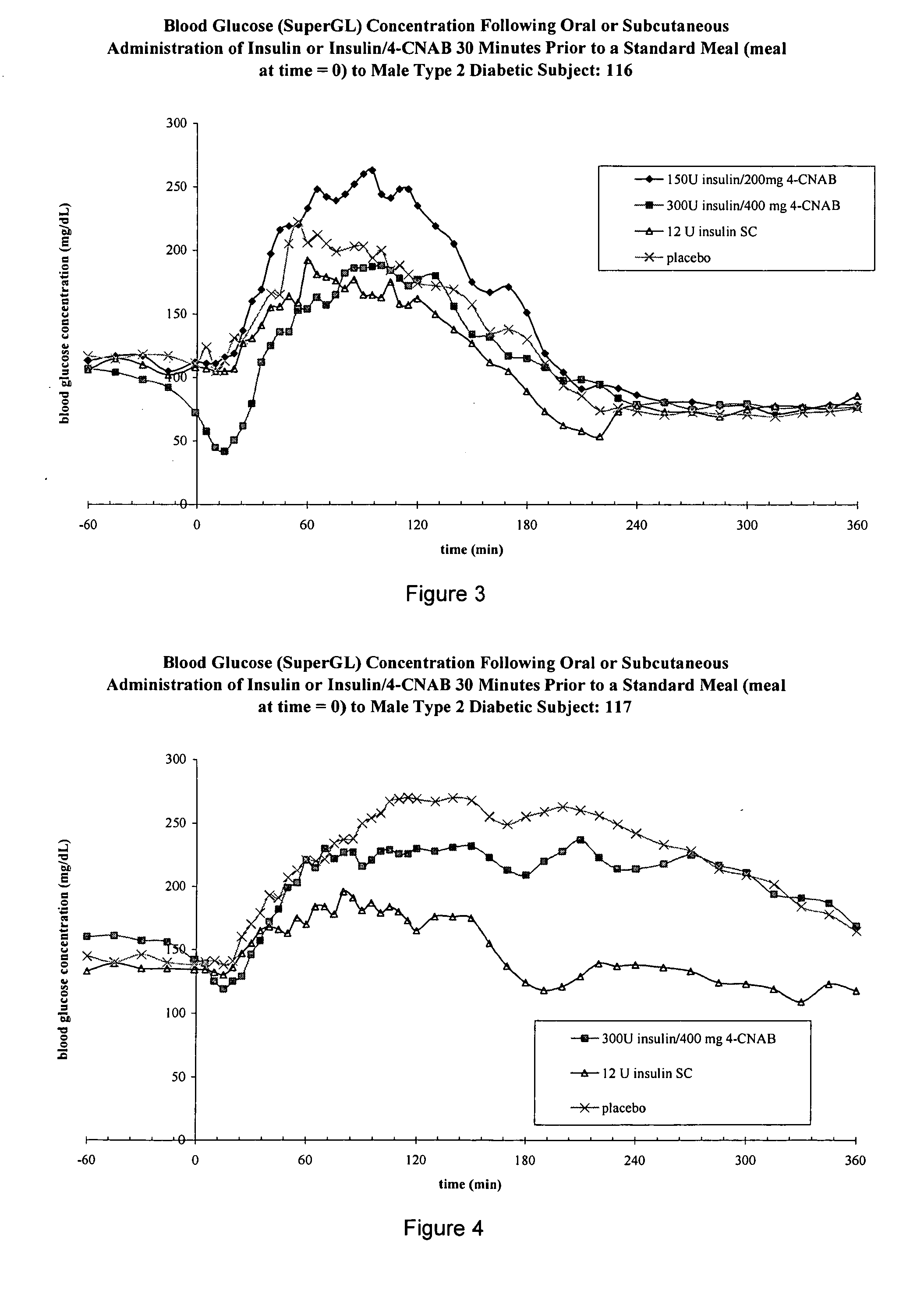
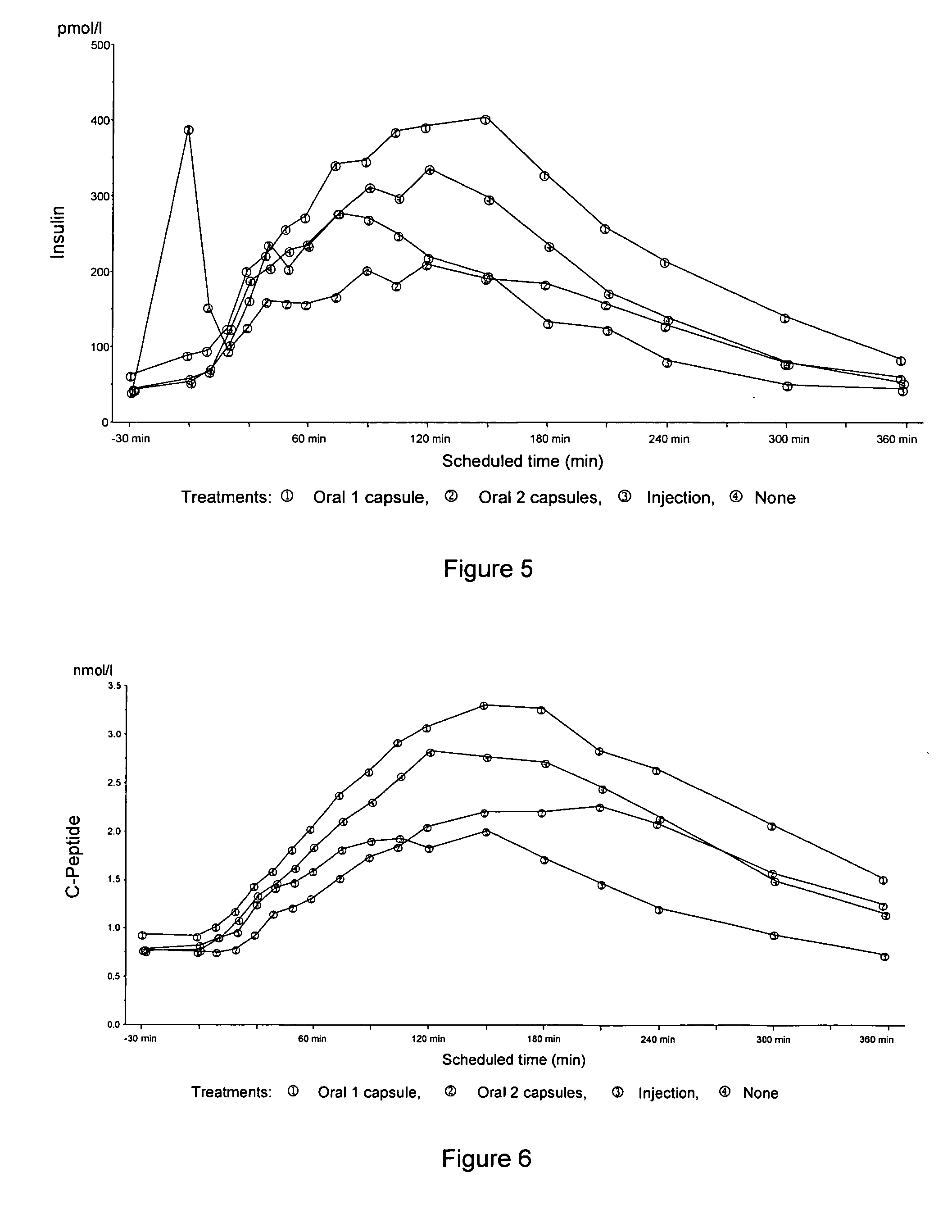
InactiveUS20050203001A1Good treatment effectPositive therapeutic effectOrganic active ingredientsPeptide/protein ingredientsLate onset diabetesHypoglycemia
Methods for treating impaired glucose tolerance and early and late stage diabetes in mammals, for prophylactically sparing β-cell function, aiding in preventing β-cell death, preventing the onset of overt diabetes in a mammal with type 2 diabetes, treating the current level of glycemic control dysfunction of a mammal with impaired glucose tolerance or diabetes, comprising orally administering insulin and a delivery agent that facilitates insulin absorption from the gastrointestinal tract at the time of or shortly before mealtime, e.g., within about 10 minutes prior to ingestion of a meal, on a chronic basis. The methods also comprise, in addition to administering a rapid-acting insulin to provide a first insulin peak, administering a slow acting insulin to provide a second insulin peak occurring at a later time but of a longer duration. These methods achieve improved glycemic control without the risks of hypoglycemia, hyperinsulinemia and weight gain and the need for frequent blood glucose monitoring that are normally associated with insulin therapy.
Owner:EMISPHERE TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com