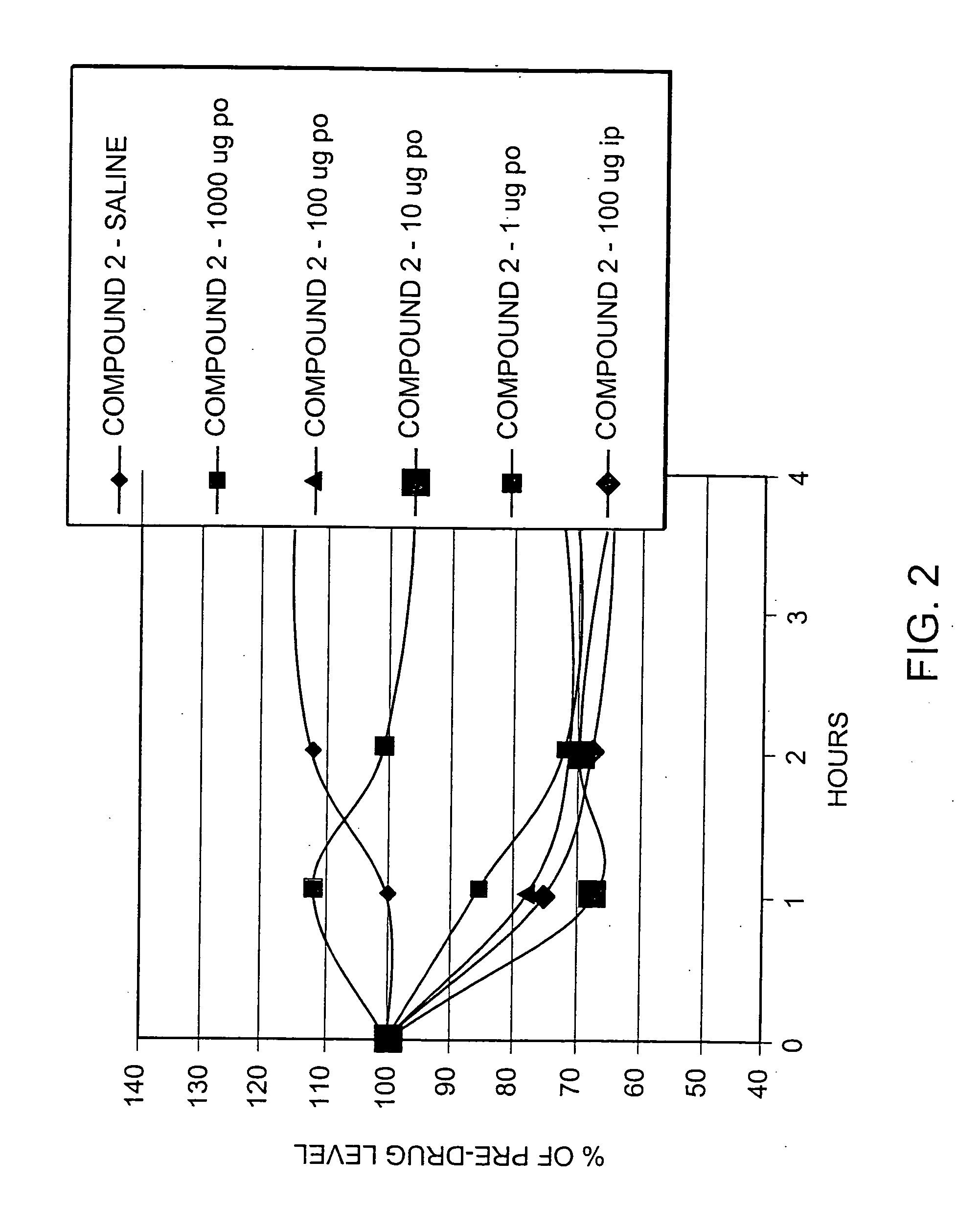
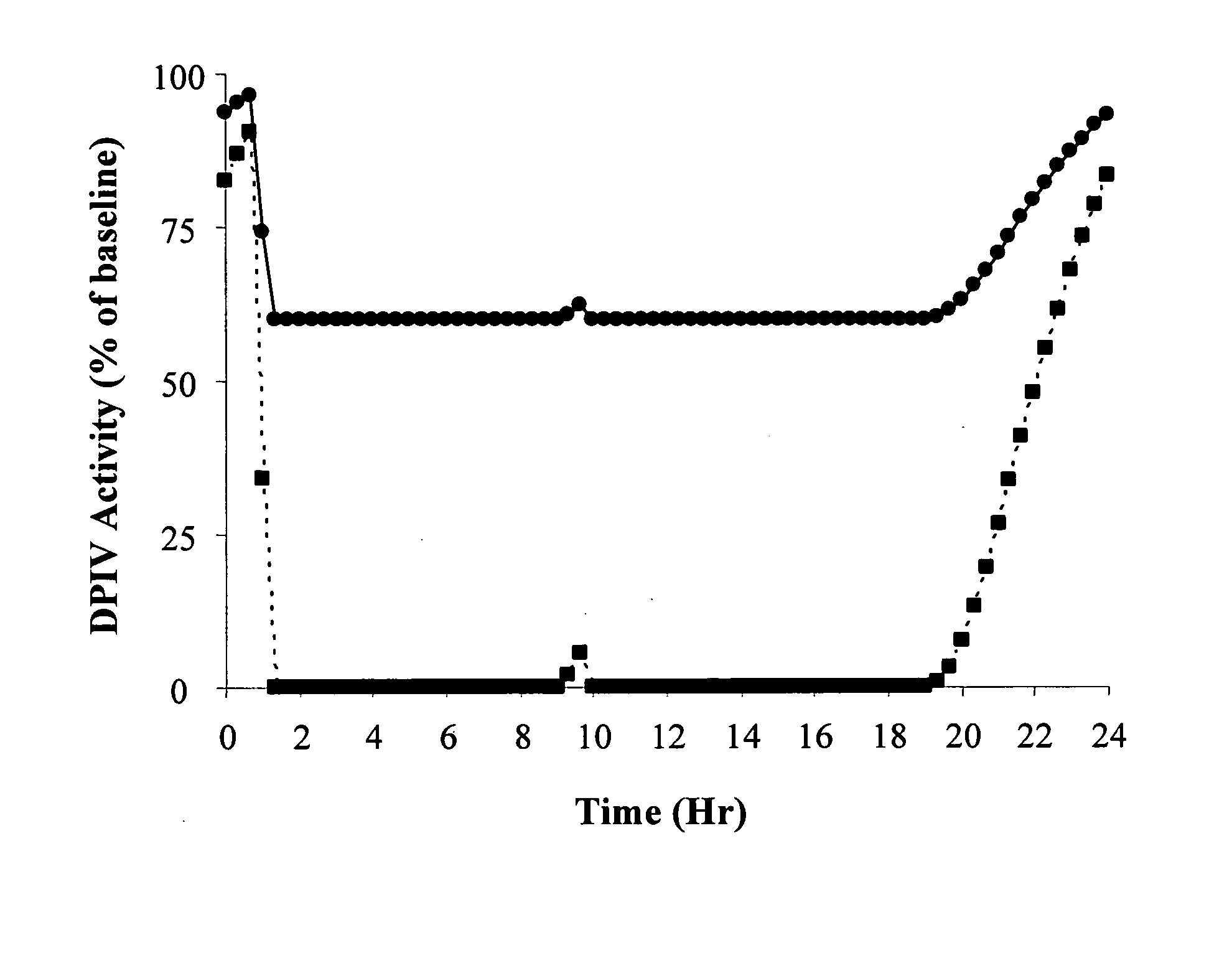
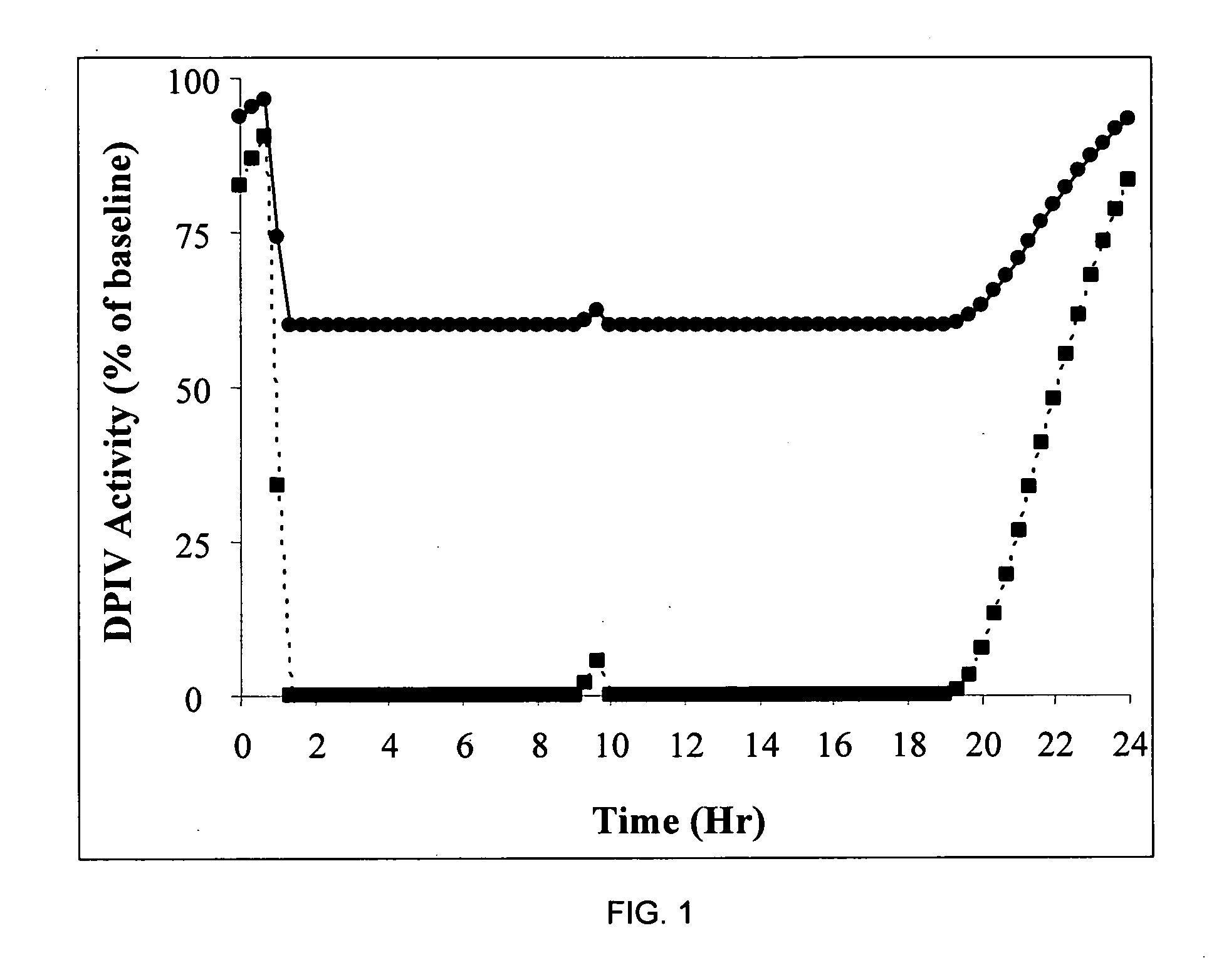
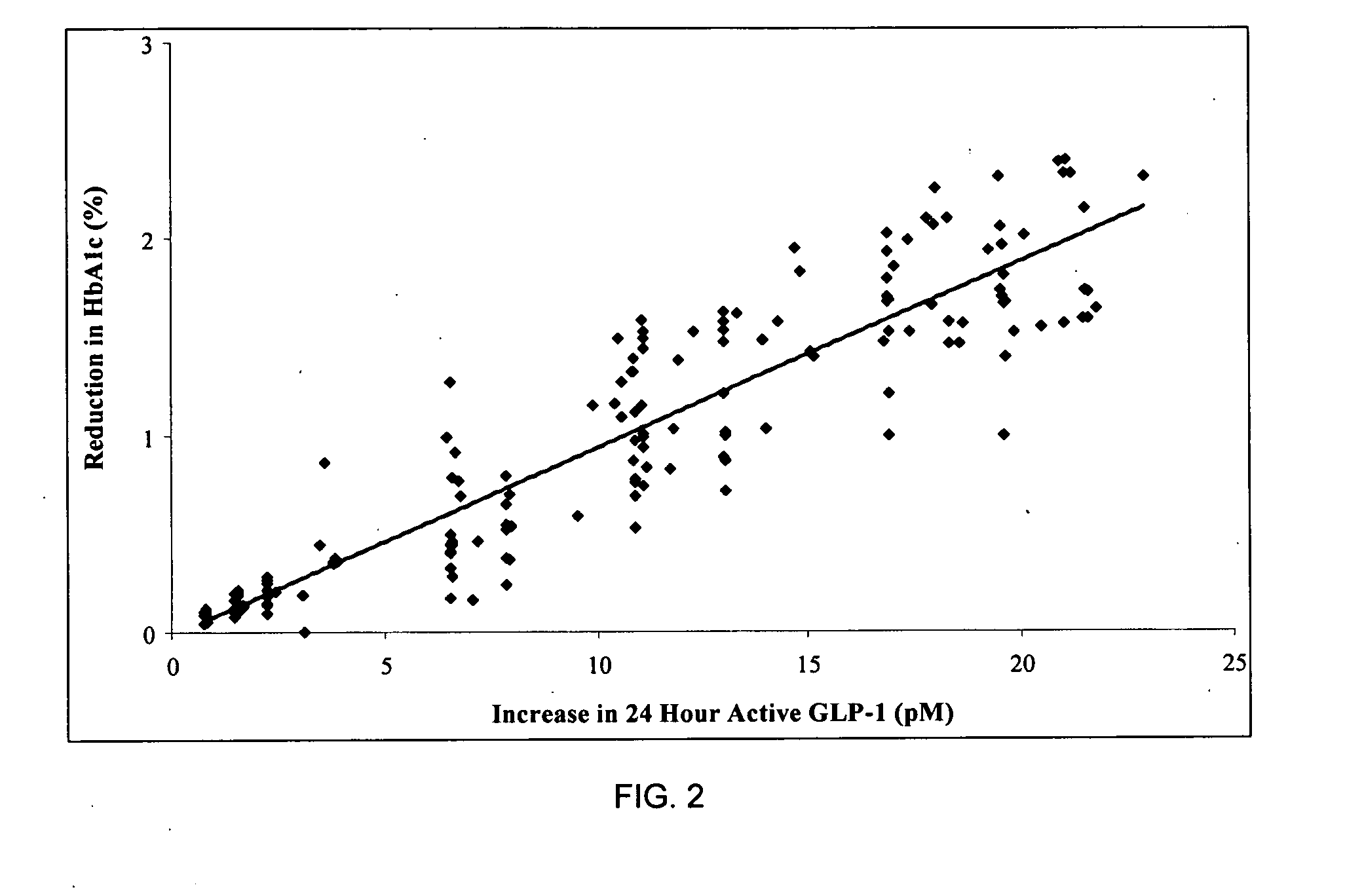
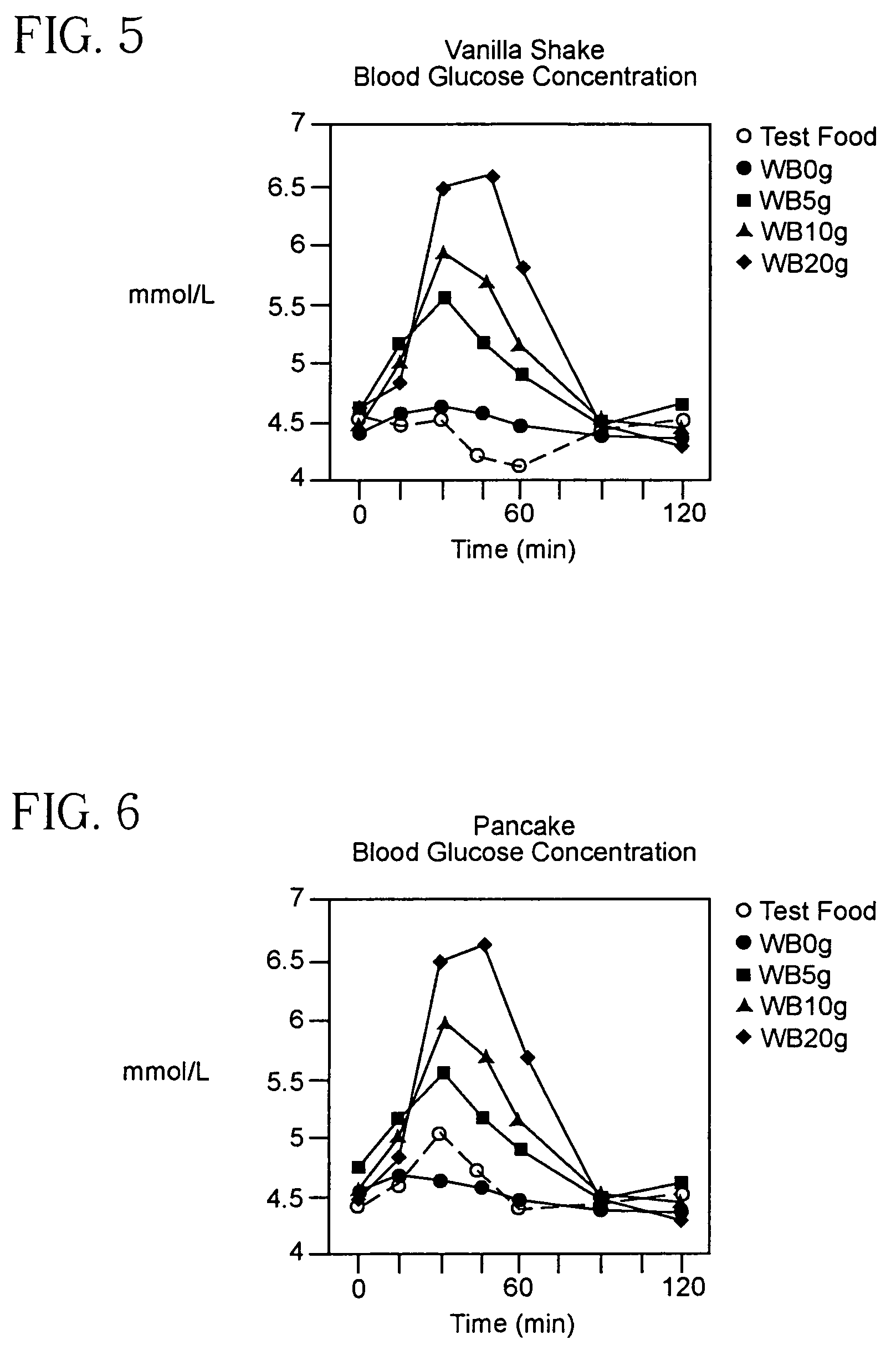
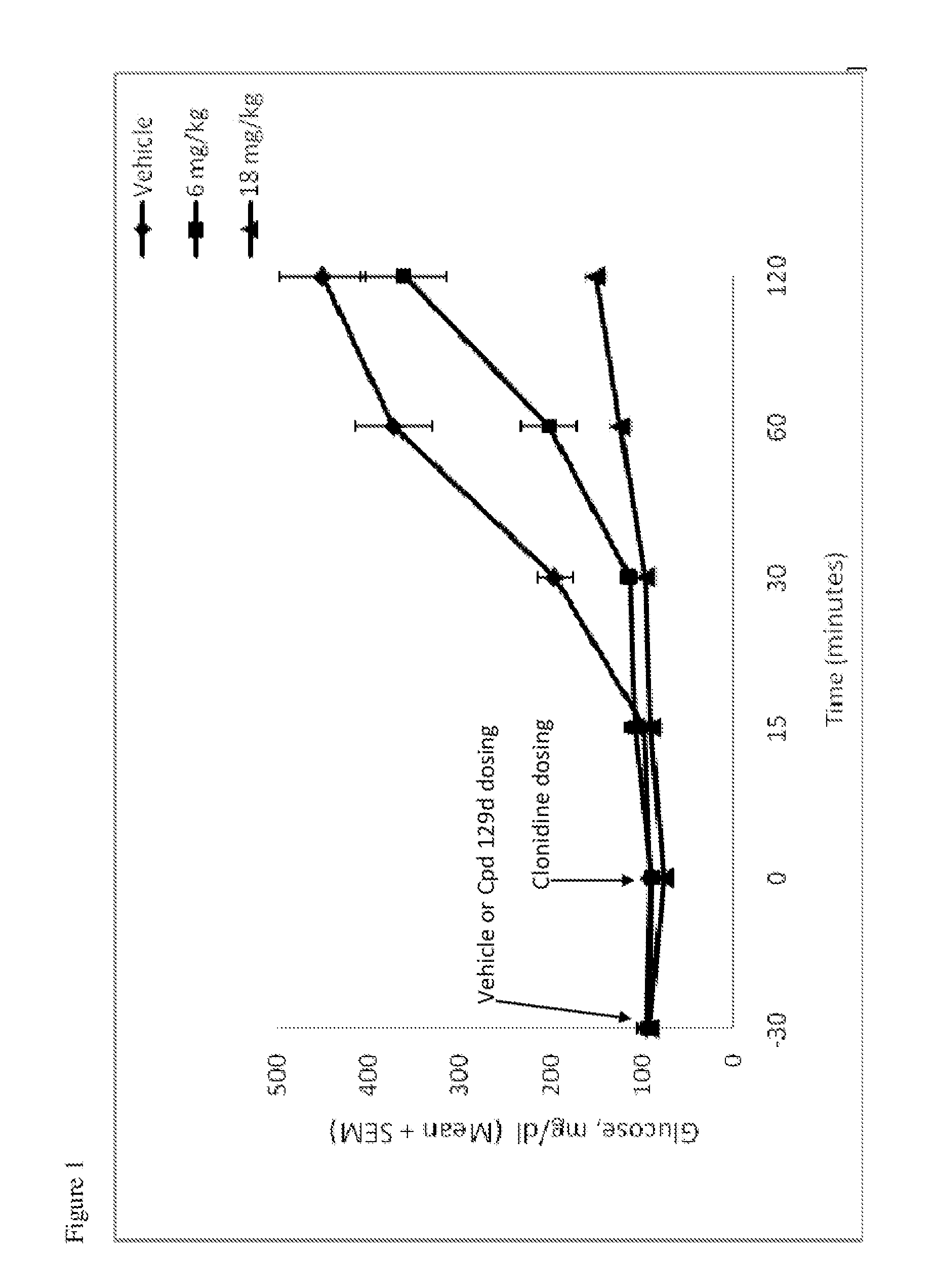
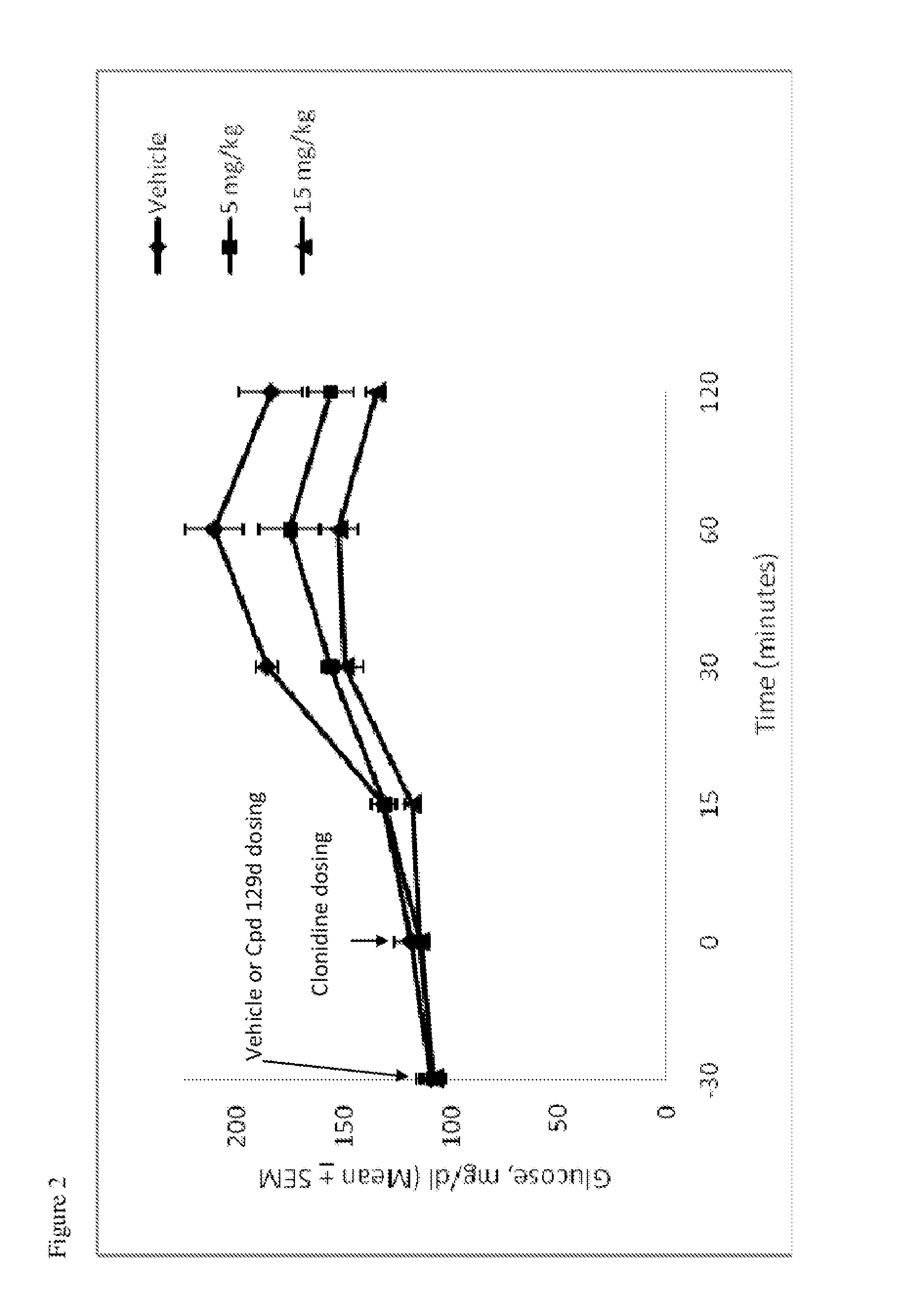
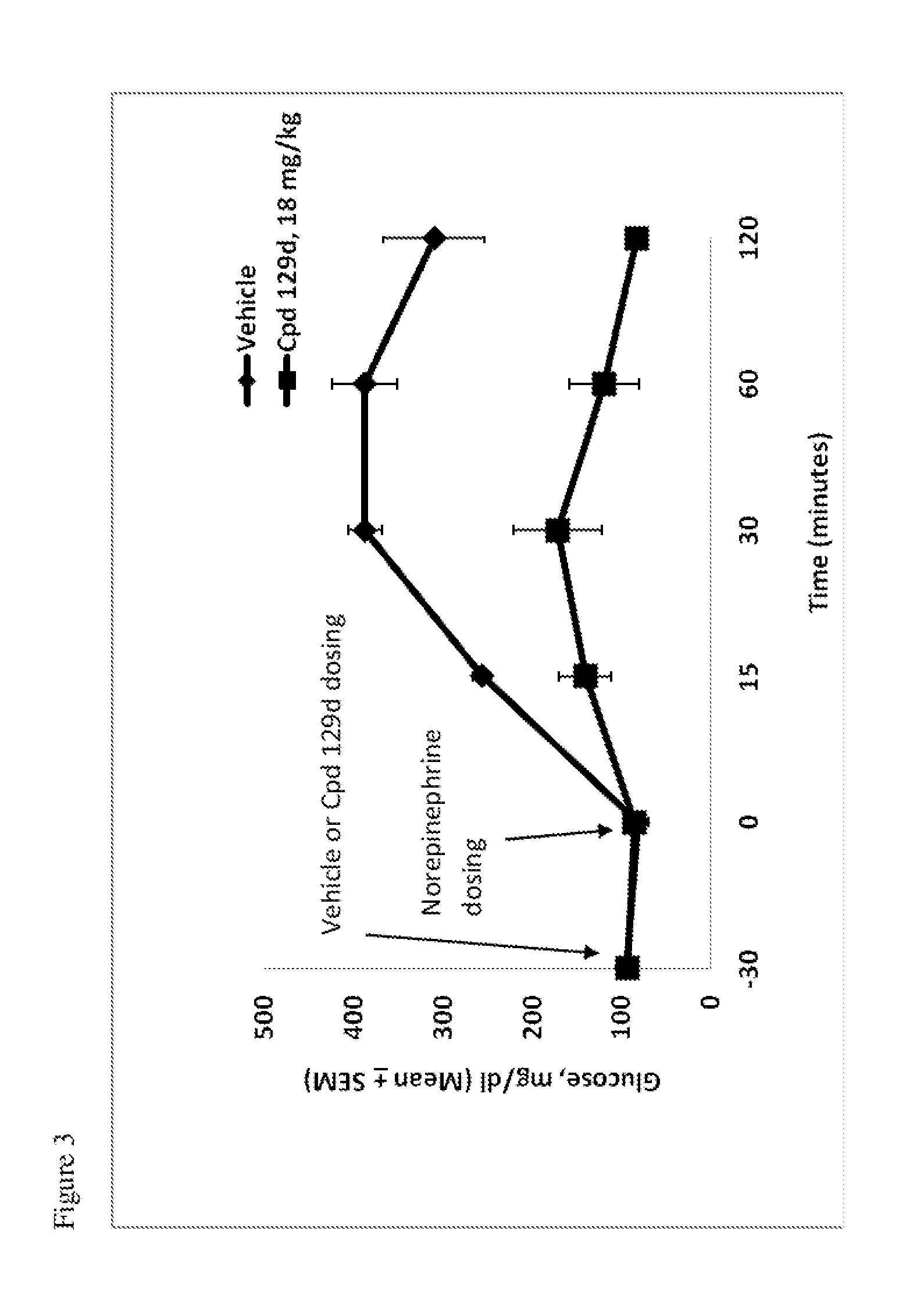
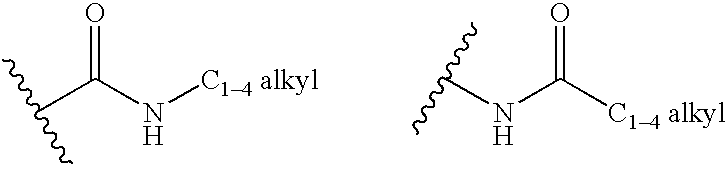Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
744results about How to "Lower blood sugar levels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
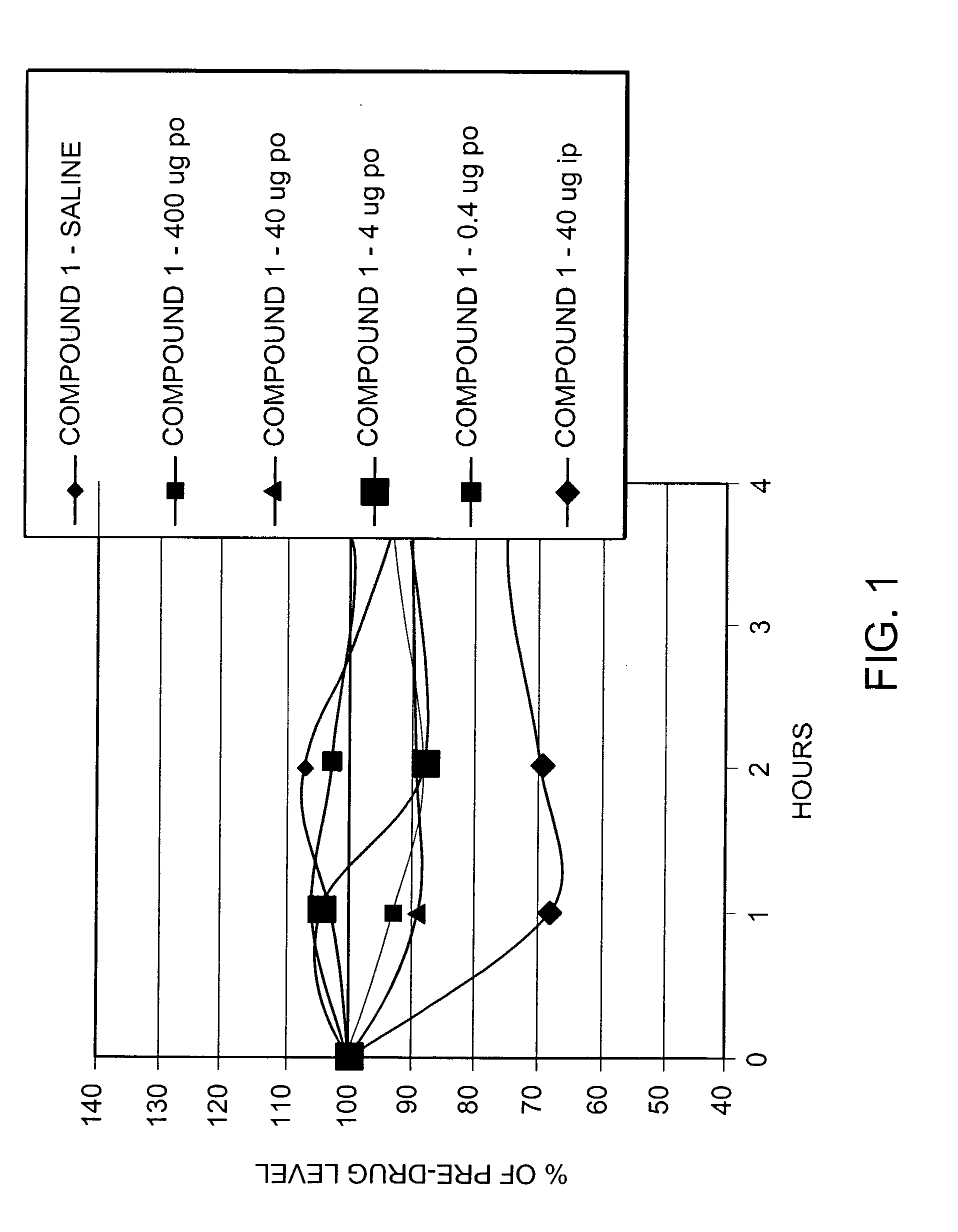
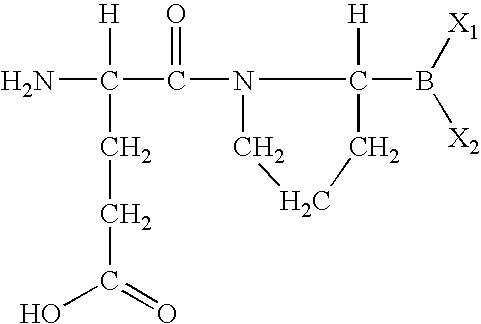
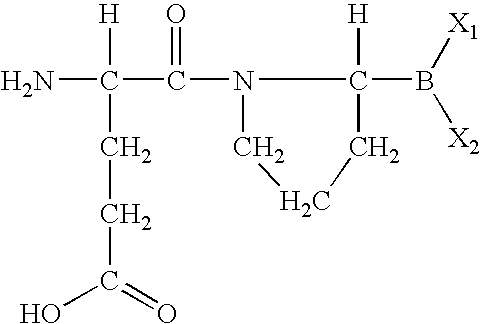
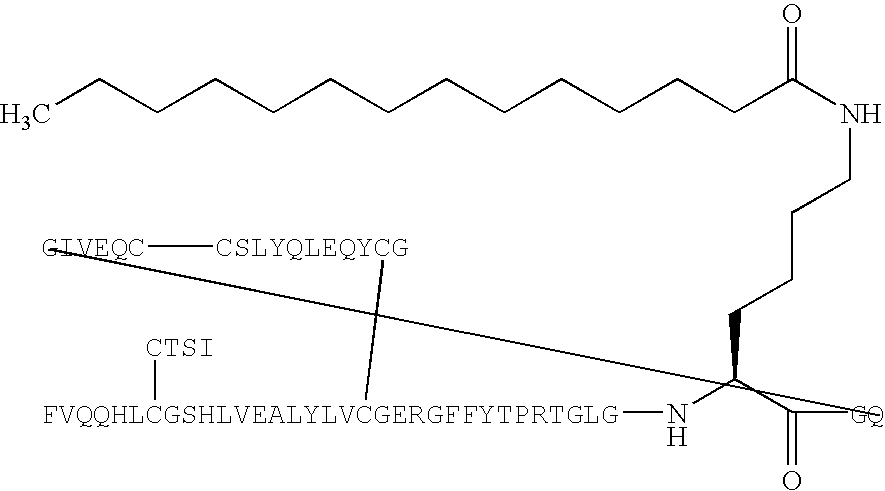
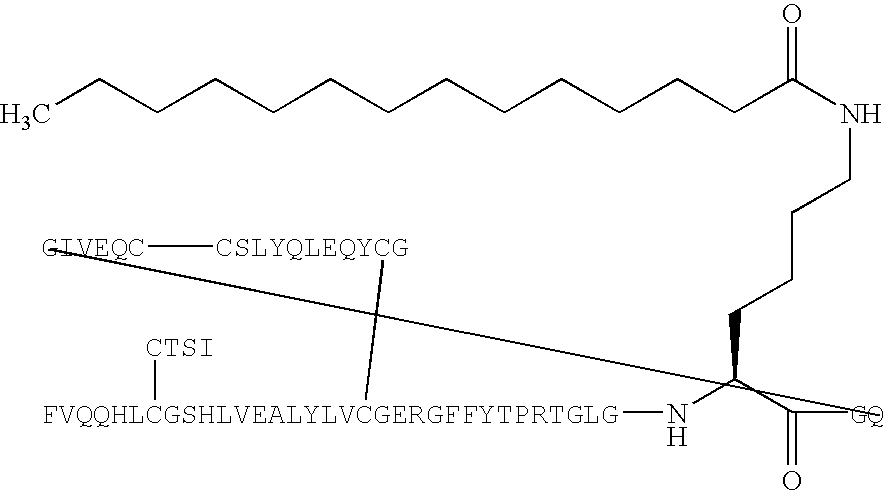
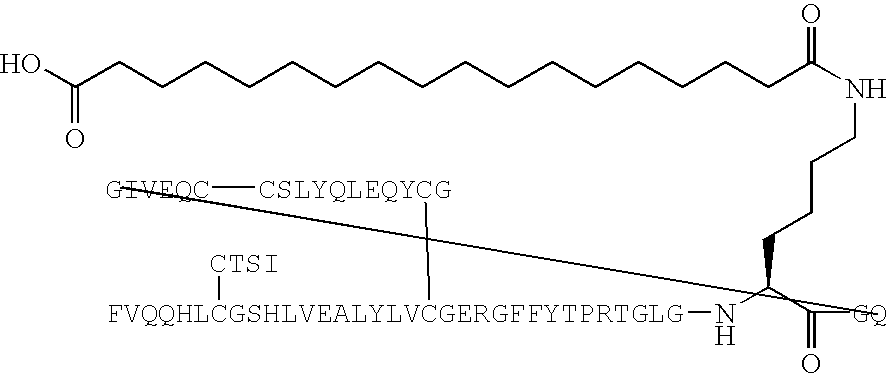
Peptide agonists of GLP-1 activity
InactiveUS6528486B1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsMetabolism disorderD-GlucoseGlycemic
The present invention relates to novel peptide conjugates which have increased stability and are useful in the treatment of excess levels of blood glucose.
Owner:ZP HLDG SPV
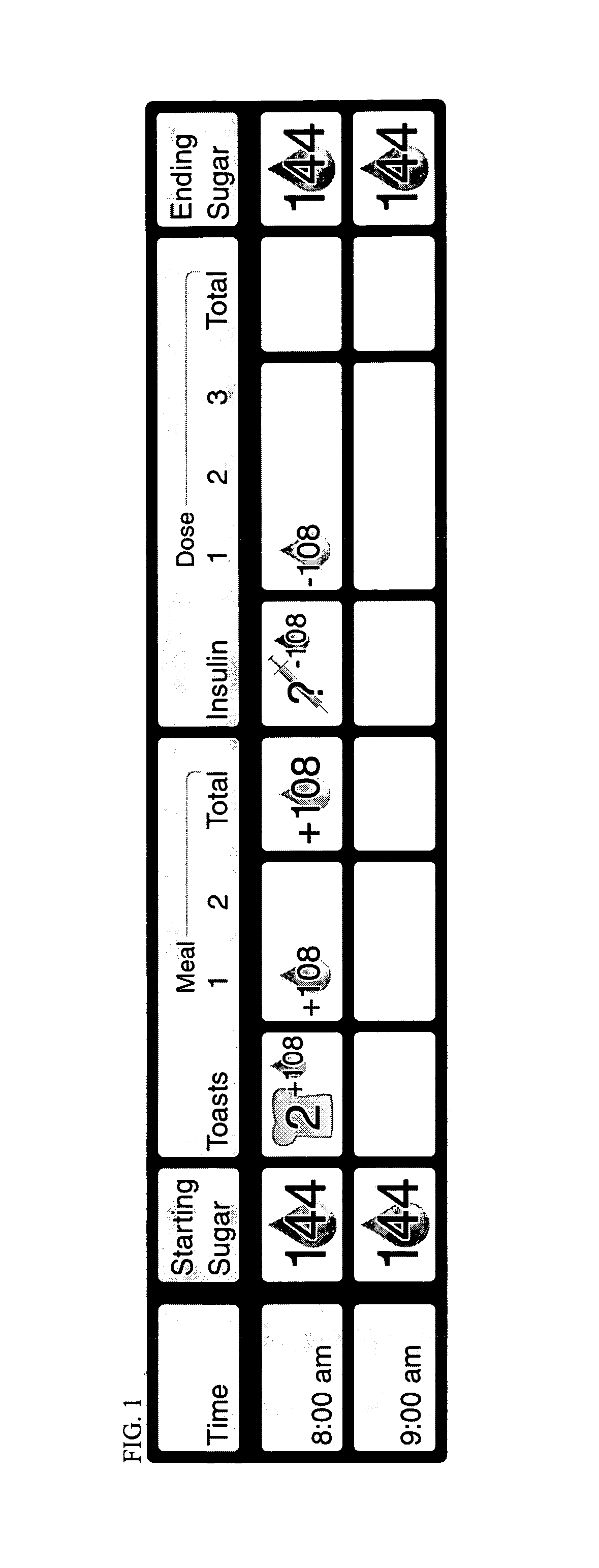
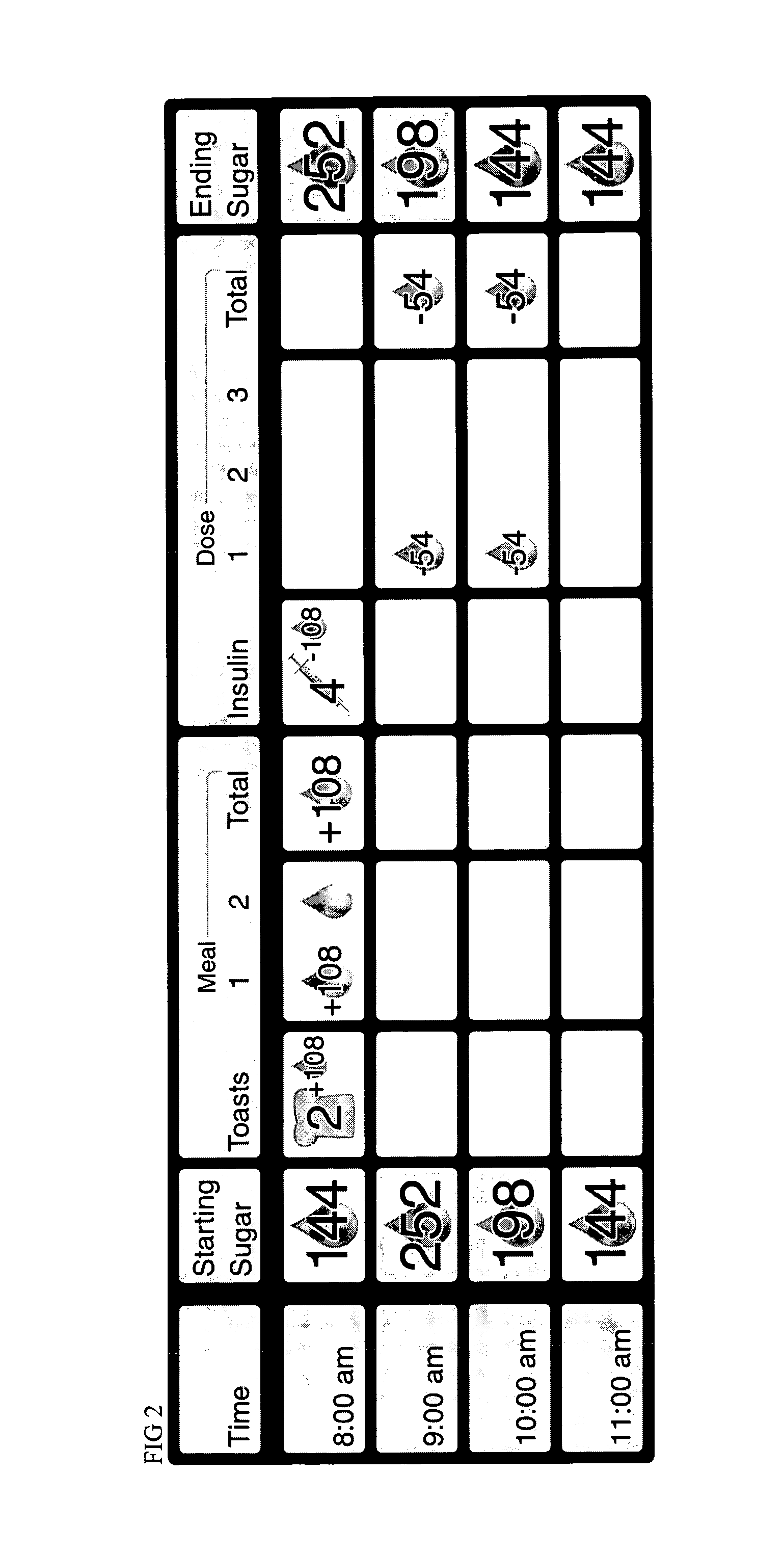
Method of food and insulin dose management for a diabetic subject
InactiveUS7137951B2Lower blood sugar levelsReduce the amount requiredPeptide/protein ingredientsDrug and medicationsINSULIN USEInsulin dose
The invention relates to a method of food and insulin dose management for a diabetic subject, comprising:providing an intended insulin unit value or an intended carbohydrate unit value representing the amount of insulin or carbohydrate intended for intake by the subject; anddetermining the balance value of either insulin units or carbohydrate units needed to balance with the provided unit value and maintain blood sugar in the subject in a target blood sugar range.
Owner:PILARSKI JOSEPH
Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level
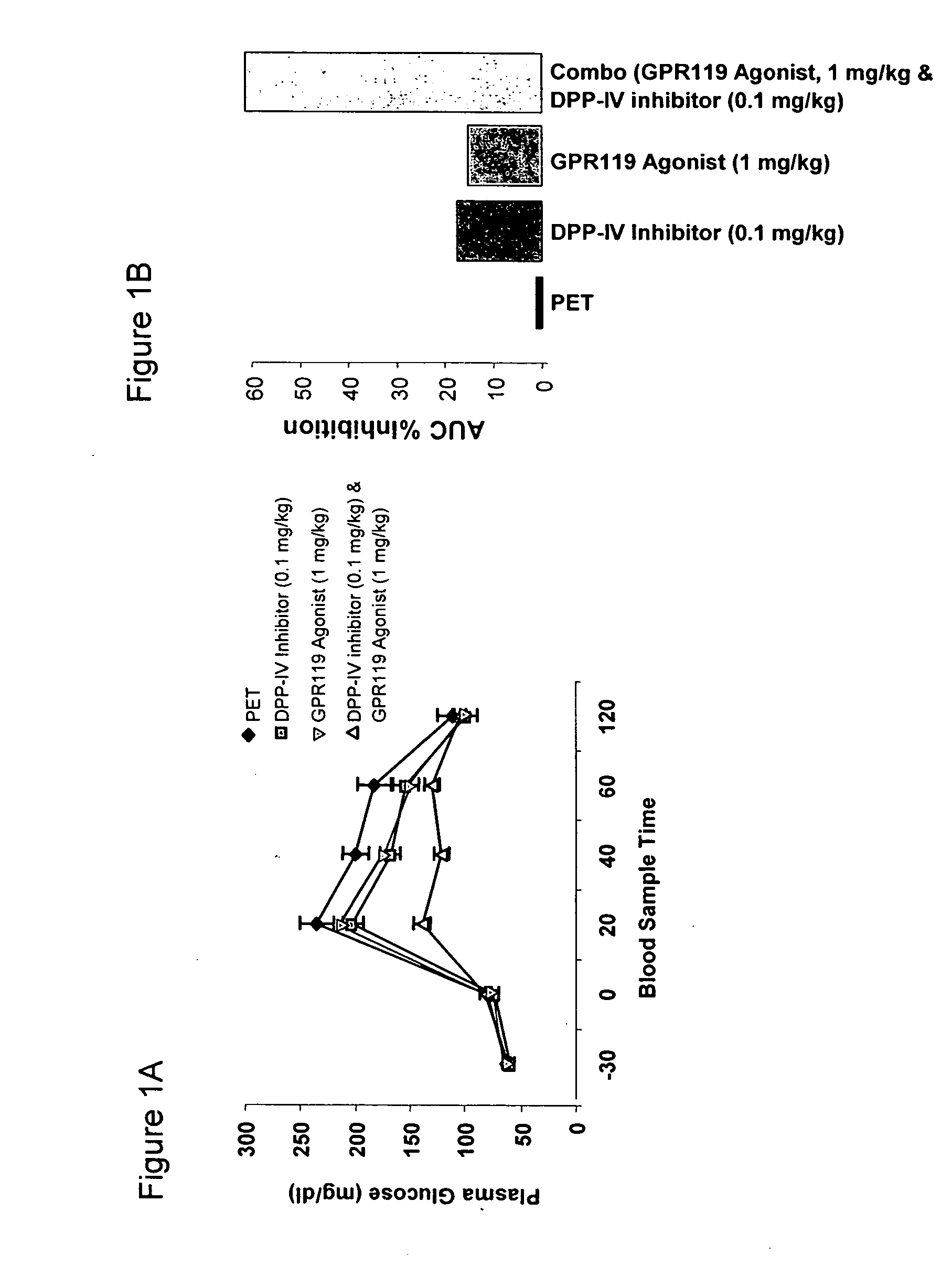
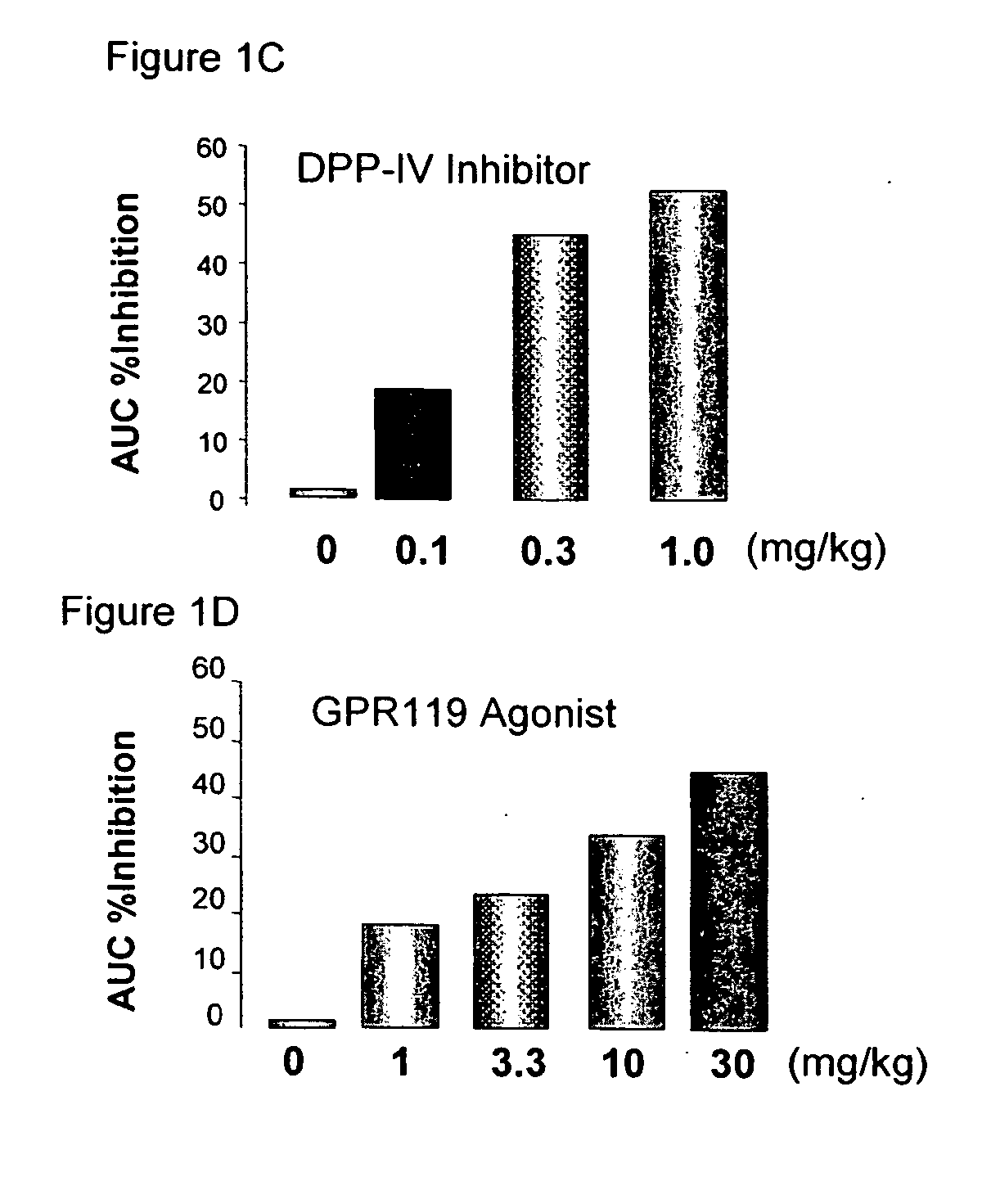
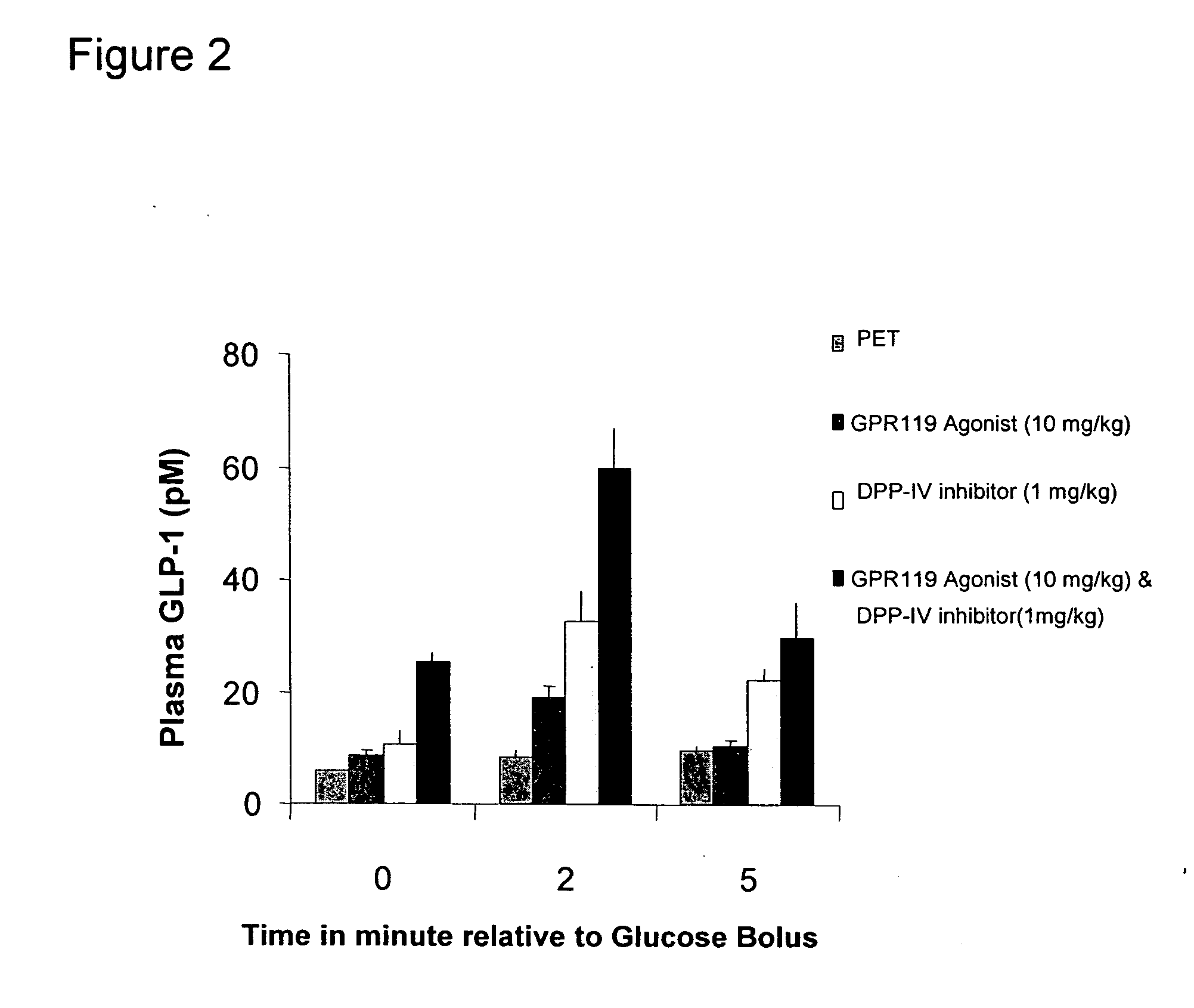
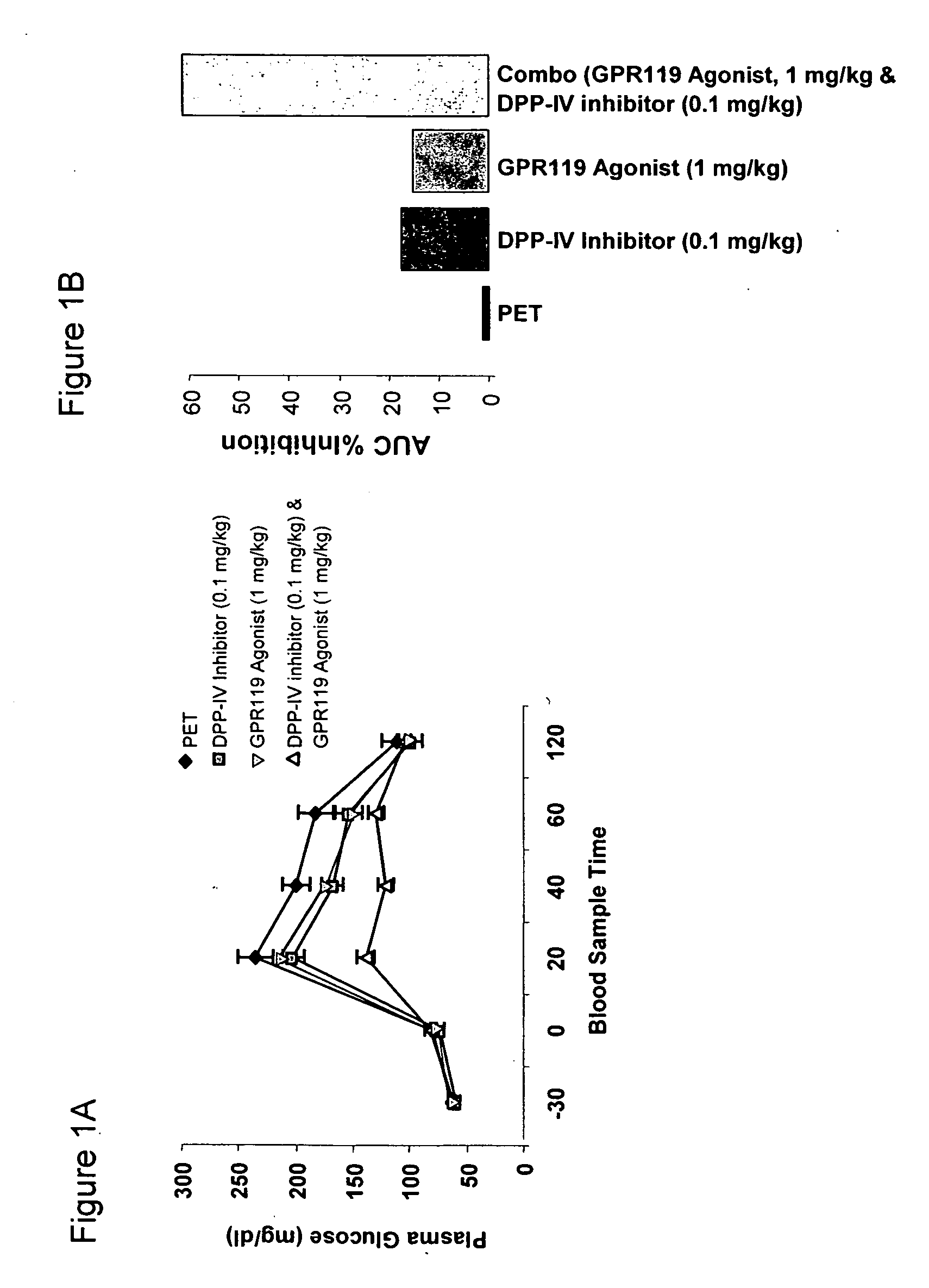
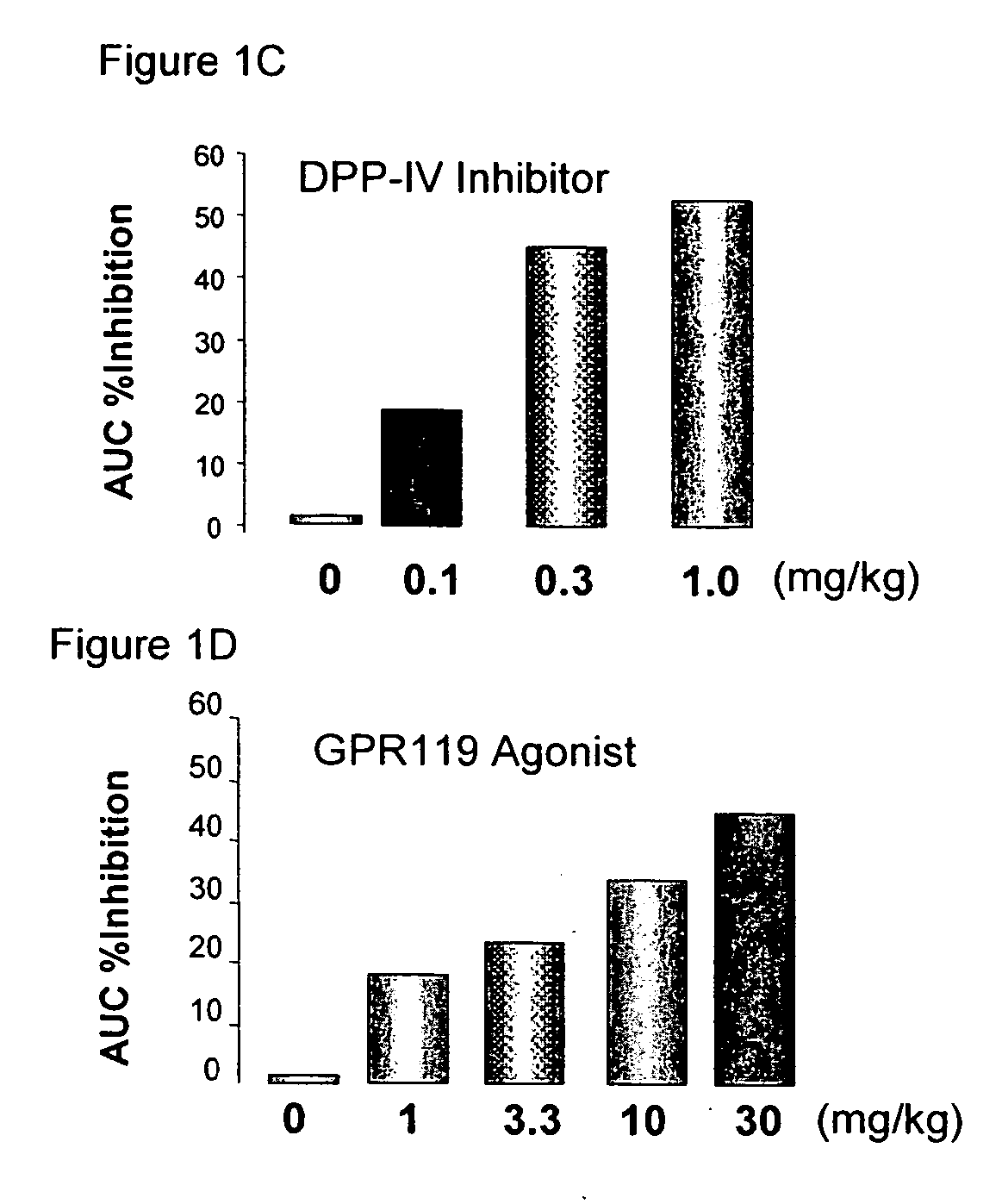
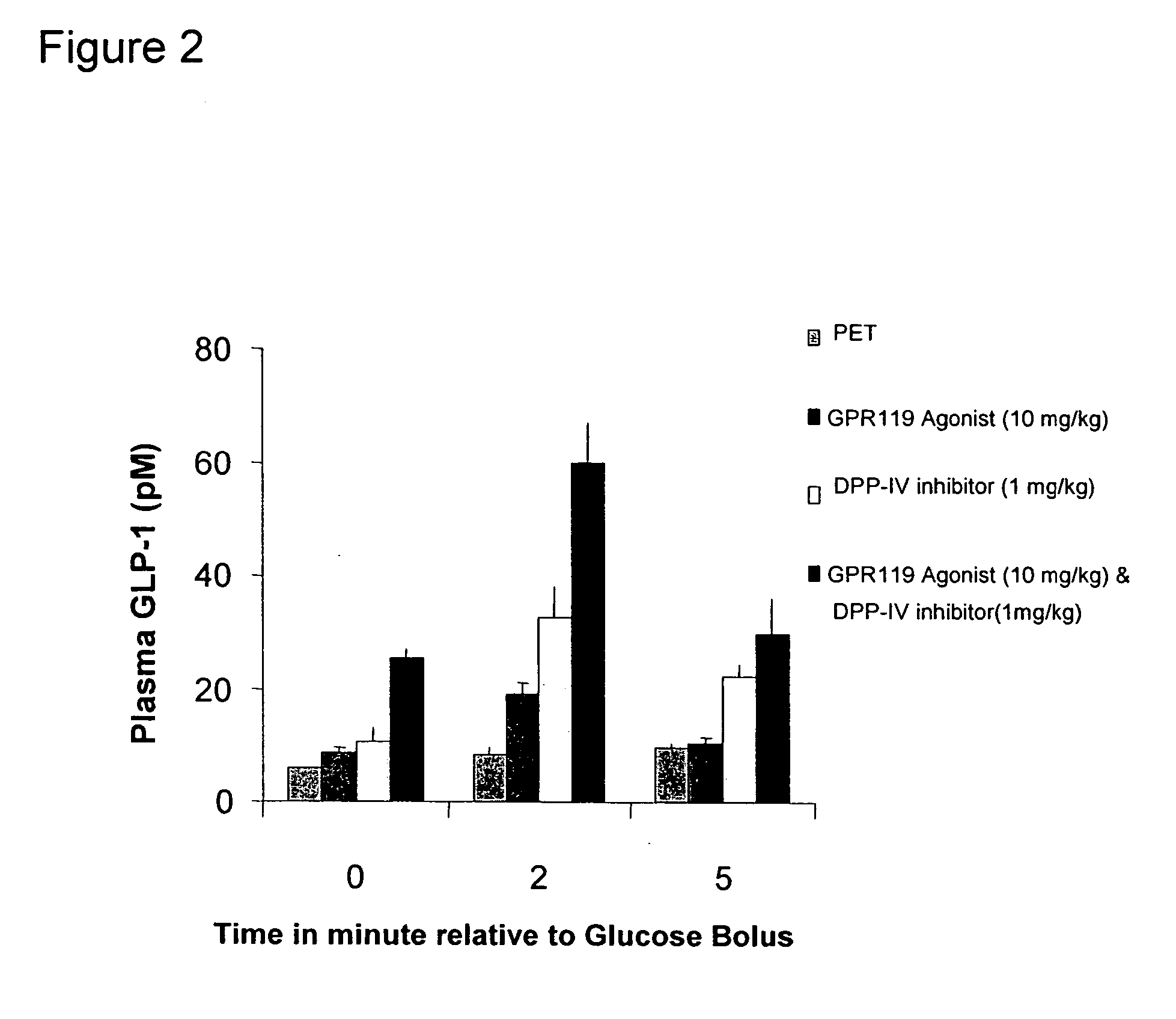
InactiveUS20060154866A1Lower blood sugar levelsImprove the level ofOrganic active ingredientsSenses disorderGpr119 agonistG protein-coupled receptor
The present invention concerns combination of an amount of a GPR119 agonist with an amount of a dipeptidyl peptidase IV (DPP-IV) inhibitor such that the combination provides an effect in lowering a blood glucose level or in increasing a blood GLP-1 level in a subject over that provided by the amount of the GPR119 agonist or the amount of the DPP-IV inhibitor alone and the use of such a combination for treating or preventing diabetes and conditions related thereto or conditions ameliorated by increasing a blood GLP-1 level. The present invention also relates to the use of a G protein-coupled receptor to screen for GLP-1 secretagogues.
Owner:ARENA PHARMA
Anti-TrkB antibodies
ActiveUS8642035B2Lower blood sugar levelsLose weightMetabolism disorderAntibody mimetics/scaffoldsNucleotideAntigen binding
The present invention provides improved antibodies or antigen-binding molecules that specifically recognize and agonize the tyrosine receptor kinase B (TrkB) receptor, and methods of their use. Also provided in the invention are polynucleotides and vectors that encode such molecules and host cells that harbor the polynucleotides or vectors.
Owner:NOVARTIS AG
Novel peptide agonists of GLP-1 activity
InactiveUS20040106547A1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsVasoactive intestinal peptideDiseaseGastric emptying
Owner:ZEALAND PHARM AS
Methods for treating diabetes
InactiveUS20060063719A1Reducing blood glucoseLower blood sugar levelsBiocideDipeptide ingredientsPhysiologyType 2 diabetes
Owner:POINT THERAPEUTICS
Administration of insulin by jet injection
InactiveUS20060106362A1Easily employedHigh level of skillJet injection syringesMetabolism disorderInsulin dependentJet injection
The invention relates to a method for minimizing mean blood glucose levels in an insulin dependent patient by administering insulin to the patient in a sufficiently fast manner to provide a difference of 50% or less between high and low blood glucose levels. Advantageously, the insulin is administered to the patient by jet injection and the high and low blood glucose levels differ by an amount that is less than that which would be obtained after injection of insulin by a conventional needle syringe. The invention also relates to a method for reducing mean blood glucose levels in an insulin dependent patient that is receiving insulin through a conventional syringe and needle arrangement. This method provides for administration of the insulin to the patient by jet injection rather than by the syringe by substituting a jet injector for the syringe.
Owner:ANTARES PHARMA
Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level
InactiveUS20070072803A1Lower blood sugar levelsImprove the level ofOrganic active ingredientsSenses disorderGpr119 agonistG protein-coupled receptor
Owner:ARENA PHARMA
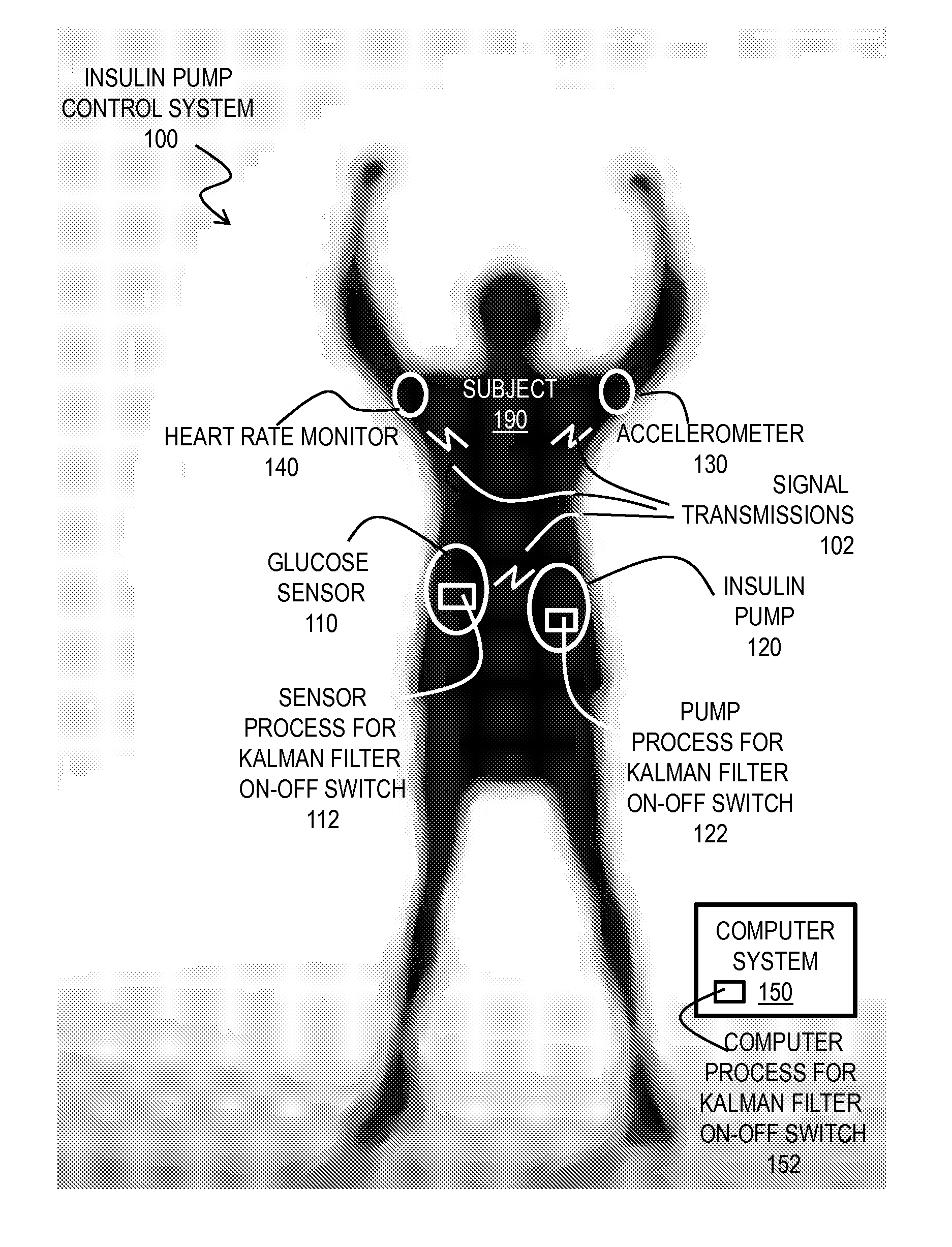
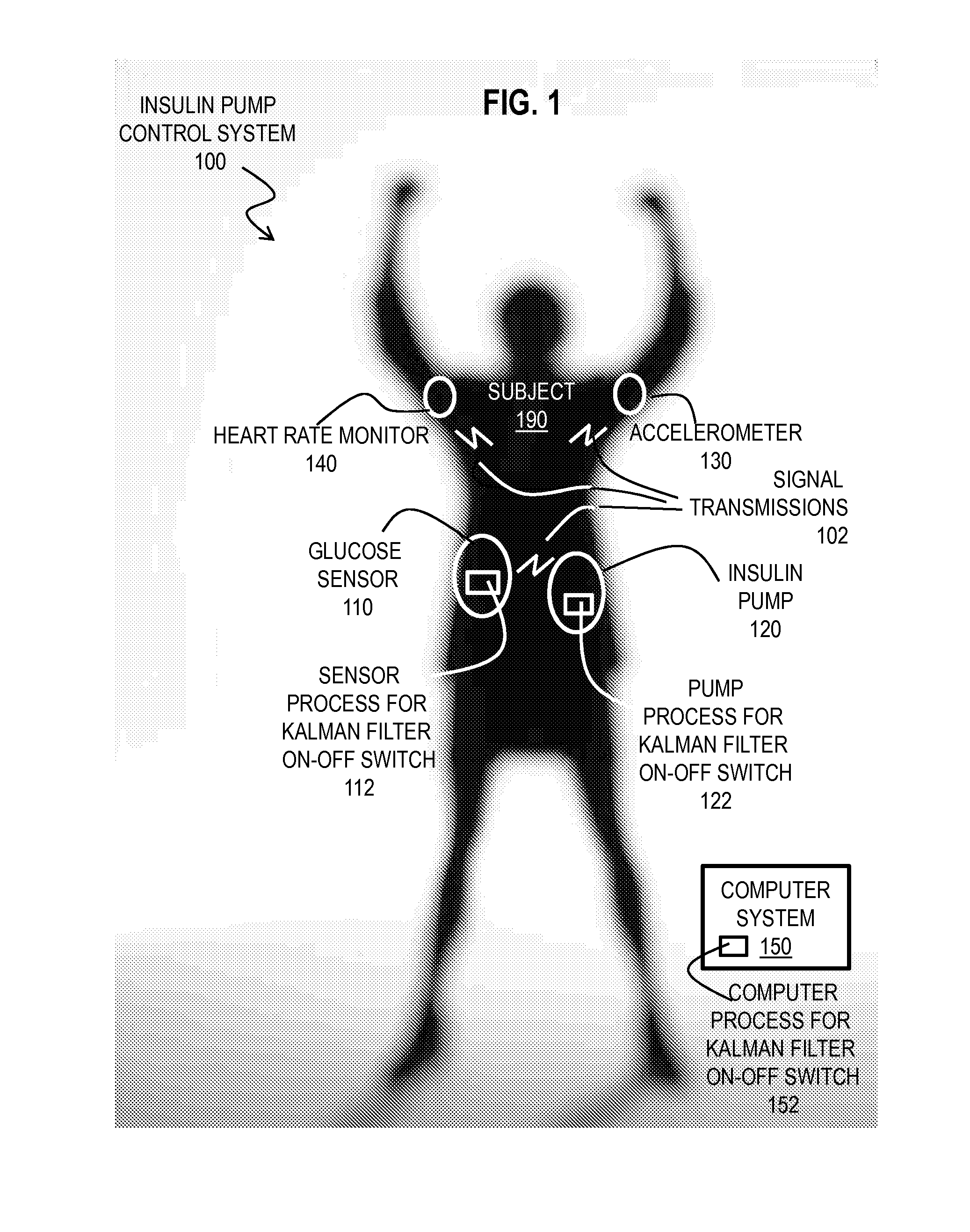
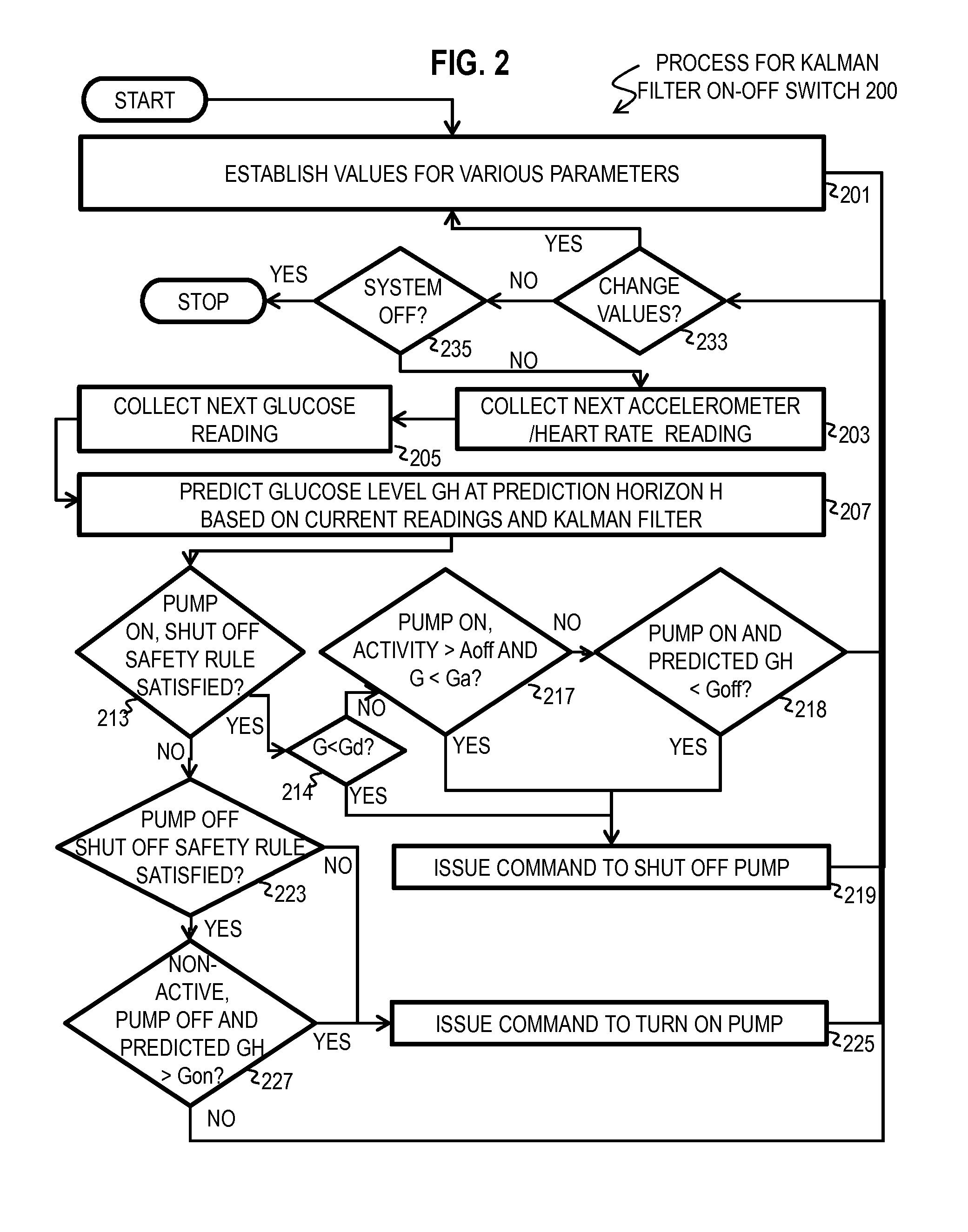
Kalman Filter Based On-Off Switch for Insulin Pump
ActiveUS20140221966A1Mitigate and prevent low blood glucose levelLower blood sugar levelsElectrocardiographyMedical devicesKaiman filterInsulin pump
Techniques for controlling an insulin pump include determining values for parameters selected from a group including a first prediction time horizon, a predicted glucose threshold (Goff) for turning the insulin pump off, a maximum shut off time within a time window, and duration of the time window. A safety rule is determined based on the maximum shut off time within the duration. Glucose readings are collected up to a current time. An expected current glucose value G and glucose temporal rate of change are determined based only on the glucose readings and a Kalman filter configured for noisy glucose readings. A glucose level (Gh1) is predicted for a future time that is the prediction time horizon after the current time. A command is issued to shut off the insulin pump if it is determined both that Gh1 is less than Goff and that the safety rule is satisfied.
Owner:UNIV OF COLORADO THE REGENTS OF +3
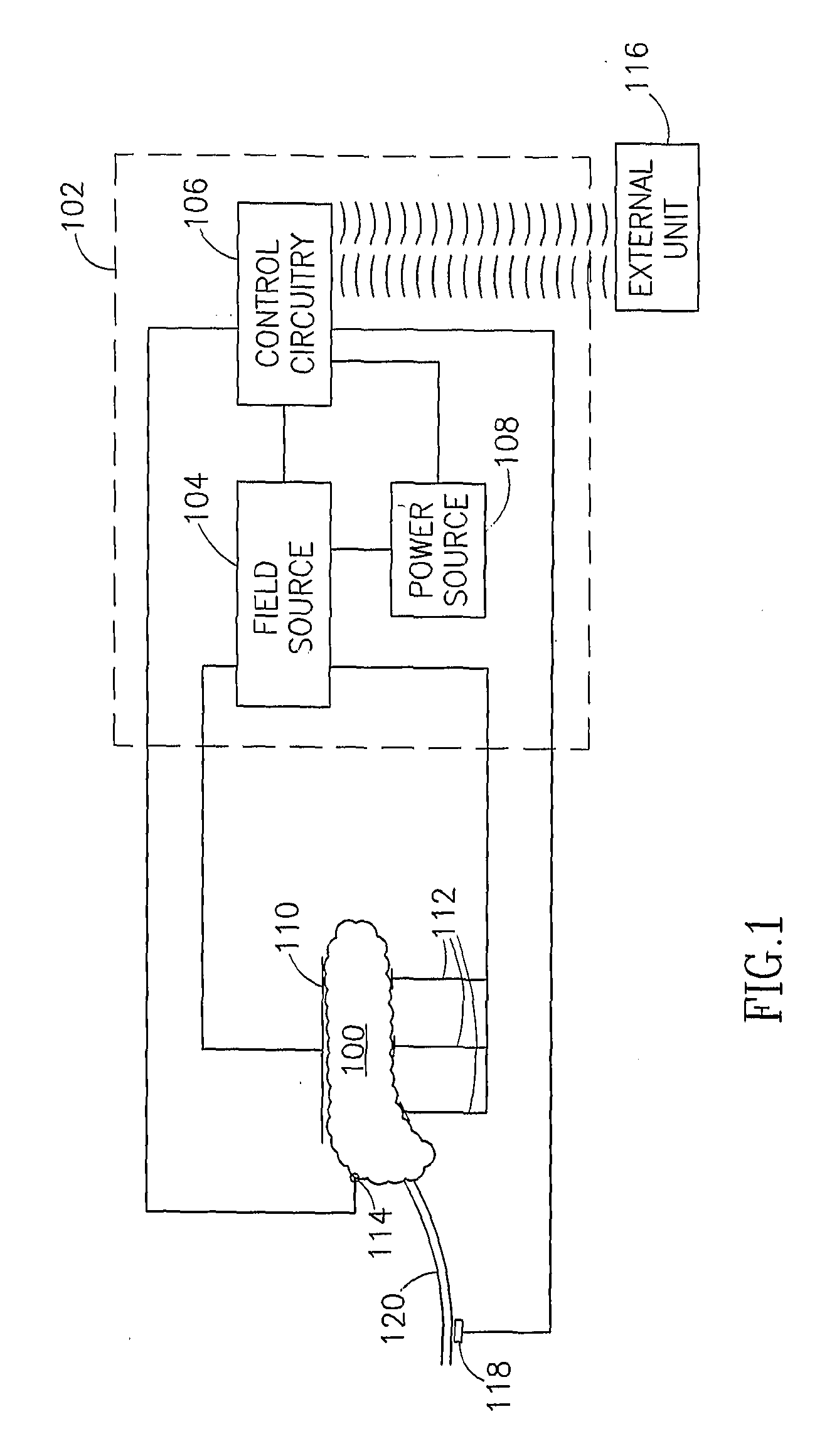
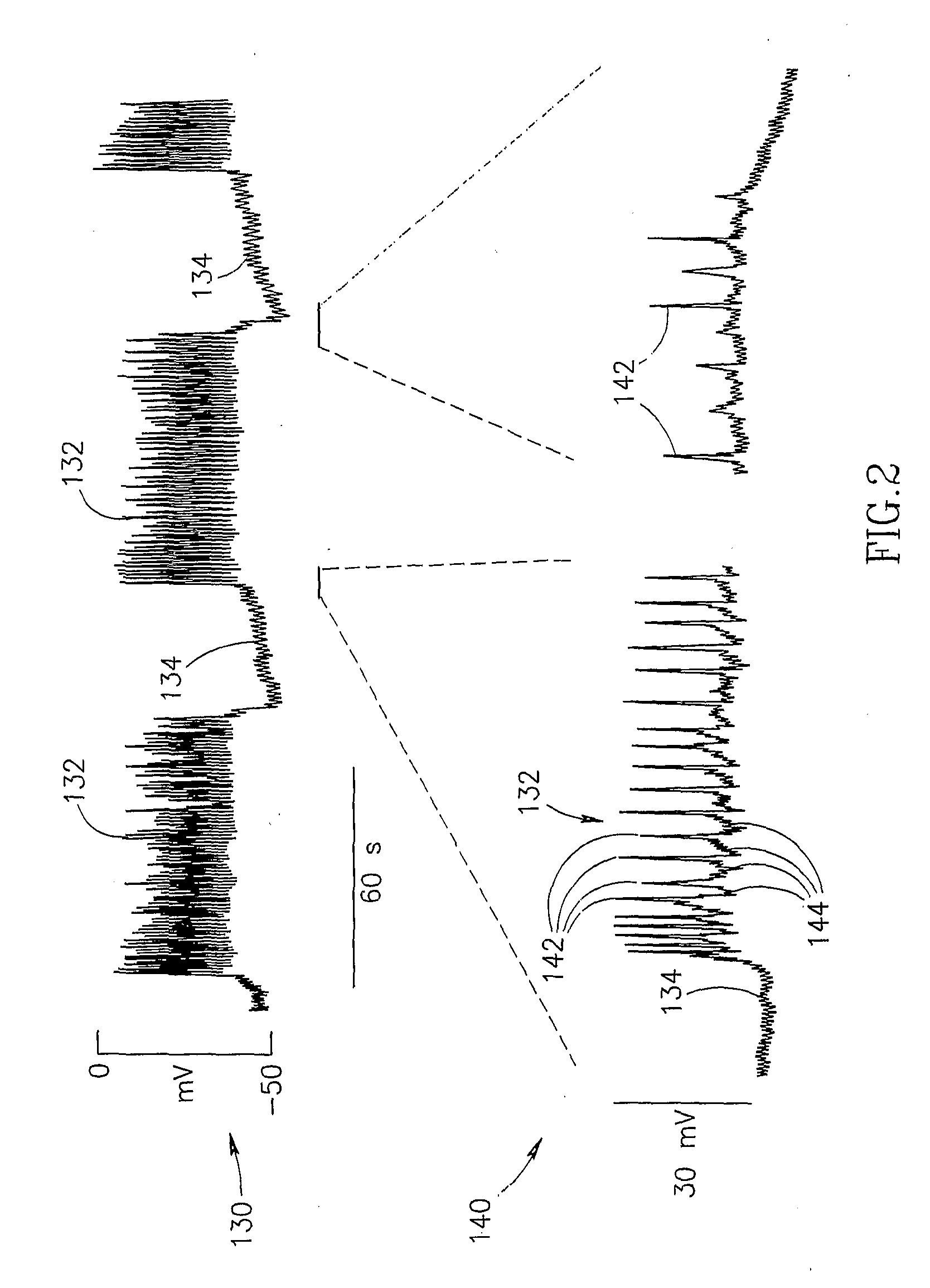
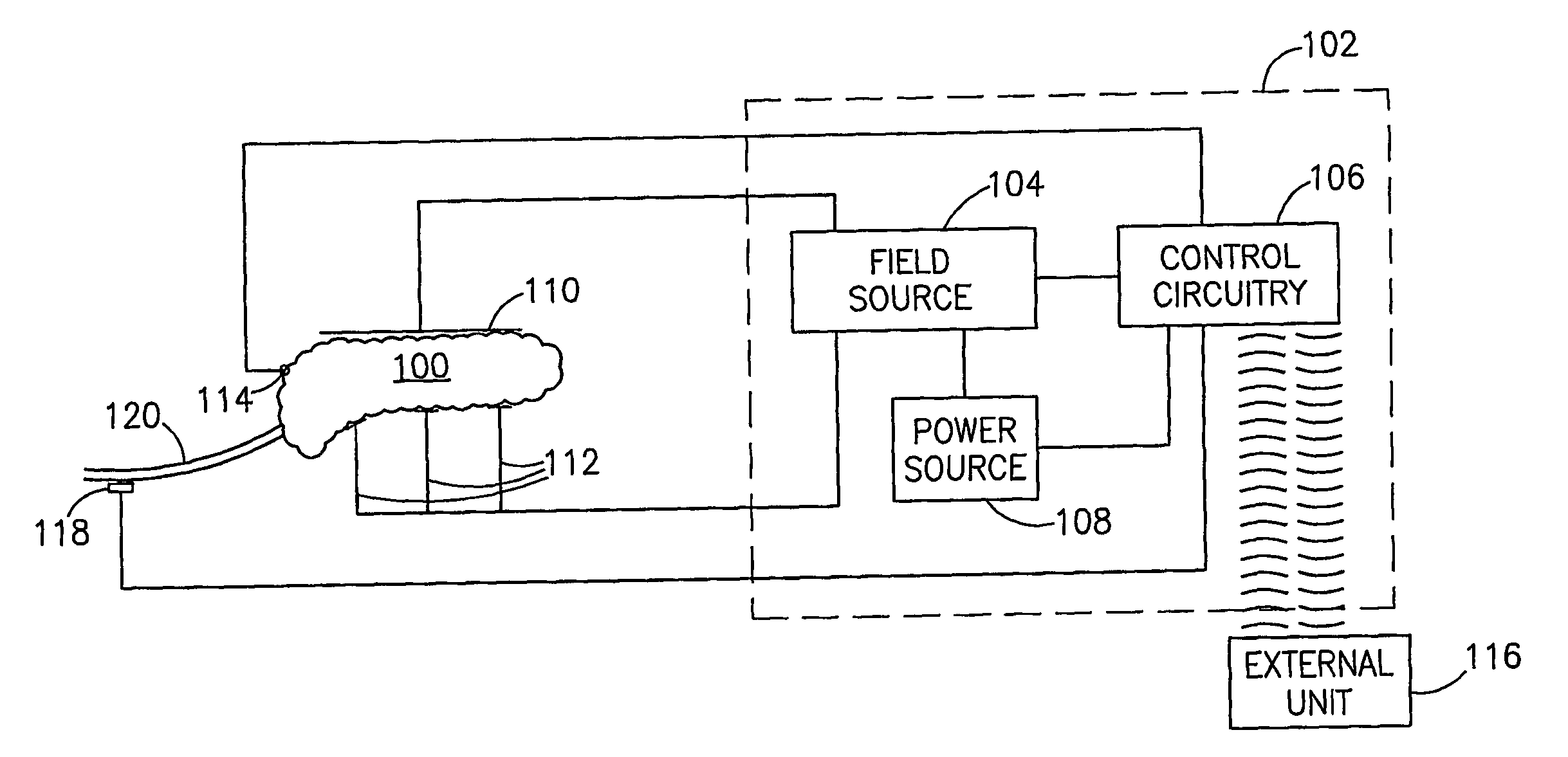
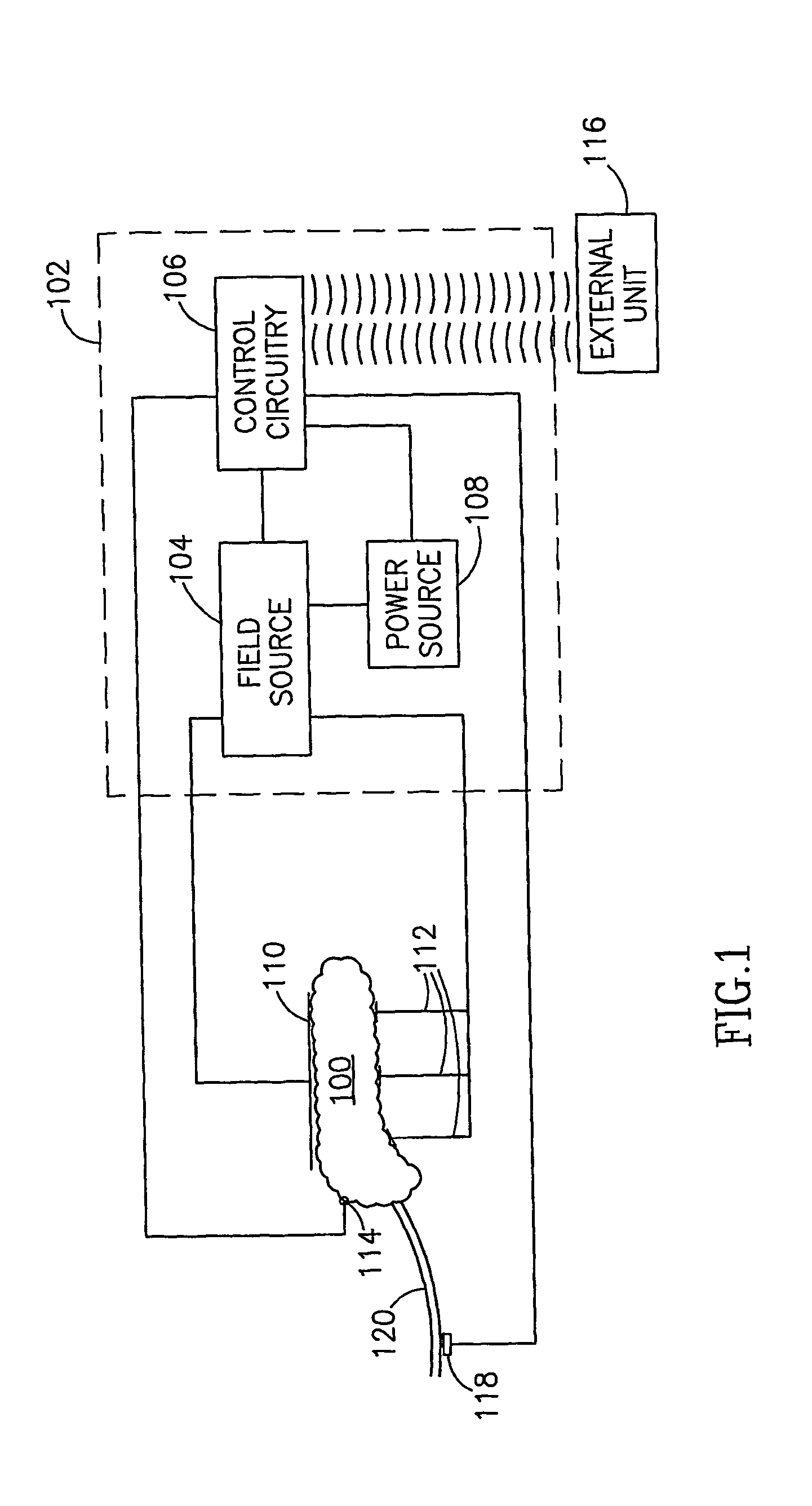
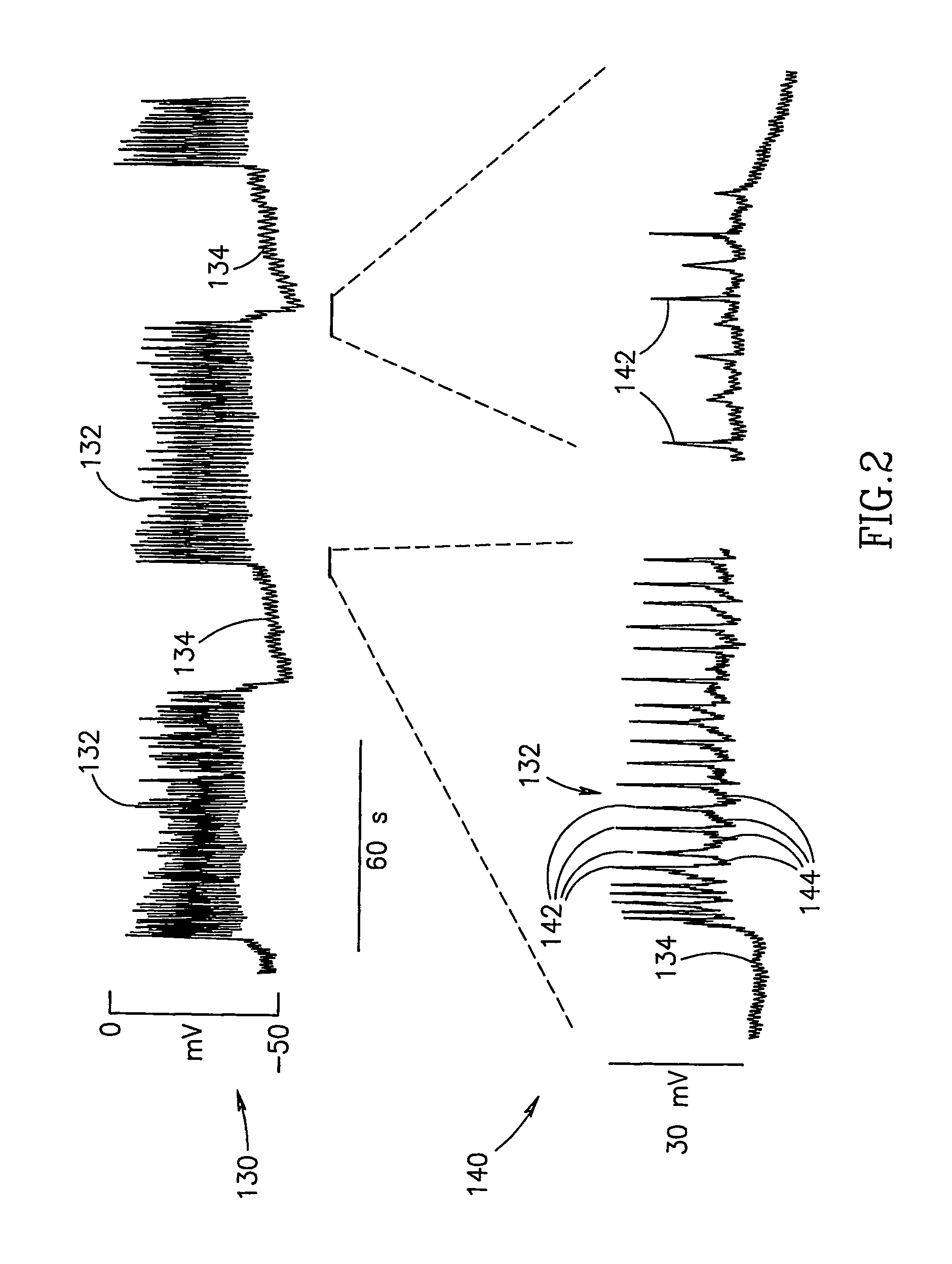
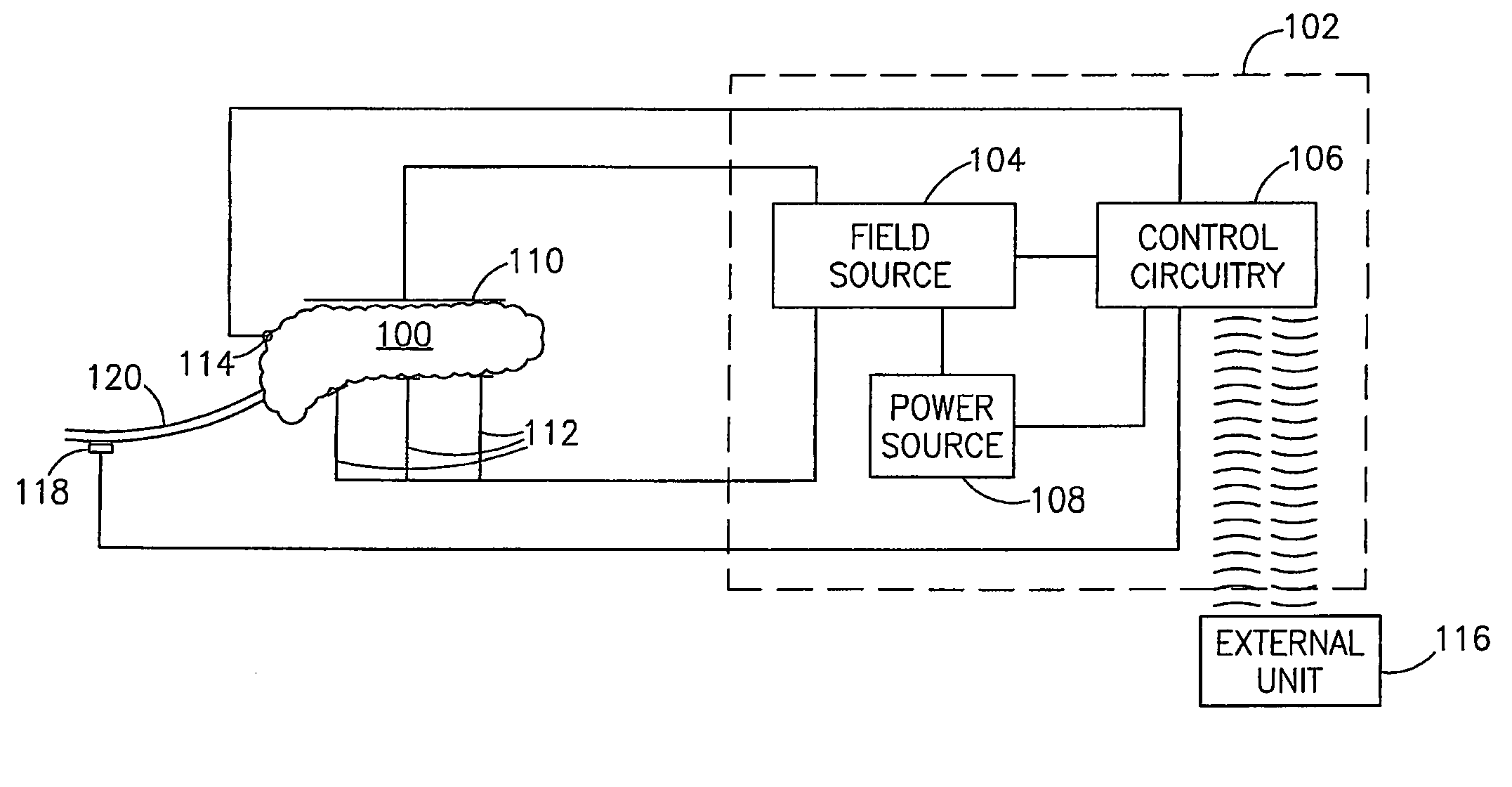
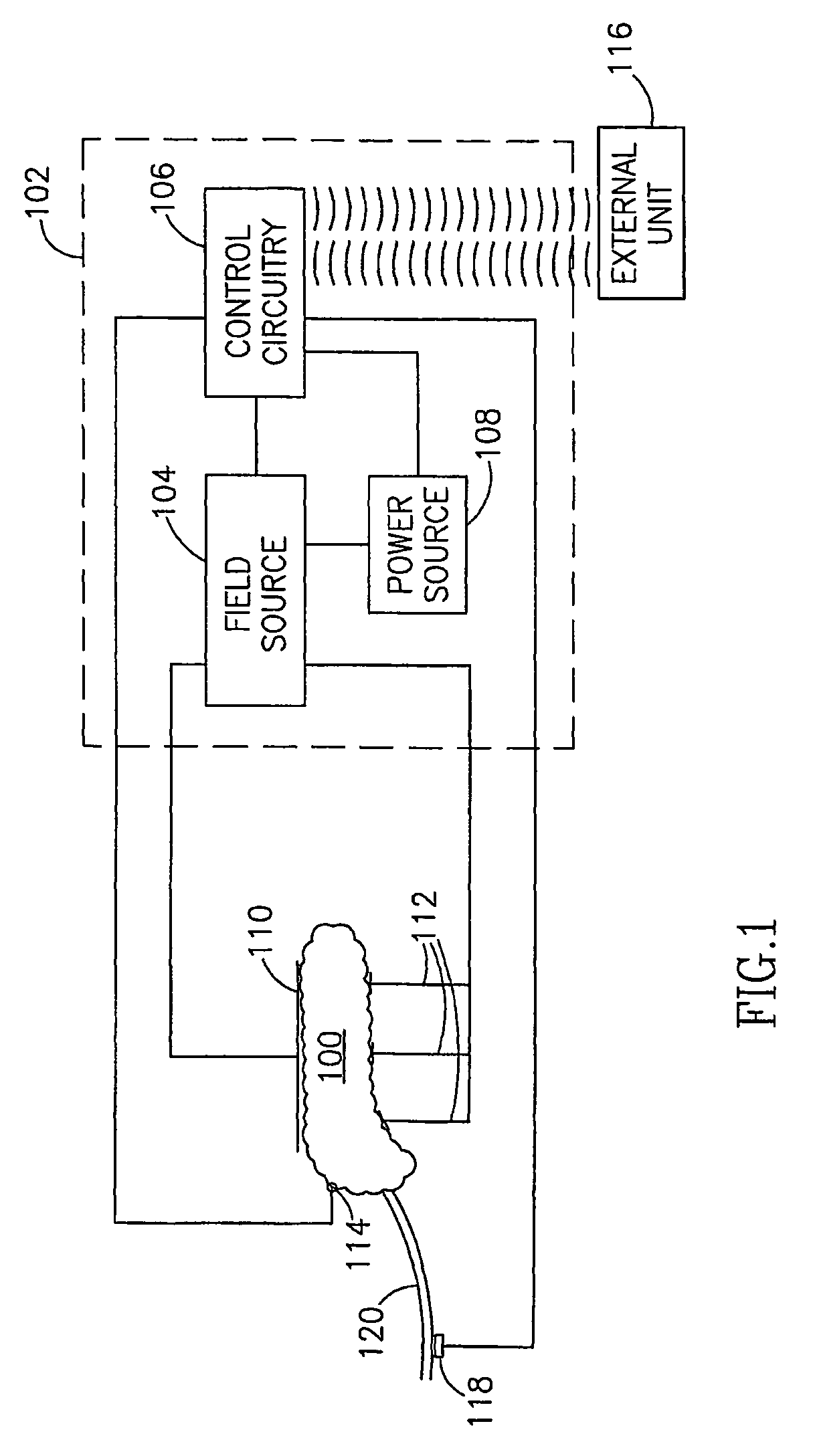
Gastrointestinal Methods And Apparatus For Use In Treating Disorders And Controlling Blood Sugar
InactiveUS20090088816A1Reduced glucose levelAvoid fatigueExternal electrodesDigestive electrodesBlood insulinPhysiology
A method of glucose level control comprising providing at least one electrode adapted to apply an electric field to a pancreas; and applying an electric field to the pancreas using said at least one electrode such that blood glucose levels are significantly reduced and blood insulin levels are not significantly increased compared to a regular insulin response in a same person.
Owner:TYLERTON INT INC
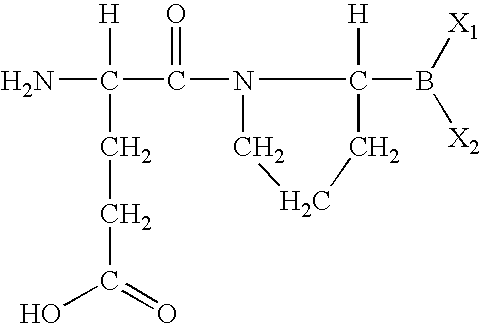
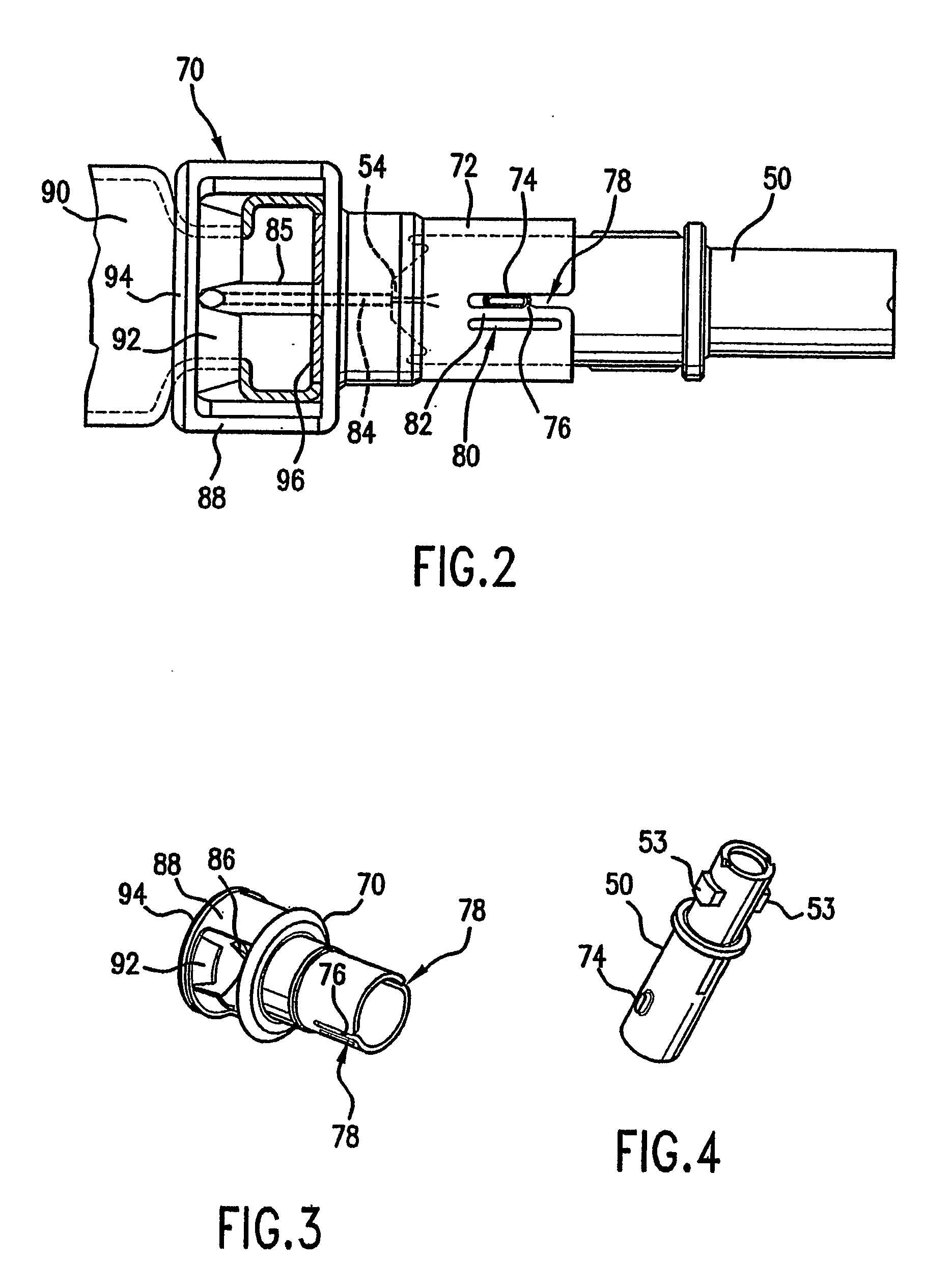
Single-chain insulin
InactiveUS20070129284A1Lower blood sugar levelsPeptide/protein ingredientsMetabolism disorderPeptidePhysical stability
The present invention is related to single-chain insulin having insulin activity comprising a B- and an A-chain or a modified B- and A-chain connected by a connecting peptide of from 6-11 amino acids. The single-chain insulins will have biological insulin activity and an IGF-1 receptor affinity similar to or lower than that of human insulin and a high physical stability. The single-chain insulin may contain at least one basic amino acid residues in the connecting peptide. The single-chain insulins may also be acylated in one or more Lys residues.
Owner:NOVO NORDISK AS
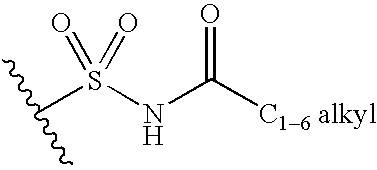
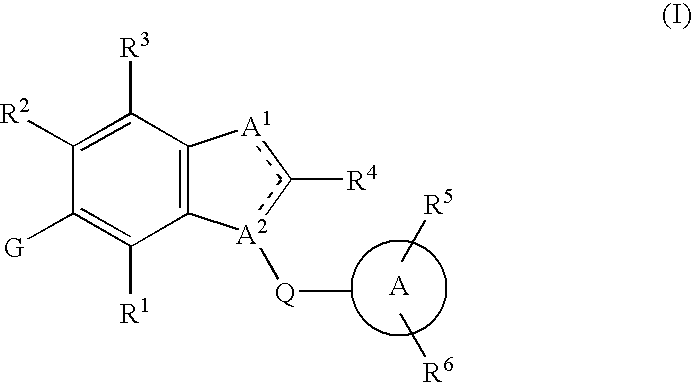
Fused heterocyclic derivative, medicinal composition containing the same, and medicinal use thereof
InactiveUS20060247179A1Good effectReduce doseBiocideAntibiotics chemistryAcute hyperglycaemiaDiabetic complication
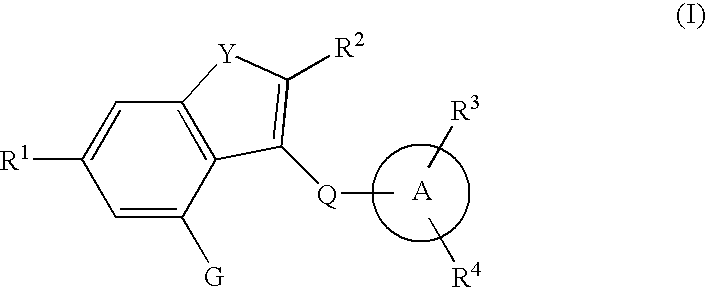
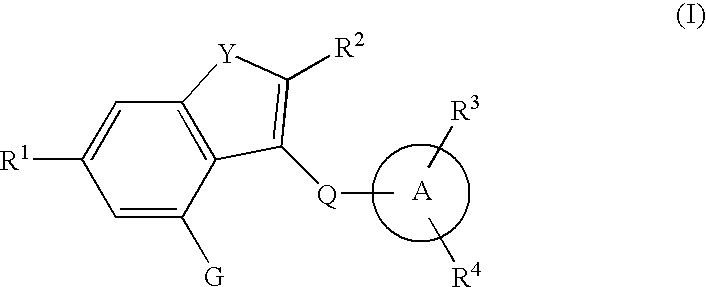
The present invention provides fused heterocyclic derivatives represented by the general formula: wherein R1 represents H, halogen, OH, etc.; R2 represents H, halogen or an alkyl group; R3 and R4 represent H, OH, halogen, etc.; Q represents alkylene, etc.; ring A represents aryl or heteroaryl; and G represents , or pharmaceutically acceptable salts thereof, or prodrugs thereof, which exhibit an excellent inhibitory activity in human SGLT and are useful as agents for the prevention or treatment of a disease associated with hyperglycemia such as diabetes, postprandial hyperglycemia, impaired glucose tolerance, diabetic complications or obesity, pharmaceutical compositions comprising the same, and pharmaceutical uses thereof.
Owner:KISSEI PHARMA
Blood glucose level control
InactiveUS20070156177A1Lower Level RequirementsIncrease insulin levelsHeart defibrillatorsInternal electrodesBlood insulinLevel insulin
A method of glucose level control comprising, providing at least one electrode adapted to apply an electric field to a pancreas; and applying an electric field to the pancreas using said at least one electrode such that blood glucose levels are significantly reduced and blood insulin levels are not significantly increased compared to a regular insulin response in a same person.
Owner:METACURE
Blood glucose level control
InactiveUS8700161B2Increase insulin levelsLower Level RequirementsImplantable neurostimulatorsDigestive electrodesBlood insulinPancreas
A method of glucose level control, comprising providing at least one electrode adapted to apply an electric field to a pancreas; and applying an electric field to the pancreas using the above-mentioned at least one electrode such that blood glucose levels are significantly reduced and blood insulin levels are not significantly increased.
Owner:TYLERTON INT INC
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Small molecules for the reduction of high blood glucose level
InactiveUS20090317372A1Lower blood sugar levelsAntibacterial agentsBiocideDiseaseAcute hyperglycaemia
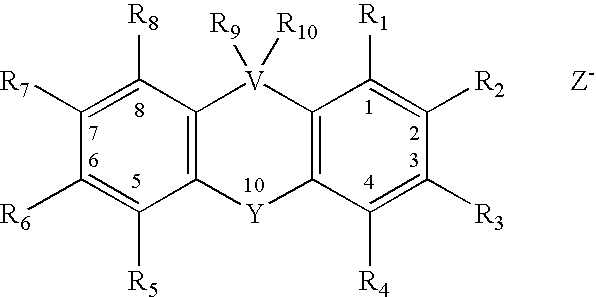
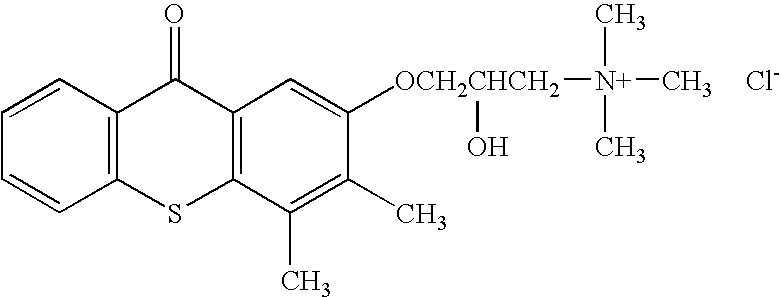
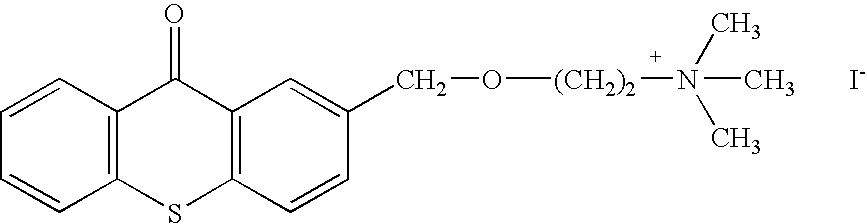
Embodiments of the present invention include the in vivo use of a family of heterocyclic compounds containing a quaternary ammonium group as exemplified by the thioxanthone and thioxanthene compounds [3-(3,4-dimethyl-9-oxo-9H-thioxanthen-2-yloxy)-2-hydroxypropyl]trimethylammonium chloride, or CCcompound1, N,N,-diethyl-N-methyl-2-[9-oxo-9H-thioxanthen-2-yl)methoxy]ethanaminium iodide, or CCcompound3, and N,N,N-trimethyl-3-(9H-thioxanthen-9-ylidene)-propane-1-aminium iodide, or CCcompound19 to reduce higher than normal blood glucose level within or close to the normal range in subjects with insulin resistance, hyperglycemia, and diabetes thereby also reducing or preventing associated diseases, complications, and pathological states.
Owner:KISS ZOLTAN
Alpha substituted carboxylic acids
InactiveUS20050187266A1Lower blood pressureLower blood sugar levelsBiocideOrganic chemistryDyslipidemiaBlood lipids
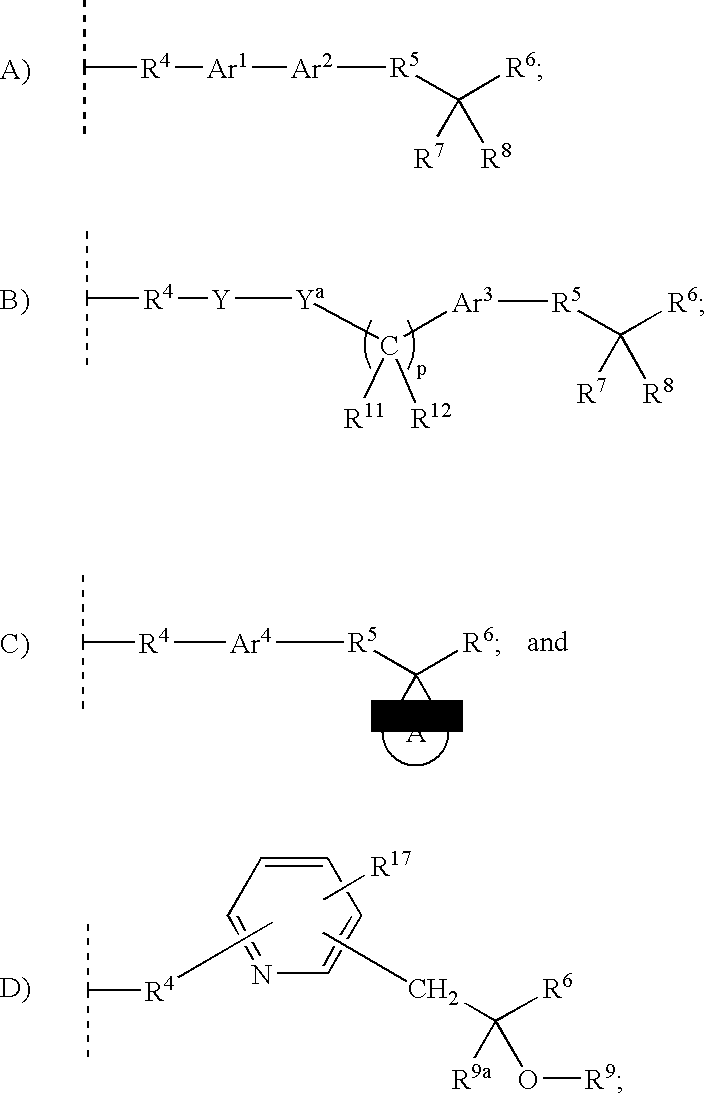
Alpha substituted carboxylic acids of formula (I): wherein R1 and R2 are as defined in the specification and R3 is wherein Y, Ar1, Ar2, Ar3, R4, R5, R6, R7, R8, R9, R9a, R10, R11, R12, R17, ring A, and p are as defined in the specification; pharmaceutical compositions containing effective amounts of said compounds or their salts are useful for treating PPAR, specifically PPAR α / γ related disorders, such as diabetes, dyslipidemia, obesity and inflammatory disorders.
Owner:AGOURON PHARMA INC +1
Phenol derivative, medicinal composition containing the same, and medicinal use thereof
InactiveUS20070185197A1Lowering excessive intracellular accumulationGood effectBiocideOrganic active ingredientsAcute hyperglycaemiaChemical structure
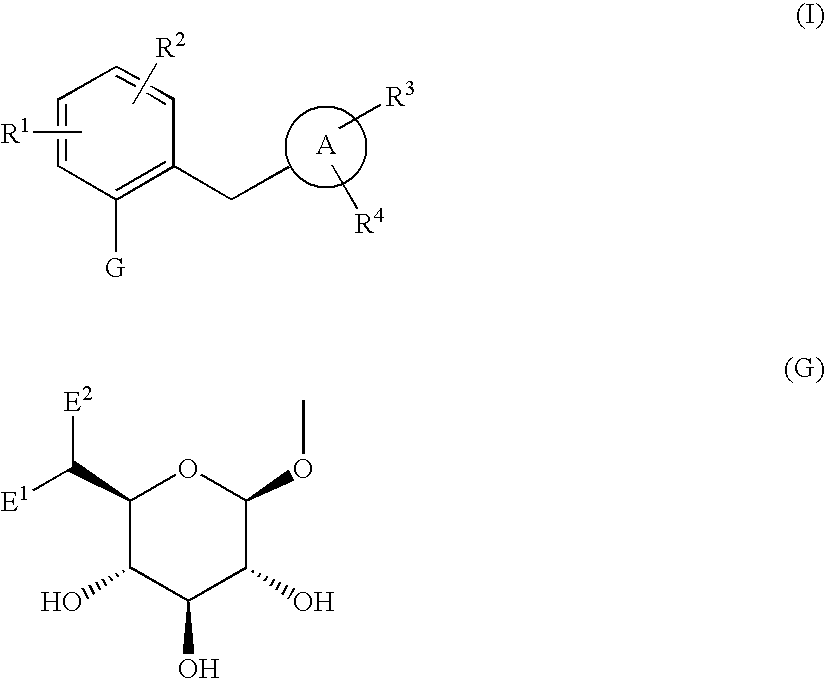
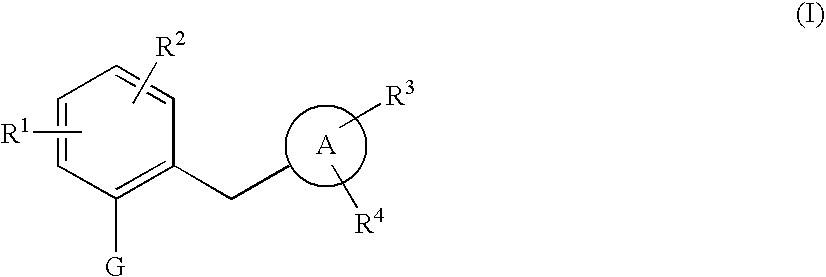
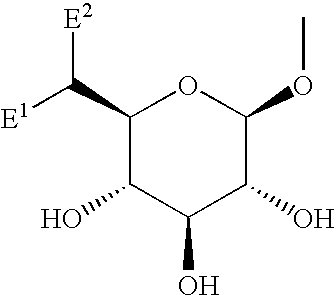
The present invention provides phenol derivatives represented by the following general formula or pharmaceutically acceptable salts thereof, or prodrugs thereof, which exhibit an inhibitory activity in human SGLT and are useful as agents for the prevention or treatment of a disease associated with hyperglycemia such as diabetes, postprandial hyperglycemia, impaired glucose tolerance, diabetic complications, obesity or the like, and pharmaceutical compositions comprising the same, and pharmaceutical uses thereof. In the chemical structure, R1 and R2 represent H, OH, NH2, etc.; R3and R4represent H, OH, a halogen atom, an optionally substituted alkyl group, etc.; ring A represents an aryl group or a heteroaryl group; G represents a group represented by the following general formula (G); E1 represents H or F; and E2 represents H, F, or a methyl group, etc.
Owner:KISSEI PHARMA
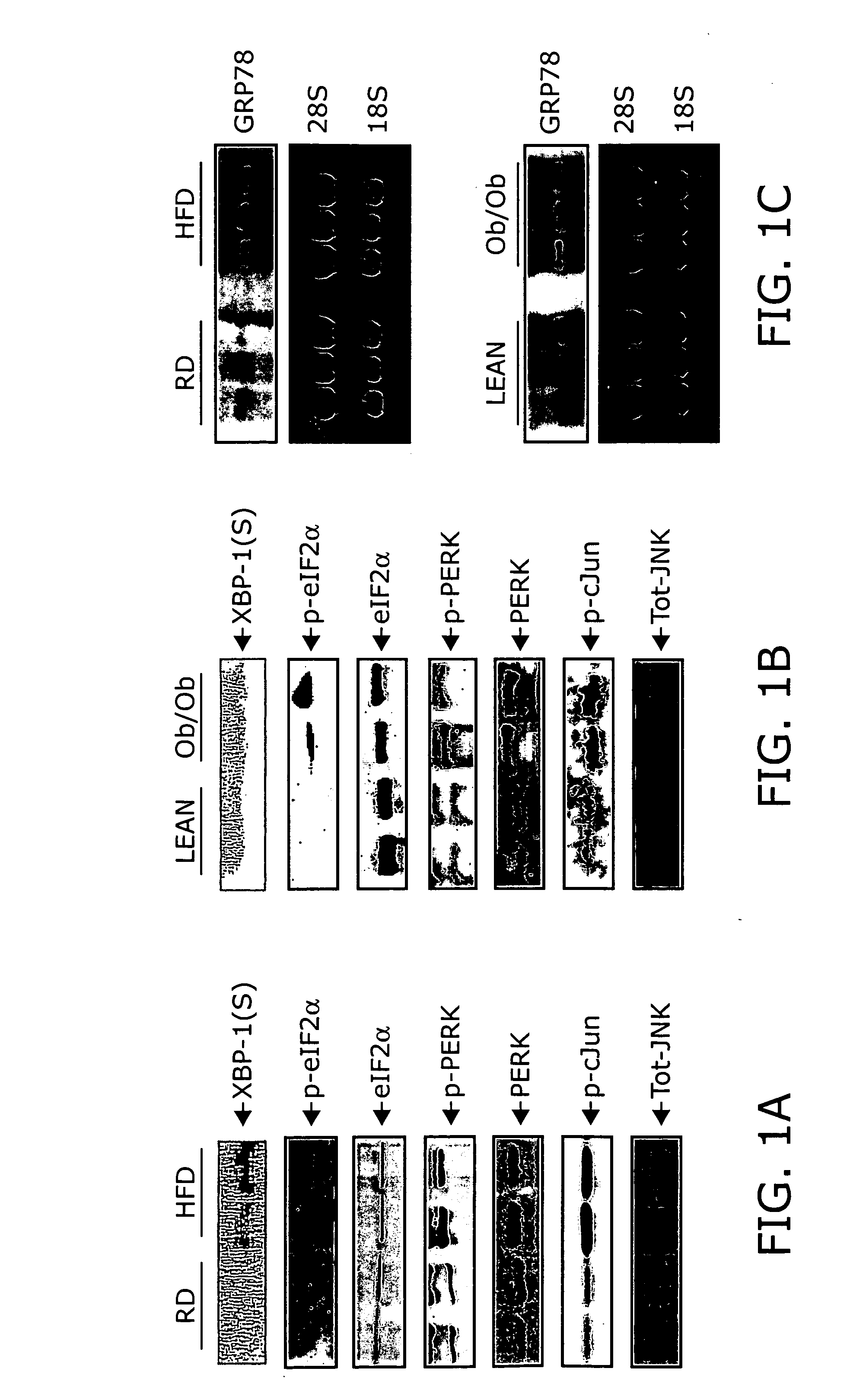
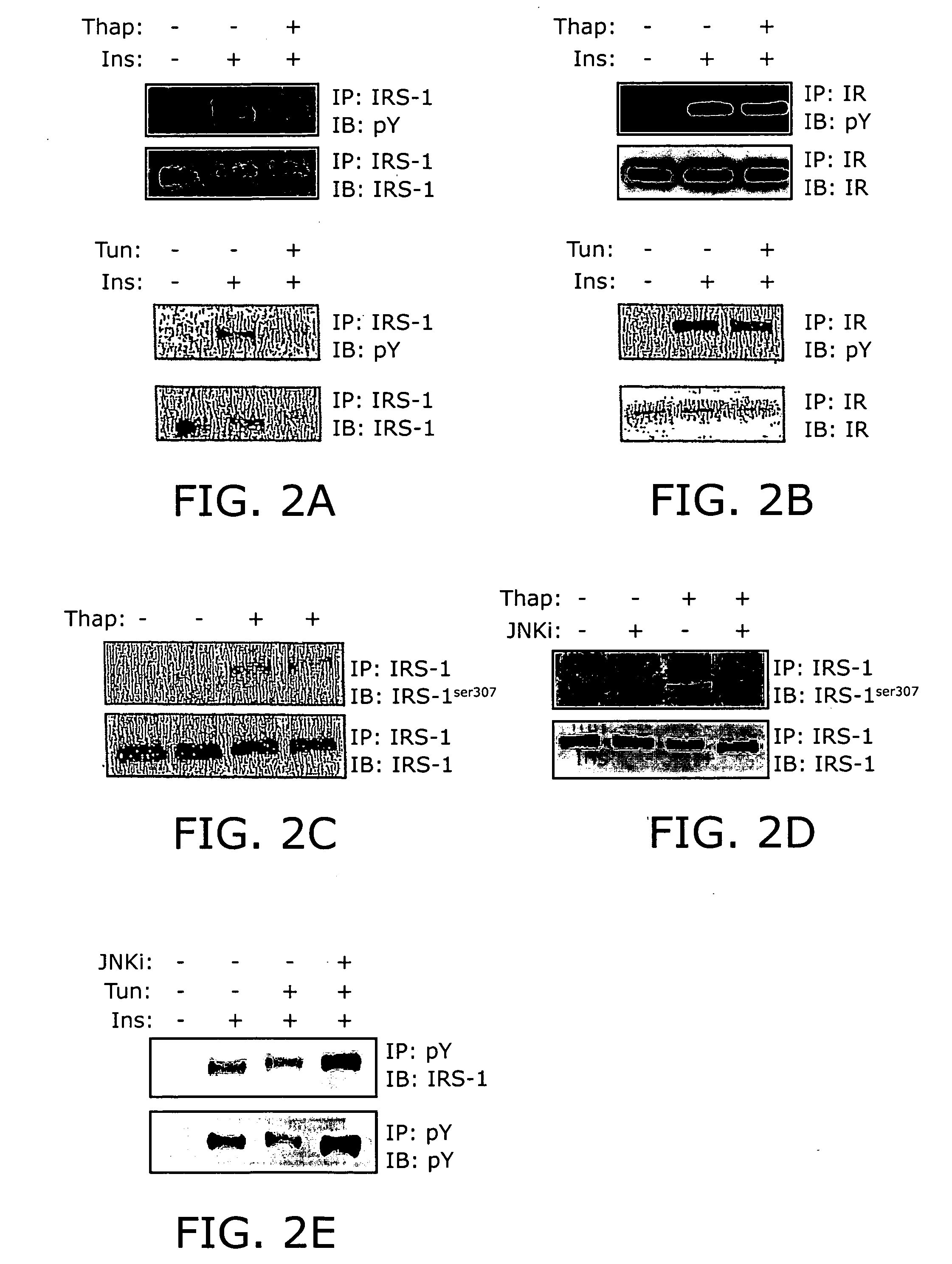
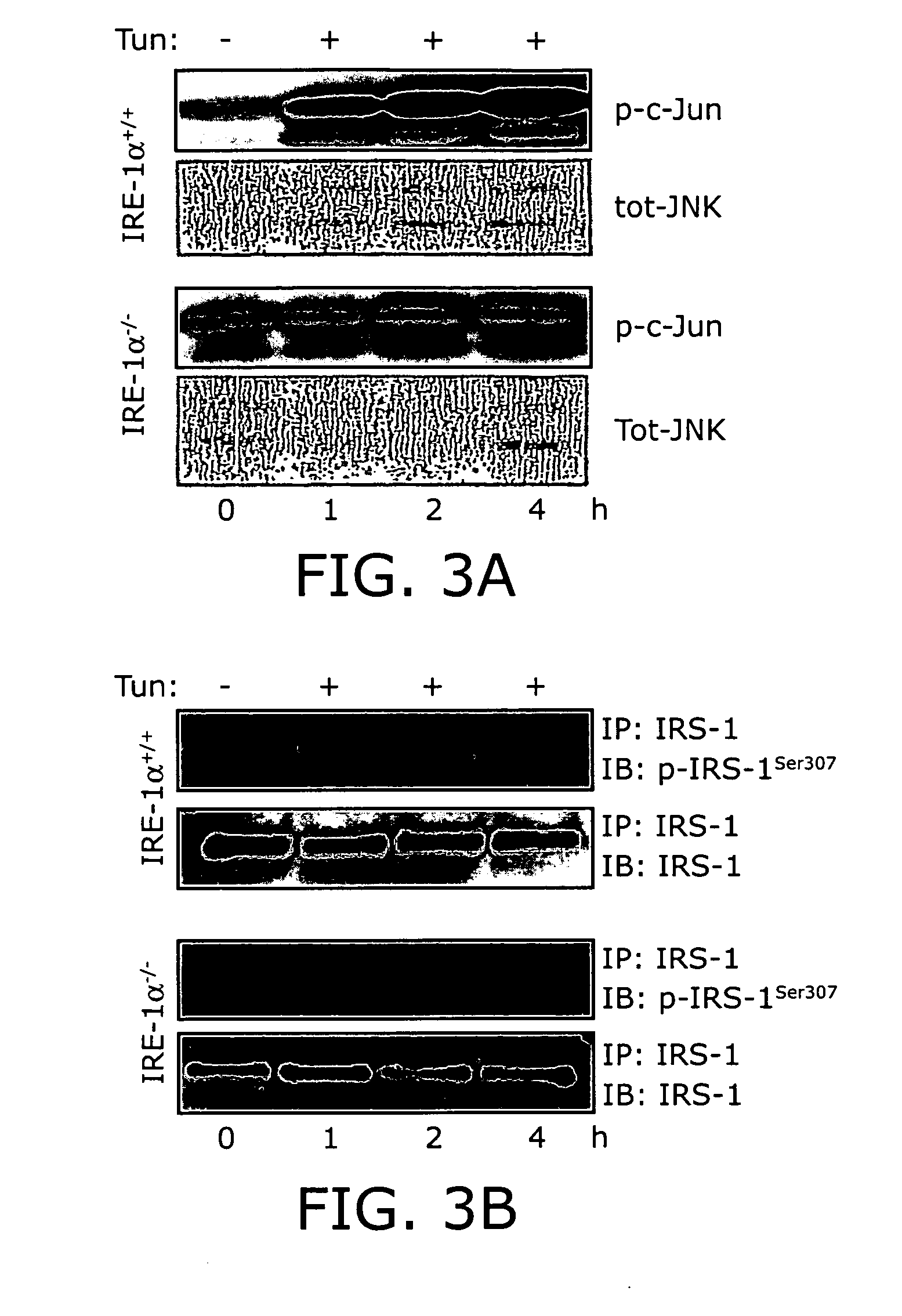
Reducing ER stress in the treatment of obesity and diabetes
InactiveUS20060073213A1Reduce ER stressIncrease insulin sensitivitySalicyclic acid active ingredientsBiocidePeripheral insulin resistanceTauroursodeoxycholic acid
Endoplasmic reticulum stress has been found to be associated with obesity. Therefore, agents that reduce or prevent ER stress may be used to treat diseases associated with obesity including peripheral insulin resistance, hypergylcemia, and type 2 diabetes. Two compounds which have been shown to reduce ER stress and to reduce blood glucose levels include 4-phenyl butyric acid (PBA), tauroursodeoxycholic acid (TUDCA), and trimethylamine N-oxide (TMAO). Other compounds useful in reducing ER stress are chemical chaperones such as trimethylamine N-oxide and glycerol. The present invention provides methods of treating a subject suffering from obesity, hyperglycemia, type 2 diabetes, or insulin resistance using ER stress reducers such as PBA, TUDCA, and TMAO. Methods of screening for ER stress reducers by identifying agents that reduce levels of ER stress markers in ER stressed cells are also provided. These agents may find use in methods and pharmaceutical compositions for treating obesity-associated diseases.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Hepatocyte growth factor for treatment of diabetes
InactiveUS6472366B2Lower blood sugar levelsLower Level RequirementsBiocidePeptide/protein ingredientsCvd riskBULK ACTIVE INGREDIENT
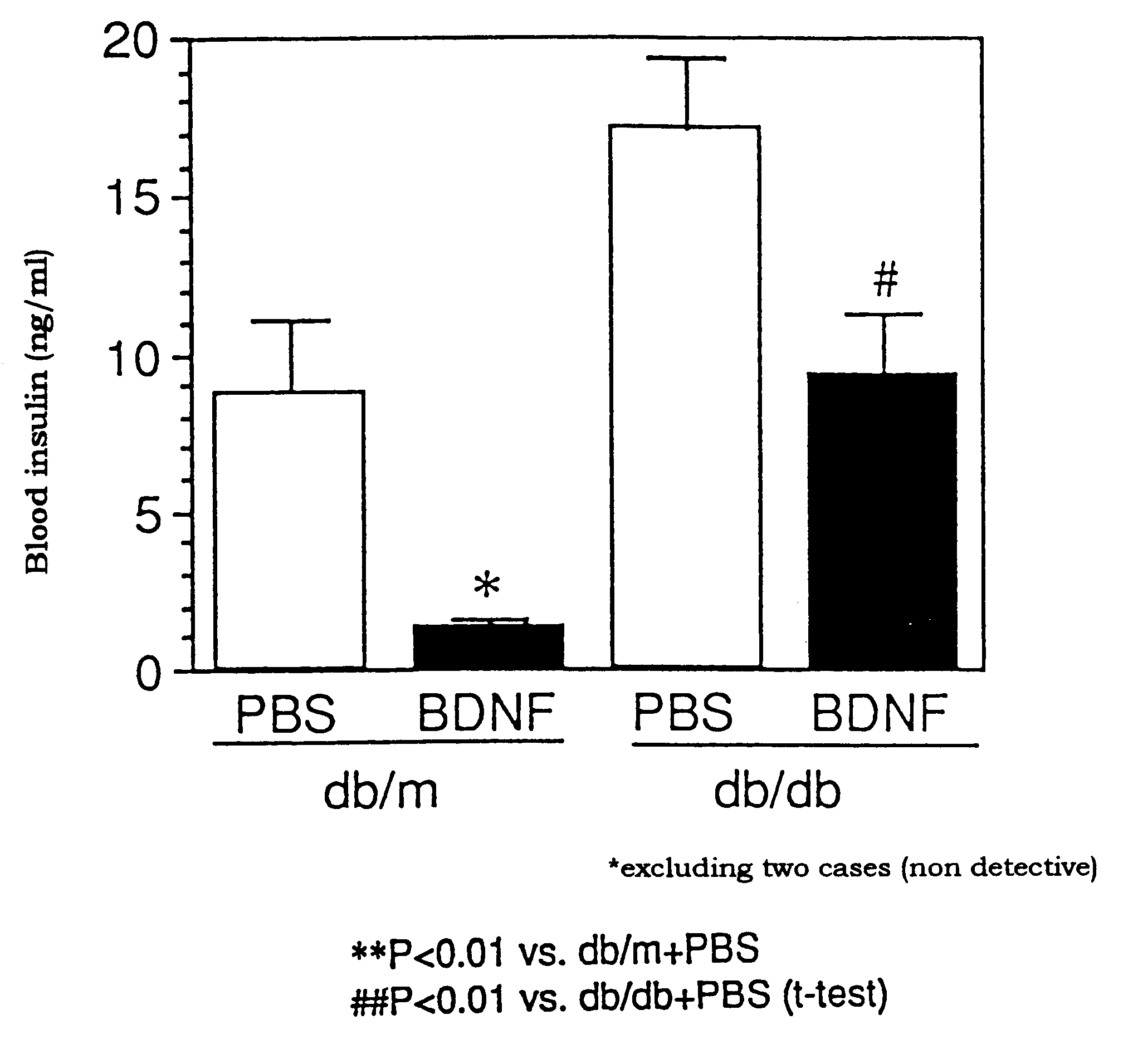
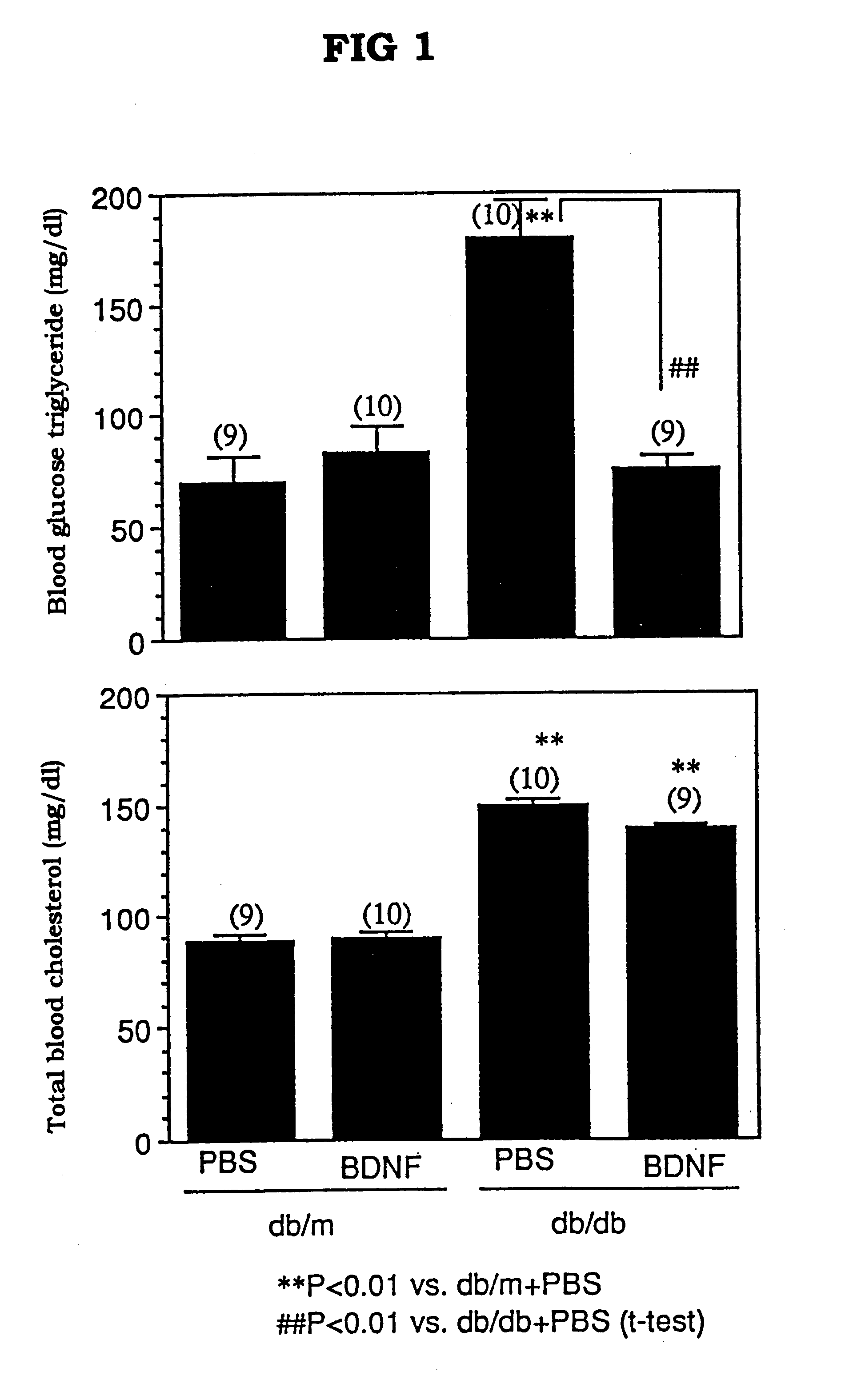
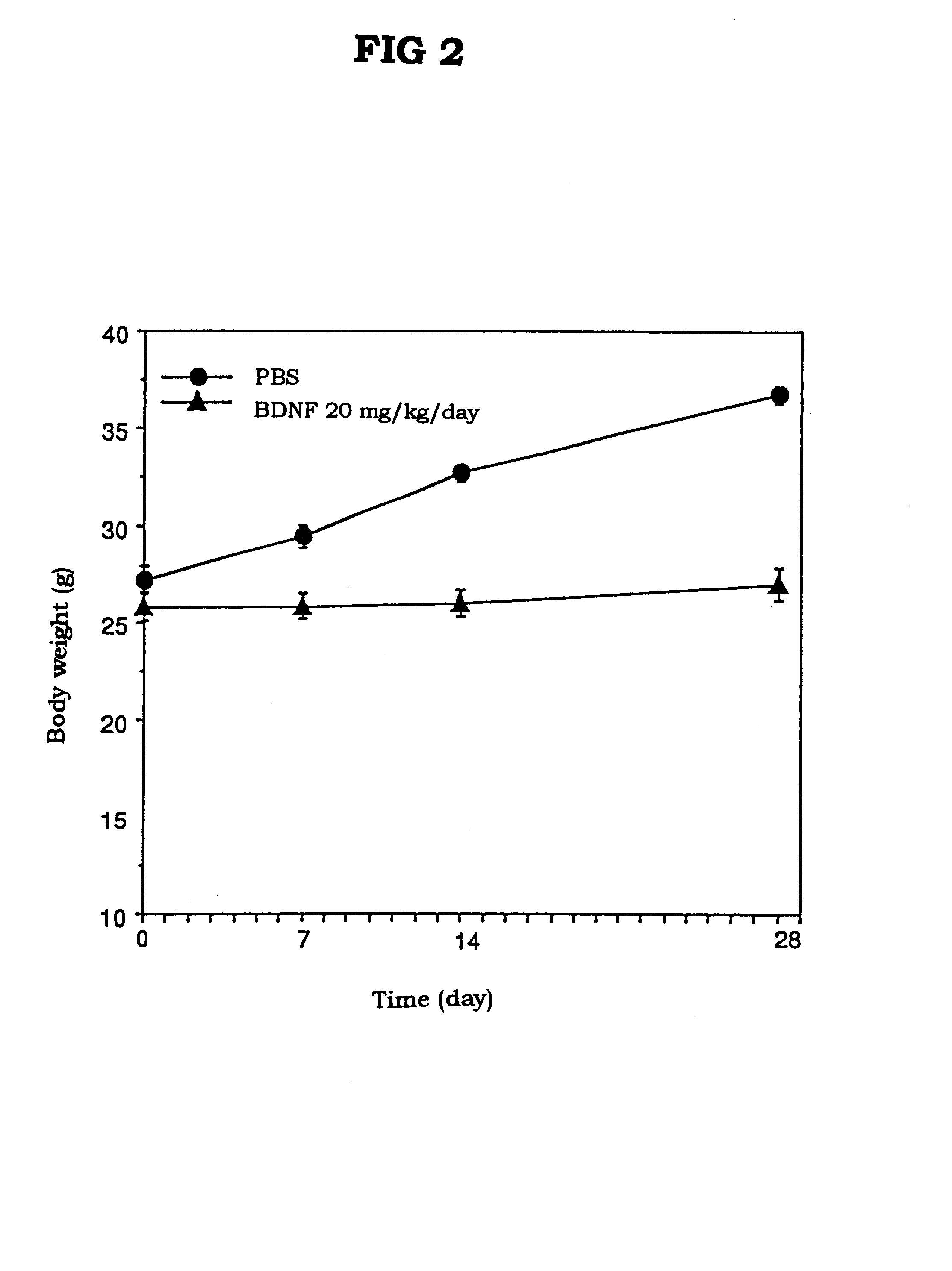
The present invention provides a therapeutic agent for treatment of diabetes and hyperlipemia, especially a therapeutic agent for treatment of type II diabetes mellitus, which comprises as the active ingredient a neurotrophic factor such as BDNF (brain-derived neurotrophic factor), ligands of trkB or trkC receptors, NGF, NT-3, NT-4 / 5, CNTF, GDNF, HGF, etc. Different from conventional oral hypoglycemic agents being mainly used in the treatment of type II diabetes mellitus, the agent of the present invention exhibit blood lipid regulating effects and body fat accumulation regulating effects, in addition to the blood glucose regulating effects. Thus, the agent of the present invention are novel, and can reduce the risk factors in diabetes accompanied by hyperlipemia or obesity, without using any other agent.
Owner:SUMITOMO PHARMA CO LTD
Hair growth compositions and methods for treating hair loss or related conditions
InactiveUS20070086972A1Mitigate and eliminate such irritationStay healthyCosmetic preparationsBiocideHair growthHair loss
The present invention relates to hair growth compositions as well as to methods for treating hair loss or related conditions.
Owner:BIRNBAUM JACOB
Peptide agonists of GLP-1 activity
InactiveUS20070111940A1Improve glucose toleranceLower blood sugar levelsPeptide/protein ingredientsVasoactive intestinal peptideGastric emptyingAgonist
Novel peptide agonists of GLP-1 activity useful for lowering blood glucose levels. The novel peptides comprise variants of the GLP-1 or the exendin-4 polypeptide sequence and are pharmacologically active and stable. These peptides are useful in the treatment of diseases that benefit from regulation of excess levels of blood glucose and / or regulation of gastric emptying, such as diabetes and eating disorders.
Owner:LARSEN BJARNE DUE +2
Treating diabetes with glucagon-like peptide-1 secretagogues
InactiveUS20070032420A1Increase basal GLP- levelDecrease DPP-IV activityOrganic active ingredientsPeptide/protein ingredientsAcute hyperglycaemiaDipeptidyl peptidase
In general this invention can be viewed as encompassing novel methods of treating diabetes and insulin resistance. The inventors have made the discovery that increasing secretion of endogenous glucagon-like peptide-1 (GLP-1) in combination with inhibiting the activity of dipeptidyl peptidase I (DPP-IV) can have a significant impact on hyperglycemia and insulin secretion in subjects suffering from diabetes and / or insulin resistance. Further the invention encompasses methods of identifying subjects having elevated secretion of GLP-1, methods of assessing sensitivity to a GLP-1 secretagogue, and methods of treating diabetes in these subjects by administering a GLP-1 secretagogue to alleviate at least one symptom of diabetes.
Owner:ENTELOS INC
Insulin formulations for insulin release as a function of tissue glucose levels
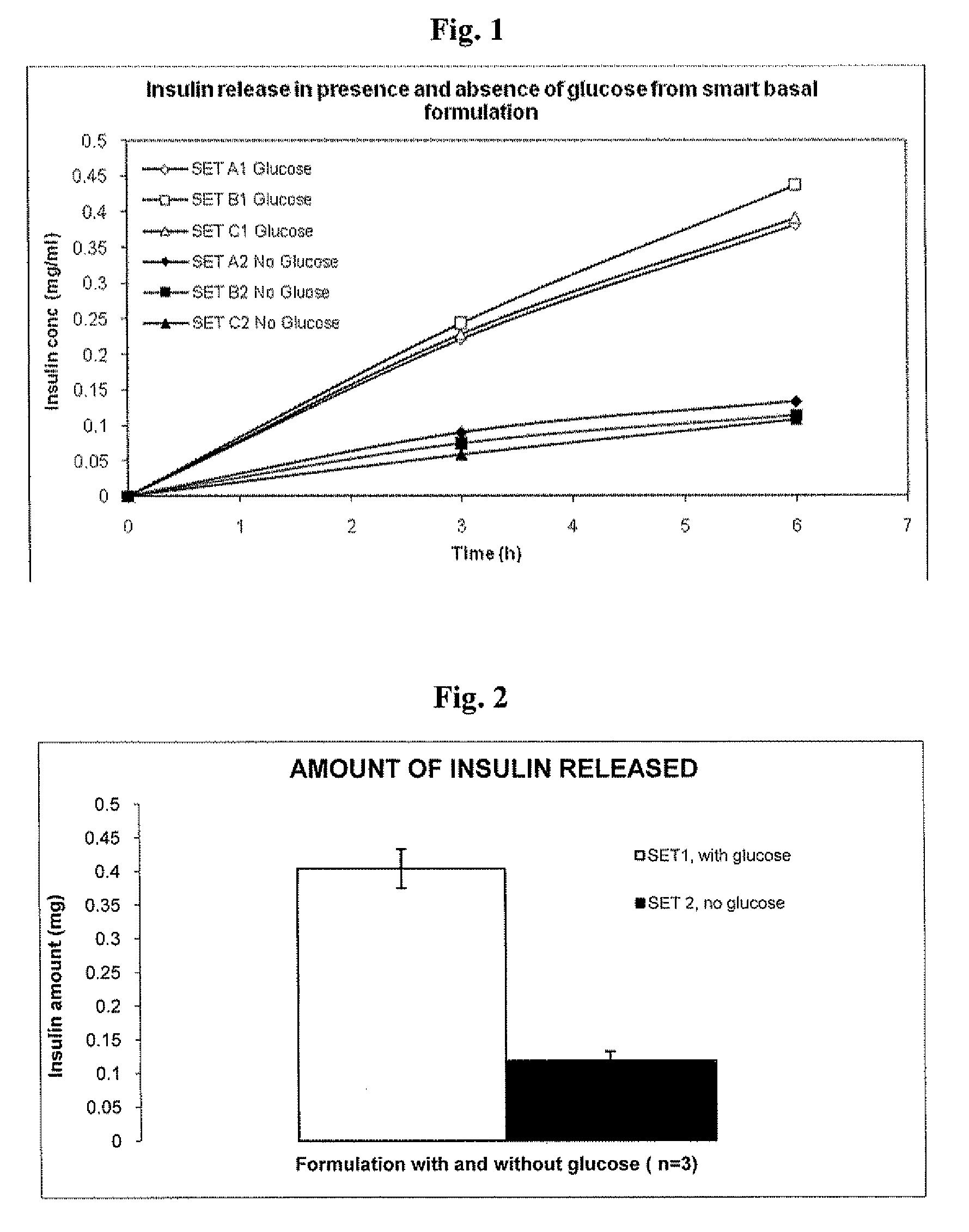
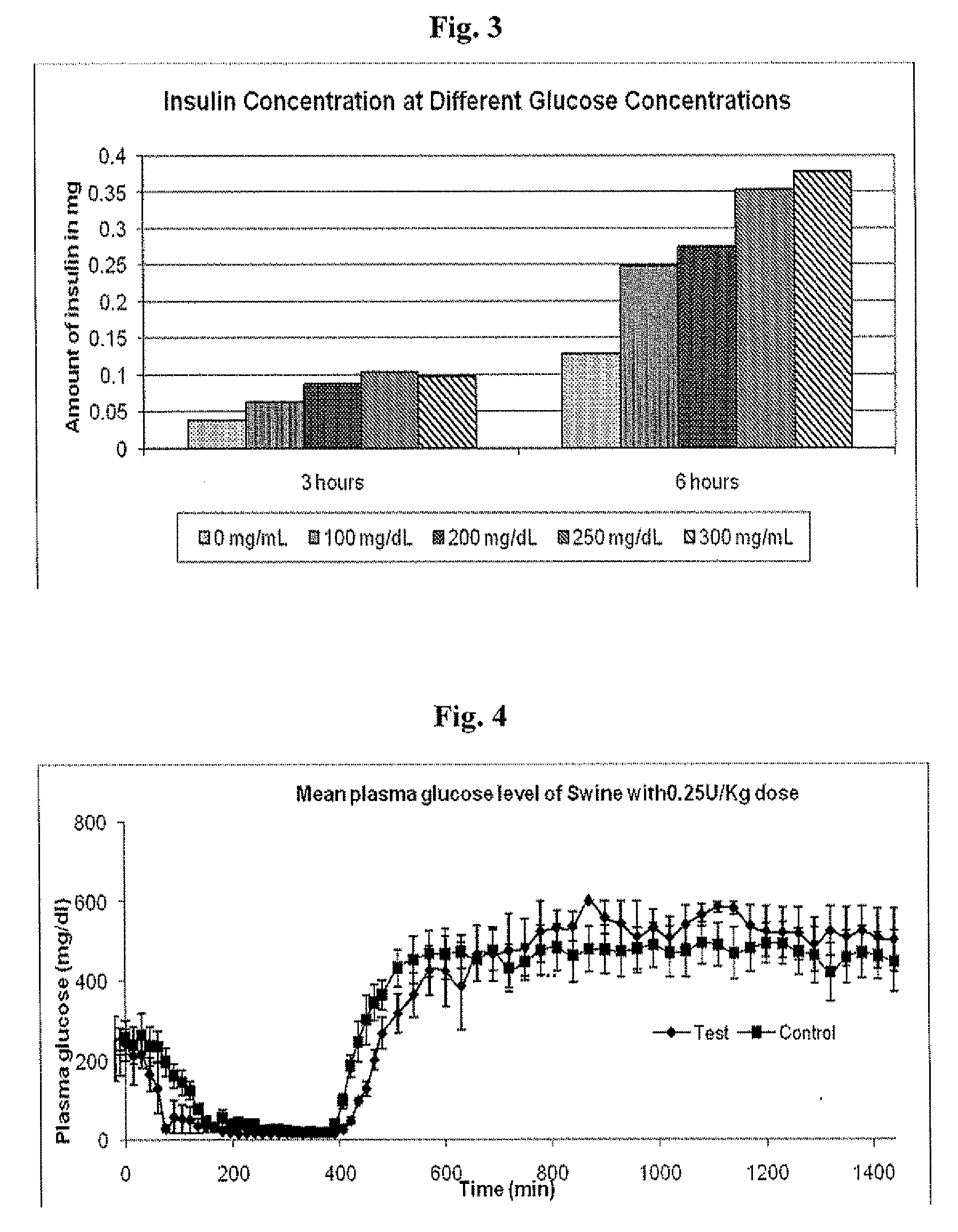
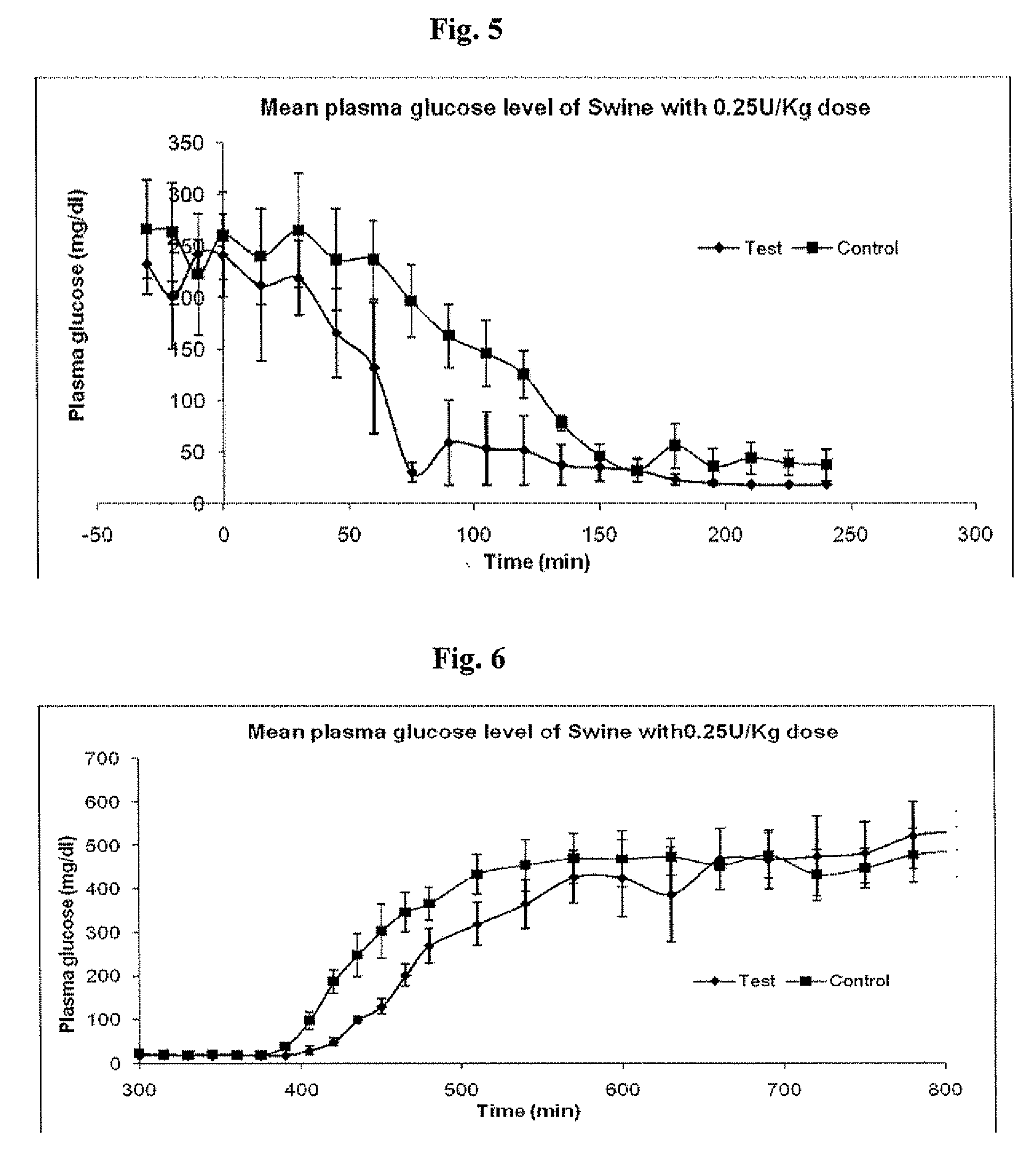
InactiveUS20090175840A1Reduce productionRaise the pHPeptide/protein ingredientsMetabolism disorderInjections insulinReducing agent
Injectable insulin formulations that are capable of modifying the amount of insulin released based on the patient's tissue glucose levels, methods for making and using these formulations are described herein. The formulation may be administered via subcutaneous, intradermal or intramuscular administration. In one preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin, an oxidizing agent or enzyme and a reducing agent or enzyme, a diluent and optionally one or more thickening agents. If a thickening agent is present in the formulation, the thickening agent increases the viscosity of the formulation following administration. Preferably the formulation contains an insulin, a diluent, glucose oxidase and peroxidase. Following administration to a patient, the insulin is released from the formulations as a function of the patient's tissue glucose level, which in turn maintains the patient's blood glucose level within an optimum range. The formulation is often referred to as a “smart” formulation since it modifies its release rate of insulin according to the patient's needs at a particular time. In a preferred embodiment, the formulation is designed to release insulin into the systemic circulation over time with a basal release profile following injection in a patient. In another embodiment, the formulation is designed to release insulin into the systemic circulation over time with a non-basal release profile following injection in a patient, such as a regular human insulin release profile or a prandial release profile.
Owner:BIODEL
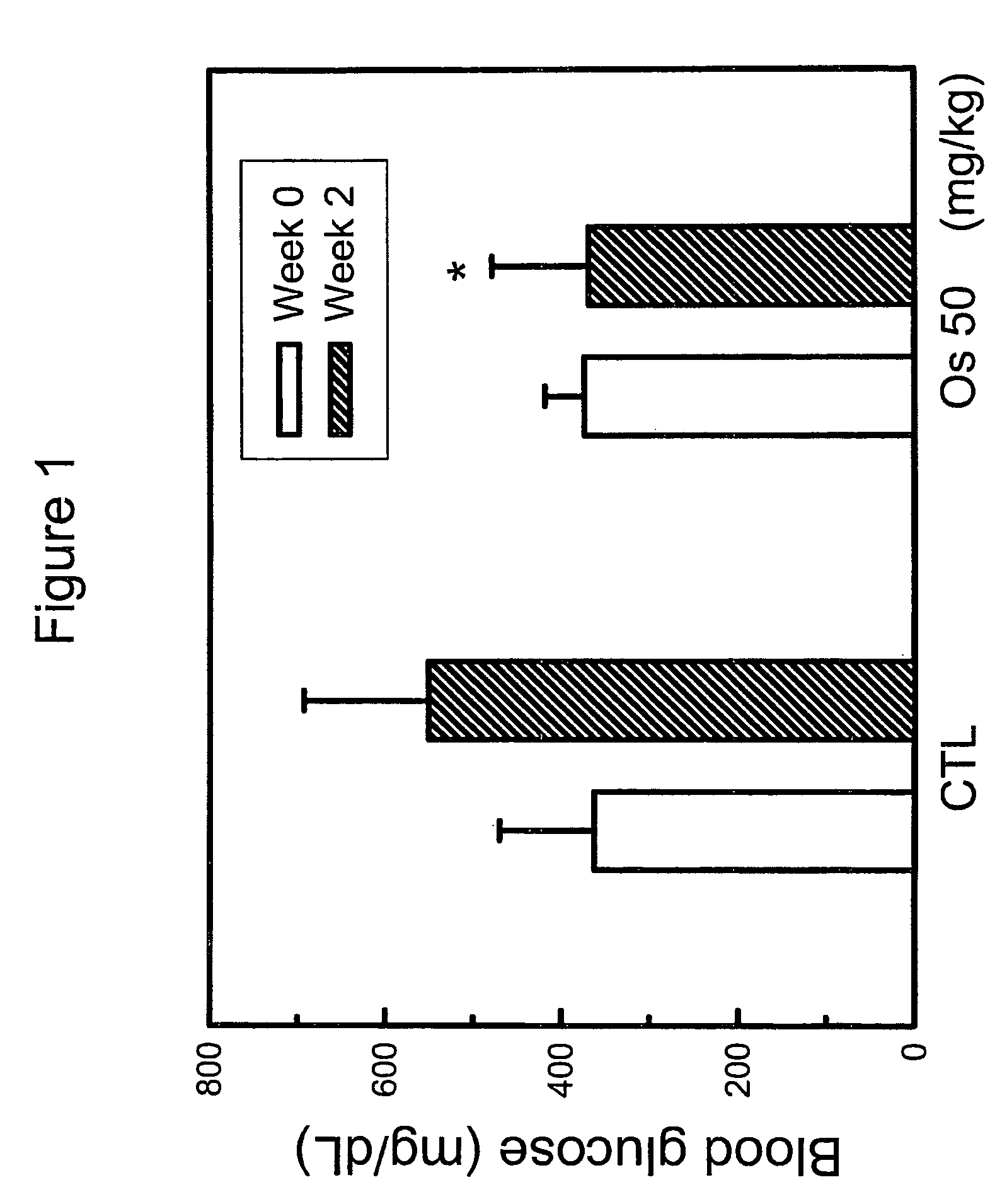
Hypoglycemic activity of osthole
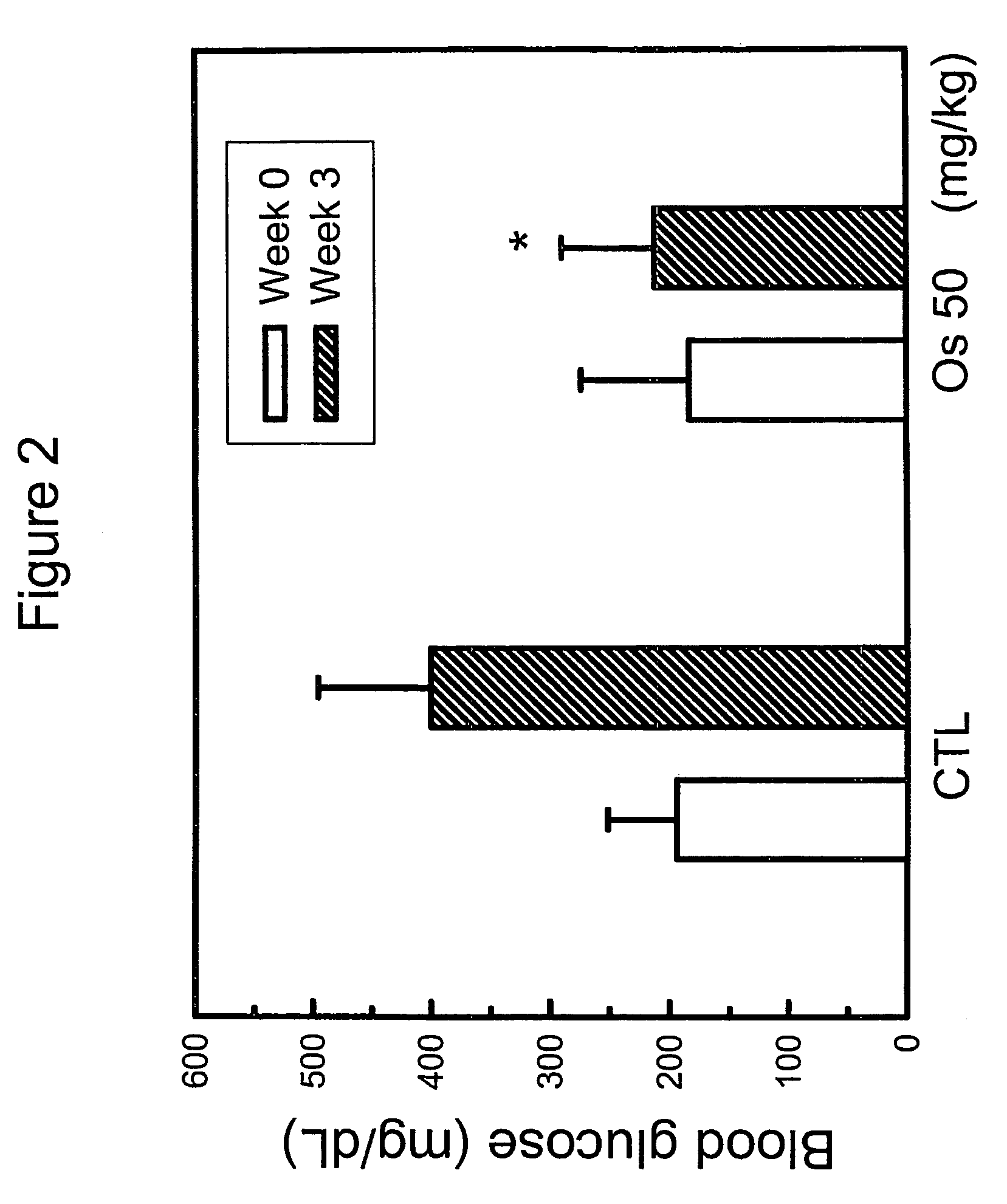
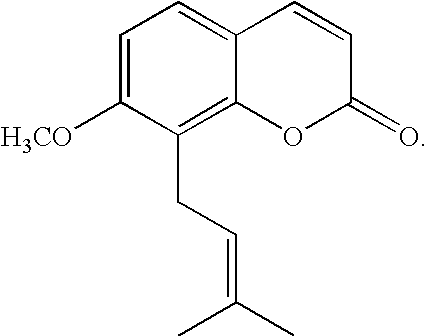
InactiveUS8198320B2Lower blood sugar levelsImprove the level ofBiocideMetabolism disorderCnidium monnieriBlood sugar
The present invention relates to a method for controlling blood glucose level and a method for the prophylaxis or treatment of diabetes mellitus and / or its complications. The present invention further relates to an anti-diabetic formulation for controlling blood glucose level and / or for the prophylaxis or treatment of diabetes mellitus and / or its complications.
Owner:TAIPEI MEDICAL UNIV
Methods and systems for determining and controlling glycemic responses
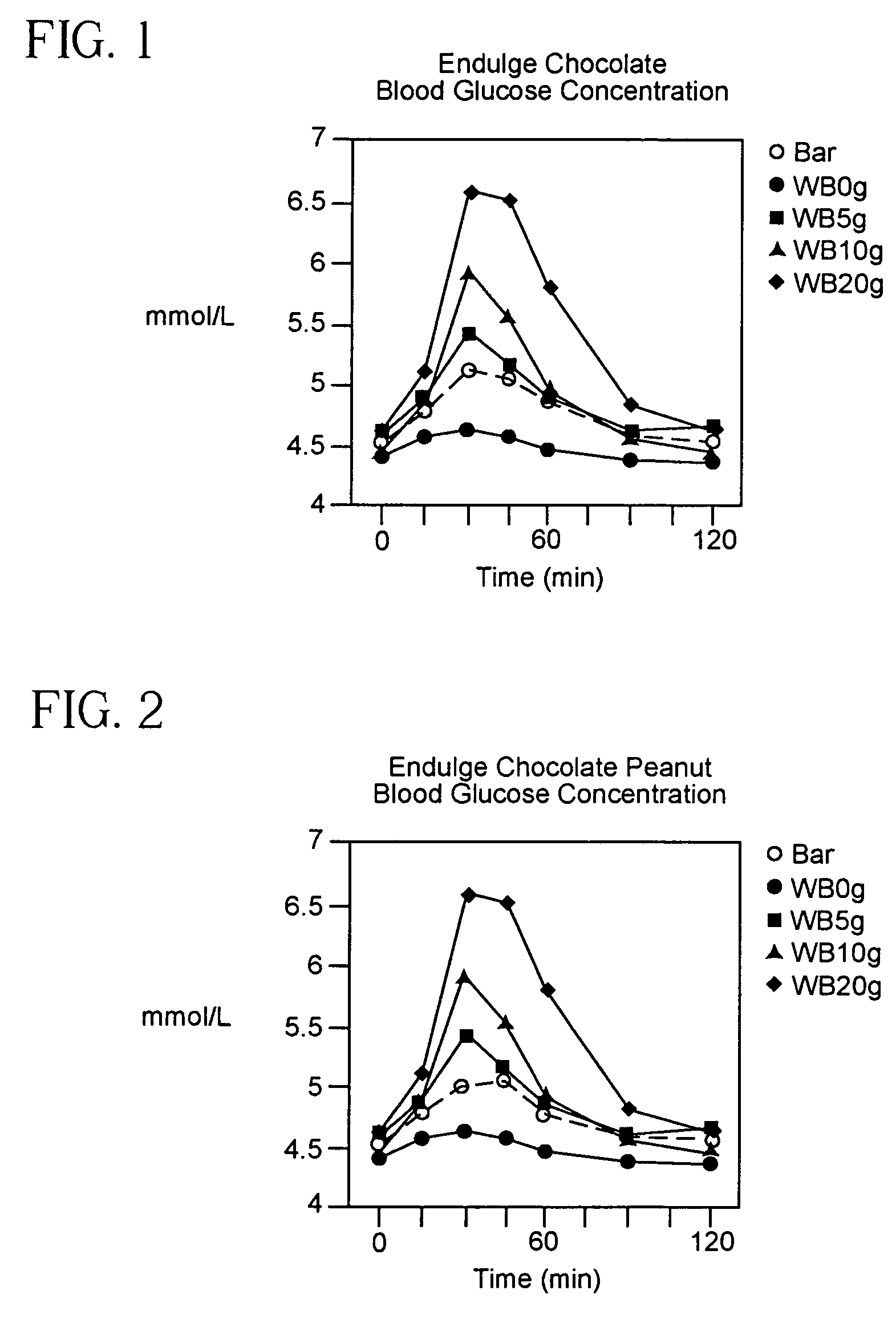
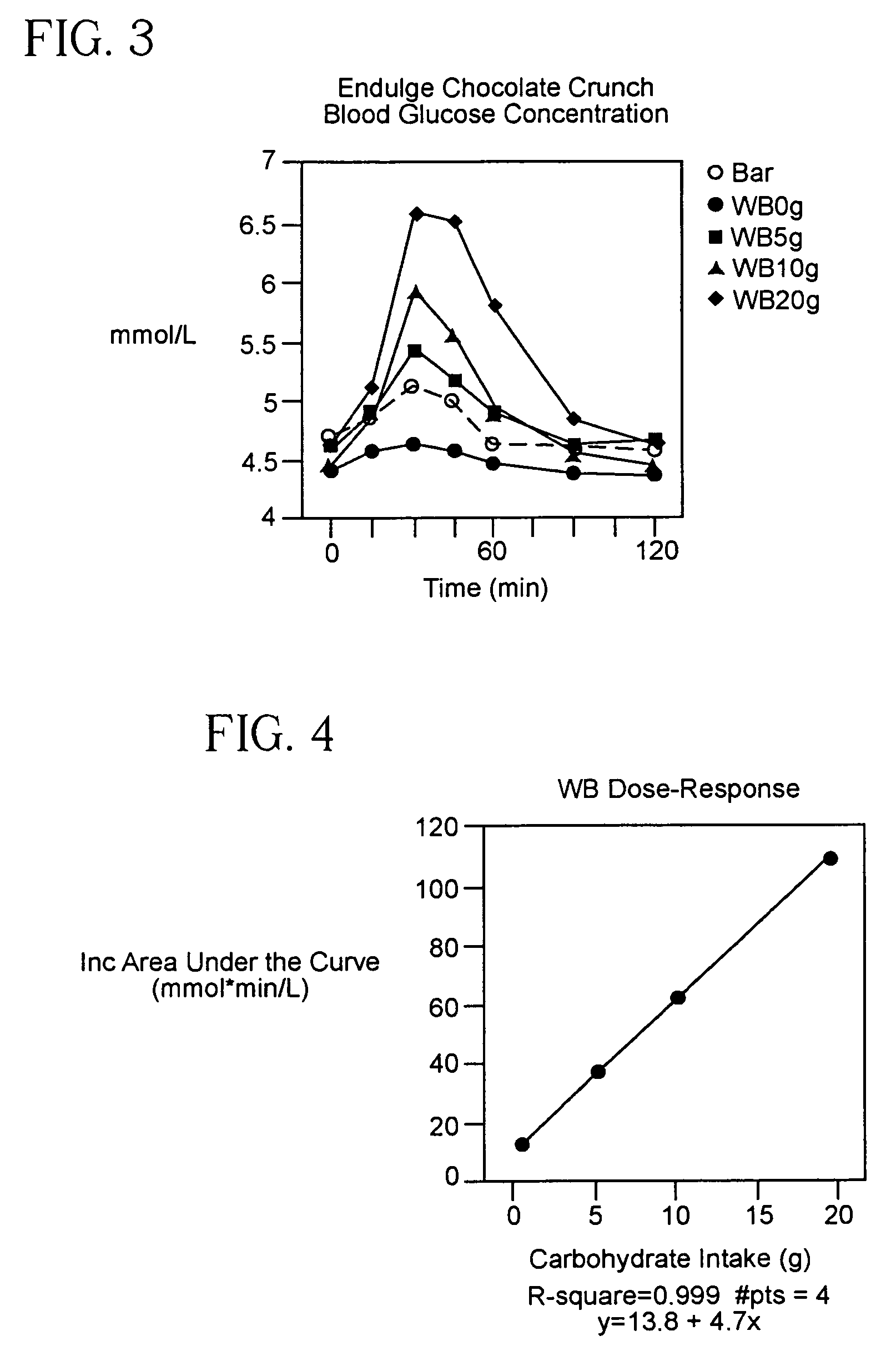
ActiveUS8571801B2Easy to implementLower blood sugar levelsMicrobiological testing/measurementBiological testingEnvironmental healthGlycemic
The present invention provides a method of determining a standard comestible Equivalent Glycemic Load of a dietary comestible comprising: (a) establishing a reliable glycemic response index for a standard comestible, wherein the index correlates glycemic response with glycemic load; (b) determining the glycemic response produced by a dietary comestible, and (c) identifying the standard comestible glycemic load from the index which is correlated with the glycemic response of the dietary comestible.
Owner:SIMPLY GOOD FOODS USA INC
Compounds and methods for treating diabetes
ActiveUS20130190294A1Lower blood sugar levelsStabilize blood sugar levelsBiocideOrganic chemistryAntagonistDisease cause
Hydrogenated pyrido[4,3-b]indoles, pyrido[3,4-b]indoles and azepino[4,5-b]indoles are described. The compounds may bind to and are antagonists of the adrenergic receptor α2A. The compounds may also bind to and are an antagonist of the adrenergic receptor α2B; or the compounds are not antagonists of the adrenergic receptor α2B and the compounds are administered in conjunction with a second agent that reduces, or is expected to reduce, blood pressure in an individual. The compounds may find use in therapy, e.g., to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. Use of the compounds to treat type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
Combination therapy for the treatment of diabetes and conditions related thereto and for the treatment of conditions ameliorated by increasing a blood GLP-1 level
InactiveUS20070072804A1Lower blood sugar levelsImprove the level ofOrganic active ingredientsSenses disorderGpr119 agonistG protein-coupled receptor
The present invention concerns combination of an amount of a GPR119 agonist with an amount of a dipeptidyl peptidase IV (DPP-IV) inhibitor such that the combination provides an effect in lowering a blood glucose level or in increasing a blood GLP-1 level in a subject over that provided by the amount of the GPR119 agonist or the amount of the DPP-IV inhibitor alone and the use of such a combination for treating or preventing diabetes and conditions related thereto or conditions ameliorated by increasing a blood GLP-1 level. The present invention also relates to the use of a G protein-coupled receptor to screen for GLP-1 secretagogues.
Owner:ARENA PHARMA
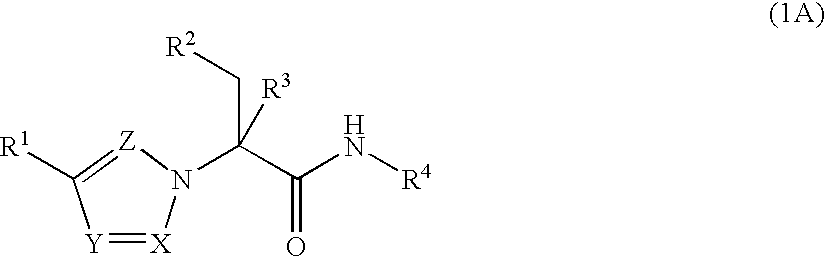
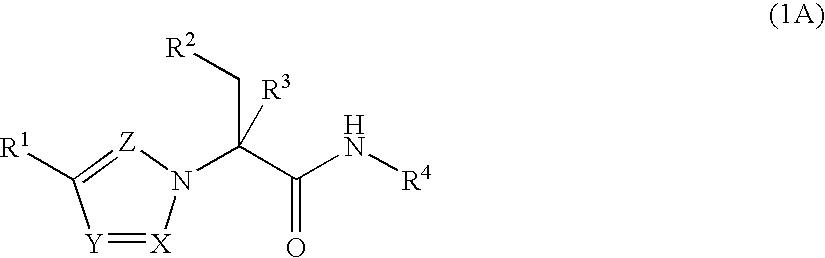
Substituted Heteroaryls
The present invention provides Formula (1A) compoundsthat act as glucokinase activators; pharmaceutical compositions thereof; and methods of treating diseases, disorders, or conditions mediated by glucokinase. X, Y, Z, R1, R2, R3, and R4 are as described herein.
Owner:PFIZER INC
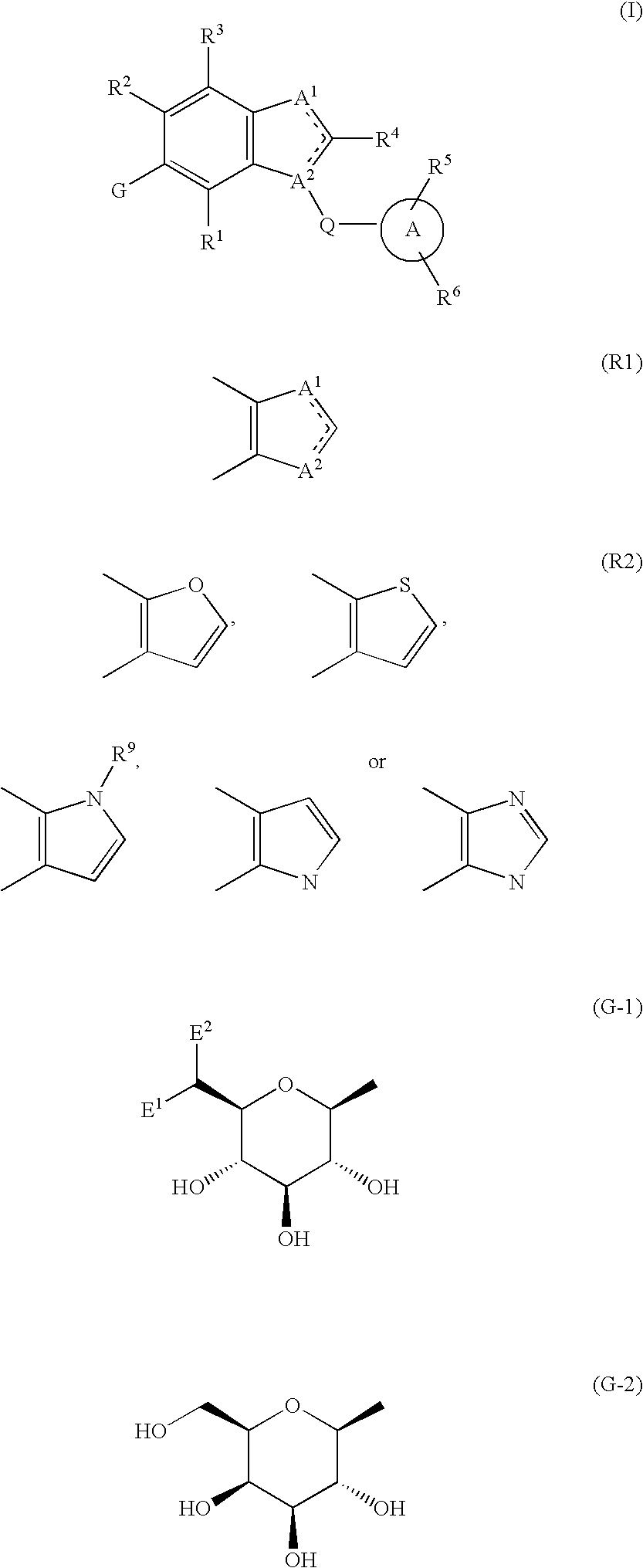
Fused heterocycle derivative, medicinal composition containing the same, and medicinal use thereof
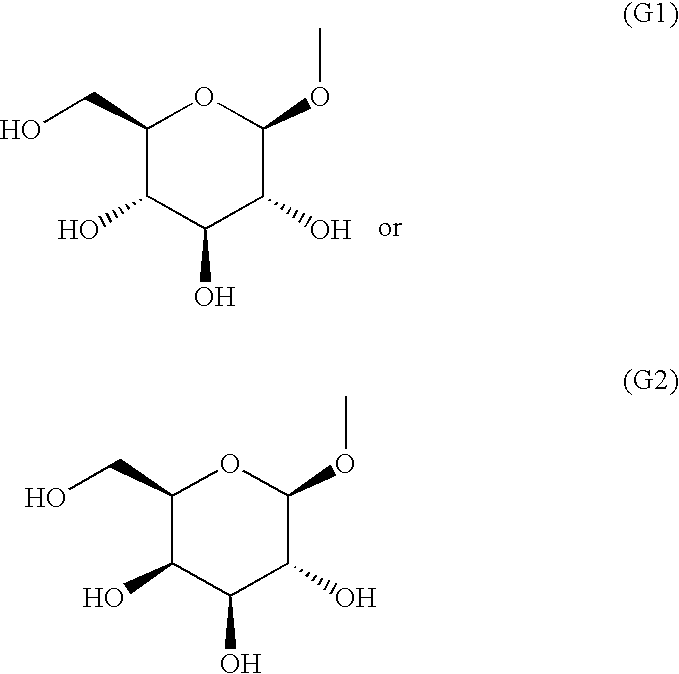
The present invention provides fused heterocyclic derivatives represented by the following general formula (I) or pharmaceutically acceptable salts thereof, or prodrugs thereof, which exhibit an inhibitory activity in human SGLT and are useful as agents for the prevention or treatment of a disease associated with hyperglycemia such as diabetes, postprandial hyperglycemia, impaired glucose tolerance, diabetic complications or obesity, in the formula R1 to R4 represent H, OH, an amino group, etc.; R5 and R6 represent H, OH, a halogen atom, an option ally substituted alkyl group, etc.; Q represents alkylene, alkenylene, etc.; ring A represents an aryl group or a heteroaryl group; the following ring (R1) represents a group represented by the following ring (R2); G represents a group represented by the following general formula (G-1) or (G-2) (E1 represents H, F or OH; and E represents H, F, a methyl group, etc.), and pharmaceutical compositions comprising the same, and pharmaceutical uses thereof.
Owner:KISSEI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com