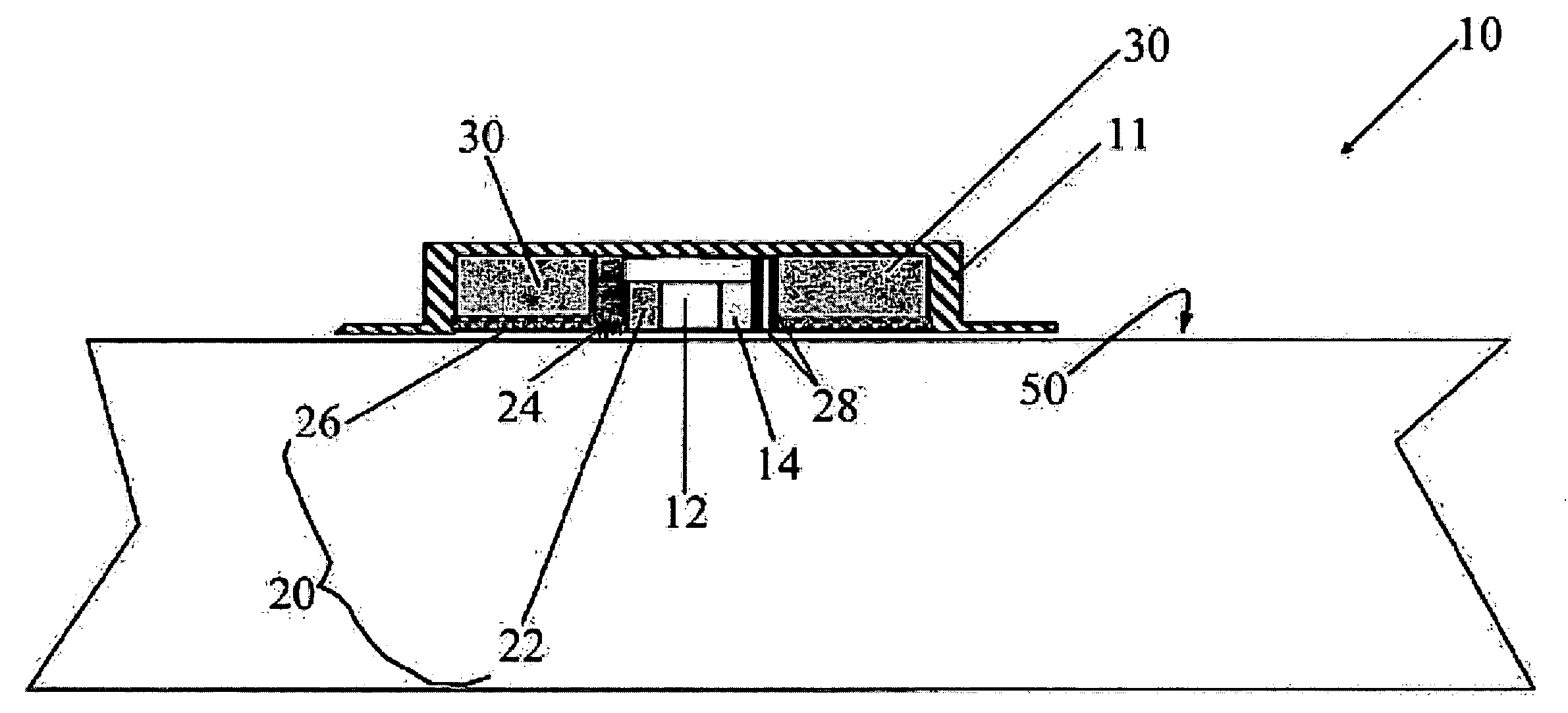
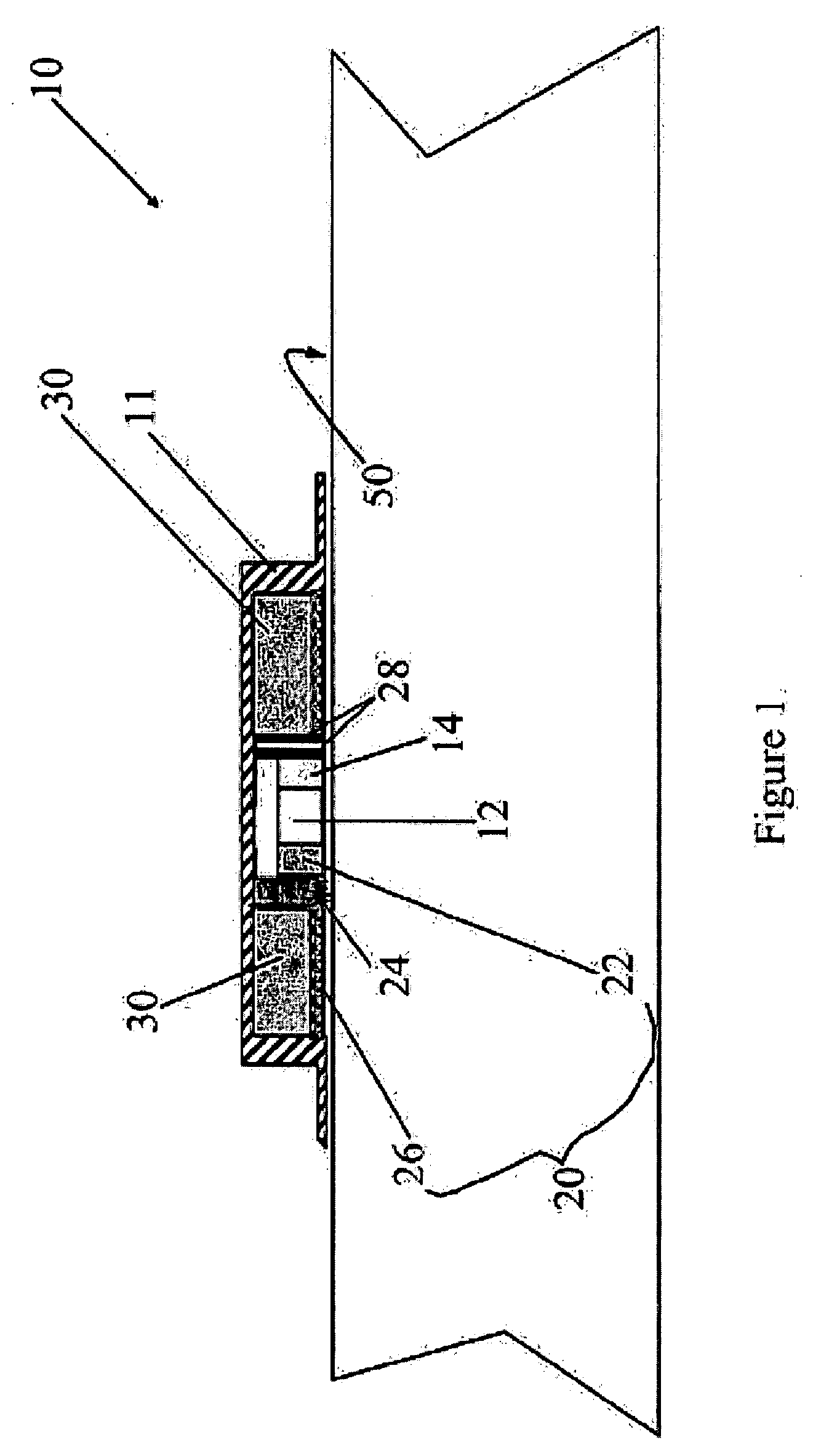
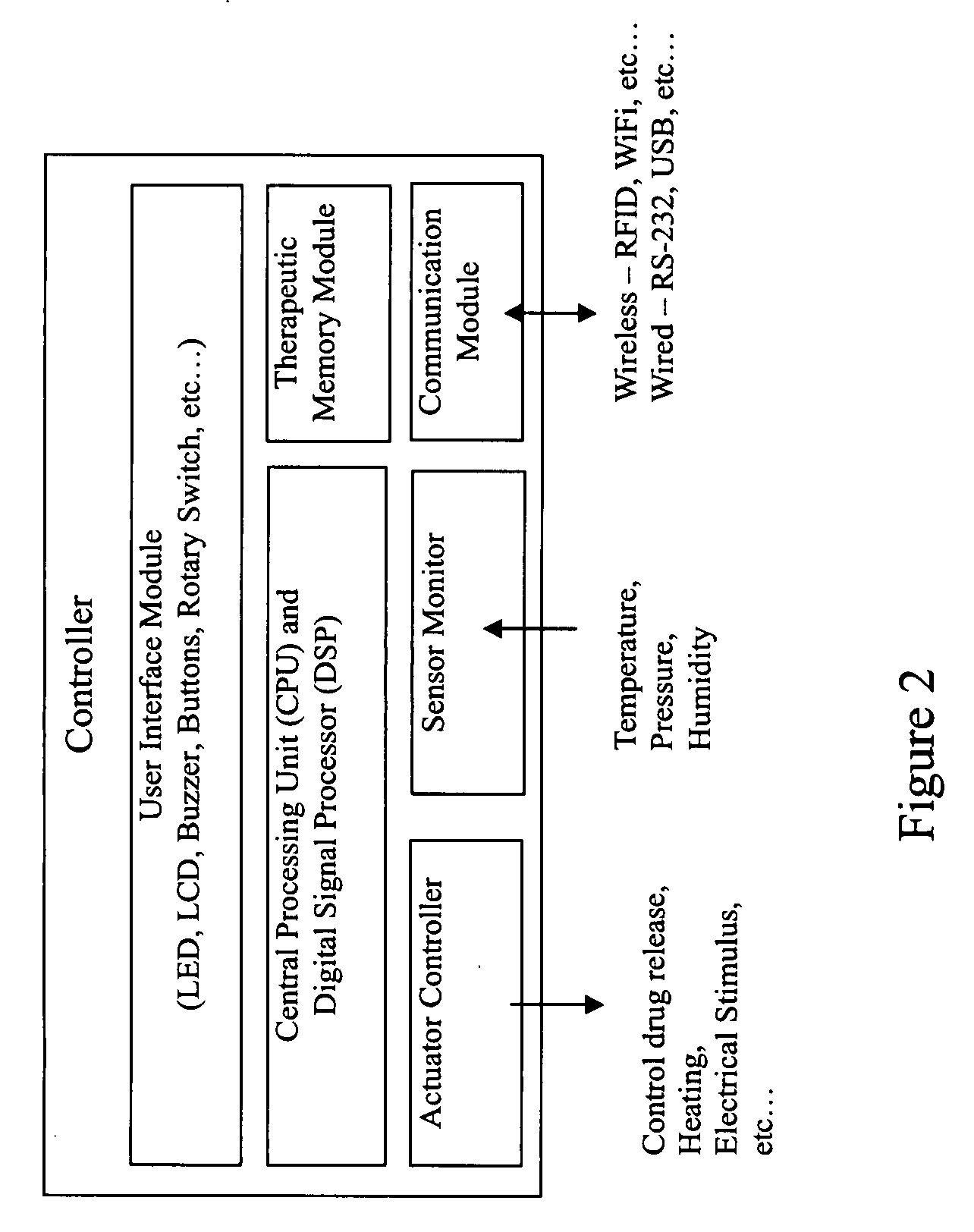
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
135 results about "Injections insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
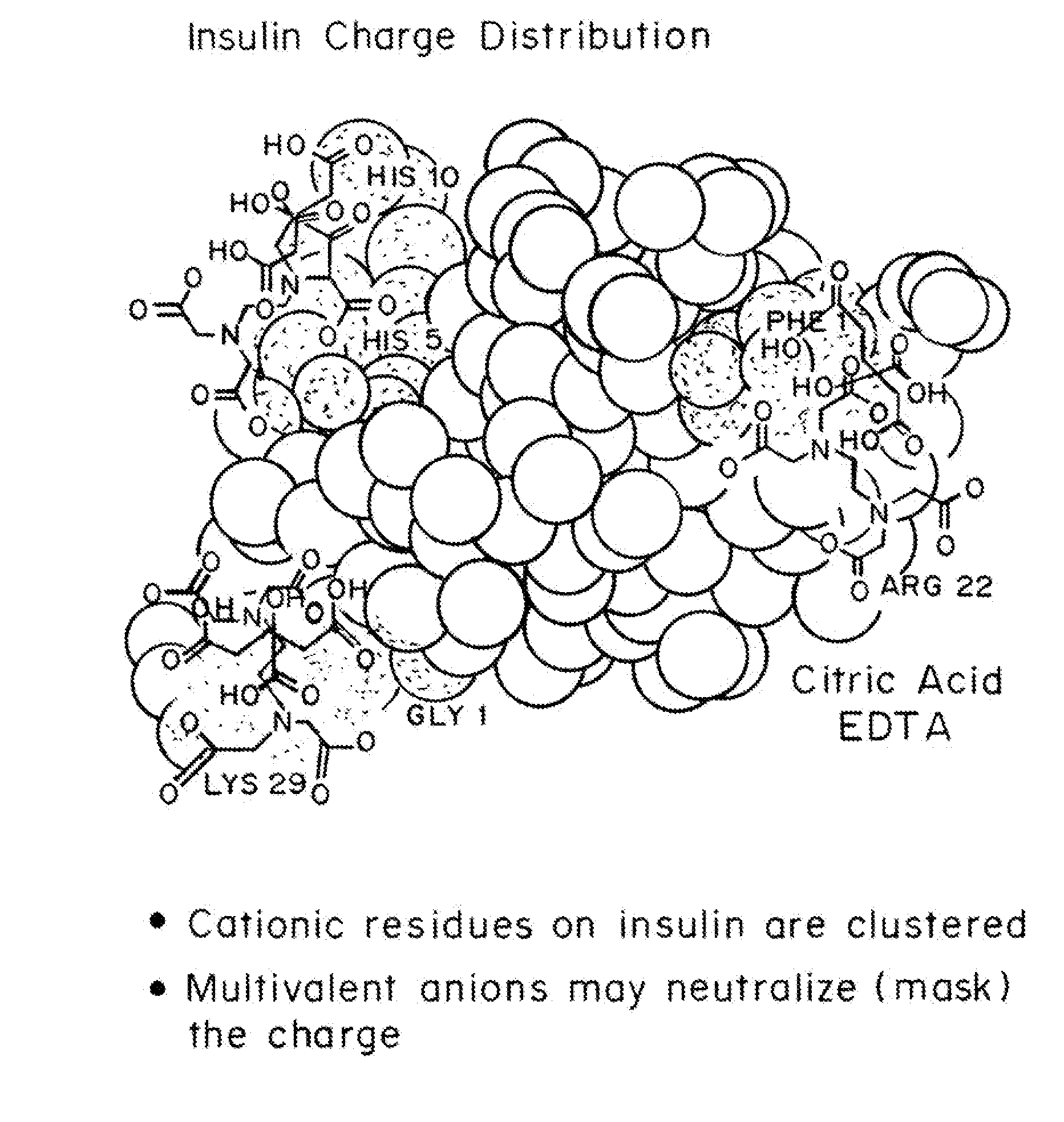
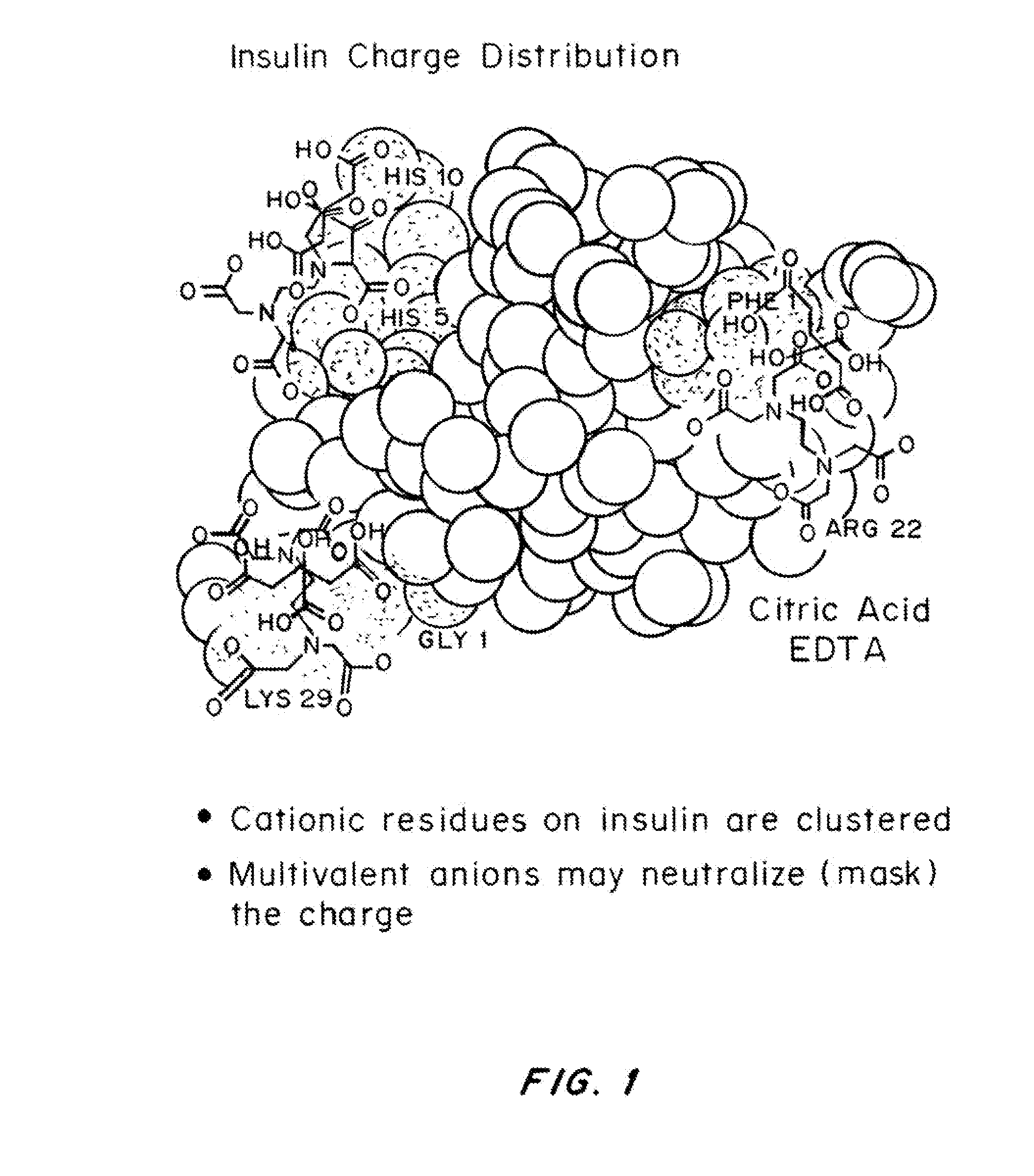
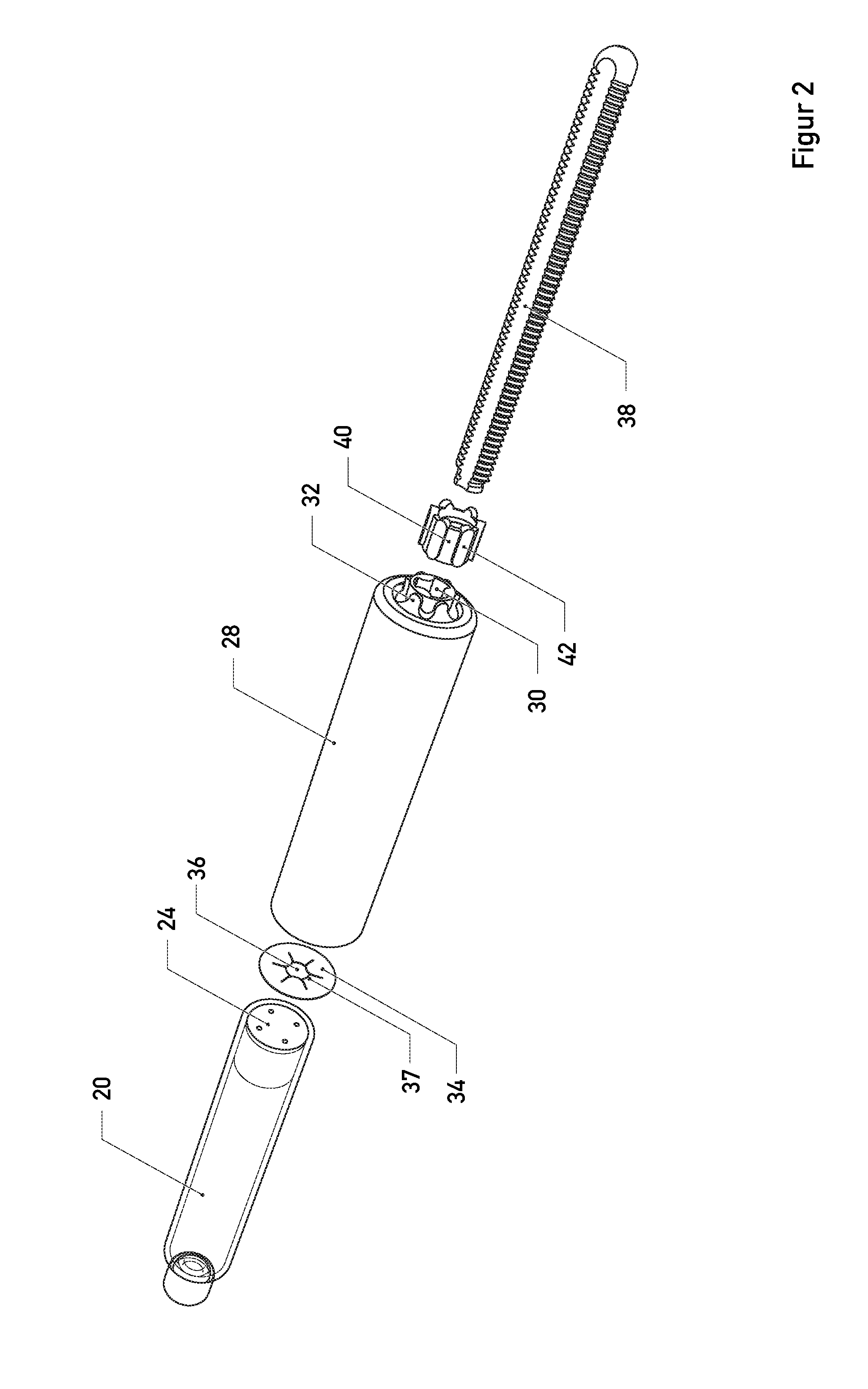
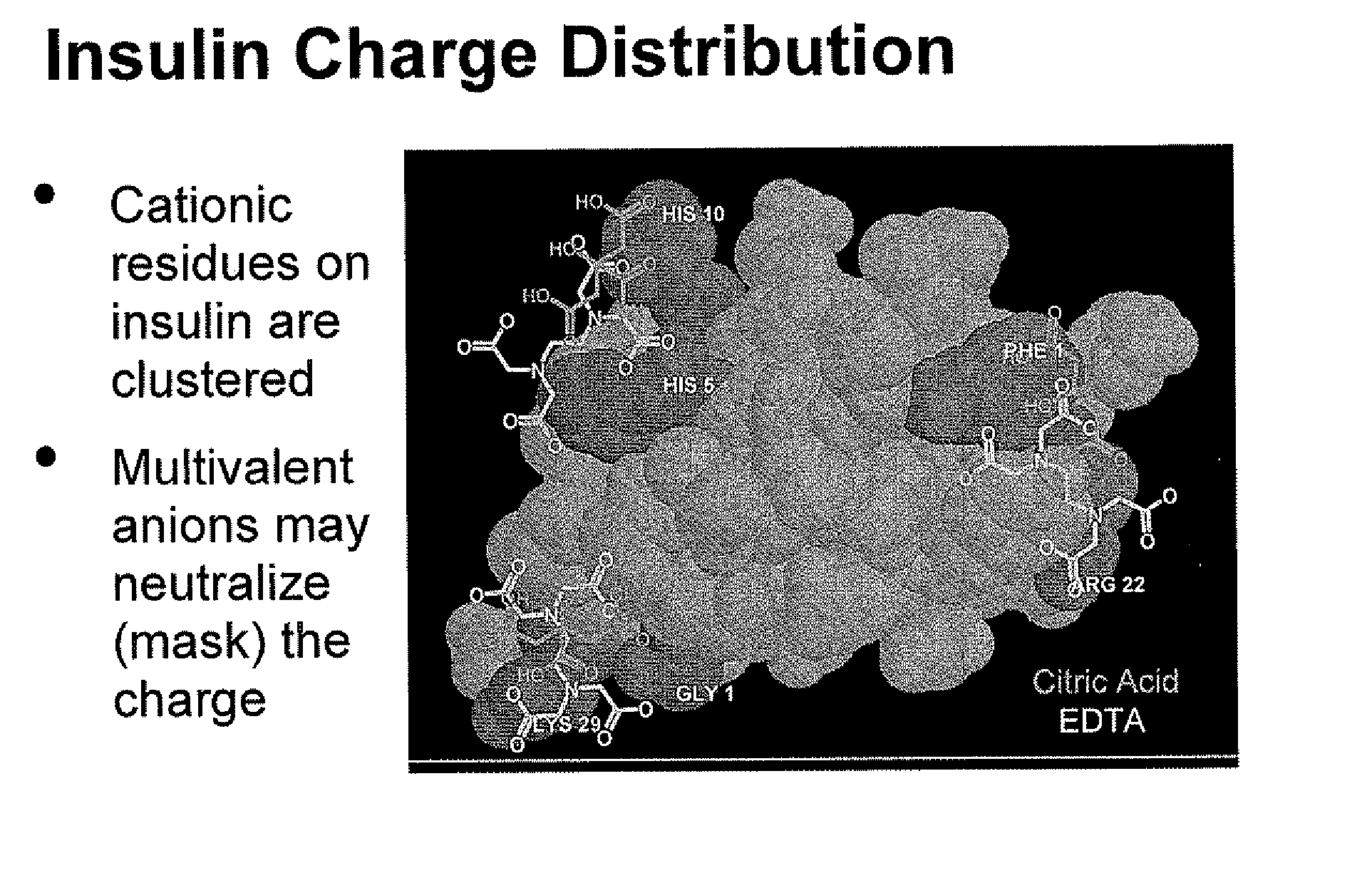
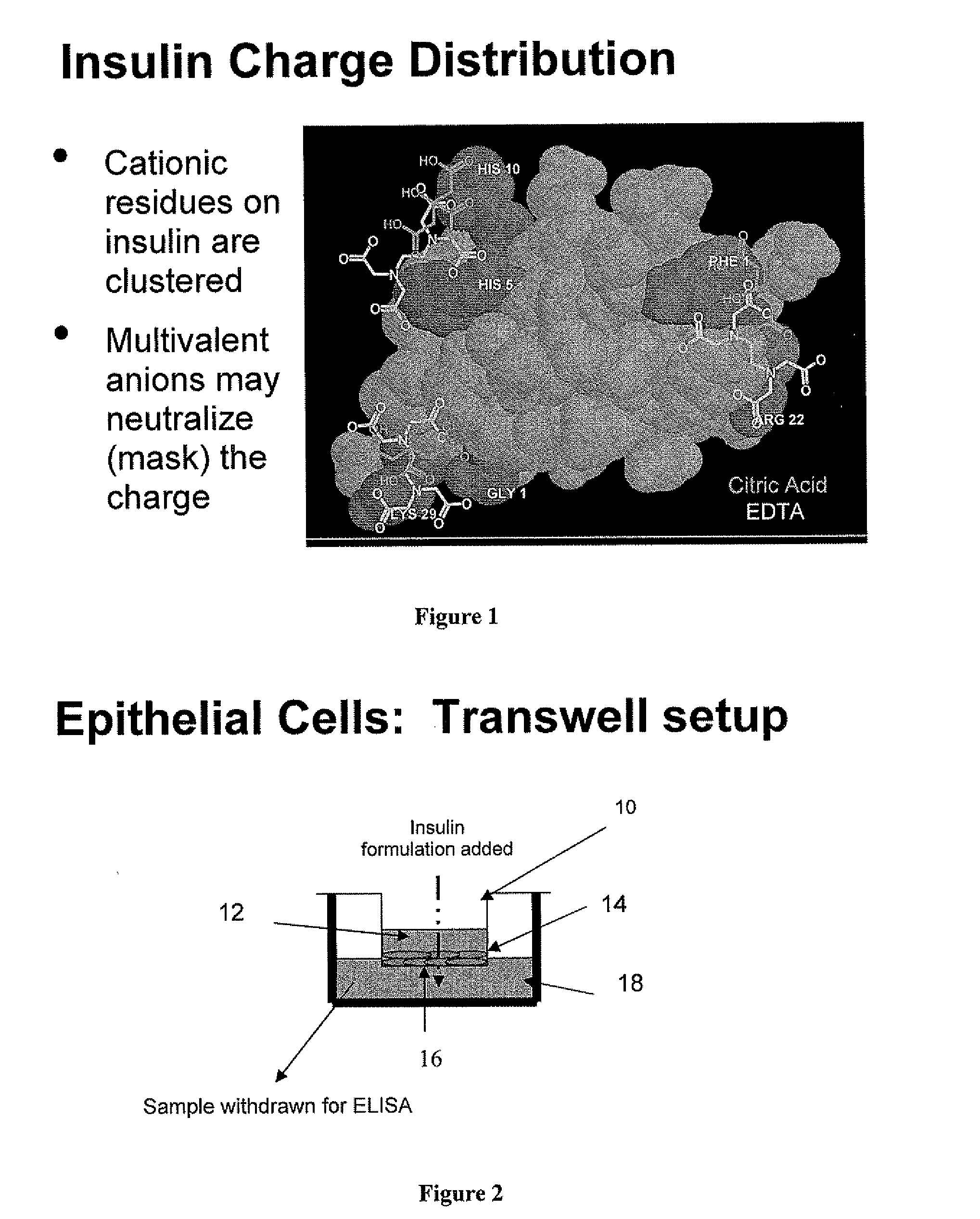
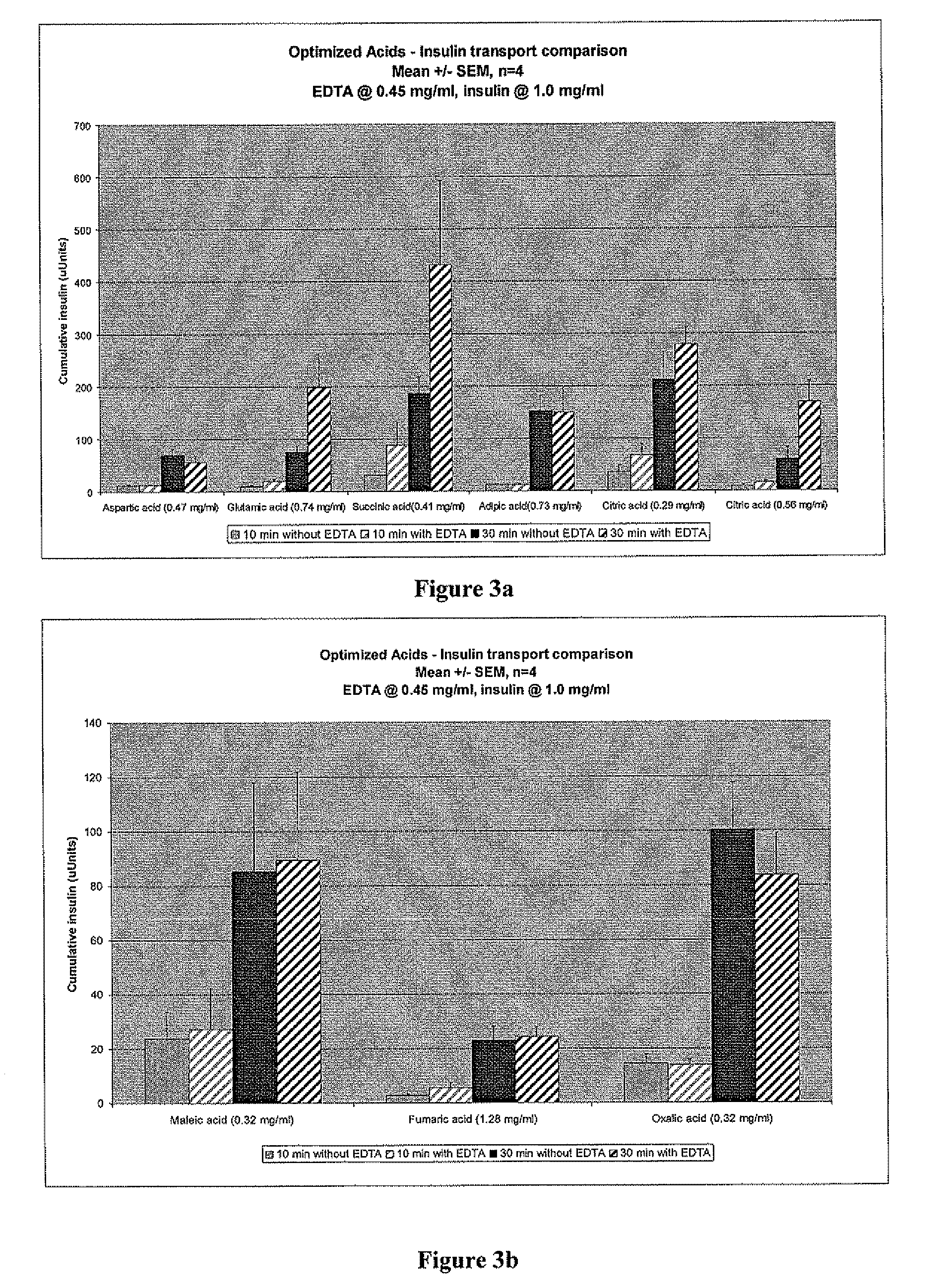
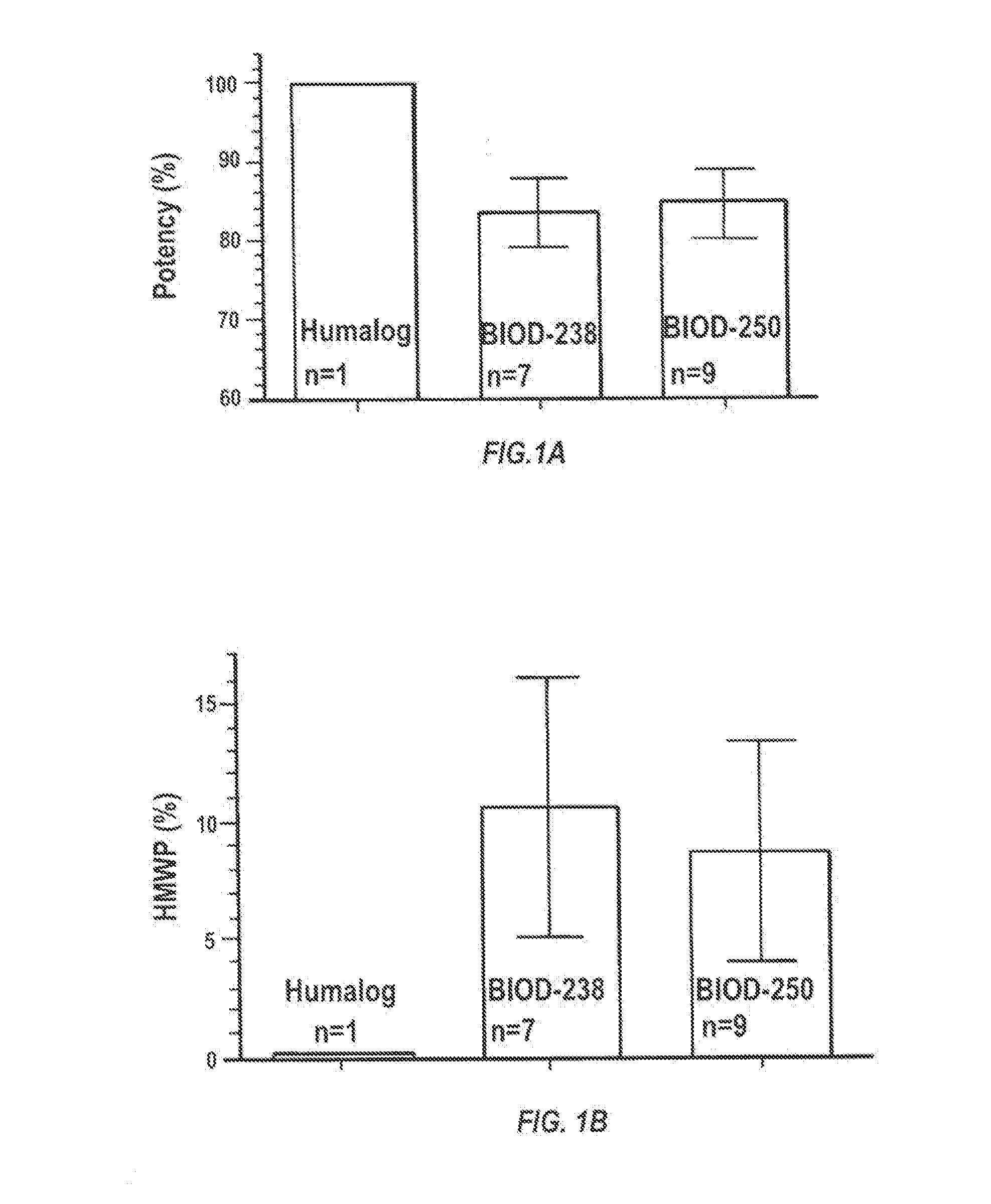
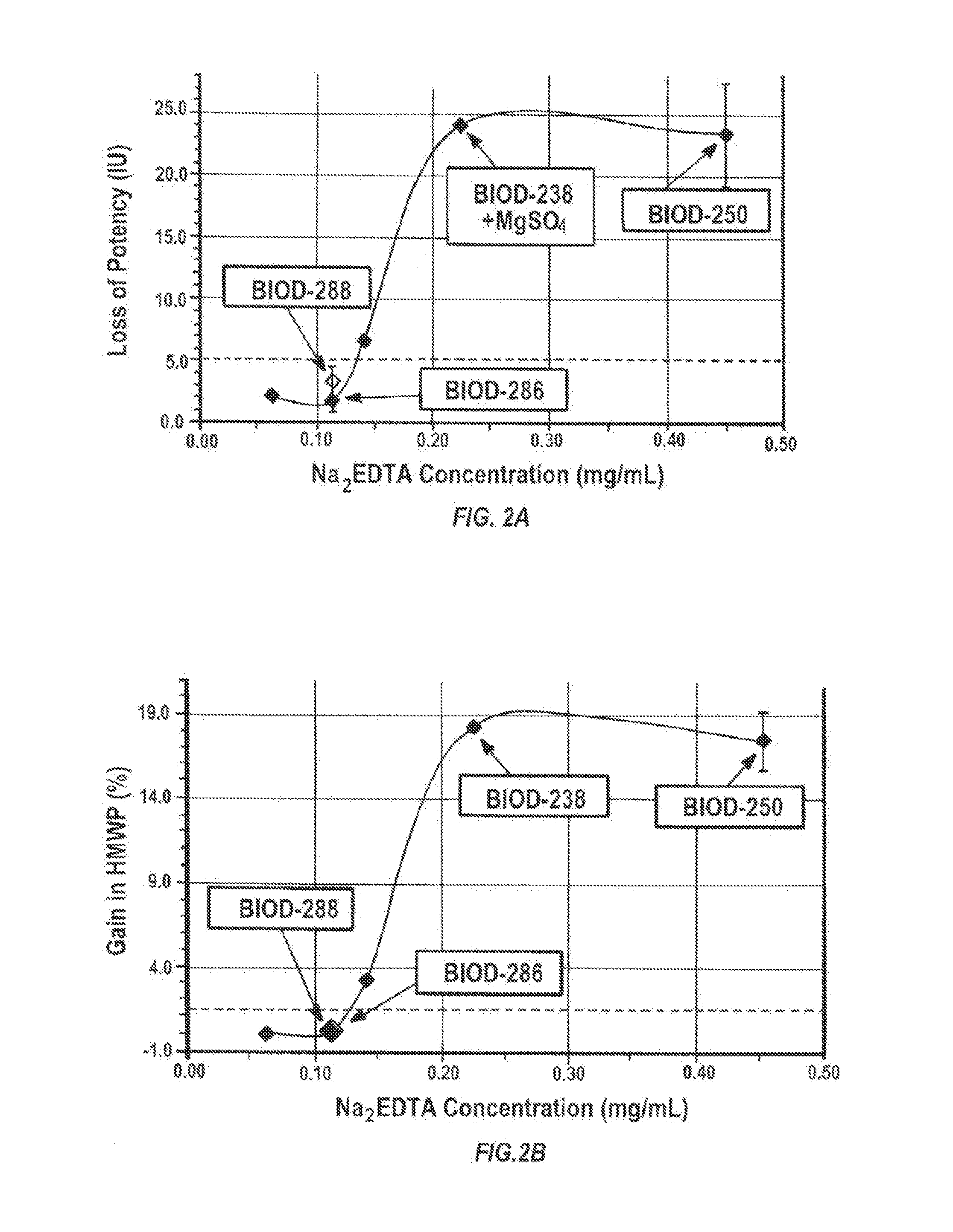
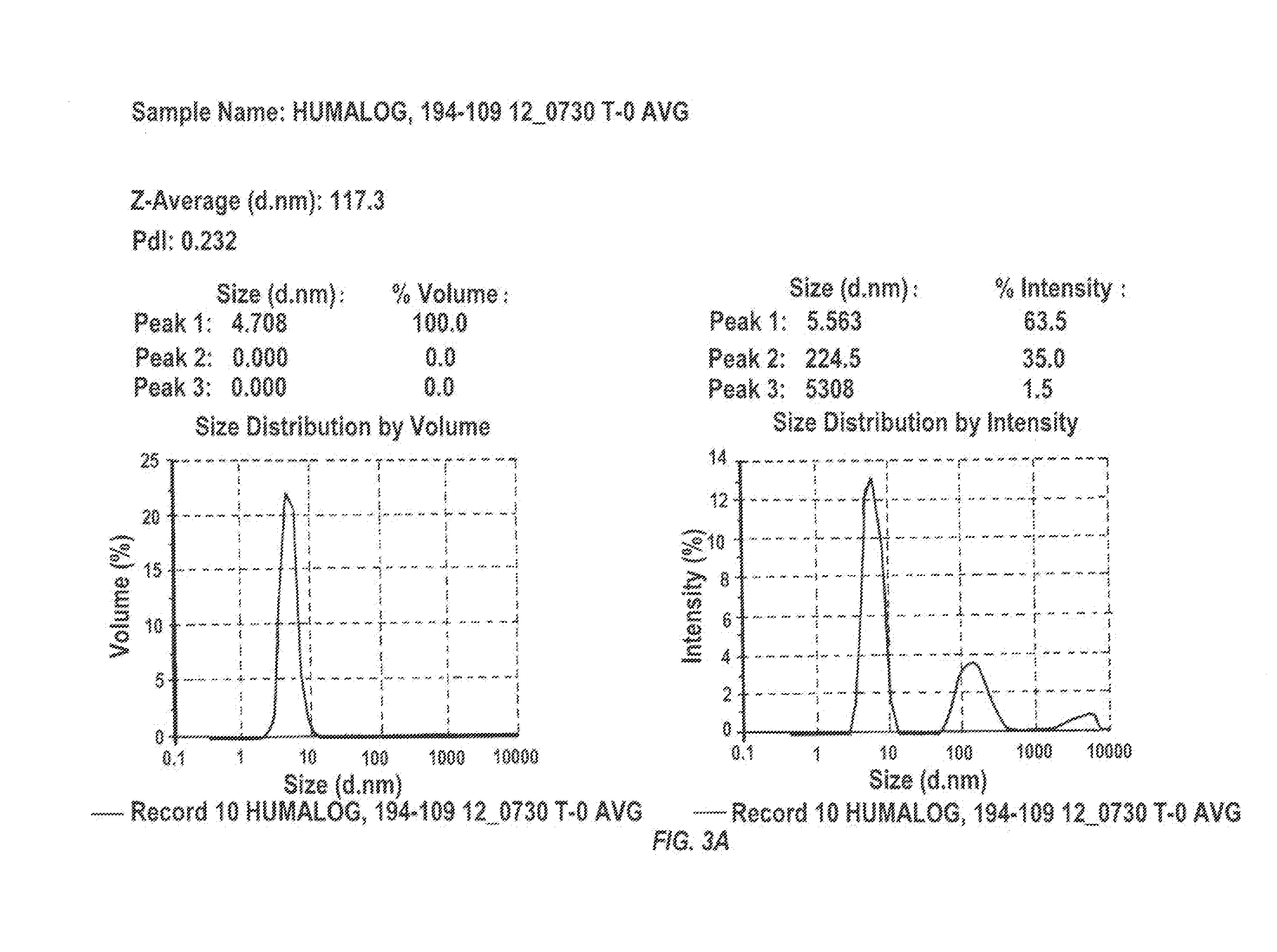
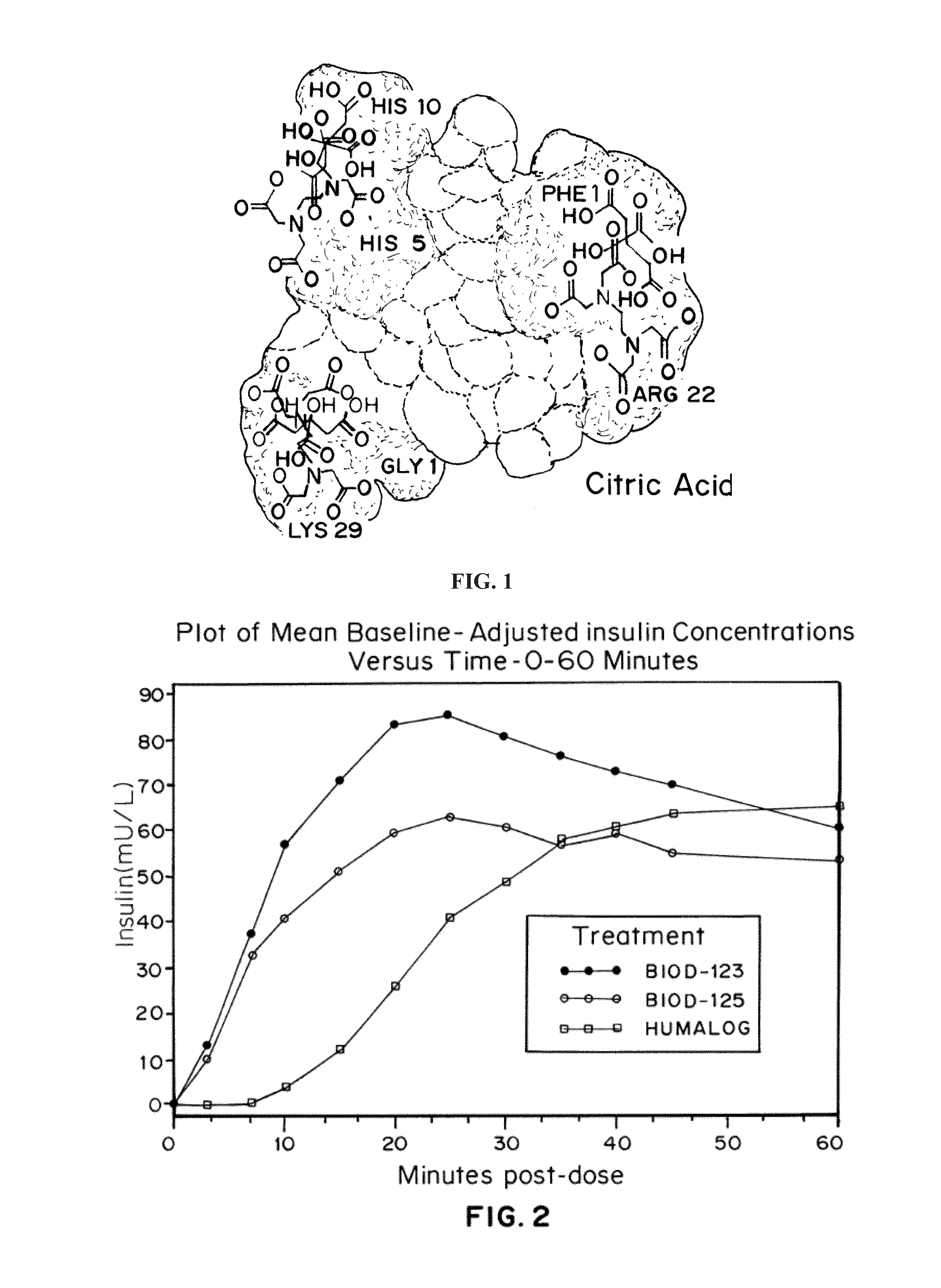
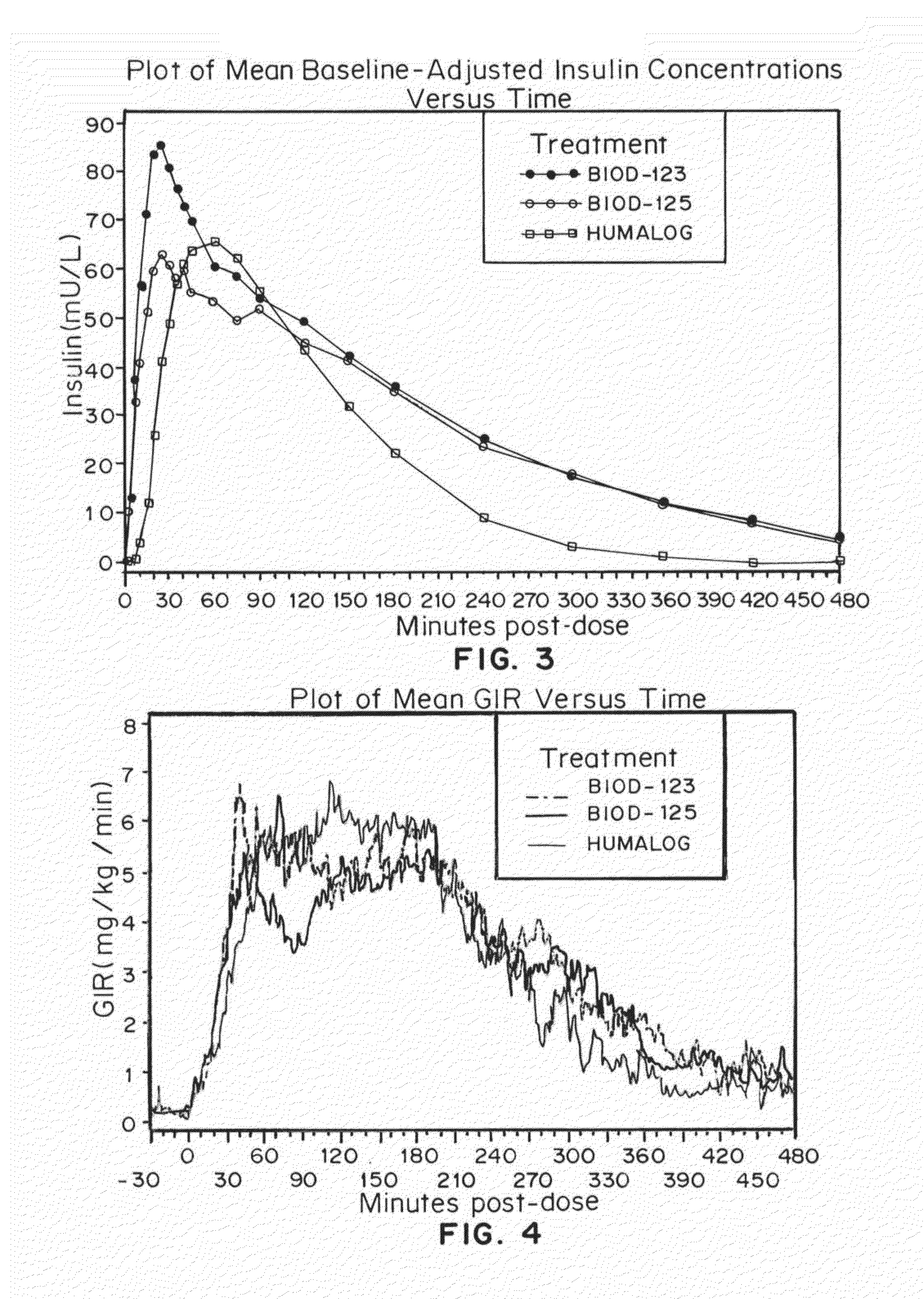
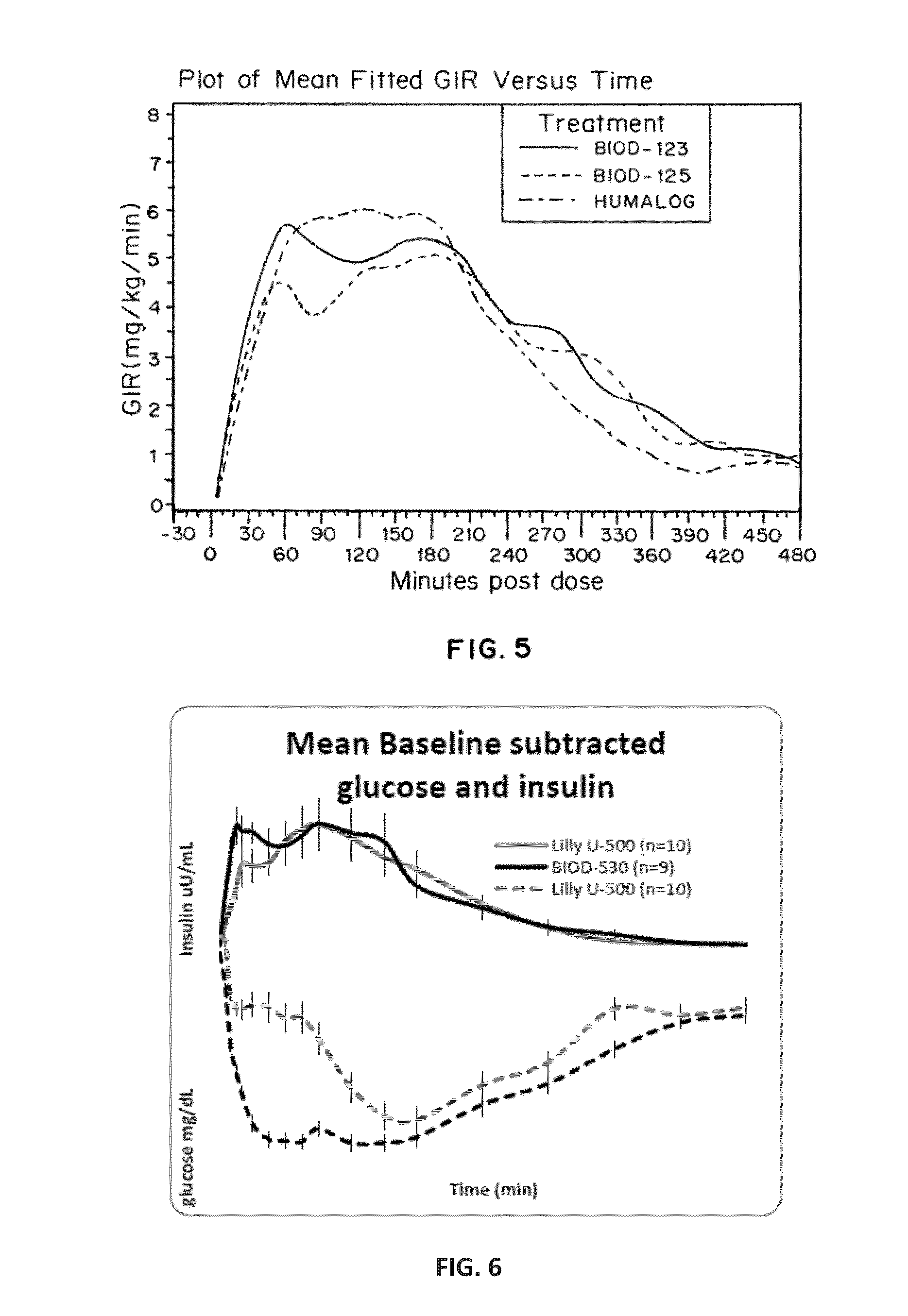
Magnesium Compositions for Modulating the Pharmacokinetics and Pharmacodynamics of Insulin and Insulin Analogs, and Injection Site Pain
ActiveUS20140113856A1Improved injection site tolerabilityPeptide/protein ingredientsMetabolism disorderEthylenediamineMagnesium salt
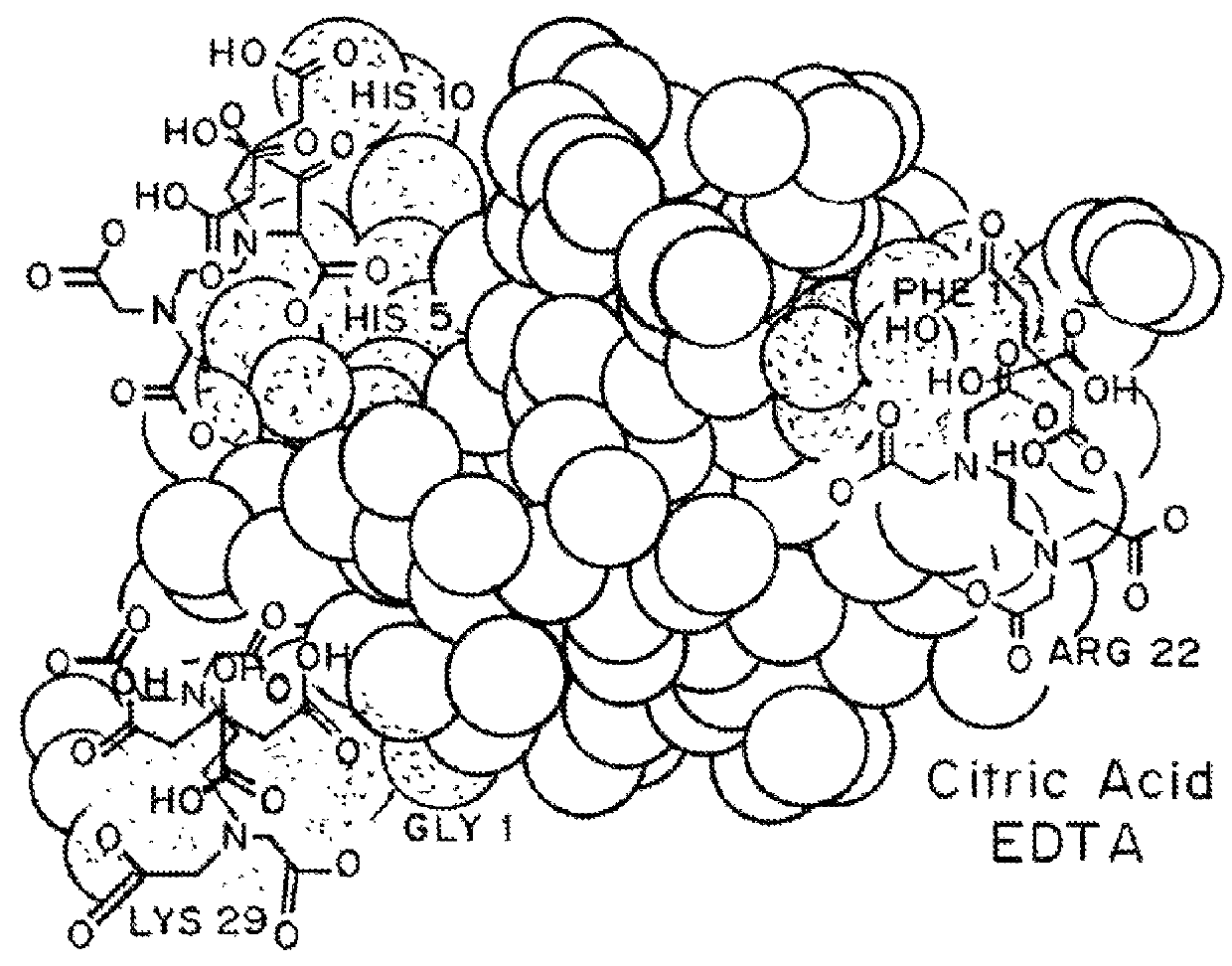
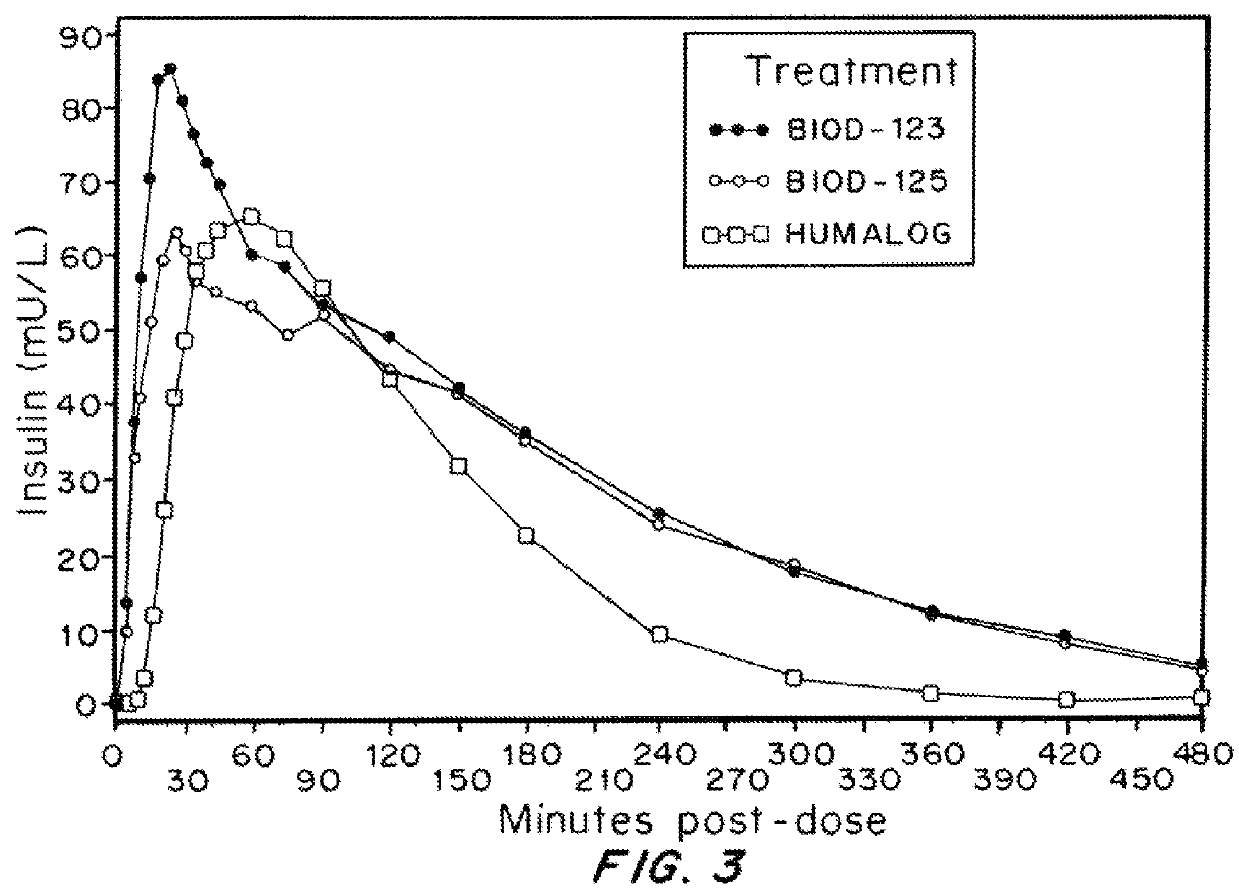
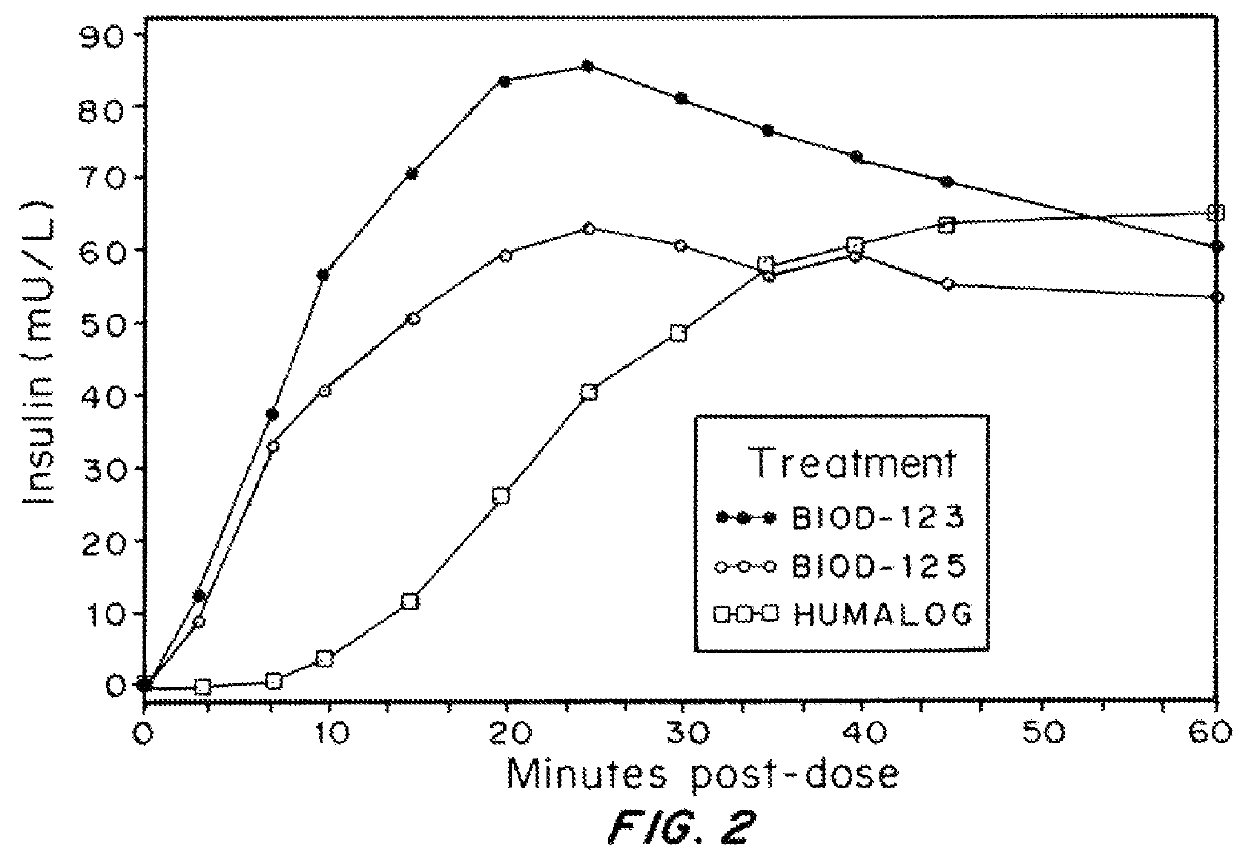
Compositions and methods for modulating injection site pain associated with rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations by mixing them with a vial containing dry powder excipients that accelerate their absorption. Devices for mixing excipient and insulin together at the time of administration, while minimizing residence time of the mixture, are also described.
Owner:ELI LILLY & CO
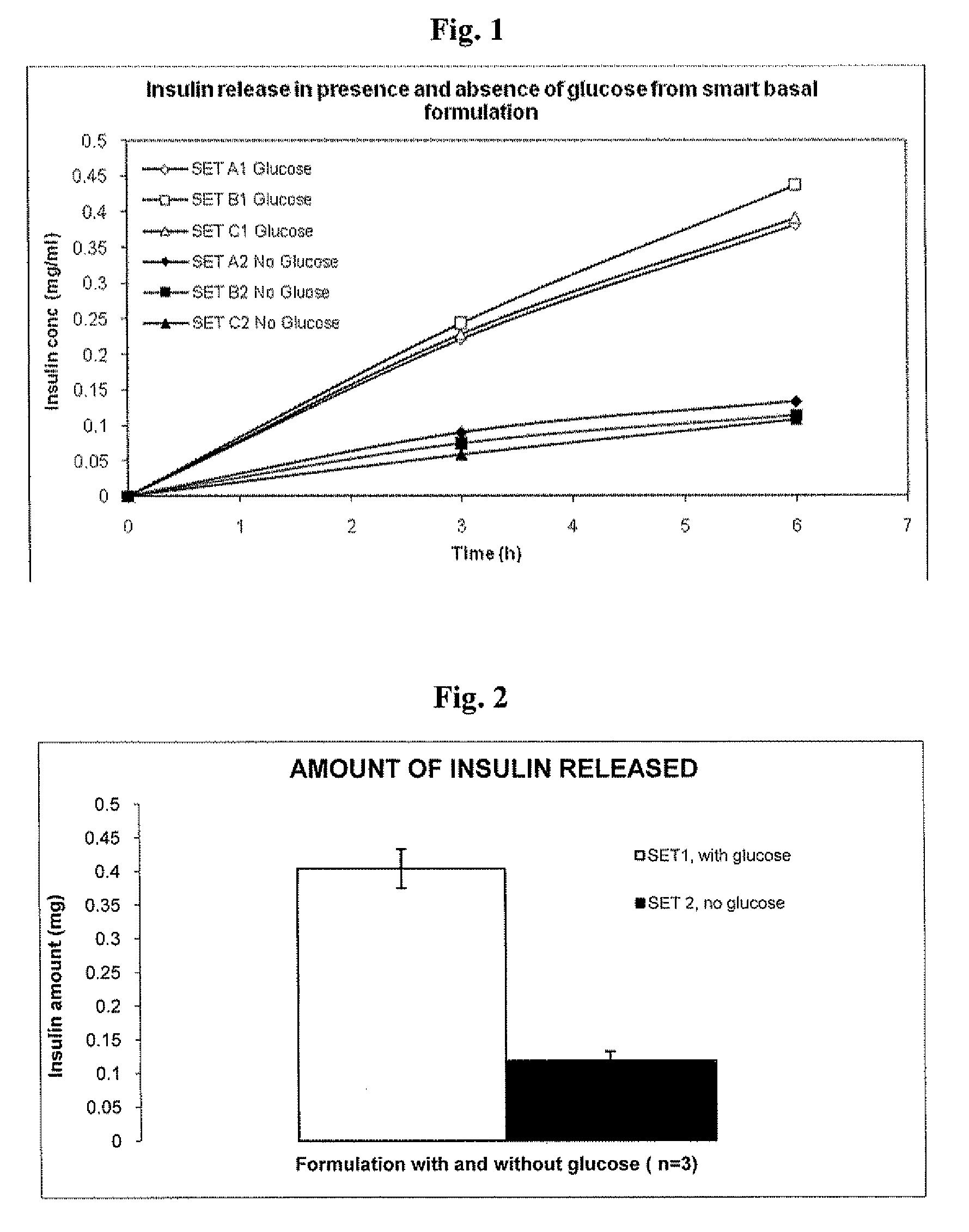
Insulin formulations for insulin release as a function of tissue glucose levels
InactiveUS20090175840A1Reduce productionRaise the pHPeptide/protein ingredientsMetabolism disorderInjections insulinReducing agent
Injectable insulin formulations that are capable of modifying the amount of insulin released based on the patient's tissue glucose levels, methods for making and using these formulations are described herein. The formulation may be administered via subcutaneous, intradermal or intramuscular administration. In one preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin, an oxidizing agent or enzyme and a reducing agent or enzyme, a diluent and optionally one or more thickening agents. If a thickening agent is present in the formulation, the thickening agent increases the viscosity of the formulation following administration. Preferably the formulation contains an insulin, a diluent, glucose oxidase and peroxidase. Following administration to a patient, the insulin is released from the formulations as a function of the patient's tissue glucose level, which in turn maintains the patient's blood glucose level within an optimum range. The formulation is often referred to as a “smart” formulation since it modifies its release rate of insulin according to the patient's needs at a particular time. In a preferred embodiment, the formulation is designed to release insulin into the systemic circulation over time with a basal release profile following injection in a patient. In another embodiment, the formulation is designed to release insulin into the systemic circulation over time with a non-basal release profile following injection in a patient, such as a regular human insulin release profile or a prandial release profile.
Owner:BIODEL
Assembly to administer insulin from a cartridge
ActiveUS20130079727A1Safe handlingEasy to handleAmpoule syringesDrug and medicationsEngineeringPharmaceutical drug
An assembly for the administration of a selected dosage of insulin or other medicaments from a cartridge by moving a plug guided in said cartridge, comprises an adapter assembly for adapting cartridges with different dimensions or contents. The adapter assembly comprises a first threaded element threaded with a thread lead and movable in a moving direction to move the plug, and a second threaded element threaded with a thread lead for setting a selected dosage, pivotably screwed to said first threaded element and limiting the movement of said first threaded element, and wherein said thread lead of said first element and said thread lead of said second threaded element are adapted to the dimensions and / or the contents of said cartridge.
Owner:EMPERRA GMBH E HEALTH TECH
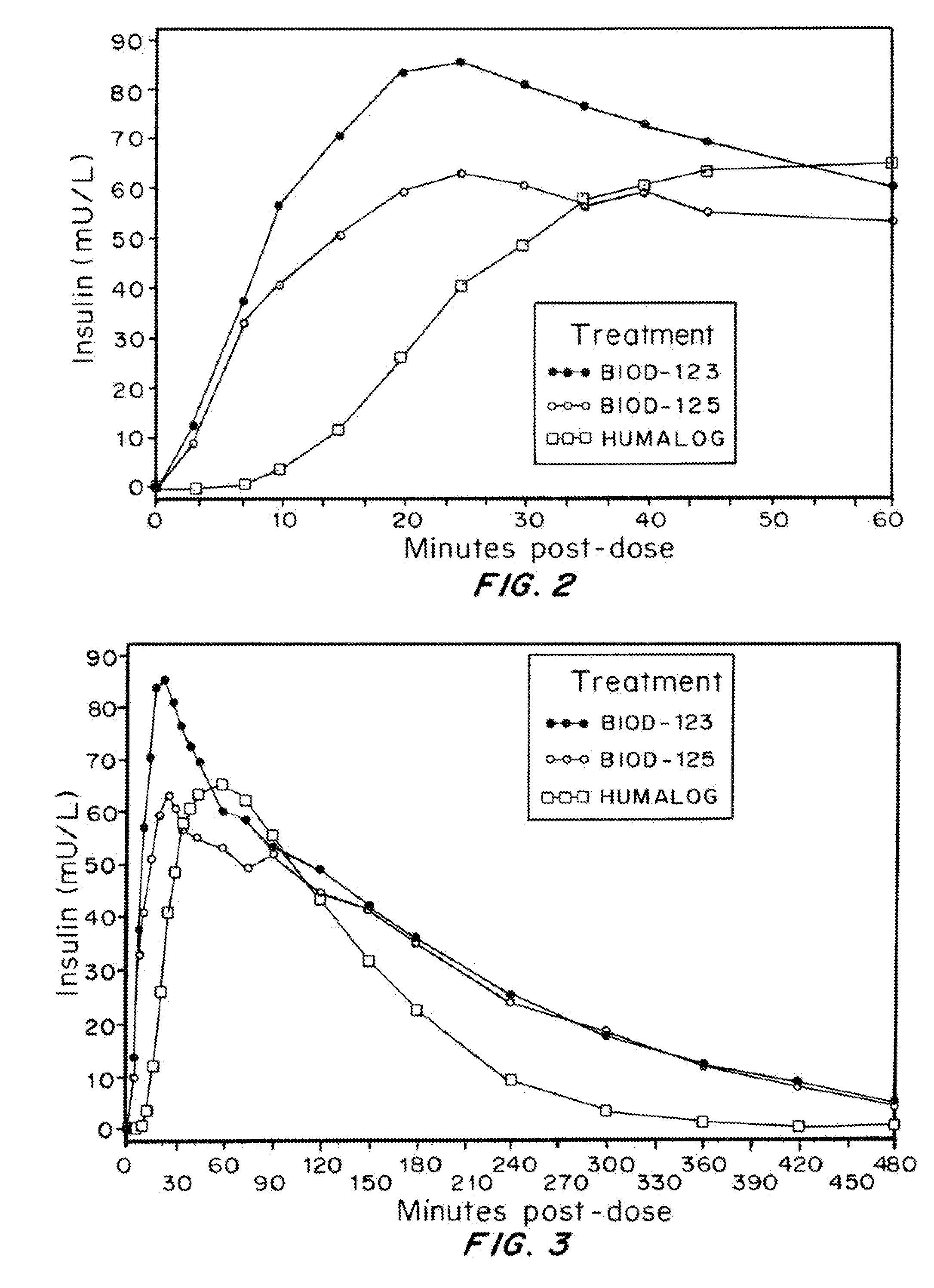
Rapid Acting Injectable Insulin Compositions
InactiveUS20080090753A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsDissolutionExcipient
Injectable insulin formulations with improved stability and rapid onset of action are described herein. The formulations may be for subcutaneous, intradermal or intramuscular administration, In the preferred embodiment, the formulations are administered via subcutaneous injection. The formulations contain insulin in combination with a chelator and dissolution agent, and optionally additional excipients. In the preferred embodiment, the formulation contains human insulin, a zinc chelator such as EDTA and a dissolution agent such as citric acid. These formulations are rapidly absorbed into the blood stream when administered by subcutaneous injection. In the preferred embodiment, the insulin is provided as a dry powder in a sterile vial. This is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water, a zinc chelator such as EDTA and a dissolution agent such as citric acid shortly before or at the time of administration. In another embodiment, the insulin is stored as a frozen mixture, ready for use upon thawing.
Owner:BIODEL
Mesenchymal stem cell injection, preparation method and application thereof in preparing medicine for treating diabetes
InactiveCN102920735AIncrease productionEasy quality controlCell dissociation methodsPeptide/protein ingredientsVitamin CSide effect
The invention discloses a mesenchymal stem cell injection, a preparation method and application of the mesenchymal stem cell injection in preparing a medicine for treating diabetes; the mesenchymal stem cells are derived from human umbilical cord and placenta; the mesenchymal stem cell injection consists of components: human mesenchymal stem cells, human albumin, low molecular heparin calcium, compound amino acid, vitamin C of 0.5% and dissolving medium; and the dissolving medium can be a compound electrolyte solution or glucose or normal saline. According to the mesenchymal stem cell injection, the preparation method and the application of the mesenchymal stem cell injection in preparing the medicine for treating the diabetes, the mesenchymal stem cell injection is used for repairing injured islet beta cells, and the blood sugar is reduced by secretion of the endogenous insulin, therefore, a purpose of foundational treating the diabetes is achieved. A disease course of the diabetes can be reversed, patients are helped in escaping out of inconvenience and toxic and side effects of taking the endogenous drug and injecting the insulin as well as serious complications caused by poor control of the blood sugar; and the 1-type and 2-type diabetes are treated thoroughly.
Owner:青岛奥克生物开发有限公司
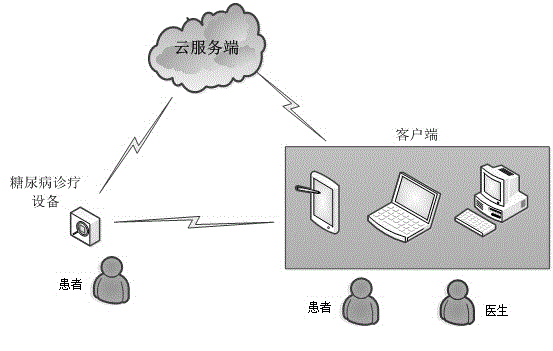
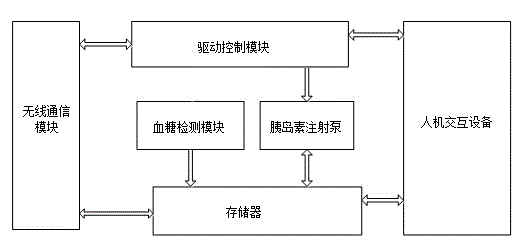
Insulin injection service system based on cloud technology
The invention provides an insulin injection service system which is more convenient to use, more reliable and capable of conducting illness state tracking and treatment management according to the actual conditions of patients and the expert knowledge of doctors. The insulin injection service system comprises diabetes diagnosing and treating equipment, a cloud server side and a client side, wherein the cloud server side is connected with the diabetes diagnosing and treating equipment and the client side through a communication network, and the diabetes diagnosing and treating equipment is in data connection with the client side. By means of the insulin injection service system, the expert knowledge of the doctors and the detail data of all the patients can be combined, and individualized treating schemes can be made. In addition, in the treating process of the patients, data can be recorded and tracked, the subsequent treating schemes can be conveniently adjusted in time, and more scientific treating plans can be made for the patients. The insulin injection service system can automatically drive an injection device to conduct injection or automatically prompt the patients to be injected, the work of the doctors is greatly reduced, and it can be ensured that the patients are injected with insulin on time every day.
Owner:广州昀锐信息科技有限公司
Long acting injectable insulin composition and methods of making and using thereof
A method of lowering blood glucose in a mammal includes injecting a therapeutically effective amount of crystallized dextran microparticles and insulin to the mammal to lower blood glucose of the mammal. The composition may be a one phase or a structured multi-phase composition for controlled release of insulin over an extended period of time.
Owner:TDC BIO S A R L
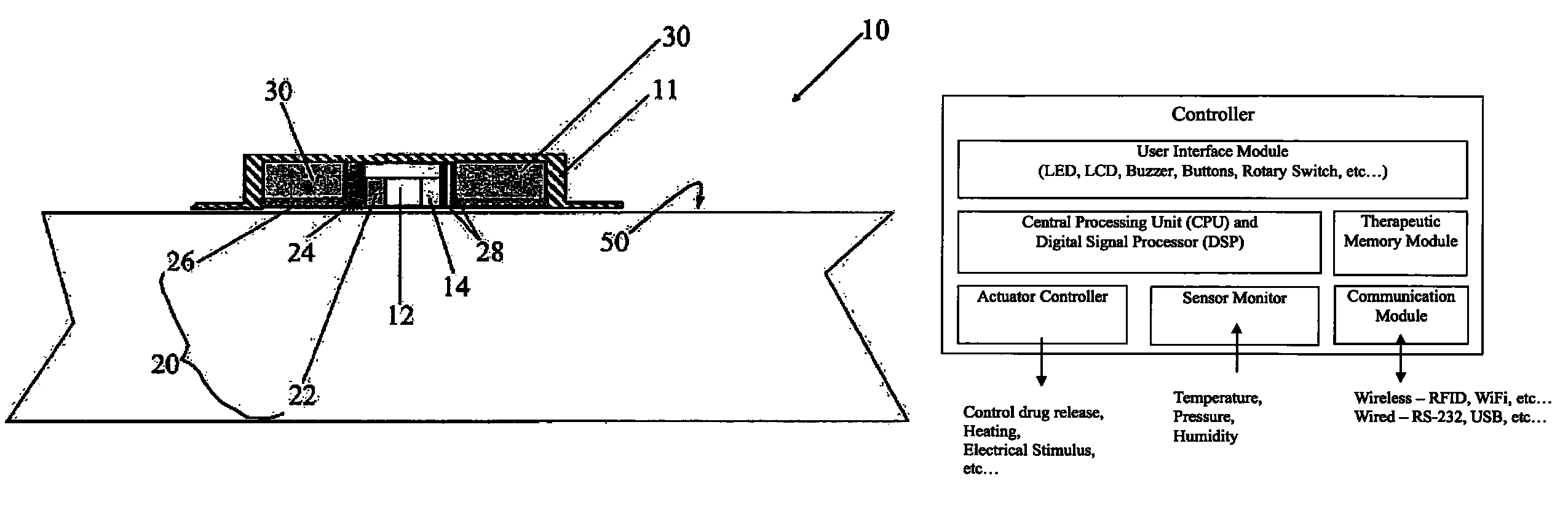
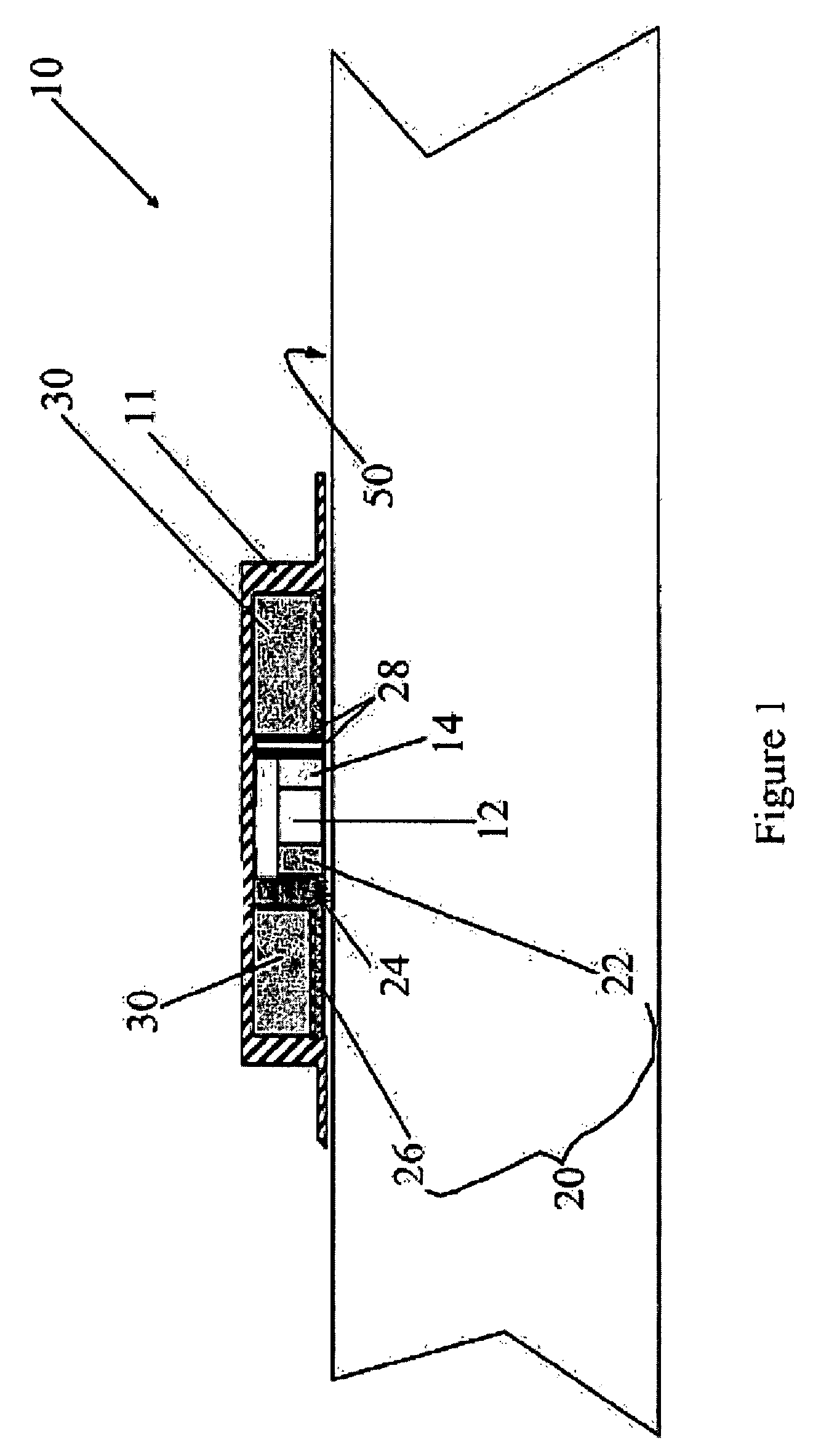
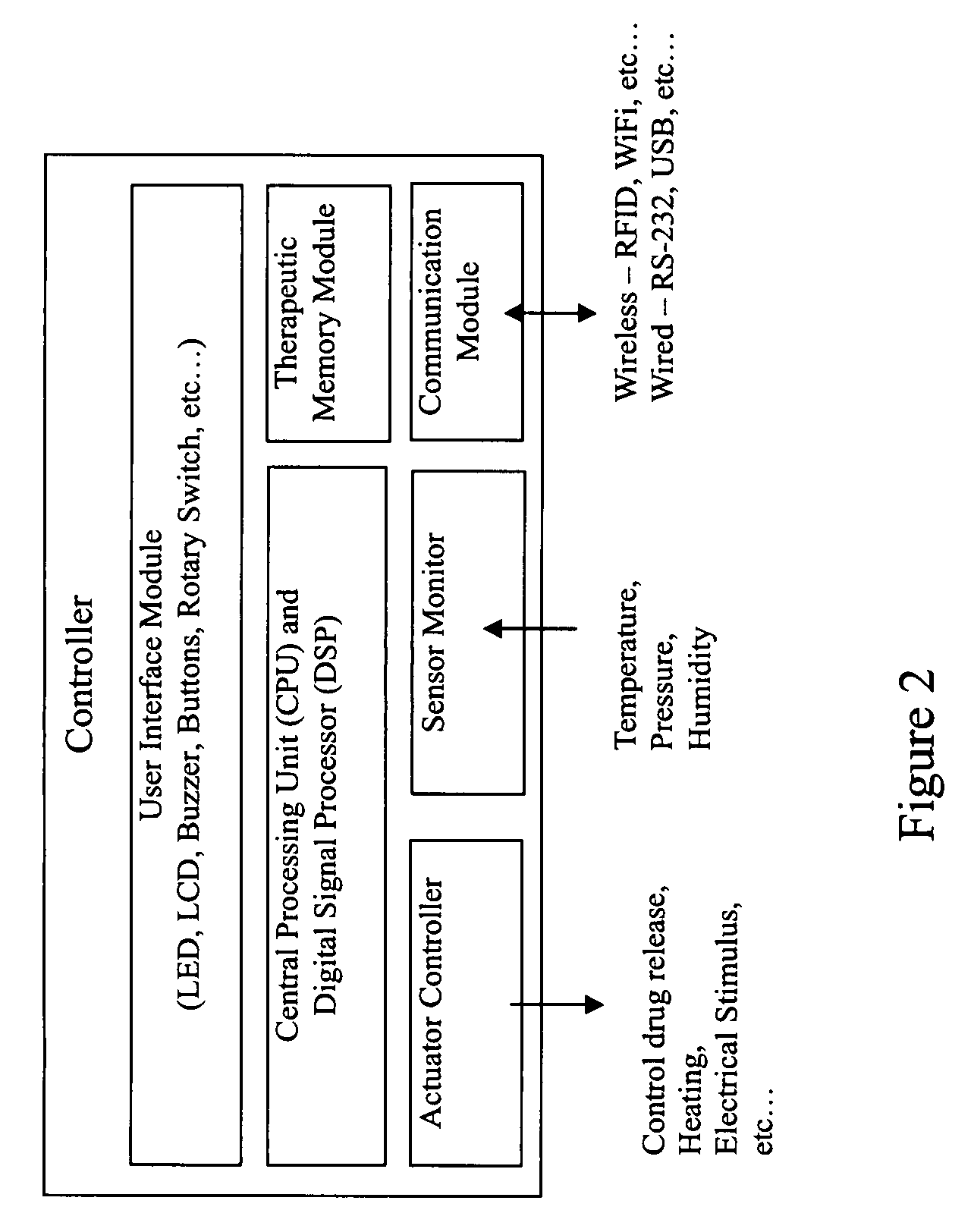
Medical device for delivering drug and/or performing physical therapy
Current methods of delivering drug usually require a certain degree of human intervention. For example, a doctor, a paramedic or a patient is required to inject insulin manually when the blood sugar level becomes low. This invention provides a medical device for use with a patient for performing at least one of the functions of delivering drug and performing physical therapy to the patient. The device of this invention includes a sensor for measuring at least one parameter from the patient, an analyzer for analyzing the parameter to determine appropriate function to be carried out on the patient by said medical device, and at least one actuator to perform at least one of the functions of delivering drug and performing physical therapy.
Owner:THE HONG KONG POLYTECHNIC UNIV
Stabilized ultra-rapid-acting insulin formulations
InactiveUS20150273022A1Quick effectPromote absorptionBiocidePeptide/protein ingredientsZinc compoundsMagnesium salt
Compositions and methods for enhancing the stability of rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, a zinc compound and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations while maintaining insulin stability.
Owner:ALBIREO PHARMA INC
Method for administering insulin to the buccal region
A mixed micellar pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an alkali metal C8 to C22 alkyl sulphate, alkali metal salicylate, a pharmaceutically acceptable edetate and at least one absorption enhancing compounds. The absorption enhancing compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, octylphenoxypolyethoxyethanol, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linolenic acid, borage oil, evening of primrose oil, trihydroxy oxo cholanyiglycine, glycerin, polyglycerin, lysine, polylysine, triolein and mixtures thereof. The amount of each absorption enhancing compound is present in a concentration of from 1 to 10 wt: / wt. % of the total formulation, and the total concentration of absorption enhancing compounds are less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA INC
Novel insulin pump
InactiveCN102836481AAccurately calculate the injection doseStable blood sugar controlFlow monitorsHuman bodyMicrocontroller
The invention discloses a novel insulin pump, which comprises a blood sugar detection system, a control system and an insulin injection system, wherein the blood sugar detection system comprises a blood sugar sensor and a wireless transmitting module which is connected with the blood sugar sensor; the blood sugar detection system can be used for continuously detecting the blood sugar content of a diabetic patient, and can be used for transmitting the blood sugar content to the control system in a wireless way; the control system comprises a wireless receiving module for receiving blood sugar data transmitted by using the blood sugar detection system, and a microcontroller system; the wireless receiving module is used for transmitting the received blood sugar data to the microcontroller module; and the dosage of insulin required be injected is obtained by calculating with a micro-controller and correcting with a model. In the novel insulin pump, a closed-loop insulin injection way is adopted innovatively, the working principle of secretion of insulin from the pancreas of a normal human body is simulated, and the blood sugar data of the patient is measured by using the blood sugar detection system. According to the novel insulin pump, the injection dosage of insulin can be adjusted in real time according to the blood sugar content of the patient, so that the aim of accurately injecting insulin is fulfilled.
Owner:HARBIN INST OF TECH
Negative pressure adsorption type hypodermic injection unit
InactiveCN101869734AQuick pullAutomatically pull outIntravenous devicesAir compressionHypodermoclysis
The invention discloses a negative pressure adsorption type hypodermic injection unit, which relates to a unit for carrying out hypodermic injection of insulin and other drugs, and consists of an adsorption device, an air compression device, a quick air releasing device and an injection syringe, wherein an absorber 1 is connected with an air compressor 3 through an air tube 2 so that the air in the absorber 1 can be quickly pumped out through the air compressor 3, negative pressure is formed in the absorber 1 so that the skin shrinks and has a bulge, and the bulge height of the skin can be adjusted through the air compressor 3. The injection syringe 4 is connected with the absorber 1 through syringe steps in a sealing way, when the skin shrinks and has the bulge, a syringe needle is automatically inserted into the skin, and then drug injection can be carried out. The quick air releasing device is formed by a rubber plug 5, when the rubber plug 5 is pulled out, the internal air pressure of the absorber 1 returns to the atmospheric pressure, the skin diastoles and returns to be smooth, and the syringe needle is automatically pulled out from the skin. The invention has the hypodermic injection characteristics of easy skin contraction, quick needle insertion, convenient injection and use, wide application scope and the like, and solves the problem that the old and the slimmer people are difficult to be injected with insulin and other subcutaneous injection drugs.
Owner:HARBIN INST OF TECH
Insulin pump
InactiveCN101138659AStabilize blood sugarReasonable designAutomatic syringesPressure infusionHuman bodyPregnancy
The insulin pump of the present invention relates to a portable electronic controlling insulin injection device, which can inject the insulin to the human body under a certain time and certain quantity. The present invention is helpful for keeping stable blood sugar for the diabetes patients. The present invention can be widely used in the 1 type diabetic patients, 2 type diabetic patients and the diabetic patients with pregnancy. The structure comprises the insulin pump body, the transparent medicine store cabin, the medicine store device, the medicine injection piston, the medicine injection pushing rod, the battery cabin, the battery, the digital display, the motor, the reducer, the motor seat and a joint of the medicine transportation. A medicine store cabin is arranged in one side of the insulin pump body. A transparent observation window is arranged outside the medicine store cabin. The medicine store tool is inserted in the medicine store cabin. A motor cabin is arranged in the back part of the medicine store cabin. The motor is arranged in the motor cabin. The reducer is connected with the motor directly. The output shaft of the reducer is provided with the thread. The hollow medicine injecting rod is provided with an inner thread. The one end of the injection rod is provided with the outer thread. The medicine injection rod sleeve is arranged outside the motor and the reducer.
Owner:无锡顶点医疗器械有限公司
Intelligent micropump for switching between insulin infusion and glucagon infusion
The invention discloses an intelligent micropump for switching between insulin infusion and glucagon infusion, belonging to the technical field of electrical equipment. The intelligent micropump can effectively stabilize the blood sugar of a patient in a safe range, and can be widely applied to type 1, type 2, special type and gestational diabetic patients. In the application, an intelligent method for simultaneously infusing insulin and glucagon based on a switching control theory is designed. The intelligent micropump for switching between the insulin infusion and the glucagon infusion is composed of a public motor, a needle and two independent sets of medicine storage and infusion systems; in a normal mode, the insulin is infused to the patient, and in a special mode, the glucagon is infused to the patient by switching the infusion system. The original function of capability of infusing one treatment medicine only is expanded that two different treatment medicaments can be respectively infused. Therefore, compared with the prior art, the multi-functional micropump has the characteristics of high integration, high safety and the like, and can distinctly improve the control effect of blood sugar.
Owner:BEIJING UNIV OF CHEM TECH
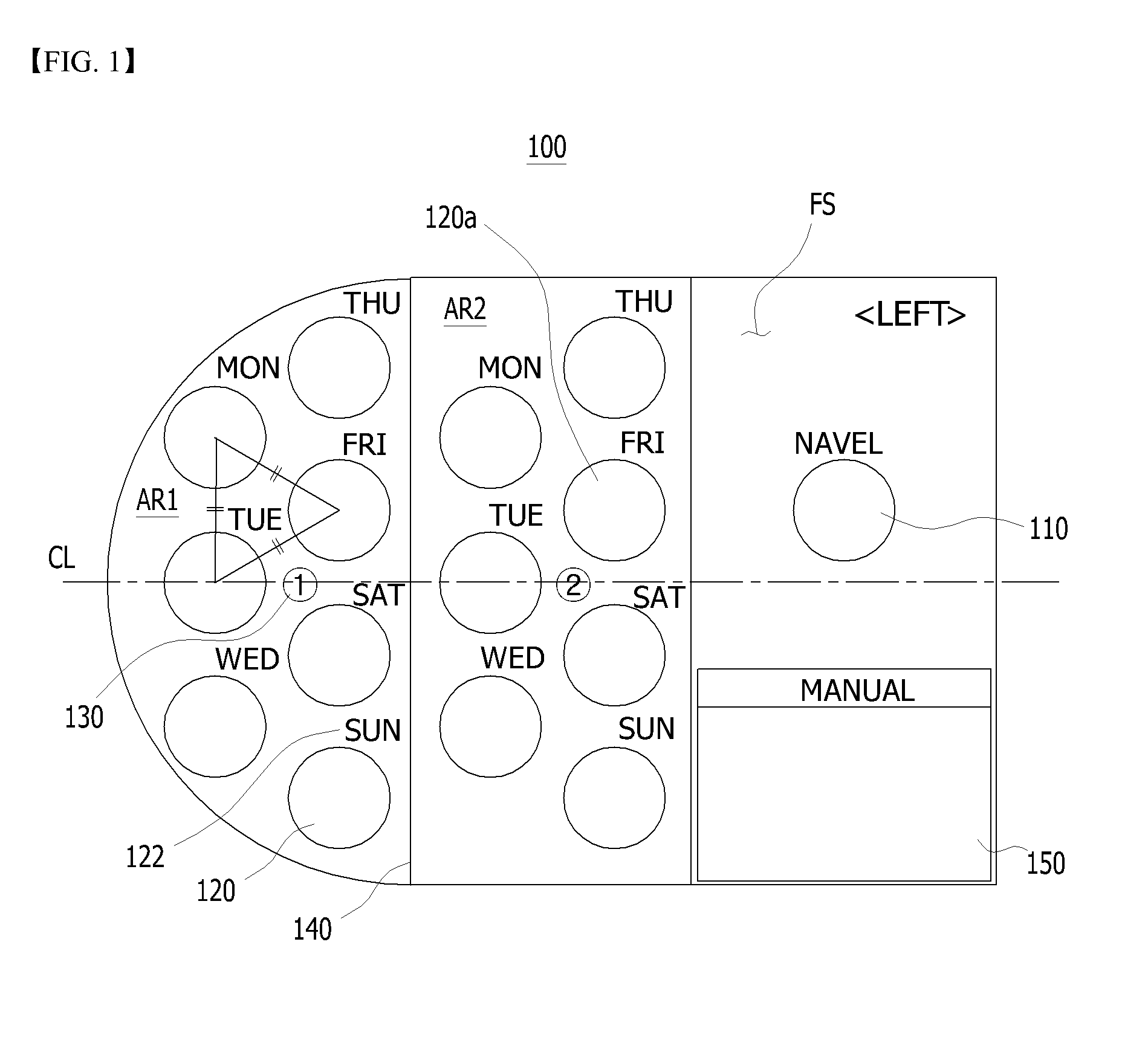
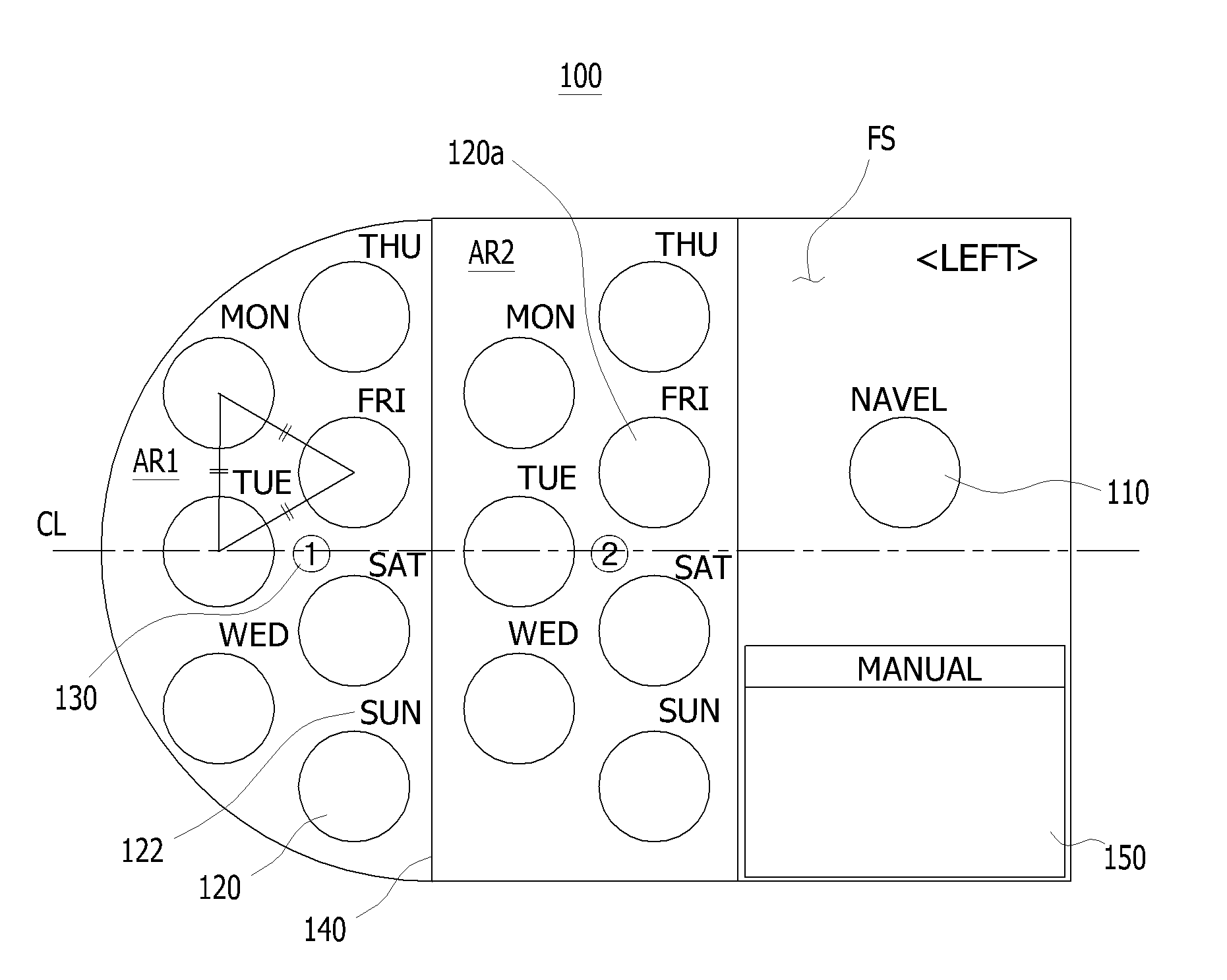
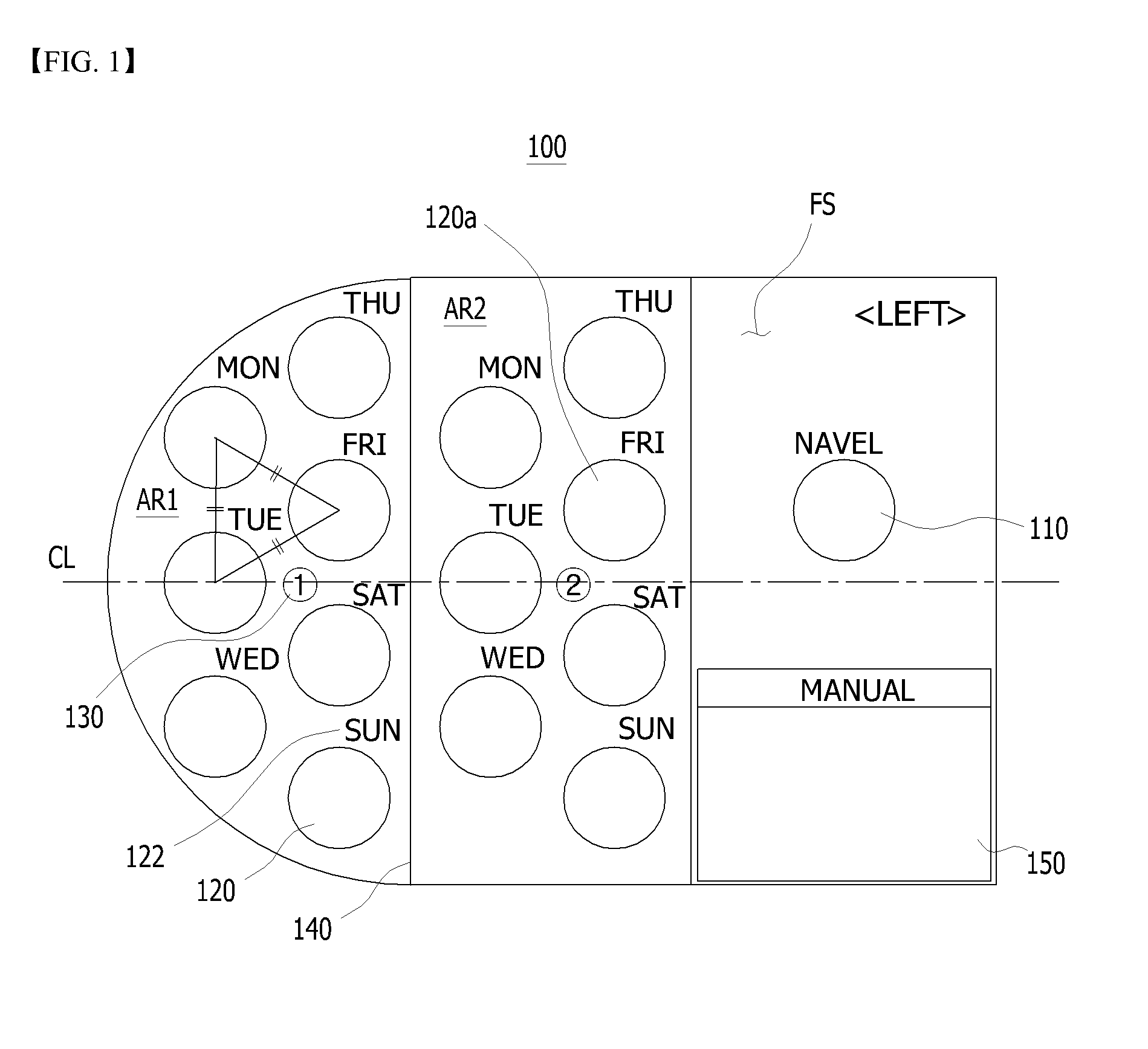
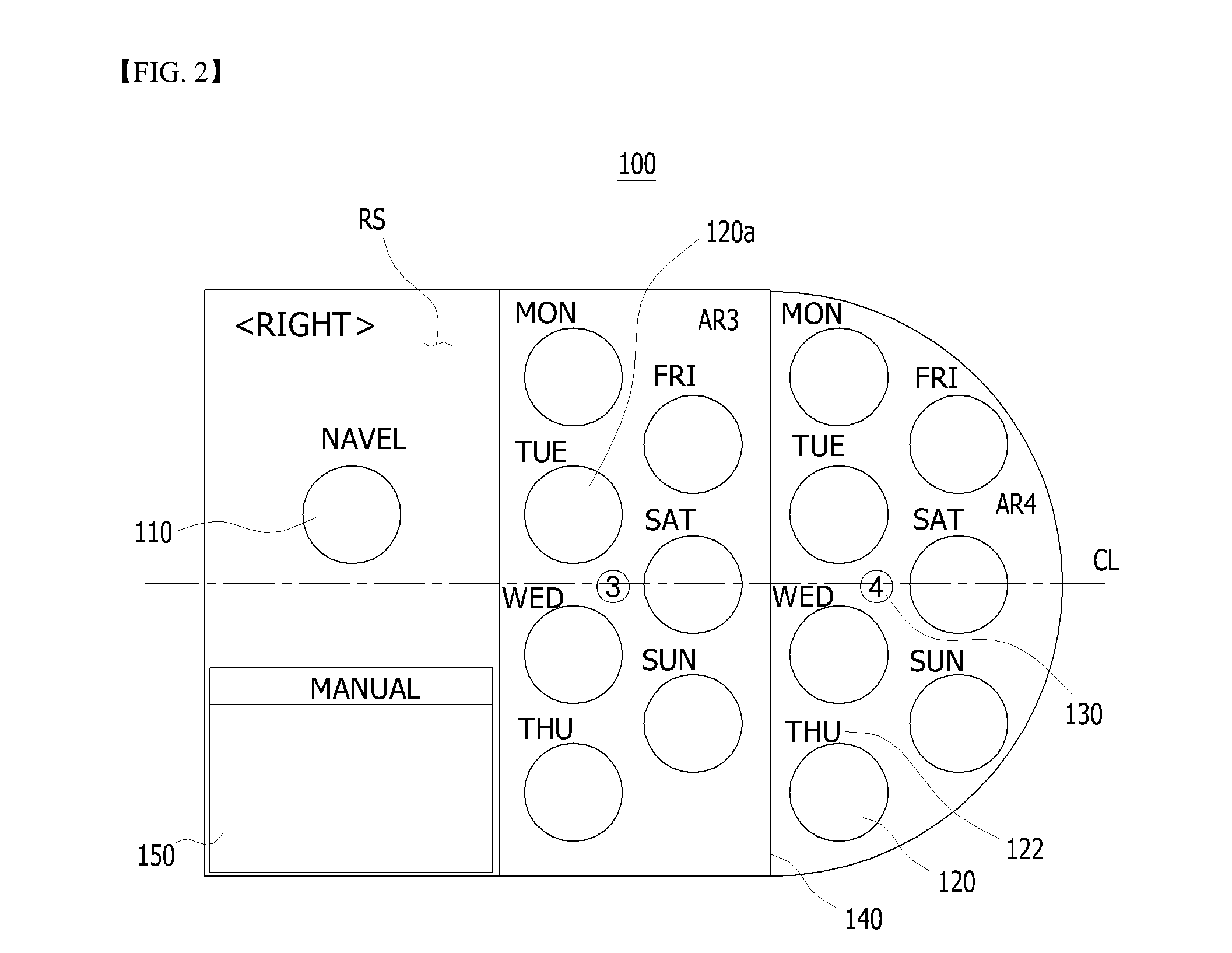
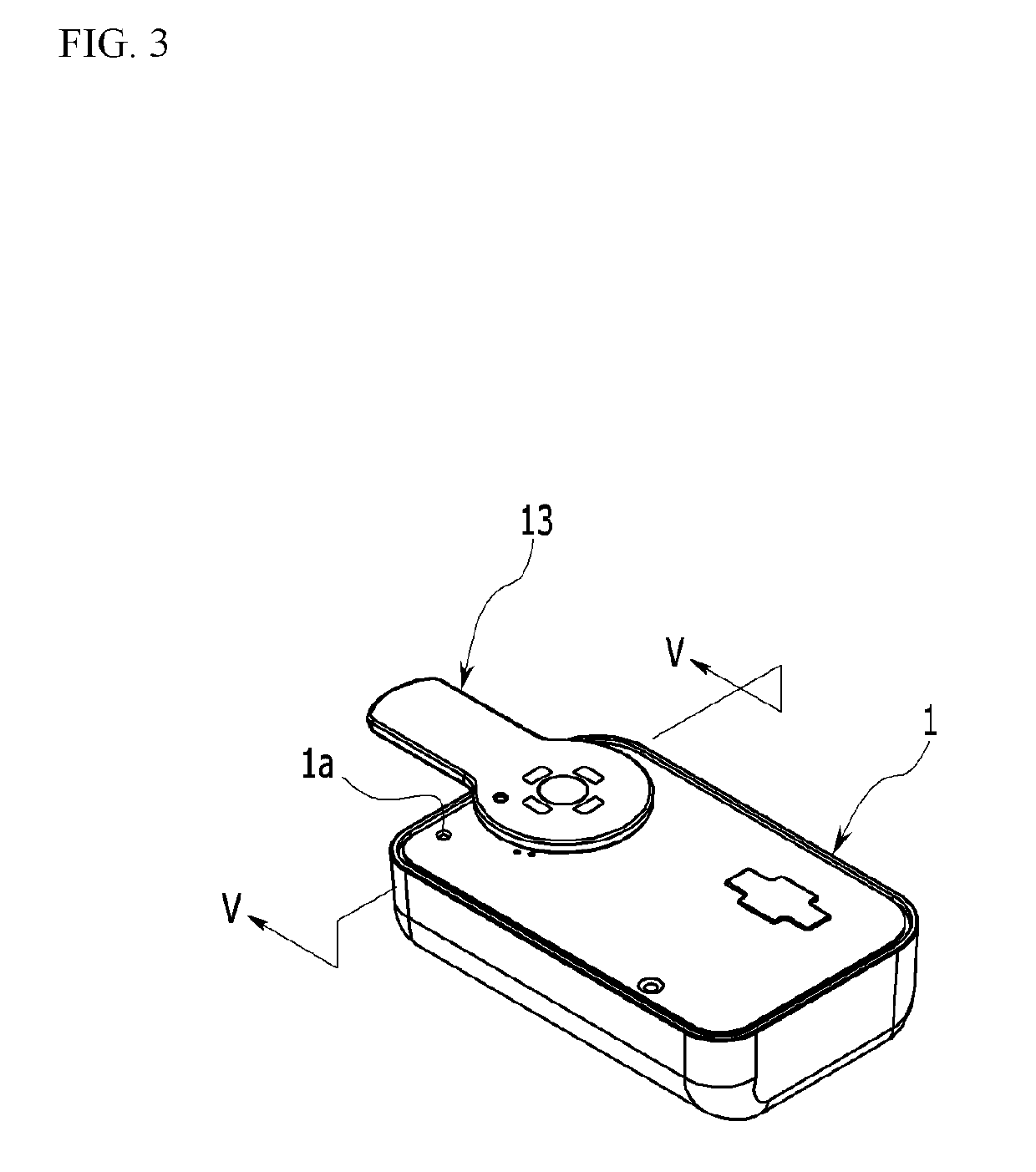
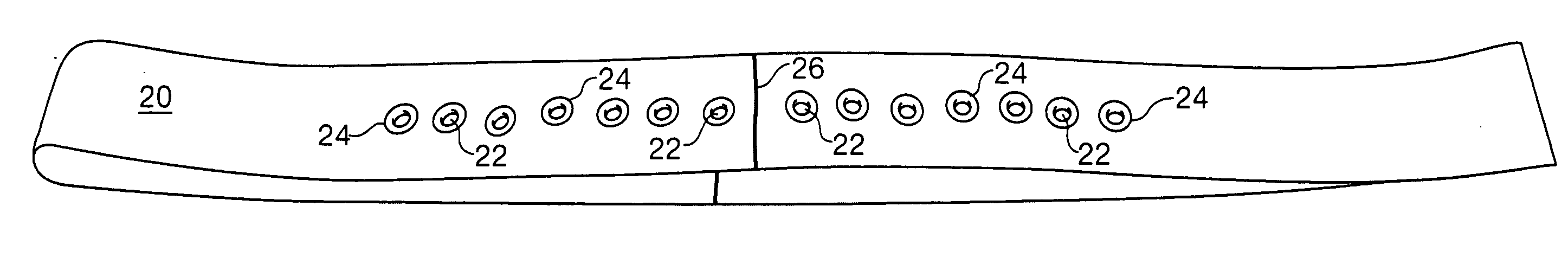
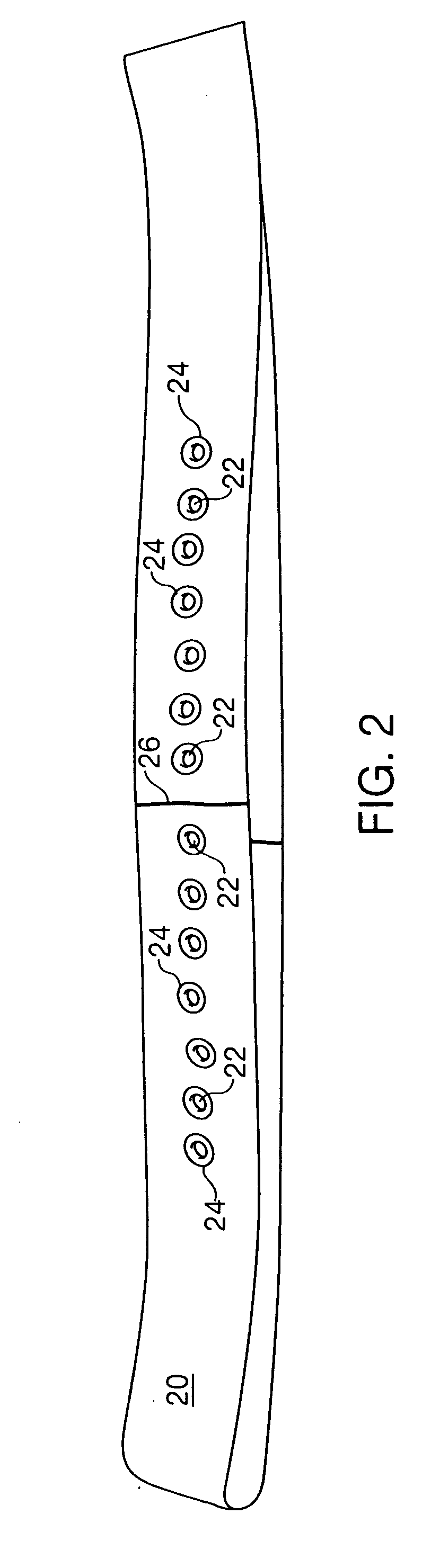
Sheet for guiding location of insulin injection
A sheet for guiding location of insulin injection has a sheet shape. The sheet has a first face and a second face opposite to the first face. A reference mark is formed at a first portion of the sheet to correspond to user's navel. A plurality of injection holes is formed at a second portion of the sheet opposite to the first portion to inject insulin, so that the insulin is injected at a left portion with respect to the reference mark by using the first face and the insulin is injected at a right portion with respect to the reference mark. A manual of the sheet is indicated on the first face and / or the second face. A day of a week is indicated corresponding to and adjacent to each of the injection holes. Thus, patient's convenience may be enhanced.
Owner:LILLY KOREA
Magnesium Compositions for Modulating the Pharmacokinetics and Injection Site Pain of Insulin
ActiveUS20140357554A1Peptide/protein ingredientsInorganic active ingredientsMagnesium saltDissolution
Compositions and methods for modulating injection site pain associated with rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations by mixing them with a vial containing dry powder excipients that accelerate their absorption. Devices for mixing excipient and insulin together at the time of administration, while minimizing residence time of the mixture, are also described.
Owner:ELI LILLY & CO
Medical device for delivering drug and/or performing physical therapy
Current methods of delivering drug usually require a certain degree of human intervention. For example, a doctor, a paramedic or a patient is required to inject insulin manually when the blood sugar level becomes low. This invention provides a medical device for use with a patient for performing at least one of the functions of delivering drug and performing physical therapy to the patient. The device of this invention includes a sensor for measuring at least one parameter from the patient, an analyzer for analyzing the parameter to determine appropriate function to be carried out on the patient by said medical device, and at least one actuator to perform at least one of the functions of delivering drug and performing physical therapy.
Owner:THE HONG KONG POLYTECHNIC UNIV
Sheet for guiding location of insulin injection
Owner:LILLY KOREA
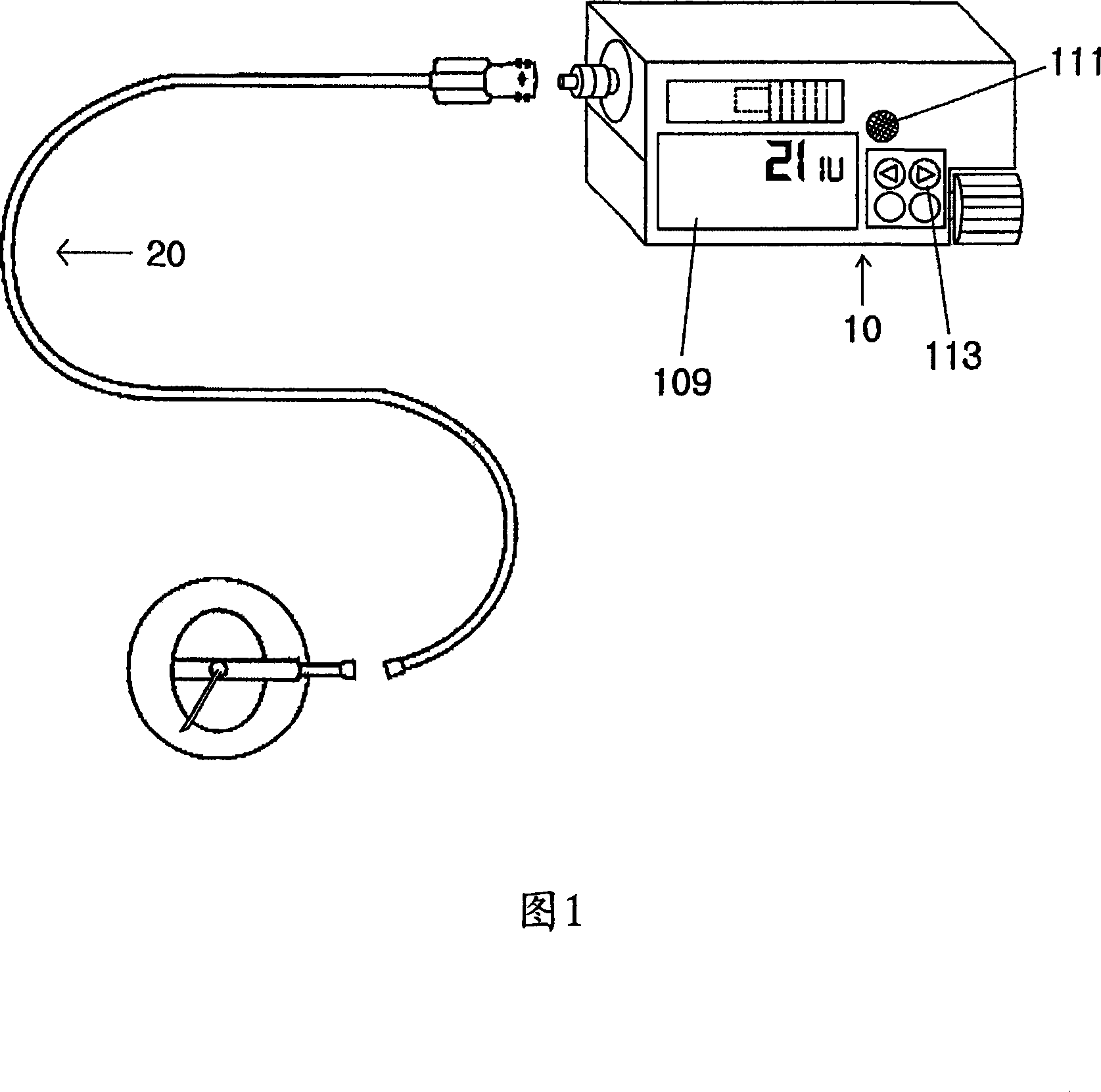
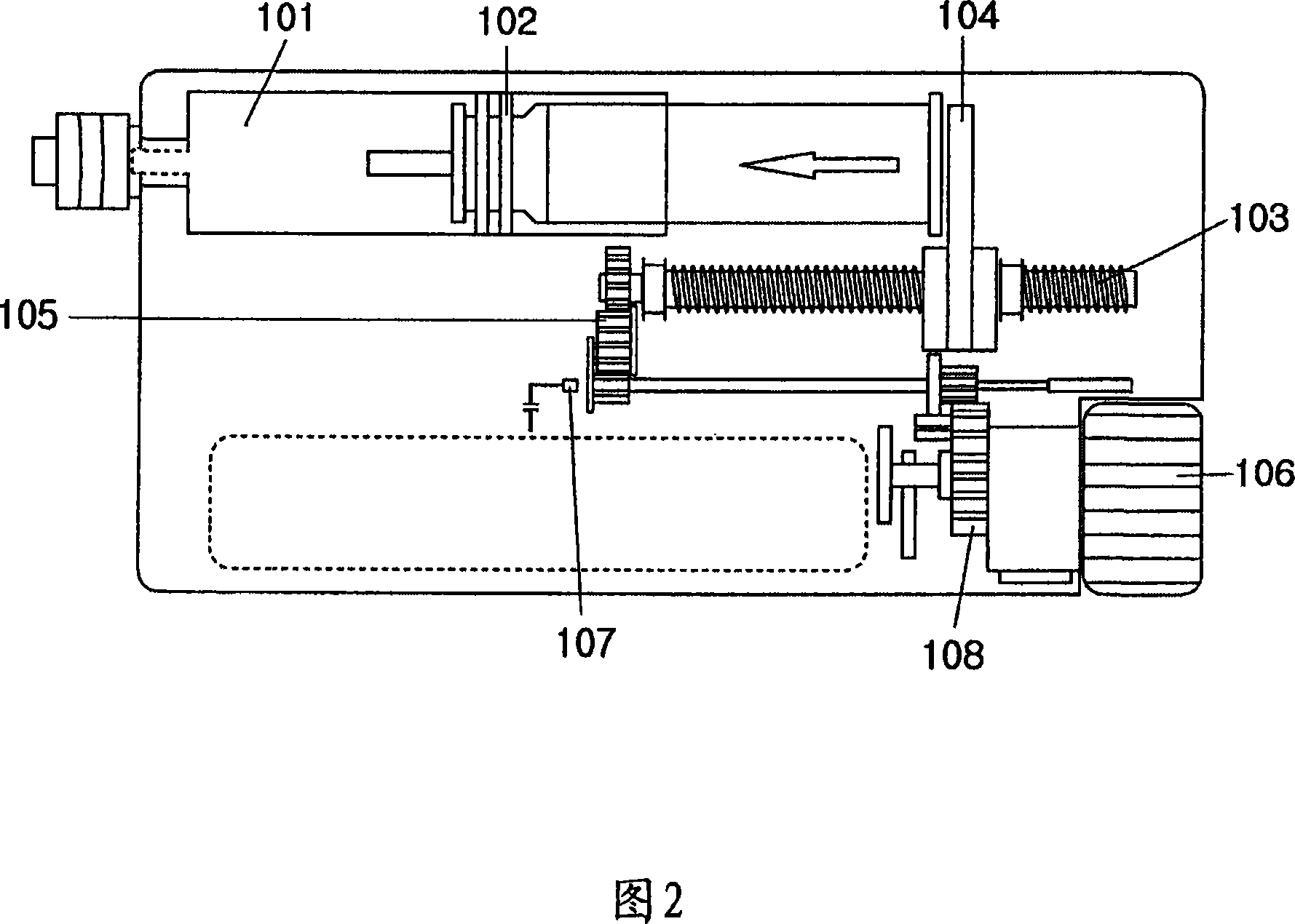
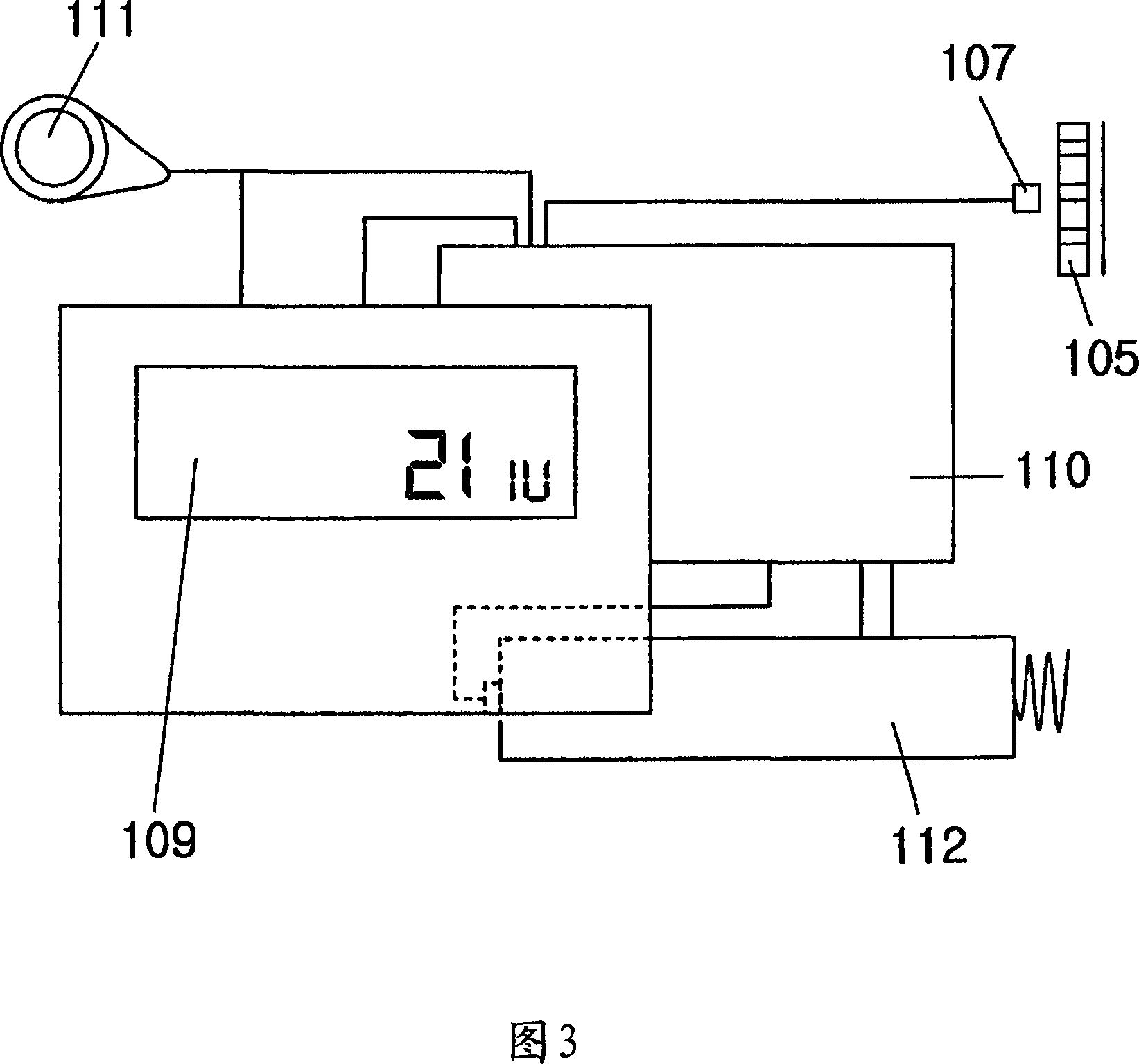
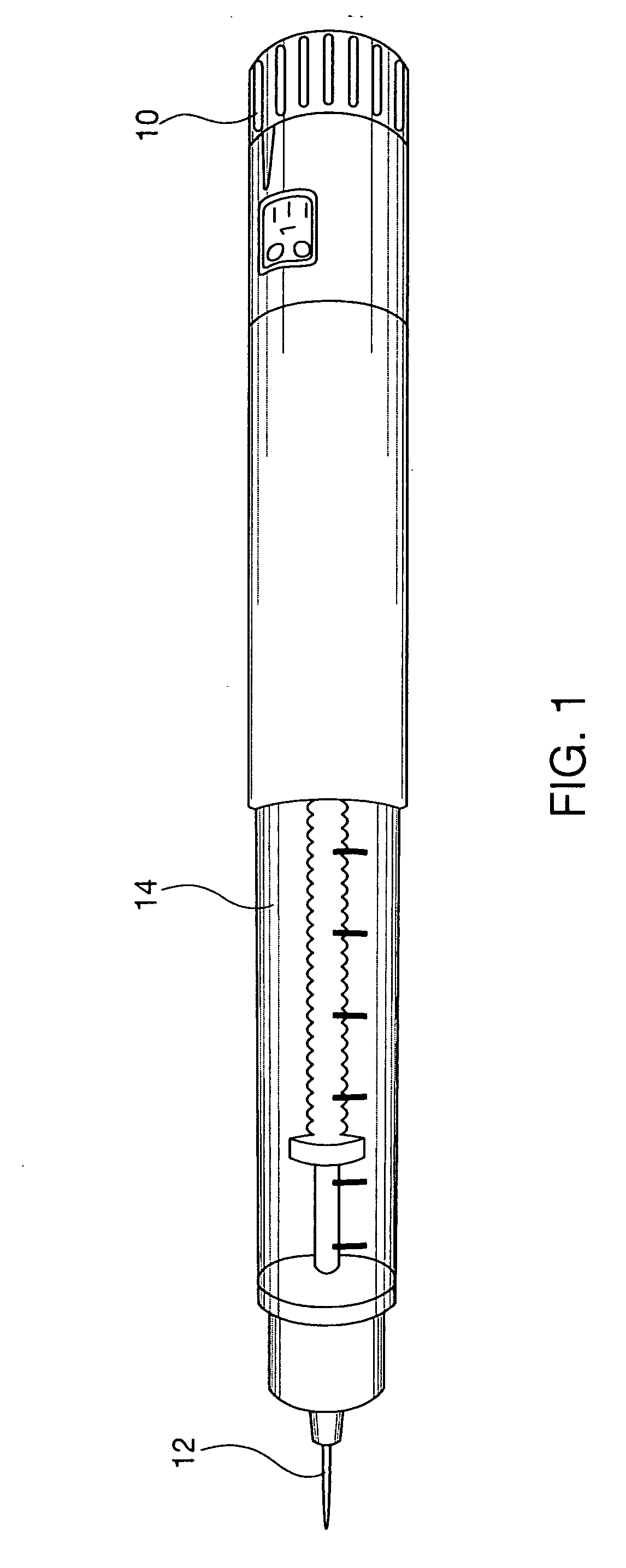
Portable mechanical insulin injection device
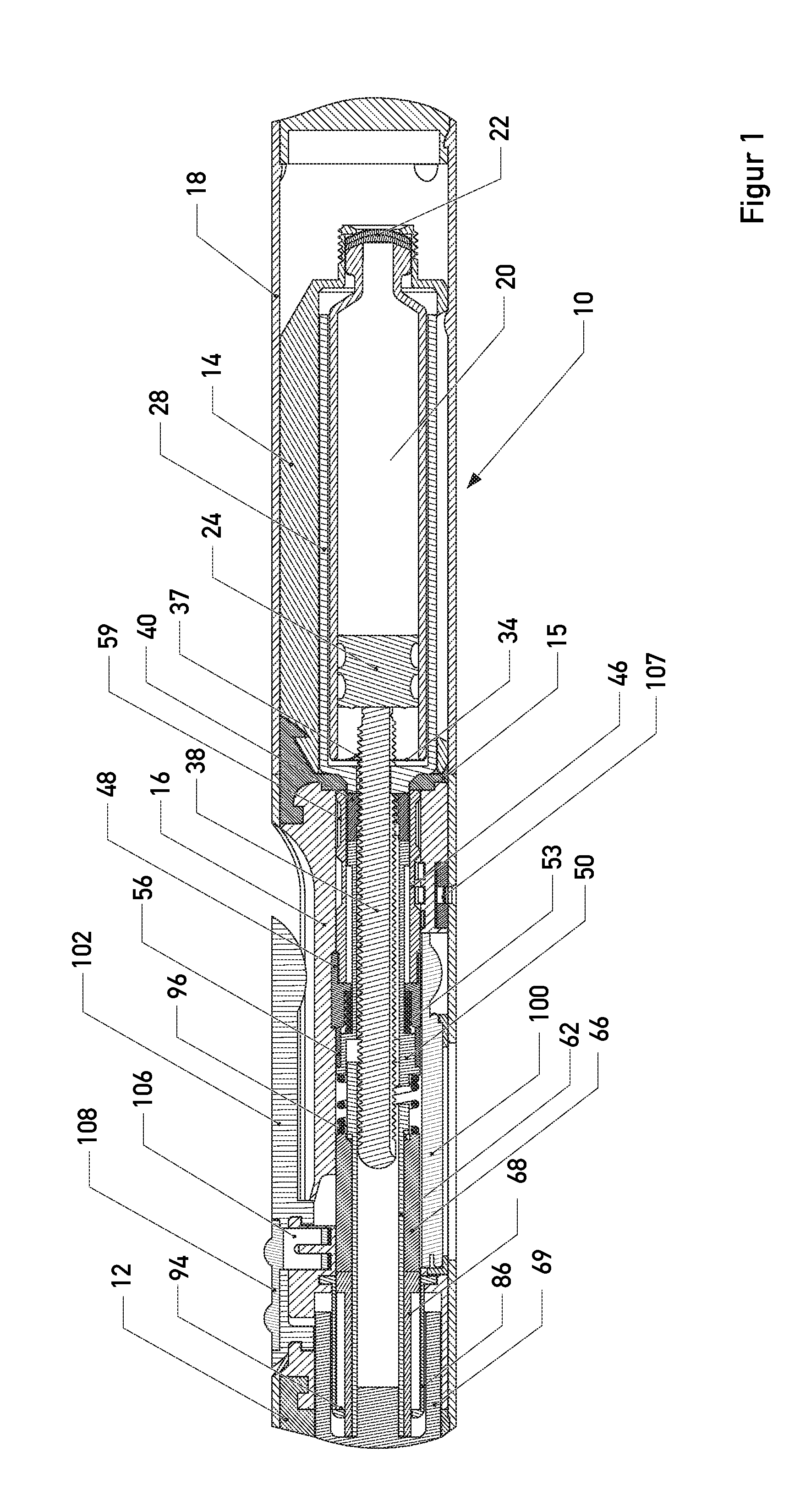
The present invention relates to a portable mechanical insulin injection device comprising i ) a mechanical insulin injection device (10) wherein said injection device is mechanically operated by hand-operated rotating part (106) whereas the amount of injected insulin and the pre-determined injection amount of insulin are electrically controlled and displayed by electrical control panel (110); and an insulin injection unit (20) wherein an insulin injection needle (211) is inserted in the skin or muscle of patient and an insulin injection connector (215) is fixed with the insulin outlet of mechanical insulin injection device (10) at the time of insulin injection.
Owner:崔奎东
Liquid medicine injection device
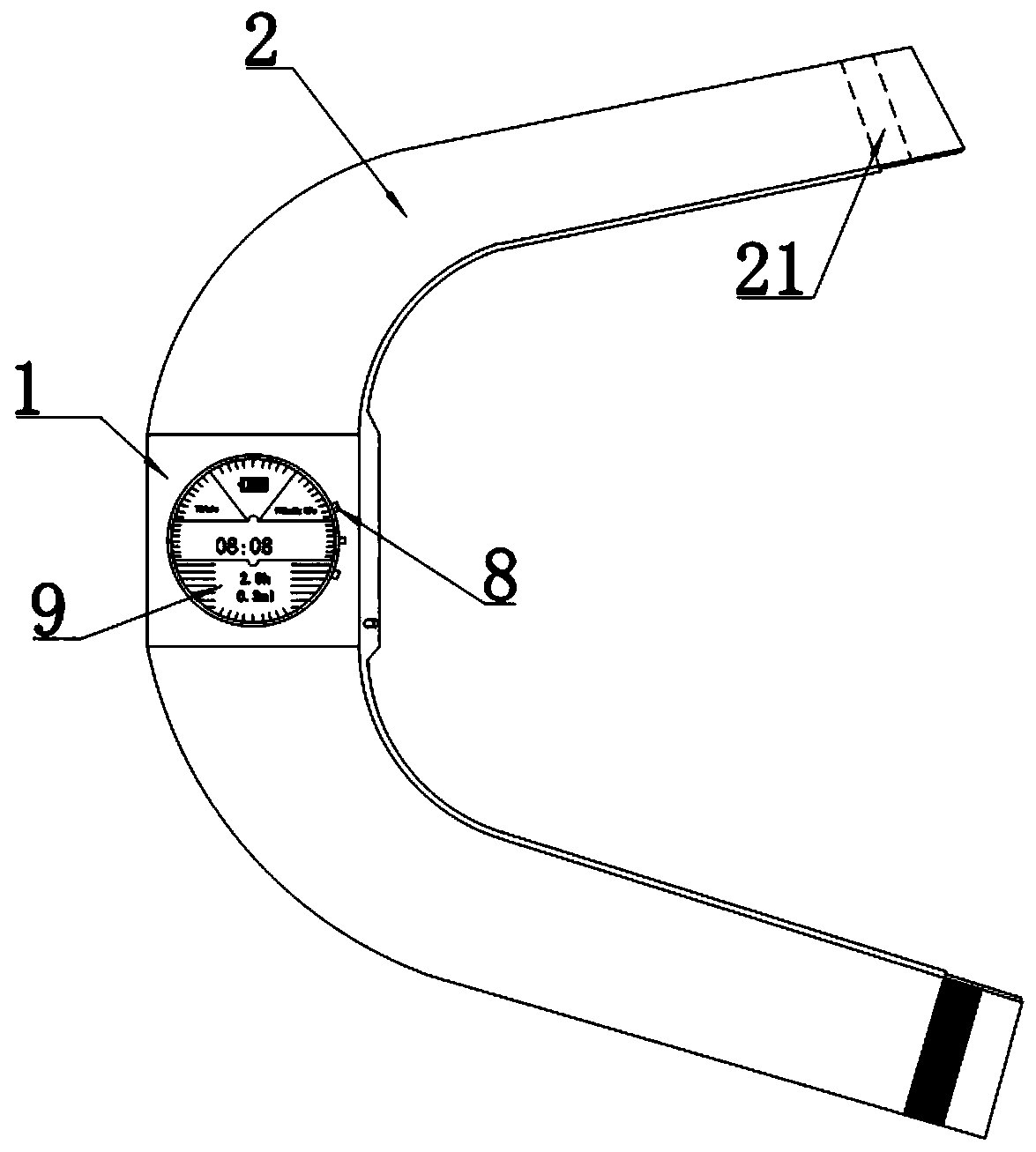
ActiveUS20190192765A1Fit closelyLow powerFlexible member pumpsMedical devicesInjection deviceBiomedical engineering
The present invention discloses a liquid medicine injection device that is attached to a patient's body to inject liquid medicine such as insulin at an appropriate time.A liquid medicine injection device according to the present invention includes: a case; a liquid medicine tank that is disposed in the case and where liquid medicine is received; an electro-osmosis pump that is connected with the liquid medicine tank and moves the liquid medicine; a needle assembly that receives the liquid medicine by being connected to the electro-osmosis pump and injects the received liquid medicine into a human body; and an adhesive member combined to the case and attached to the human body.
Owner:EOFLOE
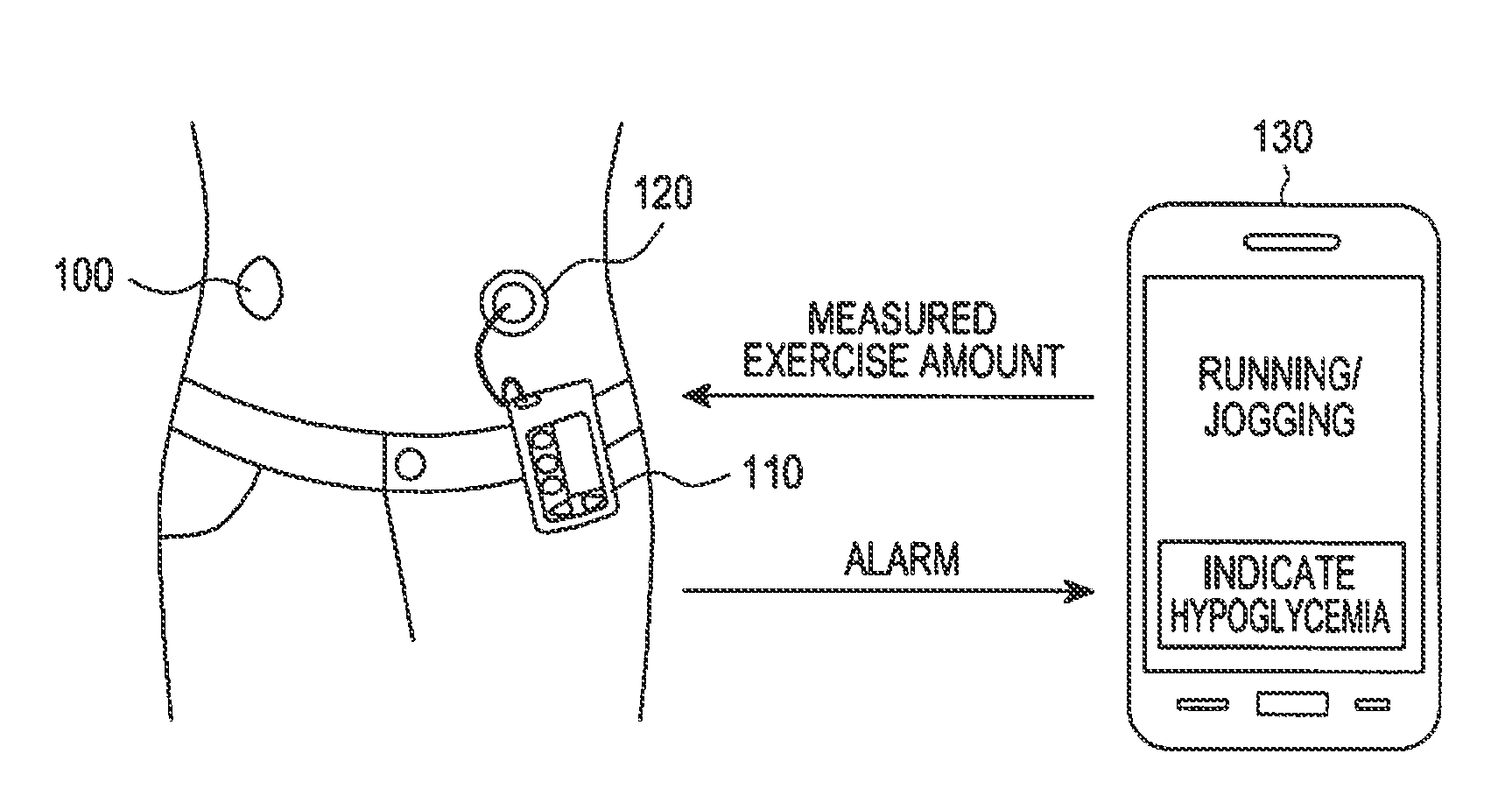
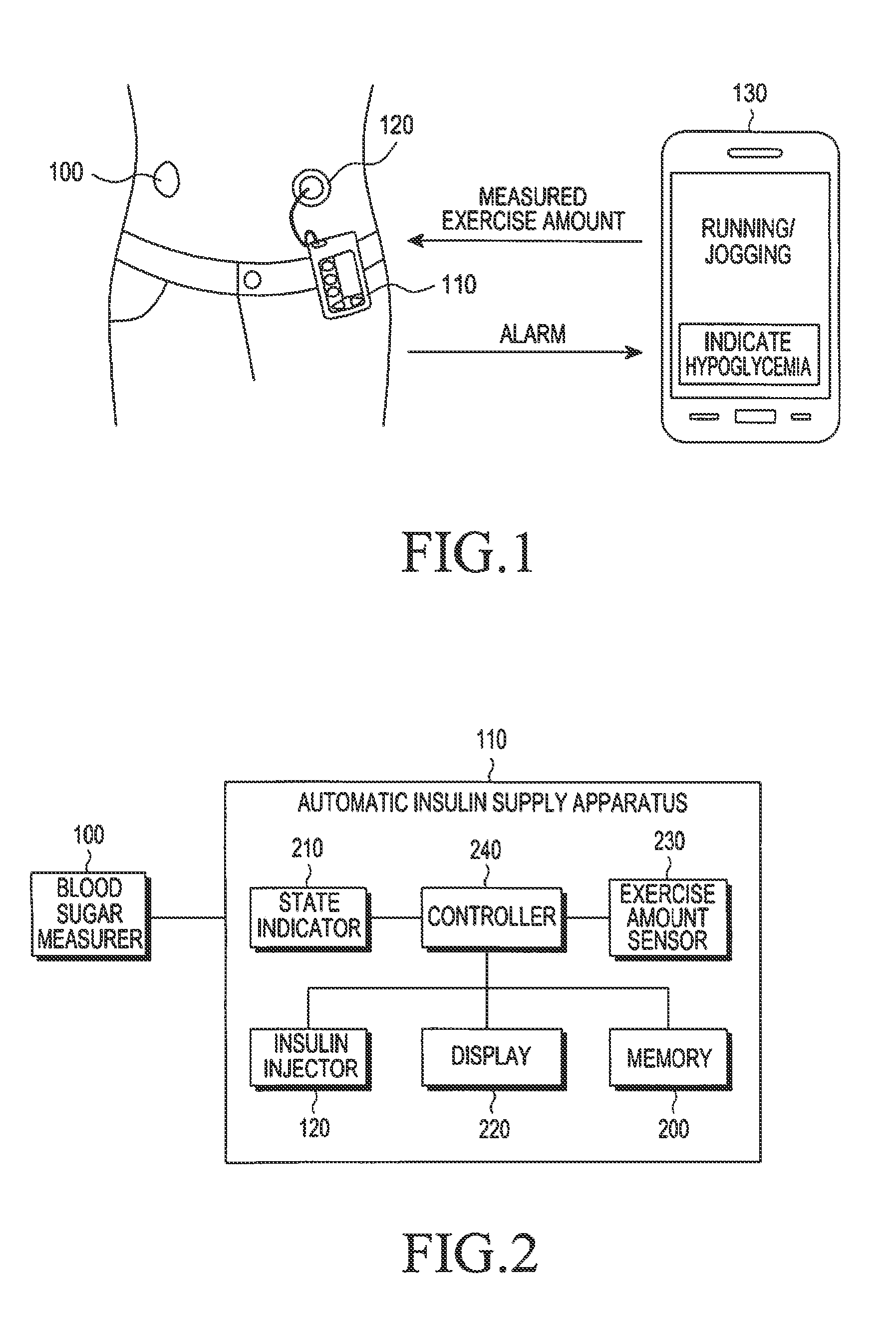
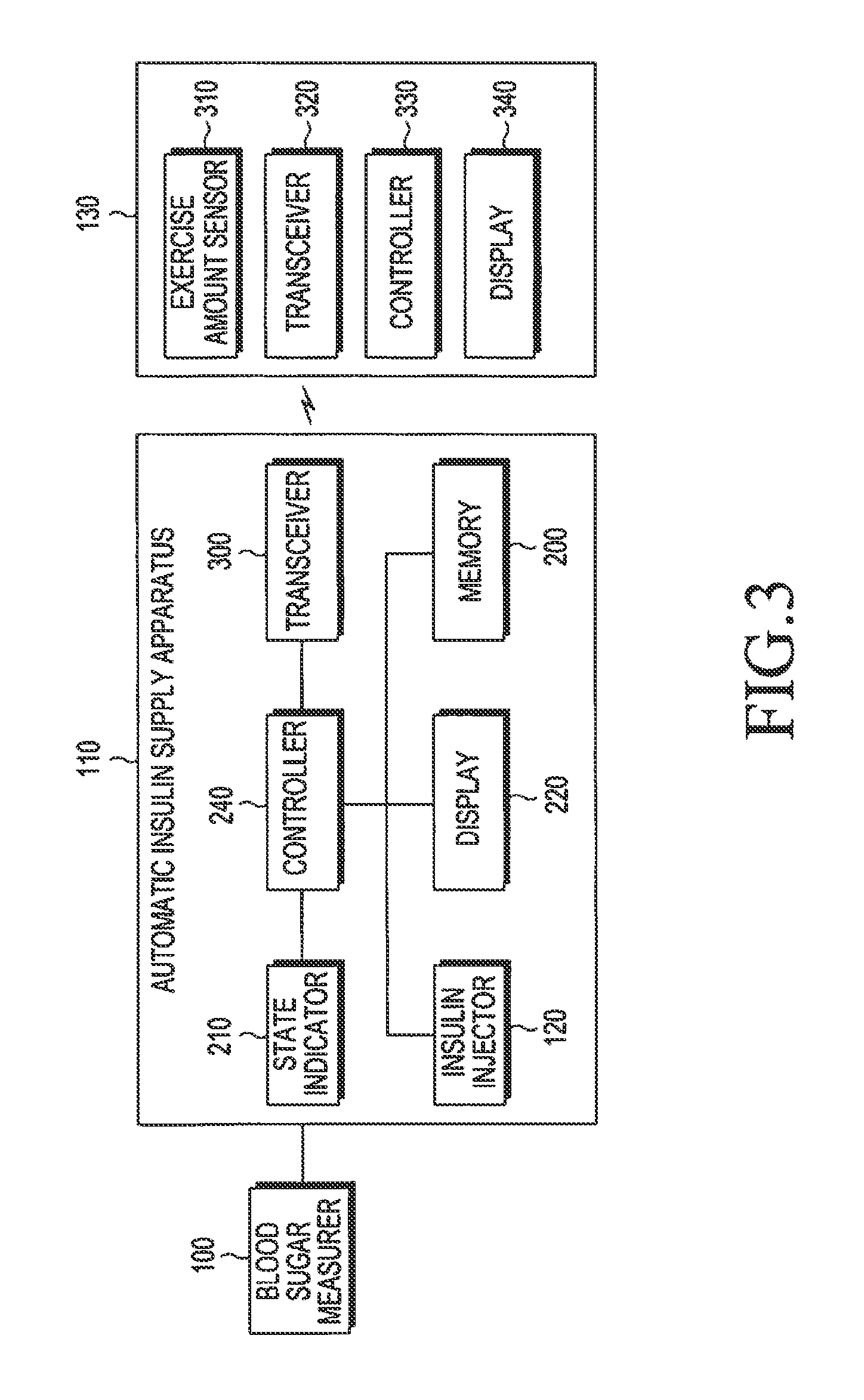
Apparatus and method for automatically supplying insulin based on amount of exercise
ActiveUS20130274183A1Physical therapies and activitiesPeptide/protein ingredientsInsulin injectionGlucose Measurement
An apparatus and method for automatically supplying insulin based on exercise amount are provided. The apparatus includes a blood sugar measurer for measuring a blood sugar level of a patient; a controller for, comparing the measured blood sugar level with a predetermined blood sugar level, if the measured blood sugar level is greater than or equal to the predetermined blood sugar level, determining whether a current time is within a blood sugar change time zone, if the measured blood sugar level is equal to or higher than the predetermined blood sugar level, and if the current time does is not within the blood sugar change time zone, acquiring exercise amount information about the patient and determining a dose of insulin to be injected inject into the patient based on the acquired exercise amount information, if the current time does not fall within the blood sugar change time zone; and an insulin injector for injecting the determined dose of insulin into the patient.
Owner:SAMSUNG ELECTRONICS CO LTD
Method for administering insulin to the buccal region
InactiveUS7070799B1Avoid stimulationCyclic peptide ingredientsEmulsion deliveryOctylphenoxy PolyethoxyethanolChamomile extract
A mixed micellar pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an alkali metal C8 to C22 alkyl sulfate, alkali metal salicylate, a pharmaceutically acceptable edetate and at least one absorption enhancing compounds. The absorption enhancing compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, octylphenoxypolyethoxyethanol, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linolenic acid, borage oil, evening primrose oil, trihydroxy oxo cholanylglycine, glycerin, polyglycerin, lysine, polylysine, triolein and mixtures thereof. Each absorption enhancing compound is present in a concentration of from 1 to 10 wt. / wt. % of the total formulation, and the total concentration of absorption enhancing compounds are less than 50 wt. / wt. % of the formulation. Methods for administering insulin to the buccal region are also disclosed.
Owner:GENEREX PHARMA INC
Apparatus for use in injecting insulin from a filled syringe
A Guide for injecting insulin in the abdomen, thigh or arm according to dimension including a wrap-around band and a series of spaced apertures along its length, with the openings of the apertures being sized to receive an insulin dispensing pin of a filled syringe, and with a reminder indicating at which site an injection was last made so as to reduce the possibility of scar tissue or abscess development for senior citizens, especially, forgetting where the most recent injection was made.
Owner:HARYLKA STANLEY +1
Insulin pump for quantitative timing administration
PendingCN110721364AAvoid underdosingGuaranteed therapeutic effectFiltering accessoriesMedical devicesInsulin injectionMedicine
The invention relates to the field of medical instruments, and discloses an insulin pump for quantitative timing administration. The insulin pump for quantitative timing administration comprises an insulin injection housing, and a binding tape connected to the housing, wherein an air sac, an insulin bag and an injection part are sequentially mounted in the insulin injection housing, the air sac isabutted with the insulin bag, the injection part and the inner wall of the insulin injection housing are in dismountable connection, the injection part comprises miniature syringes and a frame for supporting the miniature syringes, a gap is reserved between one ends facing the insulin bag, of the miniature syringes and the insulin bag, the other ends exceed the insulin injection housing, and an air inlet pipe, an inflation pipe, an air pump and a control unit for controlling timing start and close of the air pump are also arranged on the insulin injection housing. The insulin pump can be fixed to the body of a patient, can be used for injecting insulin of fixed amount within fixed time and can realize automatic insulin injection, so that a gerontal patient or patients being inconvenient for limbs can realize timely quantitative injection according to injection requirements, and the problem that the insulin is deficient in medication is solved. The treatment effect of the insulin injection can be guaranteed.
Owner:ANHUI HONGYU WUZHOU MEDICAL DEVICES CO LTD
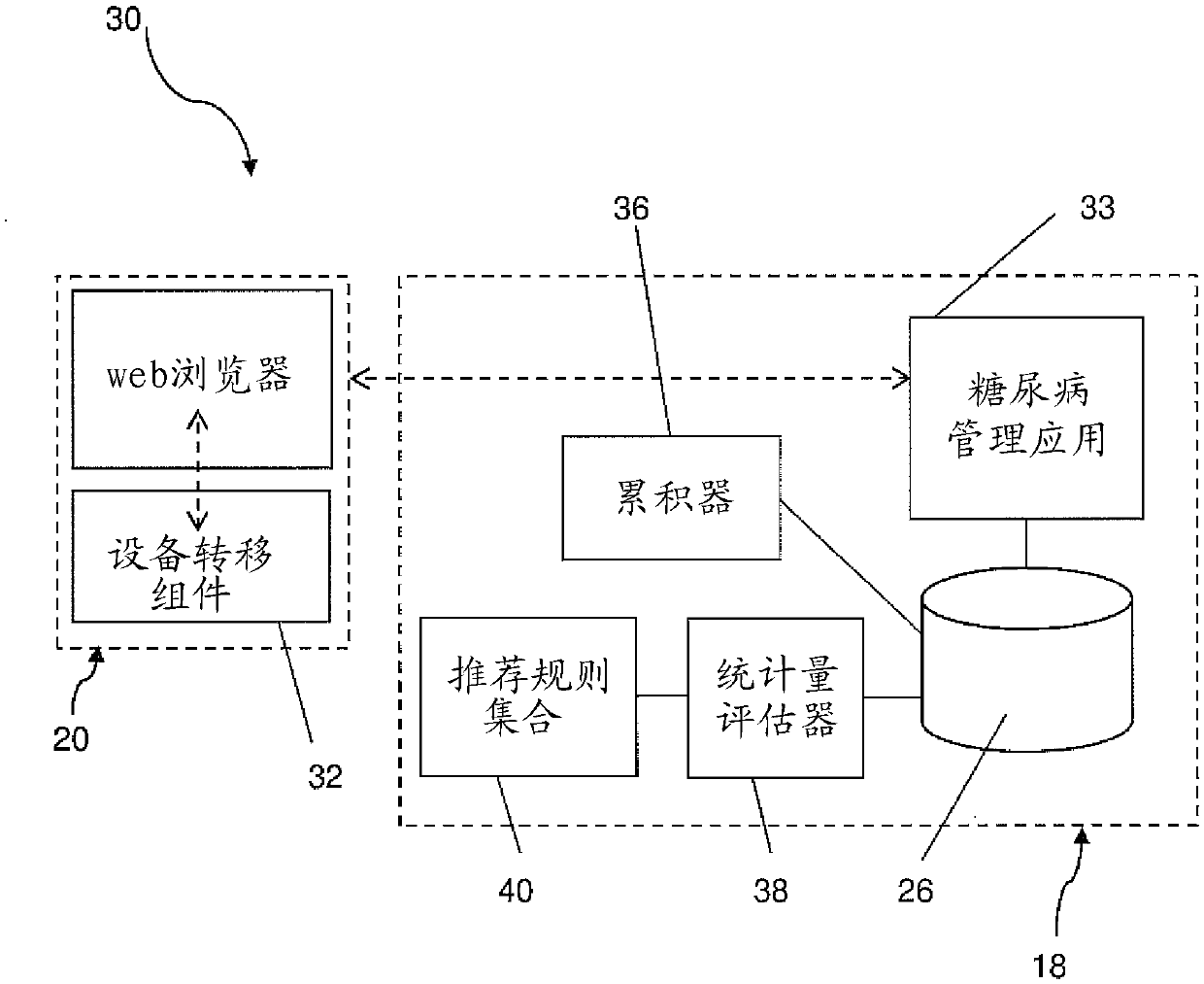
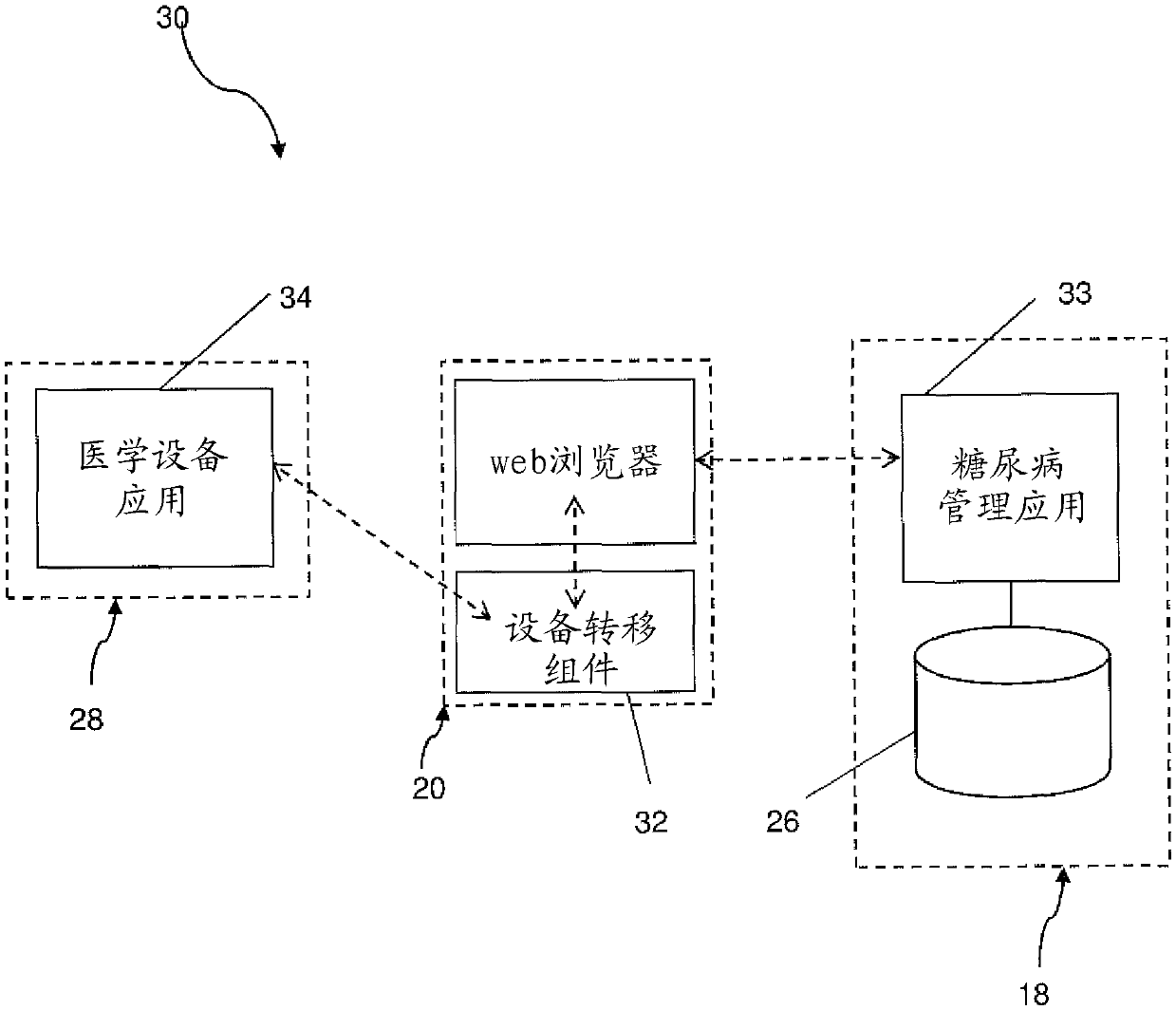
Diabetes management system with a medical device
ActiveCN105324079AEasy to manageGood treatment effectDrug and medicationsOffice automationHypodermoclysisGuideline
The invention relates to a diabetes management system (10, 10', 30, 30') for monitoring multiple daily injection insulin treatments, comprising: a portable medical device (28) associated with a patient; a data repository (26) accessible by both the patient and a health care provider; a diabetes management application (33) configured for receiving and storing, in the data repository, data from the portable medical device, the data including medical data associated with the patient and usage data associated with usage of the portable medical device, wherein the diabetes management application is configured to accumulate and evaluate statistics related to the usage data, and provide a treatment recommendation to the patient or the health care provider when the statistics meet or exceed a predetermined treatment criteria.
Owner:F HOFFMANN LA ROCHE & CO AG
Magnesium compositions for modulating the pharmacokinetics and pharmacodynamics of insulin and insulin analogs, and injection site pain
Compositions and methods for modulating injection site pain associated with rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations by mixing them with a vial containing dry powder excipients that accelerate their absorption. Devices for mixing excipient and insulin together at the time of administration, while minimizing residence time of the mixture, are also described.
Owner:ELI LILLY & CO
Protective cover for disposable injection needles
ActiveUS20150335828A1Eliminates at least mitigates riskMedical devicesInfusion needlesSyringe needleBiomedical engineering
A protective cover (4) to be used with disposable injection needles (1), such as those used for injection of insulin, which injection needles comprise a base (3) and a needle (2) fastened in the base, said protective cover comprising a hood (9) pivotably fastened on the base, said hood (9) being arranged to be pivotable, with the one and the same hand that holds a syringe on which the injection needle is arranged, between an inactive position at the side of the needle (2) of the injection needle and an active position in front of the needle (2) seen in the longitudinal direction of the needle.
Owner:LAPONIA INNOVATIO
Solid beverage for treating diabetes and preparation method thereof
InactiveCN109170470AScavenging oxygen free radicalsPromote blood circulationVitamin food ingredientsNatural extract food ingredientsPantothenic acidVitamin B6 synthesis
The invention discloses a solid beverage for treating diabetes and a prerparation method thereof. The solid beverage comprises germinant brown rice flour, small molecular peptide, cyclocarya paliurusextract, phylloporia ribis fermented mycelium, corn stigma extract, mulberry leaf extract, tartary buckwheat extract, lecithin, poria cocos extract, ampelopsis grossedentata leaf powder, calcium beta-hydroxy-beta-methylbutyrate, L-carnitine, magnesium oxide, vitamin C, taurine, zinc gluconate, niacin, vitamin E, pantothenic acid, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin A, folic acid, vitamin D3, and additives. The beverage has the effect of inhibiting the rise of blood sugar, can help diabetic patients get rid of the problem of insulin injection, can gradually replace insulin,and help the patient get rid of dependence on the insulin and western medicines, thereby avoiding the side effects of the insulin.
Owner:吉林健盛科技有限公司
Portable insulin storage and quantitative injection equipment with adjustable dosage
InactiveCN111939365AAvoid forgettingAutomatic quick exitAutomatic syringesInfusion needlesInsulin injectionInjection equipment
The invention discloses portable insulin storage and quantitative injection equipment with adjustable dosage. The portable insulin storage and quantitative injection equipment comprises a shell, wherein a working cavity is formed in the shell, an injection mechanism is arranged in the working cavity, and an insulin injector sliding barrel is fixedly arranged at the left end of the shell. The portable insulin storage and quantitative injection equipment is simple in structure and easy and convenient to operate. The portable insulin storage and quantitative injection equipment can be carried onthe arm and the abdomen, insulin can be automatically injected in a timed and quantitative mode, and a patient is prevented from forgetting to inject the insulin; besides, the injection dosage each time can be adjusted according to the actual situation, during automatic injection, the patient does not need to worry about the situation that a needle head is broken in the skin due to collision caused by emergency situations or the unknown condition that the patient is subjected to insulin injection at the moment, protection measures can be automatically started, and the needle head can automatically and rapidly leave a human body; and the temperature can be automatically adjusted, so that the insulin can be better carried and stored. The portable insulin storage and quantitative injection equipment with adjustable dosage is high in working efficiency and has high integration degree.
Owner:XINCHANG MUYUN AUTOMATION EQUIP CO LTD
Dual-quantization injector special for insulin
The invention discloses a dual-quantization injector special for insulin, which relates to an injector and is used for solving the problem that the conventional insulin injector has high manufacturing cost and cannot ensure sterile injection. The head end of a tubular bottle filled with insulin is detachably connected with a needle head cap; a needle head hole in the needle head cap is communicated with the tubular bottle filled with the insulin; one end of a piston propelling screw rod is in threaded connection with a piston, and the other end of the piston propelling screw rod is detachably connected with a cap type thrusting head; one end of the piston and one end of the piston propelling screw rod are arranged in the tubular bottle filled with the insulin; the piston is slidably connected with the tubular bottle filled with the insulin; an adjusting quantitative ring is arranged outside the tubular bottle filled with the insulin and is in threaded connection with the piston propelling screw rod; the outer wall of the piston propelling screw rod is provided with a plurality of measuring lines along the axial direction; the plurality of measuring lines are uniformly distributed along the circumferential direction of the piston propelling screw rod; and the thread pitch P of the piston propelling screw rod is equal to 2mL / n. The injector can be applied to injection of insulin.
Owner:庄青山
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com