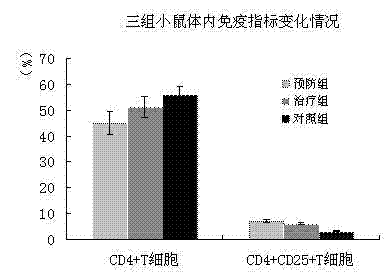
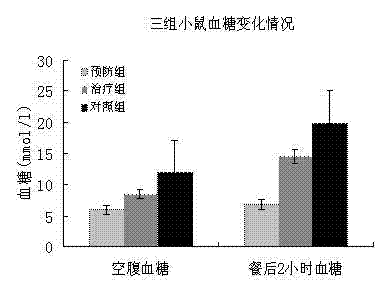
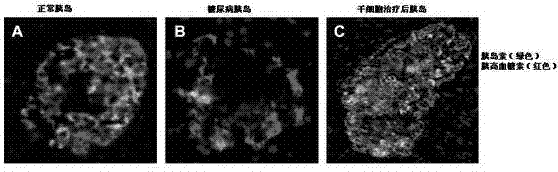
Mesenchymal stem cell injection, preparation method and application thereof in preparing medicine for treating diabetes
A technology of diabetes medicine and mesenchymal stem cells, which is applied in the field of biomedicine, can solve the problems of not fundamentally treating diabetes, not effectively repairing islet function, liver and kidney damage, etc., and achieving the effect of treating diabetes, lowering blood sugar and restoring islet function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Preparation of umbilical cord mesenchymal stem cells:
[0027] 1. The umbilical cord of a fresh full-term healthy fetus is washed with PBS buffer, which contains 100 kU / L penicillin and 100 mg / L streptomycin;
[0028] 2. Cut the 6-8cm long umbilical cord, cut the umbilical cord into 2cm long pieces, and wash it repeatedly with PBS buffer;
[0029] 3. Cut the umbilical cord tissue pieces into 2-3mm pieces 3 Small piece;
[0030] 4. Add the chopped tissue to L-DMEM medium for washing, centrifuge at 500-700g for 5 minutes, and discard the supernatant;
[0031] 5. Add the tissue block and the culture medium to the medium at a volume ratio of 2.5-3:1, mix the tissue block, inoculate it into a cell culture dish, and culture it in an incubator; the medium contains 1-10 ng / ml Basic fibroblast growth factor (bFGF) and L-DMEM medium with volume percentage 10%-15% fetal bovine serum (FBS);
[0032] 6. After 24 hours, add the culture medium described in step 5;
[0033] 7. ...
Embodiment 2
[0064] The preparation of umbilical cord and placental mesenchymal stem cells was the same as in Example 1.
[0065] For example, to prepare 100ml of stem cell injection, the injection consists of 25ml of human serum albumin stock solution (final concentration of mass volume ratio is 5%), 0.5ml of low molecular weight heparin calcium (final concentration of mass volume ratio is 0.5%), 20ml of compound amino acid (mass volume ratio of 0.5%), The volume ratio final concentration is 20%), vitamin C 0.5g (mass volume ratio final concentration is 0.5%), 54ml of 5% glucose injection and mesenchymal stem cells. Except for mesenchymal stem cells, the remaining components of the injection should be prepared in advance and pre-cooled at 4°C for later use. Mesenchymal stem cells are finally resuspended in this solution to make a single-cell suspension. The number of mesenchymal stem cells per ml of injection 1×10 7 . Mesenchymal stem cells remain in a single-cell suspension state withi...
Embodiment 3
[0067] The preparation of umbilical cord and placental mesenchymal stem cells was the same as in Example 1.
[0068] For example, to prepare 100ml of stem cell injection, the injection consists of 10ml of human serum albumin stock solution (the final concentration of mass volume ratio is 2%), 0.5ml of low molecular weight heparin calcium (the final concentration of mass volume ratio is 0.5%), 10ml of compound amino acid (mass volume ratio The volume ratio final concentration is 10%), vitamin C 0.5g (mass volume ratio final concentration is 0.5%), 0.9% normal saline injection 79ml and mesenchymal stem cells. Except for mesenchymal stem cells, the remaining components of the injection should be prepared in advance and pre-cooled at 4°C for later use. The prepared injection should be used within 1 week. The mesenchymal stem cells are finally resuspended in this solution to make a single cell suspension, and the number of mesenchymal stem cells per milliliter of injection is 2×10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com