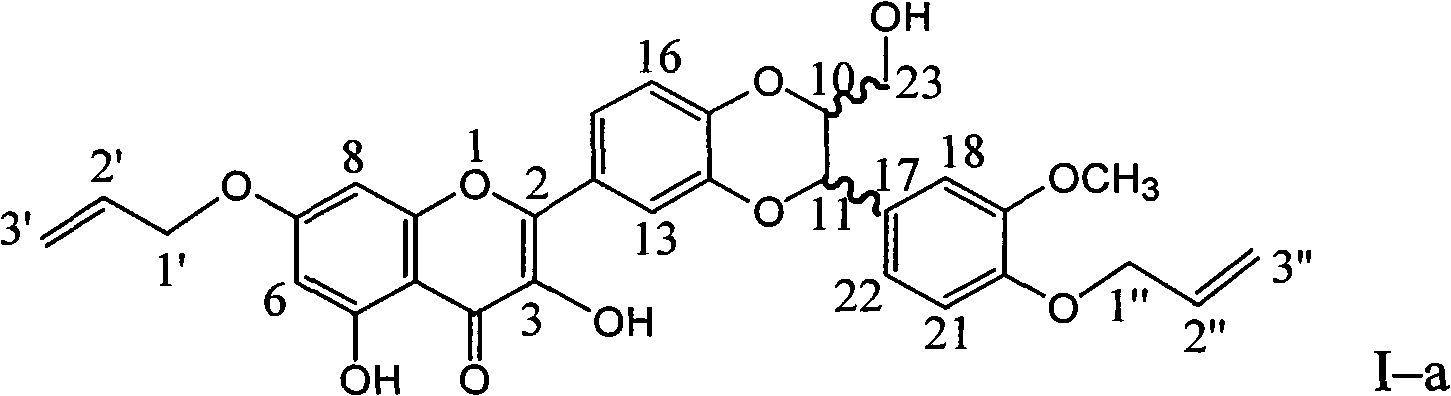
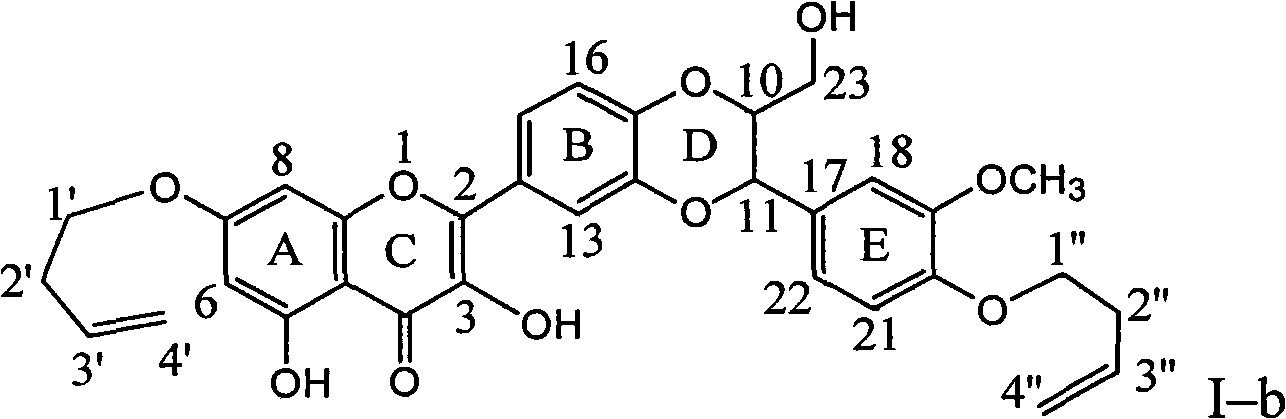
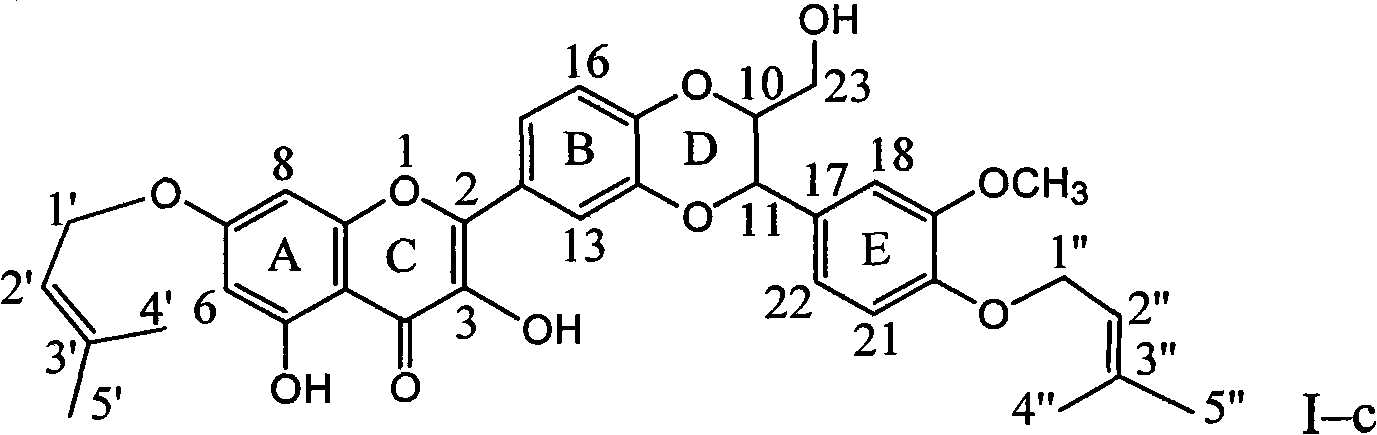
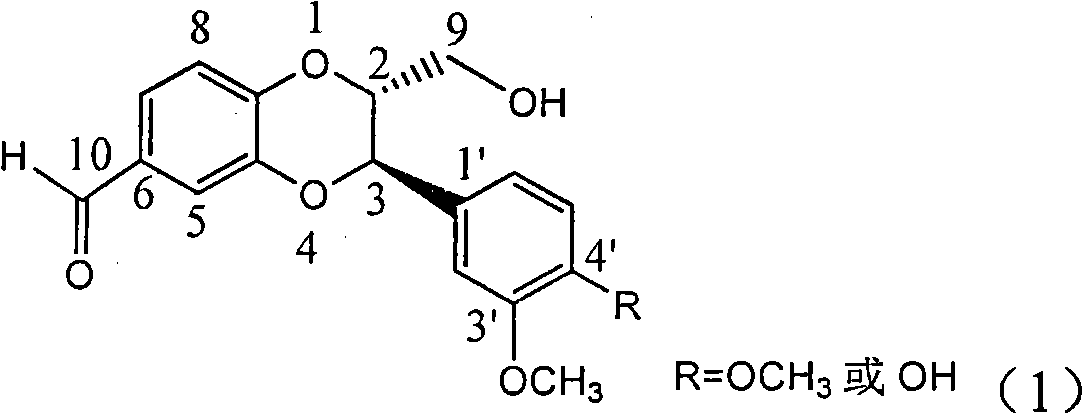
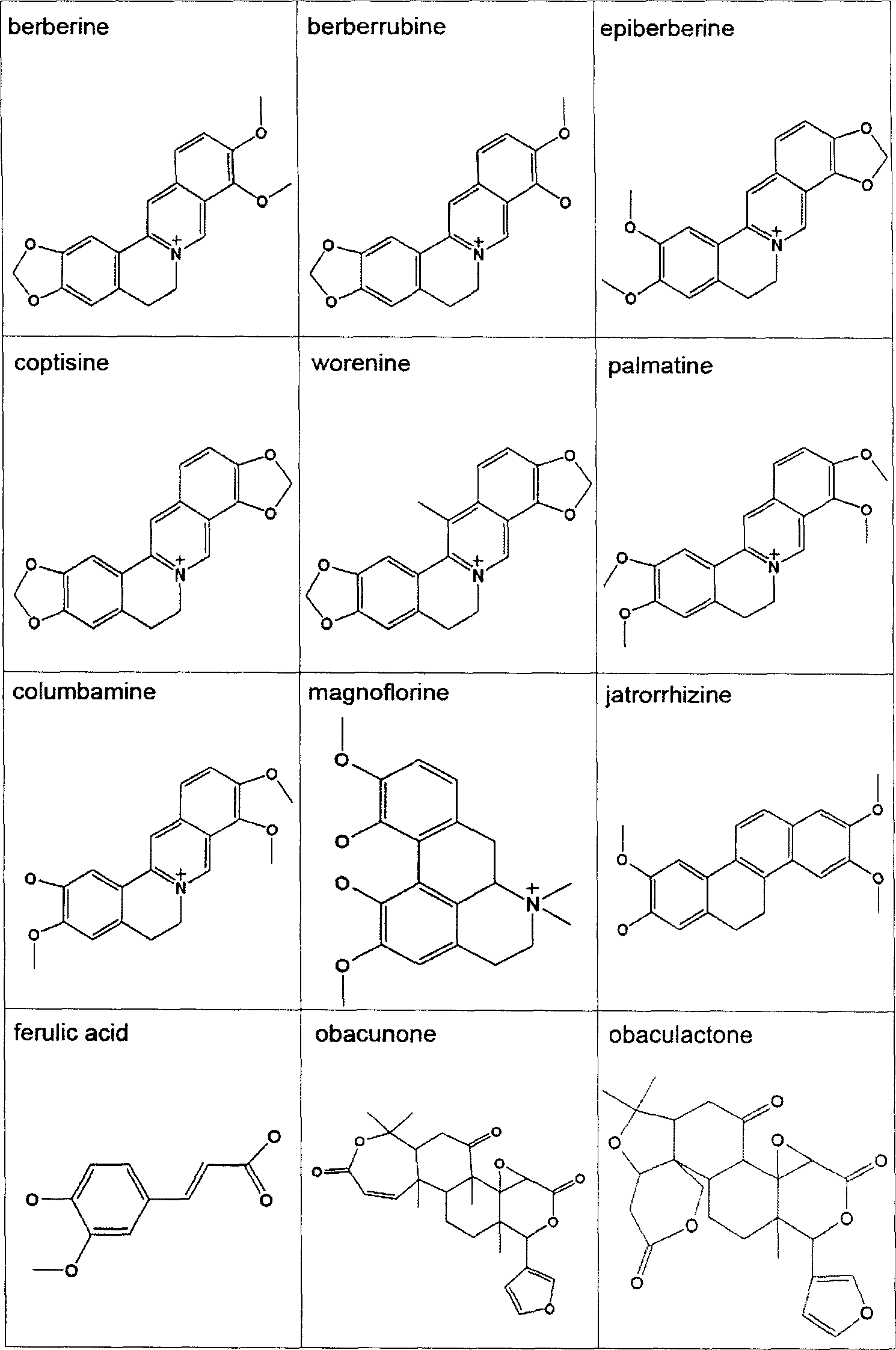
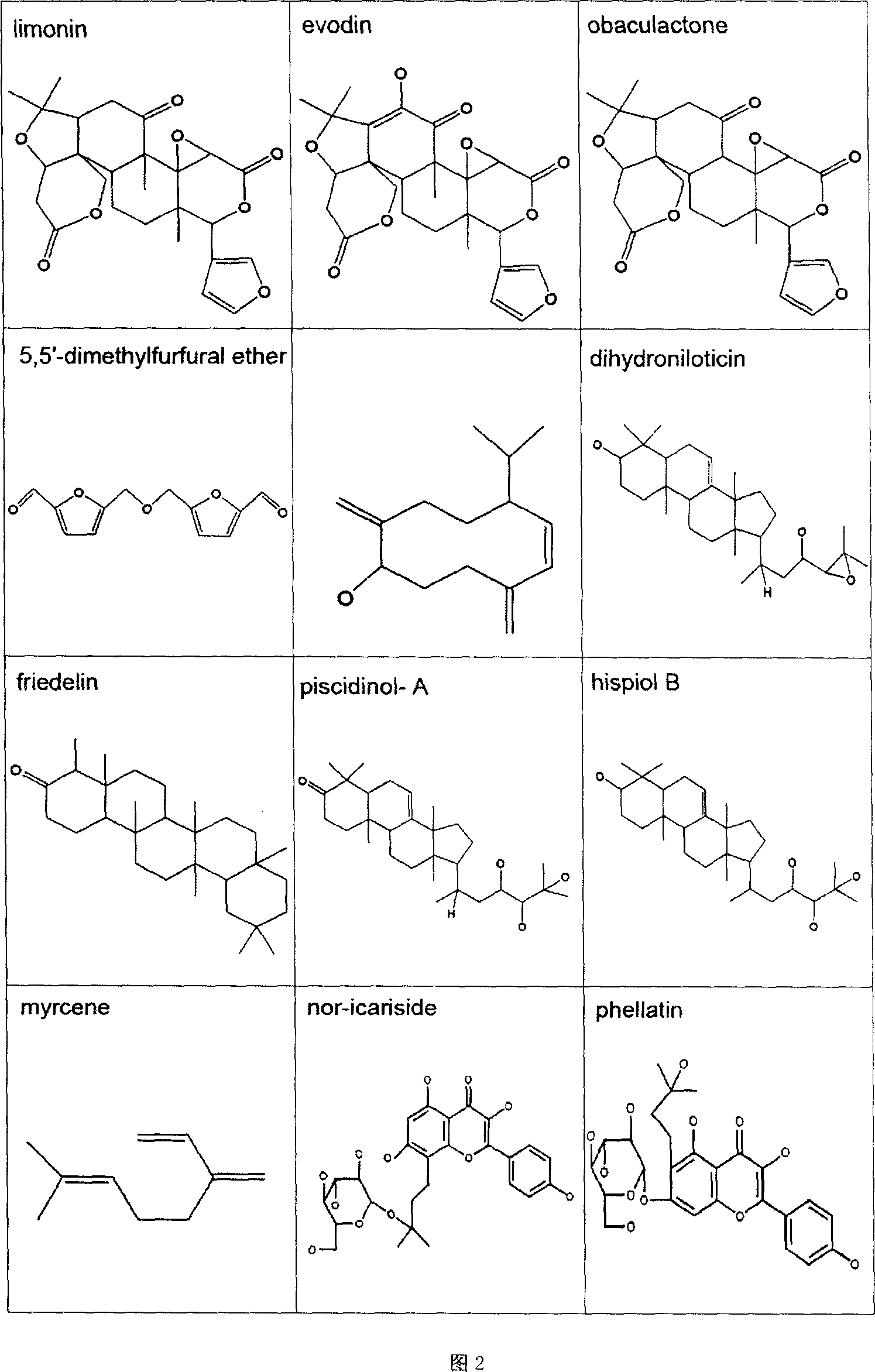
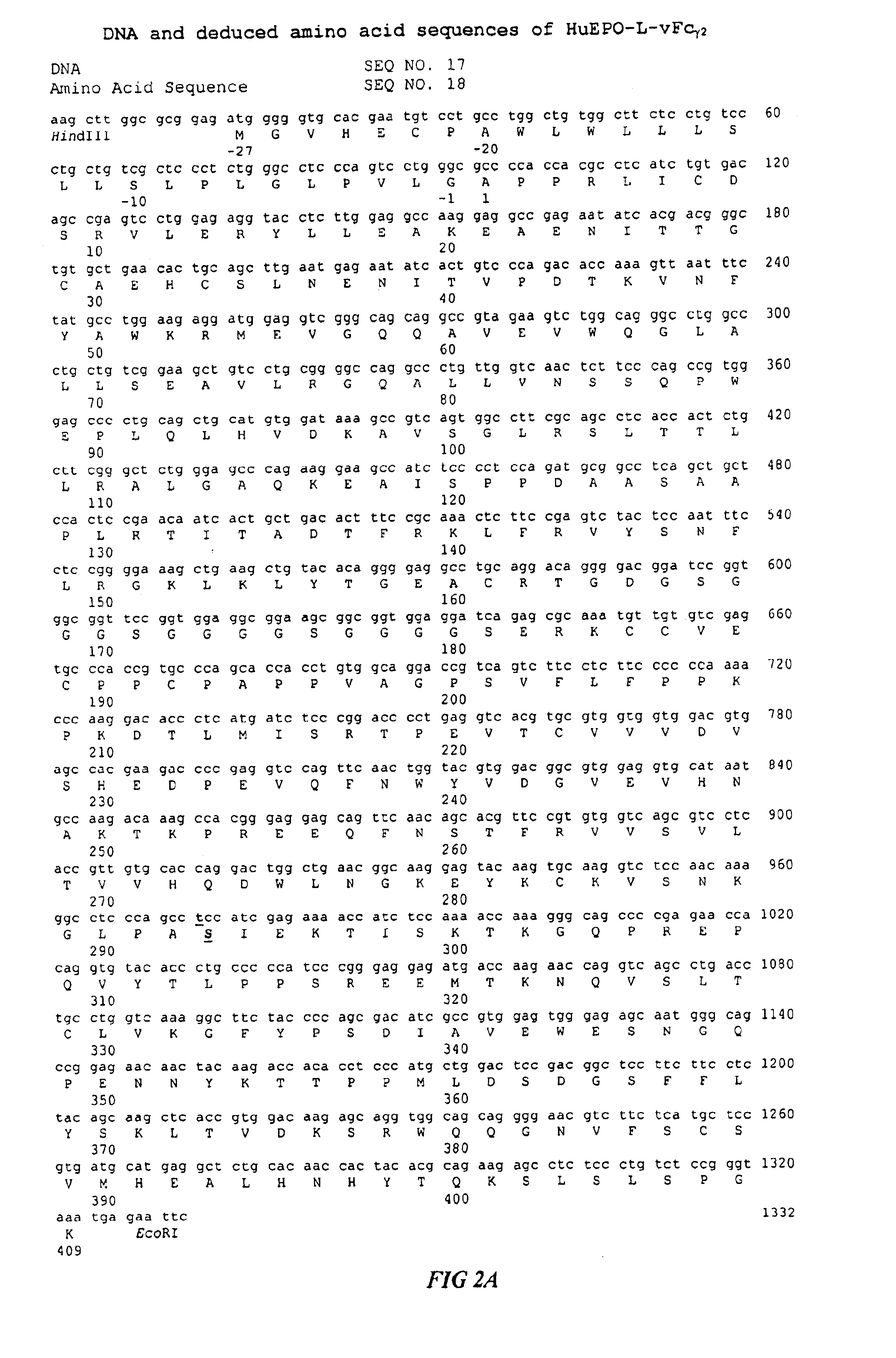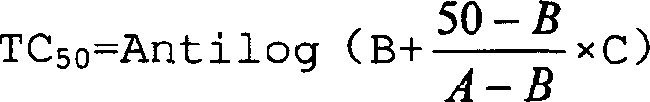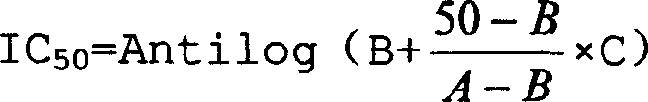Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
665 results about "Pharmacodynamic Study" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmacodynamics is the study of how a drug affects an organism, whereas pharmacokinetics is the study of how the organism affects the drug. Both together influence dosing, benefit, and adverse effects.
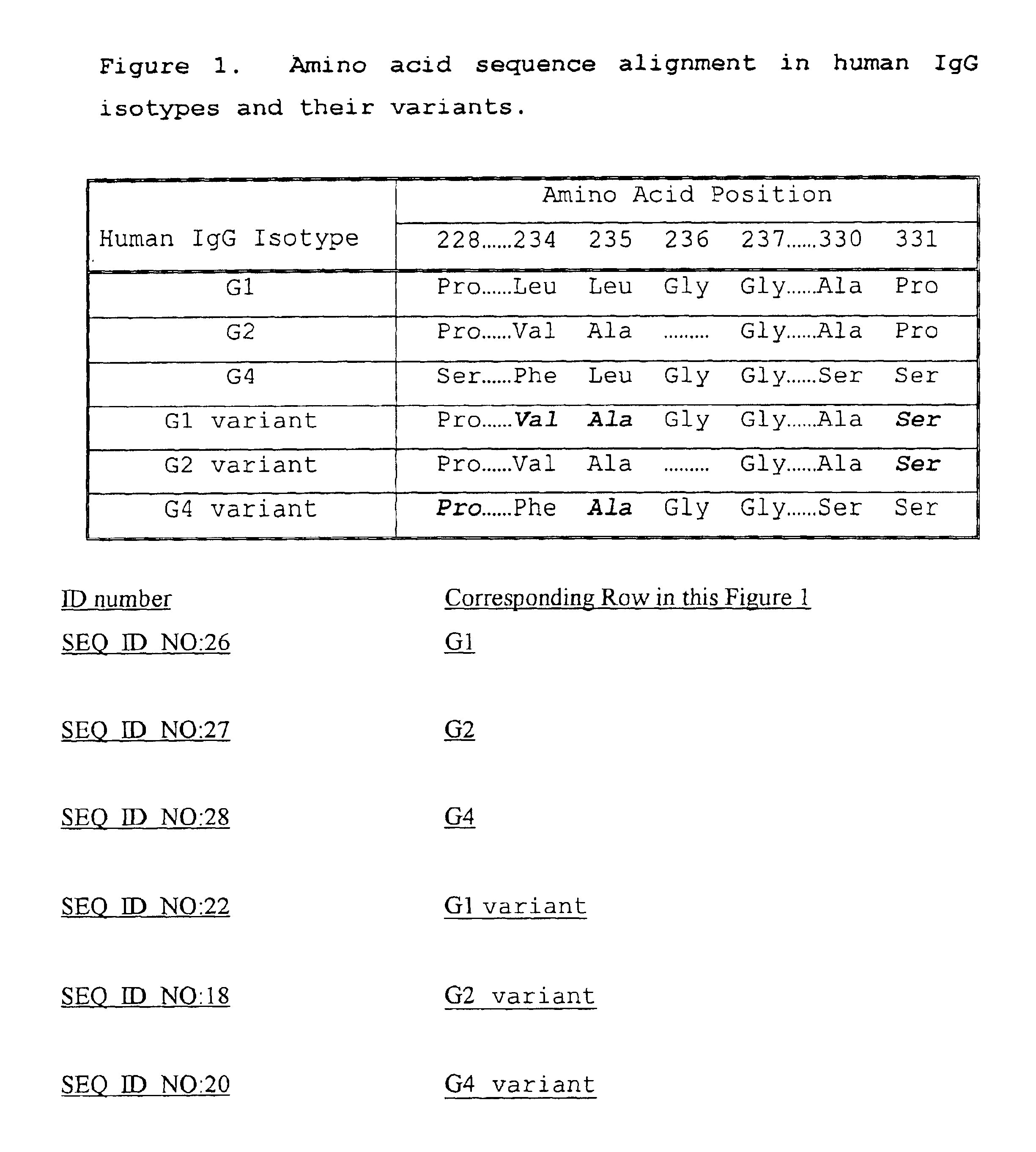
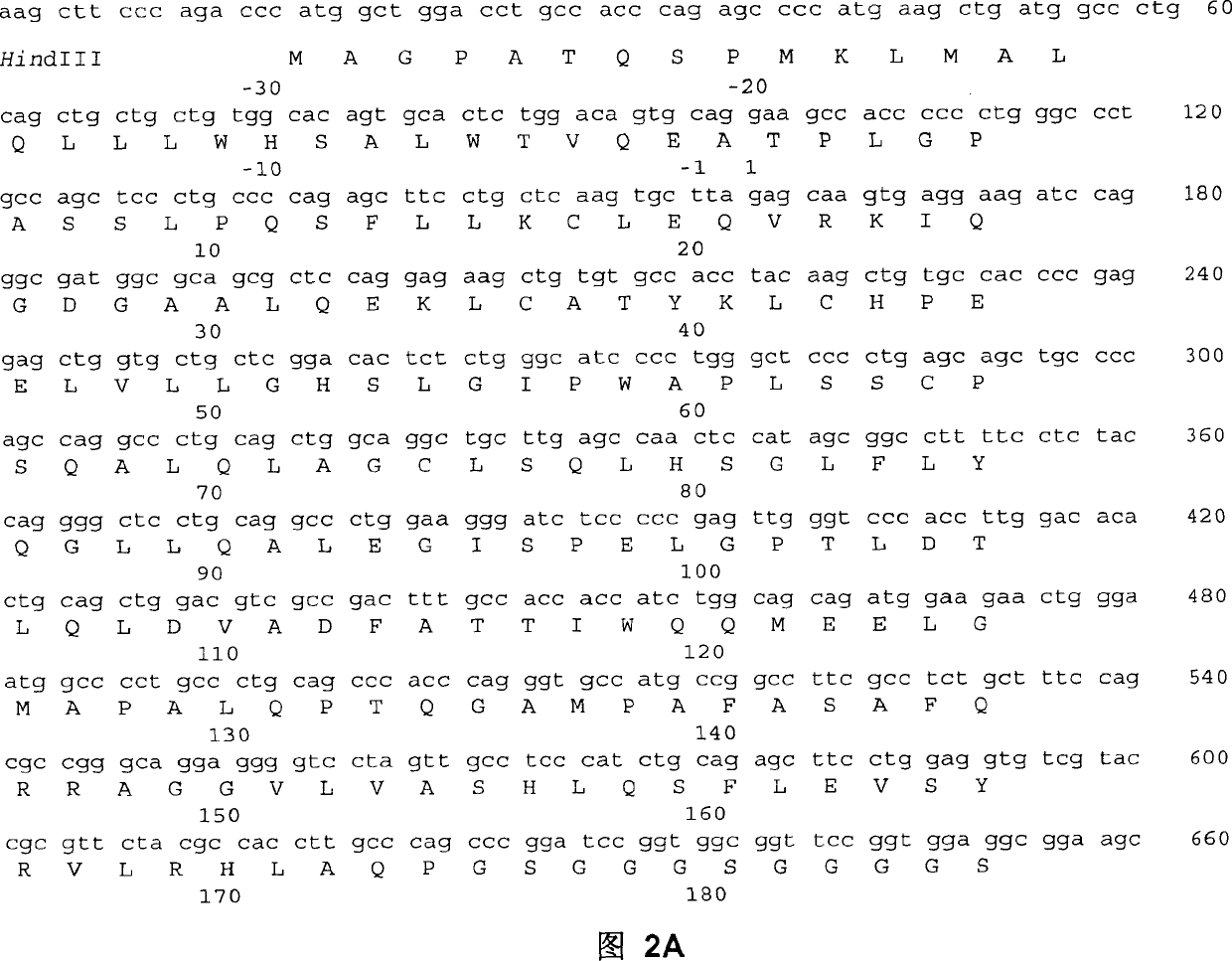
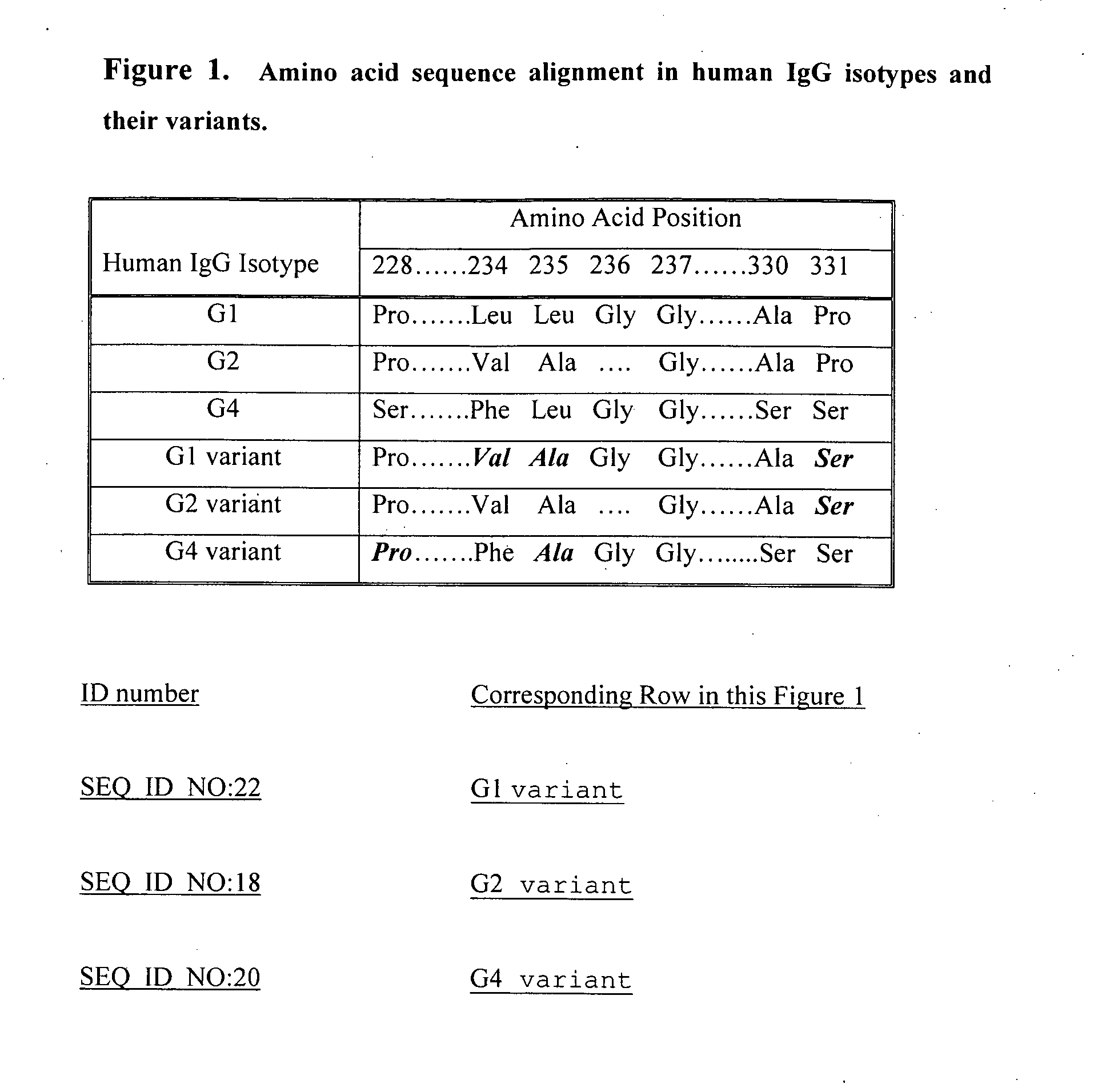
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
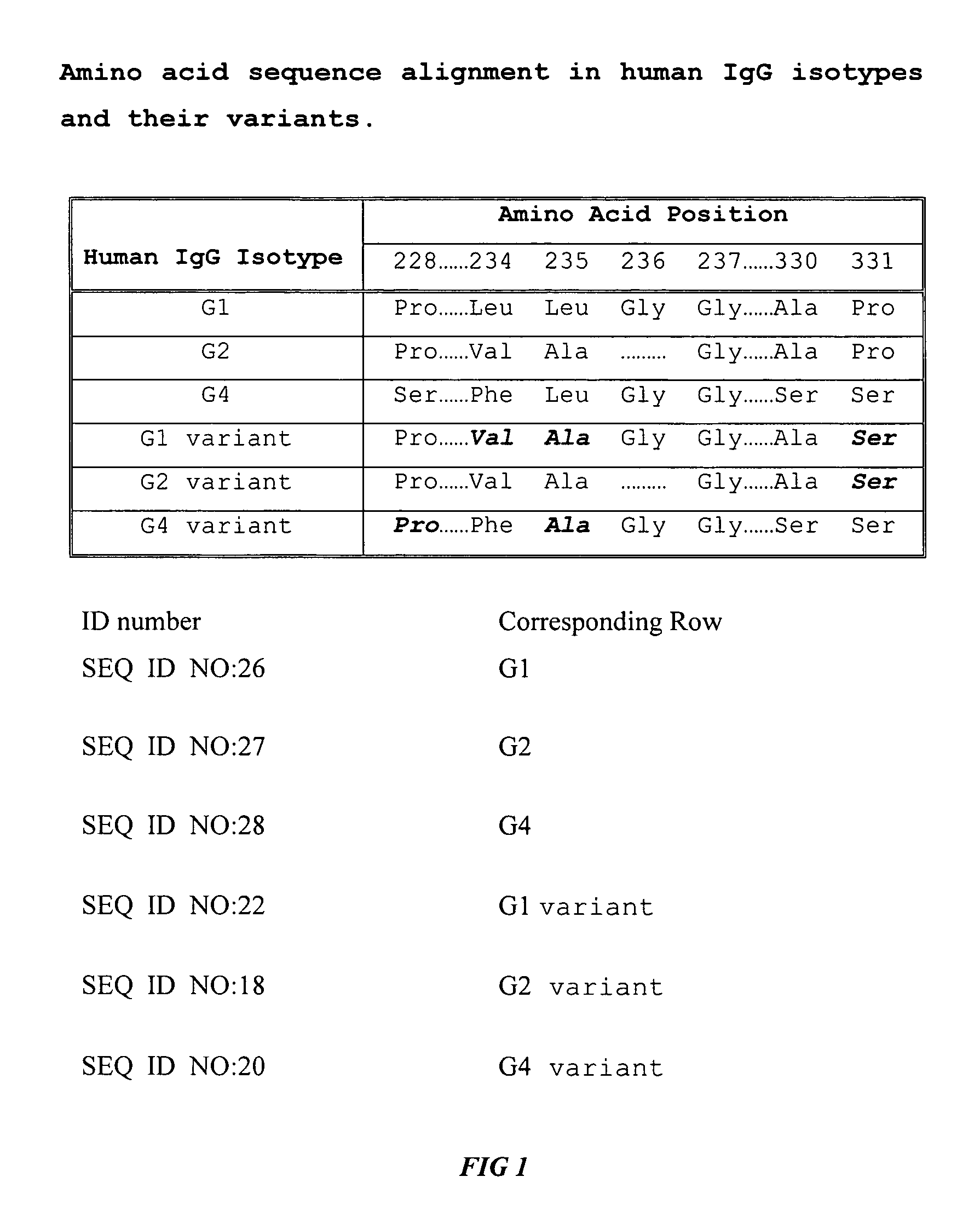
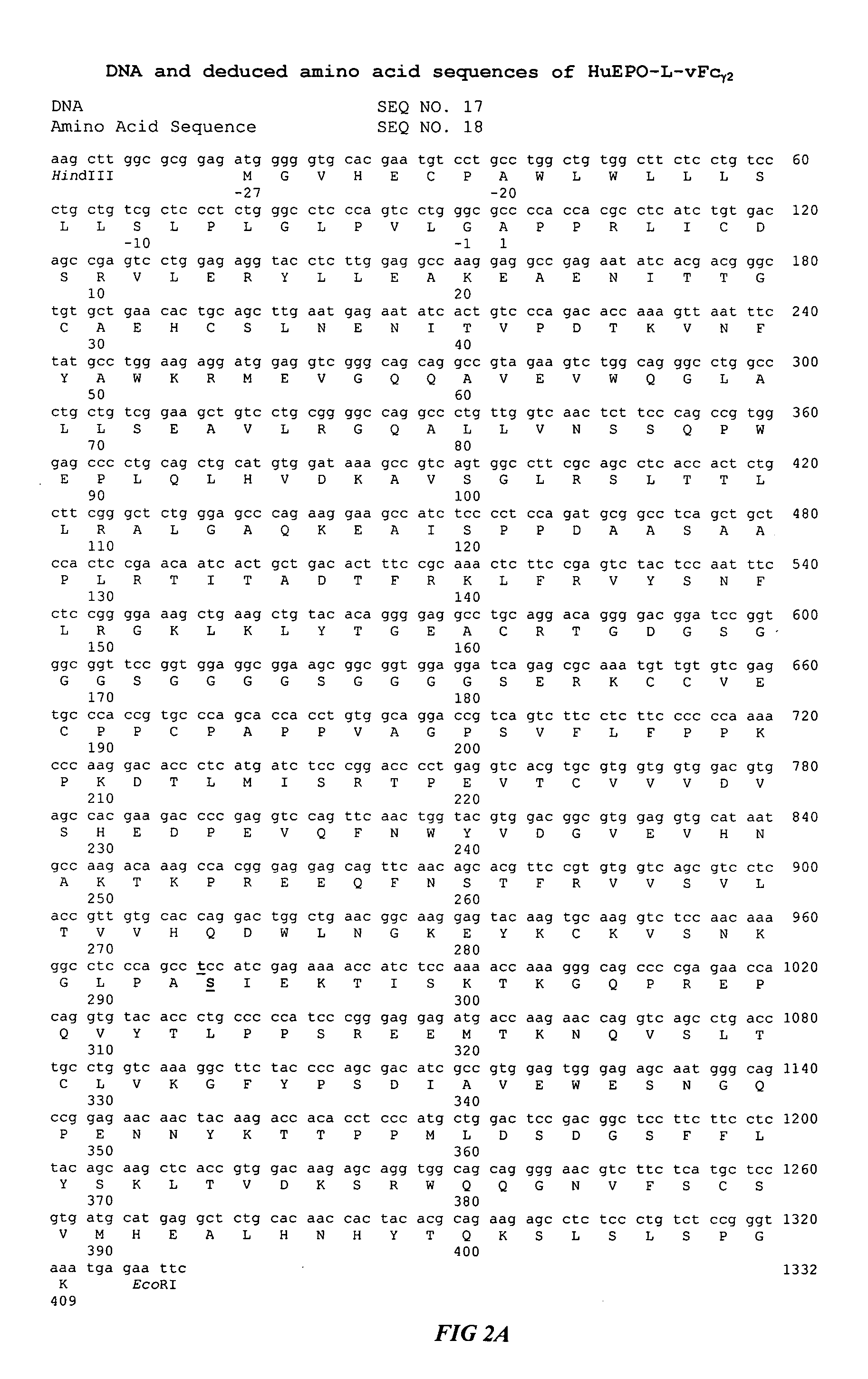
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
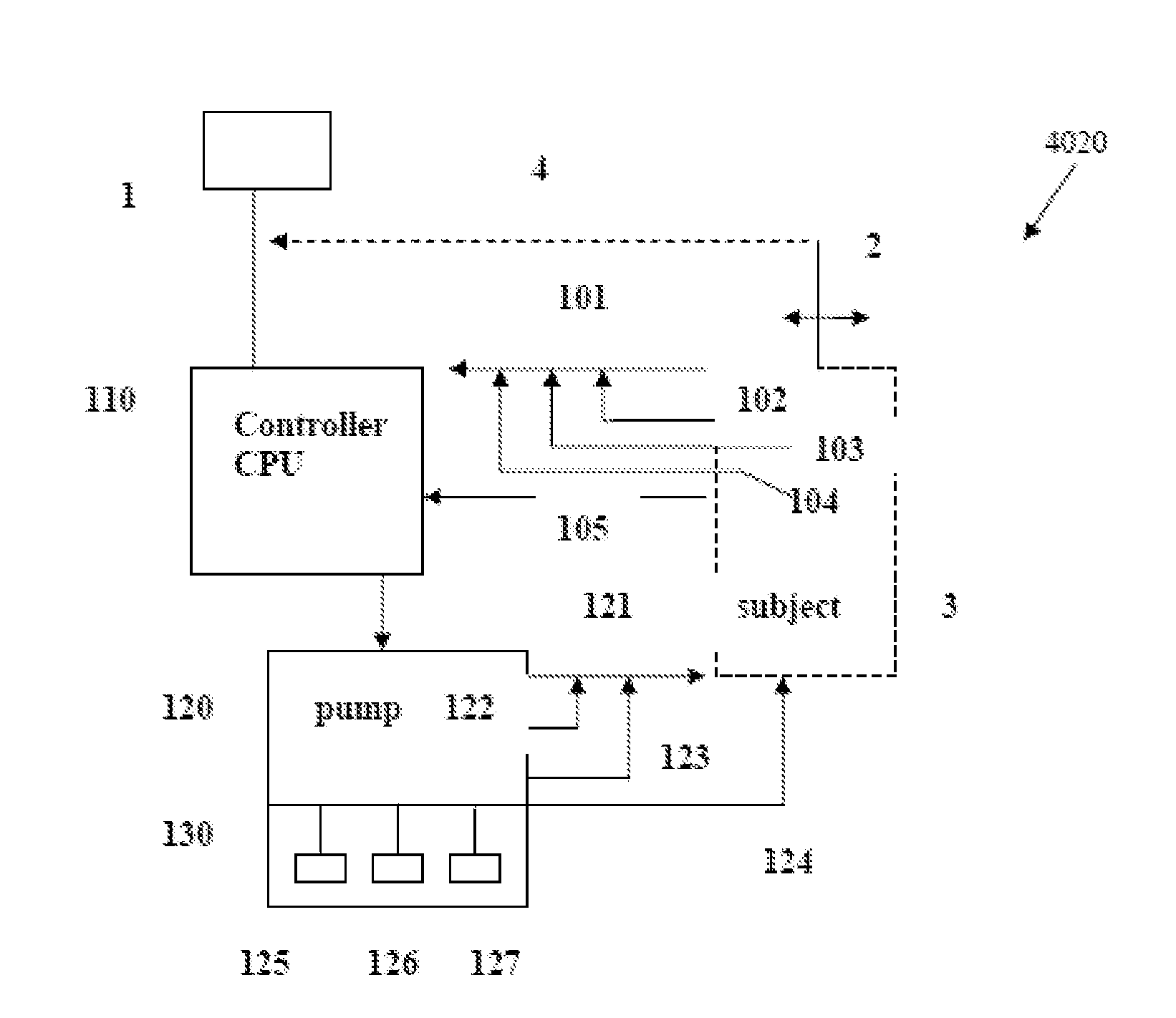
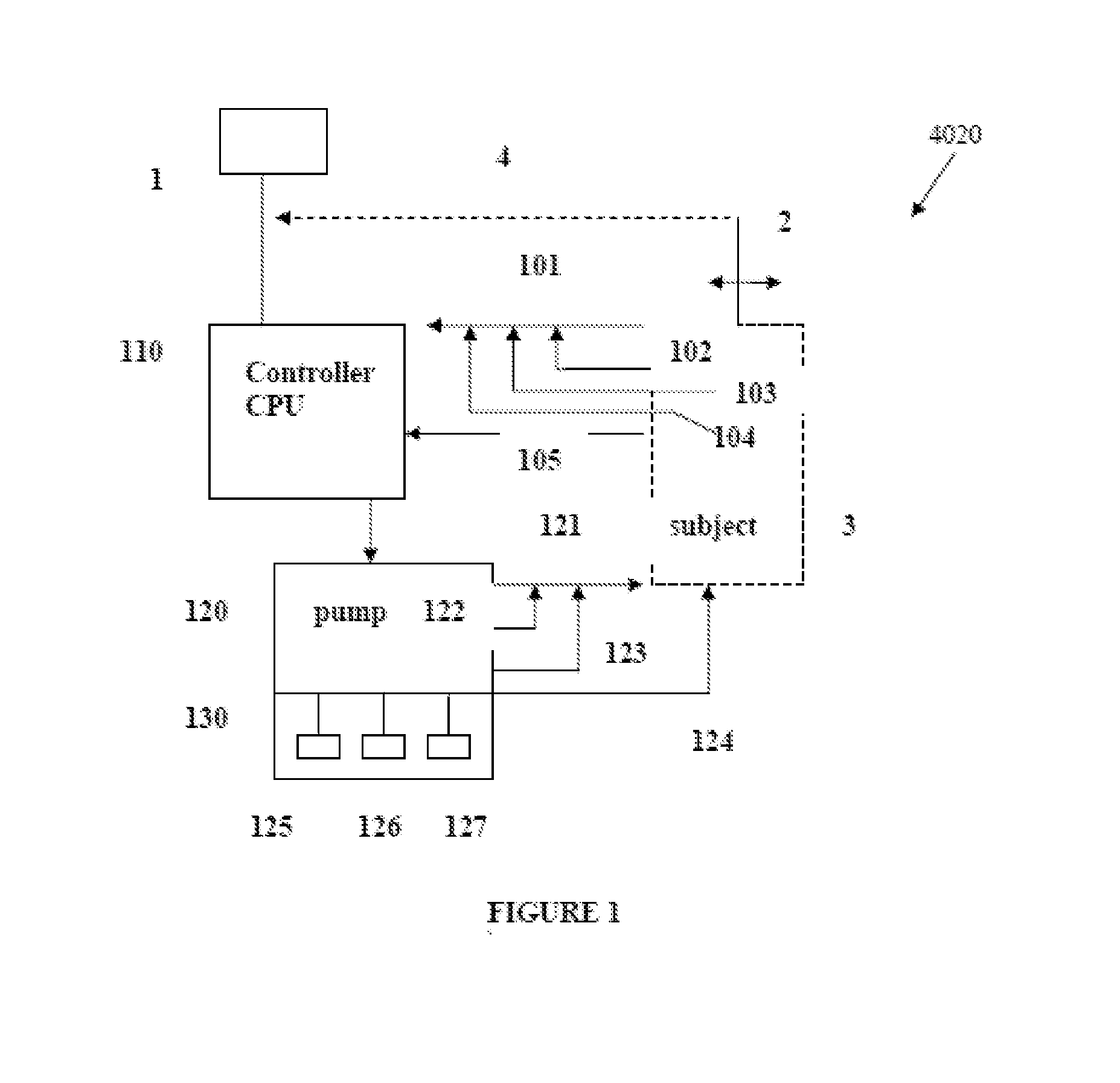
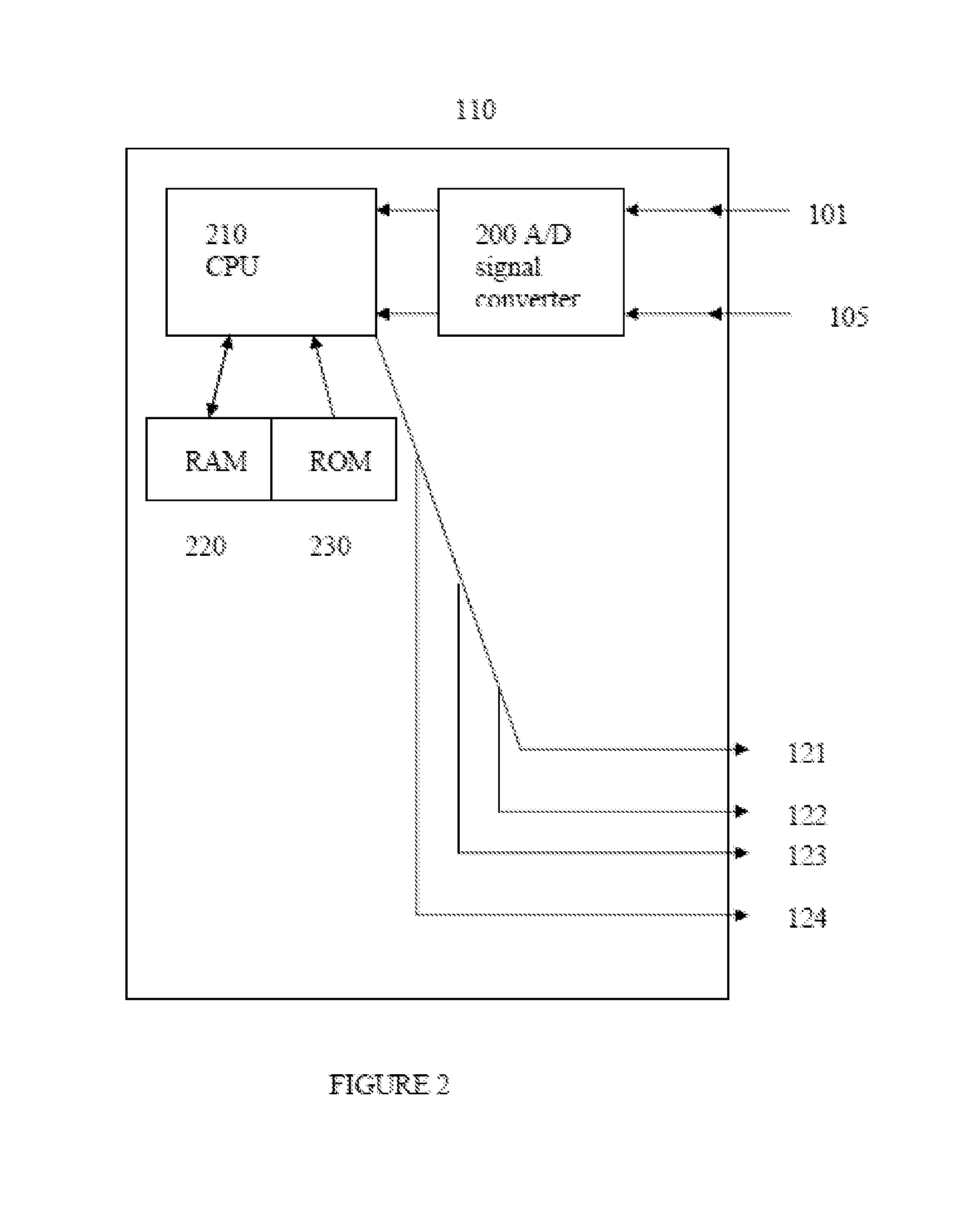
Intelligent Drug and/or Fluid Delivery System to Optimizing Medical Treatment or Therapy Using Pharmacodynamic and/or Pharamacokinetic Data
ActiveUS20130296823A1Improve securityStrengthen security controlData processing applicationsDrug and medicationsPharmacodynamic StudyDelivery system
Owner:XHALE ASSURANCE INC +1
Intra-dermal delivery of biologically active agents
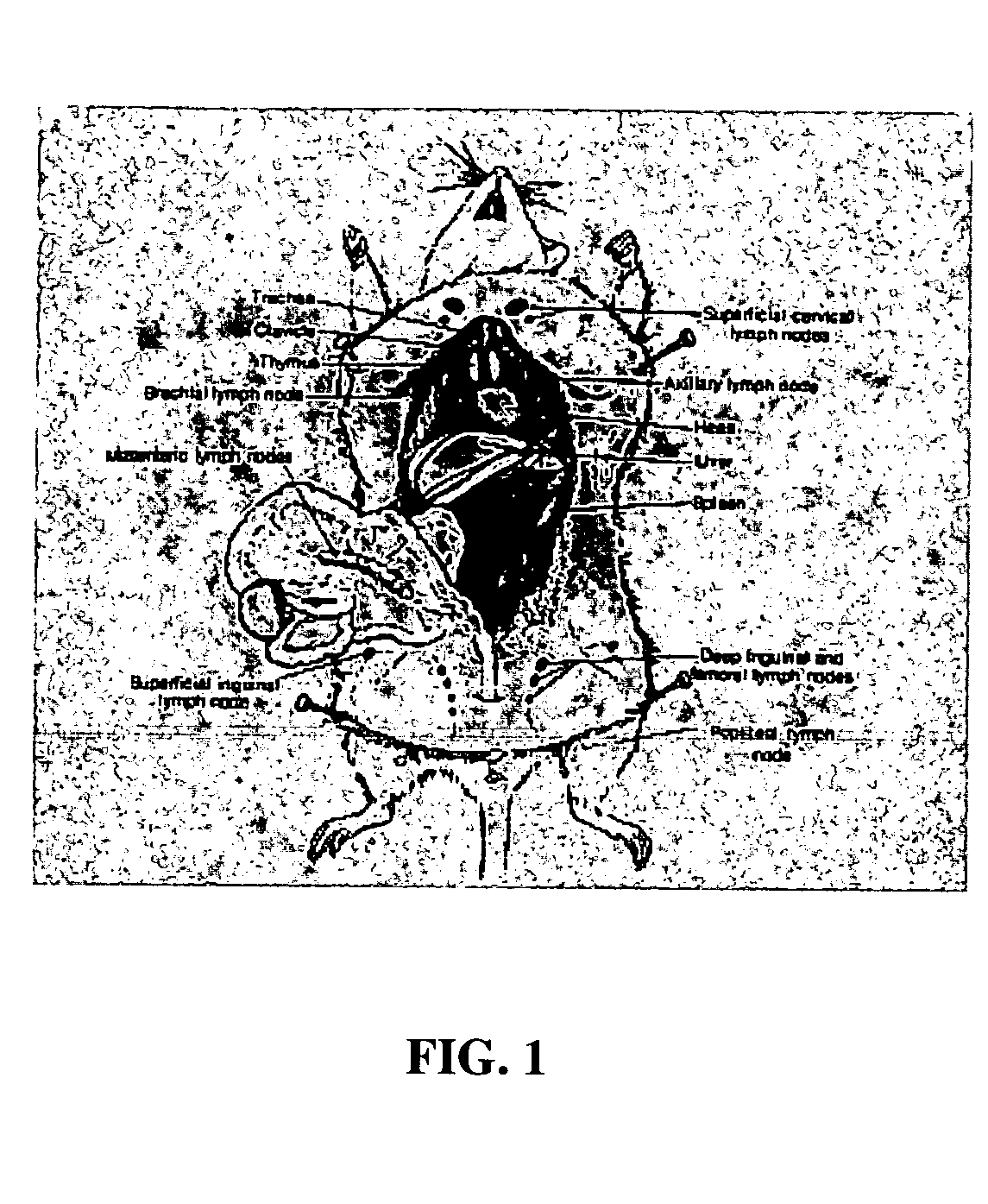
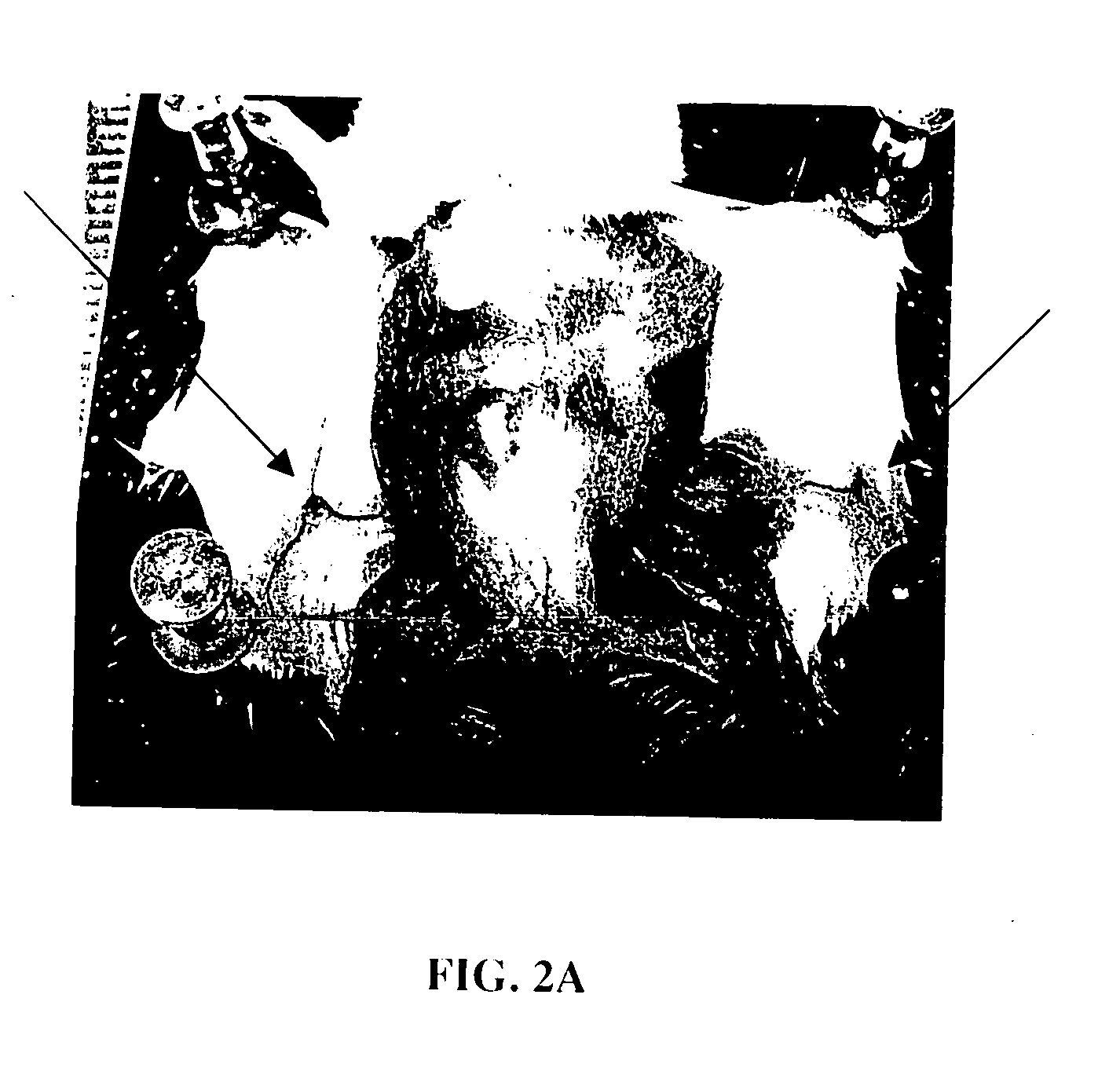
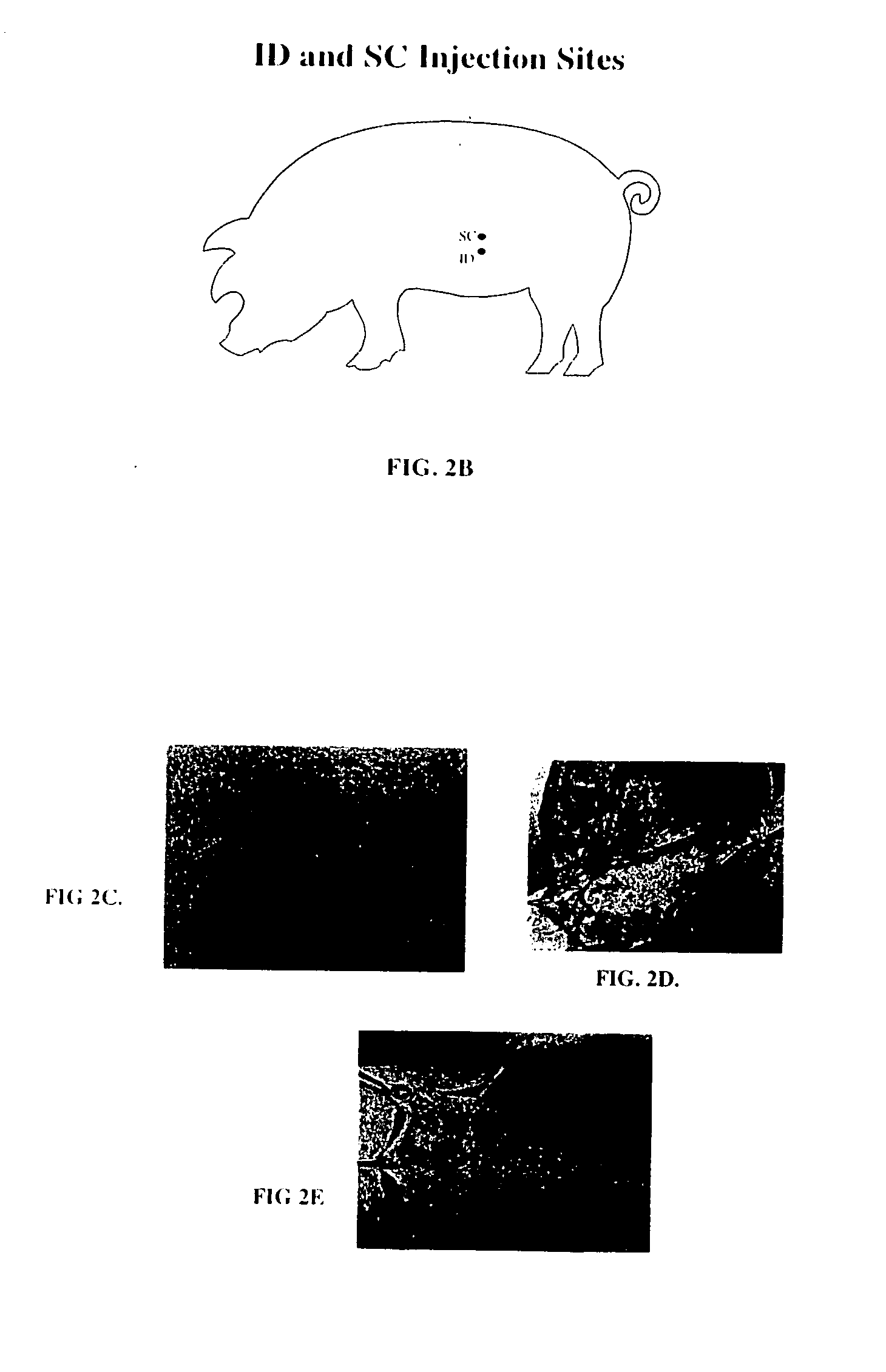
InactiveUS20050163711A1Rapid uptakeFast shippingMetabolism disorderDigestive systemLymphatic vesselDiagnostic agent
The present invention relates to methods and devices for delivering one or more biologically active agents, particularly a diagnostic agent to the intradermal compartment of a subject's skin. The present invention provides an improved method of delivery of biologically active agents in that it provides among other benefits, rapid uptake into the local lymphatics, improved targeting to a particular tissue, improved bioavailability, improved tissue bioavailability, improved tissue specific kinetics, improved deposition of a pre-selected volume of the agent to be administered, and rapid biological and pharmacodynamics and biological and pharmacokinetics. This invention provides methods for rapid transport of agents through lymphatic vasculature accessed by intradermal delivery of the agent. Methods of the invention are particularly useful for delivery of diagnostic agents.
Owner:BECTON DICKINSON & CO
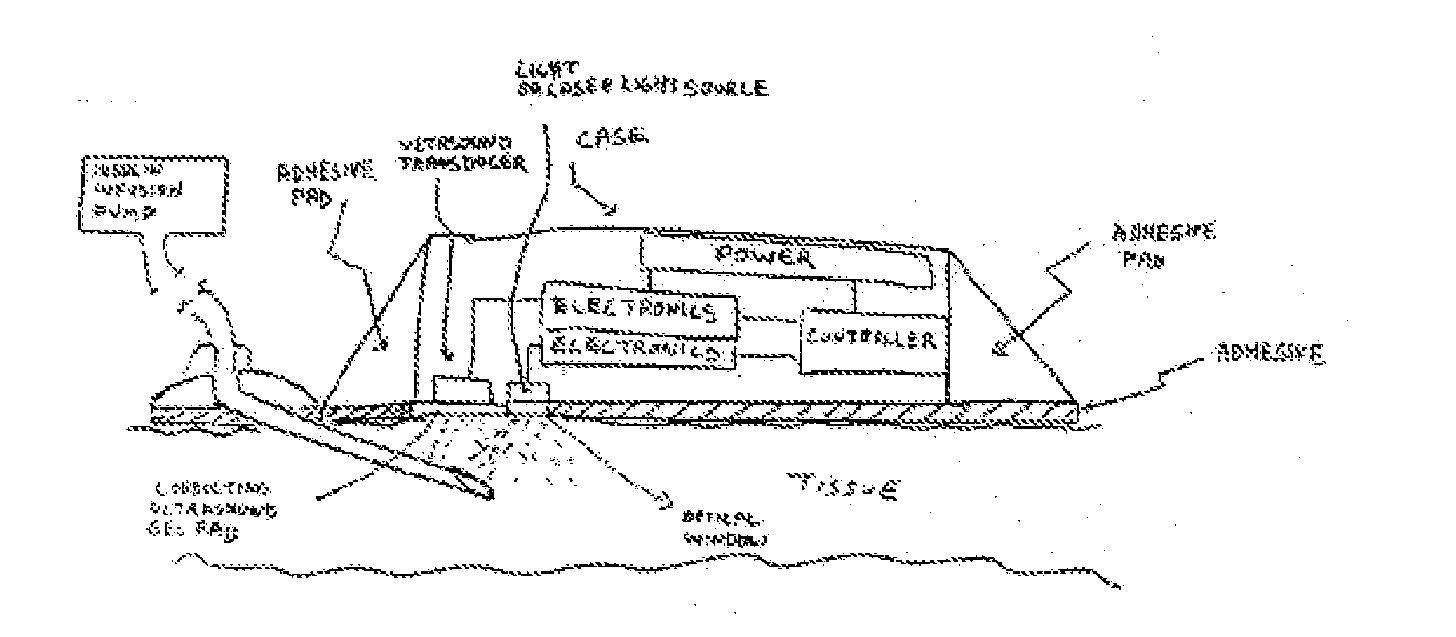
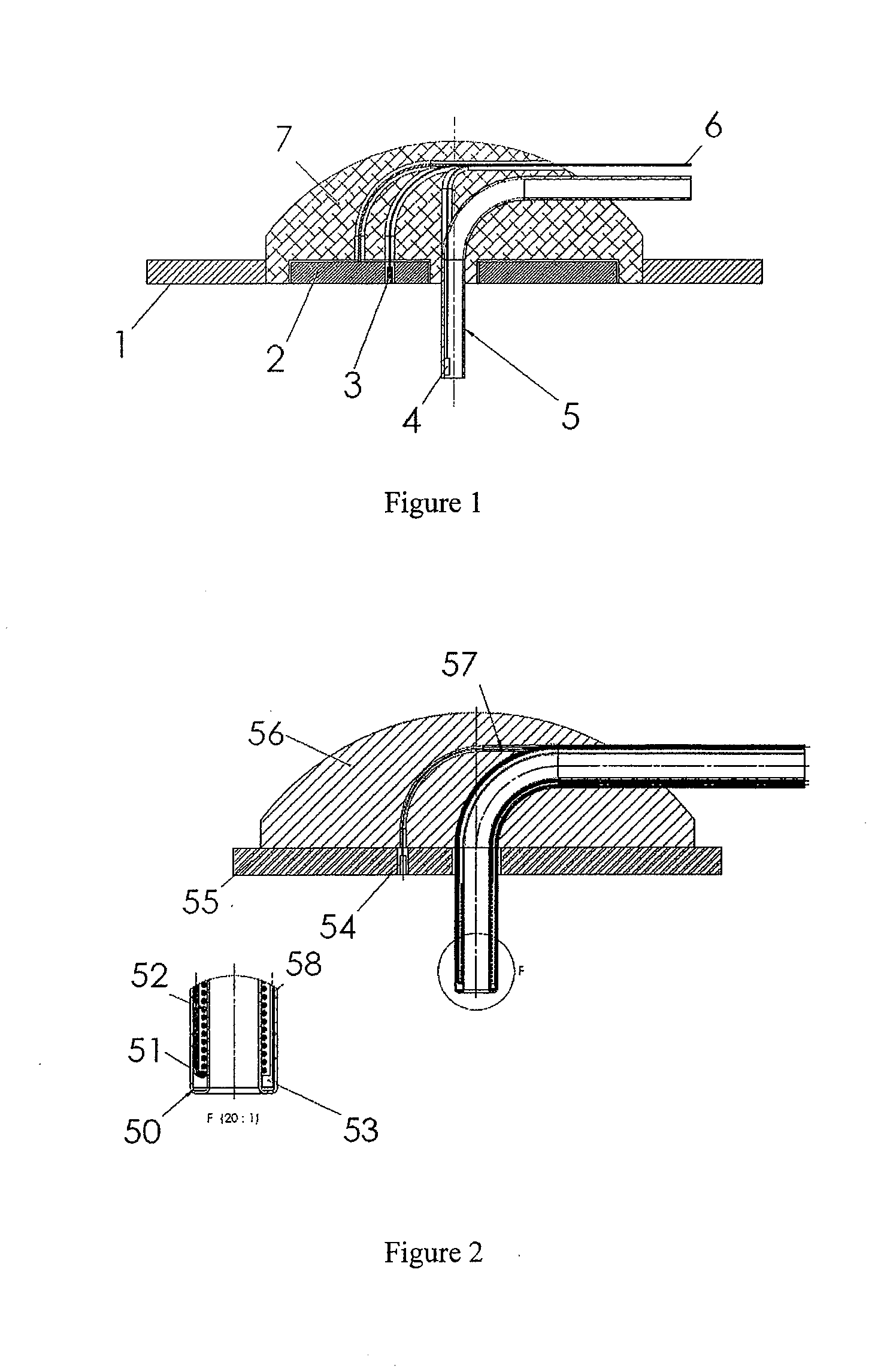
Method and device for drug delivery
ActiveUS20100152644A1Improve efficiencyImprove performanceElectrotherapyPneumatic massageInfusion catheterPharmacodynamic Study
Systems, devices and methods for delivery of a chemical substance to the body of the patient are provided. Such embodiments may include an infusion catheter configured to be inserted into tissue, a catheter securing element configured to be adhered to the skin of the patient and further configured to secure the infusion catheter to the skin, a drug delivery pump configured to infuse a drug into the infusion catheter for delivery to a drug infused region on the body of the patient, and a treatment element configured to apply a treatment to the drug infused region to improve pharmacodynamics of the drug during a period of delivery of the drug to the patient.
Owner:INSULINE MEDICAL
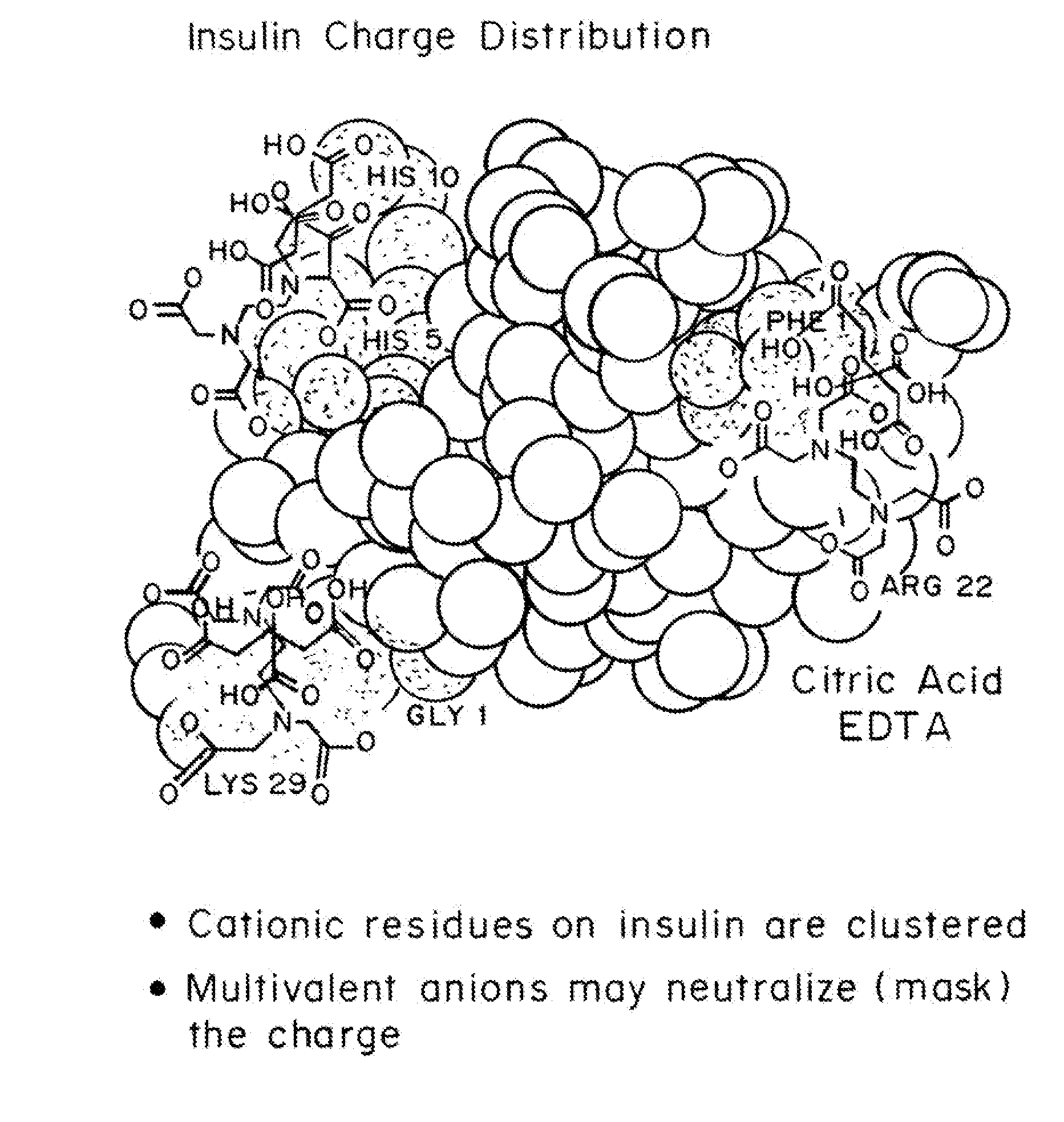
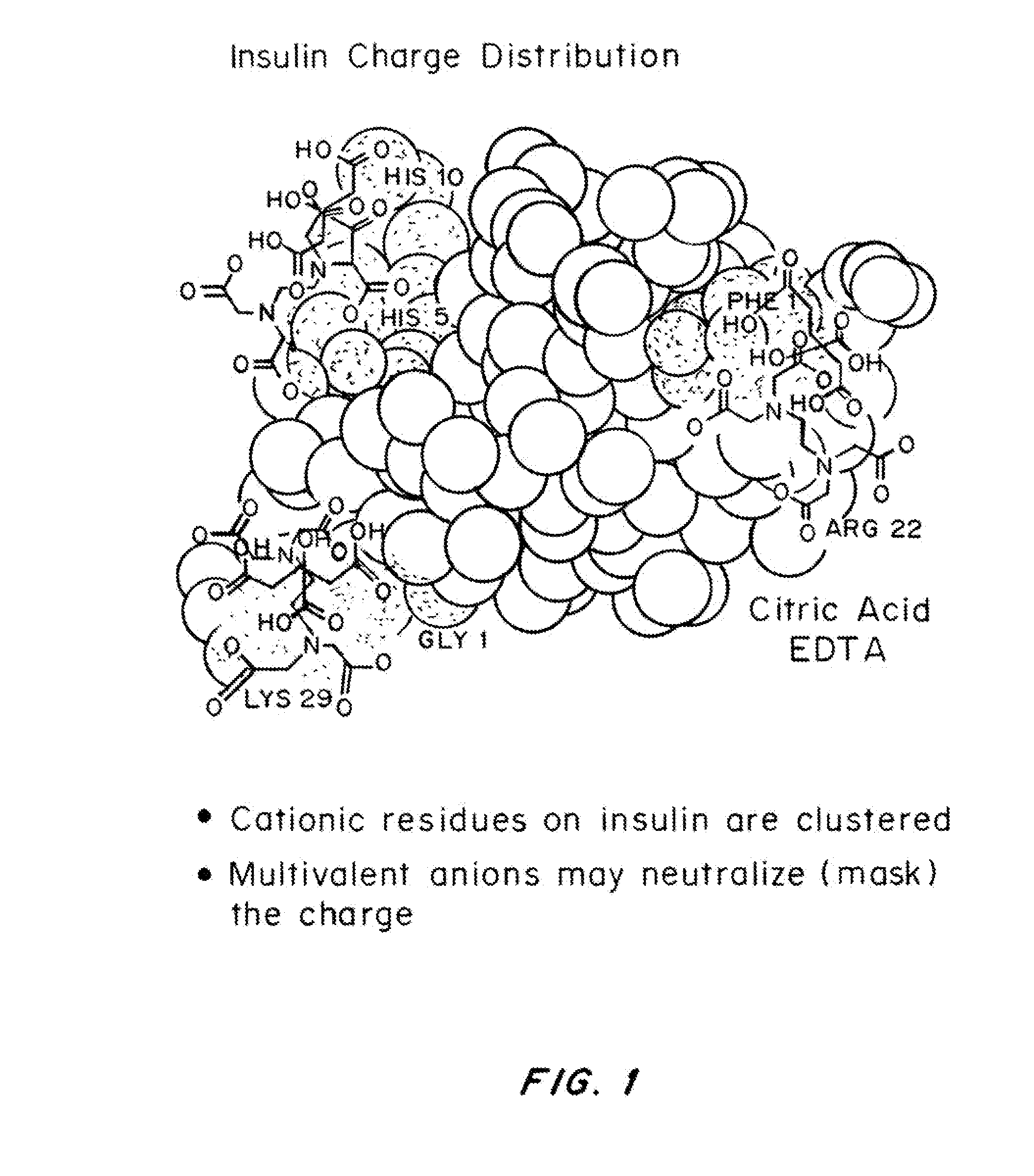
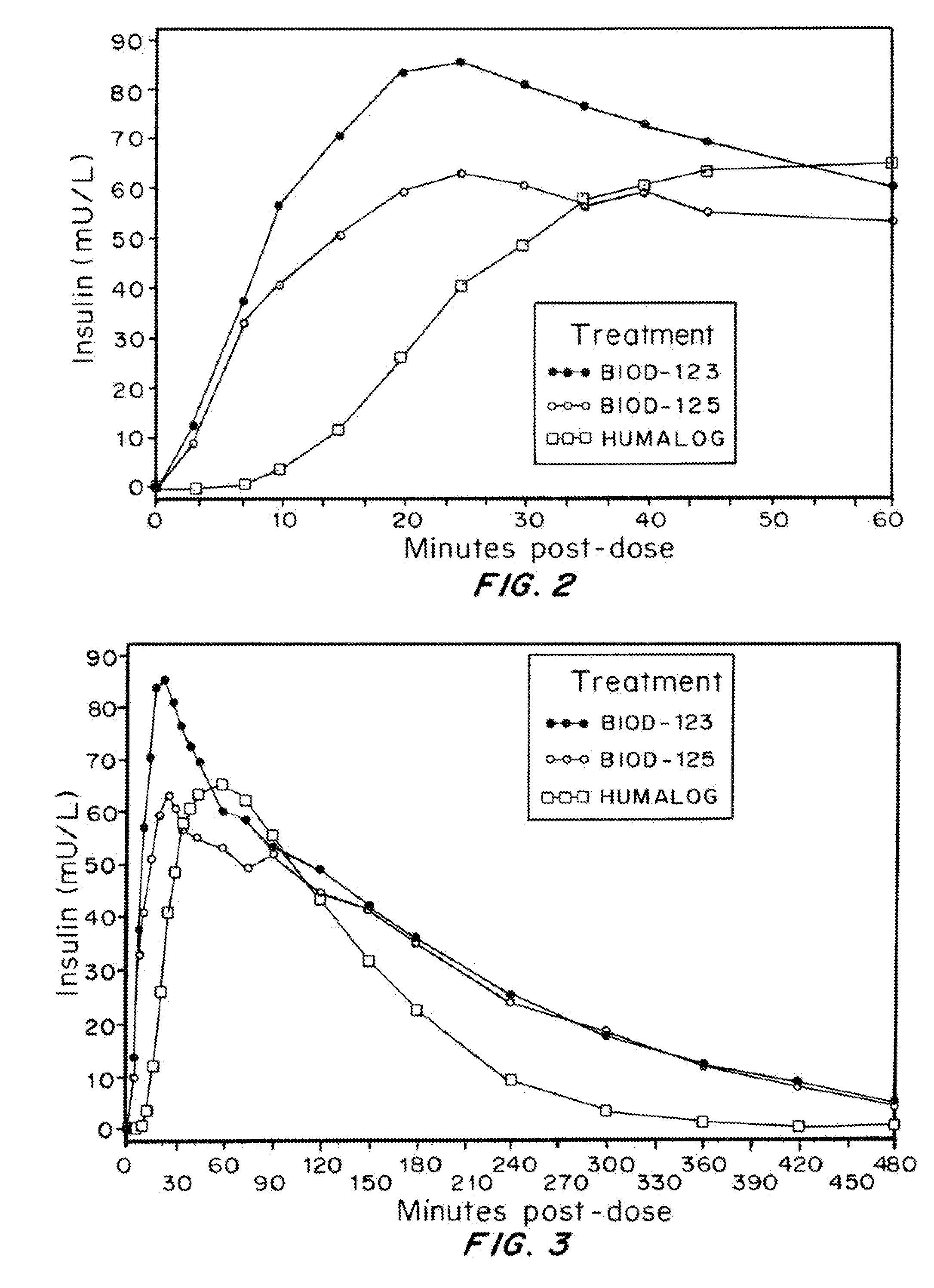
Magnesium Compositions for Modulating the Pharmacokinetics and Pharmacodynamics of Insulin and Insulin Analogs, and Injection Site Pain
ActiveUS20140113856A1Improved injection site tolerabilityPeptide/protein ingredientsMetabolism disorderEthylenediamineMagnesium salt
Compositions and methods for modulating injection site pain associated with rapid acting injectable insulin formulations have been developed for subcutaneous injection. The formulations contain insulin in combination with a zinc chelator such as ethylenediaminetetraacetic acid (“EDTA”), a dissolution / stabilization agent such as citric acid, a magnesium salt, and, optionally, additional excipients. New presentations include rapid acting concentrated insulin formulations and a way to enhance the absorption of commercially available rapid acting analog formulations by mixing them with a vial containing dry powder excipients that accelerate their absorption. Devices for mixing excipient and insulin together at the time of administration, while minimizing residence time of the mixture, are also described.
Owner:ELI LILLY & CO
Quick release type medicine phosphatide compound and medicine composition thereof
InactiveCN105457038AEnhance hydrophobic lipophilic effectImprove hydrophobicityHeavy metal active ingredientsDipeptide ingredientsPharmacodynamic StudyHydrophobic effect
The invention discloses a quick release type medicine phosphatide compound and a medicine composition thereof. In the compound, a medicine Y1 and a medicine Y2 are connected with a quick release type hydrophobic spacer arm and an enhancement type hydrophobic spacer arm through chemical bonds, the two spacer arms are cooperated to enhance the hydrophobic effect to form hydrophobic lipotropy chains, the two hydrophobic chains are connected with a phospholipid hydrophilic head to form an amphipathic molecule. The medicine composition is the quick release type medicine phosphatide compound or a combined medicine composition of the compound and a carrier acceptable in pharmacodynamics. The quick release type medicine phosphatide compound and lipidosome nano particles thereof can be used as a liquid preparation, a solid preparation, a semi-solid preparation, a sterilization preparation and a sterile preparation, are low in toxicity and can be used for efficiently treating various tumor diseases.
Owner:SOUTHEAST UNIV
Antimicrobial polymer conjugates
InactiveUS20070292404A1Improve propertiesLow immunogenicityAntibacterial agentsPeptide/protein ingredientsMicrobial agentWater soluble
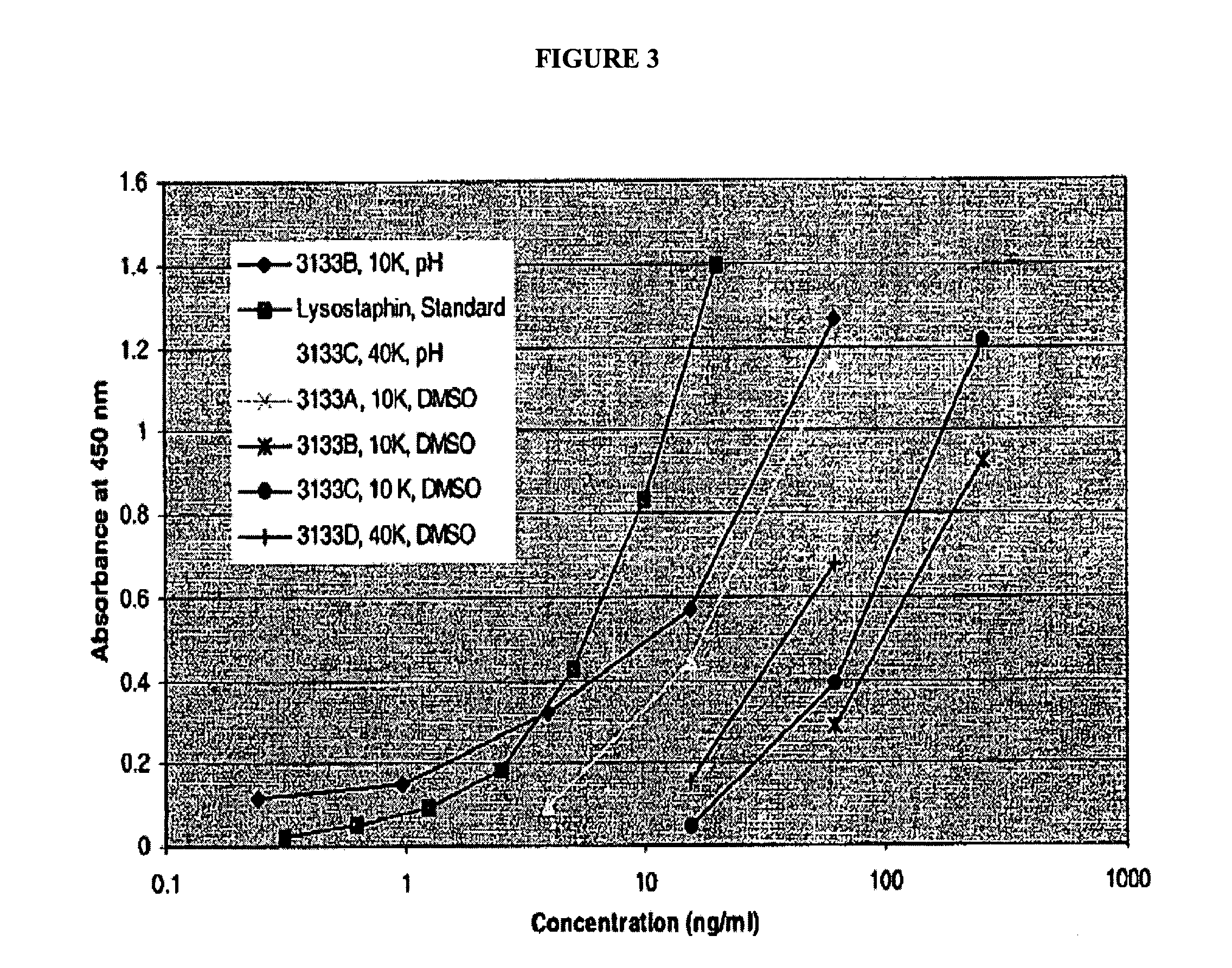
The present invention relates to the conjugation of antimicrobial agents to water-soluble polymers to improve their clinical properties in terms of their pharmacokinetics, pharmacodynamics, and reduced immunogenicity. More specifically, the present invention relates to the conjugation of antimicrobial agents such as lysostaphin to poly(alkylene oxides), such as poly(ethylene glycol) (PEG).
Owner:BIOSYNEXUS INC
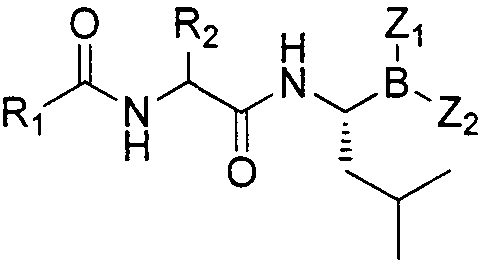
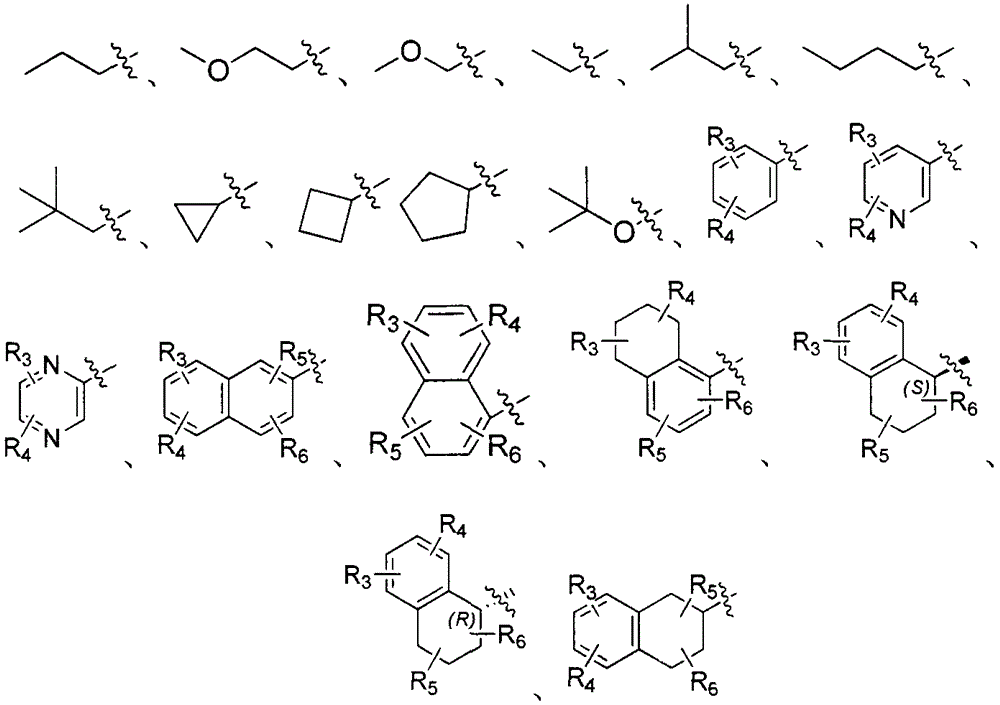
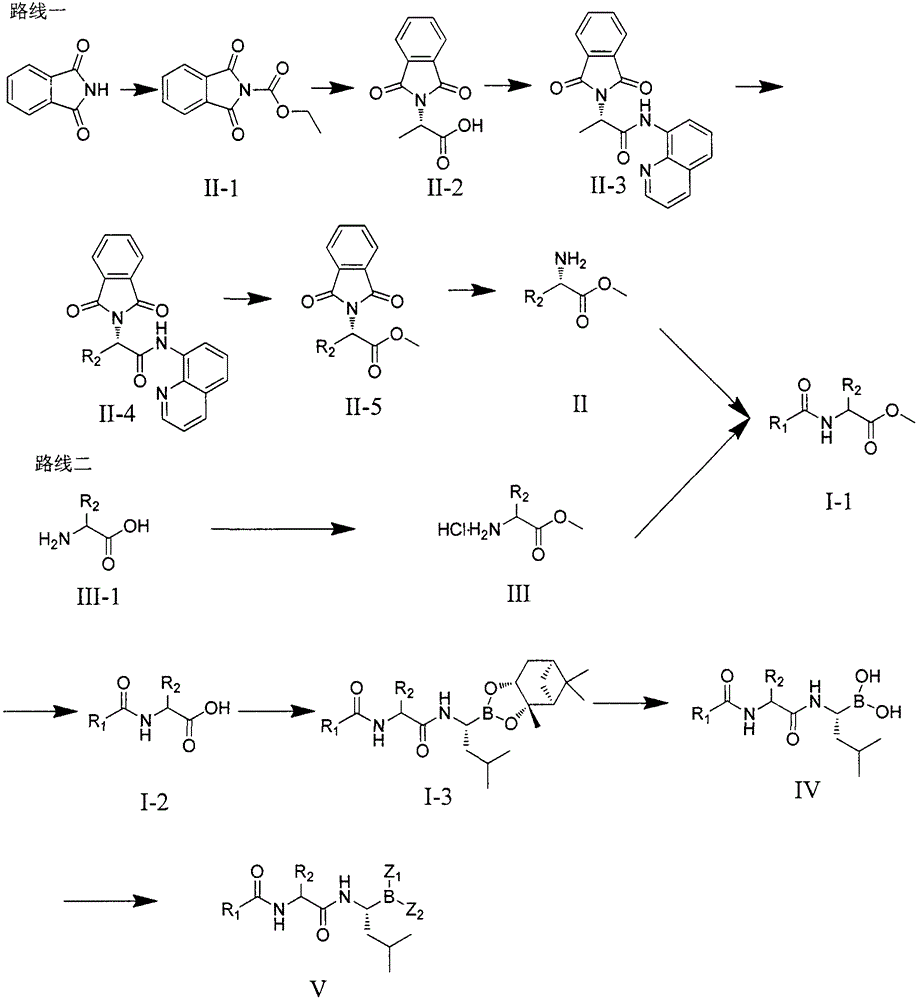
Dipeptide boric acid composed of carboxylic acid and alpha-amino acid as well as ester compound thereof, and preparation method and application of dipeptide boric acid and ester compound thereof
ActiveCN105732683AHigh yieldHigh activityBoron compound active ingredientsGroup 3/13 element organic compoundsProstate cancerProteasome inhibitor
The invention belongs to the field of drug synthesis and in particular relates to a series of novel peptide boric acids as well as an ester compound or pharmaceutical salt thereof, and a preparation method and application of the peptide boric acids as well as the ester compound or pharmaceutical salt thereof in pharmacodynamics. A structure of the peptide boric acid and the ester compound or pharmaceutical salt thereof is shown in a formula I (described in the specification). The compound provided by the invention can be used for preparing a proteasome inhibitor and can further be used for treating solid tumours and blood tumours, wherein the solid tumours are selected from non-small cell lung cancer, small cell lung cancer, lung adenocarcinoma, lung squamous carcinoma, pancreatic cancer, breast cancer, prostate cancer, liver cancer, skin cancer, epithelial cell cancer, gastrointestinal stromal tumor, nasopharynx cancer and leukemia; and the blood tumours are selected from multiple myeloma, mantle cell lymphoma and histiocytic lymphoma.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Traditional Chinese medicine preparation for treating fractures and bone injuries
InactiveCN101623415AReactivity NoneNo side effectsPowder deliveryHeavy metal active ingredientsMyrrhSide effect
The invention relates to a traditional Chinese medicine preparation for treating fractures and bone injuries. The traditional Chinese medicine preparation is characterized by being prepared from the following raw material medicines: radix notoginseng, draconis sanguis, woodlouse, safflower, peach kernel, eucommia bark, pyrite, teasel root, rhizoma cyperi, mastic, myrrh, white paeony root, rhizoma ligustici wallichii, angelica, earthworm, twotooth achyranthes root, prepared rehmannia root, astragalus root and rhizoma drynariae. The preparation method comprises the following steps: mixing the raw material medicines together, grinding the raw material medicines into a powder shape, sieving the powder into the granularity of 180 meshes, and preparing traditional Chinese medicine medicinal powder. By the clinical test of many years, the invention forms a treating prescription for treating the fractures and the bone injuries by researching the function and indication of each kind of medicine according to modern traditional Chinese medicine pharmacodynamics, carries out the clinical tests on more than one thousand cases of patients with the fractures and the bone injuries and has the effects of promoting blood circulation, relieving the pain, decreasing swelling, eliminating blood stasis, recovering wounds, promoting tissue regeneration, setting bone and treating injuries. The invention can dissipate blood stasis, promote blood circulation, diminish inflammation and alleviate pain, also has the function of tranquilizing the mind and has short treatment course, high curative effect, low treating expense and no adverse reaction and side effect, the cure rate reaches more than 90%, and the curative effect is very satisfactory to people.
Owner:葛超顺
Dracocephalum moldavica extract and dracocephalum moldavica dropping pills, and method of preparing the same
InactiveCN101219161AEasy to operateEasy to usePill deliveryCardiovascular disorderClinical efficacyDracocephalum moldavica
The invention relates to a moldavica dragonhead extract, a moldavica dragonhead dropping pill and a production method thereof, wherein, the moldavica dragonhead extract contains higher total flavonoids and luteolin and the production method of the moldavica dragonhead extract is simply operated. Pharmacodynamics test result indicates that the moldavica dragonhead dropping pill acquired from the invention has excellent curative effect to rat ischemia myocardial injury; each dose group of the moldavica dragonhead dropping pill has varying degrees of protection; Composite salviae dropping pill has similar effect with the middle-dose group of moldavica dragonhead dropping pill; all dose groups of the moldavica dragonhead dropping pill have better curative effect than Yixing Badiranjibuya Keli at ischemia myocardial; dosages of middle-dose group of moldavica dragonhead dropping pill and low-dose group of moldavica dragonhead dropping pill are only 50 percent and 25 percent of the dosage of Yixing Badiranjibuya Keli respectively, thereby greatly improving the compliance of sufferers. The moldavica dragonhead dropping pill acquired from the invention is a novel preparation of convenient use and good compliance, thus bringing into play better clinical curative effect of the moldavica dragonhead, an age-old Uighur medicinal material. The production method of the moldavica dragonhead dropping pill can be carried out easily.
Owner:XINJIANG INST OF MATERIA MEDICA
Medicine for treating bronchitis
InactiveCN101385848ASmall toxicityLow cost of treatmentRespiratory disorderPlant ingredientsVerbenaSide effect
The invention provides a medicine for treating bronchitis, which is prepared by taking the following traditional Chinese medicines as raw materials: boat-fruited stercrlia seed, bulbus fritillariae cirrhosae, tuber pinellia, dried orange peel, european verbena herb, shizandra berry, asarum, radix stemonae, white mulberry root-bark, folium eriobotryae, radix cynanchi, balloon flower, glossy privet fruit, common coltsfoot flower, liquorice, lily, fructus momordicae, heartleaf houttuynia herb, cassia twig, ophiopogon root, dried ginger, polygonatum and almond. The medicine is obtained after being tested by pharmacology and pharmacodynamics and has the efficacies on relieving cough and eliminating phlegm and preventing asthma. The prescription of the medicine in the invention is reasonable, the toxicity and side effect of the medicine is low, and the medicine for treating bronchitis has the advantages of low treatment expense, short course of treatment, rapid curative effect and high radical treatment rate. Besides, the extensive popularization of the machine can lead to good social benefit.
Owner:吴伶
7 and 20 dehydro-silybin dialky ether and preparation method and medicine use thereof
InactiveCN101565419AStrong anti-lipid peroxidationProtects chelation abilityOrganic active ingredientsNervous disorderDiseaseSuperoxide
The invention relates to a 7 and 20 dehydro-silybin dialky ether and preparation method and medicine use thereof. The compounds are capable of obviously preventing the lipid superoxide induced by the free radical from being generated; effectively protecting the injury rat adrenal pheochromocytoma cells PC12 due to hydrogen dioxide, namely with oxidation resistance injury protection function on thePC12 cells which simulate the cranial nerves; preventing the brain cells and the cranial nerves from being oxidized and controlling the neurodegenerative diseases such as senile dementia. In addition, the compounds have strong chelation on the ferrous ion. The pharmacodynamics result shows that: the compounds are anticipated to prepare the medicine for controlling the neurodegenerative diseases.
Owner:DALI UNIV
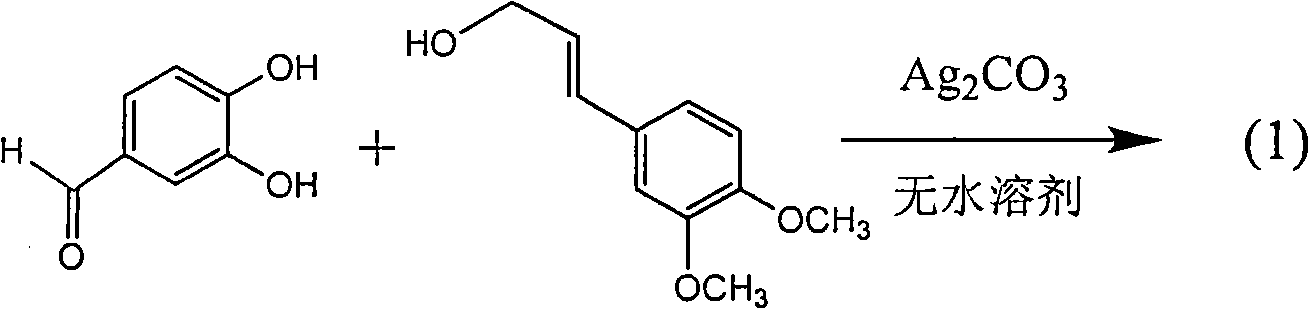
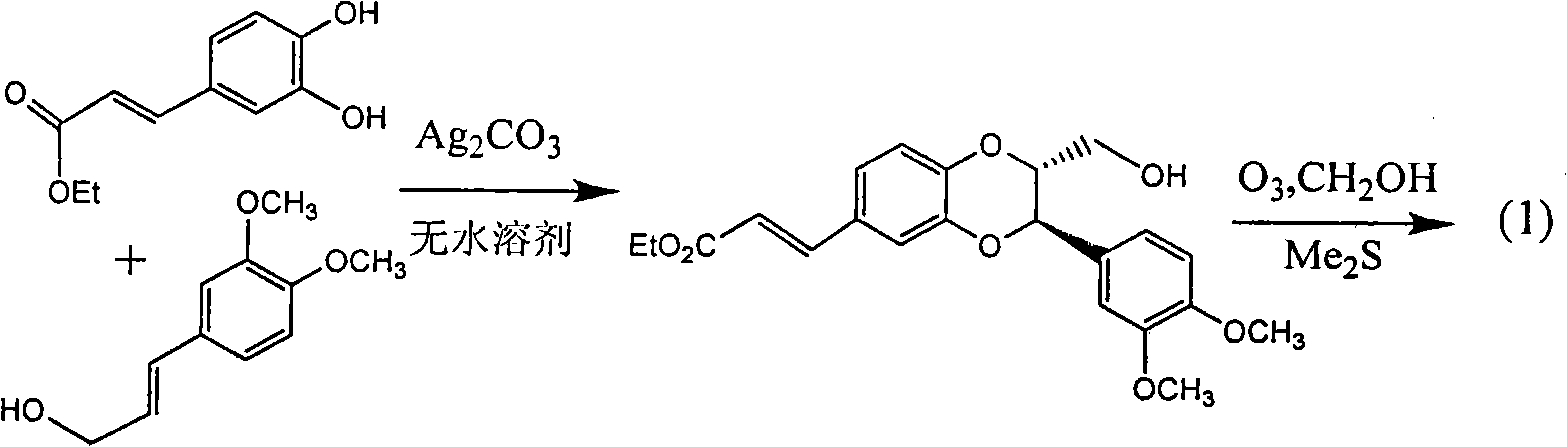
Application of benzo-phenylpropanoids in preparing drug for treating viral hepatitis B
InactiveCN101829093AEnhanced inhibitory effectExact originalityOrganic active ingredientsAntiviralsHigh concentrationDisease
The invention relates to an application of benzo-phenylpropanoids in preparing drugs for treating viral hepatitis B, in particular to two benzo-phenylpropanoids or application of pharmaceutically acceptable salt thereof in preparing drugs for inhibiting the replication of hepatitis B virus desoxyribonucleic acid (HBV DNA) and treating hepatitis B virus infection diseases. The two benzo-phenylpropanoids definitely inhibit the activity of the HBV DNA, have the replication inhibition activity on the HBV DNA at high dose (100 microgrammes / milliliter) of 1.3-2.2 times higher than the inhibition activity at the highest concentration (10000 units / milliliter) of an alpha-interferon and belong to an efficient non-nucleoside natural product inhibiting the hepatitis B viruses; pharmacodynamics results show the application of the benzo-phenylpropanoids or the pharmaceutically acceptable salt thereof capable of preparing the drugs for inhibiting the replication of the HBV DNA and treating the hepatitis B virus infection diseases in anticipation.
Owner:DALI UNIV
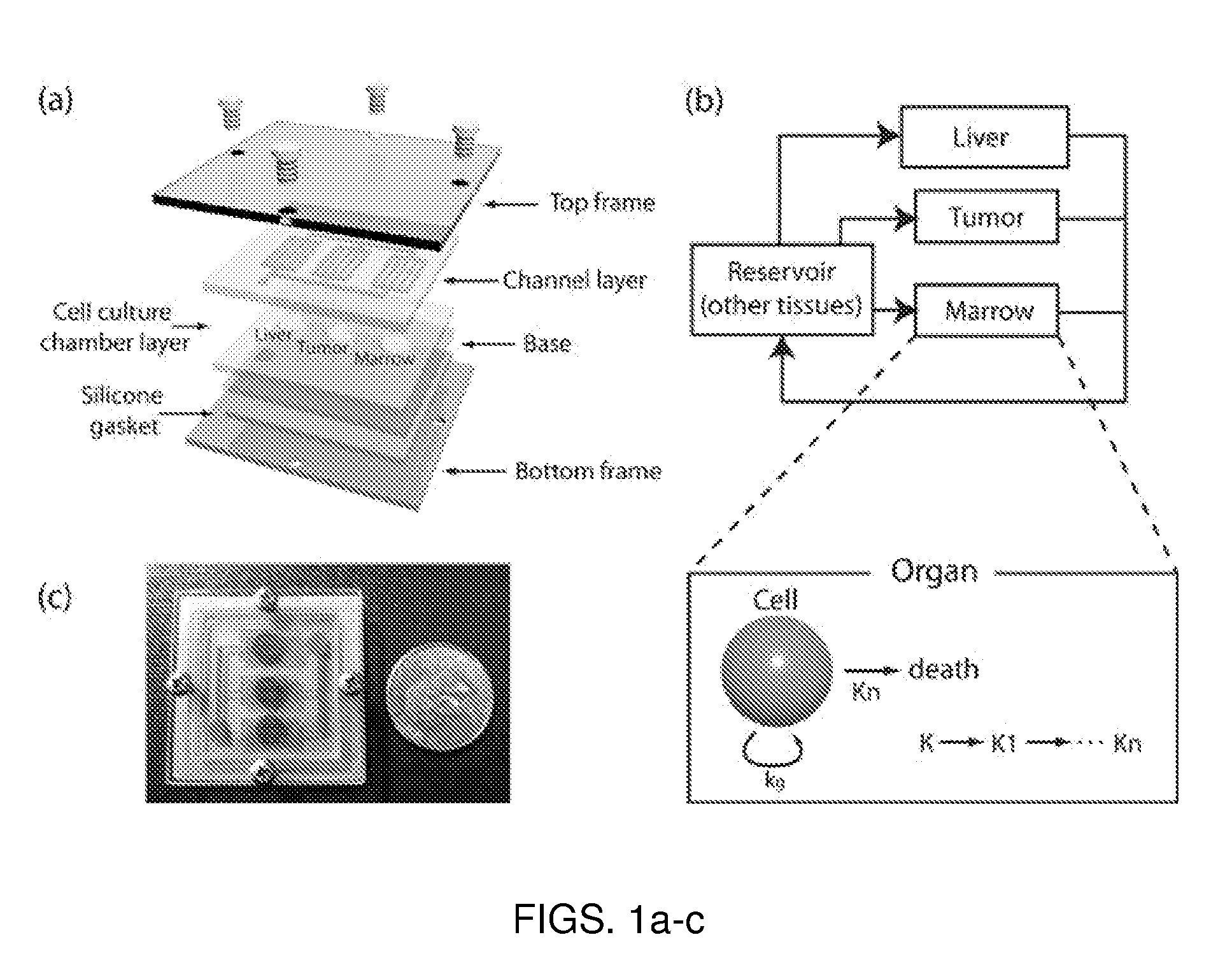
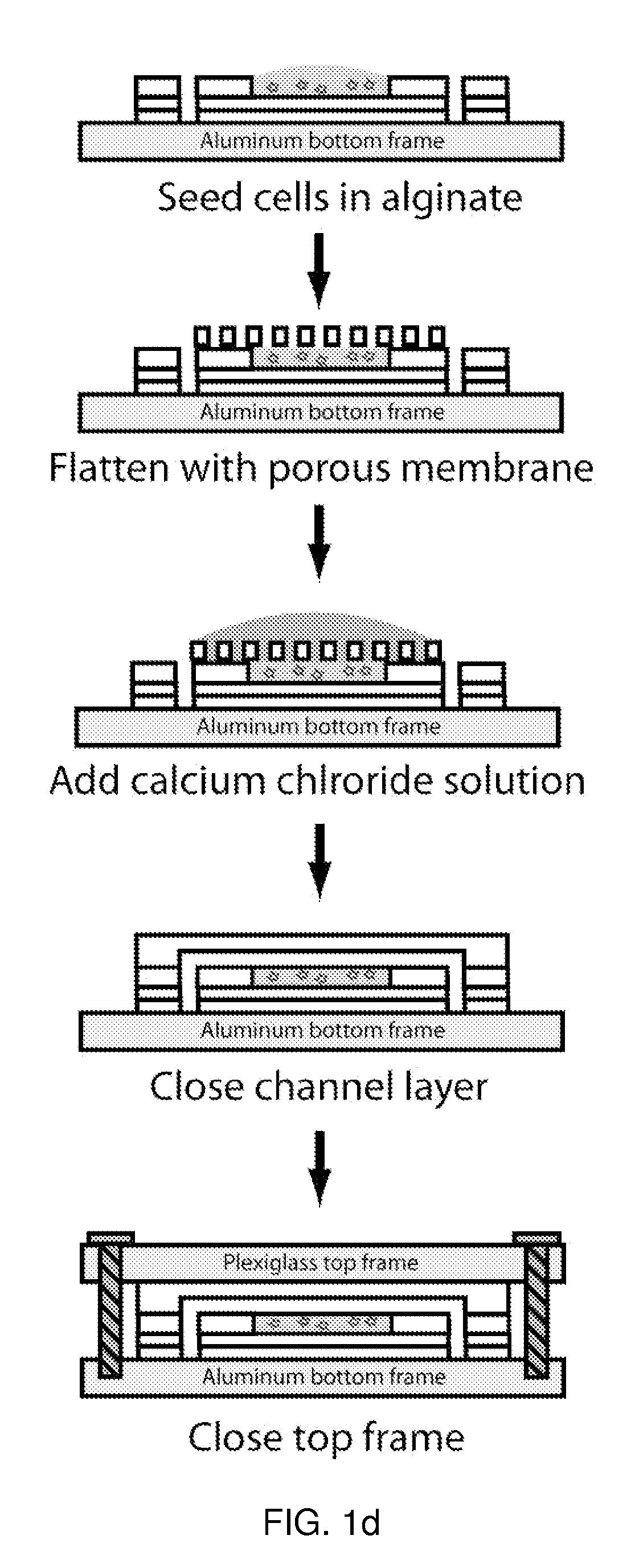
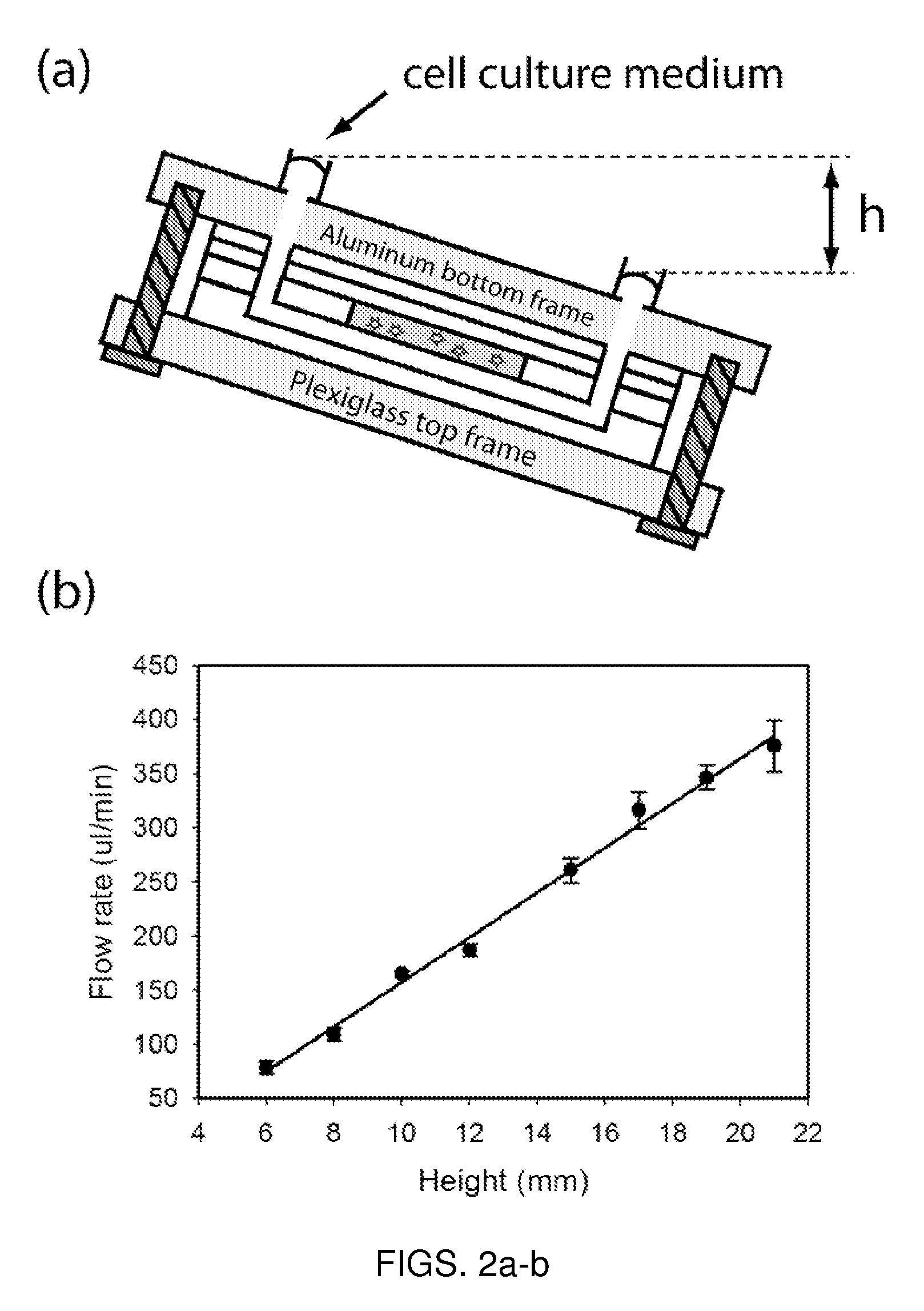
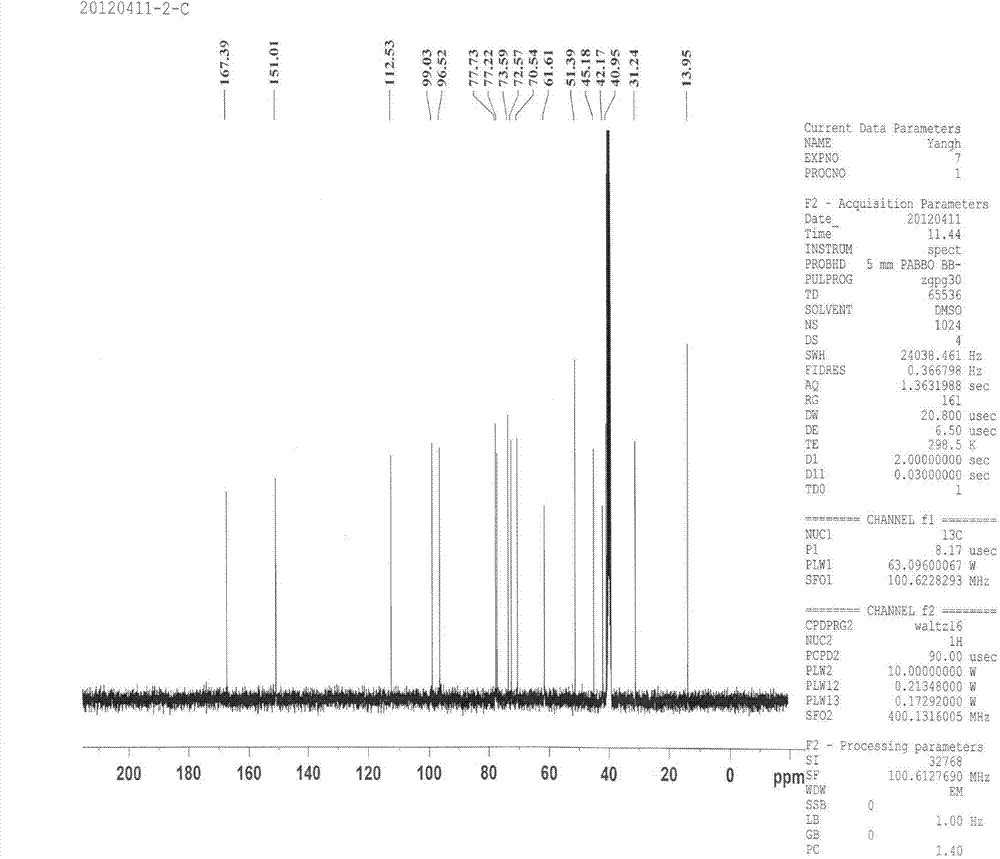
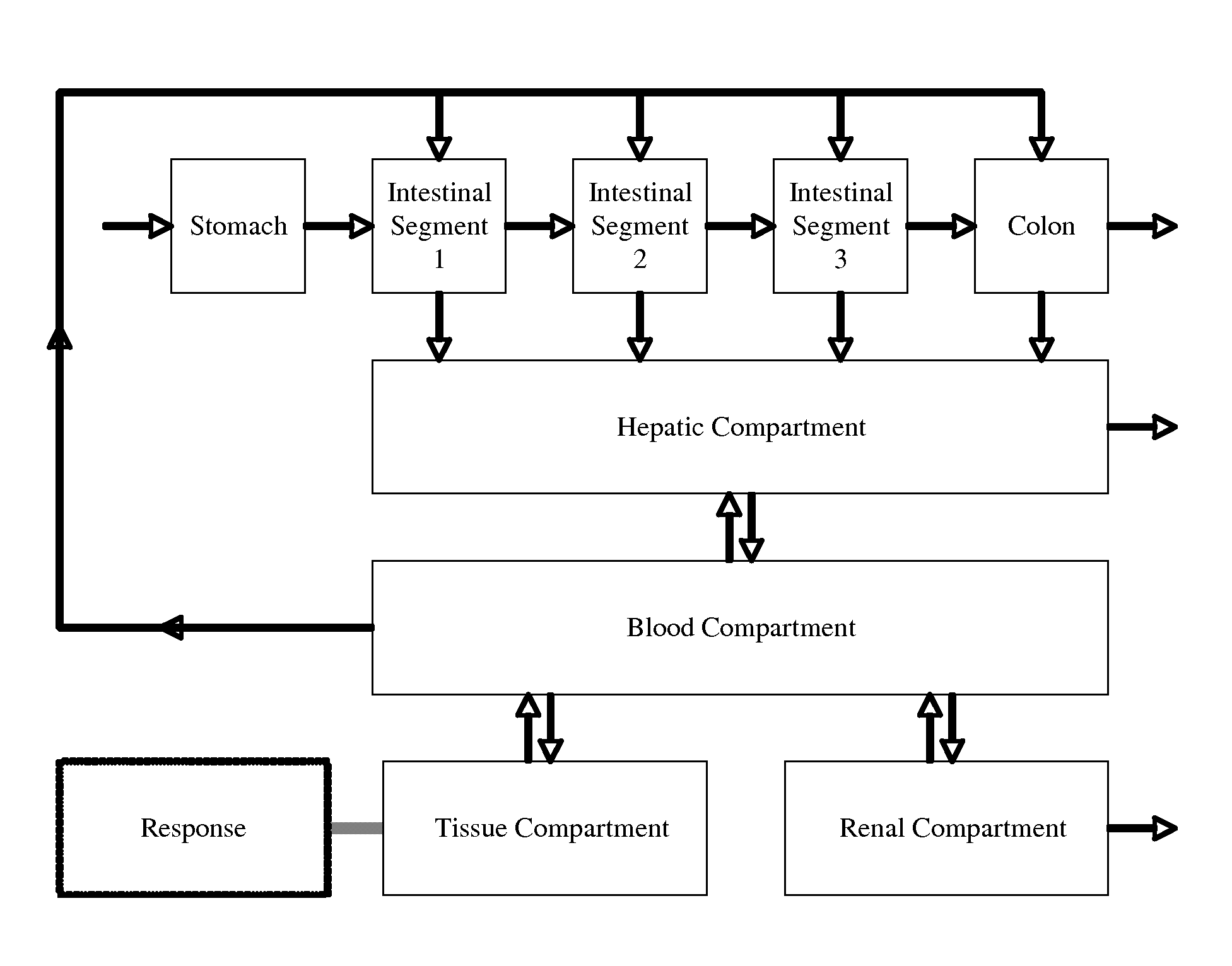
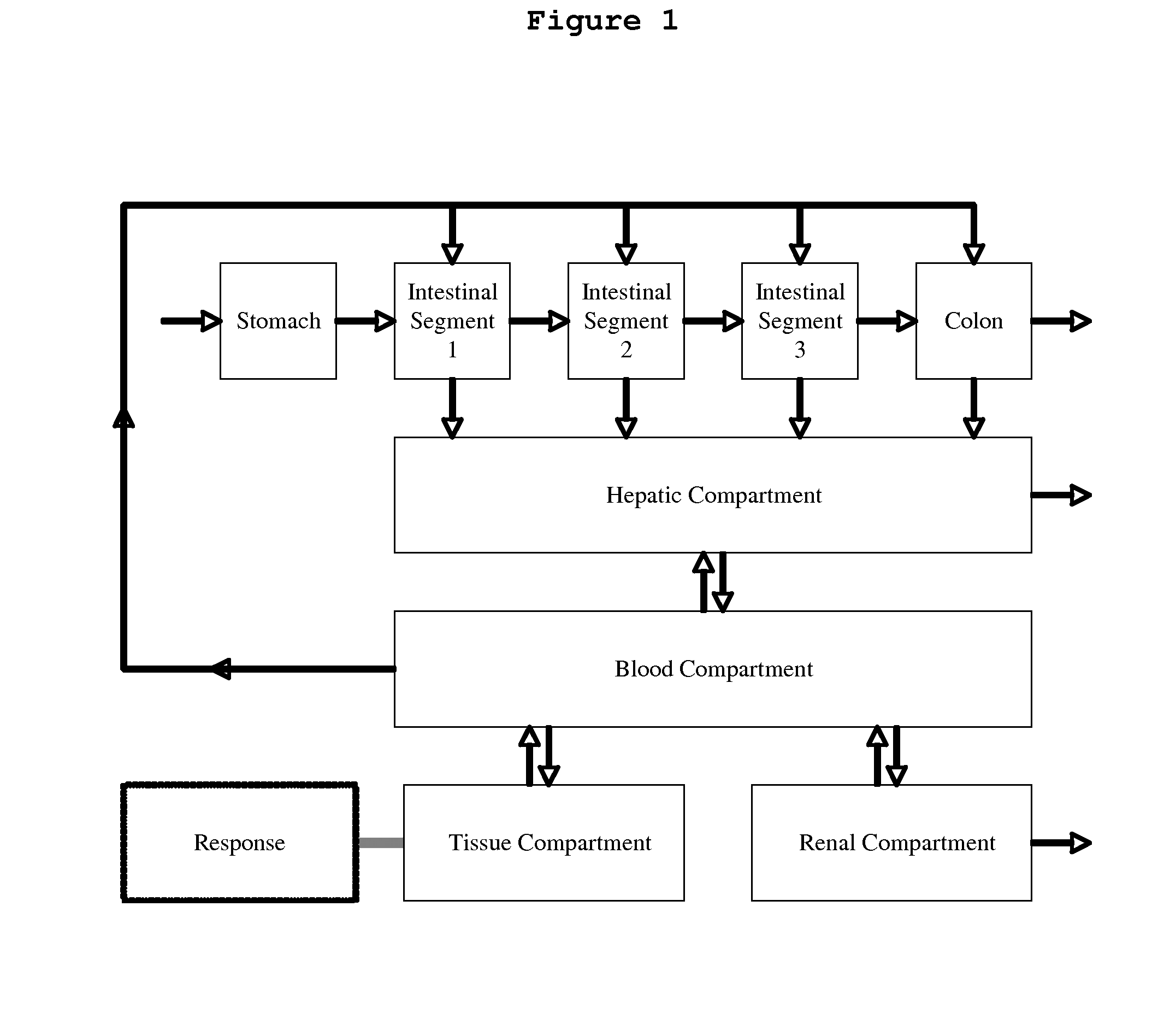
Microfluidic device for pharmacokinetic-pharmacodynamic study of drugs and uses thereof
ActiveUS8748180B2Bioreactor/fermenter combinationsCompound screeningTissues typesMicrofluidic channel
A microfluidic device for culturing cells, termed a microscale cell culture analog (μCCA), is provided. The microfluidic device allows multiple cell or tissue types to be cultured in a physiologically relevant environment, facilitates high-throughput operation and can be used for drug discovery. The microfluidic device uses gravity-induced fluidic flow, eliminating the need for a pump and preventing formation of air bubbles. Reciprocating motion between a pair of connected reservoirs is used to effect the gravity-induced flow in microfluidic channels. Bacterial contamination is reduced and high throughput enabled by eliminating a pump. The microfluidic device integrates a pharmacokinetic-pharmacodynamic (PK-PD) model to enable PK-PD analyses on-chip. This combined in vitro / in silico system enables prediction of drug toxicity in a more realistic manner than conventional in vitro systems.
Owner:CORNELL UNIVERSITY
Formulation of immunopotentiators
InactiveUS20120177681A1Improve propertiesReduce activationAntibacterial agentsBiocideRetention timeCombinatorial chemistry
Immunopotentiators can be adsorbed to insoluble metal salts, such as aluminium salts, to modify their pharmacokinetics, pharmacodynamics, intramuscular retention time, and / or immunostimulatory effect. Immunopotentiators are modified to introduce a moiety, such as a phosphonate group, which can mediate adsorption. These modified compounds can retain or improve their in vivo immunological activity even when delivered in an adsorbed form.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Recombination interferon with new space conformation and enhanced effect, its preparing method and application
ActiveCN1740197AStrong antiviral activityLittle side effectsPeptide/protein ingredientsAerosol deliveryAntigenSide effect
The invention provides recombination interferon (rSIFN-co) or the functional analog with new space conformation, enhanced effect, and low side effect. It is showed by the external pharmacodynamics, it can not only restrain the DNA replication of the hepatitis b virus, but also restrain the exudation of the surface antigen (HBsAg) and e antigen (HBeAg). The cytology toxicity is 1 / 8 of the clinical interferon and the antiviral activity is 5-20 times to the clinical interferon and has a higher, a more board spectrum and longer time biological response reaction, and prevent the tumor hyperplasia and transmission. The invention also provides the synthetic gene code, gene carrier, expression system of the synthetic gene of the recombination interferon or the functional analog. Finally, the invention also provides the preparing method and application thereof.
Owner:SUPERLAB FAR EAST LTD
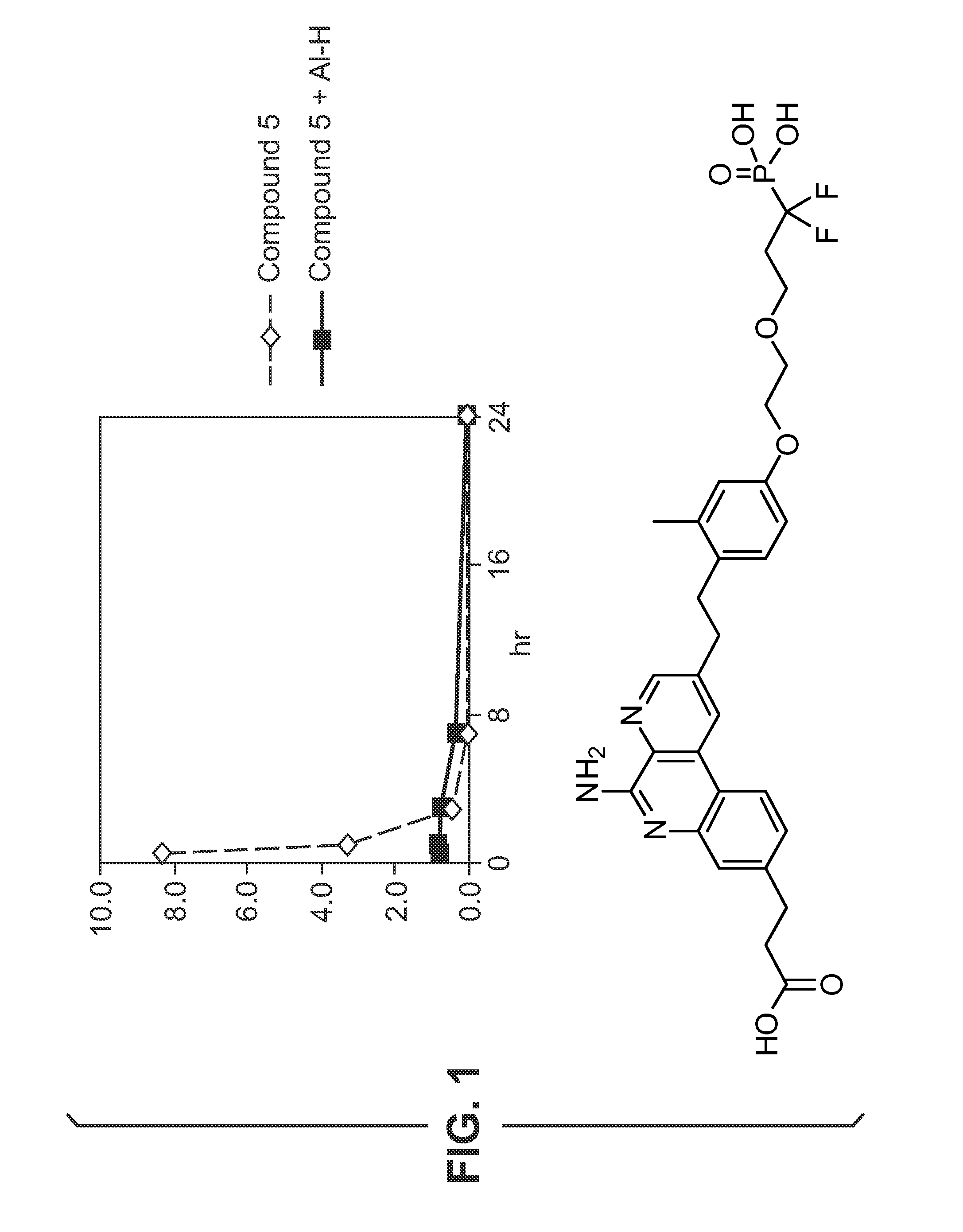
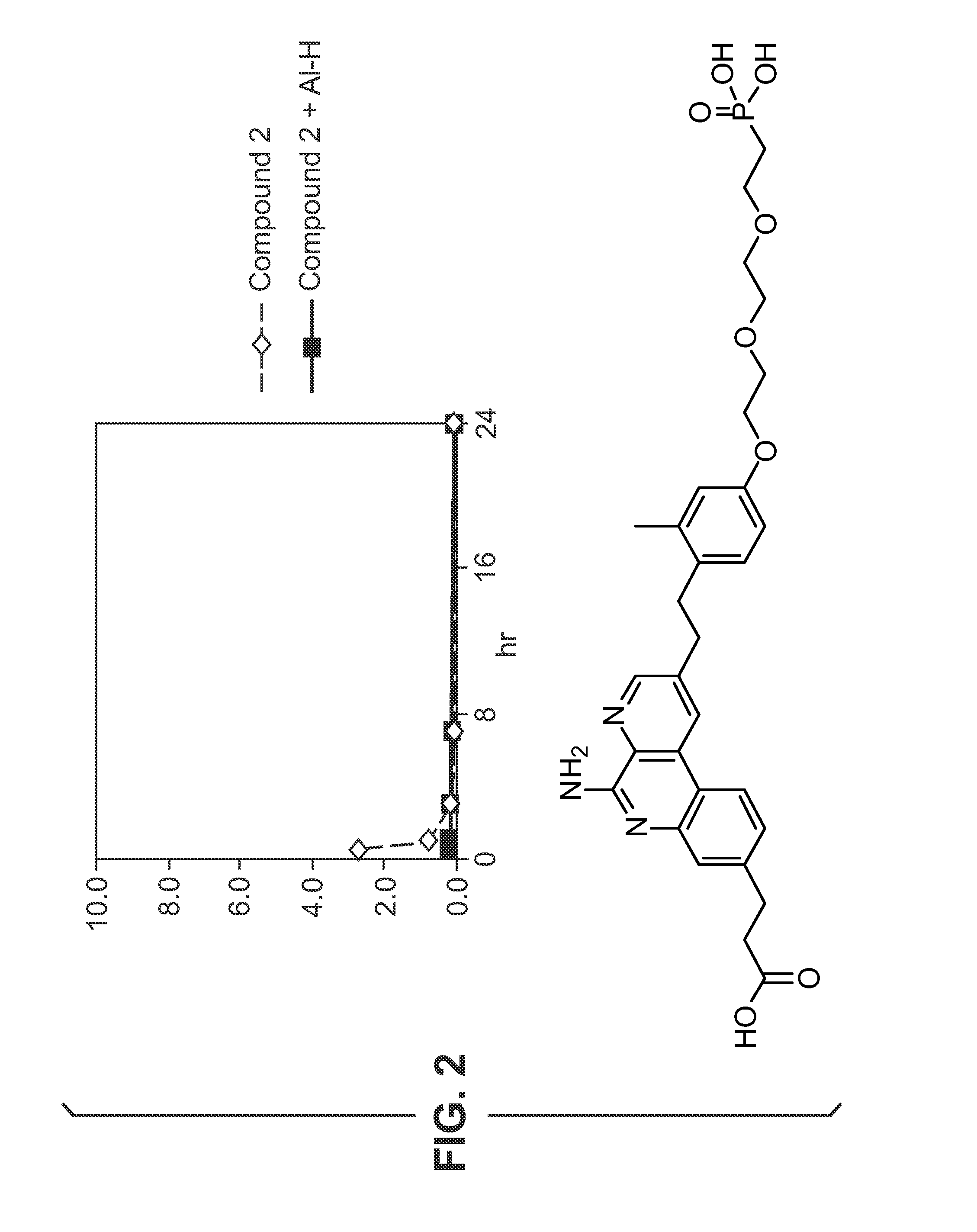
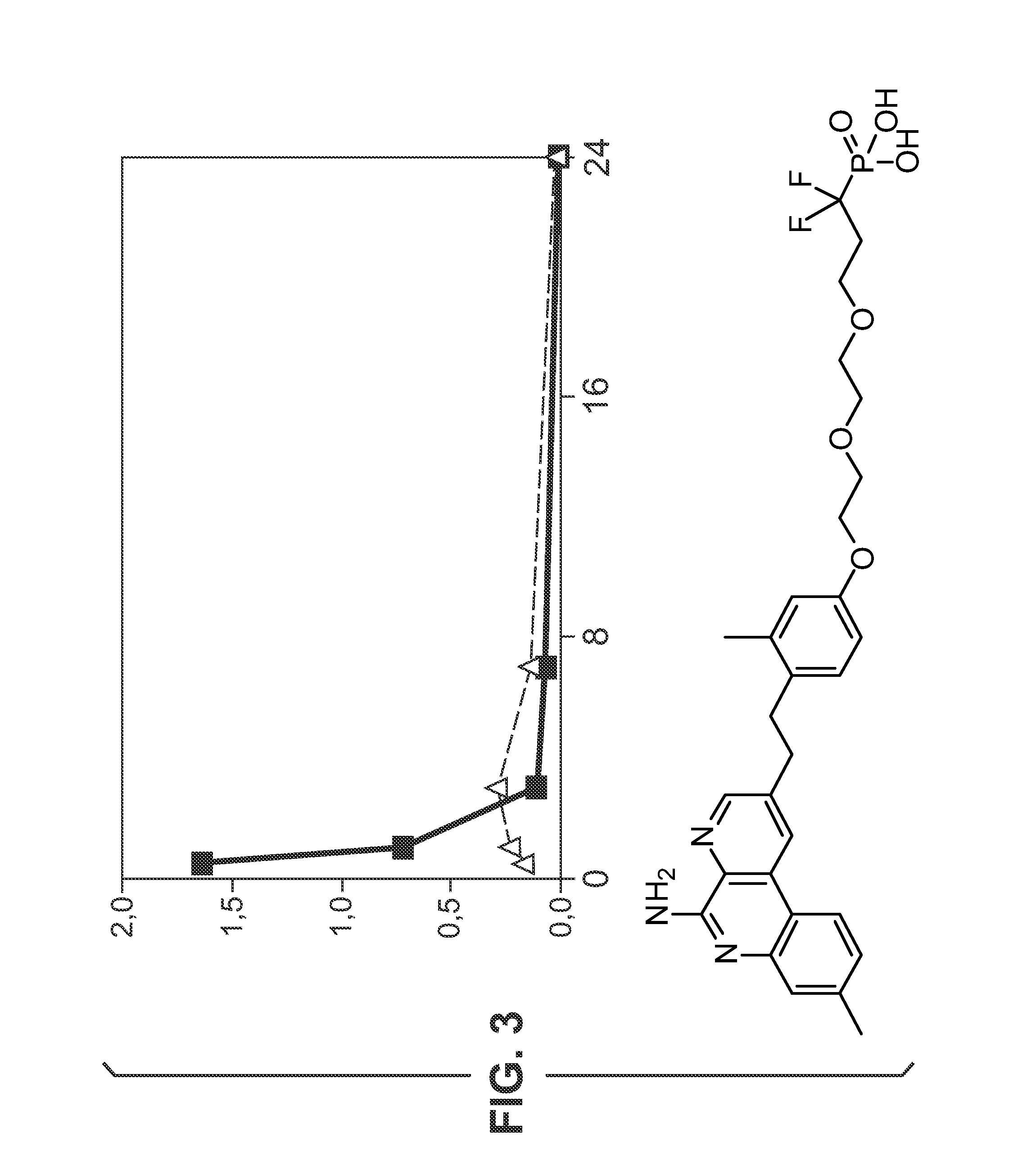
Glucose-responsive insulin conjugates
Insulin conjugates comprising an insulin molecule covalently attached to at least one bi-dentate linker having two arms, each arm independently attached to a ligand comprising a saccharide and wherein the saccharide for at least one ligand of the linker is fucose are disclosed. The insulin conjugates display a pharmacokinetic (PK) and / or pharmacodynamic (PD) profile that is responsive to the systemic concentrations of a saccharide such as glucose or alpha-methylmannose even when administered to a subject in need thereof in the absence of an exogenous multivalent saccharide-binding molecule such as Con A.
Owner:MERCK SHARP & DOHME LLC
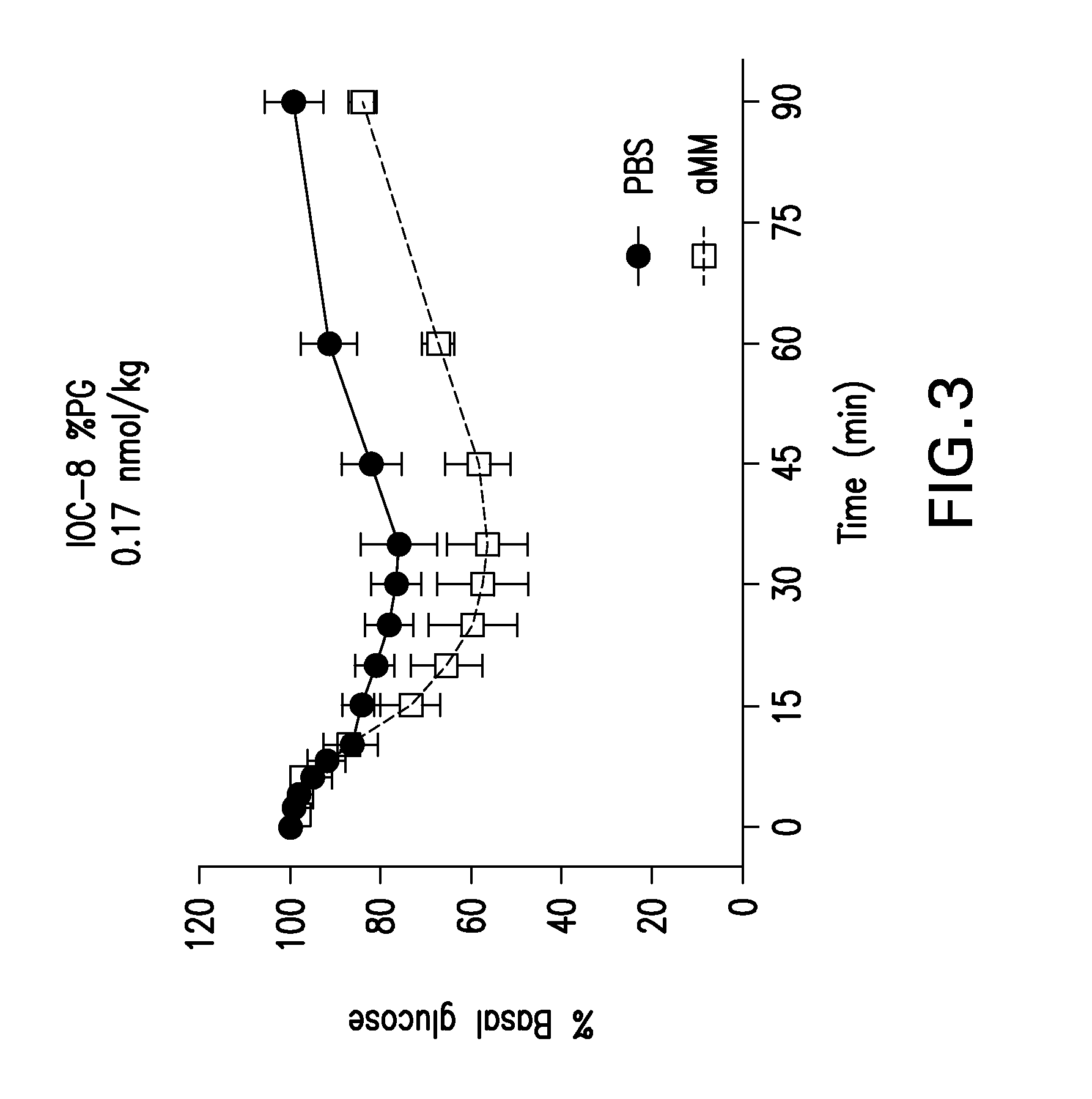
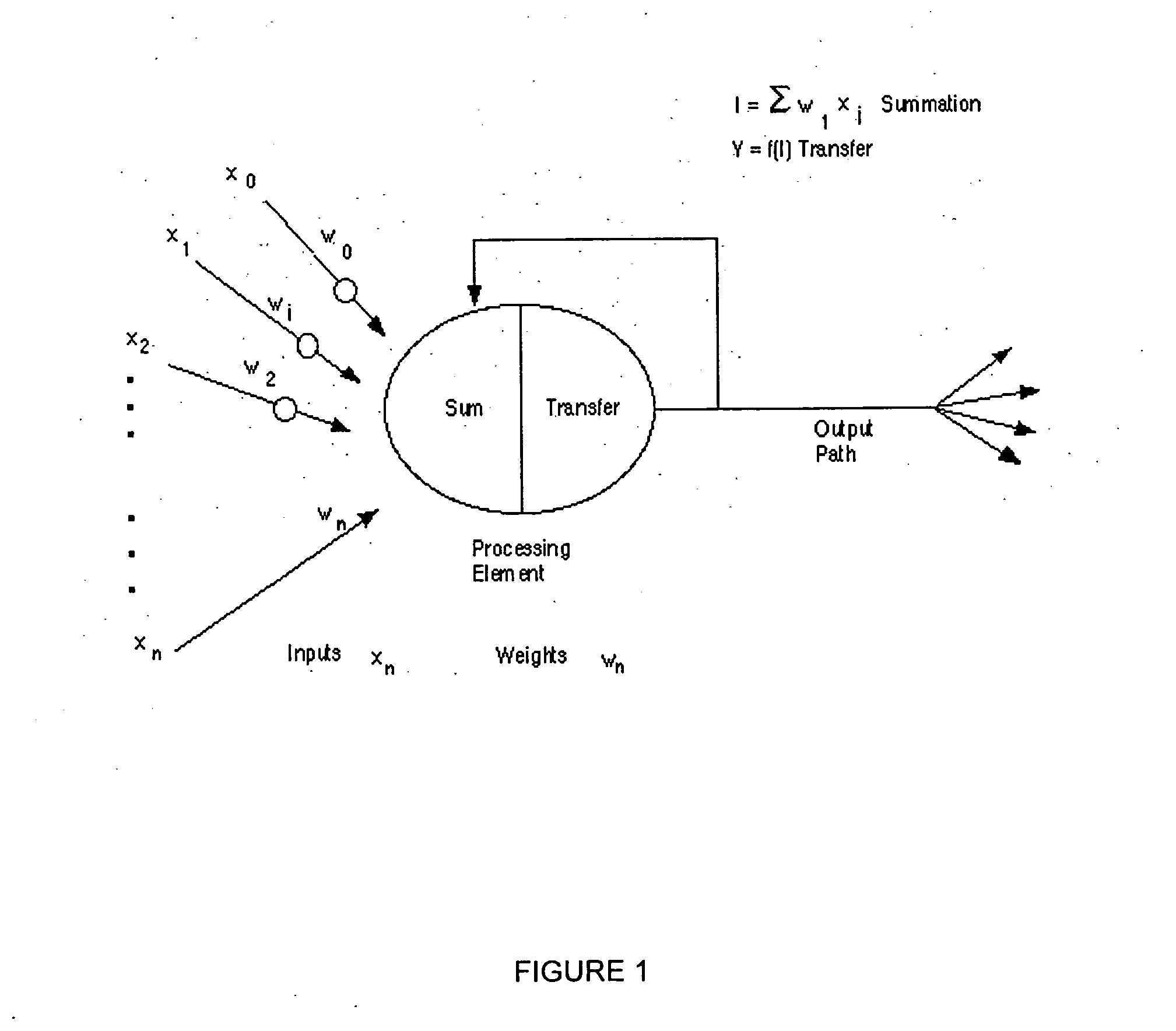
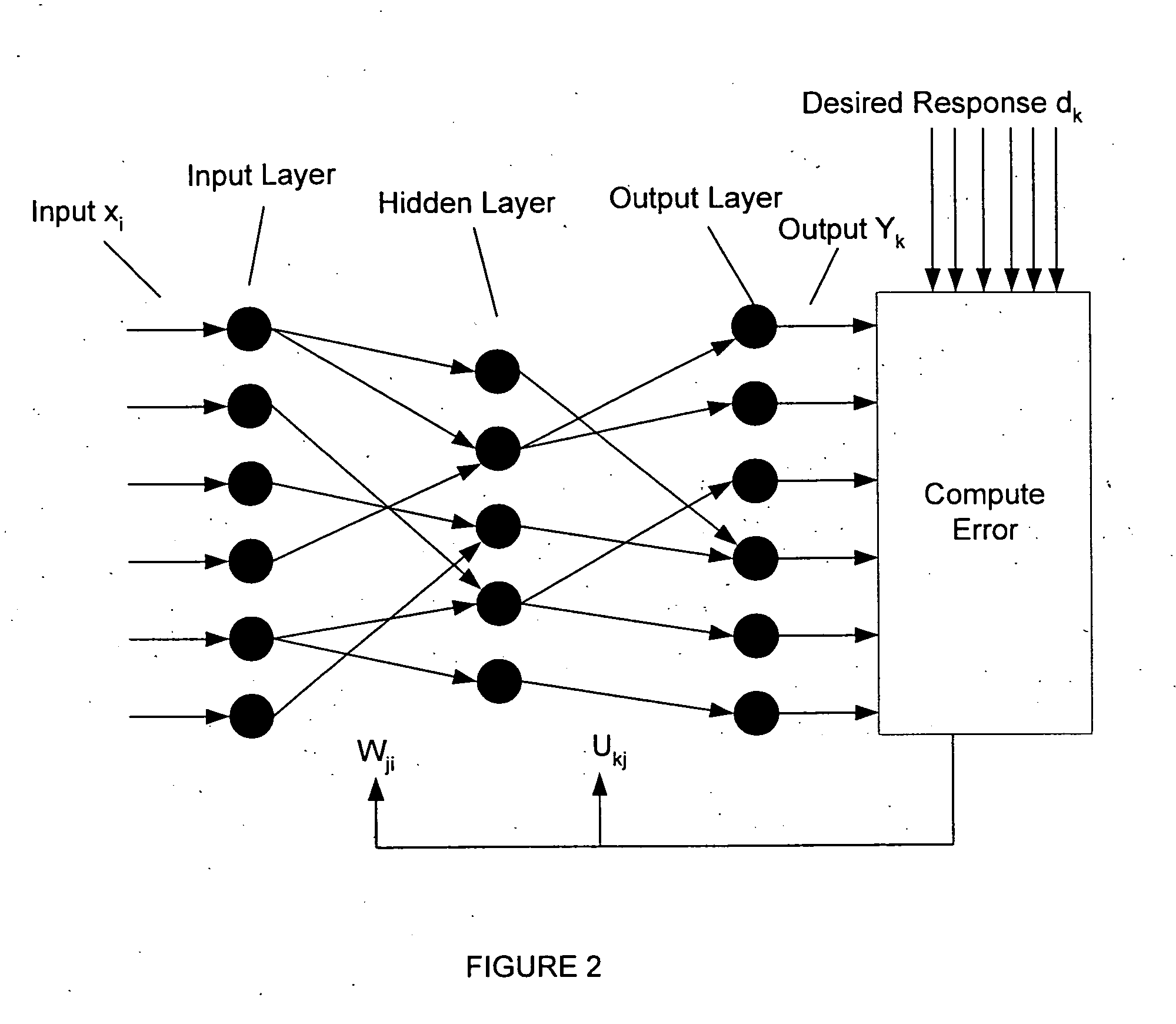
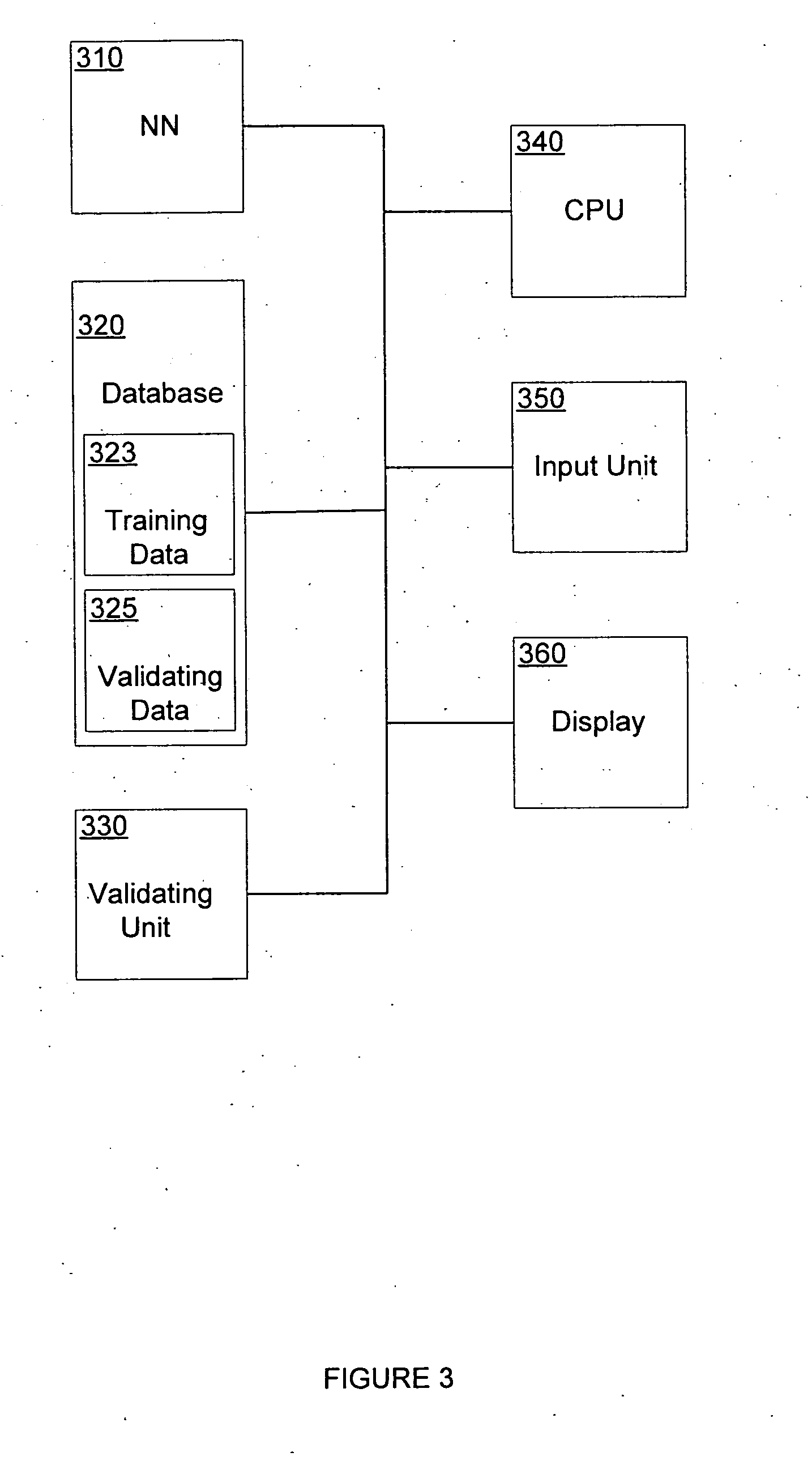
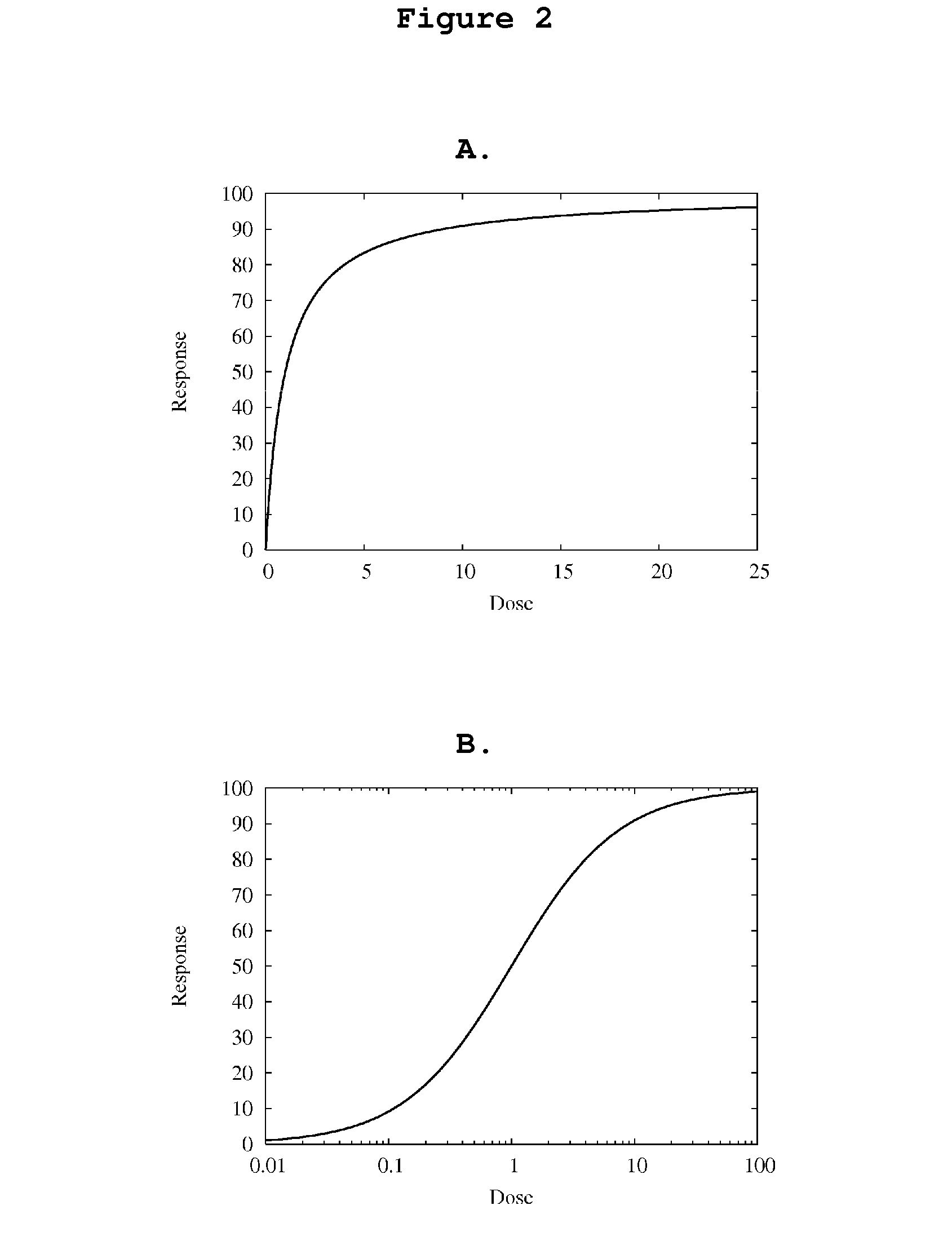
Neural network pattern recognition for predicting pharmacodynamics using patient characteristics
InactiveUS20050216200A1Data processing applicationsDrug and medicationsPatient characteristicsDrug doses
Methods are provided for predicting the effect of a drug given the drug dose and individual patient clinical characteristics. A neural network is trained on samples of clinical data including the observed drug dose and effect on patients, as well as their individual clinical characteristics. The neural network is then validated to ensure that its predictions fall within an acceptable error range. The neural network is used to predict the effect of a given drug dose for a given set of individual patient clinical characteristics. Methods are also provided for predicting the drug dose required to achieve a desired effect. Another neural network is trained on samples of clinical data including the observed drug dose and effect on patients, as well as their individual clinical characteristics. The neural network is then validated to ensure that its predictions fall within an acceptable error range. The neural network is used to predict the dose of a drug dose required to achieve a desired effect for a patient with a given set of individual clinical characteristics. The first neural network is used to generate training data for the second neural network.
Owner:UNITED STATES OF AMERICA +1
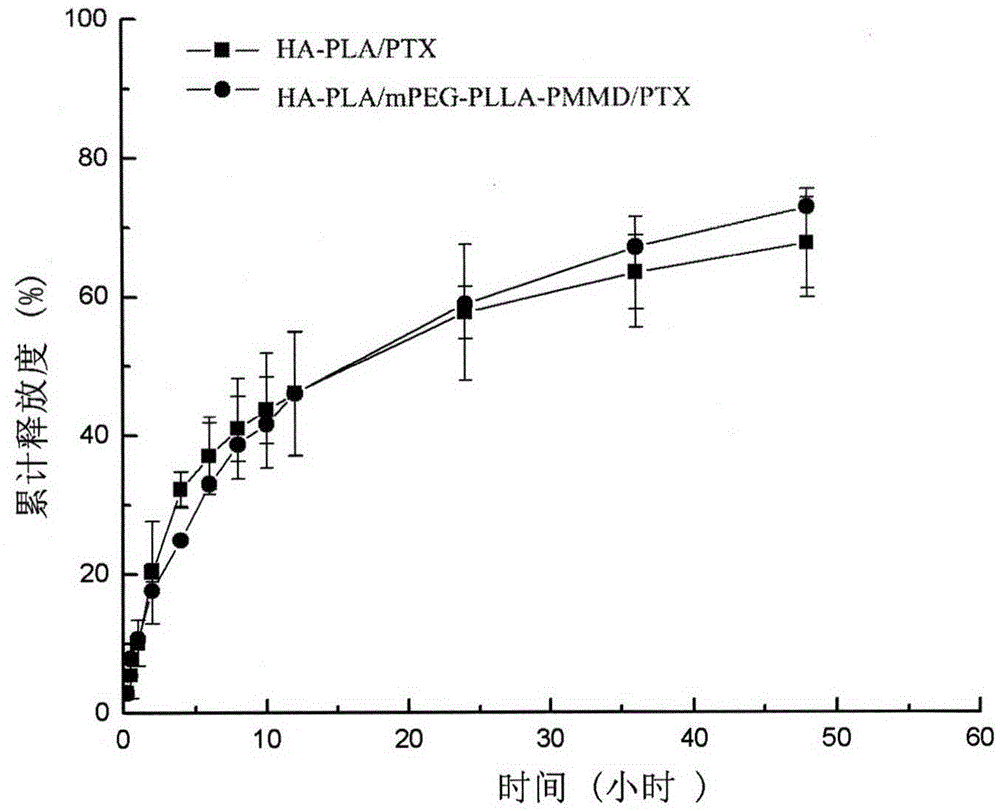
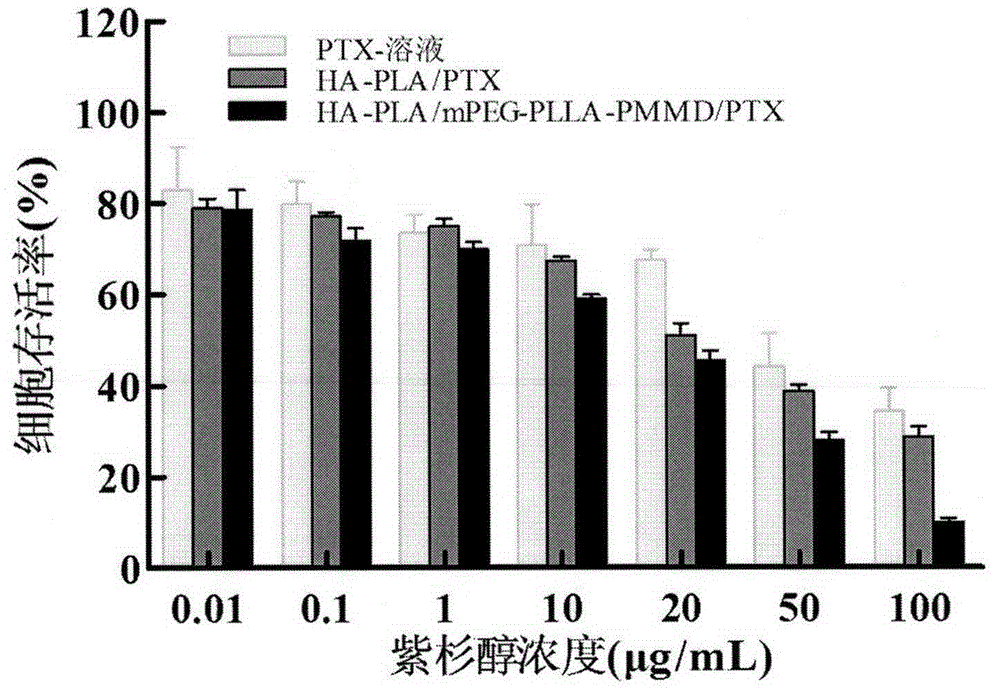
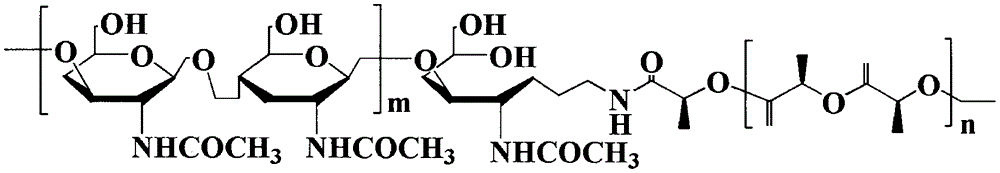
Method for synthesizing multifunctional active targeted hyaluronic acid-polylactic acid carrier and preparing anti-tumor medicinal micelle of multifunctional active targeted hyaluronic acid-polylactic acid carrier
ActiveCN104056275AExtend cycle timeSmall toxicityPharmaceutical non-active ingredientsEmulsion deliverySolubilityPolyester
The invention belongs to the fields of polymer chemistry and medicinal preparations, and particularly relates to a method for synthesizing an active targeted hyaluronic acid-polylactic acid carrier, a method for preparing an anti-tumor medicinal micelle of the active targeted hyaluronic acid-polylactic acid carrier and an application thereof. By adopting a novel self-assembly technology, amphipathic PEG (polyethylene glycol) block polyester copolymer and tumor targeted ligand hyaluronic acid-polylactic acid copolymer are self-assembled by means of the electrostatic interaction to form a multifunctional composite micelle; the solubility of insoluble tumor medicaments and the drug loading capacity and encapsulation efficiency of water-soluble anti-tumor medicines can be remarkably improved by virtue of the anti-cancer drug-loaded micelle and composite micelle composition, the medicines can be biodegraded in a body, phagocytosis of a reticuloendothelial system (RES) and excretion of a kidney can be avoided. The active targeted hyaluronic acid-polylactic acid carrier has a long-circulating effect, the multifunctional composition has a prominent advantage of tumor active targeting effect, and parameters of pharmacodynamics in vitro and in vivo of the micelle are remarkably superior to those of common anti-tumor injections. Clinically acceptable administration means of the micelle includes injection administration or mucosal administration, and preparations of the micelle can be injection, transfusion, injection lyophilized powder injections or dry powder inhalation.
Owner:CHINA PHARM UNIV
Traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis method
InactiveCN102183608AMulti-targetMultiple therapeutic pathwaysComponent separationEndogenous metabolismMetabolite
The invention provides a traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis method, which determines various endogenous metabolites in blood samples of a blank control group, a model group and a model treatment group by a GC-MS method, uses an abnormally-changed metabolite group of the model group as a biomarker group, uses an effect index with obvious callback of the abnormally-changed metabolite group after traditional Chinese medicine intervention as an efficacy marker group, determines the contents of various drugs in the blood samples of a model animal after single dose by a LC-MS method, draws a plasma concentration-time curve, calculates the pharmacokinetic parameters of each component; determines the amount of the endogenous efficacy marker group in the blood samples of the model animal single dose group by a GC-MS method, draws a time-effect curve, performs PK-PD combined analysis by combining with the plasma concentration-time curve and parameters. The invention reveals the efficacy substance base of traditional Chinese medicine in the treatment of cardiovascular diseases, and provides an effective method for traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis.
Owner:ZHEJIANG UNIV
Chemical synthesis technique of quinoxaline
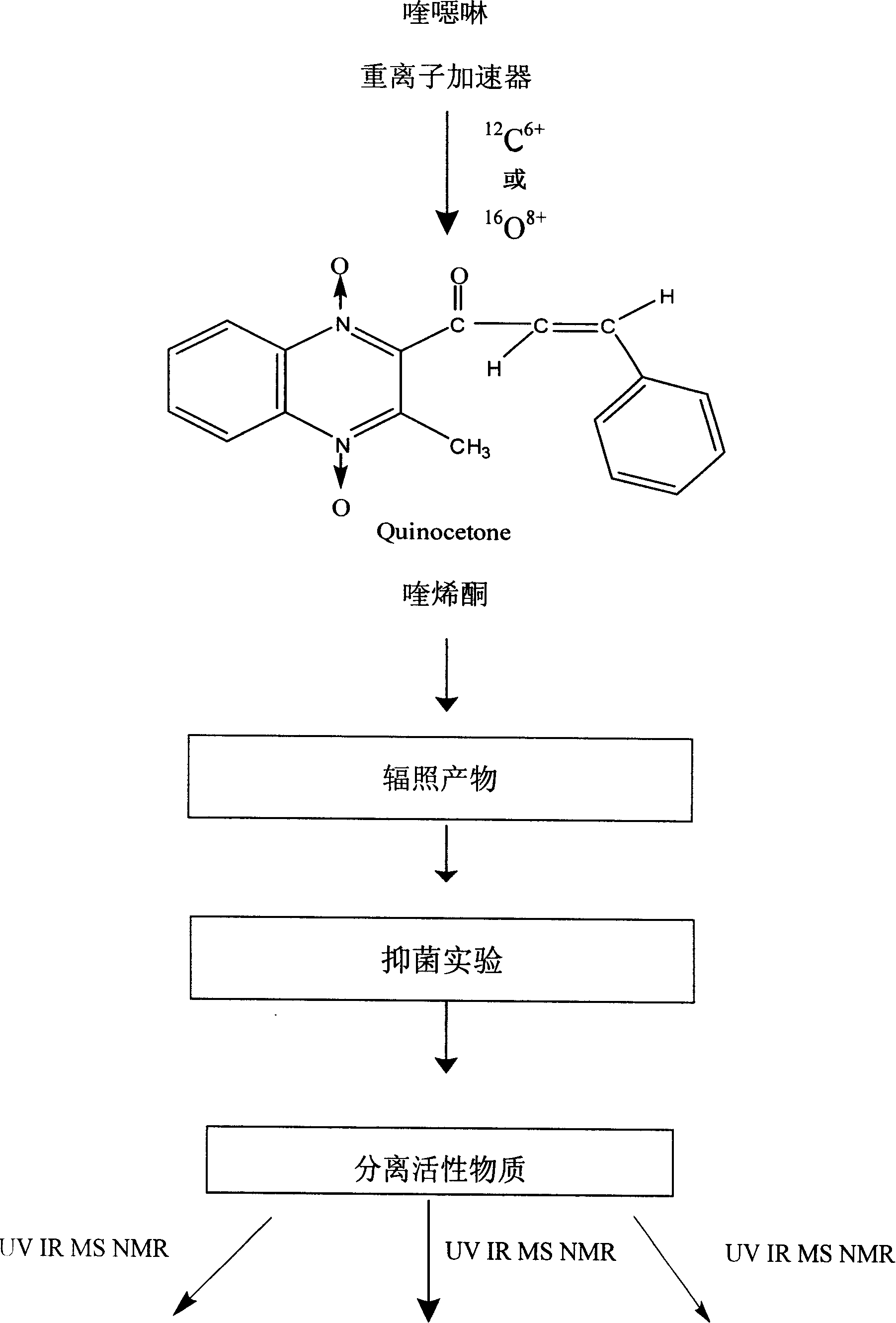
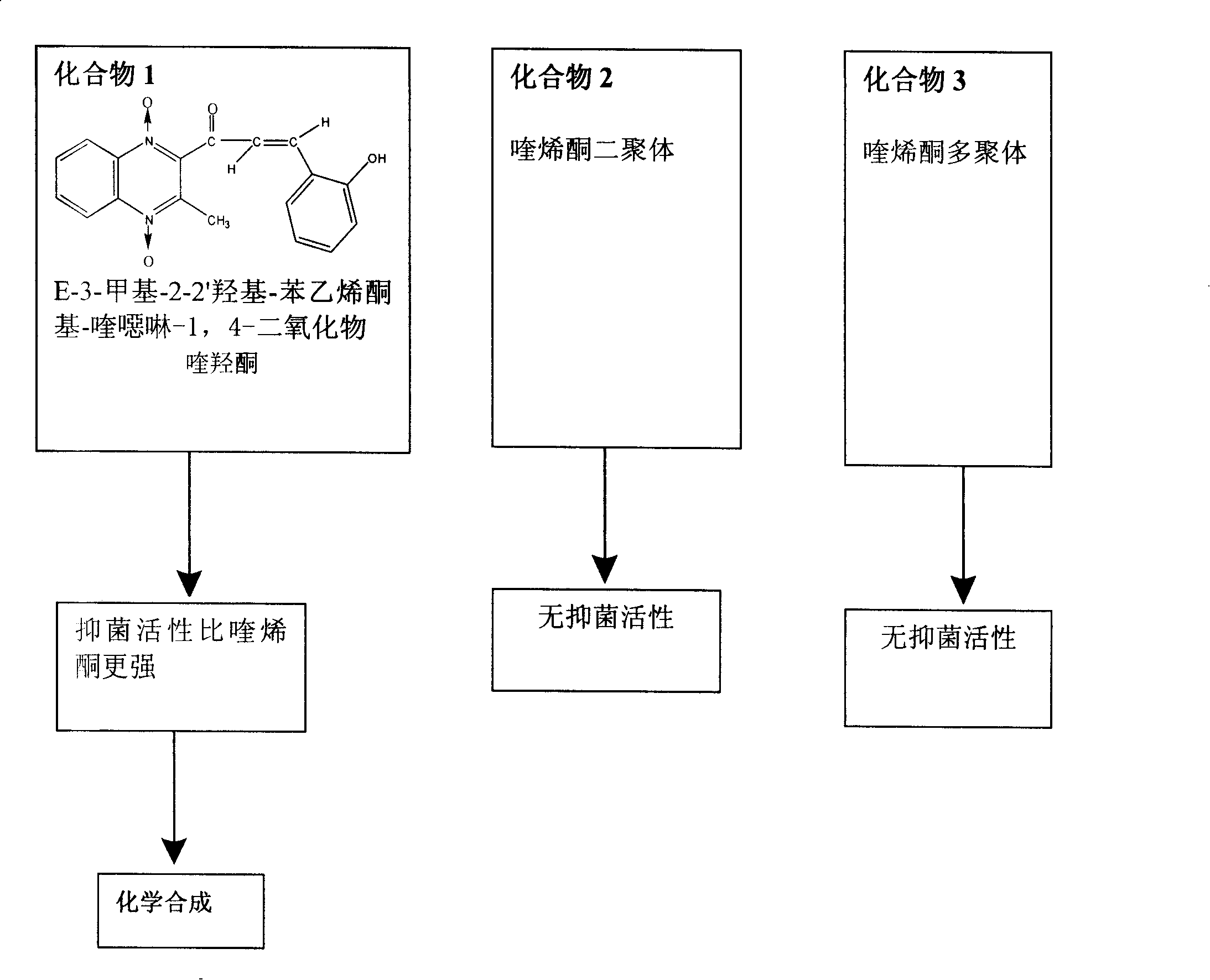
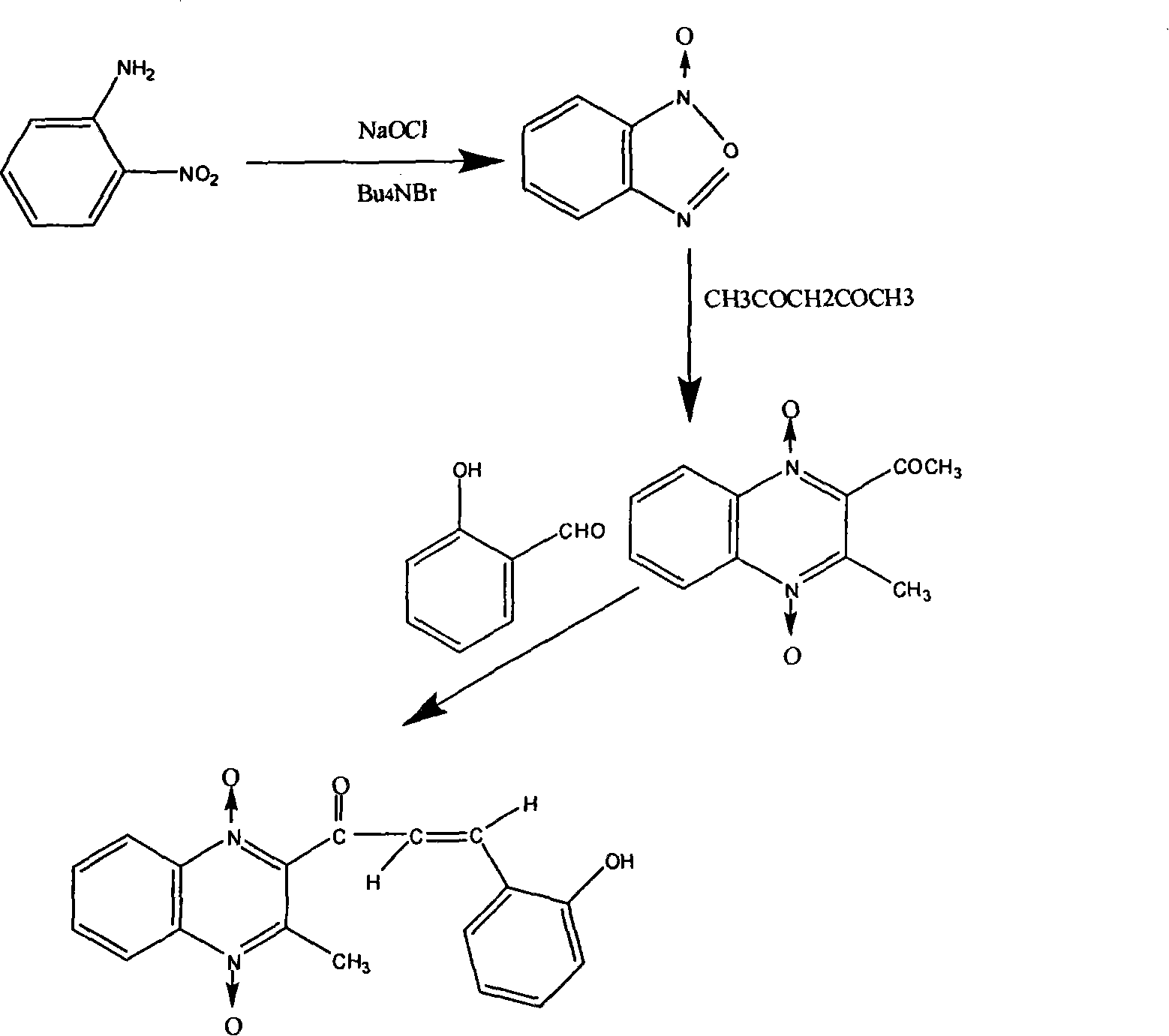
InactiveCN101182313AShorten the development cycleLow costOrganic chemistryChemical synthesisQuinoxaline
The present invention is a chemical synthesis process of quinolone, which relates to the field of veterinary drug feed additives. The existing synthesis technology is to select from various quinoxaline compound substituted derivatives, but these compounds have relatively large toxic and side effects, and simply change The side chain can no longer meet the needs. The present invention uses heavy ion beams to modify the structure of quinoxaline to produce new compound molecules. Through pharmacodynamic research, the standard sample is separated for chemical synthesis. The present invention designs three synthetic routes. Corresponding to the three chemical synthesis processes, the new compound was identified by spectrum, and it was determined to be quinolone. After further growth-promoting and toxicity tests, it showed obvious improvement and improvement compared with the lead compound quinolone. The invention has the beneficial effects of greatly shortening the development cycle and greatly saving the development cost, and shows that quinolone is a very vital veterinary drug feed additive.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Fc fusion protein of human granulocyte colony stimulin with enhanced bioactivity
InactiveCN1410450AImprove biological activityAntibody mimetics/scaffoldsColony-stimulating factorHalf-lifePharmacodynamic Study
Owner:PHARMAB
Application of active ingredient namely loganin in dogwood in preparation of medicaments for treating diabetes
InactiveCN103110651AOrganic active ingredientsMetabolism disorderChromatographic separationBULK ACTIVE INGREDIENT
The invention discloses a new medical application of loganin, and in particular relates to an application of the loganin in preparation of medicaments for treating diabetes, wherein the loganin is extracted from dogwood. The method for extracting the loganin comprises the following steps of: (1) performing water extraction and alcohol precipitation on dogwood coarse powder to obtain a dogwood water extraction and alcohol precipitation extracting solution; (2) loading the dogwood water extraction and alcohol precipitation extracting solution into a macroporous adsorption resin column, and performing enrichment to obtain dogwood iridoid glycosides; (3) performing chromatographic separation by using a silica gel column to obtain a loganin flow component; and (4) purifying to obtain a loganin monomeric compound. Pharmacodynamics experimental results prove that the loganin has the functions of reducing the content of blood sugar, serum AGEs and urokinase protein, raising the serum insulin level, relieving pathologic lesions of pancreas, kidney, liver, heart, testis and the like, and obviously improving a 'three more one less' symptom. Therefore, the loganin can be used for preparing the medicaments for treating the diabetes.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Pharmaceutical Platform Technology for the Development of Natural Products
The present invention provides a set of in vitro and in silico methodologies for predicting in vivo pharmacokinetics and pharmacodynamics of multiple components; the methodologies comprise mathematical models for solving multiple unknowns which are linearly independent and / or interacting with each other. The present invention can be applied to develop phytomedicines which contain multiple active ingredients without prior identification, isolation and purification of these components.
Owner:SINOVEDA CANADA INC
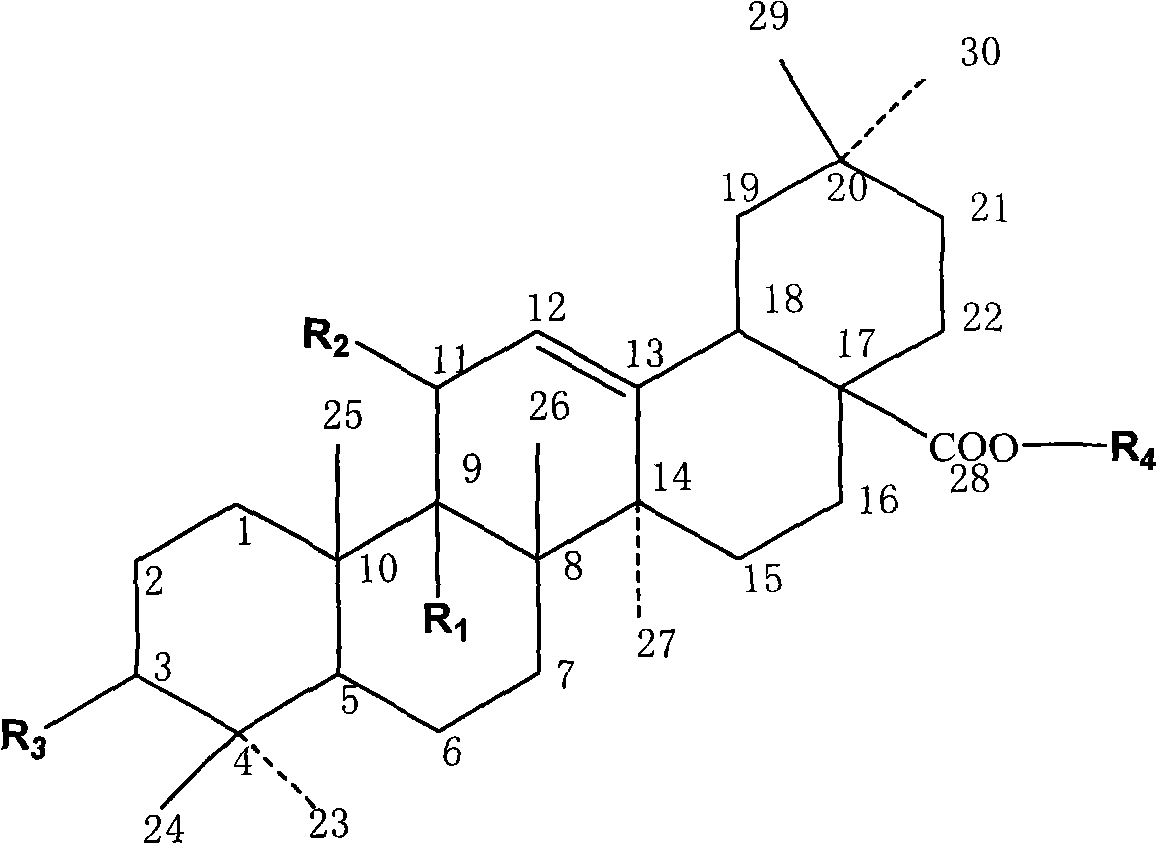
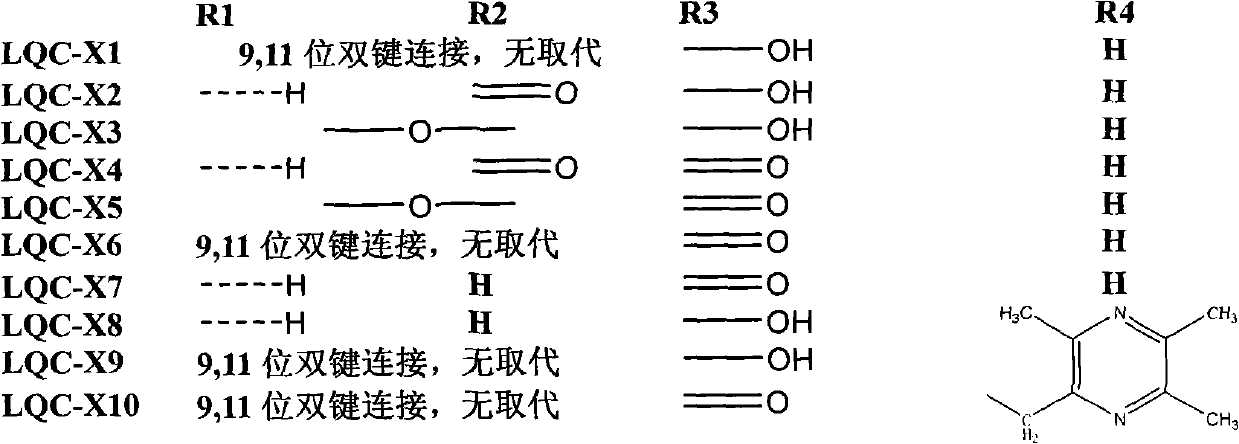
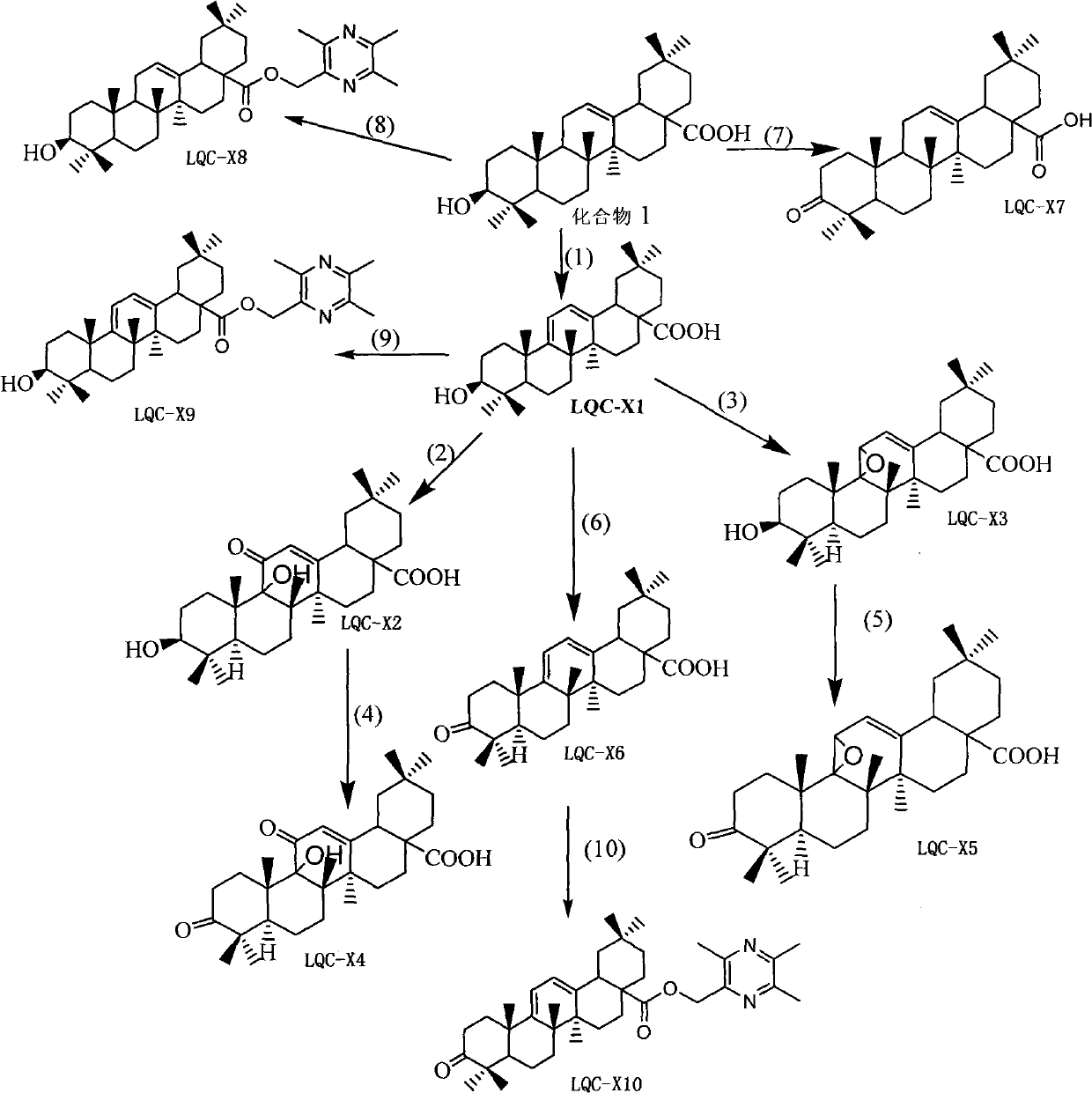
Synthesis of anti-hepatitis B medicine LQC-X and application thereof
The invention relates to the field of chemical and biological sciences, in particular to LQC-X structural formula I, and synthesis and application of intermediate of LQC-X, wherein a maximum dosage of LQC-X6 given to an mouse is 2000 mg / kg once in one day; the mouse is continuously observed for 7 days; no toxic reaction appears, and the medicine is proven to have quite high safety; and it is proved through pharmacodynamics experiments that LQC-X compounds have obvious anti-hepatitis B virus action and liver protecting and transaminase lowering action. The compounds can be used for preparing medicines for preventing and treating diseases of hepatitis B, chronic liver cirrhosis and the like. The structural formula I of LQC-X is shown in the description.
Owner:北京鸿测科技发展有限公司
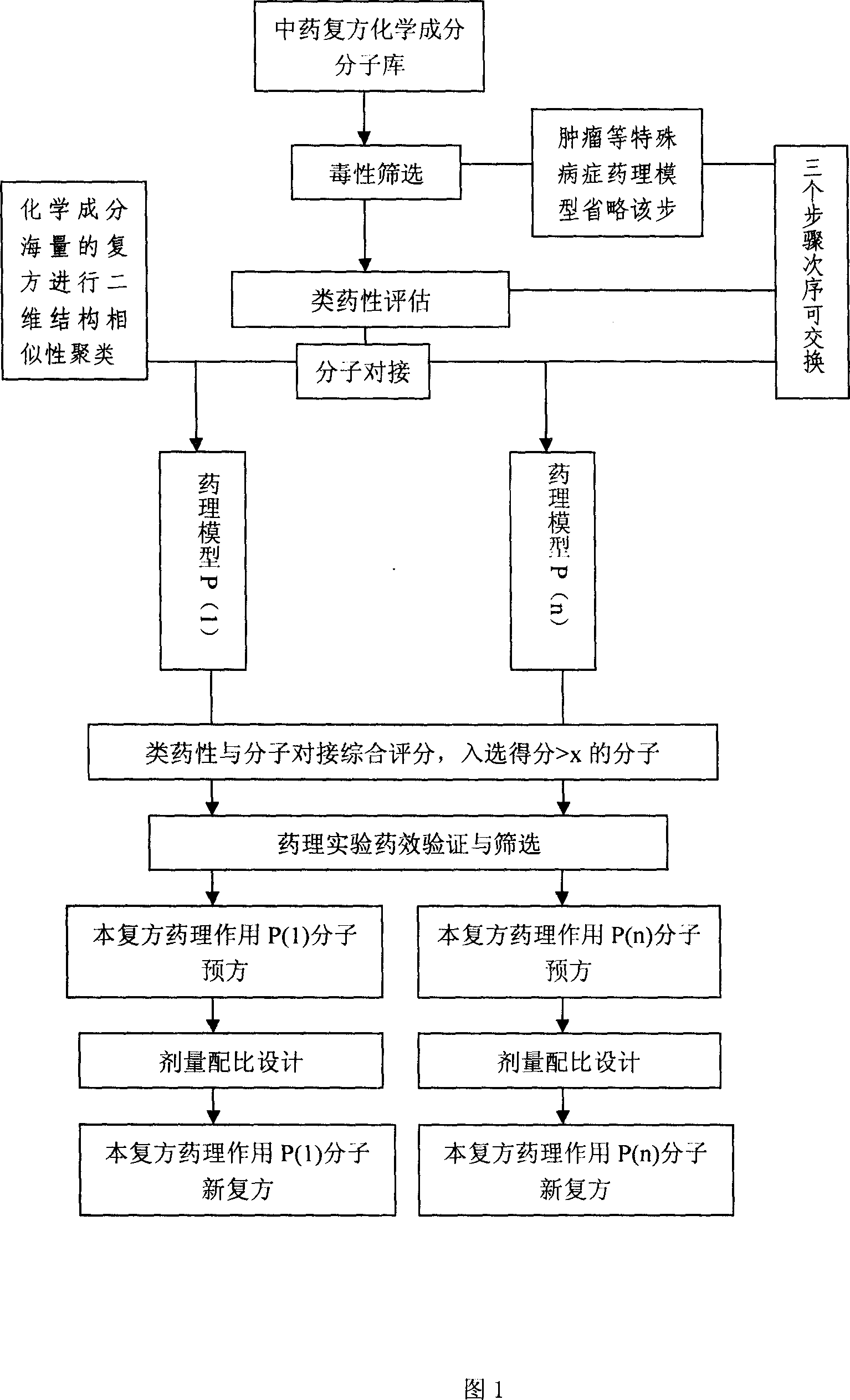
Virtual screening method for compound pesticide effect matter base of traditional chinese medicine
InactiveCN101089245AEconomic noveltyAbandon the shortcomings that are not conducive to poor compliance of preparationsLibrary screeningResearch ObjectAdditive ingredient
In this invention, first It is set that an object of study of a compound traditional Chinese medicine with its chemical compositions being clear about. The molecules of chemical constitutions of each ingredient of the Chinese medicine prescription are collected and reorganized, and setting a data base for chemical constitutions molecules of said Chinese medicine prescription. For the aim of pharmacodynamics effects of main therapeutical functions of the traditional Chinese medicine prescription with their clear therapeutical effects, clear mechanism and definited target spots, we search or set up the corresponding molecular target spots. Three procedures (without odor of priority) are: poisonousness sieving, analogous medicine properties estimation and molecular abutment decision to these molecular data base of the set pharmacodynamics models respectively. Finally we estimating all the molecules in the molecular data base, selecting-out the molecules with higher grading, to obtain the pharmacodynamics effect P(i) molecular pre-prescription, and then producing novel prescriptions.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
Centella asiatica triterpenic acid single-glucopyranoside composition, its preparation method, its quantitative analysis method and its application
The invention relates to a centella asiatica triterpenic acid single-glucopyranoside composition which is composed of ursane madecassic acid single-glucopyranoside, madecassic acid single-glucopyranoside and oleanane chebuloside II, wherein the mass ratio of ursane madecassic acid single-glucopyranoside to madecassic acid single-glucopyranoside to oleanane chebuloside II is 1:0.5-2:0.1-1, and the sum of the mass percentage content of three components is not less than 50%. The composition takes a centella asiatica extract as a substrate, the centella asiatica extract is fermented and hydrolyzed by microbes of beta-glucosidase or microbes capable of generating beta-glucosidase, and extracted by n-butanol or separated and purified by macroporous adsorption resin. The invention also provides a quantitative analysis method which is a HPLC quantitative analysis for three components by adding a proper amount of mobile phase of beta-cyclodextrin. Experimental research of pharmacodynamics proves that the composition has substantial activity for inhibiting tumor cells and fibroblast, the composition can be used for treating tumor and scar hyperplasia.
Owner:SHANGHAI NORMAL UNIVERSITY
Predicting drug-drug interactions based on clinical side effects
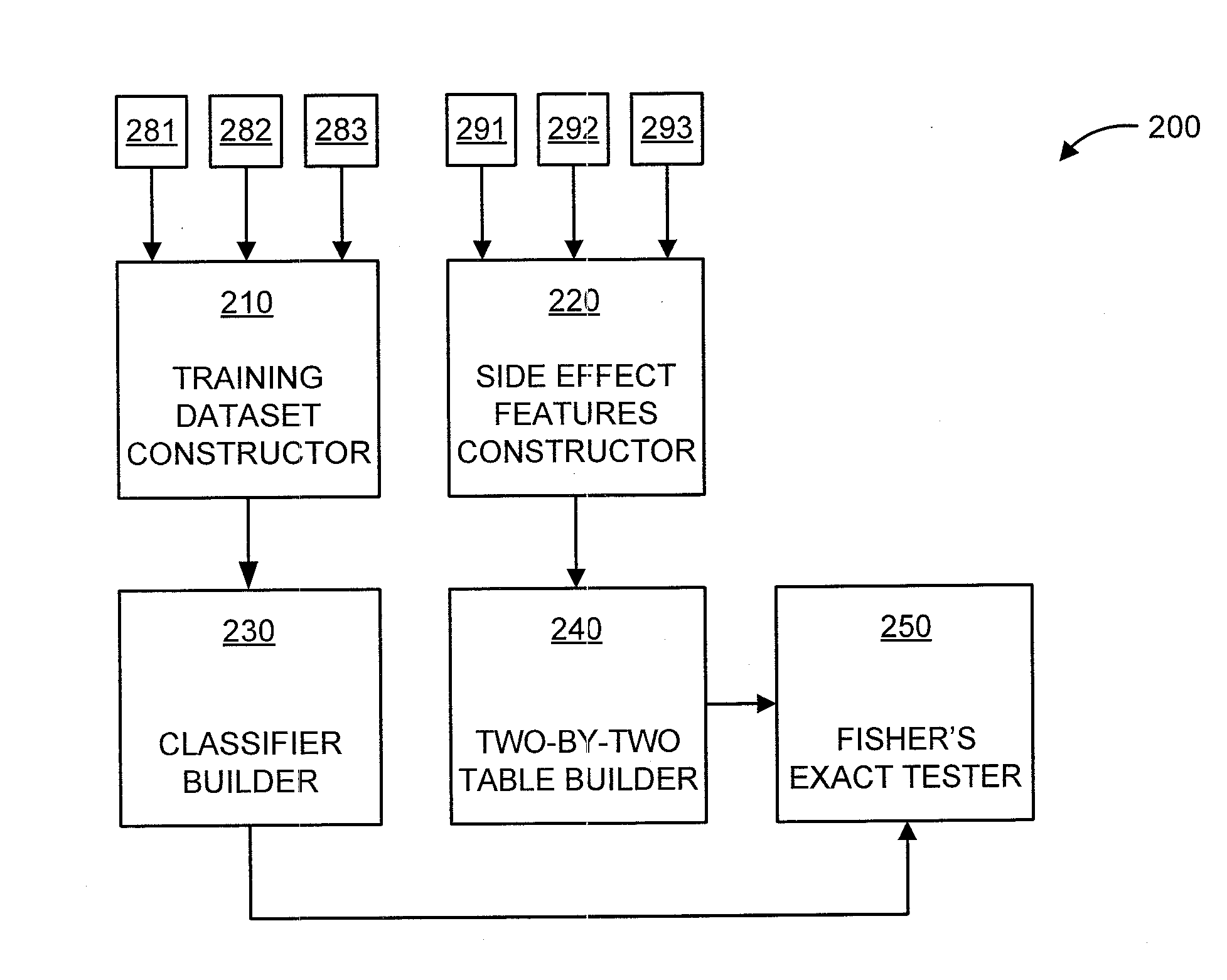
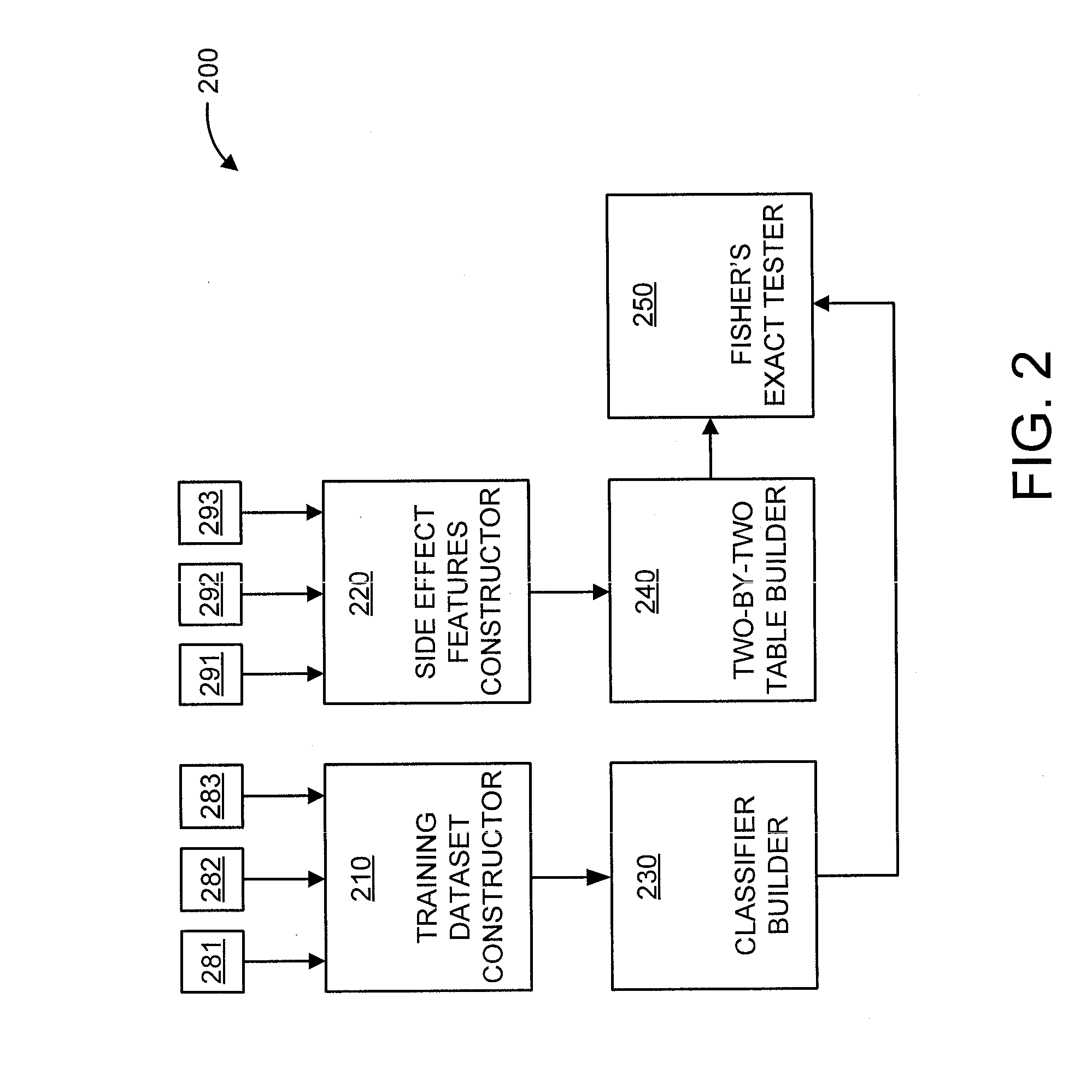
A processor-implemented method, computer program product and system are provided for predicting drug-drug interactions based on clinical side effects. The method includes constructing a drug-drug interactions training dataset that includes pharmaceutical, pharmacokinetic or pharmacodynamics drug-drug interactions from multiple data sources for each of a plurality of drugs. The method also includes constructing side effect features for each of the drugs from side effects associated with the drugs. The method further includes building, using the drug-drug interactions training dataset, a drug-drug interactions classifier that predicts adverse drug-drug interactions for drug pairs derivable from the drugs. The method additionally includes for each of the side effects, building a two-by-two table using the side effect features, and performing a Fisher's exact test using the two-by-two table to determine whether a given one of side effects is differentially shown between positive predicted drug-drug interactions and negative predicted drug-drug interactions.
Owner:IBM CORP
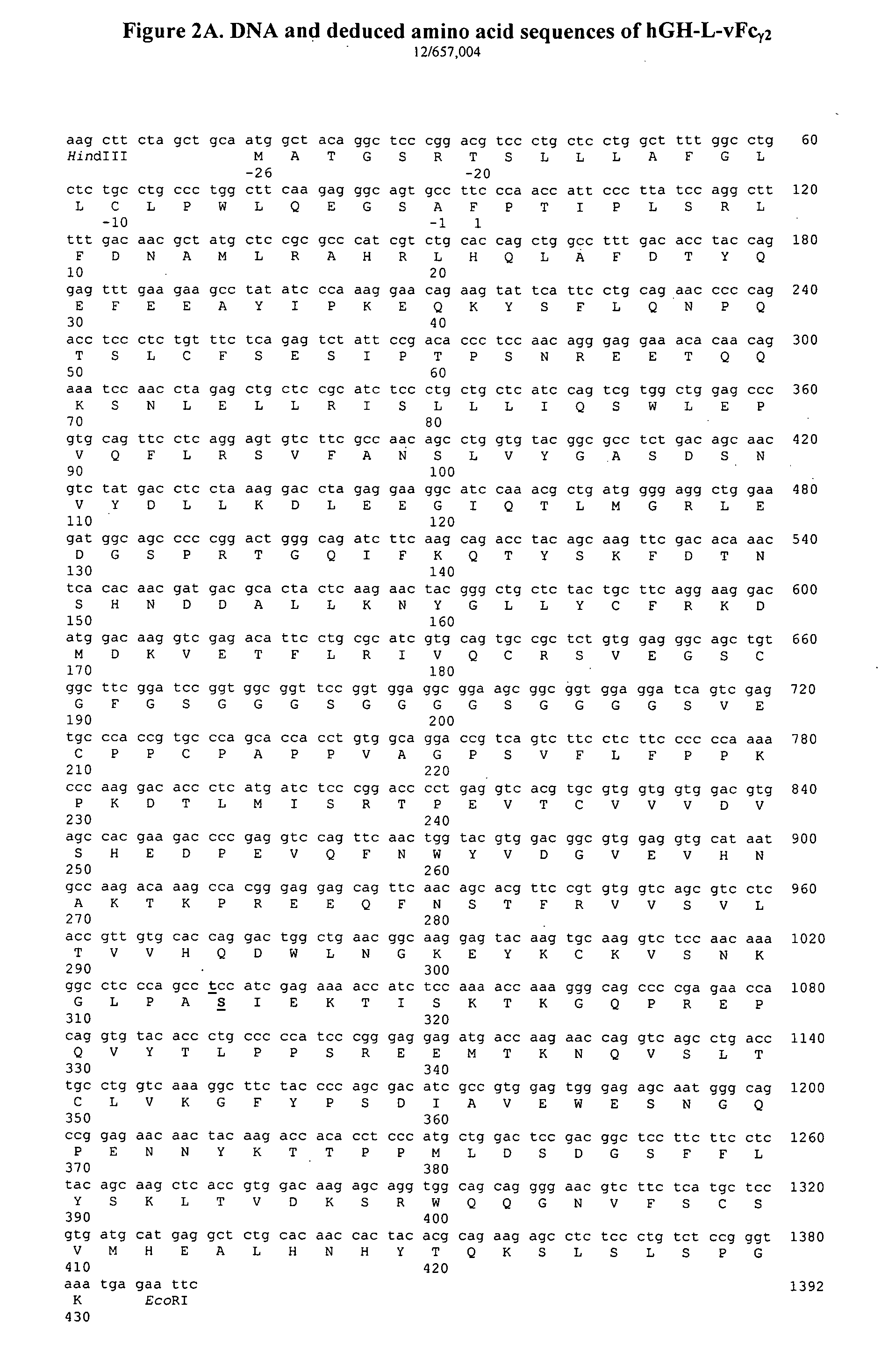
Fc fusion proteins of human growth hormone
ActiveUS20120116056A1Improve biological activityImproved pharmacodynamicsAnimal cellsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human growth hormone with good biological activities relative to rhGH on a molar basis are disclosed. The hGH-L-vFc fusion protein comprises hGH, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such hGH-L-vFc fusion proteins exhibit extended or prolonged serum half-life and / or good biological activities relative to that of rhGH on a molar basis, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com