Fc fusion protein of human granulocyte colony stimulin with enhanced bioactivity
A fusion protein and cell technology, applied in colony-stimulating factor, animal/human protein, cytokine/lymphokine/interferon, etc., can solve problems such as weak complement activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] 1. Construction of encoding hG-CSF-L-vFc γ2 fusion protein gene
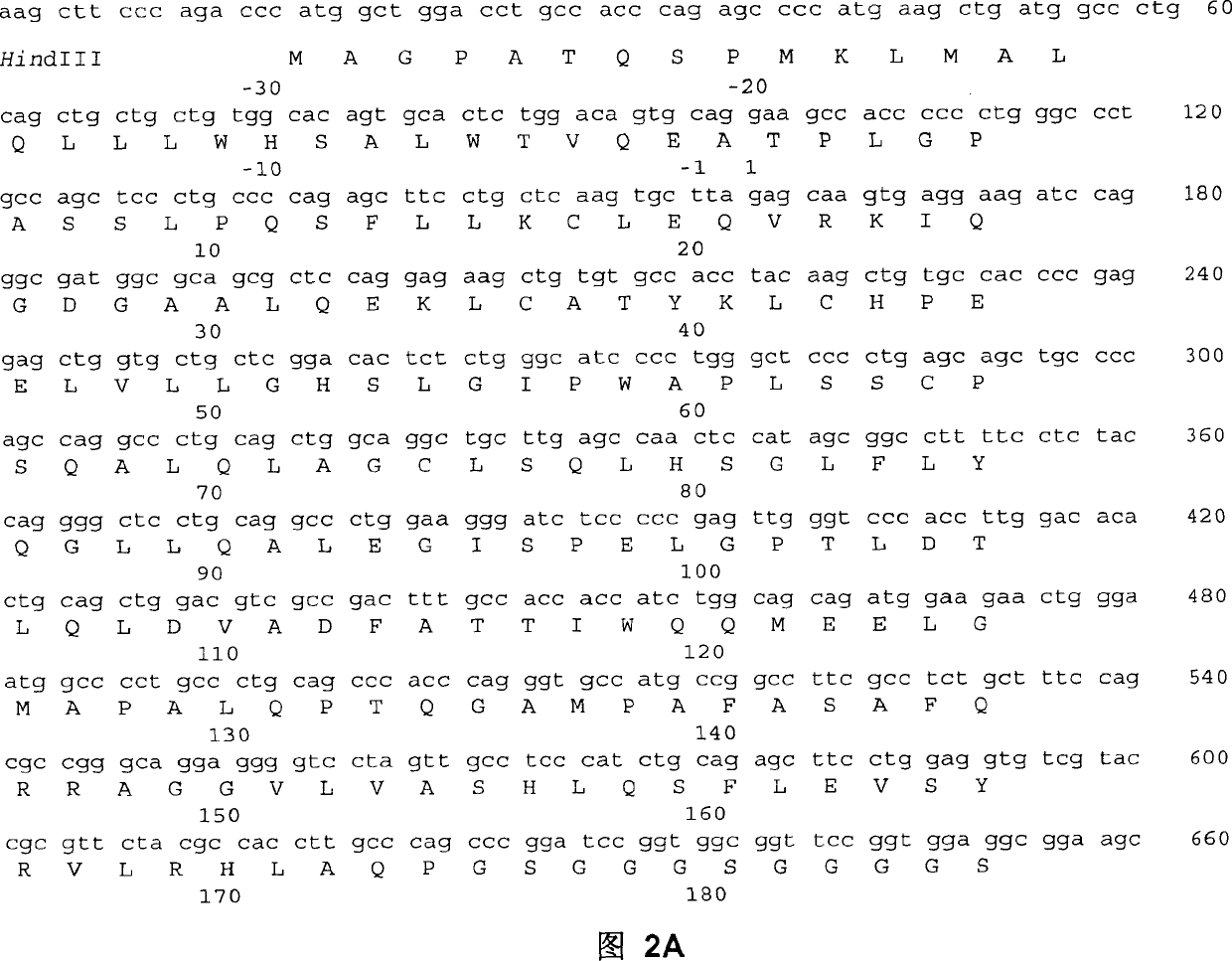
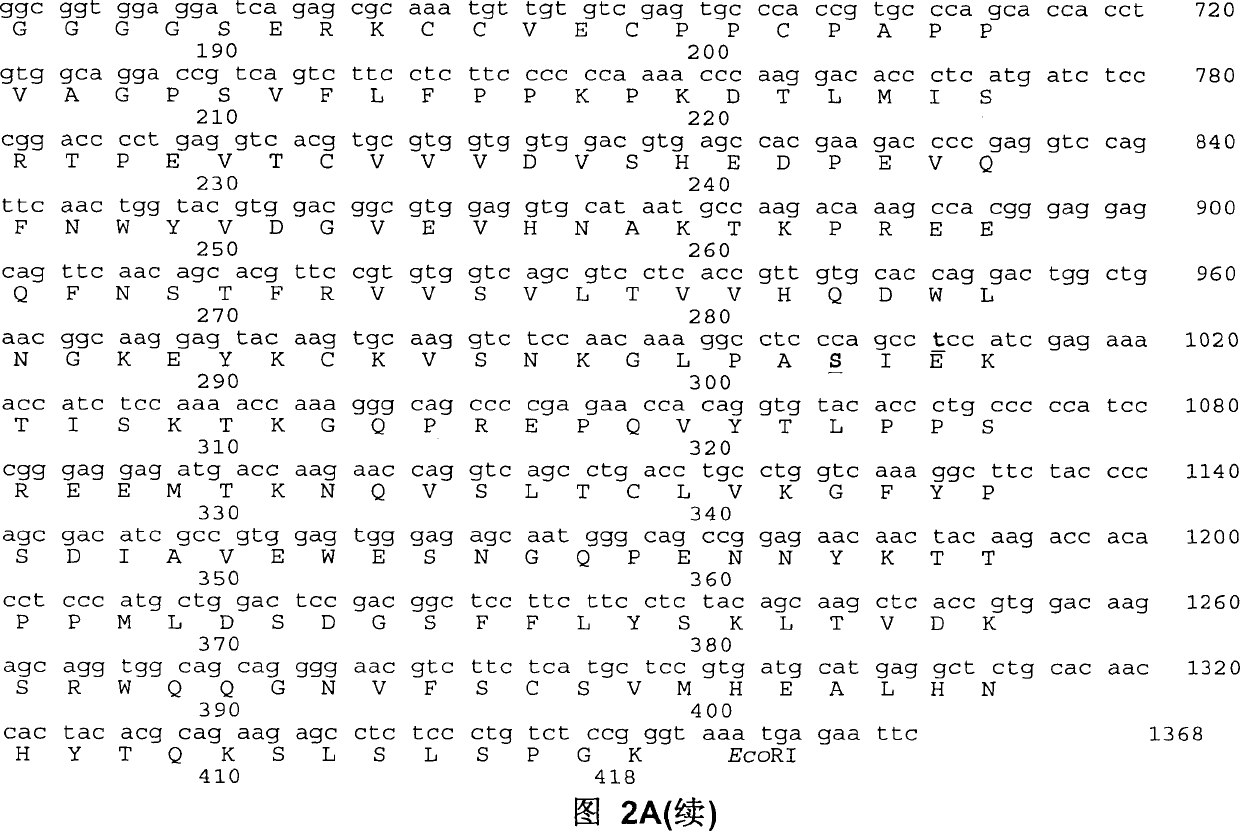
[0019] Fusion proteins are constructed from several DNA fragments. Using RNA prepared from human bladder cancer 5637 cell line, genes encoding the leader peptide and mature protein of human G-CSF were obtained by reverse transcription and polymerase chain reaction (PCR). To facilitate cloning, SEQ ID NO: 1 introducing a restriction enzyme endonuclease site (HindIII) was used as a primer for the 5' oligonucleotide. Table 1 lists the oligonucleotide sequences used to clone the hG-CSF-L-vFc fusion protein. The 3' primer (SEQ ID NO: 2) removed the G-CSF terminal codon and introduced a BamHI site. The thus obtained DNA fragment of about 600 bp in length is inserted into the HindIII and BamHI sites of an accepting vector (such as pUC19) to obtain a phGCS plasmid. The sequence of the human G-CSF gene was verified by DNA sequencing.
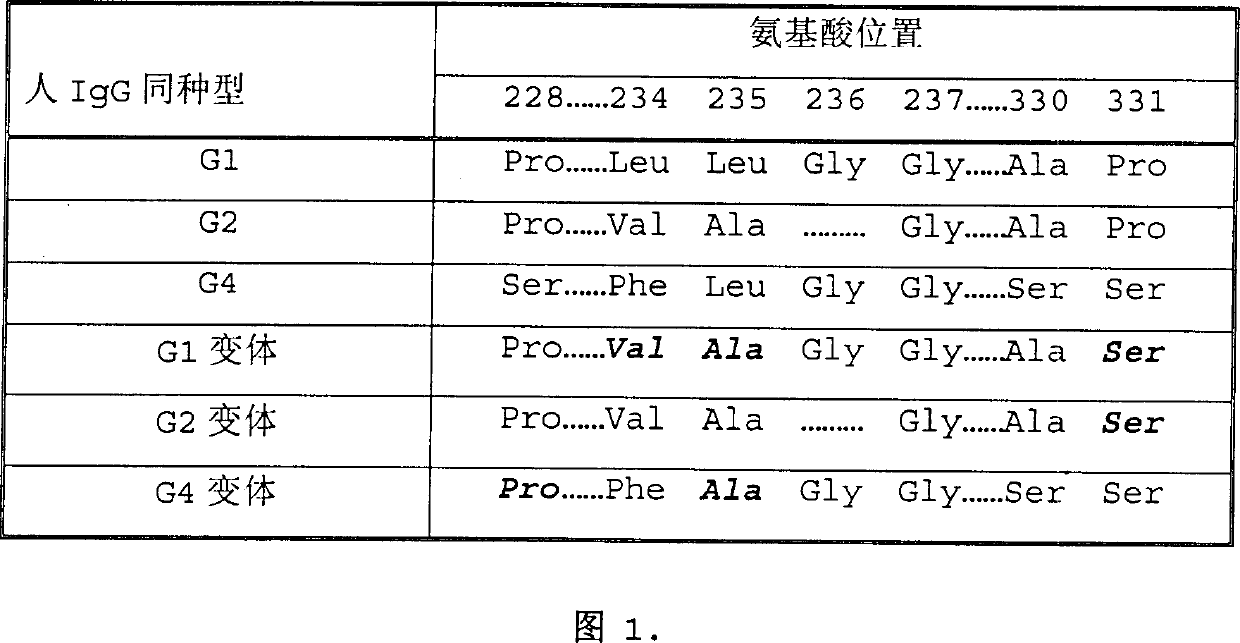
[0020] The Fc region encoding human IgG2 (Fc γ2 ) genes. The resulting F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com