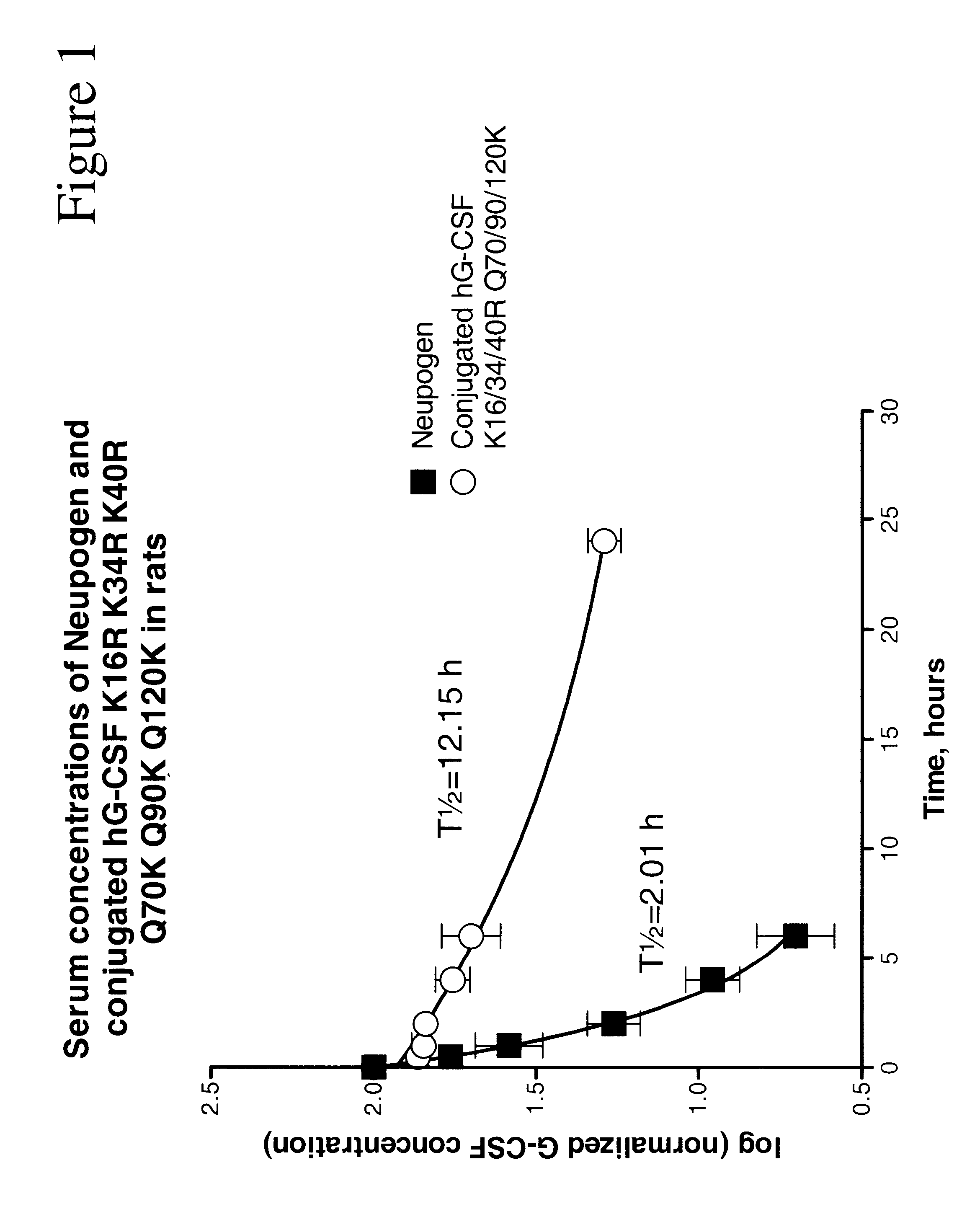
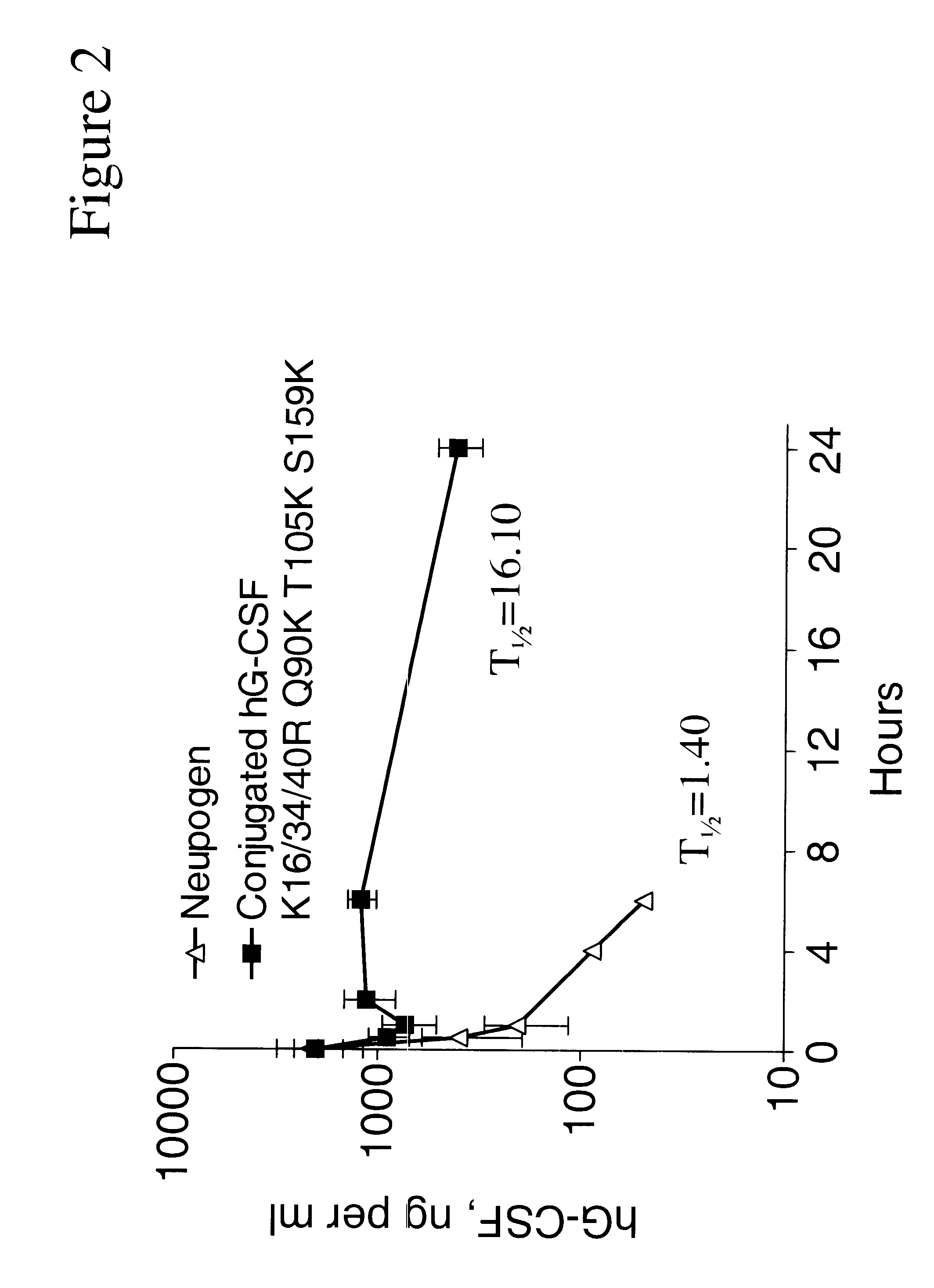
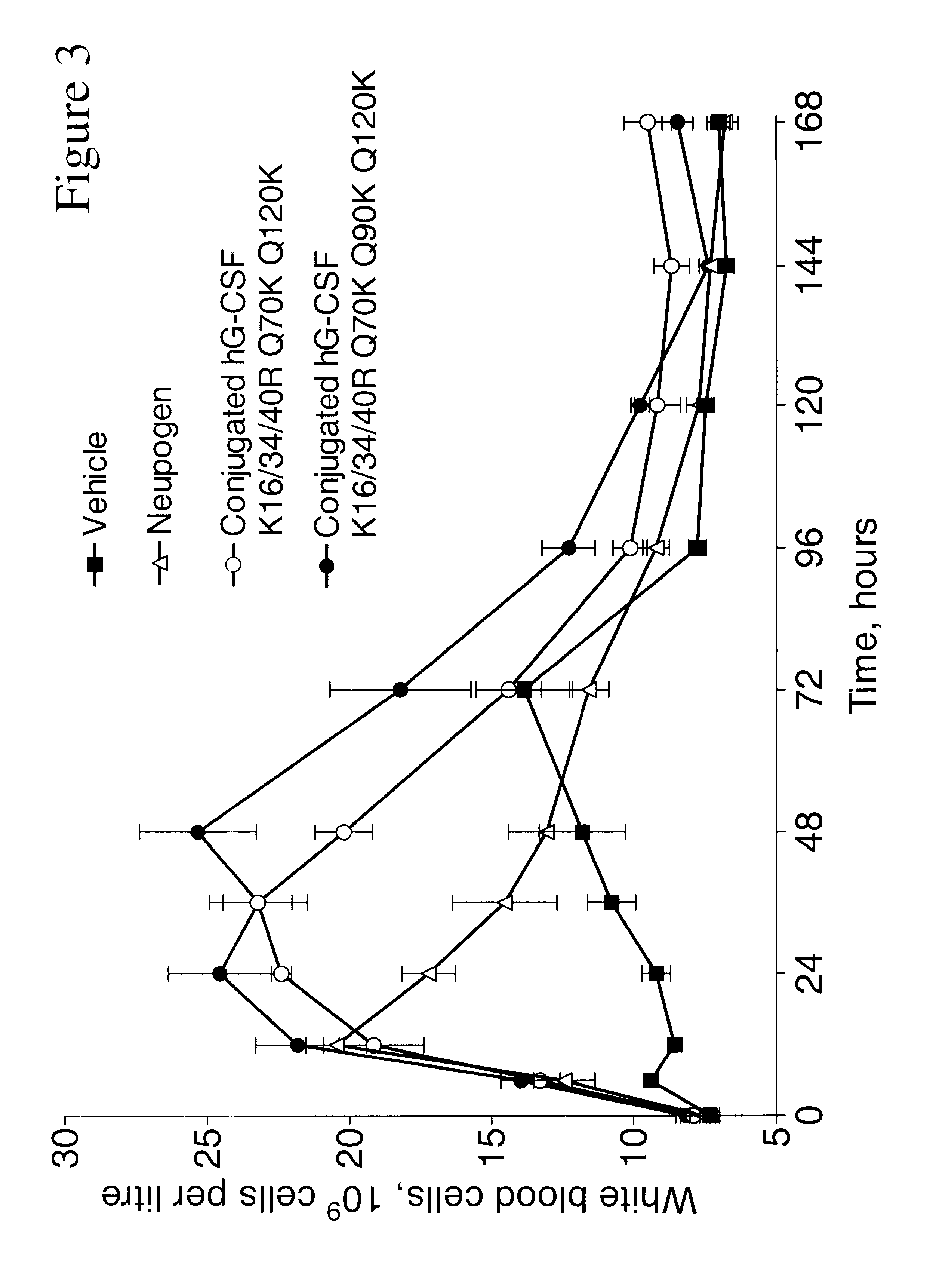
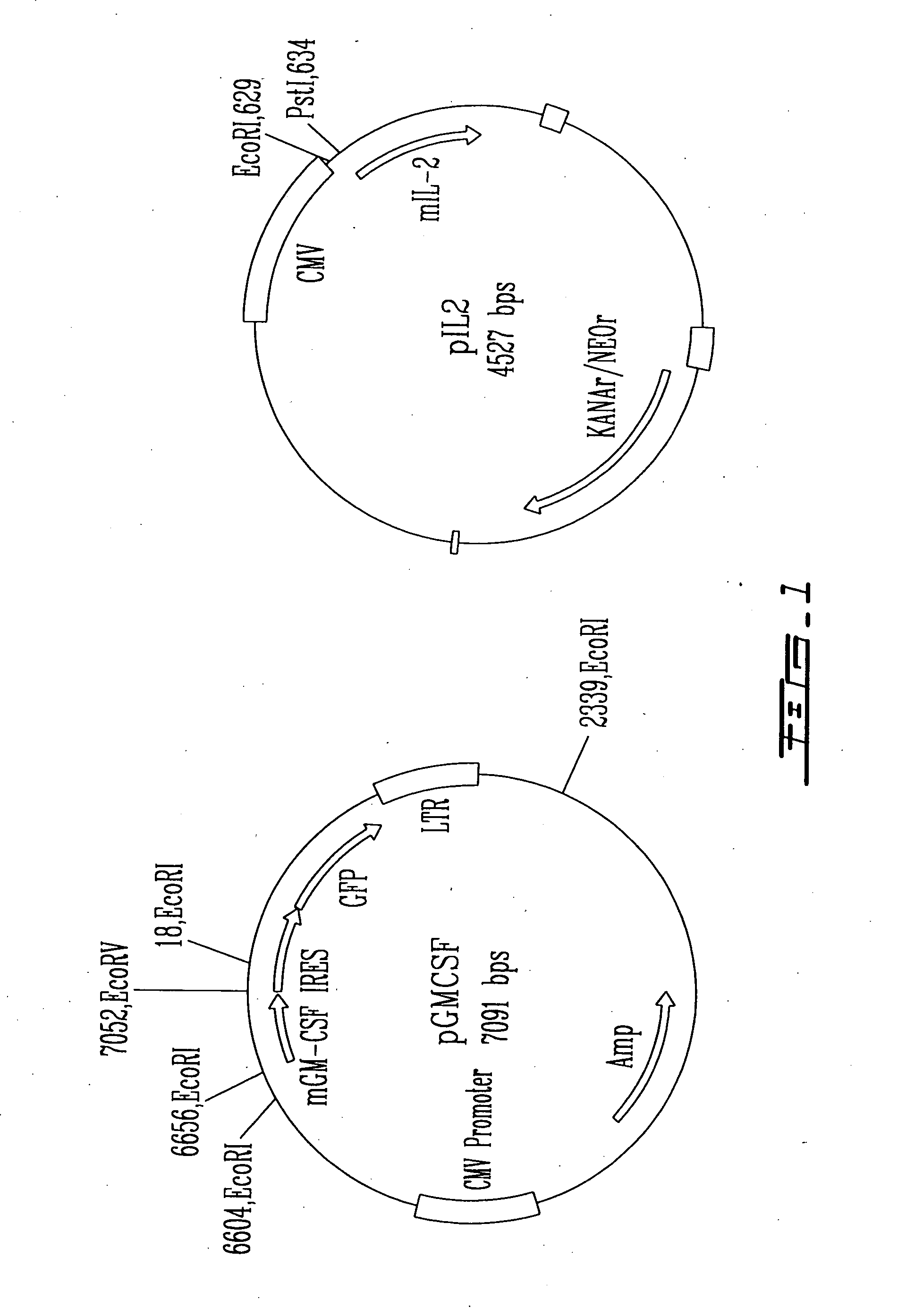
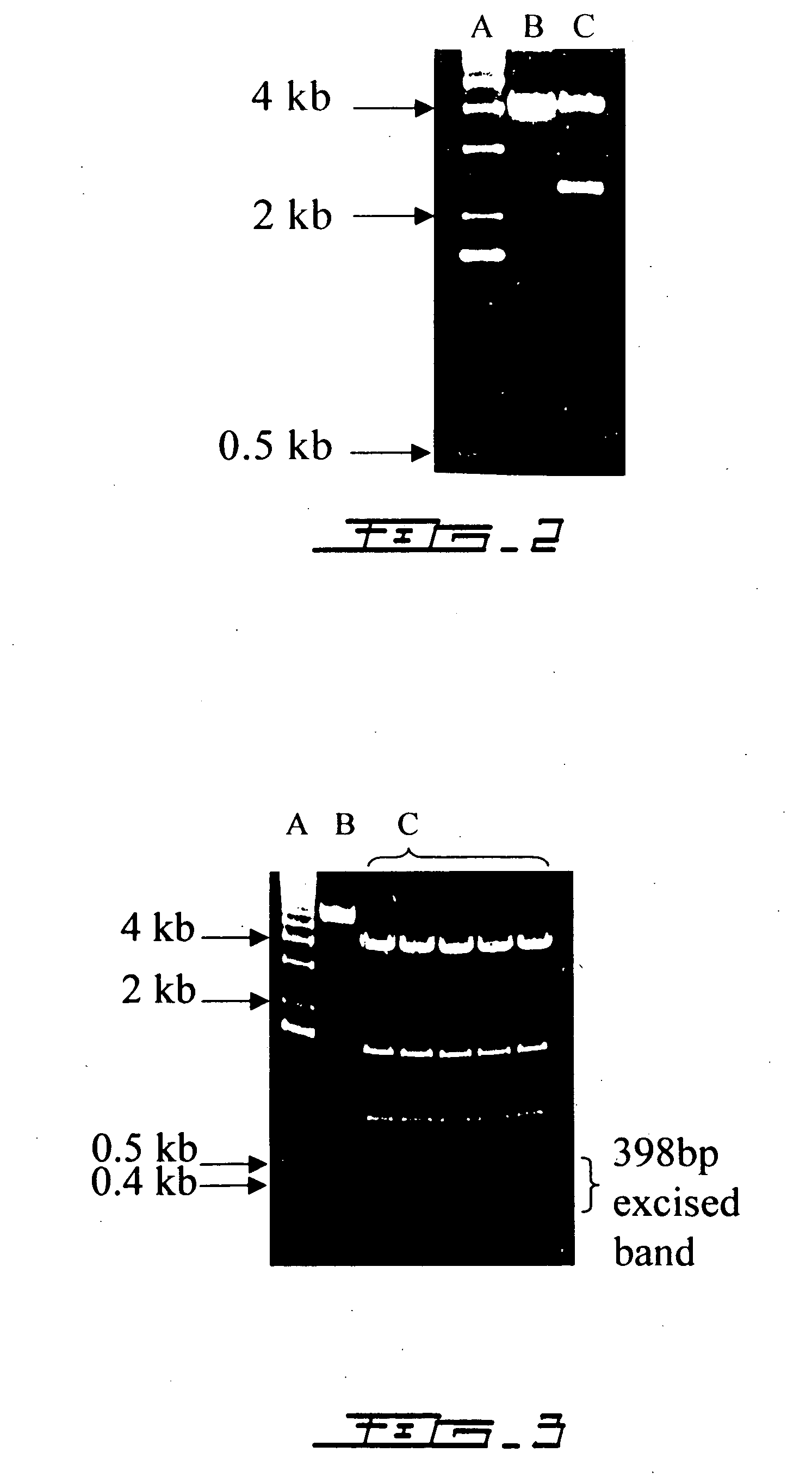
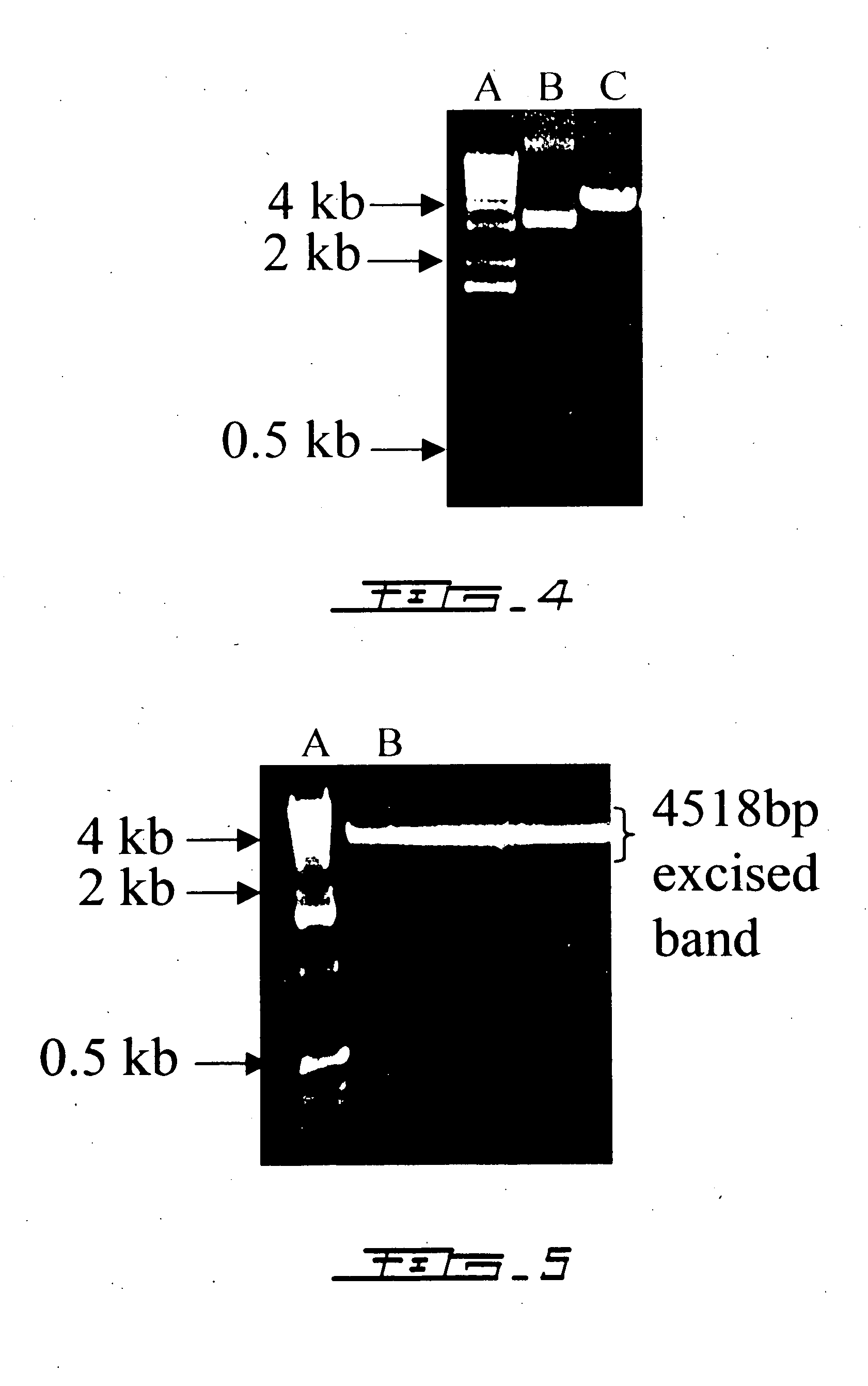
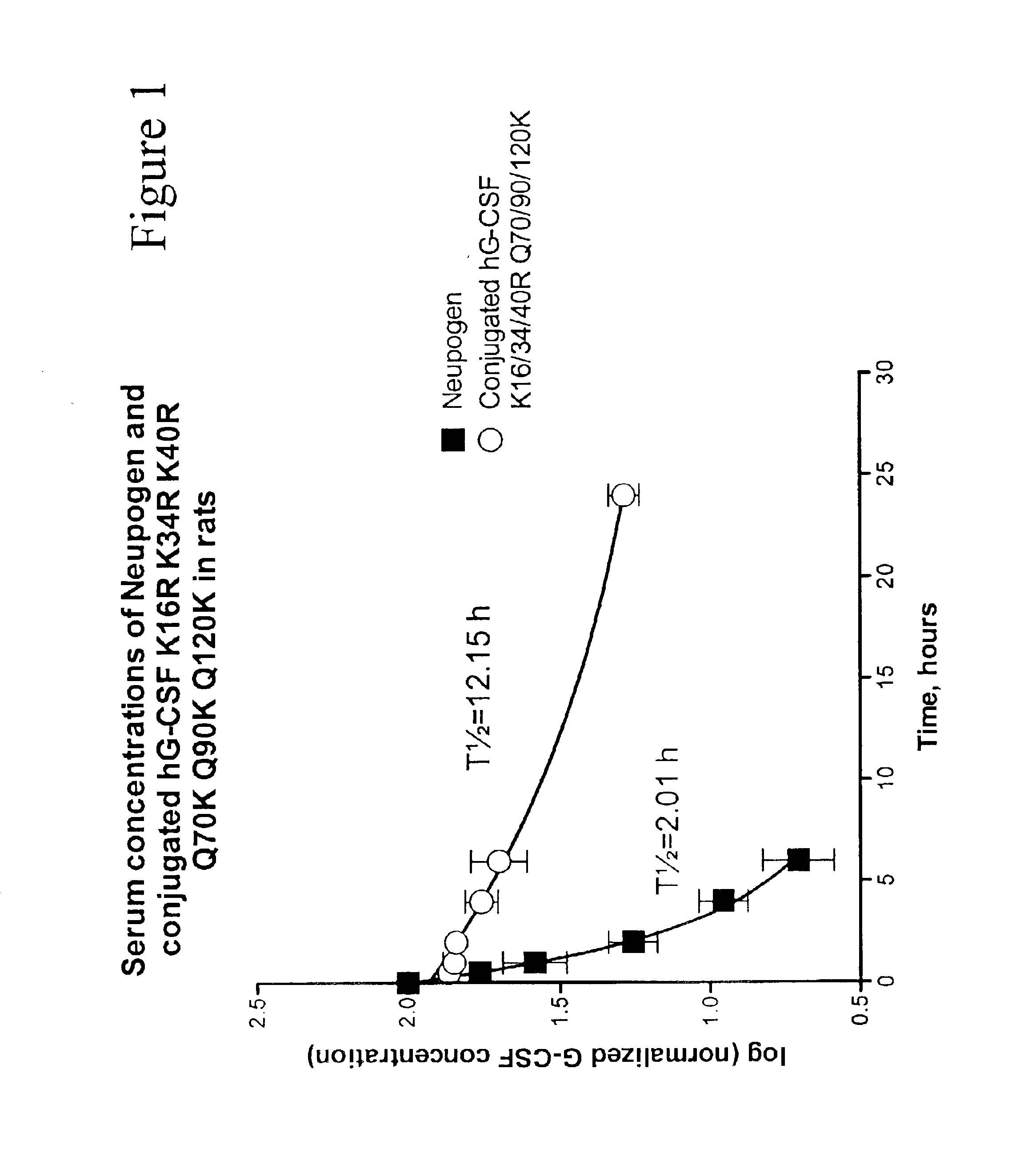
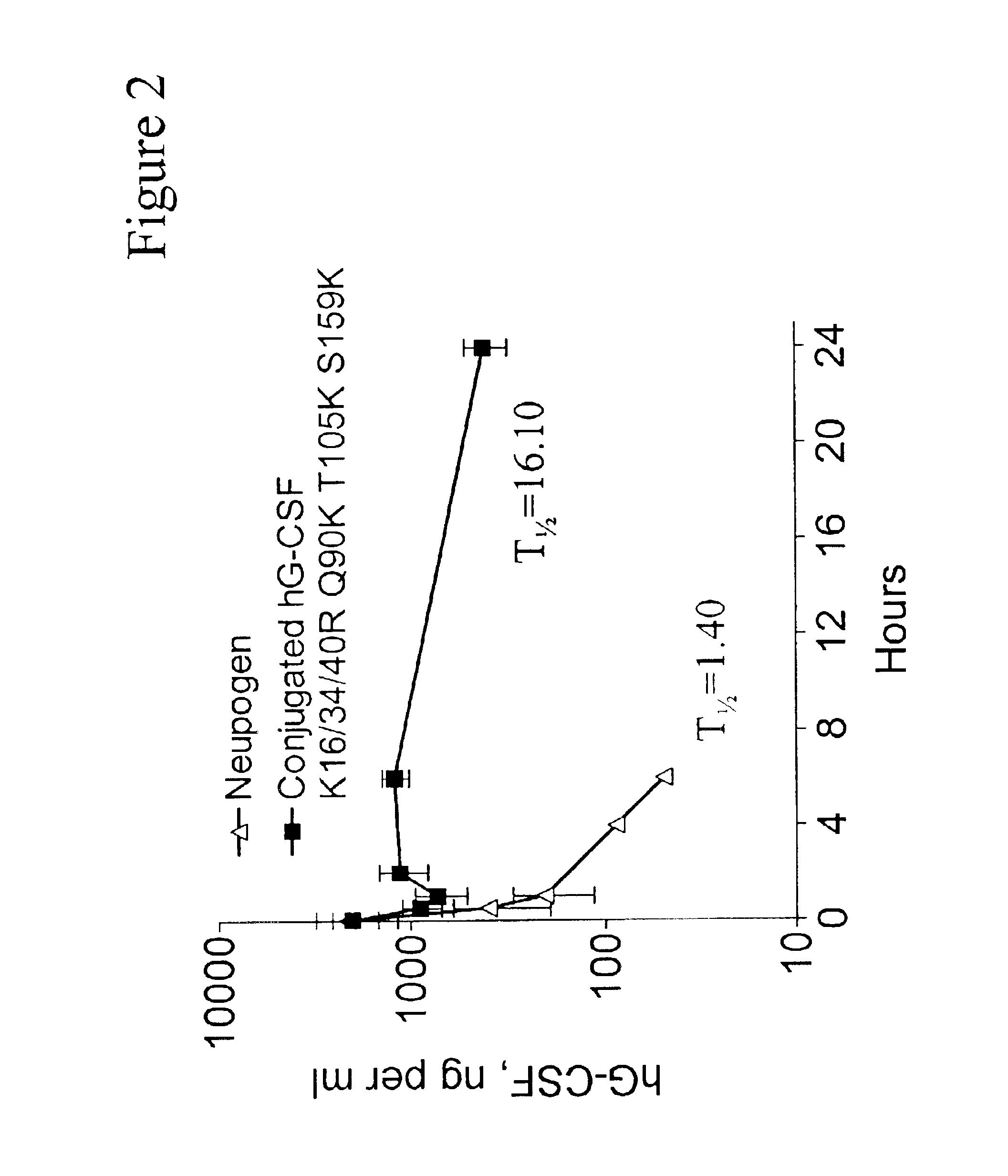
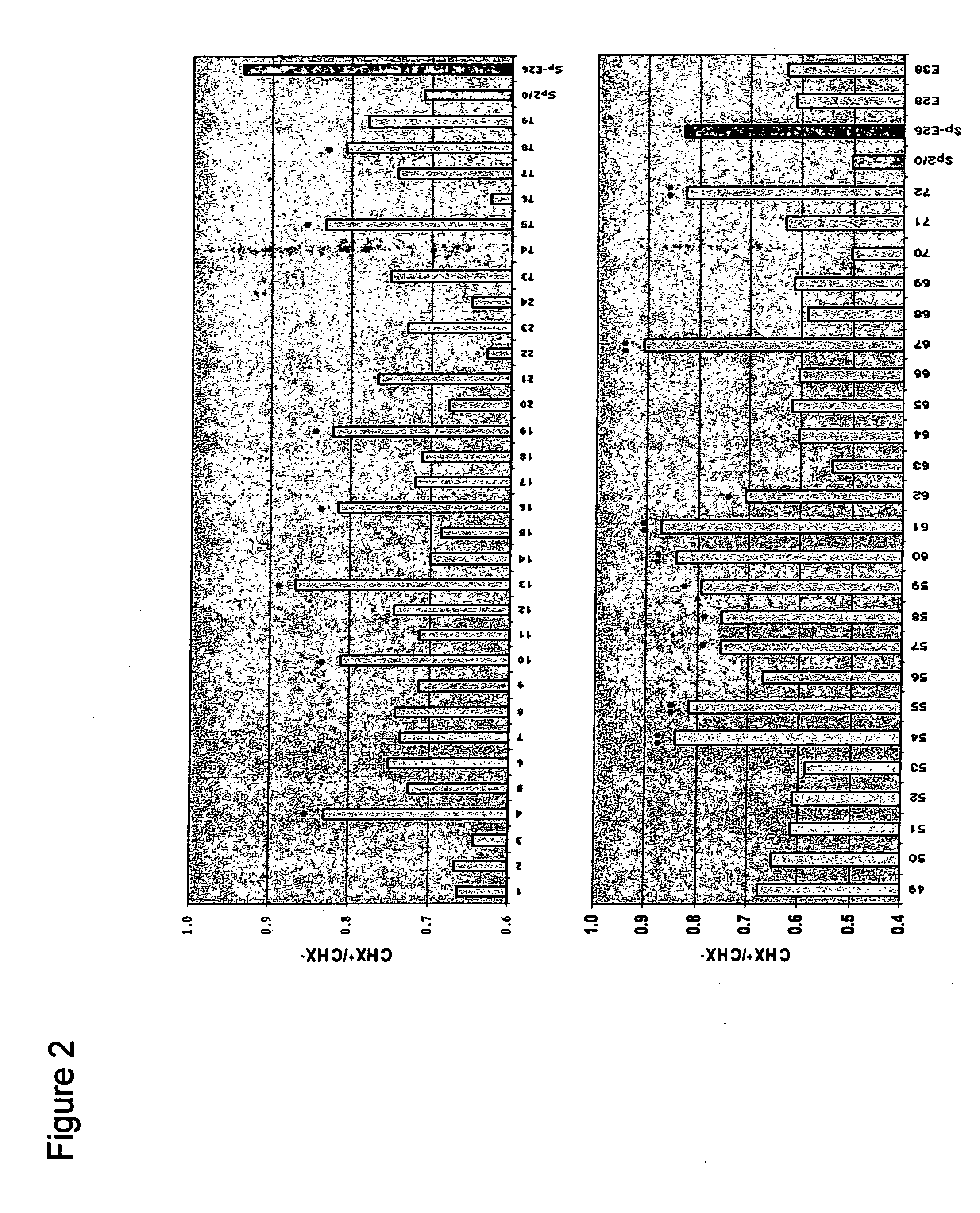
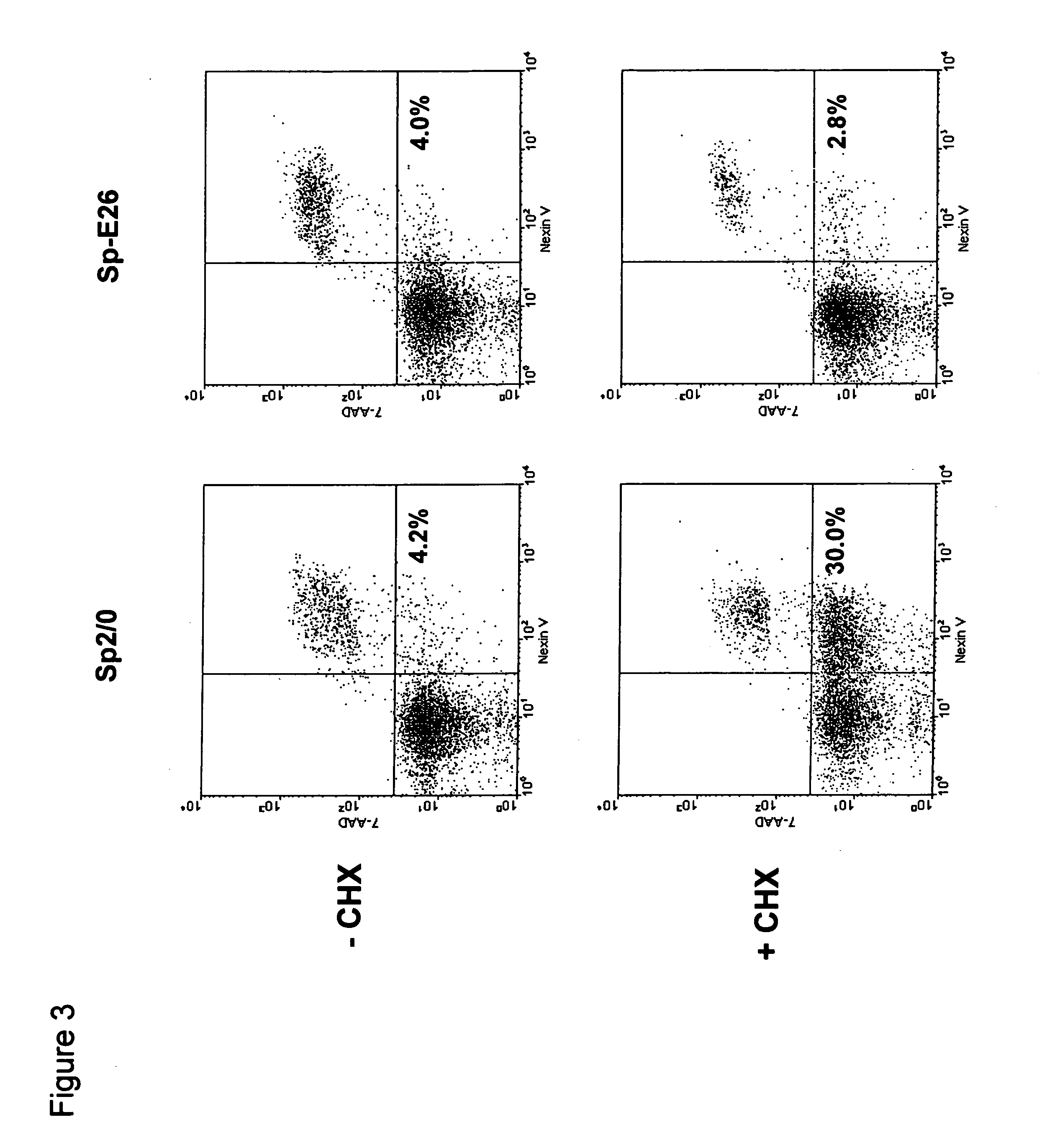
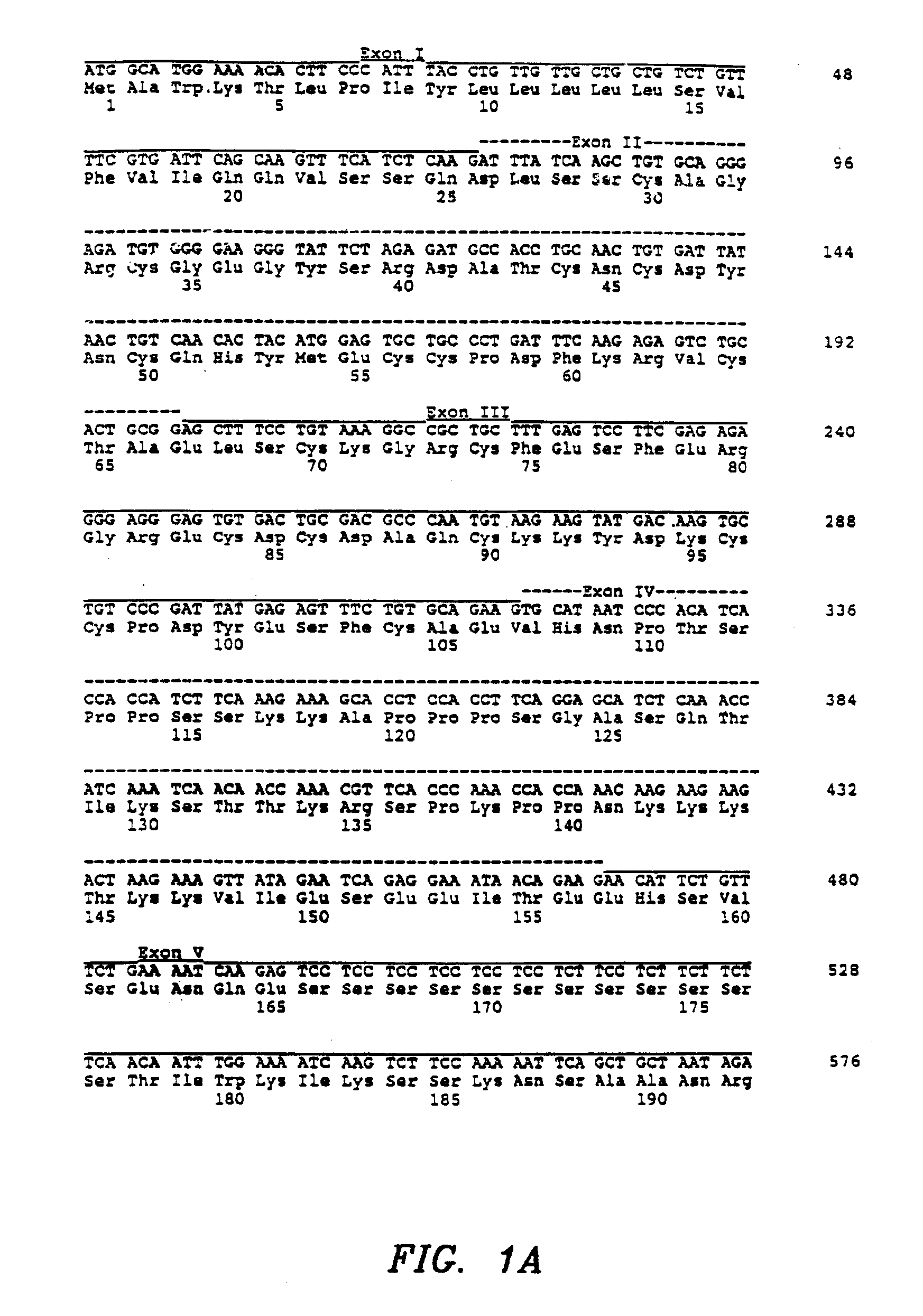
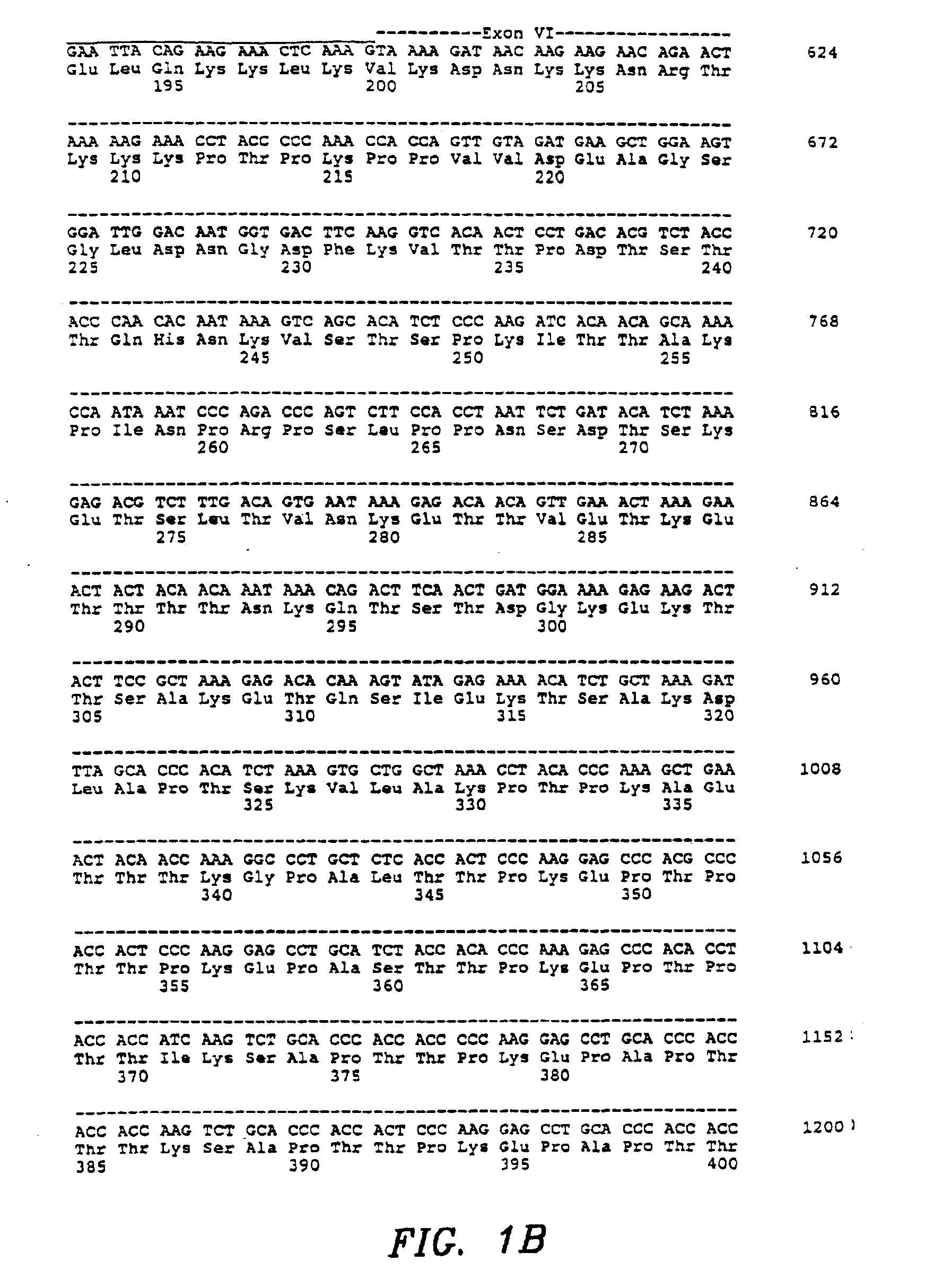
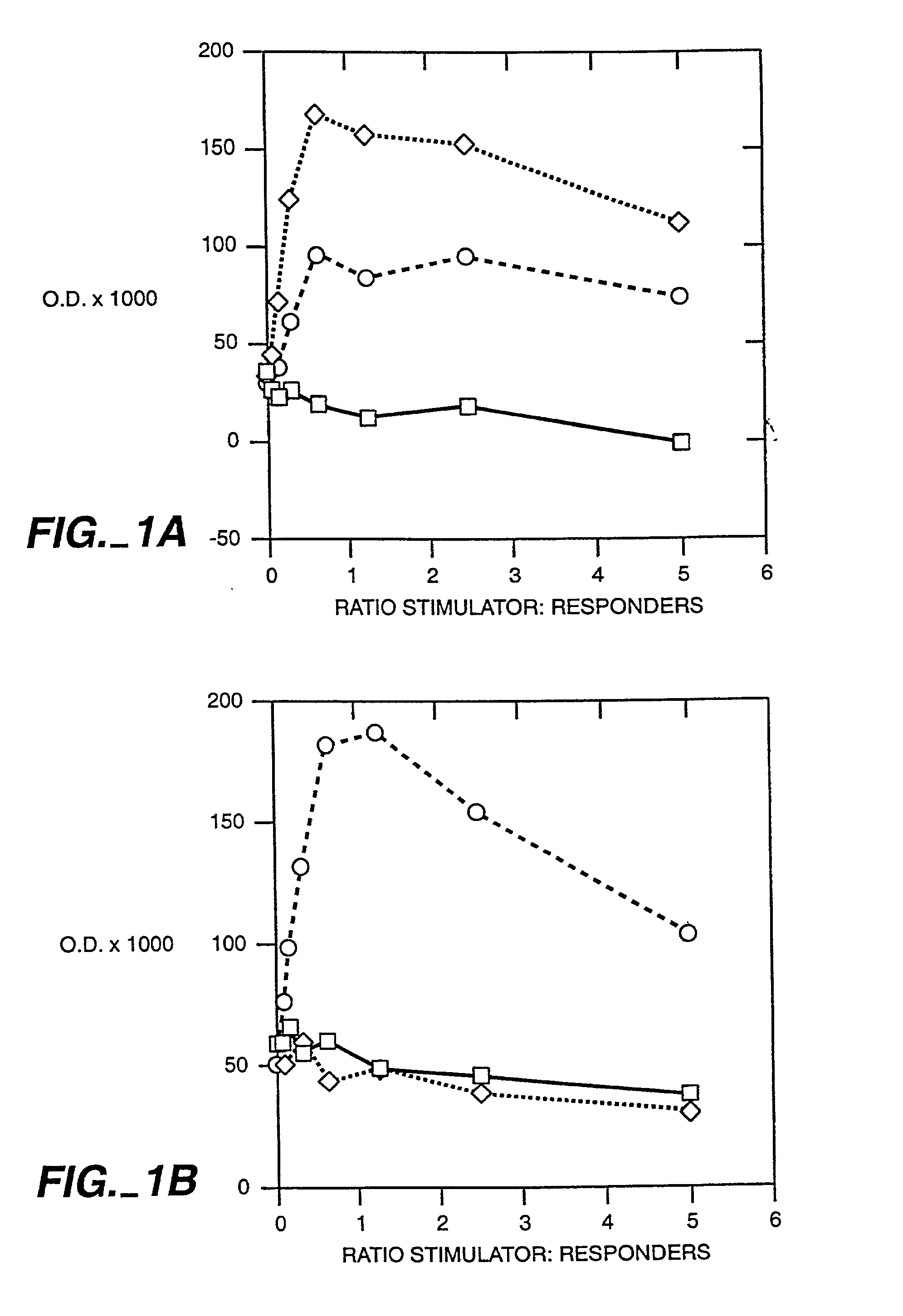
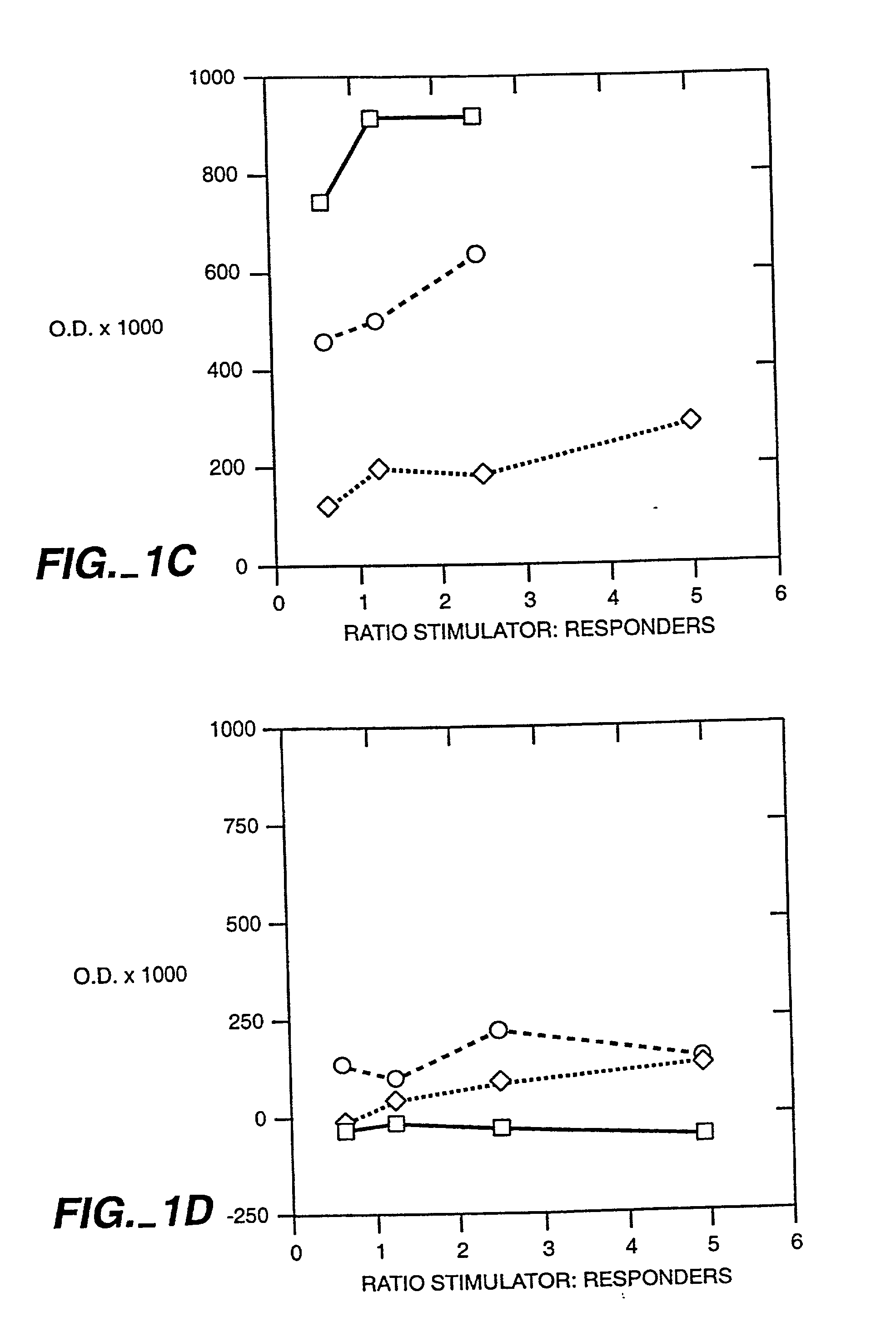
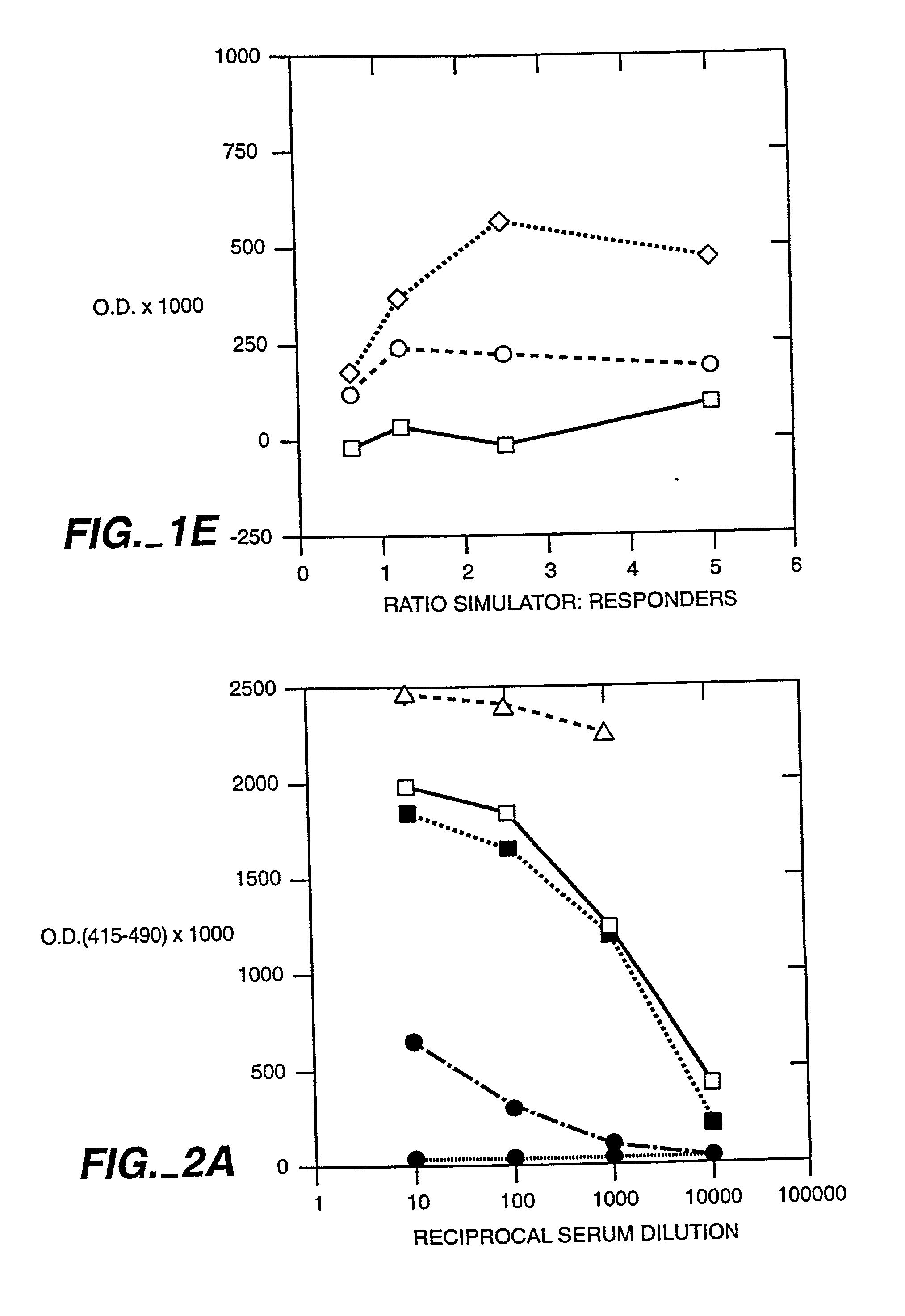
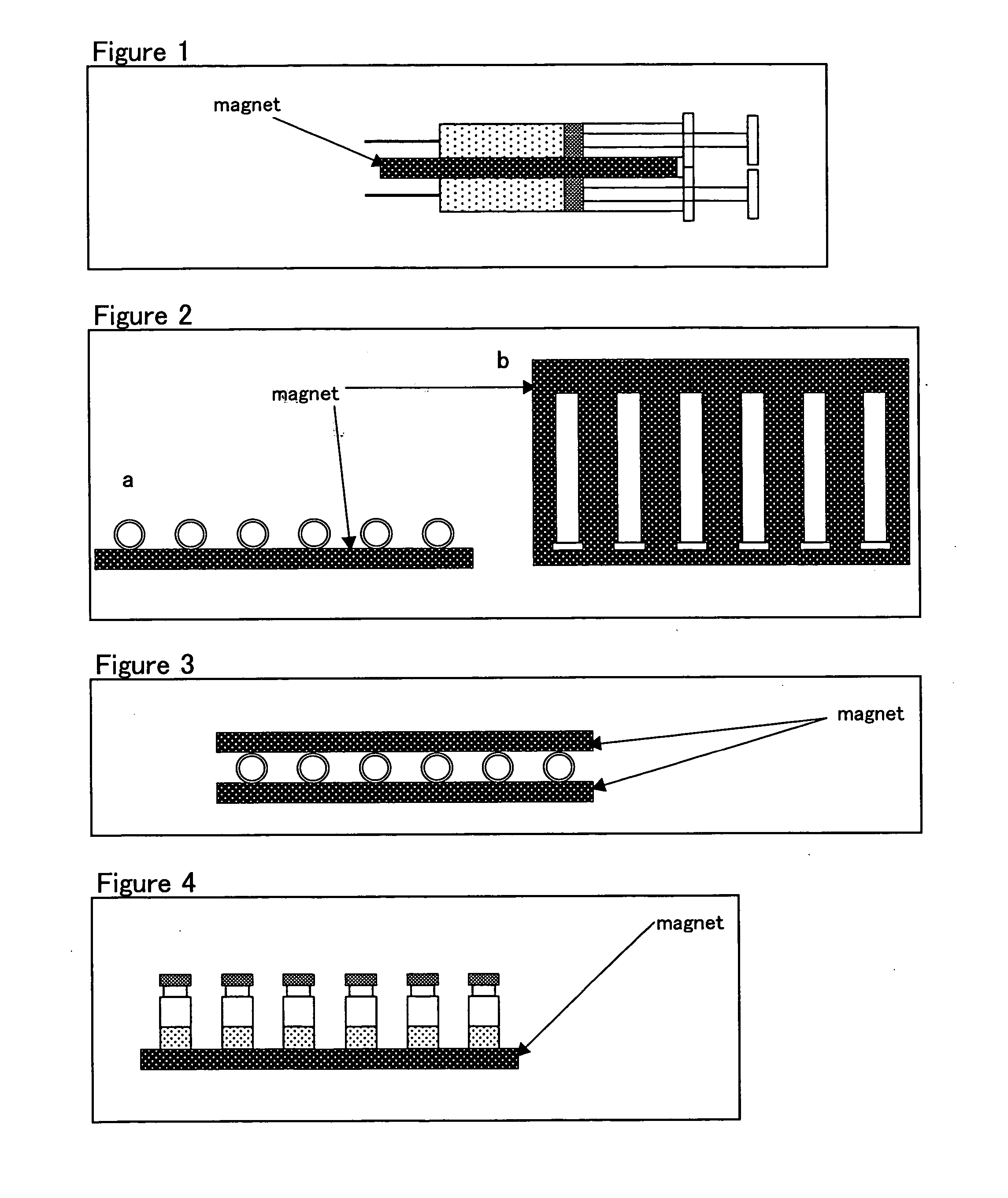
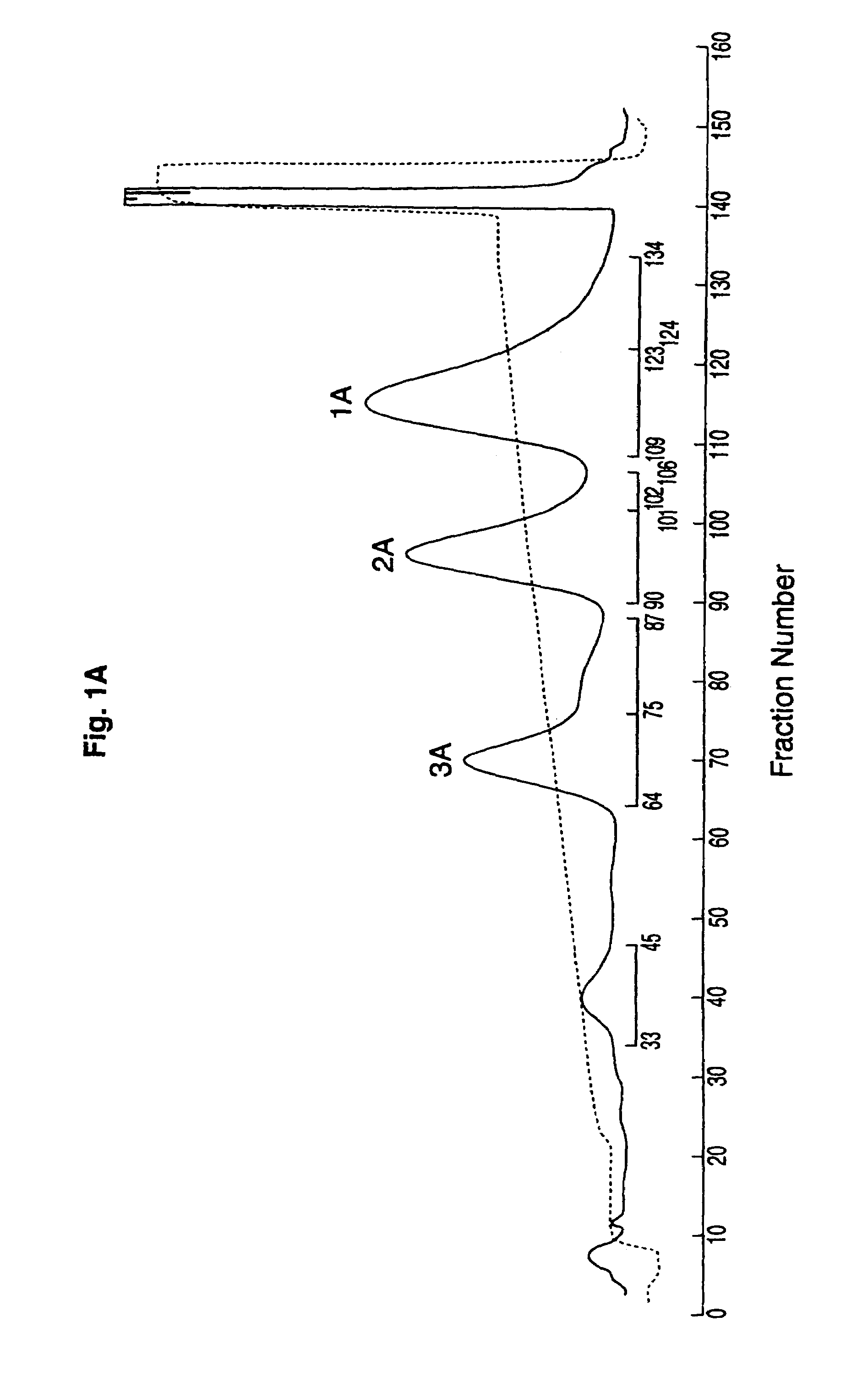
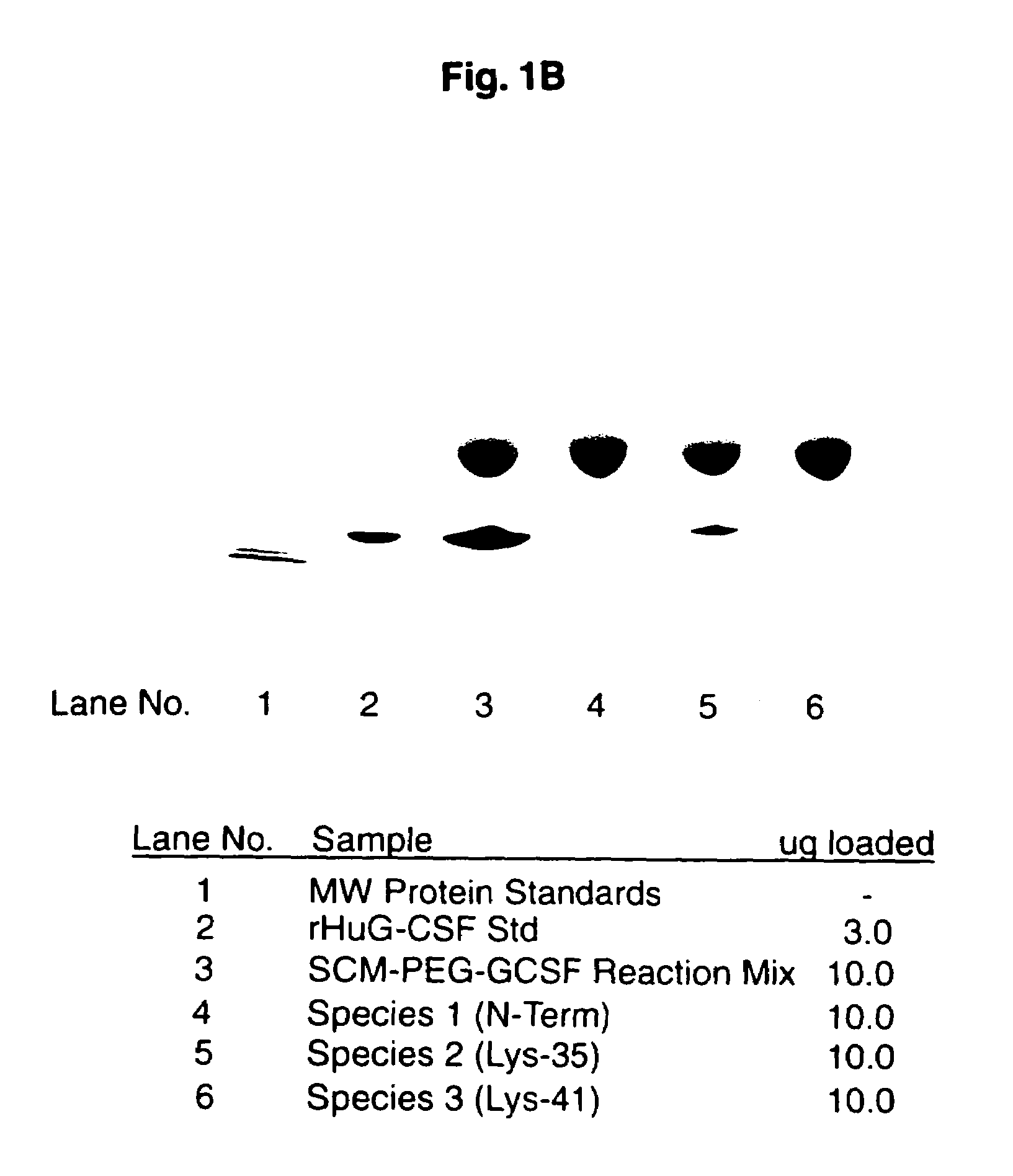
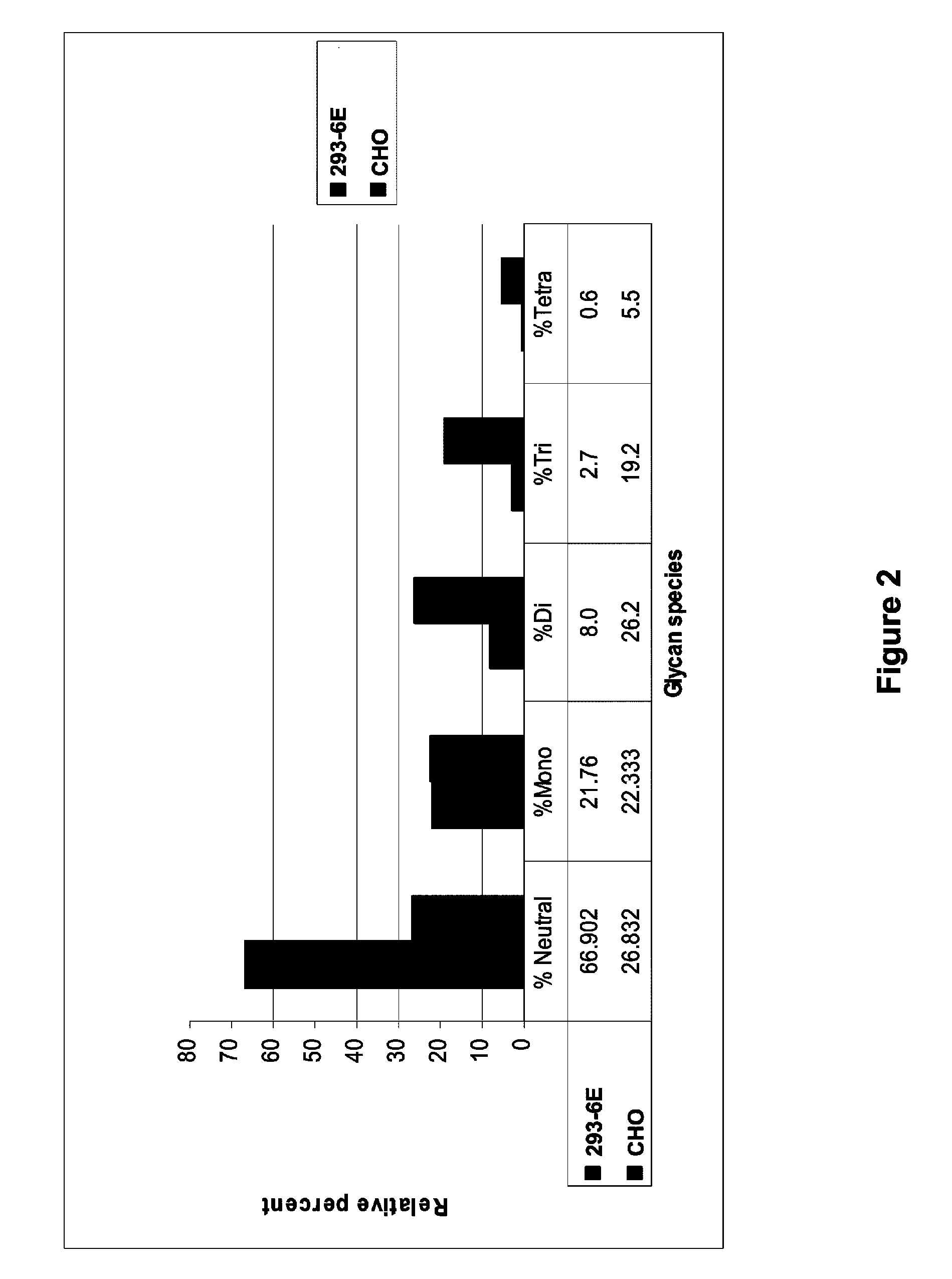
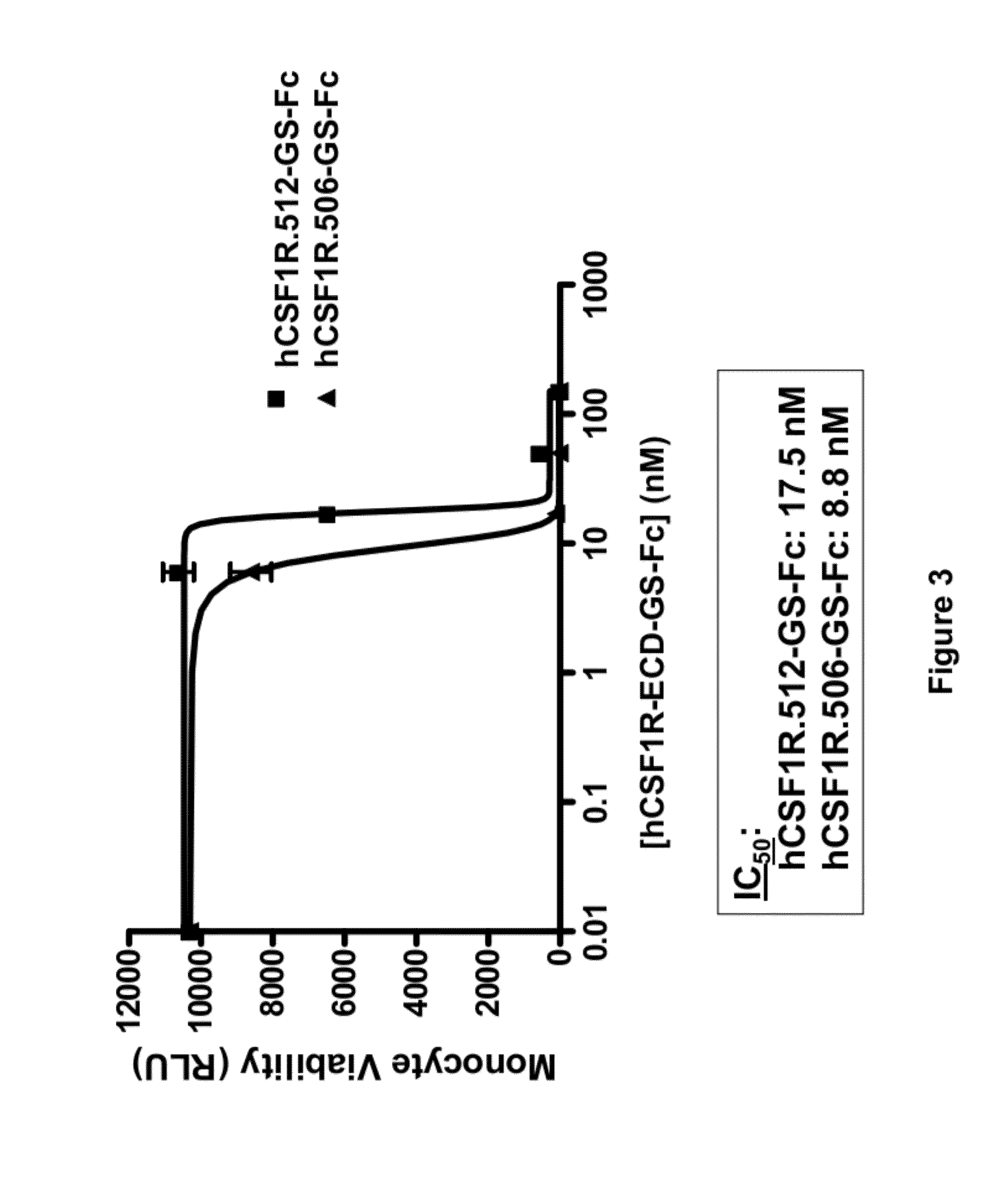
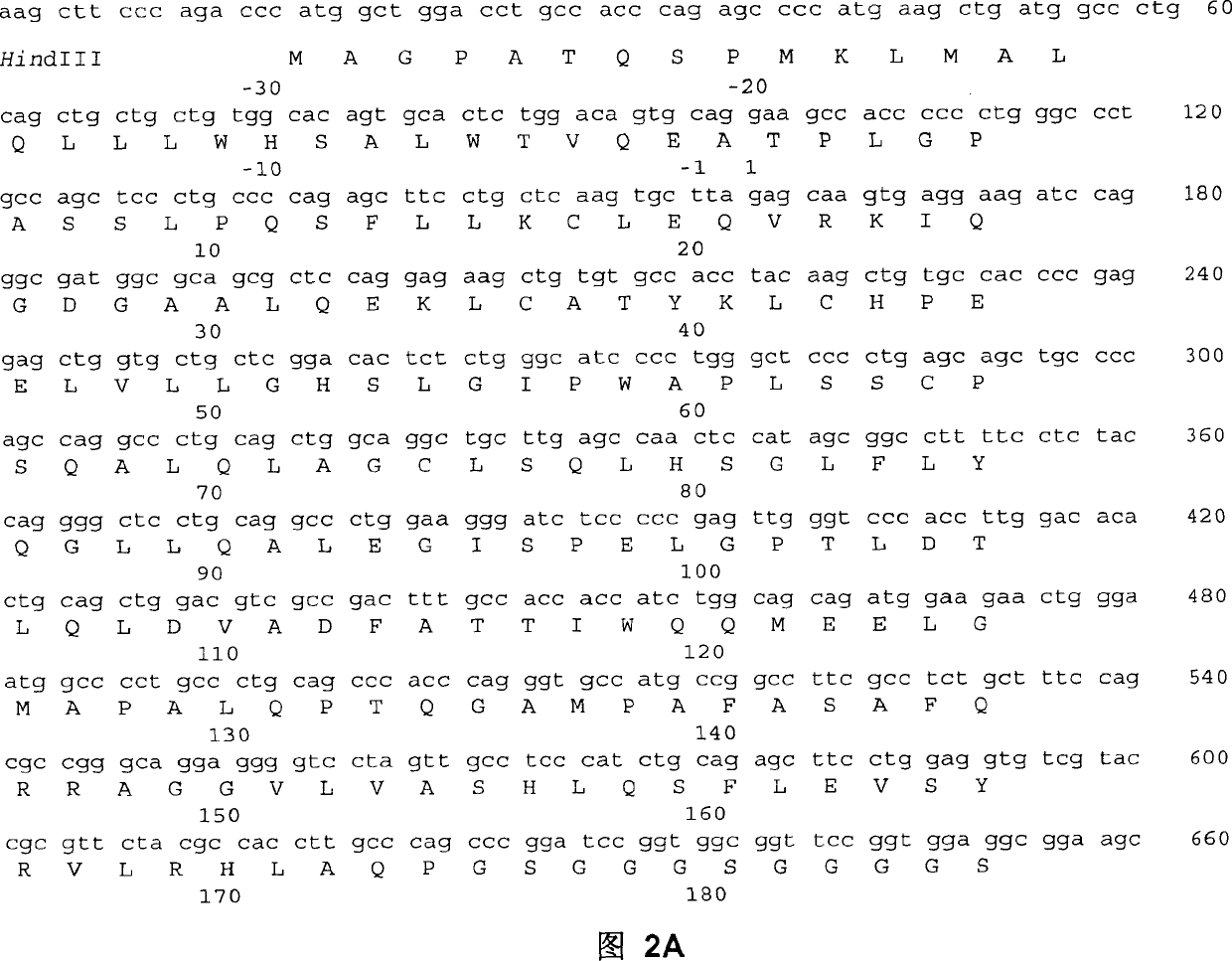
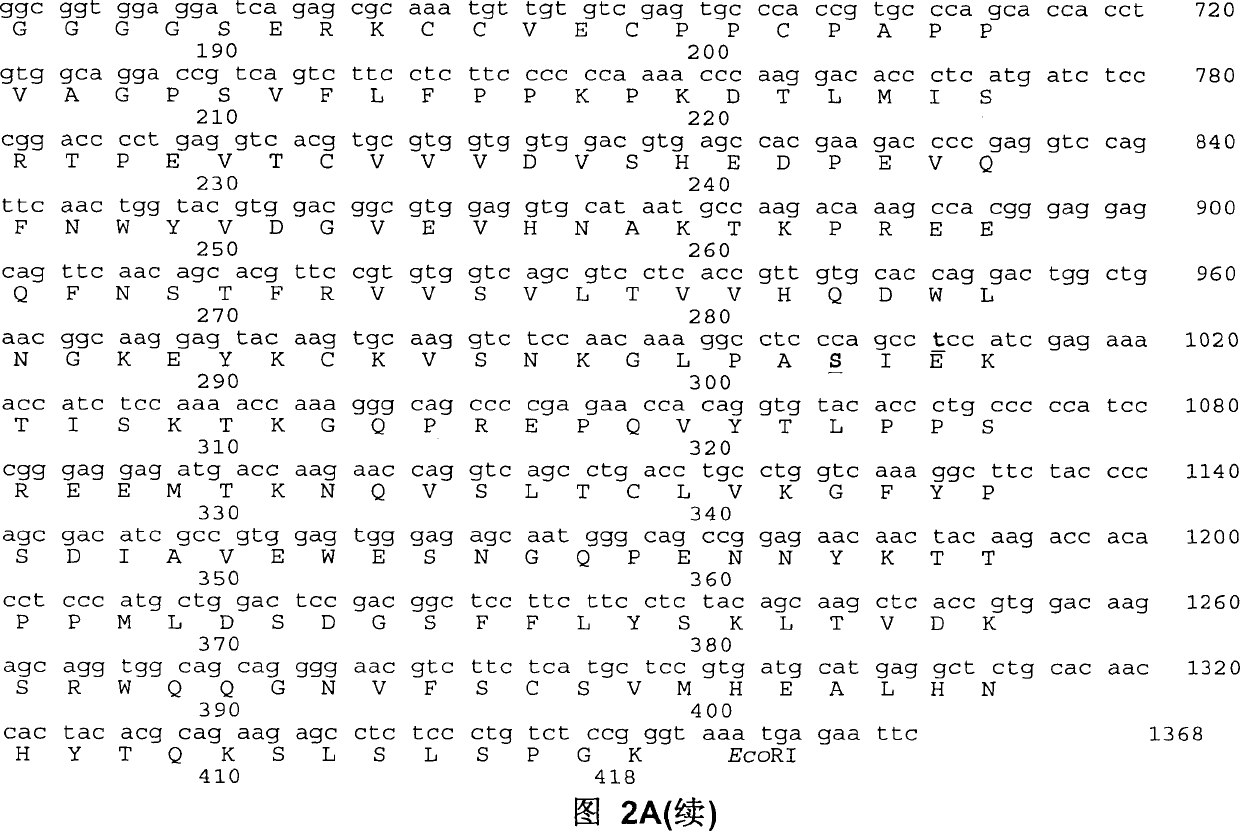
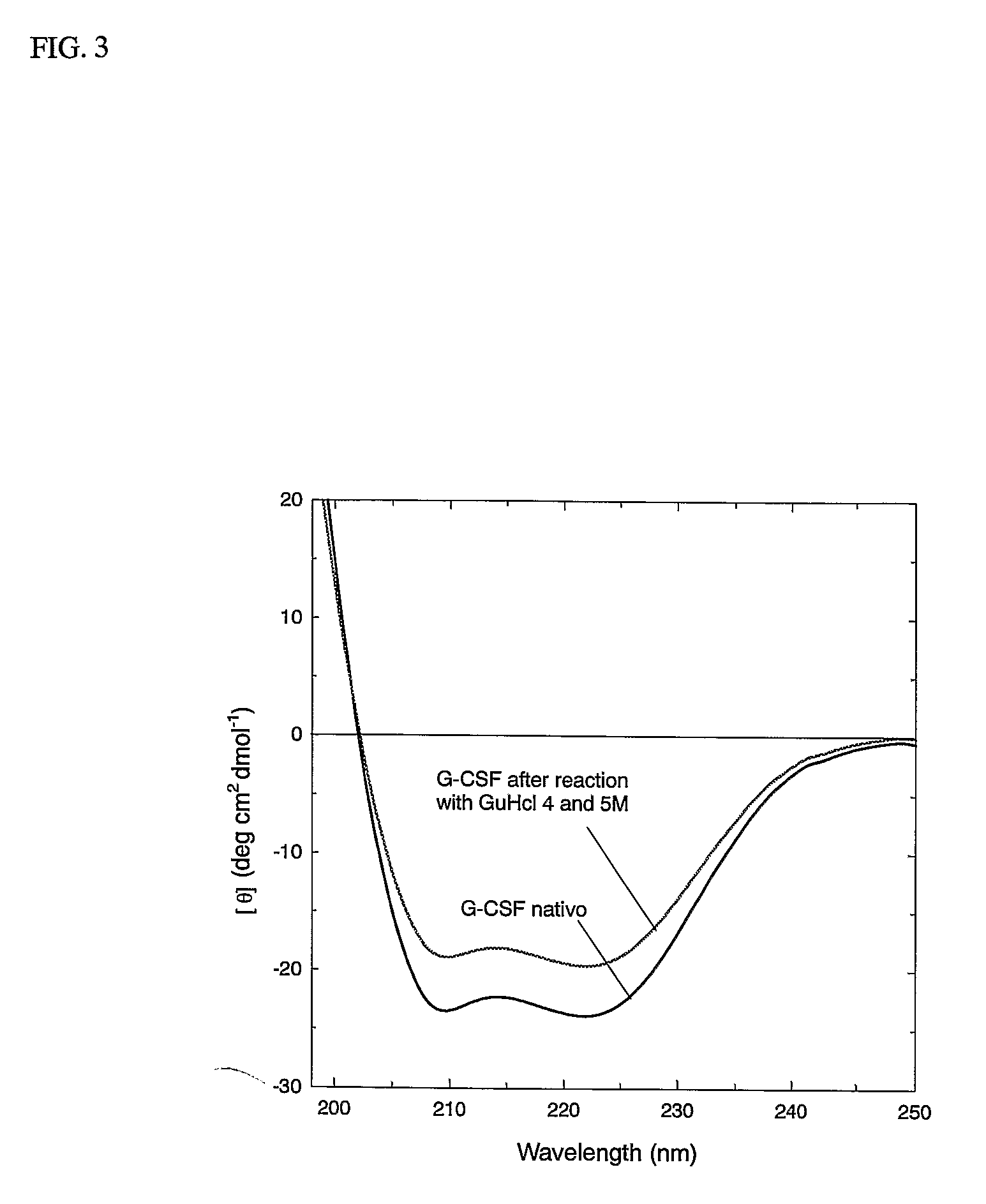
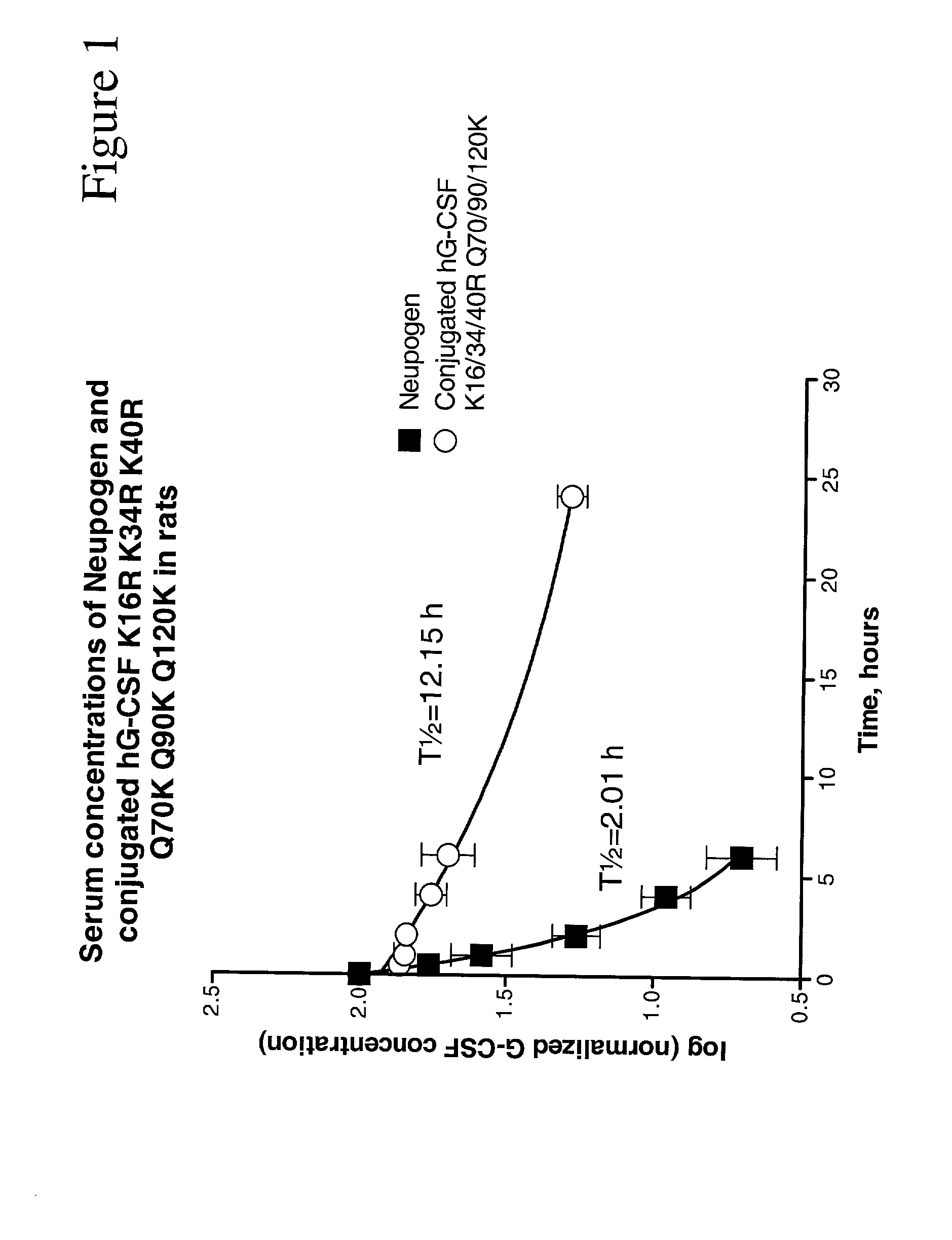
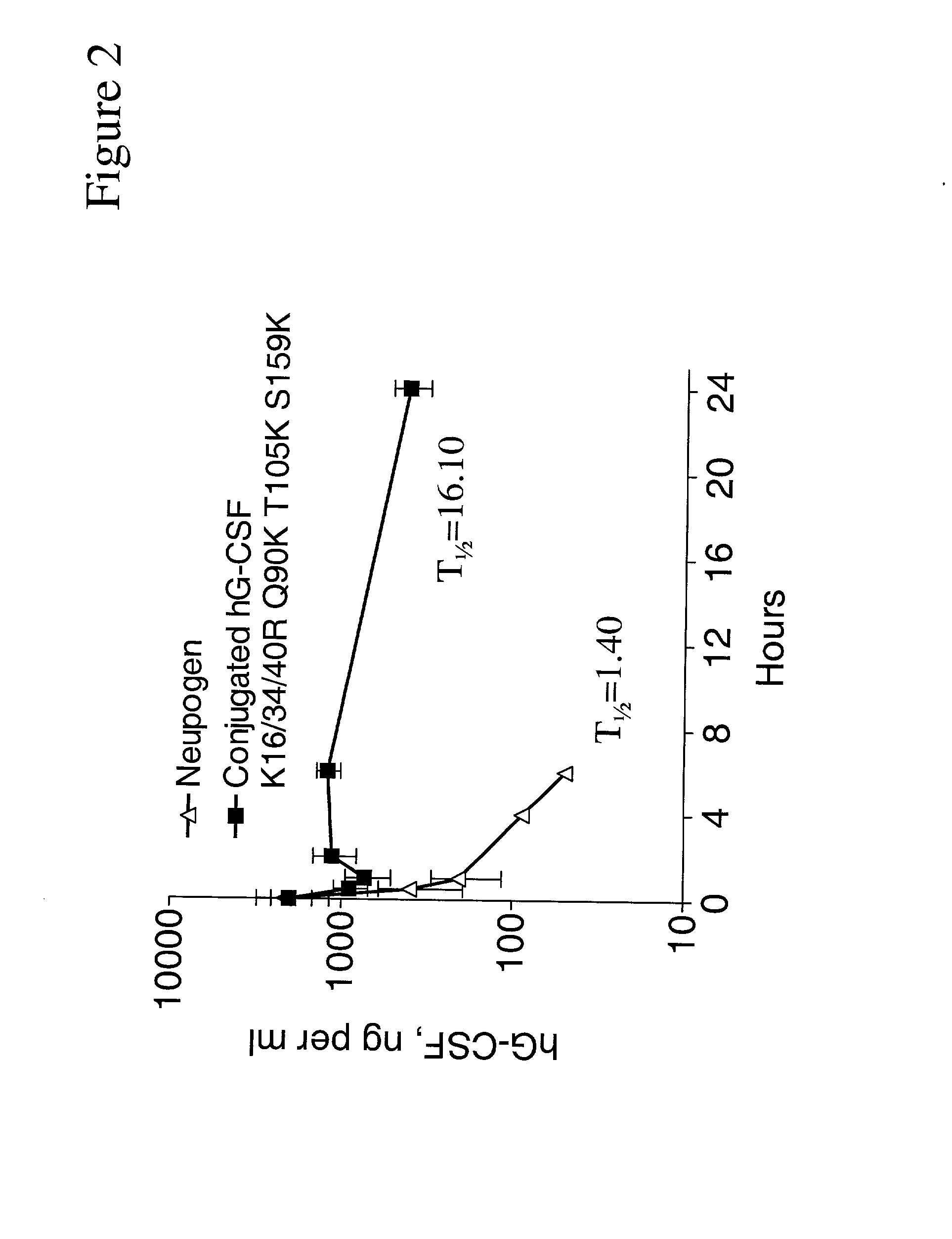
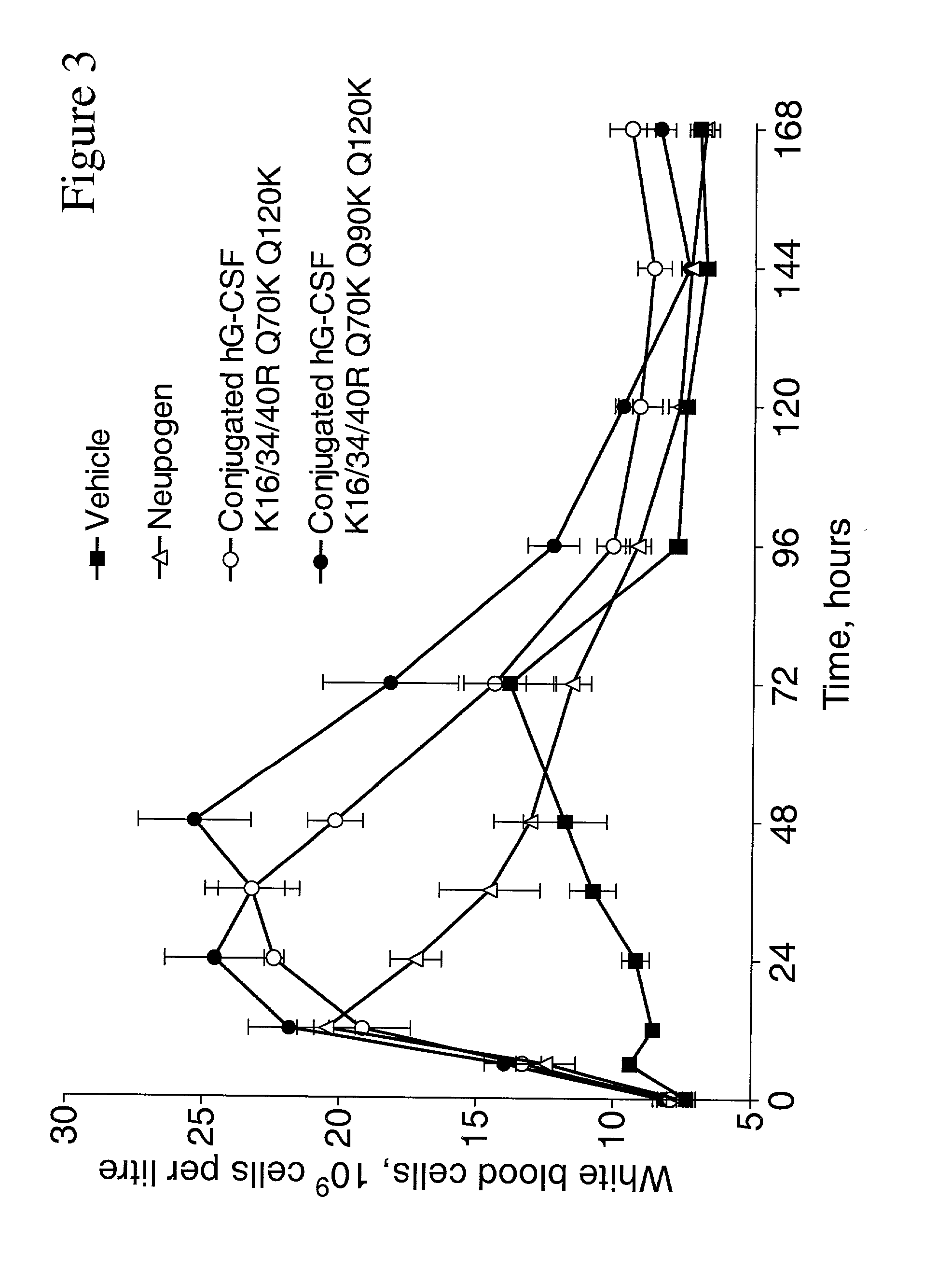
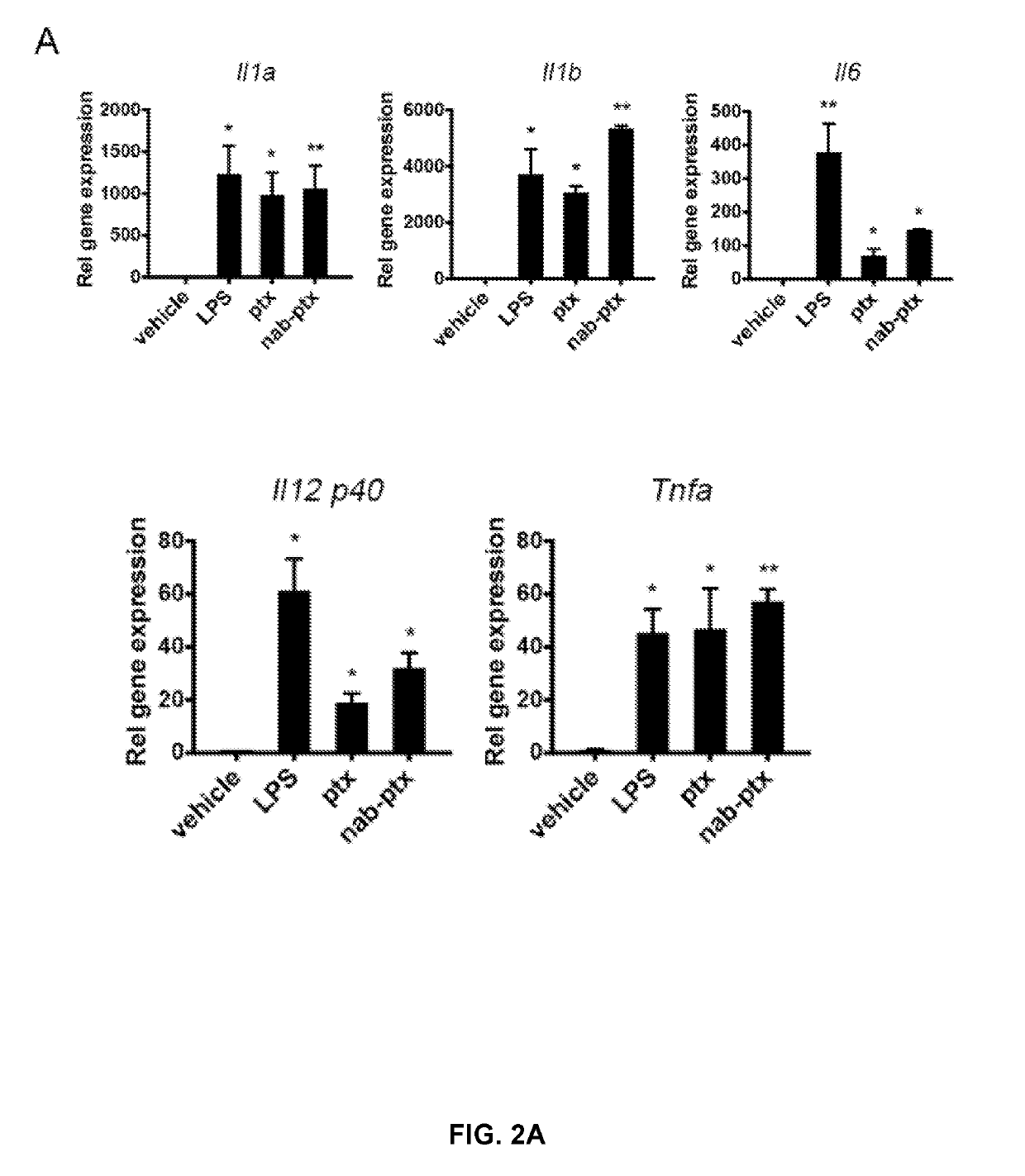
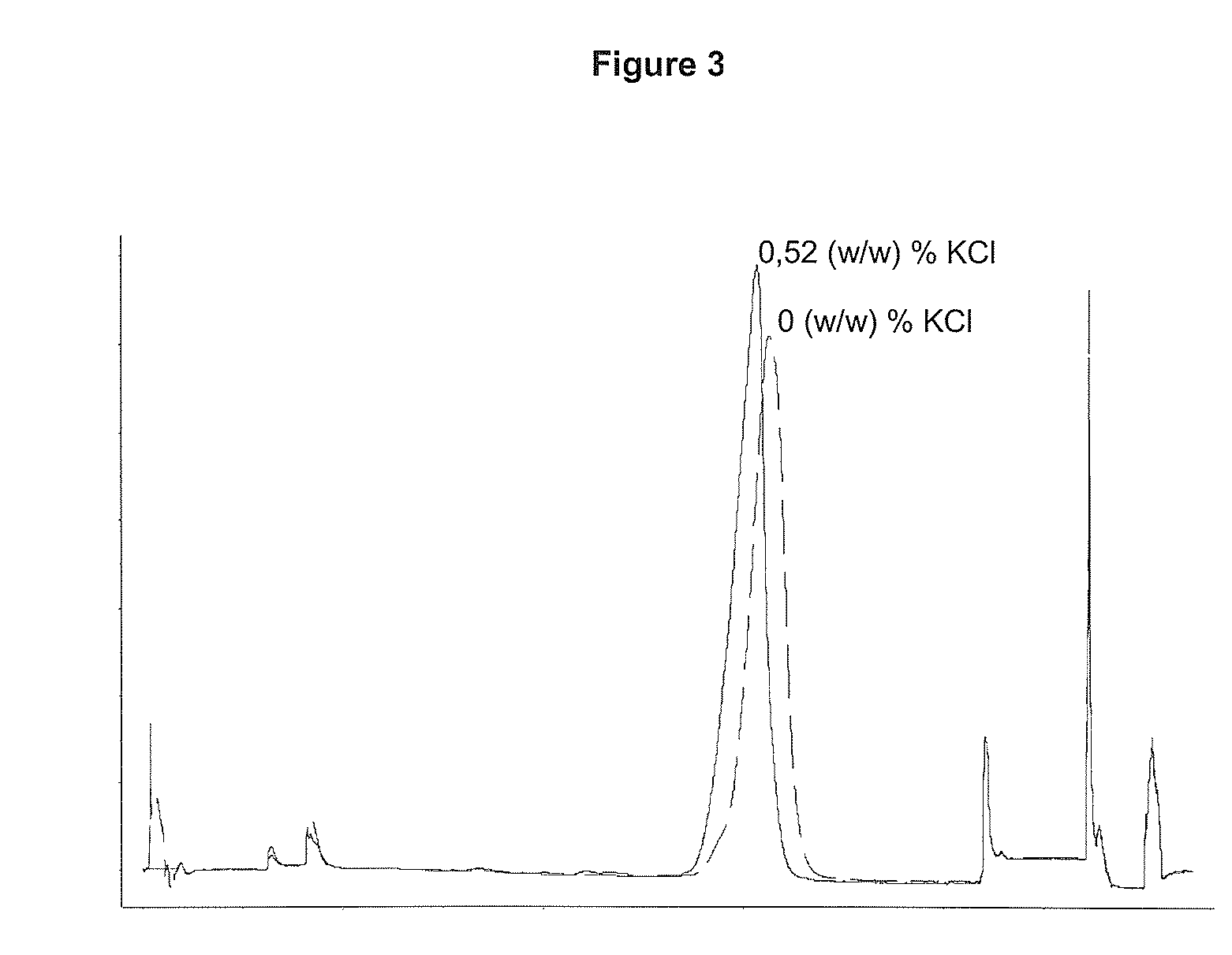
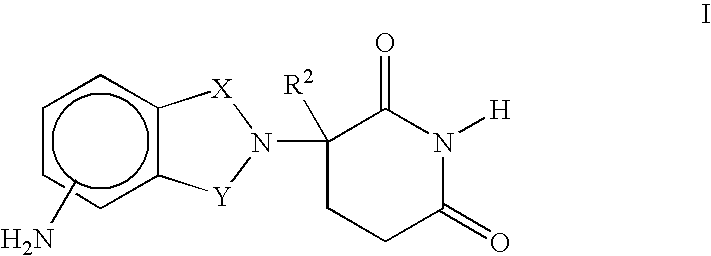
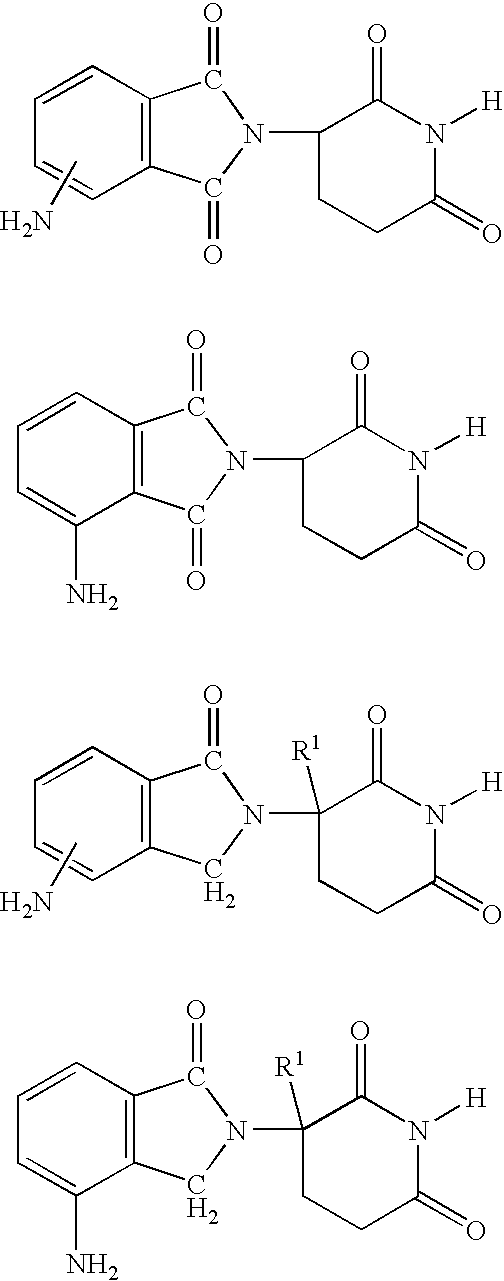
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117results about "Colony-stimulating factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
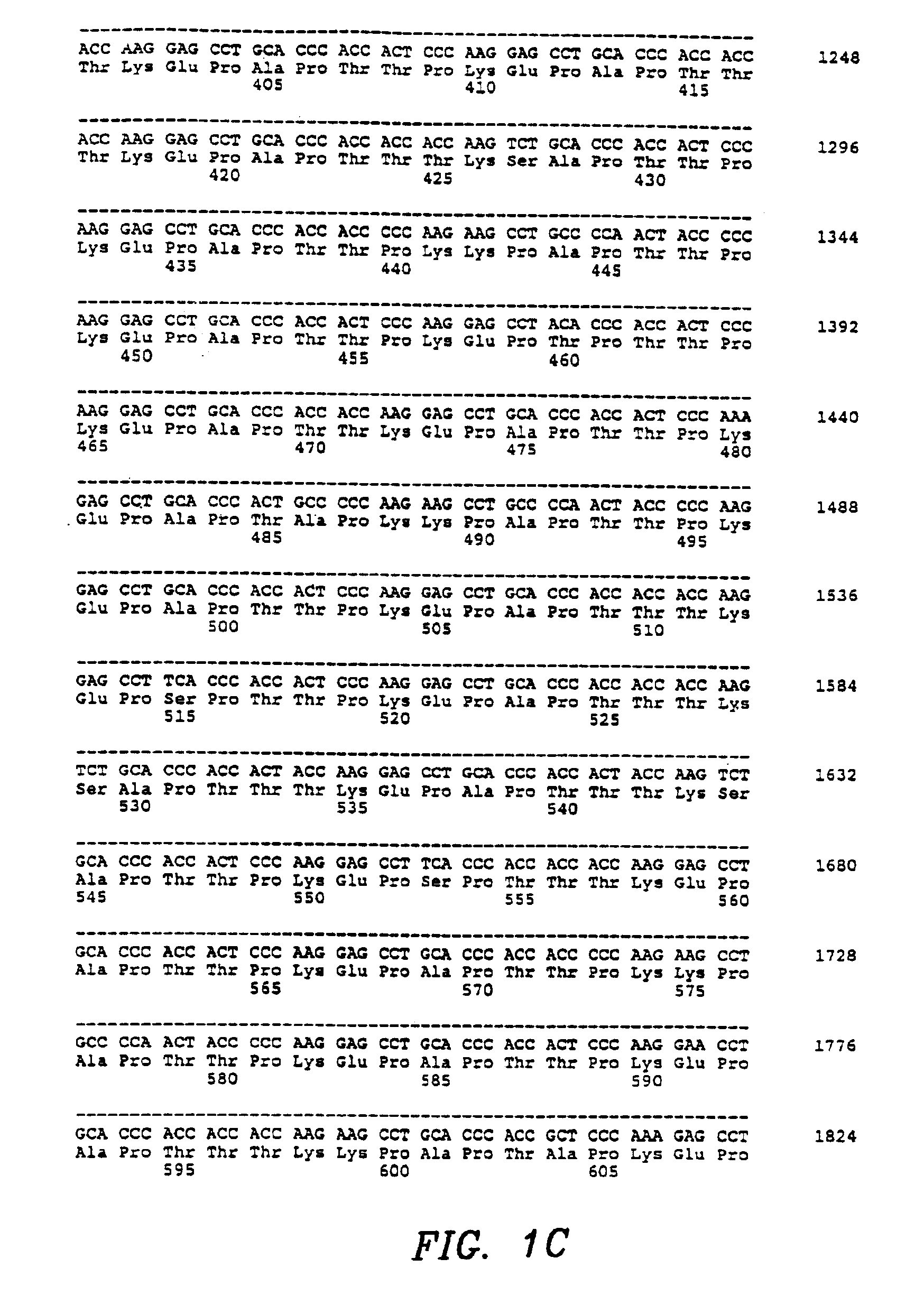
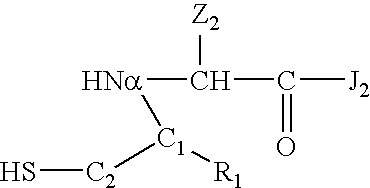
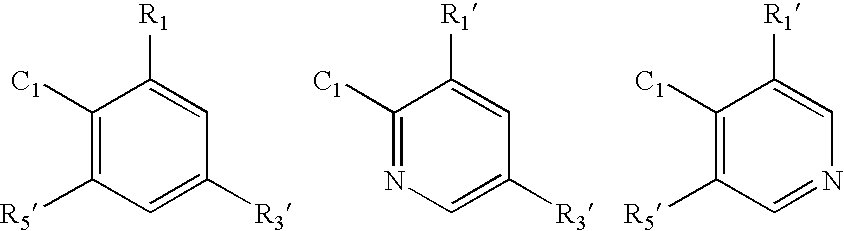
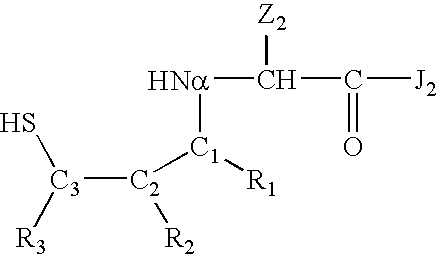
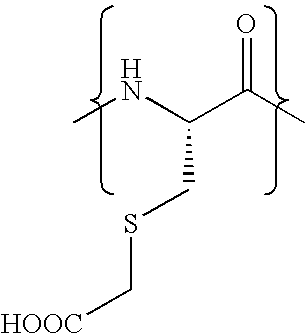
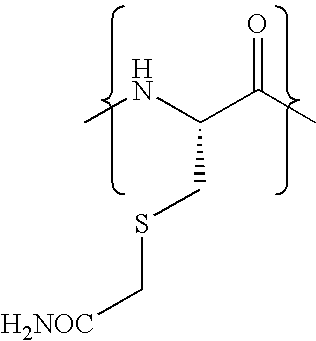
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
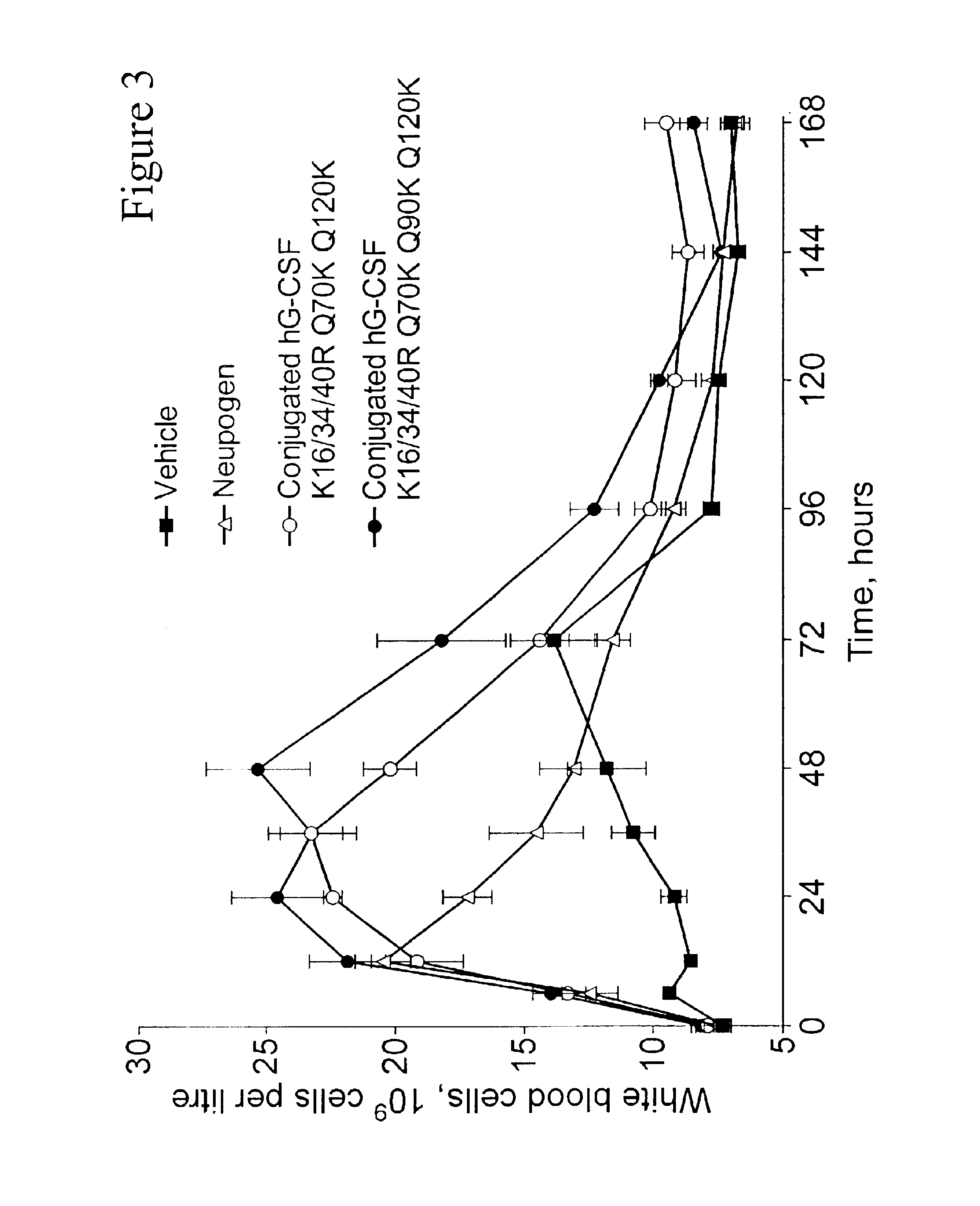
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Method of purifying protein
InactiveUS20060142549A1Efficient removalSerum immunoglobulinsColony-stimulating factorActive proteinDNA Contamination
Problems to be Solved: The present invention provides a simpler and less expensive method for purifying physiologically active proteins, especially antibodies, which can ensure removal of impurities such as DNA contaminants and viruses, and which can minimize a loss of physiologically active proteins. Means for Solving the Problems: A method for removing impurities in a physiologically active protein-containing sample, which comprises the following steps: 1) allowing the physiologically active protein-containing sample to be converted into an aqueous solution of low conductivity at a pH below the isoelectric point of the physiologically active protein; and 2) removing the resulting particles.
Owner:CHUGAI PHARMA CO LTD
Novel synthetic chimeric fusion transgene with immuno-therapeutic uses
InactiveUS20050053579A1Reducing tumorigenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsInterferon alphaWilms' tumor
The present invention relates to an immuno-therapy conjugate which comprises A-c-B wherein: A and B are different and are compounds selected from the group consisting of cytokines, chemokines, interferons, their respective receptors or a functional fragment thereof; and c is a linker consisting of a bond or an amino acid sequence containing from 1 to 100 residues. The present invention also relates to a vaccine adjuvant comprising the immuno-therapy conjugate of the present invention. The present invention further relates to a method of reducing tumor growth, for inhibiting a viral infection and for improving immune response in a patient.
Owner:GALIPEAU JACQUES +1
G-CSF conjugates
InactiveUS6831158B2Improved propertyDecrease of bioactivityPeptide/protein ingredientsPharmaceutical delivery mechanismWhite blood cellHalf-life
Owner:MAXYGEN
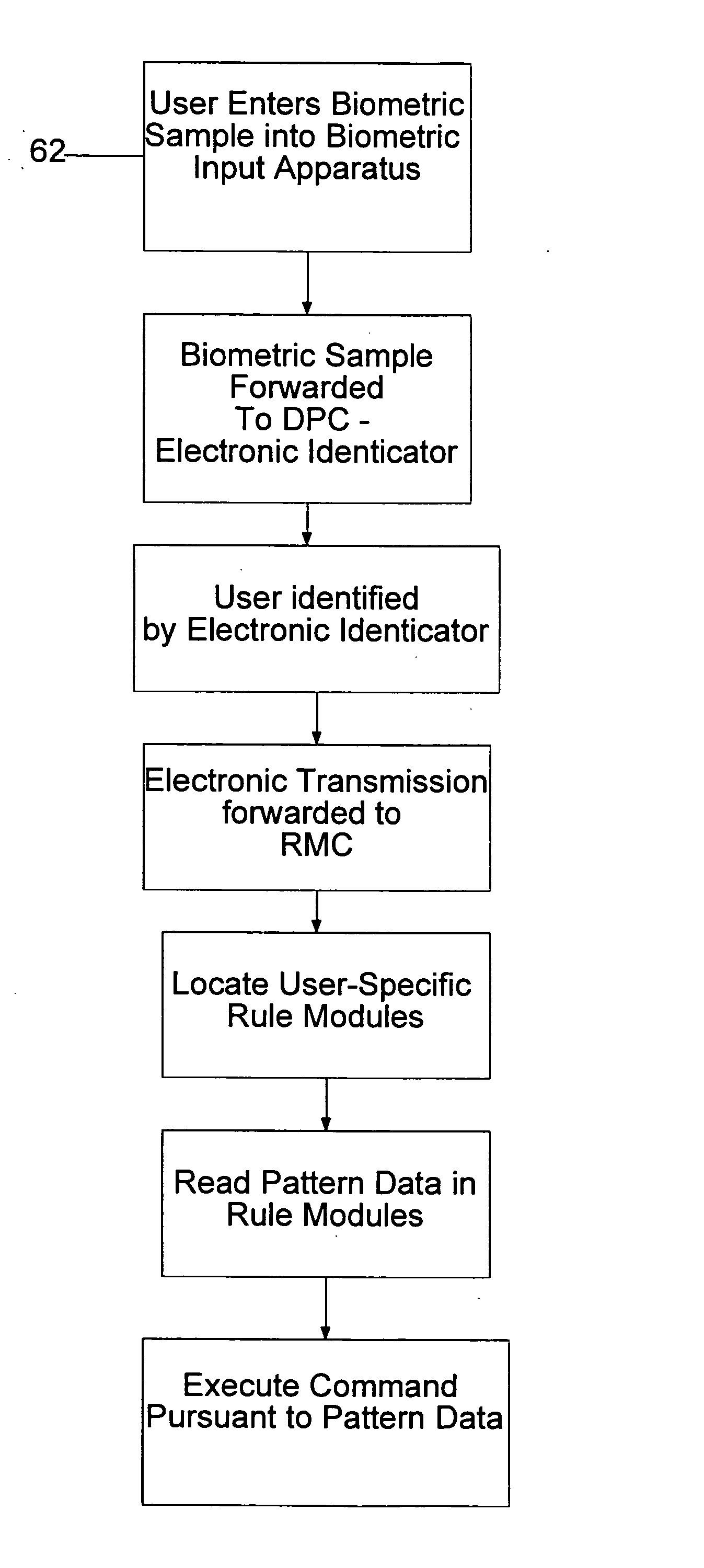
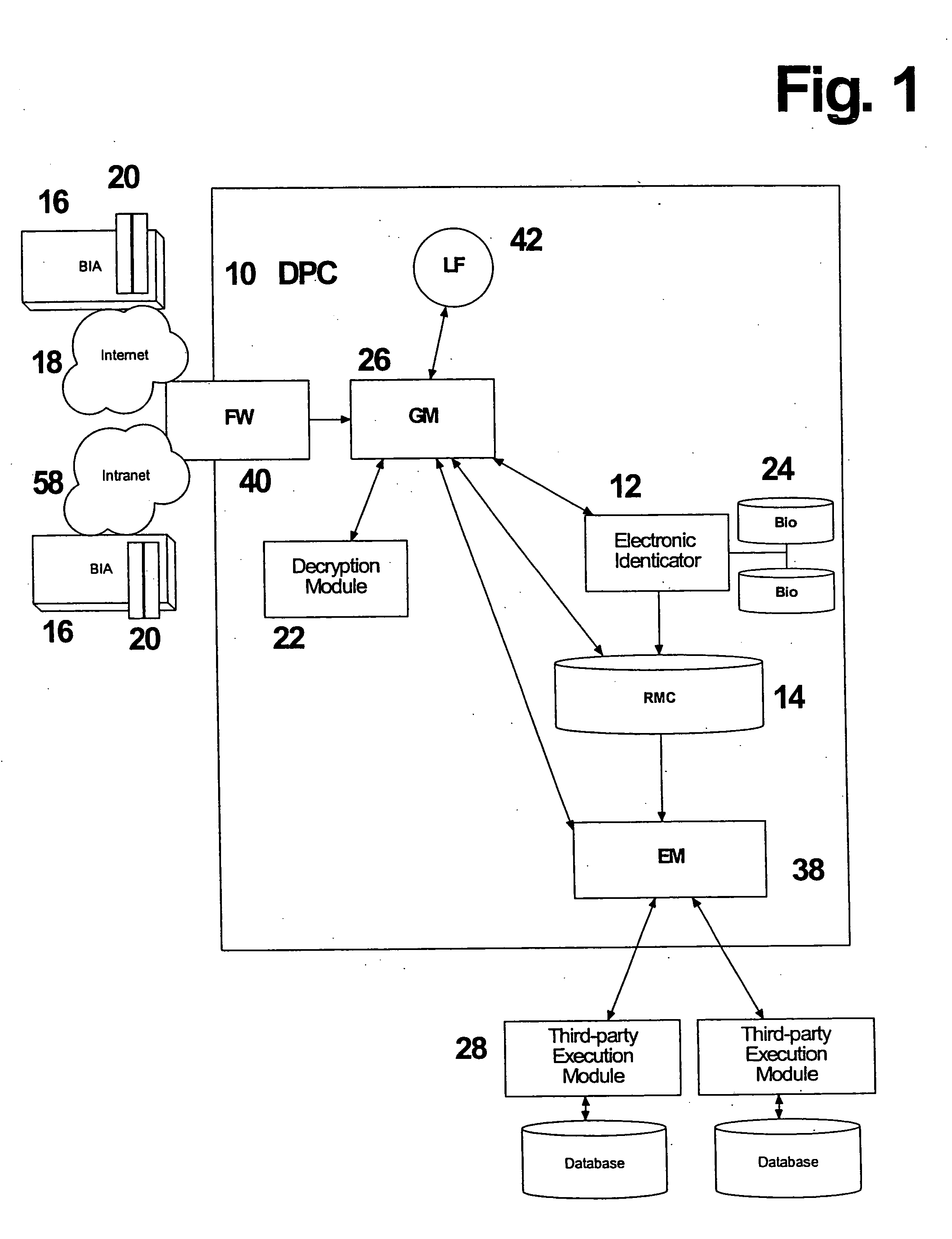
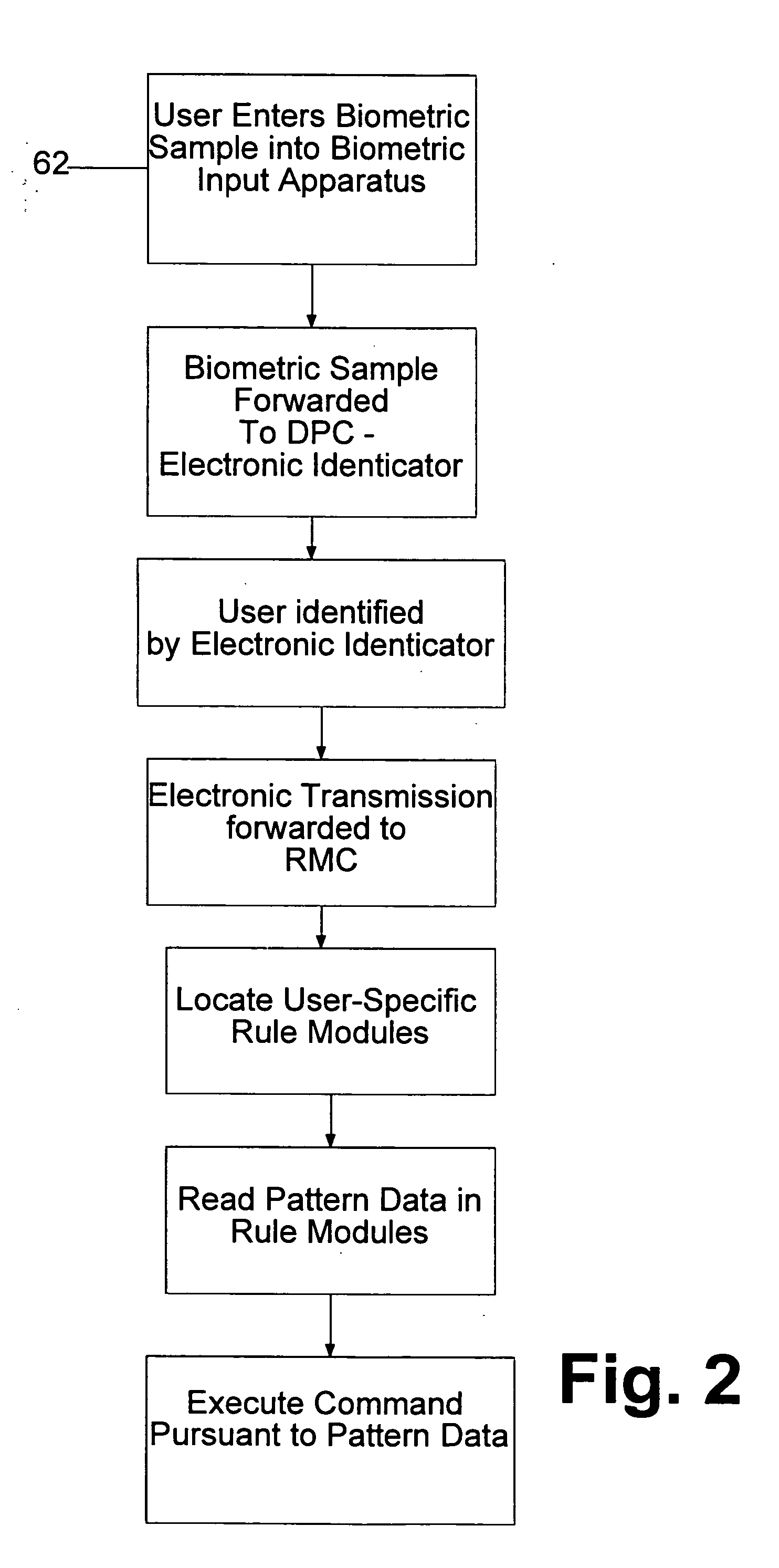
System and method for processing tokenless biometric electronic transmissions using an electronic rule module clearinghouse
InactiveUS20050289058A1Eliminate needCredit registering devices actuationColony-stimulating factorPersonalizationElectronic transmission
Herein is described a tokenless biometric method for processing electronic transmissions, using at least one user biometric sample, an electronic identicator and an electronic rule module clearinghouse. The steps for processing of the electronic transmissions comprise of a user registration step, wherein a user registers with an electronic identicator at least one registration biometric sample taken directly from the person of the user. A formation of a rule module customized to the user in a rule module clearinghouse, wherein at least one pattern data of a user is associated with at least one execution command of the user. A user identification step, wherein the electronic identicator compares a bid biometric sample taken directly from the person of the user with at least one previously registered biometric sample for producing either a successful or failed identification of the user. In a command execution step, upon successful identification of the user, at least one previously designated rule module of the user is invoked to execute at least one electronic transmission. The above-mentioned steps are conducted in a manner wherein a biometrically authorized electronic transmission is conducted without the user presenting any personalized man-made memory tokens such as smartcards, or magnetic swipe cards.
Owner:OPEN INVENTION NEWTORK LLC
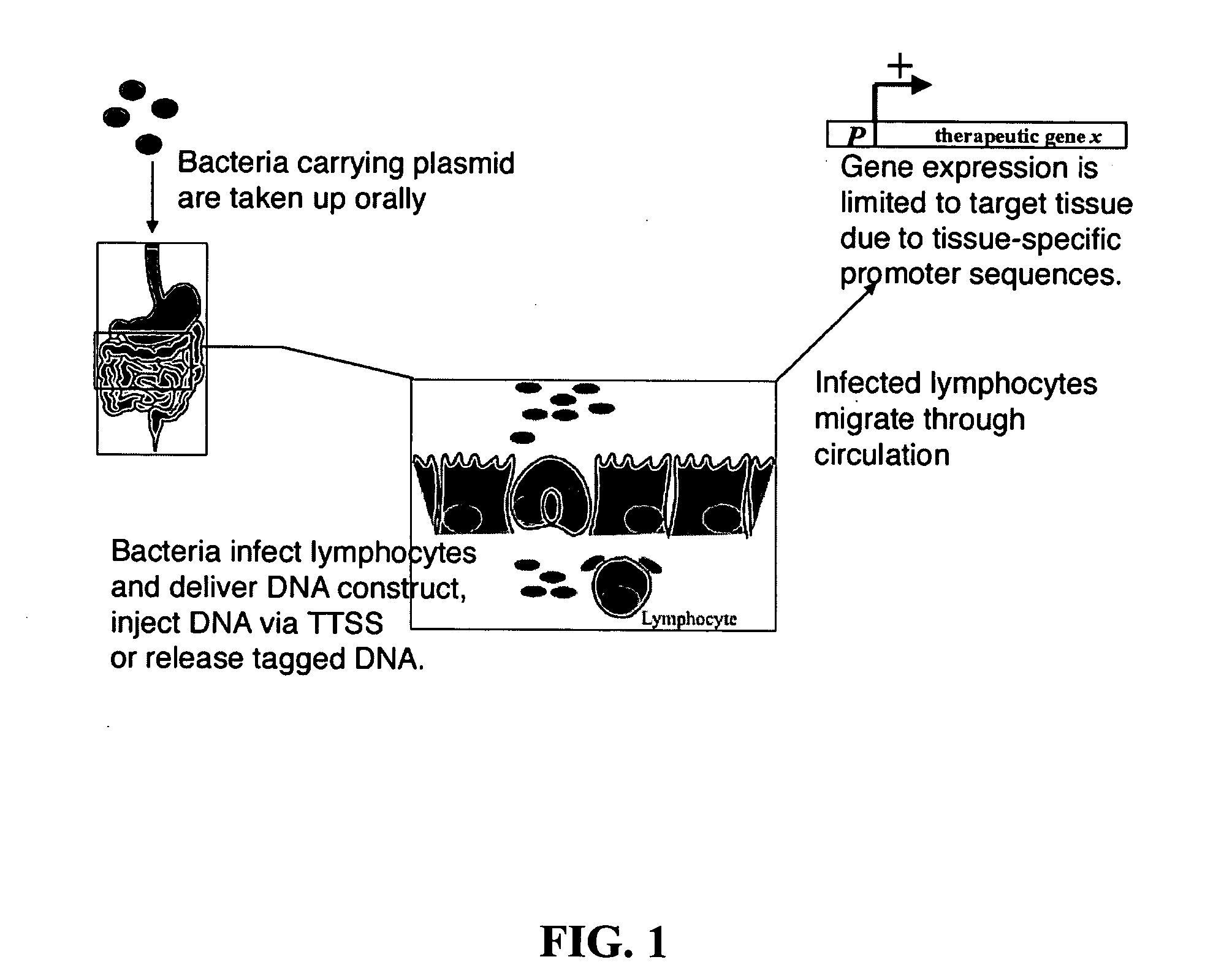
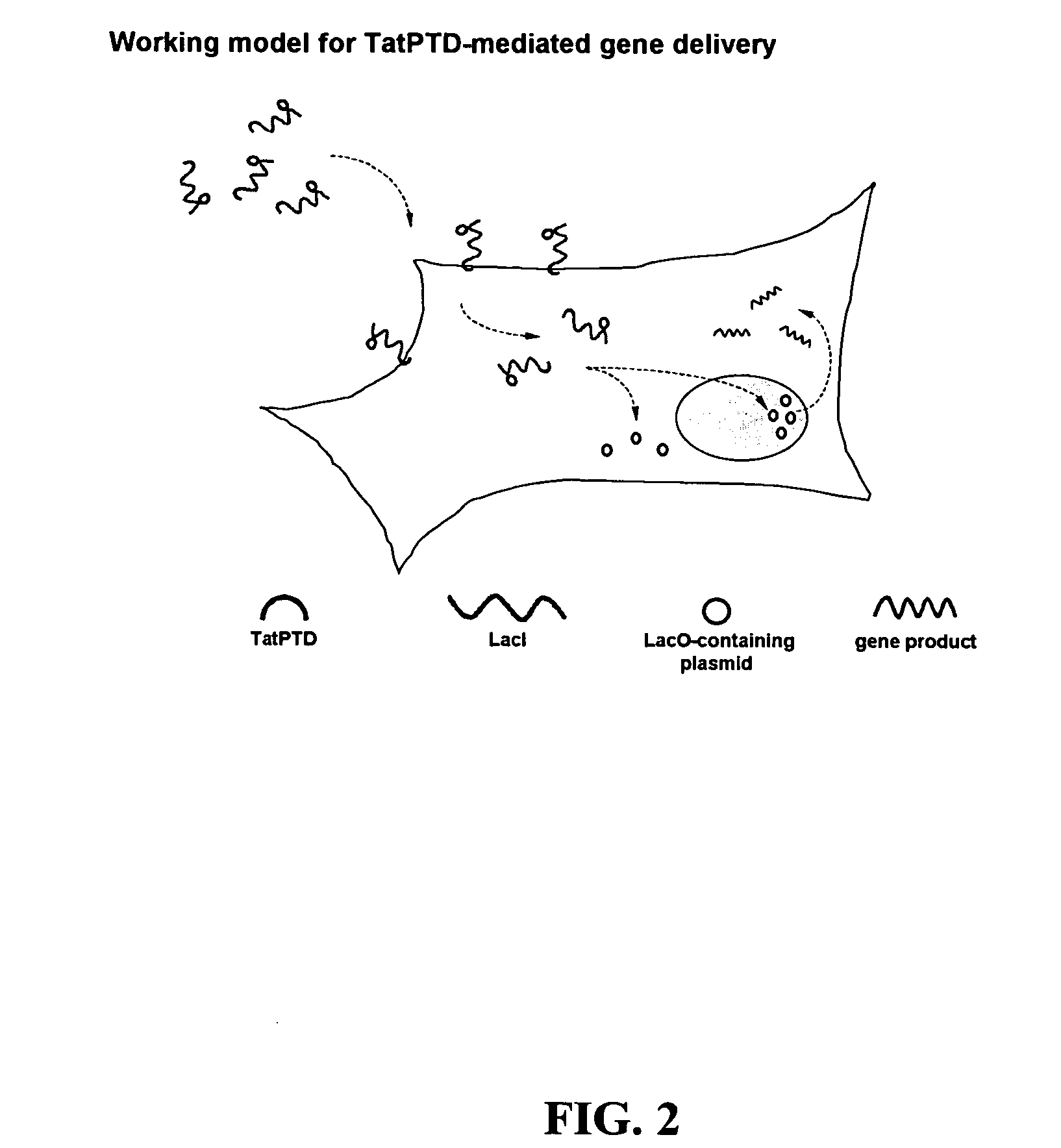
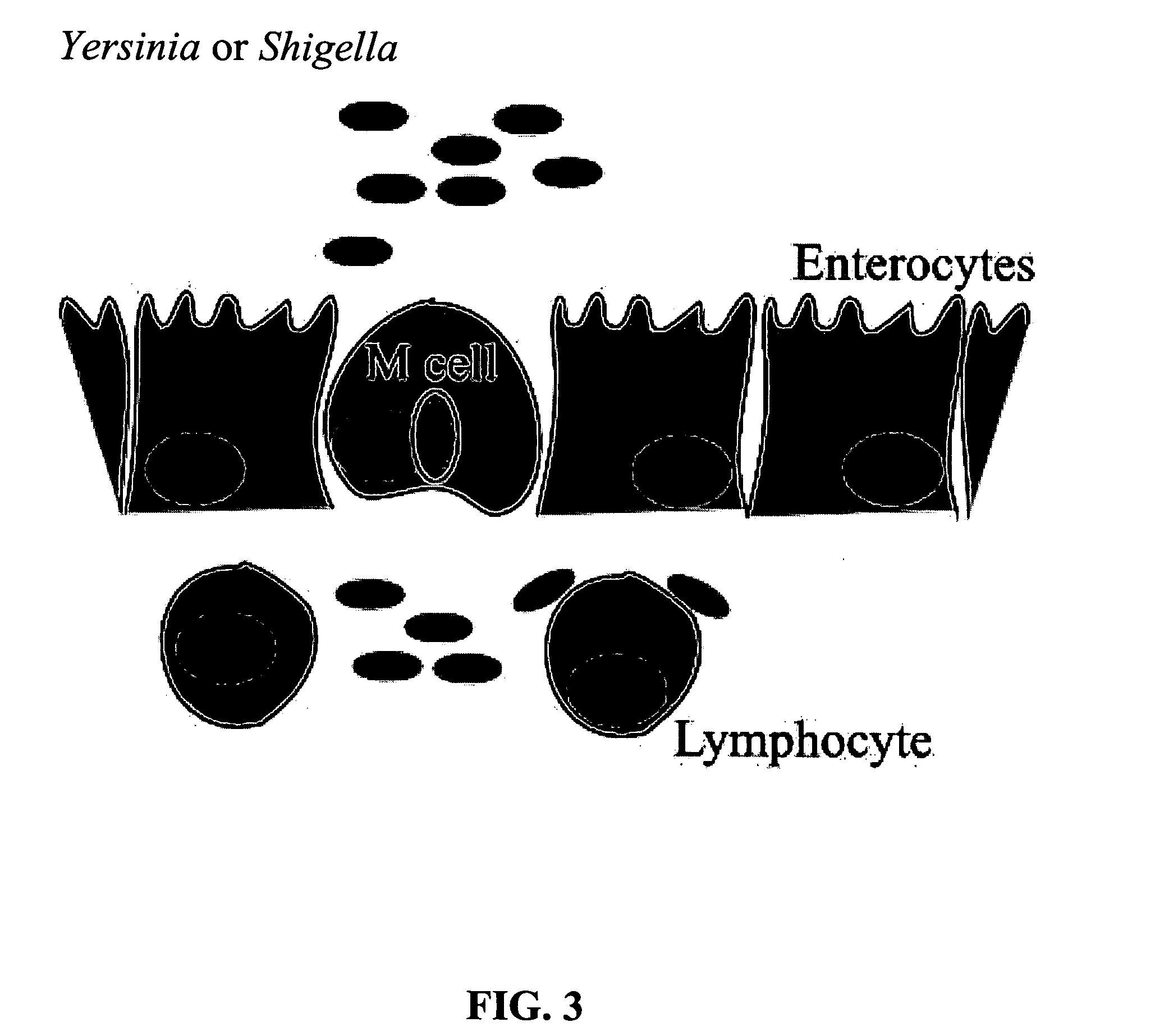
Bacterial vector systems
InactiveUS20060068469A1Efficient rapid deliveryFusion with DNA-binding domainBacteriaGene deliveryGene targets
The present invention provides bacterial vectors and fusion proteins containing a TTSS polypeptide, compositions of such fusion proteins including polynucleotides, and methods of delivering one or more genes into a target cell that involve contacting the cell with a composition that includes such a fusion protein. Compositions and methods of gene delivery that involve a bacterium and a TAT, Antp, or HSV VP22 polypeptide are also disclosed. The invention also concerns methods of delivering one or more genes into a target cell utilizing a bacterium capable of becoming internalized within the cell, wherein the bacterium includes one or more genes targeted for delivery to the cell, a gene encoding an RNA polymerase, and a gene that causes lysis of the bacterium.
Owner:RES DEVMENT FOUND
Methods and compositions for increasing longevity and protein yield from a cell culture
InactiveUS20070092947A1Increase longevity and recombinant protein yieldIncreases lifespan and viabilityFermentationInterferonsApoptosisOrganism
Disclosed herein are compositions and methods for increasing the longevity of a cell culture and permitting the increased production of proteins, preferably recombinant proteins, such as antibodies, peptides, enzymes, growth factors, interleukins, interferons, hormones, and vaccines. By transfecting cells in culture with an apoptosis-inhibiting gene or vector, cells in culture can survive longer, resulting in extension of the state and yield of protein biosynthesis. Expression of the apoptosis-inhibitor within the cells, because it does not kill the cells, allows the cells, or an increased fraction thereof, to be maintained in culture for longer periods. This invention then allows for controlled, enhanced protein production of cell lines for commercial and research uses, particularly the enhanced production of growth factors, interferons, interleukins, hormones, enzymes, and monoclonal antibodies, and the like. The method preferentially involves eukaryotic cells in culture, and more advantageously mammalian cells in culture.
Owner:IMMUNOMEDICS INC
Megakaryocyte stimulating factors
InactiveUS7030223B2Peptide/protein ingredientsMicroorganism based processesMegakaryocyteMEGAKARYOCYTE-STIMULATING FACTOR
Novel polypeptides of human megakaryocyte stimulating factors (MSFs). Pharmaceutical compositions containing same, and methods for their preparation and use are provided.
Owner:GENETICS INST INC
Polymer-modified synthetic proteins
InactiveUS20060233747A1Sugar derivativesPeptide/protein ingredientsPolymer modifiedCombinatorial chemistry
The present invention relates to methods and compositions for modifying peptides, polypeptides and proteins with polymers, especially glyco-mimetic polymers, so as to improve their biological activity or pharmacokinetic properties. The invention further provides methods and uses for such polymer-modified peptides, polypeptides and proteins. The invention is particularly suitable for use in the synthesis of polymer-modified, synthetic bioactive proteins, and of pharmaceutical compositions that contain such proteins.
Owner:AMYLIN PHARMA INC
Transgenic mammal capable of facilitating production of donor-specific functional immunity
This invention provides for transgenic non-human mammalian models of human disease, methods of making such models as well as methods of using such models to assess efficacy of therapeutic and prophylaxis treatments, to assess the antigenic potential of compounds, and other uses.
Owner:GENENCOR INT INC
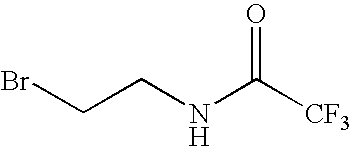
Derivatives of growth hormone and related proteins, and methods of use thereof
Owner:BOLDER BIOTECH
Method for stabilizing protein solution preparation
InactiveUS20060058511A1Suppress formationPeptide/protein ingredientsColony-stimulating factorProtein solutionActive protein
Problems to be Solved: The present invention provides a method for improving the stability of protein solution formulations by suppressing the formation of associated matter from physiologically active proteins (e.g., antibodies, enzymes, hormones, cytokines) in a solution form. Means for Solving the Problems: A method for stabilizing a protein solution formulation, which comprises storing the protein solution formulation under magnetic field lines, as well as a storage container for holding a protein solution formulation, which is equipped with a magnetic field generator.
Owner:CHUGAI PHARMA CO LTD
Pseudo-native chemical ligation
InactiveUS20060149039A1Easily employedProduct is unsuitablePeptide/protein ingredientsAntipyreticPolymer modifiedChemical ligation
The present invention concerns methods and compositions for extending the technique of native chemical ligation to permit the ligation of a wider range of peptides, polypeptides, other polymers and other molecules via an amide bond. The invention further provides methods and uses for such proteins and derivatized proteins. The invention is particularly suitable for use in the synthesis of optionally polymer-modified, synthetic bioactive proteins, and of pharmaceutical compositions that contain such proteins.
Owner:AMYLIN PHARMA INC
Fusions of cytokines and tumor targeting proteins
A conjugate of a cytokine and a tumor targeting moiety (TTM) with the provisos that when cytokine is TNF-α, TNF-β or IFN-γ, the TTM is other than a CD13 ligant; when the cytokine is IL-12, the TTM is other than an antiboy to fibronectin; when the cytokine is TNF, the TTM is other than an antibody to the transferrin receptor, and when the cytokine is TNF, IFN-γ, or IL-2 the antibody is other than an antibody to the TAG72 antigen.
Owner:MOLMED SPA
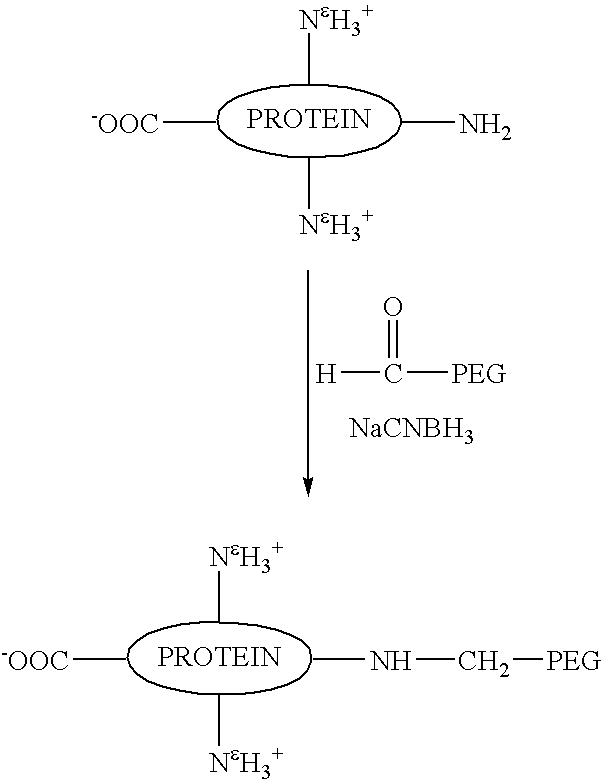
N-terminally chemically modified protein compositions and methods
InactiveUS7090835B2High proportionOrganic active ingredientsNervous disorderProtein compositionOrganic chemistry
Provided herein are methods and compositions relating to the attachment of water soluble polymers to proteins. Provided are novel methods for N-terminally modifying proteins or analogs thereof, and resultant compositions, including novel N-terminally chemically modified G-CSF compositions and related methods of preparation. Also provided is chemically modified consensus interferon.
Owner:AMGEN INC
Treatment of osteolytic disorders and cancer using CSF1R extracellular domain fusion molecules
ActiveUS8183207B2Improve propertiesGrowth inhibitionPeptide/protein ingredientsAntibody mimetics/scaffoldsColony-stimulating factorColony Stimulating Factor Receptor
Methods of using colony stimulating factor receptor (CSF1R) extracellular domain (ECD) fusion molecules for treating osteolytic bone loss, cancer metastasis, cancer metastasis-induced osteolytic bone loss, and tumor growth are provided. CSF1R ECD fusion molecules, polynucleotides encoding CSF1R ECD fusion molecules, and methods of making CSF1R ECD fusion molecules are also provided.
Owner:FIVE PRIME THERAPEUTICS
Genetically modified non-human animals and methods of use thereof
Owner:REGENERON PHARM INC +2
Fc fusion protein of human granulocyte colony stimulin with enhanced bioactivity
InactiveCN1410450AImprove biological activityAntibody mimetics/scaffoldsColony-stimulating factorHalf-lifePharmacodynamic Study
Owner:PHARMAB
Novel g-csf conjugates
InactiveUS20070166278A1Simplified mixtureSelectivity in conjugationPeptide/protein ingredientsColony-stimulating factorThiolCysteine thiolate
The present application relates to novel PEG-G-CSF conjugates in which a PEG molecule is linked to the cyteine residue in position 17 of native G-CSF primary sequence or to the cysteine residue of the corresponding position of a G-CSF analogue. The present application also describes a process for the manufacture of such conjugates, such process comprising the following steps: (i) subjecting the G-CSF protein to conditions inducing reversible denaturation of the protein, (ii) conjugation of the denatured protein obtained is step i with a thiol-reactivc PEG under denaturing conditions, (iii) subjecting the conjugates obtained in step ii to conditions promoting renaturation of the conjugate yielding biologically active G-CSF-PEG conjugate.
Owner:NEKTAR THERAPEUTICS INC
G-CSF conjugates
InactiveUS20030064922A1Improve propertiesIncrease stimulationPeptide/protein ingredientsPharmaceutical delivery mechanismPolyethylene glycolNeutrophil granulocyte
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Process for preparing purified drug conjugates
ActiveUS20110021744A1Peptide/protein ingredientsMammal material medical ingredientsOrganic chemistryChemistry
Owner:IMMUNOGEN INC
Algorithmic design of peptides for binding and/or modulation of the functions of receptors and/or other proteins
InactiveUS20050119454A1Improve the immunityImprove actionCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDiseaseProtein target
Methods of synthesizing a peptide or peptide-like molecule to a polypeptide or protein target based on mode-matching each member of a set of peptide constituents of the peptide or peptide-like molecule to peptide constituents of the target polypeptide or protein target for treatment of neurological diseases.
Owner:CIELO INST
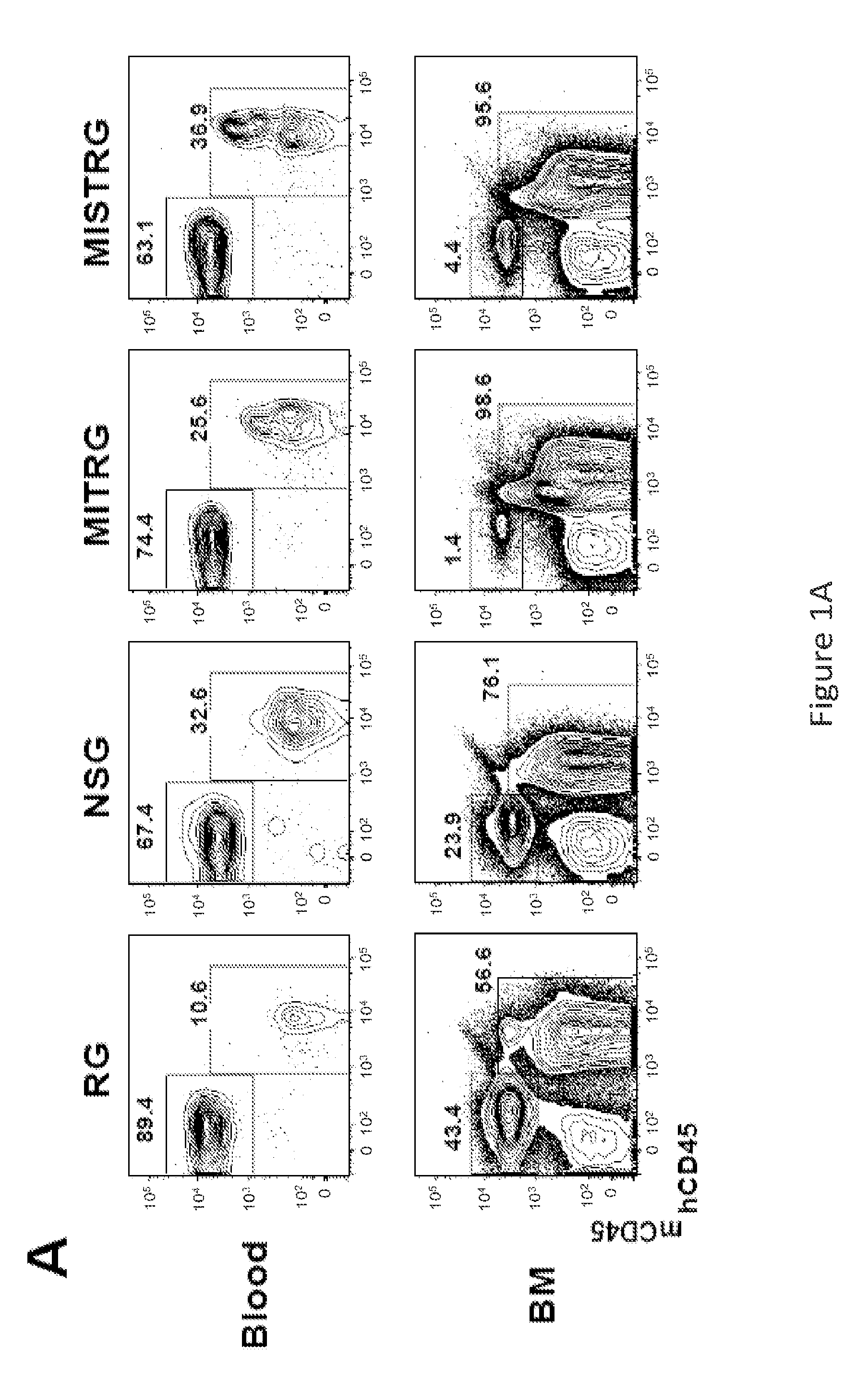
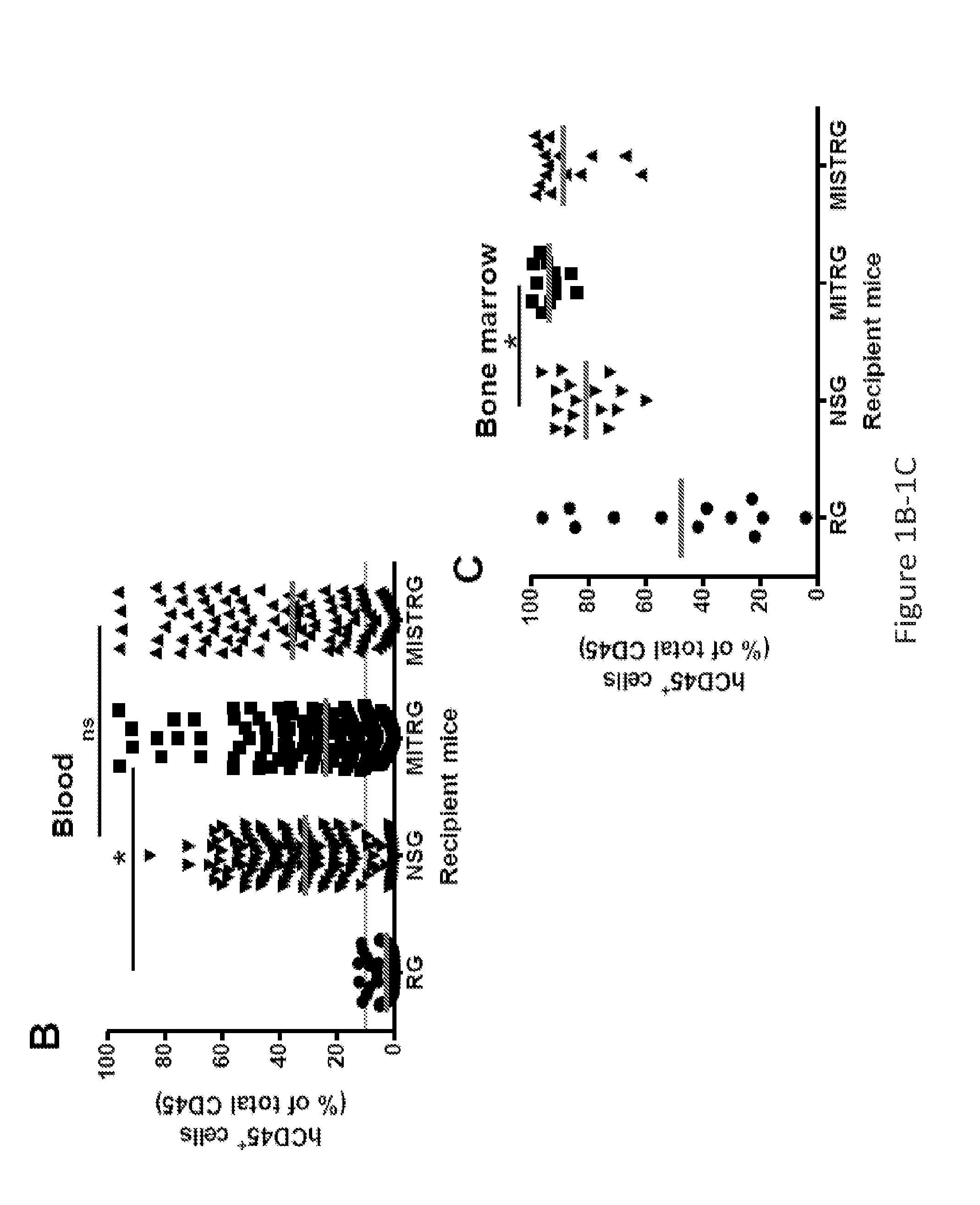
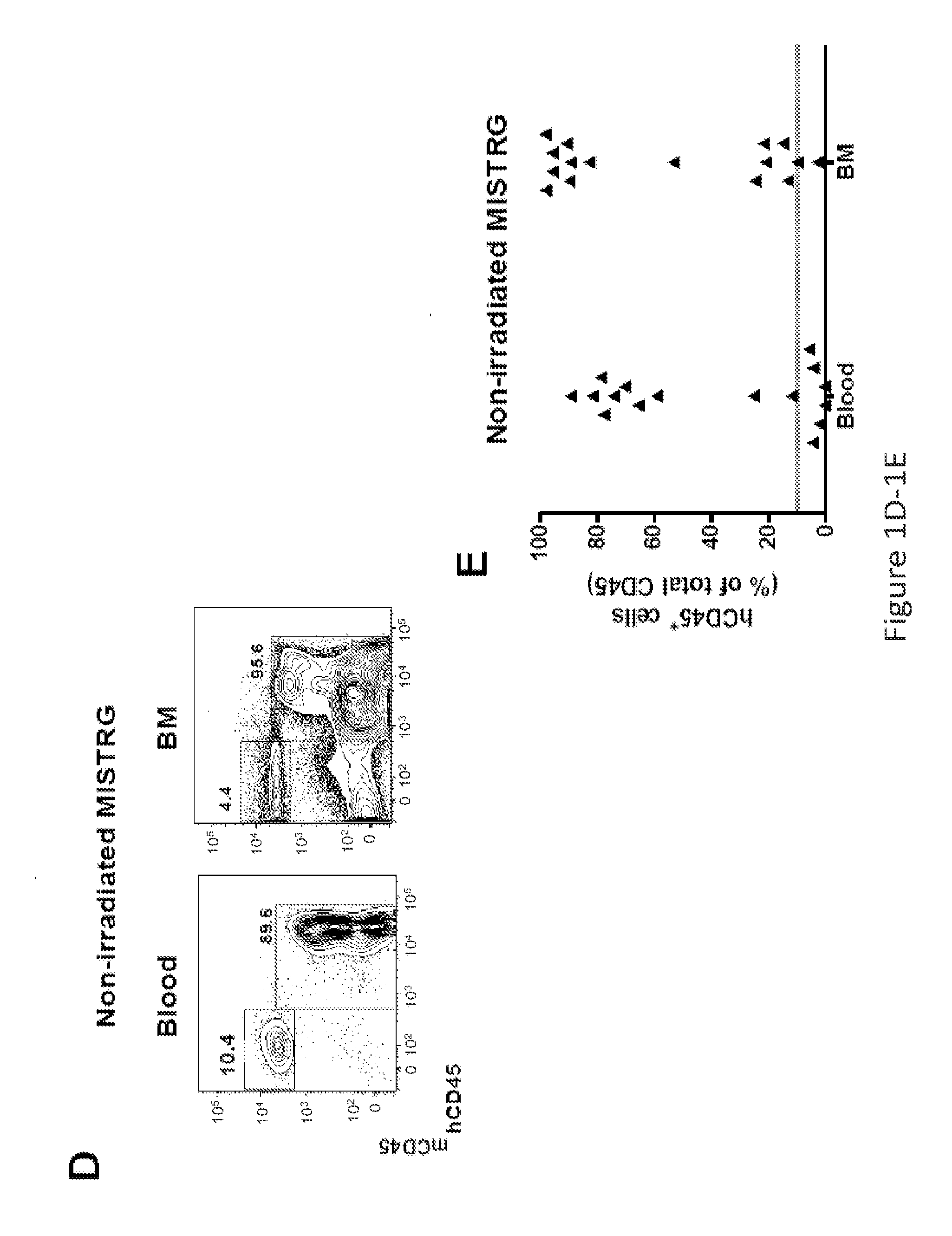
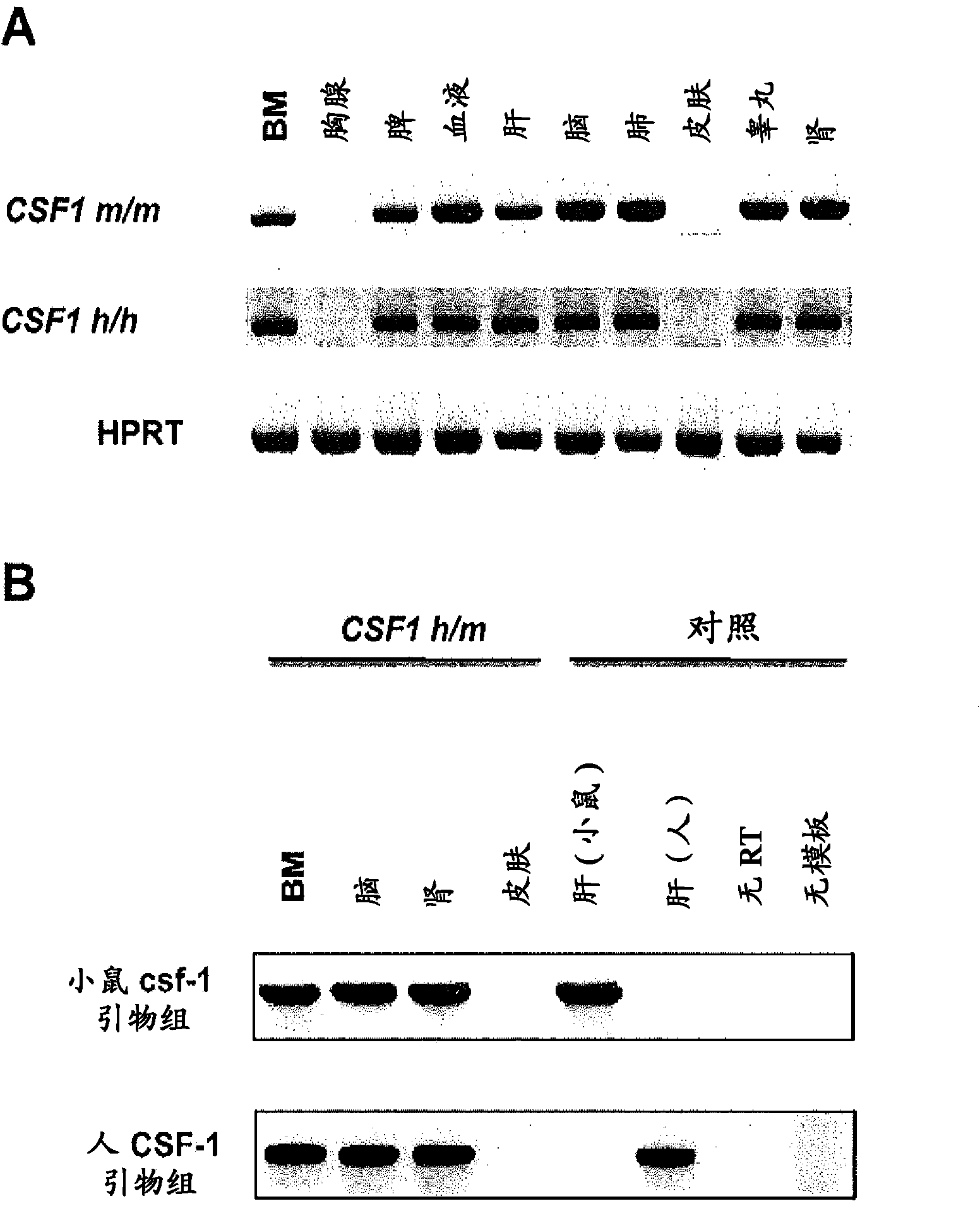
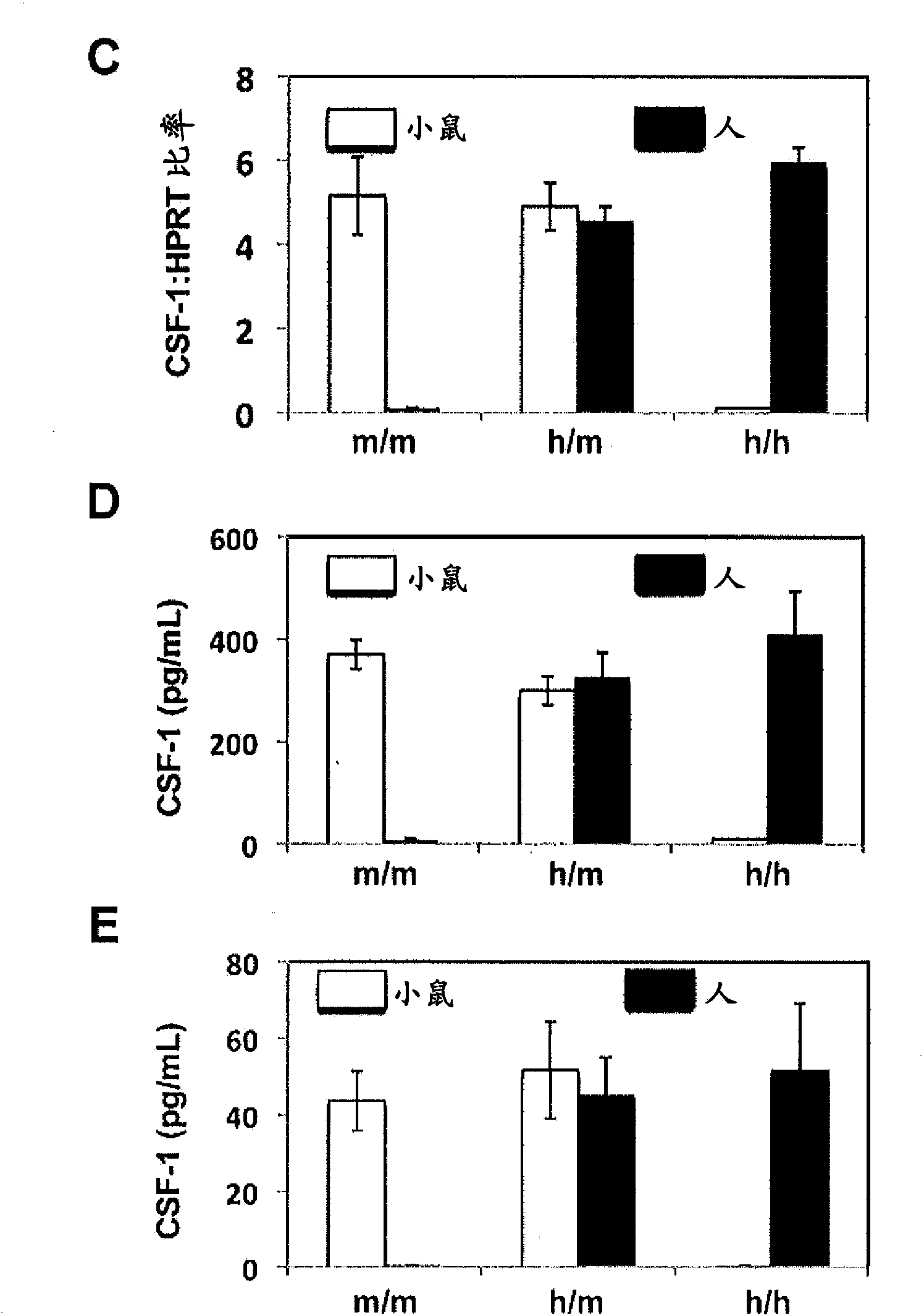
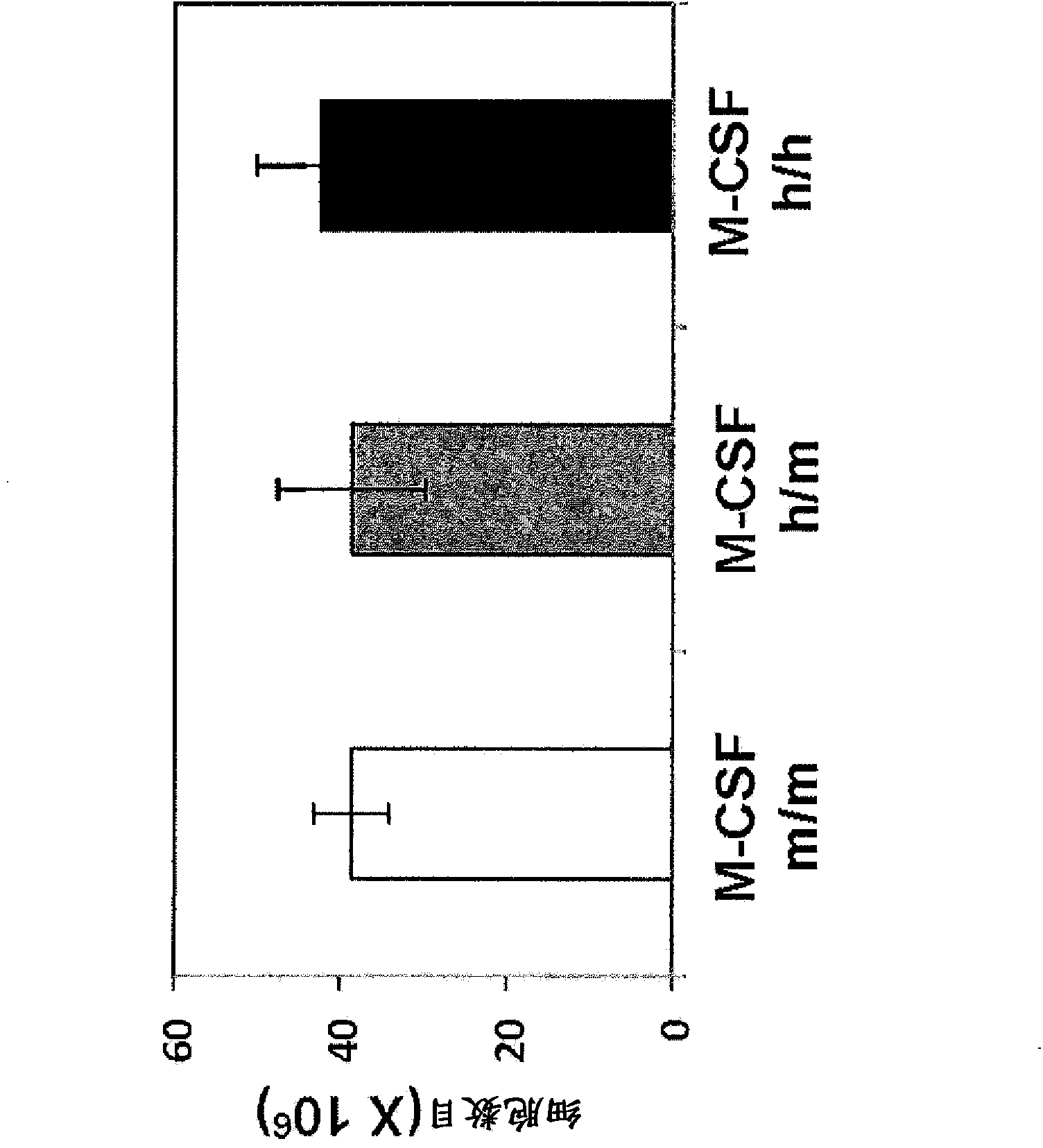
Humanized M-CSF mice
Genetically modified mice comprising a nucleic acid sequence encoding a human M-CSF protein are provided. Also provided are genetically modified mice comprising a nucleic acid sequence encoding a human M-CSF protein that have been engrafted with human cells such as human hematopoietic cells, and methods for making such engrafted mice. These mice find use in a number of applications, such as in modeling human immune disease and pathogen infection; in in vivo screens for agents that modulate hematopoietic cell development and / or activity, e.g. in a healthy or a diseased state; in in vivo screens for agents that are toxic to hematopoietic cells; in in vivo screens for agents that prevent against, mitigate, or reverse the toxic effects of toxic agents on hematopoietic cells; in in vivo screens of human hematopoietic cells from an individual to predict the responsiveness of an individual to a disease therapy, etc.
Owner:REGENERON PHARM INC +2
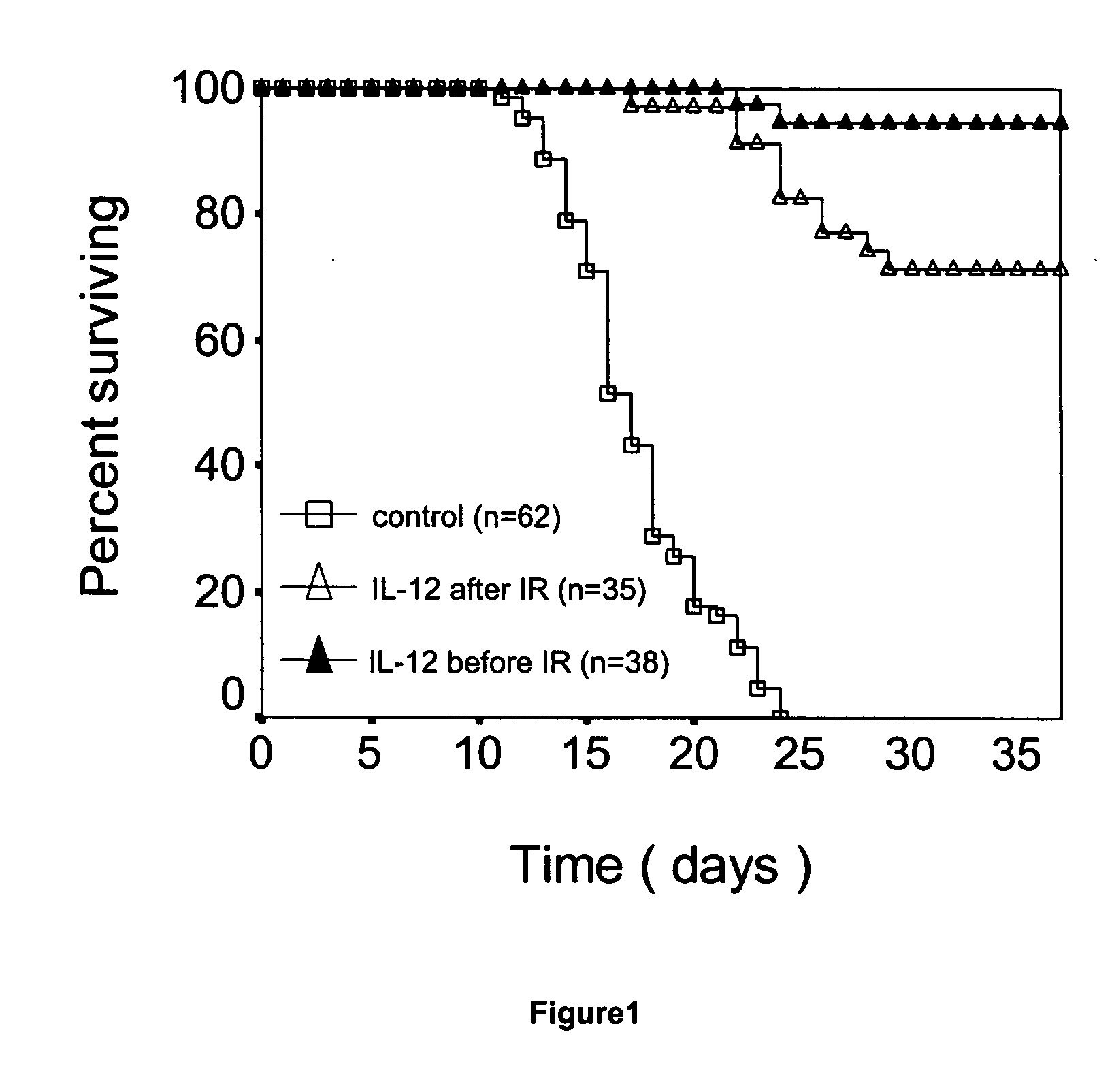
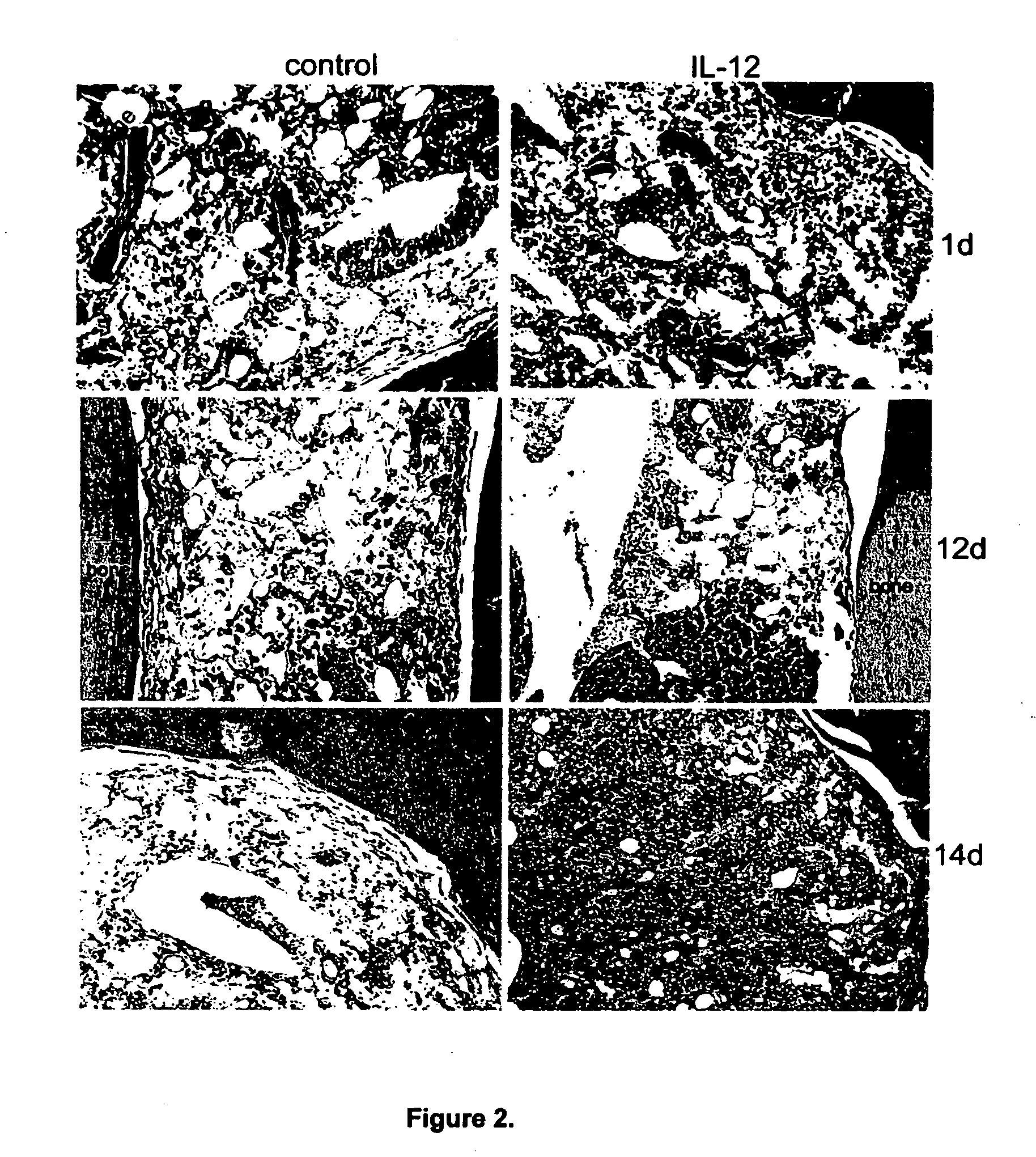
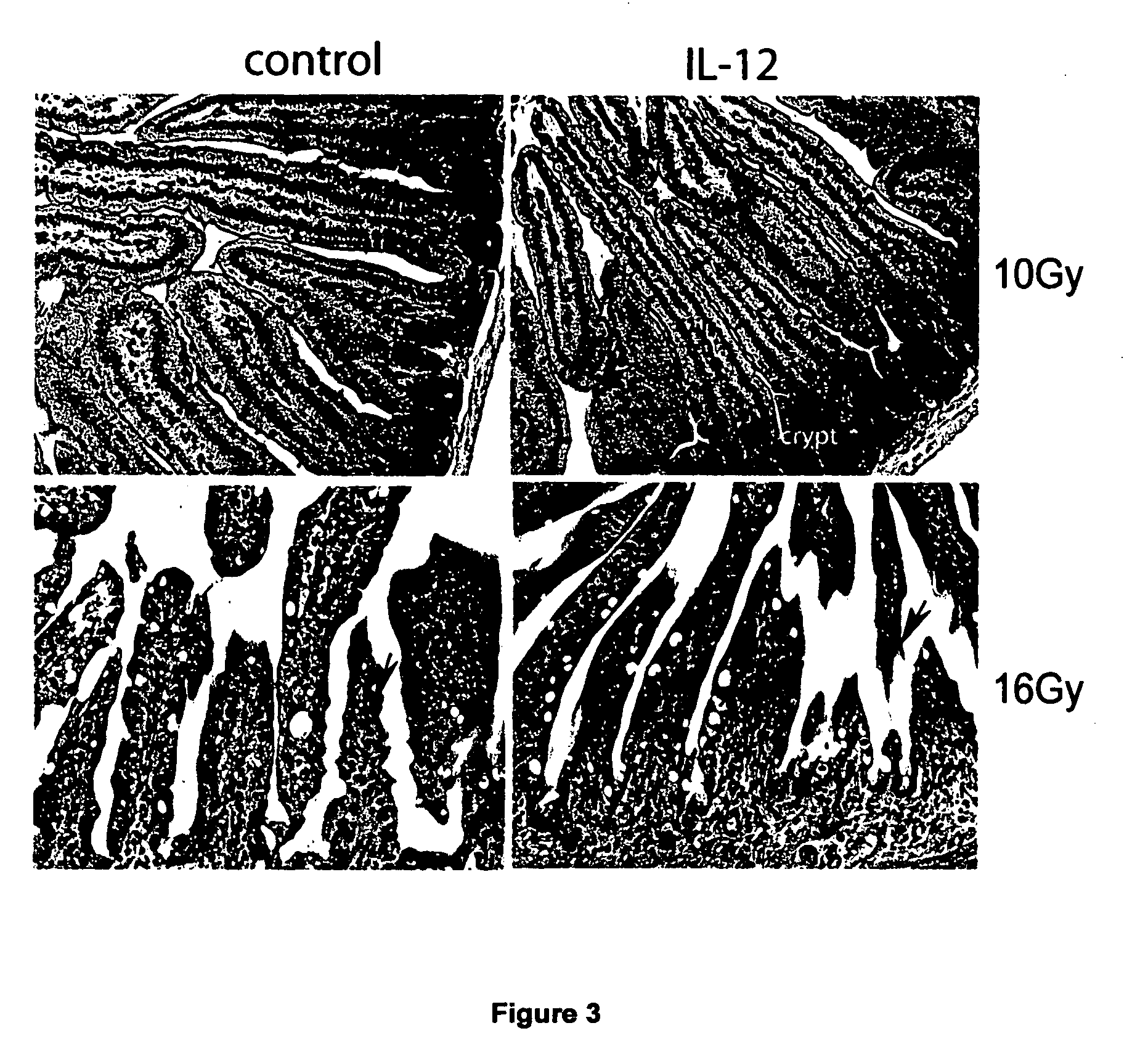
Uses of IL-12 in hematopoiesis
ActiveUS20050136034A1Reduces hematopoietic toxicityFacilitating eradicationOrganic active ingredientsPeptide/protein ingredientsWhite blood cellIn vivo
Embodiments of the present invention are directed to uses of Interleukin-12 (IL-12) in enhancing or stimulating hematopoiesis to yield hematopoietic recovery in a mammal in need. Particular embodiments of the invention are directed to uses of IL-12 as an adjuvant therapy to alleviate the hematopoietic toxicities associated with one or more treatment regimens used to combat a disease state. Other embodiments include uses of IL-12 to ameliorate various hematopoietic deficiencies. Still other embodiments are directed to uses of IL-12 for in-vivo proliferation of hematopoietic repopulating cell, hematopoietic progenitor cells and hematopoietic stem cells. Other disclosed embodiments are directed to uses of Il-12 for bone marrow preservation or recovery.
Owner:UNIV OF SOUTHERN CALIFORNIA
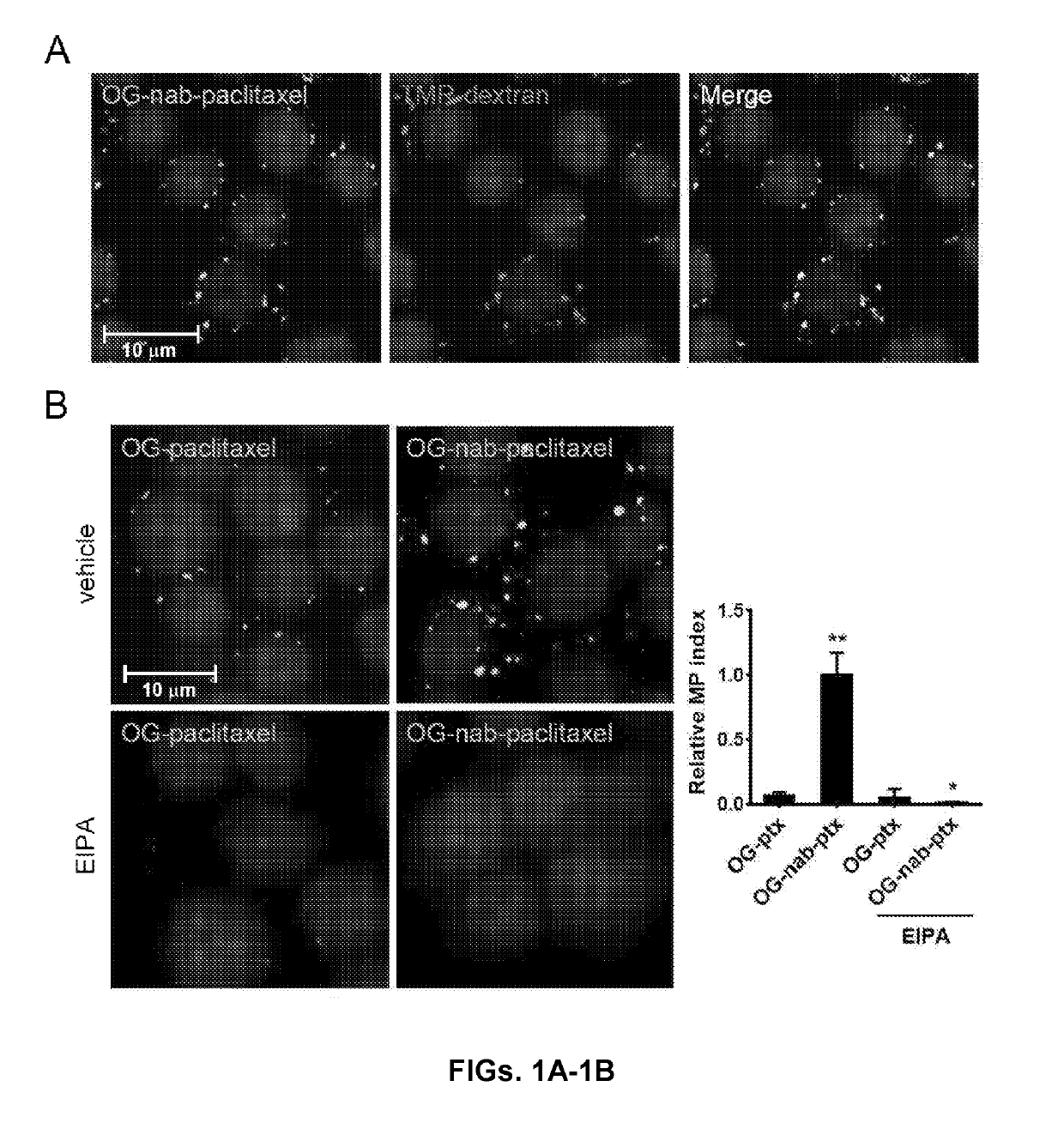
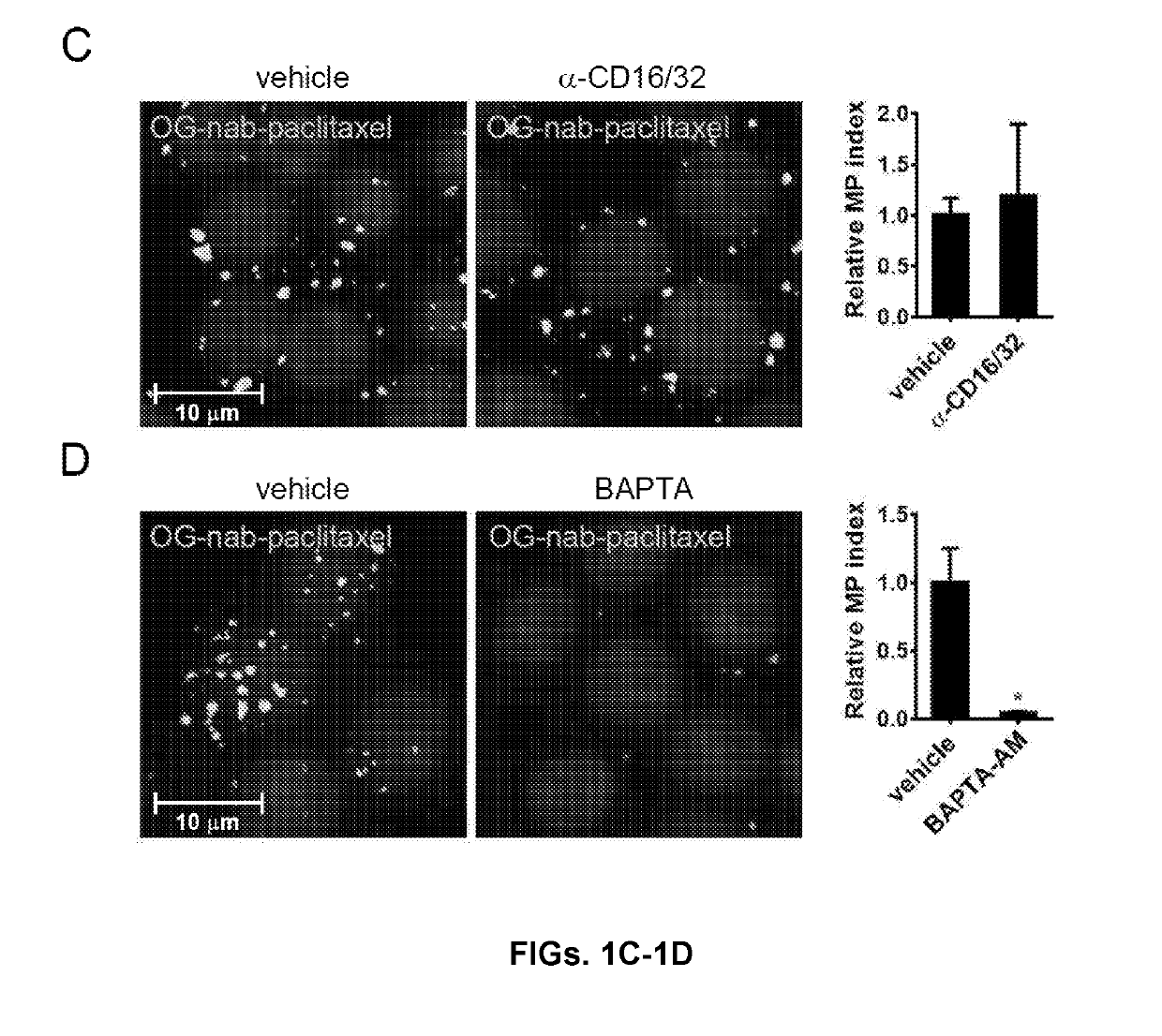
Methods and reagents for modulating macrophage phenotype
ActiveUS20190290688A1Restore immune recognitionReduced polarization effectsOrganic active ingredientsPeptide/protein ingredientsP PHENOTYPENeonatal Fc receptor
The present invention is directed to methods of inducing a phenotypic change in a population of monocytes and / or macrophages. The method includes administering to the population of monocytes and / or macrophages, a macrophage stimulating agent coupled to a carrier molecule, wherein the carrier molecule facilitates macropinocytic uptake of the agent by monocytes and macrophages in the population and is defective in neonatal Fc receptor binding, wherein the administering induces a phenotypic change in the monocytes and macrophages in the population.
Owner:NEW YORK UNIV
Purification of proteins using preparative reverse phase chromatography (RPC)
InactiveUS20090036652A1Increase separation forceColony-stimulating factorDepsipeptidesIndustrial scaleReversed-phase chromatography
The present invention provides a method for industrial-scale protein separation by reverse phase chromatography by use of a buffer system and an additional salt.
Owner:NOVO NORDISK AS
Lyophilization process
The present invention provides a process for producing a lyophilized pharmaceutical composition containing a protein. The present invention further provides a product produced by the process. The present invention further provides a process for producing an injectable pharmaceutical composition. The present invention further provides a method of treating a patient with a therapeutic protein composition.
Owner:TEVA PHARMA IND LTD
Method for the Treatment of Myelodysplastic Syndromes Using 1-Oxo-2-(2,6-Dioxopiperidin-3-Yl-)-4-Methylisoindoline
Methods of treating, preventing and / or managing myelodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com