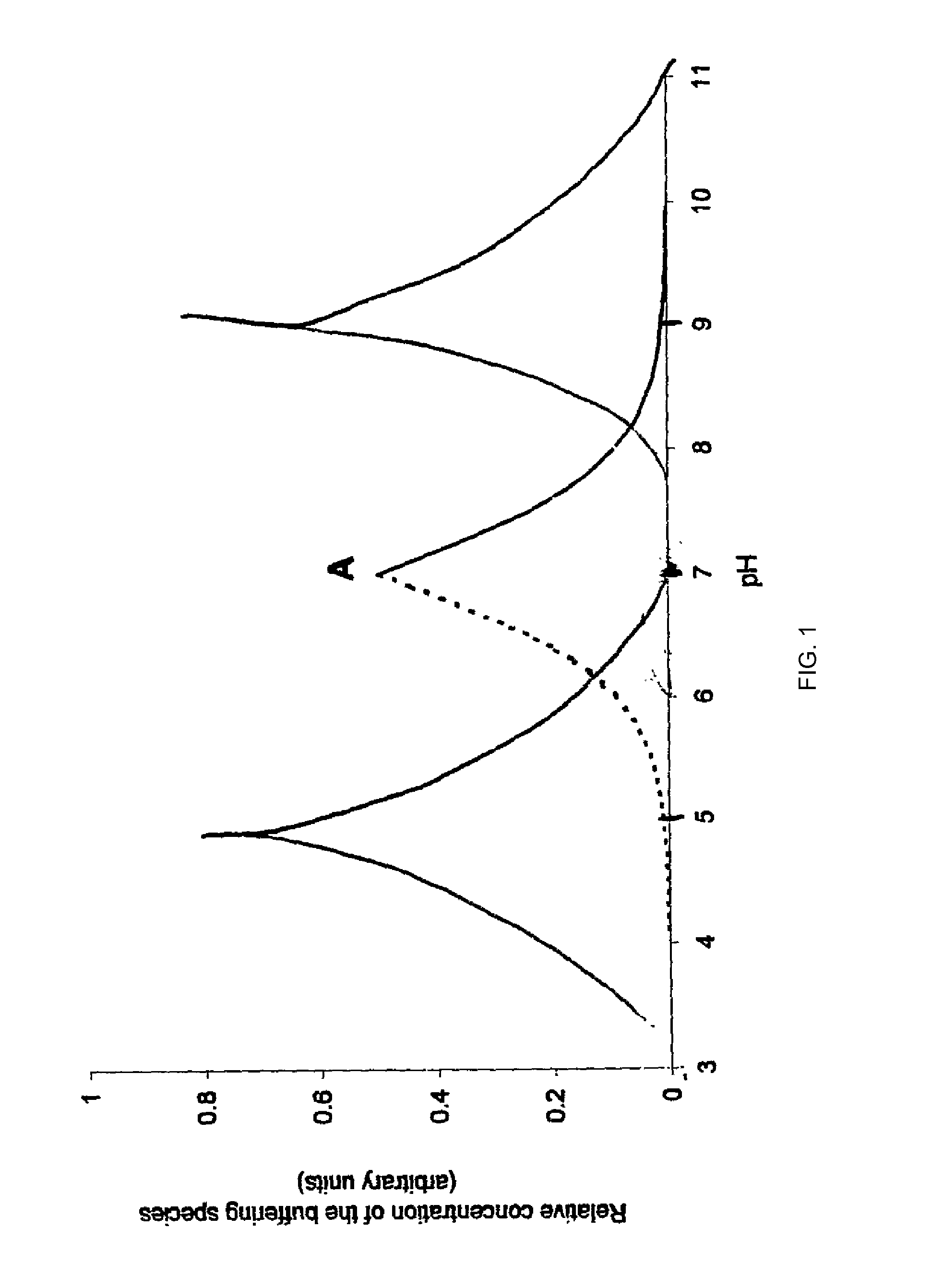
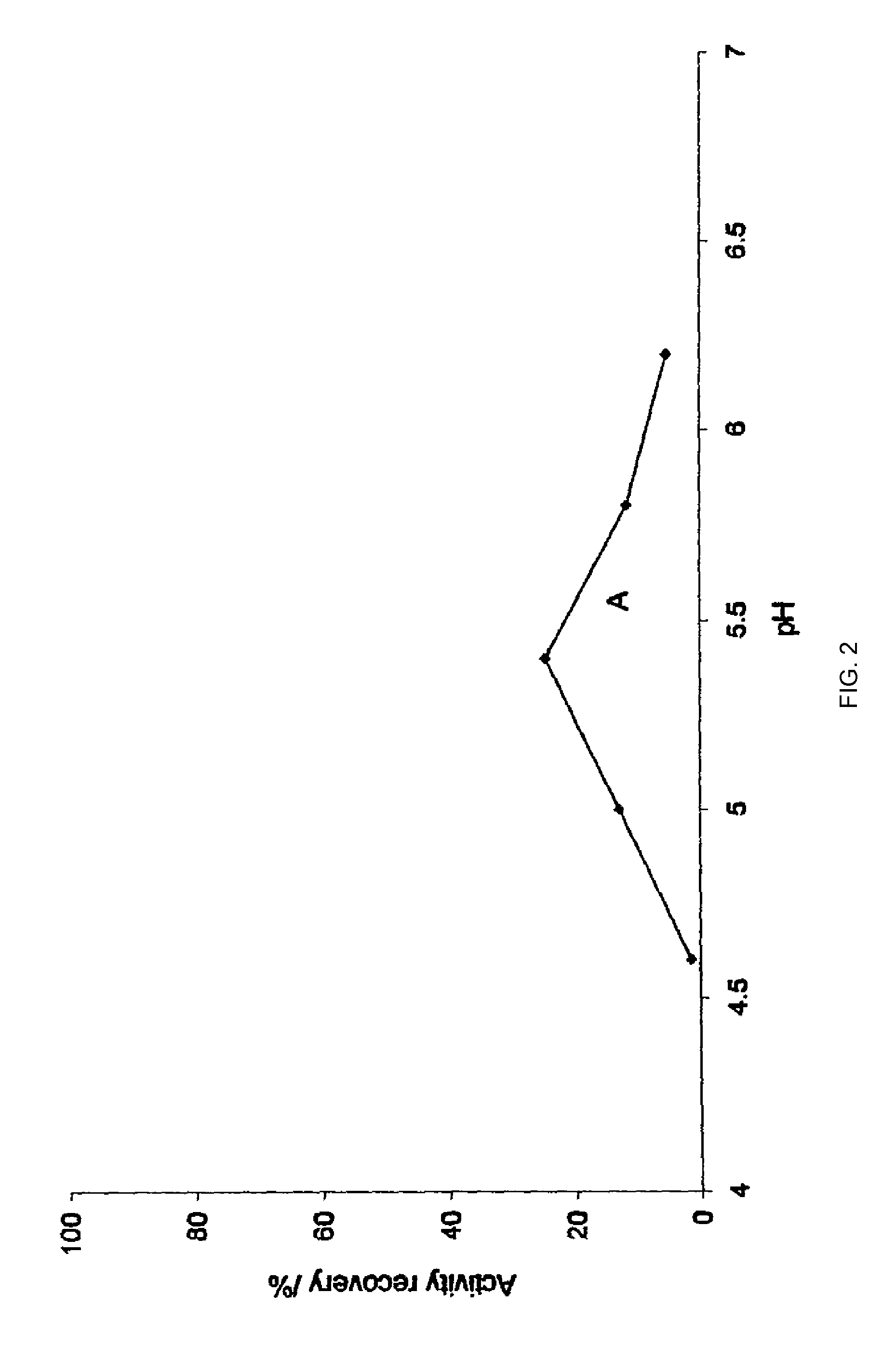
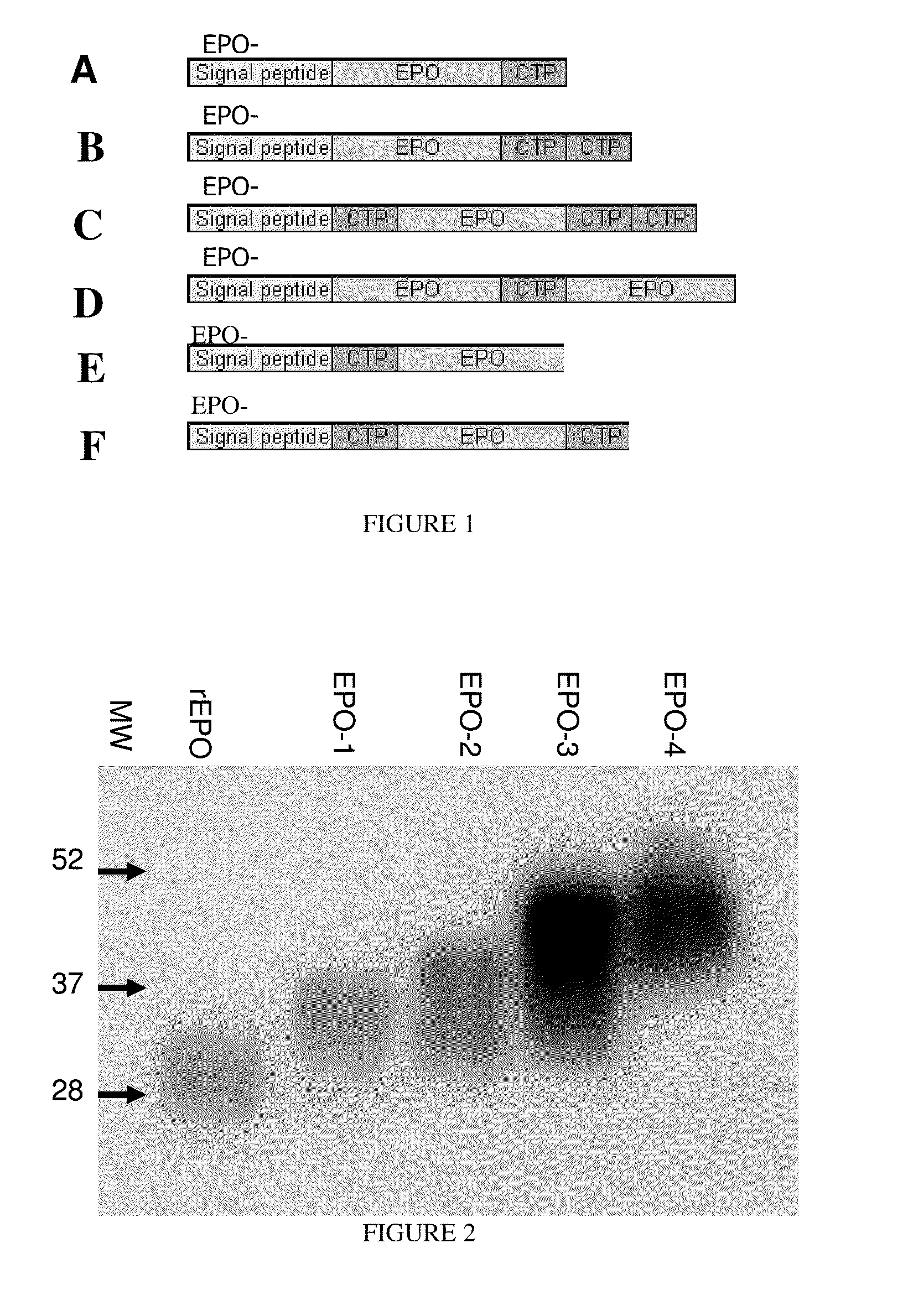
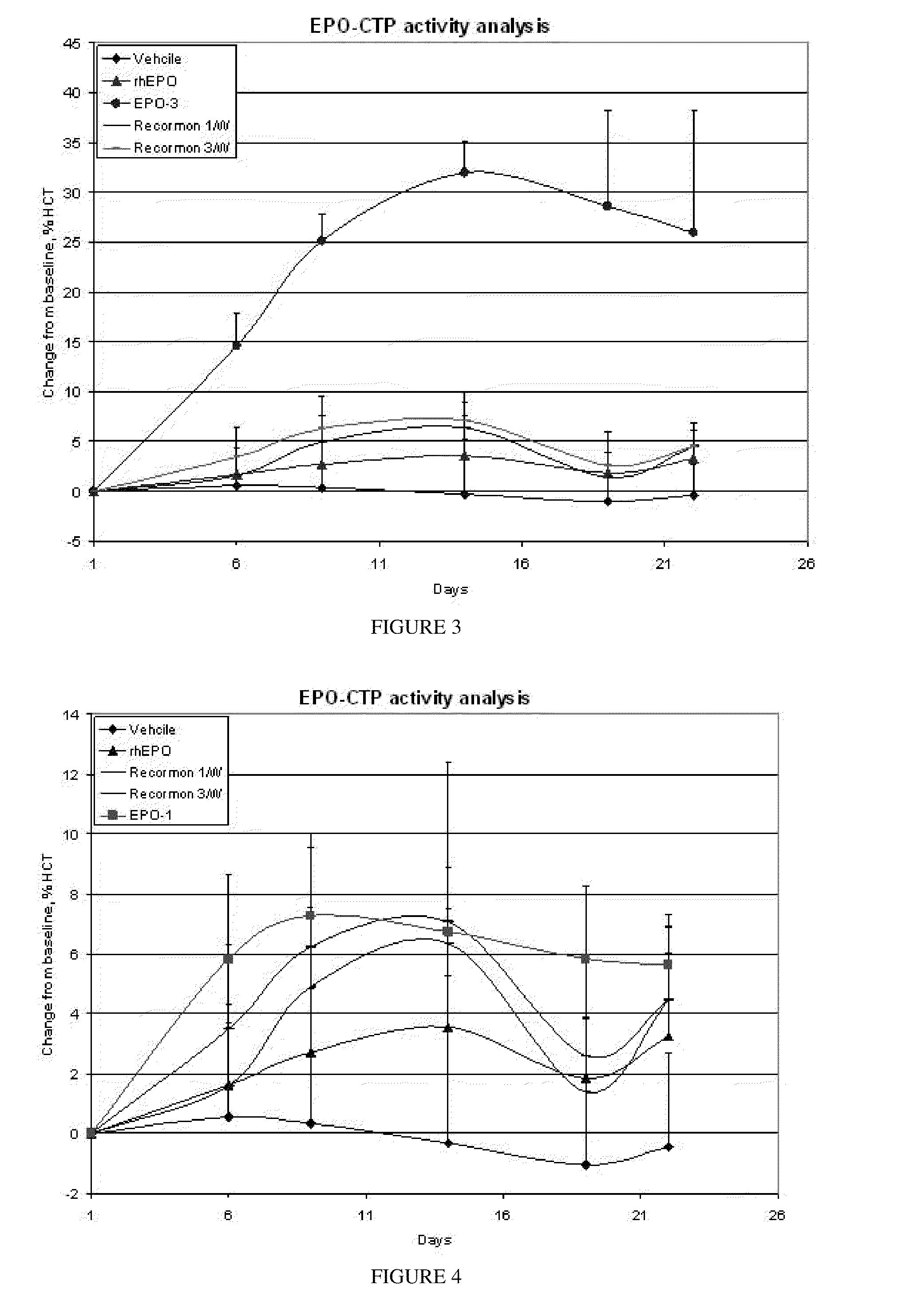
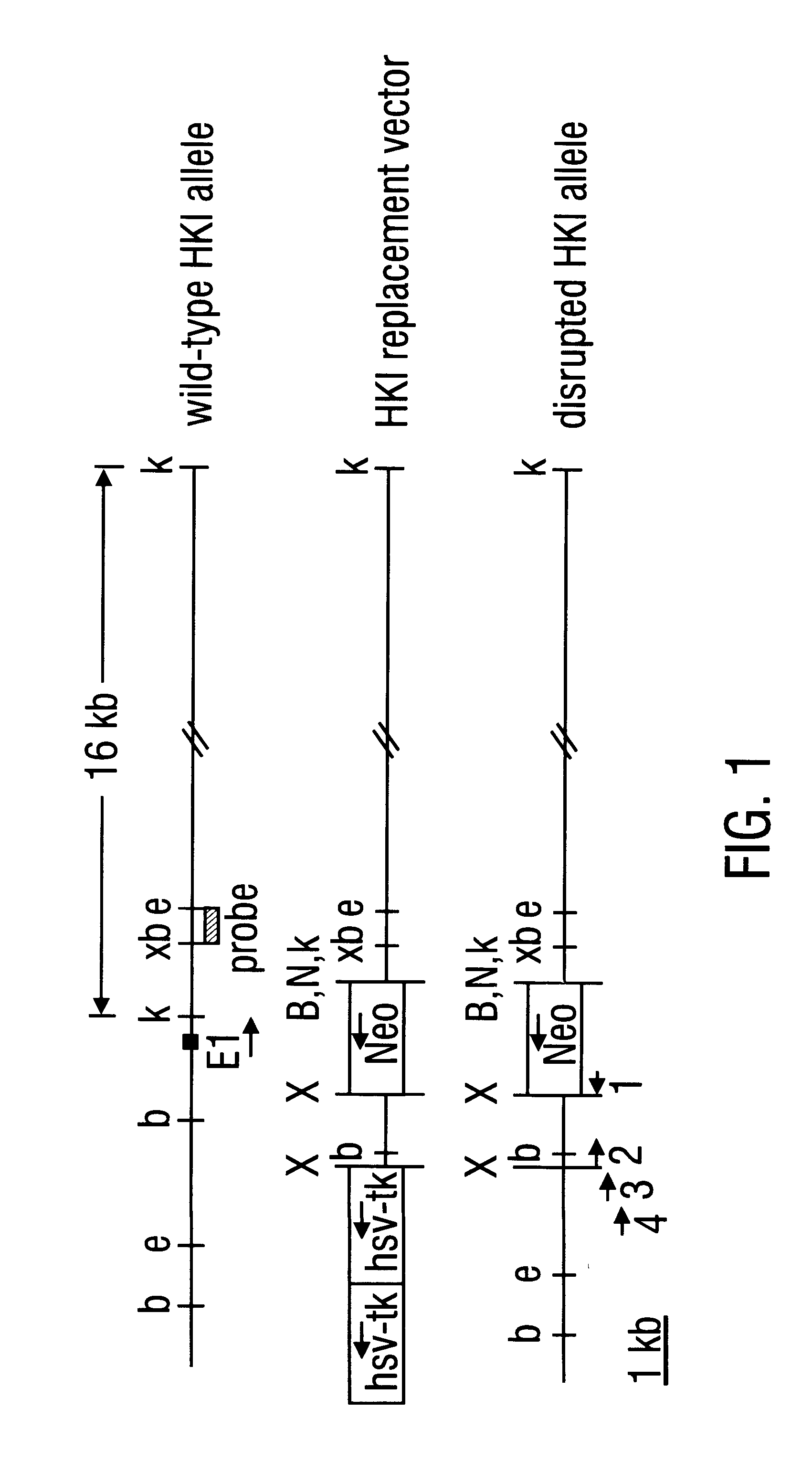
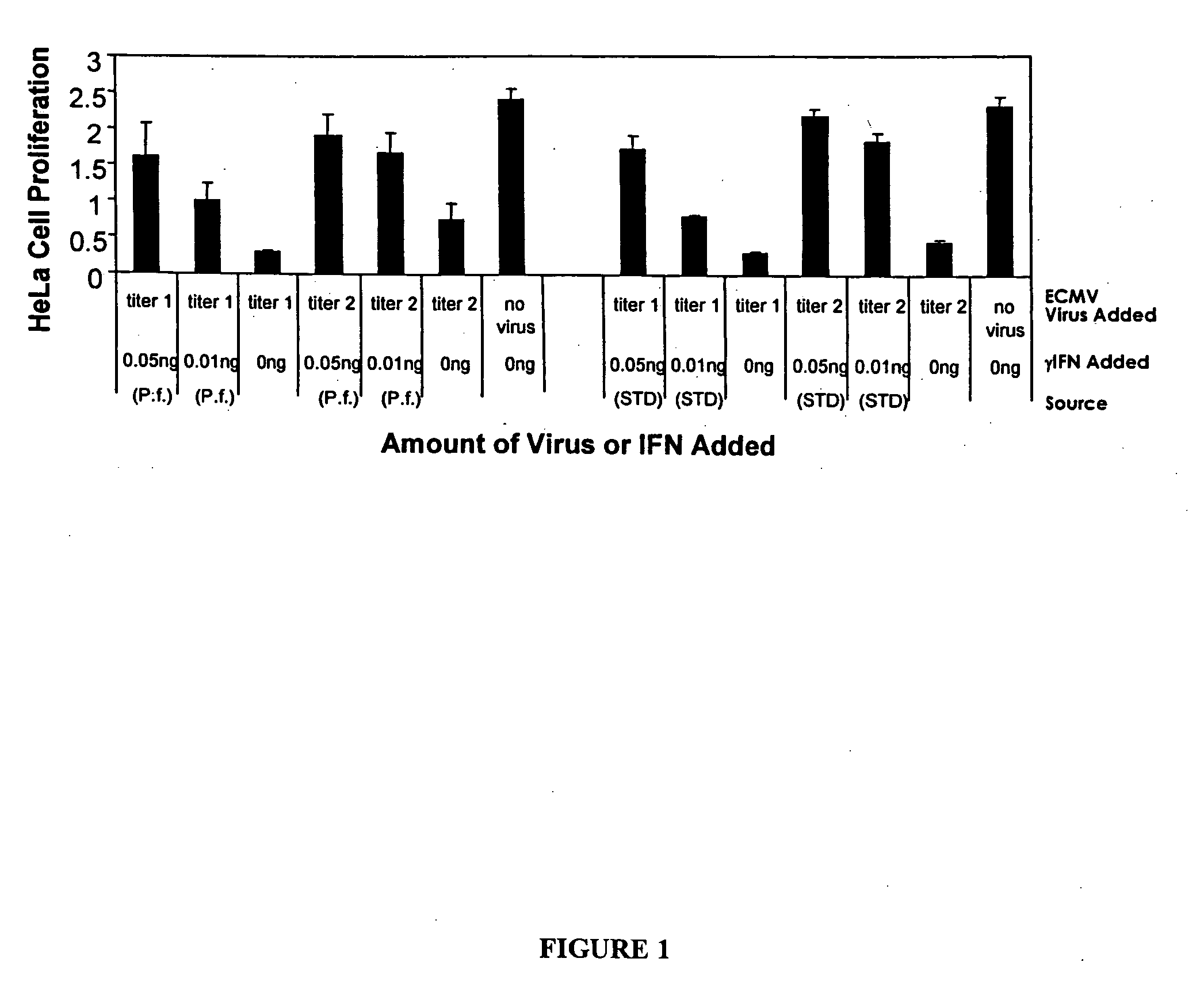
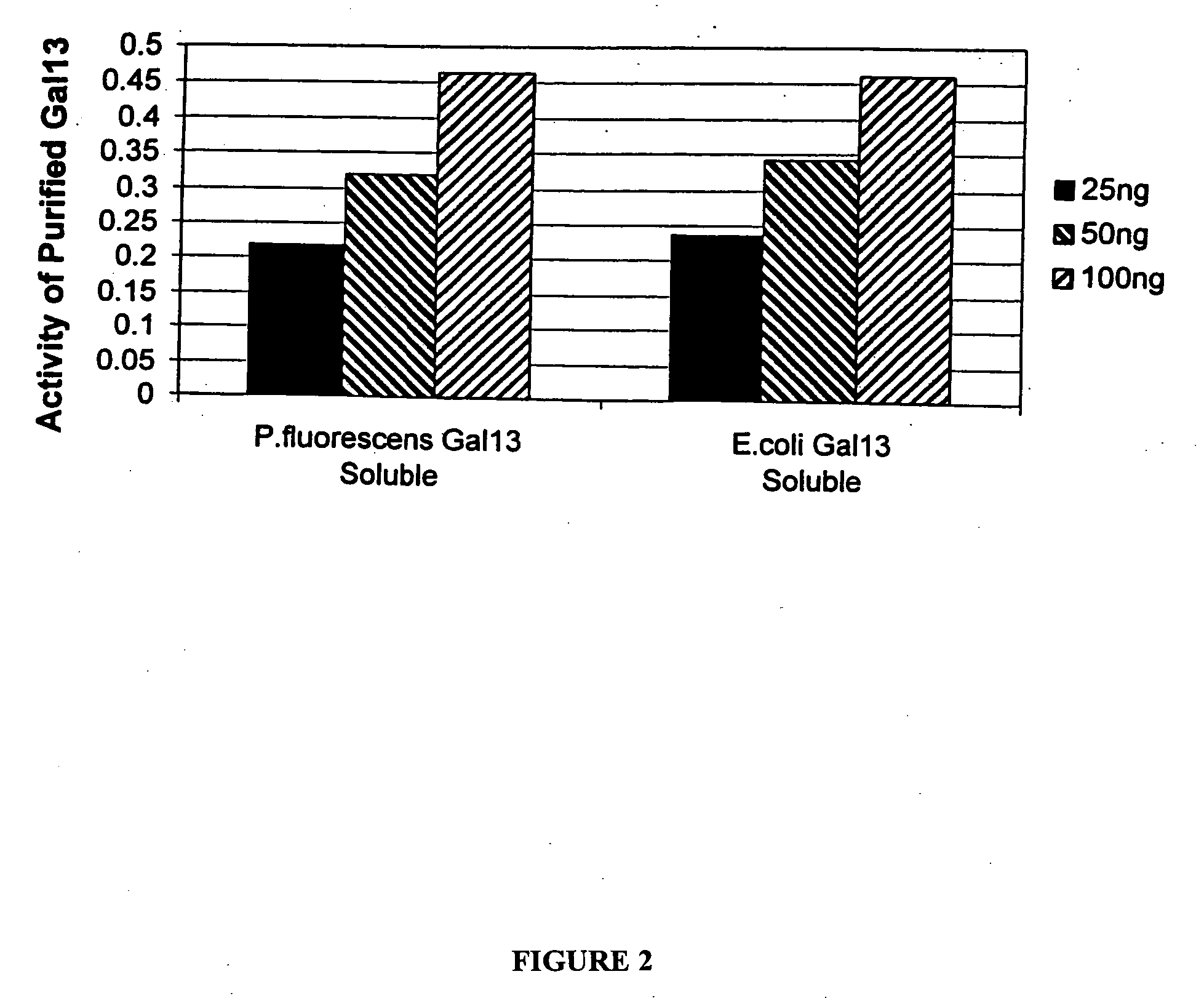
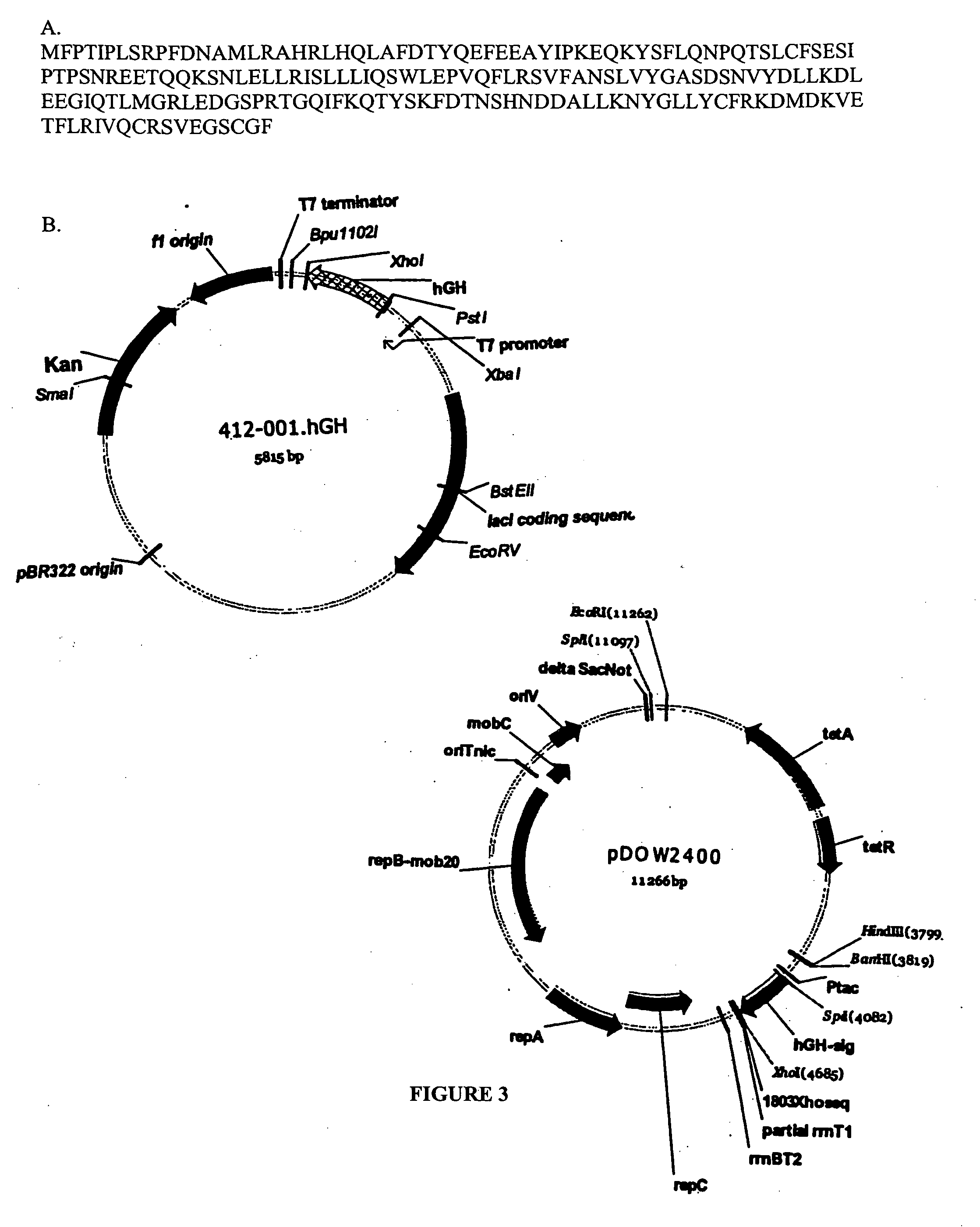
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
486results about "Growth hormones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
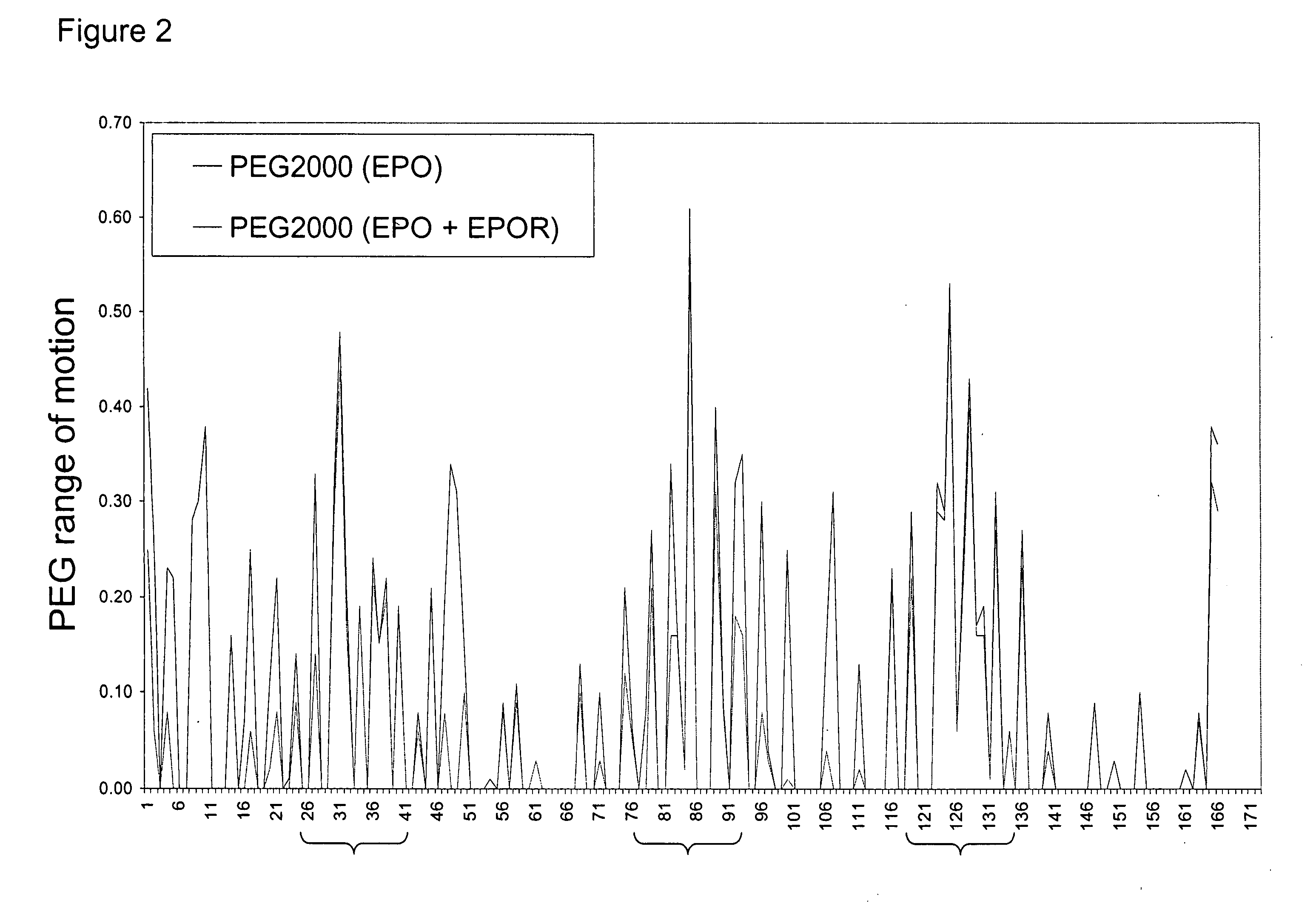
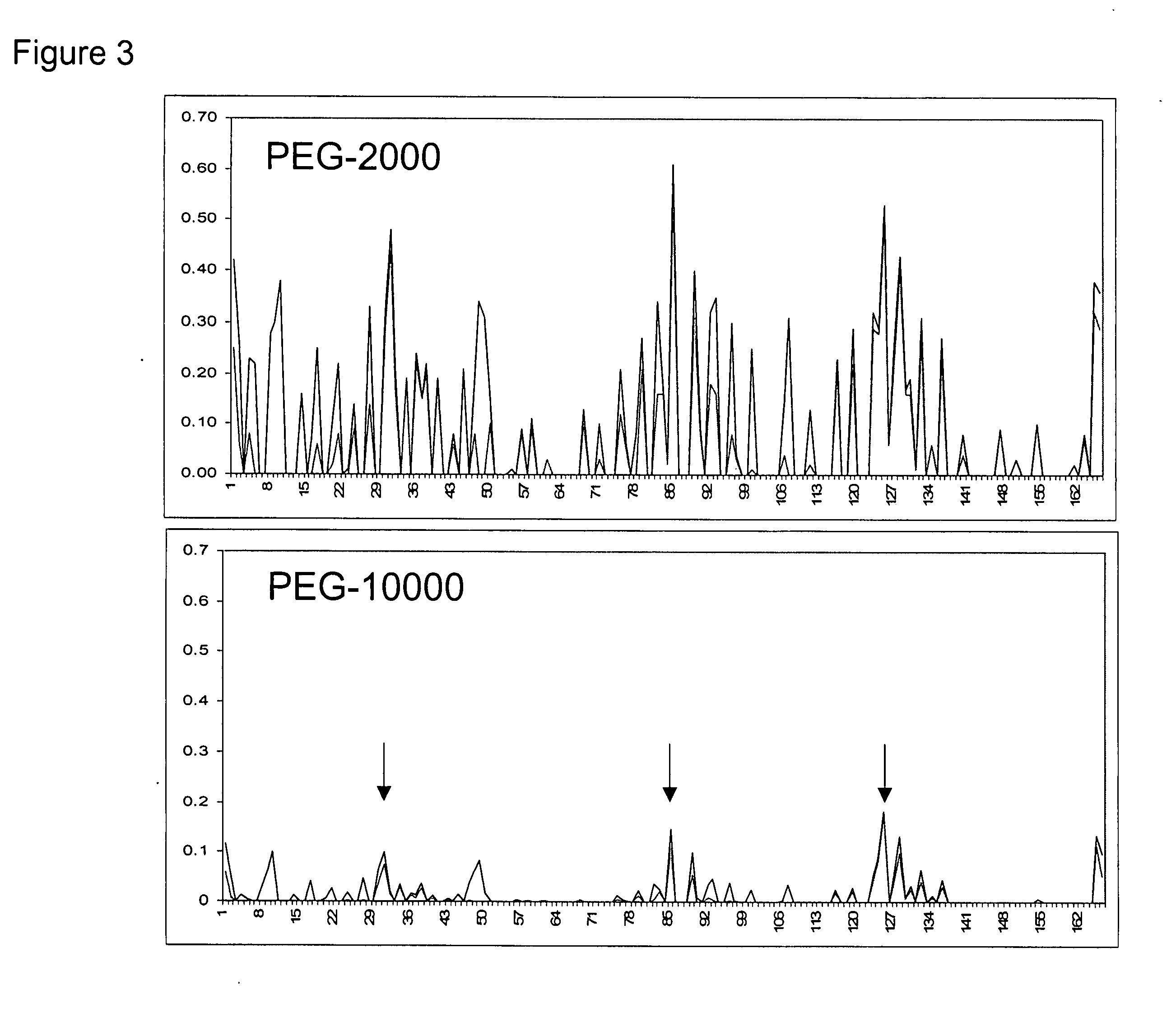
Methods for rational pegylation of proteins
The present invention relates to the use of simulation technology to rationally optimize the locations and sizes of attached polymeric moieties for modification of therapeutic proteins and the proteins generated from this method.
Owner:XENCOR
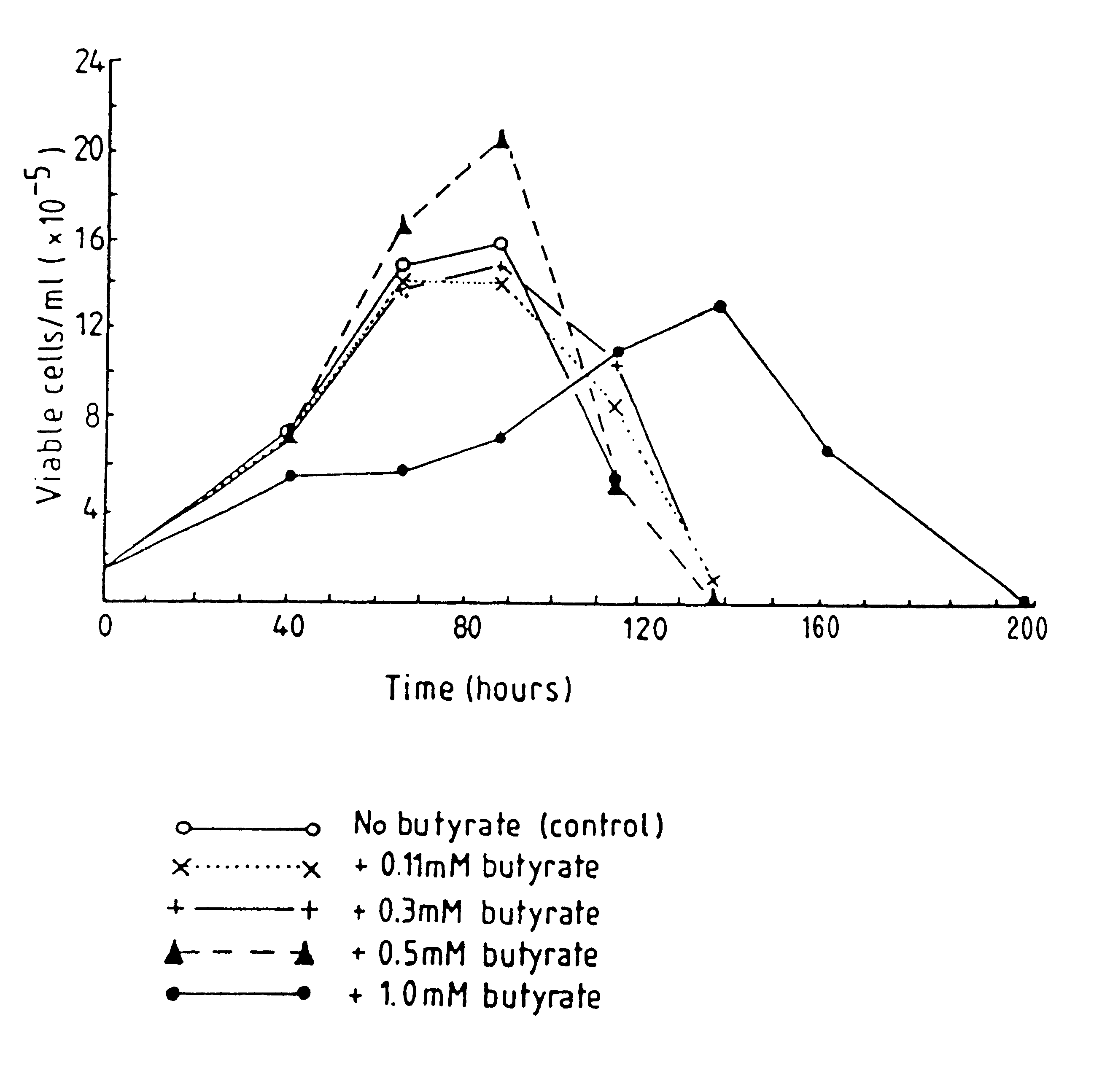
Production of proteins by cell culture
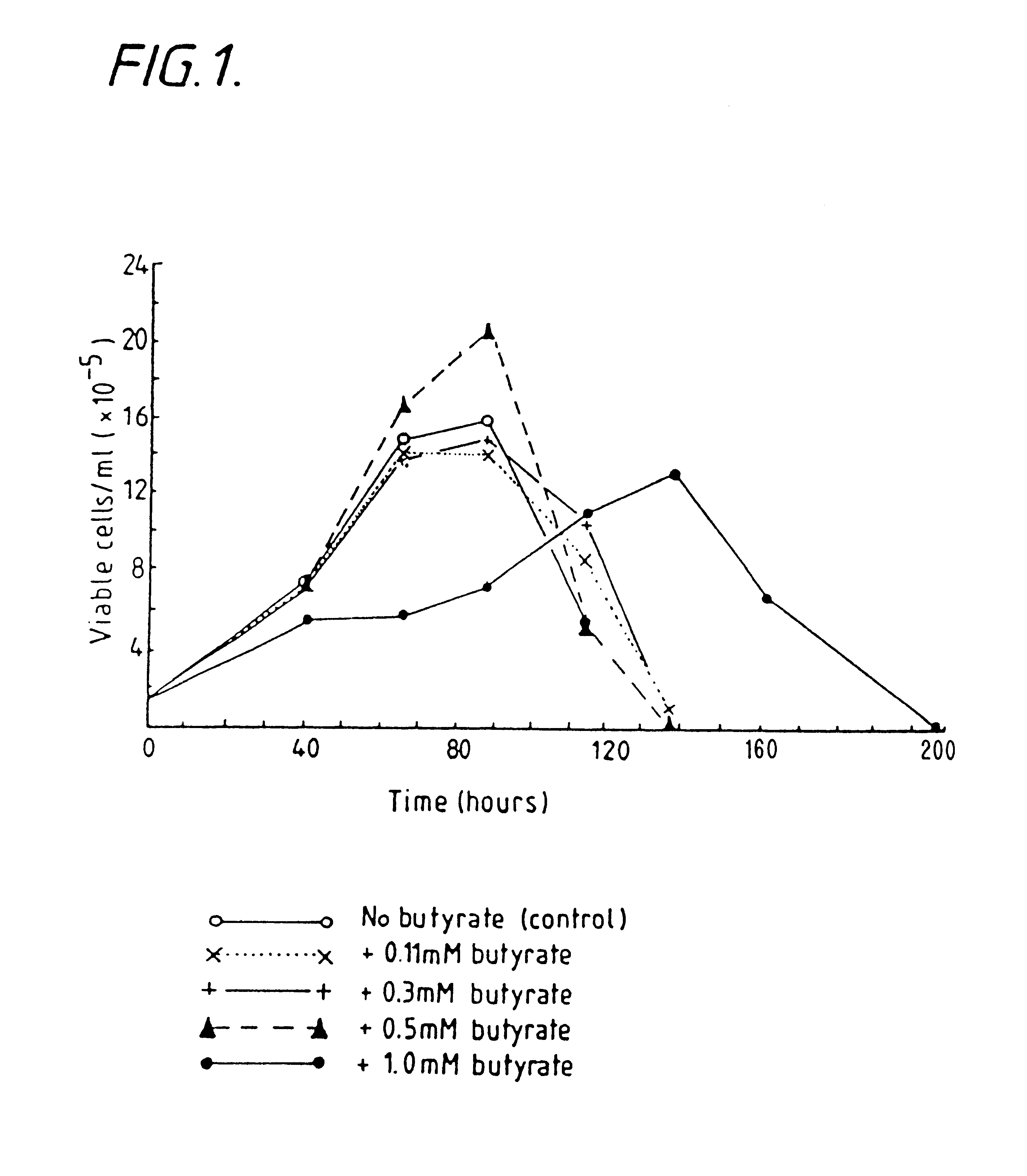
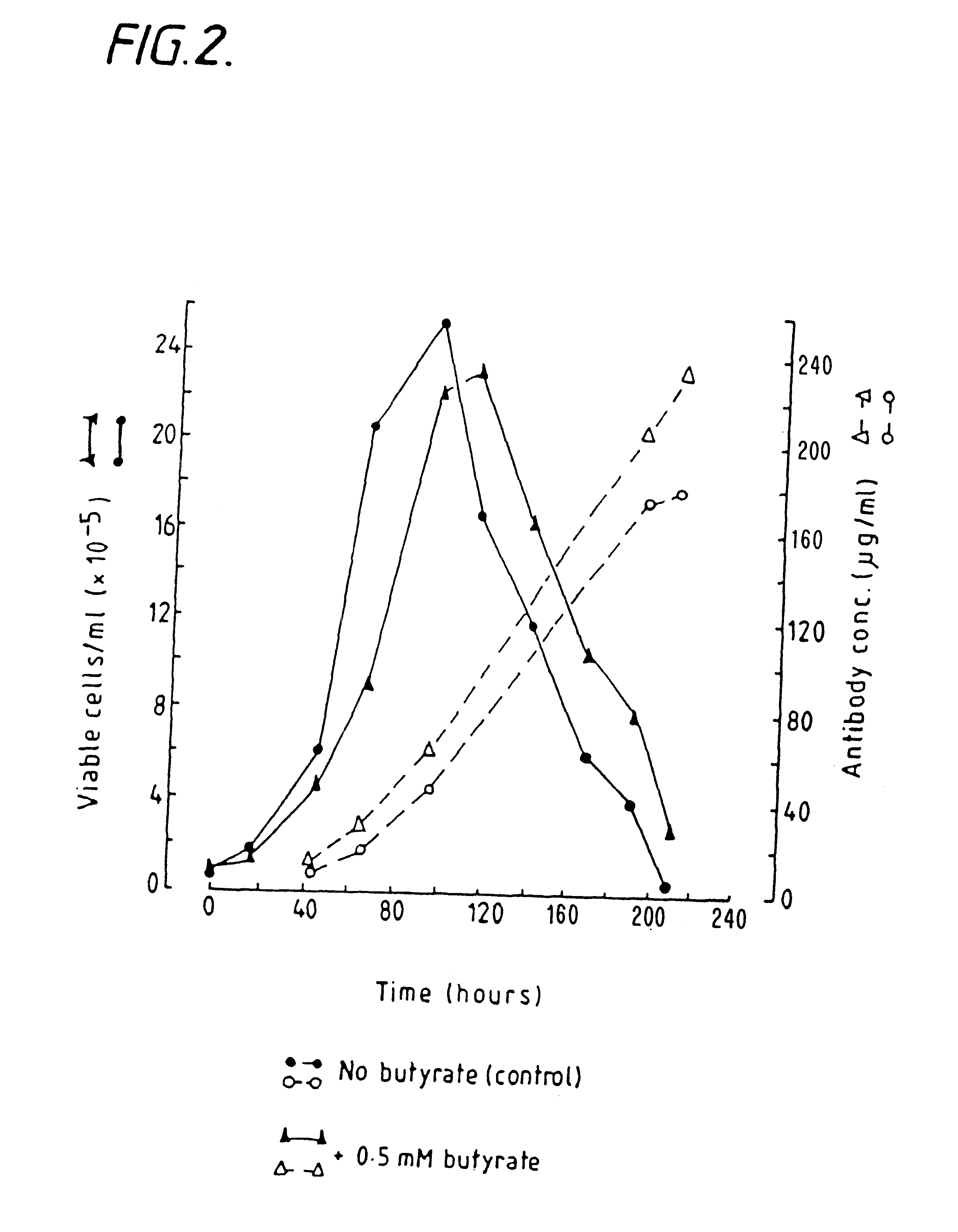
InactiveUS6413746B1High protein yieldReduce cell viabilityImmunoglobulins against blood group antigensPeptide/protein ingredients3D cell cultureBiochemistry
Methods for obtaining a protein by culture of hybridoma cells, wherein said protein is an immunoglobulin, are disclosed. The methods involve culturing animal hybridoma cells in continuous presence of an alkanoic acid or salt thereof, which enhances protein production, wherein said alkanoic acid or salt thereof is present at 2 concentration range of 0.1 mM to 200 mM.
Owner:LONZA LTD
Methods for making proteins containing free cysteine residues
The present invention relates to novel methods of making soluble proteins having free cysteines in which a host cell is exposed to a cysteine blocking agent. The soluble proteins produced by the methods can then be modified to increase their effectiveness. Such modifications include attaching a PEG moiety to form pegylated proteins.
Owner:BOLDER BIOTECH
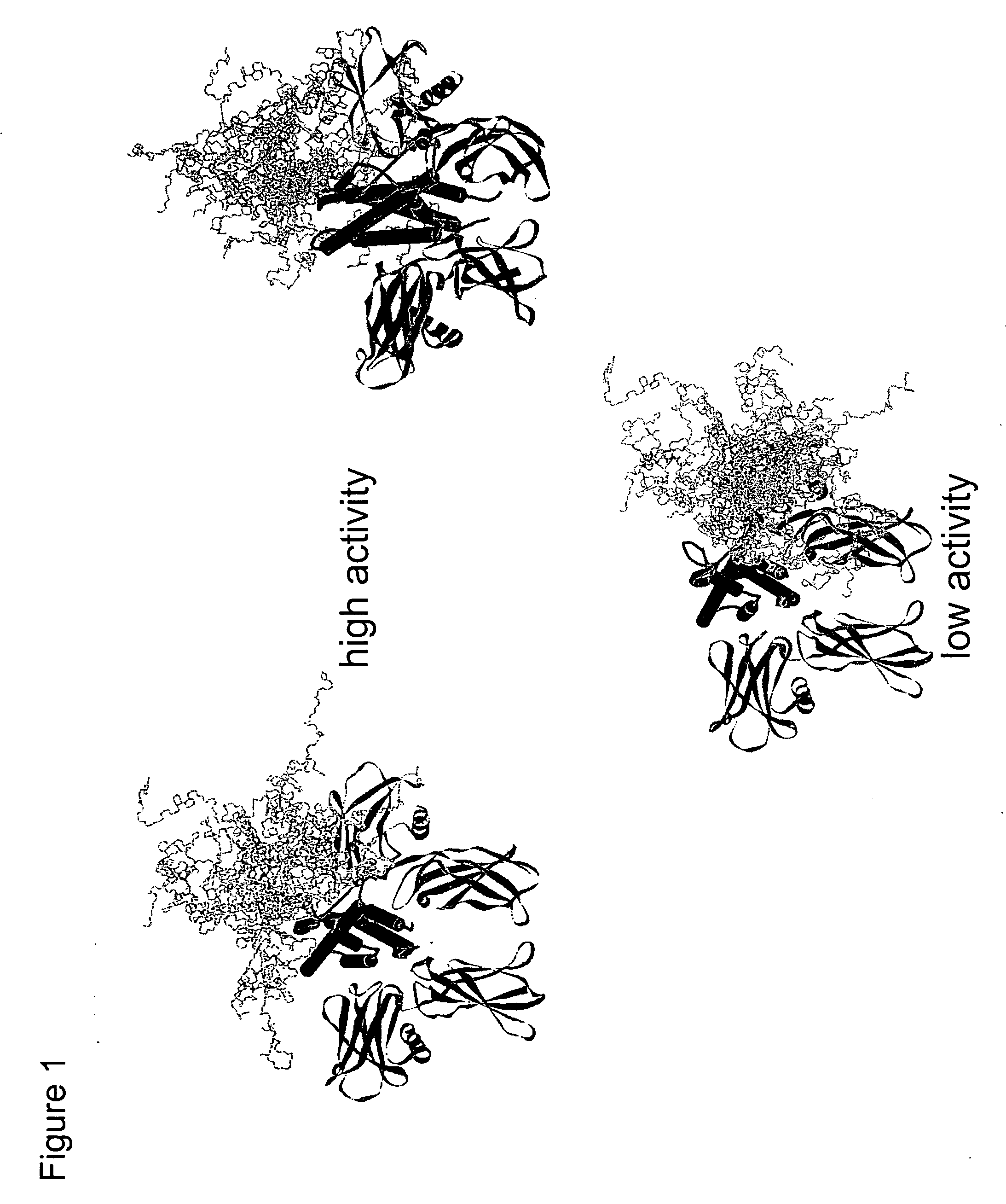
Cysteine variants of erythropoietin
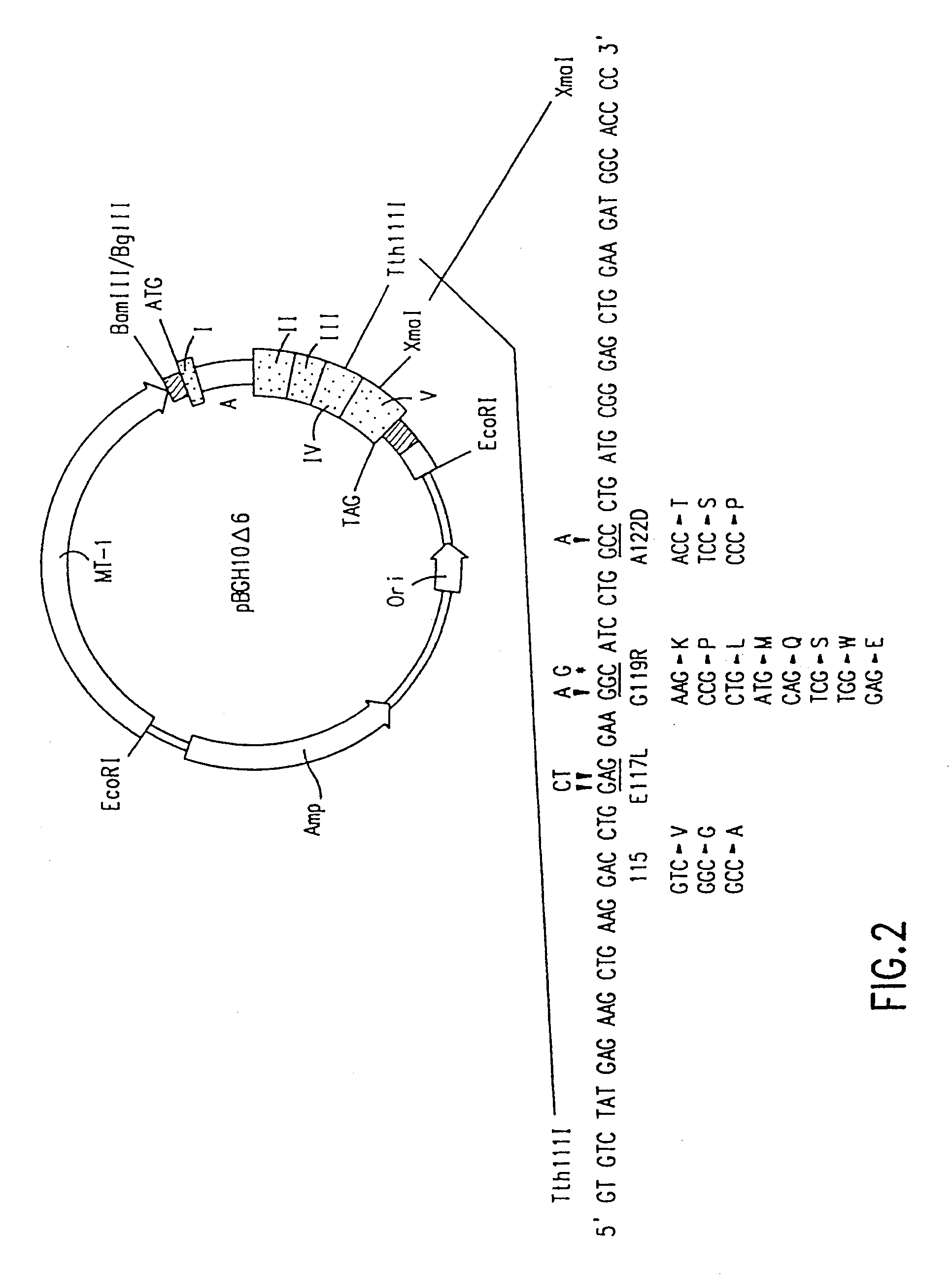
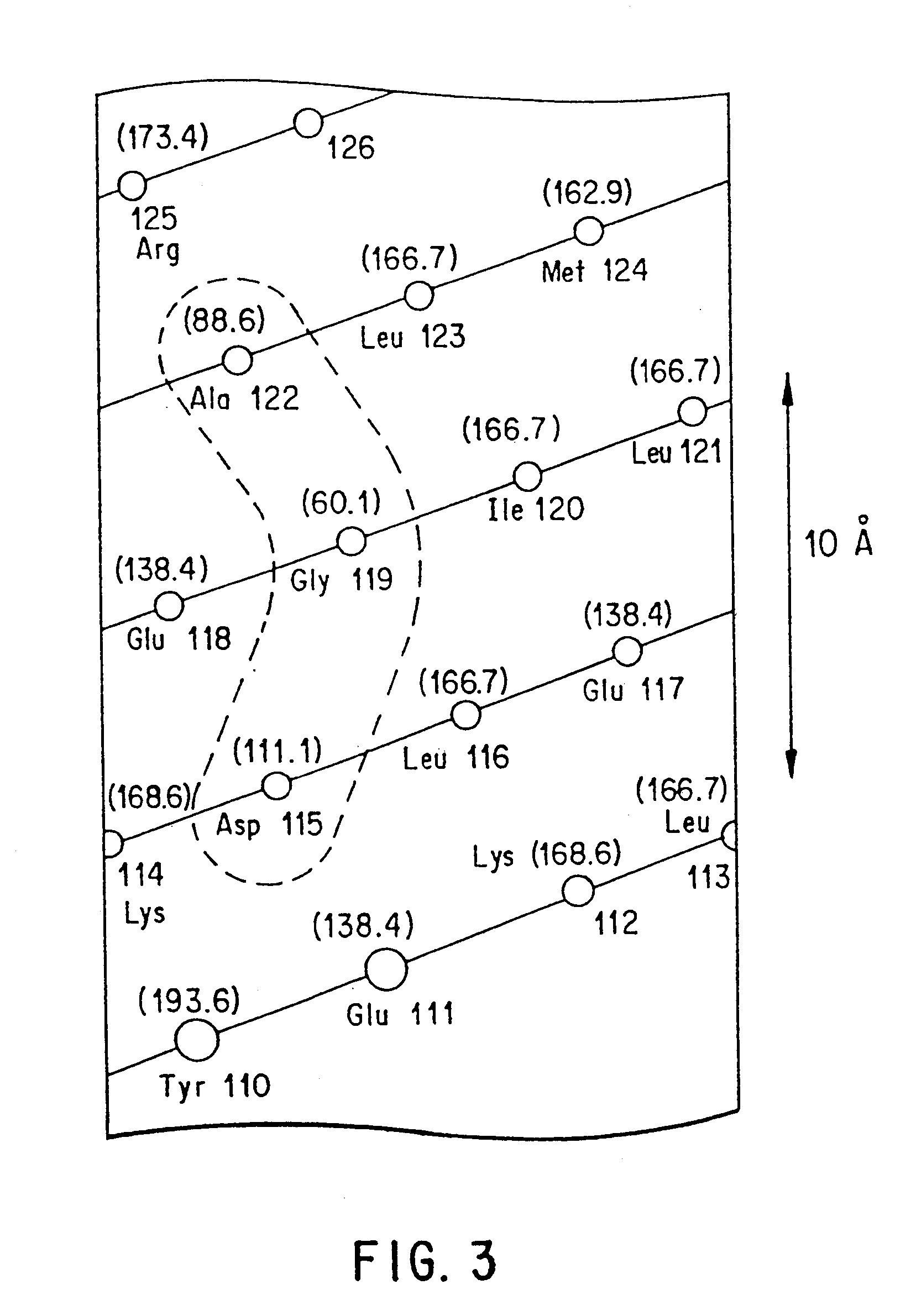
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
Cysteine variants of erythropoietin
The growth hormone supergene family comprises greater than 20 structurally related cytokines and growth factors. A general method is provided for creating site-specific, biologically active conjugates of these proteins. The method involves adding cysteine residues to non-essential regions of the proteins or substituting cysteine residues for non-essential amino acids in the proteins using site-directed mutagenesis and then covalently coupling a cysteine-reactive polymer or other type of cysteine-reactive moiety to the proteins via the added cysteine residue. Disclosed herein are preferred sites for adding cysteine residues or introducing cysteine substitutions into the proteins, and the proteins and protein derivatives produced thereby.
Owner:BOLDER BIOTECH
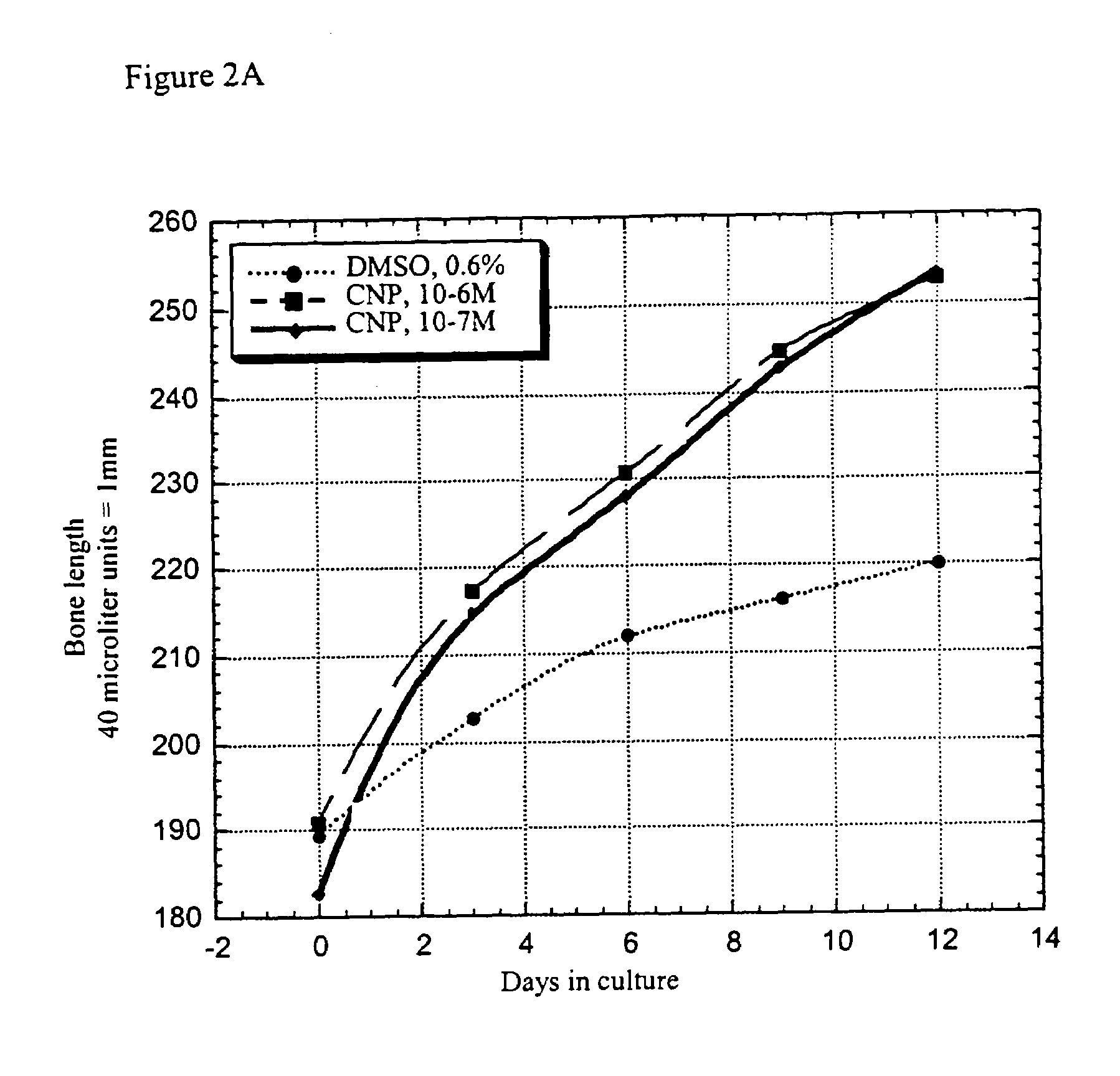
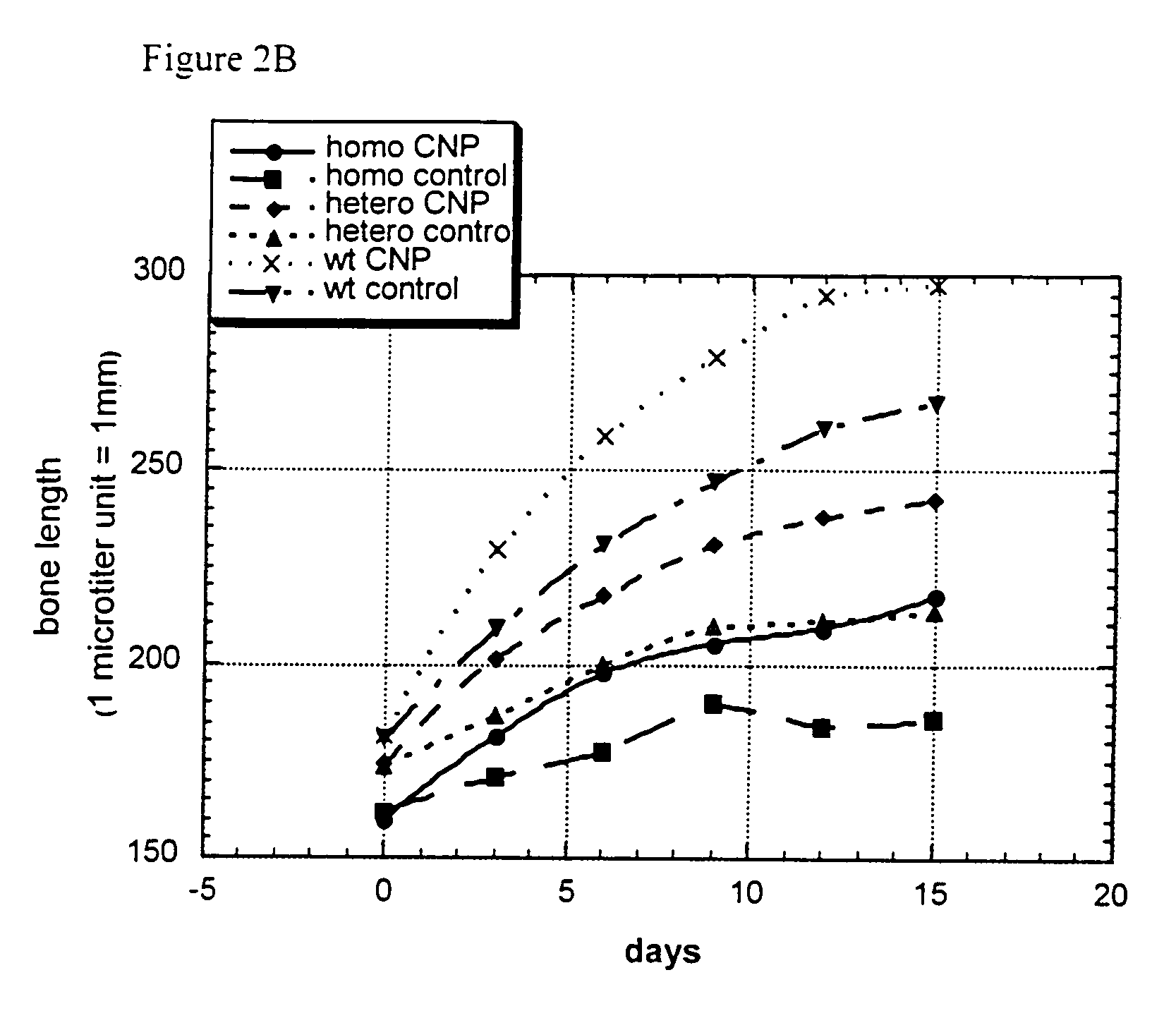
Method and composition for treatment of skeletal dysplasias
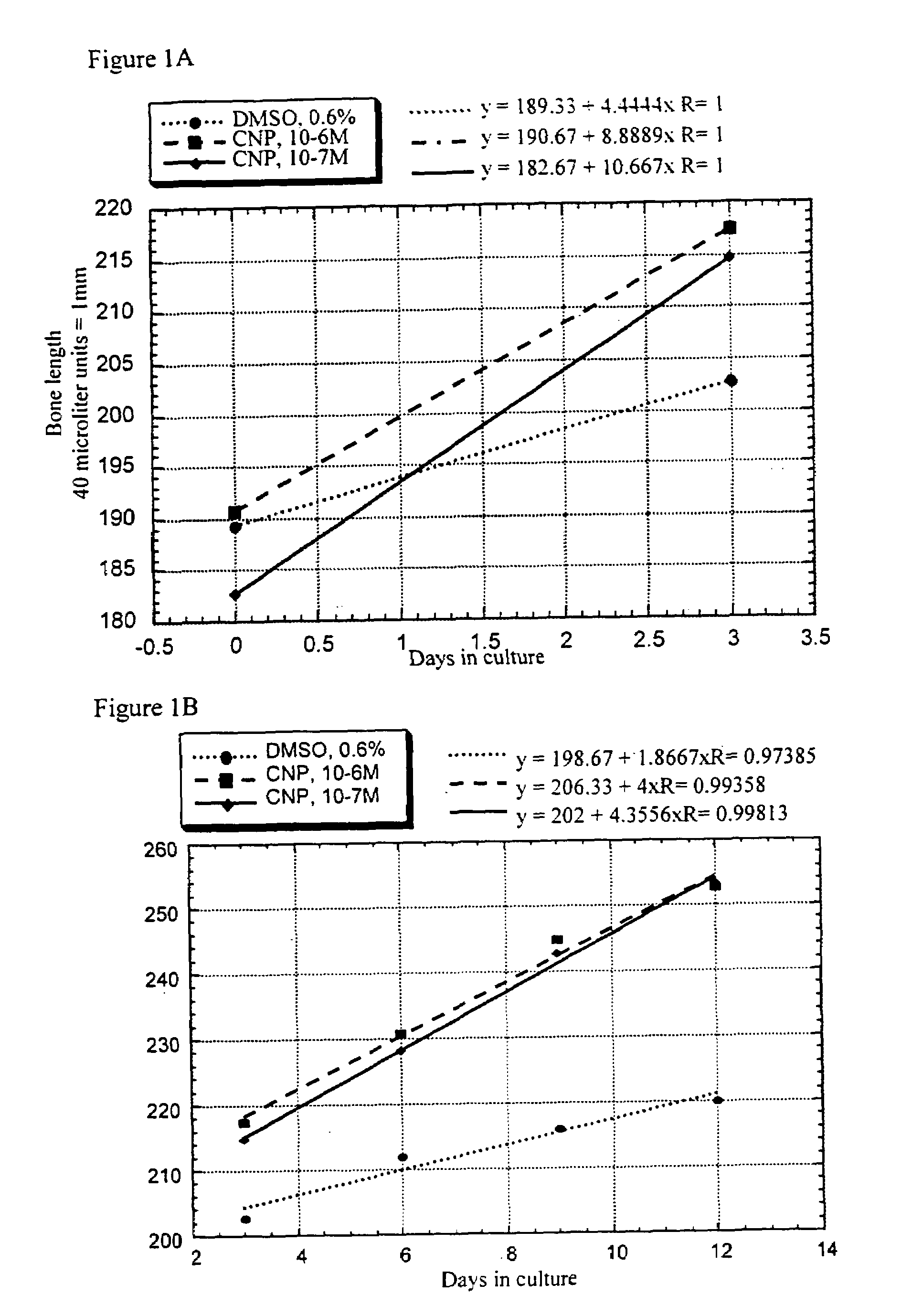
InactiveUS7276481B2Improve stabilityEnhance NP stabilizationPeptide/protein ingredientsGenetic material ingredientsBone growthBULK ACTIVE INGREDIENT
The present invention discloses pharmaceutical compositions for the treatment of skeletal dysplasias, comprising as an active ingredient at least one natriuretic peptide. Unexpectedly, it has been shown that the natriuretic factors may be effective for bone elongation in situations of abnormal bone growth especially for achondroplasia. The effects of the natriuretic peptide may be further enhanced by prolonging its residence time or action at the target site.
Owner:HEPACORE LTD
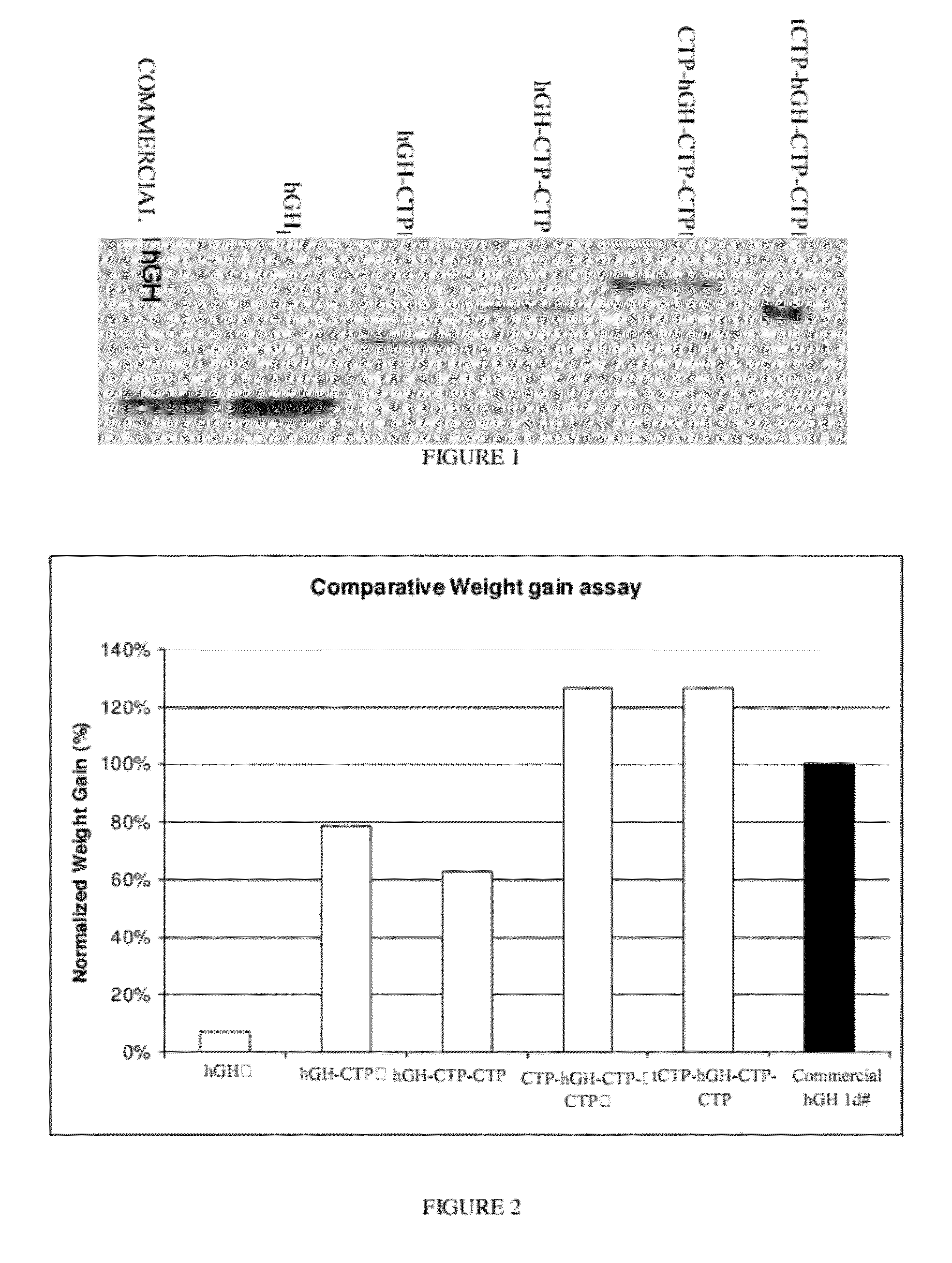
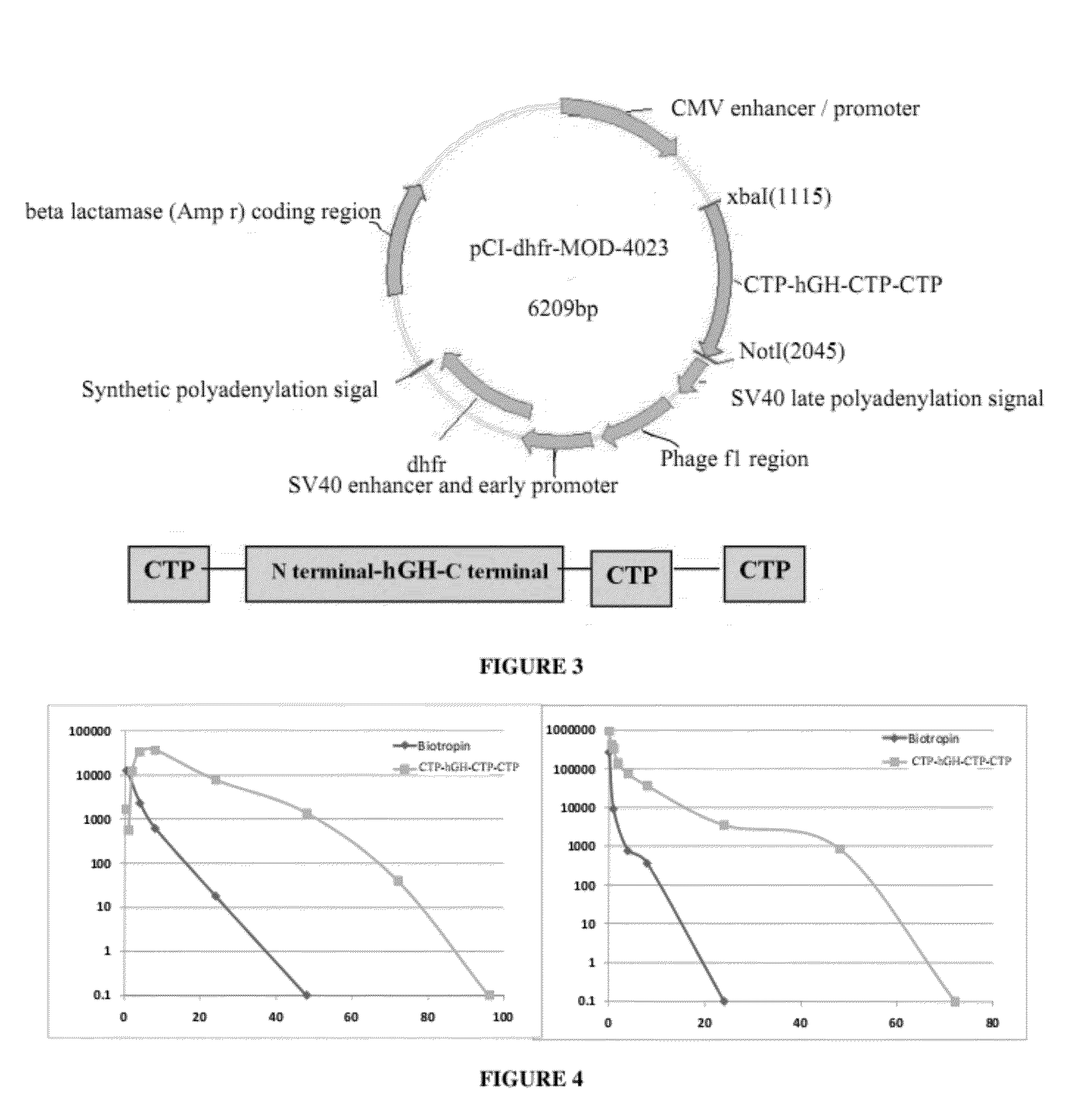
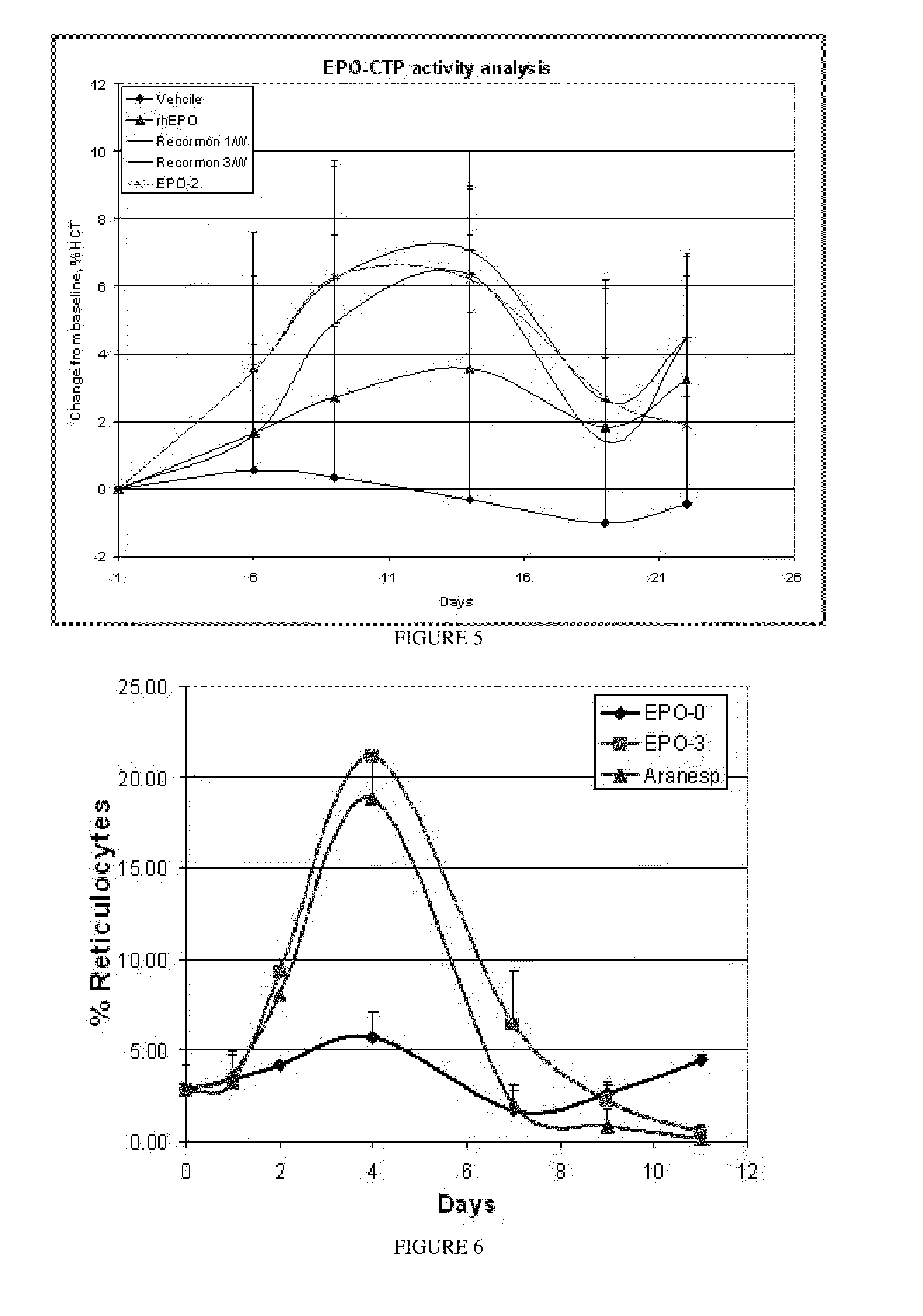
Long-acting growth hormone and methods of producing same
ActiveUS20120035101A1Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderSomatotropic hormoneNucleotide
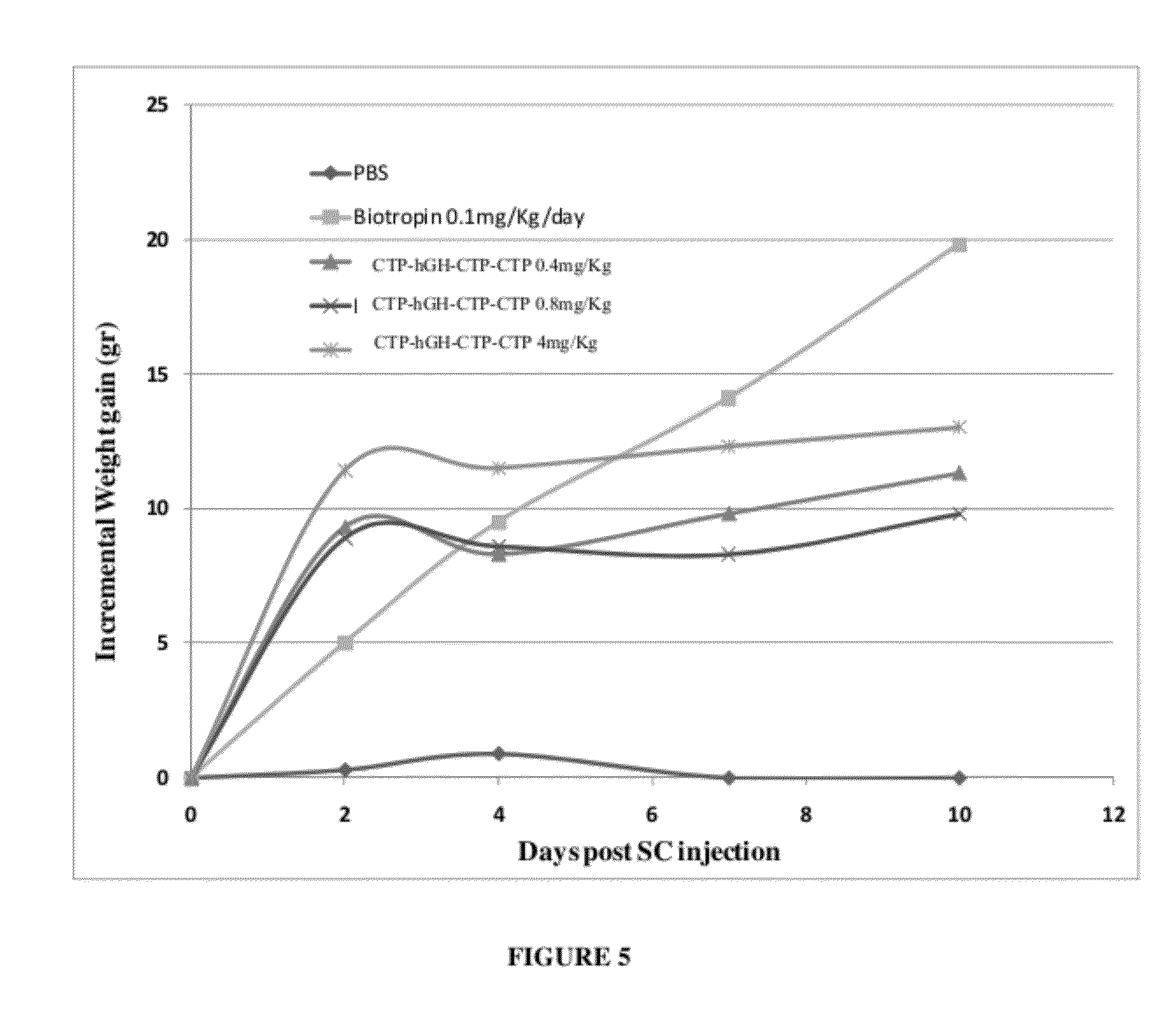
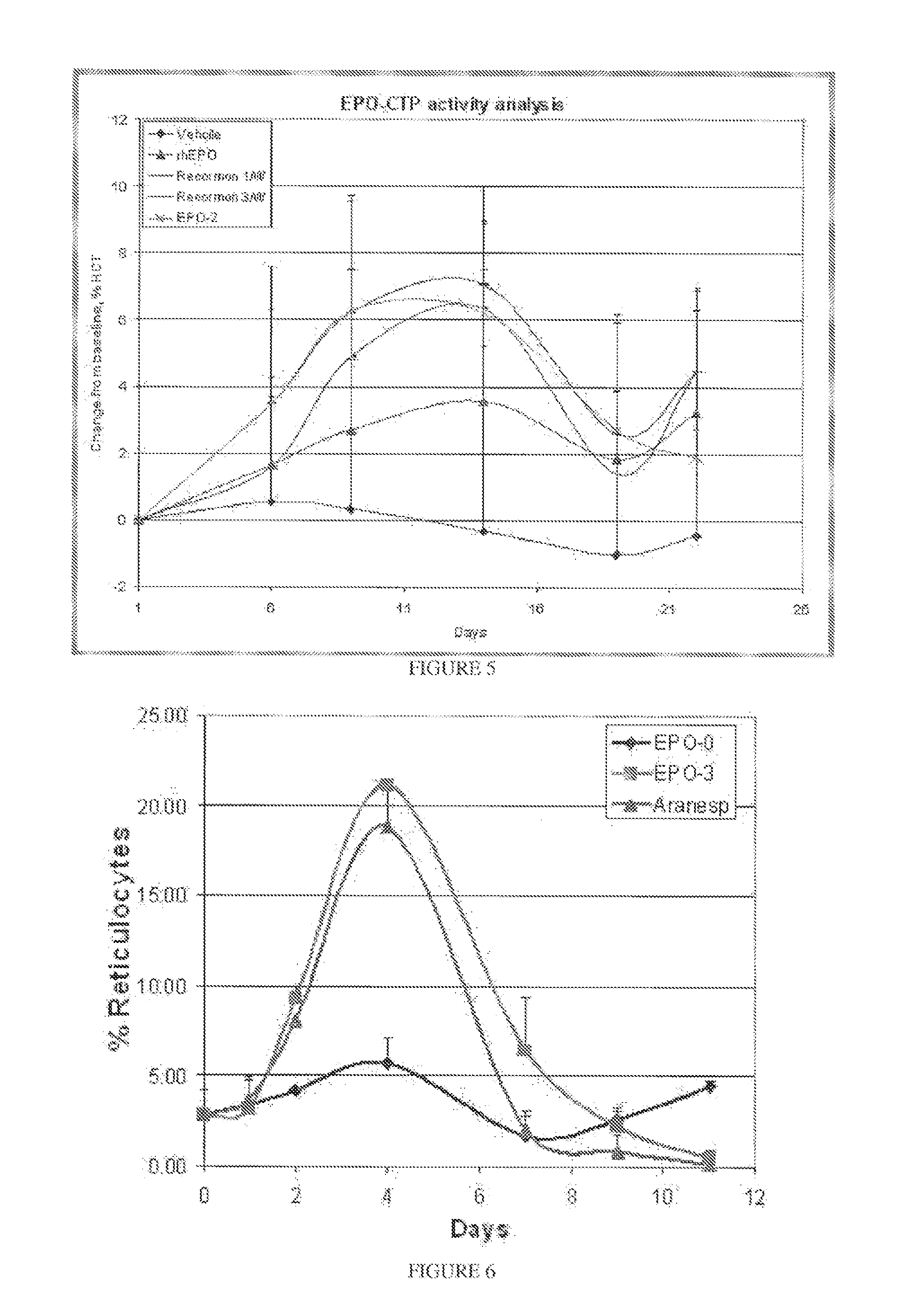
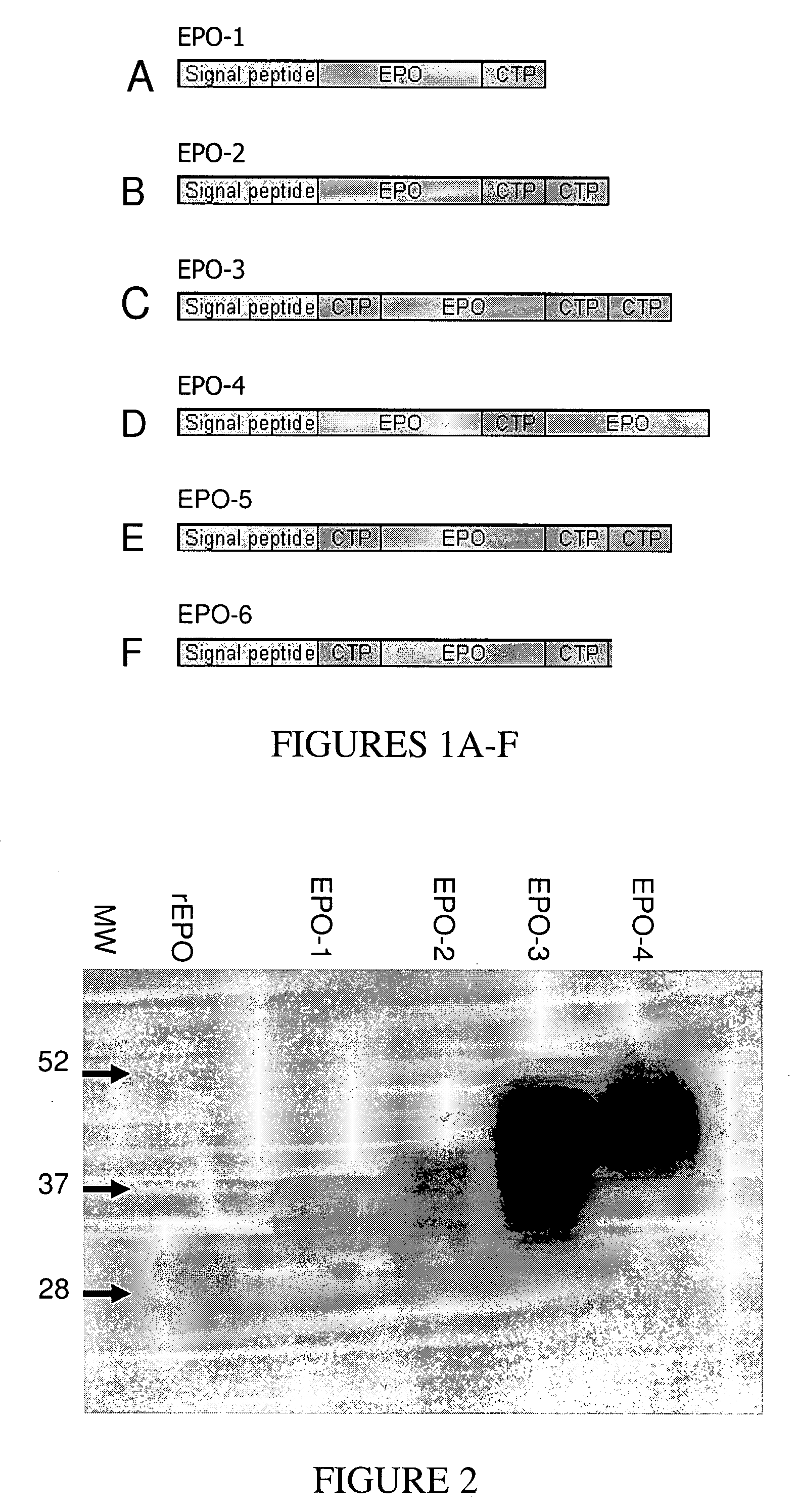
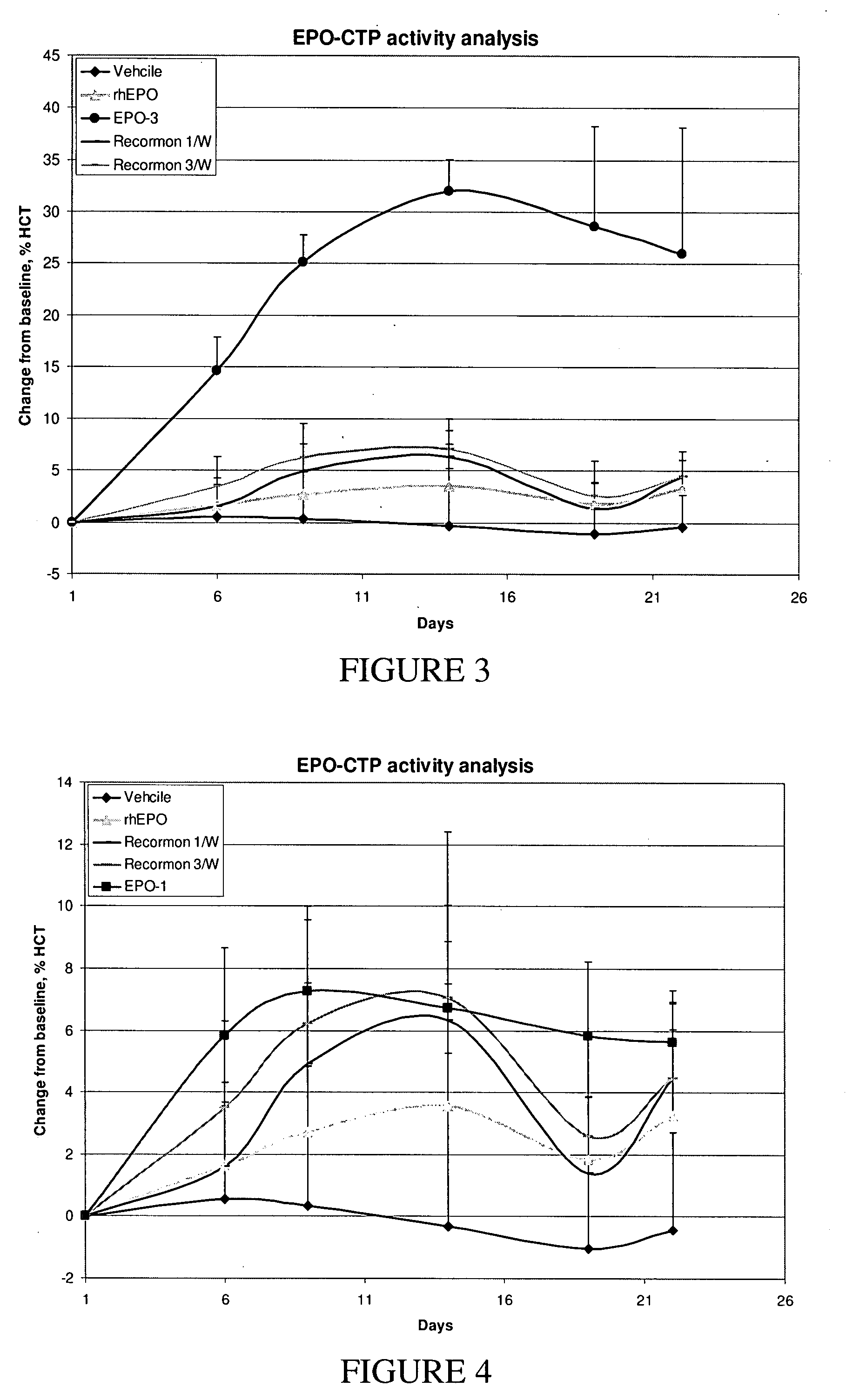
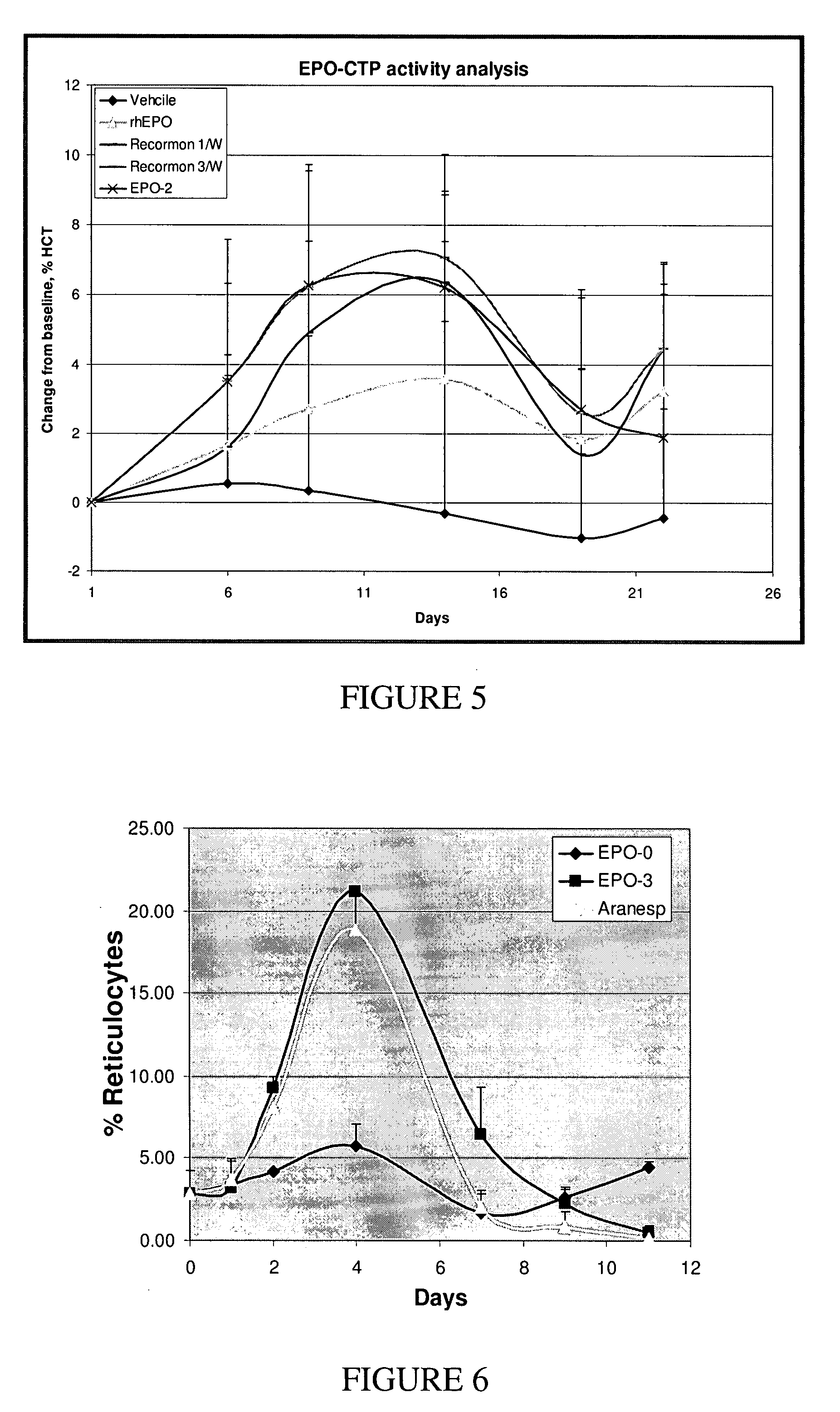
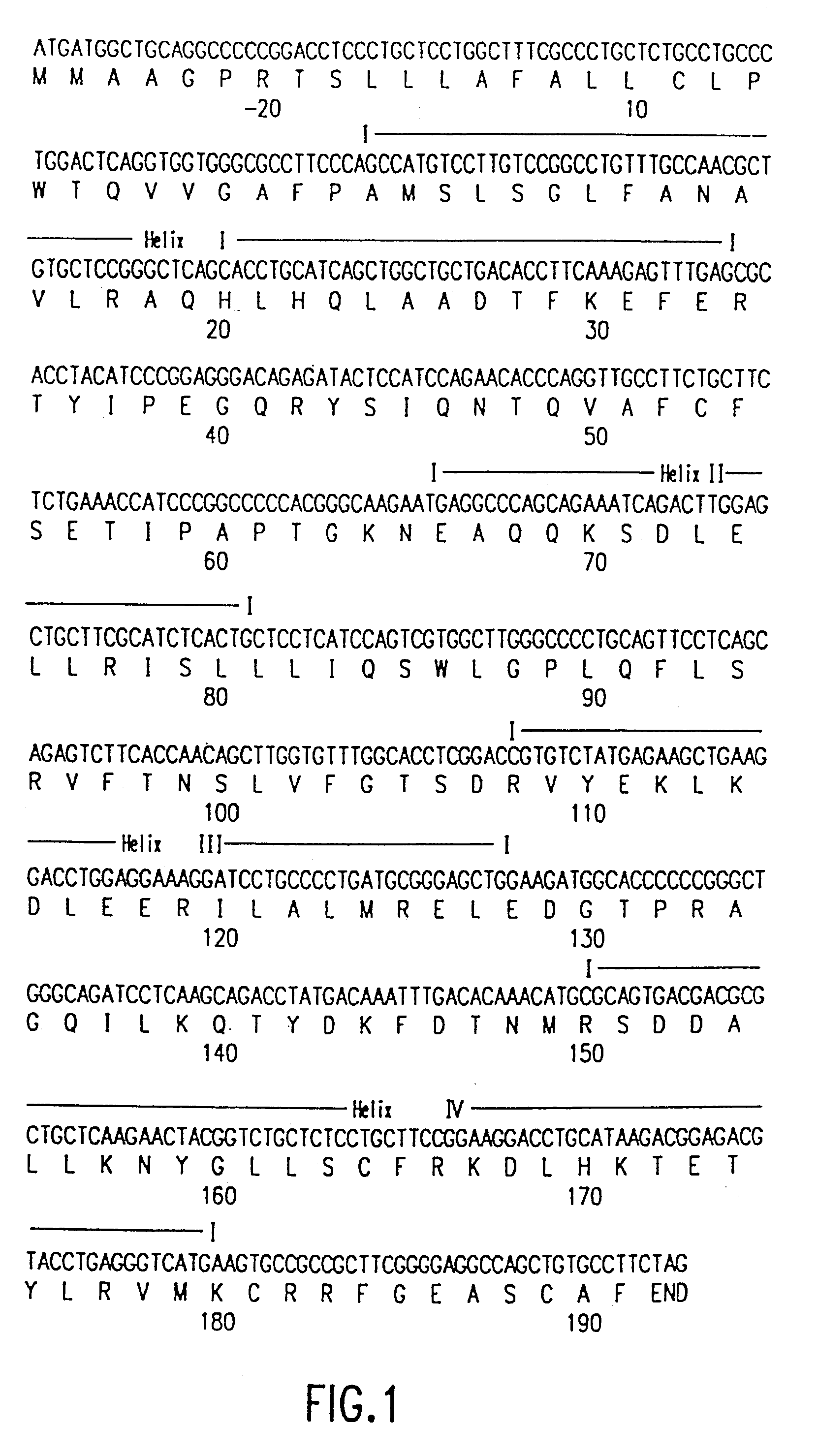
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Ghrelin binding nucleic acids
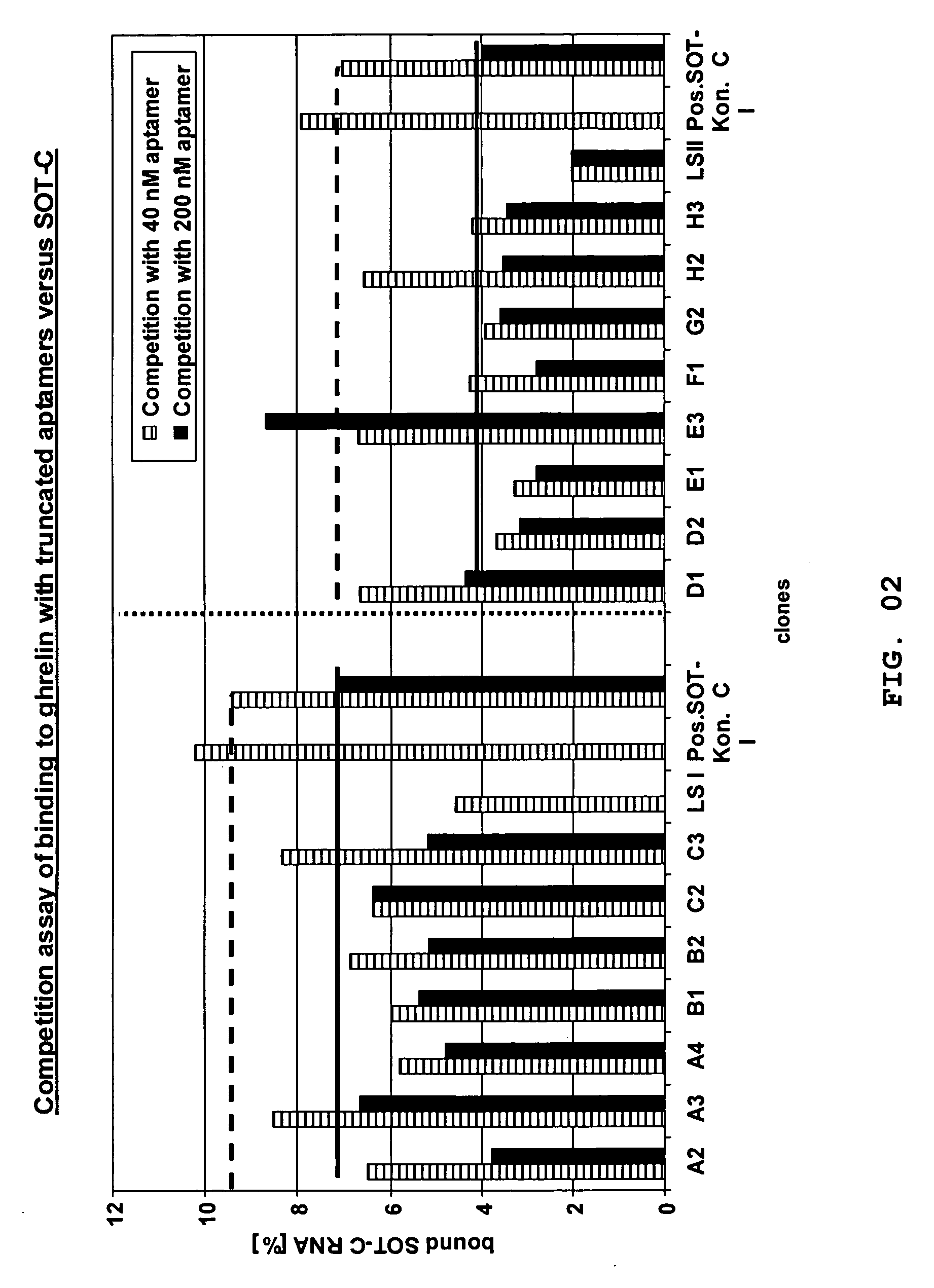
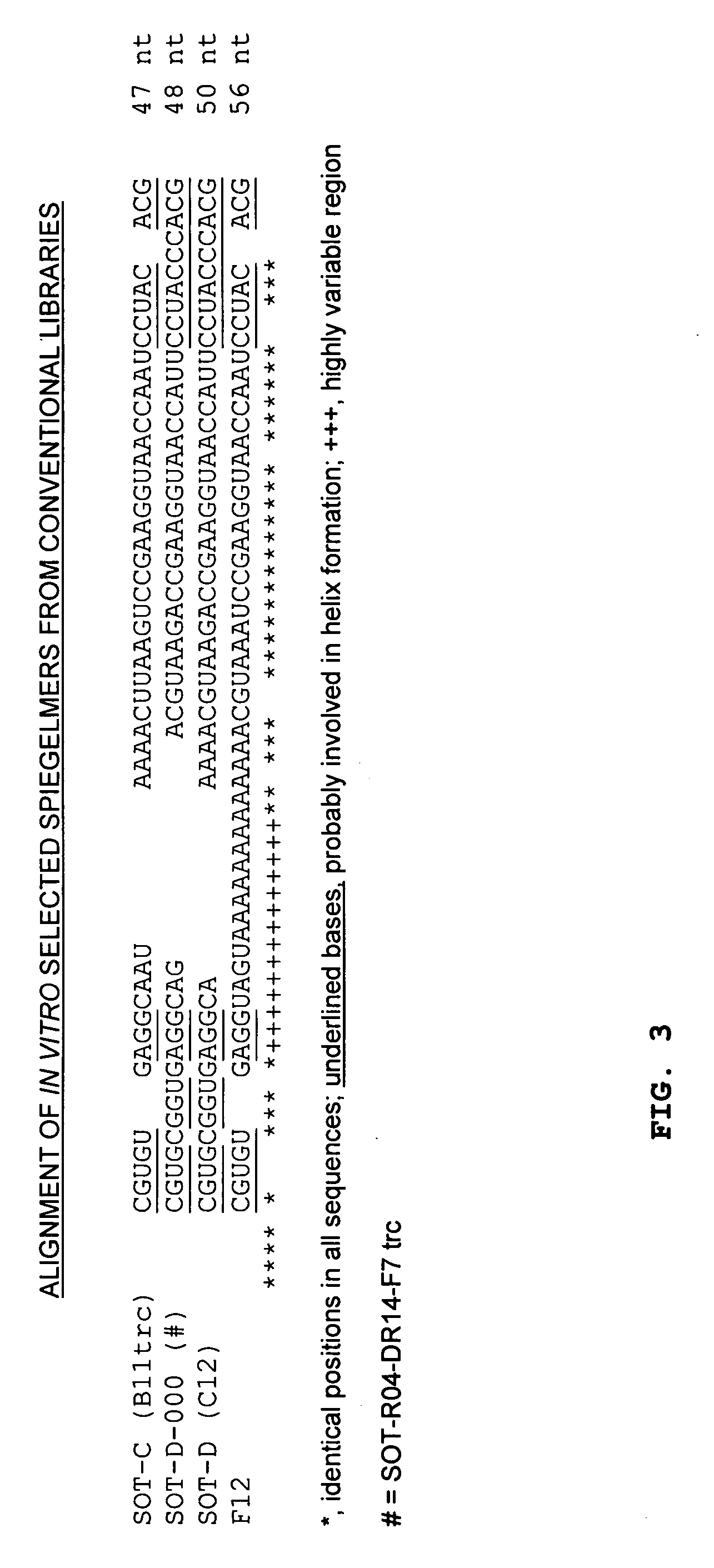
The present invention is related to a nucleic acid, preferably binding to ghrelin, whereby the nucleic acid comprises a first stretch Box A, and a second stretch Box B, whereby the first stretch Box A comprises about 25 consecutive nucleotides, the second stretch Box B comprises about six to eight consecutive nucleotides, whereby a 3′-terminal stretch of nucleotides of the first stretch Box A hybridises with the second stretch Box B, whereby upon hybridisation a first double-stranded structure is formed, whereby such first double-stranded structure comprises a bulge.
Owner:NOXXON PHARM AG
Pharmaceutical composition comprising an immunoglobulin fc region as a carrier
ActiveUS20060275254A1Prolong the action timePeptide/protein ingredientsAntibody mimetics/scaffoldsProtein targetHalf-life
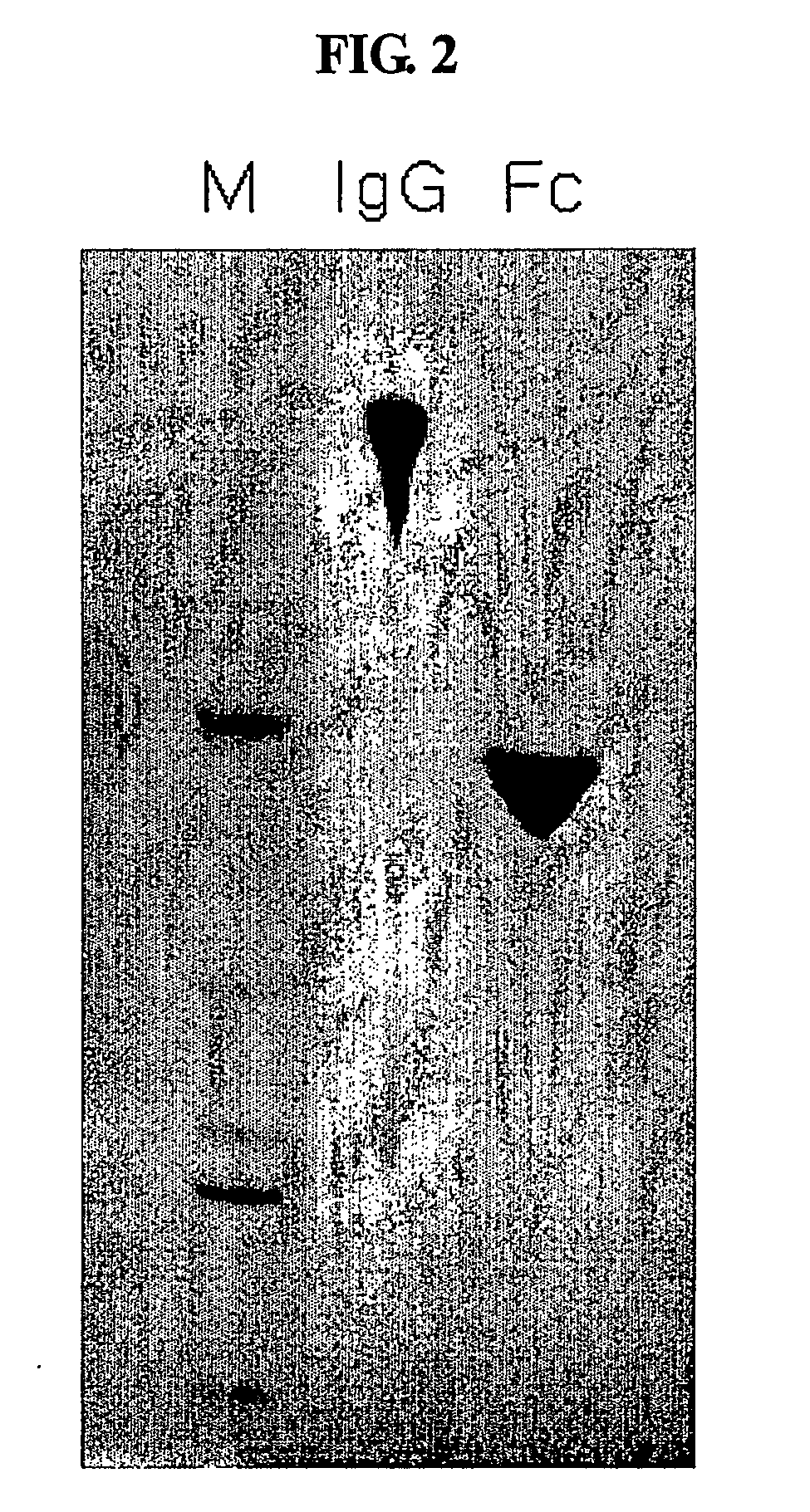
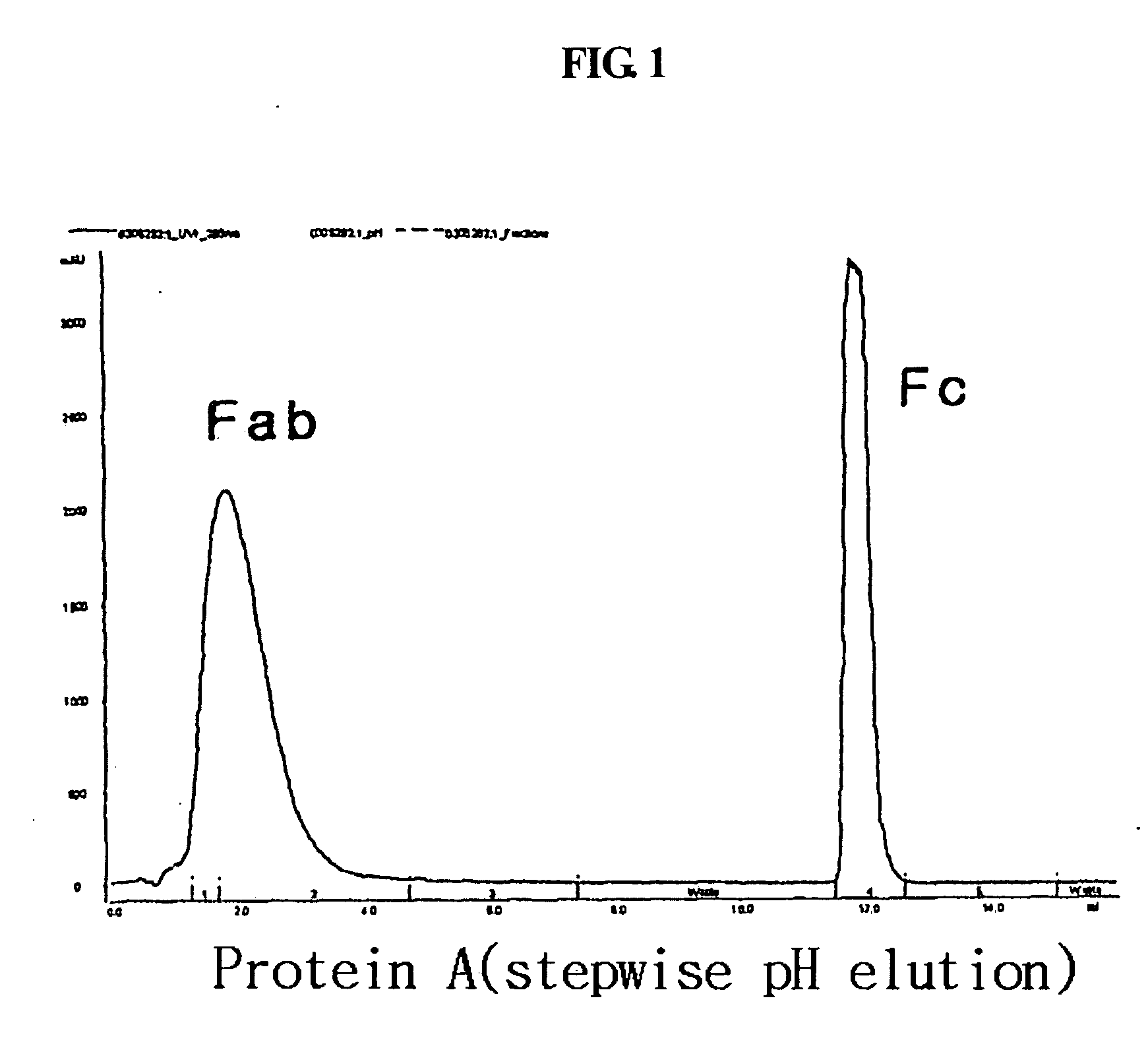
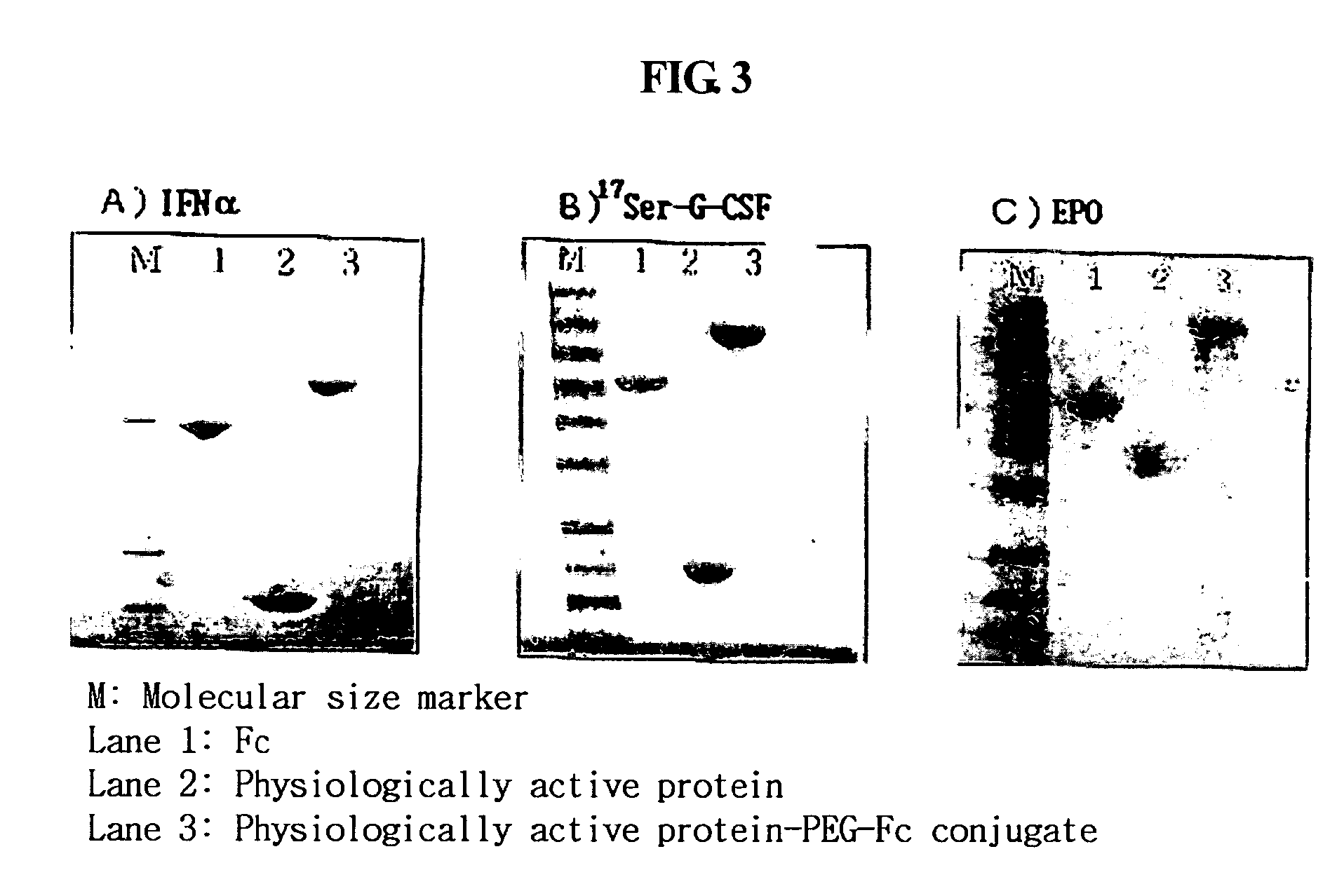
Disclosed is a novel use of an immunoglobulin Fe fragment, and more particularly, a pharmaceutical composition comprising an immunoglobulin Fe fragment as a carrier. The pharmaceutical composition comprising an immunoglobulin Fe fragment as a carrier remarkably extends the serum half-life of a drug while maintaining the in vivo activity of the drug at relatively high levels. Also, when the drug is a polypeptide drug, the pharmaceutical composition has less risk of inducing immune responses compared to a fusion protein of the immunoglobulin Fe fragment and a target protein, and is thus useful for developing long-acting formulations of various polypeptide drugs.
Owner:HANMI SCI CO LTD
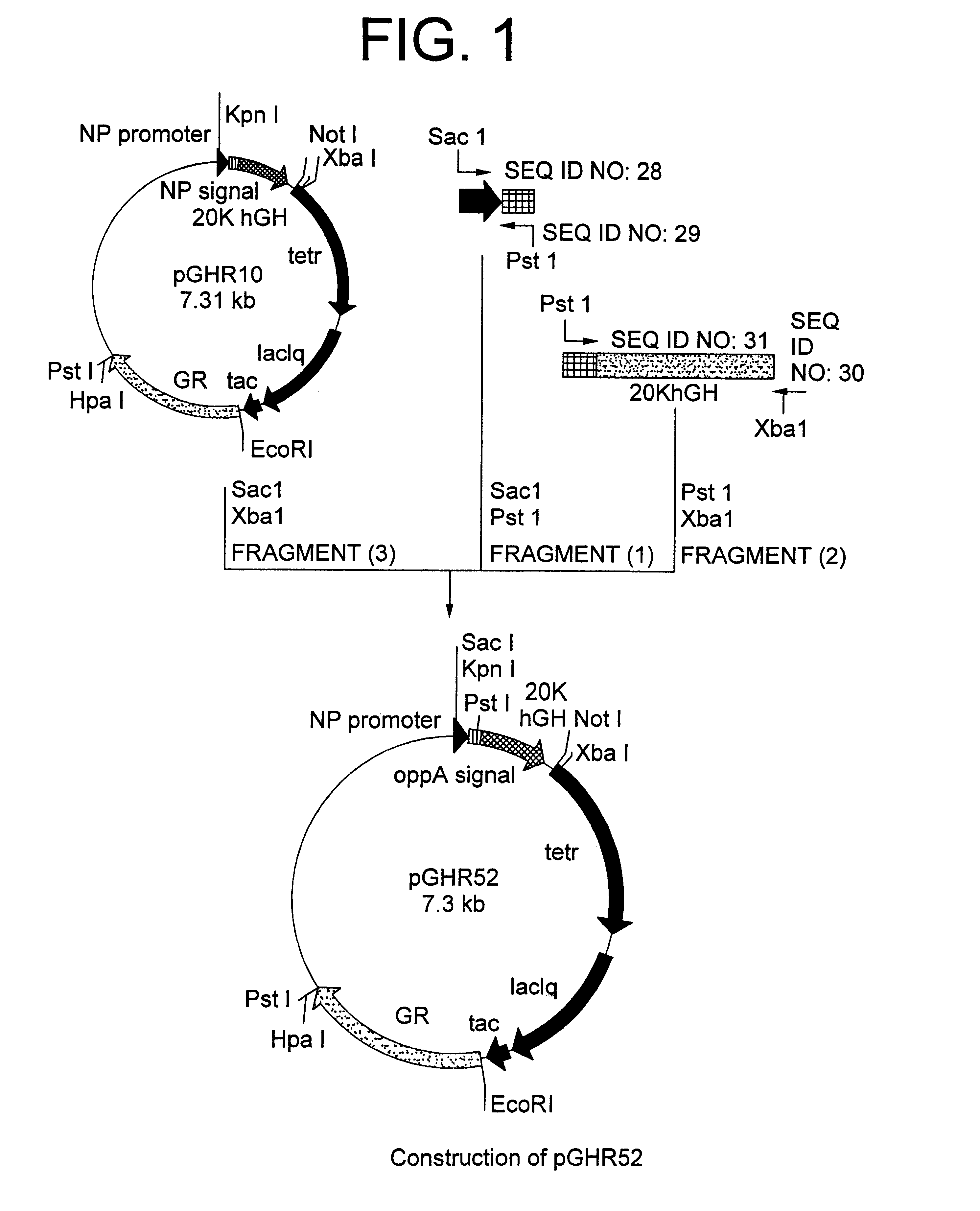
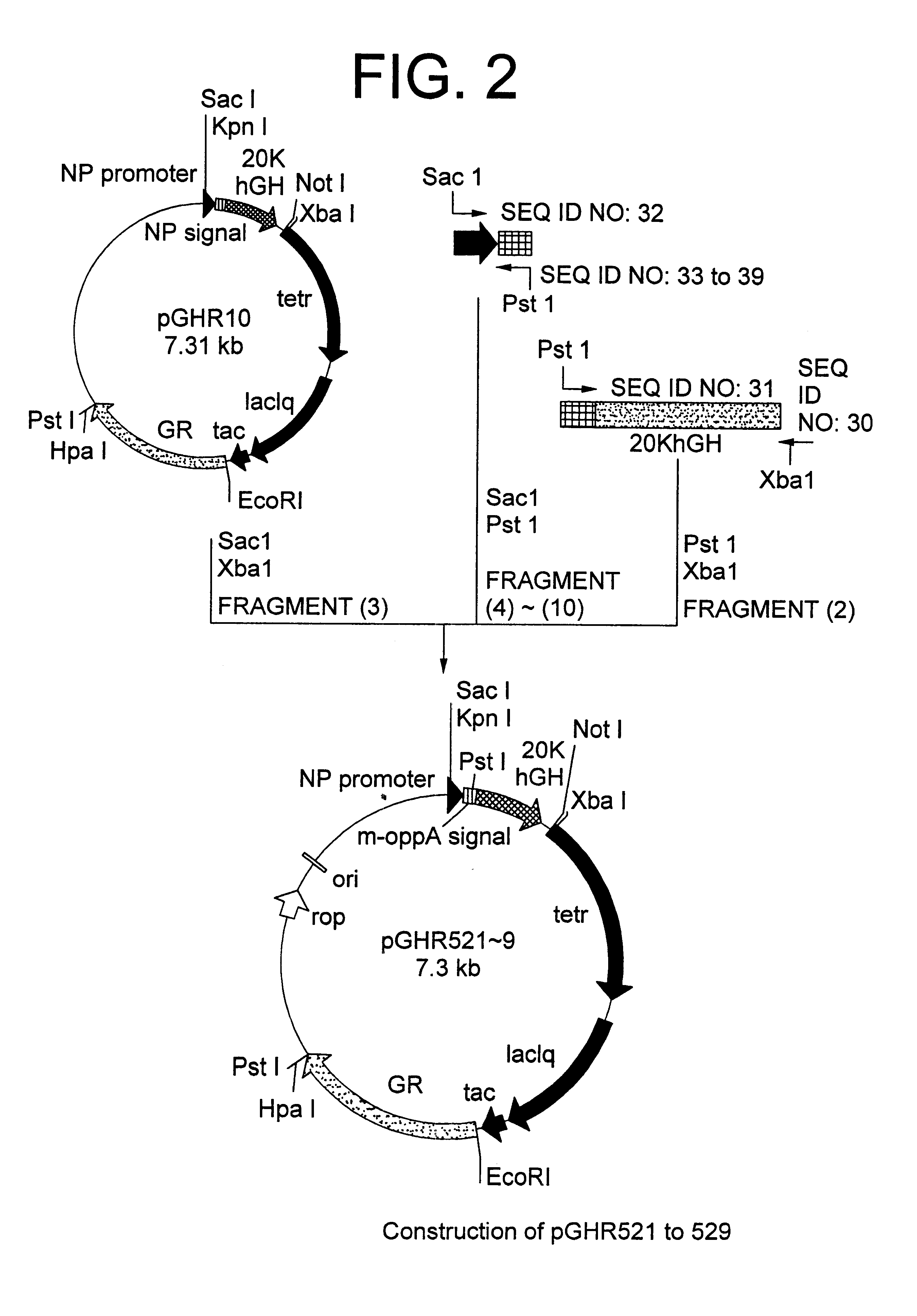
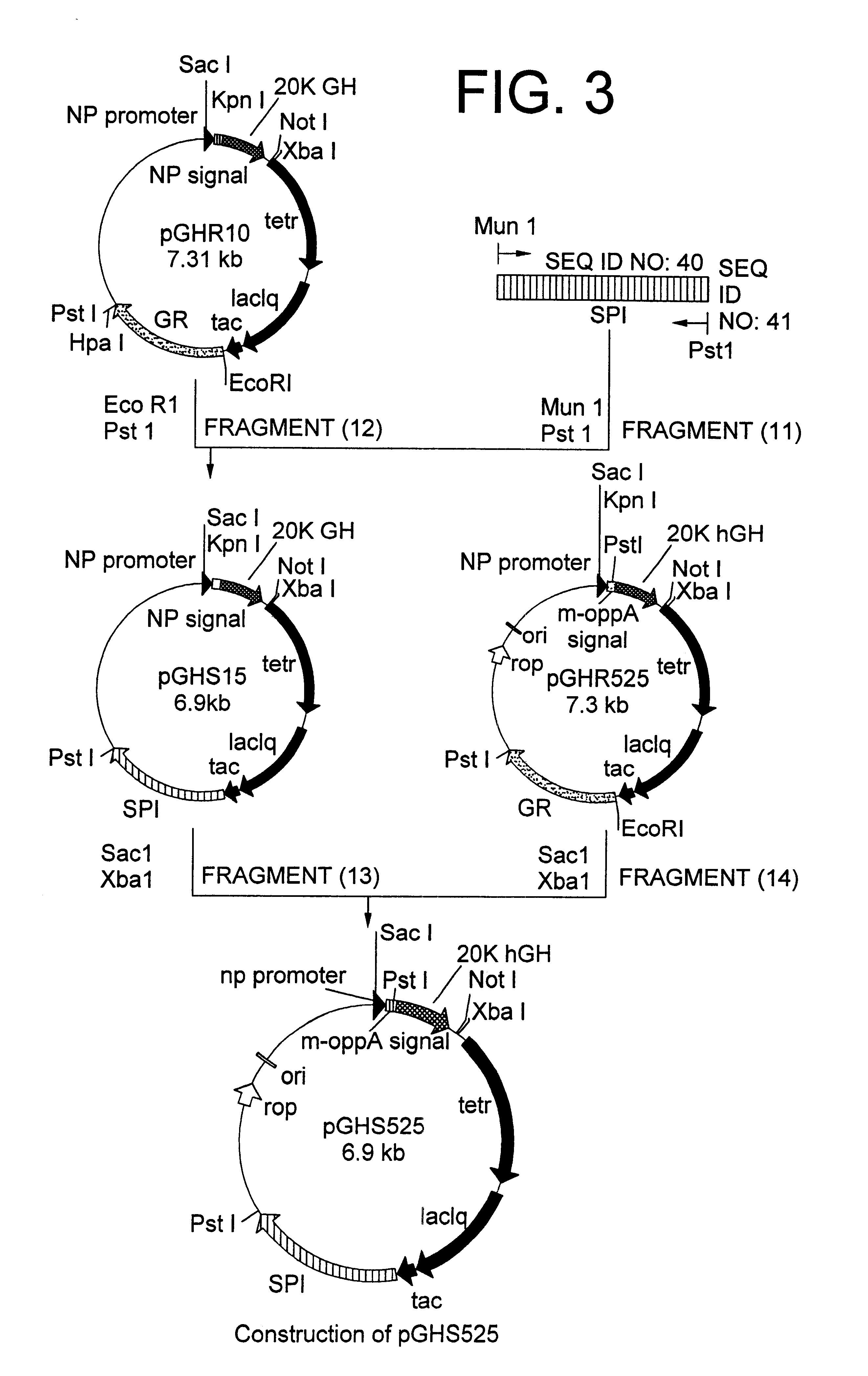
Method for secretory production of human growth hormone
InactiveUS6436674B1Increase productionDecreased tendency for lysisBacteriaHydrolasesHuman growth hormoneA-DNA
A DNA encoding 20K hGH is connected directly to a gene encoding Escherichia coli OppA protein secretion signal, or a modified form thereof, and a DNA encoding signal peptidase 1 to construct a recombinant plasmid, E. coli is transformed by the plasmid and cells of the resulting E. coli transformant strain are cultured for secretory production of the 20K hGH in the E. coli periplasm. This method enables efficient secretory production of 20K hGH and easy isolation and purification of 20K hGH from the periplasm fraction because the level of impure proteins in the E. coli periplasm is low.
Owner:MITSUI CHEM INC
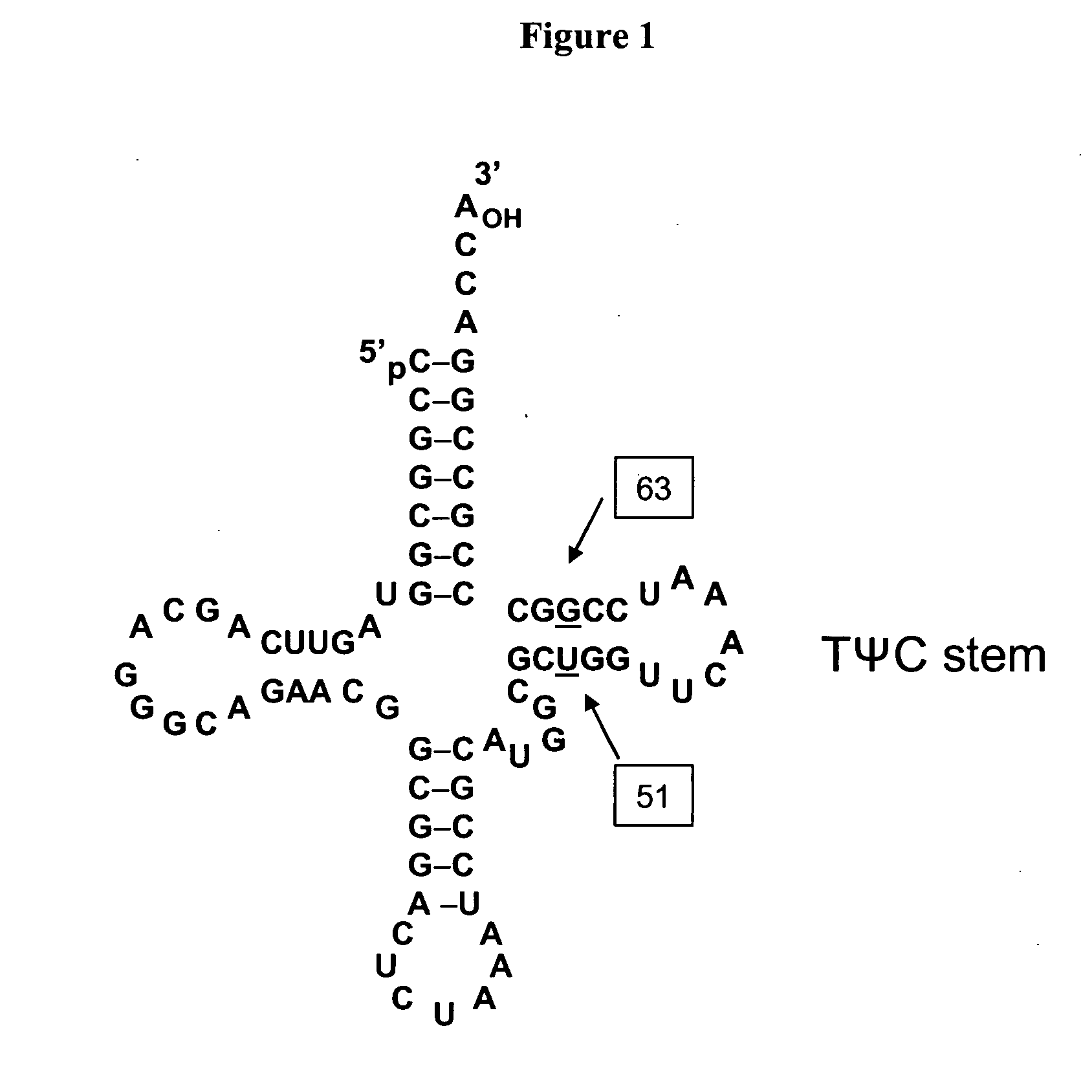
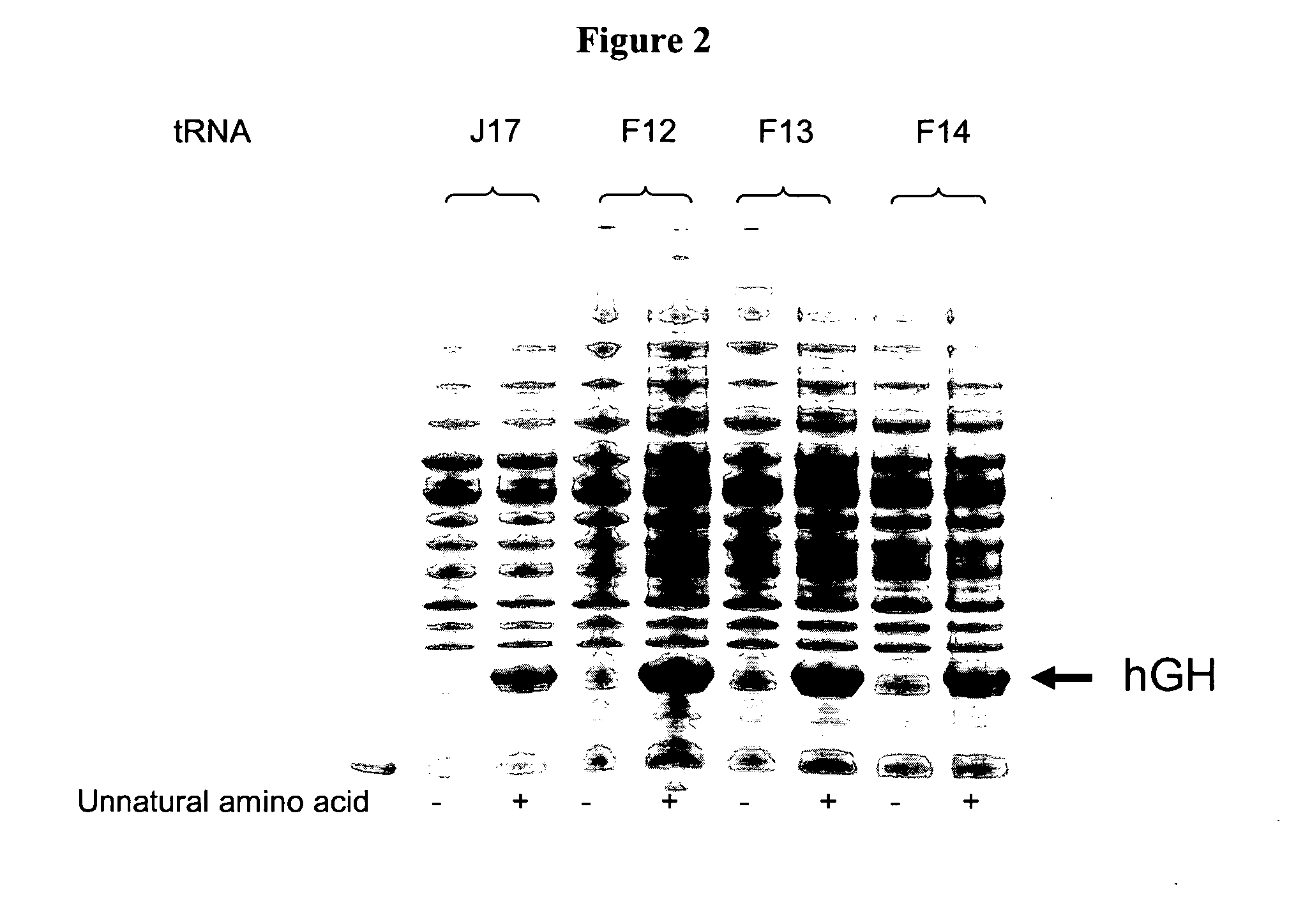
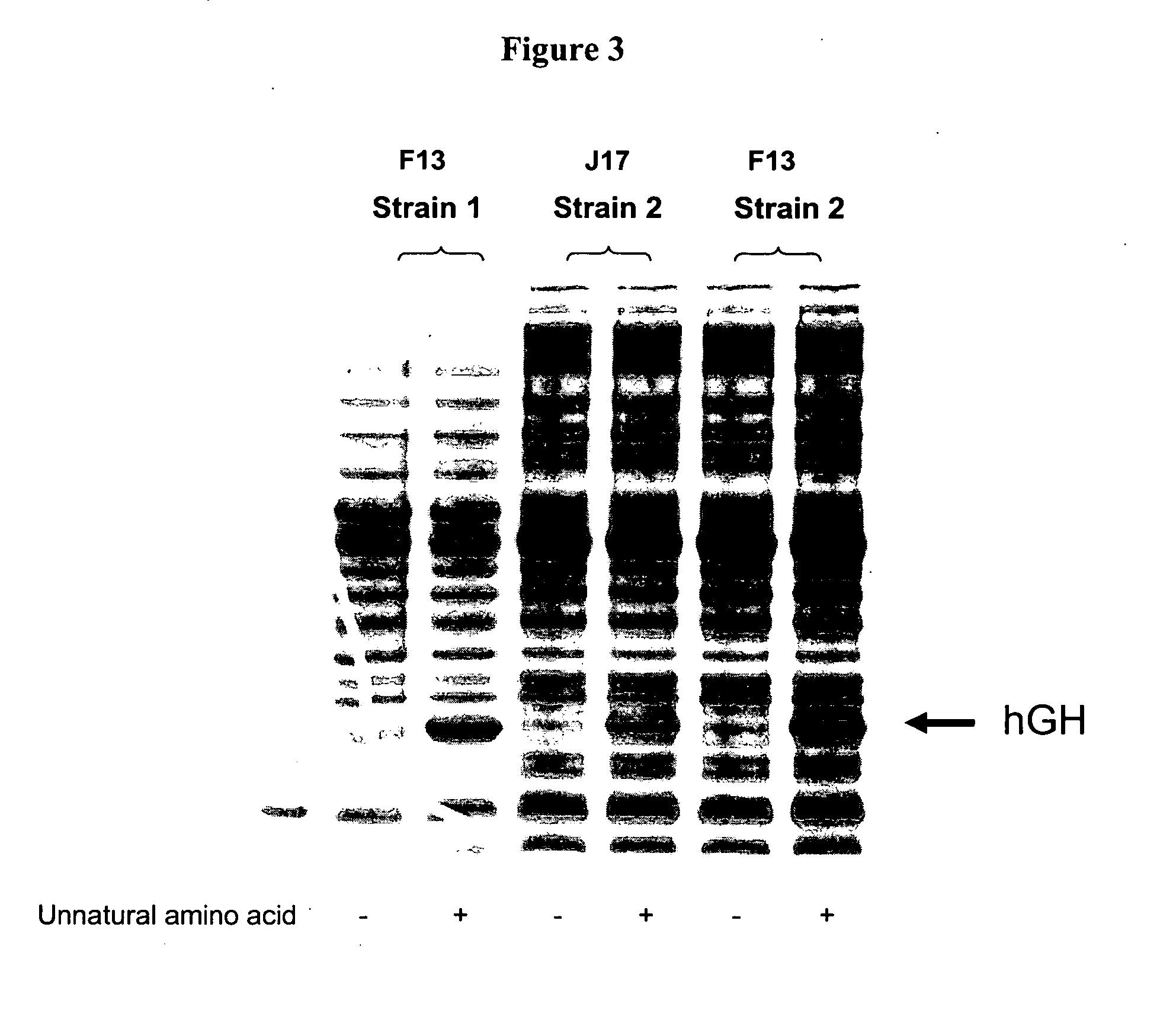
Compositions of tRNA and uses thereof
Compositions and methods of producing components of protein biosynthetic machinery that include orthogonal tRNA's, orthogonal aminoacyl-tRNA synthetases, and orthogonal pairs of tRNA's / synthetases are provided. Methods for identifying these orthogonal pairs are also provided along with methods of producing proteins using these orthogonal pairs.
Owner:AMBRX
Recombinant expression of proteins from secretory cell lines
InactiveUS6087129AIncrease productionIncrease secretionPeptide/protein ingredientsGenetic material ingredientsHeterologousHigh level expression
The present invention a provides methods for production of heterologous polypeptides using a variety recombinantly engineered secretory cell lines. The common feature of these cell lines is the absence of expression of at least one endogenous polypeptide. The host cell machinery normally used to produce the endogenous polypeptide is then usurped for the purpose of making the heterologous polypeptide. Also described are methods engineering cells for high level expression, methods of large scale protein production, and methods for treatment of disease in vivo using viral delivery systems and recombinant cell lines.
Owner:BETAGENE +1
Lipid-mediated polynucleotide administration to deliver a biologically active peptide and to induce a cellular immune response
InactiveUS7250404B2Short durationFast expressionHydrolasesMicroencapsulation basedLipid formationNucleotide
A method for delivering an isolated polynucleotide to the interior of a cell in a vertebrate, comprising the interstitial introduction of an isolated polynucleotide into a tissue of the vertebrate where the polynucleotide is taken up by the cells of the tissue and exerts a therapeutic effect on the vertebrate. The method can be used to deliver a therapeutic polypeptide to the cells of the vertebrate, to provide an immune response upon in vivo translation of the polynucleotide, to deliver antisense polynucleotides, to deliver receptors to the cells of the vertebrate, or to provide transitory gene therapy.
Owner:VICAL INC +1
Long-acting polypeptides and methods of producing and administering same
ActiveUS20130184207A1Reduce dosing frequencyIncrease the areaPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:OPKO BIOLOGICS
Protein complex using an immunoglobulin fragment and method for the preparation thereof
ActiveUS20060269553A1Improve stabilityExtended durationAntibody mimetics/scaffoldsImmunological disordersHalf-lifeImmunoglobulin Fc Fragments
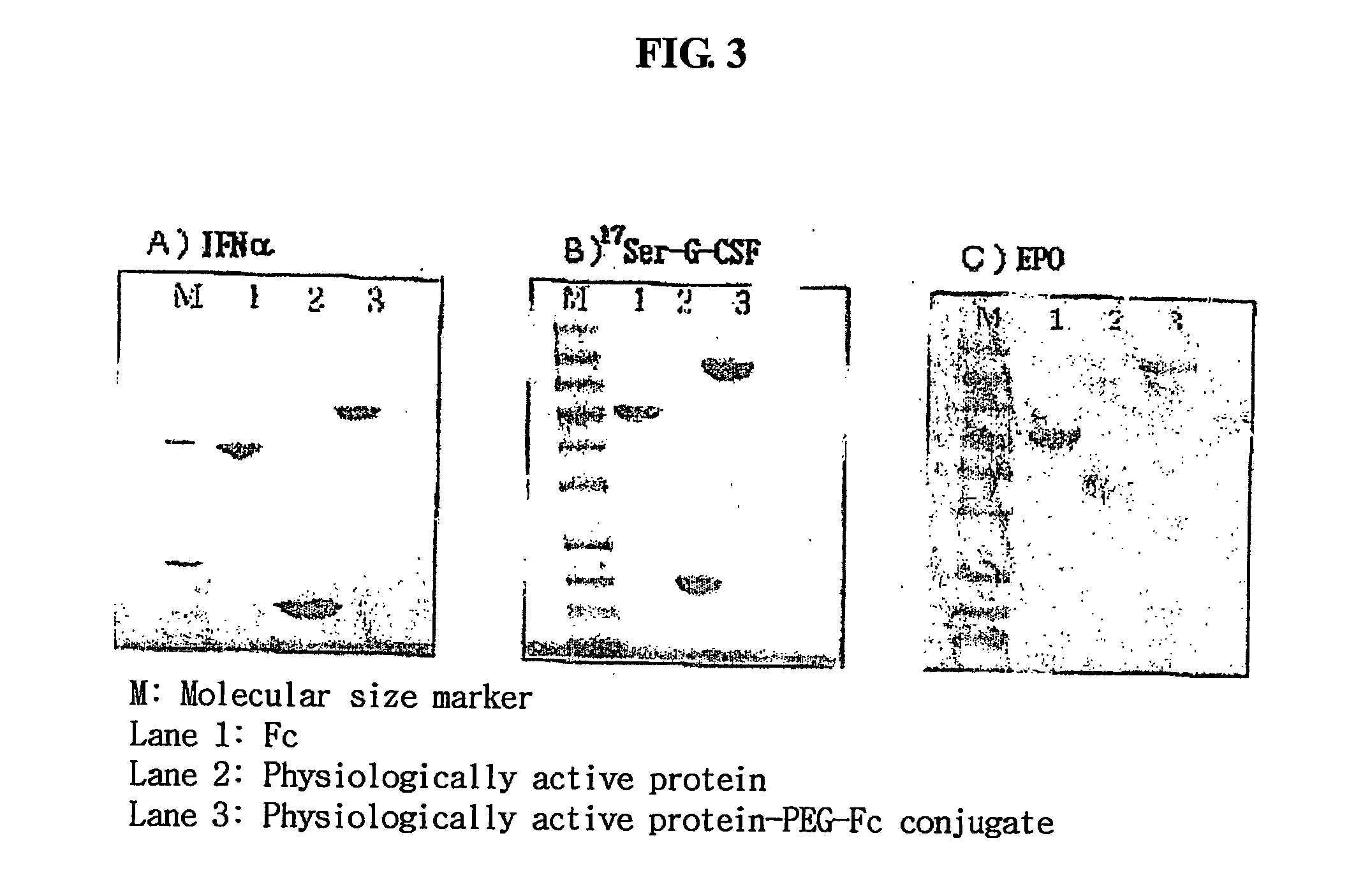
Disclosed are a protein conjugate with improved in vivo duration and stability and the use thereof. The protein conjugate includes a physiologically active polypeptide, a non-peptide polymer and an immunoglobulin Fc fragment. Since the three components are covalently linked, the protein conjugate has extended in vivo duration and enhanced stability for the physiologically active polypeptide. The protein conjugate maintains the in vivo activity at relatively high levels and remarkably increases the serum half-life for the physiologically active polypeptide, with less risk of inducing undesirable immune responses. Thus, the protein conjugate is useful for developing long-acting formulations of various polypeptide drugs.
Owner:HANMI SCI CO LTD
Long-acting polypeptides and methods of producing same
ActiveUS20070190611A1Reduce morbidityProlong lifePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides encoding same comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a peptide-of-interest are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Methods for treating acromegaly and giantism with growth hormone antagonists
The present invention relates to antagonists of vertebrate growth hormones obtained by mutation of the third alpha helix of such proteins (especially bovine or human GHs). These mutants-have growth-inhibitory or other GH-antagonizing effects. These novel hormones may be administered exogenously to animals, or transgenic animals may be made that express the antagonist. Animals have been made which exhibited a reduced growth phenotype. The invention also describes methods of treating acromegaly, gigantism, cancer, diabetes, vascular eye diseases (diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration, retinopathy of sickle-cell anemia, etc.) as well as nephropathy and other diseases, by administering an effective amount of a growth hormone antagonist. The invention also provides pharmaceutical formulations comprising one or more growth hormone antagonists.
Owner:OHIO UNIV EDISON ANIMAL BIOTECH INST
Methods for expression and purification of recombinant human growth hormone
The present invention relates generally to the production, purification, and isolation of human growth hormone (hGH). More particularly, the invention relates to the production, purification, and isolation of substantially purified hGH from recombinant host cells or culture medium including, for example, yeast, insect, mammalian and bacterial host cells. The process of the present invention is also useful for purification of hGH linked to polymers or other molecules.
Owner:AMBRX
Non-Natural Amino Acid Polypeptides Having Modified Immunogenicity
InactiveUS20090093405A1Low immunogenicityImproving immunogenicityPeptide/protein ingredientsTransferasesVaccine ImmunogenicityAmino acid
Non-naturally encoded amino acid polypeptides with modulated immunogenicity and uses thereof are provided.
Owner:AMBRX
Stabilization of aqueous compositions of proteins with displacement buffers
InactiveUS20100028372A1Improve stabilityImproves pH stabilityFactor VIIPeptide/protein ingredientsAnalytical chemistryProtein stability
An aqueous composition having increased protein stability is obtained by: a. determining a pH at which the protein has stability at the desired temperature; b. adding to the composition at least one displacement buffer wherein the displacement buffer has a pKa that is at least 1 unit greater or less than the pH of step (a); and c. adjusting the pH of the composition to the pH of step (a); wherein the aqueous composition does not comprise a conventional buffer at a concentration greater than about 2 mM and wherein the conventional buffer has a pKa that is within 1 unit of the pH of step (a).
Owner:ARECOR LTD
Long-acting polypeptides and methods of producing same
ActiveUS20090312254A1Reduce dosing frequencyImprove compliancePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides encoding same comprising one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to an amino terminus of a cytokine and two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a cytokine are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:MODIGENE LLC +1
System for expression of genes in plants
ActiveUS20050026291A1Facilitates systemic spreadReducing and eliminating riskSsRNA viruses positive-senseOther foreign material introduction processesBiotechnologyPlant virus
The present invention provides trans-complementation systems for expressing gene products in plants. In general, the invention provides systems including a carrier vector and a producer vector, both based on plant viruses. The producer vector is defective for at least one function needed for successful systemic infection of a plant, e.g., replication, cell-to-cell movement, or long distance movement. The carrier vector supplies the missing function in trans. Certain producer vectors lack a functional coat protein coding sequence, in which case the corresponding producer vector supplies coat protein in trans. The invention also provides novel plant viral vectors and methods of use, e.g., to produce polypeptides or active RNAs in plants.
Owner:IBIO
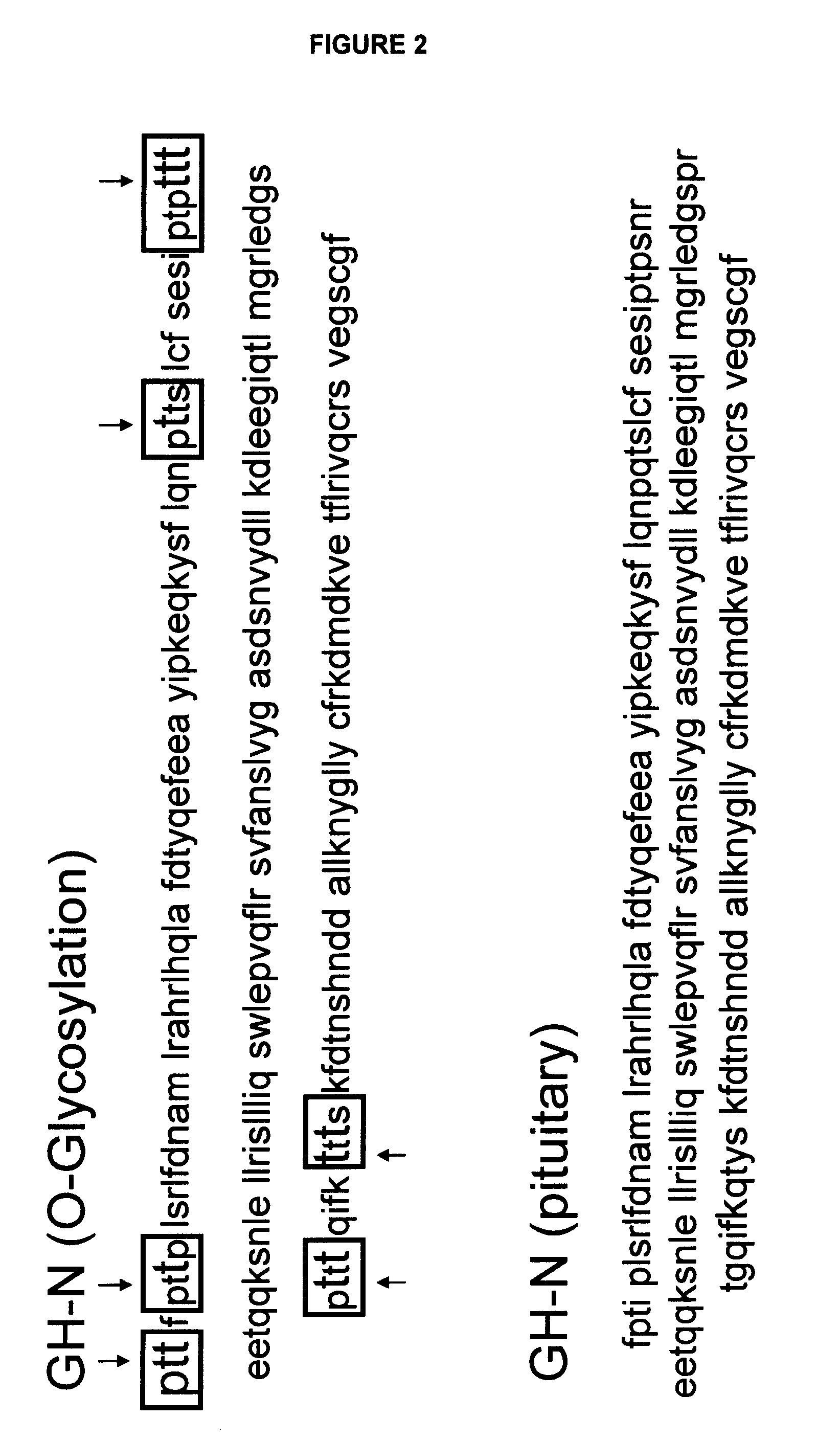
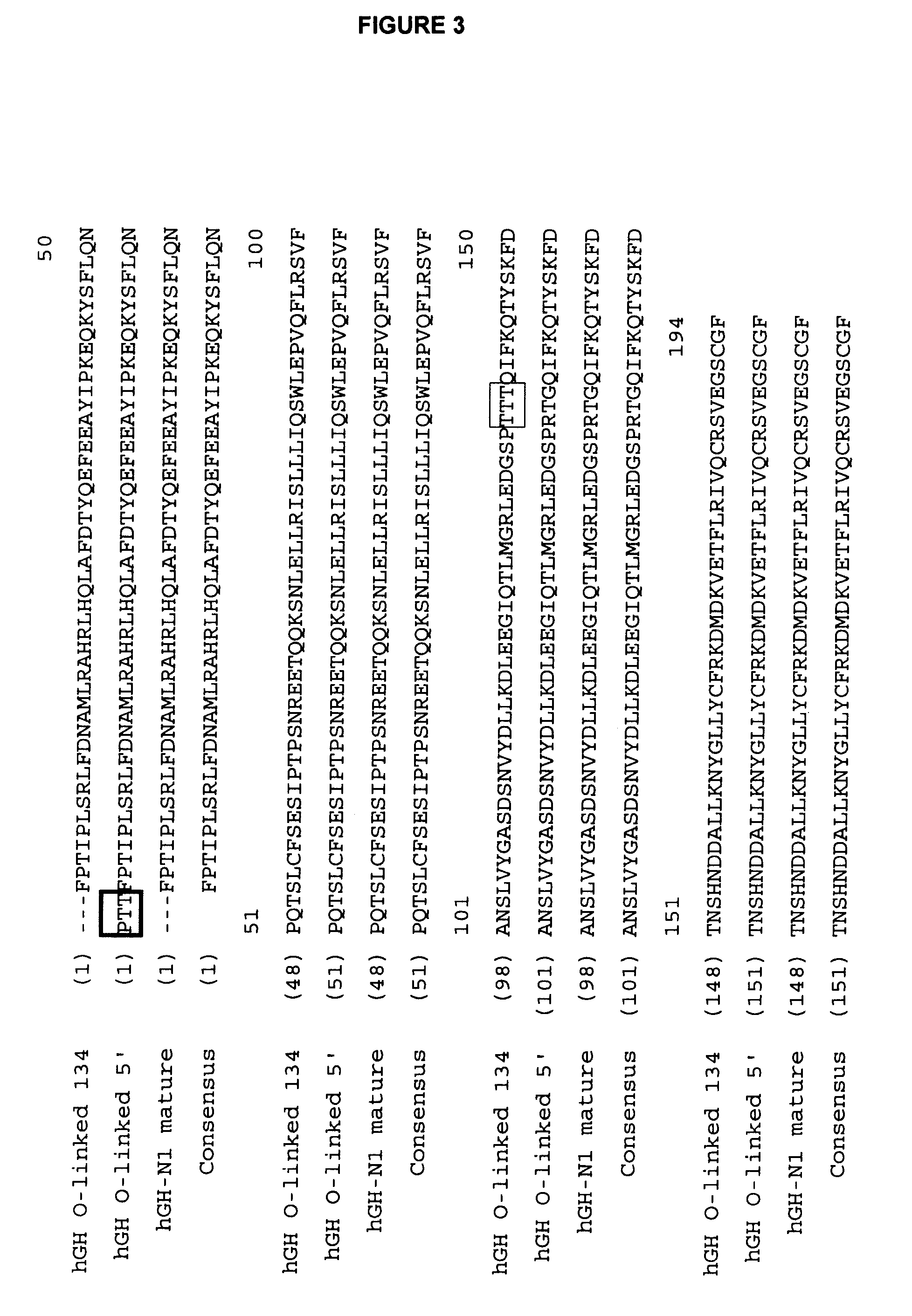
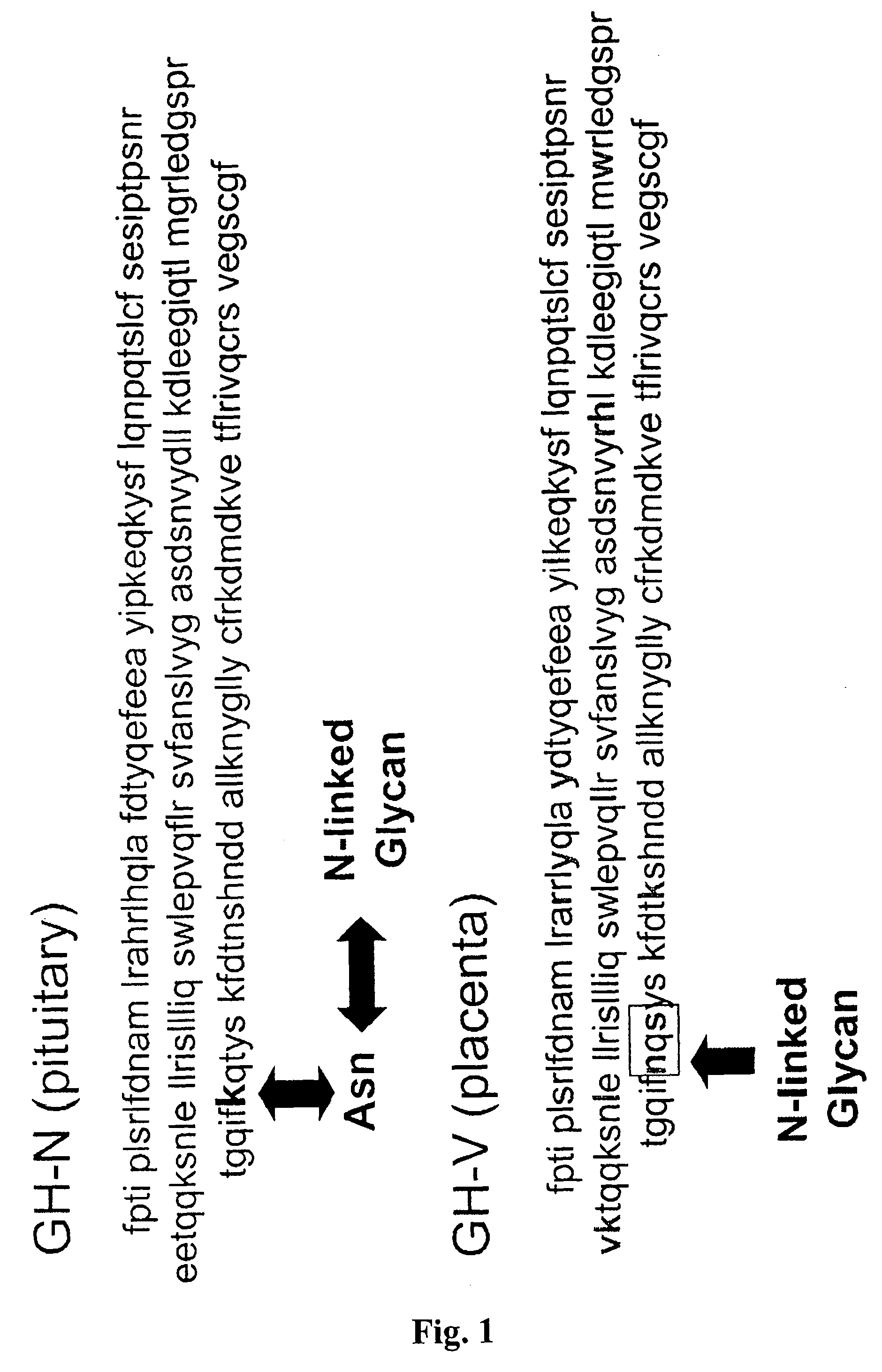
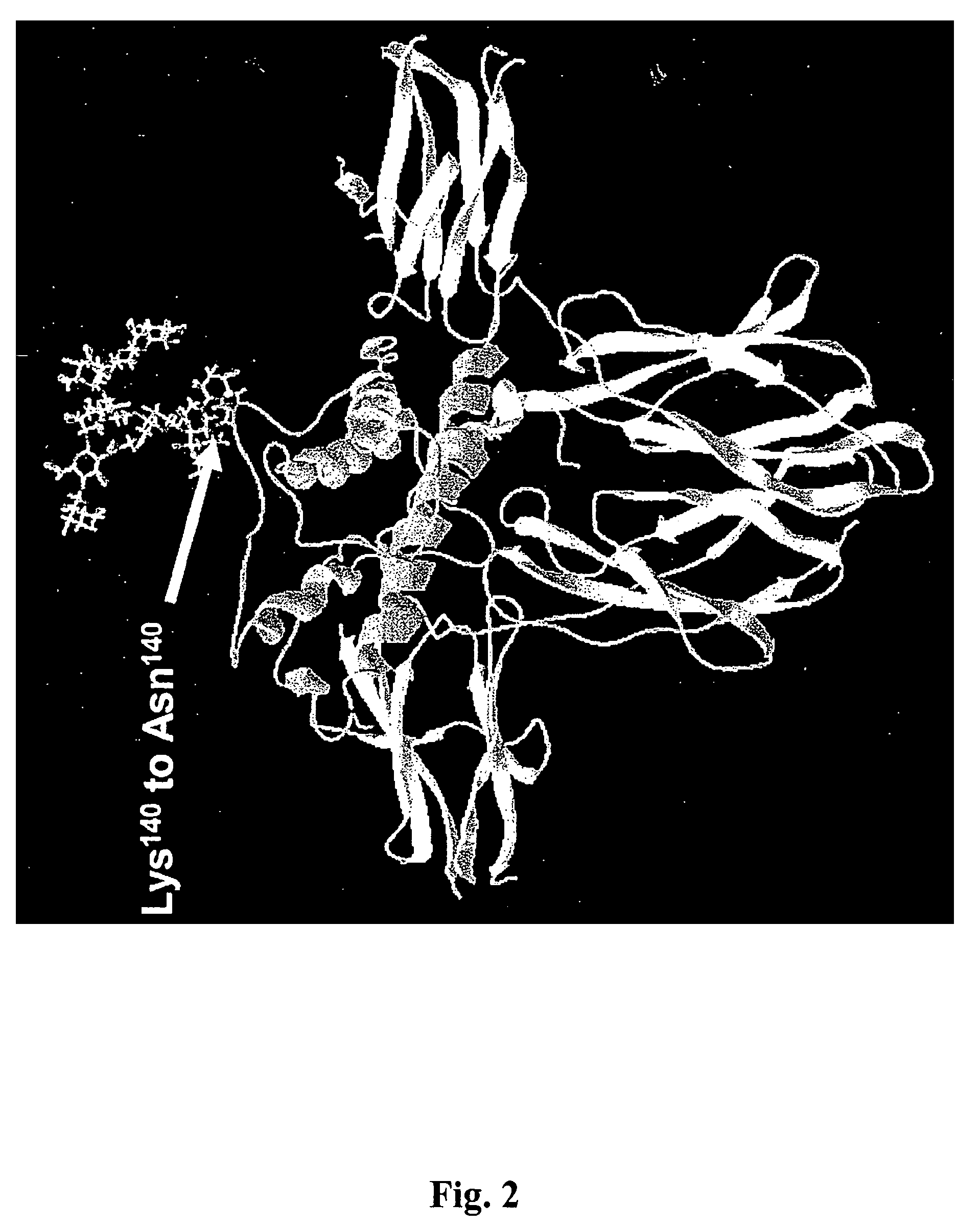
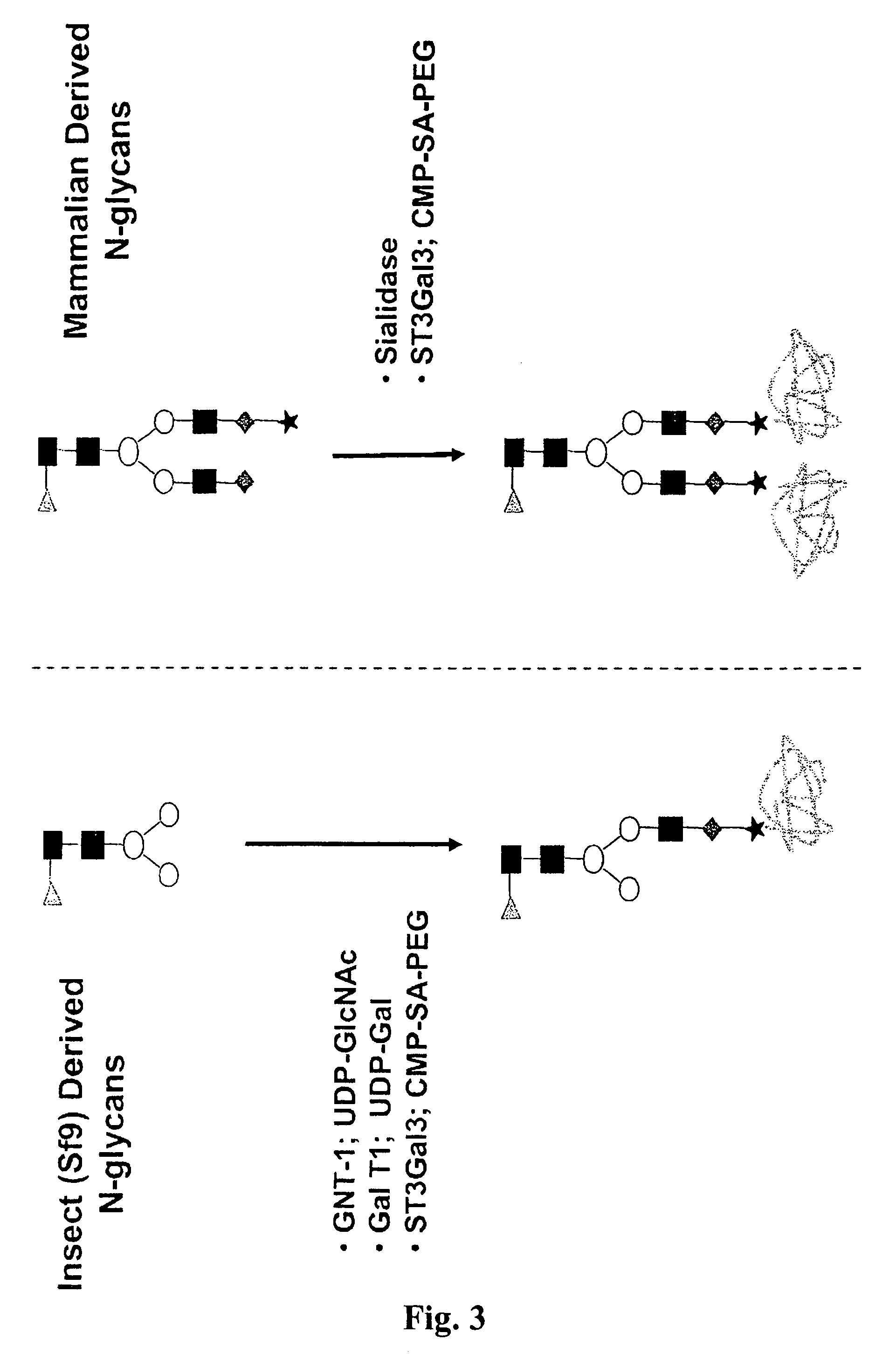
Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants
The present invention relates to protease resistant mutants of human growth hormone, which contain newly introduced proteolysis resistant mutations and N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS +1
Methods for protein refolding
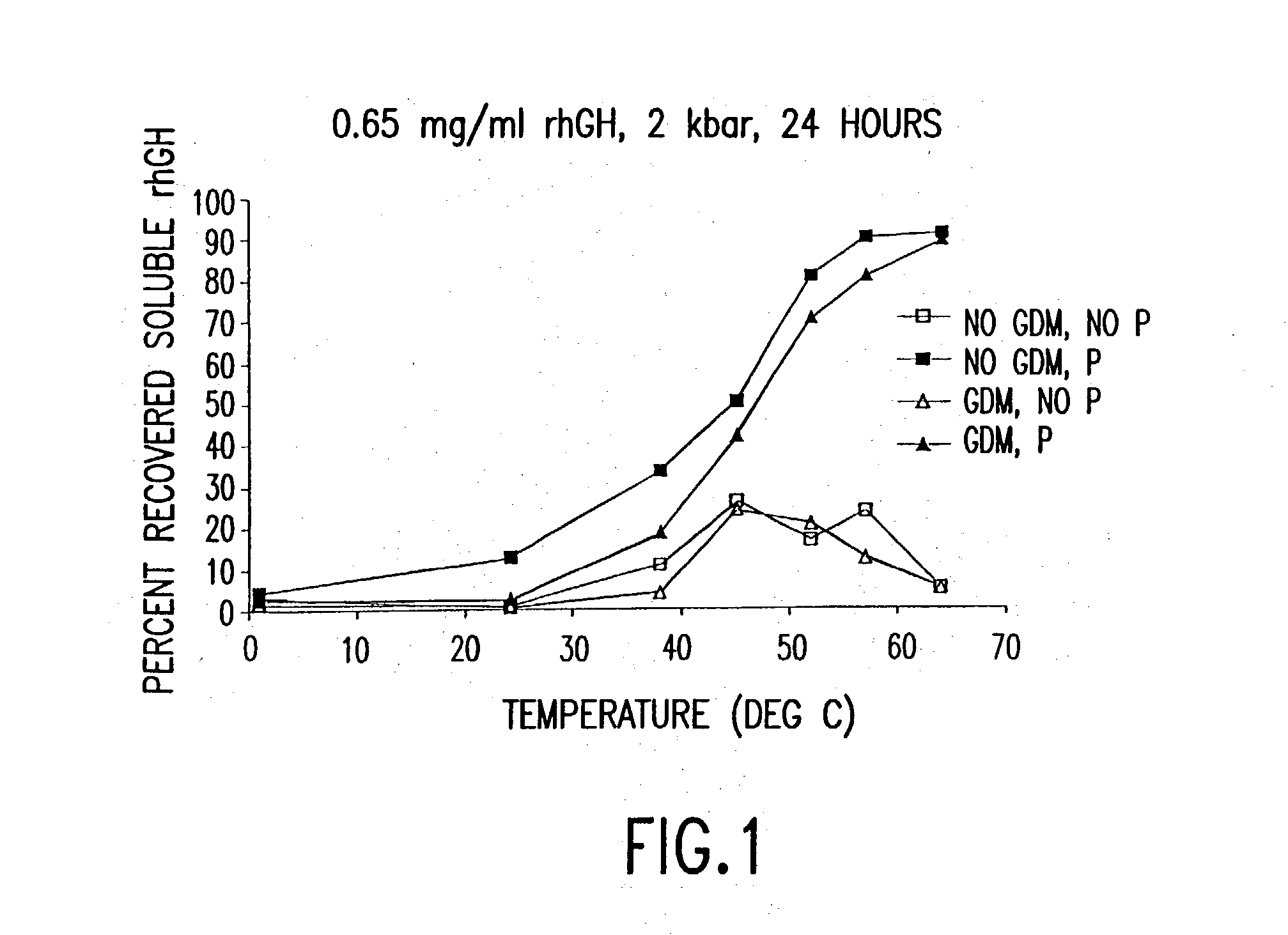
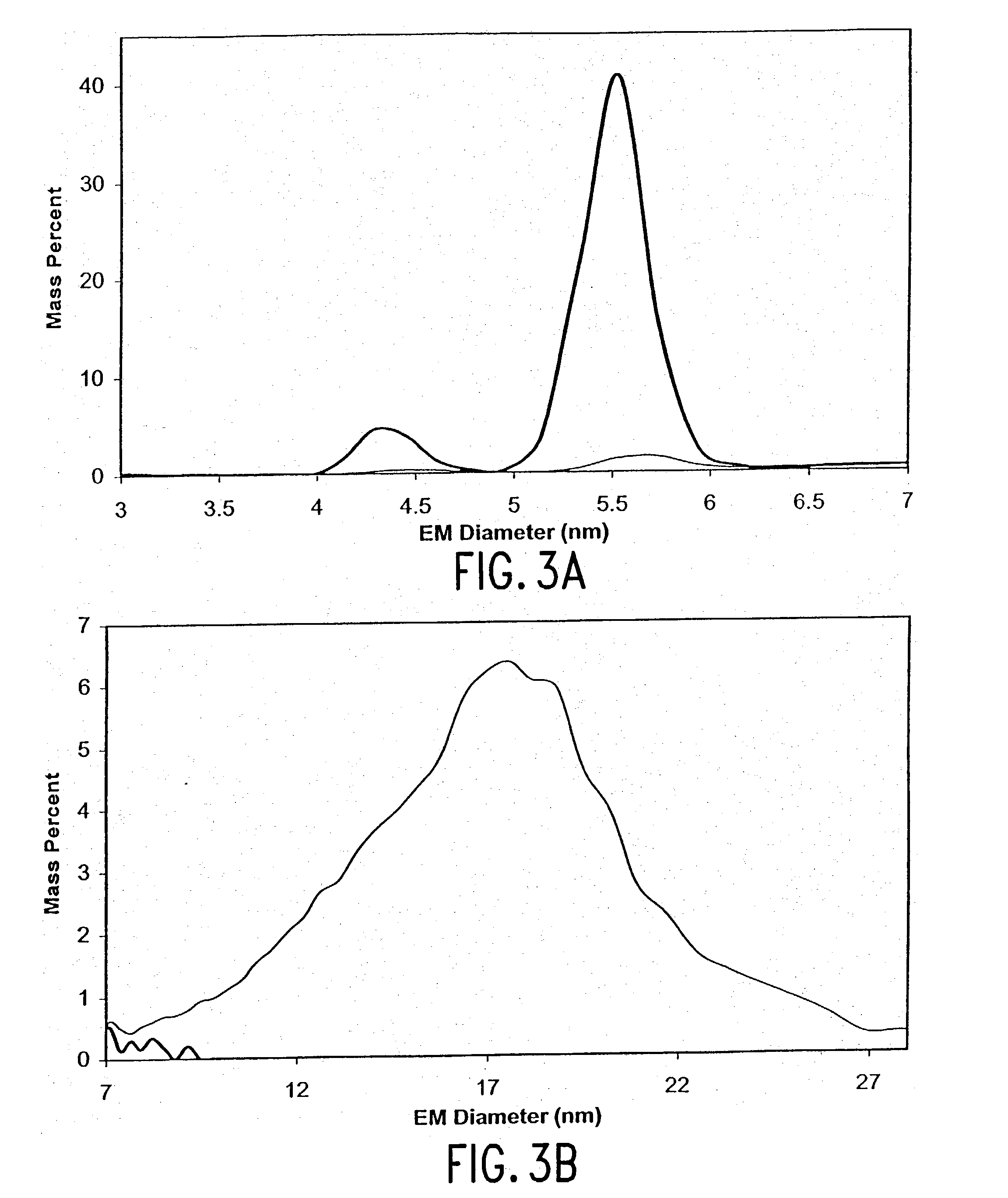
InactiveUS20040038333A1Decrease product valueDangerous anaphylactic reactionInactivation/attenuationDepsipeptidesDissolutionImproved method
The present invention discloses improved methods of disaggregating protein aggregates, and refolding denatured proteins, using high pressure. In particular, the present invention provides for the use of agitation, high temperature, "stepped" depressurization, dialysis and dilution under pressure to increase the speed and extent of aggregate dissolution and protein refolding.
Owner:BARFOLD INC
Recombinant expression of proteins from secretory cell lines
InactiveUS6194176B1Increase productionIncrease secretionPeptide/protein ingredientsGenetic material ingredientsHeterologousHigh level expression
Owner:BETAGENE +1
Expression of mammalian proteins in Pseudomonas fluorescens
The invention is a process for improved production of a recombinant mammalian protein by expression in a Pseudomonad, particularly in a Pseudomonas fluorescens organism. The process improves production of mammalian proteins, particularly human or human-derived proteins, over known expression systems such as E. coli in comparable circumstances Processes for improved production of isolated mammalian, particularly human, proteins are provided.
Owner:PELICAN TECH HLDG INC
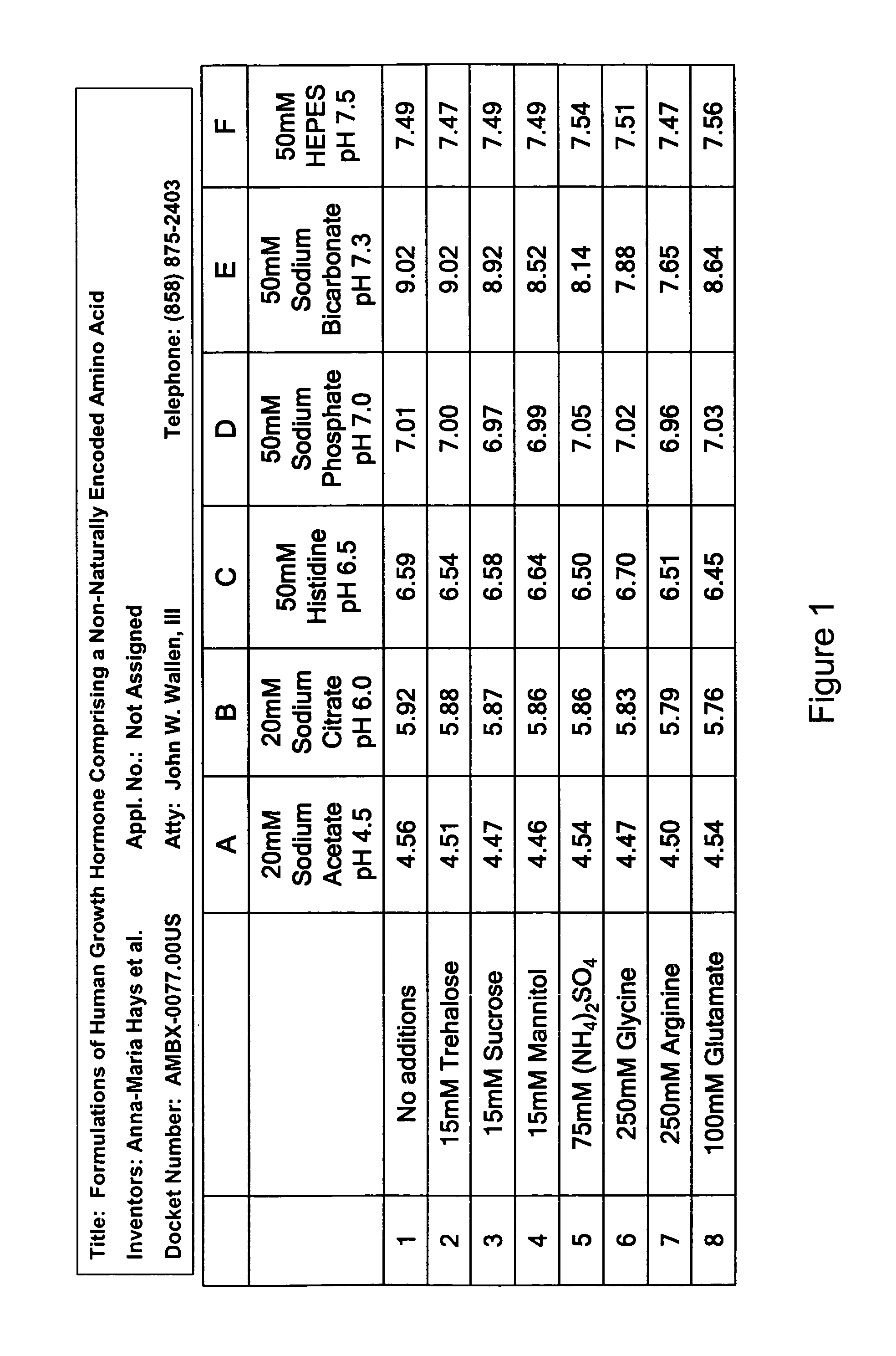
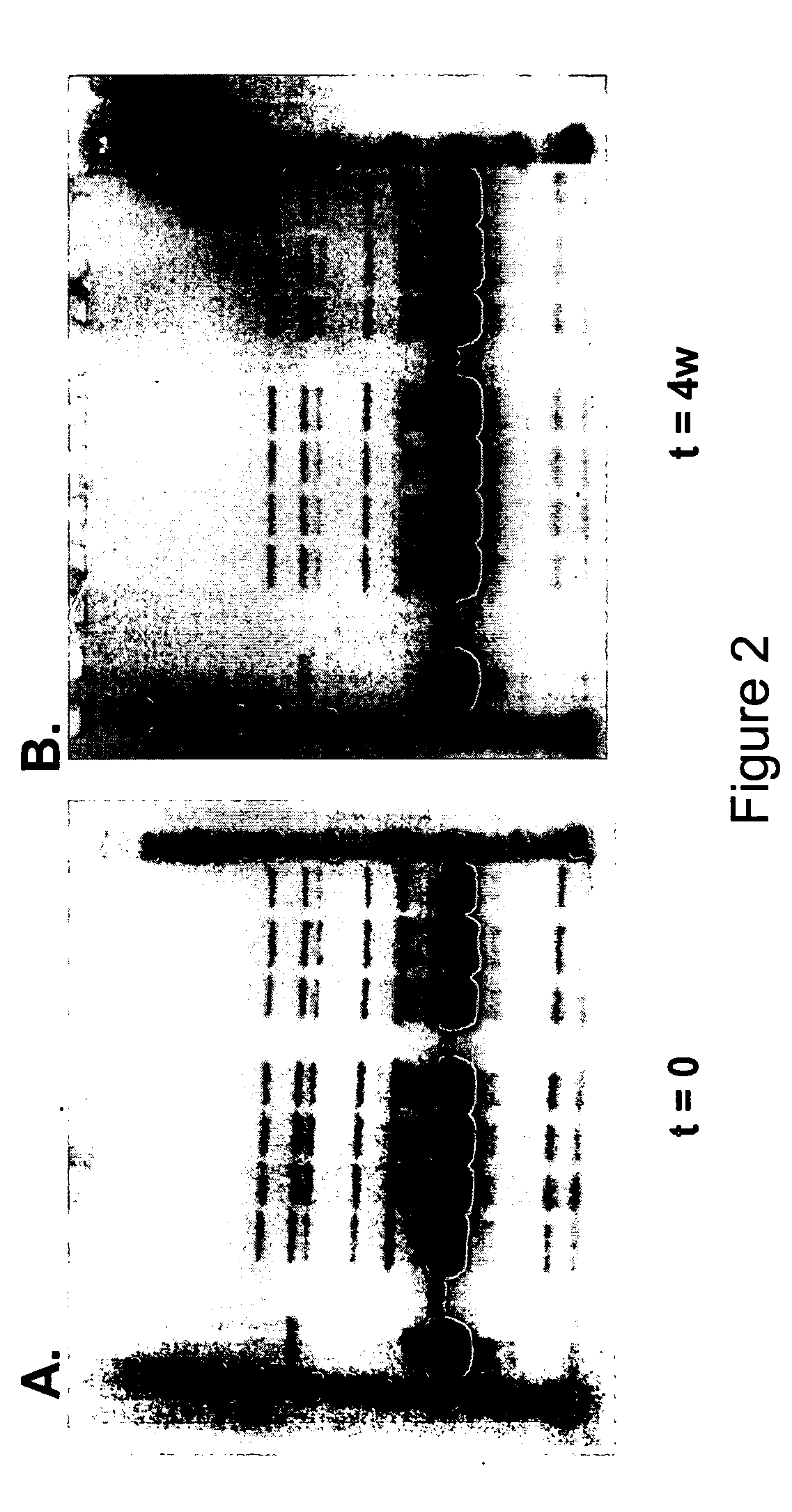
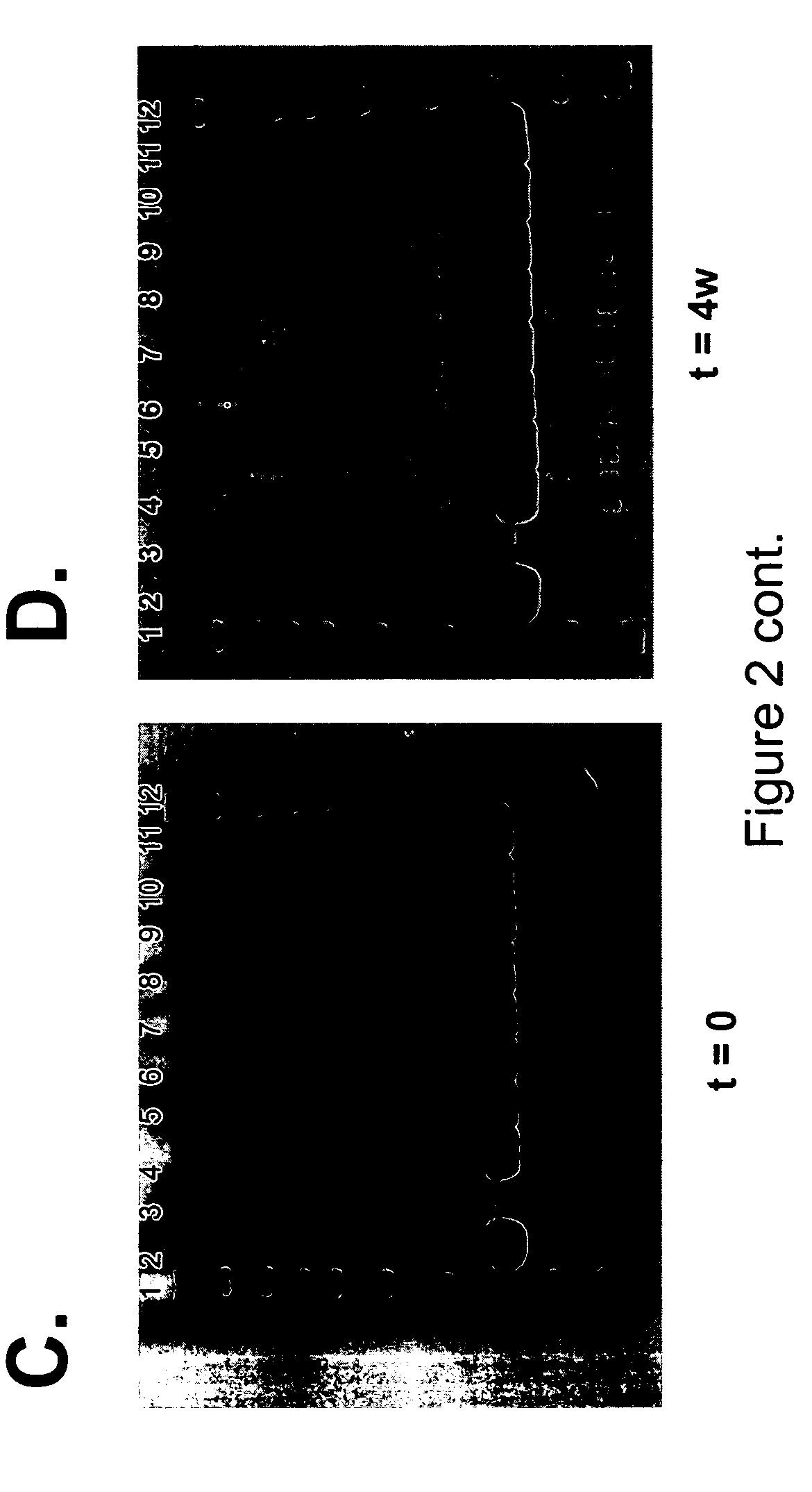
Formulations of human growth hormone comprising a non-naturally encoded amino acid at position 35
InactiveUS7816320B2Minimize formationReduced activityPeptide/protein ingredientsSkeletal disorderSomatotropic hormoneHuman growth hormone
Owner:AMBRX
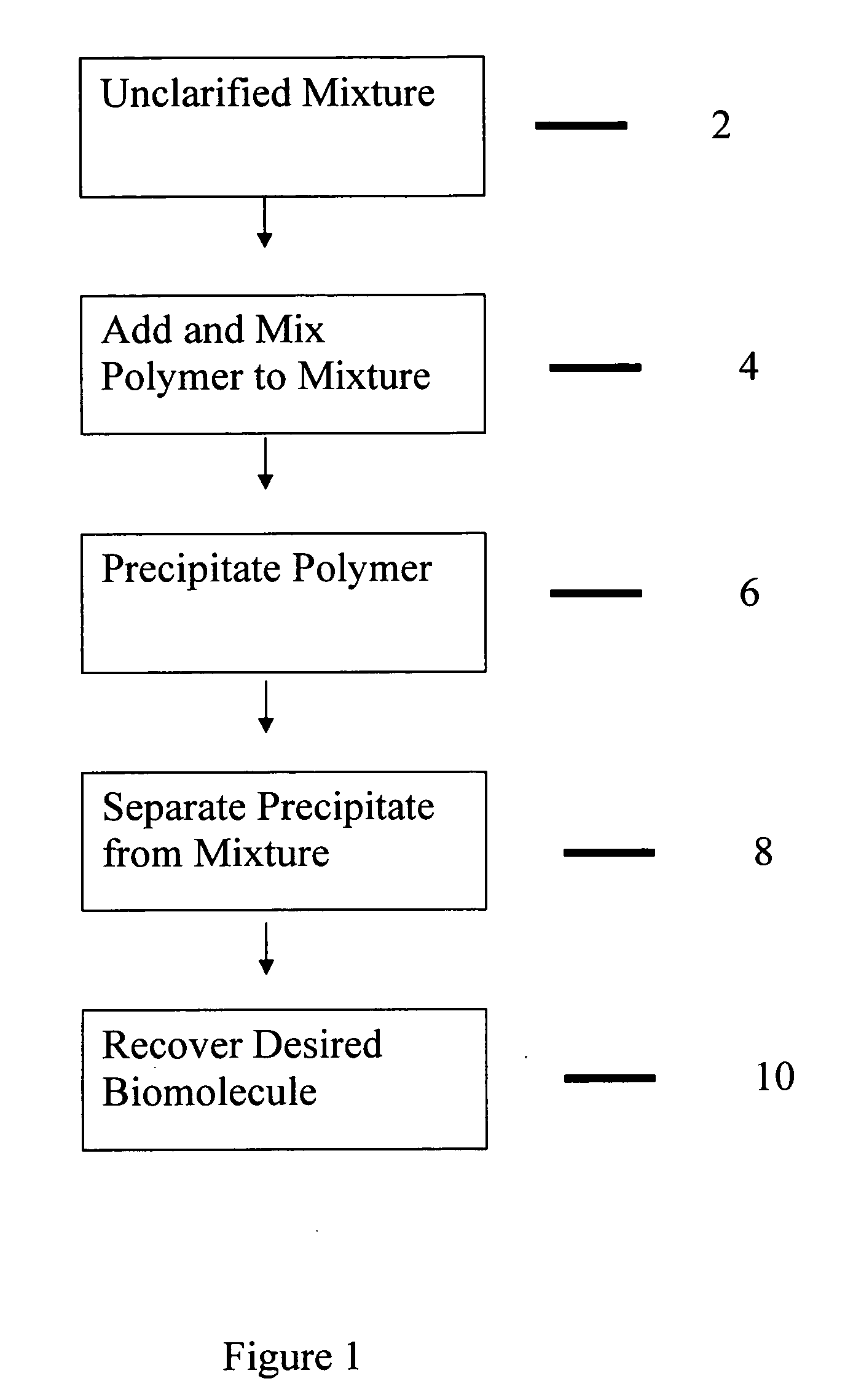
Purification of proteins
InactiveUS20110020327A1Speed up the processPeptide/protein ingredientsImmunoglobulins against growth factorsParticulatesSolid particle
The present invention relates to a bimodal polymer such as a soluble polymer capable of irreversibly binding to insoluble particulates and a subset of soluble impurities and also capable of reversibly binding to one or more desired biomolecules in an unclarified biological material containing stream and the methods of using such a material to purify one or more desired biomolecules from such a stream without the need for prior clarification. Such a polymer comprises domains of charged pendant groups such as primary, secondary, tertiary or quaternary amines, (first mode) and is rendered insoluble and precipitates out of solution simply upon complexing with oppositely charged solid particulates and a fraction of the soluble impurities in an amount sufficient to form an aggregate that can no longer be held in solution. The polymer further comprises other domains of pendant groups that are charged or uncharged, hydrophilic or hydrophobic or have a ligand that is selective for the biomolecule of interest depending on the process conditions such as pH, ionic strength, salts, and the like (second mode). When present in one mode, such as the uncharged form, said pendant groups are capable of binding to one or more desired biomolecules within the stream (protein, polypeptide, etc) in an unclarified cell broth. The precipitate can then be removed from the stream, such as by being filtered out from the remainder of the stream and the desired biomolecule is recovered such as by selective elution.
Owner:MILLIPORE CORP
Compositions and methods for the preparation of human growth hormone glycosylation mutants
InactiveUS7932364B2Peptide/protein ingredientsPeptide preparation methodsSomatotropic hormoneHuman growth hormone
The present invention relates to mutants of human growth hormone, which contain newly introduced N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS
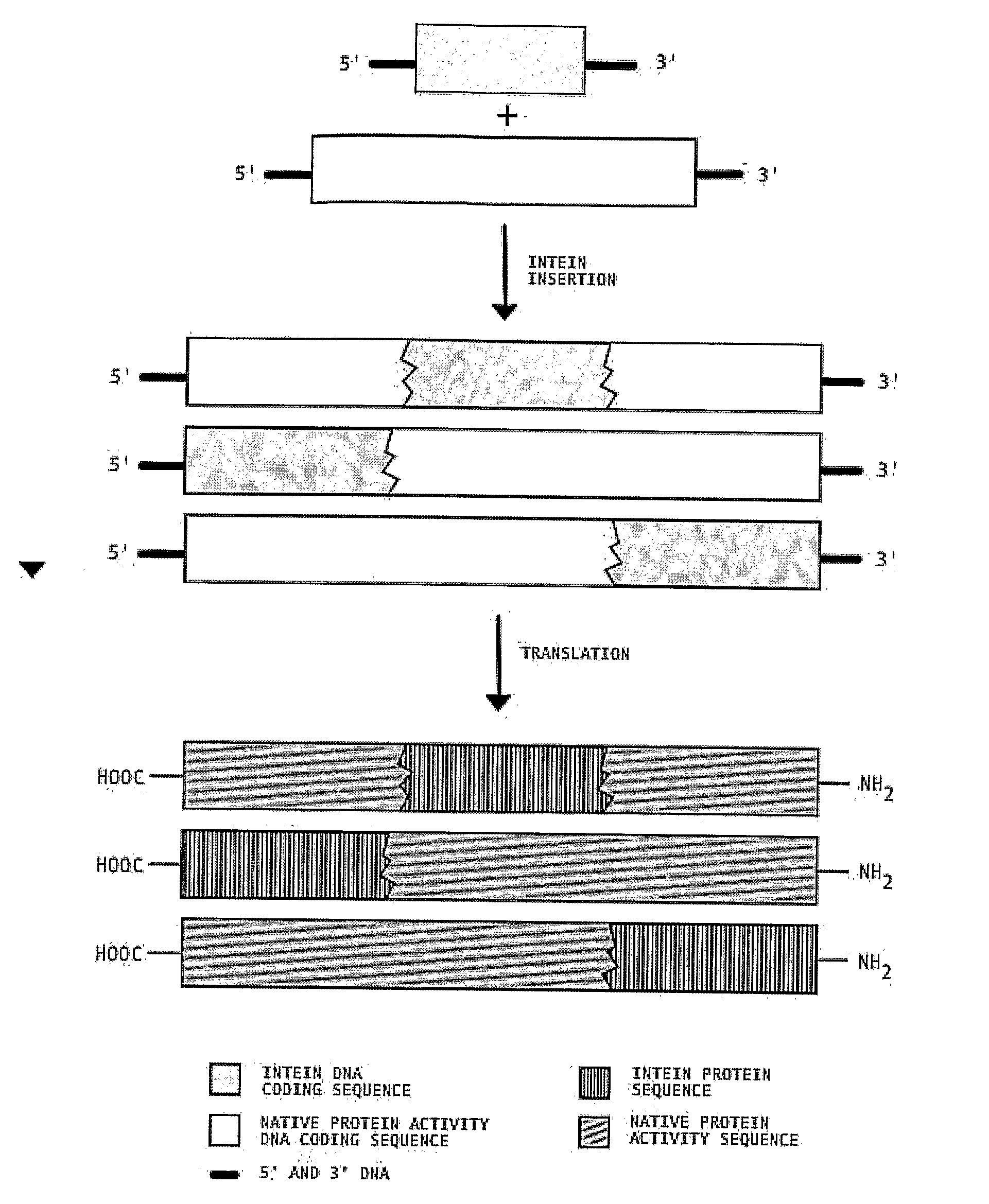
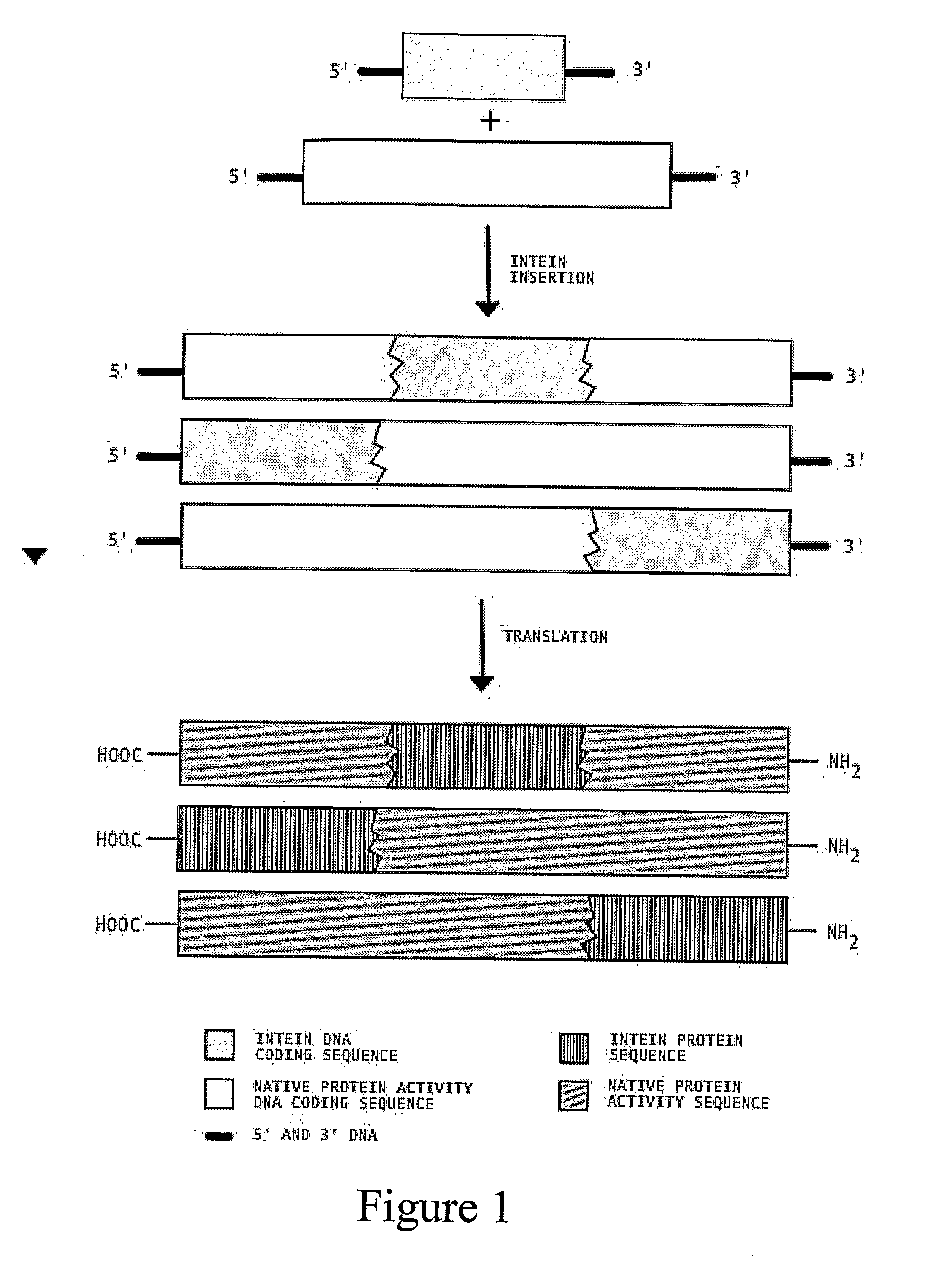
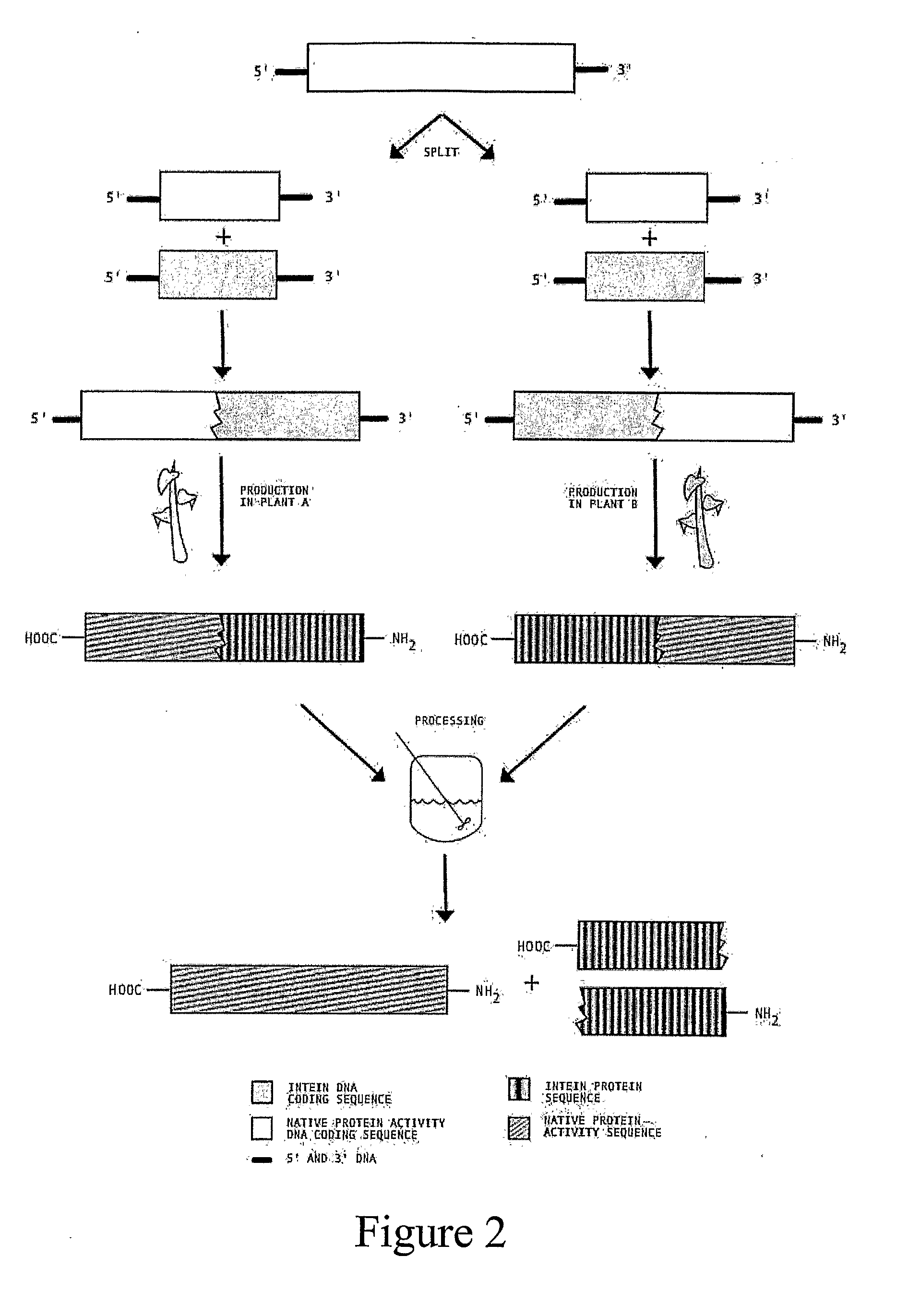
Transgenic Plants Expressing Intein Modified Proteins and Associated Processes for Bio-Pharmaceutical Production
InactiveUS20080115243A1Other foreign material introduction processesTissue cultureBiotechnologyTherapeutic protein
Transgenic plants that express CIVPS or intein modified therapeutic proteins, compositions of matter comprising them, therapeutic proteins made from the transgenic plants, methods to construct the transgenic plants containing CIVPS or intein modified therapeutic genes, methods to express CIVPS or intein modified therapeutic proteins in plants, and methods of using the transgenic plants.
Owner:AGRIVIDA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com