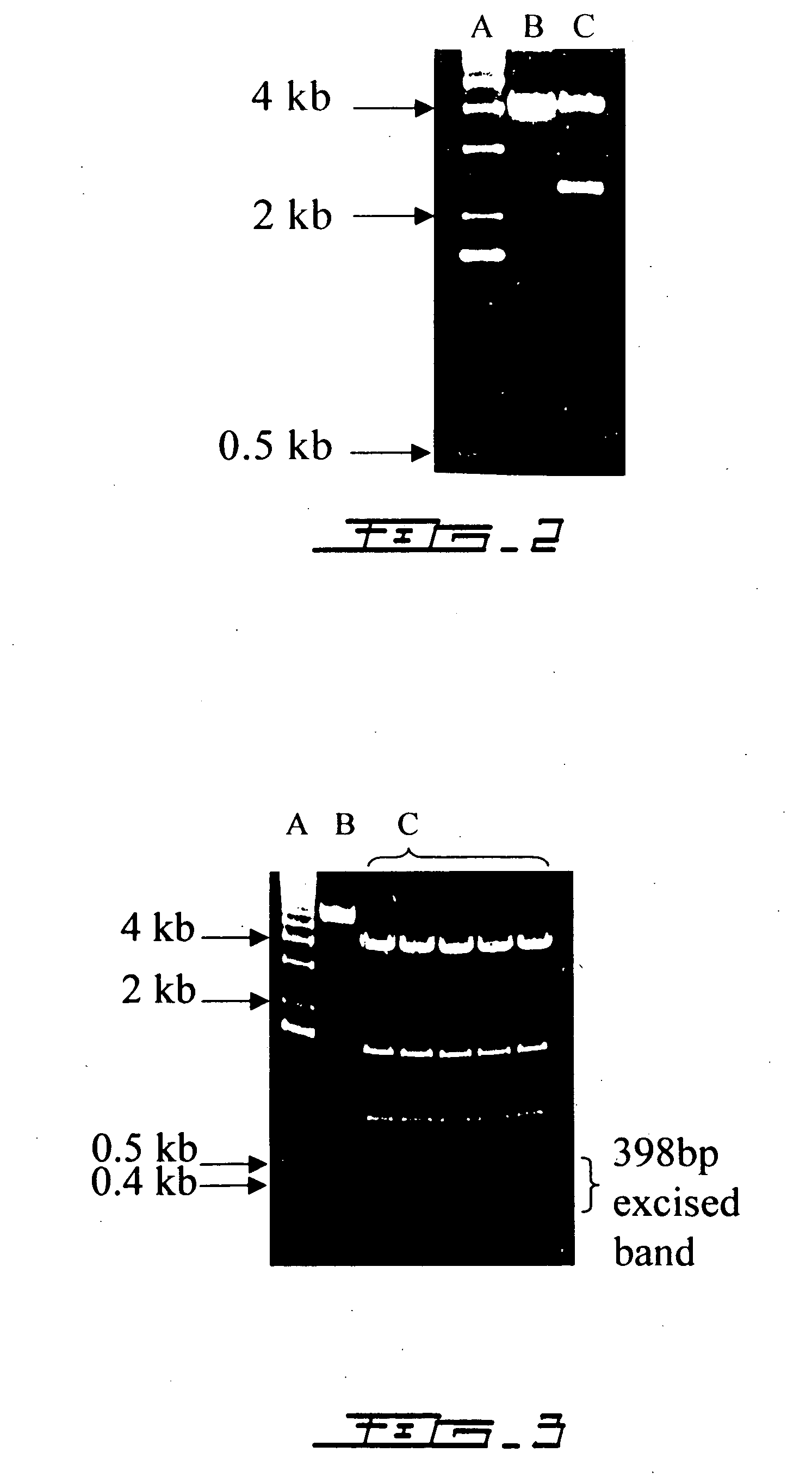
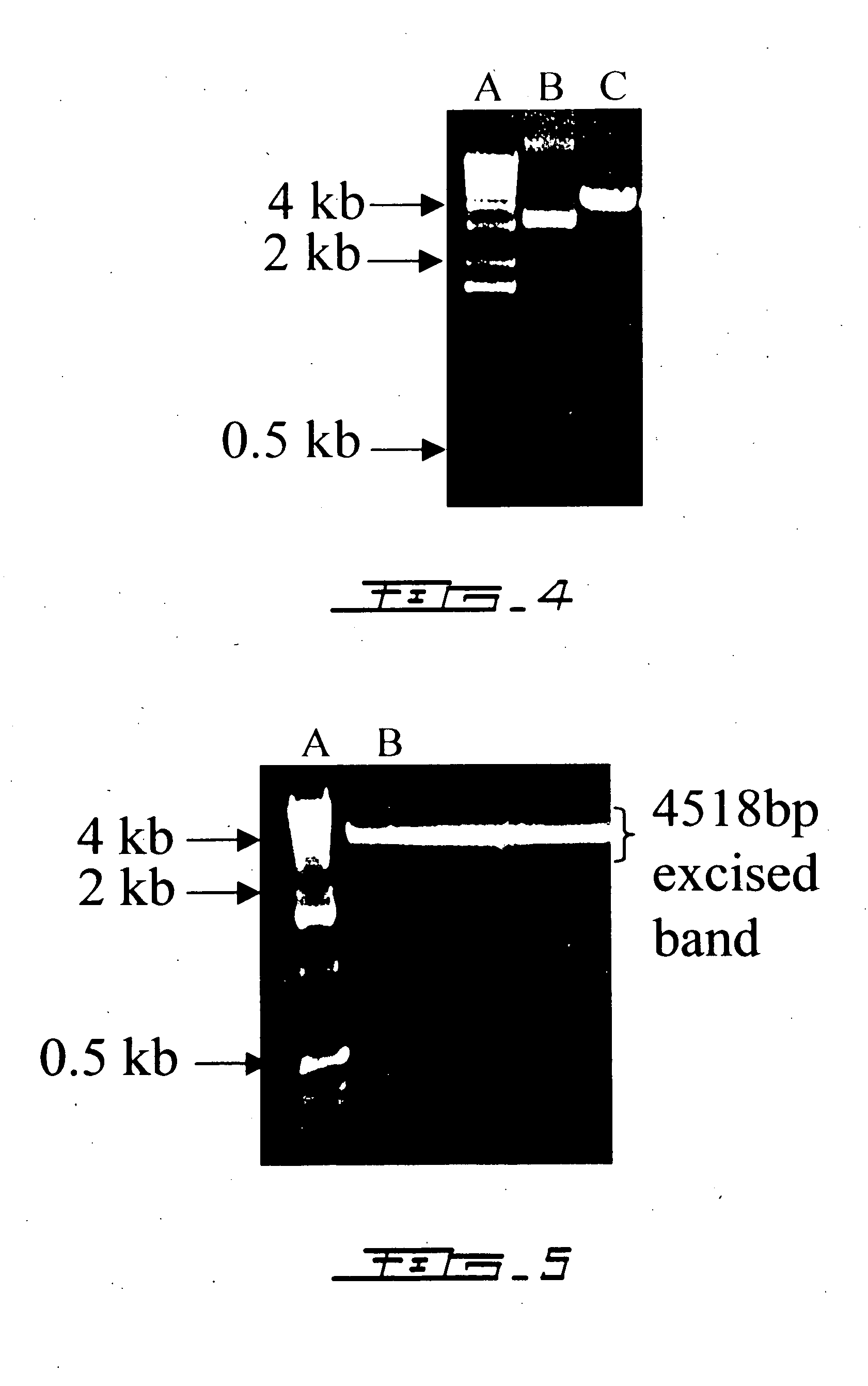
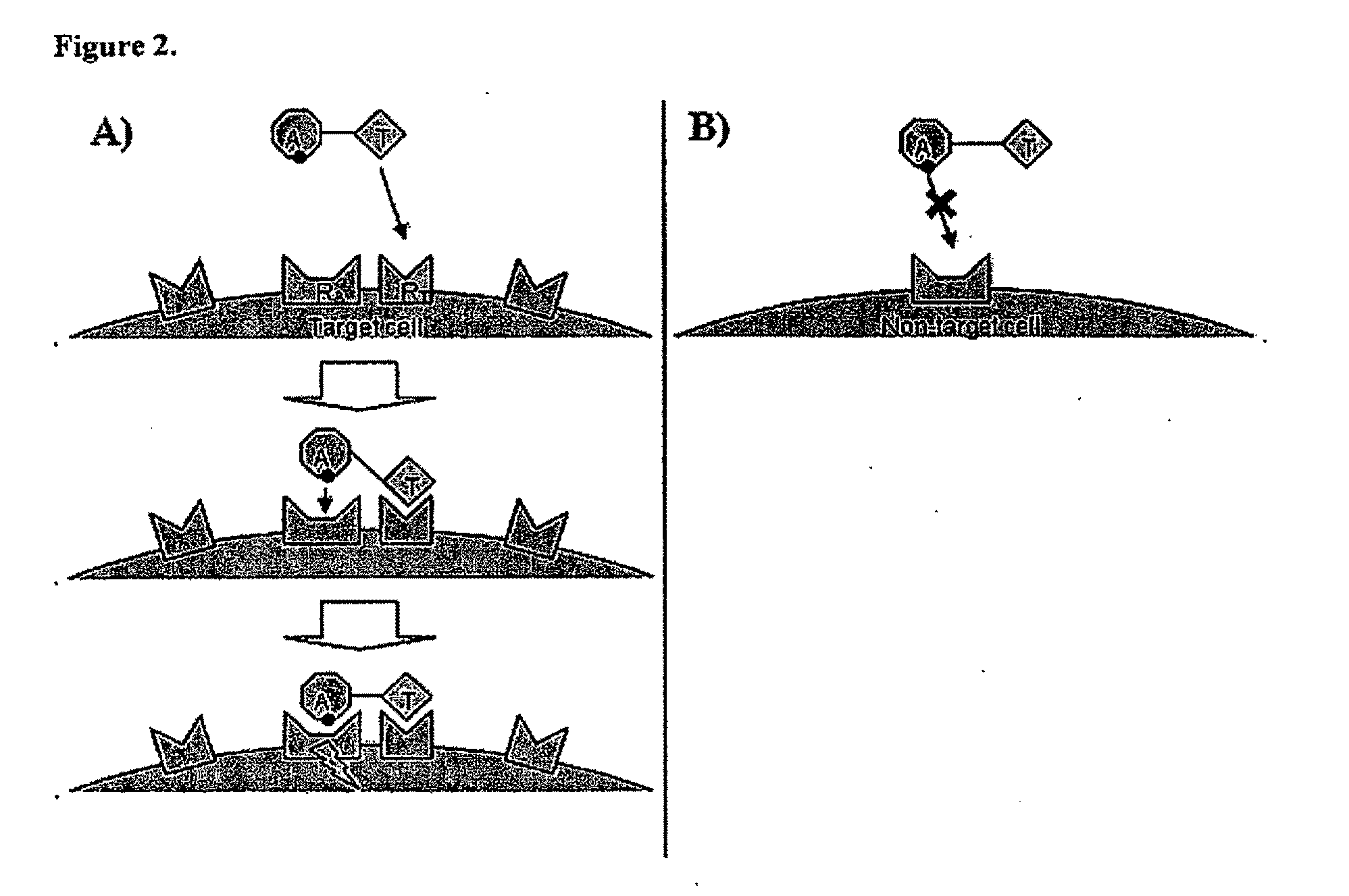
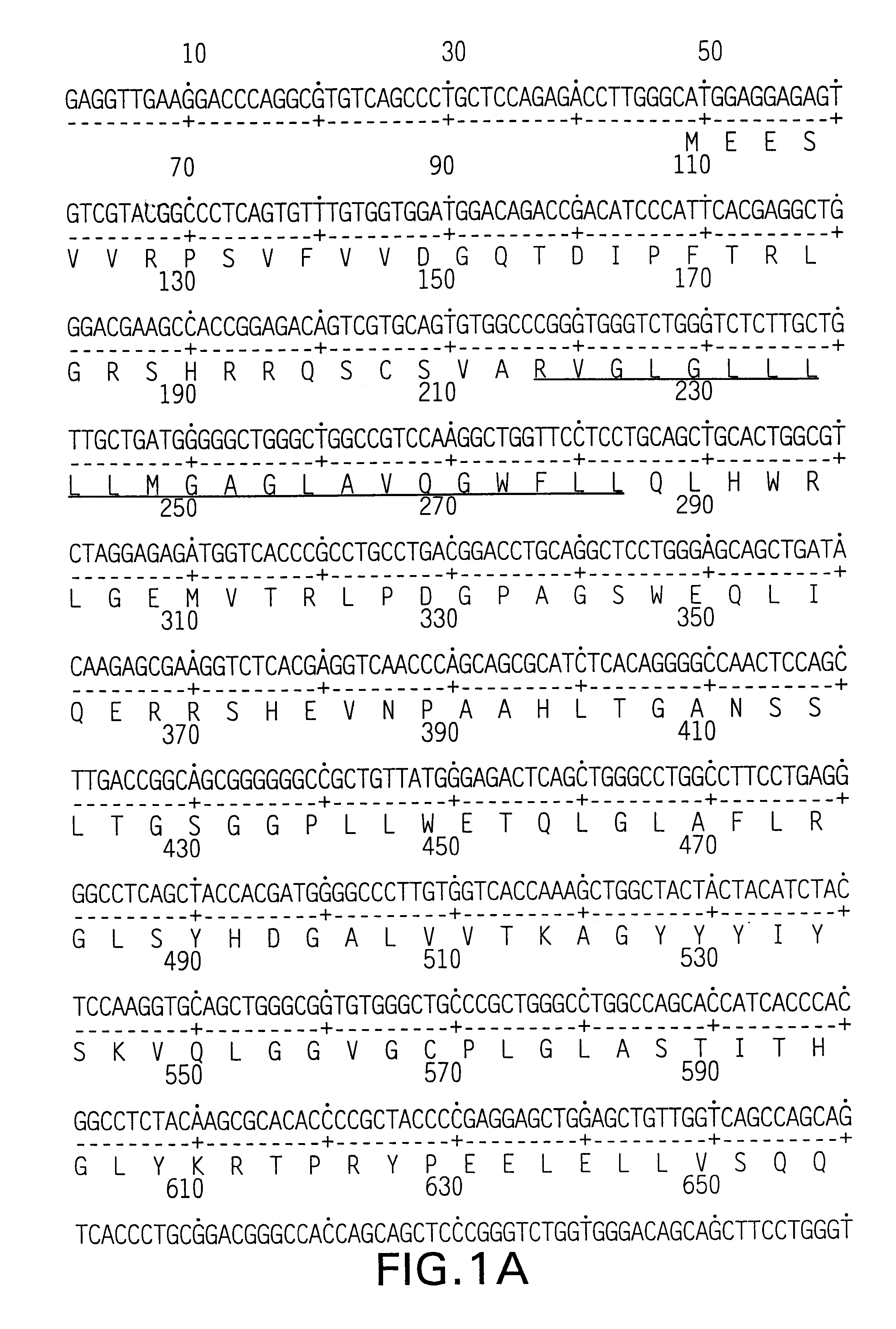
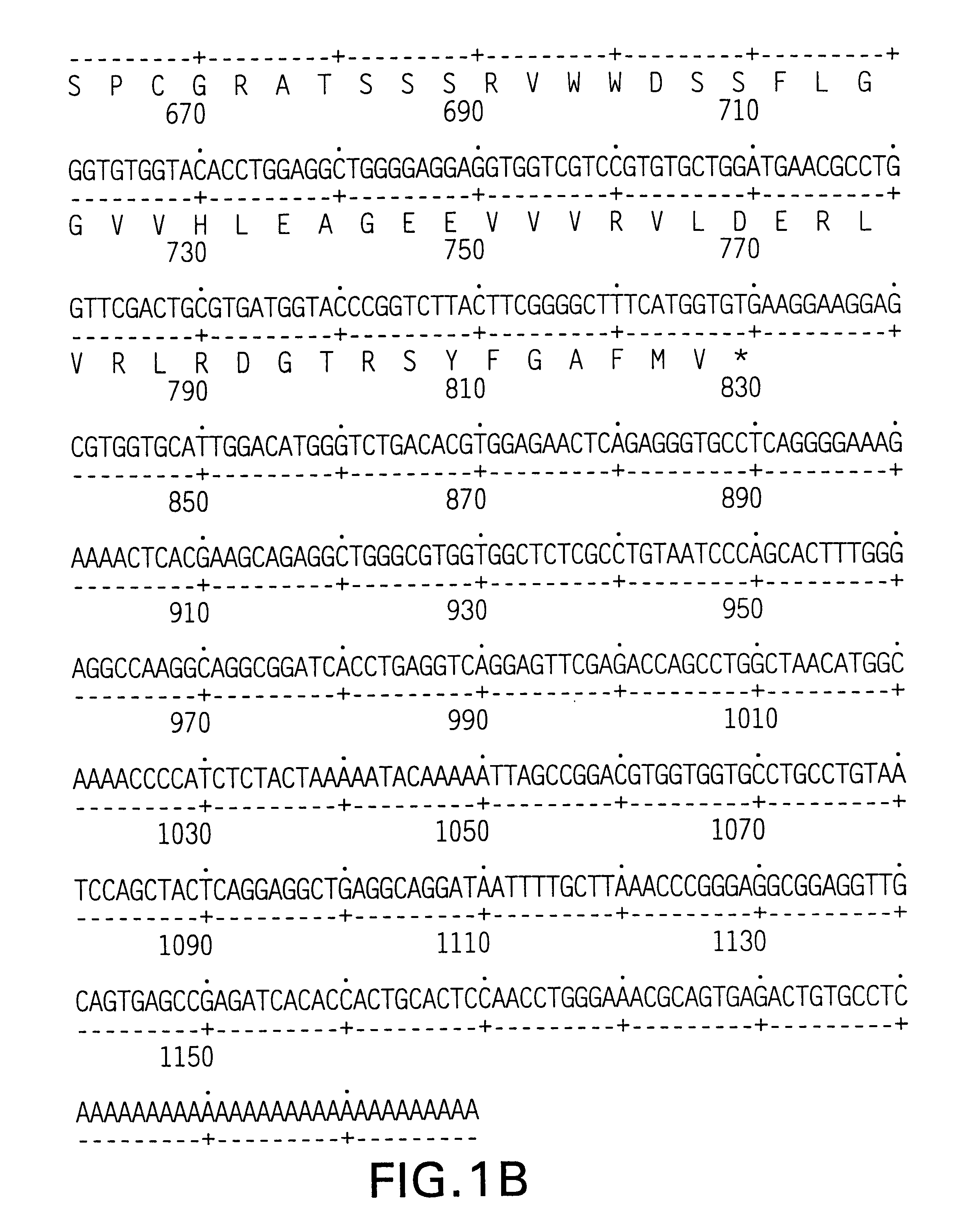
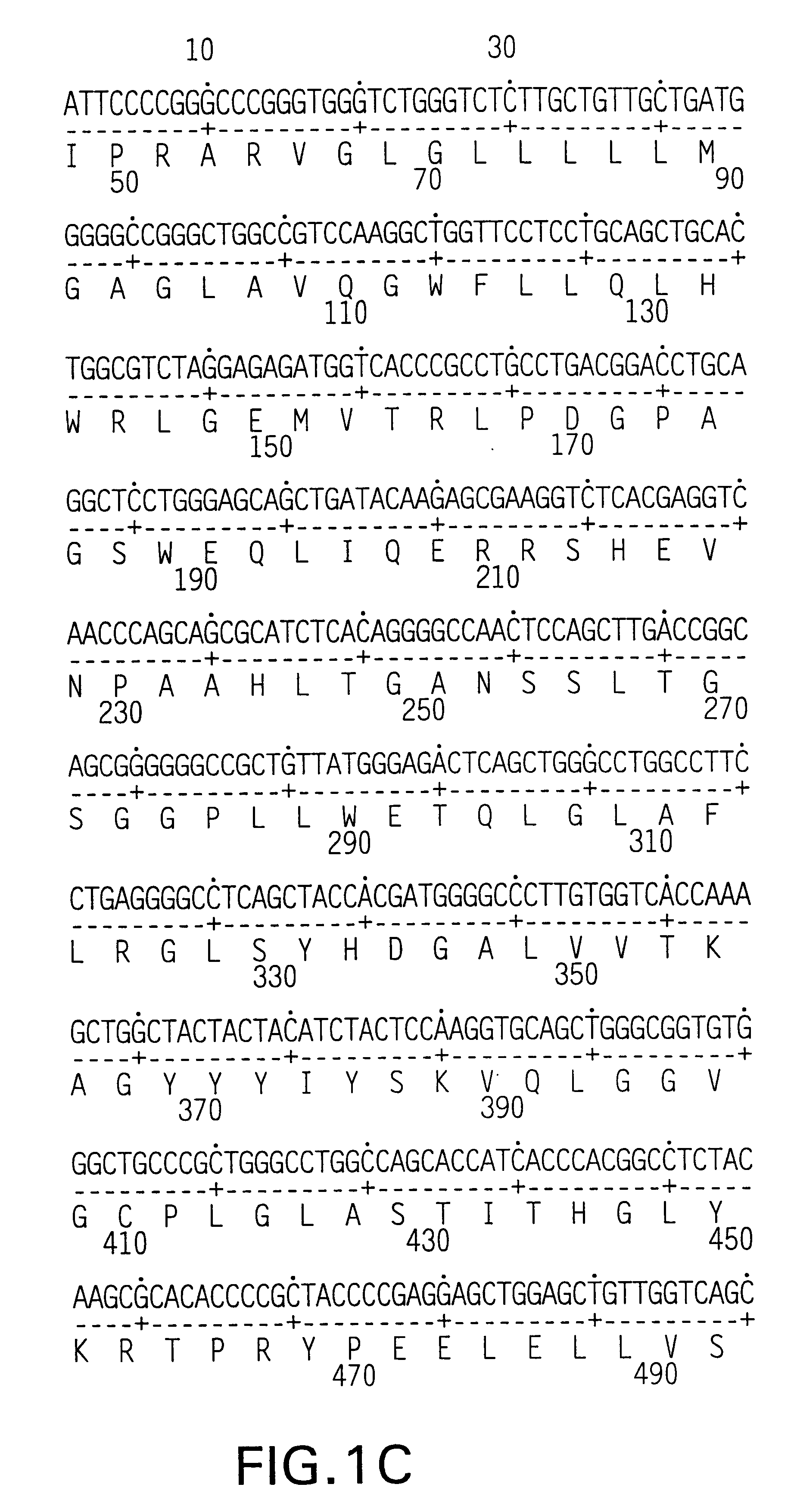
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
460results about "Tumor necrosis factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
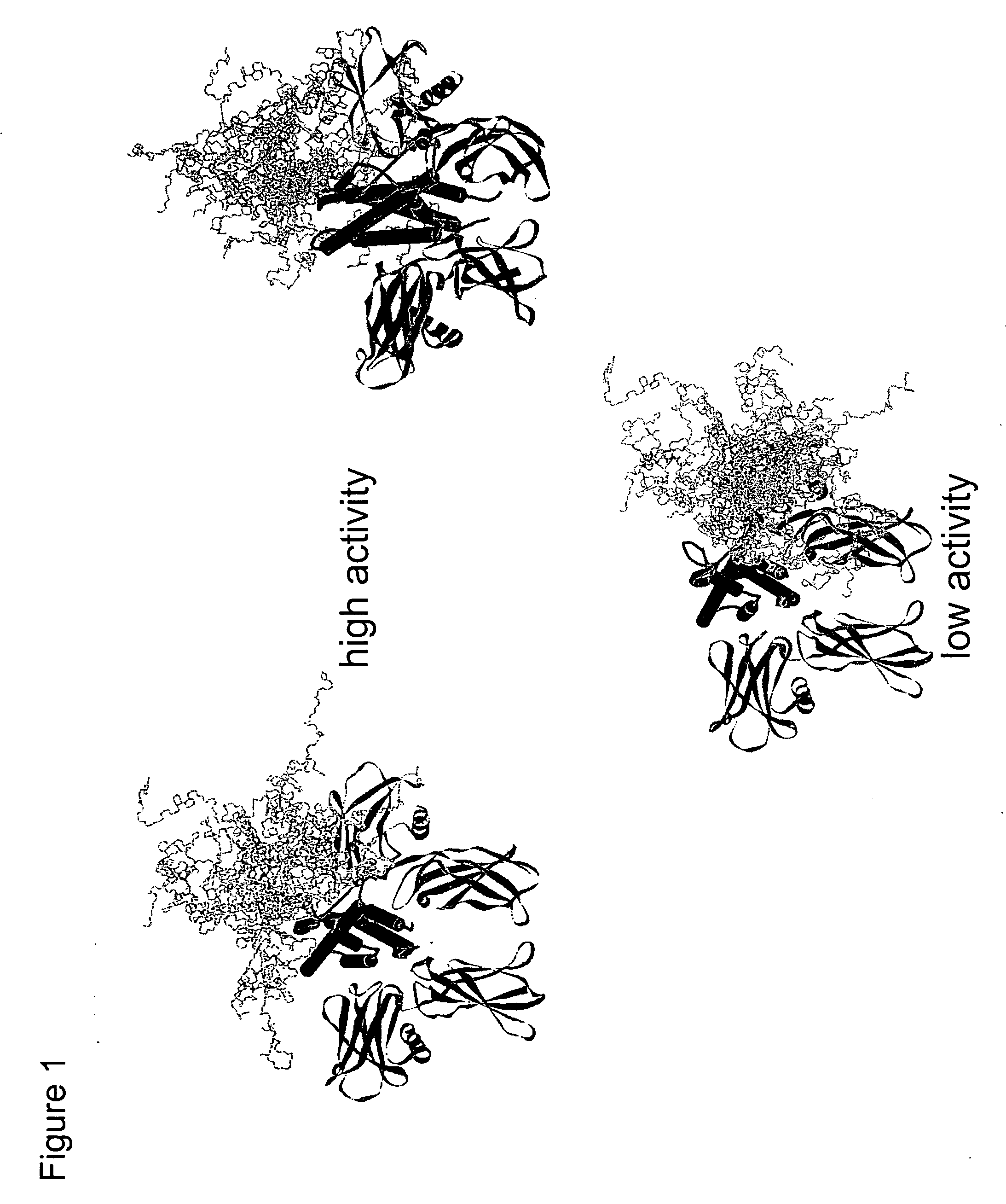
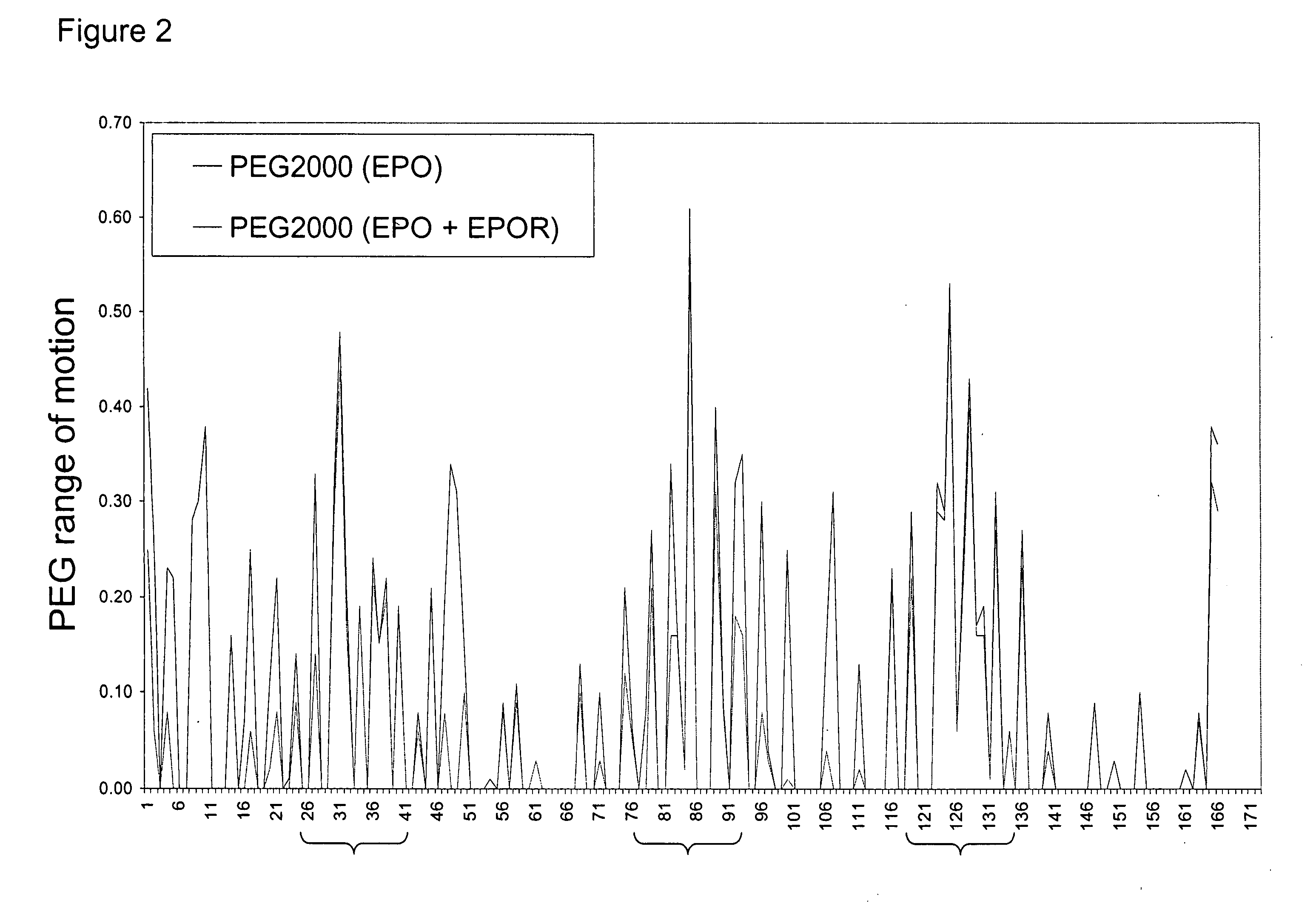
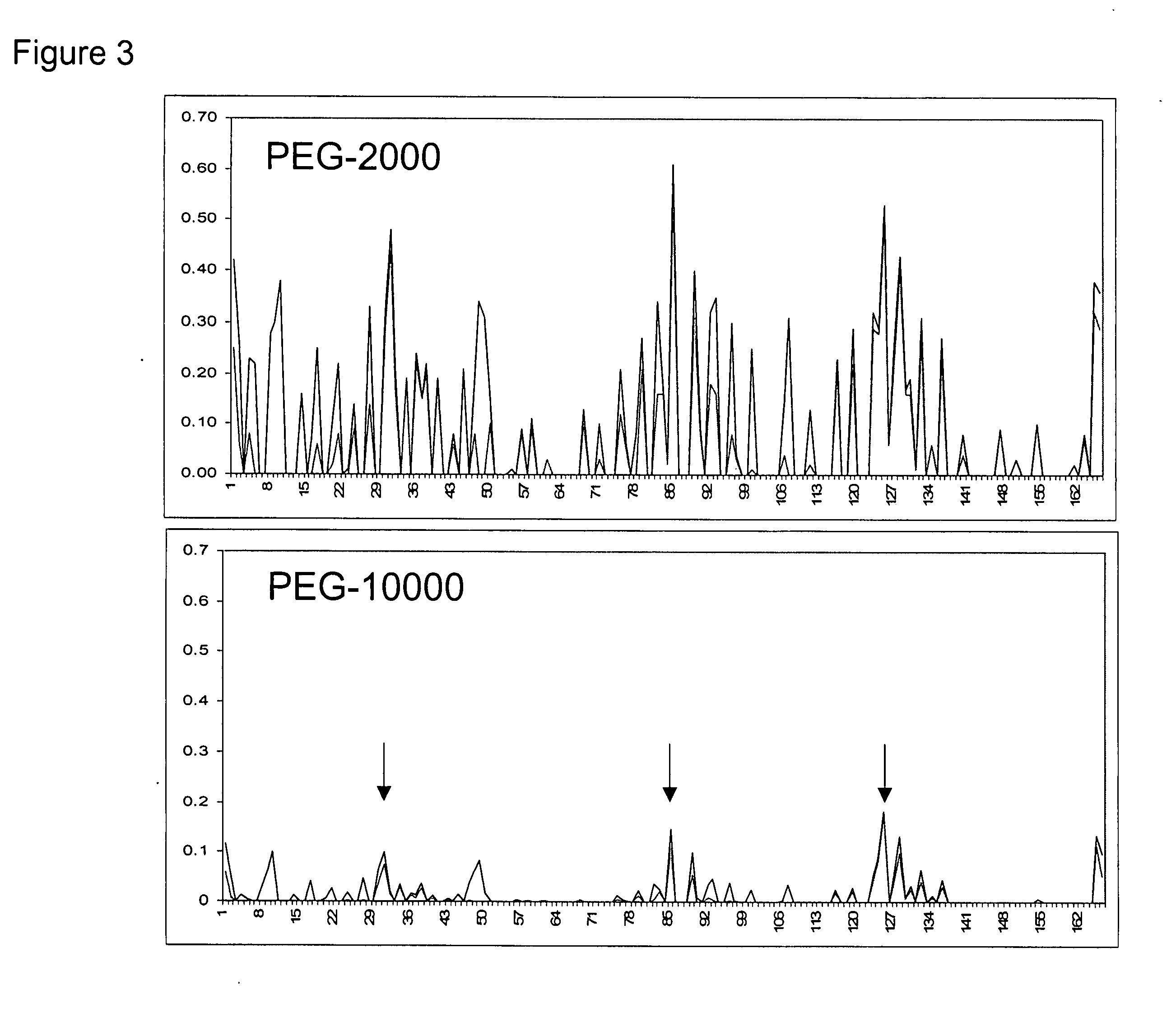
Methods for rational pegylation of proteins
The present invention relates to the use of simulation technology to rationally optimize the locations and sizes of attached polymeric moieties for modification of therapeutic proteins and the proteins generated from this method.
Owner:XENCOR
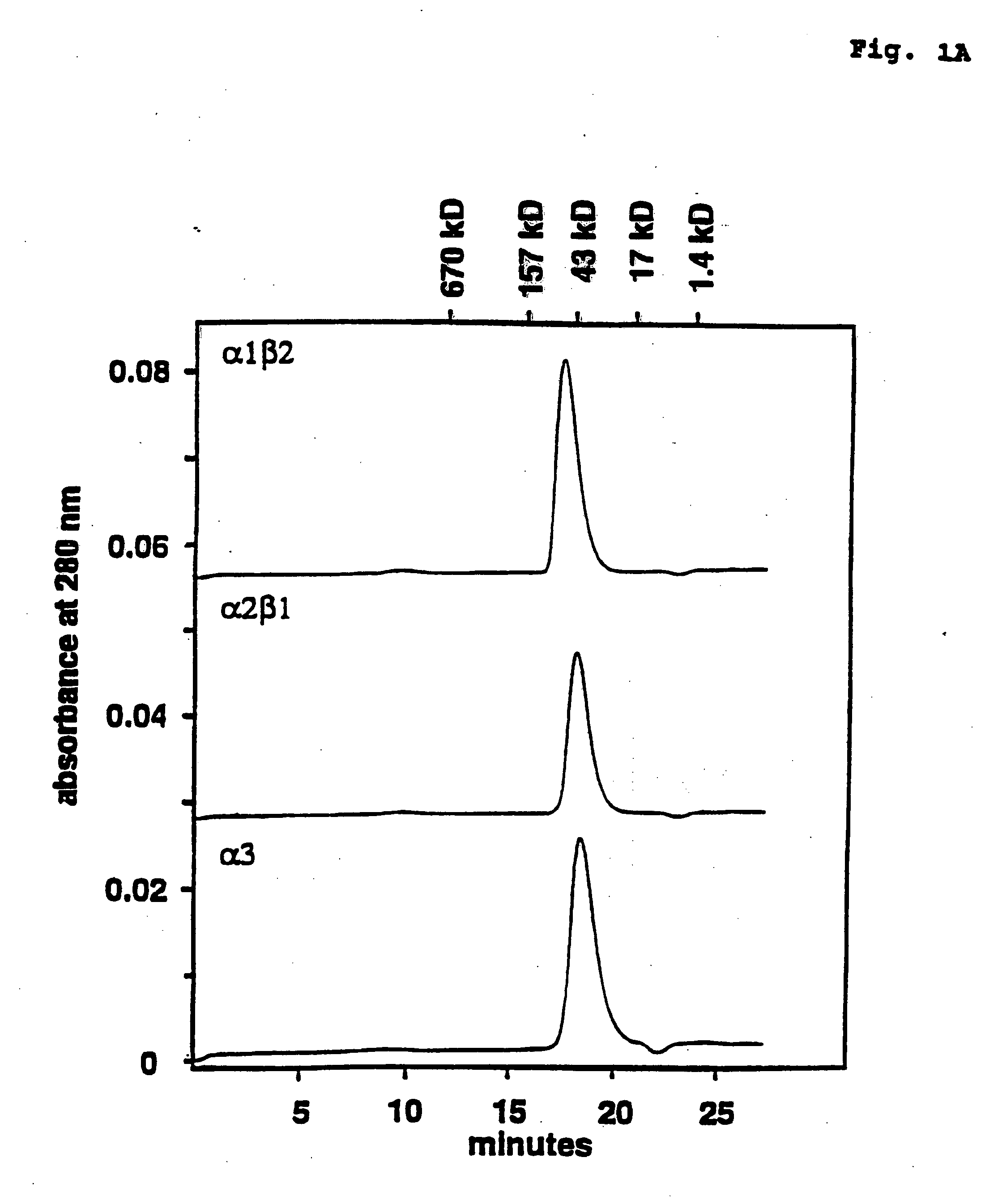
Protein scaffolds for antibody mimics and other binding proteins
InactiveUS7115396B2Easy to foldImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsWAS PROTEINAntibody
Disclosed herein are proteins that include an immunoglobulin fold and that can be used as scaffolds. Also disclosed herein are nucleic acids encoding such proteins and the use of such proteins in diagnostic methods and in methods for evolving novel compound-binding species and their ligands.
Owner:BRISTOL MYERS SQUIBB CO
Anti-TNF antibodies and peptides of human tumor necrosis factor
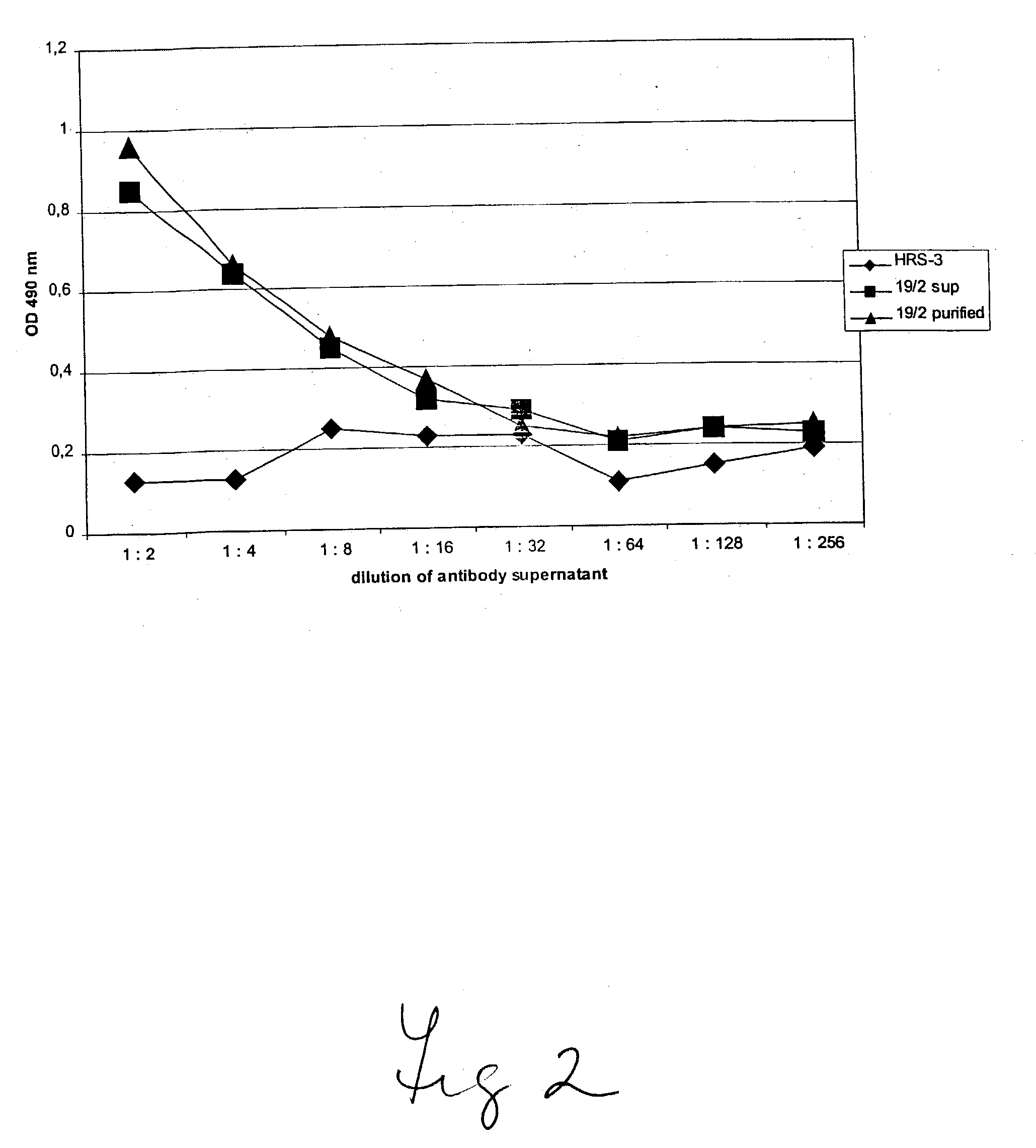
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-alpha (TNFalpha) and are useful in vivo diagnosis and therapy of a number of TNFalpha-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
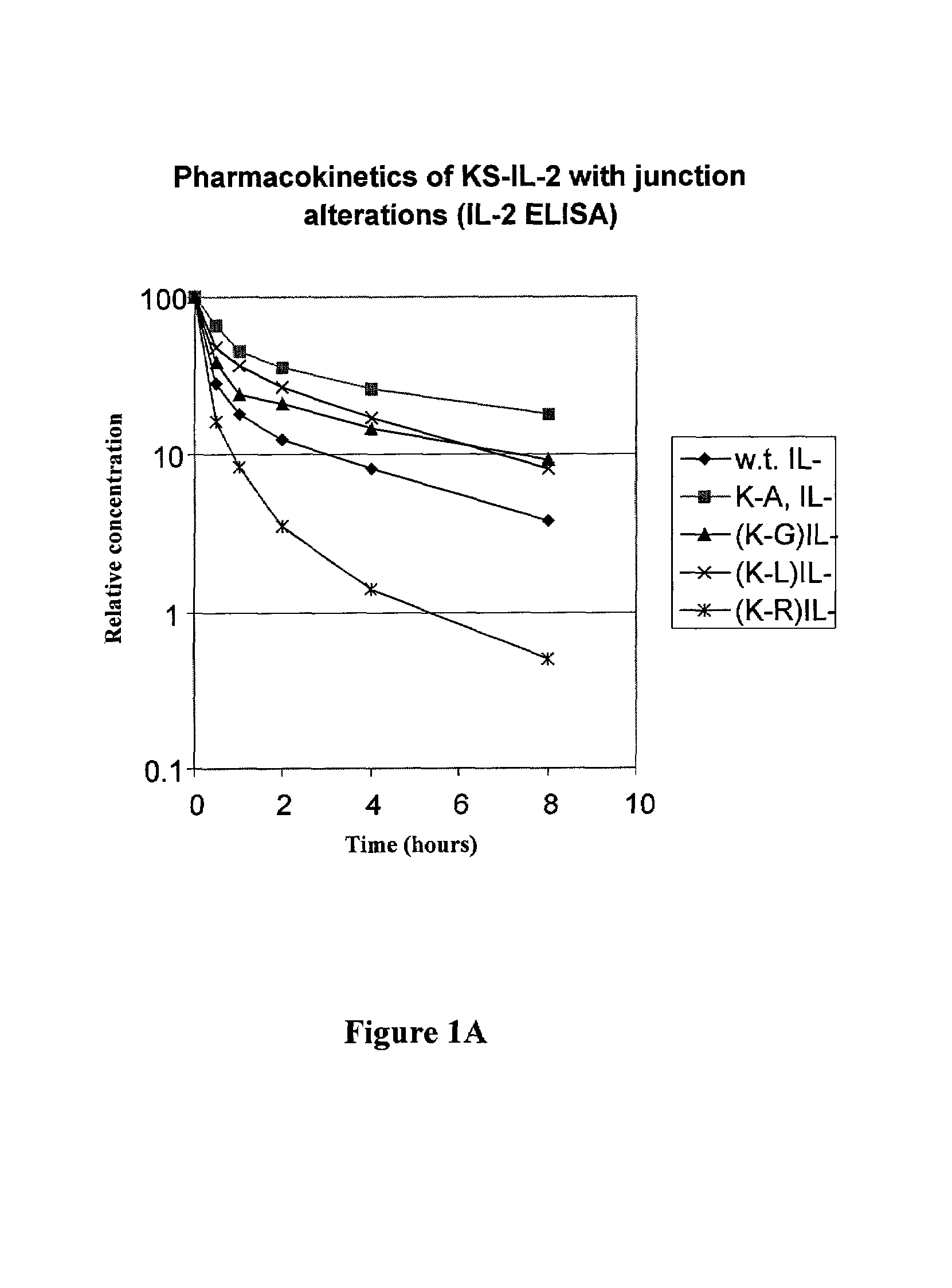
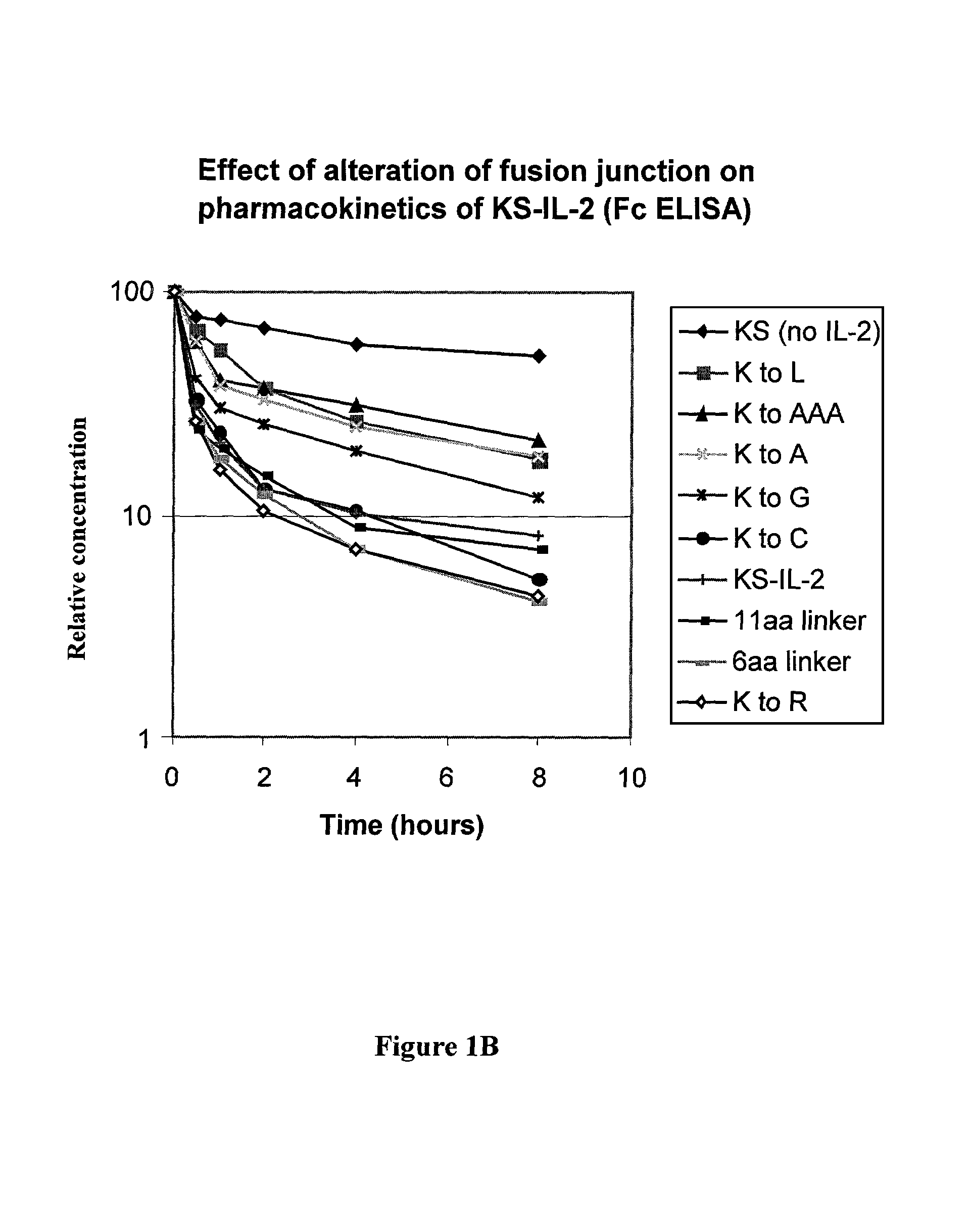
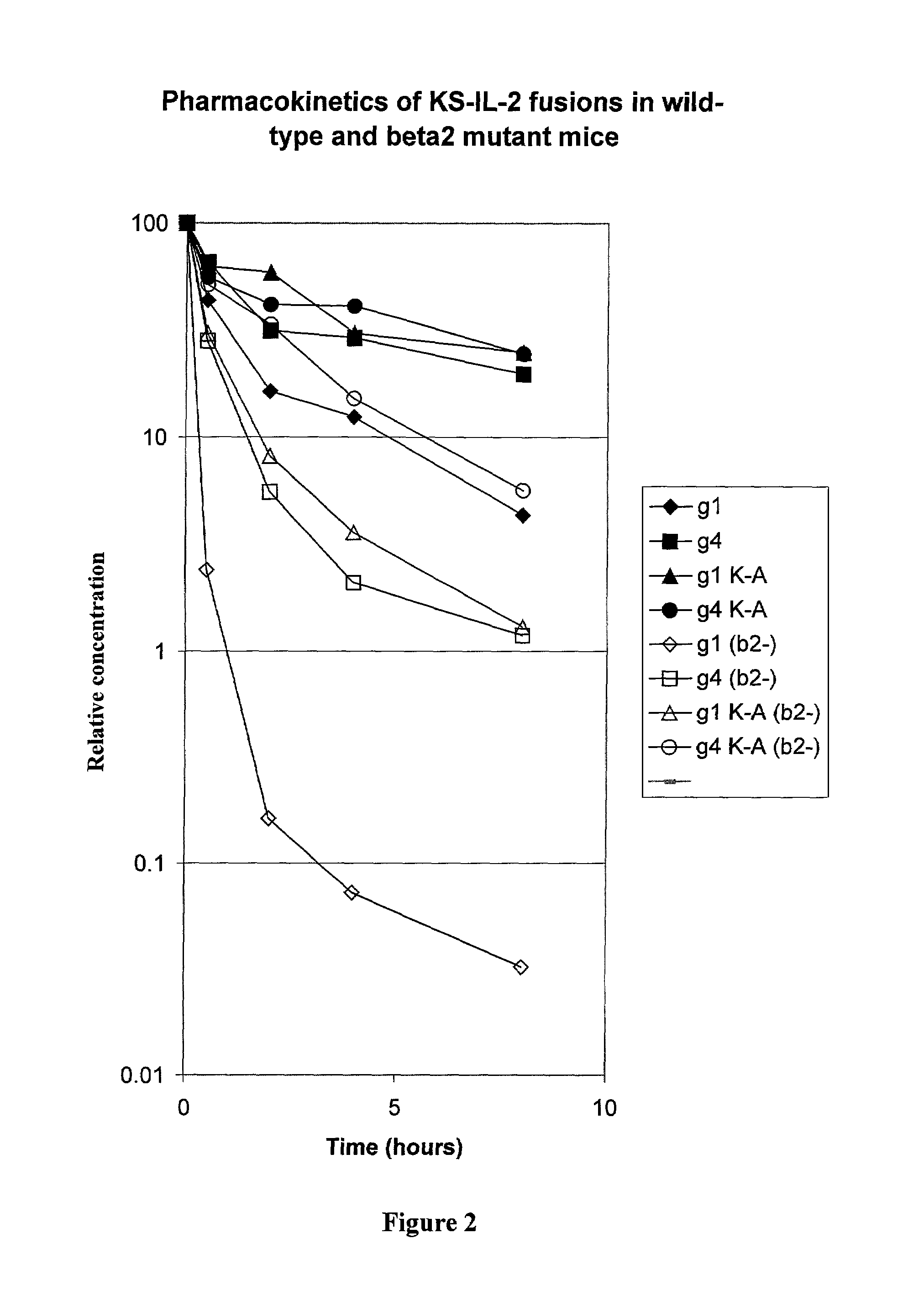
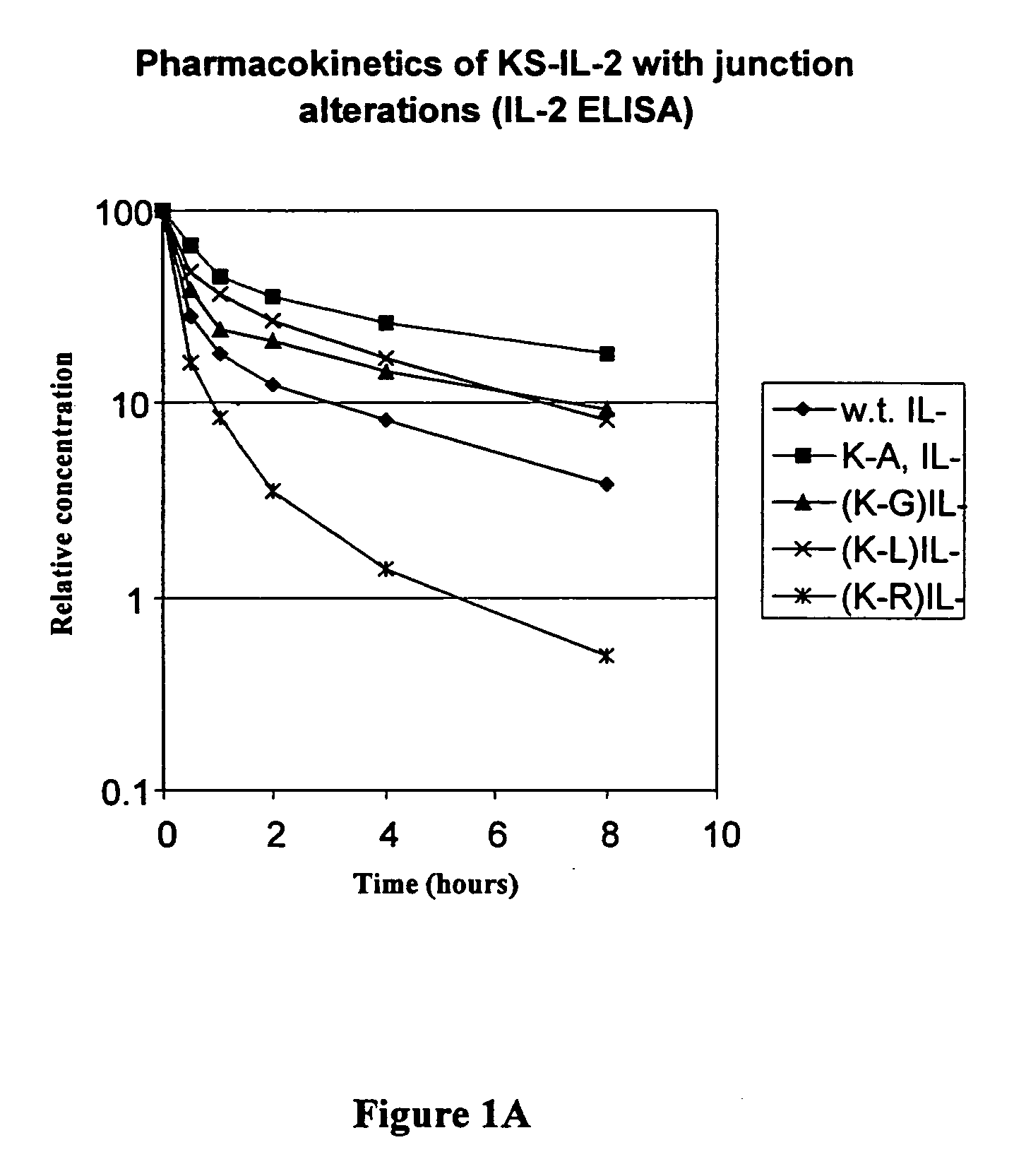
Enhancing the circulating half-life of antibody-based fusion proteins
InactiveUS7091321B2Increased serum half-lifeHigh affinityCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsLymphatic SpreadHalf-life
Disclosed are compositions and methods for enhancing the circulating half-life of antibody-based fusion proteins. Disclosed methods and compositions rely on altering the amino acid sequence of the junction region between the antibody moiety and the fused protein moiety in an antibody-based fusion protein. An antibody-based fusion protein with an altered amino acid sequence in the junction region has a greater circulating half-life when administered to a mammal. Disclosed methods and compositions are particularly useful for reducing tumor size and metastasis in a mammal.
Owner:MERCK PATENT GMBH
Method for Purifying Antibodies
ActiveUS20130171095A1Increased serum half-lifeMinimize the possibilityFactor VIIPeptide/protein ingredientsIsoelectric pointAntibody
The invention relates generally to compositions and methods for purifying the desired species from a mixture of desired heterodimer and contaminating homodimer immunoglobulin variants by modifying the isoelectric point(s) of the individual chains.
Owner:XENCOR
Soluble tumor necrosis factor receptor treatment of medical disorders
The invention pertains to methods and compositions for treating medical disorders characterized by elevated levels or abnormal expression of TNFα by administering a TNFα antagonist, such as recombinant TNFR:Fc.
Owner:IMMUNEX CORP
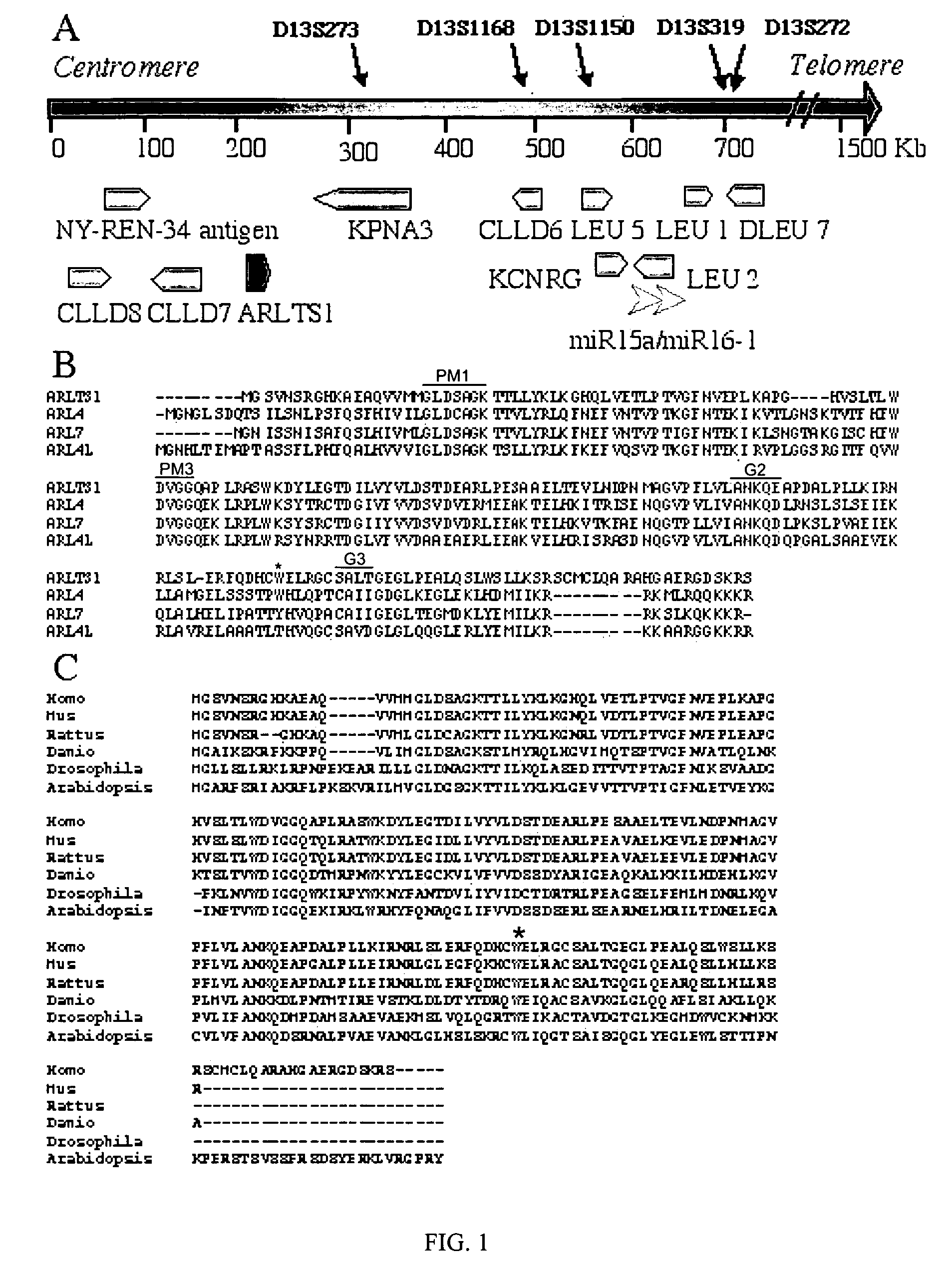
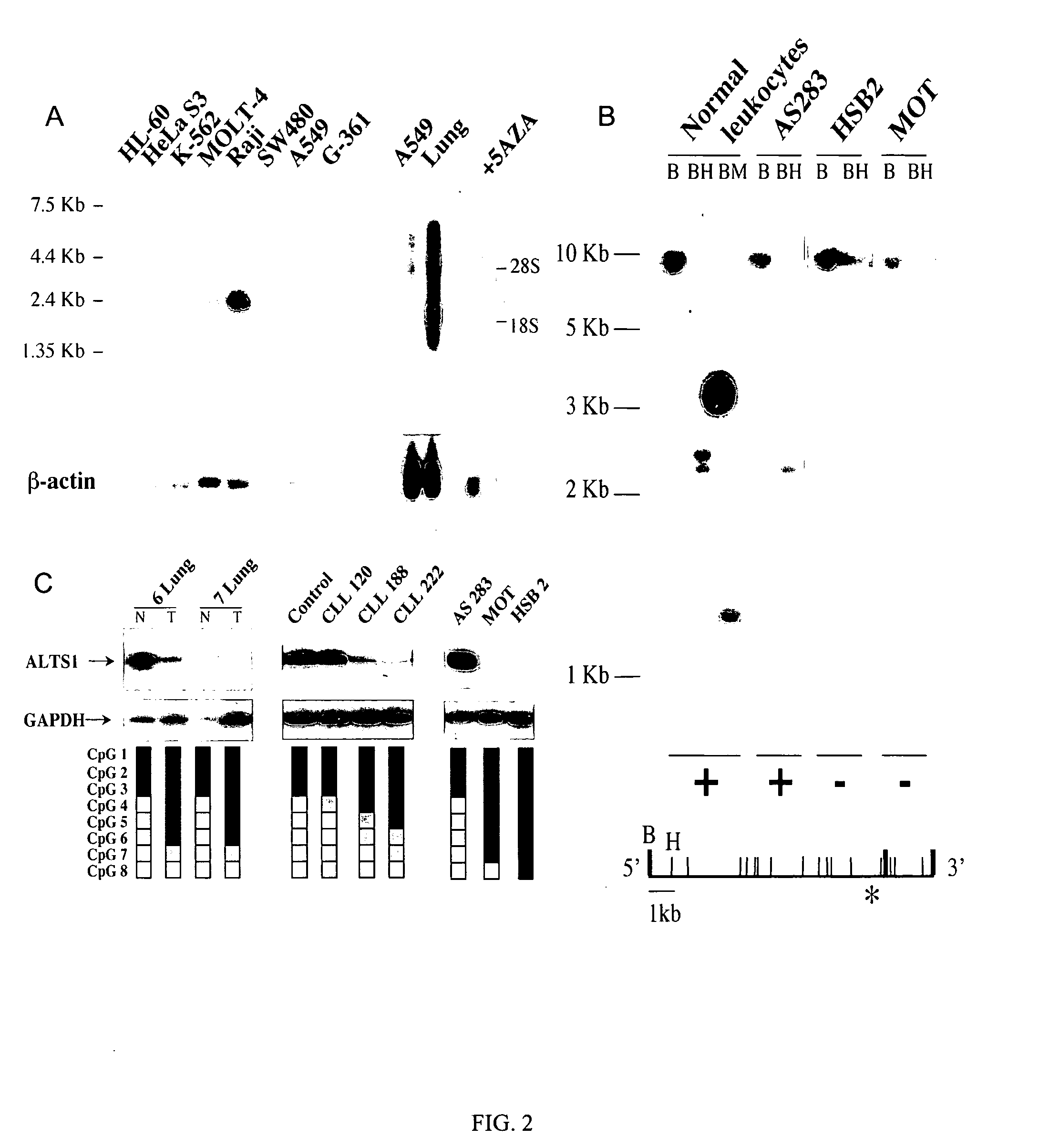
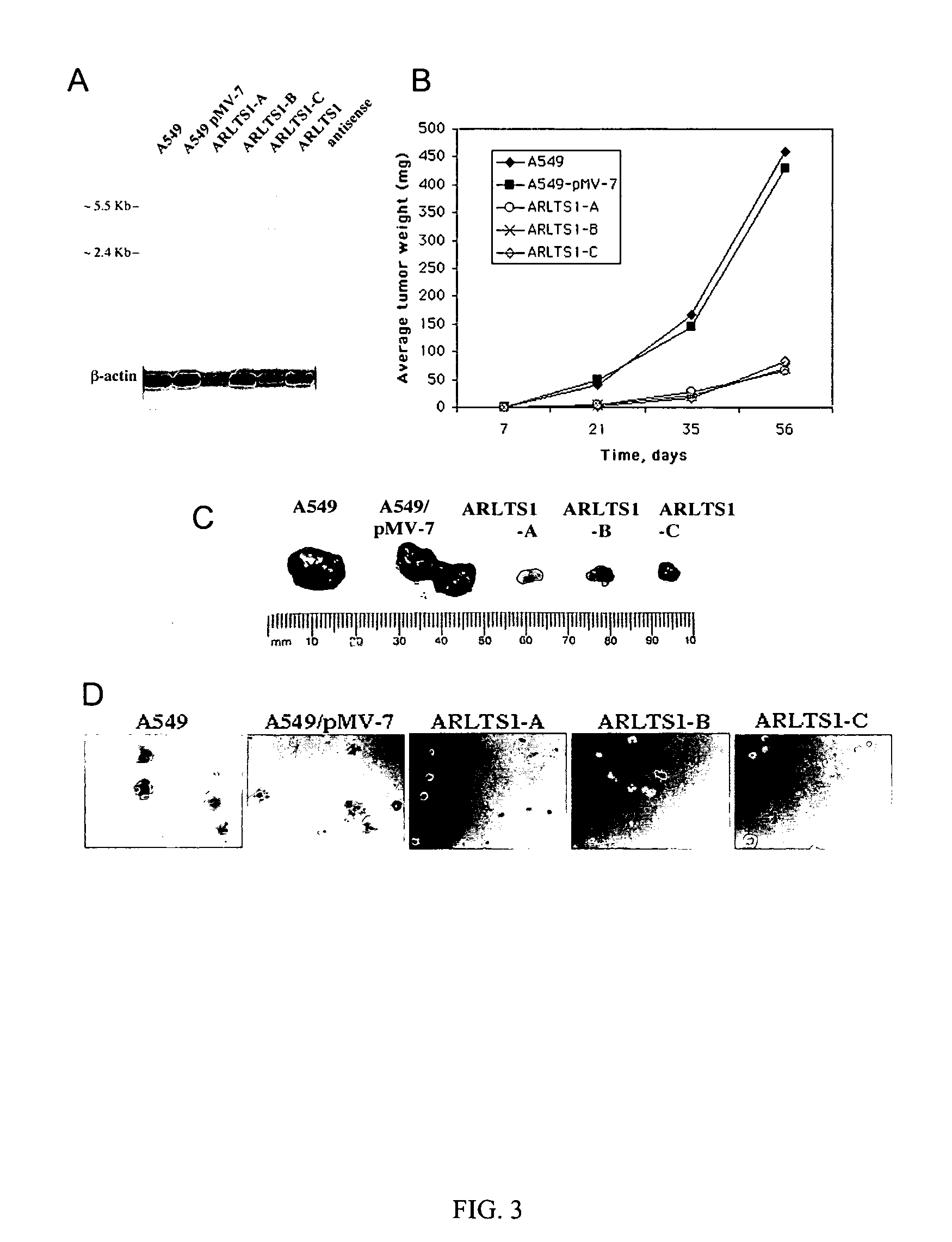
Novel tumor suppressor gene and compositions and methods for making and using the same
InactiveUS20050266443A1Sugar derivativesMicrobiological testing/measurementTumour suppressor geneGene product
The present invention relates to the identification and cloning of ARTS1, a novel tumor suppressor gene. The invention further encompasses isolated proteins encoded by ARTS1, methods of making and using the same, methods of diagnosing the presence of, or prediposition for, a cancer associated with a defective ARTS1 gene or gene product, and methods of treating or preventing cancers associated with a defective ARTS1 gene or gene product.
Owner:THOMAS JEFFERSON UNIV
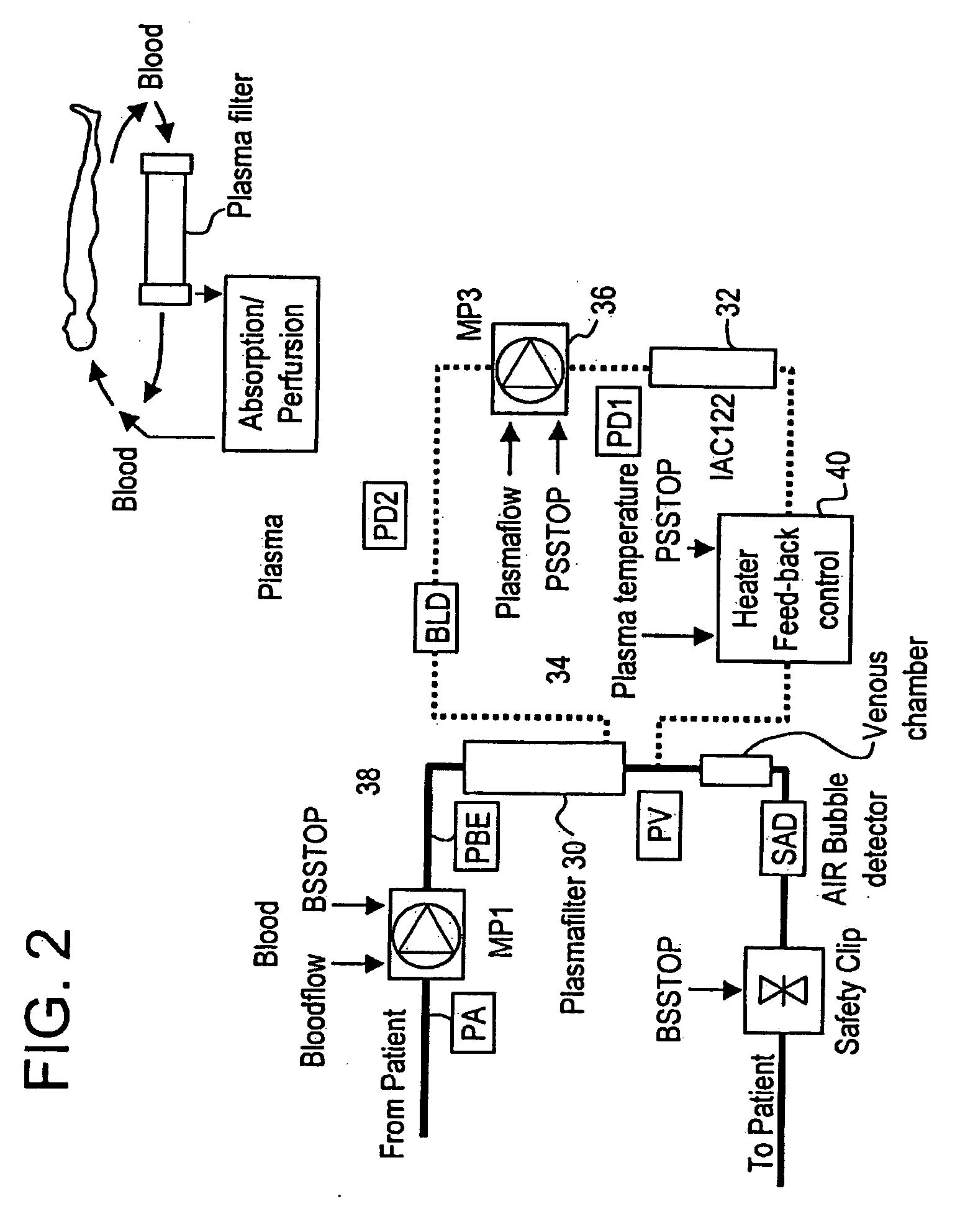
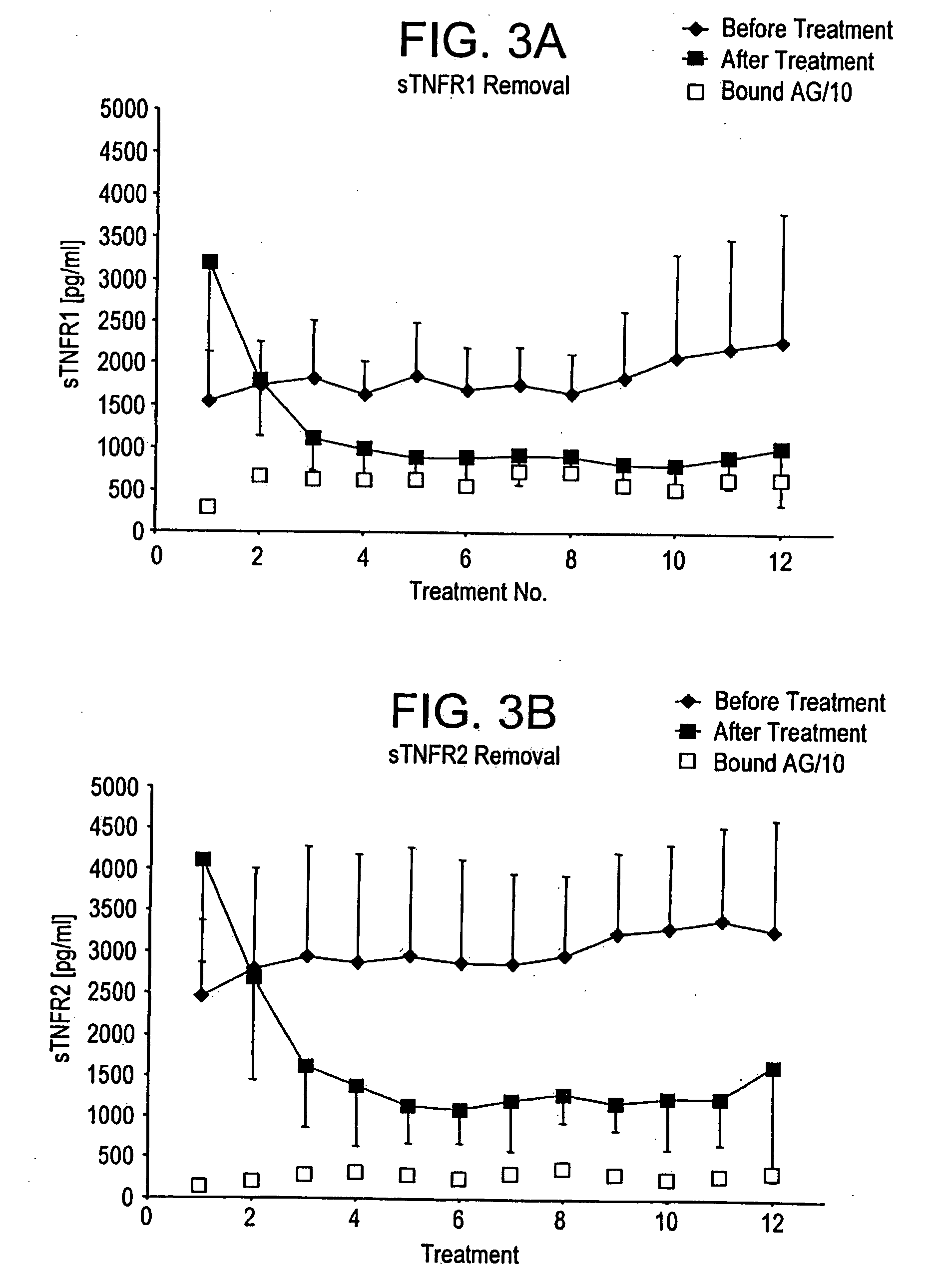
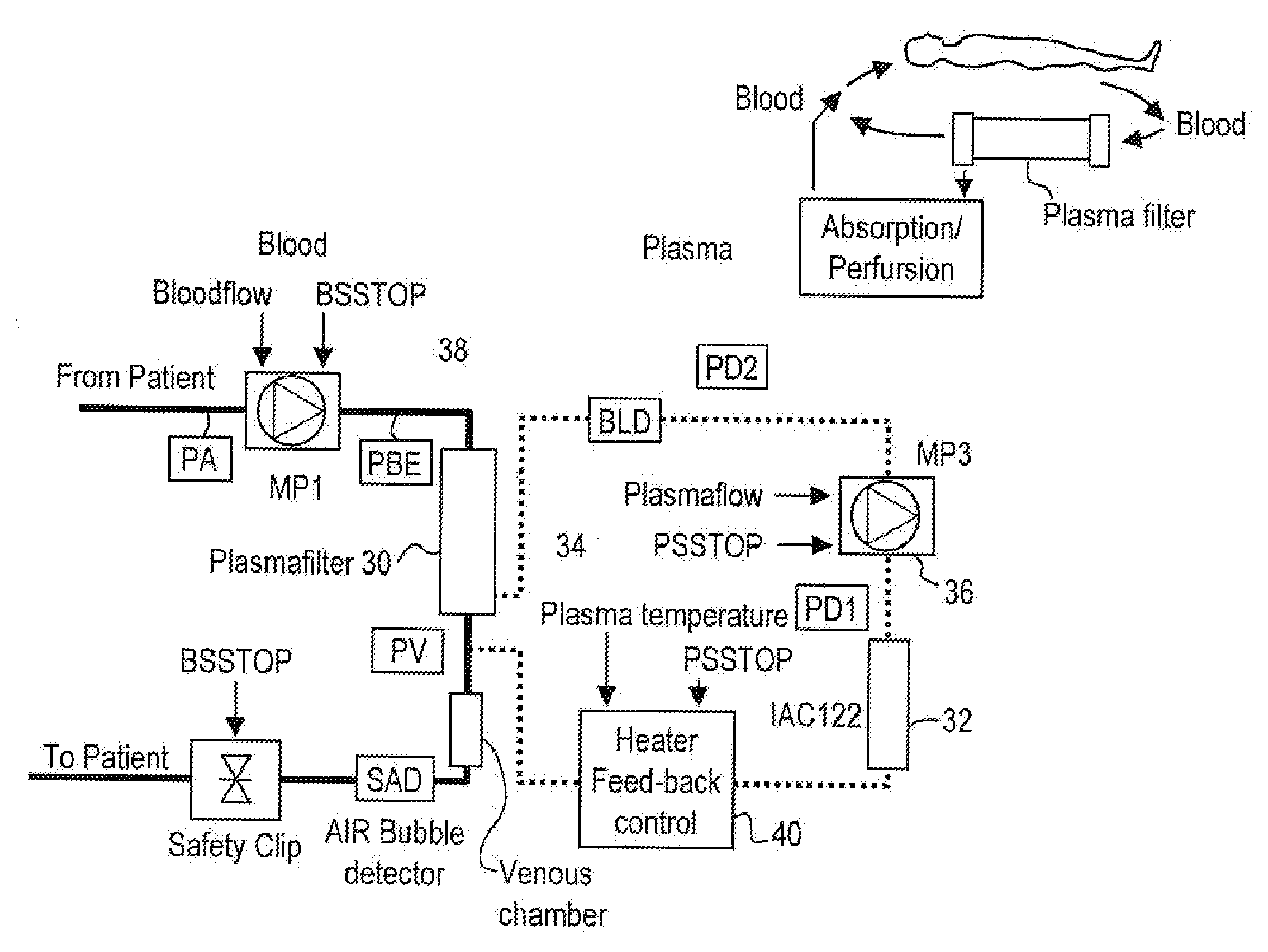
Method and system to remove soluble TNFR1, TNFR2, and IL2 in patients
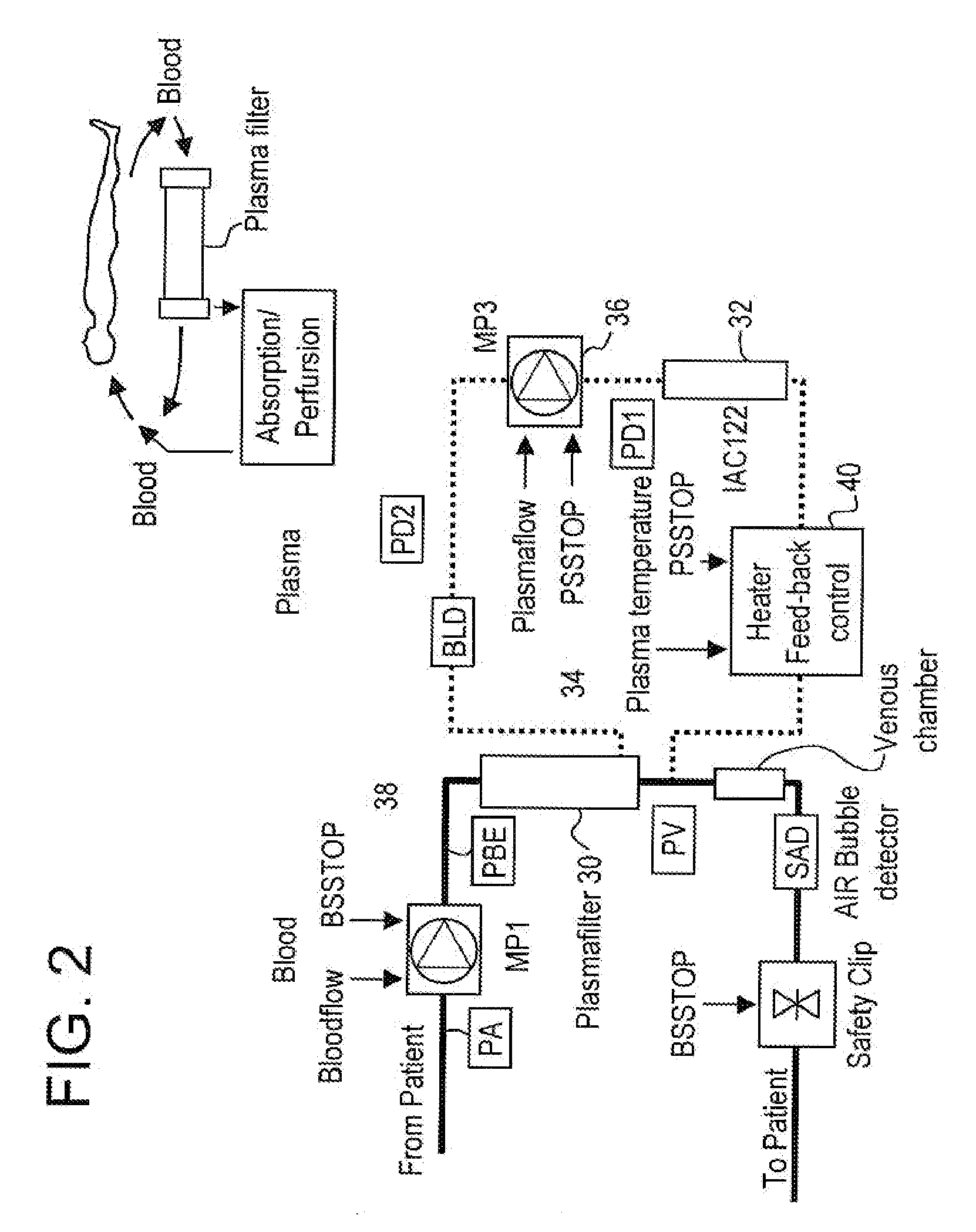
InactiveUS20050265996A1Induce remissionPeptide/protein ingredientsHaemofiltrationDiseaseAntiendomysial antibodies
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
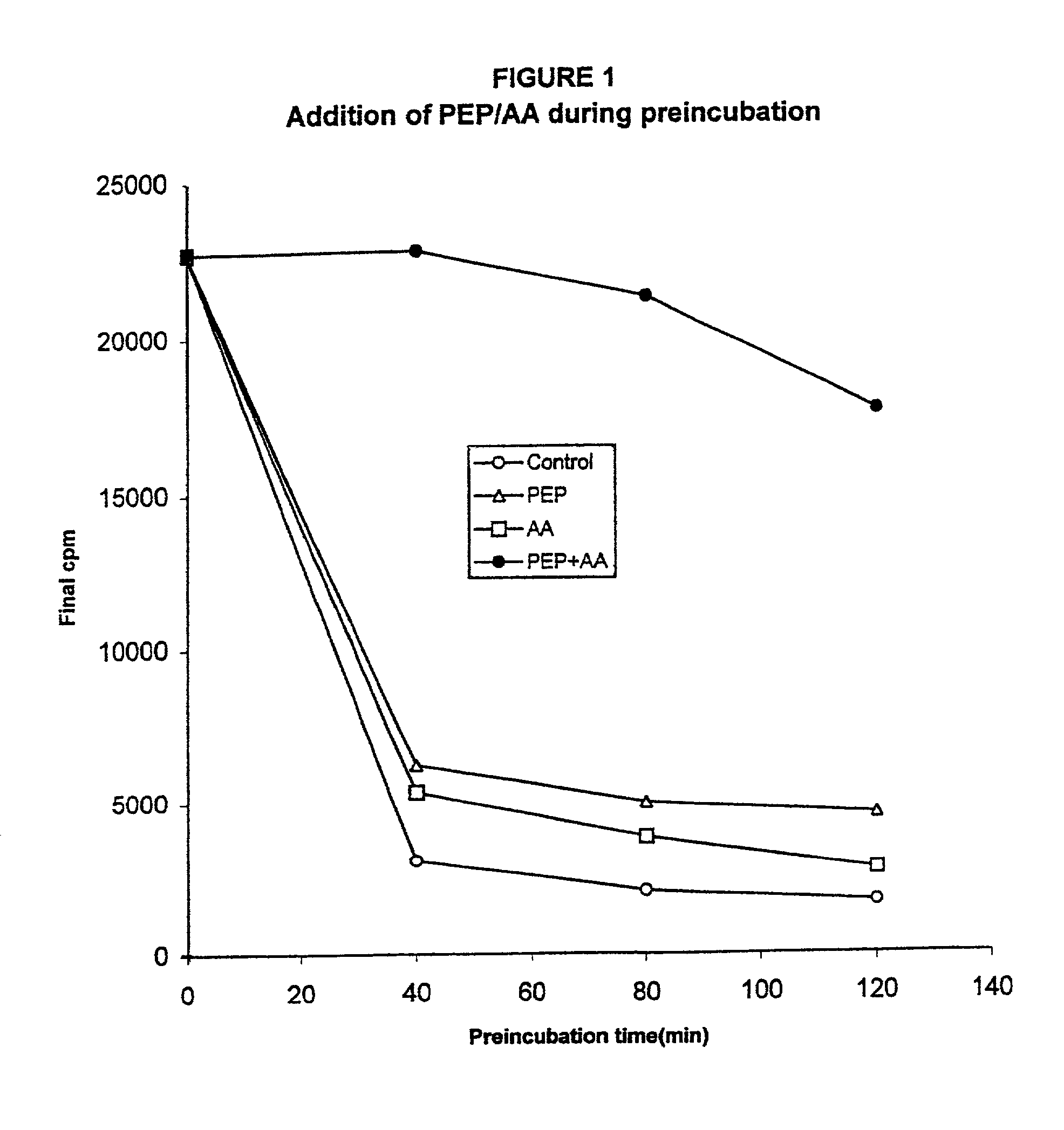
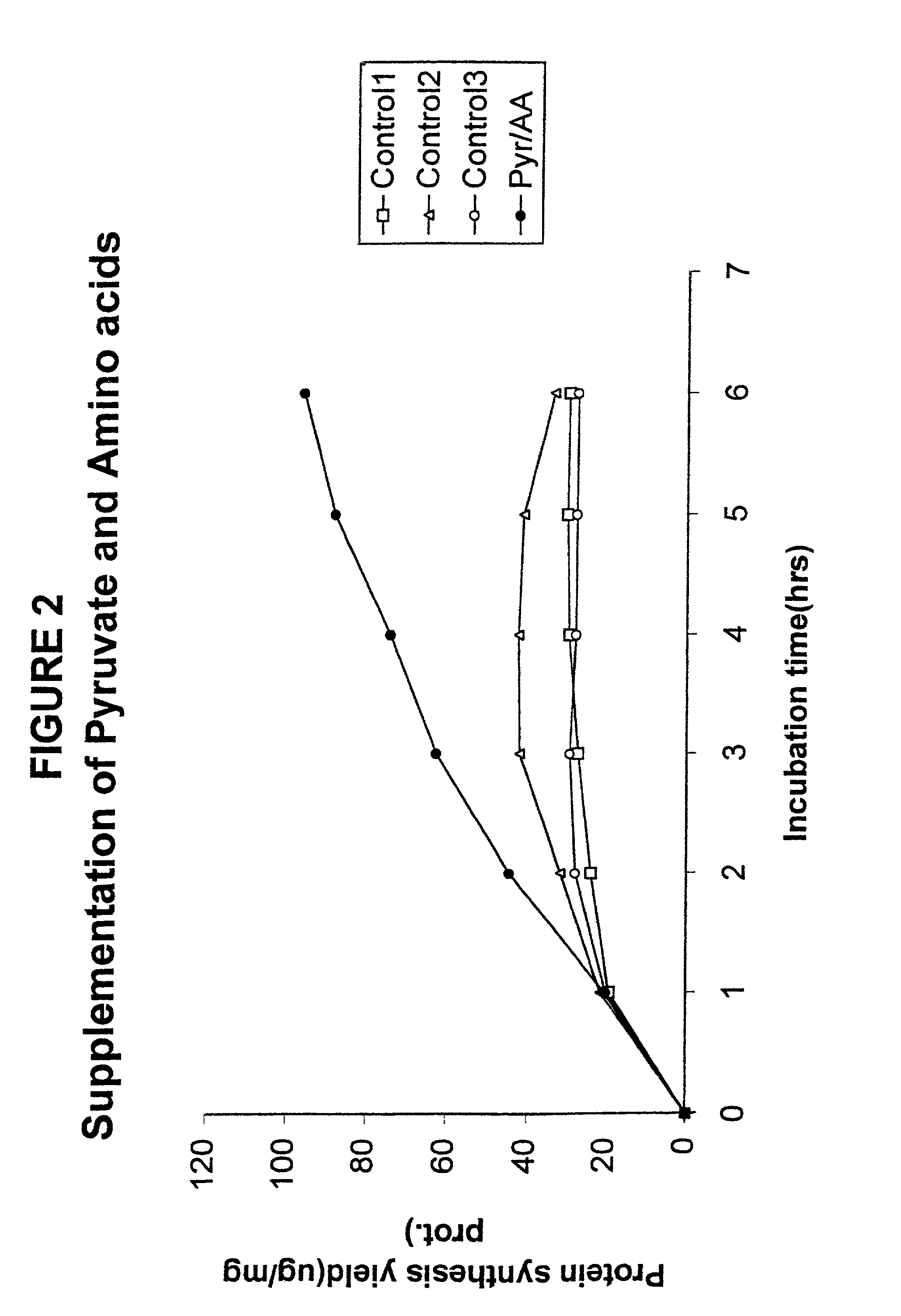
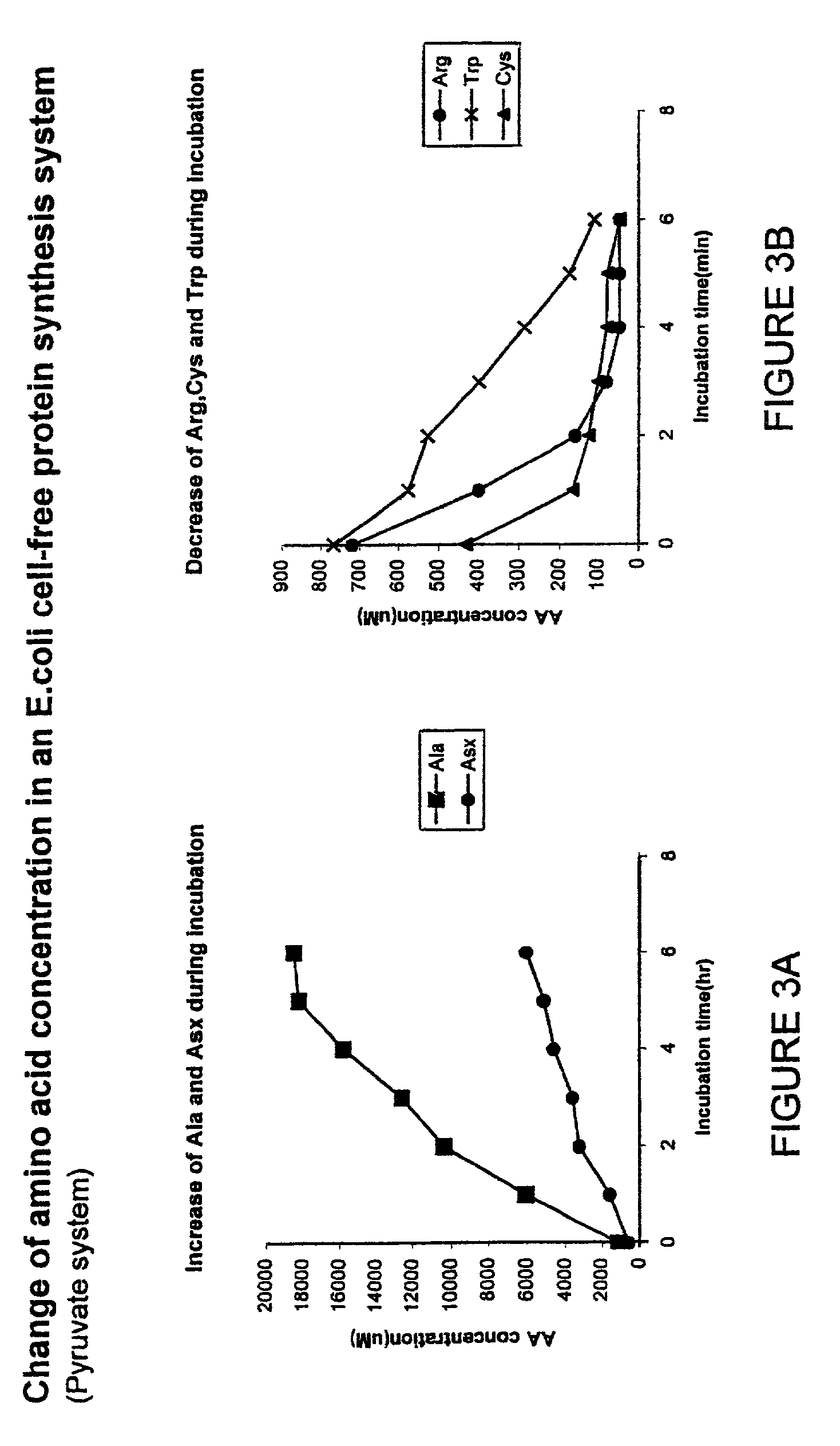
In vitro synthesis of polypeptides by optimizing amino acid metabolism
InactiveUS6994986B2Increasing protein synthesisDecrease and eliminate enzyme activityBacteriaTumor necrosis factorHigh-energy phosphateHomeostatic system
Compositions and methods are provided for the enhanced in vitro synthesis of polypeptides. In order to improve the performance of in vitro protein synthesis reactions, metabolic inhibitors, or manipulation of a source organism, is used to diminish or avoid the action of enzymes responsible for undesirable amino acids production or depletion. A homeostatic system may be used for production of ATP, where the required high energy phosphate bonds are generated in situ, e.g. through coupling with an oxidation reaction. The homeostatic energy source will typically lack high energy phosphate bonds itself, and will therefore utilize free phosphate in the reaction mix during generation of ATP. The homeostatic energy source is provided in combination with an enzyme that catalyzes the creation of high energy phosphate bonds and with an enzyme that can use that high energy phosphate bond to regenerate ATP.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Novel synthetic chimeric fusion transgene with immuno-therapeutic uses
InactiveUS20050053579A1Reducing tumorigenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsInterferon alphaWilms' tumor
The present invention relates to an immuno-therapy conjugate which comprises A-c-B wherein: A and B are different and are compounds selected from the group consisting of cytokines, chemokines, interferons, their respective receptors or a functional fragment thereof; and c is a linker consisting of a bond or an amino acid sequence containing from 1 to 100 residues. The present invention also relates to a vaccine adjuvant comprising the immuno-therapy conjugate of the present invention. The present invention further relates to a method of reducing tumor growth, for inhibiting a viral infection and for improving immune response in a patient.
Owner:GALIPEAU JACQUES +1
Chimeric activators: quantitatively designed protein therapeutics and uses thereof
ActiveUS20110274658A1Reduced cell-activating propertyAvoid and reduce unwanted side effectObesity gene productsPeptide/protein ingredientsChimerin ProteinsApoptosis
Aspects of the invention provide methods for harnessing the potential of proteins that occur naturally (e.g., in humans) and that have serious but finite toxicity. Aspects of the invention relate to a quantitative systems-biological and structural approach to design a class Mof chimeric proteins that avoid the toxicity of protein drugs while retaining their desired activities. In particular, chimeric proteins containing a variant form of a natural protein fused to a targeting moiety may be administered to a subject to target a signal (e.g., induction of apoptosis) to particular cells without having a generalized toxic effect
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Tumor necrosis factor-alpha mutants
The present invention has an object to provide a tumor necrosis factor mutant protein, particularly, a tumor necrosis factor mutant protein specific to TNF-R1 or TNF-R2; tumor necrosis factor inhibitor; or tumor necrosis factor preparation containing it as an effective ingredient, and the object is solved by providing a tumor necrosis factor mutant protein where one or more amino acid residues selected from the group consisting of 29th, 31st, 32nd, 145th, 146th and 147th, or the group consisting of 84th to 89th from the N-terminal of the amino acid sequence of SEQ ID NO:1 is / are replaced with other amino acid residue(s); a tumor necrosis factor inhibitor; and a tumor necrosis factor preparation containing it as an effective ingredient.
Owner:MAYUMI TADANORI +3
Apoptosis inducing molecule II and methods of use
The present invention relates to a novel member of the TNF-Ligand superfamily. More specifically, isolated nucleic acid molecules are provided encoding a human Apoptosis Inducing Molecule II (AIM II). AIM II polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists and antagonists of AIM II activity. Also provided are therapeutic methods for treating lymphadenopathy, aberrant bone development, autoimmune and other immune system diseases, graft versus host disease, rheumatoid arthritis, osteoarthritis and to inhibit neoplasia, such as tumor cell growth.
Owner:HUMAN GENOME SCI INC
Anti-idiotypic anti-TNF antibodies and related immunoassay methods
Anti-TNF antibodies and anti-TNF peptides, specific for tumor necrosis factor (TNF) are useful for in vivo diagnosis and therapy of a number of TNF-mediated pathologies and conditions, as well as polynucleotides coding for anti-TNF murine and chimeric antibodies, peptides, methods of making and using the antibody or peptides in immunoassays and immuno-therapeutic approaches are provided, where the anti-TNF peptide is selected from a soluble portion of TNF receptor, an anti-TNF antibody or structural analog thereof.
Owner:NEW YORK UNIV +1
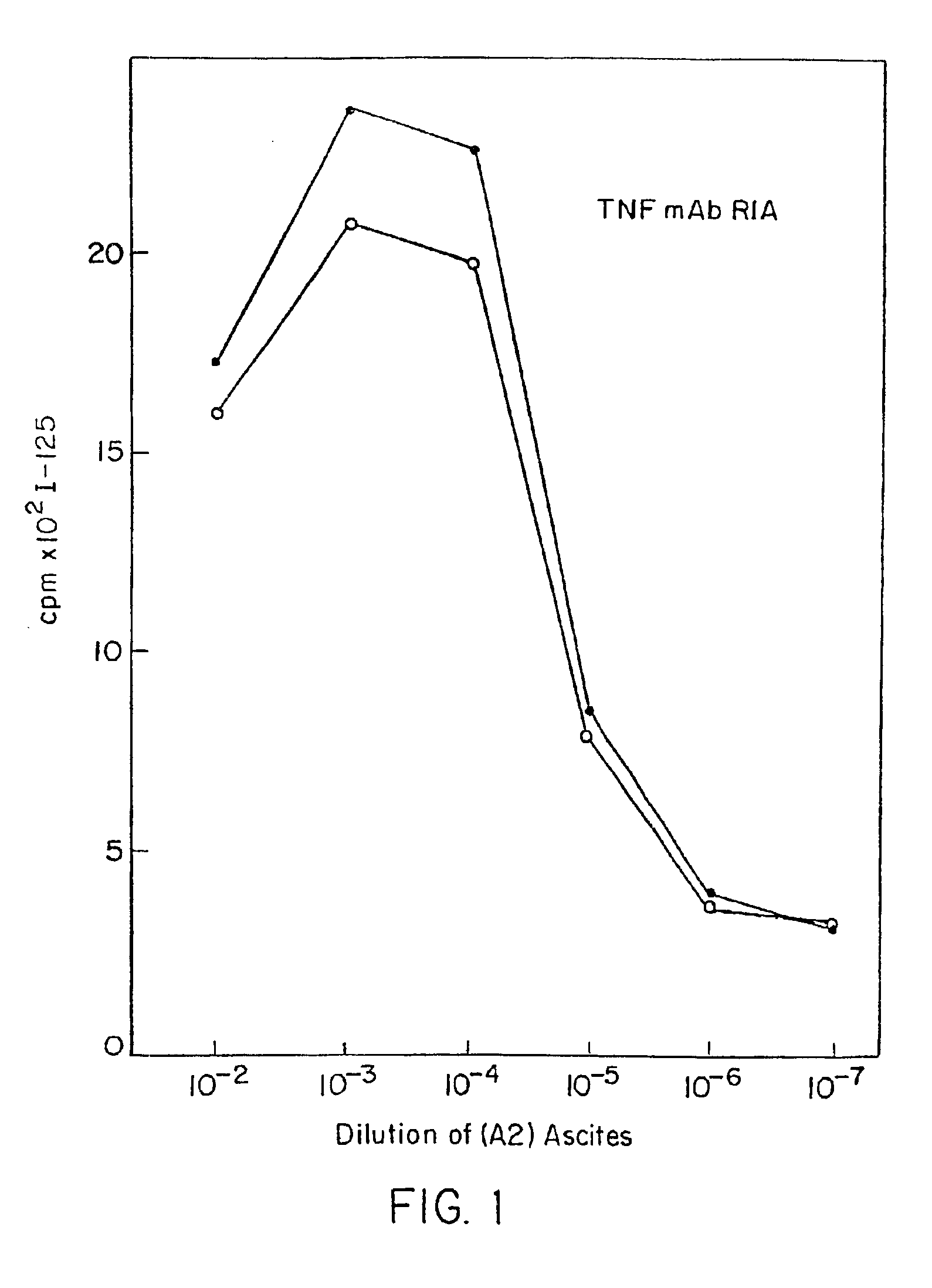
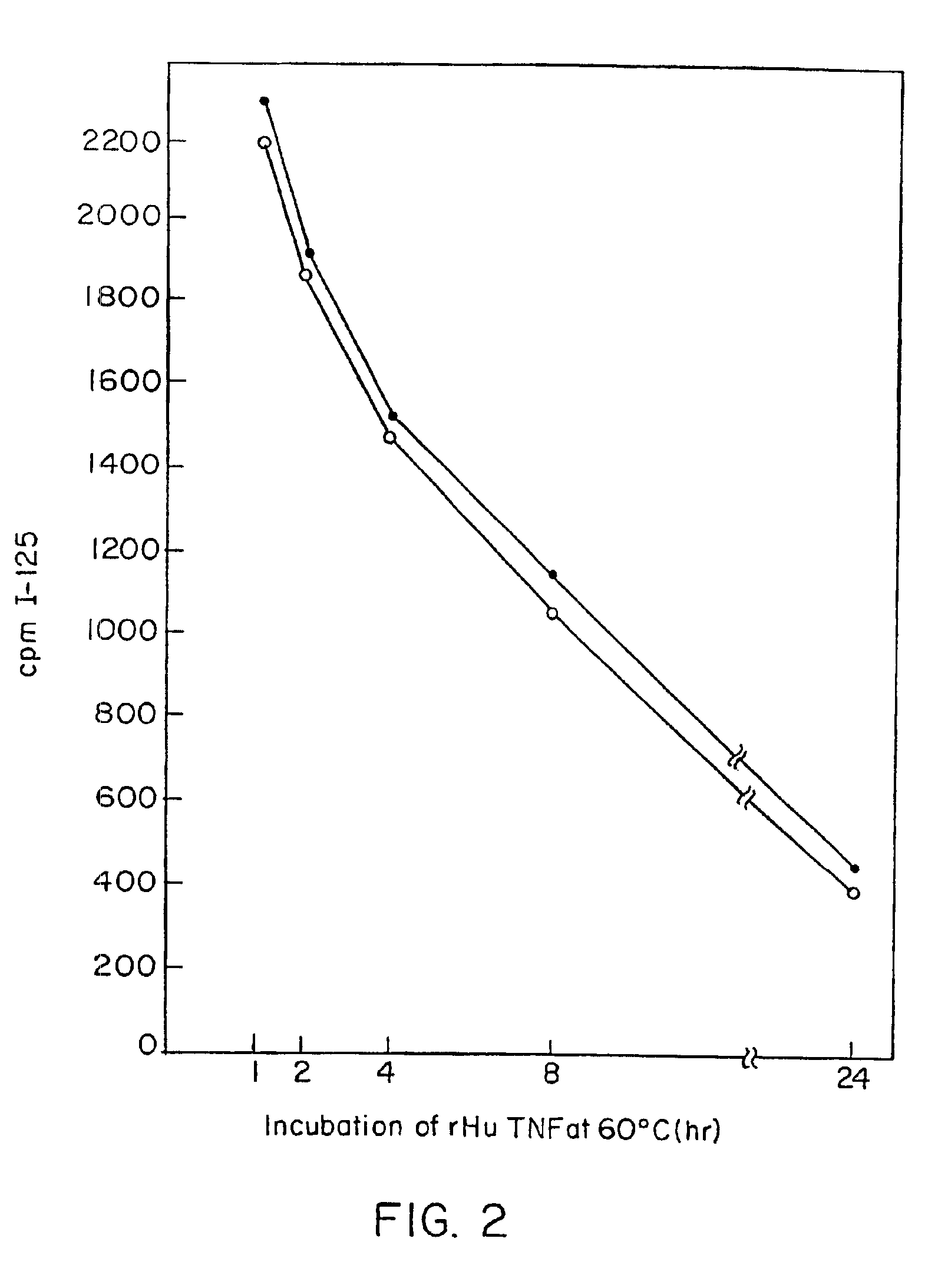
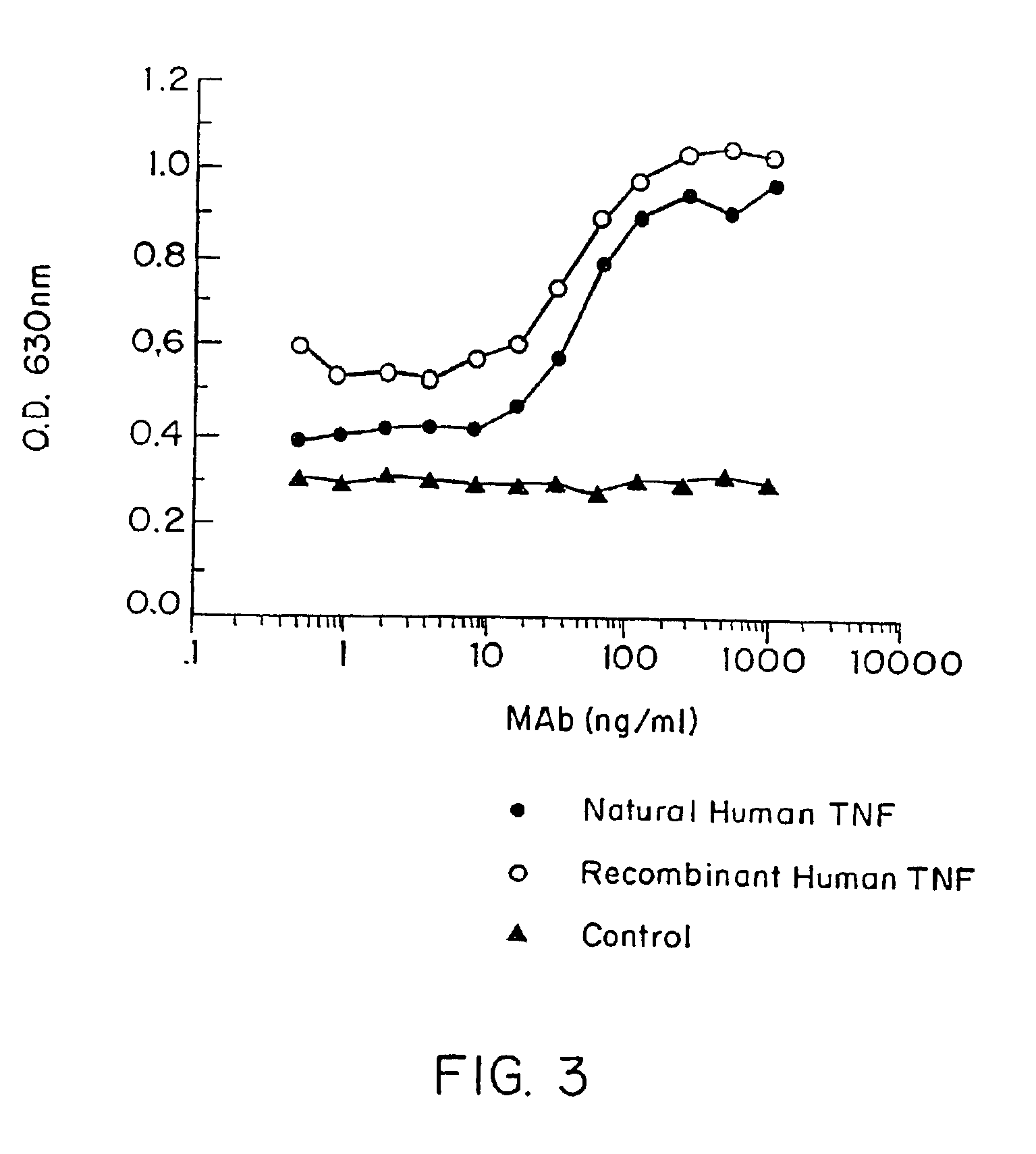
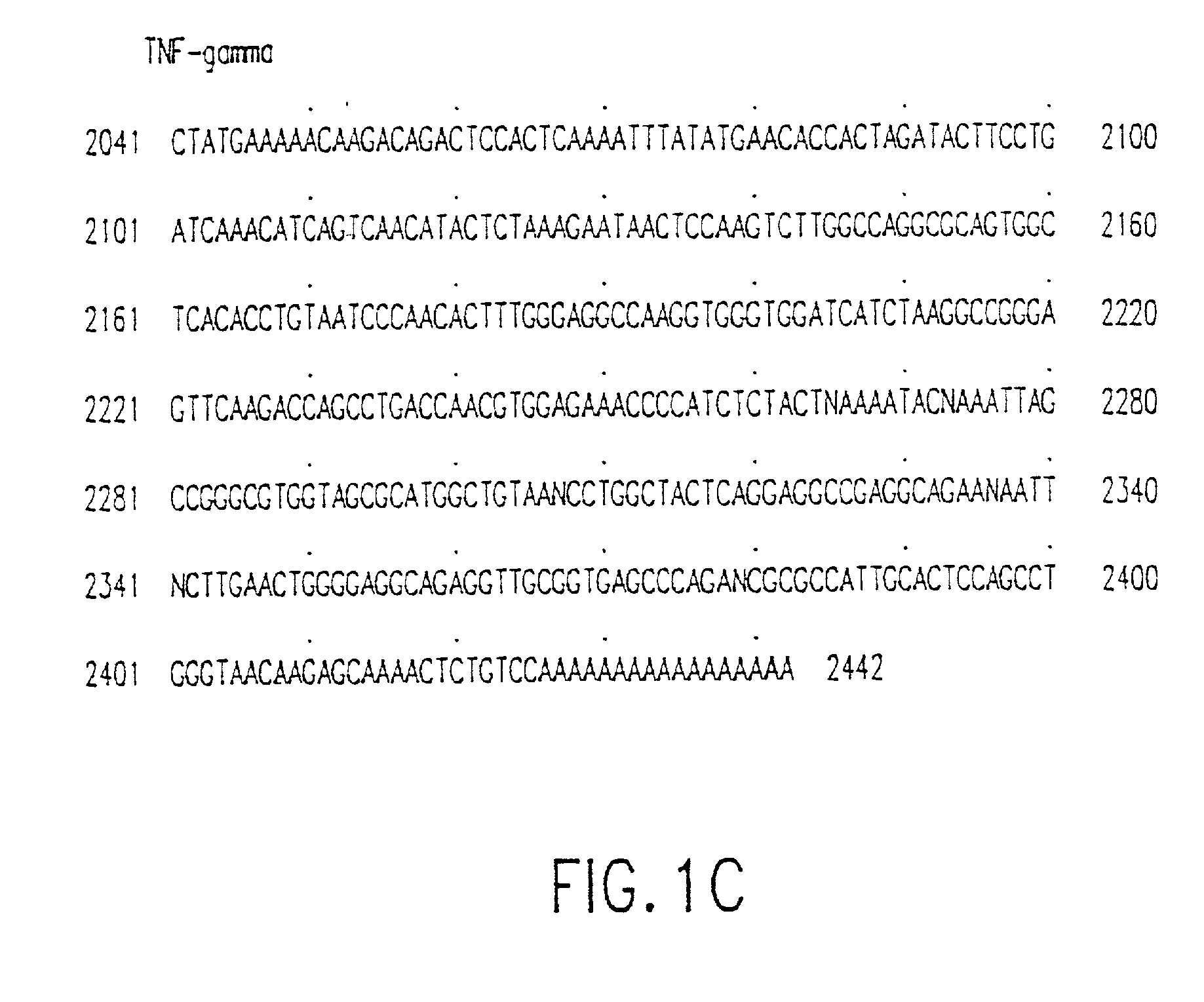
Tumor necrosis factor-gamma
InactiveUS7597886B2Increase blockingInduce inflammatory activityOrganic active ingredientsIn-vivo radioactive preparationsAbnormal tissue growthDisease
Human TNF-gamma-alpha and TNF-gamma-beta polypeptides and DNA (RNA) encoding such polypeptides and a procedure for producing such polypeptides by recombinant techniques are disclosed. Also disclosed are methods for utilizing such polypeptides to inhibit cellular growth, for example in a tumor or cancer, for facilitating wound-healing, to provide resistance against infection, induce inflammatory activities, and stimulating the growth of certain cell types to treat diseases, for example restenosis. Also disclosed are diagnostic methods for detecting a mutation in the TNF-gamma-alpha and TNF-gamma-beta nucleic acid sequences or overexpression of the TNF-gamma-alpha and / or TNF-gamma-beta polypeptides. Antagonists against such polypeptides and their use as a therapeutic to treat cachexia, septic shock, cerebral malaria, inflammation, arthritis and graft-rejection are also disclosed.
Owner:HUMAN GENOME SCI INC
Enhancing the circulating half-life of antibody-based fusion proteins
InactiveUS20060034836A1Increased serum half-lifeHigh affinityCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsLymphatic SpreadMammal
Disclosed are compositions and methods for enhancing the circulating half-life of antibody-based fusion proteins. Disclosed methods and compositions rely on altering the amino acid sequence of the junction region between the antibody moiety and the fused protein moiety in an antibody-based fusion protein. An antibody-based fusion protein with an altered amino acid sequence in the junction region has a greater circulating half-life when administered to a mammal. Disclosed methods and compositions are particularly useful for reducing tumor size and metastasis in a mammal.
Owner:MERCK PATENT GMBH
TNF super family members with altered immunogenicity
InactiveUS20060014248A1Low immunogenicitySugar derivativesTumor necrosis factorRANKL ProteinAPRIL Protein
The present invention relates to non-naturally occurring variant Tumor Necrosis Factor Super Family member proteins with reduced immunogenicity. More specifically, the present invention relates to variant BAFF, RANKL, TRAIL, CD40L and APRIL proteins with reduced immunogenicity.
Owner:XENCOR
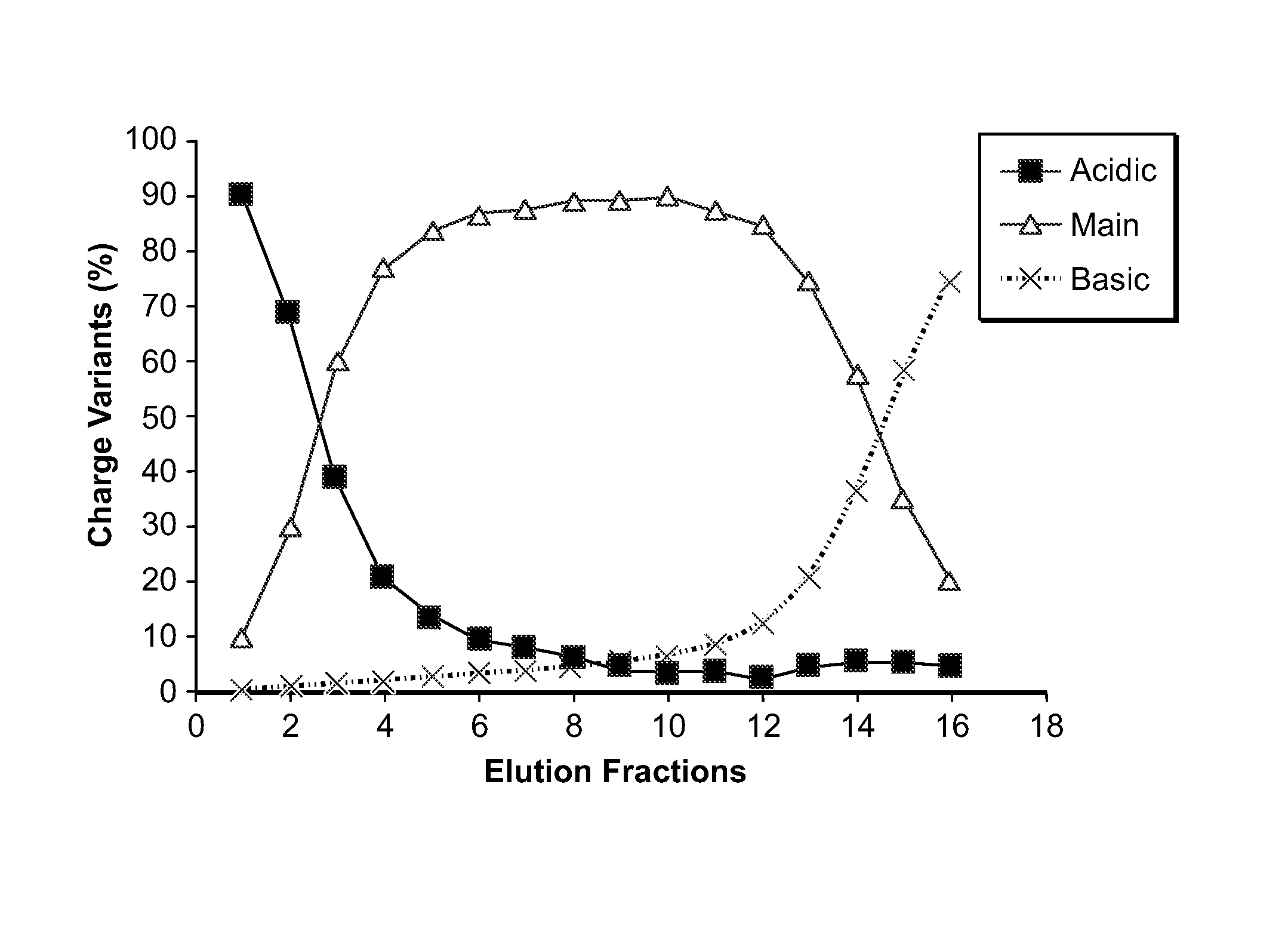
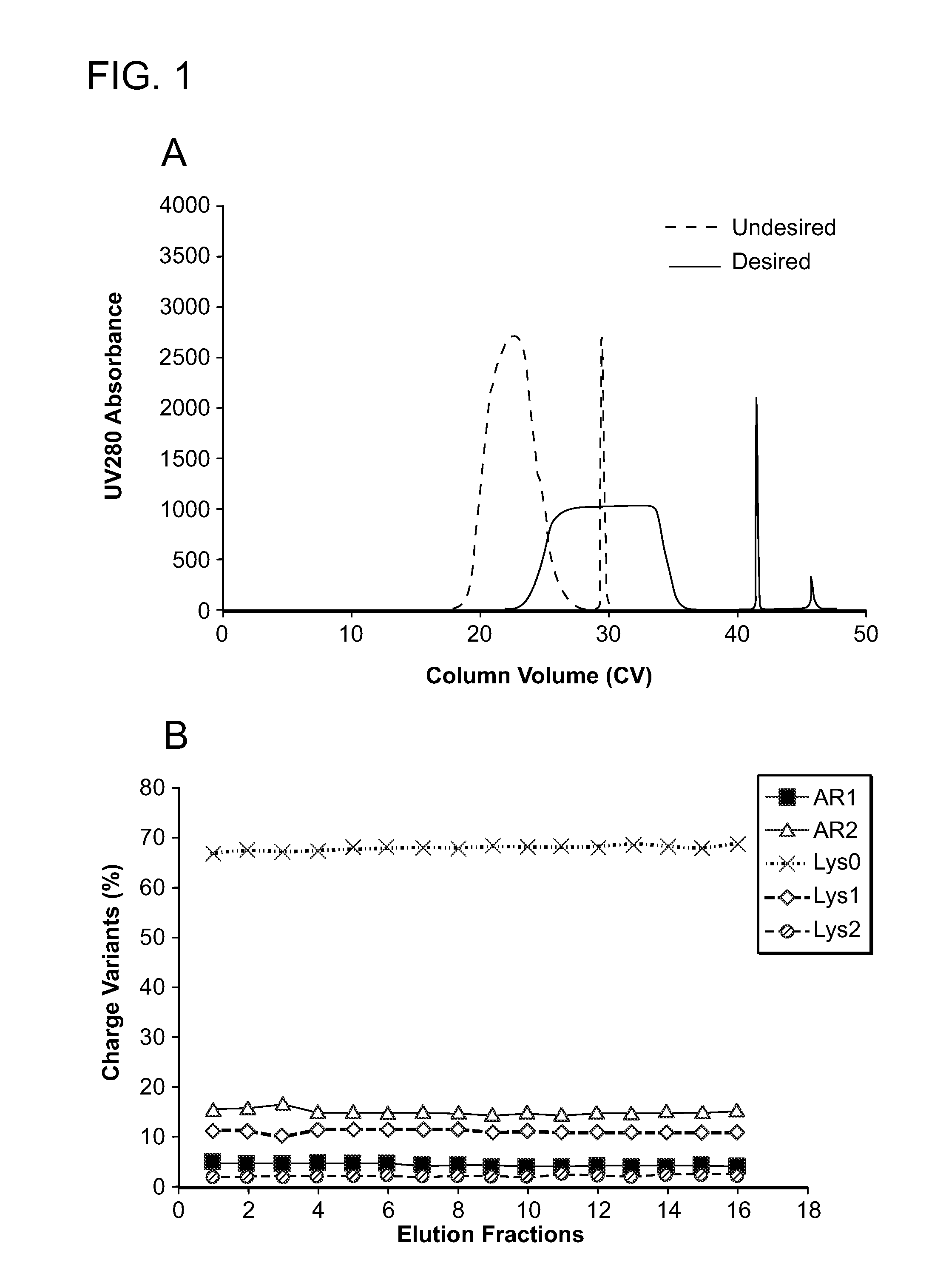
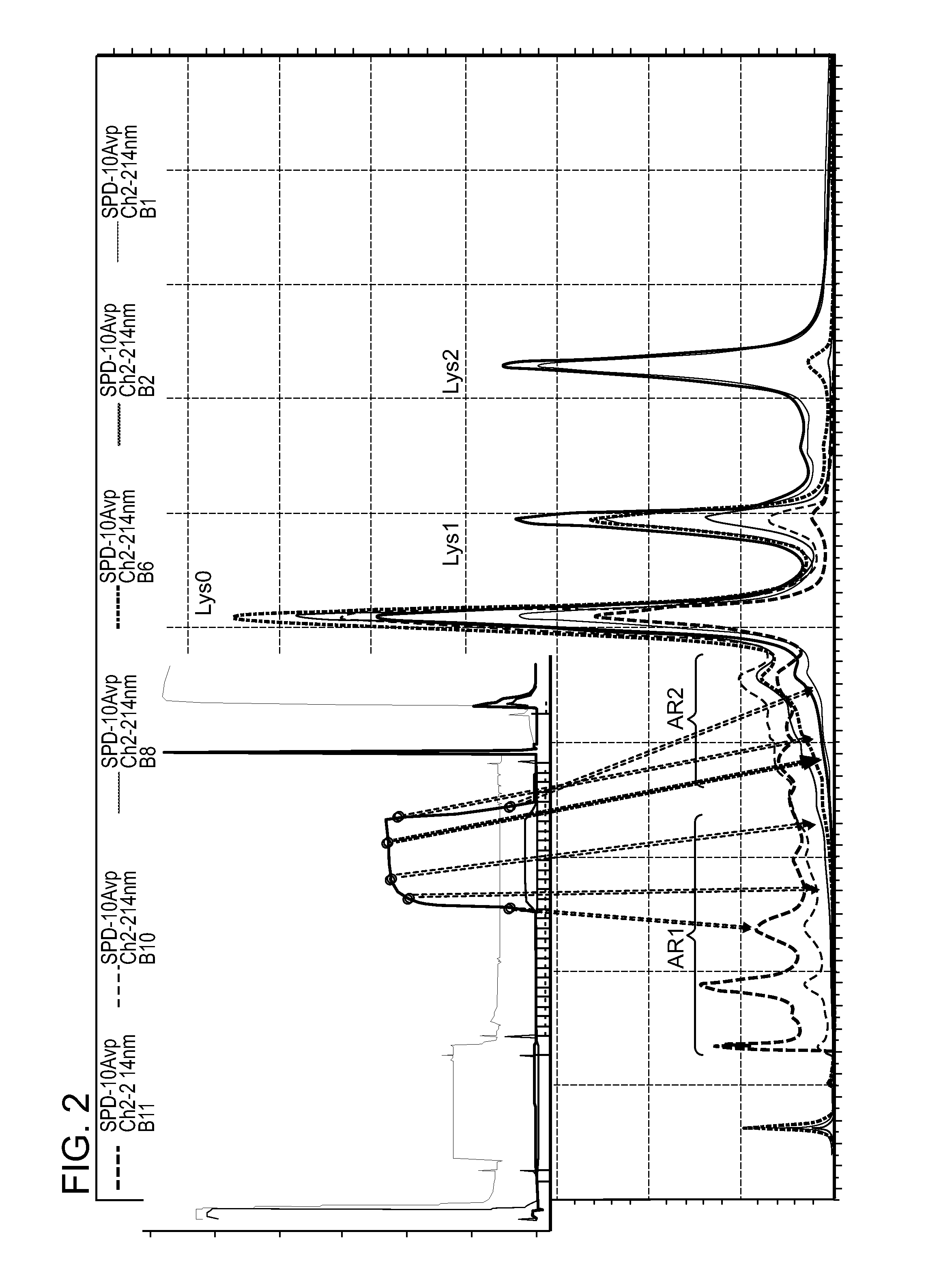
Low acidic species compositions and methods for producing and using the same using displacement chromatography
ActiveUS9017687B1Good curative effectGood biological propertiesTumor necrosis factorImmunoglobulins against cytokines/lymphokines/interferonsAntigen bindingDisplacement chromatography
The present invention relates to low acidic species (AR) compositions comprising a protein, e.g., an antibody, or antigen-binding portion thereof, and methods for producing such low AR compositions using displacement chromatography. Methods for using such compositions to treat a disorder, e.g., a disorder in which TNFα is detrimental, are also provided.
Owner:ABBVIE INC
Anti-lymphotoxin-beta receptor antibodies as anti-tumor agents
InactiveUS20050281811A1Increases the percentage of tumor cells killedPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthCross-link
This invention relates to compositions and methods useful for activating LT-β receptor signaling, which in turn elicits potent anti-proliferative effects on tumor cells. More particularly, this invention relates to lymphotoxin heteromeric complexes formed between lymphotoxin-α and multiple subunits of lymphotoxin-β, which induce cytotoxic effects on tumor cells in the presence of lymphotoxin-β receptor activating agents. Also within the scope of this invention are antibodies directed against the lymphotoxin-β receptor which act as lymphotoxin-β receptor activating agents alone or in combination with other lymphotoxin-β receptor activating agents either in the presence or absence of lymphotoxin-α / β complexes. A screening method for selecting such antibodies is provided. This invention also relates to compositions and methods using cross-linked anti-lymphotoxin-β receptor antibodies either alone or in the presence of other lymphotoxin-β receptor activating agents to potentiate tumor cell cytotoxicity.
Owner:BIOGEN MA INC
Anti-TNF antibodies and peptides of human tumor necrosis factor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-alpha (TNFalpha) and are useful in vivo diagnosis and therapy of a number of TNFalpha-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:CENTOCOR +1
Protein based tumor necrosis factor-receptor variants for the treatment of TNF related disorders
The invention relates to novel proteins with TNF-receptor antagonist activity and nucleic acids encoding these proteins. The invention further relates to TNF-receptor proteins with reduced immunogenicity and the use of these novel proteins in the treatment of TNF related disorders.
Owner:XENCOR INC
Enhanced adoptive cell therapy
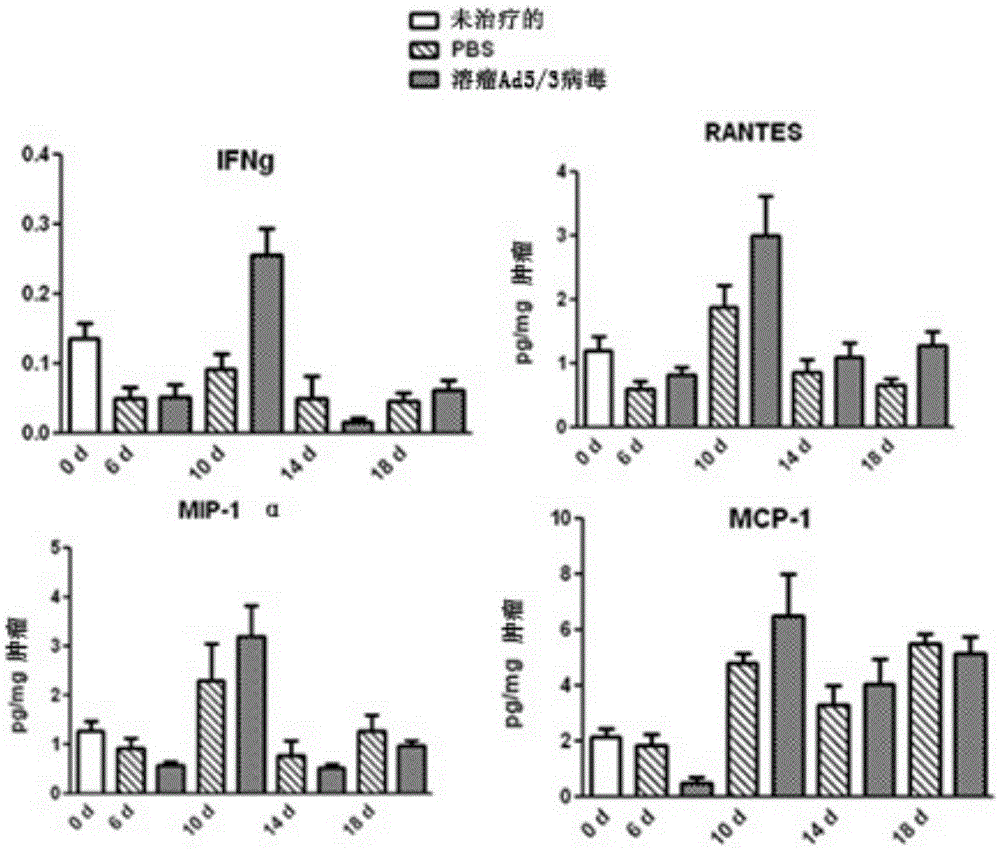
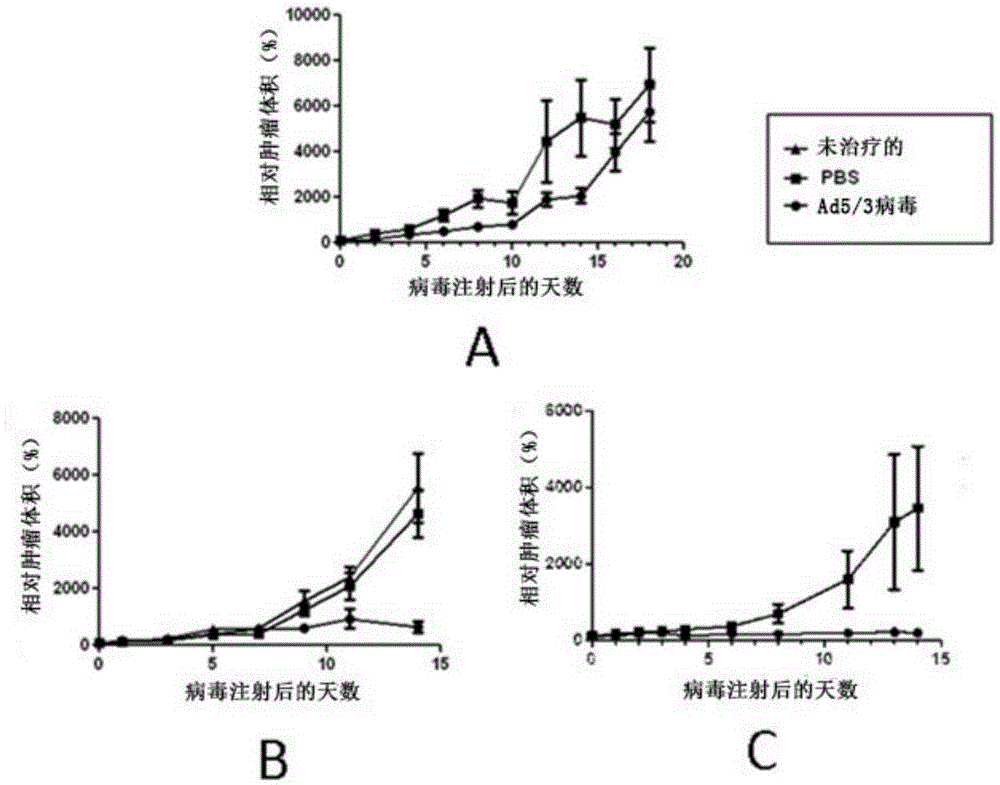
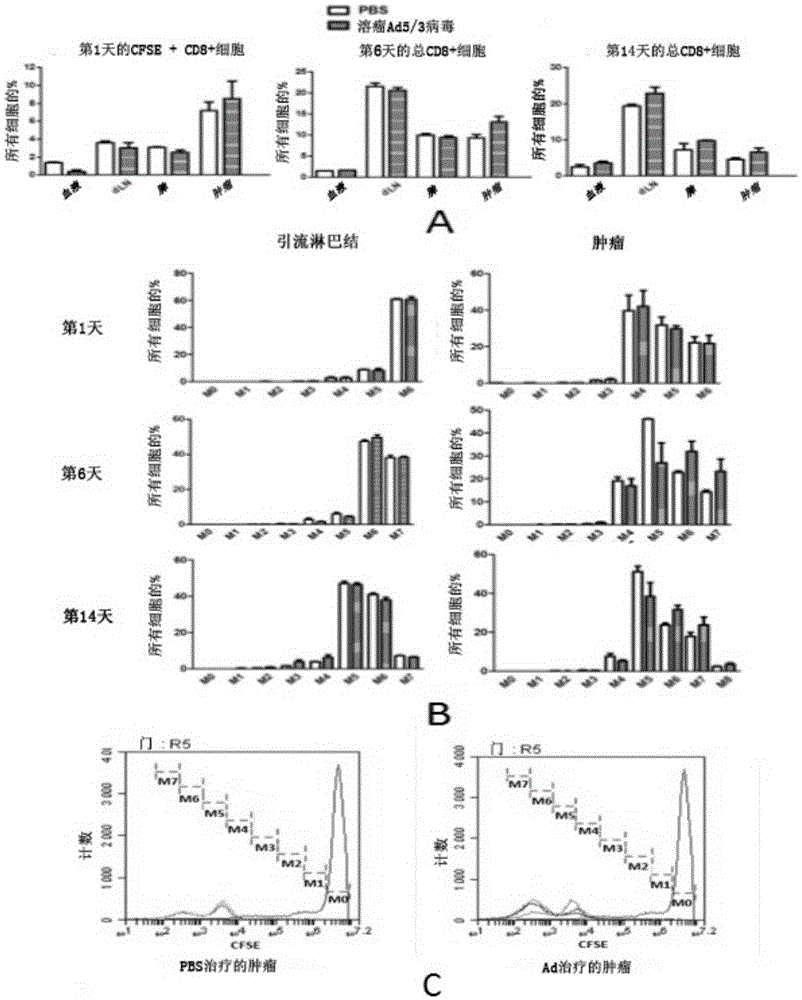
ActiveCN105307671AEnhance the popular effectEasy to handlePeptide/protein ingredientsGenetic material ingredientsAdoptive cellular therapyOncolytic adenovirus
The present invention relates to the fields of life sciences and medicine. Specifically, the invention relates to cancer therapies of humans. More specifically, the present invention relates to oncolytic adenoviral vectors alone or together with therapeutic compositions for therapeutic uses and therapeutic methods for cancer. In one aspect the present invention relates to separate administration of adoptive cell therapeutic composition and oncolytic adenoviral vectors. Furthermore, the present invention relates to a pharmaceutical kit and a pharmaceutical composition, both utilizing oncolytic adenoviral vectors.
Owner:TILT BIOTHERAPEUTICS OY
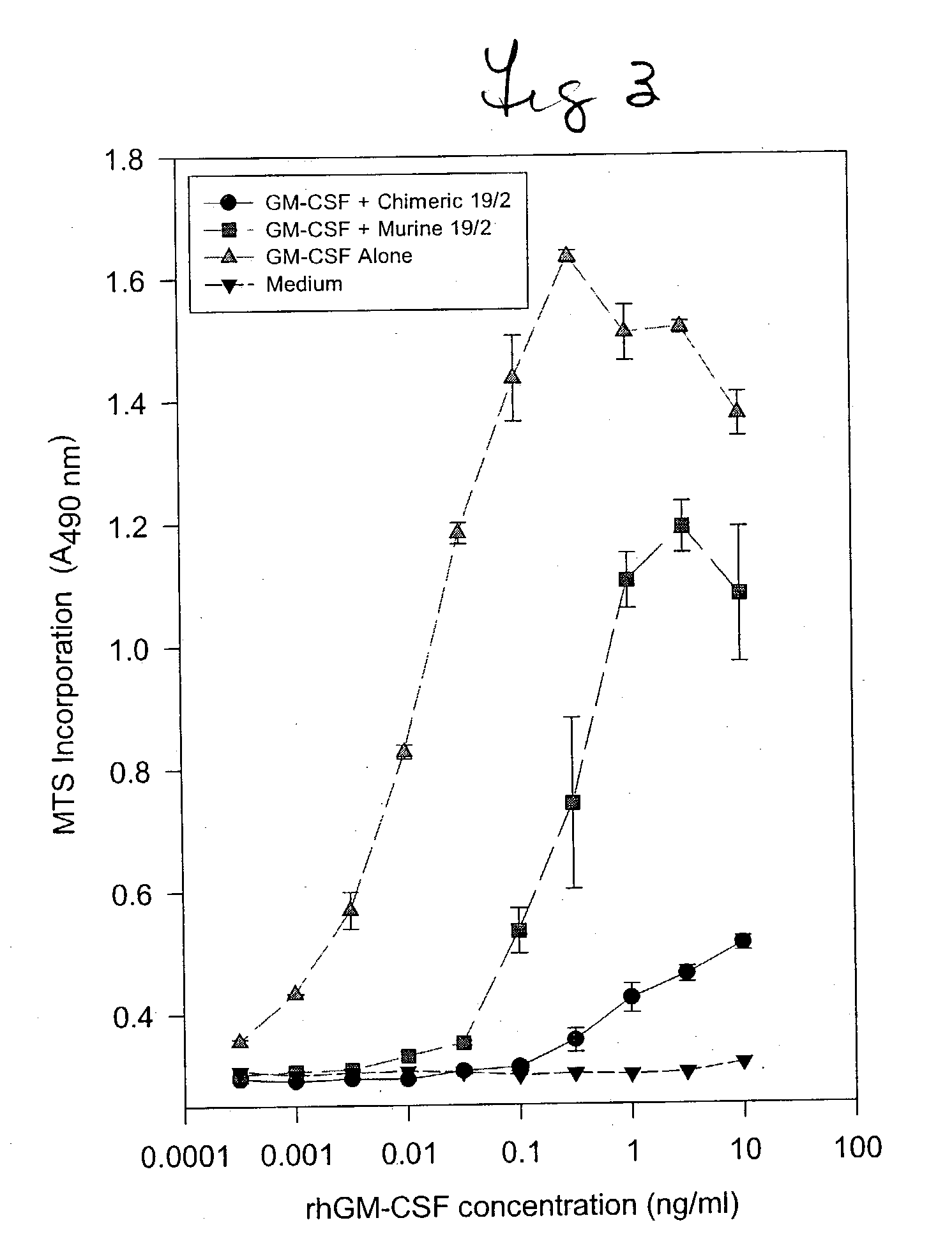
Humanized GM-CSF antibodies
Chimeric antibodies, as well as fusion proteins which comprise chimeric antibodies, are disclosed. The antibodies bind to GM-CSF, CD-30, and G250 antigen. The fusion proteins include biologically active portions of tumor necrosis factor, or full length tumor necrosis factor. Expression vectors adapted for production of the antibodies, as well as methods for manufacturing these, are also disclosed.
Owner:LUDWIG INST FOR CANCER RES
Therapeutic regimen for treating cancer
The invention provides a method for treating cancer in a human comprising (a) administering to the human a dose of a pharmaceutical composition comprising (i) a pharmaceutically acceptable carrier and (ii) an adenoviral vector comprising a nucleic acid sequence encoding a human TNF-α and operably linked to a promoter, wherein the dose comprises about 4×107 to about 4×1012 particle units (pu) of adenoviral vector, at least once in a therapeutic period comprising up to about 10 weeks, (b) administering a dose of ionizing radiation to the human over the duration of the therapeutic period, and (c) administering a dose of one or more chemotherapeutics to the human over the duration of the therapeutic period, whereby the cancer in the human is treated.
Owner:GEN VEC INC
Novel variants of CD40L protein
InactiveUS20050181994A1Reduce the binding forceSafe and effectivePeptide/protein ingredientsAntibody mimetics/scaffoldsGeneticsAgonist
The present invention relates to soluble, recombinant CD40L variant proteins that may be expressed solubly in E. coli. The variants of the present invention may substantially reduced binding to alpha IIb-beta3 integrin, act as CD40L antagonists or agonists, and methods for generating the same.
Owner:XENCOR
Colloidal metal compositions and methods
The present invention comprises compositions and methods for delivery systems of agents, including therapeutic compounds, pharmaceutical agents, drugs, detection agents, nucleic acid sequences and biological factors. In general, these vector compositions comprise a colloidal metal, derivatized PEG (polyethylene glycol) and an agent. The invention also comprises methods and compositions for making such colloidal metal compositions and for treatment of cancer.
Owner:CYTIMMUNE SCI
Increased recovery of active proteins
InactiveUS7157557B2Raise the ratioFold preciselySugar derivativesPeptide/protein ingredientsSufficient timeActive protein
The invention provides methods of increasing yields of desired conformation of proteins. In particular embodiments, the invention includes contacting preparations of a recombinant protein with a reduction / oxidation coupling reagent for a time sufficient to increase the relative proportion of a desired configurational isomer.
Owner:IMMUNEX CORP
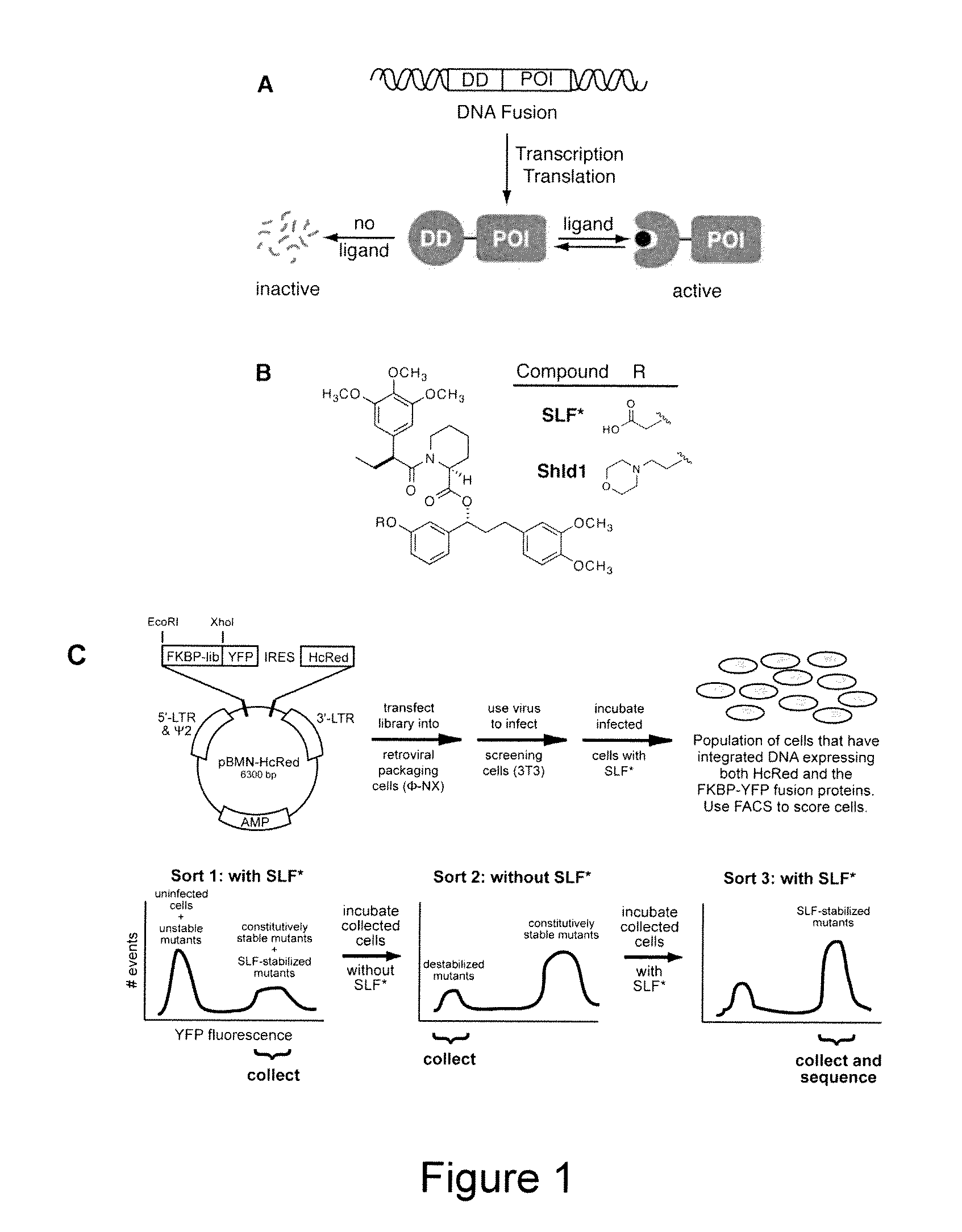
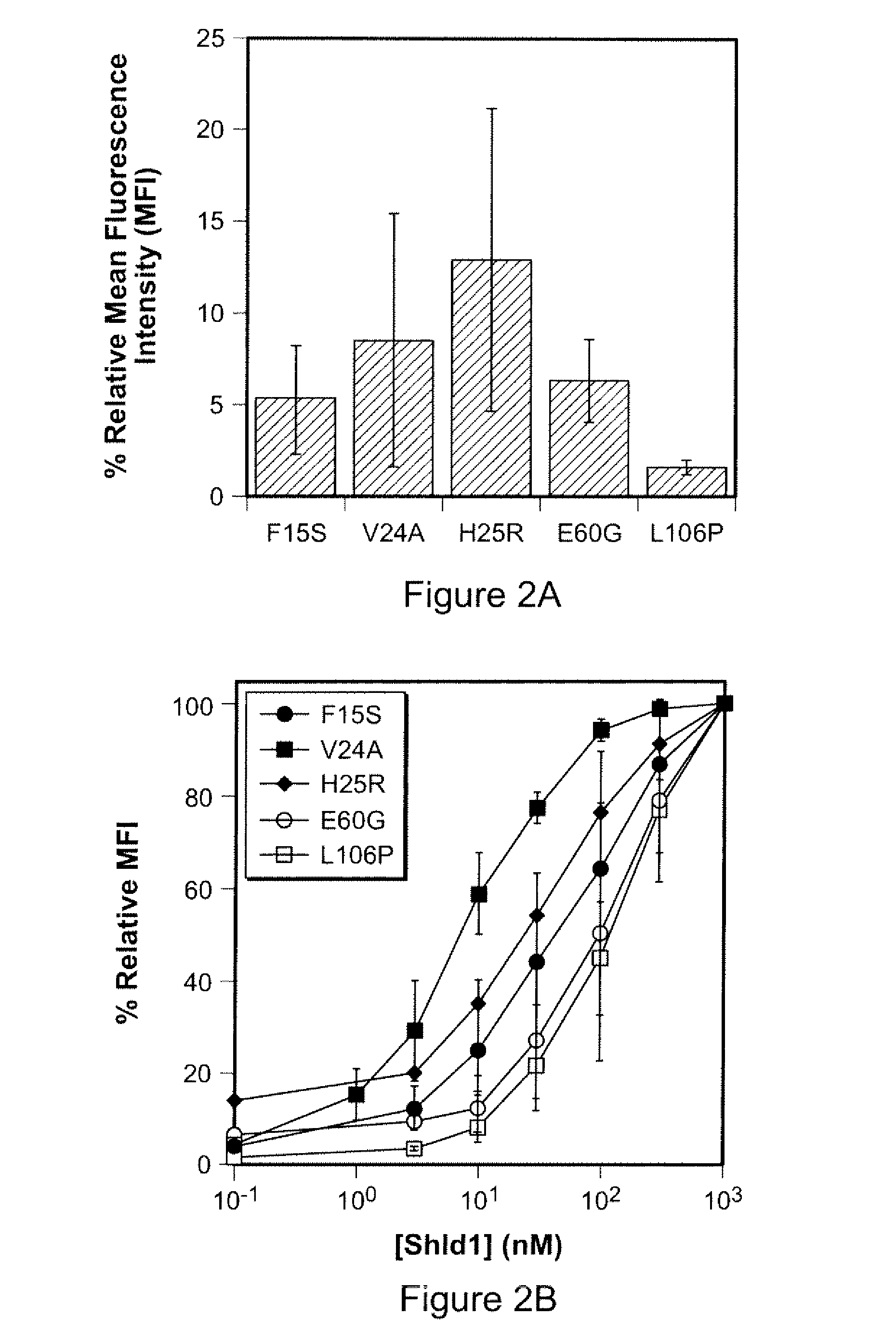
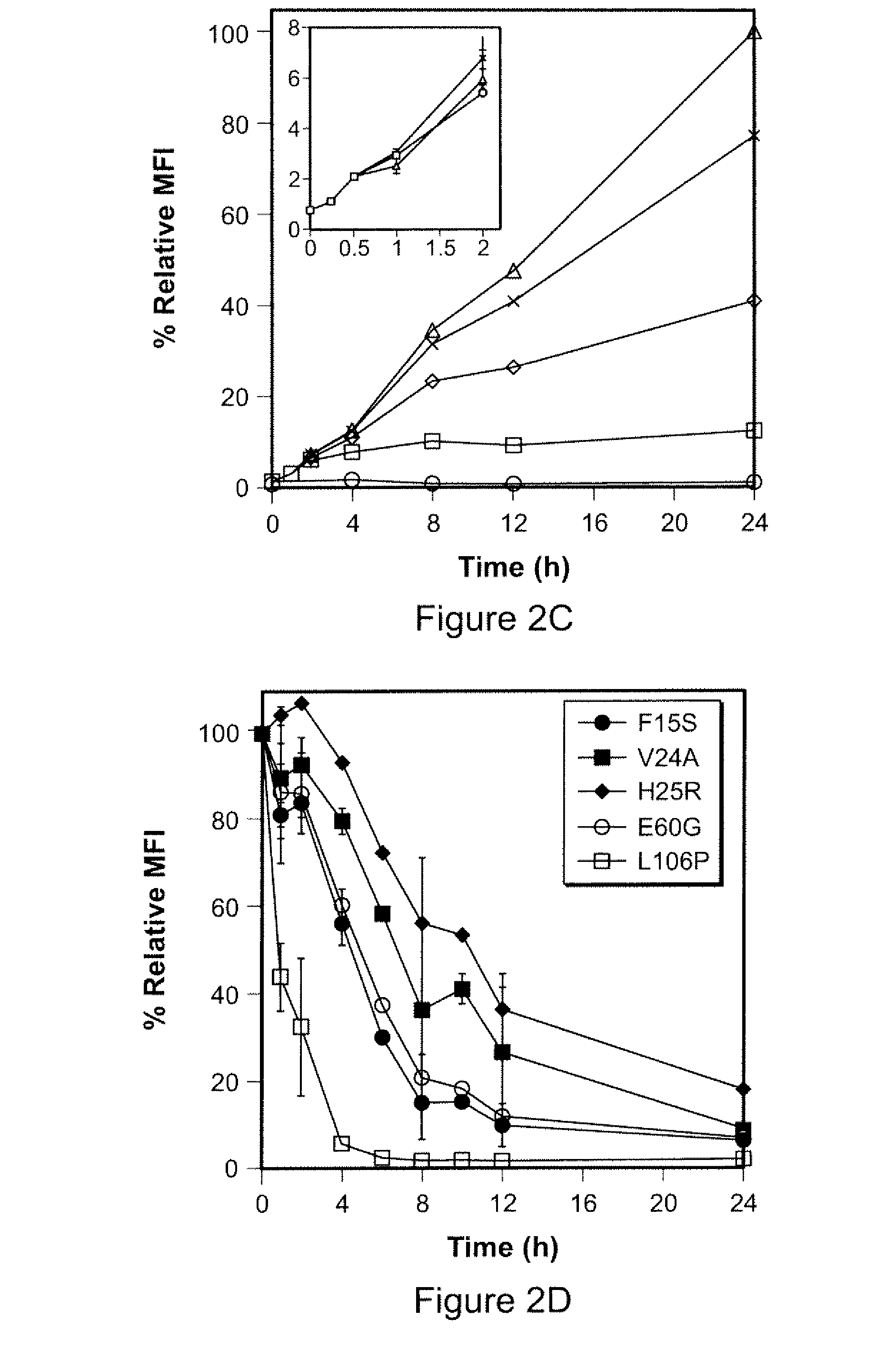
Method for regulating protein function in cells in vivo using synthetic small molecules
ActiveUS8530636B2Improve survivalInhibit tumor growthOrganic active ingredientsBiocideIn vivoProtein function
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method and system to remove soluble tnfr1, tnfr2, and il2 in patients
A method, and system, to induce remission in diseases characterized by excess production of sTNR and interleukin 2 has been developed. In the most preferred embodiment, the system consists of antibodies to sTNFR1, sTNFR2 and sIL2R immobilized in a column containing a material such as SEPHAROSE™. The patient is connected to a pheresis machine which separates the blood into the plasma and red cells, and the plasma is circulated through the column until the desired reduction in levels of sTNFR1, sTNFR2, and IL2 is achieved, preferably to less than normal levels. In the preferred method, patients are treated three times a week for four weeks. This process can be repeated after a period of time. Clinical studies showed reduction in tumor burden in patients having failed conventional chemotherapy and radiation treatments.
Owner:INNATUS CORP
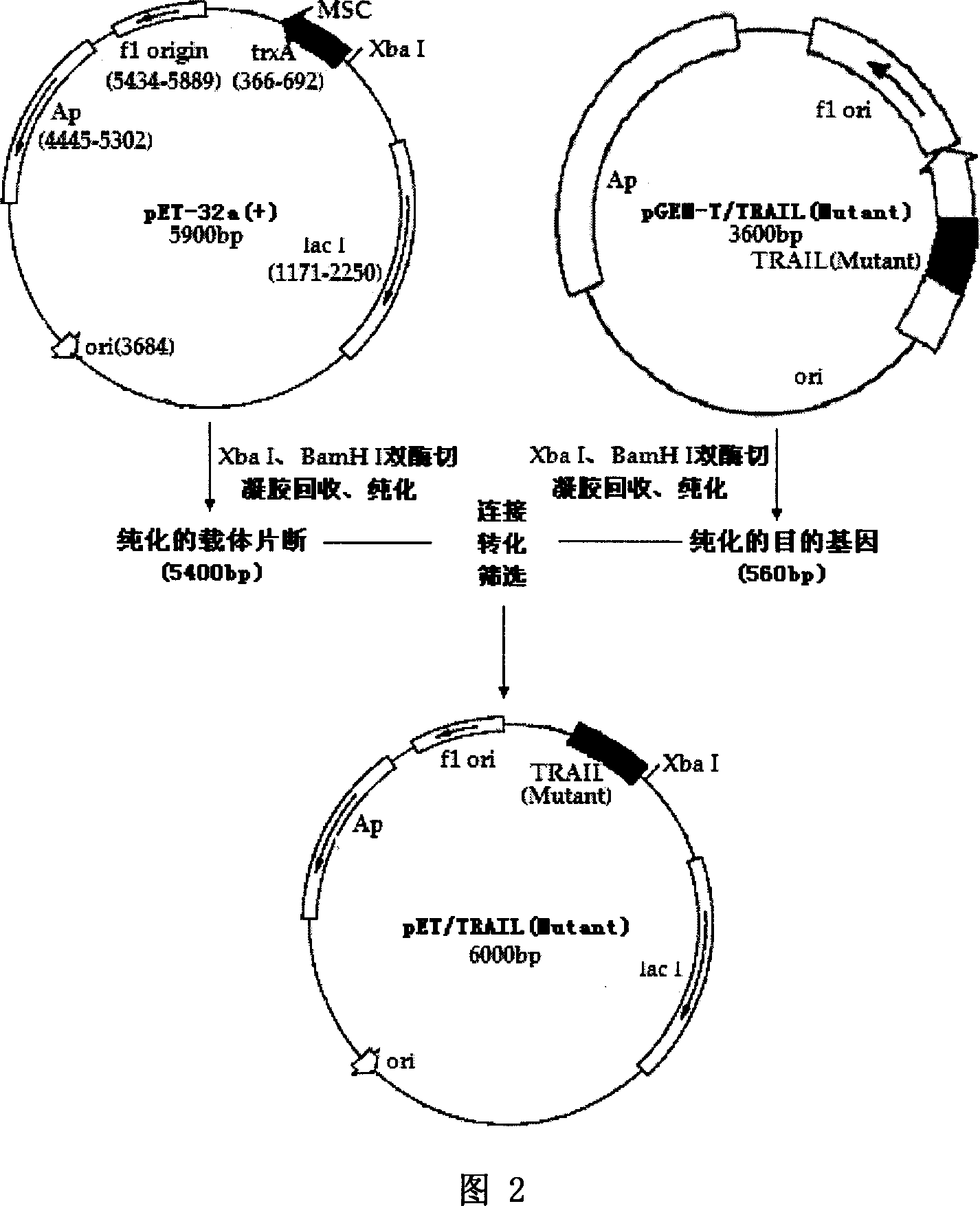
Method for preparing mutant code cDNA of apoptosis induction ligand related to human tumor necrosis factor, and application
ActiveCN1958794AHigh activityReduce use costPeptide/protein ingredientsTumor necrosis factorMutantFactor ii
This invention provides the coding cDNA of recombinant human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mutant peptides. TRAIL mutant peptides are manufactured by: constructing the expression vector containing the coding cDNA, and transferring into host cells (especially prokaryotic cells) to express TRAIL mutant peptides. The method has higher expression efficiency, increased soluble expression proportion, and higher activity of TRAIL mutant peptides than the present technique. This invention overcomes the disadvantages of low yield, high cost and low product activity faced by the present technique, and can largely reduce the utilization cost of TRAIL mutant peptides. This invention realizes the industrial production of TRAIL mutant peptides, and accelerates the application of TRAIL mutant peptides in basic research and medicine manufacture.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com