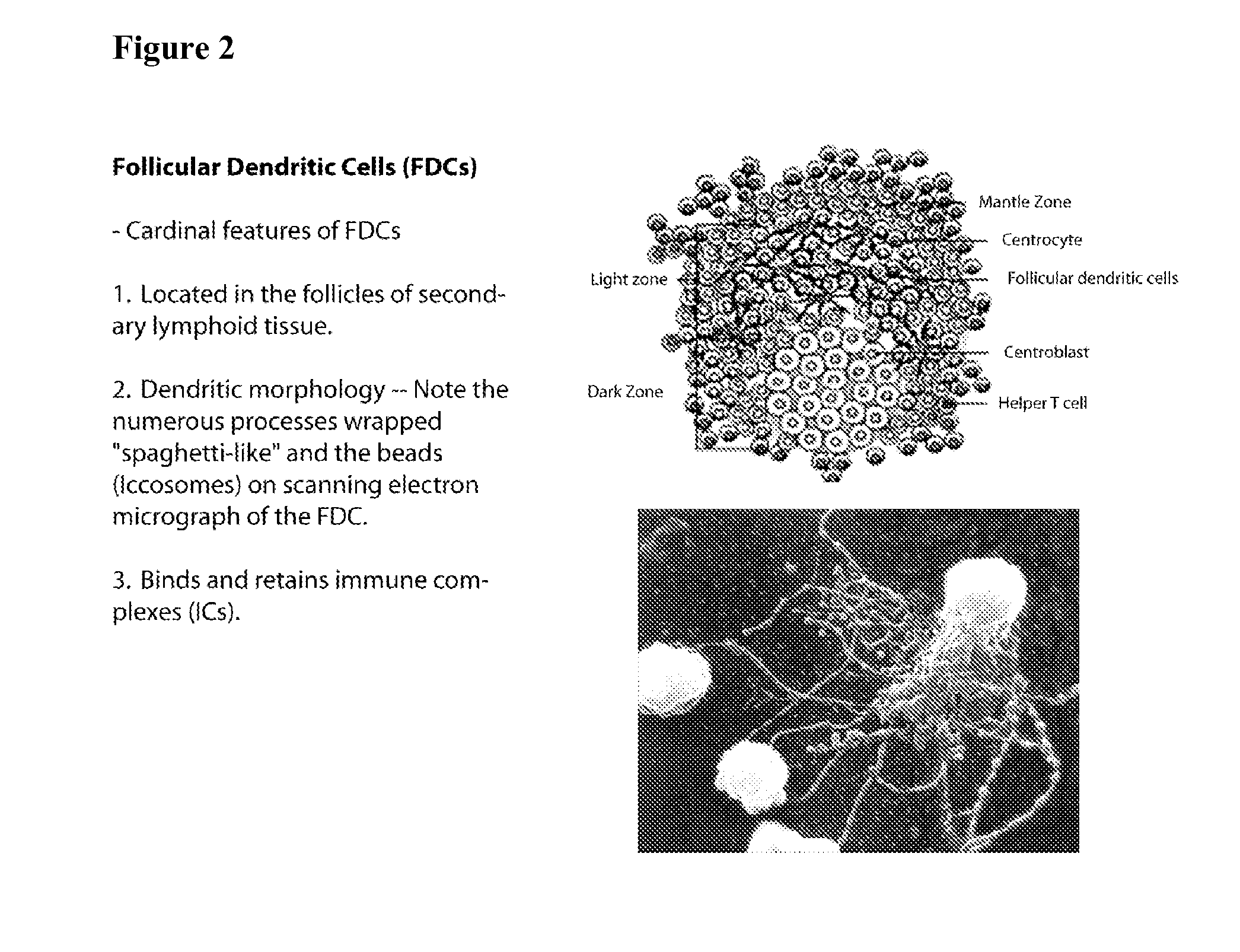
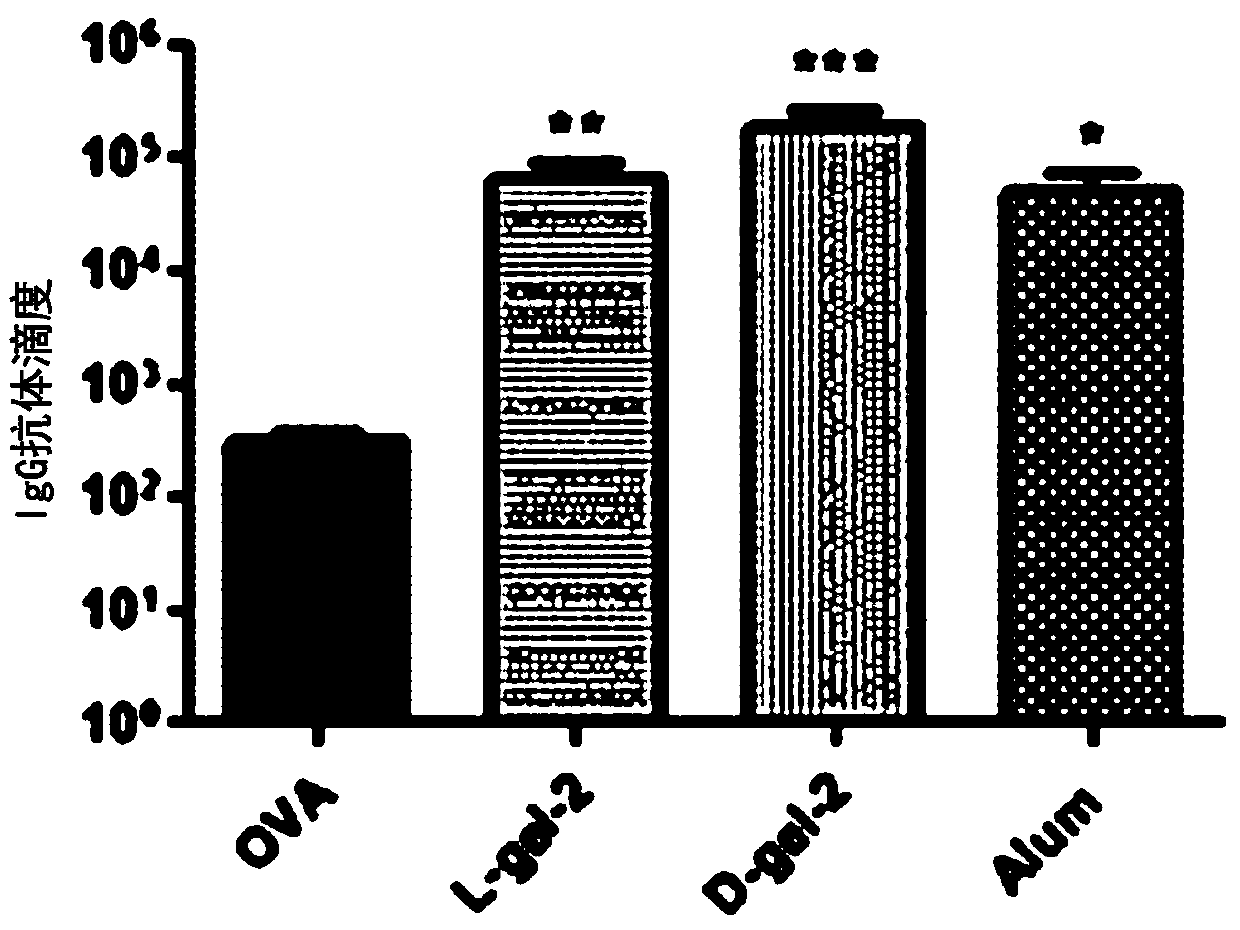
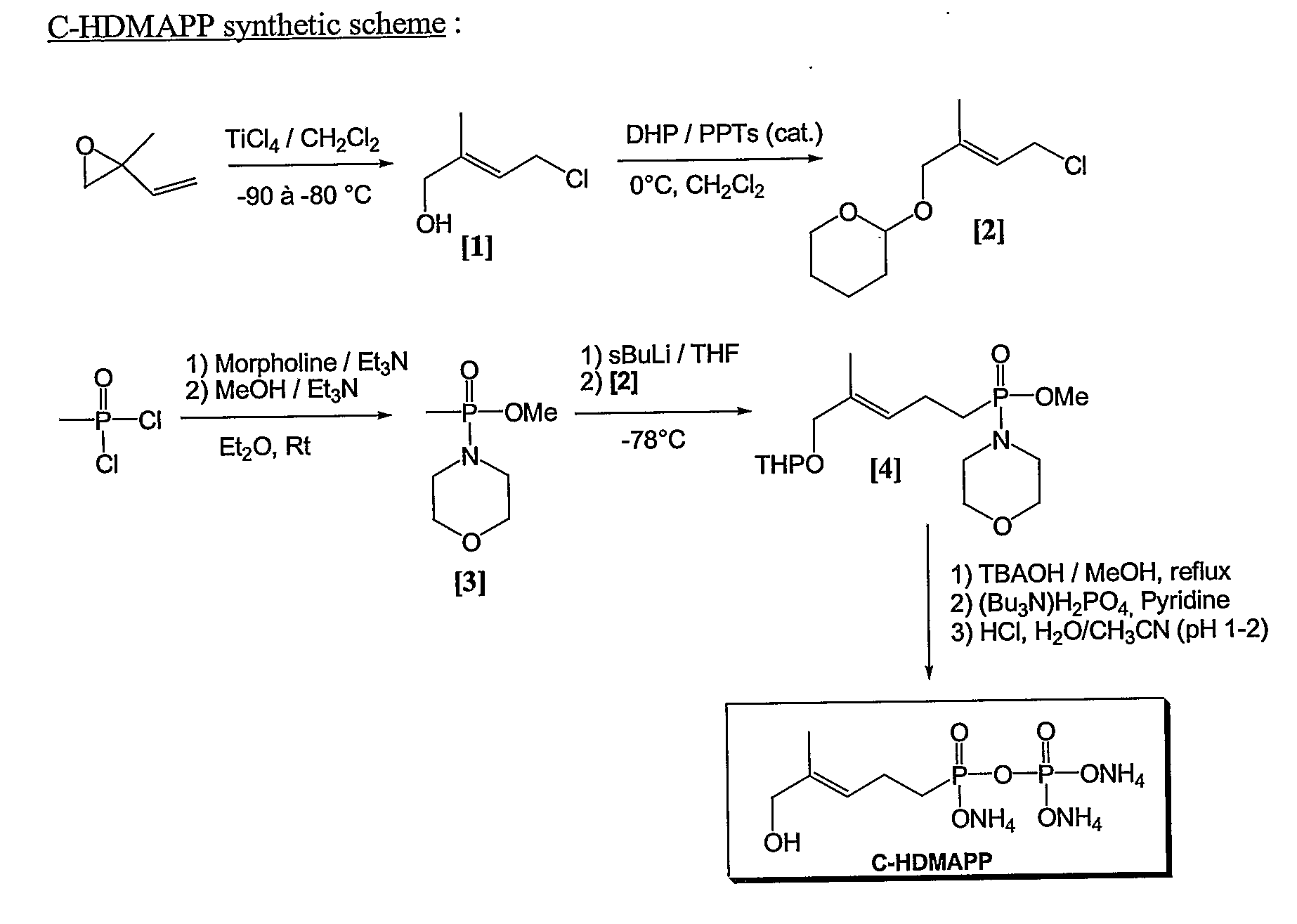
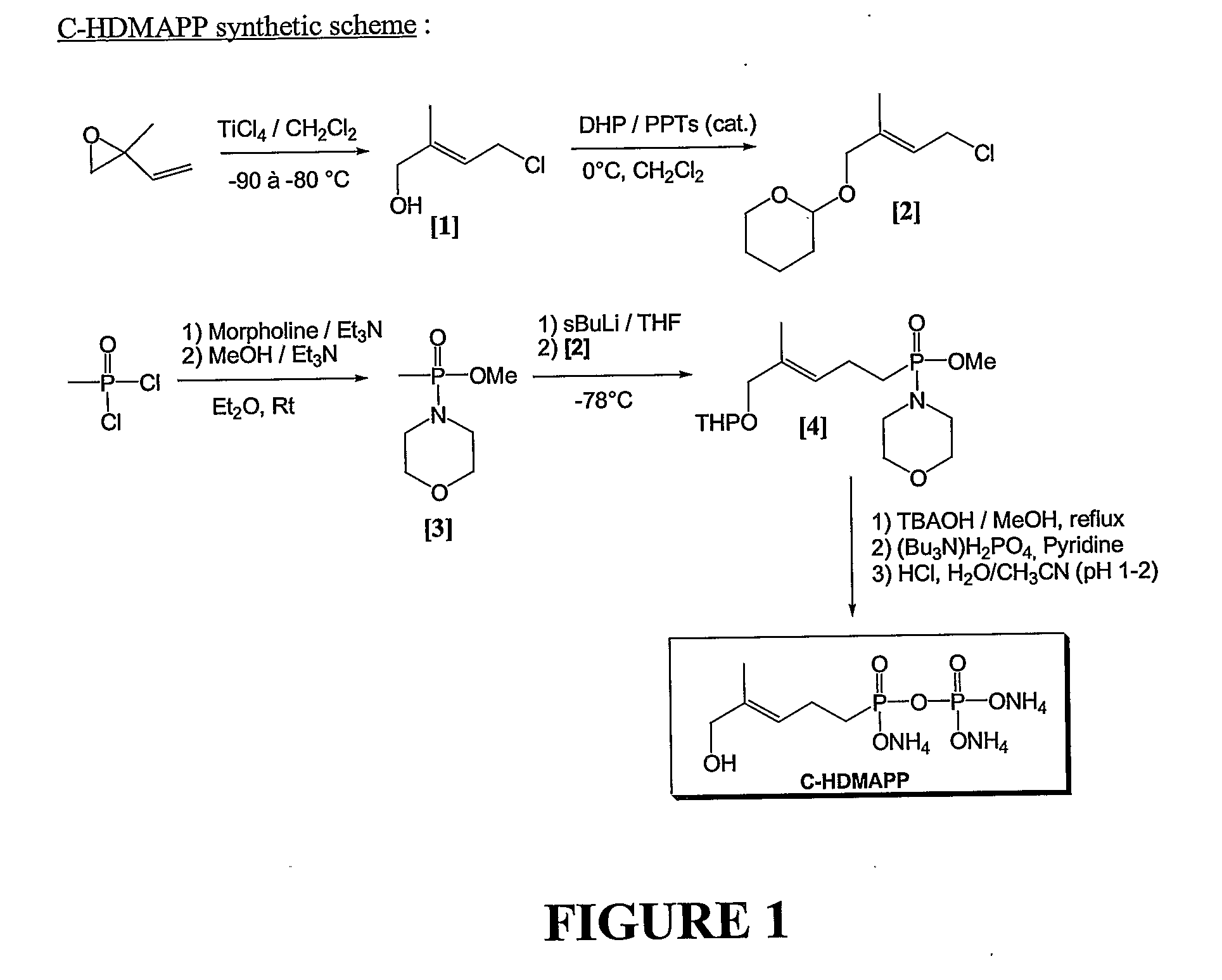
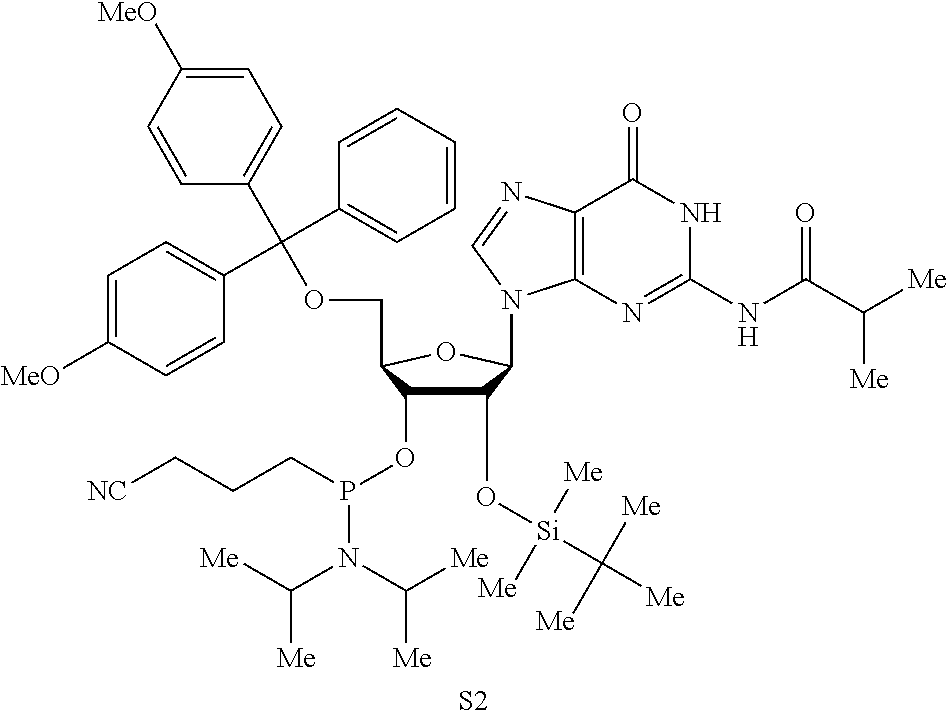
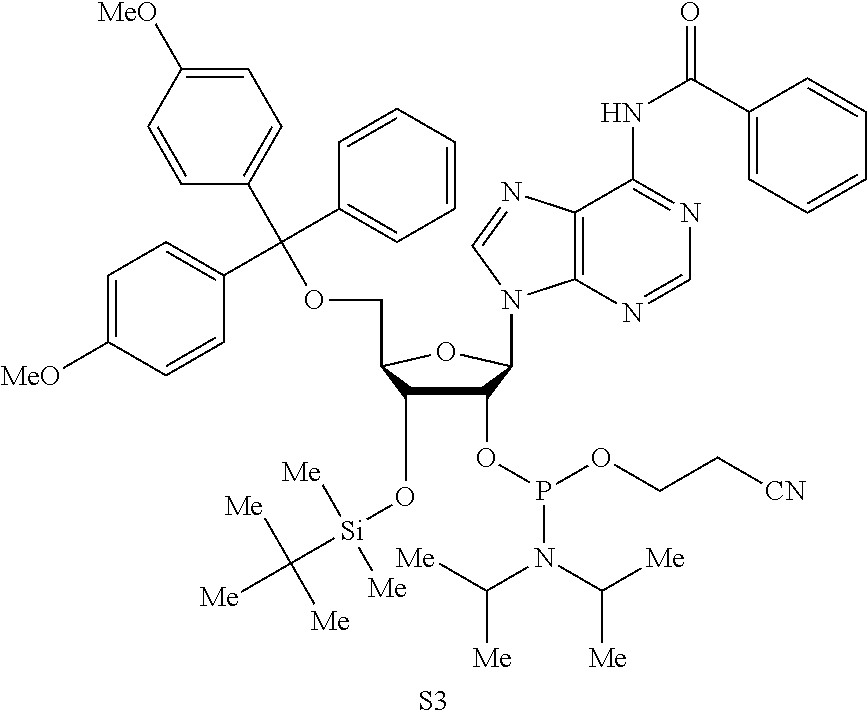
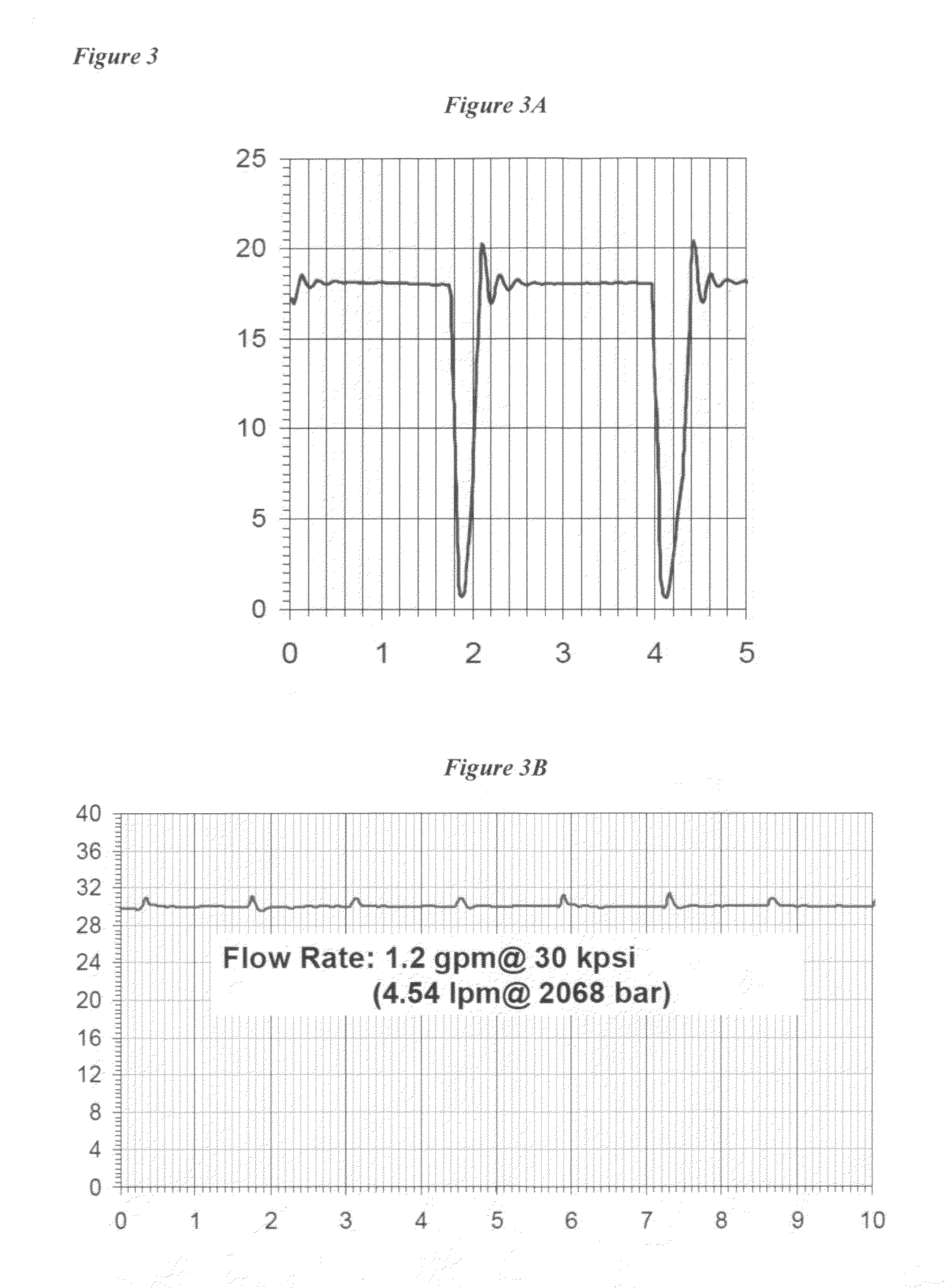
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
753 results about "Vaccine adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine adjuvants are chemical substances which are supposed to enhance the immune response to the vaccine. There are several types of adjuvants. Some of the most common adjuvants include aluminium hydroxide, aluminium phosphate and calcium phosphate.
Immunostimulatory Combinations for Vaccine Adjuvants
This invention discloses immunostimulatory combinations of Tumor Necrosis Factor Receptor Superfamily (TN-FRSF) agonists, Toll-Like Receptor (TLR) agonists, “domain present in NAIP, CIITA, HET-E, TP-I (NACHT)-Leucine Rich Repeat (LRR)” or “NLR” agonists, RIG-I-Like Helicase or “RLH” agonists, purinergic receptor agonists and cytokine / chemokine receptor agonists, together with delivery methods. The combinations, when used alone at the site of pathology, provide immunostimulation that induces host humoral and cellular immunologic responses to eliminate pathogens or neoplasms. Alternatively, when the combinations are used with a defined antigens, these combinations can induce focused humoral and cellular immunologic responses useful as prophylactic and / or ameliorative therapeutic modalities for infections and the treatment of neoplastic disorders.
Owner:RGT UNIV OF CALIFORNIA
Microfluidized oil-in-water emulsions and vaccine compositions
This invention provides submicron oil-in-water emulsions useful as a vaccine adjuvant for enhancing the immunogenicity of antigens. The present invention also provides vaccine compositions containing an antigen combined with such emulsions intrinsically or extrinsically. Methods of preparing the emulsions and vaccines are also provided by the present invention.
Owner:ZOETIS SERVICE LLC
Microfluidized oil-in-water emulsions and vaccine compositions
Owner:ZOETIS SERVICE LLC
Novel proteosome-liposaccharide vaccine adjuvant
InactiveUS20030044425A1Increase secretionUniform processSsRNA viruses negative-senseBiocideImmunotherapeutic agentCytokine
An adjuvant complex composed of bacterial outer membrane protein proteosomes complexed to bacterial liposaccharide is prepared to contain the component parts under a variety of conditions. The complex can be formulated with antigenic material to form immunogenic compositions, vaccines and immunotherapeutics. An induced immune response includes protective antibodies and / or type 1 cytokines is shown for a variety of protocols.
Owner:ID BIOMEDICAL CORP LAVAL
Novel synthetic chimeric fusion transgene with immuno-therapeutic uses
InactiveUS20050053579A1Reducing tumorigenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsInterferon alphaWilms' tumor
The present invention relates to an immuno-therapy conjugate which comprises A-c-B wherein: A and B are different and are compounds selected from the group consisting of cytokines, chemokines, interferons, their respective receptors or a functional fragment thereof; and c is a linker consisting of a bond or an amino acid sequence containing from 1 to 100 residues. The present invention also relates to a vaccine adjuvant comprising the immuno-therapy conjugate of the present invention. The present invention further relates to a method of reducing tumor growth, for inhibiting a viral infection and for improving immune response in a patient.
Owner:GALIPEAU JACQUES +1
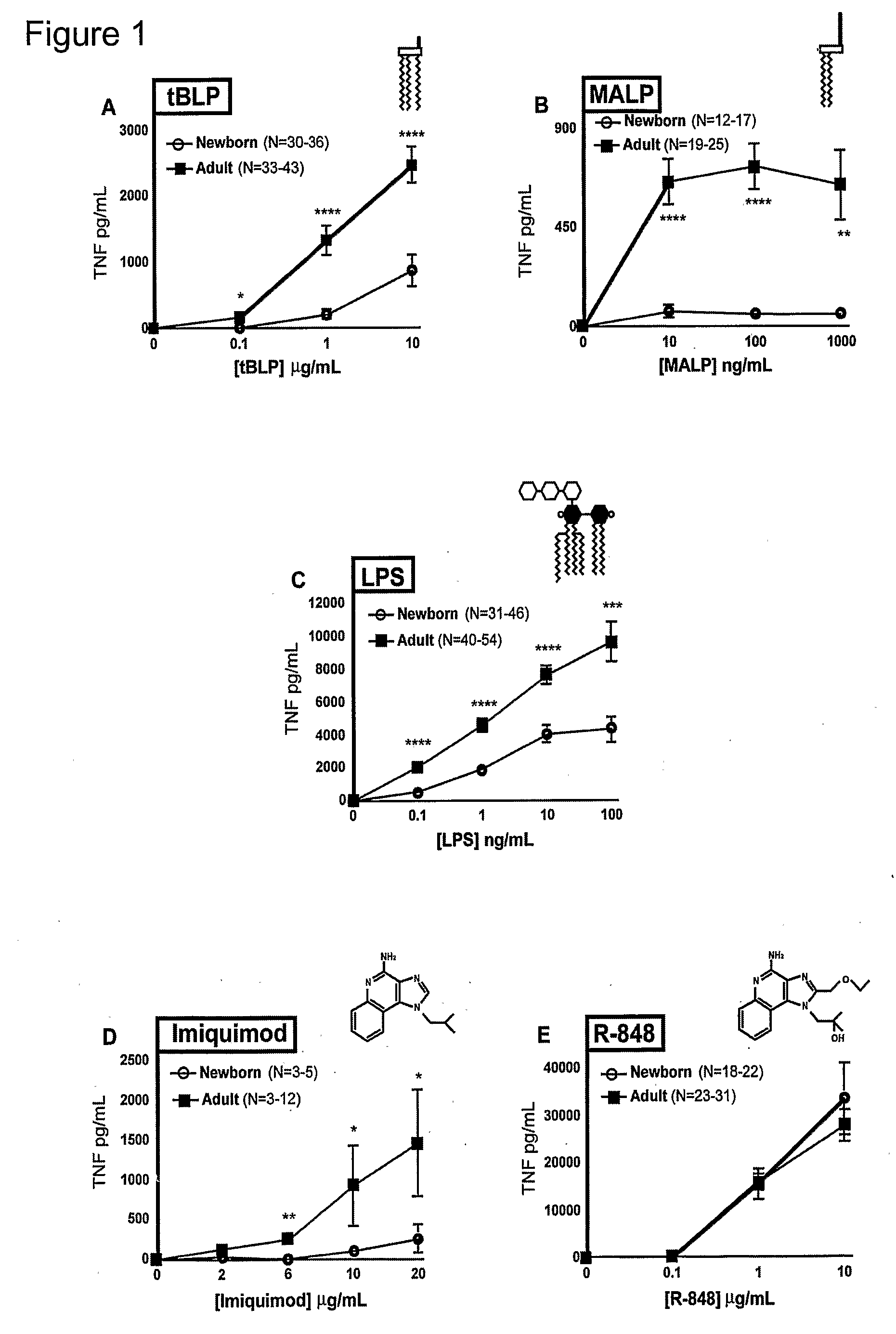
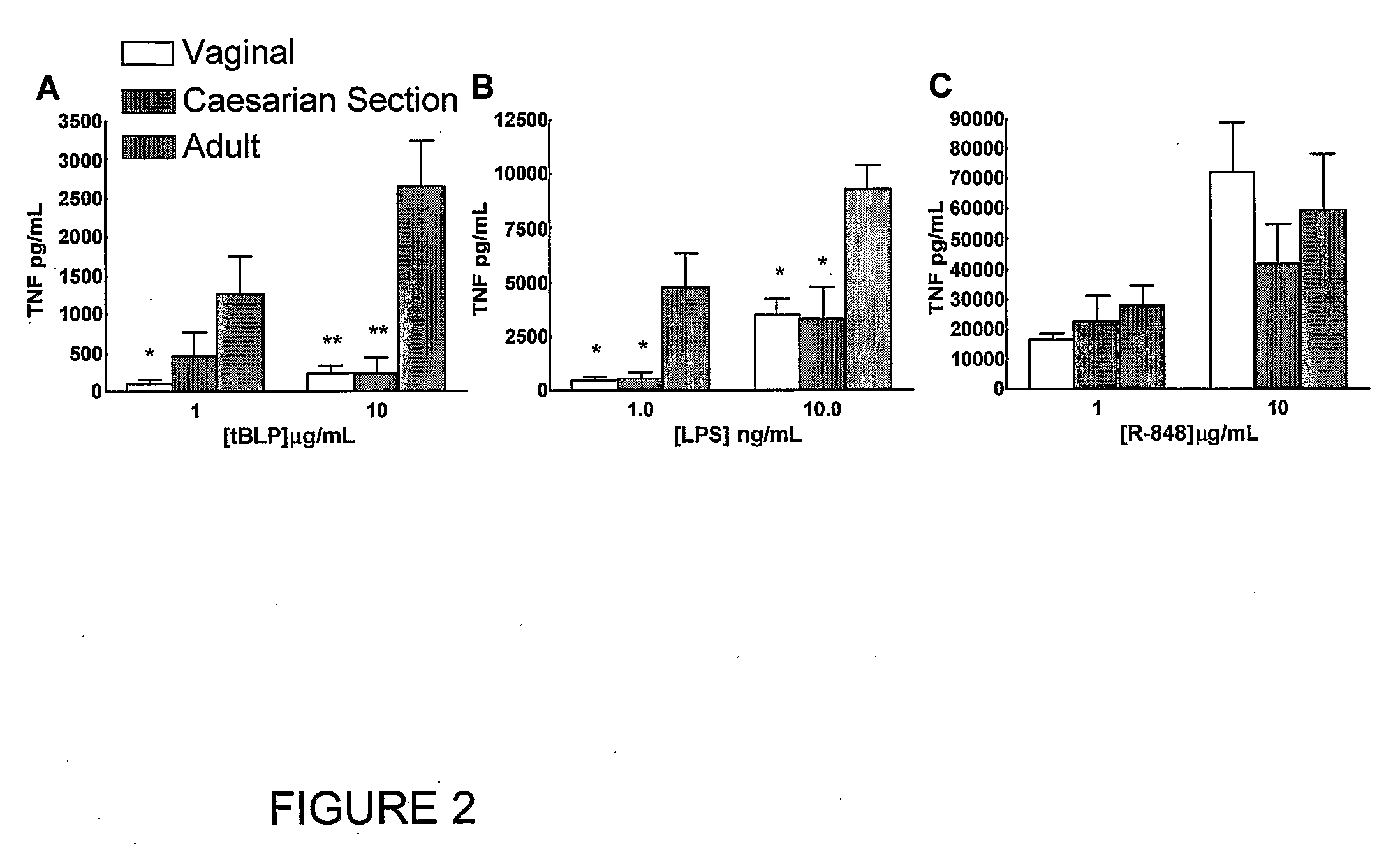
Method for Stimulating the Immune Response of Newborns
InactiveUS20080193468A1Enhance immune responsePreventing or treatingAntibacterial agentsBiocideTLR8Agonist
The present invention is based on the surprising discovery that agonists of TLR8 are uniquely efficacious in enhancing (e.g. inducing) the immune response of newborns. Thus, agonists of TLR8 serve as both vaccine adjuvants and as adjunctive therapies for acute infection in newborns, preferably the agonist is a TLR8-selective agonist. The immune response induced, or enhanced, in the neonatal host can be, for example, a cytokine immune response and / or a humoral immune response (e.g., antigen-specific).
Owner:3M INNOVATIVE PROPERTIES CO +1
High throughput screening of aptamer libraries for specific binding to proteins on viruses and other pathogens
InactiveUS20060121489A1Improve the immunityLow production costMicrobiological testing/measurementScreening processHigh-Throughput Screening MethodsVirus
The present invention includes composition and methods for making and using a combinatorial library to identify thioaptamers that bind to targets on or about pathogens. Compositions, kits and methods are also provided for the identification of pathogens, e.g., viral, bacterial or other proteins related infectious disease, as well as, vaccines and vaccine adjuvants are provided that modify host immune responses.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Oil-in-water emulsion free of surfactant and use thereof
ActiveCN104013955AThe nature is easy to controlGood biocompatibilitySsRNA viruses negative-sensePowder deliveryActive agentGlycerol
The invention discloses an oil-in-water emulsion free of surfactant. The emulsion comprises a metabolizable oil phase, a water phase and oil-water amphipathic solid particles dispersed in the water phase and having biocompatibility, wherein the oil phase comprises squalene or / and tocol; the water phase is any one or a combination of at least two of purified water, water for injection, aqueous liquid of glycerol, buffered saline liquid and clinically available infusion liquid; and the average grain diameter of the solid particles is at nanometer-to-micron grade. The emulsion can be used as a vaccine adjuvant or a medicine delivery or controlled release carrier; the properties of the emulsion can be controlled and regulated; the obtained emulsion is stable; the use of a surfactant is avoided; and harm to the human body and pollution on the environmental can be reduced.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Novel vaccine adjuvant
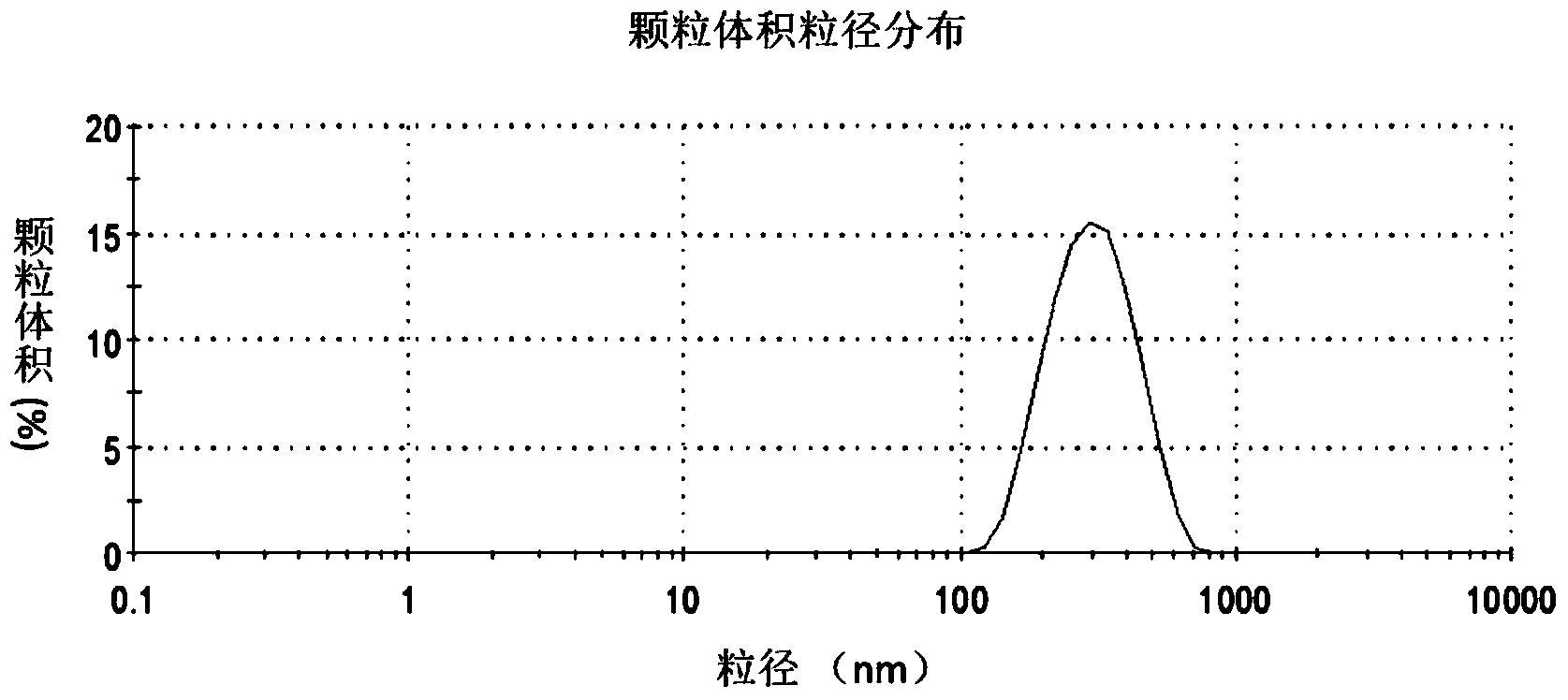
ActiveCN101428145AGood immune boosterWide range of usePowder deliveryViral antigen ingredientsEmulsionBody fluid
The invention discloses a nanometer particle adjuvant, a preparation method thereof, and the application thereof in the preparation of inactivated vaccines. According to the invention, a novel nanometer particle adjuvant is obtained through preparation by the emulsion preparation and the nanometer treatment following the dissolving of different immunopotentiator and surfactant in water and oil respectively. The adjuvant provided by the invention not only can remarkably improve the humoral immune response and the cellular immune response of various bacterial and viral inactivated vaccines, butalso has unique advantages in the preparation process, the stability, the immunity duration and the side reaction of vaccines.
Owner:BEIJING CENT BIOLOGY
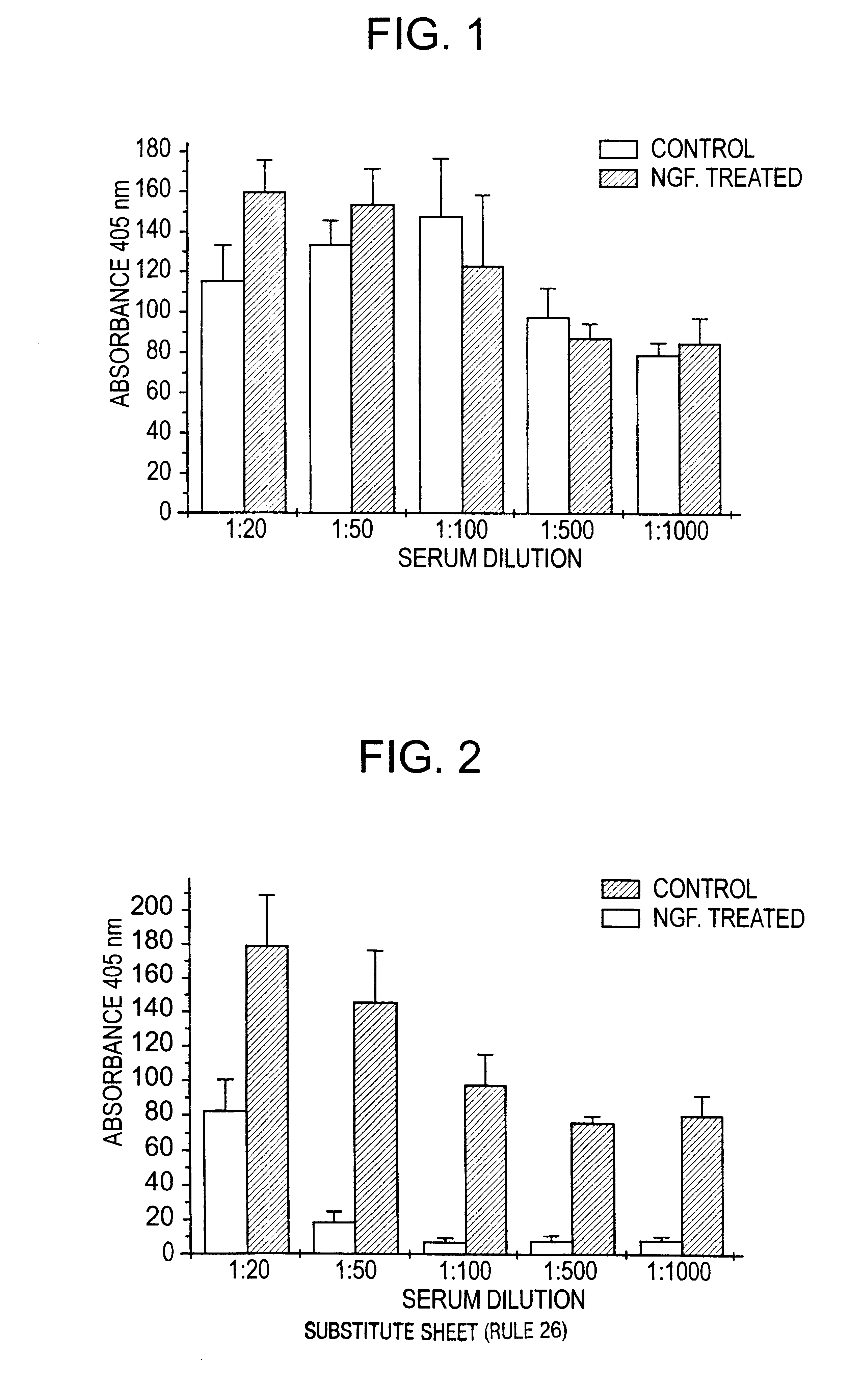
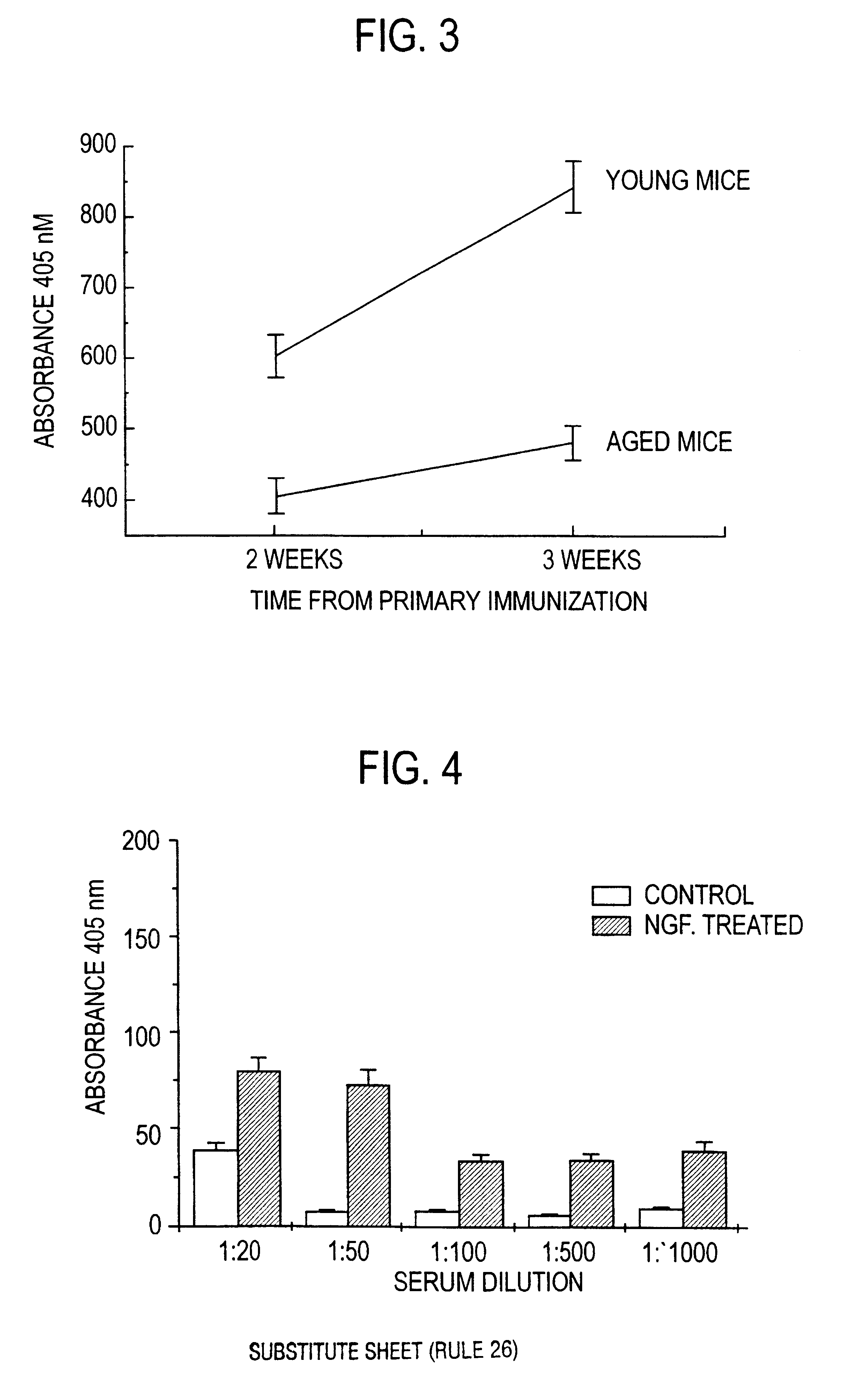
Nerve growth factor as a vaccine adjuvant
A vaccination method utilizes a pharmaceutical combination for enhancing vaccine effectiveness. The method utilizes an immune response-triggering vaccine capable of stimulating production in an immunodeficient animal antibodies to a disease-causing agent foreign to the animal. As an adjuvant, a vaccine effectiveness-enhancing amount of Nerve Growth Factor (NGF) is administered, which enhances production and affinity of the antibodies in the animal, in response to the vaccine.
Owner:PROTECHTION UNLIMITED
Models for vaccine assessment
ActiveUS20080008653A1Improve accuracyImprove predictabilityCompounds screening/testingCrankshaftsAdjuvantBiological Immunotherapy
The present invention is directed to methods for constructing and using in vivo and in vitro models of aspects of human immunity and, in particular, construction of a human immune system model for the testing of, for example, vaccines, adjuvants, immunotherapy candidates, cosmetics, drugs, biologics and other chemicals. The present invention comprises both in vivo and in vitro models of aspects of human immunity that are useful for assessing the interaction of substances with the immune system, and thus can be used to accelerate and improve the accuracy and predictability of, for example, vaccine, drug, biologic, immunotherapy, cosmetic and chemical development. The invention is also useful for the generation of human monoclonal and polyclonal antibodies.
Owner:VIRGINIA COMMONWEALTH UNIV +1
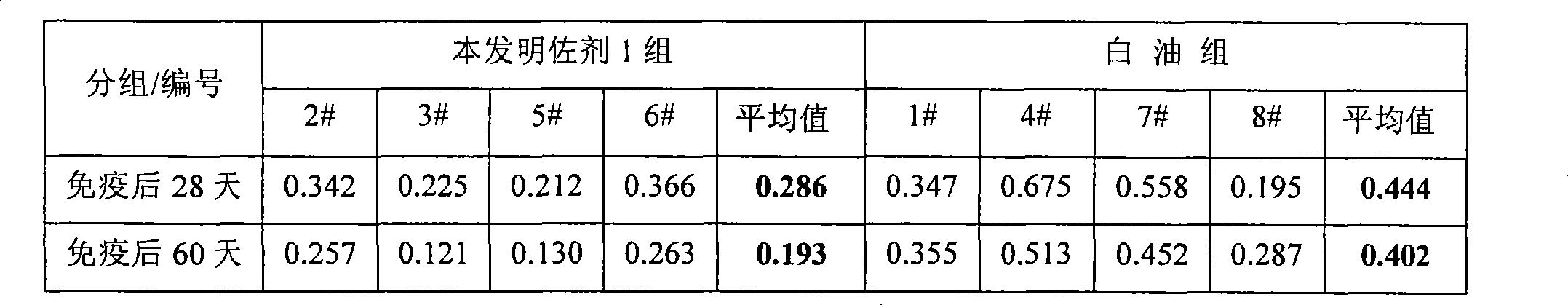
Vaccine adjuvant as well as preparation method and application thereof
ActiveCN104147599AHelp with immunityImprove the effectiveness of anti-virus protectionAntiviralsEmulsion deliveryImmune effectsMedicine
The invention discloses an oil-in-water type vaccine adjuvant as well as a preparation method and application thereof. The oil-in-water type nano-emulsion vaccine adjuvant comprises the following components in percentage by mass: 0.1-10 percent of oil phase, 0.1-10 percent of emulsifier, 0.1-3 percent of stabilizer, 0.1-3 percent of complexing agent and 0.01-10 percent of immunopotentiator. The preparation method comprises the following steps: (1) uniformly dispersing the immunopotentiator, the stabilizer and the complexing agent in water, thereby obtaining an aqueous phase; (2) mixing an oil phase and an emulsifier, thereby obtaining an oil phase; (3) slowly adding the oil phase into the aqueous phase, and continuously stirring, thereby forming a stable emulsion; (4) regulating the pH value of the emulsion, and fixing the volume to obtain a primary emulsion; and (5) performing high-speed shearing and high-pressure homogenizing on the primary emulsion. The vaccine adjuvant provided by the invention is simple in preparation, convenient to use and small in side reactions, is used for diluting vaccines, particularly swine fever live vaccines and is high in stability and good in immune effect.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Extracellular matrix materials as vaccine adjuvants for diseases associated with infectious pathogens or toxins
Disclosed are vaccines and vaccine adjuvants useful in the treatment and / or prevention of infection and diseases associated with infectious pathogens, such as tetanus, as well as diseases associated with biological toxins. Also provided are methods of preparing an adjuvant and the vaccine containing the adjuvant. Methods are also provided for vaccinating / immunizing an animal against infection and diseases associated with infectious pathogens, such as tetanus, and other diseases associated with biological toxins. Adjuvant materials are presented that are prepared from an extracellular matrix material. The adjuvants are demonstrated to enhance the immunogencity of an infectious pathogen antigen or biological toxin antigen of interest, as well as to enhance the survival of an immunized animal.
Owner:UNIV OF NOTRE DAME DU LAC +1
Adjuvant combinations of liposomes and mycobacterial lipids for immunization compositions and vaccines
ActiveUS8241610B2Good auxiliary effectEnhance immune responseAntibacterial agentsBiocideLiposomeMycobacterium
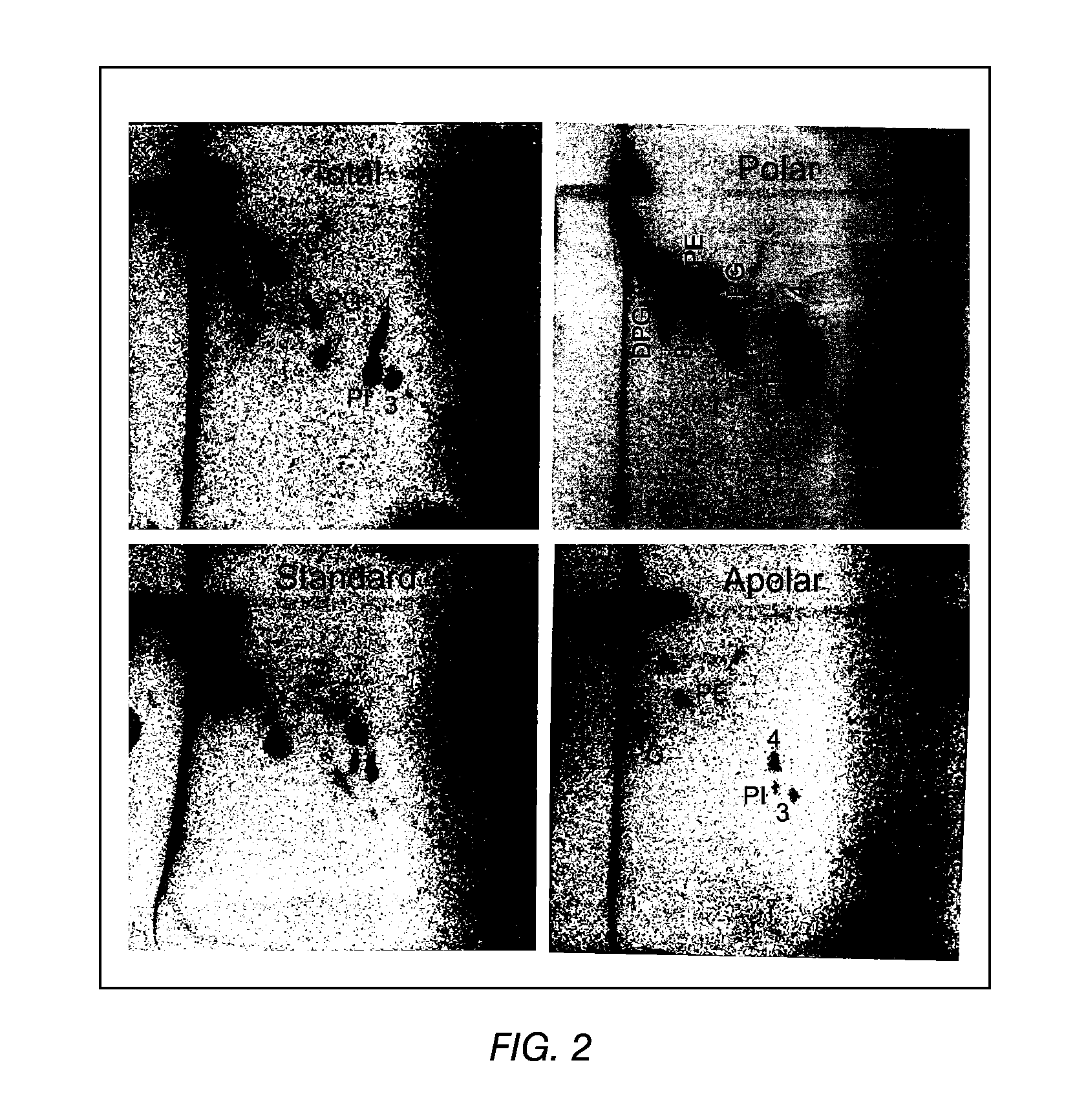
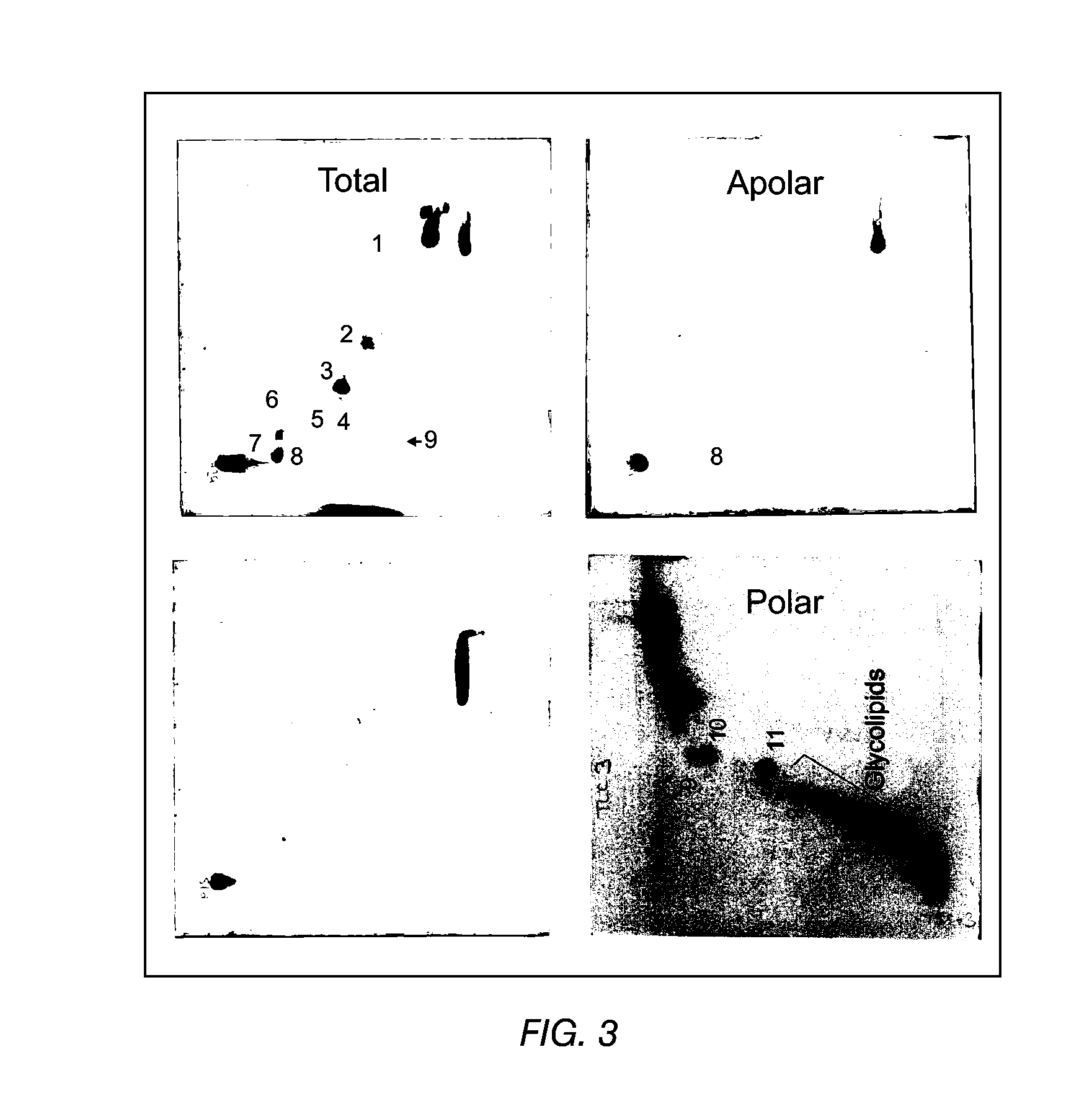
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:STATENS SERUM INST
Methods and compositions for modulating interleukin-21 receptor activity
InactiveUS7198789B2Enhance immune cell activityReduce Fc receptor bindingBiocidePeptide/protein ingredientsInfectious DisorderNatural Killer Cell Inhibitory Receptors
Methods and compositions for modulating interleukin-21 (IL-21) / IL-21 receptor (MU-1) activity using agonists or antagonists of IL-21 or IL-21 receptor (“IL-21R” or “MU-1”), are disclosed. IL-21 / IL-21R antagonists can be used to induce immune suppression in vivo, e.g., for treating or preventing immune cell-associated pathologies (e.g., pathologies associated with aberrant activity of one or more of mature T cells (mature CD8+, mature CD4+ T cells), mature NK cells, B cells, macrophages and megakaryocytes, including transplant rejection and autoimmune disorders). IL-21 / IL-21R agonists can be used by themselves or in combination with an antigen, e.g., as an adjuvant (e.g., a vaccine adjuvant), to up-regulate an immune response in vivo, e.g., for example, for use in treating cancer and infectious disorders.
Owner:GENETICS INST INC
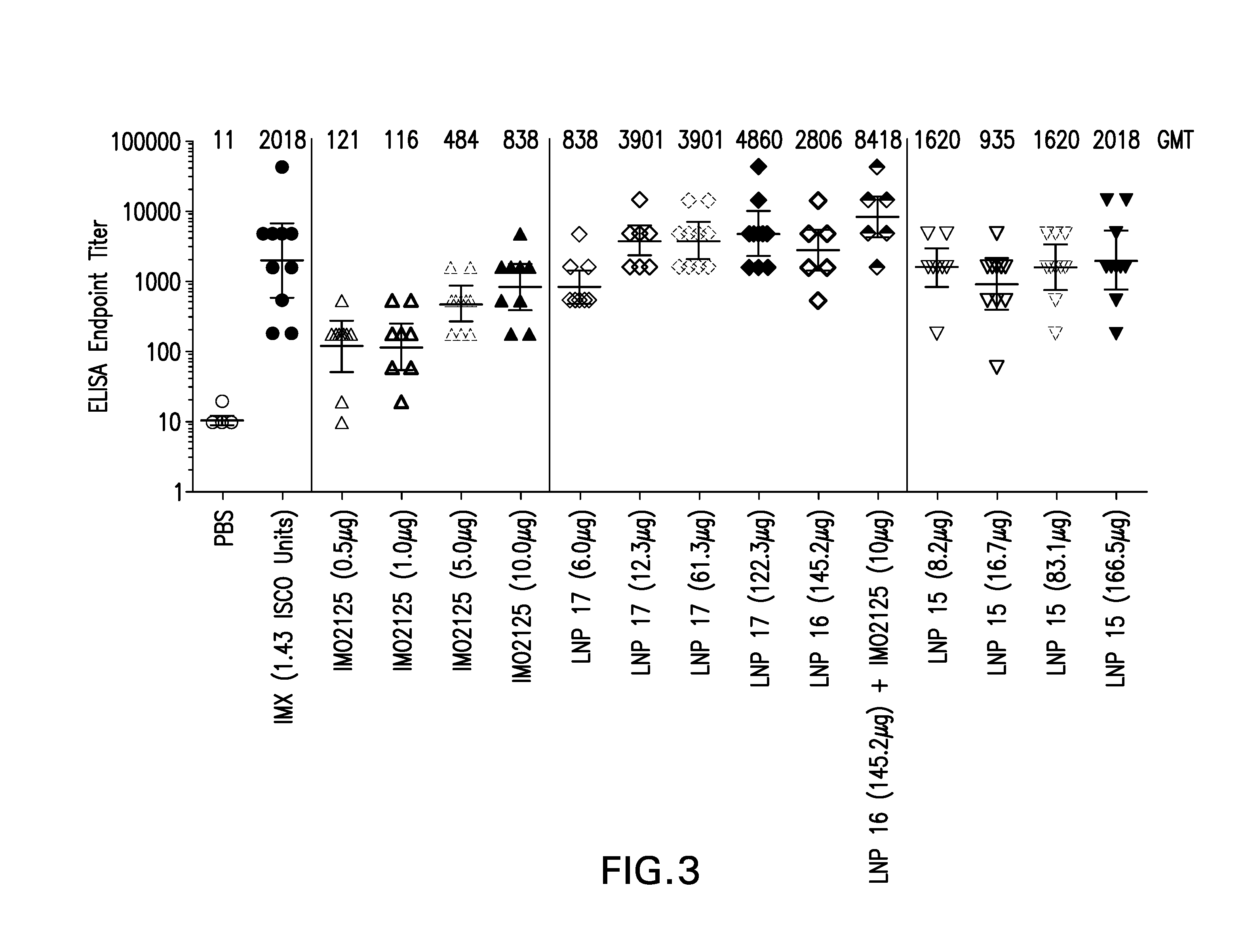
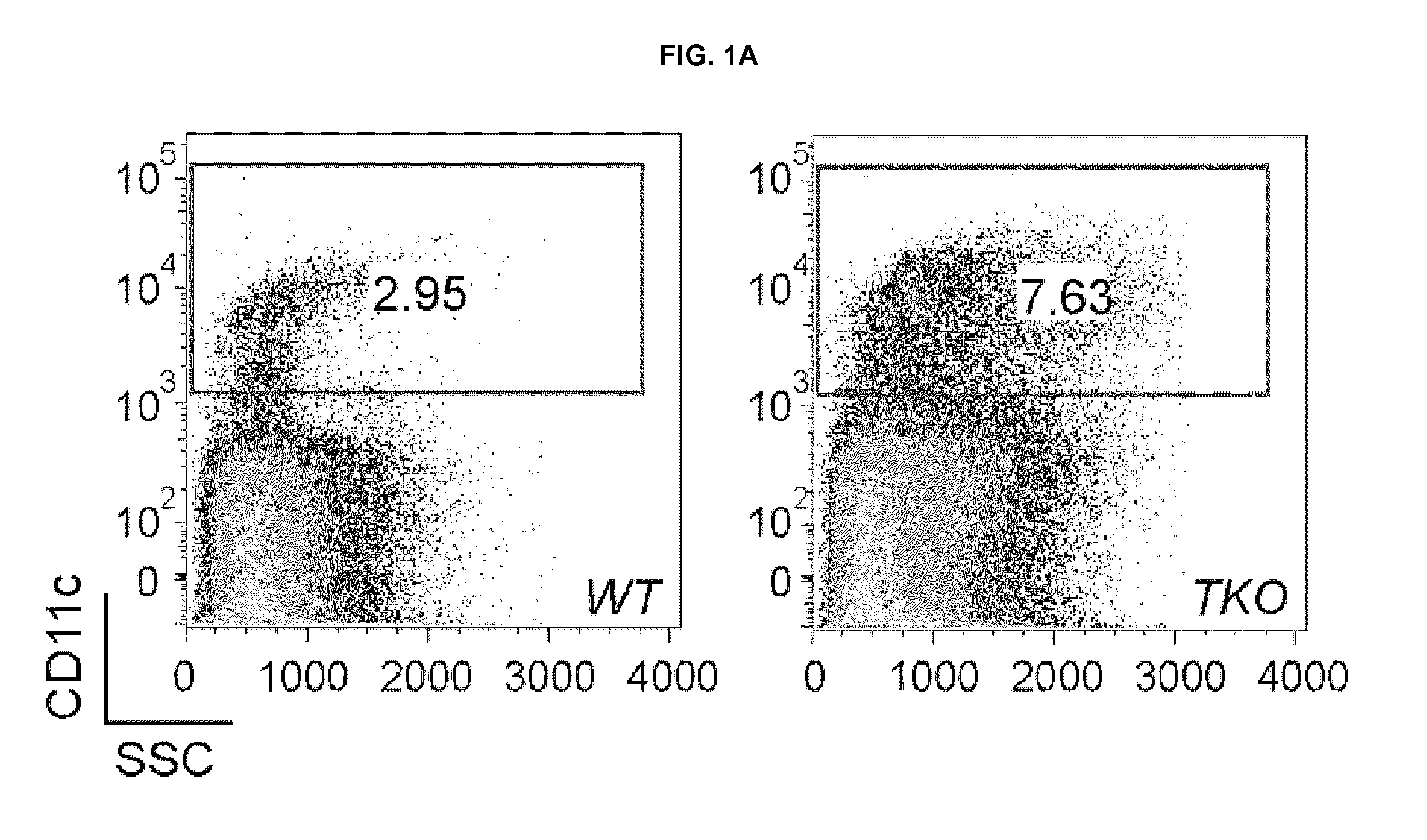
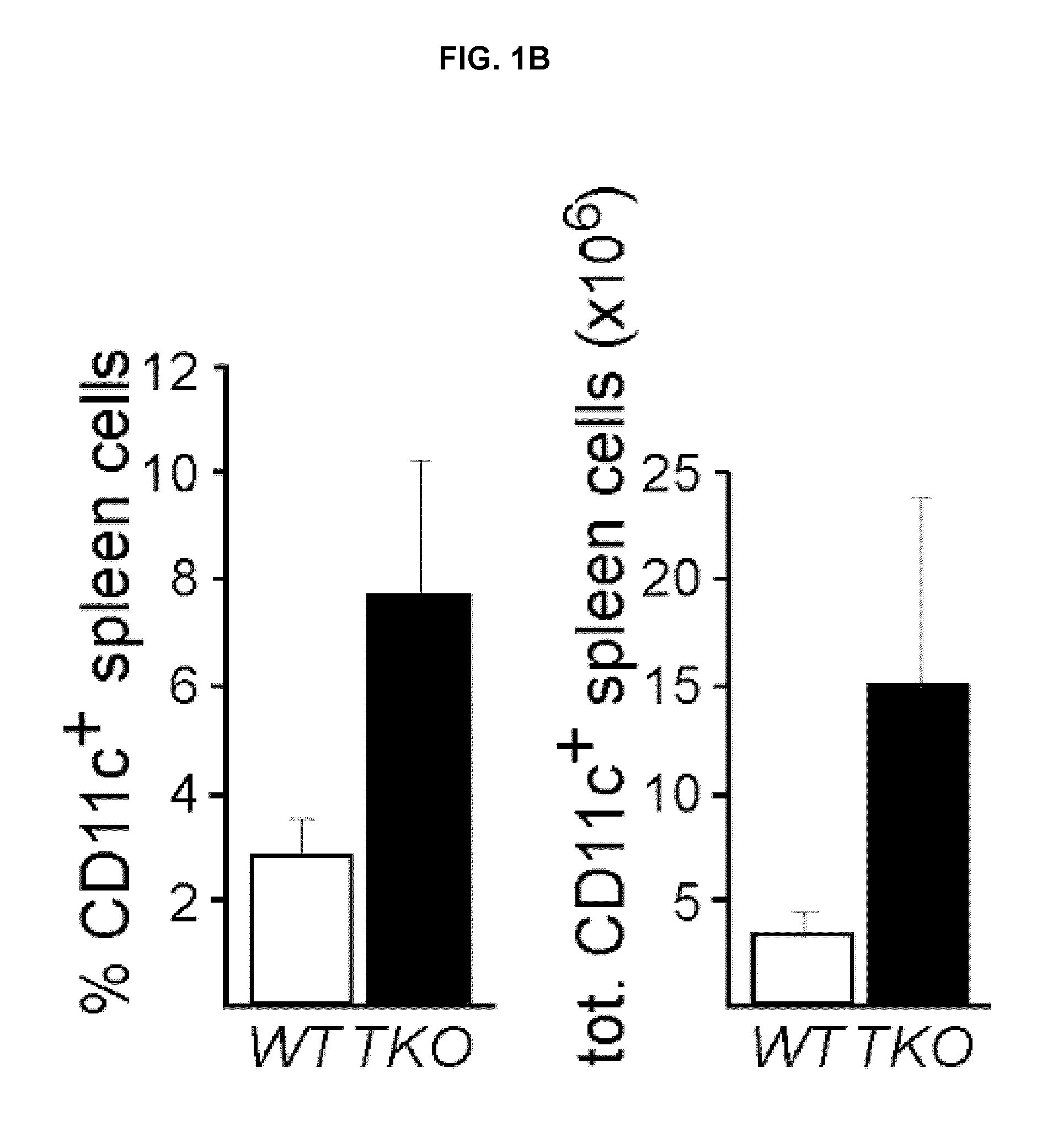
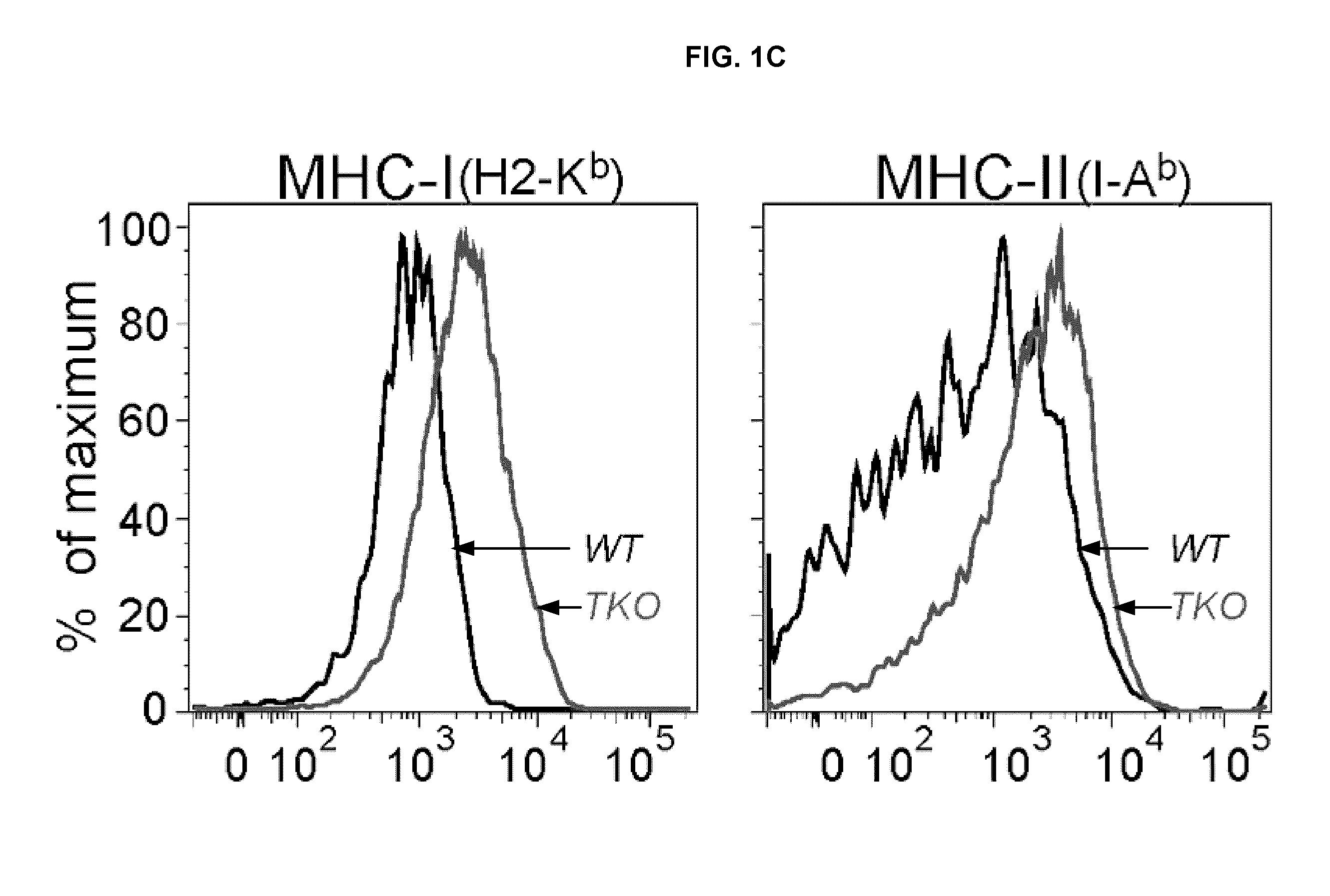
Lipid nanoparticle vaccine adjuvants and antigen delivery systems
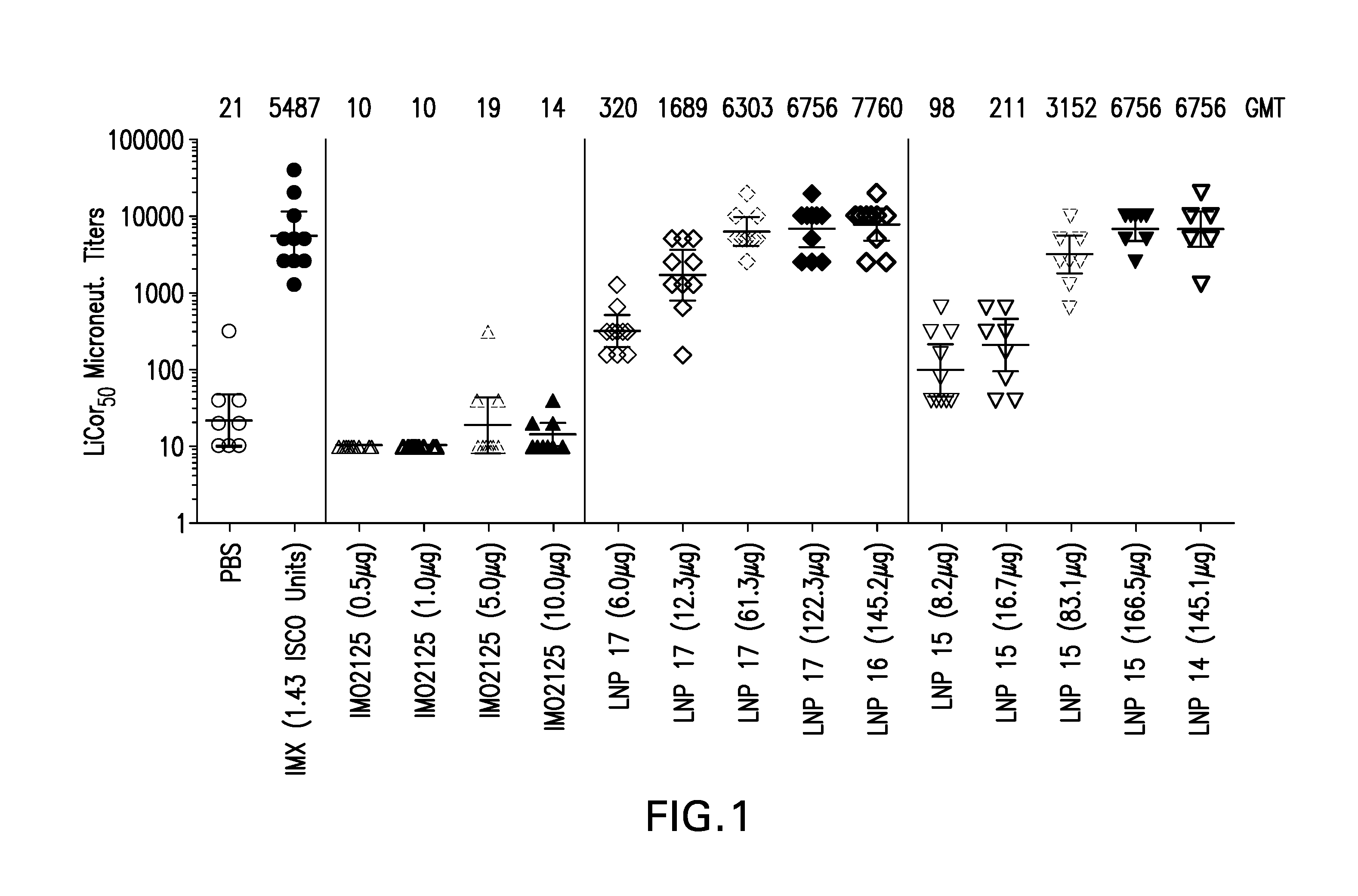
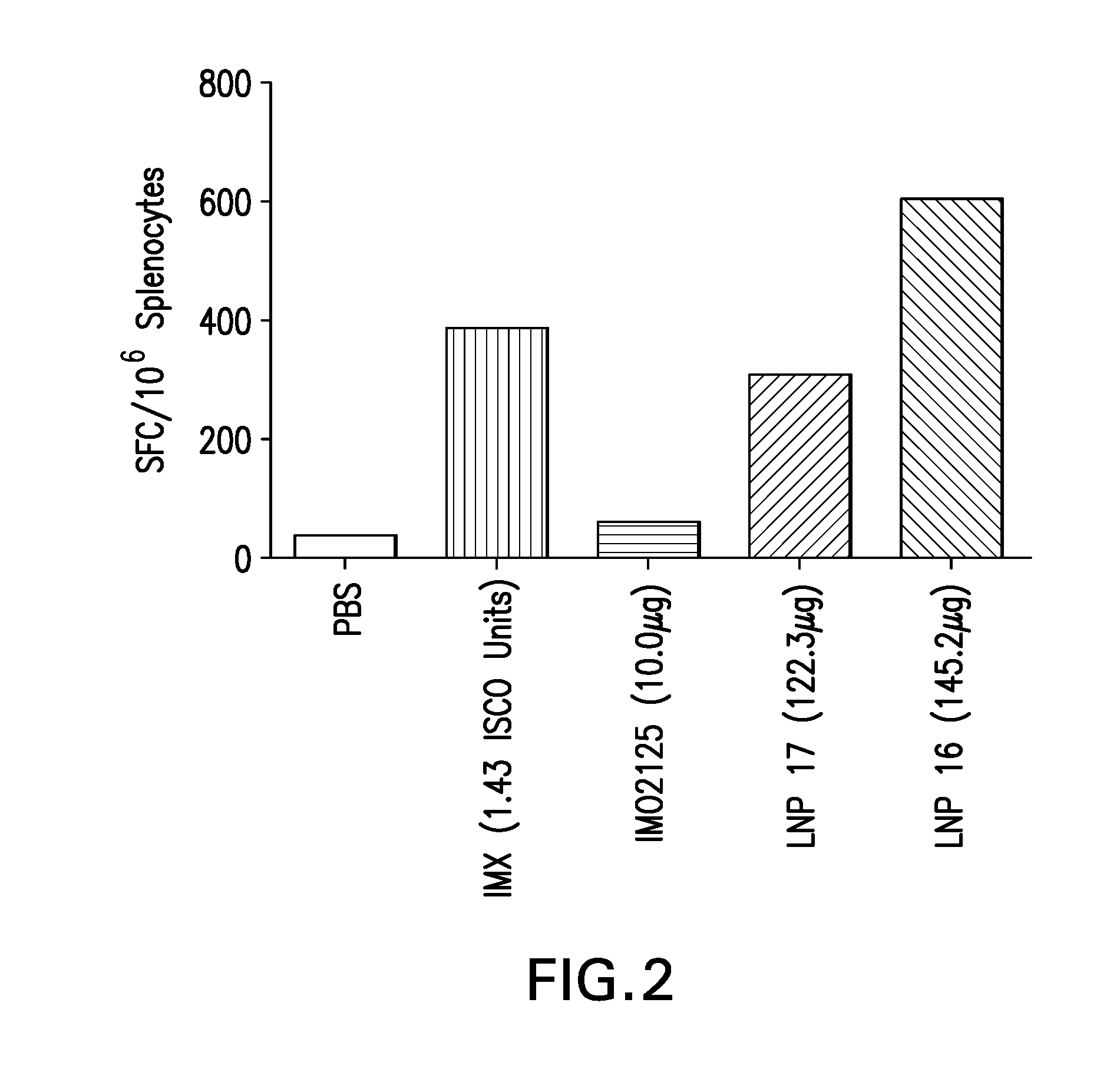
The instant invention provides for novel lipid nanoparticle (LNP) formulations, containing cationic lipids, for use as vaccine adjuvants and / or as antigen delivery systems. It is an object of the instant invention to provide LNP formulations that demonstrate enhancements in humoral and cellular immunogenicity of vaccine antigens, particularly subunit vaccine antigens, when utilized alone or in combination with immunostimulatory agents (e.g. small molecule or oligonucleotide TLR agonists). The instant invention further identifies physical and chemical properties of the LNP formulations that can be manipulated to enhance antigen efficiency and adjuvant tolerability in vivo.
Owner:MERCK SHARP & DOHME LLC
Use of tam receptor inhibitors as immunoenhancers and tam activators as immunosuppressors
This disclosure concerns compositions and methods for immunoenhancement and / or immunosuppression. In certain embodiments, the disclosure concerns methods of using a TAM receptor inhibitor for immunoenhancement, for example as a vaccine adjuvant, for the treatment of sepsis, or for treating an immunocompromised subject. Also disclosed are methods of screening for immunoenhancing agents. In other embodiments, the disclosure concerns methods of using a TAM receptor agonist for immunosuppression, for example as a treatment for an autoimmune disorder, for the treatment of an allergy, or for treating graft-versus-host disease in a subject. Also disclosed are methods of screening for immunosuppressive agents.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Novel parenteral vaccine formulations and uses thereof
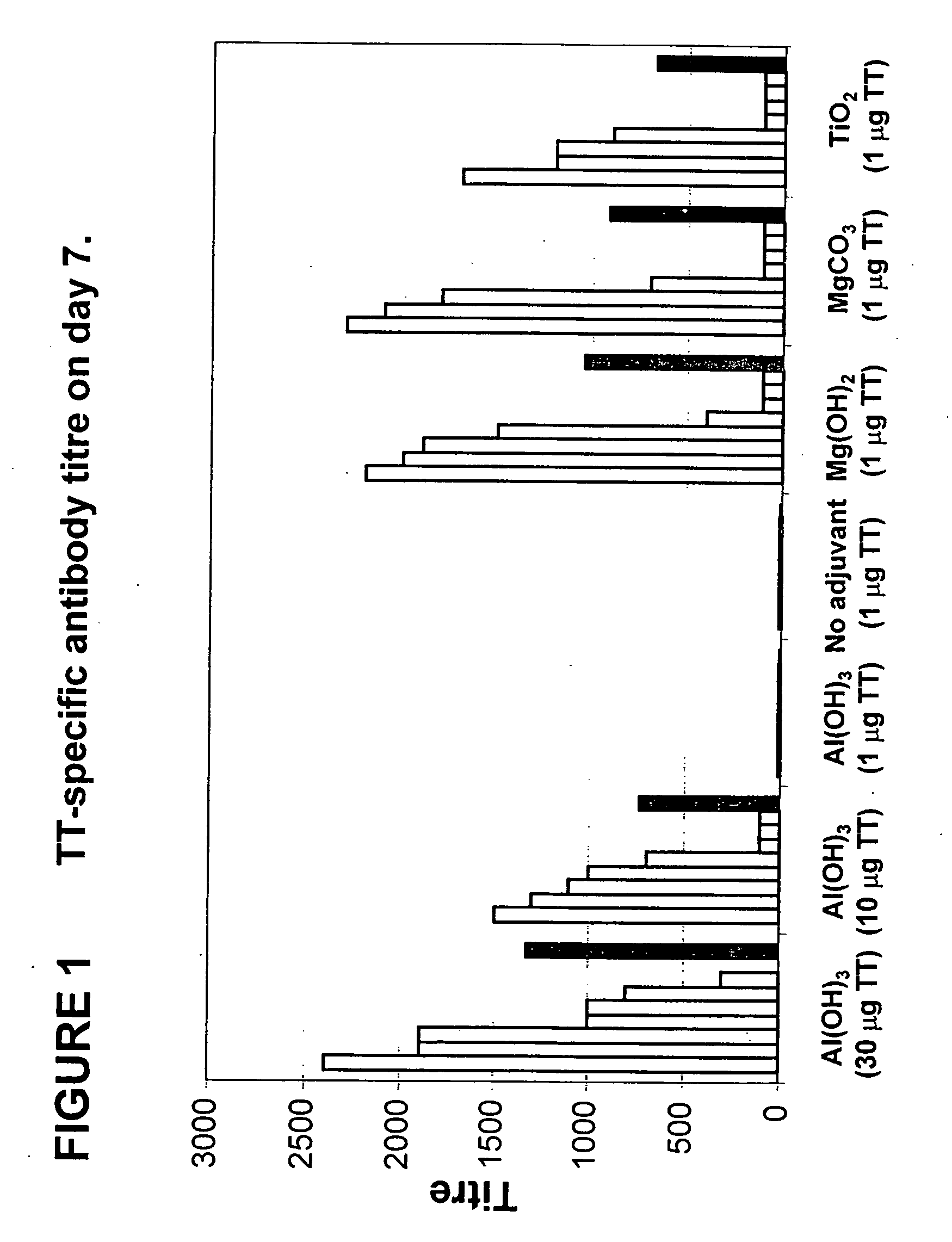
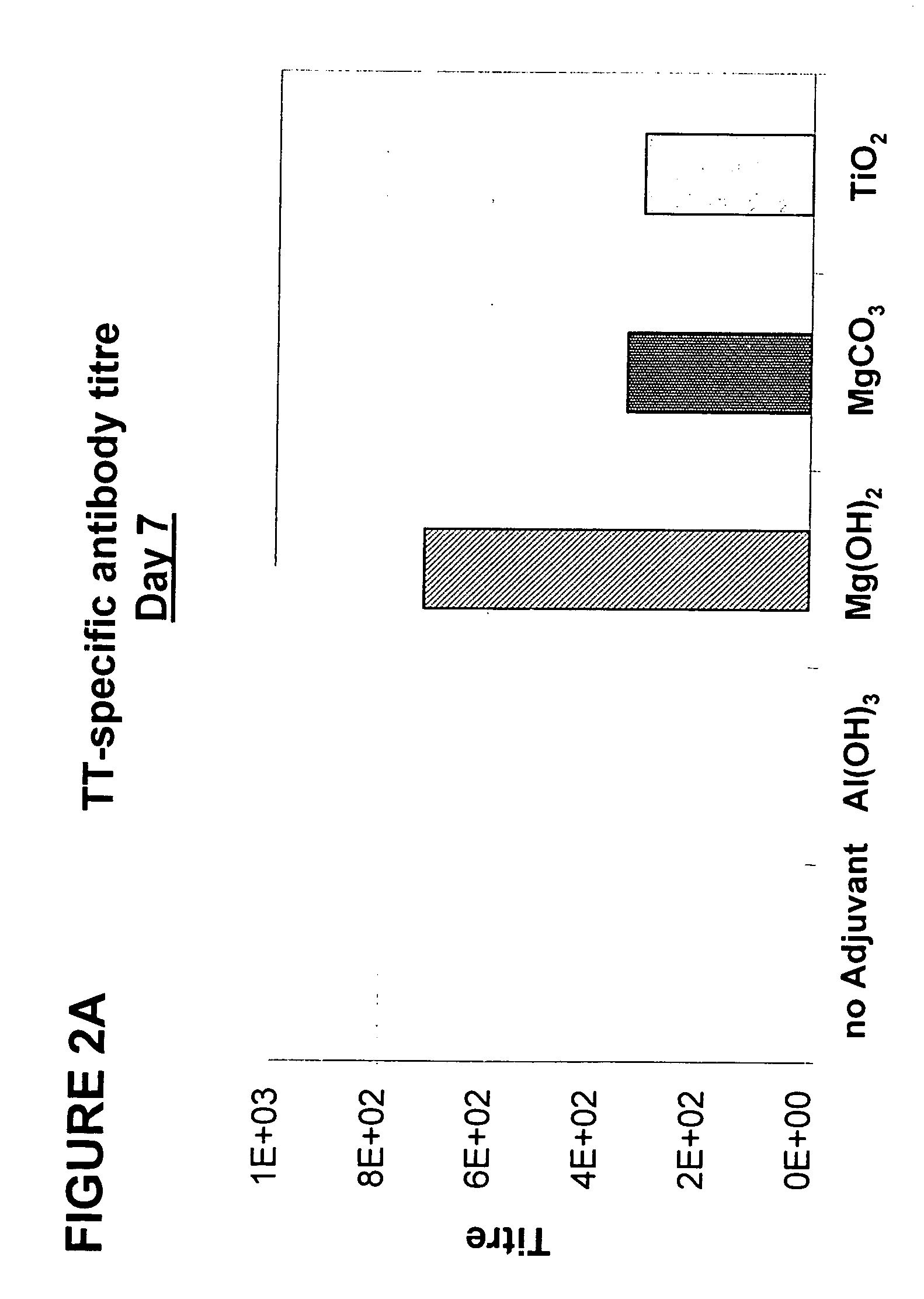
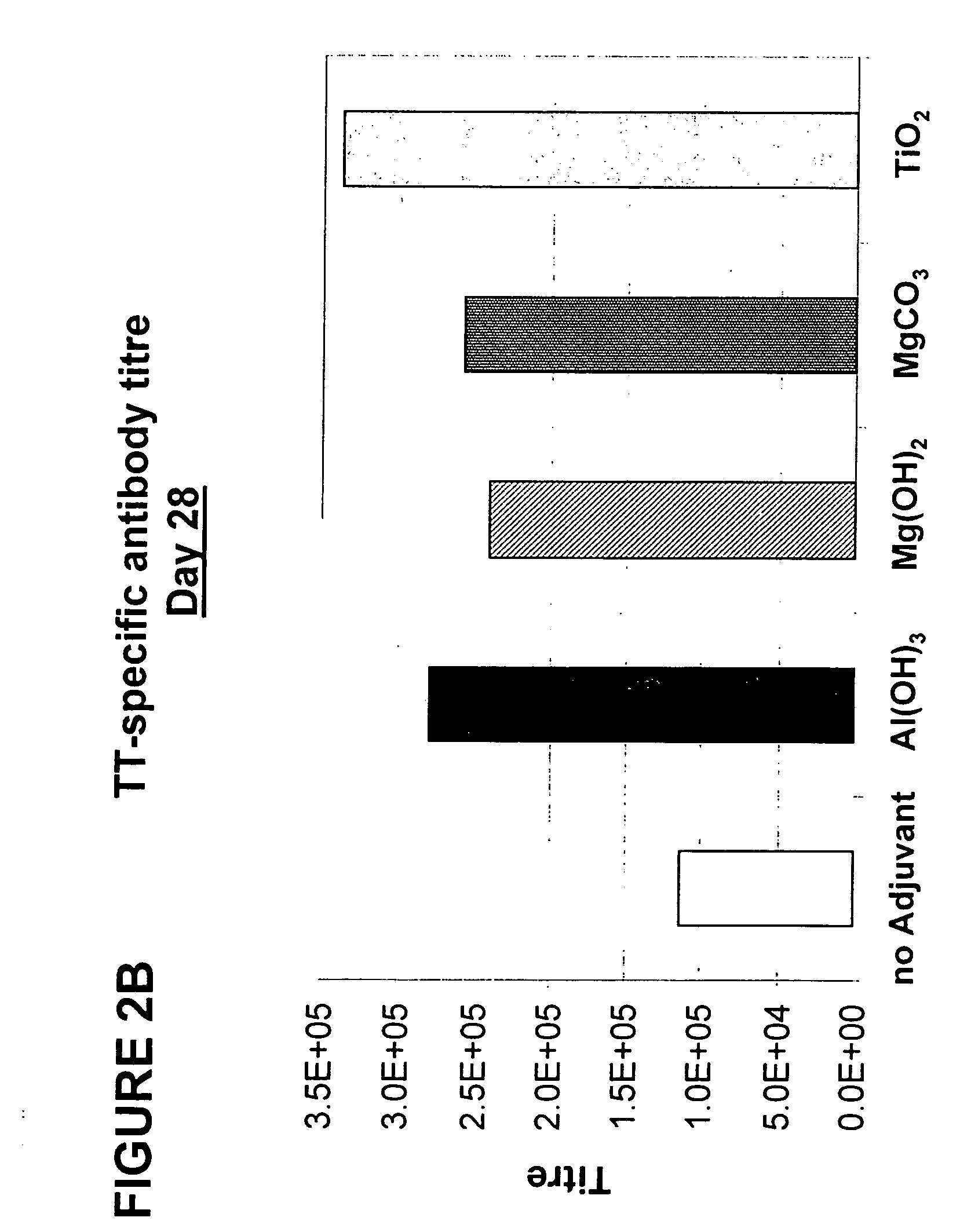
Parenteral vaccine formulations and adjuvant compositions comprising certain salts as adjuvants are disclosed. Such parenteral vaccine formulations are used for generating an immune response in a subject following administration of the vaccine formulation or the adjuvant composition. Also disclosed is the use of these salts as adjuvants in parenteral vaccine formulations and adjuvant compositions, and to vaccine adjuvants comprising such salts.
Owner:ALK ABELLO SA
Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655ASimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
Application of polypeptide hydrogel serving as protein vaccine adjuvant and protein vaccine
PendingCN105497891AGood adjuvant effectIncrease varietyVertebrate antigen ingredientsProtein s antigenBiocompatibility Testing
The invention provides an application of polypeptide hydrogel serving as a protein vaccine adjuvant and a protein vaccine. The hydrogel has good biocompatibility and can stimulate an organism to produce antigen-specific body fluid and cellular immune response very well after physically mixed with a protein antigen simply. According to the application, the polypeptide sequence is Nap-GFFY-OMe.
Owner:NANKAI UNIV
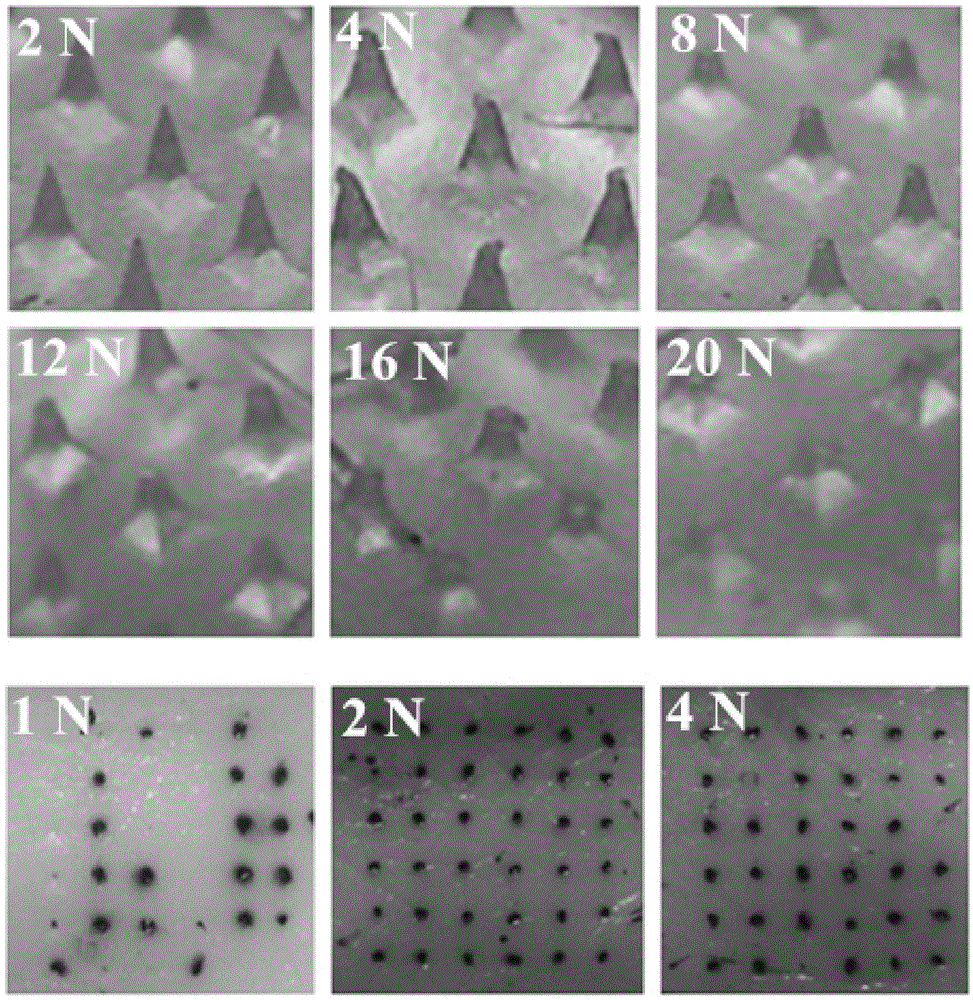
Autolytic microneedle transdermal patch and preparation method thereof
ActiveCN105311000AImproving immunogenicitySolve the problem that it is difficult to induce Th1 type cellular immunityPharmaceutical non-active ingredientsImmunological disordersTransdermal patchIntramuscular injection
The invention belongs to an autolytic microneedle in the technical field of transdermal drug delivery and especially relates to an autolytic microneedle transdermal patch and a preparation method thereof. In the invention, endogenic oligomerized hyaluronic acid and / or low-molecular-weight heparan sulfate are employed as a microneedle substrate material and a vaccine adjuvant at the same time to prepare the autolytic microneedle in which a vaccine is loaded, thereby preparing the autolytic microneedle transdermal patch in the invention. The autolytic microneedle transdermal patch can achieve effective transcutaneous immune of the vaccine and enhance humoral immune response of the vaccine. Compared with intramuscular injection immune and other autolytic microneedle transcutaneous immune in the prior art, the autolytic microneedle transdermal patch can significantly enhance cellullar immunologic response of the vaccine.
Owner:BEIJING CAS MICRONEEDLE TECH LTD
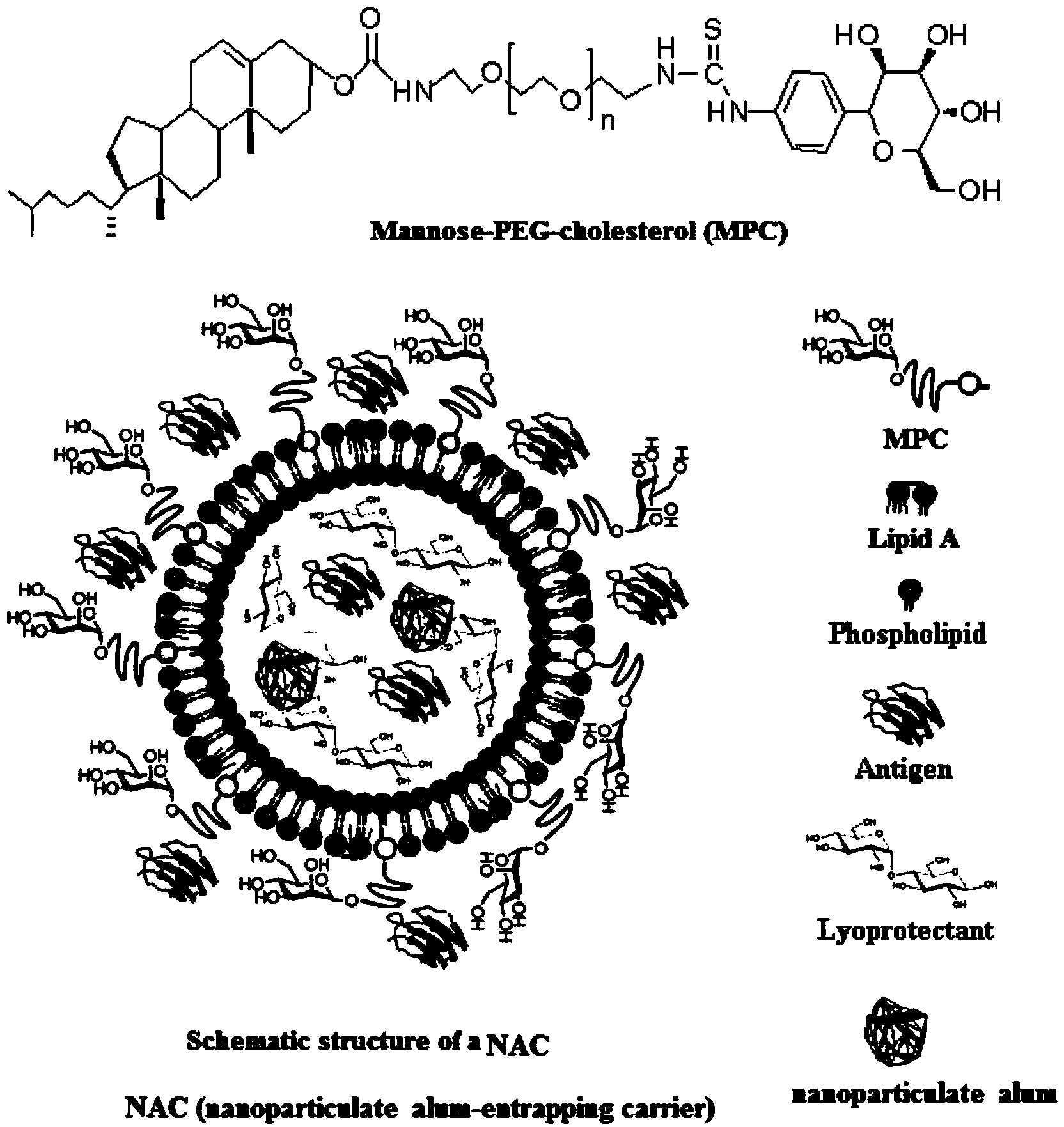
Nano aluminum-encapsulating carrier and application thereof
InactiveCN104055736AImprove stabilityEnhance internal and external stabilityAntiinfectivesEmulsion deliveryBiocompatibility TestingALUMINUM PHOSPHATE
The invention discloses a nano aluminum-encapsulating carrier and an application of the nano aluminum-encapsulating carrier in constructing a vaccine adjuvant transfer system. The nano aluminum is nanoparticles of aluminum phosphate, aluminum sulfate and aluminum hydroxide or a mixture of aluminum phosphate, aluminum sulfate and aluminum hydroxide, wherein the grain size is below 1 mu m; the carrier is a lipidosome, a lipoid, an emulsion, a nano-capsule or a micro-capsule. Construction of the vaccine adjuvant transfer system is the main purpose of the nano aluminum-encapsulating carrier. The application of the nano aluminum-encapsulating carrier in constructing the vaccine adjuvant transfer system comprises the following step: encapsulating vaccine components in the nano aluminum-encapsulating carrier; or adsorbing the vaccine components on the surface of the carrier; or purely mixing with the carrier to exert the vaccine adjuvant and the transfer function. The nano aluminum-encapsulating carrier disclosed by the invention has the advantages that the nano aluminum-encapsulating carrier is wide in application range, so that the nano aluminum-encapsulating carrier is suitable for antigens with different pathogens; the nano aluminum-encapsulating carrier is high in stability and can encapsulate antigens so as to enhance in vivo and in vitro stability; the nano aluminum-encapsulating carrier is high in safety, and the use materials have good biocompatibility; the nano aluminum-encapsulating carrier is many in inoculation and wide in way, and the carrier can be inoculated through track mucosa or subcutaneous, intracutaneous and intramuscular injection; the carrier is strong in immunosuppression induction potency.
Owner:ANHUI MEDICAL UNIV
Adjuvant Composition and Methods for Its Use
InactiveUS20070218086A1Enhance and augment immune responseReasonable yieldBacterial antigen ingredientsVirus peptidesVaccine PotencyLymphocyte
The present invention is directed to a vaccine adjuvant which improves the vaccine potency. More specifically, the present invention is directed to the use of a γδT lymphocyte activator as vaccine adjuvant to promote and enhance antigen specific immunological responses, as well as a vaccine composition comprising a γδT lymphocyte activator.
Owner:INNATE PHARMA SA
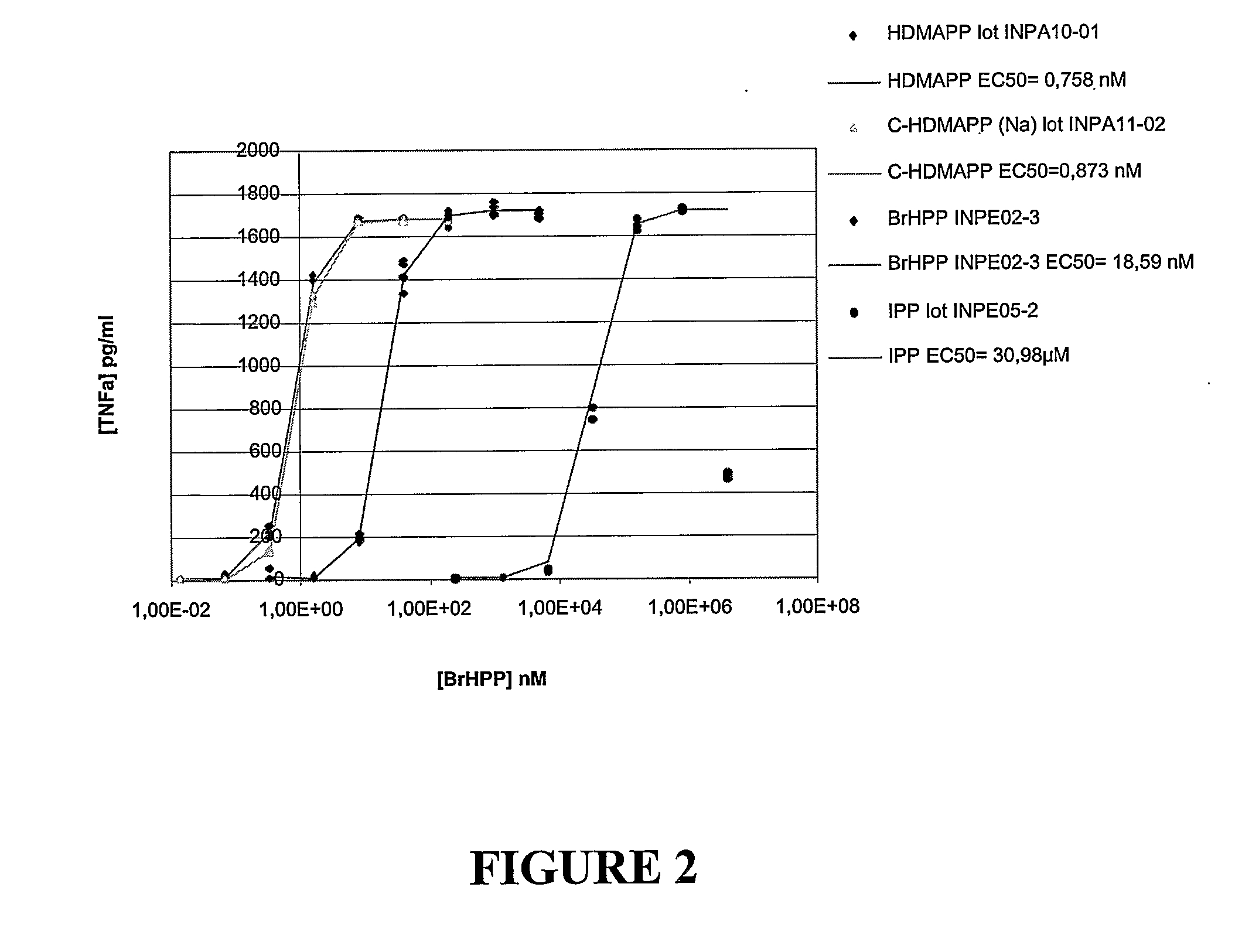
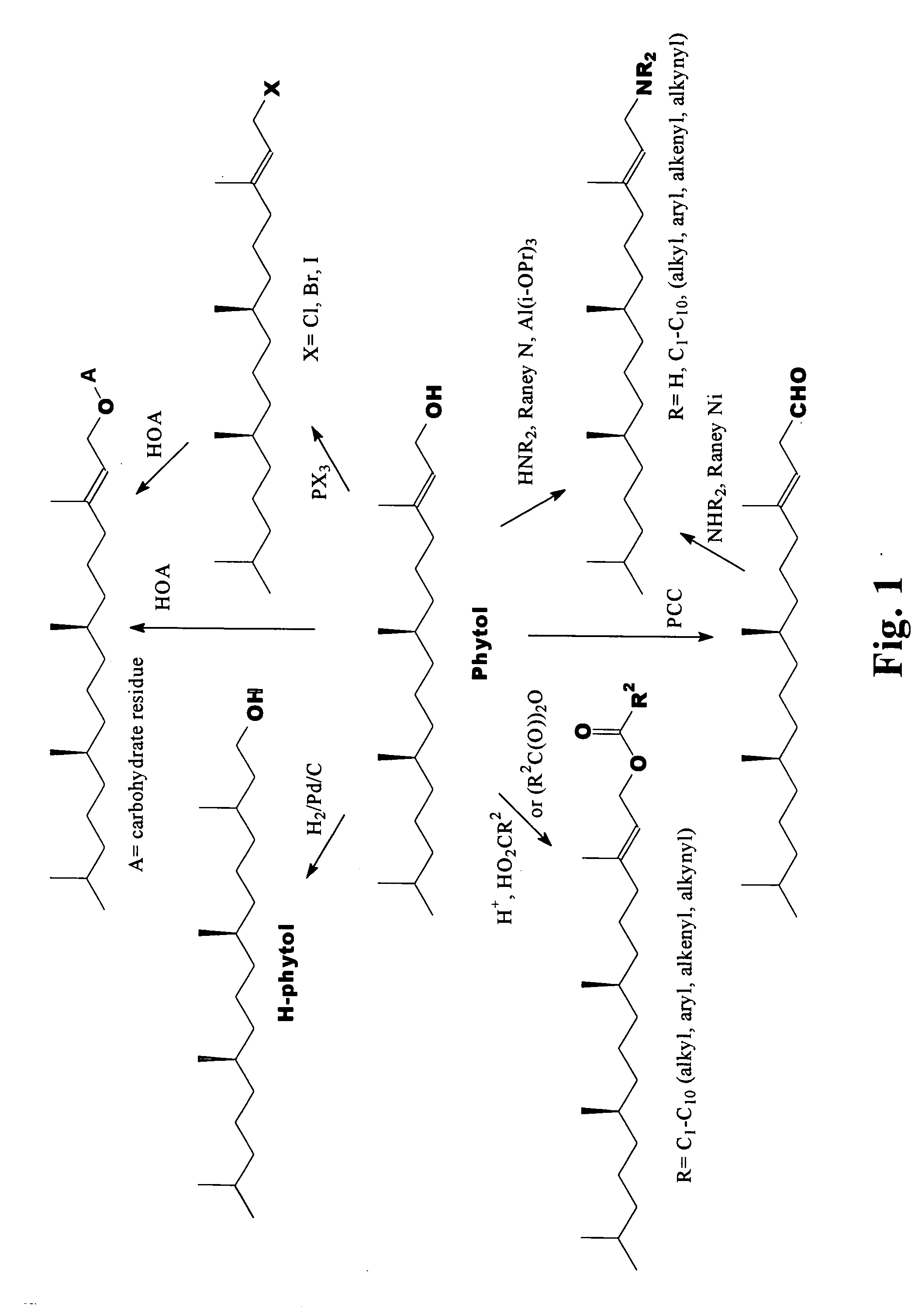
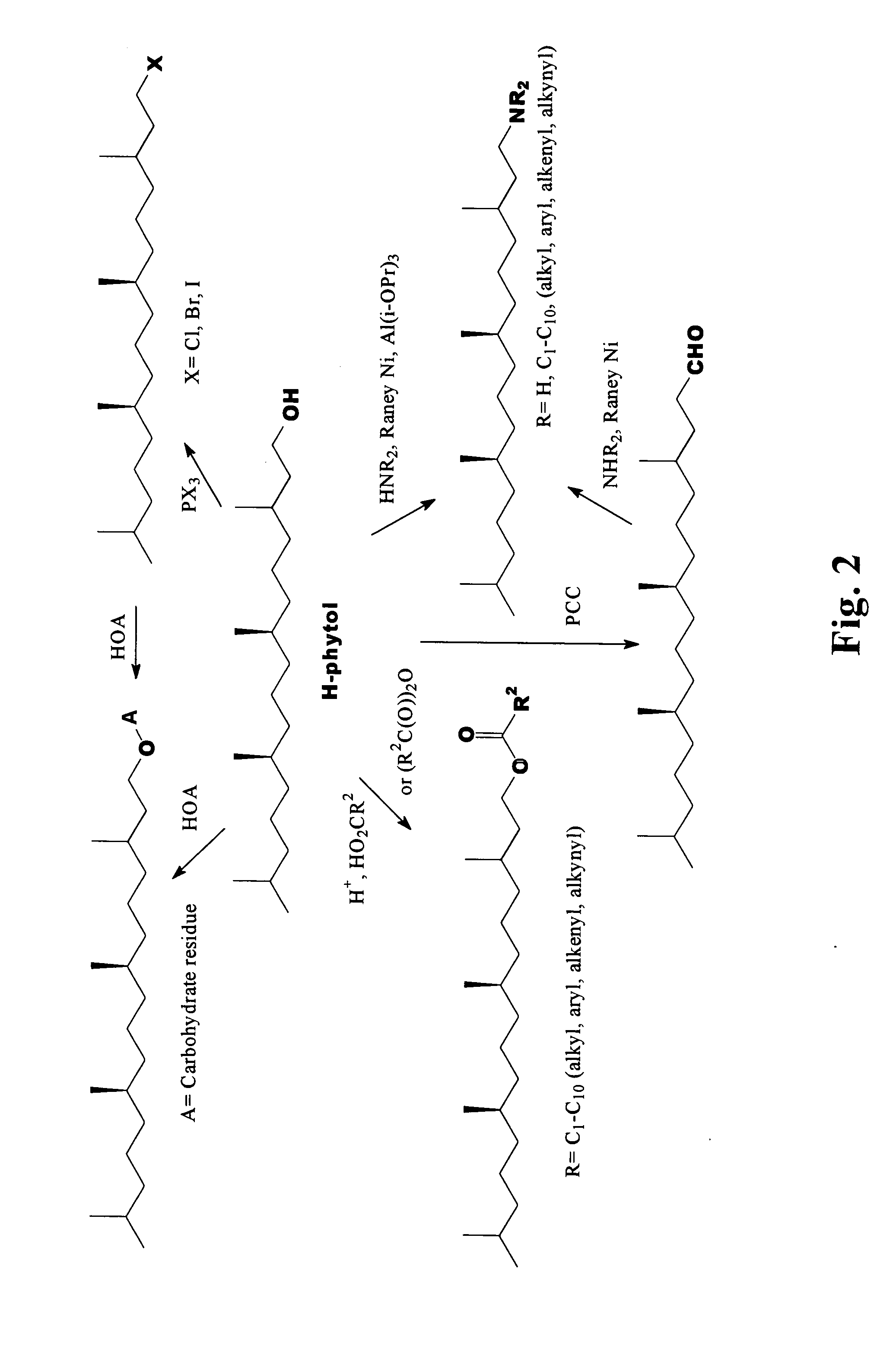
Novel phytol derived immunoadjuvants and their use in vaccine formulations
InactiveUS20050158329A1Improving immunogenicityInduce immunogenic responseBiocideHydroxy compound active ingredientsSide effectParticulate antigen
This invention relates to a novel immunoadjuvant, an adjuvant component, and vaccines containing the adjuvant component. The adjuvant includes phytol or a phytol derivative. The adjuvant component, when combined with a soluble or particulate antigen, provides a vaccine with an enhanced ability to induce both humoral and cytotoxic immune responses while displaying reduced toxicity and / or adverse side effects over vaccines that include the antigen but without the benefit of this adjuvant component.
Owner:GHOSH SWAPAN K
Polypeptide inhibiting porcine PD-1/PD-L1 pathway and application thereof
InactiveCN104761633AGood immune effectCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsBinding sitePD-L1
The invention discloses a polypeptide based on inhibiting porcine PD-1 / PD-L1 composite binding site. The immunological effect of the polypeptide after the polypeptide inhibits the porcine PD-1 / PD-L1 pathway is subjected to primary study, and basis is laid for acquiring polypeptide medicines or vaccine adjuvant for improving the immunological effect. The polypeptide is CTRRNDSGTYFCGAIYLPPKTQINESHQAKLT, and has a molecular weight of 4012.42Da.
Owner:XINXIANG UNIV
Pharmaceutical targeting of a mammalian cyclic di-nucleotide signaling pathway
ActiveUS20150343056A1Stimulate immune responseOrganic active ingredientsCompound screeningNucleotidePharmaceutical drug
Cyclic-GMP-AMP synthase (cGAS) and cyclic-GMP-AMP (cGAMP), including 2′3-cGAMP, 2′2-cGAMP, 3′2′-cGAMP and 3′3′-GAMP, are used in pharmaceutical formulations (including vaccine adjuvants), drug screens, therapies and diagnostics.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
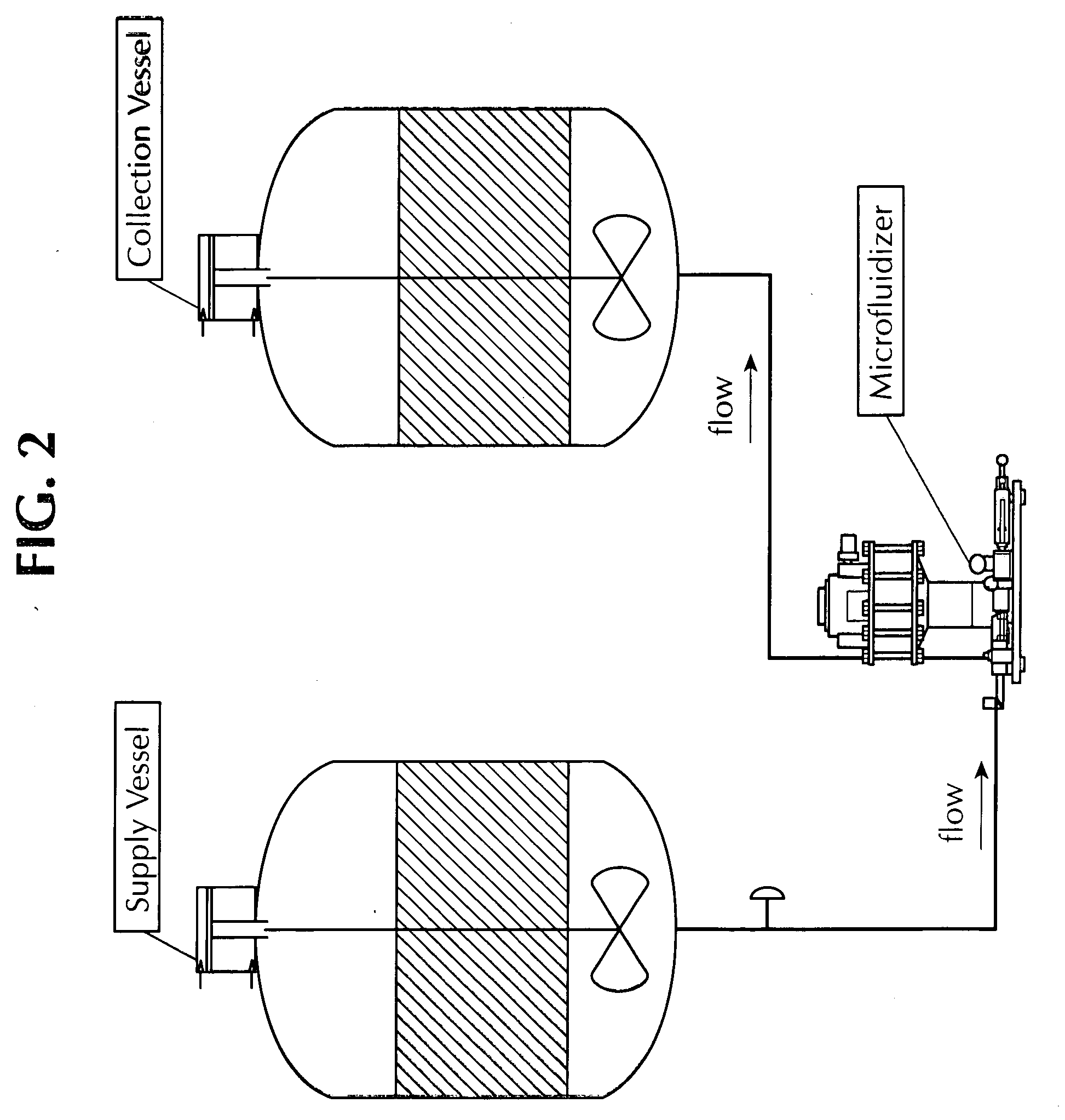
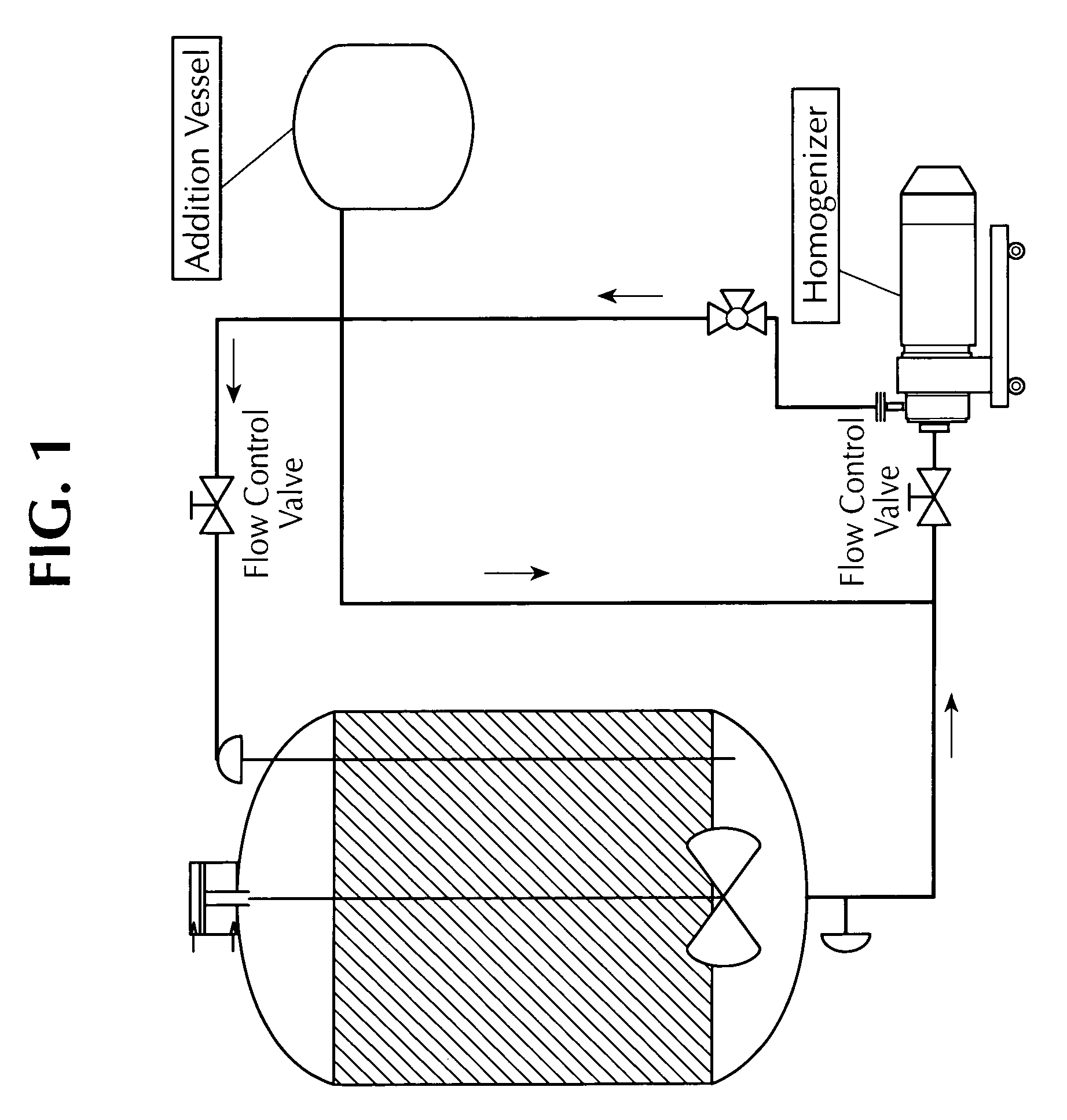
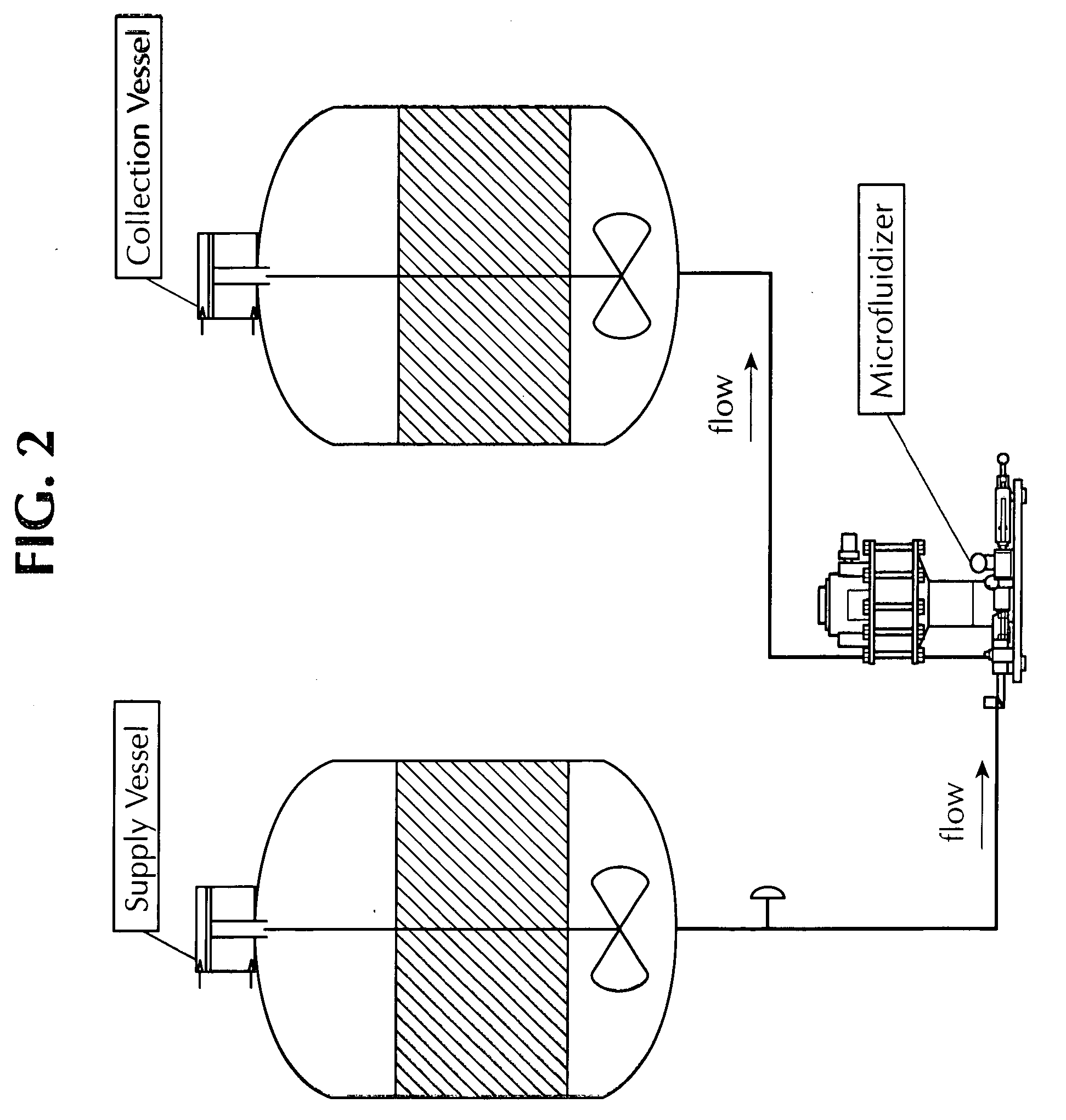
Methods for producing vaccine adjuvants
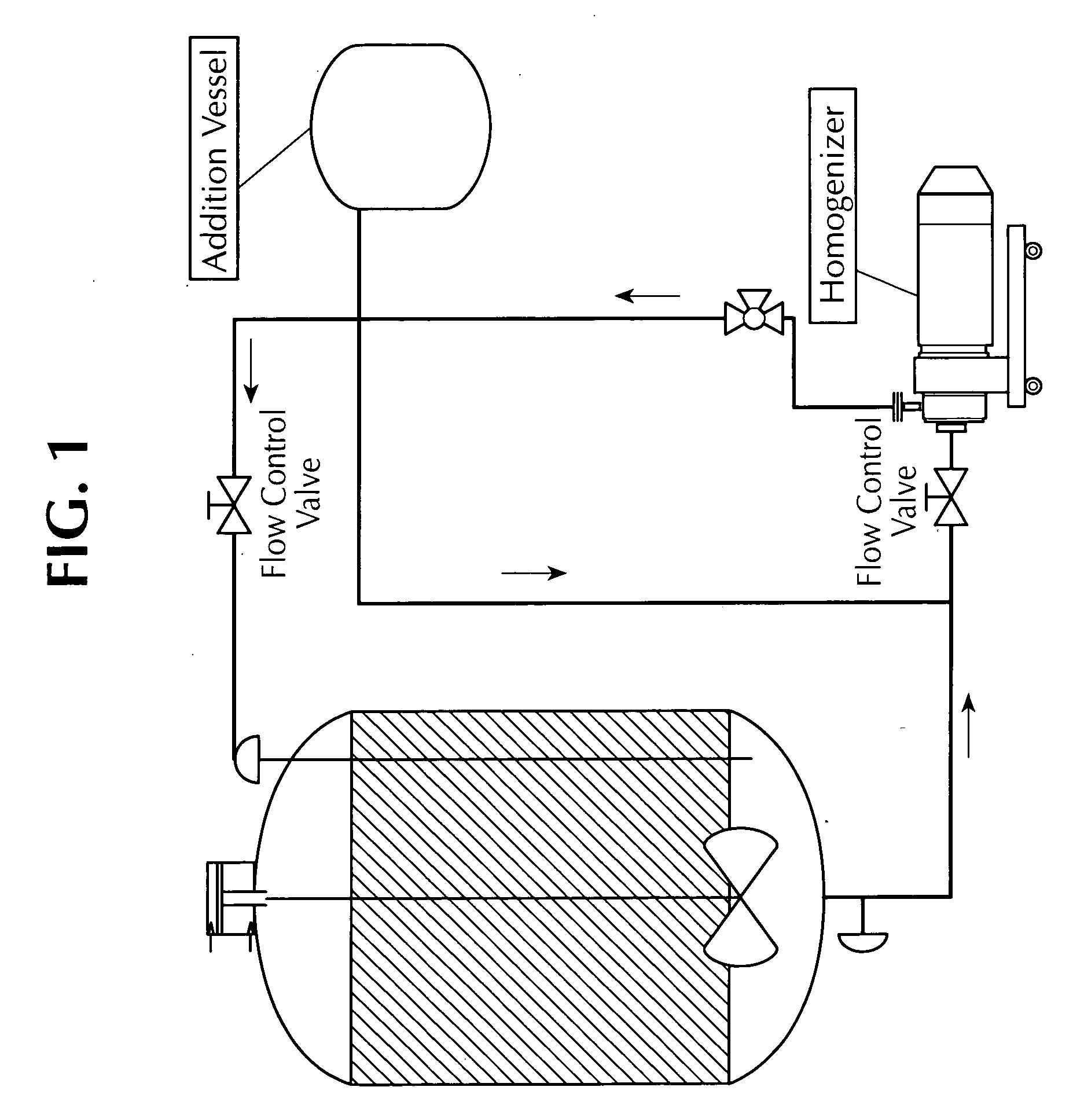
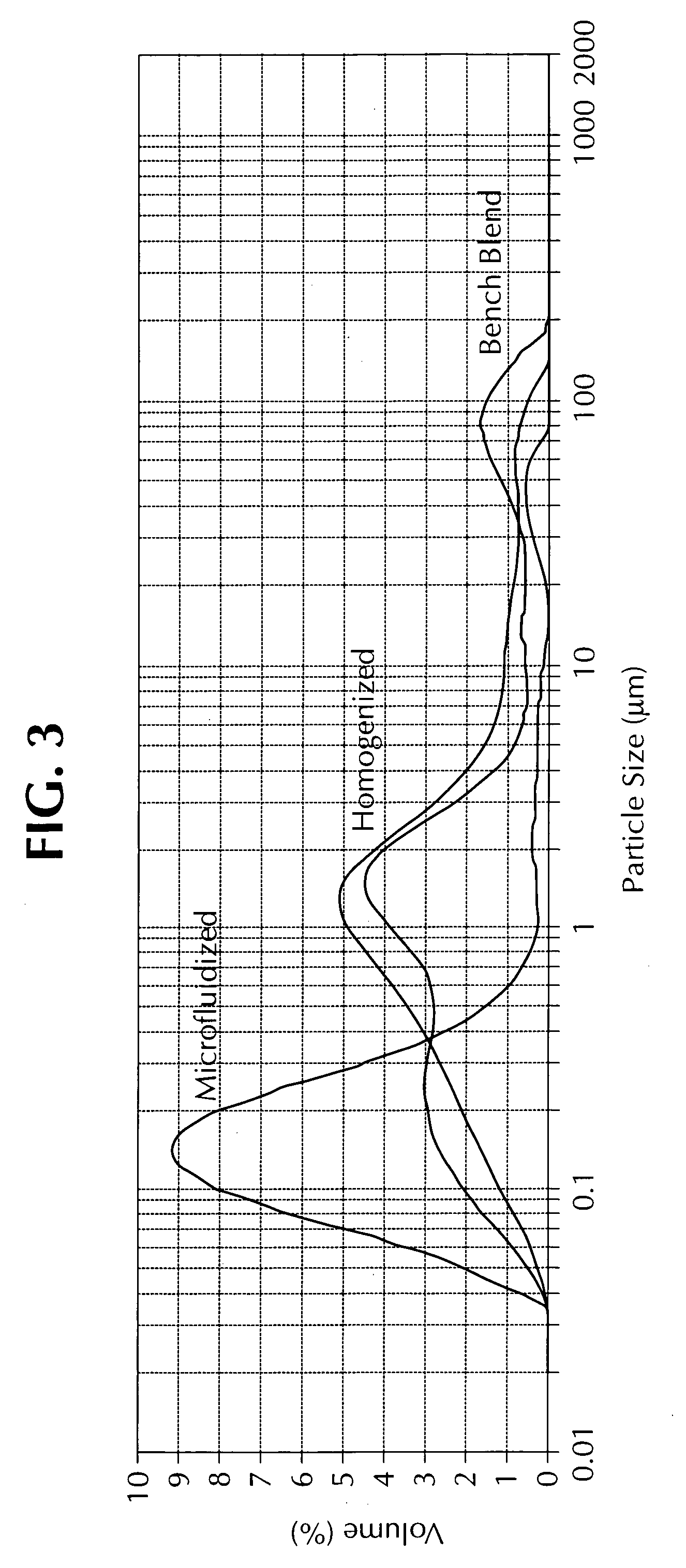
ActiveUS20110165193A1Eliminate needAvoid the needOrganic active ingredientsFlow mixersFiltrationImproved method
An improved method for the manufacture of an oil-in-water emulsion involves three procedures: (i) preparation of a preliminary emulsion; (ii) microfluidization of the preliminary emulsion to reduce its droplet size; and (iii) filtration of the microfluidized emulsion through a hydrophilic membrane.
Owner:NOVARTIS AG
Multimeric fusion proteins of the TNF superfamily ligands
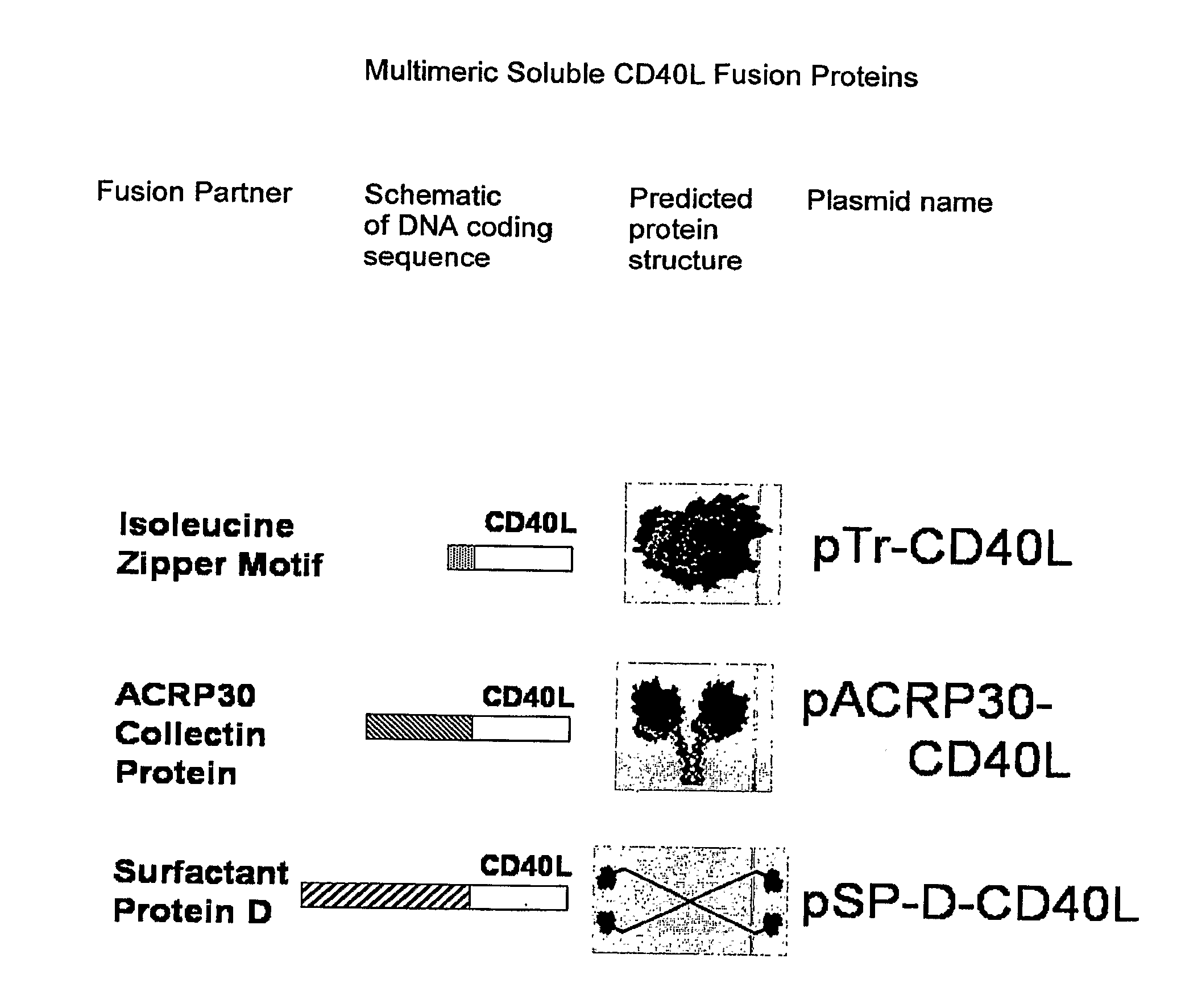
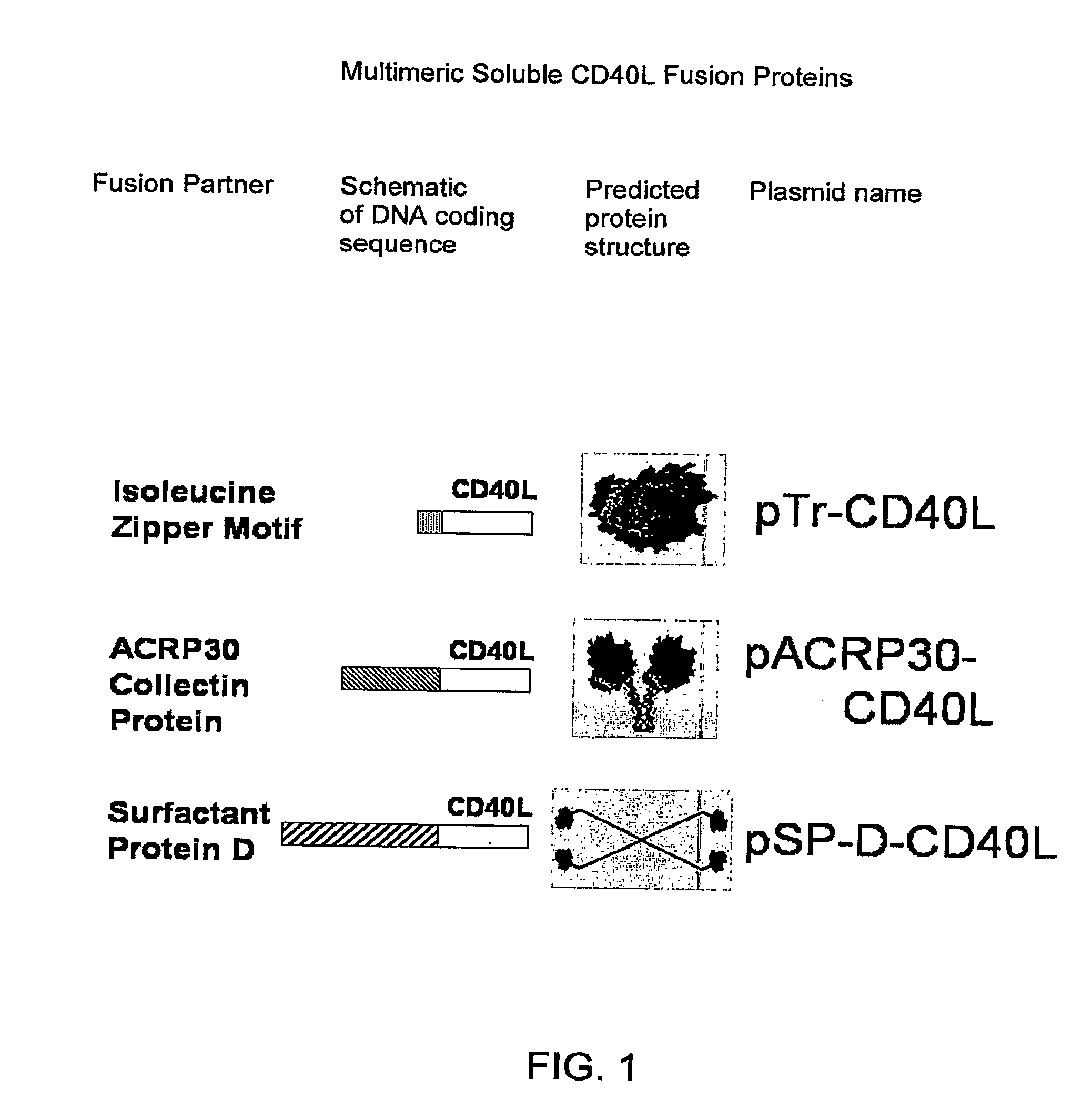
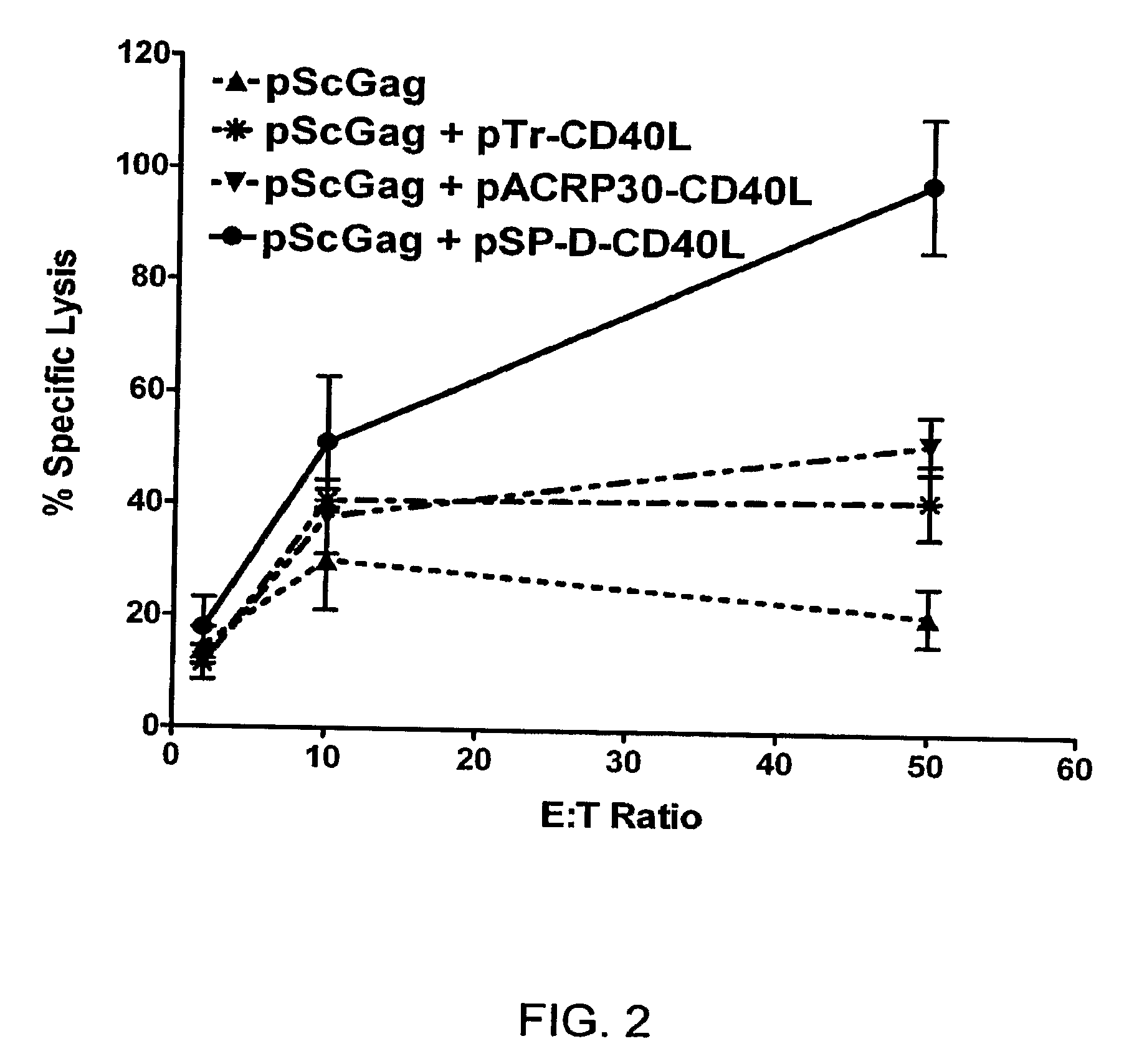
A method for constructing stable bioactive fusion proteins of the difficult to express tumor necrosis factor superfamily (TNFSF), and particularly members CD40L (CD154) and RANKL / TRANCE, with collecting, particularly pulmonary surfactant protein D (SPD) is described. Single trimers of these proteins lack the full stimulatory efficacy of the natural membrane forms of these proteins in many cases. The multimeric nature of these soluble fusion proteins enables them to engage multiple receptors on the responding cells, thereby, mimicking the effects of the membrane forms of these ligands. For CD40L-SPD, the resulting protein stimulates B cells, macrophages, and dendritic cells, indicating its potential usefulness as a vaccine adjuvant. The large size of these fusion proteins makes them less likely to diffuse into the circulation, thereby limiting their potential systemic toxicity. This property may be especially useful when these proteins are injected locally as a vaccine adjuvant or tumor immunotherapy agent to prevent them from diffusing away. In addition, these and other TNFSF-collectin fusion proteins present new possibilities for the expression of highly active, multimeric, soluble TNFSF members.
Owner:RGT UNIV OF CALIFORNIA
Self-emulsifying vaccine adjuvant and preparation thereof
InactiveCN1679933AHas self-emulsifying effectImprove stabilityEmulsion deliveryAntibody medical ingredientsMedicineOil phase
Owner:南京艾利珂药业有限公司
Adjuvant and Vaccine Compositions
InactiveUS20150044242A1Improve abilitiesImprove adsorption capacitySnake antigen ingredientsPharmaceutical non-active ingredientsSterolMedicine
Methods are provided for preparing and delivering an adjuvant for vaccines including lecithin, polymer and one or more additives. The polymer is preferably polyacrylic acid-based. The additive is preferably one or more of a glycoside and a sterol. The method of preparation includes hydrating lecithin and a polymer in saline or water and mixing the lecithin and polymer to form the adjuvant. Additives can be included prior to or after hydration of the lecithin and polymer.
Owner:ADVANCED BIOADJUVANTS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com