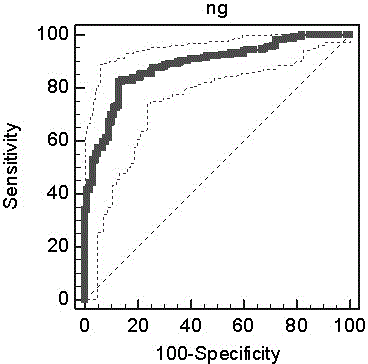
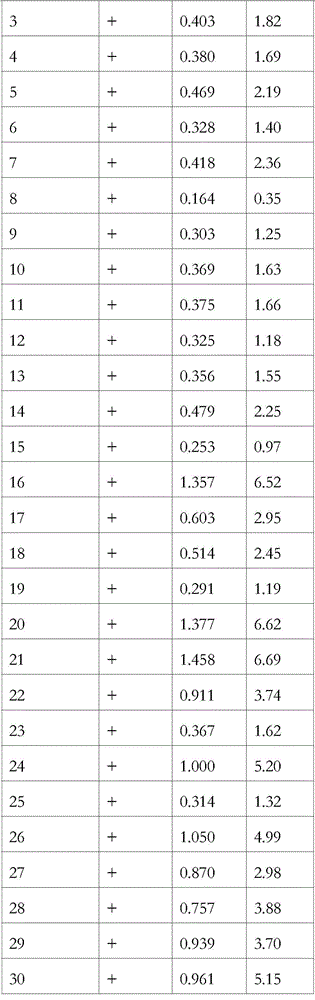
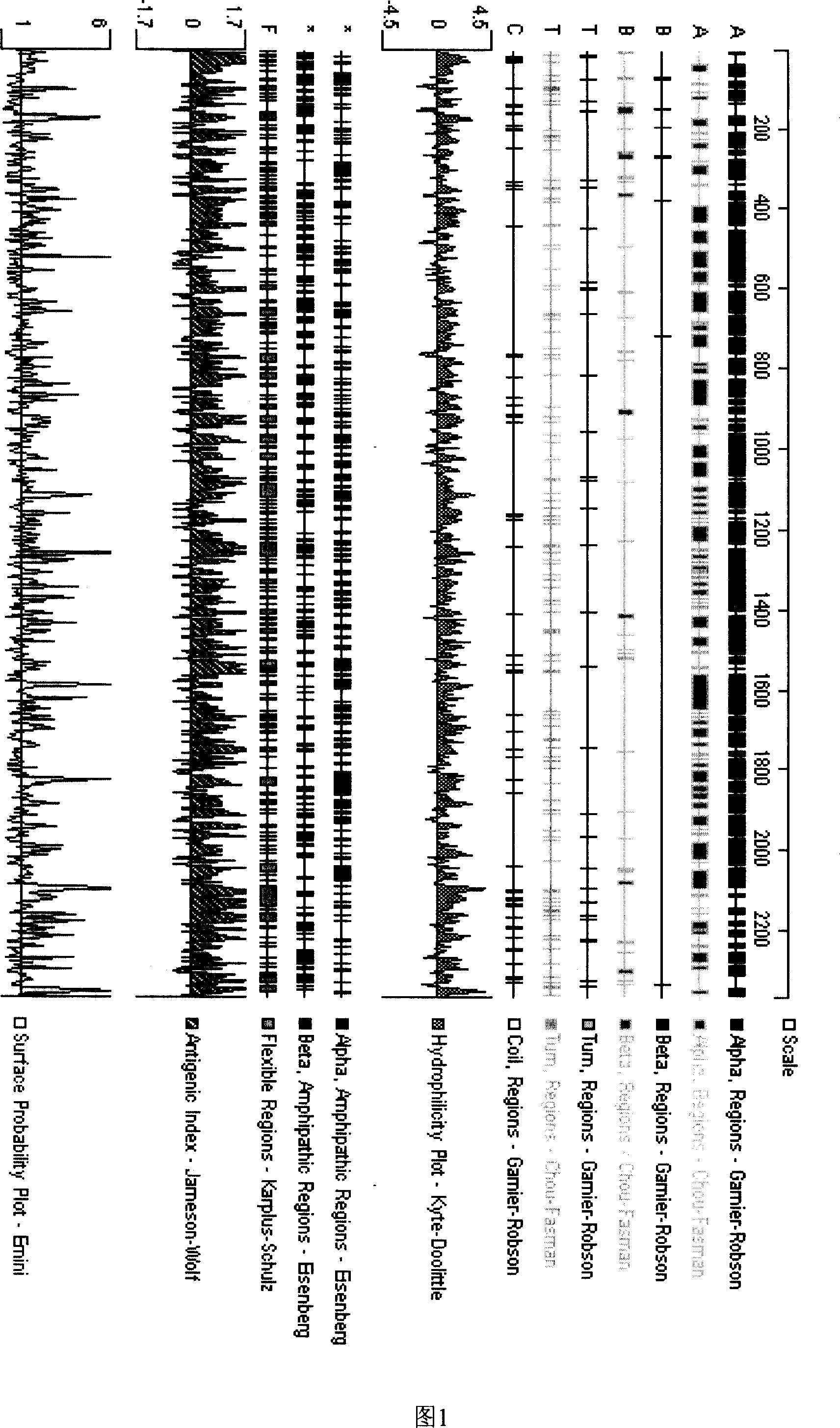
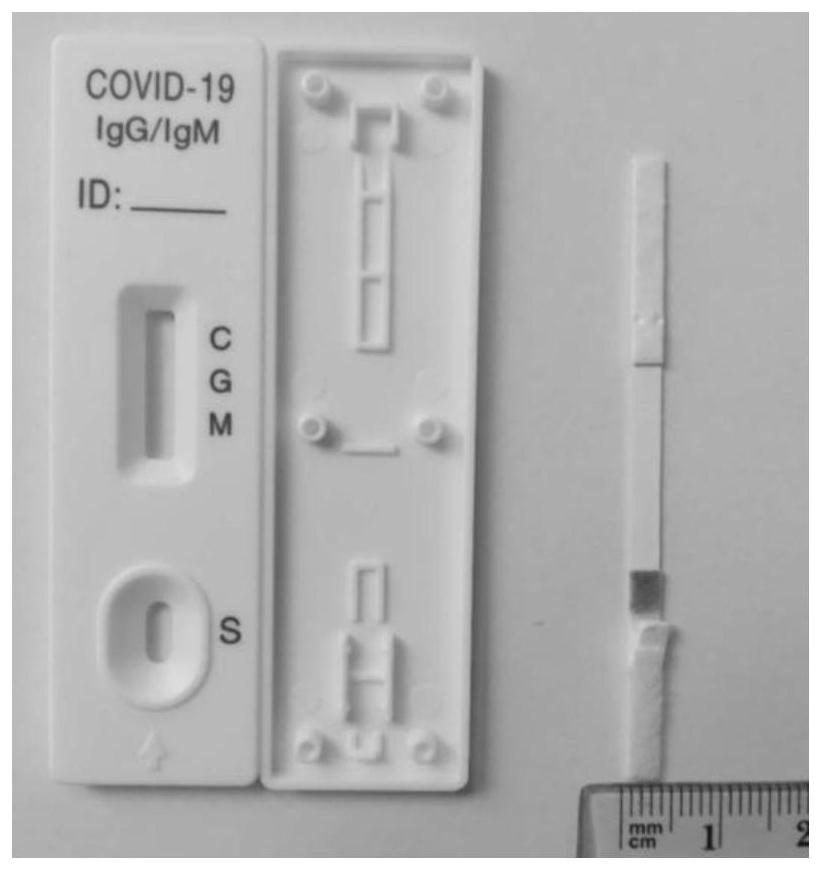
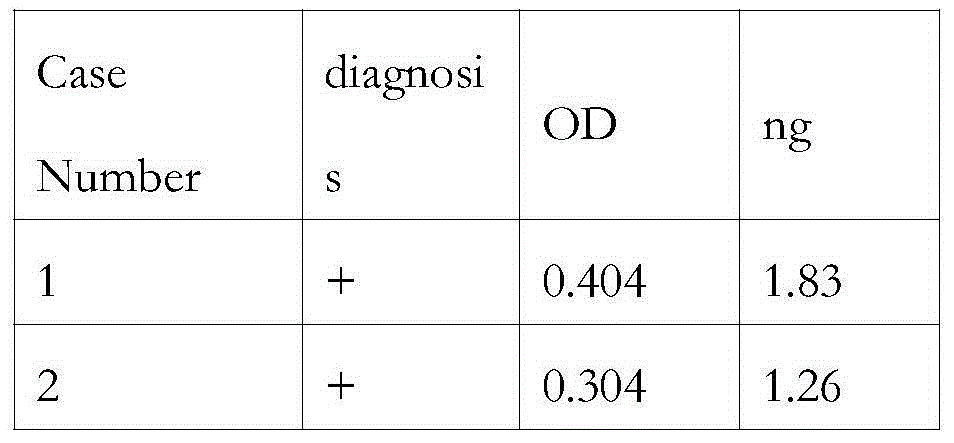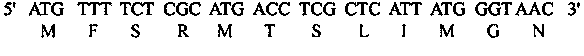Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Protein s antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
Application of polypeptide hydrogel serving as protein vaccine adjuvant and protein vaccine
PendingCN105497891AGood adjuvant effectIncrease varietyVertebrate antigen ingredientsProtein s antigenBiocompatibility Testing
The invention provides an application of polypeptide hydrogel serving as a protein vaccine adjuvant and a protein vaccine. The hydrogel has good biocompatibility and can stimulate an organism to produce antigen-specific body fluid and cellular immune response very well after physically mixed with a protein antigen simply. According to the application, the polypeptide sequence is Nap-GFFY-OMe.
Owner:NANKAI UNIV
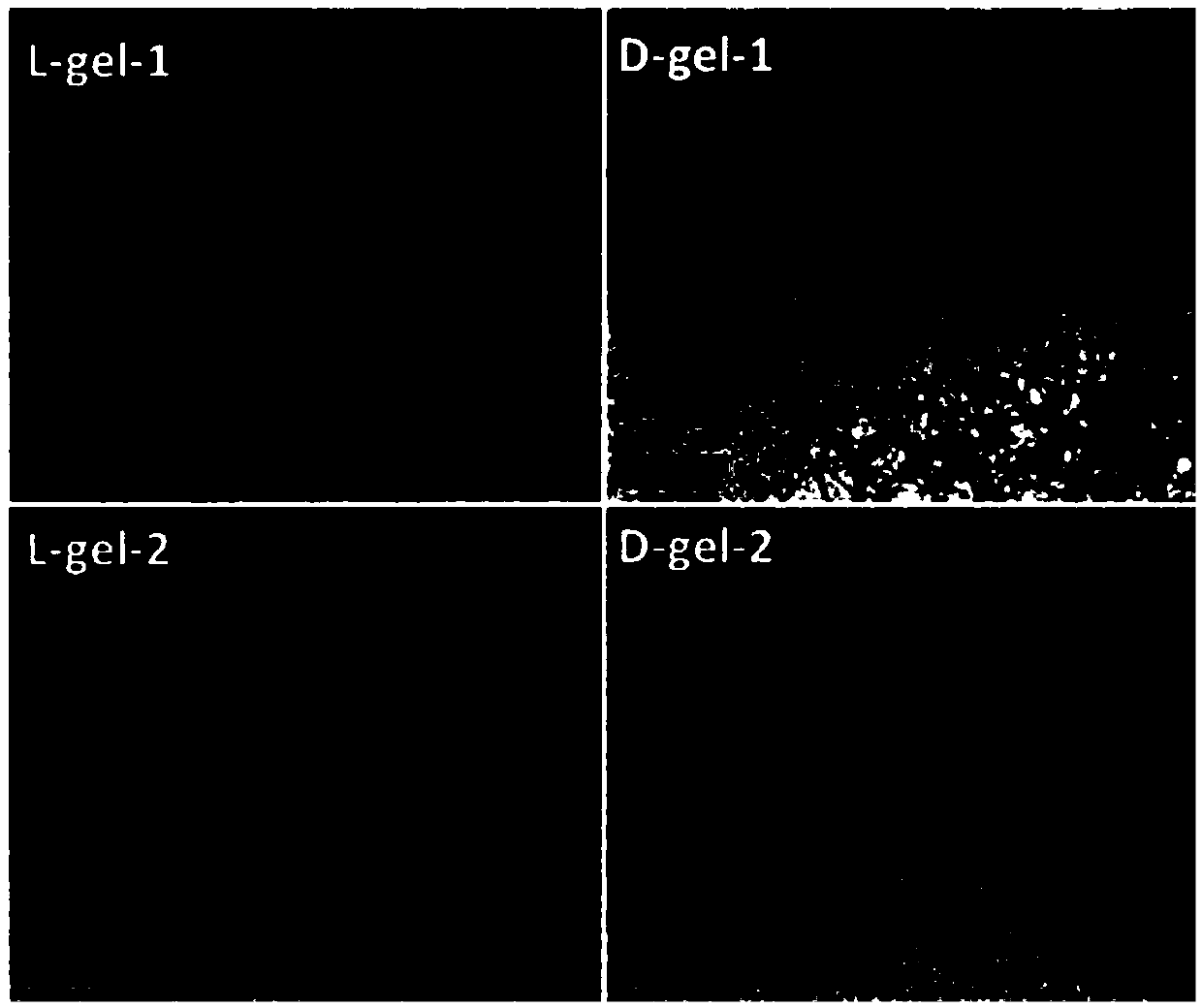
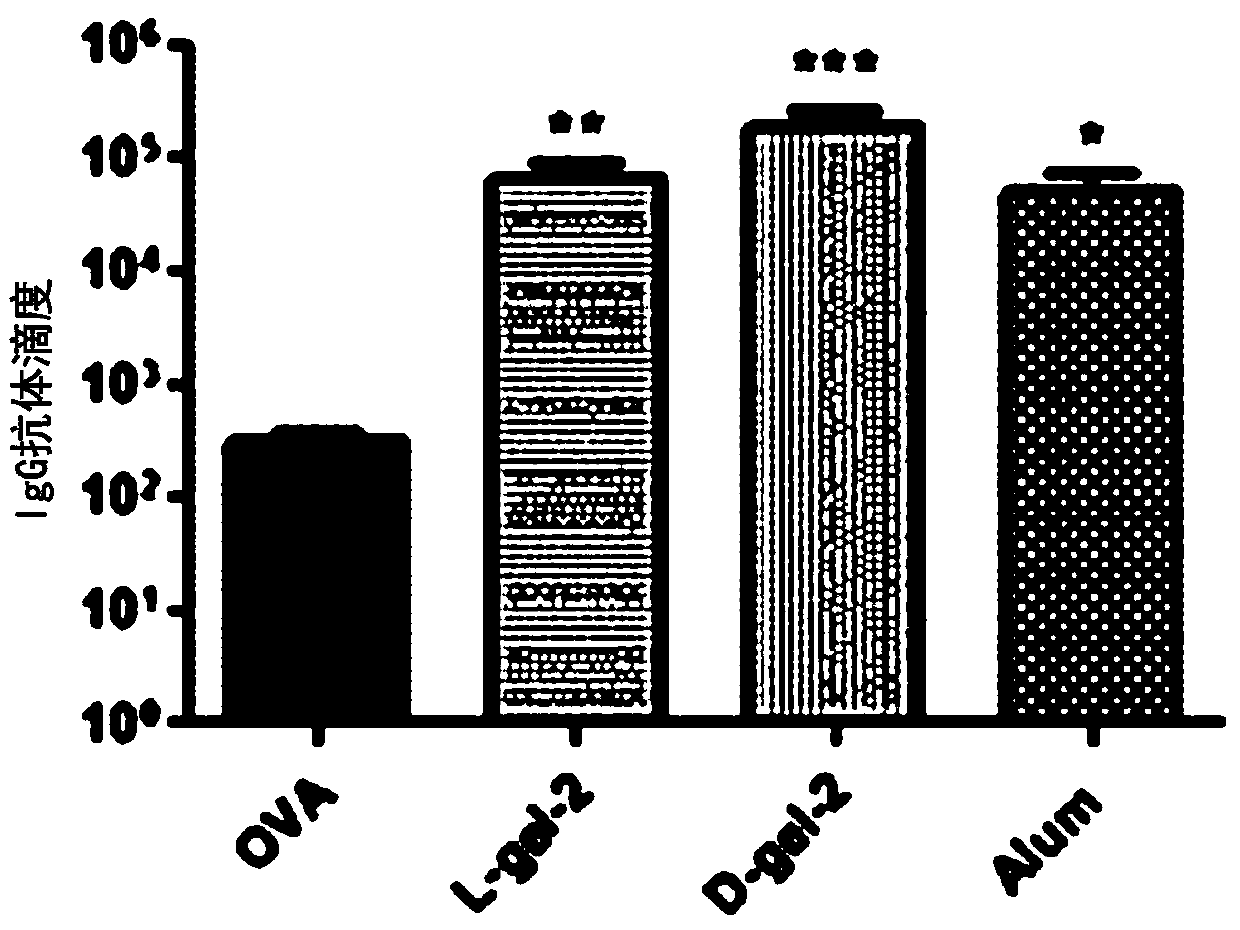
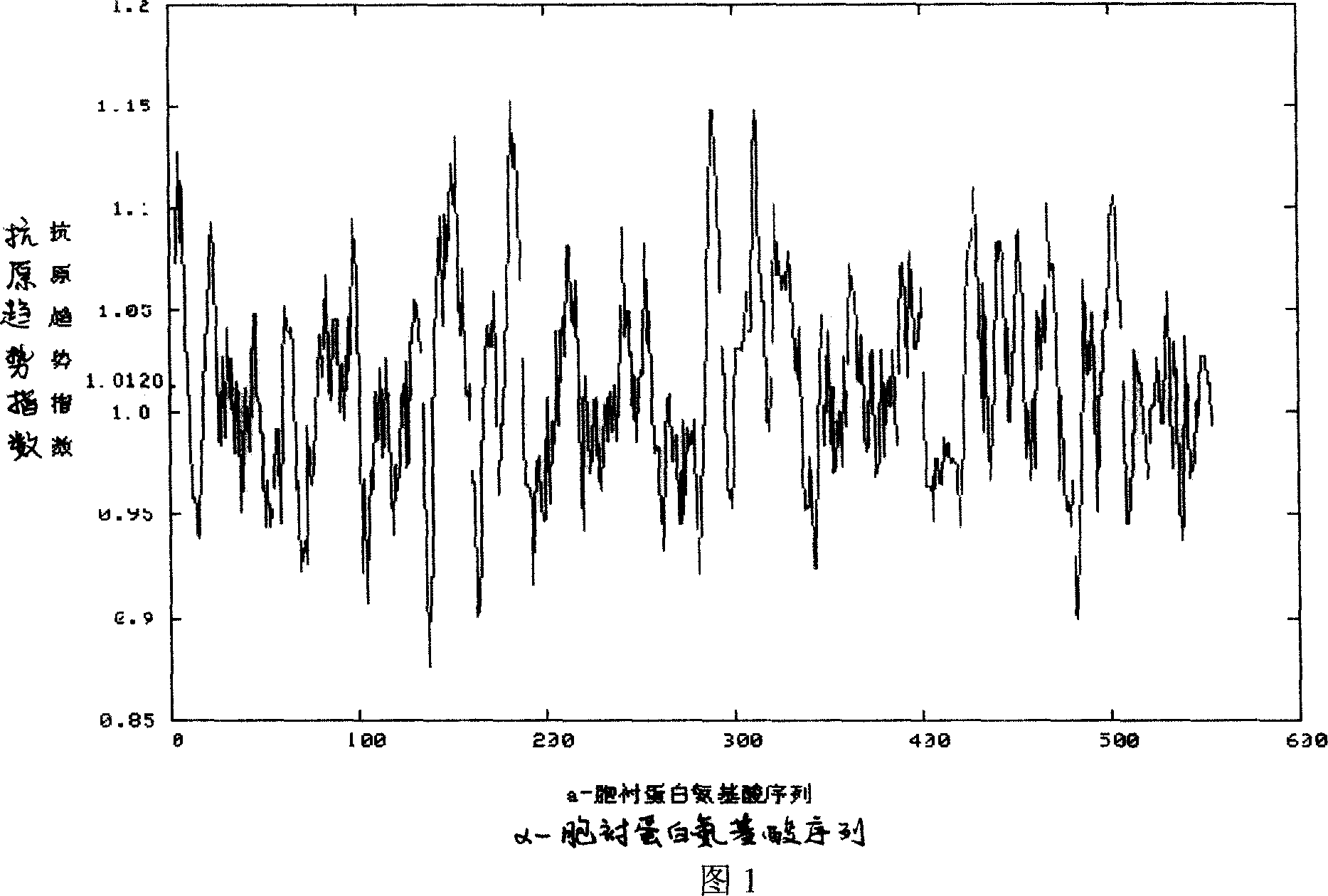
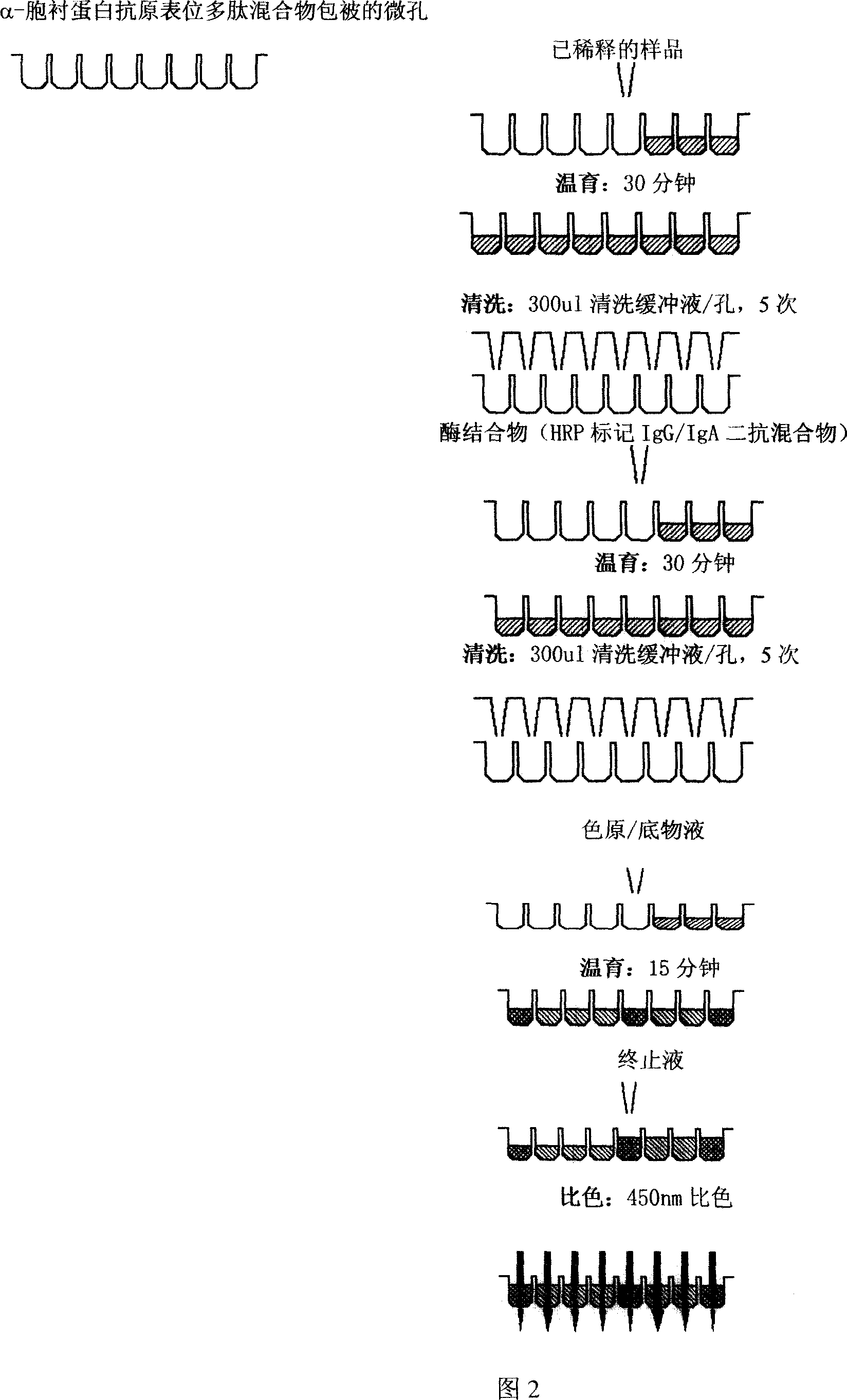
Alpha-fodrin antigen epi-position polypeptide mixture and its use
InactiveCN1979165AAdvantages of value assessmentBiological testingHybrid peptidesAntigen epitopeAntigen
The invention relates to screen for antigen epitope polypeptide, and building up of immunology detection method and the manufacturing and application for reagent for clinical diagnosis, especially for alpha-cyst albumen antigen epitope polypeptide.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
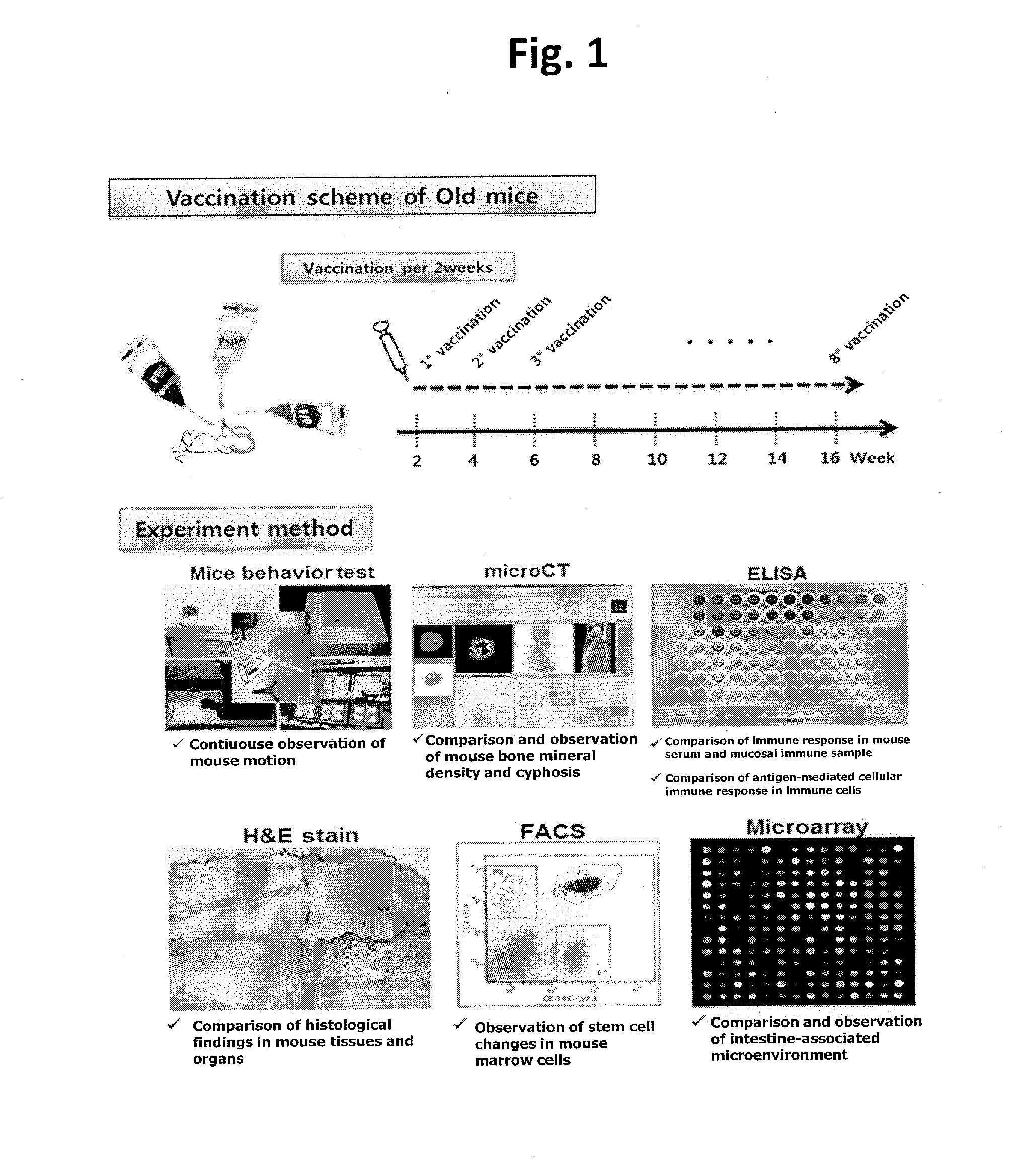
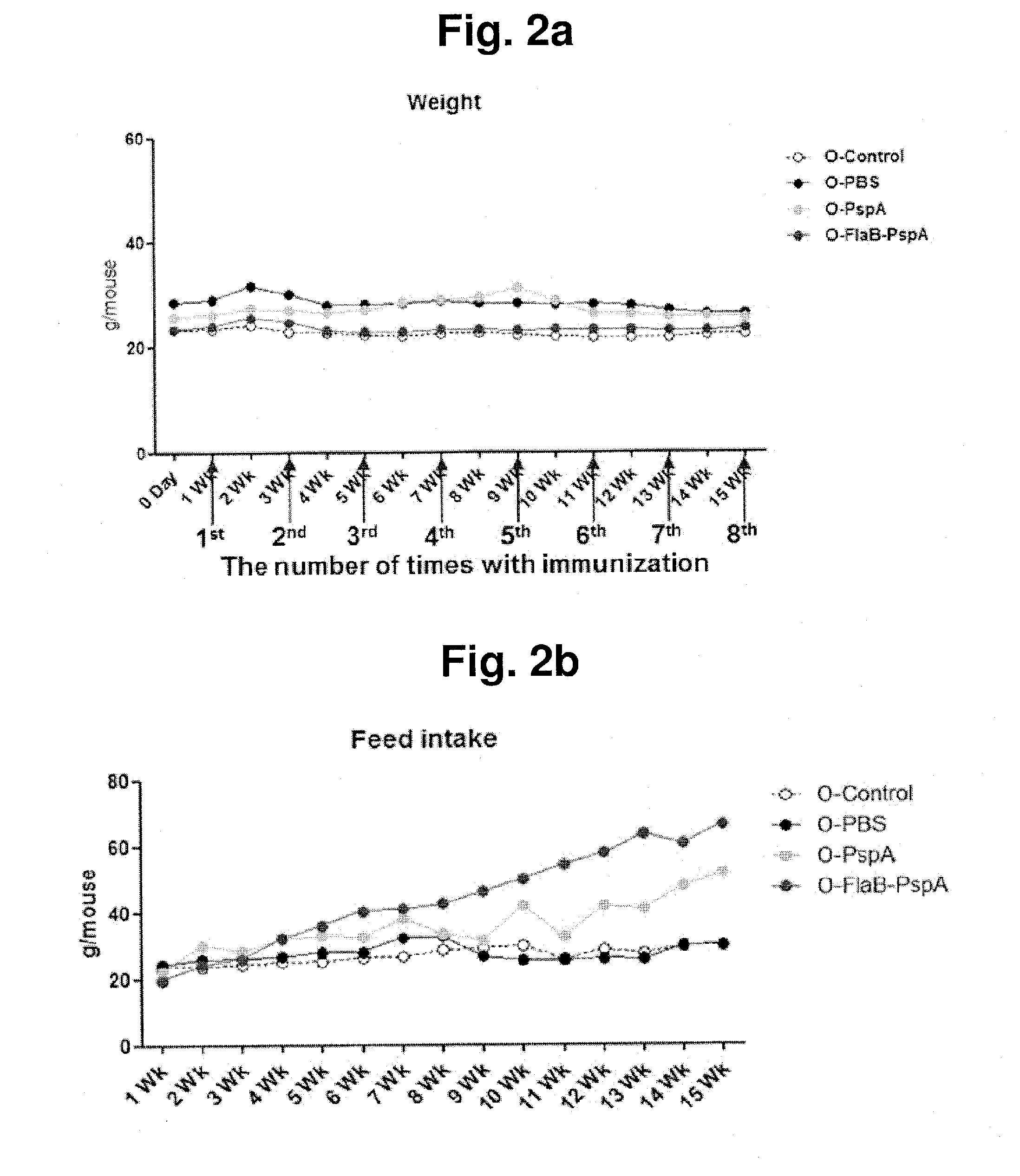
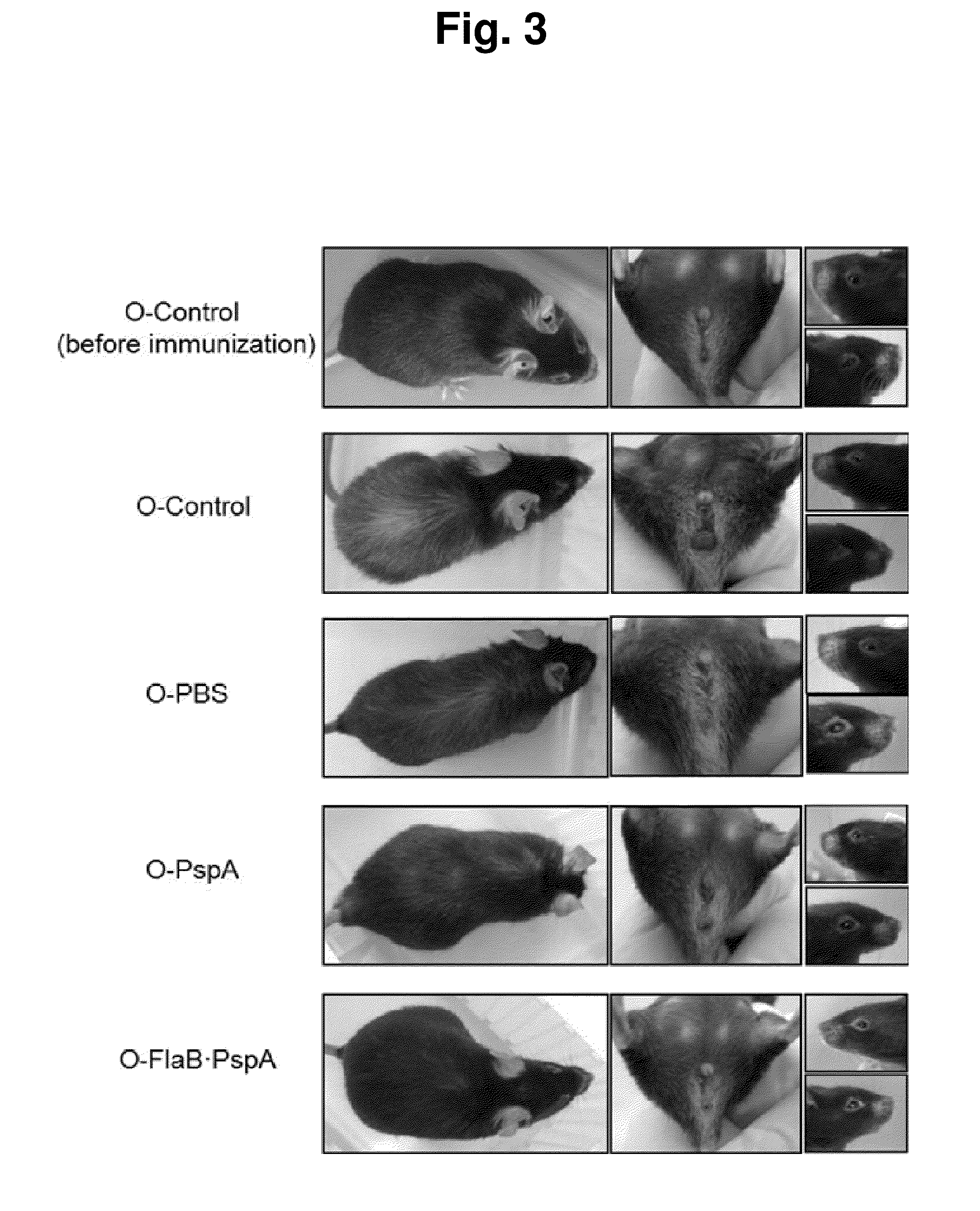
Composition comprising recombinant fusion protein of pathogenic antigen protein and flagellin of vibrio vulnificus for preventing, alleviating, or treating aging
InactiveUS20160083437A1Preventing and improving and treating agingPreventing and improving and treating metabolicBacterial antigen ingredientsPeptide/protein ingredientsFlagellinArcobacter
The present invention relates to a composition for preventing, improving, or treating aging, wherein the composition comprises a recombinant protein of flagellin, which is the constituent of Vibrio vulnificus flagella, fused with a pathogenic protein antigen, as an active component. According to the present invention, the recombinant protein of the present invention can improve external and internal aging-related malfunctions and enhance immunity. Also, the composition of the present invention can easily perform immunization through mucosal administration.
Owner:IND FOUND OF CHONNAM NAT UNIV
Tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein and application thereof
The invention discloses a tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein, namely, nonapeptide. The amino acid sequence of the nonapeptide is as follows: P5: YLGGTTGPV, or P6: YIVGFCLLV, or P7: TLTWLFAFV, or P8: GLVAGLSAV, or P9: ALGMLIAGL, or P10: MLIAGLPCL, or P11: LLCAIFAEV, or P12: RLWPTVGCL. Accordingto the invention, the HLA-A*0201 restrictive CTL epitope of a tuberculosis medicament resistance related protein antigen is predicted and analyzed by applying SYFPEITHI, BIMAS and NetCTL1.2 databasesand using an immunoinformatics mean according to a primary structure of the antigen so that the epitope peptide is obtained by virtue of selection, and the identified nonapeptide is not reported in documents. The epitope peptide is identified through an in-vitro enzyme linked immunospot (ELISPOT) experiment; and according to the result, a theoretical basis is provided for developing tuberculosis vaccine based on the medicament resistance related protein antigen and more information is provided for designing a tuberculosis polyepitope peptide vaccine based on mixed T cell epitope.
Owner:ZHENGZHOU UNIV
Indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody
The invention discloses an indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody and belongs to the technical field of serum antibody detection. The indirect ELISA method includes that serum 3-type duck hepatitis virus VP1 protein is utilized as an envelope antigen with the peridium quantity as 0.1-1mug per hole, HRP-mice anti-duck IgY is utilized as the HRP with the use concentration as 0.2-2mug / mL, and the coloration time is 10 minutes. The kit comprises a VP1 protein antigen envelope board, the HRP-mice anti-duck IgY, sample diluent, a scrubbing solution, TMB solutions A and B and a stop solution. The method and the kit can be used for detecting the serum 3-type duck hepatitis virus a antibody in duck serum and duck egg yolk, and the serum does not have cross reaction with positive serum of other viruses. Compared with a traditional neutral reaction detection method, the method has the advantage of being convenient and accurate.
Owner:WUHAN CHOPPER BIOLOGY
Prostateexosomal protein antigen, antibody and application thereof
ActiveCN104897900AThe detection method is simple and reliableBiological testingAntibody antigen reactionsProtein s antigen
The invention relates to a prostateexosomal protein (PESP) antigen, a specific corresponding antibody and application thereof, and on the basis further acquires a kit based on prostasome leakage protein quantitative indirect enzyme-linked immunoadsorption so as to effectively detect and diagnose chronic prostatitis. By means of reaction system precision detection, stability test and destructive test on the urine detection kit based on PESP antibody-antigen reaction, the reagent quality shows high batch production probability. The application is truly noninvasive, the operation is simple, and the kit has high sensitivity and specificity. The kit can alleviate the patient pain caused by chronic pelvic pain syndrome / chronic prostatitis, and also early diagnosis can further strengthen prevention and treatment of prostate cancer, therefore the kit has wide application prospect.
Owner:ONCO BIOMEDICAL TECH SUZHOU
Antigen capable of increasing CD4 + CD25 + Foxp3 + regulatory T cells and application thereof
InactiveCN101921325ASuppress inflammatory symptomsInhibitory reactivityPeptide/protein ingredientsAntipyreticAntigenDisease
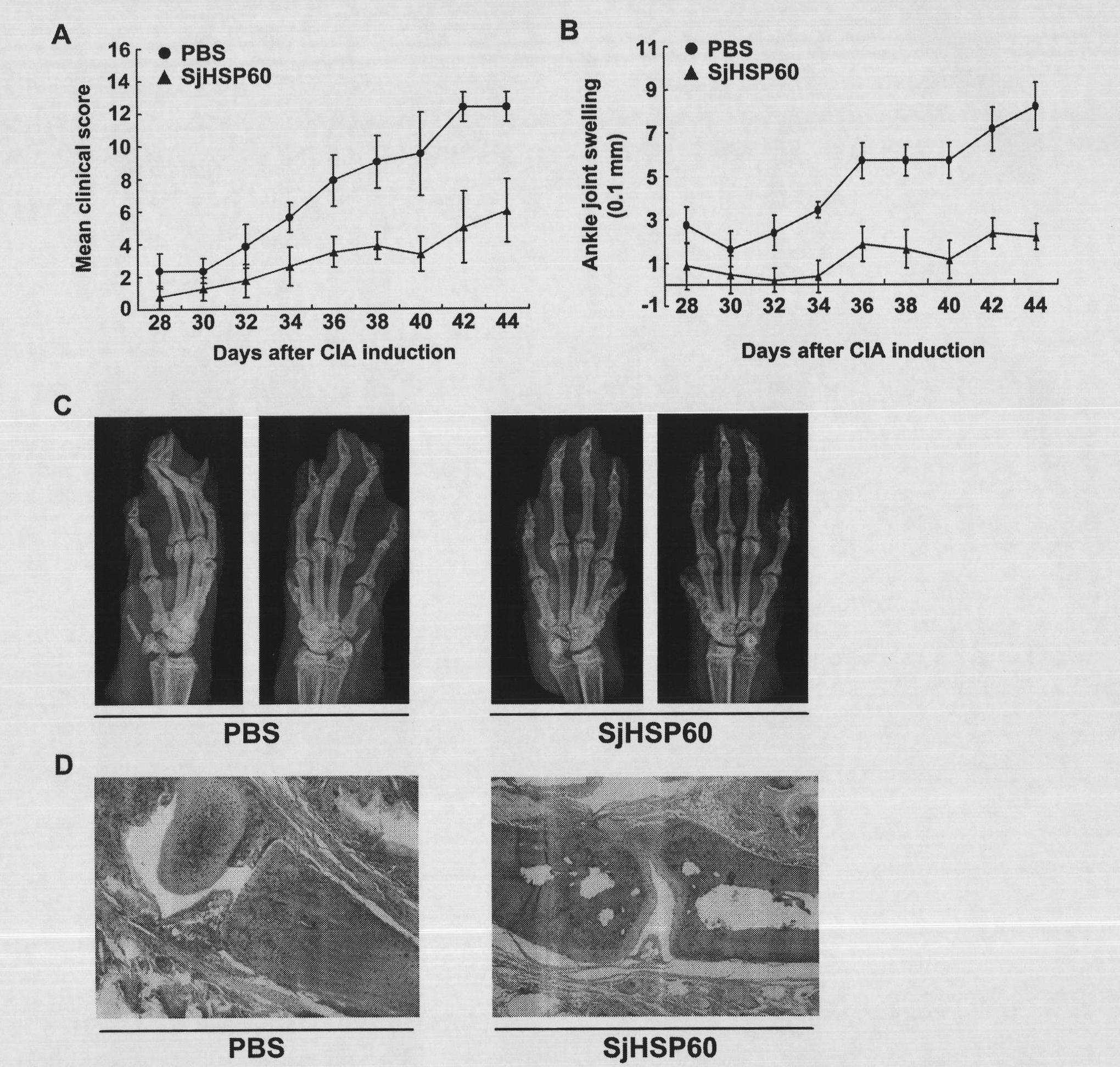
The invention belongs to the immunology field, in particular to a proteantigen molecule-Japanese blood fluke heat shock protein 60KDa (SjHSP60) which is derived from a blood fluke and is capable of increasing CD4 + CD25 + Foxp3 + regulatory T cells and application thereof. The SjHSP60 has a full-length amino acid sequence as shown in SEQ.ID.NO.1, has a series of identical or highly similar cross-reactive T cell epitopes with HSP60 infected by a host. After being used for mouse in vivo immunization or in vitro stimulus to mouse spleen and lymph gland cells, the SjHSP60 can obviously increase CD4 + CD25 + Foxp3 + Tregs. In practical application, the SjHSP60 can effectively relieve inflammatory symptoms and immunopathological effects caused by arthritis, thereby having wide prospects in the aspects of immunological suppression inducement and treatment of immunological diseases.
Owner:NANJING MEDICAL UNIV
Beta-fodrin antigen epitope polypeptide, and its screening method and use
InactiveCN1935837AAdvantages of value assessmentBiological testingAnimals/human peptidesEpitopeSerum ige
The invention relates beta-fordin antigen epitope polypeptide screening for gaining amino acid sequence corresponded with the optimum beta-fordin polypeptide. The beta-fordin antigent epitope polypeptide includes the following two that one is formed by the amino acid sequence showed in SEQ ID NO.1; another is derived from the above amino acid sequence by replacing, missing or adding one or many amino acid with antigen epitope function. It can used to specially test beta-fordin polypeptide IgG antibody for Sjogren syndrome patient. The invention also supplies the beta-fordin polypeptide IgG antibody immunology method, and its application used as antigen in Sjogren syndrome medicine preparation.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Protein chip used for detecting functional injury of central nervous system and manufacturing method therefor
InactiveCN101008644AHigh speedSpeed up the processMaterial analysisTOR activityBiological activation
This invention relates to one functional central neutral damage dialogue test protein chip and its process method, which comprises the following steps: using protein chip to fix multiple central neutral system damage generation development key function abnormal protein factor antigen and its array; the said protein antigen and its array abnormal protein factors act as key property protein factors in the neutral system for inflammation and activation reaction and cell ion balance adjust and signal transmission.
Owner:陈云 +3
Protein antigen, coding gene thereof, and application of protein antigen and coding gene thereof to identification of mycoplasma hyopneumoniae inactivated vaccine antibody and natural infection antibody
PendingCN110133284AIncreased sensitivityHigh sensitivityMicroorganism based processesDepsipeptidesBlood serumRecovery period
The invention relates to the field of biotechnology, in particular to a protein antigen, a coding gene thereof and application of the protein antigen and the coding gene thereof to identification of amycoplasma hyopneumoniae inactivated vaccine antibody and a natural infection antibody. An amino acid sequence of the protein antigen is shown as SEQ ID NO:1. A detection system disclosed by the invention can rapidly and conveniently identify the mycoplasma hyopneumoniae inactivated vaccine immune antibody and the natural infection antibody at high specificity and high sensitivity, does not needany complex instrument, can meet the current demand of identification of a mycoplasma hyopneumoniae high-immunity serum antibody and a serum antibody at a recovery period well, can be popularized andapplied on a large scale, and has a wide market prospect and great economical and social benefits.
Owner:SOUTHWEST UNIVERSITY
Reagent for detecting novel coronavirus SARS-CoV-2 antibody and application thereof
ActiveCN111647054AHigh binding activityPrevent proliferationVirus peptidesBiological material analysisReagent stripProtein s antigen
The invention discloses a reagent for detecting a novel coronavirus SARS-CoV-2 antibody and application of the reagent, and particularly relates to a polypeptide. The sequence of the polypeptide comprises one or more of partial amino acid sites corresponding to a S protein sequence and / or N protein sequence of a novel coronavirus SARS-CoV-2 shown as SEQ ID NO.1, and the polypeptide has S protein antigenicity and / or N protein antigenicity; the invention further relates to a primer composition for synthesizing the polypeptide, a preparation method of the polypeptide and application of the polypeptide in preparation of the reagent for detecting or diagnosing the novel coronavirus SARS-CoV-2, and particularly can be used for preparing a colloidal gold reagent strip and a test kit thereof. Thespecific primer designed by the invention can successfully synthesize the S protein / N protein antigen peptide with excellent binding activity, and the colloidal gold chromatography reagent strip prepared from the specific primer can rapidly and effectively detect the S protein antibody / N protein antibody of the novel coronavirus SARS-CoV-2, the generation of false negative results is avoided, thedetection efficiency is high, and epidemic diffusion can be prevented as soon as possible.
Owner:SHANGHAI SYMRAY BIOPHARMA CO LTD +1
Antituberculous CTL (Cytotoxic T Lymphocyte) epitope peptide with drug-resistant related efflux protein source for tuberculosis and application of epitope peptide
The invention discloses an antituberculous CTL (Cytotoxic T Lymphocyte) epitope peptide with a drug-resistant related efflux protein source for tuberculosis. The antituberculous CTL epitope peptide is nonapeptide, wherein the amino acid sequence of the nonapeptide is P9: ALGML IAGL. Predicative analysis is carried out on HLA-A*0210 restrictive CTL epitope of drug-resistant related protein antigen for tuberculosis by adopting immune-informatics means and SYFPEITH1, BIMAS and NetCTL1.2 databases according to the primary structure of the antigen, so that the epitope peptide is obtained by screening; and the identified nonapeptide has not been reported in any document. An in-virto ELISPOT (Enzyme-Linked Immunospot Assay) is adopted to identify the epitope peptide; and the identification result provides a theoretical basis for developing tuberculosis vaccine based on drug-resistant related protein antigen and provides more information for designing multi-epitope peptide vaccine for tuberculosi based on the mixed T cell epitope.
Owner:ZHENGZHOU UNIV
Pneumococcal vaccine containing pneumococcal surface protein a
ActiveUS20150320851A1Induce immune responseInduce protective immunityAntibacterial agentsBacterial antigen ingredientsCoccidiaProtein s antigen
A pneumococcal vaccine comprising a fusion protein at least comprising a full-length family 1 pneumococcal surface protein A (PspA) or a fragment thereof, and a full-length family 2 PspA or a fragment thereof, in particular any one of the following fusion proteins (1) to (3):(1) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 3 PspA,(2) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 4 PspA, and(3) a fusion protein at least comprising a family 1, clade 2 PspA and a family 2, clade 5 PspA,is useful as a pneumococcal vaccine comprising a single protein antigen that has broadly cross-reactive immunogenicity and can induce immune response against a wide range of pneumococcal clinical isolates.
Owner:OSAKA UNIV
COVID-19 rapid diagnosis kit and preparation method thereof
ActiveCN111398581ARapid diagnosisEasy to operateSsRNA viruses positive-senseVirus peptidesAntigenProtein s antigen
The invention discloses a COVID-19 rapid diagnosis kit. The COVID-19 rapid diagnosis kit comprises COVID-19 spike protein antigen colloidal gold. The invention also discloses a preparation method of the COVID-19 rapid diagnosis kit. The preparation method comprises the following steps: expressing and purifying COVID-19 spike protein; and preparing the COVID-19 spike protein antigen colloidal gold.The kit is simple to operate, can quickly diagnose whether a patient is infected with COVID-19 or not, and is beneficial to effective control of epidemic situations.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Composite vaccine for Alzheimer's disease prevention and treatment, and preparation method thereof
ActiveCN102895659ADoes not affect normal productionPrevent inflammatory damageNervous disorderSugar derivativesDiseaseSide effect
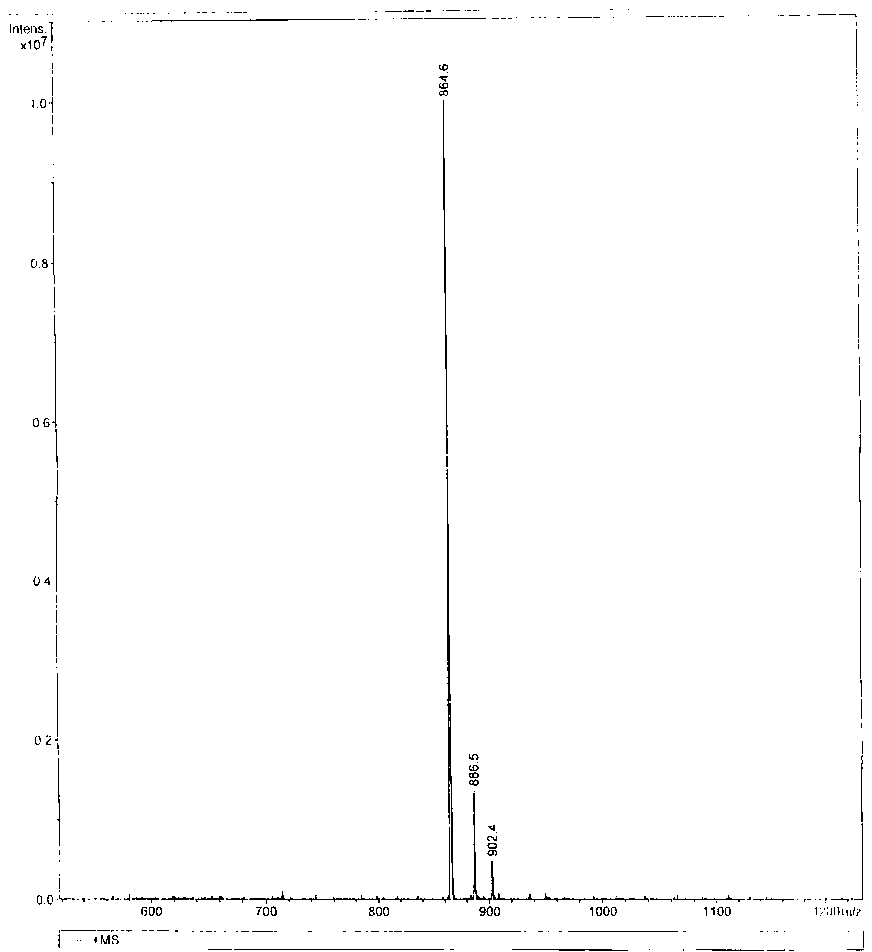
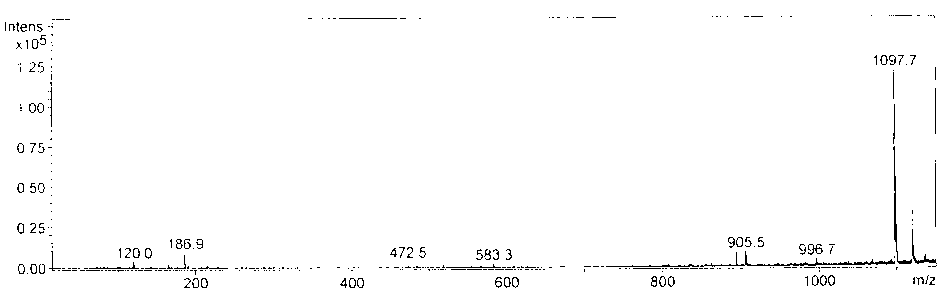
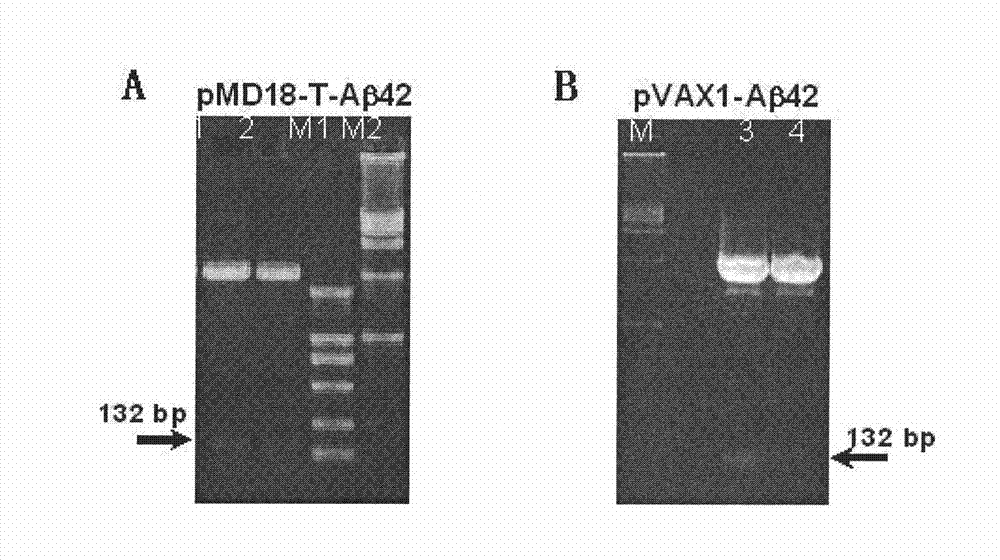
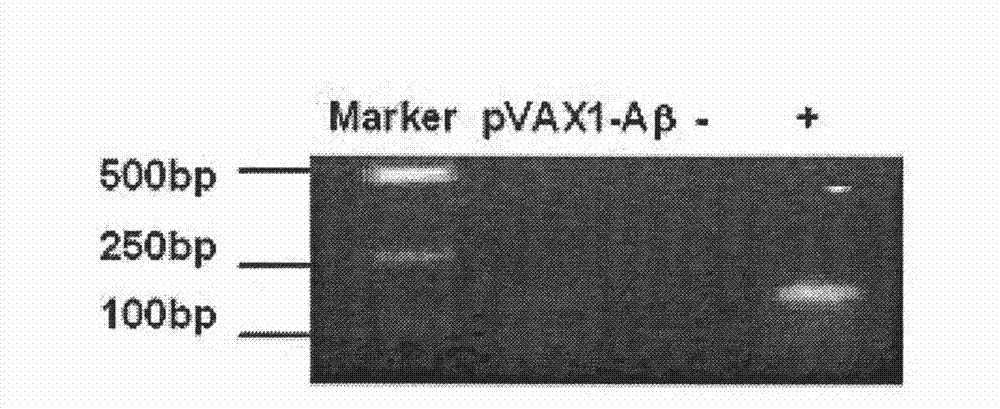
The invention belongs to the field of biological products, and relates to a composite vaccine for Alzheimer's disease prevention and treatment, wherein the composite vaccine is prepared by mixing a nucleic acid vaccine encoding Abeta42 and a protein gene engineering vaccine. Experiment results show that: Abeta42 protein antigen expressed through prokaryotic expression and pVAX-Abeta42 plasmid are adopted to co-immunize, such that the Alzheimer's disease protein vaccine in the prior art is improved, high level anti-Abeta antibody IgG can be produced, inhibition on T cell response can be induced, and the inhibition can be maintained for a fairly long time. In addition, the antibody generated through co-immunizing and for Abeta can be combined with Abeta protein fibers and nature Abeta protein precipitate in APP / PS1 Alzheimer's disease transgene incidence mice brain so as to provide Abeta precipitate removing capacity; and the vaccine is a potential, effective, and side effect-free vaccine, and can be used for Alzheimer's disease prevention and treatment.
Owner:FUDAN UNIV
Bivalent DNA vaccine of type A and type O foot-and-mouth disease virus and its preparing process
InactiveCN1557486ASafe preparationNo pathogenic effectGenetic material ingredientsAntiviralsDiseaseProtein s antigen
The present invention relates to one kind of bivalent livestock type-A and type-O foot and mouth disease DNA vaccine. It is prepared through connecting serially the coding regions of the VP1 protein antigen determinants of both type-A and type-O foot and mouth disease virus strains, and the connection with DNA segment capable of raising the immunogenicity. The preparation process includes connecting enzyme incised segment with eukaryotic expression carrier and transforming competent colibacillus cell; PCR identification to obtain positive cloning; culturing, collecting thallus, cracking cell and centrifuging; and other steps. The bivalent livestock type-A and type-O foot and mouth disease DNA vaccine is used to immunize livestock to generate antibody resisting both type-A and type-O foot and mouth disease viruses. It has no pathogenetic effect and can induce the livestock body to generate comprehensive immune response and express modified natural antigen.
Owner:XIAMEN UNIV
No-adjuvant therapeutic protein vaccine containing heat shock protein and HPV 16Z protein antigen
InactiveCN1900118AStimulate immune responseViral antigen ingredientsHybrid peptidesEscherichia coliDisease
The present invention relates to the fusion protein of heat shock protein and HPV16Z E6 / E7, the obtaining process and expression vector of the fusion protein, host with transformed expression vector, and the application of the fusion protein in medicine for preventing and treating HPV relative diseases. The fusion protein may be expressed in colibacillus in the form of inclusion body and further column renatured and chromatographically purified to reach purity over 95 %. It is used in inducing E6 and E7 specifying cellular immunity reaction in mouse body in the condition of no adjuvant and has obvious therapeutic effect on transplanted TC-1 cell tumor of mouse.
Owner:张伟
Antitumor cytotoxic T lymphocyte (CTL) epitope peptide analog derived from COX-2
The invention discloses an antitumor cytotoxic T lymphocyte (CTL) epitope peptide analog derived from COX-2. The amino acid sequence of the analog is Tyr-Leu-Ile-Gly-Glu-Thr-Ile-Lys-Leu. In the invention, according to a primary structure of an antigen, an immune informatics means is adopted, and SYFPEITHI, BIMAS and NetCTL1.2 databases are applied so as to carry out predictive analysis on HLA-A*0201 restriction CTL epitope of COX-2 protein antigen, and then an epitope peptide P321 is obtained by selection; and then 1-site and 9-site amino acids of the epitope peptide P321 are respectively replaced by using Y and L so as to obtain the epitope peptide analog P321-1Y9L. In the invention, the candidate CTL epitope peptide analog which has a strong bonding capability with a major histocompatibility complex molecular and is derived from the COX-2 can be modified and initially identified by adopting a method combining a theory and an experiment; and because the identified nonapetide is not reported in documents, a theory base is provided for developing tumor therapeutic polypeptide vaccine based on the COX-2 and a foundation is established for constructing subsequent multivalent antigen peptide vaccine according to the invention.
Owner:ZHENGZHOU UNIV
Human NOTCH1 NICD protein antigen and antibody as well as preparation methods and application thereof
InactiveCN106749617AStrong specificityHigh affinityCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsSerum igeSeparation technology
The invention relates to a specific Thr1861 site phosphorylated antigen peptide aiming at a human NOTCH1 NICD protein, an antibody, application of the antigen peptide and the antibody, and a preparation method of the antibody. The antibody is shown as SEQ ID NO:1 through an active amino acid sequence, Thr amino acid is phosphorylated and prepared as an antigen peptide immunized animal. When the antibody is prepared, antiserum is purified by utilizing an affinity purification-affinity separation technology. The preparation method has the advantages of being short in preparation period, high in antibody potency, good in recovery rate, and the like; the obtained antibody has high specificity and high affinity; the phosphorylated antibody is helpful to reveal an action mechanism of phosphorylation modification of the NOTCH1 NICD protein in the occurrence and development of tumors and other diseases, and has wide clinical application prospect in accepts of tumor diagnosis, treatment, prognosis judgment and the like.
Owner:SOUTHERN MEDICAL UNIVERSITY +1
A kind of prostatic excretory protein antigen and its antibody and application
ActiveCN104897900BThe detection method is simple and reliableImmunoglobulins against animals/humansBiological testingAntibody antigen reactionsProtein s antigen
Owner:ONCO BIOMEDICAL TECH SUZHOU
Preparation method of novel genetically recombinant pure protein antigen
InactiveCN103898139AHigh purityGood antigenicityVirus peptidesImmunoglobulins against virusesHemagglutininProtein s antigen
The invention discloses a preparation method of a novel genetically recombinant pure protein antigen and relates to the technical field of protection of hemagglutinin viruses and development of detection reagents. The preparation process comprises the following steps: I, gene recombinant expression of a hemagglutinin H7 antigen; II, preparation of the hemagglutinin H7 antigen; III, application of the hemagglutinin pure protein to animals / human bodies; and IV, application of the high purity hemagglutinin H7 antigen to detecting hemagglutinin H7-containing viruses. The novel genetically recombinant pure protein antigen has the characteristics of high purity and good antigenicity, and does not exist in form of granules and not only does not contain any genetic materials such as RNA (Ribonucleic Acid), but also does not contain other protein impurities. The novel genetically recombinant pure protein antigen can be used for preparing a high specific antibody for H7N9. Because of the protein characteristic of the H7 antigen, the chromatographic method can be used for purifying a single antigen, and the purity of SEC-HPCL (Size Exclusion Chromatography-High Performance Liquid Chromatography) is higher than 90%. The high purity H7 antigen and the highly specific H7N9 antibody can be used for developing diagnostic reagents for diagnosing H7N9 avian influenza.
Owner:SHANGHAI SIQI MEDICAL TECH
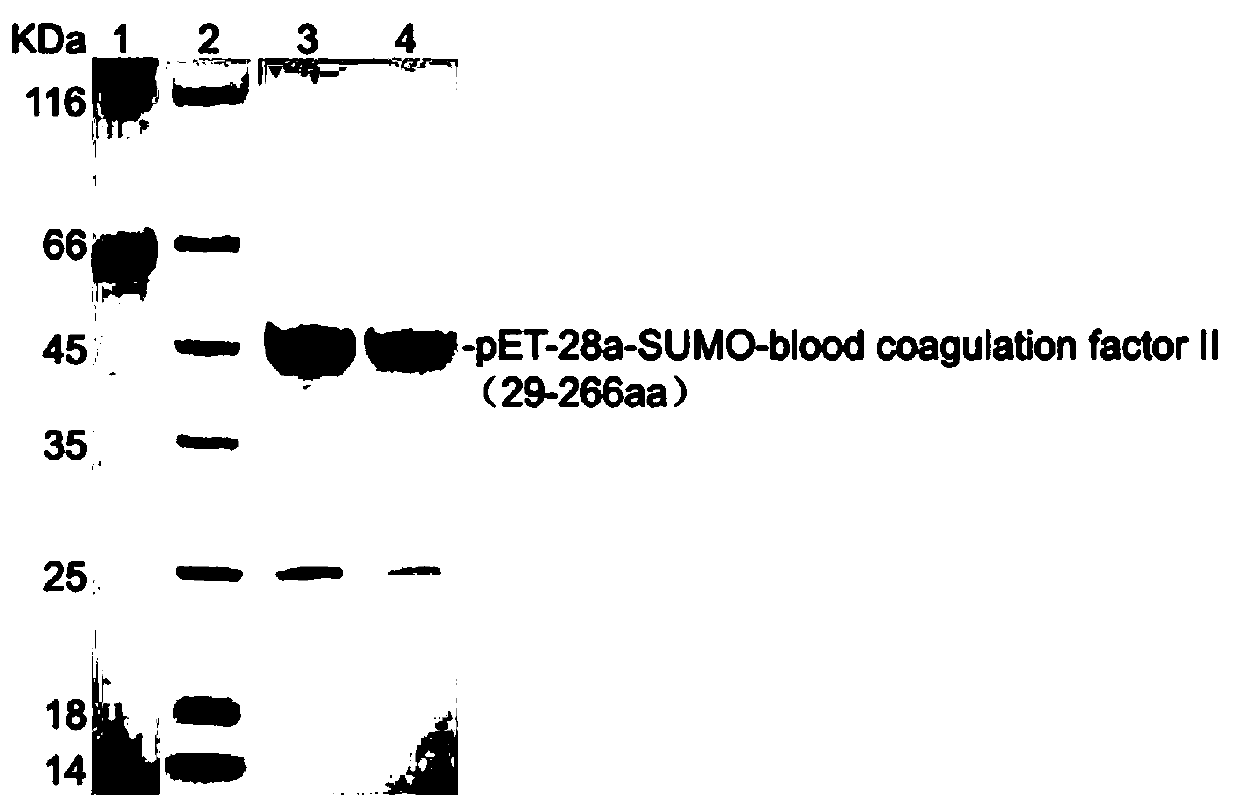
Method for preparing pET-28a-SUMO-coagulation factor II protein antigen and polyclonal antibody thereof
ActiveCN110343715AImmunoglobulins against blood coagulation factorsVector-based foreign material introductionEscherichia coliProtein s antigen
The invention provides a method for preparing a pET-28a-SUMO-coagulation factor II protein antigen and a polyclonal antibody thereof. According to the method, a grass carp coagulation factor II gene sequence is synthesized by using a grass carp coagulation factor II specific primer through a PCR amplification technology, then cloned into a pET-28a-SUMO expression vector, then delivered into E. coli Rosetta for expression and then purified to obtain the pET-28a-SUMO-coagulation factor II protein antigen, the antigen is used for repeated immunization of laboratory-scale Japanese white rabbits, blood sampling is carried out, antiserum is collected, pET-28a-SUMO-coagulation factor II protein is used as the antigen for affinity purification, and the concentrated grass crap coagulation factor IIpolyclonal antibody is obtained. The method can obtain the concentrated grass carp coagulation factor II polyclonal antibody meeting the requirements of subsequent experiments, thereby providing an important experimental reagent for the subsequent detection of the expression of the grass carp coagulation factor II.
Owner:HUNAN AGRICULTURAL UNIV
Modified seasonal flu-RSV (Respiratory Syncytial Virus) combined vaccine and preparation method thereof
ActiveCN107050446AStable and efficient connectionStrong immune responseSsRNA viruses negative-senseViral antigen ingredientsDigestionHigh pressure
The invention discloses a modified seasonal flu-RSV (Respiratory Syncytial Virus) combined vaccine and a preparation method thereof. The modified seasonal flu-RSV combined vaccine is prepared from a flu protein antigen, an RSV protein antigen and a nanoparticle carrier, wherein the surface of the nanoparticle carrier is hydroxylated; the flu protein antigen and the RSV protein antigen react with hydroxyl groups on the surface of the nanoparticle carrier and are connected to the surfaces to nanoparticles. According to the modified seasonal flu-RSV combined vaccine disclosed by the invention, the nanoparticles are used as a novel protein carrier, antigen protein is efficiently and stably connected, and the nanoparticles having a flu antigen and an RSV antigen at the same time are successfully constructed; an experiment on mice verifies that a good systemic immune response can be caused, the nanoparticles are used as the carrier and can be repeatedly synthesized, the stability is high, not only can digestion of all kinds of enzymes in a body be resisted, but also the modified seasonal flu-RSV combined vaccine has many advantages of tolerating high pressure, sterilizing and the like. By adopting the nanoparticles as the novel protein carrier, the method is simple, the operation is simple and convenient, various protein and polysaccharide antigens can be connected, too much modification is not needed, and a good application prospect is obtained.
Owner:BRAVOVAX
TNF-alpha (tumor necrosis factor-alpha) mutant protein and carrier protein CRM197 chemically coupled product and preparation method thereof
InactiveCN108126193AImproving immunogenicityProlong lifeAntipyreticAnalgesicsMutated proteinAdjuvant
The invention provides a protein antigen of a vaccine preparation targeting TNF-alpha (tumor necrosis factor-alpha) as well as a preparation method and an application of the protein antigen. Specifically, the protein antigen comprises recombination expression hTNF-alpha (human TNF-alpha) protein and CRM197 which are coupled through glutaraldehyde and inactivated through formaldehyde. The protein antigen almost has no TNF-alpha toxicity in the dosage of a vaccine, reserves the high immunogenicity and can stimulate mice to produce a remarkable immunoreaction targeting hTNF-alpha after being combined with an aluminum hydroxide adjuvant.
Owner:SHANGHAI HYCHARM
Protein antigen composition for detecting Alzheimer's disease autoantibody and application of composition
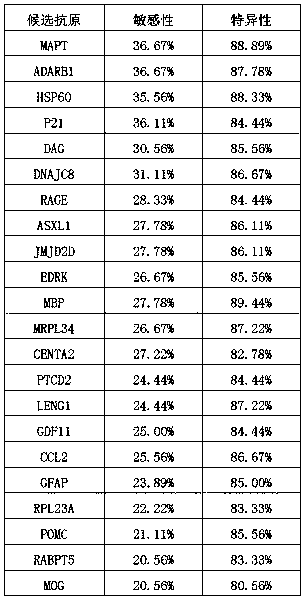
ActiveCN110850104AVarious ways to useEasy to operateImmunoglobulin superfamilyHydrolasesDiseaseHSP60
The invention belongs to the field of biological detection, in particular to a protein antigen composition for detecting an Alzheimer's disease autoantibody in a human serum sample and an applicationof the composition. The adopted technical scheme includes that the antigen composition comprises at least two protein fragments of the following proteins: MAPT, ADARB1, HSP60, P21, DAG, DNAJC8, RAGE,ASXL1 and JMJD2D. Based on the above antigen composition, a kit for diagnosis, especially early diagnosis of Alzheimer's disease or related risks can be prepared. By using the antigen composition, early Alzheimer's disease can be diagnosed pertinently, rapidly and accurately, and thus the composition has important practical significance.
Owner:SHANGHAI ZHONGQI BIOTECHNOLOGY CO LTD
Lateral flow detection device for detecting a coronavirus by immunoassay
PendingUS20210373018A1Improve the detection rateQuick checkImmunoassaysProtein s antigenIgm antibody
The present invention provides a lateral flow test device for detecting a coronavirus antibody by immunoassay; the test device includes three lateral flow test strips; a first test strip is directed to the antibody detection of a N full-length protein and / or an S full-length protein antigens / antigen; and a second test strip is directed to the antibody detection of an S-RBD-site protein antigen, and both of the first strip and the second strip are combined to detect novel coronavirus IgG and IgM antibodies, which can practically reduce the possibility of missing detection and wrong detection; further, a third test strip is directed to the detection of a neutralizing antibody at an S-RBD site to rapidly detect the neutralizing antibody having protection effect, thus further helping the prevention of missing detection and helping patients to perform self-detection and judge the situations of recovery, or vaccine immunity.
Owner:HANGZHOU BIOTEST BIOTECH CO LTD
Method for generating novel induction antibody
ActiveCN103524625AImmunoglobulins against growth factorsHybrid peptidesAntibody secretionProtein s antigen
The invention provides a method for generating a novel induction antibody, which comprises a step of preparing a eukaryotic expression vector expressing fusion antigen protein. The fusion protein consists of four parts of sequences: (1) a signal peptide sequence from a human IgE heavy-chain coded sequence; (2) an antigen protein sequence; (3) an auxiliary sequence from a mycobacterium tuberculosis cytochrome C oxidase subunit II, wherein the sequence can amplify the antibody response of the rat and mice against the antigen protein; and (4) a polypeptide sequence MFSRMTSLIMGN that can be combined with the mice FcrR II (APCTS for short), wherein the sequence can specifically guide the protein antigen to the mice antigen-presenting cell (APC) so as to improve the antigen presenting efficiency. The plasmid expressing the fusion antigen protein is directly injected into the mice muscle or skin, and the mice cells can take in DNA (deoxyribonucleic acid) and the efficiently-expressed fusion antigen protein; and then the animal is stimulated to generate a high-affinity antibody against the antigen protein. The generated antibody after being purified can be applied to the scientific research and clinical diagnosis or treatment; and the antibody secretion cell can be used for preparing a specific monoclonal antibody by use of the hybridoma technology.
Owner:SUPERVIEW BIOTECH
Duck adenovirus type I Penton protein as well as preparation method and application thereof
InactiveCN111704656ANo transmembrane domainGuaranteed neutralityVirus peptidesMicroorganism based processesCell freeFree protein
The invention belongs to the field of protein engineering, and particularly relates to duck adenovirus type I Penton protein as well as a preparation method and application thereof. The inventor, by analysis, finds that the Penton protein has the advantages of hydrophilicity, no transmembrane structural domain, no signal peptide prediction and the like, and full-length expression is selected. A Penton target fragment is obtained through artificial synthesis after sequence optimization, and the expression mode of the protein is cell-free protein expression. Compared with truncated expression ofantigen dominant epitopes of traditional Penton protein, the artificially synthesized and expressed Penton recombinant protein is high in accuracy, the neutrality of the protein is guaranteed, the trouble of high mutation rate is avoided, and the application to the development of an antibody detection technology is better facilitated. In addition, an expression system has unique advantages of saving time and improving the total yield of soluble full-length protein. Furthermore, the expression system is low in cost, large in expression quantity and high in Western Blot detection reactogenicity.
Owner:ANYANG INST OF TECH
Gene engineering bacterium for preparing Ebola virus nucleoprotein antigen and application
PendingCN108342348AEasy to manufactureIncrease productionSsRNA viruses negative-senseBacteriaSolubilityEscherichia coli
The invention discloses a gene engineering bacterium for preparing an Ebola virus nucleoprotein antigen and application. A gene engineering strain Escherichia coli Bl21(DE3) / pET-28a-EBOV-NP is inducedto express Ebola virus nucleoprotein, and the antigen is obtained through centrifugation and ultrasonic crushing. The antigen is strong in solubility, has good antigenicity and is suitable for serving as an antigen for indirect ELISA detection. The preparation method of the Ebola virus nucleoprotein antigen is simple and rapid, and the yield is large. Compared with a classical preparation method,the obtained antigen is more stable in property, and an established indirect ELISA detection method is high in sensitivity, good in specificity, short in detection period and wide in application range.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com