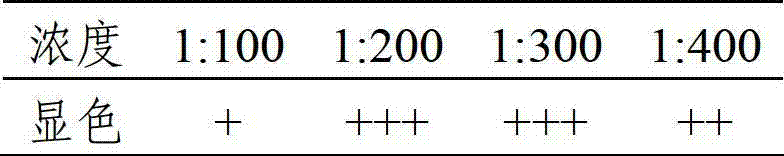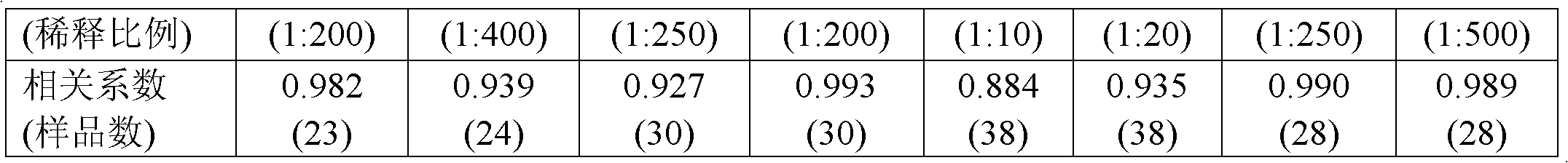Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
353 results about "Serum antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A serum antibody is an antibody derived from serum, a blood component that is collected after blood has coagulated. As it is collected after coagulation, blood serum does not actually contain blood cells or clotting factors. It does, however, include many different types of proteins that are not involved in...
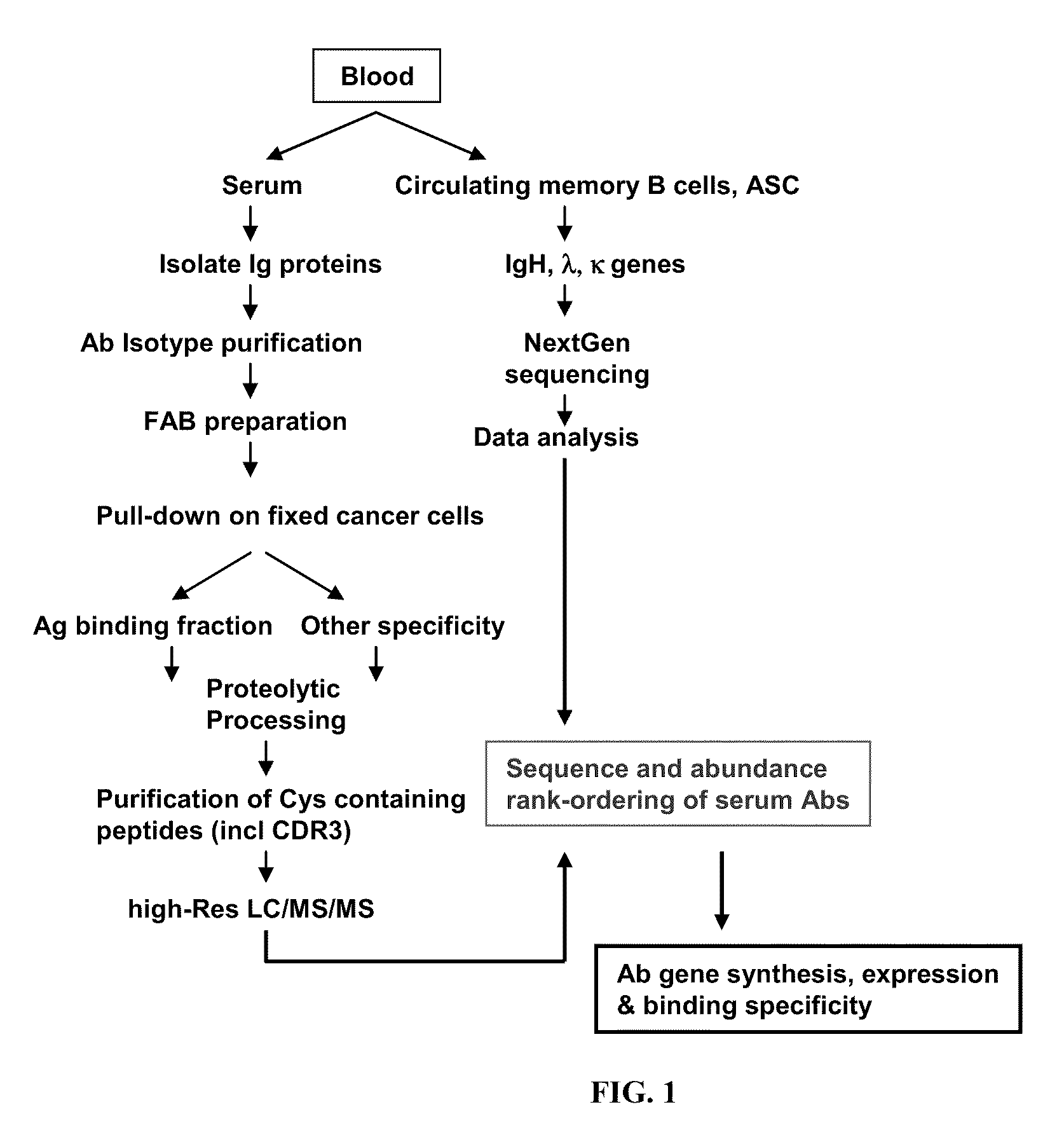
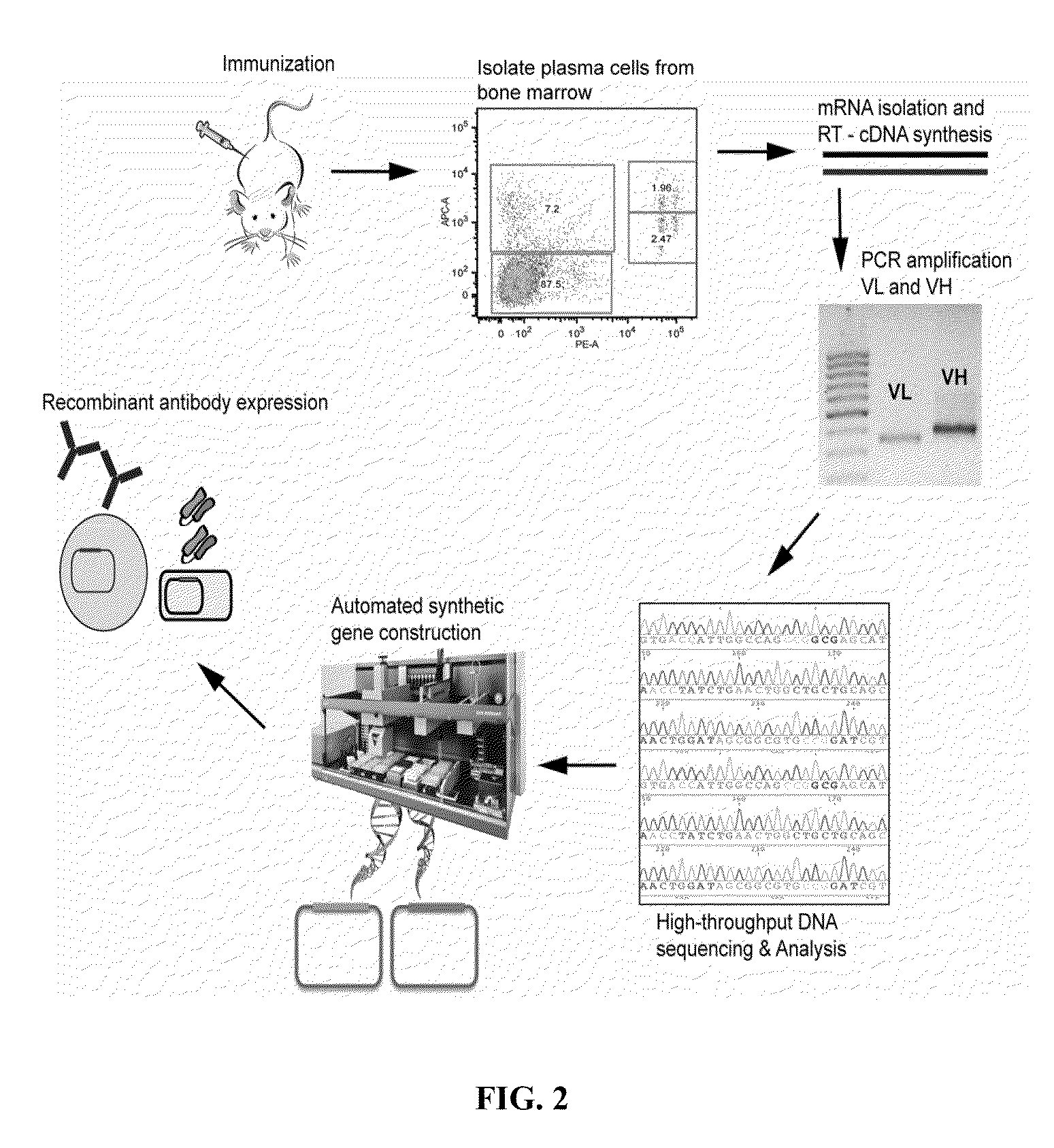
Rapid Isolation of Monoclonal Antibodies from Animals
ActiveUS20110312505A1Microbiological testing/measurementLibrary screeningSerum igeAntigen Binding Fragment
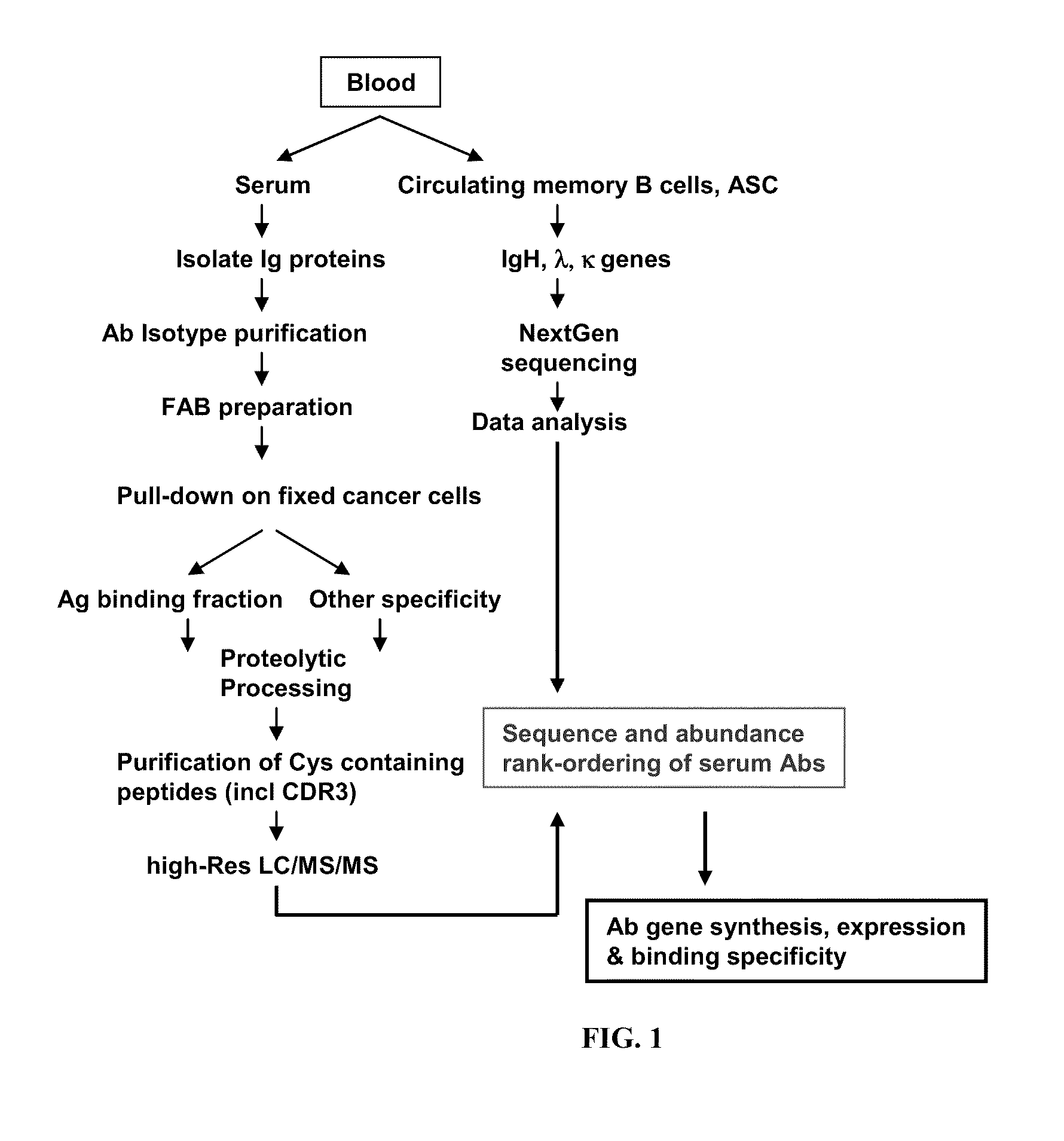
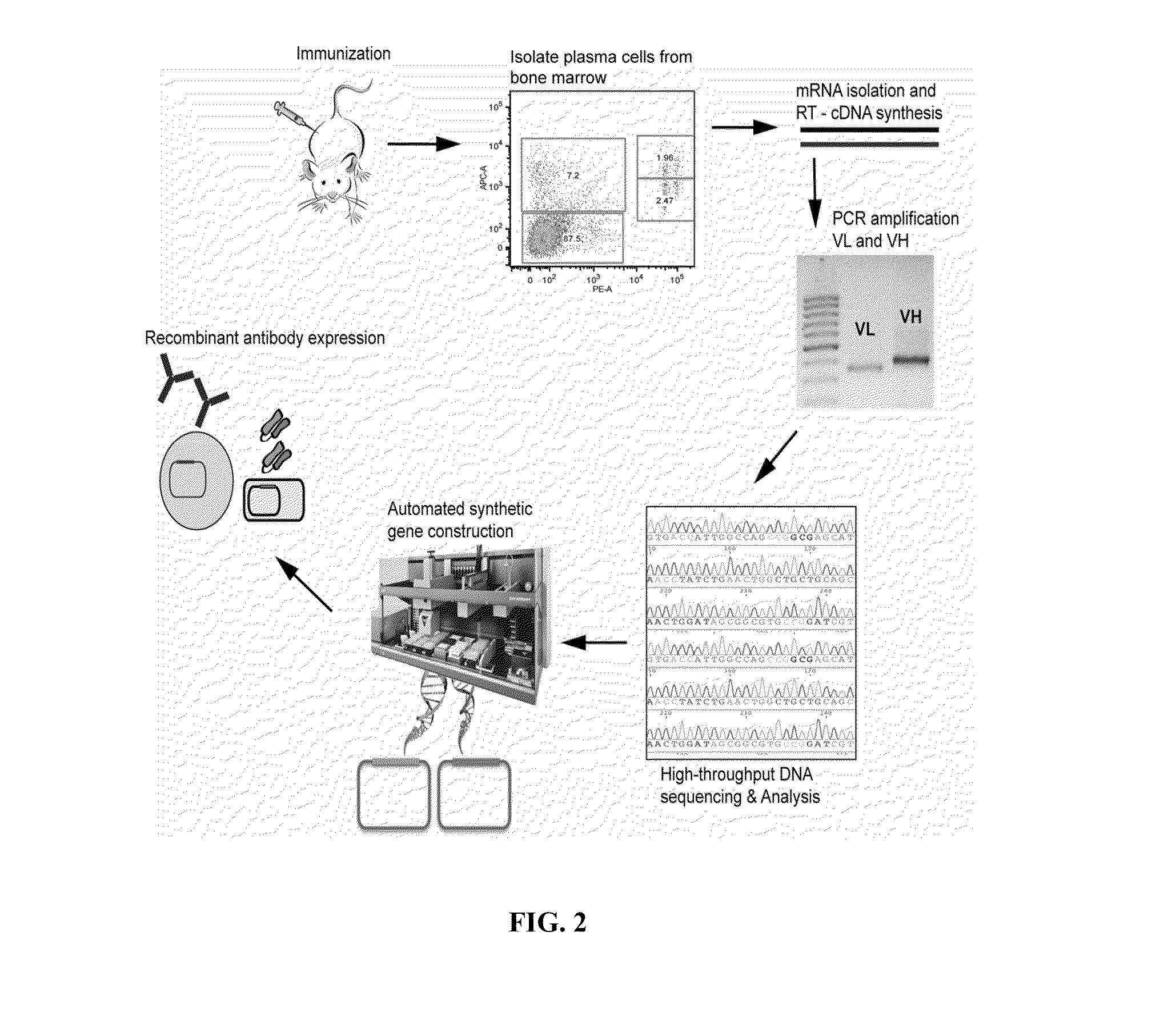
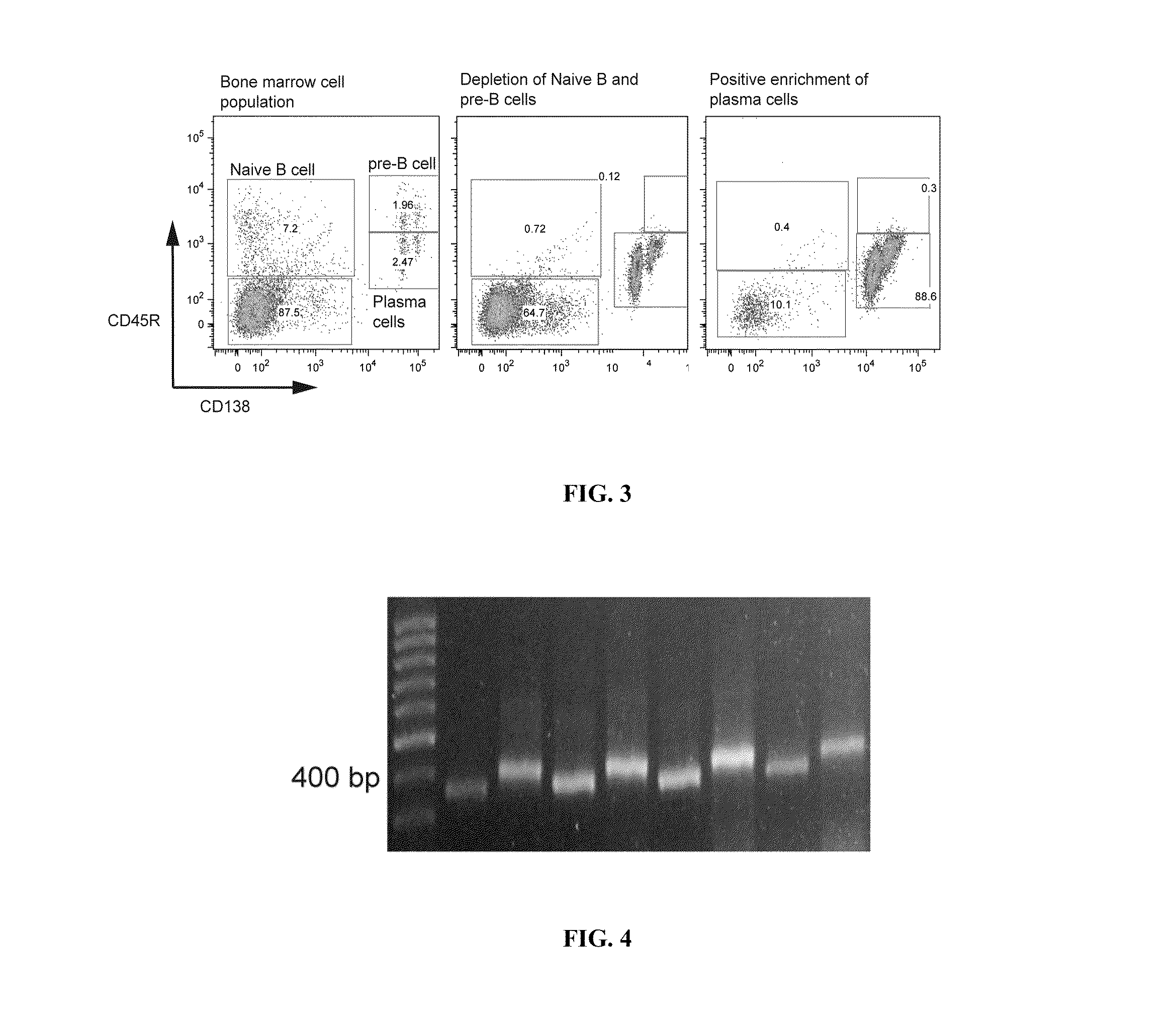
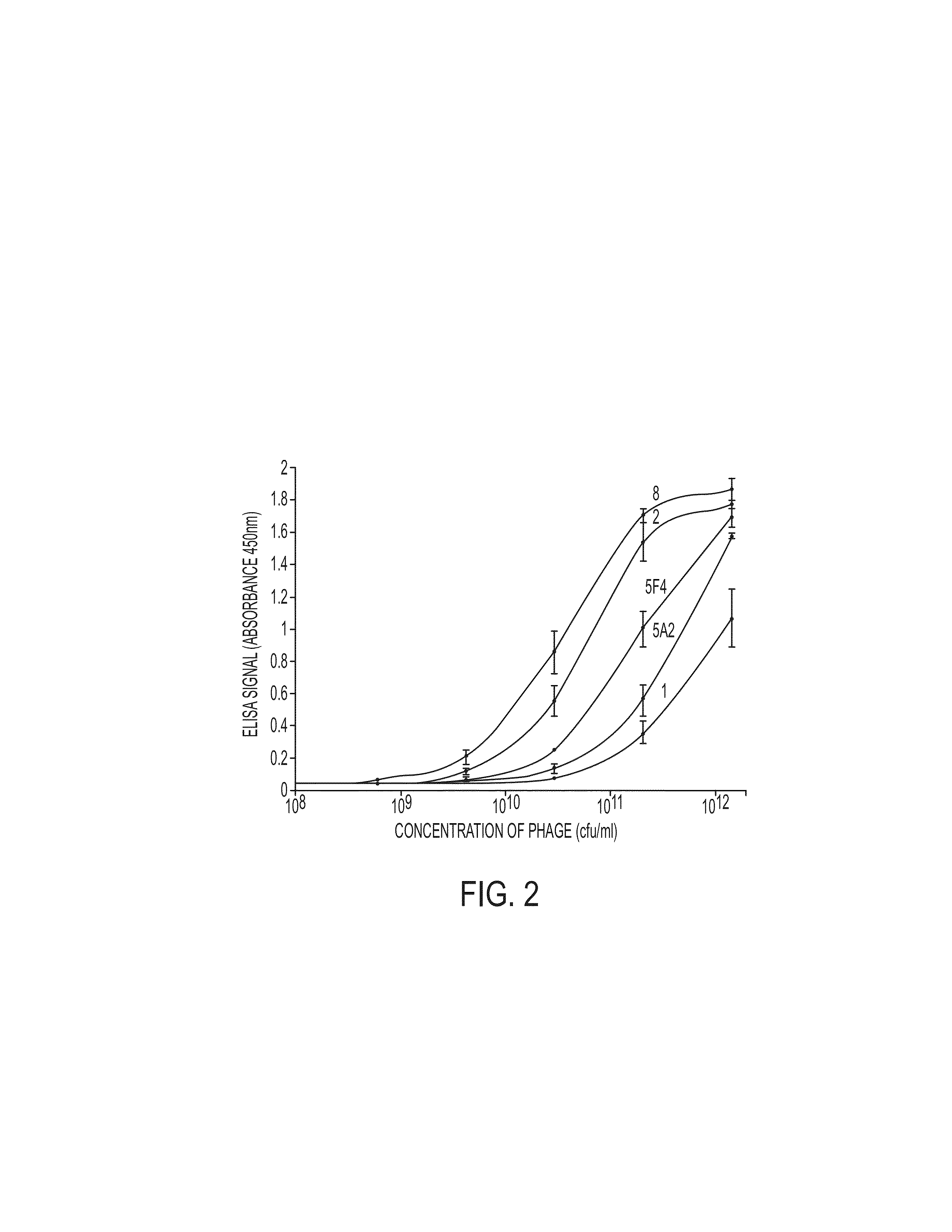
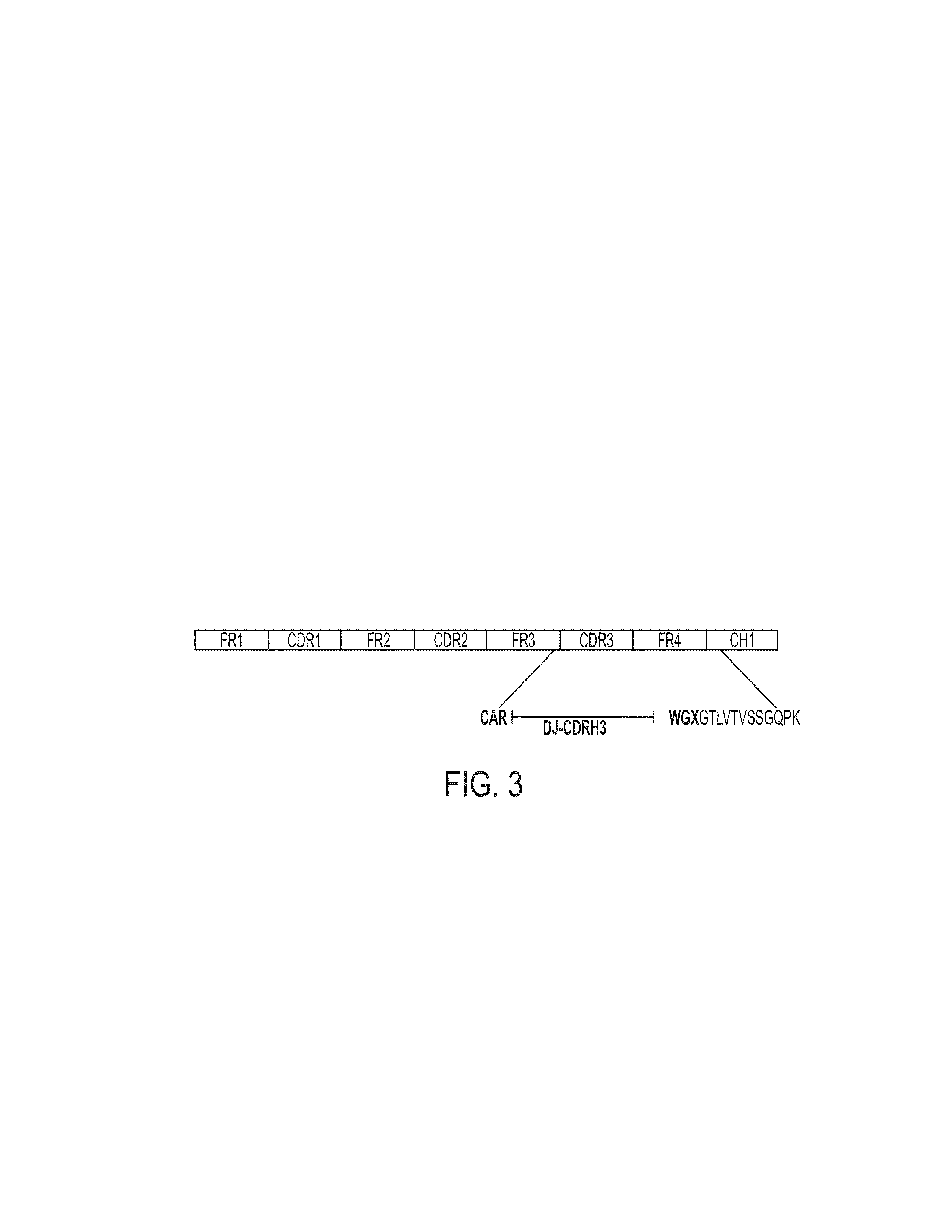
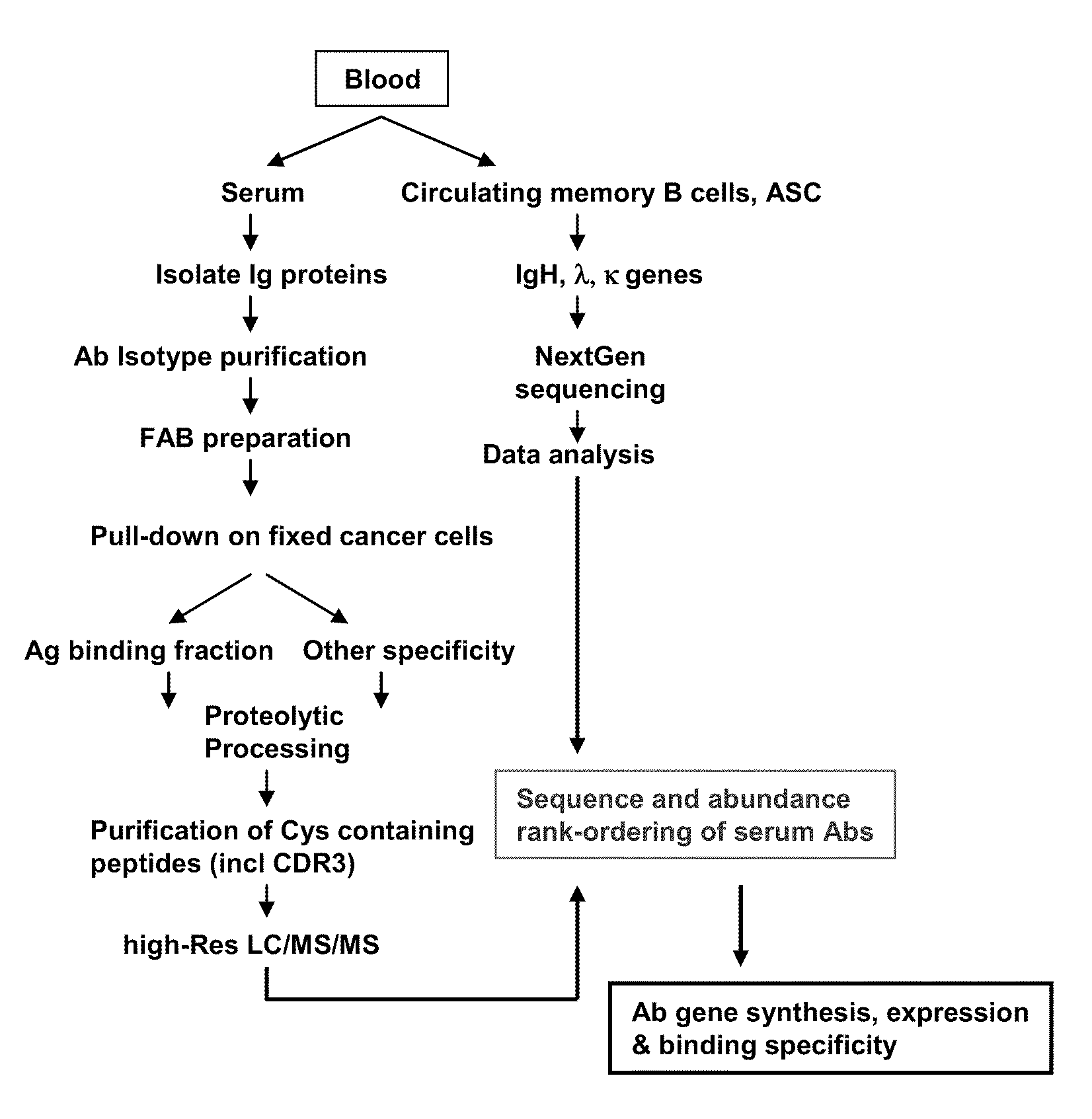
Methods and compositions for identification of candidate antigen-specific variable regions as well as generation of antibodies or antigen-binding fragments that could have desired antigen specificity are provided. For example, in certain aspects methods for determining amino acid sequences of serum antibody CDR and abundancy level are described. In some aspects, methods for determining nucleic acid sequences of antibody variable region sequences and frequency are provided. Furthermore, the invention provides methods for identification and generation of antibody or antigen-binding fragments that comprise highly-represented CDR.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
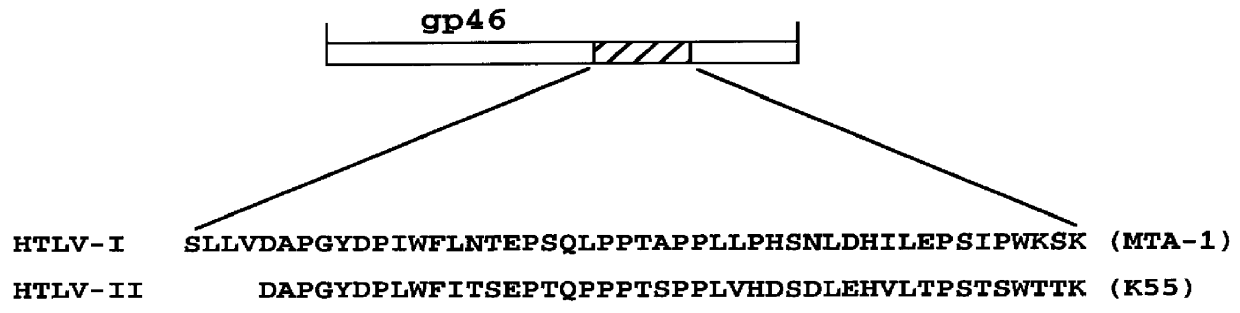
HTLV-I/HTLV-II assay and method
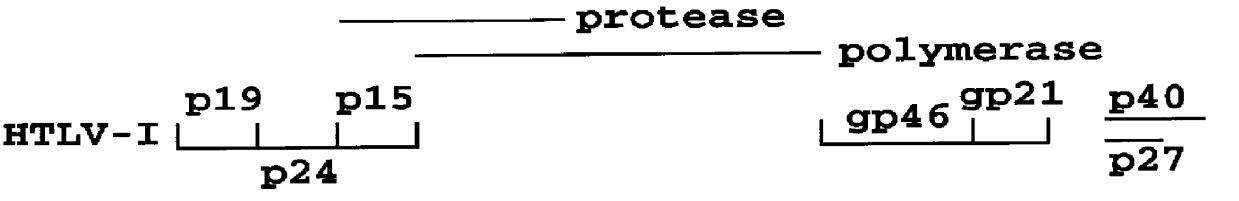
InactiveUS6110662AMicrobiological testing/measurementBiological material analysisPeptide antigenSerum samples
Method and assay kit for positively identifying HTLV-I and HTLV-II infection from human serum samples. The kit includes peptide antigens from the C-terminal regions of HLTV-I p19 and HTLV-II p21 gag proteins, and peptide antigens from the HLTV-I and HTLV-II env proteins immobilized on a solid support. After reaction of the serum sample with the solid support, an antibody-detection reagent in the kit is added to the support, to detect binding of human serum antibodies to each of the peptide antigens separately. The test allows positive identification of HTLV-I or HTLV-II when antibody binding to each HTLV-I or HTLV-II gag and env peptide antigen, respectively, is observed. Also disclosed is a kit for screening human sera for evidence of HTLV-I or HTLV-II infection.
Owner:GENELABS TECH INC +1
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Detection of measurement of antibodies to antigenic proteins in biological tissues or samples
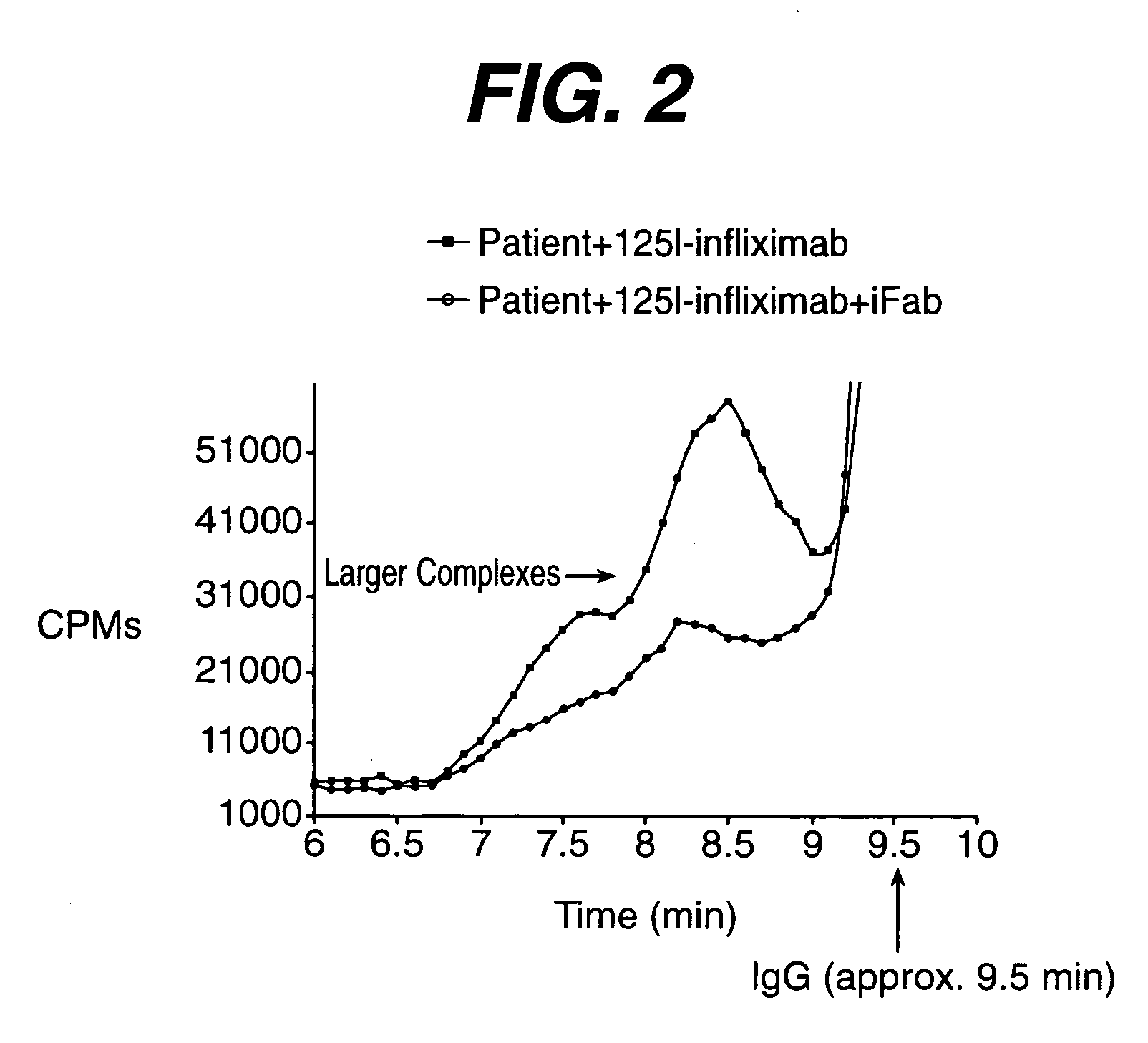
The present invention relates to methods and compositions for detecting and / or measuring serum antibodies to antigenic proteins in a sample, comprising adding a labeled antigenic protein or fragment thereof to a sample derived from serum and expected to contain serum antibodies and measuring differences in at least one characteristic between (a) a labeled serum antibody-antigenic protein complex; (b) an serum antibody-antigenic protein complex in the sample; and / or (c) displaced lableled or unlabeled serum antibody, antigenic protein or fragment thereof.
Owner:CENTOCOR
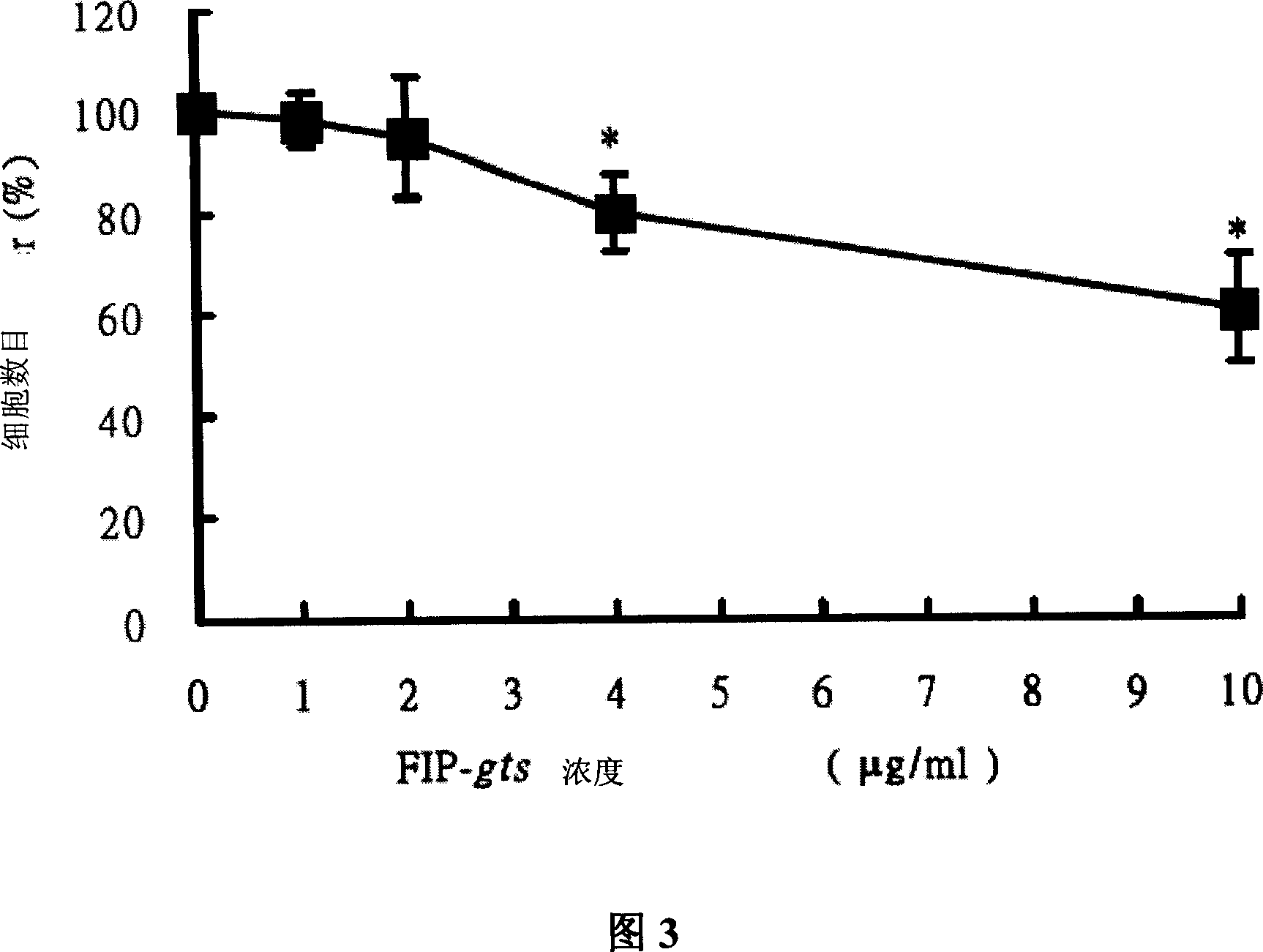
Use of fungal immunomodulatory protein
InactiveCN1939532APeptide/protein ingredientsMetabolism disorderSerum igeActivated Natural Killer Cell
This present invention relates to the use of fungal immunomodulatory protein in immunotherapy, treating or activating natural killer cells, macrophage or increasing cytokines and serum antibody.
Owner:YEASTERN BIOTECH
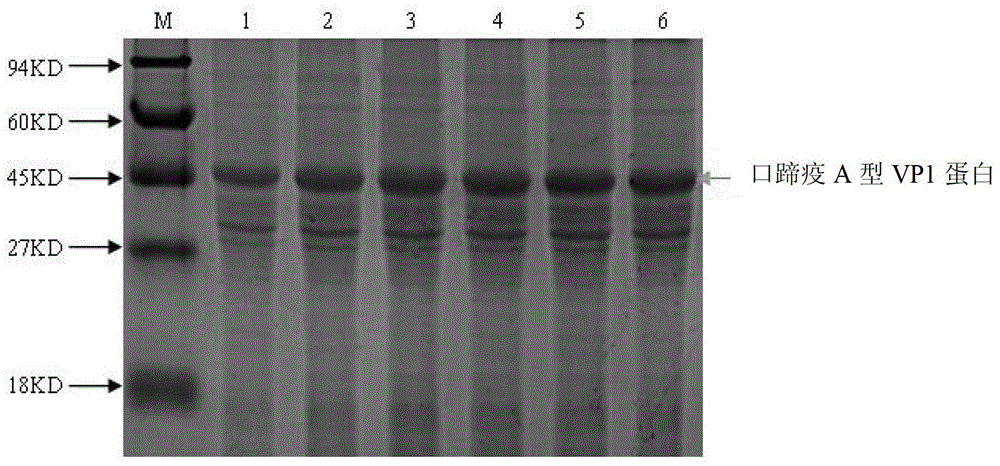
Competitive ELISA method based on foot-and-mouth disease A type VP1 protein and its monoclonal antibody
InactiveCN103554234AHigh purityImprove production efficiencySsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenDisease
The invention relates to a competitive ELISA method based on a foot-and-mouth disease A type VP1 protein and its monoclonal antibody, also relates to a preparation method of the foot-and-mouth disease A type VP1 protein, and a preparation method of the monoclonal antibody of the foot-and-mouth disease A type VP1 protein, and belongs to the technical field of animal immunological detection. In the invention, a primer pair C1 and C2 and a primer pair E1 and E2 are amplified to obtain a gene sequence of the foot-and-mouth disease A type VP1 protein, the foot-and-mouth disease A type VP1 protein is obtained by constructing an expression plasmid, introducing the expression plasmid into a prokaryotic expression host and carrying out inducible purification, the foot-and-mouth disease A type VP1 protein monoclonal antibody is obtained by treating the foot-and-mouth disease A type VP1 protein as an antigen through a hybridomas technology, and the competitive ELISA method used for detecting a foot-and-mouth disease A type antibody is established based on the foot-and-mouth disease A type VP1 protein and its monoclonal antibody. The detection method has a strong specificity and a good stability, and can be used for detecting a foot-and-mouth disease A type serum antibody. By comparing a result obtained through the detection method with a liquid phase blocking ELISA kit, the coincidence rate is 95.8%.
Owner:广西壮族自治区动物疫病预防控制中心
Supression of allergic reactions by transdermal administration of allergens conjugated to cholera toxin or fragments thereof
InactiveUS20050074462A1Suppress allergic reactionsBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantCo administration
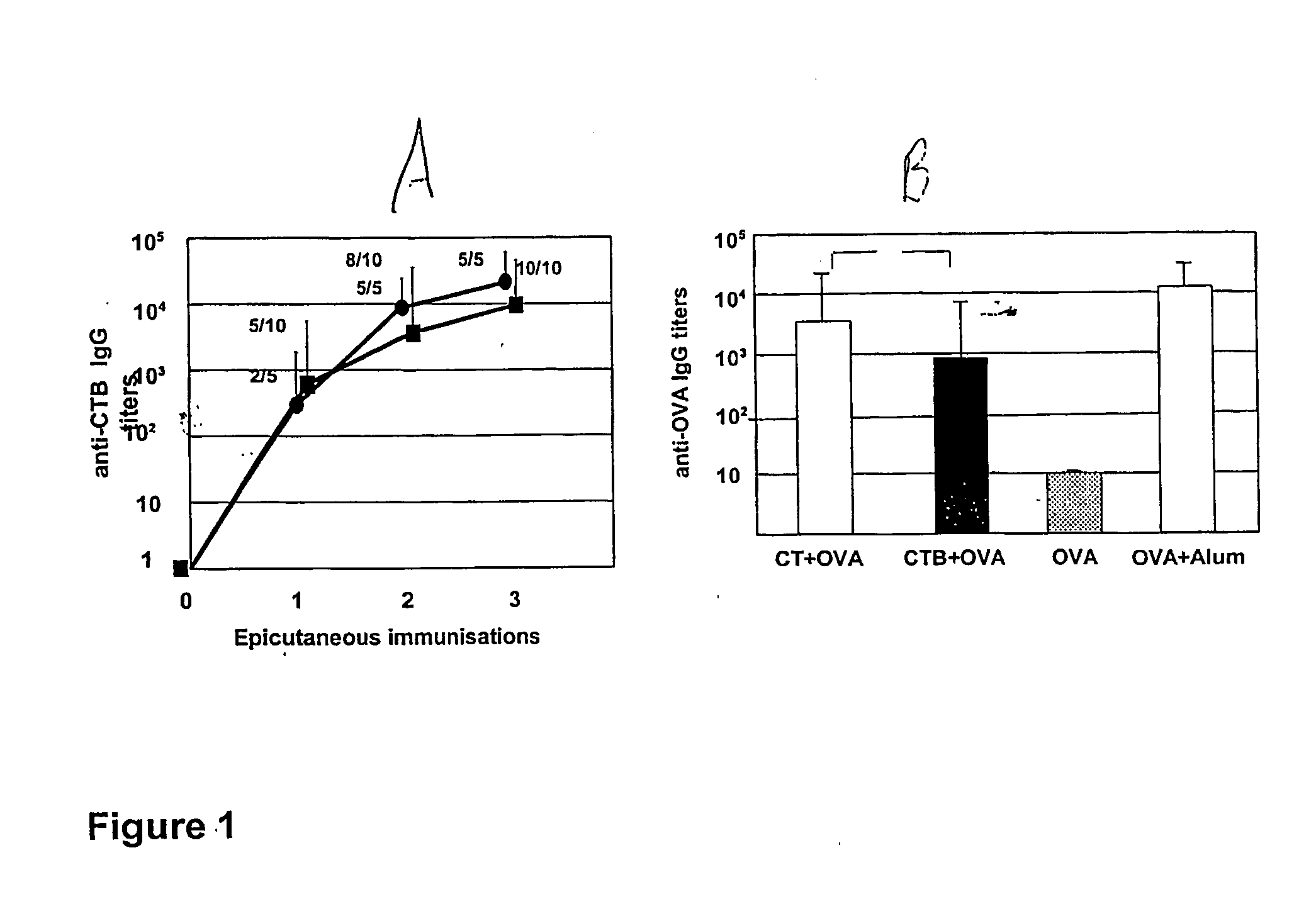
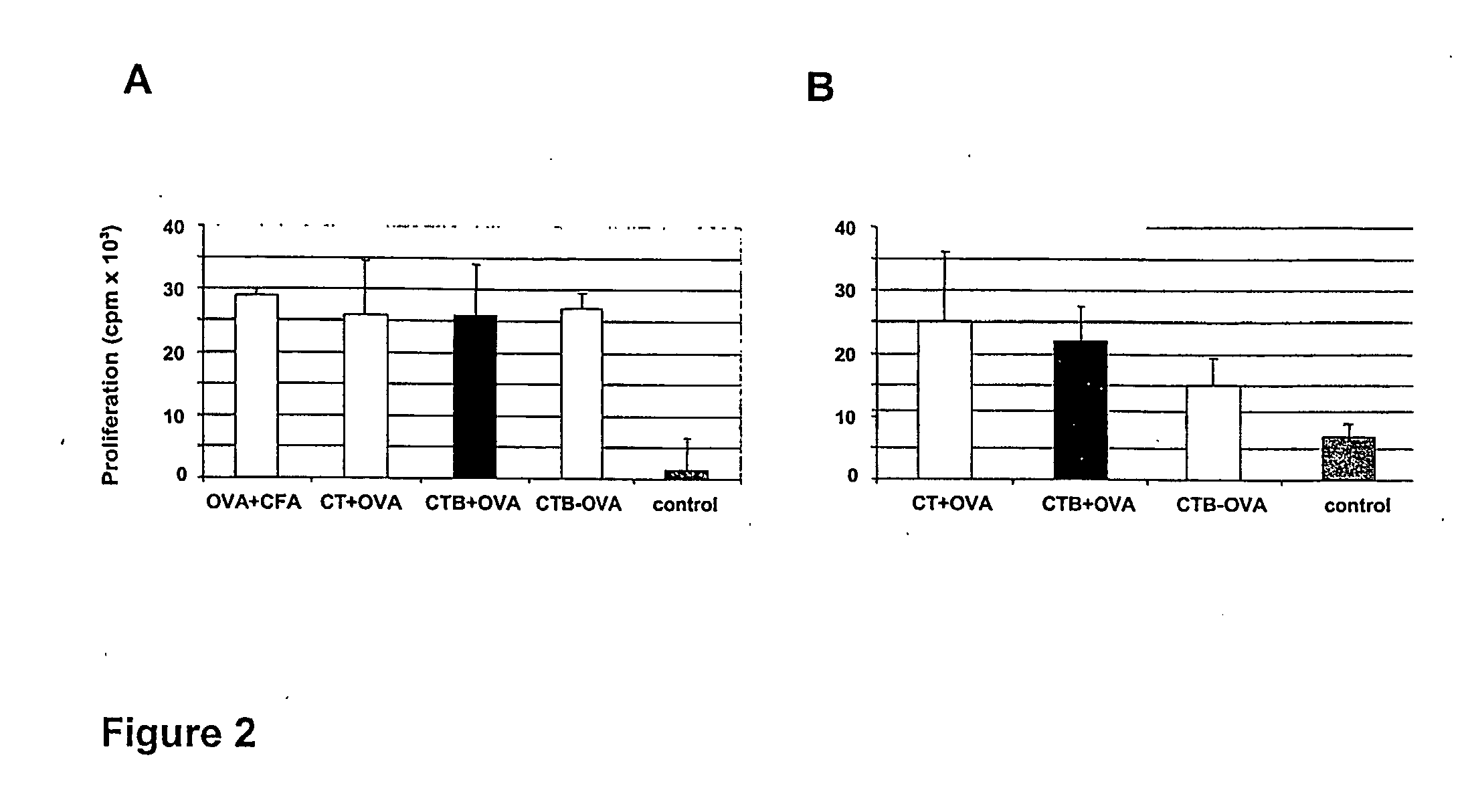
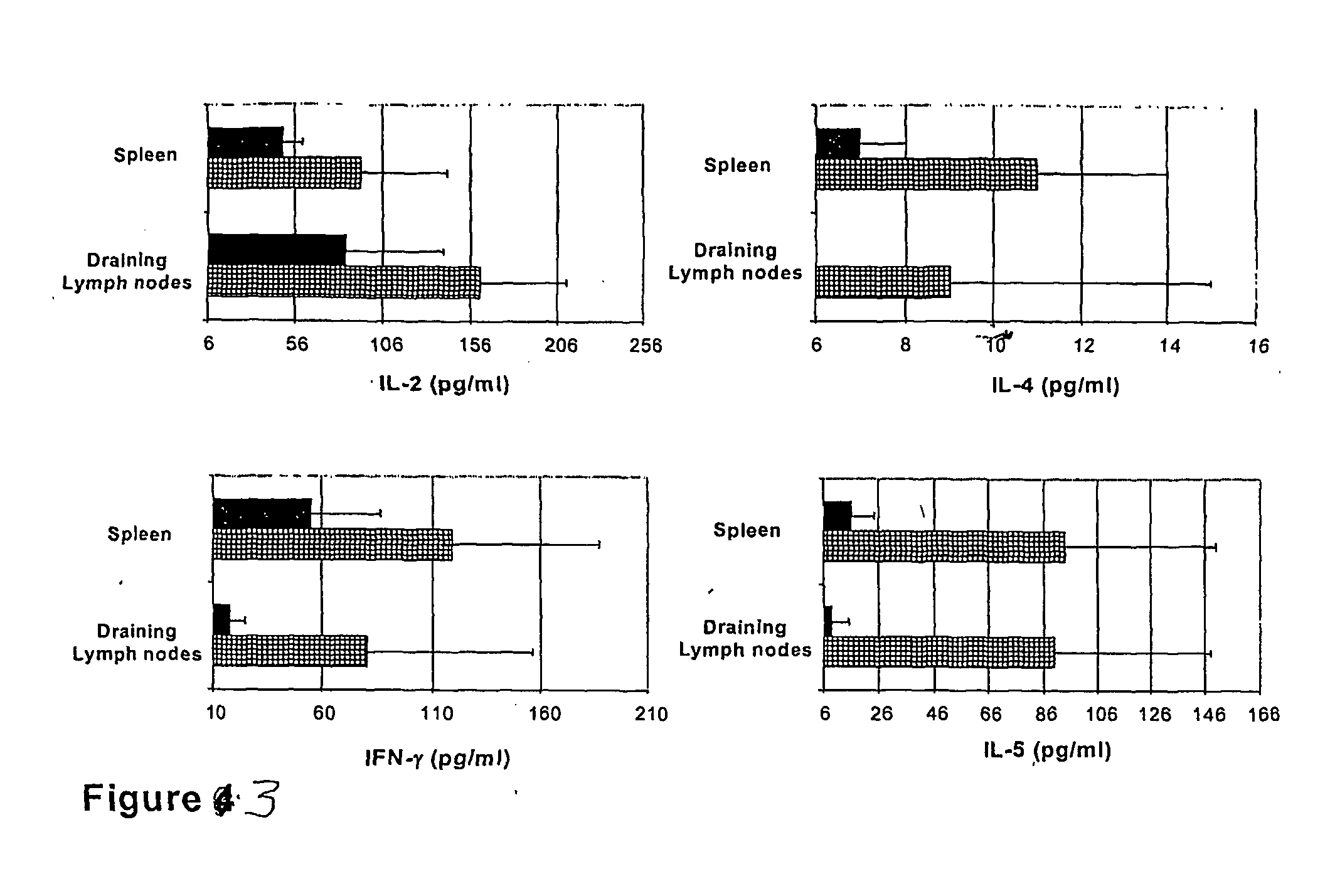
The present invention discloses the use of the non-toxic cell-binding B subunit of CT (CTB), and holotoxin CT that is devoid of ADP-ribosylating activity, as adjuvants for enhancing transcutaneous immune response to a co-administered protein allergen. It was found that topical administration of CTB to mice induced serum antibody response against itself comparable to those evoked by CT, but was inefficient at promoting systemic antibody responses against an admixed prototype protein allergen. To the contrary co-administration of either CT or CTB with allergen led to vigorous antigen-specific T cell proliferative responses in lymph nodes draining the cutaneous site of administration and at distant systemic sites. Consistent with these observations, it was found that CTB selectively potentiated Th1-driven responses without affecting Th2-dependent responses. Cutaneously applied CT enhanced serum IgE responses to a co-administered allergen, while CTB partially suppressed epicutaneously induced IgE responses to the same allergen.
Owner:DUOTOL
Methods for isolating molecular mimetics of unique Neisseria meningitidis serogroup B epitopes
InactiveUS20060035284A1Determine autoreactivityLeast riskAntibacterial agentsAnimal cellsEscherichia coliSalmonella serotype typhi
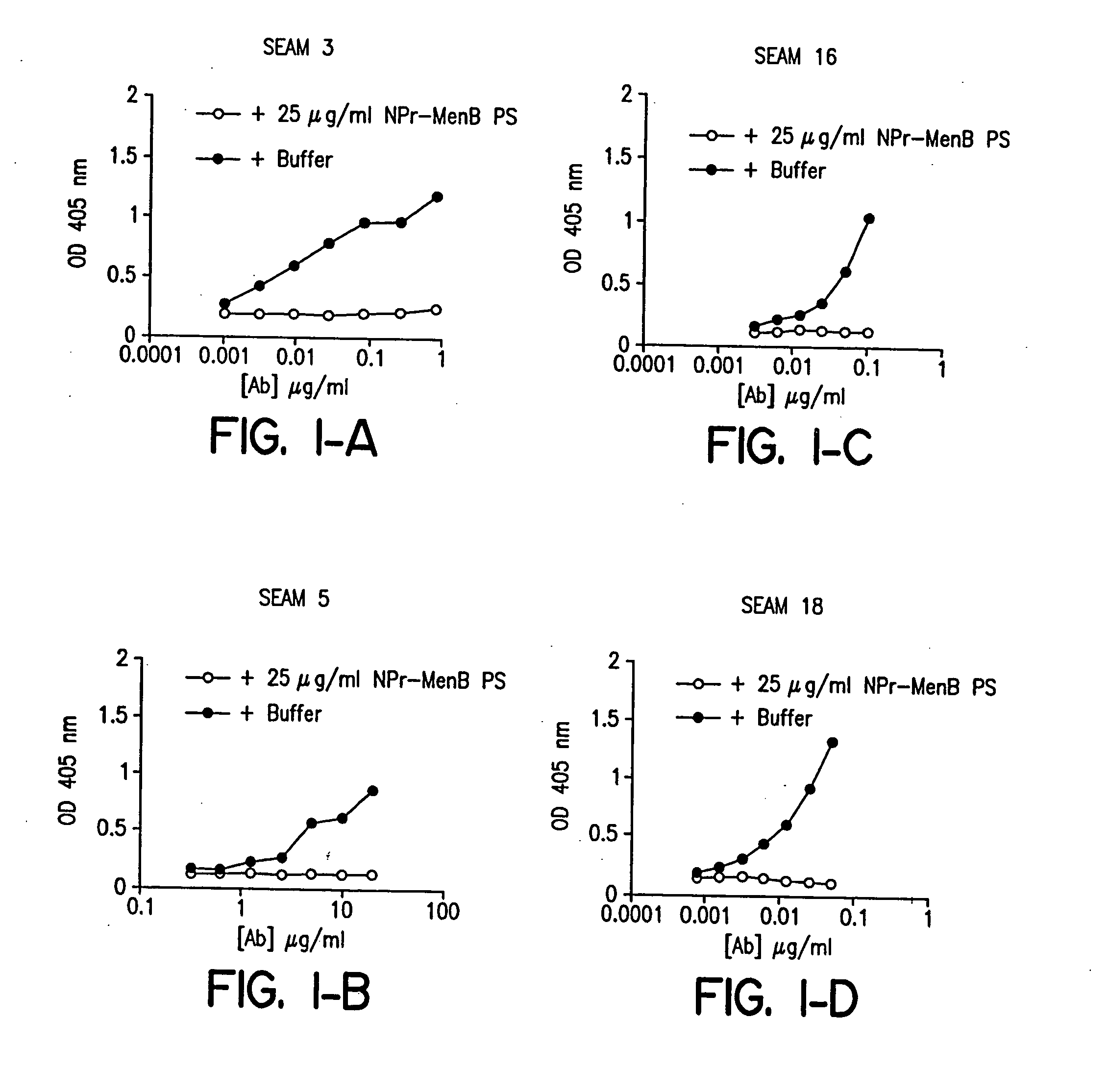
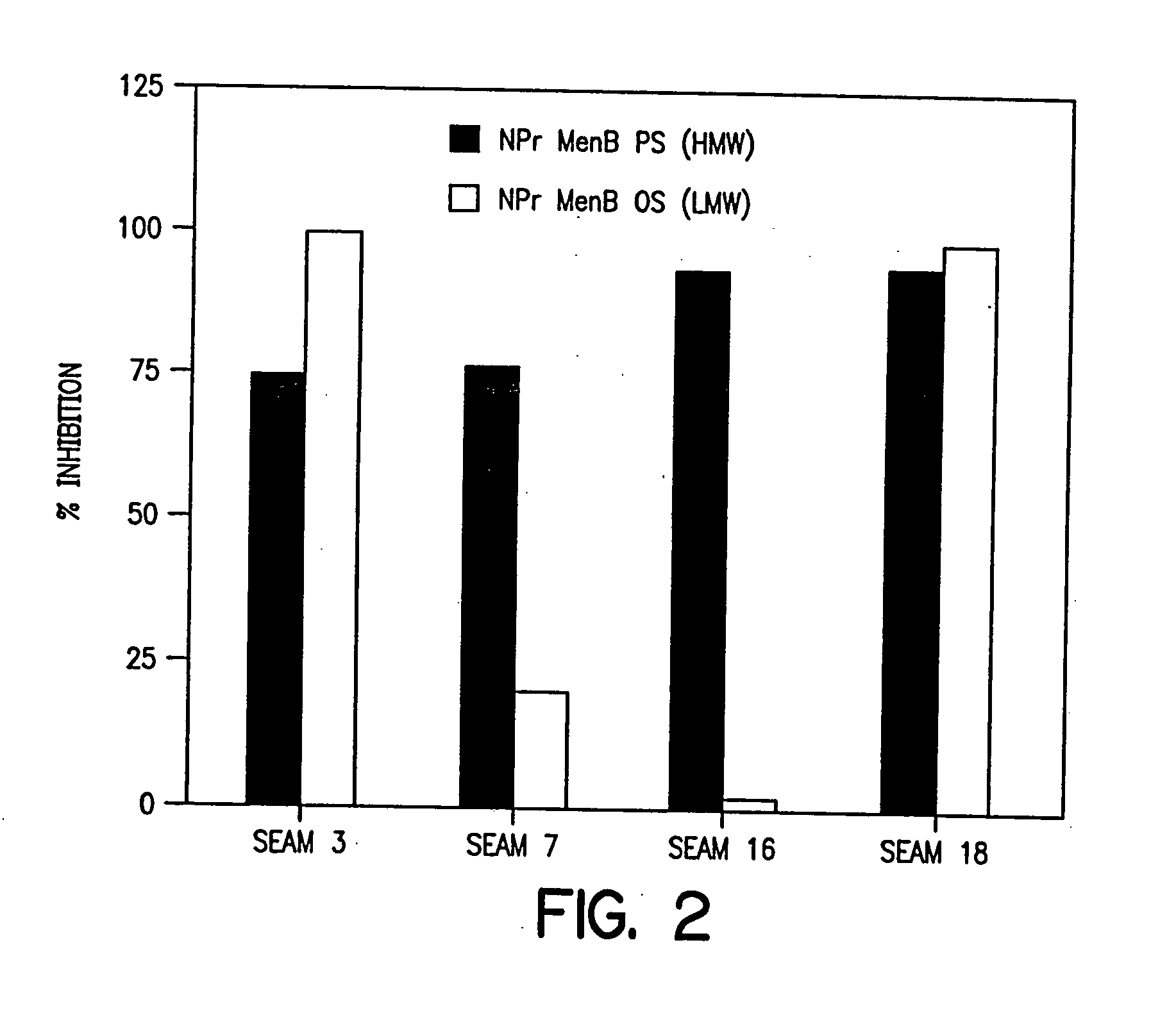
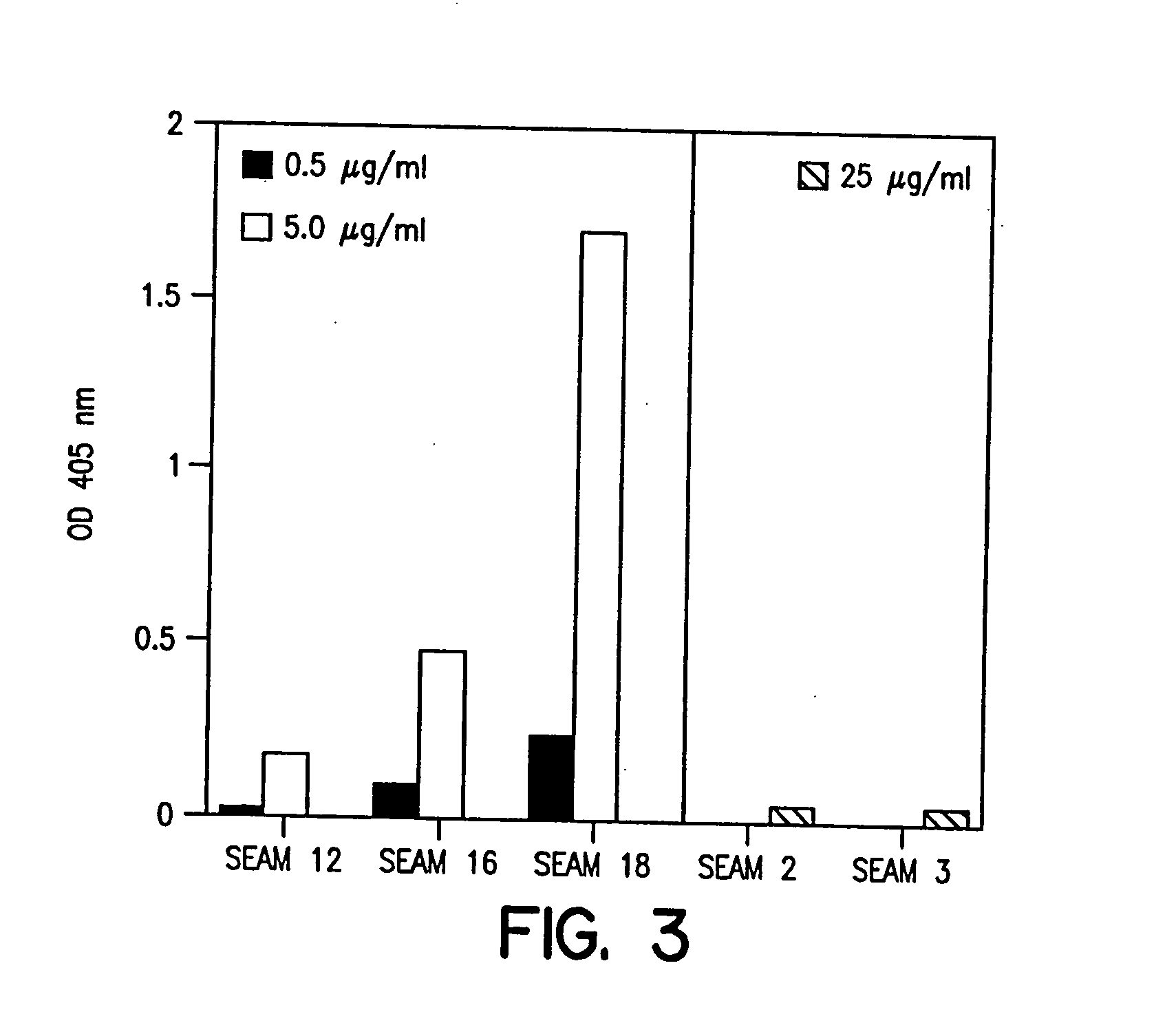
Novel bactericidal antibodies against Neisseria meningitidis serogroup B (“MenB”) are disclosed. The antibodies either do not cross-react or minimally cross-react with host tissue polysialic acid and hence pose minimal risk of autoimmune activity. The antibodies are used to identify molecular mimetics of unique epitopes found on MenB or E. coli K1. Examples of such peptide mimetics are described that elicit serum antibody capable of activating complement-mediated bacteriolysis of MenB. Vaccine compositions containing such mimetics can be used to prevent MenB or E. coli K1 disease without the risk of evoking autoantibody.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Polypeptide immunoassay kit and detection method thereof
InactiveCN102062780AStrong specificityStrong elution abilityImmunoglobulins against animals/humansMaterial analysis by electric/magnetic meansAntigenSolid phases
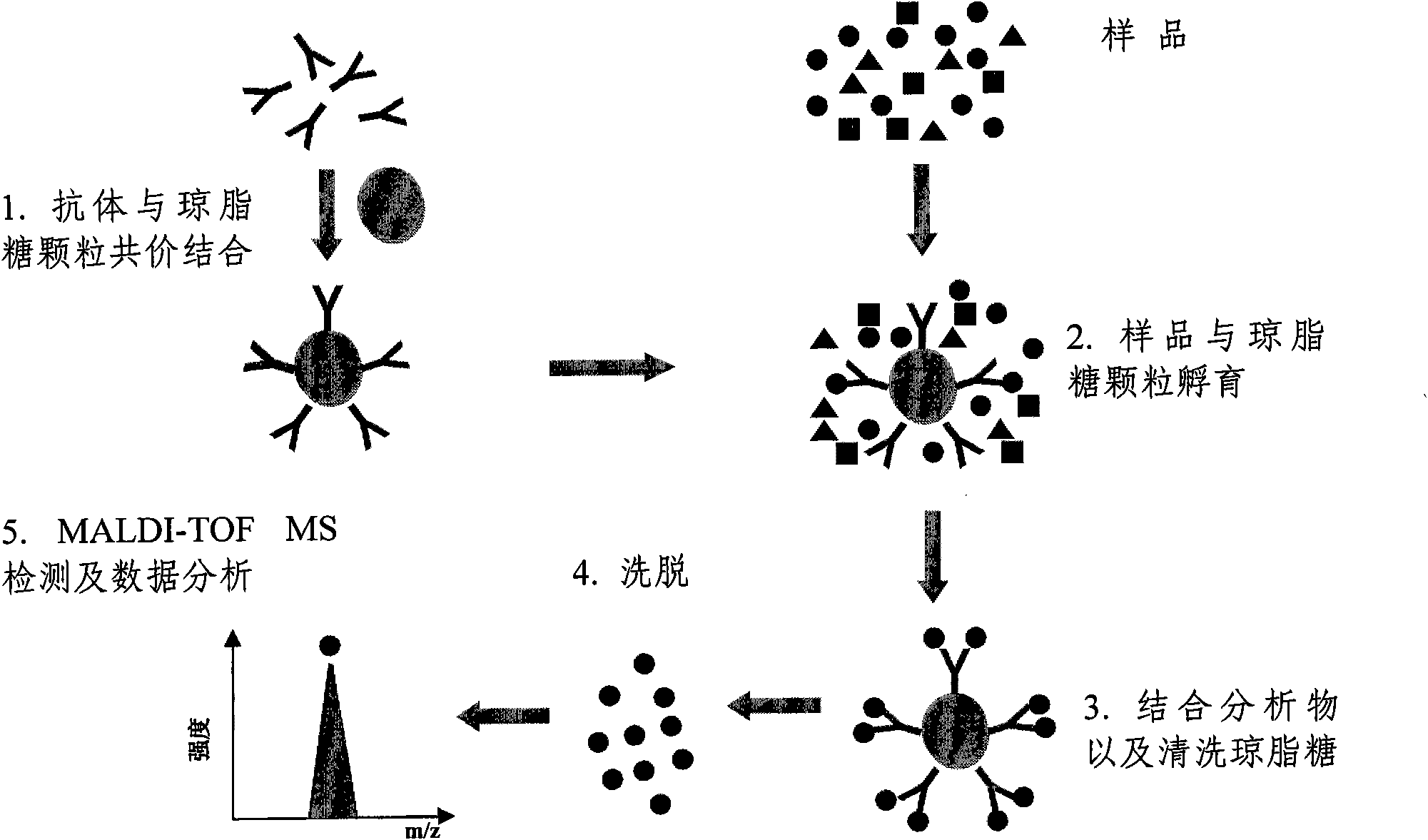
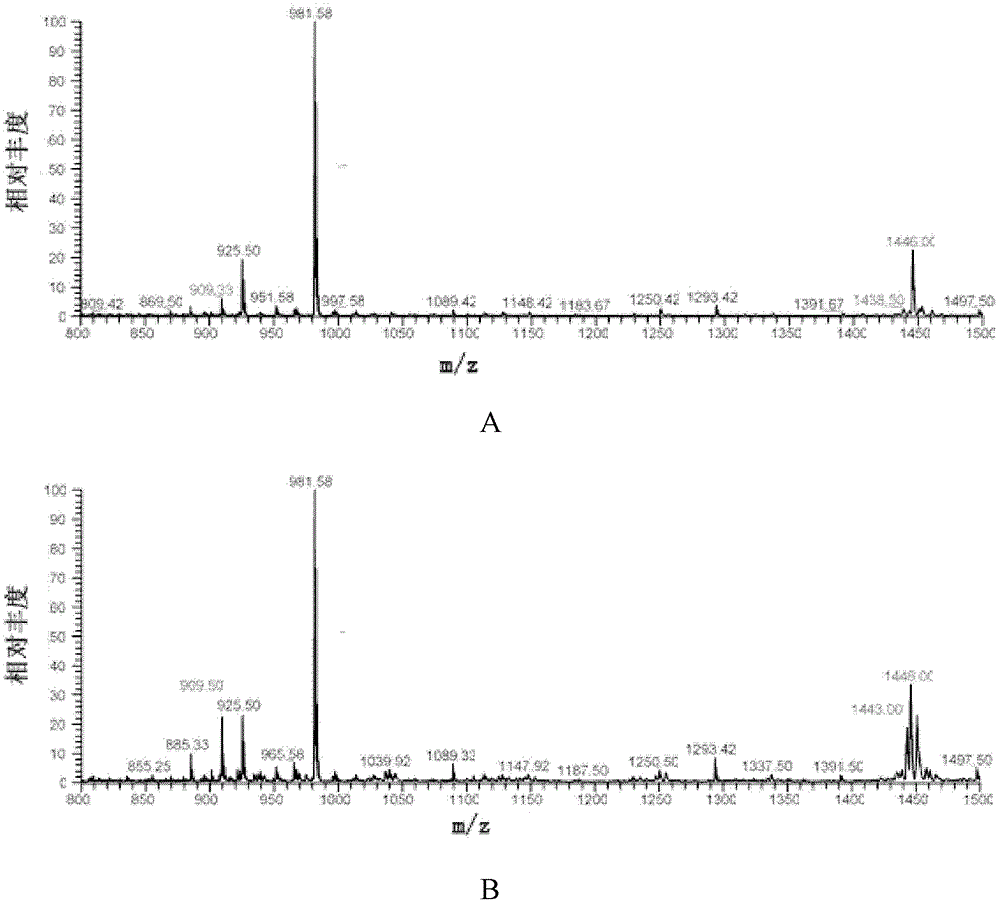
The invention relates to a polypeptide immunoassay kit, which comprises a protein G or protein A agarose serving as a solid phase carrier, a purified serum polypeptide antibody and PBS buffer solution. The invention also relates to a polypeptide immunomic spectrometry analysis method, which comprises the following steps: coupling a purified serum antibody with a proper solid carrier; allowing the coupled product to bond with a polypeptide marker antigen specifically, and separating the antigen from the carrier by using eluent; and finally, detecting polypeptide antigen in the eluent by high-pass matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOFMS). Thus, the complete immunomic spectrometry analysis method is established.
Owner:BEIJING C & N INT SCI TECH +1
Enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody, test method and application thereof
InactiveCN101839917AStrong specificityIncreased sensitivityDepsipeptidesFermentationAntigenDuck hepatitis A virus
The invention relates to an enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody and relates to a test method and application of the kit. The kit comprises an enzyme label plate coated by the recombinant VP1 (virus protein) protein, a rabbit anti-duck IgY antibody marked by horseradish peroxidase, a TMB substrate colour reagent, a positive serum, a negative serum and a kit specification. In the invention, by adopting the polymerase chain reaction, the VP1 genes are amplified from the DHV-1genome and the VP1 gene-containing recombinant expression plasmid pET32a-VP1 is constructed; the plasmid is transferred to host cells BL21 (DE3), and the in-vitro expression VP1 protein is purified by a nickel column and then used as the antigen; the enzyme-linked immunosorbent assay kit is established; the positive serum is the standard positive serum of duck hepatitis virus type-I and the negative control is the standard negative serum of duck. The test kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale popularization and application, very important application value in diagnosis of duck hepatitis virus type-I, survey of epidemiology and immunization survey and the like.
Owner:HENAN UNIV OF SCI & TECH
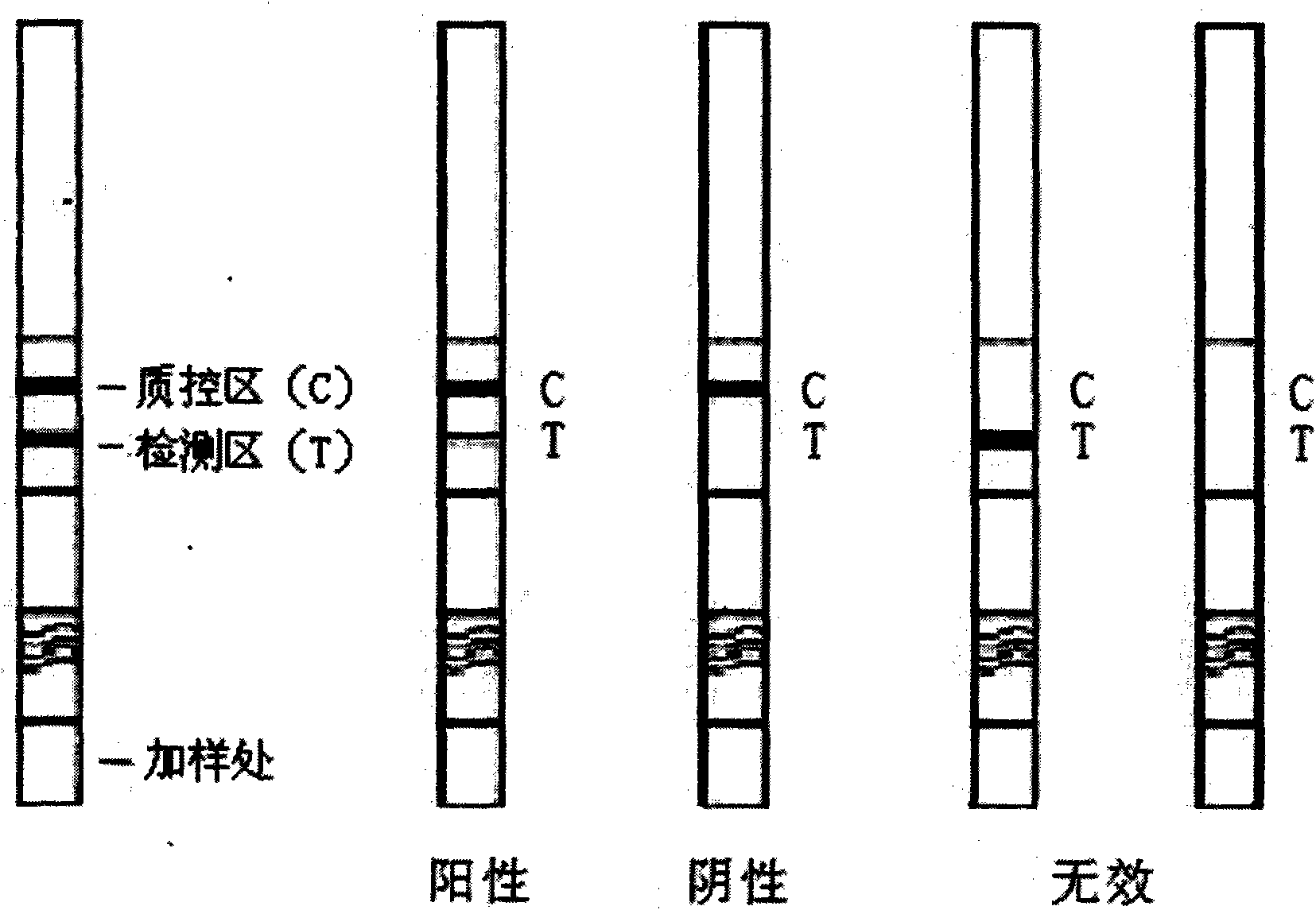
Test strip for detecting colibacillus O157:H7 mouse serum antibody by colloidal gold immunochromatography
The invention relates to a test strip for detecting colibacillus O157:H7 mouse serum antibody by colloidal gold immunochromatography, belonging to the technical field of antibody detection kits and consisting of colloidal gold cushion prepared by utilizing a colloidal gold solution the granule of which is 20nm to mark purified sheep anti-mouse IgG, nitric acid cellulose films which are respectively coated by a colibacillus O157:H7 antigen and purified mouse serum IgG and serve as a detection line and a quality control line, a detection test strip formed by assembling a sample absorbing cushionprepared from glass cellulose films and an absorbing cushion made of water absorbing filter paper, positive serum, negative serum, a serum sample diluent and a test strip operating instruction. The test strip is convenient to use, rapid and sensitive, and a detection result can be obtained within 10 minutes, and therefore, the test strip is suitable for on-site detection when infection status ofmouse O157:H7 is surveyed and traced back in animal farmer areas, food production enterprise areas and the like and use when the food safety environment risk evaluation is carried out.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Vaccines against Escherichia coli O157 infection
InactiveUS6858211B1Reduce severityPrevent and treat illnessBiocideImmunoglobulinsDiseaseEscherichia coli
This invention relates to conjugates of the O-specific polysaccharide of E. coli O157 with a carrier, and compositions thereof, and to methods of using of these conjugates and / or compositions thereof for eliciting an immunogenic response in mammals, including responses which provide protection against, or reduce the severity of, bacterial infections. More particularly it relates to the use of polysaccharides containing the tetrasaccharide repeat unit: (→3)-α-D-GalpNAc-(1→2)-α-D-PerpNAc-(1→3)-α-L-Fucp-(1→4)-β-D-Glcp-(1→), and conjugates thereof, to induce serum antibodies having bactericidal (killing) activity against hemolytic-uremic syndrome (HUS) causing E. coli, in particular E. coli O157. The conjugates, and compositions thereof, are useful as vaccines to induce serum antibodies which have bactericidal or bacteriostatic activity against against E. coli, in particular E. coli O157, and are useful to prevent and / or treat illnesses caused by E. coli O157.The invention further relates to the antibodies which immunoreact with the O-specific polysaccharide of E. coli O157 and / or the carrier, that are induced by these conjugates and / or compositions thereof. The invention also relates to methods and kits using one or more of the polysaccharides, conjugates or antibodies described above.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERIVCES THE
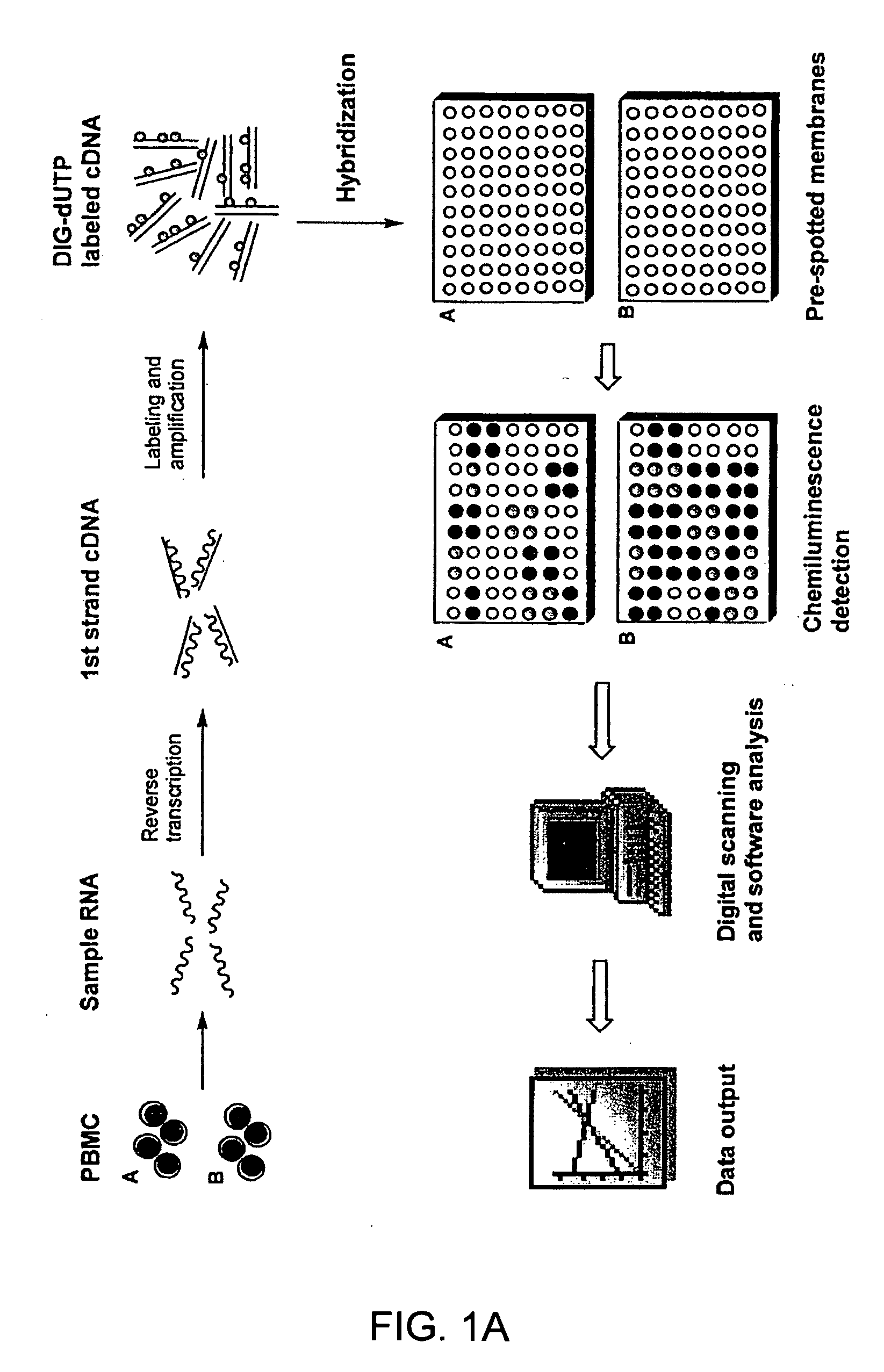
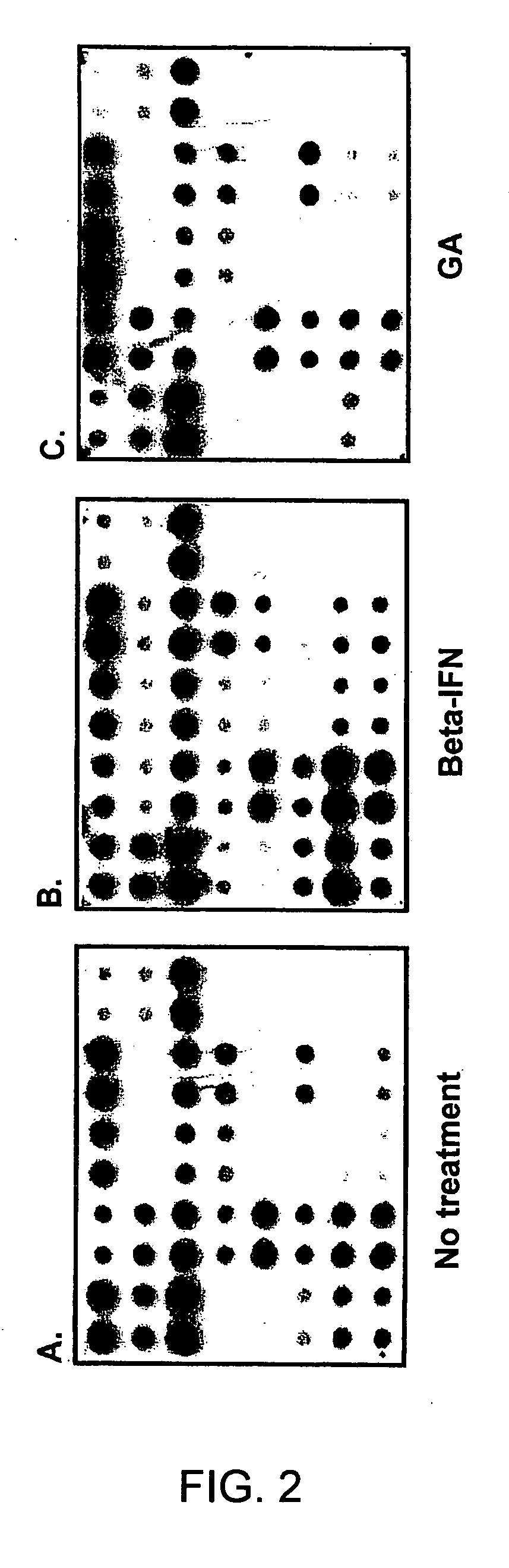
Gene expression profiling technology for treatment evaluation of multiple sclerosis
InactiveUS20050064483A1Microbiological testing/measurementNeutralizing antibodyGene expression profiling
The invention relates to gene expression profiling technology to quantitatively measure the expression profiles of genes selected based on their role in inflammation and their susceptibility to regulation by current multiple sclerosis (MS) treatment agents, beta-interferon (IFN) and glatiramer acetate (GA). The invention also provides an assay for detection of beta-IFN neutralizing antibody based on the blocking effect of serum antibodies on the known regulatory properties of beta-IFN on PBMC and evaluation of treatment responses in MS patients.
Owner:BAYLOR COLLEGE OF MEDICINE
Method of immunizing humans against Salmonella typhi using a Vi-rEPA conjugate vaccine
InactiveUS6797275B1Reduce severityPrevent typhoid feverAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
This invention relates to conjugates of the Vi polysaccharide of S. typhi with the carrier Pseudomonas aeruginosa recombinant exoprotein A (rEPA), and compositions thereof, and to methods of using of these conjugates and / or compositions thereof for eliciting an immunogenic response in humans, including responses which provide protection against, or reduce the severity of, S. typhi bacterial infections. The conjugates, and compositions thereof, are useful as vaccines to induce serum antibodies againt S. typhi and are useful to prevent and / or treat illnesses caused by S. typhi.
Owner:UNITED STATES OF AMERICA
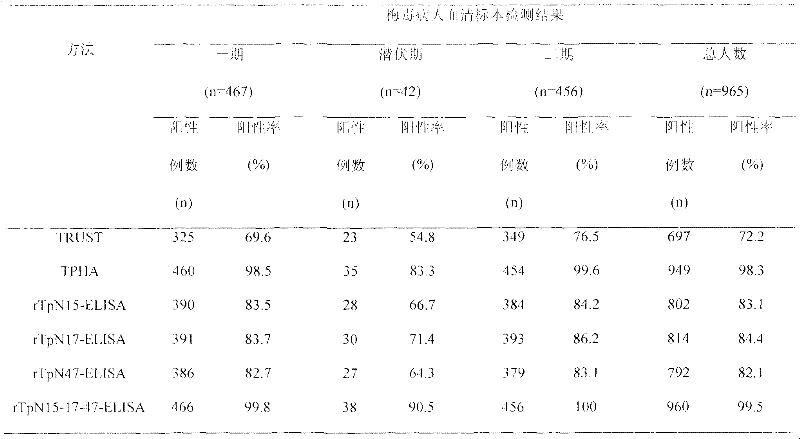
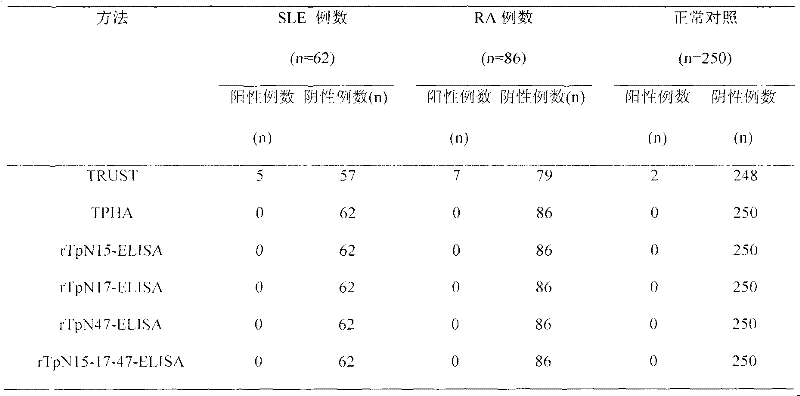
Preparation method of rTpN15-17-47-ELISA for detecting syphilis serum antibody
InactiveCN102183646AOvercome the disadvantage of high costGood antigenicityBacteriaFermentationSyphilisNucleotide
The invention relates to a syphilis serological screening or diagnosis method. The purpose is that the method has the characteristics of low price, high speed, sensitivity and specificity. The technical scheme provided by the invention is that: a preparation method of rTpN15-17-47-ELISA for detecting syphilis serum antibodies comprises the following steps in turn: constructing an artificial fusion gene tpN15-17-47 of tpN15, tpN17 and tpN47 and a prokaryotic expression system E.coliBL21DE3Pet42a-tpN15-17-47 by gene technology, purifying the recombinant expression product rTpN15-17-47 as a coating antigen, establishing rTpN15-17-47-ELISA for detecting syphilis serum antibodies. The fusion gene tpN15-17-47 has a nucleotide sequence and an amino acid sequence as shown in sequence table 5.
Owner:孙爱华 +1
PED (Porcine Epedemic Diarrhea) inactivated vaccine and preparation method thereof
ActiveCN104383528AEnhance immune responseImprove the level ofOrganic active ingredientsDipeptide ingredientsAntiendomysial antibodiesDipeptide
The invention provides a PED (Porcine Epedemic Diarrhea) inactivated vaccine and a preparation method thereof and relates to the field of biopharmacy. The PED inactivated vaccine comprises inactivated PEDV (Porcine Epedemic Diarrhea Virus), and is characterized in that the PED inactivated vaccine comprises 0.05-10 mg / mL Beta-glucosylceramide, 0.1-21 mg / mL monophosphoryl lipid A, 1.5-125 mg / mL muramyl dipeptide and 0.7-4.5 mg / mL Beta-glucan. According to the ingredients of the PED inactivated vaccine, Beta-glucosylceramide, monophosphoryl phosphoryl lipid A, muramyl dipeptide and Beta-glucan have the synergistic effect, the immune response of animals to antigens in the vaccine is significantly improved, the immune window phase is shortened, the antibody production duration of the animal body is obviously prolonged, the serum antibody level is improved, and the level of total intestinal mucosa secretory antibodies (the total SIgA) is improved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Proteomic identification of antibodies
ActiveUS20130178370A1Microbiological testing/measurementLibrary screeningAntigen Binding FragmentAntigen binding
Methods and compositions for identification of candidate antigen-specific variable regions as well as generation of antibodies or antigen-binding fragments that could have desired antigen specificity are provided. For example, in certain aspects, methods for determining amino acid sequences of serum antibody CDR3 and abundancy levels are described. In some aspects, methods for determining nucleic acid sequences of antibody variable region sequences and the frequency thereof in biological samples are provided. Furthermore, the invention provides methods for identification and generation of antibodies or antigen-binding fragments that comprise highly-represented CDR domains.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for preparing bivalent yolk antibody for preventing and treating duck virus hepatitis I and III
InactiveCN103059131ASolve the dilemma of ineffective treatment of mixed infectionsHigh purityEgg immunoglobulinsDigestive systemYolkSerum ige
The invention discloses a method for preparing a bivalent yolk antibody for preventing and treating duck virus hepatitis I and III. The bivalent yolk antibody can be used for effectively preventing and treating the duck virus hepatitis I and III single and mixed infection and solving the problems that the existing vaccines, serum antibodies and yolk antibodies are not effective to the mixed infection. In addition, the bivalent yolk antibody is extracted by adopting a caprylic acid precipitation method. The method is safe, low in cost and suitable for mass production. The prepared bivalent yolk antibody has high purity and high titer, is stable in nature and is easy for storage.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Porcine rotavirus delta VP8* subunit recombinant protein and applications thereof
InactiveCN103304642AStrong immune responseFast titerViral antigen ingredientsVirus peptidesVp4 geneInclusion bodies
The invention relates to a porcine rotavirus delta VP8* subunit recombinant protein and an encoding gene of the protein. The invention further provides a recombinant protein formed after increasing tetanus toxin T cell epitope P2 into the recombinant protein, and an encoding gene. The delta VP8* protein is 64th-site to 223th-site amino acid in the VP8* and can effectively stimulate an organism to produce specific serum antibody, humoral immune response is good, the problem that the VP4 gene can not conduct prokaryotic expression due to overlarge fragment can be overcome, the protein can be expressed as a soluble protein in vitro, so that the problem that the VP8* is expressed as an inclusion body in vitro can also be overcome; the T cell epitope P2 (830th-site to 844th-site amino acid of TT) in the tetanus toxin is induced into the delta VP8* subunit recombinant protein, so that the immunity efficacy of the protein can be greatly improved, the faster and stronger neutralizing antibody titer can be induced, and high-titer rotavirus cross neutralizing antibody can also be induced.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Rapid isolation of monoclonal antibodies from animals
ActiveUS9090674B2Serum immunoglobulinsMicrobiological testing/measurementSerum igeAntigen Binding Fragment
Methods and compositions for identification of candidate antigen-specific variable regions as well as generation of antibodies or antigen-binding fragments that could have desired antigen specificity are provided. For example, in certain aspects methods for determining amino acid sequences of serum antibody CDR and abundancy level are described. In some aspects, methods for determining nucleic acid sequences of antibody variable region sequences and frequency are provided. Furthermore, the invention provides methods for identification and generation of antibody or antigen-binding fragments that comprise highly-represented CDR.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Diagnostic marker for systemic lupus erythematosus
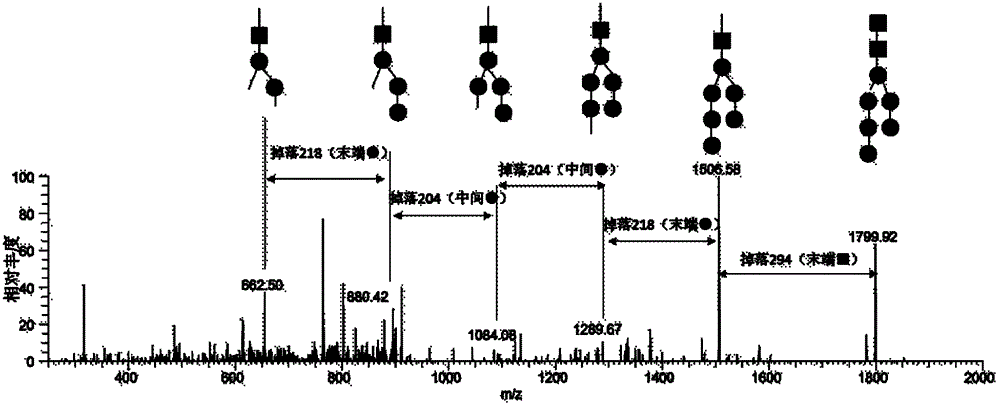
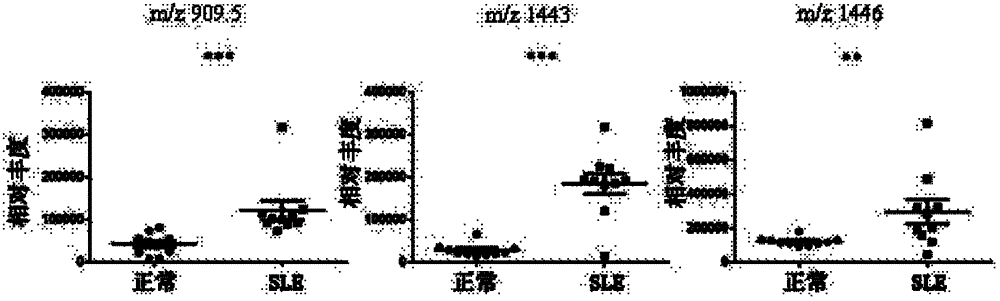
The invention relates to a diagnostic marker for systemic lupus erythematosus, and in particular discloses application of a reagent for specific detection of high mannose type N-linked carbohydrate chain level in a serum antibody to preparation of a diagnostic tool for diagnosis and / or prognosis assessment of systemic lupus erythematosus. The increase of the high mannose type N-linked carbohydrate chain level in the serum antibody is closely related to the occurrence of systemic lupus erythematosus. Therefore, the diagnostic marker can be used for early diagnosis and / or prognostic assessment of systemic lupus erythematosus, and further can be used for guiding treatment of systemic lupus erythematosus.
Owner:杭州中赢生物医疗科技有限公司
Traditional Chinese medicine astragalus polysaccharide immunopotentiator
ActiveCN101884788AGood effectNo side effectsAntibody medical ingredientsCellular immunityNewcastle disease vaccine
The invention relates to a traditional Chinese medicine immunopotentiator (an astragalus polysaccharide immunopotentiator for short) prepared from astragalus extracts and sulfated epimedium polysaccharides, which belongs to the field of immunological adjuvants of livestock and poultry. 1,000ml of the liquid medicine is prepared from 40g of astragalus and 120g of epimedium herb. The preparation method of the immunopotentiator comprises the following steps of: decocting the astragalus with water for three times, then merging the obtained filter liquor and condensing the filter liquor into 500ml of astragalus solution; extracting the epimedium polysaccharides by using the epimedium herb water decoction and ethanol precipitate method, then decorating the polysaccharides by using the chlorosulfonic acid-pyridine method, and preparing the polysaccharides into 500ml of sulfated epimedium polysaccharide solution after carrying out distilled water dialysis on the polysaccharides; and mixing the astragalus solution and the sulfated epimedium polysaccharide solution, and then filtering, sub-packaging and sterilizing to obtain the traditional Chinese medicine astragalus polysaccharide immunopotentiator. The immune experiment shows that the astragalus polysaccharide immunopotentiator has the advantage of obviously improving the proliferation of peripheral blood lymphocyte of chicken and enhancing the cellular immunity, obviously improving the potency of a serum antibody, promoting the lymphocyte proliferation, enhancing the cellular immunity and humoral immunity, and improving the immune response of a vaccine by coordinately immunizing chickling by using the Newcastle disease vaccine.
Owner:NANJING AGRICULTURAL UNIVERSITY
Compositions comprising fungal immunomodulatory protein and use thereof
InactiveUS20100009915A1Peptide/protein ingredientsMetabolism disorderActivated Natural Killer CellTumor Cell Mobility
This invention relates to a method for stimulation or an activation of immunological function directed to activate natural killer cells and macrophages or increase production of serum antibody in a patient in need of such stimulation or activation, comprising administering an isolated and / or purified polypeptide of a fungal immunomodulatory protein. This invention also relates to a method for suppressing proliferation of a cancer cell and a method for suppressing a tumor cell mobility, comprising providing to the tumor cell a purified polypeptide of a fungal immunomodulatory protein.
Owner:YEASTERN BIOTECH
Indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody
The invention discloses an indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody and belongs to the technical field of serum antibody detection. The indirect ELISA method includes that serum 3-type duck hepatitis virus VP1 protein is utilized as an envelope antigen with the peridium quantity as 0.1-1mug per hole, HRP-mice anti-duck IgY is utilized as the HRP with the use concentration as 0.2-2mug / mL, and the coloration time is 10 minutes. The kit comprises a VP1 protein antigen envelope board, the HRP-mice anti-duck IgY, sample diluent, a scrubbing solution, TMB solutions A and B and a stop solution. The method and the kit can be used for detecting the serum 3-type duck hepatitis virus a antibody in duck serum and duck egg yolk, and the serum does not have cross reaction with positive serum of other viruses. Compared with a traditional neutral reaction detection method, the method has the advantage of being convenient and accurate.
Owner:WUHAN CHOPPER BIOLOGY
Colloidal gold strip for TGEV antibody and PEDV antibody
ActiveCN103033626AObvious superiorityGuaranteed FeaturesMaterial analysisSerum igeMonoclonal antibody
The invention discloses a test strip for quickly detecting a PEDV (Porcine Epidemic Diarrhea Virus) serum antibody and a TGEV (Transmissible Gastroenteritis Virus) serum antibody and a preparation method of the test strip. The test strip comprises a support layer, a sample loading layer, a gold labeling protein release pad, a detection layer and an absorption layer, wherein a gold labeling protein and a detection line protein of the test strip are an S1 protein of a PEDV and a recombination protein of a TGEV, which are obtained by an efficient prokaryotic expression system; the recombination protein comprises an S protein AD site; and two quality control line proteins are monoclonal antibodies for the two proteins. Compared with the traditional test strip for detecting the PEDV antibody and the TGEV antibody, the test strip is high in specificity and safety and simple to operate, and judges results quickly.
Owner:兆丰华生物科技(南京)有限公司 +3
Vaccine composition, preparation method and application thereof
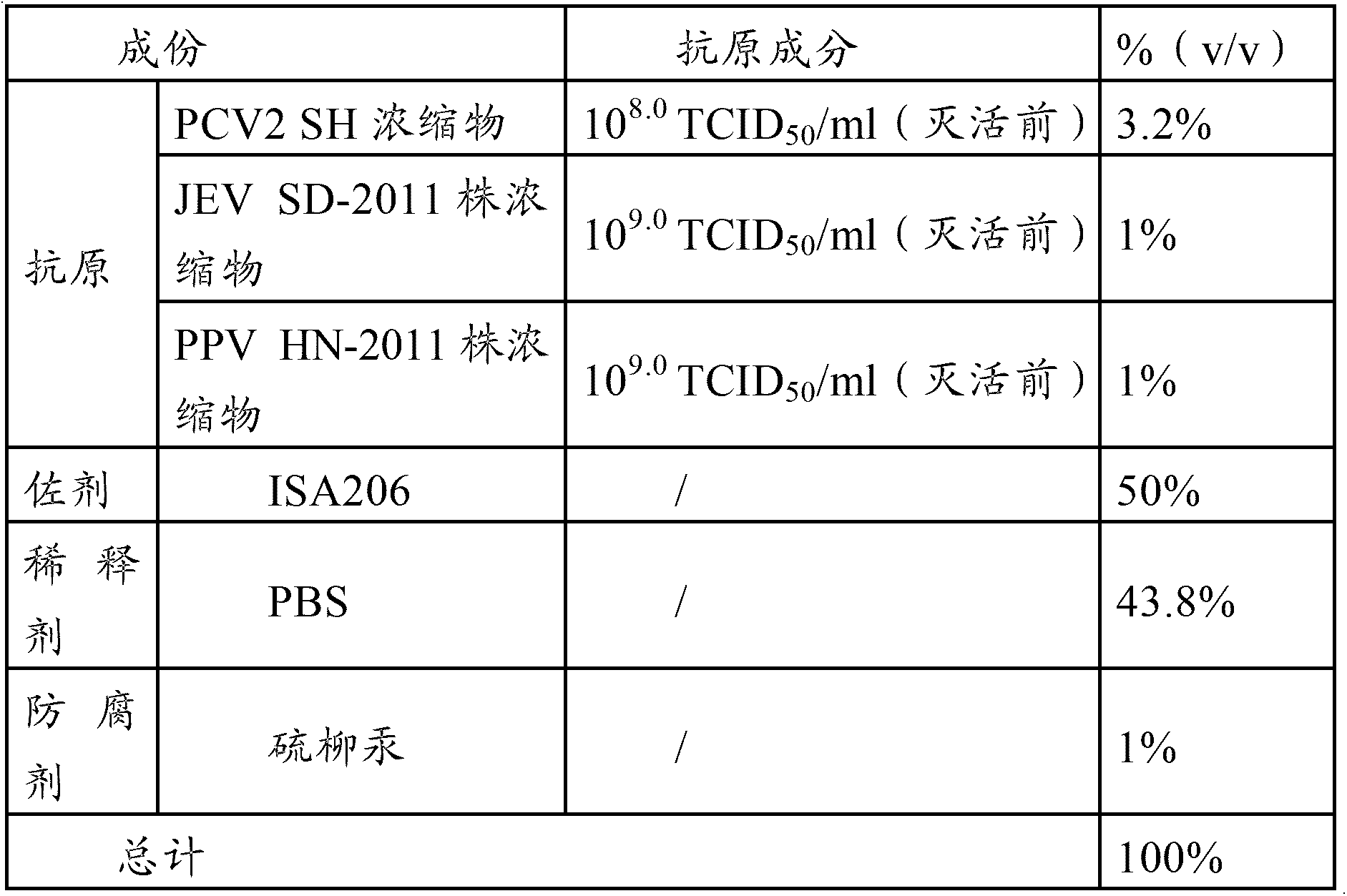
ActiveCN104043117AReduce the impactLess side effectsViral antigen ingredientsAntiviralsDiseaseSide effect
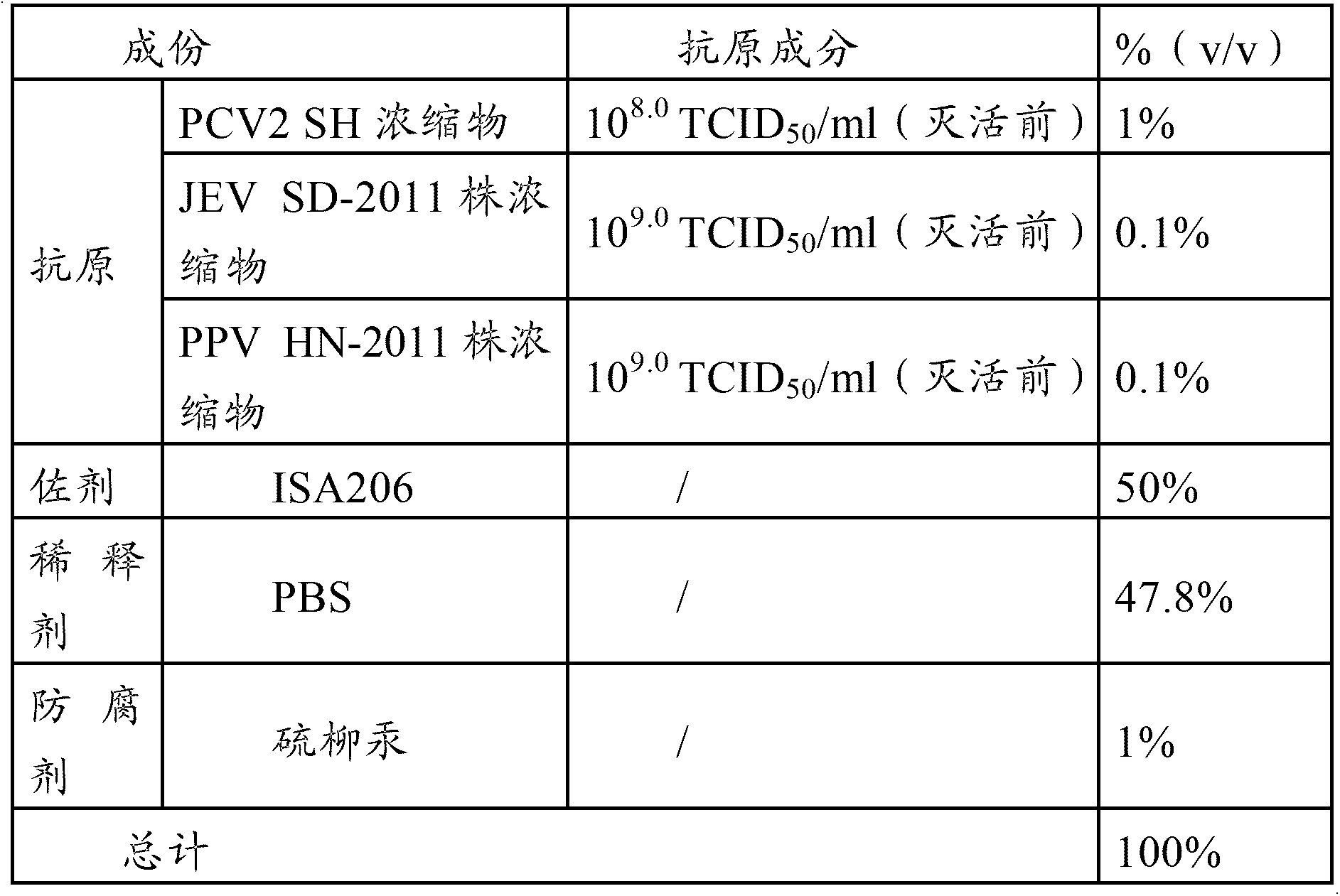
The invention provides a vaccine composition, a preparation method and an application thereof. The vaccine composition includes: an immune dose of porcine circovirus 2-type antigen, an immune dose of porcine Japanese encephalitis virus antigen, an immune dose of porcine parvovirus antigen and an adjuvant. The vaccine composition can effectively prevent and cure postweaning piglet multisystemic syndrome and sow reproduction dysfunctional diseases which are caused by 2-type porcine circovirus, porcine Japanese encephalitis virus and porcine parvovirus. An immune effect of the vaccine composition is better than that of a single vaccine. The vaccine composition is little in side effects, is high in valence of a serum antibody, has a long immune period, can save time and labor intensity, has a small stress response to a pig, can simplify immunity processes and can reduce production cost and prevention cost.
Owner:PU LIKE BIO ENG
Kit for detecting capripoxvirus serum antibody based on synthetic peptide
The invention discloses a kit for detecting a capripoxvirus serum antibody based on a synthetic peptide. A preparation method of the kit comprises the following steps of 1, designing a short-peptide aiming at a capripoxvirus main immunogene P32, 2, synthesizing a peptide fragment by a conventional peptide synthesis technology, 3, preparing the kit for detecting a capripoxvirus serum antibody based on a synthetic peptide, and 4, optimizing conditions and finishing the preparation of the kit for detecting a capripoxvirus serum antibody. A coating antigen adopted by the kit is an external synthetic immunoactive peptide fragment, reduces the danger of totvirus use and prevents virus diffusion and escape. The synthetic peptide adopted by the kit has high purity, has immunological characteristics similar to capripoxvirus particles, and can replace capripoxvirus particles and be used as an antigen for detection and thus the kit realizes sensitive, specific, safe and reliable detection of a capripoxvirus serum antibody.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method of utilizing yolk antibody instead of serum antibody to evaluate infection state of breeder flock
The invention relates to a method of utilizing yolk antibody instead of serum antibody to evaluate infection state of breeder flock. The method of the invention comprises the following steps: collecting chicken serum and eggs, respectively; respectively detecting antibody levels of serum and yolk by REV, REOV, ALV-J, ALV-AB, CAV and IBDV enzyme linked immunosorbent assay (ELISA) kits from IDEXX company; comparatively analyzing serum and yolk antibody OD value correlation coefficient by SPSS Statistics 17.0 to obtain correlation coefficient of an optimum dilution factor of yolk antibody and a serum antibody OD value according to ELISA detection. The detecting result further indicates that the positive and negative result consistent rate of yolk sample and the serum sample at the optimum yolk dilution factor is 96%-100%. The result of the invention indicates that the serum samples can be replaced by yolk when various infection states of breeder flock are detected by ELISA kits provided by IDEXX for detection with serum samples, so that the infection state of breeder flock can be detected by detecting yolk antibody level.
Owner:SHANDONG AGRICULTURAL UNIVERSITY +1
Indirect enzyme-linked immunosorbent assay (ELISA) method for detecting duck flavivirus serum antibody
The invention relates to an indirect enzyme-linked immunosorbent assay (ELISA) method for detecting a duck flavivirus serum antibody, which is characterized in that: duck flavivirus is used as an envelop antigen, and an indirect ELISA method for detecting duck flavivirus serum antibody in duck blood serum is established. The method can effectively diagnose the duck flavivirus which is prevalent at present in China and can carry out large-scale investigation of blood serum epidemiology, and a quick, simple and convenient detection method can be provided for controlling the prevention and the prevalent situation of the duck flavivirus.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
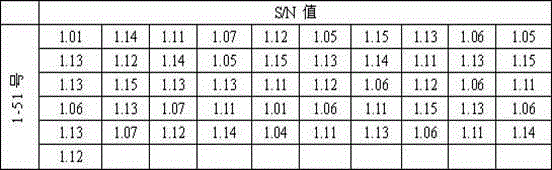
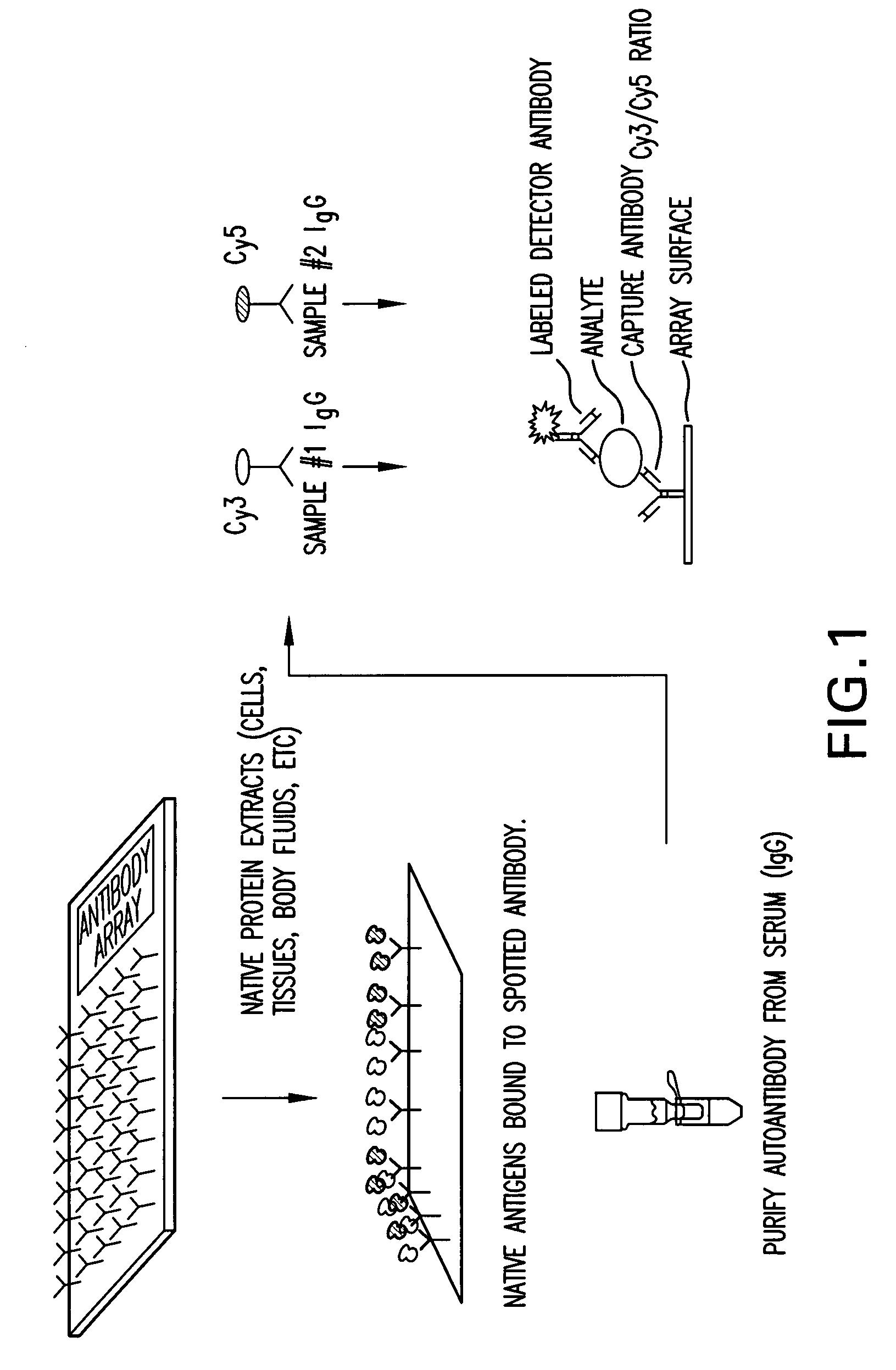
Diagnostic serum antibody profiling
The invention is directed to a microarray assay procedure that can be used for profiling the antibodies present in serum, plasma or blood. The assay may be used to identify antibodies and antigens that are characteristic of particular diseases or conditions. In addition, the invention includes specific antigens that are associated with prostate cancer, progressive benign prostate hyperplasia (BPH) and ovarian cancer.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com