Use of fungal immunomodulatory protein
A technology of immunomodulatory proteins and uses, applied in peptide/protein components, medical raw materials derived from fungi, plant/algae/fungus/moss components, etc., can solve problems such as cell apoptosis and uninvestigated molecular mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Change cell morphology
[0064] The A549 cell line belongs to human lung cancer epidermal cells, and this lung cancer cell line is rather malignant due to its high migration capability. In the present invention, the A549 lung cancer cell line is used as a cancer model to study the response of cancer cells to FIP-gts.
[0065] First, the present invention observes the morphological changes of cells treated with FIP-gts under a microscope. A549 cells were treated with FIP-gts at different concentrations (0, 1, 2, 4 and 10 μg / ml) and observed at different times and photographed. The morphological changes of the cells were recorded (Figure 1).
[0066] A549 cells were treated with 2, 4 or 10 μg / ml of FIP-gts, and the cell morphology changed significantly after 6 hours. The cell morphology changed from a state of adhering with little tentacles to round and A round and loosly-attached morphology, these round cells will float with gentle shaking of the dish. Afte...
Embodiment 2
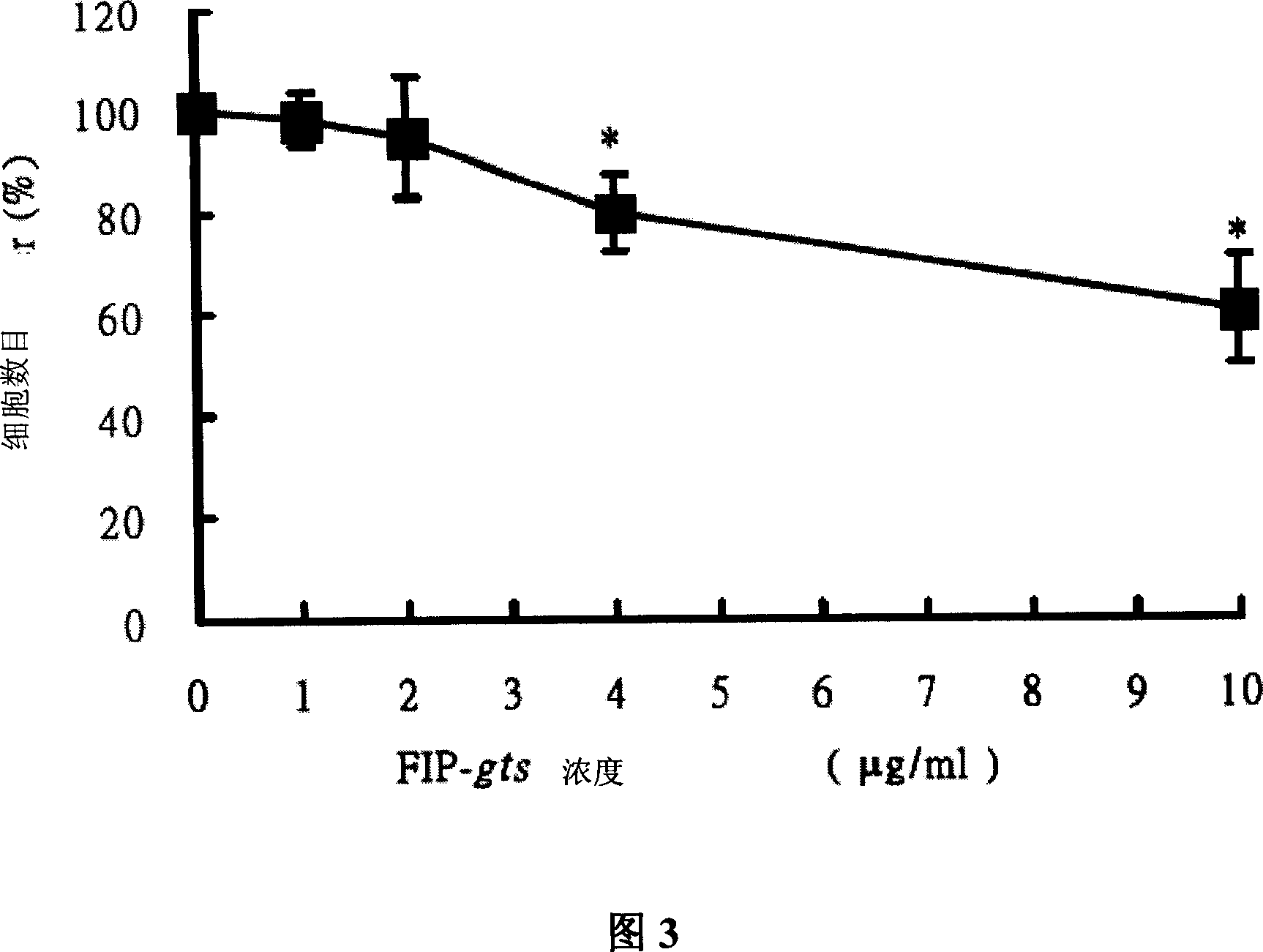
[0068] Example 2: Cell viability assay
[0069] To further confirm the above experimental results, the present invention uses trypan blue staining to check cell viability. In the present invention, the cells were treated with the same FIP-gts concentration (0, 1, 2, 4 and 10 μg / ml of FIP-gts) as the previous experiment for 48 hours, and then trypan blue was added, and the living cells would not be stained by trypan blue, In the present invention, the number of unstained cells (live cells) is calculated as the viability of cells.
[0070] The present invention was inoculated in 2 x 10 in 6 cm petri dishes 5 H1355 and A549 cells, H1355 cell line is a cell model for general research on metabolism. After culturing at 37°C for 16 hours, replace the new culture medium, and add different concentrations of FIP-gts (0, 1, 2, 4 and 10 μg / g / g / ml).
[0071]The FIP-gts drug-treated cells were collected after 48 hours, the old culture medium was removed and the cells were collected in a...
Embodiment 3
[0073] Example 3: Counting the number of cells - Colony forming
[0074] In this experiment, the cell colony forming method will be used to analyze the poisoning effect of FIP-gts on cancer cells. A549 cells were treated with different concentrations of FIP-gts (0, 0.4, 2 and 10 μg / ml) and FIP-gts drugs. After 24 hours, the treated cells were seeded with 400 cells per 6 cm culture dish for 12 days.
[0075] First, the present invention was individually inoculated in 2×10 6 cm petri dishes 5 After culturing A549 cells at 37°C for 16 hours, the culture medium was replaced with a new medium, and different concentrations of FIP-gts (0, 0.4, 1, 2 and 10 μg / ml) were added at the same time (Figure 4). After 24 hours of treatment, the cells were washed twice with 1×PBS, and the drug-treated cells were reinserted, and 1 ml of TE buffer solution was added and allowed to stand at 37° C. for 1 minute to break up the cells. Serial dilutions were used to accurately estimate the number of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com