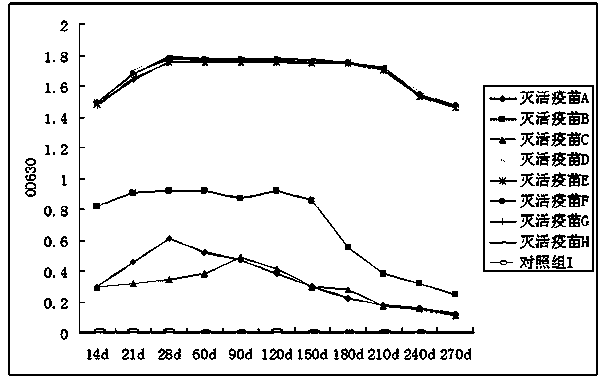
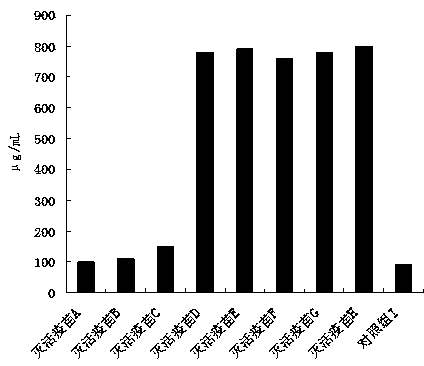PED (Porcine Epedemic Diarrhea) inactivated vaccine and preparation method thereof
A porcine epidemic diarrhea and inactivated vaccine technology, which is applied in the field of porcine epidemic diarrhea inactivated vaccines and its preparation, can solve the problems of ineffective neutralization of intestinal viruses, long antibody window period, total secretion of intestinal mucosa of susceptible animals Insufficient antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Experimental materials
[0027] Monophosphonyl lipid A, muramyl dipeptide, and β-glucan were all purchased from InvivoGen. β-glucosylceramide was purchased from Avanti Polar Lipids, USA. Six-week-old healthy SRC mice were purchased from the Experimental Animal Center of Nanjing Medical University. Epidemic diarrhea antibody detection kit was purchased from Harbin Kehua Biological Company. Mouse intestinal total SIgA ELISA detection kit was purchased from Nanjing Jiancheng Bioengineering Institute. PEDV CV777 Strain: Disclosed in the Study of Porcine Epidemic Diarrhea Inactivated Vaccine Wang Ming, Ma Siqi, etc. China Livestock and Poultry Infectious Diseases 1993 [J].
[0028] 2. Vaccine Preparation
[0029] The preparation method of inactivated vaccine A is as follows:
[0030] (1) Preparation of aqueous phase solution: the inactivated porcine epidemic diarrhea virus liquid (the strain is PEDV CV777 strain, the poison price before inactivation is 5×10 7.5 TCID...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com