Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
215 results about "Risk of infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Risk of infection is a nursing diagnosis which is defined as "the state in which an individual is at risk to be invaded by an opportunistic or pathogenic agent (virus, fungus, bacteria, protozoa, or other parasite) from endogenous or exogenous sources" and was approved by NANDA in 1986. Although anyone can become infected by a pathogen, patients with this diagnosis are at an elevated risk and extra infection controls should be considered.
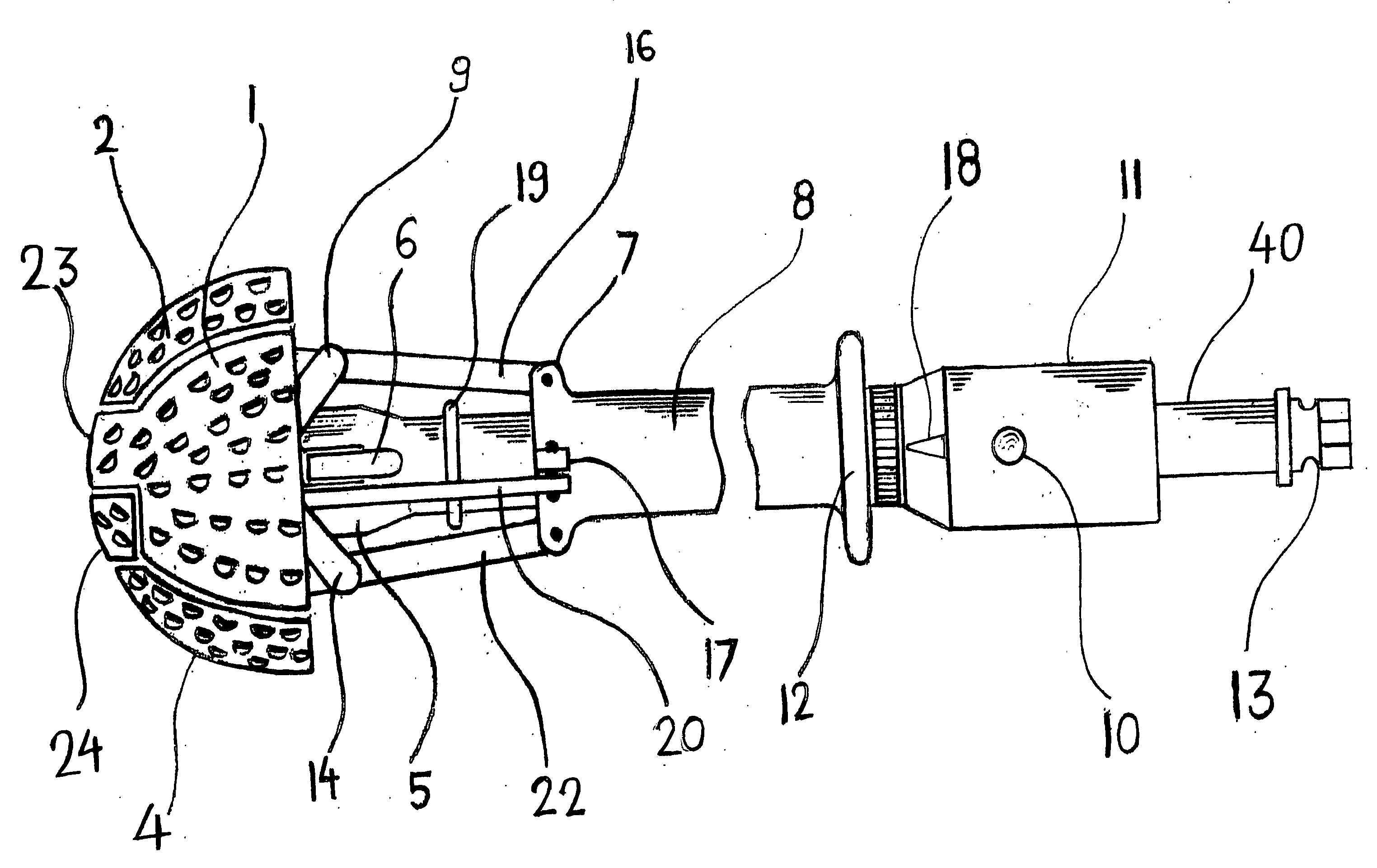
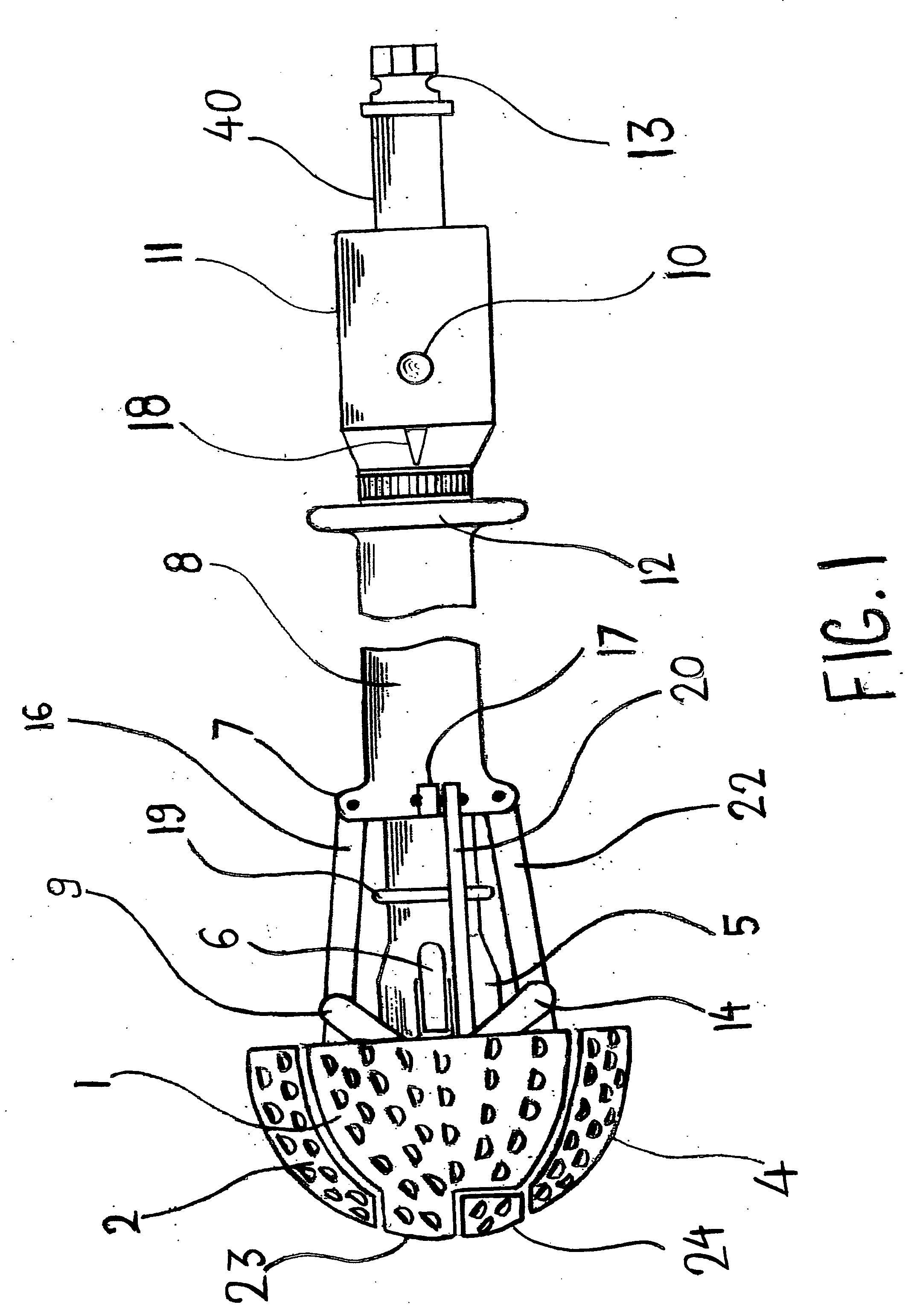
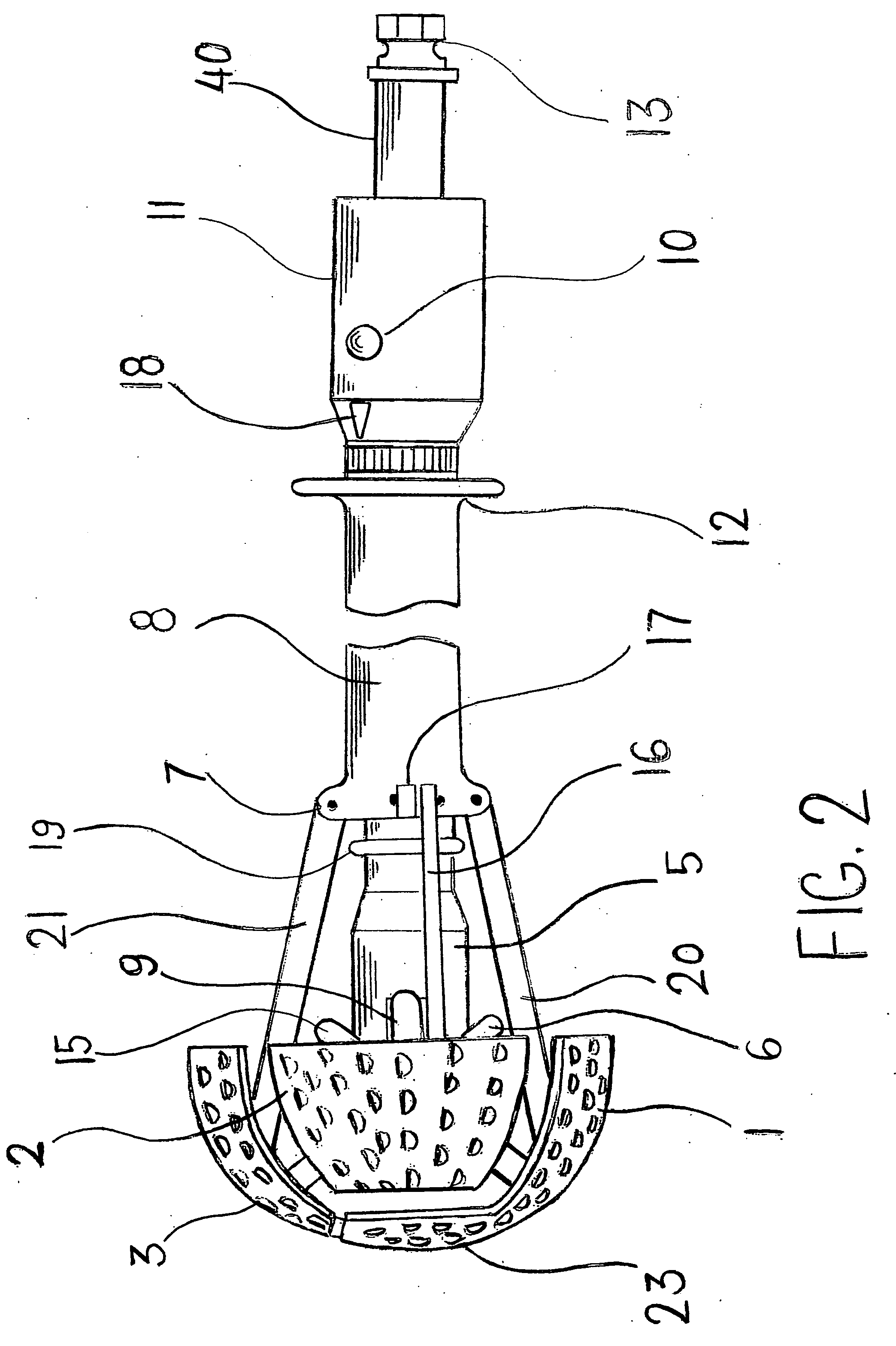
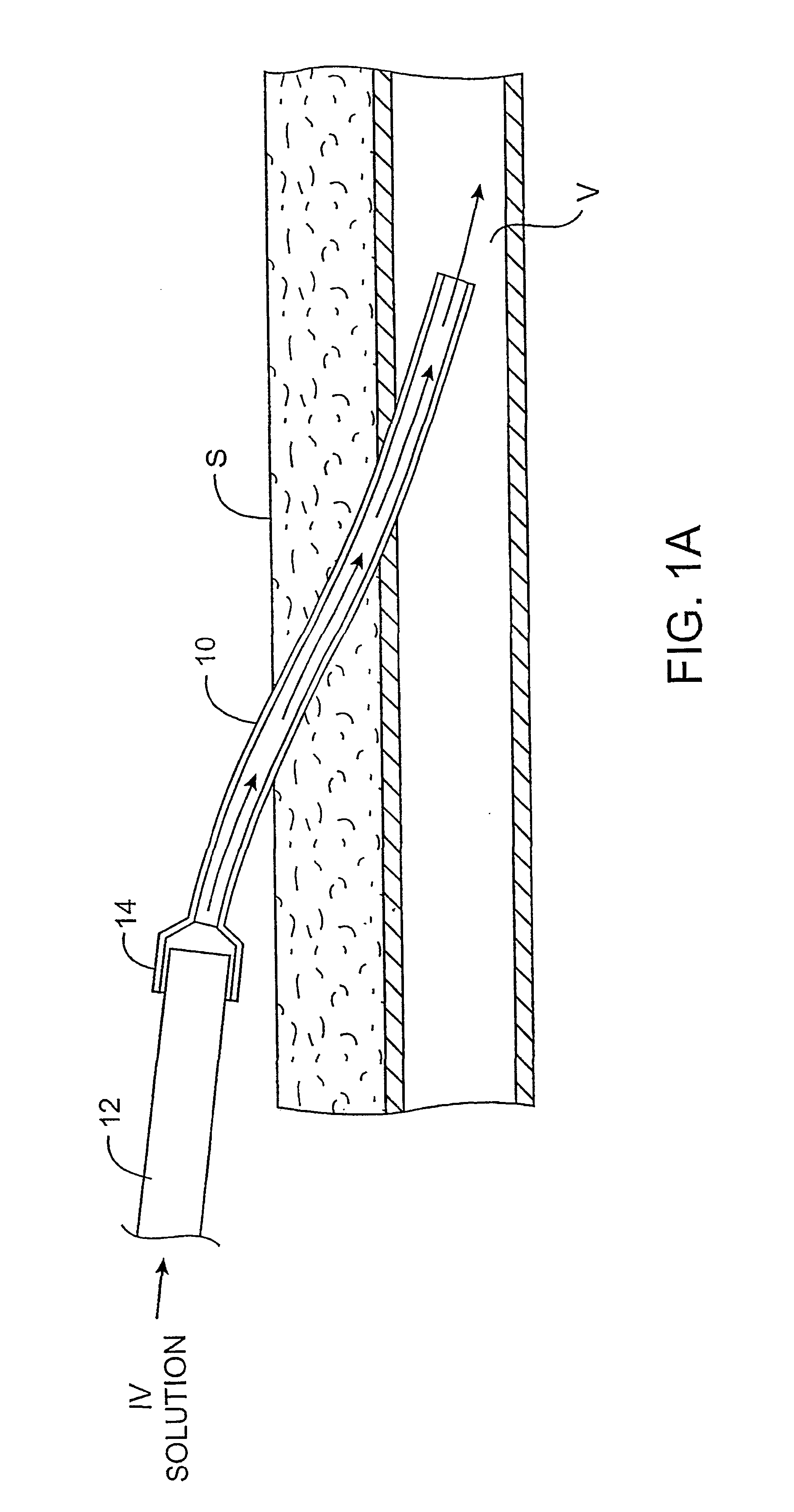
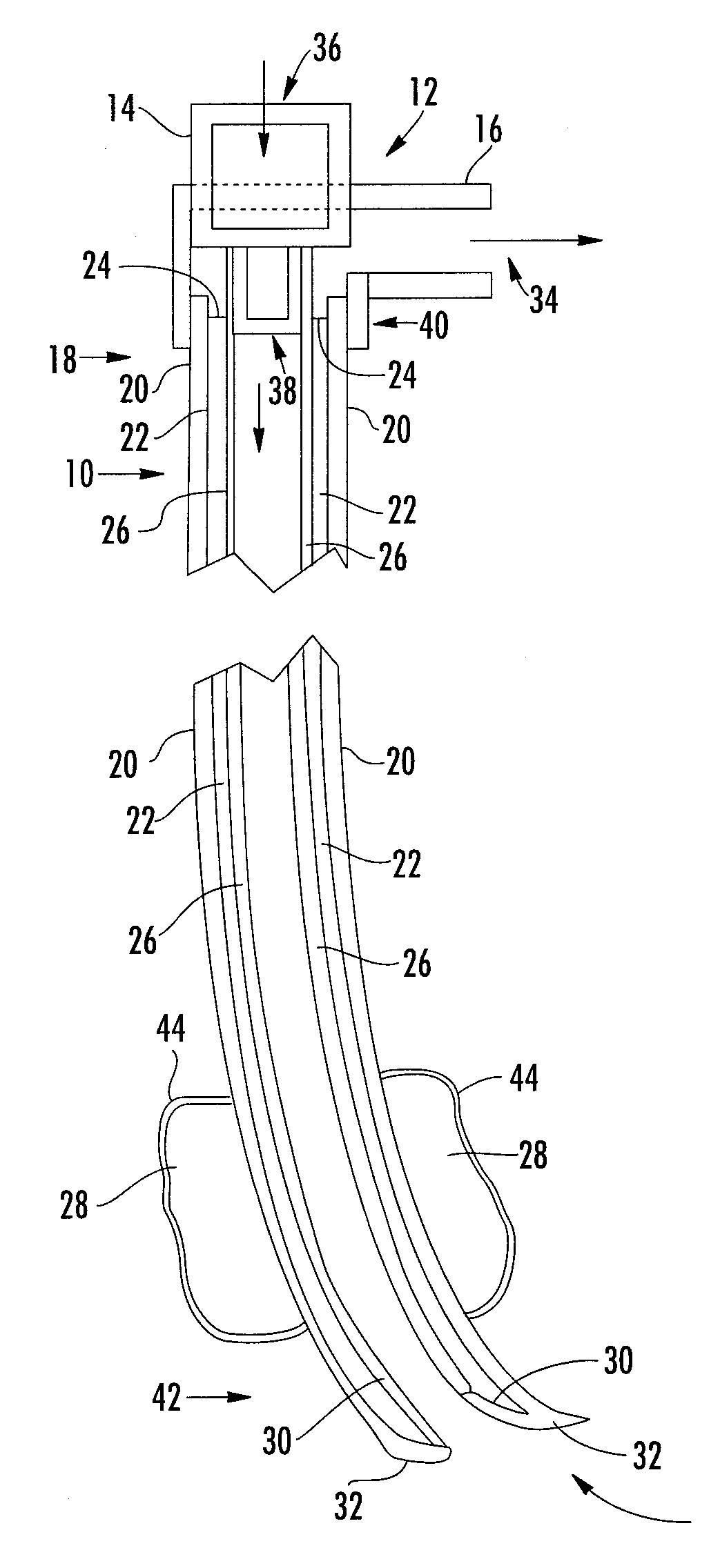
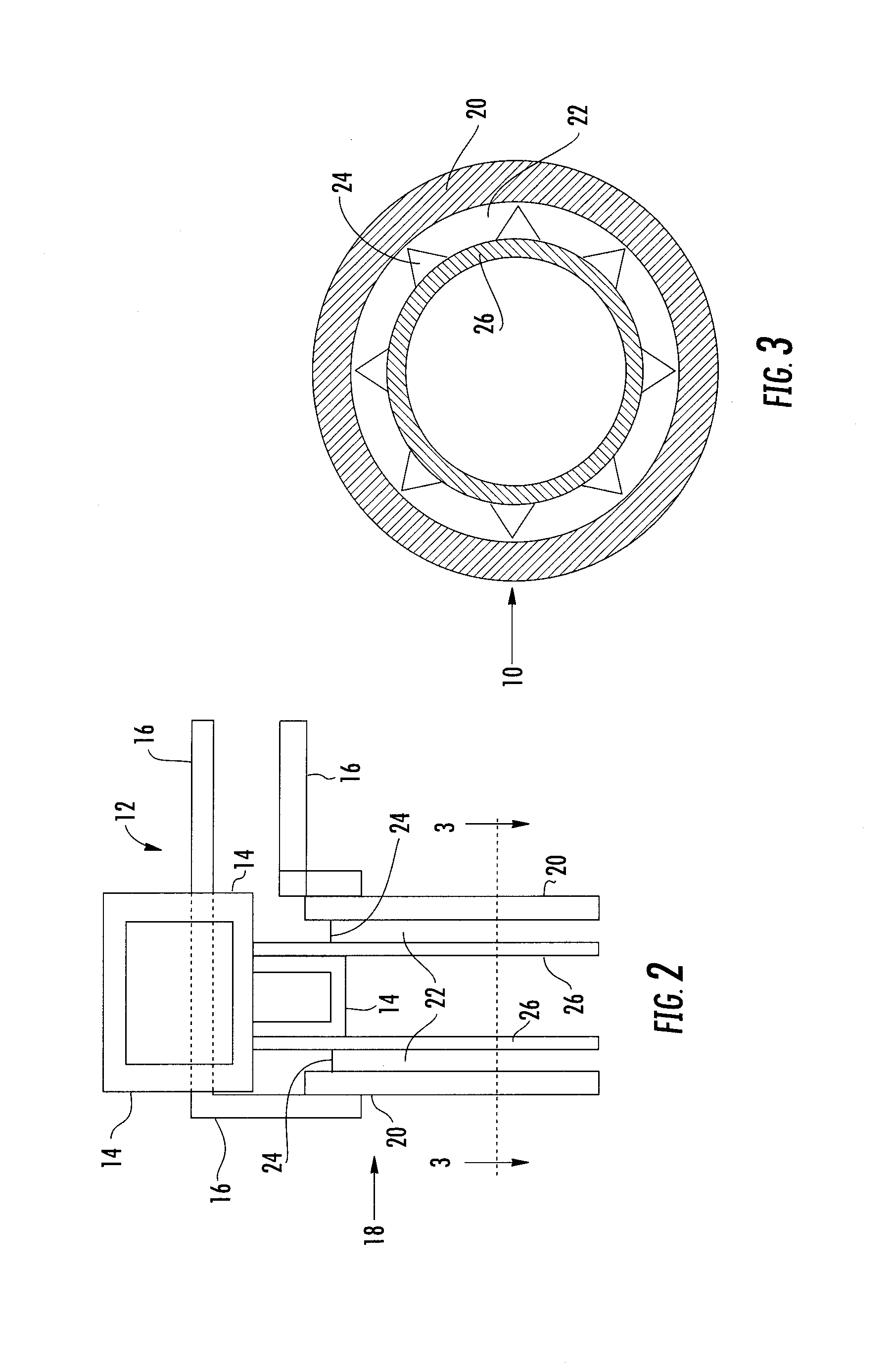
Expandable spring loaded acetabuler reamer
In accordance with the present invention an improved spring-loaded expandable acetabular reamer is described, which comprises a number of convex reaming segments symmetrically located by pair around a central core of the Reamer tool. It is also an object of the present invention to provide and improved spring-loaded reaming segment, which expand faster and requires less manipulation by the operating surgeon and staff minimizing therefore the risk of infection and damage to tissue. Furthermore, introducing large size conventional acetabular reamers with rough and sharp edges through small surgical incisions will undoubtedly cause damage to the incision edge and the surrounding soft tissues, which may ultimately result in delayed wound healing. It is therefore highly desirable to provide an improved acetabular cup, which has the capacity of expanding in diameter to replace several acetabular cups thereby reducing the number of instruments used as well as shortening the time of the procedure and reducing healing time and lessen the risk of infection.
Owner:TERMANINI ZAFER
Methods of reducing risk of infection from pathogens
InactiveUS20050080093A1Reduce the risk of infectionAntibacterial agentsBiocideNatural sourceMedicine
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided comprising administering a sodium channel blocker or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:PARION SCI DURHAM NC
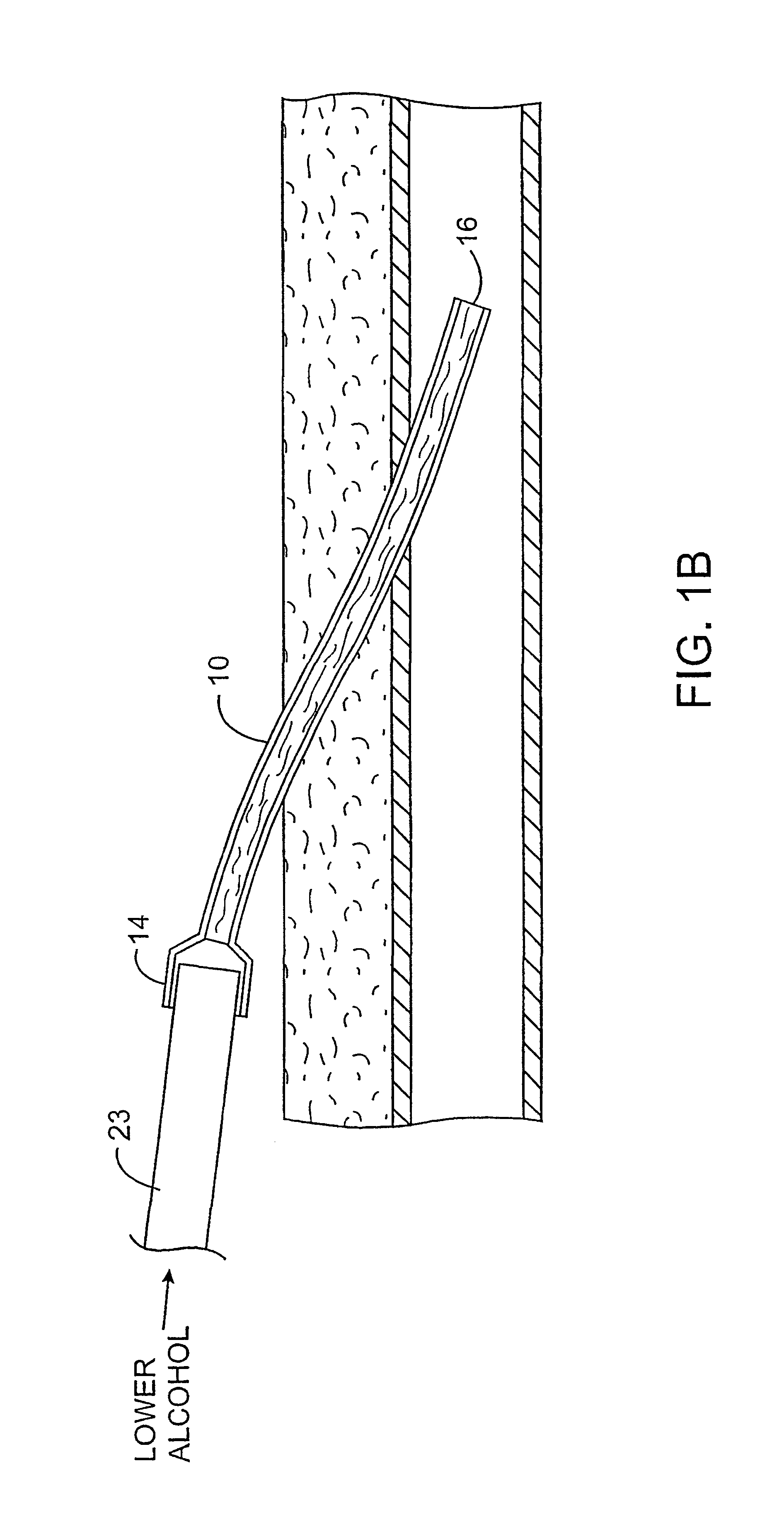
Methods and kits for locking and disinfecting implanted catheters
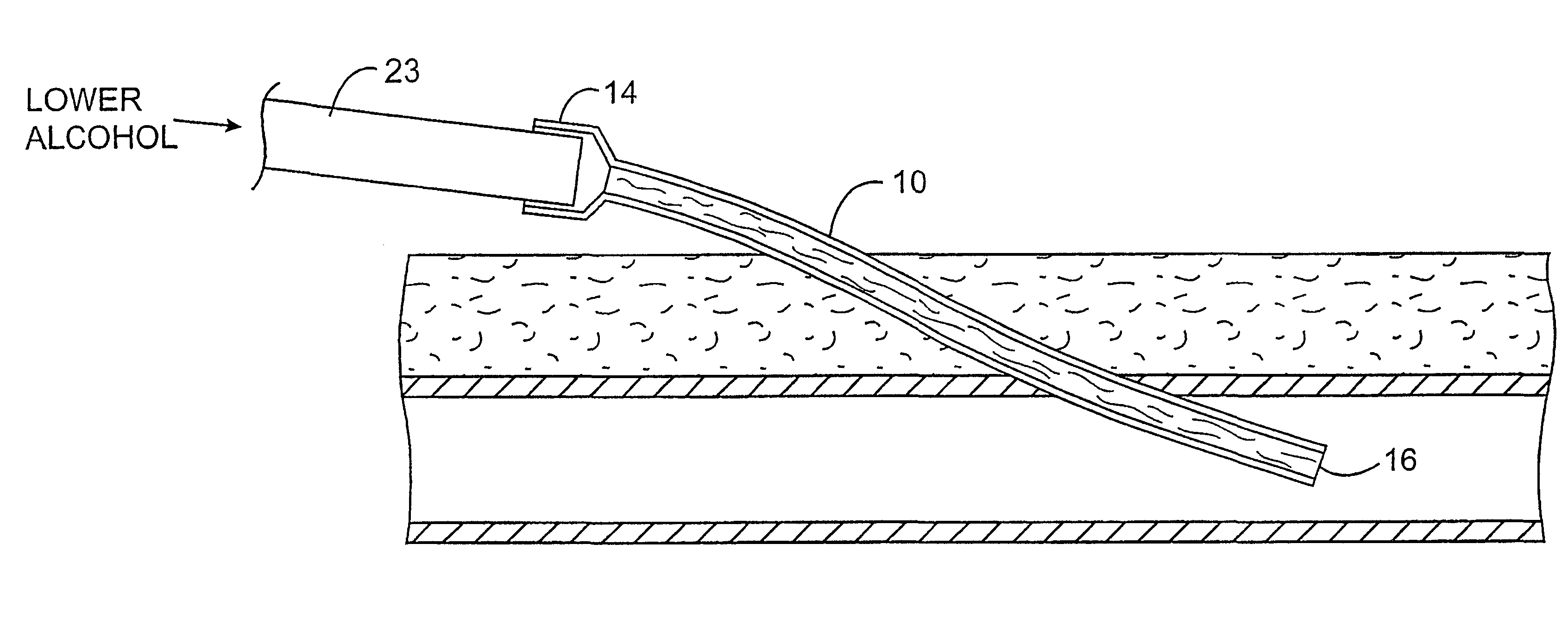
InactiveUS6592564B2Reduce riskInhibiting fouling and plugging of the lumenInfusion devicesMedical devicesAlcoholMedicine
Implanted catheters are locked with a solution comprising a lower alcohol, typically ethanol, propanol, or butanol, most preferably isopropanol. The use of an alcohol can both reduce fouling of the catheter, particularly clotting and thrombus in intravascular catheters, as well as reducing the risk of infection. The risk of infection can be further reduced by employing a catheter body which is sufficiently porous to permit the lower alcohol or other anti-microbial solution to penetrate into the catheter body and preferably through the catheter into tissue surrounding the implanted catheter.
Owner:EXCELSIOR MEDICAL
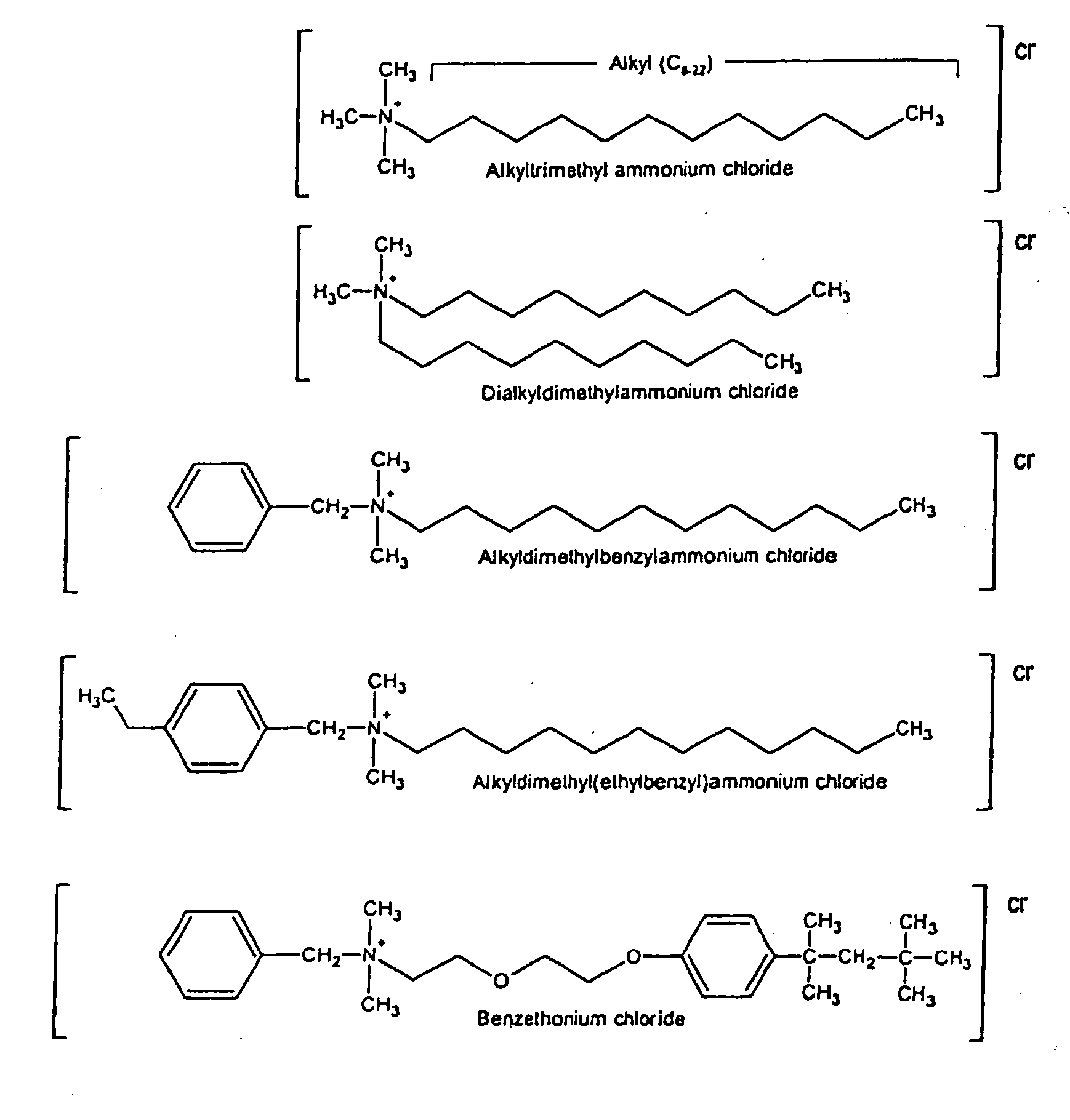
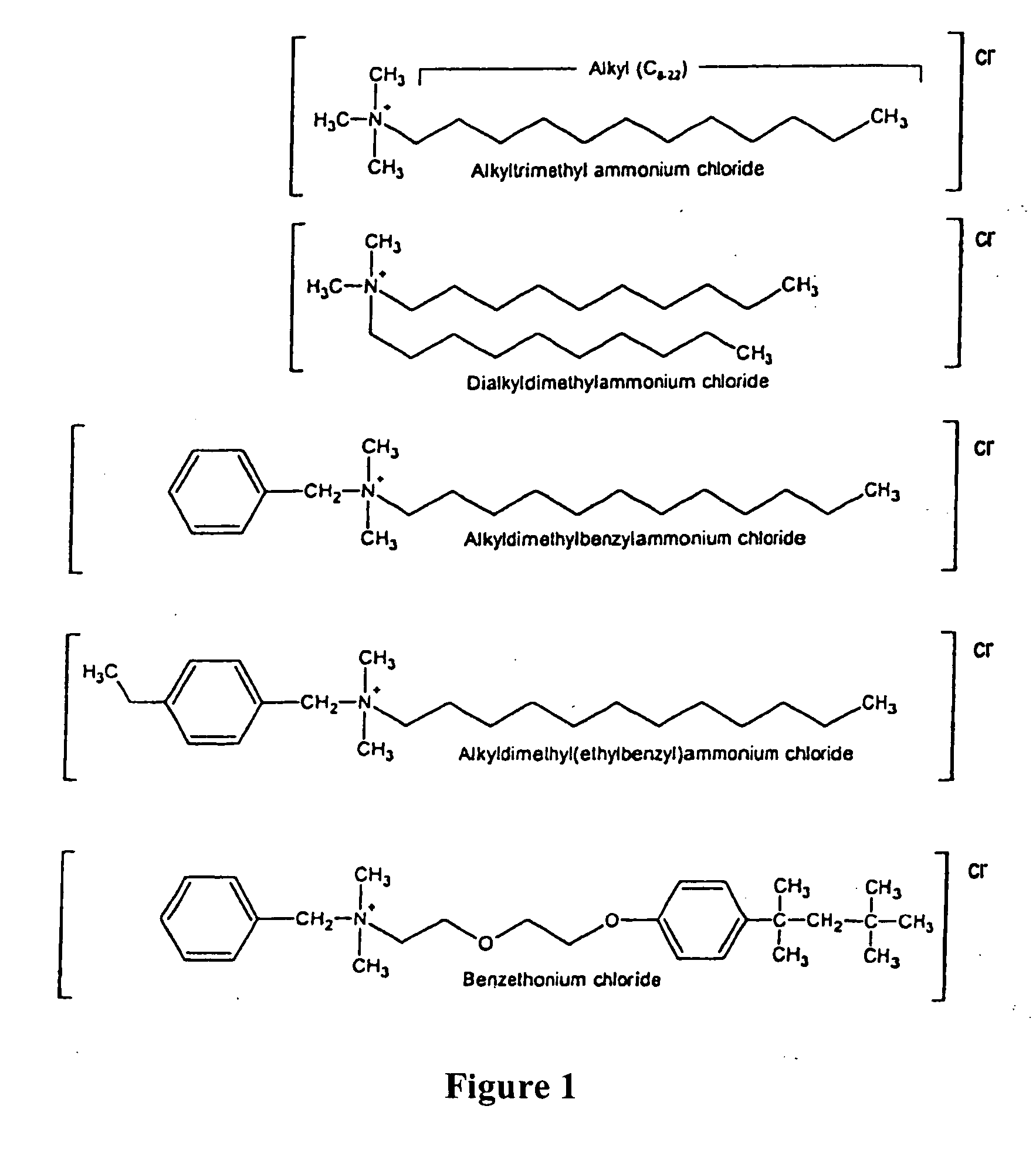
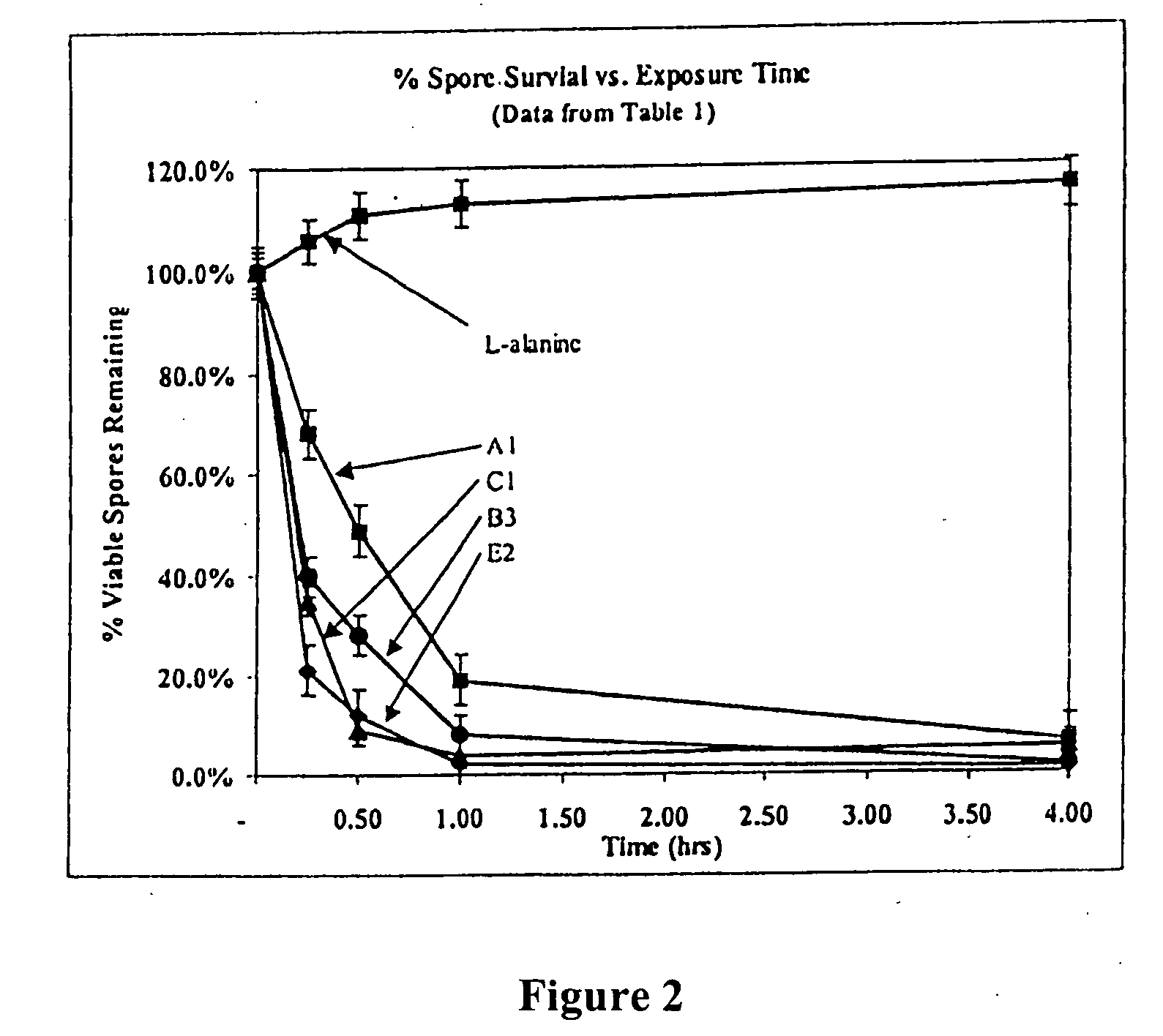
Antimicrobial and sporicidal composition
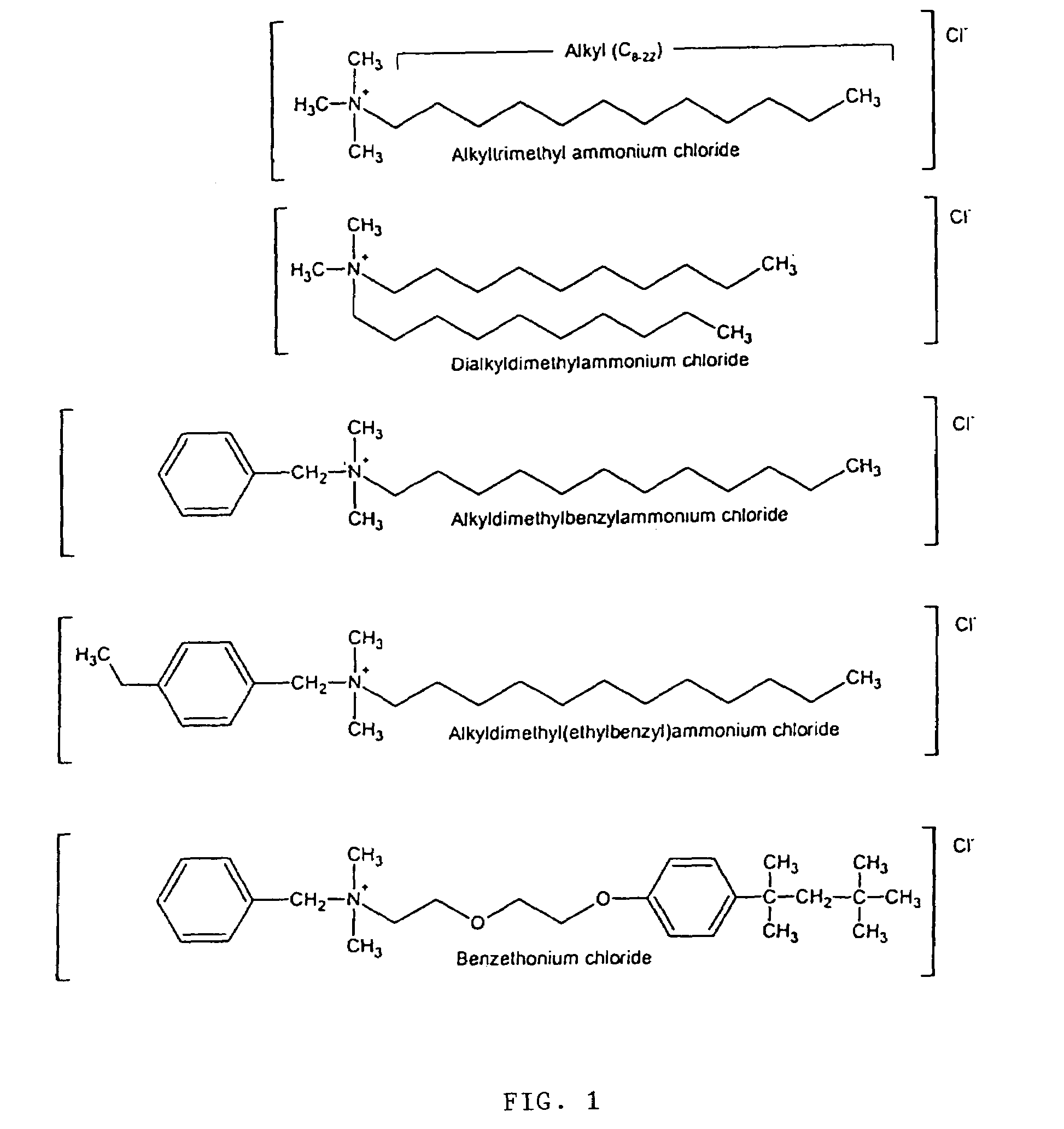
InactiveUS7192601B2High bactericidal activityAntibacterial agentsCosmetic preparationsBacteroidesAmmonium compounds
Germicidal compositions with enhanced activity towards killing microbiological spores and vetgetative cells comprising certain quaternary ammonium compounds (QACs), phenolic compounds, monohydric alcohols, hydrogen peroxide, iodine, triclocarban, triclosan or combinations thereof with one or more spore coat opening agents. The invention also provides for the application of the germicidal compositions to animate and inanimate surfaces to help kill germs and protect against the risk of infection from bacteria, molds, yeasts, fungi, viruses, and microbiological spores.
Owner:PURE IP LC
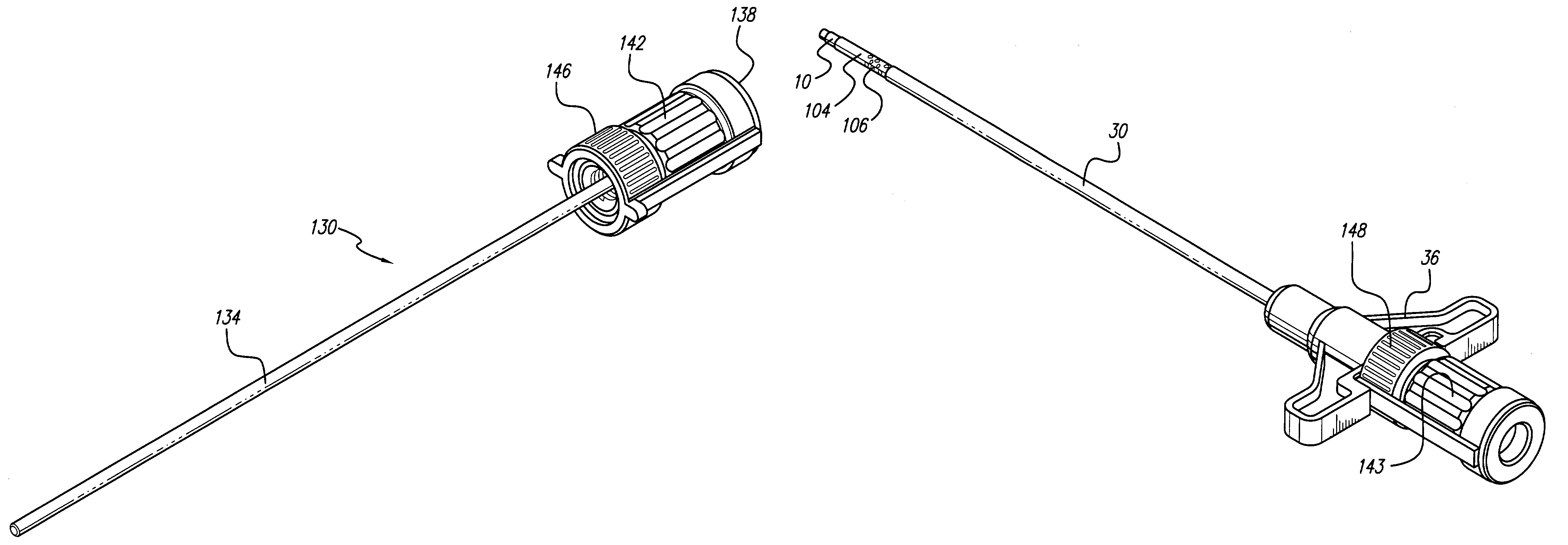
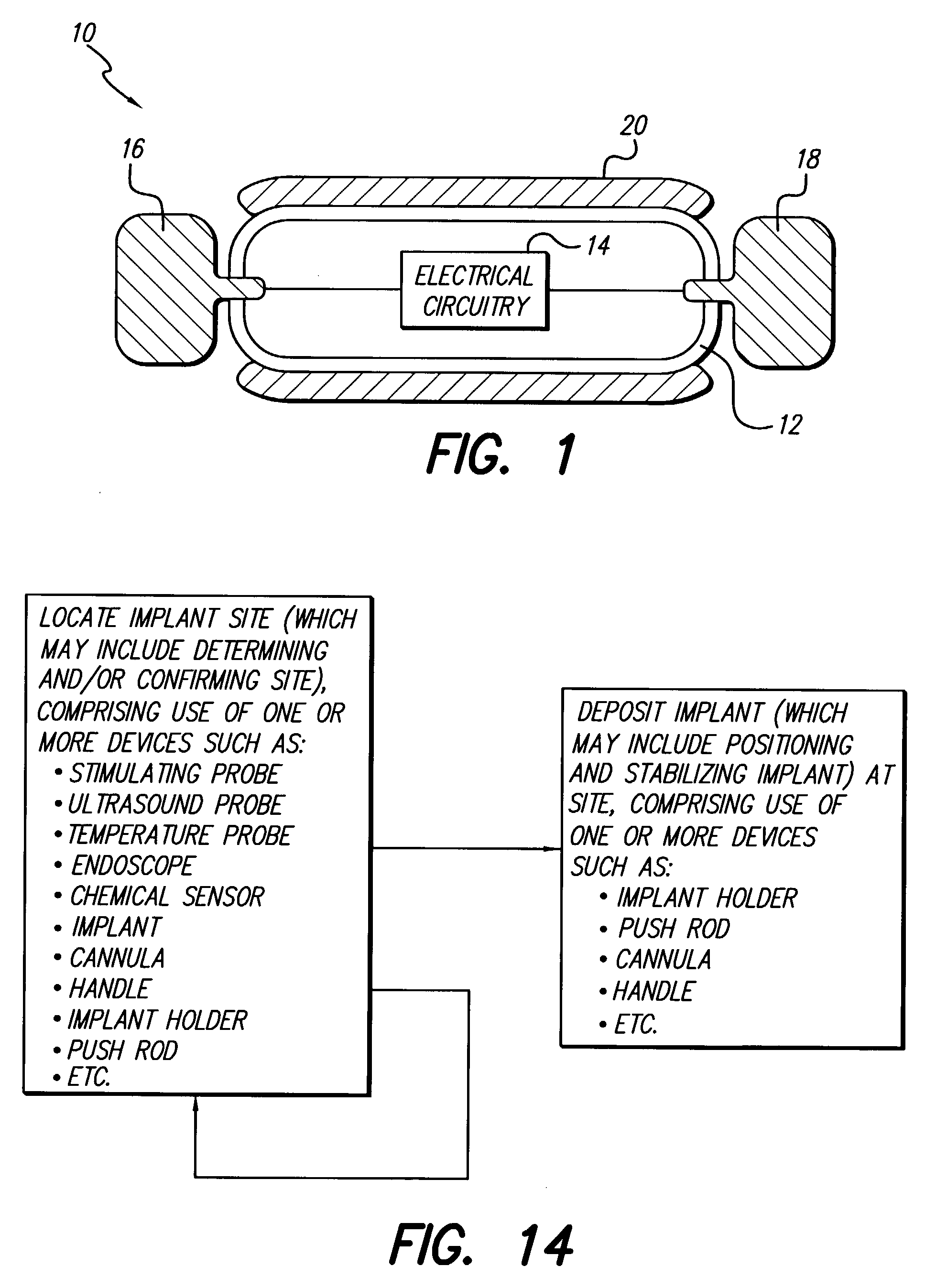
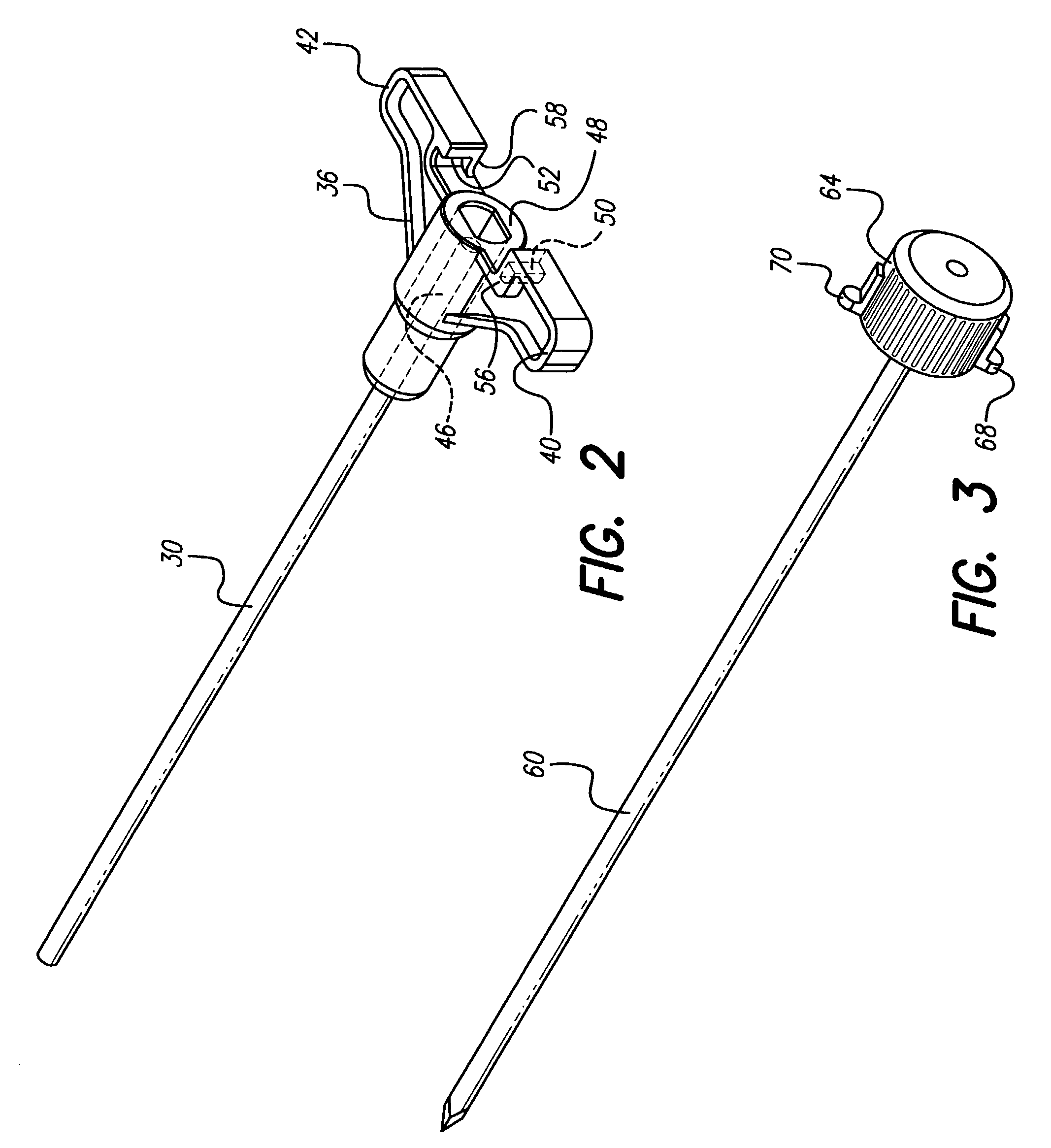
Surgical insertion tool
InactiveUS7799037B1Facilitate entryProtect and ease handlingSpinal electrodesDiagnosticsImplanted deviceOperative time
A tool is provided for facilitating determining a proper location for an implantable device or medication, and for then delivering the implant to the precise location determined with the tool. To determine the target location, the tool may include a component for testing target locations, such as a stimulating probe for simulating a miniature implantable stimulator. In one embodiment, the tool is used to test a miniature implantable stimulator prior to depositing the implant precisely at the target location. The components of the tool are configured to maintain the implant at the target location while the tool is withdrawn. In one embodiment, a push rod assembly of the tool keeps the implant in position while it retracts the implant holder from around the implant. The ergonomic and light-weight tool leads to reduced surgical time, number and size of incisions, risk of infection, and likelihood of error.
Owner:BOSTON SCI NEUROMODULATION CORP
Methods of reducing risk of infection from pathogens
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided including administering a sodium channel blocker or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:PARION SCI DURHAM NC
Endotracheal tube apparatus and method for using the same to reduce the risk of infections
InactiveUS7258120B2Cleaned and replacedReduce riskTracheal tubesRespiratory apparatusCatheterCvd risk
Disclosed herein is a novel tube-in-tube endotracheal tube apparatus that allows for replacement or cleaning of an inner (first) tube without having to re-intubate the patient. The novel endotracheal tube apparatus enables the application of continuous suction or intermittent suction. The endotracheal device also serves to decrease the incidence of ventilator-associated pneumonia (VAP).
Owner:UNIV OF FLORIDA RES FOUNDATION INC
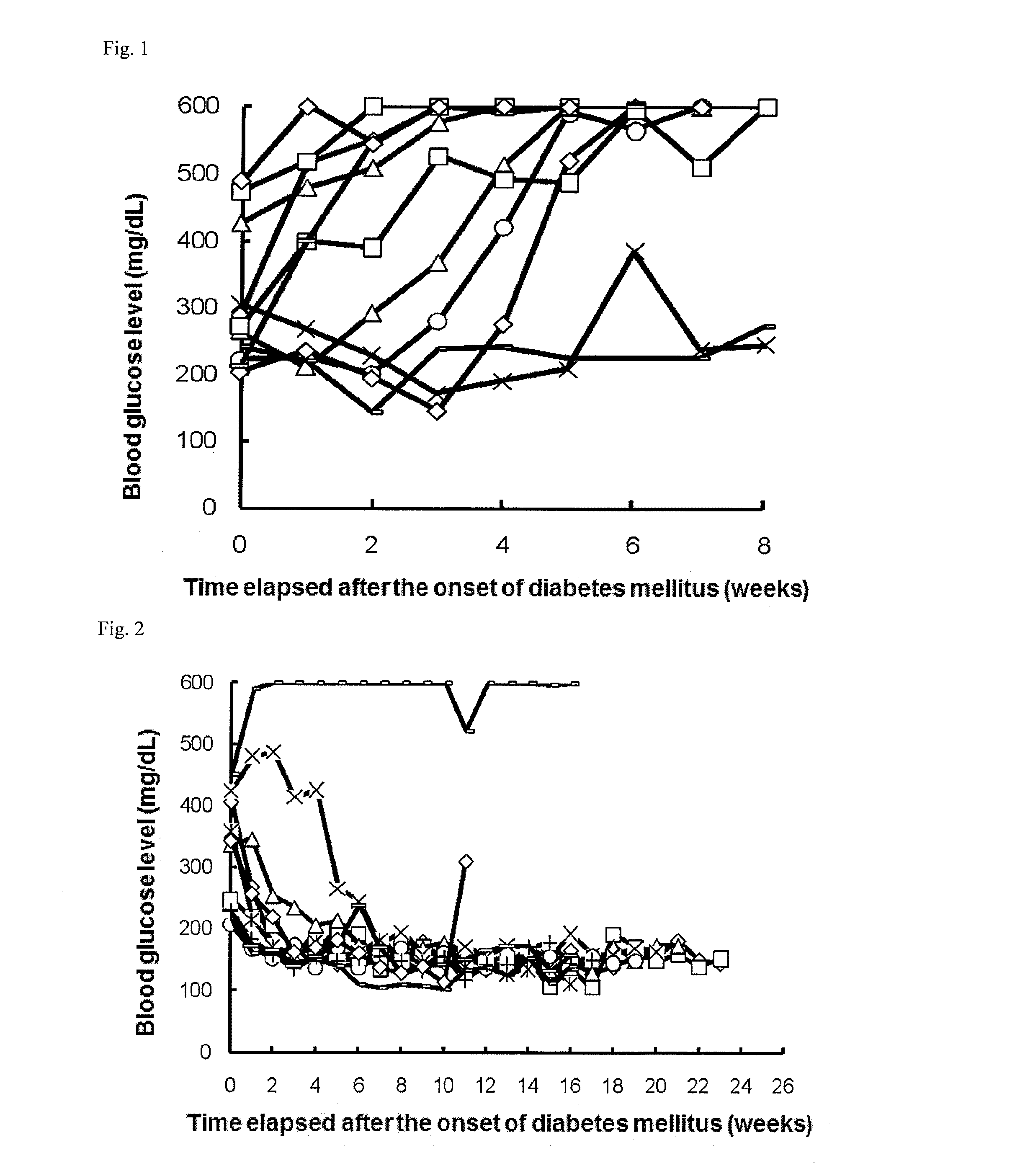
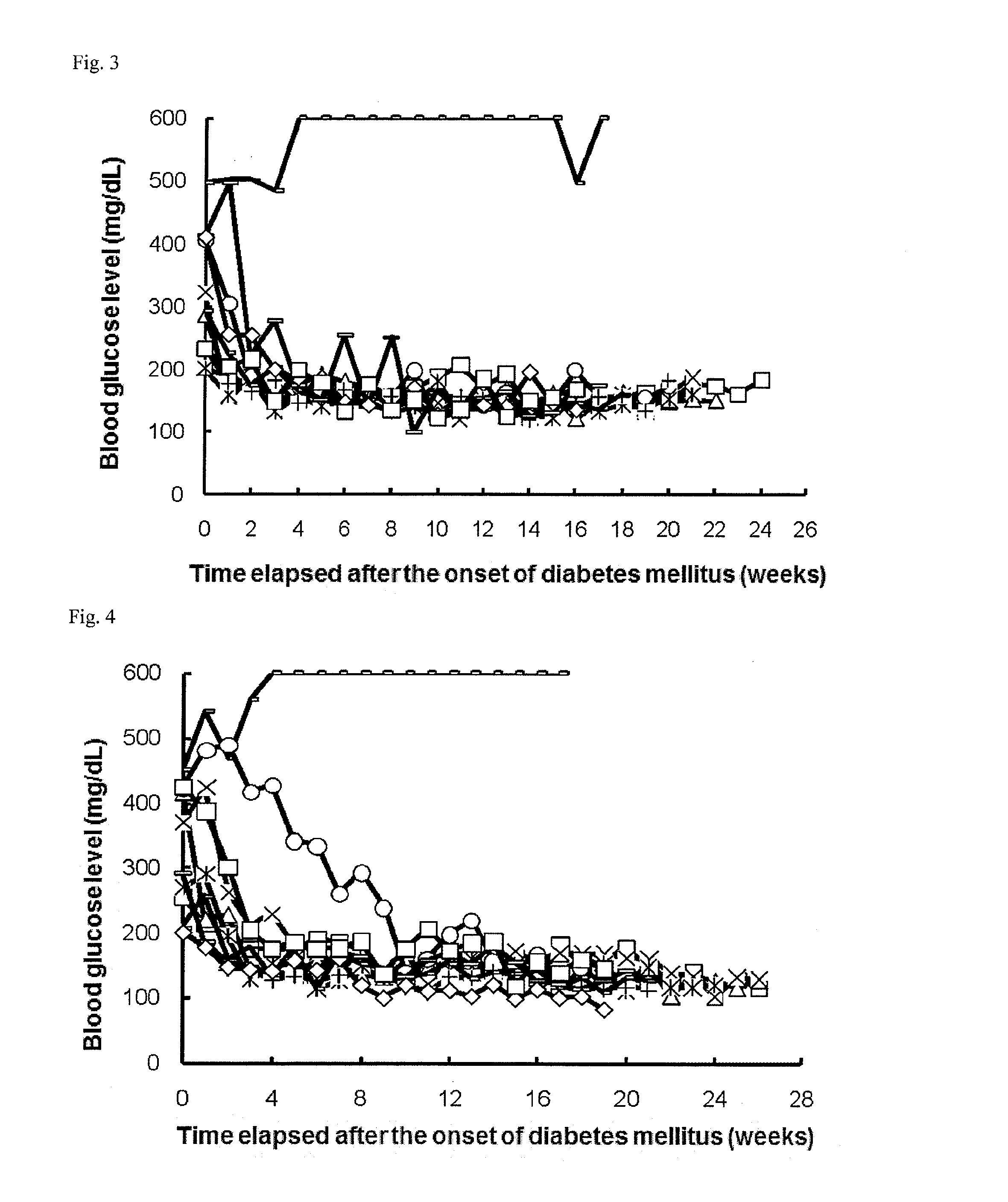
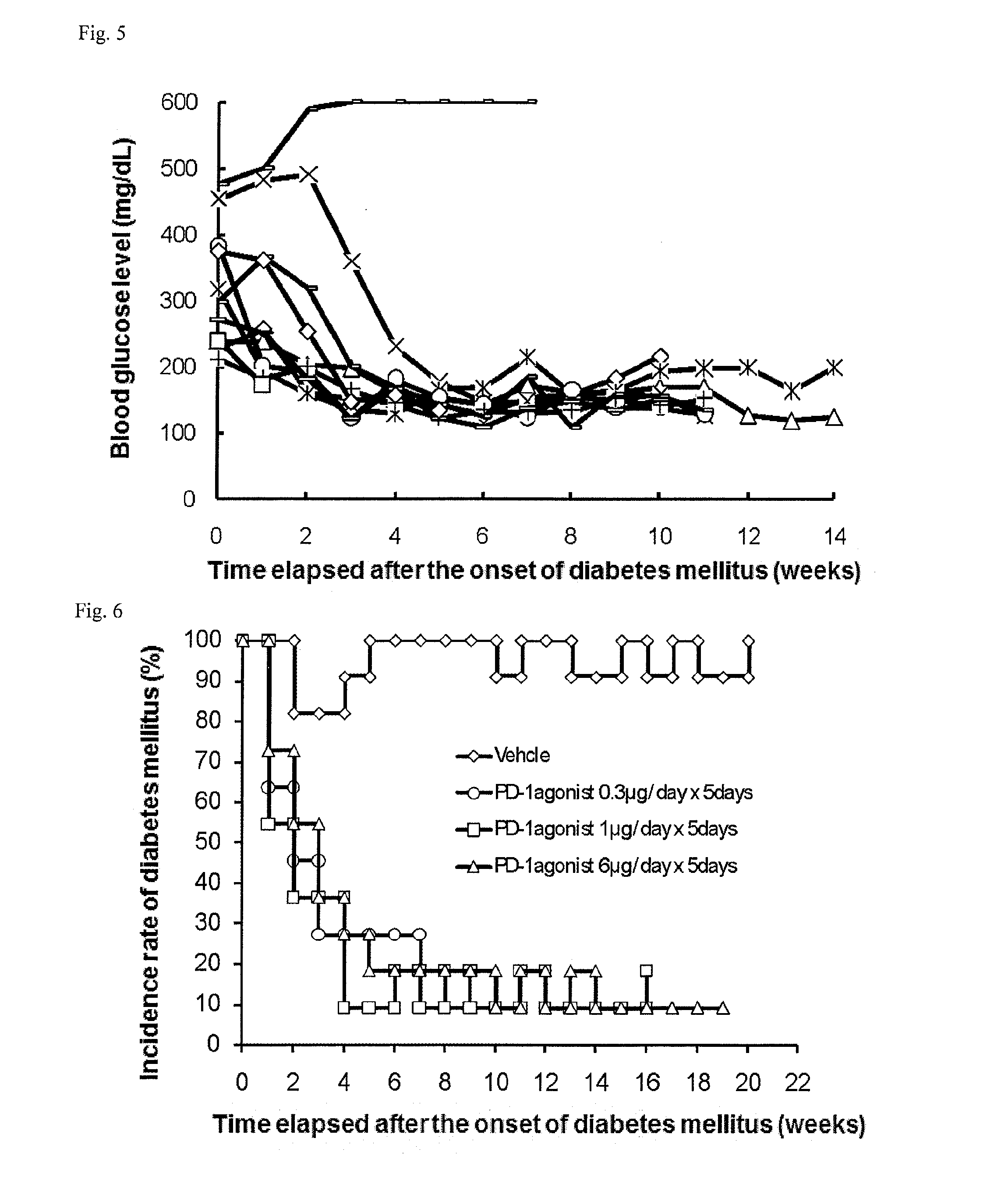
Therapeutic agent for autoimmune diseases comprising pd-1 agonist
ActiveUS20140220021A1Reduce the risk of infectionReduce administrative burdenNervous disorderAntipyreticAutoimmune diseaseActive ingredient
Provided is a prophylactic, symptom progress-suppressive, and / or therapeutic agent for an autoimmune disease. The agent lowers the risk of infections and reduces the burden of administration to patients. The prophylactic, symptom progress-suppressive, and / or therapeutic agent includes a PD-1 agonist as an active ingredient and is administered (a) 1 to 10 times within one month from the first administration, (b) in a total PD-1 agonist dose of 20 to 1250 μg / kg, and (c) without requiring administration for at least 3 months after the last administration.
Owner:ONO PHARMA CO LTD
Inhibiting the growth of bacteria in absorbent articles by adding other bacteria
InactiveUS6187990B1Avoid it happening againGrowth inhibitionBacteriaSanitary towelsBacteroidesMicroorganism
The invention relates to absorbent articles, such as diapers and like articles and is concerned with methods for preventing undesirable odors and / or preventing the growth of undesirable microorganisms when the articles are in use, and also provides an absorbent article which can be worn for long periods of time without generating undesirable odors, incurring the risk of infection or having a negative effect on skin. Another object is to amplify the presence in the wearer's urogenital zone of microbiological flora that will assist in preventing the occurrence of urinal tract infections. These objects have been achieved by adding to the absorbent articles microorganisms which exhibit antagonistic properties against present undesirable strains of microorganisms, so as to restrain the growth of these or establishing of new undesirable species.
Owner:MOLNLYCKE AB
Methods of reducing risk of infection from pathogens
InactiveUS20090253714A1Reduce the risk of infectionAntibacterial agentsOrganic active ingredientsNatural sourceMedicine
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided comprising administering amiloride, benzamil, phenamil or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:JOHNSON MICHAEL R +1
Recombinant virus vectors
InactiveUS6319703B1Risk minimizationReduced zero riskBiocidePeptide/protein ingredientsInfected cellCytopathic effect
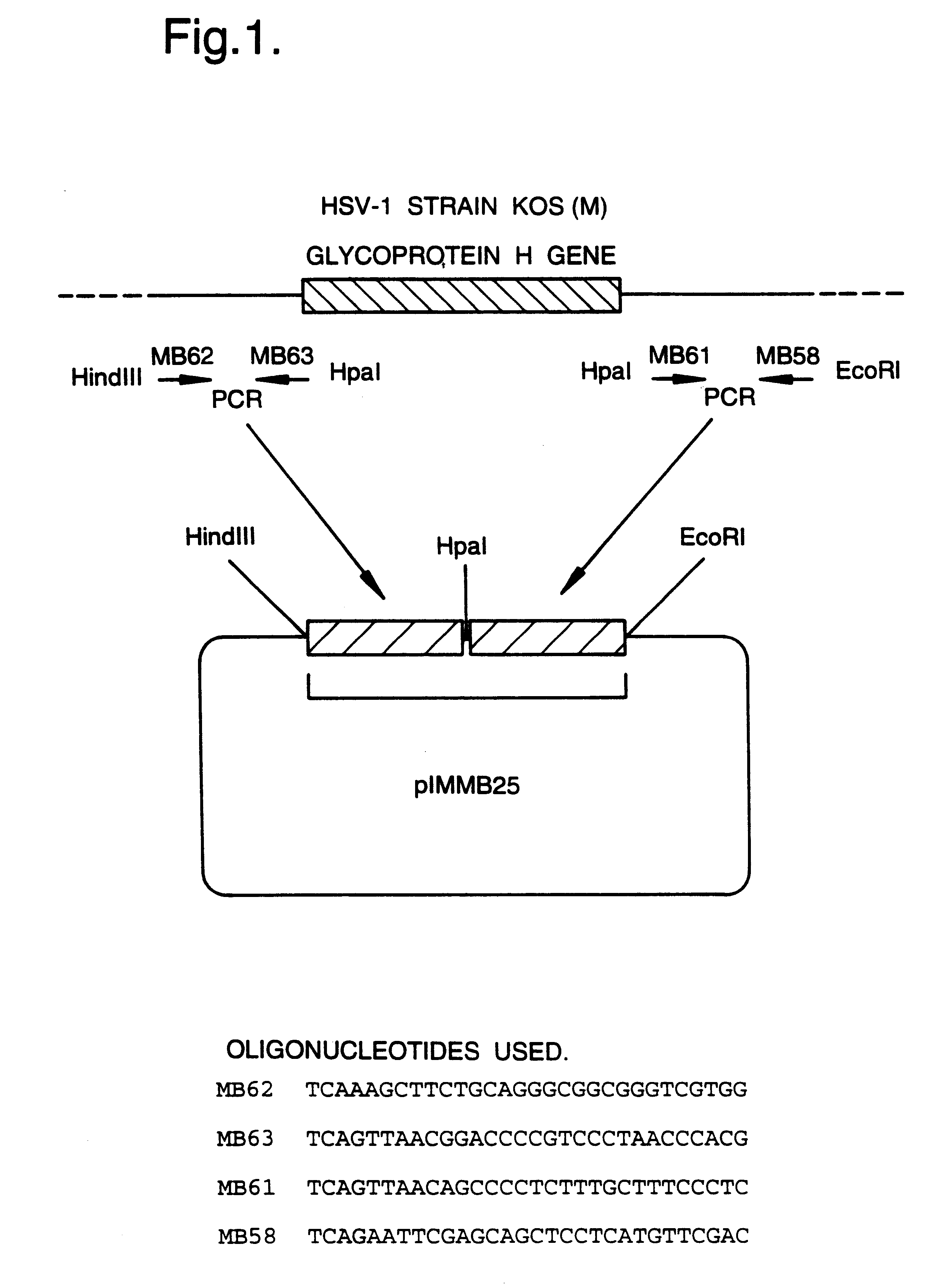
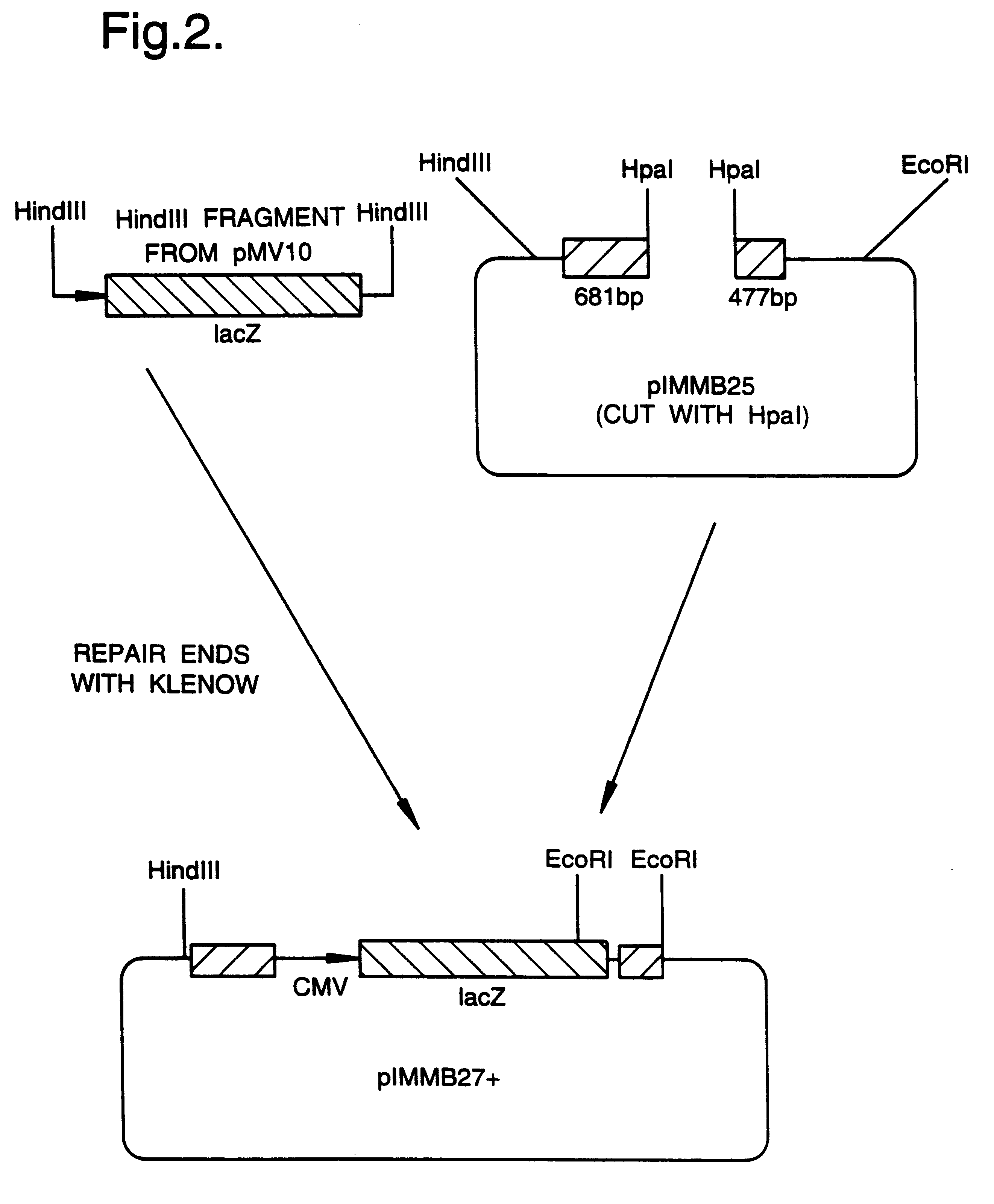
A mutant herpesvirus that can be used a recombinant virus vector includes (a) a mutation such that the mutant virus has a reduced ability in comparison with a parent type to cause lysis of an infected cell, and (b) an inactivating mutation in a gene essential for the production of infectious virus. An example is a HSV1 mutant lacking the essential glycoprotein gH gene and having a mutation impairing the function of the gene product VP16. A heterologous gene can be carried at the site of the inactivated essential gene, e.g. a gene suitable for administering gene therapy. The vector has an increased margin of safety over known herpesvirus vectors in respect of incidence of cytopathic effects and / or risk of infection.
Owner:SPECK PETER G
Attenuated Pasteurella piscicida vaccine for fish
Live-attenuated vaccines against Edwardsiella ictaluri or against Pasteurella piscicida are disclosed. Both vaccines are incapable of reversion to virulence, because both are made by deletion mutations in the aroA gene, the purA gene, or both. These vaccines may be used not only to vaccinate fish against Edwardsiella ictaluri or Pasteurela piscicida, but also to serve as vectors to present antigens from other pathogens to the fish, thereby serving as vaccines against other pathogens as well, with no risk of infection by reversion to the virulent form of the pathogen in which the antigen occurs naturally.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Therapy for enteric infections
InactiveUS20120177650A1Significant positive effectReduce the possibilityAntibacterial agentsEgg immunoglobulinsInfection riskEnteric pathogen
A method and composition for treating enteric pathogen infections in animals suffering from such infections or displaying diseases or conditions consistent with such infections or for preventing or reducing the likelihood of enteric pathogen infections in animals at risk for developing such infections.
Owner:BORODY THOMAS JULIUS
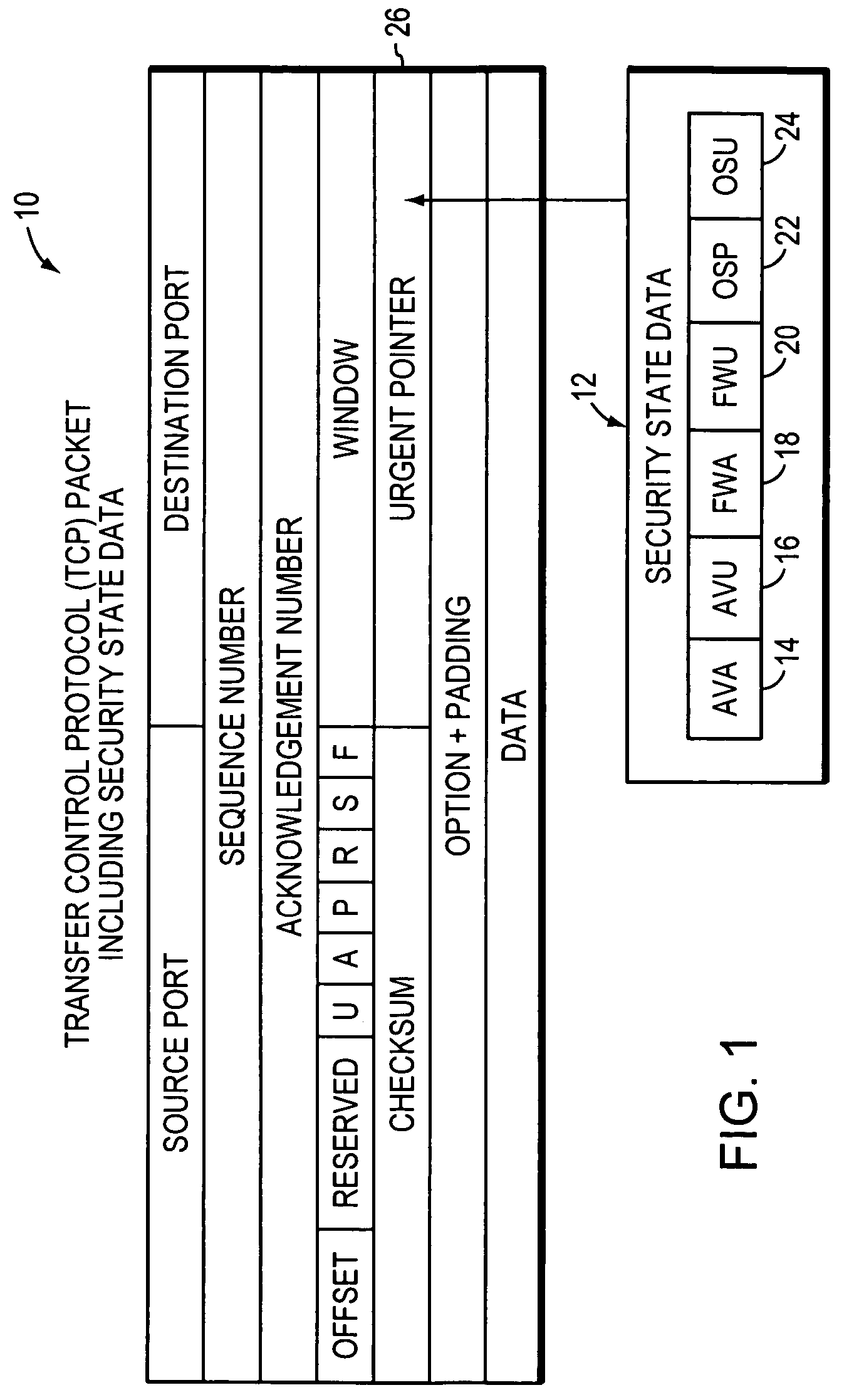
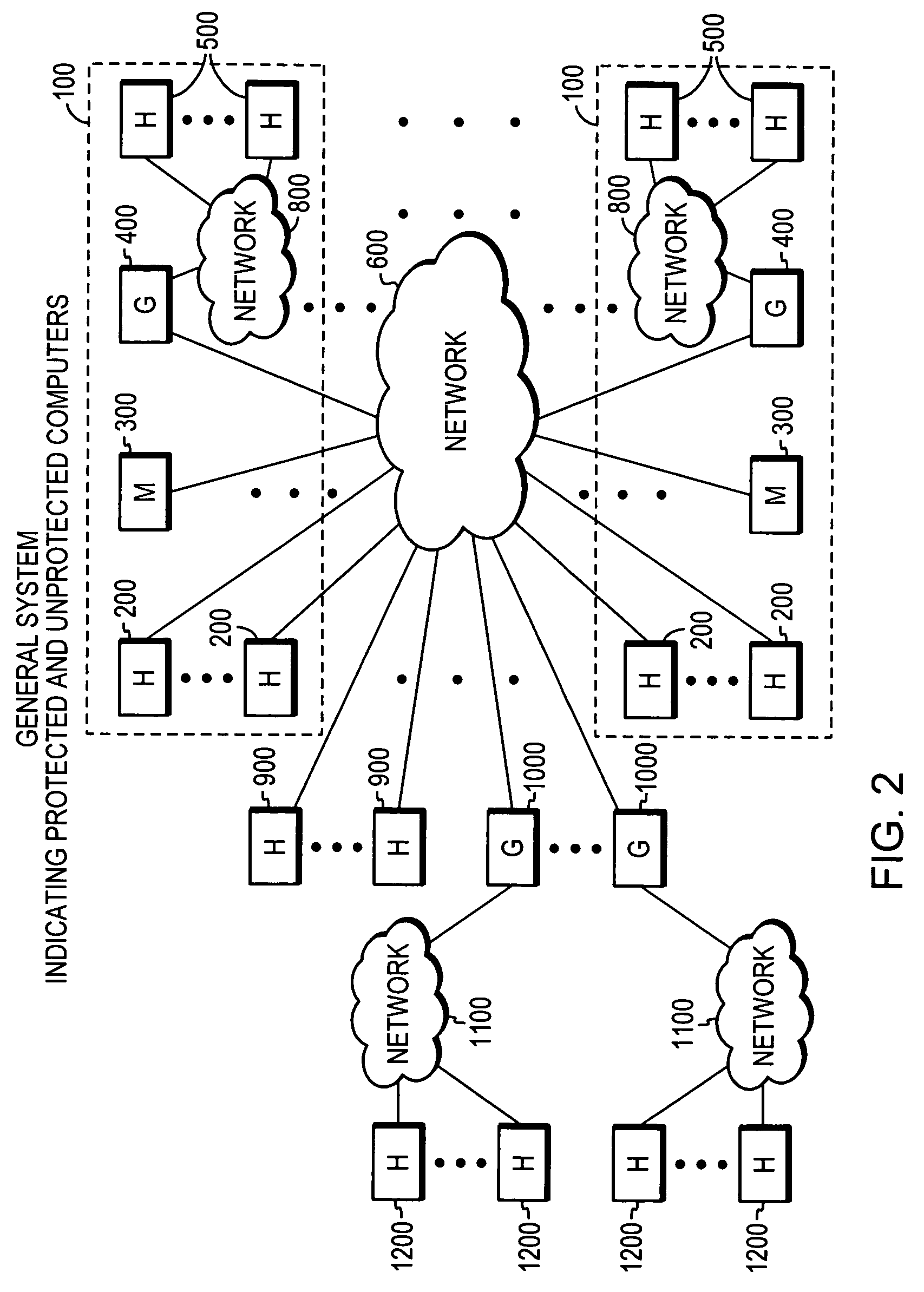
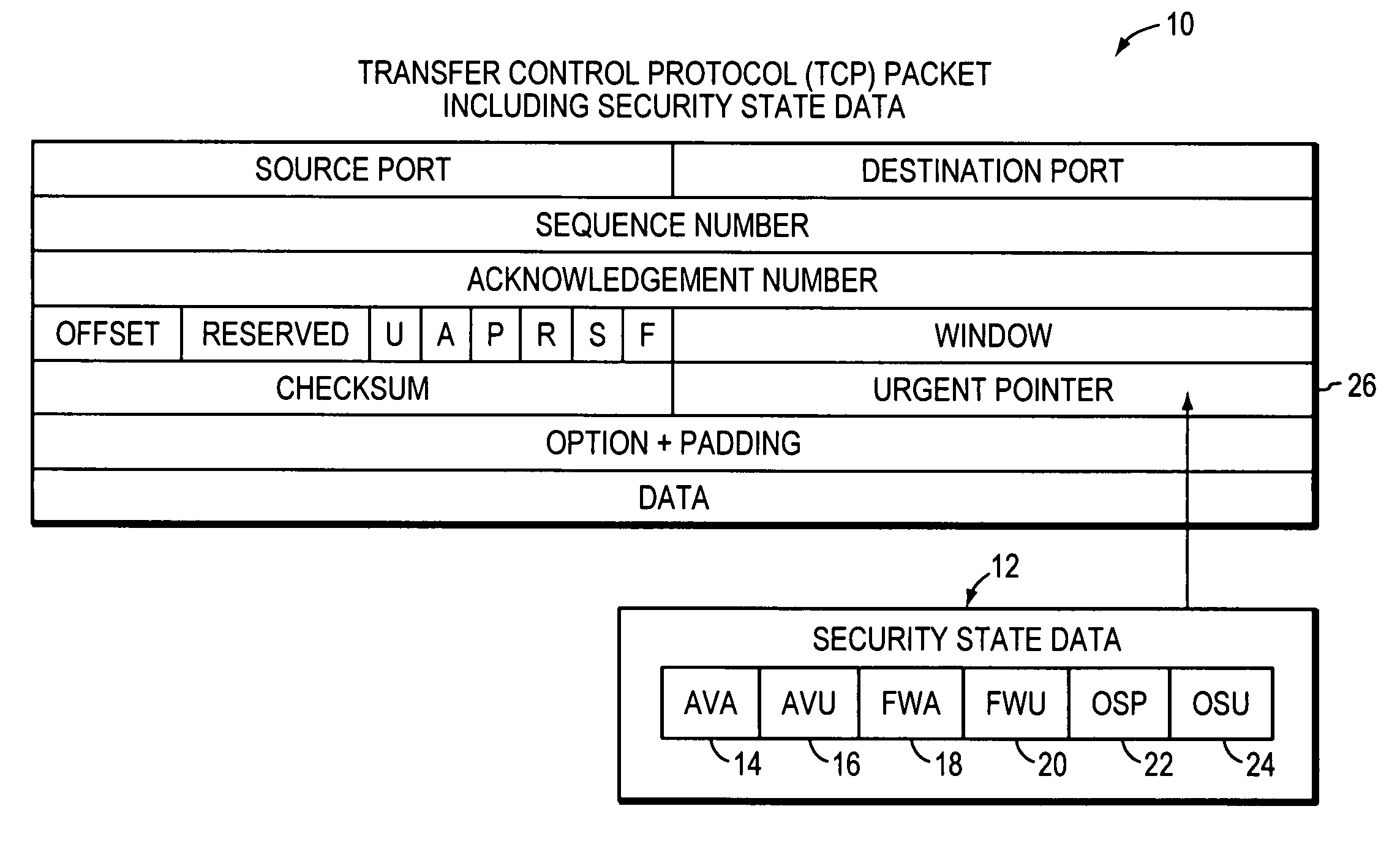
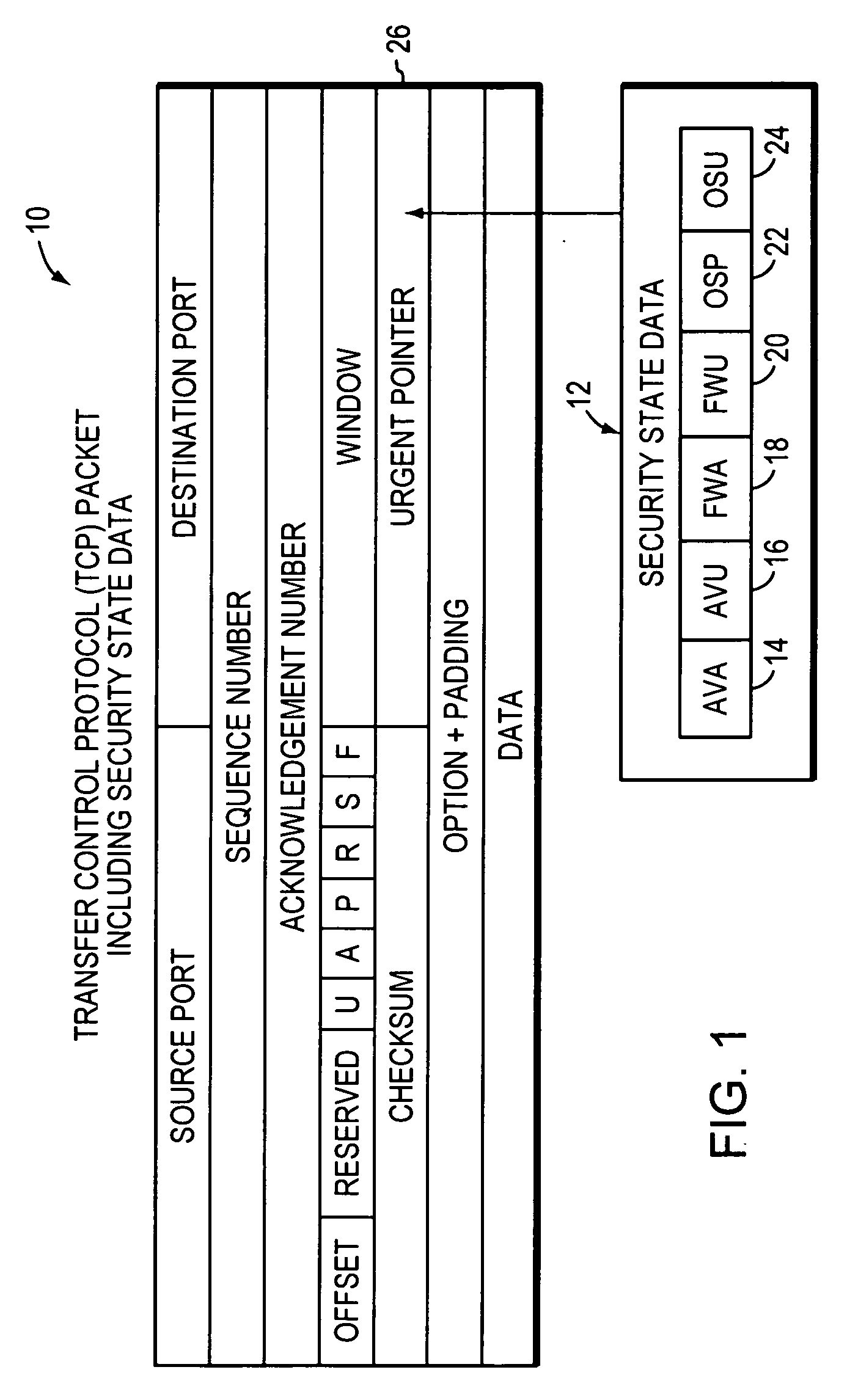
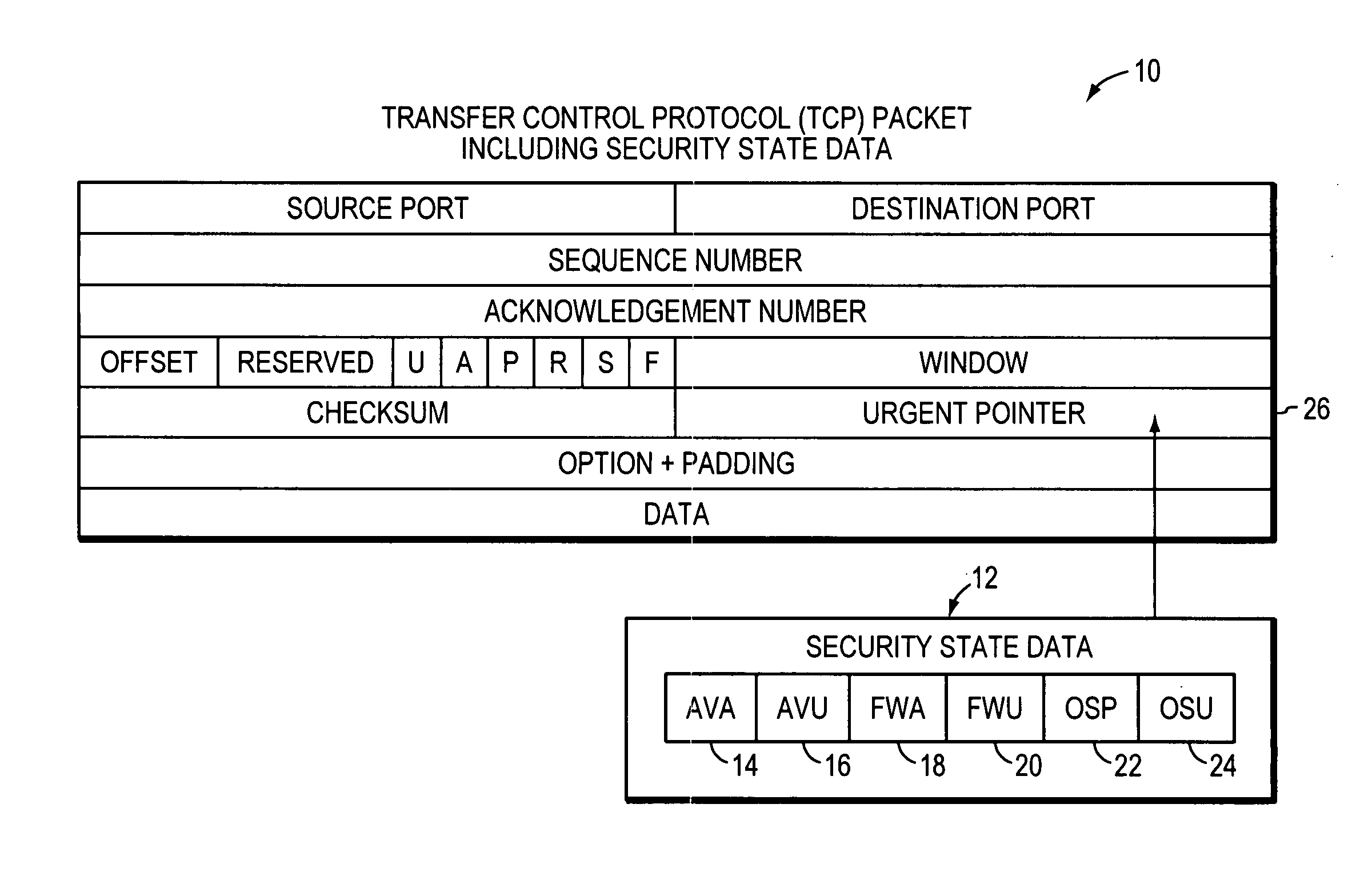
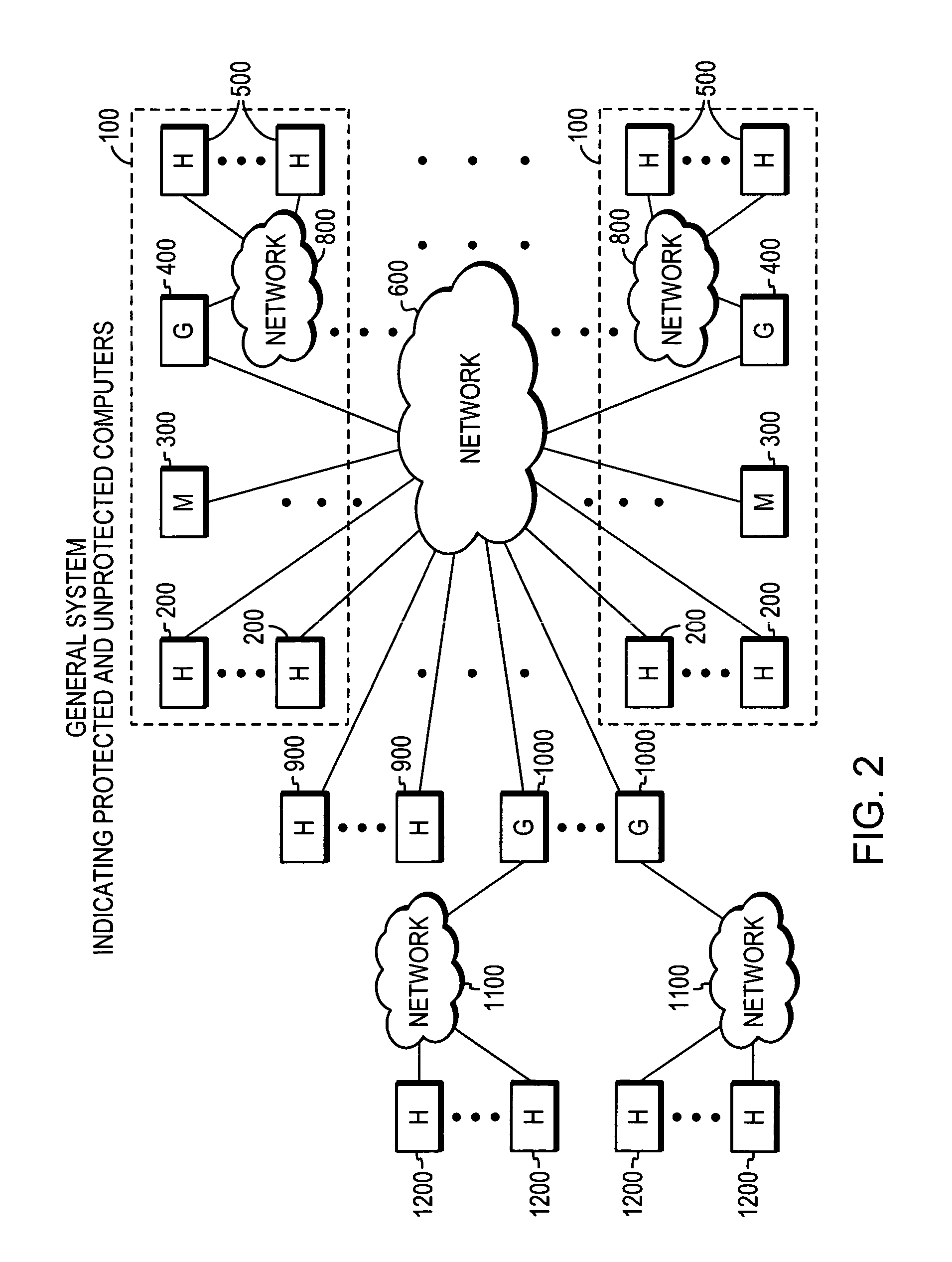
System, apparatuses, methods and computer-readable media for determining the security status of a computer before establishing a network connection
InactiveUS7591001B2Avoid infectionDigital data processing detailsUser identity/authority verificationNetwork connectionSecure state
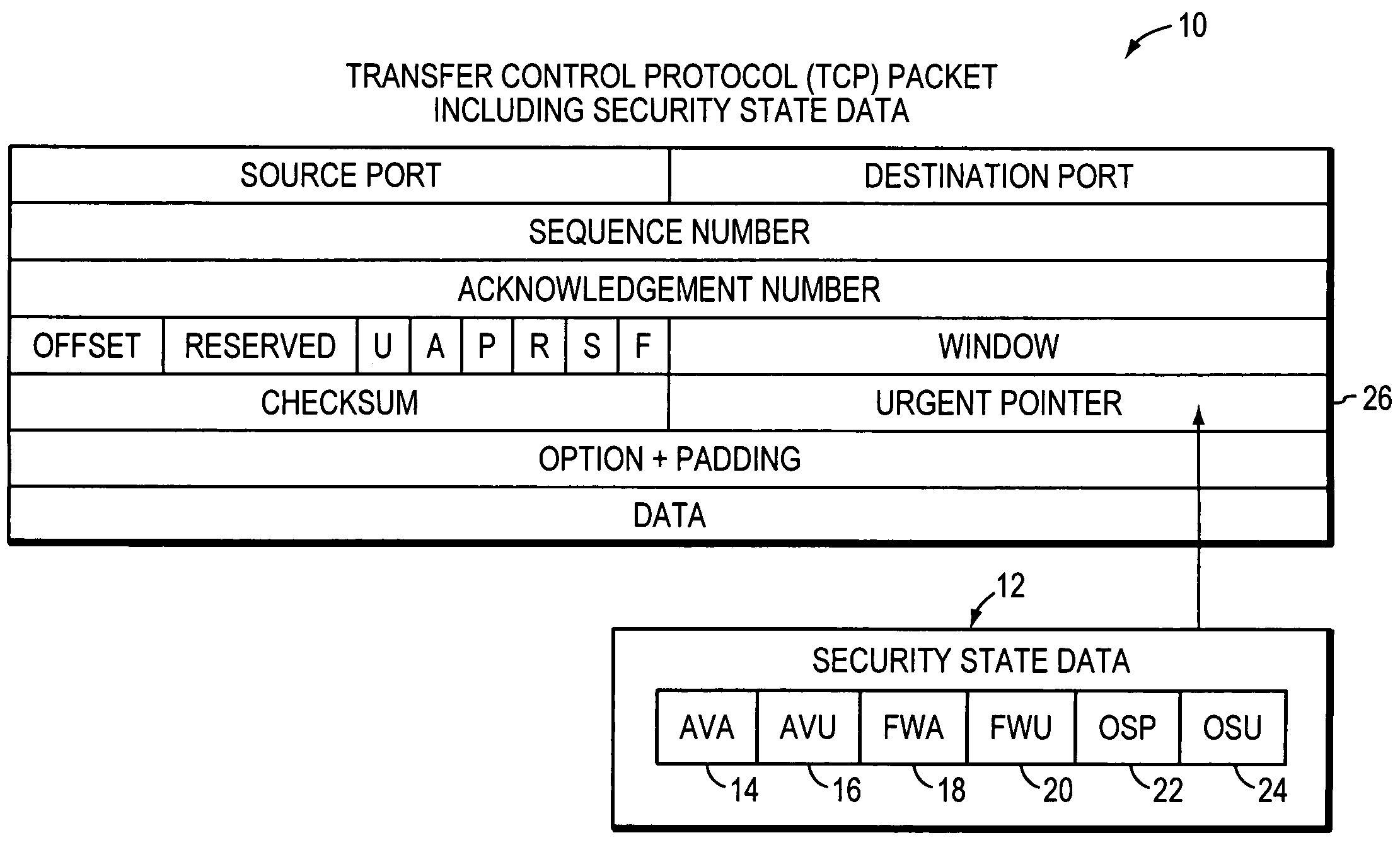
The disclosed system, apparatuses, methods, and computer-readable media can be used by a computer to establish the security status of another computer before establishing a network connection to it. Responsive to a request message, security state data indicating this status can be incorporated into a response message as one of the first few packets exchanged by computers to establish a network connection. This enables a computer to determine whether the other computer's security status is compliant with its security policy before establishing the network connection, reducing risk of infection by a virus, worm, or the like.
Owner:LIQUIDWARE LABS
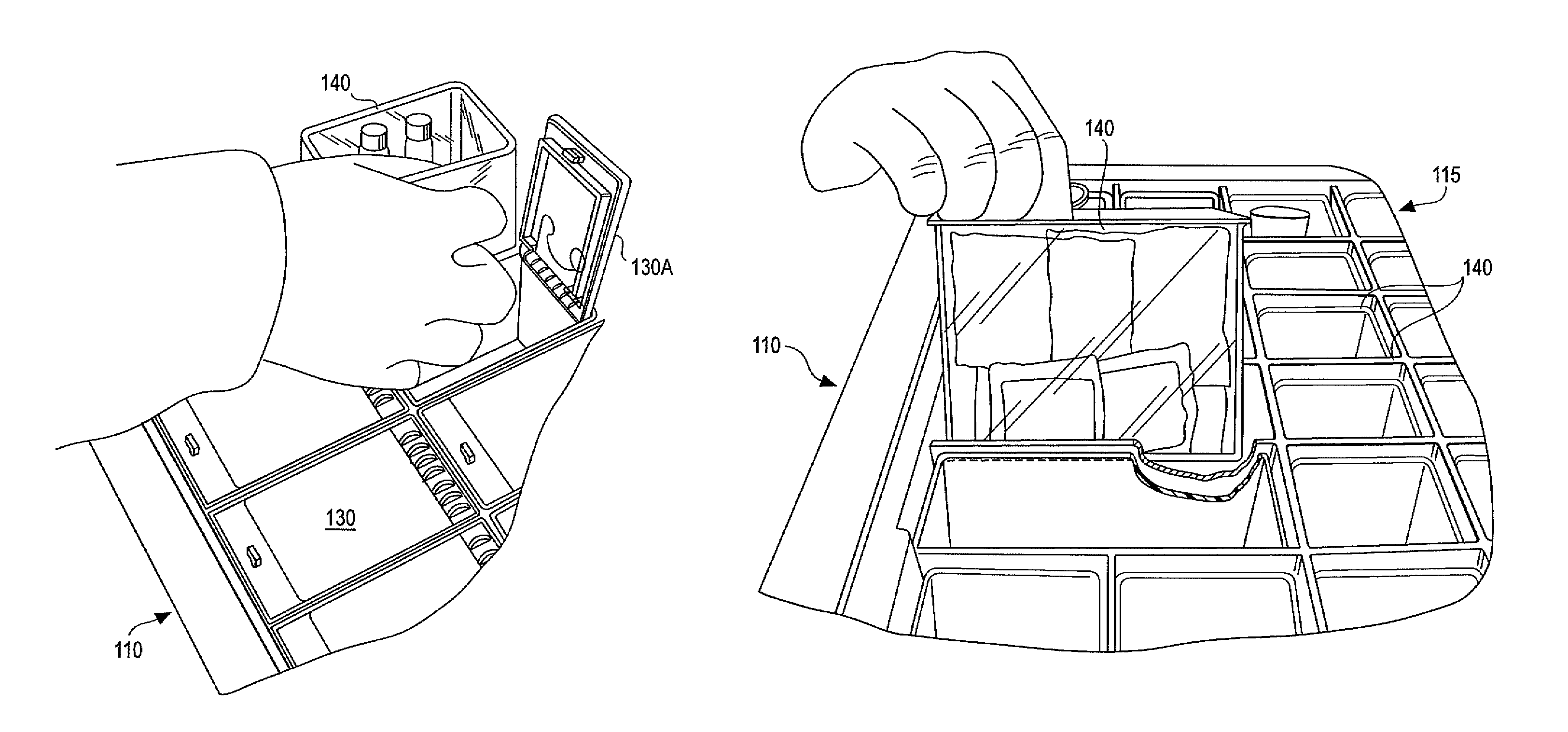
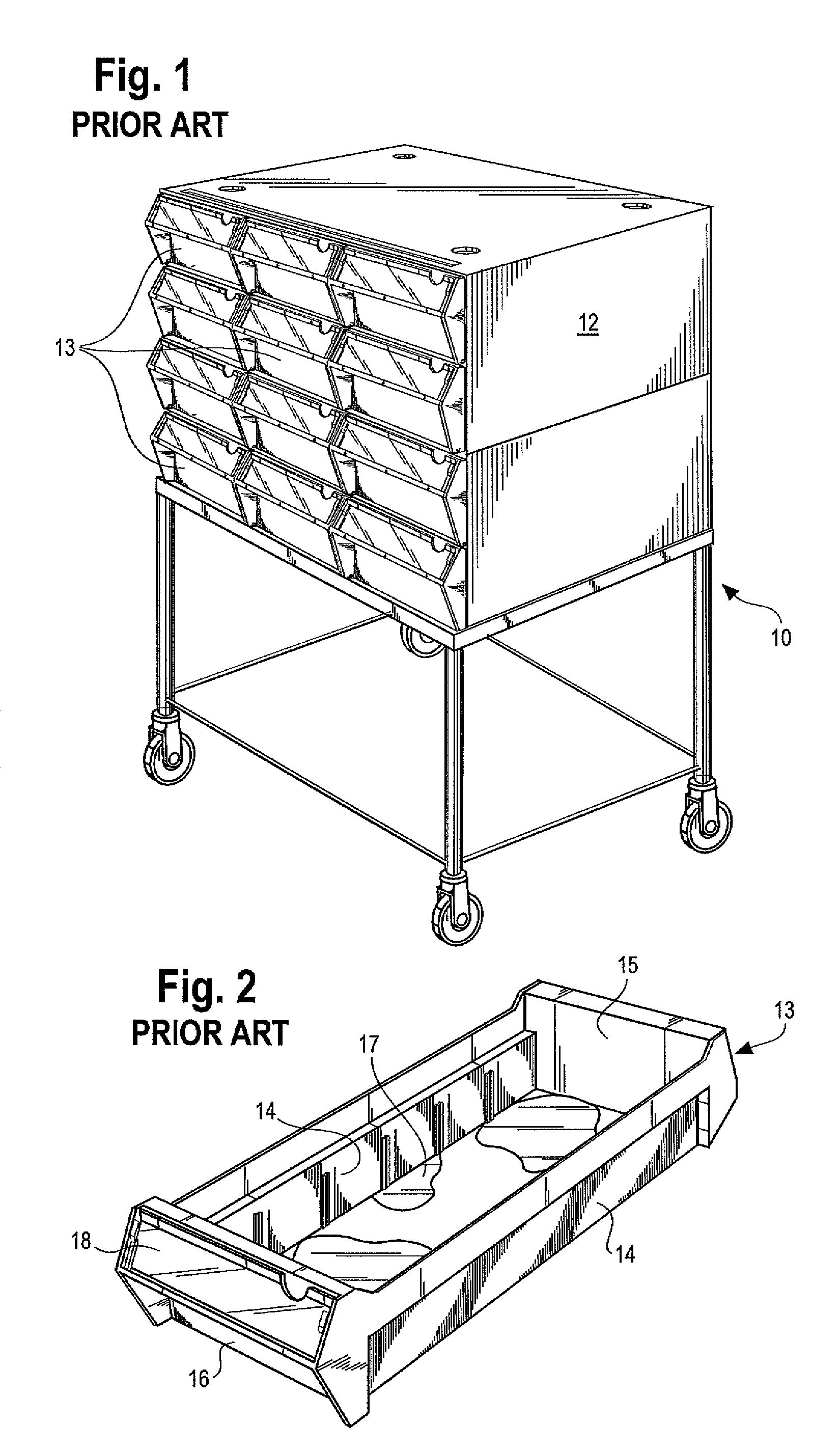
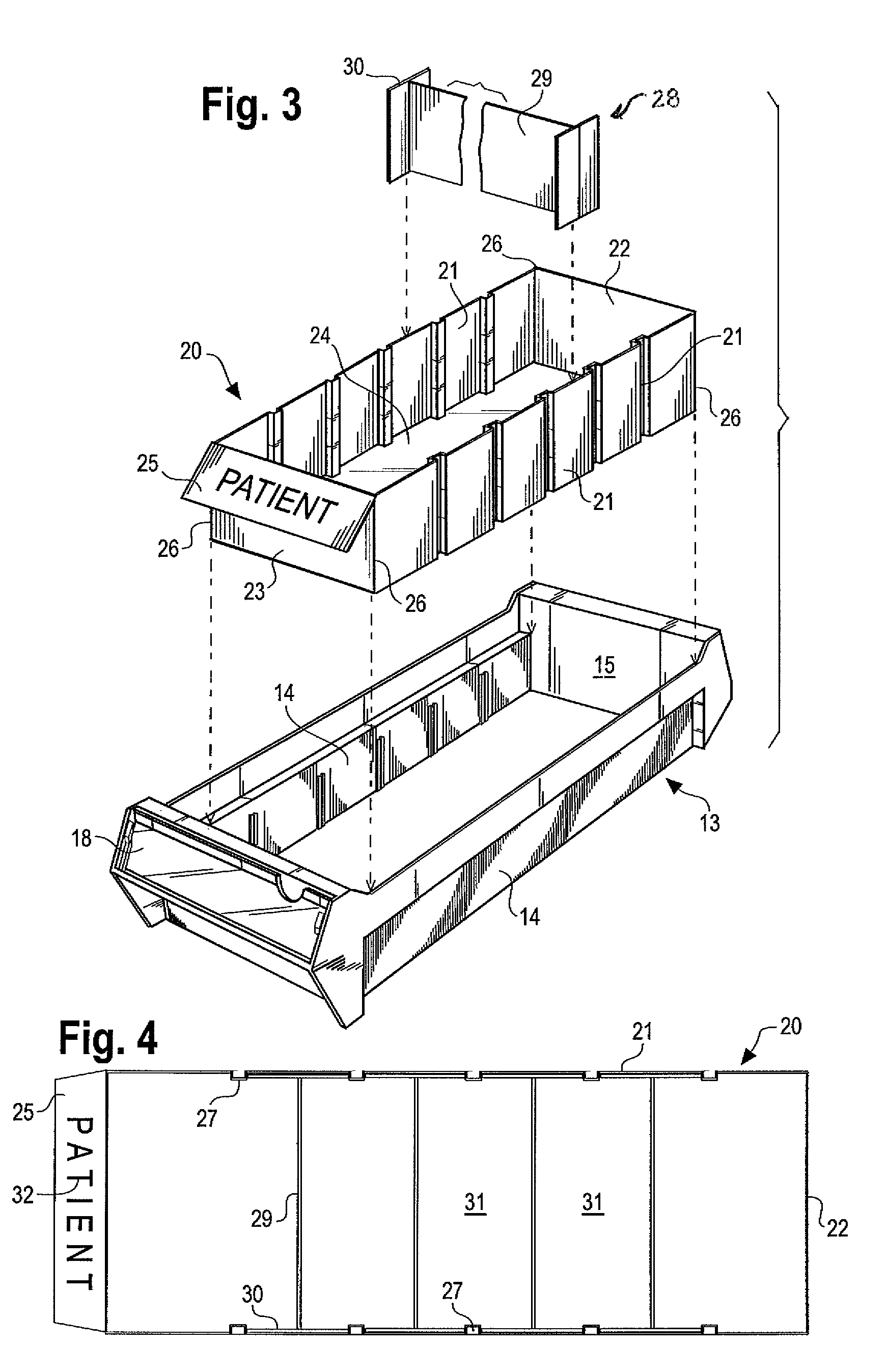
Medication cart drawer liner and method for using same to reduce nosocomial infections
InactiveUS7806488B2High strengthSave storage spaceSmall article dispensingDiagnosticsLiner dispenserCvd risk
In a medication storage cart or ADM machine housing a plurality of individual drawers or bins holding medication and / or medical equipment, the invention provides devices and a method for reducing the risk of nosocomial infection through the use of disposable liners. Disposable liners may be placed within the bins, within drawer liners, or within cubies or mini-drawers. In some applications the liners may be color-coded for a predetermined use or patient compartmentalized with dividers, or customized with special labels. The disposable liners may have a tapered form so that multiple liners may be stored in a nested stack to minimize space requirements and to be loaded into a liner dispenser system.
Owner:DBL SOLUTION
Methods of reducing risk of infection from pathogens with soluble amide and ester pyrazinoylguanidine sodium channel blockers
InactiveUS20070021439A1Reduce the risk of infectionAntibacterial agentsOrganic active ingredientsNatural sourceMedicine
Prophylactic treatment methods are provided for protection of individuals and / or populations against infection from airborne pathogens. In particular, prophylactic treatment methods are provided comprising administering a sodium channel blocker or pharmaceutically acceptable salts thereof to one or more members of a population at risk of exposure to or already exposed to one or more airborne pathogens, either from natural sources or from intentional release of pathogens into the environment.
Owner:PARION SCI DURHAM NC
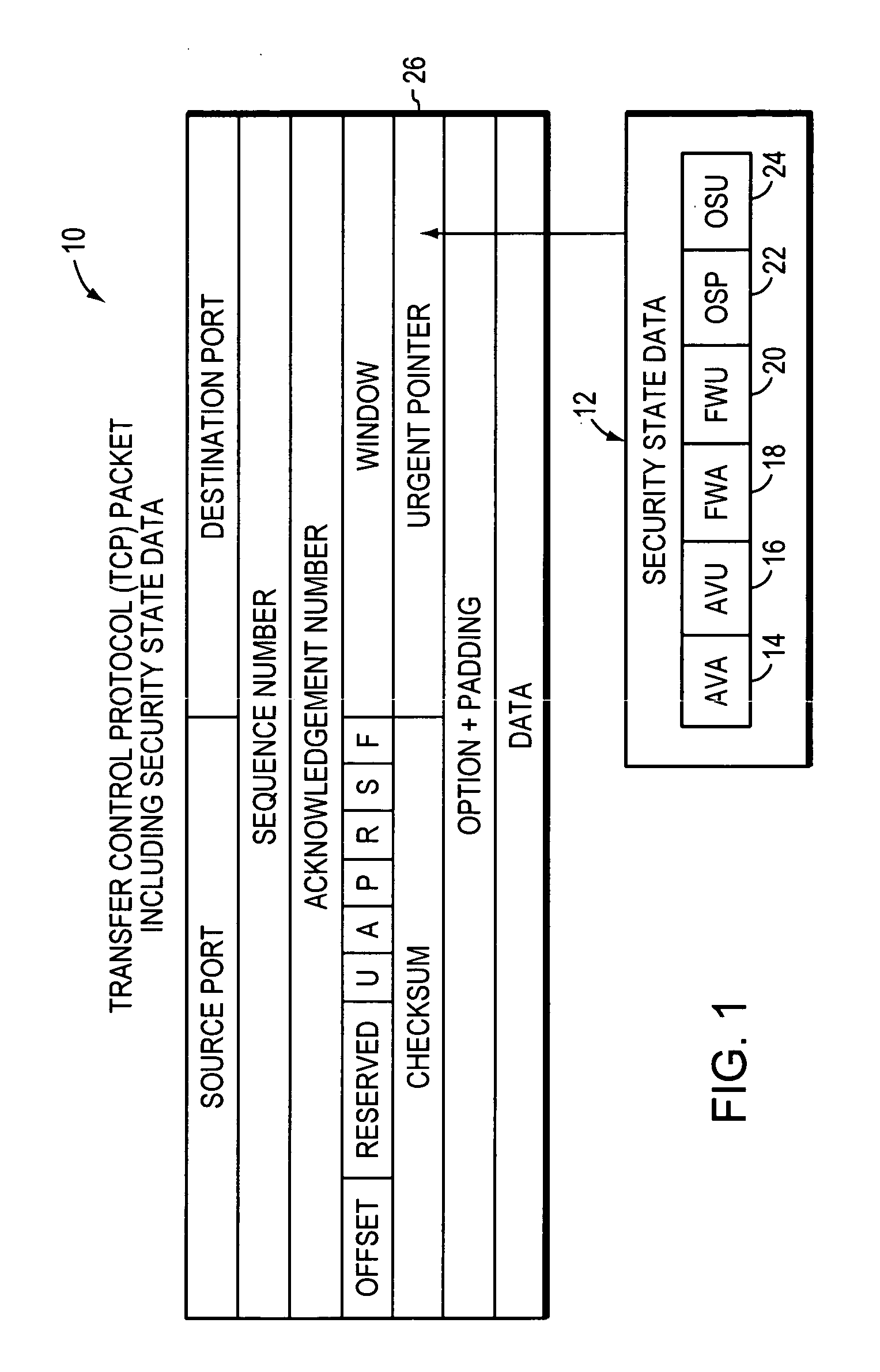
System, apparatuses, methods and computer-readable media for determining security status of computer before establishing network connection second group of embodiments-claim set II
InactiveUS20050268342A1Avoid infectionDigital data processing detailsAnalogue secracy/subscription systemsNetwork connectionSecure state
The disclosed system, apparatuses, methods, and computer-readable media can be used by a computer to establish the security status of another computer before establishing a network connection to it. Responsive to a request message, security state data indicating this status can be incorporated into a response message as one of the first few packets exchanged by computers to establish a network connection. This enables a computer to determine whether the other computer's security status is compliant with its security policy before establishing the network connection, reducing risk of infection by a virus, worm, or the like.
Owner:TRUSTED NETWORK TECH
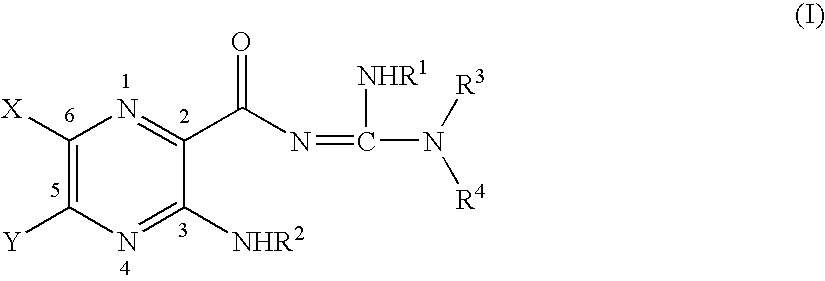
Method for reducing the risk of or preventing infection due to surgical or invasive medical procedures
The present invention relates to methods for reducing the risk of infection due to surgical or invasive medical procedures. The present invention also relates to methods for preventing infection due to surgical or invasive medical procedures.
Owner:RIB-X PHARMA
Porcine reproductive and respiratory syndrome virus and methods of use
InactiveUS7041443B2Effective treatmentSsRNA viruses positive-senseVirus peptidesRespiratory syndrome virusImmunogenicity
The present invention provides isolated European-like porcine reproductive and respiratory syndrome viruses, polynucleotides, and polypeptides. The present invention also provides methods for making antibodies to the viruses and polypeptides, methods for detecting porcine reproductive and respiratory syndrome viruses, immunogenic compositions, and methods for treating a porcine subject at risk of infection by, or displaying symptoms of, a porcine reproductive and respiratory syndrome virus infection.
Owner:RGT UNIV OF MINNESOTA
Composition and methods of making and using influenza proteins
InactiveUS20090196915A1Many symptomReduce the likelihood of infectionSsRNA viruses negative-sensePowder deliveryImmuno modulationBody fluid
The invention provides compositions of influenza proteins, such as matrix and nucleoprotein, that are presented to an individual's immune system as multimeric displays to induce an immune response. The compositions are optionally associated with any type of immunomodulatory compound (IMC) comprising an immunostimulatory sequences (ISS). The invention further provides compositions of influenza matrix and nucleoproteins that can induce cellular and / or humoral immune response. The invention also provides methods of making and using these compositions, e.g., as a vaccine, for ameliorating symptoms associated with infection with influenza virus or for reducing the risk of infection with influenza virus.
Owner:DYNAVAX TECH CORP
Method for reducing the risk of or preventing infection due to surgical or invasive medical procedures
InactiveUS20070238720A1Reducing of microbial infectionReduce riskBiocideOrganic active ingredientsPrevention infectionCvd risk
The present invention relates to methods for reducing the risk of infection due to surgical or invasive medical procedures. The present invention also relates to methods for preventing infection due to surgical or invasive medical procedures.
Owner:RIB-X PHARMA
Flange extender comprising honey
ActiveUS20170007440A1Promotes healthy skinNot compromise on comfortSurgical adhesivesColostomyIrritationEngineering
A flange extender for an ostomy bag comprises honey. In a preferred embodiment, the flange extender comprises a composition of hydrocolloid and medical grade Manuka honey and at least one release liner. Incorporation of honey into a flange extender provides additional adhesion to the skin of an ostomate and to a flange of an ostomy bag, increased wear time may possible and there may be reduced peristomal skin irritation. These advantages provide increased comfort for a user as well as reduced risk of infection through the antibacterial properties of honey.
Owner:WELLAND MEDICAL
System, apparatuses, methods and computer-readable media for determining security status of computer before establishing network connection second group of embodiments-claim set III
InactiveUS20050256957A1Avoid infectionDigital computer detailsTransmissionNetwork connectionSecure state
The disclosed system, apparatuses, methods, and computer-readable media can be used by a computer to establish the security status of another computer before establishing a network connection to it. Responsive to a request message, security state data indicating this status can be incorporated into a response message as one of the first few packets exchanged by computers to establish a network connection. This enables a computer to determine whether the other computer's security status is compliant with its security policy before establishing the network connection, reducing risk of infection by a virus, worm, or the like.
Owner:TRUSTED NETWORK TECH
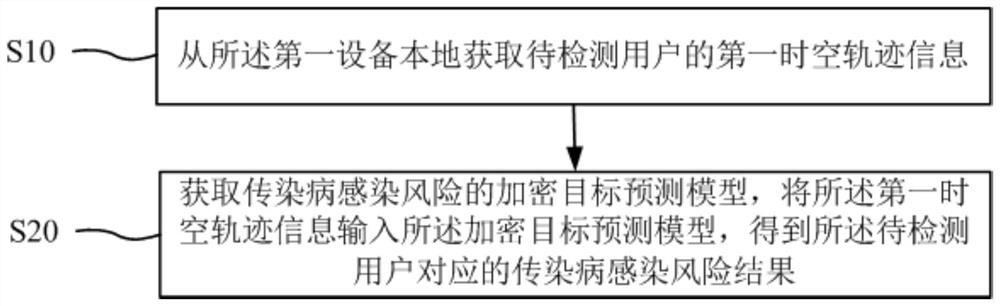
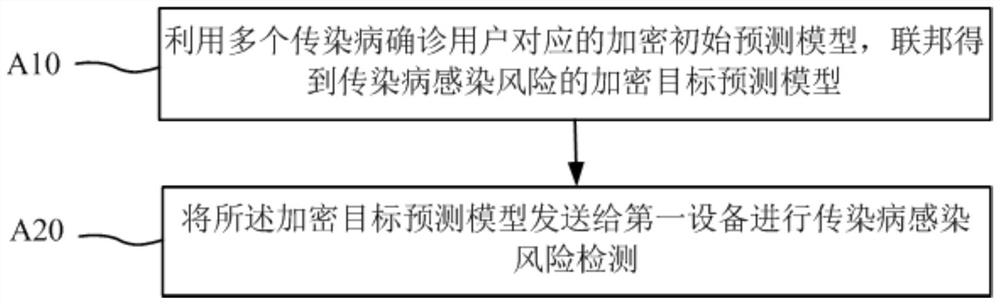
Infectious disease infection risk prediction method and device and storage medium
PendingCN111652446APrevent leakageEpidemiological alert systemsForecastingUser privacyEmergency medicine
The invention discloses an infectious disease infection risk prediction method and device and a storage medium. The method comprises the steps that of first space-time trajectory information of a to-be-detected user is obtained locally from first equipment, then an encrypted target prediction model of infectious disease infection risks is obtained, the first space-time trajectory information is input into the encrypted target prediction model, and an infectious disease infection risk result corresponding to the to-be-detected user is obtained. Obtaining; an encryption prediction model of the infectious disease infection risk is acquired by utilizing the space-time track information of the definite diagnosis user; infection risk detection of the to-be-detected user is realized; whole duringthe detection process, wherein the target prediction model is obtained based on the space-time trajectory information of the infectious disease definite diagnosis user; c. Compared with the prior art, the method has the advantages that the a model is encrypted, the unknown original space-time trajectory of the user is diagnosed, the user to be detected does not need to share the space-time trajectory information, infection risk detection is completed locally, the space-time trajectory information of the user can be protected, and leakage of user privacy is effectively avoided.
Owner:WEBANK (CHINA)
Antimicrobial and sporicidal composition
InactiveUS20080095861A1High bactericidal activityAntibacterial agentsBiocideTriclosanAmmonium compounds
Germicidal compositions with enhanced activity towards killing microbiological spores and vetgetative cells comprising certain quaternary ammonium compounds (QACs), phenolic compounds, monohydric alcohols, hydrogen peroxide, iodine, triclocarban, triclosan or combinations thereof with one or more spore coat opening agents. The invention also provides for the application of the germicidal compositions to animate and inanimate surfaces to help kill germs and protect against the risk of infection from bacteria, molds, yeasts, fungi, viruses, and microbiological spores.
Owner:PURE IP LC
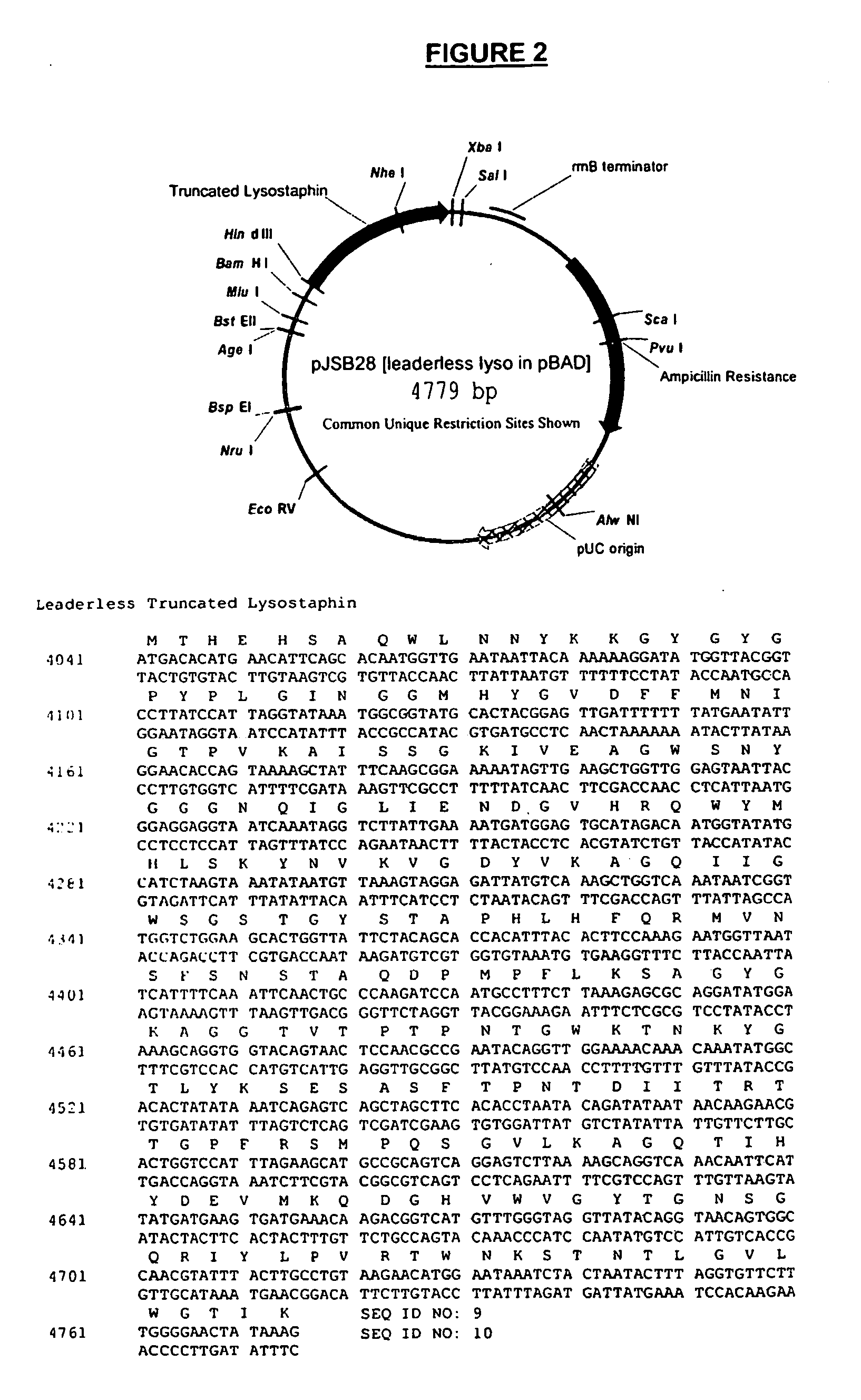
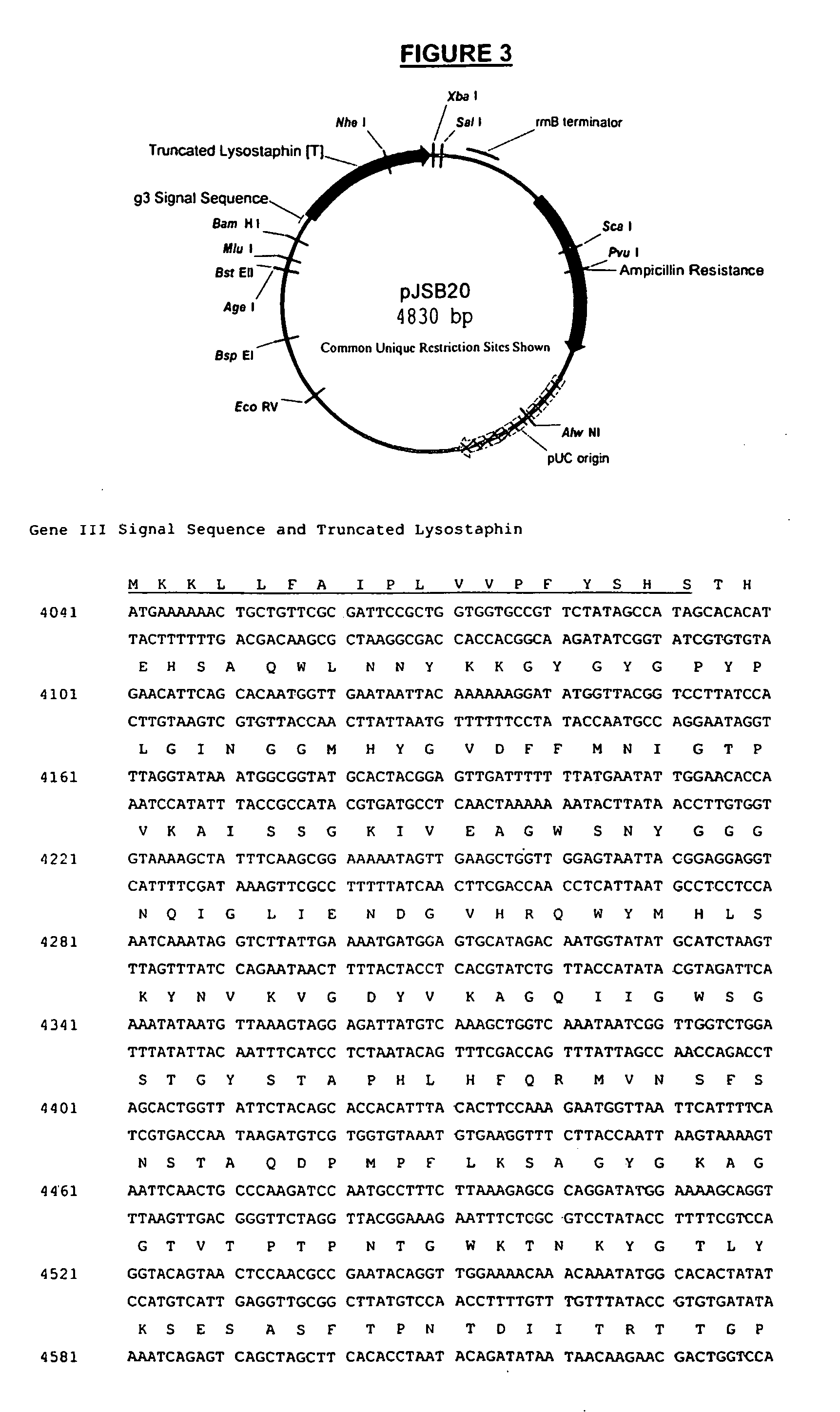
Truncated lysostaphin molecule with enhanced staphylolytic activity
InactiveUS20050118159A1Reduce individual infectionAvoid spreadingAntibacterial agentsSugar derivativesStaphylococcal infectionsStaphylococcus saprophyticus
This invention relates to the production of recombinant lysostaphin in a homogenous form through the use of recombinant DNA molecules that express homogenous lysostaphin and host cells transformed with these DNA molecules. This invention also relates to the production of truncated forms of lysostaphin. The resulting lysostaphin preparations can be administered to those at infected or risk for infection by staphylococcal bacteria.
Owner:BIOSYNEXUS INC
Therapeutic agent for autoimmune diseases comprising PD-1 agonist
ActiveUS9701749B2Reduce the risk of infectionReduce administrative burdenNervous disorderAntipyreticDiseaseAutoimmune responses
Owner:ONO PHARMA CO LTD
Amnion and chorion wound dressing and uses thereof
An improved wound dressing is described. The wound dressing contains an allograft having at least one layer of human amnion and chorion tissues that has a size and shape appropriate for covering a wound, a base sheet for securing the allograft over the wound, and optionally a support layer to protect the allograft. Methods of preparing the wound dressing and using it in wound healing are also described. The products and methods improve the performance of the wound healing, e.g., by accelerating wound healing, reducing scar formation, inflammation and risk of infection.
Owner:LIVENTA BIOSCI
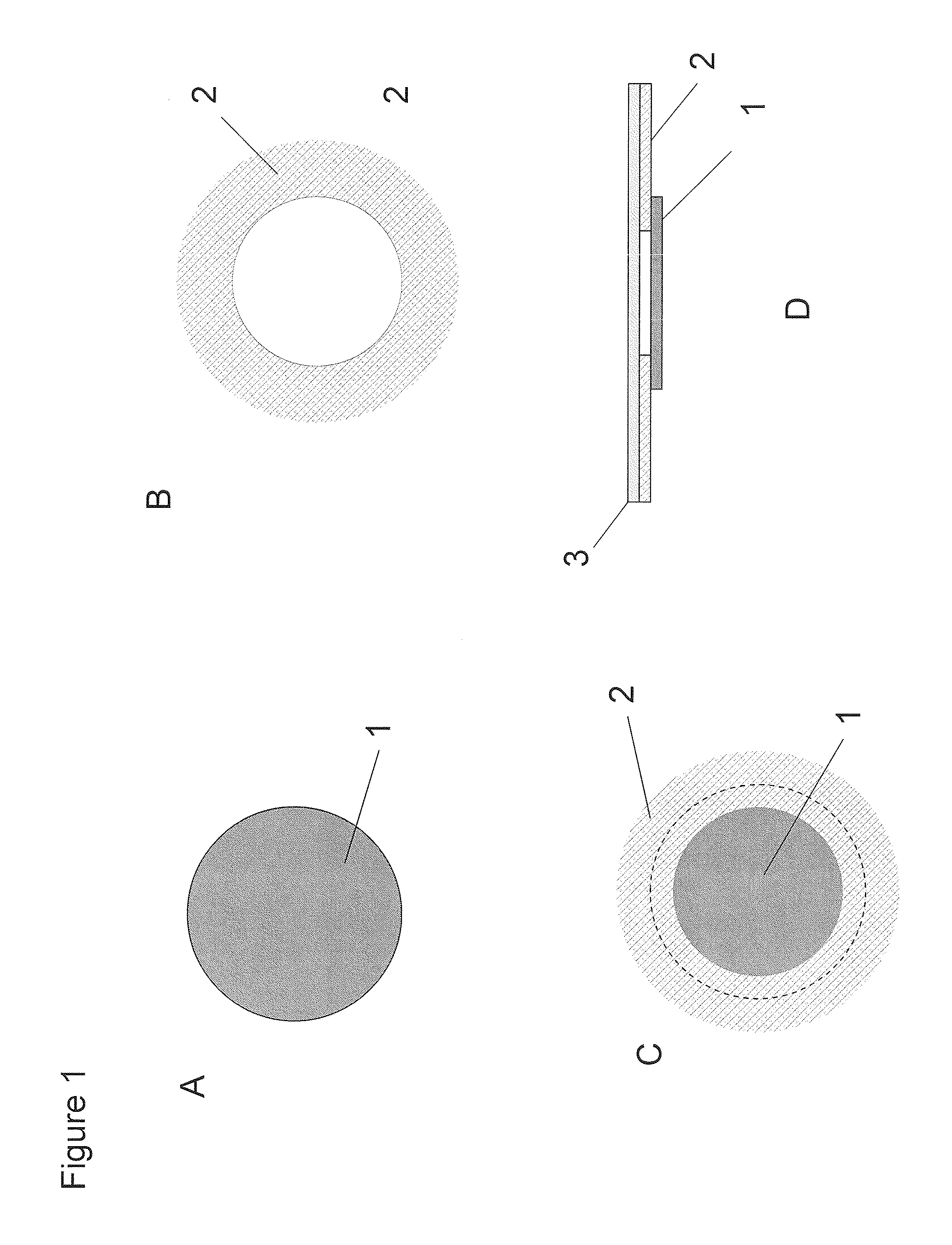
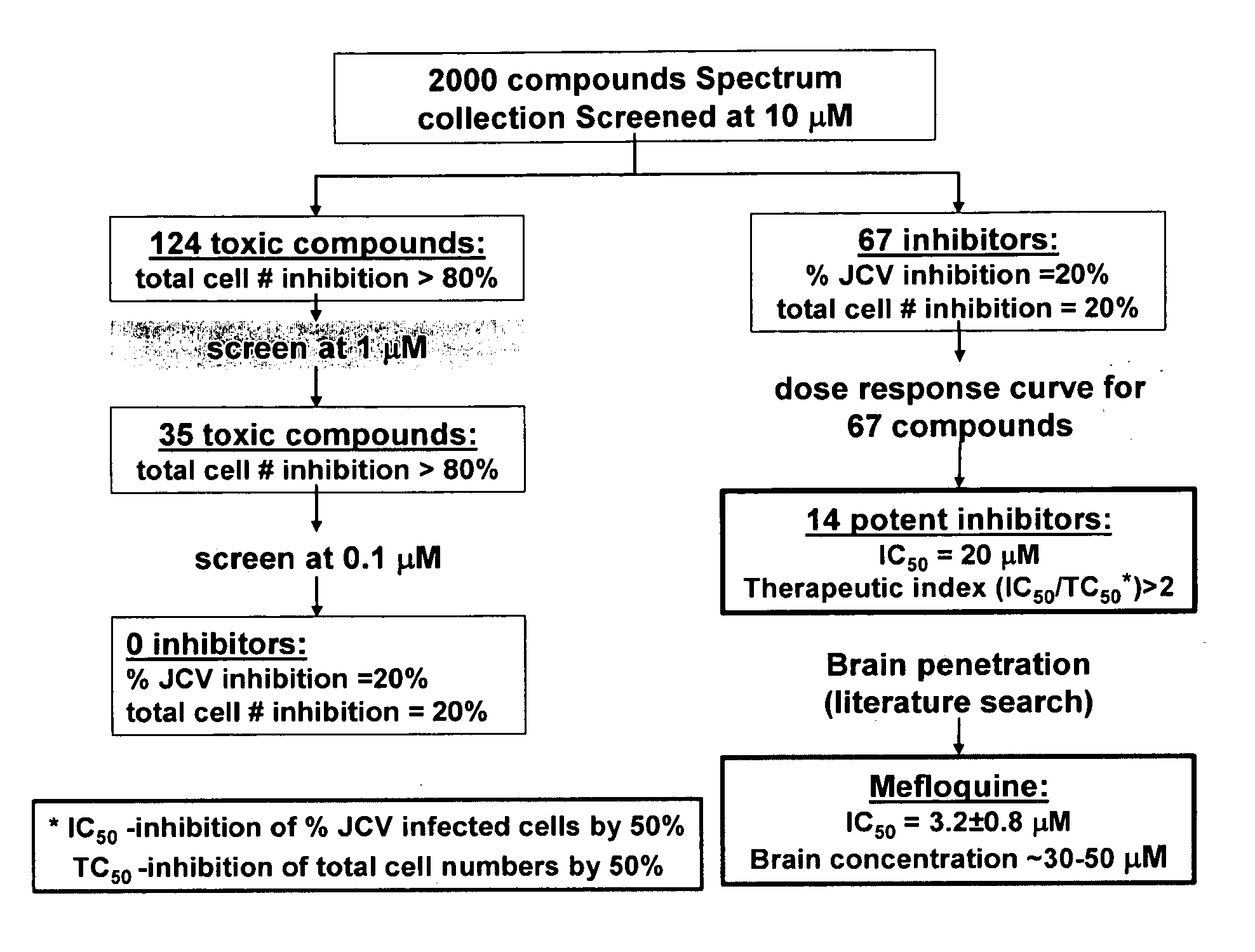
Compositions and methods for the treatment of progressive multifocal leukoencephalopathy (PML)
ActiveUS20130183289A1Reduce riskBiocideOther blood circulation devicesPolyomavirus JCViral infection
The invention relates to compositions, methods, and kits for treating subjects infected by or at risk of infection with a DNA virus (e.g., a JC Virus or a BK virus). Aspects of the invention are useful to prevent or treat DNA virus associated conditions (e.g., PML) in subjects that are immunocompromised. Compositions are provided that inhibit intracellular replication of DNA viruses.
Owner:BIOGEN MA INC
Closed type throat swab sampling device
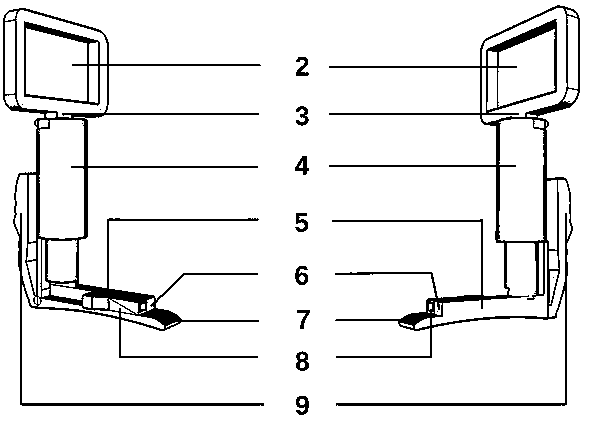
PendingCN111084643AGet it right and fastEfficient acquisitionBronchoscopesLaryngoscopesDiseaseSurgery
The invention discloses a closed type throat swab sampling device. According to the device, the upper end of a handle is connected with a display screen through a connecting shaft, the lower part of the handle is connected with a long plate, the top end of the long plate is connected with a tongue depressor, and the long plate is provided with a sampling cavity and a camera; a fixed valve is arranged at the joint of the long plate and the handle; and a sealing cover is fixedly or movably sleeved on the long plate, and an elastic band is arranged at the middle hole to be tightly bonded with thelong plate. The invention has the advantages that: the device can be used to solve the problem of how to safely and correctly collect throat swab specimens by medical staff for infectious respiratorydiseases, particularly for patients with corona virus disease 2019 at present. Oral and nasal parts of the patient are properly covered in a closed manner, thus avoiding an extremely high risk of infection since the medical staff directly face respiratory tract pathogenic bacteria when collecting throat swab specimens of patients; and a real-time image in the oral cavity is displayed in vitro byleading-in the camera with a light source to the oral cavity, and the medical staff can correctly and quickly acquire an ideal throat swab specimen under the guidance of the real-time image.
Owner:杨人强
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com