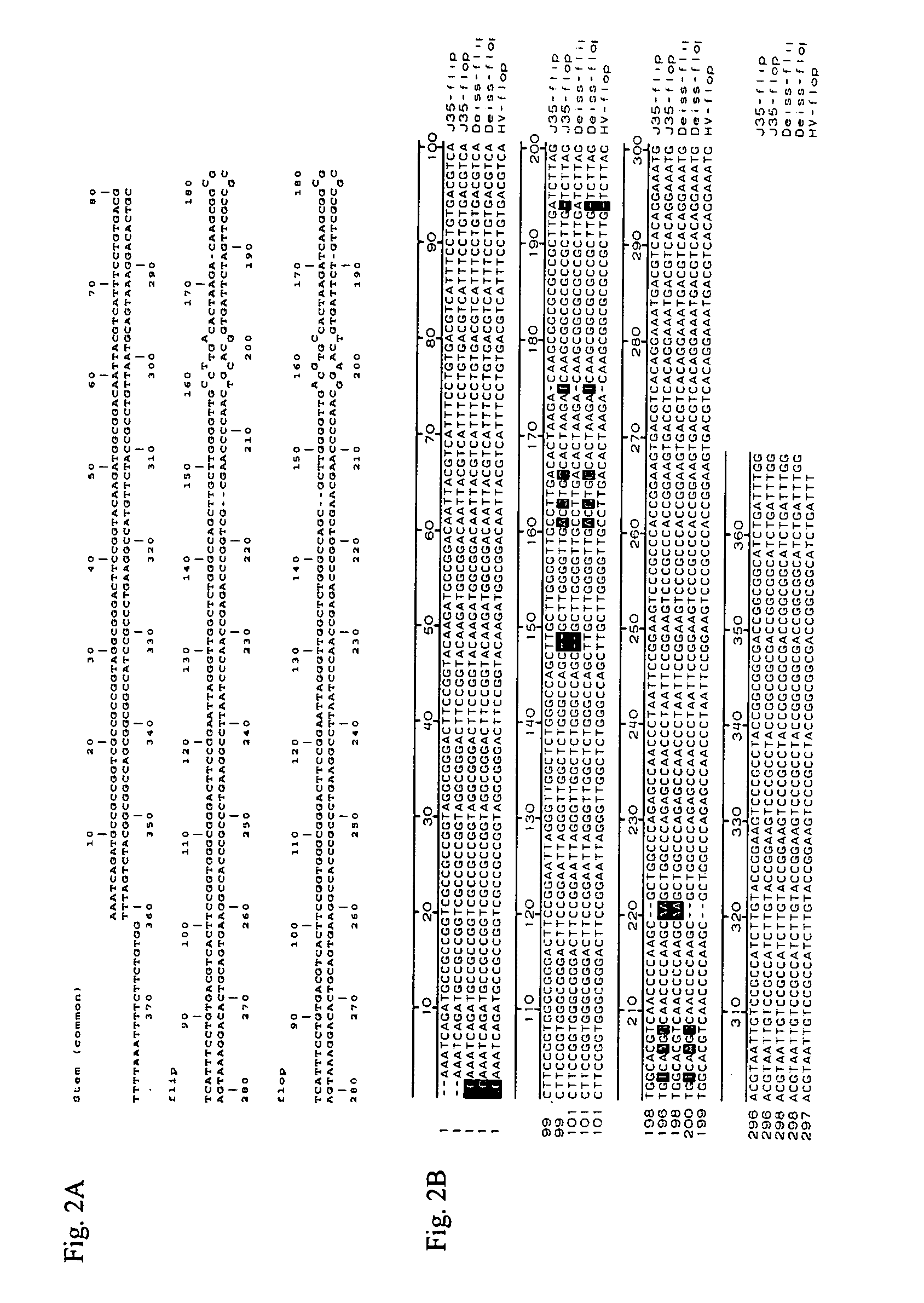
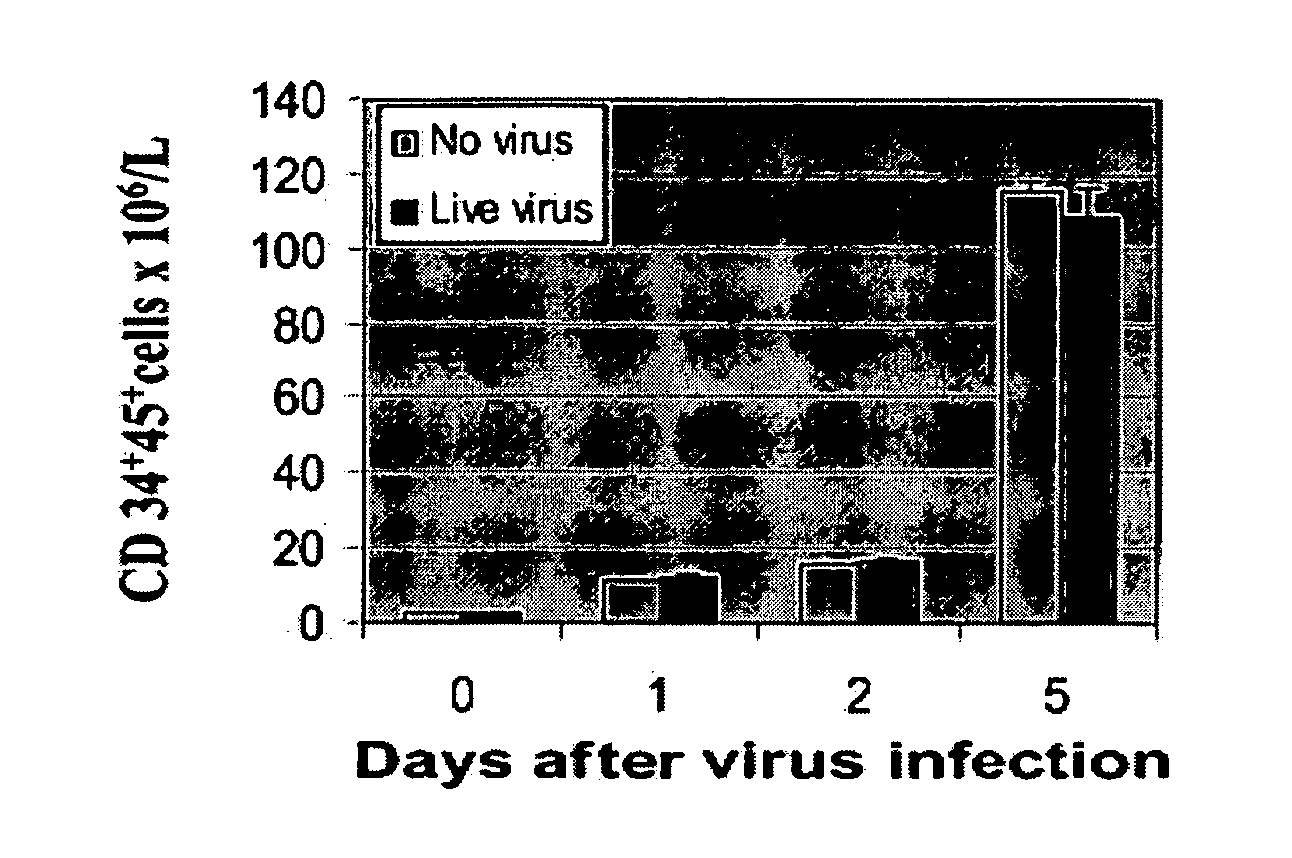
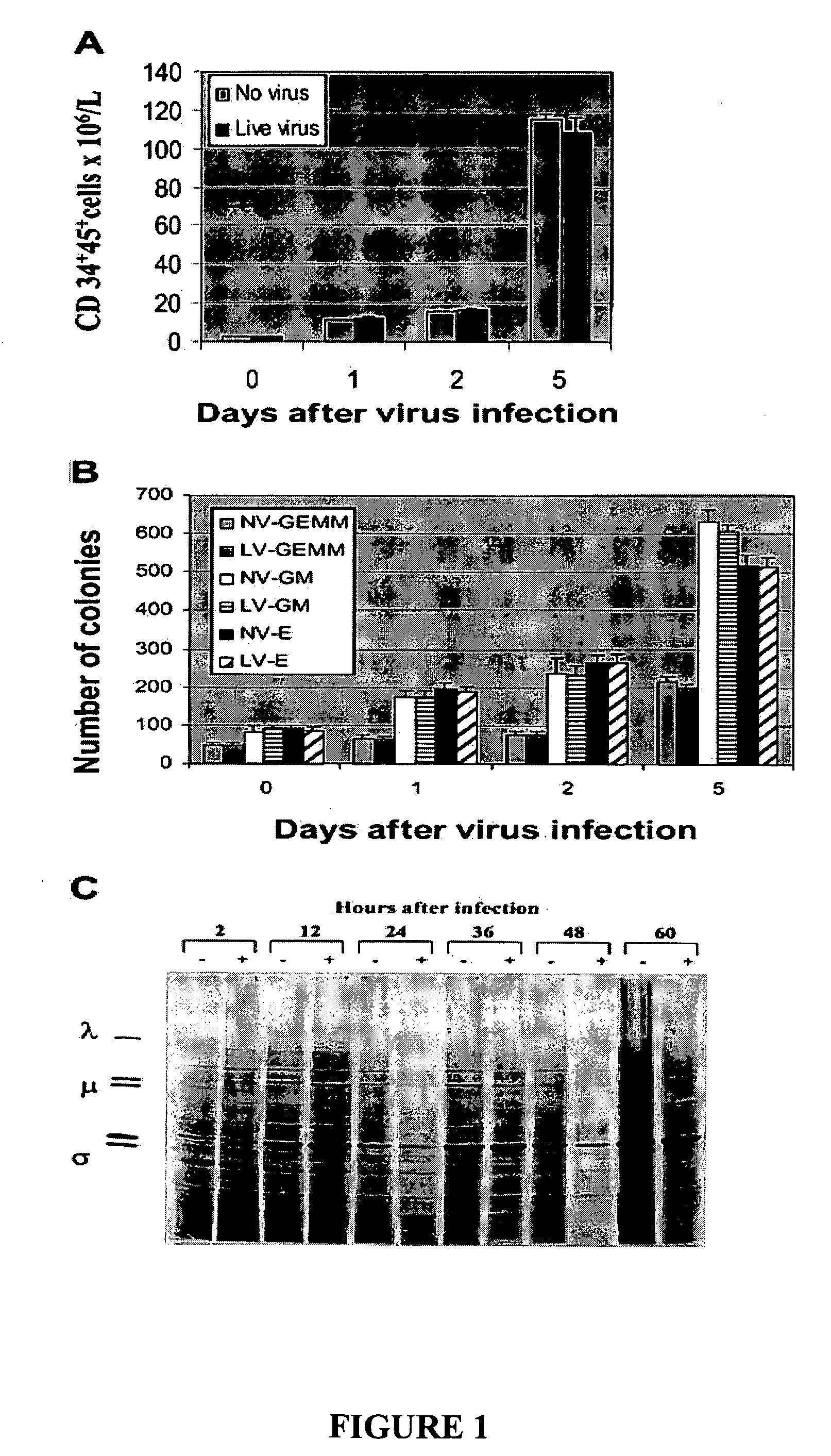
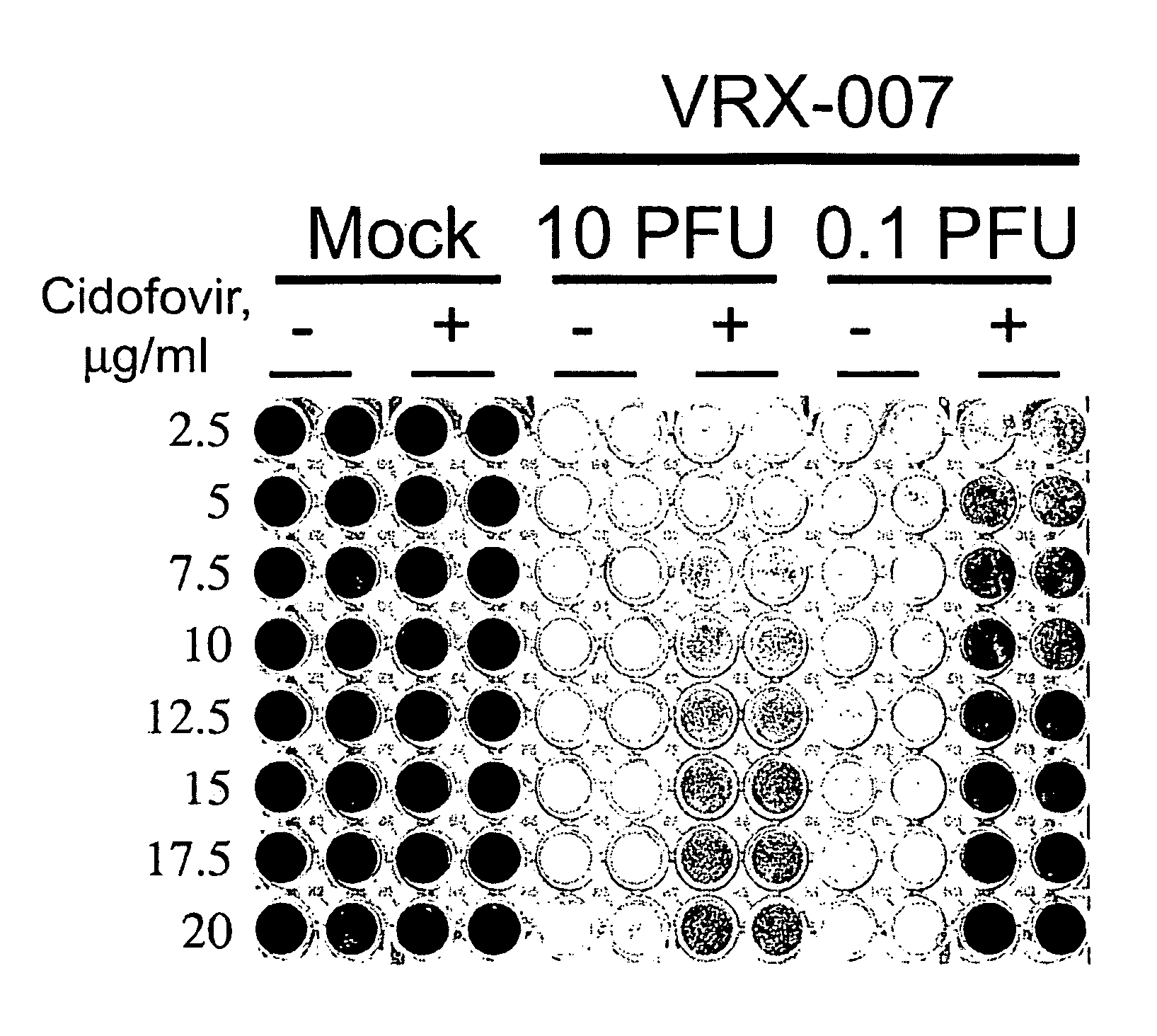
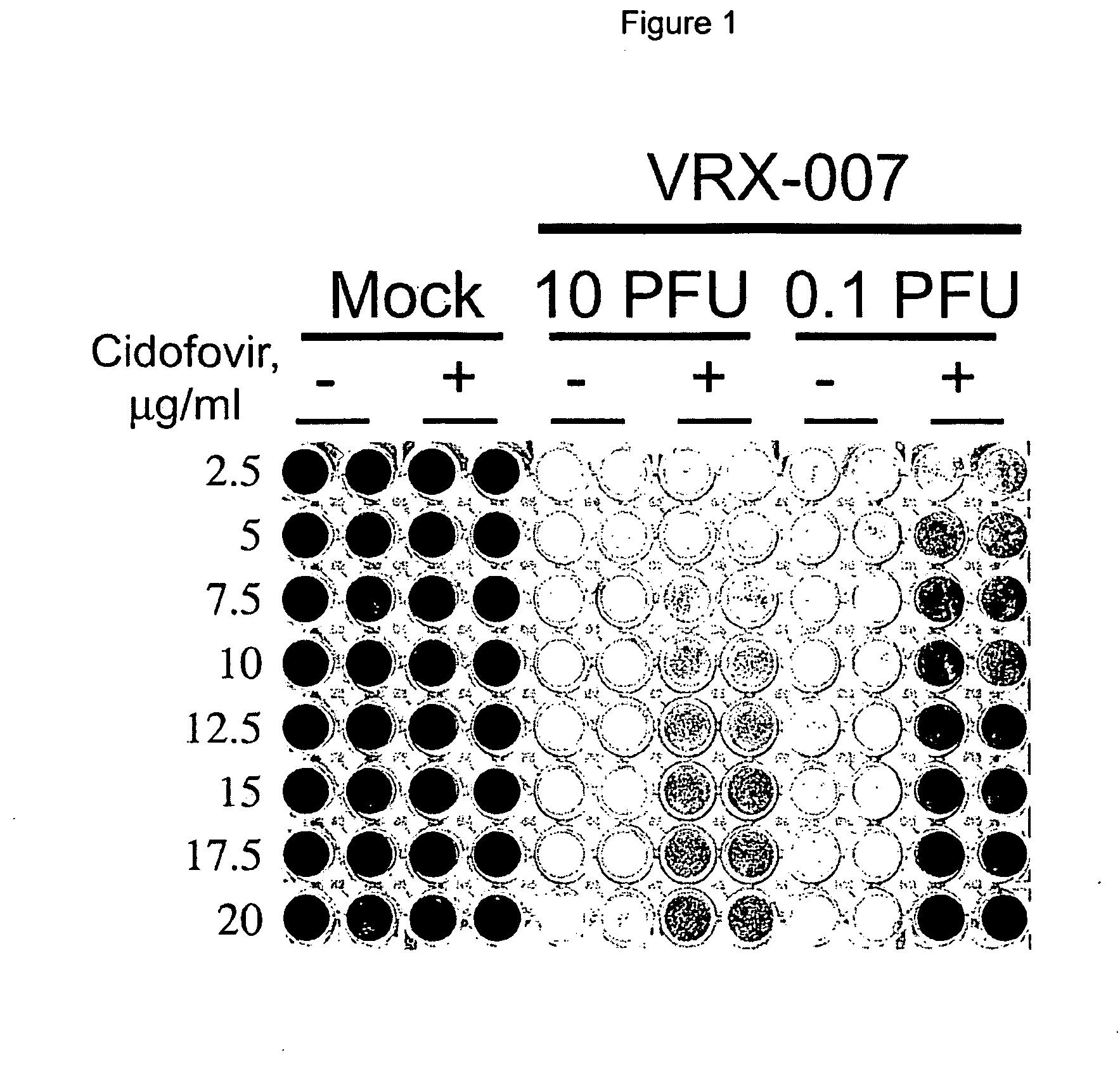
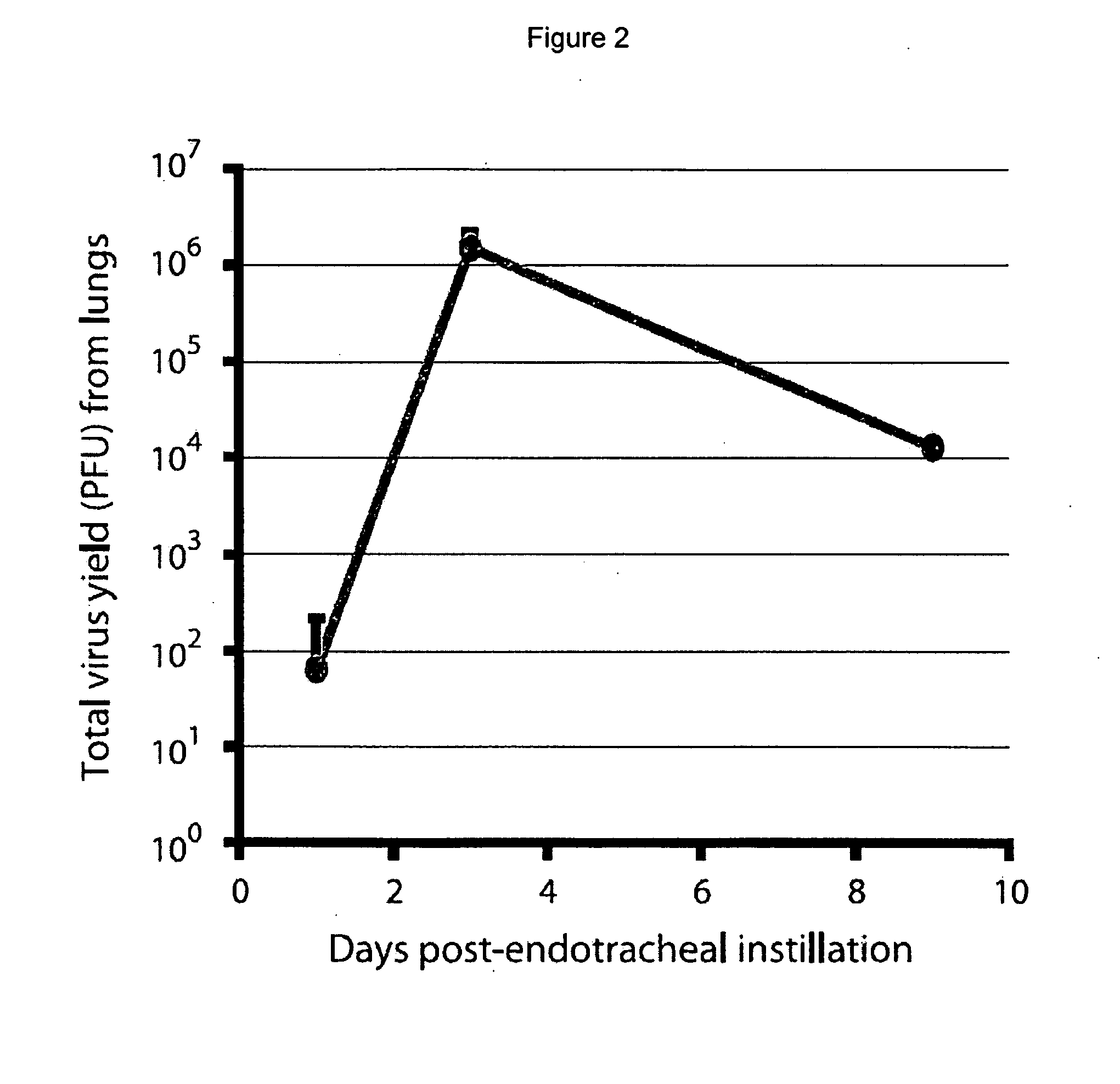
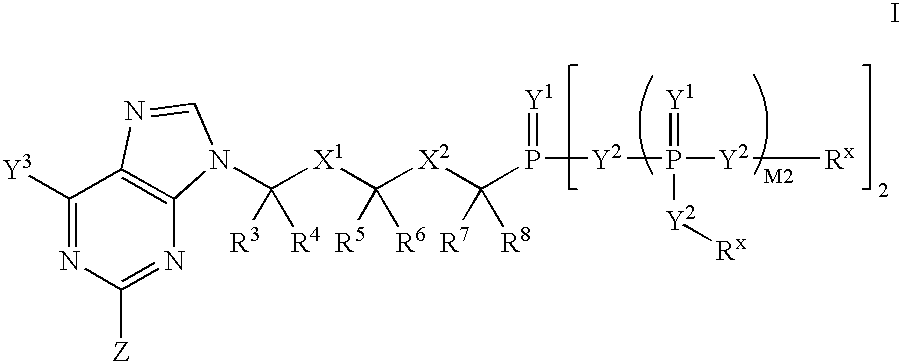
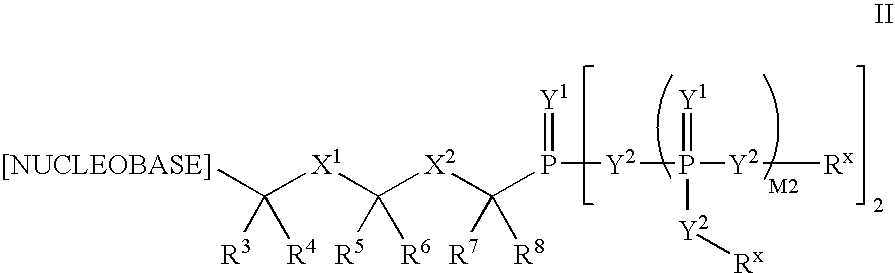Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
195 results about "Infectious virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
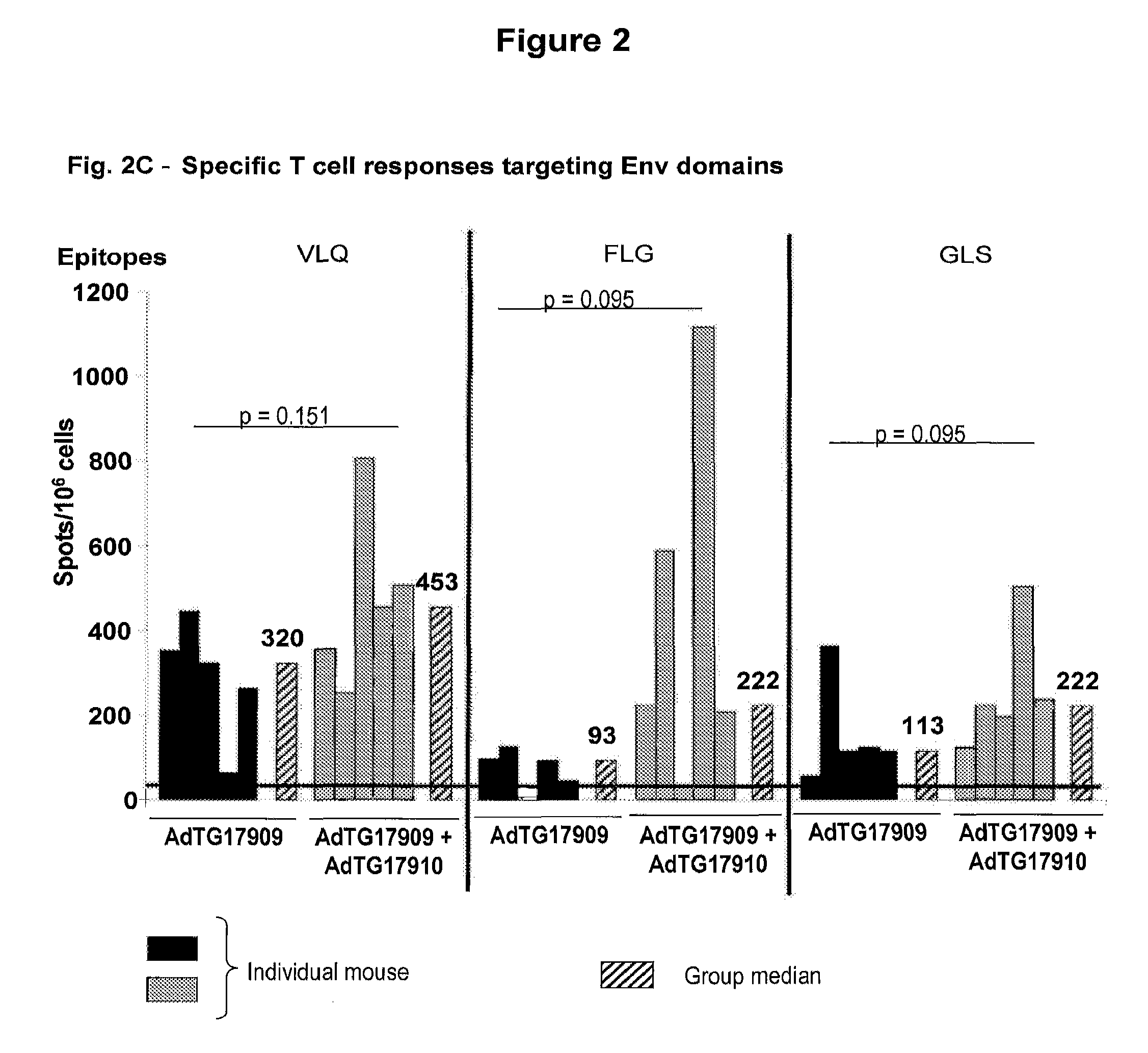
Viruses cause familiar infectious diseases such as the common cold, flu and warts. They also cause severe illnesses such as HIV/AIDS, smallpox, and Ebola. Viruses are like hijackers. They invade living, normal cells and use those cells to multiply and produce other viruses like themselves.
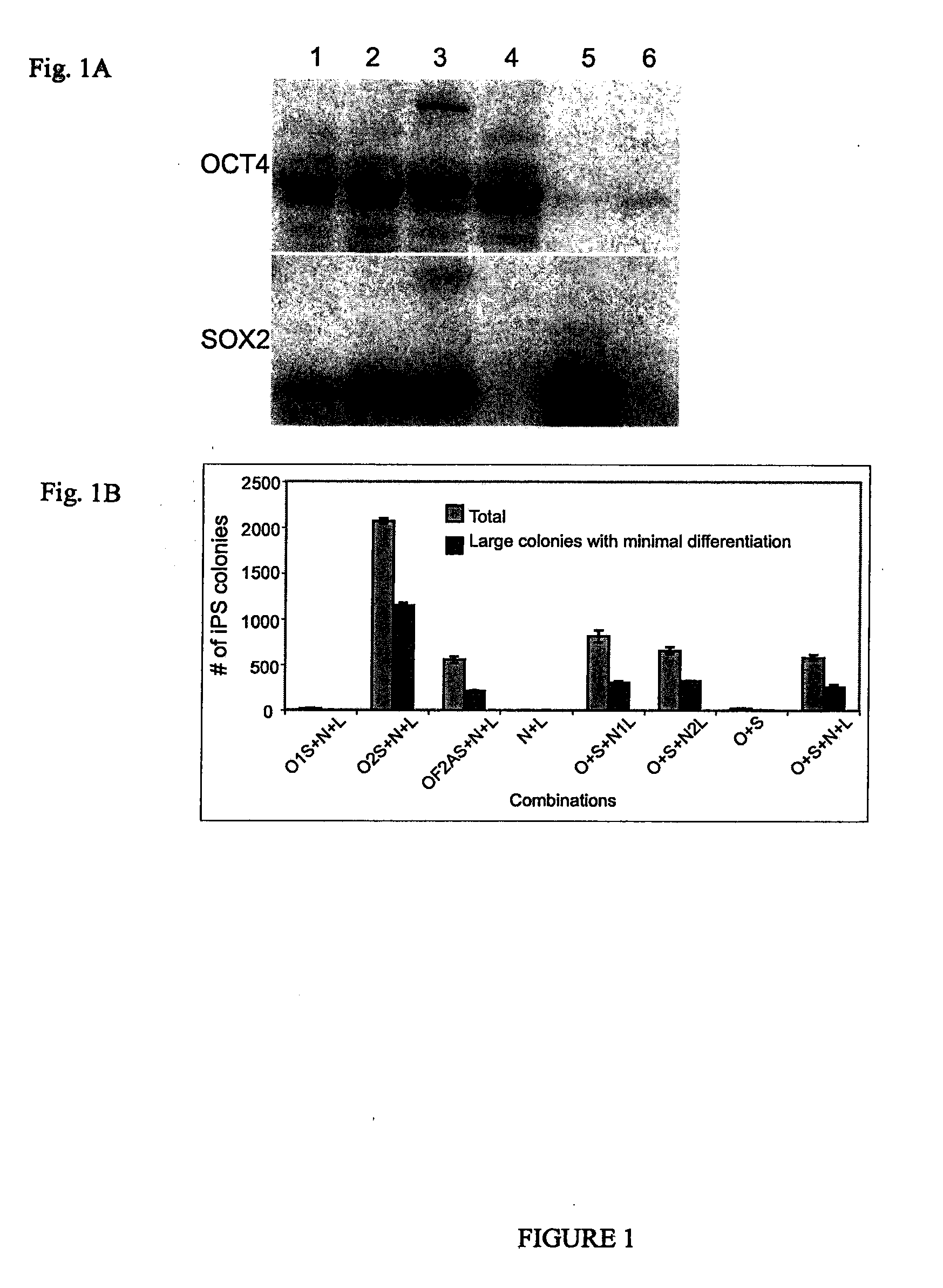
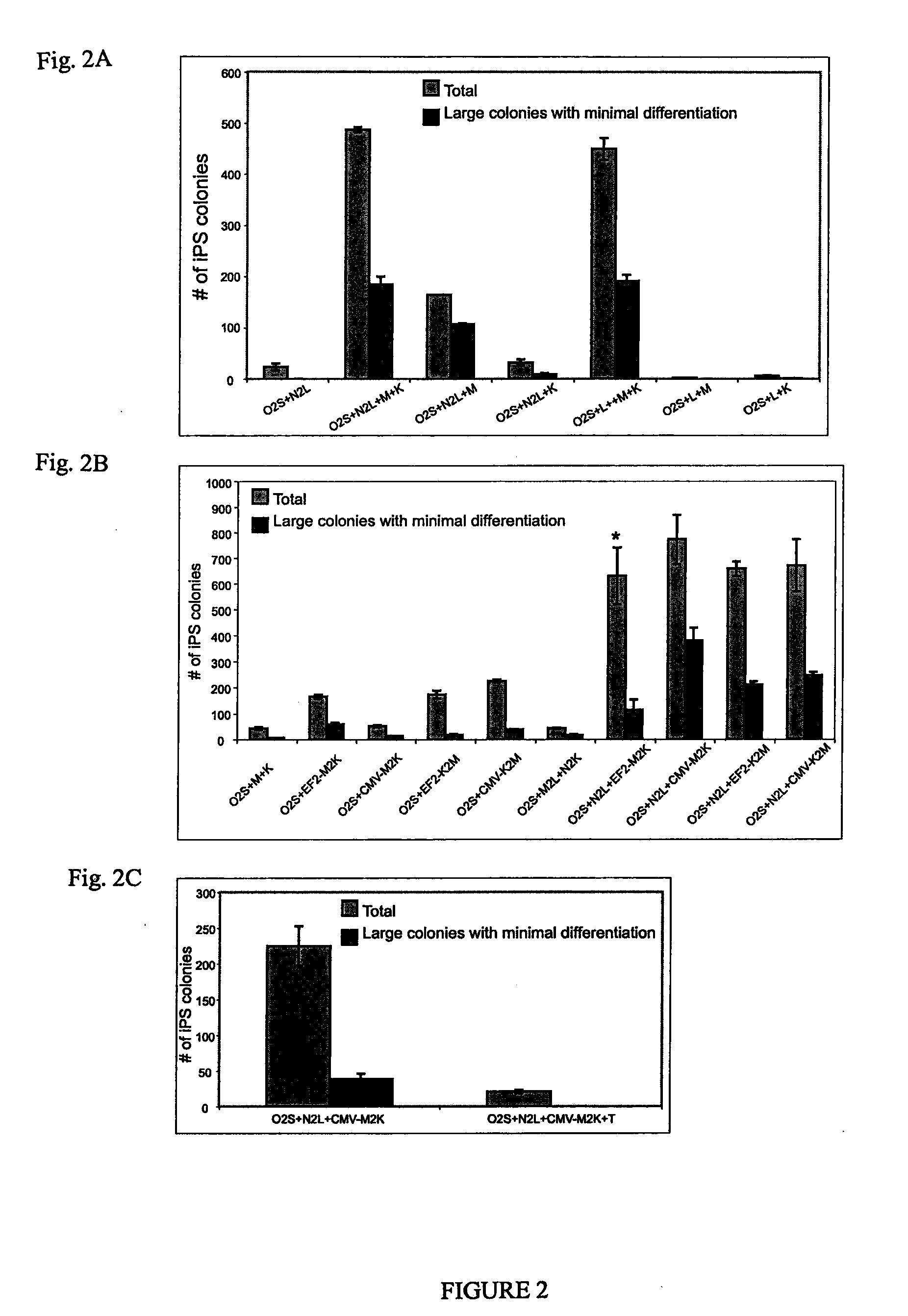
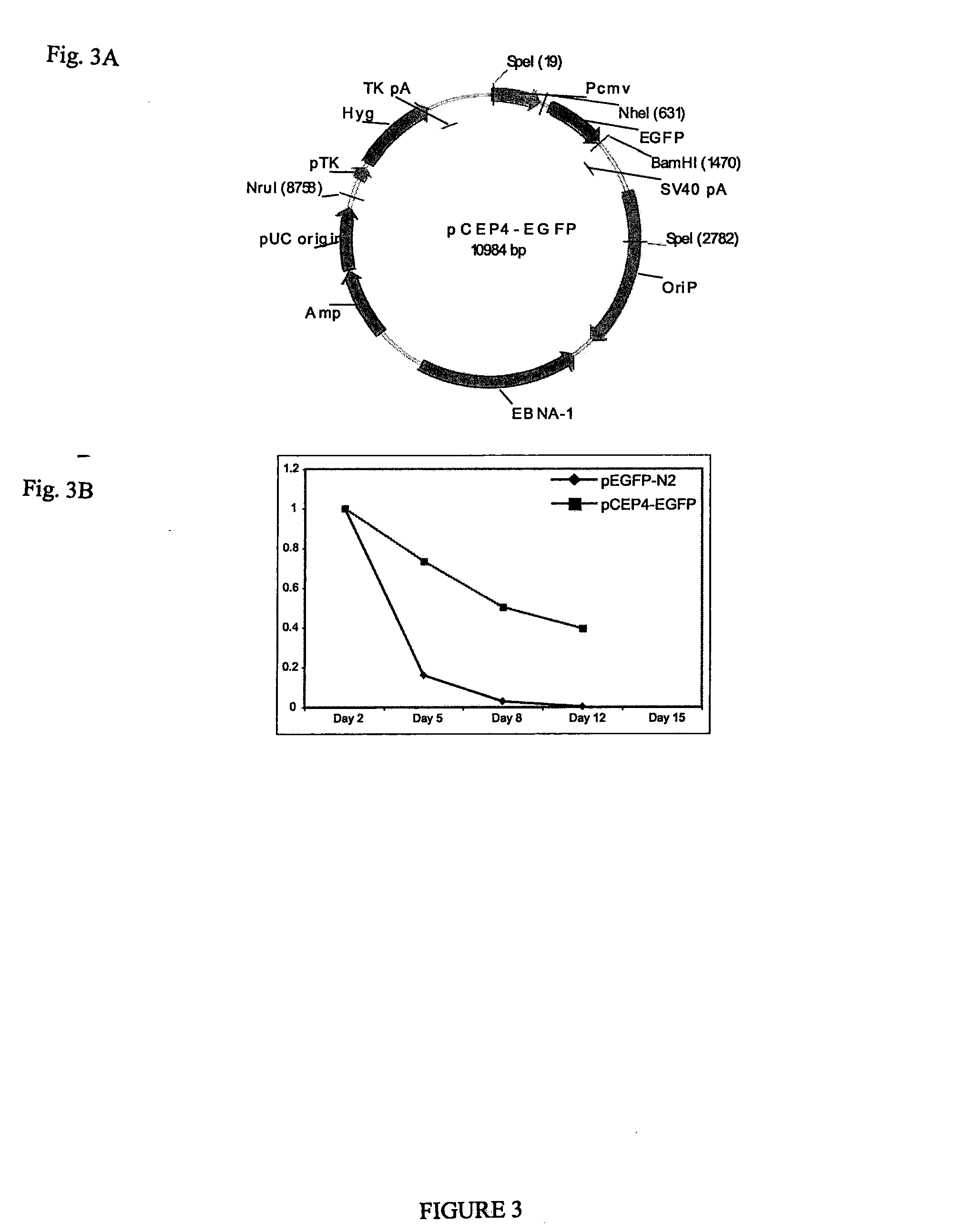
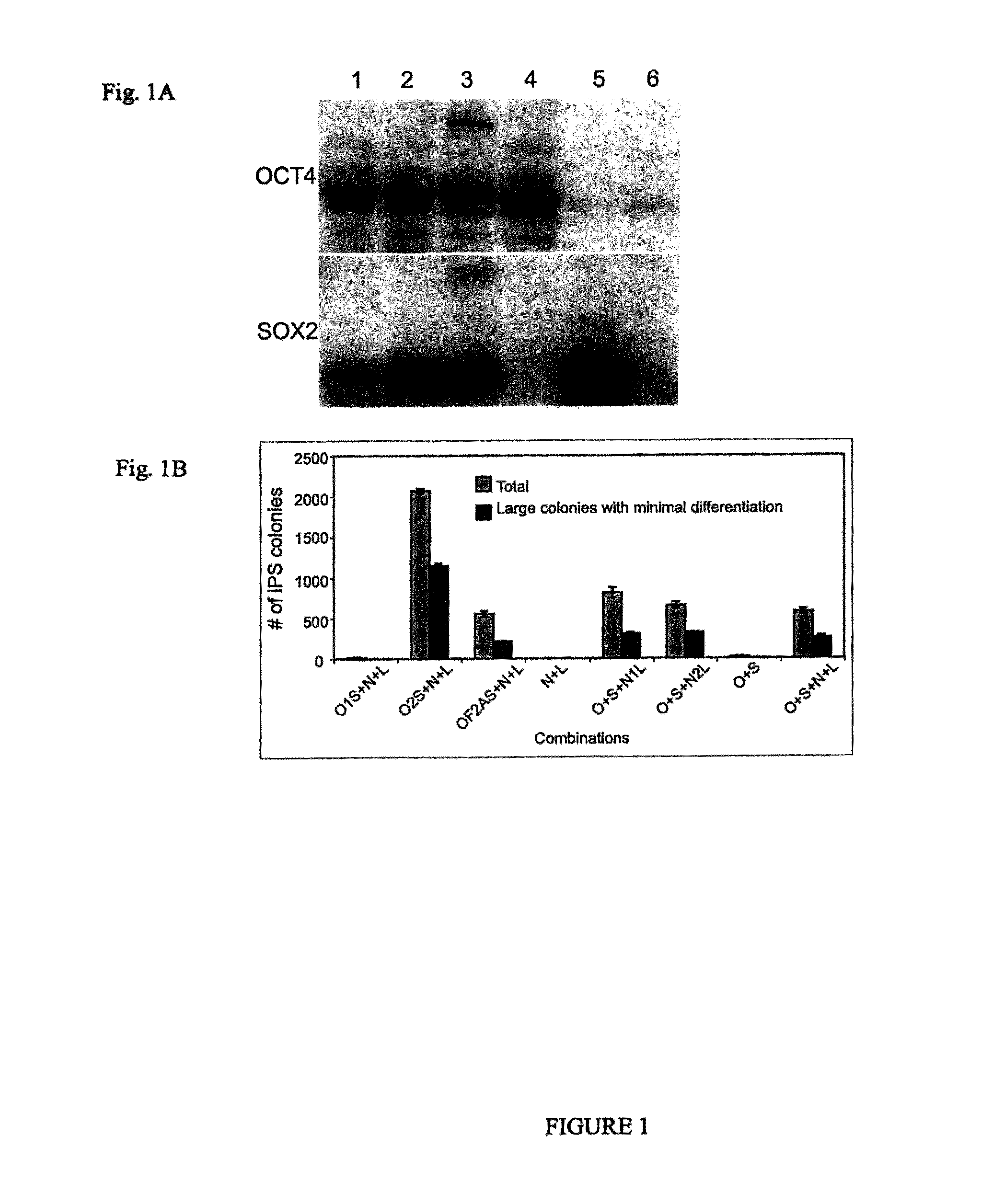
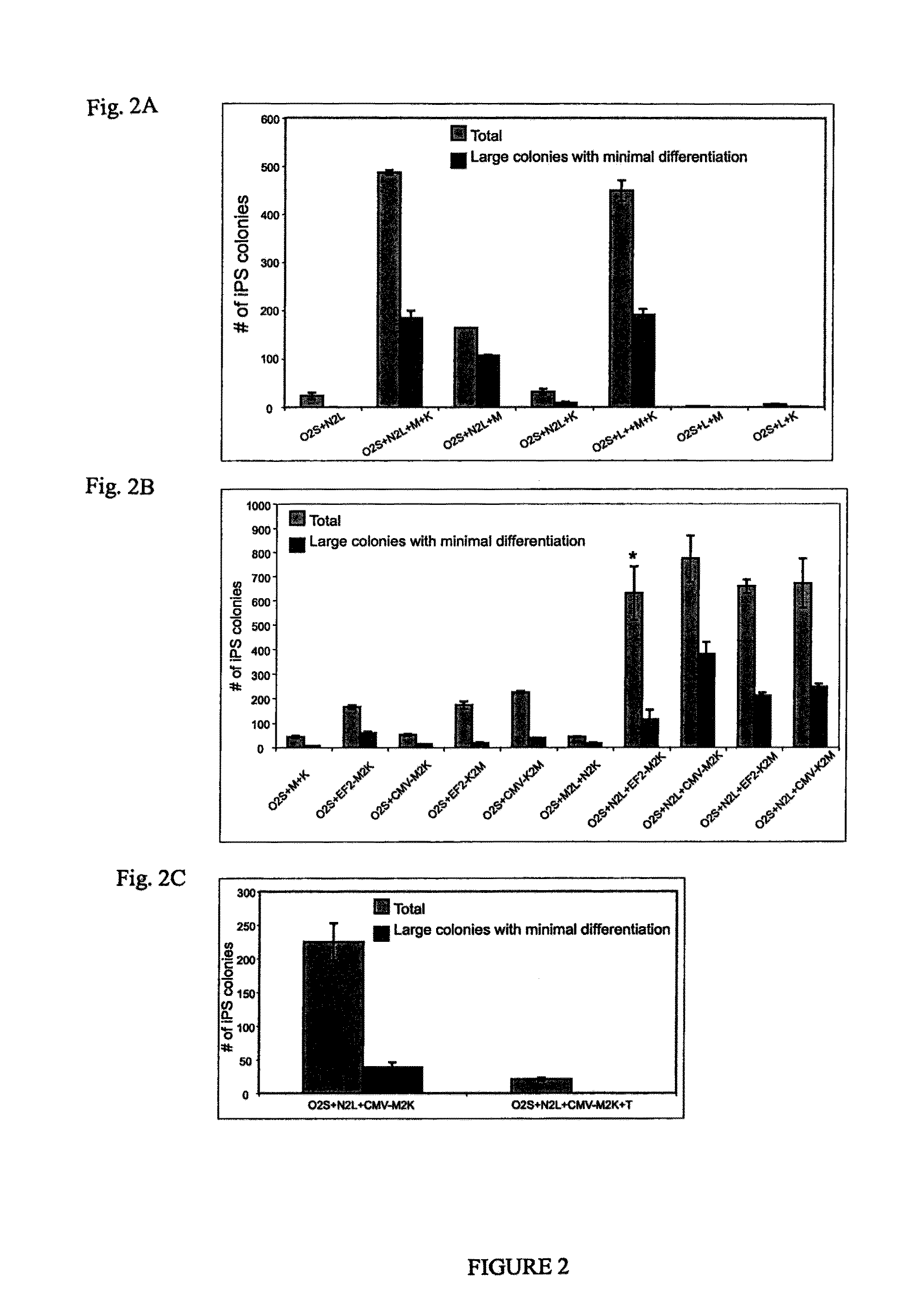
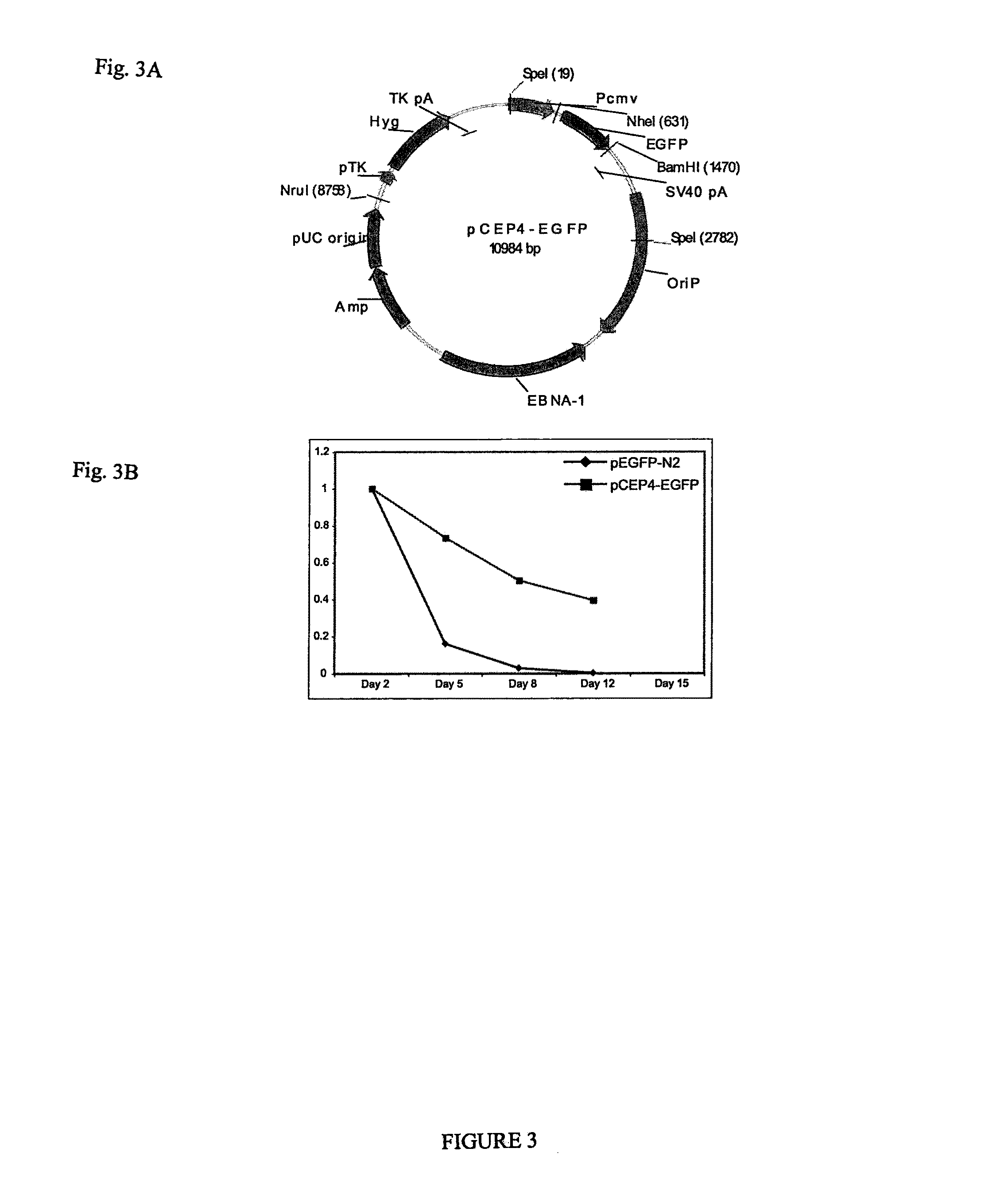
Pluripotent stem cells obtained by non-viral reprogramming
ActiveUS20100184227A1Genetically modified cellsArtificial cell constructsInduced pluripotent stem cellInfectious virus
Methods for reprogramming primate somatic cells to pluripotency using an episomal vector that does not encode an infectious virus are disclosed. Pluripotent cells produced in the methods are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
OCT4 and SOX2 with SV40 T antigen produce pluripotent stem cells from primate somatic cells
Methods for reprogramming primate somatic cells to pluripotency using an episomal vector that does not encode an infectious virus are disclosed. Pluripotent cells produced in the methods are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
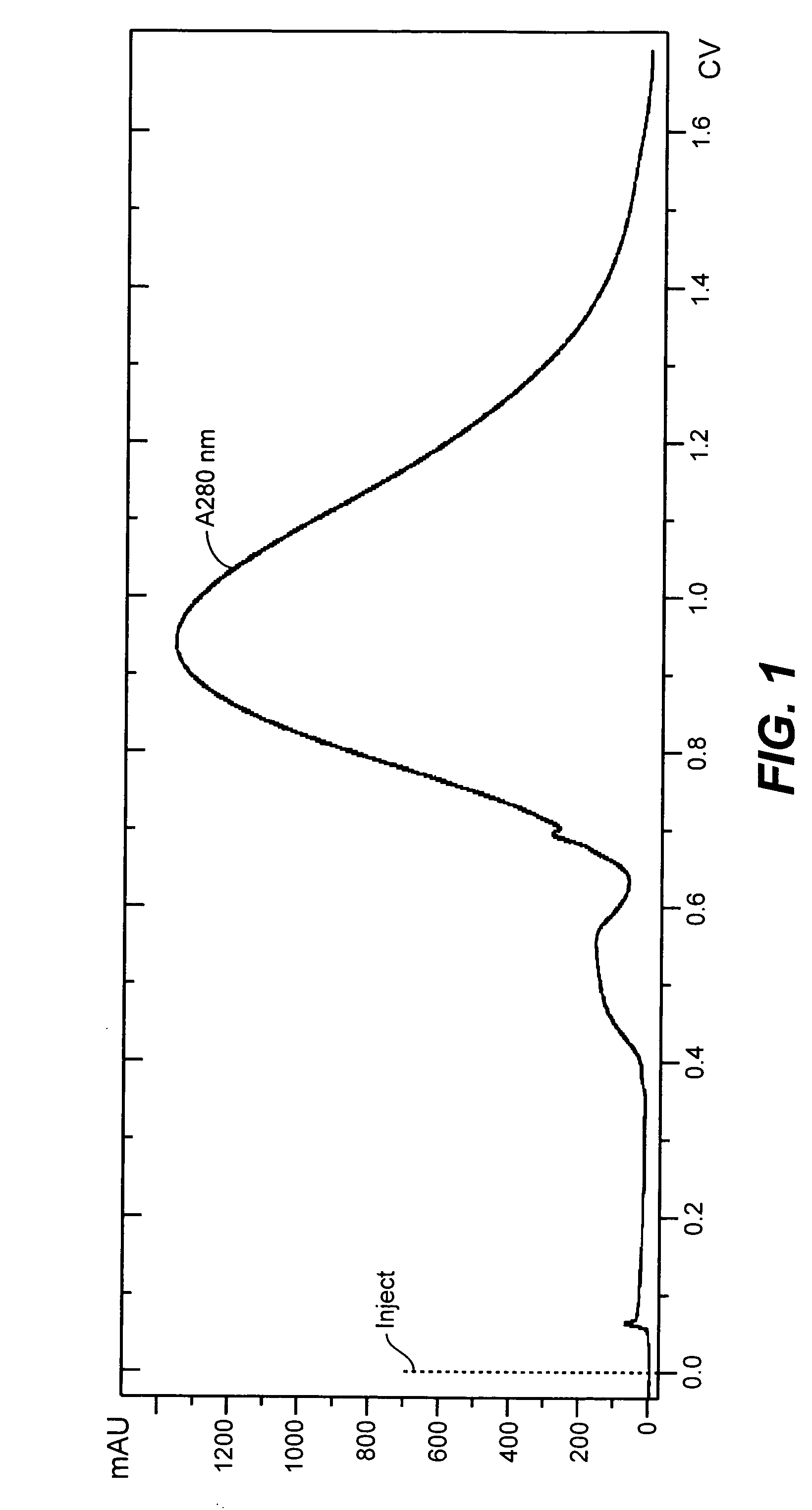
Viral purification methods
The present invention is directed to an improved method of purifying virus, particularly reovirus. Infectious virus can be extracted from a cell culture with a detergent to produce high titers of virus, and the virus can then be purified by simple steps such as filtration and column chromatography. Viruses and compositions comprising the viruses prepared according to the present invention are also provided.
Owner:ONCOLYTICS BIOTECH
Viral purification methods
ActiveUS7223585B2Simple methodViral antigen ingredientsGenetic material ingredientsPurification methodsFiltration
The present invention is directed to an improved method of purifying virus, particularly reovirus. Infectious virus can be extracted from a cell culture with a detergent to produce high titers of virus, and the virus can then be purified by simple steps such as filtration and column chromatography. Viruses and compositions comprising the viruses prepared according to the present invention are also provided.
Owner:ONCOLYTICS BIOTECH
Method of extracting virus from cell culture
InactiveUS7186542B2High virus titersConveniently performedViral antigen ingredientsMicroorganism based processesInfectious virusBiomedical engineering
The present invention is directed to a method of extracting virus, particularly reovirus, from a culture of cells. Infectious virus can be extracted from the culture with a detergent at a convenient temperature such as 25° C. or 37° C. to produce high virus titers. Both ionic and non-ionic detergents can be used in the present invention.
Owner:ONCOLYTICS BIOTECH
Recombinant virus vectors
InactiveUS6319703B1Risk minimizationReduced zero riskBiocidePeptide/protein ingredientsInfected cellCytopathic effect
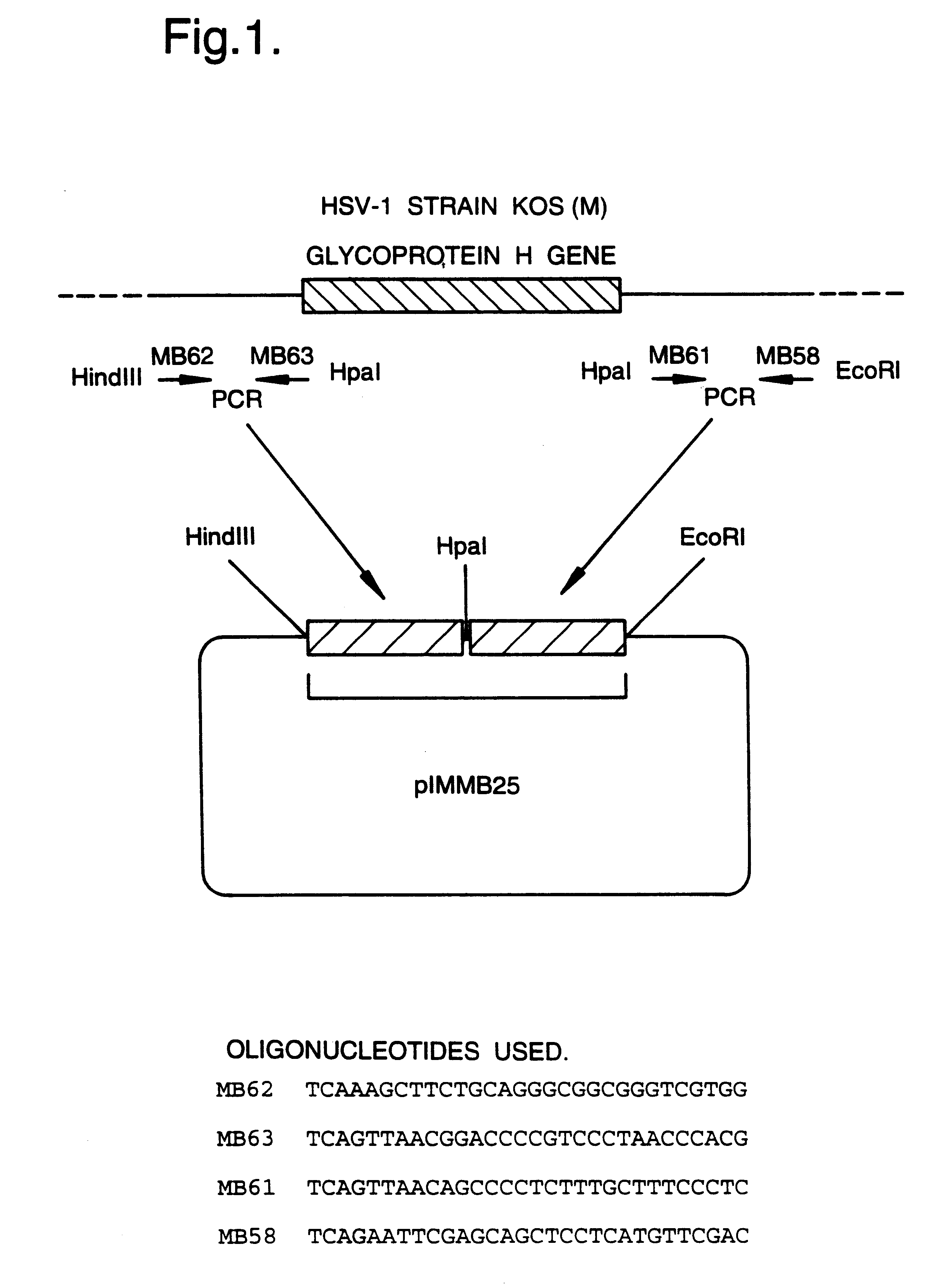
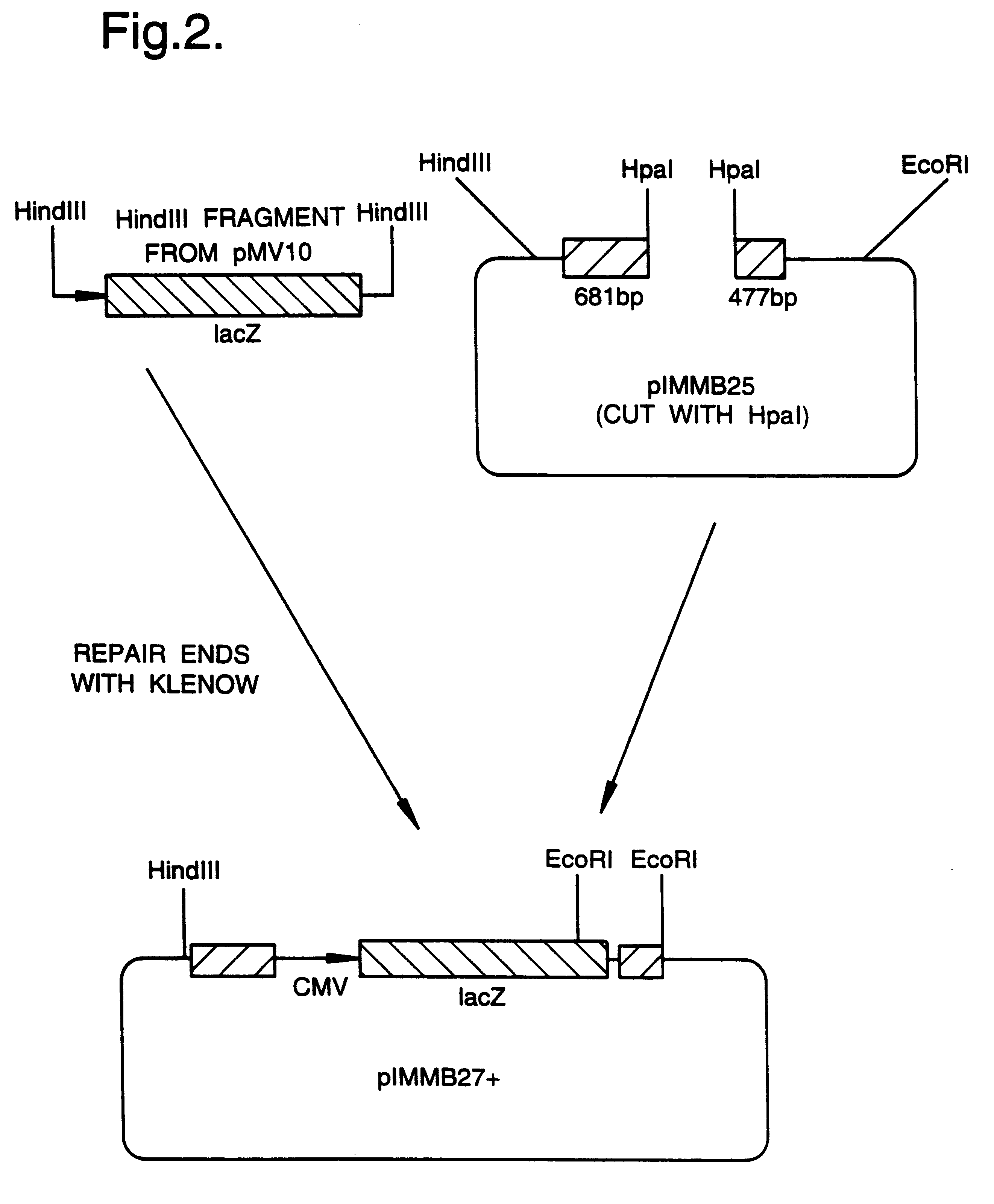
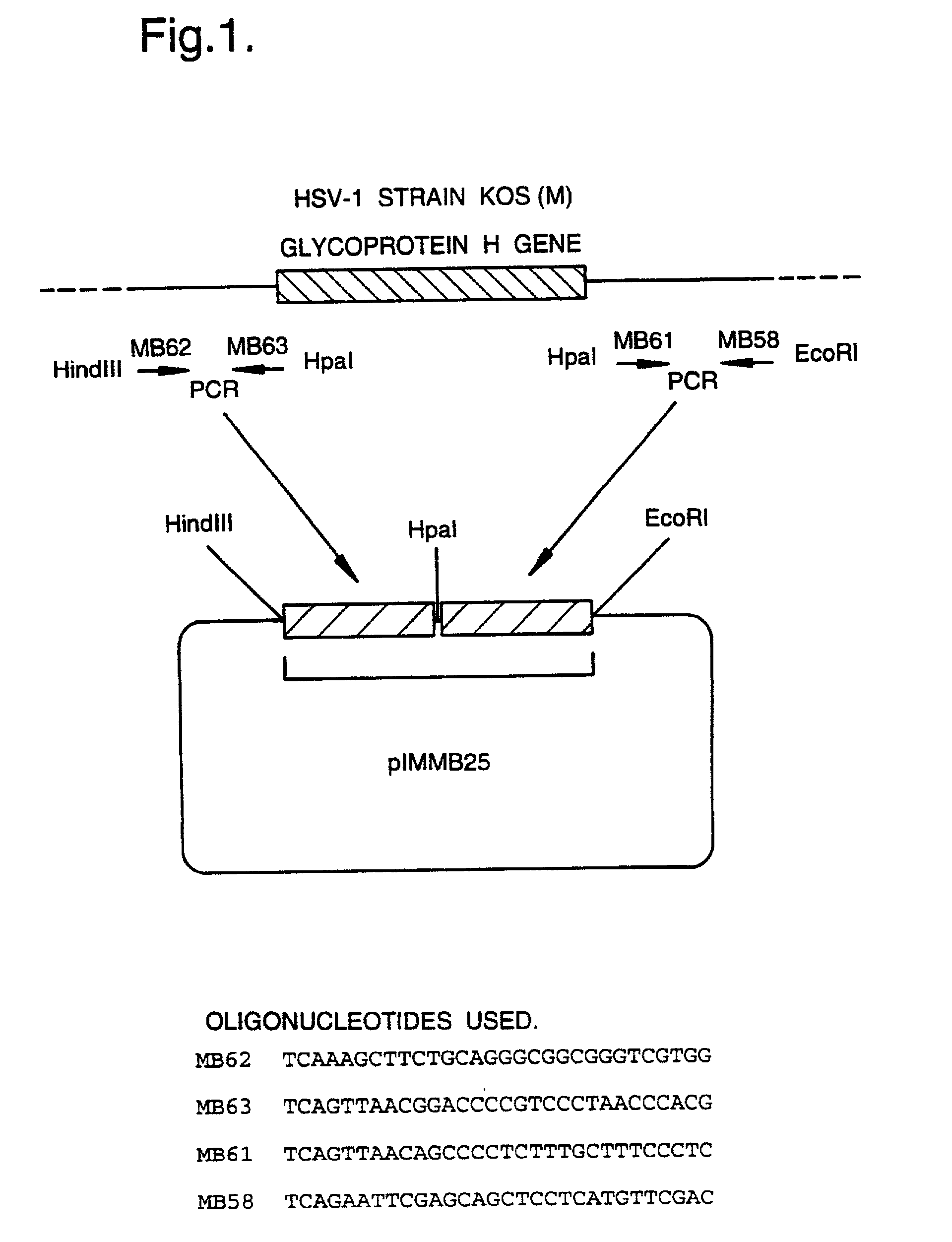
A mutant herpesvirus that can be used a recombinant virus vector includes (a) a mutation such that the mutant virus has a reduced ability in comparison with a parent type to cause lysis of an infected cell, and (b) an inactivating mutation in a gene essential for the production of infectious virus. An example is a HSV1 mutant lacking the essential glycoprotein gH gene and having a mutation impairing the function of the gene product VP16. A heterologous gene can be carried at the site of the inactivated essential gene, e.g. a gene suitable for administering gene therapy. The vector has an increased margin of safety over known herpesvirus vectors in respect of incidence of cytopathic effects and / or risk of infection.
Owner:SPECK PETER G
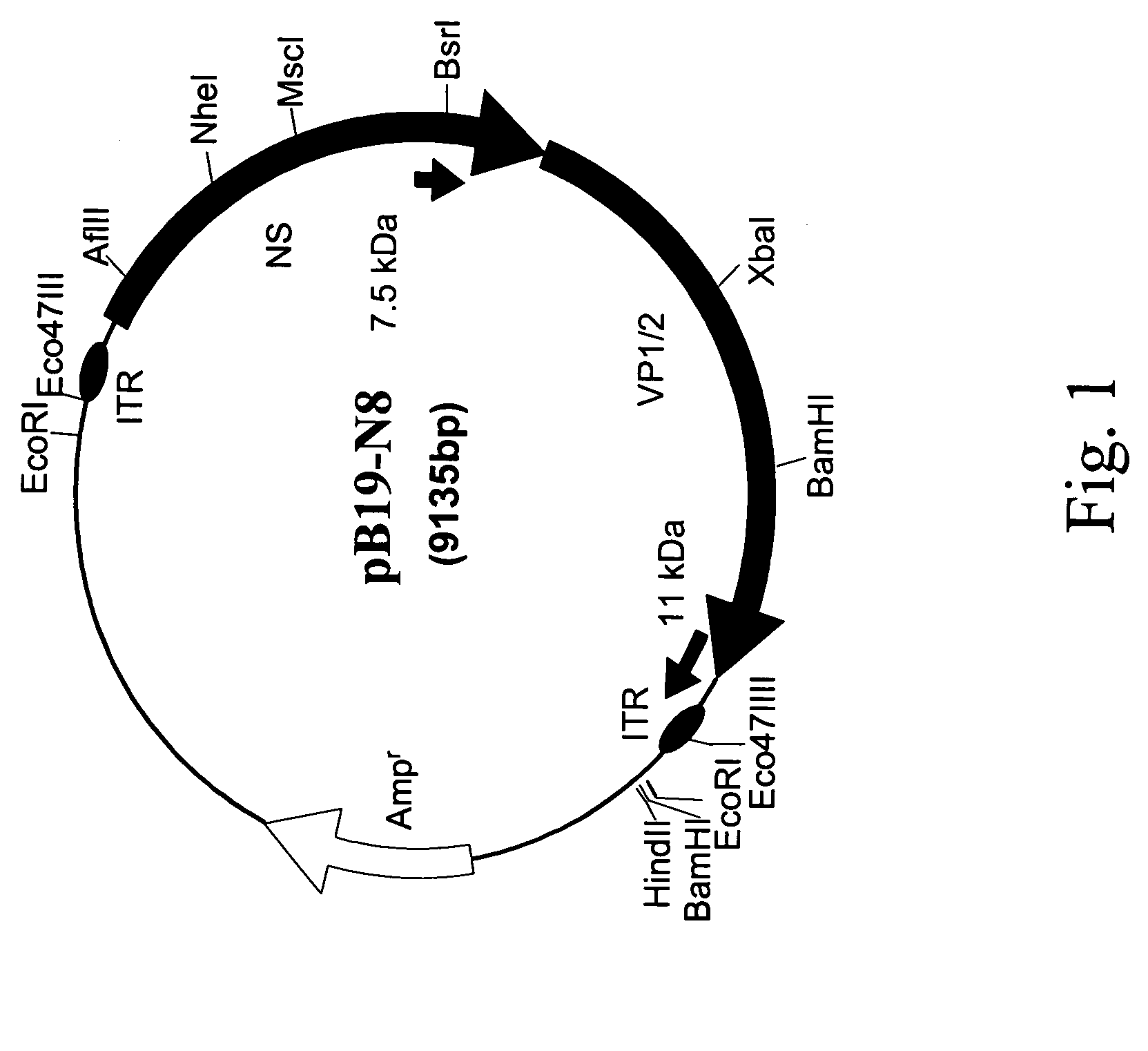
Infectious clone of human parvovirus B19 and methods
ActiveUS20060008469A1Reduce vector sizeEliminate undesired restriction siteSugar derivativesViral antigen ingredientsInfectious virusParvovirus B19 Infections
The invention relates to infectious clones of parvovirus B19, methods of cloning infectious B19 clones, and methods of cloning viral genomes that have secondary DNA structures that are unstable in bacterial cells. A B19 infectious clone and methods of producing B19 infectious clones are useful for producing infectious virus. Infectious virus is useful for identifying and developing therapeutically effective compositions for treatment and / or prevention of human parvovirus B19 infections.
Owner:INSTITUT NAT DE RECH SCI +1
Combination of transplantation and oncolytic virus treatment
InactiveUS20050214266A1Assess effectGuaranteed accuracyBiocideGenetic material ingredientsInfectious virusPre treatment
Oncolytic viruses can be used to purge cellular compositions to remove undesired neoplastic cells before the cellular compositions are used for transplantation. The present invention relates to the use of a virus to pre-treat a subject prior to delivery into the subject a transplant that has been purged with the same virus. This pre-treatment serves to elicit an immune response in the subject against the virus, thereby protecting the subject from infections by the virus after receiving the transplant, which likely contains infectious viruses.
Owner:ONCOLYTICS BIOTECH
Viral Purification Methods
Owner:ONCOLYTICS BIOTECH
Electronic cigarette with antibacterial layer cigarette holder
InactiveCN103504481AImprove the bactericidal effectProtection of rights and interestsAntibacterial agentsTobacco devicesElectronic cigaretteInfectious virus
The invention belongs to the technical field of electronic cigarettes, and particularly relates to an electronic cigarette holder with an antibacterial layer. According to the technical scheme, a food-grade antibacterial agent for the infectious disease of digestive tract and the respiratory infectious virus or pathogenic bacteria wraps the electronic cigarette holder, and a medical slow release formulation is mixed in the antibacterial agent. The antibacterial layer of the cigarette holder specially conducts killing on the infectious disease of digestive tract and the respiratory infectious virus or pathogenic bacteria, wherein the infectious disease of digestive tract and the respiratory infectious virus or pathogenic bacteria are easily bred on residual saliva. The antibacterial agent can be appropriately and slowly released, the aim of timely killing the virus or the pathogenic bacteria can be achieved, and meanwhile the electronic cigarette holder has the better economical efficiency.
Owner:HONGTA TOBACCO GRP
Method for Producing Viral Vaccines
ActiveUS20090060950A1Reduce in quantityReduced responseSsRNA viruses negative-senseViral antigen ingredientsInfectious virusViral Vaccine
The present invention provides a method for the manufacture of a preparation comprising virus antigens comprising a) inoculation of cells with infectious virus in a fluid, b) propagation of said virus in said cells, c) collecting said propagated virus, d) inactivating said collected virus, and e) treating said inactivated virus with a detergent, resulting in a preparation comprising viral antigens.
Owner:RESILIENCE GOVERNMENT SERVICES INC
Composition for treating hbv infection
ActiveUS20120251569A1Good curative effectAvoid infectionFungiVirusesHepatitis B immunizationInfectious virus
The present invention provides a composition comprising hepatitis B virus (HBV) component(s), and which may be either nucleic acid- or polypeptide-based as well as nucleic acid molecules and vectors encoding such HBV component(s). It also relates to infectious viral particles and host cells comprising such nucleic acid molecules or vectors. It also provides composition and kits of parts comprising such nucleic acid molecules, vectors, infectious viral particles or host cells and the therapeutic use thereof for preventing or treating HBV infections.
Owner:TRANSGENE SA
Efficient cell culture system for hepatitis C virus genotype 5A
Owner:HVIDOVRE HOSPITAL
Method for detecting FMDV (Foot and Mouth Disease Virus) through real-time fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction)
InactiveCN102220436AReduce pollutionGuaranteed inspection resultsMicrobiological testing/measurementMicroorganism based processesInfectious virusReverse transcription polymerase chain reaction
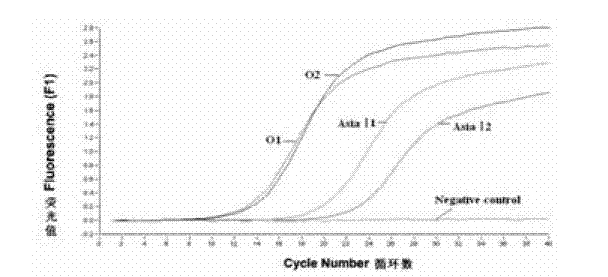
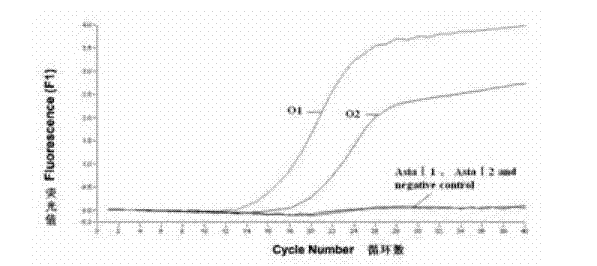
The invention discloses a method for detecting an FMDV (Foot and Mouth Disease Virus) group and O type and AsiaI type thereof through real-time fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction). By carrying out specificity and sensitivity verification on the method established by the invention, the result shows that the FMDV can be well set apart from other Artiodactyla infectious viruses as well as O type and AsiaI type, and the sensitivity can be up to 10 copies of a template. The method established by the invention is characterized in that the whole reaction process is a closed pipe operation, thereby maximally reducing the pollution to the system. The whole process sequentially including the acquisition of a sample to be detected, the extraction of nucleic acid, the preparation of a fluorescent quantitative RT-PCR system and the representation of a reaction result can be completed within 2.5 hours, and the time required for detection is greatly shortened on the premise of ensuring the detection result.
Owner:珠海出入境检验检疫局检验检疫技术中心
Recombinant virus vectors
InactiveUS20020037575A1Free from riskUseful persistenceBiocidePeptide/protein ingredientsHeterologousInfected cell
A mutant herpesvirus that can be used as a recombinant virus vector comprises (a) a mutation such that the mutant virus has a reduced ability in comparison with a parent type to cause lysis of an infected cell, and (b) an inactivating mutation in a gene essential for the production of infectious virus. An example is a HSV1 mutant lacking the essential glycoprotein gH gene and having a mutation impairing the function of gene product VP16. A heterologous gene can be carried at the site of the inactivated essential gene, e.g. a gene suitable for administering gene therapy. The vector has an increased margin of safety over known herpesvirus vectors in respect of incidence of cytopathic effects and / or risk of reversion.
Owner:SPECK PETER G
Novel dustproof, antibacterial and antiviral nano-fiber mask
ActiveCN111235756AEasy to stay dryIsolation erosionElectro-spinningNon-woven fabricsMicrosphereEngineering
The invention belongs to the technical field of ultrafiltration materials and sanitary protection, and particularly relates to a novel dustproof, antibacterial and antiviral nano-fiber mask. The maskcomprises a mask main body, and is characterized in that the mask main body sequentially comprises a non-woven fabric layer, a multi-layer drug-carrying composite fiber membrane layer and a non-wovenfabric layer from inside to outside, wherein the multi-layer drug-carrying composite fiber membrane layer comprises at least three layers, the inner layer and the outer layer are melt blown layers orelectrostatic spun fiber membrane layers, and the middle layer is a loaded drug-carrying nano microsphere fiber membrane layer. The multi-layer drug-carrying composite fiber membrane layer used in themask can enhance the slow-release performance and the anti-virus and antibacterial properties of a drug through multiple mesoporous structures formed by drug-carrying nano microspheres and differentmaterials, the function of filtering particles and smell in air is enhanced, and the mask has relatively strong killing and inhibiting effects on public health safety, respiratory systems and infectious viruses.
Owner:广东云曌医疗科技有限公司
Inactivated vaccines for aids and other infectious diseases
InactiveUS7033813B2Bioreactor/fermenter combinationsBiological substance pretreatmentsManufacturing technologyBACTERIAL INFECTIOUS DISEASES
Presented herein is a description for the manufacturing of inactivated HIV for use in vaccines against AIDS, as well as other inactivated viruses for other infectious diseases. This invention incorporates methods for inactivating infectious virus particles while retaining protein integrity and antigenicity. The methods utilize critical, near-critical or supercritical fluids with or without polar cosolvents. This invention would allow for the creation of HIV vaccines from genetically attenuated HIV strains for a greater degree of product safety, and from combinations of different HIV strains for broader protection. This HIV vaccine manufacturing technology is inexpensive, amenable to large-scale processing and portable, i.e. it can be readily implemented in a host country site. This invention can be utilized for other viral and bacterial infectious diseases, such as influenza and hepatitis.
Owner:APHIOS
Screening tool for antiviral agents
A method is provided for screening anti-adenovirus agents. The method includes reducing the activation of the immune system of a small mammal, administering a human adenovirus vector to the small mammal, monitoring the tumor cells in the mammal, and analyzing infectious virus units within the tumor cells and the organs of the small mammal. Specifically, the immune system of the small mammal is suppressed using cyclophosphamide. The small mammal may be, but is not limited to, one of the following: mice, rabbits, cotton rats, hamsters, rats, and other small rodents.
Owner:SAINT LOUIS UNIVERSITY
Nucleobase phosphonate analogs for antiviral treatment
ActiveUS20050059637A1Increasing cellular accumulationImprove retentionBiocideOrganic active ingredientsHigh concentrationAcquired immunodeficiency
The present invention provides novel compounds with activity against infectious viruses. The compounds of the invention may inhibit retroviral reverse transcriptases and thus inhibit the replication of the virus. They are useful for treating human patients infected with a human retrovirus, such as human immunodeficiency virus (strains of HIV-1 or HIV-2) or human T-cell leukemia viruses (HTLV-I or HTLV-II) which results in acquired immunodeficiency syndrome (AIDS) and / or related diseases. The present invention also relates generally to the accumulation or retention of therapeutic compounds inside cells. The invention is more particularly related to attaining high concentrations of active metabolite molecules in HIV infected cells. Intracellular targeting may be achieved by methods and compositions which allow accumulation or retention of biologically active agents inside cells. Such effective targeting may be applicable to a variety of therapeutic formulations and procedures.
Owner:GILEAD SCI INC
Composition for treating hbv infection
ActiveUS20130011435A1Promote productionEasy to purifyBacteriaVirus peptidesHenipavirus InfectionsCyrtanthus elatus virus A
The present invention provides a composition comprising hepatitis B virus (HBV) component(s), and which may be either nucleic acid- or polypeptide-based as well as nucleic acid molecules and vectors encoding such HBV component(s). It also relates to infectious viral particles and host cells comprising such nucleic acid molecules or vectors. It also provides composition and kits of parts comprising such nucleic acid molecules, vectors, infectious viral particles or host cells and the therapeutic use thereof for preventing or treating HBV infections.
Owner:TRANSGENE SA
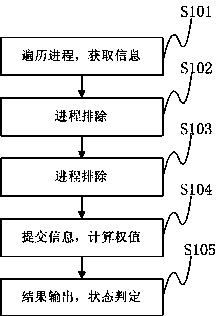
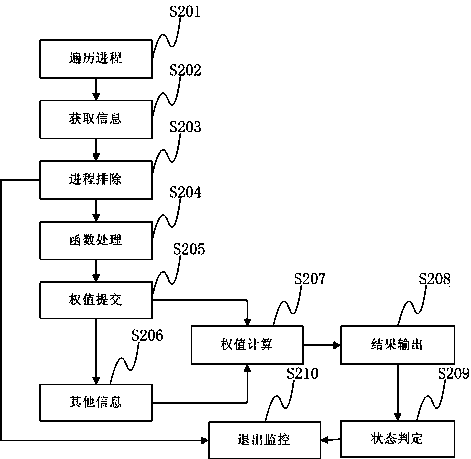
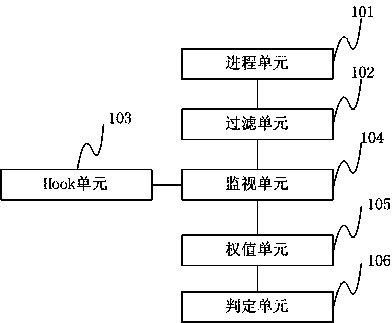
Method and device for preventing infectious virus infection
ActiveCN103353930AReduce monitoringGuaranteed operating efficiencyPlatform integrity maintainanceNumerical rangeMonitoring system
The invention discloses a method for preventing the infectious virus infection, which comprises the following steps: traversing all processes of a current system and processes newly created by a monitoring system; acquiring all process information in the systems, and filtering the processes during the operation; carrying out hook processing on functions in a security policy, and recording or duplicating relevant document information of the functions before invoking; monitoring the process after filtering, carrying out weighting processing on the information of the invoked function, and carrying out the weighting processing on document information related with the processes after finishing the processes; calculating the sum of the weight of the function information of the progresses and the document information, and outputting the judgment result of the progresses of infectious malicious codes corresponding to the sum according to the given numerical range in the security policy. The invention further discloses a device for preventing infectious virus infection. The method and the device can monitor the operation of the infectious malicious codes, judge the operation schedule of the malicious codes according to the monitoring of the operation state of the infectious malicious codes, and recover the infected document information.
Owner:BEIJING ANTIY NETWORK SAFETY TECH CO LTD
Reverse Genetics System
The complete genomic sequence of maize fine streak virus (MFSV), a negative-strand RNA virus that infects plants and insects, is disclosed. The inventors have characterized the MFSV genome and identified the leader and trailer sequences, seven open reading frames as well as the functions of some of the encoded proteins, and the gene junction sequences and their functions. Using various functionally important components of the MFSV genome, the inventors have demonstrated that a reverse genetics system of using cloned cDNA to produce infectious viruses can be developed for plant negative-strand RNA viruses. Methods of using the reverse genetic system to produce wild-type or recombinant plant negative-strand RNA viruses, to express genes of interest, and to screen for agents that may affect the production and function of the viruses are disclosed. Further disclosed are various nucleic acid constructs, vectors, genetically engineered cells, and kits useful in the reverse genetics system.
Owner:WISCONSIN ALUMNI RES FOUND
Primer set and kit for quickly detecting various melon viruses
InactiveCN101906485AQuick checkThe detection is accurate, simple and economicalMicrobiological testing/measurementDNA/RNA fragmentationInfectious virusMelon (food)
The invention relates to a primer set and a kit for quickly detecting various melon viruses. By applying specificity primer pairs SEQ ID NO.1-10 designed according to nucleotide conserved region sequences of 5 viruses, a multiple RT-PCR system is optimized in aspects such as primer concentration, Mg2+ concentration, Taq DNA polymerase concentration, dNTPs concentration, annealing temperature and the like which influence multiple RT-PCR (M-PCR) amplification, and the primer set and a kit capable of simultaneously detecting field samples complexly infected by five viruses CMV, SqMV, TMV, WMV and ZYMV from watermelon leaf tissues are established. The primer set and the kit for detecting complex infectious viruses of melons have the advantages of saving time, reducing cost and improving efficiency.
Owner:NORTHWEST A & F UNIV
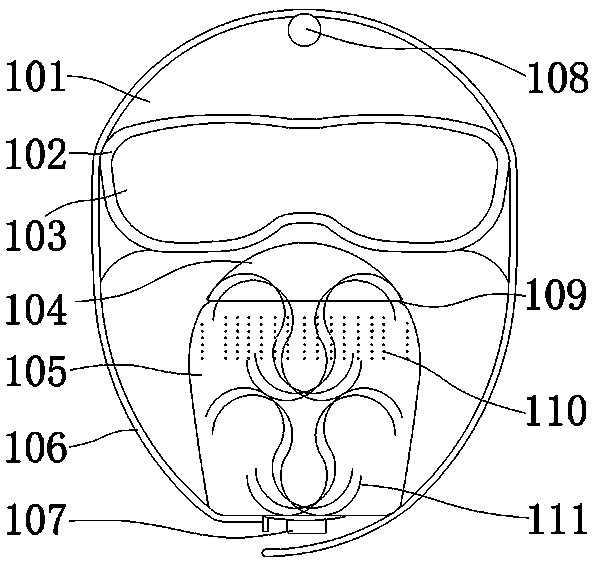
Air supply and ventilation multi-purpose protective mask and air supply device
The invention innovatively designs an air supply and ventilation protective mask. A miniature air supply fan is installed adopting a combined plug connection manner, and is externally connected with aportable mobile power supply to obtain electric power driving operation, the air in a mask shell is driven to flow, at the same time, the air impedance is overcome to the maximum extent, and multiple-layer or thickened air filters, namely the air inlet filter plate and the air outlet filter plate, is adapted to enhance the filtering protection function. Moreover, the mask shell does not directlycontact the face of a user, so that the user can breathe more smoothly and easily, moisture is taken away by the airflow, air filters away from the mouth nose of the user cannot be wetted, wearing ismore comfortable, and the protection is more safe and long-acting. According to the air supply and ventilation protective mask, by assembling an upper cover plate, a lower cover plate and goggles, theair filter plates are replaced and renewed to block the invasion of harmful air, substances or infectious viruses to the human body, and multiple safety and health protection are realized; and the air supply and ventilation protective mas can also be used by removing components such as the goggles, the air filters, sealing strips and the air supply fan. The air supply and ventilation protective mask has the characteristics of multi-scene application and reuse.
Owner:于卫华
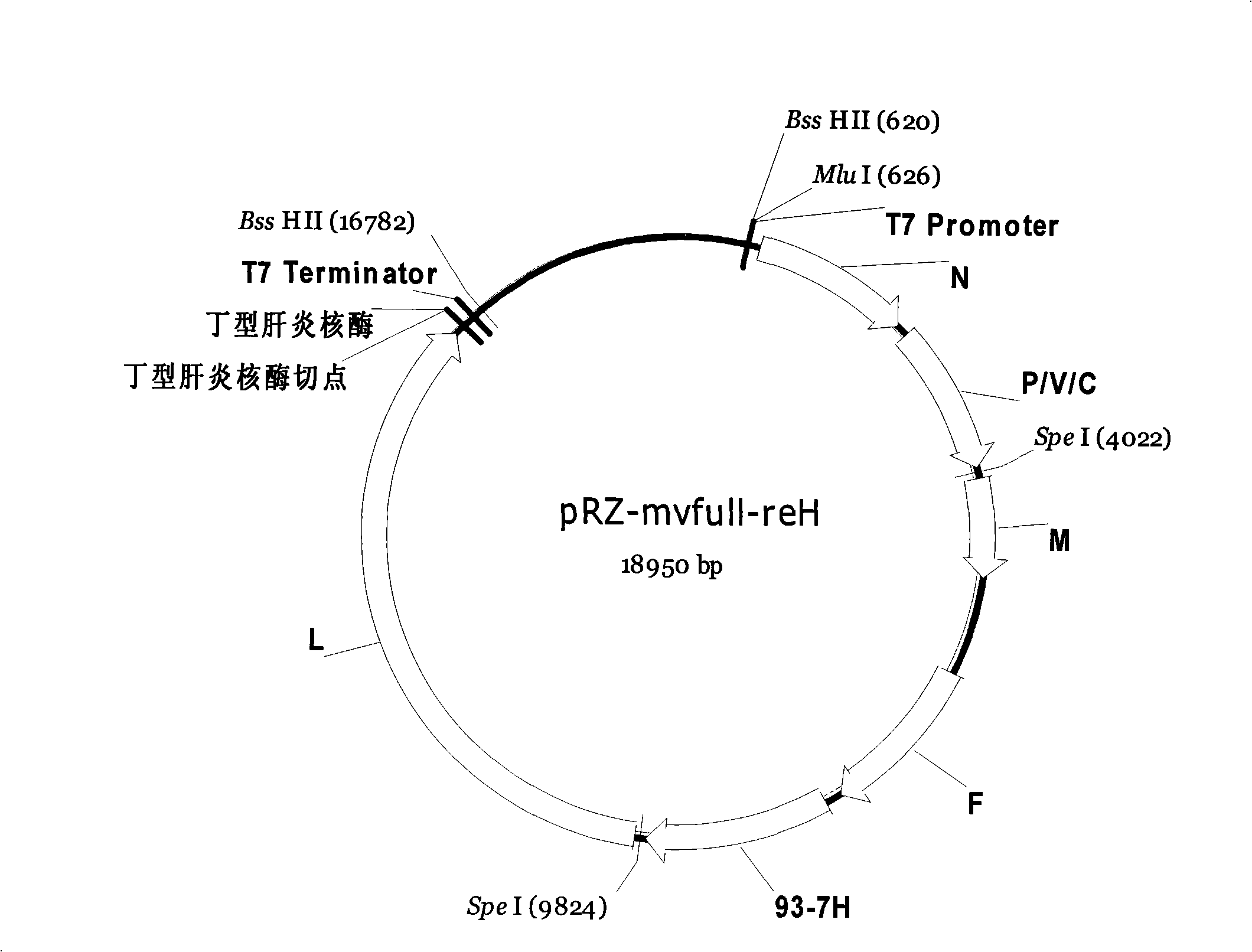
Modified measles virus whole gene cDNA clone and infectious virus preparation
InactiveCN101323857AHas development valueGood for Universal Vaccine ValueViruses/bacteriophagesGenetic engineeringHemagglutininGenotype
The invention provides an entire-gene cDNA clone of modified measles virus, which is obtained from the substitution and transformation of main surface antigen genes (H gene) of main existing epidemic strains (genotype of H1 and representative strain of China93-7) in China to the full-length cDNA clones of measles virus strain CC-47. The invention adopts the RNA virus reverse genetic technique to carry out the rescue of modified CC-47 virus on the cDNA level so as to obtain modified infective virus, thus providing great convenience for the research on the replication, the proliferation and the package of novel virus stains. In addition, the novel produced infective virus has the hemagglutinin antigen of an epidemic strain and has more suitable application value of the hoplivac.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Efficient cell culture system for hepatitis c virus genotype 5a
InactiveUS20110021611A1Organic active ingredientsSsRNA viruses positive-senseSequence analysisSerial passage
The present inventors developed 5a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 5a reference strain SA13. Compared to the J6 / JFH control virus, after transfection of in vitro transcripts in Huh7.5 cells, production of infectious viruses was delayed. However, in subsequent viral passages efficient spread of infection and HCV RNA titers as high as for J6 / JFH were obtained. Infectivity titers were at all time points analyzed comparable to J6 / JFH control virus. Sequence analysis of recovered 5a / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in p7, NS2 and / or NS3. Infectivity of the 5a / 2a viruses was CD81 and SR-BI dependant, and the recombinant viruses could be neutralized by chronic phase sera from patients infected with genotype 5a. Conclusion: The developed 5a / 2a viruses provide a robust in vitro tool for research in HCV genotype 5, including vaccine studies and functional analyses of an increasingly important genotype in South Africa and Europe.
Owner:HVIDOVRE HOSPITAL
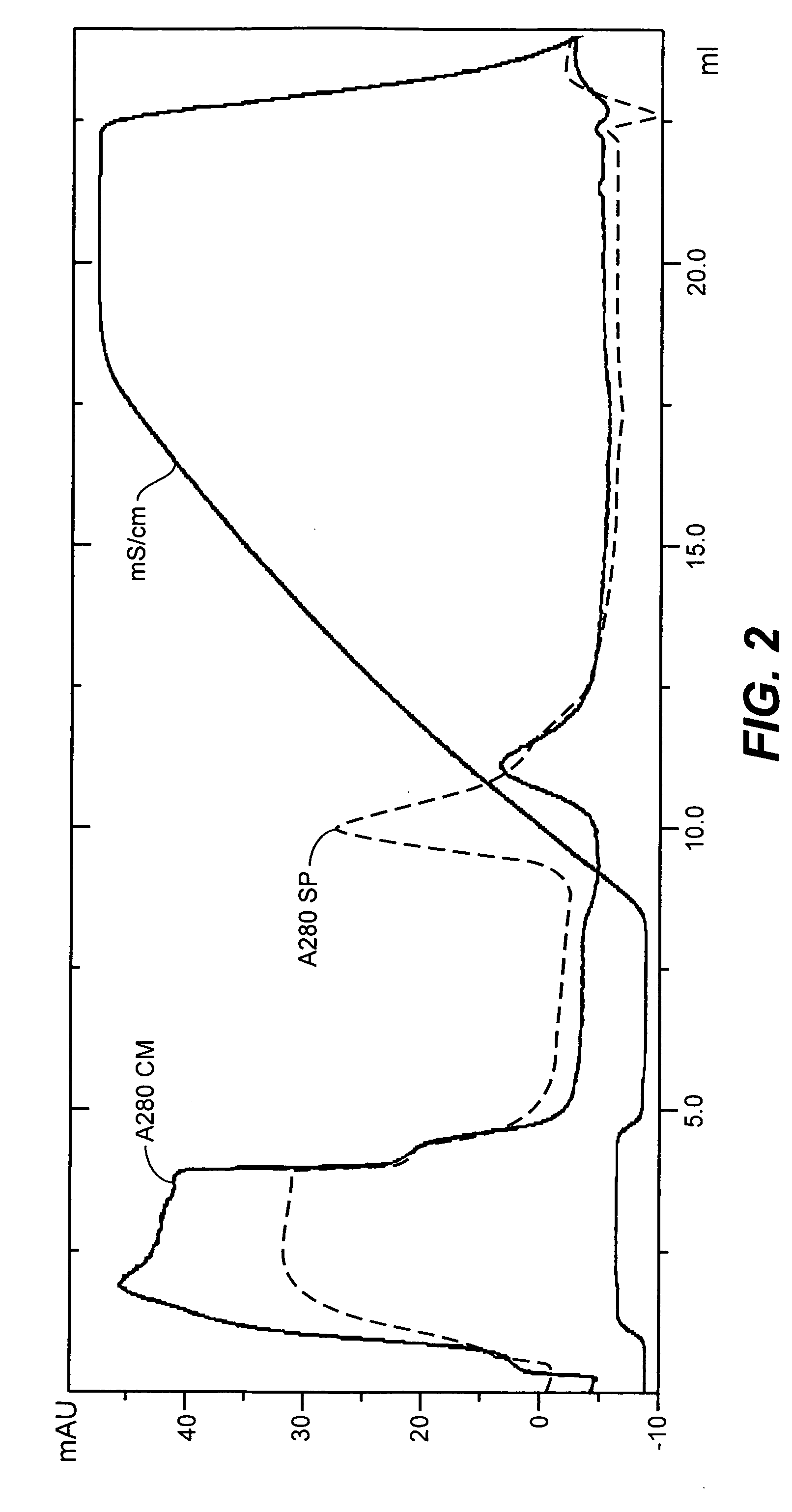
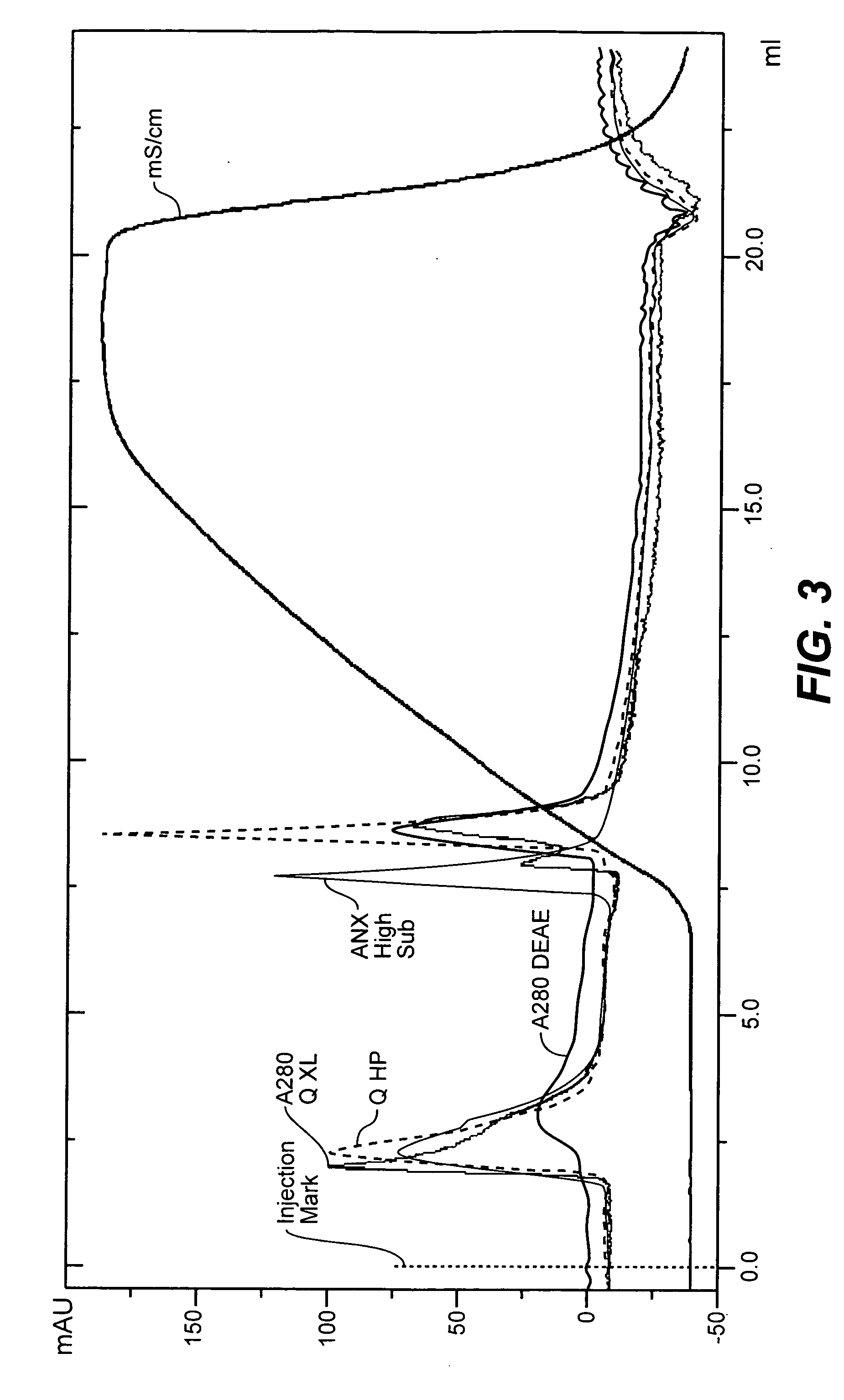
Chromatography based purification strategies for viruses
ActiveUS10894079B2SsRNA viruses negative-senseSsRNA viruses positive-senseMeasles virus IgGInfectious virus
The present invention provides purification strategies for sterically demanding, i.e. large and pleomorphic, infectious virus particles or VLPs derived therefrom, preferably having a measles virus scaffold to yield fractions or compositions with a significantly reduced content of contaminating host cell DNA and a reduced content of further process-related impurities. Further provided are methods of propagating and purifying infectious virus particles having a measles virus scaffold suitable to provide a preparation having a strongly reduced content of contaminating host cell DNA and a reduced content of further process-related impurities for immunogenic or anti-tumor purposes. In addition, immunogenic and vaccine compositions based on the above methods are provided. Finally, there are provided immunogenic or vaccine compositions produced by the disclosed methods, which are suitable for use in immunogenic or prophylactic vaccination treatment of a subject in need thereof.
Owner:MERCK SHARP & DOHME LLC
Method of extracting virus from cell culture
InactiveUS20050095692A1Increase temperatureHigh virus titersViral/bacteriophage medical ingredientsMicroorganism based processesInfectious virusTiter
The present invention is directed to a method of extracting virus, particularly reovirus, from a culture of cells. Infectious virus can be extracted from the culture with a detergent at a convenient temperature such as 25° C. or 37° C. to produce high virus titers. Both ionic and non-ionic detergents can be used in the present invention.
Owner:ONCOLYTICS BIOTECH
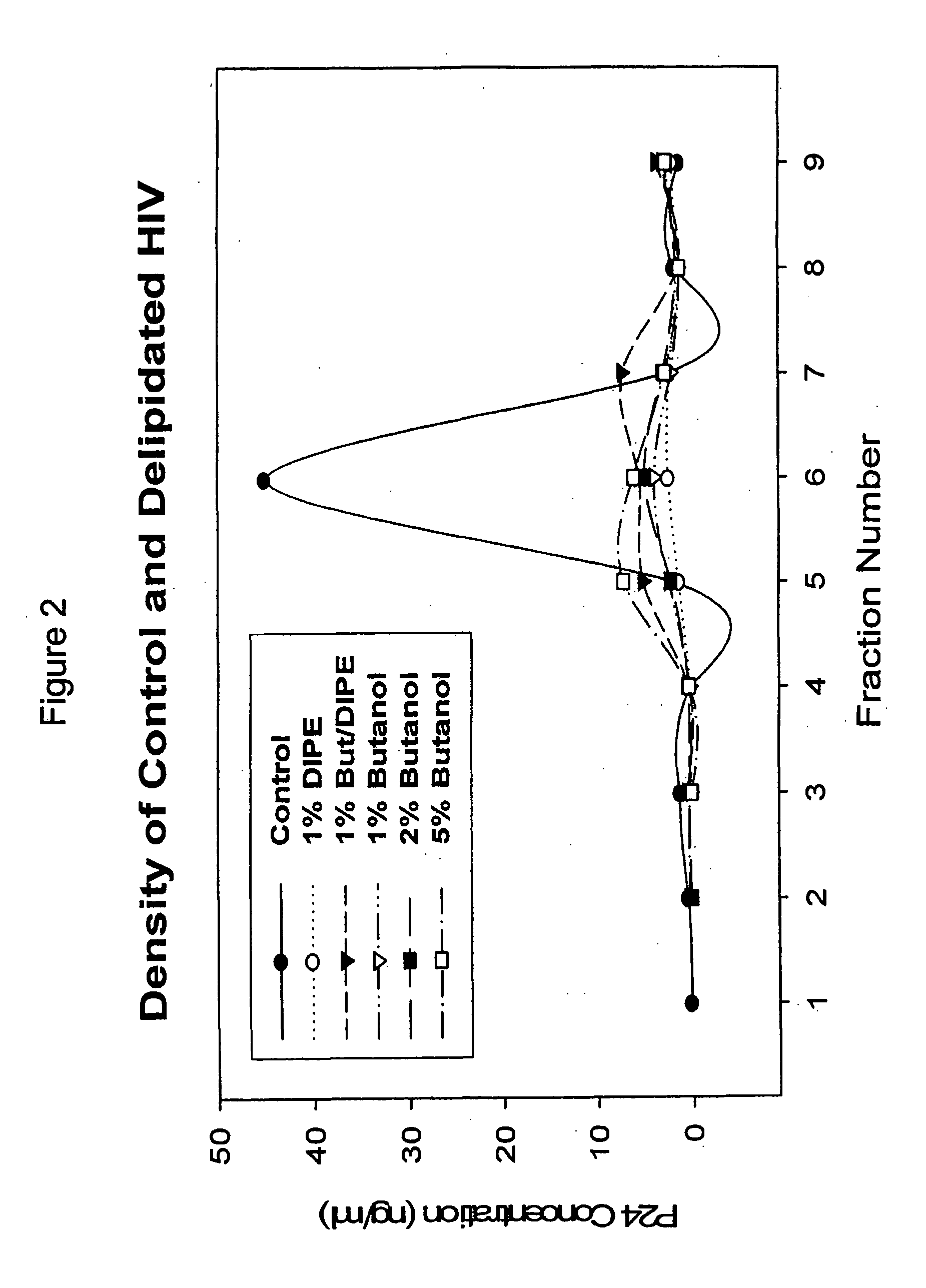
Modified viral particles with immunogenic properties and reduced lipid content useful for treating and preventing infectious diseases
InactiveUS20070031923A1The process is simple and effectivePositive immunologic responseSsRNA viruses positive-senseViral antigen ingredientsLipid formationViral Vaccine
Described is a composition and method for reducing the occurrence and severity of infectious diseases, especially infectious diseases in which lipid-containing infectious viral organisms are found in biological fluids, such as blood. The present invention employs solvents useful for extracting lipids from the lipid-containing infectious viral organism thereby creating immunogenic modified, partially delipidated viral particles with reduced infectivity. The present invention provides delipidated viral vaccine compositions, such as therapeutic vaccine compositions, comprising these modified, partially delipidated viral particles with reduced infectivity, optionally combined with a pharmaceutically acceptable carrier or an immunostimulant. The vaccine composition is administered to a patient to provide protection against the lipid-containing infectious viral organism or, in case of a therapeutic vaccine, to treat or alleviate infection against the lipid-containing infections viral organism. The vaccine compositions of the present invention include combination vaccines of modified viral particles obtained from one or more strains of a virus and / or one or more types of virus.
Owner:LIPID SCI +1
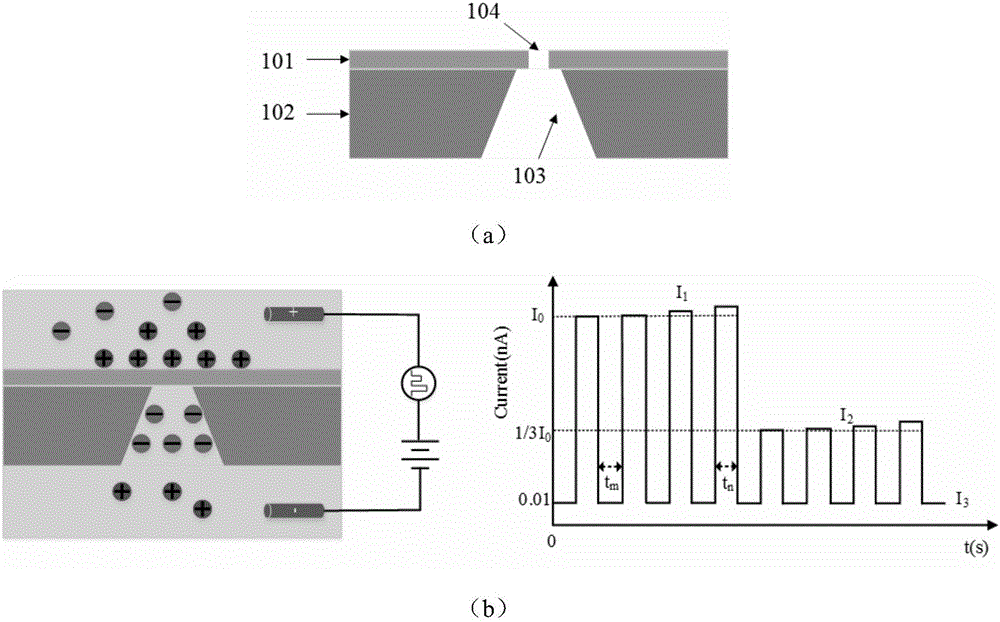
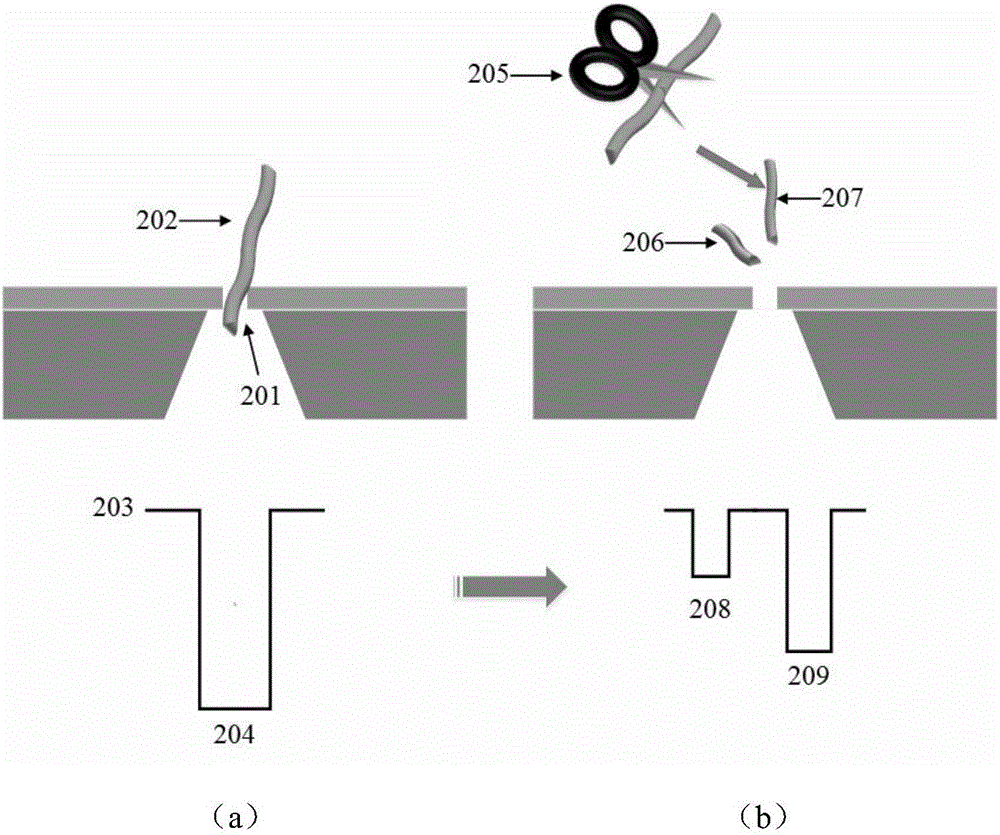
HIV-1 protease detection method based on solid state nanopore
InactiveCN106443008AJudgment activityJudgment concentrationBiological material analysisBiological testingProtein targetProtein precursor
Owner:重庆中科德馨生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com