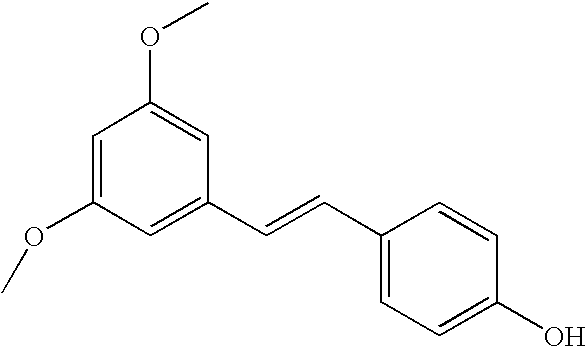
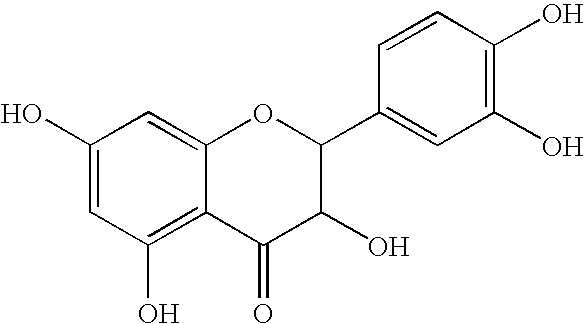
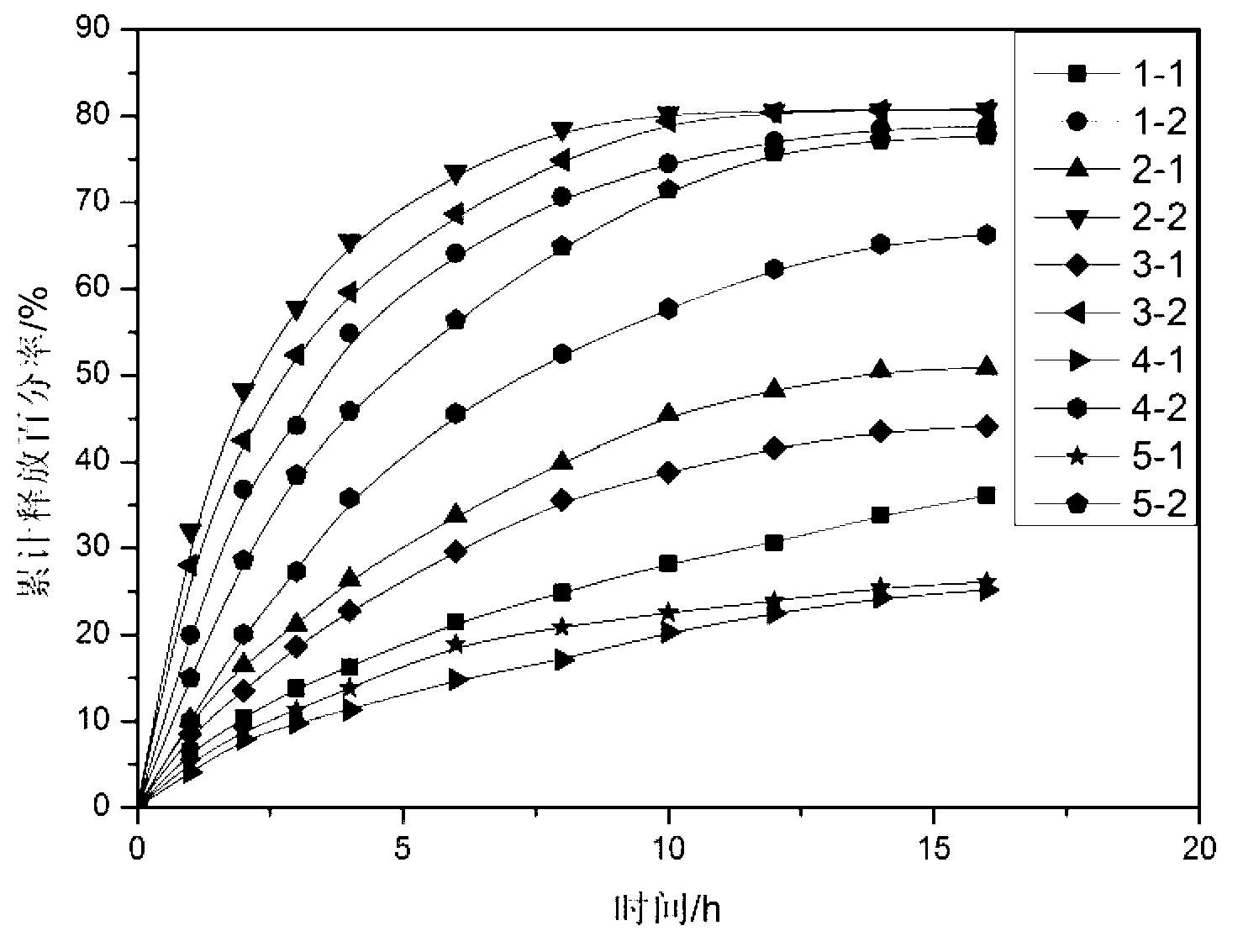
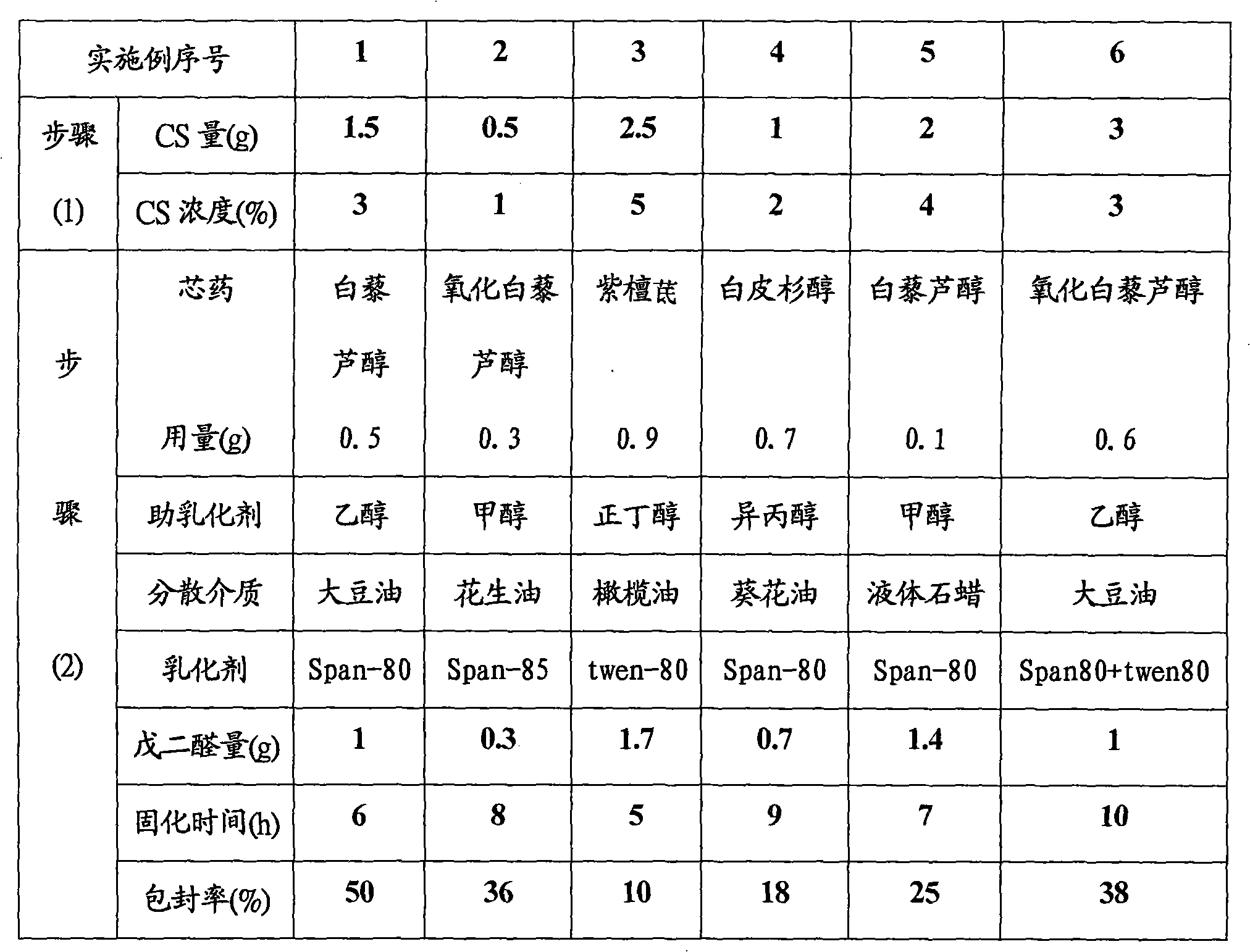
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
168 results about "Slow Release Formulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
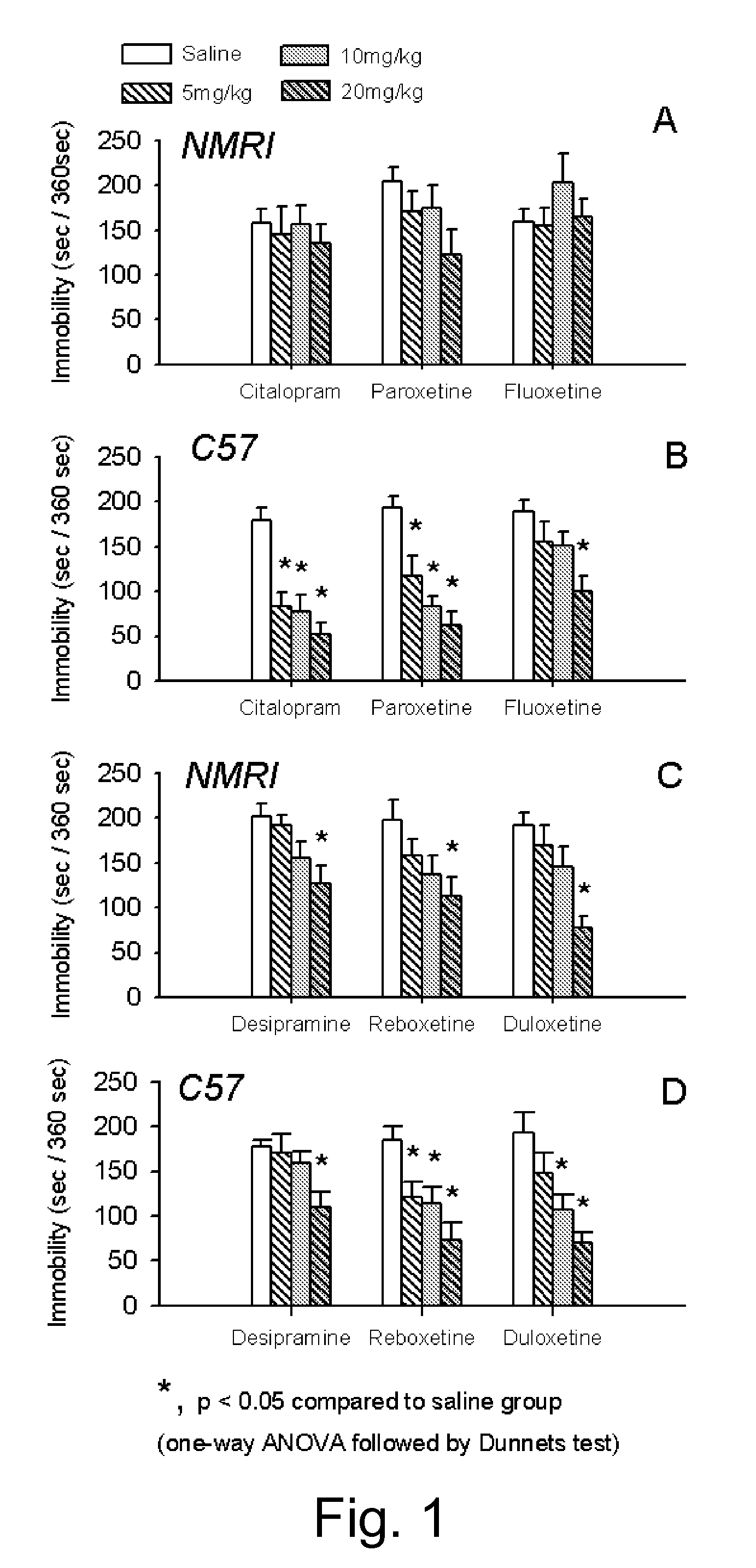
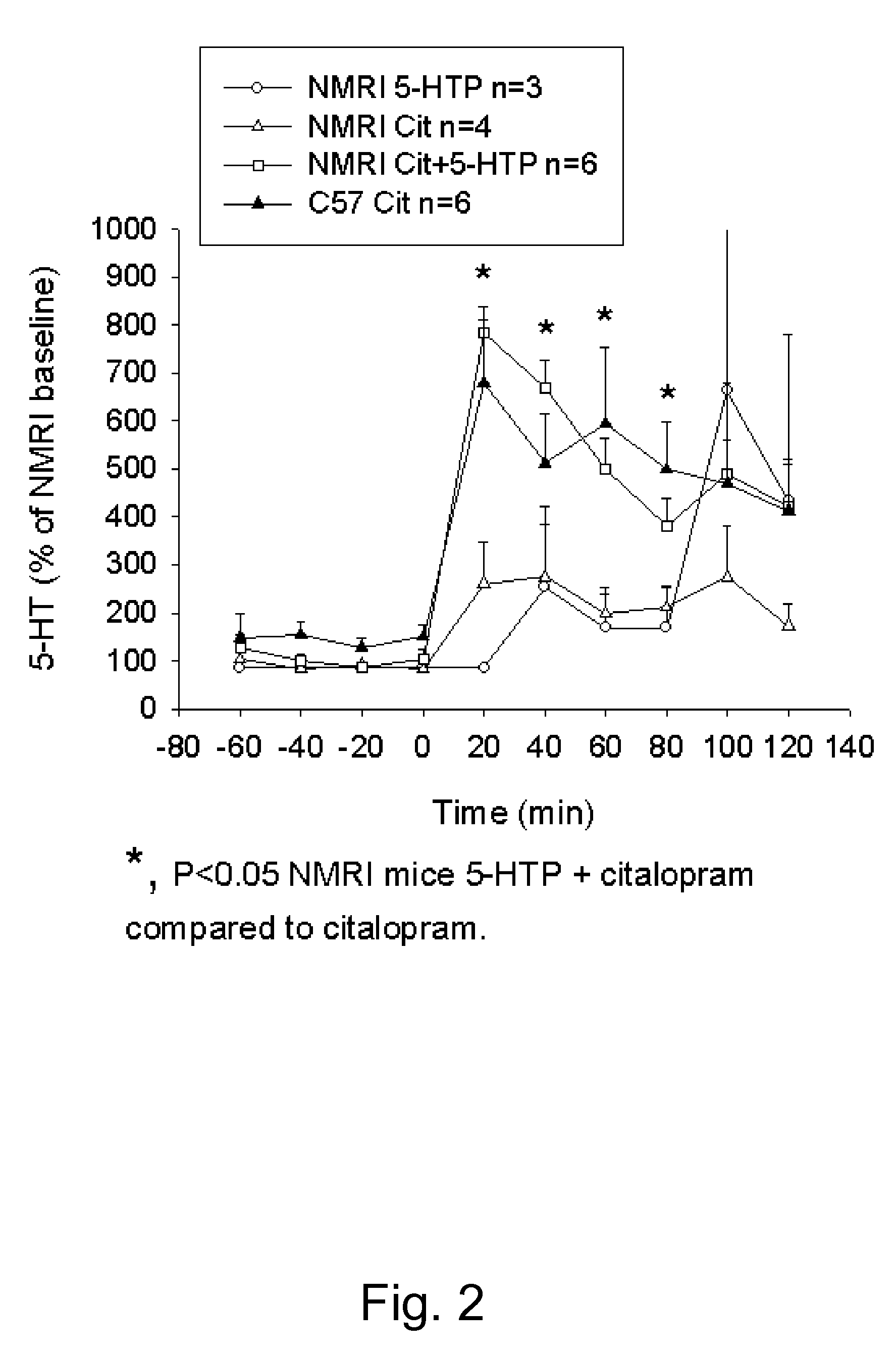
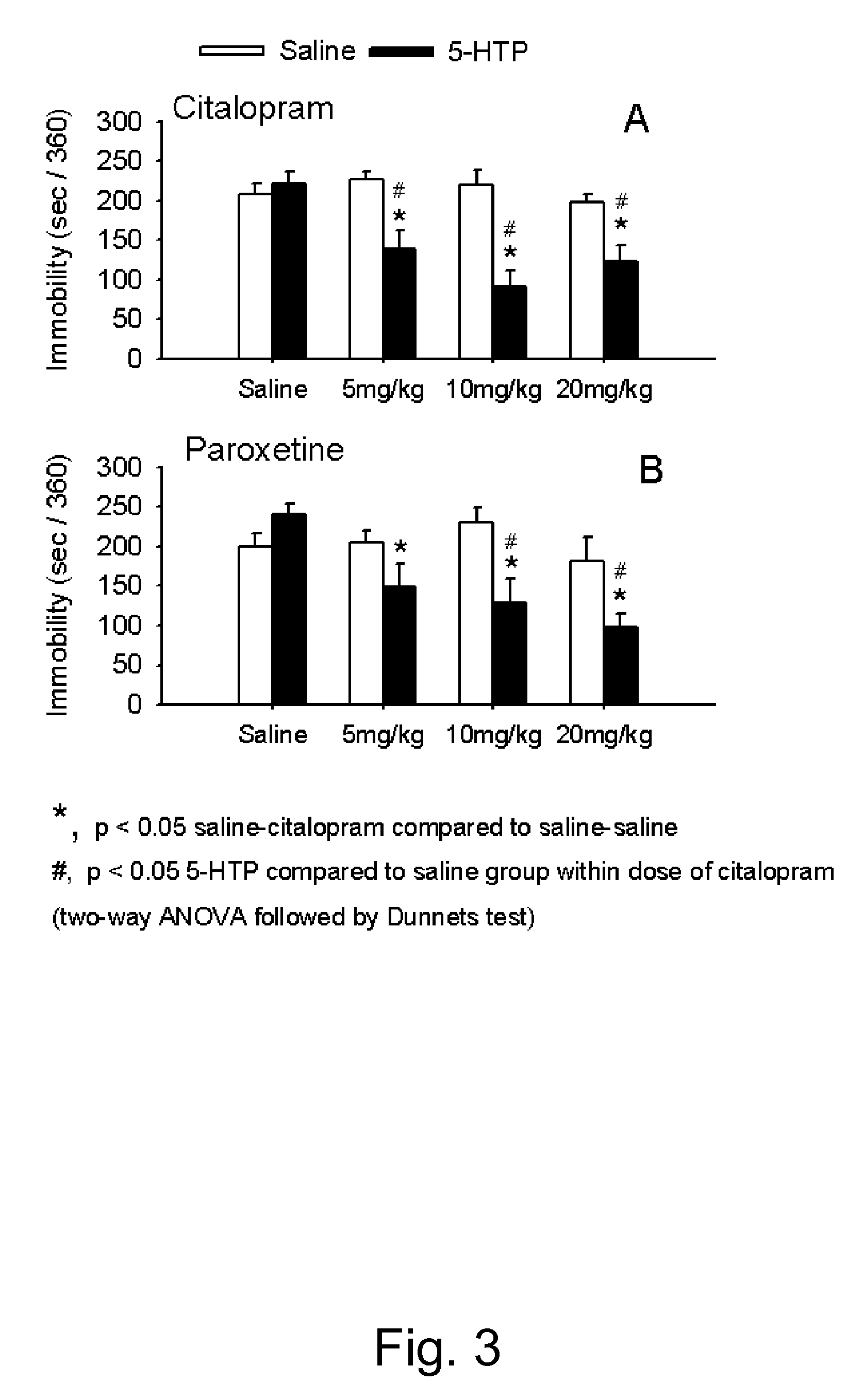
Pharmaceutical compositions of 5-hydroxytryptophan and serotonin-enhancing compound
ActiveUS20100298379A1Improve the level ofImprove usabilityBiocideNervous disorderBipolar mood disorderRecurrent anxiety
This invention relates to novel pharmaceutical compositions comprising a therapeutically effective amount of a slow-release formulation of 5-hydroxytryptophan (5-HTP) and a serotonin-enhancing compound. The pharmaceutical compositions for use according to the invention are contemplated particularly useful for combating CNS disorders, including depressive disorders, bipolar disorders, anxiety disorders, obesity and pain.
Owner:DUKE UNIV
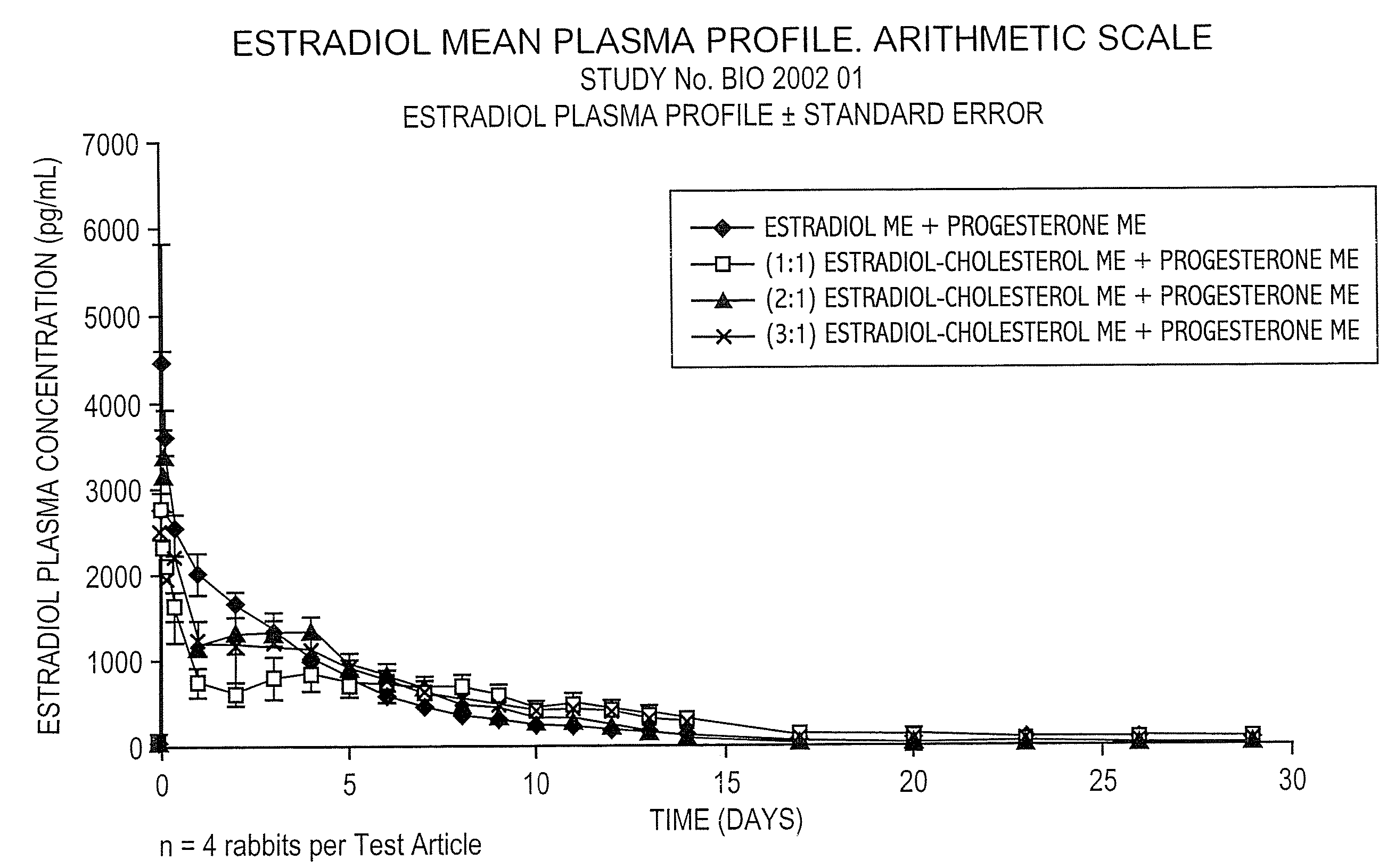
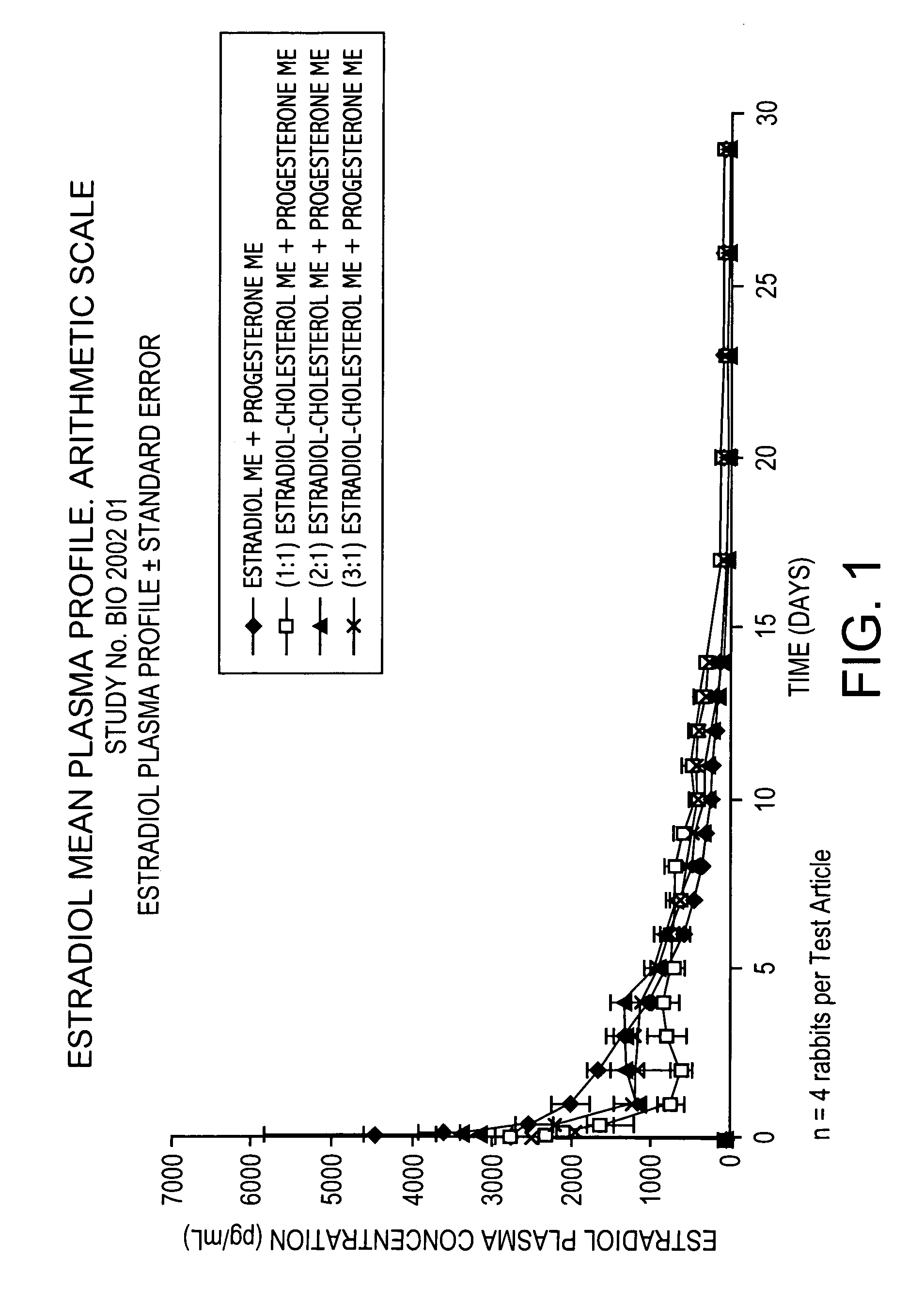
Pharmaceutical formulation for contraception and hormone-replacement therapy
InactiveUS20090081303A1Extended dwell timeAvoid disadvantagesPowder deliveryOrganic active ingredientsRegimenCholesterol
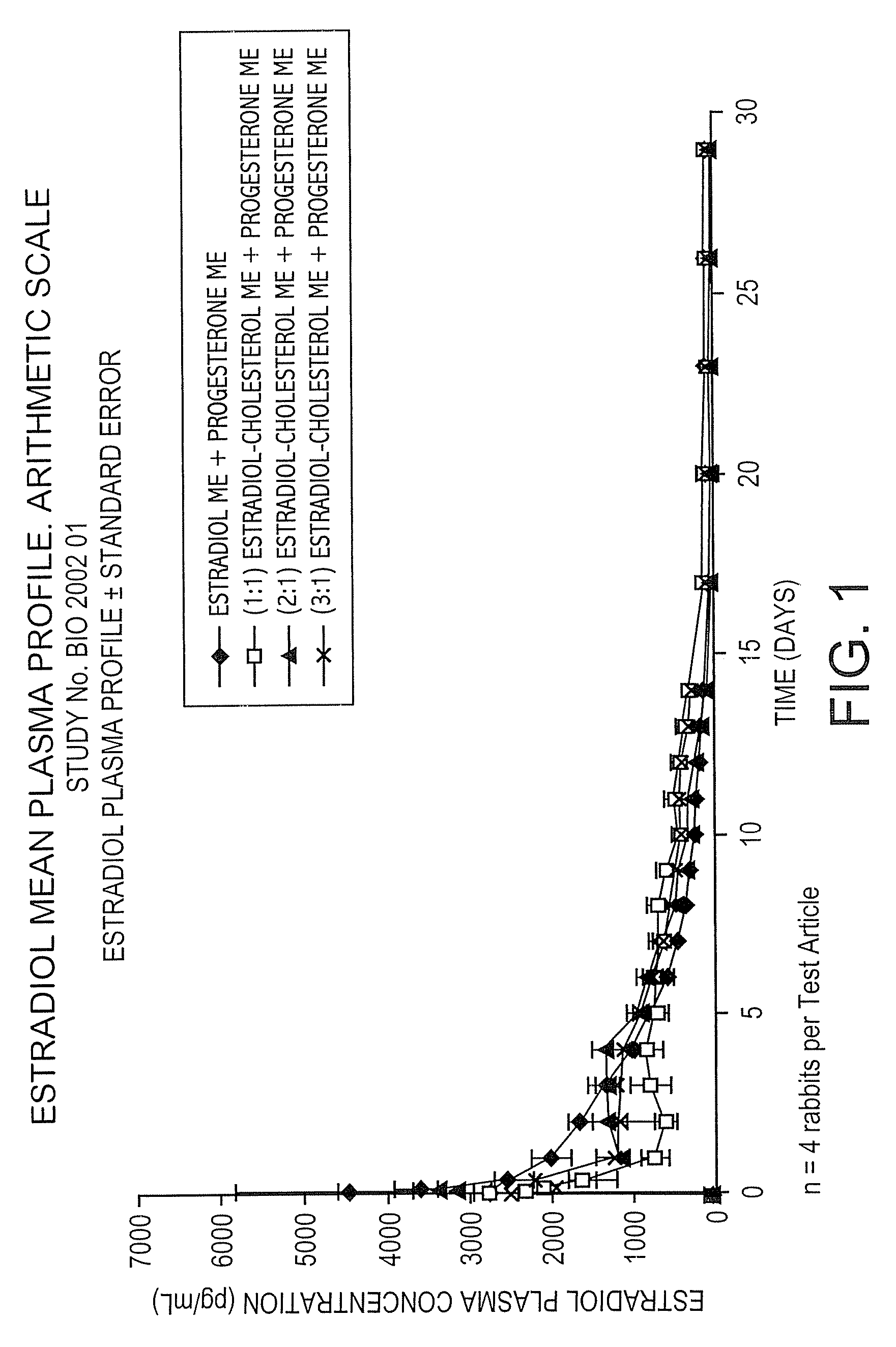
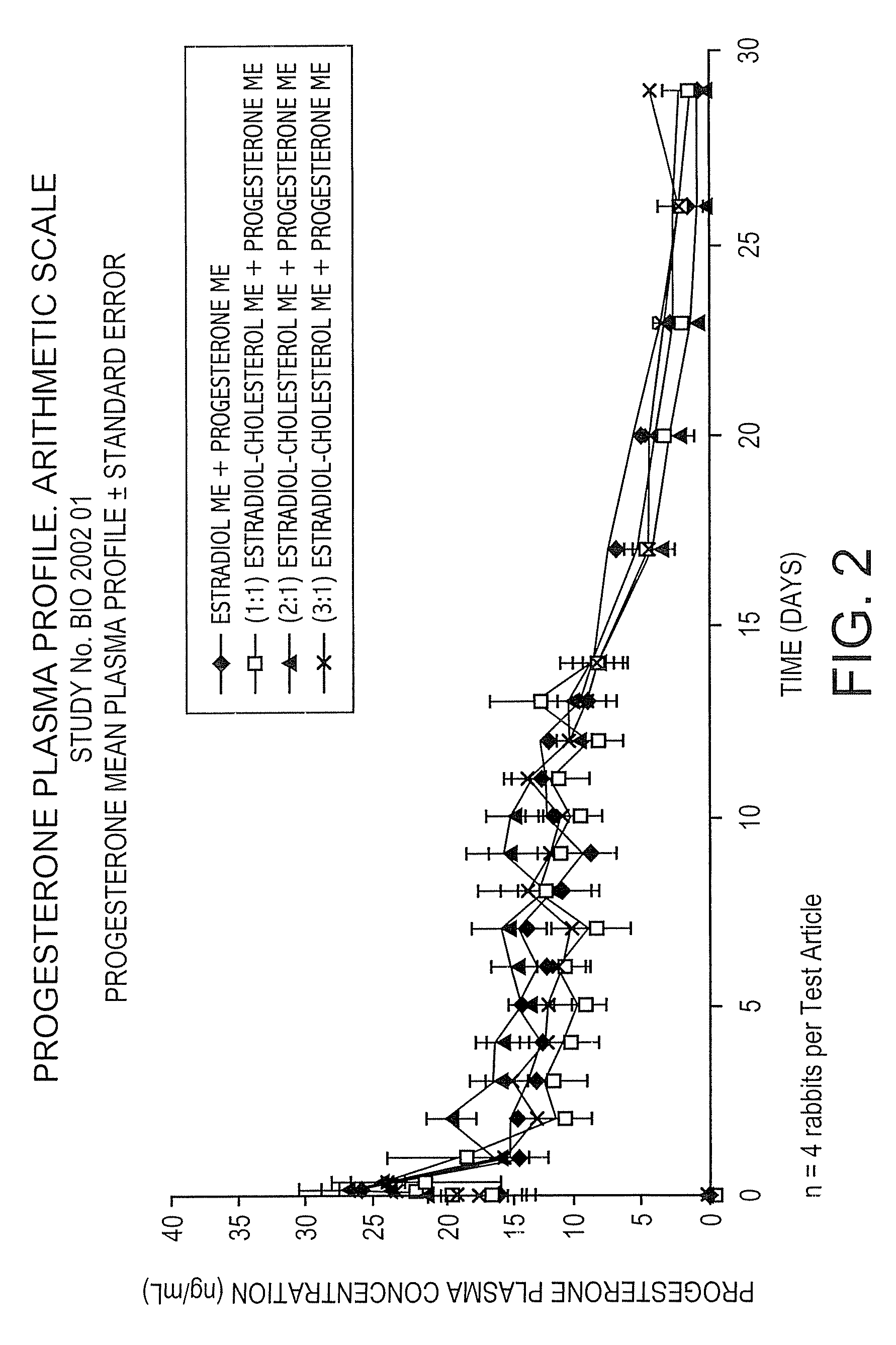
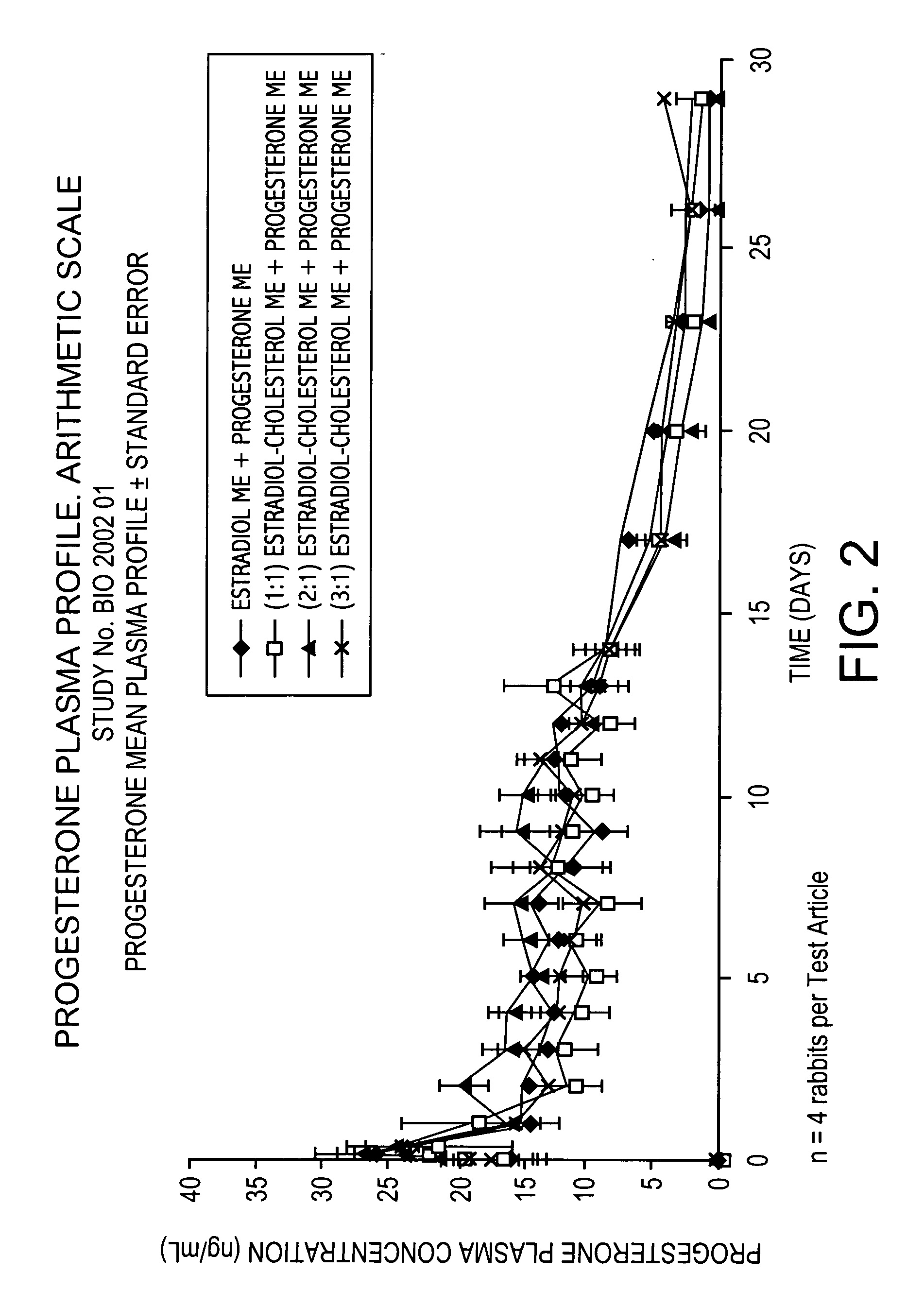
The present invention provides slow release estradiol-progesterone formulations that can be used in either contraception or hormone replacement therapies. The formulations comprise shaped particles of estradiol that is in a hemicrystalline form that exhibits especially low dissolution rates. The shaped particles comprise estradiol compounded in a 1:1 molar ratio with cholesterol, and are administered in combination with progesterone. The slow release formulations of the present invention afford the dual advantages of a low dose estradiol formulation with a low frequency administration regimen. The formulations can be parenterally administered once a month or less often.
Owner:SKENDI FINANCE
Active component of red sage root, its slowly releasing medicine and medical purpose, and its preparing process
An active component of red sage root, its slow-releasing agent, its medicinal application, and its preparing process are disclosed. In its effective region, the content of general solviolic acid is more than 80% and the content of salviolic acid A is more than 10%. Said slow-releasing agent is prepared through extracting in water or alcohol ic water, getting a supernatant, removing alcohol, dissolving in water, then washing to remove inpurities, eduting with alcohol, recovering alcohol eluent and drying. It can be used to prepare the medicine to prevent and cure cardiovascular and cerebrovascular diseases.
Owner:SHENYANG PHARMA UNIVERSITY
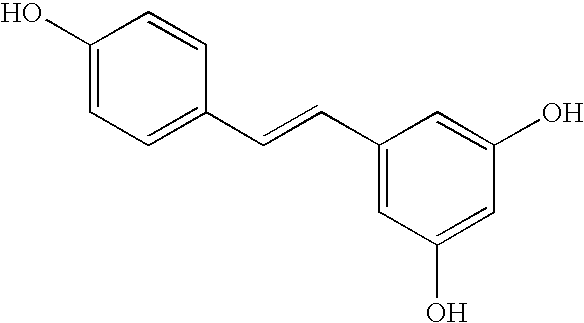
Anti-aging composition containing resveratrol and method of administration
InactiveUS20090163580A1Improve athletic performanceBiocideNervous disorderActive agentExercise performance
Formulations and methods of treatment and putative prevention for aging (anti-aging composition) and for diseases or conditions of all reactive oxygen species-dependant illnesses, such as Alzheimer's disease, Parkinson's disease, diabetes mellitus, cardiovascular disease, cancer, hepatitis, and disorders associated with estrogen deficiencies including osteoporosis and breast cancer and for improving athletic performance of humans include resveratrol and two (2) or more of the following features or additional active ingredients: (1) slow release formulation of resveratrol; (2) pterostilbene; (3) quercetin; (4) fisetin, and (5) naringenin. Slow release is defined for the purposes of the present invention as releasing 95% of the active agent or agents in eight (8) hours through normal human gastrointestinal absorption.
Owner:NATROL
Porous temperature-sensitive hydrogel slow release formulation and preparation method thereof
The invention relates to a porous temperature-sensitive hydrogel slow release formulation and a preparation method thereof. The preparation method comprises the steps of taking poly(N-isopropylacrylamide) porous temperature-sensitive hydrogel as a carrier, loading drugs in the hydrogel, thereby realizing controlled release of the drug by utilizing the temperature sensitivity characteristic of the poly(N-isopropylacrylamide). When the temperature is lower than the LCST (Lower Critical Solution Temperature) of the hydrogel, the drug release is slow, and when the temperature is higher than the LCST, the release speed is accelerated. The preparation method can be used for preparing temperature-sensitive sustained-release pesticide and insecticide, the drug use frequency can be reduced by utilizing the temperature-sensitive sustained-release property, and the effects of improving the pesticide effect and reducing the toxicity can be achieved. The porous temperature-sensitive hydrogel sustained-release inhibitor is fast to react, and simple in technique, has good temperature-sensitive characteristic and sustained-release property, is convenient to use, safe and efficient, thereby having wide application prospects.
Owner:SHANDONG UNIV
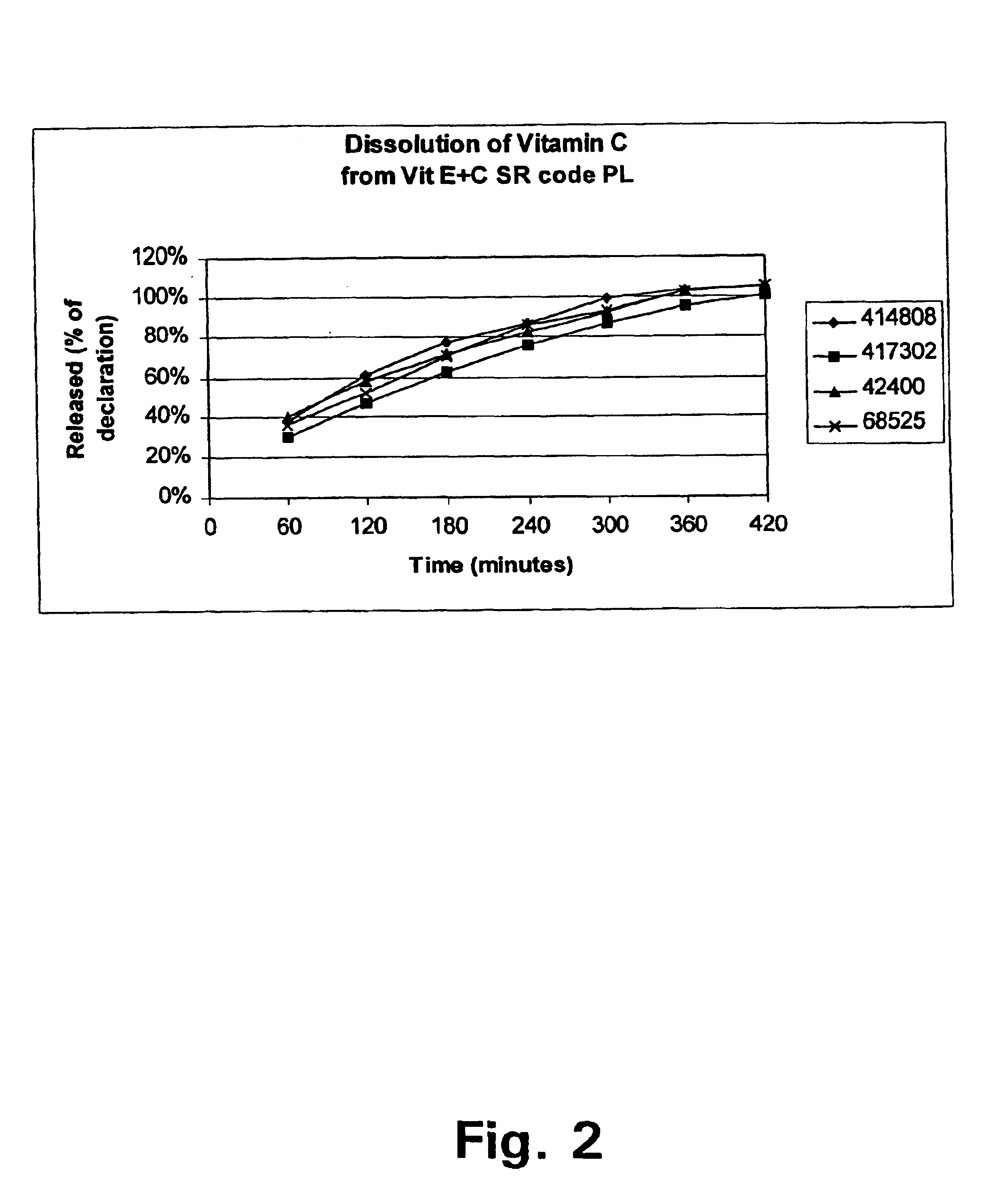
Pharmaceutical delivery system for vitamin C and vitamin E and use of a combination of vitamin C and E for preventing or treating conditions involving oxidative stress
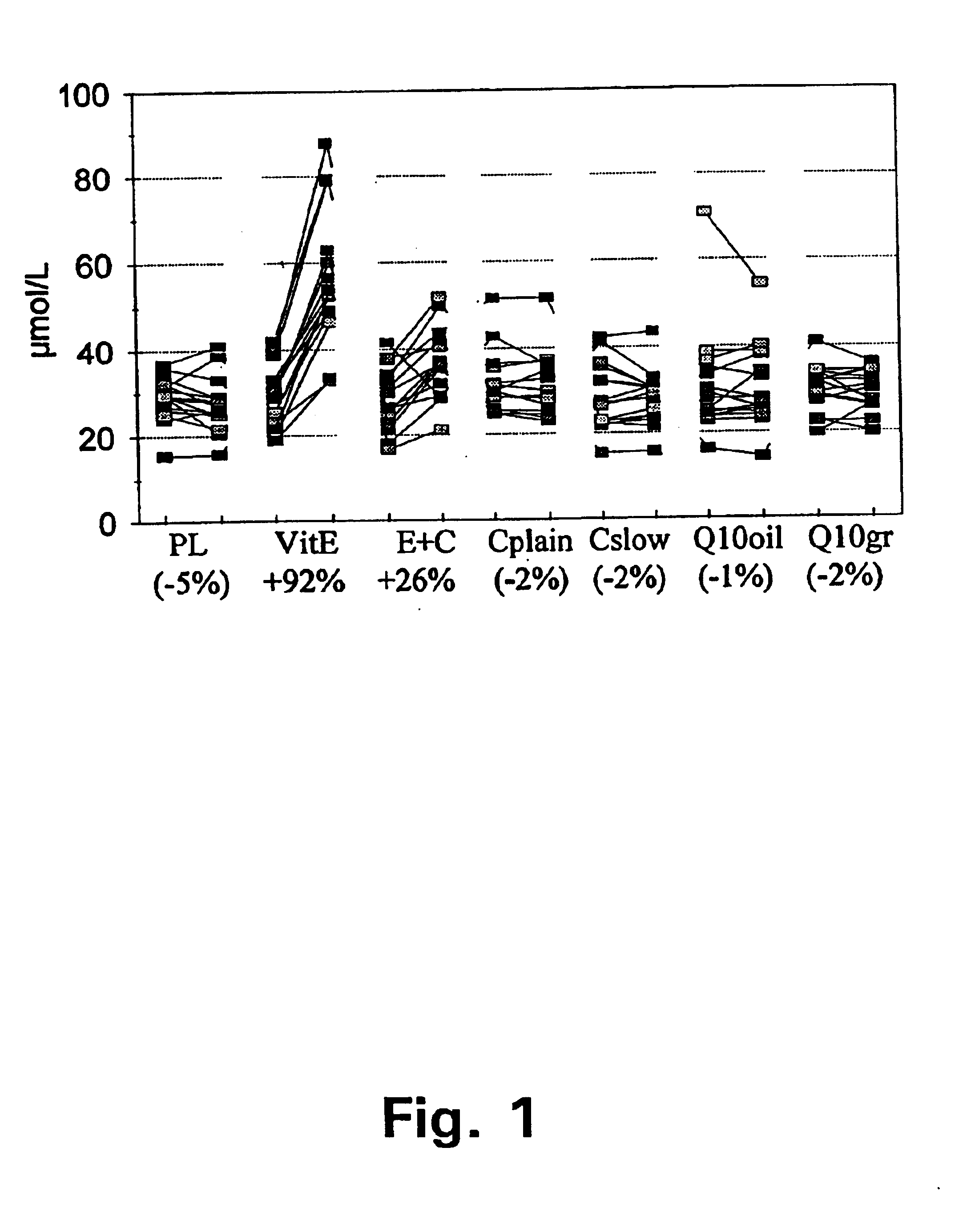
A pharmaceutical delivery system comprising a slow-release formulation of vitamin C (ascorbic acid) and a plain-release formulation of vitamin E (tocopherol) has been found to raise and maintain the concentrations of these vitamins in the blood plasma to a ratio of approximately 2.2:1. The steady-state concentration and ratio of these antioxidants has been found to be critical in the prevention and treatment of oxidative stress related disorders such as arteriosclerosis and diabetes and neural degenerative disorders such as Alzheimer's Disease.
Owner:AS FERROSAN
Environment responsive gelling copolymer
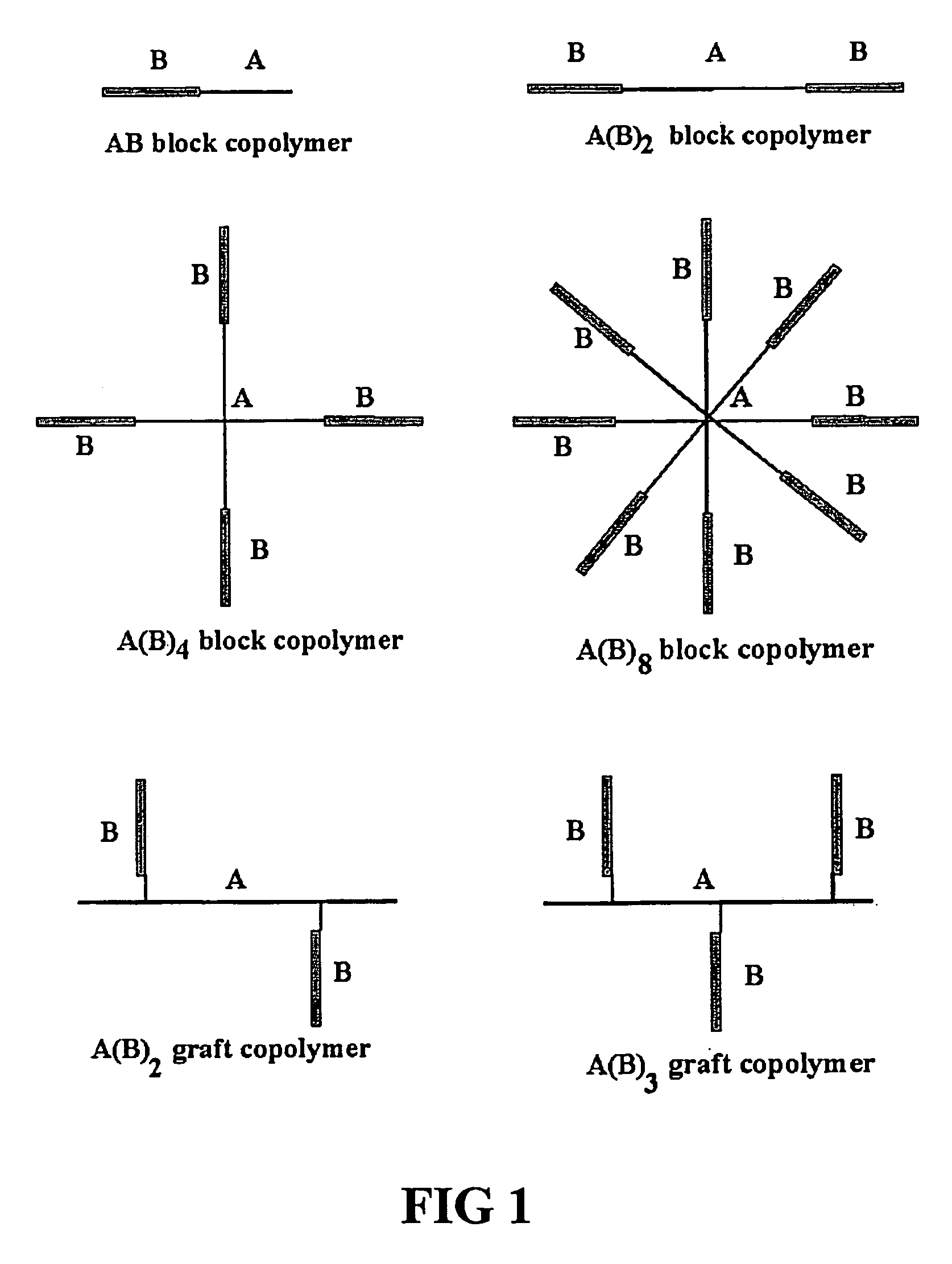
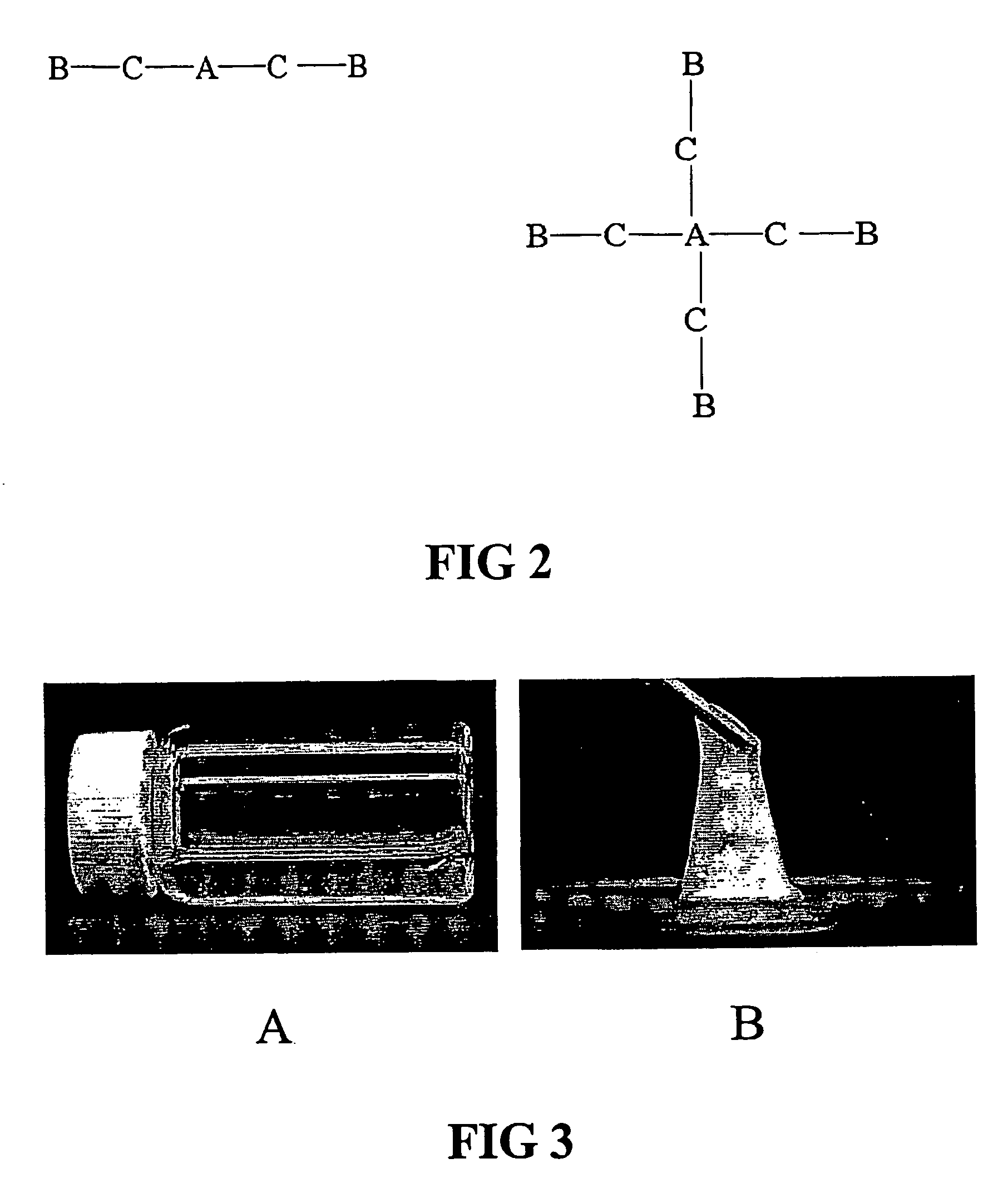
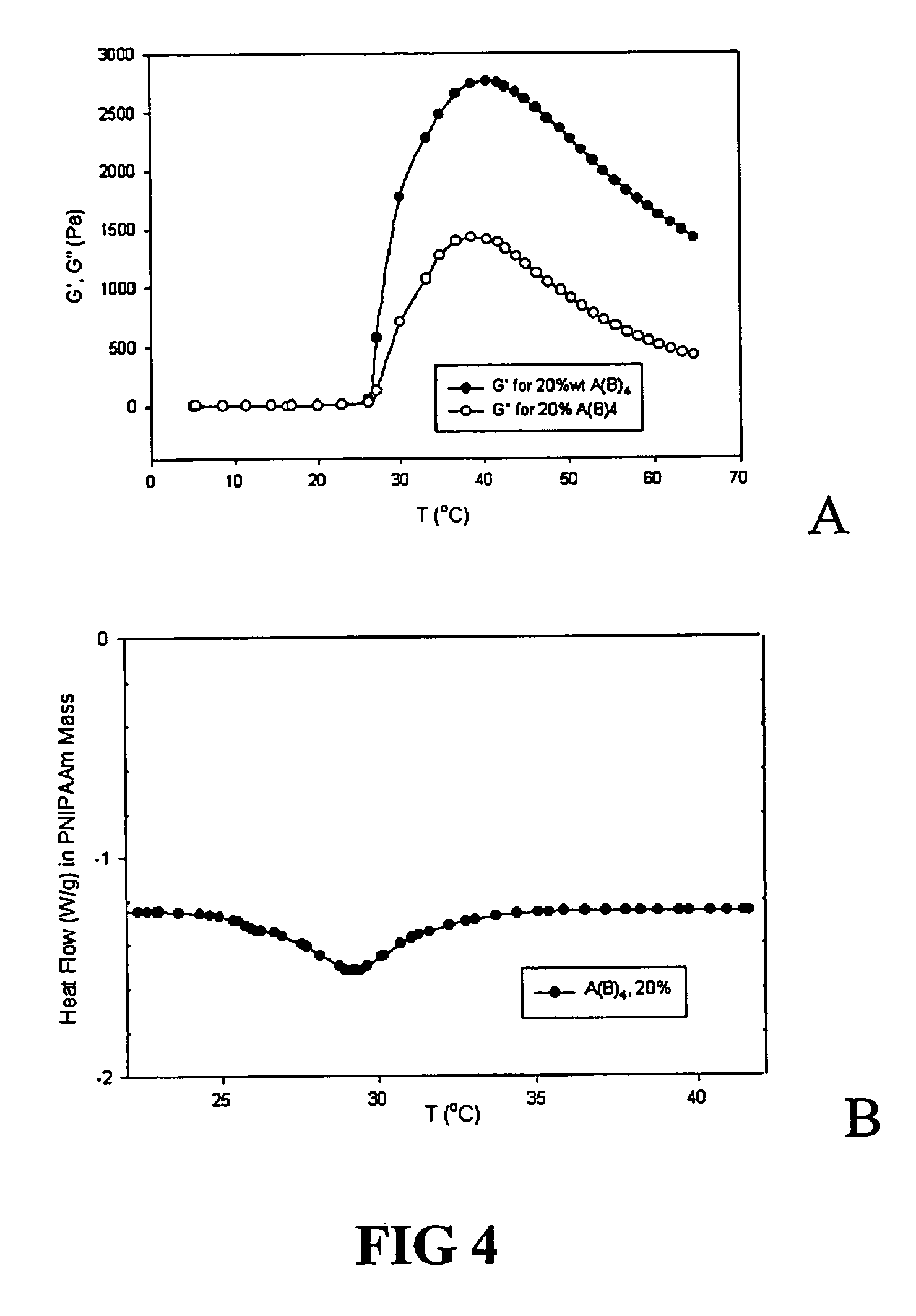
The invention relates to a gelable copolymer composition comprising a copolymer in a solvent. The copolymer has the structure A(B)n, wherein the core (A) is soluble in the solvent, the arms (B) are convertible between soluble and insoluble in the solent depending on an environmental condition, and n>1. The composition forms a gel under environmental conditions in which B is insoluble, through formation of B aggregates. Block copolymers comprising polyethylene glycol (PEG) and poly(N-iso-propylacrylamide) (PNIPAAm) having a liquid form at ambient temperature under aqueous conditions and a gel form at body temperature are disclosed. Copolymer compositions according to the invention can be used to form in situ implants useful in slow-release formulations of biologically active molecules
Owner:CHENG YU LING +1
In-situ phase change gel slow release system taking phospholipid as substrate and preparation method thereof
ActiveCN102526753AGood slow releaseImprove liquidityAerosol deliveryOintment deliveryIntramuscular injectionPhospholipid
The invention provides an in-situ phase change gel slow release system taking a phospholipid as a substrate, and provides a preparation method thereof. A high-concentration phospholipid slow release preparation is prepared from a high-concentration (50-85 percent) phospholipid, a bioactive ingredient, ethanol solutions of different concentrations and / or injection oil with a simple method, and has the characteristics of high biocompatibility, small untoward effect, remarkable slow release effect and suitability for various administration forms such as hypodermic injection, intramuscular injection, external administration and the like; the amount of a coated medicament can be conveniently adjusted according to the clinical dosage of a medicament; and the in-situ phase change gel slow release system has a wide application prospect.
Owner:成都师创生物医药科技有限公司
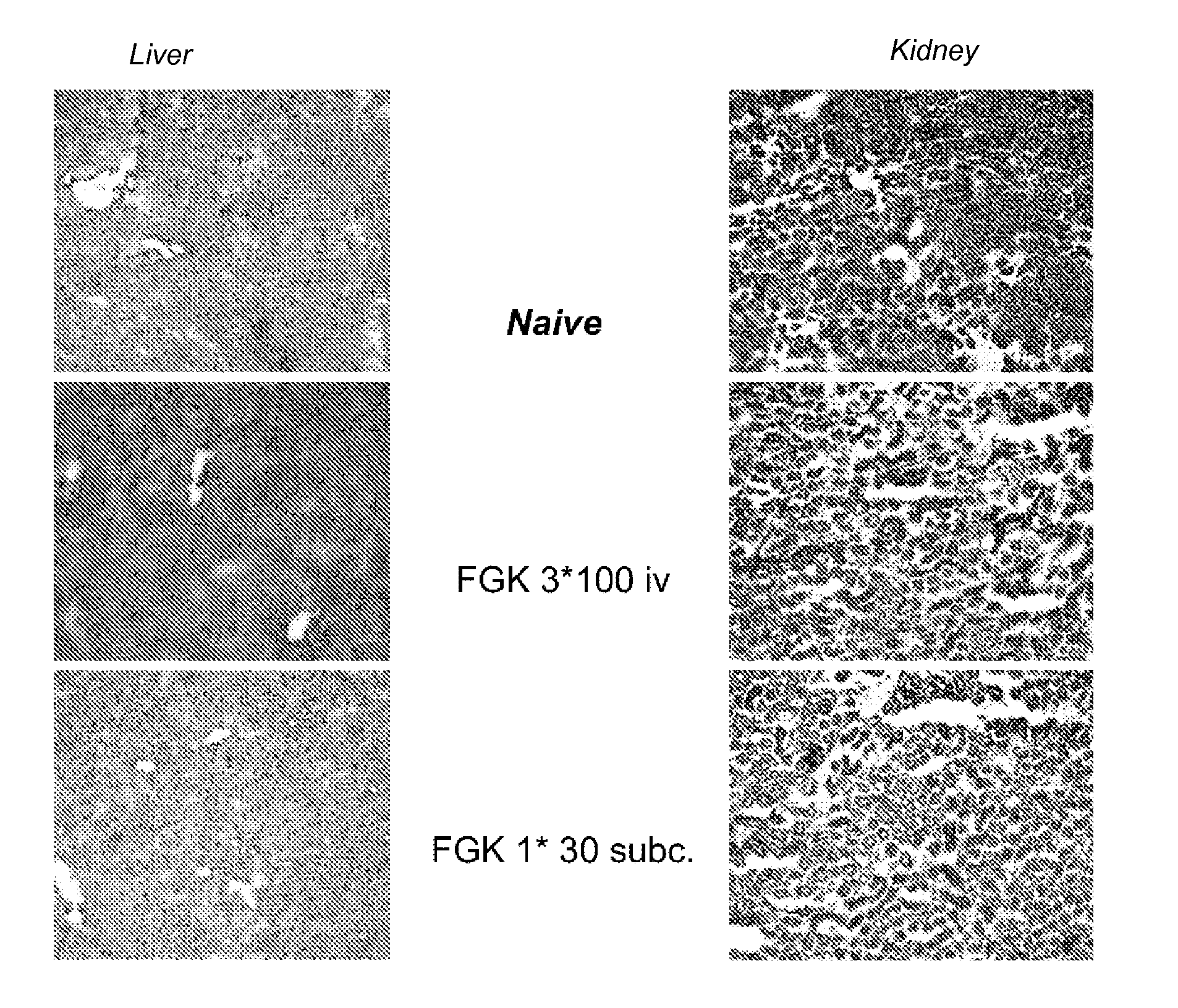
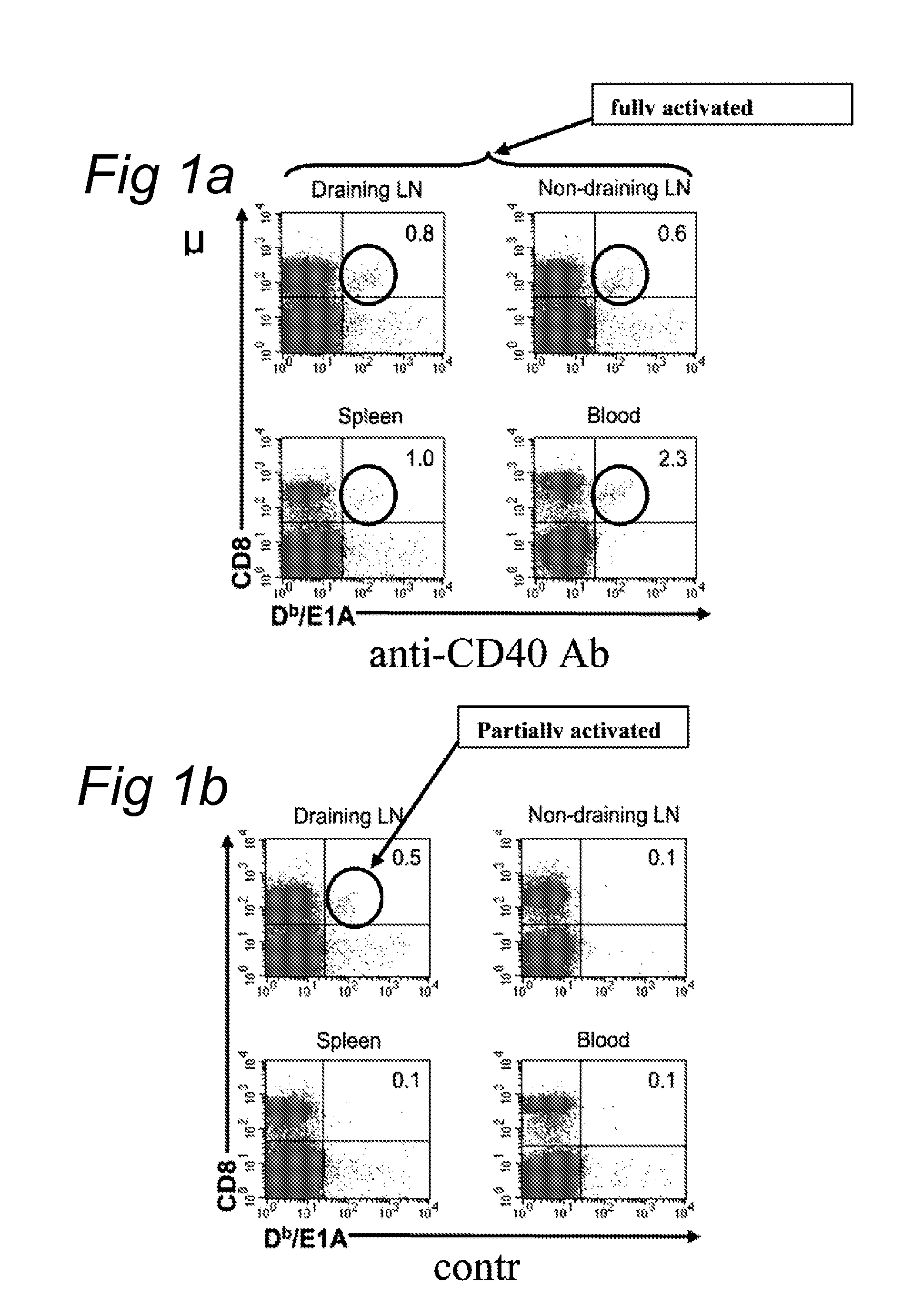
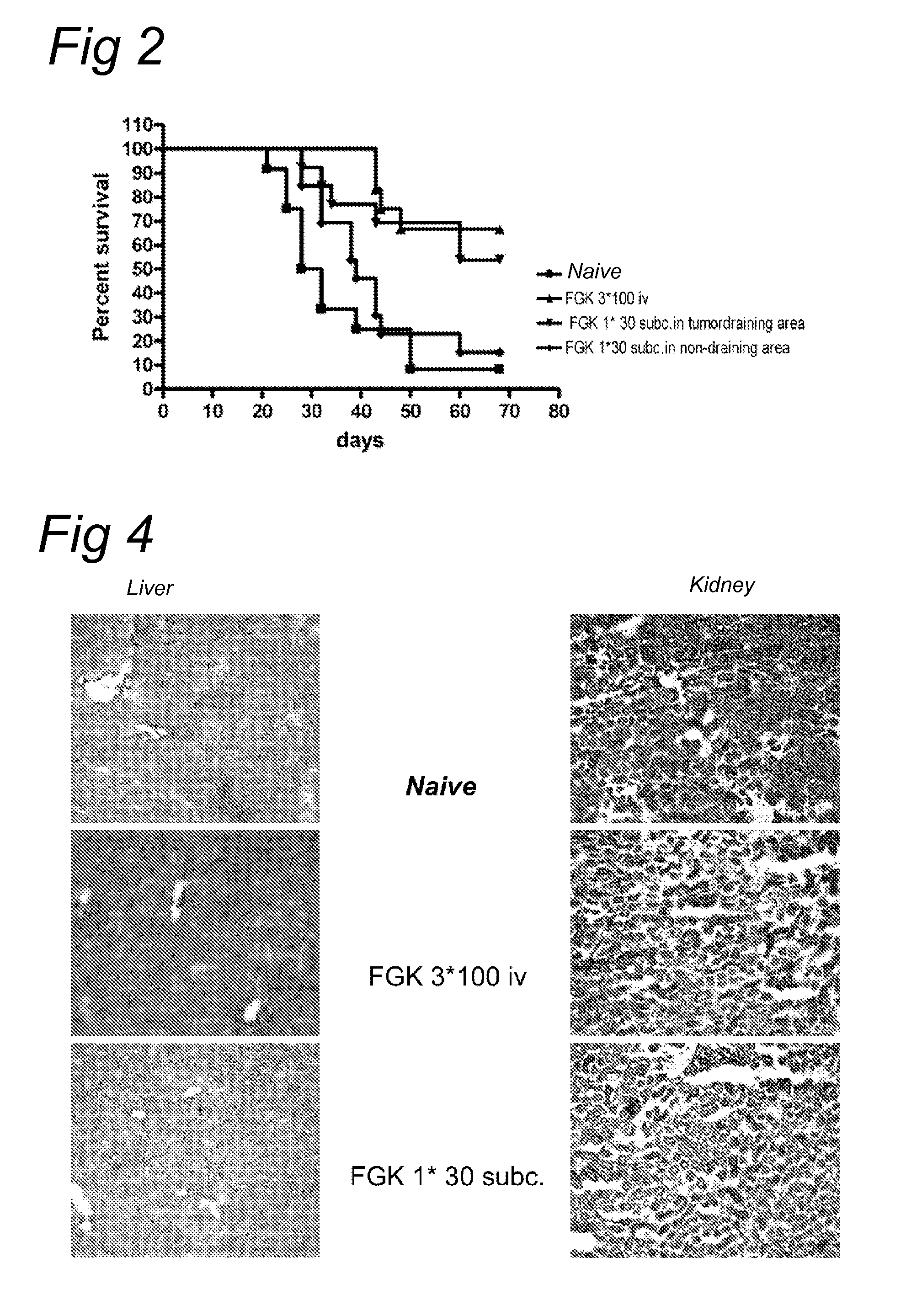
Delivery of a cd40 agonist to a tumor draining lymph node of a subject
InactiveUS20110311525A1Reduce doseLow immunogenicityOrganic active ingredientsPeptide/protein ingredientsAgonistSlow Release Formulation
The invention relates to the use of a CD40 agonist for treating cancer, a pre-malignant disorder or an infectious disease, wherein a CD40 agonist is locally administered and targeted to a tumor draining lymph node of a subject. Optionally, a CD40 agonist is formulated in a slow-release formulation. Optionally, a CTL-activating peptide is further administered.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Electronic cigarette with antibacterial layer cigarette holder
InactiveCN103504481AImprove the bactericidal effectProtection of rights and interestsAntibacterial agentsTobacco devicesElectronic cigaretteInfectious virus
The invention belongs to the technical field of electronic cigarettes, and particularly relates to an electronic cigarette holder with an antibacterial layer. According to the technical scheme, a food-grade antibacterial agent for the infectious disease of digestive tract and the respiratory infectious virus or pathogenic bacteria wraps the electronic cigarette holder, and a medical slow release formulation is mixed in the antibacterial agent. The antibacterial layer of the cigarette holder specially conducts killing on the infectious disease of digestive tract and the respiratory infectious virus or pathogenic bacteria, wherein the infectious disease of digestive tract and the respiratory infectious virus or pathogenic bacteria are easily bred on residual saliva. The antibacterial agent can be appropriately and slowly released, the aim of timely killing the virus or the pathogenic bacteria can be achieved, and meanwhile the electronic cigarette holder has the better economical efficiency.
Owner:HONGTA TOBACCO GRP
Pesticide fertilizer granular formulation
The invention relates to a pharmaceutical granular formulation containing weedicide and fertilizer for agricultural production. By total weight of the pharmaceutical granular formulation, the compositions and the contents of raw materials are: 0.01 to 10 percent of bensulfuron-methyl, 0.2 to 30 percent of pretilachlor or quinclorac, and 5 to 45 percent of macro-element N, 1 to 10 percent of macro-element P and 1 to 15 percent of macro-element K in the fertilizer, and 0.1 to 5 percent of microelement zinc and 0.1 to 5 percent of microelement boron in the fertilizer, and 0.1 to 5 percent of slow release formulation, 0.05 to 5 percent of safener, 0.5 to 5 percent of dispersing auxiliary, and the balance being stuffing. The pharmaceutical granular formulation effectively guarantees scientific release of farm chemical and the fertilizer, and has double effects of weed removal and fertilizer application.
Owner:GUANGDONG ZHONGXUN AGRI TECH
Slow-release preparation containing beta-lactamase inhibitor and cephalosporin and its use
The present invention relates to a slow-released preparation containing beta-lactamase inhibitor and cephalosporin. Said slow-released preparation can be made into antibiotic slow-released injection or slow-released implant preparation. Said injection is formed from slow-released microsphere and solvent, the slow-released microsphere contains slow-released auxiliary material and beta-lactamase inhibitor with antibacterial effective dose and cephalosporin, the solvent is special one containing suspension adjuvant of carboxymethyl cellulose sodium, etc. and its viscosity is 100 cp-3000 cp (20 deg.C-30 deg.C). The slow-released auxiliary material is selected from EVAc, polylactic acid copolymer, sebacic acid copolymer, albumin glue and gelatin, etc. The slow-released implant preparation is prepared by using slow-released microsphere or adopting melting process. Said invention also provides its application method. and can obtain obvious therapeutic effect for curing various infective diseases.
Owner:JINAN SHUAIHUA PHARMA TECH
Duloxetine hydrochloride delayed release formulations
Delayed release formulations of duloxetine hydrochloride and methods for its manufacture are described. A preferred formulation includes an inert core, a drug layer comprising duloxetine hydrochloride, a separating layer and an enteric layer comprising at least one of methacrylic acid copolymer and hydroxypropyl methyl cellulose phthalate.
Owner:TEVA PHARM USA INC
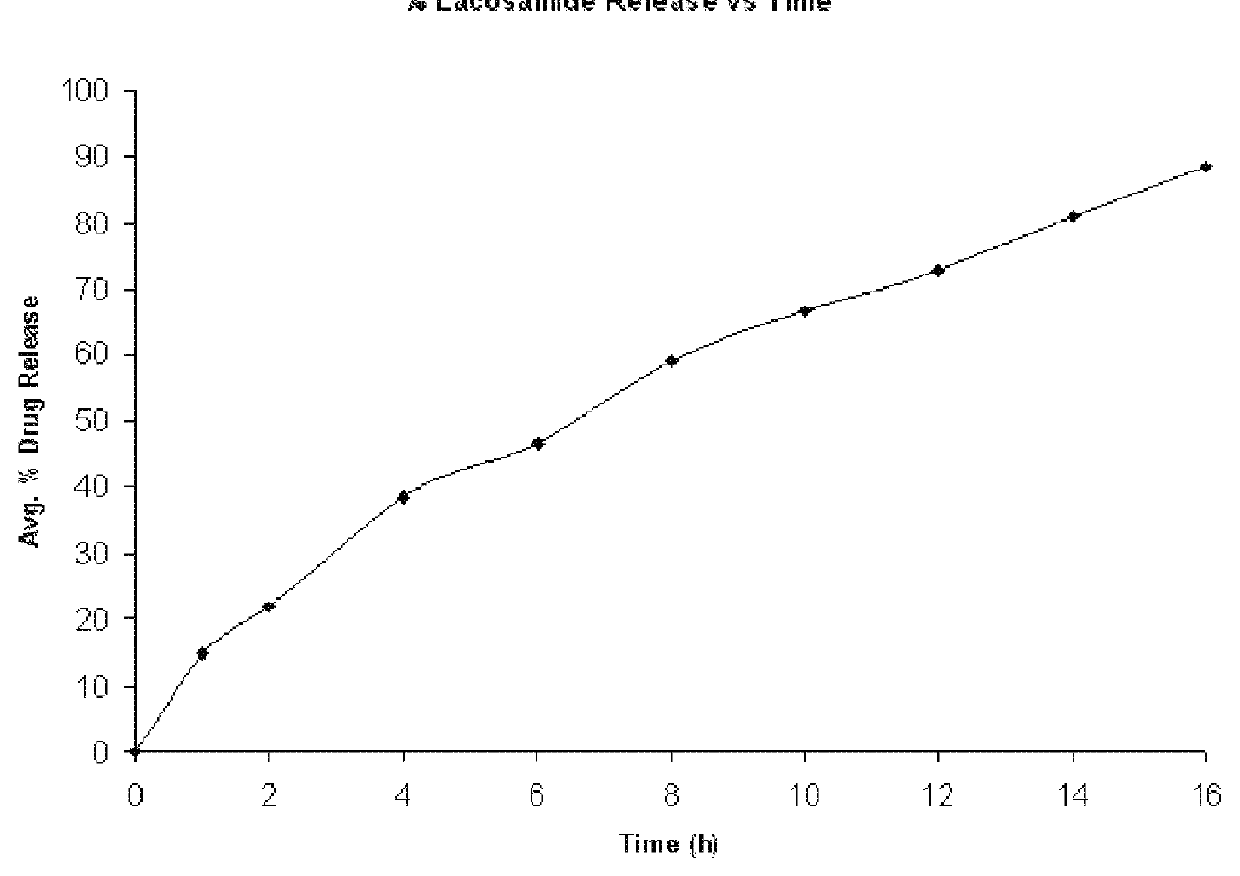
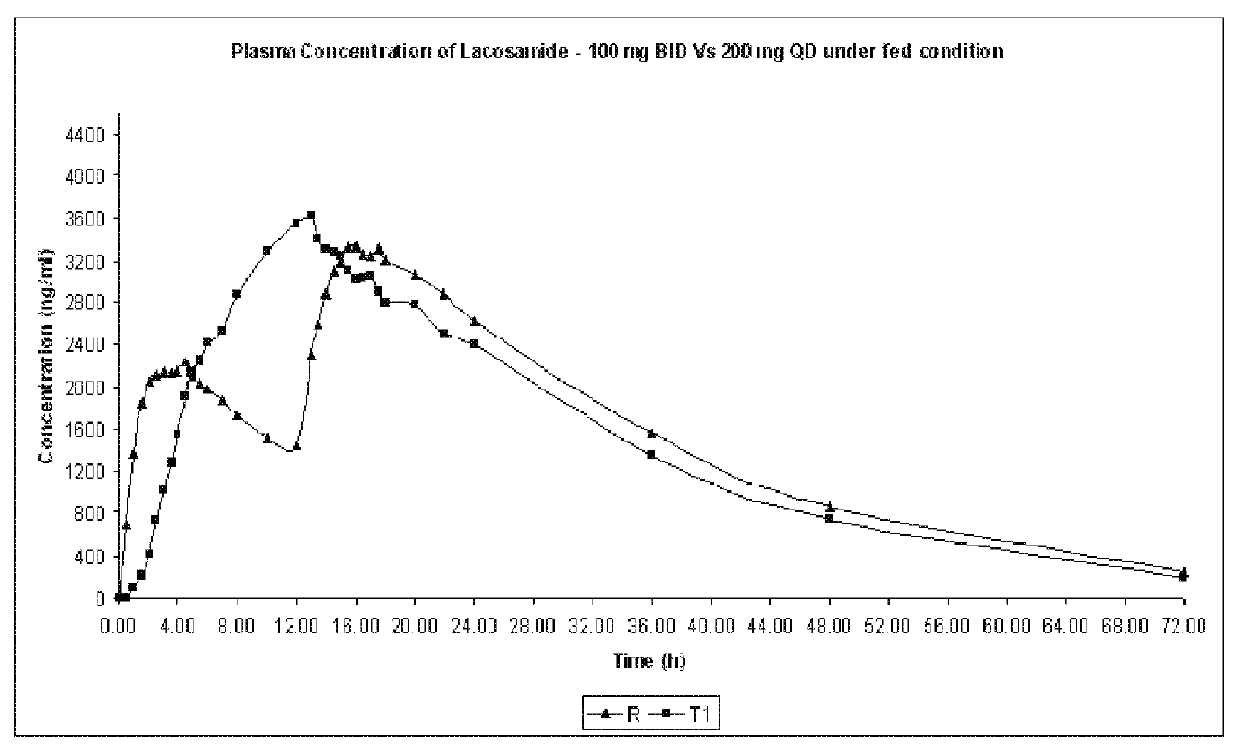
Modified release formulation of lacosamide
The present invention provides a modified release formulation of lacosamide. The modified release formulation of the present invention comprising lacosamide and modified release polymer provides modified release of lacosamide with minimal Cmax to Cmin peak to trough variation over a period of at least 12 hrs.
Owner:LUPIN LTD
Ranolazine hydrochloride slow-release preparation and its preparing method
ActiveCN1891218AMinimized changes in plasma concentrationsRelease completelyOrganic active ingredientsCardiovascular disorderAdhesiveDiluent
The present invention relates to a ranolazine hydrochloride delayed-release preparation and its preparation method. Said ranolazine hydrochloride delayed-release preparation contains 25-95 wt% of ranolazine hydrochloride and one or several kind of medicinal inert auxiliary materials, including delayed-release matrix, adhesive, disintegrating agent, diluent, lubricating agent, flow aid or moistening agent.
Owner:QILU PHARMA HAINAN
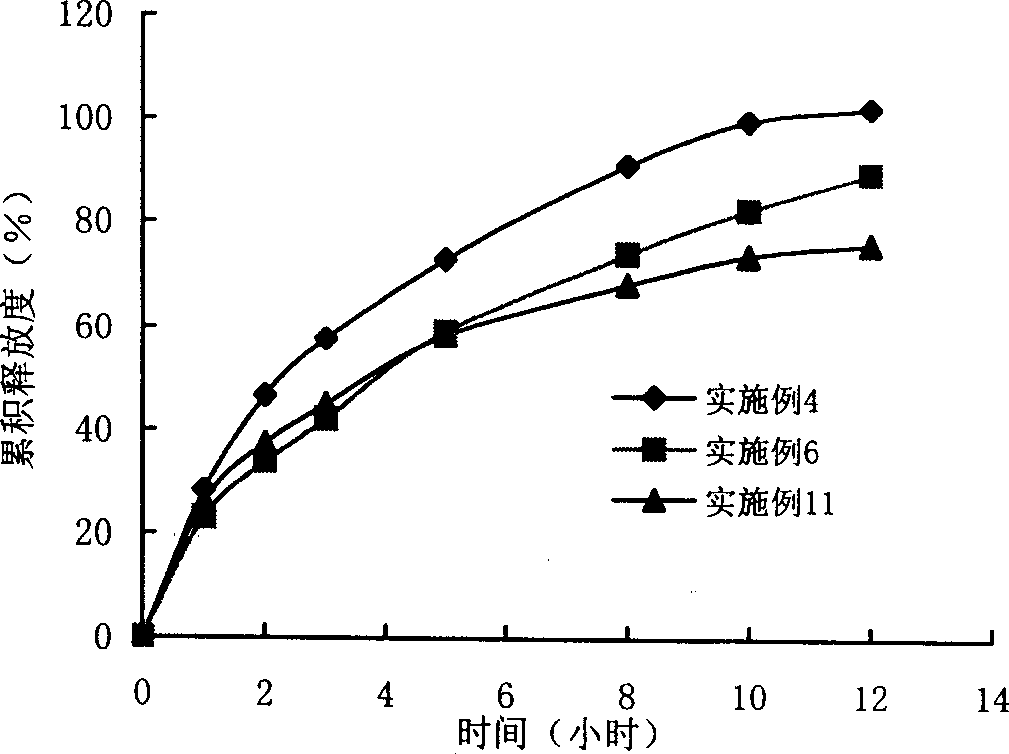
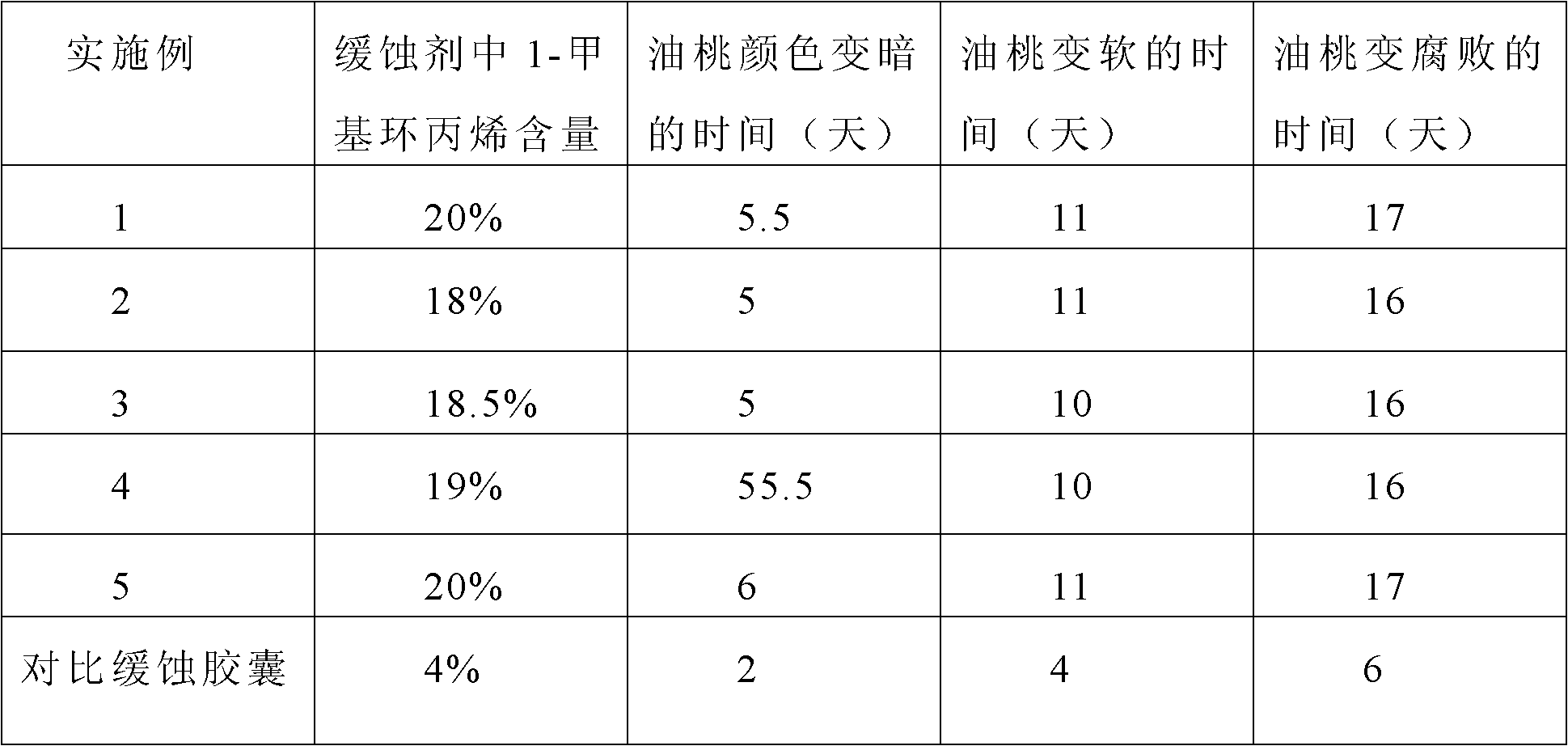
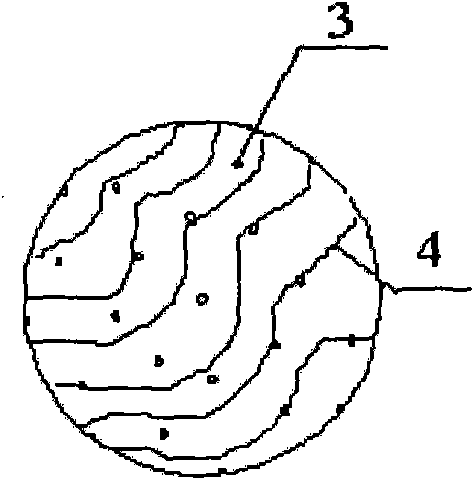
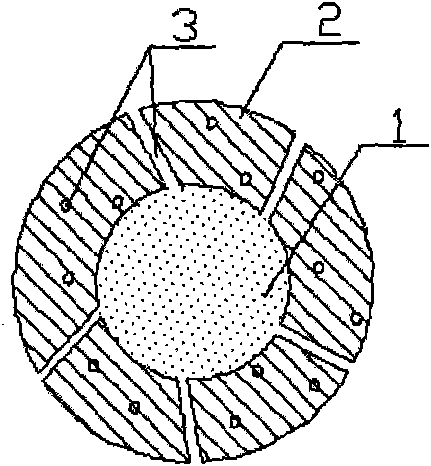
1-methyl cyclopropene slow release formulation and preparation method thereof
InactiveCN102217671AExtended release timeLong release timeFruit and vegetables preservationMicroballoon preparationRelease time1-Methylcyclopropene
The invention discloses a 1-methyl cyclopropene slow release formulation and a preparation method thereof. The slow release formulation consists of 1-methyl cyclopropene as a core material and a covering film wrapping outside the core material, and the selected covering film is gelatin. The preparation method of the 1-methyl cyclopropene slow release formulation sequentially includes the following steps: dissolving the gelatin in water to formulate solution with the weight percentage of 2-10% and controlling the temperature of the solution between 1-4 DEG C; adding 1-methyl cyclopropene and stirring to form a gelatin-coated 1-methyl cyclopropene microemulsion; adding precipitating agent so that the microemulsion precipitates in the system and continuing to stir for 20-30min; adding a film-solidifying agent to solidify the covering film; fitering and then washing the solidified product to obtain a slurry 1-methyl cyclopropene microcapsule; and adding a separating agent in the slurry 1-methyl cyclopropene microcapsule so that the microcapsule disperses. The product is high in content of effective substances and long in slow release time. The preparation method is simple in technology and convenient in operation.
Owner:WUHAN SHUANGQI TECH DEV
Thermosensitive hydrogel collagenase formulations
InactiveUS20160000890A1Acceptable injectabilityReduce in quantityPeptide/protein ingredientsSkeletal disorderWater basedUse medication
It is an object of the present disclosure to provide a formulation for injectable and topical collagenase, which will have extended residence time for the drug at the therapeutic targeted area for the indication being treated. It is a further object of the disclosure to provide a slow release formulation for collagenase, which is compatible with the active ingredient and does not adversely affect its activity. Still a further object of the disclosure is to provide an injectable formulation for collagenase which can be effectively administered to a patient with a small size needle without exhibiting pre-gelation, which would interfere with the ability to deliver the required dose for treatment. Still a further object of the disclosure is to provide a water-based topical formulation for collagenase which will be more compatible with other topically used medications to achieve better results.
Owner:BIOSPECIFICS TECH CORP
Health-care fat substitute, preparation method and application thereof
ActiveCN102972774ASimple operation processThe operation process is easy to controlFood preparationFlavorAdditive ingredient
The invention provides a health-care fat substitute, a preparation method thereof and an application thereof. The health-care fat substitute is formed by using water-soluble alginate as main material, and compounding gels, slow release formulations, thickening stabilizer and nutritional ingredients through an appropriate formula or a proper processing method. The fat substitute prepared by the preparation method provided by the invention is a glucolipid solid with certain hardness and elasticity, has good fat sensory characteristic and can be extensively applied to partially substituting fat in low-fat meat products; the fat substitute has the health-care functions of reducing blood sugar, detoxifying and beautifying, can better improve the mouthfeel of the food to keep the flavor of the food when being applied to the food product. Moreover, the invention has the advantages of a simple process, and easy control in operation.
Owner:青岛明月海祥营养食品有限公司
Chitosan coating medicine slow release microsphere and preparation method thereof
InactiveCN101618021AGood biocompatibilityLow toxicityHydroxy compound active ingredientsDigestive systemChitosan coatingAlcohol
The invention discloses a chitosan coating medicine slow release microsphere and a preparation method thereof. The chitosan coating medicine slow release microsphere comprises a condensate capsule shell of chitosan and glutaric dialdehyde, core medicine resveratrol, oxidative resveratrol, pterostilbene or white cedar alcohol, wherein the core medicine resveratrol, the oxidative resveratrol, the pterostilbene or the white cedar alcohol are coated into the capsule shell. The preparation method is simple and easy, the preparation cost is low, the microsphere envelop rate is 10-50 percent, the microsphere is round and has good dispersivity, the stability of the original medicine is increased, and the chitosan coating medicine slow release microsphere realizes the function of medicine slow control and release and can be conveniently matched with other medicines. The chitosan coating medicine slow release microsphere is suitable for preparing a slow release formulation of medical resveratrol, oxidative resveratrol, pterostilbene and white cedar alcohol.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
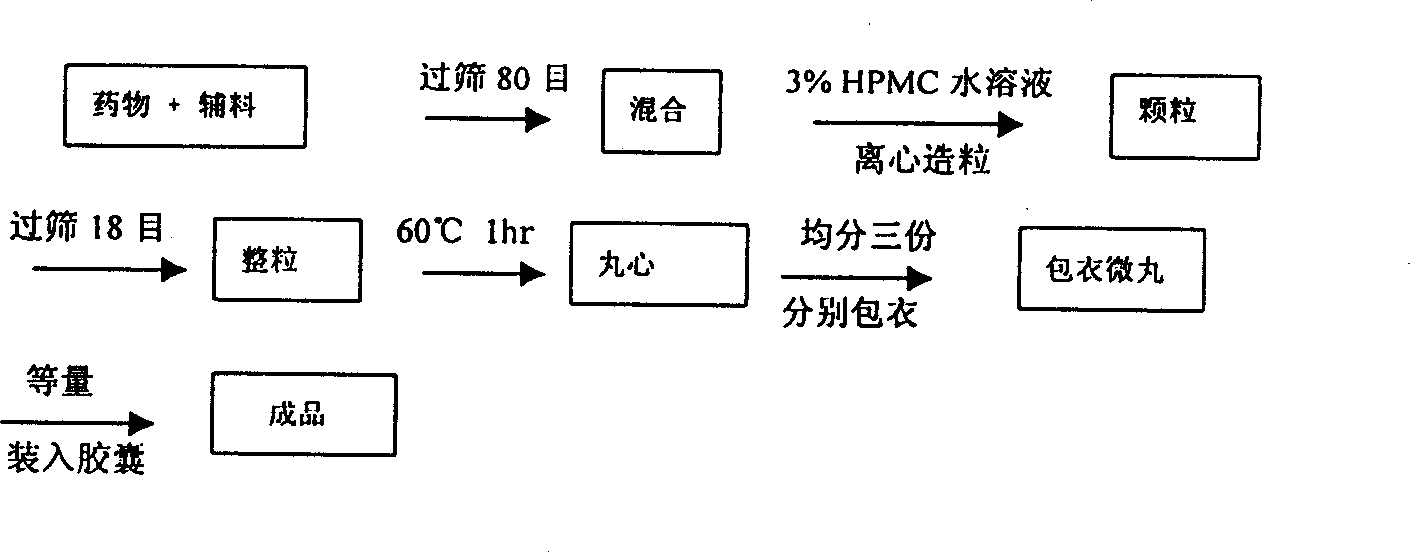
Traditional Tibetan medicine Ruyizhenbao composite preparation and preparation method thereof
ActiveCN102430090AFast absorptionStable active ingredientsOrganic active ingredientsAntipyreticMedicinal herbsCaplet Dosage Form
The invention discloses a Ruyizhenbao medicine composite, which is prepared by adopting the following steps of essential oil extraction and inclusion, ethanol extraction, water extraction, fluid extract drying, medicinal material fine powder crushing, expensive and fine medicine fine powder preparation and the like. Pharmaceutically conventional auxiliary materials are added according to a conventional process to be prepared into clinically acceptable dosage forms such as: powders, capsules, tablets, oral liquid, condensed pills, granular formulations, pills, pellets and slow release preparations. The invention is characterized in that the Ruyizhenbao composite is prepared by taking phytochemical components as the material base and the pharmacodynamics activity as the guidance by adoptinga modern extraction preparation method. The medicinal preparation prepared by the method disclosed by the method has the advantages of high absorption rate, stable pharmacodynamic components, high bioavailability and the like under the condition that the pesticide effects of the raw preparation are remained.
Owner:JINHE TIBETAN MEDICINE
Production method of melamine formaldehyde-polyvinyl formal fertilizer slow-release formulation
ActiveCN101353282AImprove adhesionGood flexibilityFertilizer mixturesMelamine formaldehydeSlow Release Formulation
The invention discloses a production method for a melamine formaldehyde-polyvinyl alcohol formaldehyde fertilizer slow-release agent, which comprises the following steps: the preparation of melamine formaldehyde polymer solution, the preparation of polyvinyl formal solution, and the preparation of a slow-release agent. The production method makes use of the characteristics of the good water resistance of the melamine formaldehyde and sol-water glass, and the good caking property and flexibility of the polyvinyl formal to produce an endoplasmic slow-release agent with good combination with urea or compound fertilizer paste. The production method has the advantages of simple process, short production period, and the like; compared with slow-release urea or slow-release compound fertilizer by the secondary processing and production, the method can save energy and reduce transportation.
Owner:CNOOC FUDAO
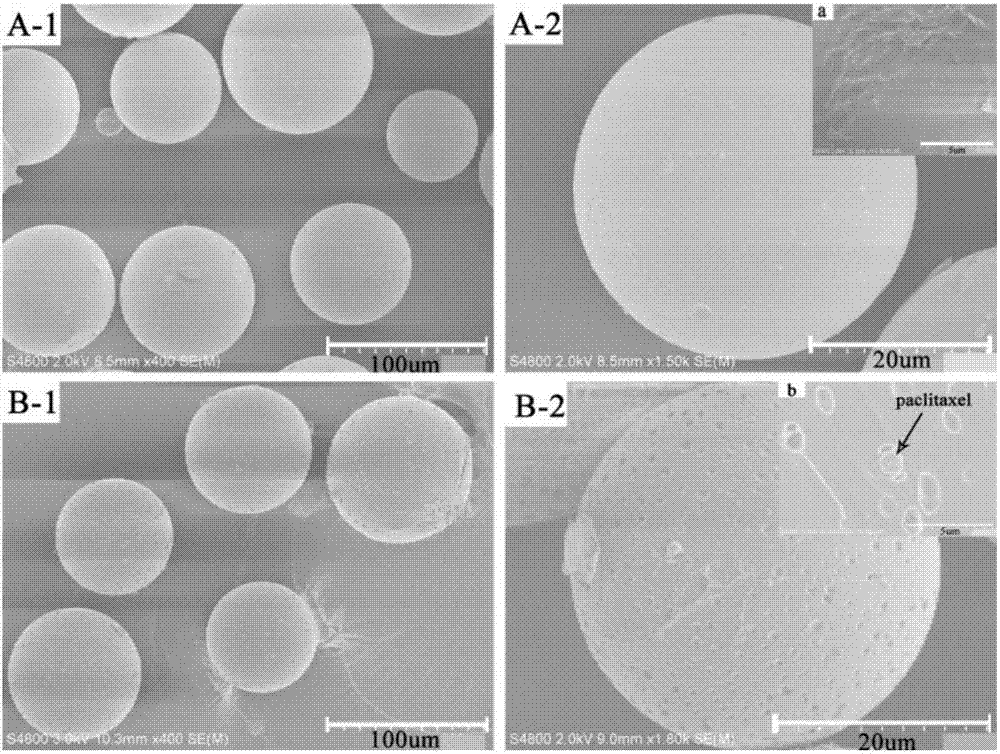
Preparation method of taxol-loading polylactic acid-hydroxyacetic acid microspheres
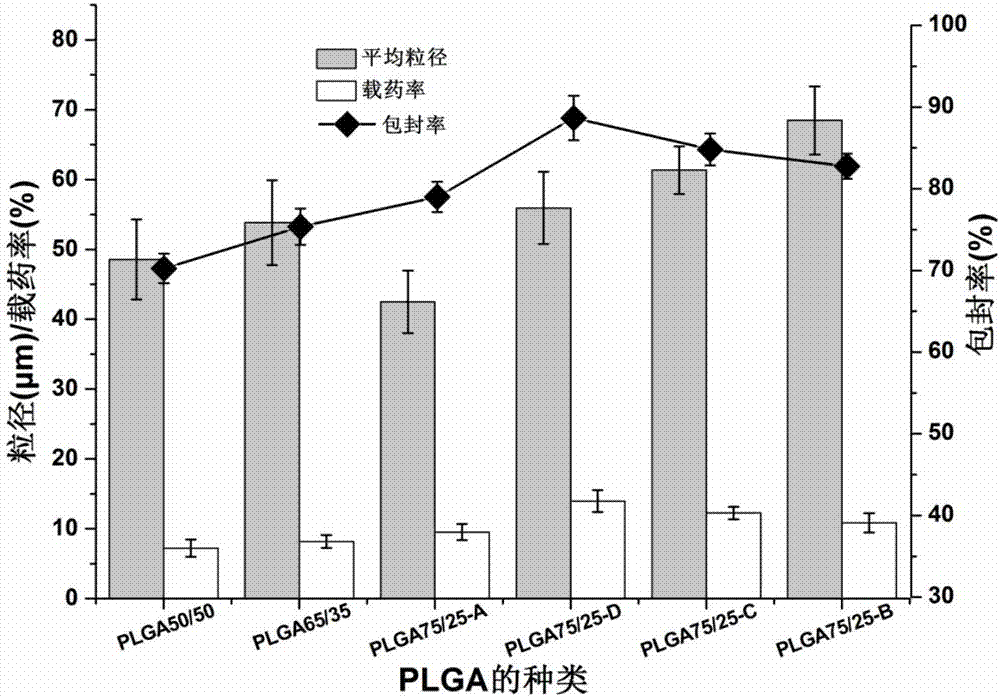
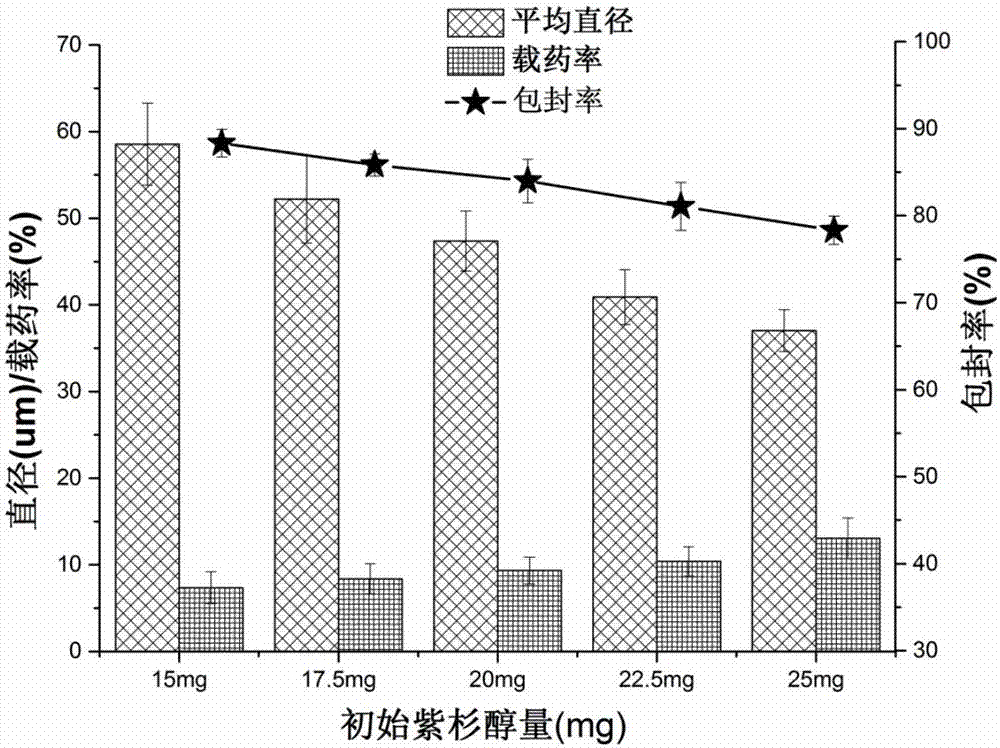
InactiveCN107049984ARound shapeSmall particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSide effectPolyvinyl alcohol
The invention belongs to the field of preparation of targeted therapeutic microspheres of slow release formulation and in particular relates to a preparation method of taxol-loading polylactic acid-hydroxyacetic acid (PLGA) microspheres. The preparation method comprises the following steps: dissolving a polylactic acid-hydroxyacetic acid copolymer in an organic solvent, and adding a taxol drug, wherein the polymer and the taxol drug are uniformly dissolved to obtain an organic phase; dropwise adding the organic phase into a fresh polyvinyl alcohol aqueous solution and curing and forming microspheres; and performing centrifugal separation on the cured drug-loading microspheres to obtain the uniformly dispersed taxol-loading polylactic acid-hydroxyacetic acid (PLGA) microspheres. The taxol-loading targeted therapeutic PLGA microspheres prepared by the preparation method have an ideal drug loading ratio and encapsulation efficiency while guaranteeing the drug stability of taxol, can reach an effect of slow release, maintain the optimum drug concentration in vivo, prolong the action time of the drug and reduce the side effects brought by burst release of the drug, and have important theoretical value and actual application prospect.
Owner:WUHAN UNIV OF TECH
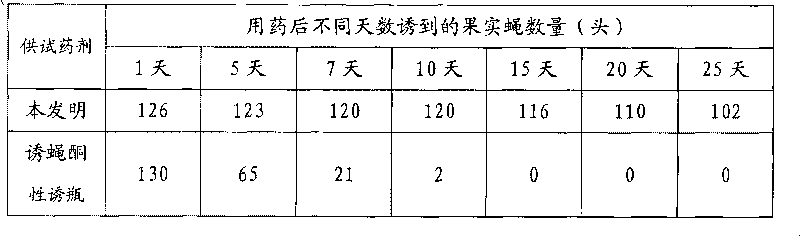
Preparation method of sexual attraction slow-release formulation for fruit flies
The invention relates to a preparation method of a sexual attraction slow-release formulation for fruit flies. By weight parts, the preparation method comprises the following steps: (1) dissolving 1-50 parts of methyl eugenol or cue-lure and 0.1-20 parts of toxic ingredients in 100 parts of organic solvent, for standby application, wherein the toxic ingredients include trichlorphon, abamectin, and emamectin benzoate or chlopyrifos; (2) stirring 1000 parts of polyatomic alcohol with molecular weight smaller than 3000 for 15-100 minutes at the temperature of 35-75 DEG C and vacuum degree of minus 0.1MPa; and (3) adding the standby solution of the step (1) in the polyatomic alcohol of the step (2), continuing stirring to be uniform, then adding 3000-10000 parts of isocyanate, continuing stirring for 120 minutes at the temperature of 35-75 DEG C and vacuum degree of minus 0.1MPa, stopping vacuum-pumping, and then obtaining pasty sexual attraction slow-release formulation for the fruit flies. The sexual attraction slow-release formulation prepared by the method is mainly used for monitoring fruit fly population density or preventing and controlling fruit fly pests in large area.
Owner:HUBEI GREAT BIOTECH
Pharmaceutical formulation for contraception and hormone-replacement therapy
InactiveUS20050025827A1Minimize and eliminate undesirable side effectAvoiding undesirable hormone imbalanceOrganic active ingredientsCosmetic preparationsRegimenCholesterol
The present invention provides slow release estradiol-progesterone formulations that can be used in either contraception or hormone replacement therapies. The formulations comprise shaped particles of estradiol that is in a hemicrystalline form that exhibits especially low dissolution rates. The shaped particles comprise estradiol compounded in a 1:1 molar ratio with cholesterol, and are administered in combination with progesterone. The slow release formulations of the present invention afford the dual advantages of a low dose estradiol formulation with a low frequency administration regimen. The formulations can be parenterally administered once a month or less often.
Owner:SKENDI FINANCE
Sustaining agent of Duosuo theosine and its preparation
A slow-release doxofylline is composed of a fast-release component prepared from doxofylline (10-60%) and fast-release assistant and a slow-release component prepared from doxofylline (40-90%) and slow-release assistant. Its preparing process is also disclosed.
Owner:HEFEI HEYUAN PHARM TECH CO LTD
Pesticidal disease protecting and nutrient pesticide fertilizer and application thereof, and pesticidal disease protecting and nutrient pesticide fertilizer slow release formulation and application thereof
The invention relates to the field of pesticides and fertilizers, in particular to a pesticidal disease protecting and nutrient pesticide fertilizer and application thereof, and a pesticidal disease protecting and nutrient pesticide fertilizer slow release formulation and application thereof. The pesticidal disease protecting and nutrient pesticide fertilizer comprises 0.001 to 12 parts of thiamethoxam, 0.001 to 5 parts of thifluzamide, 20 to 60 parts of effective fertilizer composition, 0.01 to 10 parts of secondary element fertilizer effective composition, 0.001 to 5 parts of trace element fertilizer effective composition, and 0.001 to 1 part of beneficial element fertilizer effective composition in part by mass, wherein the effective fertilizer composition is selected form N, P2O5 and K2O; the secondary element fertilizer effective composition is selected from calcium, magnesium, silicon or sulfur; the trace element fertilizer effective composition is selected from boron, zinc, manganese, molybdenum, iron or copper; the beneficial element fertilizer effective composition is selenium. The pesticide and fertilizer can effectively prevent and cure aphid in wheat field and banded sclerotial blight, can reduce the loss of wheat field caused by insect pest after being applied, and has good yield-increasing effect.
Owner:LIANBAO CROP TECH
Methods and compositions
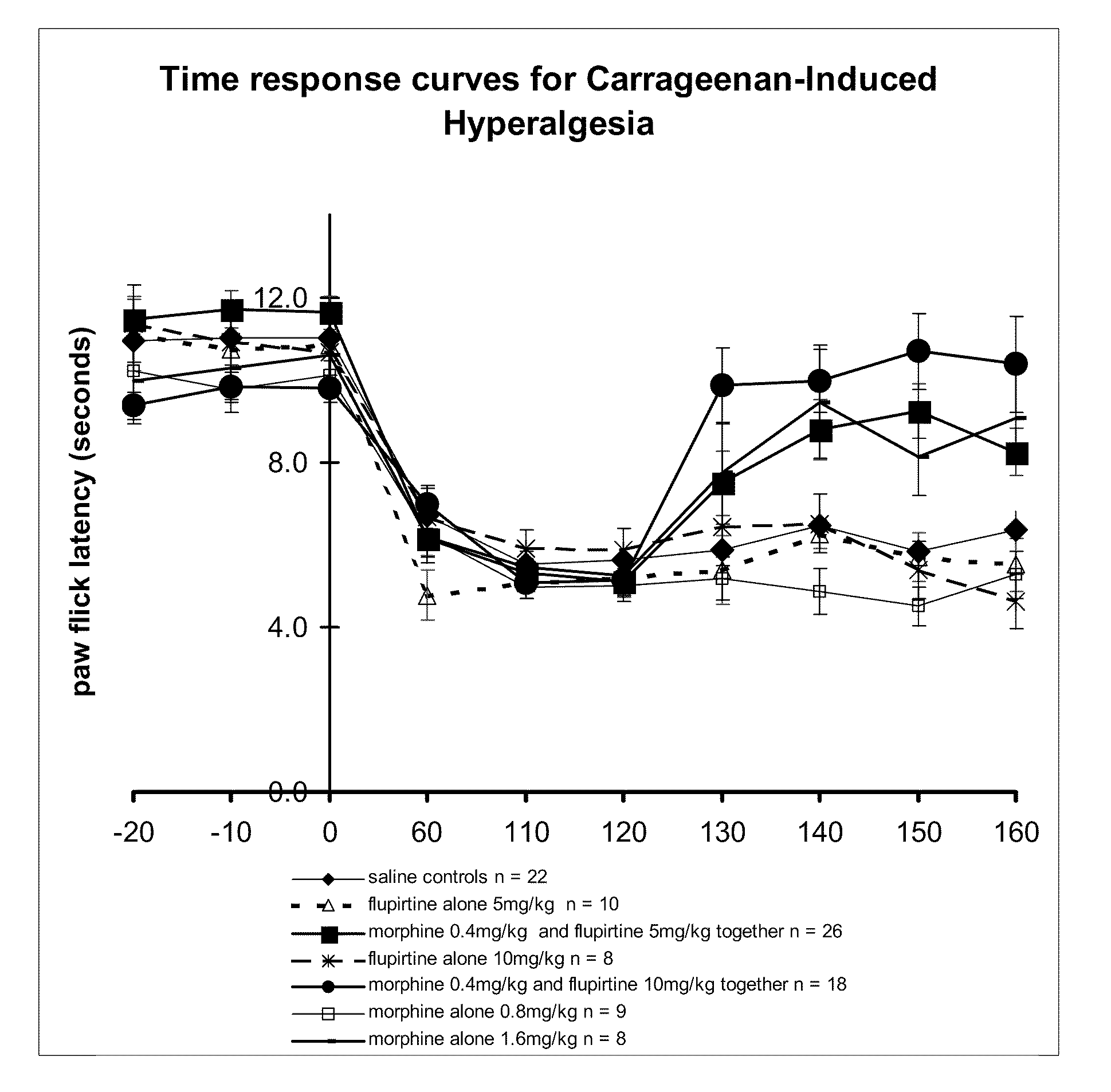
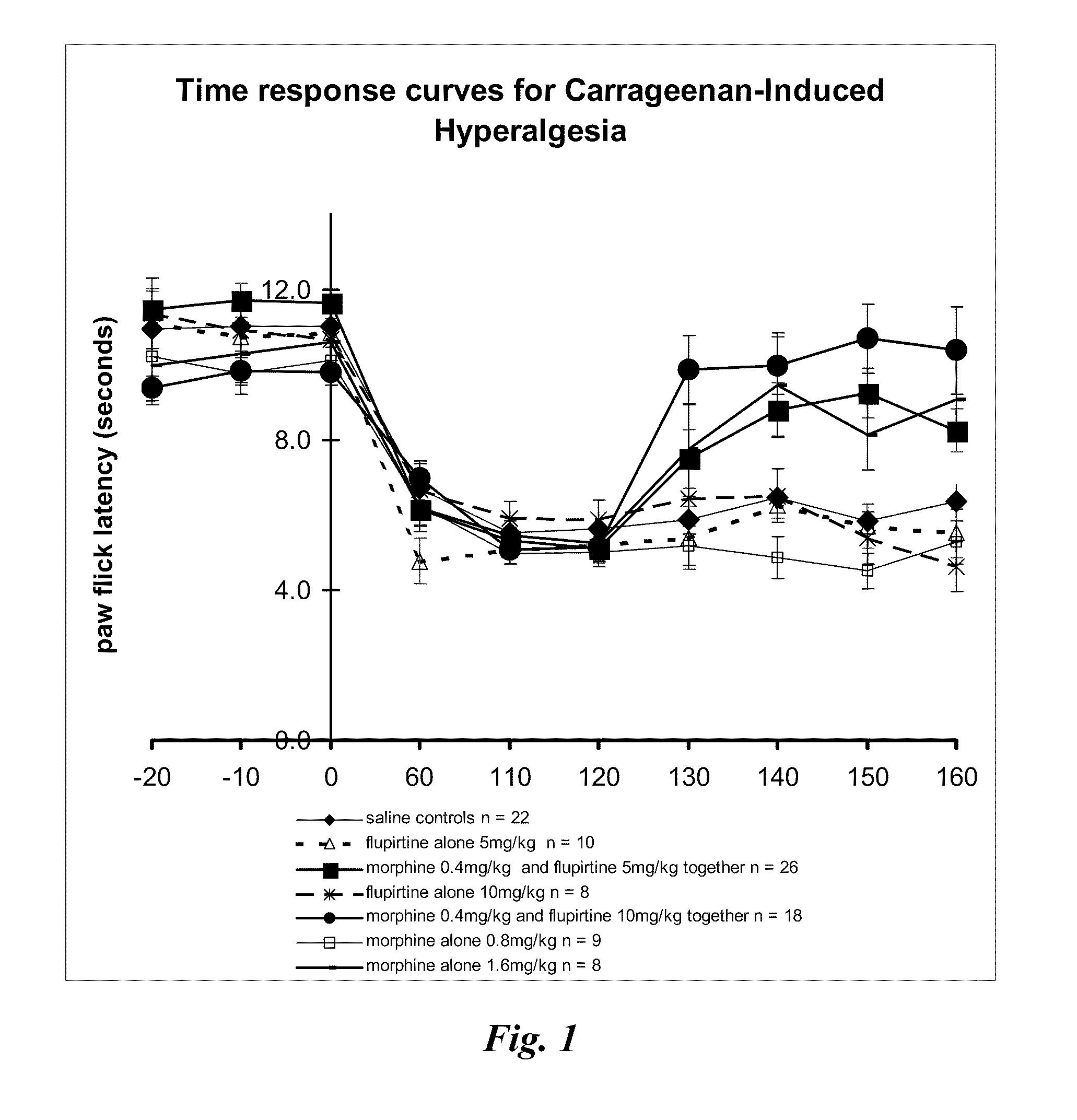
InactiveUS20110092482A1Lower Level RequirementsReduce of sensationBiocideNervous disorderDiseaseSlow Release Formulation
The present invention relates generally to the field of pain management, and in particular, the management of neuropathic or inflammatory pain including a neuropathic or inflammatory component of nociceptive pain. More particularly, the present invention provides methods and compositions which treat, alleviate, prevent, diminish or otherwise ameliorate the symptoms of neuropathic or inflammatory pain. The present invention further contemplates combination therapy involved in the treatment of pain in association with the treatment of a particular disease condition or pathology. The present invention further also provides sustained and slow release formulations, tamper-proof deliver systems and stents, catheters and other mechanical devices coated with formulations which permit sustained or slow release of active ingredients involved in pain management.
Owner:RELEVARE AUST
Preparation method of pH sensitiveness raphanin chitosan microsphere
InactiveCN103006572AFacilitated releaseImprove stabilityPharmaceutical non-active ingredientsGranular deliveryCross-linkRaphanin
The invention discloses a preparation method of a pH sensitiveness raphanin chitosan microsphere. The preparation method comprises the following steps: 1) dissolving chitosan powder into an acetic acid solution; 2) taking a raphanin water solution and adding into the acetic acid solution; and 3) dropping a sodium tripolyphosphate solution into the mixture liquid obtained in the step 2) so as to obtain the raphanin-chitosan microsphere. The preparation method of the pH sensitiveness raphanin chitosan microsphere is reasonable in design, and the microsphere is prepared by using an ionic cross-linking method in a mode that the chitosan is taken as a carrier and the sodium tripolyphosphate is taken as a cross-linking agent, the experiment is simple and mild, and no organic solvents are used, so that the method is safe and environmental-friendly, a drug carrying microsphere solution of which the average particle size is 500 nm is successfully prepared, and moreover the prepared microsphere solution is good in stability and has no great change in particle size after being placed for a long time; under the acidic condition, the raphanin can be released more easily, and the higher the pH value is, the longer the releasing time is, and therefore the microsphere is an ideal slow release formulation of the raphanin.
Owner:ZHEJIANG KUIRUI AGRI DEV CO LTD
Slow-release preparation containing beta-lactamase inhibitor and its use
InactiveCN1850046AEasy to operateGood repeatabilityAntibacterial agentsAntisepticsDiseaseTreatment effect
The present invention relates to a slow-released preparation containing beta-lactamase inhibitor. Said slow-released preparation can be made into antibiotic slow-released injection or slow-released implant preparation. Said injection is formed from slow-released microsphere and solvent, said slow-released microsphere contains slow-released auxiliary material and beta-lactamase inhibitor with antibacterial effective dose and penicillin antibiotics, the solvent is special solvent containing suspension adjuvant of carboxymethyl cellulose sodium, etc. its viscosity is 100 cp-3000 cp (20 deg.C-30deg.C); the slow-released auxiliary material is selected from EVAc, polylactic acid copolymer, sebacic acid copolymer, albumin glue and glatin, etc. The slow-released implant preparation is prepared by using slow-released microsphere or adopting melting process. Said invention also provides its application range and can obtain obvious therapeutic effect for curing various infective diseases.
Owner:JINAN SHUAIHUA PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com