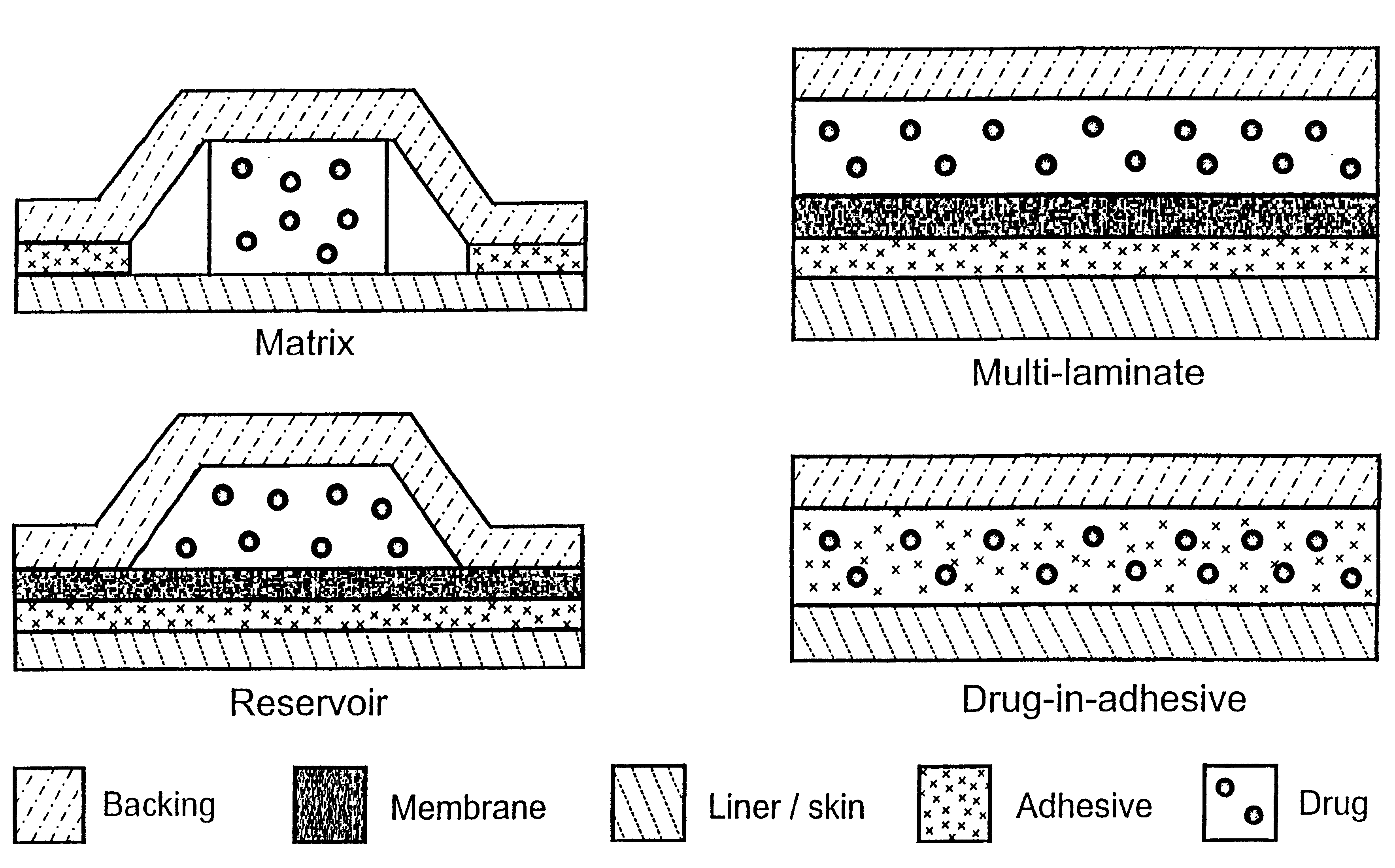
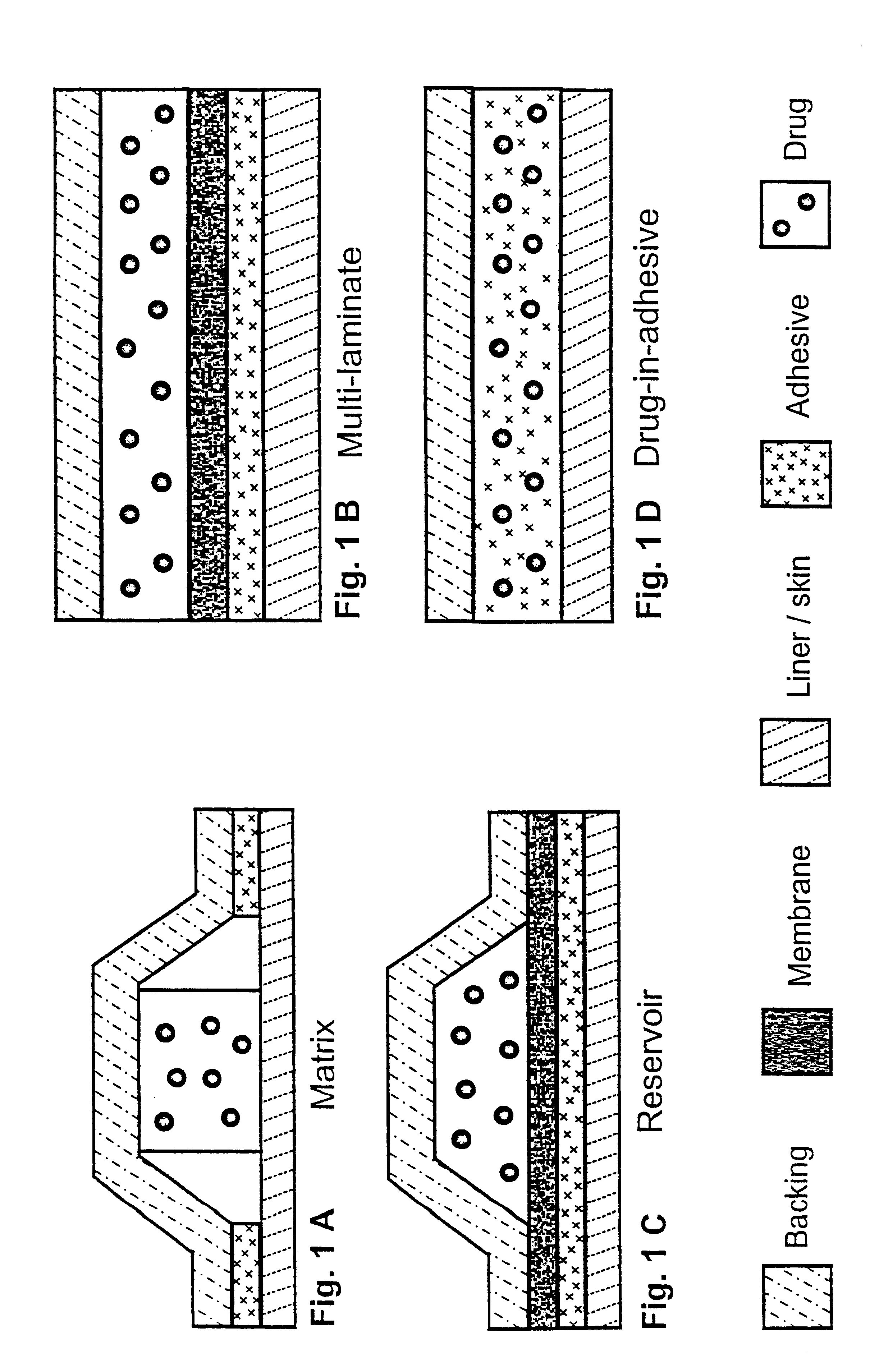
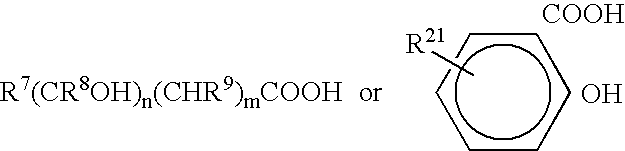Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
20980results about "Ointment delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
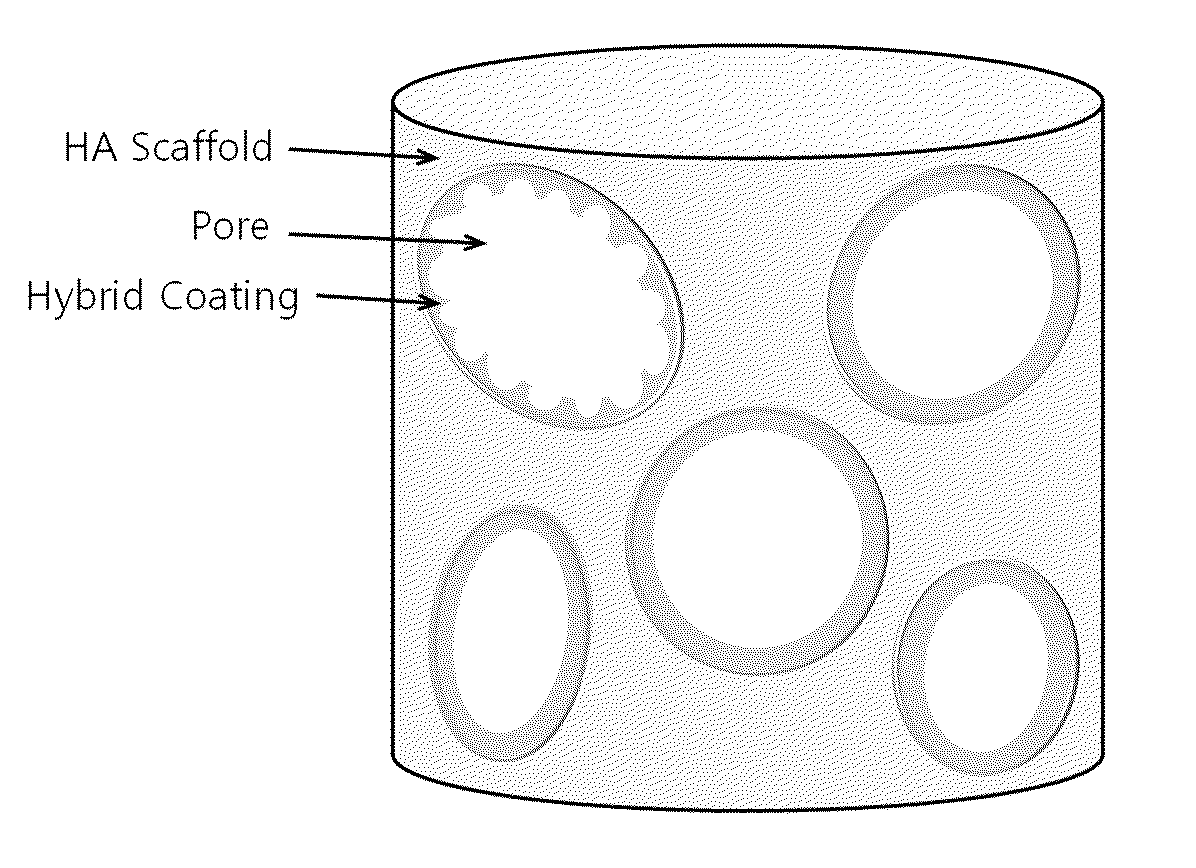
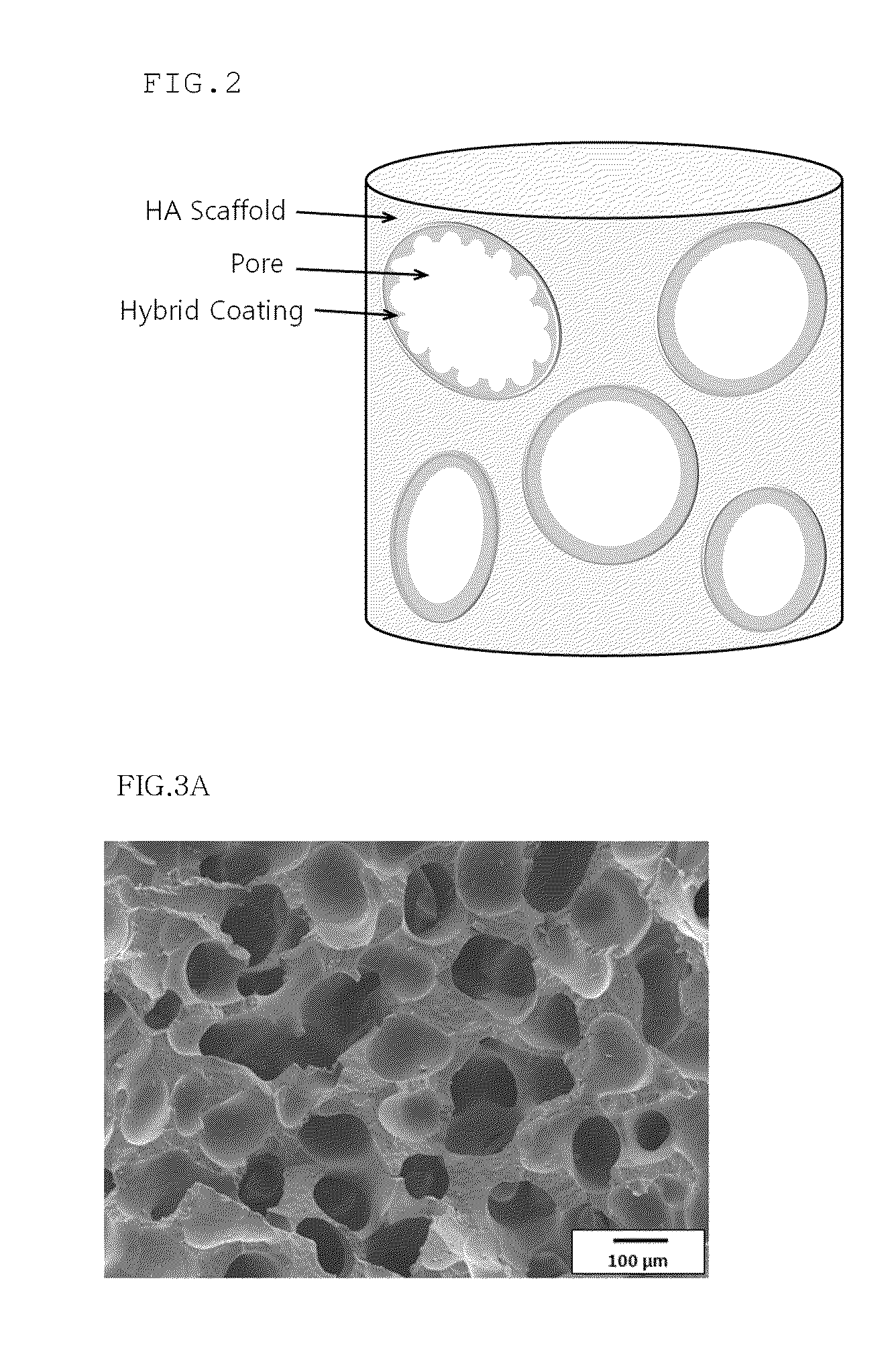
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
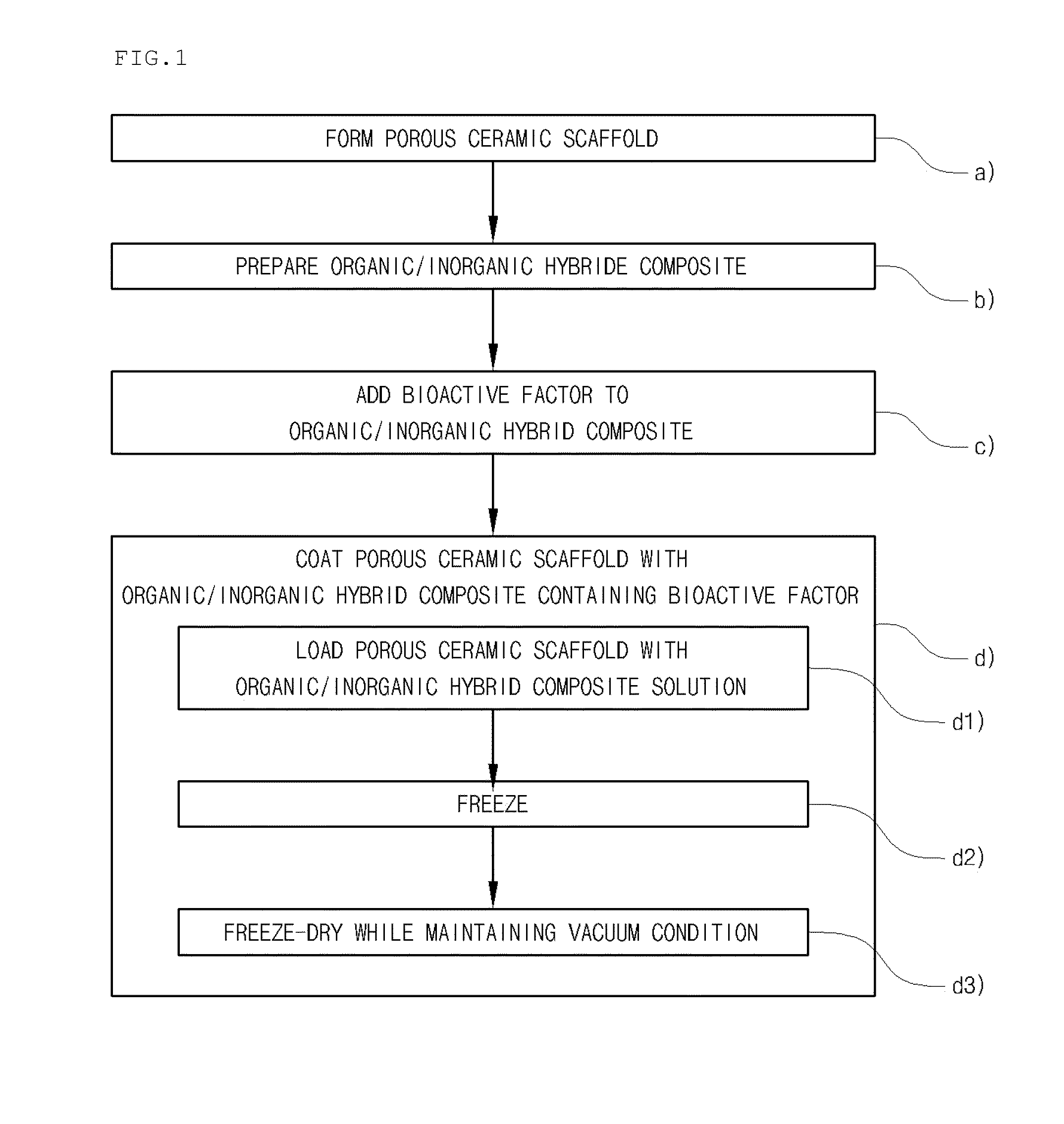
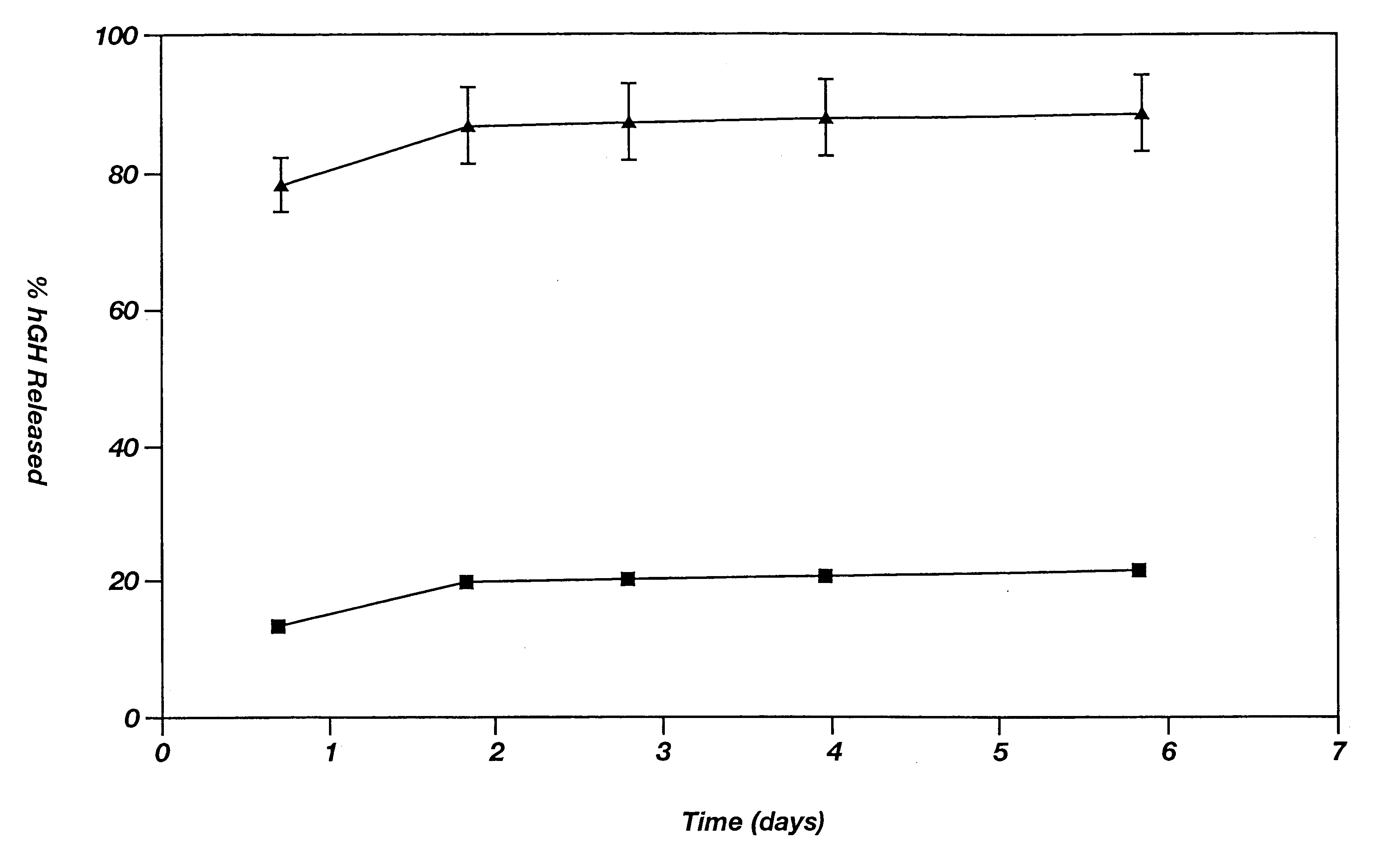
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
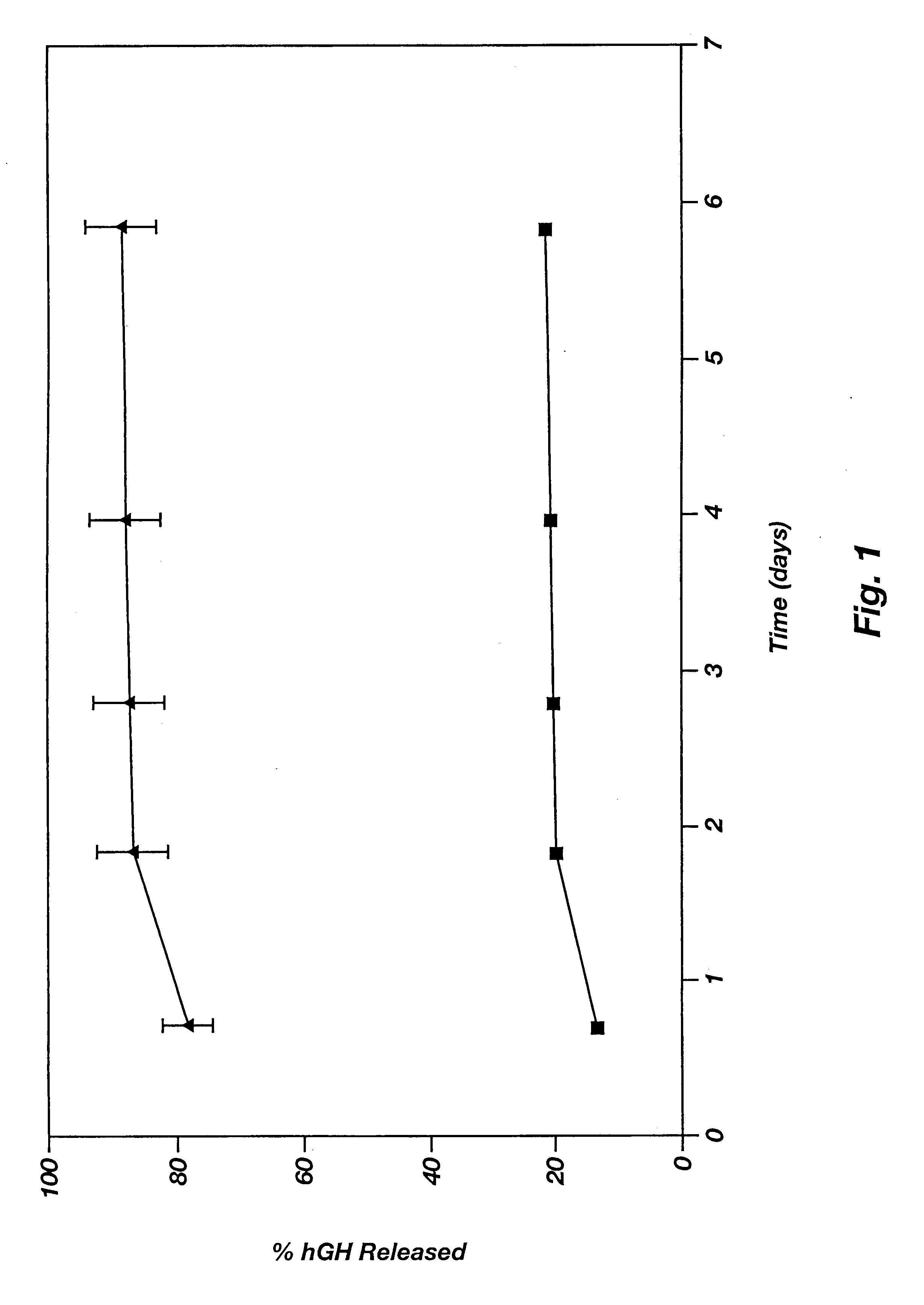
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
N-acetyl aldosamines, n-acetylamino acids and related n-acetyl compounds and their topical use
Compositions comprising N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl compounds are useful to alleviate or improve various cosmetic conditions and dermatological disorders, including changes or damage to skin, nail and hair associated with intrinsic aging and / or extrinsic aging, as well as changes or damage caused by extrinsic factors. N-acetyl-aldosamines, N-acetylamino acids, and related N-acetyl composition may further comprise a cosmetic, pharmaceutical or other topical agent to enhance or create synergetic effects.
Owner:TRISTRATA TECH
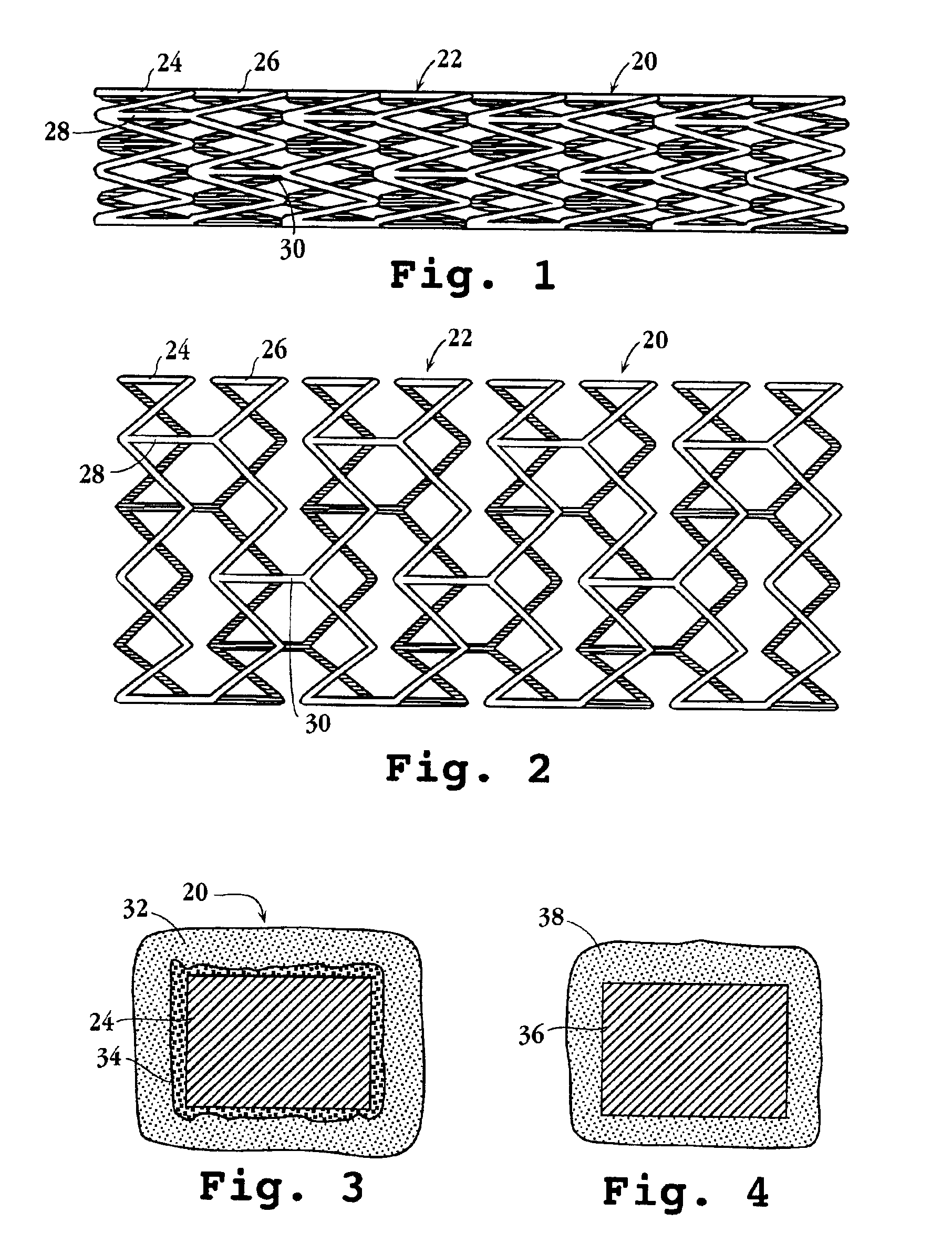
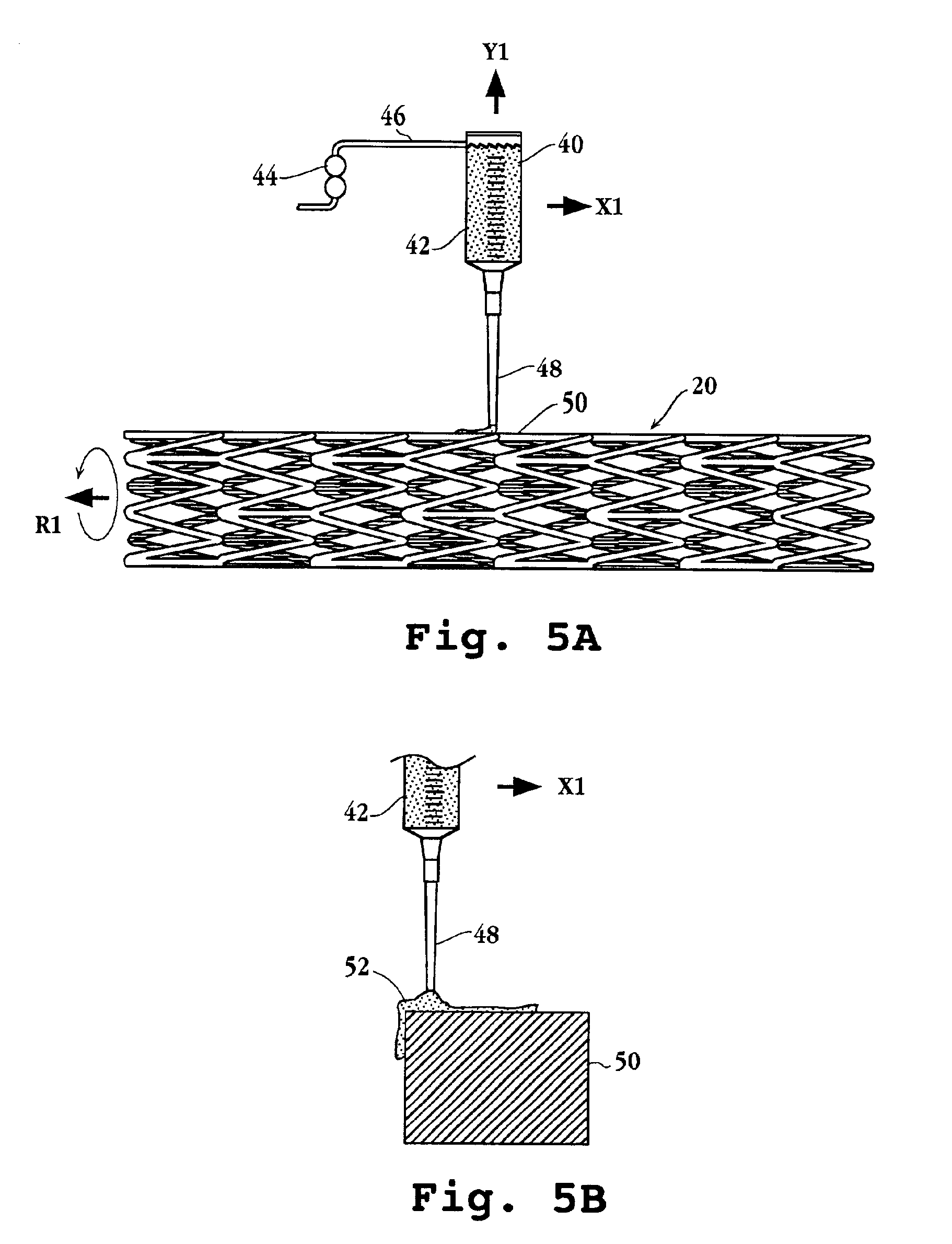
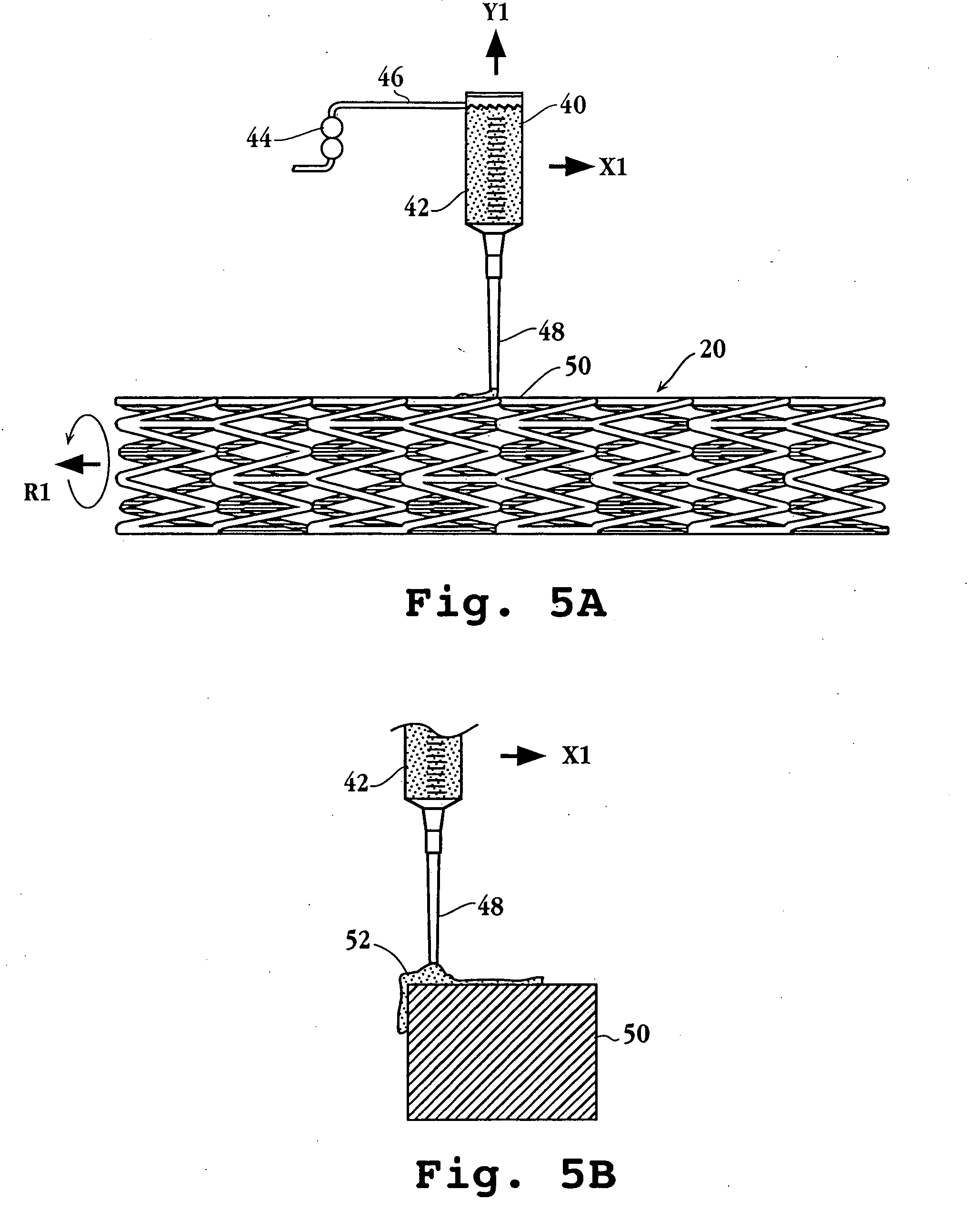
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Cationic antiseptic compositions and methods of use
ActiveUS20060051385A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideAmmonium compoundsCetylpyridinium
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including a cationic antiseptic such as biguanides and bisbiguanides such as chlorhexidine and its various salts including but not limited to the digluconate, diacetate, dimethosulfate, and dilactate salts; polymeric quaternary ammonium compounds such as polyhexamethylenebiguanide; silver and various silver complexes; small molecule quaternary ammonium compounds such as benzalkoium chloride and alkyl substituted derivatives; di-long chain alkyl (C8-C18) quaternary ammonium compounds; cetylpyridinium halides and their derivatives; benzethonium chloride and its alkyl substituted derivatives; and octenidine. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Compositions and methods for treating contracture
InactiveUS20050186261A1Prevent and minimize contracture formationPrevent relapseBiocideMuscular disorderPsychiatryContracture
A method for treating contracture is provided that includes administering to a patient in need thereof a composition that includes a therapeutic agent effective in treating contracture. Compositions, devices, and kits for use in treating contracture are also described.
Owner:ANGIOTECH INT AG (CH)
Poly(vinyl alcohol) hydrogel
InactiveUS6231605B1Exemption stepsLow biocompatibilityOrganic active ingredientsBone implantBearing surfaceSacroiliac joint
The present invention relate to a poly(vinyl alcohol) hydrogel construct having a wide range of mechanical strengths for use as a human tissue replacement. The hydrogel construct may include a tissue scaffolding, a low bearing surface within a joint, or any other structure which is suitable for supporting the growth of tissue.
Owner:GEORGIA TECH RES CORP
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
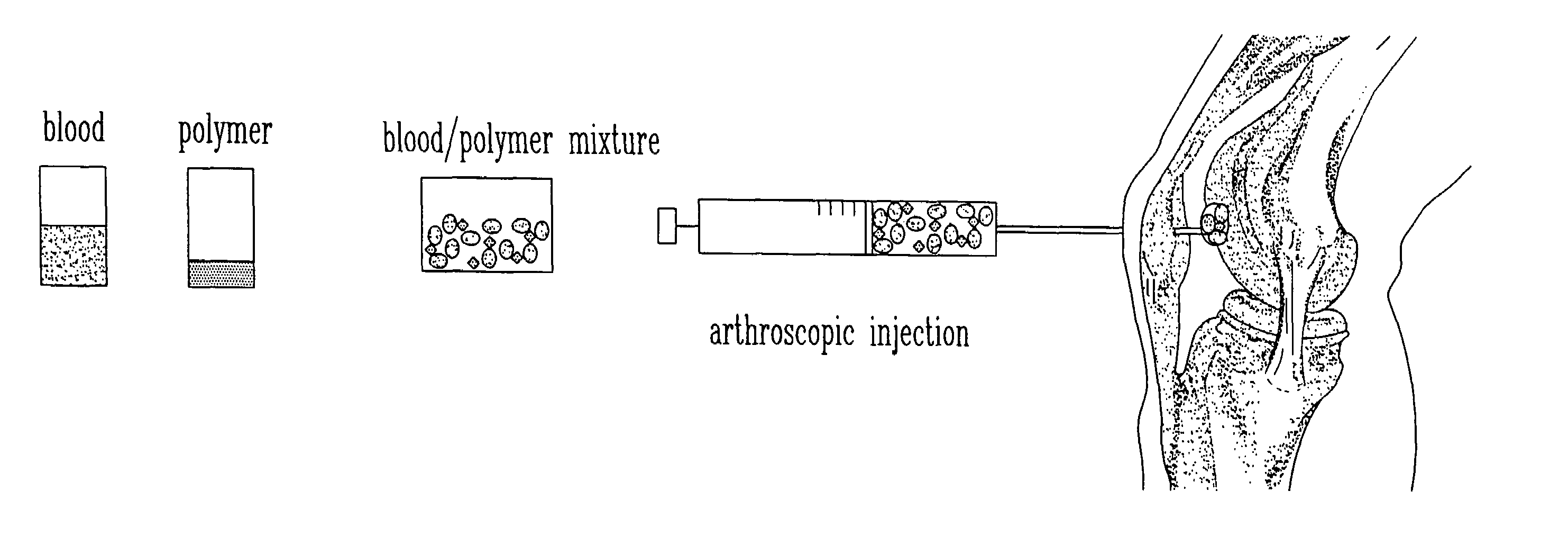
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS7148209B2Add supportImprove coagulation/solidificationBiocidePeptide/protein ingredientsAbnormal tissue growthRepair tissue
Owner:SMITH & NEPHEW ORTHOPAEDICS
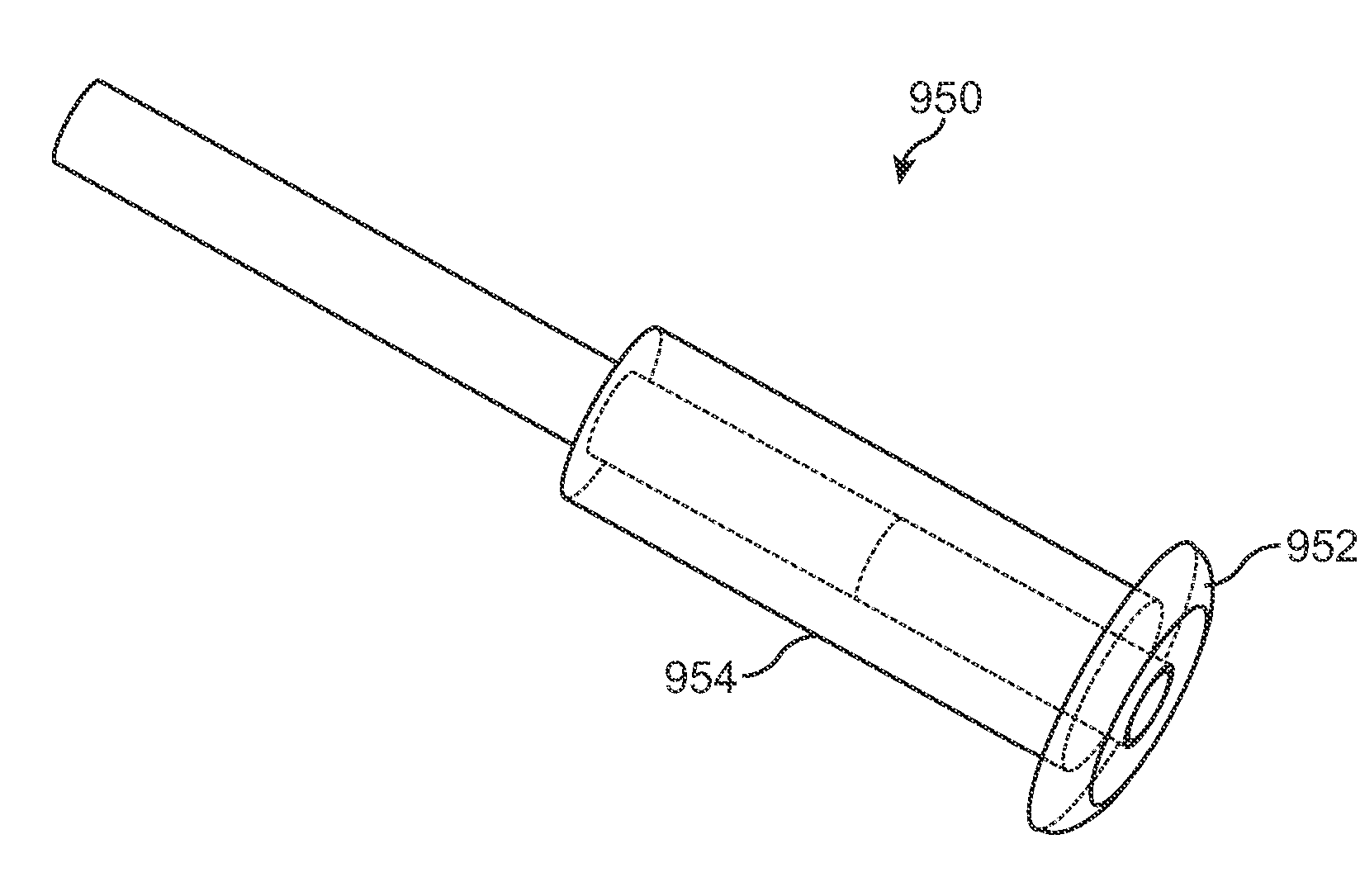
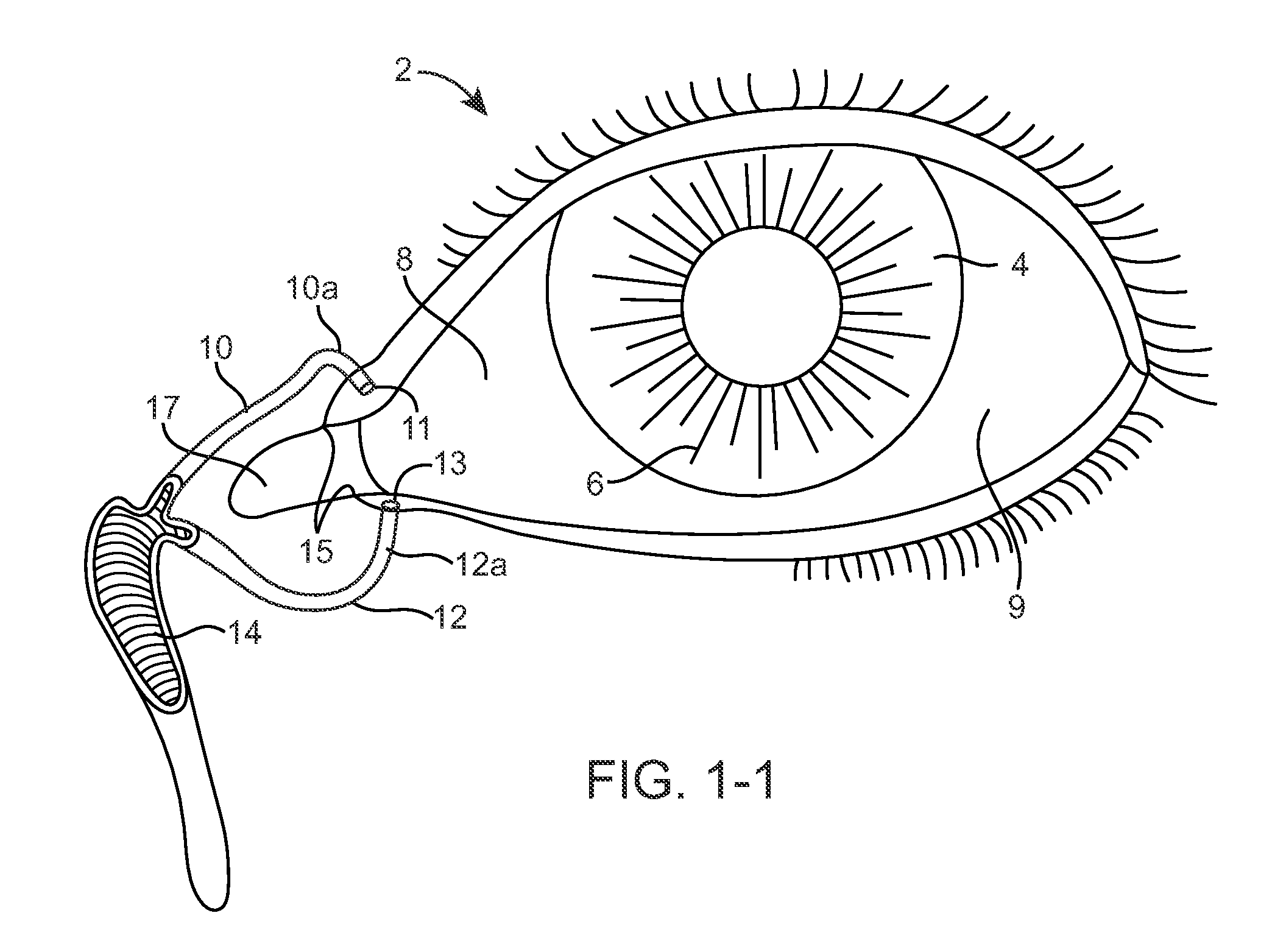
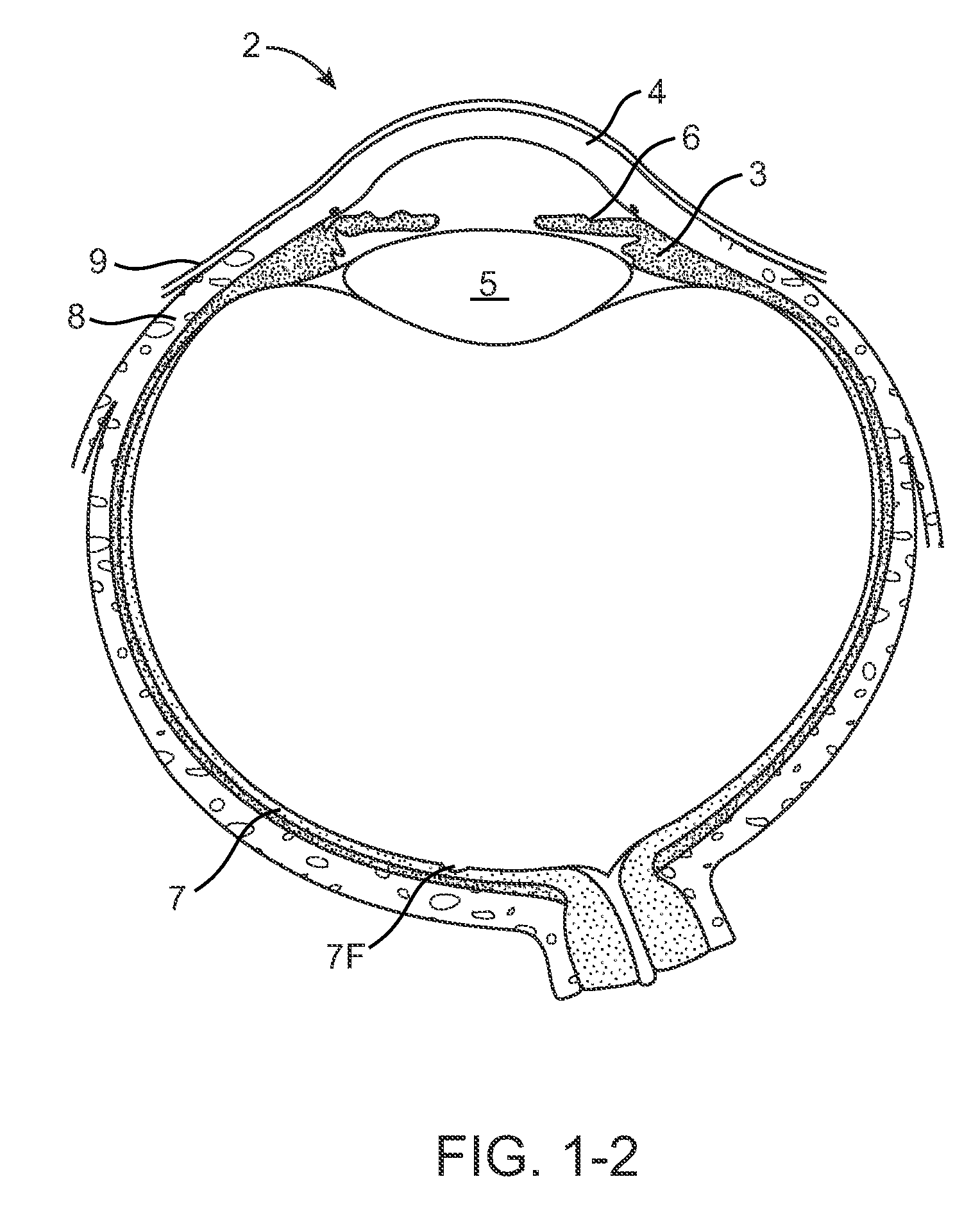
Nasolacrimal Drainage System Implants for Drug Therapy
ActiveUS20070243230A1Reduce deliveryAvoid flowAntibacterial agentsSenses disorderShape-memory alloyImplanted device
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
Isolation, cultivation and uses of stem/progenitor cells
The present invention relates to a method for isolating stem / progenitor cells from the amniotic membrane of umbilical cord, wherein the method comprises separating the amniotic membrane from the other components of the umbilical cord in vitro, culturing the amniotic membrane tissue under conditions allowing cell proliferation, and isolating the stem / progenitor cells from the tissue cultures. The isolated stem cell cells can have embryonic stem cell-like properties and can be used for various therapeutic purposes. In one embodiment, the invention relates to the isolation and cultivation of stem cells such as epithelial and / or mesenchymal stem / progenitor cells under conditions allowing the cells to undergo mitotic expansion. Furthermore, the invention is directed to a method for the differentiation of the isolated stem / progenitor cells into epithelial and / or mesenchymal cells.
Owner:CELLRESEARCH CORP PTE LTD
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
ActiveUS20070269487A1Avoid expulsionInhibition releaseAntibacterial agentsPowder deliveryEffective treatmentBiomedical engineering
Owner:MATI THERAPEUTICS
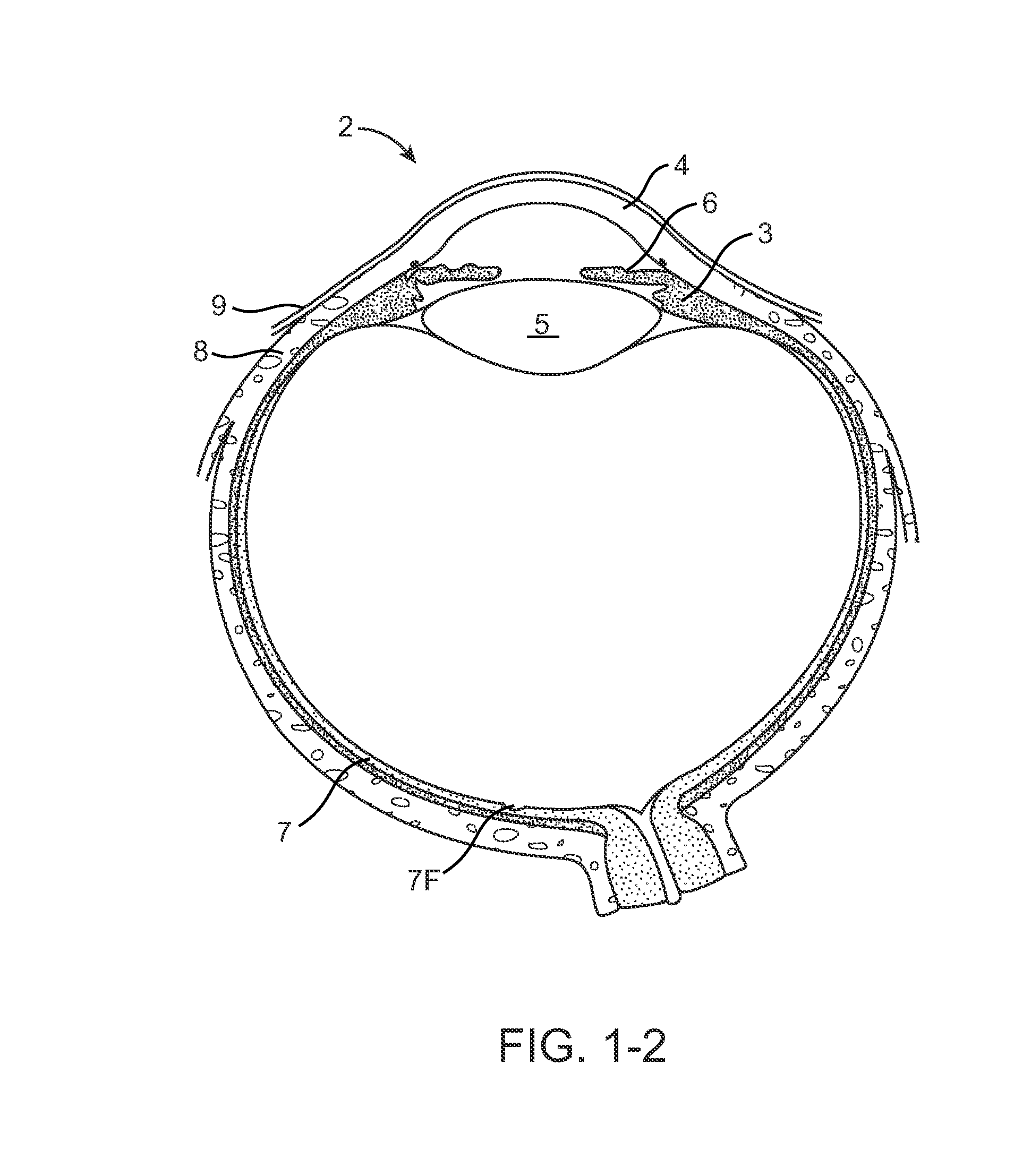
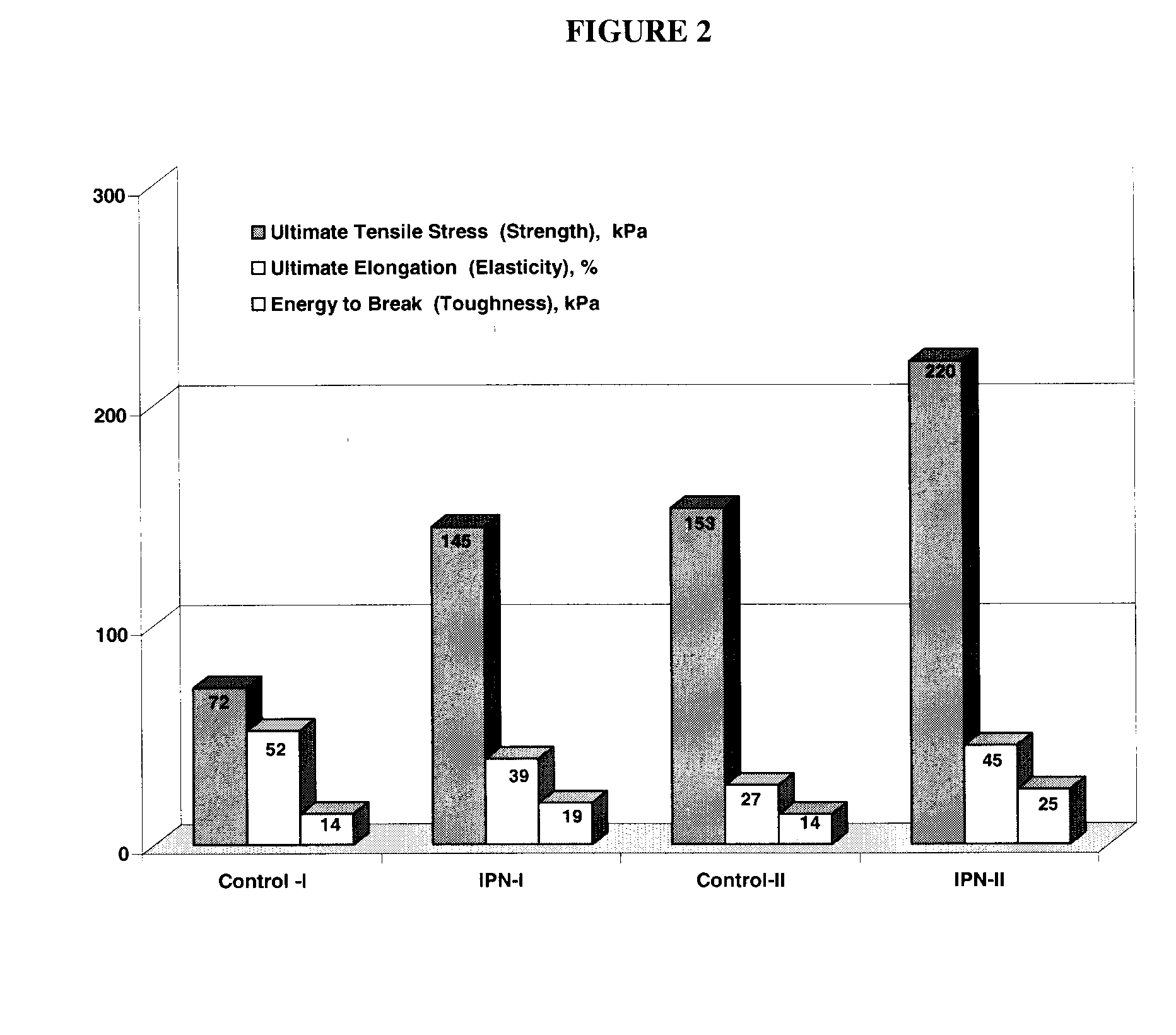
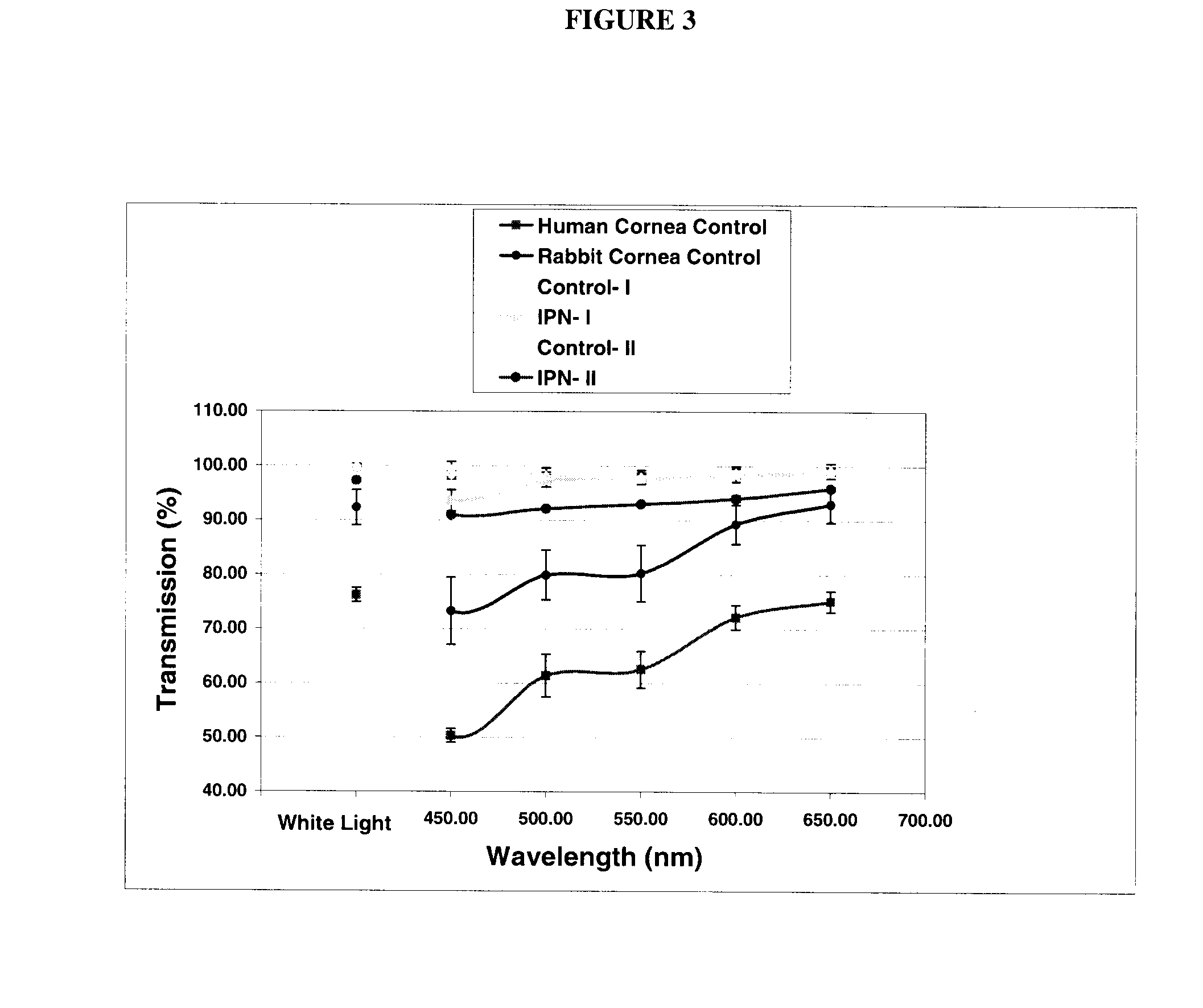
Interpenetrating Networks, and Related Methods and Compositions
The present invention provides interpenetrating polymeric networks (IPNs), and related methods and compositions. The hydrogel material of this invention comprises an interpenetrating network of two or more polymer networks, wherein at least one of the polymer networks is based on a biopolymer. Also provided is a method of producing the hydrogel material comprising, combining a first polymeric network with a second polymeric network, wherein the first polymeric network or the second polymeric network is based on a biopolymer. The present application also discloses devices manufactured from the IPN hydrogel material and uses thereof.
Owner:OTTAWA HOSPITAL RES INST +1
Oral care compositions containing combinations of anti-bacterial and host-response modulating agents
InactiveUS20070053849A1Potent anti-inflammatory activityPromote progressAntibacterial agentsCosmetic preparationsWhole bodyOral bacterial infection
The present invention encompasses topical oral care compositions comprising the combination of an anti-bacterial agent with an anti-inflammatory agent in an orally acceptable carrier for effective treatment and prevention of bacteria-mediated diseases and conditions in the oral cavity and for modulating host reaction to bacterial pathogens present in the oral cavity and to the toxins, endotoxins, inflammatory cytokines and mediators released by or prompted by these pathogens. The present invention also encompasses methods of use of these compositions comprising topical application to the oral cavity. The benefits of the present compositions and methods extend beyond treating and preventing oral bacterial infections in the oral cavity to promoting whole body or systemic health.
Owner:THE PROCTER & GAMBLE COMPANY
Capsaicinoid gel formulation and uses thereof
InactiveUS20060148903A1Relieve painReduce the amount requiredBiocideNervous disorderSurgical sitePost-Procedural Pain
The present invention provides capsaicinoid gel formulations and methods for relieving pre- and post-surgical pain at a site in a human or animal by administering at a surgical site in a human or animal in need thereof a dose of capsaicinoid gel in an amount effective to attenuate post-surgical pain at the surgical site, the dose of capsaicin ranging from 100 μg to 10,000 μg.
Owner:ALGORX PHARMA INC
Topical nitric oxide donor compositions
InactiveUS6287601B1Reduced and failing organ functionPoor appetitePowder deliveryBiocideLipid formationEquine Species
Compositions and methods of the topical treatment of equine laminitis are disclosed. In particular, combinations of a fast acting nitric oxide (NO) donor, a sustained acting NO donor and an NSAID mixed in a lipid-based carrier are described. The application of such combinations to the affected areas, e.g., the hoofs and surrounding tissues, of an equine afflicted with laminitis provides relief from the debilitating effects of this painful, often life-threatening condition.
Owner:STREHKEHN INT LTD
Topical Pharmaceutical Foam Composition
InactiveUS20070154402A1Reduced intensity of colorReduce odor intensityAntibacterial agentsBiocideAlcohol freeActive agent
A stable topical alcohol-free aerosol foam containing one or more keratolytic agents is provided. The foam-forming formulation is an emulsion which contains an HFA propellant and one or more keratolytic agents. The emulsion has an oil phase and an aqueous, i.e. water-containing, phase. The active agent(s) may be present in either phase of the emulsion or dispersed in the emulsion. The oil phase may consist at least in part of the HFA propellant. Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and / or other excipients. The foam is stable on the skin, for example, for at least 5 minutes at body temperature, preferably at least 20 minutes at body temperature, and disappears into the skin upon rubbing or after prolonged standing. In one embodiment, the formulation contains an HFA propellant which does not contain additional co-solvents or co-propellants. The formulations demonstrate reduced intensity of the odor and / or color associated with the keratolytic agent(s) as compared to conventional formulations containing keratolytic agents.
Owner:PRECISION DERMATOLOGY
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS20020076441A1Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentPharmaceutical drug
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
Methods of synthesis and use of chemospheres
InactiveUS20110104052A1Prevents immunoclearanceExtended half-lifePowder deliveryBiocideDiagnostic agentComputed tomography
The present invention provides, in general, compositions comprising a hydrogel and an agent, for example a therapeutic agent or an imaging agent, for locoregional delivery. In certain preferred embodiments of the invention, the hydrogel compositions are detectable by Magnetic Responance and CT Scan and are used for locoregional delivery of therapeutic agents, for example chemotherapeutic agents. The invention also features polymer matrix compositions comprising nanoparticles that can be loaded after polymerization with bioactive agents, for example a diagnostic agent or therapeutic agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
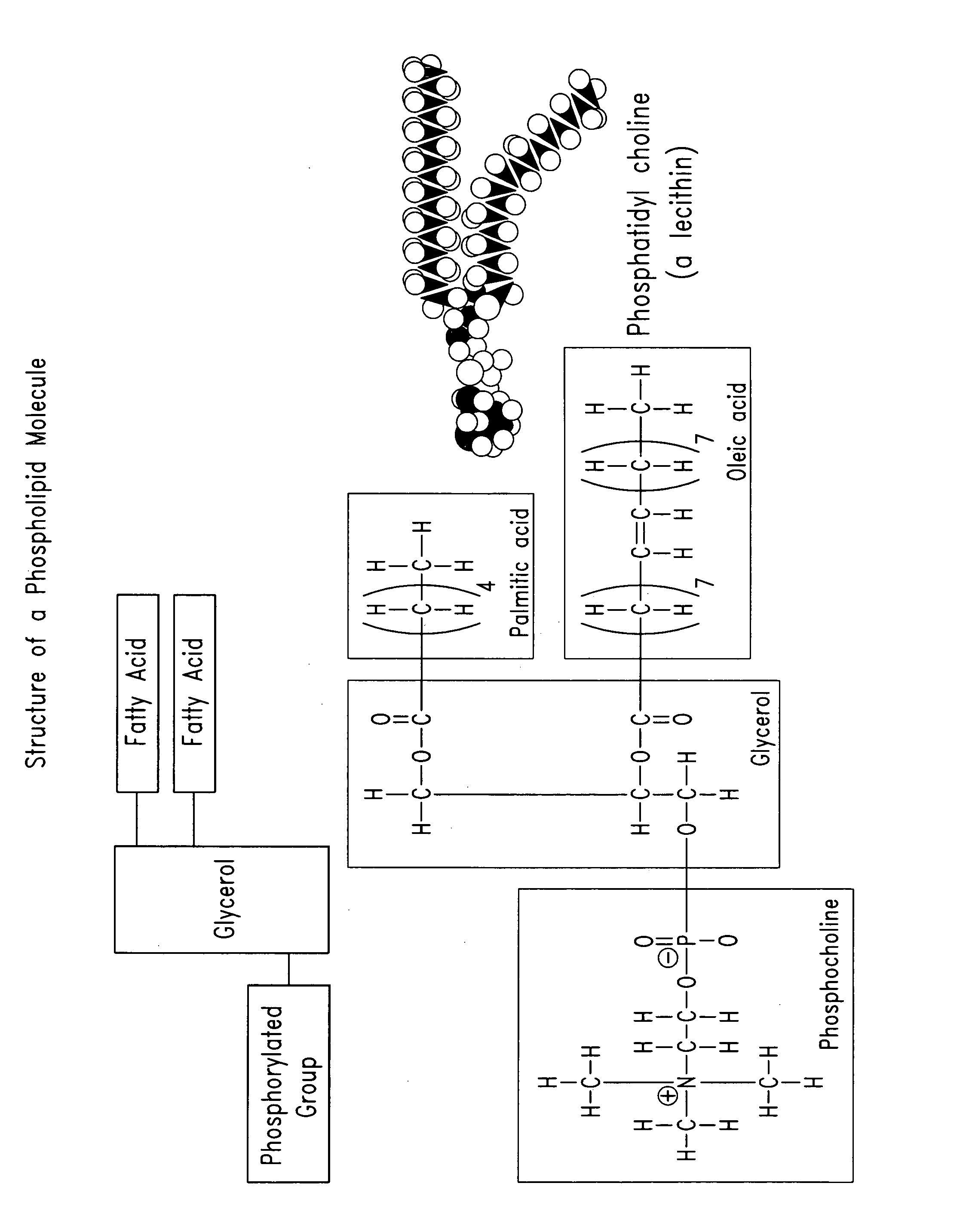
Phospholipid compositions and methods for their preparation and use
The present invention provides compositions that comprise a phospholipid component (that contains one or more phospholipids) and a pharmaceutically acceptable fluid carrier, where the phospholipid component is in the range from about 10% to about 90% of the total weight. Optionally, the compositions may further comprise non-phospholipid filler materials, where the amount of the non-phospholipid filler materials is in the range from about 5% to about 50% of the total weight. In certain embodiments, the compositions may be injectable, non-liposomal, and / or in form of a gel or a paste. The compositions of the present invention are useful for repairing and augmenting soft and / or hard tissues or for sustained local drug delivery.
Owner:ENCORE THERAPEUTICS
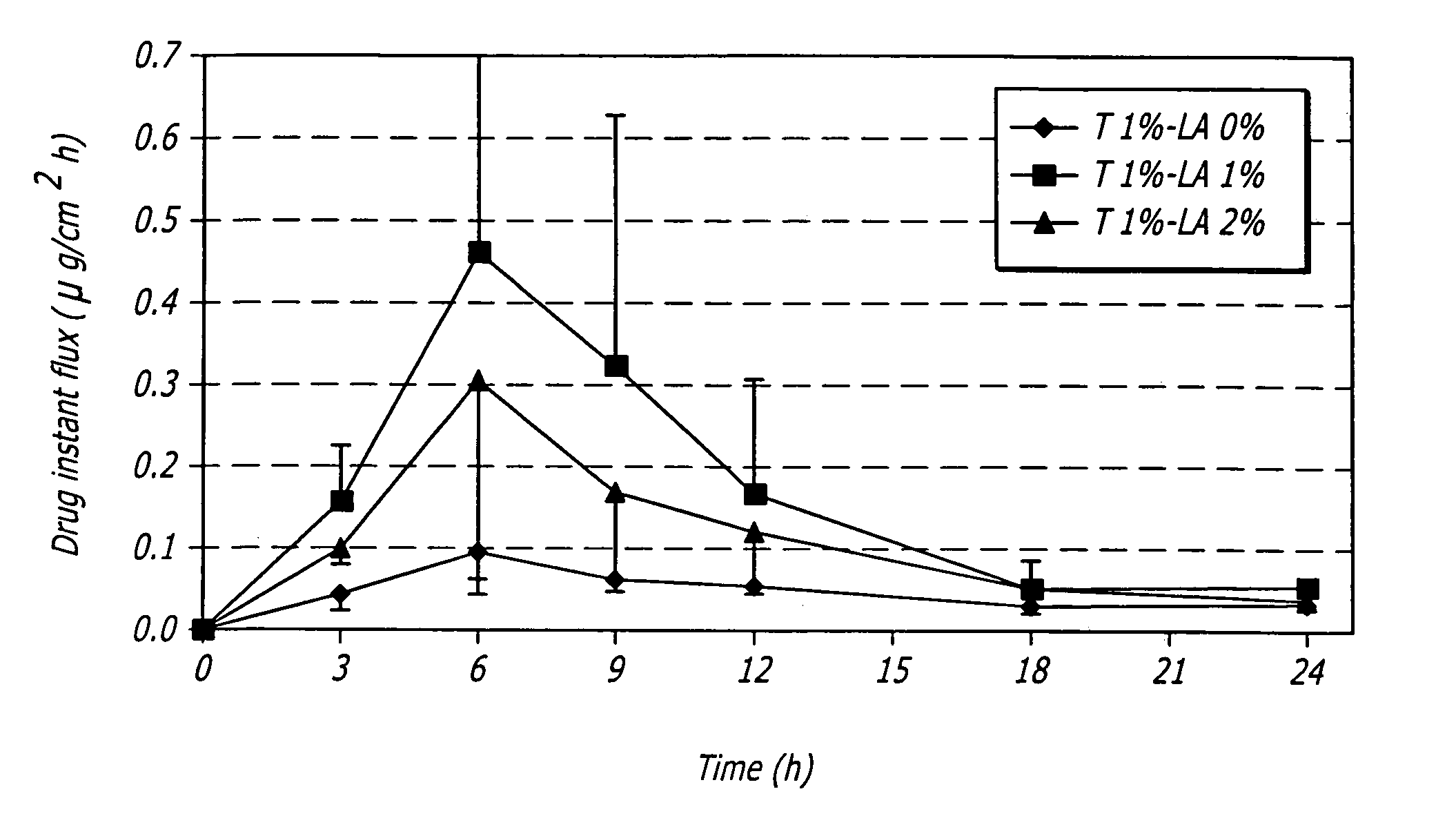
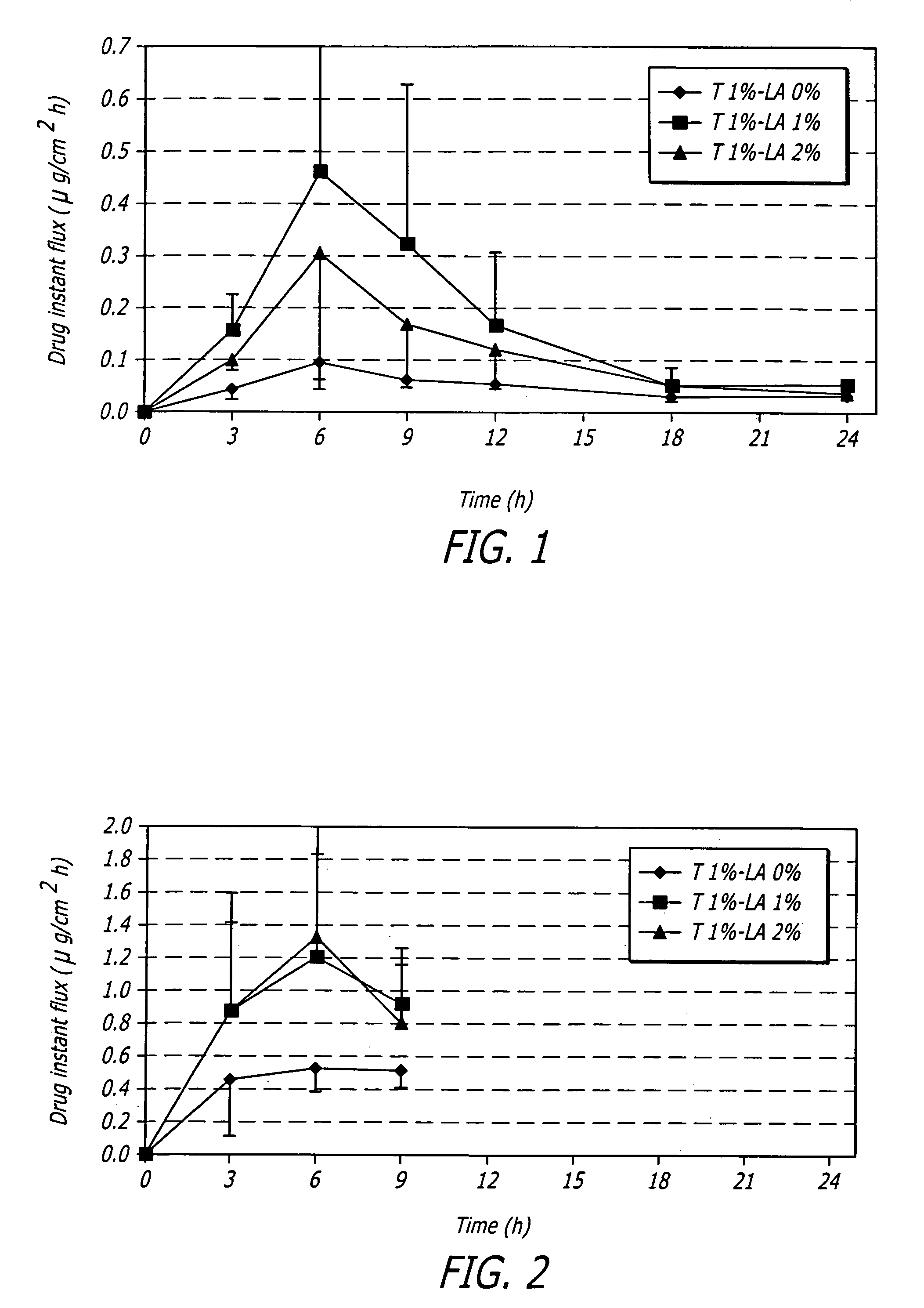
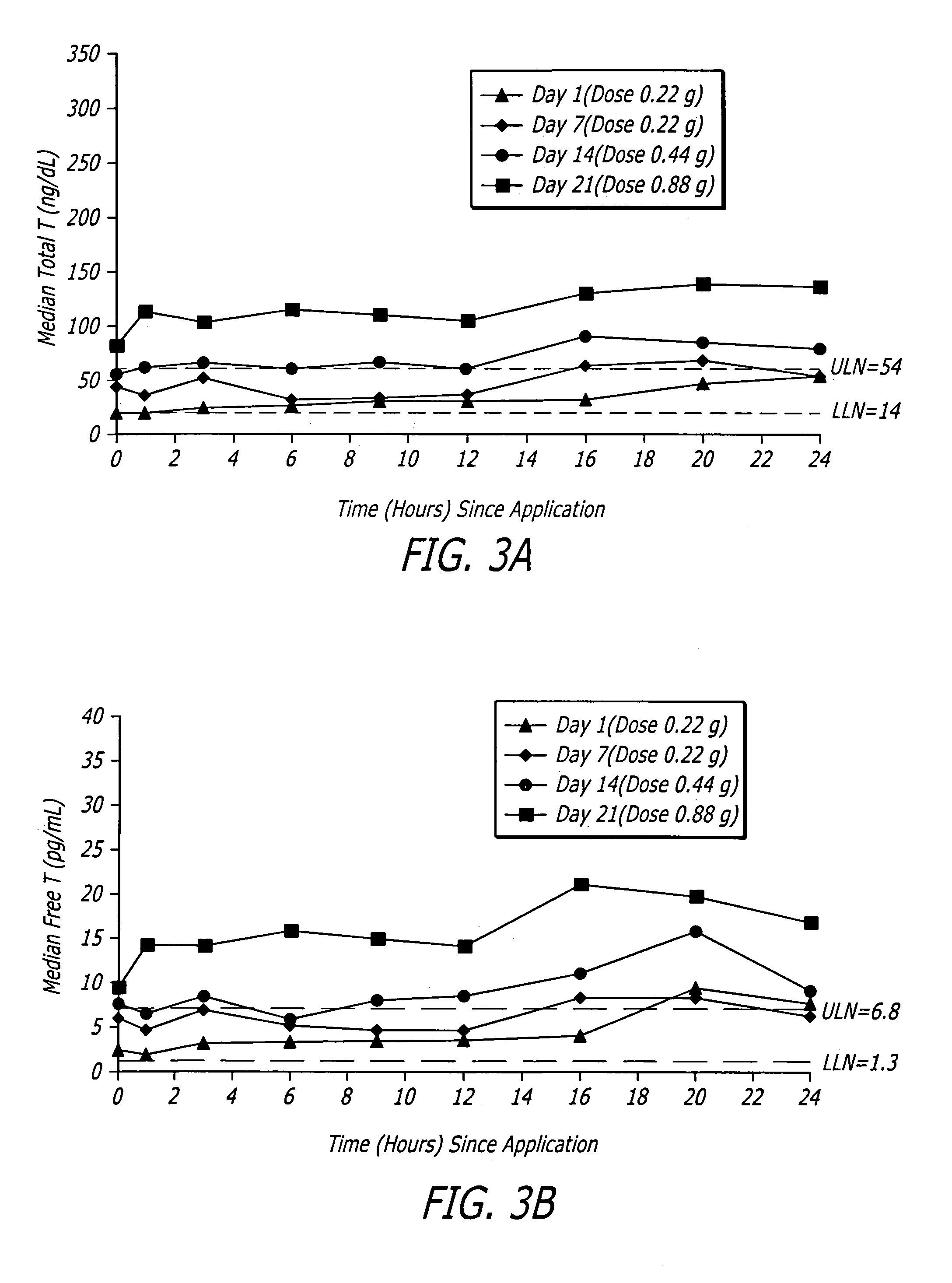
Pharmaceutical composition and method for transdermal drug delivery
InactiveUS20050020552A1Increase concentrationImproves transdermal penetrationOrganic active ingredientsAerosol deliveryIsostearic acidHormones regulation
A pharmaceutical composition for transdermal administration of a hormone (e.g., testosterone), which includes isostearic acid as a penetration enhancer, and methods utilizing same for treating medical conditions in which elevating a hormone serum level is beneficial are disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
Sprayable formulations for the treatment of acute inflammatory skin conditions
A topical spray or foam, methods of making the formulation, and methods of use thereof, has been developed. In one preferred embodiment, the composition includes one or more active agents and exhibits both antibacterial activity and antifungal activity. Excipients such as chemical disinfectants, anti-pruritic agents to minimize itching, and skin protective compounds may be added. The composition may be formulated to be dispensed as a spray or foam and the spray or foam may be administered either by a hand pump or by an aerosolizing propellant. A second single phase formulation has also been developed. The formulation comprises a first drug which is water soluble or hydrophilic and a second drug which is lipid soluble or hydrophobic, wherein at least one of the drugs is bound to an ion-exchange resin. The use of binding resins, such as ion-exchange resins, allows drugs with incompatible solvent requirements to be prepared in a single-phase formulation.
Owner:COLLEGIUM PHARMA INC
Imiquimod cream formulation
InactiveUS20070264317A1Composition is stablePowder deliveryBiocideExcipientPharmaceutical preservatives
Owner:AGIS INDUSTRIES (1983) LTD
Sustained release delivery system
InactiveUS6335035B1Low back painRelief the painPowder deliveryOrganic active ingredientsMedicineDelivery system
Owner:GLYCOBIOSCI +1
Formulations for transdermal or transmucosal application
InactiveUS7198801B2Improve permeabilityOrganic active ingredientsBiocideLong chain fatty acidIrritation
The present invention relates generally to formulations for transdermal or transmucosal administration of an active agent. The invention is a substantially malodorous-free and irritation free transdermal formulation which is substantially free of long chain fatty alcohols, long-chain fatty acids, and long-chain fatty esters.
Owner:ANTARES PHARMA IPL
Transdermally administered tolterodine as anti-muscarinic agent for the treatment of overactive bladder
InactiveUS6517864B1Achieve effectClinical efficacyOrganic active ingredientsAerosol deliveryMuscarinic antagonistMetabolite
Device for transdermal administration of tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, optionally together with pharmaceutically acceptable carrier(s) to a human being or an animal in order to achieve an effect against overactive bladder. Use of a compound having an effect against overactive bladder comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s), for the manufacture of a composition to be administered transdermally for achieving an effect against overactive bladder. Method for achieving an effect against overactive bladder in a living body by transdermal administration of a compound comprising tolterodine, optionally encompassing salts, prodrugs and metabolites thereof, and optionally together with pharmaceutically acceptable carrier(s).
Owner:MCNEIL AB +1
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20040102429A1Minimize and prevent irritationReduce transmissionAntibacterial agentsOrganic active ingredientsHigh concentrationFungicide
The addition of low concentrations of combinations of water-soluble organic salts of zinc to gels, creams, lotions or ointments can increase the ability of these products to reduce or prevent exogenous irritants from causing irritation of the underlying substrate. The addition of low concentrations of combinations of water-soluble organic zinc salts to these gels, creams, lotions or ointments also can reduce the irritation of skin or mucous membranes caused by the addition of potentially-irritating substances such as spermicides, microbicides, fungicides or other therapeutic agents to the gel, cream, lotion or ointment. The advantages of this anti-irritant approach over others, which generally employ high concentrations of single zinc salts, are the reduced potential for zinc toxicity, the reduced potential for toxicity related to zinc itself, and the preservation of the desirable biological properties of potentially-irritating therapeutic substances added to the gel, cream, lotion or ointment.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Fluidic Tissue Augmentation Compositions and Methods
InactiveUS20070212385A1Quality improvementExtending and improving qualityBiocideCosmetic preparationsBiologyTissue augmentation
Owner:KYTHERA BIOPHARMLS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com