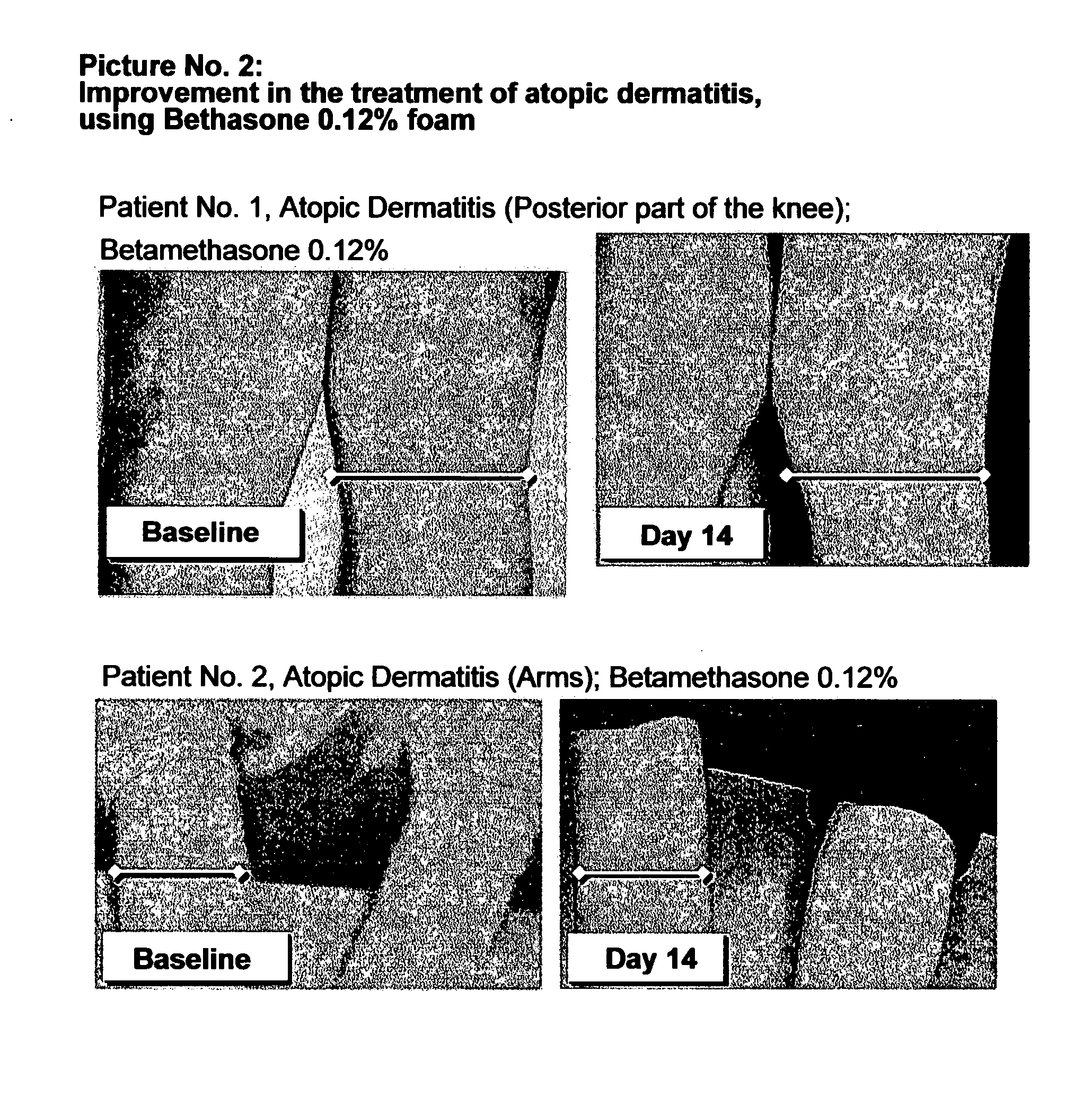
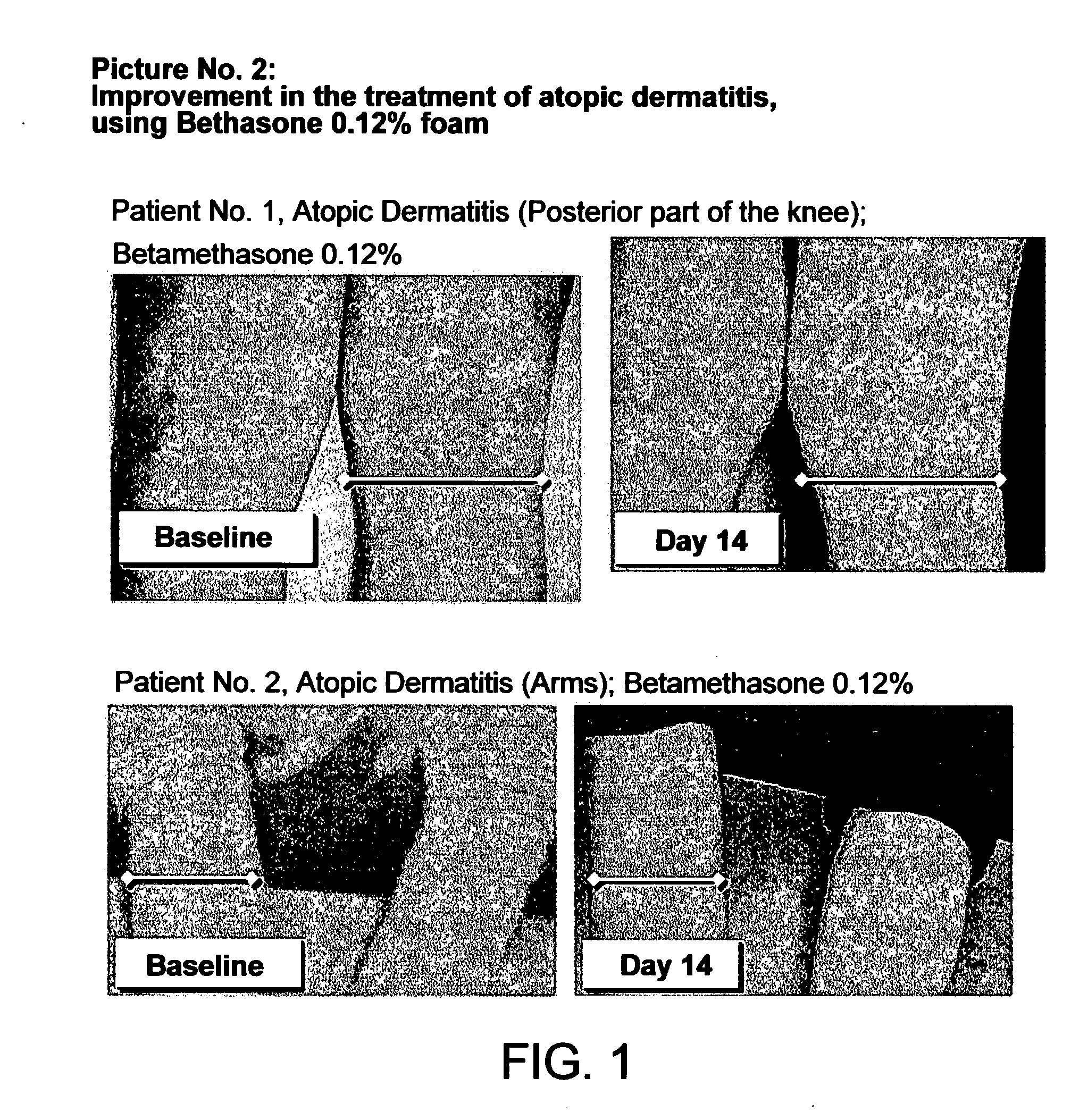
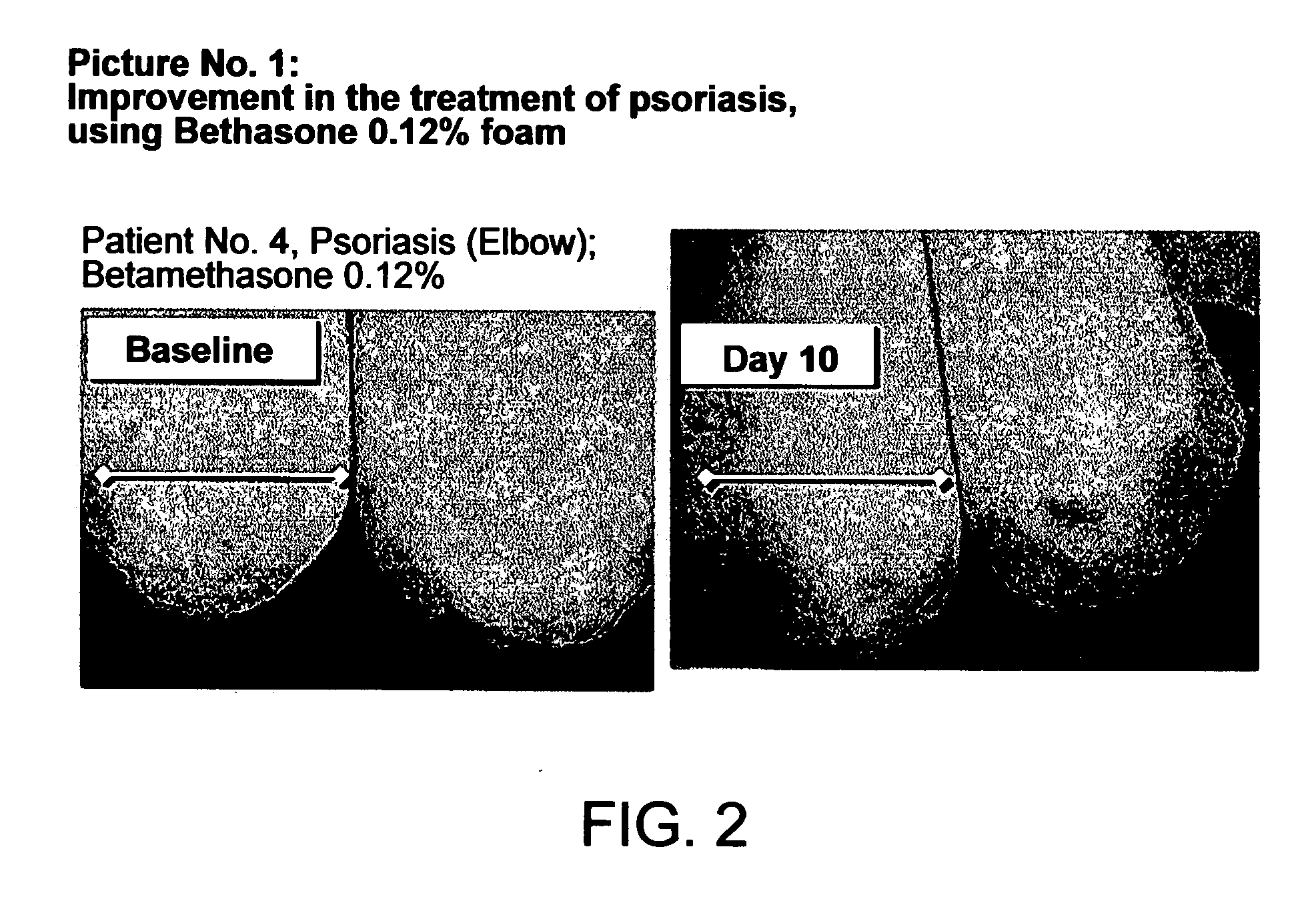
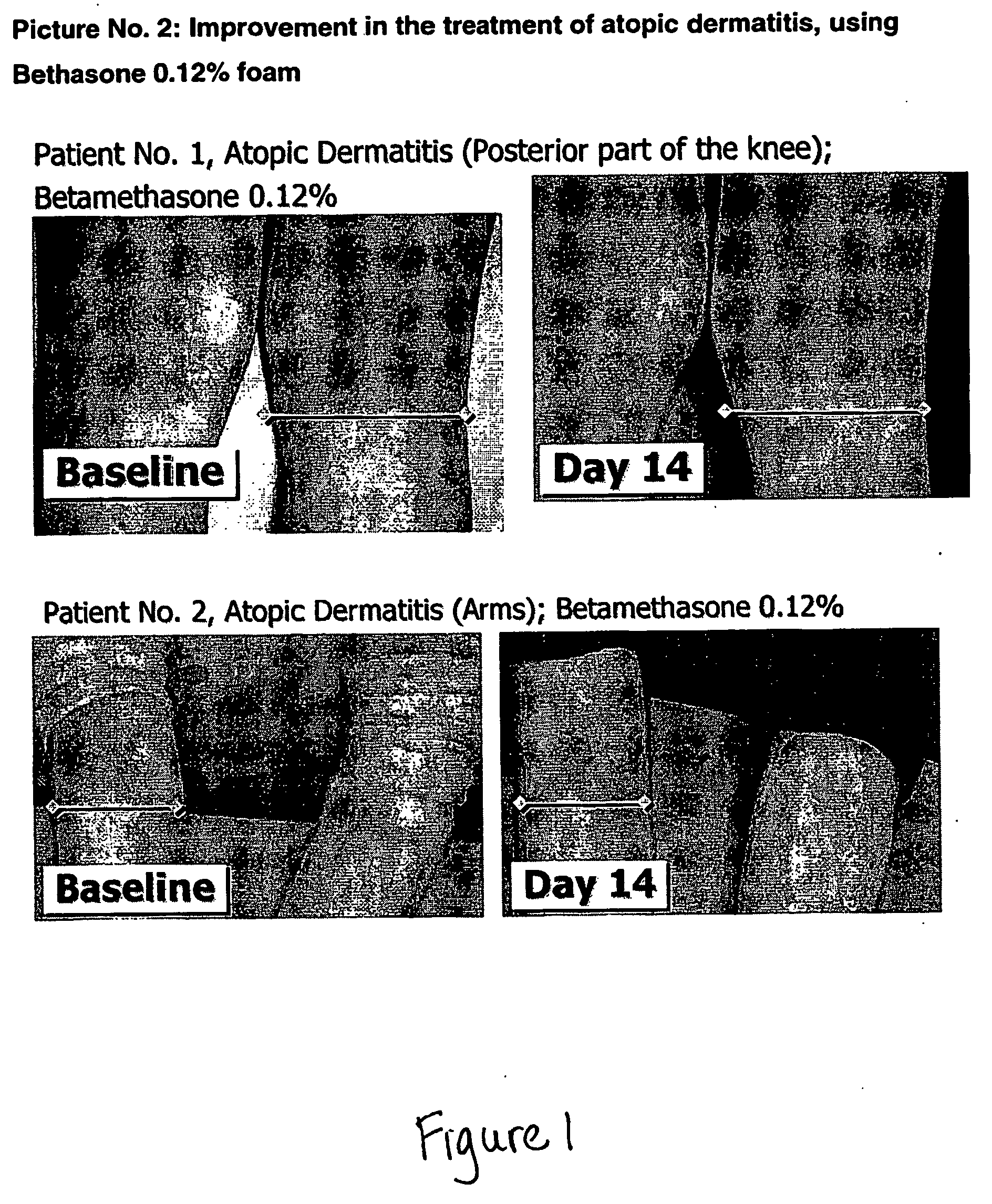
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
356 results about "Alcohol free" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
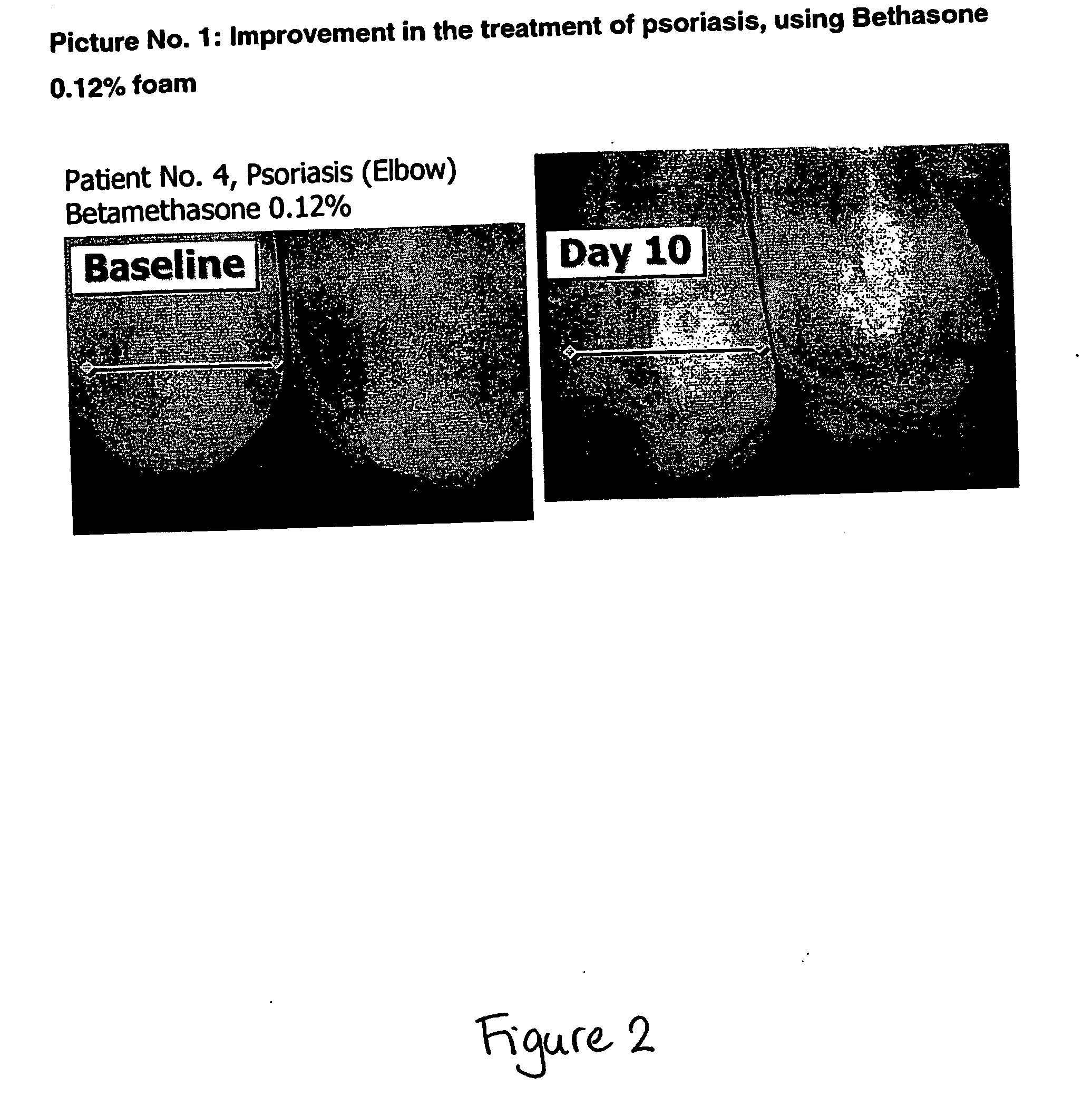
Film forming foamable composition
InactiveUS20060193789A1Improve solubilityReduce deliveryCosmetic preparationsBiocideAlcohol freeFilm-forming agent
A foamable composition, includes (1) about 6% to about 70% by weight of at least one organic carrier; (2) about 0.1% to about 5% by weight of at least one surface-active agent; (3) about 0.01% to about 5% by weight of at least one film forming agent; (4) water; and (5) about 3% to about 25% by weight of the total composition of at least one liquefied or compressed gas propellant. The composition is substantially alcohol free and is used in treating, alleviating or preventing a disorder.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Foam carrier containing amphiphilic copolymeric gelling agent
InactiveUS20050069566A1Broaden applicationEvenly distributedAntibacterial agentsCosmetic preparationsAlcohol freeWater soluble
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier including water, a hydrophobic solvent, a surface-active agent and a gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Cosmetic and pharmaceutical foam
InactiveUS20080031907A1Efficient ConcentrationReduce sensitivityAntibacterial agentsBiocideAlcohol freeVegetable oil
The invention relates to uses of an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent as a flame retardant or flame resistant foam. The hydrophobic solvent is preferably mineral oil; medium chain triglycerides; isopropyl myristearate or octyl dodecanol, silicone oil or vegetable oil or mixtures thereof. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, also making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil-soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Cosmetic and pharmaceutical foam
InactiveUS20060140984A1Lower yield strengthRubbing easy and efficientCosmetic preparationsBiocideAlcohol freeAdjuvant
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Topical Pharmaceutical Foam Composition
InactiveUS20070154402A1Reduced intensity of colorReduce odor intensityAntibacterial agentsBiocideAlcohol freeActive agent
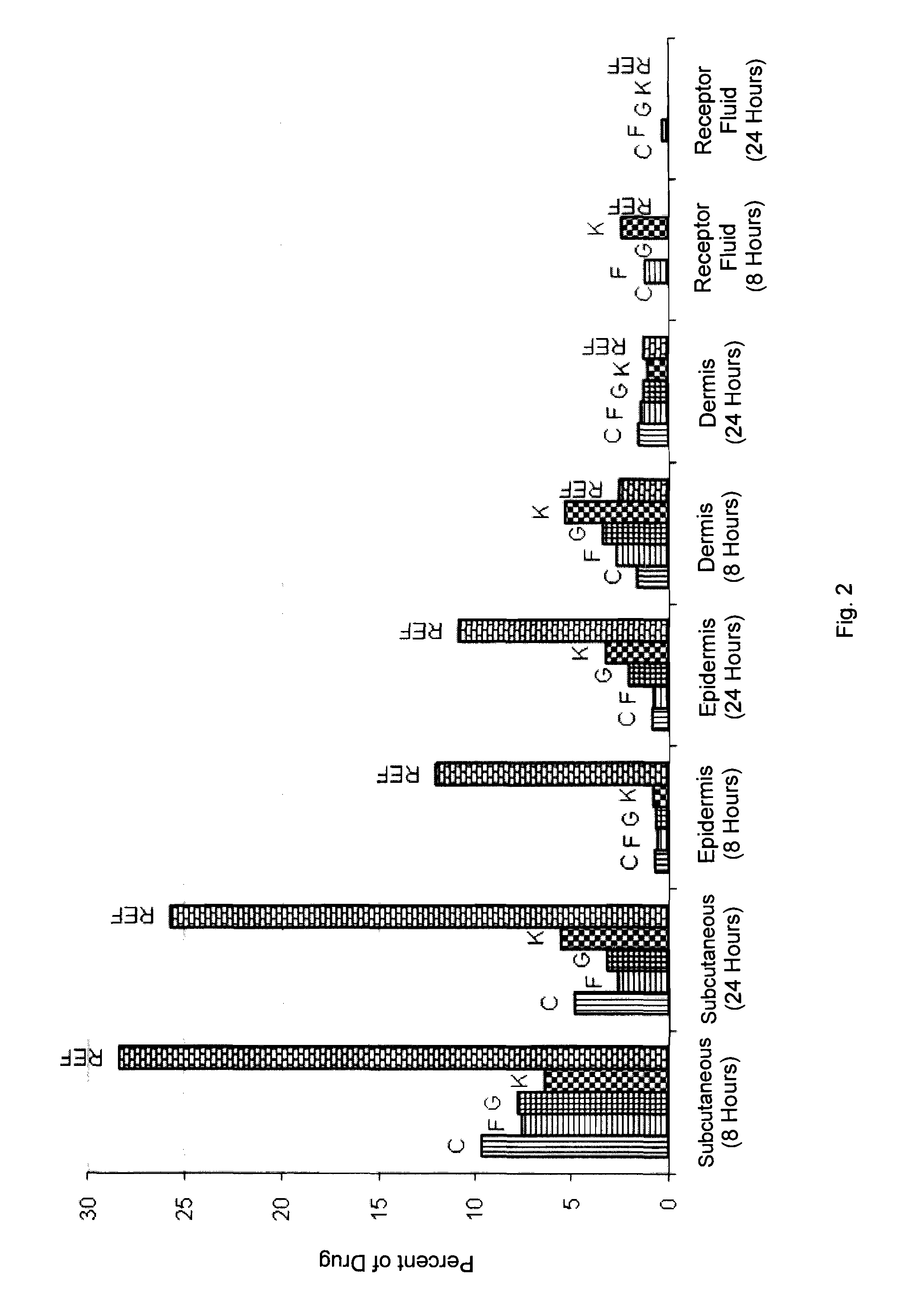
A stable topical alcohol-free aerosol foam containing one or more keratolytic agents is provided. The foam-forming formulation is an emulsion which contains an HFA propellant and one or more keratolytic agents. The emulsion has an oil phase and an aqueous, i.e. water-containing, phase. The active agent(s) may be present in either phase of the emulsion or dispersed in the emulsion. The oil phase may consist at least in part of the HFA propellant. Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and / or other excipients. The foam is stable on the skin, for example, for at least 5 minutes at body temperature, preferably at least 20 minutes at body temperature, and disappears into the skin upon rubbing or after prolonged standing. In one embodiment, the formulation contains an HFA propellant which does not contain additional co-solvents or co-propellants. The formulations demonstrate reduced intensity of the odor and / or color associated with the keratolytic agent(s) as compared to conventional formulations containing keratolytic agents.
Owner:PRECISION DERMATOLOGY
Body cavity foams
The invention relates to an alcohol-free cosmetic or therapeutic foam carrier comprising water, a hydrophobic organic carrier, a foam adjuvant agent, a surface-active agent and a gelling agent. The cosmetic or therapeutic foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble therapeutic and cosmetic agents.
Owner:VYNE THERAPEUTICS INC
Foam incorporating eutetic mixture
InactiveUS20050075407A1Broaden applicationEvenly distributedCosmetic preparationsBiocideAlcohol freeMedicine
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a surface-active agent a gelling agent and a combination of active agents, which creates, upon admixing, a eutectic mixture. The foam carrier further comprises active agents and excipients with therapeutic properties.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Alcohol-free transdermal analgesic composition and processes for manufacture and use thereof
InactiveUS7052715B2Reduced shelf lifeImprove permeabilityOrganic active ingredientsBiocideAlkaneAlcohol free
The instant invention is directed toward a dermal delivery system composition comprising an aqueous base vehicle including American Emu oil, Isopropyl Palmitate (PROTACHEM IPP), PEG-8 (a polyethylene glycol available under the tradename PROTACHEM 400), methylsulfonylmethane (MSM) and SEPIGEL 305 (a combination including polyacrylamide / C13–C14 Iso-paraffin and Laureth-7), in combination with an analgesic composition, such as ibuprofen, and to processes for the manufacture and use thereof.
Owner:ALL NATURAL FMG
Penetrating pharmaceutical foam
ActiveUS20050074414A1Broaden applicationEvenly distributedAntibacterial agentsBiocideAlcohol freeActive agent
The invention relates to an alcohol-free cosmetic or pharmaceutical foam composition comprising water, a hydrophobic solvent, a surface-active agent, a gelling agent, an active component selected from the group of urea, hydroxy acid and a therapeutic enhancer and a propellant. The foam further comprises active agents and excipients with therapeutic properties having enhanced skin penetration.
Owner:VYNE THERAPEUTICS INC
Foamable Composition
InactiveUS20130189196A1Improve solubilityReduce deliveryBiocideCosmetic preparationsAlcohol freeFilm-forming agent
Owner:FOAMIX PHARMACEUTICALS LIMITED
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
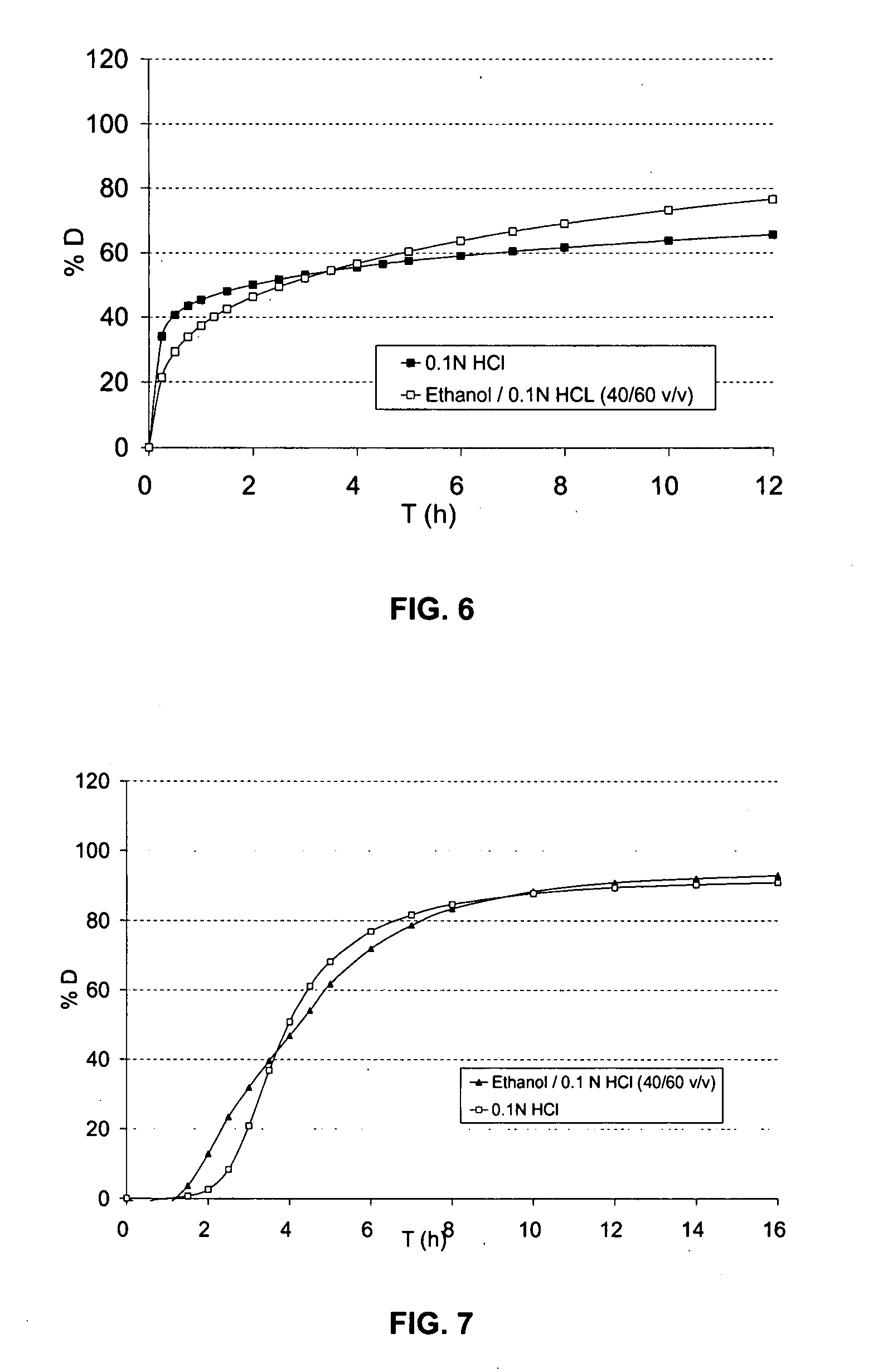
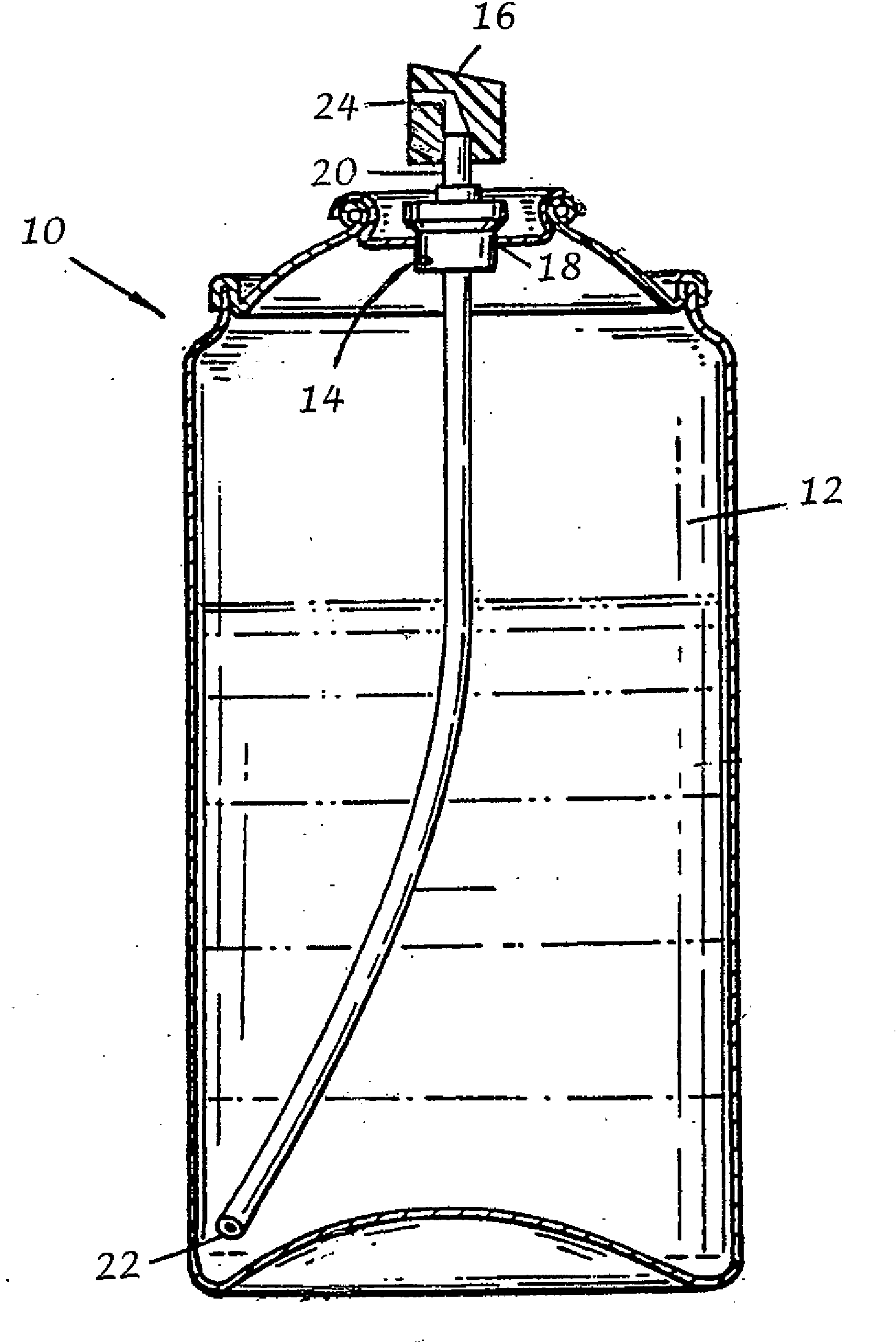
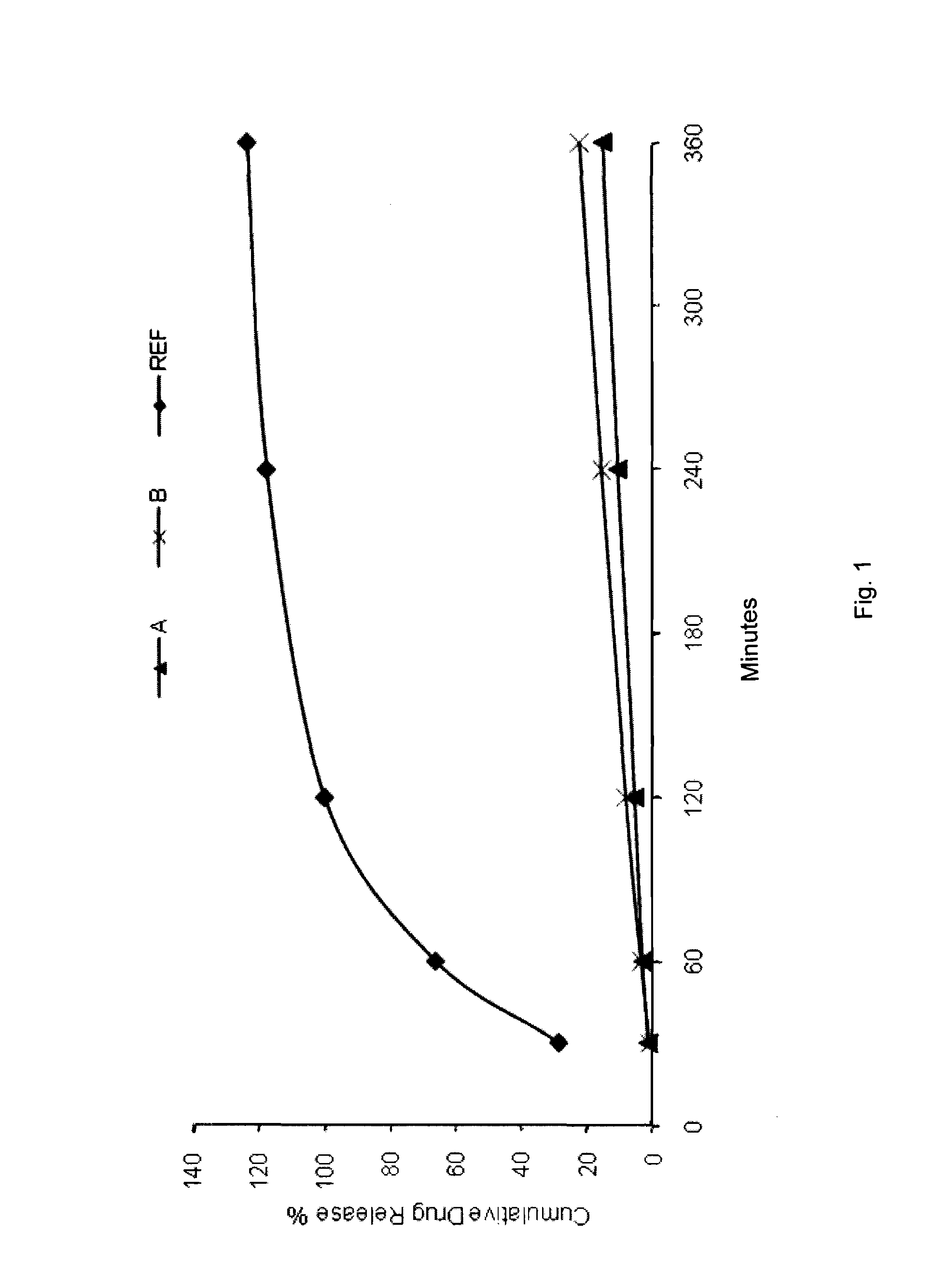
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
Topical Pharmaceutical Foam Composition
InactiveUS20120128598A1Reduce intensityMinimizing the skin sensitizing reactionsAntibacterial agentsAntimycoticsAlcohol freeMedicine
Owner:PRECISION DERMATOLOGY
Transdermal, alcohol-free, pharmaceutical compositions
An alcohol-free, transdermal drug delivery composition administered via a metered spray drug delivery device is described herein. The non-occlusive transdermal drug delivery composition includes a therapeutically effective amount of at least one physiologically active agent or prodrug thereof, an effective amount of at least one dermal penetration enhancer; and at least one non-volatile liquid. The transdermal drug delivery composition is administered to a dermal or mucosal surface of an animal needing the same using a metered spray device capable of delivering a fine spray of substantially uniform particle size to minimize the required drying time therefor.
Owner:LUMARA HEALTH IP
Alcohol-free microemulsion composition
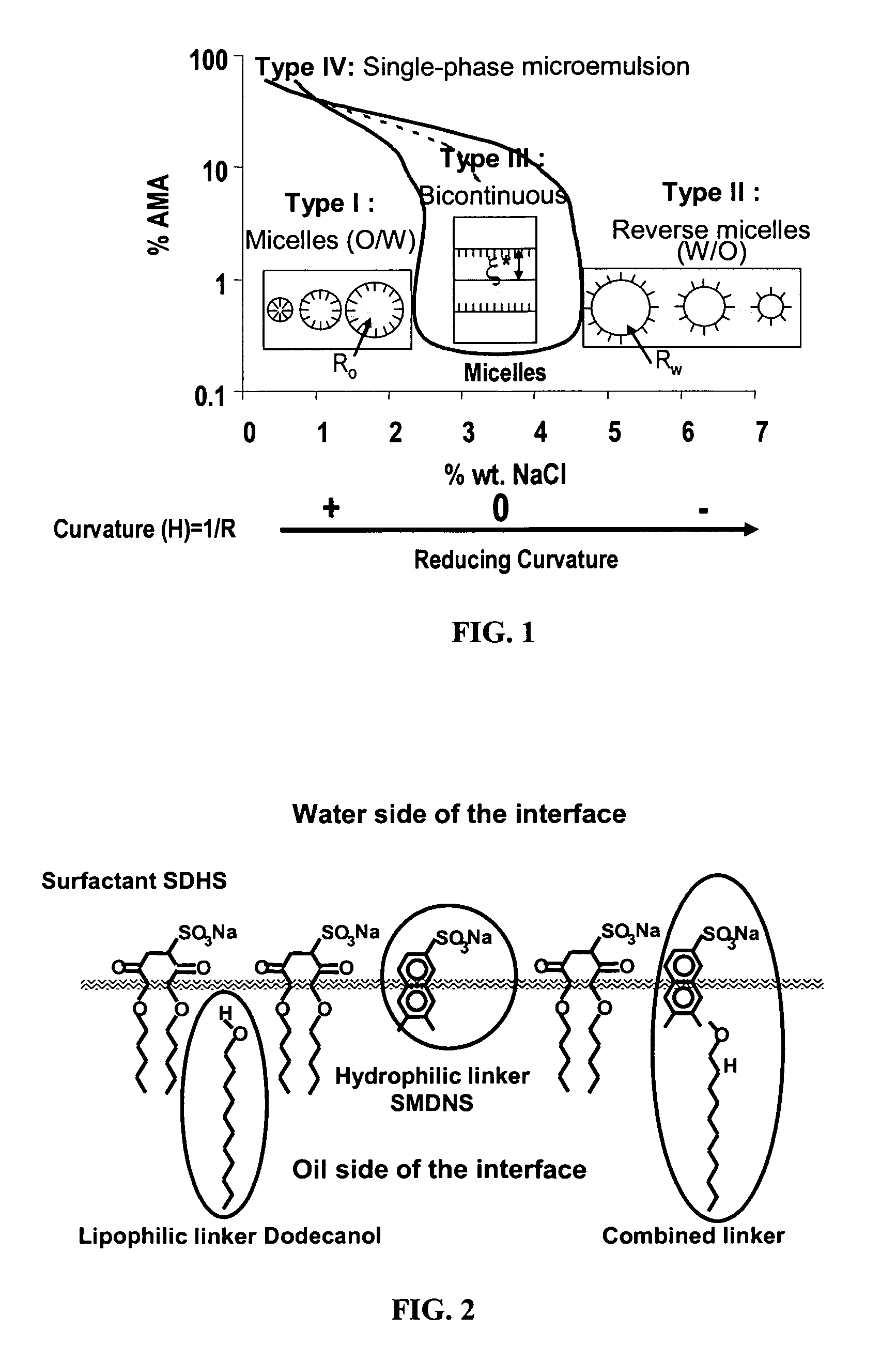
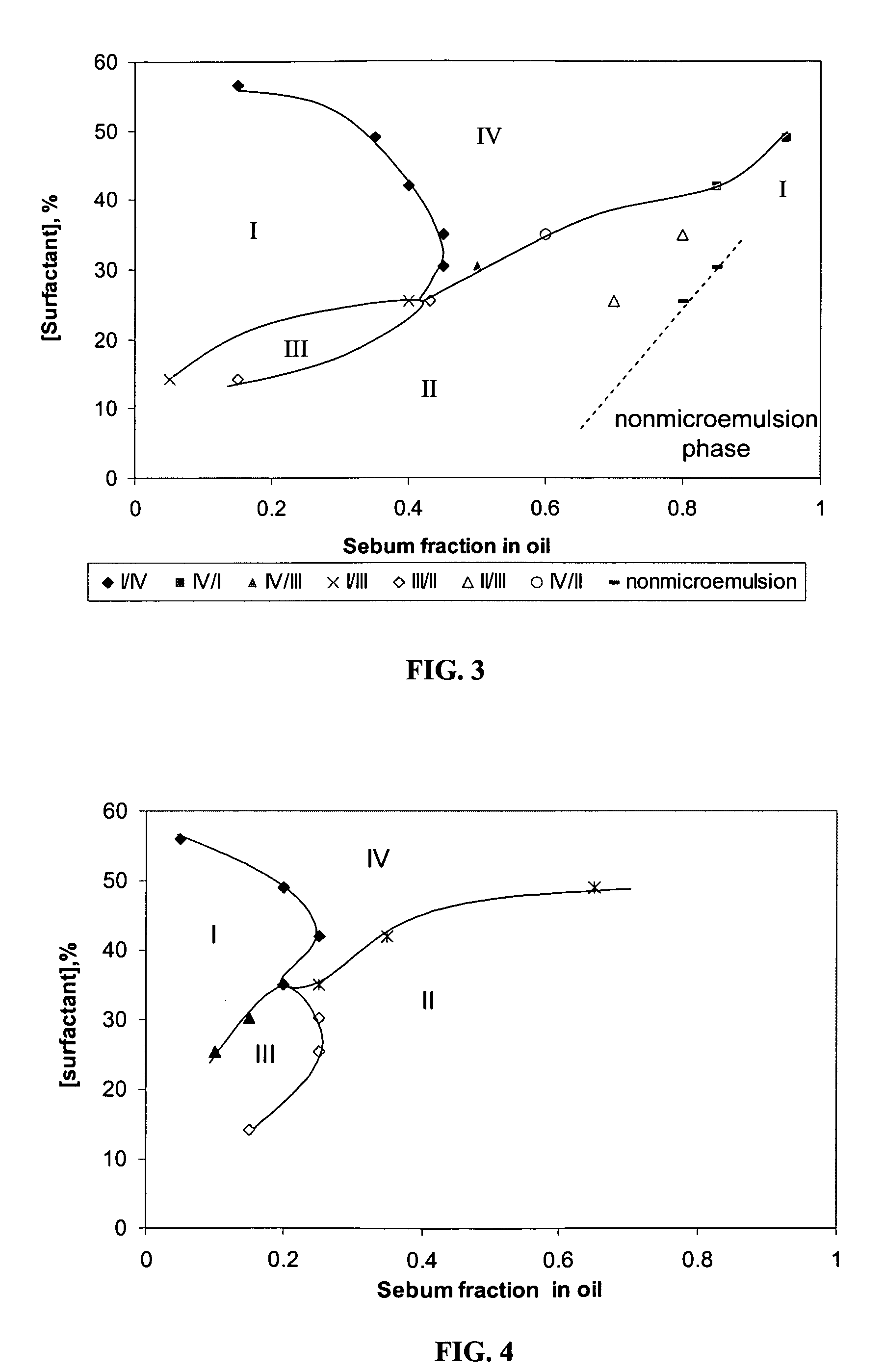
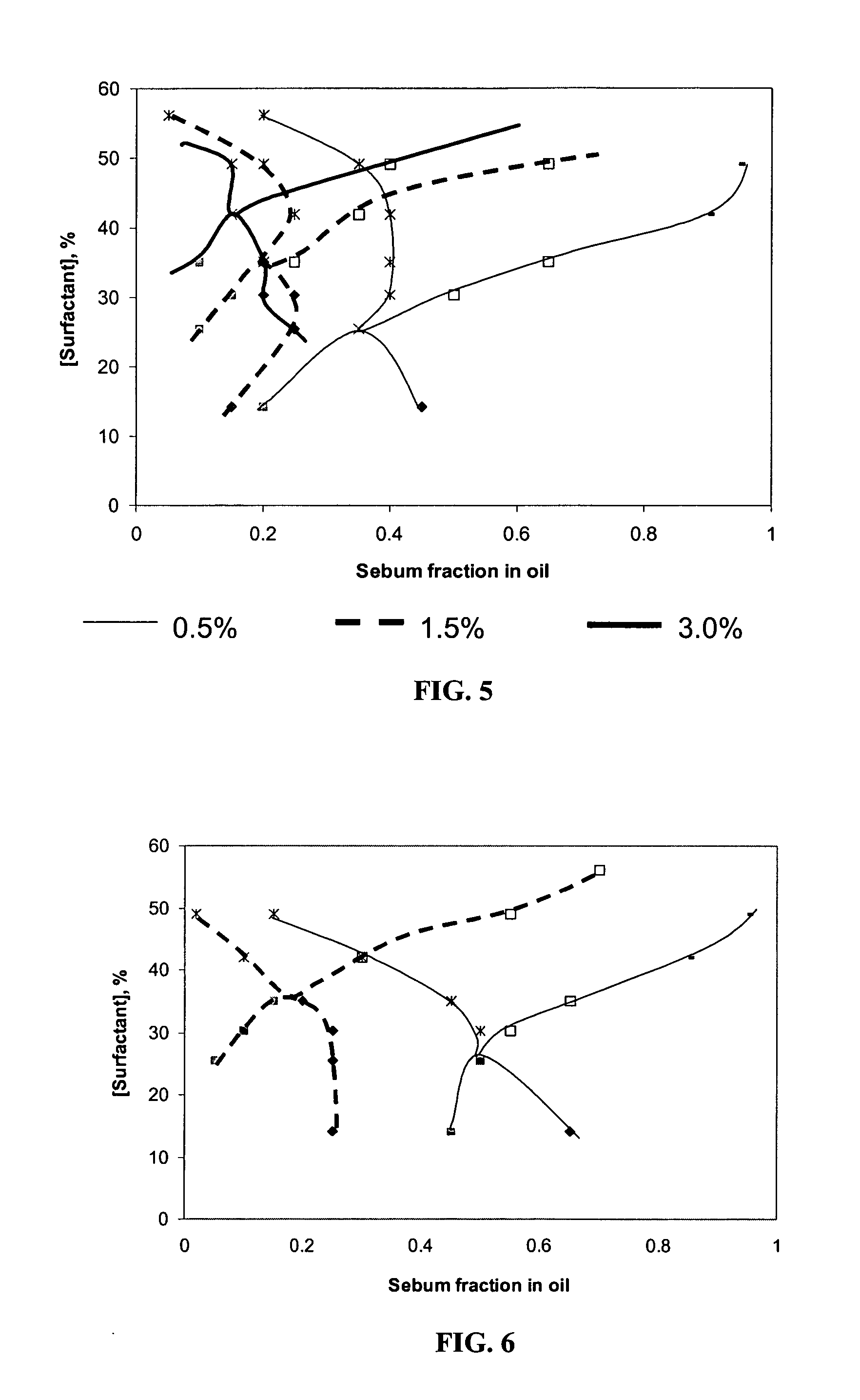
InactiveUS20060165739A1Better cleansingPromote absorptionCosmetic preparationsHair cosmeticsLipid formationAlcohol free
The present invention concerns compositions that comprise alcohol-free microemulsions and methods for their use that include a surfactant, a lipophilic linker, and / or a hydrophilic linker. These compositions can be used, for example, in cosmetic or hair applications. In certain aspects, compositions of the invention have the ability to microemulsify sebum while providing enhanced cleansing of cosmetic products from the skin or hair. In addition, the compositions have the ability to enhance the penetration of skin or hair active ingredients, such as emollients, humectants, anti-oxidants, lipids, vitamins, botanicals, dyes, tanning compounds, etc.
Owner:MARY KAY INC
Alcohol-free fountain solution with high viscosity and low surface tension
InactiveCN103935150AHigh effective contentReduce pollutionPrinting pre-treatmentAlcohol freeAqueous alcohol
The invention discloses alcohol-free fountain solution with high viscosity and low surface tension. The alcohol free fountain solution with high viscosity and low surface tension comprises the following components by weight, 30-55 parts of alcohol solvents, 2-6 parts of buffer agents, 3-8 parts of wetting agents, 1-4 parts of surface active agents, 0.3-2.2 parts of antifoaming agents, 0.5-3 parts of thickening agents, 0.5-1.2 parts of anticorrosion and bactericidal agents, 1-3 parts of penetrating agents, 0-2 parts of electrolyte and 20-45 parts of viscosity modifier water. The fountain solution is free of volatile solvents containing alcohol and isopropyl alcohol and pollution to the environment, the surface tension ranges from 4*10-2N / m to 7*10-2N / m, pH ranges from 4.8 to 5.5, the heat conduction rate ranges from 800mus / cm to 1200mus / cm, and therefore, quality requirements of the fountain solution can be met better.
Owner:苏州禾川化学技术服务有限公司
Alcohol-free concentrated fountain solution and preparation method thereof
InactiveCN102303465AStable concentrationPrint color difference is smallPrinting pre-treatmentAlcohol freePhysical well being
The invention provides an alcohol-free concentrated fountain solution and a preparation method thereof. The alcohol-free concentrated fountain solution is prepared from the following components based on 100 parts by weight: 0.5-5 parts of low-foam wetting agent, 8-20 parts of pH value regulator, 3-20 parts of printing plate protective agent, 5-10 parts of a water film regulator, 0.2-1.0 part of decontamination intensifier, 0.1-1.0 part of bactericide, 0.1-1.2 parts of defoamer and the balance of water. The concentrated fountain solution provided by the invention is environmentally-friendly, provides guarantee for the health of workers, and can improve the quality of the printing products.
Owner:冯星枢 +1
Alcohol-free fountain solution condensate
InactiveCN101524931AGood reproducibilityParameter stabilityPrinting pre-treatmentAlcohol freePhosphoric acid
The invention discloses an alcohol-free fountain solution condensate. The process for preparing the alcohol-free fountain solution condensate comprises the following steps: proportionally weighing fatty alcohol-polyoxyethylene ether, coco-glucoside, Arabic gum, citric acid, sulfonylation dextrosamine, gluconic acid sodium salt, phosphoric acid and disodium hydrogen phosphate; dissolving the solid materials in water into solution, and diluting liquid materials in water; respectively putting the materials into a reaction kettle; and stirring for 30 to 45 minutes to obtain the alcohol-free fountain solution condensate. The alcohol-free fountain solution condensate does not contain alcohol or isopropanol, and can be used after being diluted by water into 2-3 percent solution. Fountain solution prepared from the alcohol-free fountain solution condensate not only ensures that the printing has high definition, vivid effect and wide application, but also has no harm to human body and no pollution to the environment. Compared with market fountain solution which consumes large amount of alcohol or isopropanol or alcohol-reduced fountain solution, the alcohol-free fountain solution condensate greatly reduces the cost.
Owner:JIEXING ENVIRONMENTAL PROTECTION TECH INVESTMENT SHANGHAI
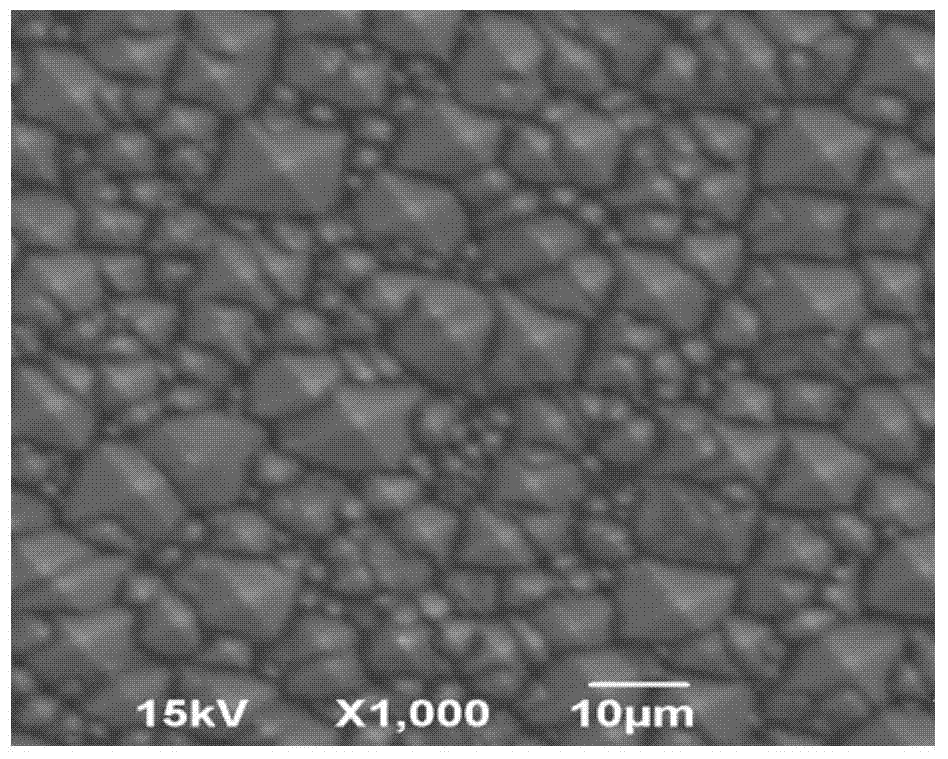
Alcohol-free alkaline texturing solution for mono-crystalline silicon wafer, texturing method for mono-crystalline silicon wafer, solar cell and manufacturing method for solar cell
InactiveCN103614778AReduce reflectivityIncreased anisotropyAfter-treatment detailsFinal product manufactureAlcohol freeAbsorption capacity
The invention discloses an alcohol-free alkaline texturing solution for a mono-crystalline silicon wafer, a texturing method for the mono-crystalline silicon wafer, a solar cell and a manufacturing method for the solar cell. The alcohol-free alkaline texturing solution for the mono-crystalline silicon wafer comprises an alkaline solution and a texturing additive, wherein the texturing additive is an alcohol-free additive. According to the alcohol-free alkaline texturing solution, isopropanol which is harmful to human bodies and the environment adopted in the conventional alkaline texturing solution is abandoned; the corrosion depth of the surface of a silicon wafer can be controlled within the range of 5 to 7.5 mu m easily by performing surface texturing on the mono-crystalline silicon wafer obtained by performing linear cutting on a diamond by only adopting the alcohol-free alkaline texturing solution consisting of the alkaline solution and the alcohol-free additive; meanwhile, the corrosion speed is guaranteed; the anisotropy of corrosion is enhanced; pyramid structures on the surface of the silicon wafer obtained after the texturing are small and uniform in size, so that the reflectivity of the surface of the silicon wafer is reduced by about 1 percent; the light absorption capacity of the surface of the silicon wafer is increased; the conversion efficiency of the solar cell is improved.
Owner:YINGLI ENERGY CHINA
Topical gels compositions
Topical alcoholic gel compositions are disclosed that are useful for delivering therapeutic levels of an NSAID to target in and below the skin. The compositions comprise a topically active drug, an alcoholic solvent, a polymeric thickener, and optionally a keratolytic agent. In one embodiment, excellent viscosity for dermal application is attained without the need of a step for neutralizing the pH of the composition. Alcoholic and alcohol-free topical compositions comprising an NSAID prodrug are also disclosed. The compositions are particularly useful for the treatment of pseudofolliculitis barbae.
Owner:SPANN WADE MONIQUE +1
Clear sunscreen gels and methods of use thereof
The present invention relates to an alcohol-free transparent sunscreen composition containing at least one chemical ultra-violet (UV) light absorber, at least one solvent and at least one diphenyl silicone elastomer gellant. Preferably, the solvent is selected from the group consisting of dimethicone, dicaprylyl carbonate, lauryl lactate, neopentyl glycol diheptanoate, glycerin, and combinations thereof, and the diphenyl silicone elastomer gellant contains diphenylsiloxy phenyl trimethicone. Such a transparent sunscreen composition can be used to form a stable and aesthetically appeal clear gel for protecting skin against harmful effects of UV radiation.
Owner:ELC MANAGEMENT LLC
Composition for treating oral cavity and Mucousal infections
The present invention provides a composition of matter for treating oral cavity infections and mucosal infections, said composition comprising: at least one anti-microbial drug; and at least one essential oil, in combination with a substantially, alcohol-free carrier system, said carrier system being selected from an isotonic system and a moderately hypertonic system, wherein the final composition isotonicity is between 140 and 480 miliosmolar.
Owner:J P M E D
Alcohol-free printing fountain solution and preparation method thereof
The invention discloses an alcohol-free printing fountain solution and a preparation method of the alcohol-free printing fountain solution. The alcohol-free printing fountain solution comprises, by weight, 10-15% of propylene glycol monomethyl ether, 2-4% of ethylene glycol, 4-8% of a citric acid, 0.5-1.5% of sodium hydroxide, 15-25% of glycerinum, 0.1-0.3% of sodium carboxymethyl cellulose, 0.05-0.1% of lauroyl diethanol amine, 0.4-0.6% of liquid tea saponin, 0.3-0.6% of dodecyl dimethyl benzyl ammonium chloride, 0.5-1% of dodecyl glucoside, 0.05-0.1% of 2-ethyl hexanol and 50-55% of water. The alcohol-free printing fountain solution does not contain isopropyl alcohol and is low in toxicity, safe, environmentally friendly, good in printing effect and low in cost, thereby having huge economic benefit and social benefit.
Owner:安徽唯宝印刷科技有限公司
Alcohol-free transdermal insulin composition and processes for manufacture and use thereof
The instant invention is directed toward a dermal delivery system composition comprising an aqueous base vehicle including American Emu oil, Isopropyl Palmitate (PROTACHEM IPP), PEG-8 (a polyethylene glycol available under the tradename PROTACHEM 400), methylsulfonylmethane (MSM) and SEPIGEL 305 (a combination including polyacrylamide / C13-C-Iso-paraffin and LAURETH 7, in combination with a therapeutically effective amount of at least one species of insulin, and to processes for the manufacture and use thereof.
Owner:ALL NATURAL FMG
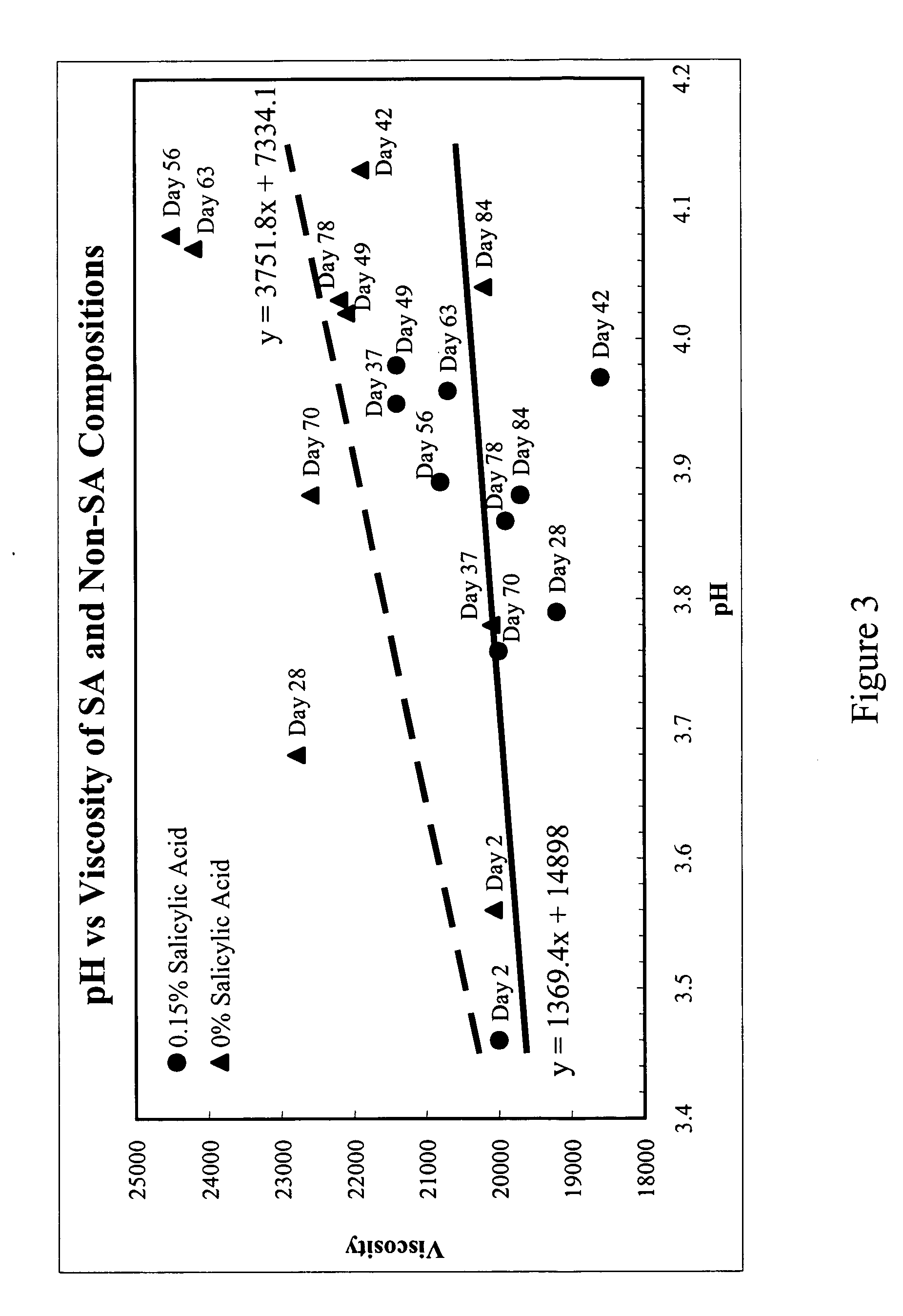
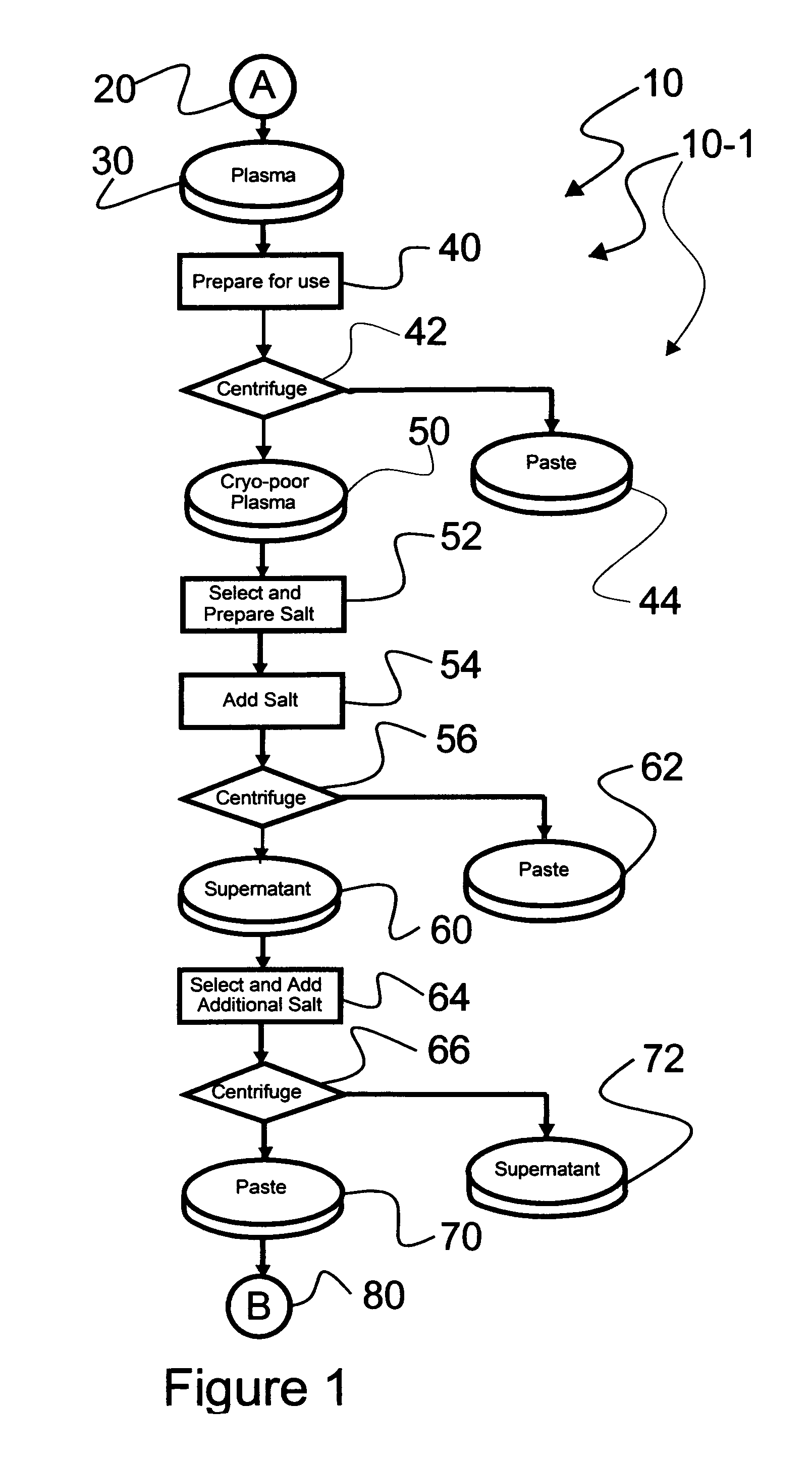
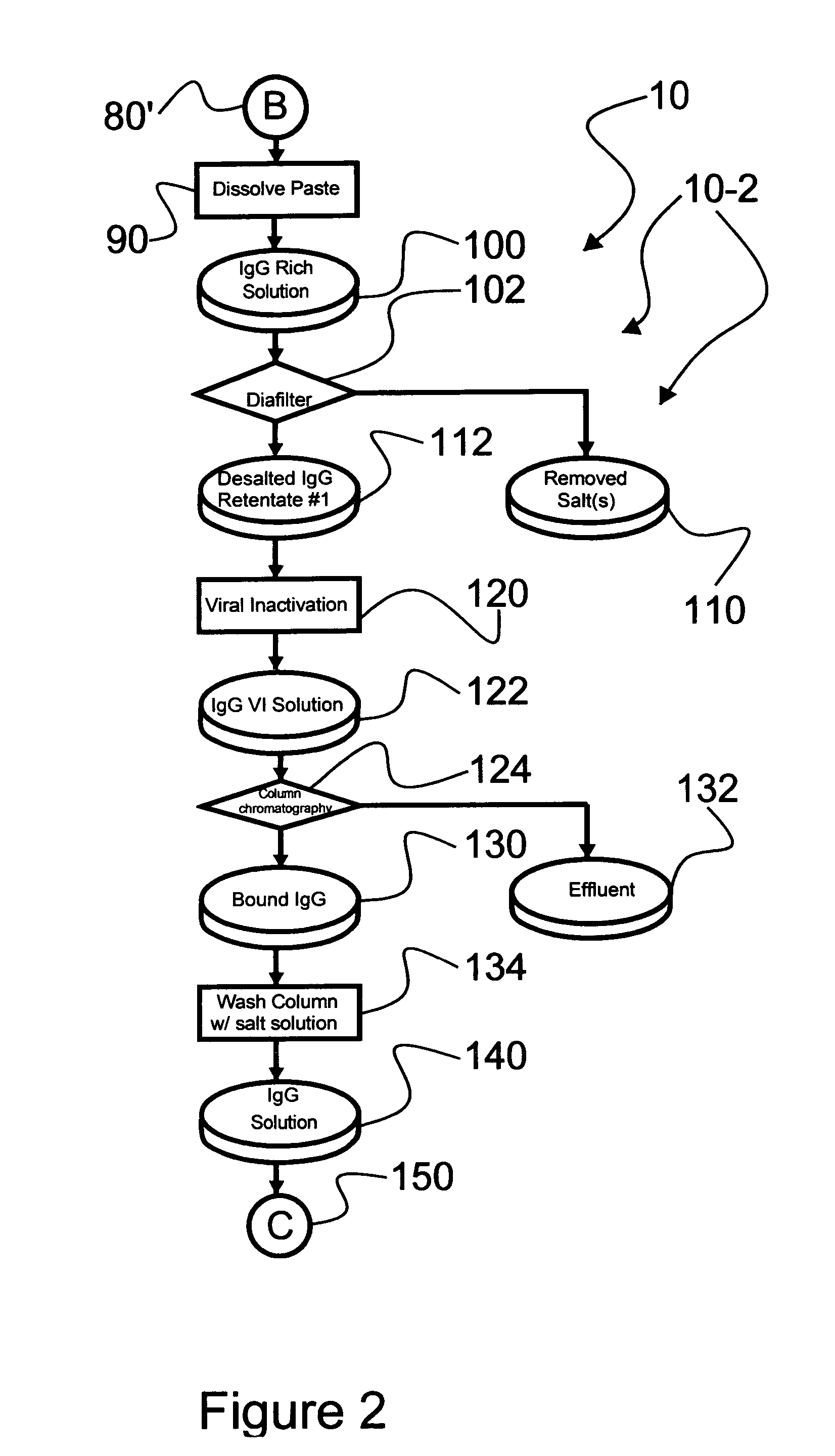
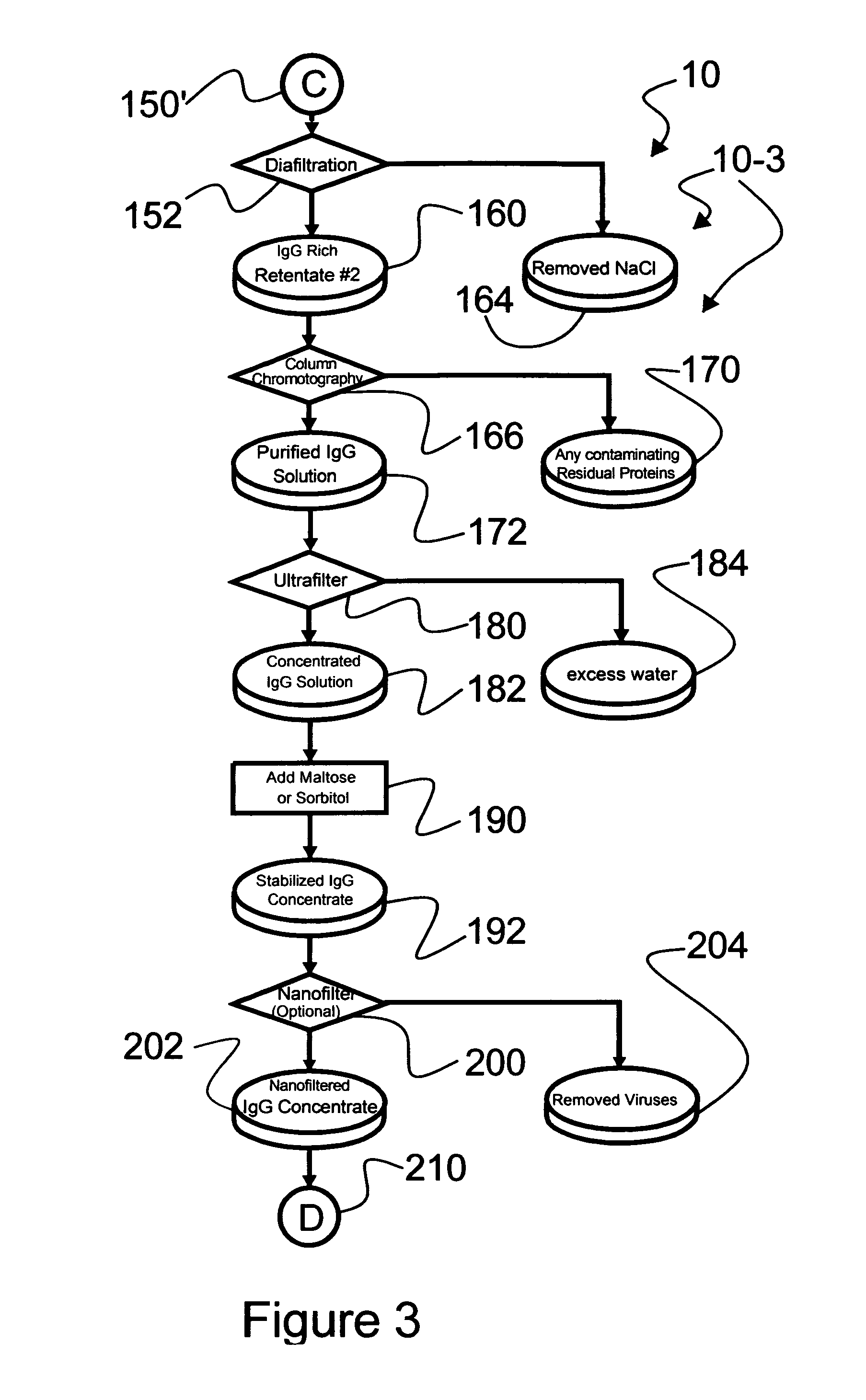
Ultra-high yield intravenous immune globulin preparation
ActiveUS7879332B2Quick restoration of the internal water moleculeReduce formationBiocideMedical devicesSodium acetateAlcohol free
An efficacious large-scale alcohol-free plasma fractionation production process which produces a high-yielding, non-denatured, double viral-inactivated intravenous human immune gamma globulin (IgG) product. The process employs one or more salts from a group of salts comprising sodium citrate, sodium acetate, sodium gluconate, ammonium sulfate, sodium chloride, sodium sulfate and ammonium chloride in two initial fractionation steps, followed by diafiltration to remove those salts employed. A process which employs alcohol via the process of the disclosed inventive method is also disclosed.
Owner:PLASMA TECH LLC
Method for preparing silica aerogel composite material by adopting chloride-free and alcohol-free process
The invention discloses a method for preparing a silica aerogel composite material by adopting a chloride-free and alcohol-free process. The method comprises the following steps: S1, adopting a water-based silicon source as a raw material, regulating the pH value of sol with a chloridion-free acid, adding an additive after desalting, and uniformly stirring to obtain a first mixed solution; S2, immersing a fiber reinforced material into the first mixed solution, and completing gel curing; S3, standing the obtained composite gel material so as to obtain an aged composite gel material; S4, placing the aged composite gel material in a chloride-free and alcohol-free displacer to realize uniform solution infiltration; S5, performing surface methylsilane modification on the composite gel by usinga chloride-free surface modifier; S6, drying the modified composite gel, thereby obtaining the silica aerogel composite material. The invention provides a method for preparing the silica aerogel composite material without any chloride ion or alcohol substance in the formula.
Owner:SHENZHEN AEROGEL TECH CO LTD
Monocrystalline silicon wafer alcohol-free texturing process and texturing additive
ActiveCN104576831AReduce CODReduce the cost of cashmereAfter-treatment detailsFinal product manufactureAlcohol freePotassium hydroxide
The invention relates to a monocrystalline silicon wafer alcohol-free texturing process and a texturing additive. Firstly, a silicon wafer is placed in preprocessing liquid so as to be preprocessed for 60 s to 300 s, and the silicon wafer is then placed in texturing liquid for texturing. The monocrystalline silicon texturing process includes the steps that deionized water is heated to 70 DEG C to 90 DEG C, sodium hydroxide or potassium hydroxide is added, and monocrystalline silicon texturing corrosive liquid is acquired. When the texturing additive is adopted for texturing, isopropanol or ethyl alcohol is not needed, tiny, even and dense pyramid texturing faces can be acquired, texturing cost is reduced, and environmental pollution is avoided. The preprocessing process is added before the texturing step, the silicon wafer acquired after texturing can be cleaner, rework caused by white spot fingerprints and the like can be reduced, and certain practical value is achieved.
Owner:JIANGSU SHUNFENG PHOTOVOLTAIC TECH CO LTD
Alcohol-free transdermal insulin composition
InactiveUS7291591B2Promote recombinationPromote absorptionPeptide/protein ingredientsDepsipeptidesAlcohol freeMedicine
The instant invention is directed toward a dermal delivery system composition comprising an aqueous base vehicle including Emu oil, at least one fatty acid alkyl ester, polyethylene glycol, and a gelling agent, in combination with a therapeutically effective amount of at least one species of insulin, and to processes for the manufacture and use thereof.
Owner:ALL NATURAL FMG
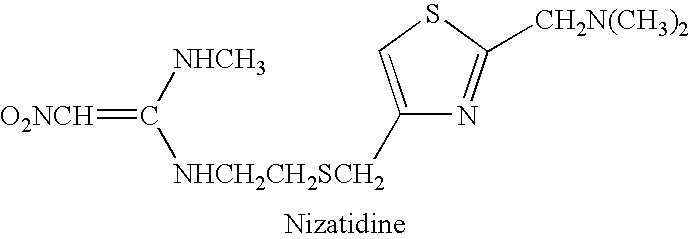
Composition, system and method of treatment of gastrointestinal disorders with nizatidine oral solution
InactiveUS20060094760A1Faster gastric secretionMore responsiveBiocideAnimal repellantsAlcohol freeMedicine
An alcohol-free, oral solution of nizatidine treats gastric and intestinal disorders. Oral doses of solution, which are equivalent to 150 mg twice daily, or 300 mg once daily, pill form of conventional nizatidine are orally administered and have a bioequivalency greater than 70%. The oral solution allows a wider population to obtain nizatidine treatment, particularly children, and the elderly, who have difficulty ingesting pills, can take the oral solution. Also, adolescents and younger children, in particular, can be treated with an alcohol-free oral solution.
Owner:BRAINTREE LAB
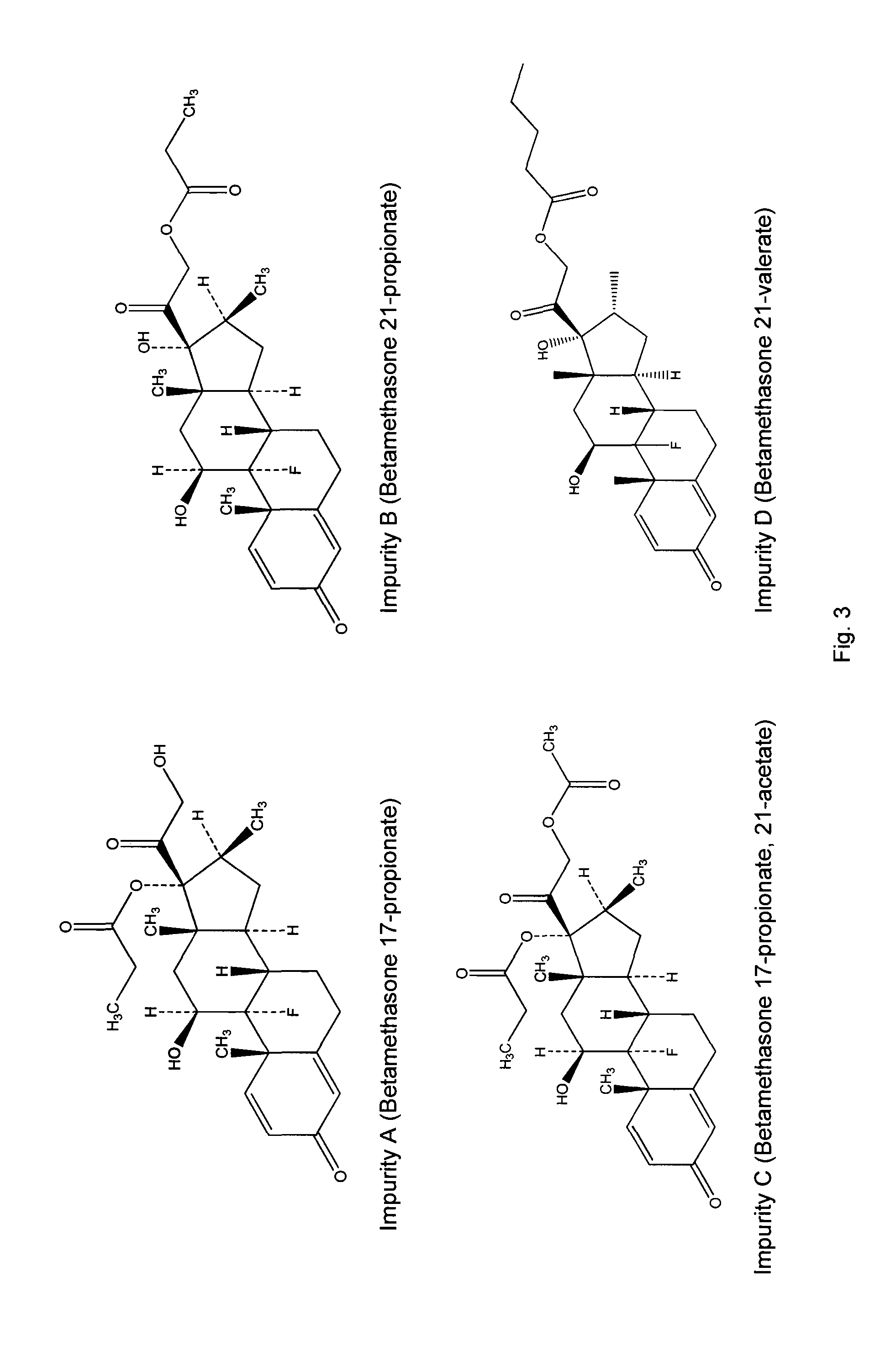
Topical formulations comprising a steroid
ActiveUS20120214776A1Improve usabilityLong duration of actionOrganic active ingredientsSpray nozzlesDiseaseAlcohol free
The application provides formulations for the topical administration of an active agent comprising at least one steroid, in the form of topical sprays that are propellant-free, and / or substantially non-foaming, and / or alcohol-free. The present application also provides processes for preparing such compositions and methods of using them in management of skin diseases or disorders such as psoriasis, dermatoses, and other associated skin diseases or disorders.
Owner:PRIMUS PHARM INC
Fourth generation alcohol-free additive not additionally added in suede preparation of monocrystalline silicon
InactiveCN103290484AReduce pollutionImprove photoelectric conversion rateAfter-treatment detailsAlcohol freeGlycerol
The invention relates to a fourth generation alcohol-free additive not additionally added in suede preparation of monocrystalline silicon. The fourth generation alcohol-free additive comprises the following main components: a dispersing agent, a humectant and deionized water, wherein the dispersing agent is one of or a combination of more of styrene sodium sulfonate, benzyl naphthalenesulfonate formaldehyde condensate, sodium polyaluminate, sodium hexametahposphate, sodium methylene dinaphthalenesulfonate, sodium polysilicate, sodium lignin sulfonate and sodium polyacrylate; and the humectant is one of or a combination of more of sodium hyaluronate, D5-panthenol, glycerol, propanediol, polyethylene glycol, polypropylene glycol, honey and collagen. When the suede preparation additive is added into a suede preparation solution, clean and uniform monocrystalline silicon suede can be quickly prepared, the photoelectric conversion rate is improved, the production cost is reduced, and the environmental pollution is reduced.
Owner:JINGJIANG JINGYI CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com