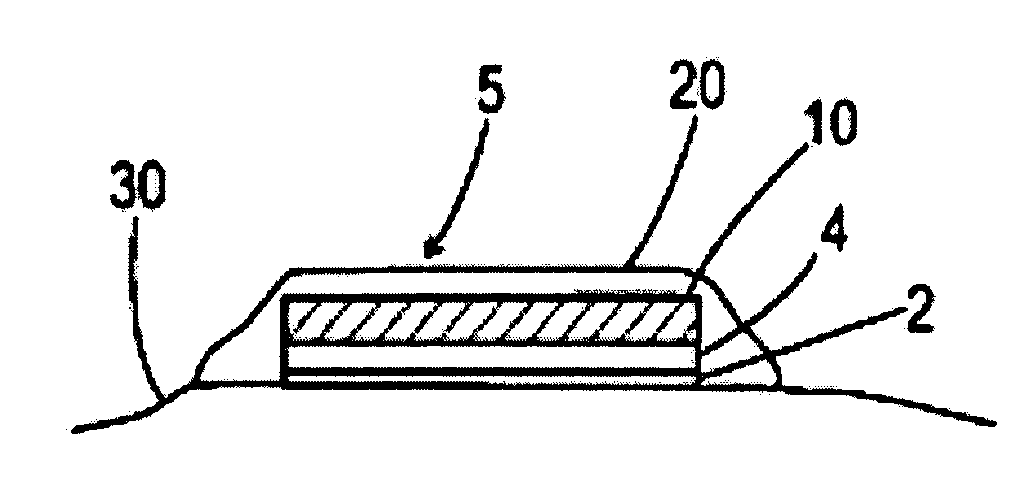
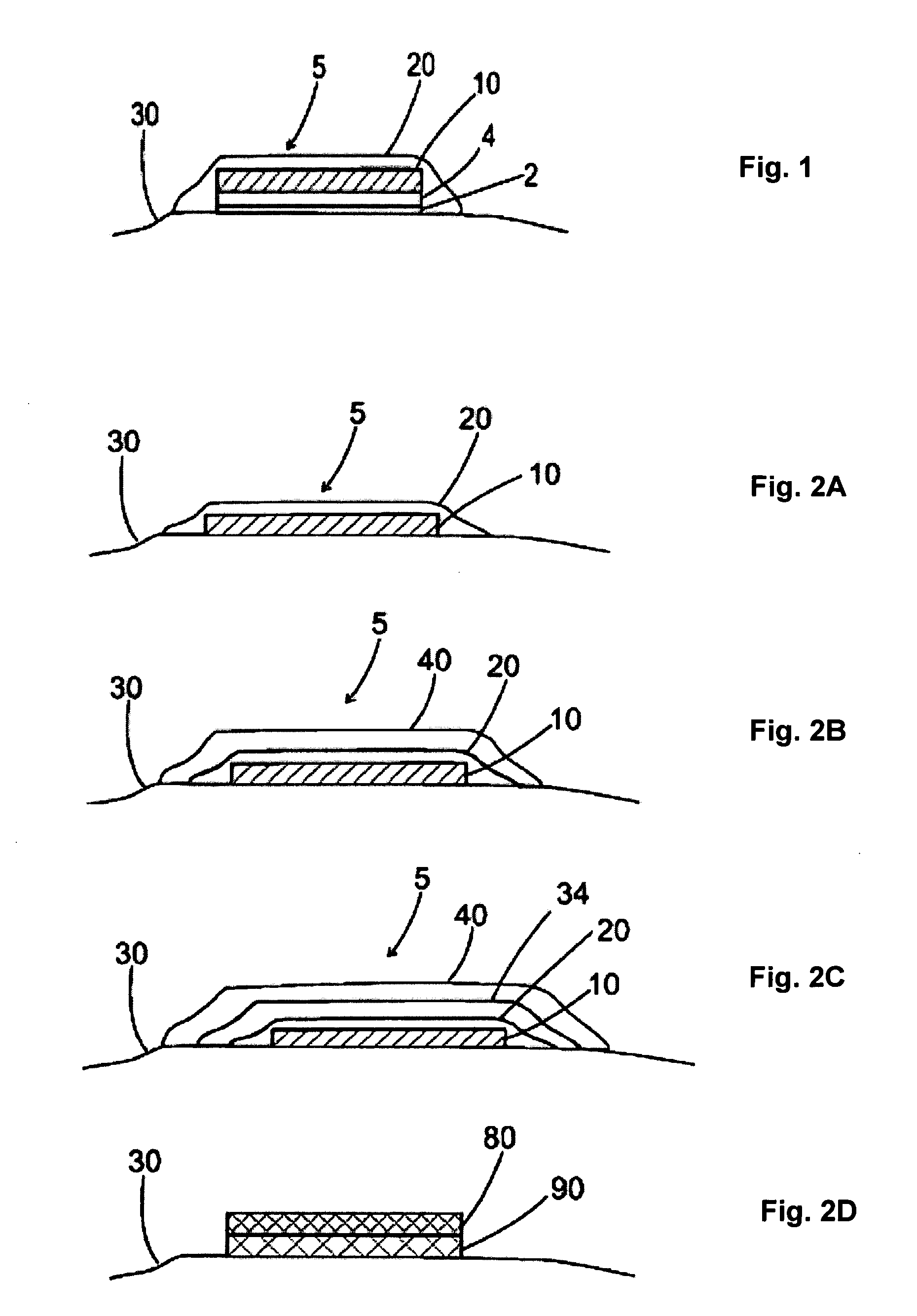
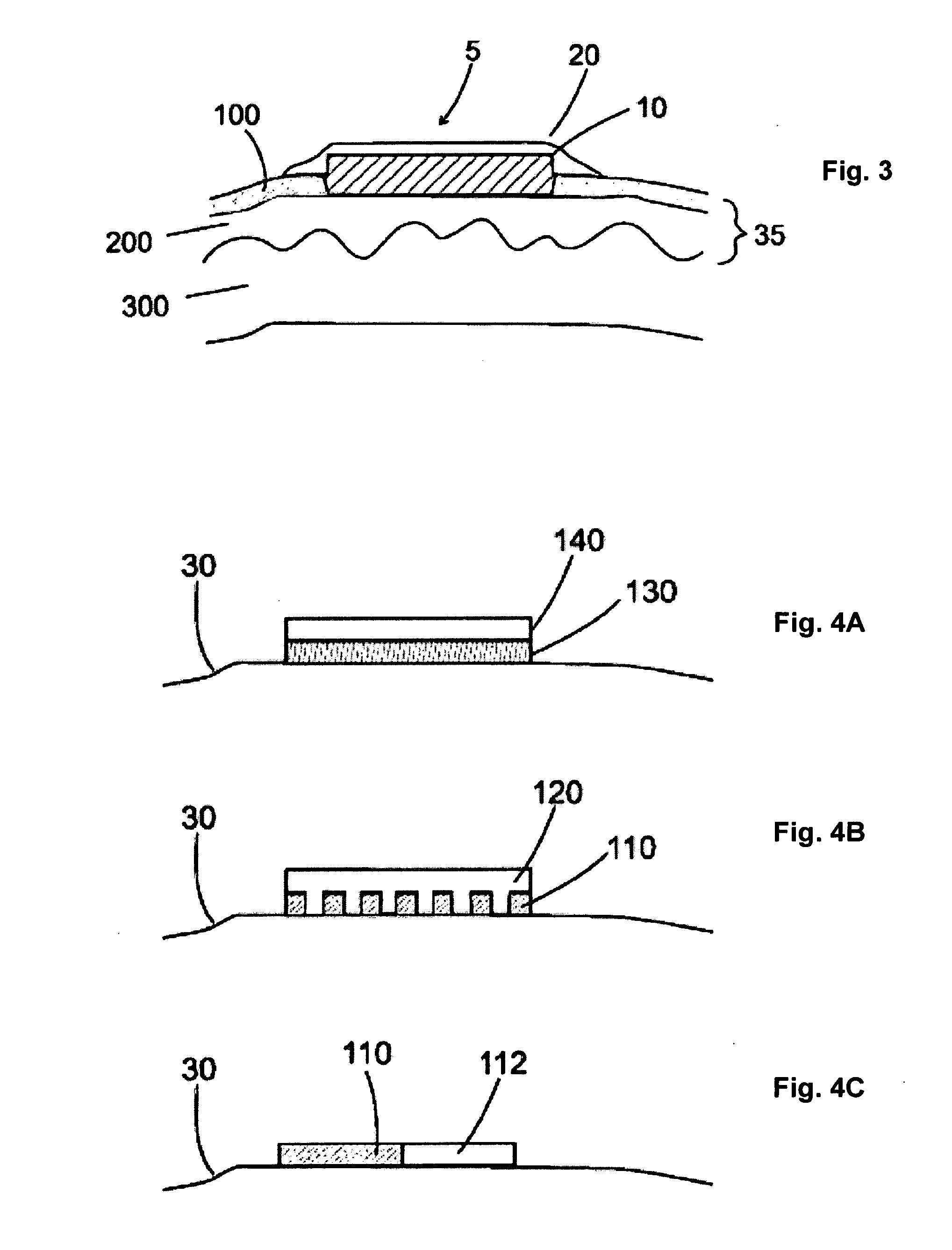
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
992 results about "Stratum corneum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
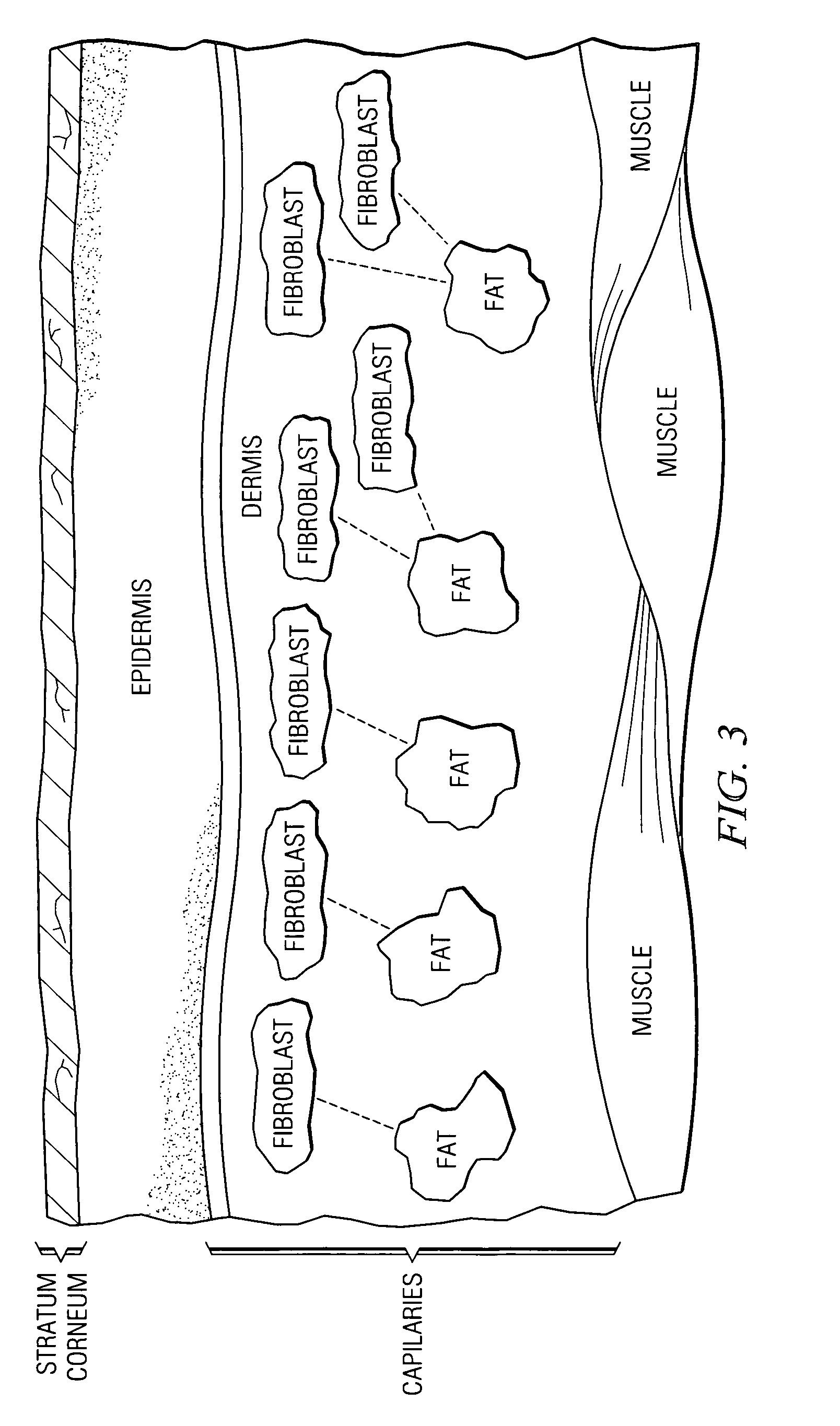
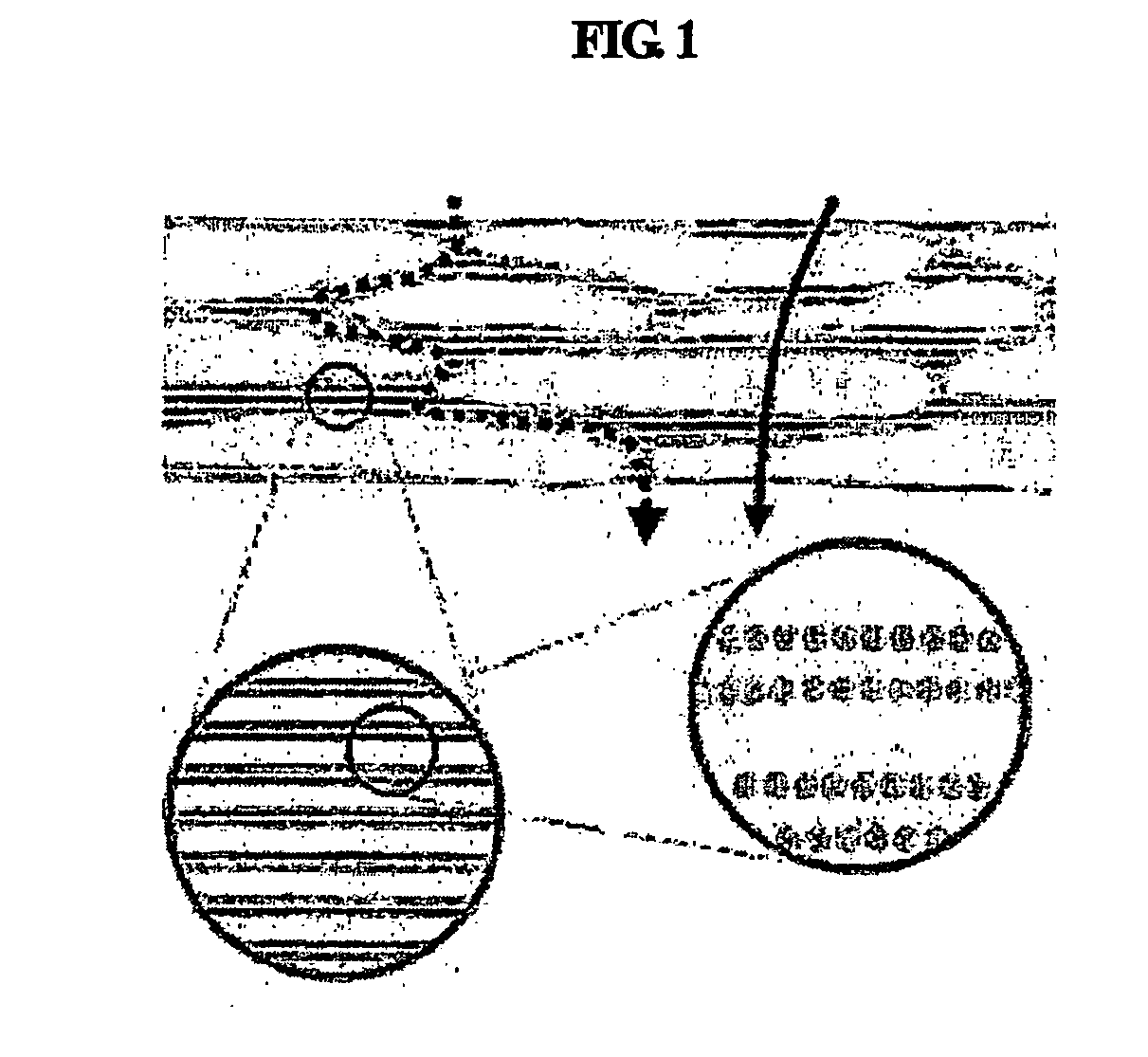
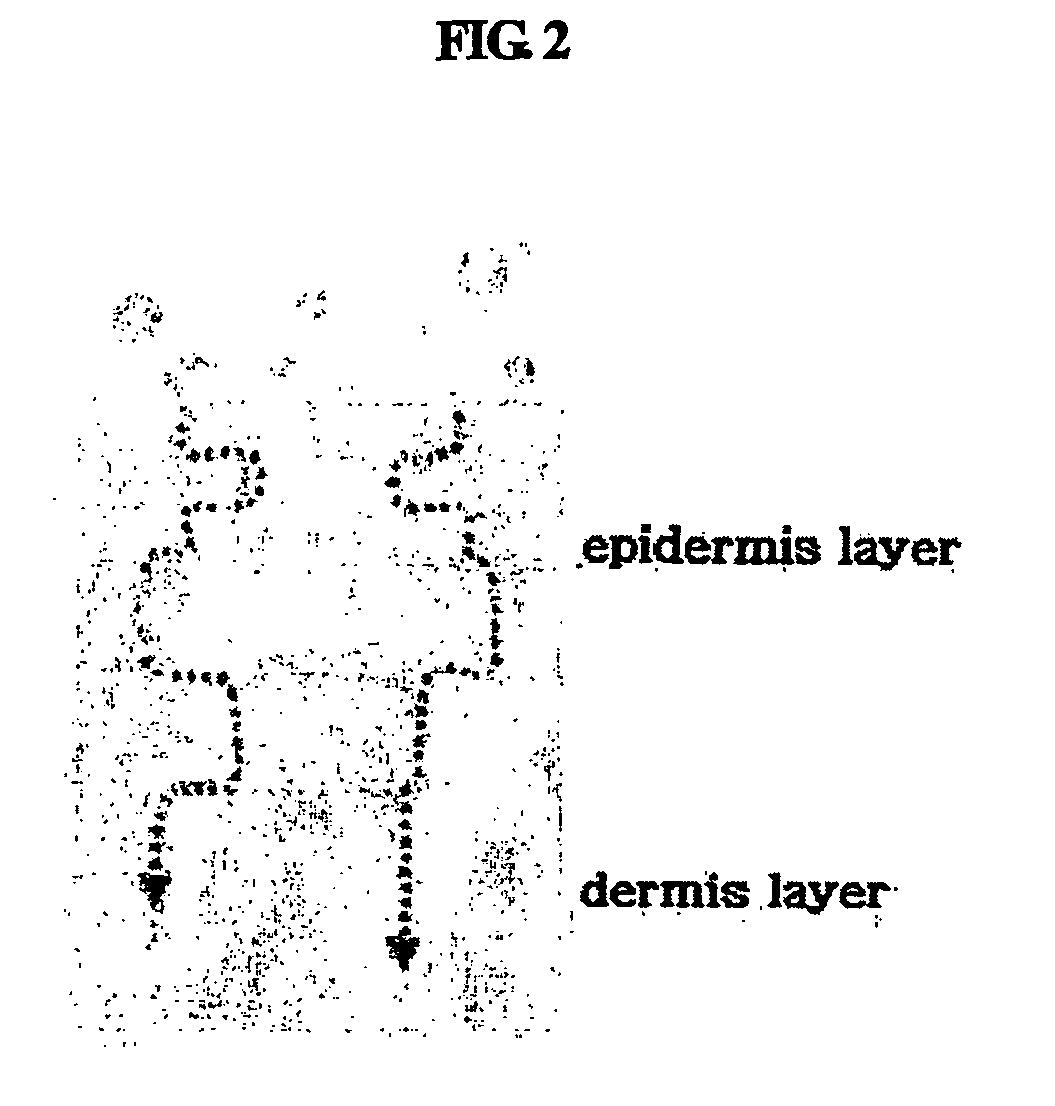
The stratum corneum (Latin for 'horny layer') is the outermost layer of the epidermis. There has been a long-standing belief in dermatology that the stratum corneum consisted of dead cells (corneocytes), devoid of biological activity and function. The stratum corneum is now understood to be live tissue that performs protective and adaptive physiological functions including mechanical shear, impact resistance, water flux and hydration regulation, microbial proliferation and invasion regulation, initiation of inflammation through cytokine activation and dendritic cell activity, and selective permeability to exclude toxins, irritants, and allergens. This layer is composed of 15–20 layers of flattened cells with no nuclei and cell organelles. Their cytoplasm shows filamentous keratin. These corneocytes are embedded in a lipid matrix composed of ceramides, cholesterol, and fatty acids. Their properties depend on the component ratio of the three major components.
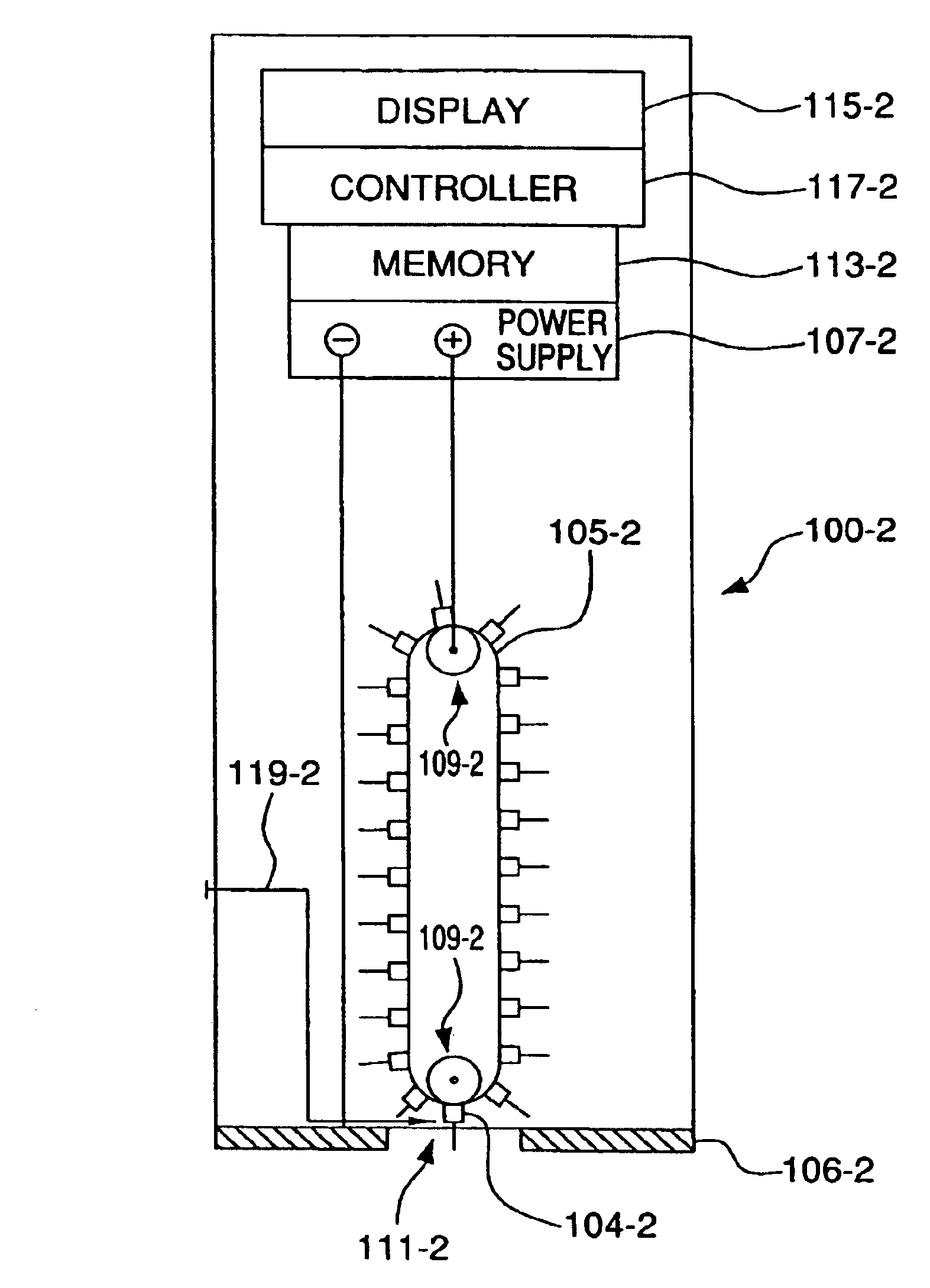
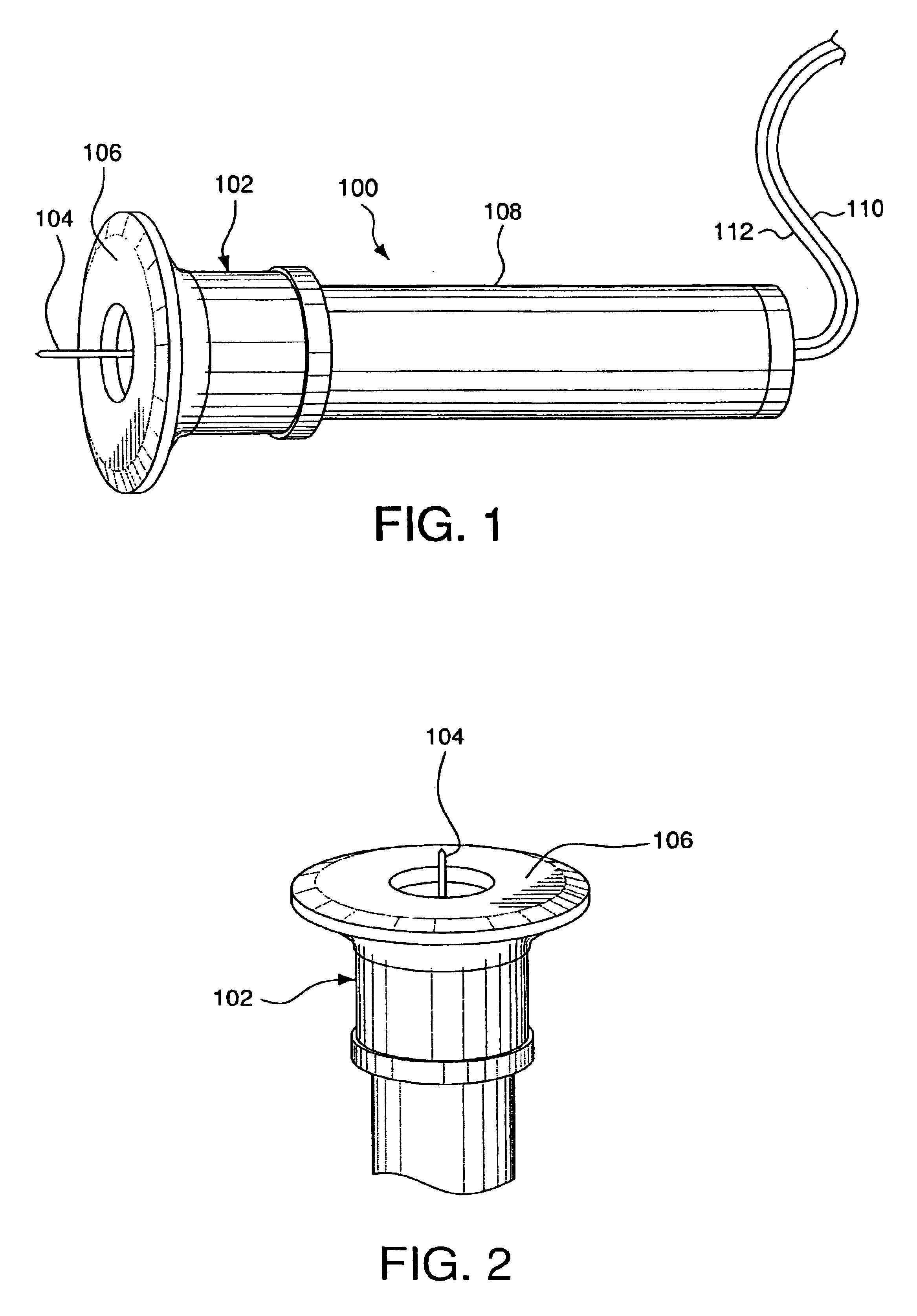
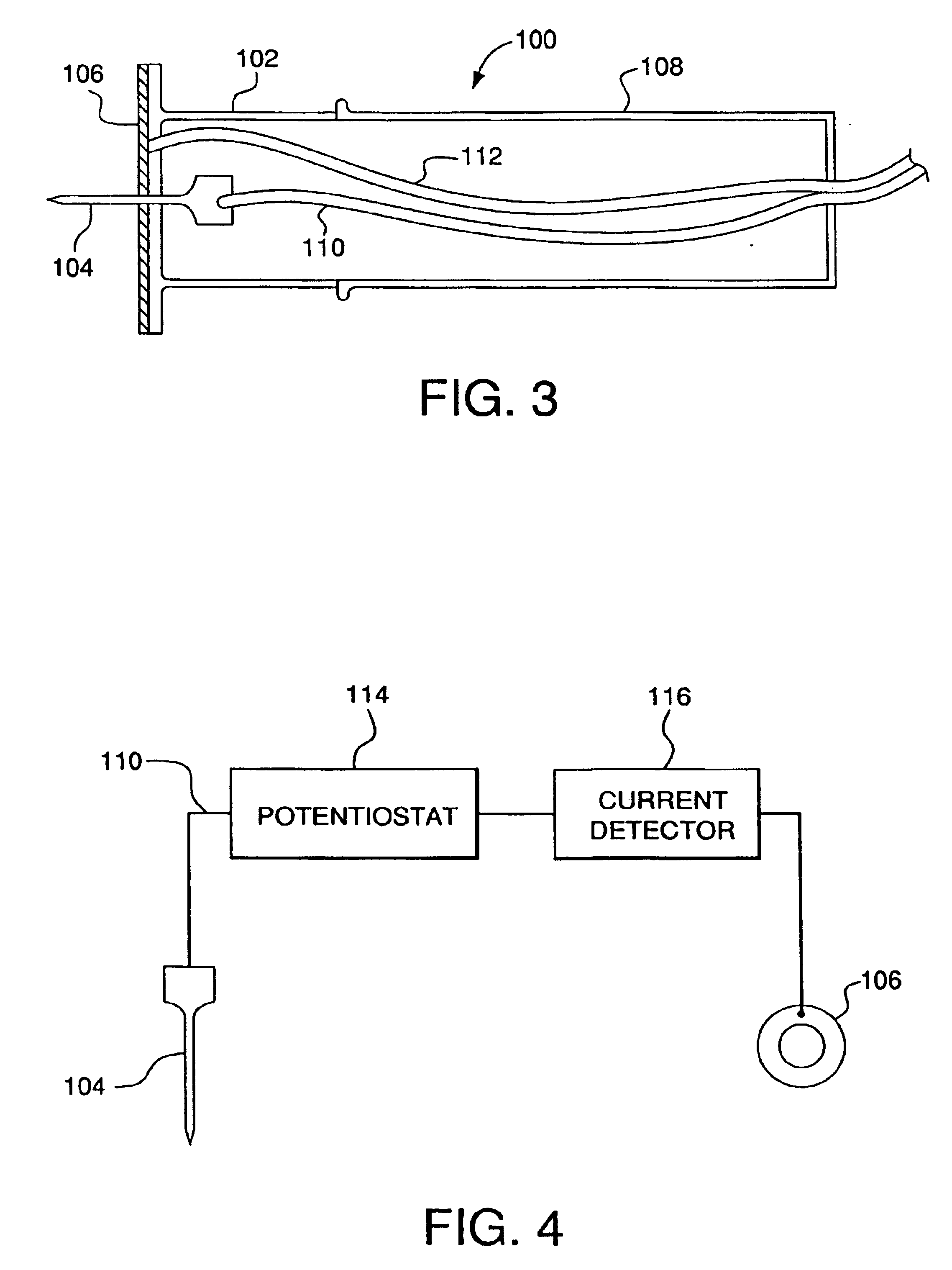
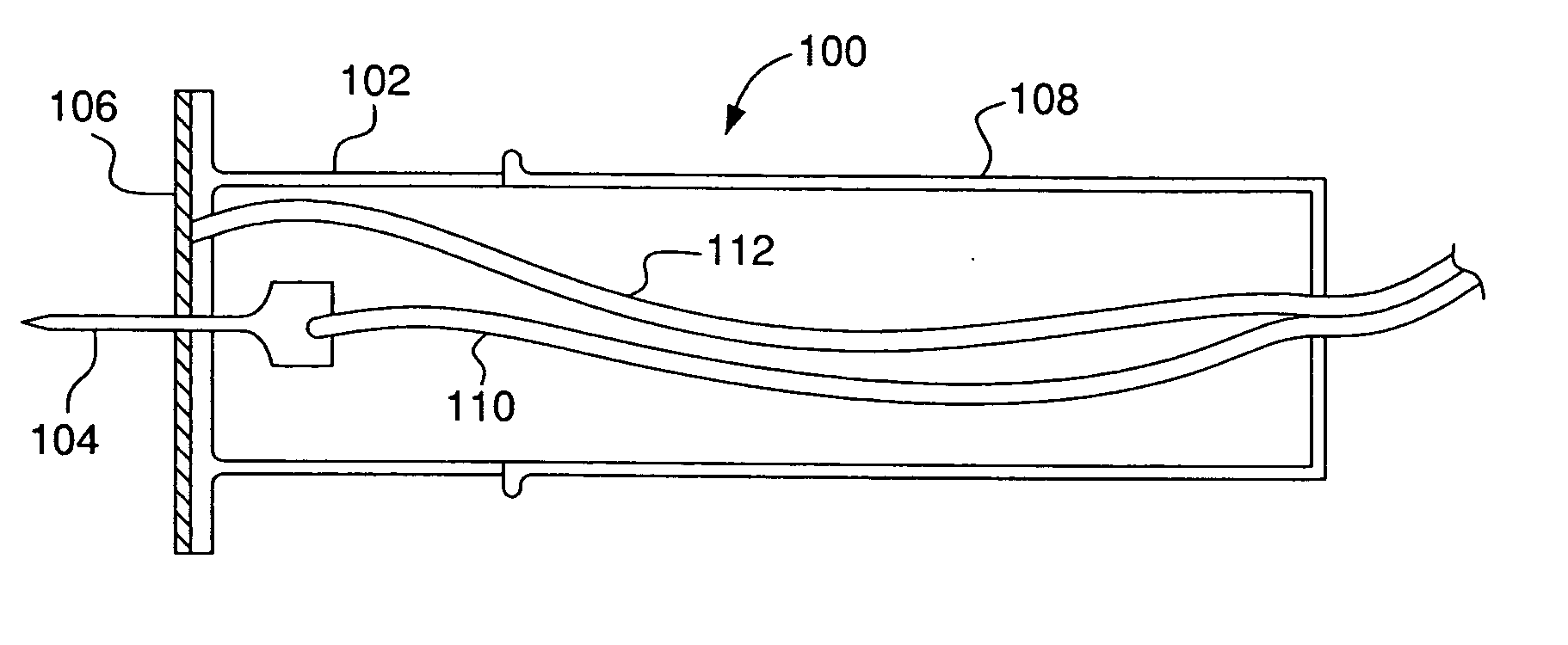
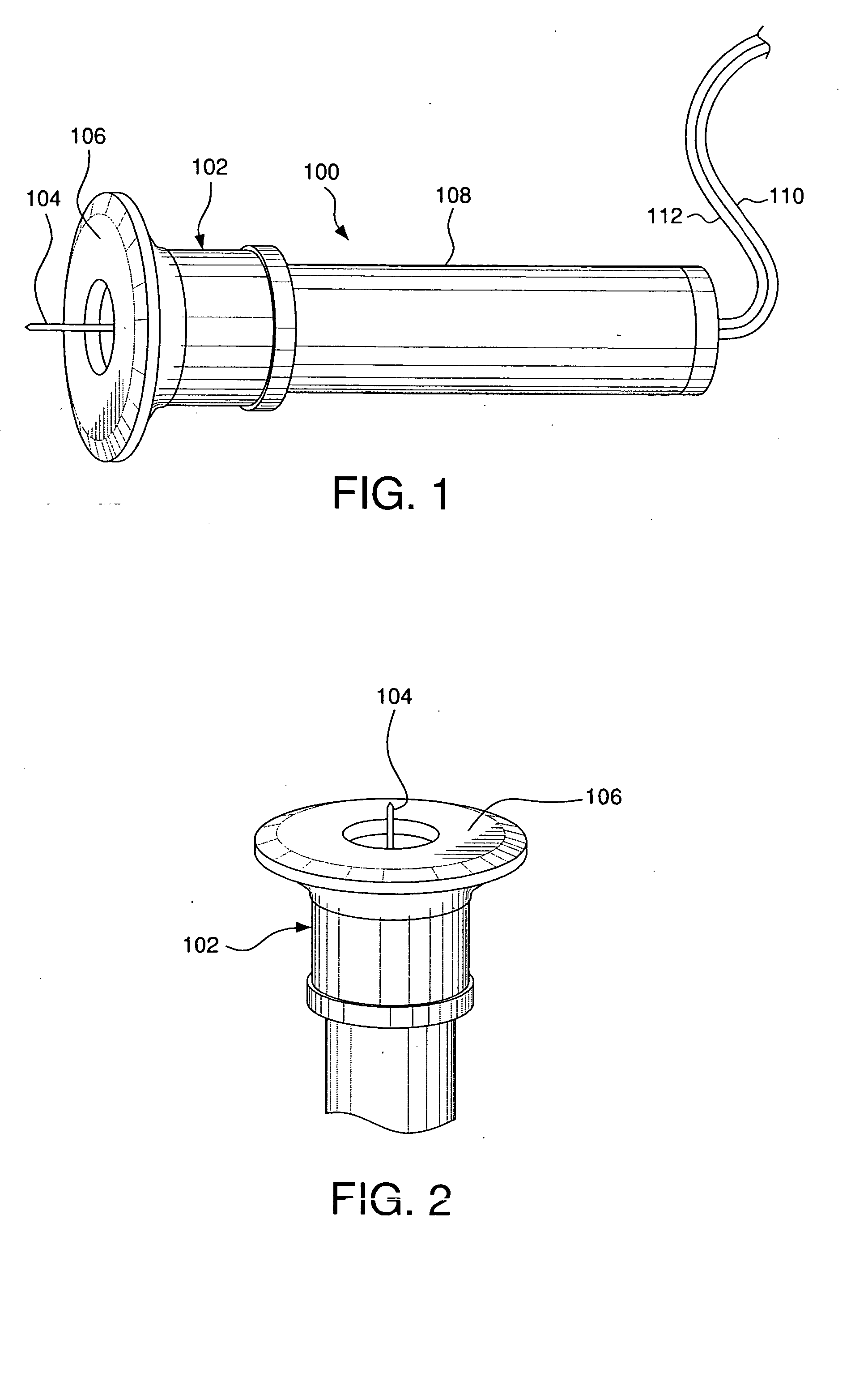
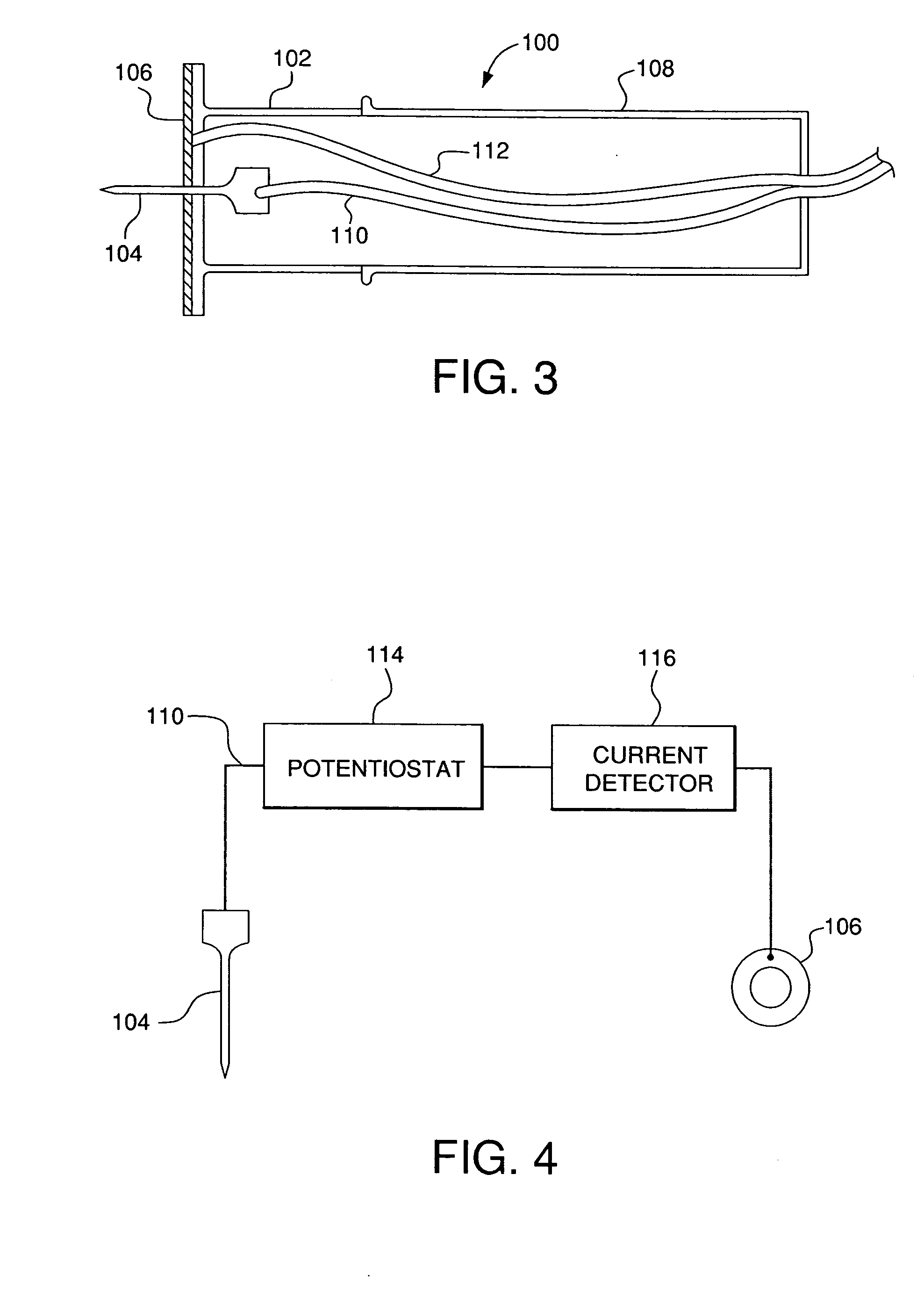
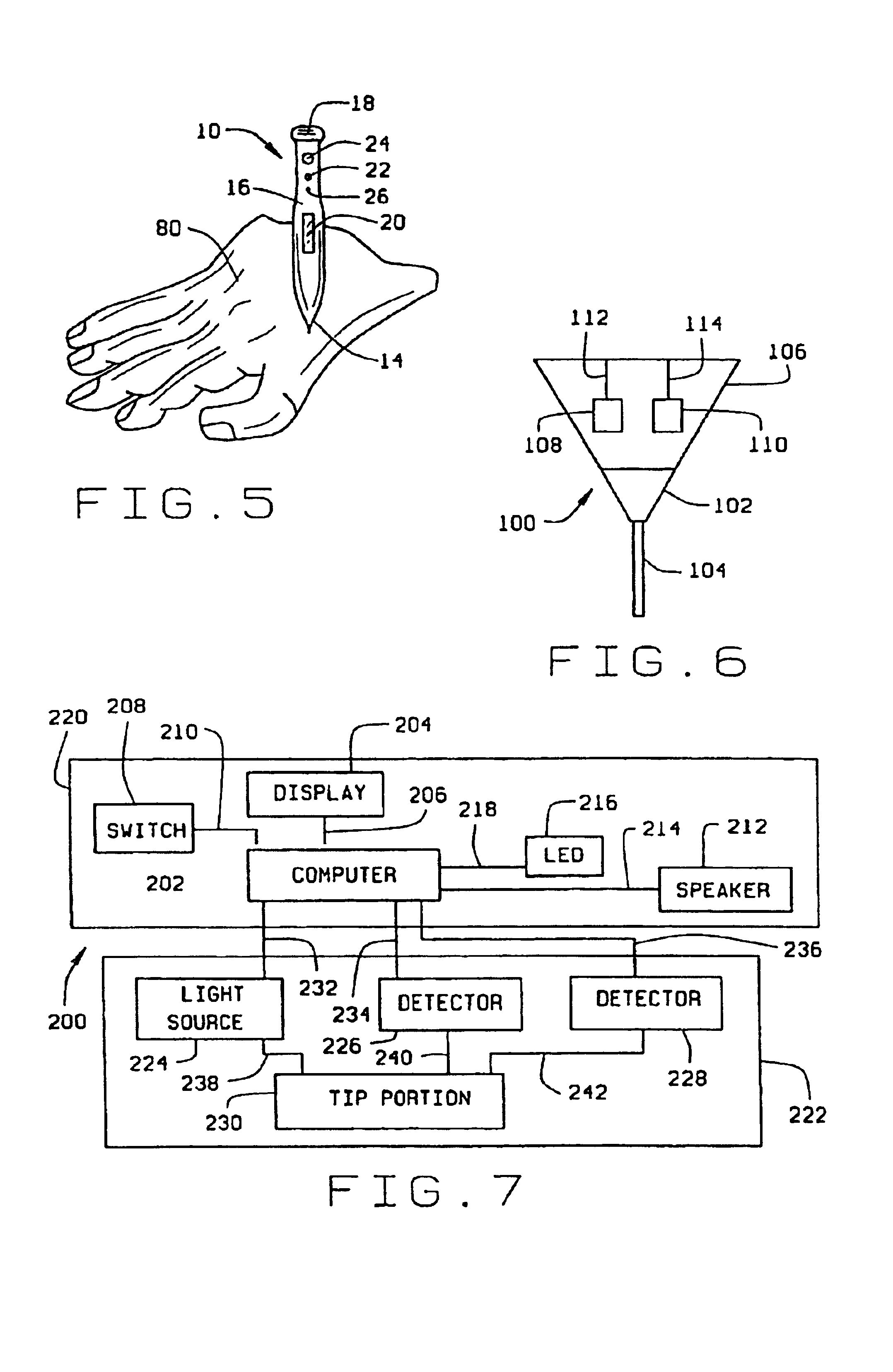
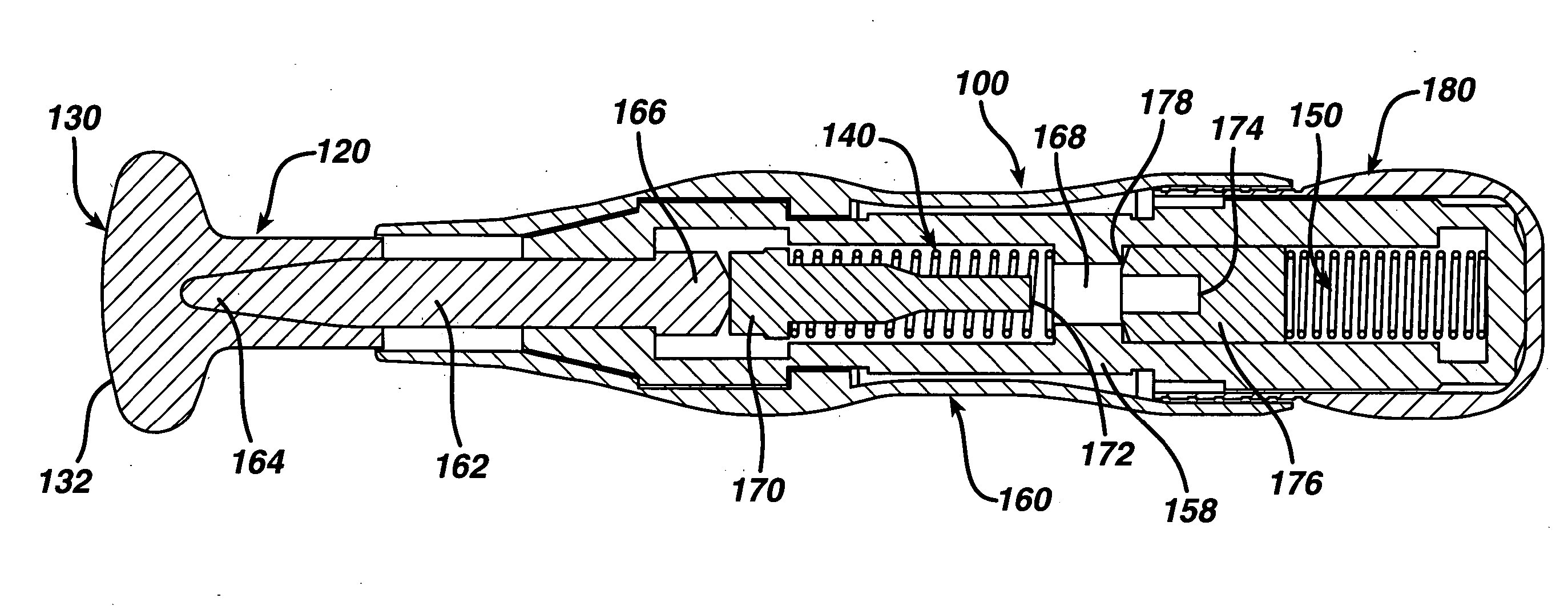
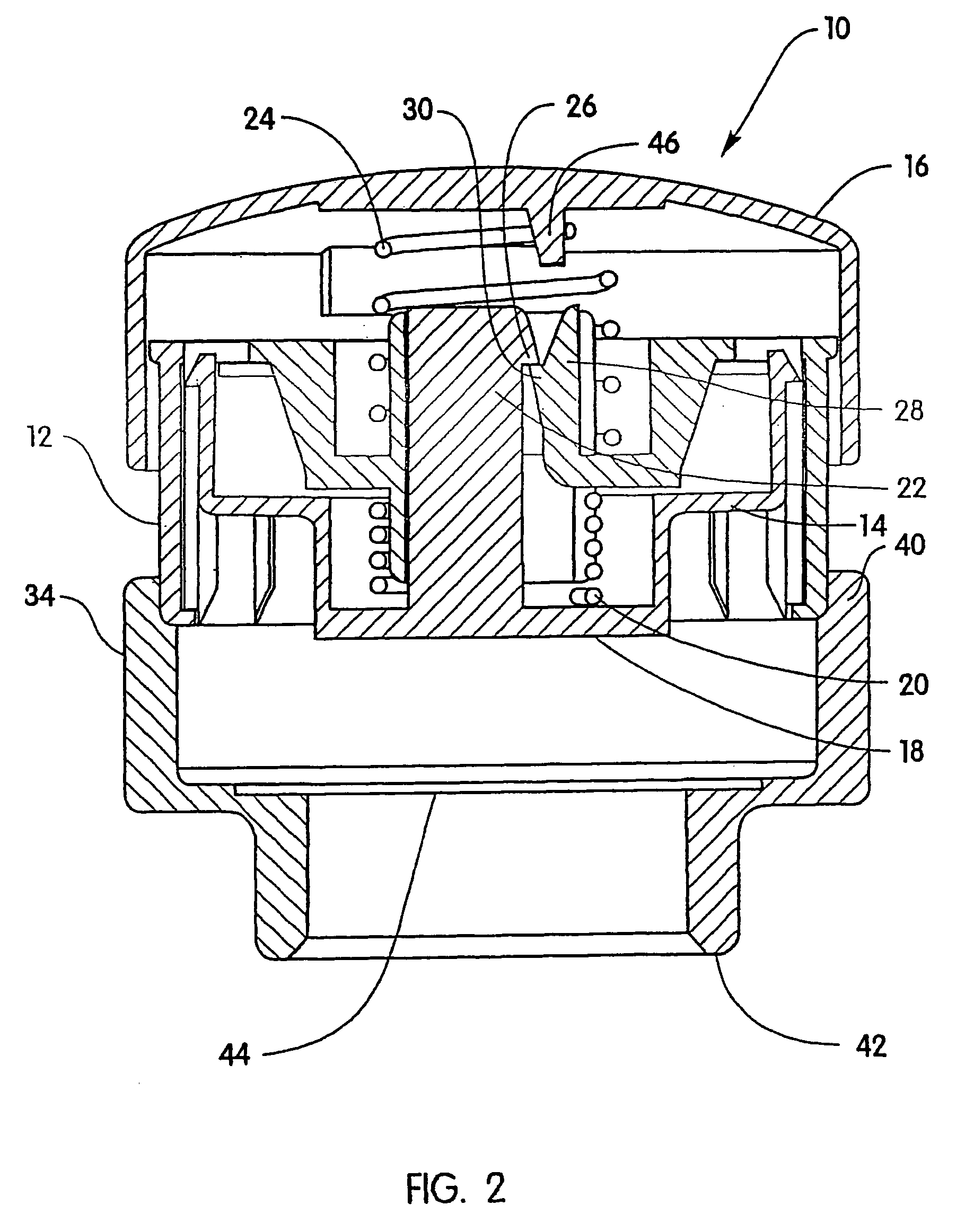
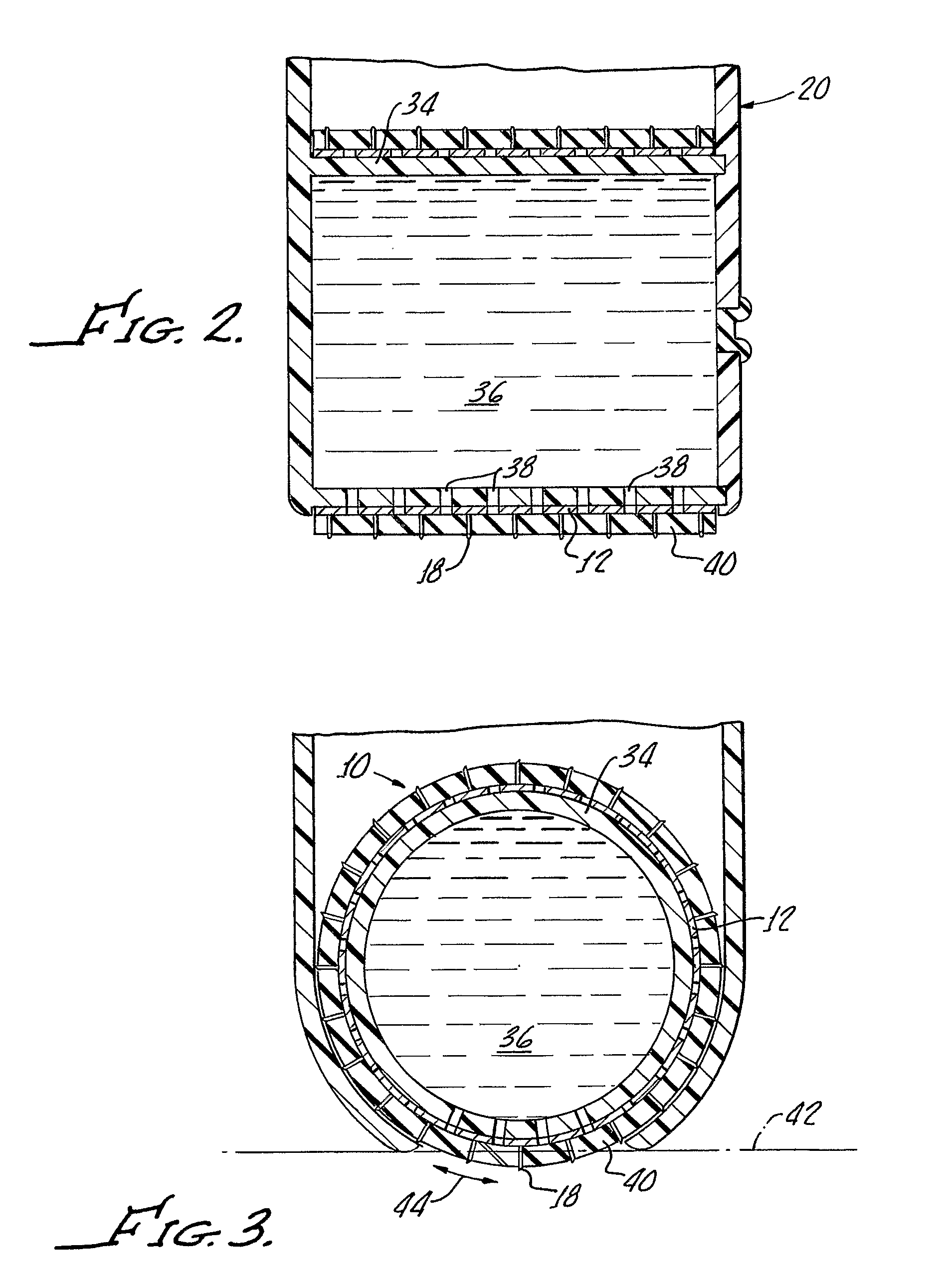
Minimally-invasive system and method for monitoring analyte levels
InactiveUS6952604B2Improve responseImprove signal and performanceCatheterSensorsAnalyteStratum corneum
A minimally-invasive analyte detecting device and method for using the same. The system and method employ a device having an active electrode optionally coated with a substance, and a counter-electrode that is configured at least partially surround the active electrode. The configuration of the auxiliary electrode and active electrode improves the current flow through the device and increases the sensitivity of the device. When the device is placed against the patient's skin, the active electrode is adapted to enter through the stratum corneum of a patient to a depth less than a depth in the dermis at which nerve endings reside. An electric potential is applied to the active electrode and the analyte level is determined based on the amount of current or charge flowing through the device.
Owner:BECTON DICKINSON & CO
Minimally-invasive system and method for monitoring analyte levels
A minimally-invasive analyte detecting device and method for using the same. The system and method employ a device having an active electrode optionally coated with a substance, and a counter-electrode that is configured at least partially surround the active electrode. The configuration of the auxiliary electrode and active electrode improves the current flow through the device and increases the sensitivity of the device. When the device is placed against the patient's skin, the active electrode is adapted to enter through the stratum corneum of a patient to a depth less than a depth in the dermis at which nerve endings reside. An electric potential is applied to the active electrode and the analyte level is determined based on the amount of current or charge flowing through the device.
Owner:BECTON DICKINSON & CO
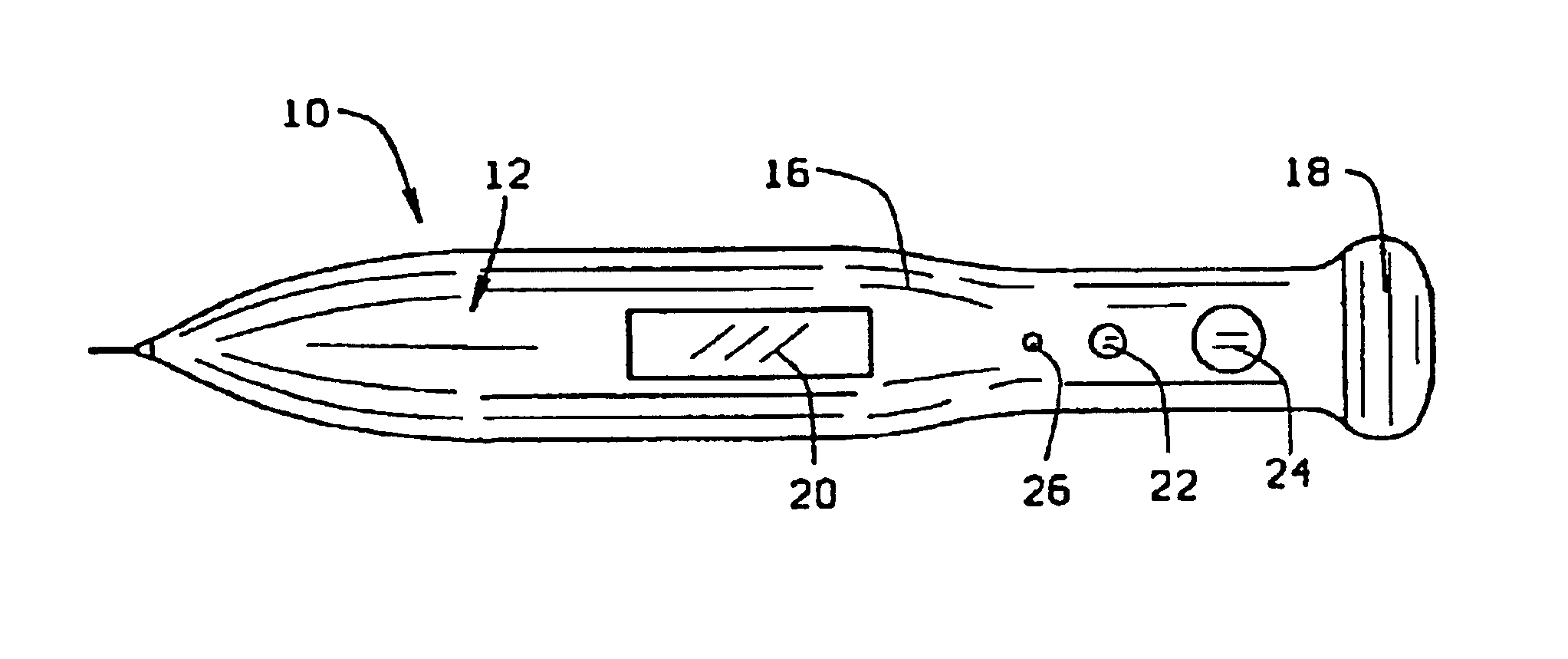
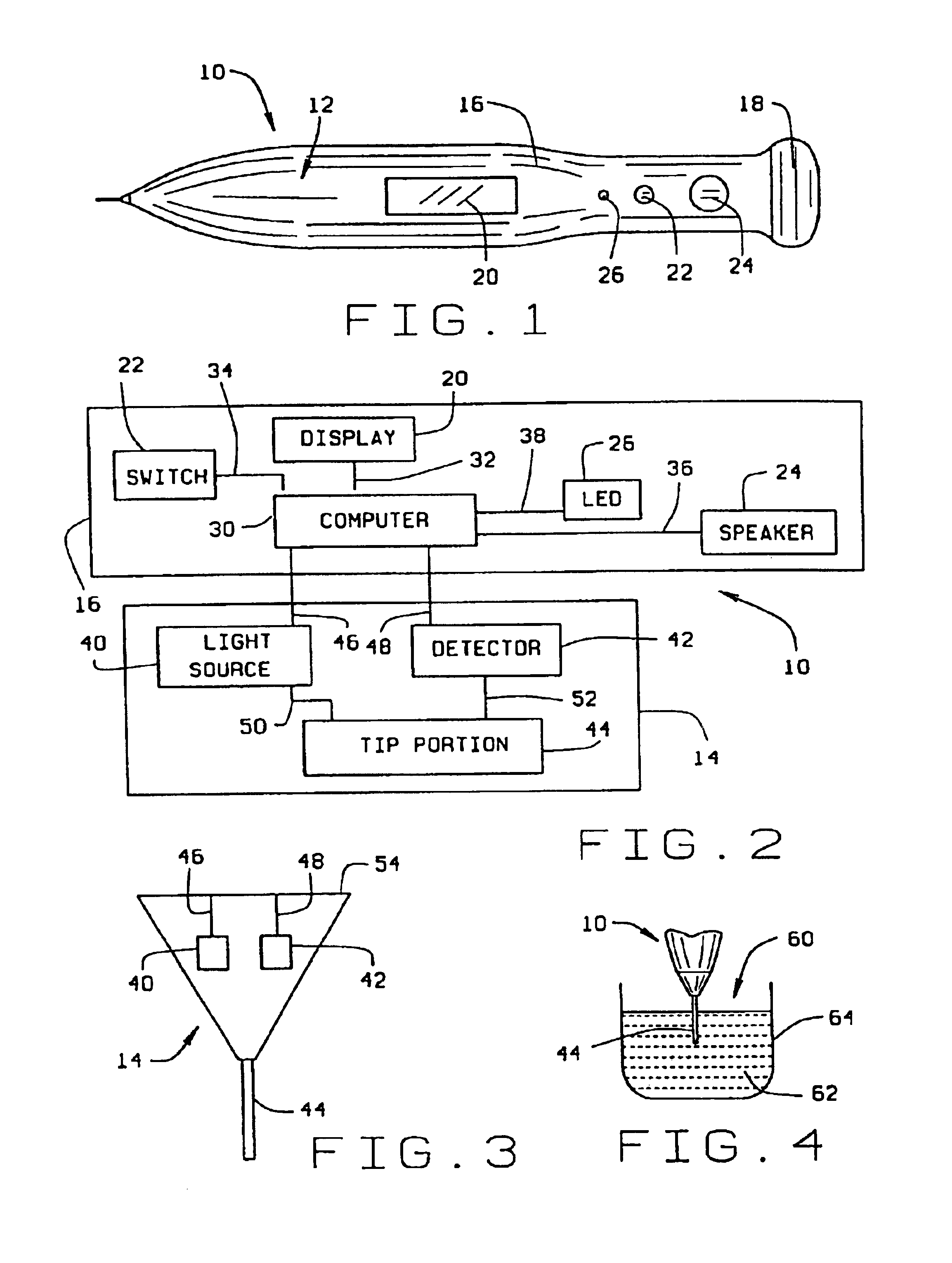
Micro-invasive method for painless detection of analytes in extracellular space
InactiveUS6904301B2Reduces and eliminates delay timeAvoid destructionAdditive manufacturing apparatusSurgeryAnalyteStratum basale
A method of detecting at least one analyte in extra-cellular spaces includes the step of inserting a microprobe through the stratum corneum toward the stratum basale of the skin of a subject into extra-cellular spaces containing interstitial fluid having at least one analyte to be detected, said microprobe having a diameter at its tip no larger than approximately 10-50 microns. The method further includes optically testing for a predetermined analyte in the extra-cellular space adjacent the distal end of the microprobe without drawing a sample of the interstitial fluid. Preferably the microprobe body includes a sensor layer covering the distal optical tip of the microprobe body, the sensor layer being adapted to interact with a predetermined analyte to be detected in the interstitial fluid, and an optical detector responsive to interaction of the sensor layer with the predetermined analyte to signal detection of said predetermined analyte.
Owner:BECTON DICKINSON & CO
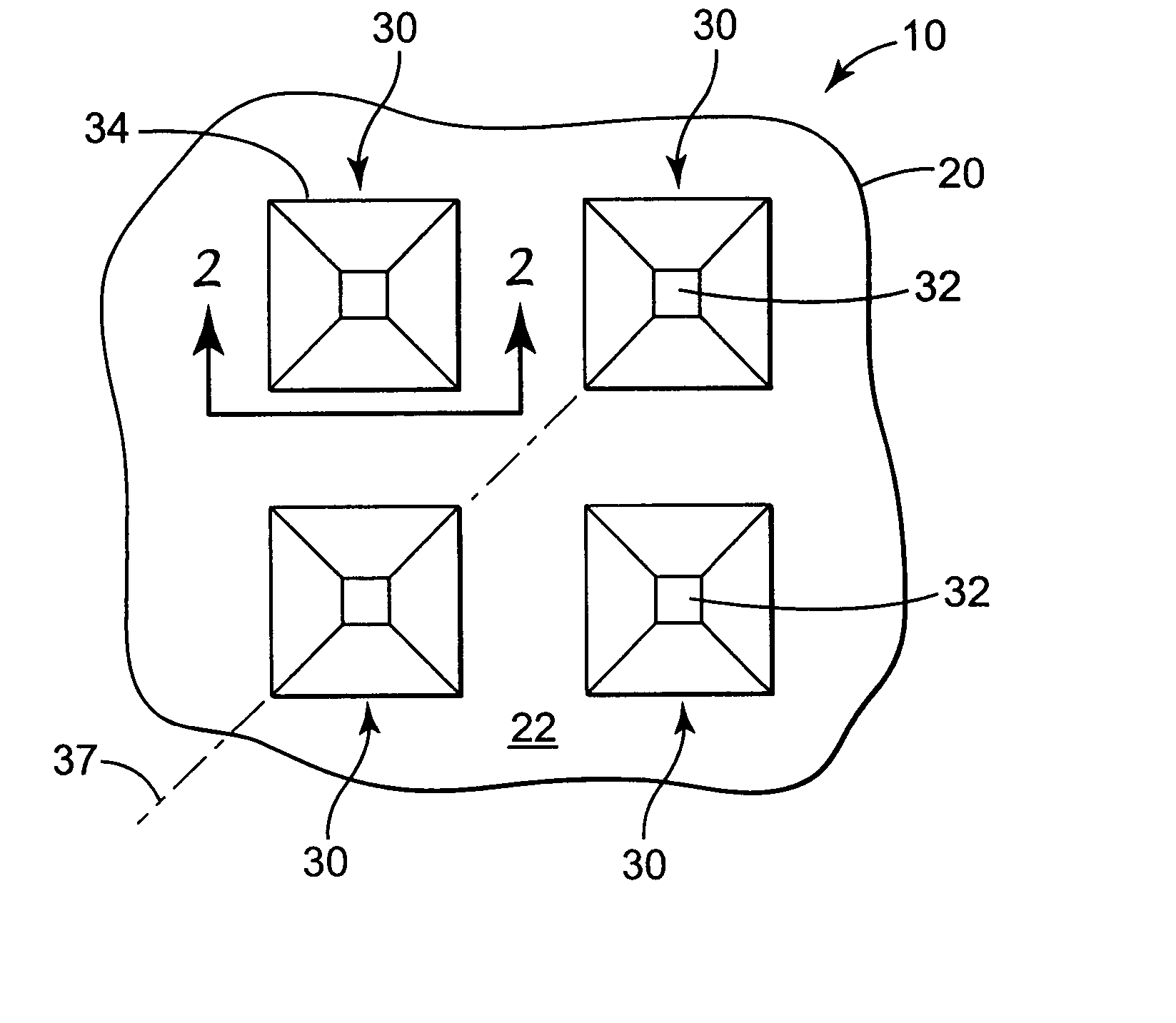
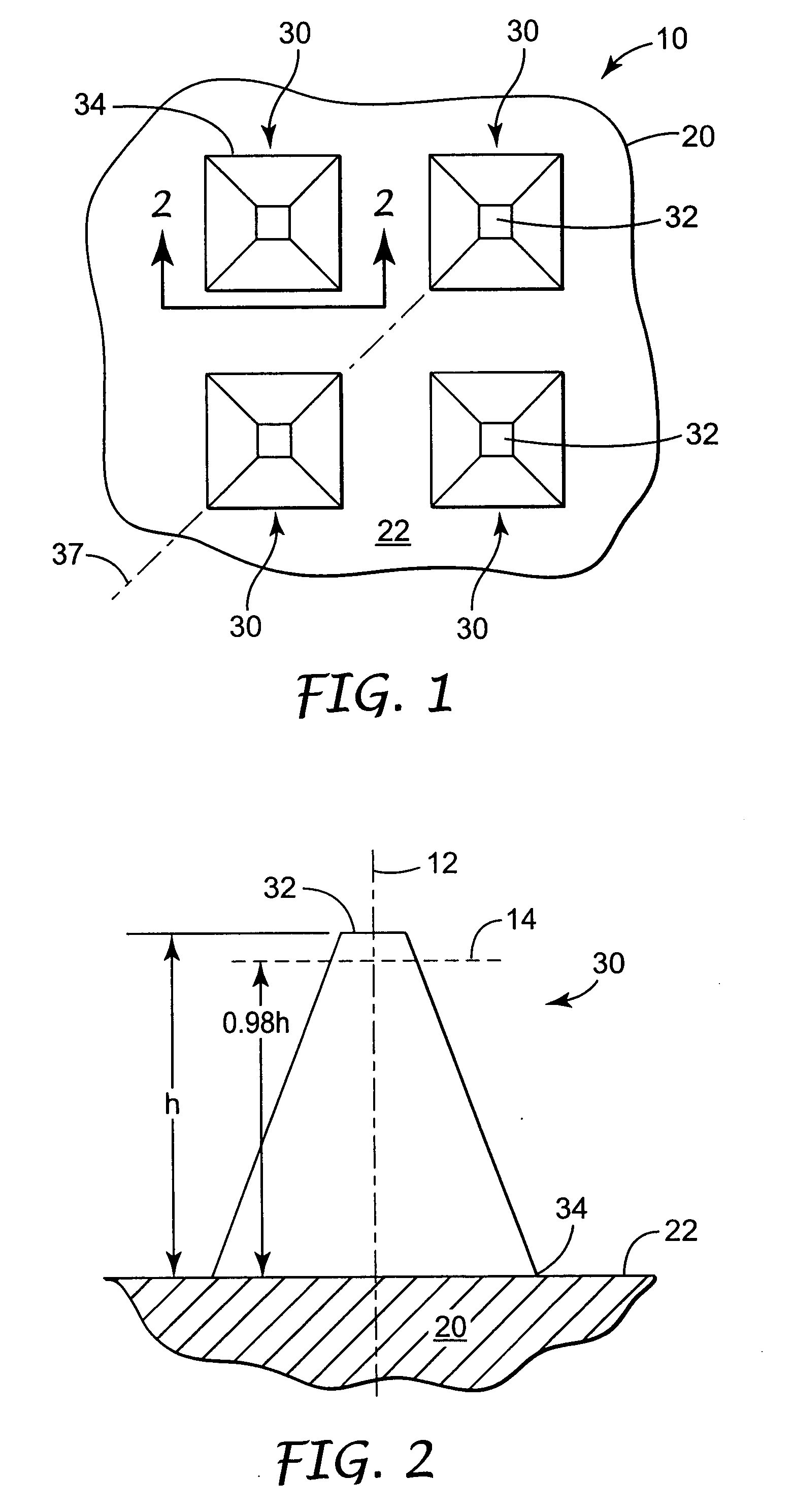
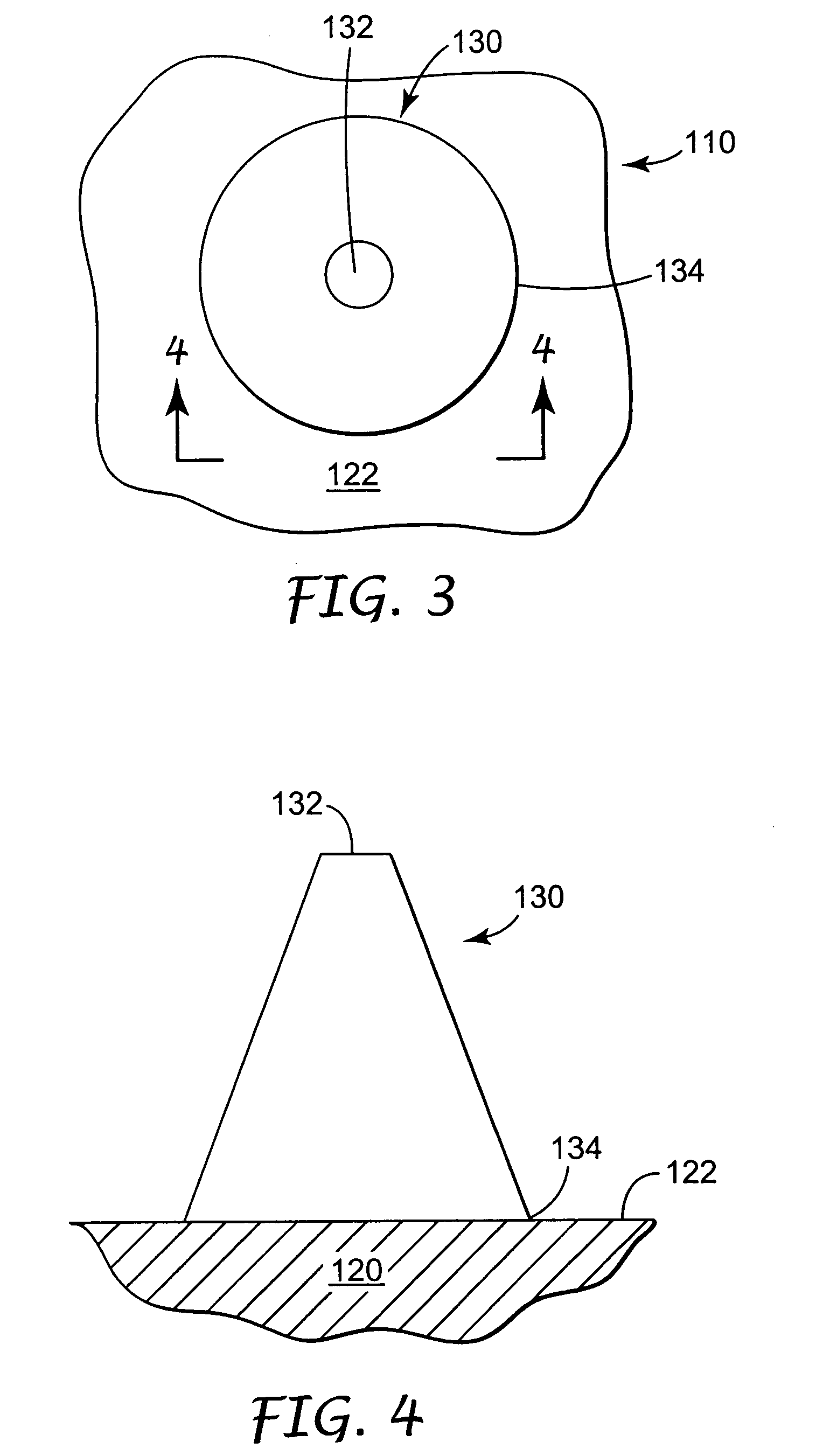
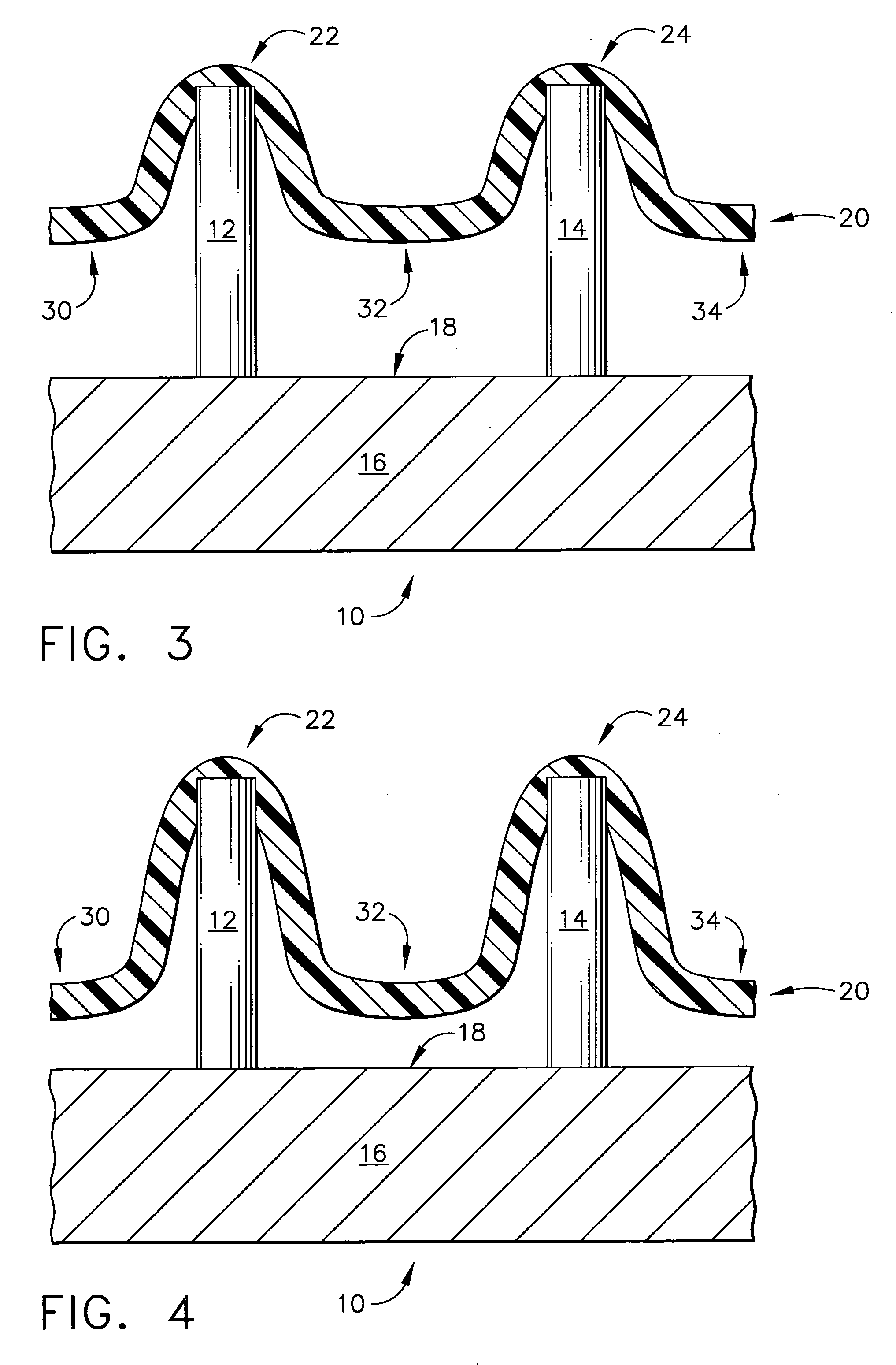
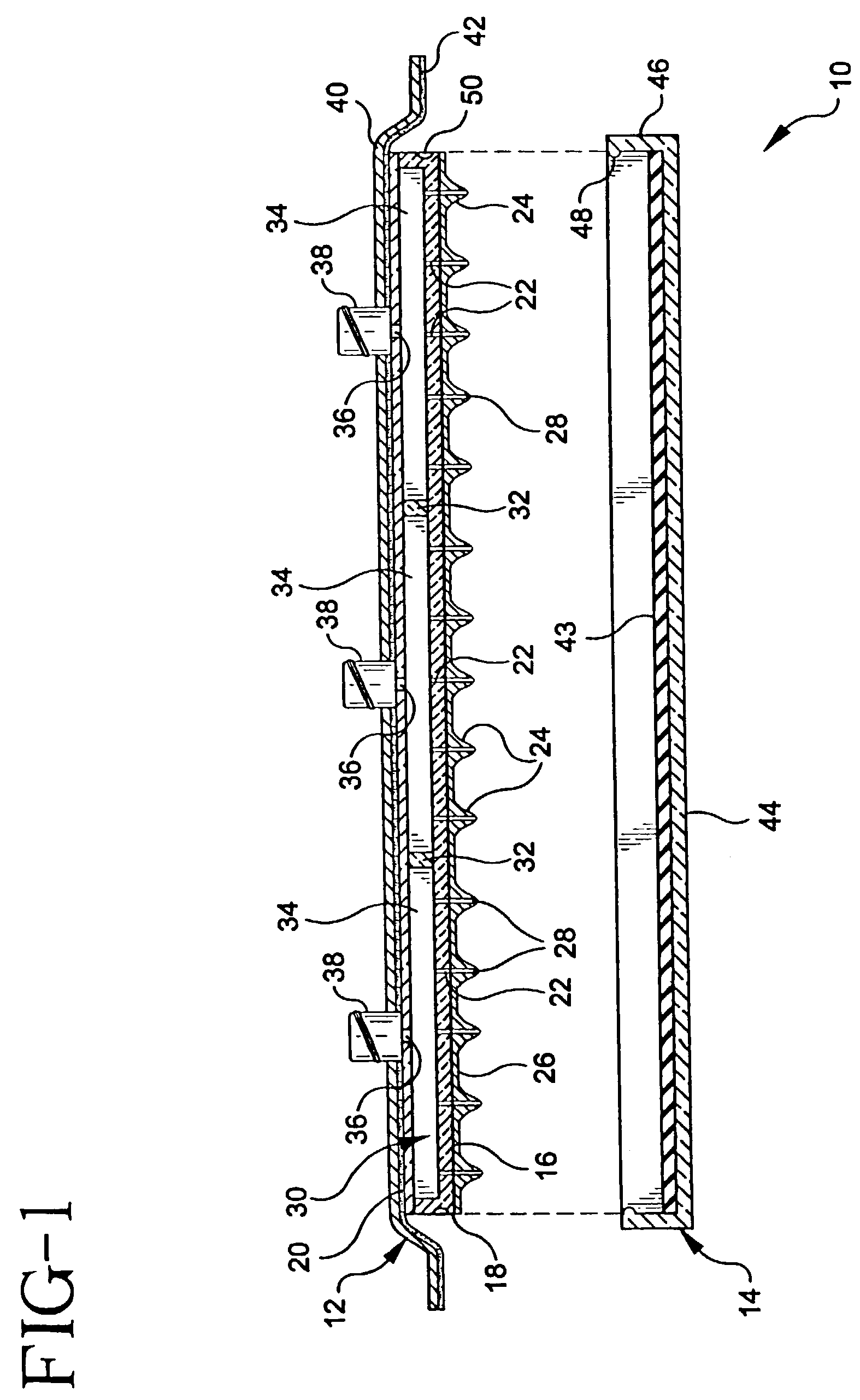
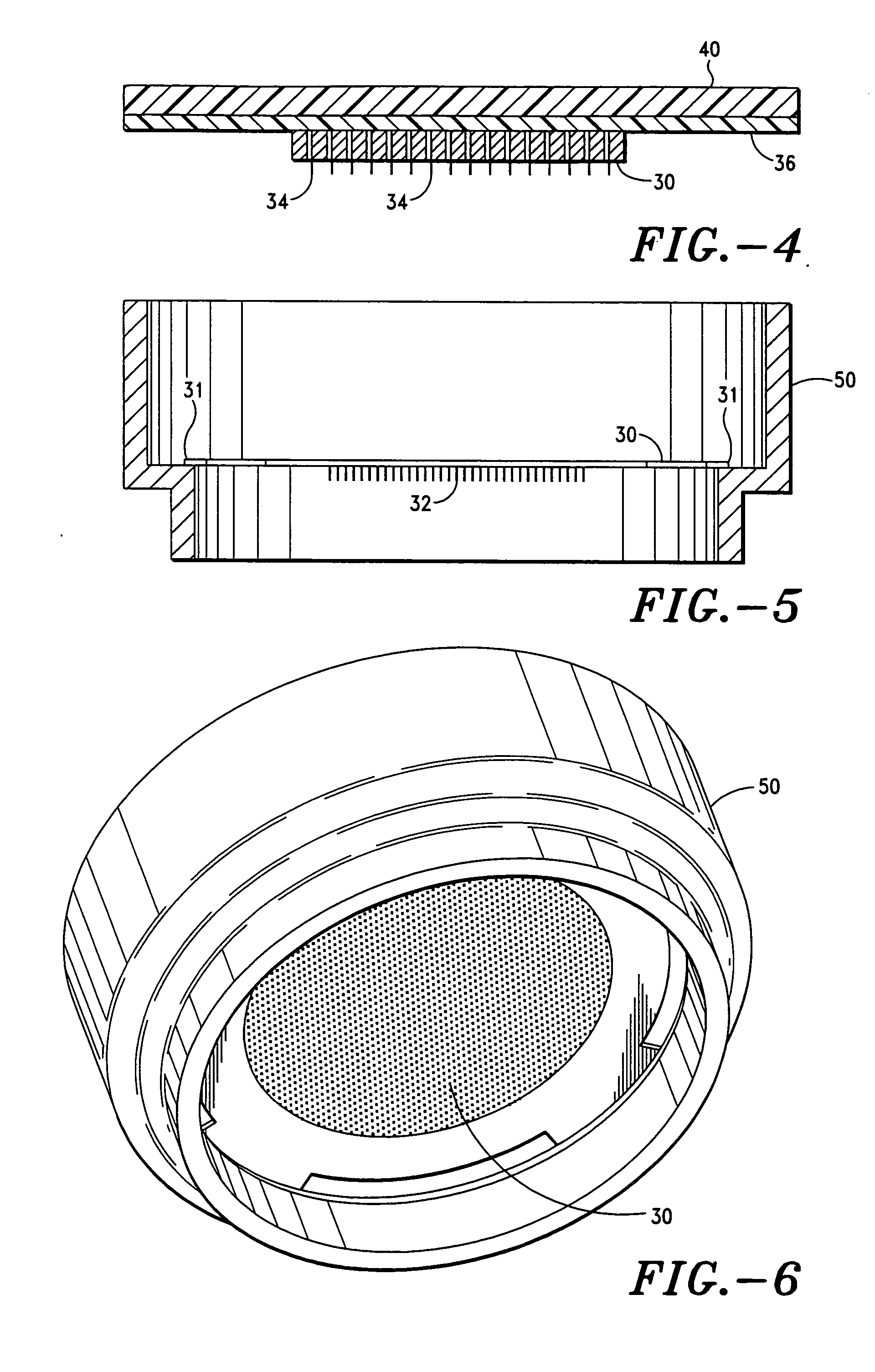
Microneedle devices and microneedle delivery apparatus
InactiveUS20050261631A1Reduces tip fractureEffective perforationSurgical needlesMicroneedlesStratum corneumPain experience
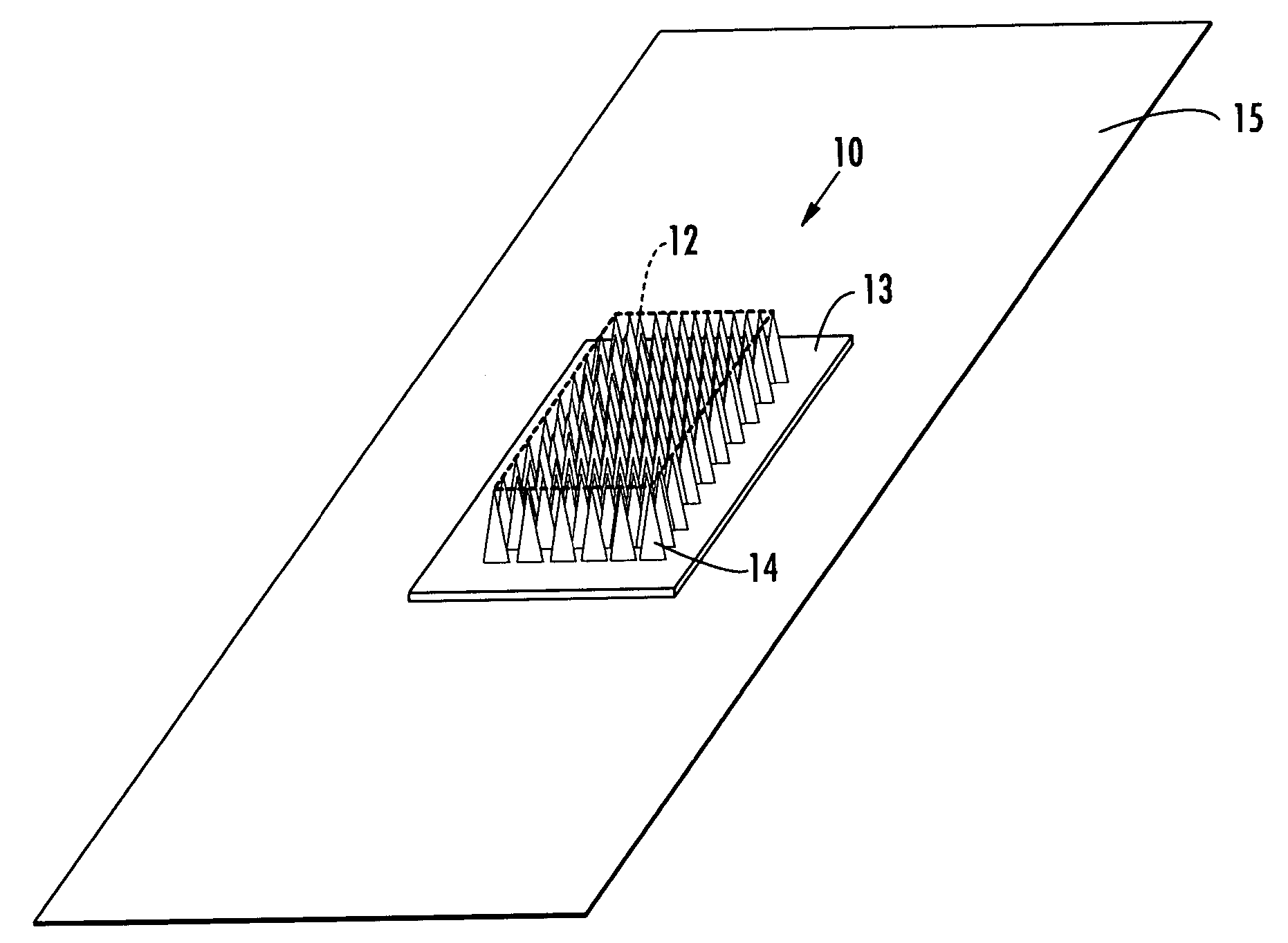
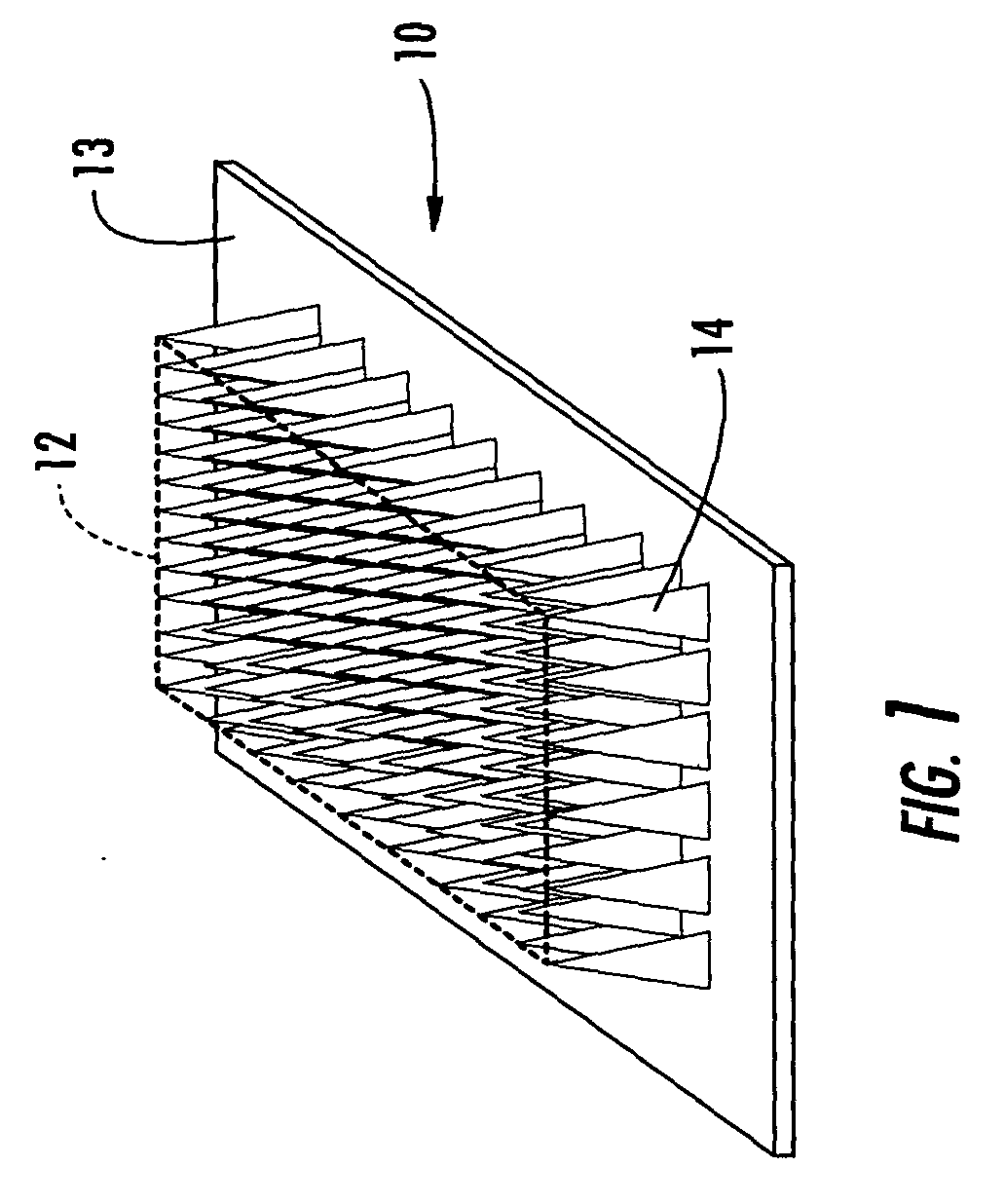
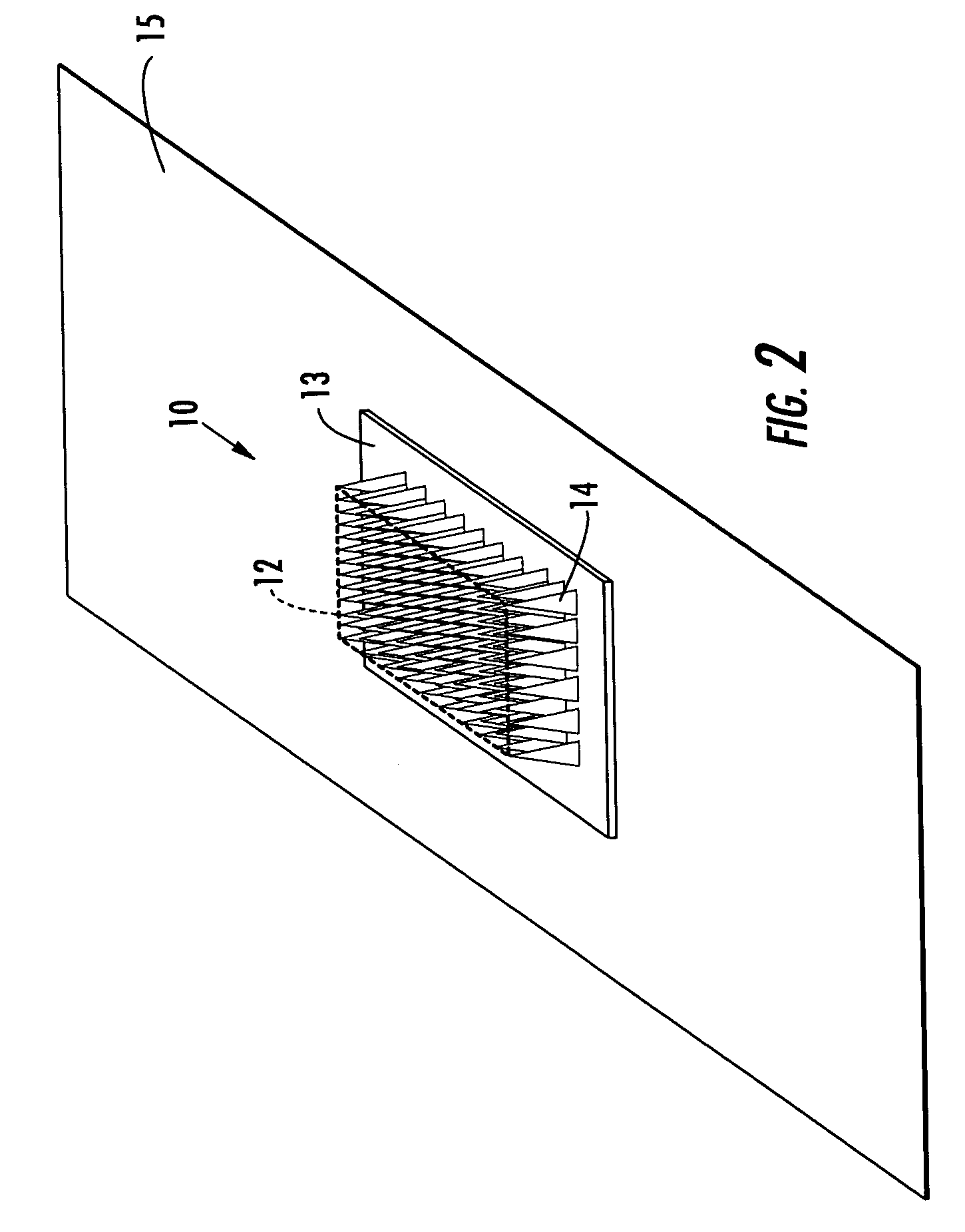
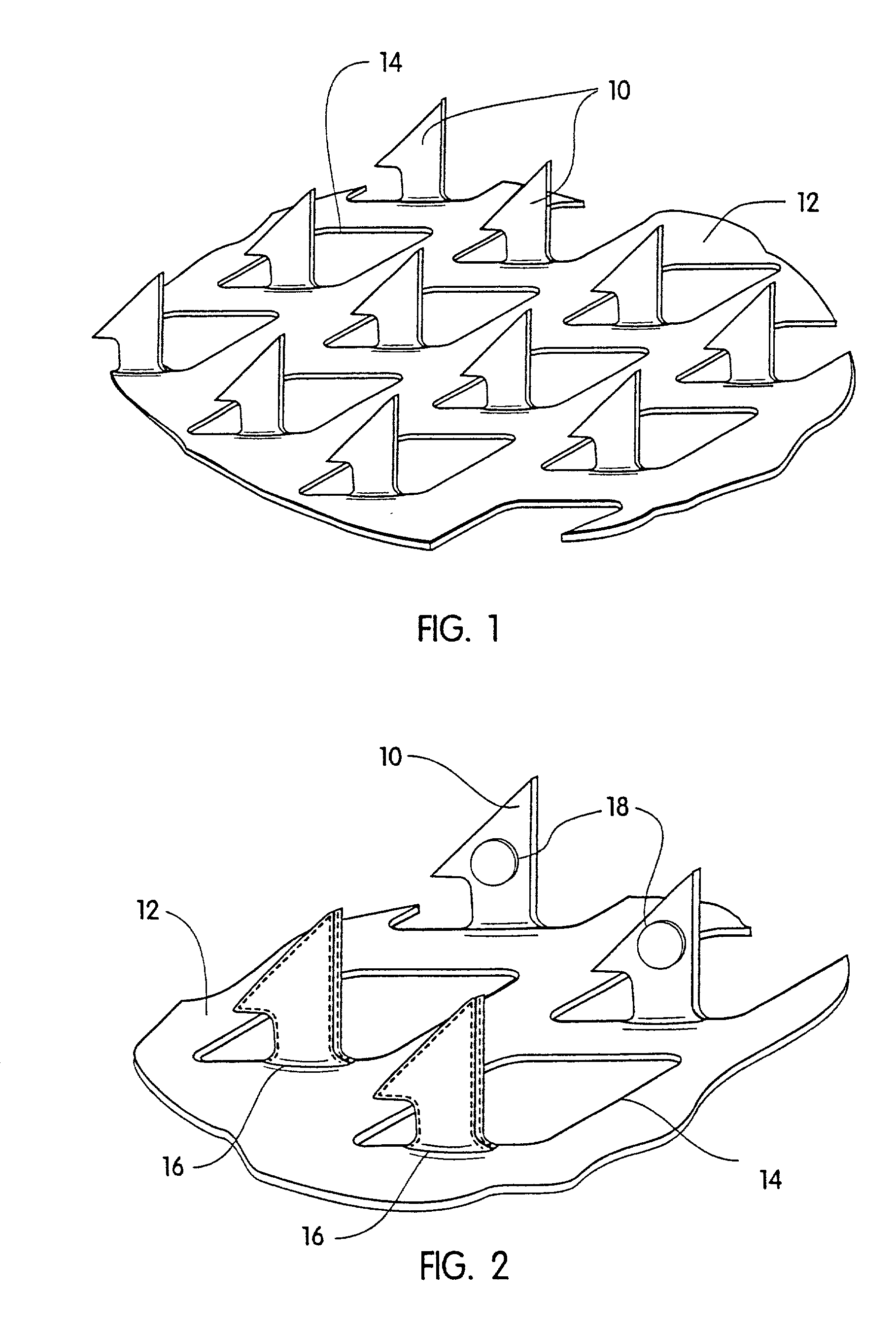
Microneedle devices with microneedles having a truncated tapered shape are disclosed. The microneedles of microneedle devices may also have a controlled aspect ratio. Microneedle delivery apparatus are disclosed that include drivers designed to deliver microneedles at velocities that may enhance perforation of the stratum corneum while limiting the sensation of pain experienced at the delivery site.
Owner:3M INNOVATIVE PROPERTIES CO
Applicator for applying functional substances into human skin
An applicator for applying functional substances, such as cosmetic powder, food color marking, India ink effect marks, or drugs into human skin, having a base, a plurality of microneedles fixed to and projecting from the base a distance only sufficient to penetrate into the stratum corneum or dermis, with the microneedles being of a material that is capable of disintegration and dispersion into the stratum corneum or dermis, such as maltose. The needles contain the functional substance for delivery into the stratum corneum or dermis. The microneedles are of a length approximately 0.5 to 500 μm when used to apply a functional substance to the stratum corneum, or are of a length of approximately 500 to 5,000 μm when used to apply a functional substance to the dermis.
Owner:TOBINAGA YOSHIKAZU +1
Transdermal drug delivery devices having coated microprotrusions
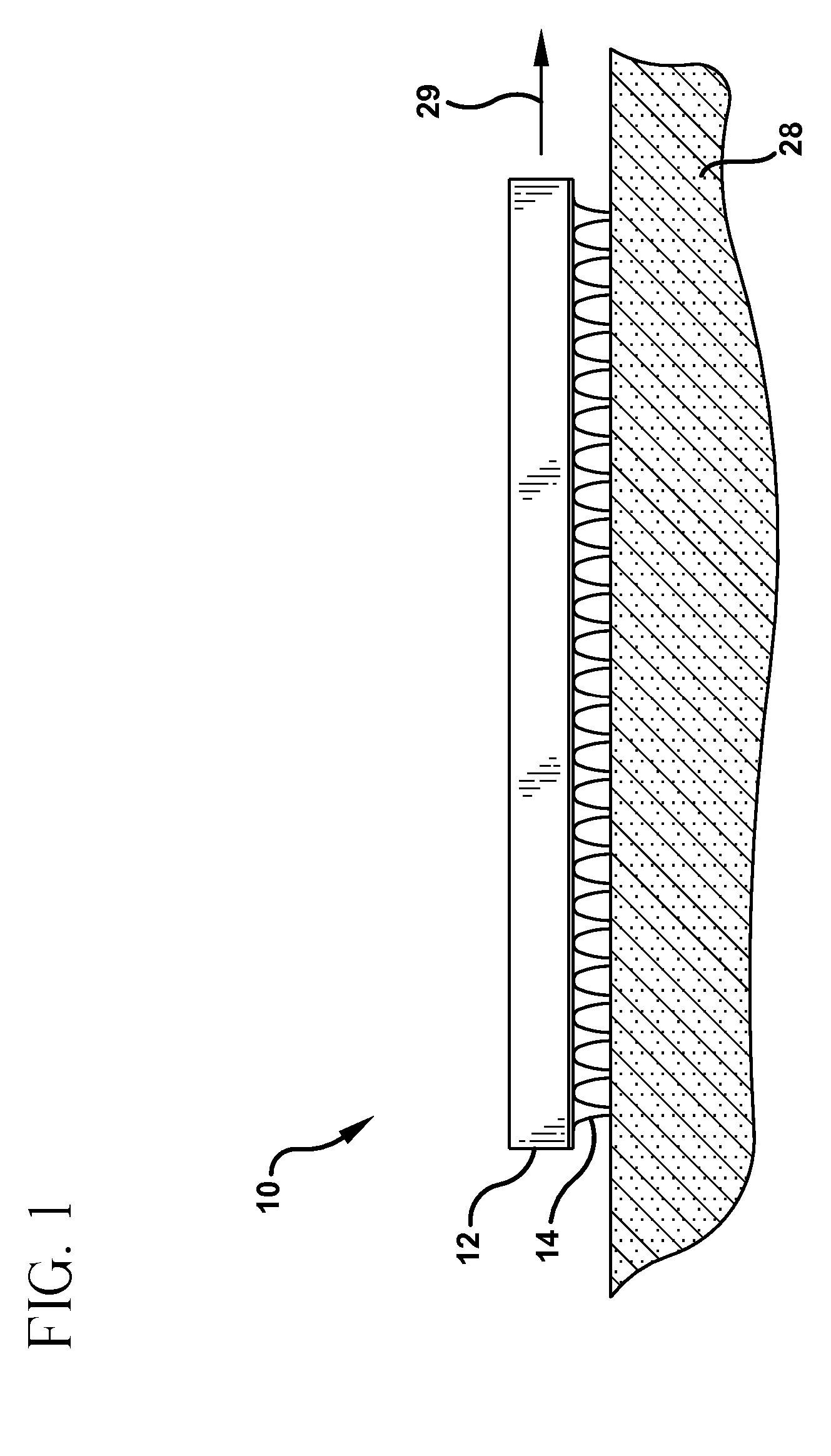
A device (12) and method are provided for percutaneous transdermal delivery of a potent pharmacologically active agent. The agent is dissolved in water to form an aqueous coating solution having an appropriate viscosity for coating extremely tiny skin piercing elements (10). The coating solution is applied to the skin piercing elements (10) using known coating techniques and then dried. The device (12) is applied to the skin of a living animal (e.g., a human), causing the microprotrusions (10) to pierce the stratum corneum and deliver a therapeutically effect dose of the agent to the animal.
Owner:ALZA CORP
Intracutaneous microneedle array apparatus
InactiveUS6931277B1Facilitate biological fluid samplingIncrease transdermal flow rateElectrotherapyMicroneedlesStratum corneumEngineering
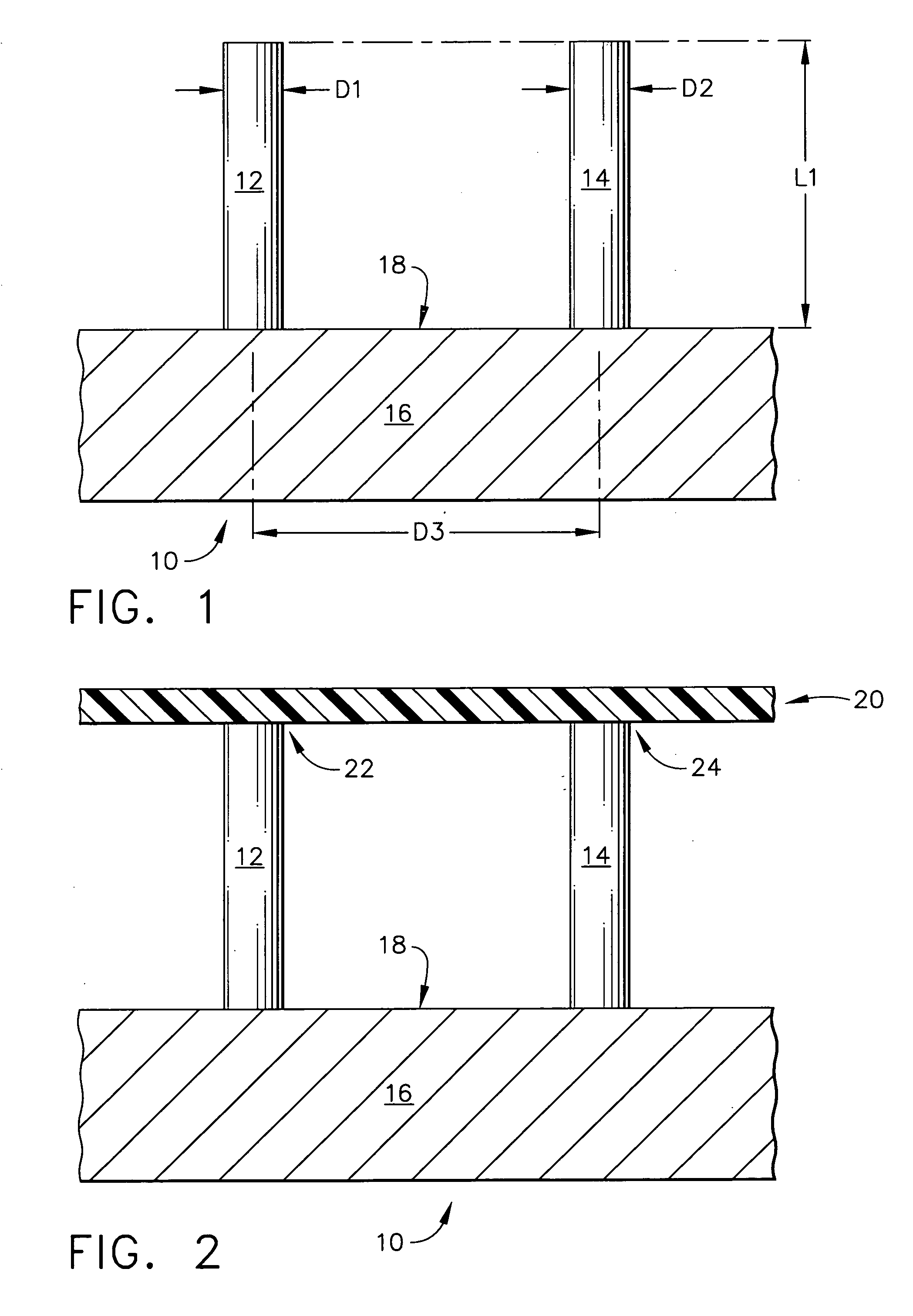
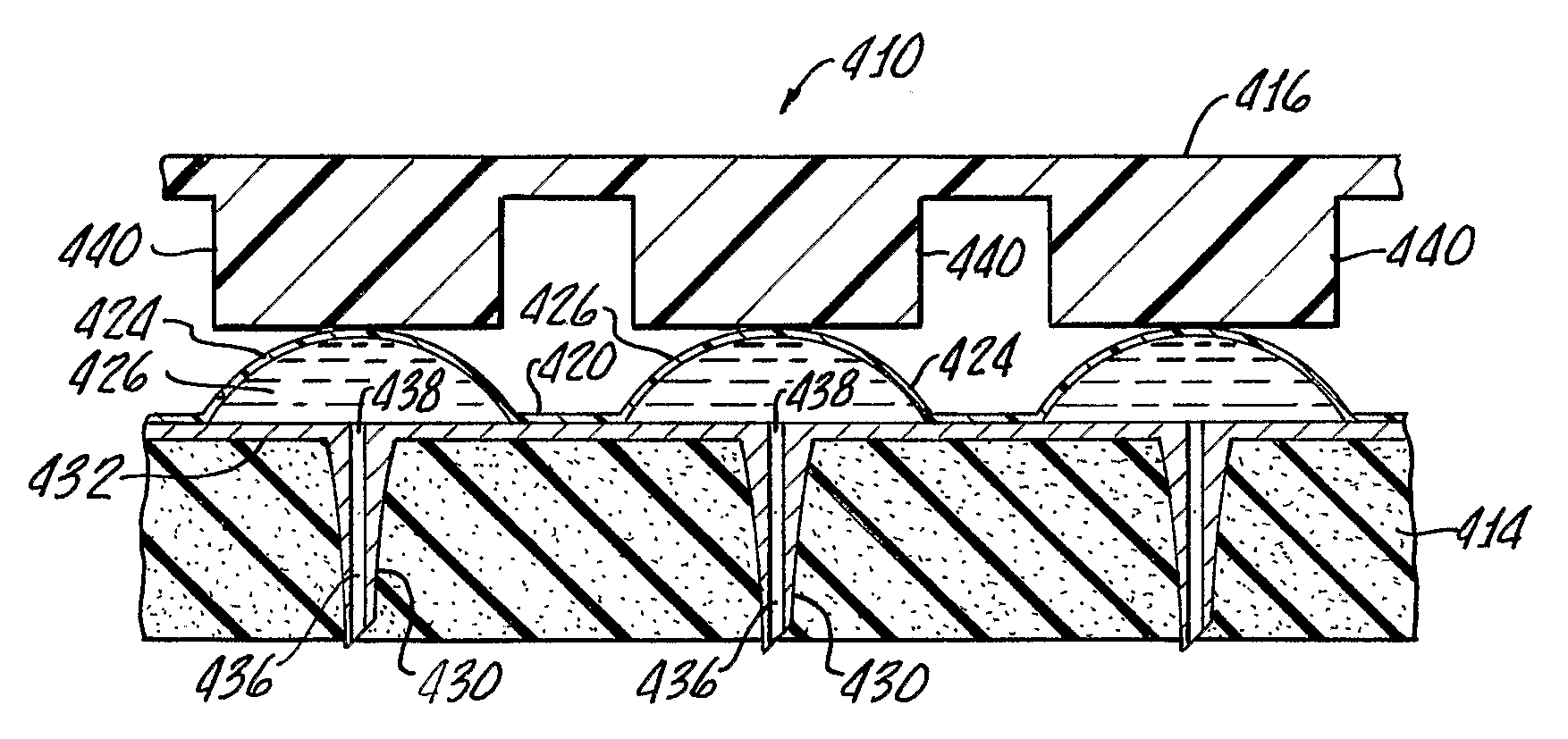
Improved microneedle arrays are provided having a sufficiently large separation distance between each of the individual microneedles to ensure penetration of the skin while having a sufficiently small separation distance to provide high transdermal transport rates. A very useful range of separation distances between microneedles is in the range of 100–300 microns, and more preferably in the range of 100–200 microns. The outer diameter and microneedle length is also very important, and in combination with the separation distance will be crucial as to whether or not the microneedles will actually penetrate the stratum corneum of skin. For circular microneedles, a useful outer diameter range is from 20–100 microns, and more preferably in the range of 20–50 microns. For circular microneedles that do not have sharp edges, a useful length for use with interstitial fluids is in the range of 50–200 microns, and more preferably in the range of 100–150 microns; for use with other biological fluids, a useful length is in the range of 200 microns–3 mm, and more preferably in the range of 200–400 microns. For circular microneedles having sharp side edges, a useful length for use with interstitial fluids is in the range of 50–200 microns, and more preferably in the range of 80–150 microns; for use with other biological fluids, a useful length is again in the range of 200 microns–3 mm, and more preferably in the range of 200–400 microns. For solid microneedles having a star-shaped profile with sharp edges for its star-shaped blades, a useful length for use with interstitial fluids is in the range of 50–200 microns, and more preferably in the range of 80–150 microns; for use with other biological fluids, a useful length is again in the range of 200 microns–3 mm, and more preferably in the range of 200–400 microns, while the radius of each of its blades is in the range of 10–50 microns, and more preferably in the range of 10–15 microns.
Owner:CORIUM INC
Method and apparatus for the transdermal administration of a substance
InactiveUS6960193B2Efficient managementNo painSurgeryMicroneedlesStratum corneumBiomedical engineering
A transdermal delivery device includes a plurality of microneedles for injecting a substance such as a pharmaceutical agent into or below the stratum corneum of the skin. The device has housing formed from a top and bottom wall to define a chamber for containing a pharmaceutical agent. An inlet port is provided in the top wall of the housing for supplying the pharmaceutical agent to the chamber and directing the agent to the microneedles. The housing can have a Luer lock type fitting for coupling with a syringe having a Luer lock collar to inject the pharmaceutical agent into the housing. The housing can be divided into a plurality of chambers by an internal wall for supplying different agents simultaneously or sequentially to a patient. The microneedles have a length of about 5–250 microns and generally about 50–100 microns.
Owner:BECTON DICKINSON & CO
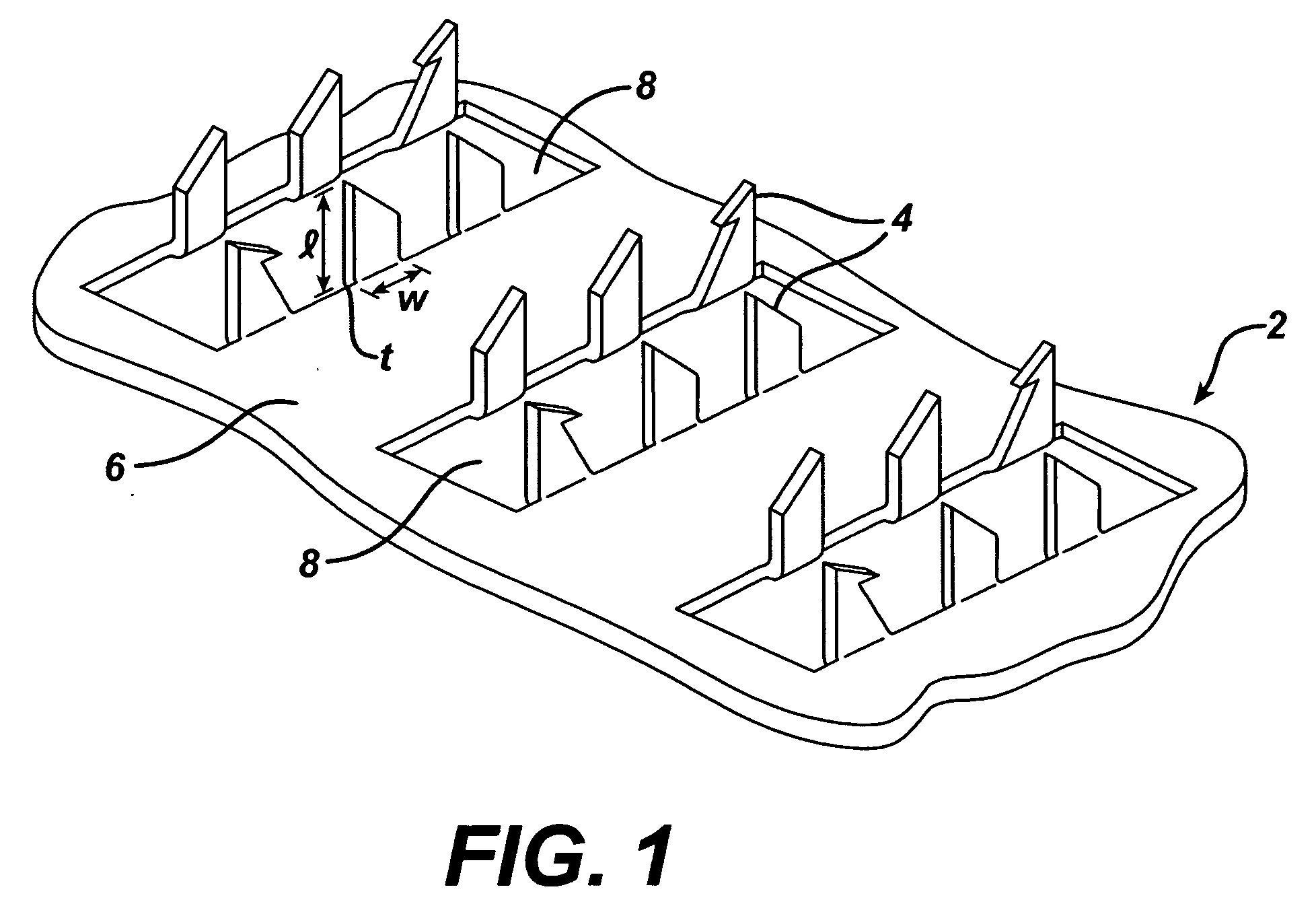
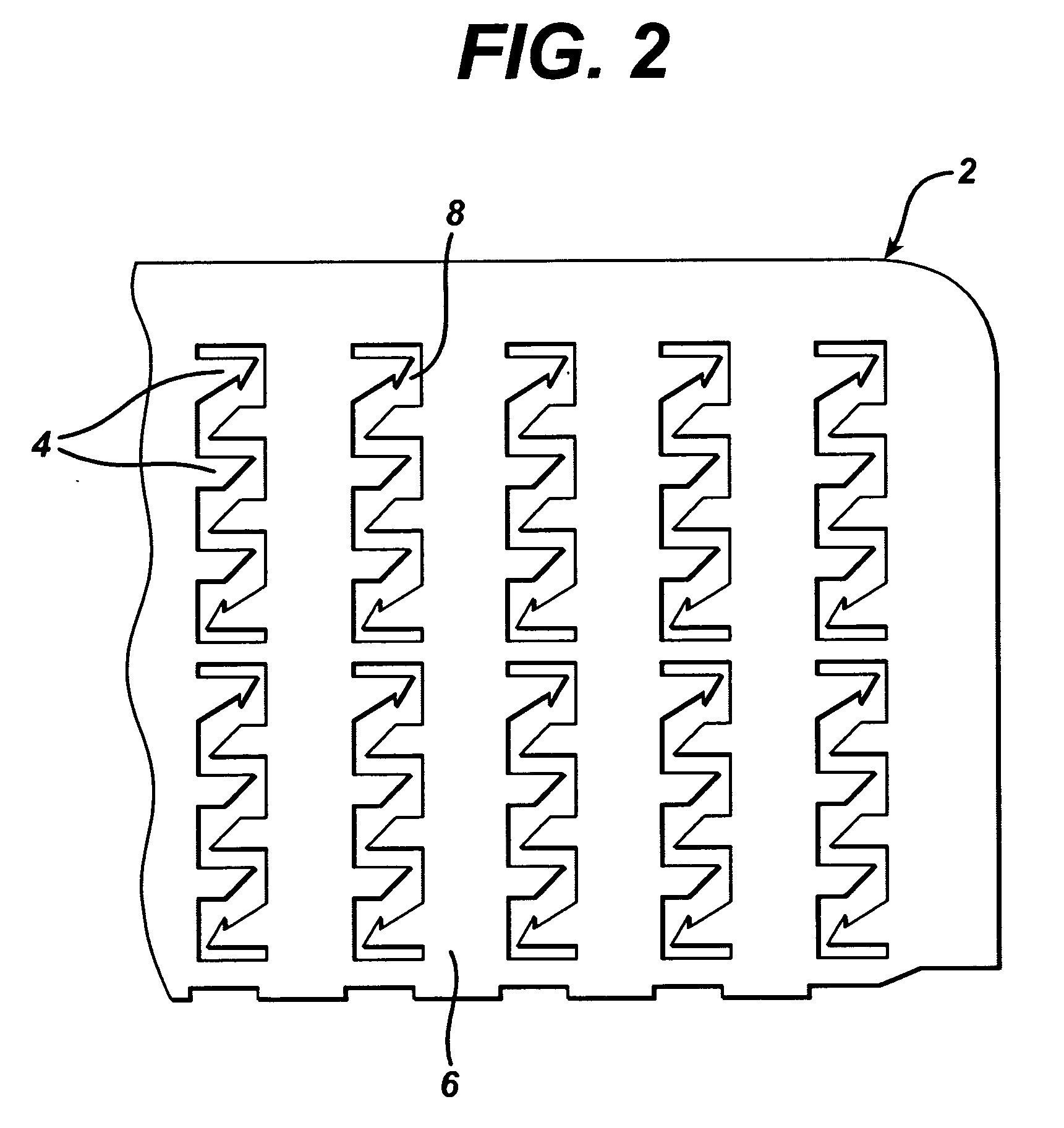
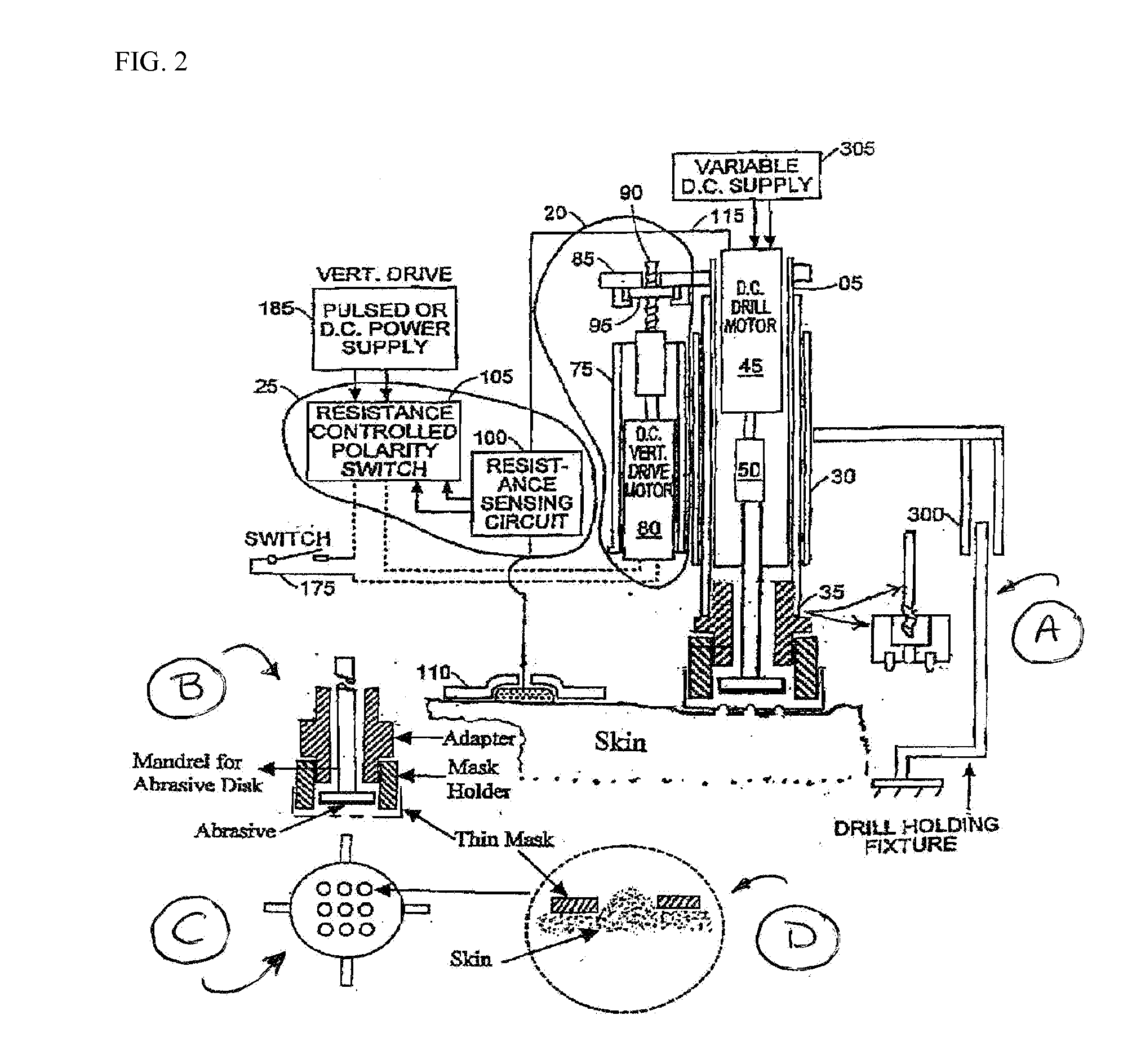
Skin abrader for biomedical electrode
InactiveUS6136008AEfficiently and effectively removesEfficiently and effectively removedElectrocardiographySurgical furnitureStratum corneumBiomedical engineering
A skin abrader for a biomedical electrode is disclosed wherein the abrader has a geometrically structured surface abrasive selected to provide sufficient abrasive effect to easily remove a portion of the stratum corneum of mammalian skin with minimal skin irritation, in order to reduce skin impedance encountered during diagnosis or monitoring of a mammalian patient.
Owner:BANK ONE N A +1
System and method for transdermal delivery
InactiveUS20060036209A1Improve transdermal deliveryReduce deliveryElectrotherapyMicroneedlesElectricityActive agent
A system and method for transdermally delivering a biologically active agent comprising one or more electrodes having stratum corneum-piercing projections and a circuit that delivers an electrical signal to the electrodes to electroporate a cell membrane. Preferably, the system is configured to generate homogeneous electrical fields and, more preferably, to generate spherically or semispherically symmetrical electric fields. Methods of the invention include applying a first electric signal to facilitate transdermal transport of the agent and applying a second electric signal to facilitate intracellular transport of the agent.
Owner:ALZA CORP
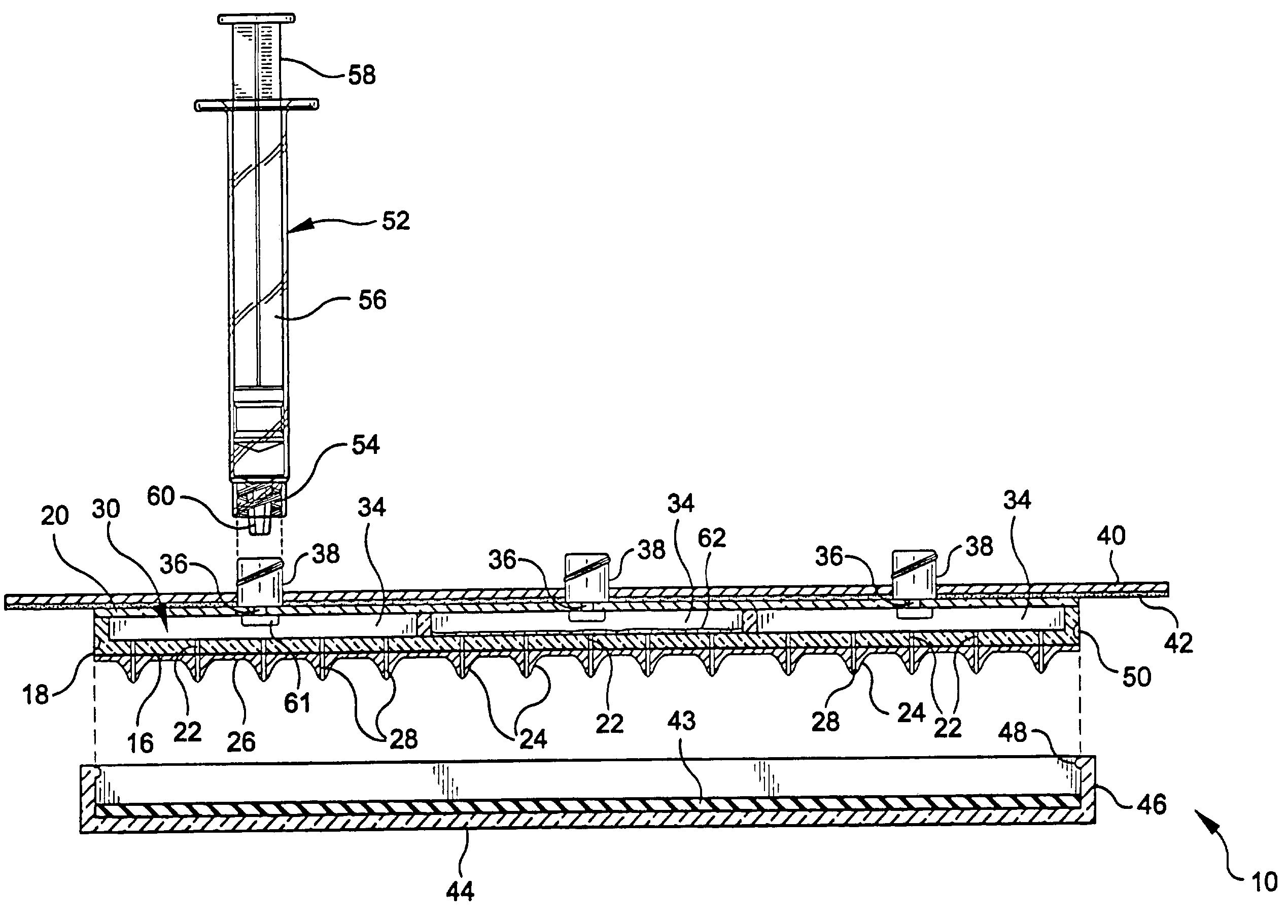
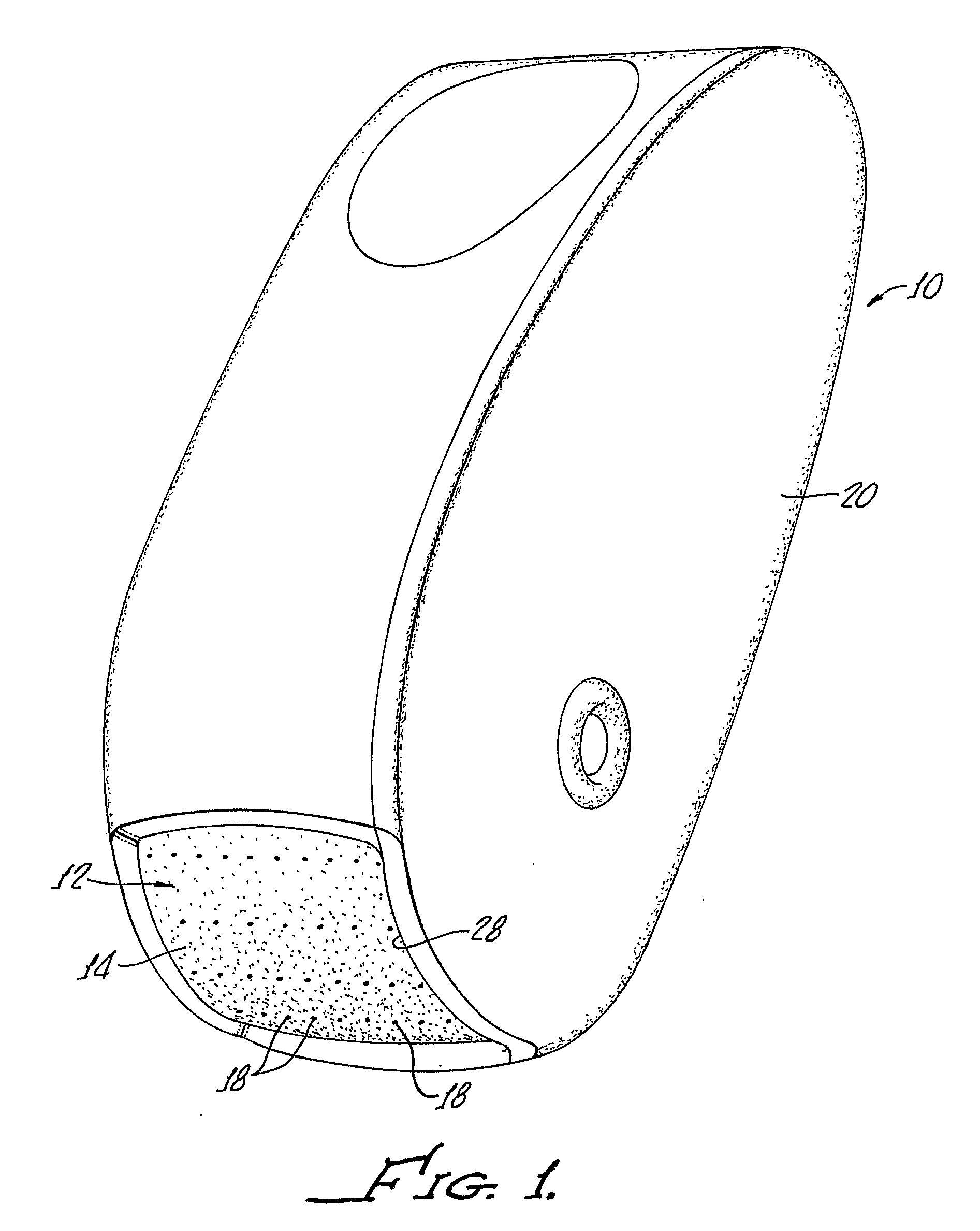
Apparatus, system, and method to deliver optimal elements in order to enhance the aesthetic appearance of the skin
ActiveUS20070073217A1Eliminate and greatly reduce disadvantageEliminate and greatly reduce and problemMicroneedlesSurgeryFiberGrowth Agents
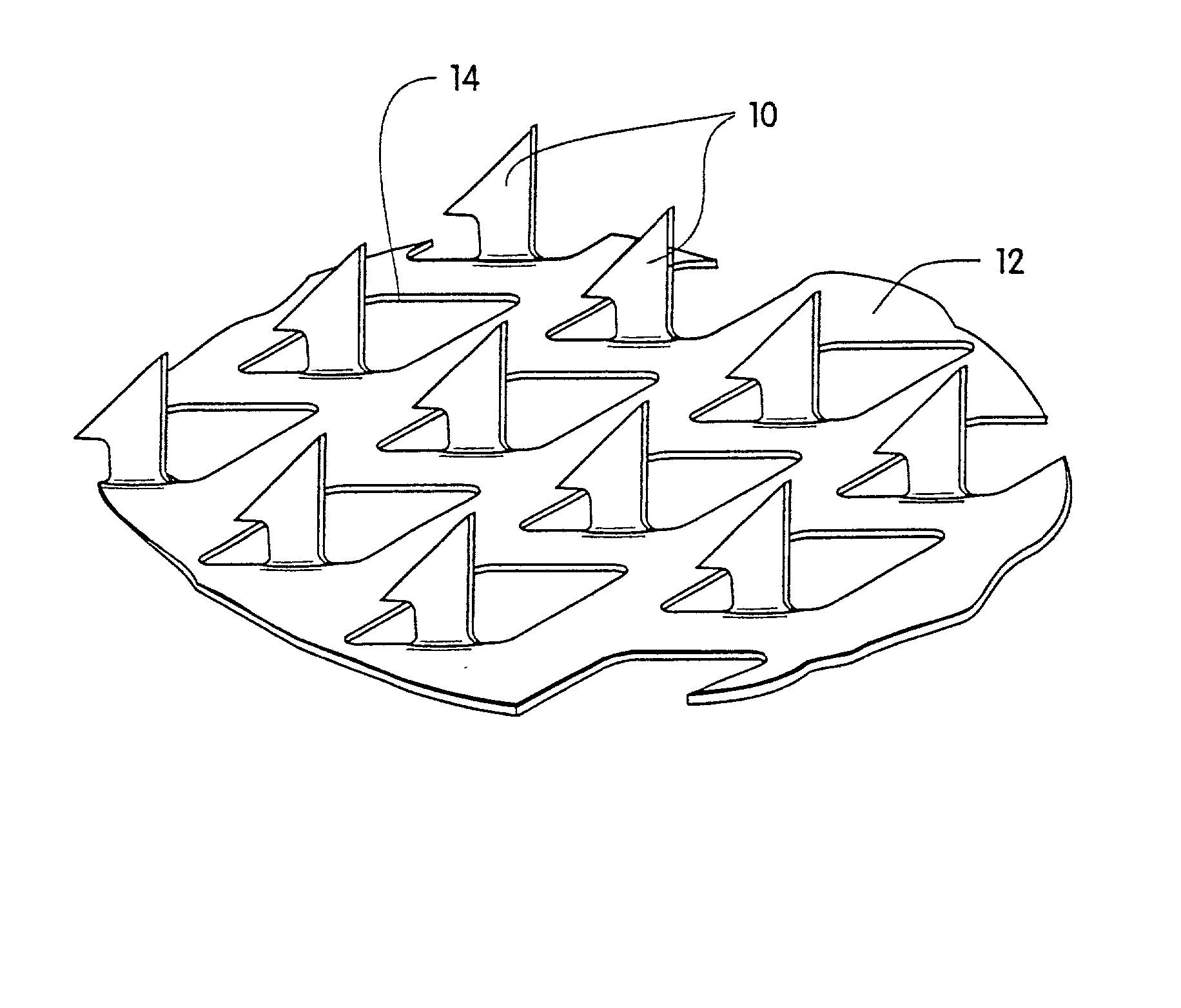
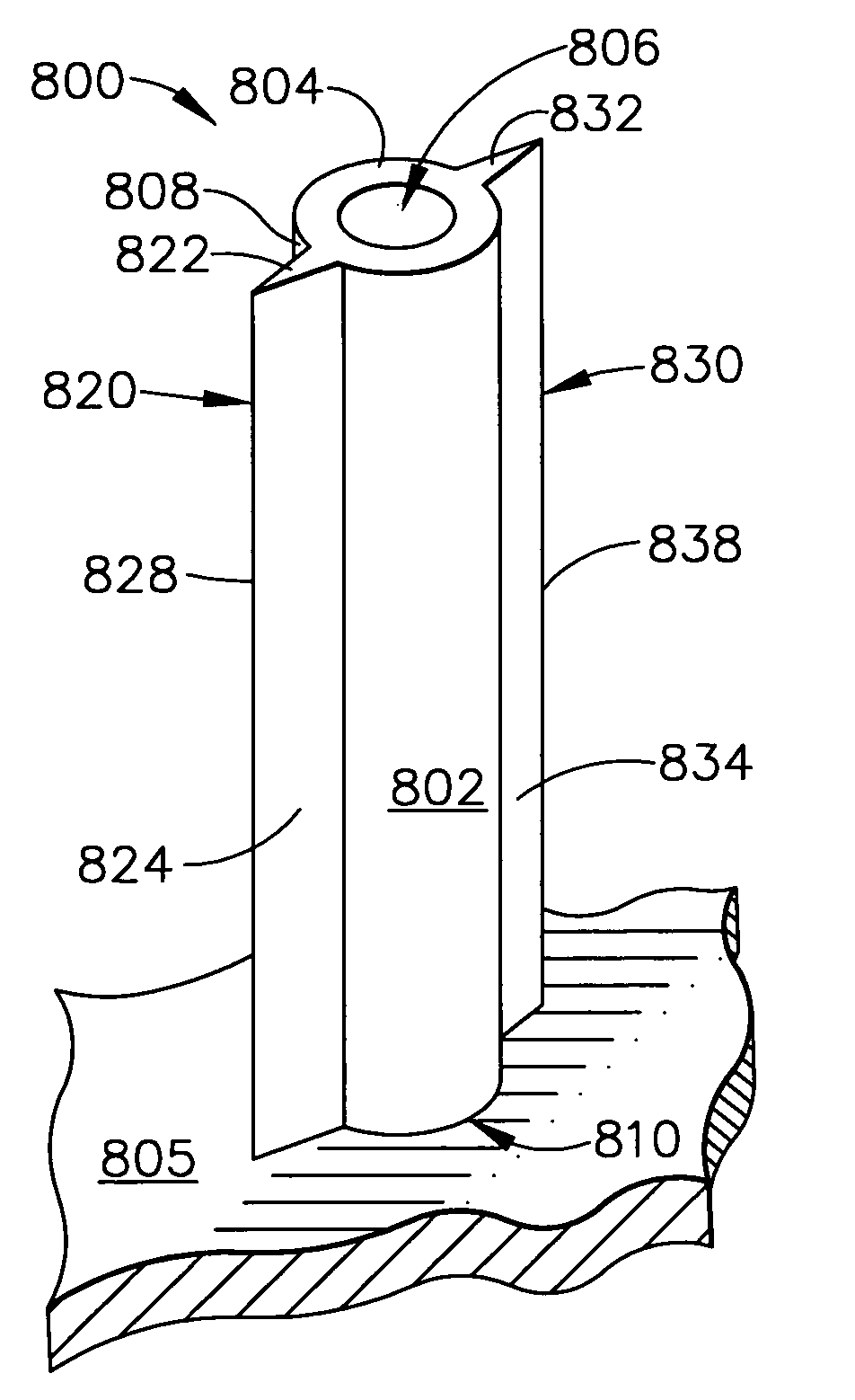
An apparatus for delivering a bioactive material to a subterranean layer of a skin architecture is provided that includes a head including one or more needles that are operable to penetrate a stratum corneum of a skin. A bioactive material is disposed on one or more of the needles, whereby movement of the head operates to pick up the bioactive material and to deliver a portion of the bioactive material to a selected location, the selected location being a dermis, or an epidermis, or both the dermis and the epidermis. In more particular embodiments, the bioactive material is a macromolecule substance that is part of a group of substances, the group consisting of a protein, a vitamin, a gene, a growth agent, a drug, and a peptide. The needles create an injury that triggers collagen production from one or more fibroblasts in the skin.
Owner:BEAUTY BIOSCI
Method of treating acne with stratum corneum piercing device
The invention features a method of treating acne by piercing the stratum corneum of skin in need of such treatment with a stratum corneum-piercing device that contains at east one stratum corneum-piercing microprotrusion and a compressible cover such that the compressible cover substantially encases the at least one stratum corneum-piercing microprotrusion, and wherein upon contacting the skin with the compressible cover, the at least one stratum corneum-piercing microprotrusion protrudes from the compressible cover and pierces the stratum corneum of the skin.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Transdermal delivery of therapeutic agent
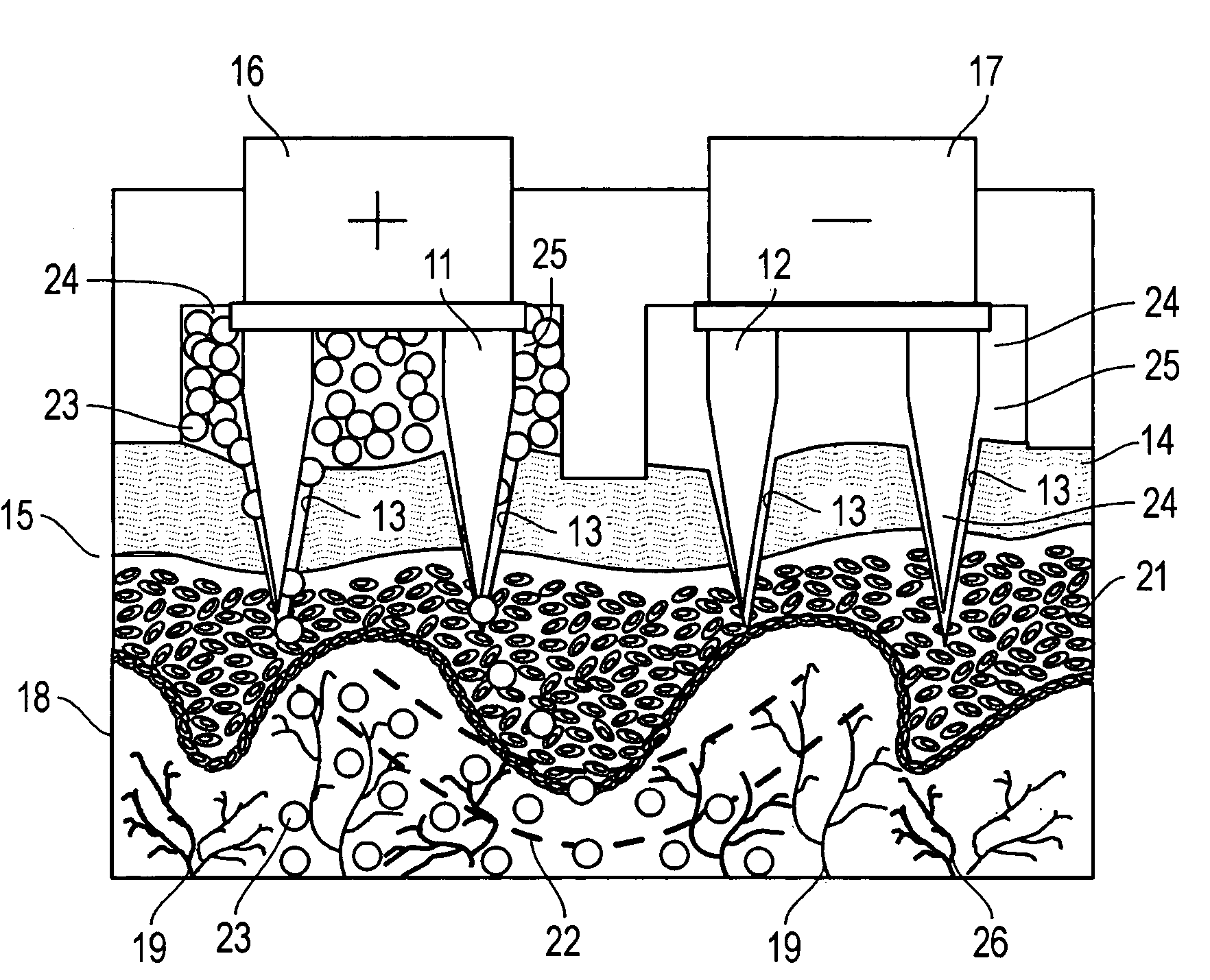
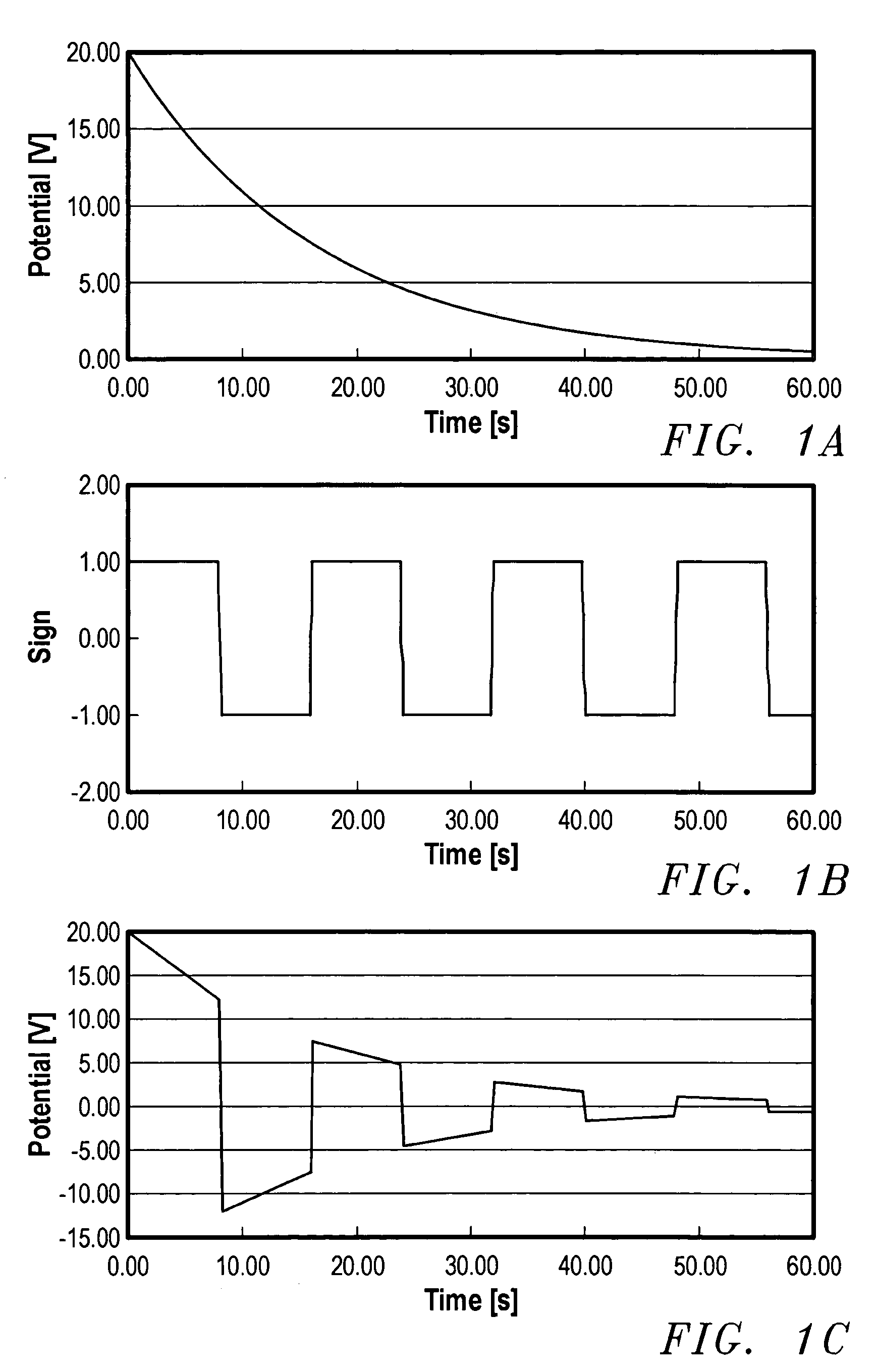
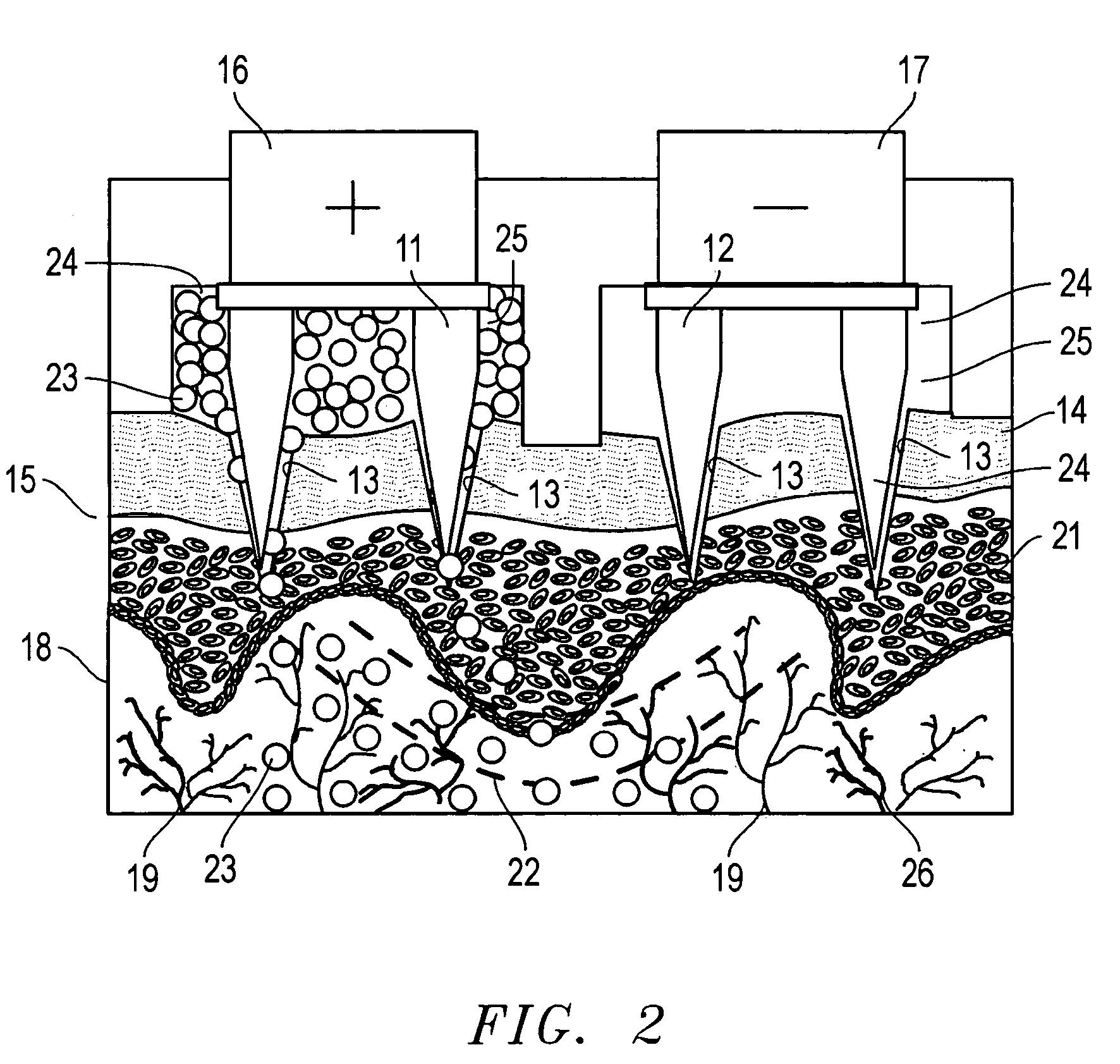
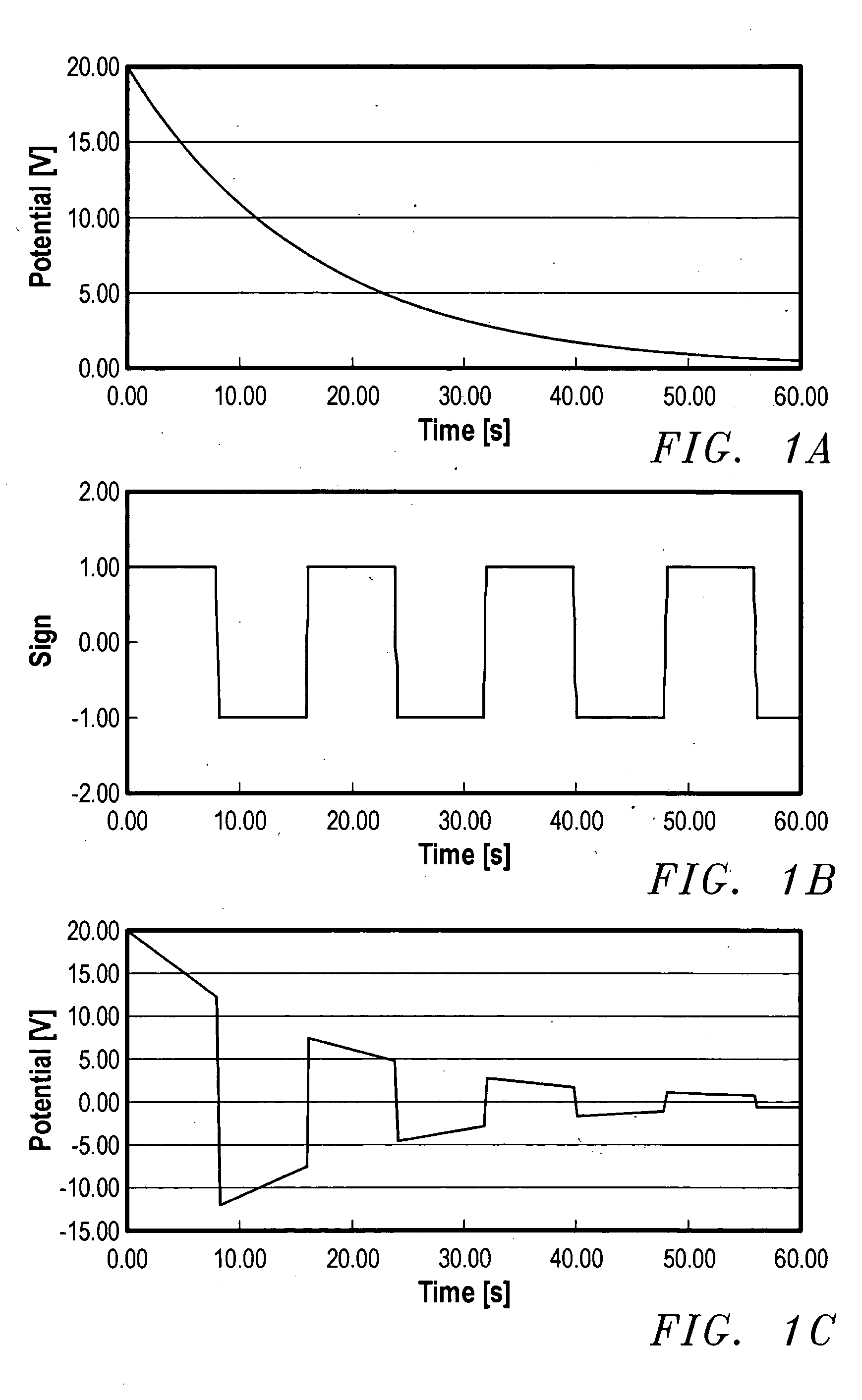
A device for the transdermal delivery of a therapeutic agent to a biological subject that includes a first electrode comprising a first array of electrically conductive microprojections for providing electrical communication through a skin portion of the subject to a second electrode comprising a second array of electrically conductive microprojections. Additionally, a reservoir for holding the therapeutic agent surrounding the first electrode and a pulse generator for providing an exponential decay pulse between the first and second electrodes may be provided. A method includes the steps of piercing a stratum corneum layer of skin with two arrays of conductive microprojections, encapsulating the therapeutic agent into biocompatible charged carriers, surrounding the conductive microprojections with the therapeutic agent, generating an exponential decay pulse between the two arrays of conductive microprojections to create a non-uniform electrical field and electrokinetically driving the therapeutic agent through the stratum corneum layer of skin.
Owner:LYNNTECH
Composition and apparatus for transdermal delivery
InactiveUS20050106209A1High viscosityImprove stabilityBiocidePeptide/protein ingredientsActive agentCounterion
A formulation for coating a transdermal delivery device having a plurality of stratum corneum-piercing microprojections, the formulation including a biologically active agent and at least one viscosity-enhancing counterion. Preferably, the formulation has a viscosity in the range of about 20-200 cp.
Owner:ALZA CORP
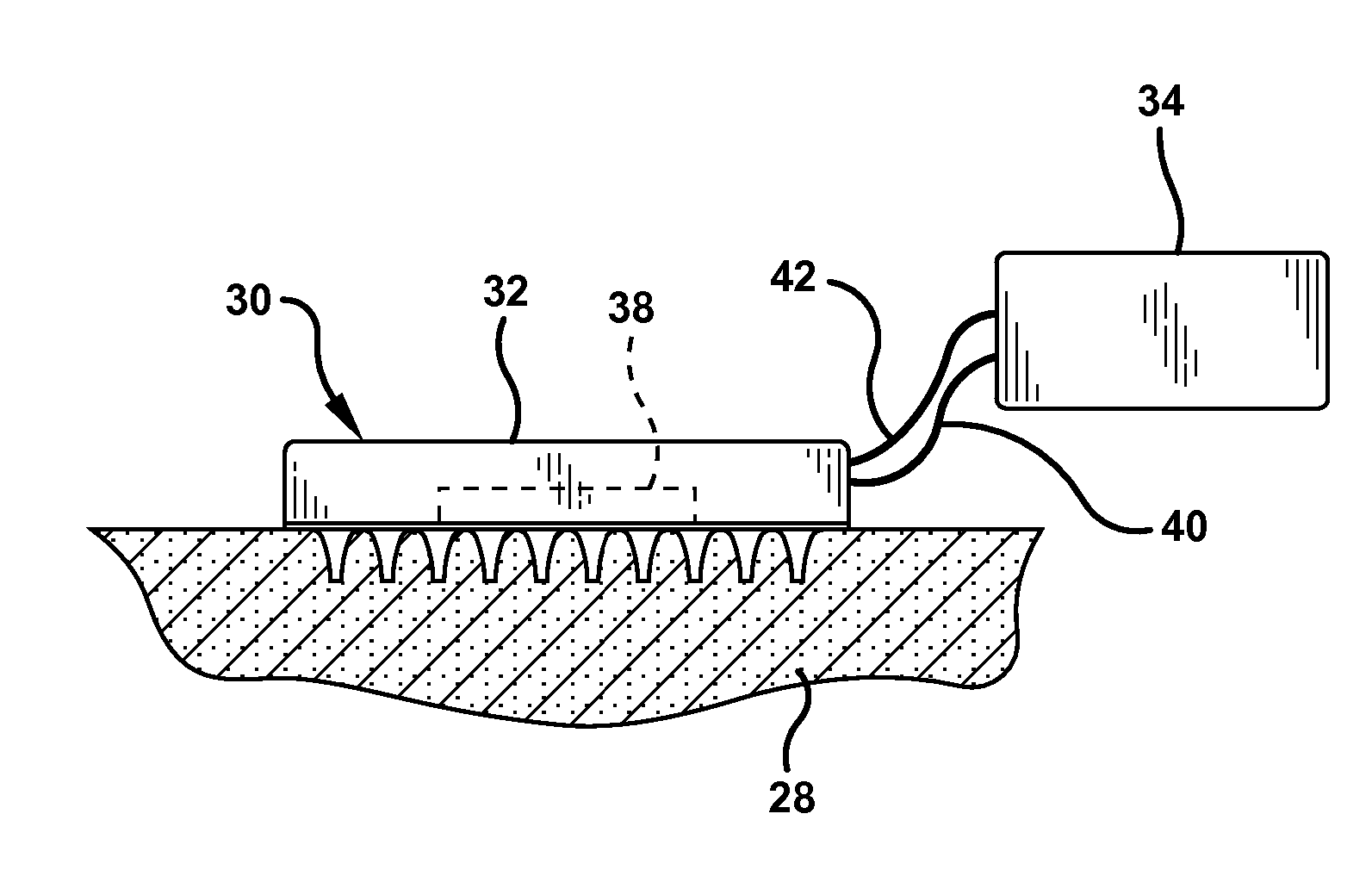
Apparatus and method for piercing skin with microprotrusions
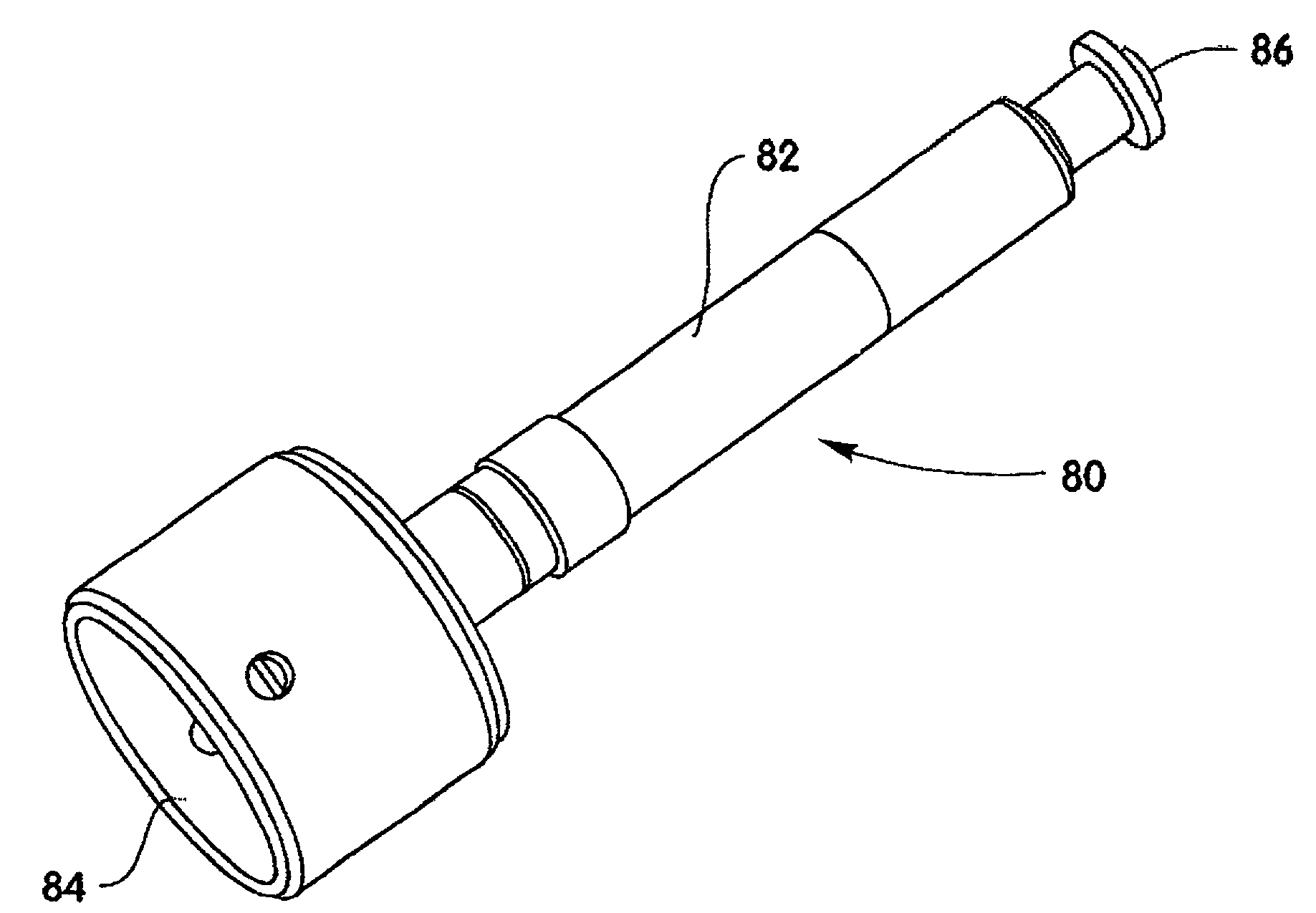
A method and device are described for applying a microprotrusion member (44) including a plurality of microprotrusions (90) to the stratum corneum with impact. The method and device are used to improve transport of an agent across the skin for agent delivery or sampling. The applicator (10, 60, 80) causes the microprotrusion member (44) to impact the stratum corneum with a certain amount of impact determined to effectively pierce the skin with the microprotrusions (90). The preferred applicator (10, 60, 80) impacts the stratum corneum with the microprotrusion member (44) with an impact of at least 0.05 joules per cm2 of the microprotrusion member (44) in 10 msec or less.
Owner:ALZA CORP
Device, systems, methods and tools for continuous glucose monitoring
The present invention includes methods and apparatus for continuous glucose monitoring of a patient. In one aspect a glucose monitor includes a plurality of substantially cylindrical tissue piercing elements adapted to pierce the stratum corneum and enter the epidermis, allowing for the diffusion of glucose from the interstitial fluid into the glucose monitors described herein. In another aspect of the invention, a glucose monitor includes a deformed substrate layer defining a plurality of tissue piercing elements.
Owner:ARKAL MEDICAL
Drug-delivery patch comprising a dissolvable layer and uses thereof
InactiveUS20090010998A1Improve stabilitySsRNA viruses negative-senseBacterial antigen ingredientsStratum corneumBiomedical engineering
The present invention provides a drug-delivery patch having at least one dissolvable layer comprising an active material and an adhesive backing or cover. The present invention also provides a method of transdermally vaccinating an animal by ablating an area of the stratum corneum of the animal and applying the patch described herein to the area.
Owner:ROCKY MOUNTAIN BIOSYST
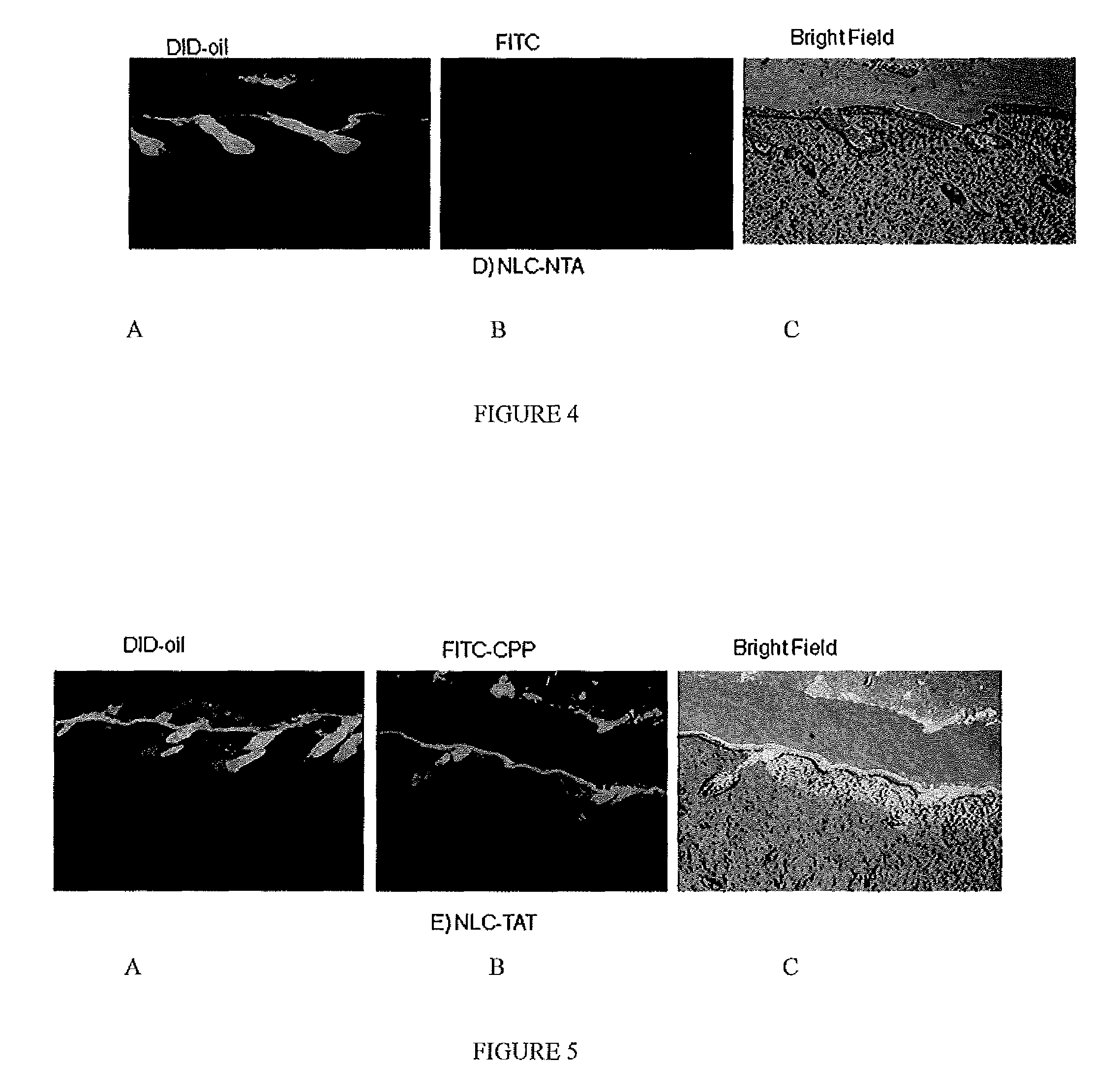
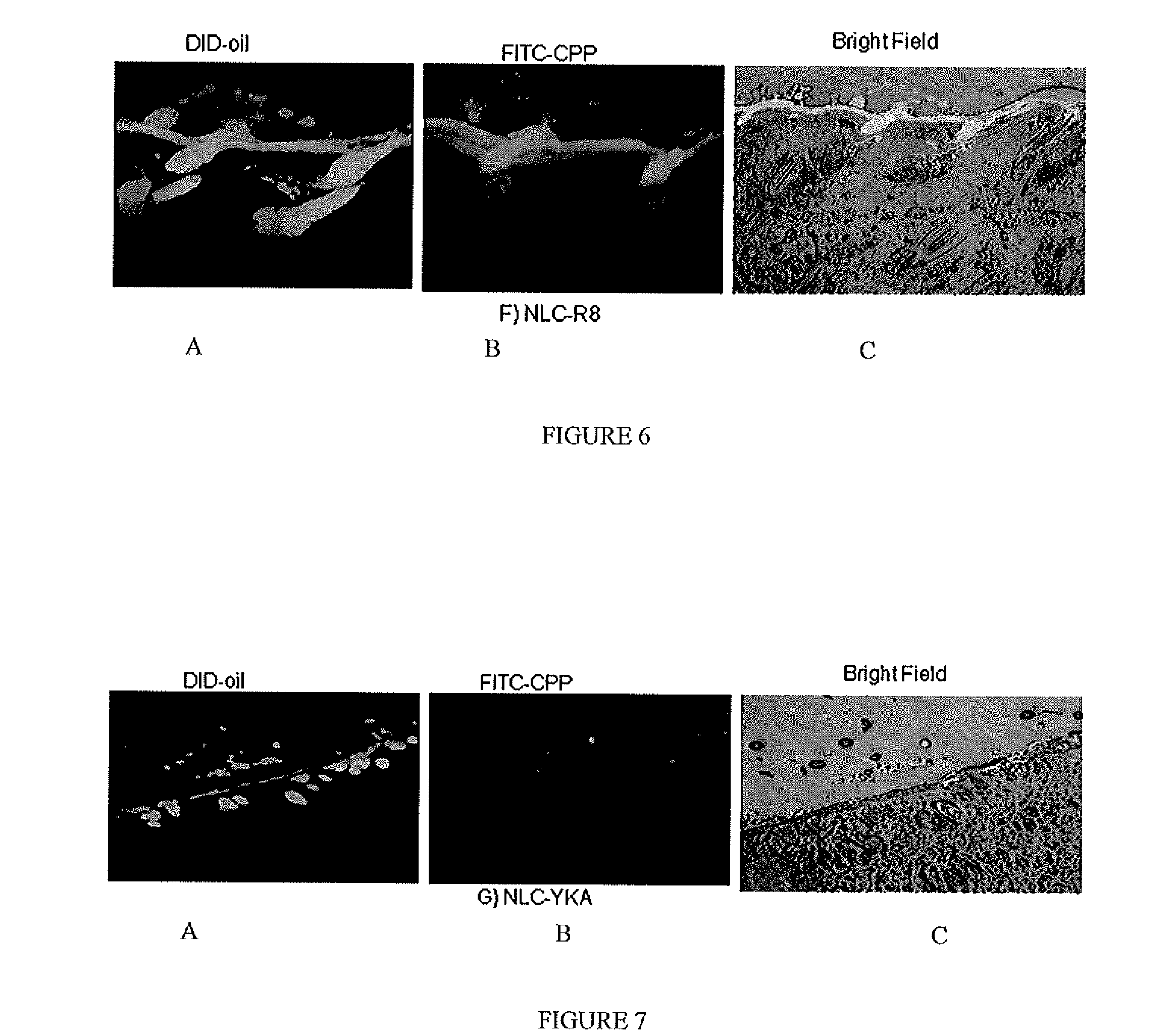
Nanoparticle formulations for skin delivery
Methods and formulations for treating a condition of the skin by delivering therapeutic formulations to the skin that translocates active substances across the stratum corneum barrier to a targeted skin tissue. The methods and formulations comprise active substances encapsulated within surface modified nanostructured lipid carrier nanoparticles.
Owner:FLORIDA A&M UNIVERSITY
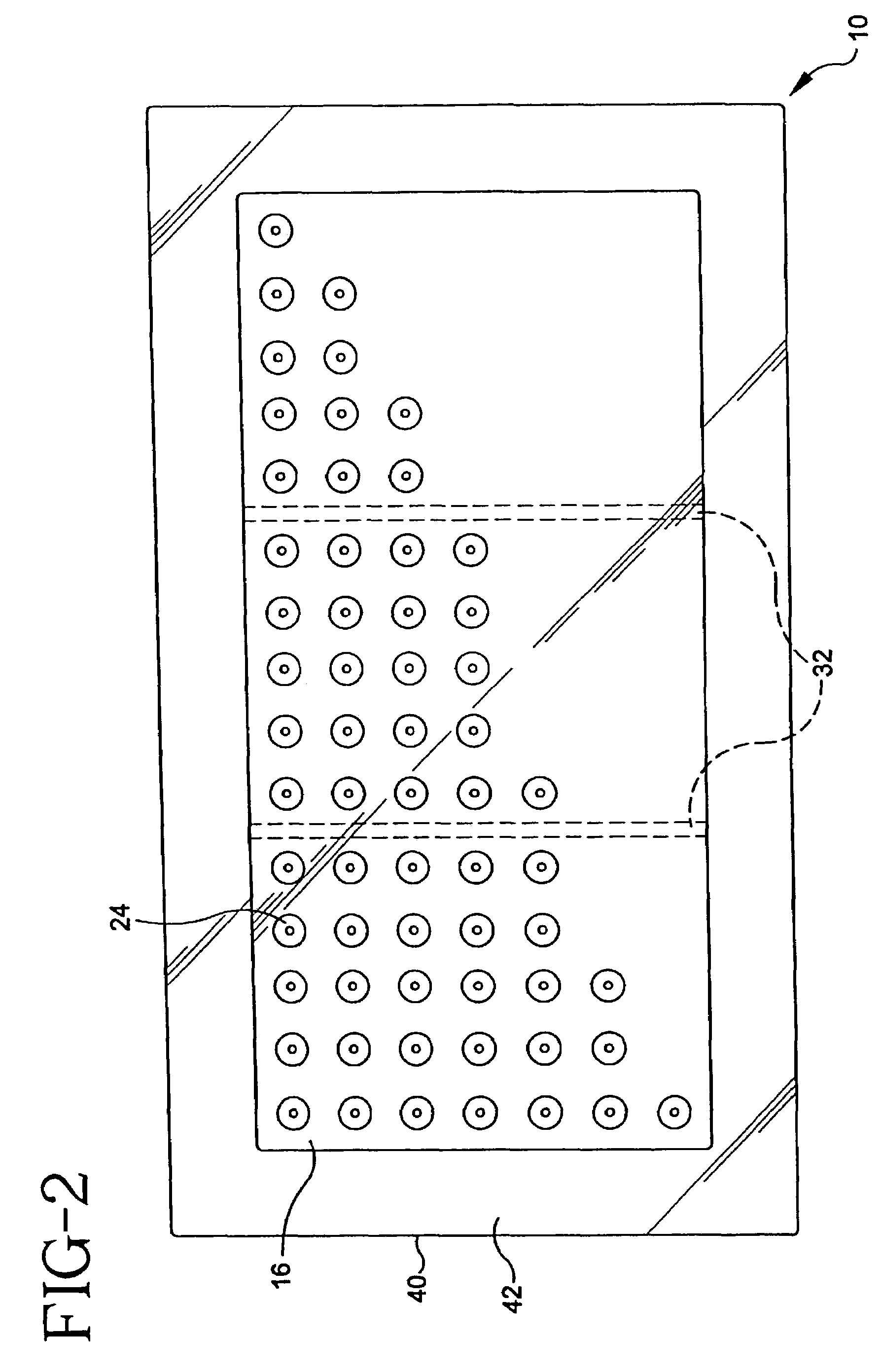
Multi-site injection system
A multi-site injection system (10) in accordance with the present invention generally includes a plurality of medicament delivering needles / microprotrusions (18) along with a support therefore. A supply of medicament (36) is provided and a means provided for supplying the medicament to the plurality of needles / microprotrusions in order to effect delivery into a stratum corneum (42) of a user.
Owner:ALLERGAN INC
Transdermal delivery of therapeutic agent
A device for the transdermal delivery of a therapeutic agent to a biological subject that includes a first electrode comprising a first array of electrically conductive microprojections for providing electrical communication through a skin portion of the subject to a second electrode comprising a second array of electrically conductive microprojections. Additionally, a reservoir for holding the therapeutic agent surrounding the first electrode and a pulse generator for providing an exponential decay pulse between the first and second electrodes may be provided. A method includes the steps of piercing a stratum corneum layer of skin with two arrays of conductive microprojections, encapsulating the therapeutic agent into biocompatible charged carriers, surrounding the conductive microprojections with the therapeutic agent, generating an exponential decay pulse between the two arrays of conductive microprojections to create a non-uniform electrical field and electrokinetically driving the therapeutic agent through the stratum corneum layer of skin.
Owner:LYNNTECH
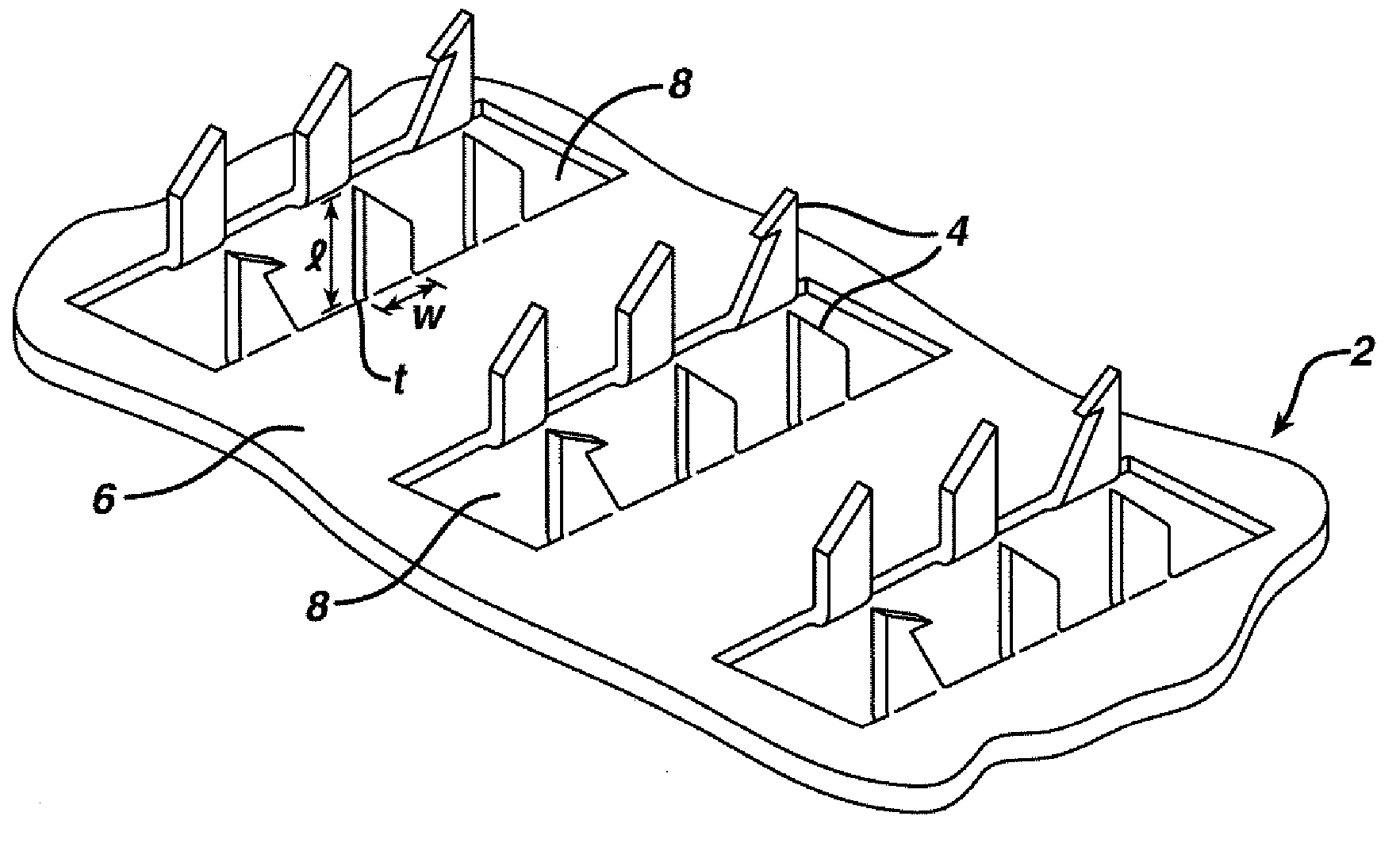
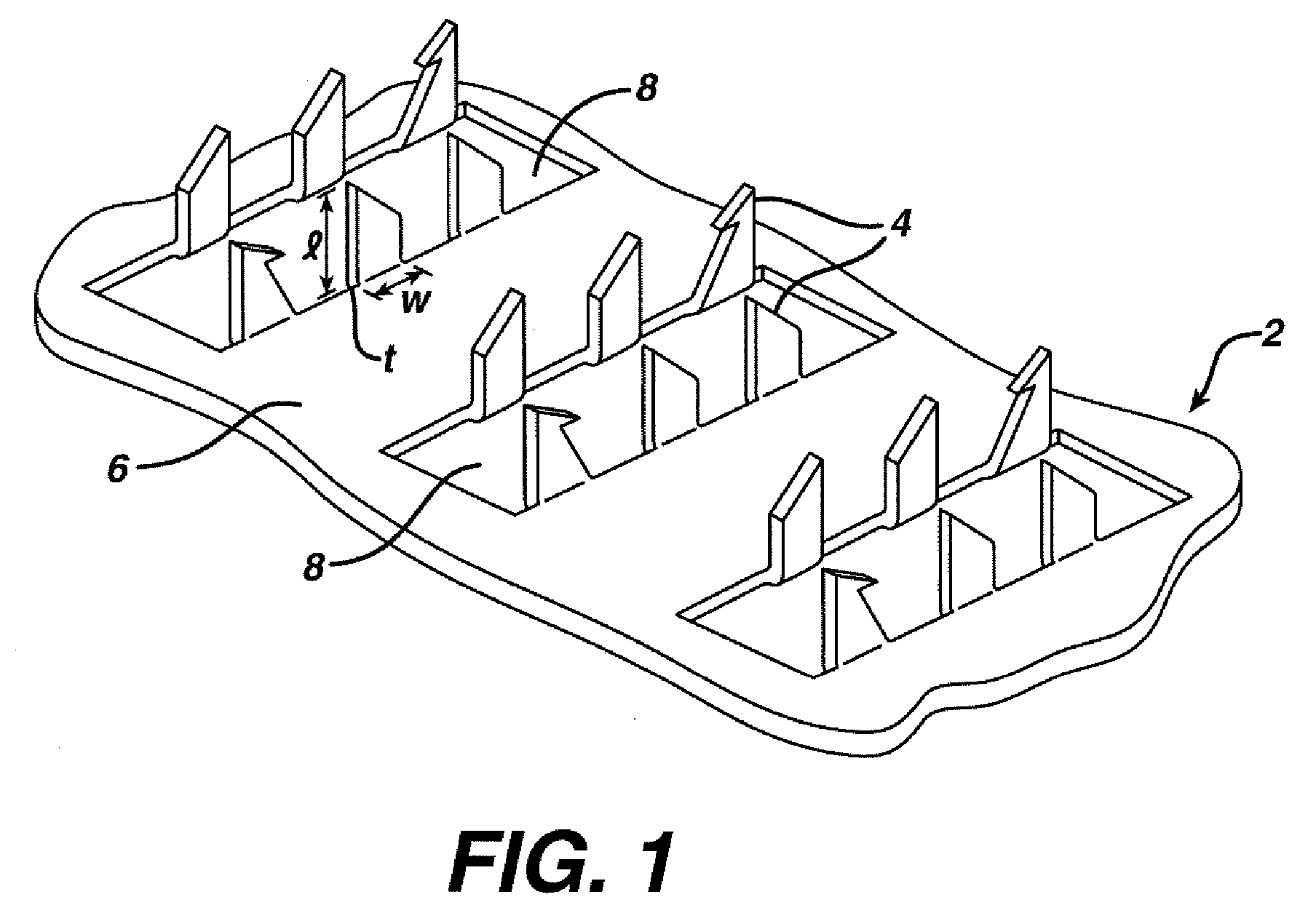
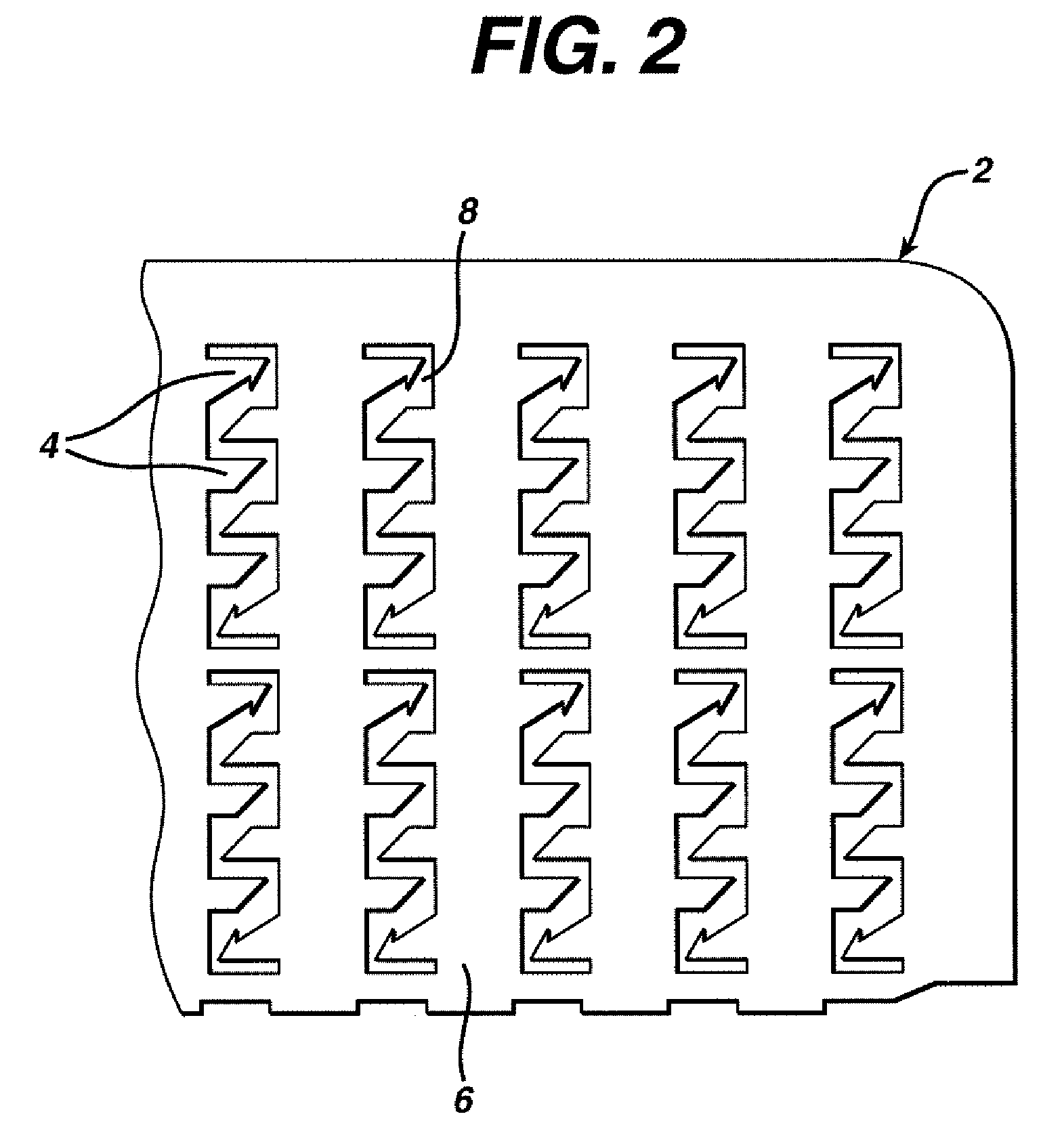
Method and device for abrading skin
InactiveUS20060264893A1Effective penetrationMinimal irritationElectrotherapyMicroneedlesIrritationStratum corneum
A device includes a plurality of microneedles for abrading the stratum corneum of the skin to form a plurality of grooves in the tissue having a controlled depth and width. The microneedles have a length of about 5-250 microns and generally about 5-200 microns. The device is rubbed over the skin to prepare an abraded site after which a transdermal delivery or sampling device is applied to the abraded delivery site. The abrasion increases the permeability of the skin and the rate of delivery and extraction of a substance without pain or irritation to the patient.
Owner:BECTON DICKINSON & CO
Method of treating ACNE with stratum corneum piercing patch
The invention features a method of treating acne by piercing the stratum corneum of skin in need of such treatment with a stratum corneum-piercing device that contains at least one stratum corneum-piercing microprotrusion and a compressible cover such that the compressible cover substantially encases the at least one stratum corneum-piercing microprotrusion, and wherein upon contacting the skin with the compressible cover, the at least one stratum corneum-piercing microprotrusion protrudes from the compressible cover and pierces the stratum corneum of the skin.
Owner:WU JEFFREY M +5
Controllled delivery system of antifungal and keratolytic agents for local treatment of fungal infections
A topical sustained release delivery system for delivery of antifungal agents to the finger or toenails achieving high penetration through the nails by combining the antifingal agent with a keratolytic agent and a humectant. The pharmaceutical sustained release topical preparation is provided in a varnish or spray form for treating the nail and surrounding tissues, where the active ingredient is an antifungal agent, a keratolytic agent, or preferably a combination of an antifuingal and a keratolytic agent. The composition may further comprise an antibacterial, an antiviral, an antipsoriatic agents, or combinations thereof.
Owner:TARO PHARMA INDS
Multiple-layered liposome and preparation method thereof
InactiveUS20070082042A1Good skin permeabilityImprove stabilityDermatological disorderLiposomal deliverySterolIntercellular space
Disclosed are multilayered liposomes for transdermal absorption and a method of preparing the liposomes. The multilayered liposomes are prepared using a mixture of oil-phase components comprising squalane, sterols, ceramides, neutral lipids or oils, fatty acids and lecithins, is 200 to 5000 nm in particle size, and is capable of entrapping a physiologically active substance. The multilayered liposomes entrap a larger amount of a physiologically active substance and are structurally stable when encapsulating the physiologically active substance, compared to unilamellar liposomes. Also, they are prepared by a simple and cost-effective process not using a high-pressure homogenizer but using a general homo mixer. Further, since the multilayered liposomes are prepared in a larger size than the intercellular spaces in the stratum corneum, they overcome the tension of surrounding cells when passing through the intercellular spaces and are thus able to penetrate into the dermal layer, compared to nano-sized unilamellar liposomes. Thus, the multilayered liposomes are useful for enhancing the transdermal absorption of physiologically active substances.
Owner:BIOSPECTRUM
Frequency assisted transdermal agent delivery method and system
InactiveUS20050153873A1Adequate buffering capacityHigh viscosityOrganic active ingredientsElectrotherapyActive agentBiocompatible coating
Owner:ALZA CORP
Transdermal delivery of beneficial substances effected by a hostile biophysical environment
InactiveUS20090221536A1Good for healthImprove physical functionBiocideNervous disorderSexual functionArginine
The present invention generally relates to the transdermal delivery of substances and, in some embodiments, to the transdermal delivery of beneficial substances by a hostile biophysical environment. In one aspect, various methods for the transdermal delivery of beneficial substances are disclosed. By creating a hostile biophysical environment, beneficial substances may be delivered, according to certain embodiments, through the stratum corneum of the skin into the body. Beneficial substances include, but are not limited to, pharmaceutical agents, drugs, vitamins, co-factors, peptides, dietary supplements, and others. The beneficial effects disclosed include, for instance, relief of pain and inflammation, prevention and healing of ulcers of the skin, relief of headache, improved sexual function and enjoyment, growth of hair on the scalp, improving muscle size and / or function, removing body fat and / or cellulite, treating cancer, treating viral infections and others. A hostile biophysical environment may also be used in conjunction with systems and methods for increasing local blood flow, according to one set of embodiments. For example, by using a nitric oxide donor such as L-arginine, local blood flow may be increased, e.g., by transdermally delivering the nitric oxide precursor. The nitric oxide donor may be the sole cause of increased blood flow, or it may be supplemented with an adjunct such as theophylline.
Owner:STRATEGIC SCI & TECH
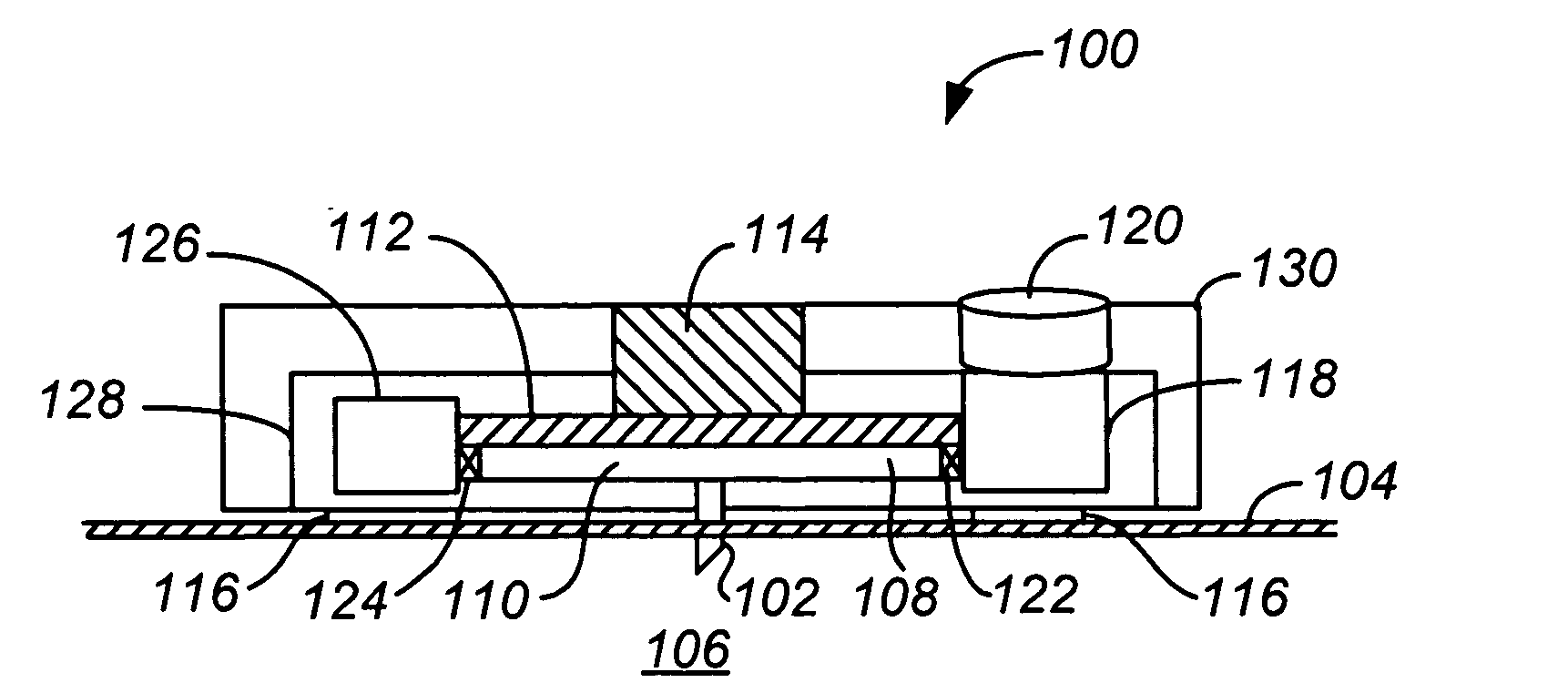
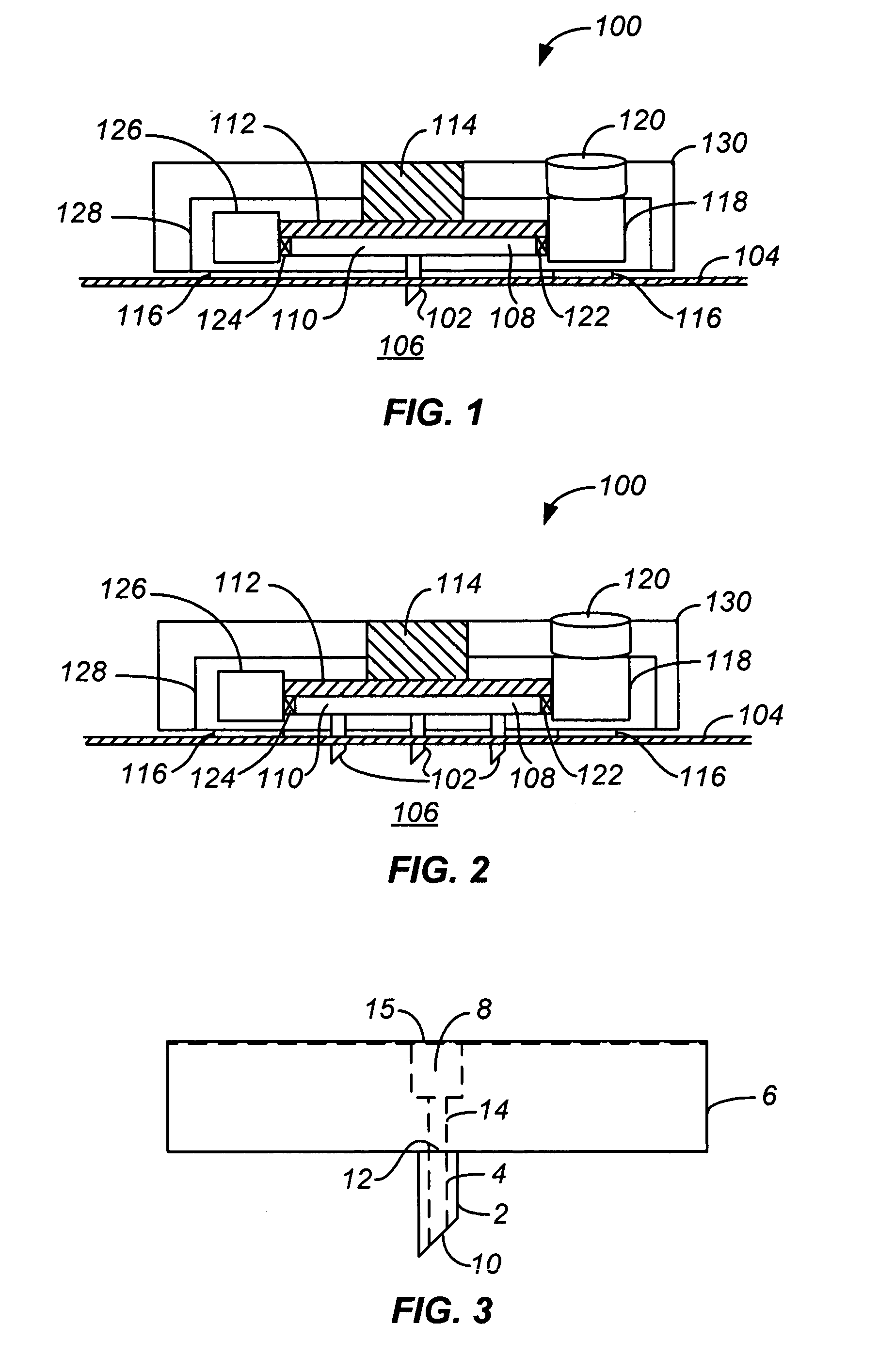
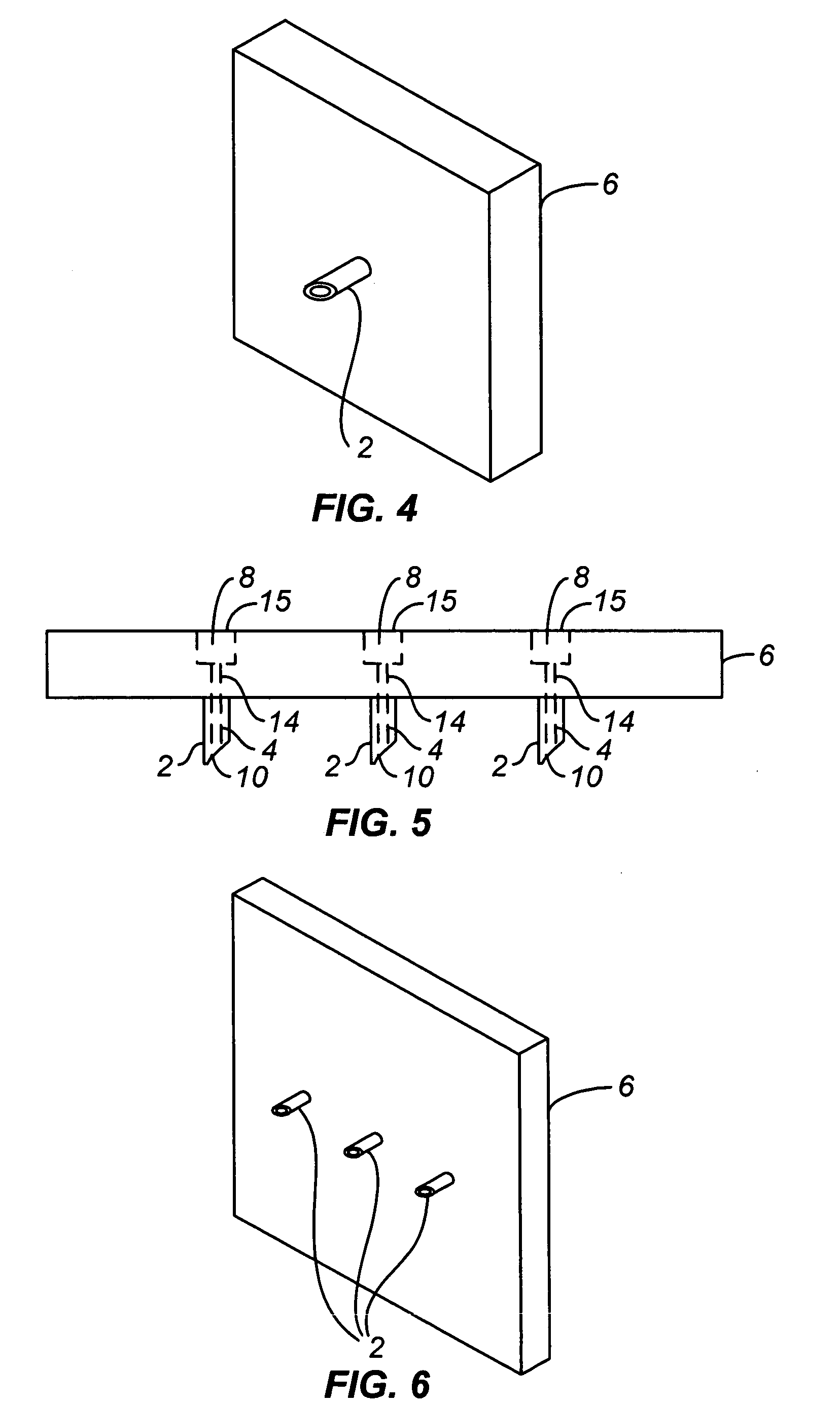
Handheld transdermal drug delivery and analyte extraction
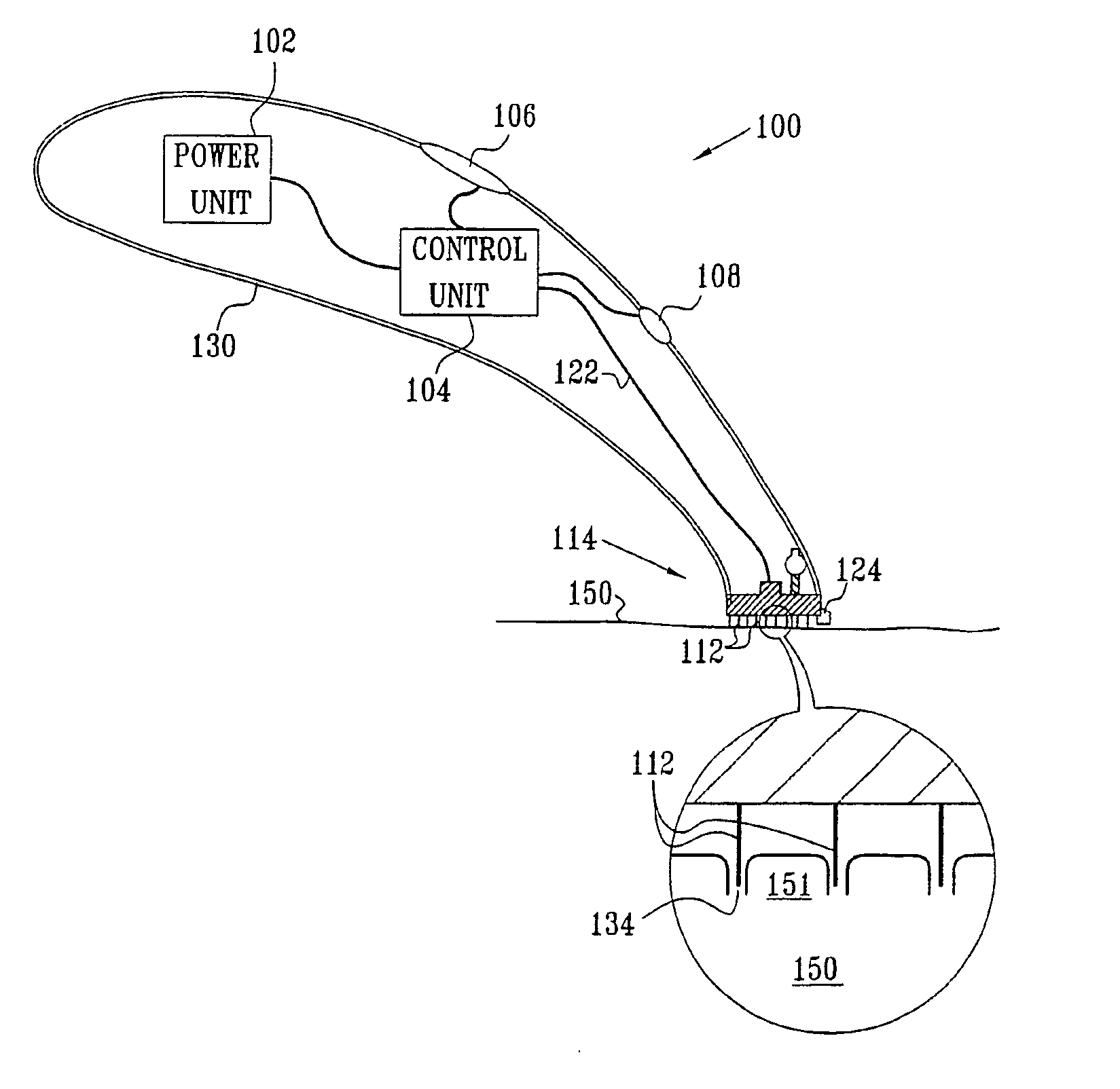
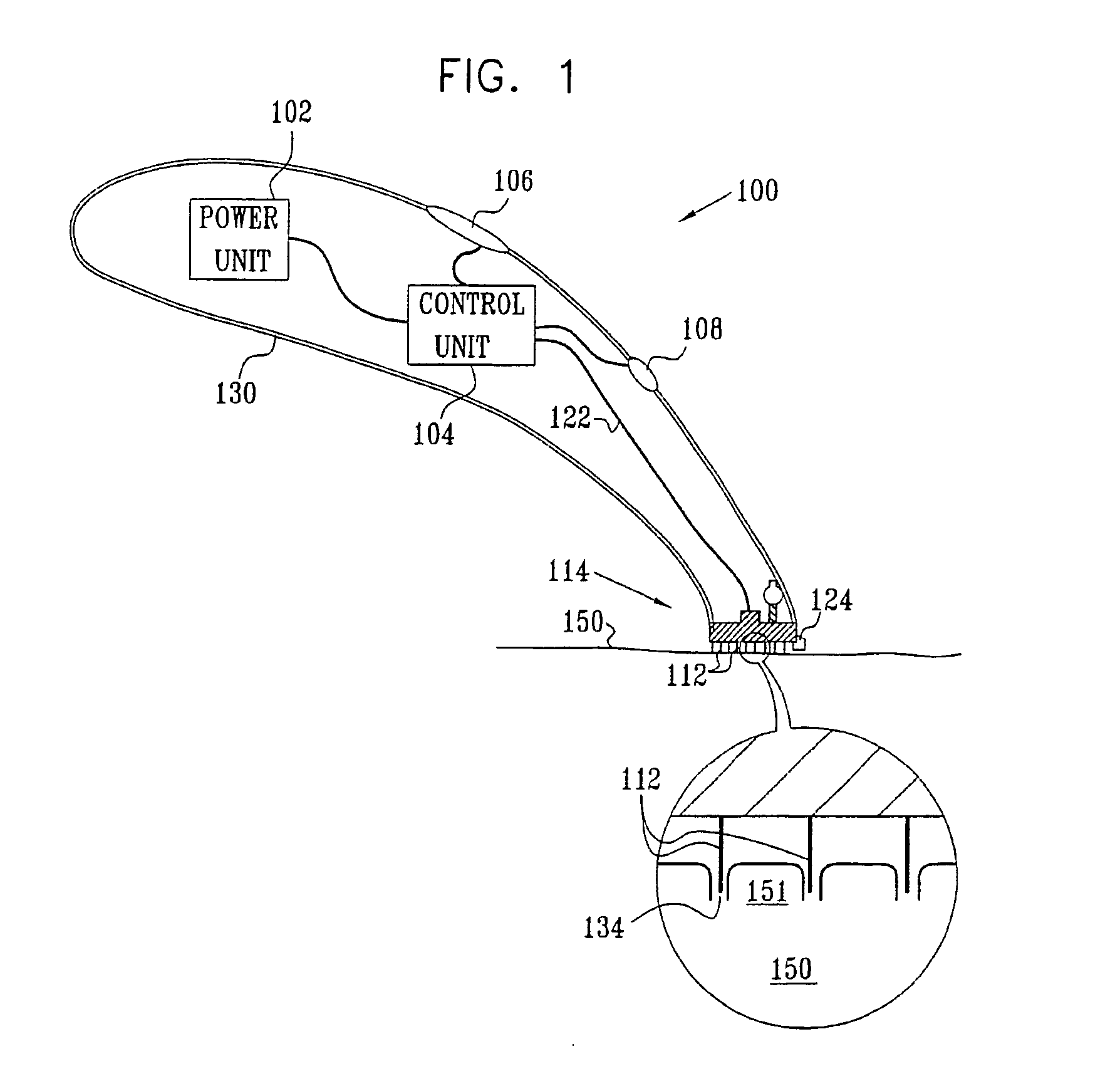
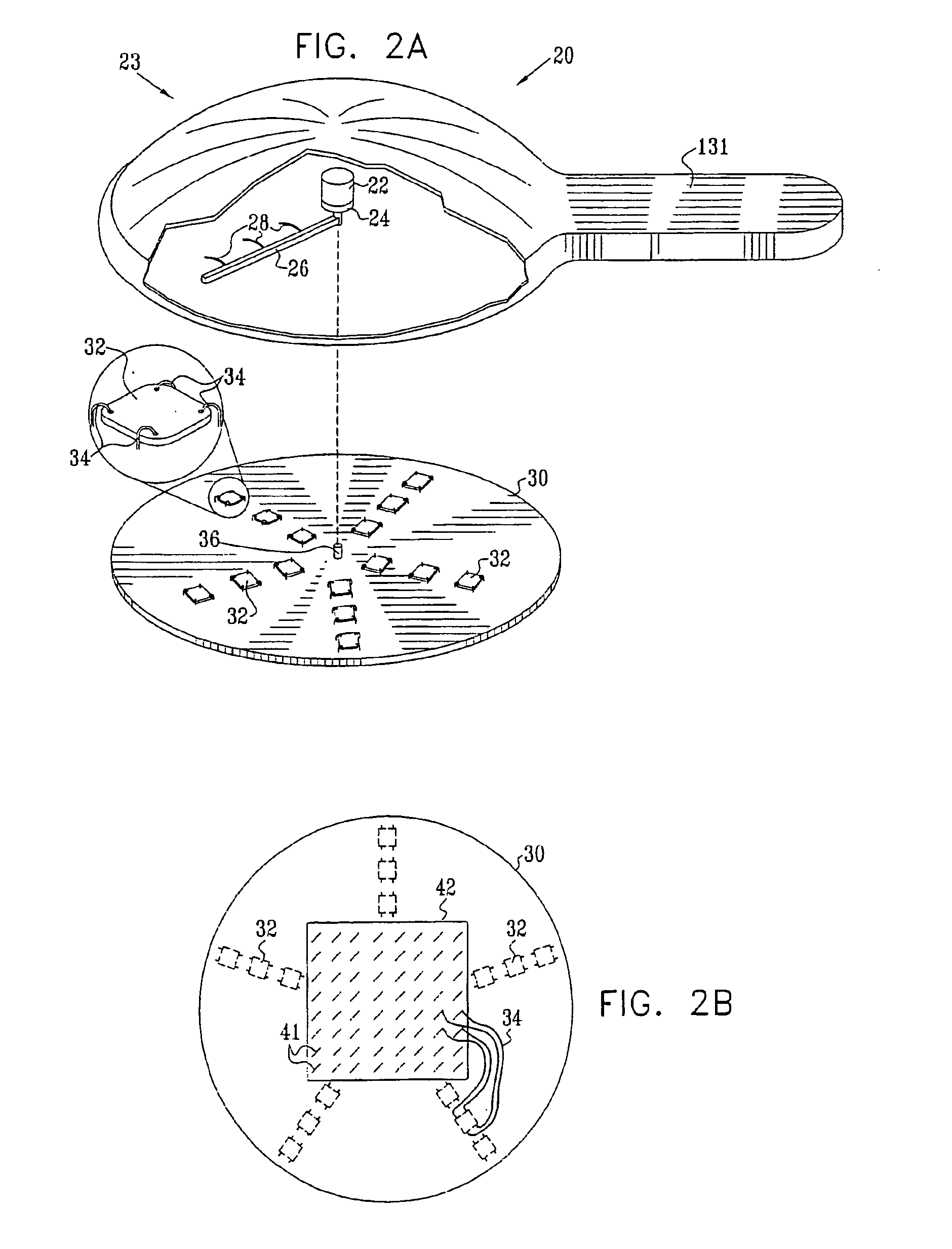
InactiveUS20050119605A1Facilitate transdermal transportMaximize minimum distanceElectrotherapyMedical devicesContact padAnalyte
Apparatus (20) for application to skin of a subject is provided. The apparatus includes a board (30) having a first surface and a second surface, the first surface including a plurality of ablation electrodes (41), which are adapted to be applied to the skin, and the second surface including one or more contact pads (32), each one of the contact pads electrically coupled to at least one of the ablation electrodes. The apparatus further includes one or more driving electrodes (28). An energy applicator (e.g., motor 22), coupled to the driving electrodes, is adapted to pass the driving electrodes over the contact pads. A power source (e.g., power unit 102) is adapted to drive a current from the driving electrodes, to the contact pads, and to the ablation electrodes. The current is capable of ablating at least a portion of stratum corneum of the skin in a vicinity of the ablation electrodes, so as to facilitate transdermal transport of a substance.
Owner:TRANSPHARMA MEDICAL
Transdermal delivery rate control using amorphous pharmaceutical compositions
InactiveUS20050175680A1High propensity toward skin irritationDecrease in percutaneous absorption efficiencyOrganic active ingredientsNervous disorderMedicineActive agent
A pharmaceutical composition for transdermal delivery comprising one or more physiologically active agents; one or more dermal penetration enhancers; and a volatile pharmaceutically acceptable carrier comprising a volatile solvent; and wherein the physiologically active agent and dermal penetration enhancer form an amorphous deposit upon evaporation of the volatile carrier, said amorphous deposit forming a reservoir within the stratum corneum; and (A) wherein the composition has a release rate profile of physiologically active agent so as to provide a ratio of the maximum concentration (Cmax) to the average concentration (Cavg) for the physiologically active agent over the dosage interval within the range of 1 to 10.
Owner:ACRUX DDS
Method of treating acne with stratum corneum piercing device
The invention features a method of treating acne by piercing the stratum corneum of skin in need of such treatment with a stratum corneum-piercing device including a microprotrusion member having a skin-contacting surface and plurality of stratum corneum-piercing microprotrusions thereon.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Method and apparatus for the formation of multiple microconduits
Disclosed is an apparatus that creates a number of microconduits, i.e., small holes in the stratum corneum, the outermost layer of human skin tissue, to provide a pathway therethrough, which can be used, for example, for transdermal drug delivery.
Owner:GALDERMA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com