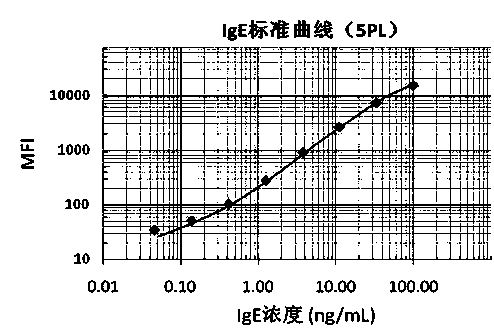
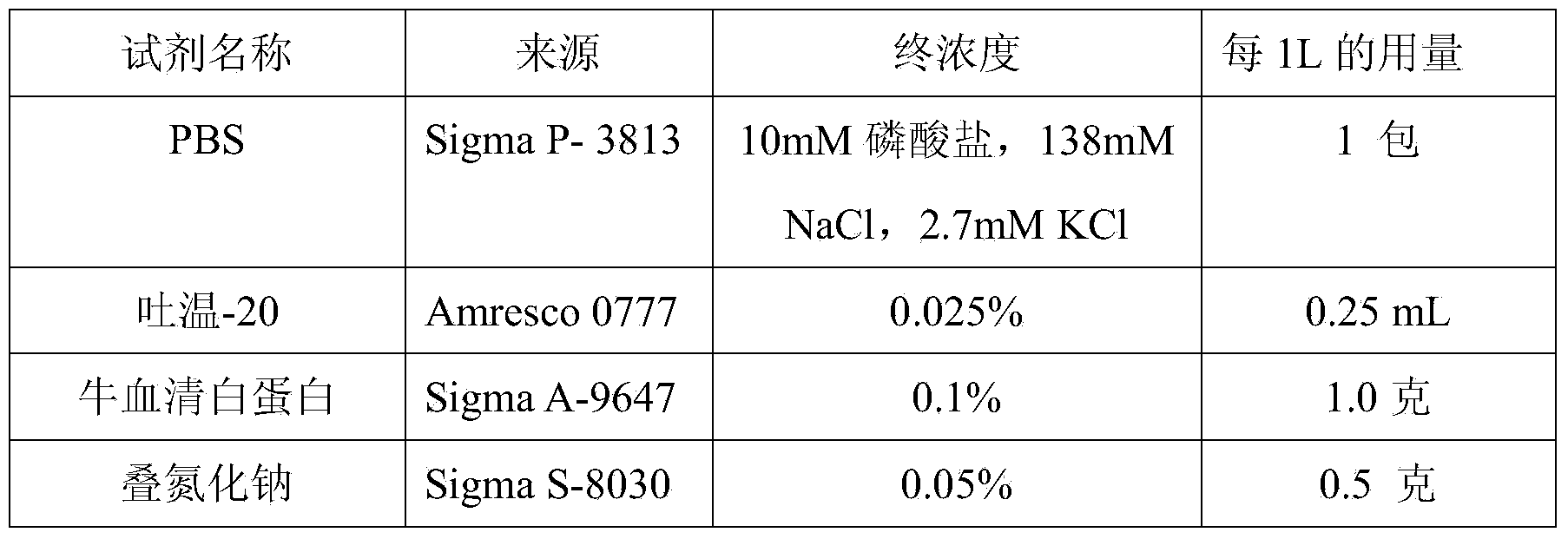
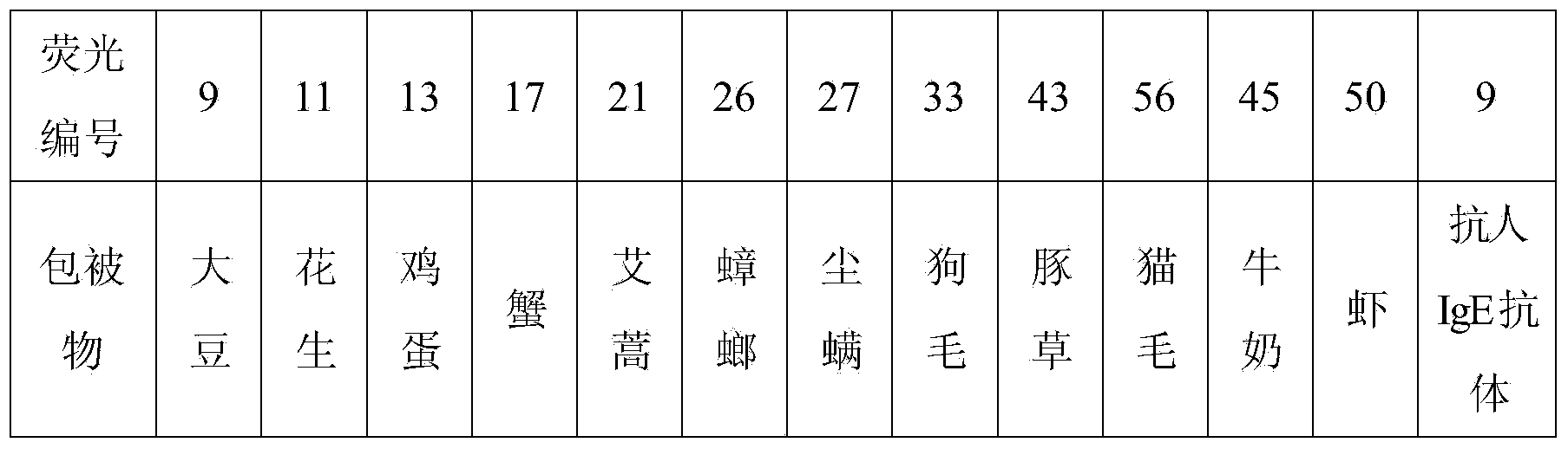
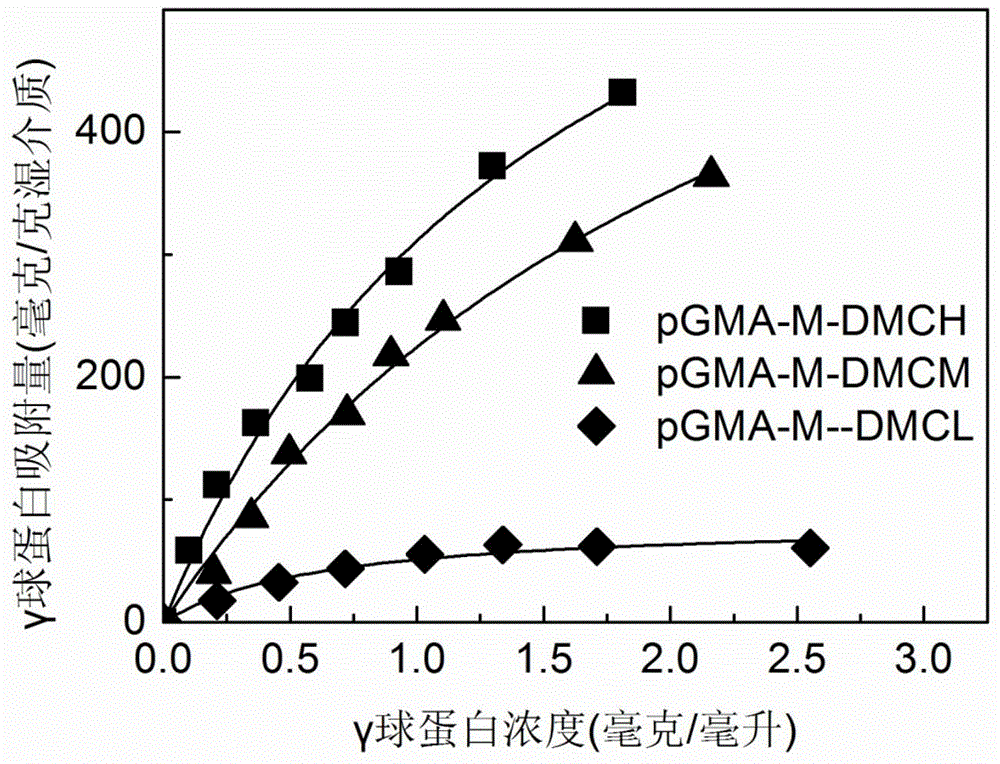
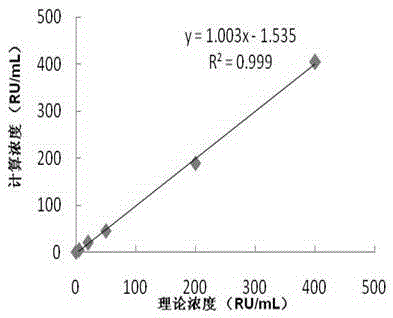
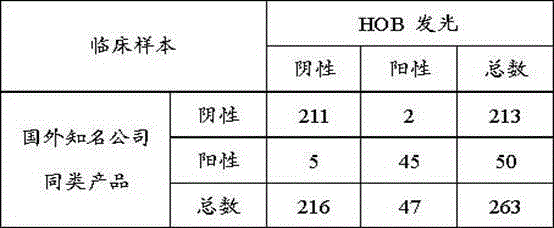
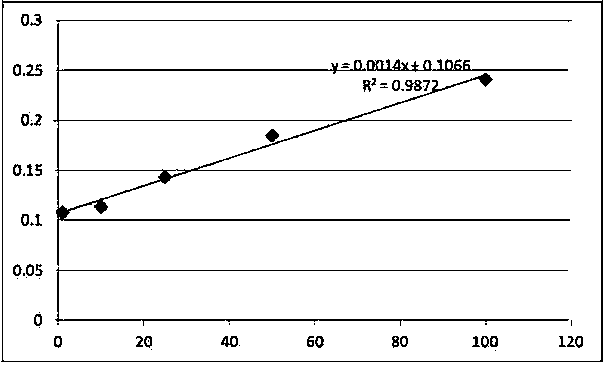
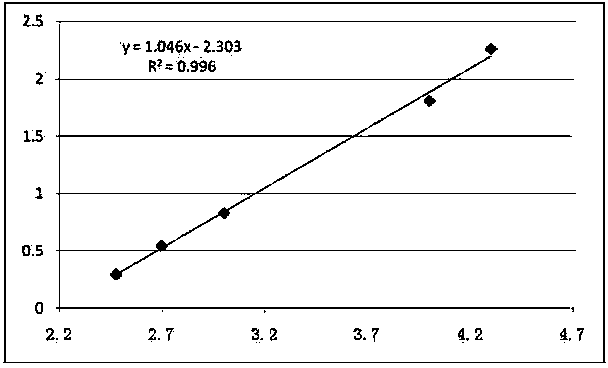
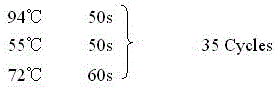Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
88 results about "Gamma globulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gamma globulins are a class of globulins, identified by their position after serum protein electrophoresis. The most significant gamma globulins are immunoglobulins (antibodies), although some immunoglobulins are not gamma globulins, and some gamma globulins are not immunoglobulins.
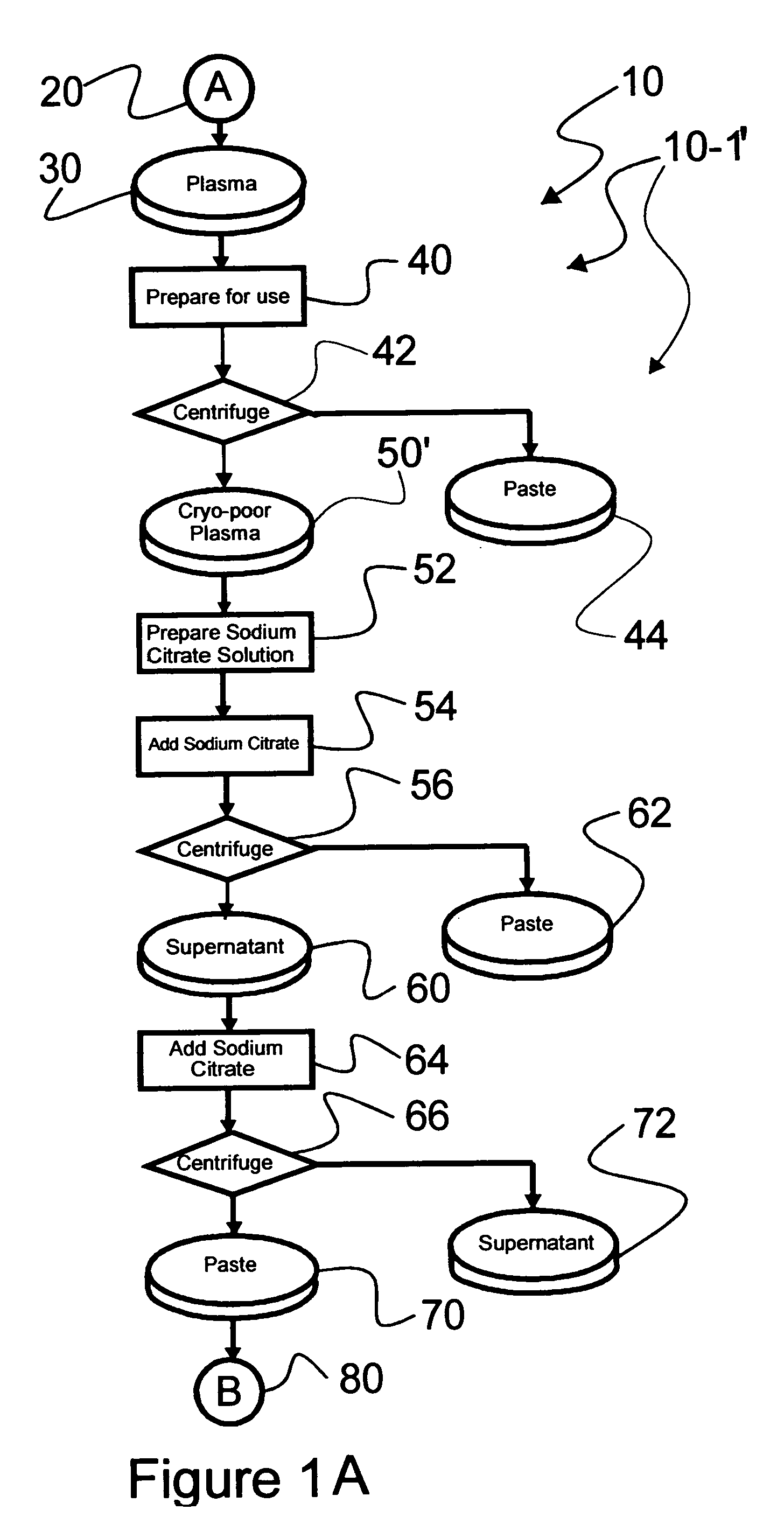
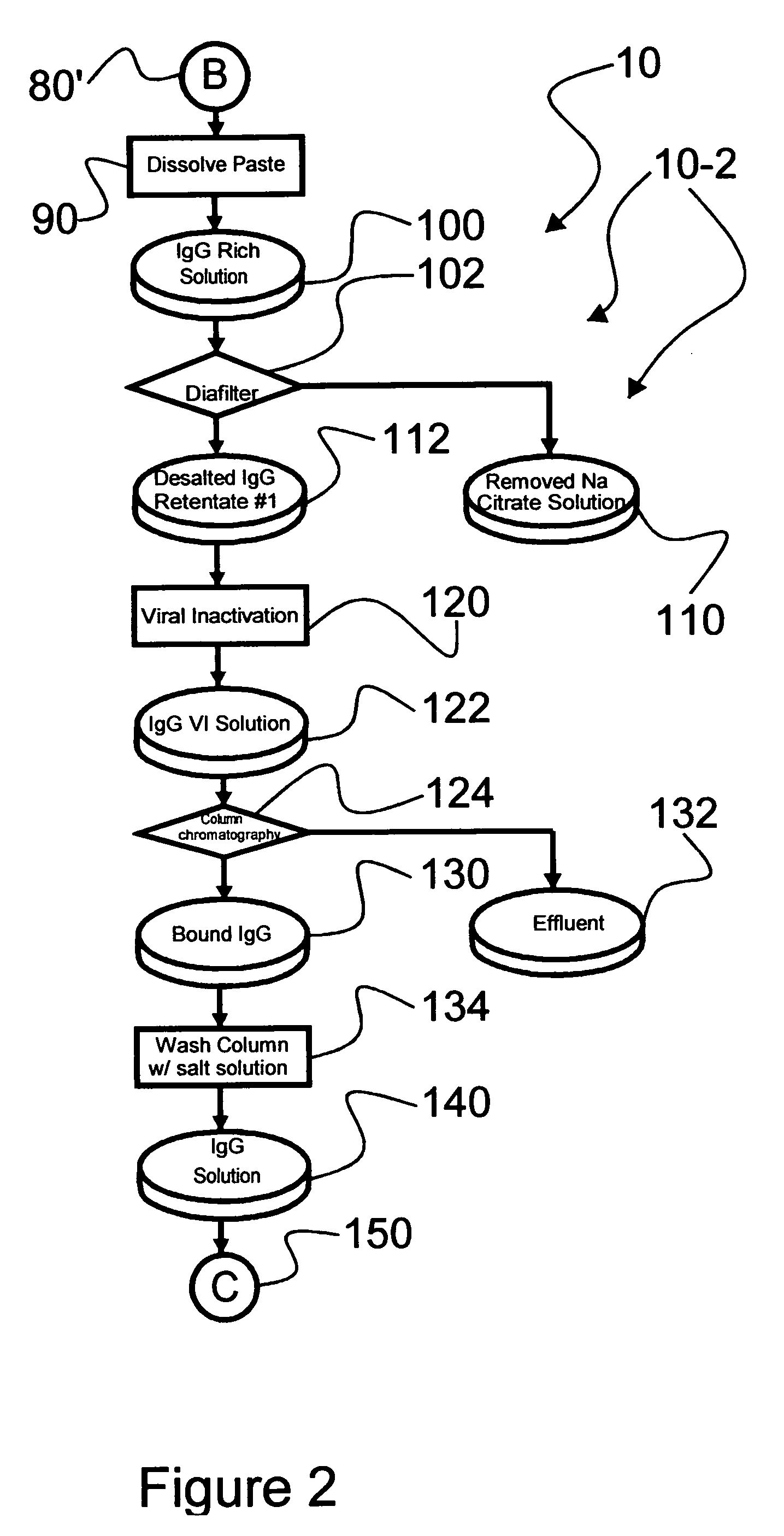
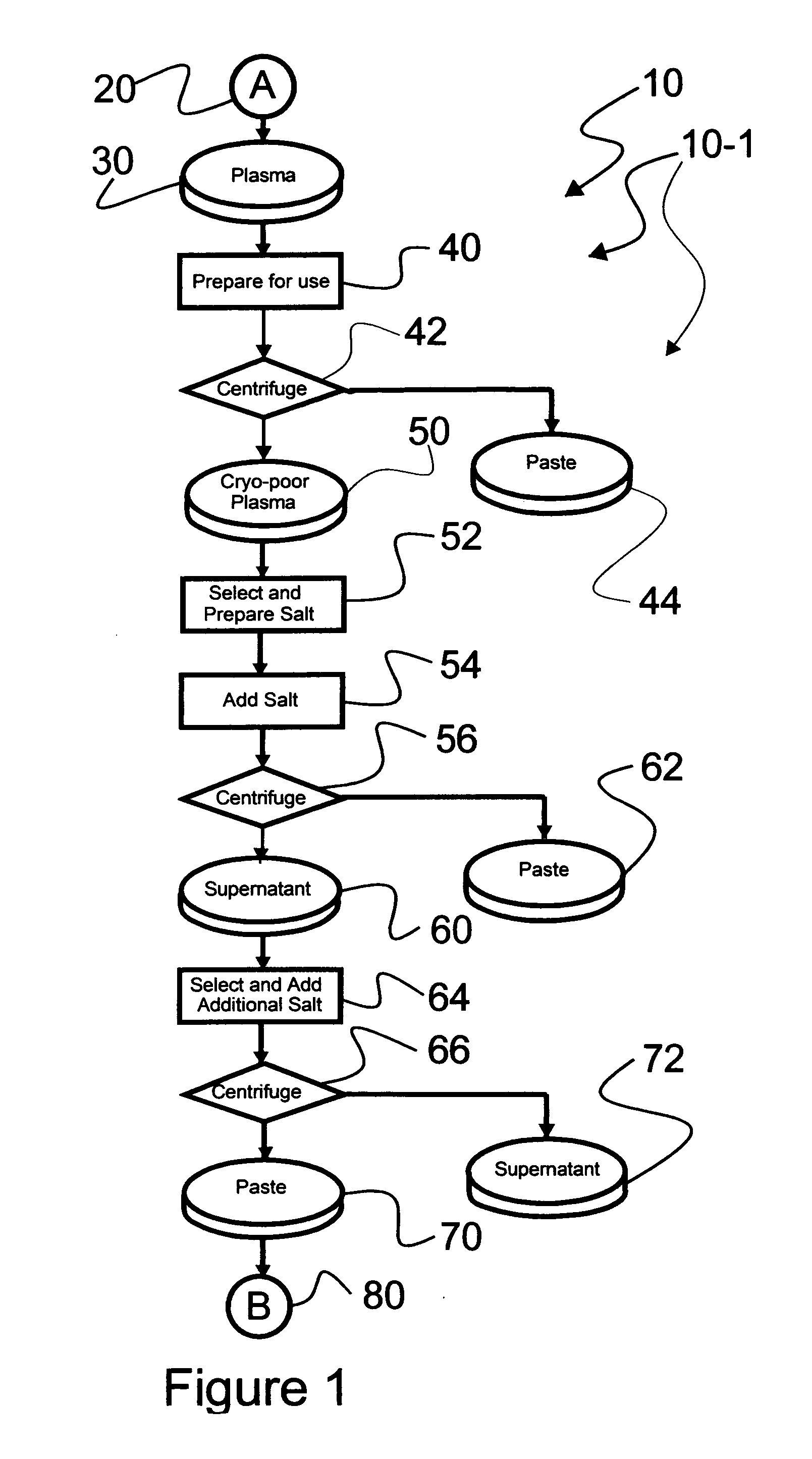
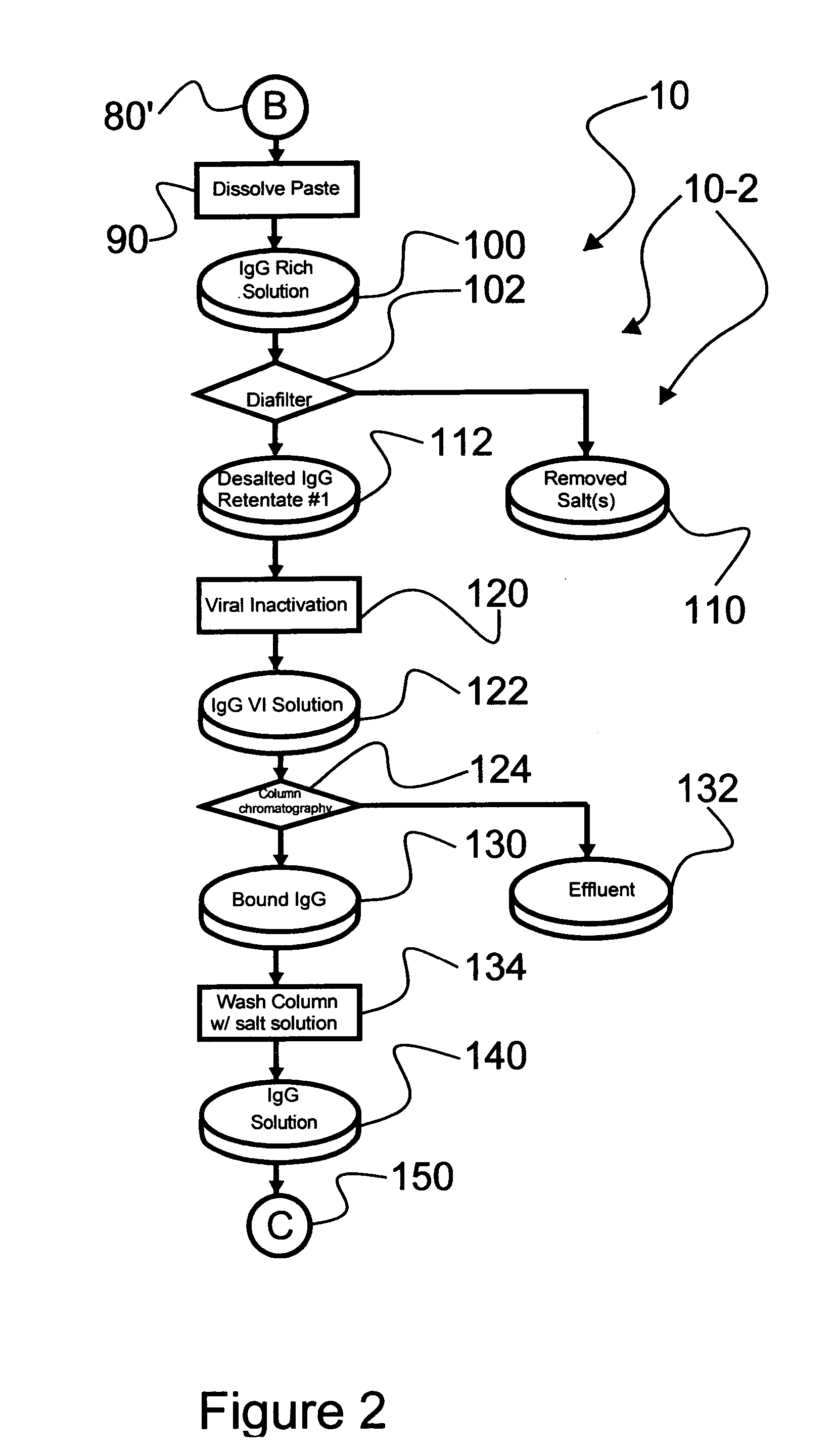
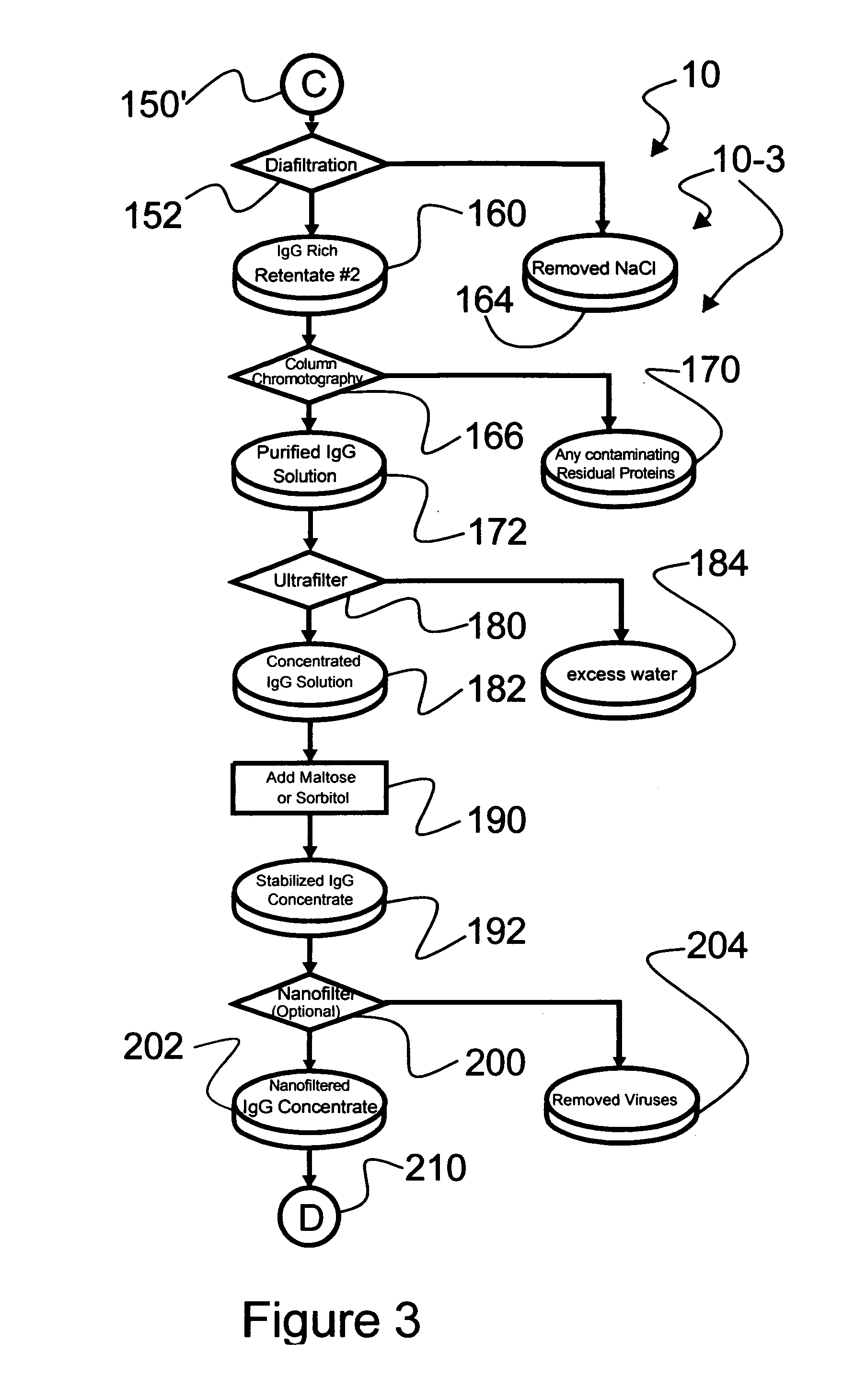
Chromatographic method for high yield purification and viral inactivation of antibodies
InactiveUS6955917B2Minimizes post virus treatment manipulationYield maximizationPeptide/protein ingredientsSerum immunoglobulinsLipid formationLow ionic strength
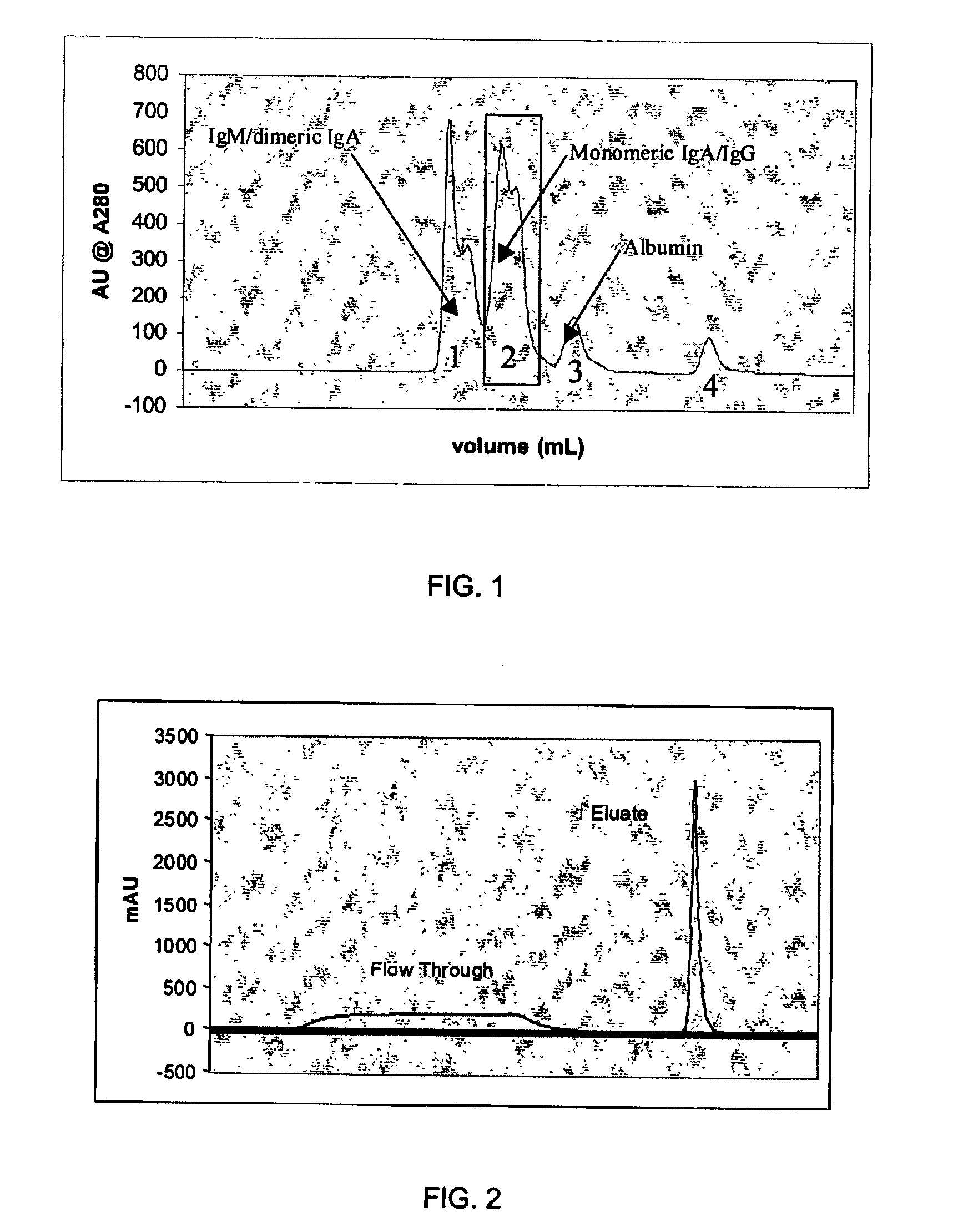
An improved process for the purification of antibodies from human plasma or other sources is disclosed. The process involves suspension of the antibodies at pH 3.8 to 4.5 followed by addition of caprylic acid and a pH shift to pH 5.0 to 5.2. A precipitate of contaminating proteins, lipids and caprylate forms and is removed, while the majority of the antibodies remain in solution. Sodium caprylate is again added to a final concentration of not less than about 15 mM. This solution is incubated for 1 hour at 25° C. to effect viral inactivation. A precipitate (mainly caprylate) is removed and the clear solution is diluted with purified water to reduce ionic strength. Anion exchange chromatography using two different resins is utilized to obtain an exceptionally pure IgG with subclass distribution similar to the starting distribution. The method maximizes yield and produces a gamma globulin with greater than 99% purity. The resin columns used to obtain a high yield of IgG retain IgM and IgA. IgA and IgM may be eluted from these resins in high yield and purity.
Owner:BAYER HEALTHCARE LLC
Traditional Chinese medicine composition for treating chronic hepatitis and preparation method thereof
InactiveCN101757561AAchieve standardizationAchieve scaleAnthropod material medical ingredientsDigestive systemMedicinal herbsImmune complex deposition
The invention discloses a new traditional Chinese medicine composition for treating chronic hepatitis and a preparation method thereof. The traditional Chinese medicine composition mainly comprises the following Chinese medicinal herbs of dried orange peel, rhizoma cyperi, pericarpium citri reticulatae viride, radix scrophulariae, root of rehmannia, folium isatidis, root of kudzu vine, houttuynia cordata, indigo naturalis, peach kernel, red flower, radix paeoniae alba, Tuckahoe, rhizoma alismatis, oriental wormwood, desmodium, polygonum cuspidatum, root of red-rooted salvia, radix bupleuri, angelica sinensis, herba lycopi, earthworm, goldthread, felwort and root bark of the peony tree. The traditional Chinese medicine composition can be prepared into a common oral preparation according to a conventional traditional Chinese medicine preparation method. The invention can remarkably improve the symptoms of mild acratia, inappetence, abdominal distension, pain over the liver and the like of chronic persisting hepatitis, and can improve the symptoms of asthenia, poor appetite, abdominal distension, semiliquid, pain over the liver, poor complexion, poorer health, manpower reduction, hepatomegaly accompanied with haphalgesia and rap pain, and splenomegaly of the chronic active hepatitis and the symptoms of jaundice, spider angioma, liver palms, acne and the like caused by the chronic hepatitis. The invention can also improve the symptoms of long-term obvious dysfunction of liver, ALT continuous increase or repeated fluctuation, albumin reduction, globulin increase, gamma globulin or IgG increase, time extension of protrombin time, capability of positive reaction in self antibody and rheumatoid factors, capability of circulating immune complex increase, capability of addiments C3 and C4 reduction and the like, and has accurate remarkable clinical treatment effect and rapid effect taking.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Ultra-high yield intravenous immune globulin preparation
ActiveUS7879332B2Quick restoration of the internal water moleculeReduce formationBiocideMedical devicesSodium acetateAlcohol free
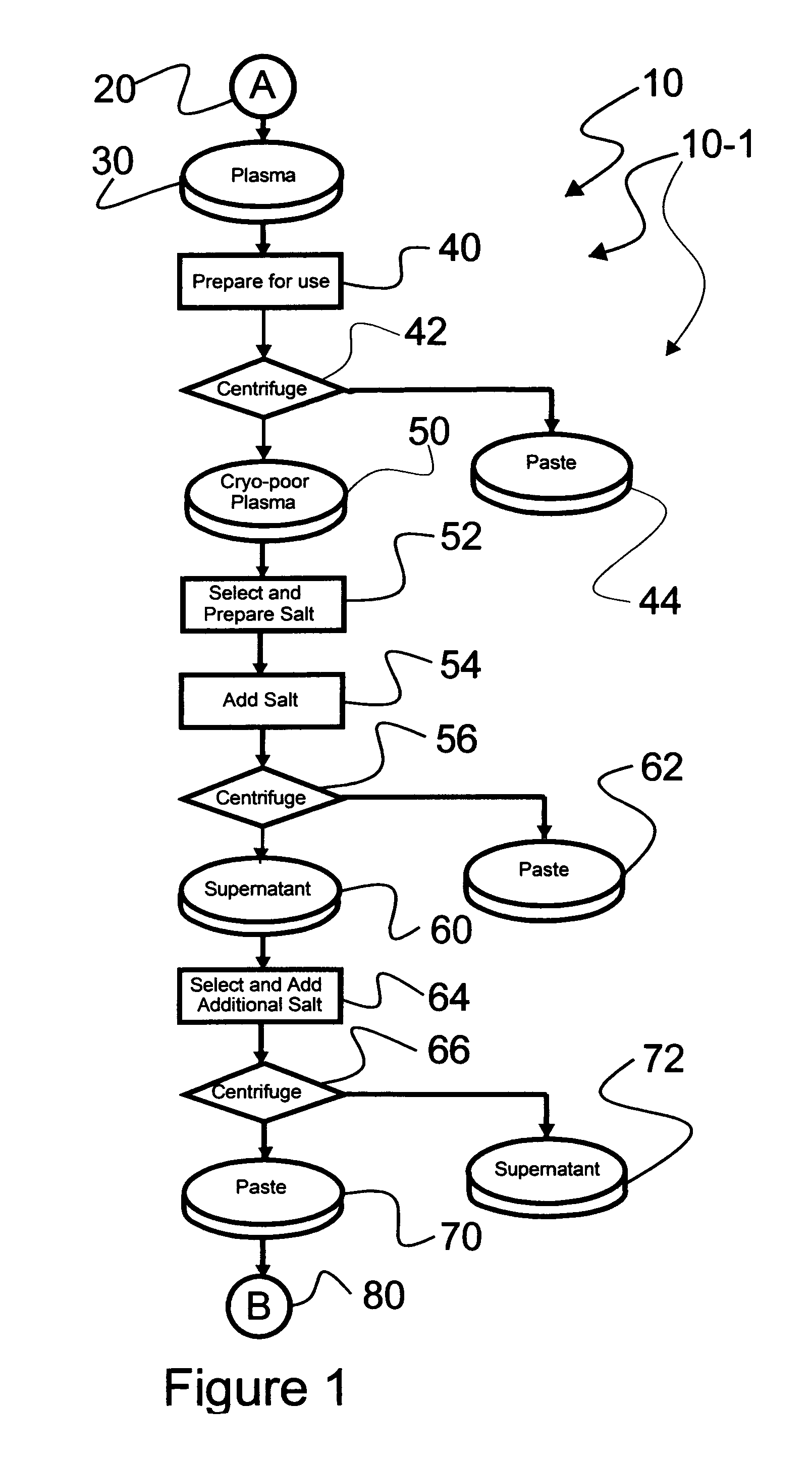
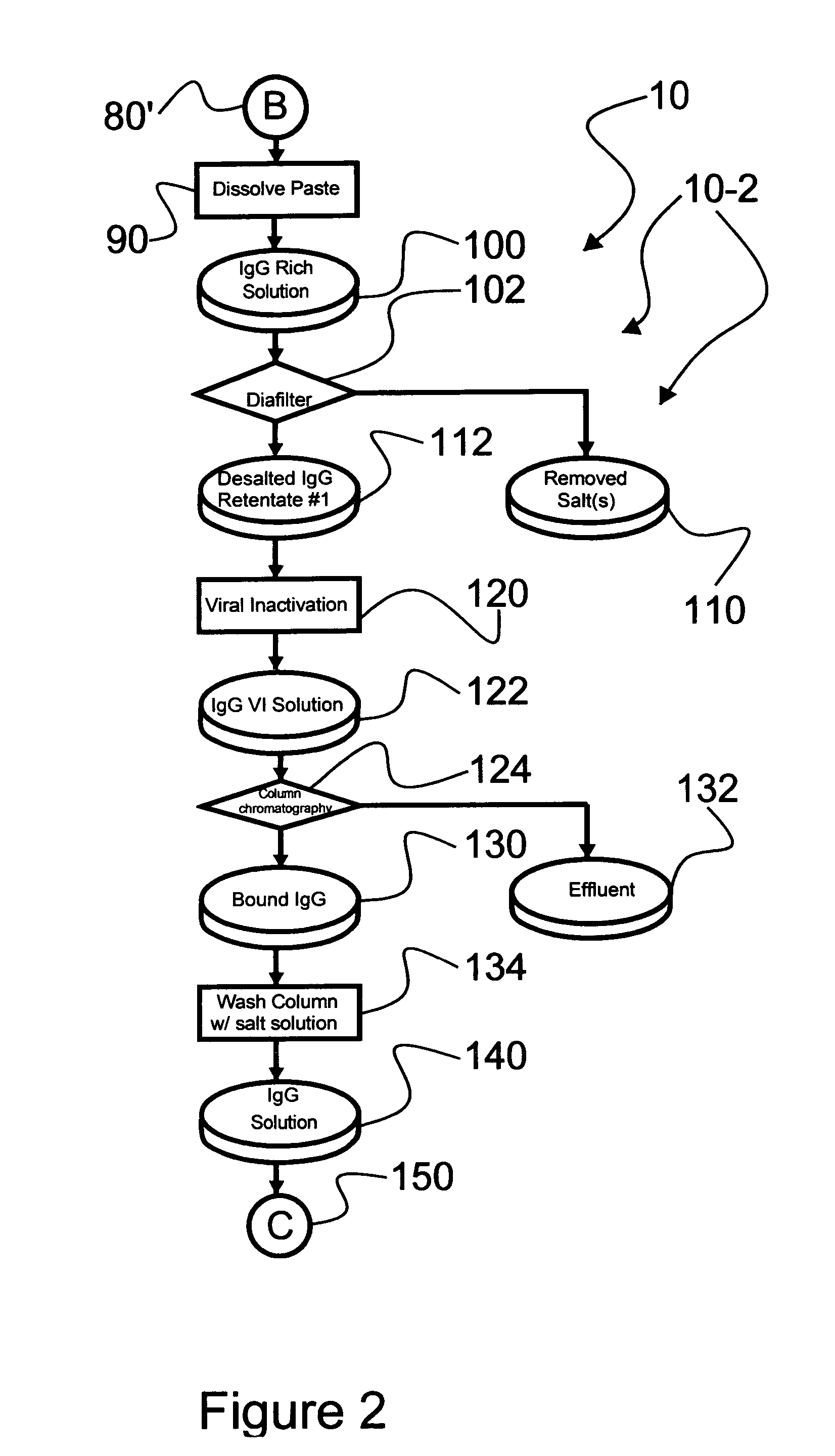
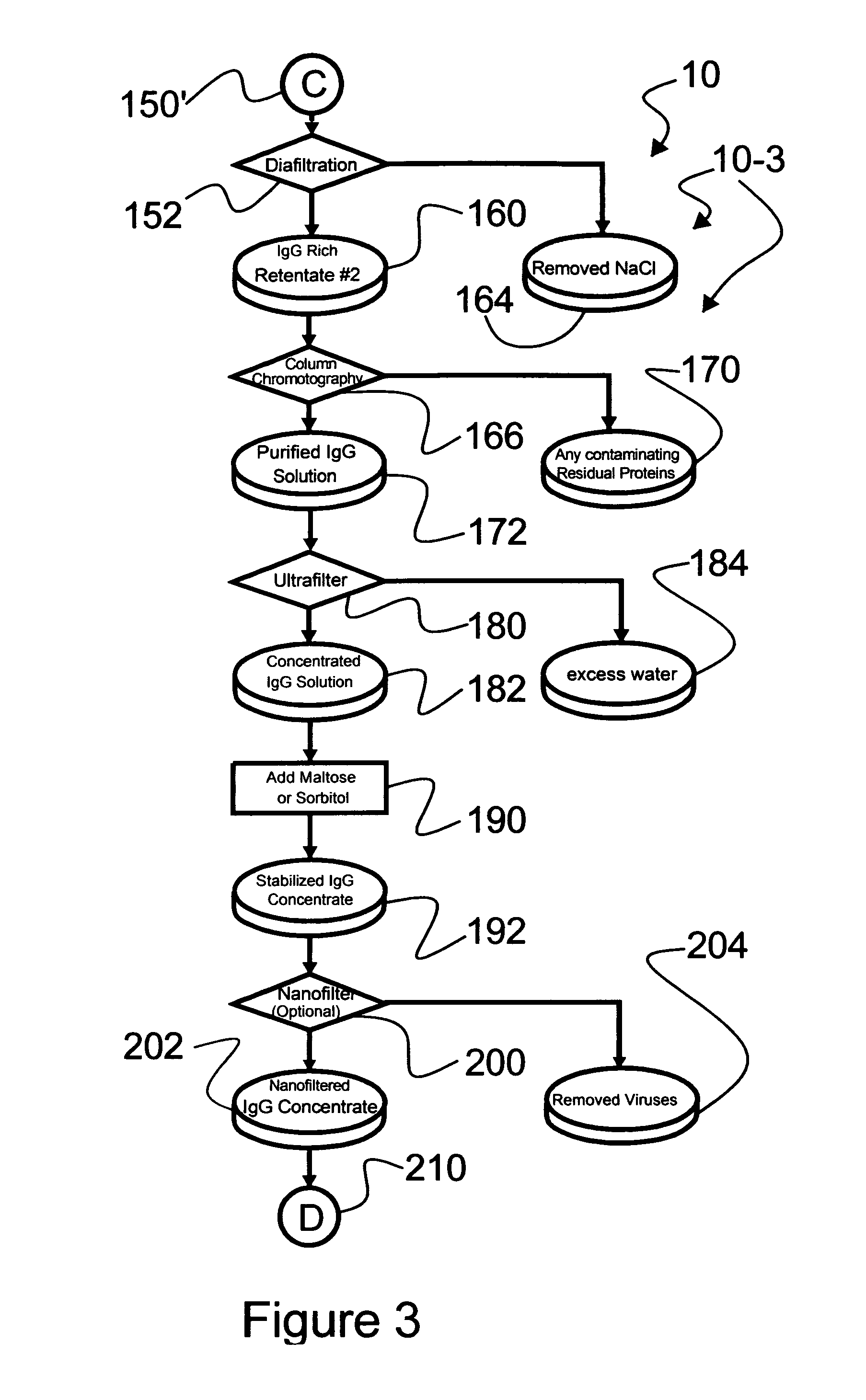
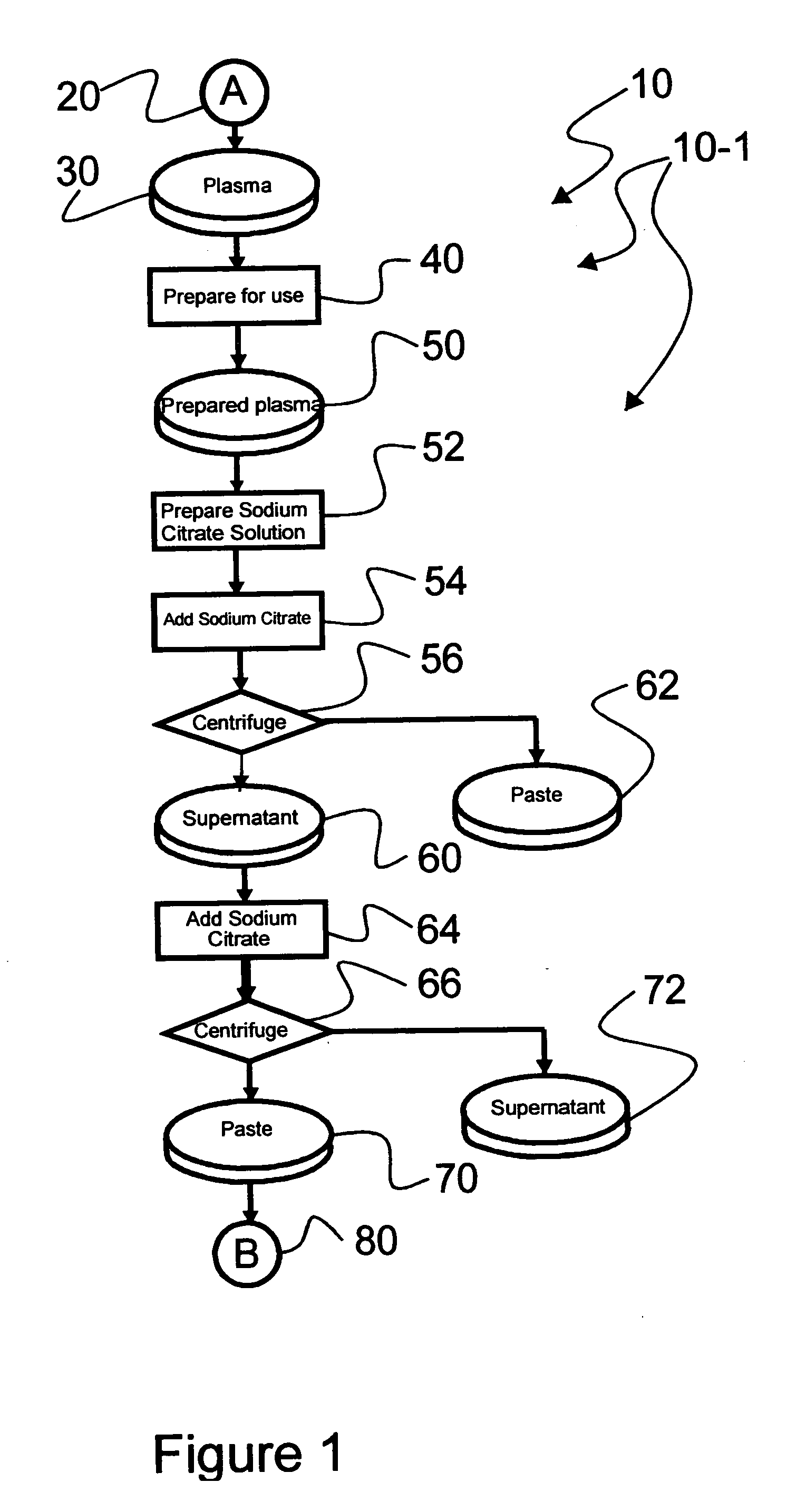
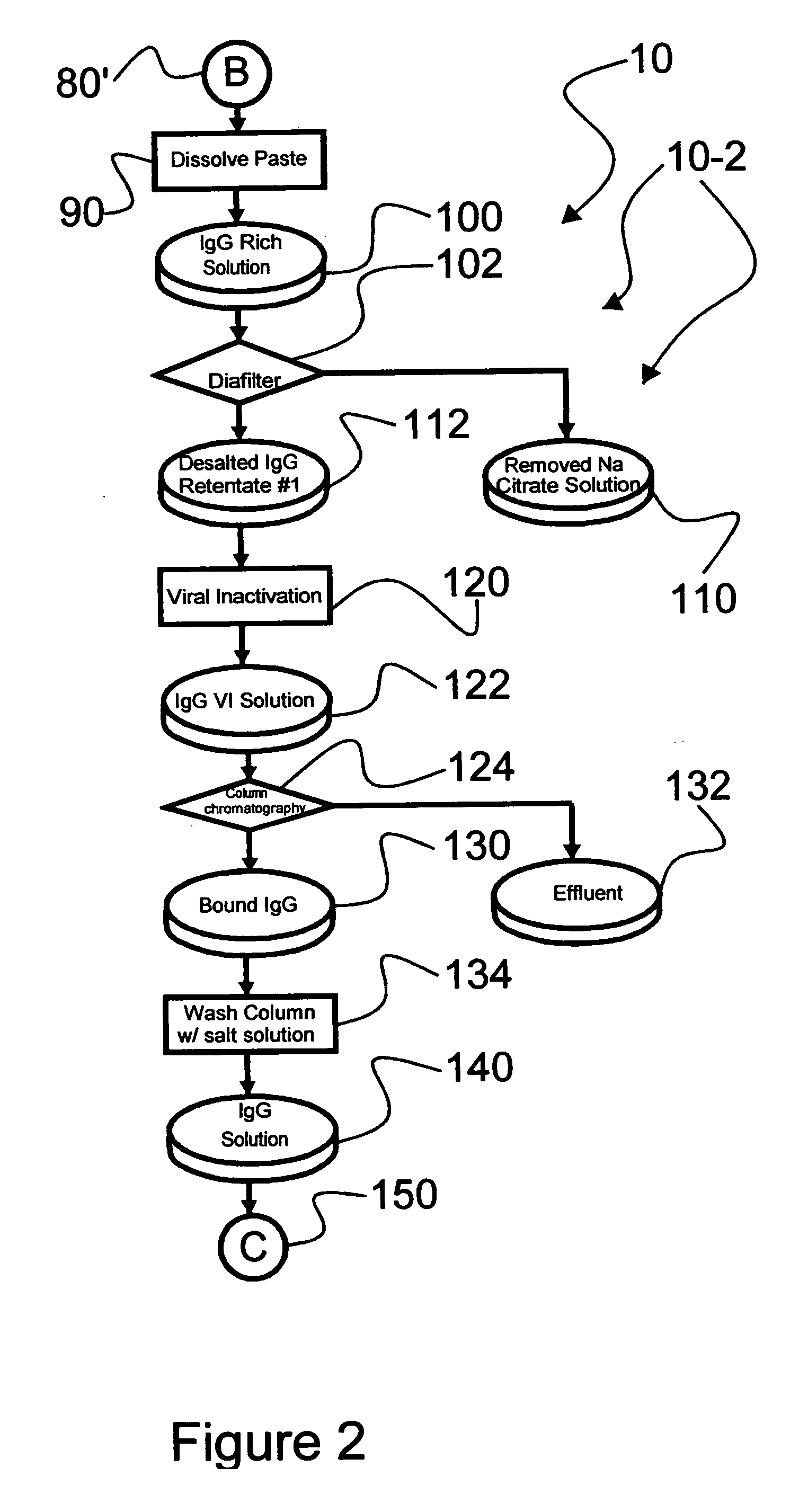
An efficacious large-scale alcohol-free plasma fractionation production process which produces a high-yielding, non-denatured, double viral-inactivated intravenous human immune gamma globulin (IgG) product. The process employs one or more salts from a group of salts comprising sodium citrate, sodium acetate, sodium gluconate, ammonium sulfate, sodium chloride, sodium sulfate and ammonium chloride in two initial fractionation steps, followed by diafiltration to remove those salts employed. A process which employs alcohol via the process of the disclosed inventive method is also disclosed.
Owner:PLASMA TECH LLC
Method for determining anti-nucleosome antibody IgG (intravenous gamma globulin) and reagent device
The invention provides a method for realizing immunization detection for an anti-nucleosome antibody IgG (intravenous gamma globulin) based on the enzyme-linked immunoassay principle and a reagent device therefor; and an analytic method, the reagent device and an assorted reagent are independently, individually and disposably used for detecting the anti-nucleosome antibody IgG based on enzyme-linked immunoassay. Various reagents needed by enzyme-linked immunoassay for the anti-nucleosome antibody IgG are contained in one analytic device; and by using the method, the related immunology detection can be conveniently carried out according to the using requirements of detection items so as to provide better basis for clinical application.
Owner:SHENZHEN YHLO BIOTECH
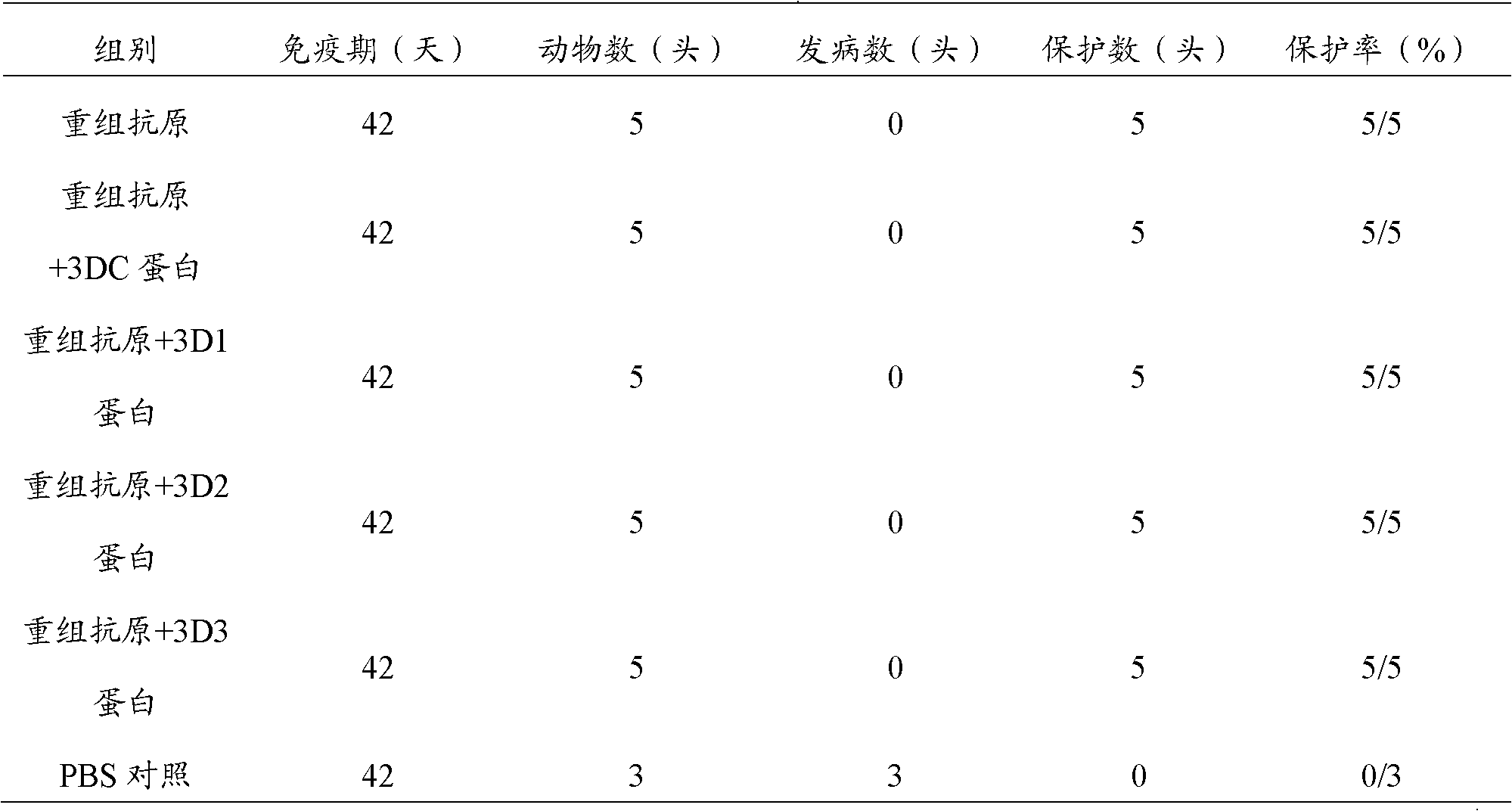
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
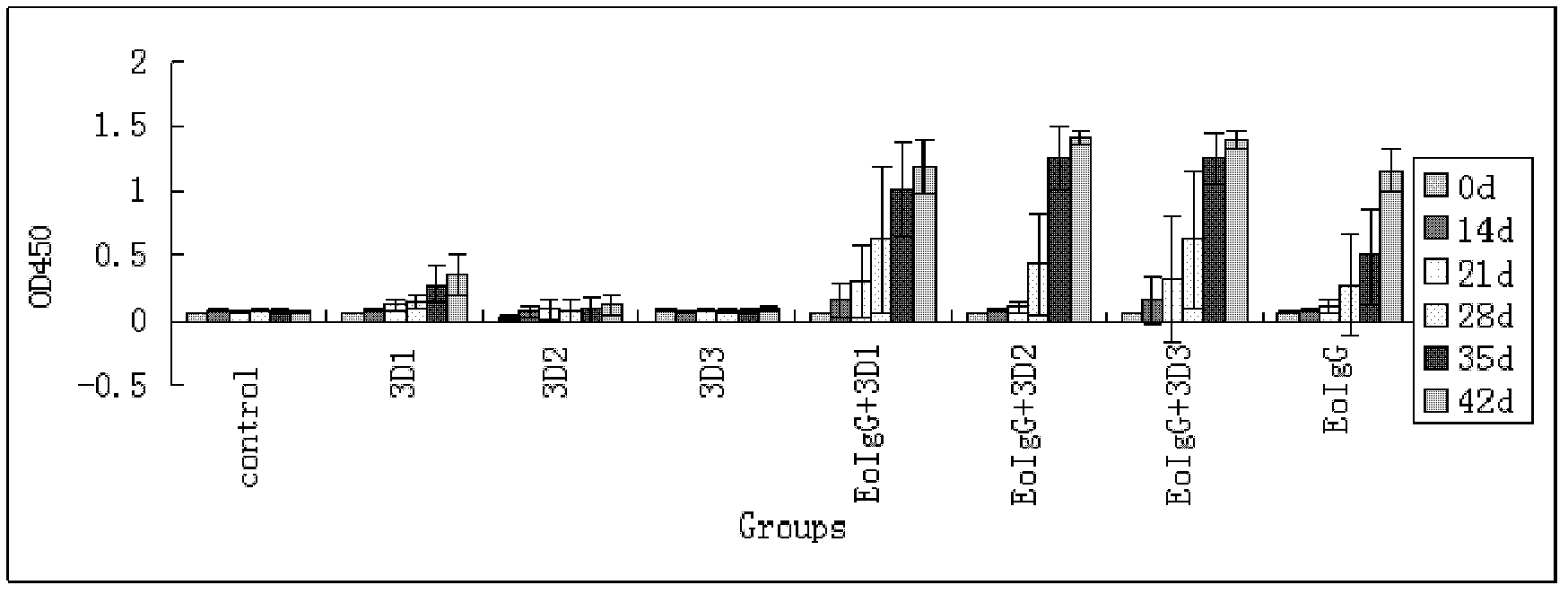
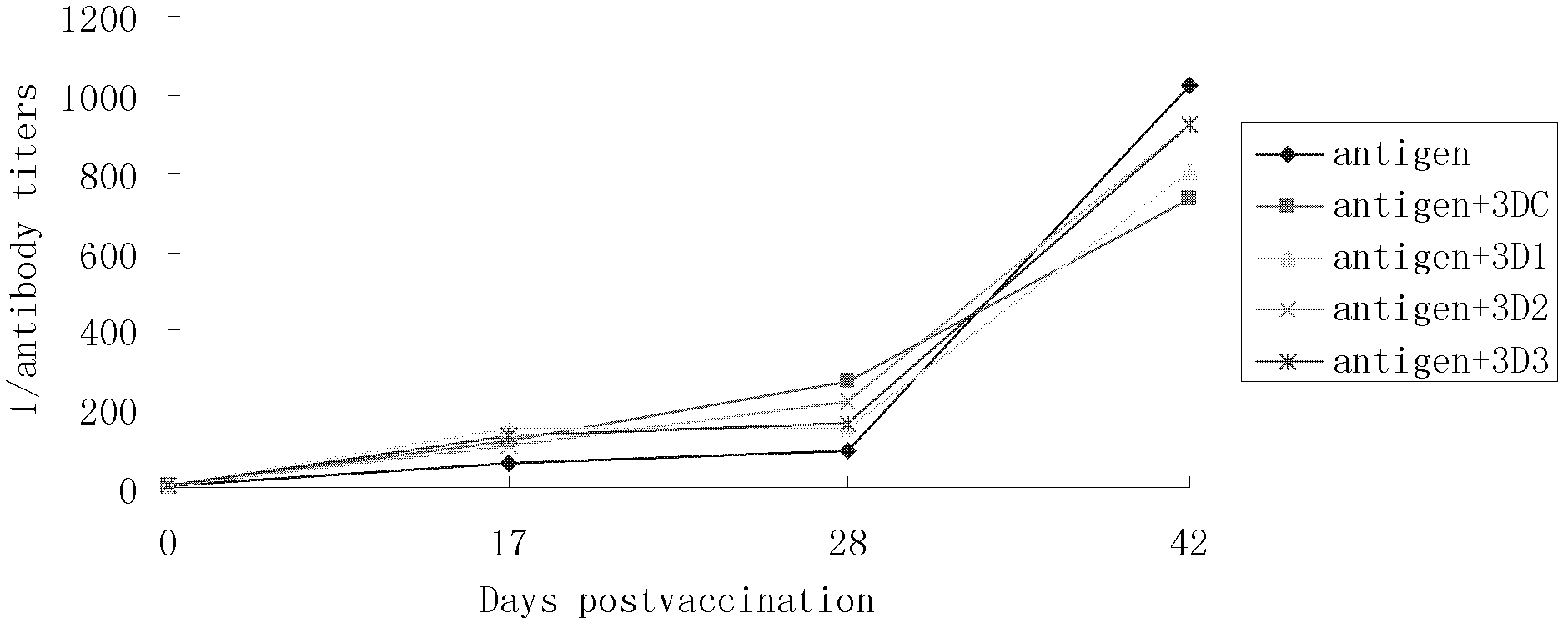
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Ultra-high yield intravenous immune globulin preparation
An efficacious large-scale alcohol-free plasma fractionation production process which produces a high-yielding, non-denatured, double viral-inactivated intravenous human immune gamma globulin (IgG) product. The process employs sodium citrate in two initial fractionation steps, followed by diafiltration to remove sodium citrate.
Owner:PLASMA TECH LLC
Immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, detection card component produced by same and method for preparing same
ActiveCN102680702AHigh sensitivityImprove signal-to-noise ratioBiological testingFluorescence/phosphorescencePorphyrinIMMUNE FLUORESCENCE
The invention discloses an immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, a detection card component produced by the same and a method for preparing the same. The test strip component comprises a test strip and a platinum porphyrin marked specific antibody independently packed. The test strip comprises a bottom lining, a water absorption pad, coating analysis film and a sample pad. The coating analysis film is provided with a detection line and a quality control line, a specific antibody coated by the detection line is a C-reactive protein resistance monoclonal antibody, and a specific antibody coated by the quality control line is a rabbit intravenous gamma globulin (IgG) antibody; the detection card component comprises a test strip, a card box composed of a cover plate and a back plate and a latinum porphyrin marked specific antibody independently packed. The test strip component for detecting C-reactive protein in body fluid has the advantages of being simple in operation, rapid, sensitive, good in specificity and the like, and having good clinical application prospect.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Liquid phase chip for detecting allergen specific antibody and preparation method of liquid phase chip
ActiveCN103454412AComprehensive clinical testingAccurate clinical detection meansFluorescence/phosphorescenceBiotin-streptavidin complexFluorescence
The invention discloses a liquid phase chip for detecting an allergen specific antibody. The liquid phase chip mainly comprises (1) a plurality of common allergen probes, comprising 12 fluorescence coded microspheres respectively coated with mite, cockroach, ragweed, felon herb, dog hairs, cat hairs, soybean, peanut, milk, egg, shrimp and crab allergen extracts; (2) a biotin labeling detection antibody; (3) a streptavidin phycoerythrin; (4) a total IgE (immunoglobulin E) probe used when semiquantitative or quantitative detection is carried out on the allergen specific IgE antibody, namely, a fluorescence coded microsphere coated with a rat anti-human IgE monoclonal antibody. The invention also discloses a preparation method of the liquid phase chip probe. The liquid phase chip disclosed by the invention is mainly used for detecting the allergen specific IgE antibody in a serum sample, also for detecting an allergen specific IgG4 (Intravenous Gamma Globulin 4) antibody and a total IgE antibody, and has the advantages of few used samples, parallel detection of a plurality of indexes, high flux, high sensitivity and the like.
Owner:NANJING BOMINDA BIO TECH
Method for preparing high-capacity protein chromatographic medium through atom transfer radical polymerization
InactiveCN105001376AEasy to recycleMild elution conditionsCation exchanger materialsOther chemical processesAdsorption equilibriumGlycidyl methacrylate
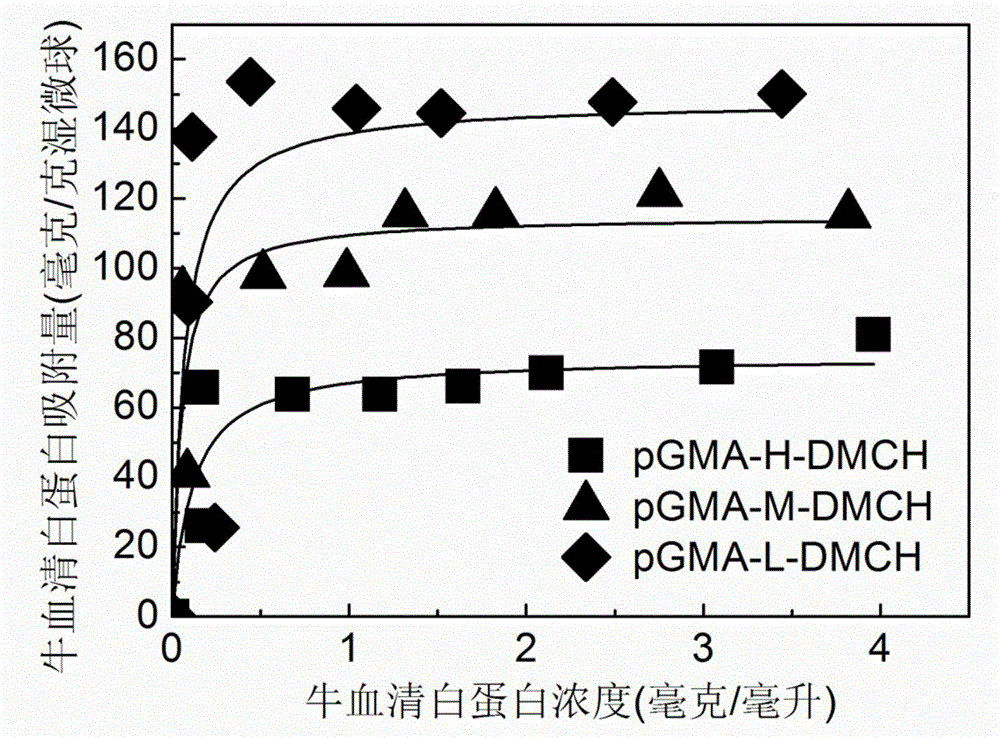
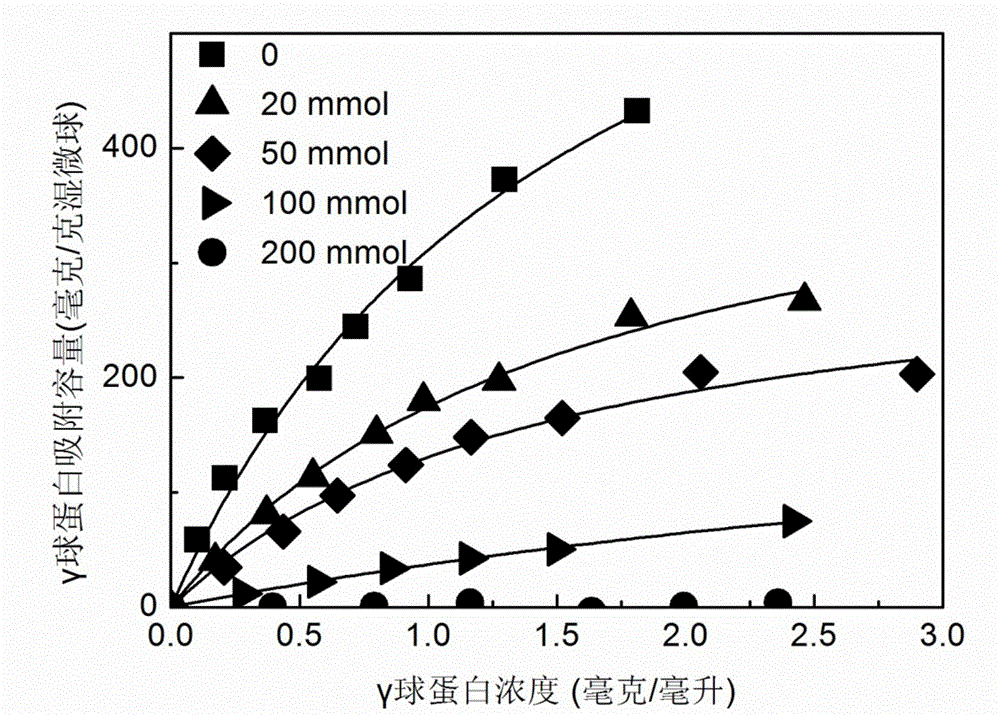
The invention relates to a method for preparing high-capacity protein ion-exchange chromatographic medium through atom transfer radical polymerization. The method comprises a bromination procedure of coupling a glycidyl methacrylate microsphere with the microsphere of an initiator 2-bromoisobutyryl bromide and a synthesis procedure of initiating polymerization of monomeric compounds on the surface of the microspheres through free radical transfer so as to obtain the ion-exchange polymer grafted chromatographic medium. The method prepares the polymer grafted ion-exchange chromatographic medium through atom transfer radical polymerization technology; the synthesized polymer grafted ion-exchange chromatographic medium has controllable density and chain length of graft polymer; reaction conditions are mild; the selection range of the monomeric compounds used as monomer is wide; gamma globulin saturated adsorption capacity of the synthesized polymer grafted ion-exchange chromatographic medium can reach above 800 mg / g of a wet medium, so the chromatographic medium has excellent adsorptivity; and adsorption equilibrium can be realized within 5 min in a protein solution with a concentration of 1 mg / mL, so the chromatographic medium has wide application prospects.
Owner:TIANJIN UNIV
New process for the industrial-scale purification of gamma globulins from human plasma for industrial applications
ActiveUS20120316323A1High yieldImprove stabilityPeptide preparation methodsImmunoglobulinsAnion-exchange chromatographyBlood plasma
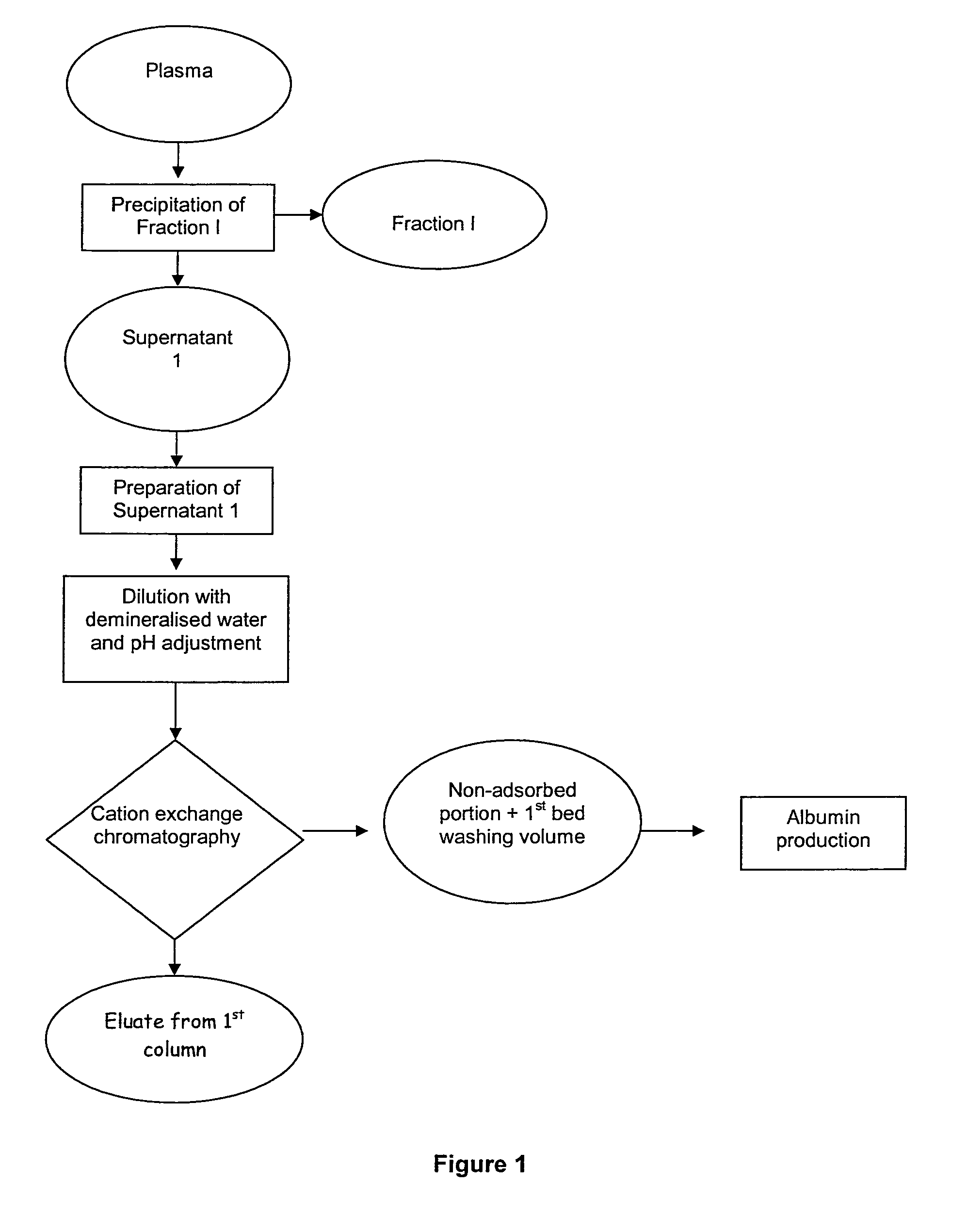
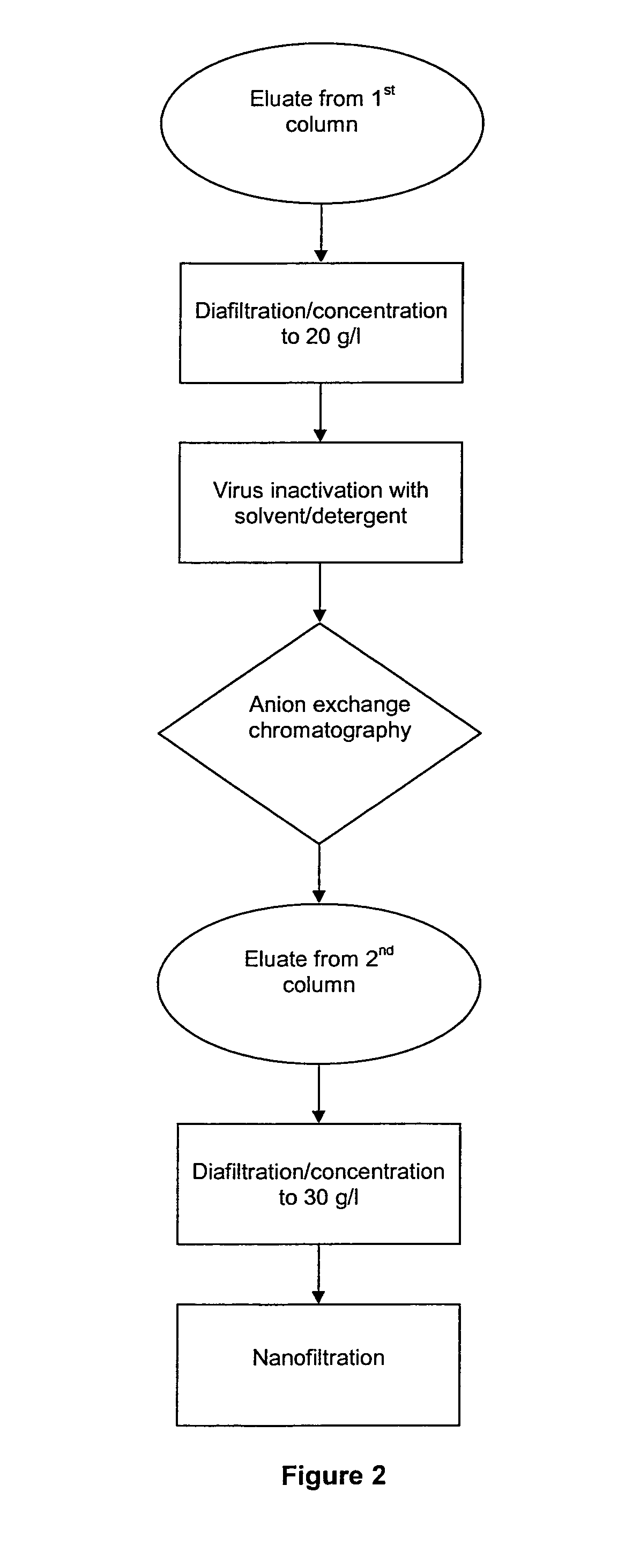
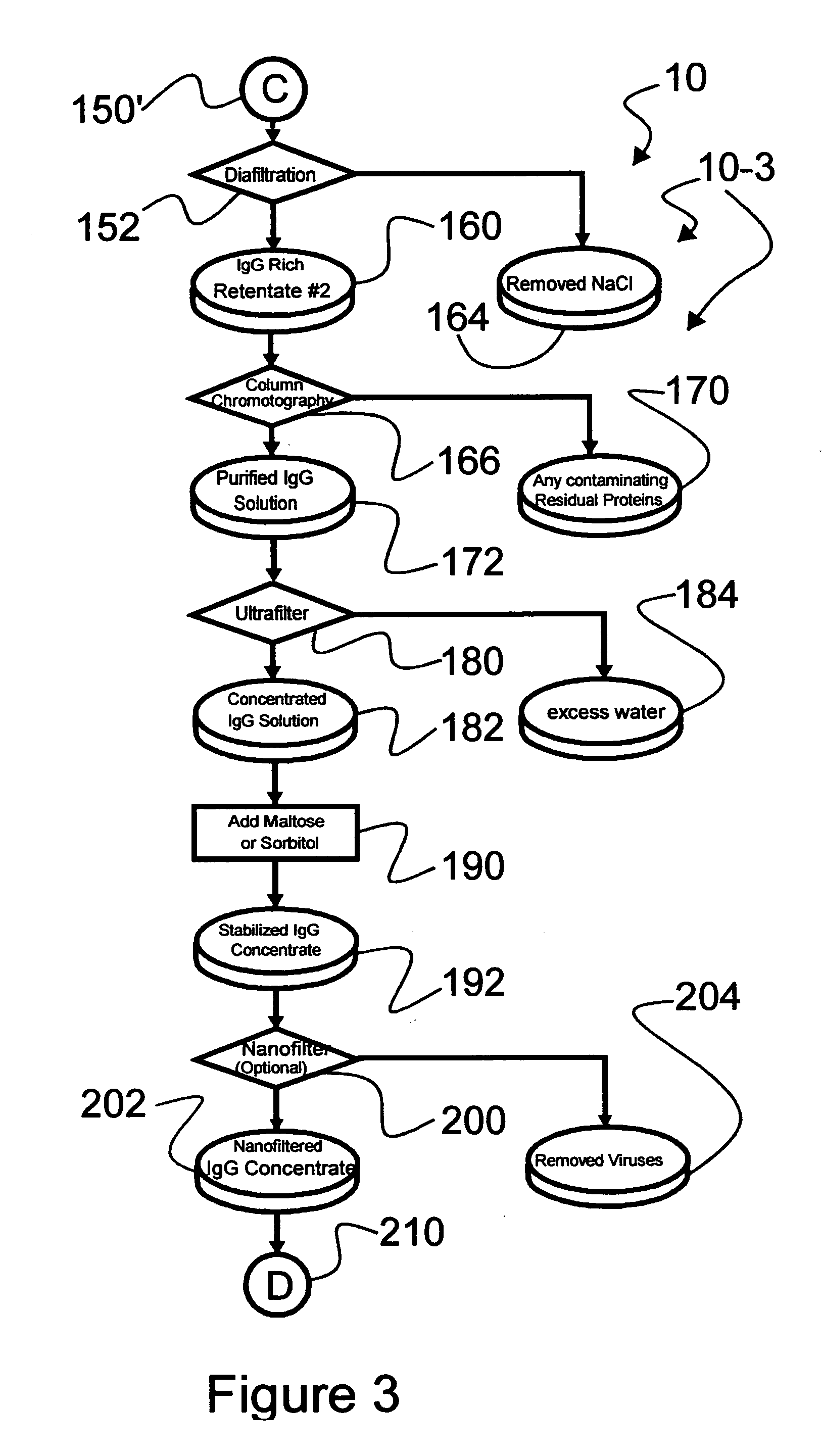
The invention relates to a novel, industrial-scale process for the purification of gamma-immunoglobulins (IgG) starting from plasma or fractions thereof. The method involves two chromatographic steps, i.e. a cation exchange capture chromatography, and then a polishing anion exchange chromatography, ensuring a highly purified end product, which contains no aggregates, and high yields. The process also involves a virus inactivation step by means of a solvent / detergent treatment to inactivate the viruses with a lipid envelope, and a virus removal step by nanofiltering to ensure the removal of the non-enveloped viruses.
Owner:KEDRION
Ultra-high yield intravenous immune globulin preparation
ActiveUS20070049734A1Quick restoration of the internal water moleculeReduce formationImmunoglobulins against animals/humansMedical devicesSodium acetateAlcohol free
An efficacious large-scale alcohol-free plasma fractionation production process which produces a high-yielding, non-denatured, double viral-inactivated intravenous human immune gamma globulin (IgG) product. The process employs one or more salts from a group of salts comprising sodium citrate, sodium acetate, sodium gluconate, ammonium sulfate, sodium chloride, sodium sulfate and ammonium chloride in two initial fractionation steps, followed by diafiltration to remove those salts employed. A process which employs alcohol via the process of the disclosed inventive method is also disclosed.
Owner:PLASMA TECH LLC
Methods of using immunoglobulin (Ig) compositions
InactiveUS20040241102A1Increase weightPromote effectivePowder deliverySerum immunoglobulinsDiseaseAntigen
The invention is directed to methods of using Ig compositions to prevent and / or treat humans, livestock and / or domesticated animals suffering from a variety of disorders. The Ig composition comprises a concentrated amount of one or more immunoglobulins selected from the group consisting of alpha, beta, and gamma globulins. Preferably, the Ig composition comprises other antibodies identified as providing an immune response and / or immune factors such as complement. The Ig composition preferably includes immunoglobulins specific to a variety of antigens.
Owner:CENT BIOMEDIA
Gold-labeled test strip for rapid detection of chromium ions as well as preparation method and application thereof
InactiveCN103412125AFast detection of chromium ionsShorten detection timeMaterial analysisCellulosePhysical chemistry
The invention discloses a gold-labeled test strip for rapid detection of chromium ions as well as a preparation method and an application thereof. The gold-labeled test strip is characterized in that a bottom layer is a support layer, a middle layer is an absorption layer, a protection film is fixed on the absorption layer, the absorption layer is sequentially provided with a sample pad, a gold-labeled antibody binding pad, and a cellulose membrane layer from a test end as well as a water absorption pad at a handle end, detection prints printed by a carrier protein solution coupled with the chromium ions are arranged on the cellulose membrane layer, and contrast prints printed by rabbit-anti-mouse or goat-anti-mouse IgG (Intravenous Gamma Globulin) antibody solution are arranged on the cellulose membrane layer; colloidal gold-labeled chromium ion monoclonal antibodies are coated in the gold-labeled antibody binding pad. The gold-labeled test strip can be used for rapidly detecting pollution residues of the chromium ions in soil, water and food, has the advantages of specificity, sensitivity, rapidness, simplicity, convenience, visual and intuitional result and the like, not only can be used for screening large-batch samples, but also can be used for rapidly detecting small-batch samples and is wide in applicable range.
Owner:HENAN INST OF SCI & TECH
Magnetic bead method for quickly purifying antibody
InactiveCN103275217AMore purifiedImprove efficiencyPeptide preparation methodsImmunoglobulinsMagnetic beadMedicine
The invention relates to the biological and pharmaceutical industry, and discloses a magnetic bead method for quickly purifying an antibody. The method is characterized in that by using an affinity chromatography principle, carboxyl magnetic beads are activated, and then connected with stphylococcl protein A (SPA) capable of being combined with Fc (fragment crystalline) of IgG (intravenous gamma globulin) molecules of human and various mammals; and obtained Protein A magnetic beads can be used for purifying the antibody in serum, ascites or a cell culture fluid. The Protein A magnetic beads are high in specificity, quick and convenient in the aspect of antibody purification, and can be widely applied to the biological and pharmaceutical industry.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Method for quantitatively detecting protein through quantum dot resonant scattering
InactiveCN102565020AGood correlationImprove stabilityFluorescence/phosphorescenceProtein detectionBovine serum albumin
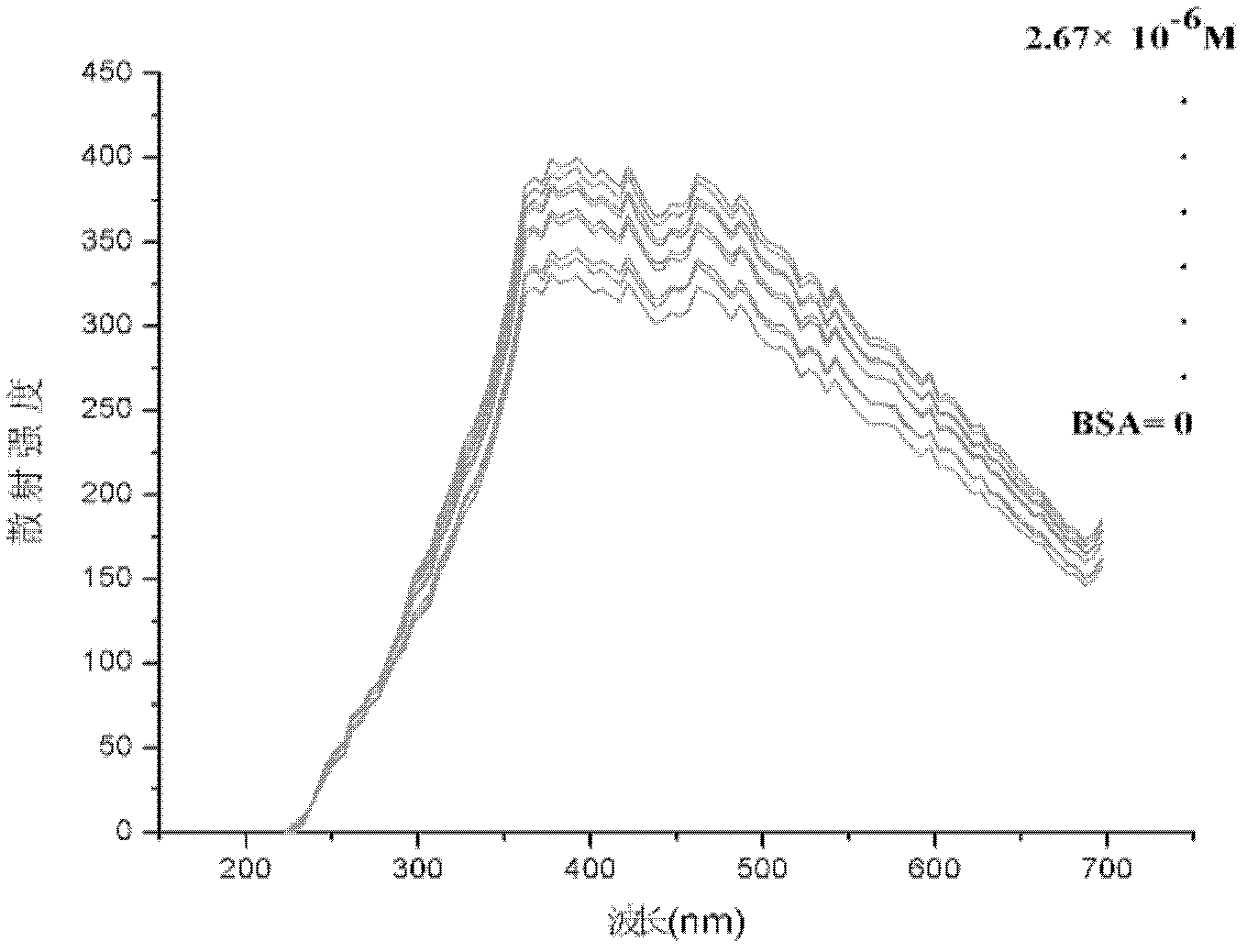
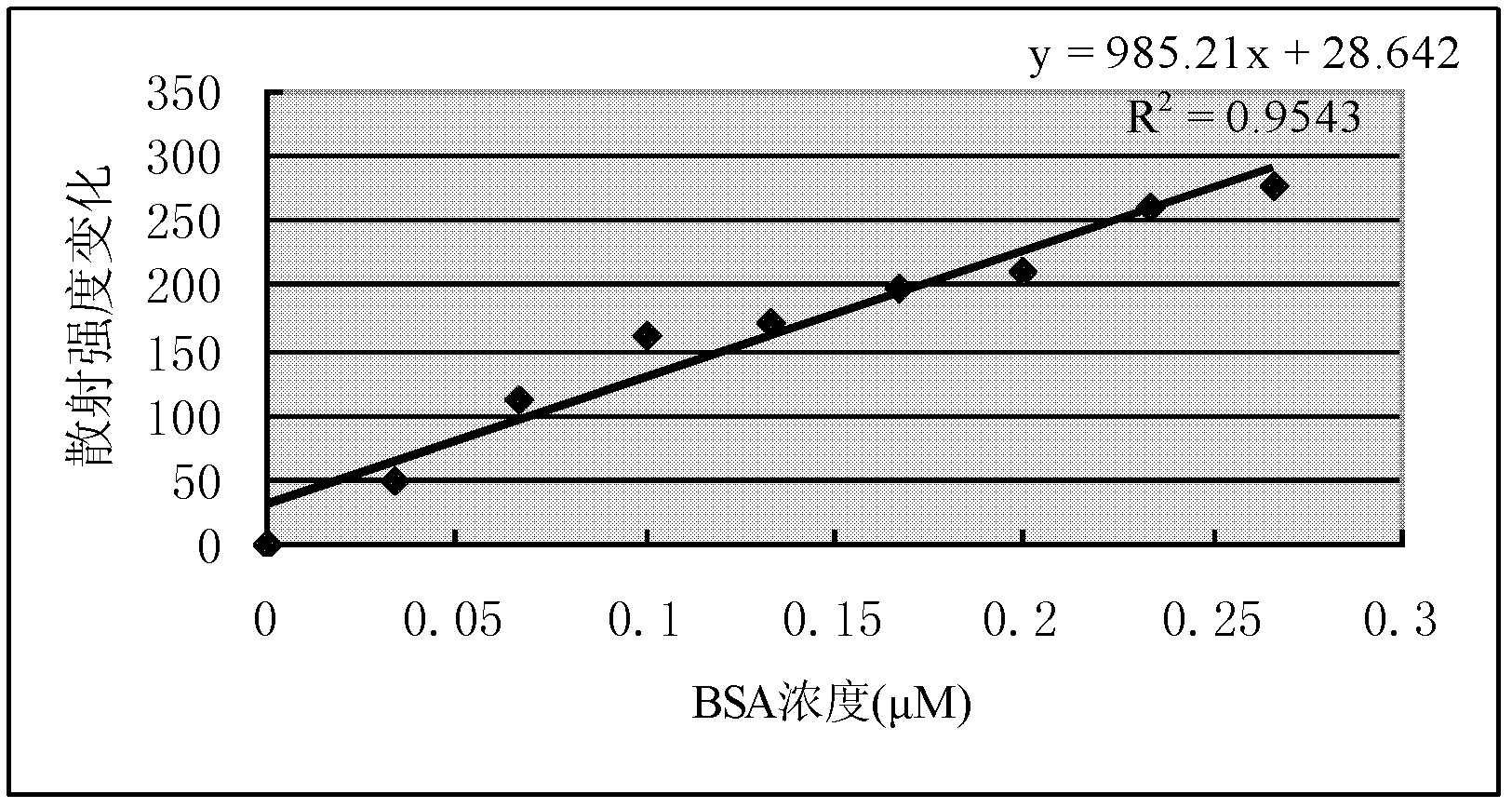
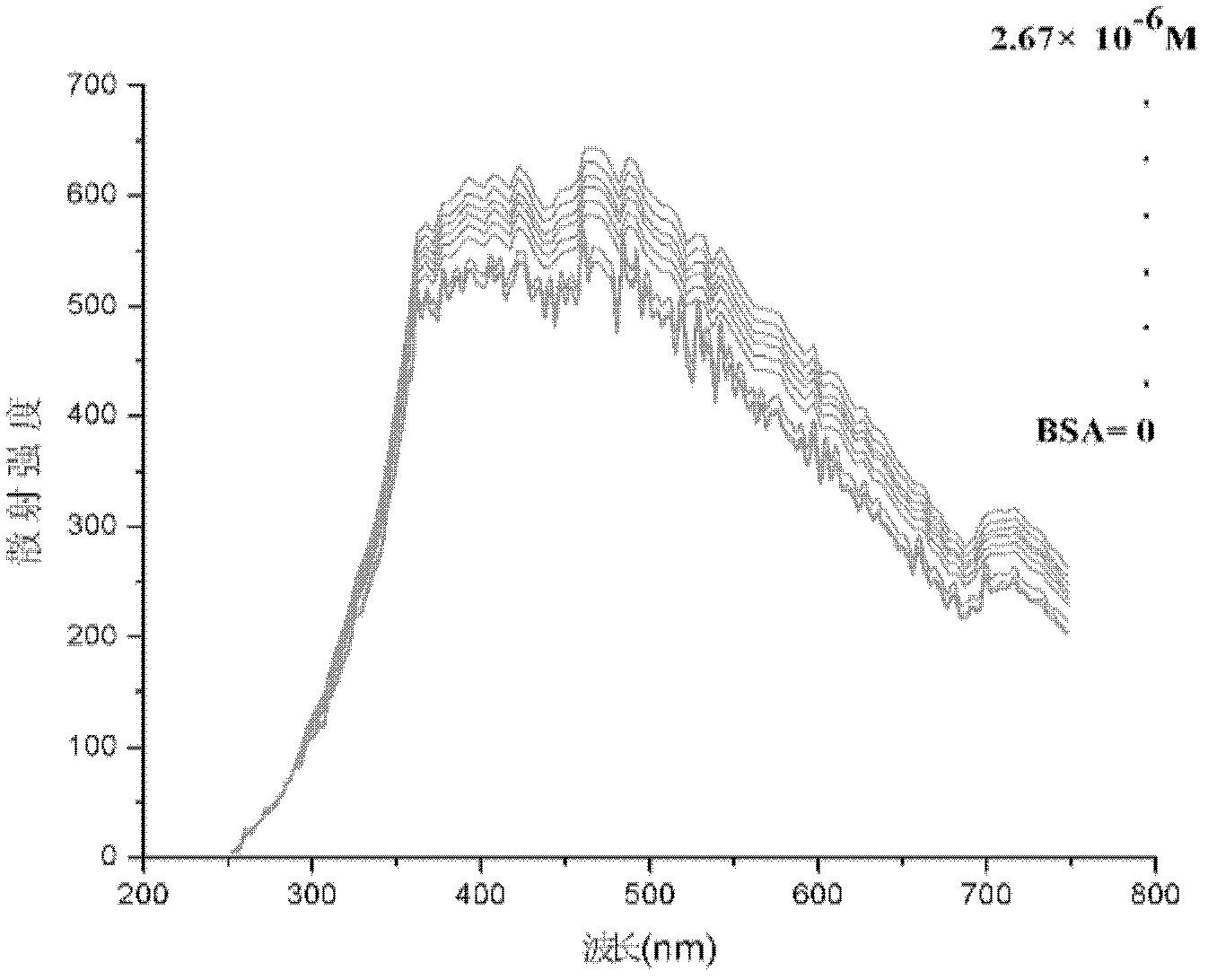
The invention discloses a method for quantitatively detecting proteins through quantum dot resonant scattering. The method is a supplement to protein detection methods. The proteins are adsorbed by using quantum dots through an electrostatic effect, resonant scattering light can be generated within a visible light wavelength range to detect standard bovine serum albumins (BSA) and gamma-globulins, a standard curve is drawn by taking the concentration (mol / L) of the proteins as a horizontal coordinate and the highest-intensity change of scattering wavelength as a vertical coordinate, and the concentration of the proteins can be calculated through a regression equation through scattering intensity change. Moreover, the quantum dots with different grain sizes are additionally used for detecting the BSA and the gamma-globulins, good correlation between the concentration of the proteins and the scattering intensity is found, the correlation coefficient reaches 0.95 to 0.99, the gradient reaches 107, the sensitivity is higher, the stability is high, the detection time does not cause a great influence on the result and a new practical method is developed for quantitatively detecting the concentration of biological macromolecular proteins.
Owner:SHANGHAI NORMAL UNIVERSITY
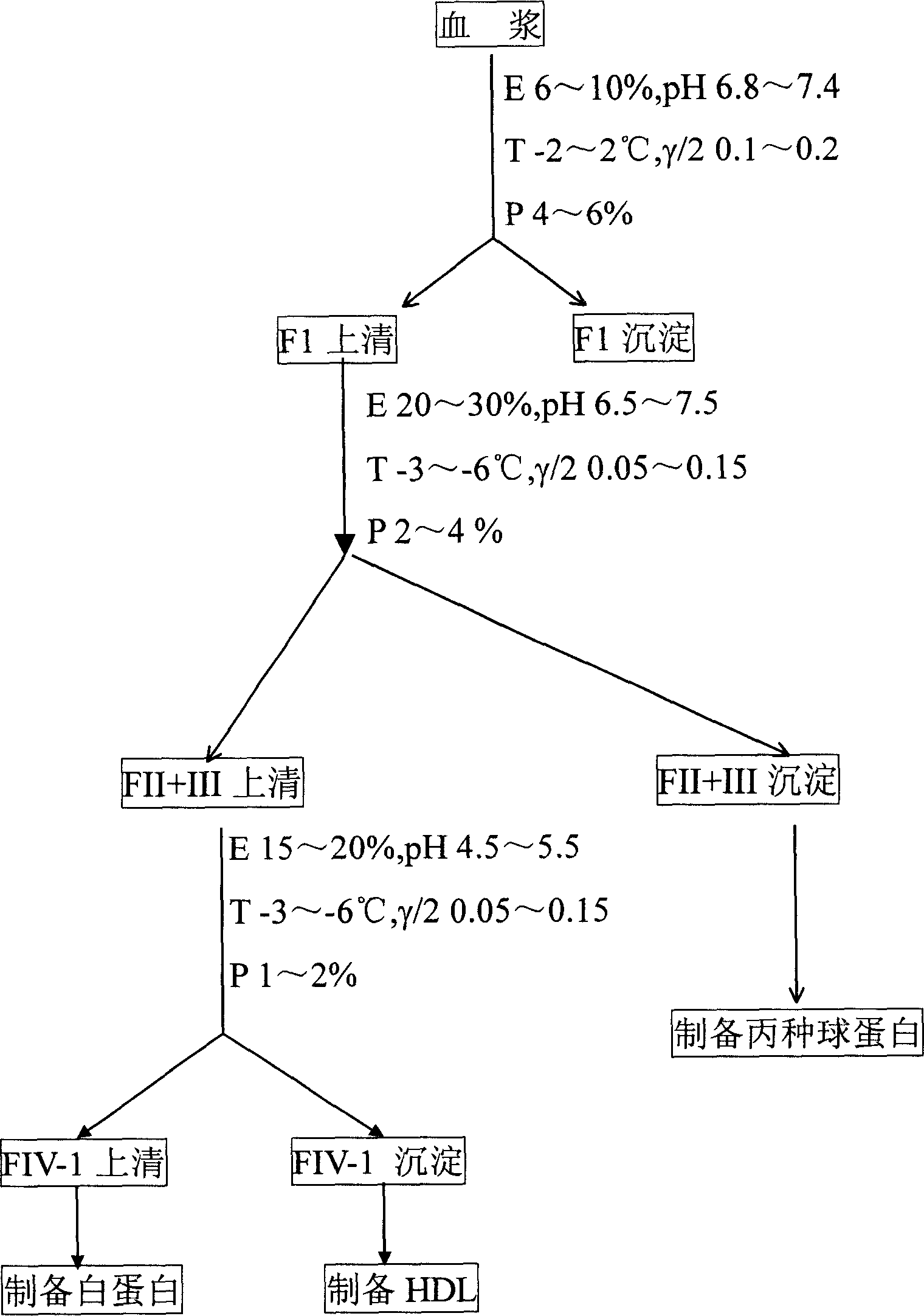
Method for recovering alhumin from deposited components I+II+III from cold ethanol method
ActiveCN102311496AEfficient recyclingGuaranteed recyclingSerum albuminPeptide preparation methodsBiotechnologyProtein solution
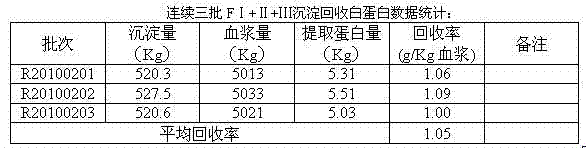
The invention relates to a method for recovering alhumin from deposited components I+II+III from the cold ethanol method. Traditional recovery methods have defects of less treating capacity of a chromatography column, large investment in solid-liquid centrifugation equipment and high energy consumption; in addition, by traditional recovery methods, only gamma globulin of the components is extracted during the extraction process, and an FII supernatant still contains much alhumin, thus influencing the extraction efficiency. The technical scheme adopted in the invention comprises the following steps of: (1) preparation of an F1 supernatant; (2) preparation of an FIII supernatant; (3) preparation of a crude alhumin solution; (4) extraction of FIII alhumin deposit; (5) preparation of V deposit; (6) impurity removal of a protein solution; and (7) preparation of an alhumin product. By the adoption of the method provided by the invention, alhumin remained in the deposited components I+II+IIIis effectively recovered, thus increasing the extraction amount of alhumin per ton blood plasma raw material by 0.5-1.0 kilograms, raising the total yielding rate of alhumin and reducing the production cost.
Owner:XIAN HUITIAN BLOOD PROD CO LTD
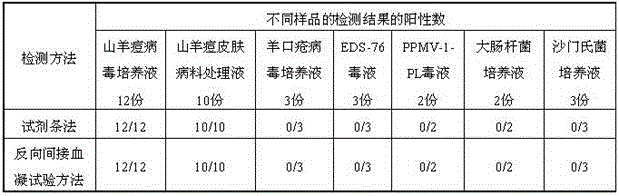
Colloidal gold test strip based on goat pox virus and preparation method thereof
The invention relates to a colloidal gold test strip based on a goat pox virus. The colloidal gold test strip comprises a PVC (Polyvinyl Chloride) rubber plate, a nitrocellulose membrane, a colloidal gold mat, a sample mat and absorbent paper, wherein the sample mat adheres to one end of the PVC rubber plate; the absorbent paper adheres to the other end of the PVC rubber plate; the colloidal gold mat and nitrocellulose membrane adhere to the middle part of the PVC rubber plate in sequence; the sample mat adheres to one end of the golden standard mat; the nitrocellulose membrane adheres to the other end of the colloidal gold mat; the nitrocellulose membrane adheres to the absorbent paper; the colloidal gold mat is coated with an anti-GTPV-P32 protein monoclonal antibody marked with colloidal gold; the nitrocellulose membrane is provided with a detection line which is linearly coated with an anti-GTPV-ORF122 protein monoclonal antibody at the concentration of 1mg / mL, and a quality control line which is linearly coated with a goat anti-mouse IgG (Intravenous Gamma Globulin) antibody at the concentration of 1mg / mL in sequence along the flow direction of a sample. Meanwhile, the invention further discloses a preparation method of the diagnosis strip. The test strip has the characteristics of high specificity, high stability, easiness in operation and rapid detection, and is suitable for field detection at the occurrence of animal epidemic diseases.
Owner:GANSU ANIMAL HUSBANDRY & VETERINARY MEDICINE INST
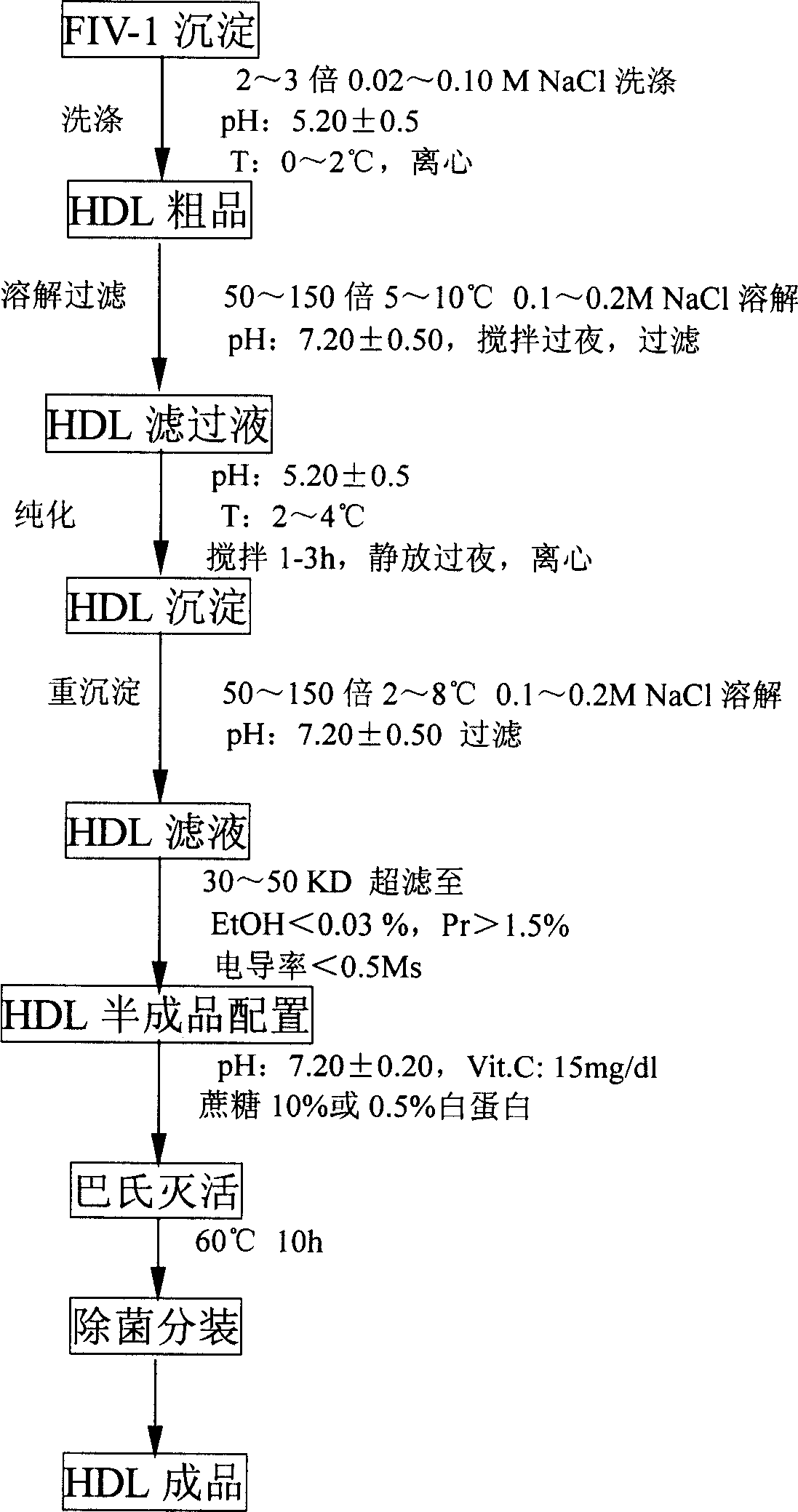
Human blood high density lipoprotein and its preparation method and use
ActiveCN1699419ATake advantage ofSafe preparationPeptide/protein ingredientsAnimals/human peptidesOrgan dysfunctionHigh-density lipoprotein
The invention relates to a human high density lipoprotein and product, preparation process and use thereof, which comprises producing human plasma albumin and gamma globulin by employing low-temperature ethanol method, using discarded FIV-1 deposition as raw material, extracting HDL from FIV-1 through separation and purification steps. The prepared human high density lipoprotein and product can be applied for the preparation of medicament for treating multiple organ disturbance syndrome (MODS) and cardiovascular diseases such as atherosclerosis (AS).
Owner:江永忠 +3
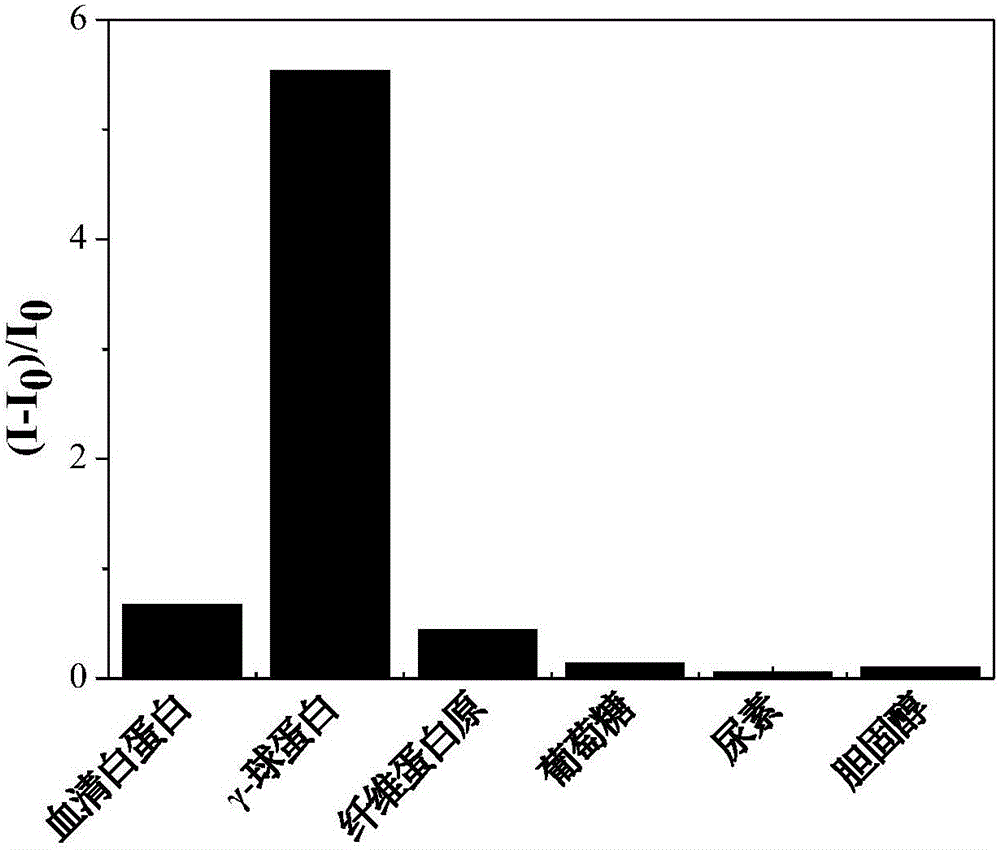
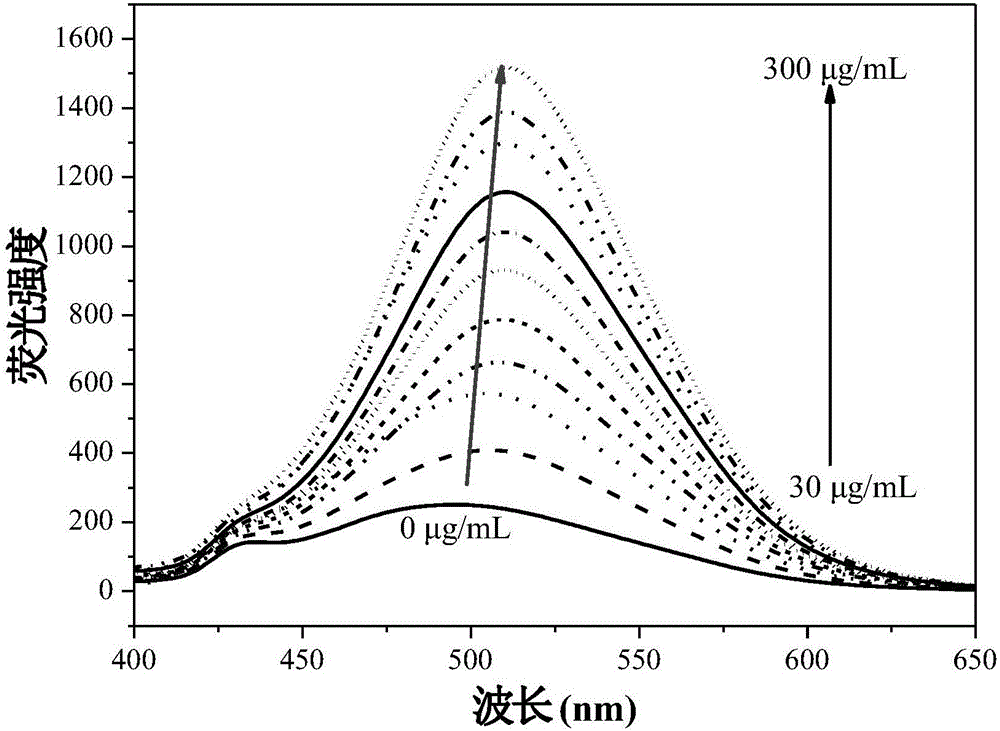
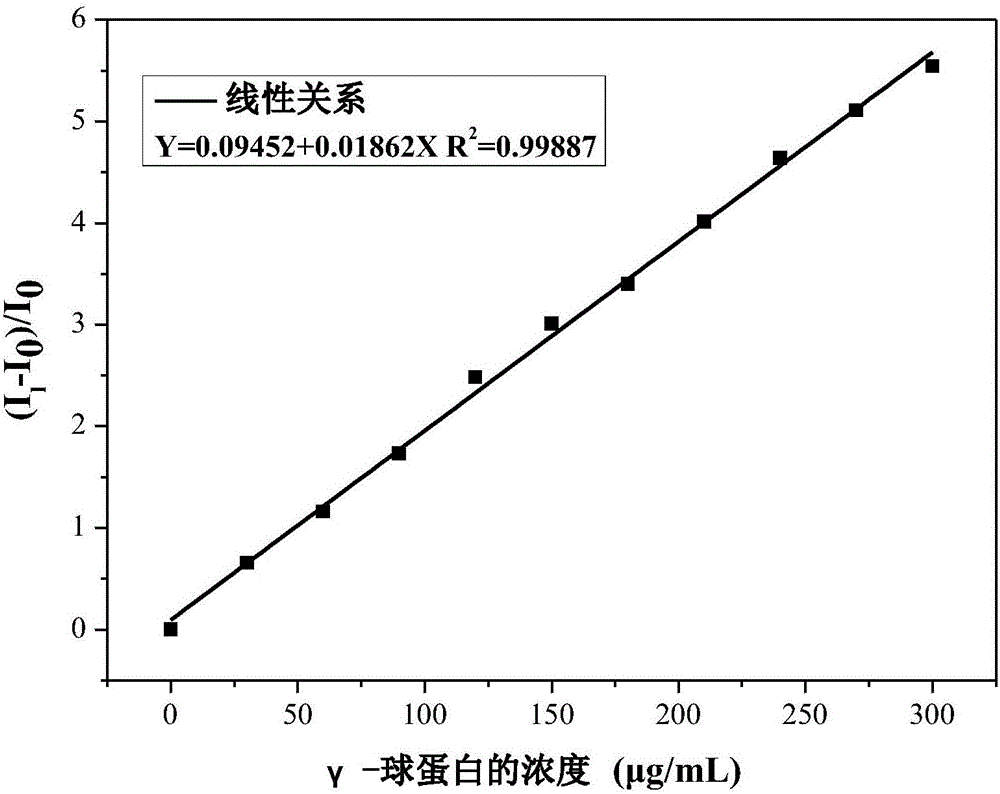
Fluorescent reagent for detecting trace gamma-globulin, as well as preparation method and application thereof
InactiveCN105778894AFluorescent signal enhancementHigh sensitivityOrganic chemistryFluorescence/phosphorescencePotassium hexafluorophosphateSolvent
The invention discloses a fluorescent reagent for detecting trace gamma-globulin, as well as a preparation method and application thereof, and belongs to the field of fluorescent bio-sensors. The fluorescent reagent is prepared from TABD-Py-PF6 and a good solvent. The preparation method comprises the following steps: dissolving (1Z,3Z)-1,4-di(4-methoxycarbonyl) phenyl-1,4-dibromo-1,3-butadiene in the good solvent, adding 4-pyridine phenylboronic acid, a catalyst and a basic salt, and reacting to obtain TABD-Py; dissolving the TABD-Py in a solvent, adding iodomethane to synthesize iodate, adding potassium hexafluorophosphate, and reacting to obtain TABD-Py-PF6; dissolving the TABD-Py-PF6 in the good solvent to obtain the fluorescent reagent. The fluorescent reagent does not need participation of metal ions, has a specific lighting-type fluorescent response on gamma-globulin, has high and quick sensitivity on gamma-globulin detection, and has high selectivity. The fluorescent reagent has an excellent visible detection signal, can be completely separated from high-precision instruments according to actual requirement, and can meet current requirement for detecting the gamma-globulin in serum to a great extent.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Novel functionalized gold nanorod immune probe as well as preparation method and application of gold nanorod biological chip
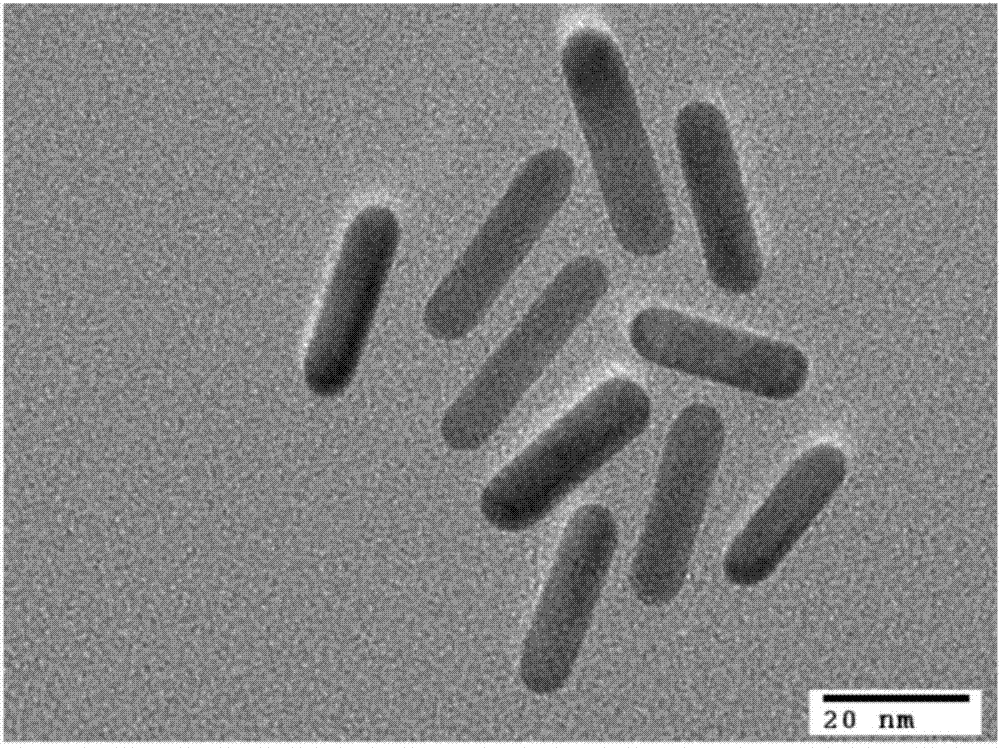
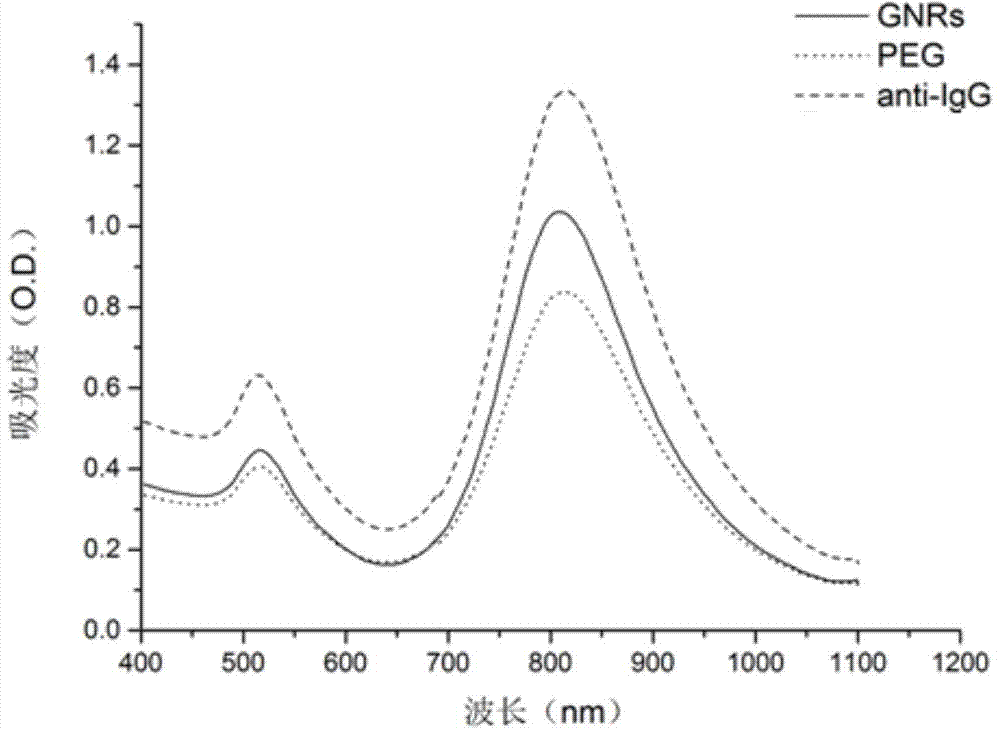
InactiveCN103575875ANot easy to accumulateThe result is stableMaterial analysisGold nanorodGamma globulin
The invention discloses a novel functionalized gold nanorod immune probe as well as a preparation method and an application of a gold nanorod biological chip. The preparation method comprises the following steps of modifying a human IgG (Intravenous Gamma Globulin) antibody by a Traut reagent; coupling the modified IgG antibody with a sulfydryl having high affinity with gold; and covalently binding the modified IgG antibody with a gold nanorod directly through -SH. The operation method is simple, the conditions are mild, the functionalization of the gold nanorod can be quickly realized, the functionalized gold nanorod fixed on a slide can be used as an unmarked biological chip for specifically detecting a human IgG antigen which can be obtained according to an offset dose-effect relationship curve of a nanorod vertical plasma absorption peak, and each nano vertical plasma absorption peak offset is capable of detecting the human IgG antigen at 137 pM. Therefore, the gold nanorod immune probe prepared by the method and used for antigen-antibody detection has the advantages of simple operation, high detection sensitivity, good specificity and a small number of needed instruments and equipment and is clinically popularized possibly.
Owner:ZHENJIANG NO 1 PEOPLES HOSPITAL +1
Method for determining antiproteinase 3 antibody IgG (intravenous gamma globulin) and reagent device
The invention provides a method for realizing immunization detection of antiproteinase 3 antibody IgG (intravenous gamma globulin) based on the enzyme-linked immunoassay principle and a reagent device therefor; and an analytic method, the reagent device and an assorted reagent are independently, individually and disposably used for detecting the antiproteinase 3 antibody IgG based on enzyme-linked immunoassay. Various reagents needed by the enzyme-linked immunoassay for the antiproteinase 3 antibody IgG are contained in one analytic device; and by using the method, the related immunology detection can be conveniently carried out according to the using requirements of detection items so as to provide better basis for clinical application.
Owner:SHENZHEN YHLO BIOTECH
Enzyme-linked immuno sorbent assay (ELISA) kit created on the basis of hog cholera virus recombinant protein nopaline synthase (NS2)
The invention relates to an enzyme-linked immuno sorbent assay (ELISA) kit created on the basis of hog cholera virus recombinant protein nopaline synthase (NS2). The ELISA kit comprises an elisa plate wrapped by the hog cholera virus recombinant protein NS2, a ten-time concentration detergent, a serum diluent, a standard serum, an anti-swine Intravenous gamma globulin (IgG) enzyme-labeled antibody, an nzyme-labeled antibody diluent, a sealing agent, a color developing agent A, a color developing agent B and a stop solution. The ELISA kit can detect hog cholera virus antibodies. The method created by the ELISA kit has specificity, sensitivity and operability. In a sensitivity test, the coincidence rate between the method of the ELISA kit and an immunofluorescence technical method is 82%, and the method of the ELISA kit is more sensitive than the immunofluorescence technical method. The detection rate of other etiological agents through the method of the ELISA kit is zero. Compared with the hemagglutination inhibition (HI) method, the method of the ELISA kit has the advantages of being fast and low in cost, and having easily judging results, and can carry out detection work after hog cholera lapinized virus vaccine immunity.
Owner:ZHENGZHOU HOUYI PHARMA
Method for detecting human myocardial troponin T through cytometric bead array
The invention discloses a method for detecting human myocardial troponin T through cytometric bead array. The method comprises the following three steps of: activating a bead, coupling a monoclonal antibody and detecting the human myocardial troponin T, specifically, firstly, activating the beads; secondly, preparing the monoclonal antibody of the carboxylation bead coupled mouse anti human myocardial troponin T; thirdly, capturing the human myocardial troponin T in a specimen; and finally, adding multiple antigenic and fluorescein isothiocyanate labeled donkey anti goat intravenous gamma globulin G of goat anti human myocardial troponin T, and detecting the fluorescence intensity of fluorescein isothiocyanate (FITC) on the bead on a flow cytometry to indirectly detect the content of the human myocardial troponin T in the specimen. The monoclonal antibody coupled bead, the polyclonal antibody of the goat anti human myocardial troponin T, and the fluorescein isothiocyanate labeled donkey anti goat intravenous gamma globulin (IgG) can be prepared into a commodity kit, which can detect the human myocardial troponin T at any time. The method is simple and feasible; and by the method, the human myocardial troponin T can be quickly detected with convenience and the basis for disease diagnosis is provided.
Owner:贵州省临床检验中心
Secondary sicca syndrome epitope and application thereof
The invention discloses a secondary sicca syndrome epitope and an application thereof and belongs to the technical field of immunological diagnoses. The epitope is a polypeptide, and the amino acid sequence of the epitope is shown as SEQ ID NO: 1 in the sequence table. By means of an enzyme-linked immunosorbent assay (ELISA), reaction conditions of the epitope polypeptide and patient serums are detected; and according to the detection, the epitope polypeptide can be specifically combined with intravenous gamma globulin (IgG) in the patient serums instead of reacting with serums of healthy persons. The epitope polypeptide can be used for preparing drugs for diagnosing secondary sicca syndromes.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Immunofluorescence test strip component for quickly and quantitatively detecting myocardial creatine kinase isozyme, detection card component comprising same and preparation method
An immunofluorescence test strip component for quickly and quantitatively detecting myocardial creatine kinase isozyme comprises a test strip and specific antibodies with platinum-porphyrin marks, the specific antibodies are used with the test strip and are independently packed, the test strip comprises a bottom liner, an absorbent pad, an envelope analysis membrane and a sample pad, the absorbent pad, the envelope analysis membrane and the sample pad are sequentially jointed on the bottom liner, a detection line and a quality control line are disposed on the envelope analysis membrane, the specific antibody enveloped by the detection line is a monoclonal antibody resisting the myocardial creatine kinase isozyme, the specific antibody enveloped by the quality control line is a rabbit IgG (intravenous gamma globulin) antibody, and accordingly the specific bodies with the platinum-porphyrin marks are the monoclonal antibody resisting the myocardial creatine kinase isozyme and the antibody resisting the rabbit IgG. The invention further discloses a preparation method for the detection test strip component, a detection card comprising the test strip component and a preparation method of the detection card. The detection test strip component can simply, quickly and sensibly detect the level of the myocardial creatine kinase isozyme in a human body, and is used for diagnosing acute myocardial infarction.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Anti-LKM (liver-kidney microsomal) 1 antibody detection kit and detection method
The invention discloses an anti-LKM (liver-kidney microsomal) 1 antibody detection kit and a detection method. The kit comprises biotinylation LKM-1 antigen, a calibrator, a quality control material, an AP (alkaline phosphatase)-anti-human IgG (intravenous gamma globulin) antibody, a magnetic particle reagent, a chemiluminescent substrate and a cleaning liquid. According to the detection method, the reagent in the kit is used for detection of the sample. With the adoption of the scheme, a full-automatic magnetism particulate chemiluminescent detection method is adopted for selection of an alkaline phosphatase AP-adamantane AMPPD system, and the kit has the advantages of good stability, high sensitivity, good repeatability and the like; meanwhile, the detection time is greatly shortened, the total time required for one test is about 50 minutes, the operation is simple and convenient, and full-automatic detection is really realized.
Owner:SUZHOU HAOOUBO BIOPHARML
High-sensitivity food allergen detecting method and detecting kit
ActiveCN103616520AStrong enrichment abilityHigh sensitivityBiological material analysisBiological testingBiotechnologyFood allergen
The invention discloses a high-sensitivity food allergen detecting method and a detecting kit. The detecting method comprises the following steps: (1) coupling food allergen proteins with magnetic beads, wherein food allergens include but are not limited to food allergen proteins of aquatic products, milk, eggs, meat, cereals, nuts, fruits, vegetables and beans; (2) building a standard curve, namely diluting an anti-food-allergen IgG (Intravenous Gamma Globulin) antibody standard substance in a gradient manner, immunologically reacting the diluted standard substance with the food allergen protein coupled magnetic beads, performing signal amplification by an anti-human IgG secondary antibody, and performing linear regression according to an average absorbance value and corresponding concentration to build the standard curve; and (3) screening food allergens, namely immunologically reacting the food allergen protein coupled magnetic beads with serum of an allergy patient, performing signal amplification by the anti-human IgG secondary antibody, and then determining the types of the food allergens as well as the concentration of the corresponding antibody. The range of sensitivity is not higher than 2pg / mL, and the method and the kit can be applicable to detection on allergens of genetically modified foods.
Owner:浙江天科高新技术发展有限公司 +1
Ultra-high yield intravenous immune globulin preparation
InactiveUS20070049732A1Reduce energy costsHigh yieldSerum albuminImmunoglobulins against animals/humansAlcohol freeFractionation
An efficacious large-scale alcohol-free plasma fractionation production process which produces a high-yielding, non-denatured, double viral-inactivated intravenous human immune gamma globulin (IgG) product. The process employs sodium citrate in two initial fractionation steps, followed by diafiltration to remove sodium citrate.
Owner:PLASMA TECH LLC
Receptor-based immunochromatography test strip for detecting Beta-lactam antibiotics and preparation method of test strip
ActiveCN103983775AEnables multi-residue detectionRapid Field DetectionMaterial analysisBiotechnologyCellulose
The invention discloses a receptor-based immunochromatography test strip for detecting Beta-lactam antibiotics and a preparation method of the test strip, which belong to the technical field of immunological detection. The test strip comprises a PVC (polyvinyl chloride) back lining, wherein the front end of the PVC back lining is provided with a sample pad which is connected with the front end of a cellulose nitrate membrane; the rear end of the cellulose nitrate membrane is connected with a water absorption pad; a combined microporous board is enveloped with a mixture of an anti-Beta-lactam antibiotic receptor-colloidal gold marker and a mouse negative serum-colloidal gold marker; the cellulose nitrate membrane is sequentially enveloped with an ampicillin-BSA (Bovine Serum Albumin)) detection line and a goat anti mouse IgG (Intravenous Gamma Globulin) control line. By virtue of the immunochromatography suitable for on-site detection, the multi-residue detection on at least fifteen Beta-lactam antibiotics can be achieved; the detection is fast and only needs 5-10 minutes; the test strip is convenient to carry, suitable for on-site detection, and simple and convenient to operate without needing professionals.
Owner:无锡迪腾敏生物科技有限公司 +1
Reagent card for semiquantitatively detecting salbutamol
InactiveCN102507944AStrong specificityImprove accuracyMaterial analysisAntigenGas chromatography–mass spectrometry
The invention relates to a reagent card for semiquantitatively detecting salbutamol. The reagent card comprises a shell and a test paper strip which is arranged in the shell; the test paper strip comprises a substrate polyvinyl chloride (PVC) plate, a detection reaction part which is adhered to the central part of the substrate PVC plate, a colloidal gold pad and a water adsorption pad which are adhered to left and right ends of the detection reaction part respectively, and a sample pad which is adhered to the left end of the colloidal gold pad; and the detection reaction part is a nitrocellulose membrane; and the surface of the detection reaction part is sequentially provided with more than two detection lines coated with conjugated antigen for detecting the salbutamol, and a quality control line coated with goat anti mouse intravenous gamma globulin (IgG) from left to right, wherein the concentration of conjugated antigen is gradually reduced. The reagent card is high in specificity and accuracy, and any concentration range out of the detection limit of the reagent card for semiquantitatively detecting the salbutamol can be detected according to actual requirements. The correlation of the detection result of the reagent card, and detection results of gas chromatography-mass spectrometry (GC-MS), high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS) and other methods fully meet the requirements, special operation staff are not needed, and the reagent card is suitable for field detection and is high in detection speed.
Owner:BIOLOGY INST OF HEBEI ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com