Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1599 results about "Antibody detection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody detection is carried out in laboratories and in the field to examine antibody and antigen bondings and identify an antibody by its particular color change when an enzyme reacting substrate molecule is linked to it while still bound to the antigen.
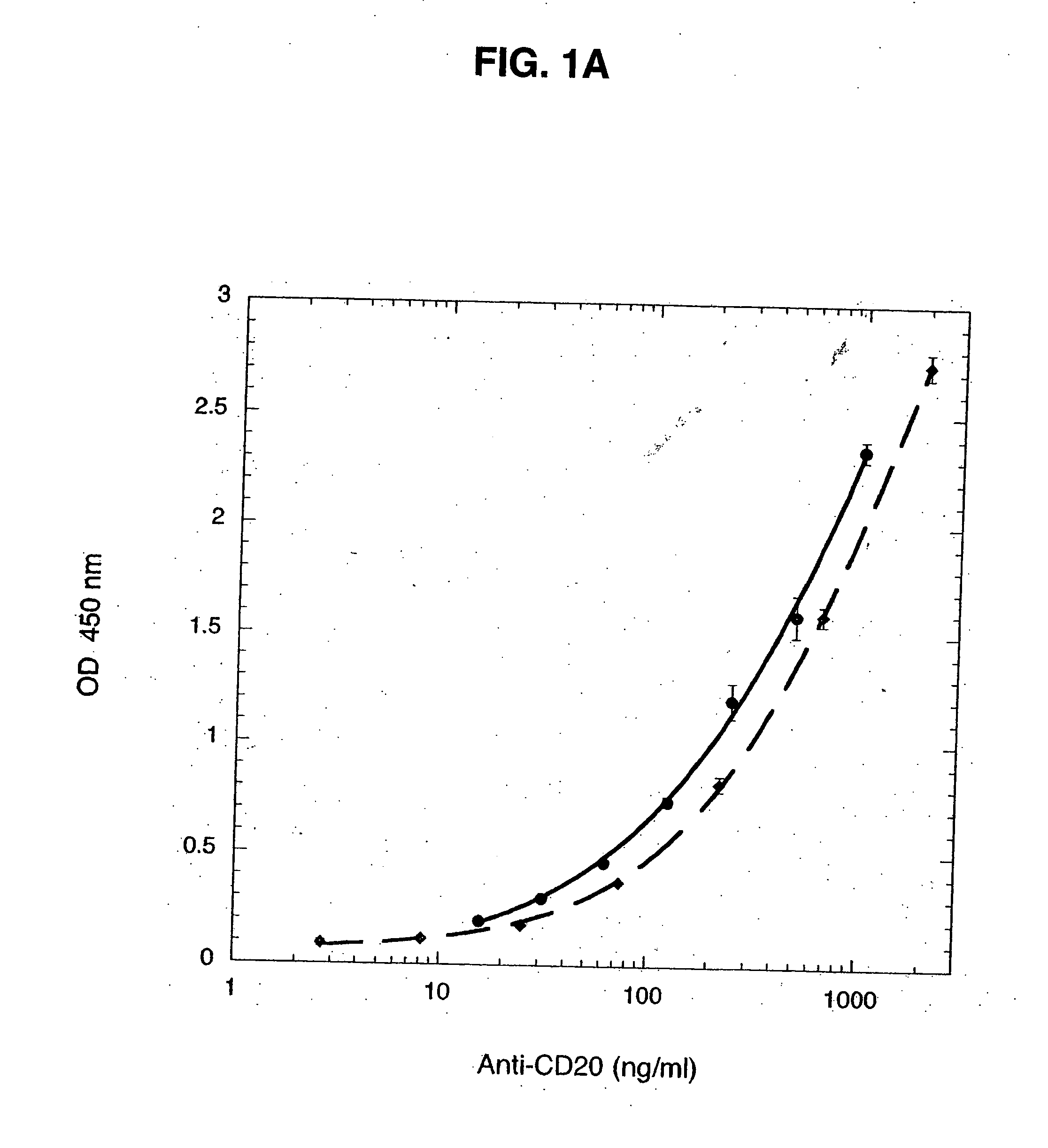
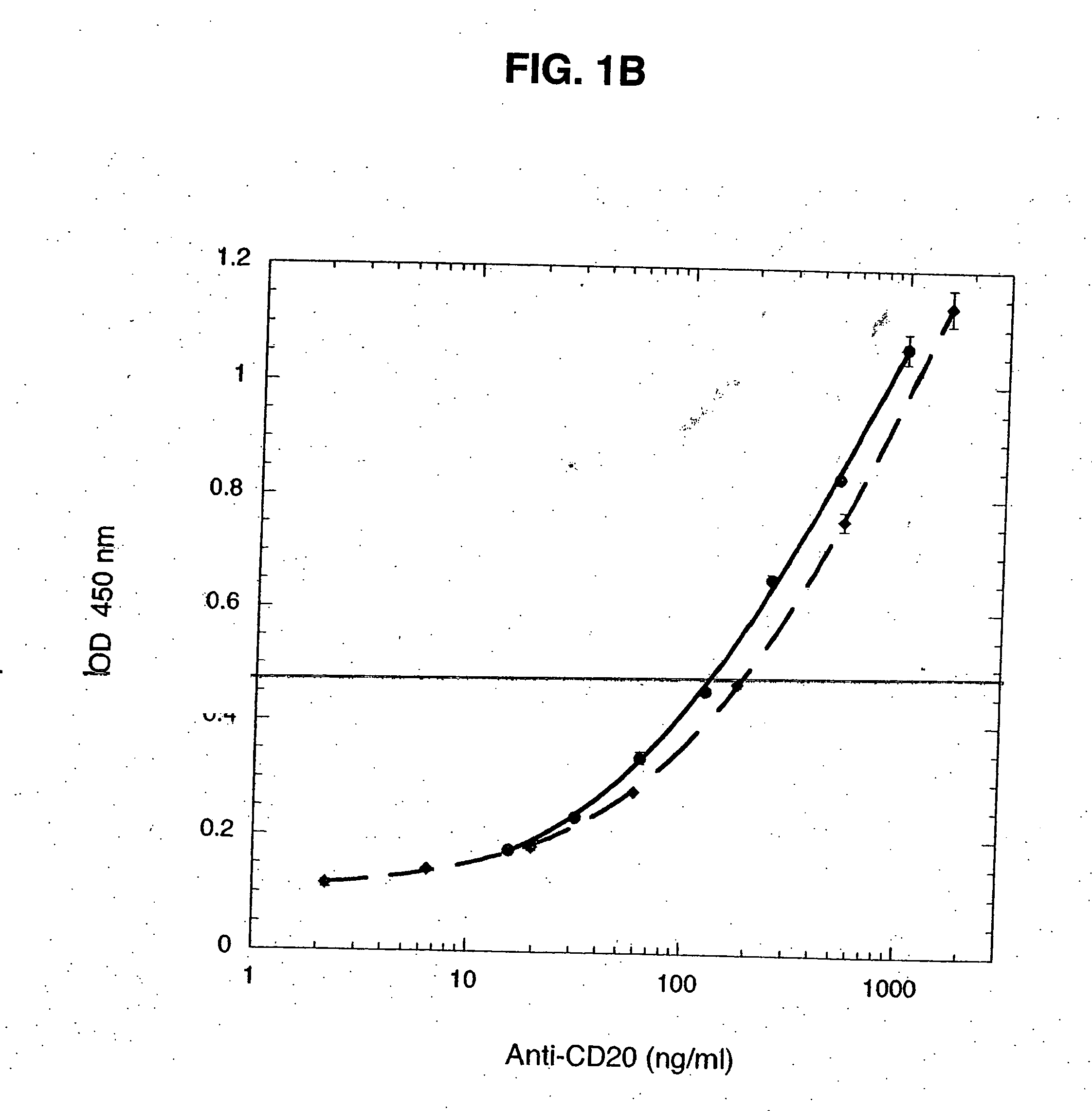
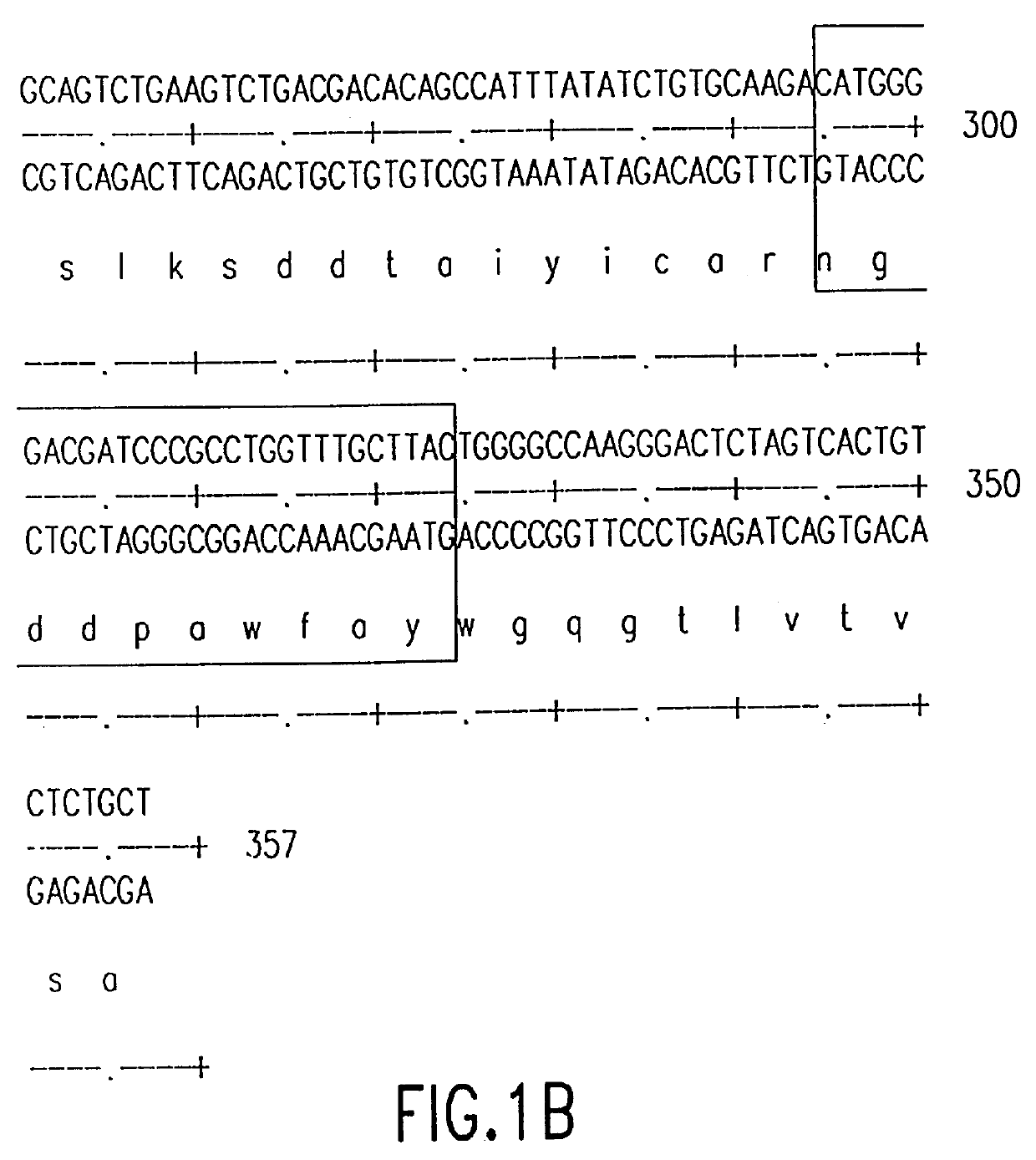
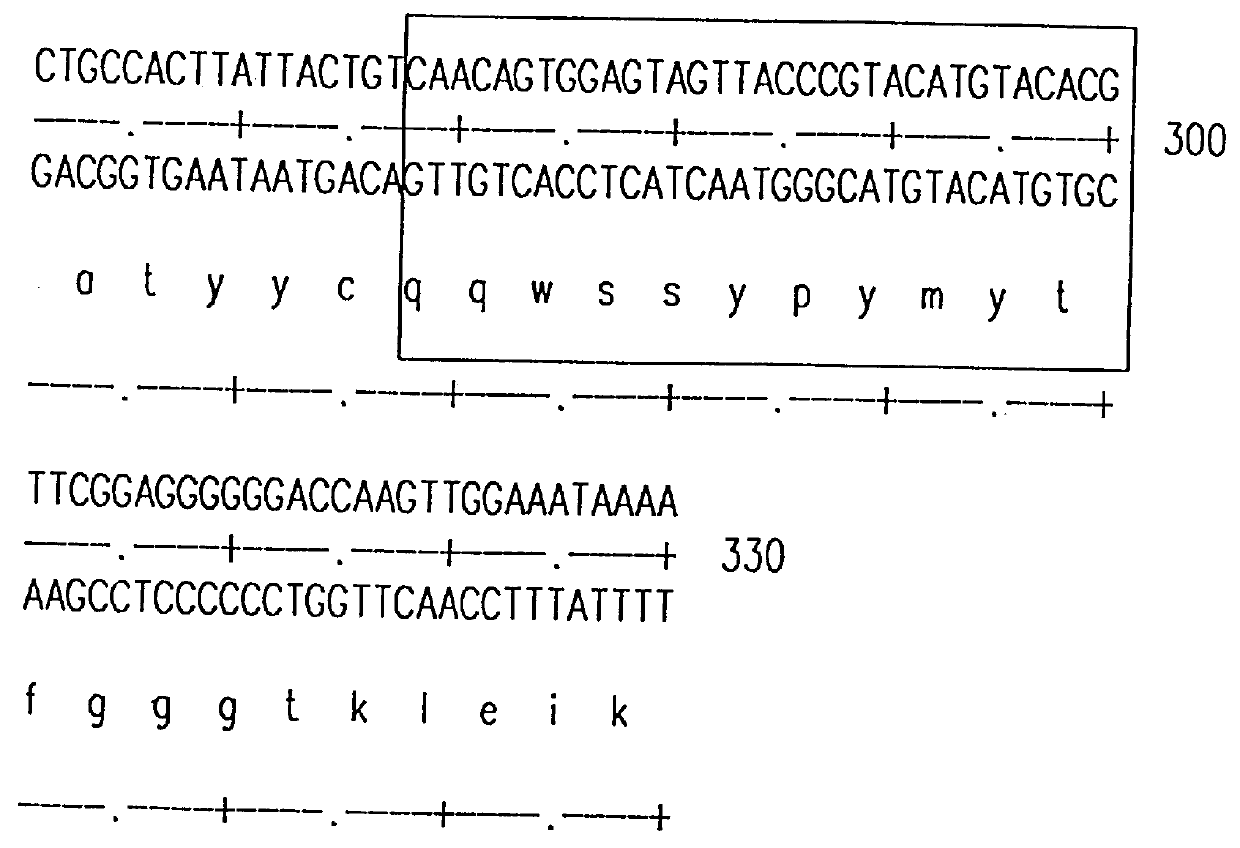
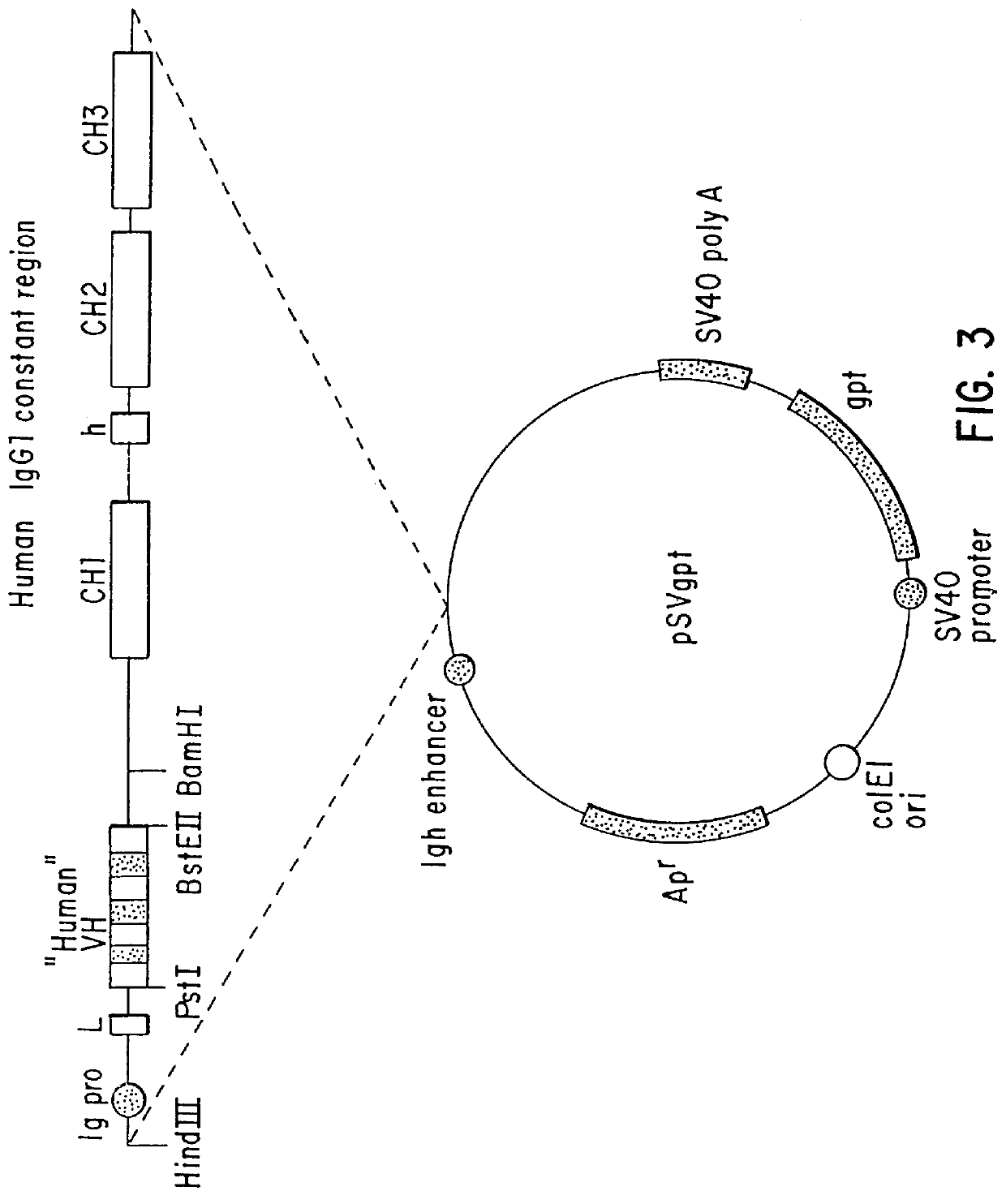
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM') of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM' allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
Method for screening taste-modulating compounds
The invention provides nucleic acid and amino acid sequences for a novel family of taste transduction G-protein coupled receptors, antibodies to such receptors, methods of detecting such nucleic acids and receptors, and methods of screening for modulators of taste transduction G-protein coupled receptors.
Owner:RGT UNIV OF CALIFORNIA +1
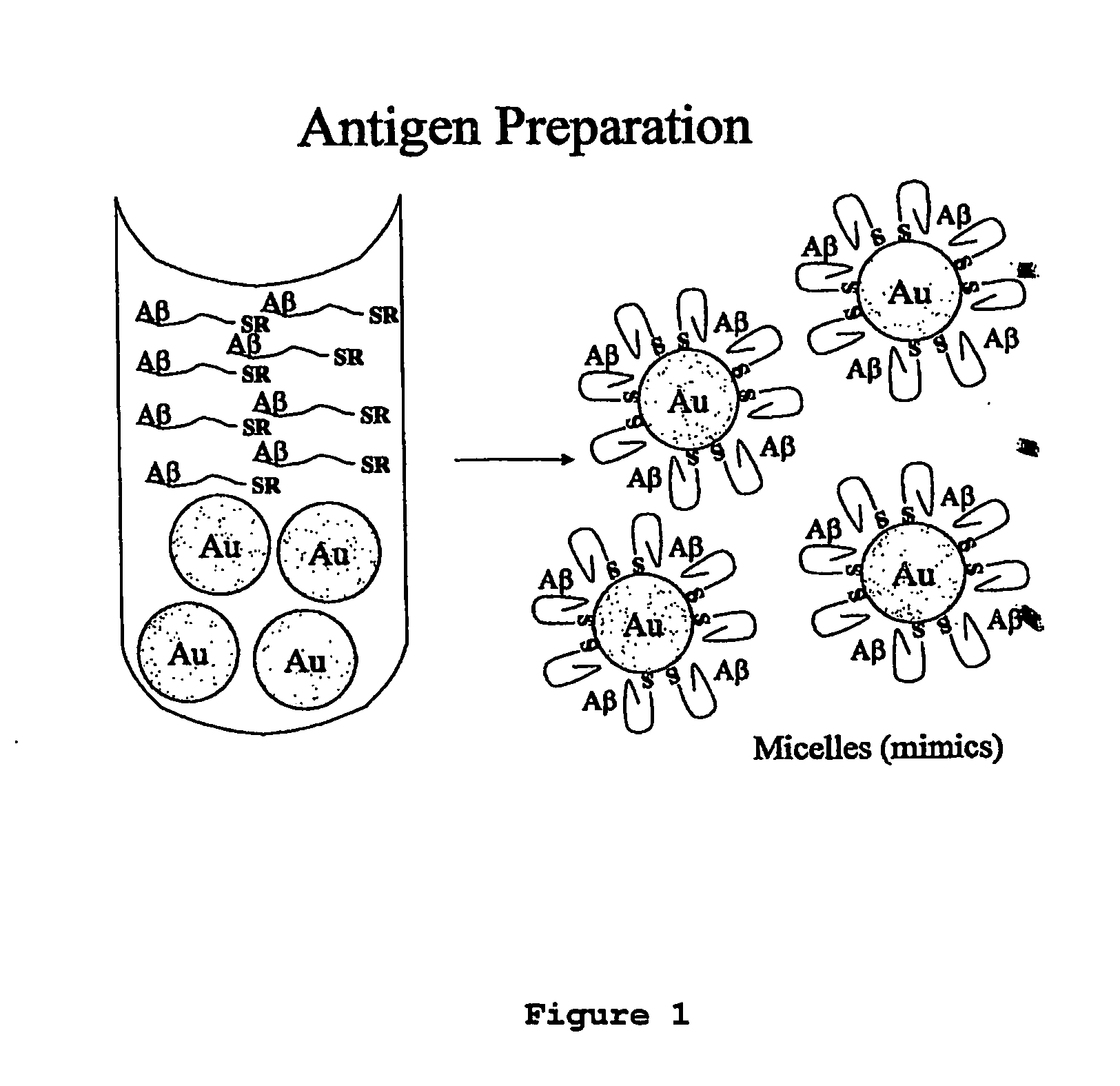
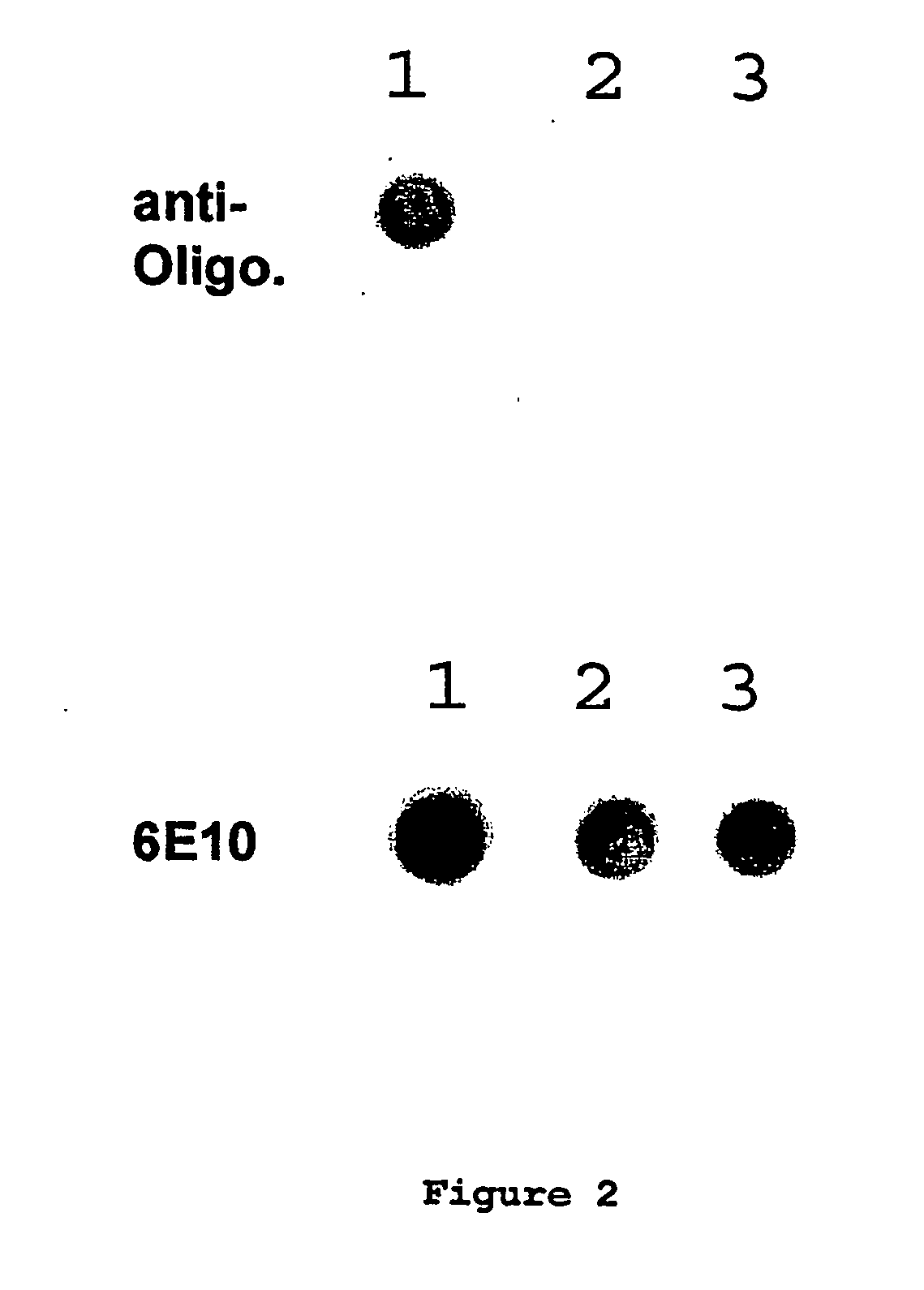
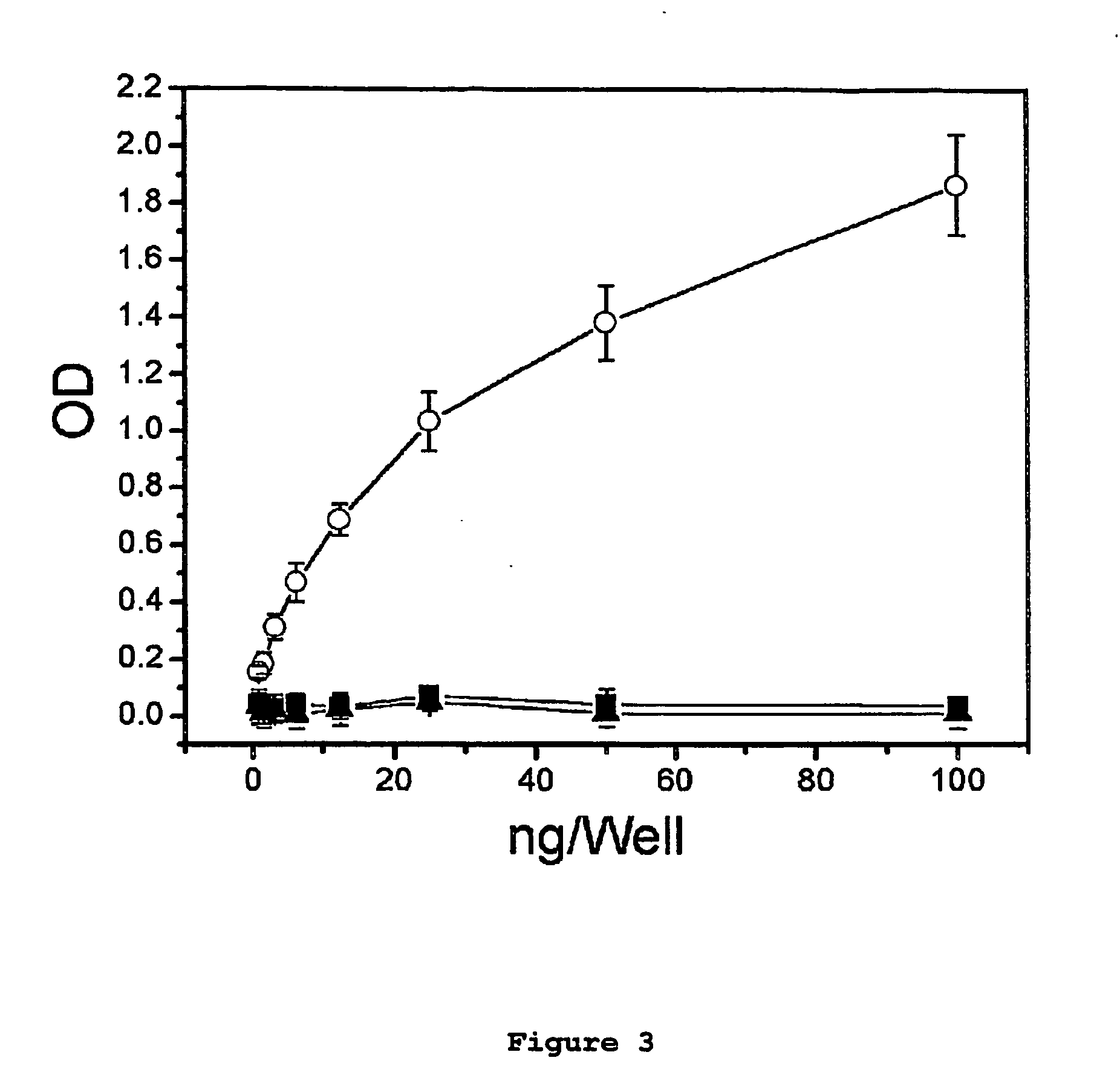
Immunogens and corresponding antibodies specific for high molecular weight aggregation intermediates common to amyloids formed from proteins of differing sequence
ActiveUS20060280733A1Low toxicityNervous disorderPeptide/protein ingredientsHigh molecular massAmyloid disease
Compositions of matter that comprise one or more conformational epitopes found on amyloid peptide aggregates, antibodies to such epitopes and methods for making and using the compositions, eptitopes and / or antibodies. The invention includes synthetic or isolated compositions that contain or consist of certain conformational epitopes that are found on peptide aggregates (e.g., toxic peptide aggregates) present in human or veterinary patients who suffer from, or who are likely to develop, amyloid diseases (e.g., Alzheimer's Disease). The invention includes methods for the detection, treatment and prevention of diseases in humans or animals, using such compositions. The invention further includes antibodies which bind to the conformational epitopes as well as methods for making such antibodies and methods for the detection, treatment and prevention of diseases and / or identification of potential therapies (e.g., drug screening) using such antibodies.
Owner:RGT UNIV OF CALIFORNIA
Monitoring of gene expression by detecting hybridization to nucleic acid arrays using anti-heteronucleic acid antibodies
InactiveUS6232068B1Nucleotide librariesMicrobiological testing/measurementHybridization probeHybridization Array
Owner:TULARIK INC +1
Detection kit of IgM/IgG antibodies of novel coronavirus (SARS-CoV-2)
ActiveCN111187354AImprove response accuracyOvercoming cumbersome detection operationsSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeCoronavirus antibody
The invention provides a detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), and relates to the technical field of biology. According to the detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), the IgM / IgG antibodies of novel coronavirus (SARS-CoV-2) are detected through adoption of a colloidal gold capture method, a colloidal gold indirect method and a colloidal gold double antigen sandwich method, wherein adopted antigens are highly active recombinant antigens which are obtained through fusion expression of N protein fragments and S protein dominant epitope fragments, and colloidal gold is labeled indirectly, so that steric hindrance is reduced, and the activity, reaction consistency and accuracy of the antigens are increased. Since a sample pad is pretreated, the interference of complex components in a blood sample to a reaction can be reduced greatly, the detection kit has no requirements for instruments during detection, simple and flexible operation, direct and manual interpretation of results and a short test time, the results can be interpreted in only 15 minutes, and the test results are accurate.
Owner:北京新创生物工程有限公司
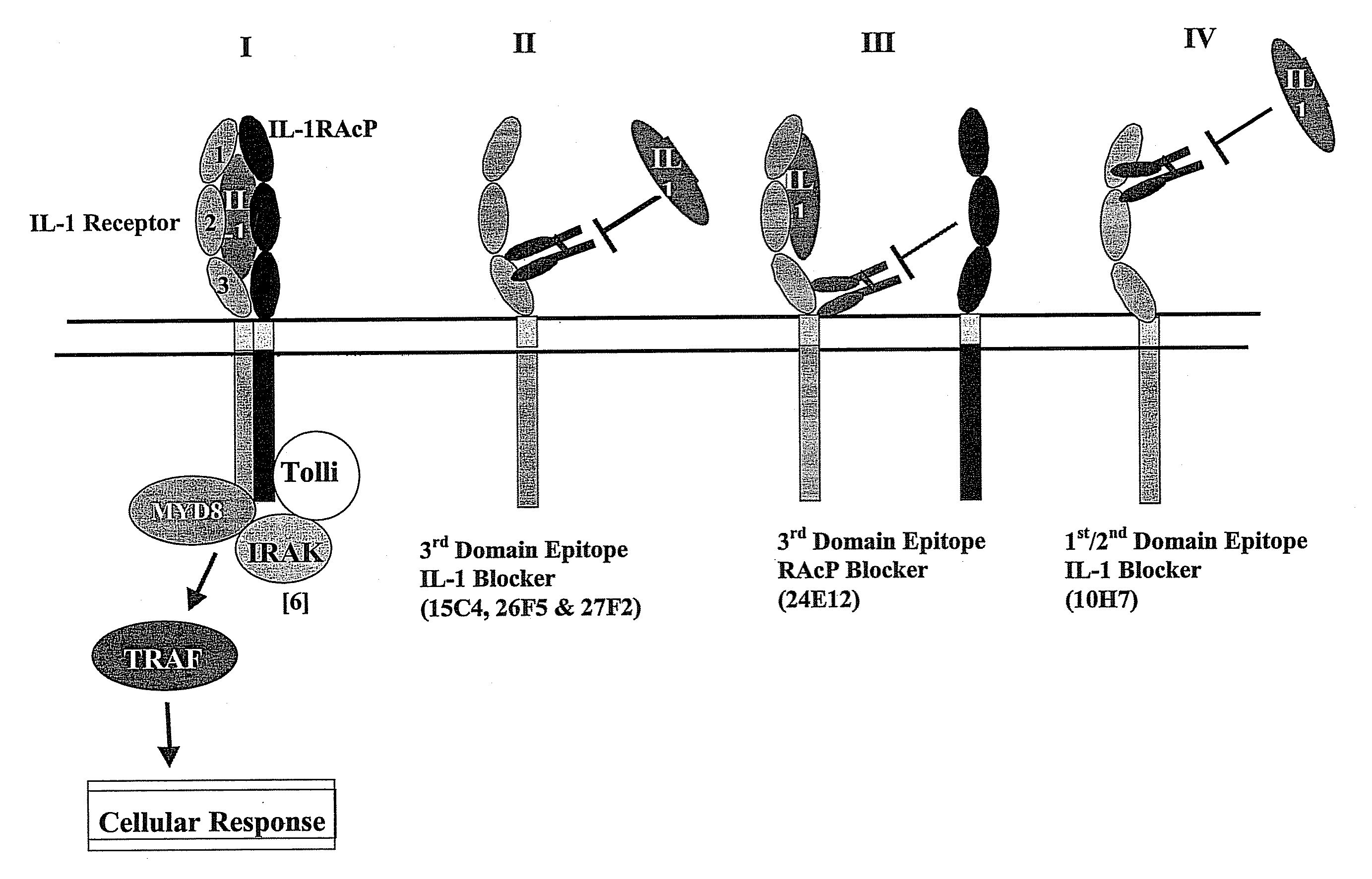
Therapeutic human anti-IL1-R1 monoclonal antibody
Antibodies that interact with interleukin-1 receptor type 1 (IL-1R1) are described. Methods of treating IL-1 mediated diseases by administering a pharmaceutically effective amount of antibodies to IL-1R1 are described. Methods of detecting the amount of IL-1R1 in a sample using antibodies to IL-1R1 are described.
Owner:ER SQUIBB & SONS INC
Milk allergen test plate and preparation method thereof
ActiveCN102636650AReduce medical costsSave medical resourcesBiological testingBeta lactoglobulin AbImmune complex deposition
The invention discloses a milk allergen test plate, which belongs to the gold immunochromatography detection. According to the milk allergen test plate disclosed by the invention, two ends of a PVC (polrvinyl chloride) base plate are respectively provided with a to-be-tested sample zone and an absorption zone. Colloidal gold mark antigens prepared by respectively marking colloidal gold into casein, beta lactoglobulin and alpha lactalbumin and mixing are orderly arranged between the sample zone to be tested and the absorption zone, and a nitrocellulose membrane is respectively provided with a detection zone coated with mixed milk allergen and a quality control zone coated with anti-beta lactoglobulin antibodies. In detection, color lines are formed in the detection zone and the quality contol zone when an immune complex is formed by specific milk antibodies contained in samples. If the samples do not contain specific milk antibodies, the detection zone does not display color, and only one color line is formed in the quality control zone. The milk allergen test plate disclosed by the invention with the design has the advantages of strong pertinence to the allergen detection, simplicity in operation, low cost and high sensitivity and the like, and can prevent the phenomenon of missed diagnosis in the single antibody detection. The milk allergen test plate is applied for rapidly screening patient allergic to milk, and is especially suitable for being used by primary medical treatment units.
Owner:江苏迈源生物科技有限公司
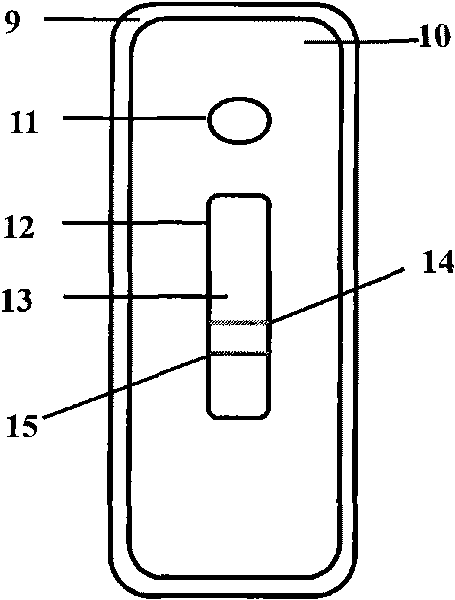
Device for simultaneously carrying out blood group determination, serum cross-check and antibody detection test
ActiveUS7745228B2Practical and easily mannerFunction increaseBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteQuantitative determination
This invention relates to a device for the simultaneous qualitative or quantitative determination of several analytes in a liquid sample. The device comprises a membrane with a charging zone, for the application of the liquid sample, at least two indicator zones which can interact with the analyte(s) and at least one absorption region, which accepts the fluid after passing through the indicator zones, whereby the indicator zones lie between the charging zone and an absorption region, characterized in that the flow directions (flow tracks) are essentially parallel from the application zone through each indicator zone to an absorption region and at least two different flow tracks are present. The invention further relates to a method for the determination of several analytes or derivatives thereof in a liquid sample, comprising: application of the sample to the charging zone of a membrane of the device, whereby said sample is present in sufficient amounts to permit the sample fluid to flow in the direction of the absorption region through the indicator zones and to permit the analytes or derivatives thereof in the liquid sample to form a complex in the indicator zone.
Owner:GRIFOLS DIAGNOSTIC SOLUTIONS INC
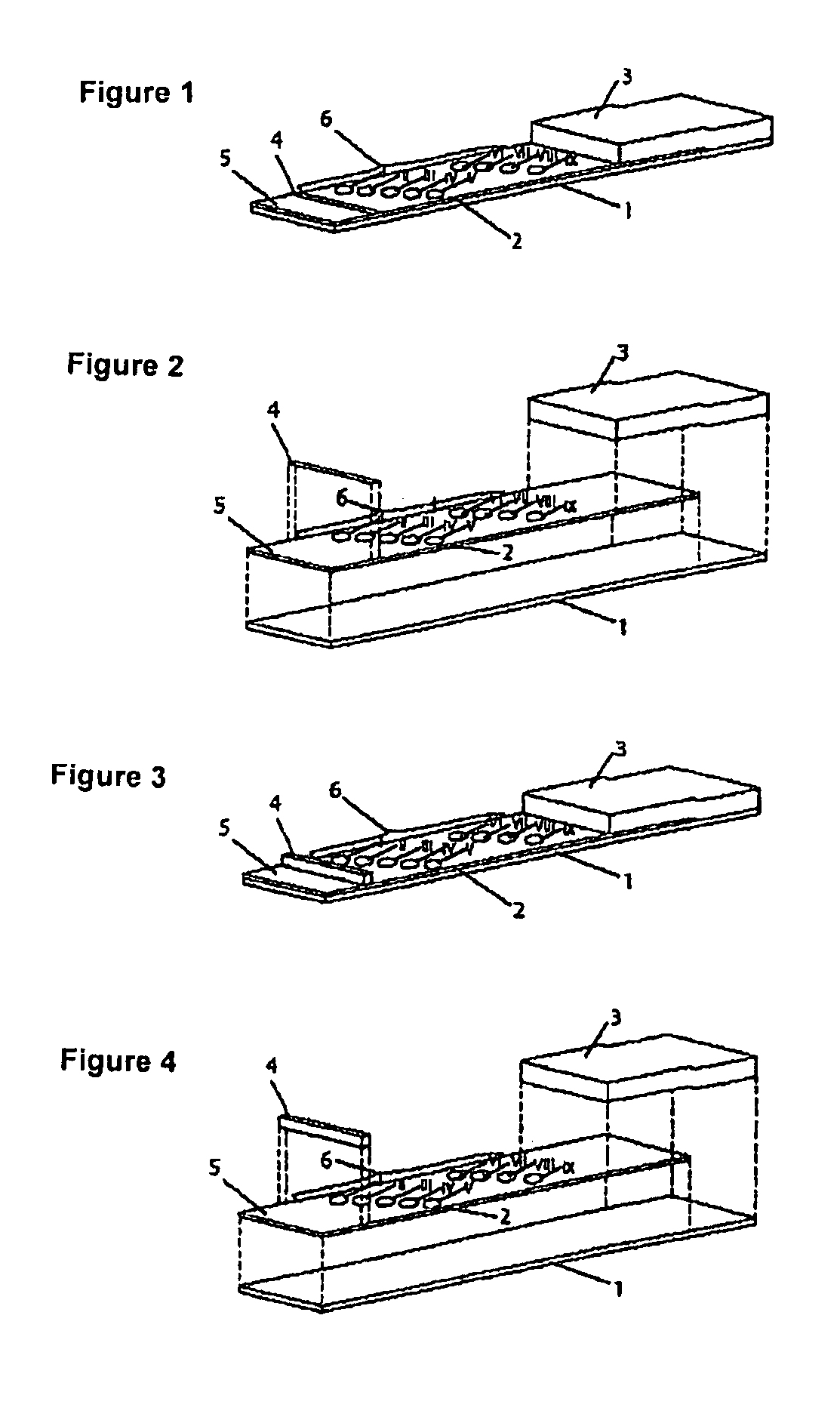
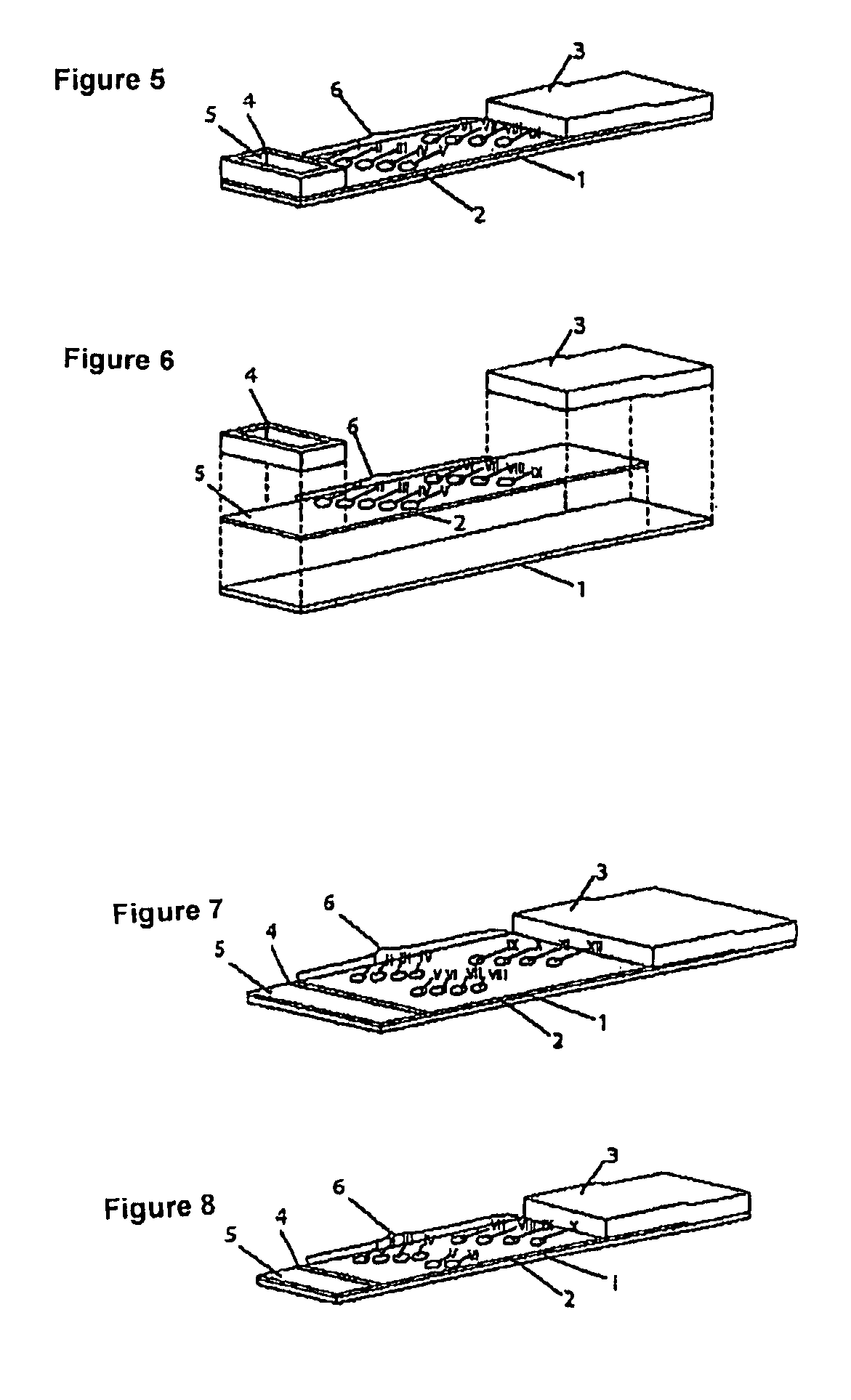
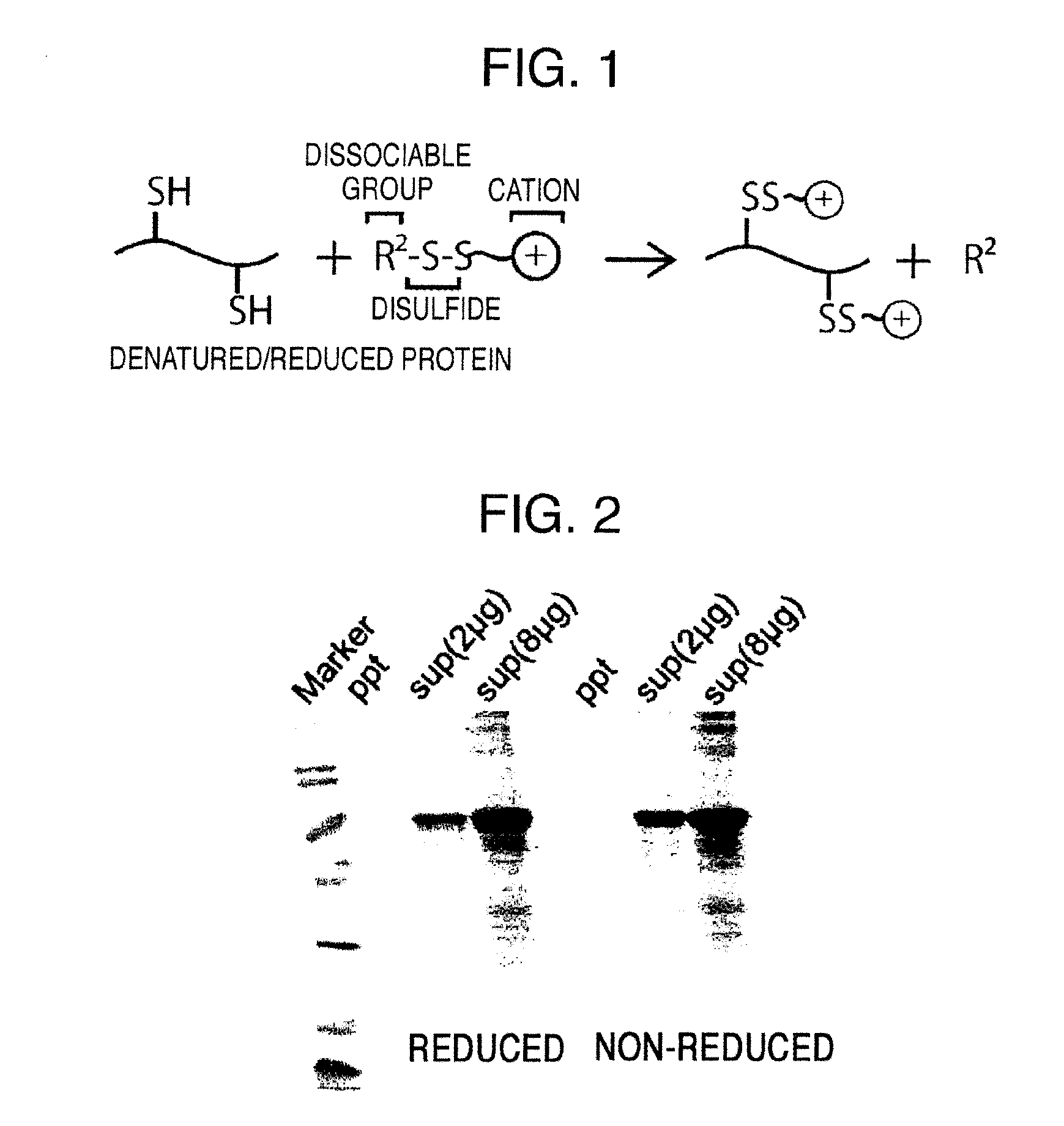
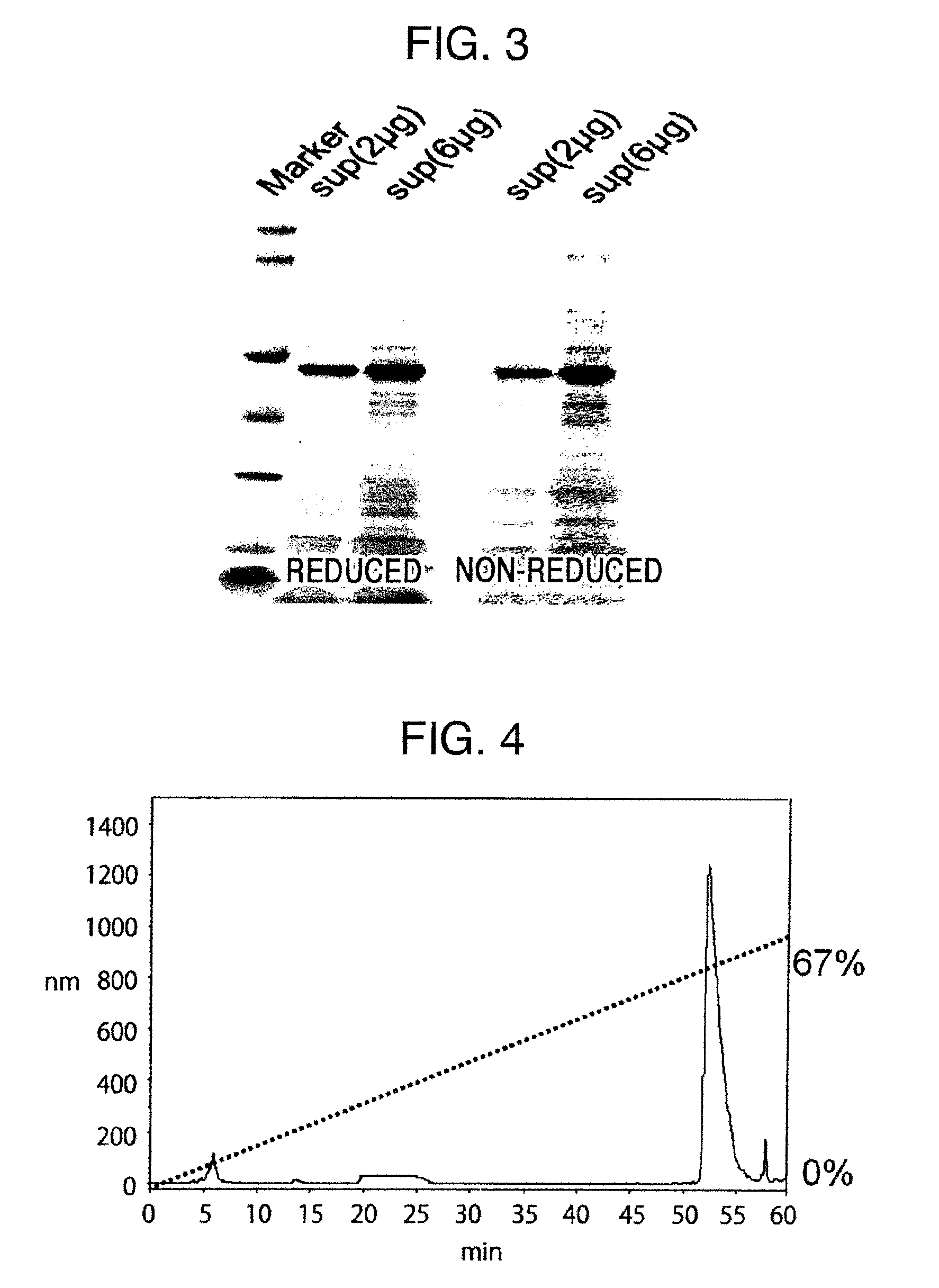
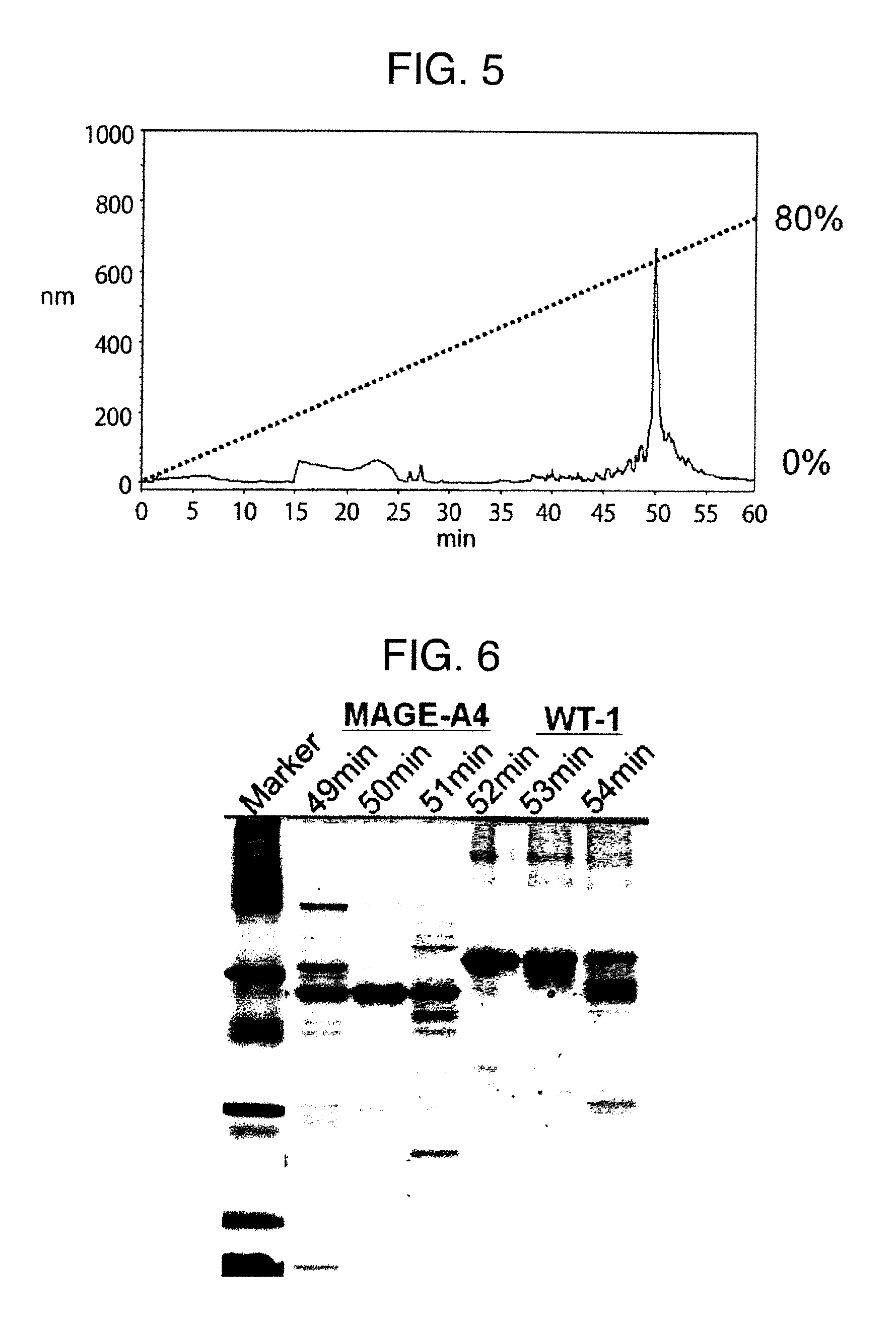
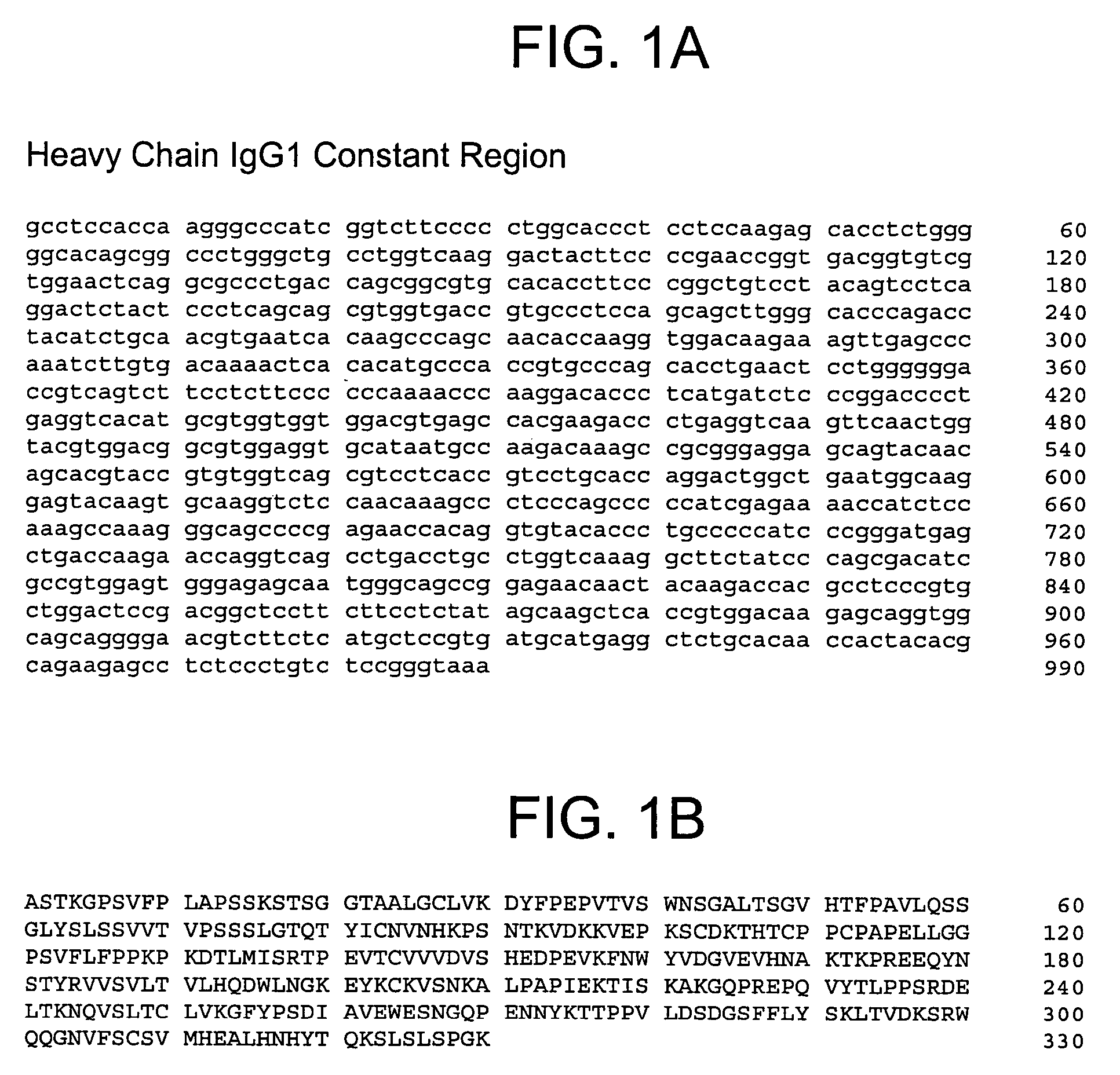
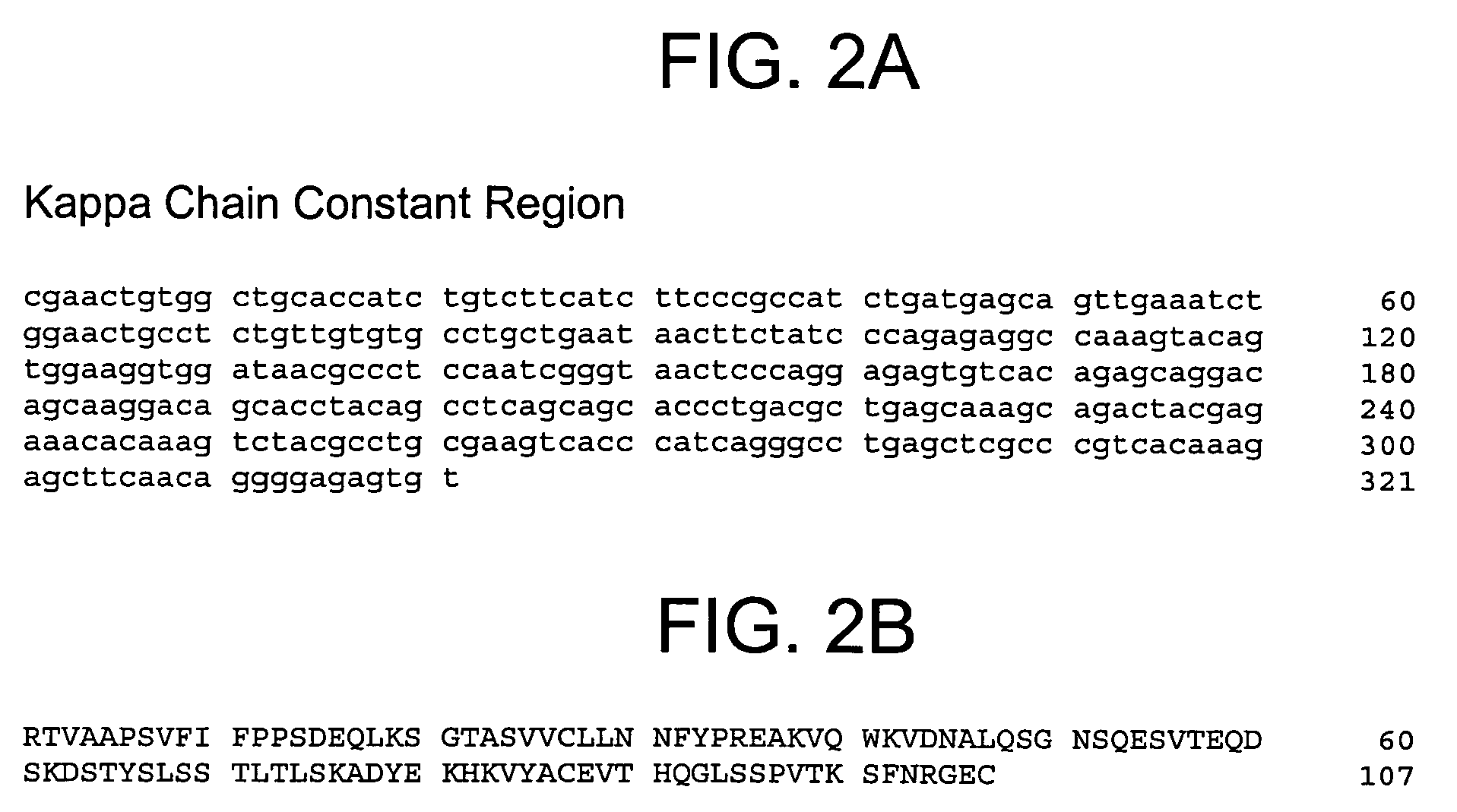
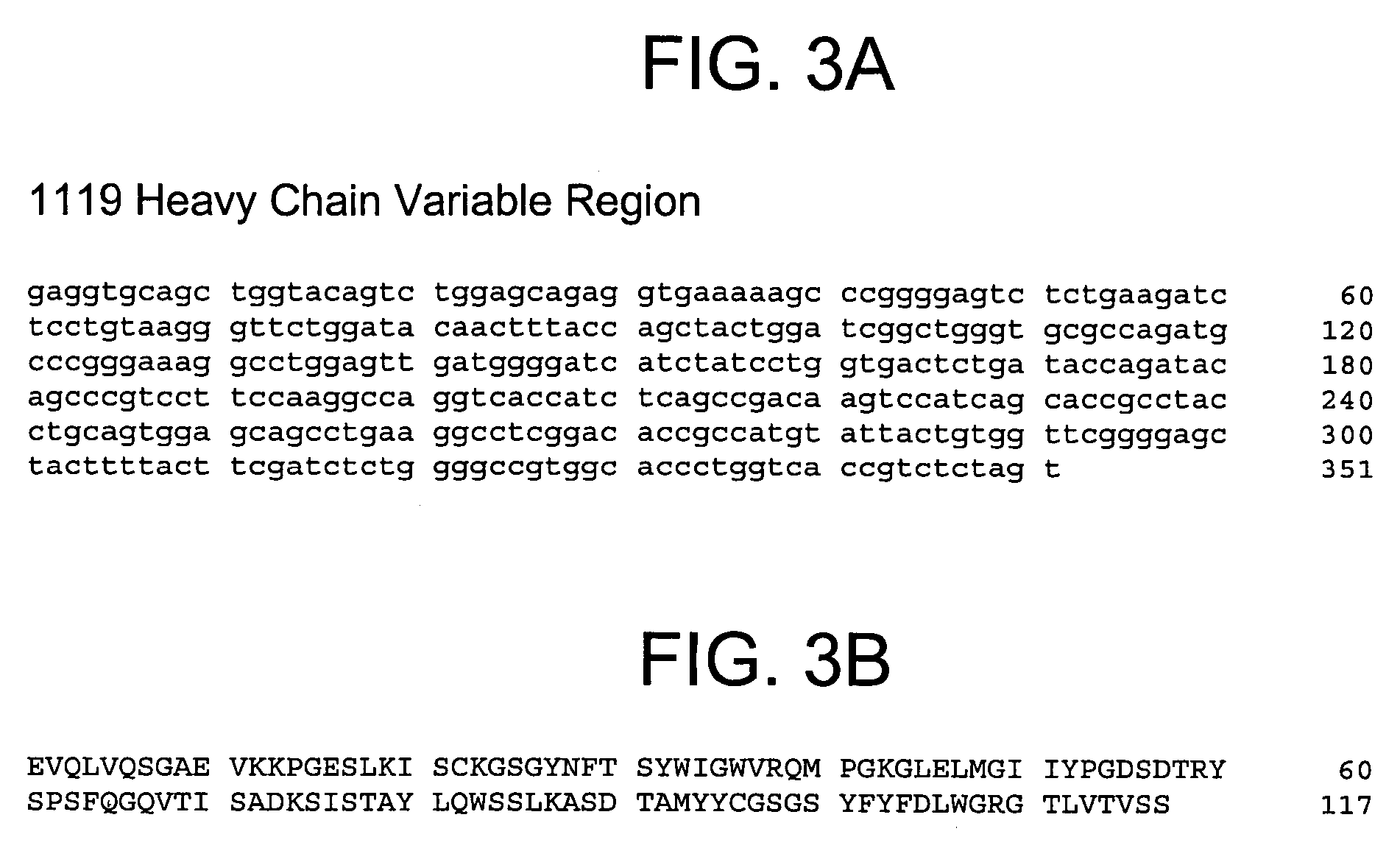
Method for producing reagent for antibody detection and use thereof
ActiveUS20150064801A1Efficiently solubilizeEffective recoveryTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsProtein moleculesAntigenic protein
The present invention provides the following: a method for efficiently producing a reagent for detecting an antibody that specifically binds with an insoluble antigen protein present in a liquid sample; a reagent for antibody detection produced by the production method; and a use of the antibody. In a step for solubilizing an antigen protein, it is possible to efficiently solubilize and recover the antigen protein by using a cationizing agent; therefore, when compared to conventional methods, it is possible to efficiently produce a reagent for detecting an antibody that has bound to multiple antigen protein molecules in a carrier.
Owner:FUTAMI JUNICHIRO +1
Human anti-IFN-γ neutralizing antibodies as selective IFN-γ pathway inhibitors
ActiveUS7335743B2Prevents and antagonizes inhibitionNot inhibit or modulate the biological activityNervous disorderAntipyreticNeutralizing antibodyDisease cause
This invention provides antibodies that interact with or bind to human interferon-gamma (IFN-γ) and methods for treating IFN-γ mediated diseases by administering a pharmaceutically effective amount of antibodies to IFN-γ. Methods of detecting the amount of IFN-γ in a sample using antibodies to IFN-γ are also provided.
Owner:ER SQUIBB & SONS INC
Human anti-OPGL neutralizing antibodies as selective OPGL pathway inhibitors
Monoclonal antibodies and hybridomas producing them that interact with osteoprotegerin ligand (OPGL) are provided. Methods of treating osteopenic disorders by administering a pharmaceutically effective amount of antibodies to OPGL are also provided. Methods of detecting the amount of OPGL in a sample using antibodies to OPGL are further provided.
Owner:ER SQUIBB & SONS INC
HIV antibody and antigen combined rapid detection reagent kit
ActiveCN101266246AImprove the detection rateShorten the windowMaterial analysisDiseaseDirect observation
The invention relates to biology applied technology field, especially relates to a immune chromatography assay for quickly detecting the human immunologic deficiency disease (HIV) antibody and antigen. The assay comprises two independent test papers A and B and plastic case C for storing the test paper, wherein the test paper A is used for detecting the HIV antibody and the test paper B is used for detecting the HIV p24 antigen. The test paper A and test paper B are parallely encased in the plastic case to constitute the assay. On detecting, the detected sample is added on the sample pad of the test paler and the immunoreaction result is directly observed to perform detection. The assay is used for screening or clinical diagnosis of the HIV infection and at the same time the HIV antigen and antibody are detected, the simpler antibody detection can effectively reduce the window phase for detecting the HIV, with features of quick reaction, easy operation, economy and practicality, suitable for insitu detecting.
Owner:天津中新科炬生物制药股份有限公司
Assay for antibodies
InactiveUS20060099662A1Reduce non-specific stickingReduce backgroundBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsHuman patientElisa method
The presence and quantity of an antibody of interest in a patient's bloodstream or other biological sample can serve as an important clinical or other analytical or diagnostic tool. ELISA methods, and kits for such assays, as well as anti-idiotypic antibodies and hybridomas producing them, are developed to detect levels of the antibody in biological samples, which are from, for example, animal models and human patients.
Owner:GENENTECH INC
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
Biomarkers for oxidative stress
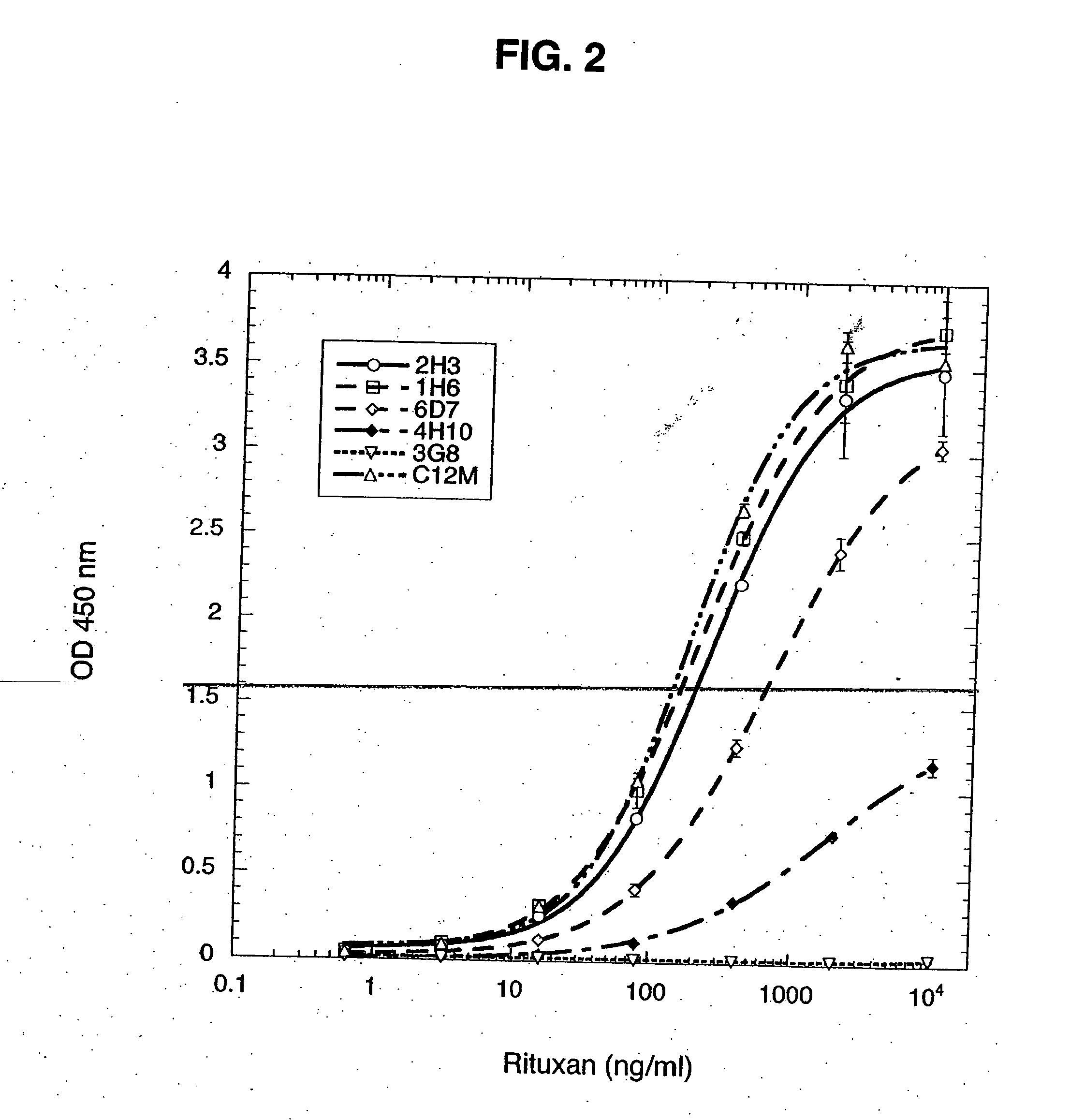
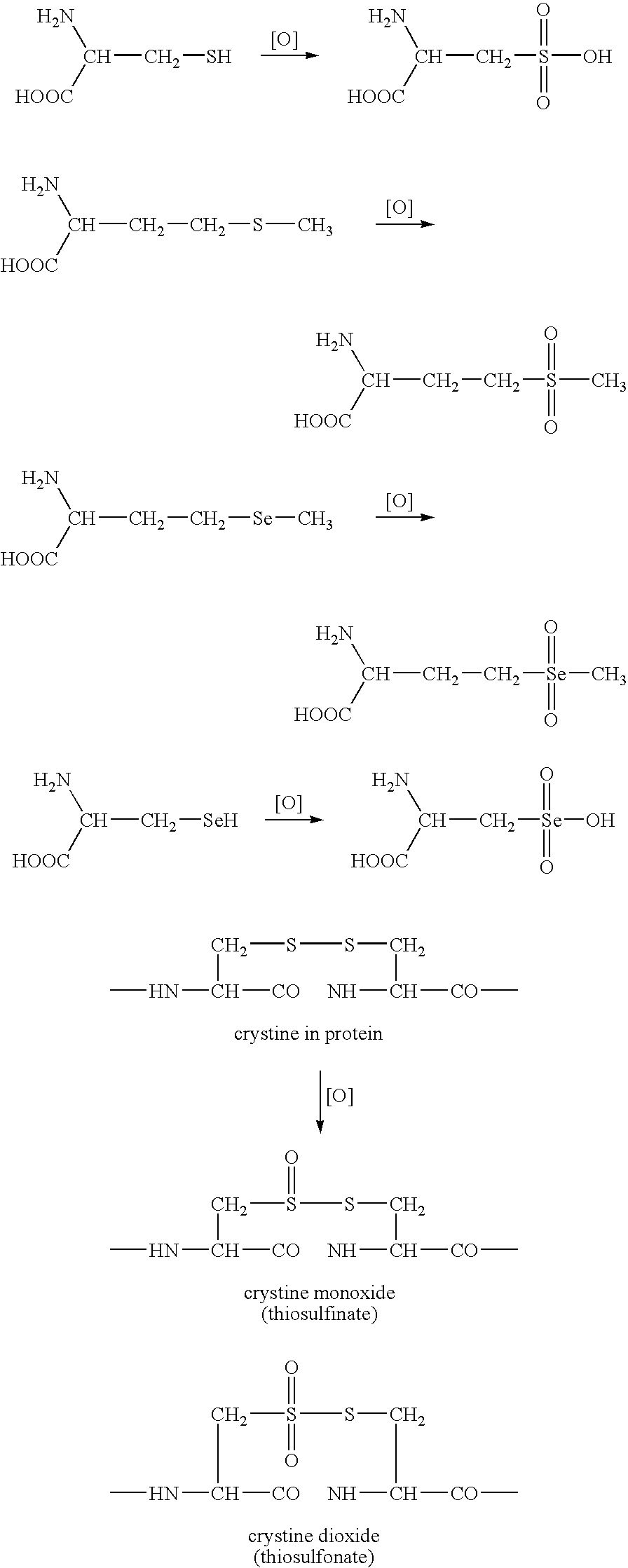
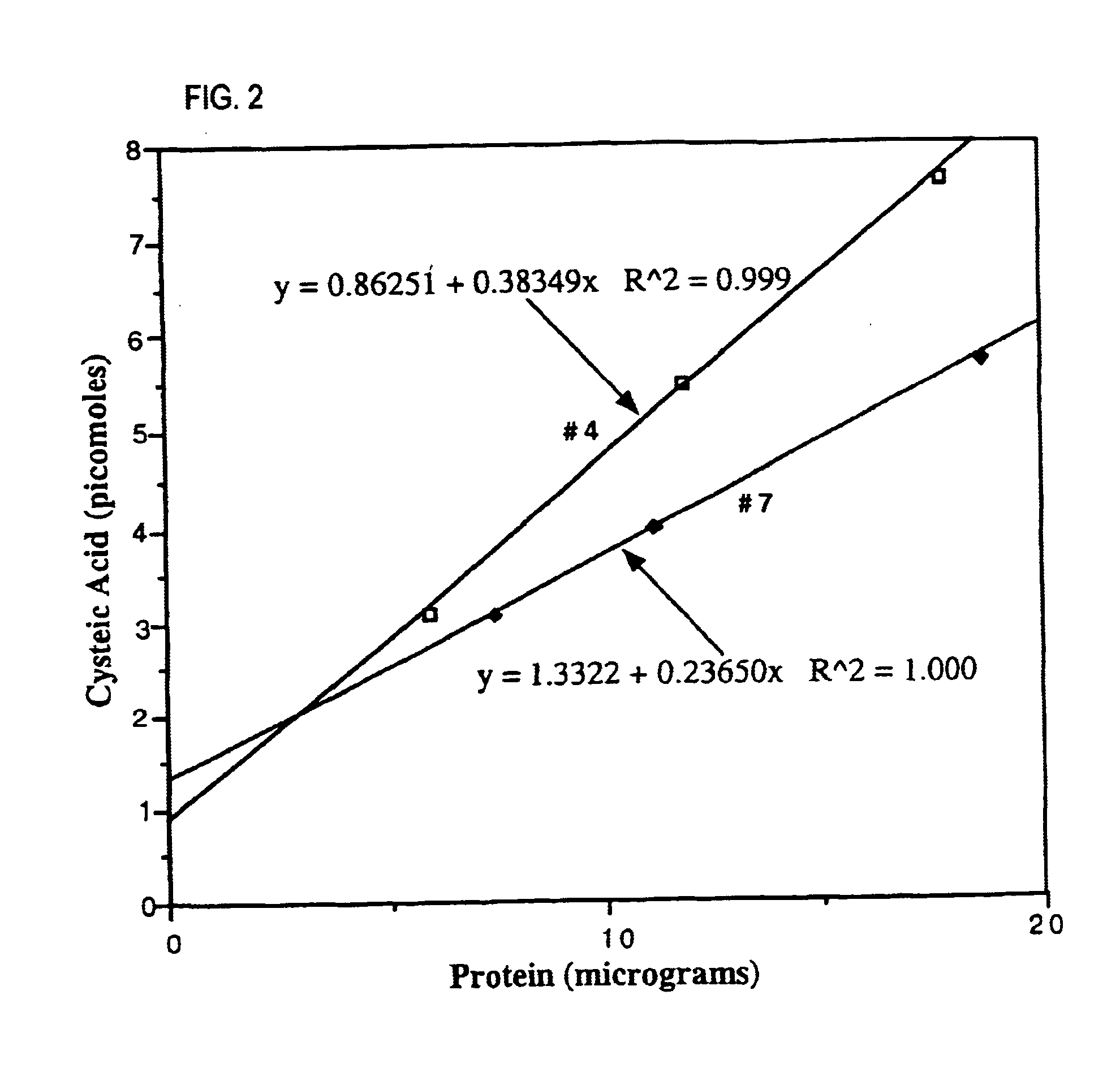
This invention related generally to methods of detecting and quantifying biomarkers of oxidative stress in proteins. The biomarker may be any amino acid that has undergone oxidation (or other modification, e.g. chloro-tyrosine, dityrosine). Emphasis is given herein on oxidized sulfur- or selenium-containing amino acids (SSAA). The biomarker of oxidative stress in proteins may be detected with an antibody that binds to oxidized amino acids, specifically oxidized sulfur- or selenium-containing amino acids. The antibody may be monoclonal or polyclonal. The presence of biomarker or amount of biomarker present in a sample may be used to aid in assessing the efficacy of environmental, nutritional and therapeutic interventions, among other uses.
Owner:EMORY UNIVERSITY
Sandwich method for detecting double antigen by antibody indirectly marked with nanometer granule and kit thereof
The invention provides an antibody detection double-antigen sandwich method using nanoparticles. A nanoparticle label and a label between the labeled antigens implement an indirect labeling by combining the label on an antigen and a ligand which is labeled on the nanoparticle label and can specifically recognize the label, wherein the labeled antigen is a gene engineering recombined antigen. Withthe indirect labeling manner, the sensitivity and the specificity of the method can be remarkably improved.
Owner:FAPON BIOTECH INC
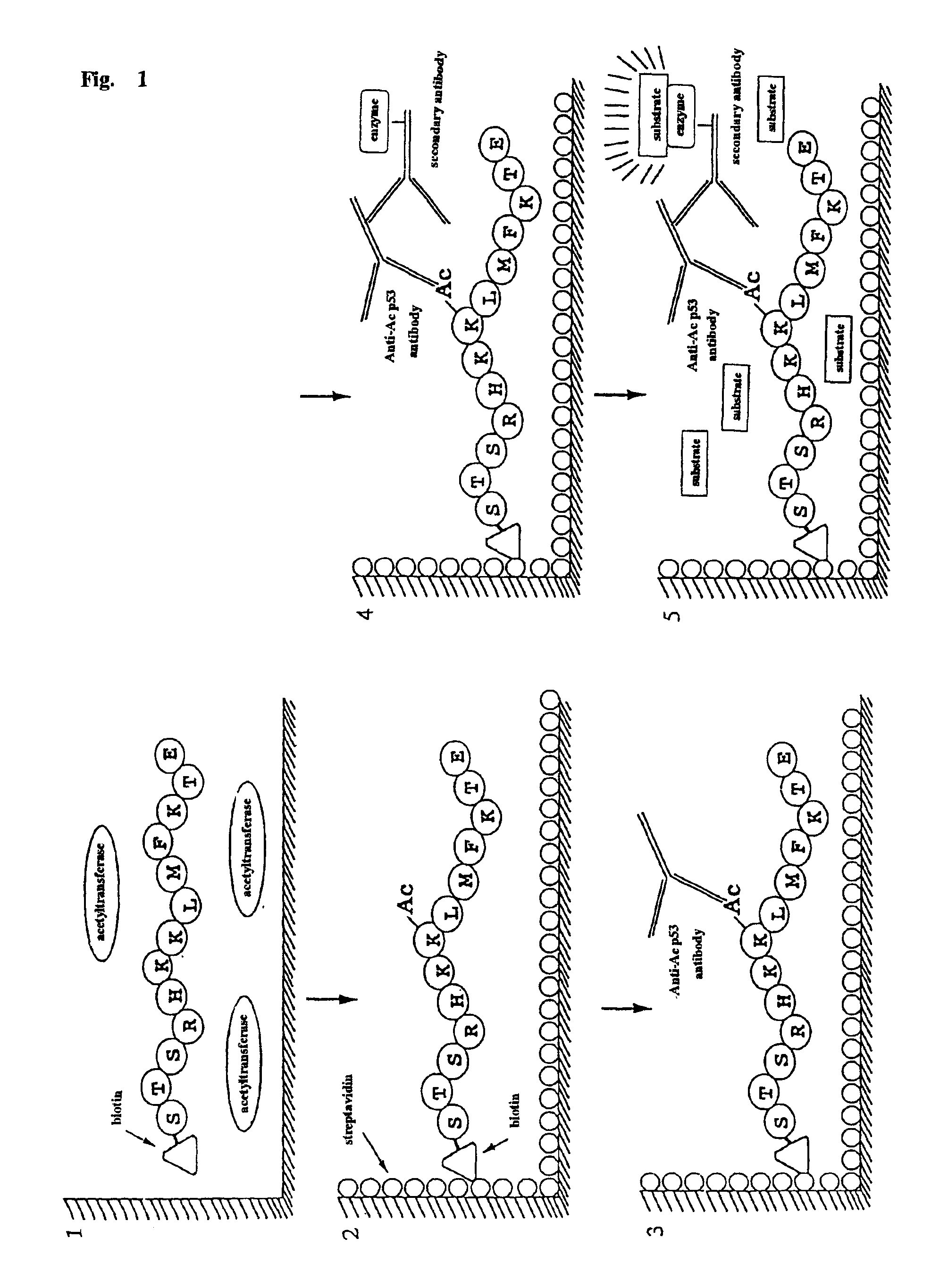
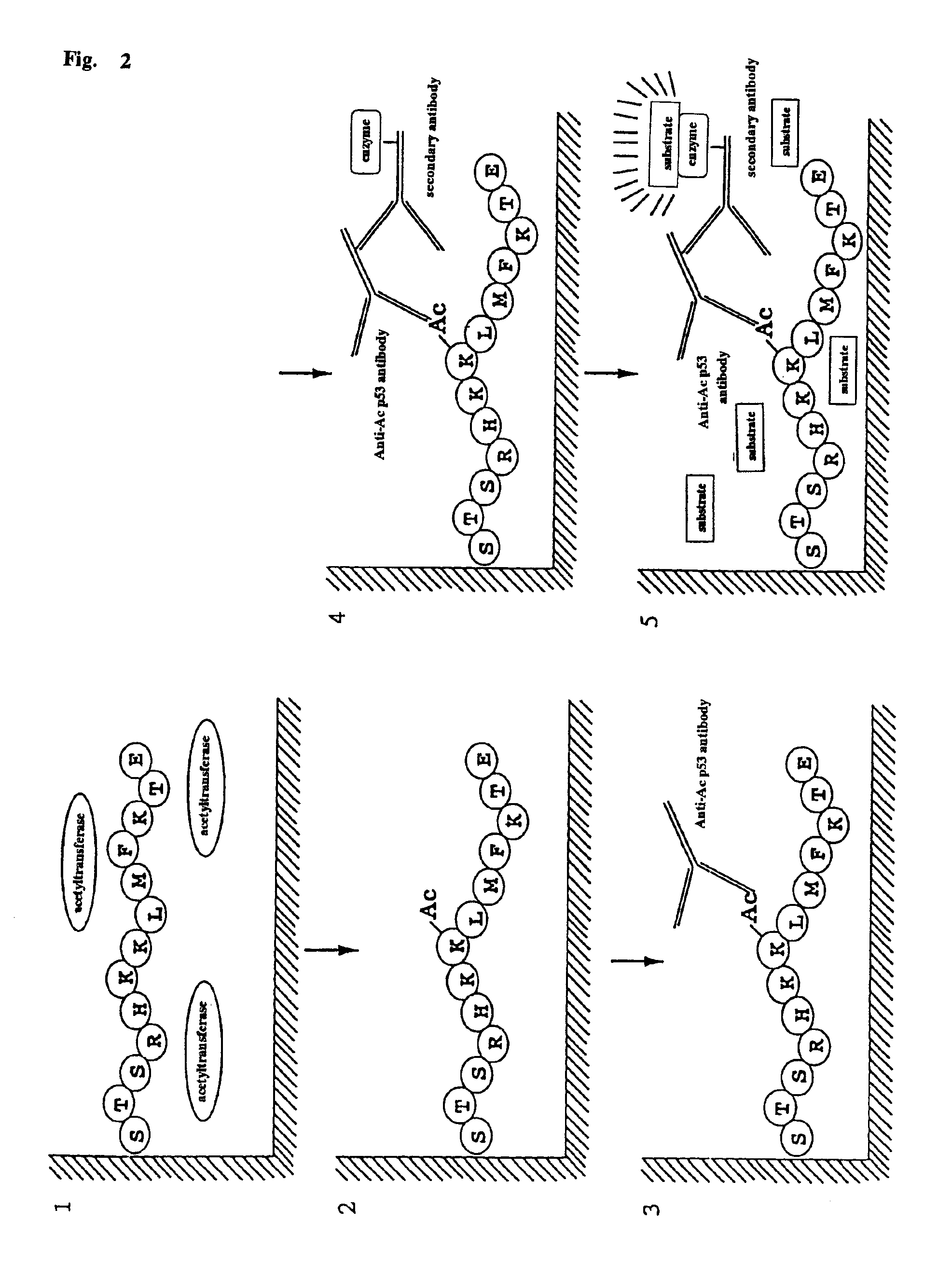
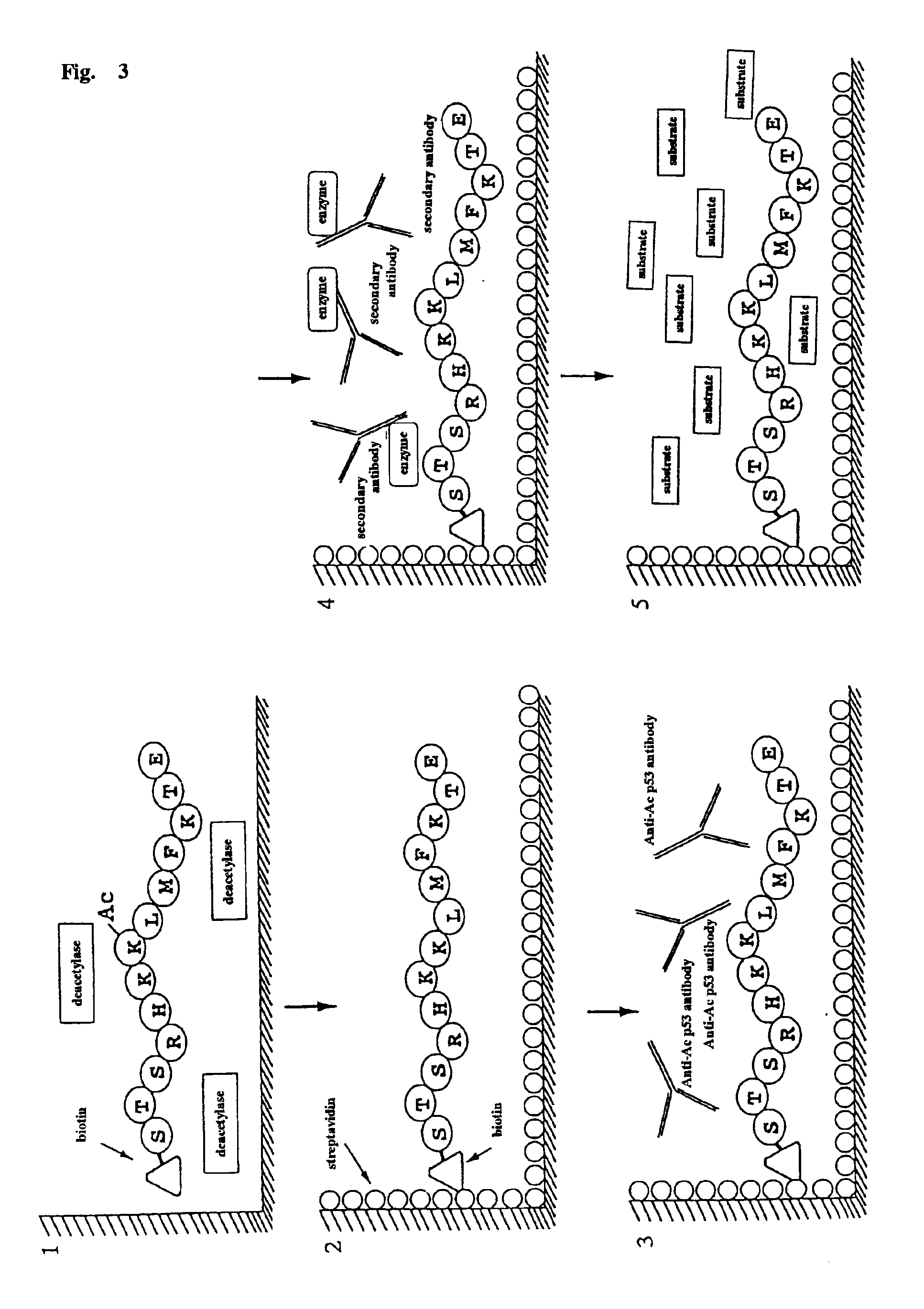
Method for detecting acetyltransferase and deacetylase activities and method for screening inhibitors or enhancers of these enzymes
InactiveUS6884597B1Easy to detectConducive to screeningCompound screeningApoptosis detectionPeptide substrateAcetyltransferase
A method for simply and conveniently detecting acetyltransferase and deacetylase activities of proteins by executing an acetylation reaction of a peptide substrate with an acetyltransferase, or a deacetylation reaction of an acetylated peptide substrate with a deacetylase, and after the completion of these reactions, detecting the acetyl group bound to the peptide substrate by using an anti-acetylated peptide antibody. This system for detecting acetyltransferase and deacetylase activities using the anti-acetylated peptide antibody enables screening inhibitors or enhancers of acetyltransferase and deacetylase. A system for screening deacetylase inhibitors or acetyltransferase enhancers using cultured cells is also provided.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
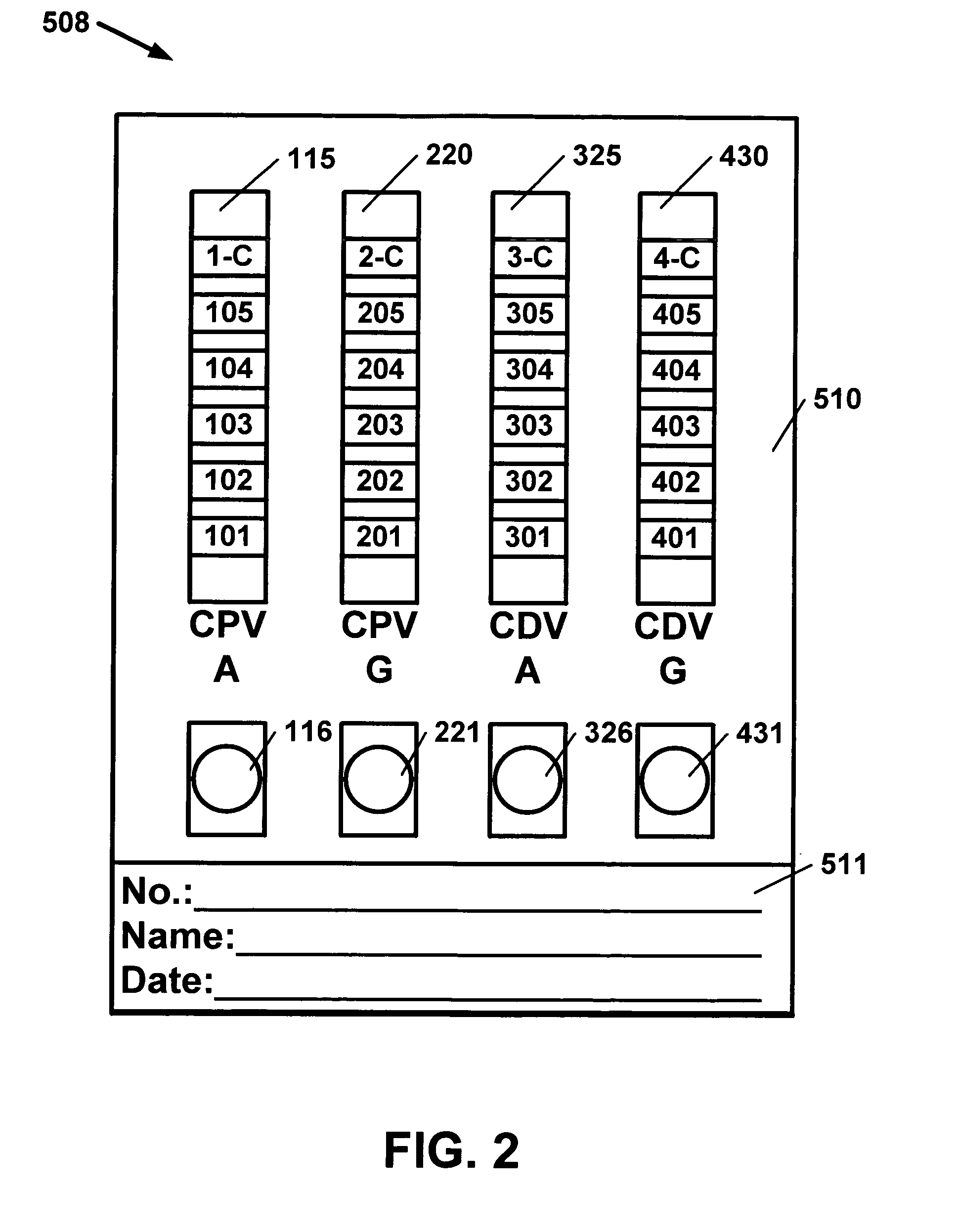
Antibody detection method and device for a saliva sample from a non-human animal
InactiveUS20130309656A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSaliva sampleCanine distemper virus CDV
A rapid test apparatus, system, and method of use utilizing lateral flow immunoassay (LFIA) detection of a selected ligand in a liquid sample from a body fluid such as saliva in a pet in which antibodies and their complimentary antigens are used with detection-nanoparticles to provide a visual or measurable end point indicator in which the method measures the exposure to viruses in the canine from Canine Parvovirus (CPV) and / or Canine Distemper virus (CDV).
Owner:DAVIS DAVID C
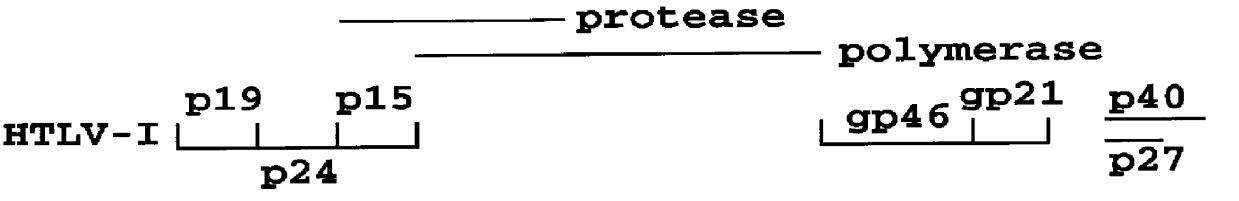
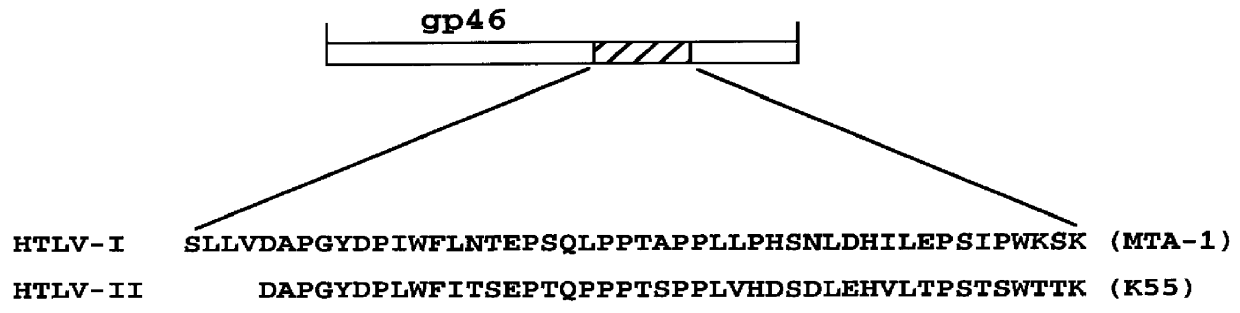
HTLV-I/HTLV-II assay and method
InactiveUS6110662AMicrobiological testing/measurementBiological material analysisPeptide antigenSerum samples
Method and assay kit for positively identifying HTLV-I and HTLV-II infection from human serum samples. The kit includes peptide antigens from the C-terminal regions of HLTV-I p19 and HTLV-II p21 gag proteins, and peptide antigens from the HLTV-I and HTLV-II env proteins immobilized on a solid support. After reaction of the serum sample with the solid support, an antibody-detection reagent in the kit is added to the support, to detect binding of human serum antibodies to each of the peptide antigens separately. The test allows positive identification of HTLV-I or HTLV-II when antibody binding to each HTLV-I or HTLV-II gag and env peptide antigen, respectively, is observed. Also disclosed is a kit for screening human sera for evidence of HTLV-I or HTLV-II infection.
Owner:GENELABS TECH INC +1
Monoclonal antibody specific for the extracellular domain of prostate specific membrane antigen
InactiveUS7476513B2VirusesIn-vivo radioactive preparationsImmunoenzymatic assayProstate specific membrane
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM′) of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM′ allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
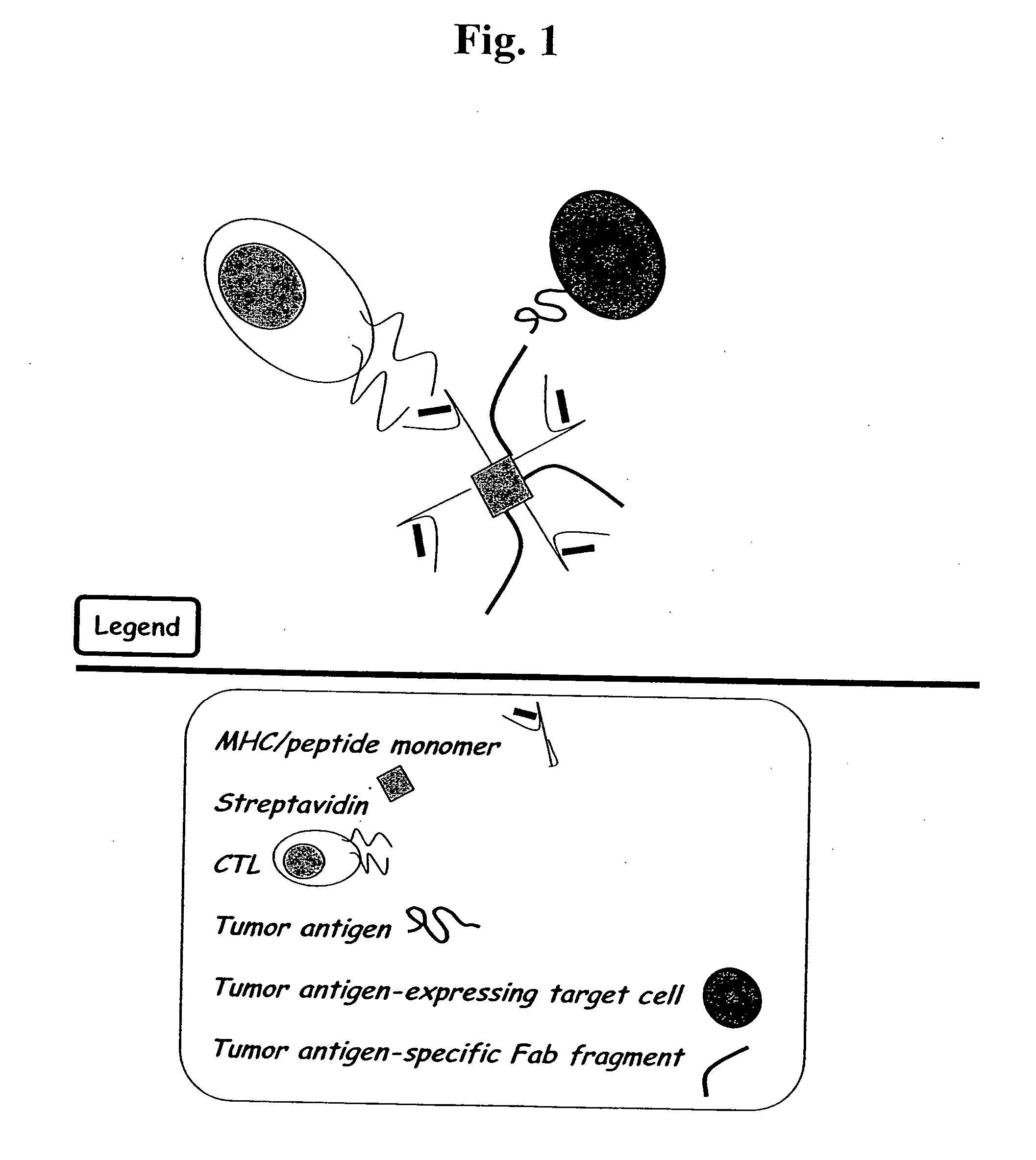
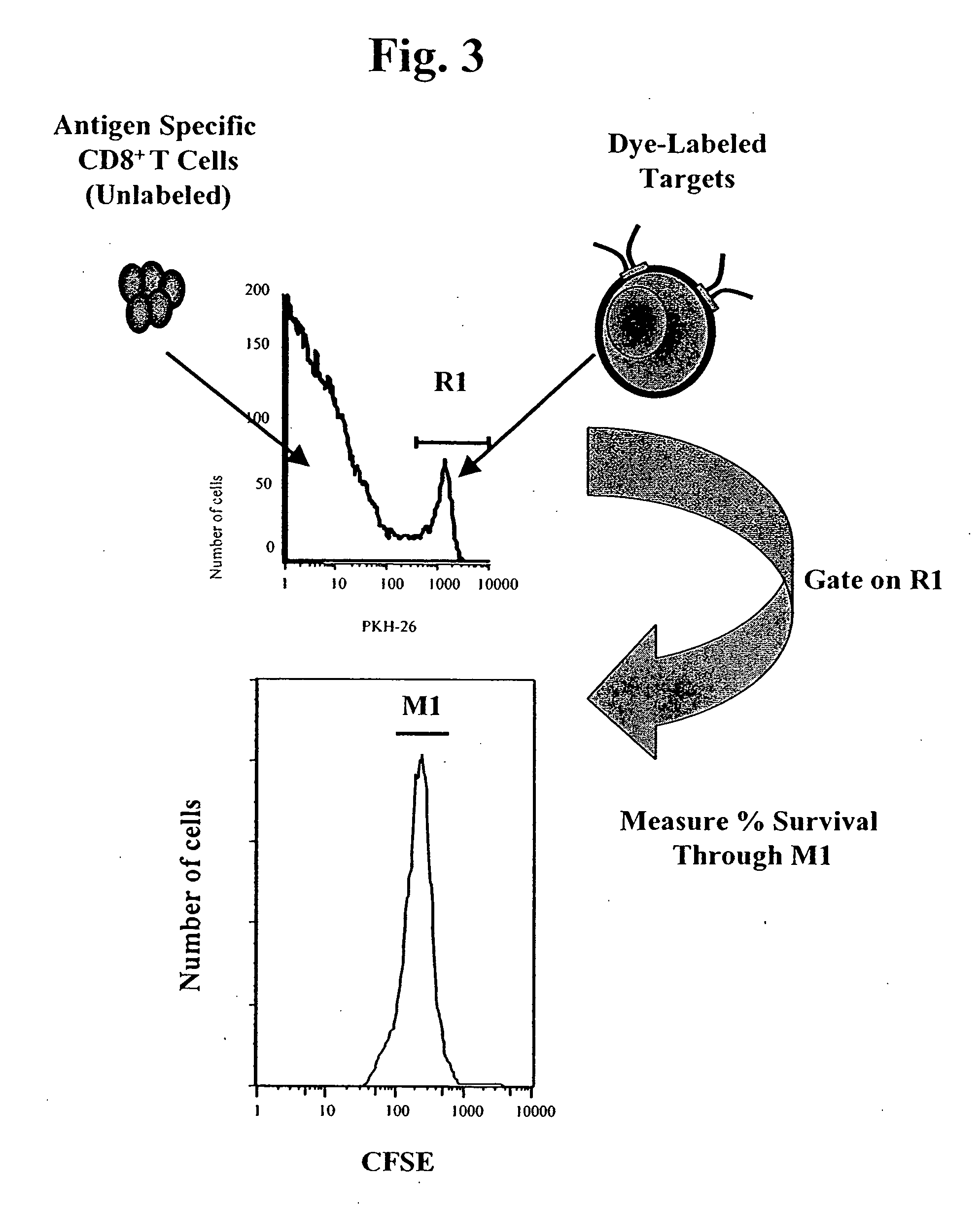
MHC bridging system for detecting CTL-mediated lysis of antigen presenting cells
InactiveUS20050287611A1Reduce signalingBiological material analysisBlood/immune system cellsLysisFluorescence
A bridging assay that utilizes a multivalent MHC binding molecule to enumerate the number of antigen-specific CTLs in a particular sample and also determines the functional capability of the CTL population in the sample is provided. In one embodiment, the assay is used to measure the effector function of any tetramer-positive CTL using a single non-MHC-containing target cell line that is adapted to form an antibody bridge with the tetramer. Furthermore, effector function and enumeration can be measured by flow cytometry, and additional markers residing on either effector or target cell populations may be detected using antibodies coupled with other fluorochromes. The tetramer bridging assay will allow investigators to easily determine the lytic capacity and antigenic specificity of CTLs using a commercially available reagent in a non-radioactive assay.
Owner:BECKMAN COULTER INC
Rapid vaccinia antibody detection device, method and test kit
InactiveUS20040002063A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeTest sample
The invention relates to a rapid vaccinia antibody detection device, method and test kit for the detection of vaccinia antibody in a serum, plasma or whole blood test sample utilizing a purified vaccinia cell lysate as the capture antigen. The detection device operates on the basis of a 2-step flow-through format in use with a push buffer.
Owner:MEDMIRA
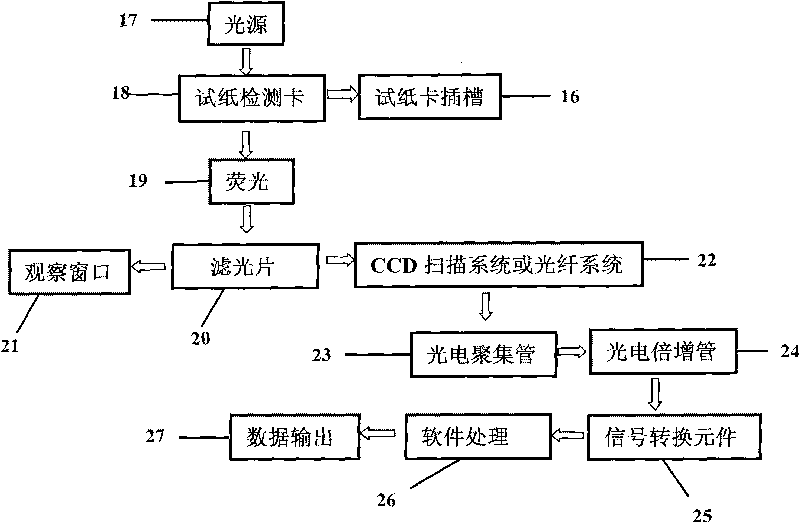
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
InactiveCN101726596ASimple and fast operationHigh sensitivityBiological testingLuminescent compositionsCelluloseHepatitis B virus
The invention discloses a fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and a method for preparing the same. The testing card comprises a hepatitis b surface antigen test paper strip, a hepatitis b e surface antigen test paper strip, a hepatitis b surface antibody test paper strip, a hepatitis b e surface antibody test paper strip, and a hepatitis b core antibody test paper strip. Each test paper strip is formed by overlapping and bonding filter paper, a sample pad, a glass fiber film spray-coated with fluorescent microspheres, a cellulose nitrate film and water absorption paper on a bottom plate by glue in sequence, wherein the cellulose nitrate film is coated with antigens serving as a testing area and anti-rabbit antibodies serving as a quality control area; and during a test, after emitted fluorescent light passes a filter, the emitted spectrum is collected, accumulated and multiplied by the CCD scanning technology and then converted into a numerical signal, the numerical signal is multiplied by a correction factor, and the strength of the corrected fluorescent light is substituted in a standard curve of a fluorescence analyzer, so that the concentrations of the five indexes of hepatitis b of the sample can be automatically worked out. The test of hepatitis b viruses by the testing card has the characteristics of specificity, sensitivity, simpleness and accuracy.
Owner:WUXI ZODOLABS BIOTECH
Therapeutic Human Anti-IL-1R1 Monoclonal Antibody
Antibodies that interact with interleukin-1 receptor type 1 (IL-1R1) are described. Methods of treating IL-1 mediated diseases by administering a pharmaceutically effective amount of antibodies to IL-1R1 are described. Methods of detecting the amount of IL-1R1 in a sample using antibodies to IL-1R1 are described.
Owner:ER SQUIBB & SONS INC
Method for detecting cancers
The invention provides for the production of several humanized murine antibodies specific for the antigen LK26, which is recognized by the murine antibody LK26. This antigen is expressed in all choriocarcinoma, teratocarcinoma and renal cancer cell lines whereas it is not expressed on cell lines of leukaemias, lymphomas, neuroectodermally-derived and epithelial tumour cell lines (excepting a small subset of epithelial cell lines). Furthermore, whereas renal cancer cell lines express the LK26 antigen, normal renal epithelial cells do not. Similarly, with the exception of the trophoblast, all normal adult and fetal tissues tested are negative for the LK26 phenotype. The invention also provides for numerous polynucleotide encoding humanized LK26 specific antibodies, expression vectors for producing humanized LK26 specific antibodies, and host cells for the recombinant production of the humanized antibodies. The invention also provides methods for detecting cancerous cells (in vitro and in vivo) using humanized LK26 specific antibodies. Additionally, the invention provides methods of treating cancer using LK26 specific antibodies.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
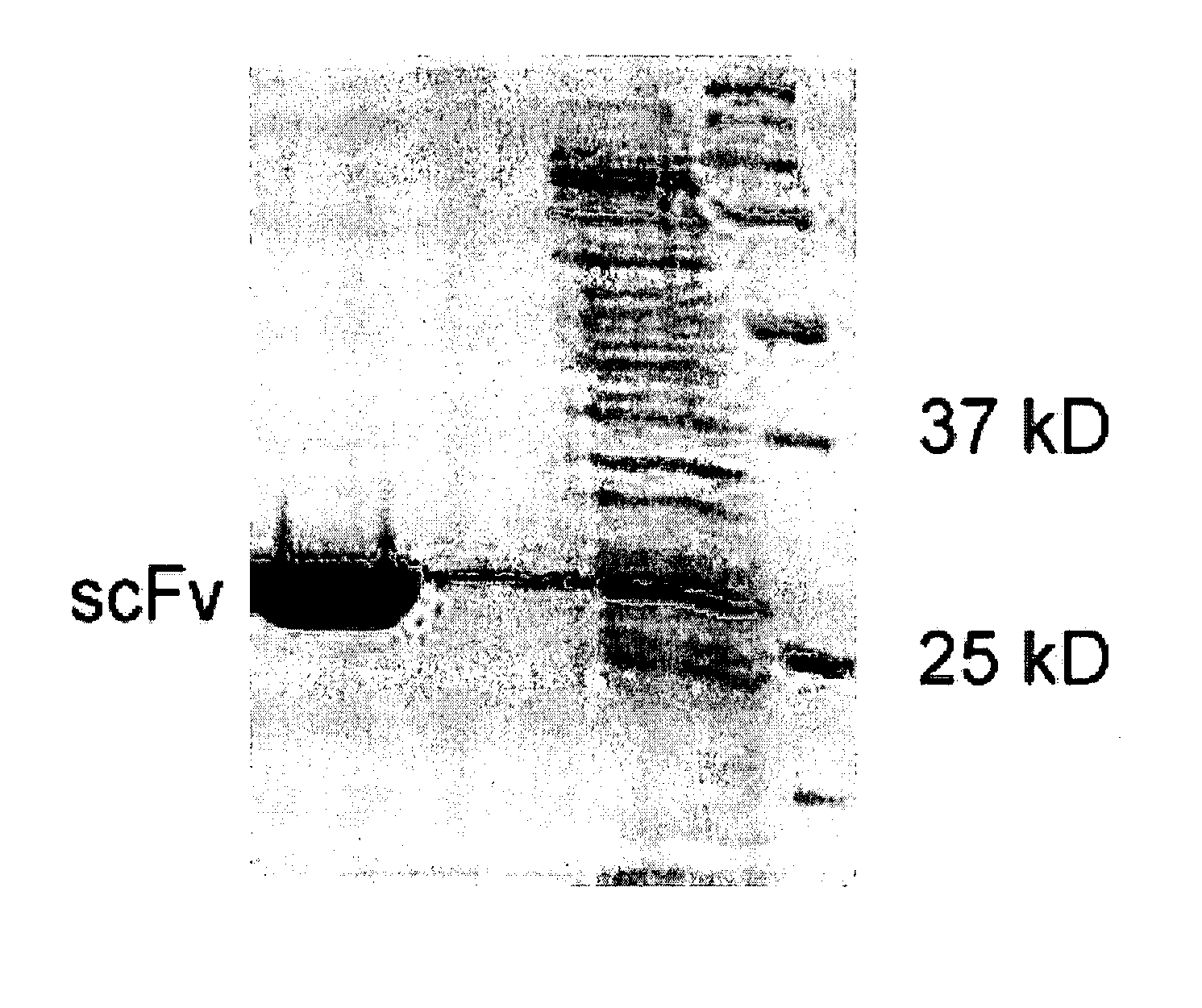
Recombinant antibodies to sclerotinia antigens
InactiveUS20080104734A1Sugar derivativesImmunoglobulins against animals/humansSclerotiniaAnti fungal
The invention is directed to recombinant antibodies which bind to Sclerotinia sclerotiorum antigens and comprise a single chain variable fragment (scFv). The antigen may be selected from SSPG1d or a portion thereof, aspartyl protease or a portion thereof, or whole Sclerotinia sclerotiorum mycelium. The invention also provides an antibody linked to an anti-fungal polypeptide. The invention extends to nucleic acid sequences encoding the antibodies, and expression vectors comprising the nucleic acid sequences. The invention is also directed to transgenic plants, seeds, tissues or cells transformed with the expression vectors. Methods for producing a transgenic plant that is resistant to Sclerotinia sclerotiorum, and for detecting Sclerotinia sclerotiorum in a biological sample utilizing an antibody which binds to Sclerotinia sclerotiorum antigen, and immunoassay kit for same are also provided.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
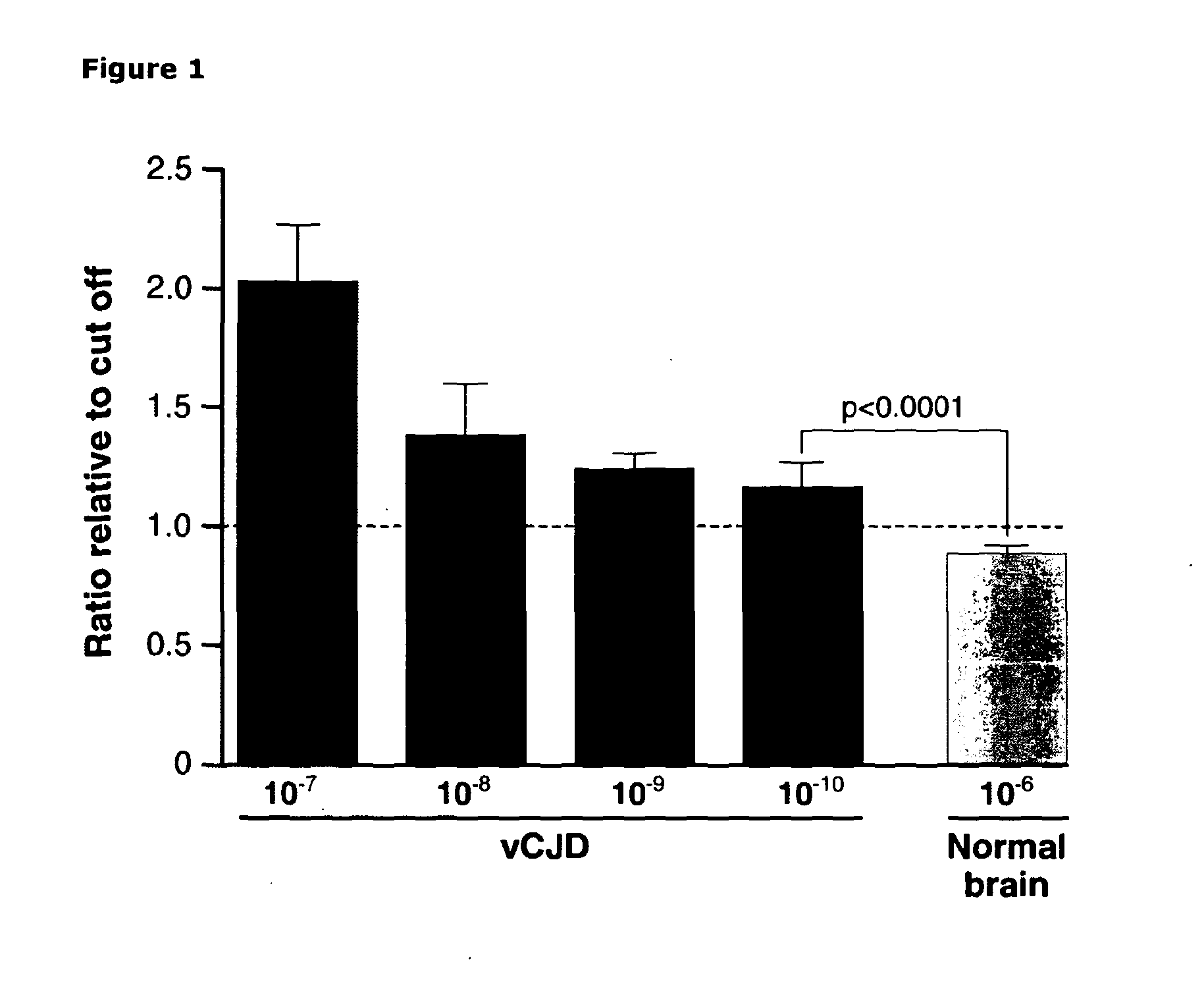
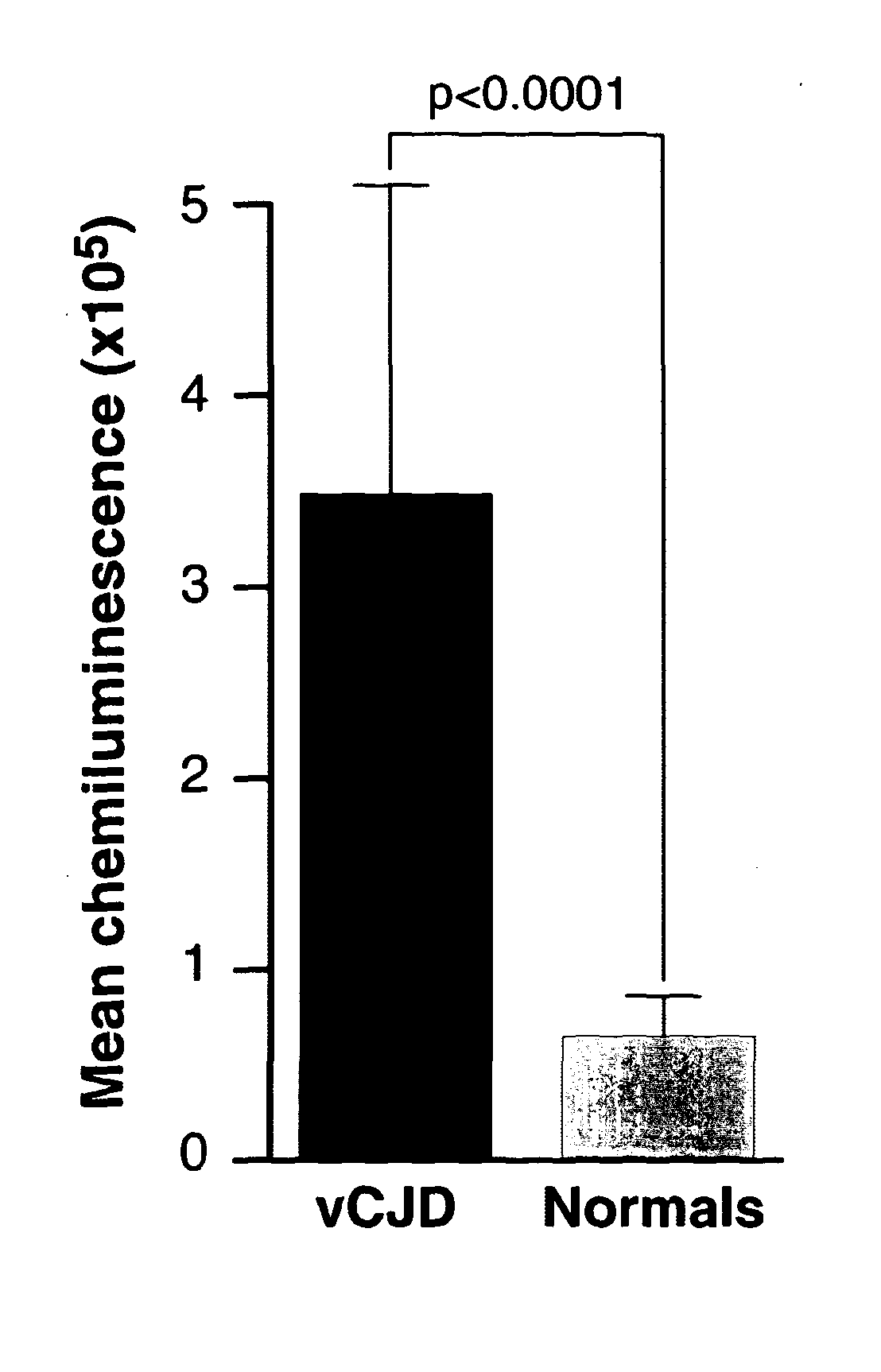
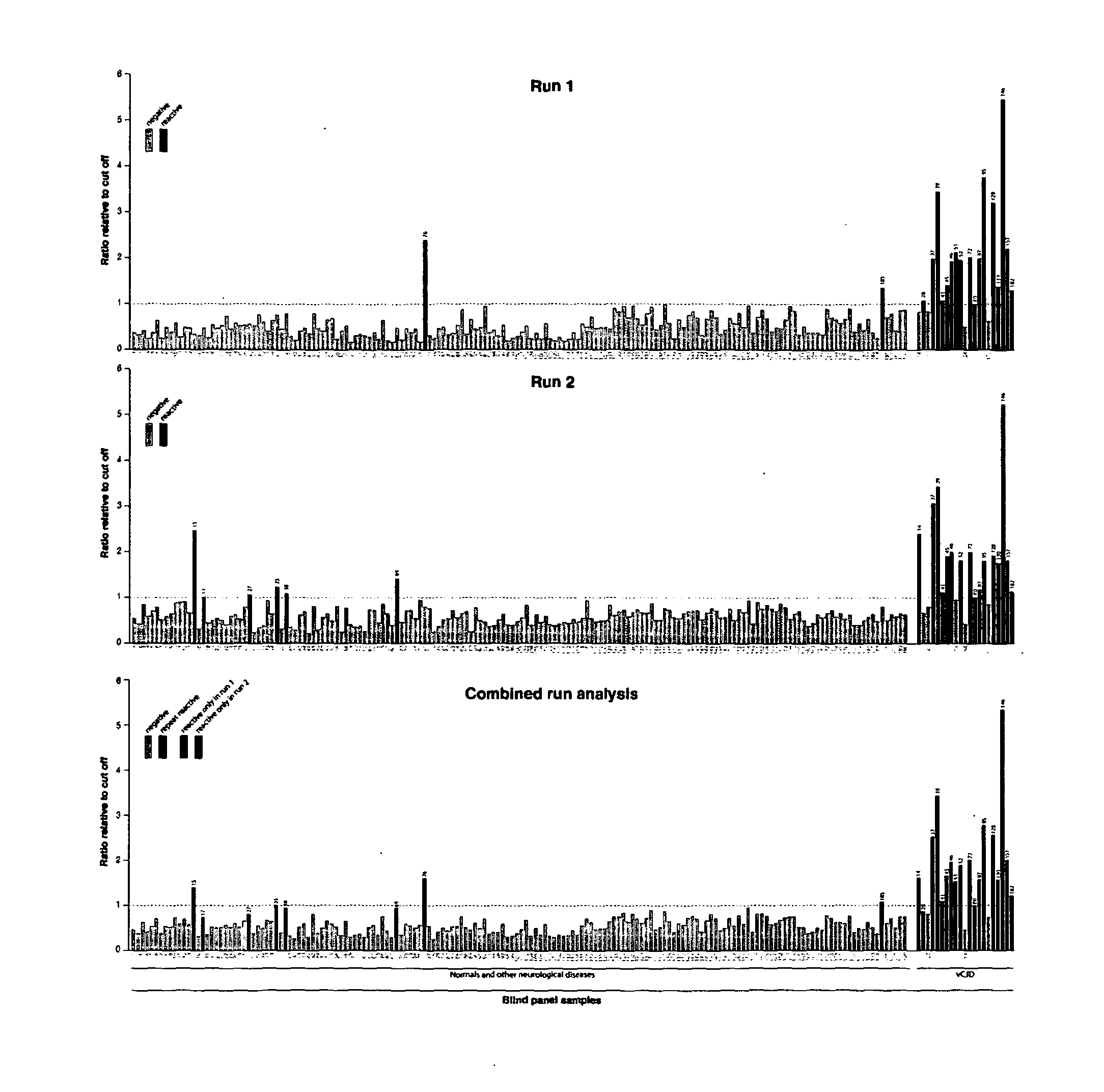
Assay for Prions
InactiveUS20130196356A1Avoid treatmentPrevent steppingDisease diagnosisBiological testingBovine serum albuminUrine production
The invention relates to a method for detection of abnormal PrP in a sample of blood or urine, said method comprising: (a) diluting the sample with buffer to comprise final concentrations of (i) 10 mM to 500 mM buffer agent; (ii) 1% to 10% w / v bovine serum albumin; and (iii) 1% to 8% w / v CHAPS; (b) adding steel particles and incubating to allow PrP binding; (c) washing the steel particles to remove diluted sample; and (d) detecting abnormal PrP captured on the steel particles using antibody capable of binding said abnormal PrP. The invention also provides compositions and kits.
Owner:D GEN
Recombinant antibodies to sclerotinia antigens
The invention is directed to recombinant antibodies which bind to Sclerotinia sclerotiorum antigens and comprise a single chain variable fragment (scFv). The antigen may be selected from SSPG1d or a portion thereof, aspartyl protease or a portion thereof, or whole Sclerotinia sclerotiorum mycelium. The invention also provides an antibody linked to an anti-fungal polypeptide. The invention extends to nucleic acid sequences encoding the antibodies, and expression vectors comprising the nucleic acid sequences. The invention is also directed to transgenic plants, seeds, tissues or cells transformed with the expression vectors. Methods for producing a transgenic plant that is resistant to Sclerotinia sclerotiorum, and for detecting Sclerotinia sclerotiorum in a biological sample utilizing an antibody which binds to Sclerotinia sclerotiorum antigen, and immunoassay kit for same are also provided.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Human Anti-OPGL Neutralizing Antibodies As Selective OPGL Pathway Inhibitors
Monoclonal antibodies and hybridomas producing them that interact with osteoprotegerin ligand (OPGL) are provided. Methods of treating osteopenic disorders by administering a pharmaceutically effective amount of antibodies to OPGL are also provided. Methods of detecting the amount of OPGL in a sample using antibodies to OPGL are further provided.
Owner:AMGEN INC +1
Immune body detecting biochip using piezo-electricity thin film acoustic wave device
InactiveCN101246162ASpecific antigen contentAnalysing solids using sonic/ultrasonic/infrasonic wavesBiological testingBiological materialsBiochip
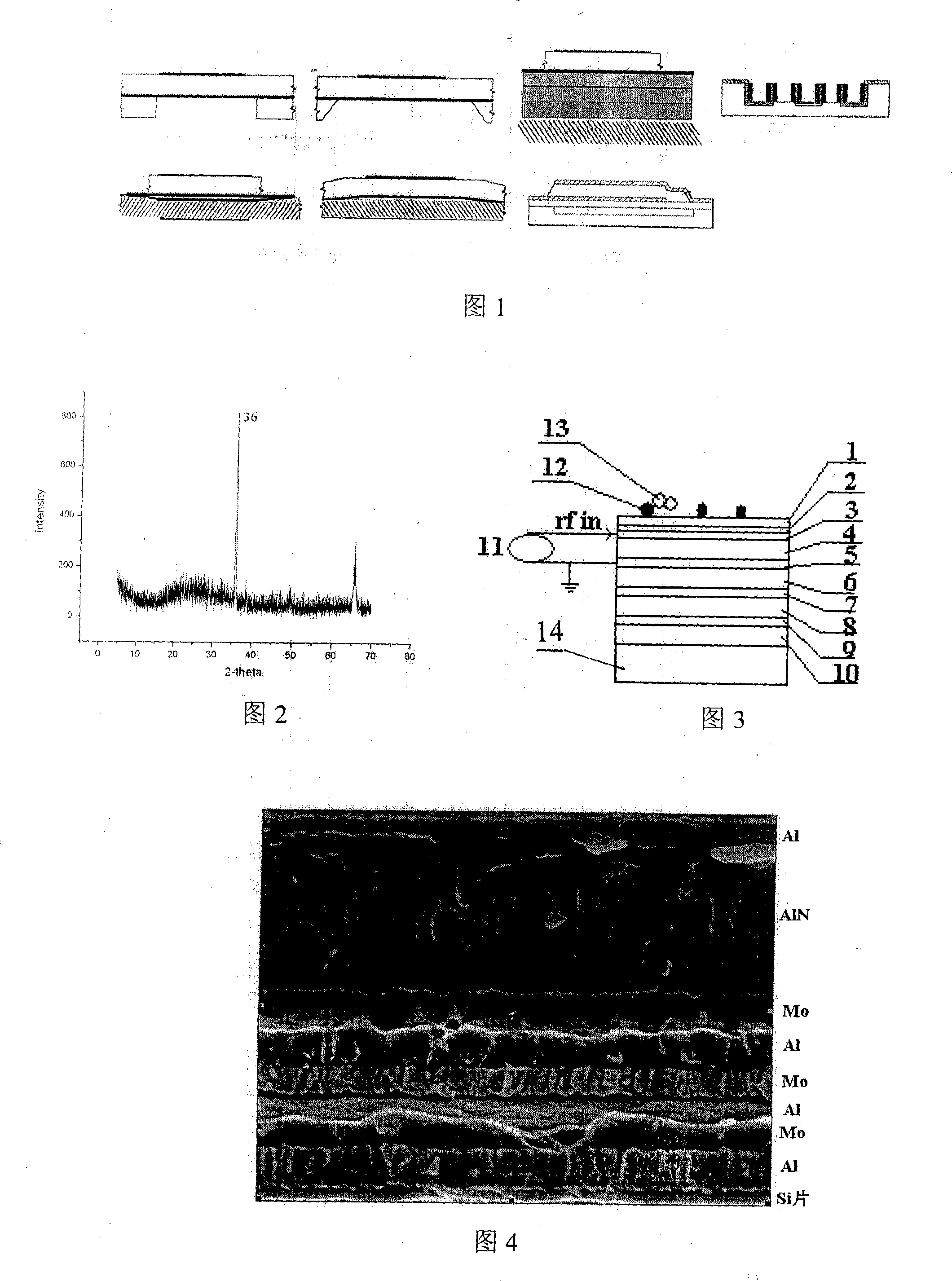
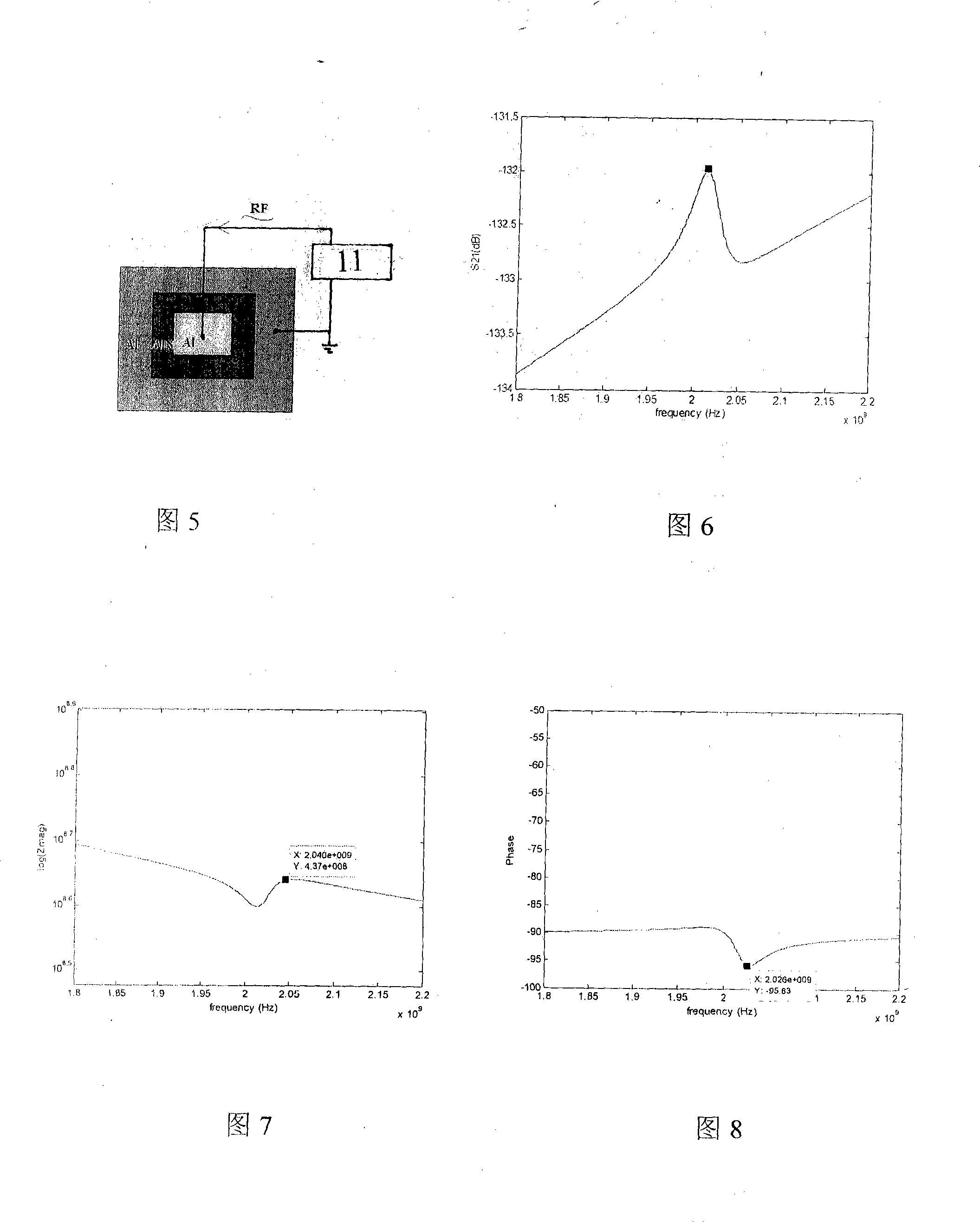
The invention belongs to the field of antibody detecting biochip which is based on piezoelectric effect, especially relates to a antibody detecting biochip using piezoelectric film acoustic wave device; including resonator FBAR which is equipped with upper electrode, bottom electrode and bragg reflection layer whole reflection film, the character of it resides in that: the piezoelectric film is equipped between the upper electrode and bottom electrode on resonator, the bragg reflection layer whole reflection film includes loudness acoustic impedance and undertone impedance, the loudness is composed of metal Mo, the undertone is composed of metal Al; the FRAB working frequency is 0.1-10GHz, the range of Q value is 100-2000; metal layer is planted on upper electrode, protein and antibody tissue are attached on the metal layer; the invention can measure resonance frequency migration parameters of antibody tissue and antigen, then specific antigen content is obtained; the sensor can be integrated on the chip, at the same time can sense micro antibody material, and creates favorable conditions for measuring trace amount of biological materials in body fluid.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com