Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
795 results about "Urine production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
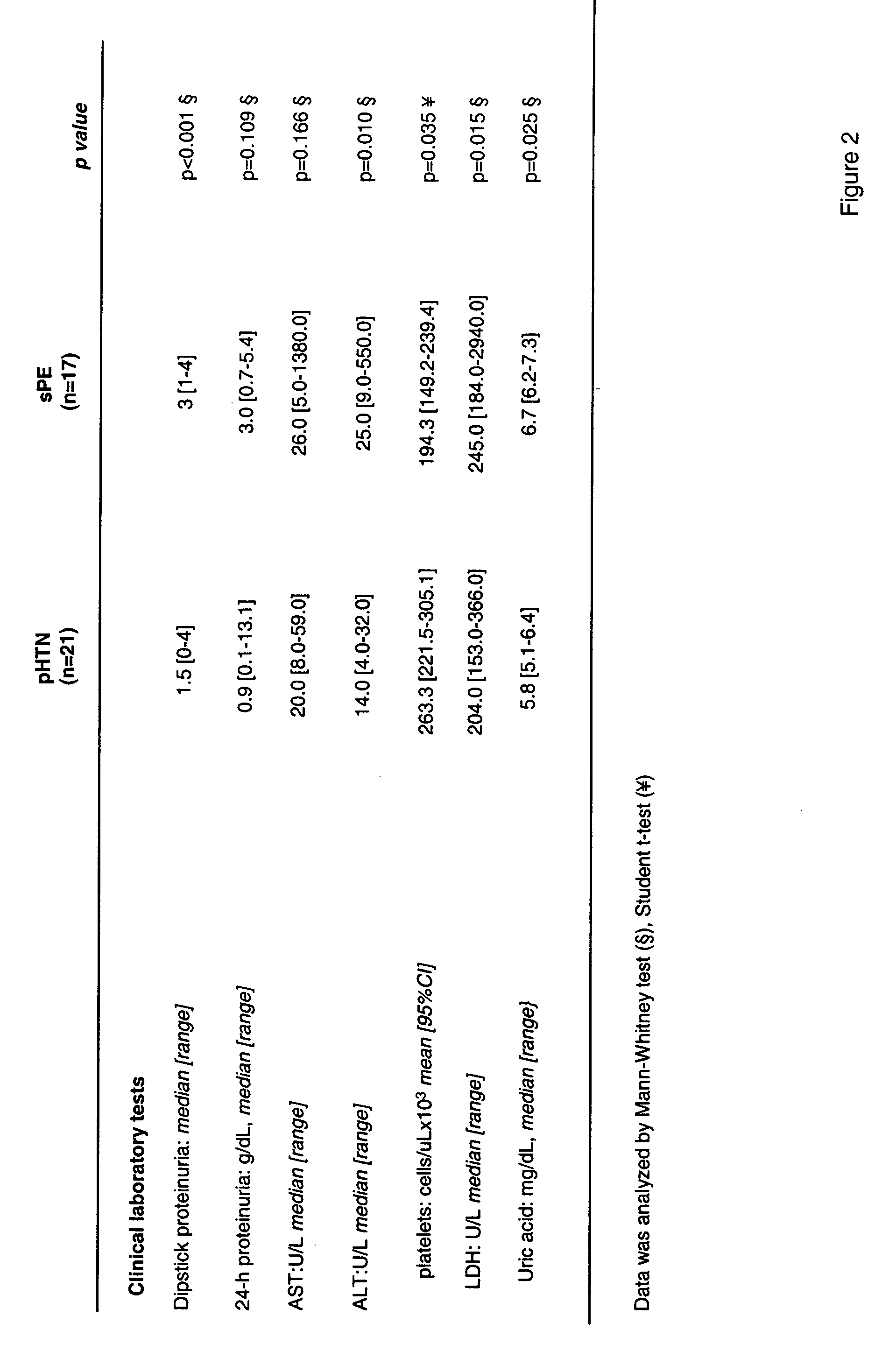
Producing too much or too little urine needs medical attention. Polyuria is a condition of excessive production of urine (> 2.5 L/day), oliguria when < 400 mL are produced, and anuria one of < 100 mL per day.
Methods, compositions, and automated systems for separating rare cells from fluid samples
InactiveUS7166443B2Aid in diagnosis and prognosisBioreactor/fermenter combinationsBiological substance pretreatmentsCancer cellRed blood cell
The present invention recognizes that diagnosis and prognosis of many conditions can depend on the enrichment of rare cells from a complex fluid sample. In particular, the enrichment of fetal cells from maternal samples, such as maternal blood samples, can greatly aid in the detection of fetal abnormalities or a variety of genetic conditions. In addition, the present invention recognizes that the enrichment of rare malignant cells from patient samples, can aid in diagnosis, prognosis, and development of therapeutic modalities for patients. The invention includes microfabricated filters for filtering fluid samples and methods of enriching rare cells of fluid samples using microfabricated filters of the present invention. The invention also includes solutions for the selective sedimentation of red blood cells (RBCs) from a blood sample and methods of using selective RBC sedimentation solutions for enriching rare cells of a fluid sample. Yet another aspect of the invention is an automated system for processing a fluid sample that includes: at least one filtration chamber that includes a microfabricated filter; automated means for directing fluid flow through at least one filtration chamber of the automated system, and means for collecting enriched rare cells. The present invention also includes methods of using automated systems for separating rare cells from fluid samples. Preferred fluid samples are blood, effusion, or urine samples, and rare cells that can be enriched from such sample include nucleated red blood cells and cancer cells.
Owner:AVIVA BIOSCI
Analyte test system for determining the concentration of an analyte in a physiological or aqueous fluid
InactiveUS20050196747A1Cheap productionReliable resultsBioreactor/fermenter combinationsBiological substance pretreatmentsSmall sampleQuality control system
This invention provides a device for determining the concentration of an analyte like glucose, cholesterol, free fatty acids, triglycerides, proteins, ketones, phenylalanine or enzymes, in a physiological or aqueous fluid like blood, serum, plasma, saliva, urine, interstitial and / or intra-cellular fluid, the device having an integrated calibration and quality control system suitable for dry reagent test strips with a very small sample volume of about 0.5 μL based on to a new sample distribution system. The production of the inventive analyte test element involves only a small number of uncomplicated production steps enabling an inexpensive production of the strips.
Owner:EGOMEDICAL SWISS
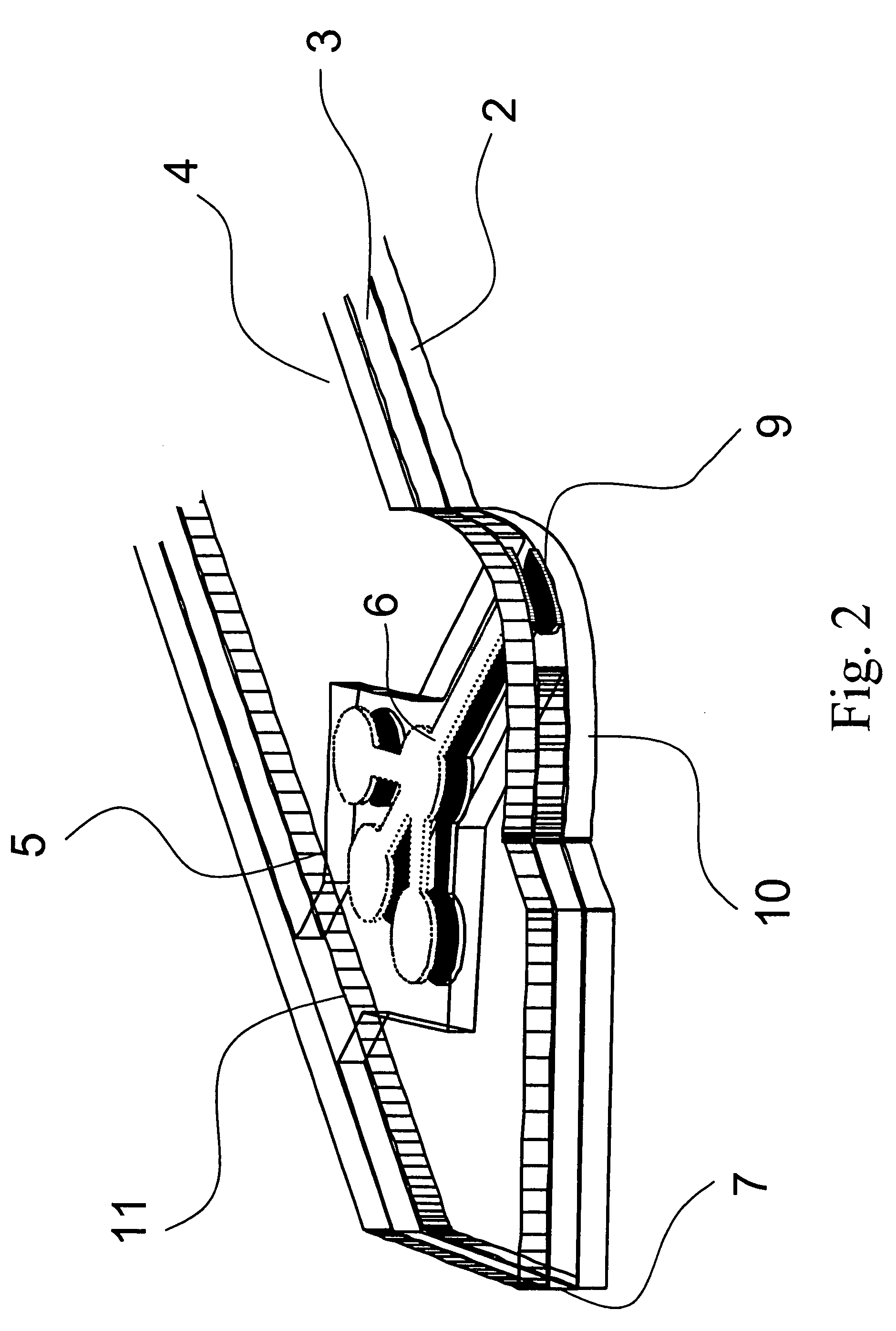
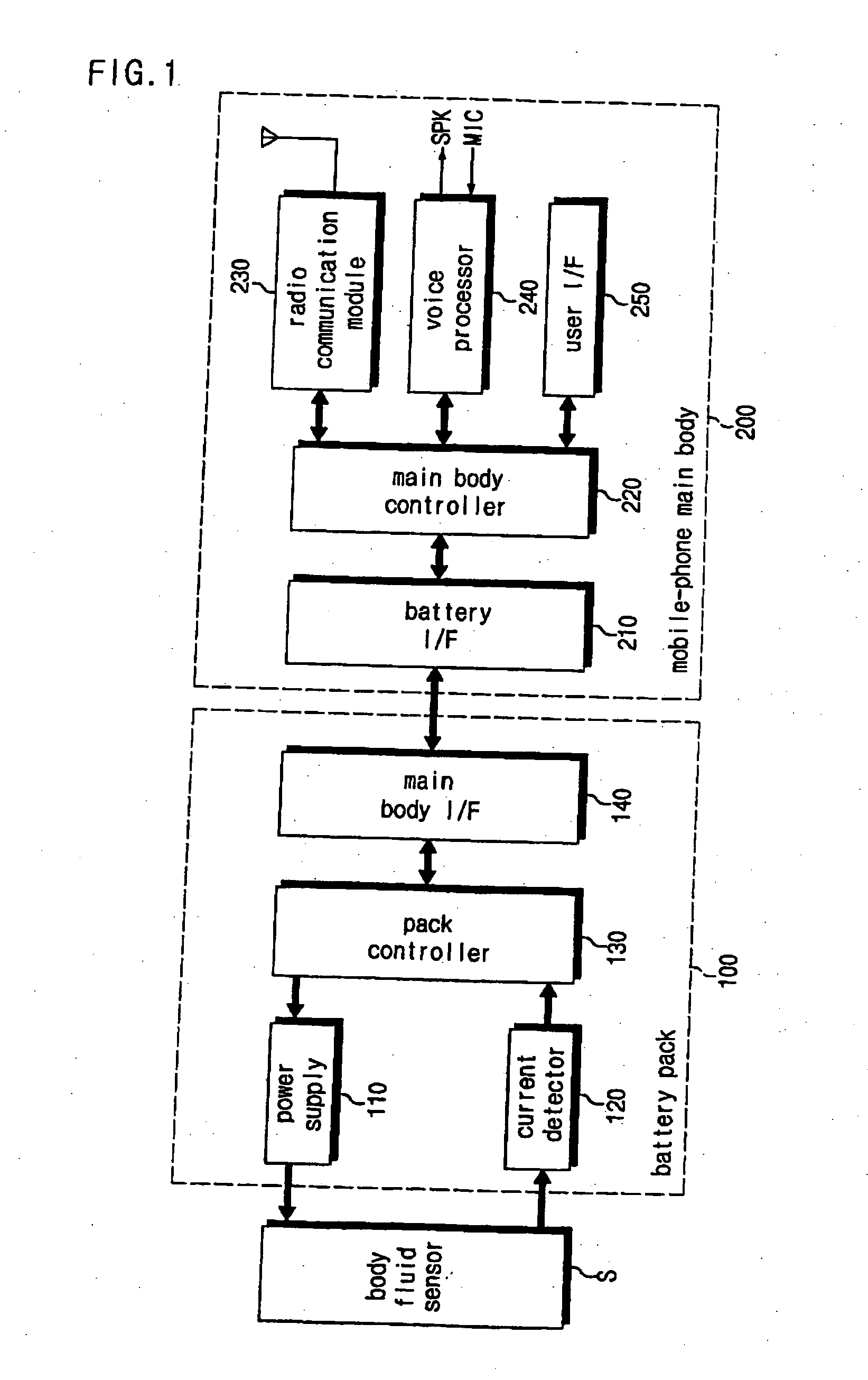
Battery pack of a mobile communication terminal to be capable of reading output of bio-sensors and self-diagnosis system
A battery pack for self-diagnosis and a system using the same, which can read a data value from a body fluid sensor (referred to as a biosensor) reacting with body fluid such as urine, and indicate a measurement value of a test item such as a blood glucose level, a cholesterol level or etc. The battery pack includes: a power supply for supplying electric power to a working electrode of the body fluid sensor; a current detector for detecting the amount of electric current flowing into the working electrode; a battery pack controller for controlling an electric power supply operation for the working electrode, reading, from a memory, the test item-based measurement value corresponding to the detected current amount, and outputting the read measurement value; and an interface for carrying out an interface function so that the test item-based measurement value outputted from the battery pack controller can be sent to the main body of the mobile communication terminal.
Owner:SEYFARTH SHAW
Measuring system
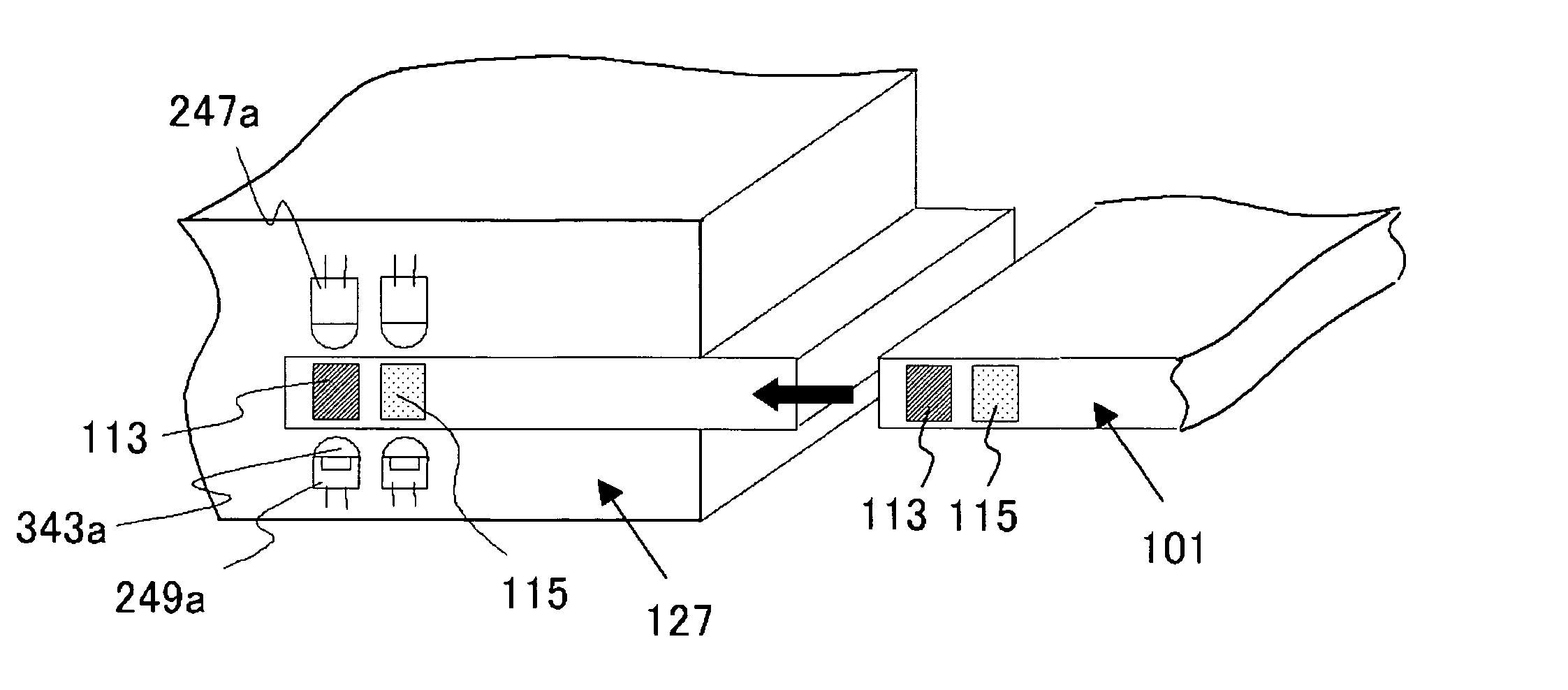
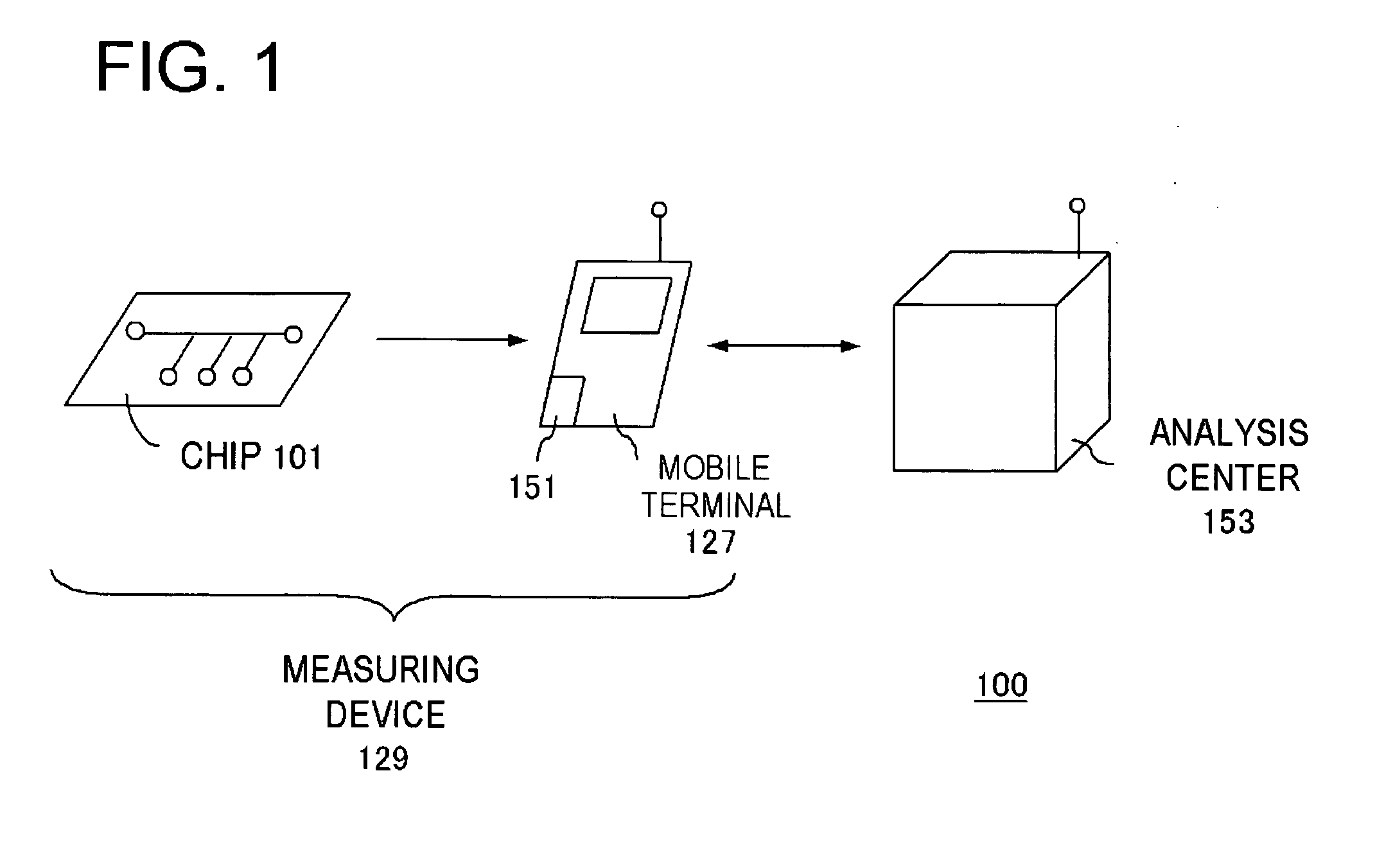
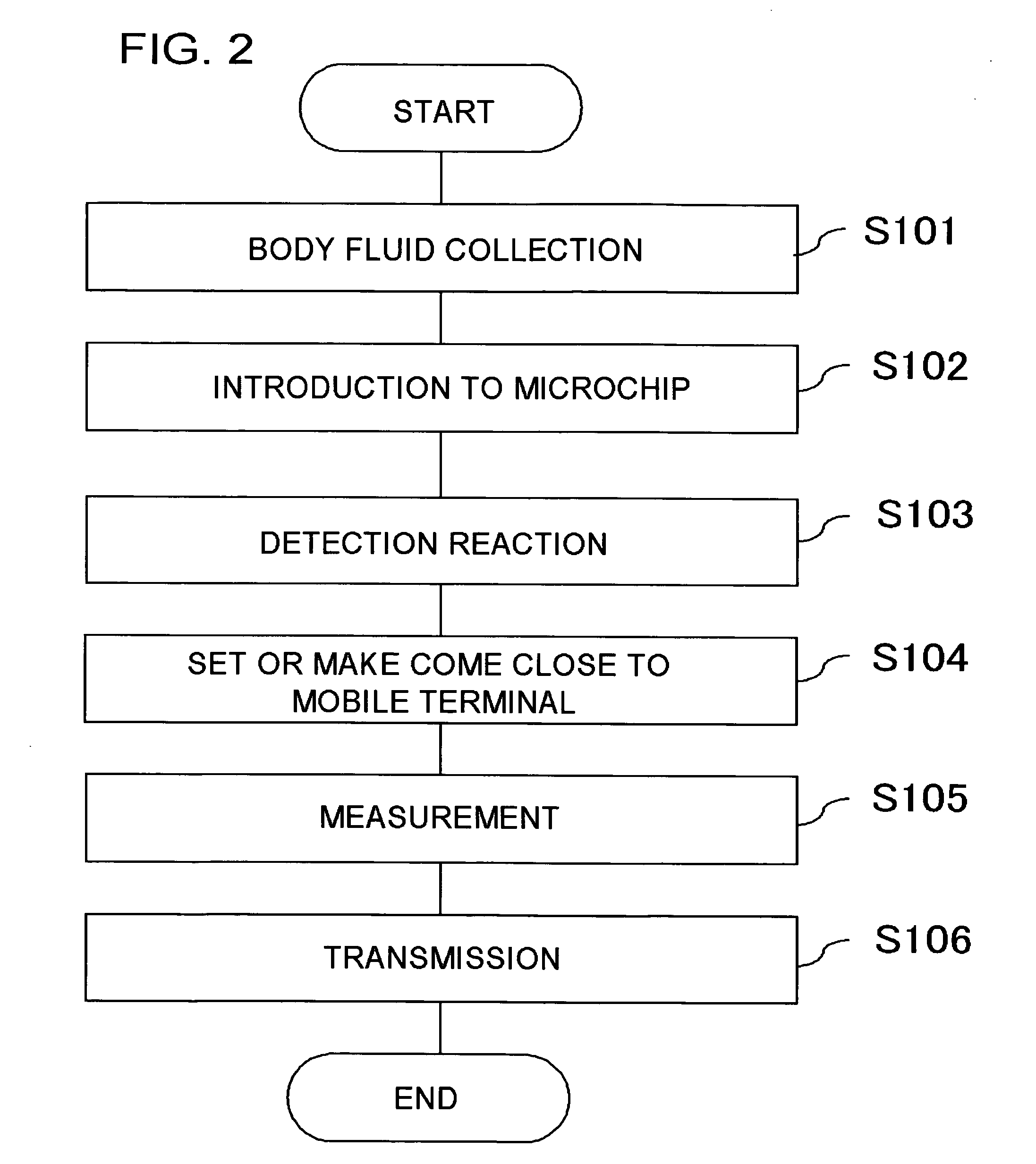
InactiveUS20060292039A1Easy to checkWithdrawing sample devicesMaterial analysis by optical meansAnalysis centerEngineering
It becomes possible for a user to check his / her own health state at a desired place without visiting inspection agencies. Furthermore, it becomes possible for a user to conveniently check his / her own health state. First, a user collects body fluid to be a measurement object, such as blood, saliva, and urine. Then, the collected body fluid is introduced to the chip (101) as a sample. Then, the sample is made to act on a detection reagent which acts on a specific component in the sample to generate a predetermined detection reaction. This chip (101) is set to the mobile terminal (127). A measurement unit (151) of the mobile terminal (127) measures an amount of a specific component in the sample by means that product of reaction is quantitated by an optical method or the like. The mobile terminal (127) transmits the measurement value to an analysis center (153).
Owner:NEC CORP
Cartridge for diagnostic assays
InactiveUS20060165558A1Gap be createAnalysis using chemical indicatorsLaboratory glasswaresFlow cellMagnetic tape
A cartridge is provided for receiving a sample, including but not limited to blood, urine, and the like. The cartridge includes an input port or dock for receiving a sample from a sample container. A first chamber is in fluid communication with the input port. A flow cell is in fluid communication with the first chamber. The flow cell contains at least one reagent. The reagent can be a calibrant, a fluid containing reactant, a fluid not containing a reactant, a sample, and the like. A pressure port is configured to be coupled to a pressure source, including but not limited to a syringe pump, and the like. A vent port is also provided. The cartridge is configured to maintain fluids in a sealed manner.
Owner:FASTRAQ
Catheters with suction capability and related methods and systems for obtaining biosamples in vivo
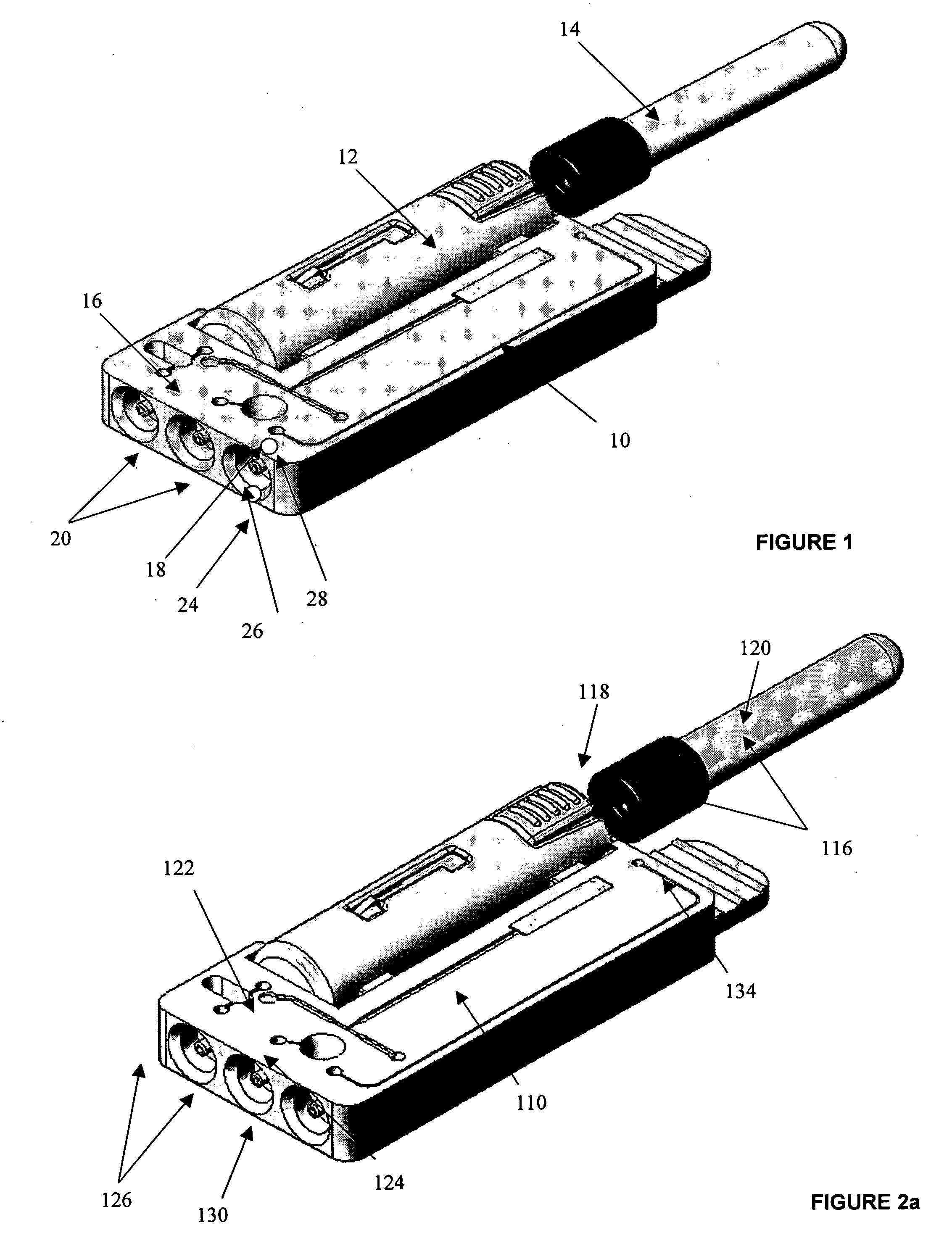
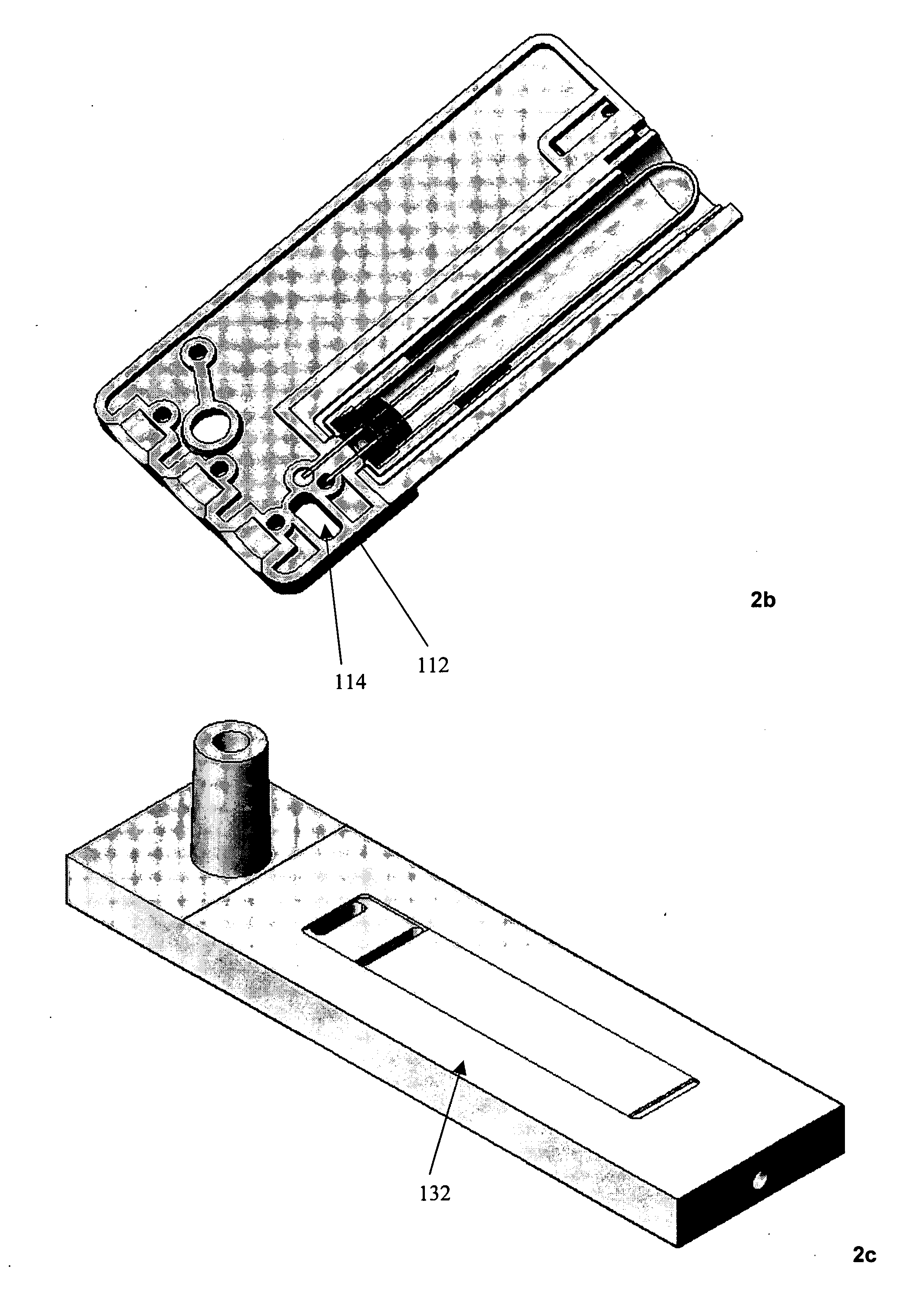
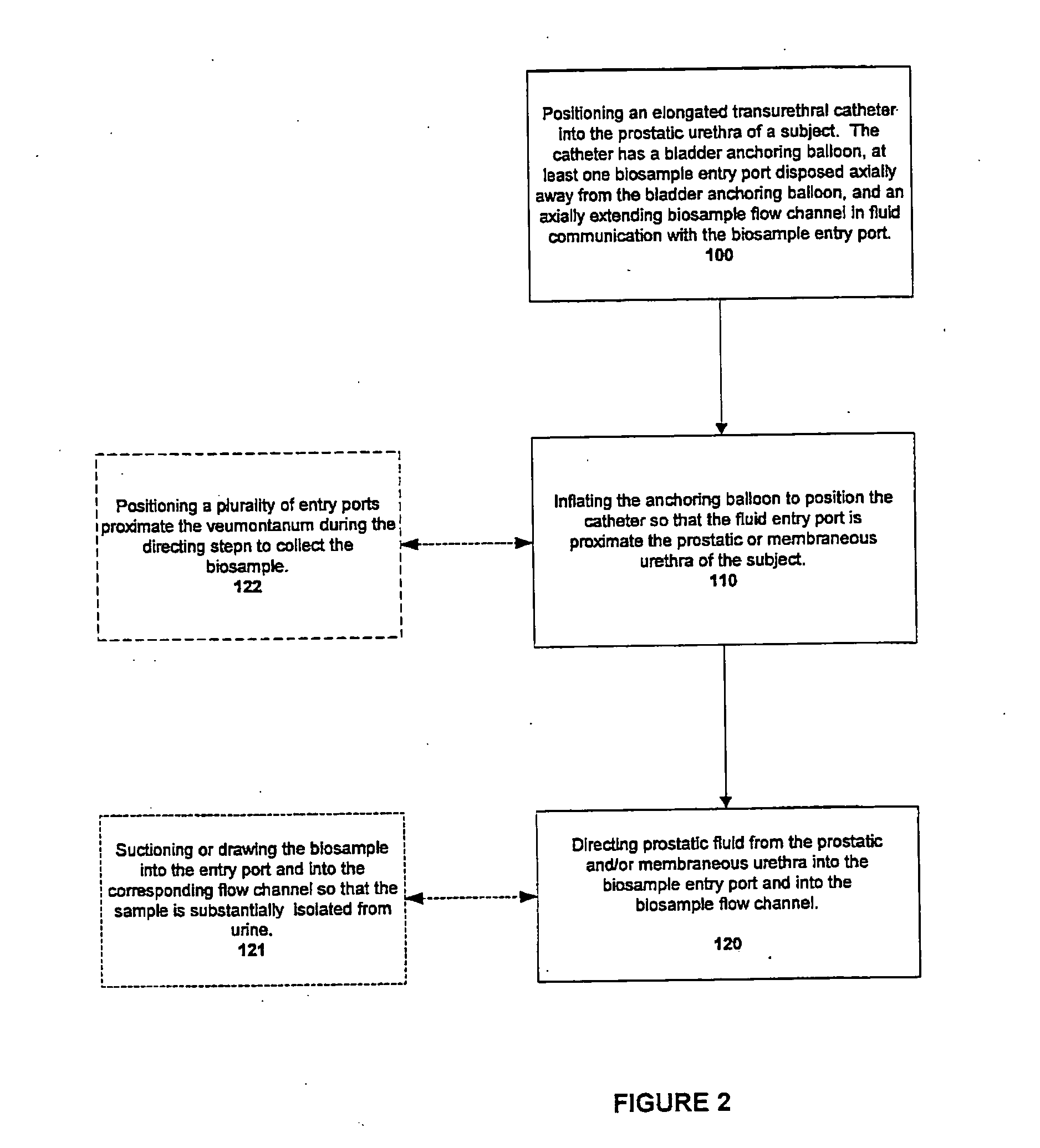
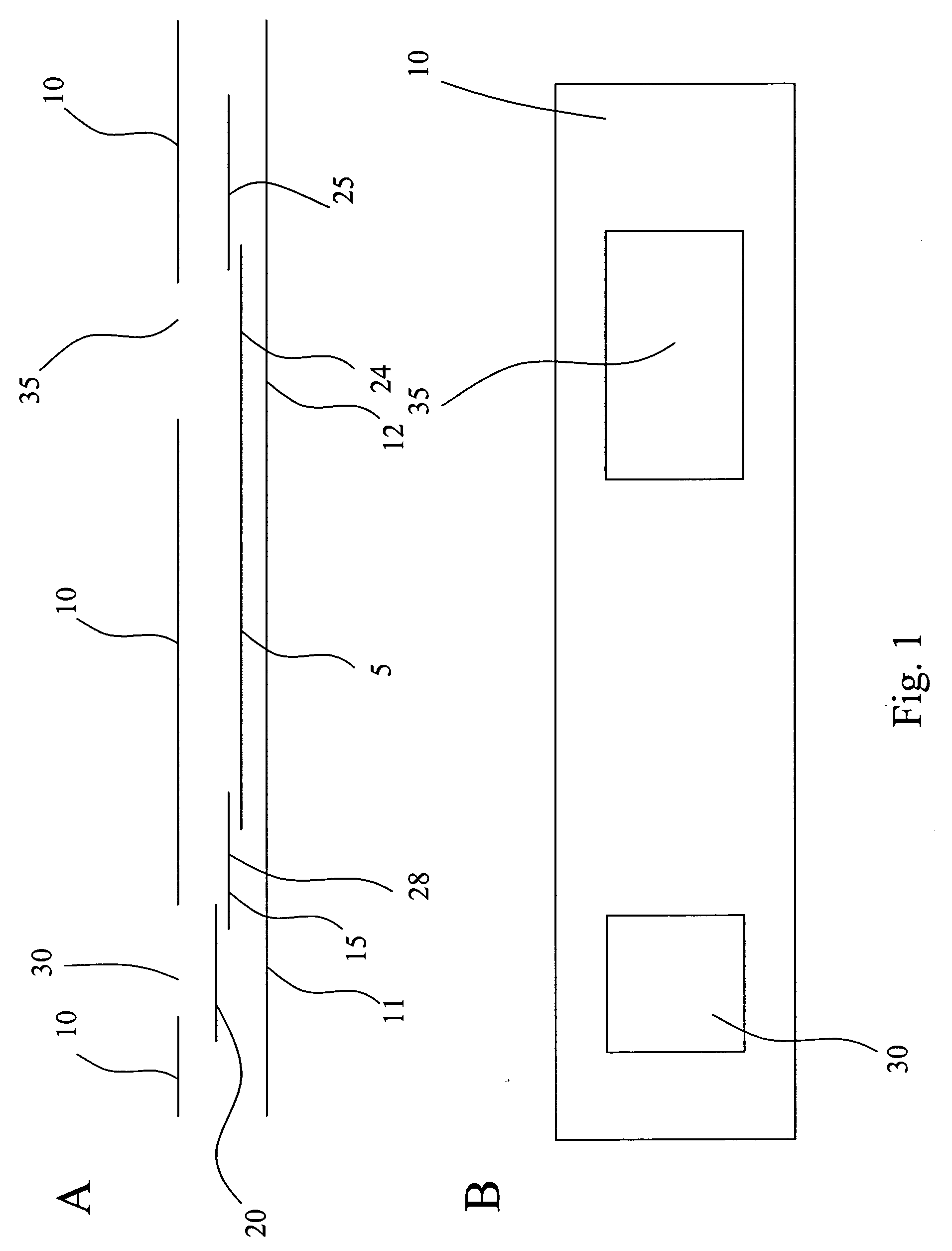
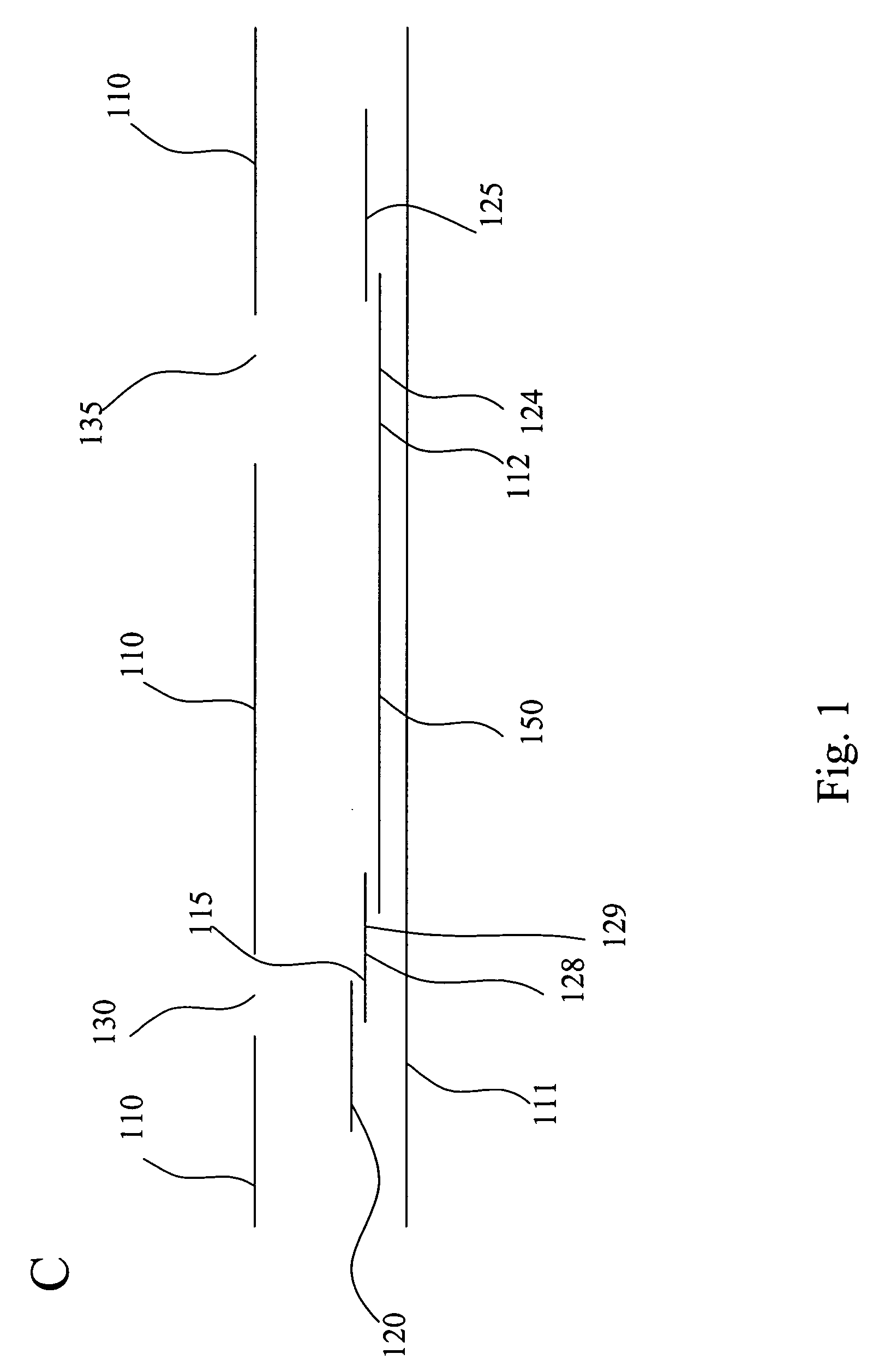
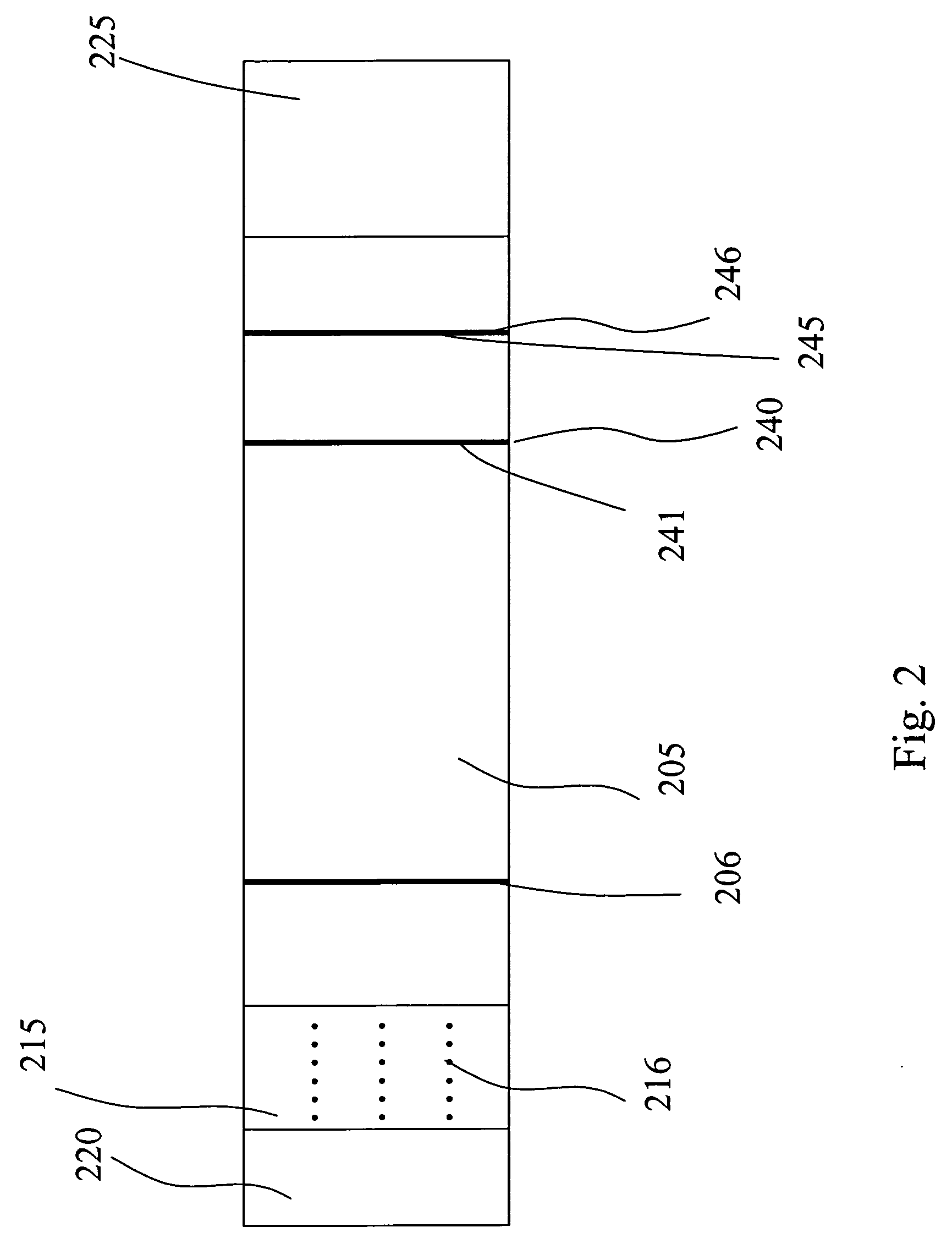
InactiveUS20050054994A1Convenient treatmentImprove responsivenessStentsSurgical needlesUrethraProstatic fluid sample
Methods, systems, and computer program products for obtaining a sample in vivo and / or treating a subject include positioning an elongated transurethral catheter in the prostatic urethra of a subject, the catheter having a bladder anchoring balloon, at least one biosample entry port disposed axially away from the bladder anchoring balloon, and an axially extending biosample flow channel in fluid communication with the biosample entry port held internally in the catheter. The anchoring balloon is inflated to position the catheter so that the fluid entry port is proximate the prostatic urethra of the subject and prostatic fluid is suctioned from the prostatic urethra into the biosample entry port and into the biosample flow channel. The catheter can include a thermal treatment balloon and / or dilatation balloon and the suctioned sample can be obtained concurrently with, during, or proximate in time to the applied treatment. The catheter can be configured to allow urine to drain therethrough during the treatment / collection of the sample while keeping the urine isolated from the collected prostatic fluid sample.
Owner:WIT IP CORP
Diagnostic assay device
This invention relates to assays for an analyte in a liquid sample such as a body fluid. More particularly, the invention relates to a method and apparatus for the detection of a ligand in a body fluid such as urine or blood, which includes an immobilization zone for interfering agents in a sample.
Owner:SEKISUI DIAGNOSTICS
Method and system for microfluidic manipulation, amplification and analysis of fluids, for example, bacteria assays and antiglobulin testing
A microfluidic system for isolation and amplification of DNA or RNA from aqueous solutions and detection of the DNA or RNA on a lateral flow detection strip, including a disposable microfluidic card for use in analysis of bacteria in platelets and an analysis of sexually transmitted diseases (STD) in urine. The card will include an embedded membrane that filters out cells and cellular debris. Any biological debris on the membrane will be lysed and the DNA or RNA amplified via PCR amplification protocol, including appropriate reagents and thermal cycling conditions. The amplified DNA or RNA are transferred to a lateral flow detection strip for a visual diagnostic read out. An alternate embodiment includes a microfluidic card for use in typing antiglobulin assays.
Owner:PERKINELMER HEALTH SCIENCES INC
Production of hSA-linked butyrylcholinesterases in transgenic mammals
InactiveUS20060253913A1Promote formationImprove stabilityAnimal cellsVectorsMammalButyrylcholinesterase
The present invention provides methods for the large-scale production of recombinant butyrylcholinesterase fused to human serum albumin in cell culture, and in the milk and / or urine of transgenic mammals. The recombinant butyrylcholinesterase-albumin fusion protein of this invention can be used to treat and / or prevent organophosphate pesticide poisoning, nerve gas poisoning, cocaine intoxication, and succinylcholine-induced apnea.
Owner:PHARMATHENE
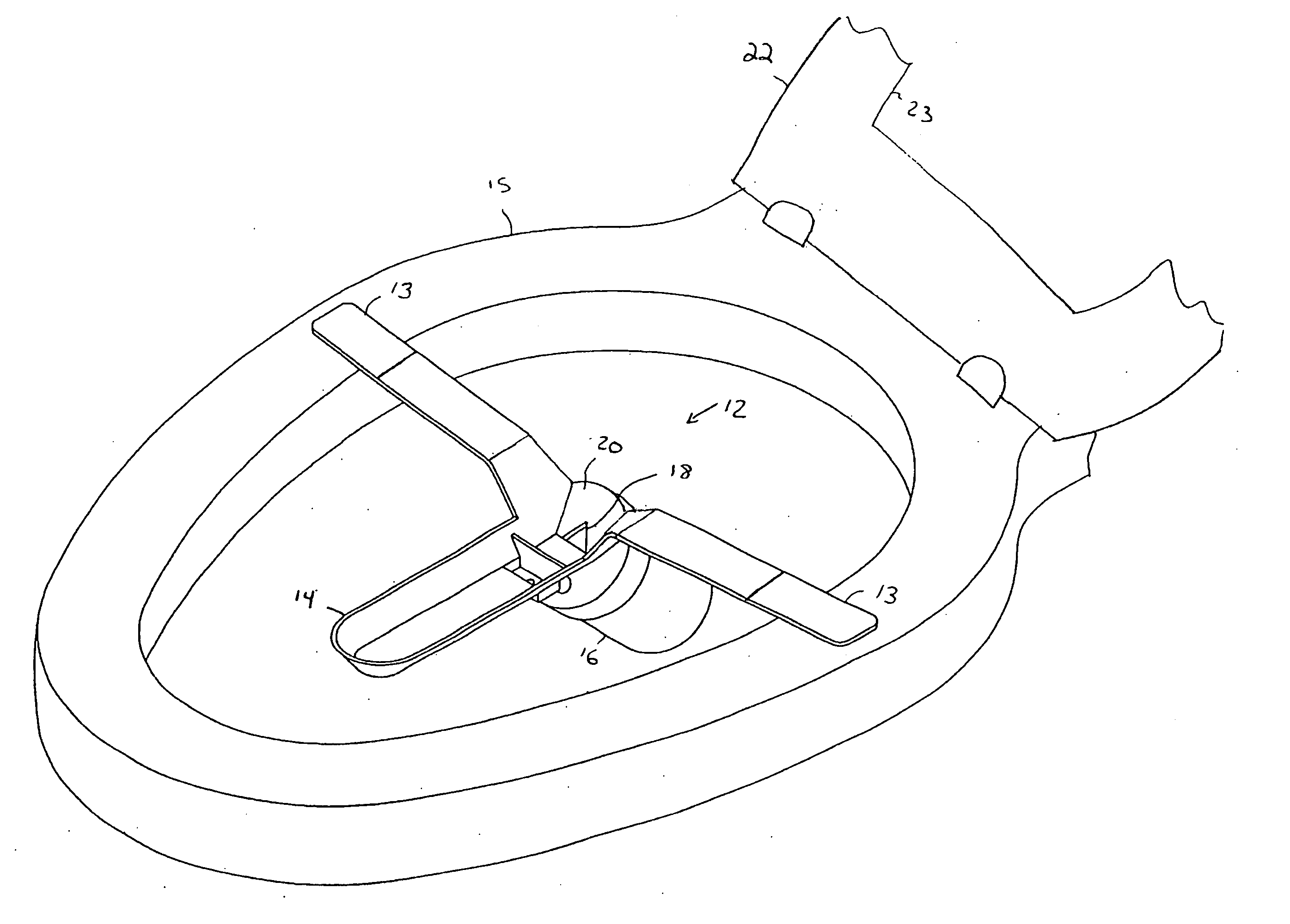
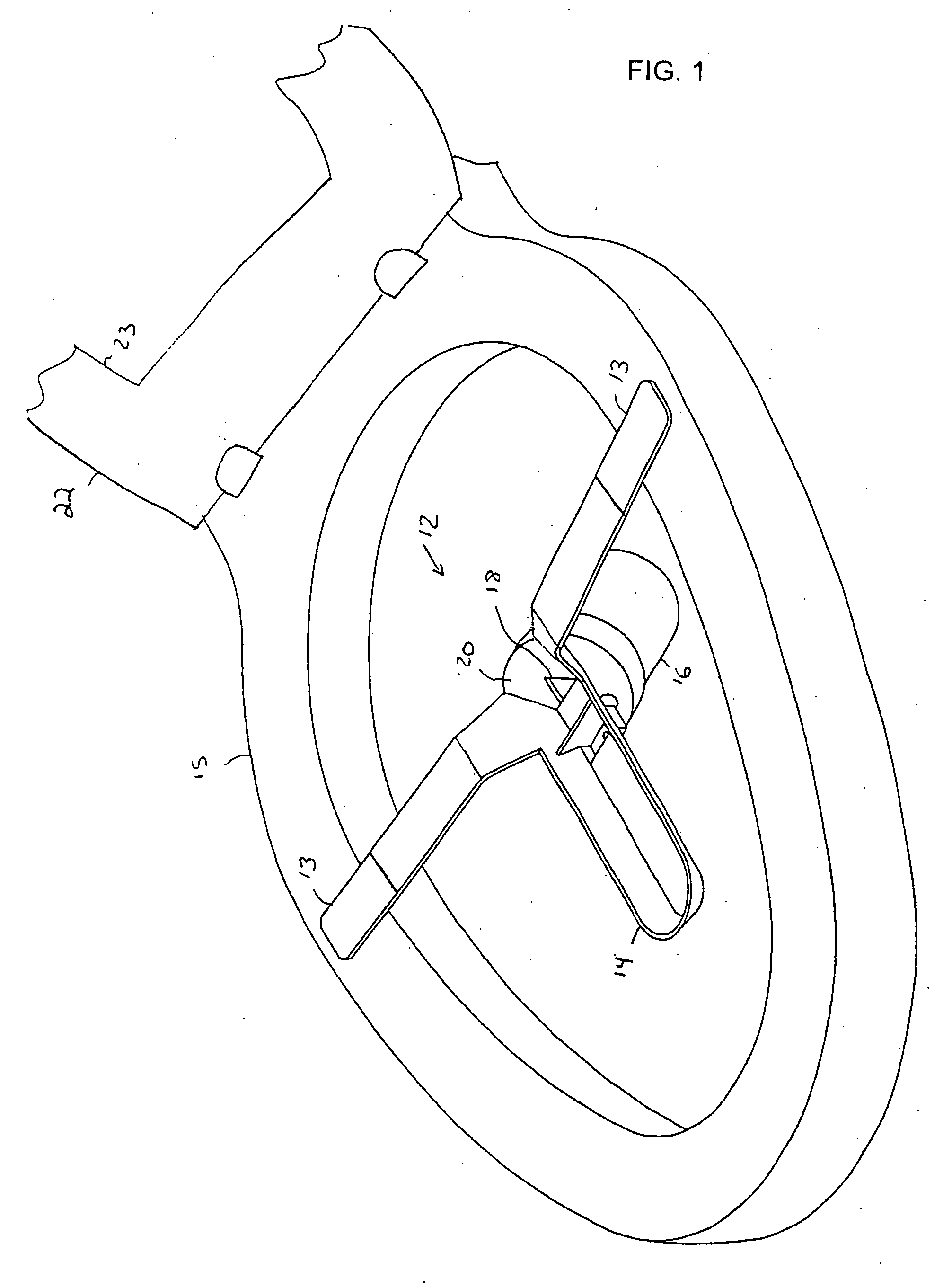
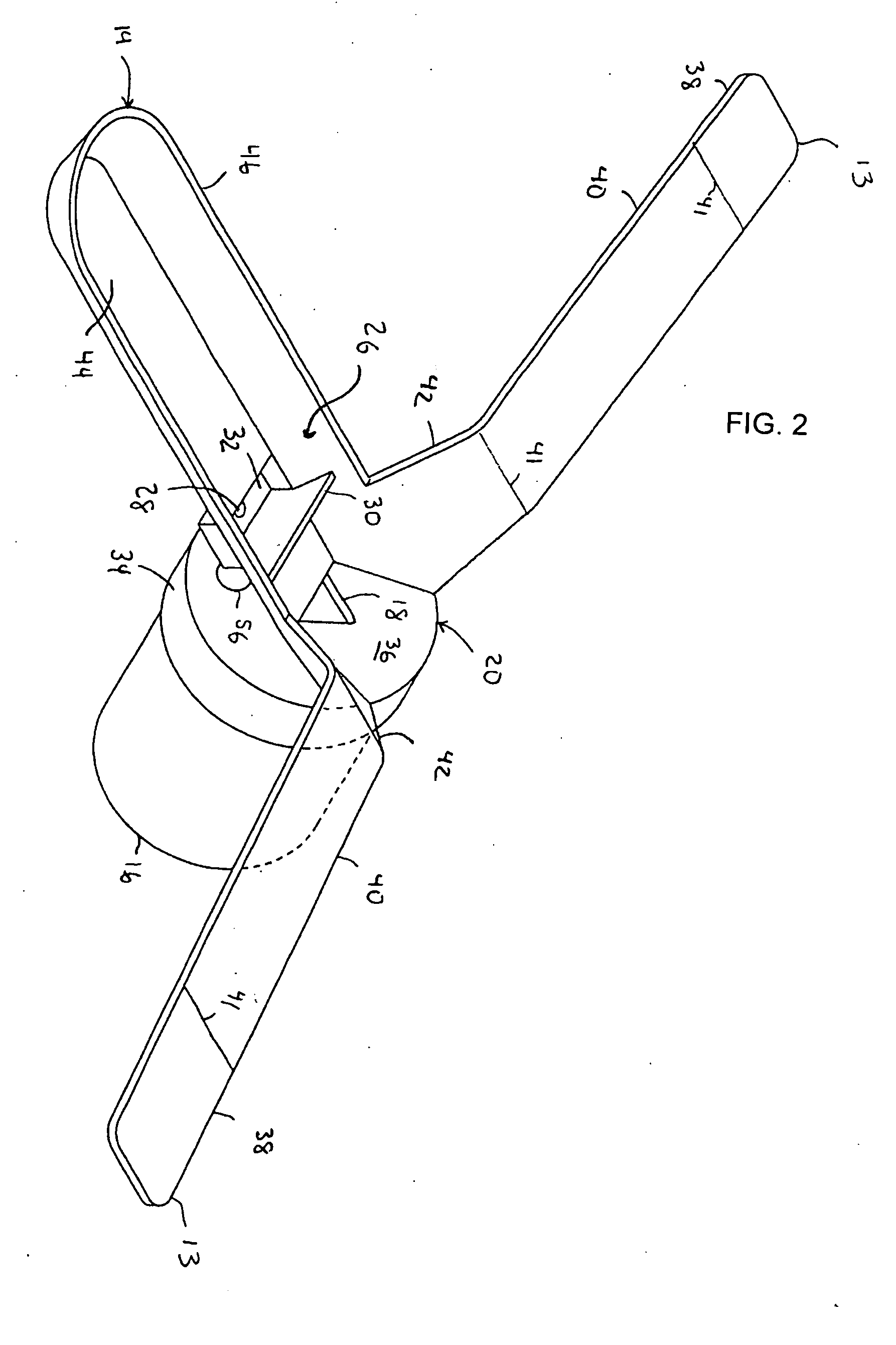
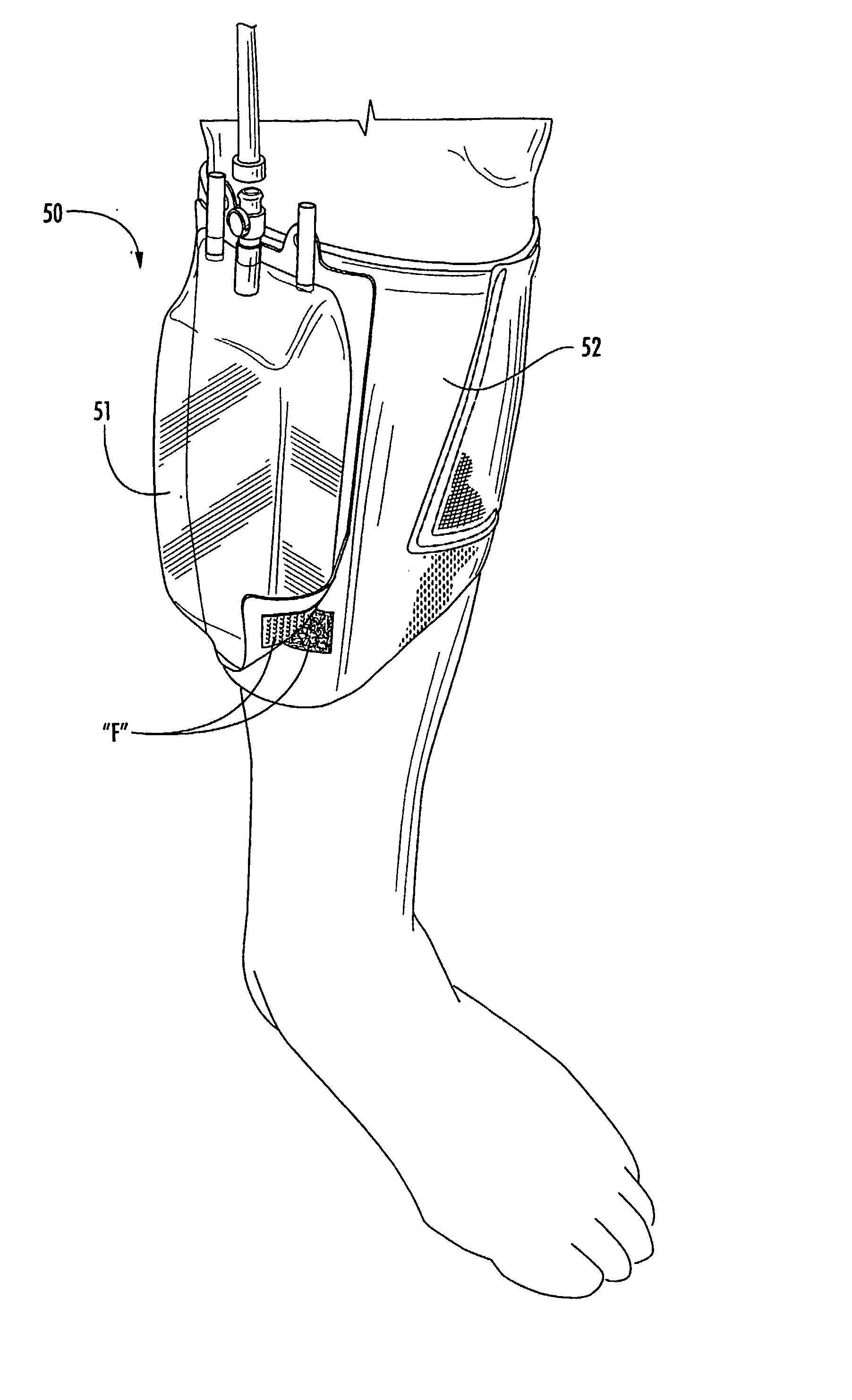
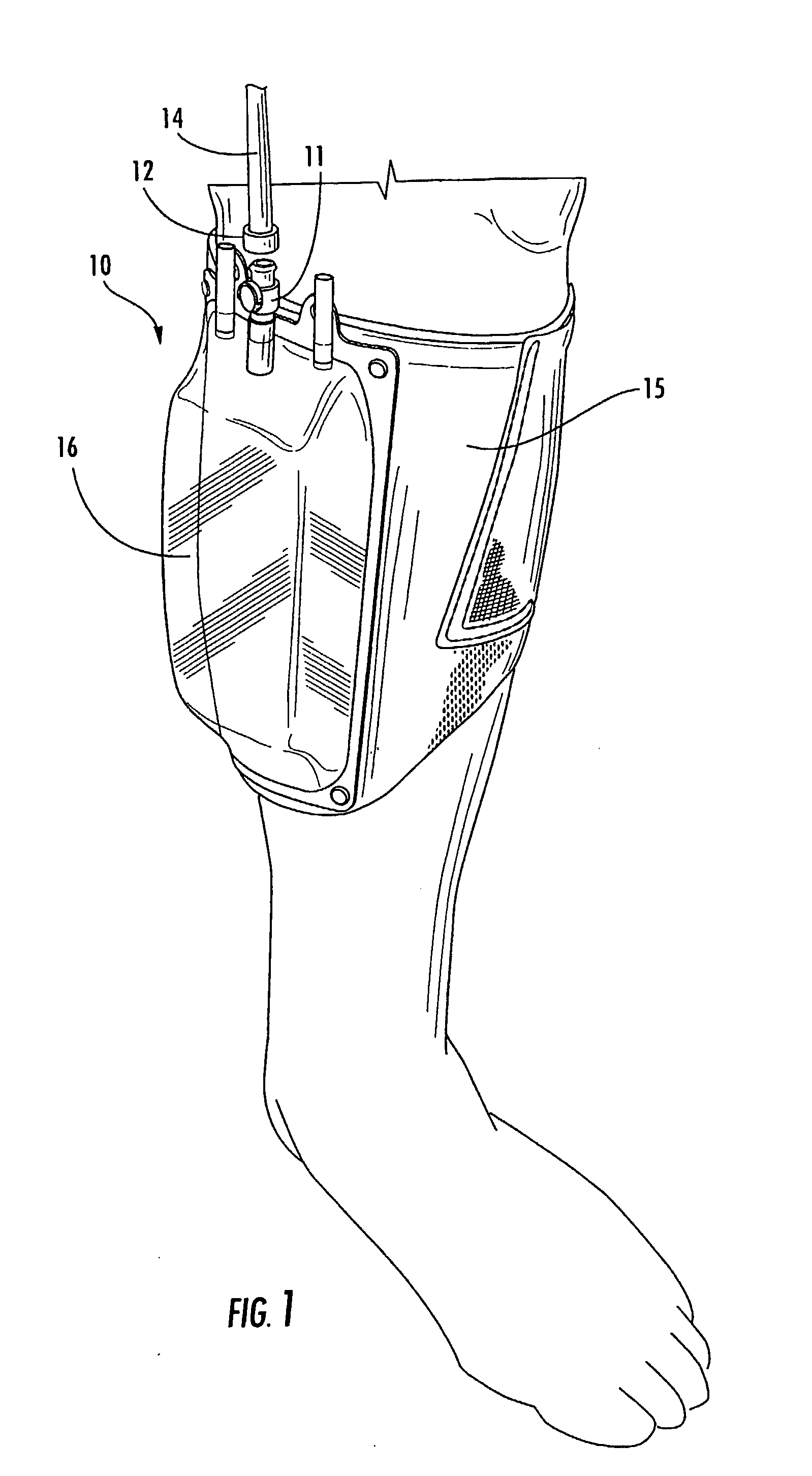
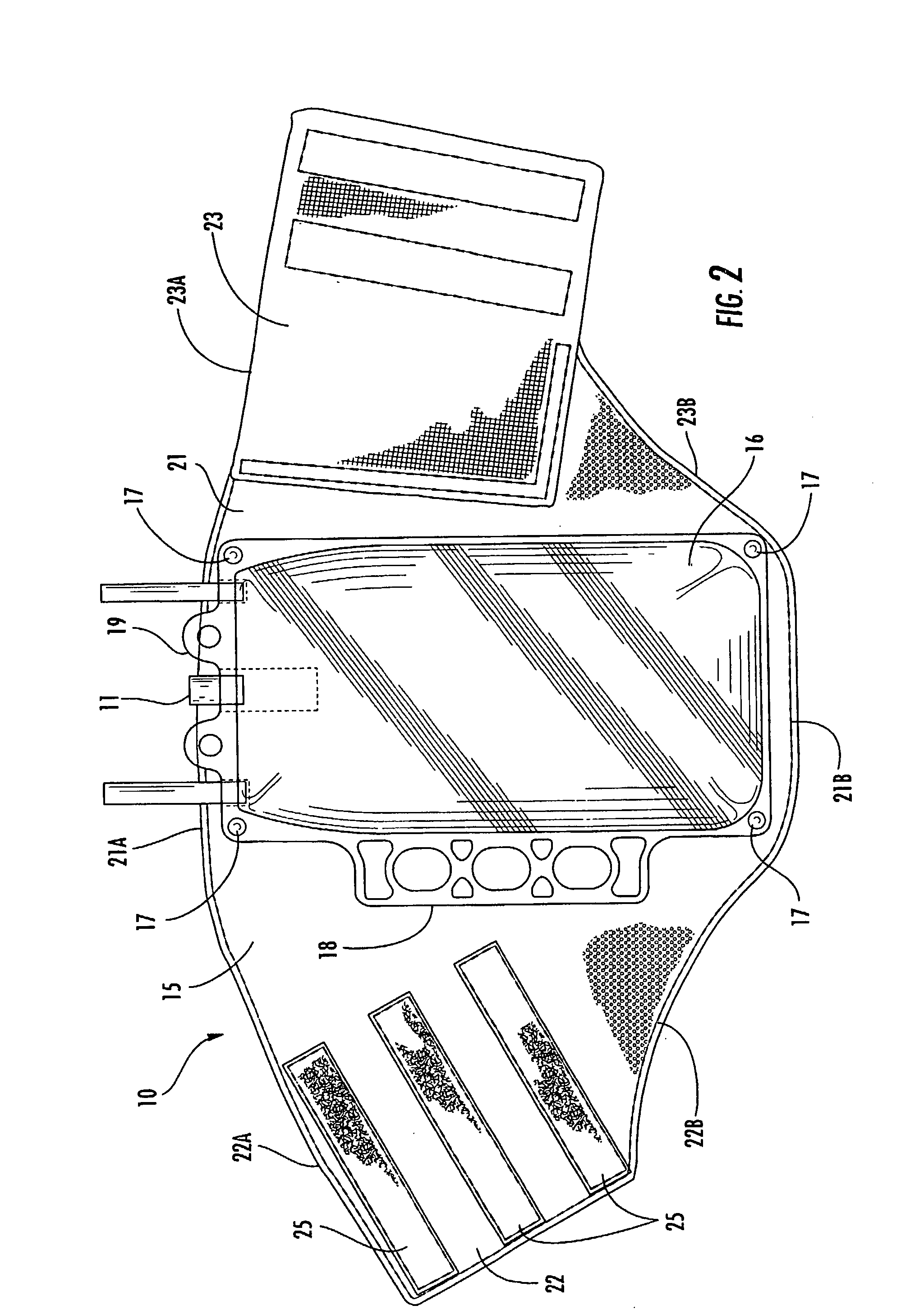
Multi-functional and modular urine collection system
ActiveUS20070010797A1Reduce retentionImprove wettabilityWound drainsMedical devicesCollection systemUrine Collections
Multi-functional urine collection devices, embodiments of which can include a self-expanding container having a receptacle for receiving urine from the tubing, a pump for moving urine through the tubing and into a receptacle, extendable tubing that may be shortened and / or lengthened, and / or one or more meters for monitoring, measuring, transmitting or storing a characteristic from the urine.
Owner:CR BARD INC
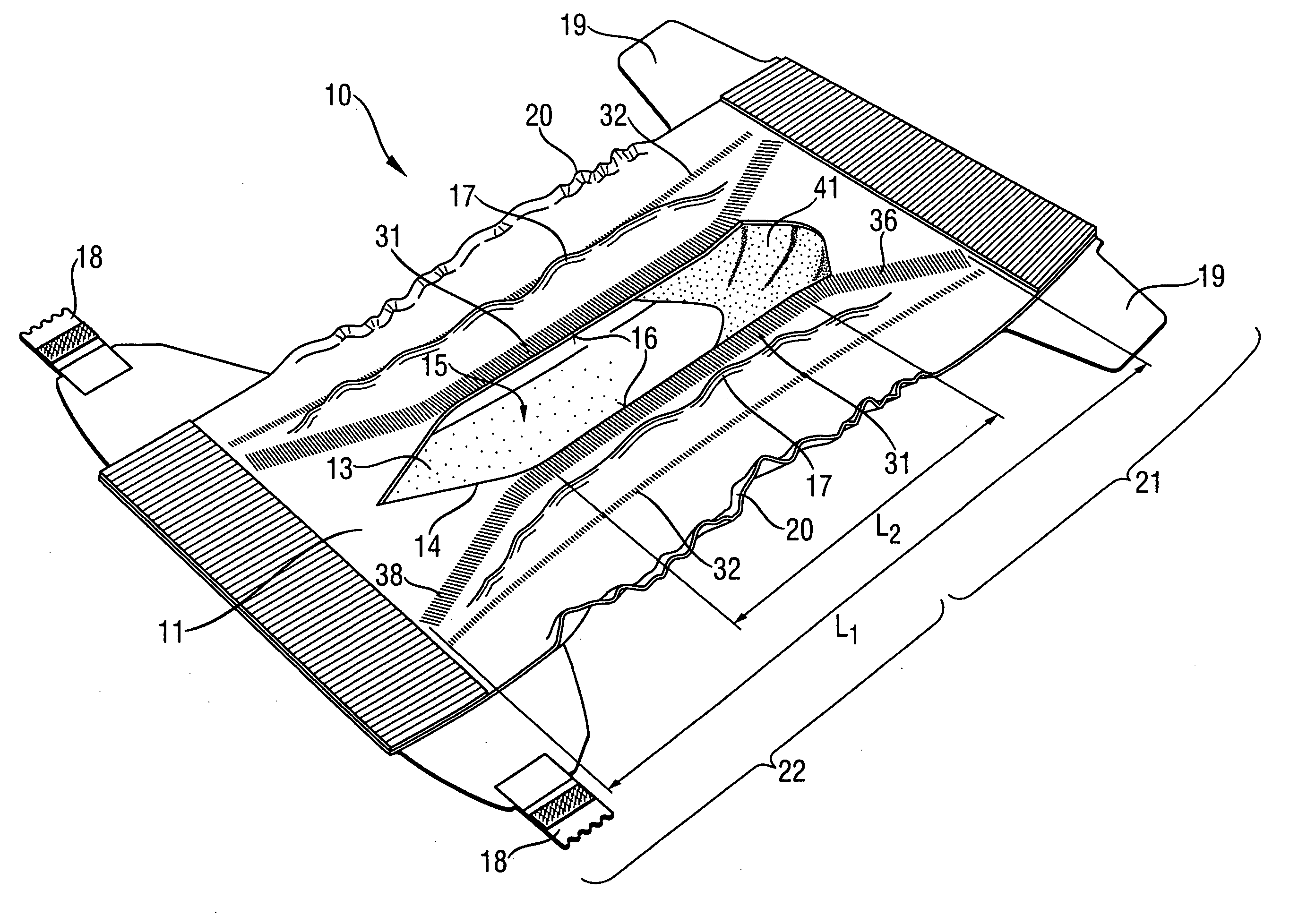
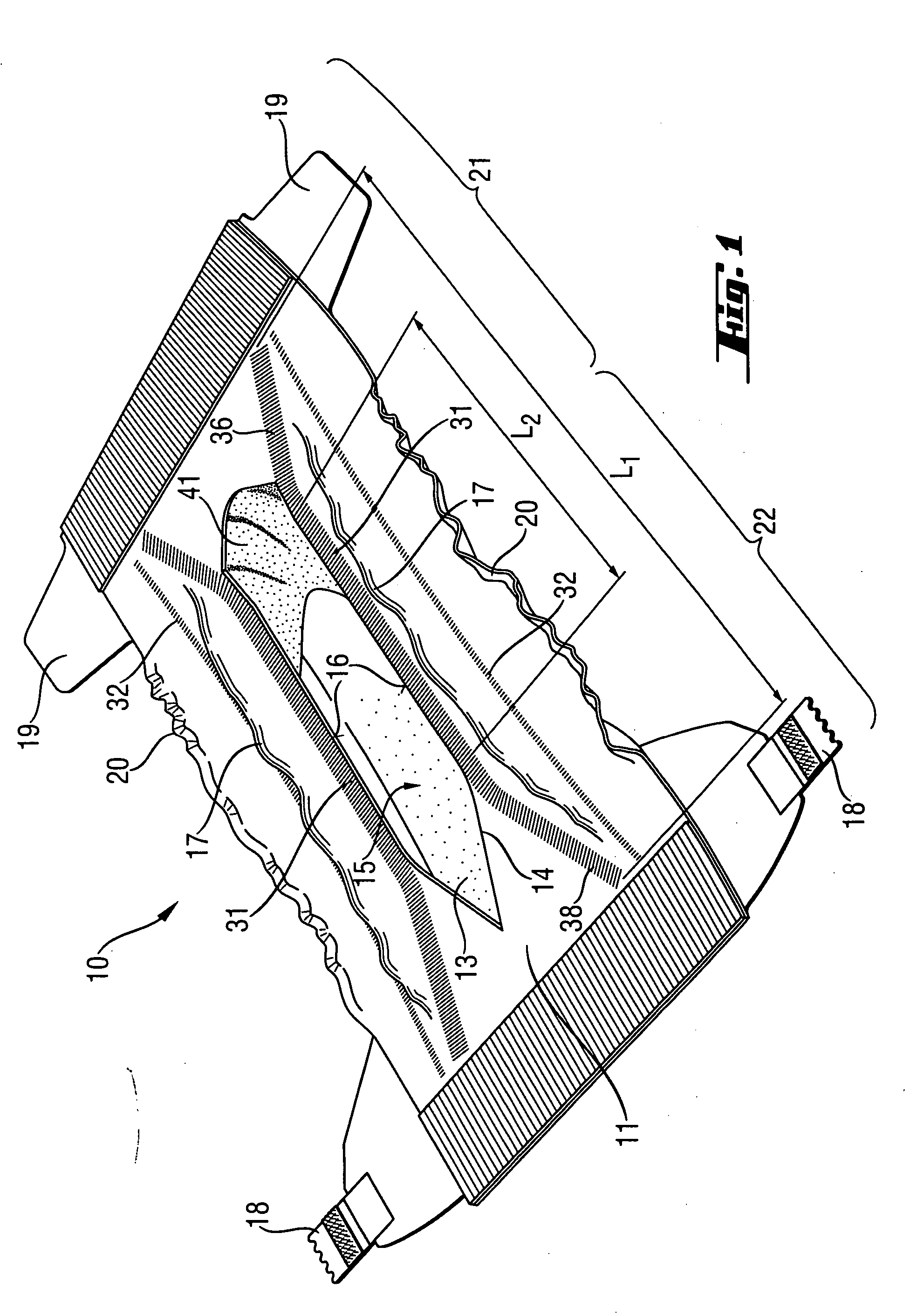
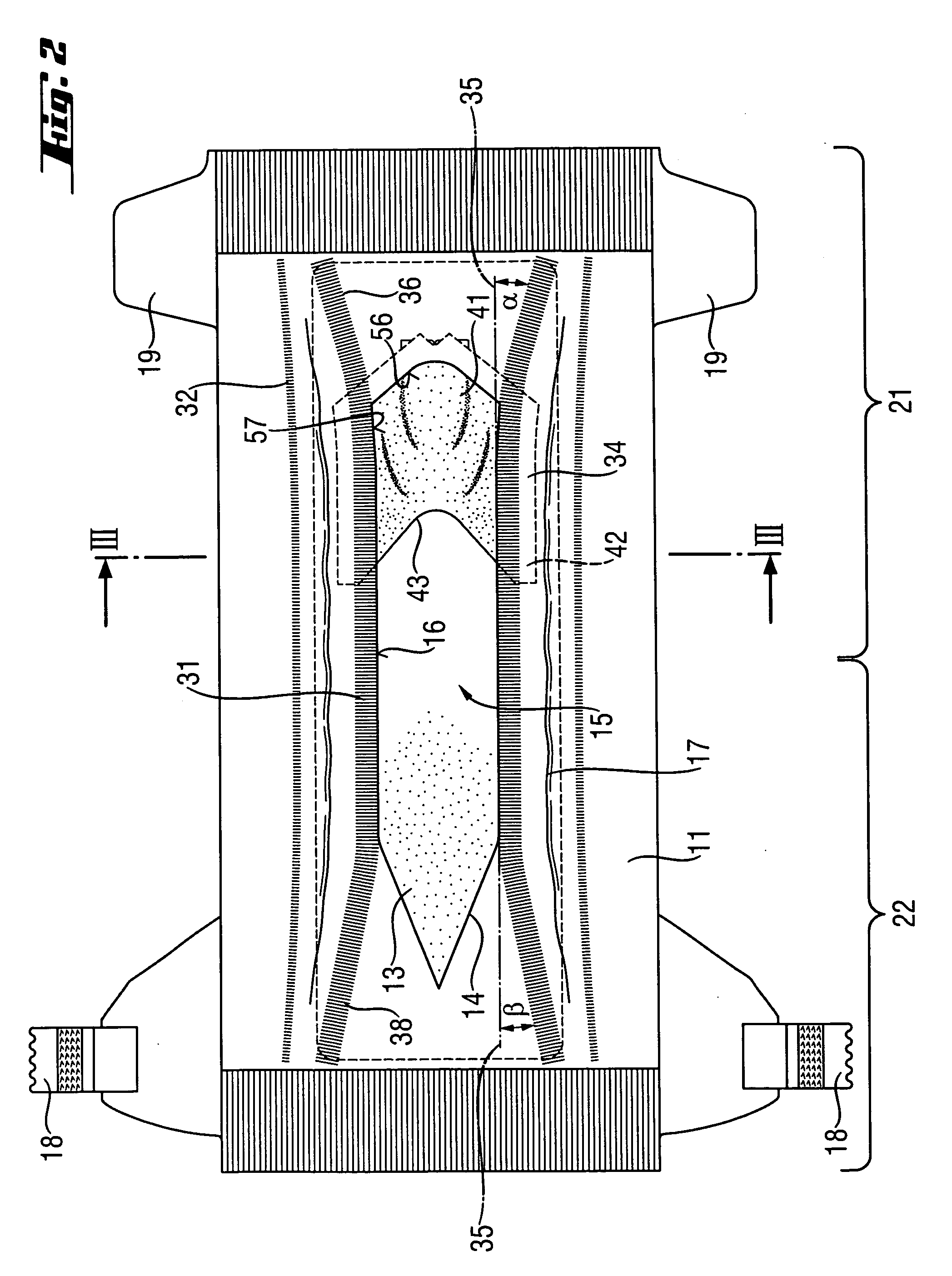
Absorbent article with urine-permeable coversheet
This invention is directed to an absorbent article, preferably a diaper or training pants, having a backsheet, an absorbent core and a topsheet, provided with at least one opening adapted to receive fecal material, comprising also a genital coversheet, which in use covers the genitals, and which is positioned in, under or above part of the opening, such that a void space can be created between the genital coversheet and the absorbent core and such that a void space is present between the topsheet and the absorbent core.
Owner:THE PROCTER & GAMBLE COMPANY
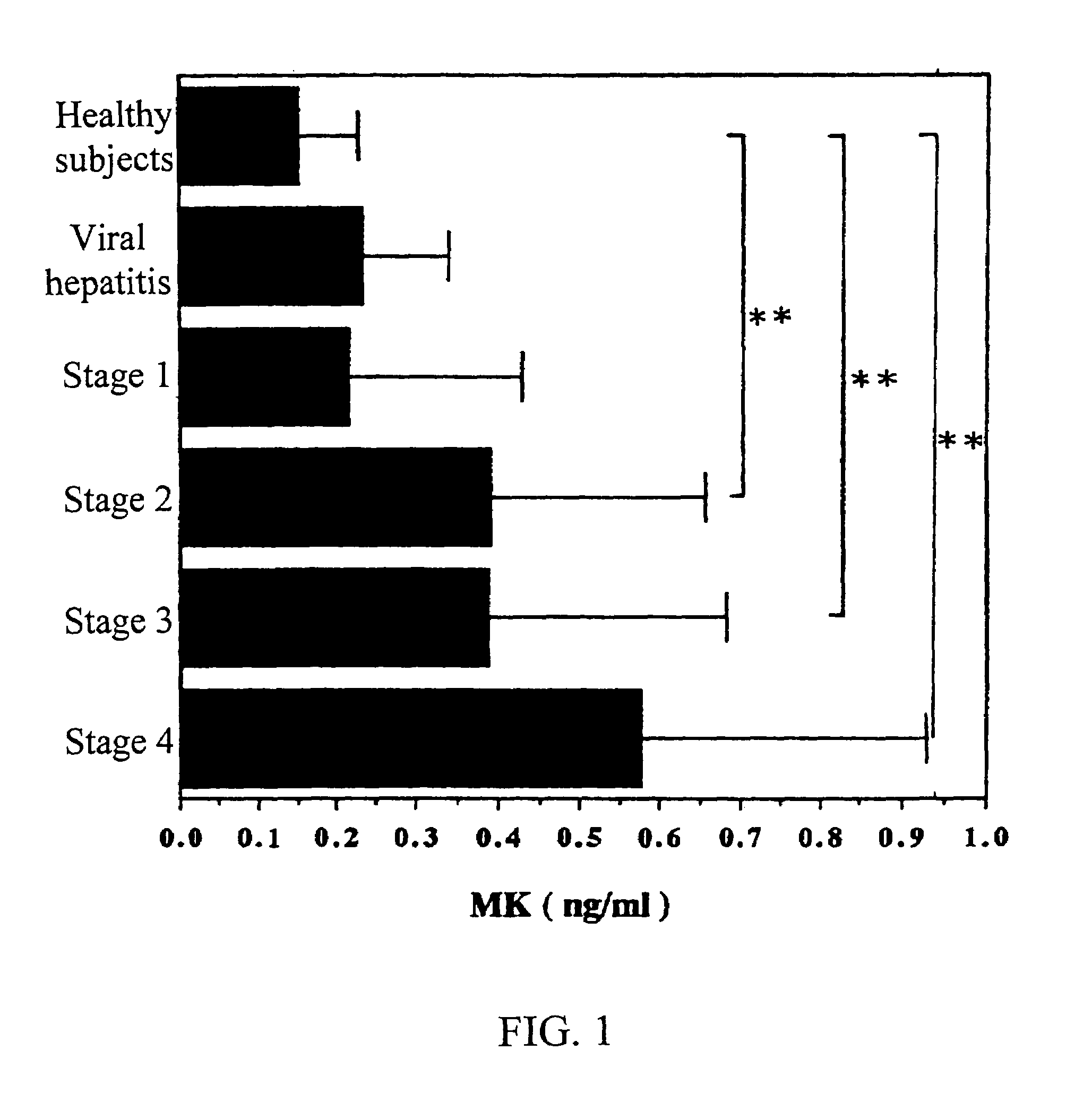
Methods for detecting early cancer
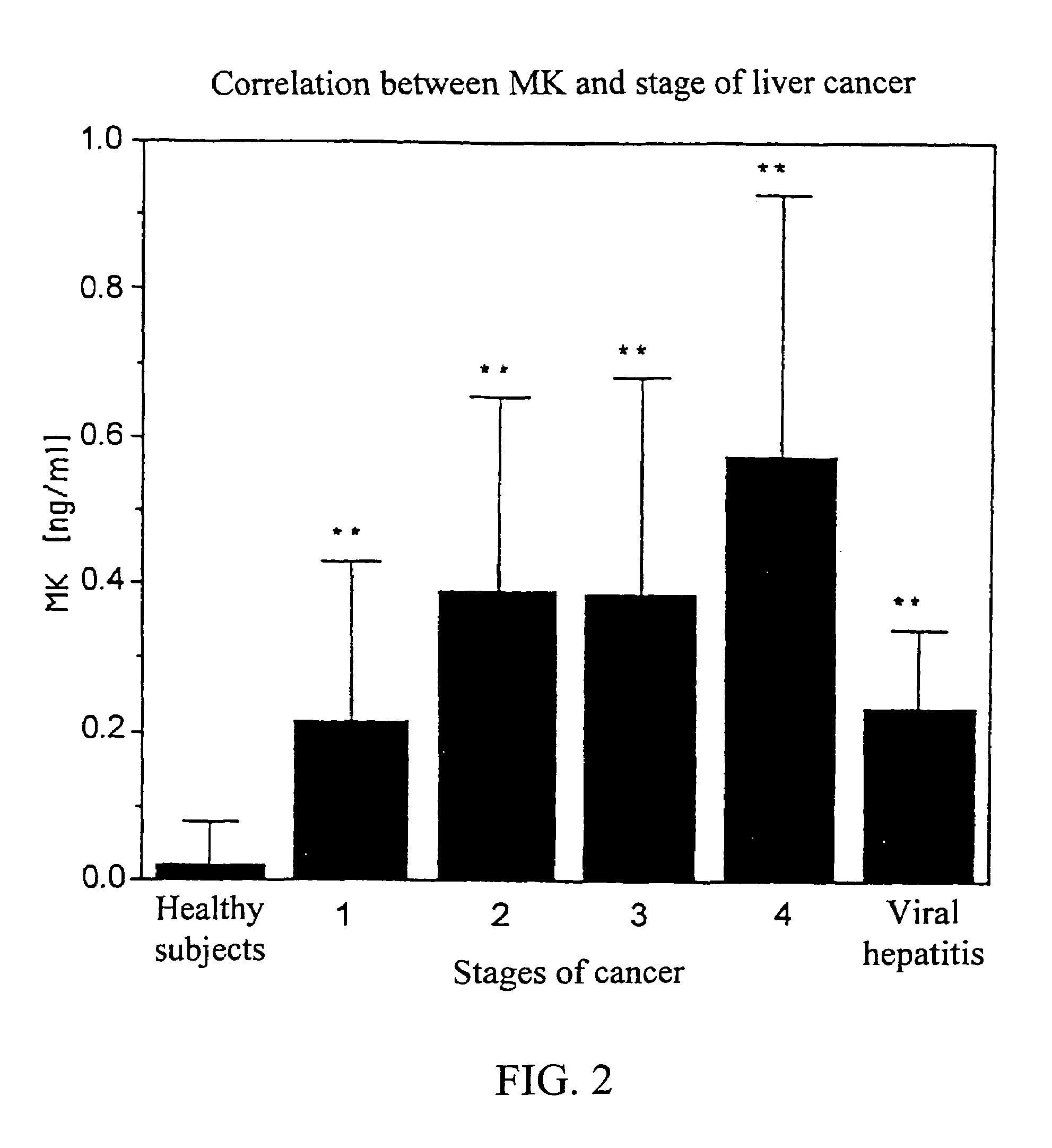
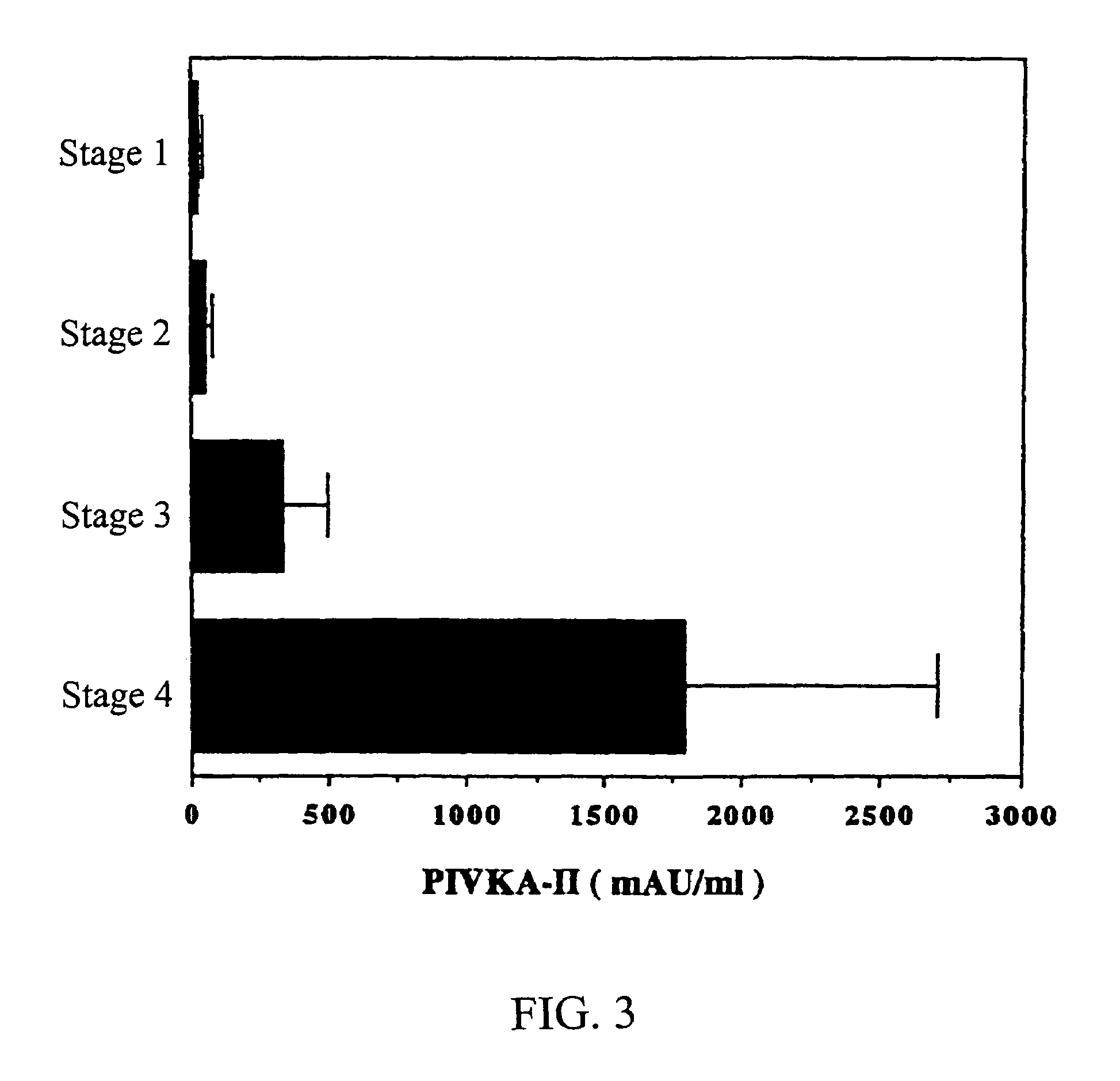
InactiveUS7090983B1Easy to implementSensitive highPeptide/protein ingredientsMicrobiological testing/measurementBacteriuriaUrine production
MK (midkine) was found to rise in the blood or urine of patients with various types of cancers at early stage. Based on this finding, a method for detecting early cancer, comprising the step of measuring MK in blood or urine was completed.
Owner:MEDICAL THERAPIES
Potty training device
ActiveUS20070151009A1Simple and inexpensive solutionEffective blockingWater closetsFluid dynamicsPotty trainingPorous sheet
A potting training device for preventing a potty-training child's urine stream from passing through the opening formed between the toilet seat and the toilet bowl. The device is made of a flexible, water-resistant, preferably non-porous sheet material, having a folding seam separating a urine-deflecting surface and a plurality of attachment tabs. An adhesive material is applied to the surface of each attachment tab and attaches the attachment tabs to the underside of the toilet seat. The sheet material, in its unattached flat configuration, is manipulated into a curved configuration that matches the curvature of the toilet seat, and is attached thereto. The potty training device is also not visible to the observer unless the toilet seat is lifted up, in a vertical position or viewed from a position to the rear of the attachment point when the toilet seat is down.
Owner:CONRAD JOSEPH MICHAEL III
Disposable absorbent article having a visibly highlighted wetness sensation member
InactiveUS20050096612A1Increase awarenessFacilitate opportunityOther accessoriesDiagnostic recording/measuringPotty trainingControl layer
A disposable absorbent article including a wetness sensation member and visible highlighting indicating the presence of the wetness sensation member to facilitate an opportunity for the toilet training of the wearer. The wetness sensation member includes a permeable layer and a flow control layer. Urine deposited on the wetness sensation member can penetrate through the permeable body-facing layer in a z direction away from the wearer to the flow control layer. The flow control layer retards the passage of the urine through the wetness sensation member in the z direction while supporting the movement of the urine in an x-y plane to increase the wetted area contacting the wearer's skin and thereby enhance the wearer's awareness that urination has occurred. The visible highlighting is visible when viewing a body-facing surface of the article and may be associatively correlated with an externally visible marking and / or with the concept of toilet training.
Owner:THE PROCTER & GAMBLE COMPANY
Methods for diagnosis and prognosis of epithelial cancers
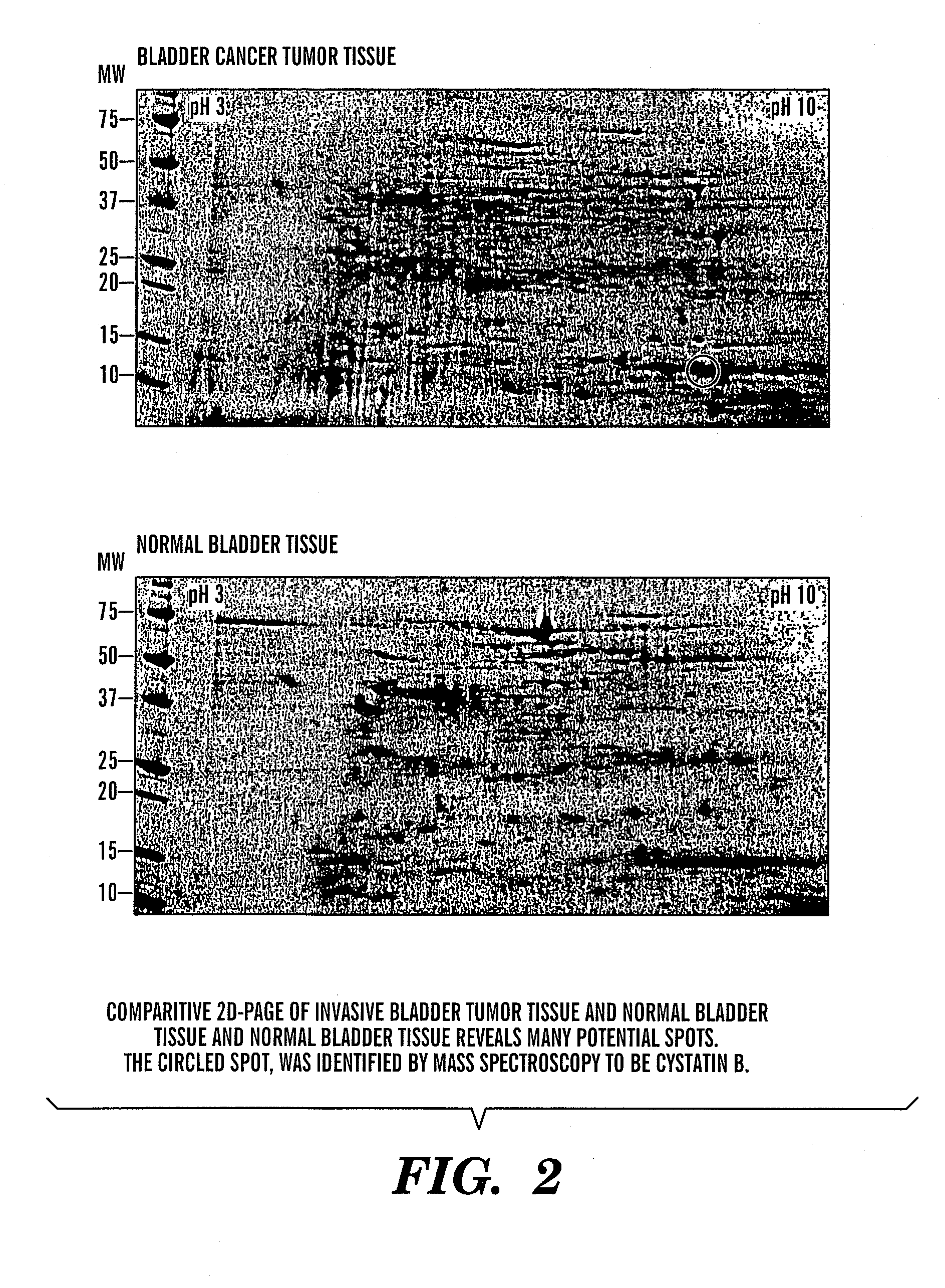
InactiveUS20080064047A1Easy diagnosisQuick and easy and safeBiological material analysisBiological testingBladder cancer patientCystatin B
The present invention is based on the discovery that three proteins, Cystatin B, Chaperonin 10, and Profilin are present in the urine of patients with bladder cancer, a cancer of epithelial origin. Accordingly, the present invention is directed to methods for prognostic evaluation of cancers of epithelial origin and to methods for facilitating diagnosis of cancers of epithelial origin by monitoring the presence of these markers in biological samples. The invention is also directed to markers for therapeutic efficacy.
Owner:THE GENERAL HOSPITAL CORP +1
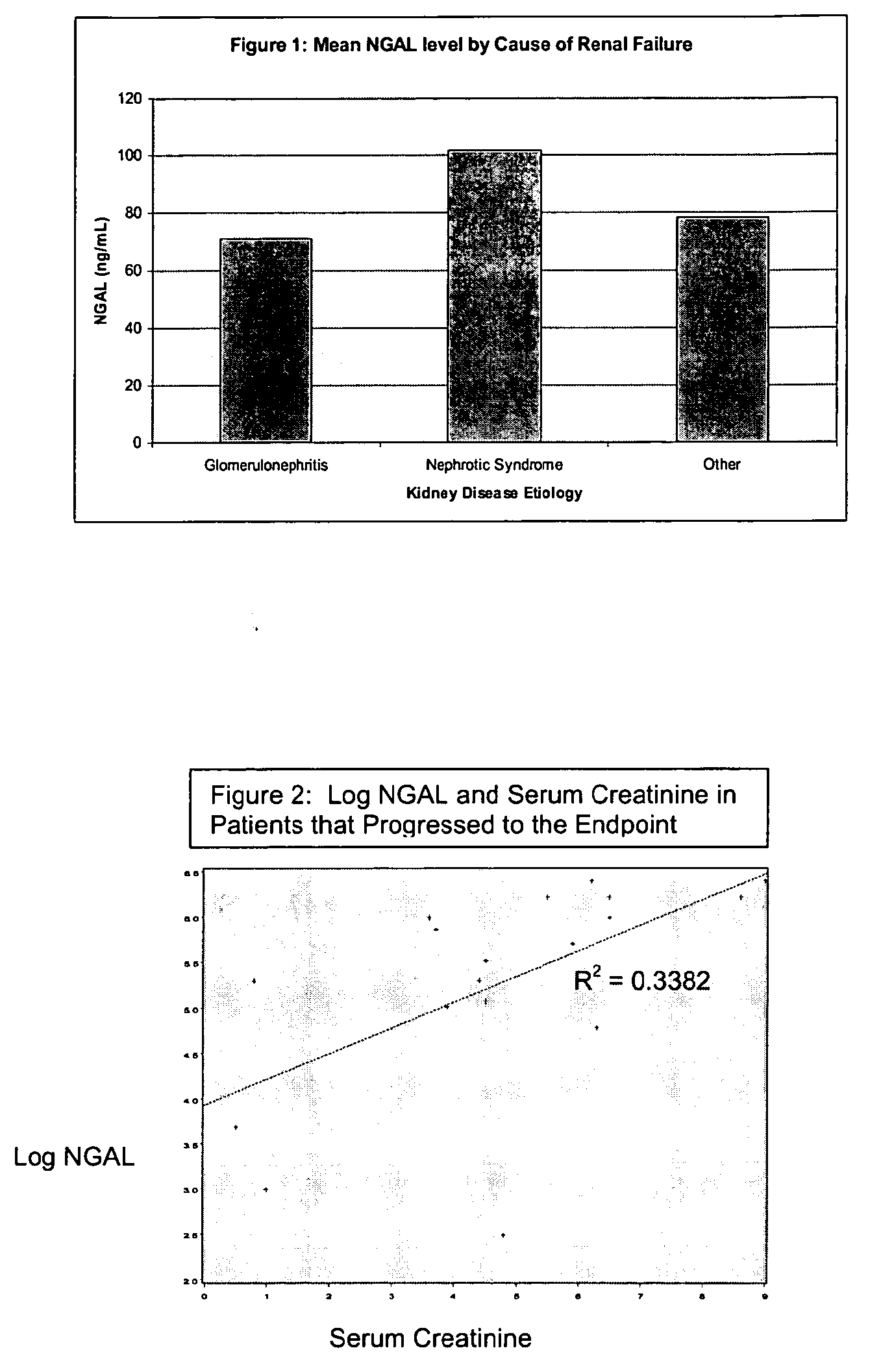
Diagnosis and monitoring of chronic renal disease using ngal
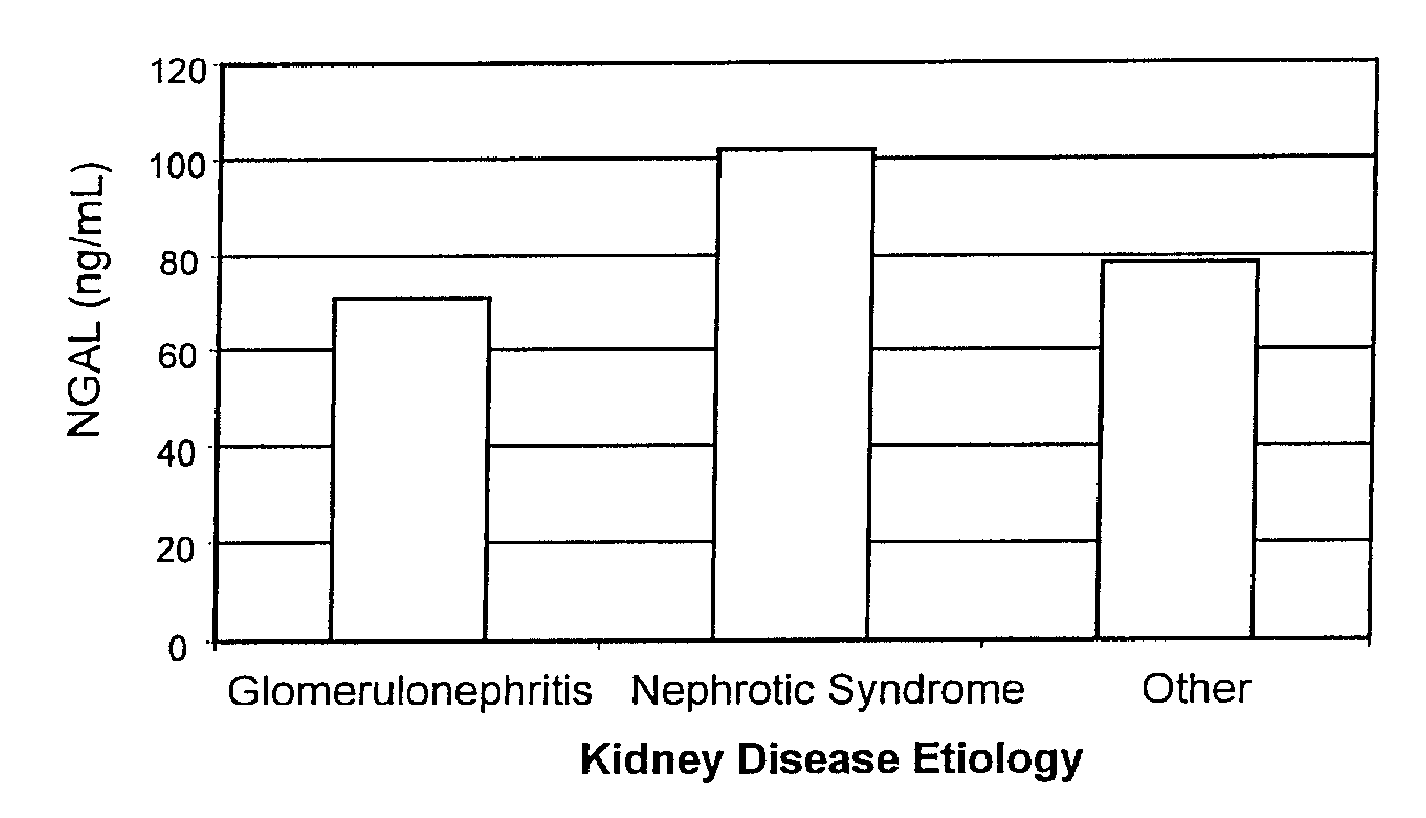
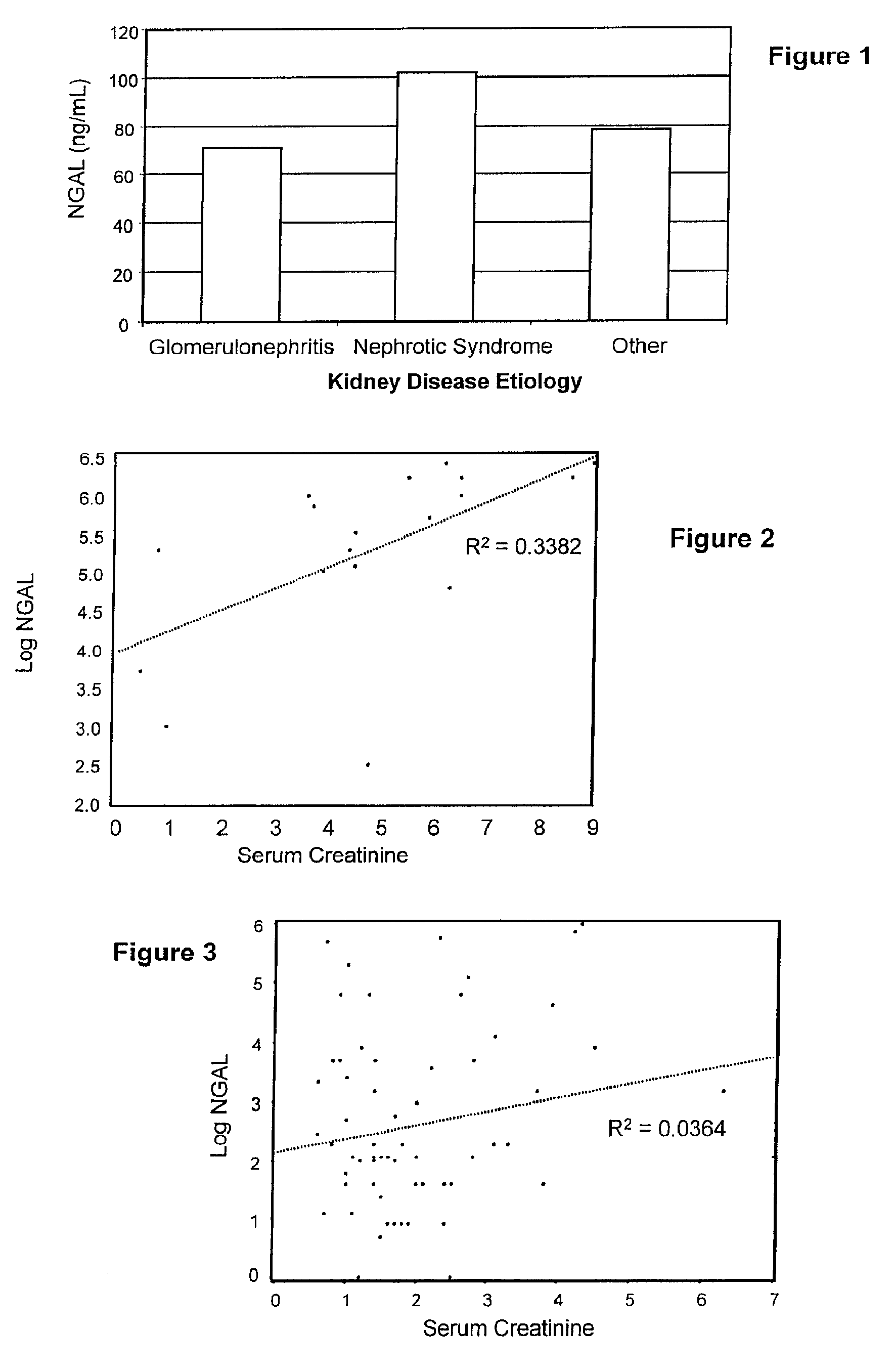
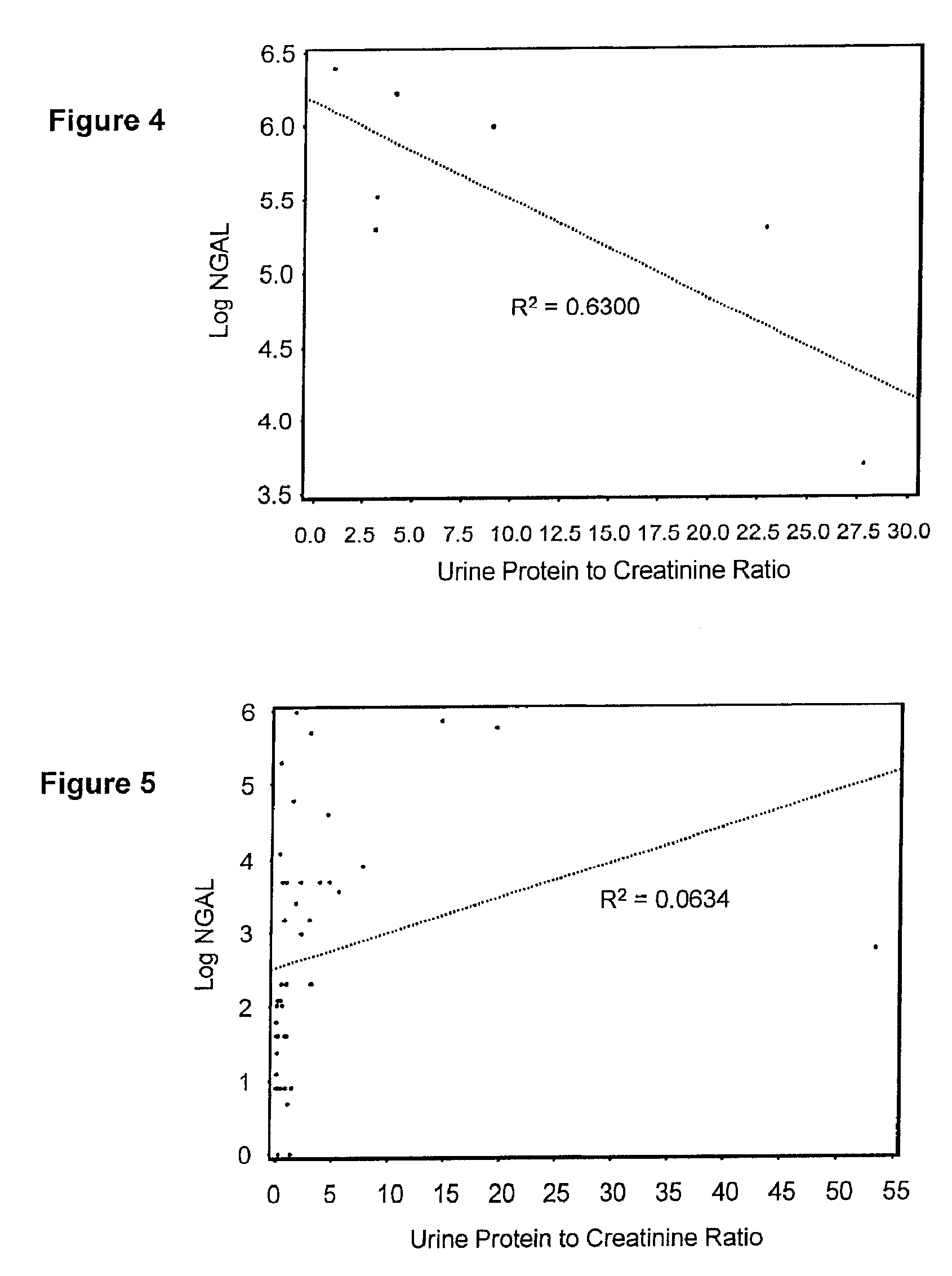
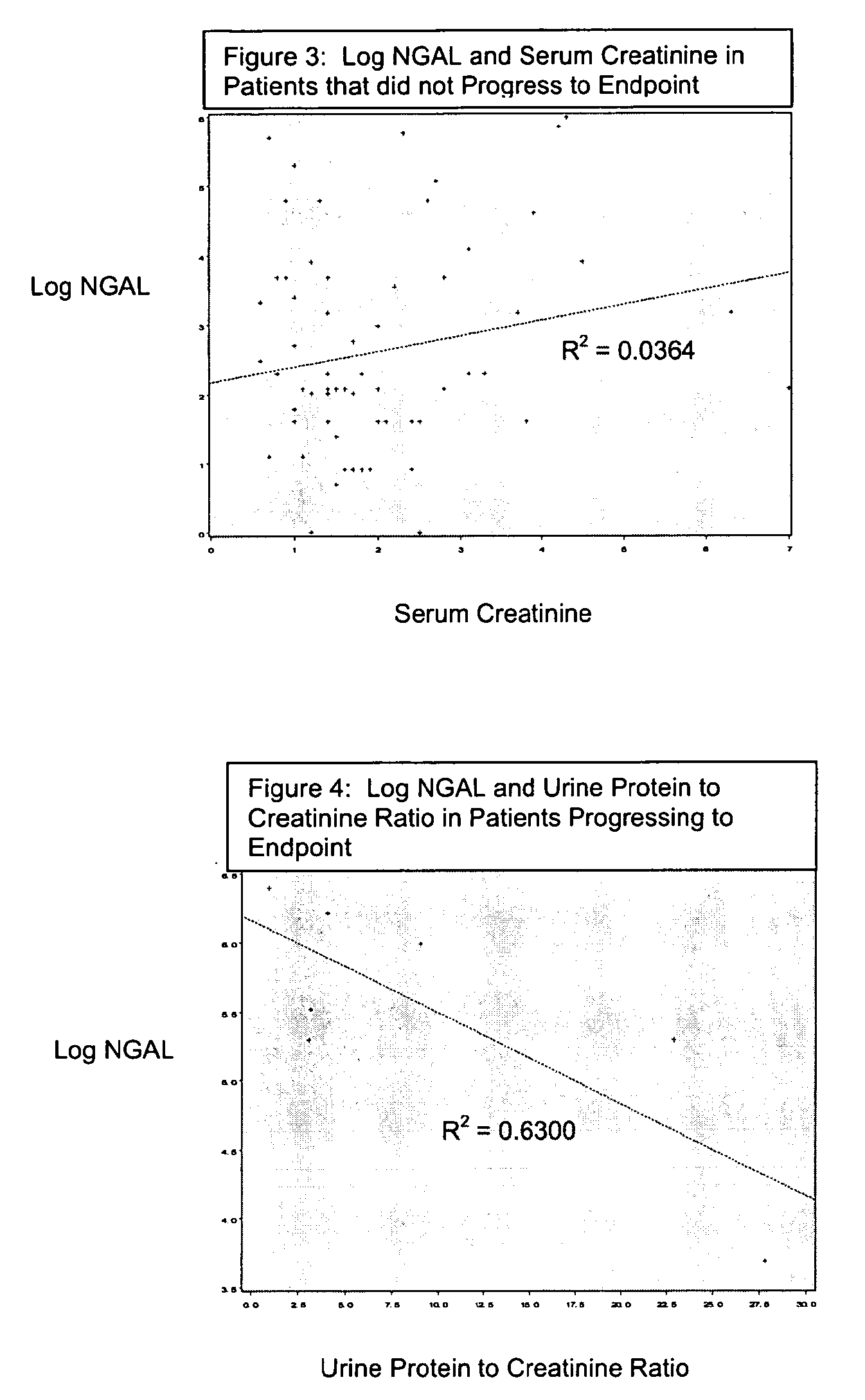
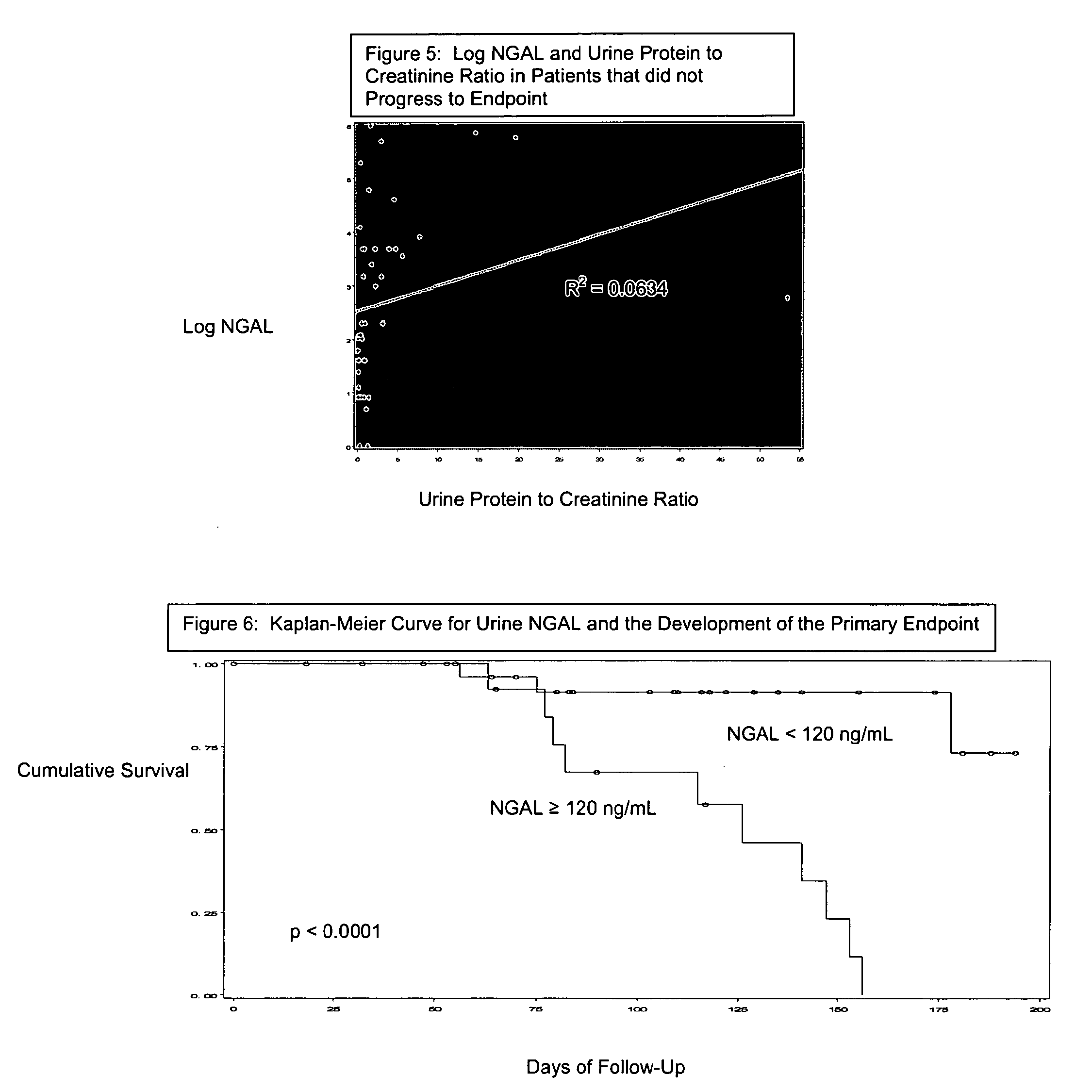
InactiveUS20080014644A1Monitor effectivenessEarly detectionDisease diagnosisBiological testingRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Medical valve and method to monitor intra-abdominal pressure
ActiveUS20070038143A1Reduce flow rateLevel controlEngine diaphragmsUrinary catheterHospitalized patients
An apparatus for monitoring the intra-abdominal pressure of a hospitalized patient includes a urinary catheter connected to a urine valve providing selectable communication between a discharge end of the urinary catheter and either a drain or a fluid source. Preferably, the urine valve is adapted for remote actuation and has a housing adapted to resist patient discomfort from leg-valve contact. Plumbing structure desirably maintains fluid supply and drain conduits in a substantially parallel arrangement to assist routing those conduits between a patient's legs. When the urine valve is oriented to permit communication with the fluid source, an infusion pump may be used to infuse a known quantity of fluid through the urine valve and into the patient's bladder. A pressure transducer desirably is connected in-circuit to indicate the fluid's pressure. To facilitate the infusion process, an automatic flow control device may be included in a fluid supply path and arranged to permit repetitive operation of a syringe to inject a bolus of fluid into the patient's bladder. Subsequent to a period of time in which to make a pressure measurement, preferred embodiments of the urine valve automatically return to a bladder draining position.
Owner:CONVATEC TECH INC
Particulate water absorbing agent with water-absorbing resin as main component
InactiveUS20070066167A1Satisfactory performancePractical to useSynthetic resin layered productsAbsorbent padsParticulatesSaline water
The present invention provides a water absorbing agent which maintains excellent water absorbing properties for a long time, even when urine composition of human urine varies depending. A particulate water absorbing agent comprising a water-absorbing resin obtained by crosslinking polymerization of an unsaturated monomer, which exhibits Centrifuge retention capacity in a physiological saline solution of not lower than 32 g / g, mass median particle size (D50) of 200 to 400 μm, ratio of particles with diameter of smaller than 150 μm of 0 to 2% by weight, and increased extractables by deterioration of 0 to 15% by weight and extractables for one hour in deterioration test liquid of 0.1 to 30% by weight.
Owner:NIPPON SHOKUBAI CO LTD
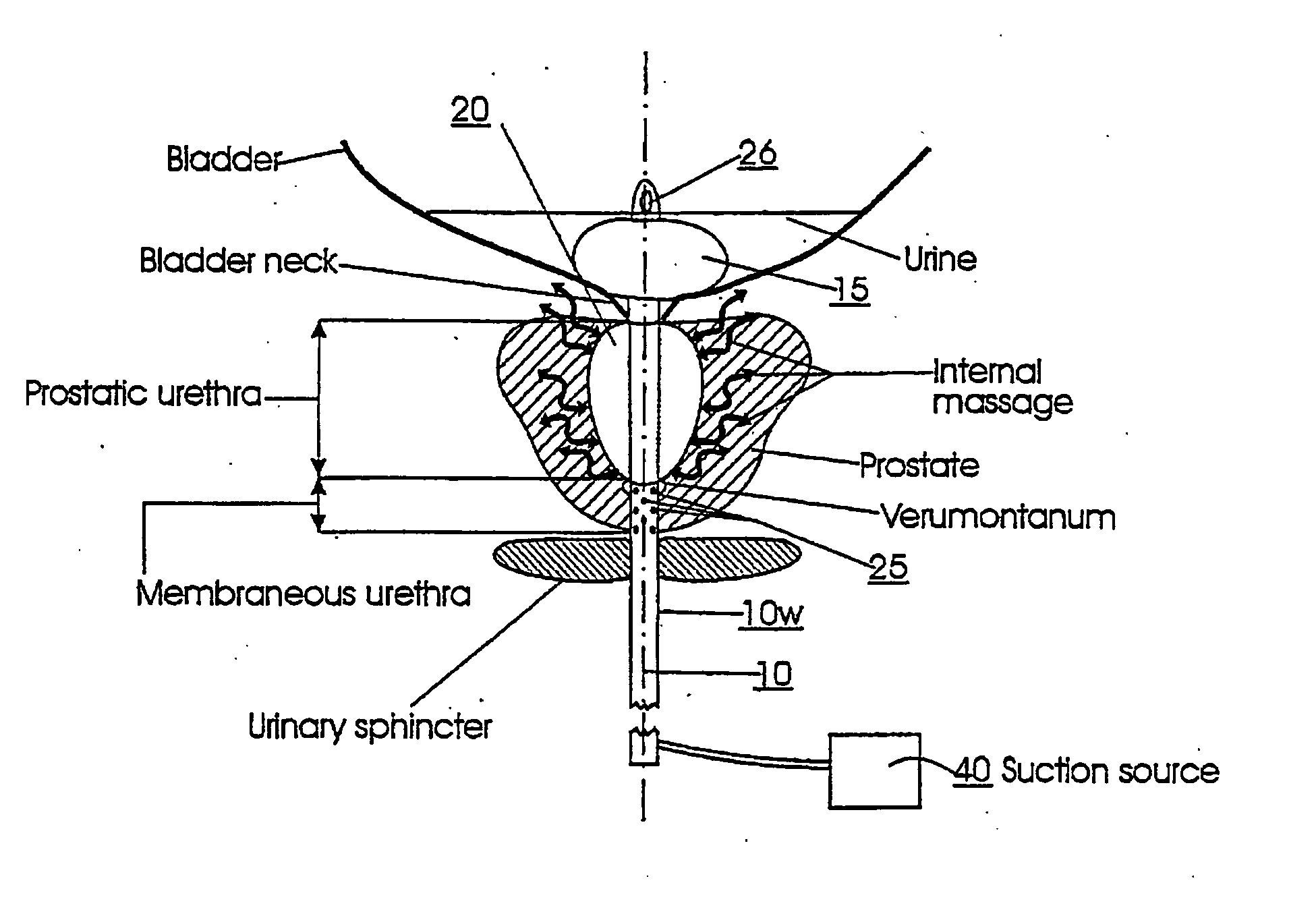
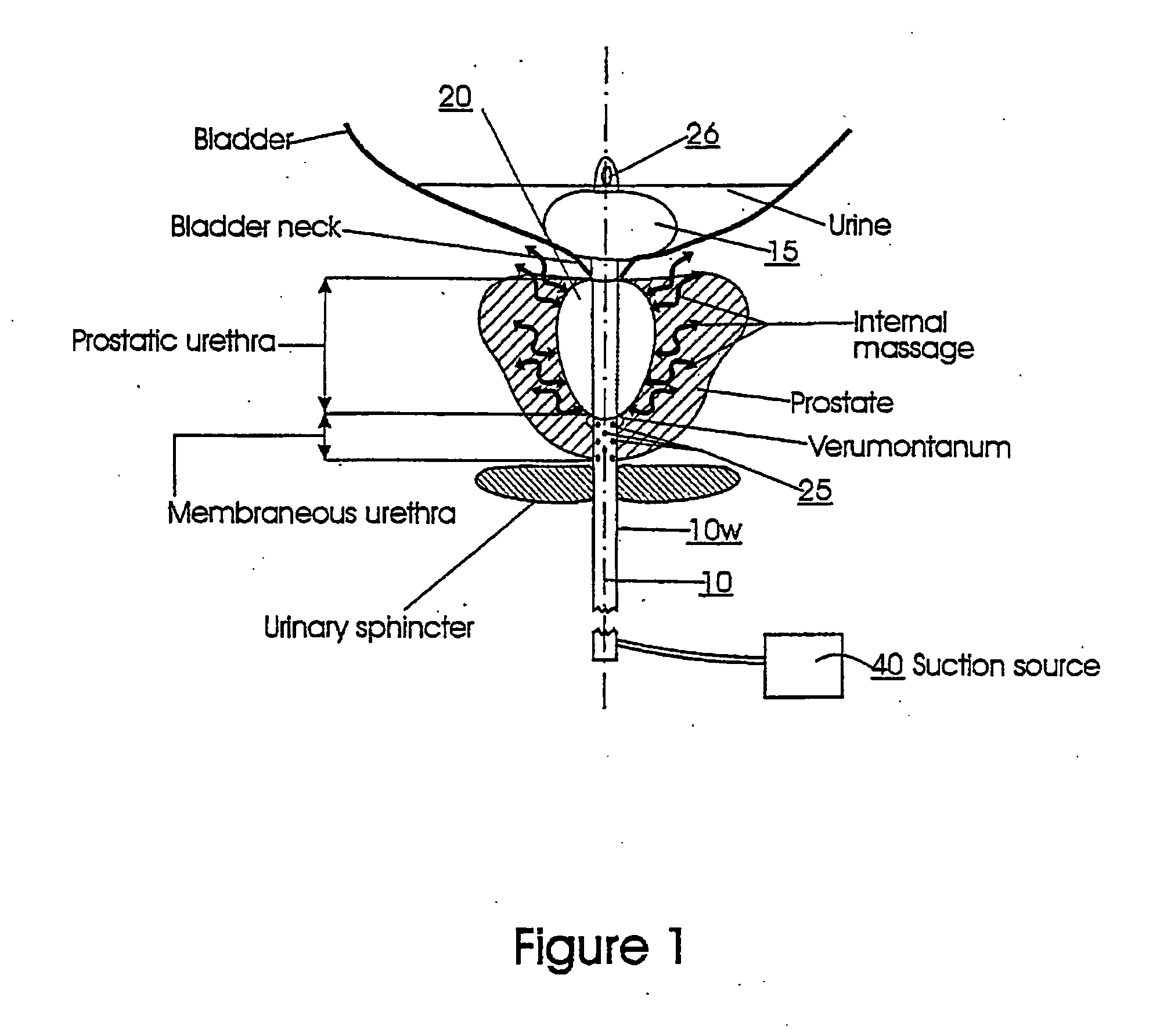
Device for maintaining a passage for urine through the prostate
PCT No. PCT / SE96 / 00607 Sec. 371 Date Nov. 12, 1997 Sec. 102(e) Date Nov. 12, 1997 PCT Filed May 9, 1996 PCT Pub. No. WO96 / 35395 PCT Pub. Date Nov. 14, 1996A device for maintaining a passage through the prostate gland (13) after treatment of a catheter (10) inserted through the urethra into the prostate gland, the catheter (10) being provided with means (11) for heating the prostate gland. A sleeve (12) is received over the catheter (10) so as to follow the catheter (10) during insertion into a desired position within the prostate gland, and the sleeve (12) is formed to remain in the desired position when the catheter is removed from the prostate gland, thereby maintaining a passage having a predetermined lower inner diameter through the prostate gland when tissue in the prostate gland swells.
Owner:PROSTALUND OPERATIONS
Systems and methods for characterizing kidney diseases
The present invention relates to methods of diagnosing, predicting and monitoring kidney disorders. In particular, the present invention relates to the diagnosis, prediction and monitoring of kidney disorders by detection of cytokines, cytokine-related compounds and chemokines in urine. The present invention further relates to methods and compositions for assessing the efficacy of agents and interventions used to treat kidney disorders.
Owner:RENOVAR
Urine sample collection device
InactiveUS20060184064A1Easy to useReduce manufacturing costSurgeryBathroom accessoriesUrine CollectionsUrine collection device
Owner:SAMPLE RITE
Fluid collection and aspiration unit for management of urinary incontinence
A flexible unit for collecting and transporting liquid to a collection point or to an area of use, and especially for facilitating management of urinary incontinence is provided which includes a flexible pad adapted to be positioned under an incontinent patient or worn under an undergarment. The pad is provided with an outer liquid permeable polymeric film layer, an outer liquid impermeable polymeric film layer and an intermediate cellular layer made up of a series of individual, spaced, thin wall, liquid impermeable polymeric cells. An aspiration assembly is connected to the pad for removing urine from the interior of the pad, which collects in the spaces between the individual cells. A disposable porous sheet, preferably comprising Dry-Weave® material, is releasably positioned over the outer permeable film layer of the pad. The pad may be sanitized and reused multiple times with only replacement of the Dry-Weave® sheet being required.
Owner:MAHNENSMITH MICHAEL
Urine receiver and urine collection processing system implementing urine receiver
InactiveUS20060015081A1Good adhesionPrevent leakageNon-surgical orthopedic devicesSuction devicesUrine leakageSkin surface
A urine receiver which is sanitary, easy to attach, and furthermore, prevents urine leakage even when a wearer repeatedly changes positions is provided. A urine receiver 10 is implemented in a urine collection processing system wherein urine discharged from the wearer is suctioned into a urine tank via a urethral tube. The urine receiver 10 comprises, at the least: a liquid permeable, air-impermeable sheet 21 which is placed opposite of and covering the urethral meatus of the wearer; a leak-proof sheet 22 which is placed on the surface of the air-impermeable sheet 21 opposite to the urethral meatus and bonds to the outer border of the air-impermeable sheet 21; a suction part 26 which is provided between the air-impermeable sheet 21 and the leak-proof sheet 22 and to which the urethral tube 11 is connected; and a gathers part 16 for sealing the space between the air-impermeable sheet 21 and the wearer's skin surface which is provided on the outer border part of the air-impermeable sheet 21 on the urethral meatus side.
Owner:UNI CHARM CORP
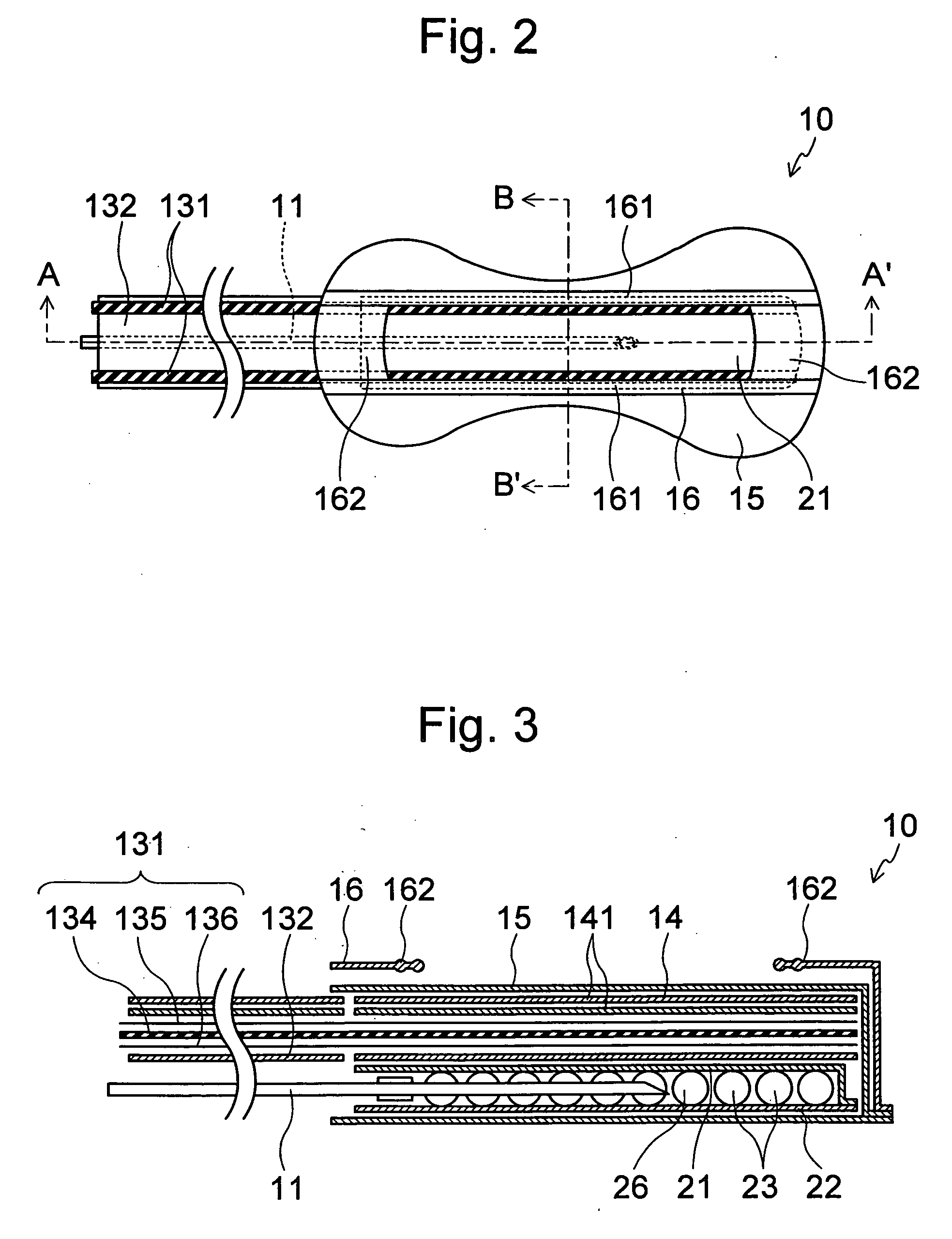
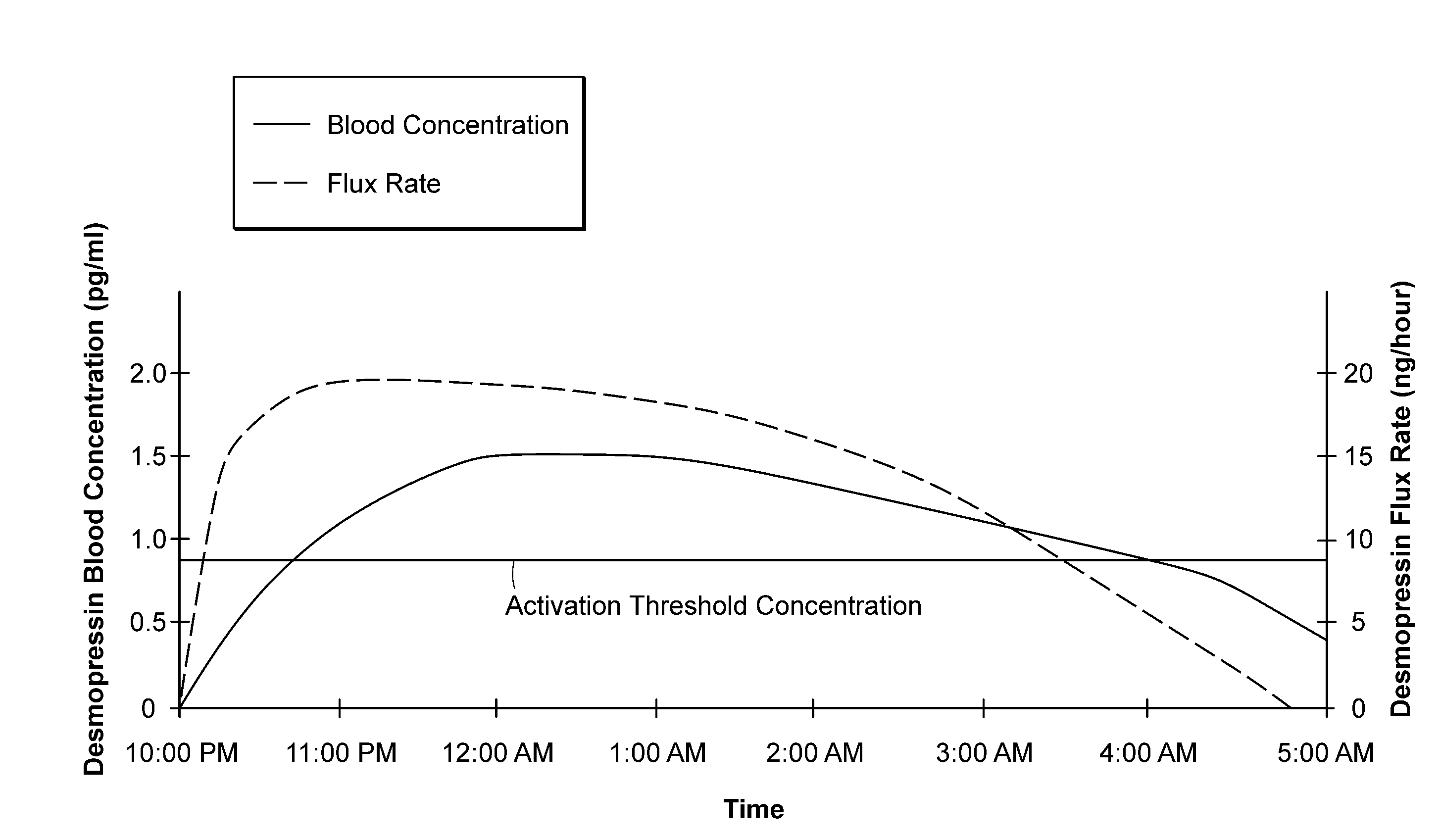
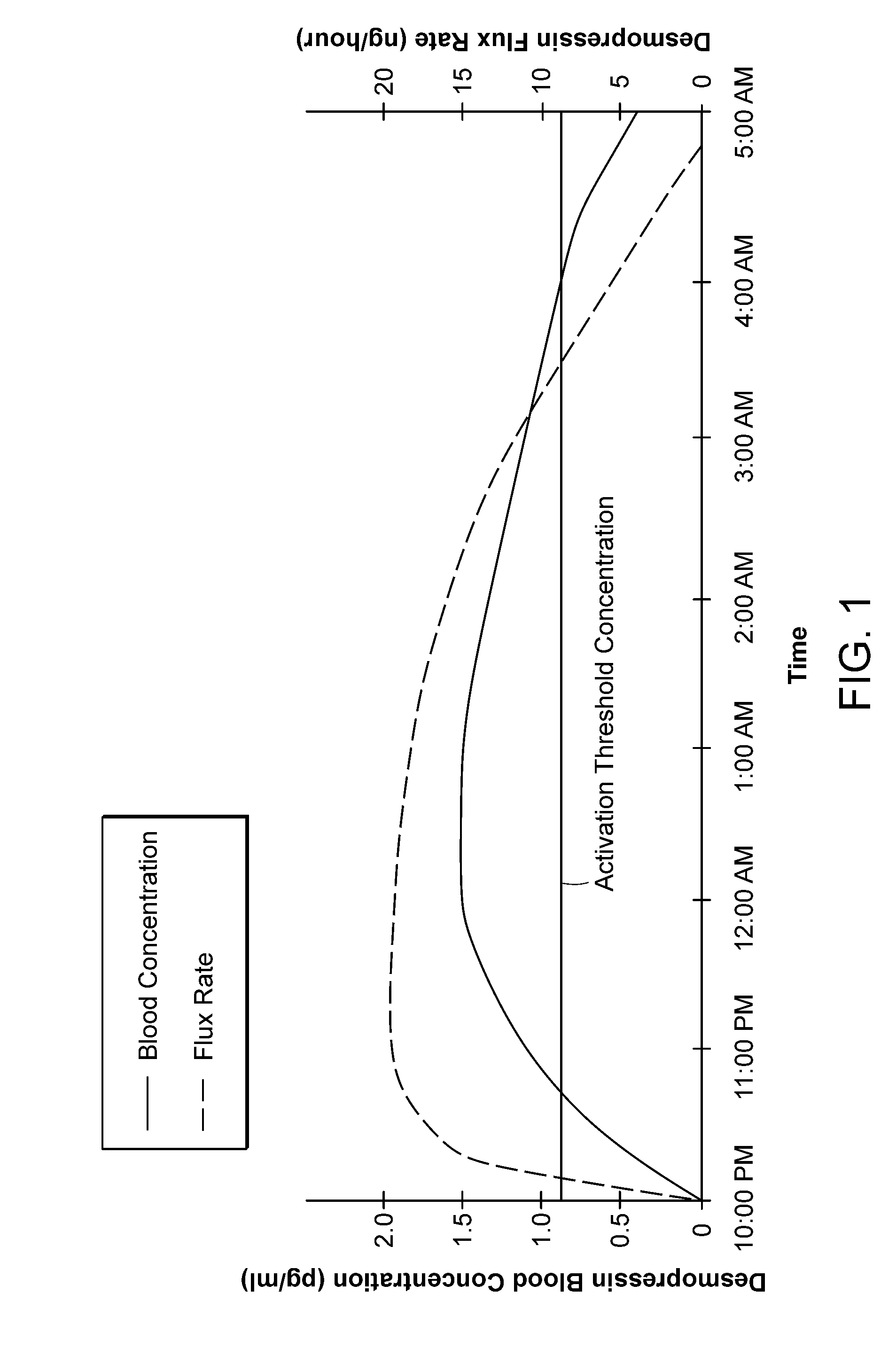
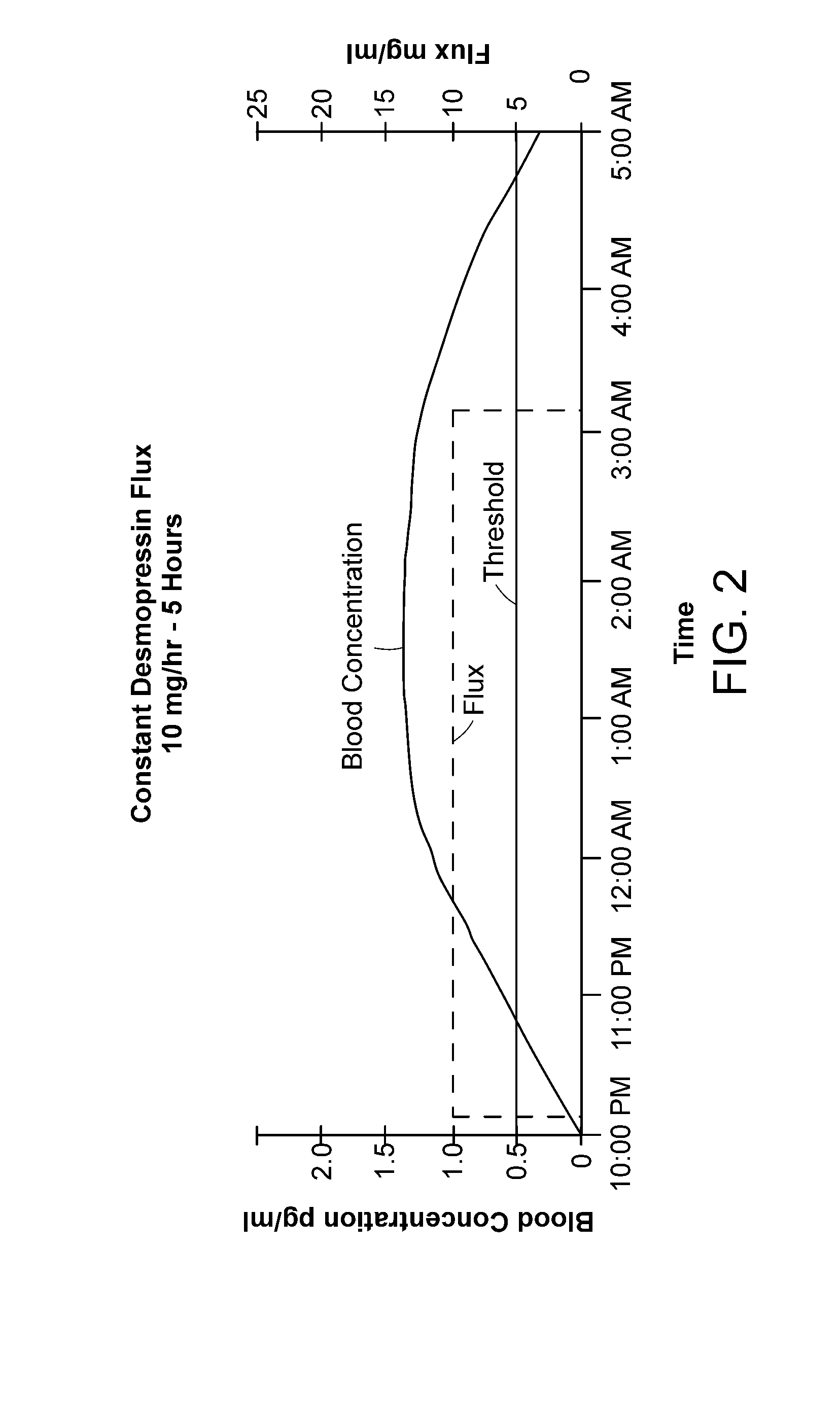
Methods and devices for desmopressin drug delivery
InactiveUS20090042970A1Reduce urine productionRestore normal urine productionBiocidePowder deliveryDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Apparatus for monitoring intra-abdominal pressure
InactiveUS20070255167A1Increase speedFacilitate making pressure measurementEqualizing valvesSafety valvesUrinary catheterHospitalized patients
An improved apparatus for monitoring the intra-abdominal pressure of a hospitalized patient includes a urinary catheter connected to a urine valve having selectable communication positions between a discharge end of the urinary catheter and either a drain or a fluid source. Preferably, the urine valve has a housing adapted to resist patient discomfort from leg-valve contact. One operable protective housing may be embodied as a separate tray component. Plumbing structure desirably maintains fluid supply and drain conduits in a substantially parallel arrangement to assist routing those conduits between a patient's legs. When the urine valve is oriented for communication to the fluid source, an infusion pump may be used to introduce a known quantity of fluid through the urine valve and into the patient's bladder where the fluid's pressure can be measured. Desirably, a double check valve is included in a fluid supply path and arranged to permit repetitive operation of a syringe to inject a bolus of fluid into the patient's bladder. Subsequent to making a pressure measurement, the urine valve is returned to the bladder draining position.
Owner:CONVATEC TECH INC
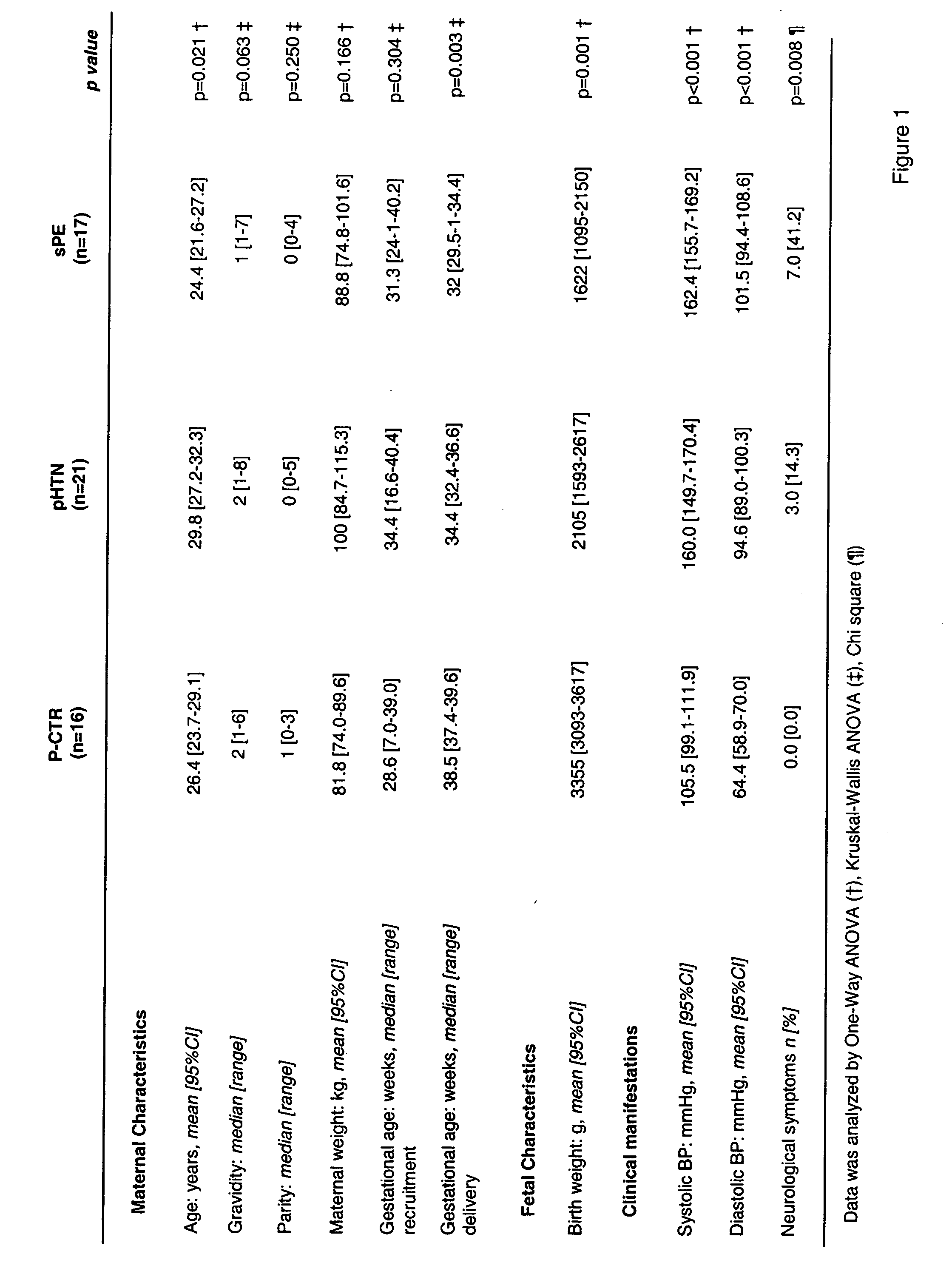
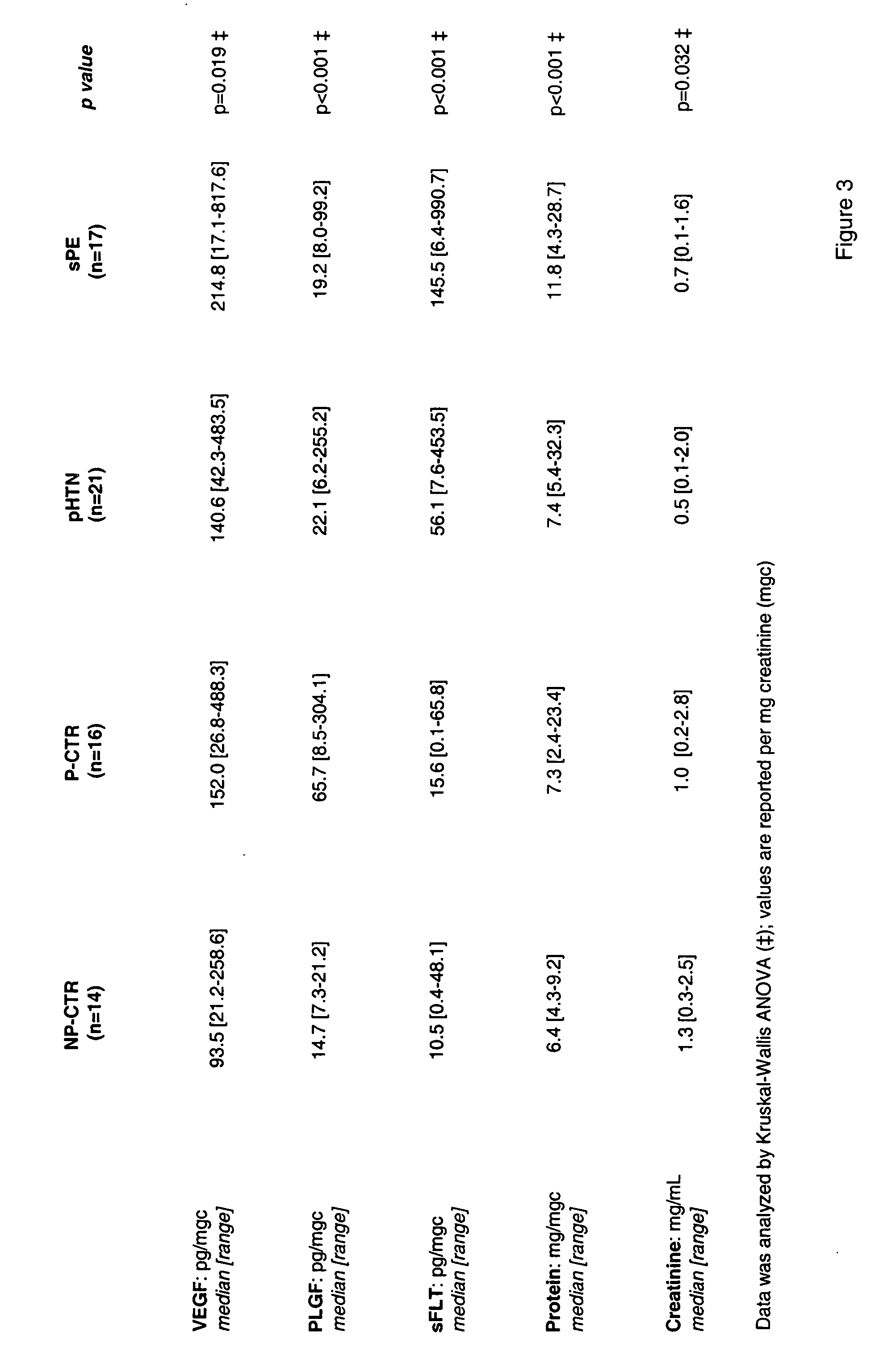
Diagnosis of preeclampsia
ActiveUS20060183175A1Reduce needRaise the possibilityPeptide/protein ingredientsPeptide sourcesDiseaseThird trimester
The present invention provides methods and compositions related to the detection and / or monitoring of the levels of angiogenic factors, specifically VEGF, PlGF and sFlt-1, in urine samples obtained from pregnant women and the effects of such levels on the risk of developing complications of pregnancy, including hypertensive disorders such as preeclampsia, in the first, second, and / or third trimester of pregnancy. The present invention also provides kits for identifying and screening patients at risk of developing a complication of pregnancy, such as preeclampsia.
Owner:YALE UNIV
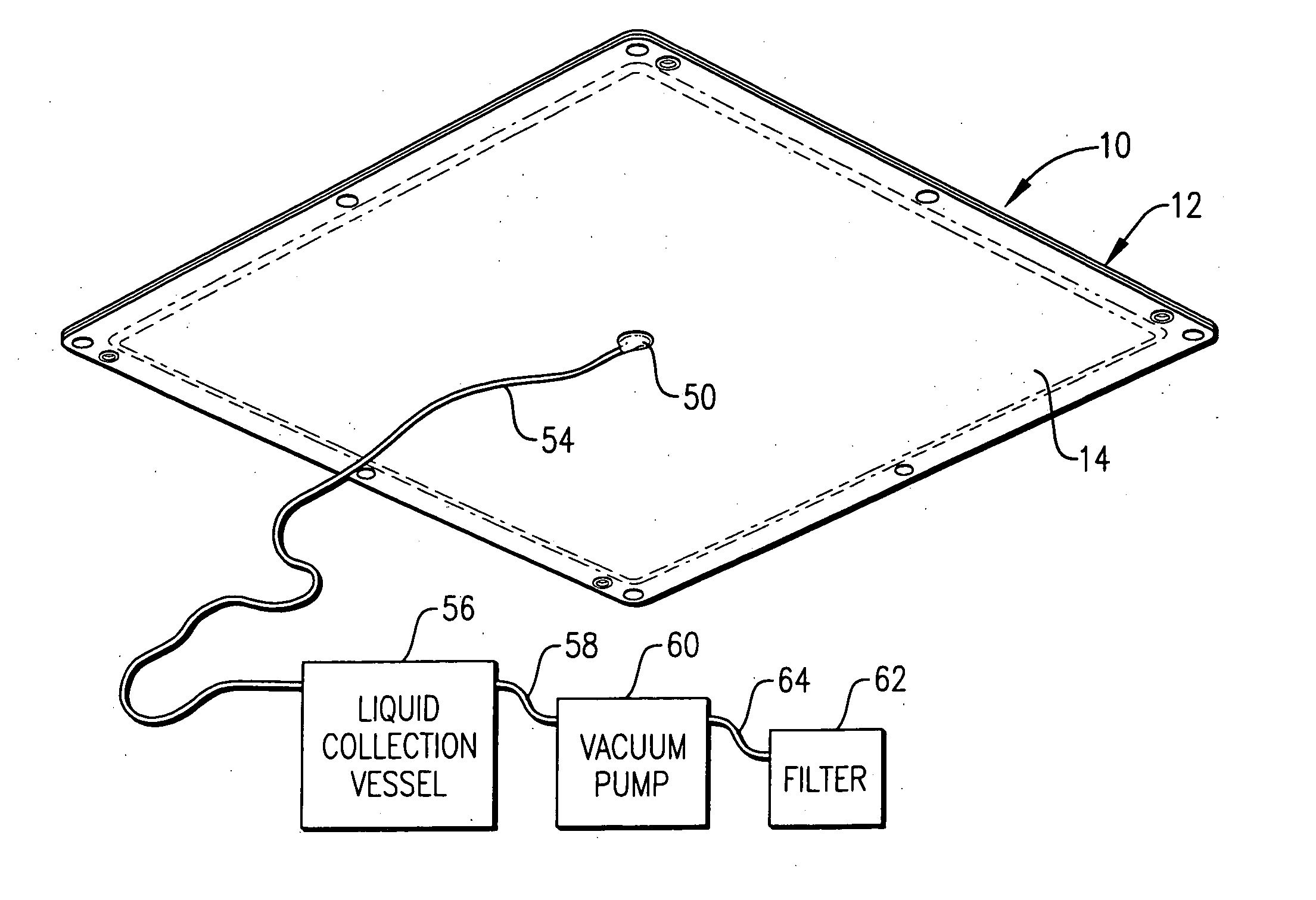
Device for collecting, testing and storing fluids
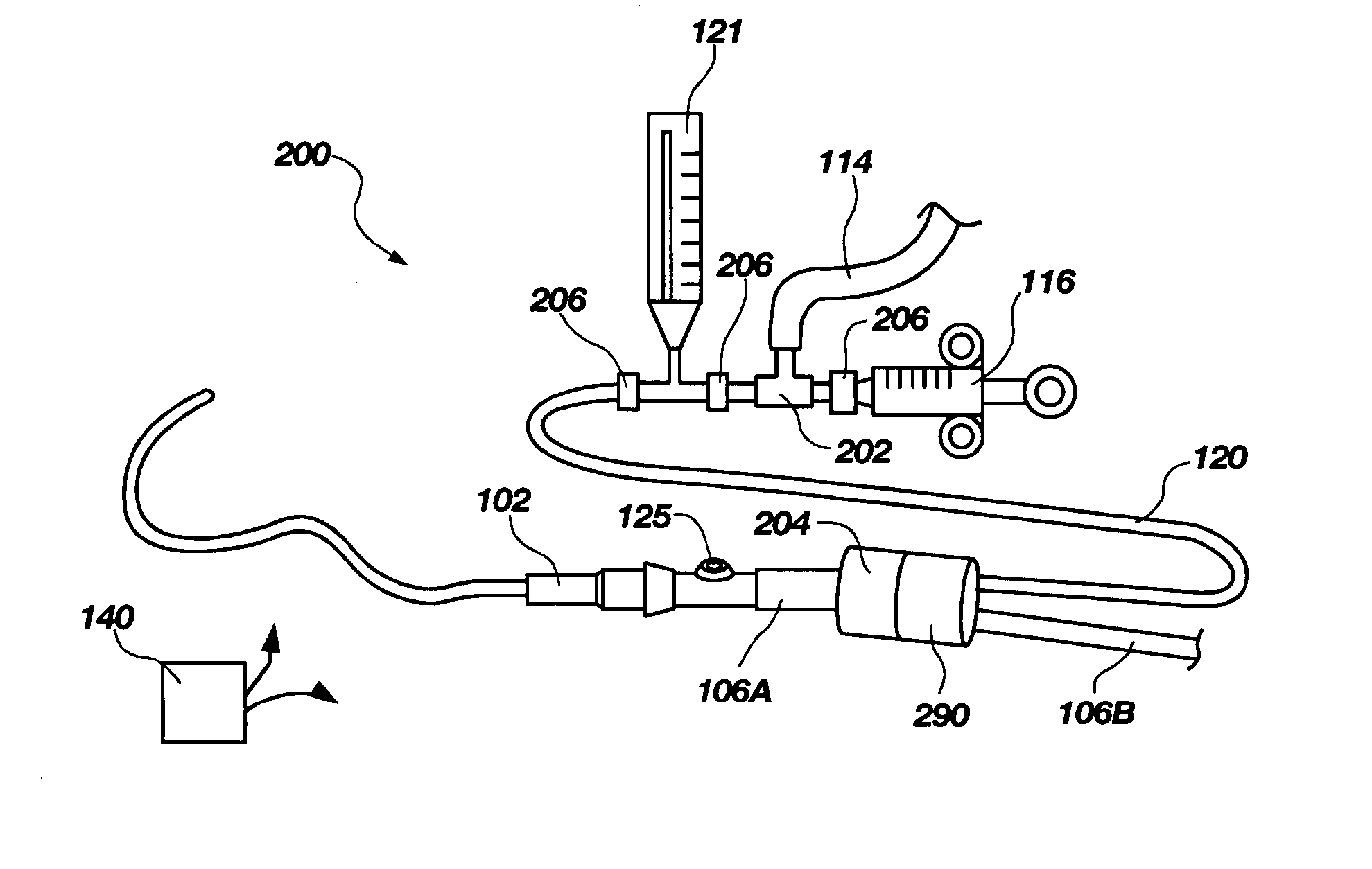
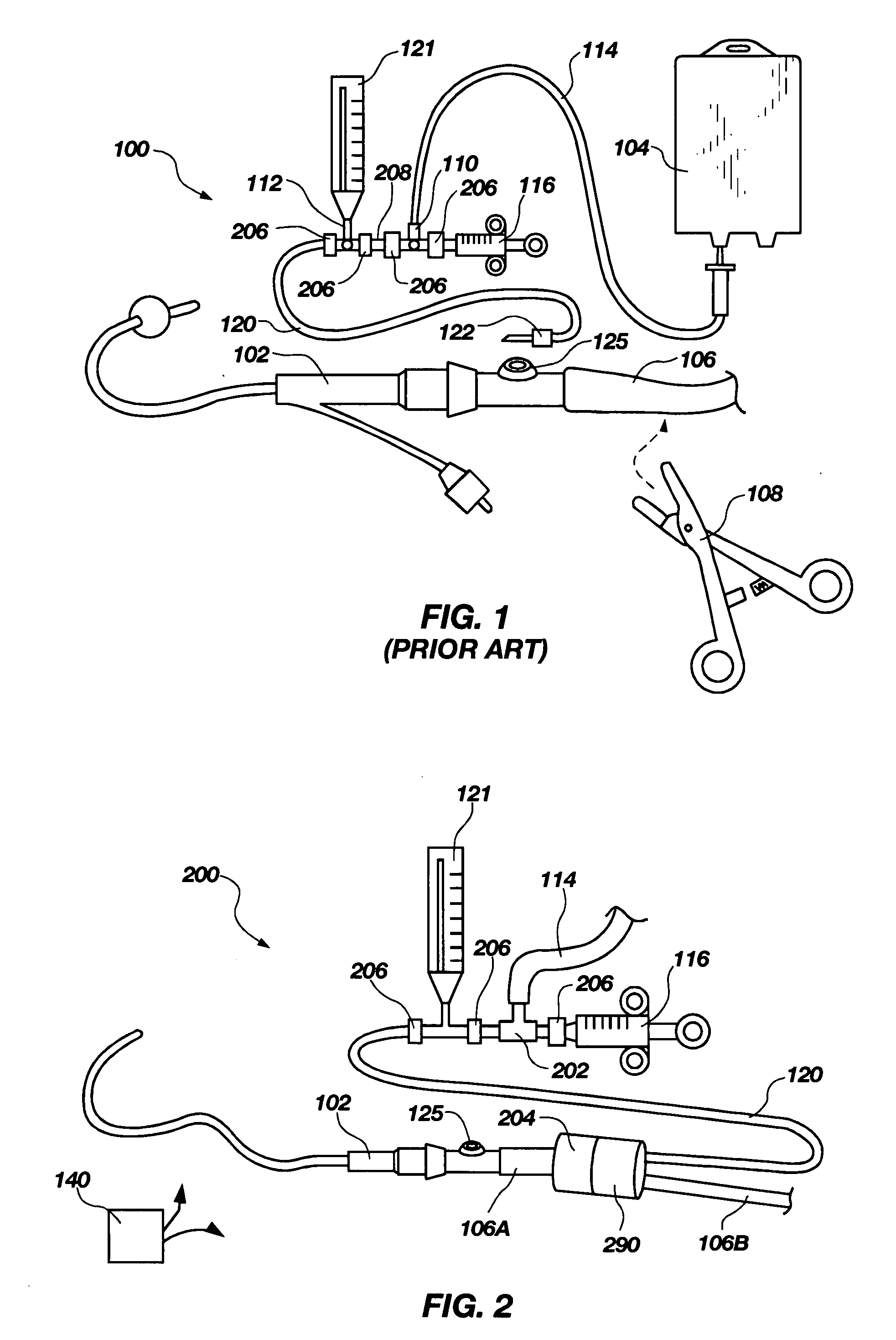
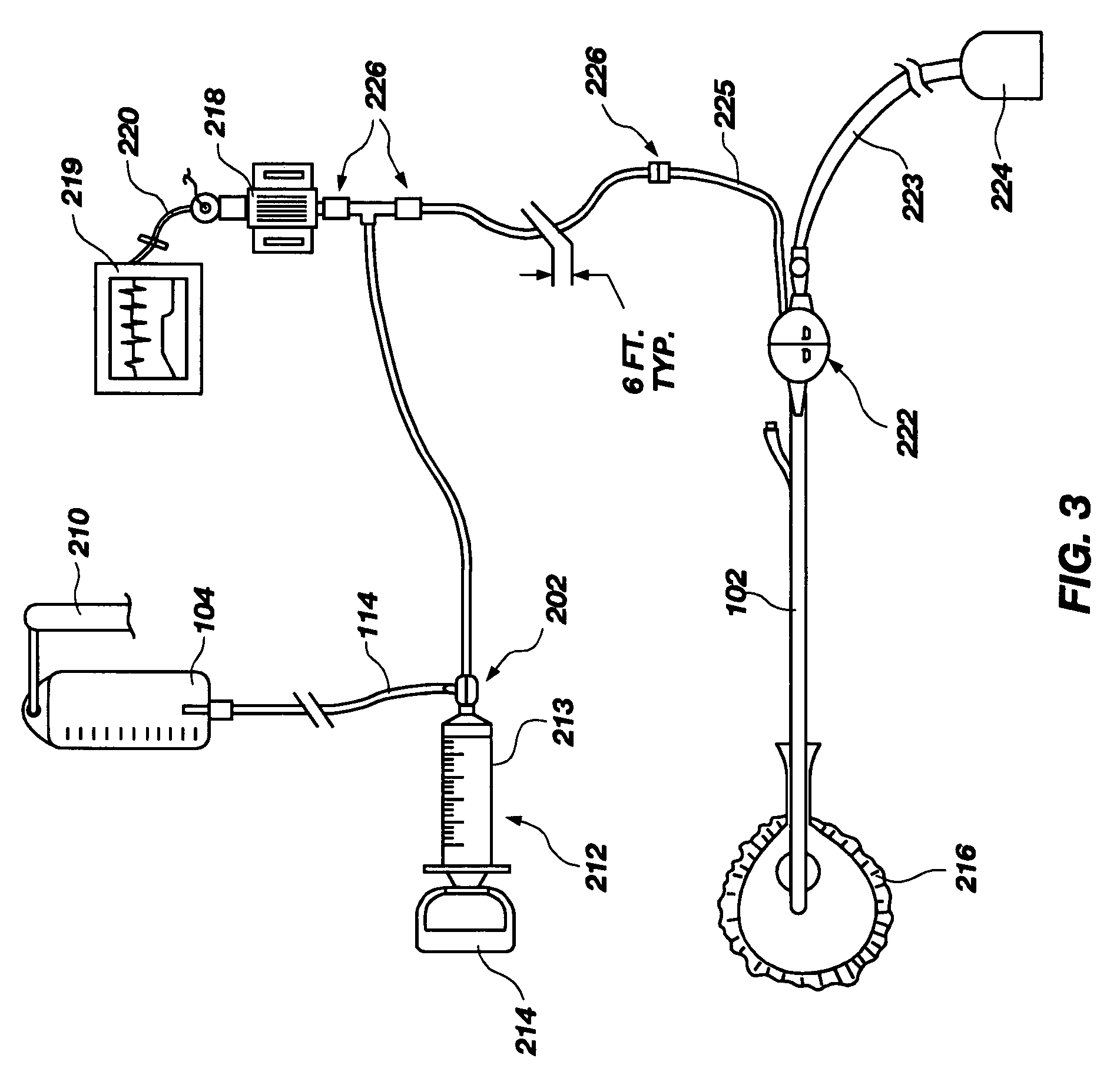
ActiveUS20060280650A1Preventing fluid communicationAnalysis using chemical indicatorsVaccination/ovulation diagnosticsBody fluidUrine production
A device for collecting, testing and storing fluids includes a container and a removable cap. Body fluids, such as urine, are collected in the container. The cap defines a test strip holder that receives one or more drug test strips and an adulteration strip. An opening defined through a bottom of the cap provides fluid communication between the container and the cap. When tilted, fluid from the container enters the cap through the hole. A variety of plugs may be employed to block the opening and thereby prevent fluid communication between the container and the cap after fluid has entered the cap. The remaining uncontaminated and untested body fluid is stored in the container and thus made available for further confirmation testing. Associated methods for collecting, testing and storing fluids with a single device are also provided. A method of manufacturing the foregoing test device is also provided.
Owner:BRANAN MEDICAL
Urine Sensor
InactiveUS20070035405A1Lose their capabilityReliable detectionAbsorbent padsAlarmsUrine productionTissue skin
Here is disclosed a urine receiver. The urine receiver comprises a container member adapted to suck urine discharged from a wearer of the urine receiver and a urine sensor provided with a pair of electrodes interposed between the container member and the wearer's skin and serving to detect the urine. The paired electrodes covered with insulating coating. The coating is formed with through-holes adapted to expose a limited area of the electrodes.
Owner:ALFRED E MANN INST FOR BIOMEDICAL ENG AT THE UNIV OF SOUTHERN CALIFORNIA +1
Flexible Bag Wrap Adapted For Use In An Incontinence Management System
InactiveUS20080004560A1Growth inhibitionOptimize allocationFeet bandagesColostomyYarnUrine production
A flexible bag wrap is adapted for use in an incontinence management system. The bag wrap includes a flexible base adapted for wrapping around a body part of a user. The base includes a three-dimensional fabric spacer. The fabric spacer has an inside facing for residing nearest the skin of the user, an outside facing opposite the inside facing, and an intermediate spacer yarn interconnecting the inside and outside facings. A collection bag is carried by the base adjacent the outside facing of the fabric spacer, and is adapted for collecting urine excreted by the user.
Owner:MISKIE MARK
Detection of NGAL in chronic renal disease
Methods of assessing the ongoing kidney status in a subject afflicted with chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in fluid samples over time is disclosed. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine and serum as a result of chronic renal tubule cell injury. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com