Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Prostatic urethra" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
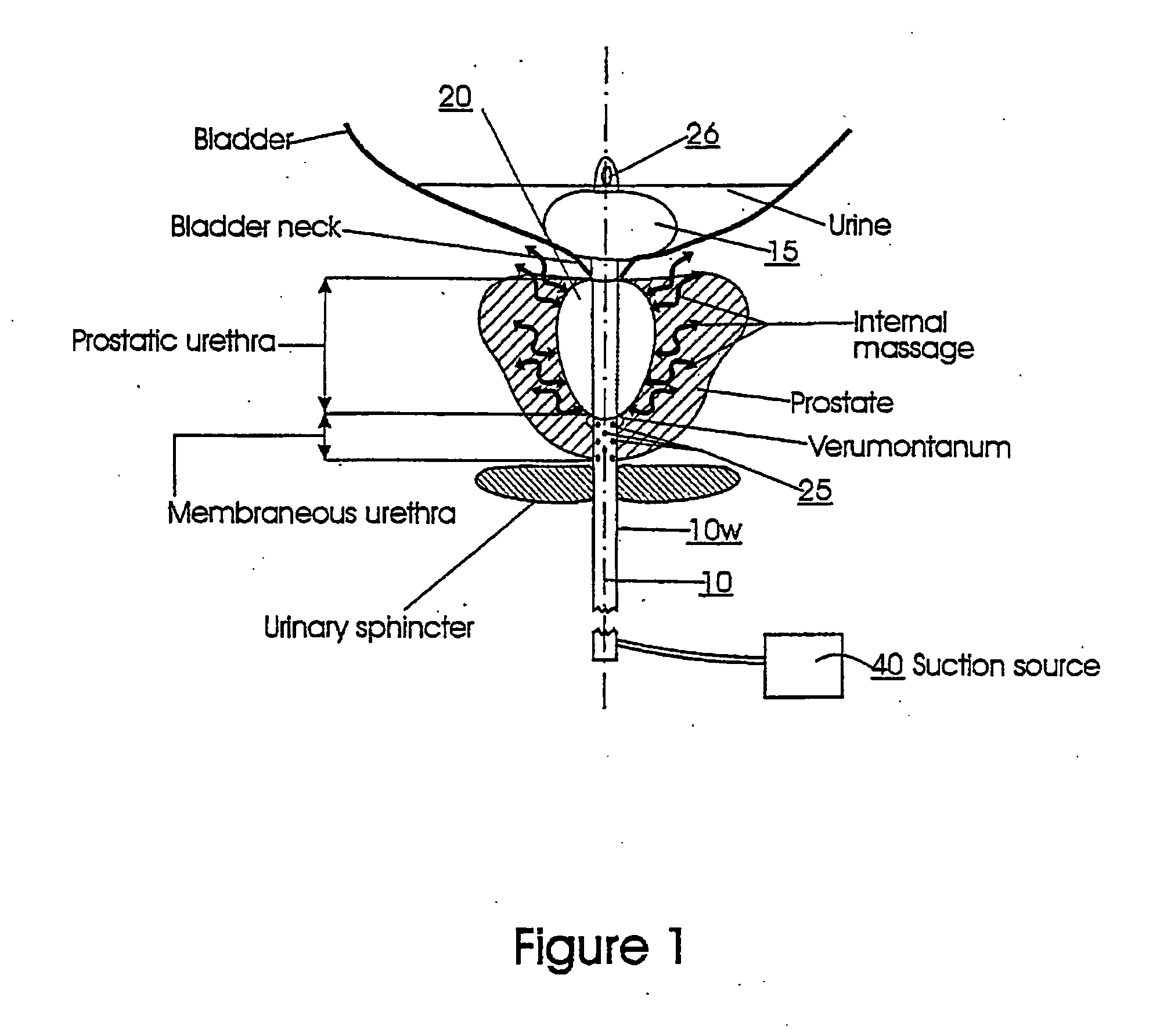
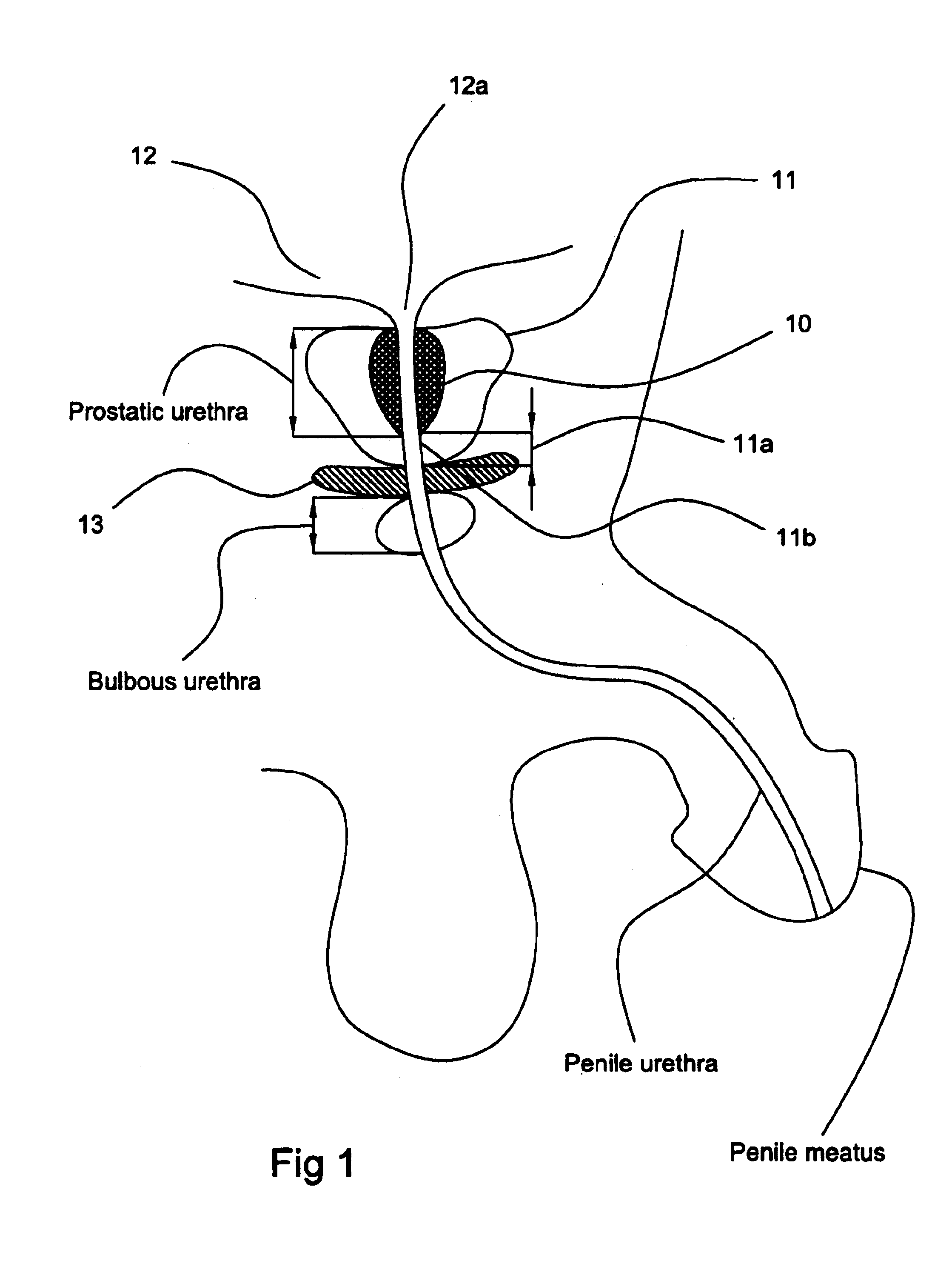
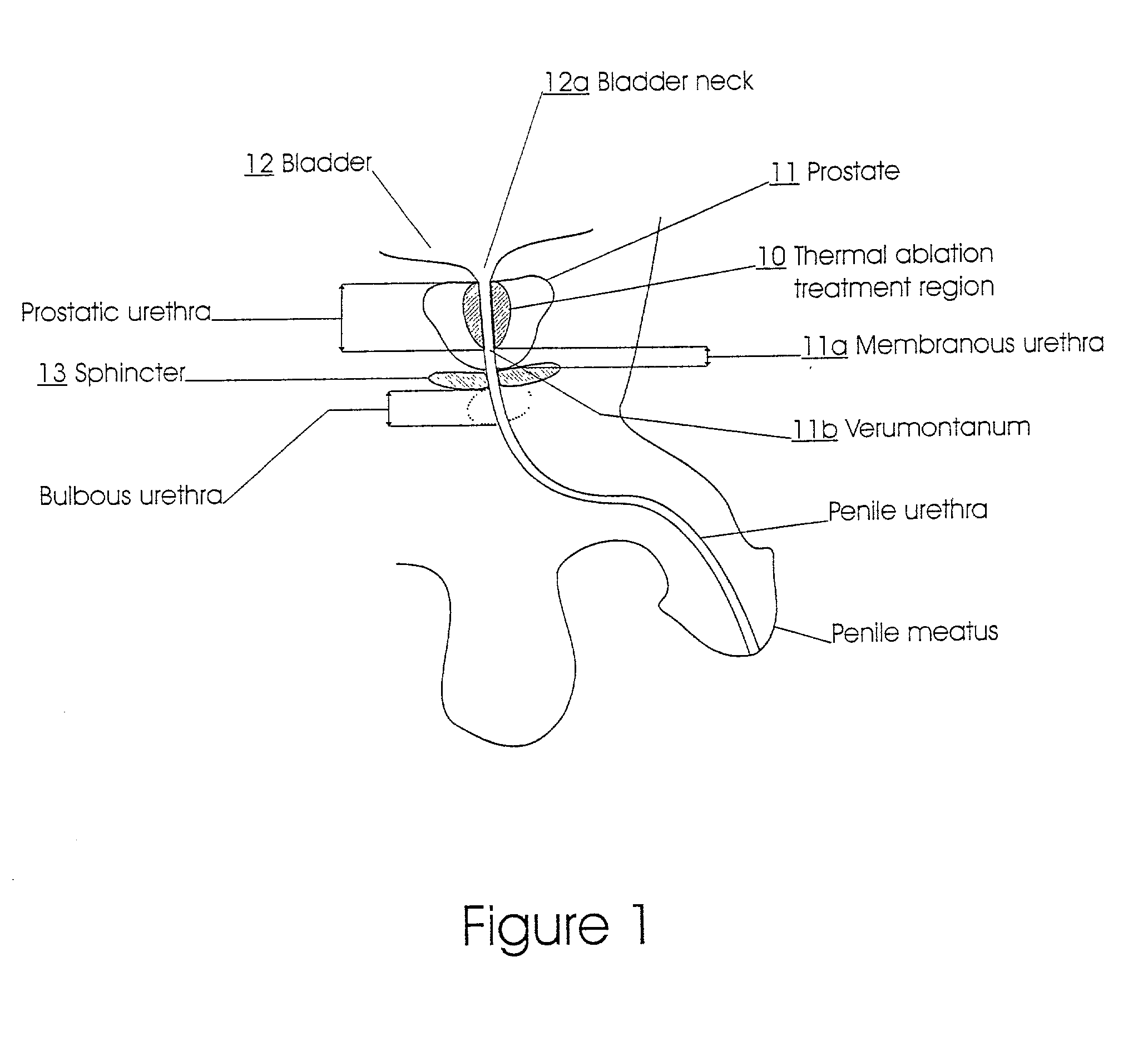
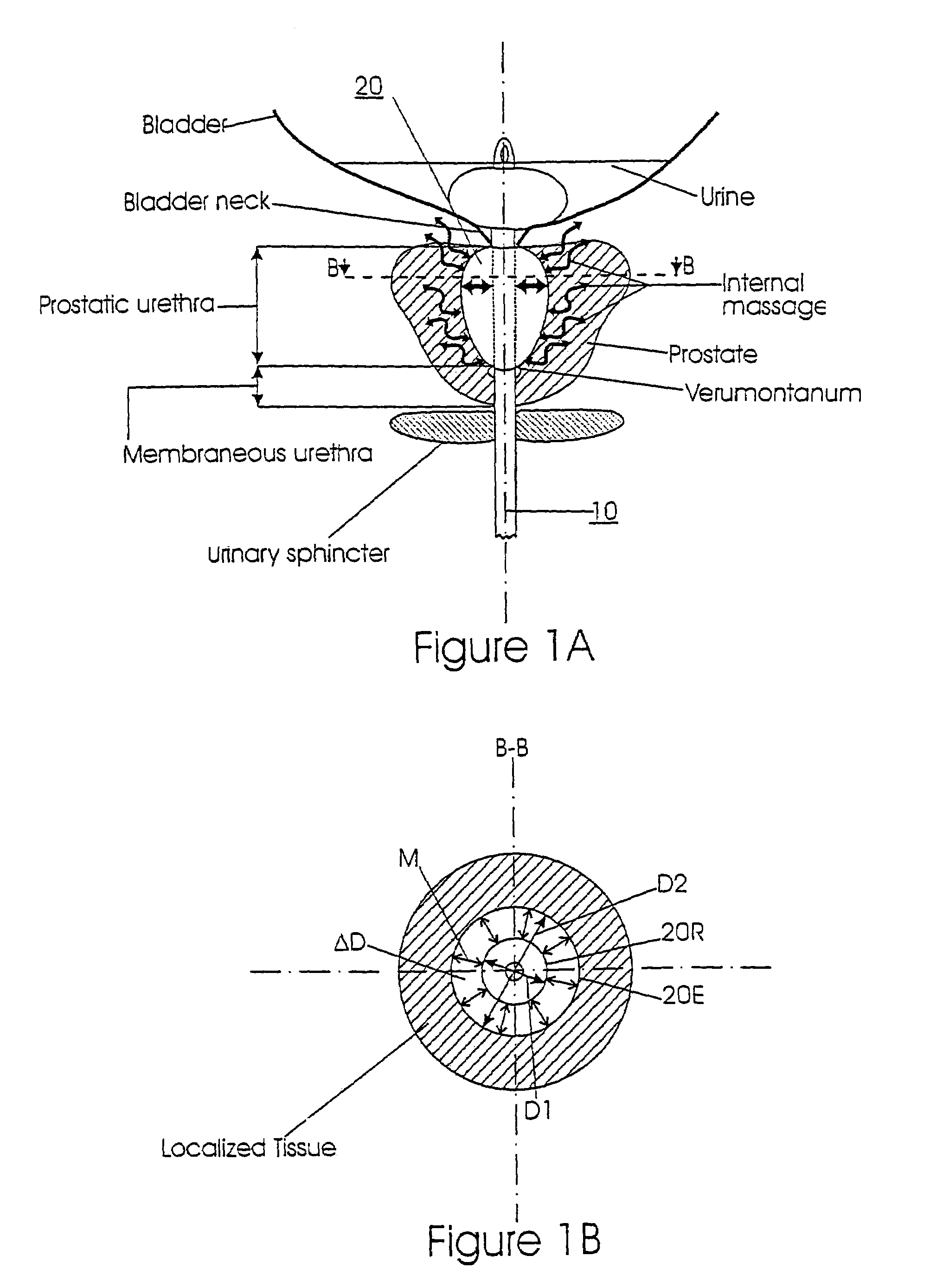
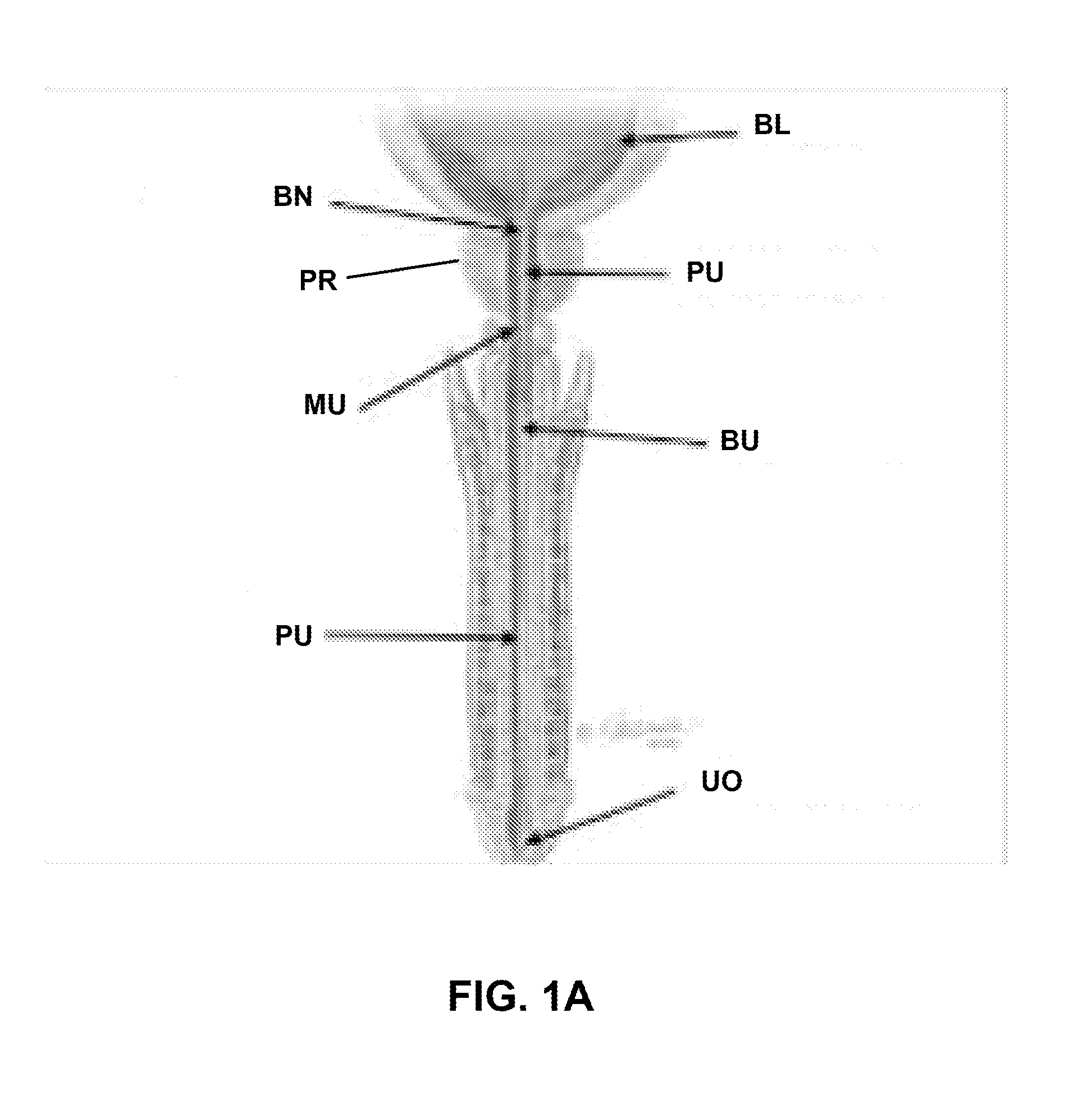
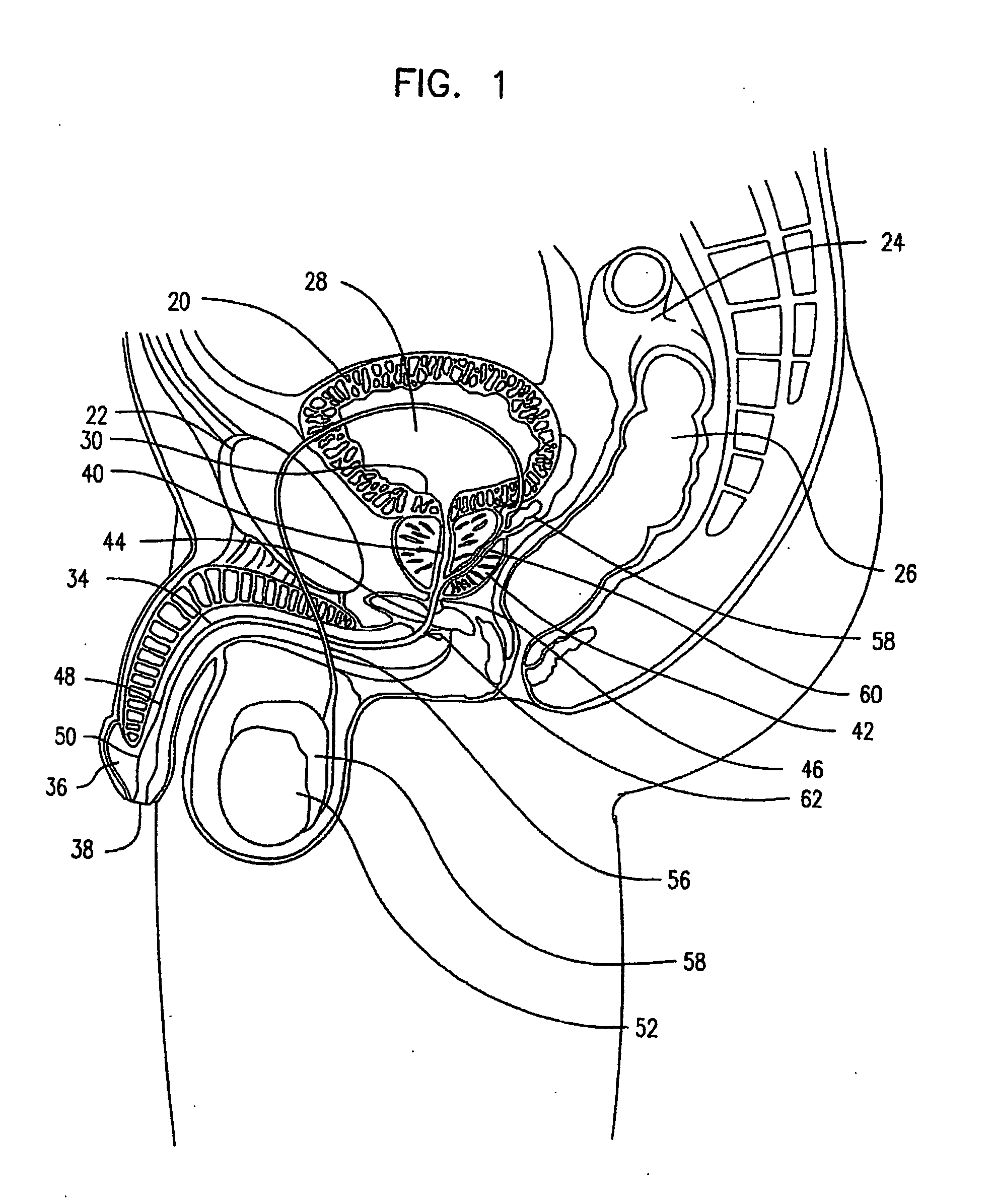
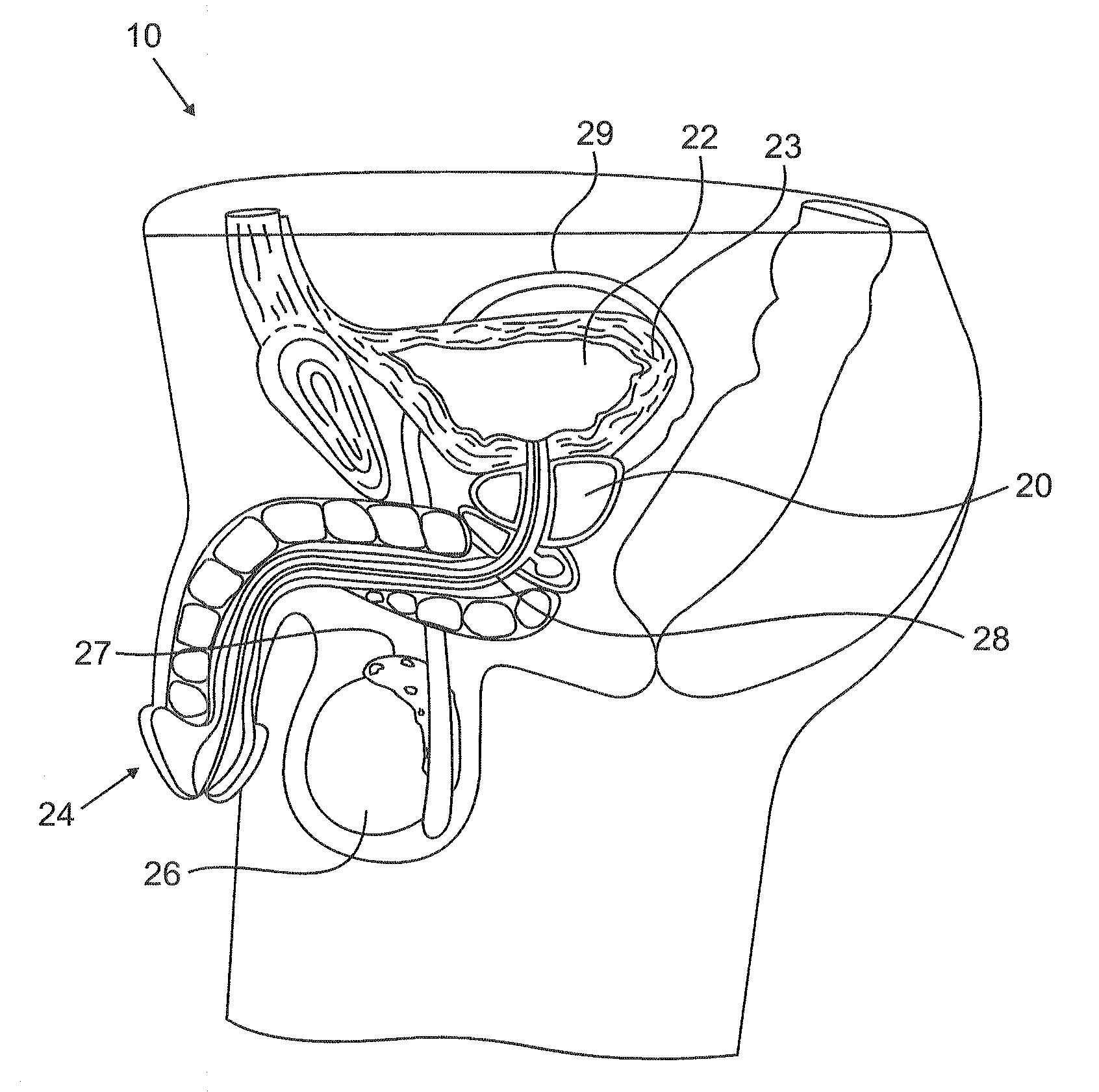
The prostatic urethra, the widest and most dilatable part of the urethra canal, is about 3 cm long. It runs almost vertically through the prostate from its base to its apex, lying nearer its anterior than its posterior surface; the form of the canal is spindle-shaped, being wider in the middle than at either extremity, and narrowest below, where it joins the membranous portion.
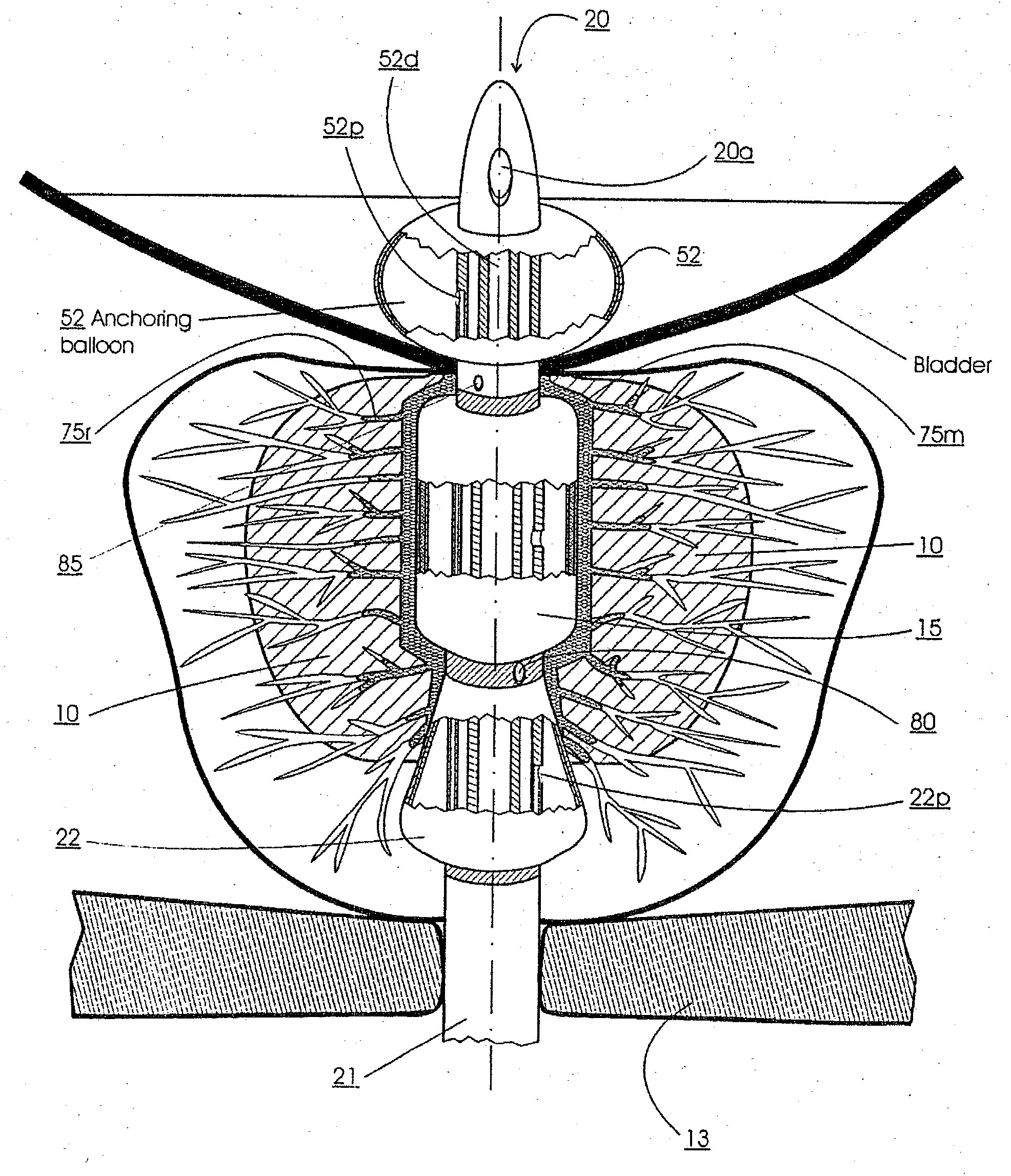
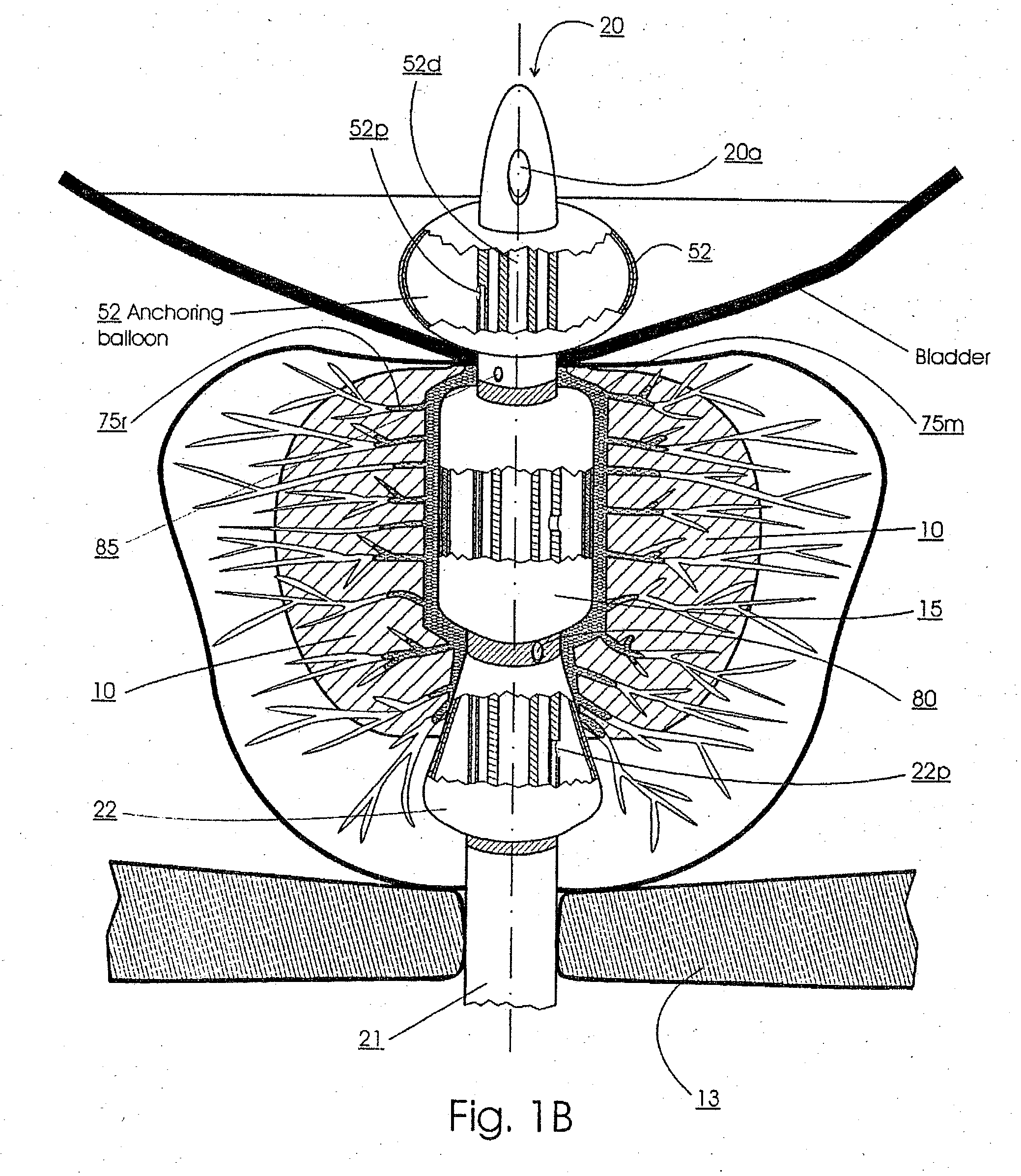
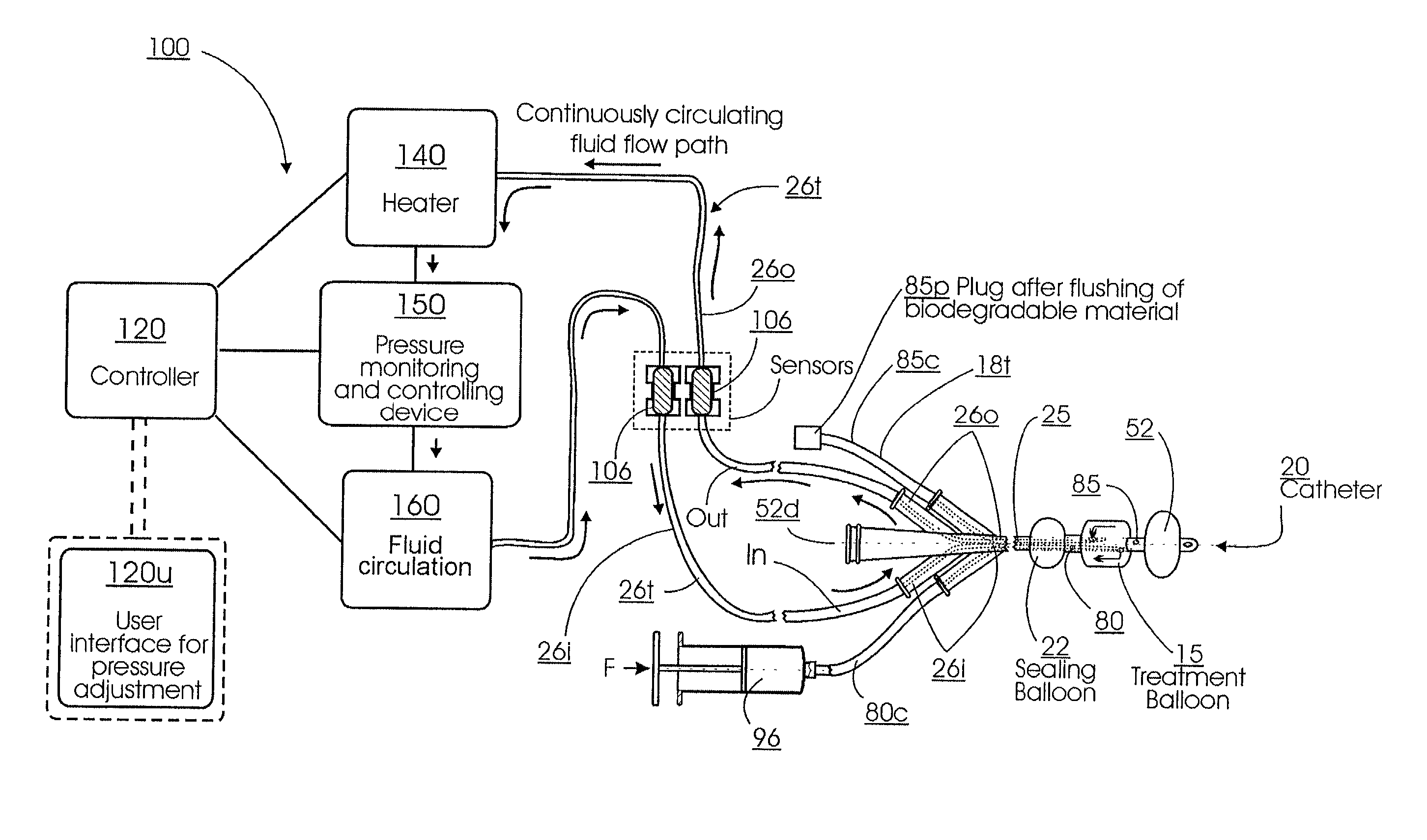
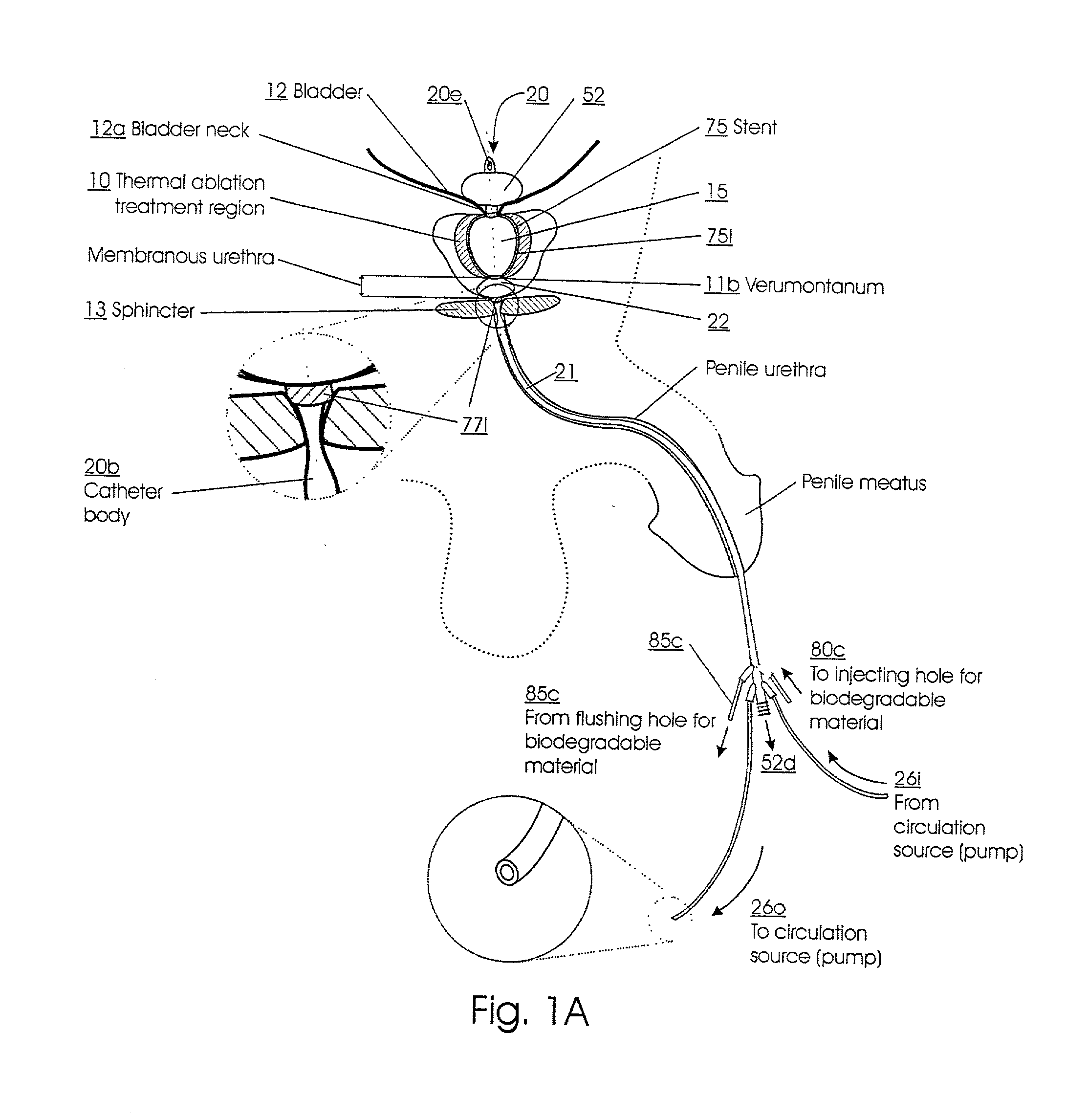
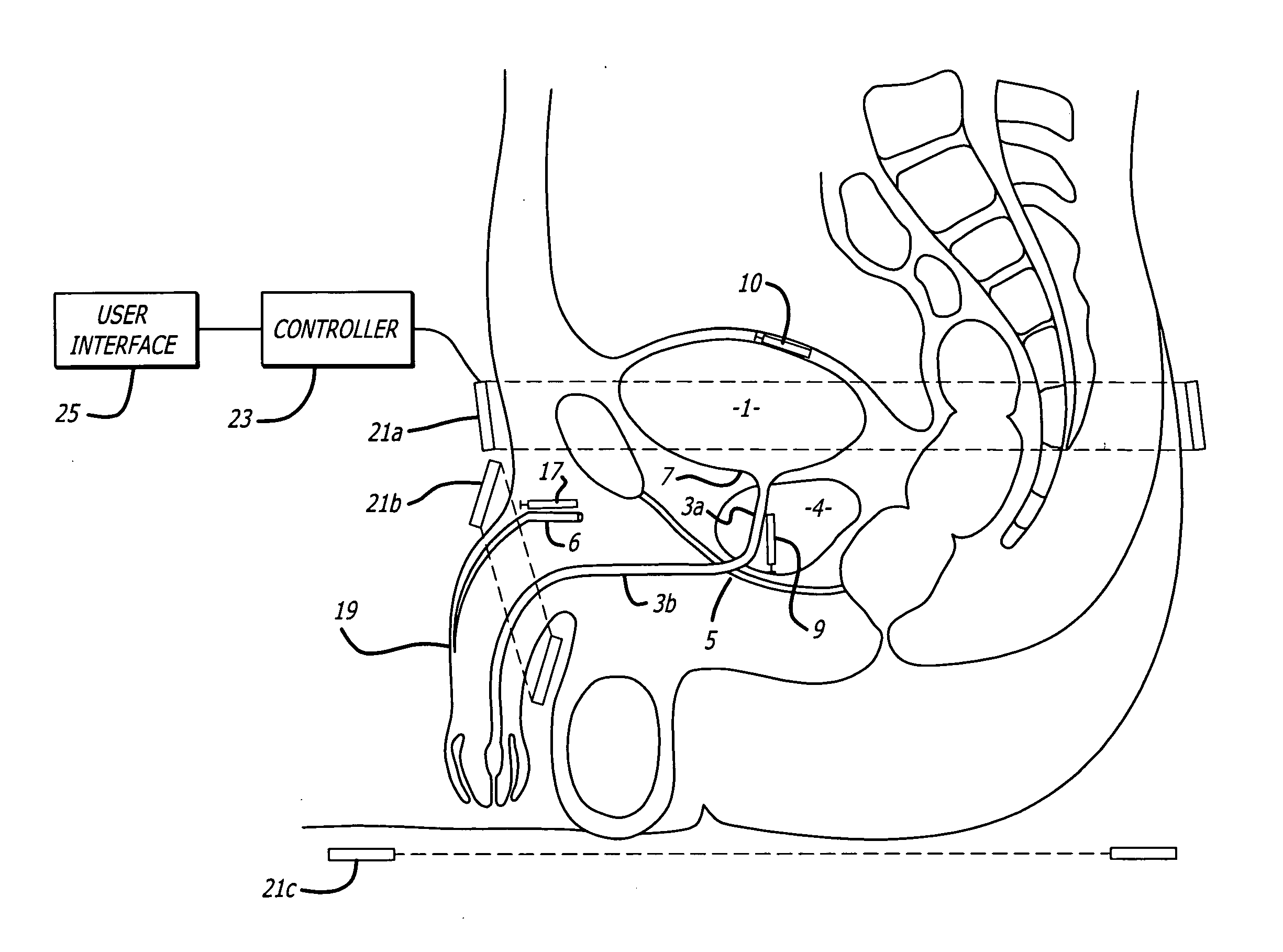
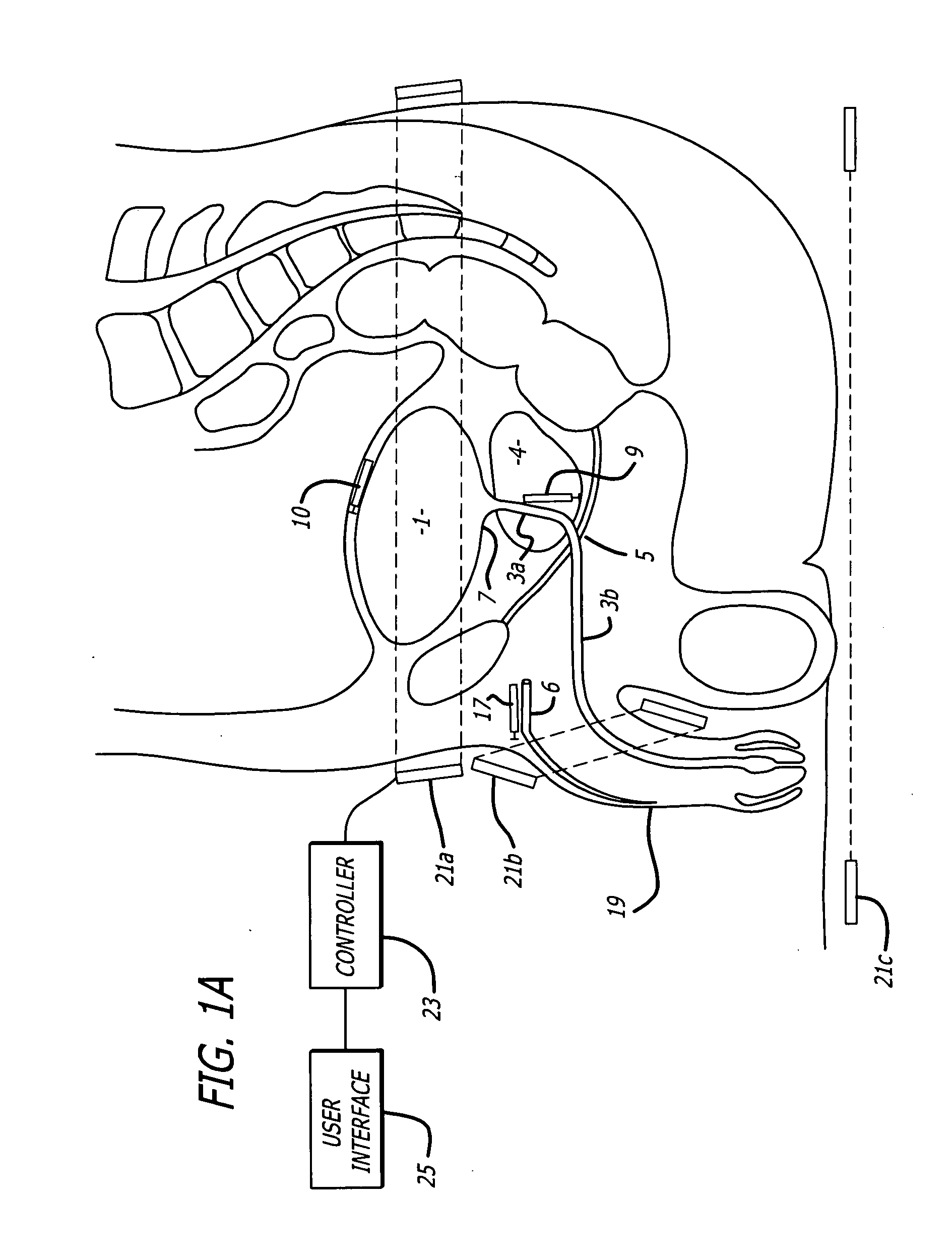
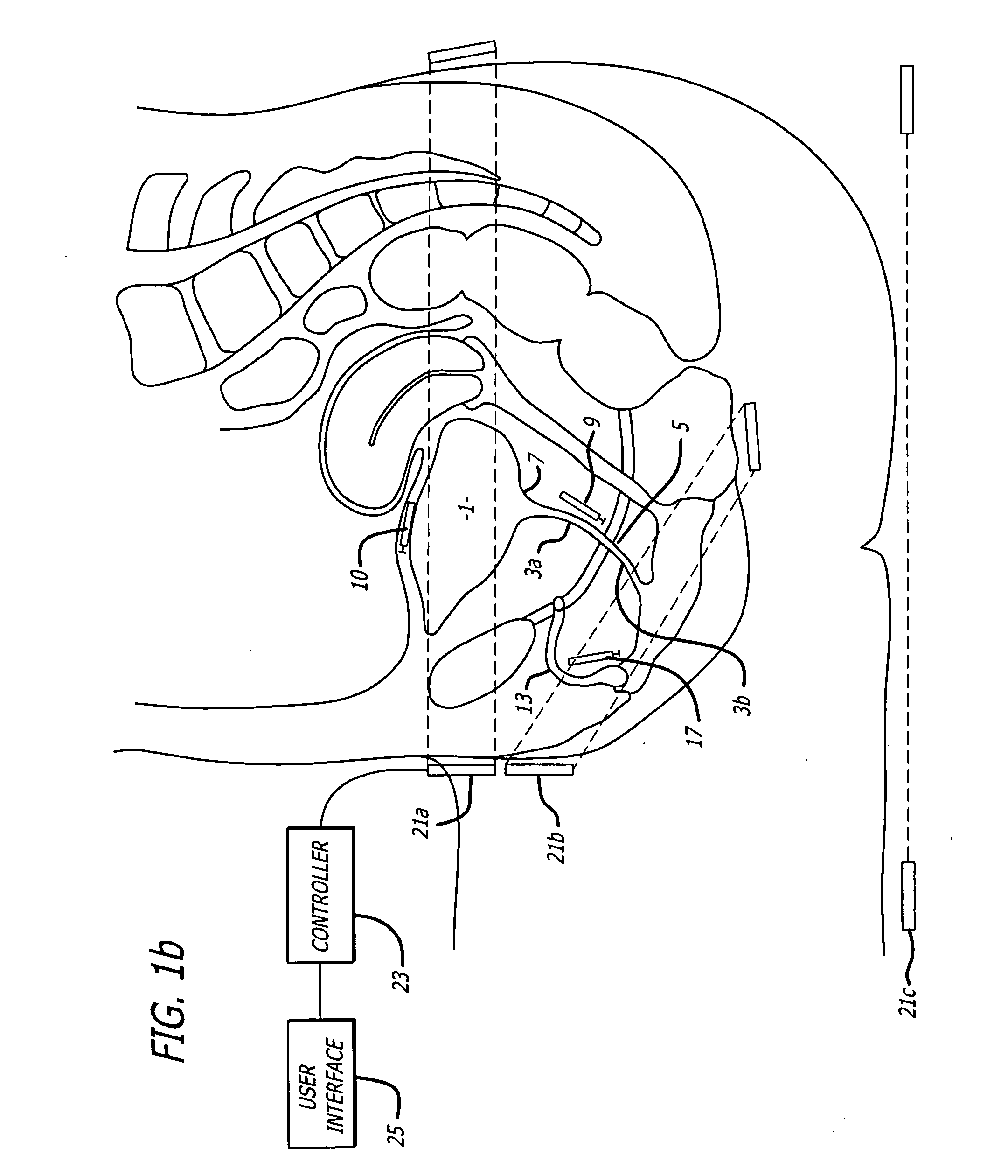
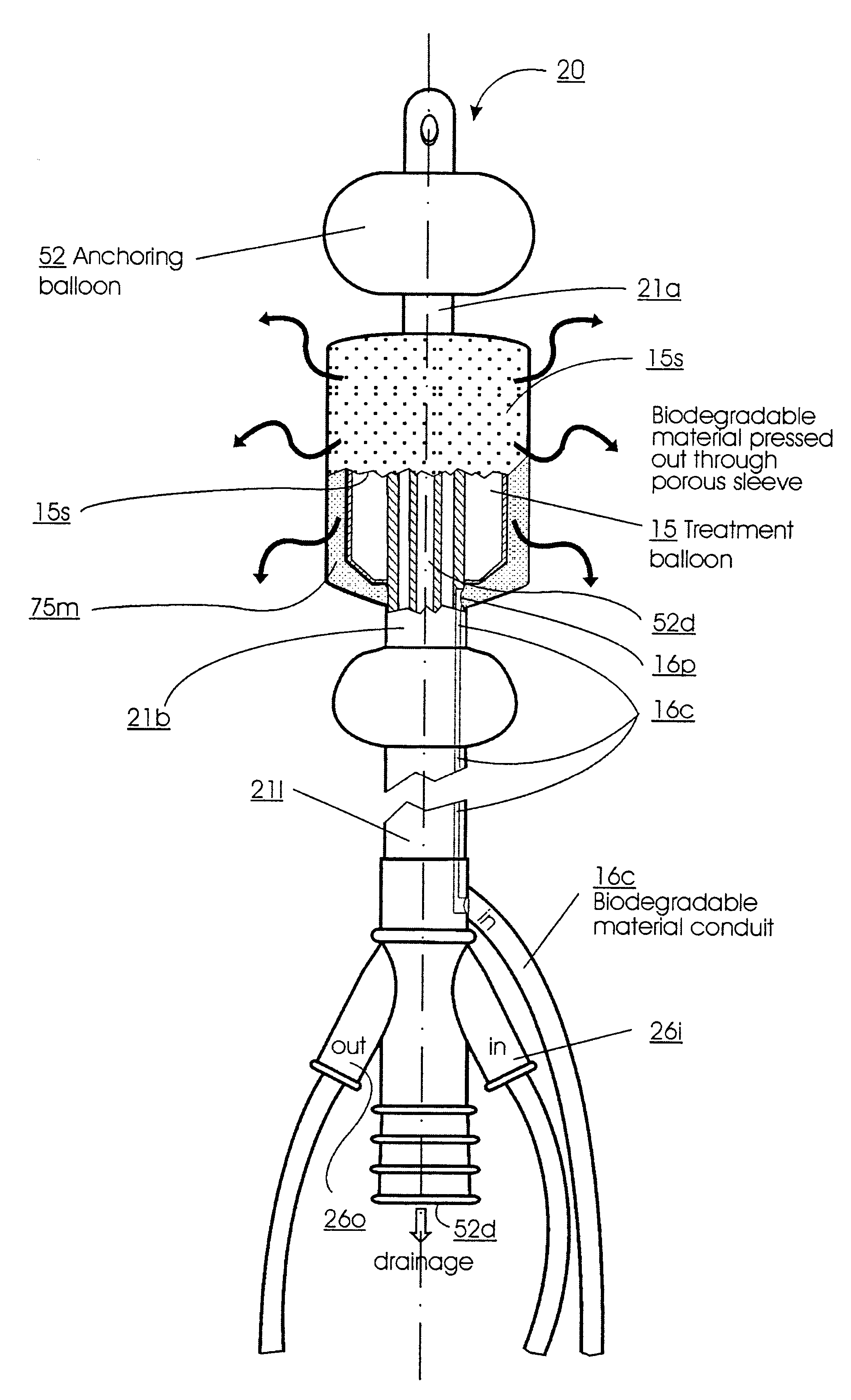
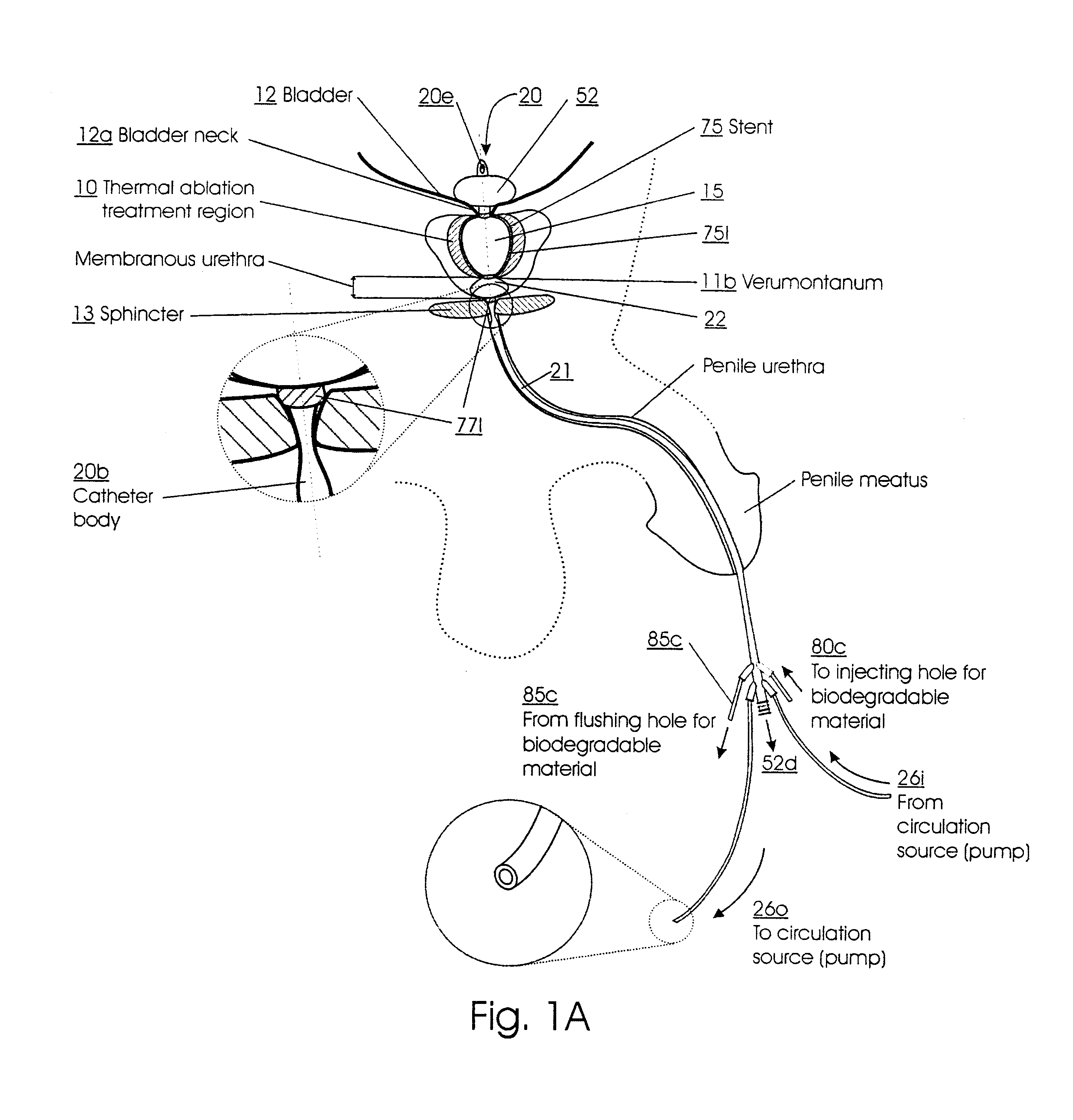
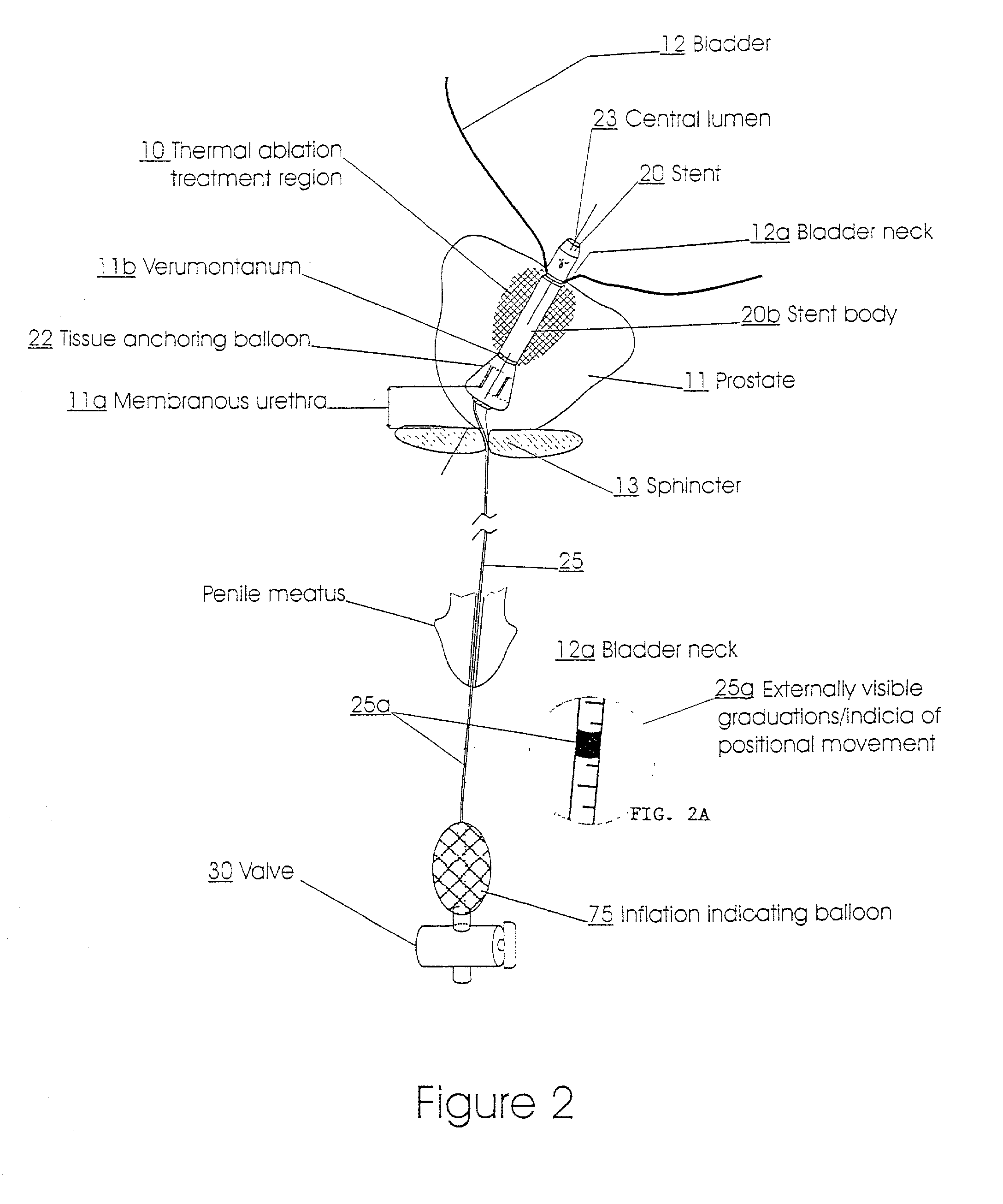
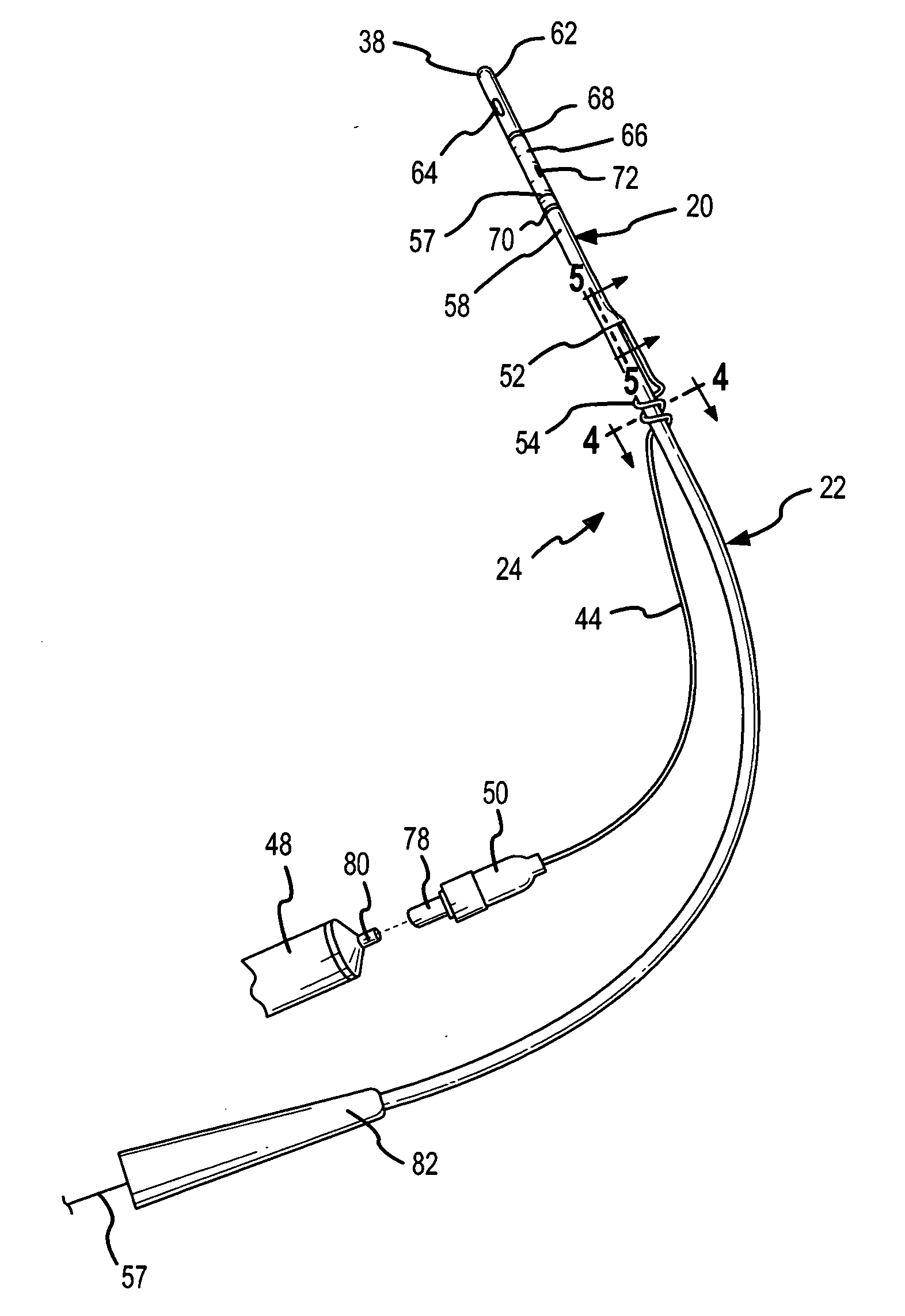
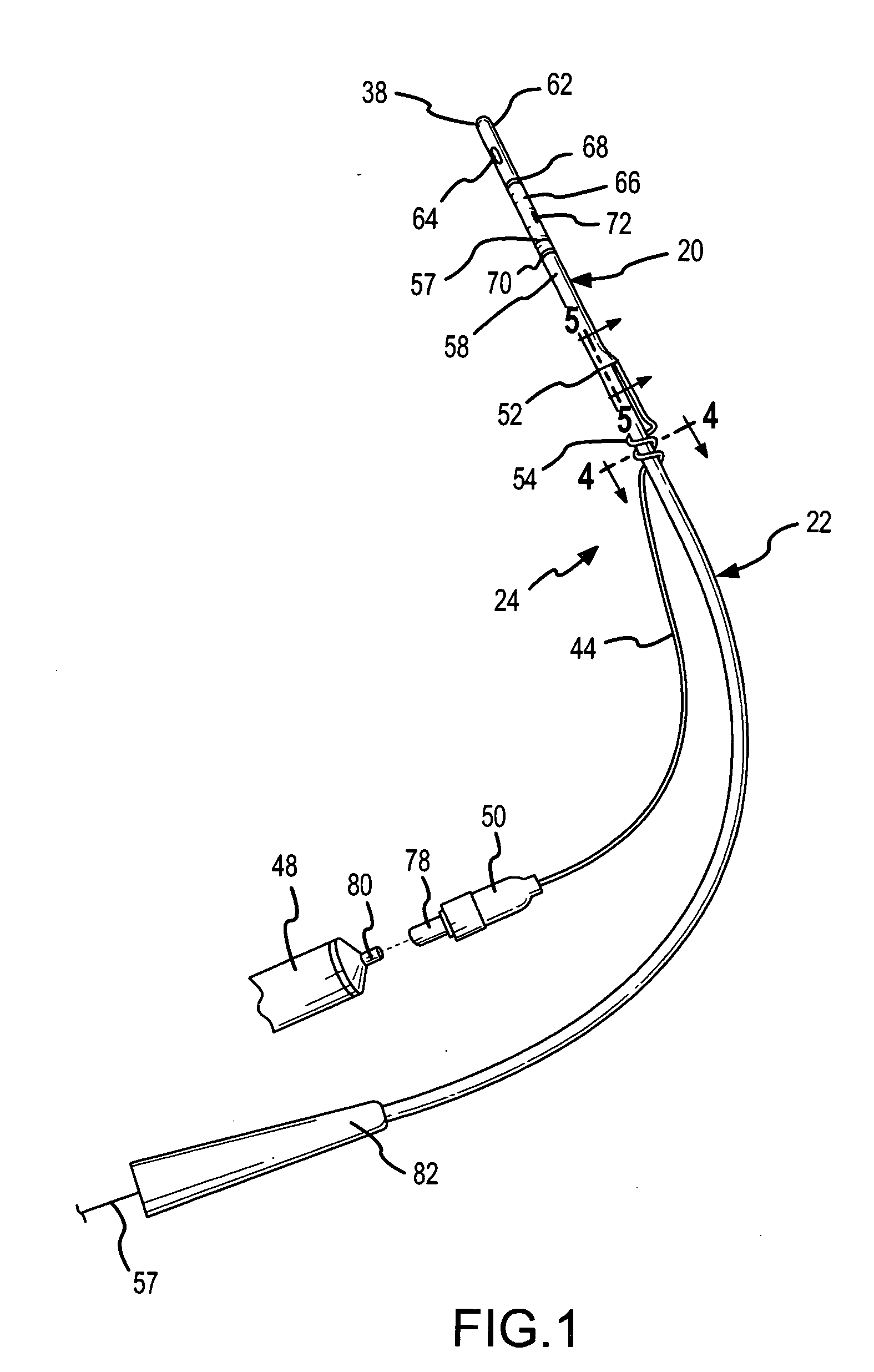
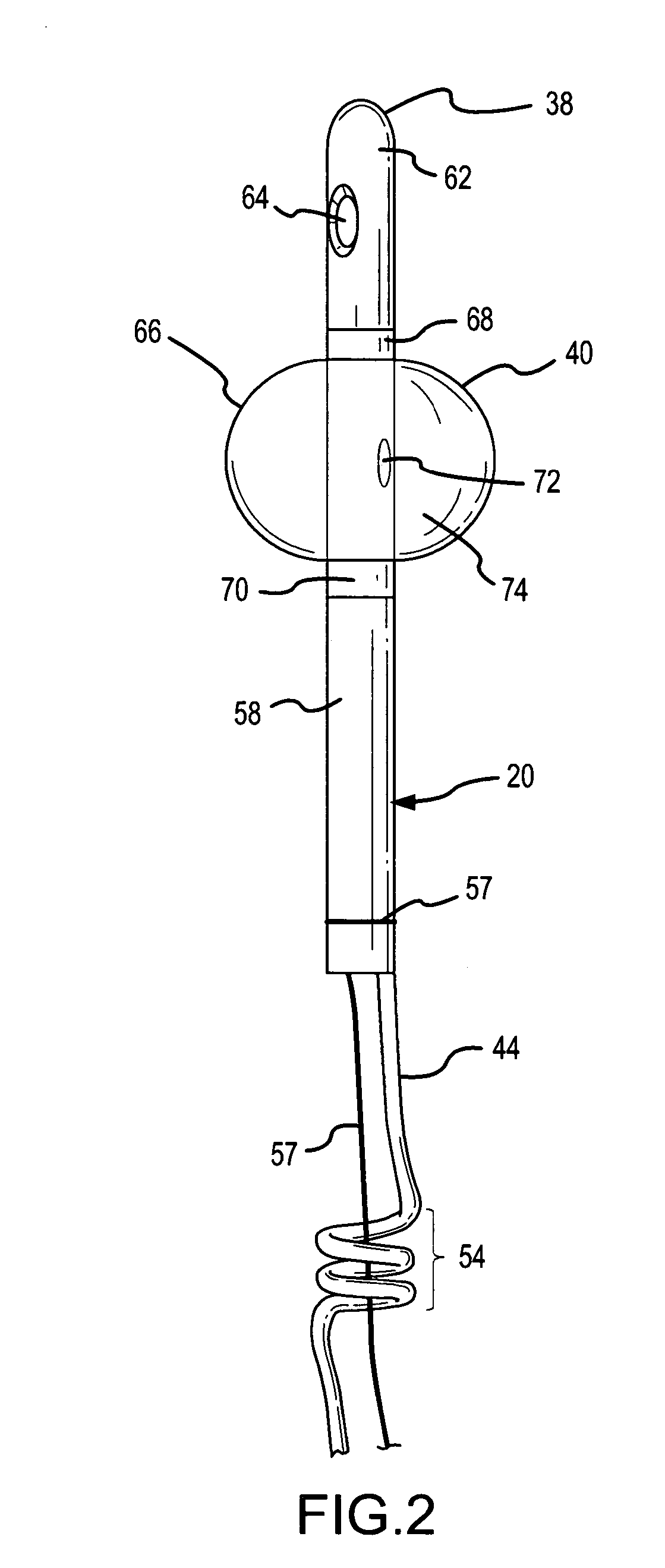
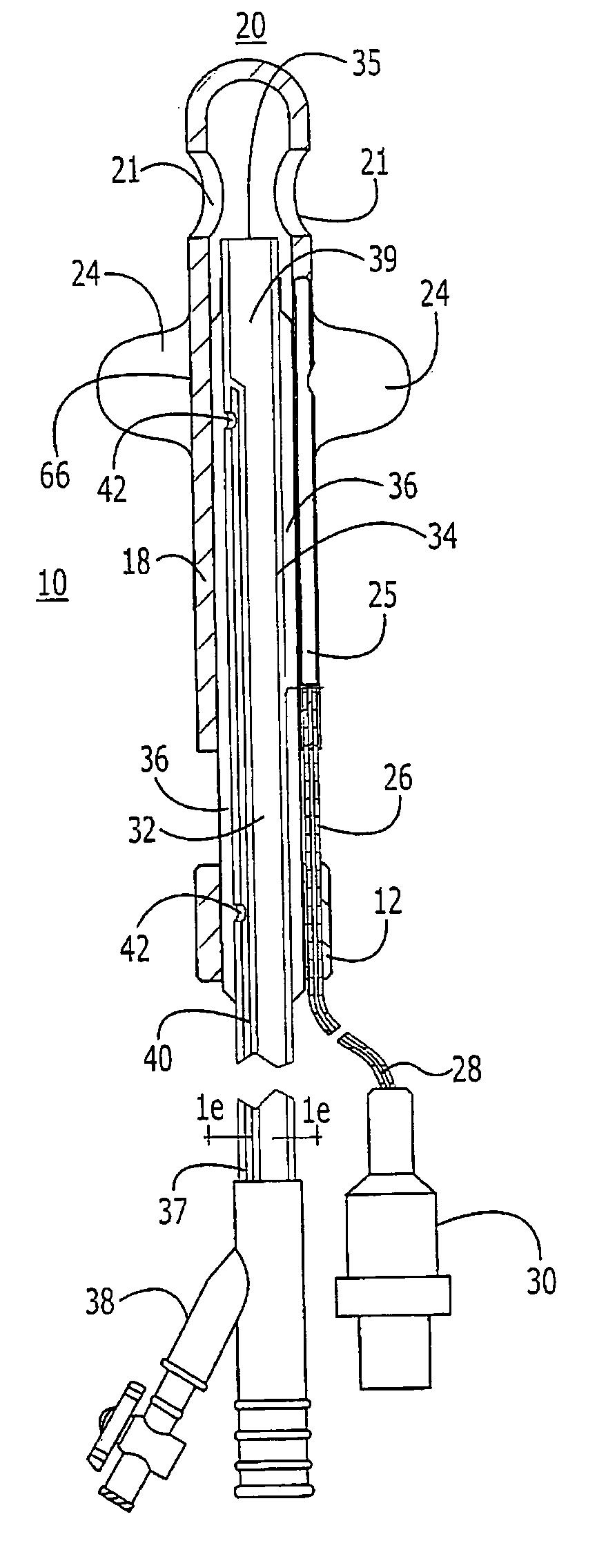
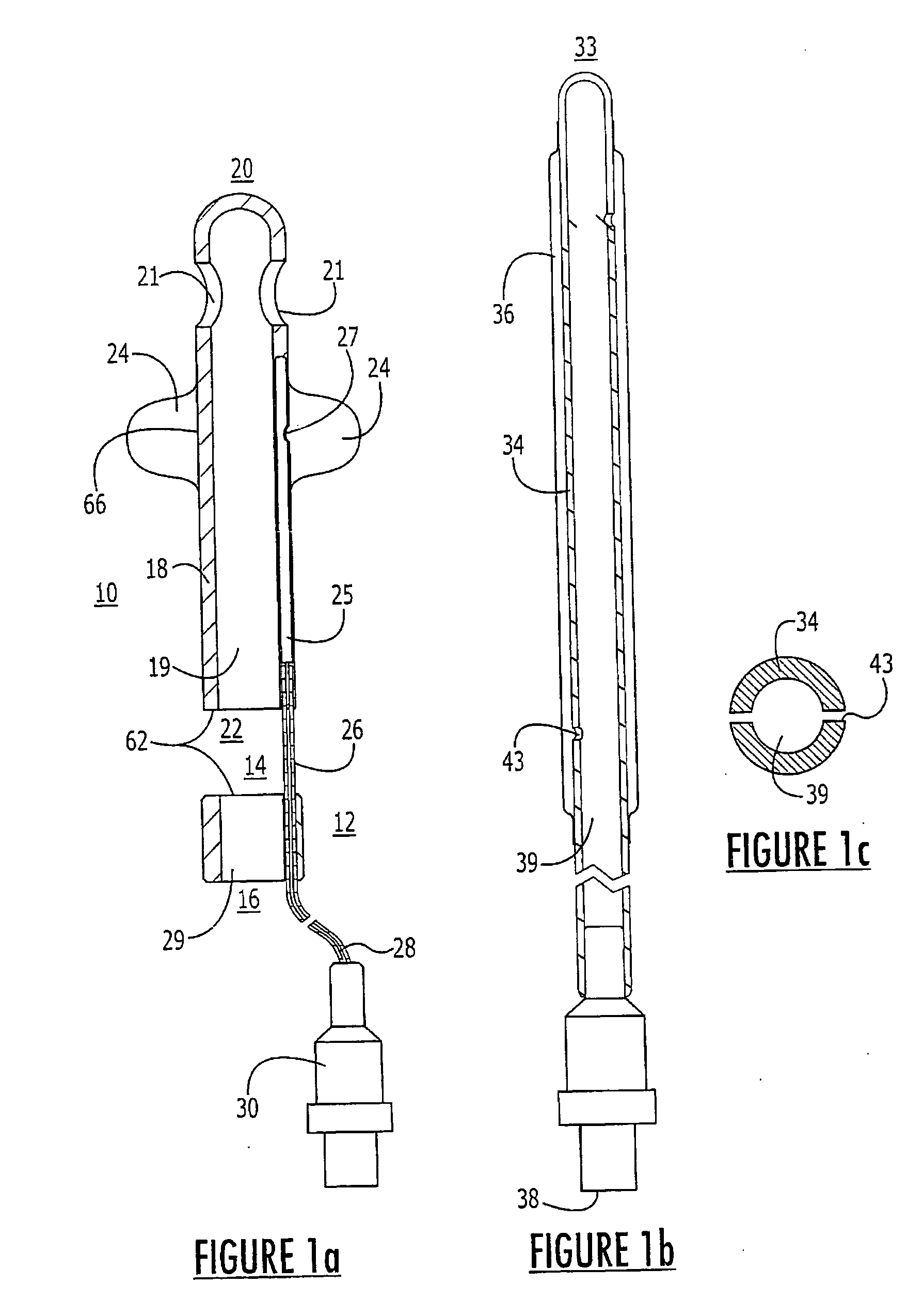
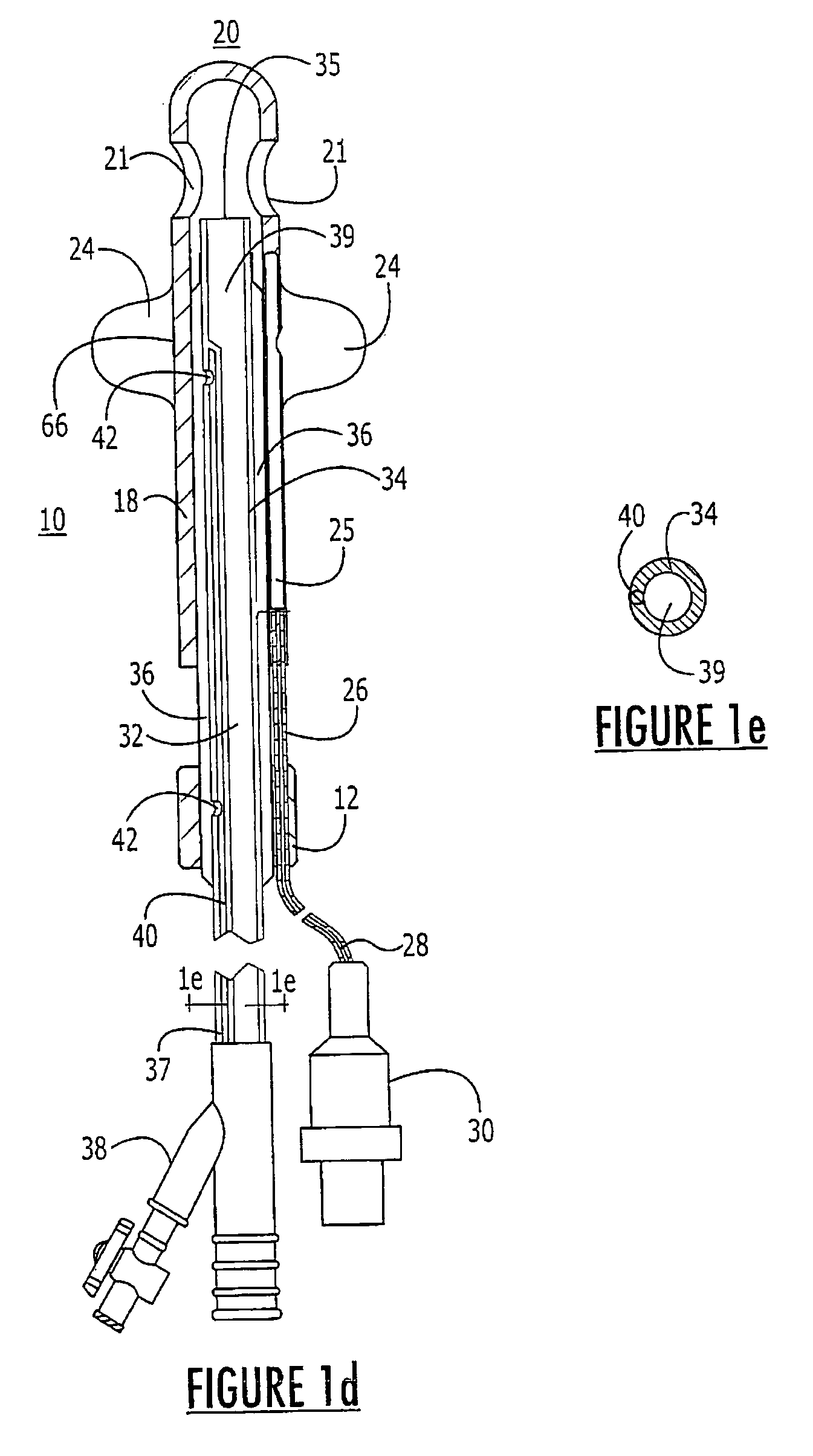
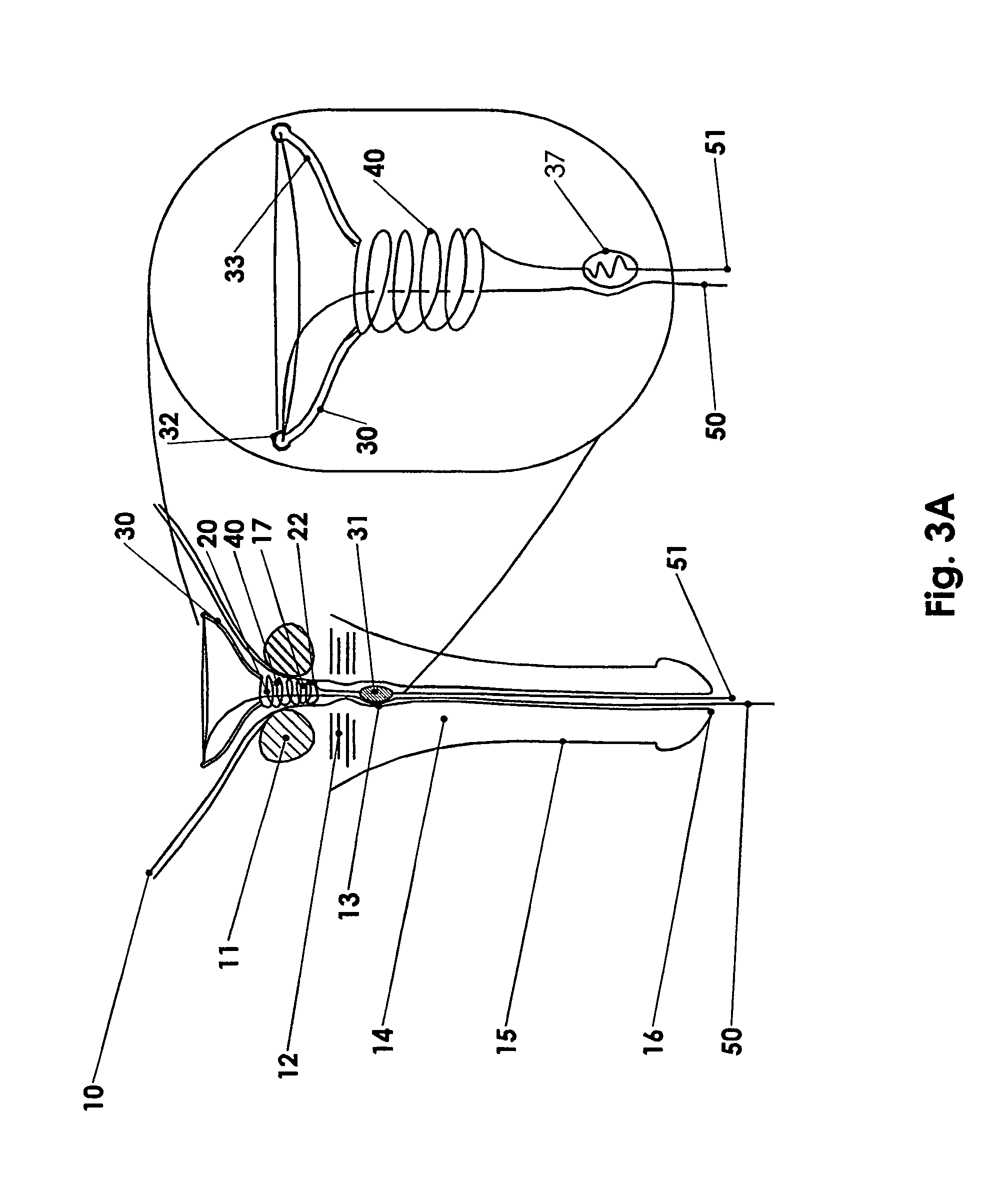
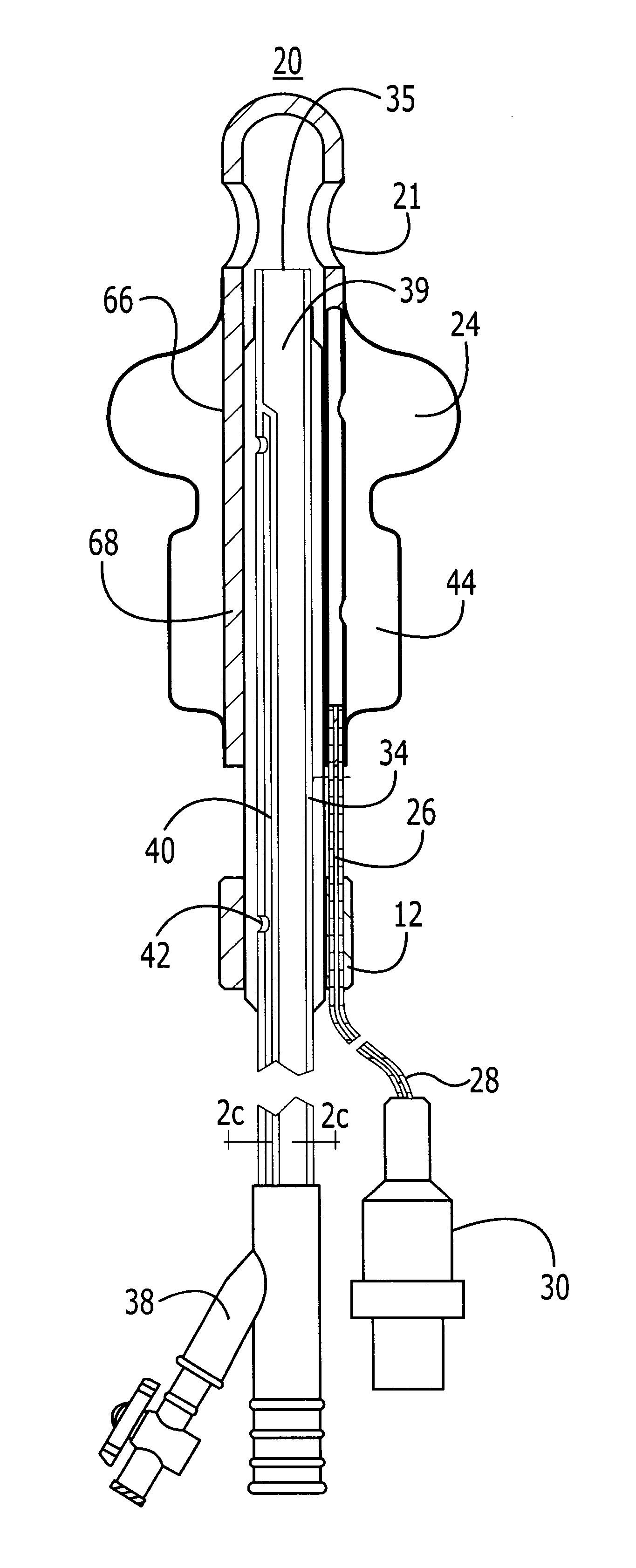
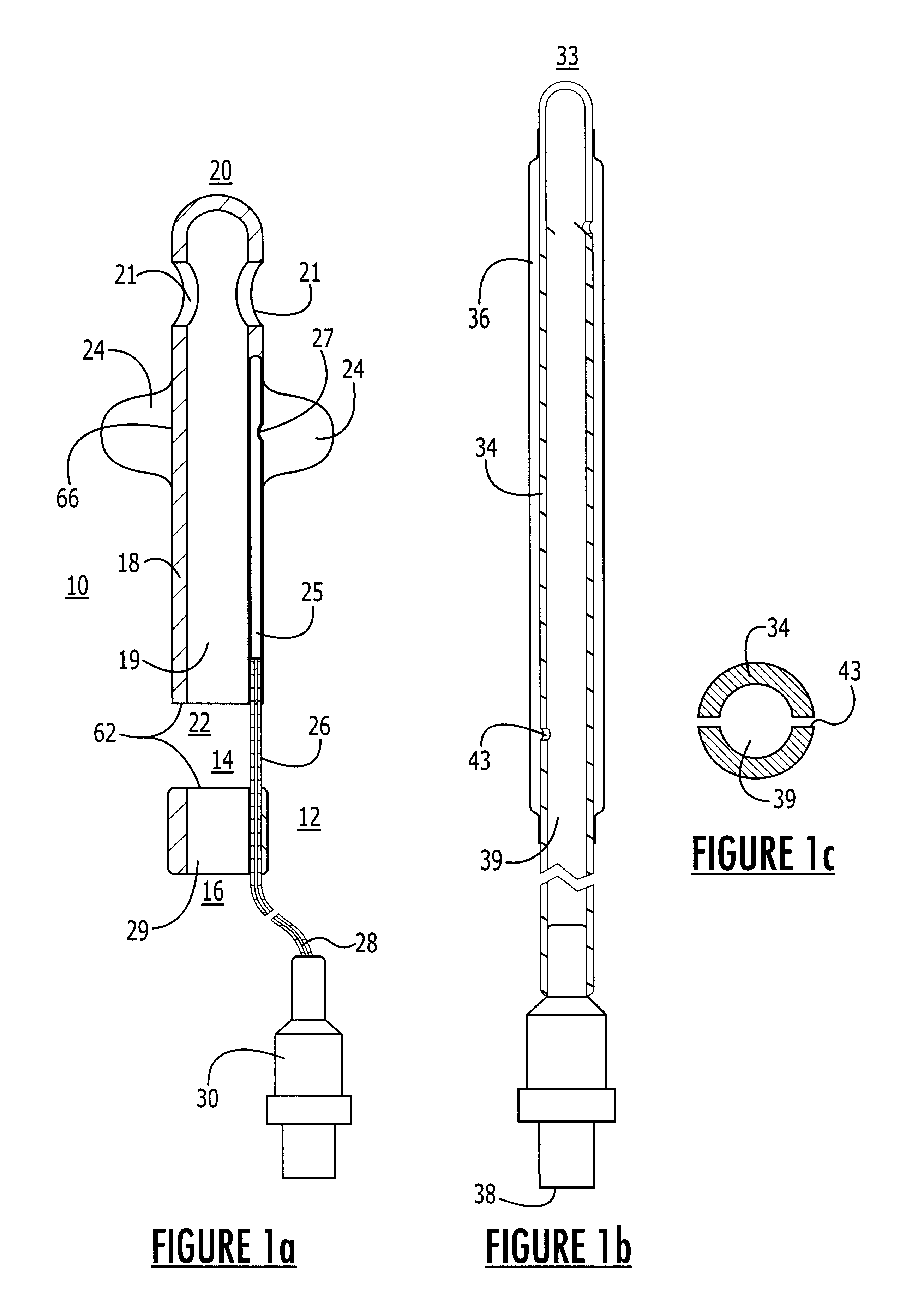
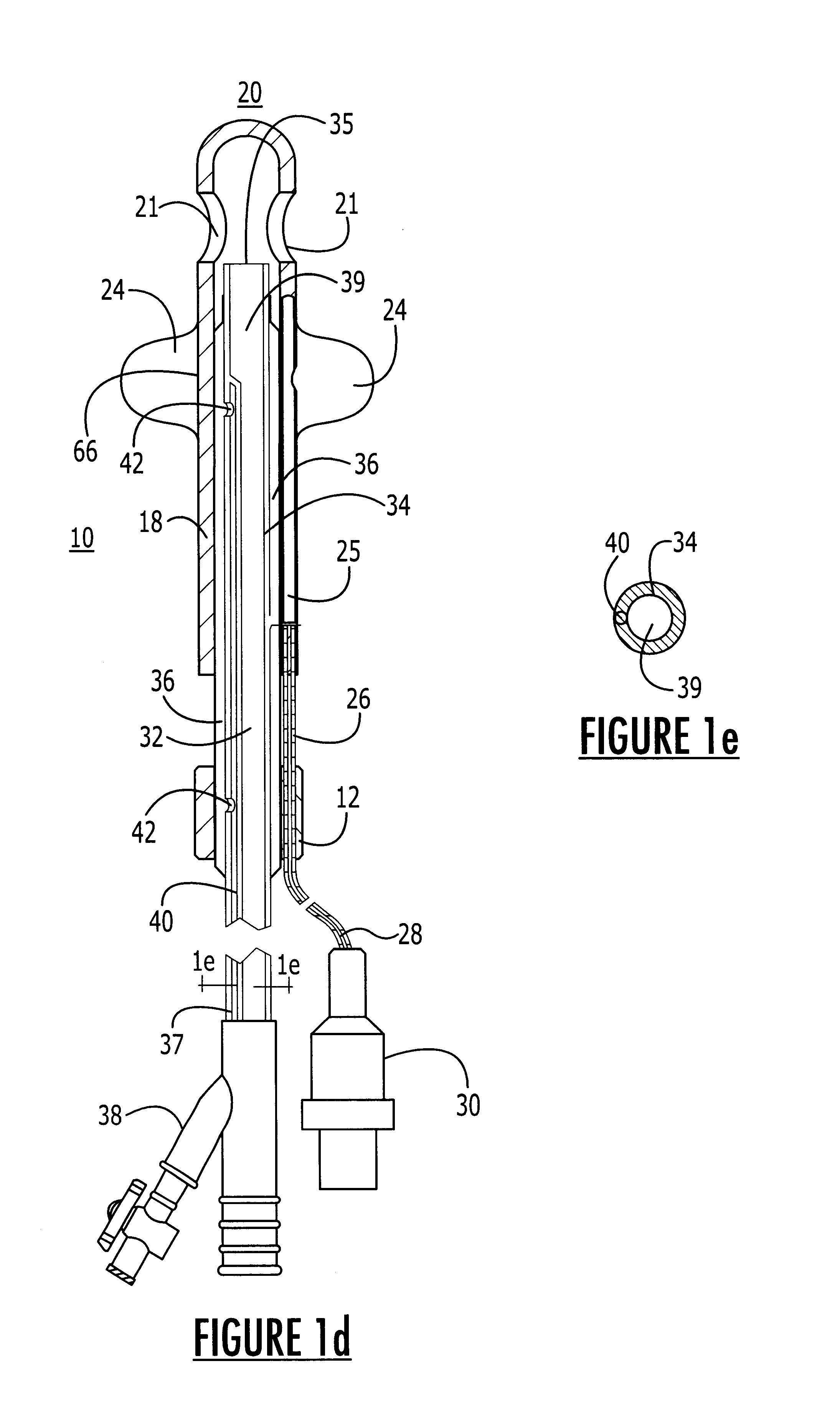
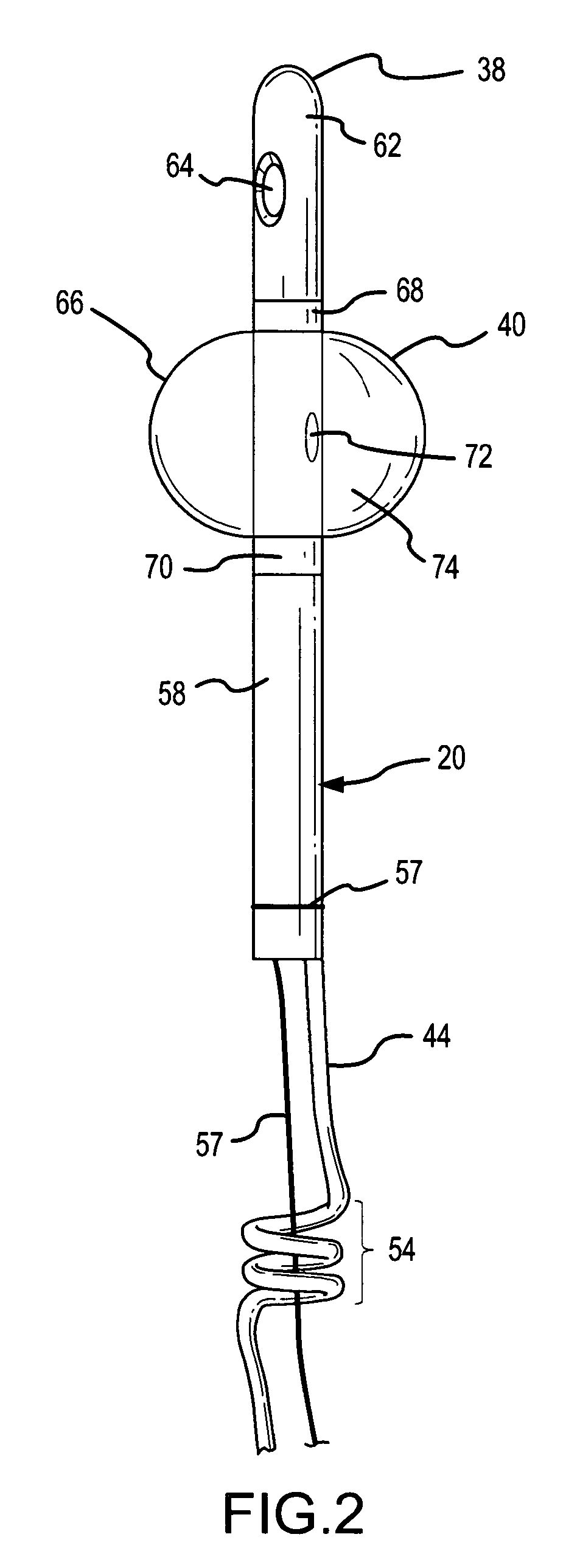
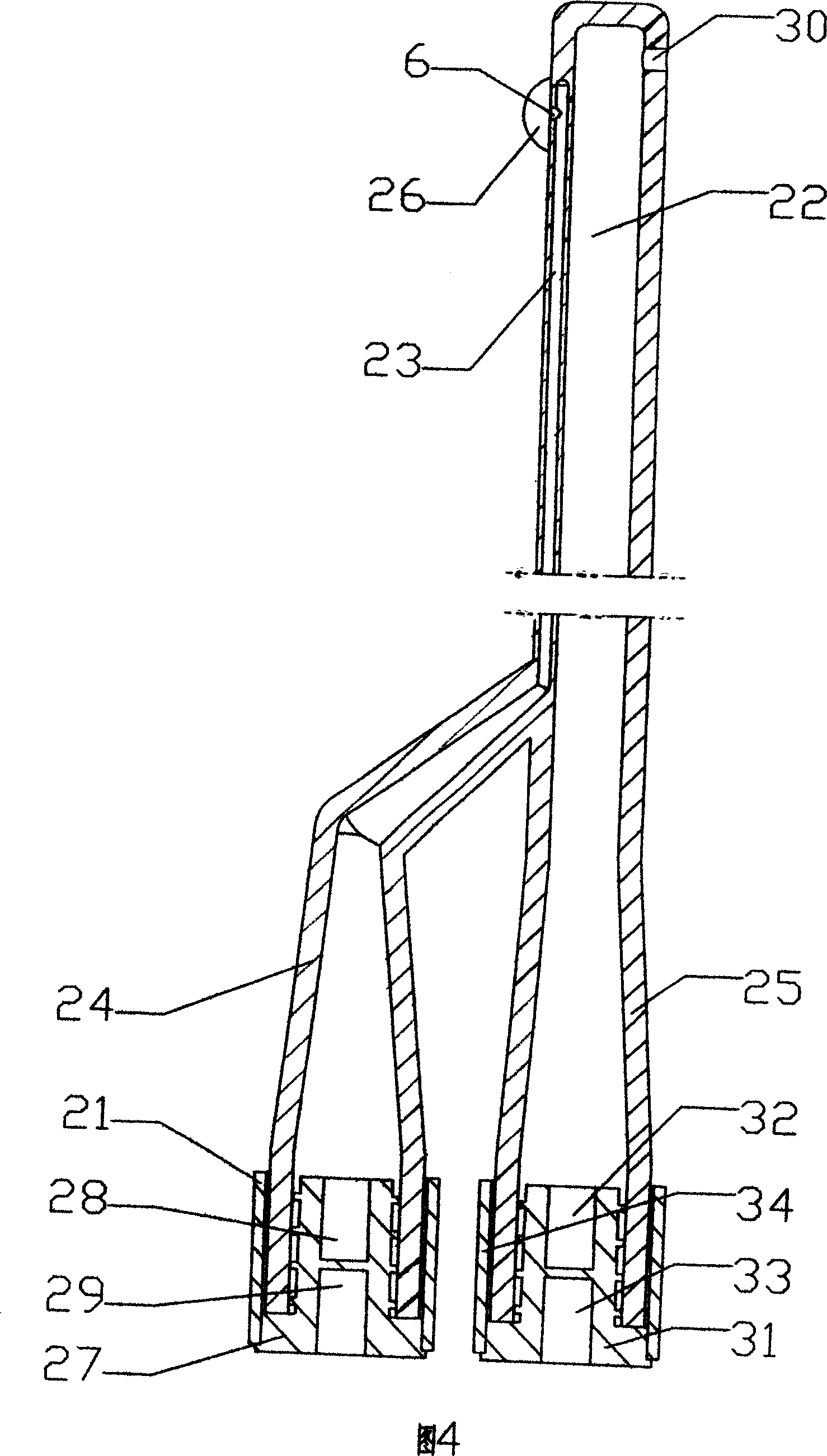
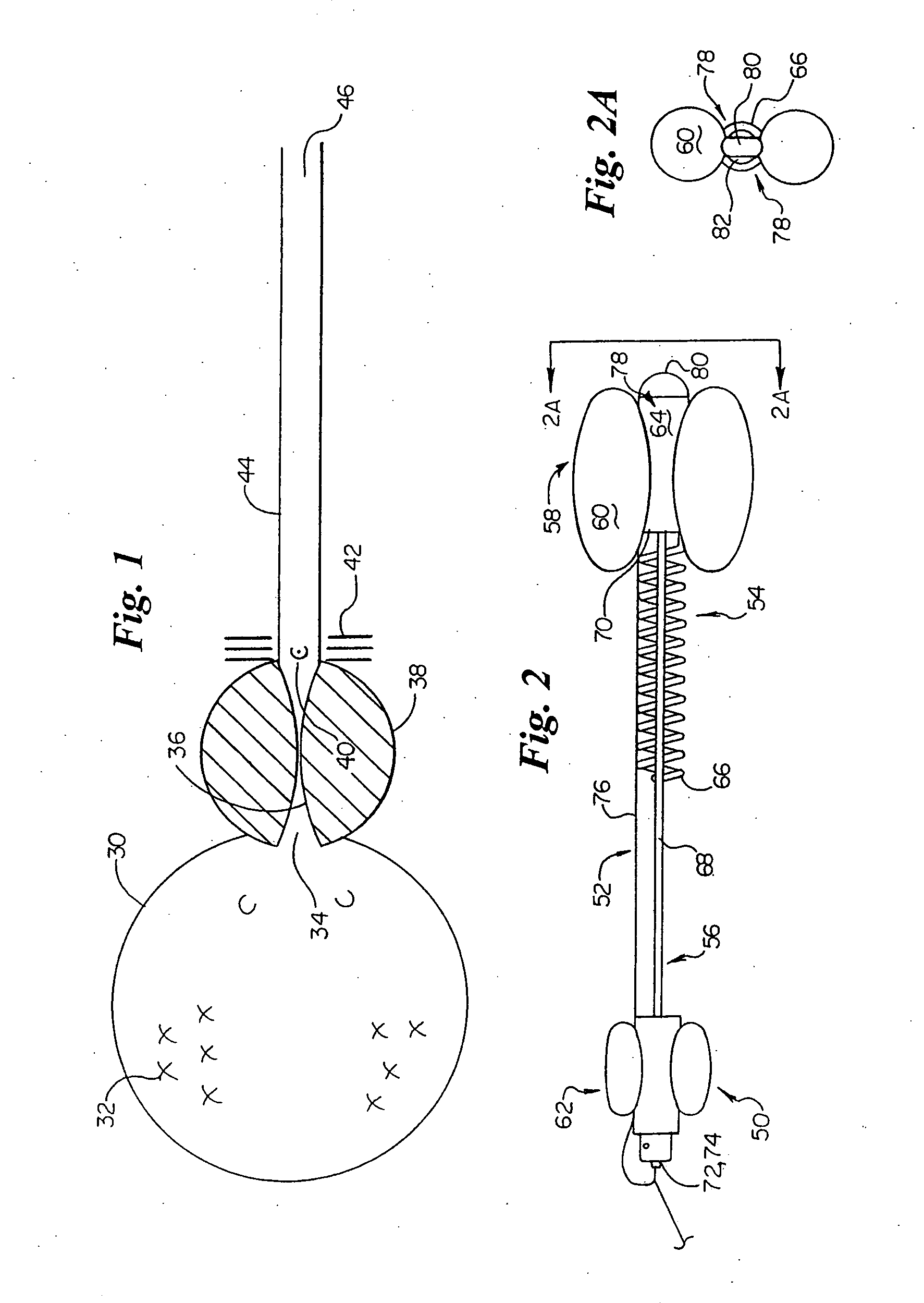
Method for treating the prostate and inhibiting obstruction of the prostatic urethra using biodegradable stents
InactiveUS20040230316A1Avoid obstructionReduce widthBalloon catheterSurgical instruments for heatingUrethraBiodegradable scaffold
Methods of treating the prostate include administering a thermal ablation therapy and inhibiting the obstruction or closure of the prostatic urethral opening by forming a biodegradable stent in situ in the subject such that the stent attaches to the walls of the prostatic urethra. A related method of treating BPH includes thermally treating or ablating localized tissue in the prostate with a treatment catheter and inserting flowable stent material via the treatment catheter into the prostate (either before, during, or after the thermal treatment), forming the flowable stent material so that it defines a stent that remains in position after removal of the treatment catheter to inhibit the closure of the urinary passage. Associated stents and catheters are included.
Owner:WIT IP CORP
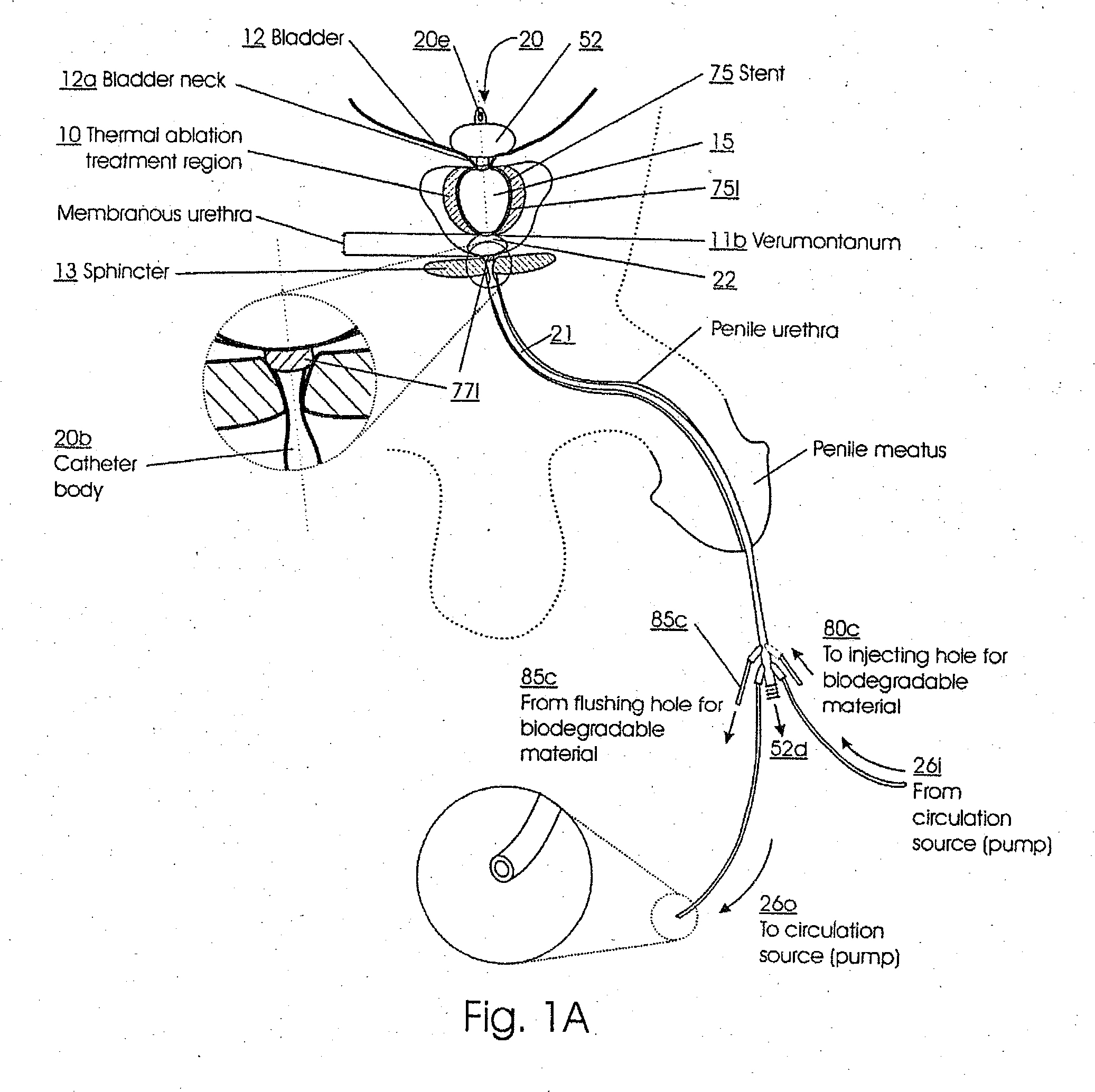
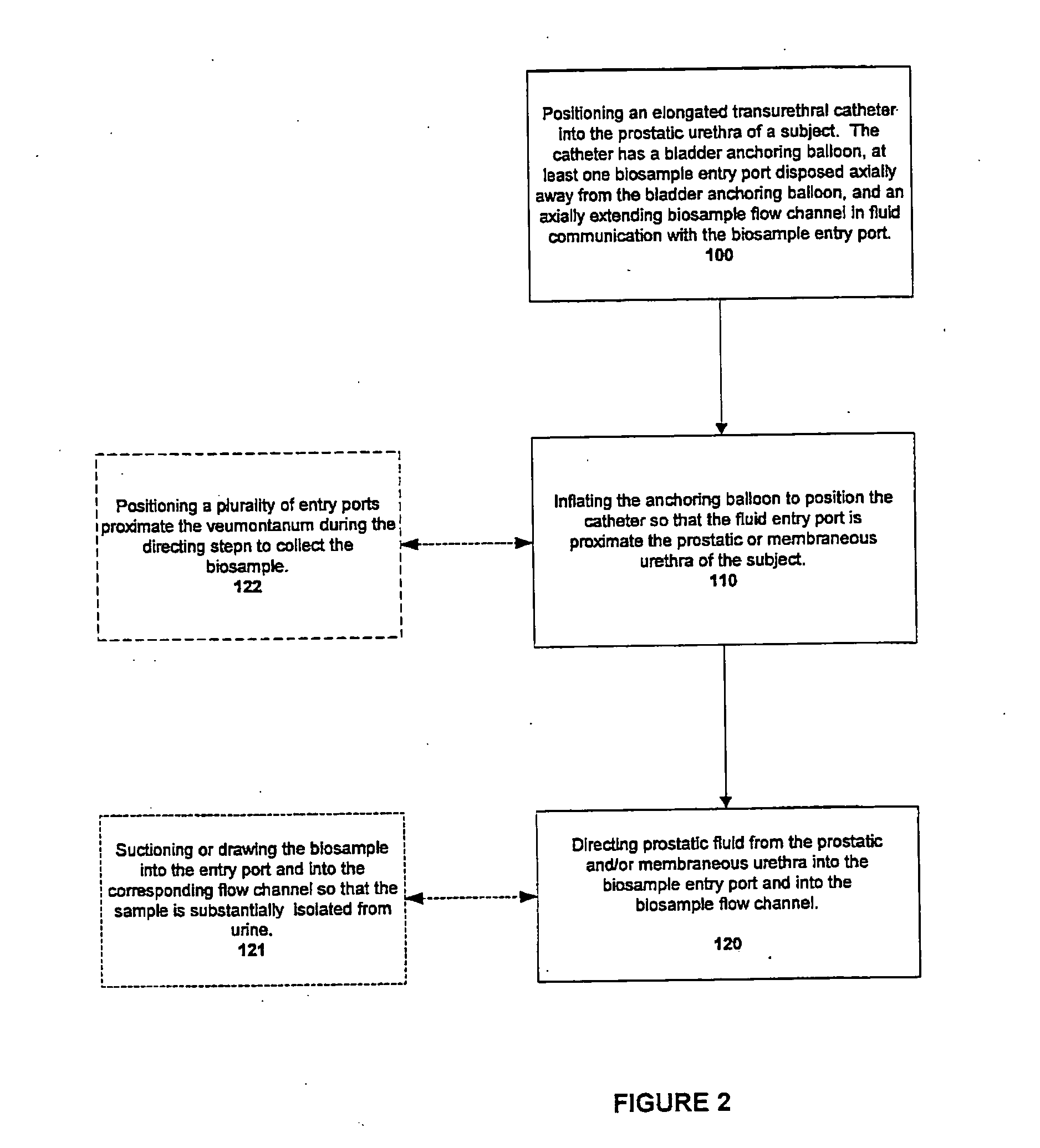
Catheters with suction capability and related methods and systems for obtaining biosamples in vivo
InactiveUS20050054994A1Convenient treatmentImprove responsivenessStentsSurgical needlesUrethraProstatic fluid sample
Methods, systems, and computer program products for obtaining a sample in vivo and / or treating a subject include positioning an elongated transurethral catheter in the prostatic urethra of a subject, the catheter having a bladder anchoring balloon, at least one biosample entry port disposed axially away from the bladder anchoring balloon, and an axially extending biosample flow channel in fluid communication with the biosample entry port held internally in the catheter. The anchoring balloon is inflated to position the catheter so that the fluid entry port is proximate the prostatic urethra of the subject and prostatic fluid is suctioned from the prostatic urethra into the biosample entry port and into the biosample flow channel. The catheter can include a thermal treatment balloon and / or dilatation balloon and the suctioned sample can be obtained concurrently with, during, or proximate in time to the applied treatment. The catheter can be configured to allow urine to drain therethrough during the treatment / collection of the sample while keeping the urine isolated from the collected prostatic fluid sample.
Owner:WIT IP CORP
Methods for treating the prostate and inhibiting obstruction of the prostatic urethra using biodegradable stents
InactiveUS20020082610A1Avoid obstructionReduce widthStentsEar treatmentUrethraBiodegradable scaffold
Methods of treating the prostate include administering a thermal ablation therapy and inhibiting the obstruction or closure of the prostatic urethral opening by forming a biodegradable stent in situ in the subject such that the stent attaches to the walls of the prostatic urethra. A related method of treating BPH includes thermally treating or ablating localized tissue in the prostate with a treatment catheter and inserting flowable stent material via the treatment catheter into the prostate (either before, during, or after the thermal treatment), forming the flowable stent material so that it defines a stent that remains in position after removal of the treatment catheter to inhibit the closure of the urinary passage. Associated stents and catheters are included.
Owner:JPMORGAN CHASE BANK AS ADMINISTATIVE AGENT
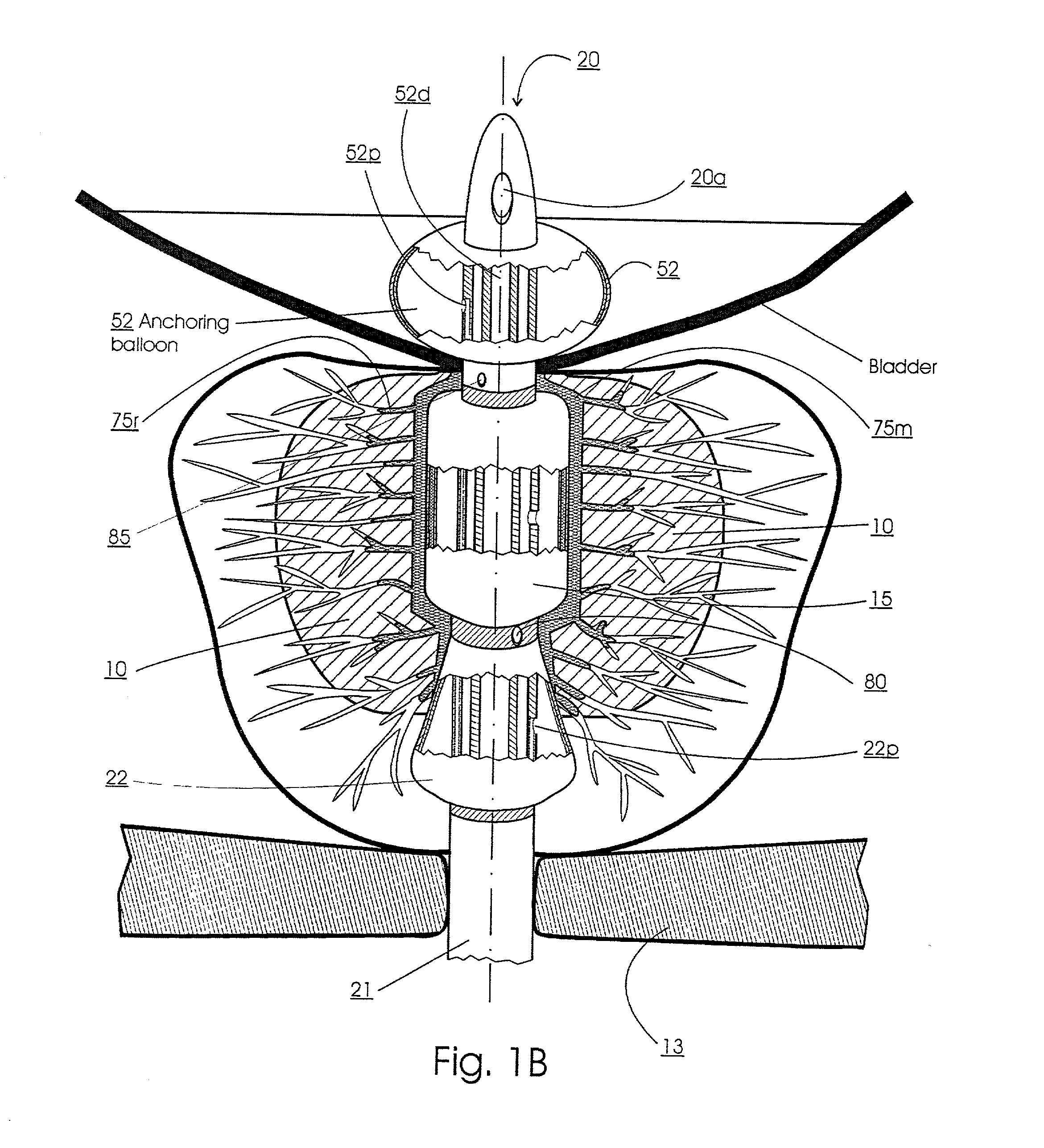
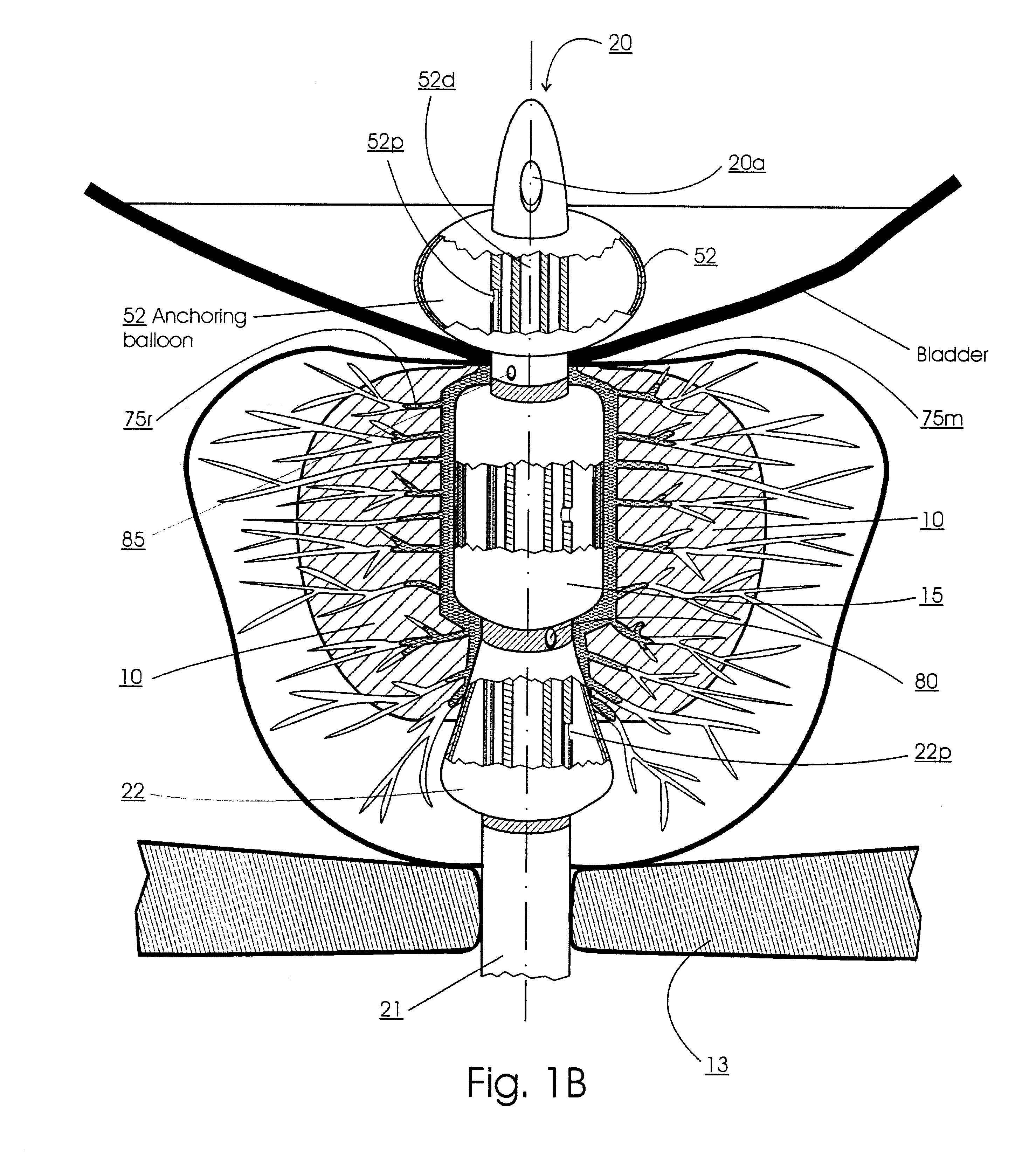
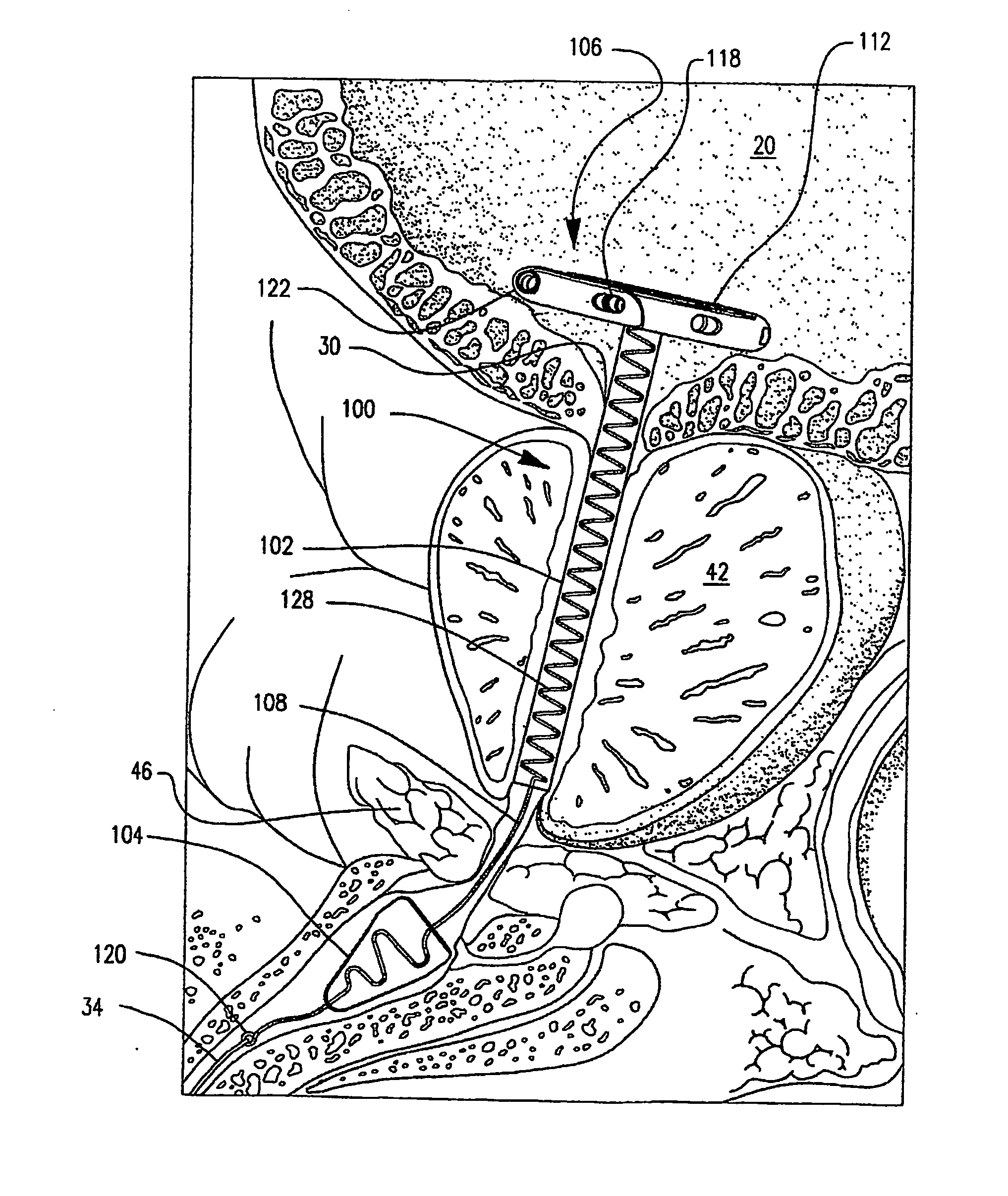
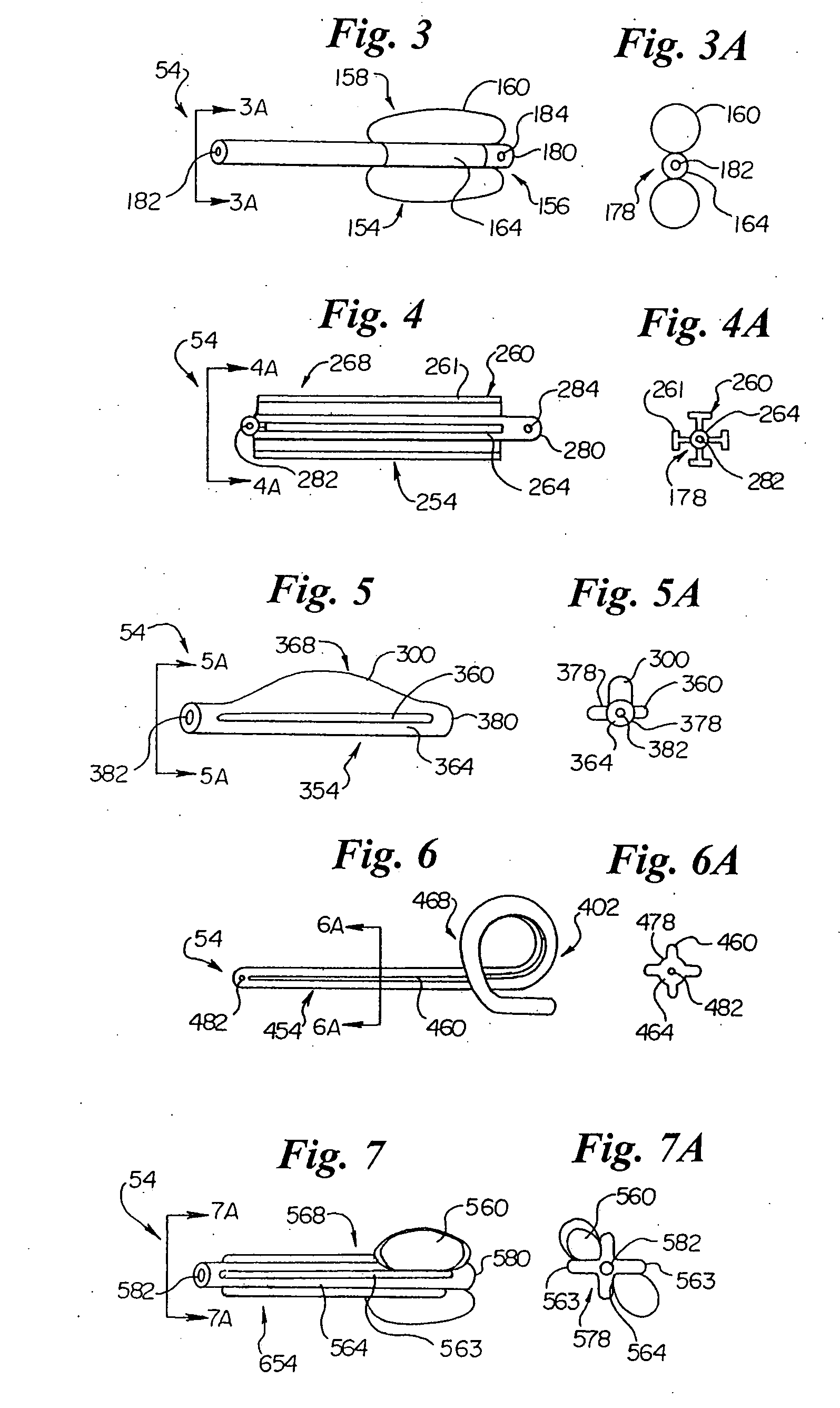
Prostatic stent with localized tissue engaging anchoring means and methods for inhibiting obstruction of the prostatic urethra
A prostatic stent is configured as a unitary body, which is adapted to reside above the sphincter when in position in a subject and allow normal functioning of the sphincter. The stent includes and elongated and substantially narrow conduit, which extends through the sphincter and outside the body of the subject. The conduit is sized and constructed to allow normal operation of the sphincter. The stent also includes and upper and / or an intermediate inflatable portions and may include a second conduit for the introduction of medication to the stent.
Owner:JPMORGAN CHASE BANK AS ADMINISTATIVE AGENT
Method and apparatus for the treatment of urinary tract dysfunction
InactiveUS20050055063A1Accurately localized and precisely gradedAvoid damageElectrotherapyArtificial respirationSphincterSpinal cord
Owner:ALFRED E MANN INST FOR BIOMEDICAL ENG AT THE UNIV OF SOUTHERN CALIFORNIA
Methods for treating the prostate and inhibiting obstruction of the prostatic urethra using biodegradable stents
InactiveUS6682555B2Improves Structural IntegrityEar treatmentMedical devicesUrethraBiodegradable scaffold
Owner:JPMORGAN CHASE BANK AS ADMINISTATIVE AGENT
Prostatic stent with localized tissue engaging anchoring means and methods for inhibiting obstruction of the prostatic urethra
InactiveUS20020032486A1Avoid obstructionInhibit migrationStentsBalloon catheterSphincterCatheter device
A prostatic stent is configured as a unitary body which is adapted to reside above the sphincter when in position in the subject includes an elongated tube which extends through the sphincter and outside the body of the subject. The stent is configured to allow natural operation of the sphincter when in position in the subject. The unitary body stent includes a lower inflatable portion which expands to contact tissue and help hold the stent in a desired location during a chronic use period of about 2-14 days. The stent can also include an upper and / or intermediate inflatable portions. A method of inhibiting the obstruction or closure of the prostatic urethral opening includes positioning the stent in the subject such that the unitary body of the stent is in the prostate and resides above the sphincter. A method treating BPH includes thermally ablating localized tissue in the prostate and inserting a post-treatment catheter into the prostate (preferably after an initial healing period) to allow the treated tissue to contour therearound and to maintain the urinary passage open. The stent is configured as a unitary body stent adapted to reside above the sphincter of the subject and to allow substantially normal operation of the sphincter. The stent can include a lower inflatable portion which engages, when expanded, with tissue below the localized treatment region about the membranous urethra (between the sphincter and the verumontanum). The post-treatment catheter is configured to reside in the subject for a period of about 2-14 days after delivery of the thermal ablation treatment or therapy.
Owner:JPMORGAN CHASE BANK AS ADMINISTATIVE AGENT
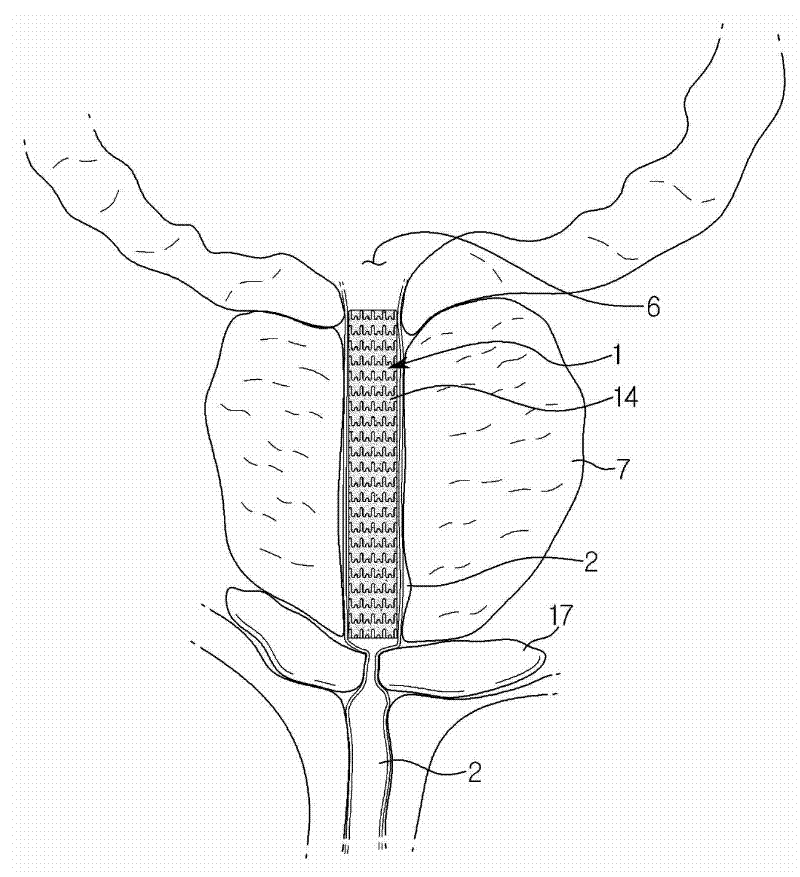
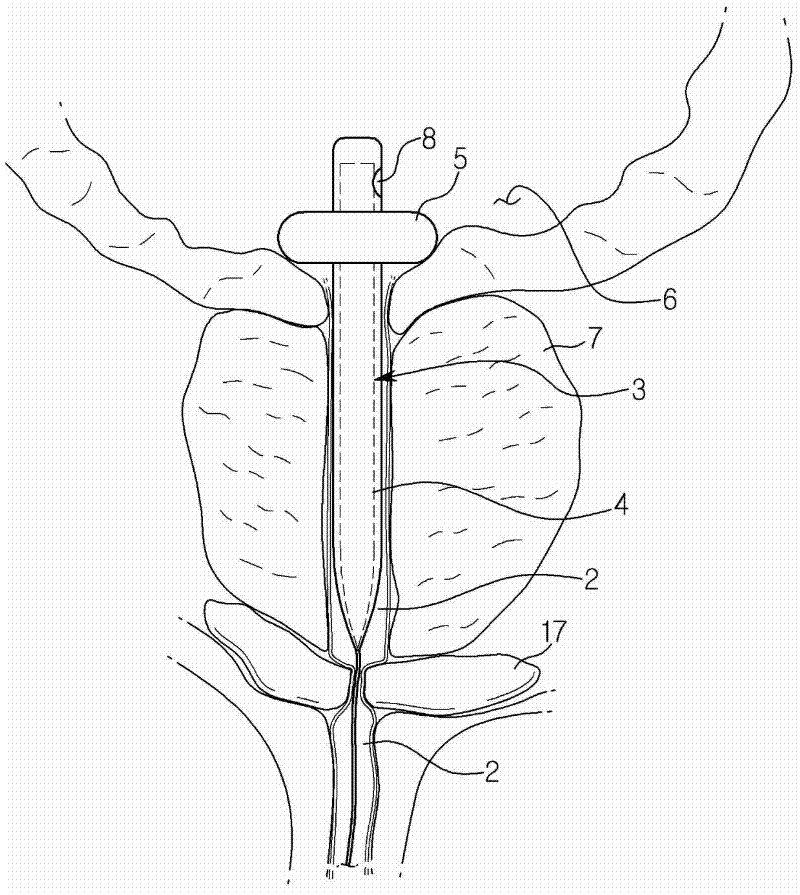
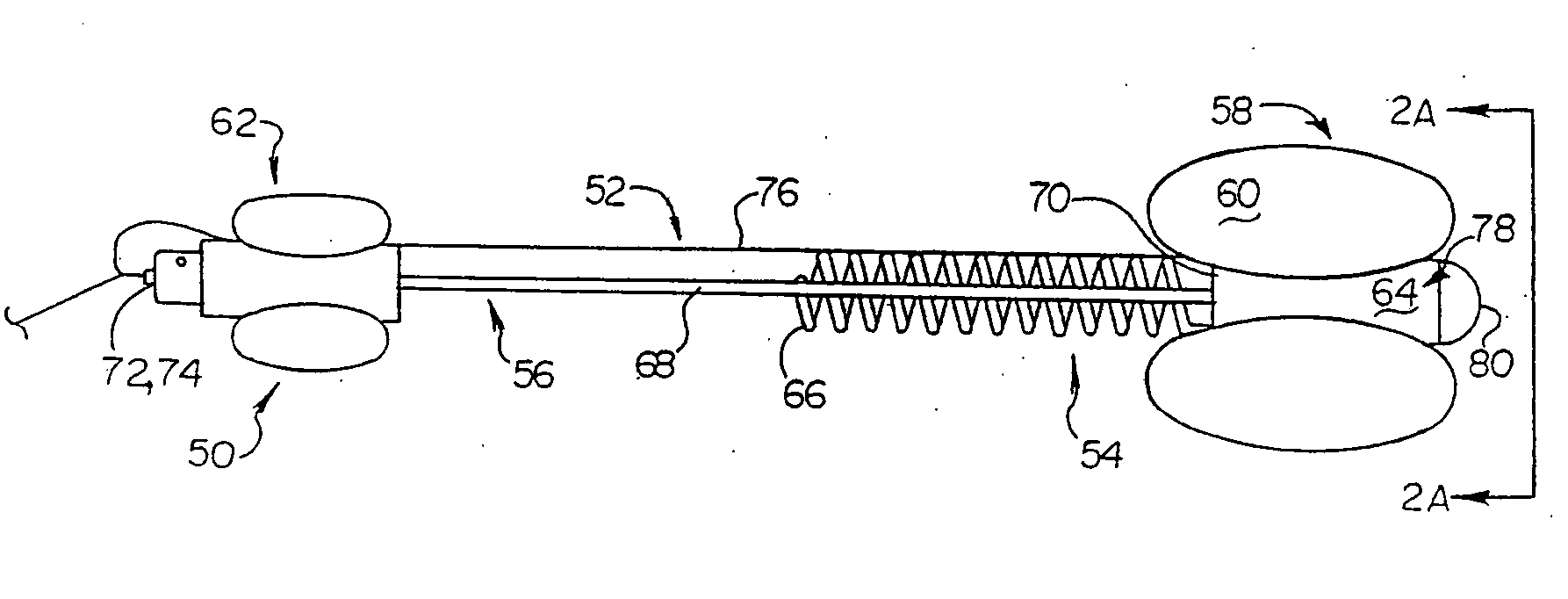
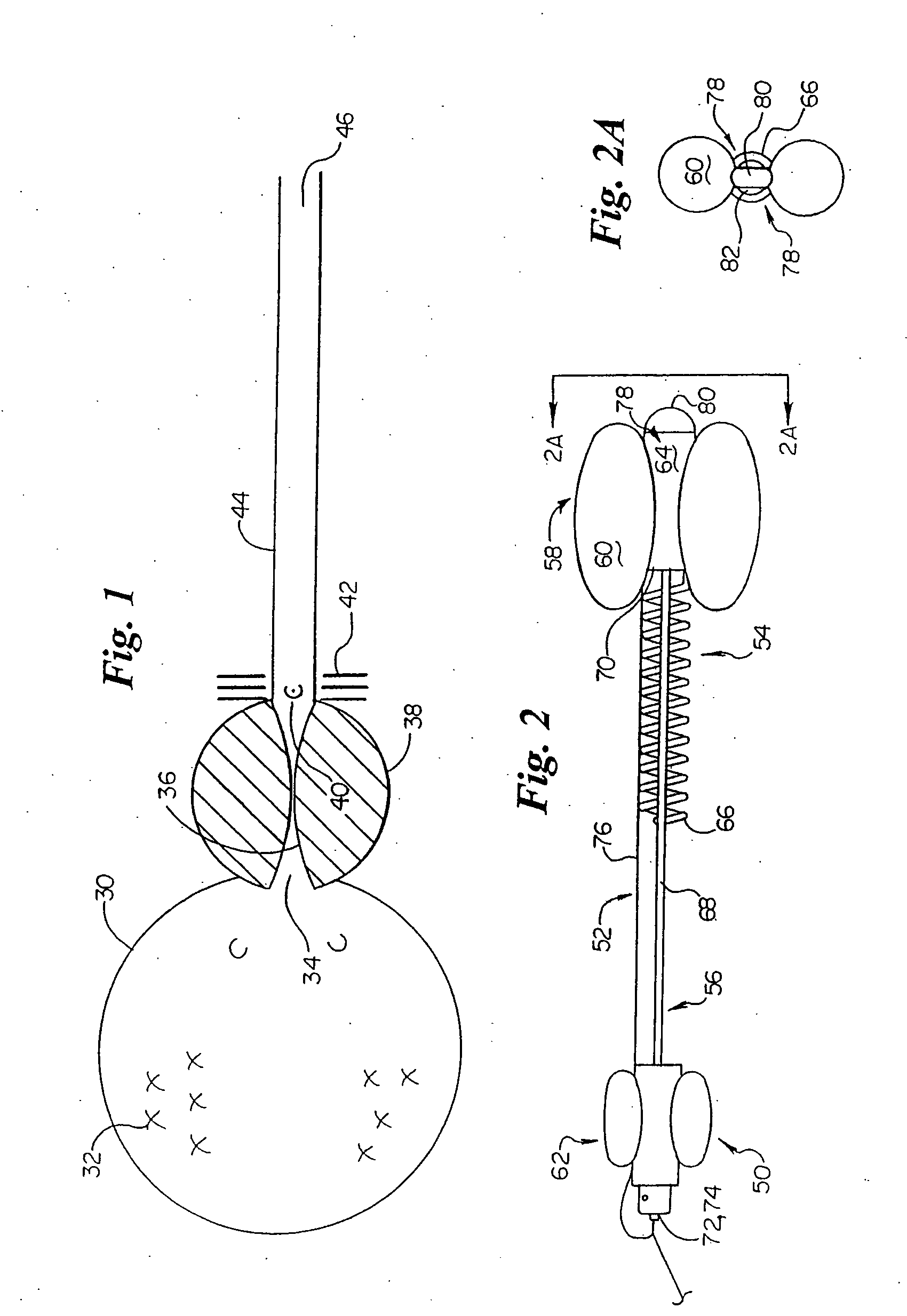
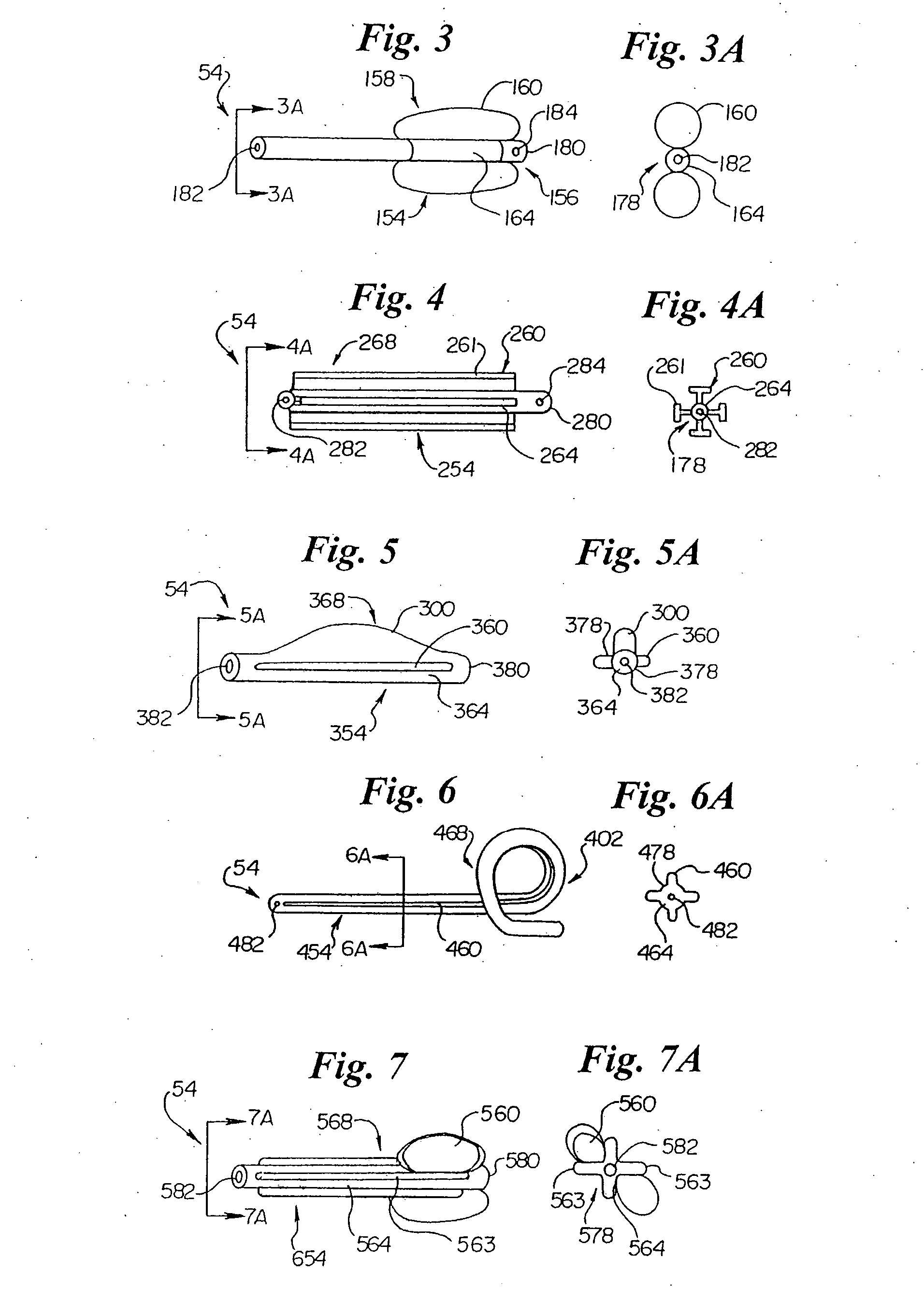
Endourethral device and method
InactiveUS7108655B2Inhibit migrationExclude influenceLighting and heating apparatusSurgeryUrethraIrrigation bladder
An endourethral device is provided having an elongate member having proximal and distal segments, the elongate member positionable within a lower urinary tract so as to at least partially traverses a prostatic urethra. A proximal anchor, adapted to abuttingly engage portions of a bladder neck so as to at least proximally anchor the device, is supported at least indirectly by the proximal segment of said elongate member. The proximal anchor includes bladder engaging elements radially extending from the proximal segment of said elongate member, urine being freely dischargable about at least the proximal segment so as to substantially bathe the bladder neck therewith.
Owner:SRS MEDICAL SYST INC
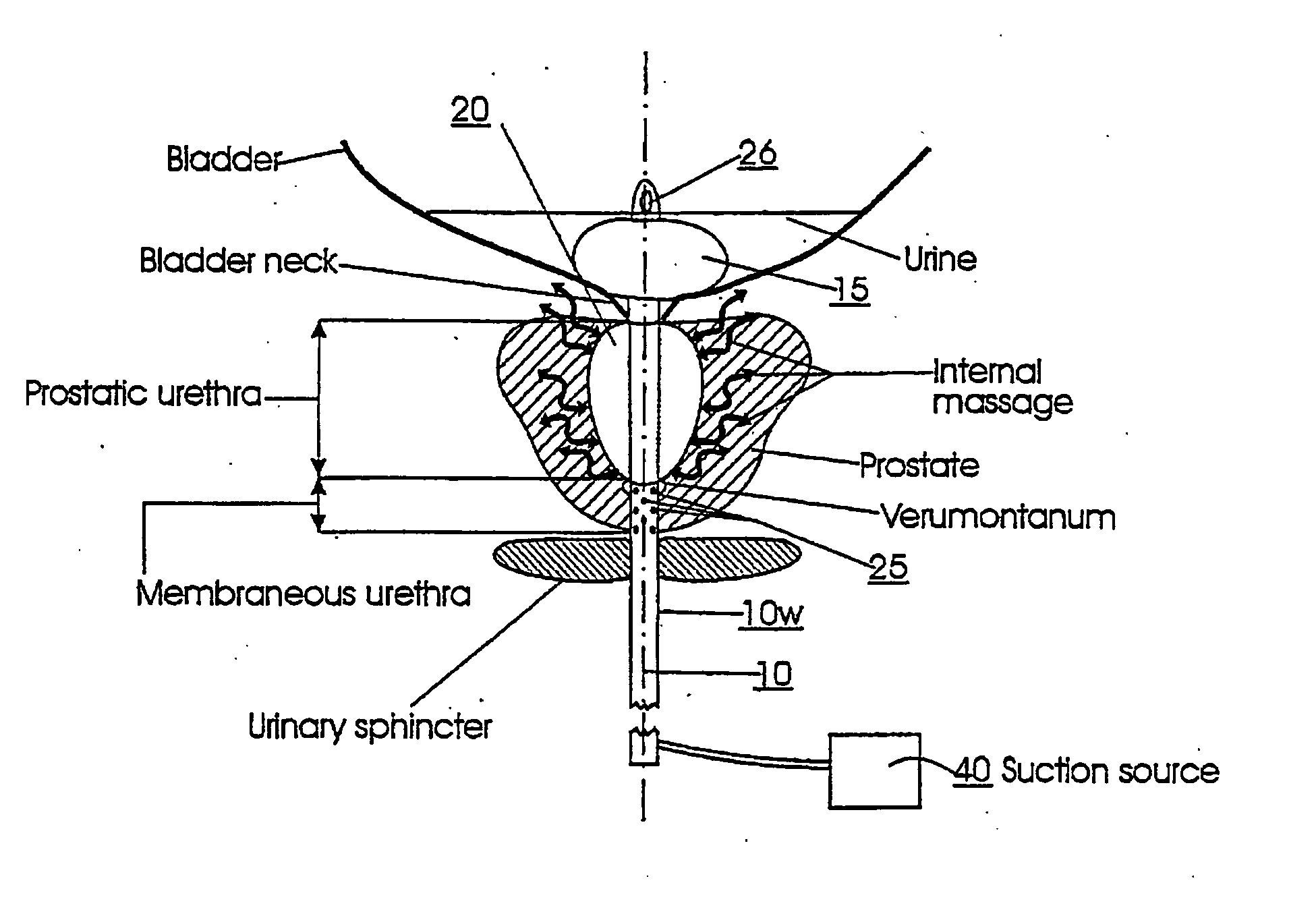
Methods for treating prostatitis
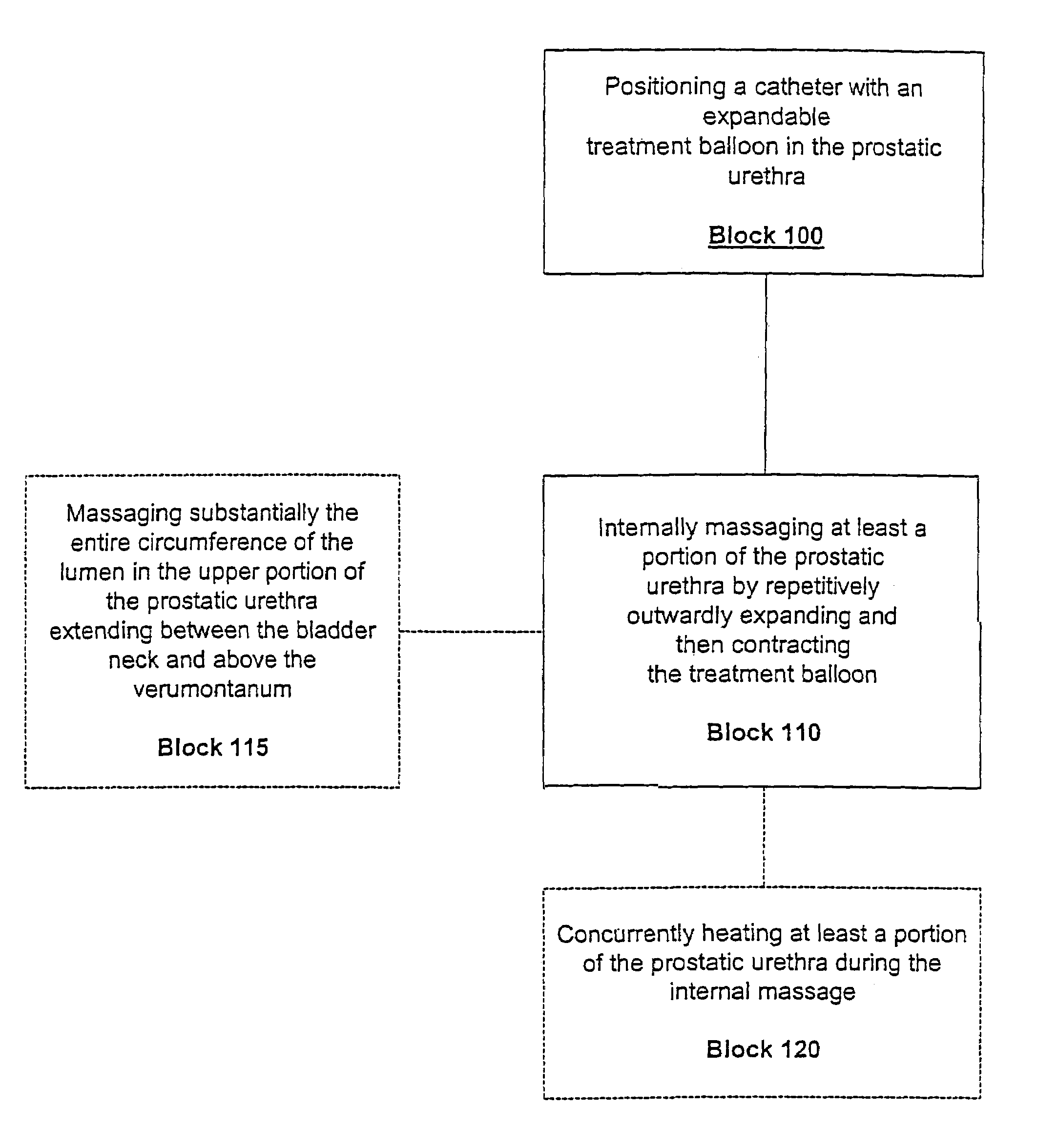
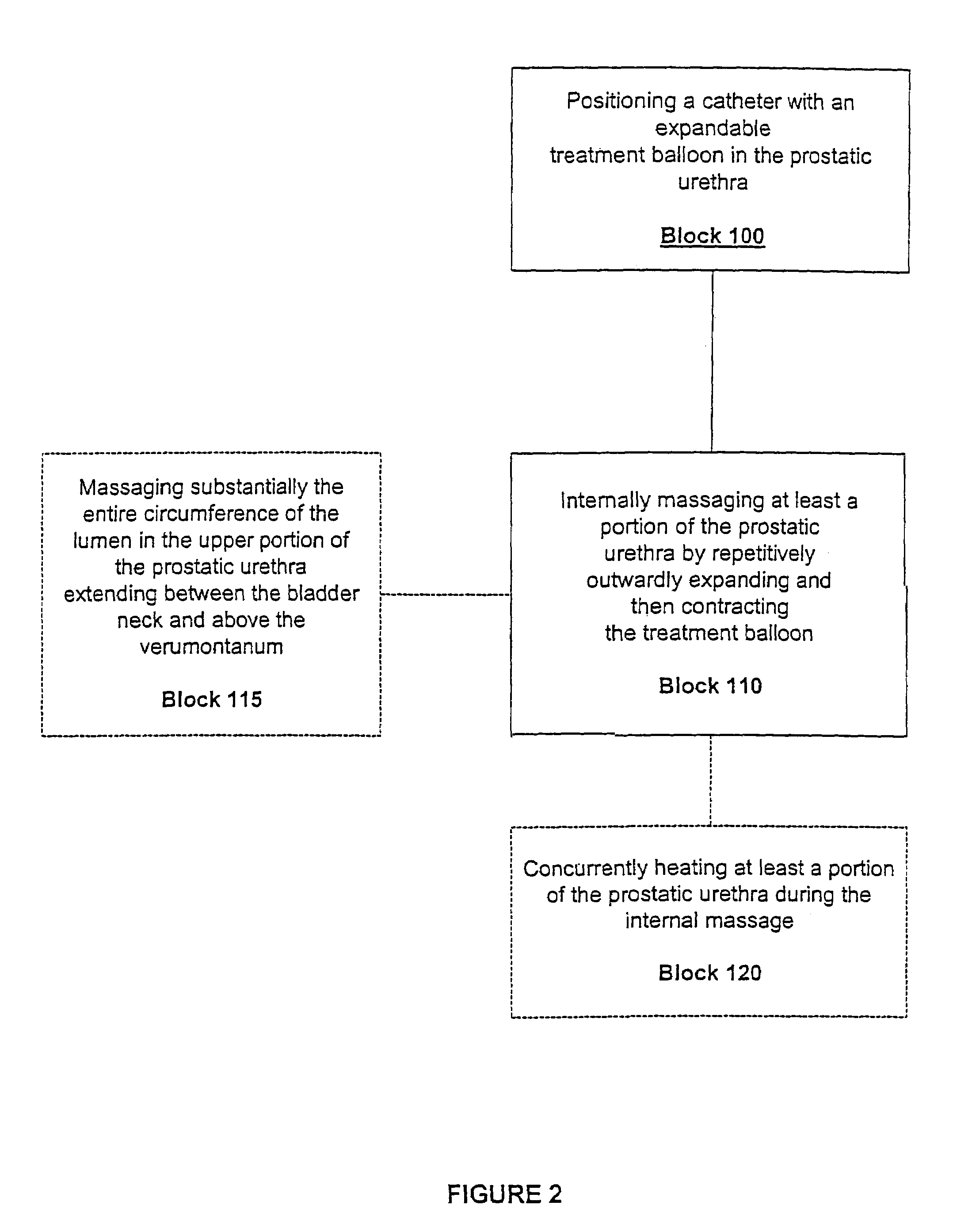
InactiveUS7014652B2Raise the possibilityLess sensitiveOrganic active ingredientsAntipyreticMassage therapiesProstatic urethra
Methods, systems, and computer program products for providing thermal therapies or internal thermal massage therapy for treating prostatitis by expanding and contracting a treatment balloon with inflation media and currently applying heat to a portion of the prostatic urethra.
Owner:WIT IP CORP
Indwelling body lumen expander
Indwelling body lumen expanders which allow for the maintenance and patency of a body lumen, such as the prostatic urethra to relieve urethral obstruction, are described. Because the one or more expanders are configured to be relatively larger in diameter than the diameter of the body lumen, the expanders may become invaginated into the lumen wall thus enabling the prostheses to avoid fluid exposure which in turn prevent the prostheses from becoming encrusted or calcified.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
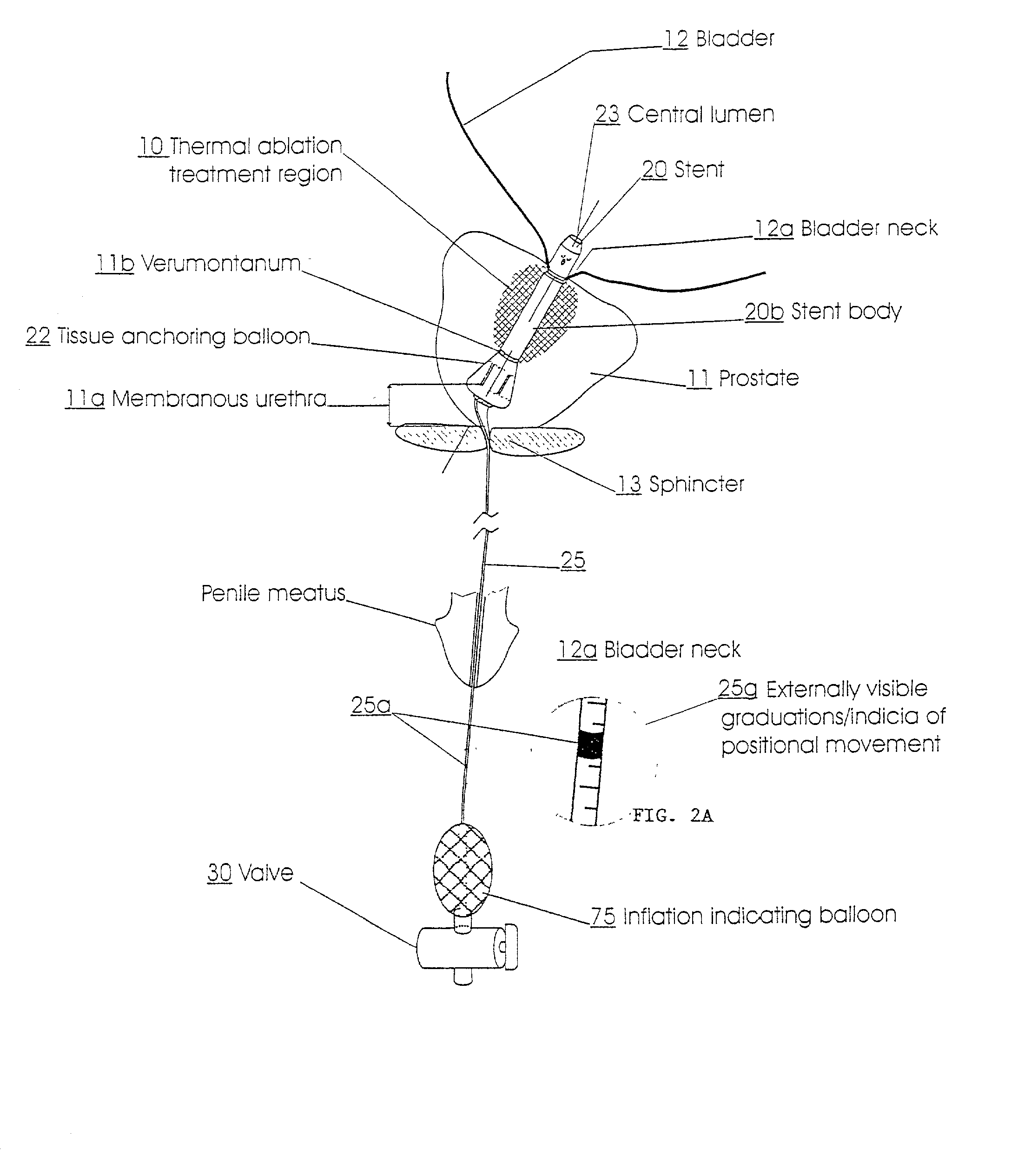
Self-adjusting endourethral device & methods of use
InactiveUS20100241240A1Permit urinationFacilitate urinationGuide needlesWound drainsUrethraUrethral stent
A self adjusting and / or positioning indwelling urethral device is provided. The device generally includes a prostatic urethral stent body and a urethral anchoring element. The prostatic urethral stent body includes a preconfigured end portion for anchored receipt within a bladder with the urethral anchoring element extending from the prostatic urethral stent body via a linkage. Engagement of the proximal anchor, delimited by the preconfigured end portion of the stent body, with the bladder is no or minimal impact upon the trigone region.
Owner:ABBEYMOOR MEDICAL INC
Partial-length indwelling urinary catheter and method permitting selective urine discharge
A partial-length catheter, or an extendable tube or sleeve member of the catheter, is selectively movable within the prostatic urethra to open a urine drainage passageway through and obstructed portion of the prostatic urethra or to open the external urinary sphincter muscle and thereby discharge urine from the bladder. A control element is manipulated at a position exterior of the urinary canal to selectively move the catheter or the extendable tube or sleeve member, thereby selectively controlling urine discharge. The catheter may also be used to diagnose urinary retention problems caused by a weak bladder or a prostatic obstruction.
Owner:PROSTALUND
Urological medical devices for release of prostatically beneficial therapeutic agents
InactiveUS20080233167A1High quantity of drugAvoid the needAntibacterial agentsNervous disorderDiseaseGynecology
According to an aspect of the invention, urological medical devices are provided, which comprise a prostatically beneficial agent selected from alpha-adrenergic blockers, antispasmodic agents, anticholinergic / antimuscarinic agents, calcium channel blockers, anti-inflammatory agents, hormone-affecting agents, anti-cancer agents, and combinations thereof, among others. The urological medical devices are adapted for implantation or insertion into a subject's urinary tract, whereupon at least a portion of the prostatically beneficial agent is released into the subject's prostatic urethra. The release profile of the prostatically beneficial agent is effective to treat a prostatic disorder, for example, benign prostate hypertrophy, prostate cancer or prostatitis, among others. Other aspects of the invention are directed to treating prostatic disorders.
Owner:BOSTON SCI SCIMED INC
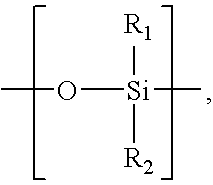
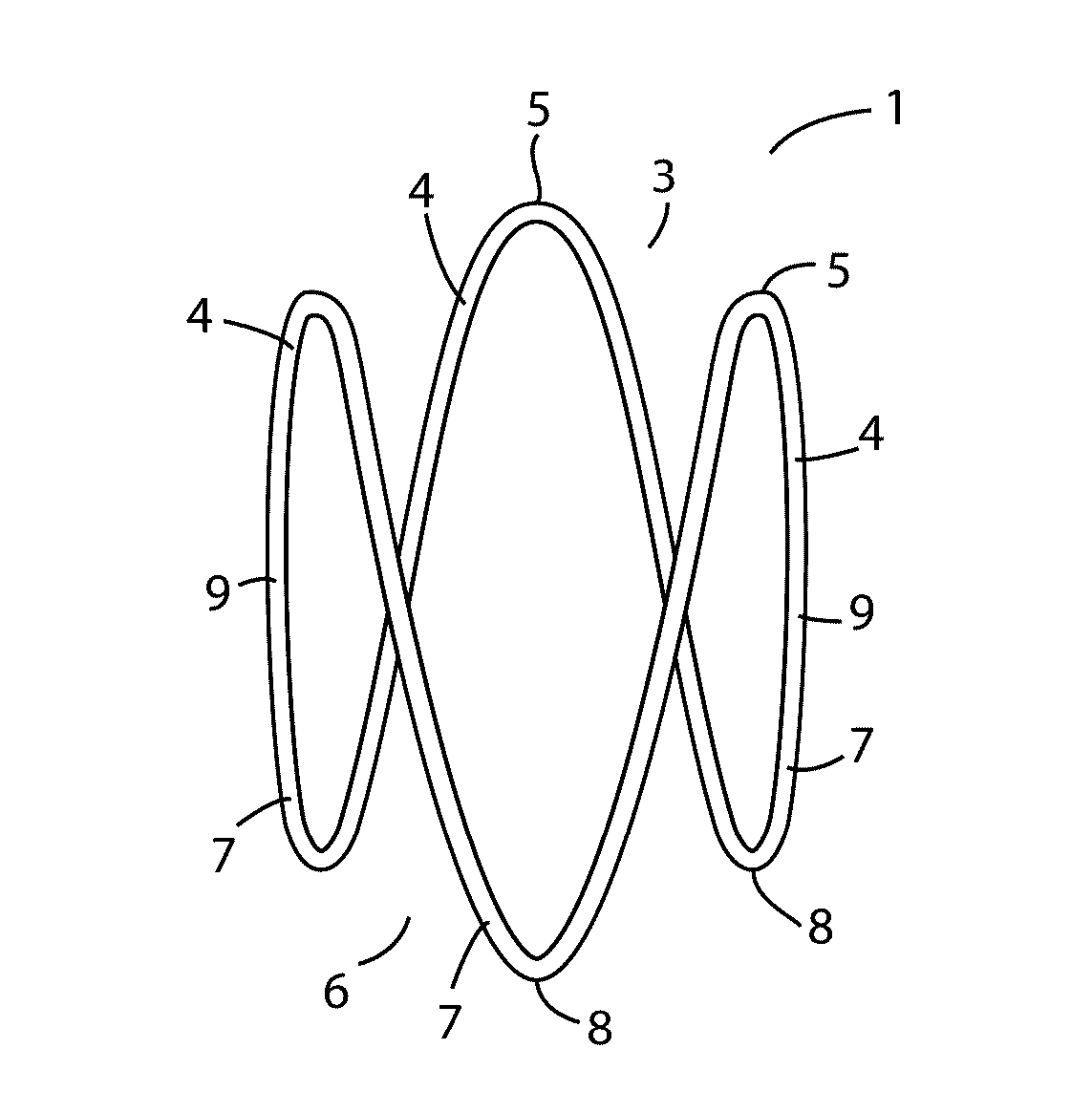
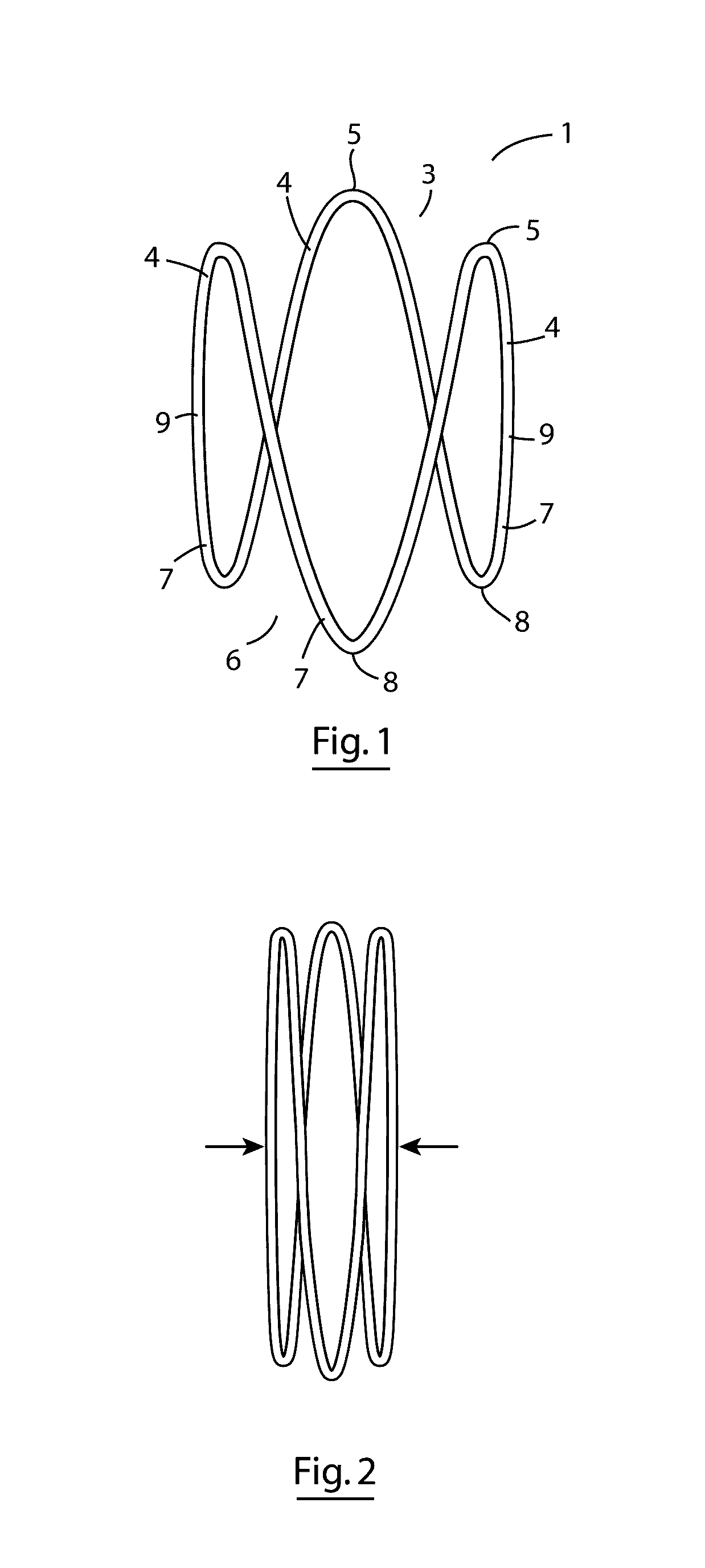
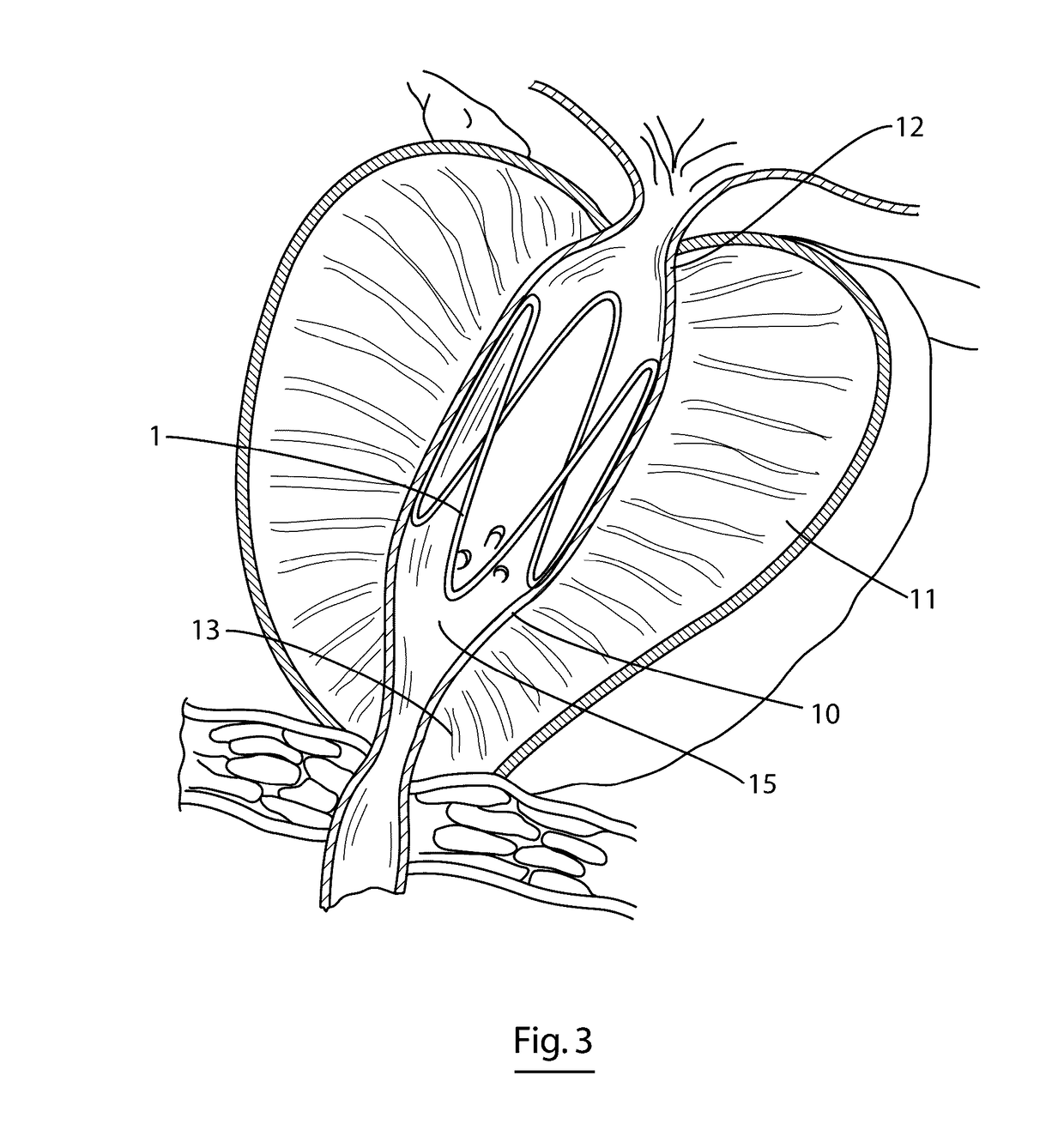
Implantable biocompatible expander suitable for treatment of constrictions of body lumen
An implantable biocompatible expander suitable for implantation into a urinary duct, comprises an elongated sinusoidal ring comprising at least two proximal prongs and at least two distal prongs, wherein the expander is resiliently deformable from a relaxed radially expanded orientation to a radially contracted orientation suitable for transluminal delivery through the urinary duct. The expander is configured to exert an outward radial force against a wall of the urinary duct when in-situ within the urinary duct. In particular, the expander is suitable for treatment of benign prostatic hyperplasia and configured for implantation into the prostatic urethra between, and substantially spanning the prostatic urethra between, the bladder neck and external sphincter.
Owner:TRINITY COLLEGE DUBLIN
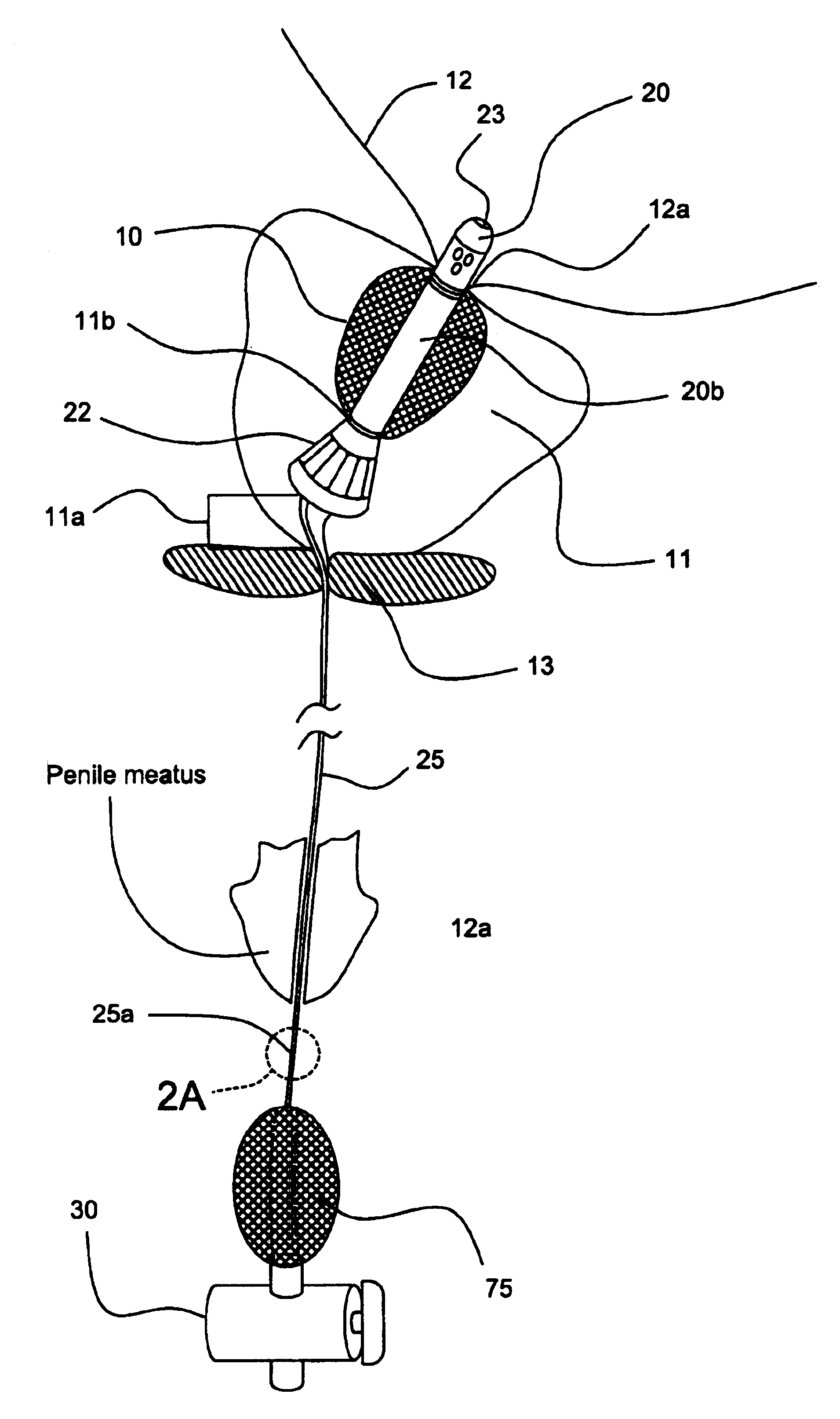
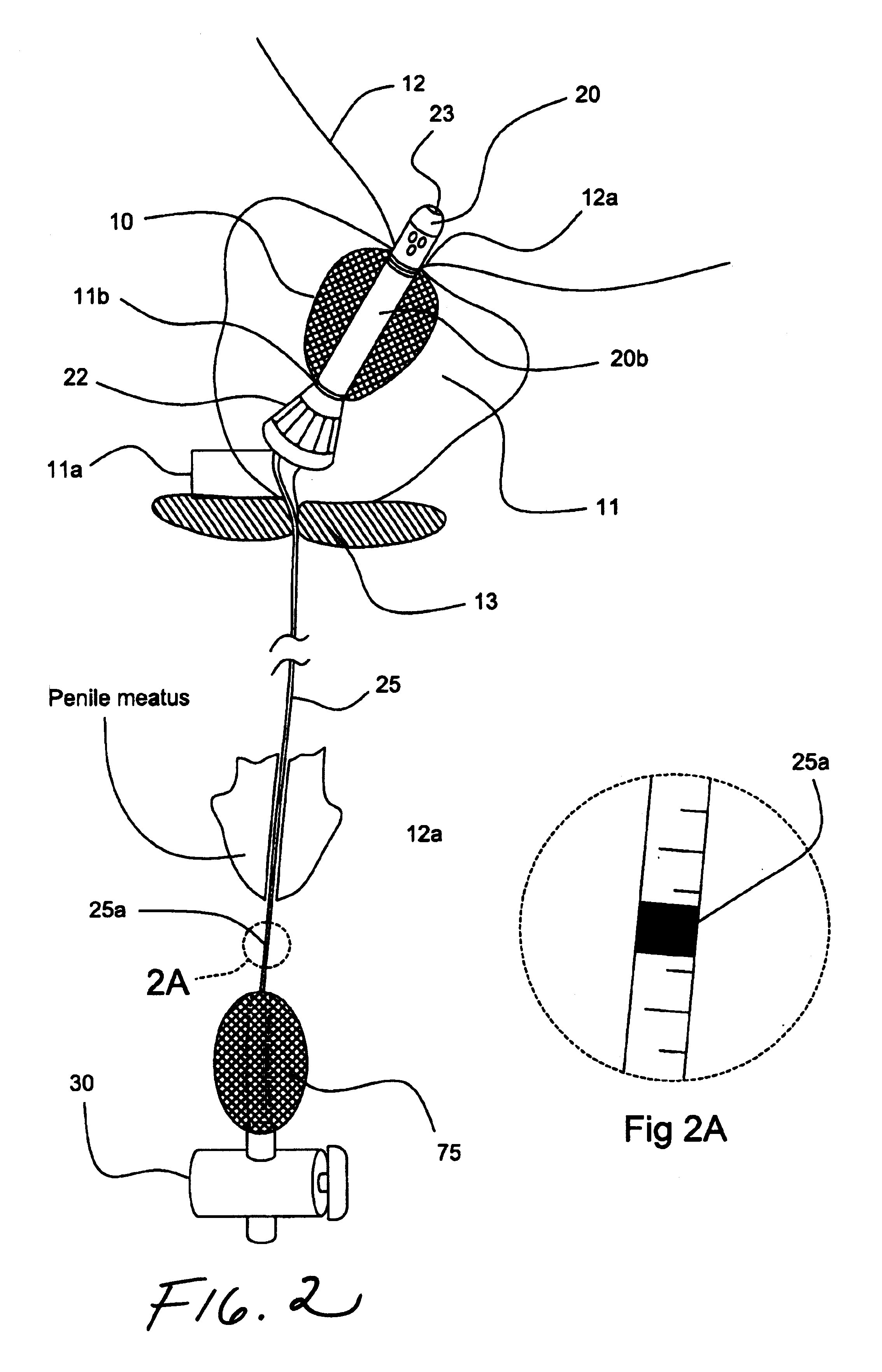
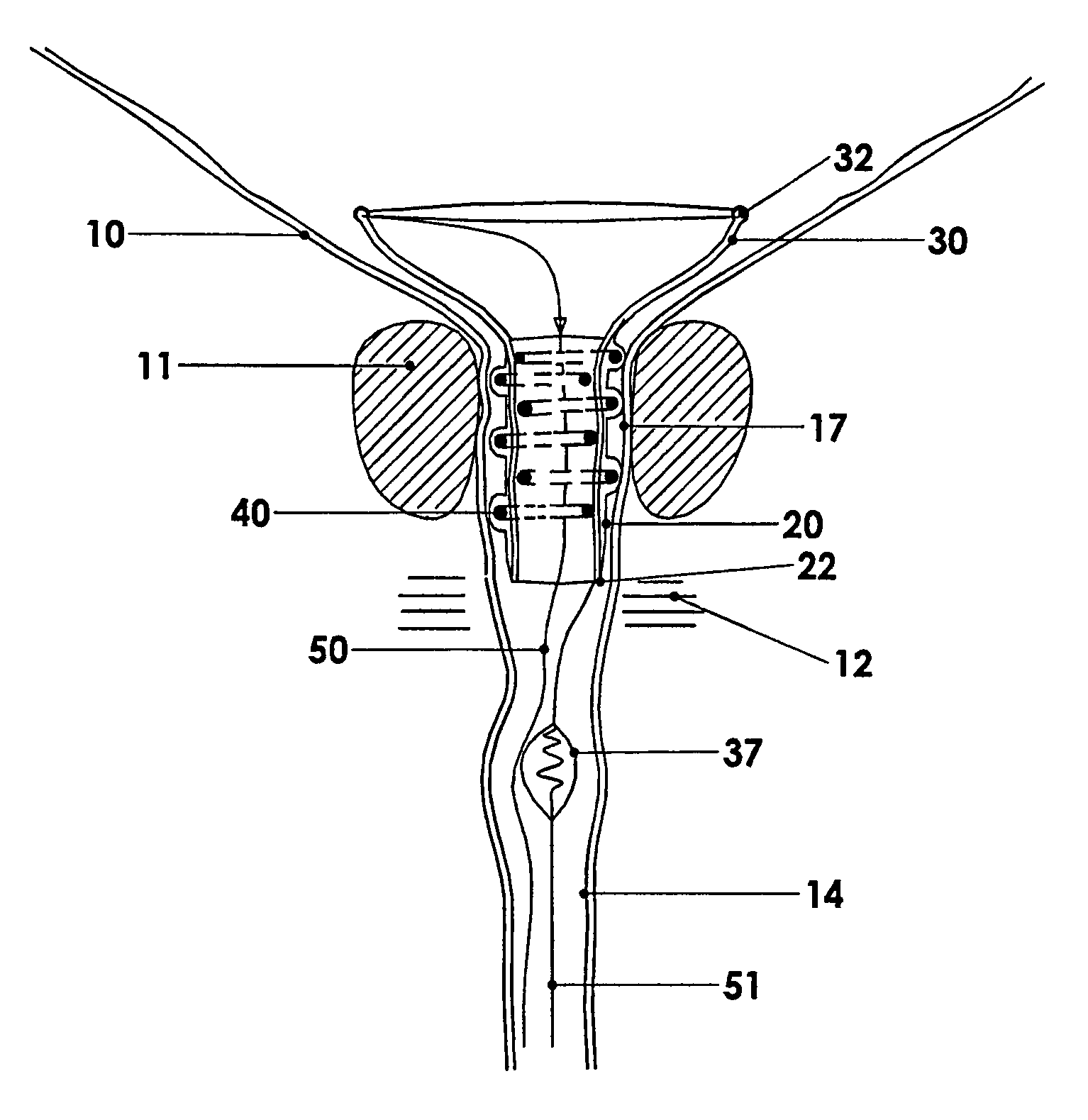
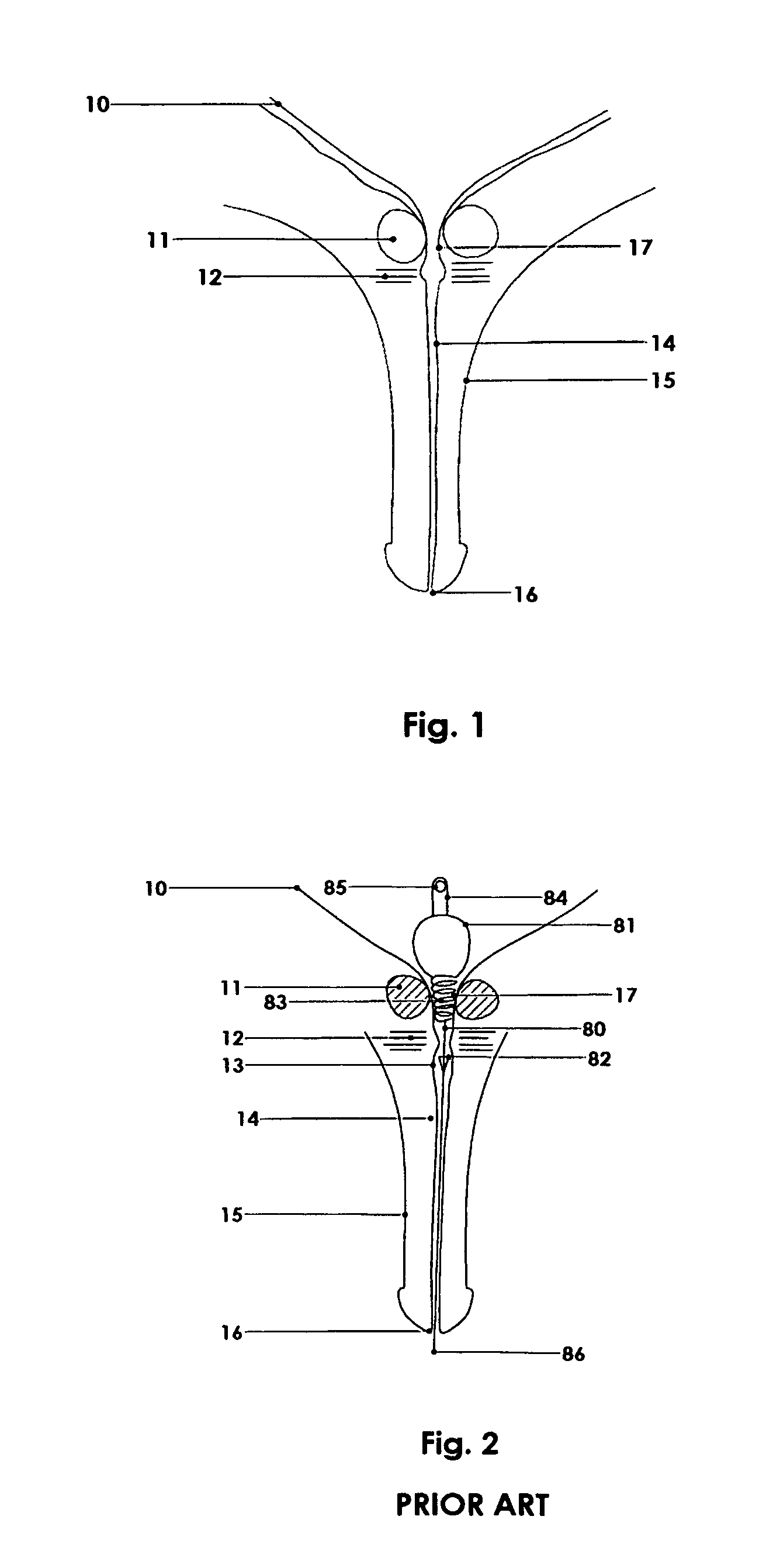
Urethral catheter and guide
An indwelling catheter for insertion into a patient's urinary tract is provided that includes first and second tubular members that enable drainage of physiological fluids. An inflatable balloon is attached to the second tubular member. The first and second tubular members are interconnected by a connecting tube forming a gap between the first and second tubular members. The connecting tube is in fluid communication with the balloon to enable the balloon to be inflated via the connecting tube. When the balloon is anchored within the urinary bladder the second tubular member is located within the prostatic urethra with one end in close proximity to the sphincter and the other end protruding into the urinary bladder. The first tubular member is located within the penile urethra with one end in close proximity to the sphincter so that the connecting tube traverses the sphincter permitting voluntary control of the sphincter.
Owner:ESHEL UZI +2
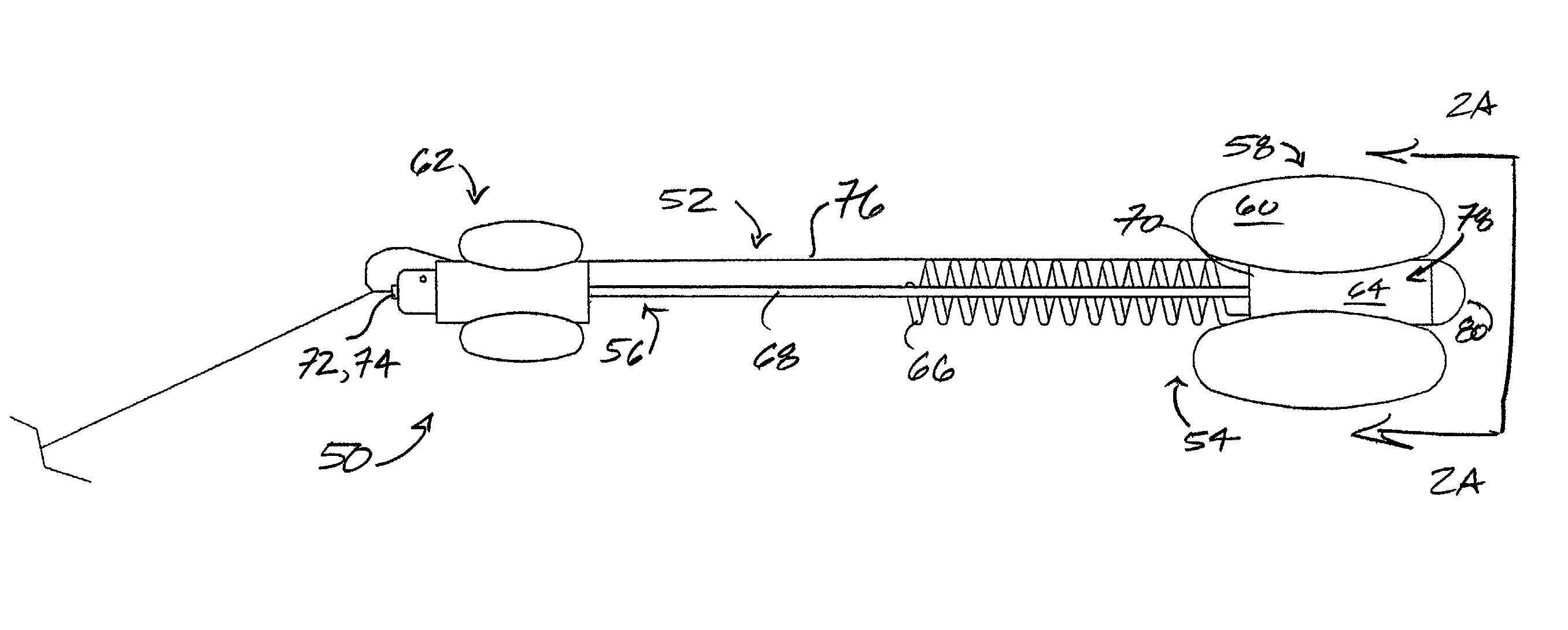
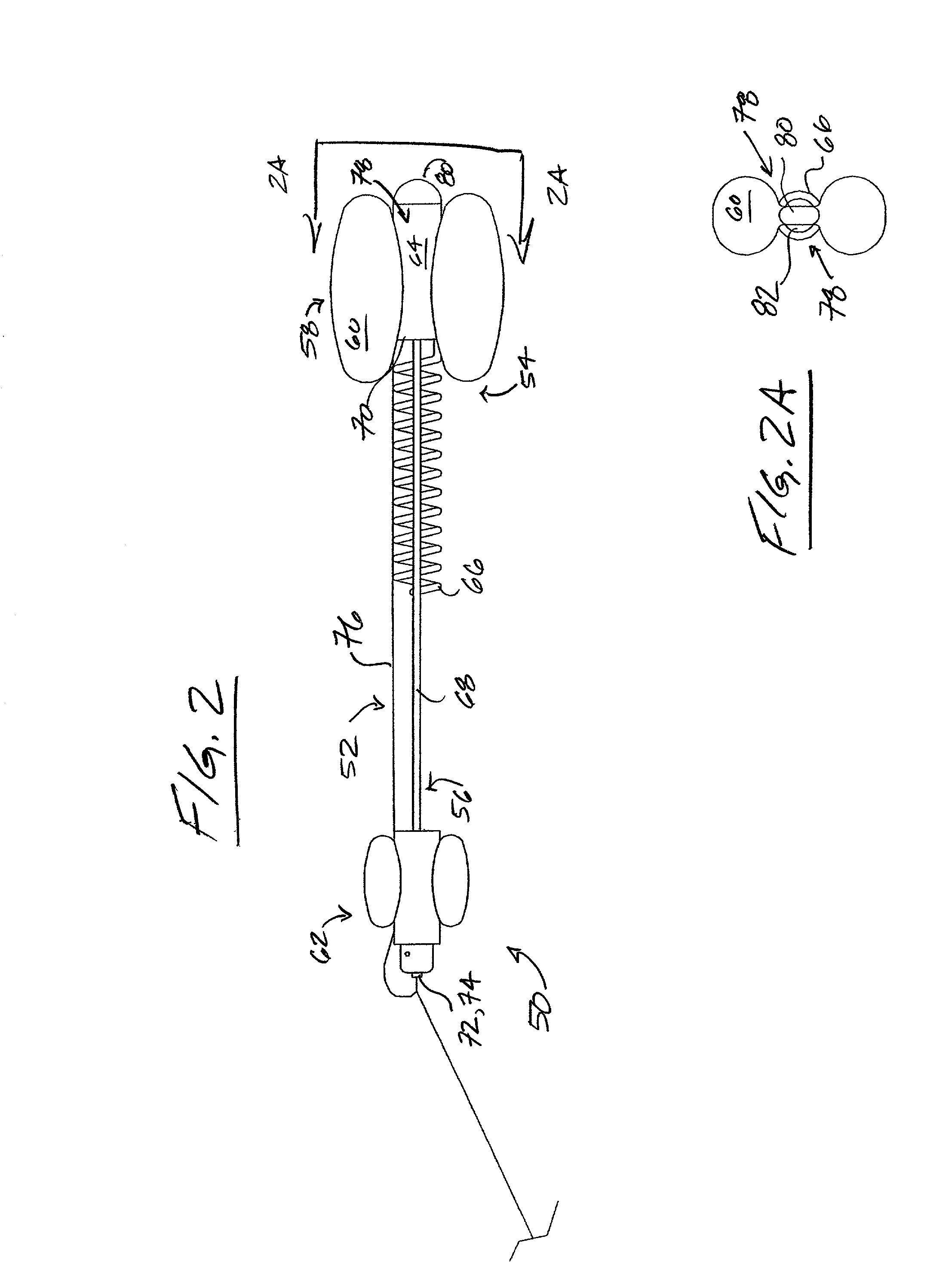
Balloon Catheter
A balloon catheter is provided for the implantation of a stent in a mammalian duct or cavity, comprising an elongate distal section and an expandable first balloon accommodating the section, further comprising means for the supply of a pressure medium for the expansion of the balloon, and means for heating the pressure medium, the catheter being provided with an elongate stent mounted onto the balloon. The catheter contains second means for establishing outwardly directed expansion of the stent at a sight or location selected from the two ends of the stent so that the stent will remain in position as implanted after removal of the catheter from the duct or cavity; and a method for the implantation of a stent in a human prostatic urethra.
Owner:WALLSTEN MEDICAL
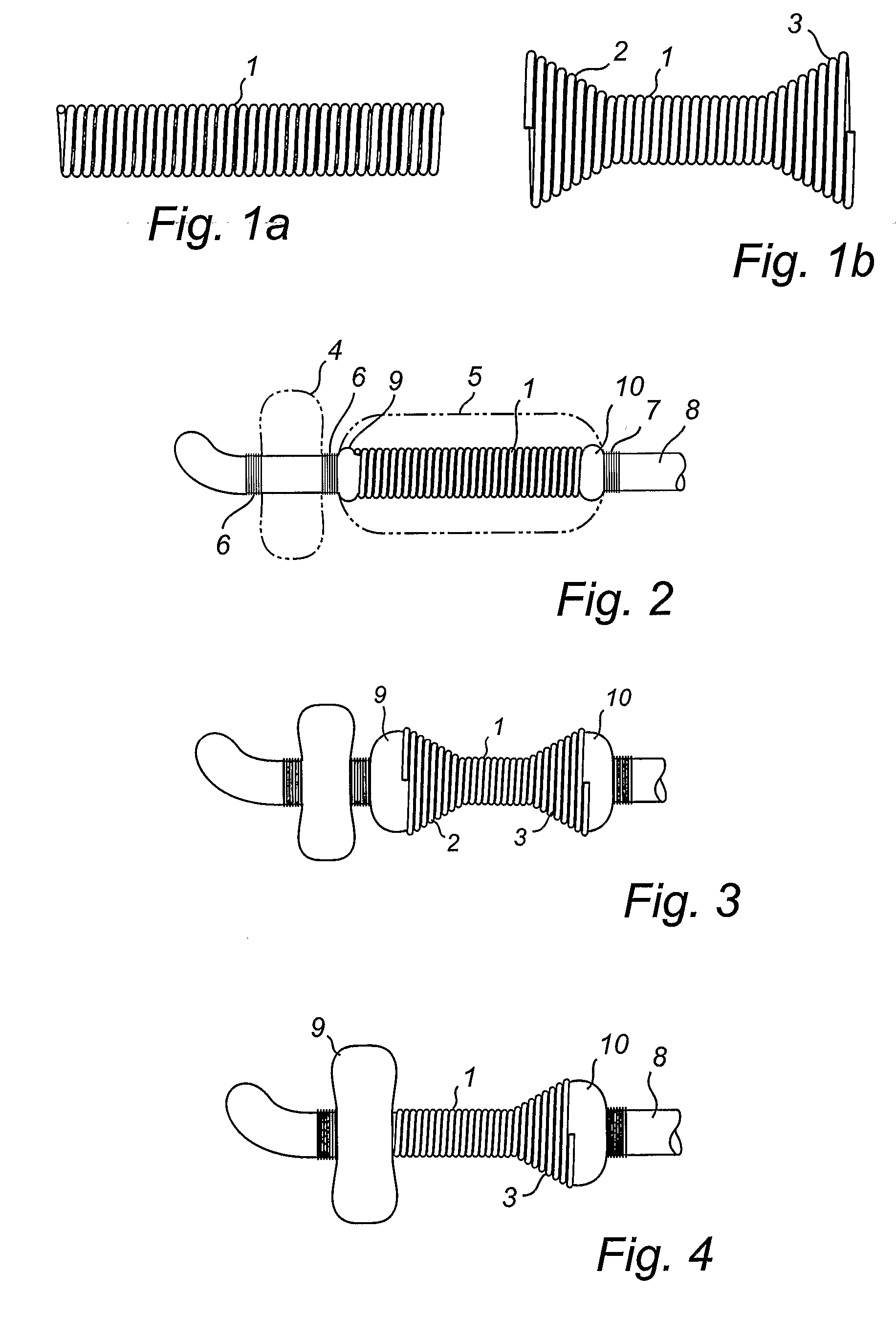
Temporary prostatic stent for benign prostatic hyperplasia
InactiveUS8465551B1Easy to drainPrevent inward movementStentsCatheterInsertion stentProstate hyperplasia
This invention relates to a temporary indwelling prostatic stent which provides a passage for urine through the prostatic urethra and which enable the patient to void the bladder at will. This temporary prostatic stent consists of a coiled or braided made out of metal or plastic section which spans the prostatic urethra, wings composed of memory alloy allowing an anchoring means in the bladder, and an anchoring means below the external sphincter, and a retrieval string to facilitate removal of the stent from the patient.
Owner:WIJAY BANDULA +2
Urethral stent for the prostate
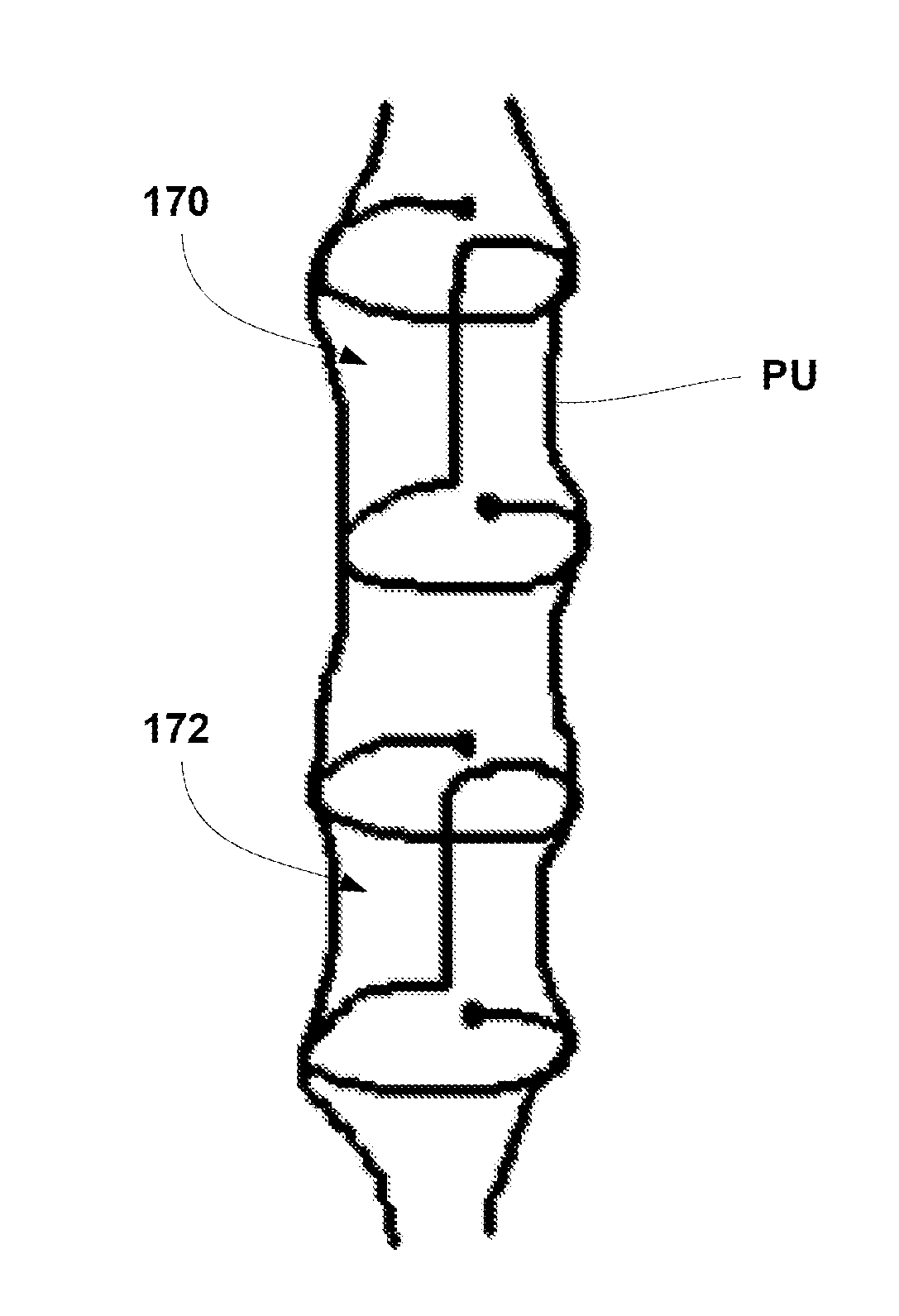
A urethral stent for the prostate including a first stent section placed into an upper portion of the urethra passing through the prostate, a second stent section placed into a lower portion of the urethra below a urinary sphincter muscle, the first and second stent sections being formed of a shape-memory alloy wire, and at least one connection filament is provided at a middle portion of the urethra passing through the urinary sphincter muscle so as to connect the first and second stent sections, thereby preventing the stent from moving to the upper or lower portions of the urethra.
Owner:TAEWOONG MEDICAL CO LTD +1
Urethral catheter and guide
An indwelling catheter for insertion into a patient's urinary tract is provided that includes first and second tubular members that enable drainage of physiological fluids. An inflatable balloon is attached to the second tubular member. The first and second tubular members are interconnected by a connecting tube forming a gap between the first and second tubular members. The connecting tube is in fluid communication with the balloon to enable the balloon to be inflated via the connecting tube. When the balloon is anchored within the urinary bladder the second tubular member is located within the prostatic urethra with one end in close proximity to the sphincter and the other end protruding into the urinary bladder. The first tubular member is located within the penile urethra with one end in close proximity to the sphincter so that the connecting tube traverses the sphincter permitting voluntary control of the sphincter.
Owner:WIT IP CORP
Partial-length indwelling urinary catheter and method permitting selective urine discharge
Owner:PROSTALUND
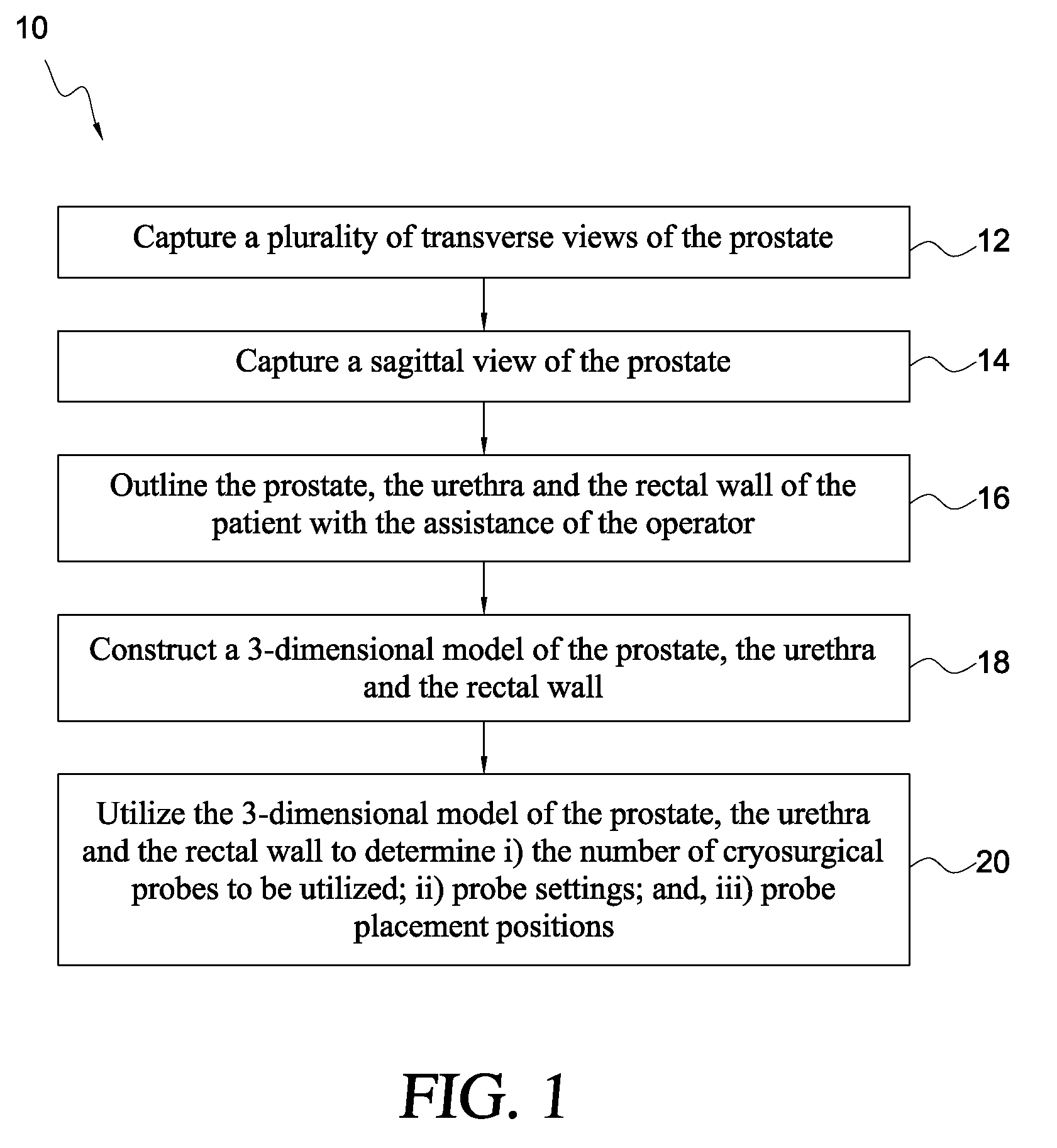
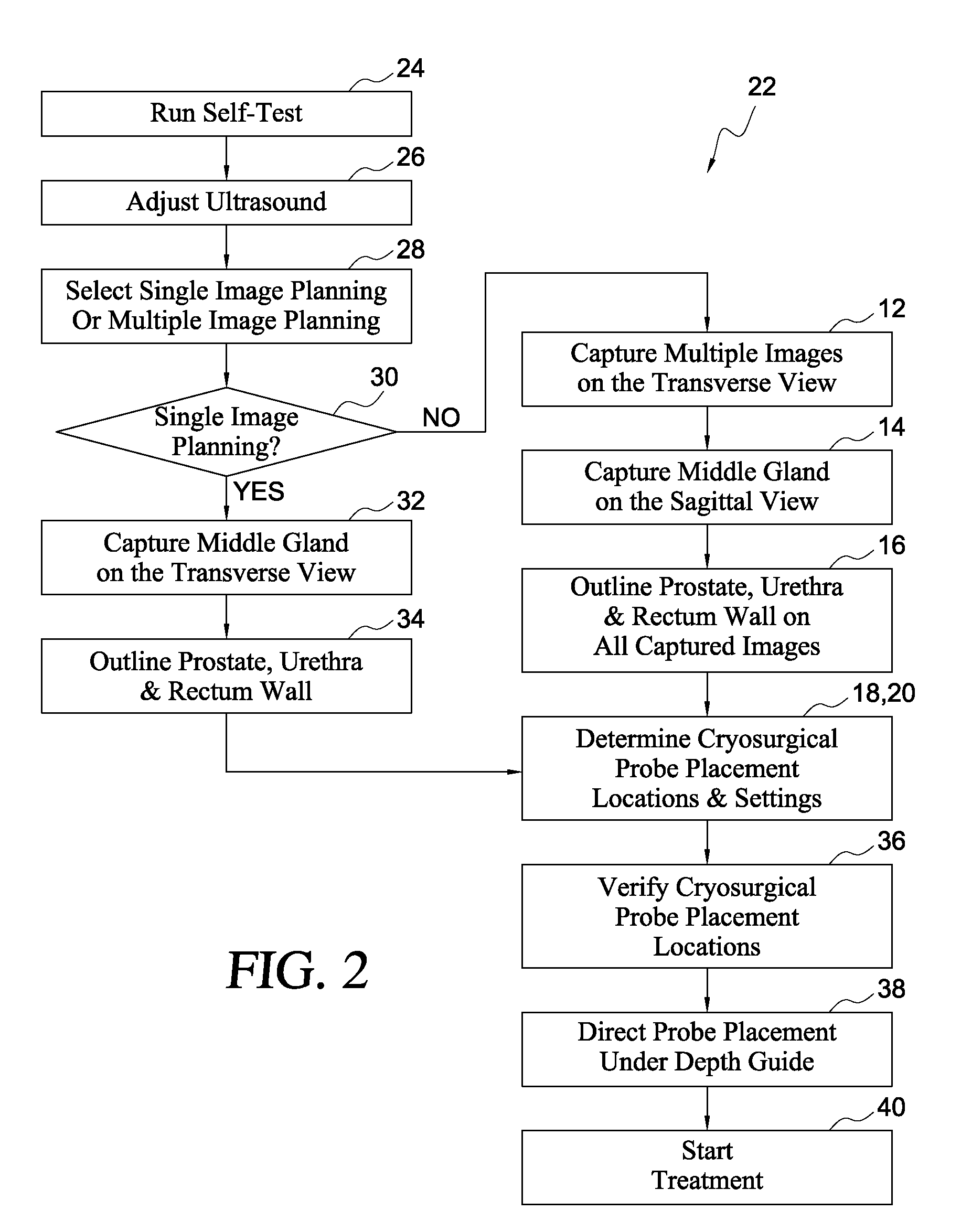
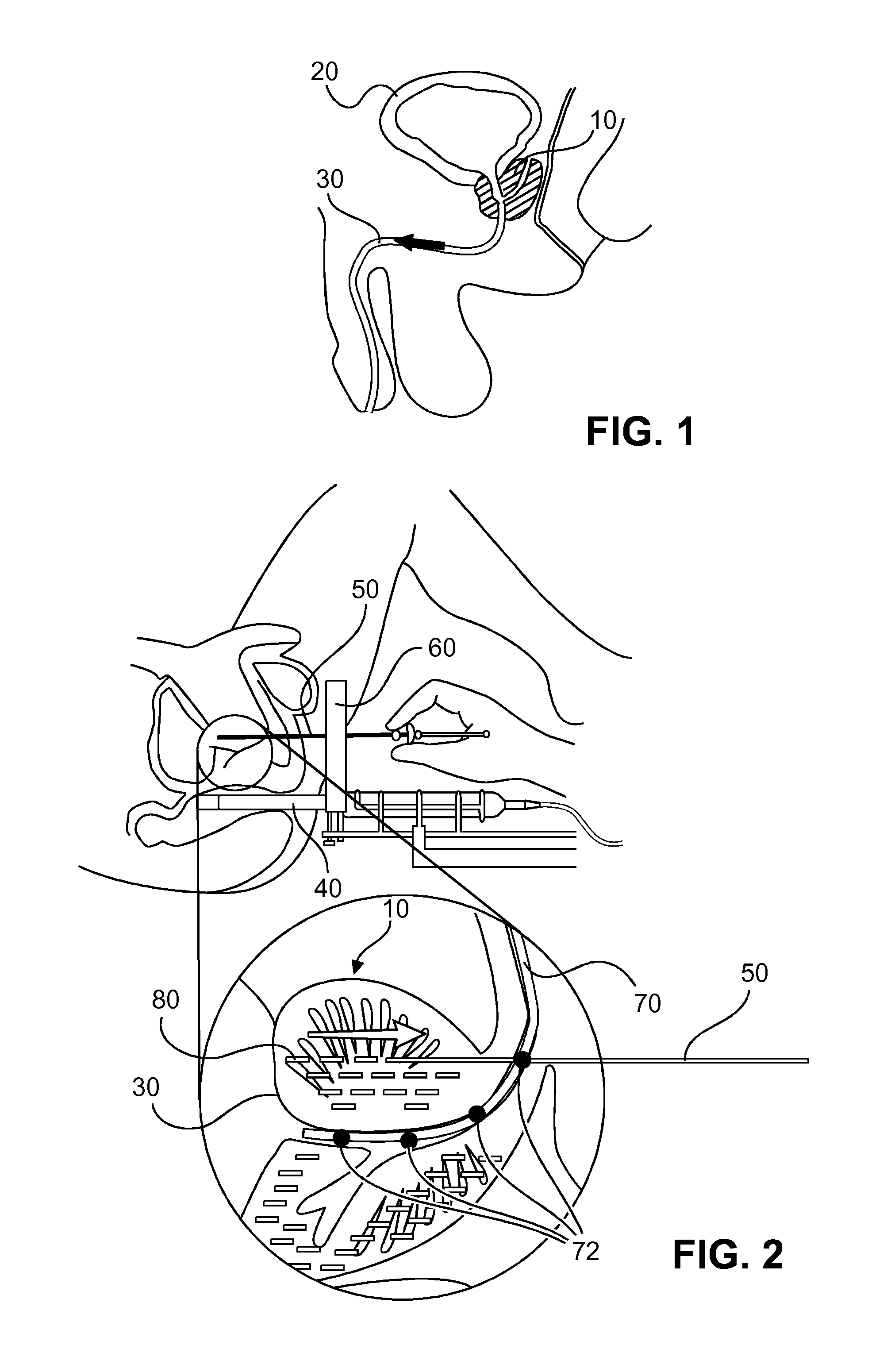
Variable cryosurgical probe planning system
ActiveUS8187260B1Ultrasonic/sonic/infrasonic diagnosticsHealth-index calculationSagittal planeUrethra
A cryosurgical system for assisting an operator in placing and operating cryosurgical probes in the prostate of a human patient. The cryosurgical system includes a computer system being programmed with software capable of performing the following steps: a) capturing a plurality of transverse views of the prostate; b) capturing a sagittal view of the prostate; c) outlining the capsule of the prostate, the urethra and the rectal wall of the patient with the assistance of the operator, utilizing the captured plurality of transverse views and the captured sagittal view; d) constructing a 3-dimensional model of the prostate, the urethra and the rectal wall utilizing the outlines of step c), above; and, e) utilizing the 3-dimensional model of the prostate, the urethra and the rectal wall to determine i) the number of cryosurgical probes to be utilized; ii) probe settings; and, iii) probe placement positions. The resultant ice thus produced by the cryosurgical probes is optimized for a specific patient.
Owner:VARIAN MEDICAL SYSTEMS
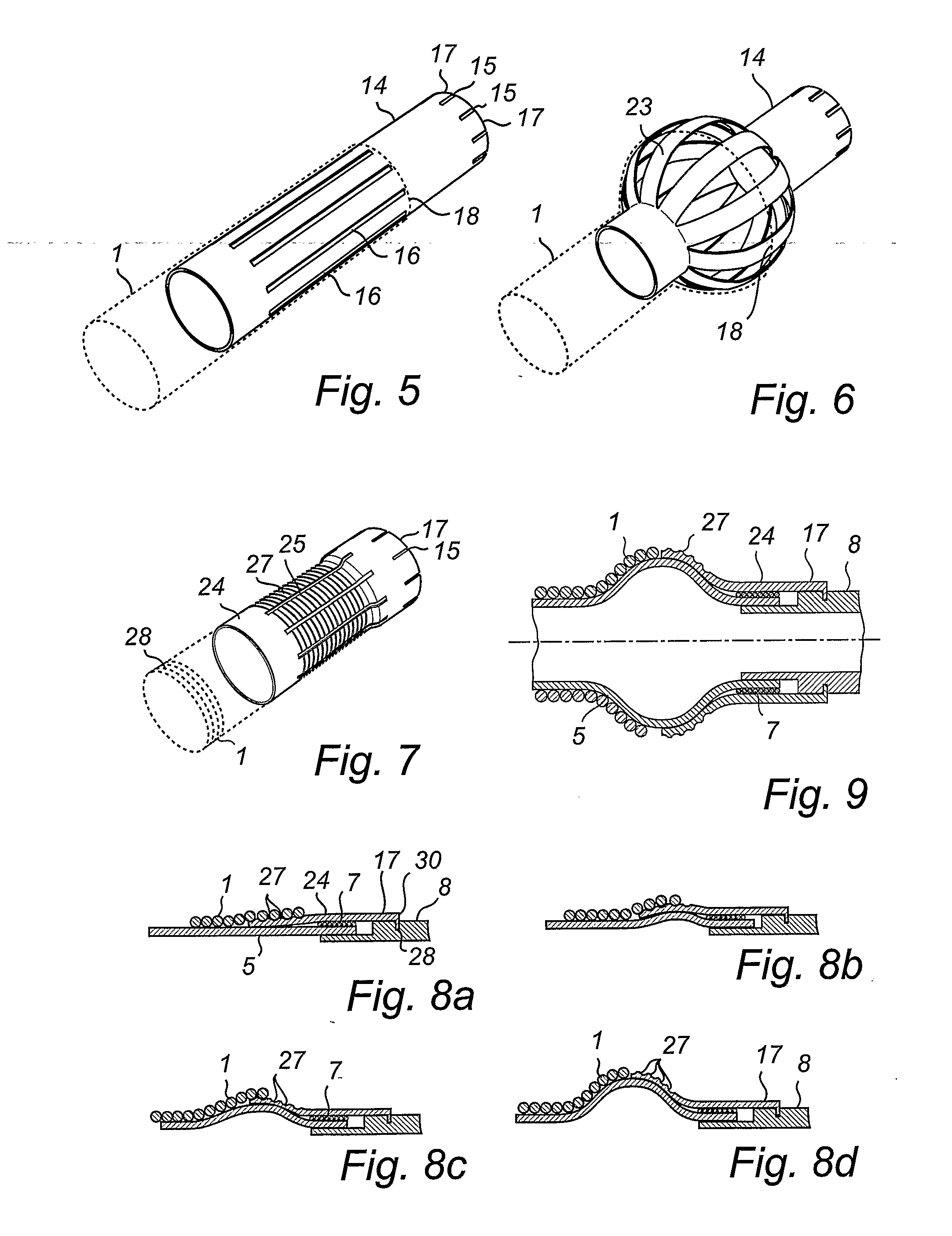
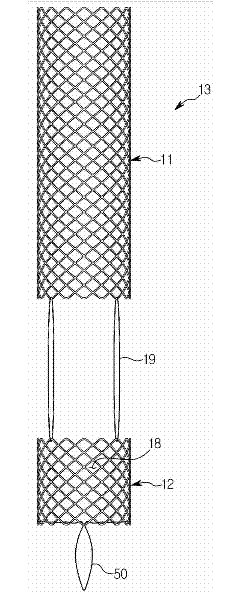
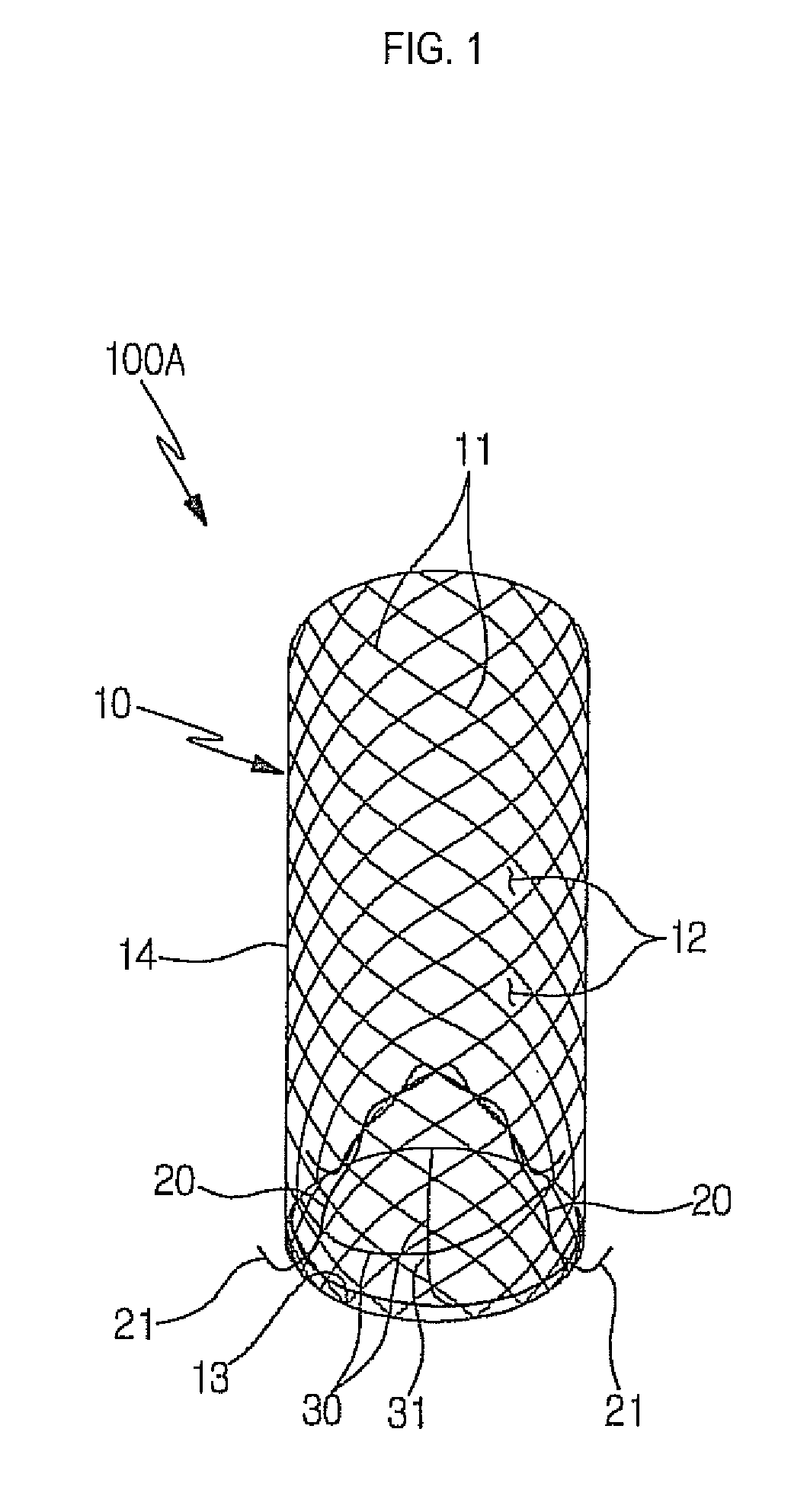
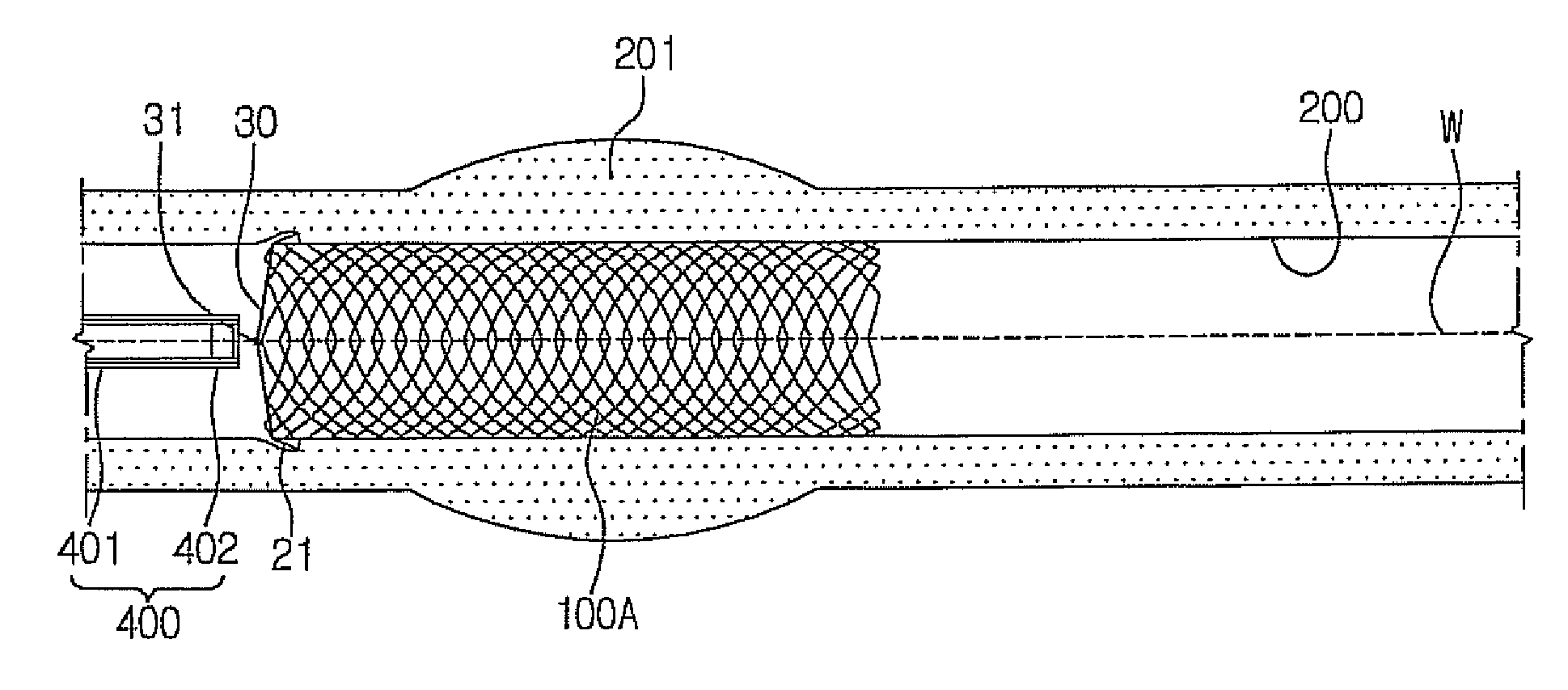
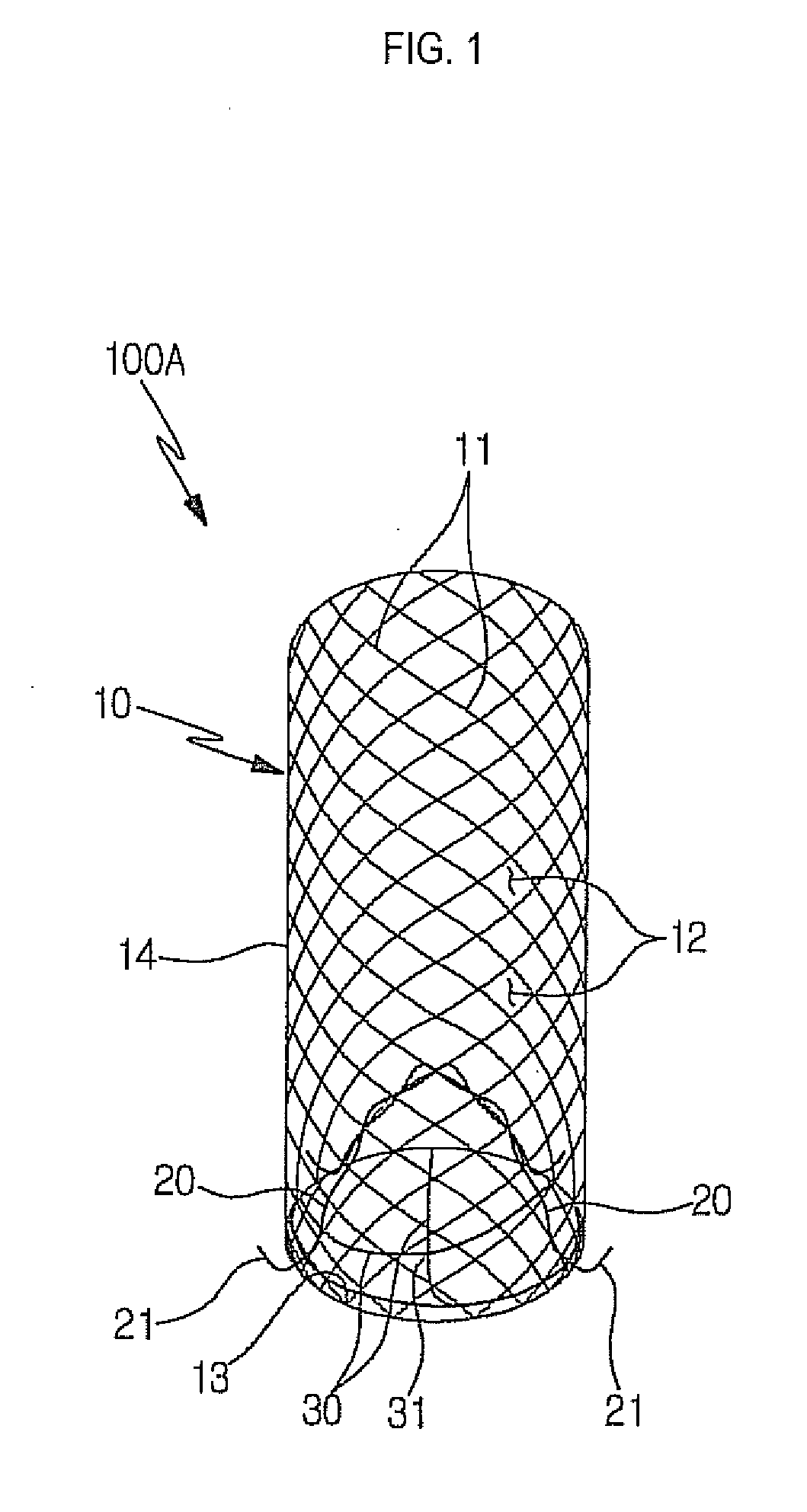
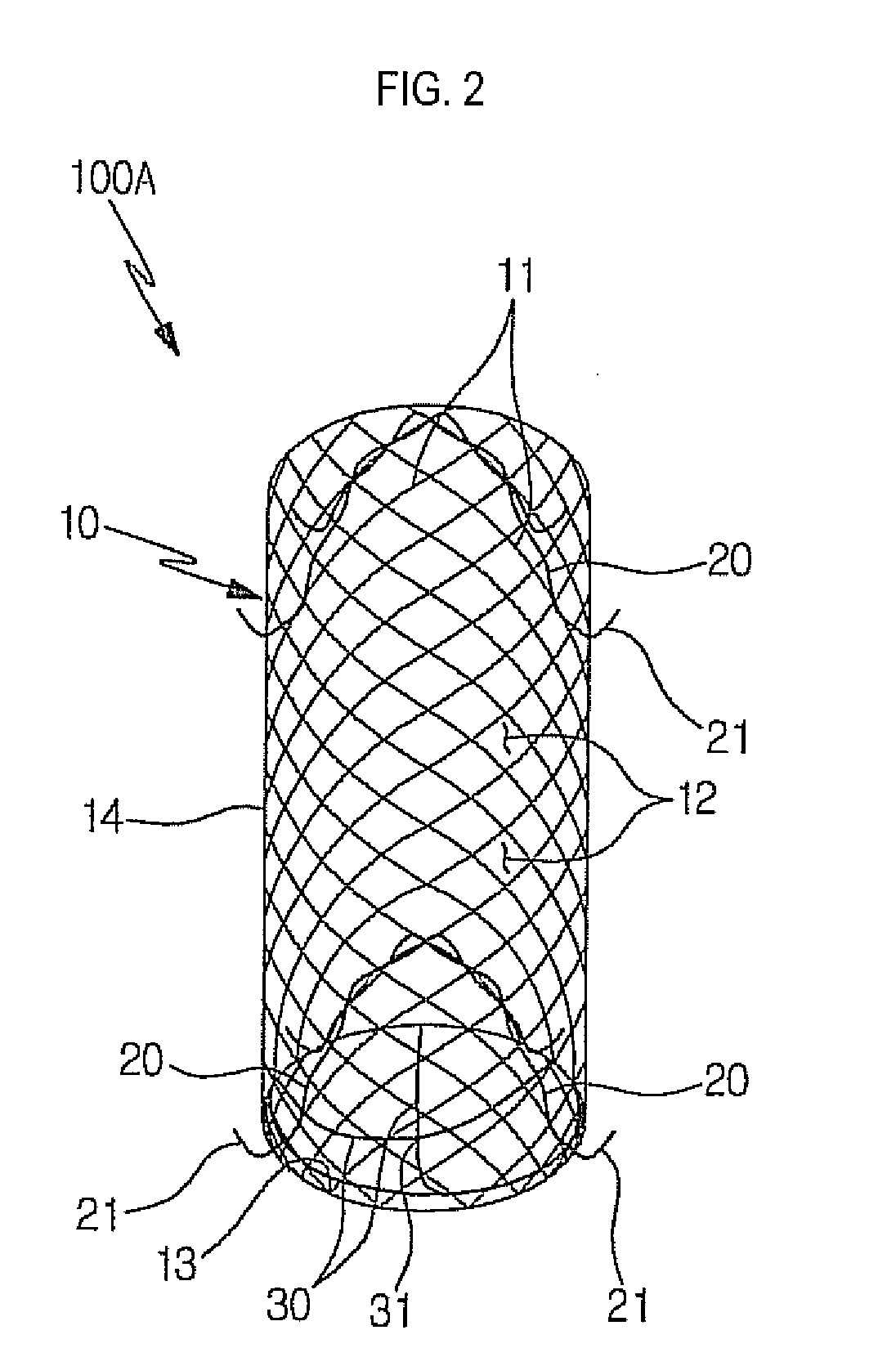
Stent for prostatic urethra expansion
Disclosed herein is a stent for prostatic urethra expansion which does not generate bladder stones, is removable without causing injury on a urethra, and does not generate atrophy of a prostatic urethra even after removal. The stent for prostatic urethra expansion include a stent unit including a cylindrical body with space parts formed by knotting or crossing shape memory alloy wires and bending terminals formed at both ends of the cylindrical body, a pair of hook wires passing through the space parts and knotted to the shape memory alloy wires, both ends thereof being wound on the bending terminals and then being bent upwardly to produce hooks, and a pair of hanging strings arranged in opposite directions to form a hanging knot. The stent for prostatic urethra expansion does not move into the bladder, and expands and maintains a lumen of the stenosed prostatic urethra, thereby reducing post-operative recovery time.
Owner:TAEWOONG MEDICAL CO LTD +2
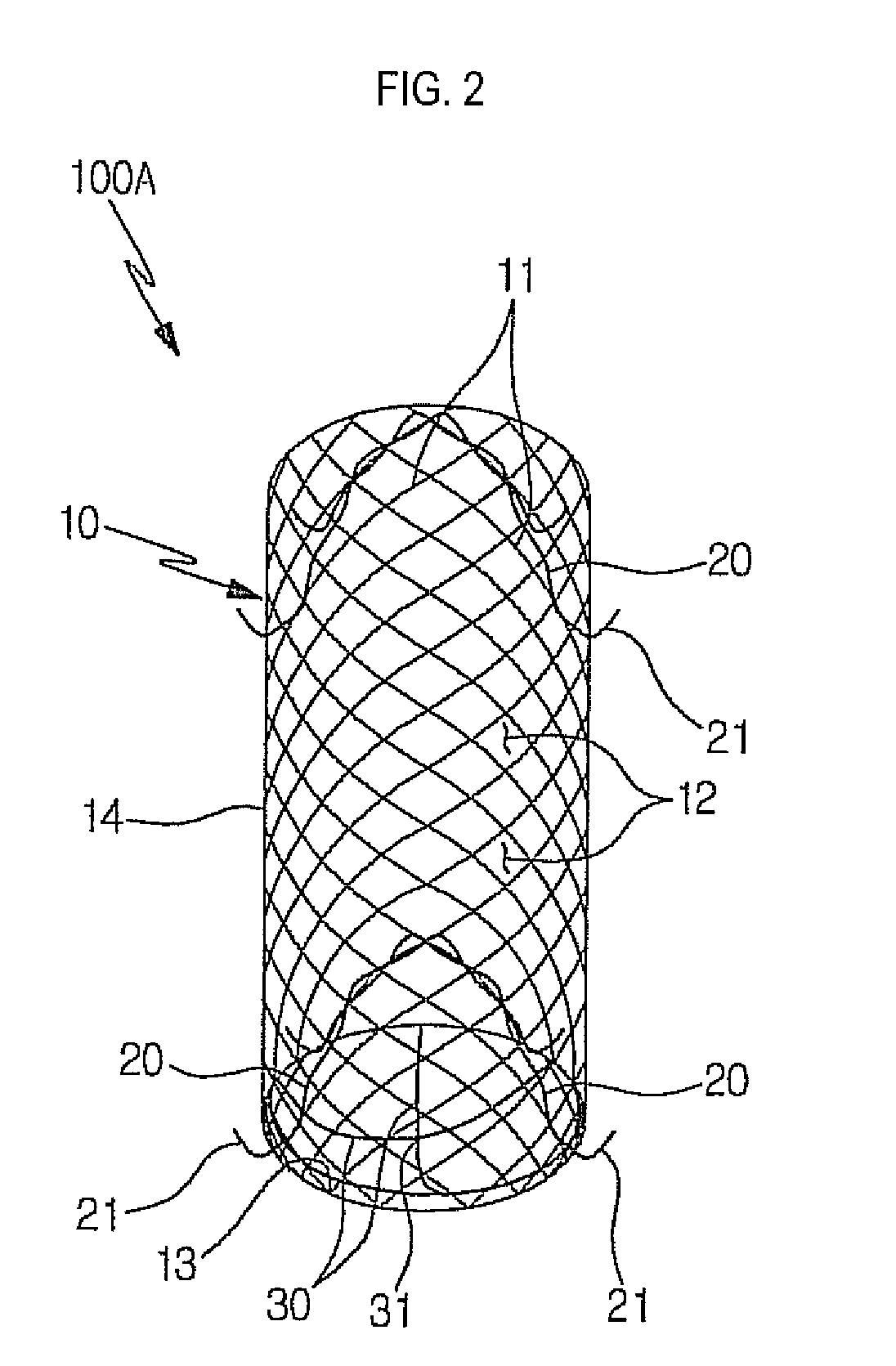
Stent for prostatic urethra expansion
Disclosed herein is a stent for prostatic urethra expansion which does not generate bladder stones, is removable without causing injury on a urethra, and does not generate atrophy of a prostatic urethra even after removal. The stent for prostatic urethra expansion include a stent unit including a cylindrical body with space parts formed by knotting or crossing shape memory alloy wires and bending terminals formed at both ends of the cylindrical body, a pair of hook wires passing through the space parts and knotted to the shape memory alloy wires, both ends thereof being wound on the bending terminals and then being bent upwardly to produce hooks, and a pair of hanging strings arranged in opposite directions to form a hanging knot. The stent for prostatic urethra expansion does not move into the bladder, and expands and maintains a lumen of the stenosed prostatic urethra, thereby reducing post-operative recovery time.
Owner:TAEWOONG MEDICAL CO LTD +2
A dilating device for the prostatic urethra
A dilating device for the prostatic urethra comprises: at least three, laterally connected ridges, wherein each ridge is configured to longitudinally engage with a different substantially longitudinal groove of the prostatic urethra of a patient, the at least three laterally connected ridges are configured to laterally compress to enable insertion into the prostatic urethra in a compressed configuration, and the at least three laterally connected ridges are configured to laterally expand to a normally-open configuration upon deployment within the prostatic urethra, to exert a radially outwards force that dilates the prostatic urethra.
Owner:BUTTERFLY MEDICAL
Prostate urethra medicine-infusion washing drainage tube
The invention is the prostate urethra medicine injection and washing drainage tube, which comprises a tube body, two tube cavities of the prostate urethra part medicine injection and washing drainage tube cavity and a bulboperineal urethra balloon tube cavity are arranged in the tube body; the front part of the tube body is provided with a bulboperineal urethra balloon wrapped around the tube body, the opening on the front end of the bulboperineal urethra balloon tube cavity is communicated with the bulboperineal urethra balloon, and the opening on the tail end thereof is arranged on the tail end of the tube body and is communicated with the outside of the tube body, the tube port of the opening on the tail end is provided with a one-way rubber plug; the opening on the front end of the prostate urethra part medicine injection and washing drainage tube cavity is arranged before the bulboperineal urethra balloon near the tube body wall on the front end of the tube body and is communicated with the outside of the tube body, the opening on the tail end thereof is arranged on the tail end of the tube body and is communicated with the outside of the tube body, the tube port of the opening on the tail end is provided with a one-way rubber plug; the opening on the front end of the prostate urethra part medicine injection and washing drainage tube cavity is 1.0cm-3.0cm far away from the opening on the front end of the bulboperineal urethra balloon tube cavity. The invention cancels the vesical cervix balloon tube cavity and the vesical cervix balloon in the catheter of the prior art, which reduces the diameter of the catheter, relieves the pain of the patient, and the sealing function is better. The invention saves materials, simplifies production art, and reduces production cost, thus the treatment charge is largely reduced for patients. Therefore, the invention is more favorable for clinic promotion compared with the prior art.
Owner:乌鲁木齐爱尚佳音医药有限公司
Endourethral device & method
InactiveUS20060195008A1Inhibit migrationExclude influenceLighting and heating apparatusSurgeryUrethraDistal segment
An endourethral device is provided having an elongate member having proximal and distal segments, the elongate member positionable within a lower urinary tract so as to at least partially traverses a prostatic urethra. A proximal anchor, adapted to abuttingly engage portions of a bladder neck so as to at least proximally anchor the device, is supported at least indirectly by the proximal segment of said elongate member. The proximal anchor includes bladder engaging elements radially extending from the proximal segment of said elongate member, urine being freely dischargable about at least the proximal segment so as to substantially bathe the bladder neck therewith.
Owner:SRS MEDICAL SYST INC
Endourethral device & method
ActiveUS20060116547A1Inhibit migrationExclude influenceLighting and heating apparatusSurgeryUrethraDistal segment
An endourethral device is provided having an elongate member having proximal and distal segments, the elongate member positionable within a lower urinary tract so as to at least partially traverses a prostatic urethra. A proximal anchor, adapted to abuttingly engage portions of a bladder neck so as to at least proximally anchor the device, is supported at least indirectly by the proximal segment of said elongate member. The proximal anchor includes bladder engaging elements radially extending from the proximal segment of said elongate member, urine being freely dischargable about at least the proximal segment so as to substantially bathe the bladder neck therewith.
Owner:SRS MEDICAL SYST INC
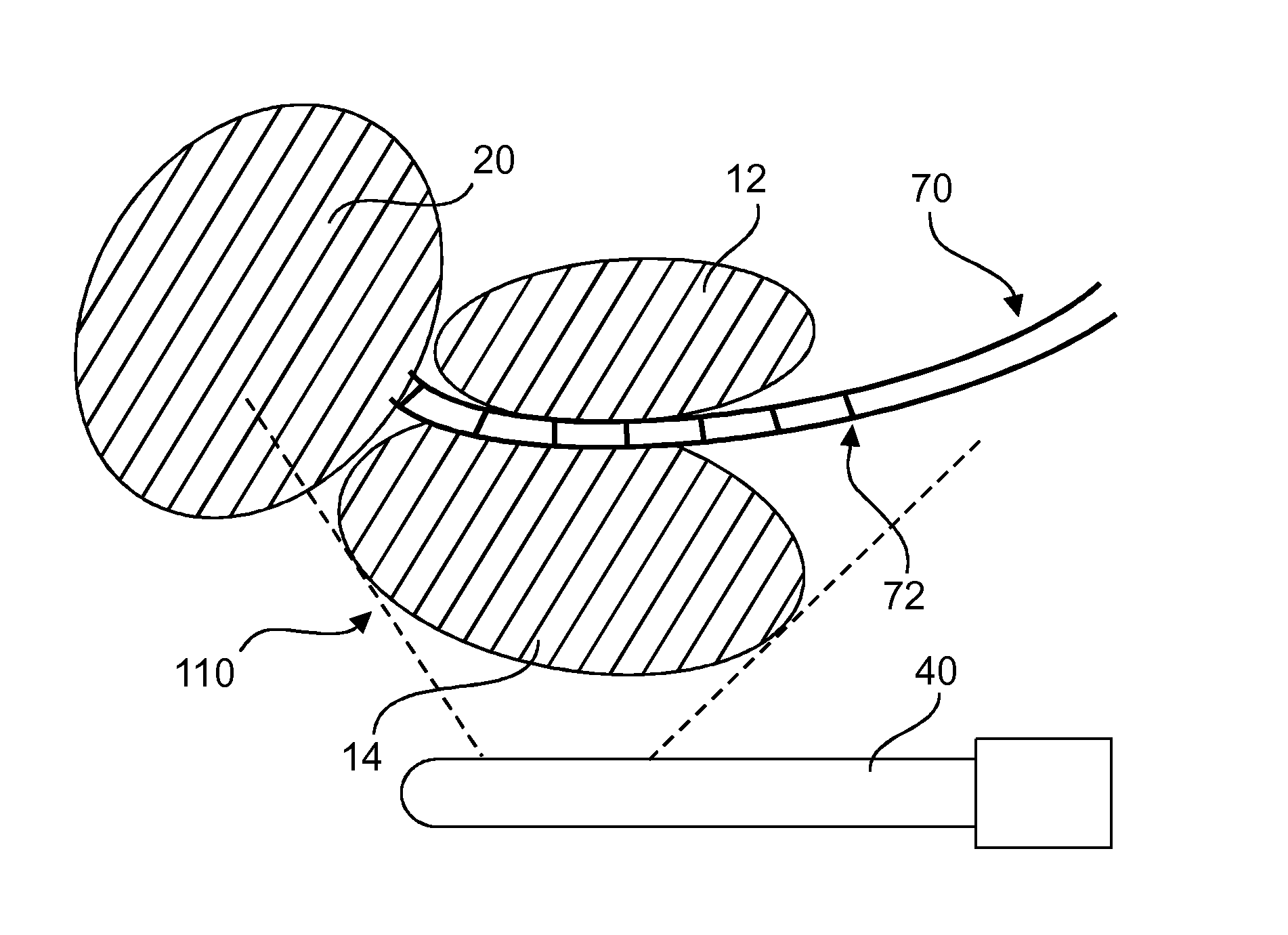
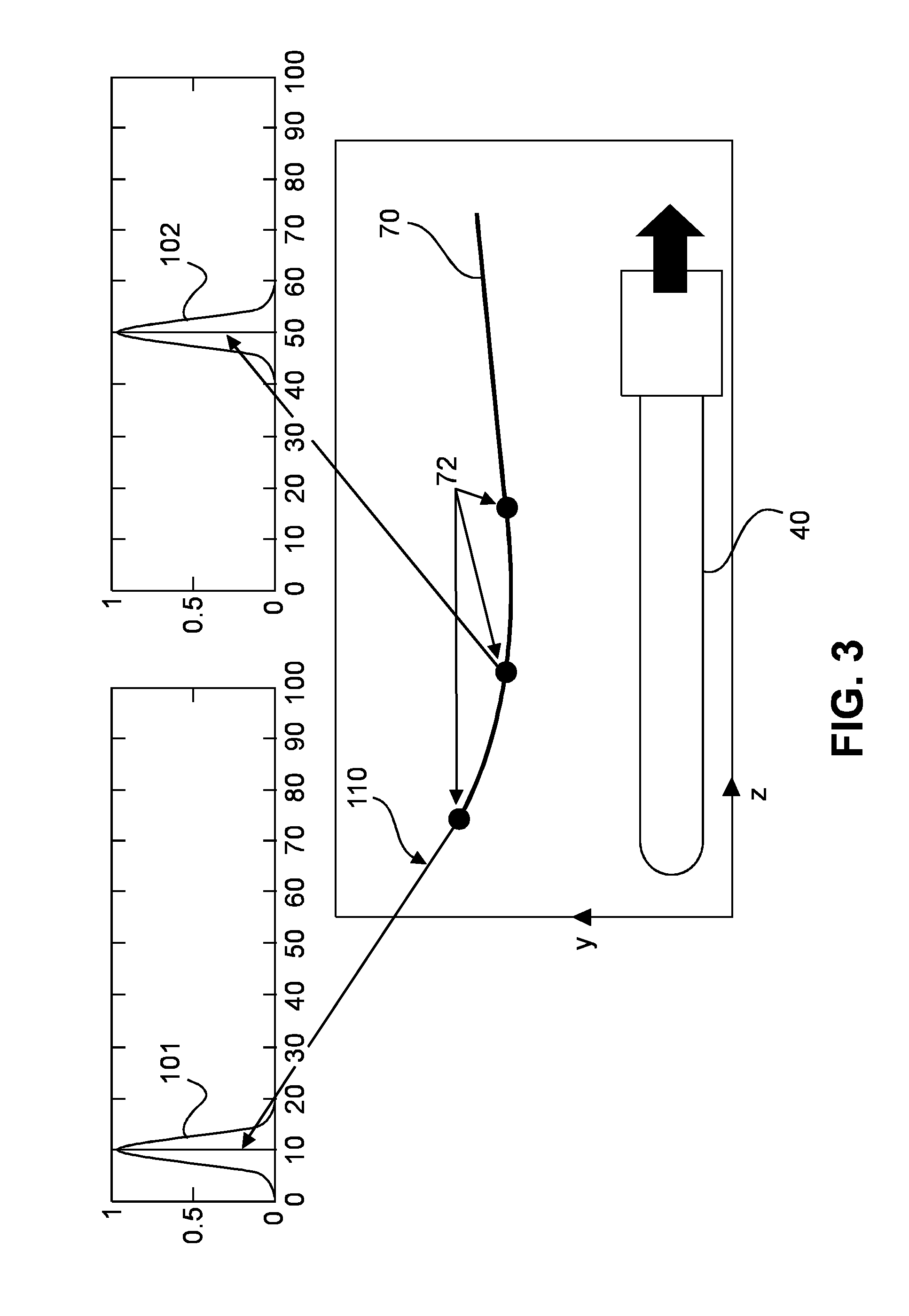
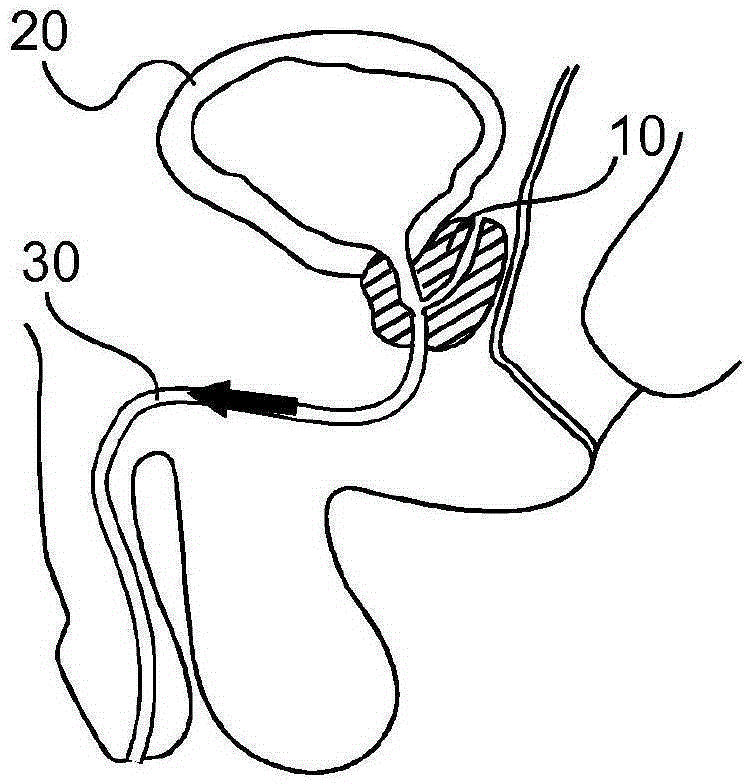
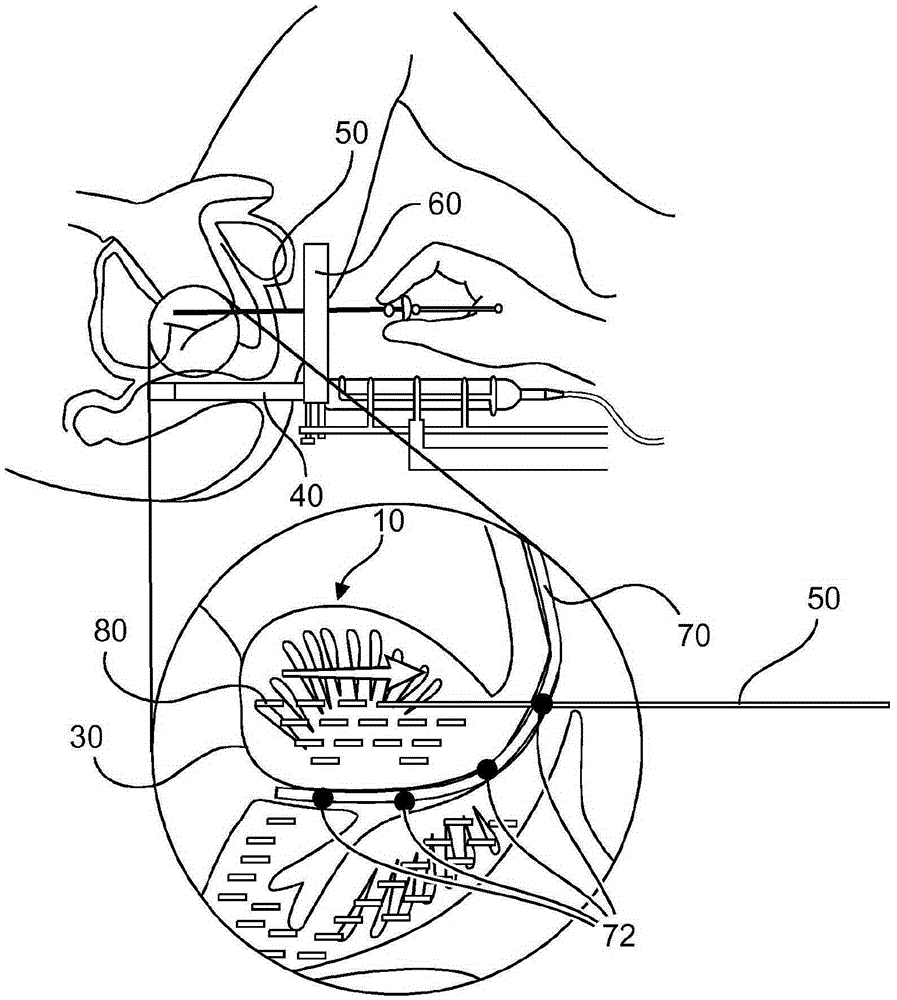
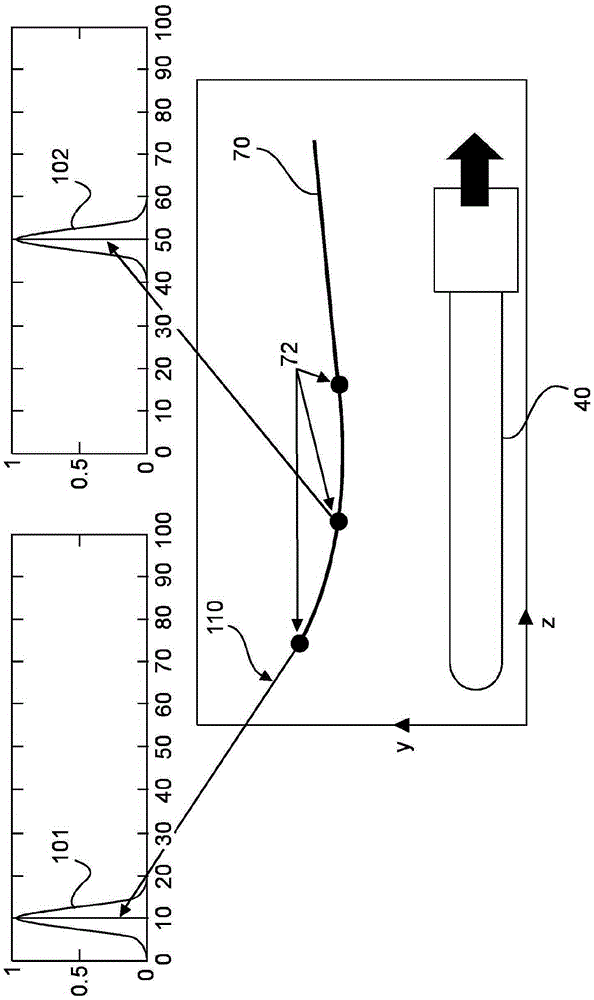
Method and system for localizing body structures
InactiveUS20160183910A1Better clinical outcomeHigh accuracy in localizationOrgan movement/changes detectionWound drainsUrethraTrus image
The invention relates to a system and method in which a Foley catheter (70) or other medical tool which is equipped with ultrasound (US) sensor(s) (72) is inserted into the prostatic urethra. Based on analysis of the US signal received by these US sensors (72) as the US beams from a transrectal US (TRUS) probe (40) or other ultrasound probe sweep the field of view, it is possible to precisely detect and track these US sensors (72) in the same frame of reference as the TRUS images, thereby precisely delineating the Foley catheter and the course of the prostatic urethra. During the procedure, before each seed is dropped, the delivered dose to the prostatic urethra can be computed based on real-time tracking and segmentation of prostatic urethra and dose radiation based on previously dropped seeds and if necessary, the procedure can be re-planned automatically.
Owner:KONINKLJIJKE PHILIPS NV
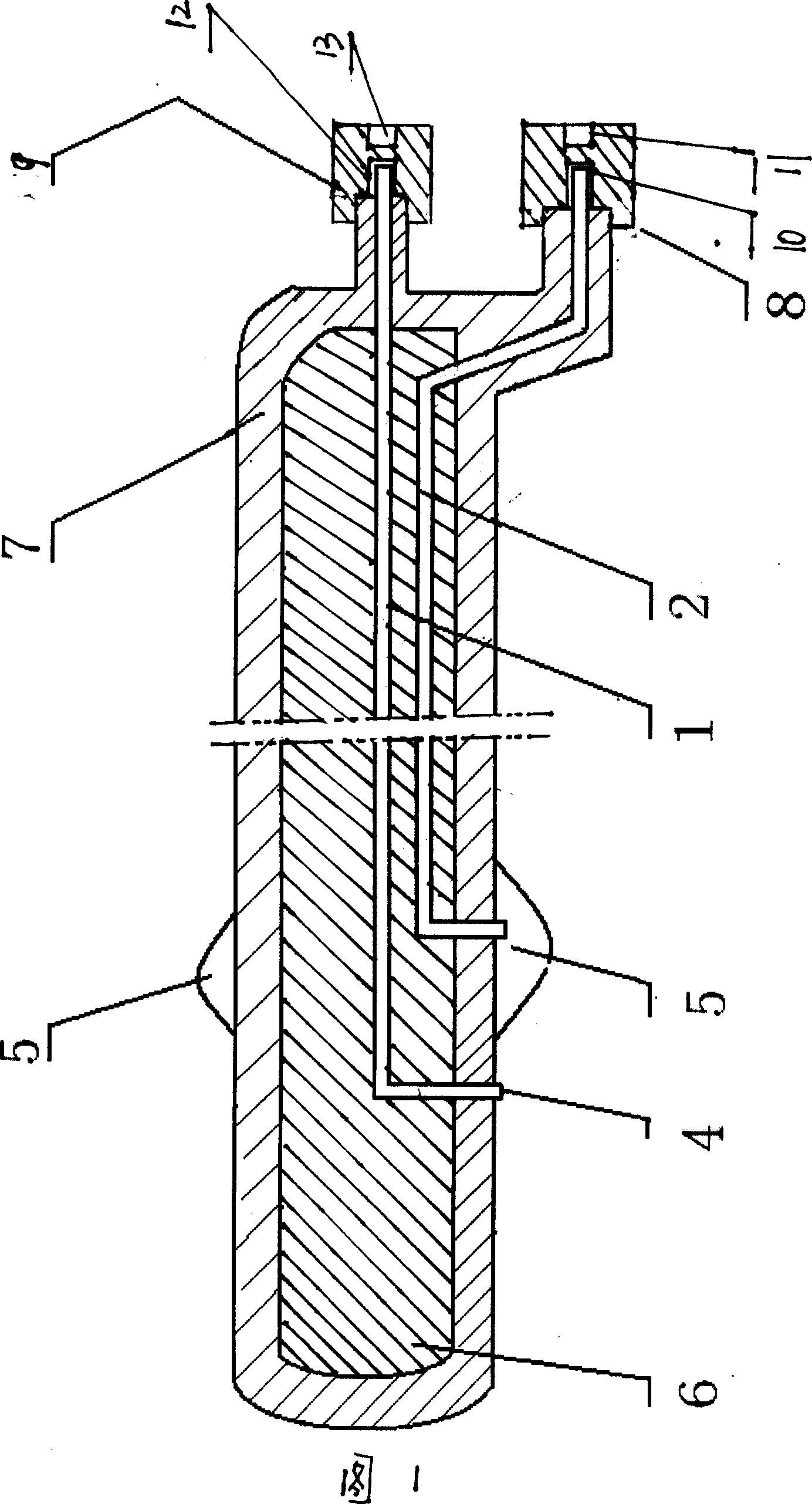
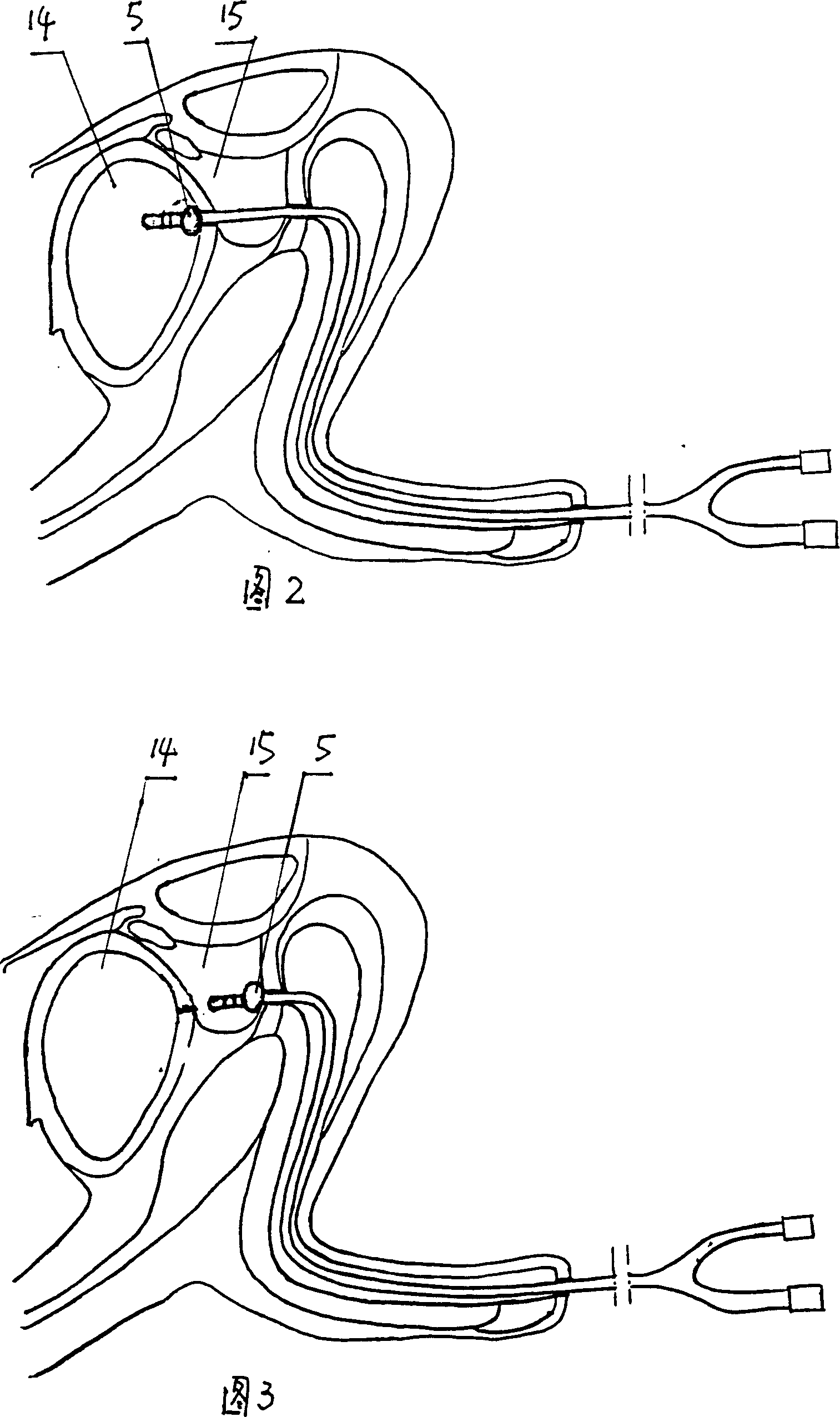
Magnetic probe steel assembly for urethral reunion operation
The invention discloses a magnetic probe steel assembly for urethral reunion operation. The magnetic probe steel assembly comprises first probe steel and second steel, wherein two ends of the first probe steel are a first handheld end and a first magnetic end; the first magnetic end is used for extending in from anterior urethra, passing through a bulb of urethra part and arriving at a membranousurethra breaking position; the end of the first magnetic end has magnetism; a channel allowing a guide wire to pass through is formed in an inner cavity of the first probe steel along the direction ofthe first probe steel; two ends of the second probe steel are a second handheld end and a second magnetic end; the second magnetic end is used for entering prostatic urethra through bladder and arriving at the membranous urethra breaking position; and the end of the second magnetic end has magnetism. According to the magnetic probe steel assembly disclosed by the invention, through cooperation ofthe first probe steel and the second probe steel, an operator is convenient to operate; and through mutual attraction of the first magnetic end and the second magnetic end, the situation that under the attraction of the second probe steel, the first probe steel enters the bladder, and urethral reunion operation is completed is guaranteed.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method and system for localizing body structures
The invention relates to a system and method in which a Foley catheter (70) or other medical tool which is equipped with ultrasound (US) sensor(s) (72) is inserted into the prostatic urethra. Based on analysis of the US signal received by these US sensors (72) as the US beams from a transrectal US (TRUS) probe (40) or other ultrasound probe sweep the field of view, it is possible to precisely detect and track these US sensors (72) in the same frame of reference as the TRUS images, thereby precisely delineating the Foley catheter and the course of the prostatic urethra. During the procedure, before each seed is dropped, the delivered dose to the prostatic urethra can be computed based on real-time tracking and segmentation of prostatic urethra and dose radiation based on previously dropped seeds and if necessary, the procedure can be re-planned automatically.
Owner:KONINKLJIJKE PHILIPS NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com