Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76results about How to "Monitor effectiveness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
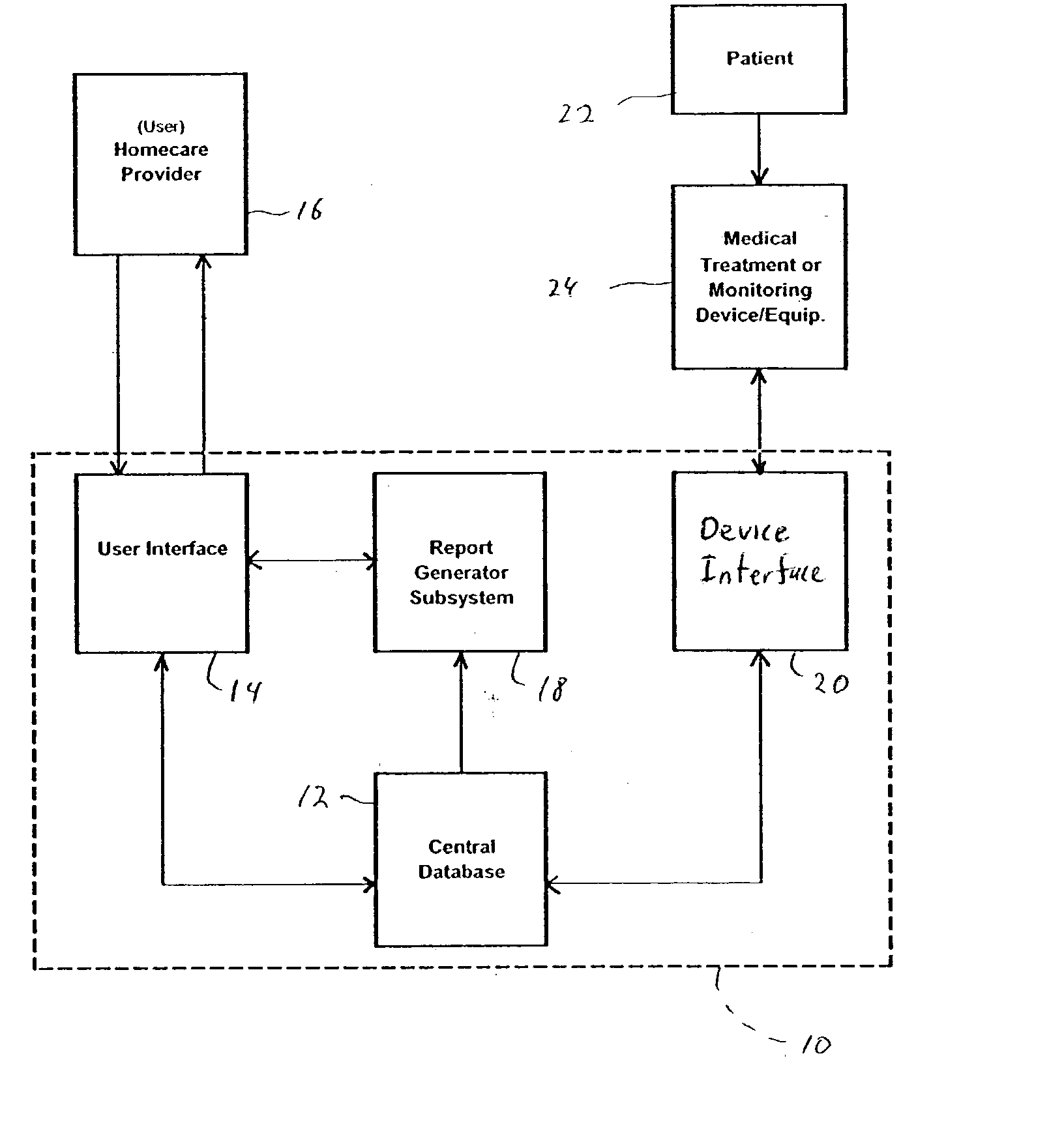
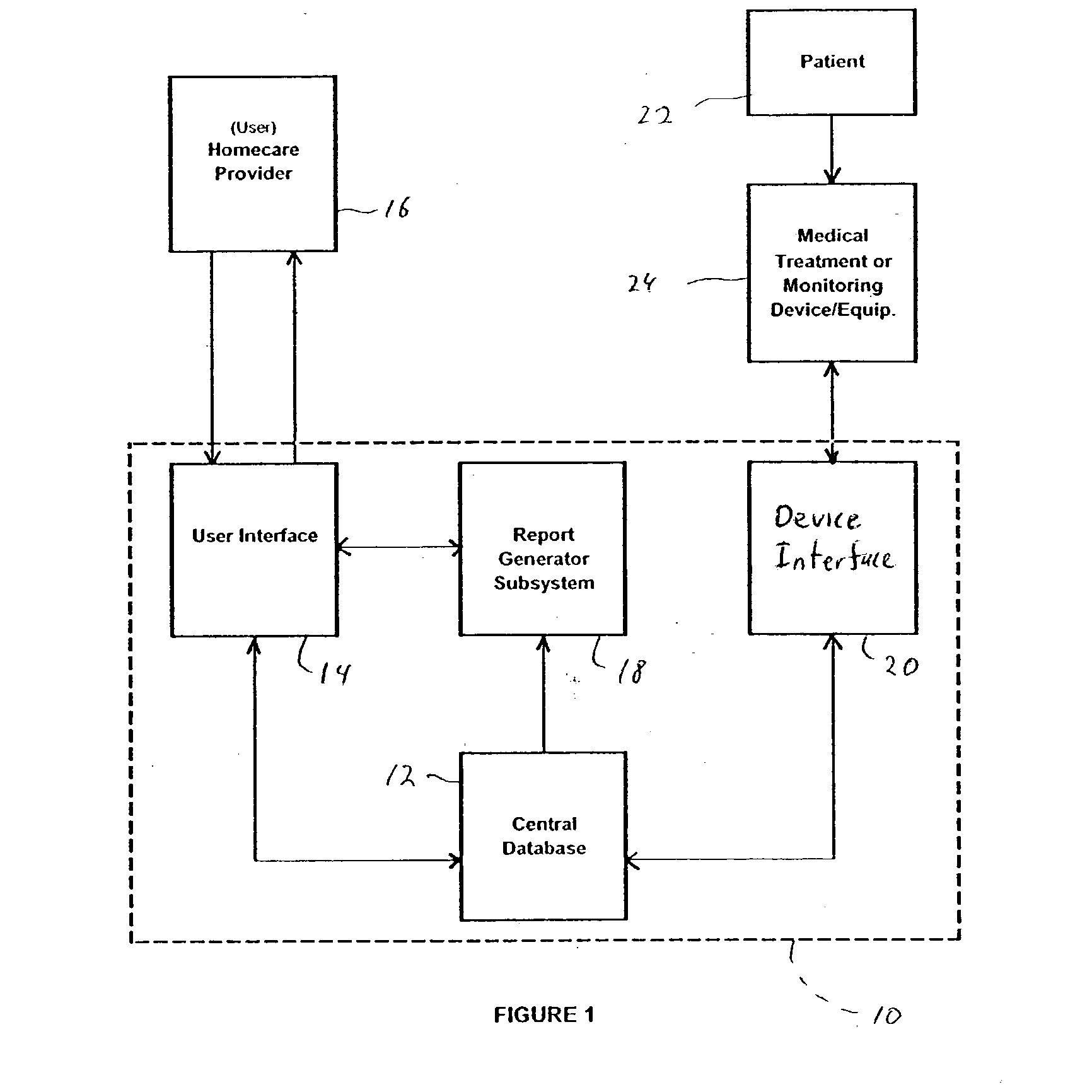
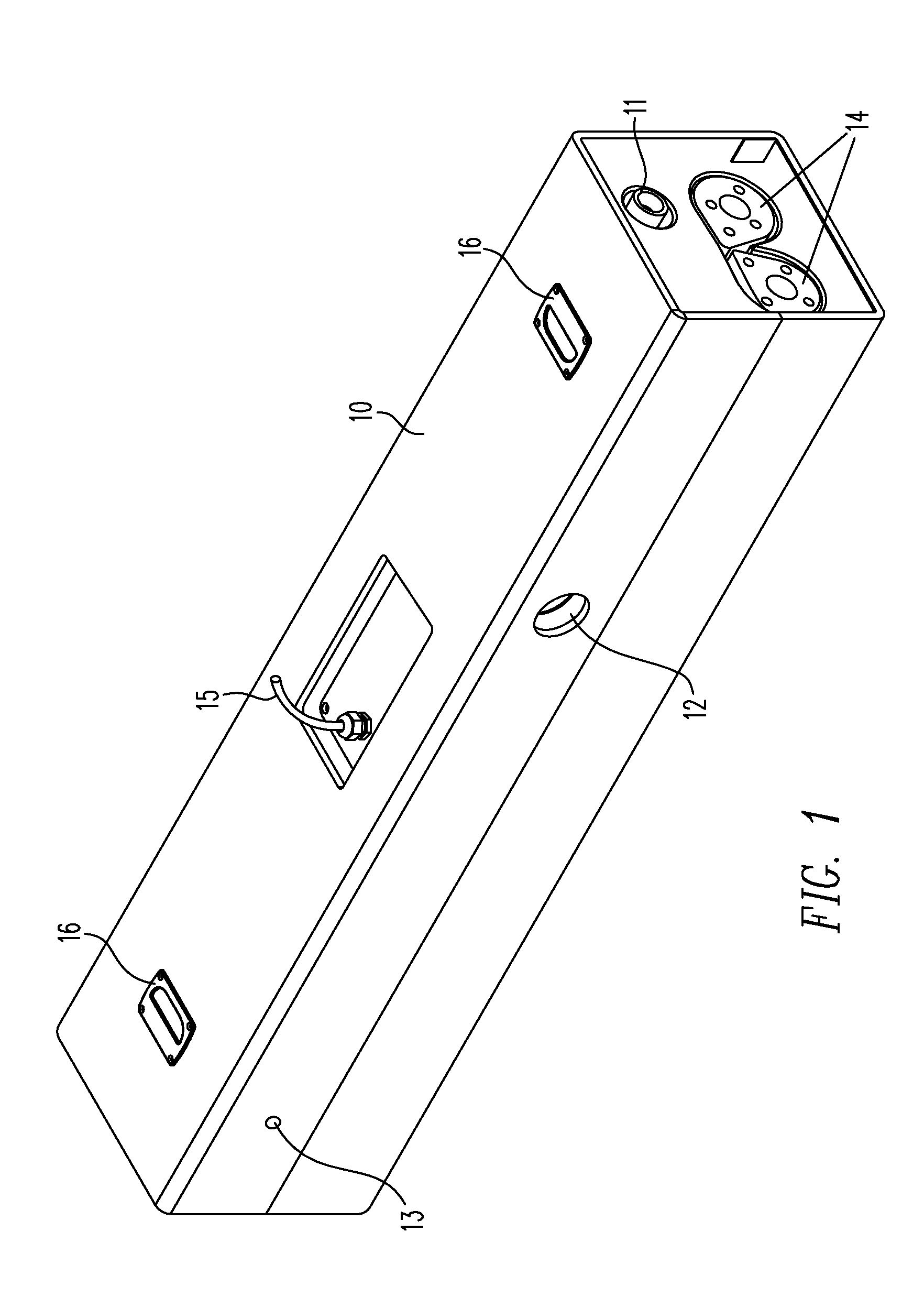
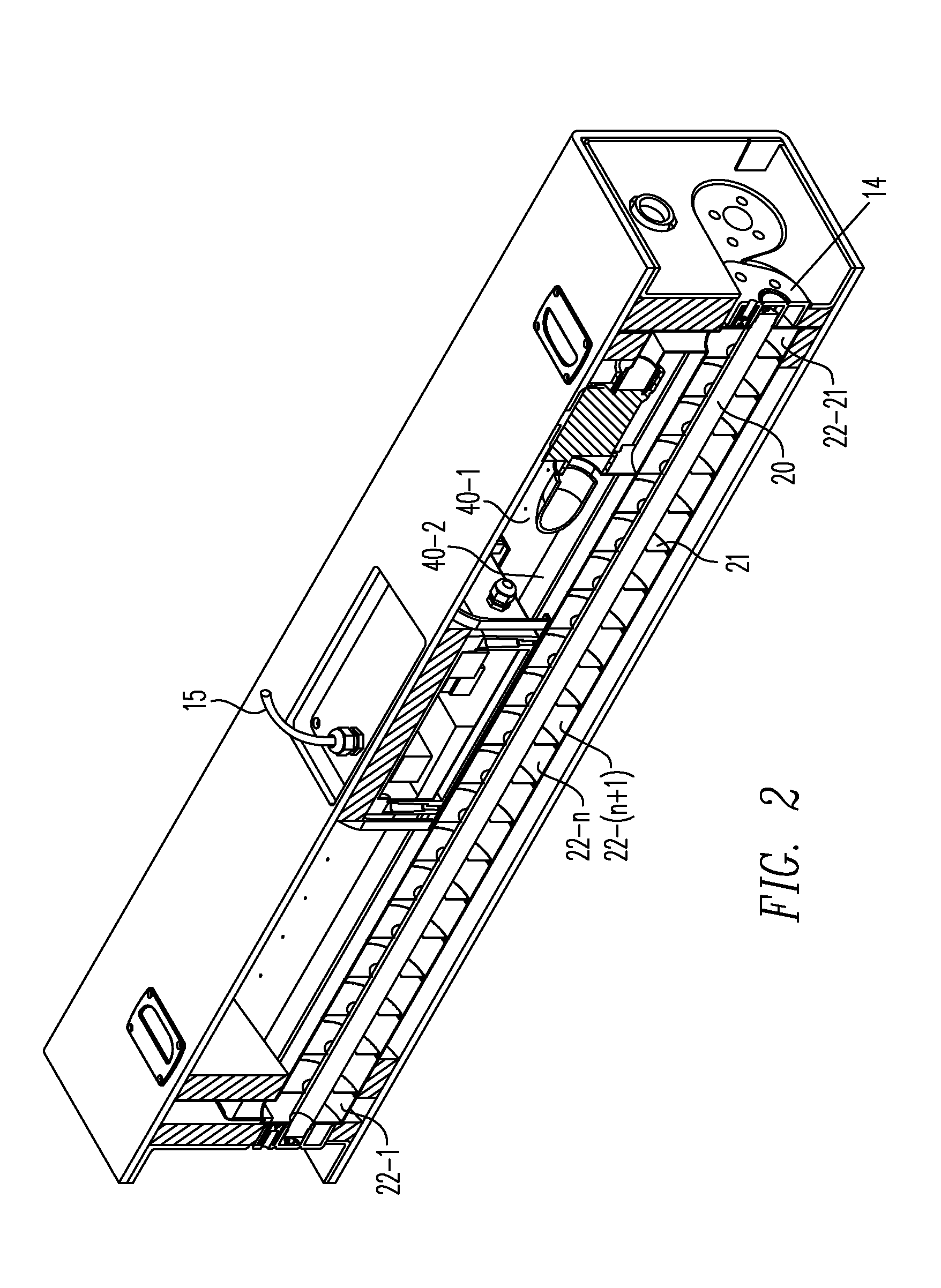
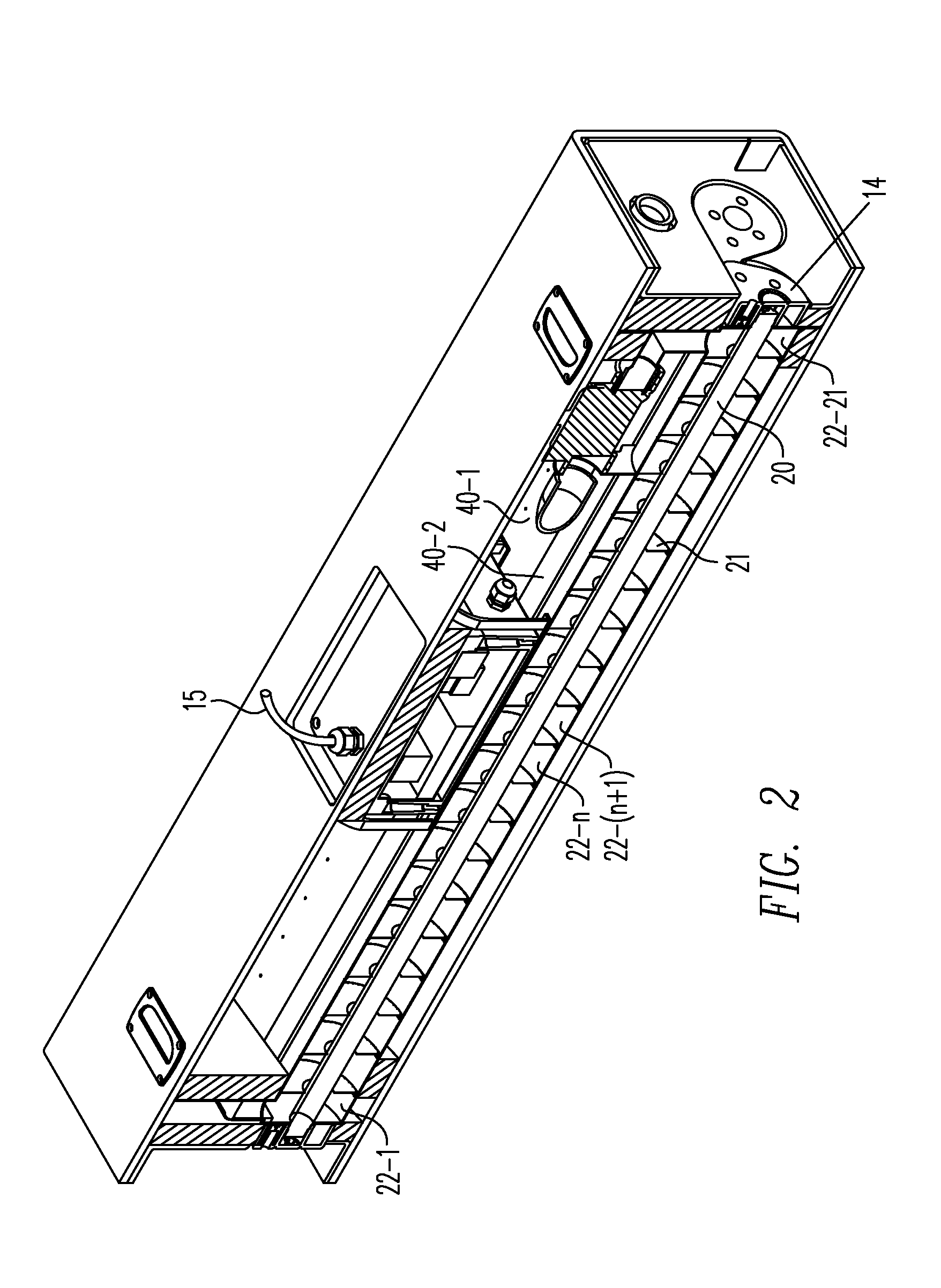
Method for managing medical information and medical information management system
InactiveUS20030208465A1None of data is presentedEffective trackingMechanical/radiation/invasive therapiesDigital data processing detailsUser inputData field
A computer-implemented method for managing medical information that includes the steps of: providing a central database of information having disparate data fields containing data, and performing an action based upon the data in the disparate data fields. The method includes of sorting, providing warnings or reminders, searching, organizing, transmitting, and presenting data from the database. The medical information management system includes a central database resident on a computing system. The central database has multiple disparate data fields containing data pertaining to patient-related information. A user interface communicates with the central database for accepting user input and transmitting system output. A visual display also communicates with the user interface to display the disparate data fields in selected or selectable formats. The system can include a report generating module, a device interface, and a compliance calculation module, all of which are in communication with the central database.
Owner:RIC INVESTMENTS LLC
Ultraviolet water purification system
ActiveUS20090084734A1Inhibits ultraviolet disinfectionImprove efficiencySamplingExhaust apparatusWireless mesh networkClosed loop feedback
An Ultraviolet-C (UVC) based portable water purification system employing a novel array of baffles increases the efficiency per unit energy of irradiating UVC light in the eradication of pathogens in the water. Closed loop feedback allows monitoring the application of UVC light power to ensure high levels of pathogen eradication. This system is capable of eradicating a wide range of waterborne bacteria, viruses, protozoa, helminthes, yeast, and mold found in natural freshwater sources worldwide. By adding pre- or post-filters, the system can remove harmful organic compounds, pesticides, inorganic compounds and heavy metals from the water. The system can also be used to eradicate pathogens in fluids other than water. As a feature of this invention, a communications systems that can reach geographically dispersed populations at low cost without the need to install costly wired communications infrastructure is combined with and powered by the water purification system. In one embodiment, a packet radio system is provided to create nodes in a wireless mesh communications system to provide voice, data, video and internet communications using an array of the water purifiers to create a wireless mesh network.
Owner:WATER OF LIFE
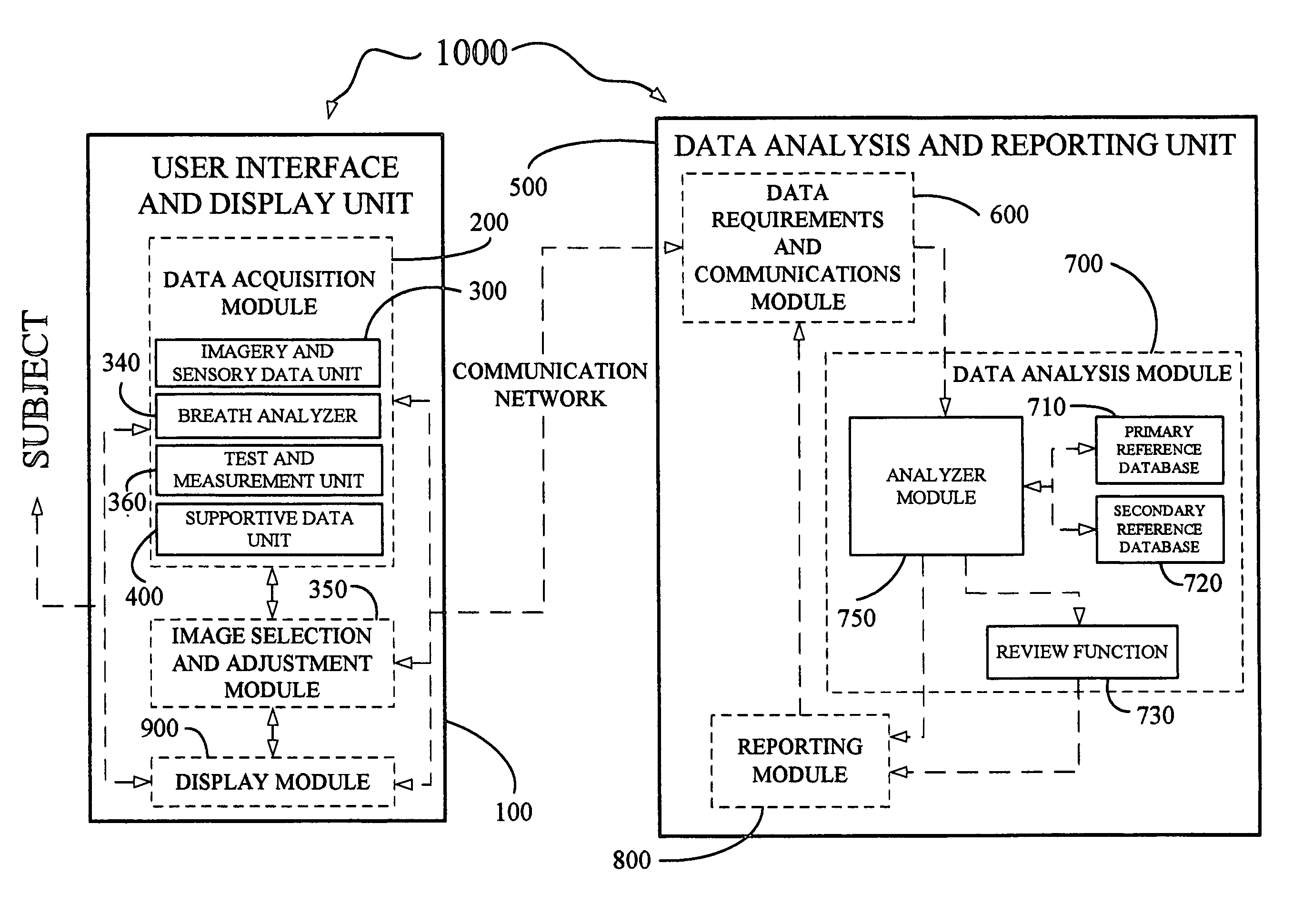
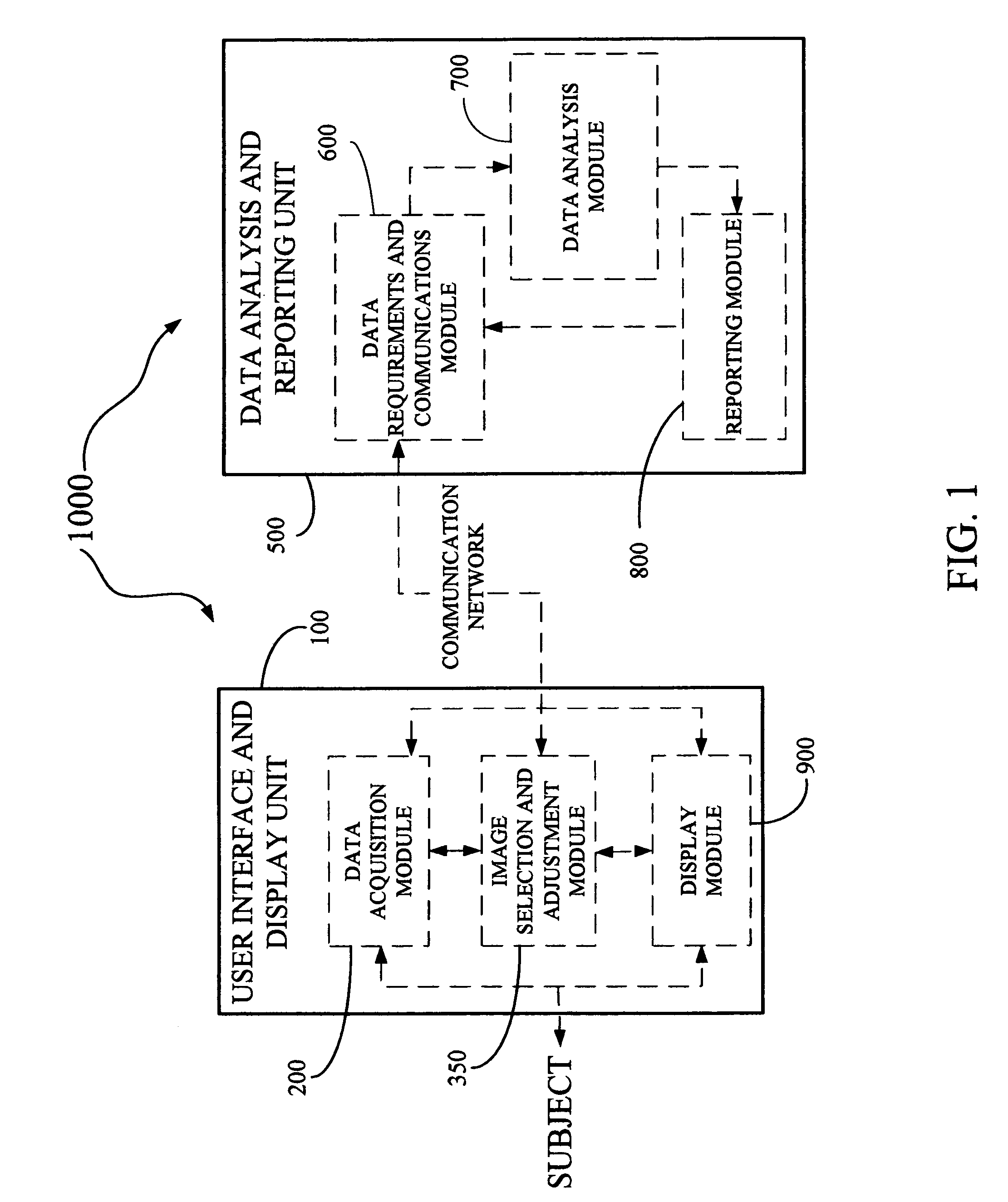
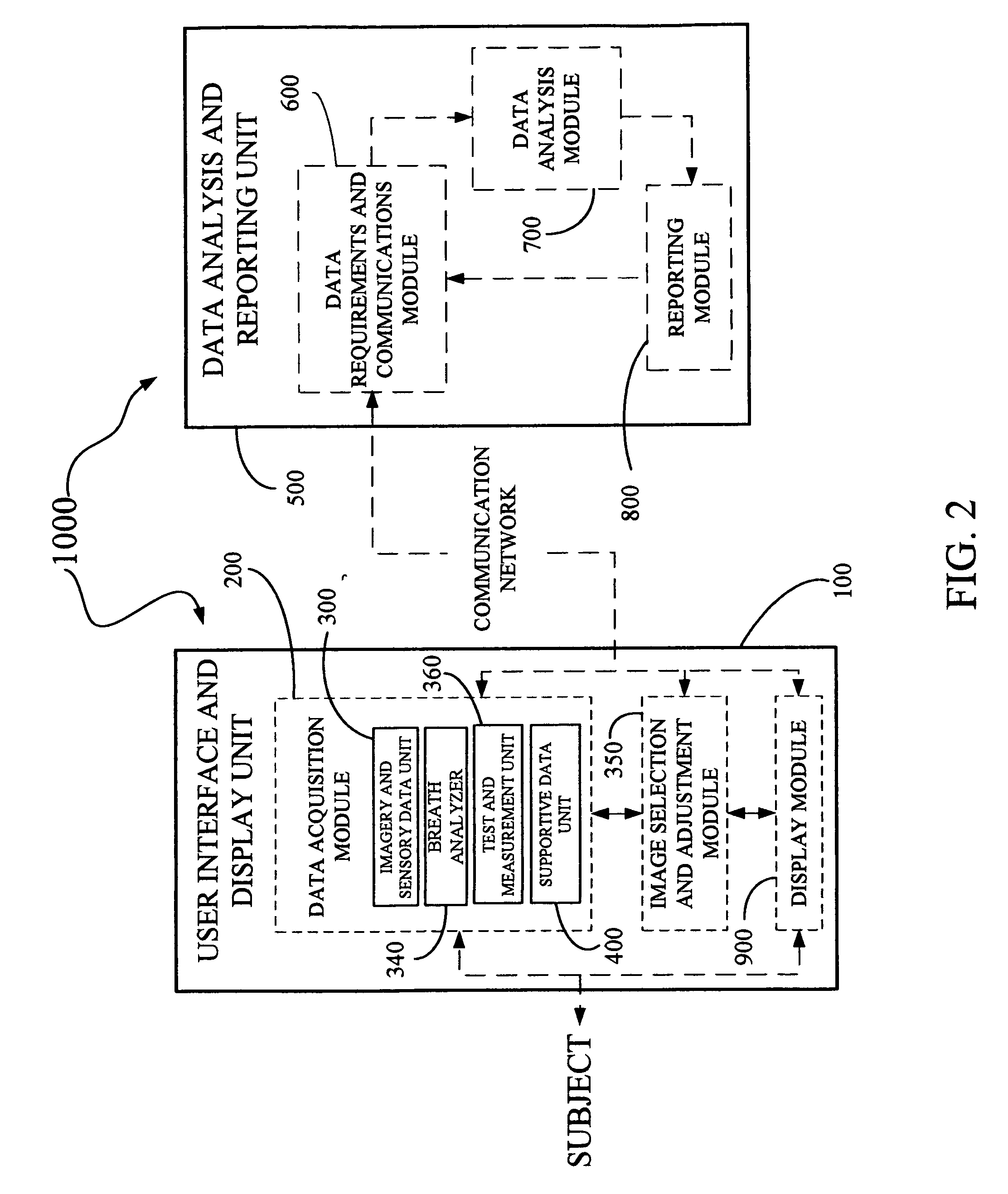
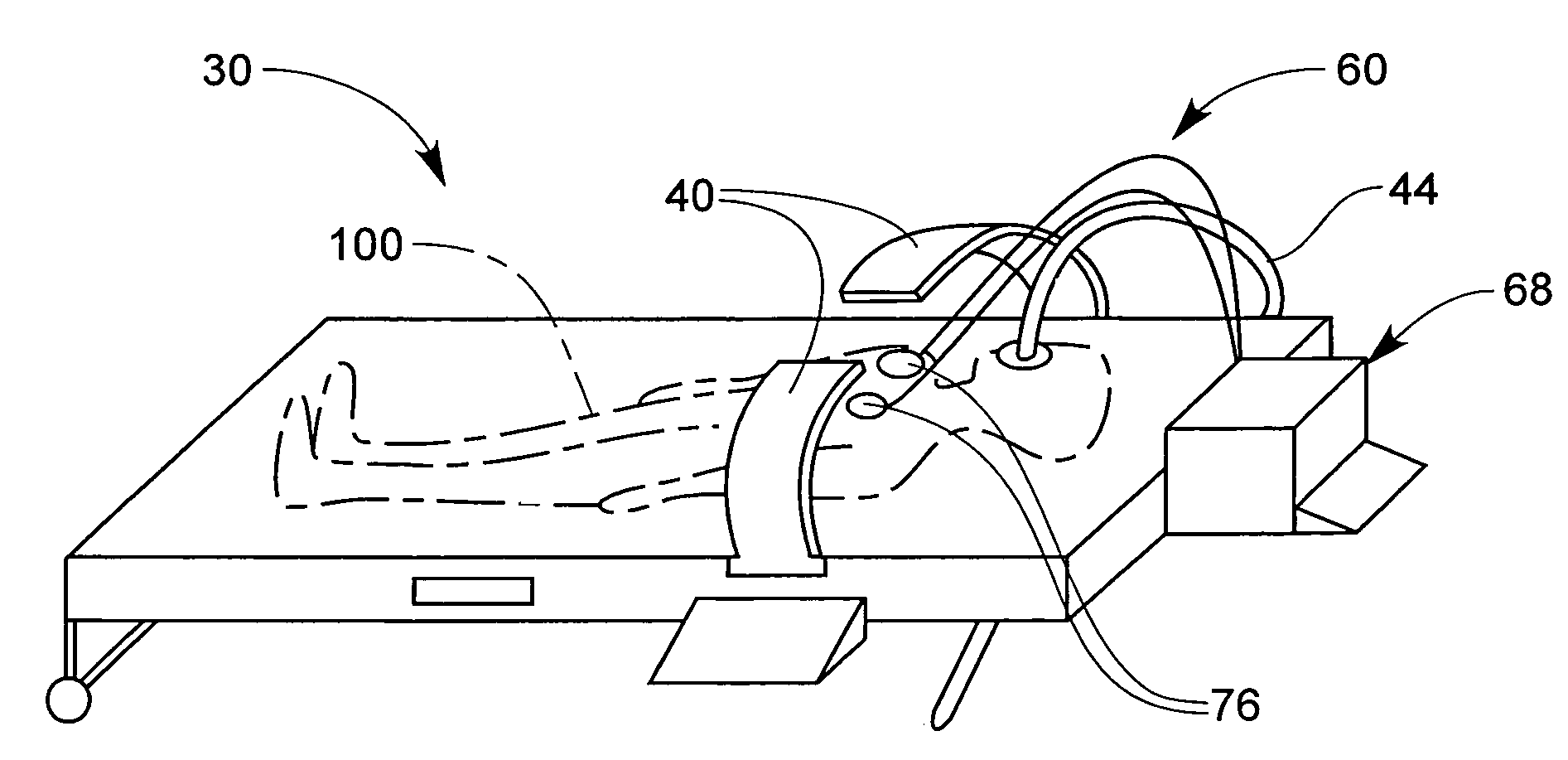
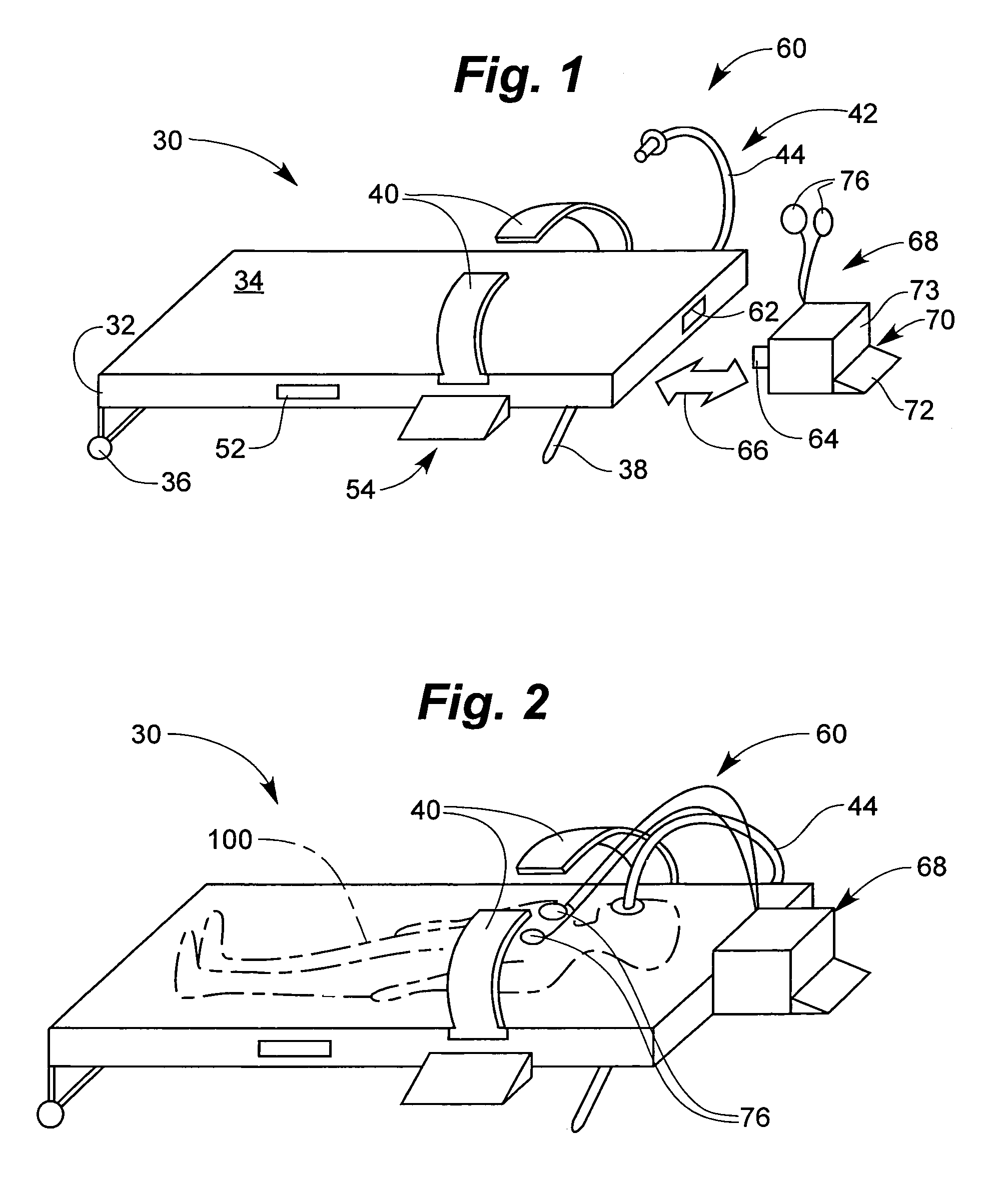
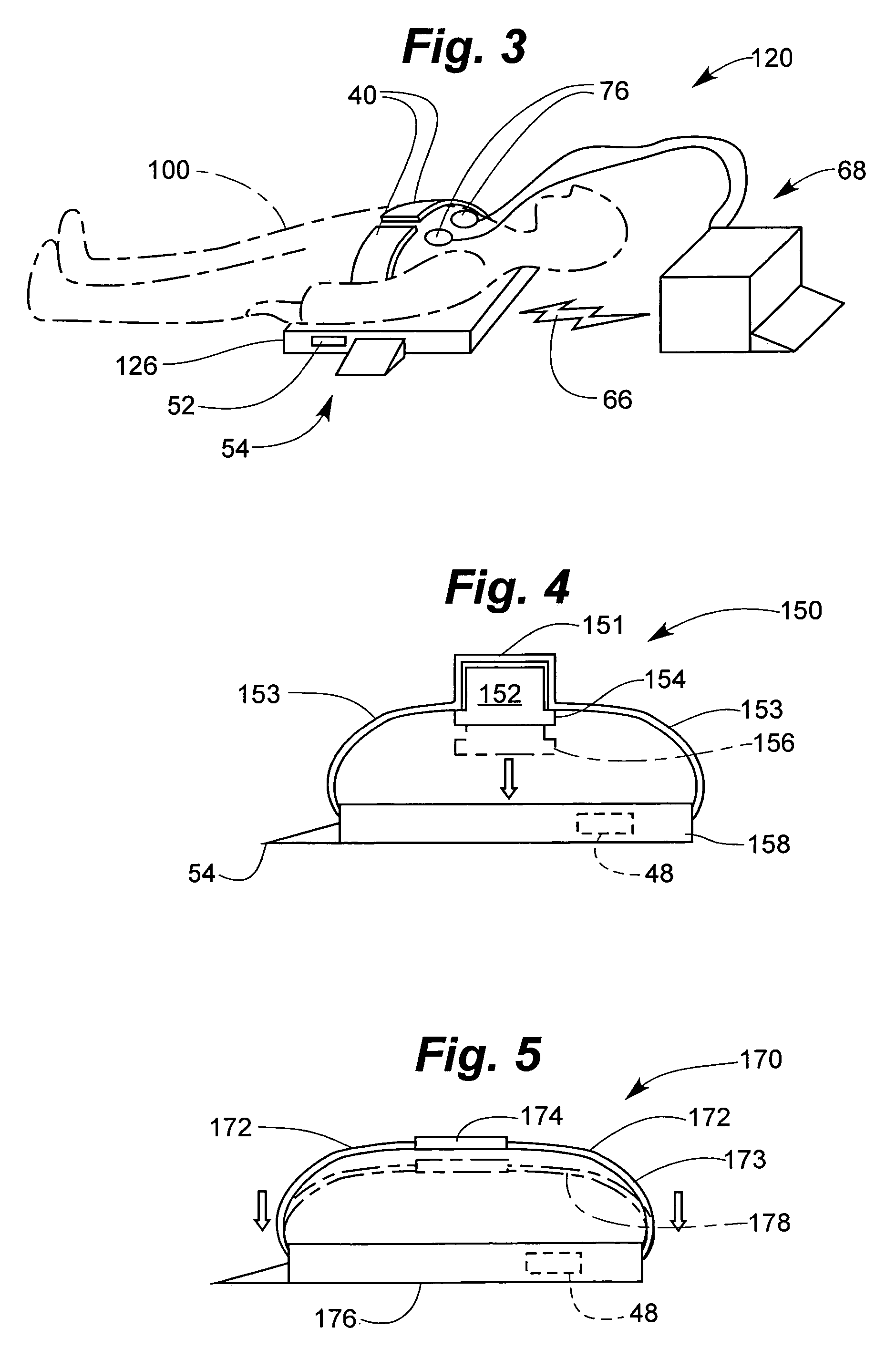
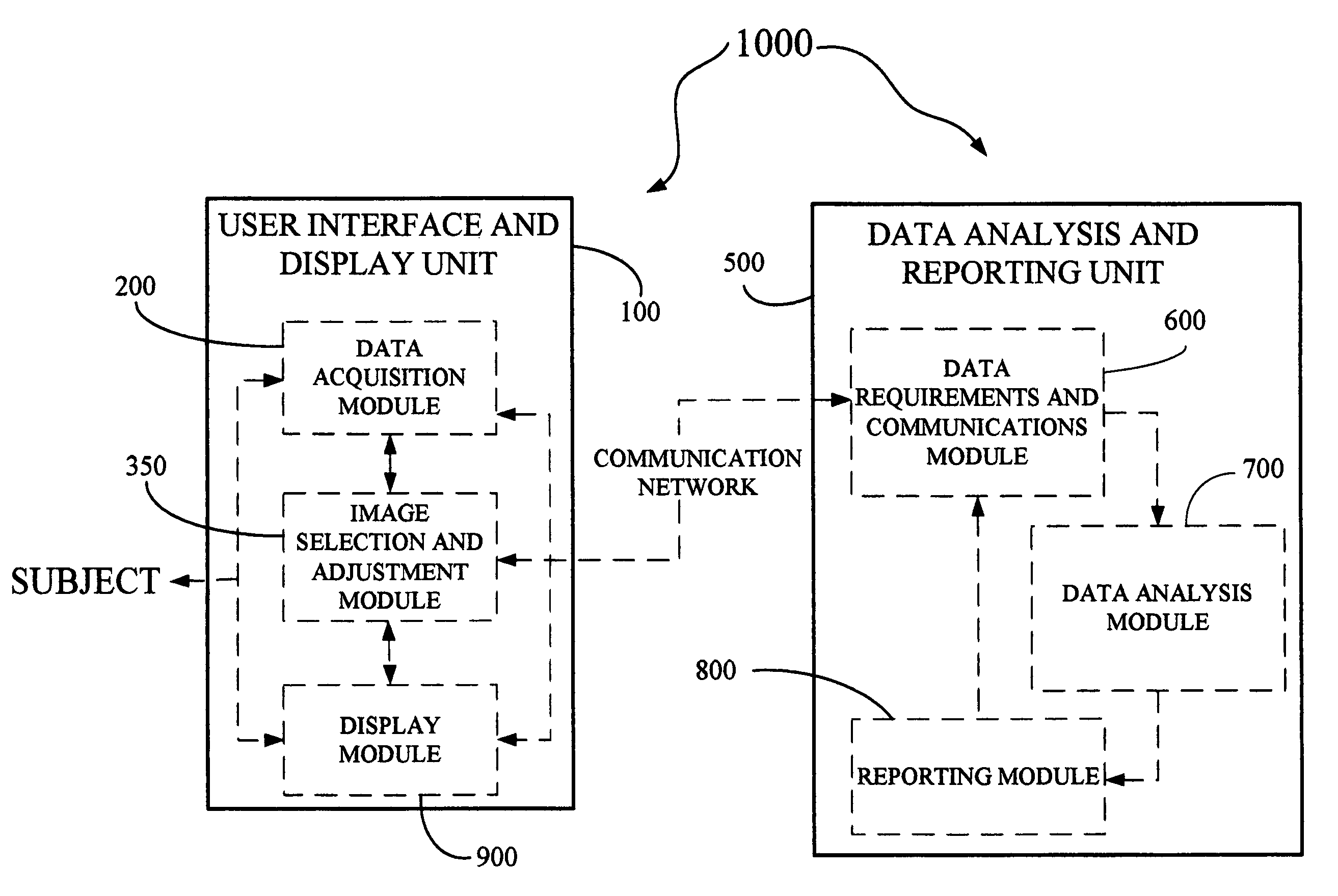
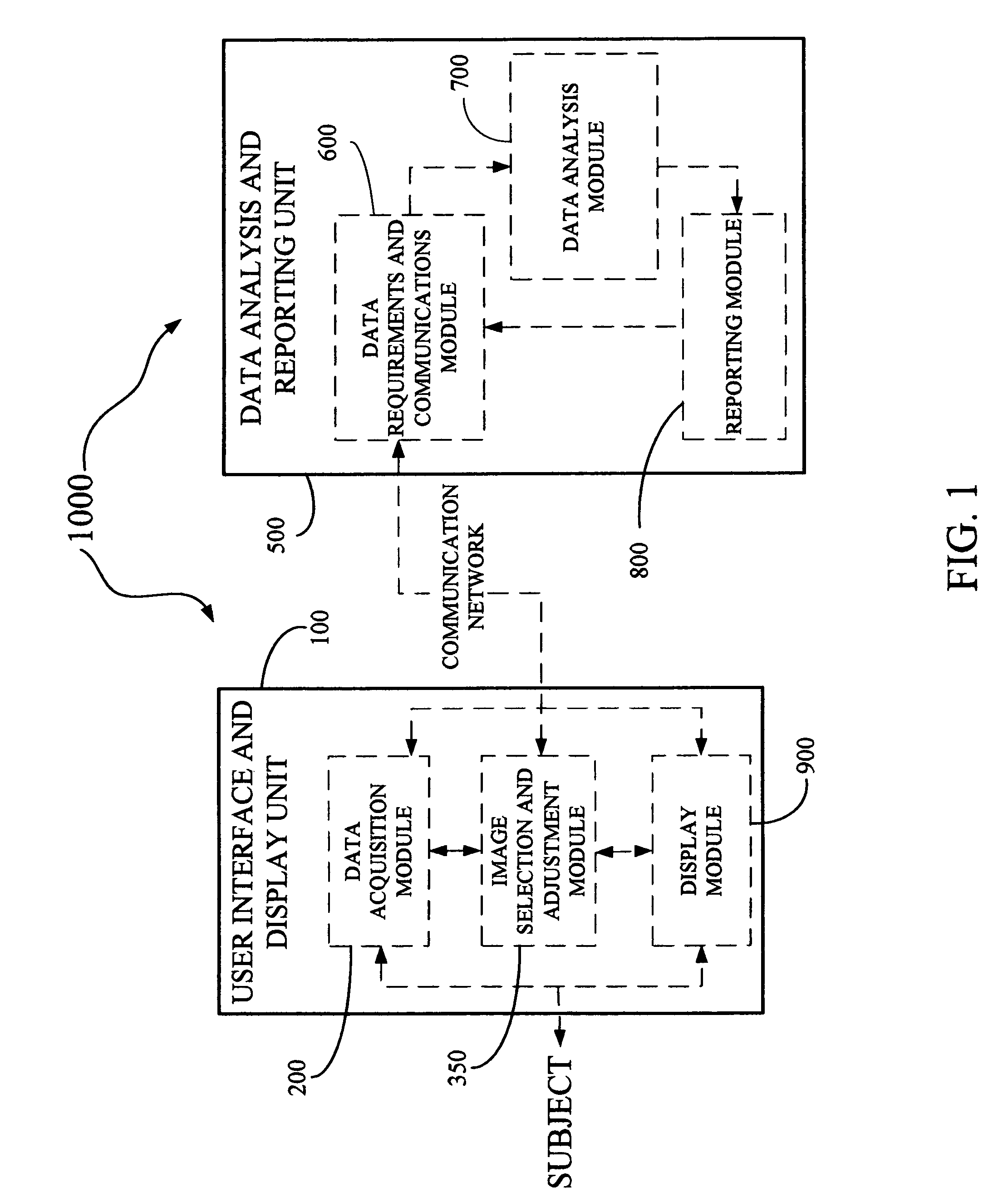
Derma diagnostic and automated data analysis system
ActiveUS20080161661A1Monitor effectivenessDiagnostics using lightDiagnostics using spectroscopySubject specificData analysis system
Disclosed is a derma diagnostic and automated data analysis system configured to acquire an array of data pertaining to a subject and their skin disorders. This data is subjected to a comprehensive analysis that culminates in diagnoses, treatments, monitoring activity and health strategy implementation. The derma diagnostic and automated data analysis system comprises a user interface and display unit, capable of acquiring a range of subject data, and communicatively coupled to a remotely located data analysis and reporting unit. The data analysis and reporting unit is capable of receiving and assessing the acquired data, using multiple analytical processes that culminate in a highly probable diagnosis and an effective treatment that is documented in a report and submitted to the subject. The diagnoses, treatments and supporting data are then permanently archived and made available for retrieval.
Owner:GIZEWSKI THEODORE M
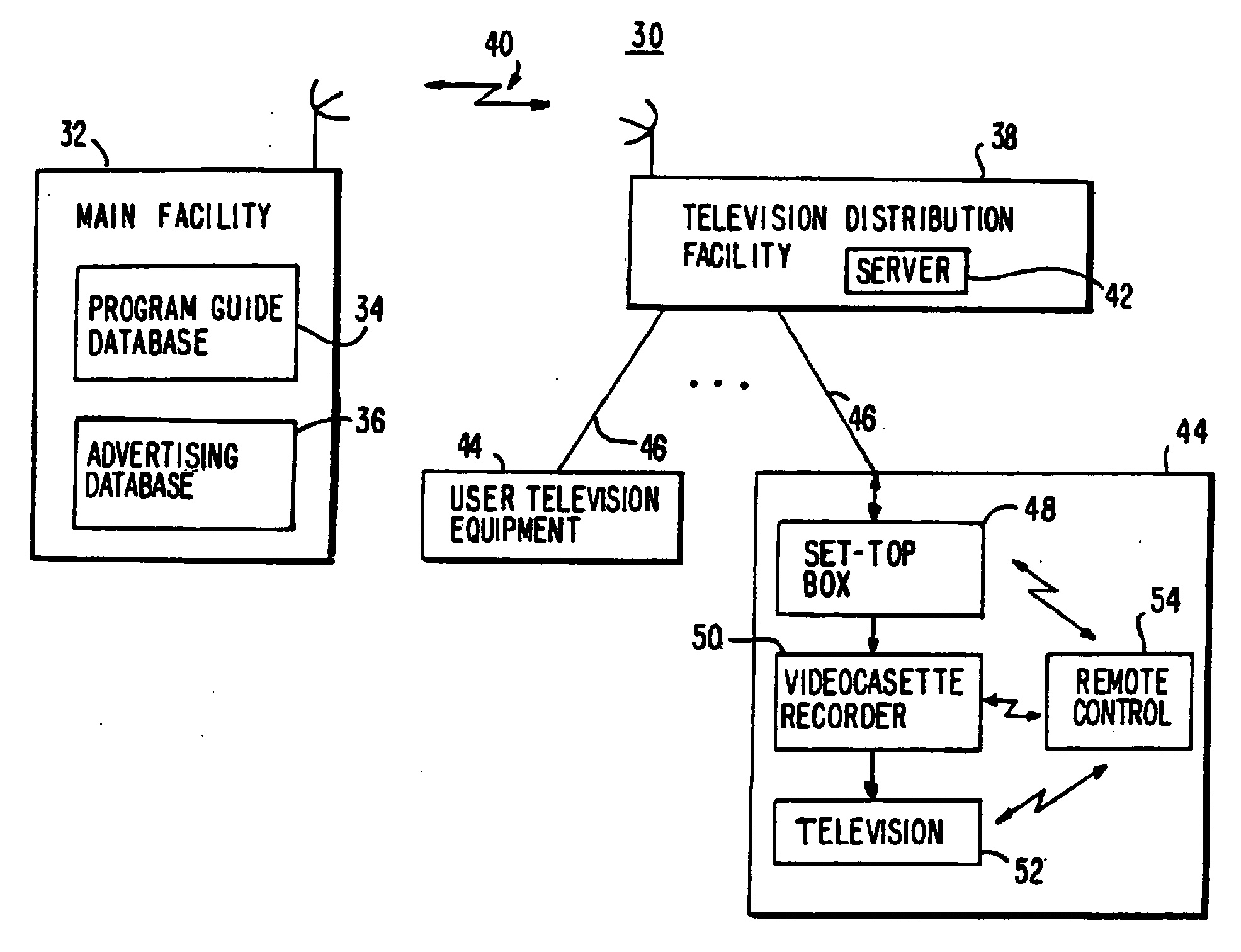
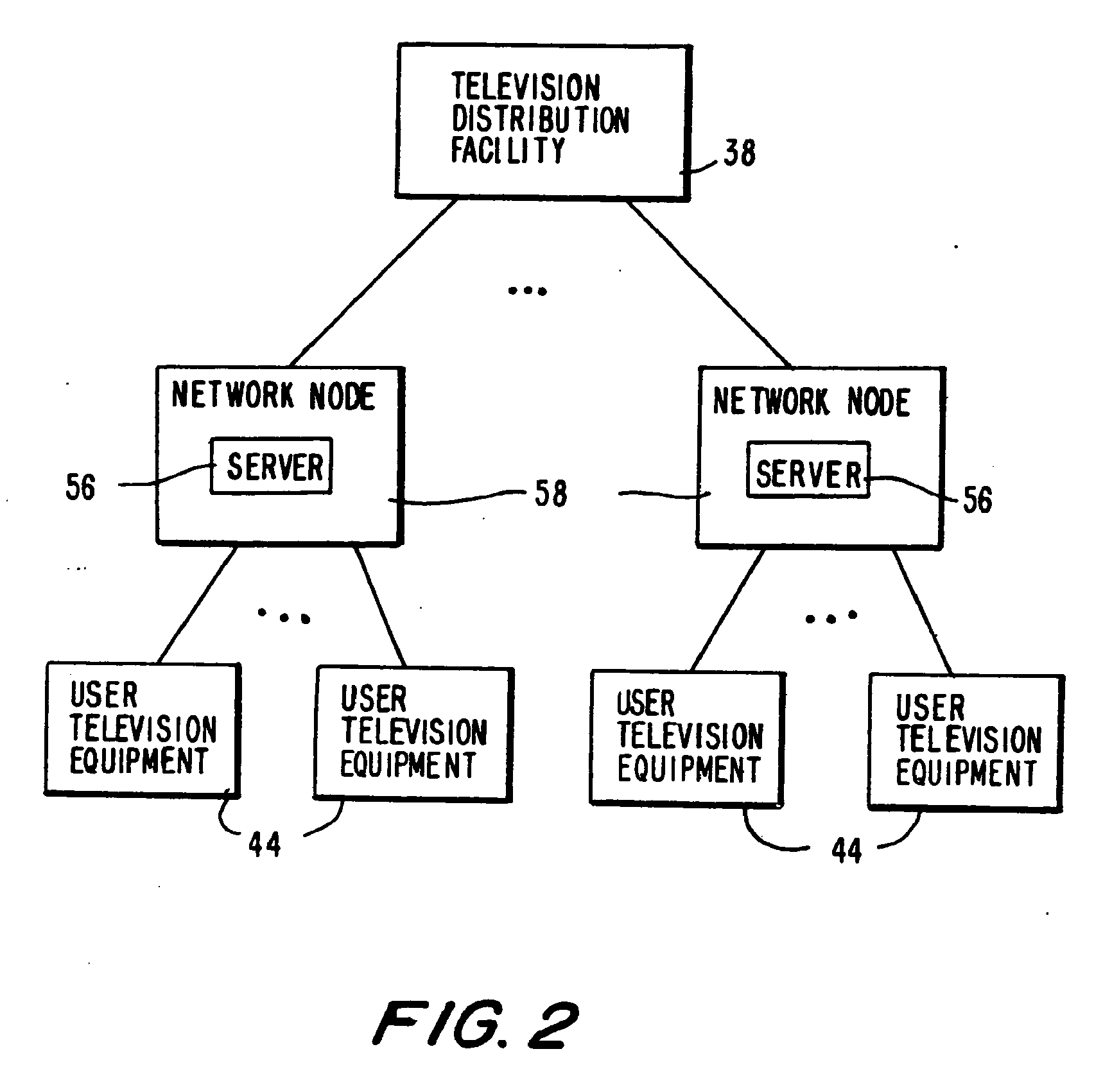
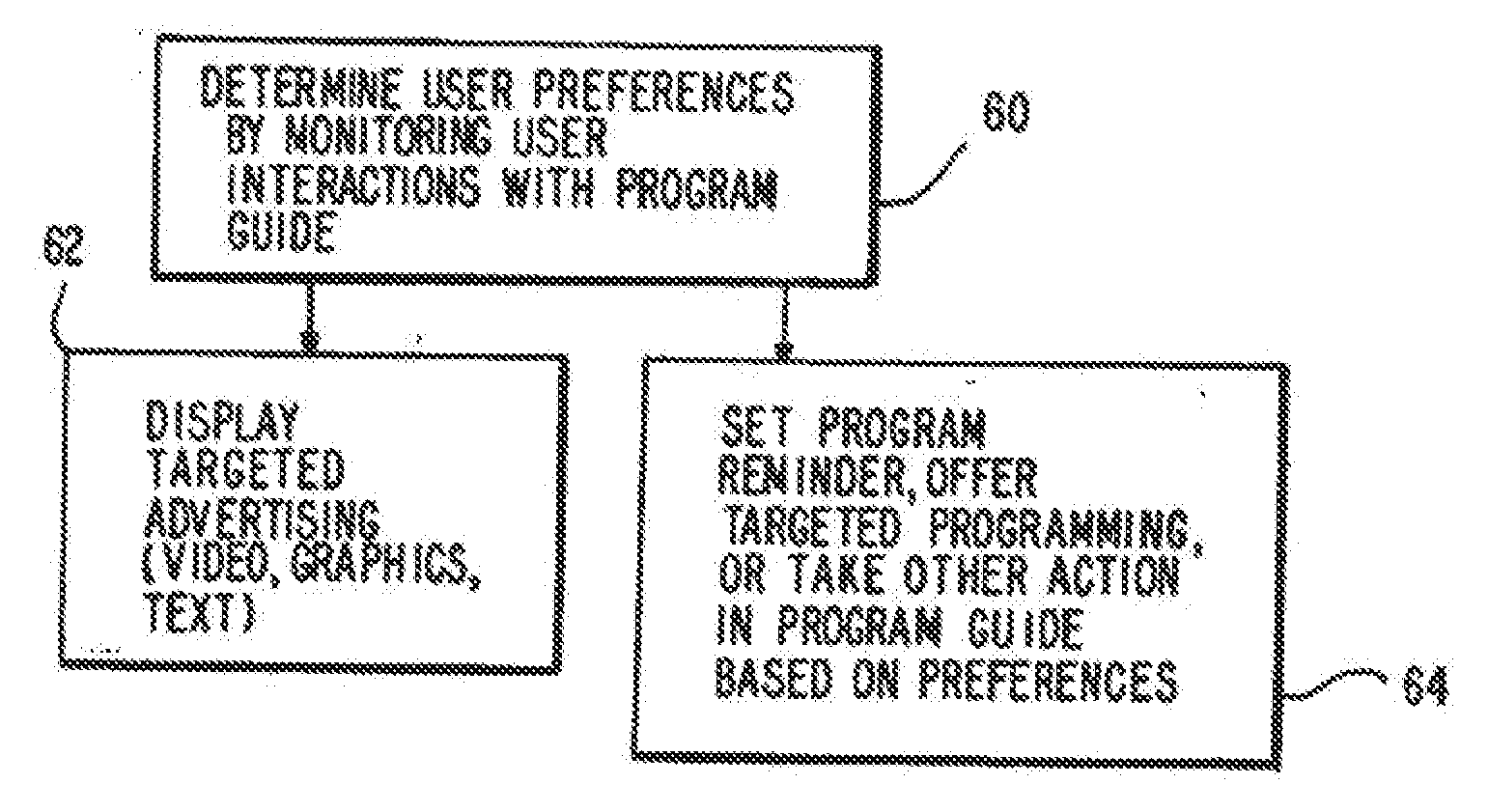
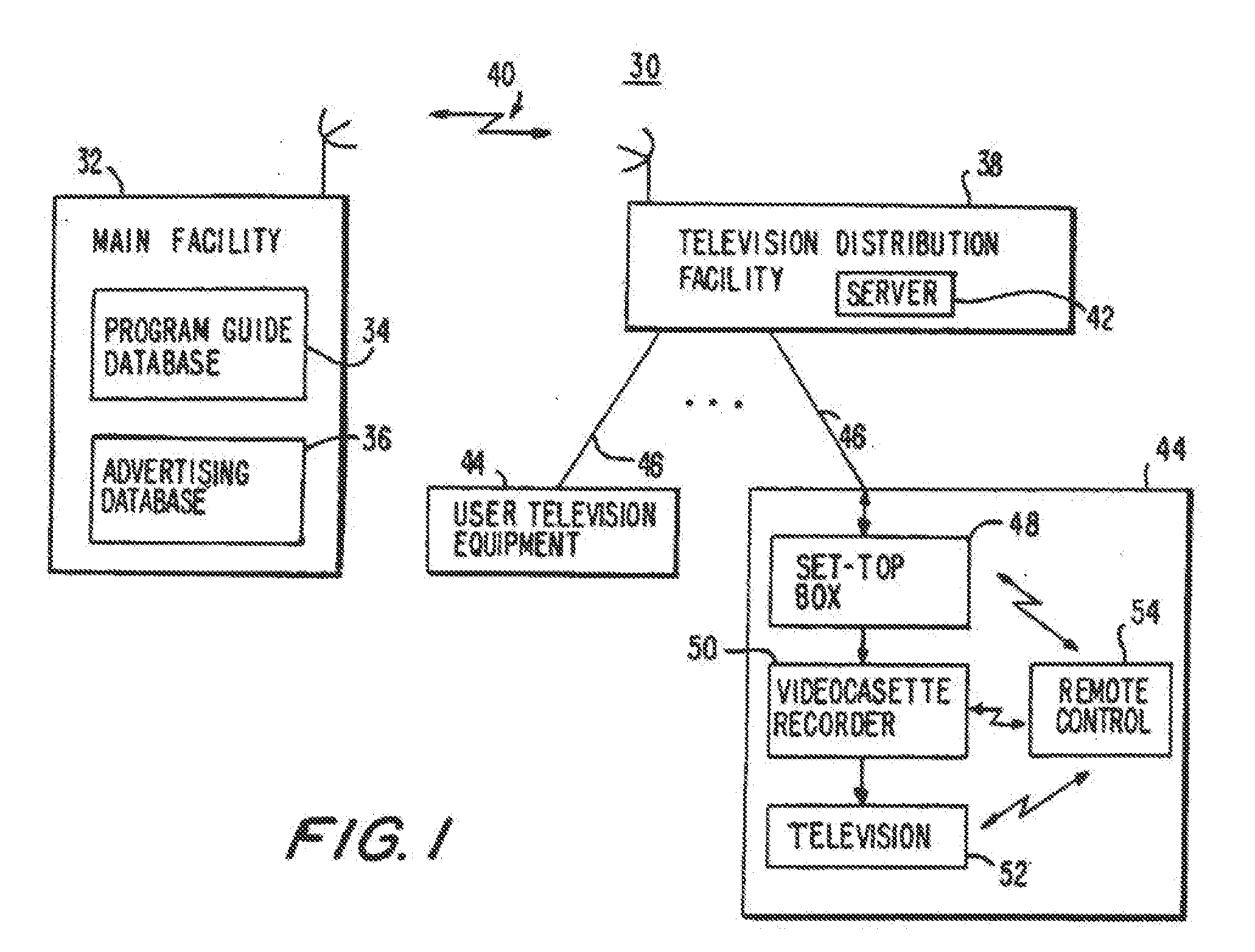
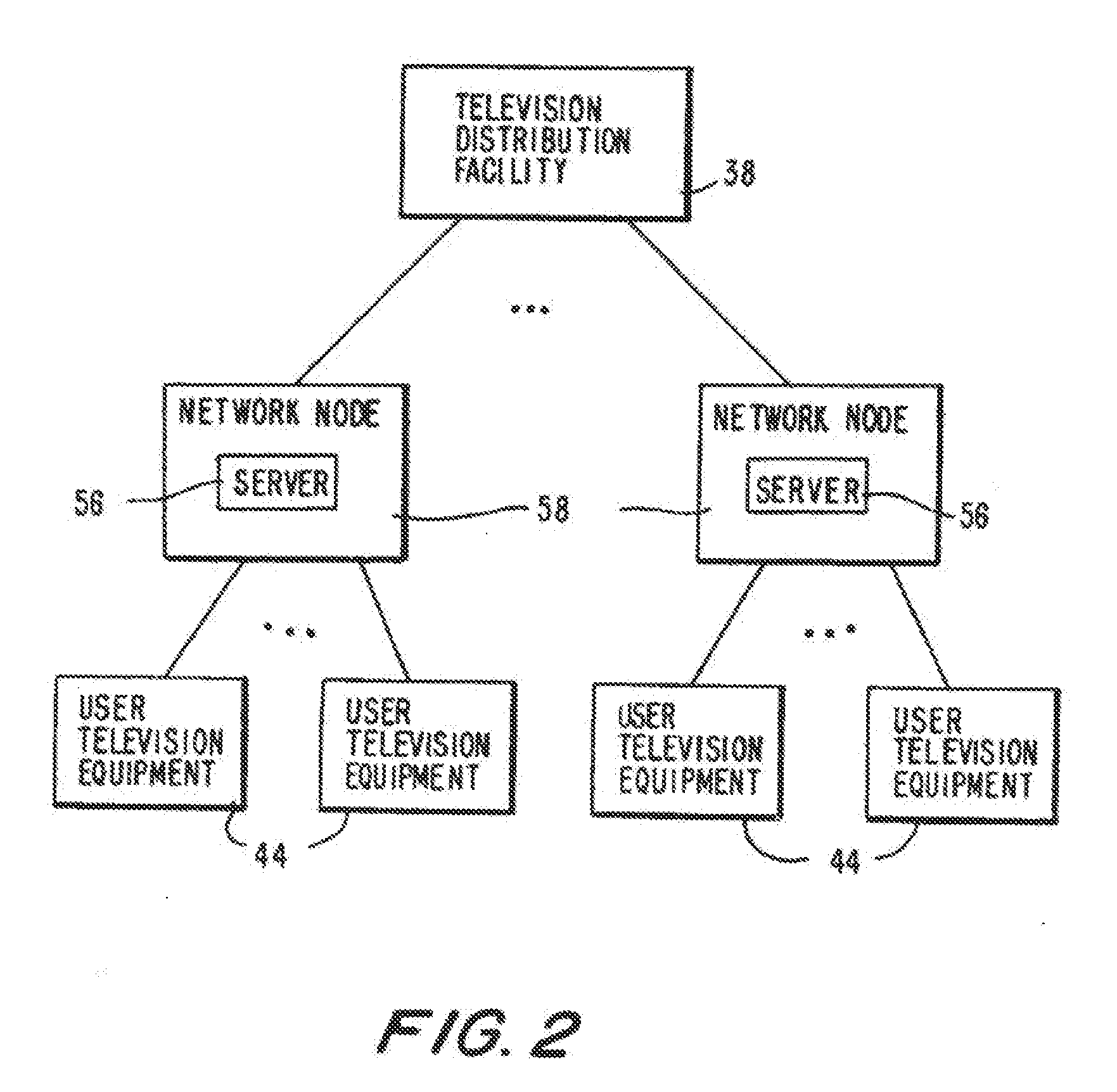
Program guide system with targeted advertising
InactiveUS20100175078A1Monitor effectivenessTelevision system detailsAnalogue secracy/subscription systemsInteractive televisionTarget–action
An interactive television program guide system is provided in which targeted advertisements may be presented to a user and targeted actions taken in the program guide based on the user's interests. The program guide monitors the user's interactions with the program guide to determine the user's interests. Interactions that may be monitored include interactions that indicate the categories of programming that interest the user (e.g., movies, sports, children's programming, etc.), setting a reminder for a program, purchasing a program, requesting information on a program, browsing program listings for a particular time or channel, etc.
Owner:ROVI GUIDES INC
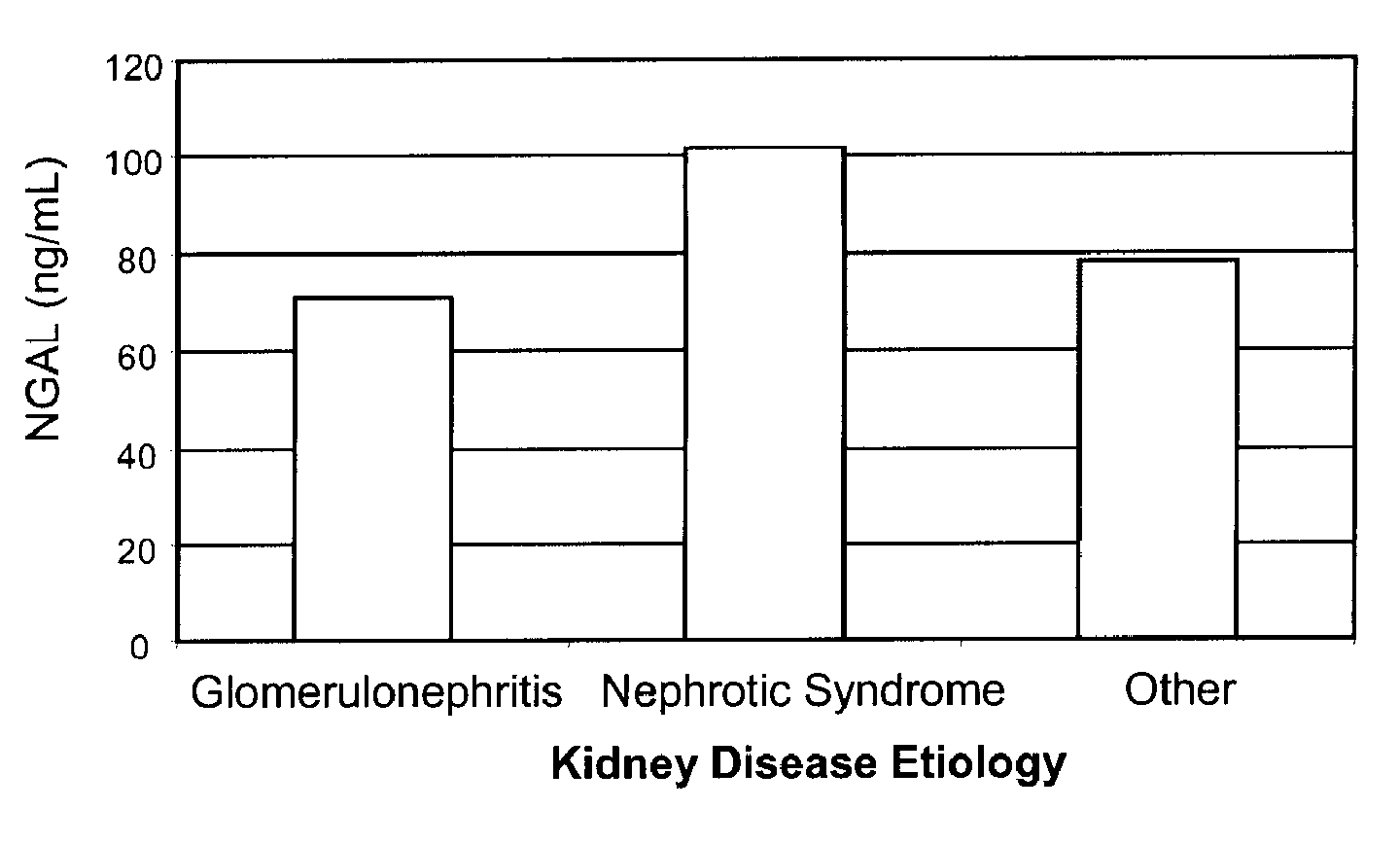
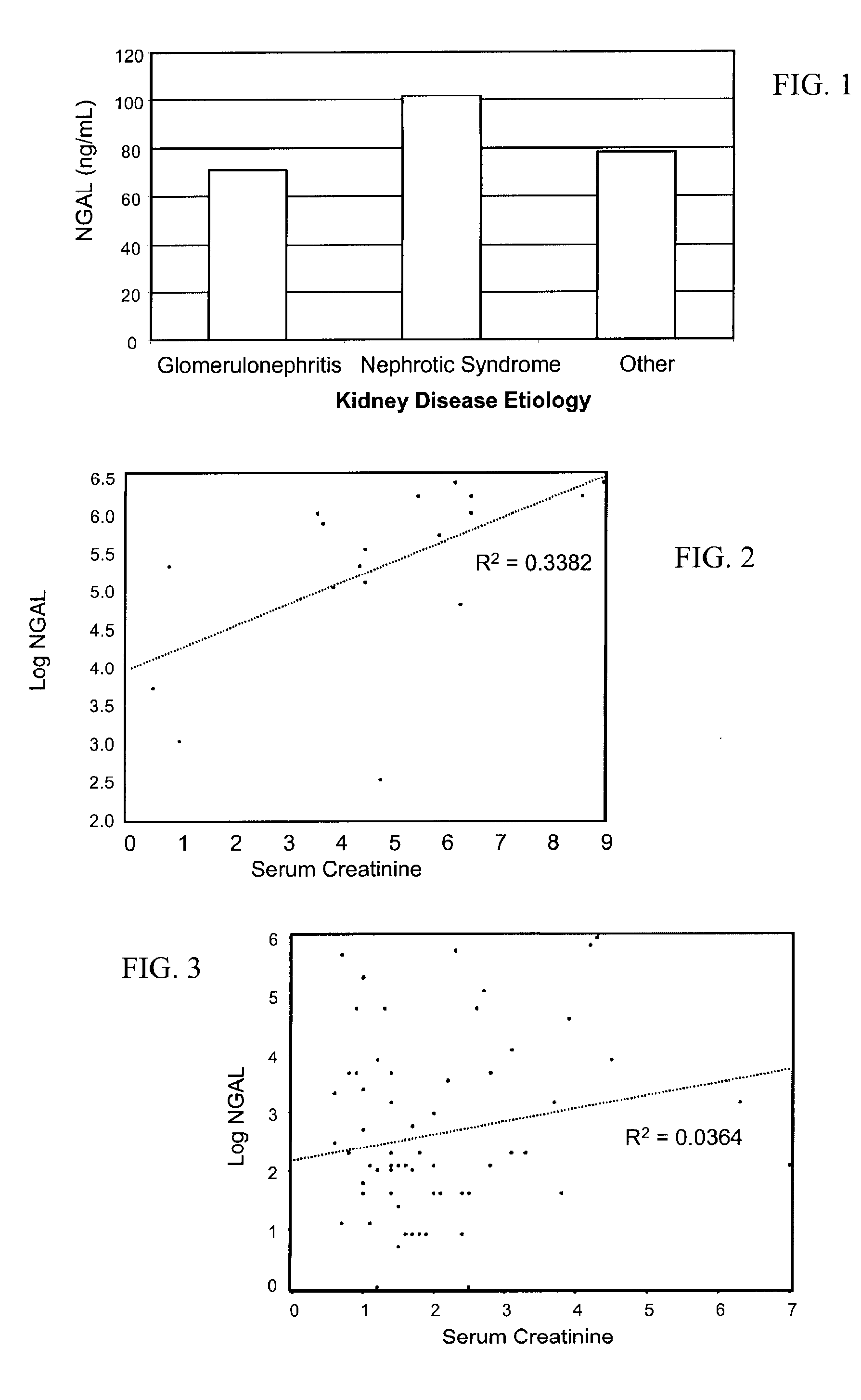
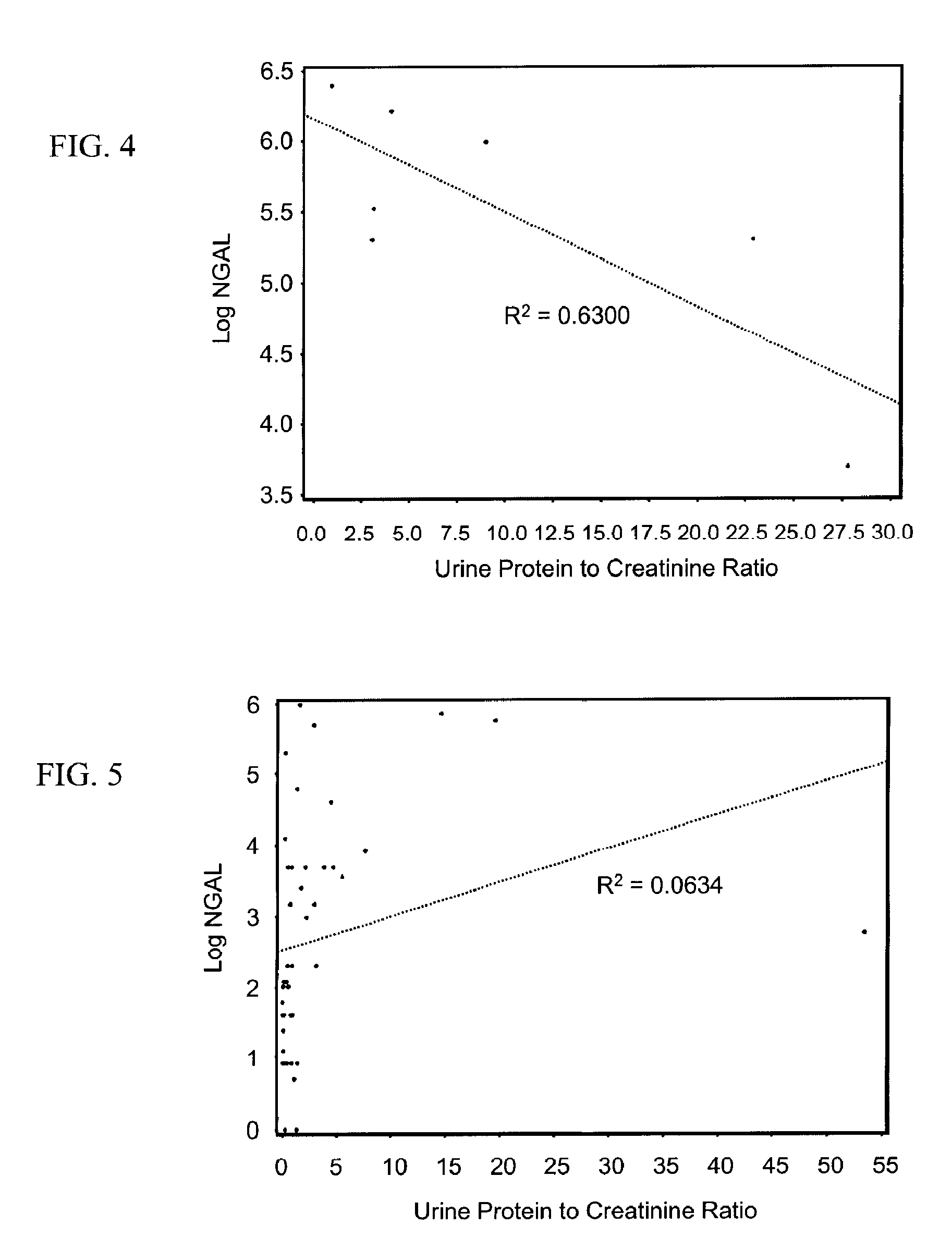
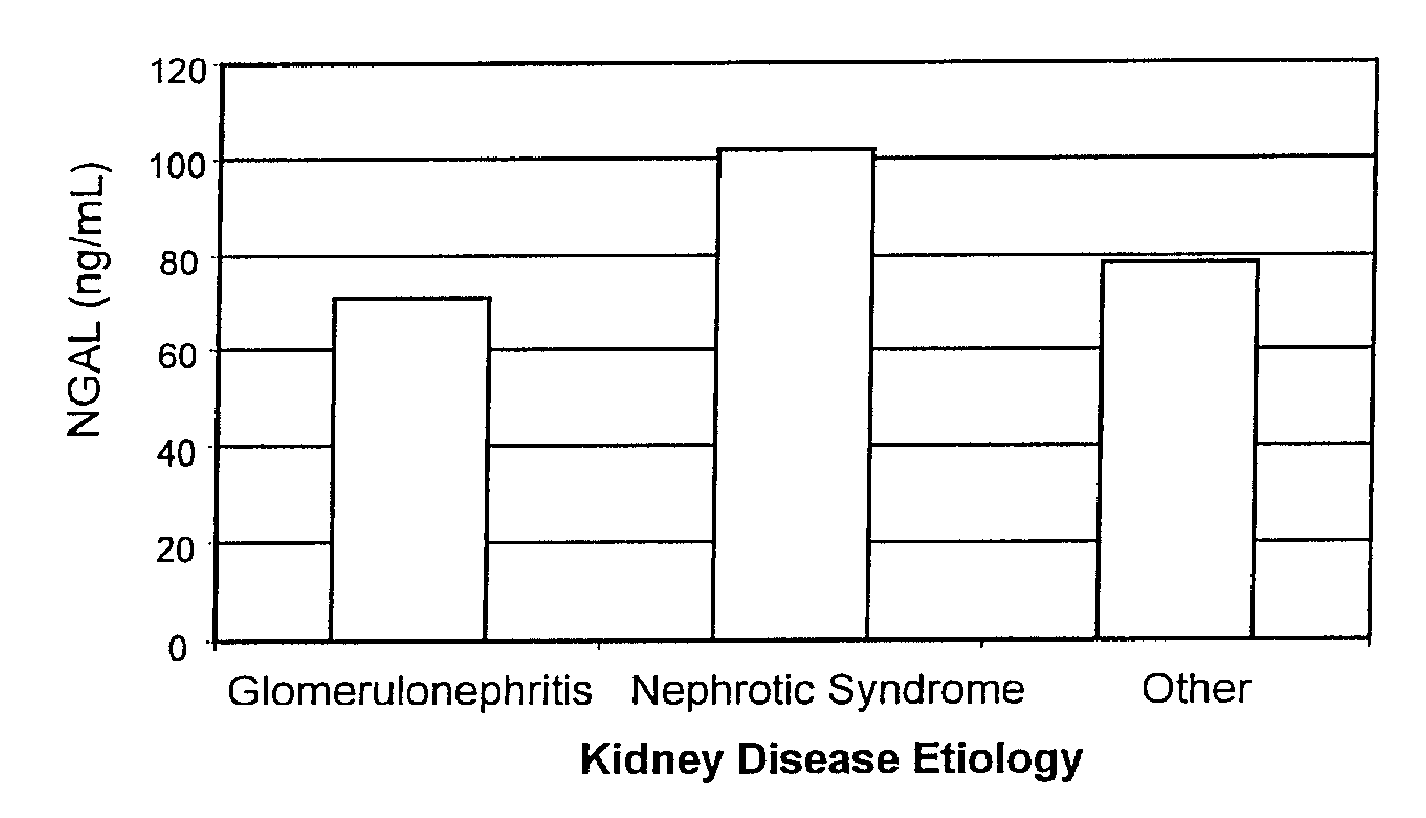
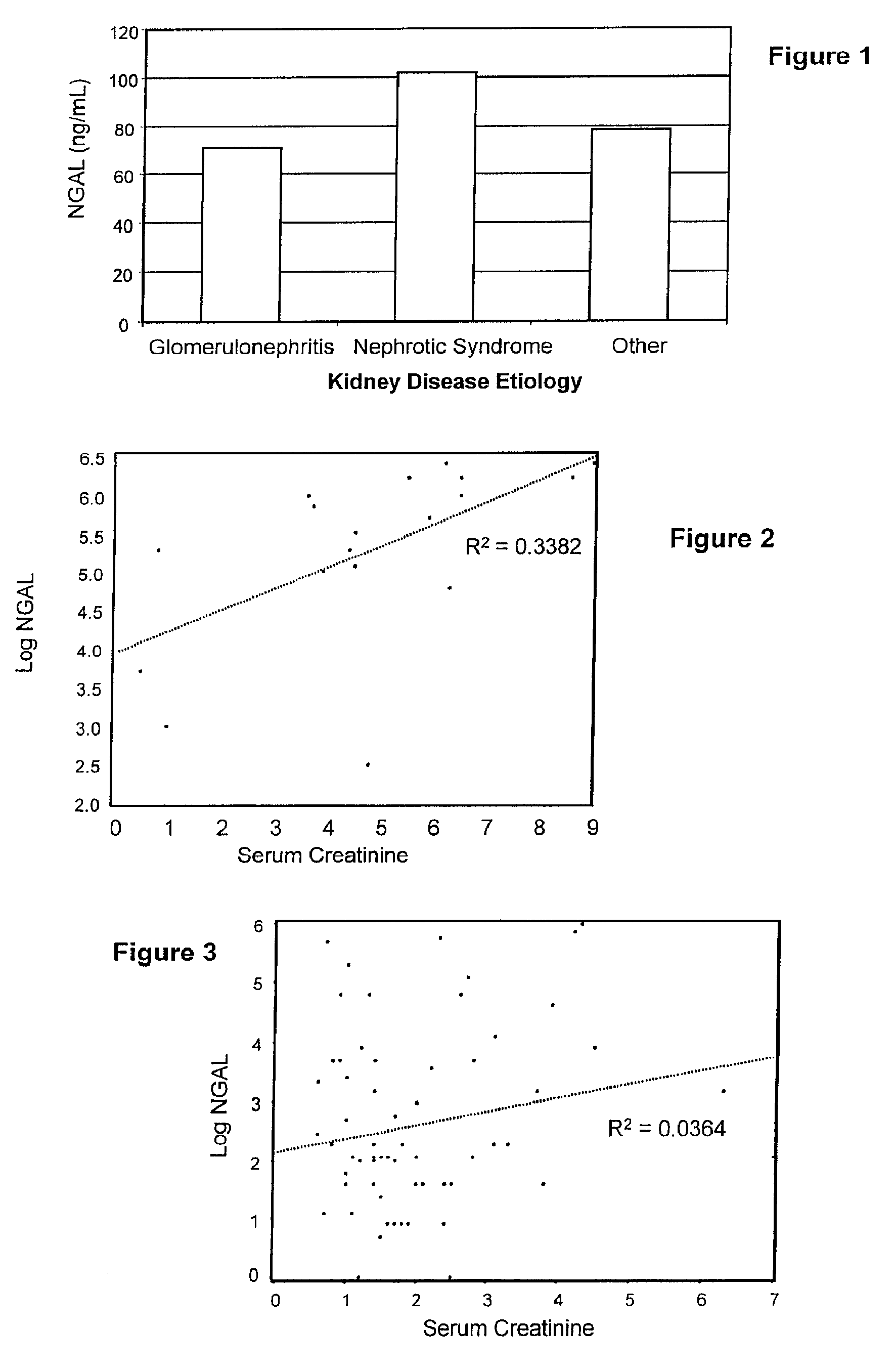
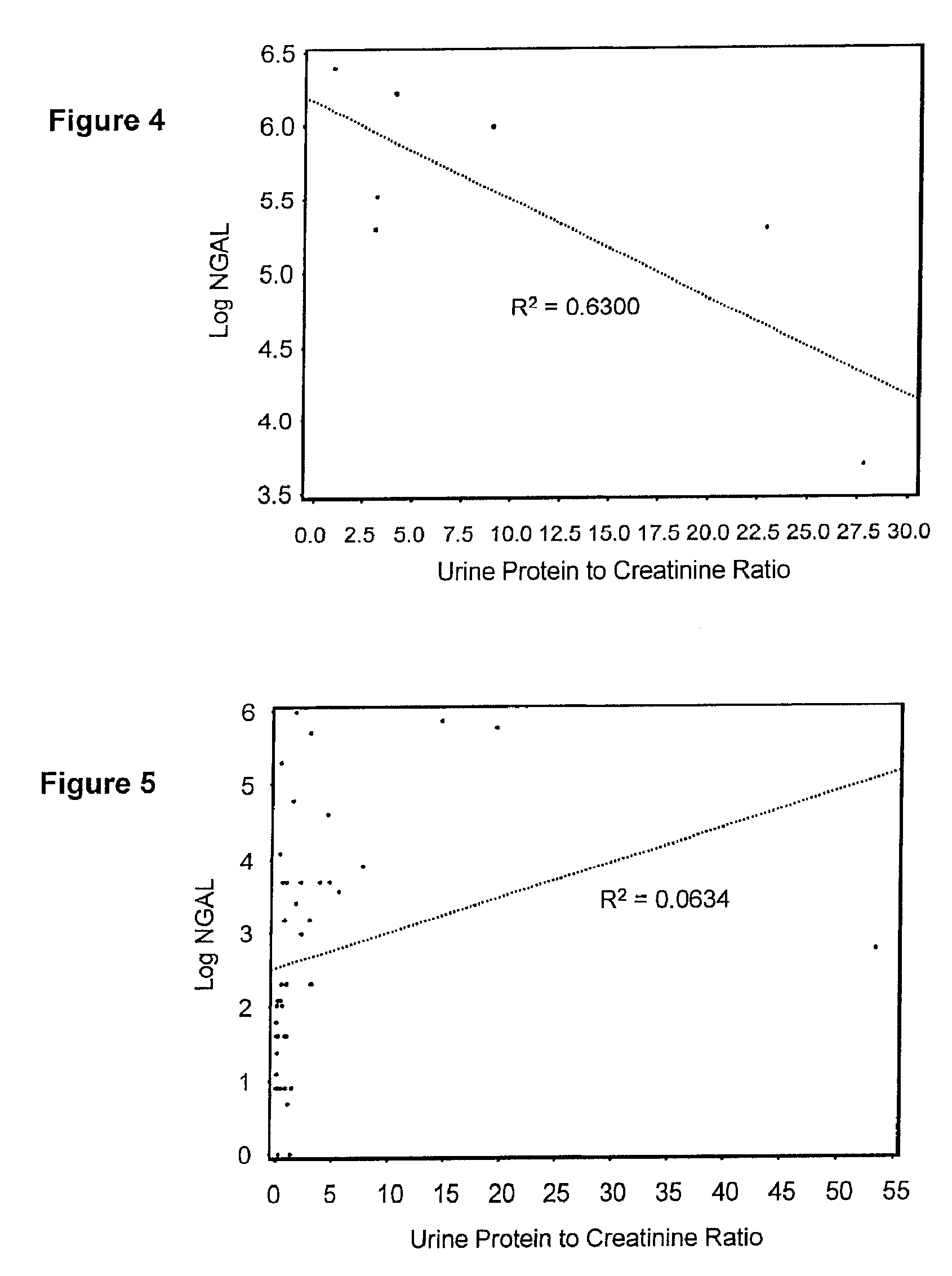
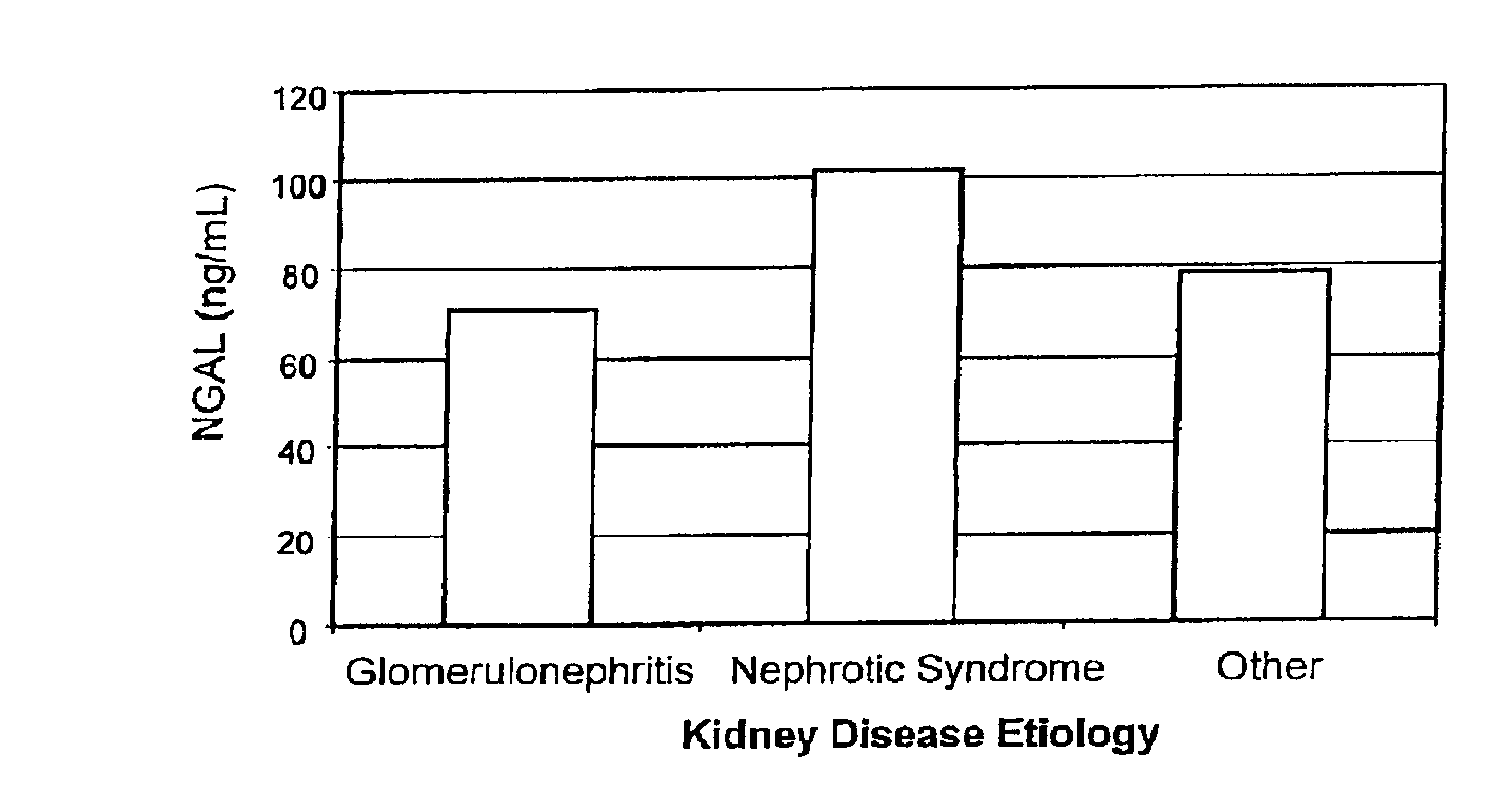
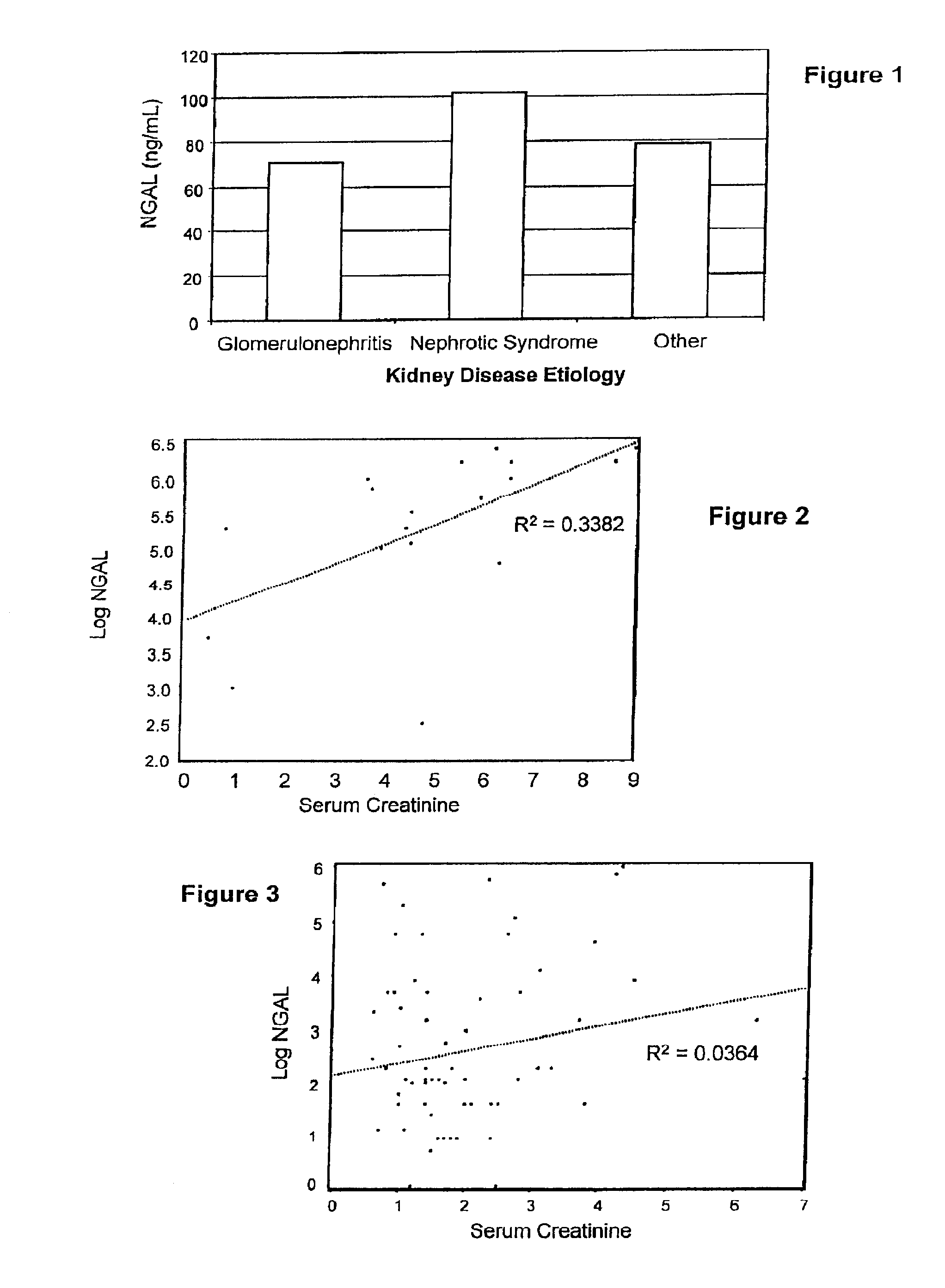
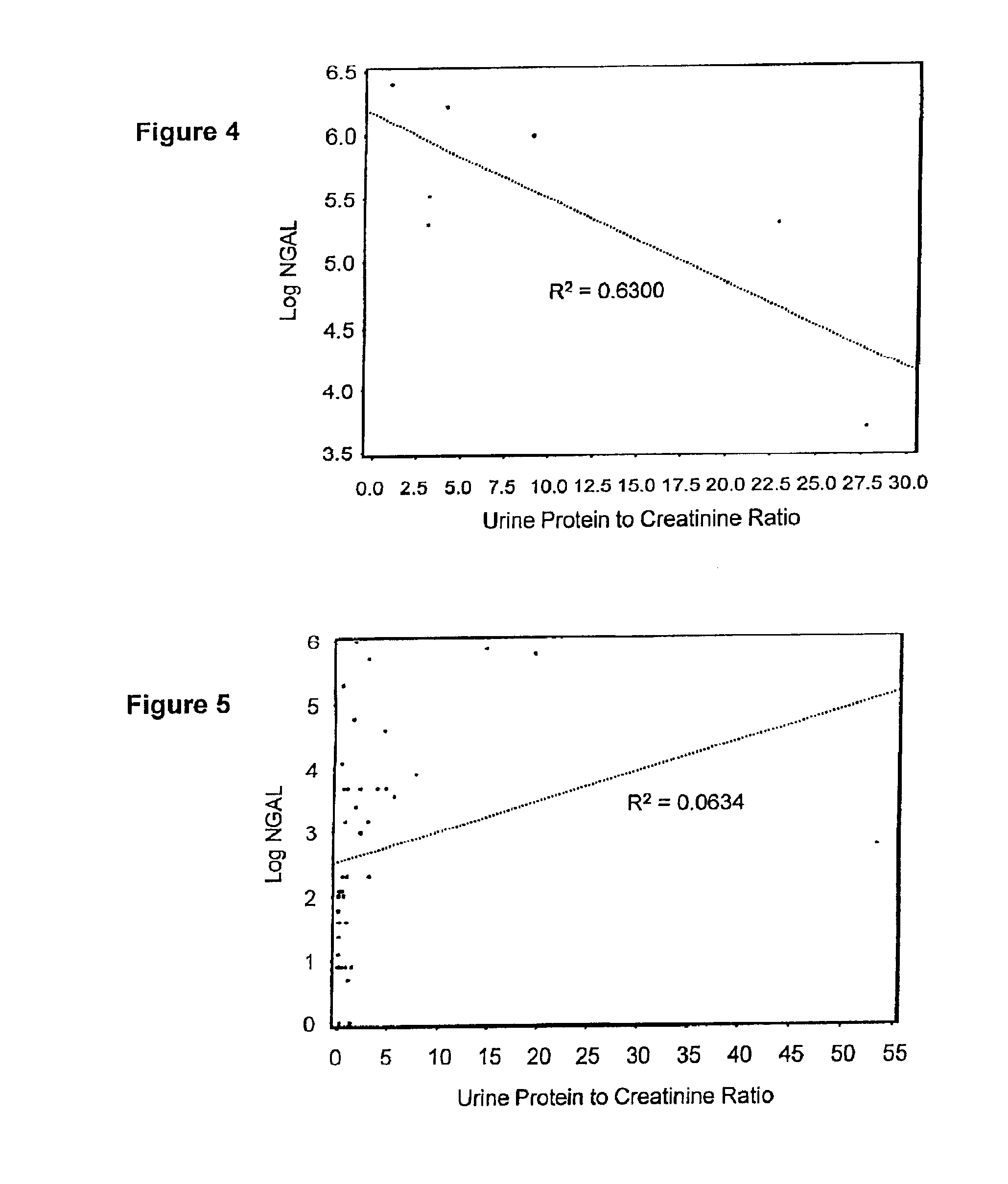
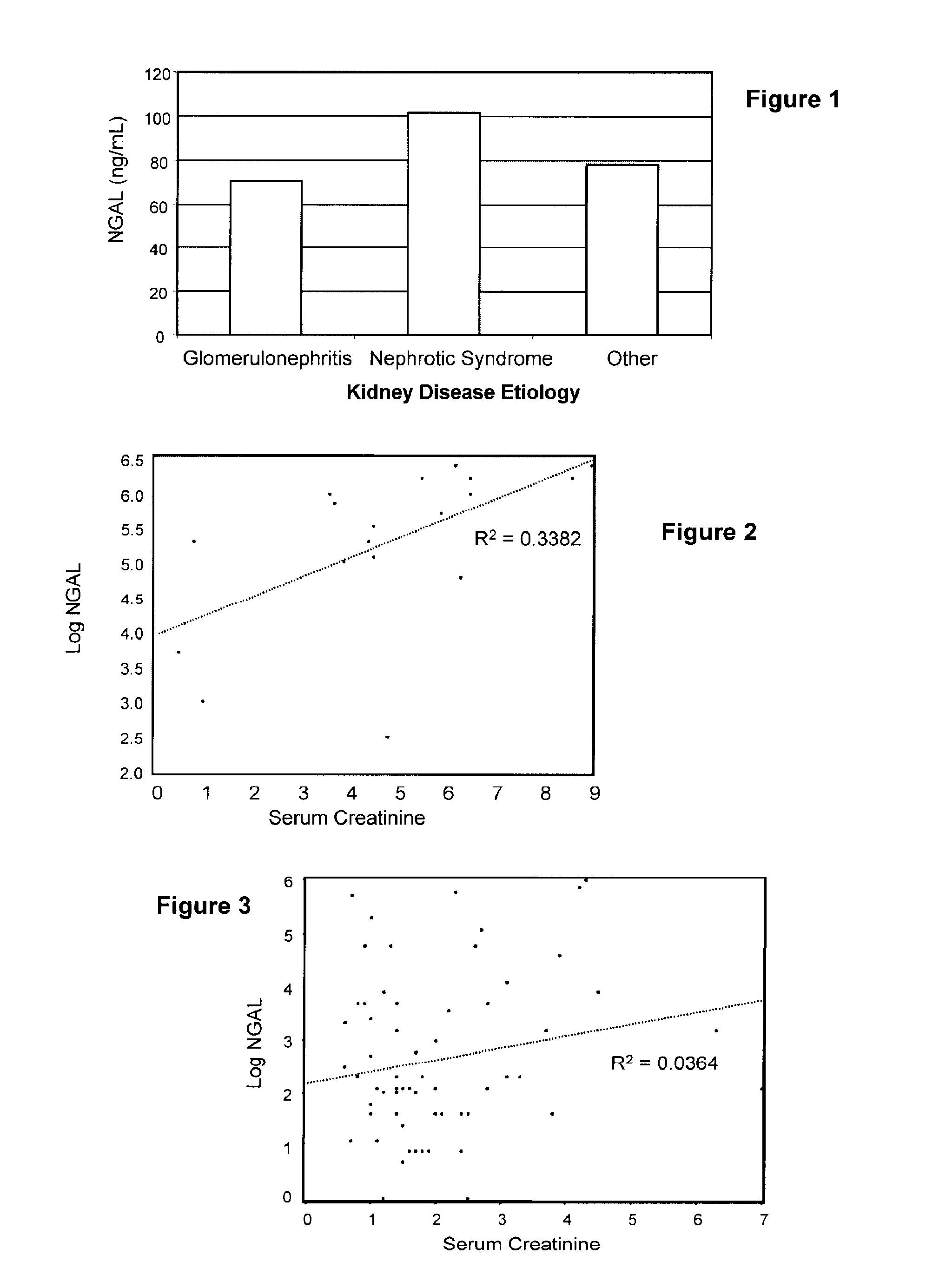
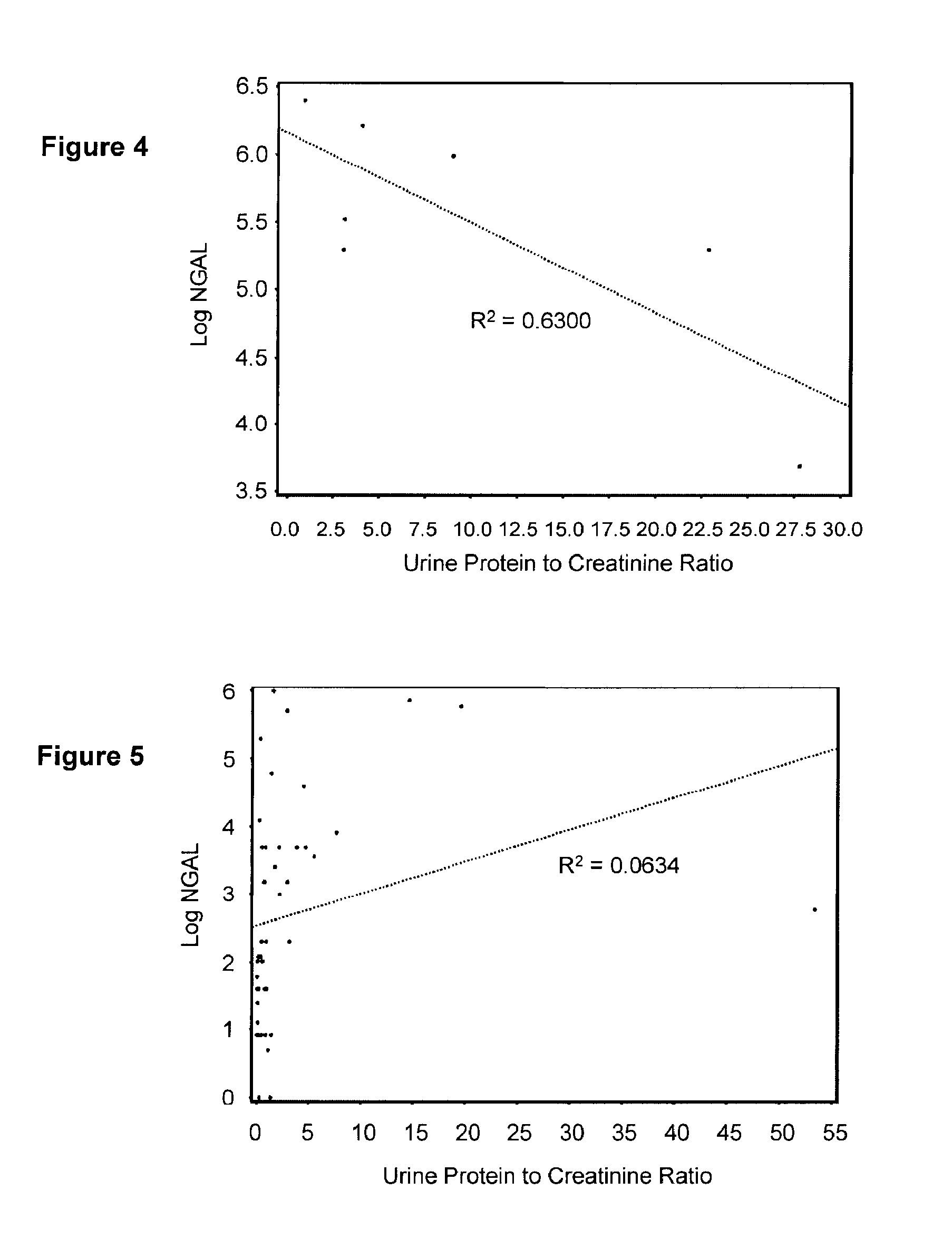
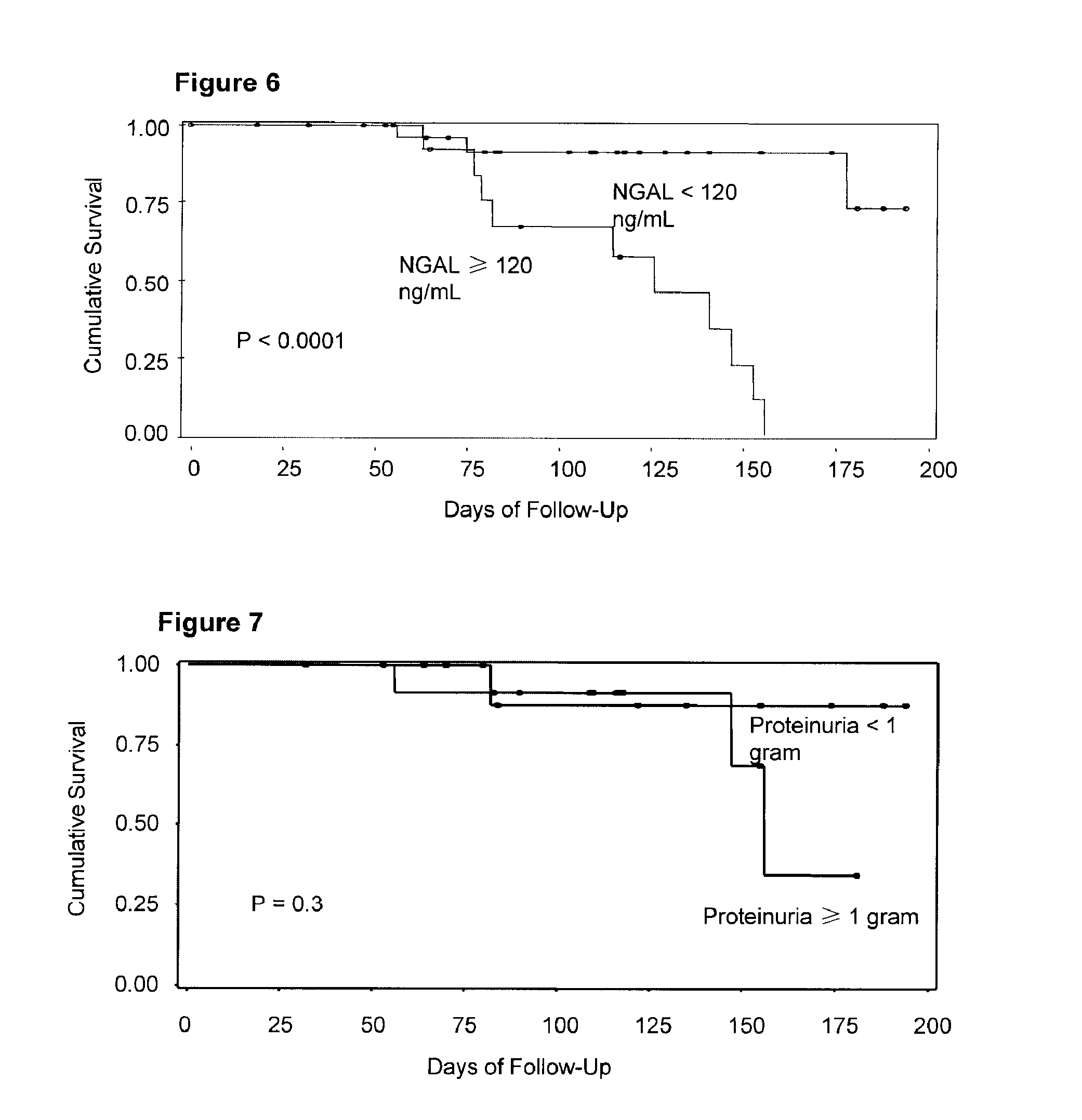
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20080090304A1Difficult to levelImprove the level ofDisease diagnosisBiological testingRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Interactive display system
InactiveUS20110288938A1Low costSuppression of theftCommerceBurglar alarm by hand-portable articles removalInteractive displaysSecurity level
The present disclosure is directed to a multiple security level display apparatus. The display apparatus may comprise a storage area; a display area adjoining the storage area; a product exhibit device for supporting at least one product, the product exhibit device being movable between a display position at least substantially within the display area, and a storage position within the storage area; a consumer-product interface for temporarily receiving at least one product removed from the product exhibit device; a movable storage area cover for separating the display area and the storage area; and a digital identification system configure for at least one of: controlling access to the display area; controlling a position of the product exhibit device; conducting an inventory including the at least one product; and monitoring a proximity of the at least one product.
Owner:STORE KRAFT
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20080014644A1Monitor effectivenessEarly detectionDisease diagnosisBiological testingRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
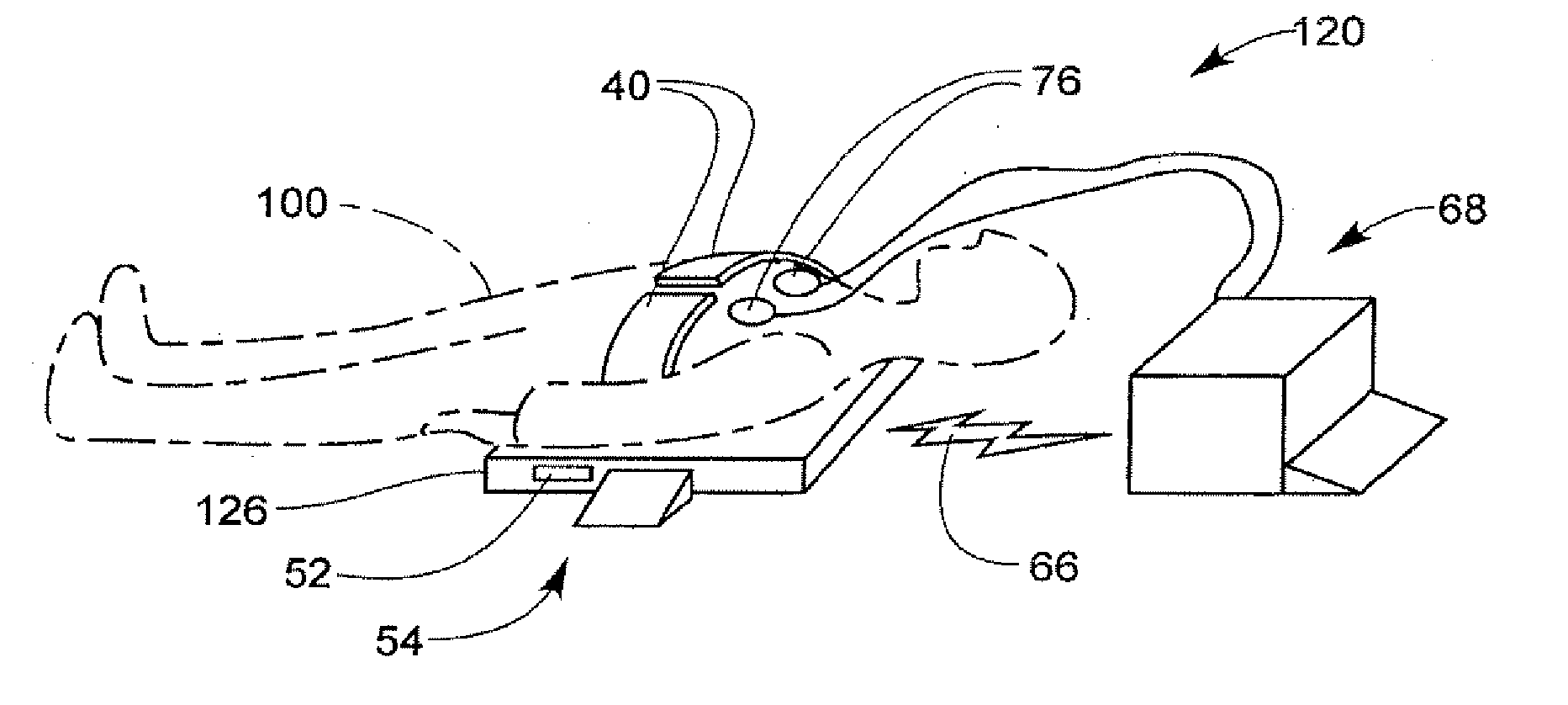
Cooperating defibrillators and external chest compression devices
ActiveUS7308304B2Optimize timingEffectiveness of chest compression could be monitoredRespiratorsDiagnosticsElectricityComputer module
Devices, methods, and software implementing those methods for providing communicating external chest compression (ECC) devices and defibrillation (DF) devices, where the ECC and DF devices can be physically separate from each other. Both ECC and DF devices are able to operate autonomously, yet able to communicate with and cooperate with another device when present. Some ECC and DF devices are adapted to be physically and / or electrically coupled to each other. One ECC device includes a backboard, a chest compression member, a communication module, controller, and at least one sensor, electrode lead or electrode. One DF device includes a defibrillator module, a controller, and a communication module that can communicate with the ECC communication module. The communicating ECC and DF devices may deliver ECC, pacing, defibrillation, ventilation, and cooling therapies, and may deliver instructions to human assistants, in a coordinated and cooperative fashion.
Owner:PHYSIO CONTROL INC
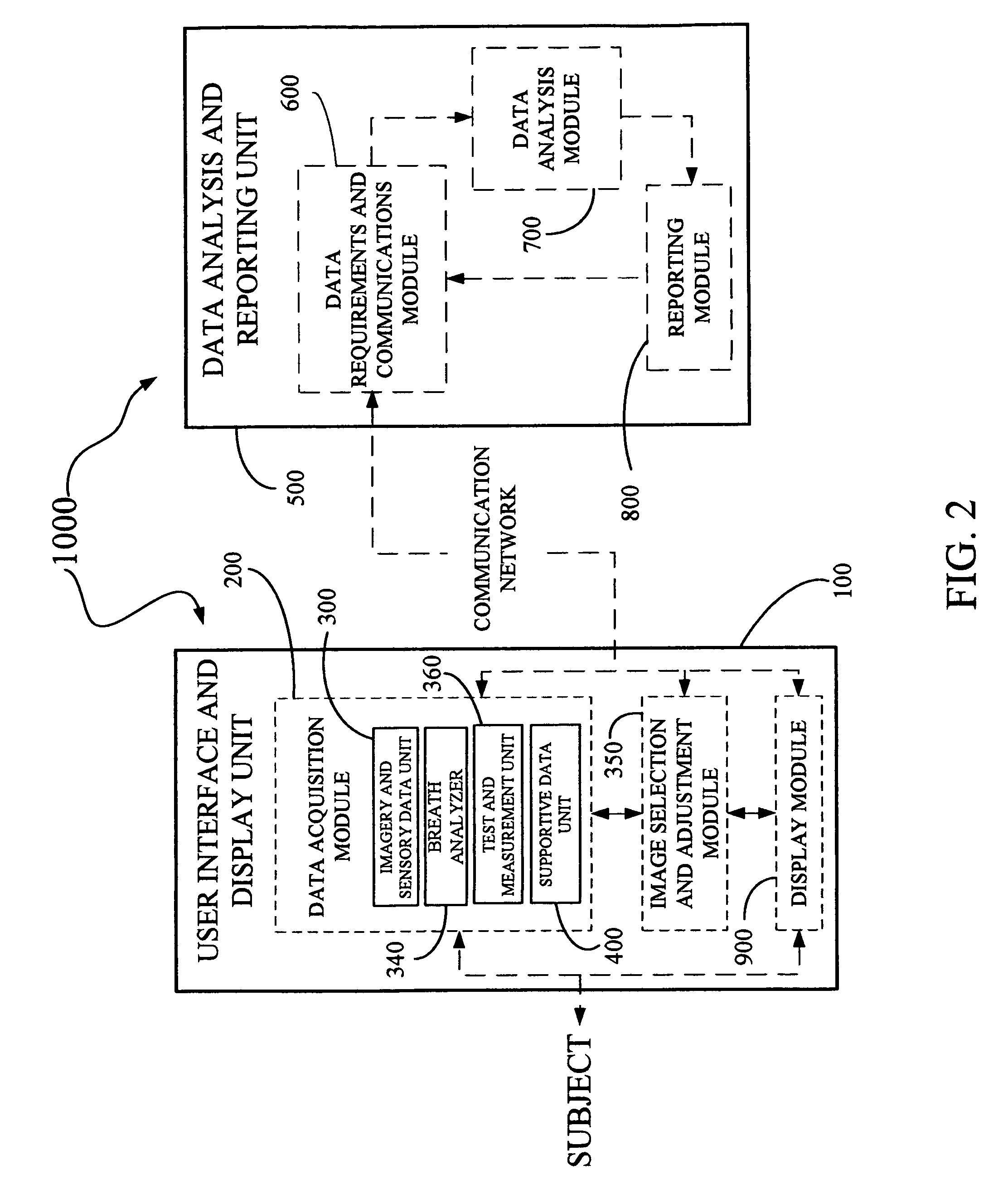
Derma diagnostic and automated data analysis system
ActiveUS8109875B2Monitor effectivenessDiagnostics using lightDiagnostics using spectroscopyData analysis systemData profiling
Disclosed is a derma diagnostic and automated data analysis system configured to acquire an array of data pertaining to a subject and their skin disorders. This data is subjected to a comprehensive analysis that culminates in diagnoses, treatments, monitoring activity and health strategy implementation. The derma diagnostic and automated data analysis system comprises a user interface and display unit, capable of acquiring a range of subject data, and communicatively coupled to a remotely located data analysis and reporting unit. The data analysis and reporting unit is capable of receiving and assessing the acquired data, using multiple analytical processes that culminate in a highly probable diagnosis and an effective treatment that is documented in a report and submitted to the subject. The diagnoses, treatments and supporting data are then permanently archived and made available for retrieval.
Owner:GIZEWSKI THEODORE M
Rapid multiple panel of biomarkers in laboratory blood tests for TIA/stroke
InactiveUS6896872B2Diagnosing the progression of TIA orMonitor effectivenessImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsNR1 NMDA receptorNMDA receptor
A methods, kits and compositions for diagnosing a central nervous system disorder, particularly transient ischemic attack or stroke, comprising measuring the level of NR2A and / or NR2B NMDA receptor or fragment thereof, in a biological sample from a human subject, and optionally measuring other biomarkers such as homocysteine and glutamate. The method is particularly useful for identifying individuals that are at risk for stroke, and for diagnosing stroke in an emergency room setting.
Owner:CIS BIOTECH
Ultraviolet water purification system
ActiveUS7862728B2Improve efficiencyEffective treatmentSamplingExhaust apparatusNODALWireless mesh network
An Ultraviolet-C (UVC) based portable water purification system employing a novel array of baffles increases the efficiency per unit energy of irradiating UVC light in the eradication of pathogens in the water. Closed loop feedback allows monitoring the application of UVC light power to ensure high levels of pathogen eradication. This system is capable of eradicating a wide range of waterborne bacteria, viruses, protozoa, helminthes, yeast, and mold found in natural freshwater sources worldwide. By adding pre- or post-filters, the system can remove harmful organic compounds, pesticides, inorganic compounds and heavy metals from the water. The system can also be used to eradicate pathogens in fluids other than water. As a feature of this invention, a communications systems that can reach geographically dispersed populations at low cost without the need to install costly wired communications infrastructure is combined with and powered by the water purification system. In one embodiment, a packet radio system is provided to create nodes in a wireless mesh communications system to provide voice, data, video and internet communications using an array of the water purifiers to create a wireless mesh network.
Owner:WATER OF LIFE
Program guide system with targeted advertising
InactiveUS20100319013A1Monitor effectivenessTelevision system detailsAnalogue secracy/subscription systemsProgram planningInteractive television
An interactive television program guide system is provided in which targeted advertisements may be presented to a user and targeted actions taken in the program guide based on the user's interests. The program guide monitors the user's interactions with the program guide to determine the user's interests. Interactions that may be monitored include interactions that indicate the categories of programming that interest the user (e.g., movies, sports, children's programming, etc.), setting a reminder for a program, purchasing a program, requesting information on a program, browsing program listings for a particular time or channel, etc.
Owner:UNITED VIDEO PROPERTIES
Insulin-mediated glucose uptake monitor
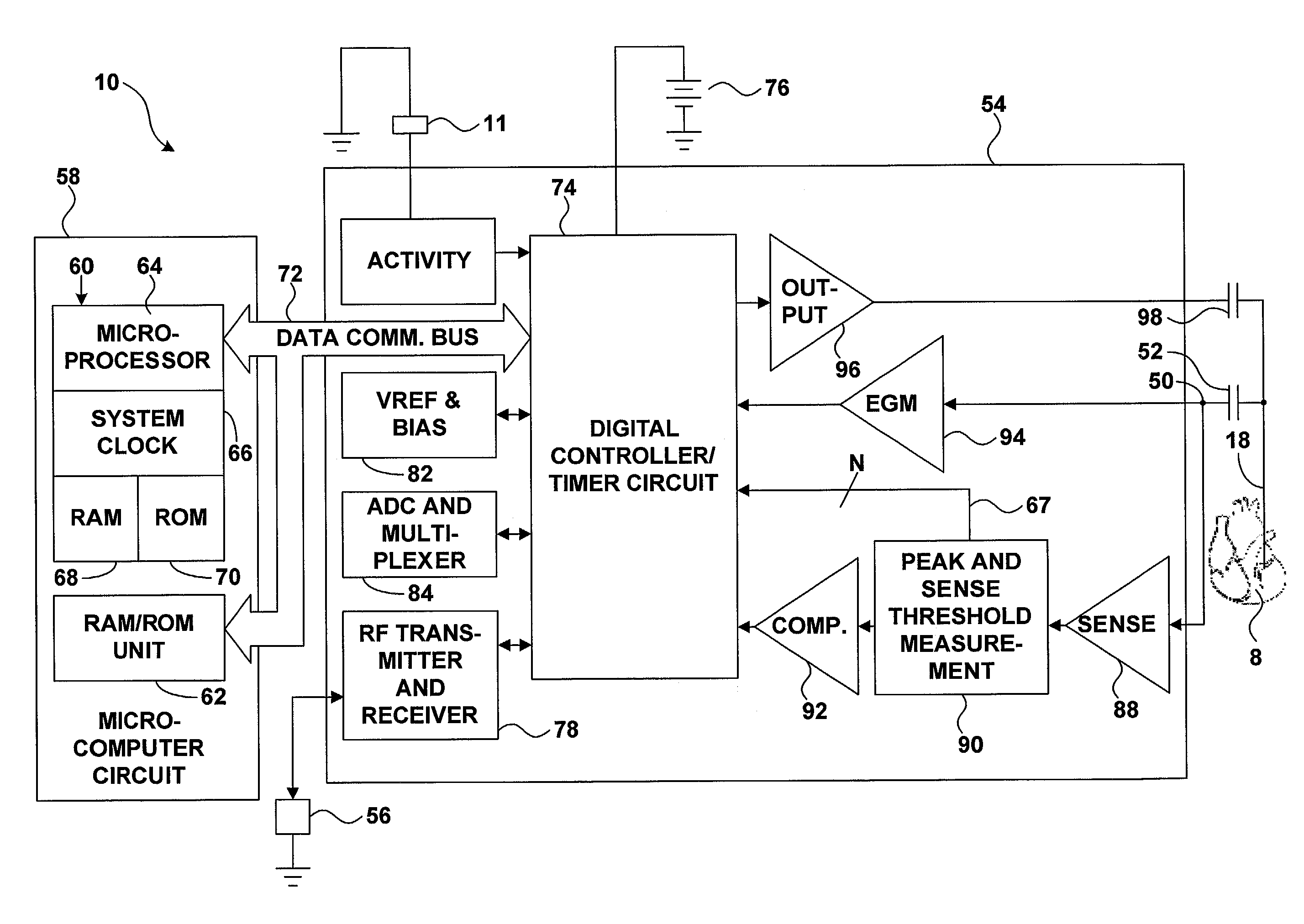
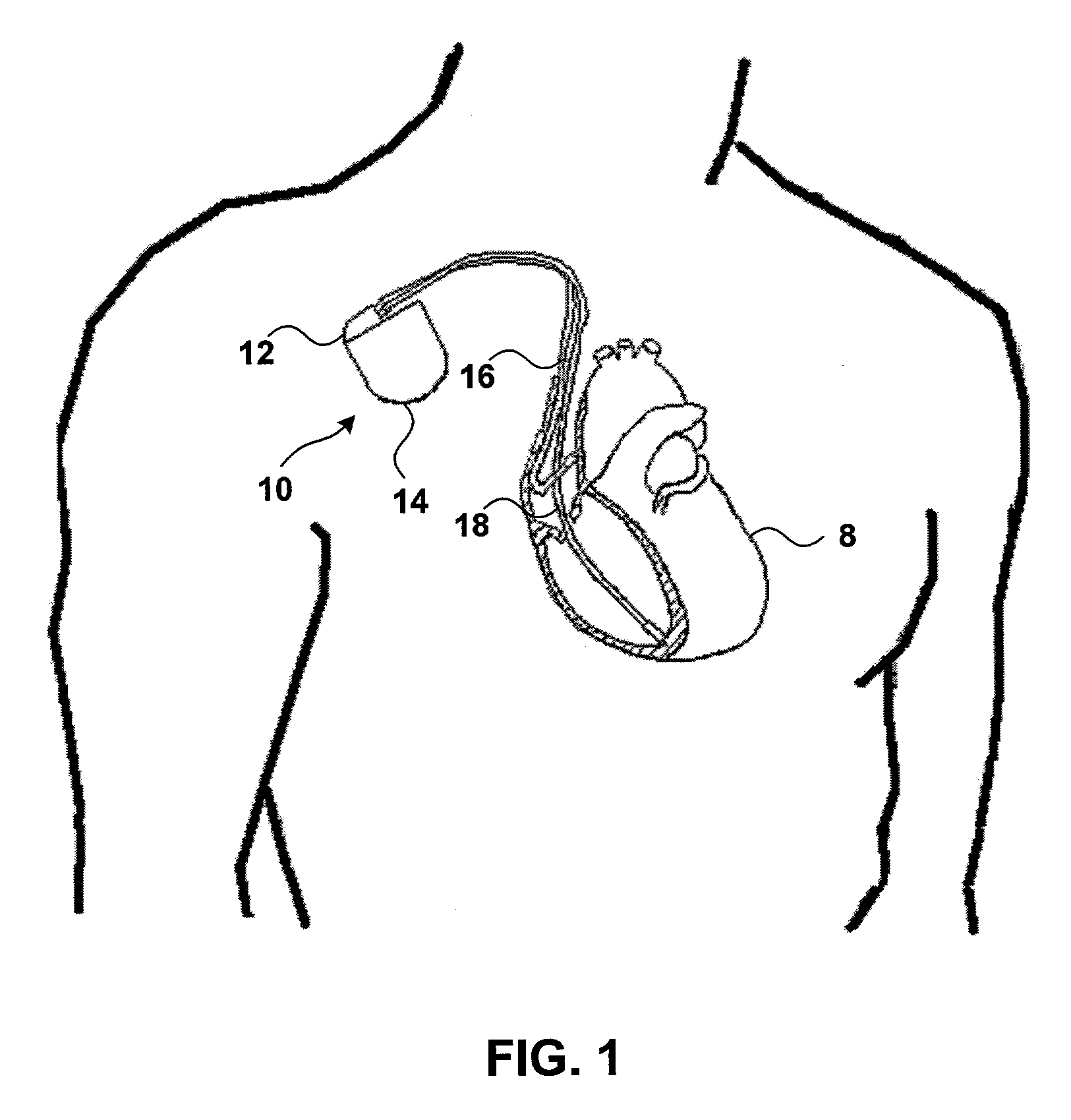
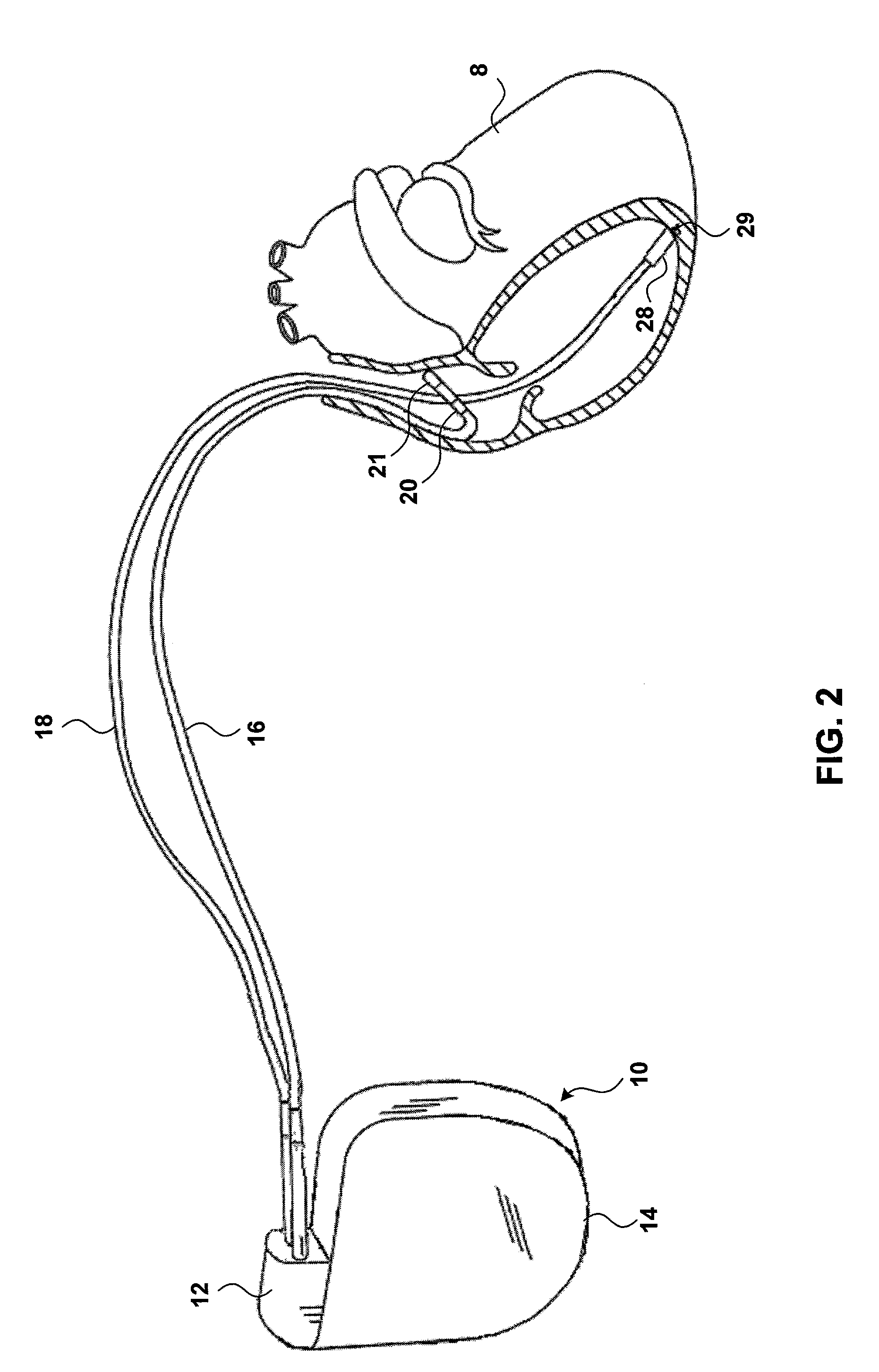
InactiveUS7069078B2Progression of diseaseJustified medically and economicallyHeart stimulatorsDiagnostic recording/measuringIGT - Impaired glucose toleranceCardiac pacemaker electrode
An implanted medical device may detect the onset of impaired glucose tolerance or Type II diabetes. The implanted medical device may have additional functionality. For example, the implanted medical device may be a pacemaker or a pressure monitor, but may also monitor insulin-mediated glucose uptake by processing electrical signals from the heart. An implanted medical device that monitors insulin-mediated glucose uptake may be implanted in a patient who has not been diagnosed with impaired glucose tolerance or Type II diabetes, and may give the patient early warning if these conditions develop.
Owner:MEDTRONIC INC
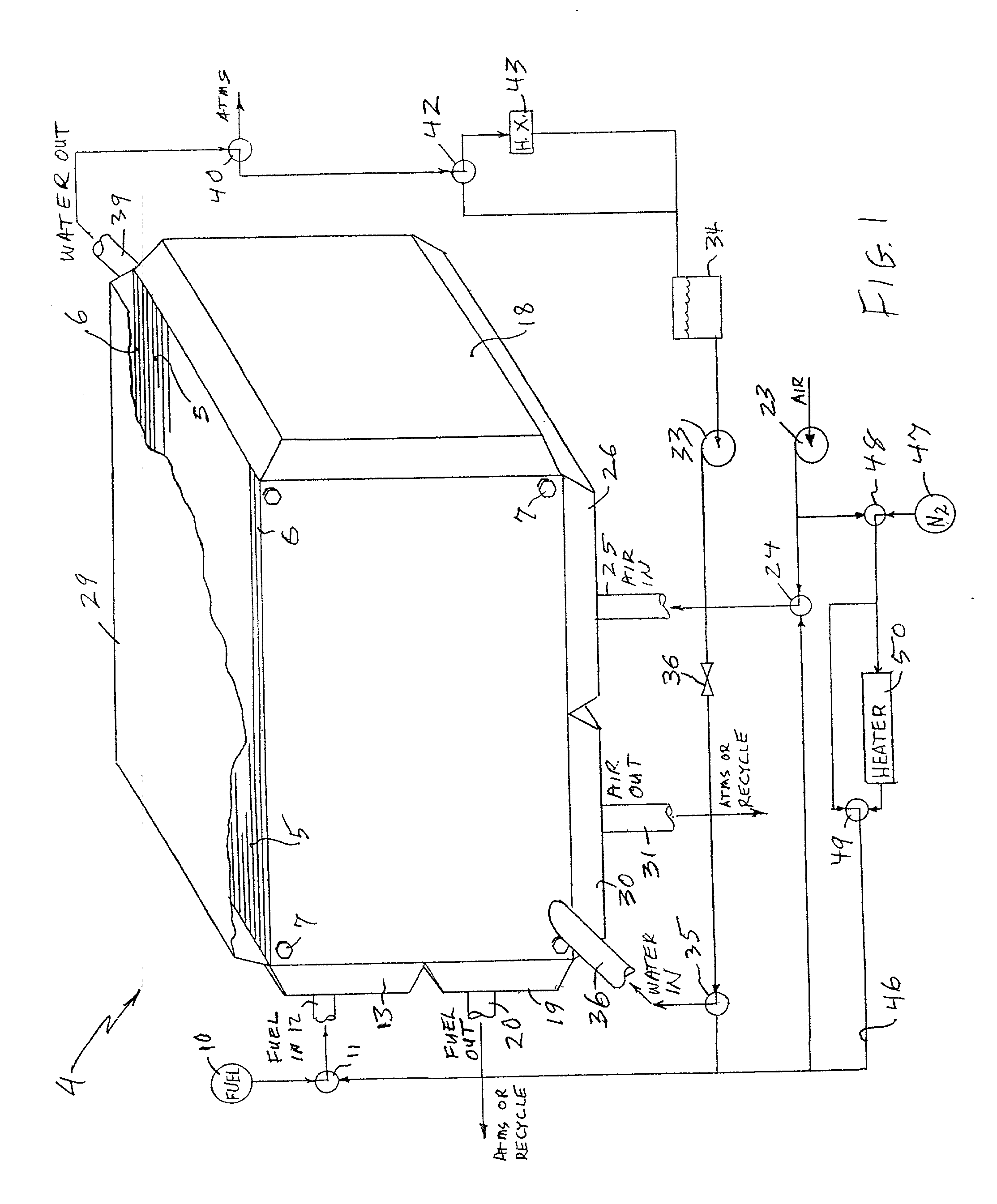
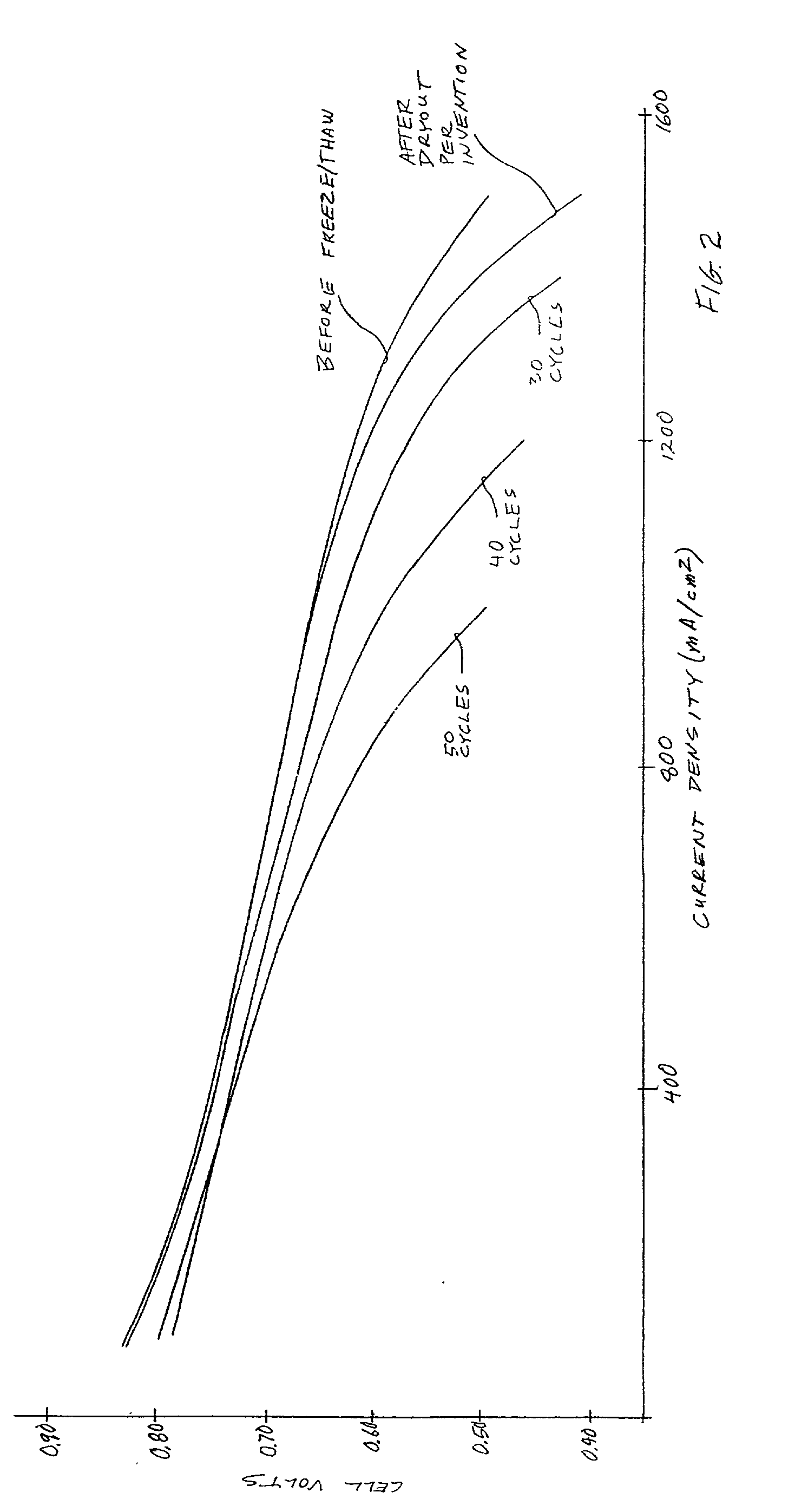
Performance recovery process for PEM fuel cells
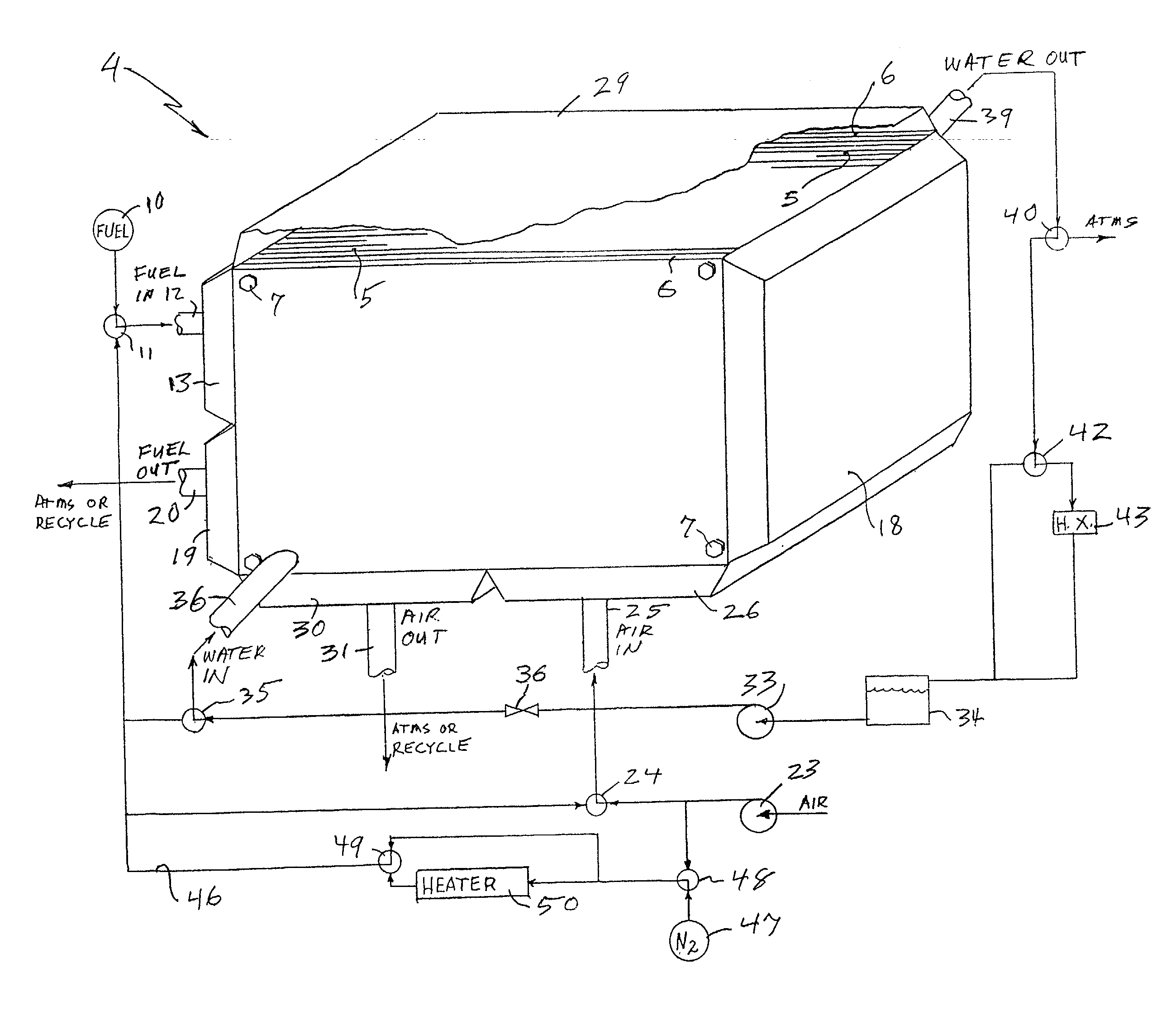
InactiveUS20030180586A1Restore performanceMonitor effectivenessFuel cell auxillariesSolid electrolyte fuel cellsThermodynamicsFuel cells
Recovery of PEM fuel cell performance is achieved by evacuating (61, 62) or by flowing water absorbing gas (46) through, or both, the fuel flow field (12, 13, 19, 20), the air flow field (25, 26, 30, 31), and the water flow field (36, 39), while resistance of the individual cells, or of the fuel cell stack, is measured; the dry out process is continued until the resistance of the cells (or the resistance per cell, measured across the fuel cell stack as a whole), has increased by at least 5 to 1 (preferably 10 to 1) over the normal resistance of the cells. The water absorbing gas may be air (23) or nitrogen (47); it may be at ambient temperature or heated (50).
Owner:AUDI AG
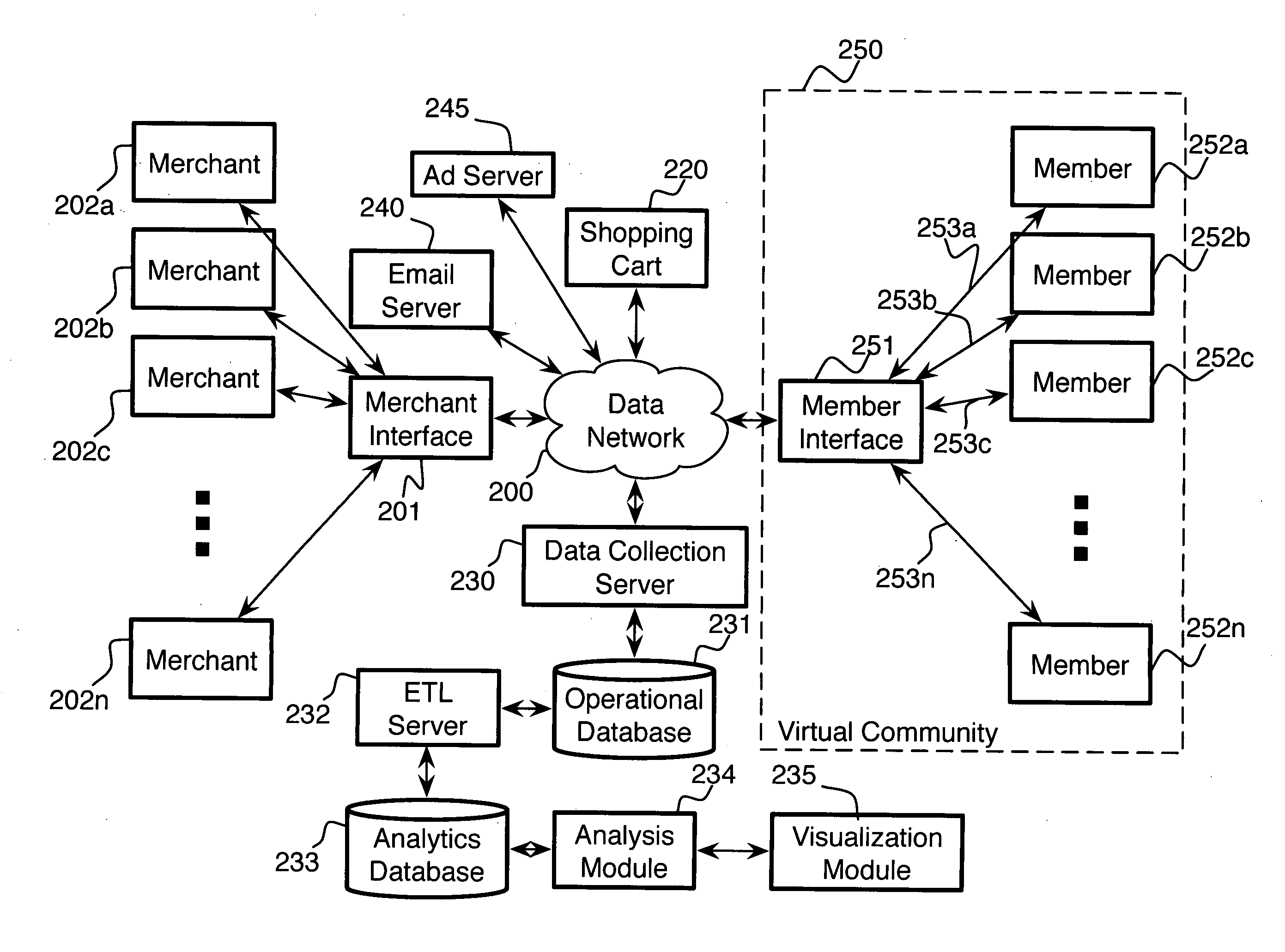
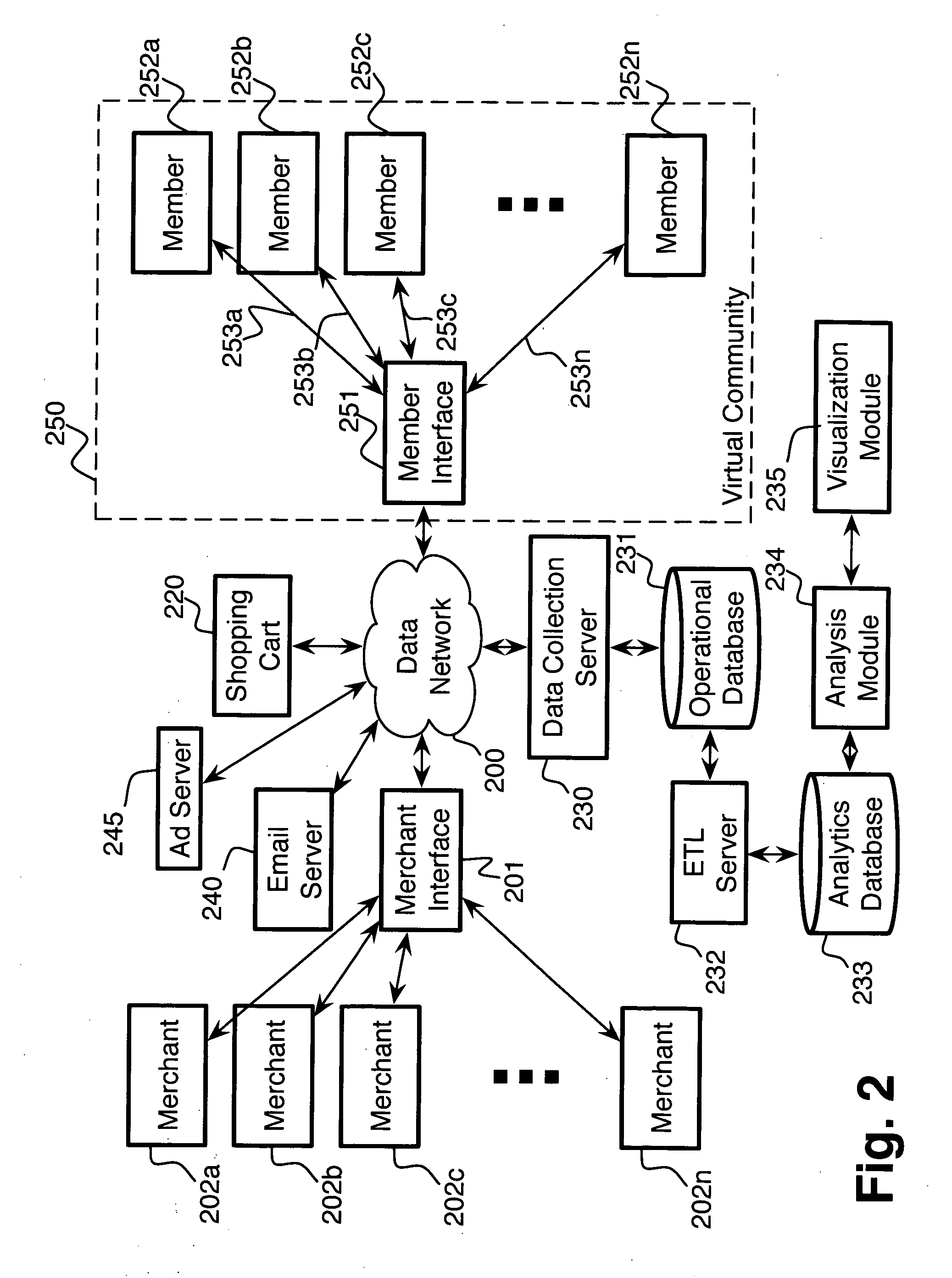
System and method for managing, monitoring and reporting on a plurality of online incentive programs
A system and method for centralizing the creation, approval, monitoring and managing of a plurality of incentive reward or spiff programs. The system preferably includes a host system that allows program managers, an administrator and participants to access the system in connection with spiff programs. The system also may monitor and report on the effectiveness of the spiff programs to permit the program manager to increase its return on investment. Once a spiff program is approved, registered participants are automatically enrolled in the program. Upon the entering or receipt of sales information, the system automatically begins the claims process for all eligible products. Centralizing the available spiff programs allows the participants to combine rewards and simplifies tax issues. The users may also view various reports on the spiff programs and directly communicate with one another.
Owner:MARKETING TECH CONCEPTS
System and method for analyzing endorsement networks
InactiveUS20100332312A1Monitor effectivenessDigital data processing detailsOffice automationCollections dataData harvesting
A system for analysis of endorsement networks, comprising a data collection server adapted for collecting event data over a data network from a plurality of components associated with an endorsement network, one or more database servers coupled to the data collection server and adapted to store event data pertaining to the endorsement network, and an analysis module coupled to at least one of the database servers, and wherein the analysis module retrieves data pertaining to the endorsement network from at least one of the databases and conducts analysis of said data sufficient at least to determine the graph structure of a significant portion of the endorsement network, is disclosed.
Owner:PURE VERTICALS
Rapid multiple panel of biomarkers in laboratory blood tests for TIA/stroke
InactiveUS20030096331A1Volume andDiagnosing the progression of TIA orPeptide preparation methodsDepsipeptidesNR1 NMDA receptorNMDA receptor
A methods, kits and compositions for diagnosing a central nervous system disorder, particularly transient ischemic attack or stroke, comprising measuring the level of NR2A and / or NR2B NMDA receptor or fragment thereof, in a biological sample from a human subject, and optionally measuring other biomarkers such as homocysteine and glutamate. The method is particularly useful for identifying individuals that are at risk for stroke, and for diagnosing stroke in an emergency room setting.
Owner:CIS BIOTECH
Method of therapeutic drug monitoring
InactiveUS20080152592A1Facilitated DiffusionMonitor effectivenessVaccination/ovulation diagnosticsSensorsMetaboliteContinuous monitoring
A method of using a diffusion-based, continuous-monitoring system to monitor the effectiveness of delivering a drug includes creating and maintaining a diffusion channel in an area of skin. The levels of the drug, metabolite, or affected substance of the drug are continuously monitored in the area of the skin for a desired duration via a diffusion-based, continuous-monitoring device. The levels of the drug, the metabolite, or affected substance is analyzed to determine the effectiveness of delivering the therapeutic drug.
Owner:ASCENSIA DIABETES CARE HLDG AG
Proteins, genes and their use for diagnosis and treatment of Schizophrenia
InactiveUS20020142303A1Monitor effectivenessCell receptors/surface-antigens/surface-determinantsSugar derivativesDrug developmentTherapeutic treatment
The present invention provides methods and compositions for screening, diagnosis and prognosis of Schizophrenia, for monitoring the effectiveness of Schizophrenia treatment, identifying patients most likely to respond to a particular therapeutic treatment and for drug development. Schizophrenia-Associated Features (SFs), detectable by two-dimensional electrophoresis of cerebrospinal fluid, serum or plasma are described. The invention further provides Schizophrenia-Associated Protein Isoforms (SPIs) detectable in cerebrospinal fluid, serum or plasma, preparations comprising isolated SPIs, antibodies immunospecific for SPIs, and kits comprising the aforesaid.
Owner:OXFORD GLYCOSCI UK
Novel genes and markers in type 2 diabetes and obesity
ActiveUS20070292412A1Reducing and minimizing debilitating effectDiagnosis can be and efficient and safeOrganic active ingredientsNervous disorderDiseaseNovel gene
Genes, SNP markers and haplotypes of susceptibility or predisposition to T2D and subdiagnosis of T2D and related medical conditions are disclosed. Methods for diagnosis, prediction of clinical course and efficacy of treatments for T2D, obesity and related phenotypes using polymorphisms in the risk genes are also disclosed. The genes, gene products and agents of the invention are also useful for monitoring the effectiveness of prevention and treatment of T2D and related traits. Kits are also provided for the diagnosis, selecting treatment and assessing prognosis of T2D. Novel methods for prevention and treatment of metabolic diseases such as T2D based on the disclosed T2D genes, polypeptides and related pathways are also disclosed.
Owner:DSM IP ASSETS BV
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20100234765A1Difficult to levelImprove the level ofDisease diagnosisDiagnostic recording/measuringRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:BARASCH JONATHAN MATTHEW +3
Cooperating defibrillators and external chest compression devices
ActiveUS20080114406A1Optimize timingEffectiveness of chest compression could be monitoredRespiratorsDiagnosticsComputer hardwareElectricity
Owner:PHYSIO CONTROL INC
Internal-reference-containing HCMV fluorescence quantitative PCR detection kit
InactiveCN102115794AMonitor effectivenessReduce false negative rateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceHuman cytomegalovirus
The invention relates to an internal-reference-containing human cytomegalovirus (HCMV) fluorescence quantitative polymerase chain reaction (PCR) detection kit. A detection primer and a detection probe are designed for a UL122 gene of an HCMV genome, a competitive internal reference amplification system is introduced by designing an internal reference probe and an internal reference male die and exists in the same reaction tube with an HCMV deoxyribonucleic acid (DNA) detection system, and the probability of false negative caused by the failure of the kit or experiment misoperation and the like can be effectively reduced by introducing the internal reference amplification system. The kit can perform qualitative and quantitative detection on HCMV DNA quickly and simply, and has high specificity and sensitivity.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Diagnosis and monitoring of chronic renal disease using ngal
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:BARASCH JONATHAN MATTHEW +3
Genes and markers in type 2 diabetes and obesity
ActiveUS7901885B2Reducing and minimizing debilitating effectDiagnosis can be and efficient and safeOrganic active ingredientsNervous disorderGene productHaplotype
Genes, SNP markers and haplotypes of susceptibility or predisposition to T2D and subdiagnosis of T2D and related medical conditions are disclosed. Methods for diagnosis, prediction of clinical course and efficacy of treatments for T2D, obesity and related phenotypes using polymorphisms in the risk genes are also disclosed. The genes, gene products and agents of the invention are also useful for monitoring the effectiveness of prevention and treatment of T2D and related traits. Kits are also provided for the diagnosis, selecting treatment and assessing prognosis of T2D. Novel methods for prevention and treatment of metabolic diseases such as T2D based on the disclosed T2D genes, polypeptides and related pathways are also disclosed.
Owner:DSM IP ASSETS BV
Biomarkers for insulin resistance and beta-cell dysfunction
ActiveUS20100130402A1A large amountMinimal numberBiocideOrganic active ingredientsPancreasInsulin resistance
The invention provides compositions and methods for determining insulin resistance and / or pancreatic β-cell dysfunction in a subject. The invention also provides compositions and methods for treating a subject according to the insulin resistance and / or pancreatic β-cell dysfunction in the subject.
Owner:PHARMACT HEALTHCARE +1
Measurement of mutation load using the p53 gene in human cells from paraffin embedded tissues
InactiveUS20070020648A1Assessing cancer riskAssessing prognosisMicrobiological testing/measurementFermentationCarcinogenMutated protein
A method for determining mutation load in a somatic cell is determined by mutation analysis of the p53 gene. The p53 gene has been found to be a useful indicator of predisposition to spontaneous mutations or prior carcinogen exposure. Cells that contain mutated p53 tend to accumulate the mutant protein. Thus, DNA from a cell identified by p53 accumulation is amplified and the amplification product further analyzed for mutations in the p53 gene.
Owner:SOMMER STEVEN S +2
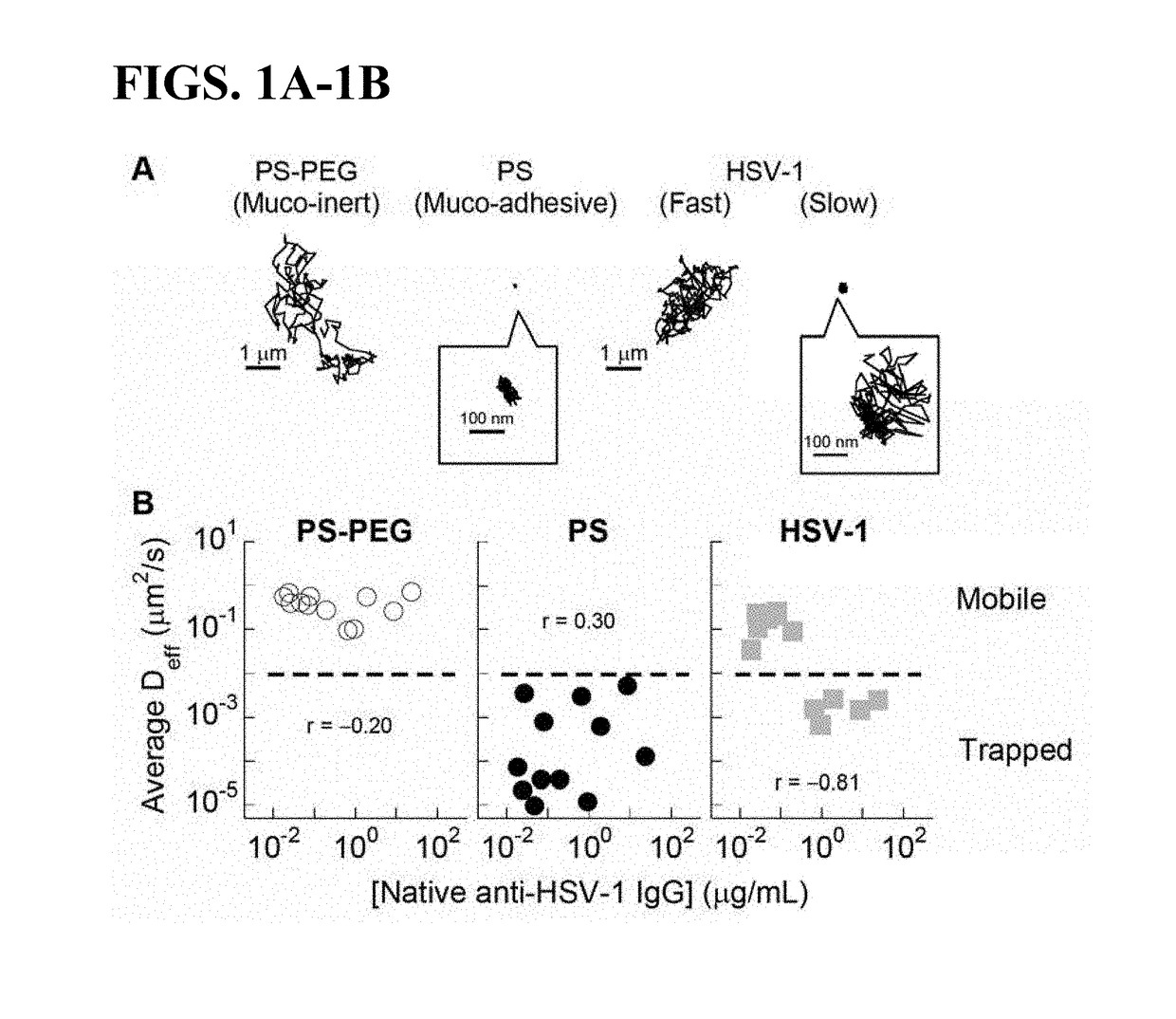
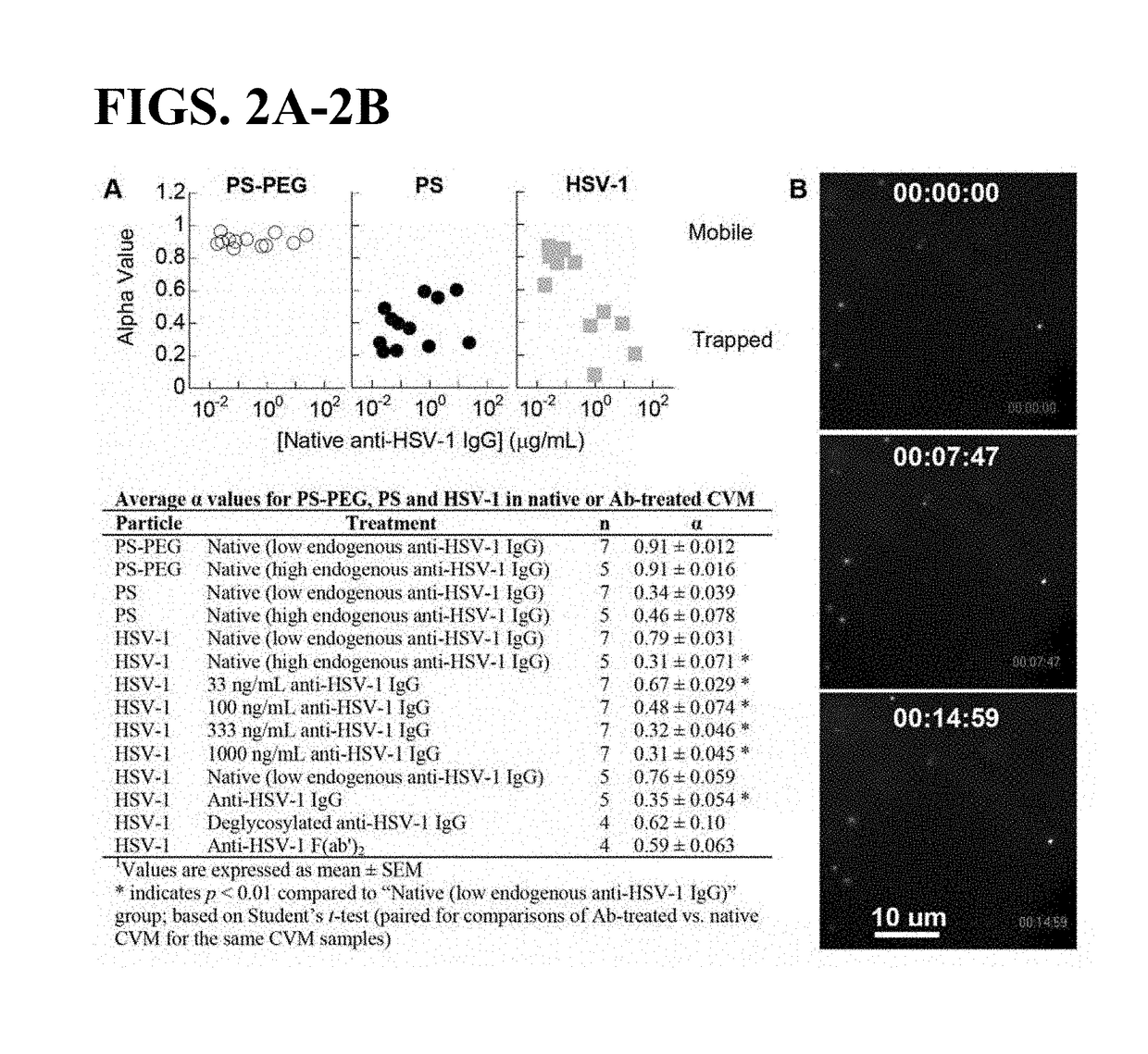
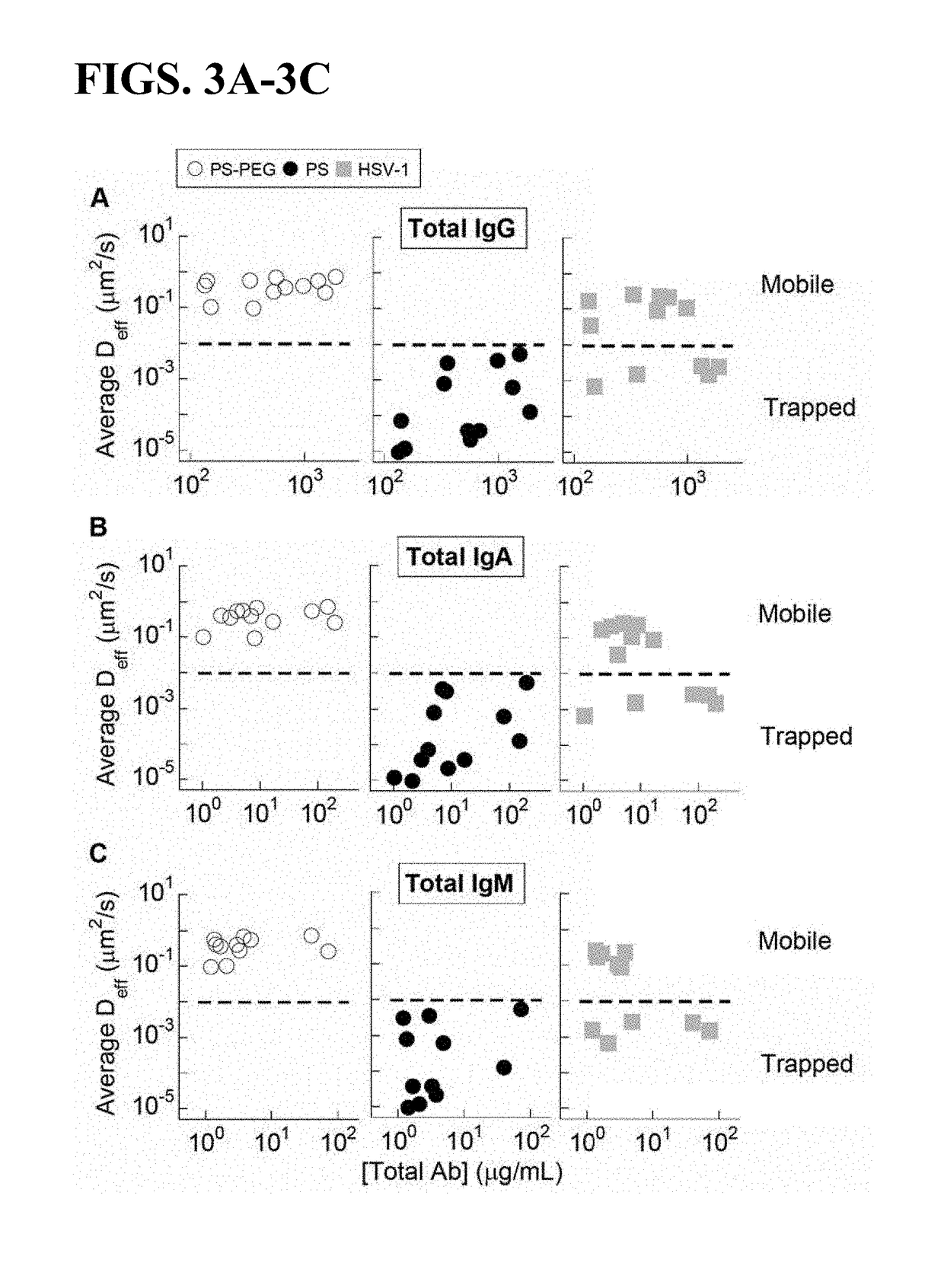
Compositions and methods for inhibiting pathogen infection
ActiveUS20190023769A1Diffusion slowDiffusion fastImmunoglobulins against virusesAntiviralsSubject matterSecretion
The presently-disclosed subject matter relates to antibodies, compositions, and methods for inhibiting and treating virus infection in the respiratory tract and virus transmission through the respiratory tract. In particular, the presently-disclosed subject matter relates to inhibiting and treating virus infection in a subject using compositions and antibodies that trap viruses in mucus of the respiratory tract, thereby inhibiting transport of virus across or through mucus secretions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
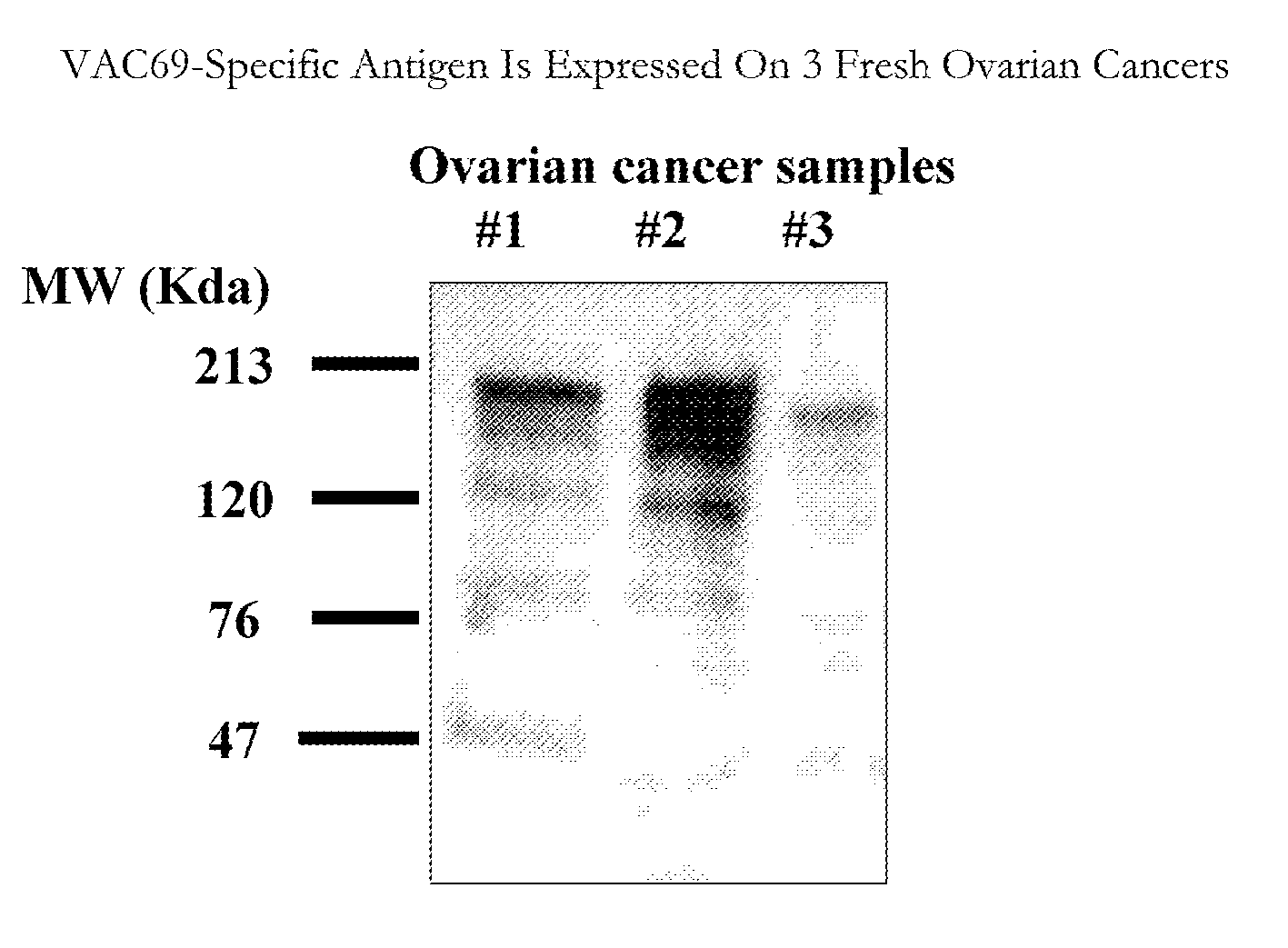
Ovarian cancer cell and myeloma cell surface glycoproteins, antibodies thereto, and uses thereof
InactiveUS20070269372A1Effectiveness of anti-cancer therapies can be monitoredAuxiliary diagnosisUltrasonic/sonic/infrasonic diagnosticsHybrid cell preparationEpitopeCell Surface Antigens
The present invention is directed to cell surface antigens found on myeloma cells and on ovarian cancer cells that are recognized by monoclonal antibodies, and antibody binding fragments thereof, as described. The monoclonal antibodies of the invention are capable of being used for therapeutic, screening, diagnostic and cell purification purposes. A representative and exemplified monoclonal antibody of the present invention recognizes and binds to an epitope common to a surface antigen that is expressed on multiple myeloma cells and to a surface antigen that is expressed on ovarian cancer cells. The function of this monoclonal antibody both in vivo and in vitro is demonstrated.
Owner:CAERUS THERAPEUTICS INC
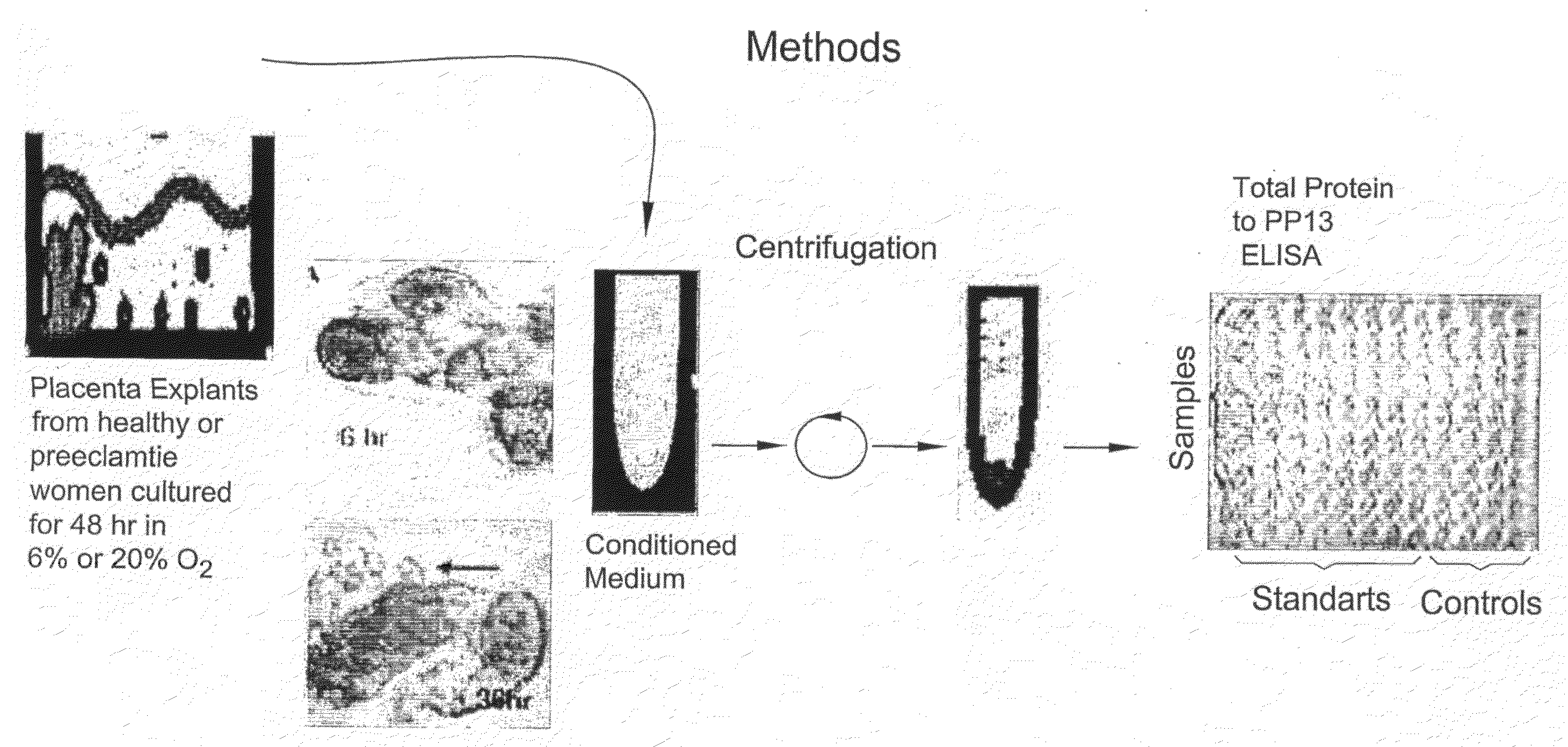
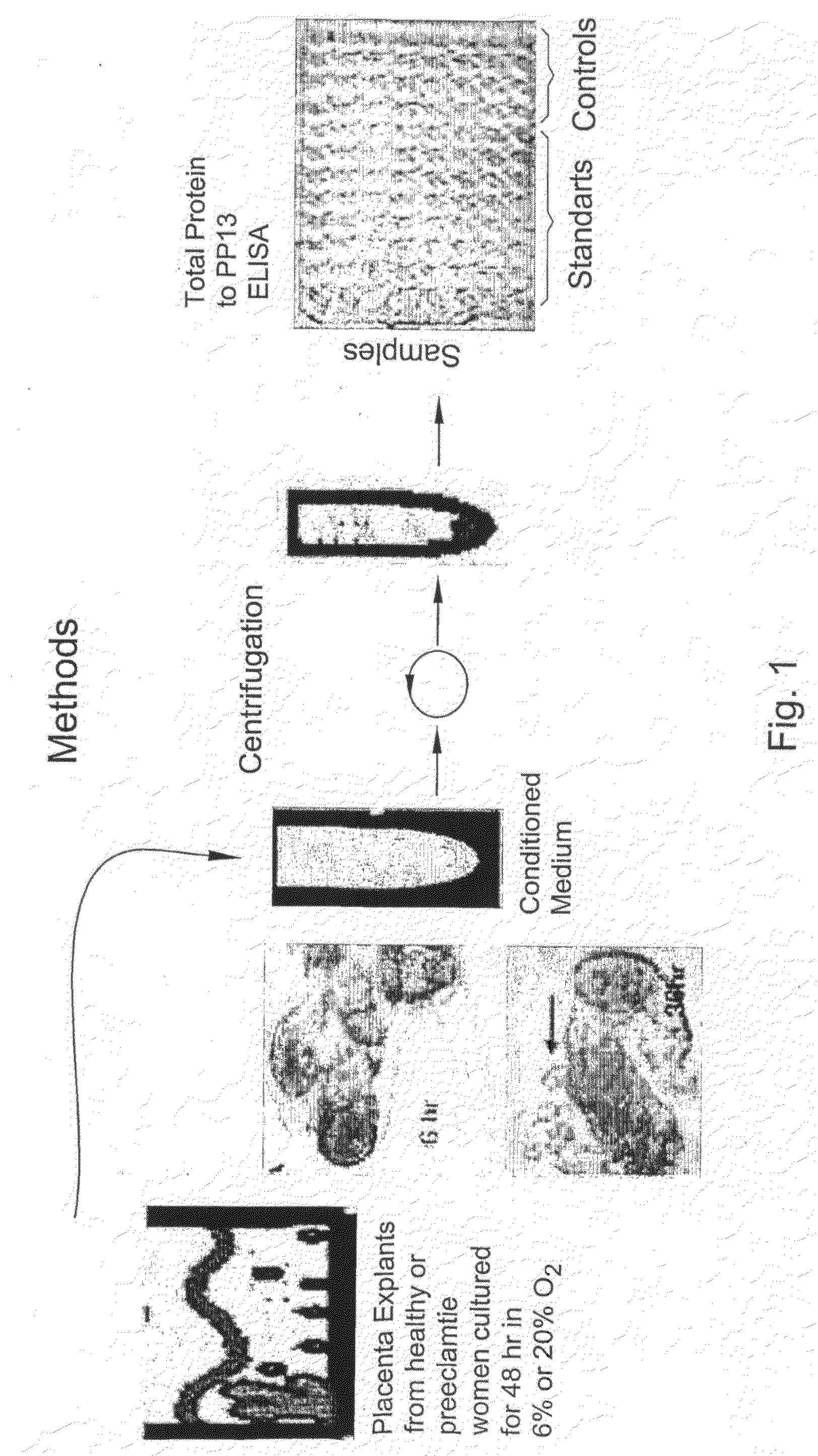
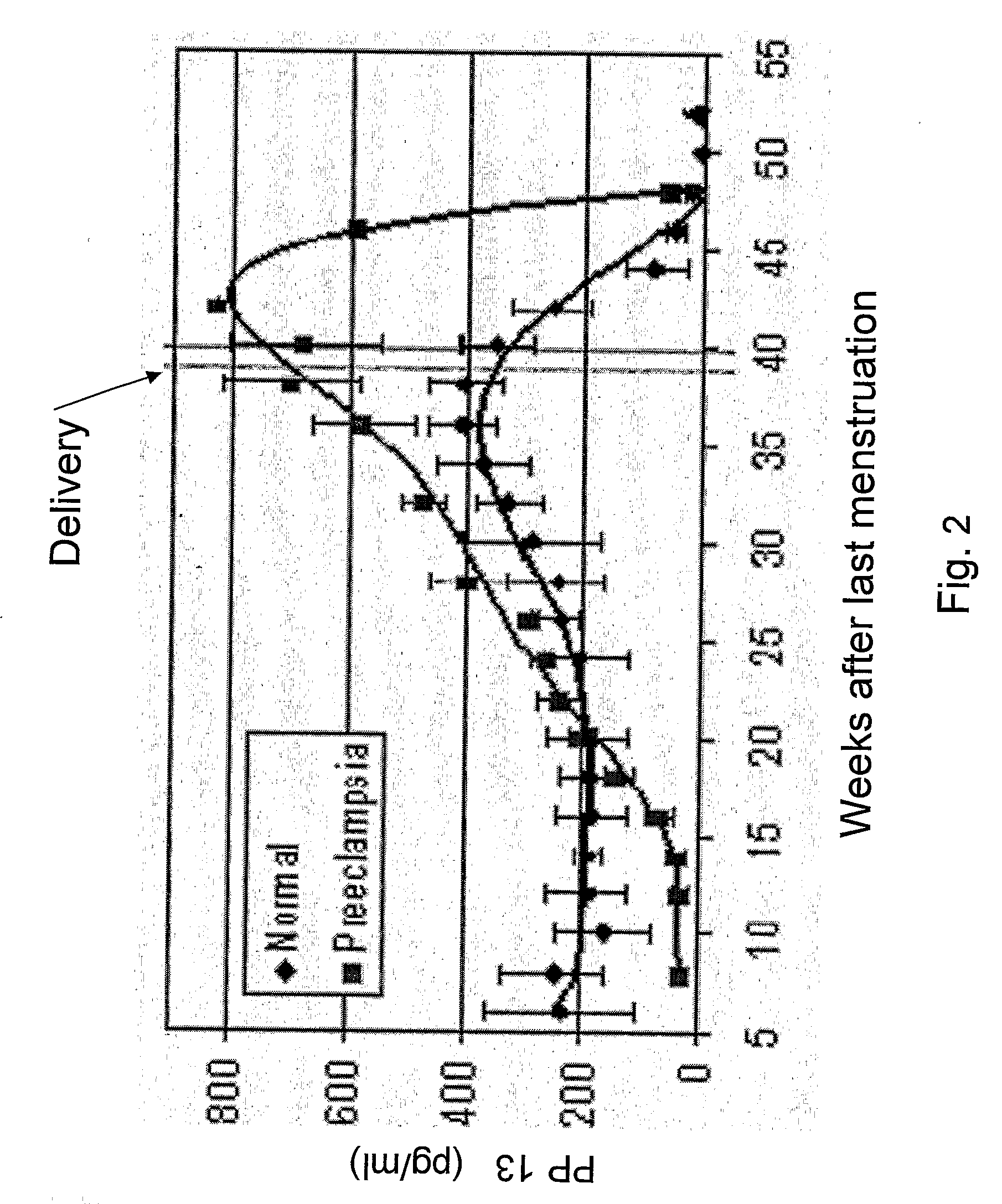
Method for Determining the Effectiveness of a Treatment for Preeclampsia
InactiveUS20080131867A1Monitor effectivenessReduce/remove riskMicrobiological testing/measurementDead animal preservationObstetricsNormal level
A method for determining the effectiveness of a treatment for preeclampsia of a pregnant woman at risk for preeclampsia, the method comprising: (a) determining a first concentration of placental protein 13 (PP13) in a bodily substance of the woman obtained prior to the treatment; (b) determining a second concentration of PP13 in a bodily substance of the woman obtained after initiation of the treatment; and (c) comparing the first and second concentrations to a corresponding normal level of PP13 and, based on the comparison, determining the effectiveness of the treatment. Diagnostic kits for practicing the method are also disclosed.
Owner:DIAGNOSTIC TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com