Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3681 results about "Somatic cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A somatic cell (from the Greek σῶμα sôma, meaning "body") or vegetal cell is any biological cell forming the body of an organism; that is, in a multicellular organism, any cell other than a gamete, germ cell, gametocyte or undifferentiated stem cell.
RNA preparations comprising purified modified RNA for reprogramming cells
ActiveUS20110143397A1Promote growthArtificial cell constructsCell culture active agentsSingle strandSomatic cell
The present invention provides compositions and methods for reprogramming somatic cells using purified RNA preparations comprising single-strand mRNA encoding an iPS cell induction factor. The purified RNA preparations are preferably substantially free of RNA contaminant molecules that: i) would activate an immune response in the somatic cells, ii) would decrease expression of the single-stranded mRNA in the somatic cells, and / or iii) active RNA sensors in the somatic cells. In certain embodiments, the purified RNA preparations are substantially free of partial mRNAs, double-stranded RNAs, un-capped RNA molecules, and / or single-stranded run-on mRNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods and compositions for organ and tissue functionality
Materials and methods for treating tissue defects in human or animal tissues using implantable cells are described. Further, culture techniques and factors for enhancing these procedures, and cell survival and adaptation are described. Many of the tissue defects may be treated with autologous cells, while applications involving non-autologous cells or stem cells are also described.
Owner:DASK TECH
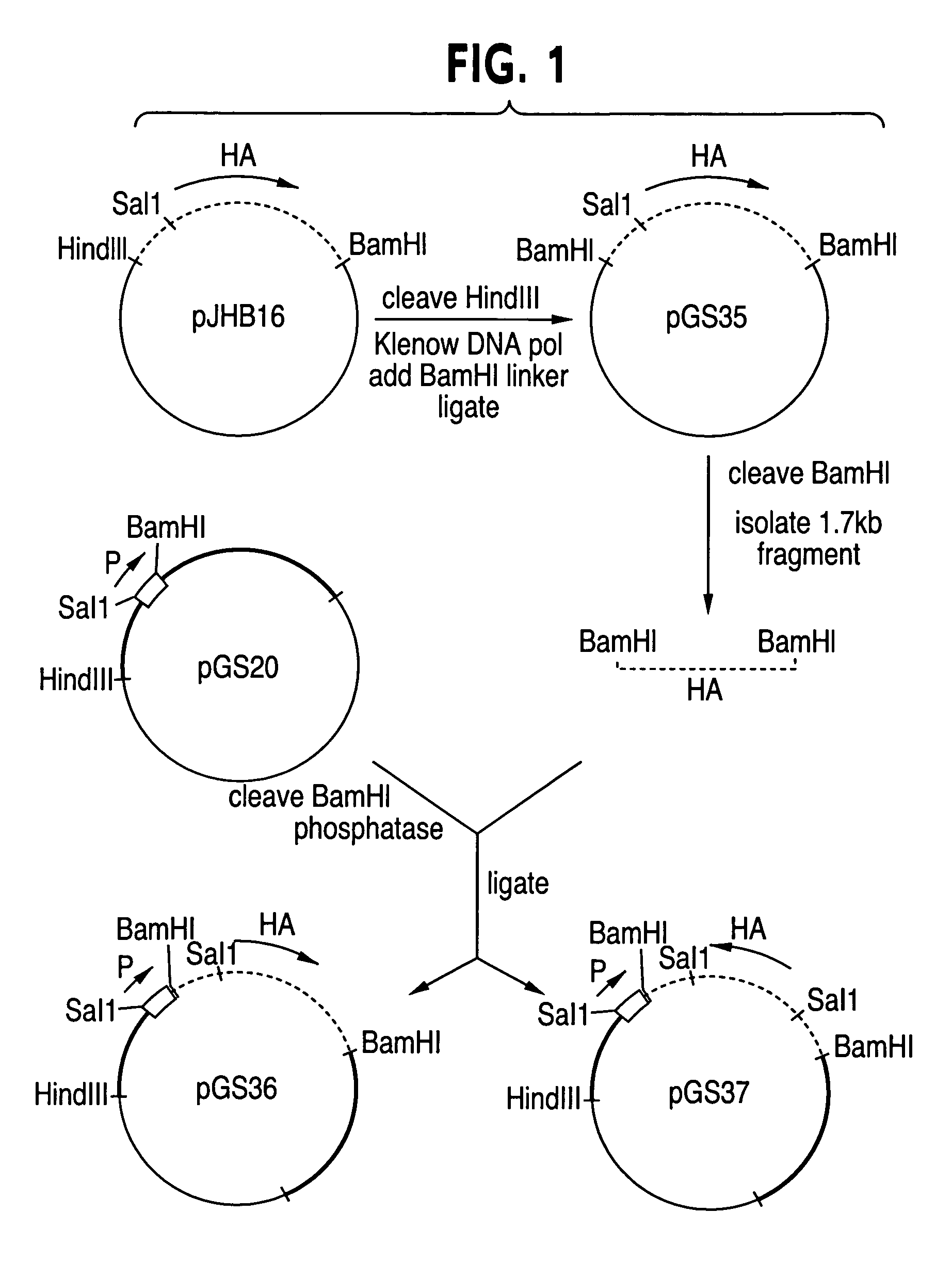
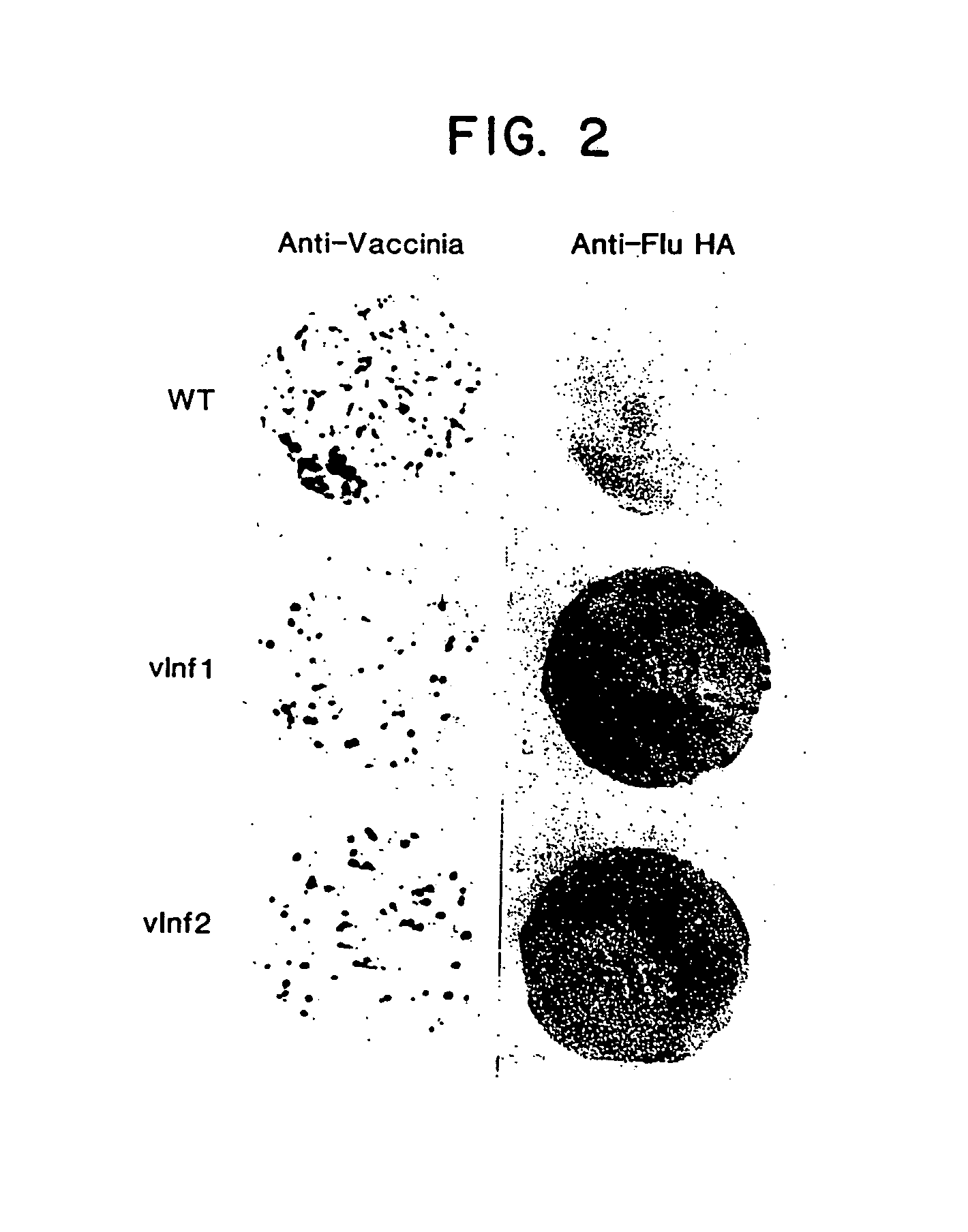
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Use of RNA for reprogramming somatic cells
InactiveUS20110065103A1Promotes reprogrammingBlood/immune system cellsDrug compositionsReprogrammingSomatic cell
The present invention provides methods for de-differentiating somatic cells into stem-like cells without generating embryos or fetuses. More specifically, the present invention provides methods for effecting the de-differentiation of somatic cells to cells having stem cell characteristics, in particular pluripotency, by introducing RNA encoding factors inducing the de-differentiation of somatic cells into the somatic cells and culturing the somatic cells allowing the cells to de-differentiate.
Owner:BIONTECH AG +2
Somatic cell reprogramming
ActiveUS20080233610A1High nucleus to cytoplasm ratioImprove effectivenessNervous disorderVirusesSomatic cellCell biology
The present invention relates to methods for reprogramming a somatic cell to pluripotency by administering into the somatic cell at least one or a plurality of potency-determining factors. The invention also relates to pluripotent cell populations obtained using a reprogramming method.
Owner:WISCONSIN ALUMNI RES FOUND
Pluripotent stem cells derived without the use of embryos or fetal tissue
InactiveUS20030113910A1New breed animal cellsArtificial cell constructsPluripotential stem cellGerm layer
Owner:STEMA
Mutational analysis of plasma DNA for cancer detection
ActiveUS20140100121A1Accurate parameterLevel of heterogeneity of tumorsSequential/parallel process reactionsMicrobiological testing/measurementMutation frequencyBlood plasma
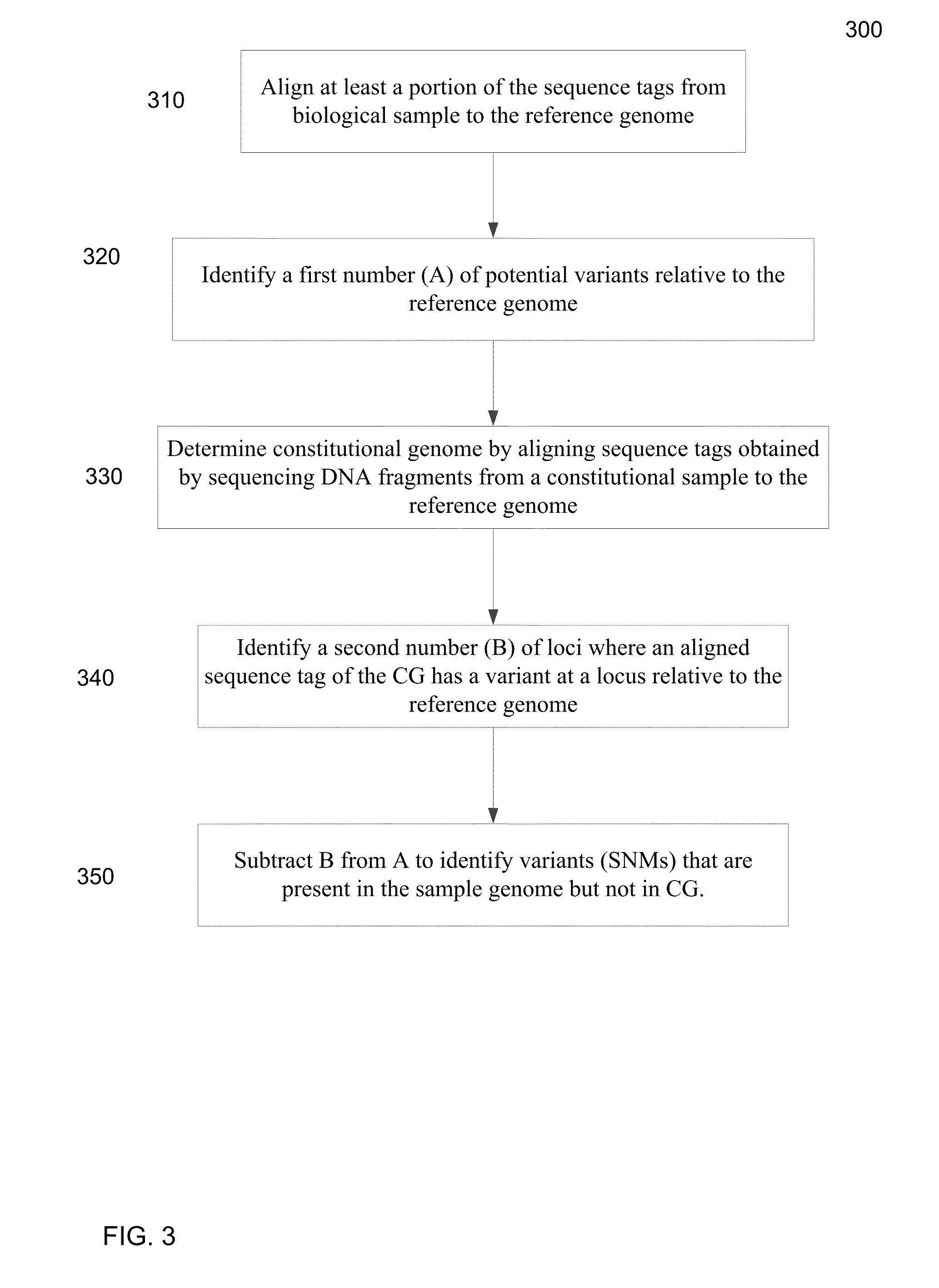
A frequency of somatic mutations in a biological sample (e.g., plasma or serum) of a subject undergoing screening or monitoring for cancer, can be compared with that in the constitutional DNA of the same subject. A parameter can derived from these frequencies and used to determine a classification of a level of cancer. False positives can be filtered out by requiring any variant locus to have at least a specified number of variant sequence reads (tags), thereby providing a more accurate parameter. The relative frequencies for different variant loci can be analyzed to determine a level of heterogeneity of tumors in a patient.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Circulating Mutant DNA to Assess Tumor Dynamics
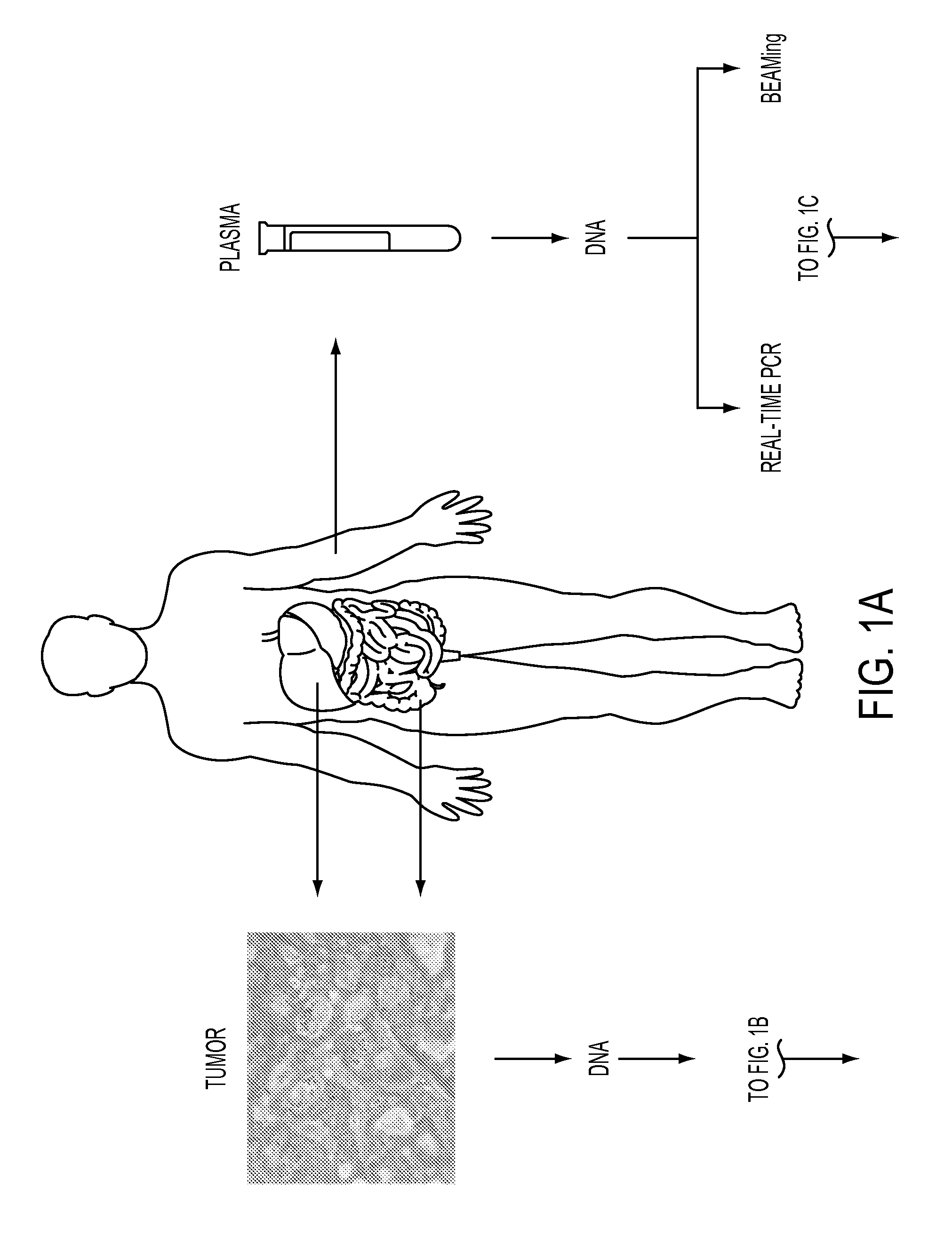
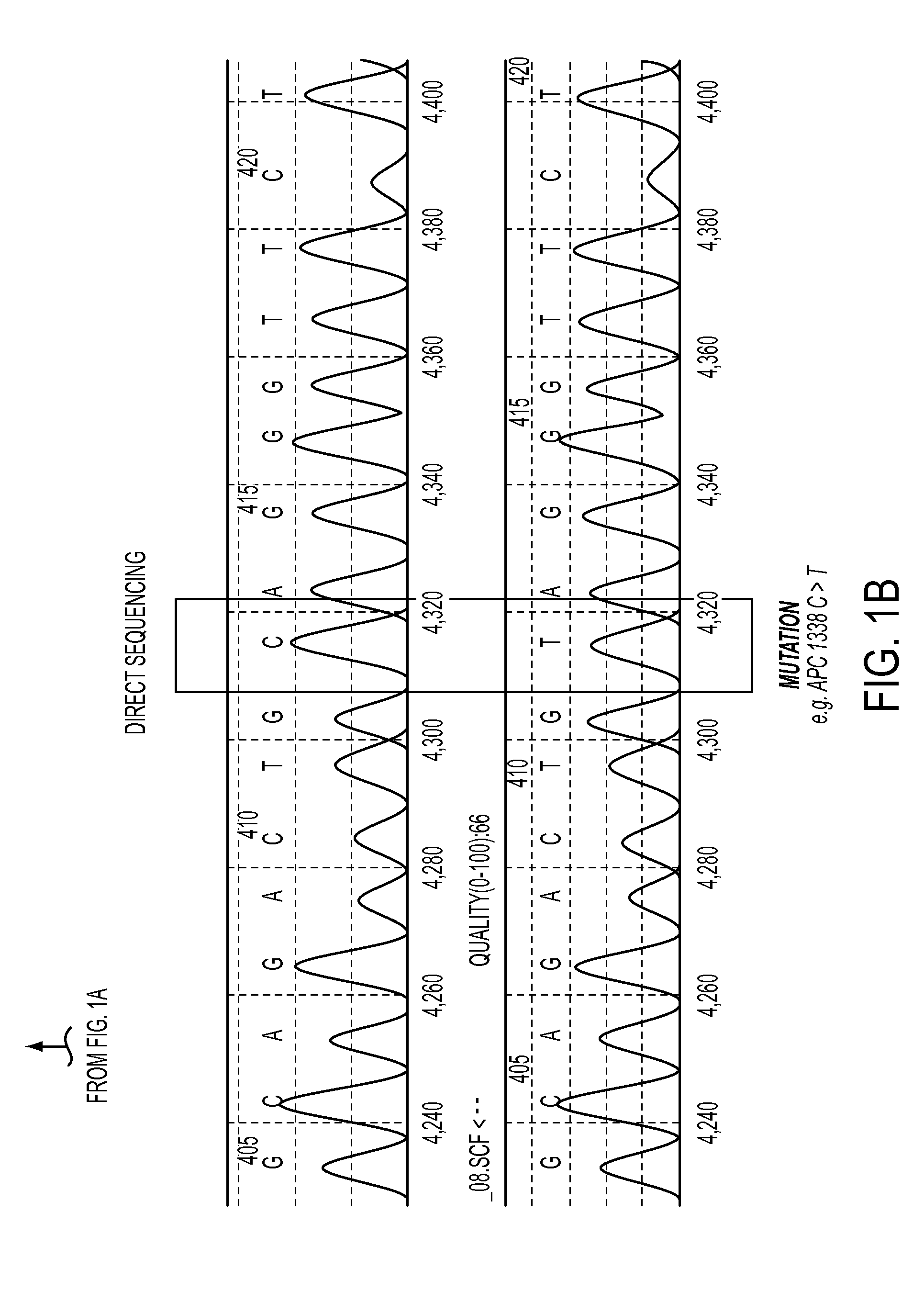
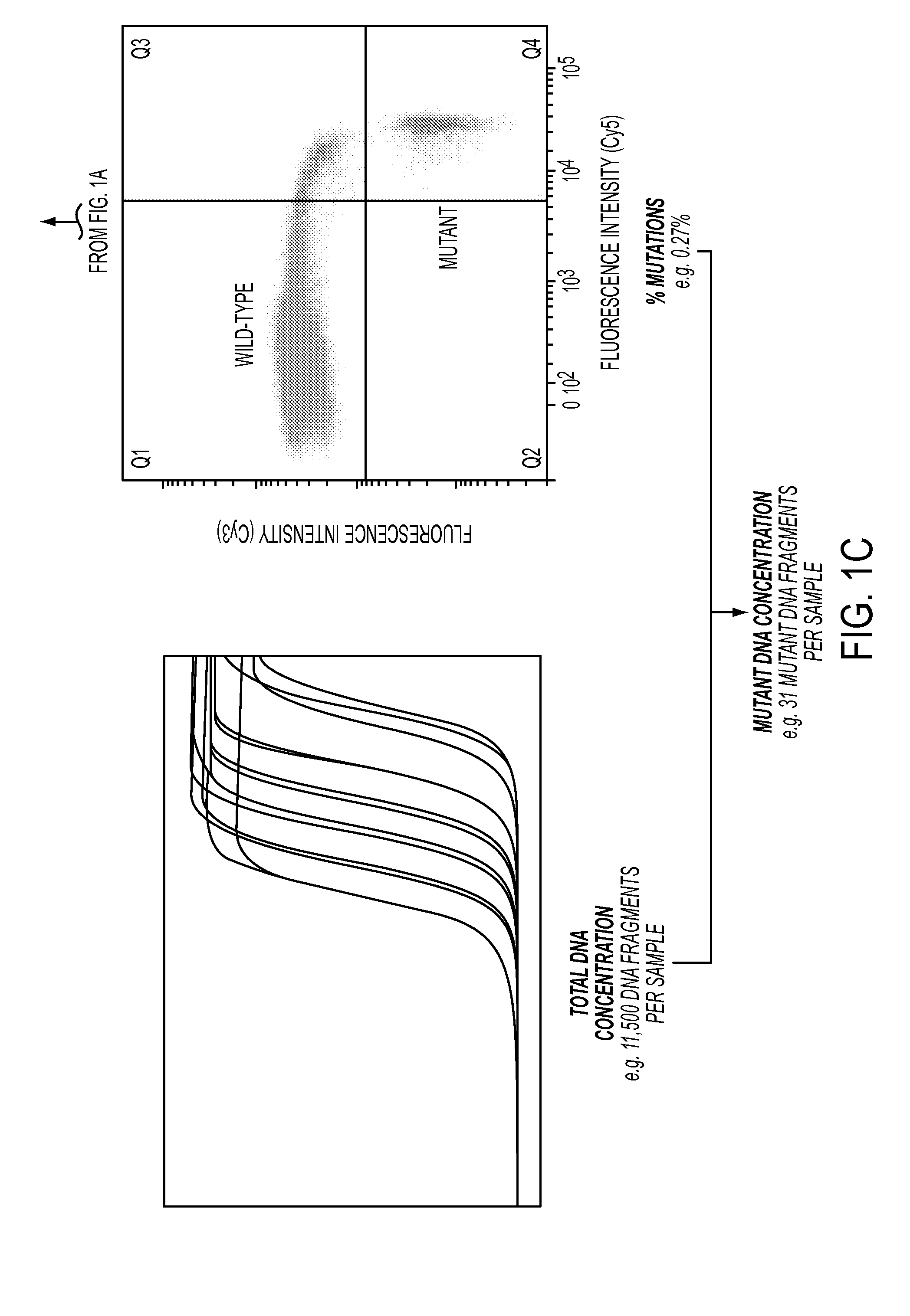
DNA containing somatic mutations is highly tumor specific and thus, in theory, can provide optimum markers. However, the number of circulating mutant gene fragments is small compared to the number of normal circulating DNA fragments, making it difficult to detect and quantify them with the sensitivity required for meaningful clinical use. We apply a highly sensitive approach to quantify circulating tumor DNA (ctDNA) in body samples of patients. Measurements of ctDNA can be used to reliably monitor tumor dynamics in subjects with cancer, especially those who are undergoing surgery or chemotherapy. This personalized genetic approach can be generally applied.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for reprogramming somatic cells
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Compositions and methods for reprogramming mammalian cells
InactiveUS20130189741A1Maintain integrityReduce activationHydrolasesArtificial cell constructsSufficient timeSomatic cell
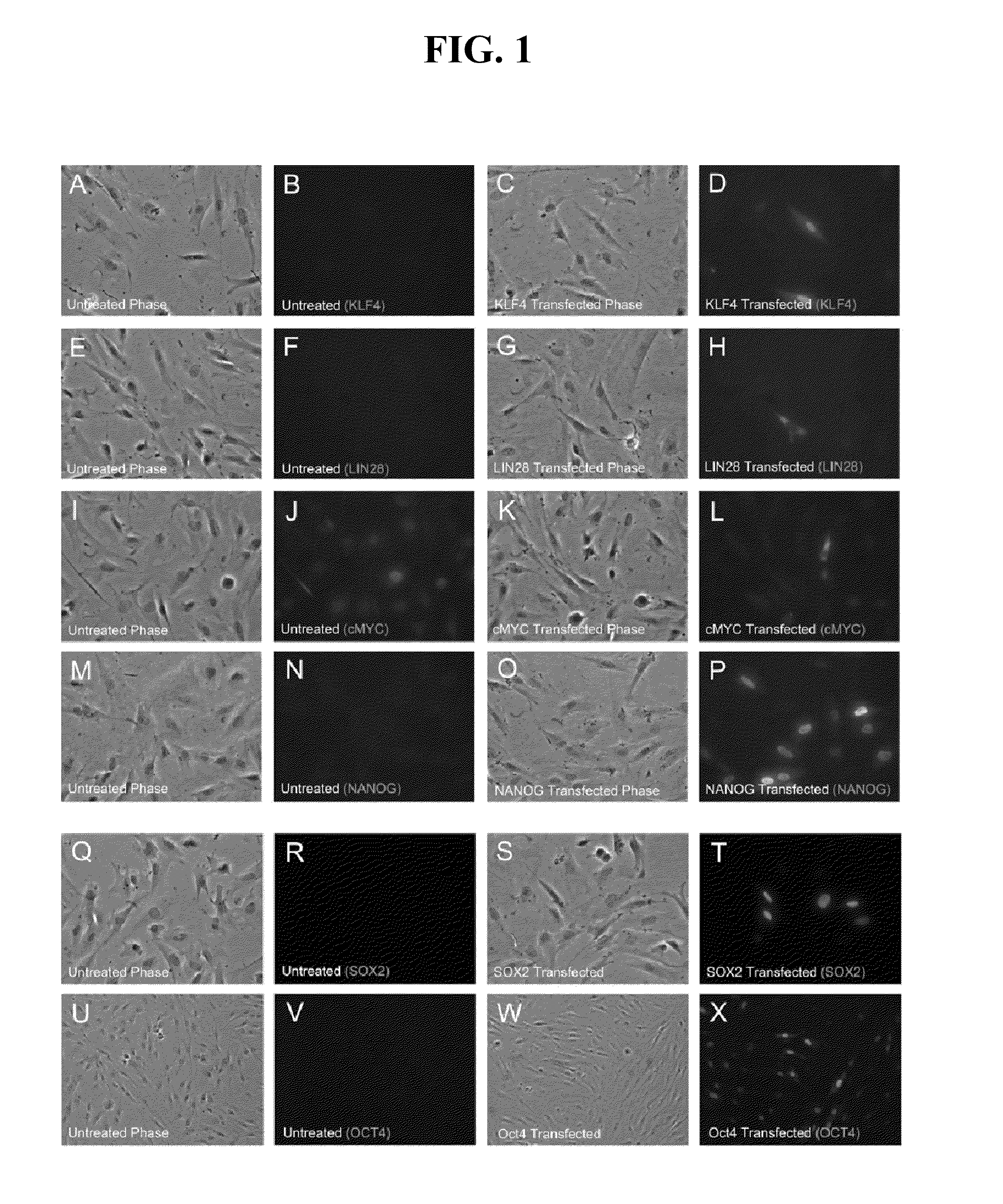
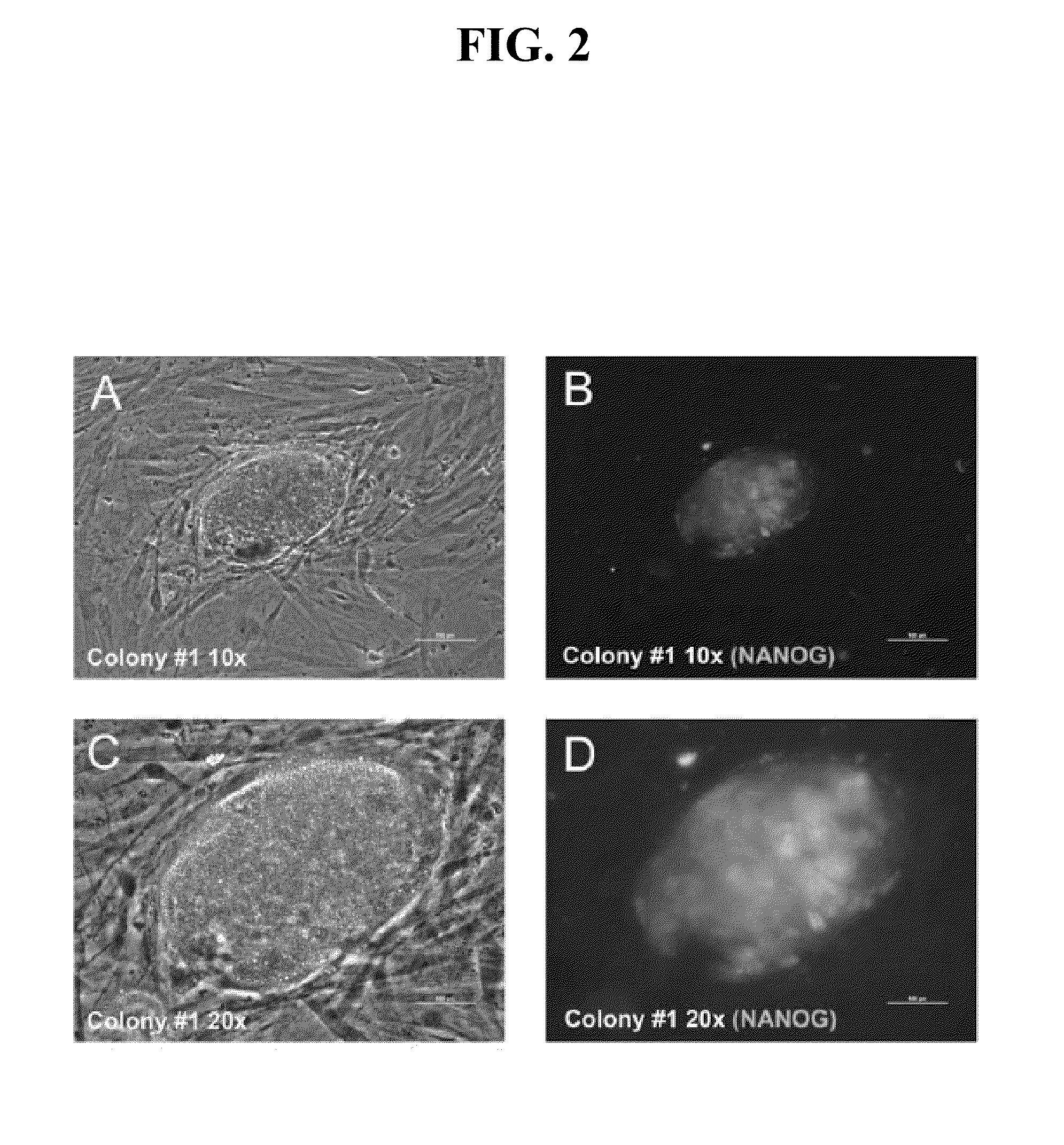
The present invention relates to methods for changing the state of differentiation of a eukaryotic cell, the methods comprising introducing mRNA encoding one or more reprogramming factors into a cell and maintaining the cell under conditions wherein the cell is viable and the mRNA that is introduced into the cell is expressed in sufficient amount and for sufficient time to generate a cell that exhibits a changed state of differentiation compared to the cell into which the mRNA was introduced, and compositions therefor. For example, the present invention provides mRNA molecules and methods for their use to reprogram human somatic cells into pluripotent stem cells.
Owner:CELLSCRIPT
Methods for the production of stably-transformed, fertile wheat employing agrobacterium-mediated transformation and compositions derived therefrom
InactiveUS7026528B2Tissue cultureOther foreign material introduction processesBiotechnologySomaclonal variation
Disclosed are processes for producing stably transformed fertile wheat a system of transforming wheat via Agrobacterium. This invention provides methods transforming a variety of explants, such as freshly isolated or pre-cultured immature embryos, embryogenic callus and suspension cells. Also disclosed are methods for recovering transgenic plants after transformation within a short period of time, if the explants are regenerable at the time of transformation. Thus the frequency of somaclonal variation associated with prolonged in vitro culture period is significantly reduced. The transformation frequency using this system is comparable to or better than published methods using other systems, such as microprojectile bombardment.
Owner:MONSANTO TECH LLC
Drug conjugate composition
ActiveUS7374762B2Snake antigen ingredientsAntibody ingredientsDrug conjugationAntiendomysial antibodies
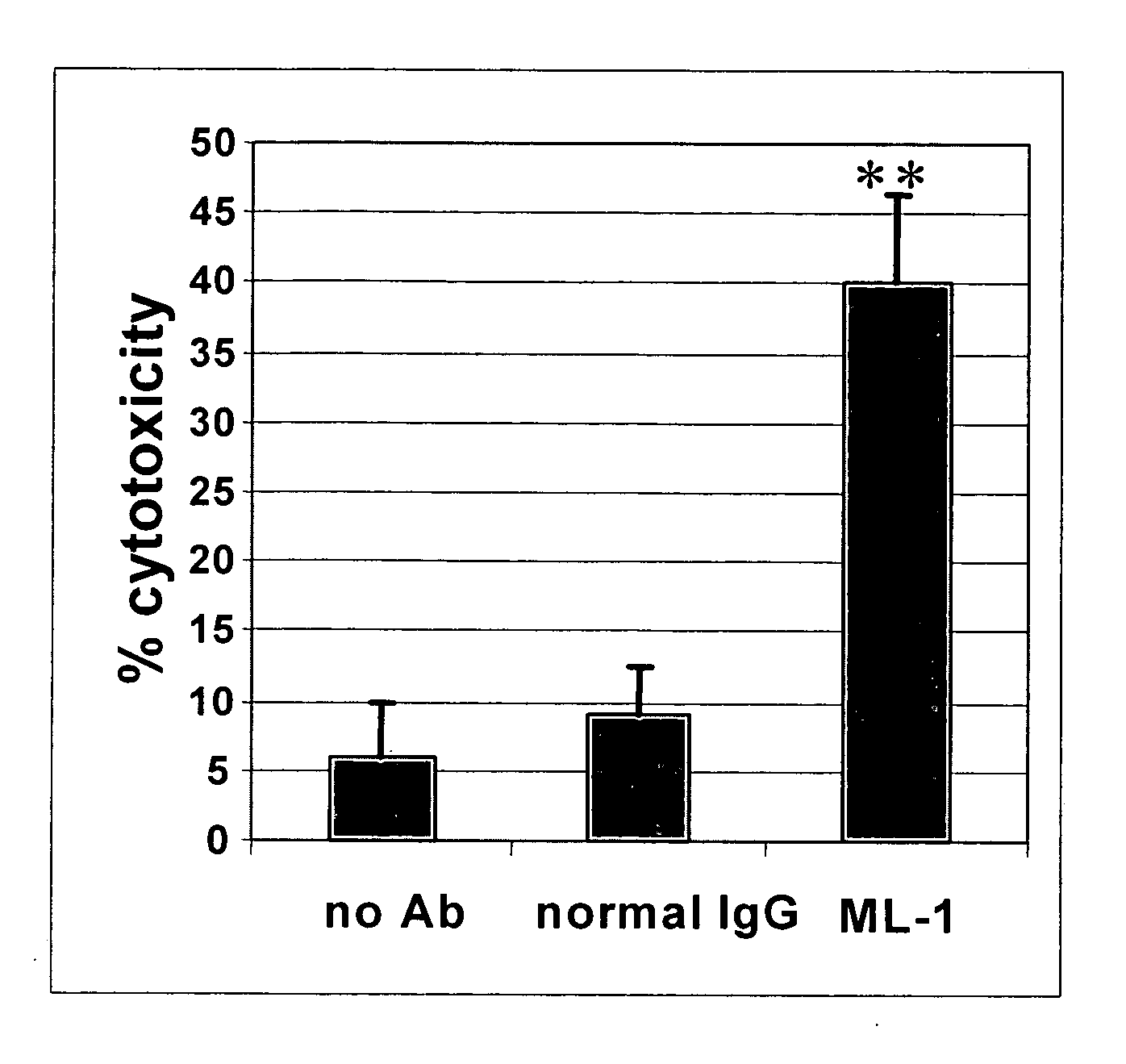
The invention provides a liquid composition and a lyophilized composition comprising a therapeutically effective amount of a conjugate comprising an antibody chemically coupled to a maytansinoid. The invention further provides a method for killing a cell in a human comprising administering to the human either of the compositions such that the antibody binds to the surface of the cell and the cytotoxicity of the maytansinoid is activated, whereby the cell is killed.
Owner:IMMUNOGEN INC
Generation of human embryonc stem-like cells using intronic RNA
ActiveUS20080293143A1Stable and relatively long-term effectDelivery stabilityOther foreign material introduction processesElectrical/wave energy microorganism treatmentReprogrammingMammal
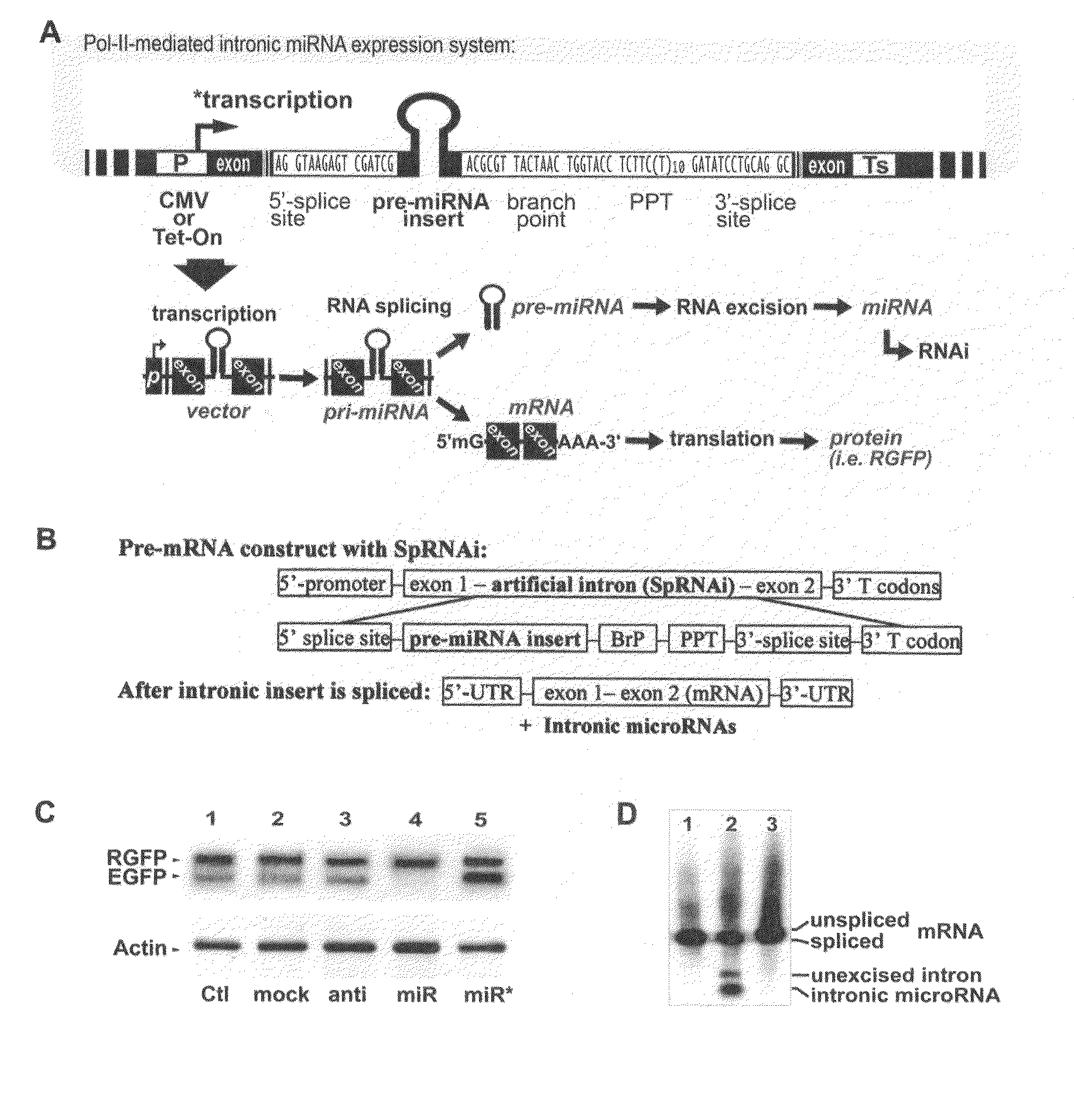
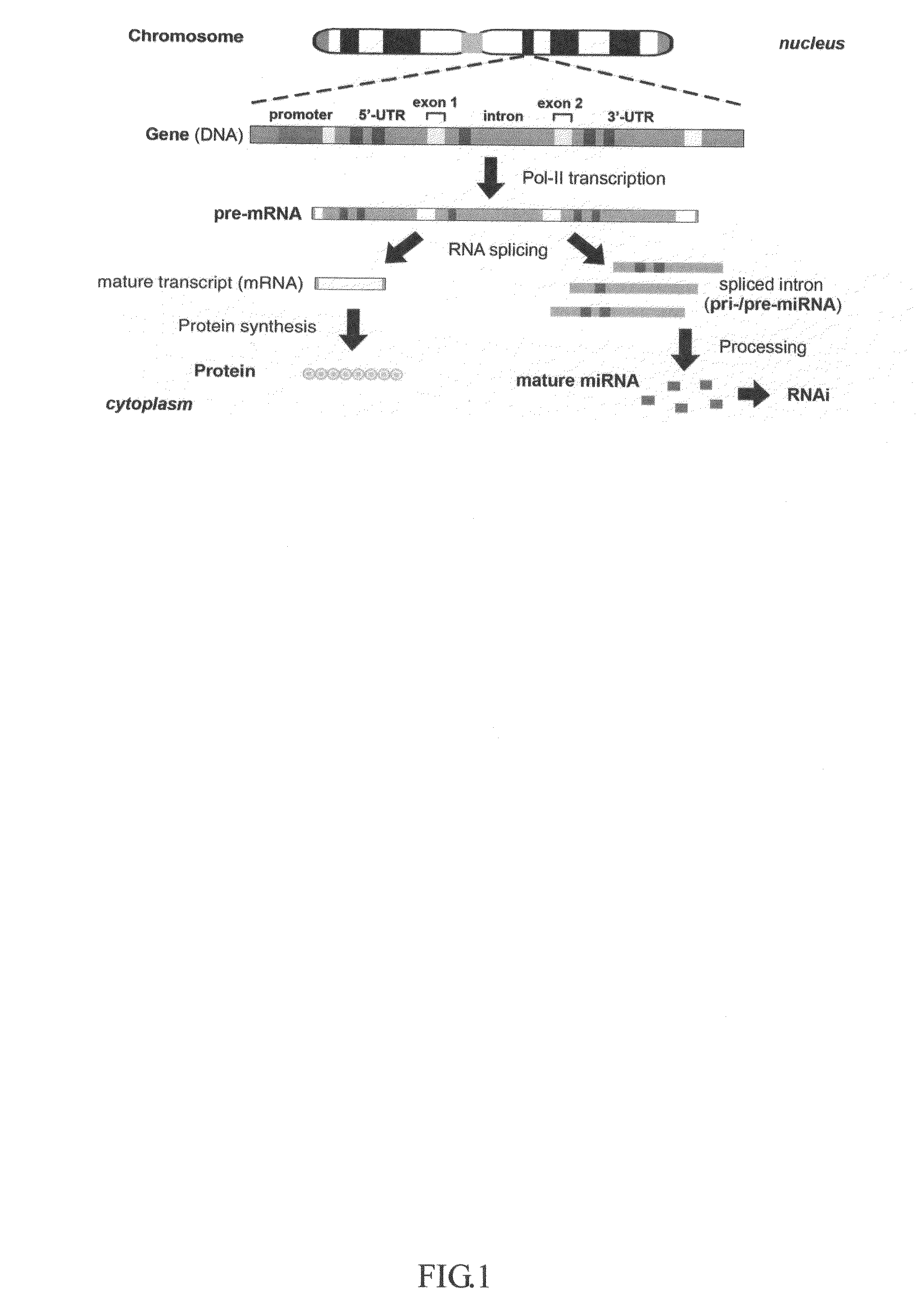
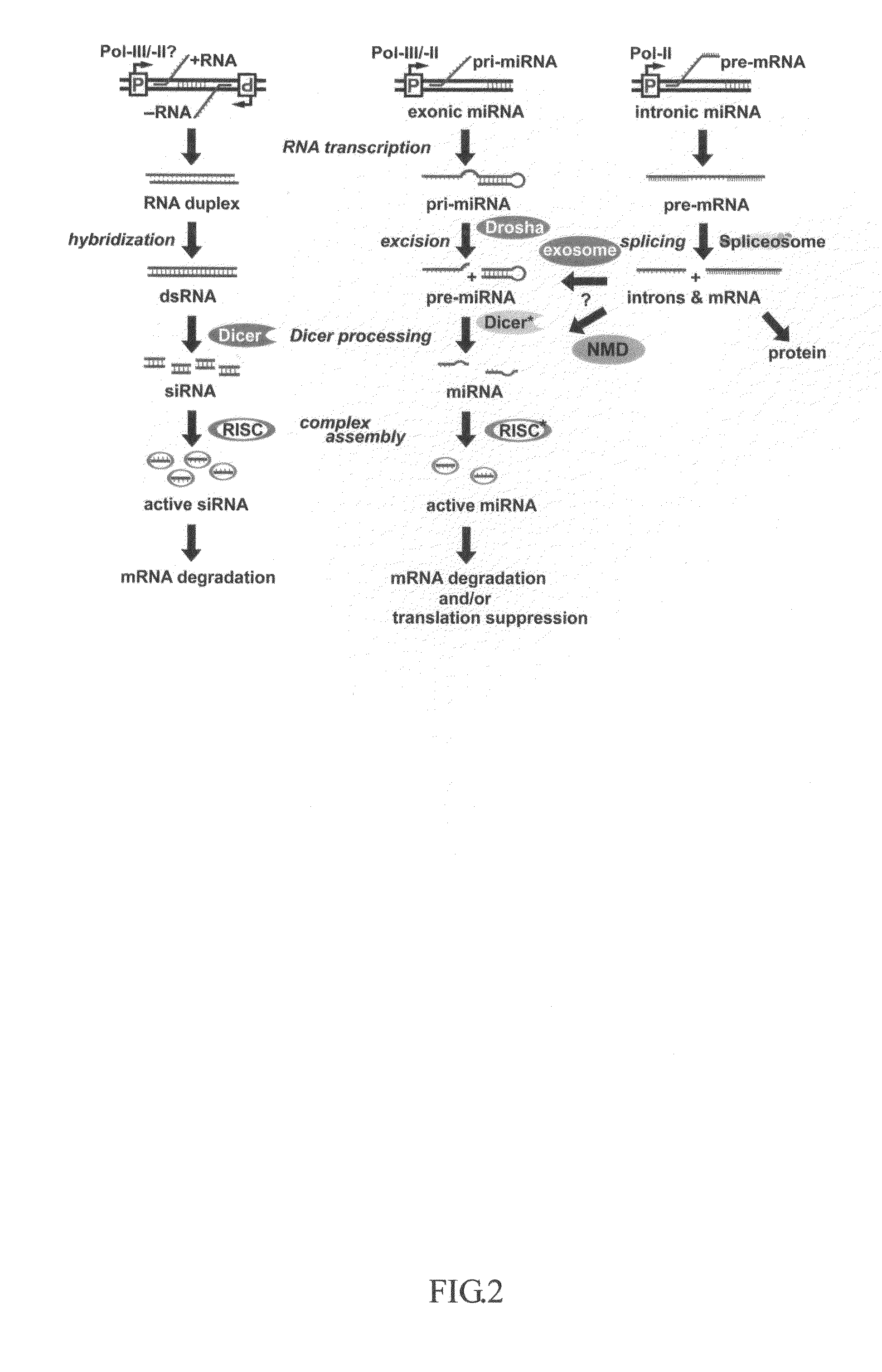
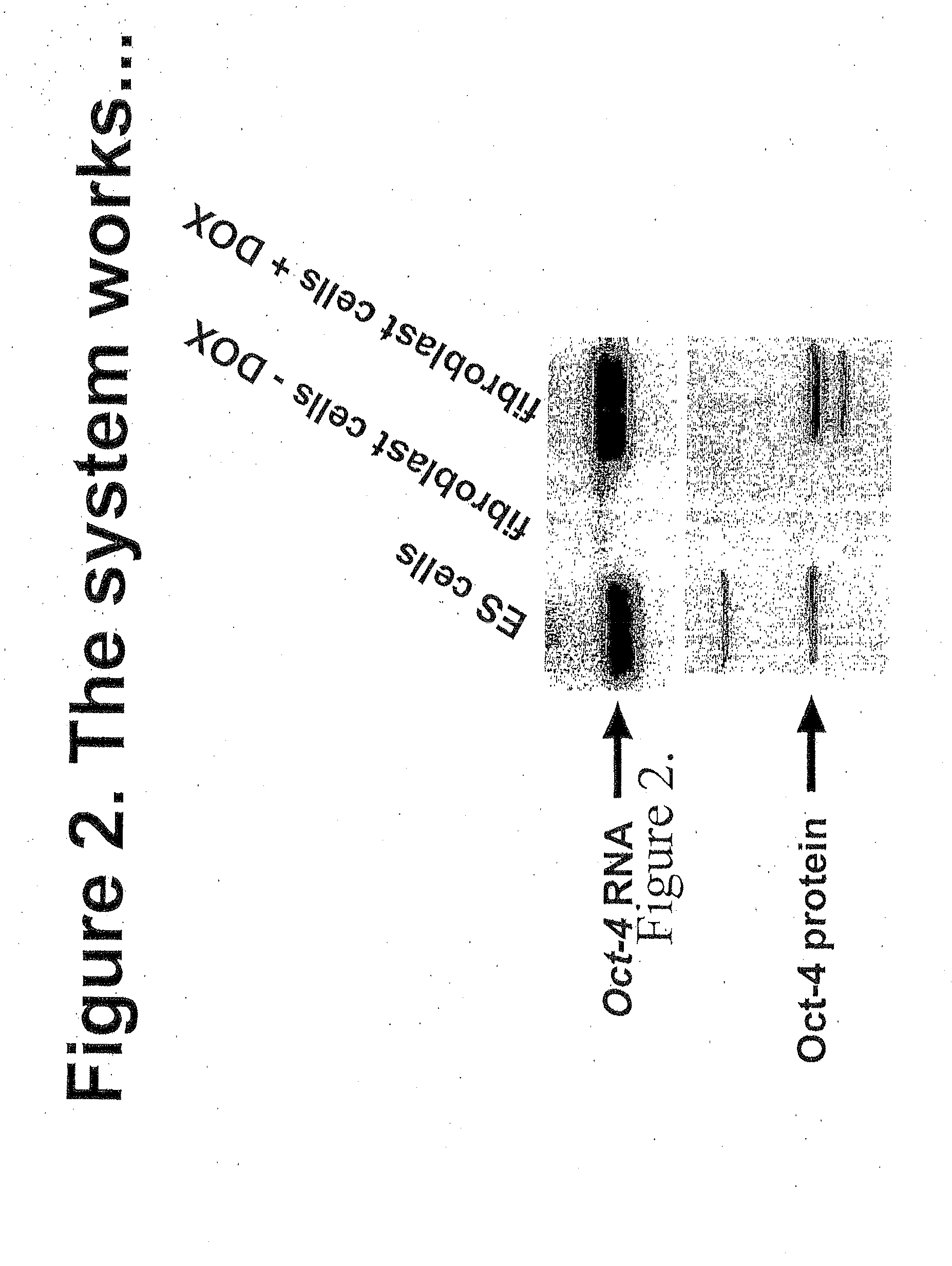
This invention generally relates to a method for developing, generating and selecting human embryonic stem (hES)-like pluripotent cells using transgenic expression of intronic microRNA-like RNA agents. More particularly, the present invention relates to a method and composition for generating a non-naturally occurring intron and its intronic components capable of being processed into mir-302-like RNA molecules in mammalian cells and thus inducing certain specific gene silencing effects on differentiation-related and fate-determinant genes of the cells, resulting in reprogramming the cells into a pluripotent embryonic stem (ES)-cell-like state. The ES-like cells so obtained are strongly express hES cell markers, such as Oct3 / 4, SSEA-3 and SSEA-4, and can be guided into various tissue cell types by treating certain hormones and / or growth factors under a feeder-free cell culture condition in vitro, which may be used for transplantation and gene therapies. Therefore, the present invention offers a simple, effective and safe gene manipulation approach for not only reprogramming somatic cells into ES-like pluripotent cells but also facilitating the maintenance of pluripotent and renewal properties of ES cells under a feeder-free cell culture condition, preventing the tedious retroviral insertion of four large transcription factor genes into one single cell as used in the previous iPS methods.
Owner:MELLO BIOTECH +1
Antibodies with immune effector activity and that internalize in folate receptor alpha-positive cells
InactiveUS20060239910A1Improve anti-tumor activityEnhanced antibody-dependent cellular cytotoxicityOrganic active ingredientsNanomedicineDrug specific IgEFolate Receptor Alpha
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
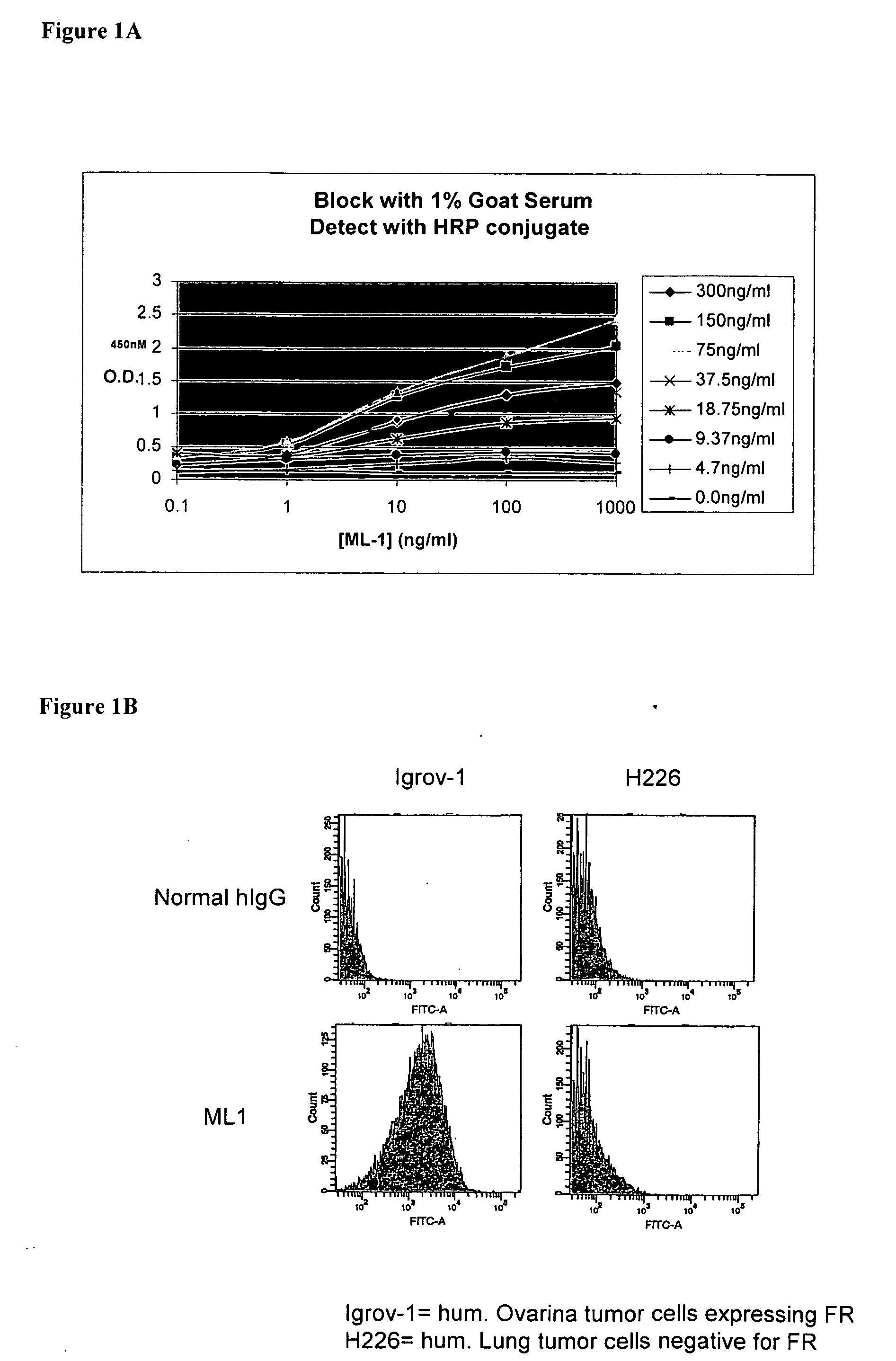
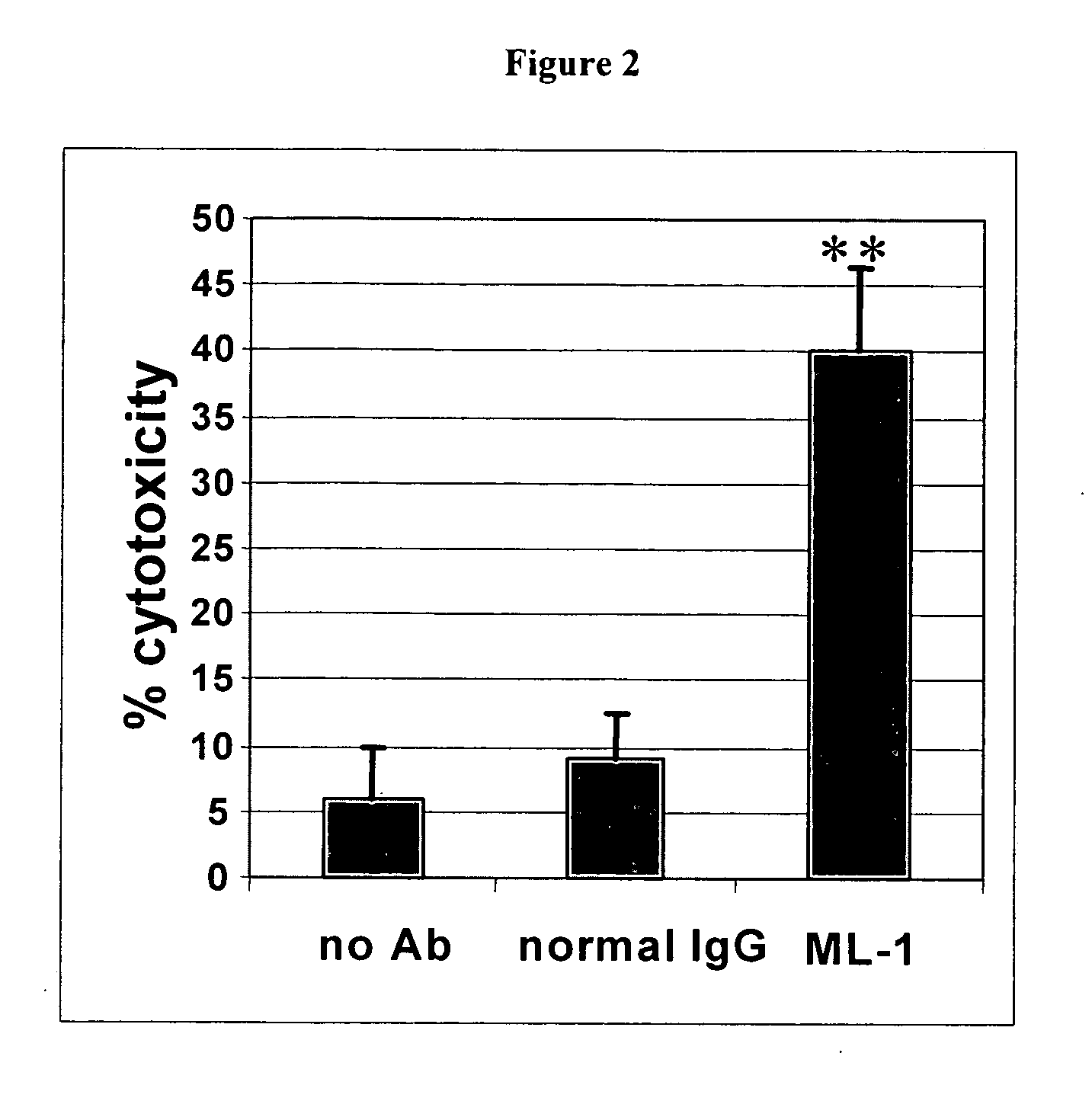
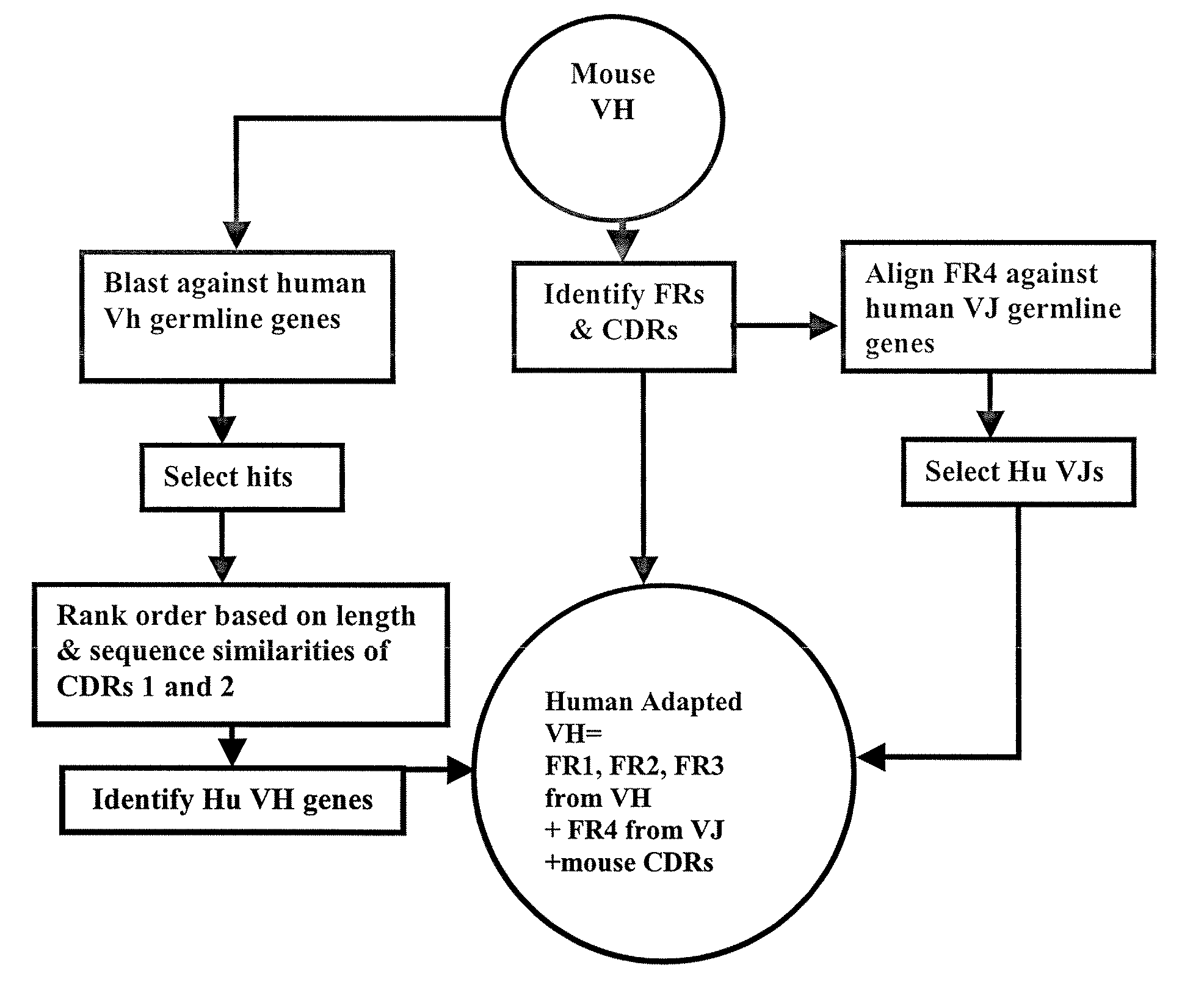
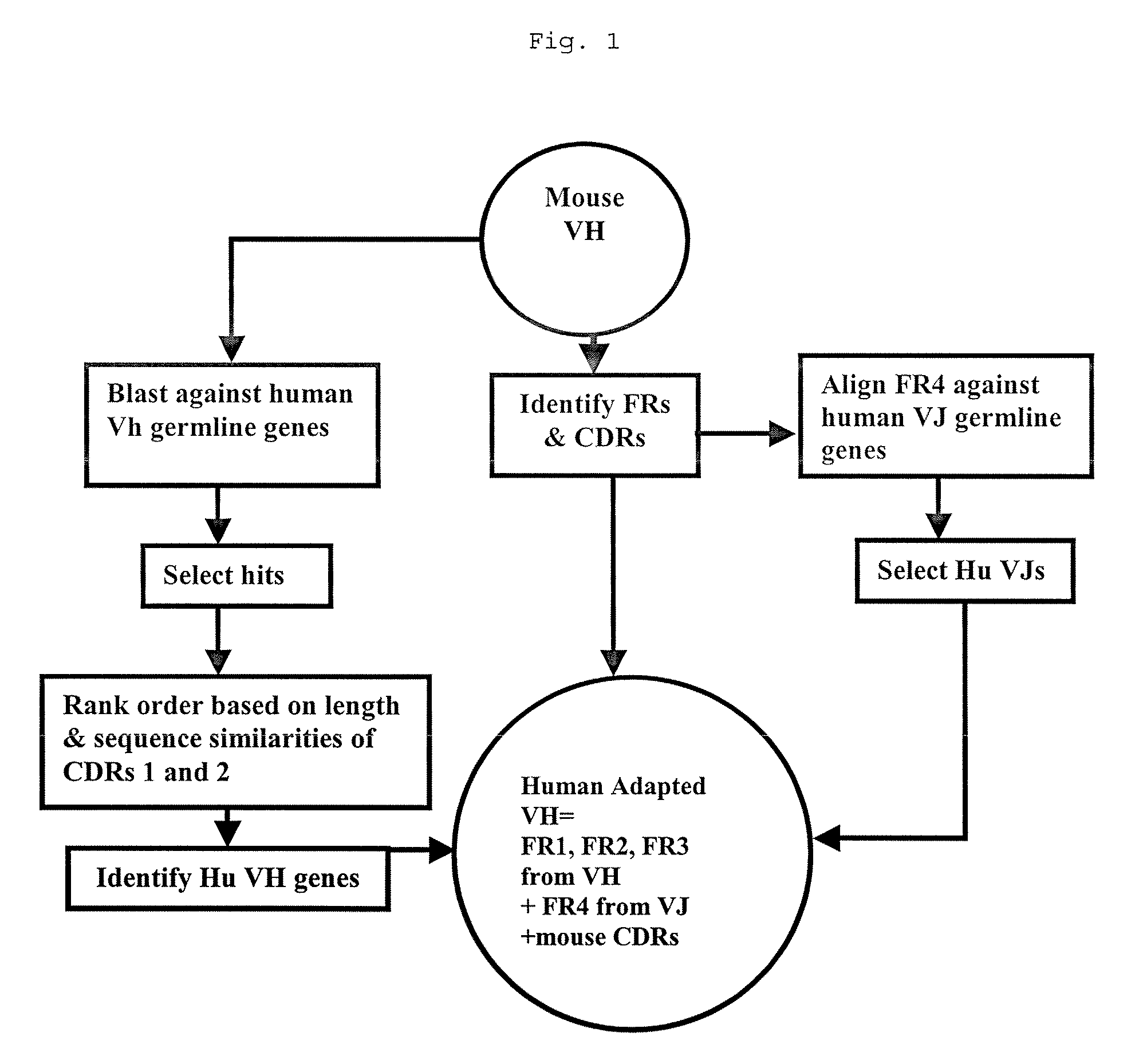
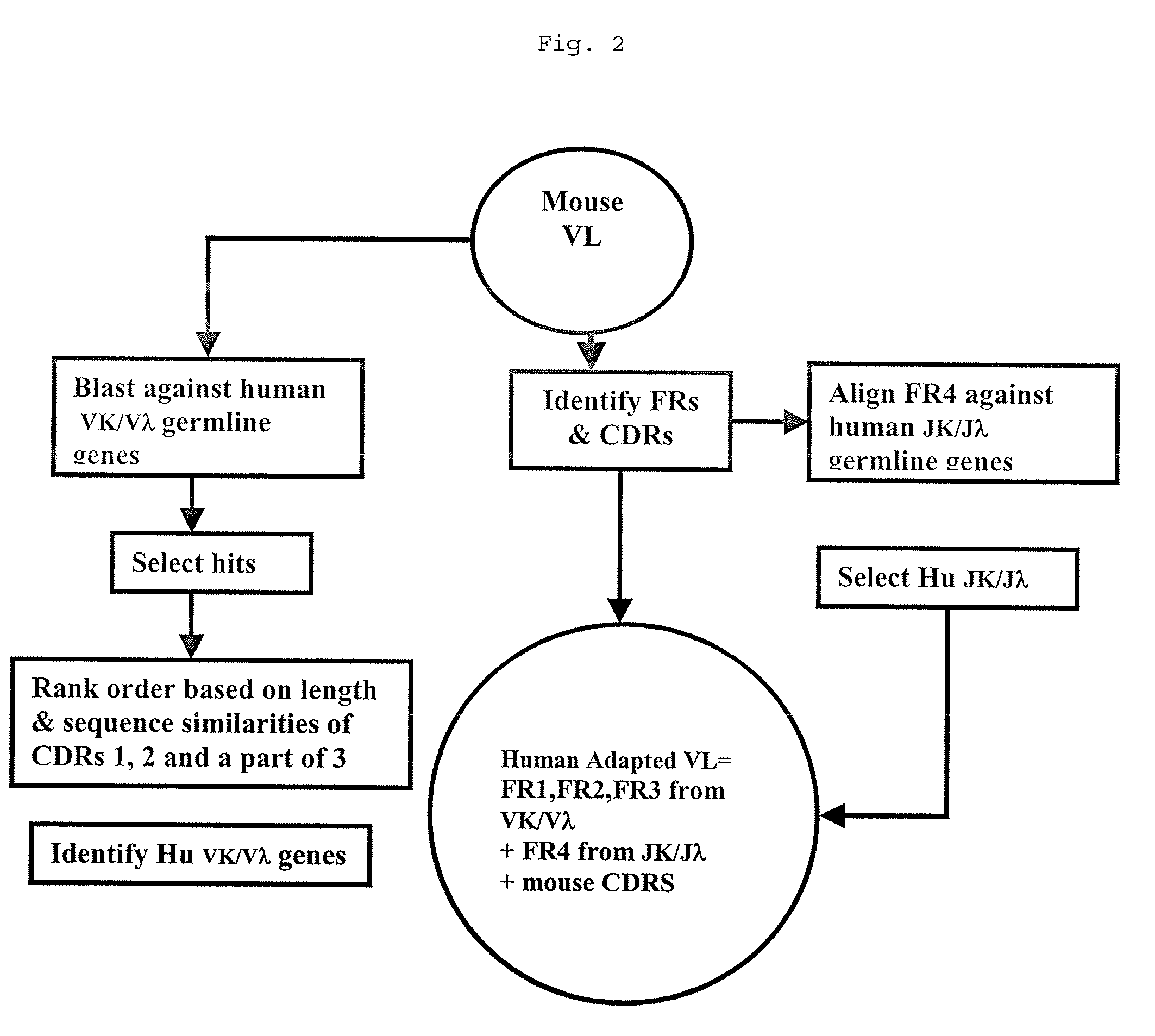
Methods for Use in Human-Adapting Monoclonal Antibodies
Methods useful for human adapting non-human monoclonal antibodies are disclosed. The methods select candidate human antibody framework sequences from a database of human germline genes or human sequences with somatic mutations.
Owner:JANSSEN BIOTECH INC
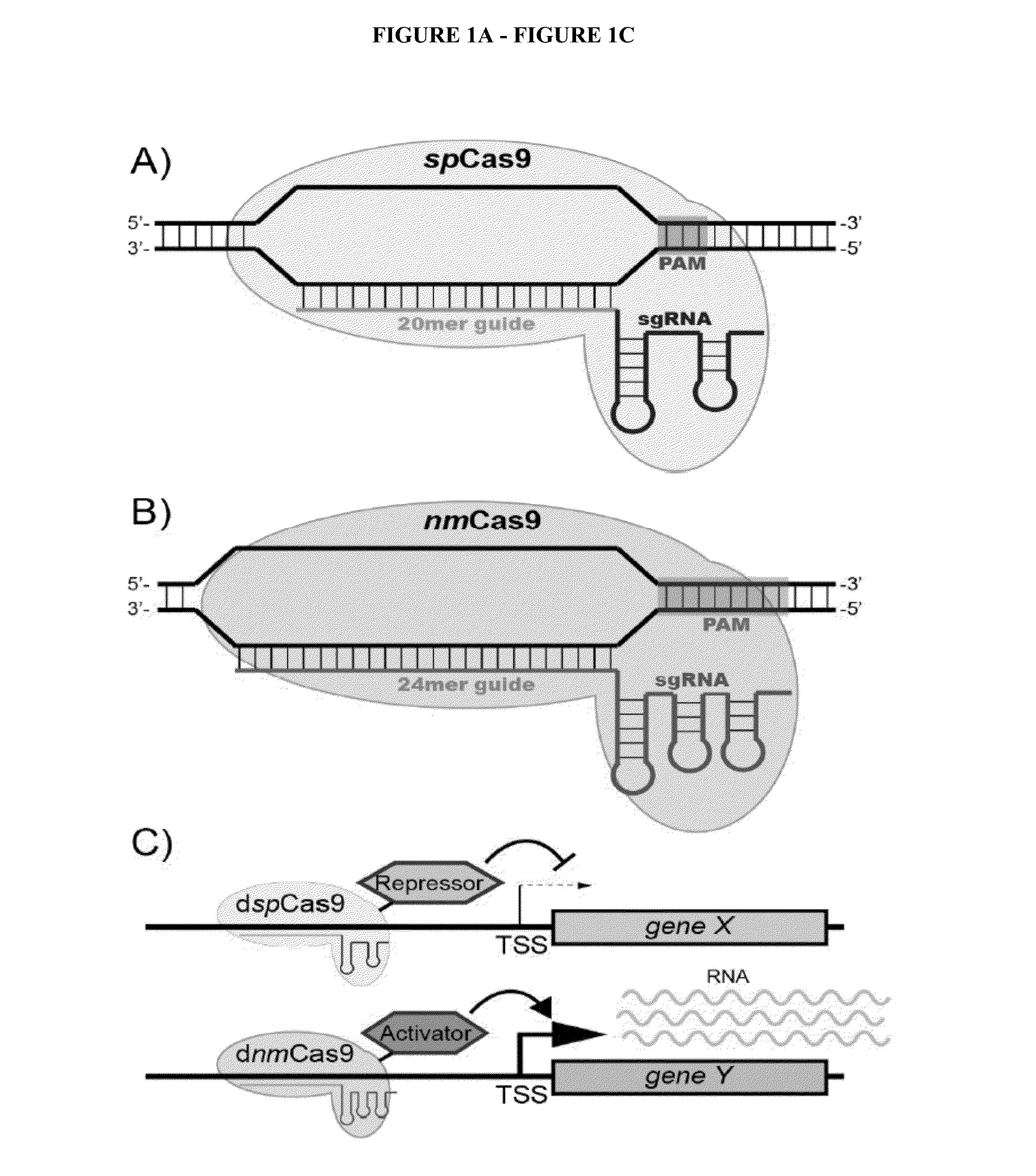
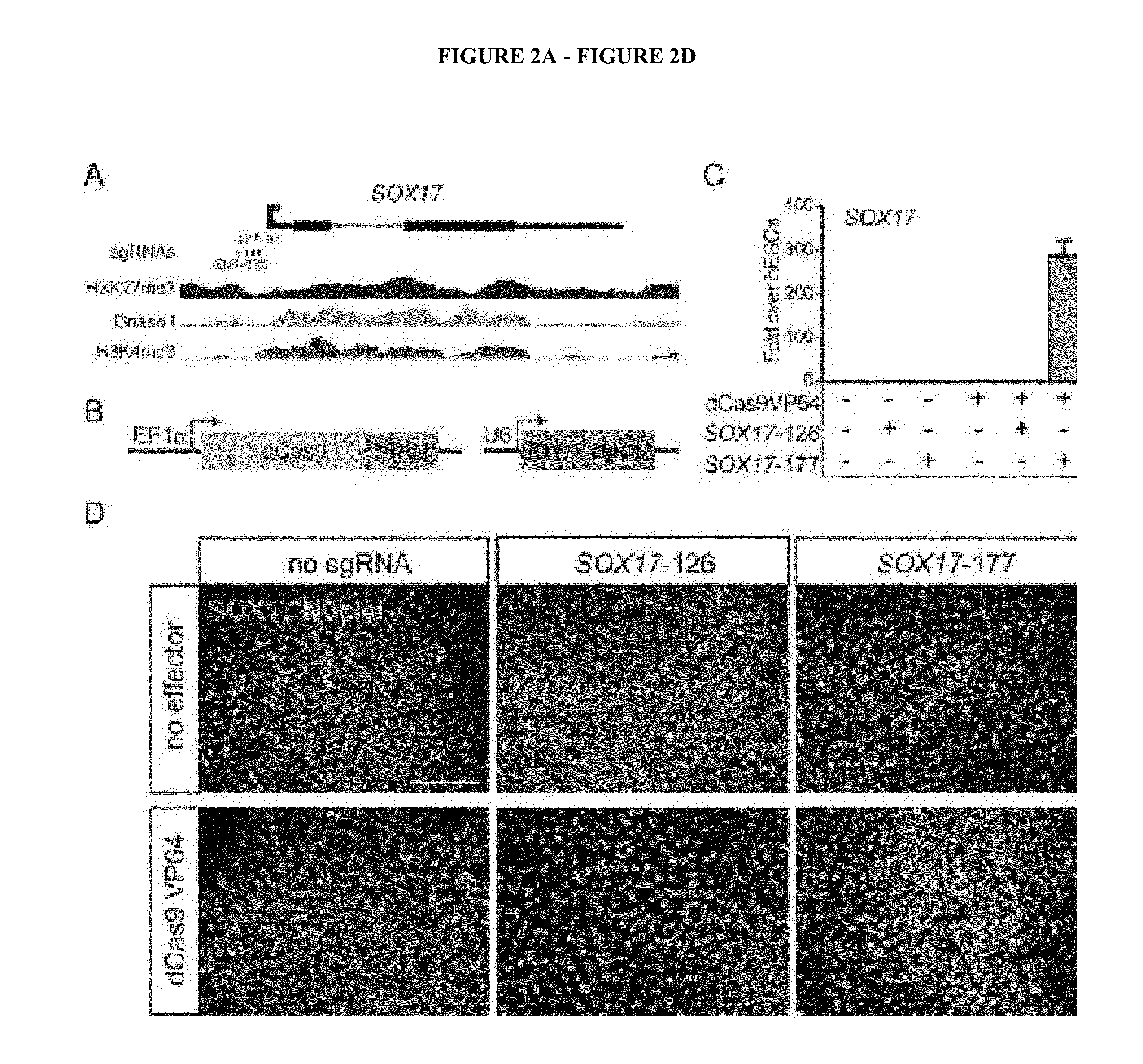
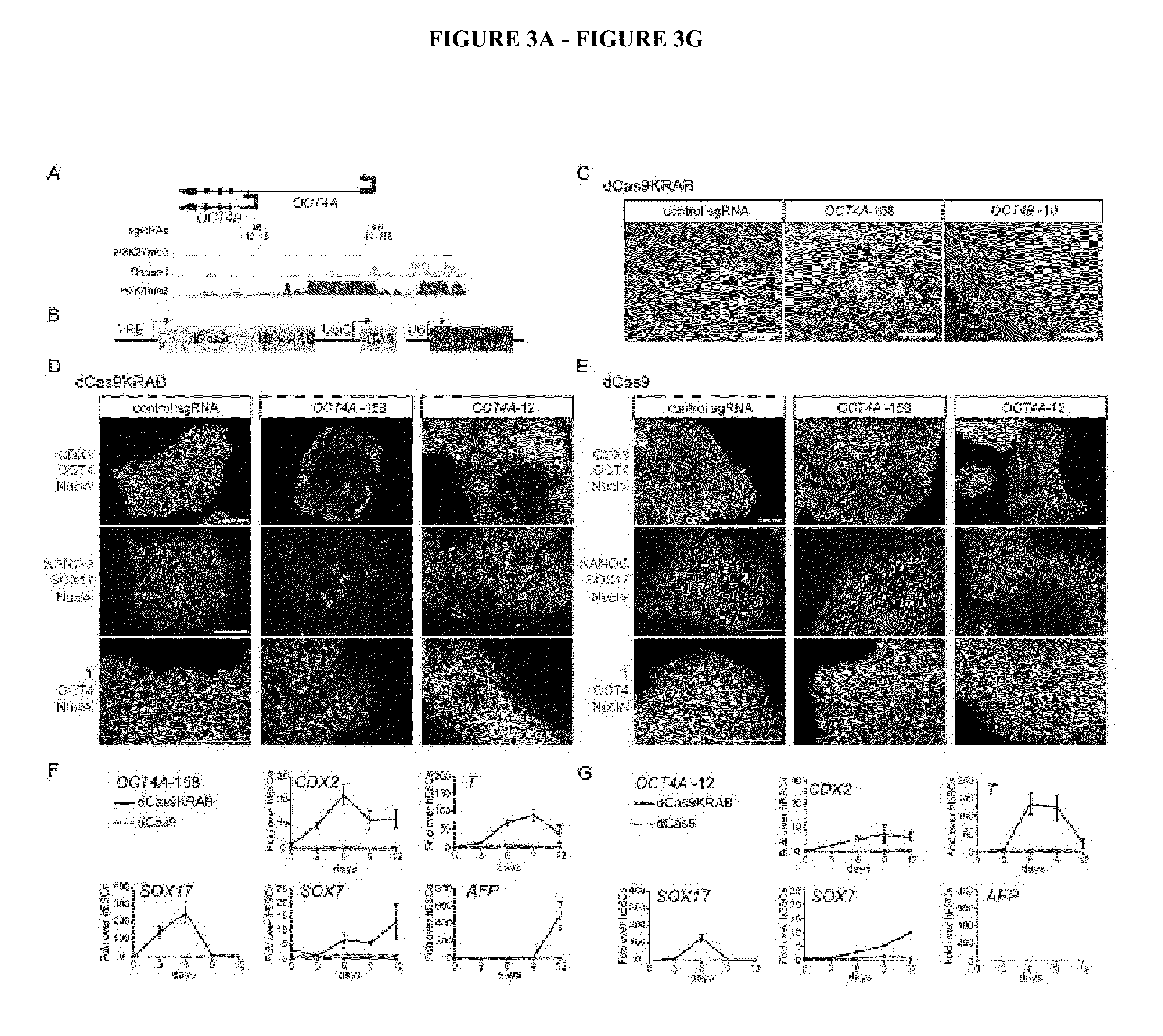
Cas9 effector-mediated regulation of transcription, differentiation and gene editing/labeling
InactiveUS20150191744A1Prevent degradationHydrolasesMicrobiological testing/measurementProgenitorReprogramming
The present disclosure relates to methods of and systems for modifying the transcriptional regulation of stem or progenitor cells to promote their differentiation or reprogramming of somatic cells. Further, the labeling and editing of human genomic loci in live cells with three orthogonal CRISPR / Cas9 components allow multicolor detection of genomic loci with high spatial resolution, which provides an avenue for barcoding elements of the human genome in the living state.
Owner:UNIV OF CENT FLORIDA RES FOUND INC +1
Reprogramming of somatic cells
The disclosure relates to a method of reprogramming one or more somatic cells, e.g., partially differentiated or fully / terminally differentiated somatic cells, to a less differentiated state, e.g., a pluripotent or multipotent state. In further embodiments the invention also relates to reprogrammed somatic cells produced by methods of the invention, to uses of said cells, and to methods for identifying agents useful for reprogramming somatic cells.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Methods for reprogramming somatic cells
The invention provides methods for reprogramming somatic cells to generate multipotent or pluripotent cells. Such methods are useful for a variety of purposes, including treating or preventing a medical condition in an individual. The invention further provides methods for identifying an agent that reprograms somatic cells to a less differentiated state.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Method of nuclear transfer
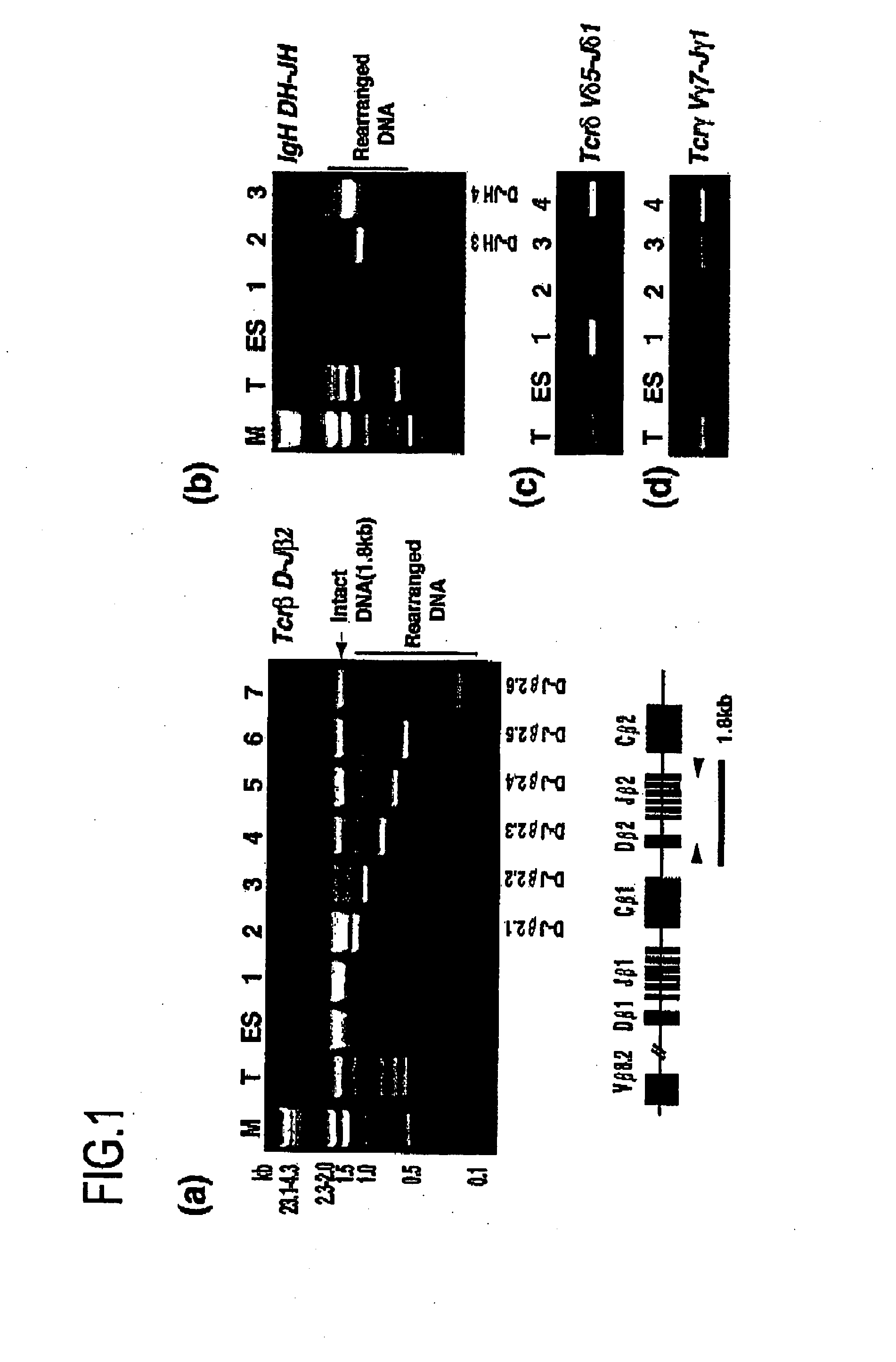
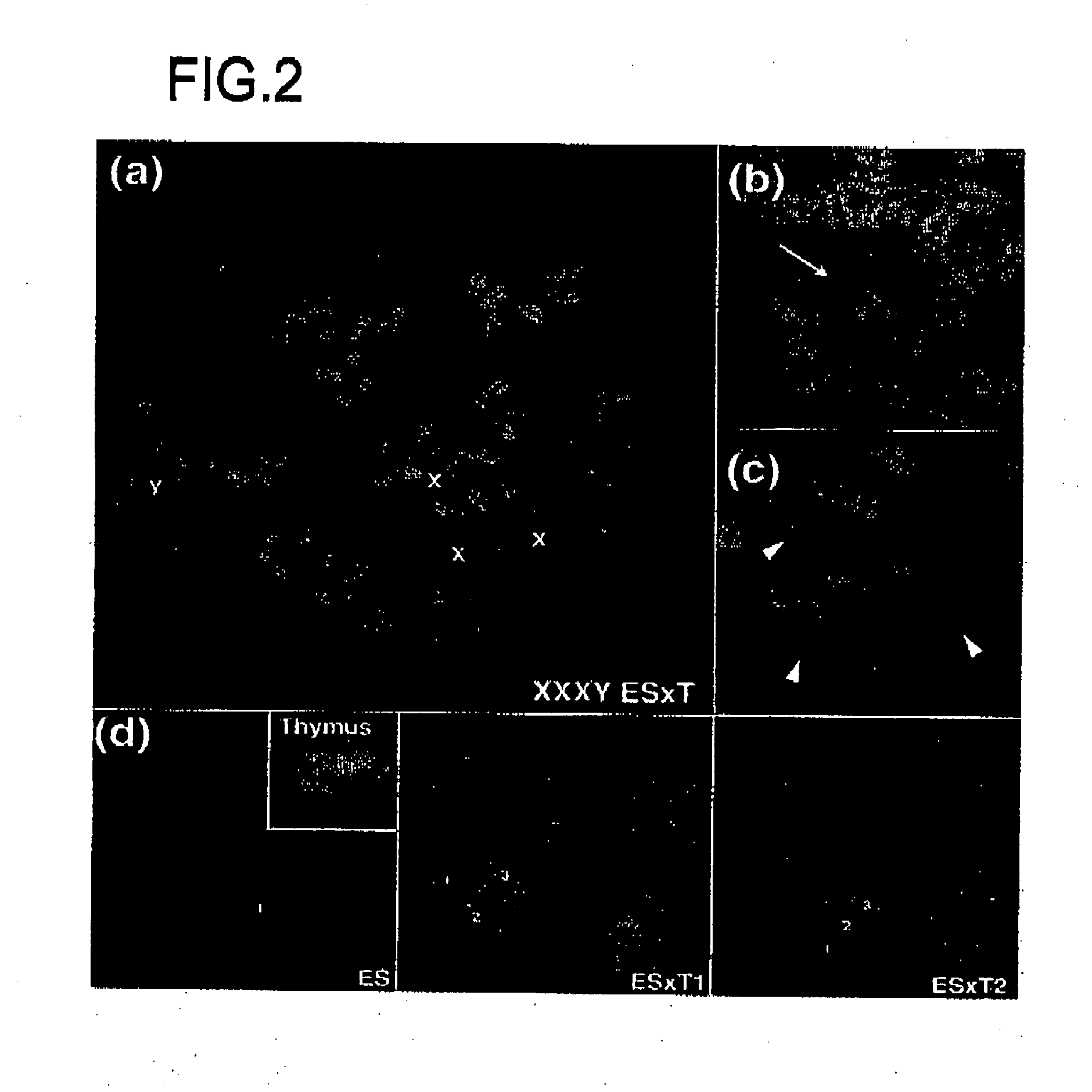
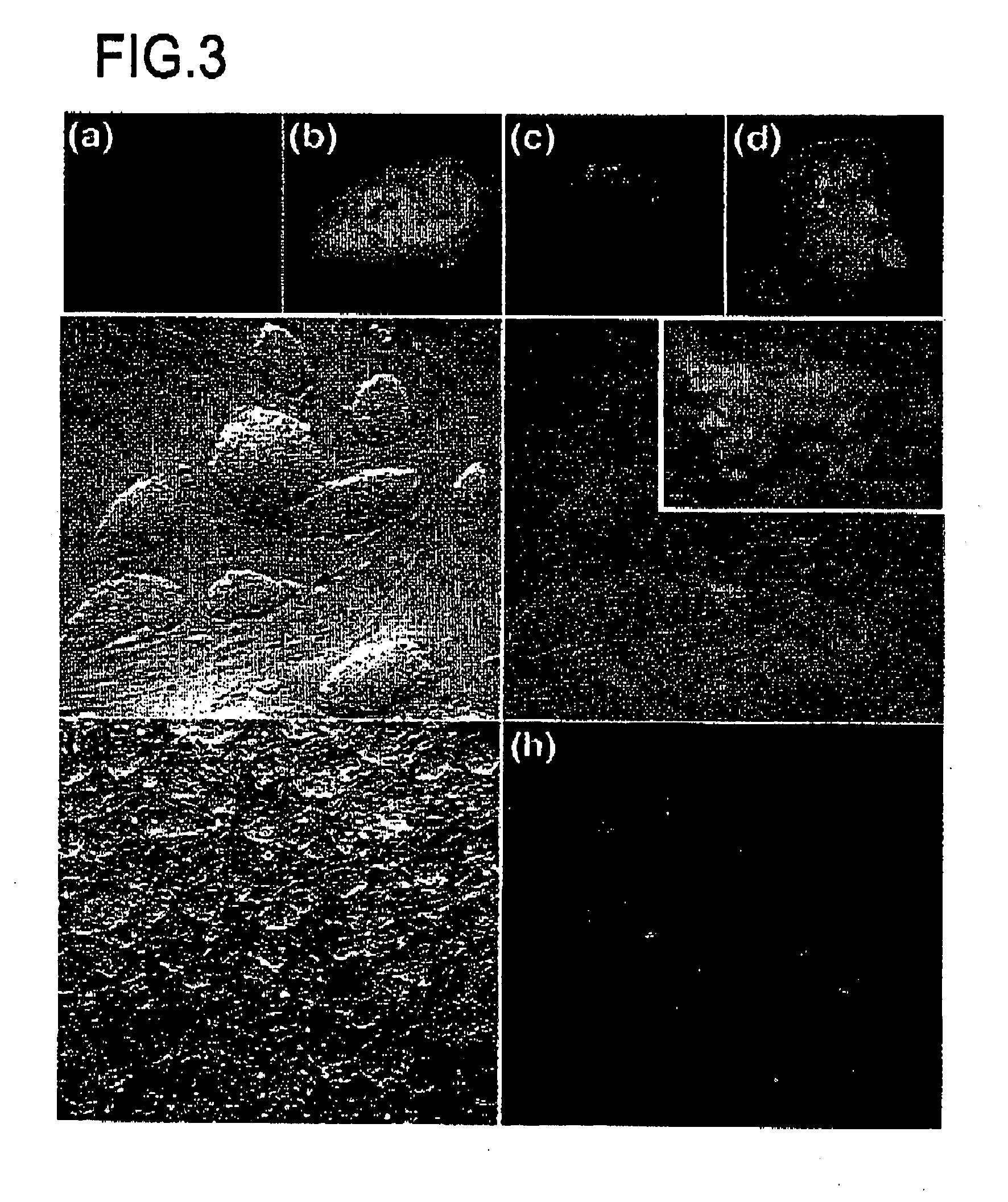
InactiveUS20080092249A1Increase the number of cellsImprove pregnancy rateRecombinant DNA-technologyFermentationNuclear transferSomatic cell
The present invention relates to nuclear methods and embryos developed therefrom. In particular, the present invention relates to a method of nuclear comprising the step of transferring a somatic cell nuclei into a zona pellucida-free, enucleated oocyte.
Owner:MONASH UNIV
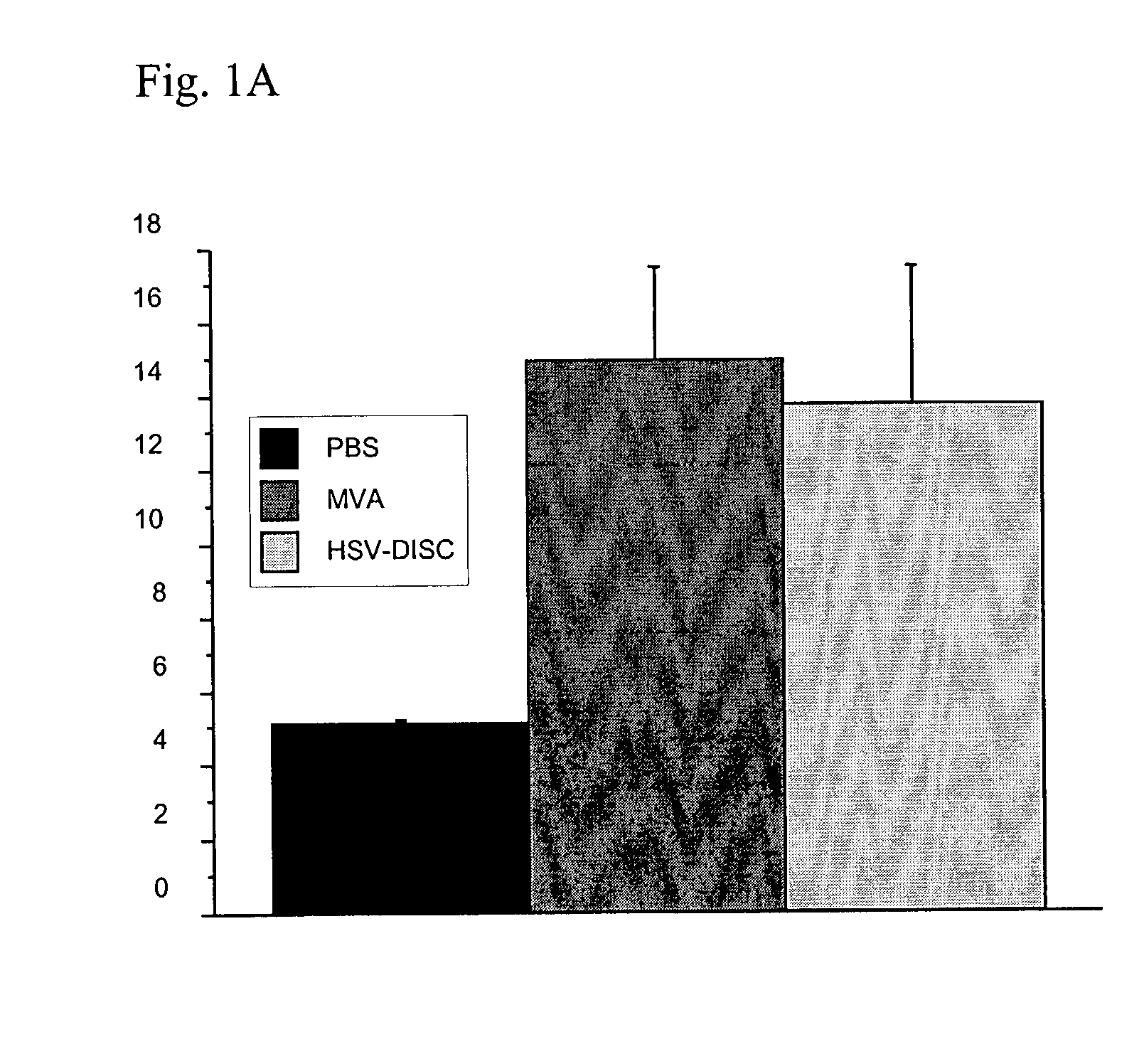
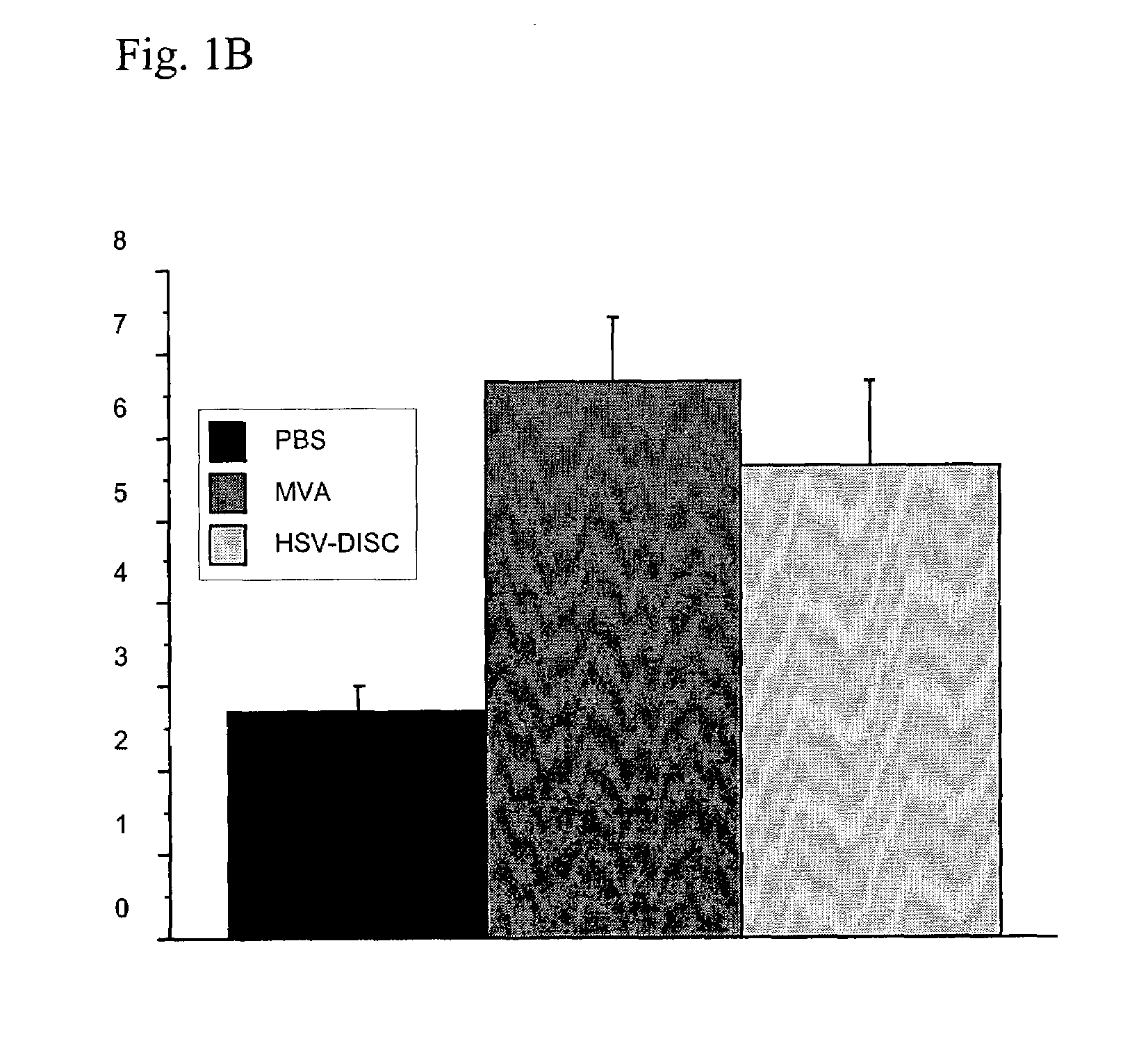
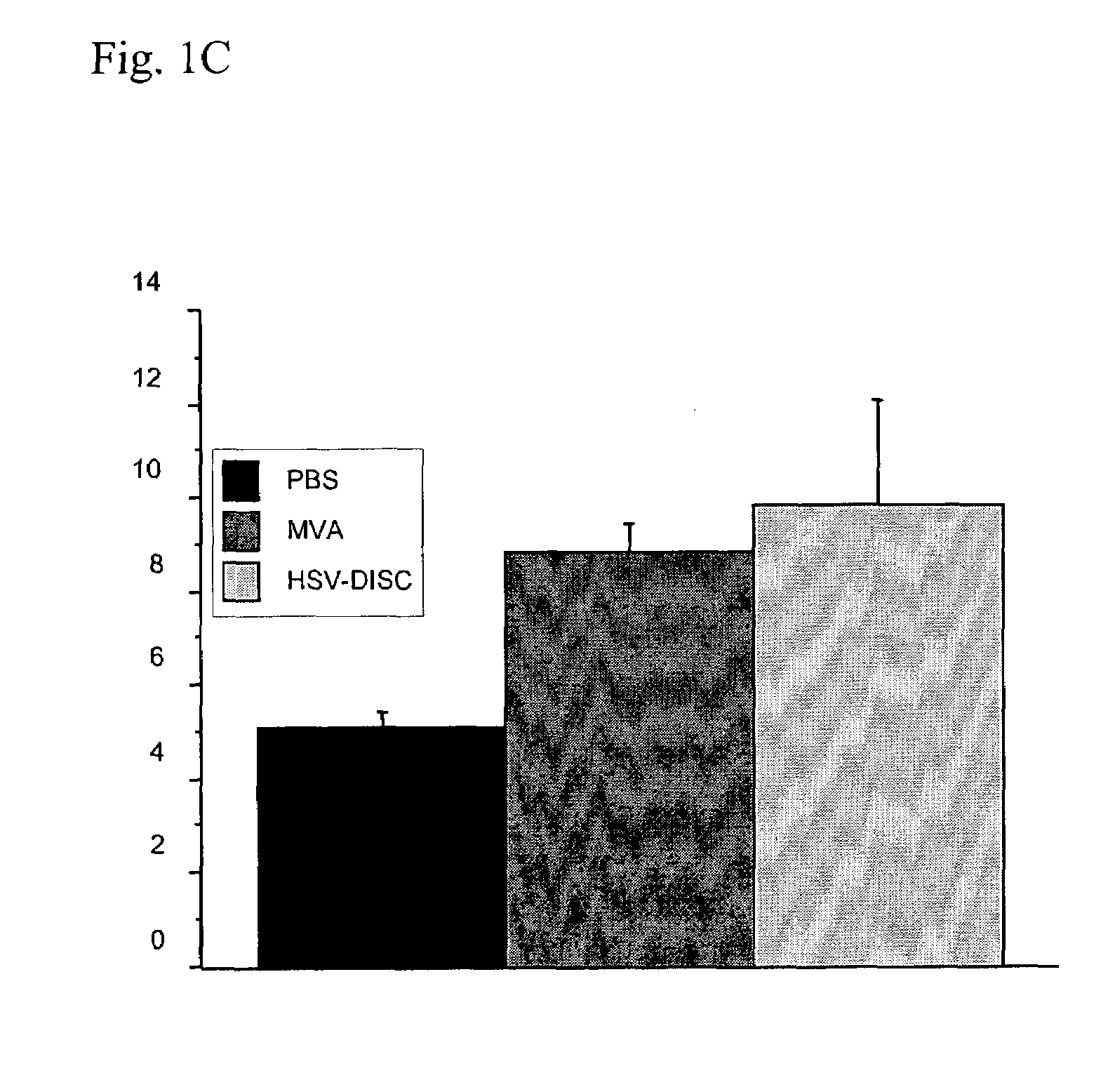
Modified vaccinia virus ankara for the vaccination of neonates
Owner:BAVARIAN NORDIC AS
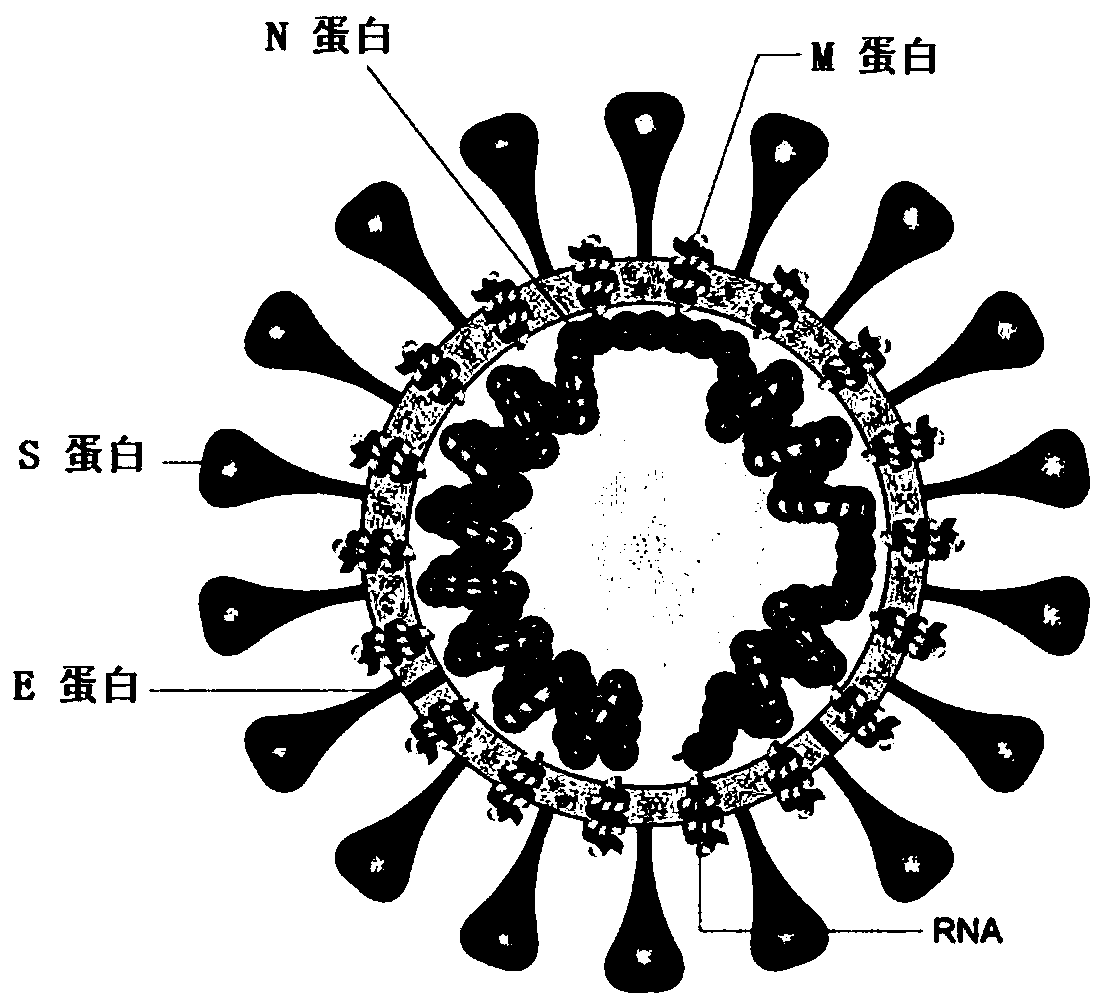
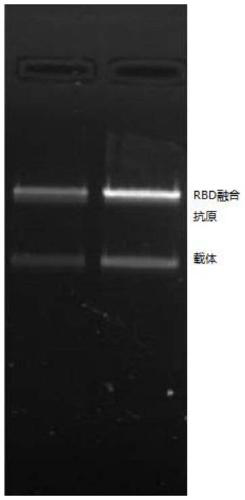
SARS-CoV-2 vaccine and preparation method thereof
ActiveCN111217917AEnter to helpImproving immunogenicityPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenDisease
The invention relates to a preparation method for a vaccine capable of treating and / or preventing SARS-CoV-2 infection or COVID-19 diseases. The core antigen of the vaccine comprises the RBD (receptor binding zone) fusion protein of the SARS-CoV-2, and a vaccine form comprises an RBD fusion protein subunit vaccine, an RBD fusion protein mRNA vaccine or an RBD fusion protein adenovirus vector vaccine. The above vaccine immunizes an organism, and immune reaction for treating and / or preventing the SARS-CoV-2 infection can be generated so as to be used for treating and / or preventing COVID-19. The invention also relates to an RBD fusion gene, the RBD fusion protein, a carrier, a cell, a preparation method, a treatment method or a pharmacy purpose of the SARS-CoV-2.
Owner:CANSINO BIOLOGICS INC
Compositions and methods for reprogramming eukaryotic cells
The present invention relates to methods for changing the state of differentiation of a eukaryotic cell, the methods comprising introducing mRNA encoding one or more reprogramming factors into a cell and maintaining the cell under conditions wherein the cell is viable and the mRNA that is introduced into the cell is expressed in sufficient amount and for sufficient time to generate a cell that exhibits a changed state of differentiation compared to the cell into which the mRNA was introduced, and compositions therefor. For example, the present invention provides mRNA molecules and methods for their use to reprogram human somatic cells into pluripotent stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
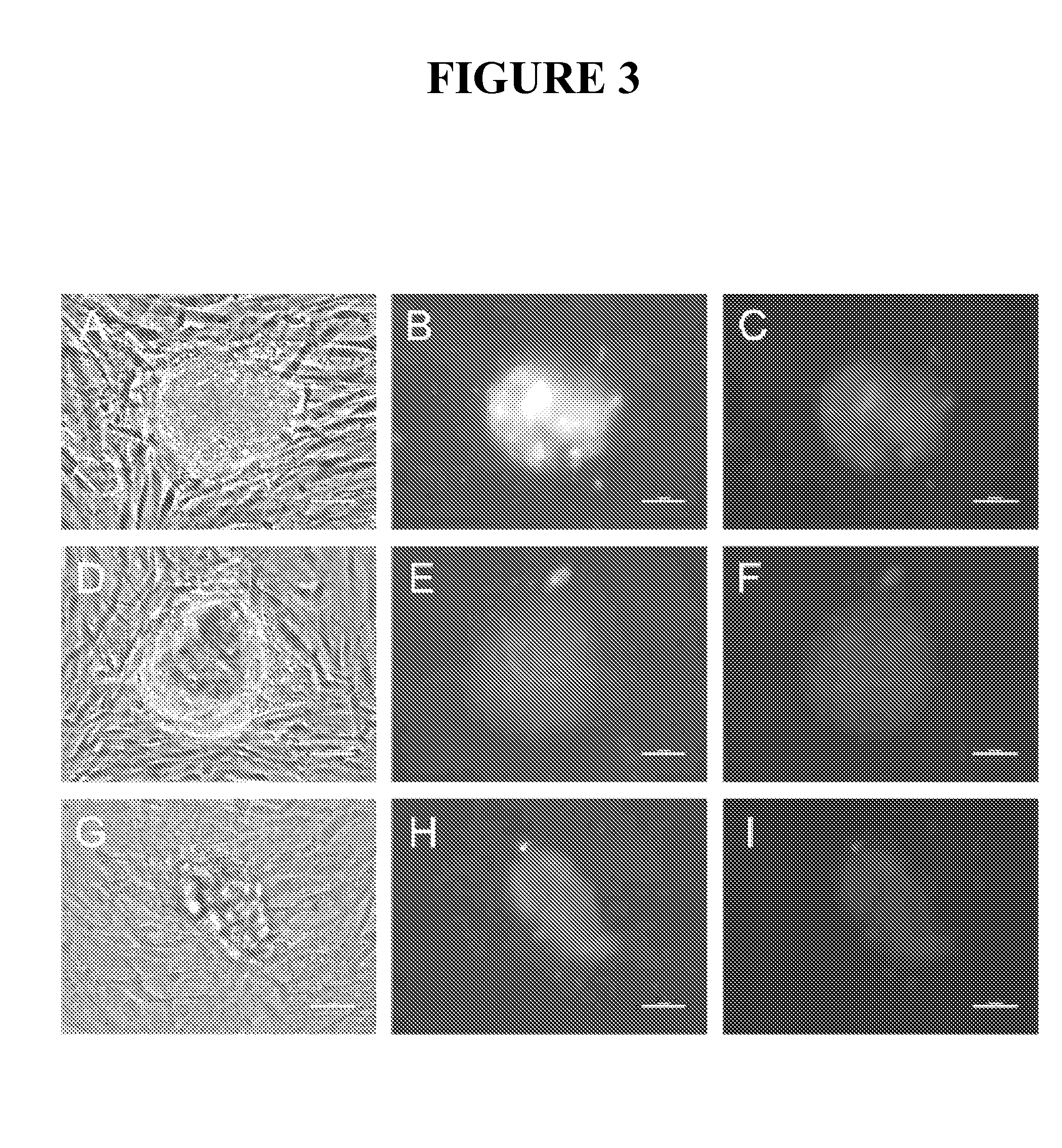
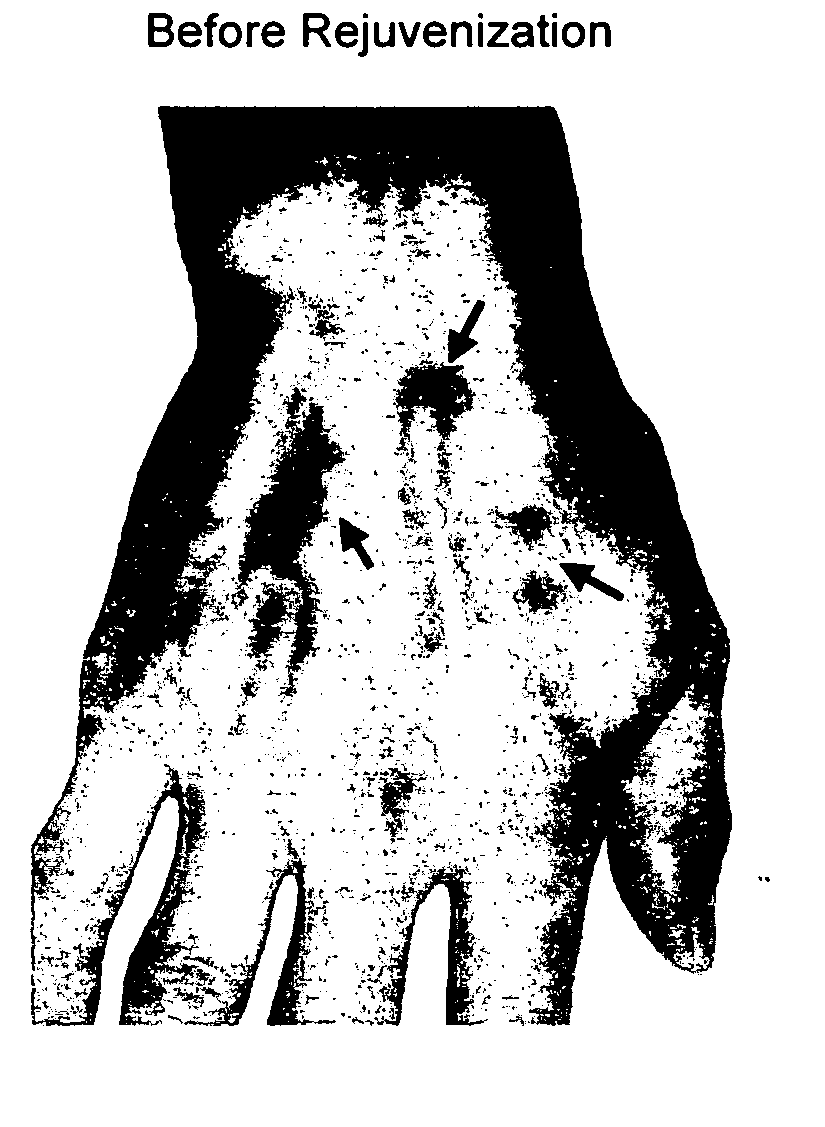
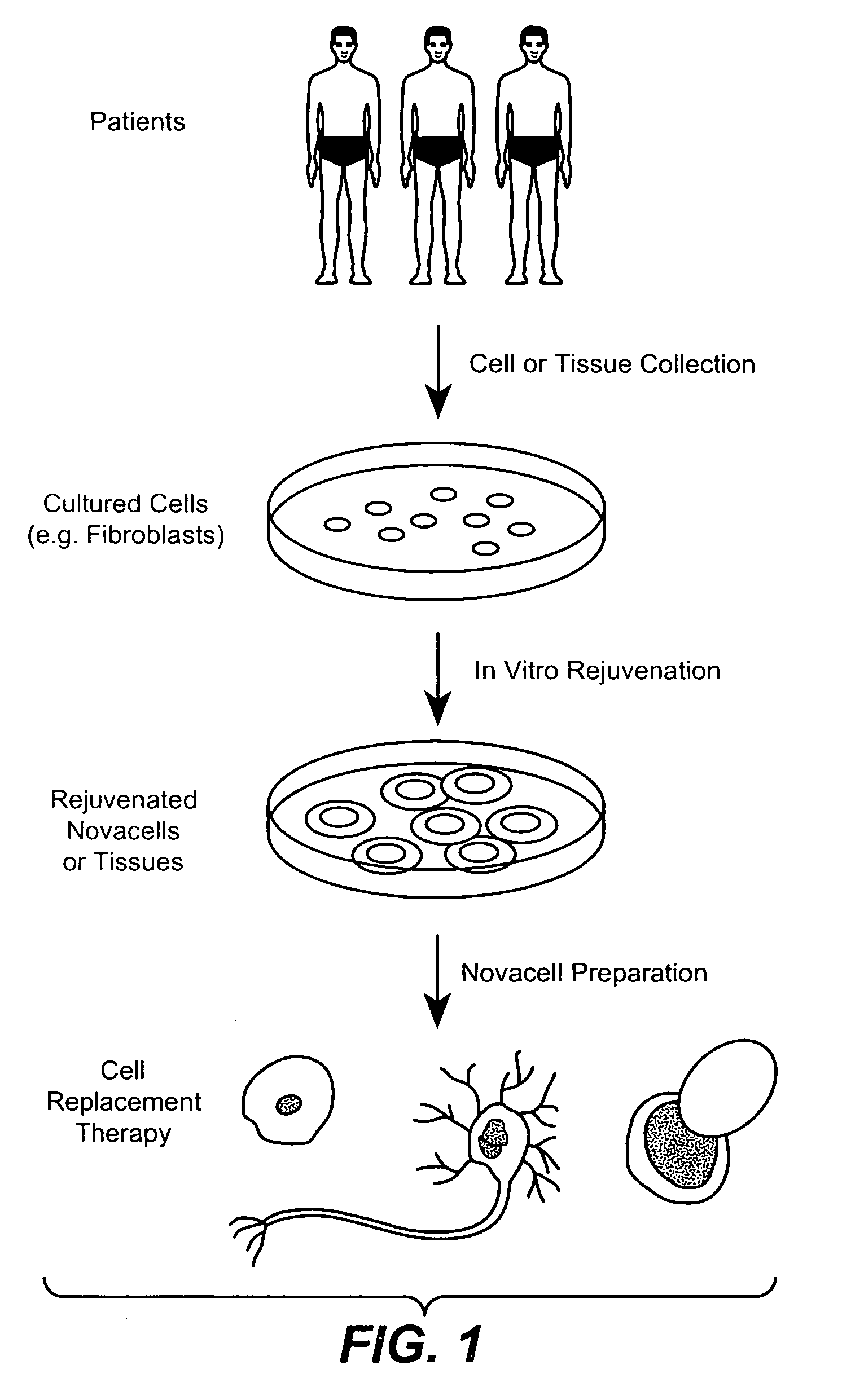
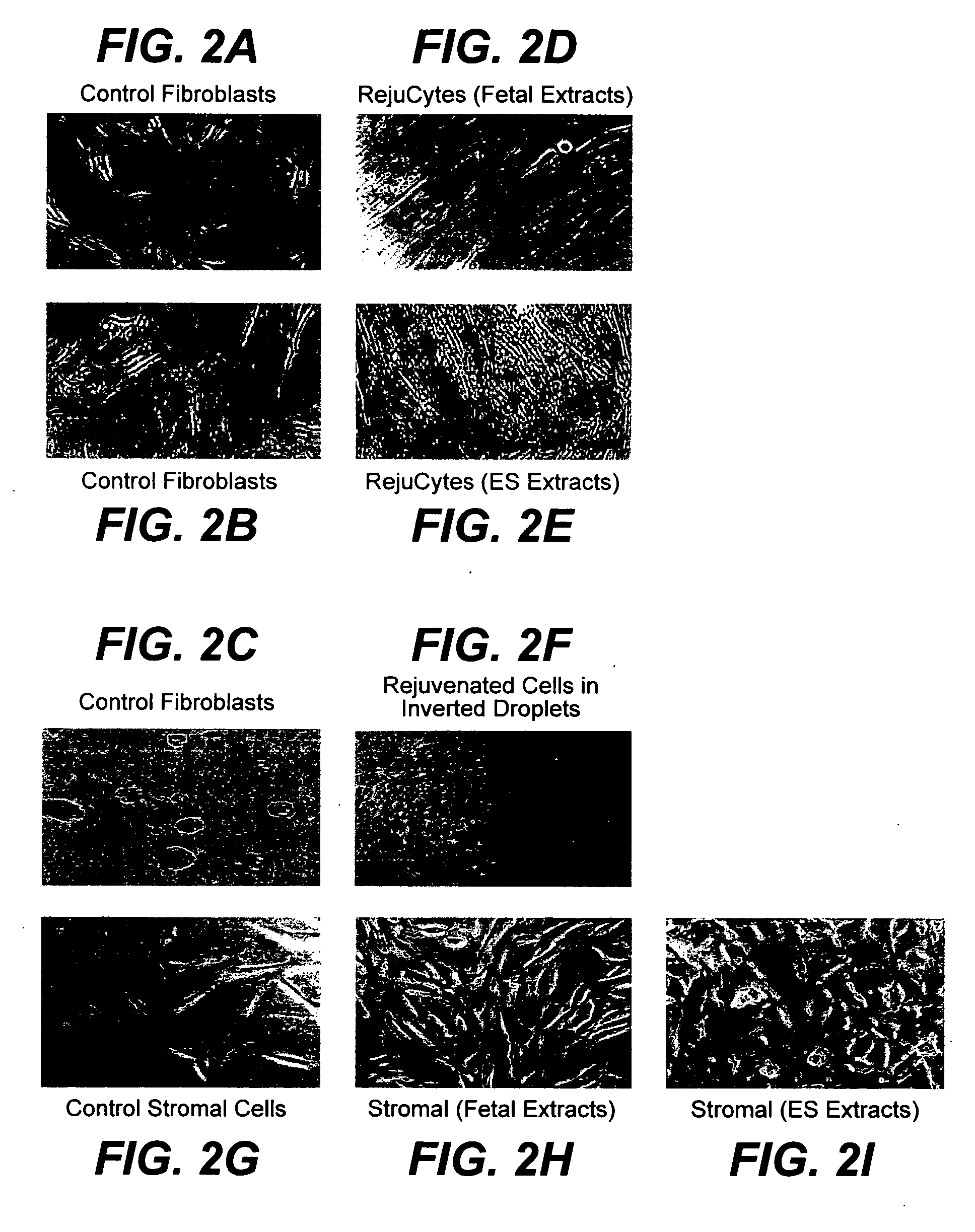
Methods for rejuvenating cells in vitro and in vivo
Owner:HU JIFAN
Isolation Of Novel AAV'S And Uses Thereof
ActiveUS20100186103A1Determine effectAvoid time-consume and costly processVectorsSpecial deliveryAdeno associate virusBinding site
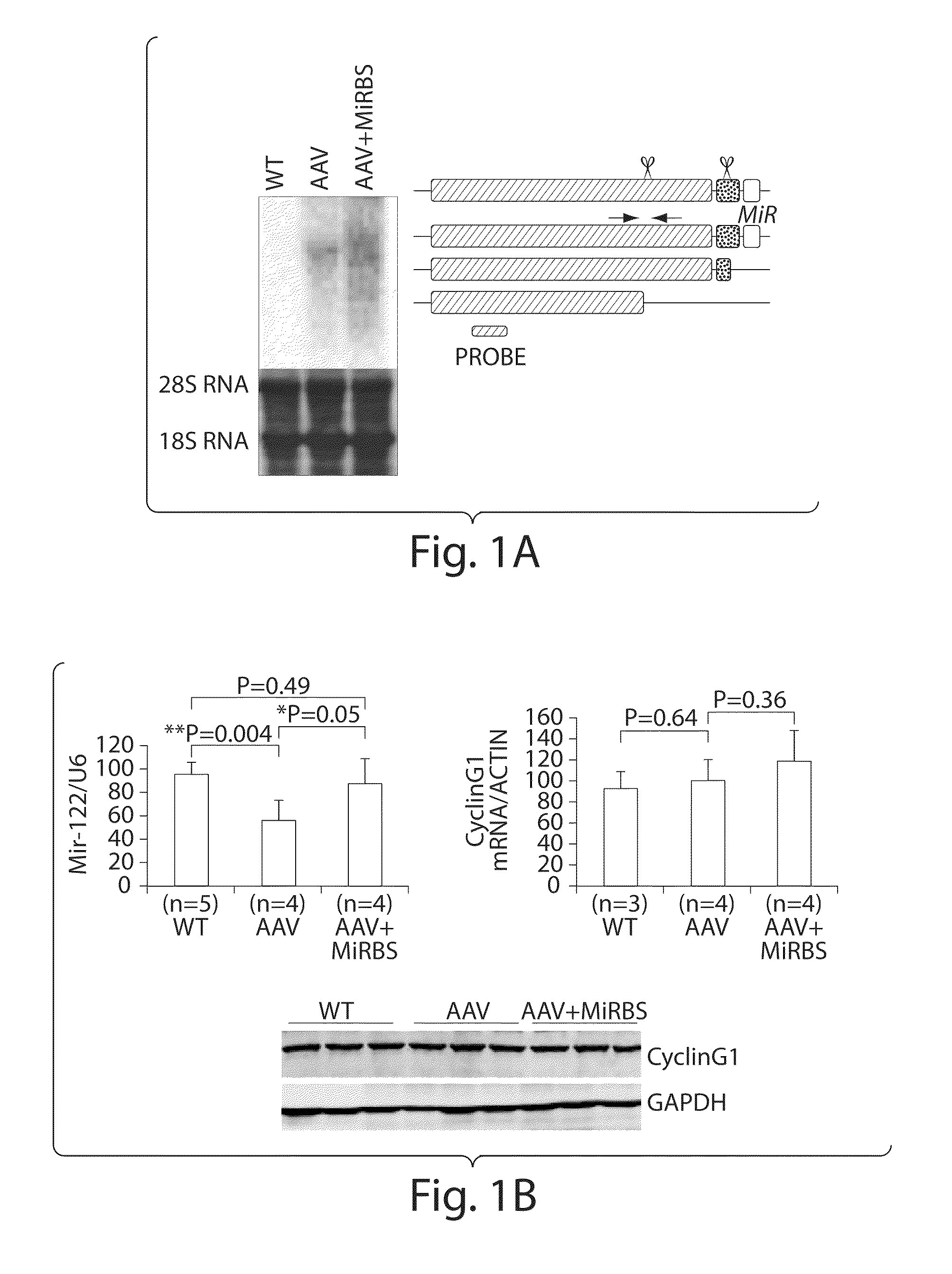
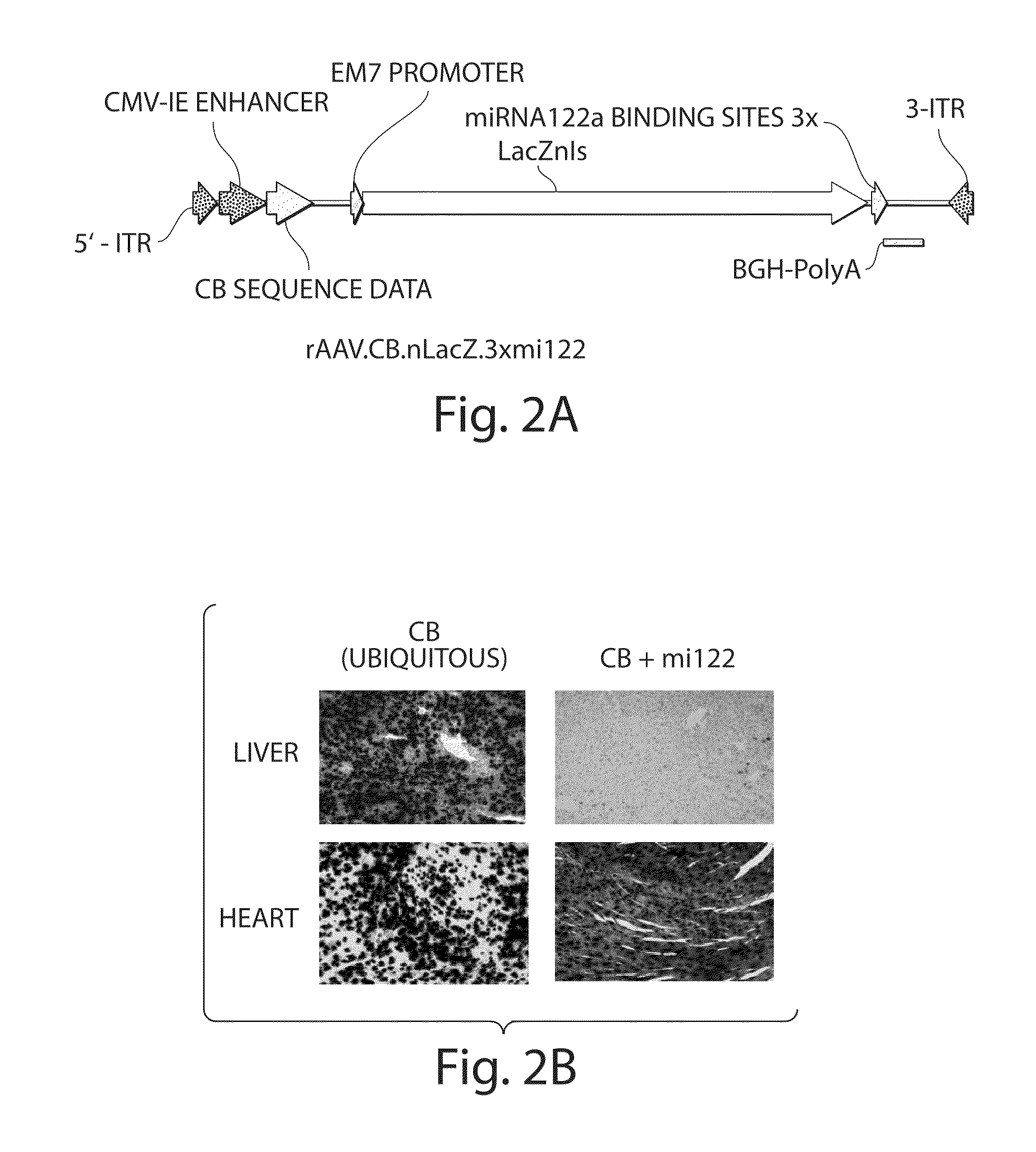
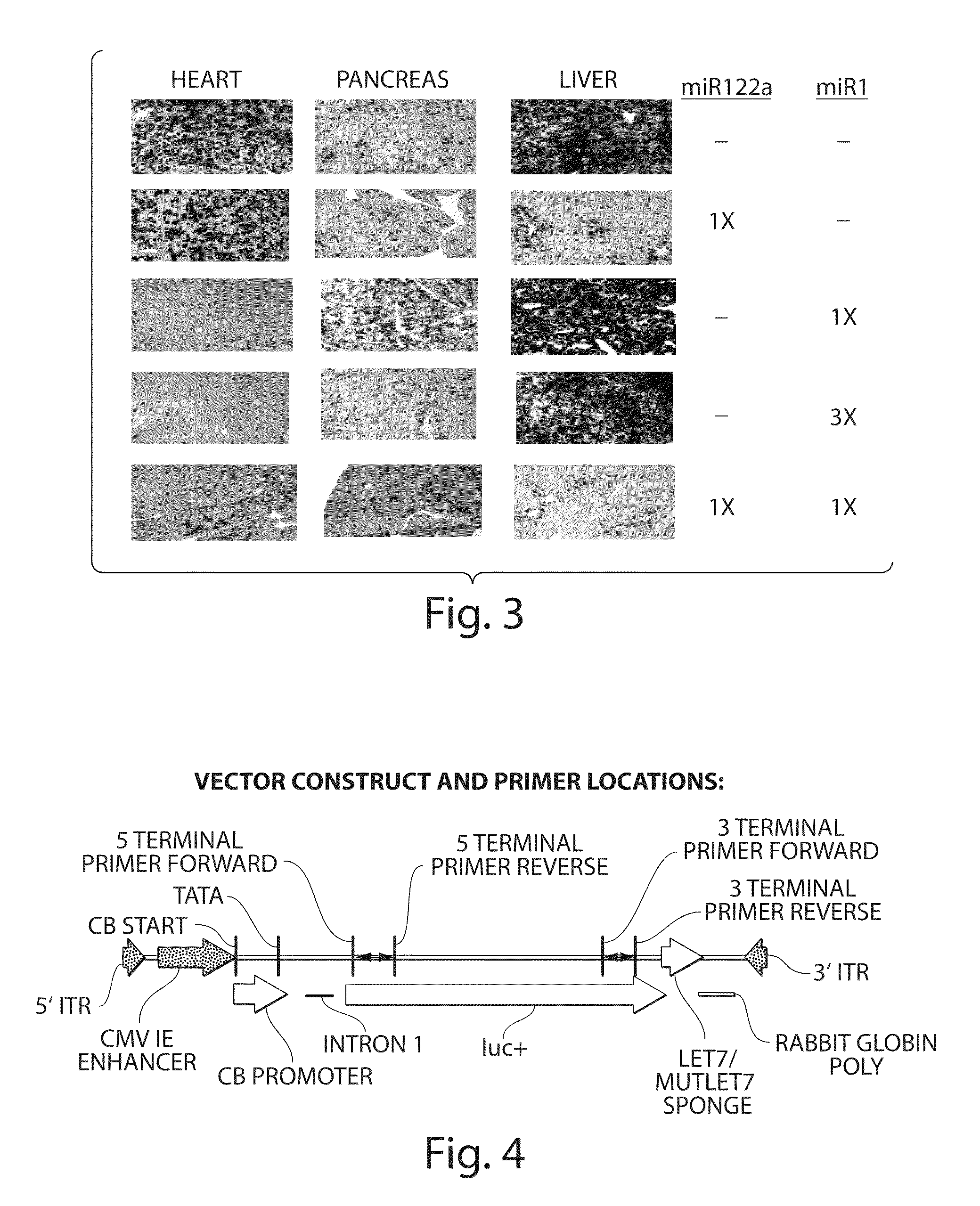
The invention in some aspects relates to isolated nucleic acids, compositions, and kits useful for identifying adeno-associated viruses in cells. In some aspects, the invention provides kits and methods for producing somatic transgenic animal models using recombinant AAV (rAAV) to an animal having at least one transgene that expresses a small interfering nucleic acid or at least one binding site for a miRNA.
Owner:UNIV OF MASSACHUSETTS
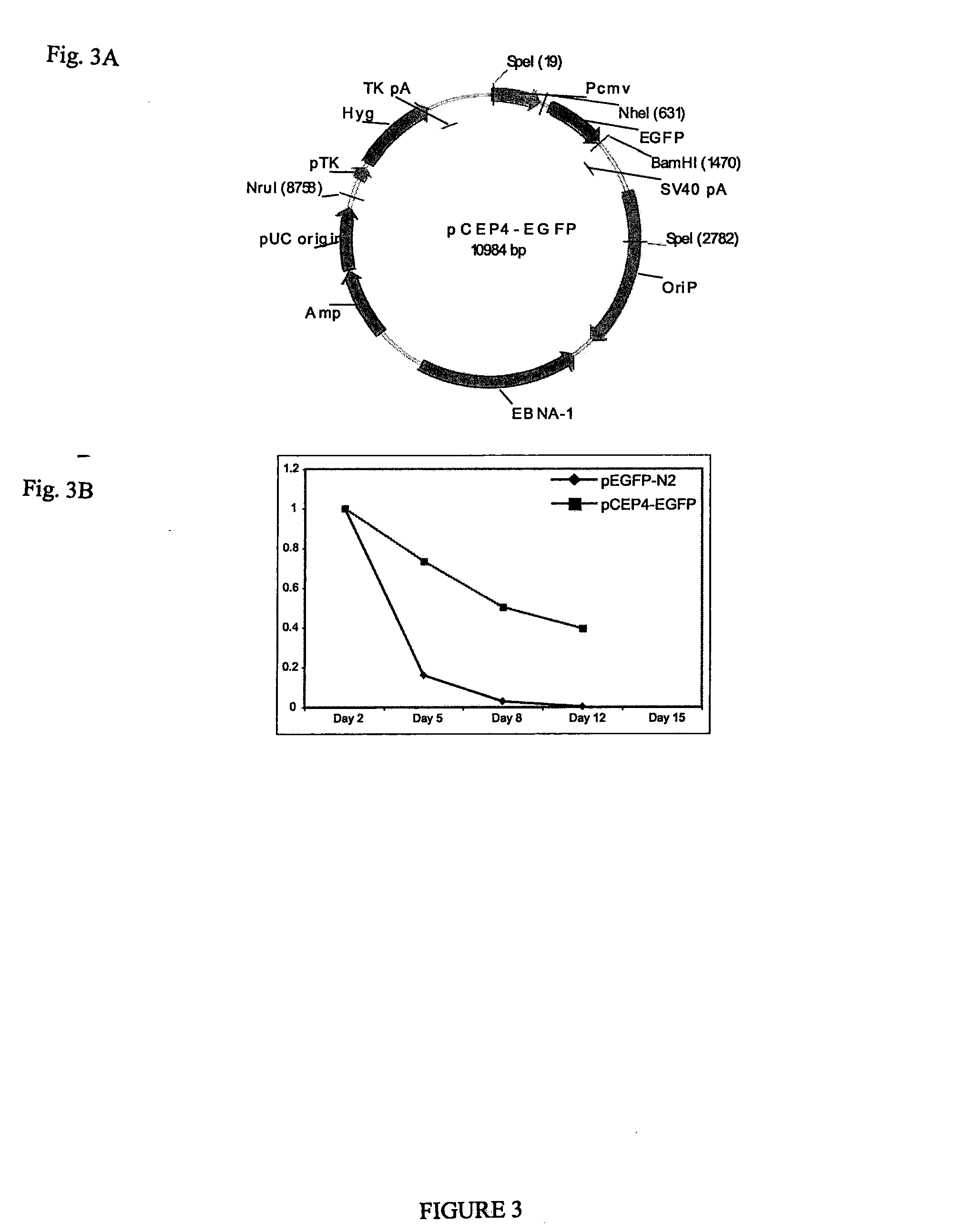
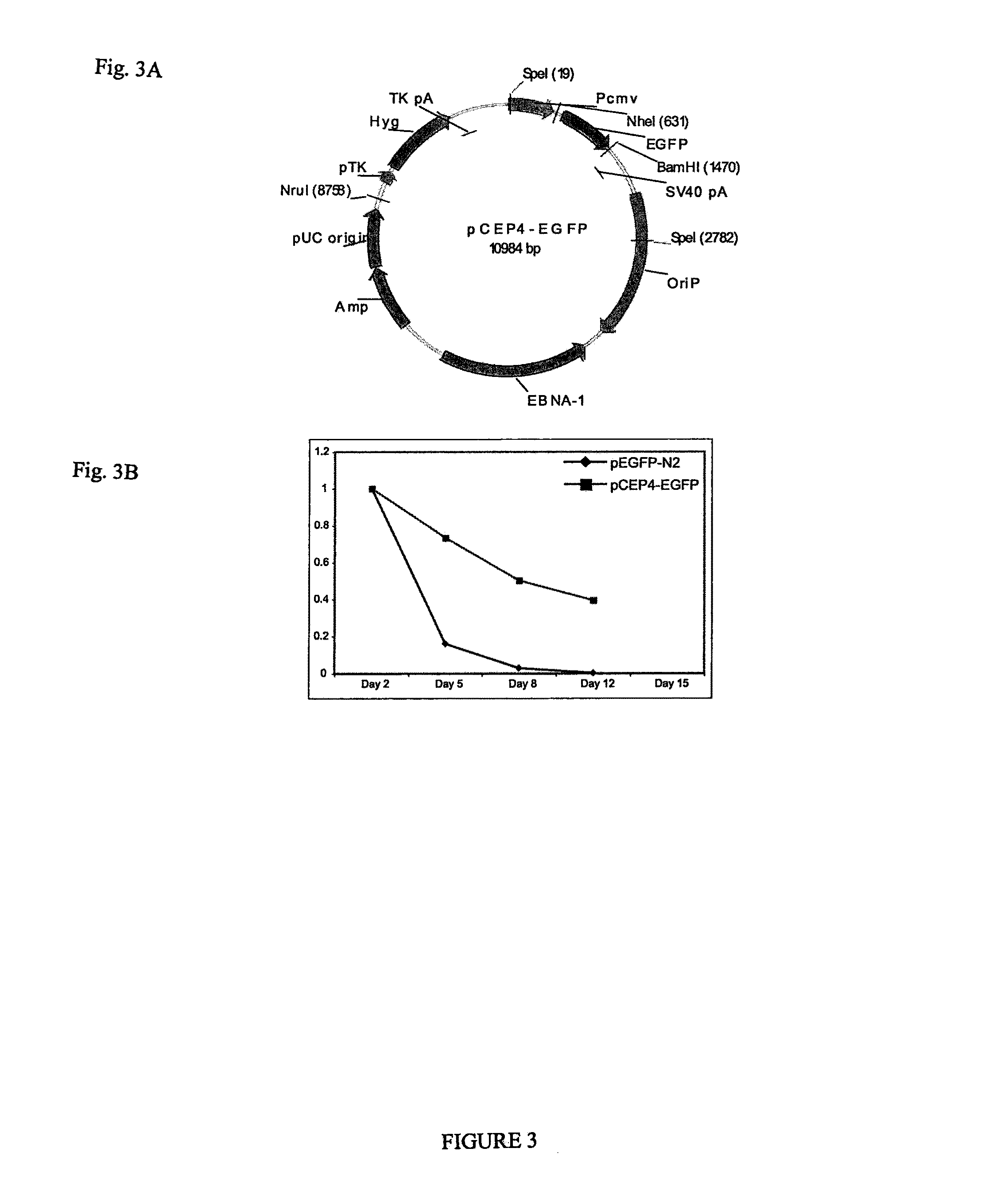
Pluripotent stem cells obtained by non-viral reprogramming
ActiveUS20100184227A1Genetically modified cellsArtificial cell constructsInduced pluripotent stem cellInfectious virus
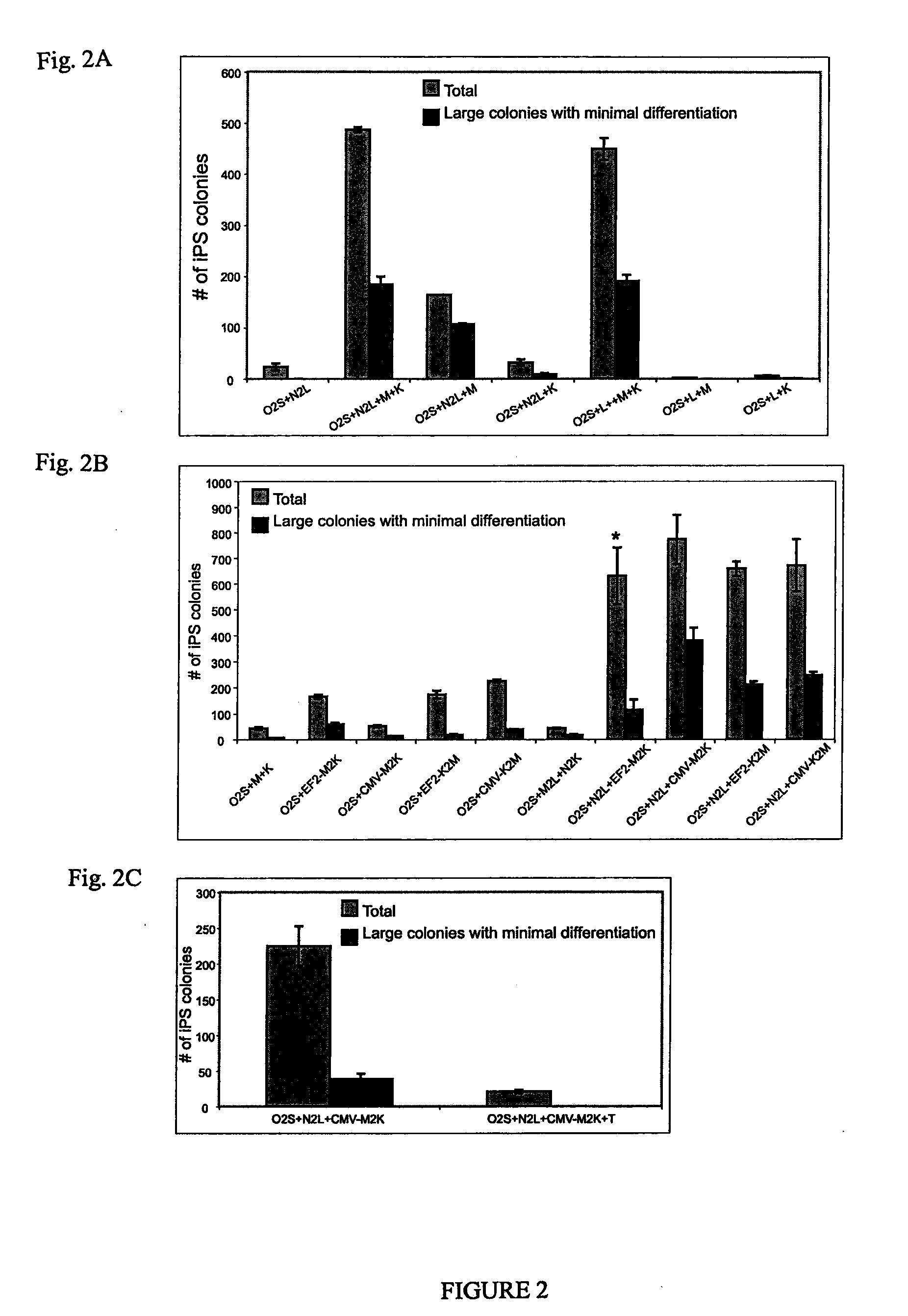
Methods for reprogramming primate somatic cells to pluripotency using an episomal vector that does not encode an infectious virus are disclosed. Pluripotent cells produced in the methods are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
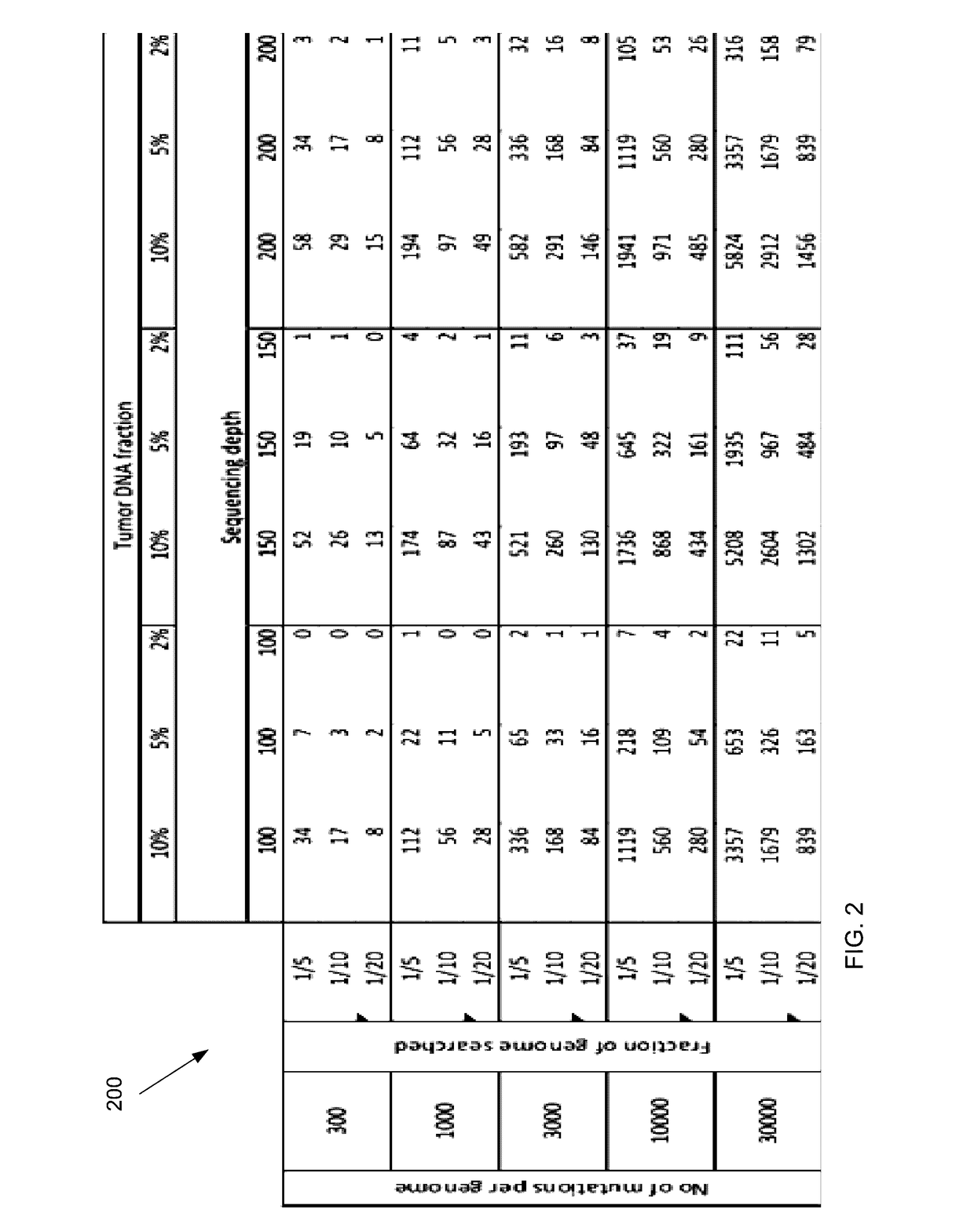
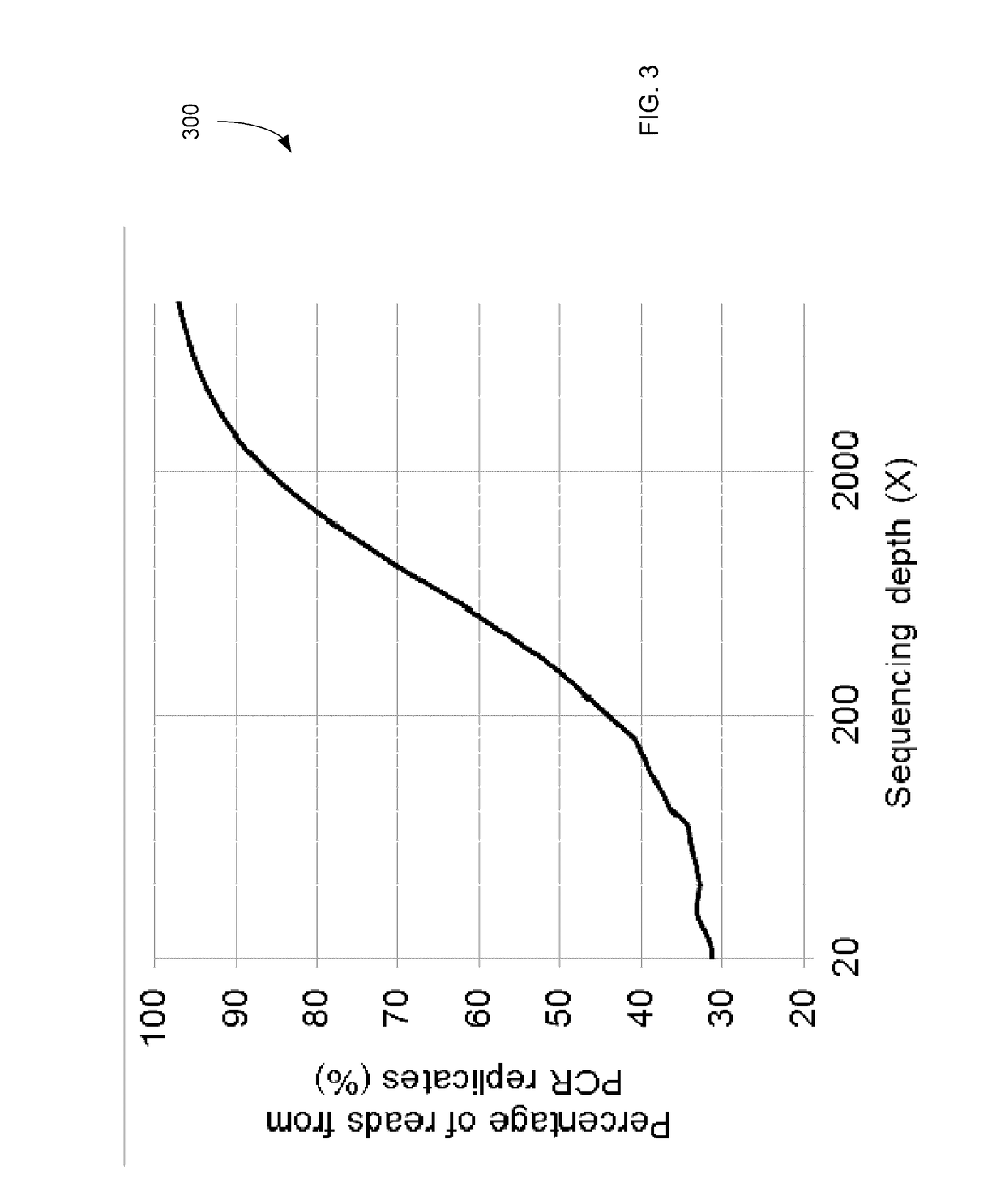
Detecting mutations for cancer screening and fetal analysis
ActiveUS20170073774A1Accurate detectionMicrobiological testing/measurementProteomicsCell freeEmbolization Therapy
Embodiments are related to the accurate detection of somatic mutations in the plasma (or other samples containing cell-free DNA) of cancer patients and for subjects being screened for cancer. The detection of these molecular markers would be useful for the screening, detection, monitoring, management, and prognostication of cancer patients. For example, a mutational load can be determined from the identified somatic mutations, and the mutational load can be used to screen for any or various types of cancers, where no prior knowledge about a tumor or possible cancer of the subject may be required. Embodiments can be useful for guiding the use of therapies (e.g. targeted therapy, immunotherapy, genome editing, surgery, chemotherapy, embolization therapy, anti-angiogenesis therapy) for cancers. Embodiments are also directed to identifying de novo mutations in a fetus by analyzing a maternal sample having cell-free DNA from the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
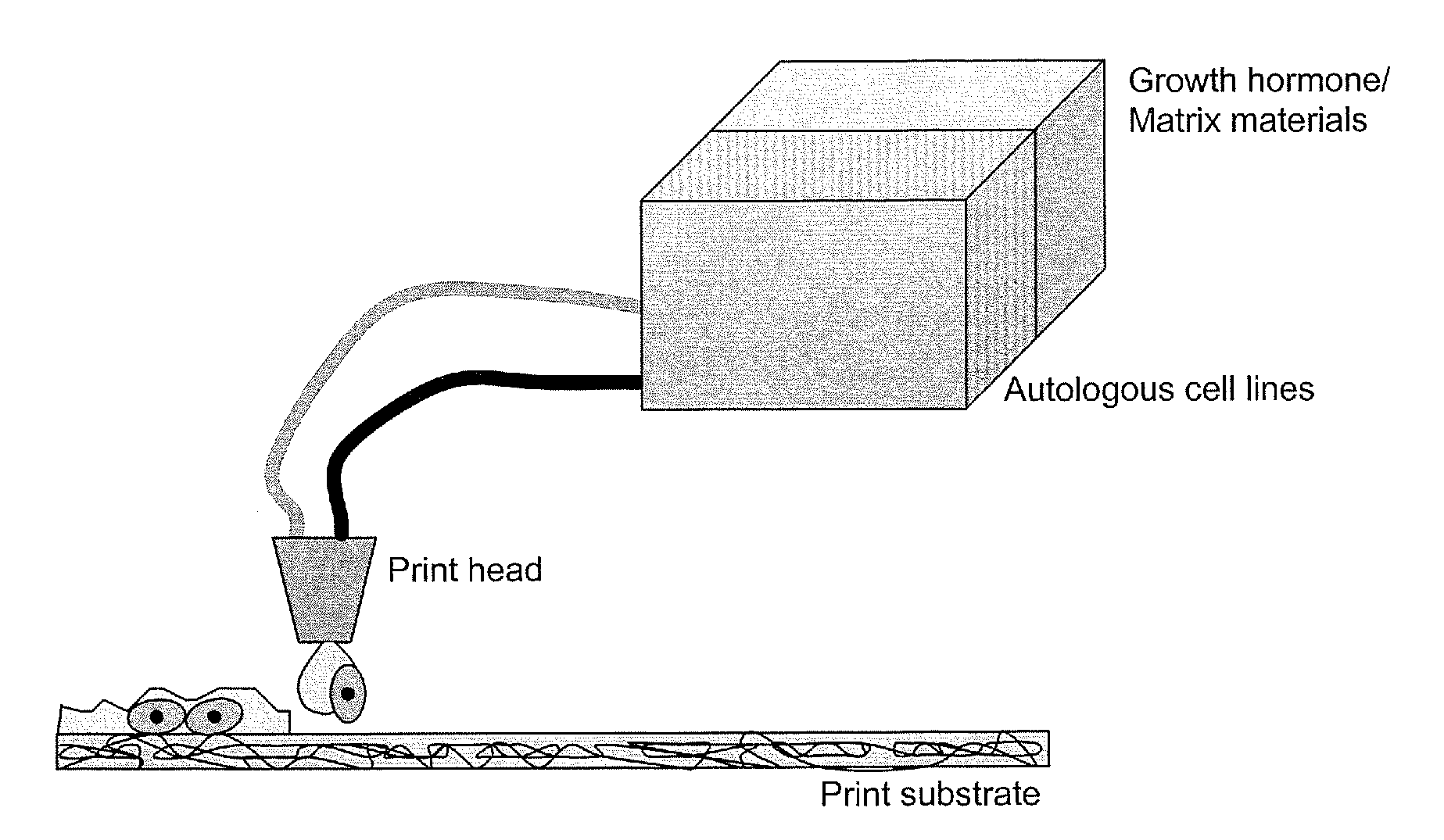
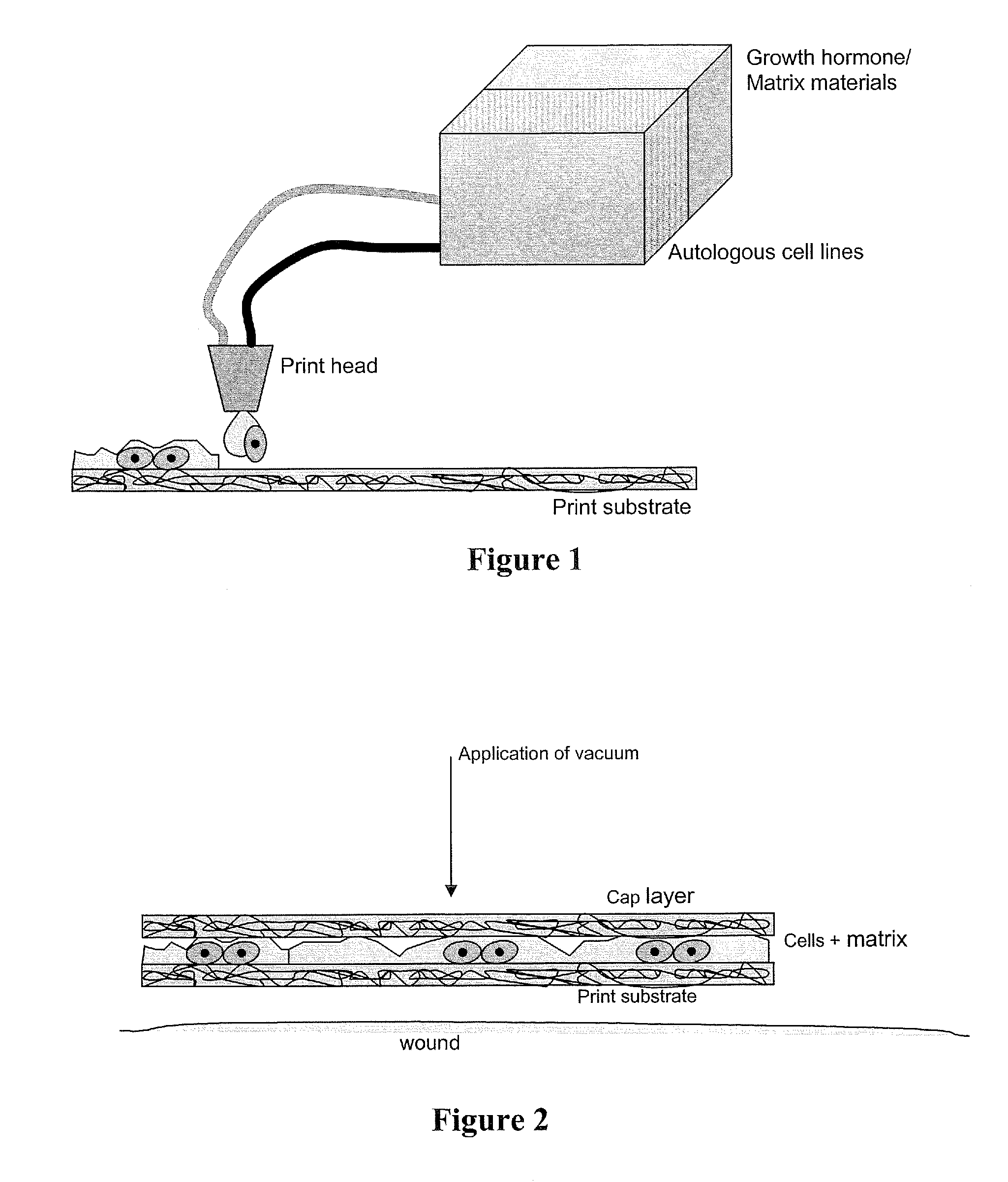
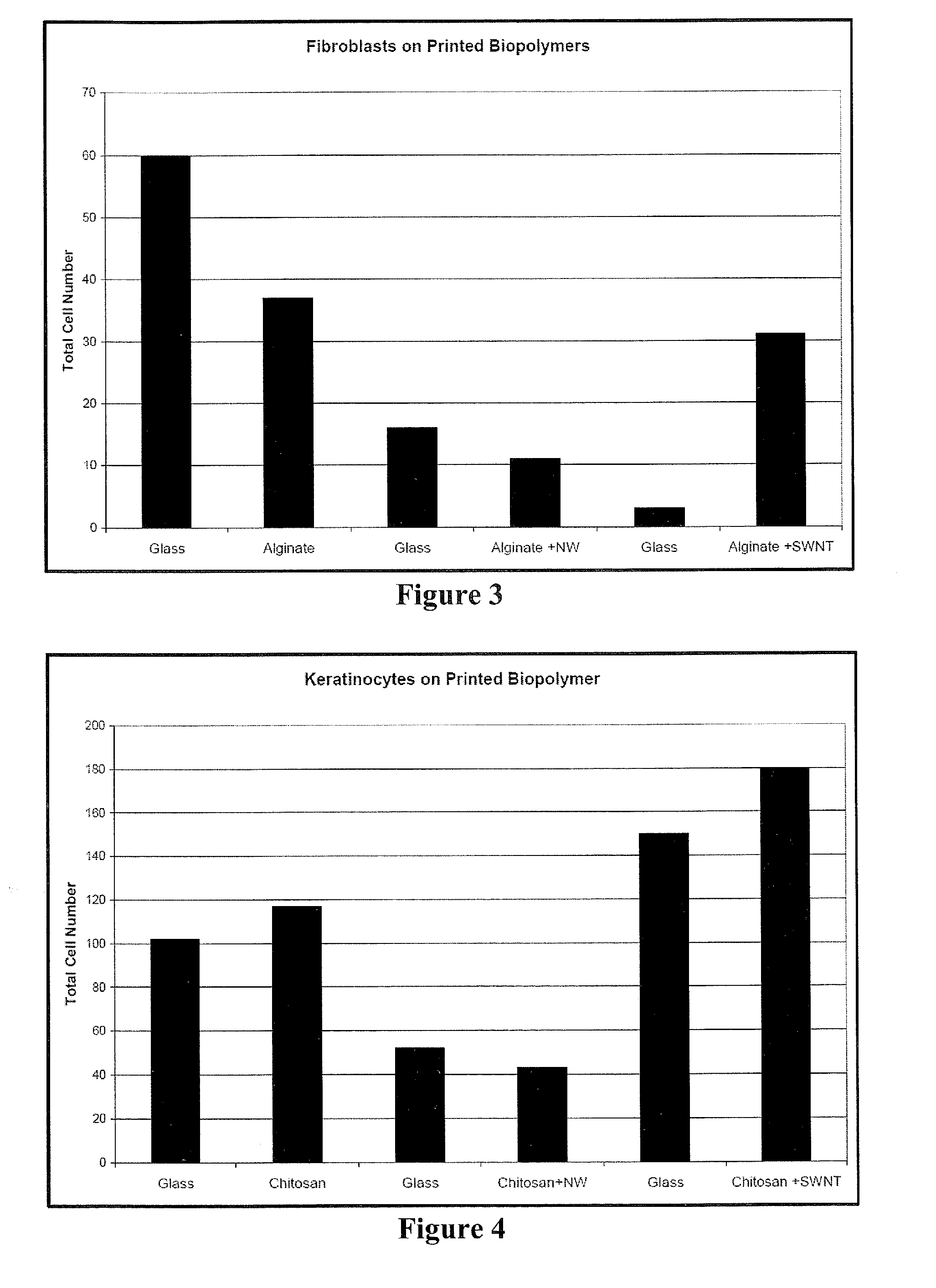
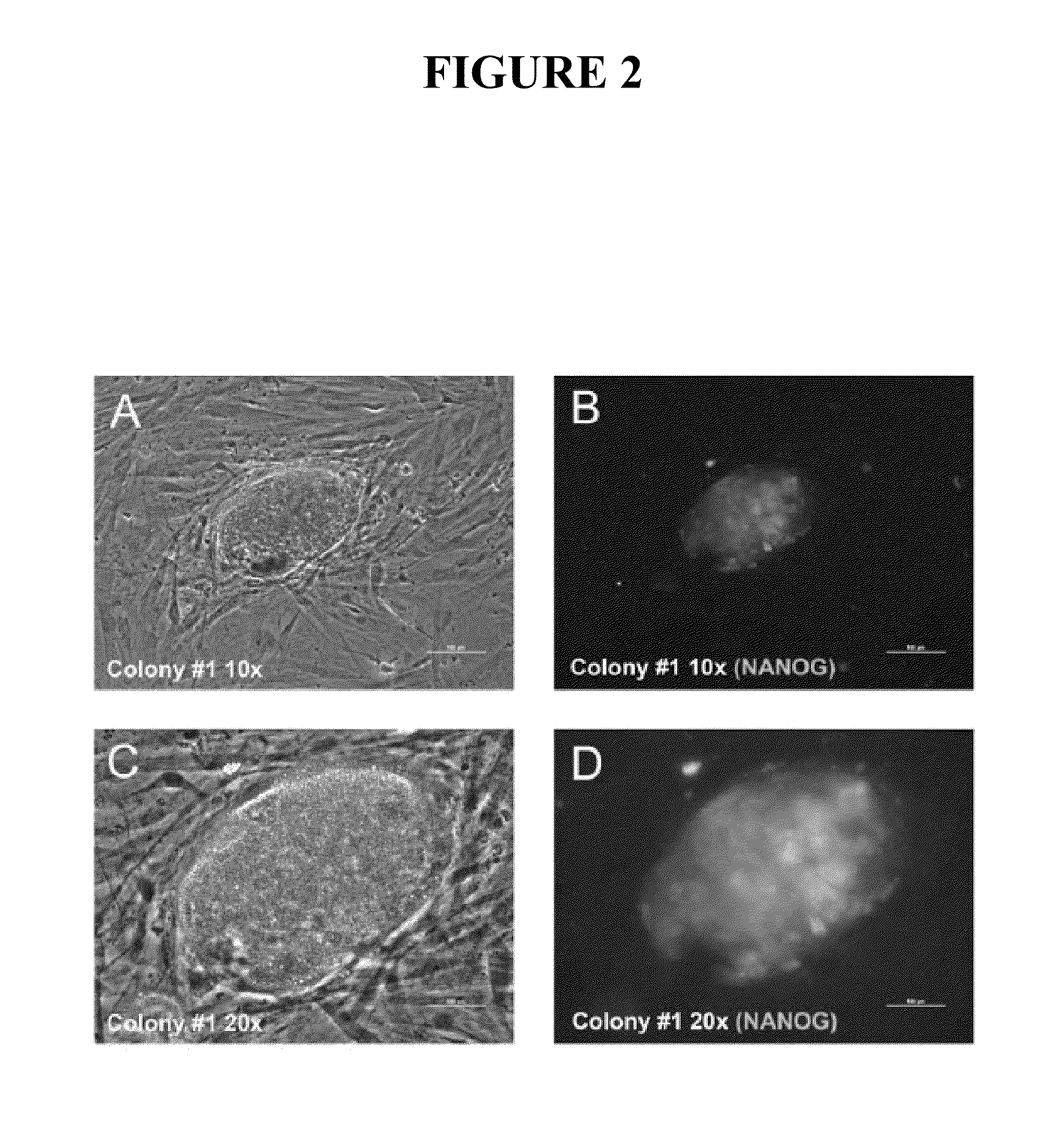
Methods and compositions for printing biologically compatible nanotube composites of autologous tissue
A method of carrying out an autologous tissue implant in a subject in need thereof is carried out by: (a) forming an autologous tissue implant from autologous cells collected from a subject (e.g., by ink-jet printing, the autologous cells and the scaffold, separately or together), and then (b) implanting the autologous tissue implant in said subject.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Compositions and methods for reprogramming eukaryotic cells
The present invention relates to methods for changing the state of differentiation of a eukaryotic cell, the methods comprising introducing mRNA encoding one or more reprogramming factors into a cell and maintaining the cell under conditions wherein the cell is viable and the mRNA that is introduced into the cell is expressed in sufficient amount and for sufficient time to generate a cell that exhibits a changed state of differentiation compared to the cell into which the mRNA was introduced, and compositions therefor. For example, the present invention provides mRNA molecules and methods for their use to reprogram human somatic cells into pluripotent stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method of screening reprogramming factor, reprogramming factor screened by the method, method of using the reprogramming factor, method of differentiating undifferentiated fused cells and method of constructing cell, tissues and organs
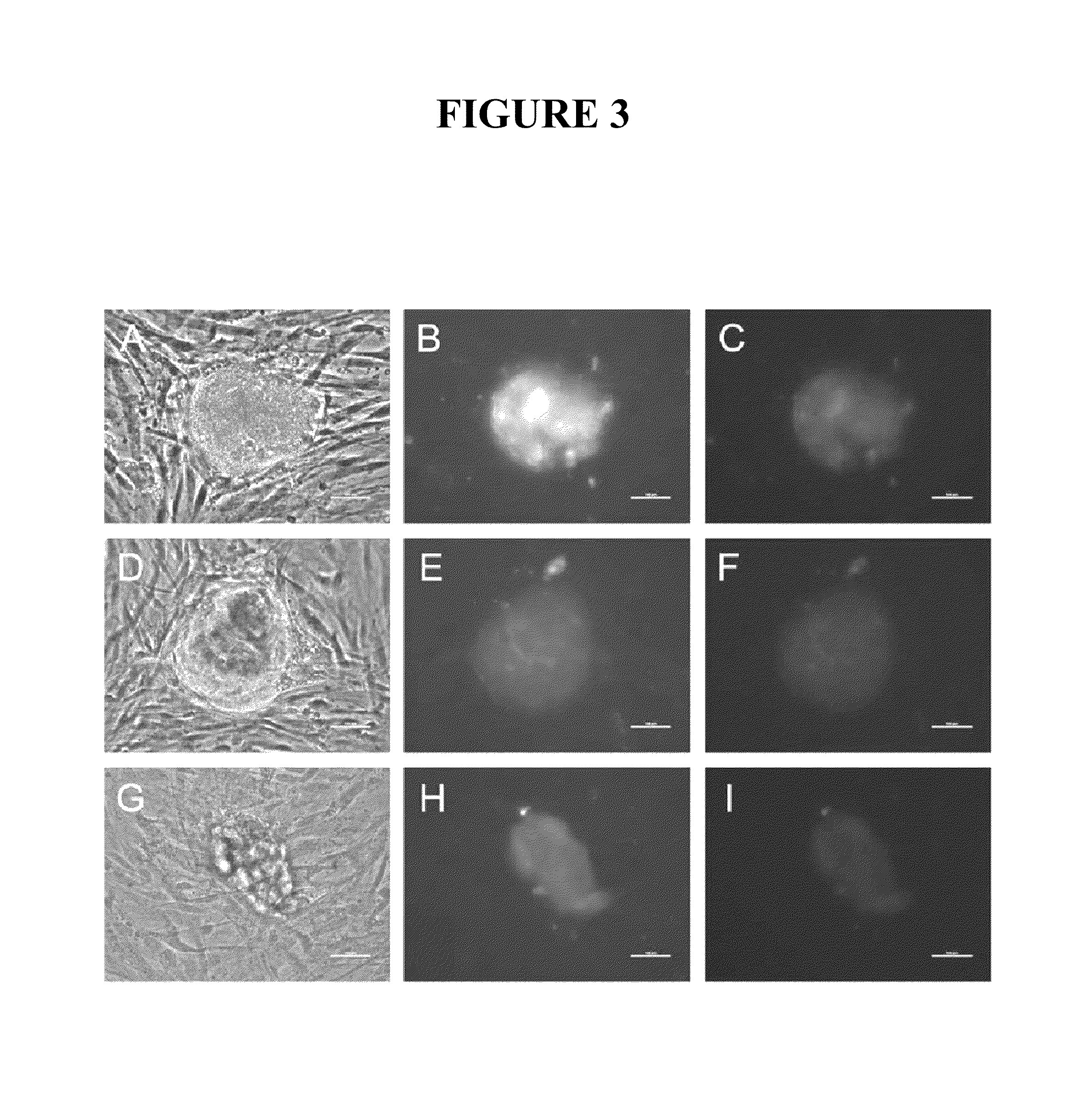
InactiveUS20050130144A1Rejection reaction in the recipient against the transplantation graft can be more efficiently suppressedCompound screeningApoptosis detectionMethod screeningSomatic cell
By exposing somatic cells to a component derived from ES cells to act on somatic cells and detecting the activity thereof, the reprogramming agent can be screened. Further, by exposing somatic cells to a component including a reprogramming agent or an isolated reprogramming agent, the somatic cells can be reprogrammed. Furthermore, according to the present invention, since the tetraploid cells have proliferating capability and pluripotency, such cells can be differentiated and the cells, tissues or organs, which can be used for transplantation, can be produced.
Owner:REPROCELL +1
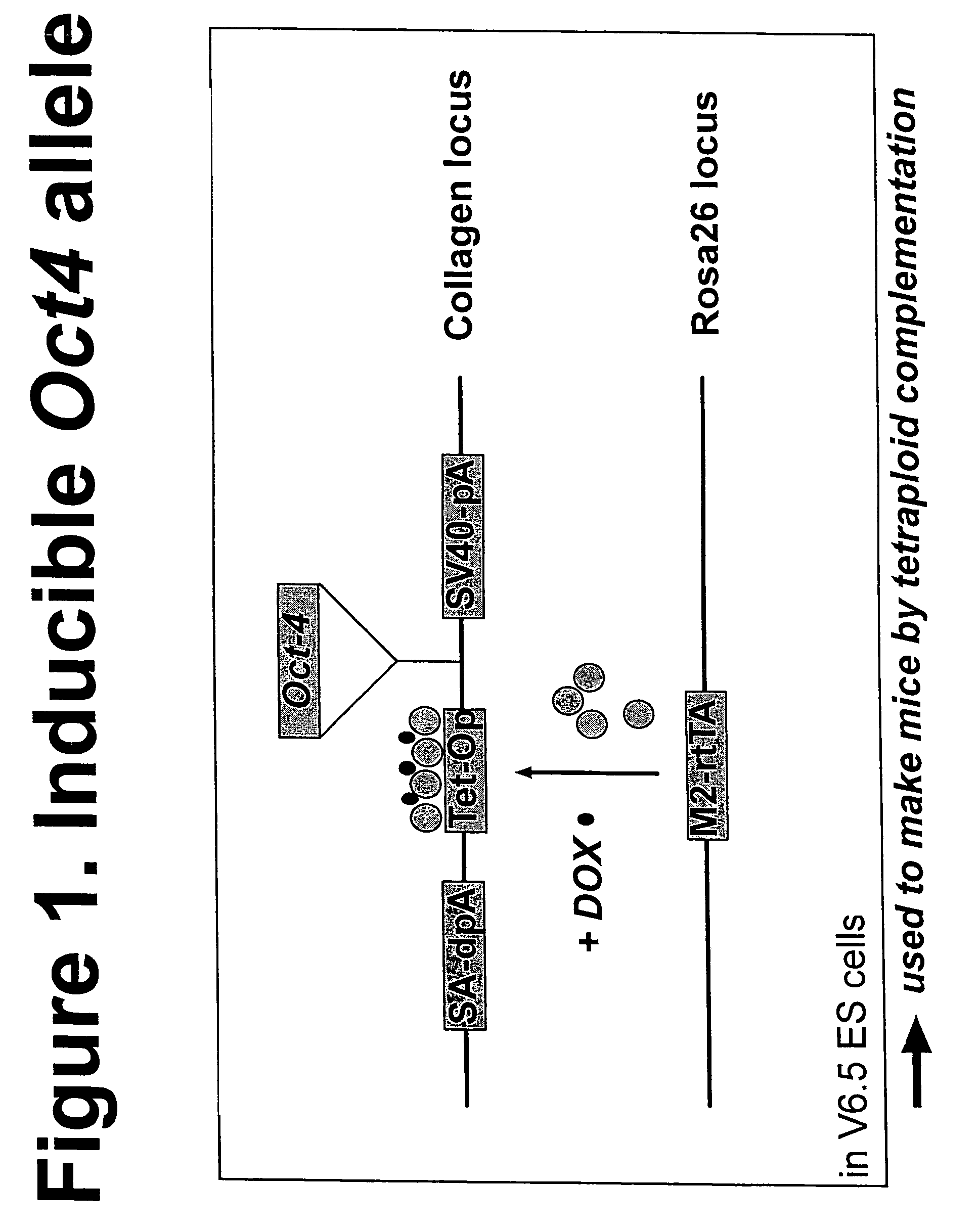
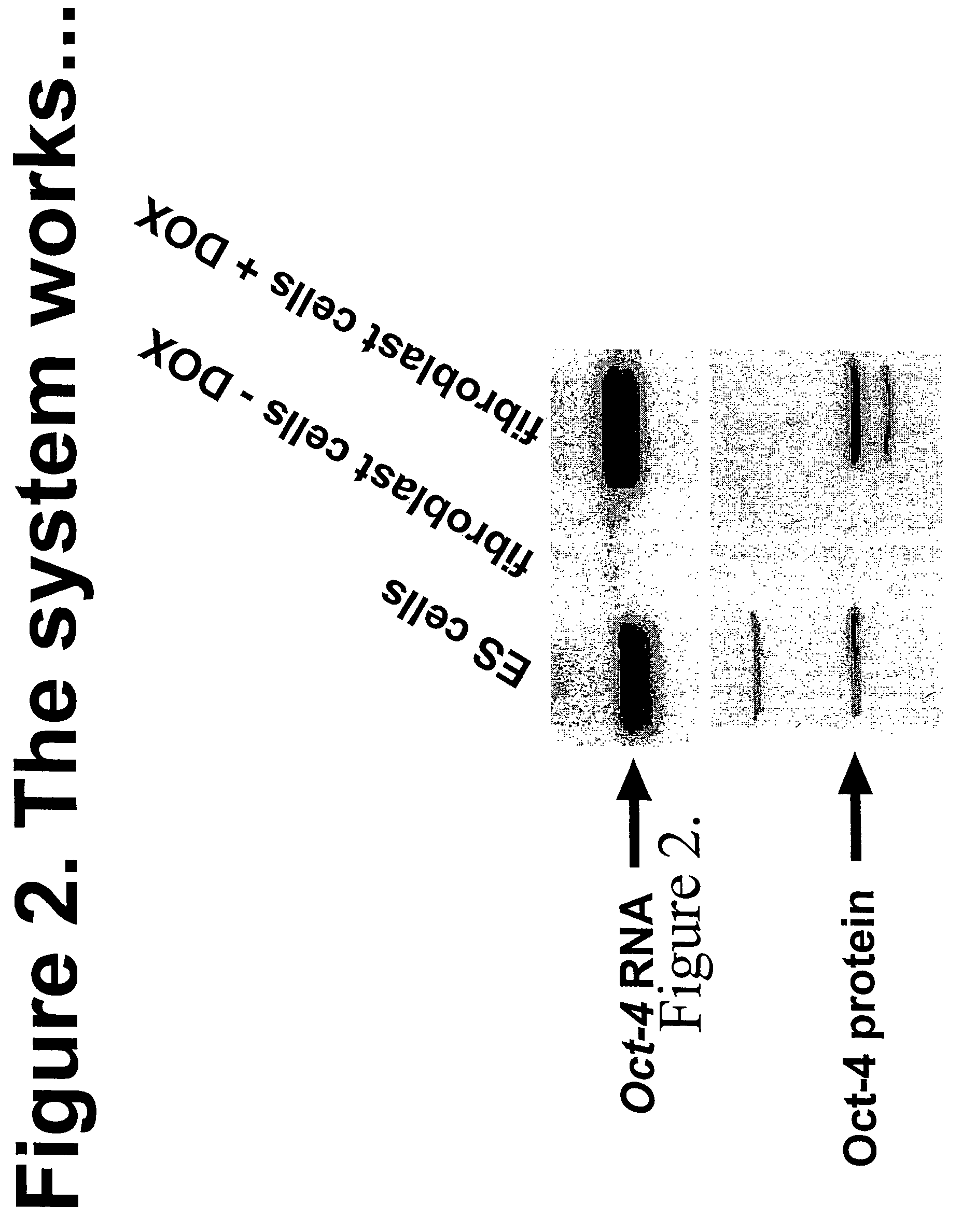
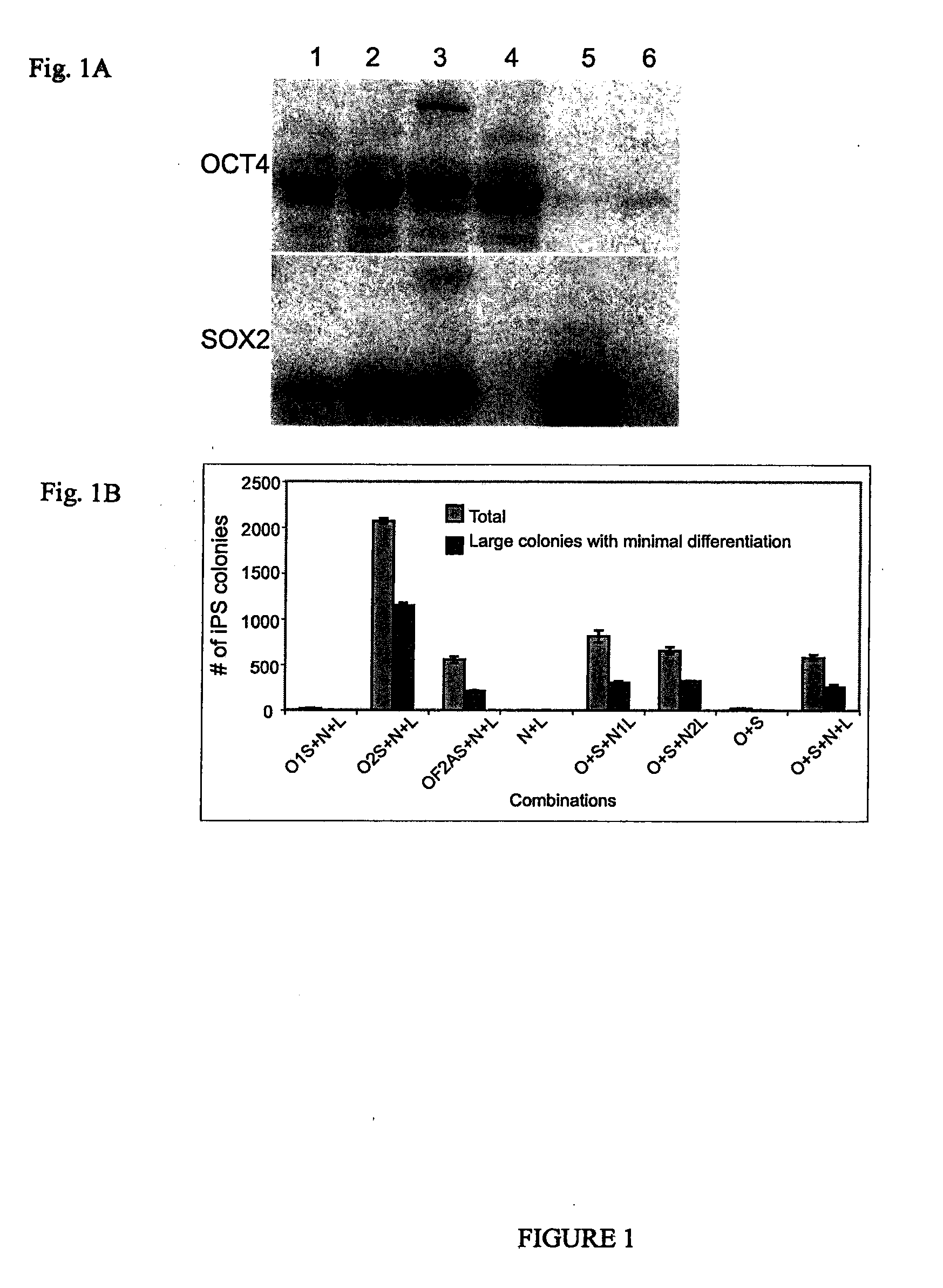
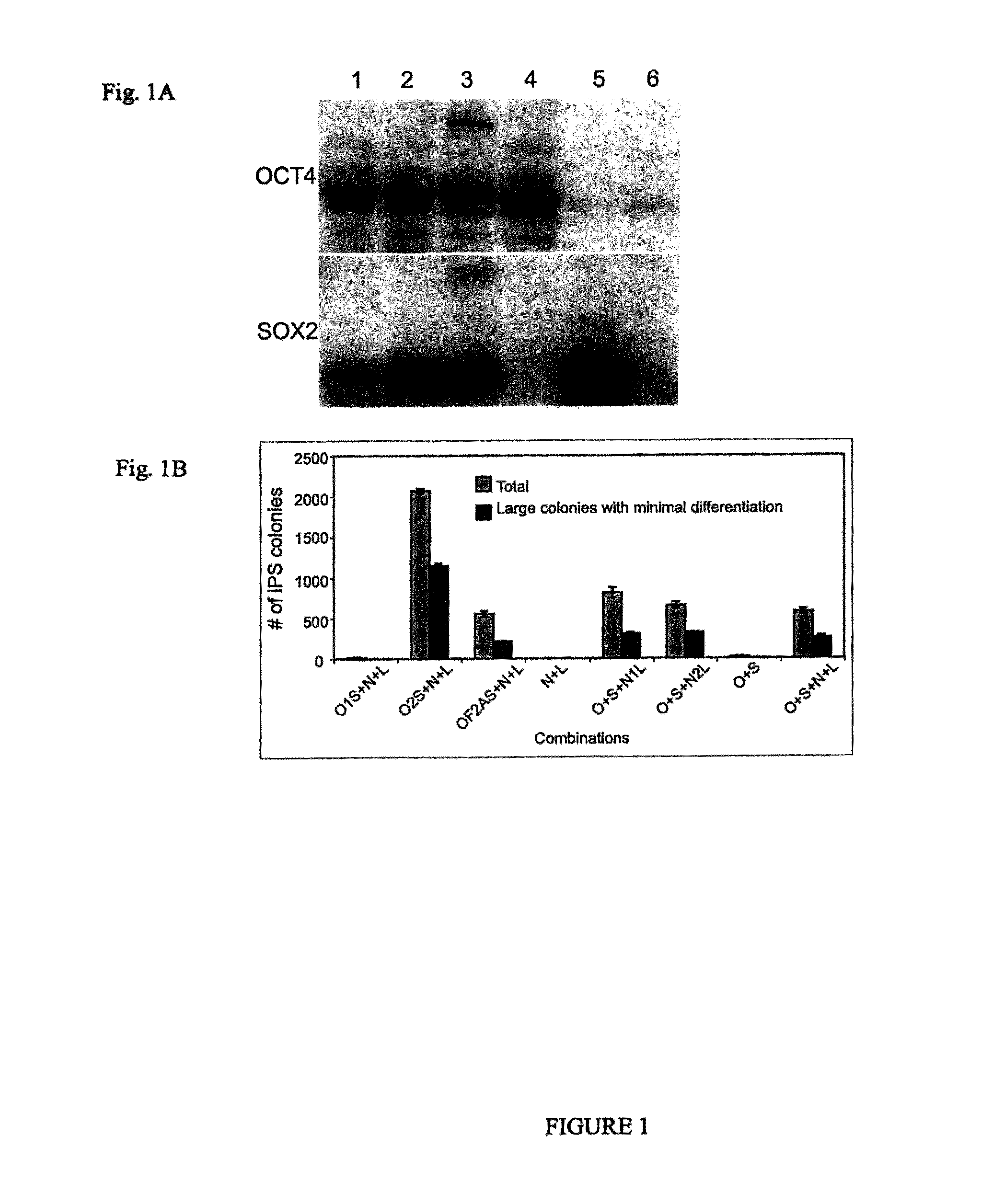
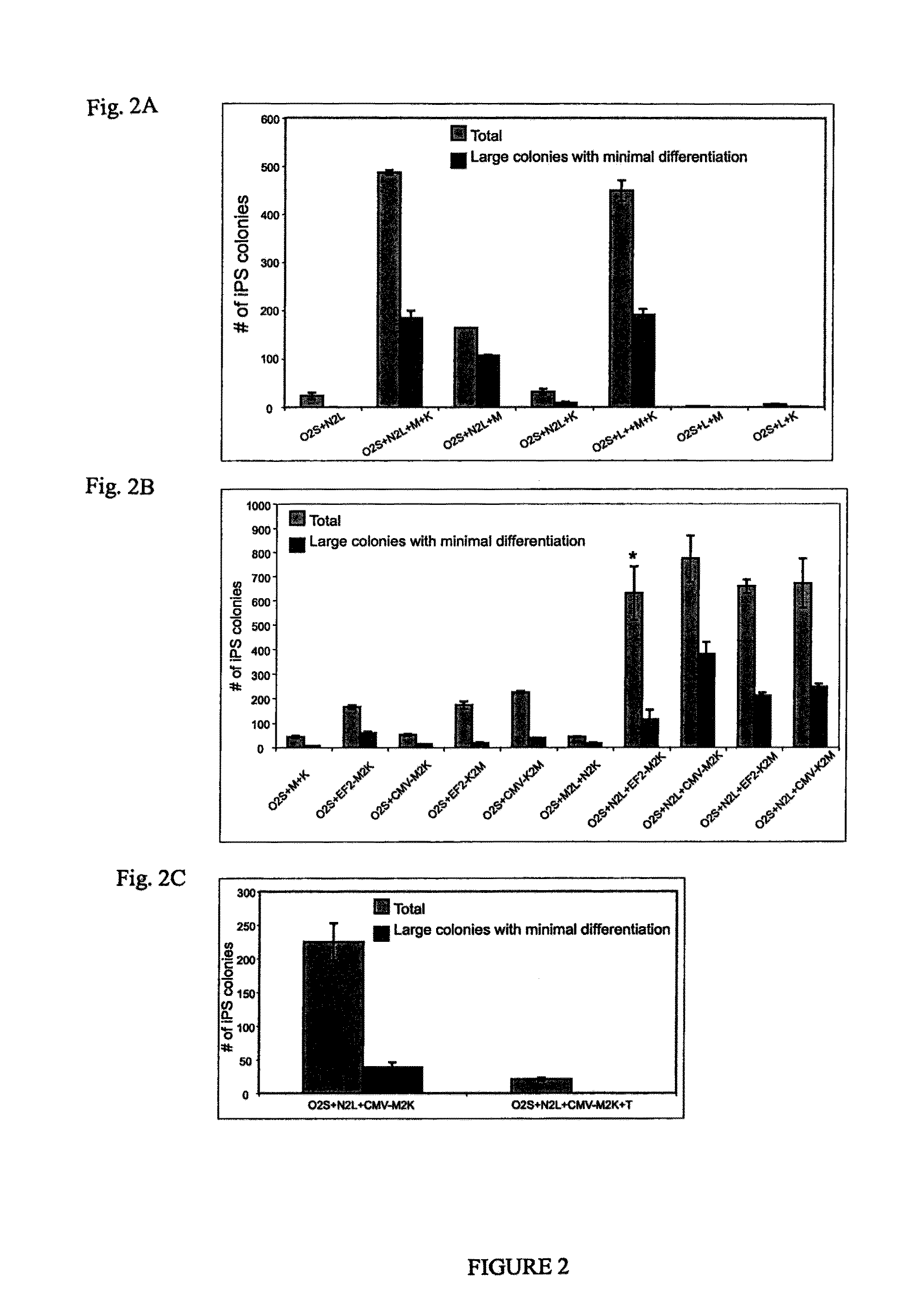
OCT4 and SOX2 with SV40 T antigen produce pluripotent stem cells from primate somatic cells
Methods for reprogramming primate somatic cells to pluripotency using an episomal vector that does not encode an infectious virus are disclosed. Pluripotent cells produced in the methods are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com