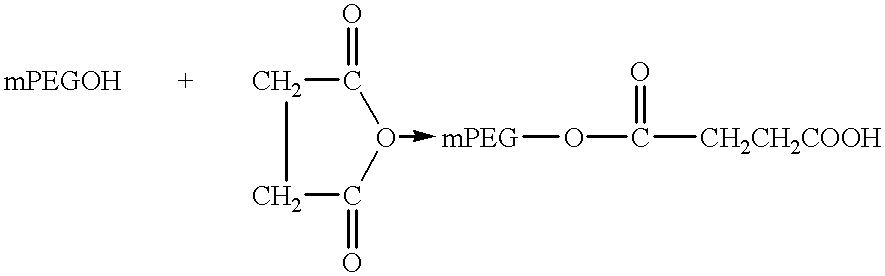
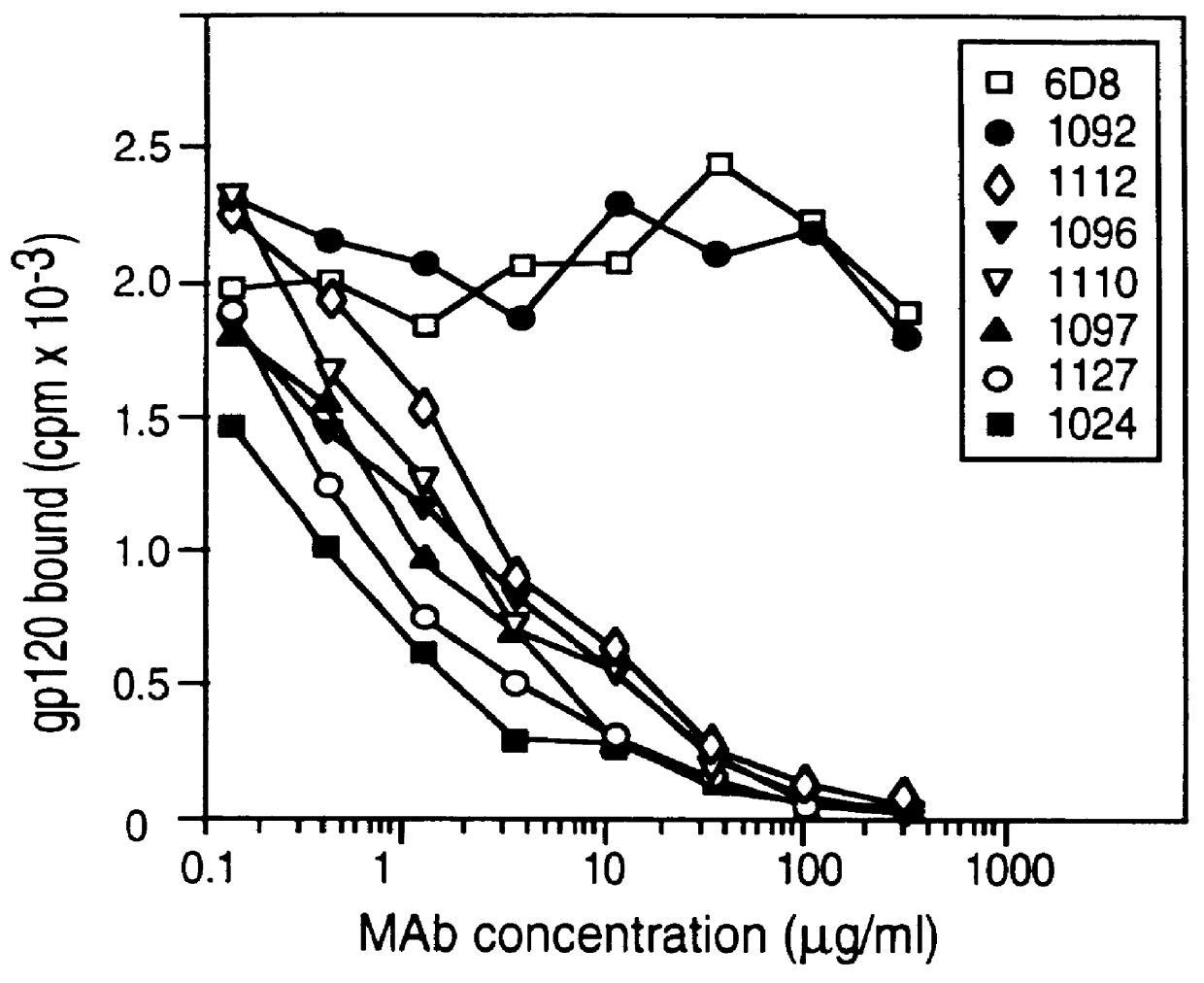
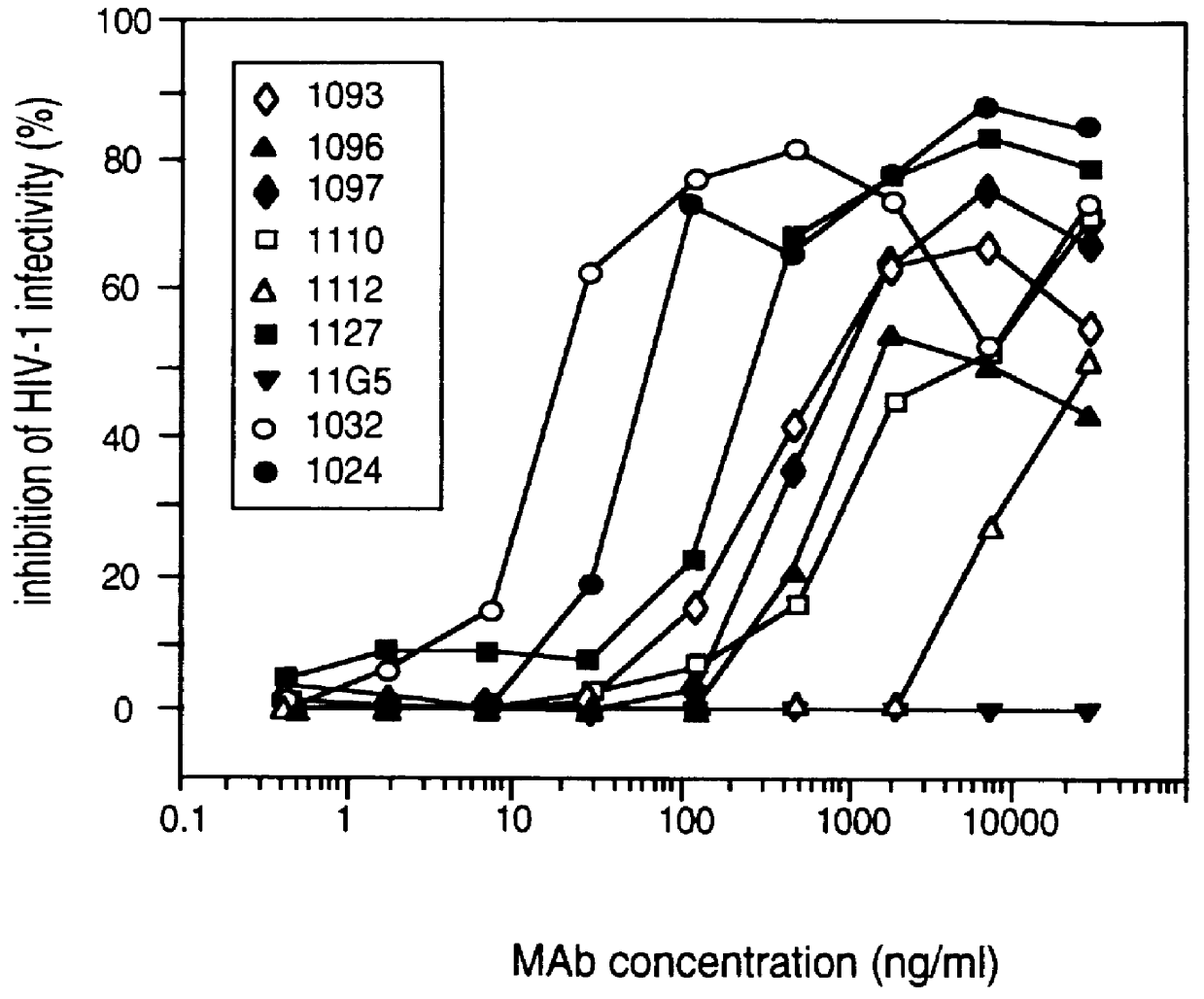
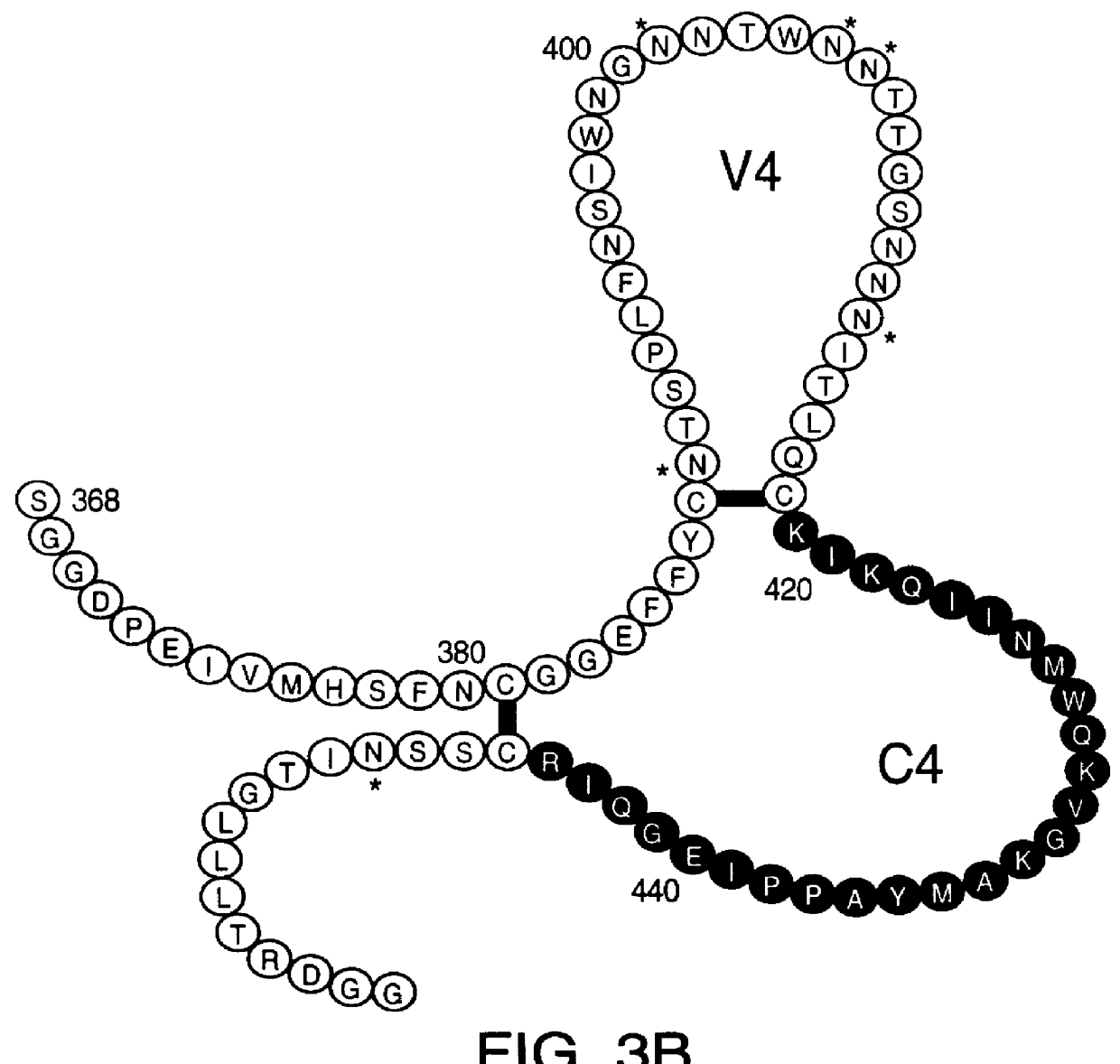
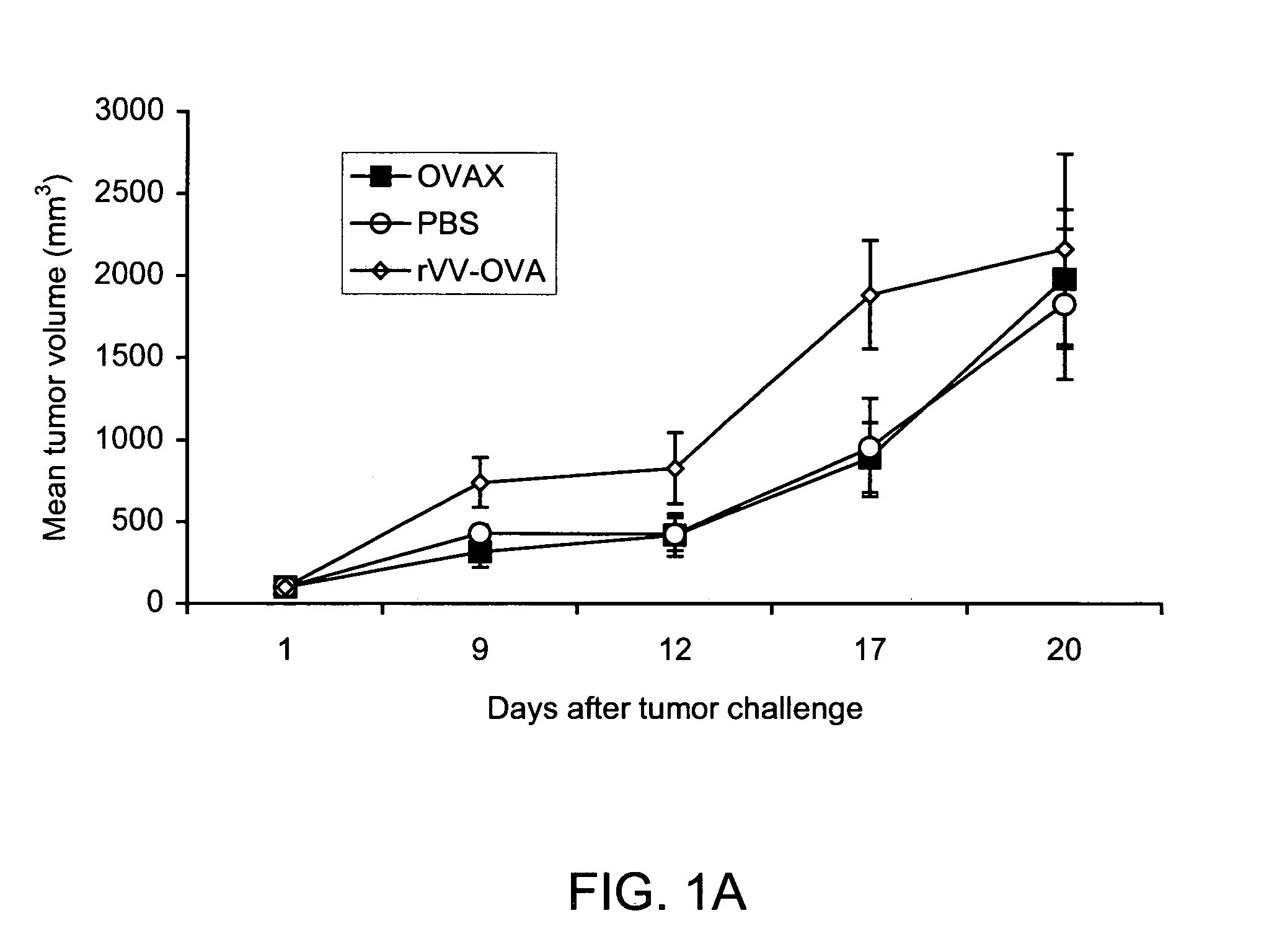
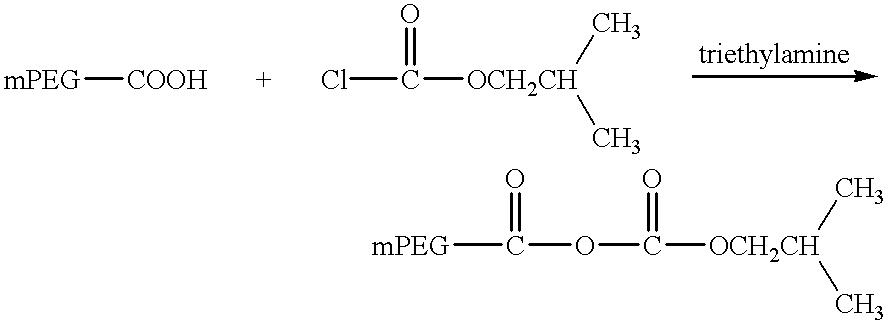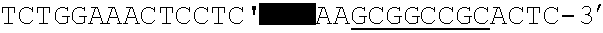Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3529results about "Snake antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Wound closure material
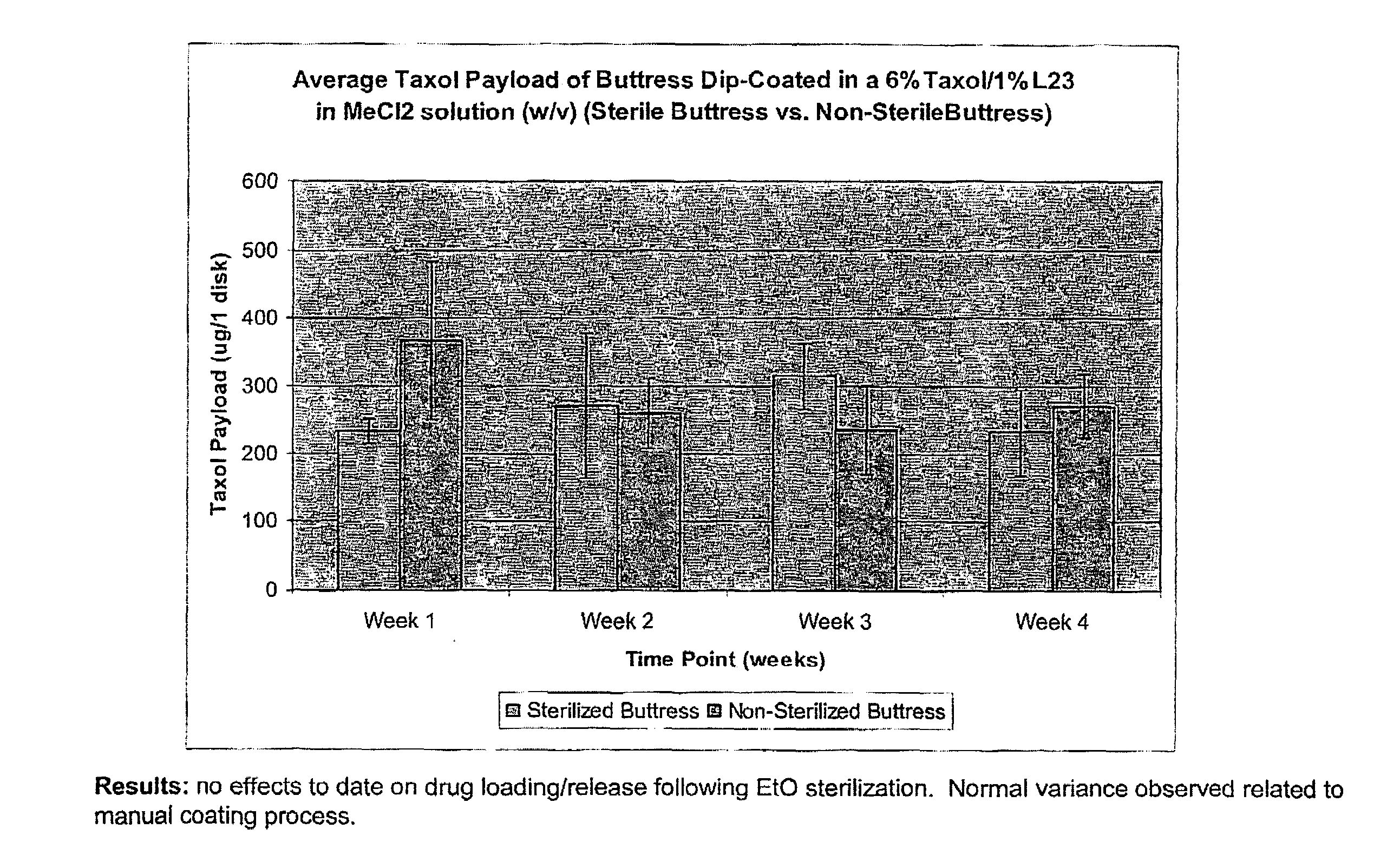
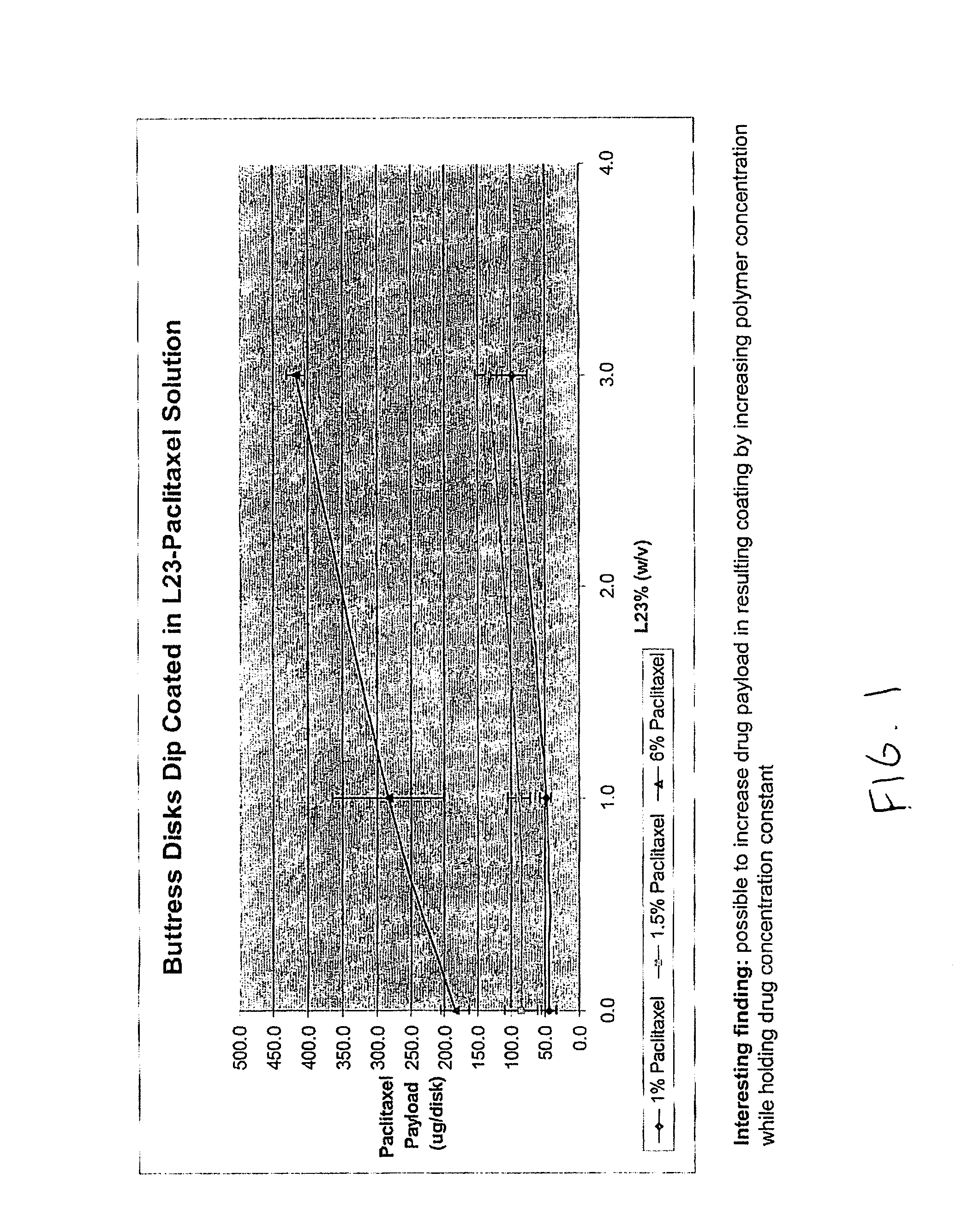
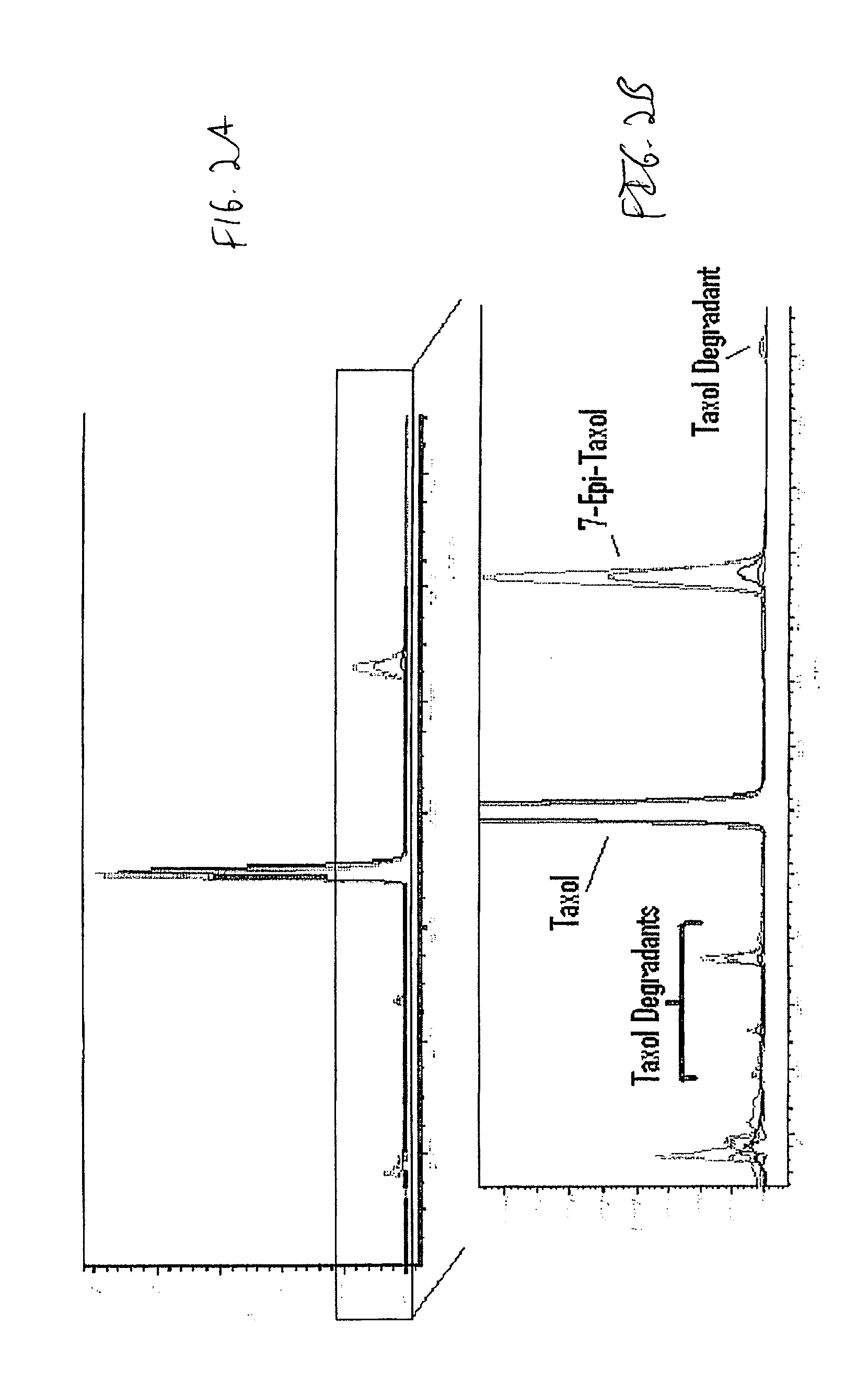
InactiveUS20100076489A1Prevent leakageSuture equipmentsHeavy metal active ingredientsButtressBiomedical engineering
Articles are provided having no orientation or a multi-directional orientation. Such articles may be in the form of films, ribbons, sheets, and / or tapes and may be utilized as buttresses with a surgical stapling apparatus or as reinforcing means for suture lines. The articles may be produced with a polymeric material having an agent, such as a chemotherapeutic agent or a radiotherapeutic agent, incorporated therein or applied as a coating thereon.
Owner:TYCO HEALTHCARE GRP LP
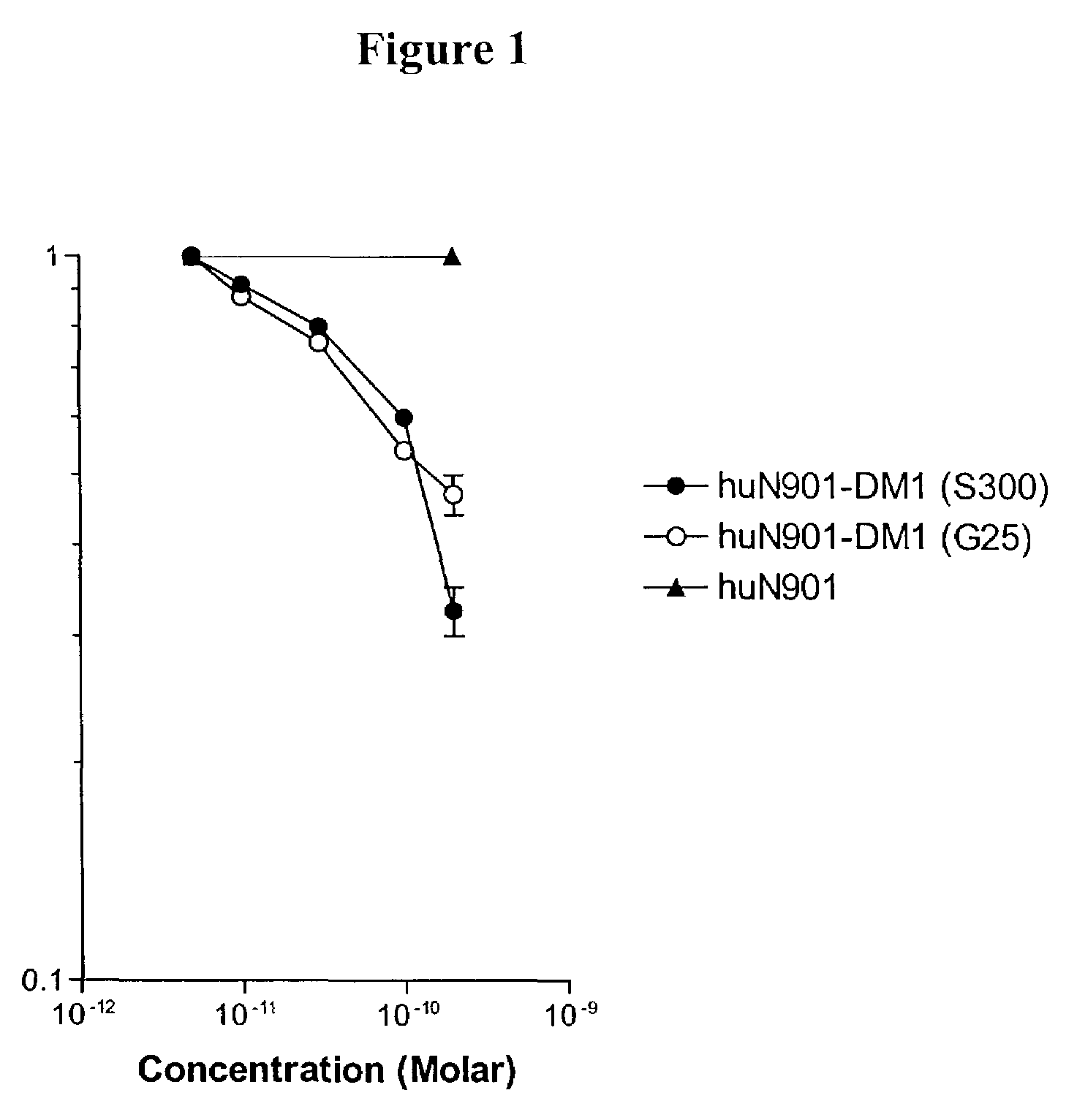
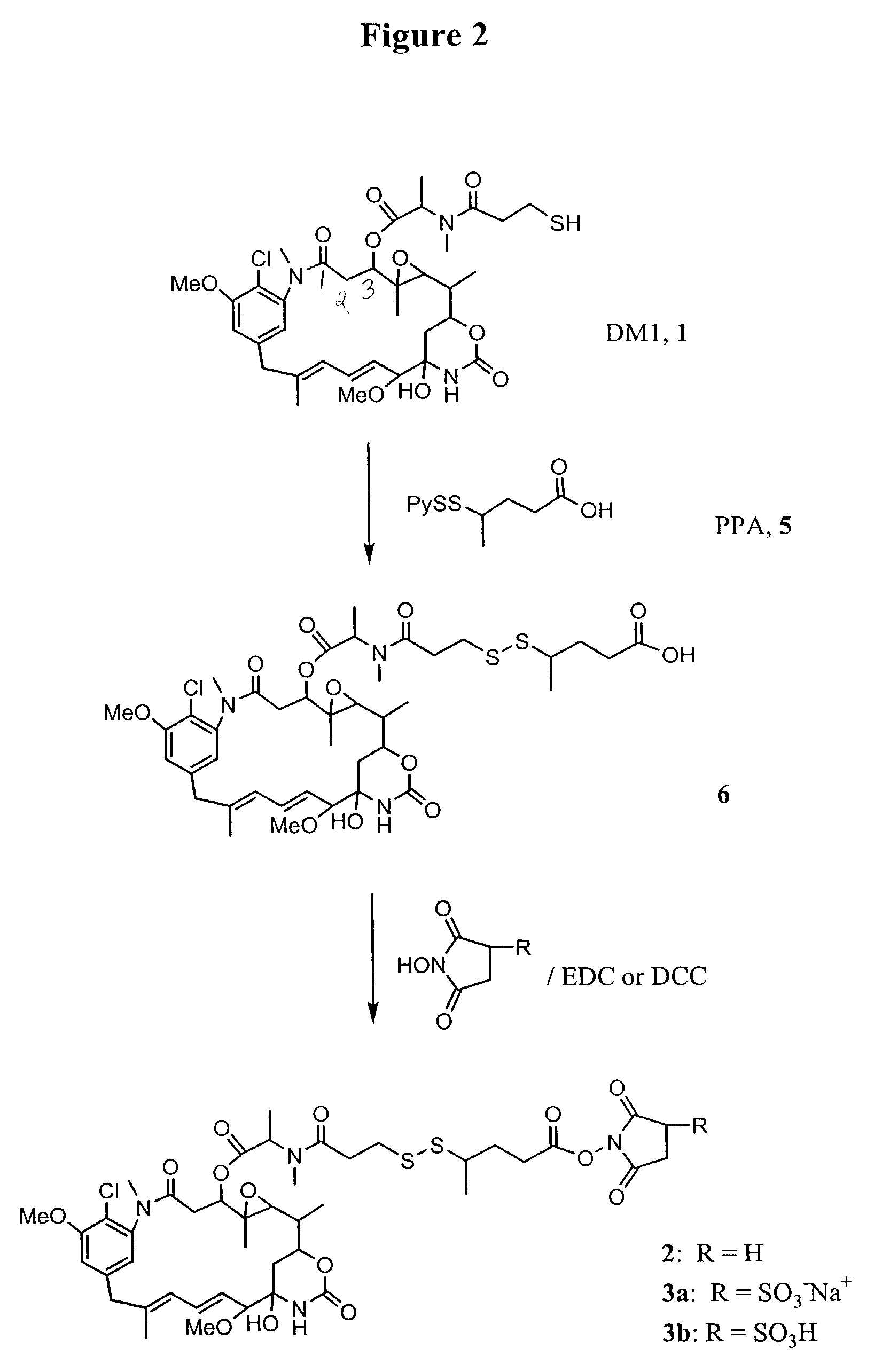
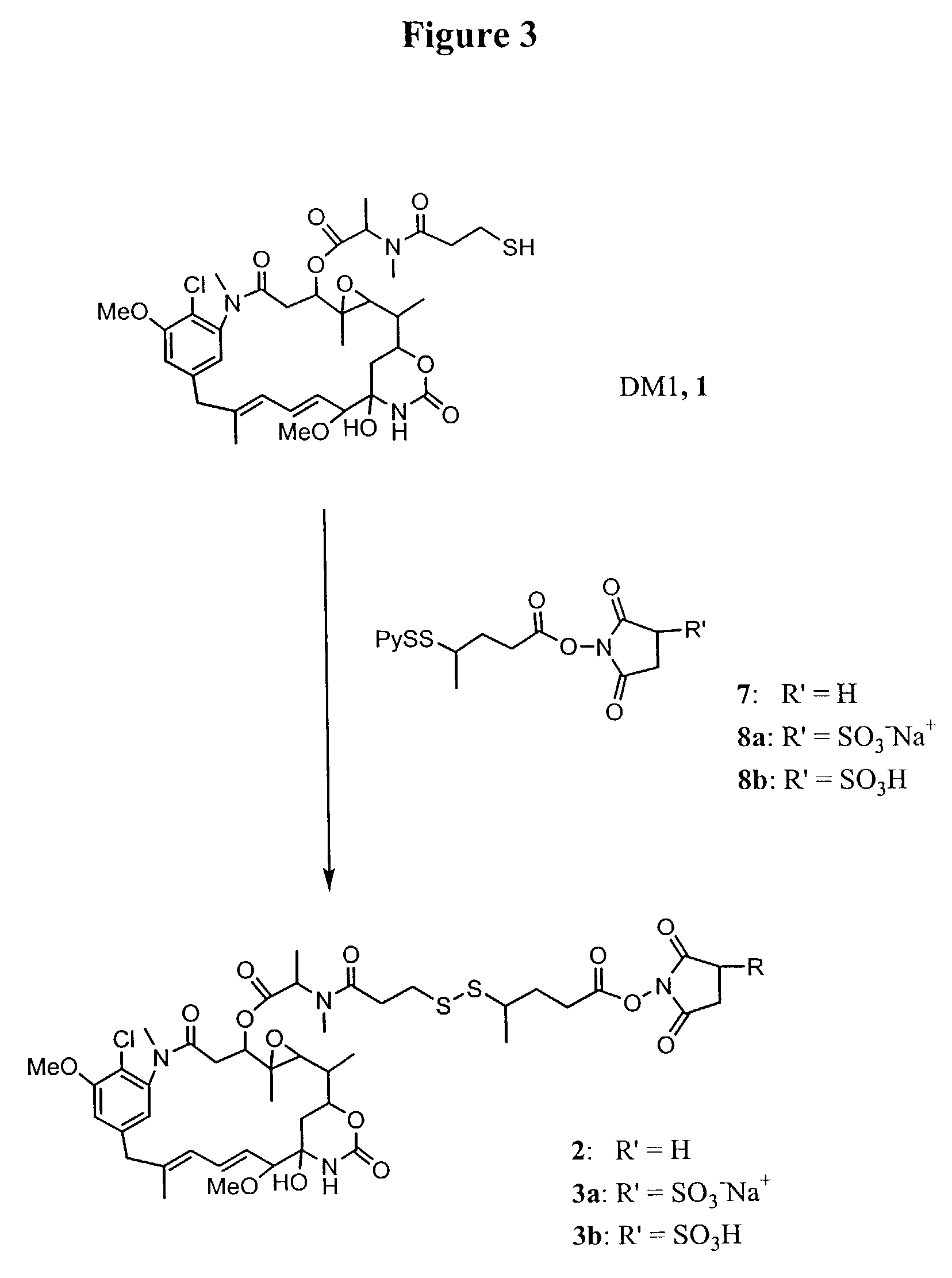
Methods for preparation of cytotoxic conjugates of maytansinoids and cell binding agents
InactiveUS7368565B2Increase productionMinimizes time processing timeAntibacterial agentsOrganic active ingredientsCell bindingCytotoxicity
The present invention discloses a one-step process for the production of cytotoxic conjugates of maytansinoids and cell binding agents. Maytansinoids having a disulfide linker that bears a reactive moiety are linked to cell binding agents, such as antibodies, without prior modification of the cell binding agent. These conjugates are useful as therapeutic agents which are delivered specifically to target cells and are cytotoxic.
Owner:IMMUNOGEN INC
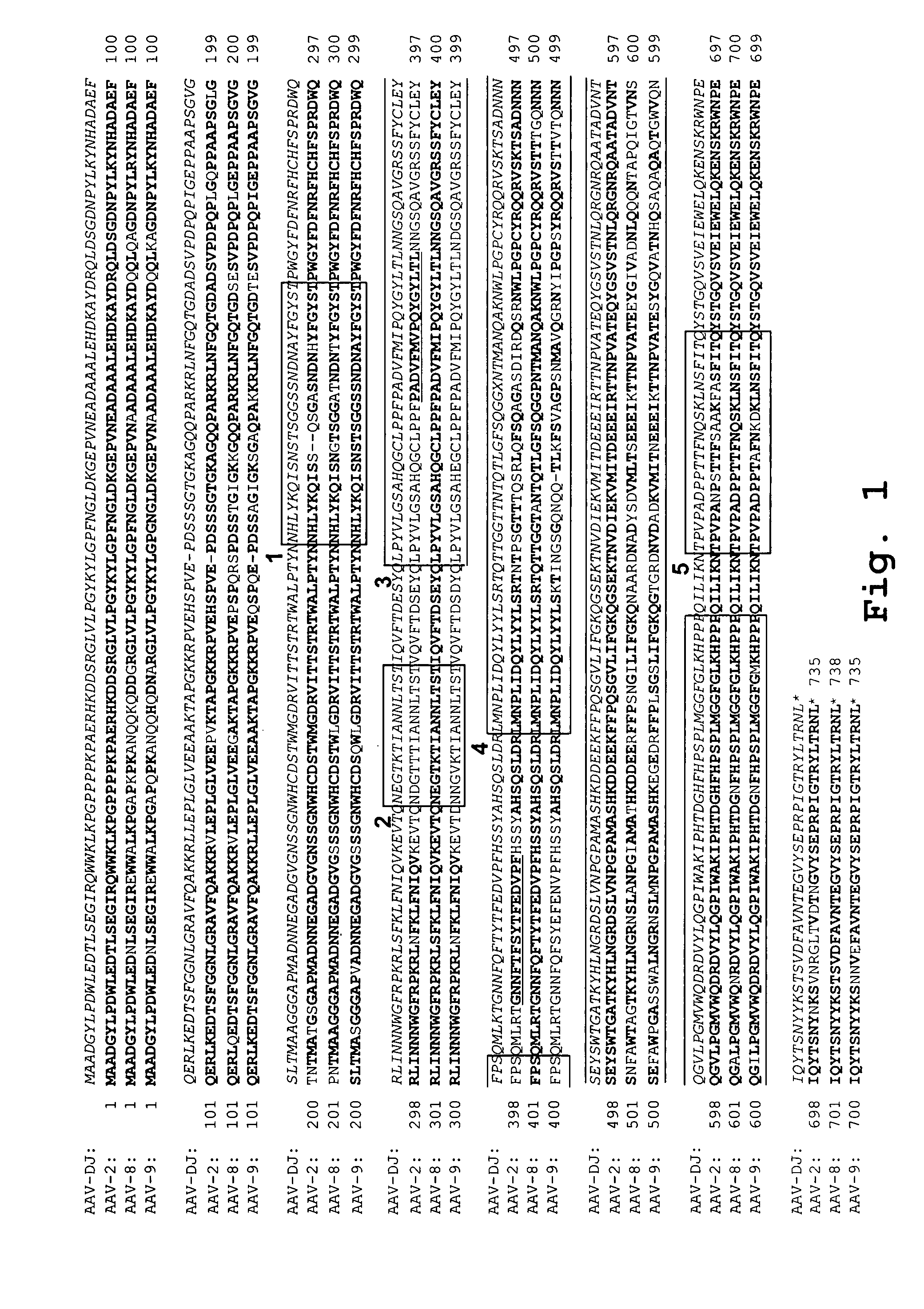
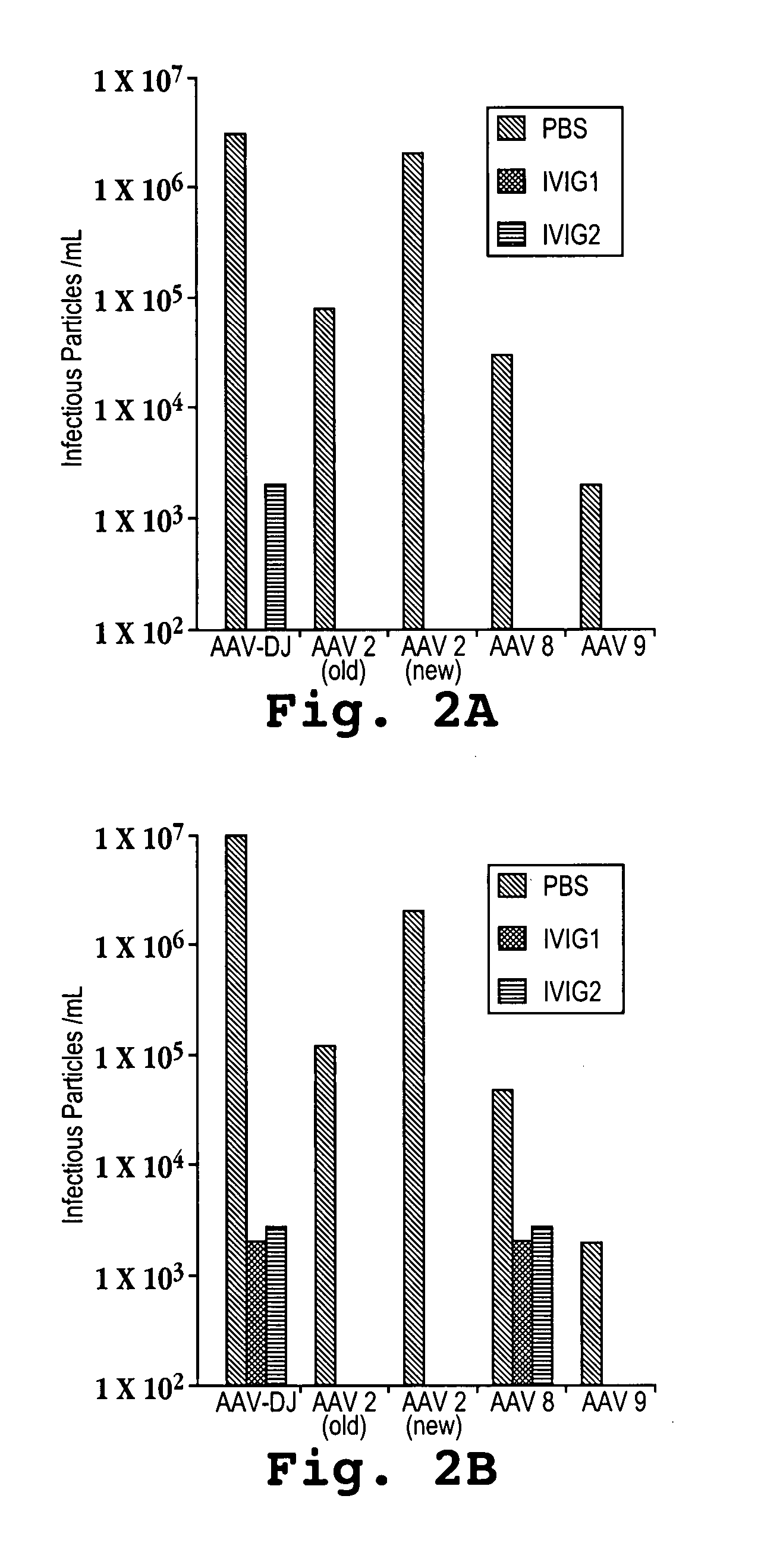
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
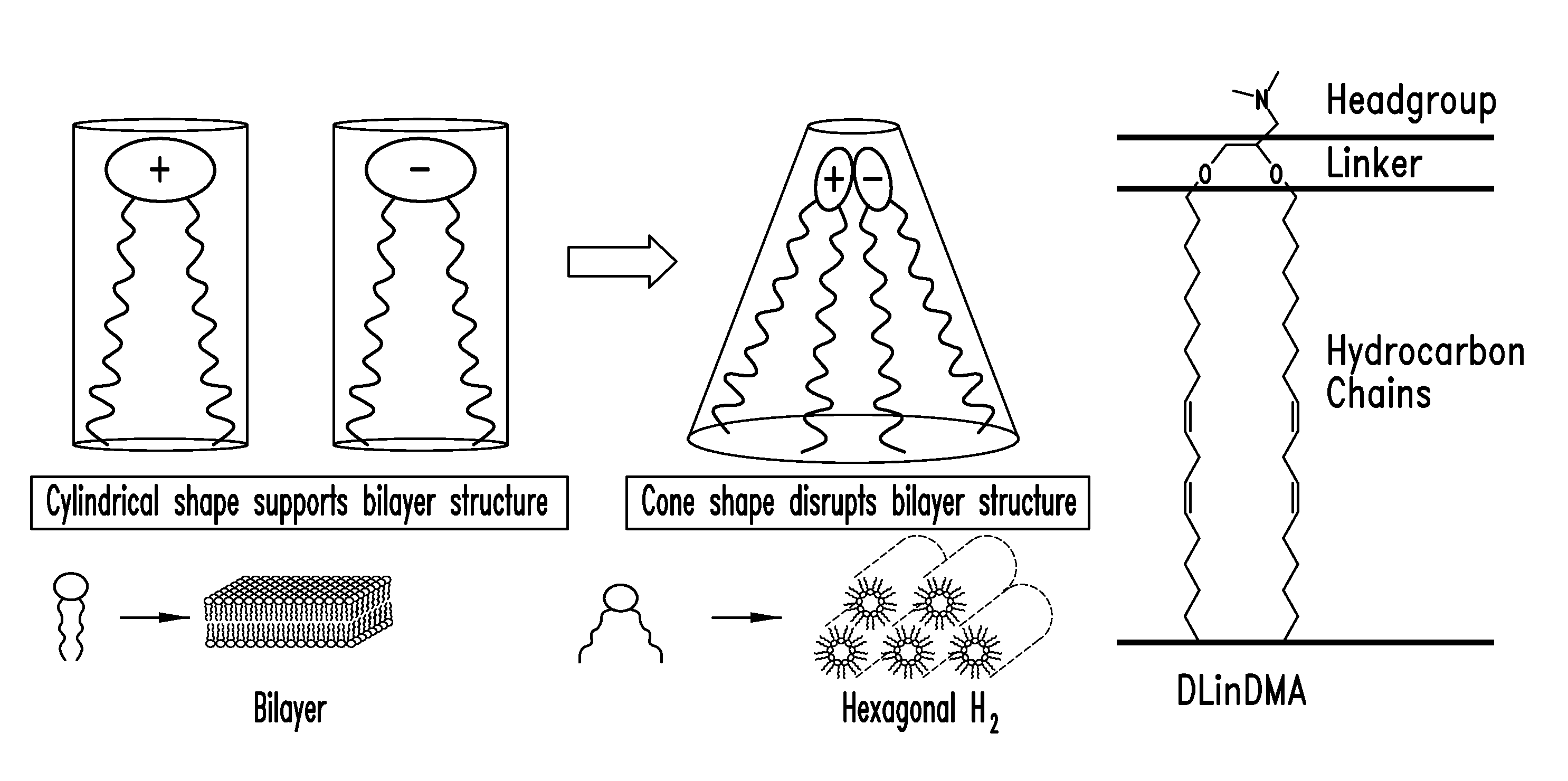
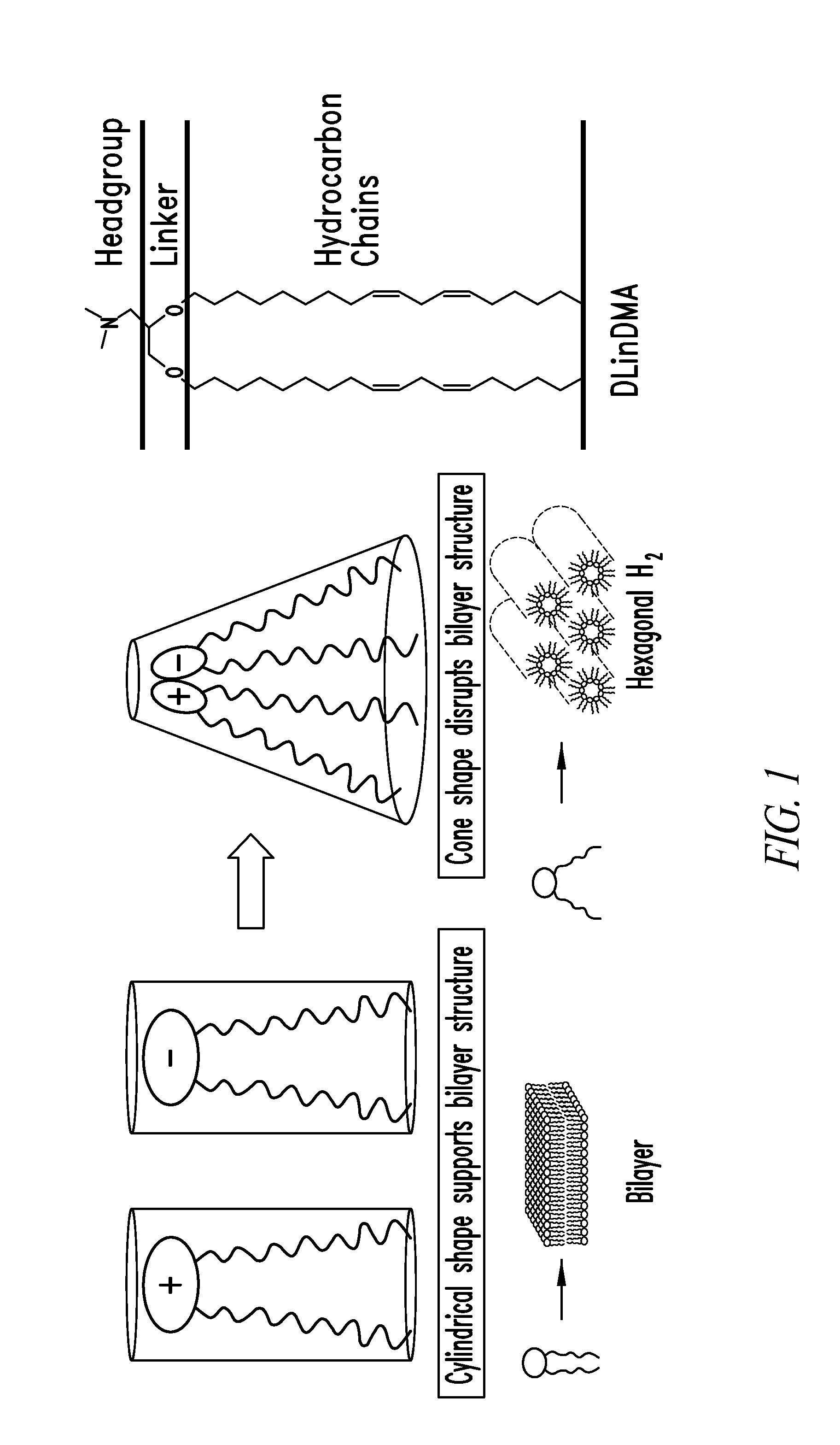
Amino lipids and methods for the delivery of nucleic acids
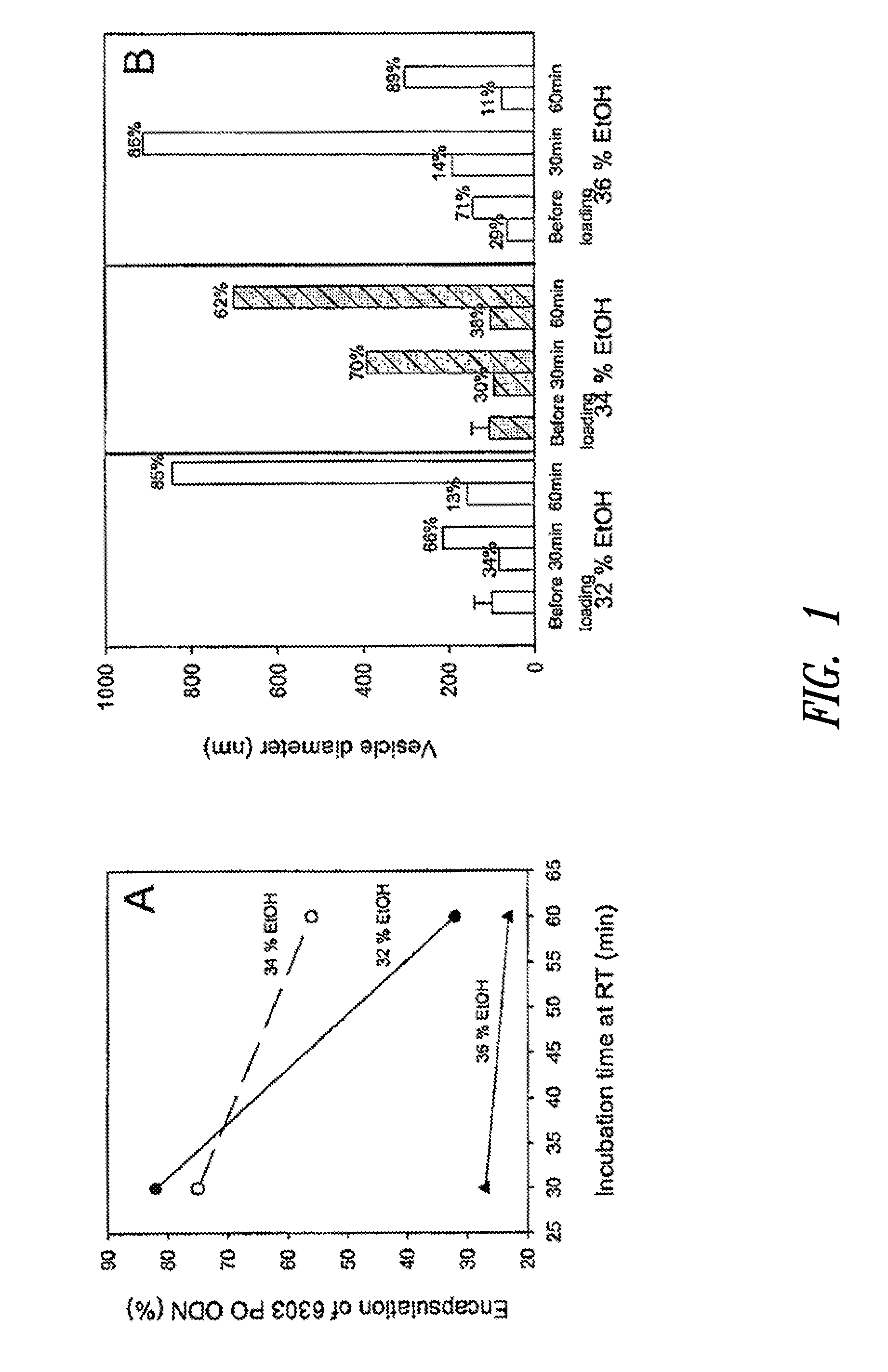
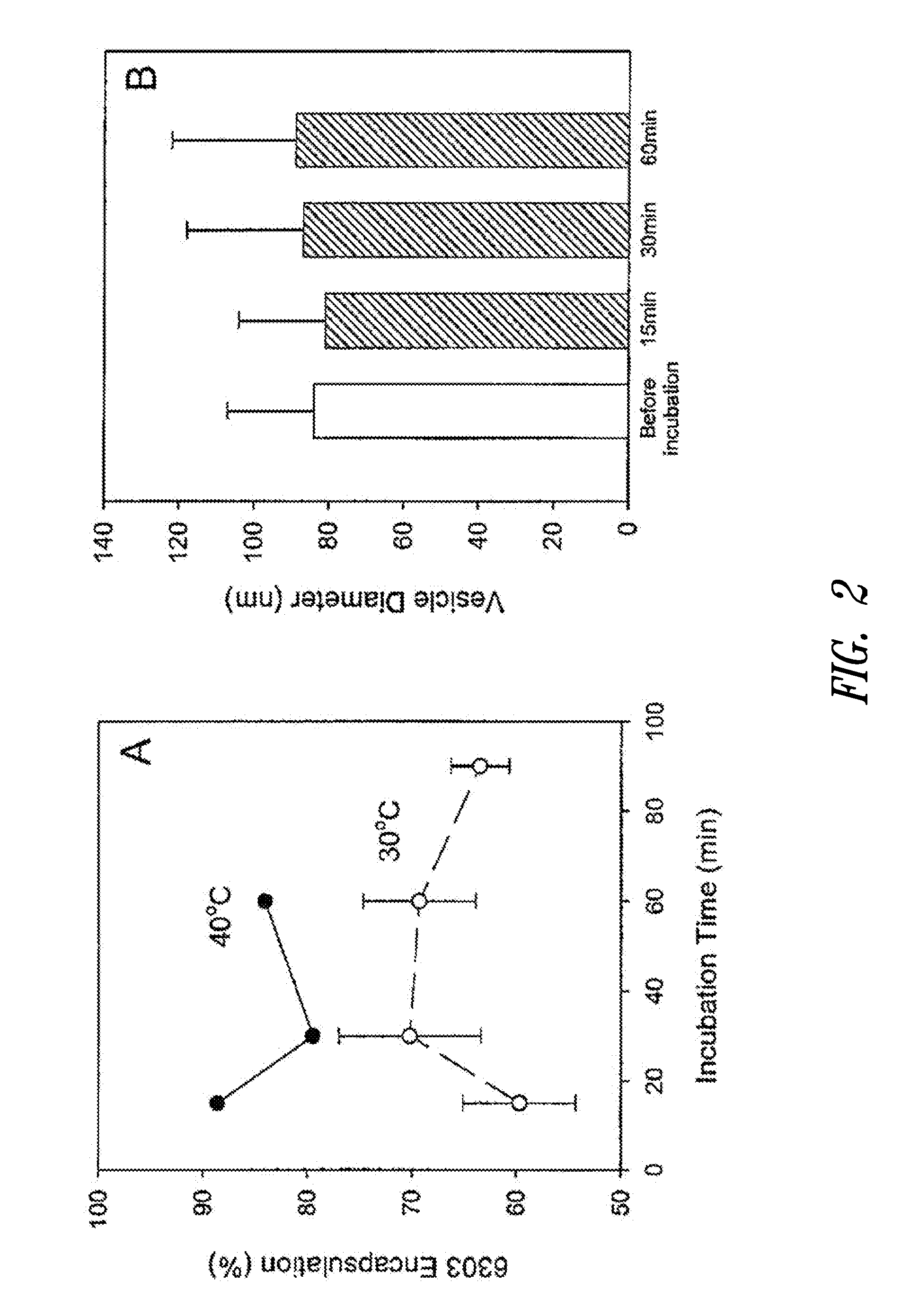
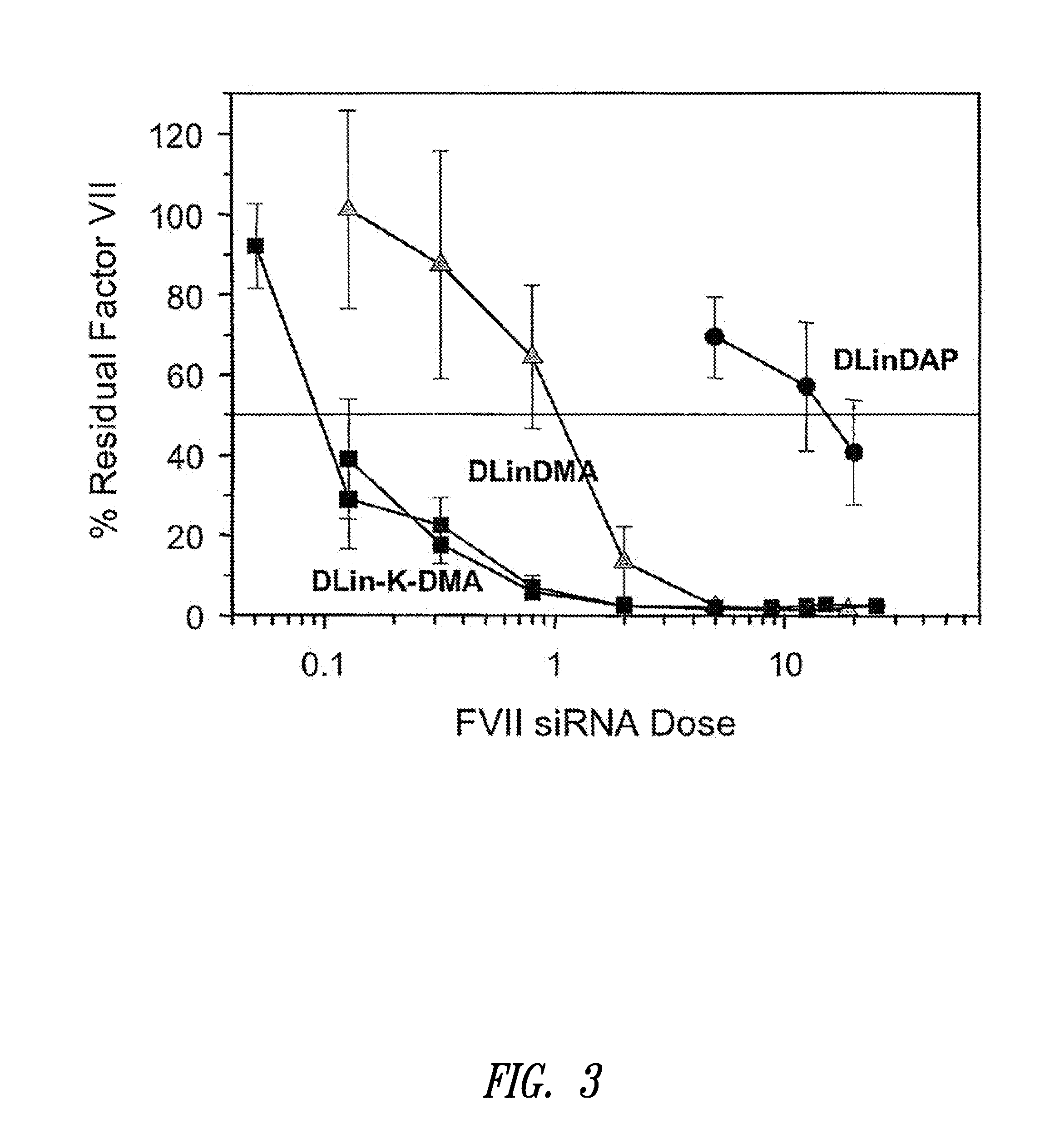
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
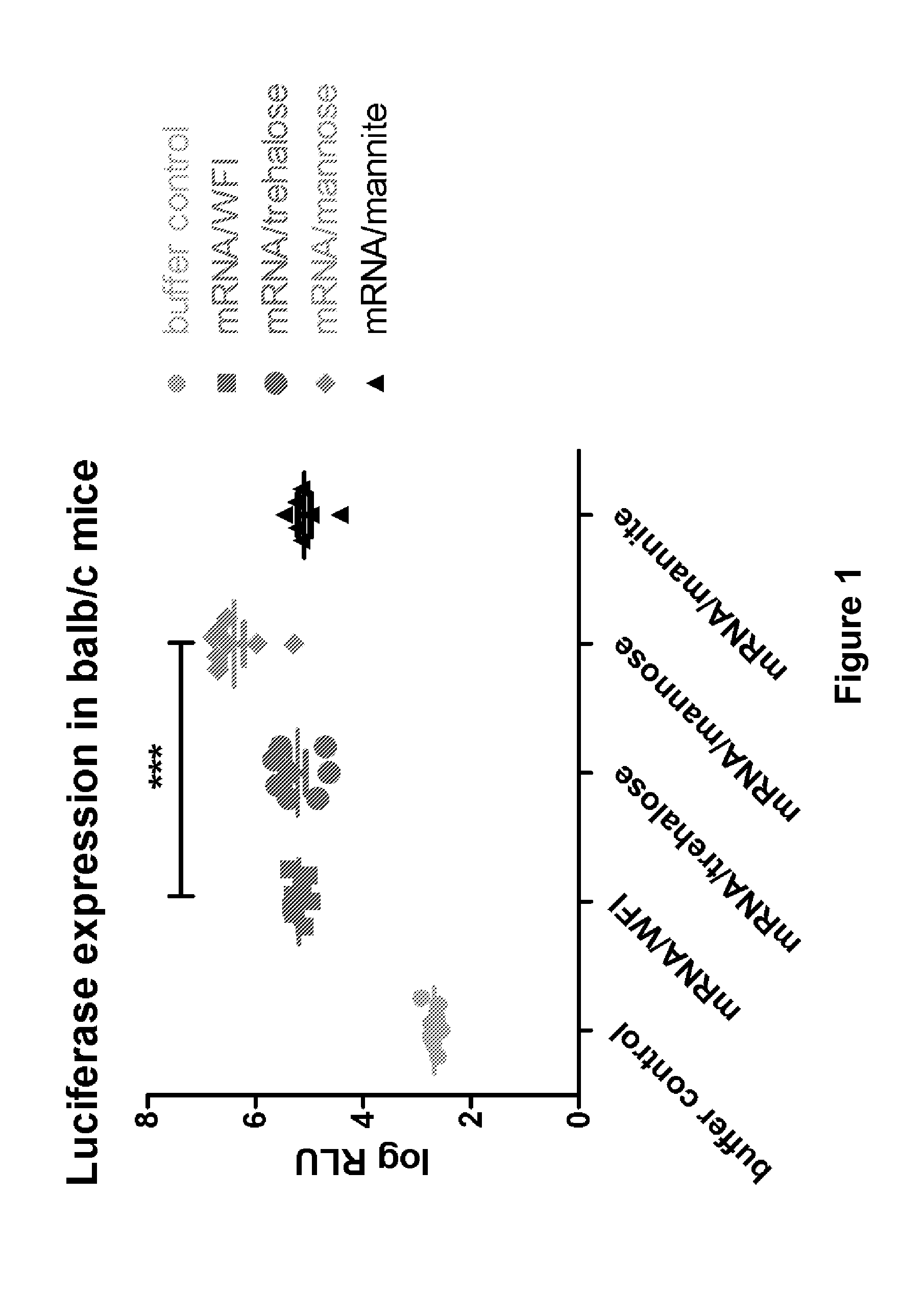
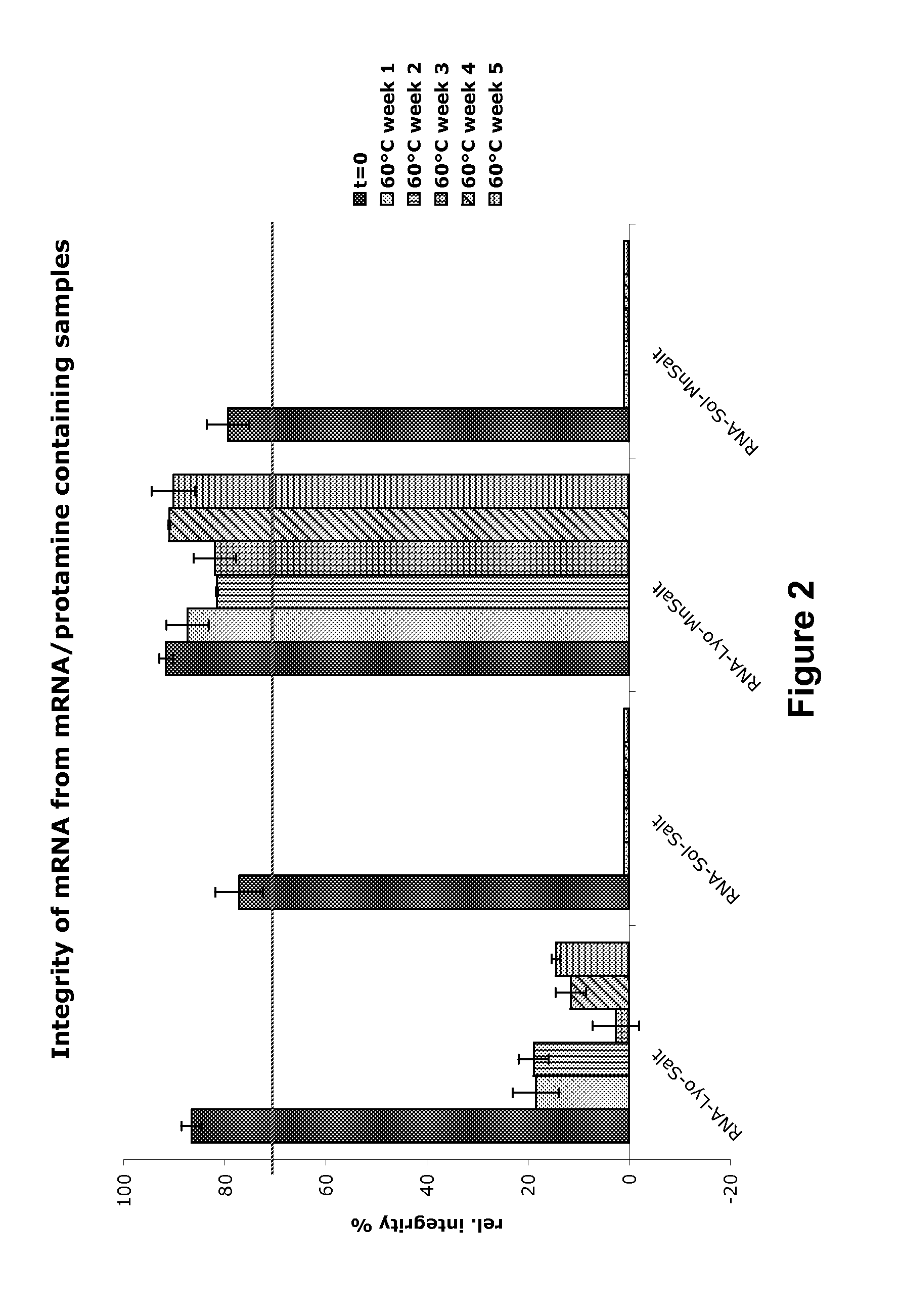
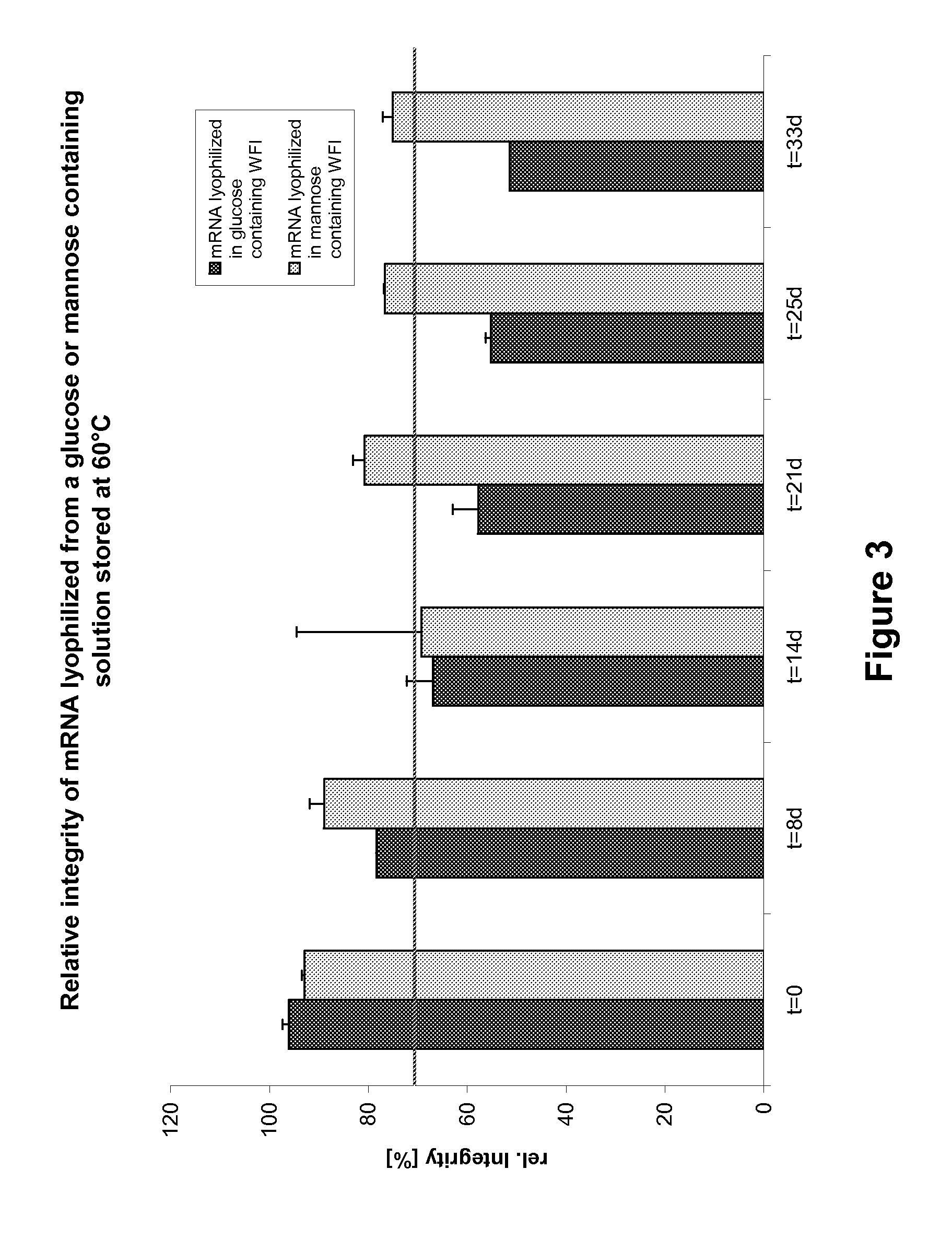
Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids
InactiveUS20120258046A1High transfection efficiencyEnhance protein expressionOrganic active ingredientsGenetic material ingredientsIn vivoTransfection
The present invention is directed to (the use of) a solution containing at least one nucleic acid (sequence) and free mannose for lyophilization, transfection and / or injection, particularly of RNA and mRNA. The inventive solution exhibits a positive effect on stabilization of the nucleic acid (sequence) during lyophilization and storage but also leads to a considerable increase of the transfection efficiency of a nucleic acid. It thus also increases in vivo expression of a protein encoded by such a nucleic acid upon increased transfection rate. The present invention is furthermore directed to a method of lyophilization using the mannose-containing solution, to pharmaceutical compositions, vaccines, kits, first and second medical uses applying such a mannose-containing solution and / or a nucleic acid (sequence) lyophilized or resuspended with such a solution.
Owner:CUREVAC SE
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Single-chain antigen-binding proteins capable of glycosylation, production and uses thereof
The present invention relates to single-chain antigen-binding molecules capable of glycosylation. Compositions of, genetic constructions coding for, and methods for producing monovalent and multivalent single-chain antigen-binding molecules capable of glycosylation are described and claimed. Composition of, genetic constructions coding for, and methods for producing glycosylated monovalent and multivalent single-chain antigen-binding molecules capable of polyalkylene oxide conjugation are described and claimed. The invention also relates to methods for producing a polypeptide having increased glycosylation and the polypeptide produced by the described method. Uses resulting from the multifunctionality of a glycosylated / polyalkylene oxide conjugated antigen-binding protein are also described and claimed.
Owner:ENZON PHARM INC
Pancreatic cancer associated antigen, antibody thereto, and diagnostic and treatment methods
The present invention is directed to an antigen found on the surface of rat and human pancreatic cancer cells and provides antibodies of high specificity and selectivity to this antigen as well as hybridomas secreting the subject antibodies. Methods for both the diagnosis and treatment of pancreatic cancer are also provided. This tissue marker of pancreatic adenocarcinoma, an approximately 43.5 kD surface membrane protein designated PaCa-Ag1, is completely unexpressed in normal pancreas but abundantly expressed in pancreatic carcinoma cells. Moreover, a soluble form of PaCa-Ag1 exists, having a molecular weight about 36 to about 38 kD, that is readily identified in sera and other body fluids of pancreatic cancer patients, using a subject antibody.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Vaccine Nanotechnology
ActiveUS20100233251A1Modulating immune systemEnhance and suppress and direct and immune responseNervous disorderAntipyreticDiseaseNanocarriers
Owner:MASSACHUSETTS INST OF TECH +4
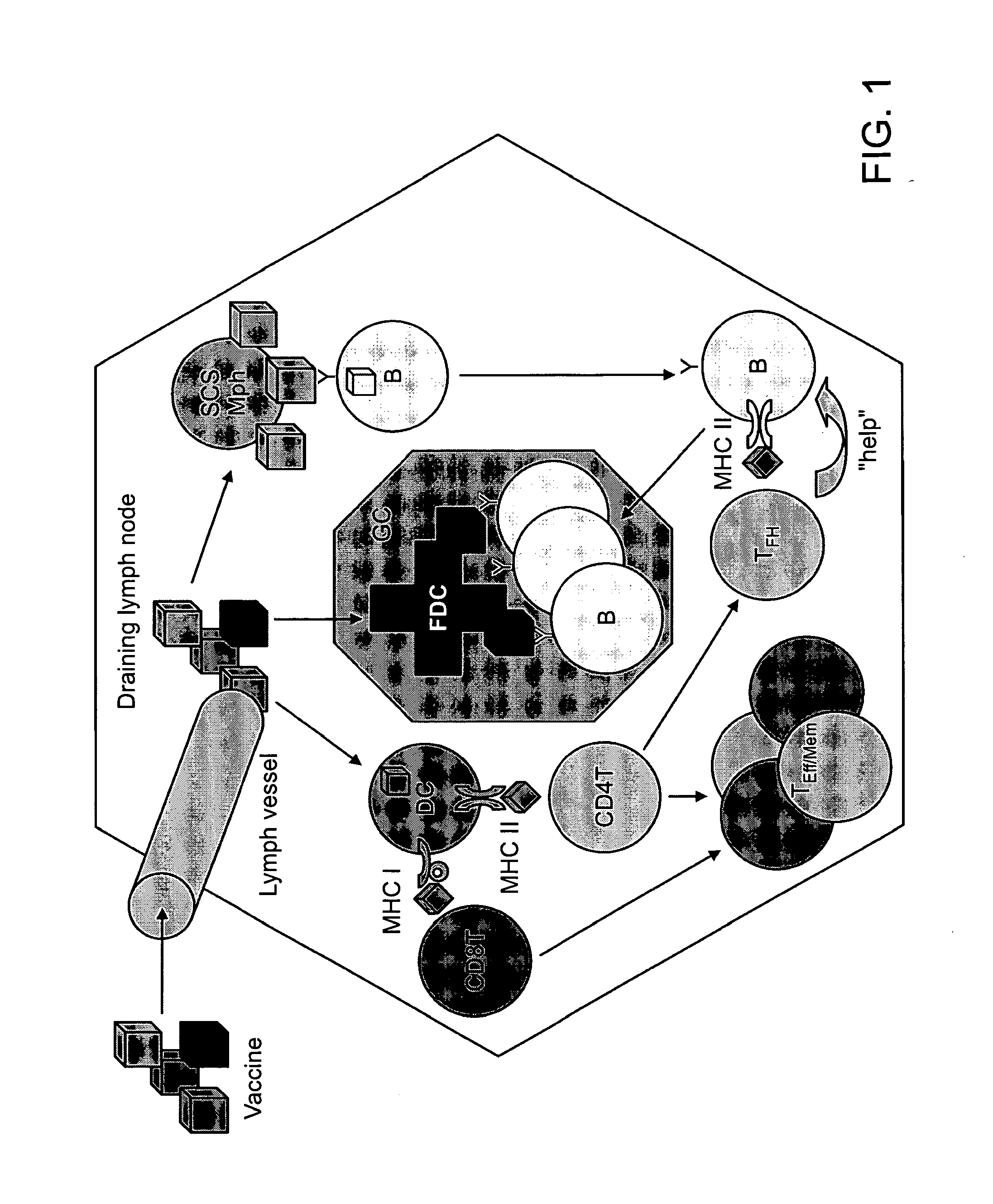
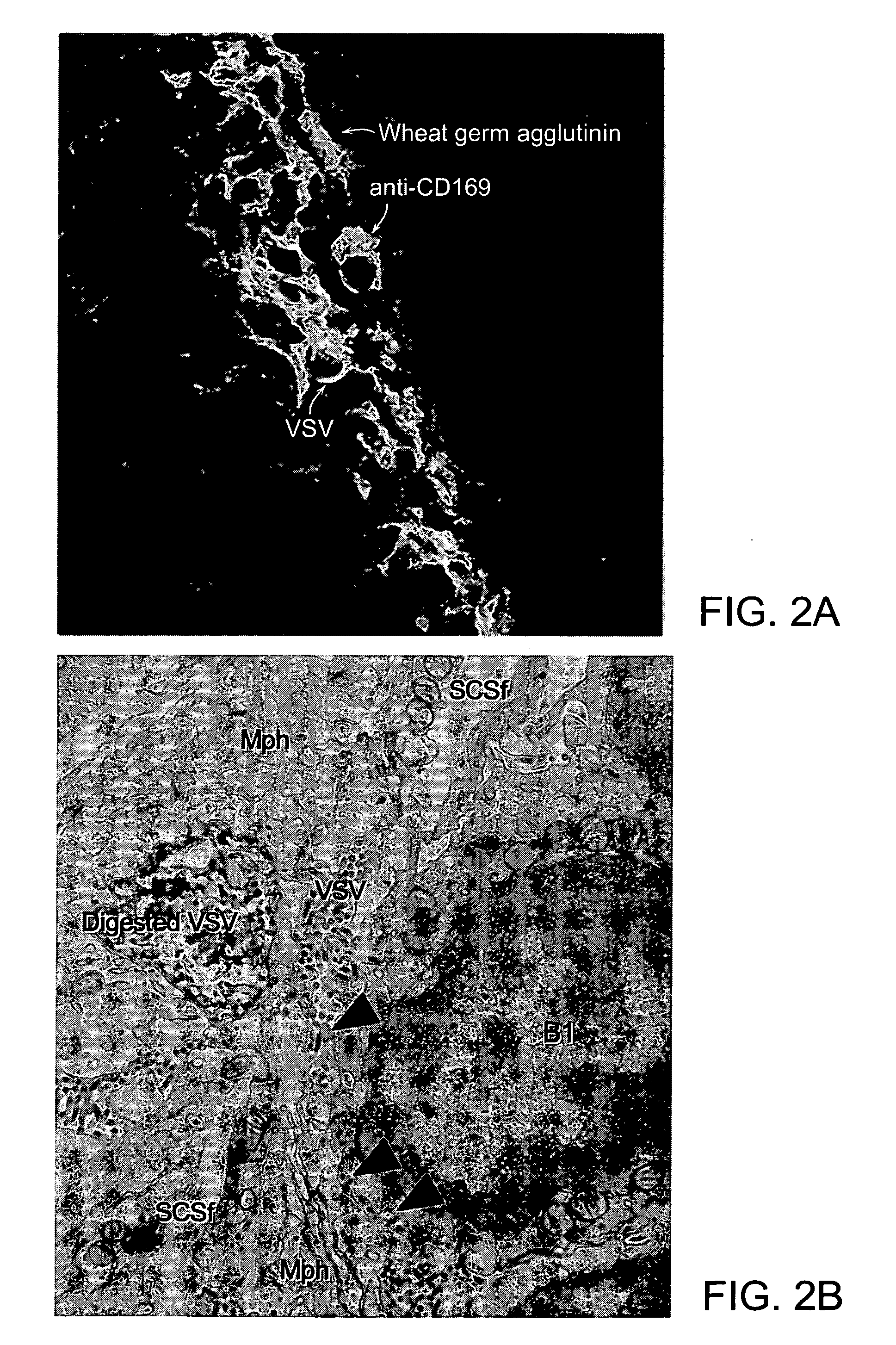
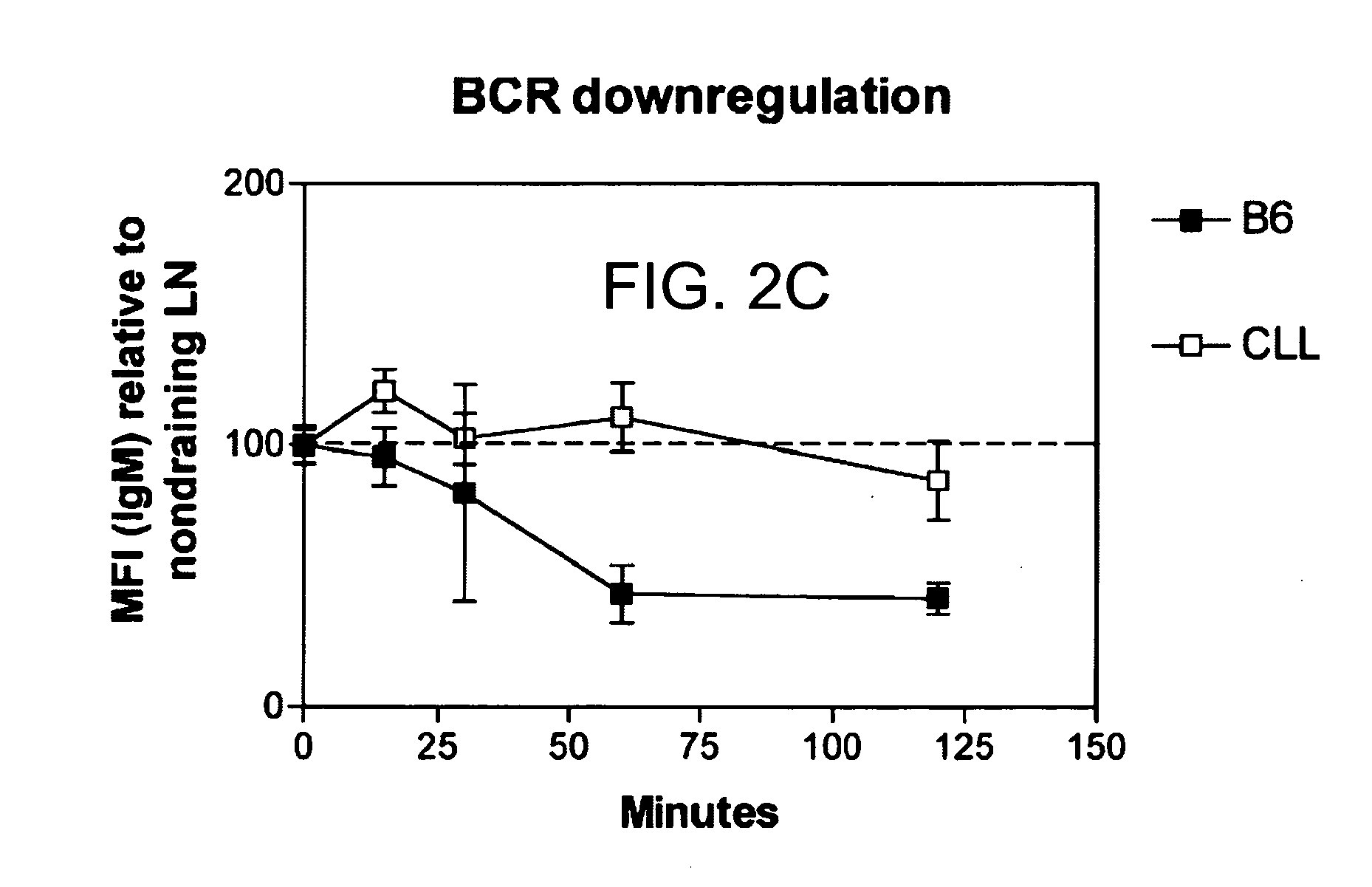
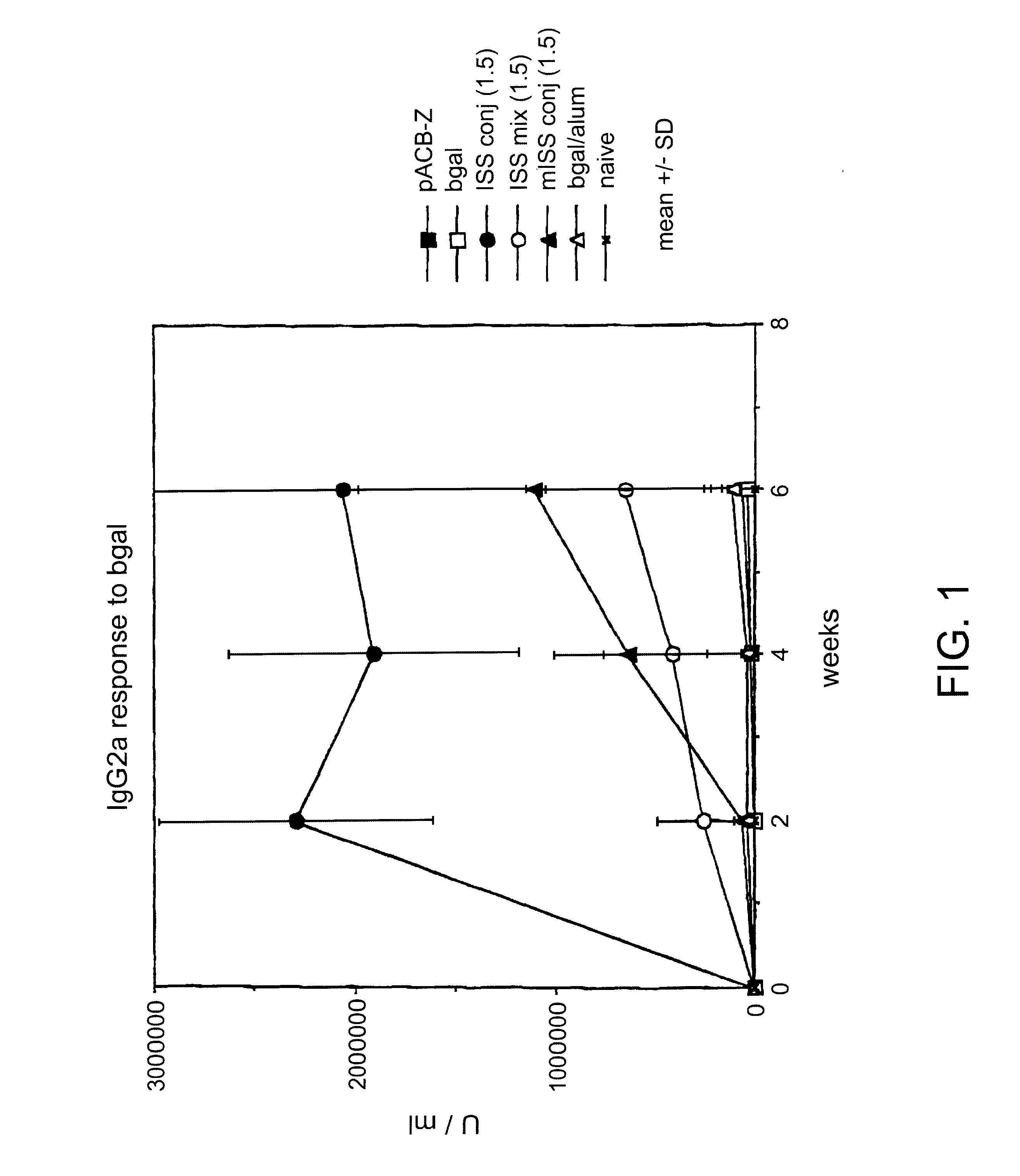
Immunostimulatory polynucleotide/immunomodulatory molecule conjugates
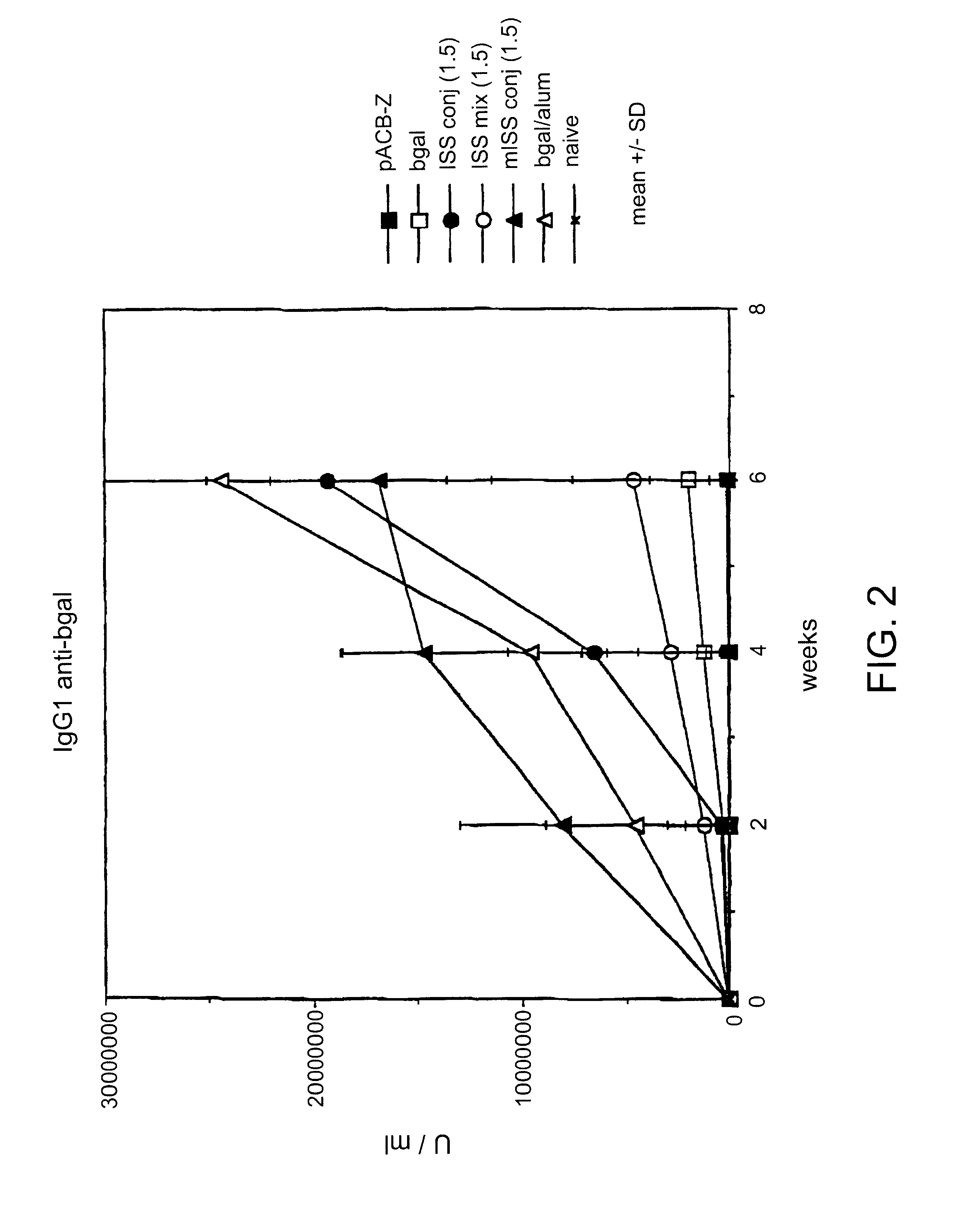
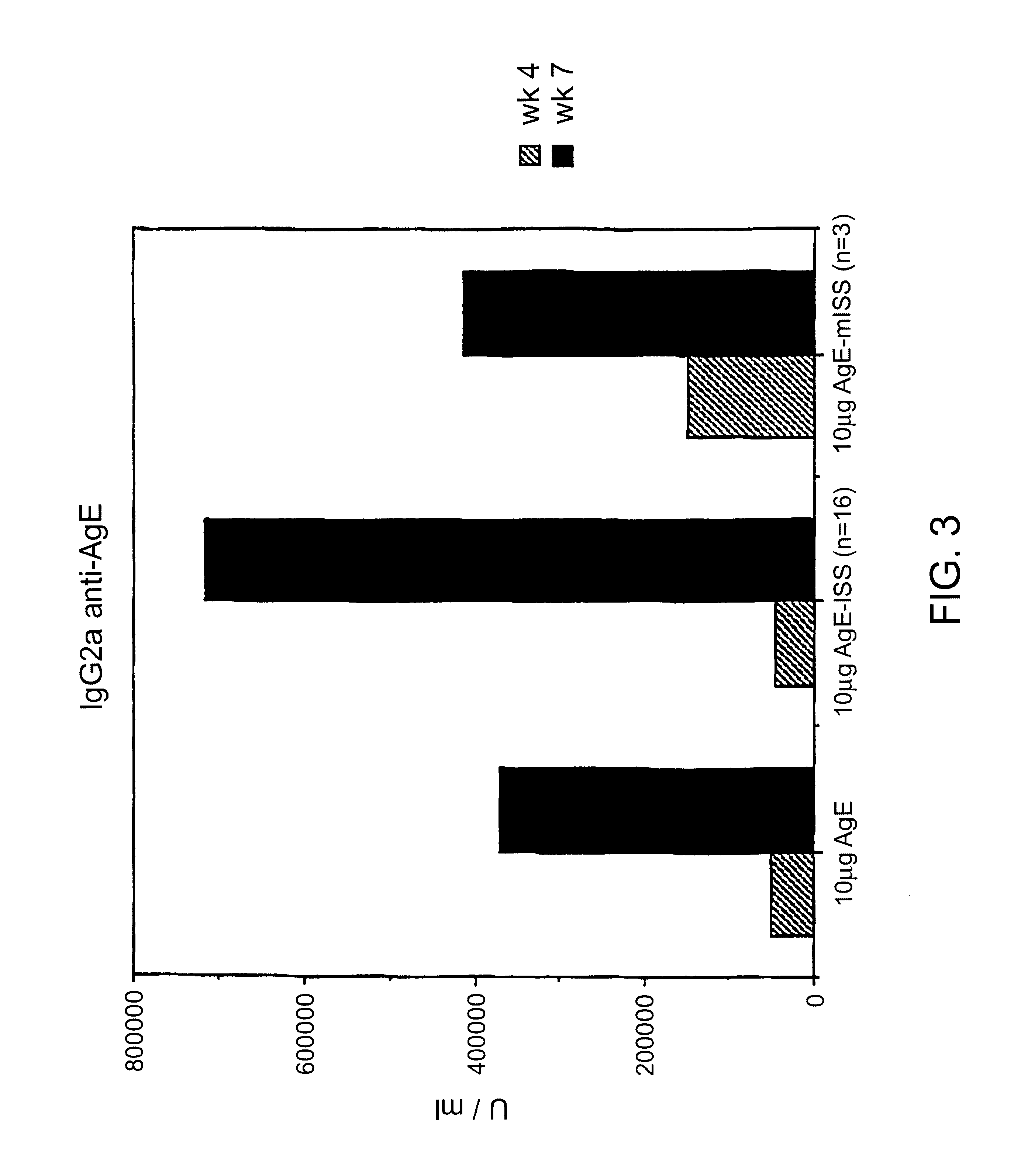
InactiveUS6610661B1Boost magnitudeBoost both humoral (antibody)Peptide/protein ingredientsGenetic material ingredientsAntigenAdjuvant
Immunostimulatory polynucleotide-immunomodulatory molecule conjugate compositions are disclosed. These compositions include a polynucleotide that is linked to an immunomodulatory molecule, which molecule comprises an antigen and may further comprise immunomodulators such as cytokines and adjuvants. The polynucleotide portion of the conjugate includes at least one immunostimulatory oligonucleotide nucleotide sequence (ISS). Methods of modulating an immune response upon administration of the polynucleotide-immunomodulatory conjugate preparation to a vertebrate host are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
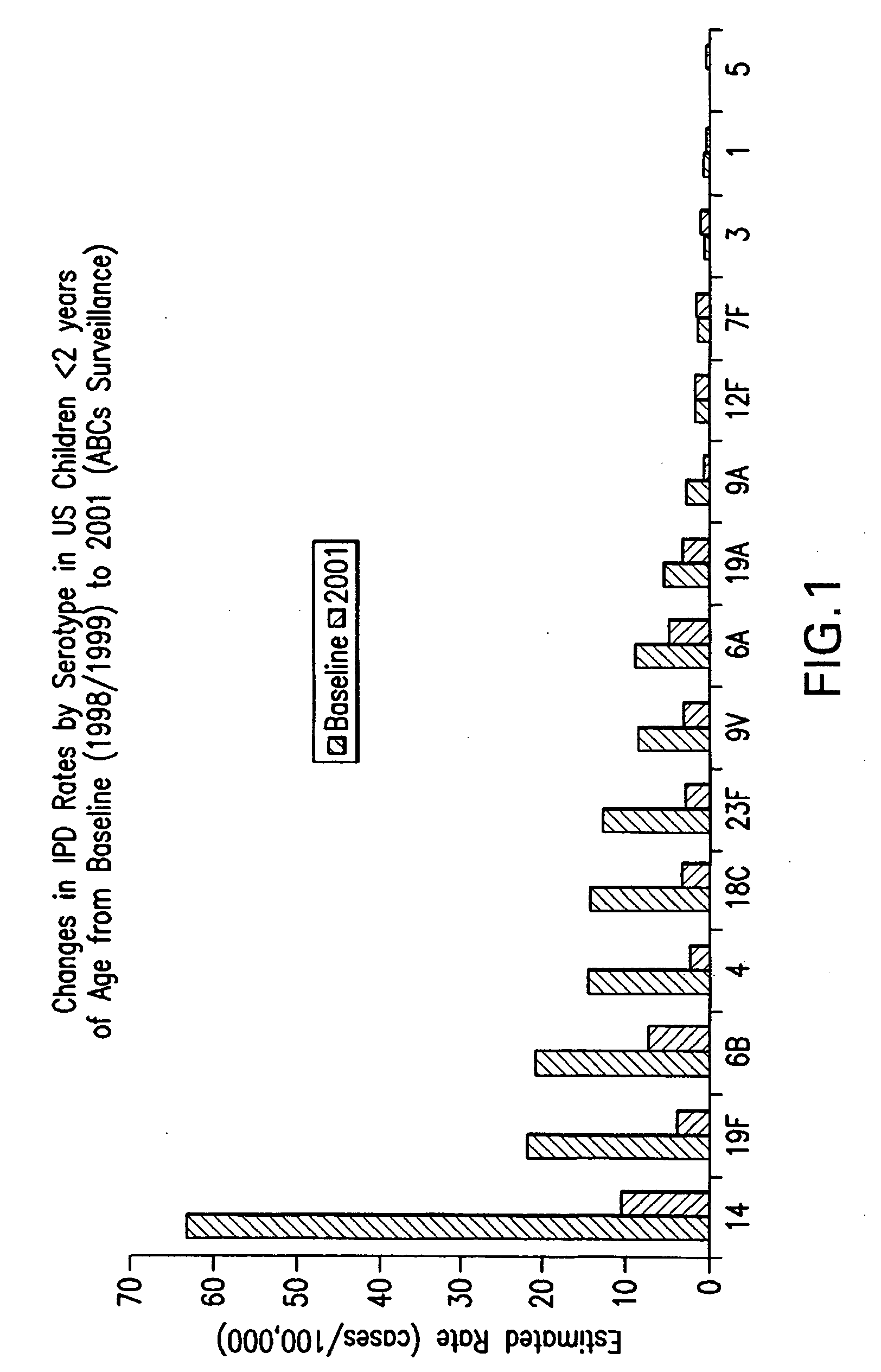
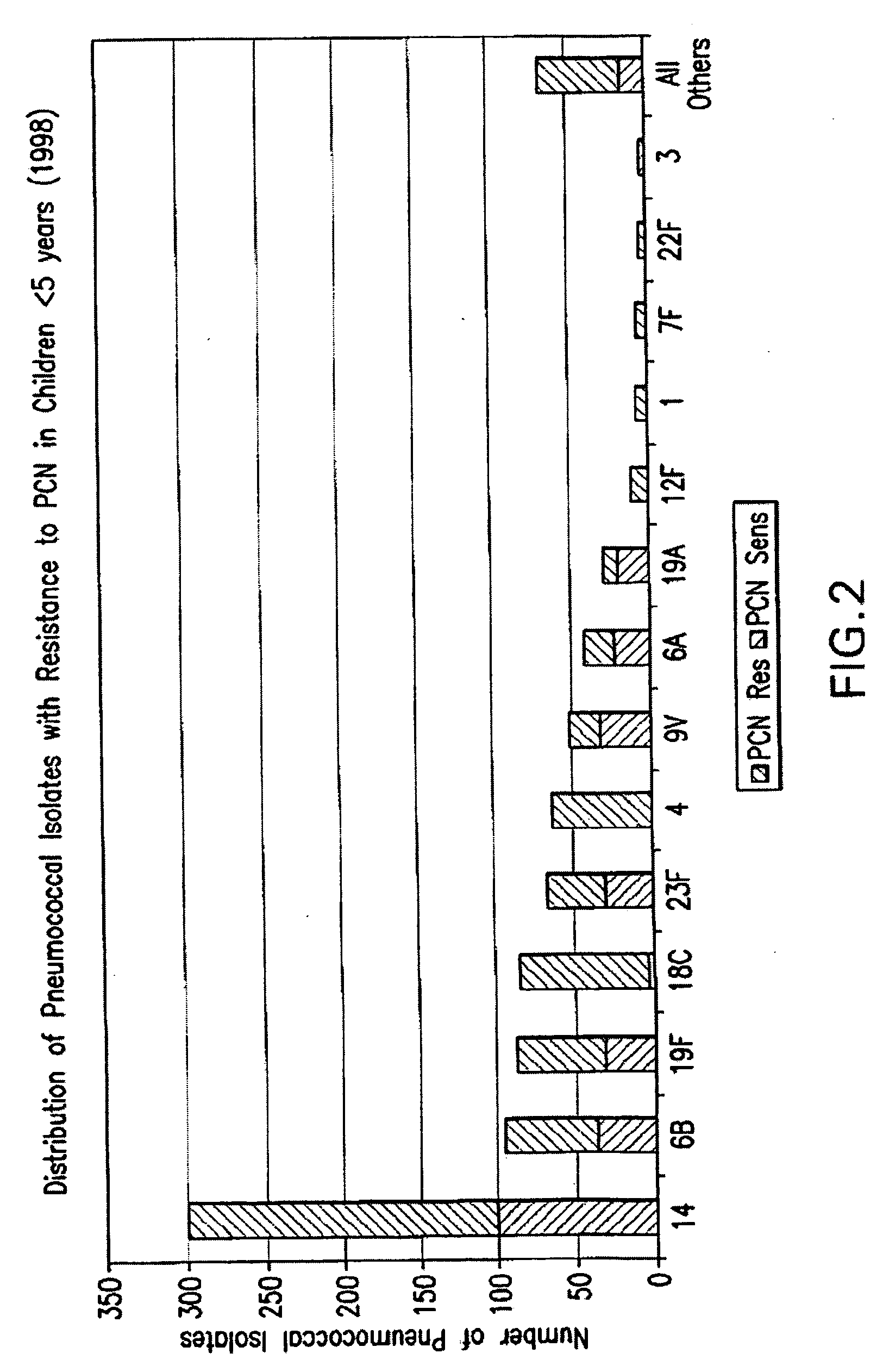
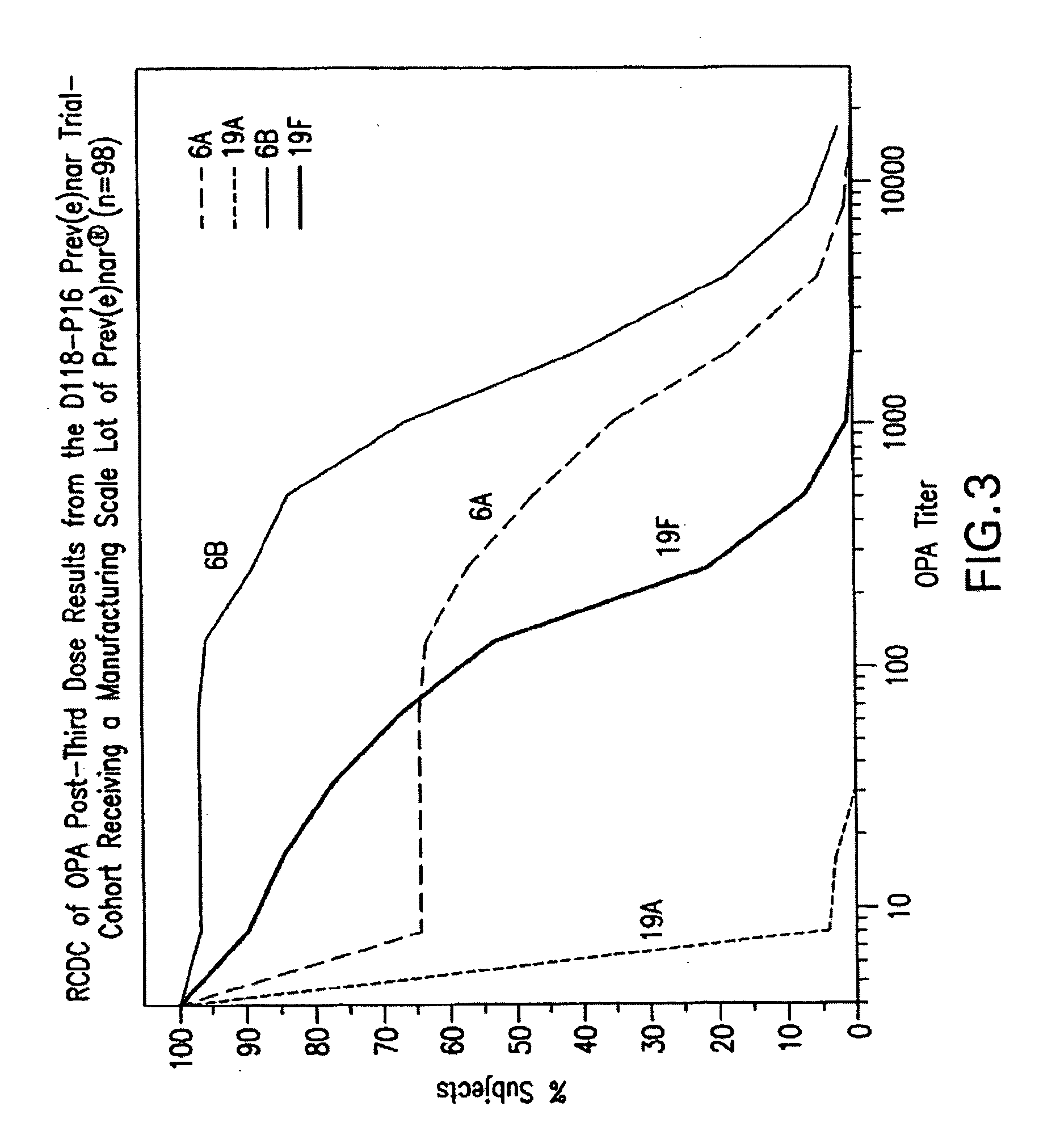
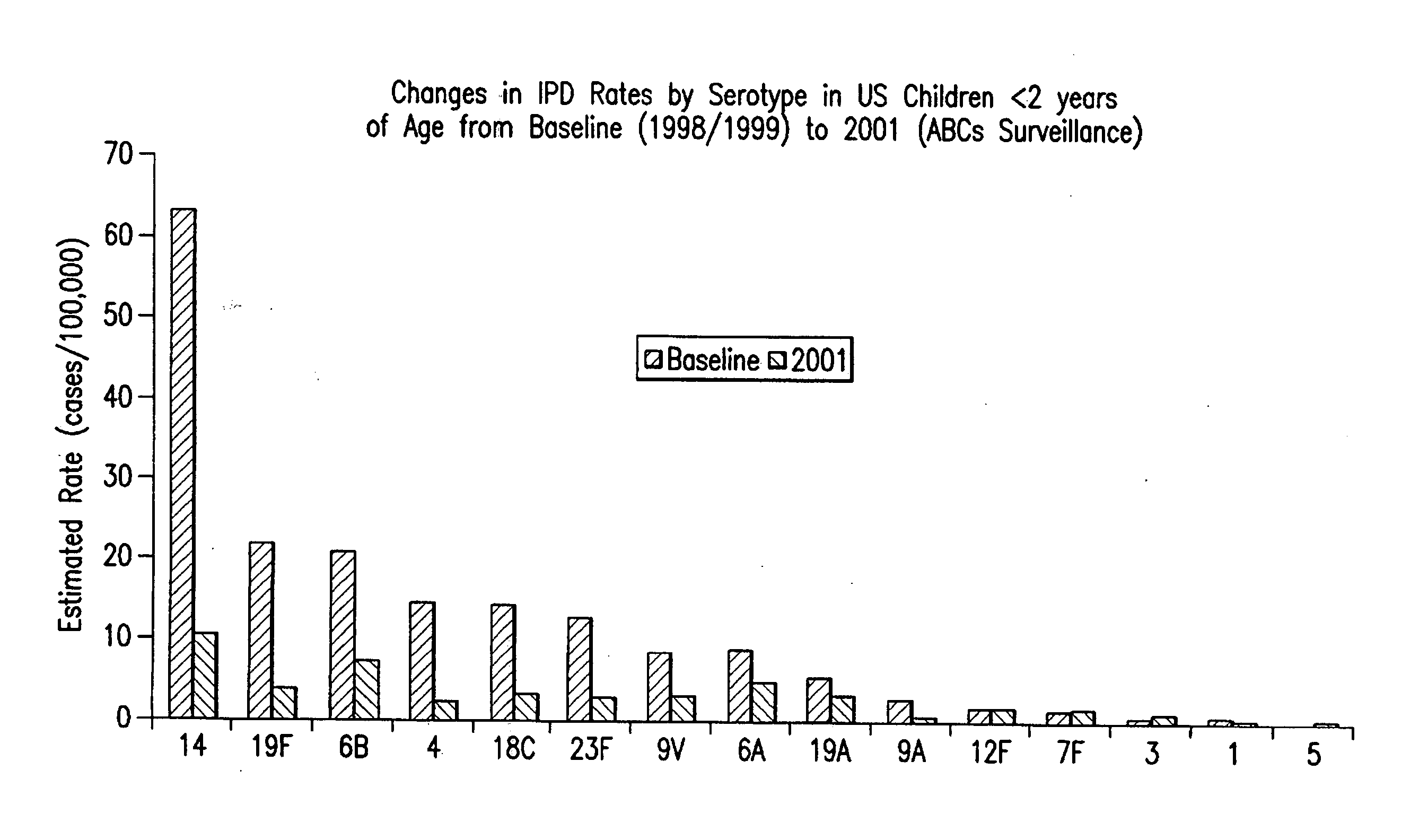
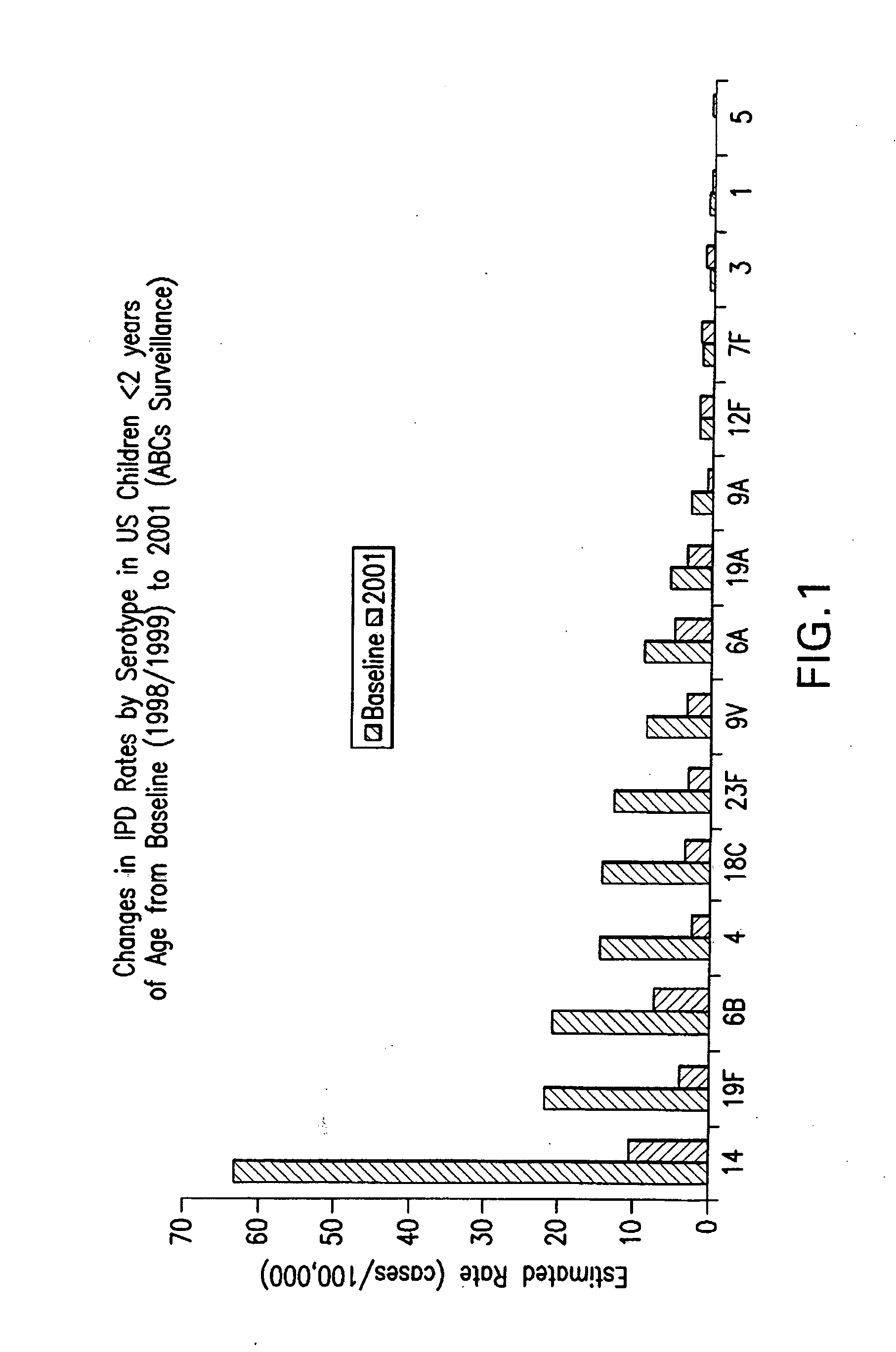
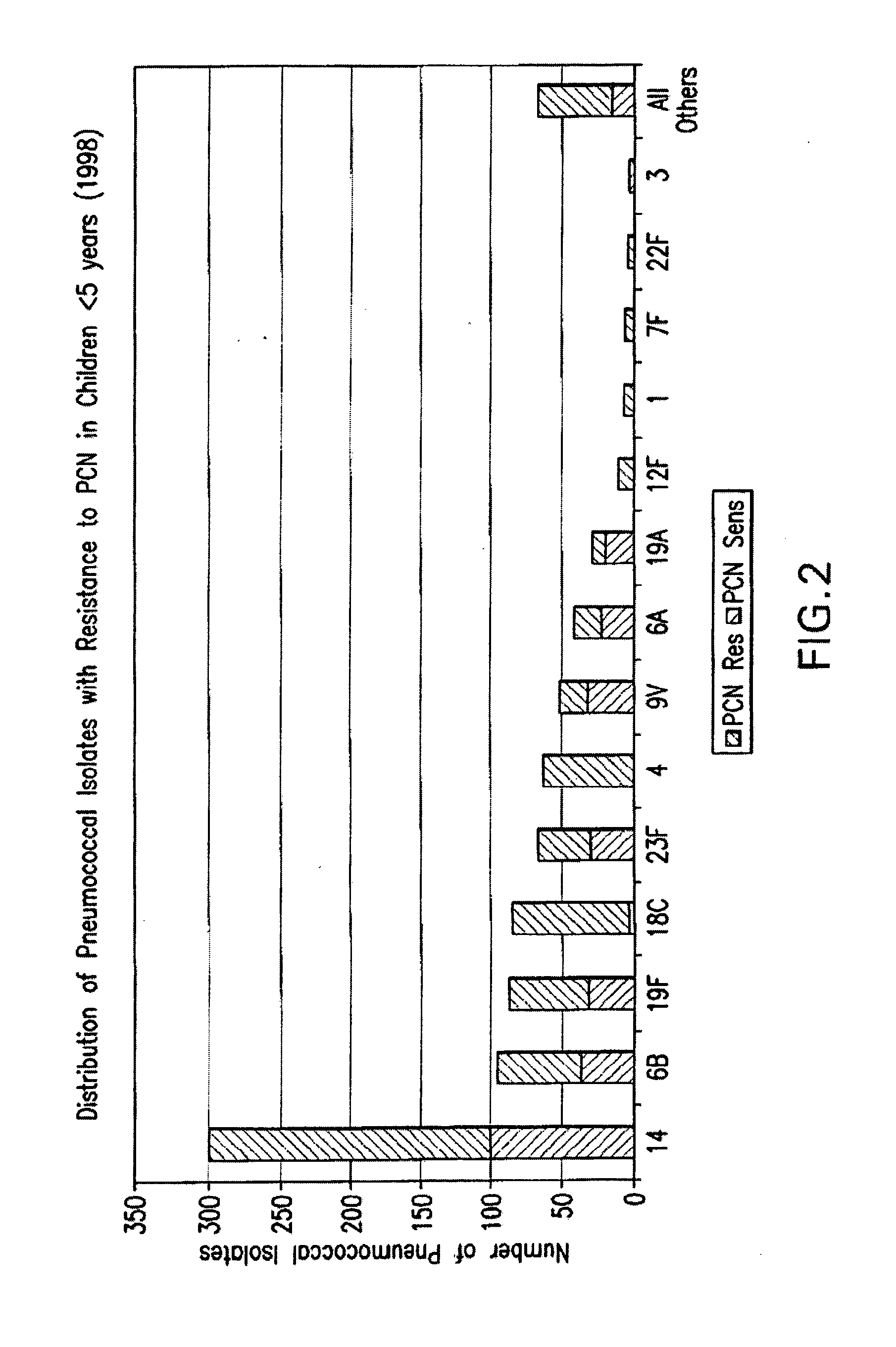
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
InactiveUS20060251684A1Reduce infectivityReduce morbiditySsRNA viruses negative-senseAntibacterial agentsPathogenic microorganismOrganic solvent
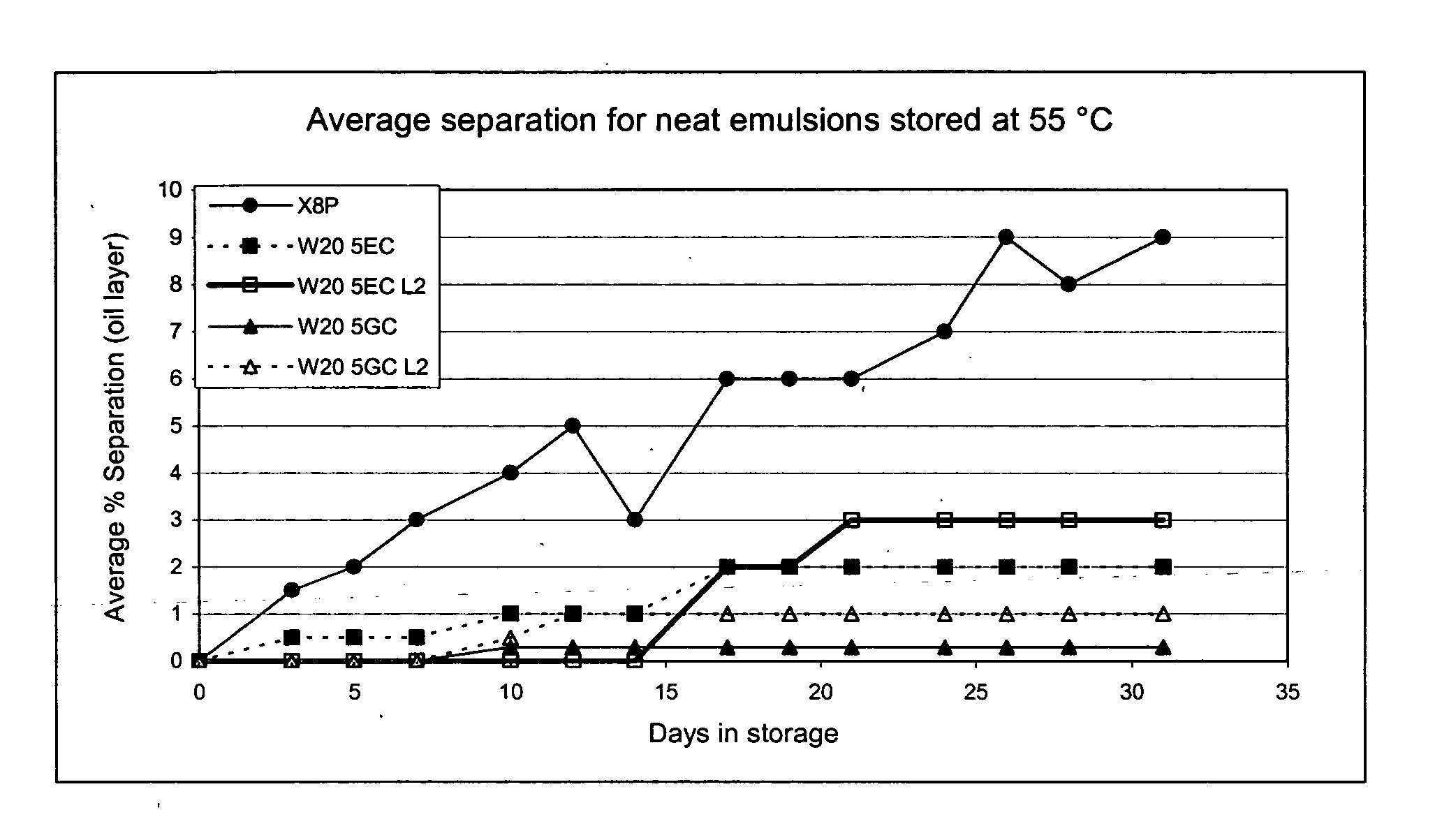
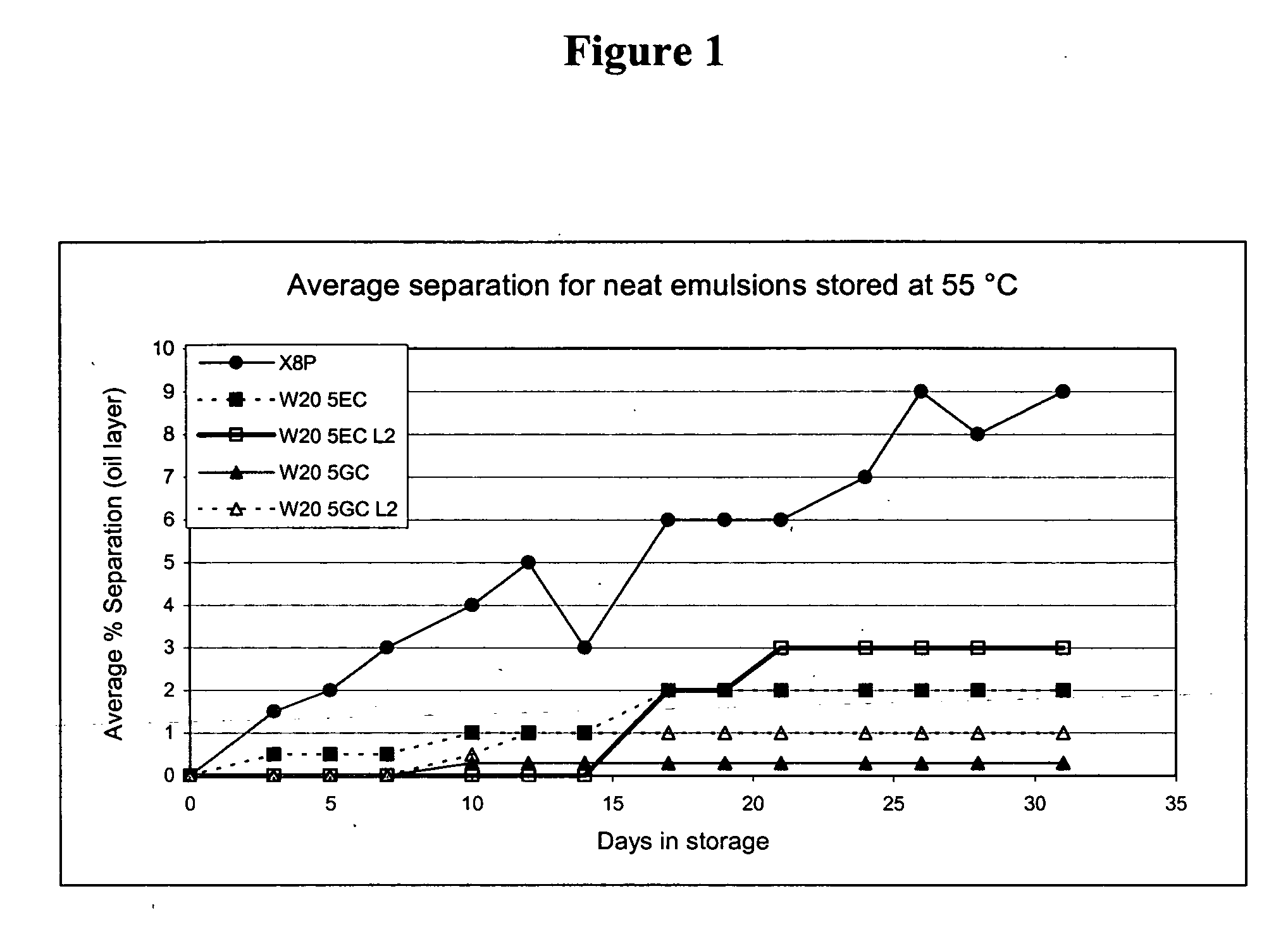
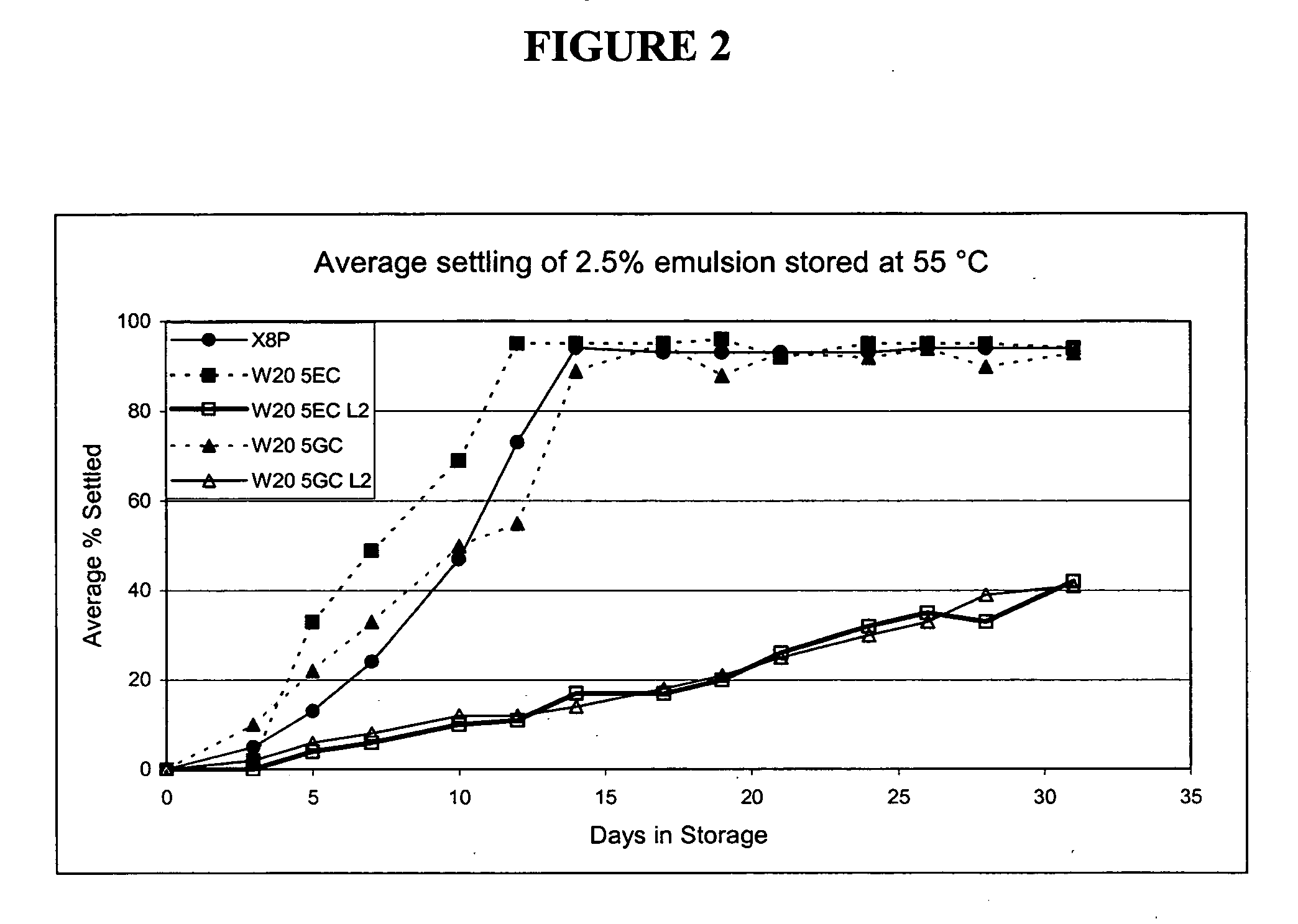
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
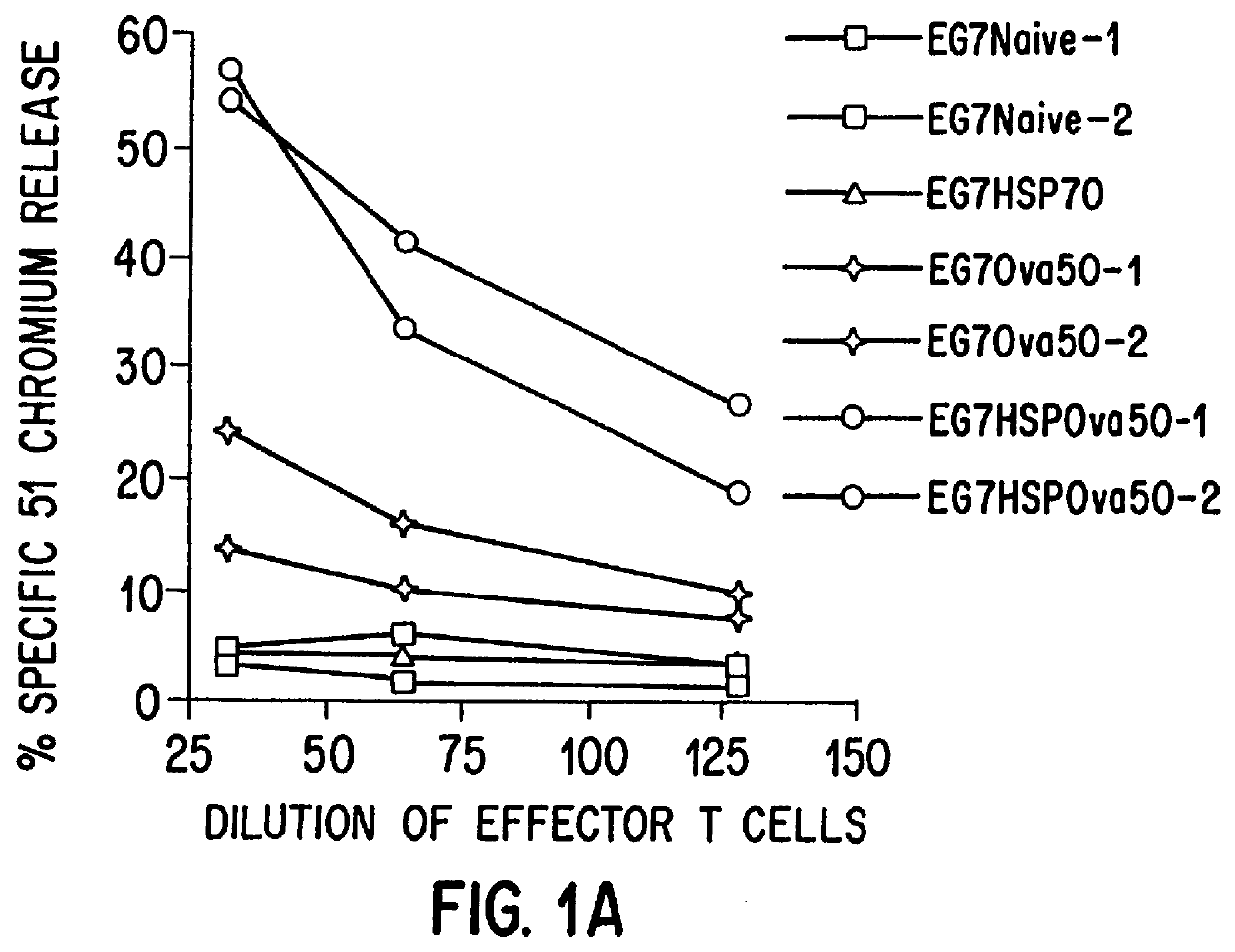
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
Compositions and methods for the delivery of nucleic acids
InactiveUS20110117125A1Reduce particle aggregationReduce selection requirementsAntibacterial agentsOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:THE UNIV OF BRITISH COLUMBIA +2
Emulsions and methods of making nanocarriers
This invention relates, in part, to methods of using emulsions for making synthetic nanocarriers and the synthetic nanocarriers formed by such methods.
Owner:SELECTA BIOSCI
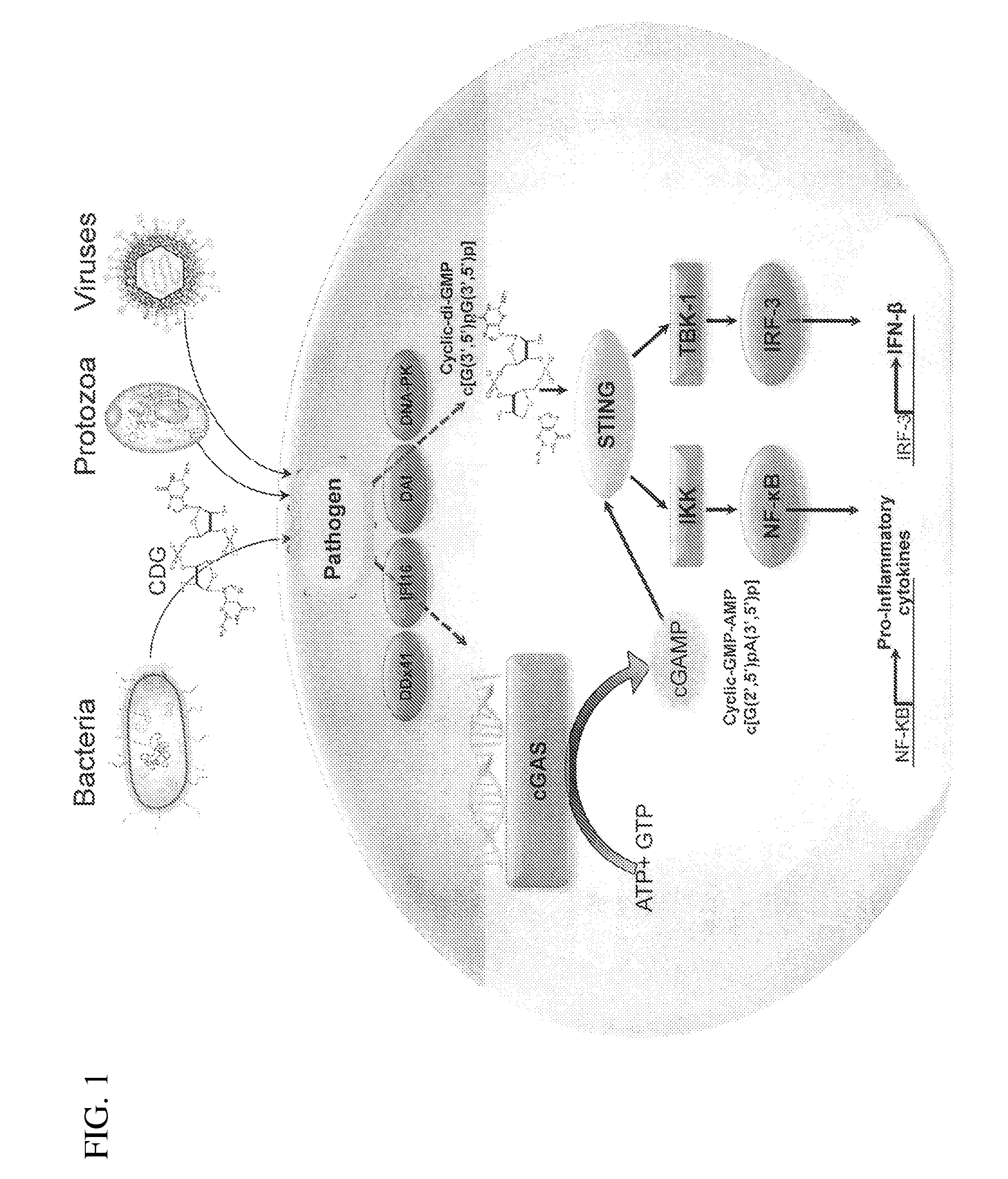
Compositions and methods for activating stimulator of interferon gene-dependent signalling
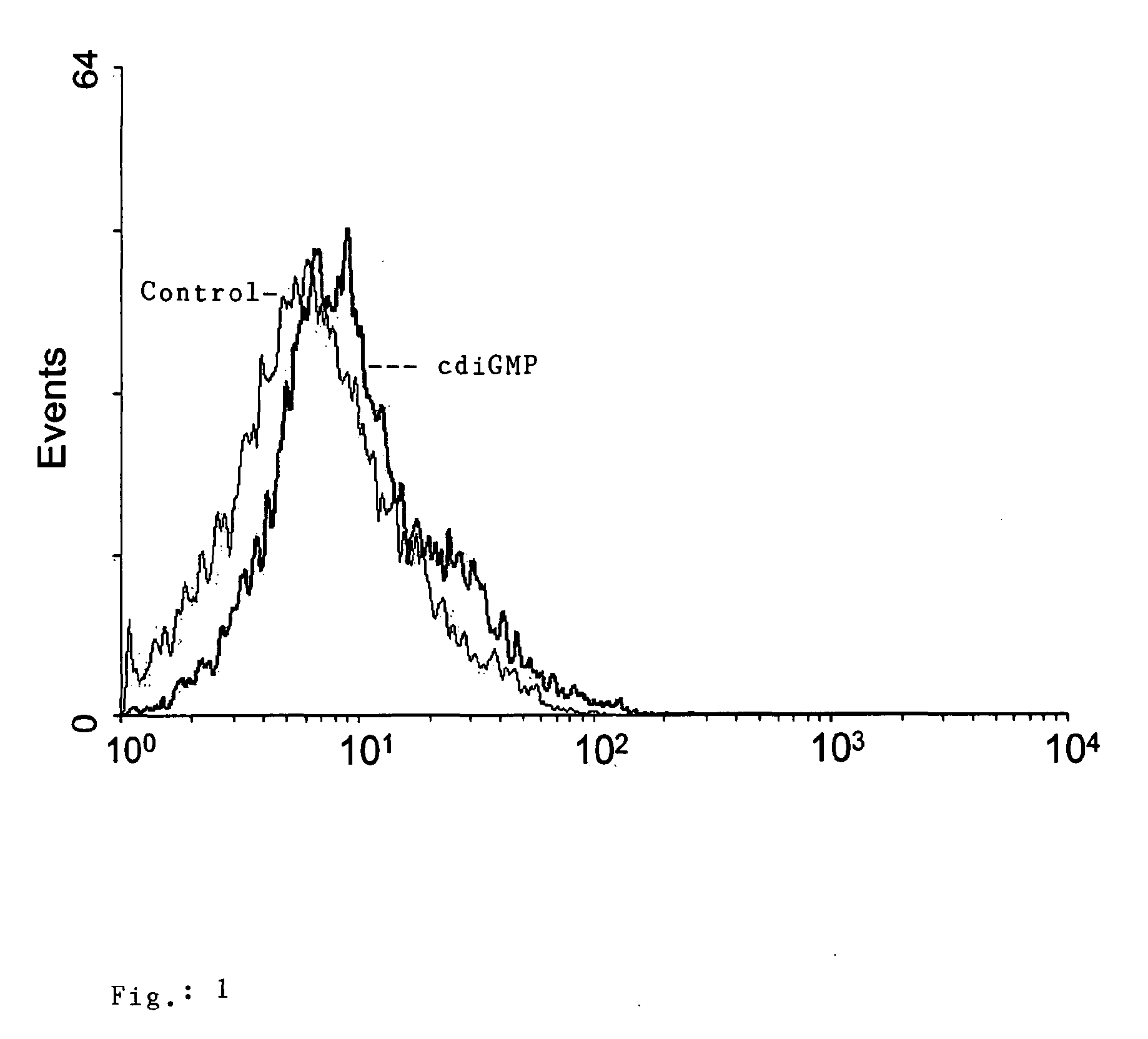
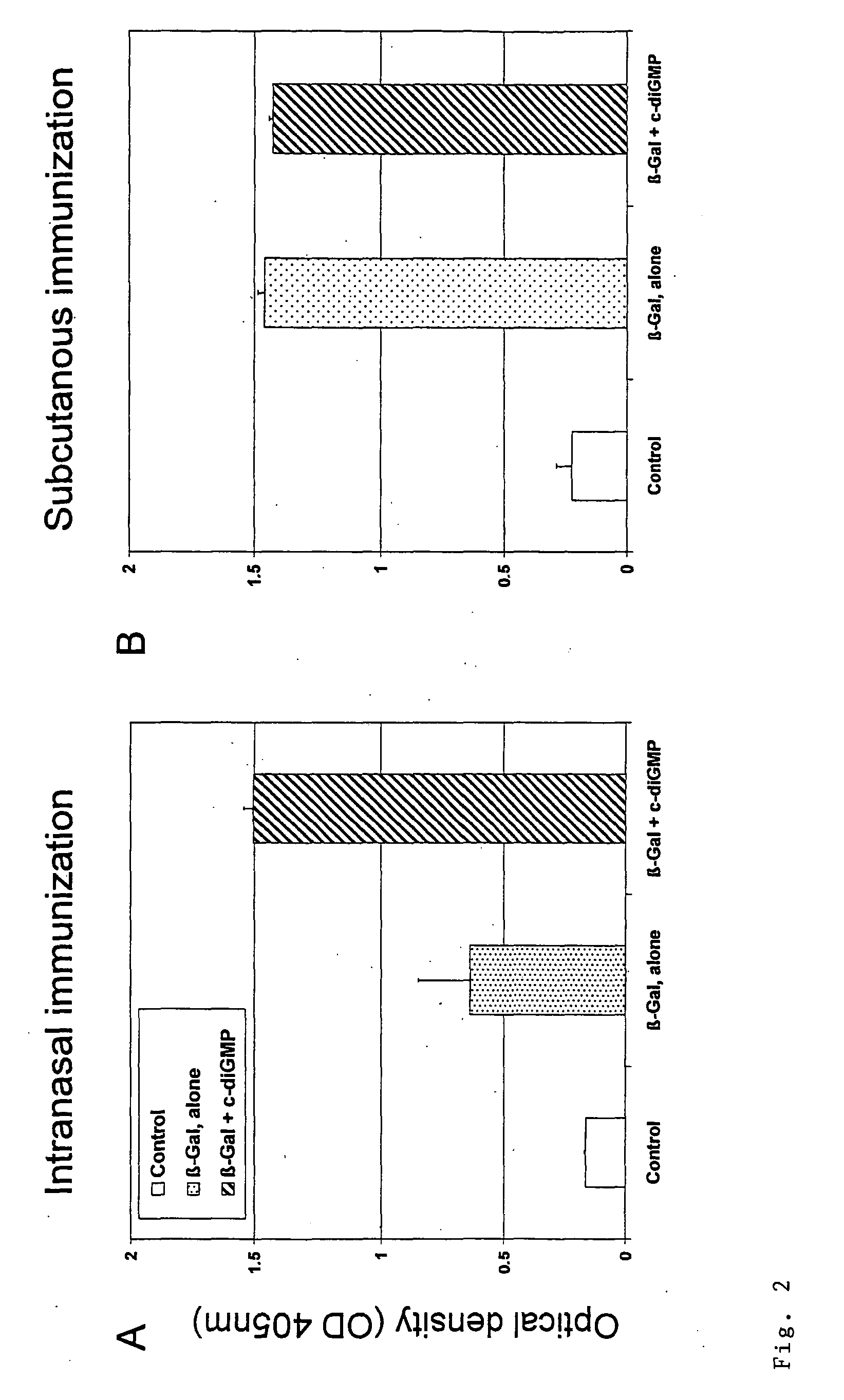
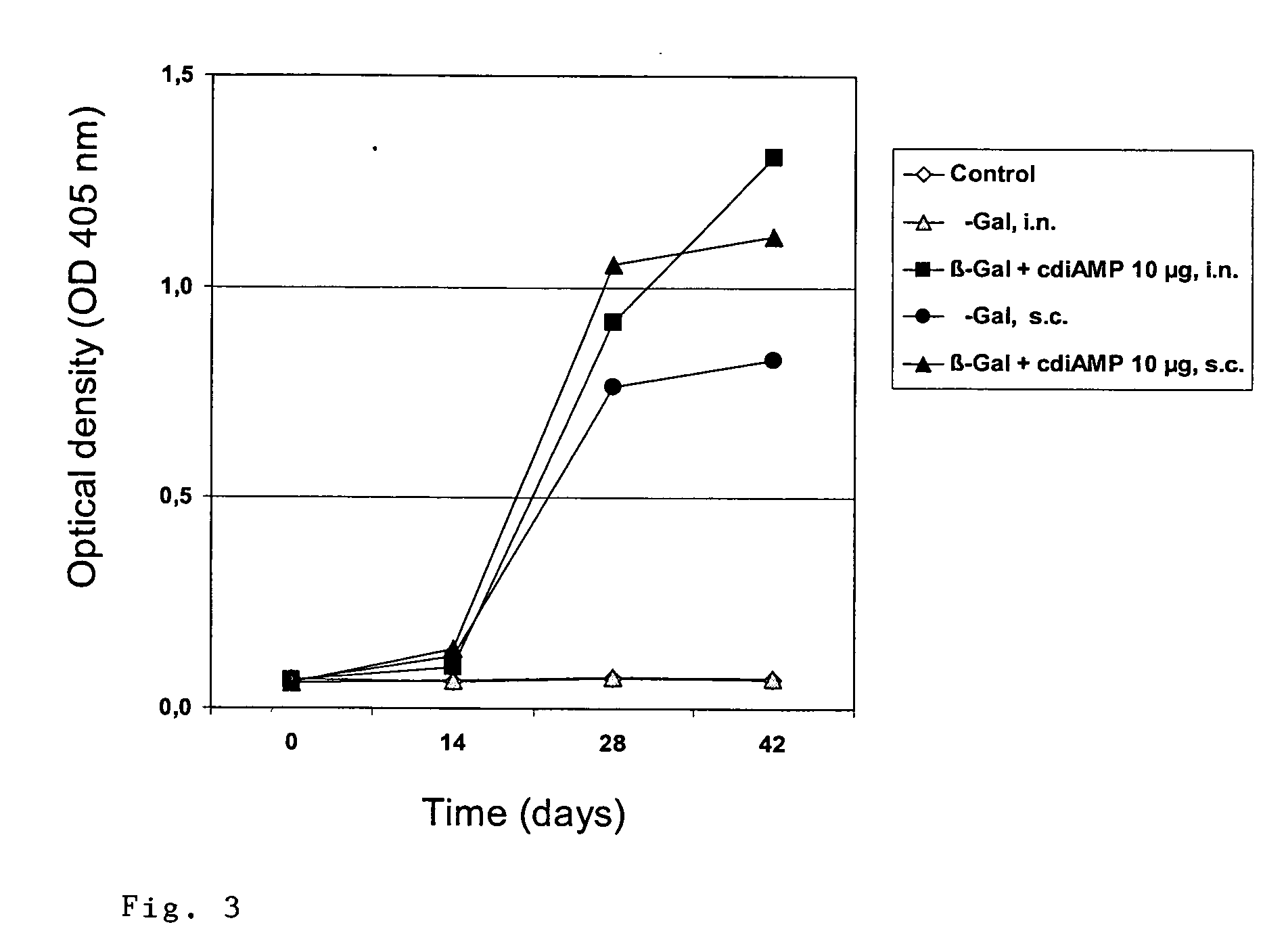
The present invention provides highly active cyclic-di-nucleotide (CDN) immune stimulators that activate DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides induce STING-dependent type I interferon production, wherein the cyclic purine dinucleotides present in the composition are substantially pure 2′,5′,2′,5′ and 2′,5′,3′,5′ CDNs, and preferably Rp,Rp stereosiomers thereof.
Owner:RGT UNIV OF CALIFORNIA +1
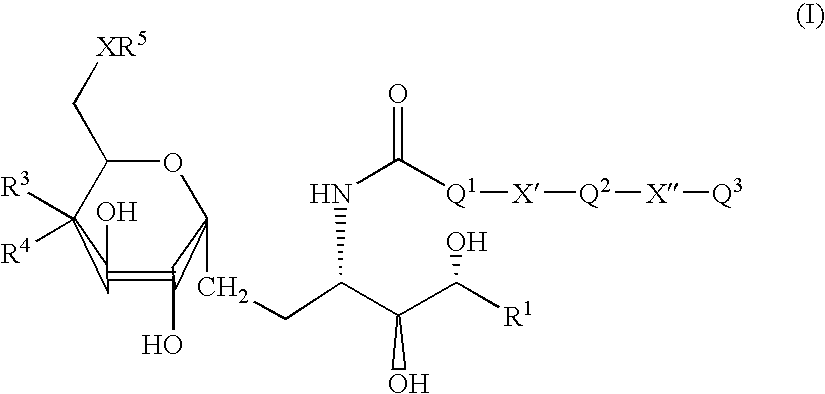
Synthetic glucopyranosyl lipid adjuvants
Compounds, particularly, glucopyranosyl lipid adjuvant (GLA) compounds, having the following structure (I) are provided:or a pharmaceutically acceptable salt thereof, wherein L1, L2, L3, L4, L5, L6, L7, L8, L9, L10, Y1, Y2, Y3, Y4, R1, R2, R3, R4, R5, R6, are as defined herein. Pharmaceutical compositions, vaccine compositions, and related methods for inducing or enhancing immune responses, are also provided.
Owner:ACCESS TO ADVANCED HEALTH INST
HIV envelope polypeptides
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
Methods for using tetanus toxin for beneficial purposes in animals (mammals)
Methods of using tetanus toxin to modulate or control neural functions or nonneural cellular activities at selected sites in animals, particularly in mammals, and more particularly in humans, are provided. Pharmaceutical formulations to modulate neural functions or non-neural cellular activities of an animal at selected sites in animals, particularly in mammals, and more particularly in humans are also provided. Uses of tetanus toxin in preparation of medicaments for methods of treating clinical disorders or symptoms of animals, particularly mammals and more particularly humans are also provided.
Owner:SANDERS
Yeast-dendritic cell vaccines and uses thereof
Owner:UNIV OF COLORADO THE REGENTS OF +1
Compositions and methods for modulating immune responses
ActiveUS20100151000A1Lower Level RequirementsDecreases functional activityPowder deliveryAntipyreticDiseaseAutoimmune responses
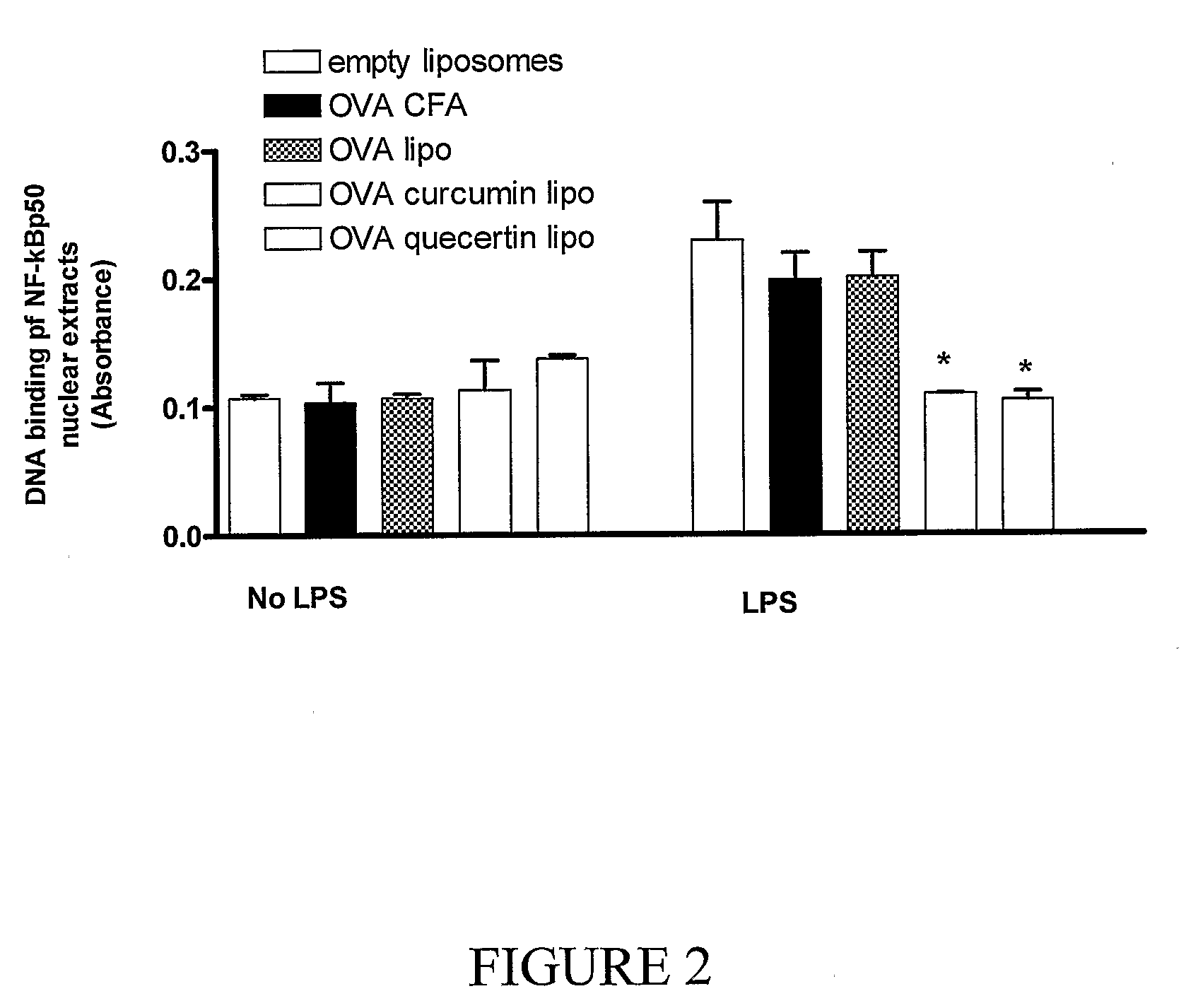
This invention discloses methods and compositions for modulating immune responses, which involve particulate delivery of agents to immune cells, wherein the agents comprise an inhibitor of the NF-κB signaling pathway and an antigen that corresponds to a target antigen. The methods and compositions of the present invention are particularly useful in the treatment or prophylaxis of an undesirable immune response associated with the target antigen, including autoimmune diseases, allergies and transplantation associated diseases.
Owner:THE UNIV OF QUEENSLAND
Targeting of Antigen Presenting Cells with Immunonanotherapeutics
ActiveUS20100129392A1Improve responsePowder deliverySnake antigen ingredientsNanocarriersImmunotherapeutic agent
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides nanocarriers capable of stimulating an immune response in T cells and / or in B cells. The invention provides nanocarriers that comprise an immunofeature surface. The nanocarriers are capable of targeting antigen presenting cells when administered to a subject. The invention provides pharmaceutical compositions comprising inventive nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive nanocarriers and pharmaceutical compositions thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Adjuvant in the form of a lipid-modified nucleic acid
The present invention relates to an immune-stimulating adjuvant in the form of a lipid-modified nucleic acid, optionally in combination with further adjuvants. The invention relates further to a pharmaceutical composition and to a vaccine, each containing an immune-stimulating adjuvant according to the invention, at least one active ingredient and optionally a pharmaceutically acceptable carrier and / or further auxiliary substances and additives and / or further adjuvants. The present invention relates likewise to the use of the pharmaceutical composition according to the invention and of the vaccine according to the invention for the treatment of infectious diseases or cancer diseases. Likewise, the present invention includes the use of the immune-stimulating adjuvant according to the invention in the preparation of a pharmaceutical composition for the treatment of cancer diseases or infectious diseases.
Owner:CUREVAC GMBH
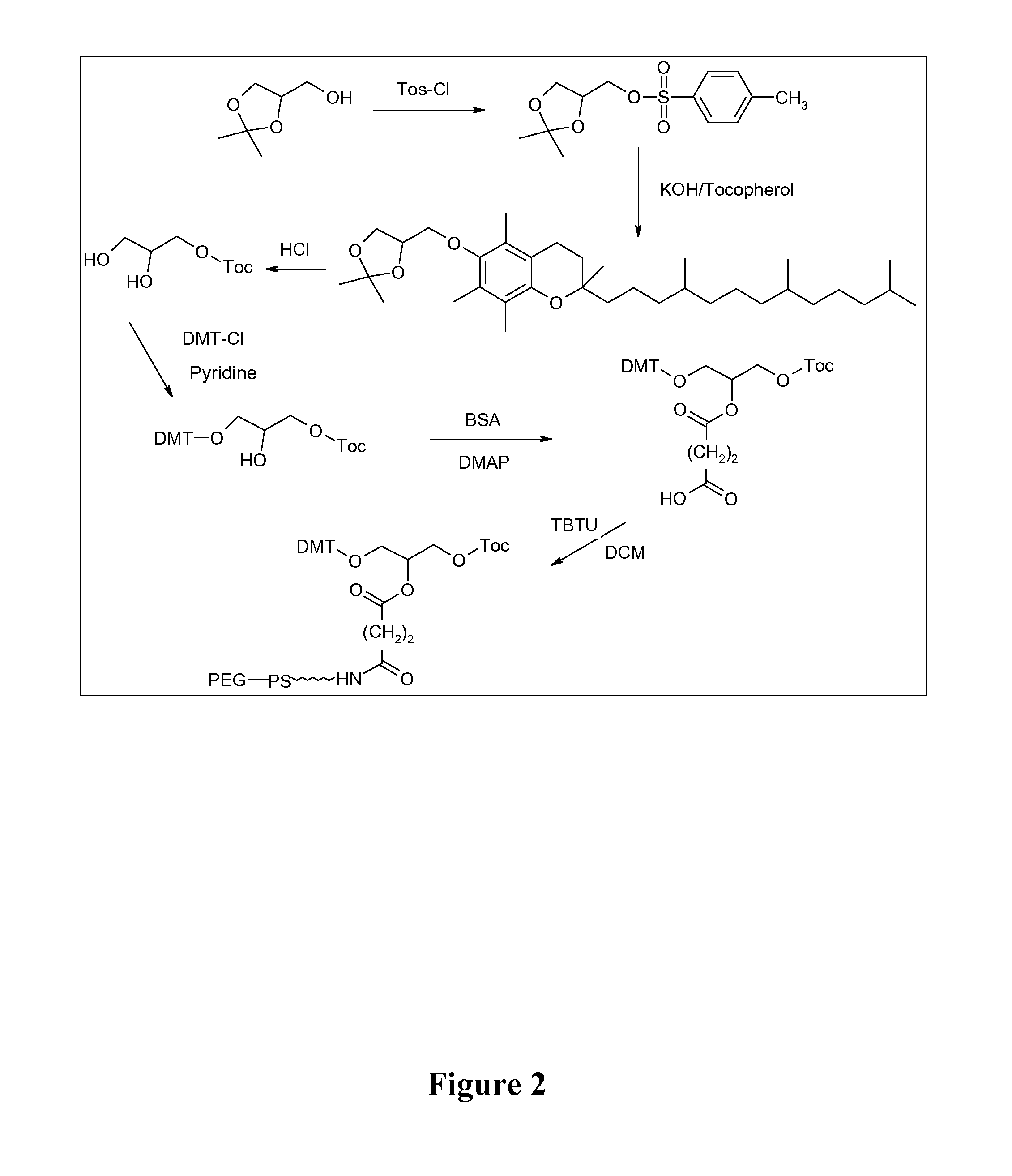
Cyclic-Dinucleotides and Its Conjugates as Adjuvants and Their Uses in Pharmaceutical Compositions
ActiveUS20080286296A1Severe formLess immunomodulatory effectOrganic active ingredientsAntipyreticDiseaseAutoimmune responses
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumors, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
Reagents and methods for inducing an immune response to prostate specific antigen
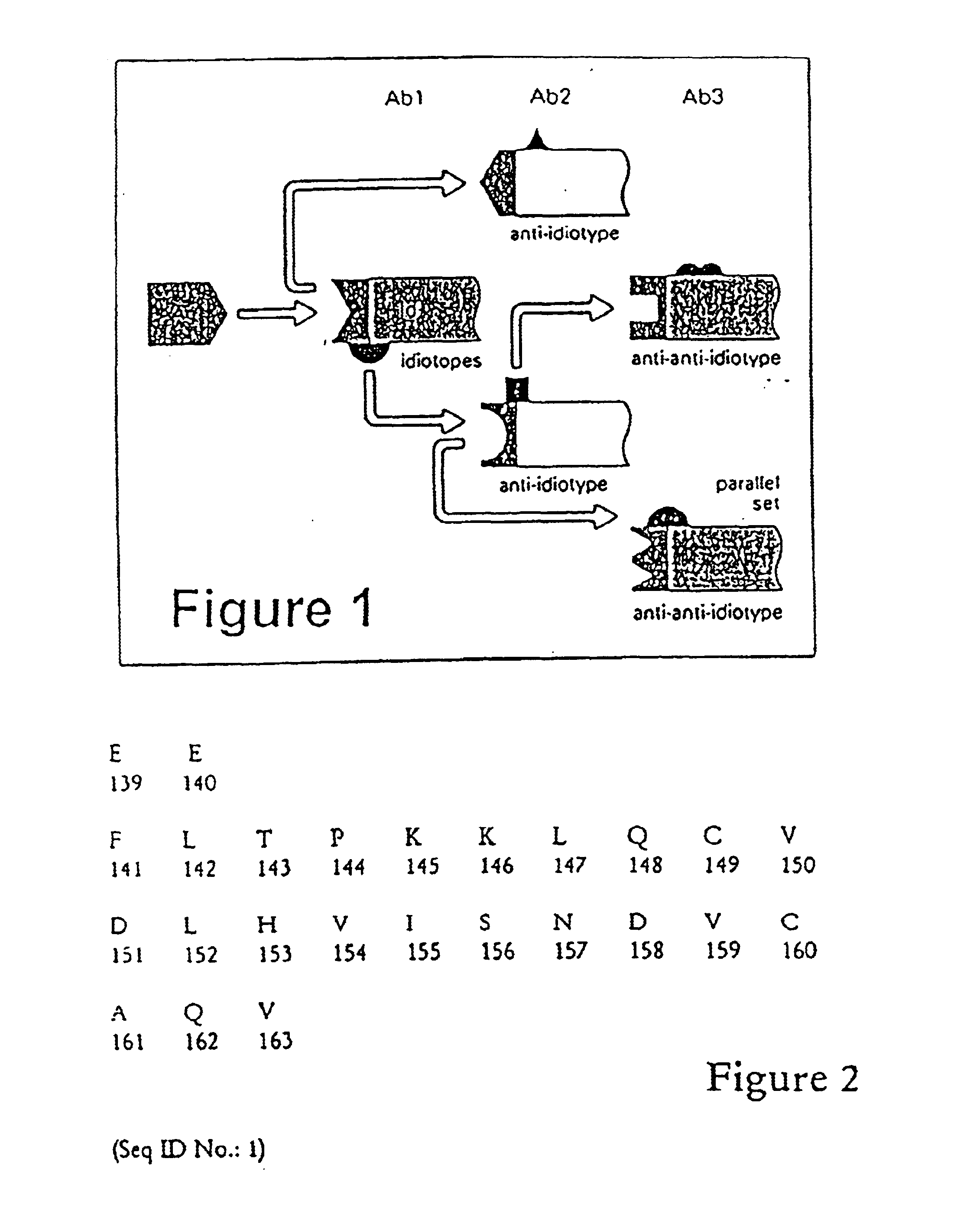
InactiveUS6881405B2Sensitive to proteolytic degradationIncrease productionSnake antigen ingredientsAntibody ingredientsProstate-specific antigenAntibody
Owner:ONCOQUEST INC
Use of synthetic glycolipids as universal adjuvants for vaccines against cancer and infectious diseases
InactiveUS20050192248A1Enhancement and extension of durationBiocideOrganic active ingredientsDiseaseAdjuvant
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes a synthetic glycolipid compound of Formula I, as described herein. According to the present invention, the use of a compound of Formula I as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com