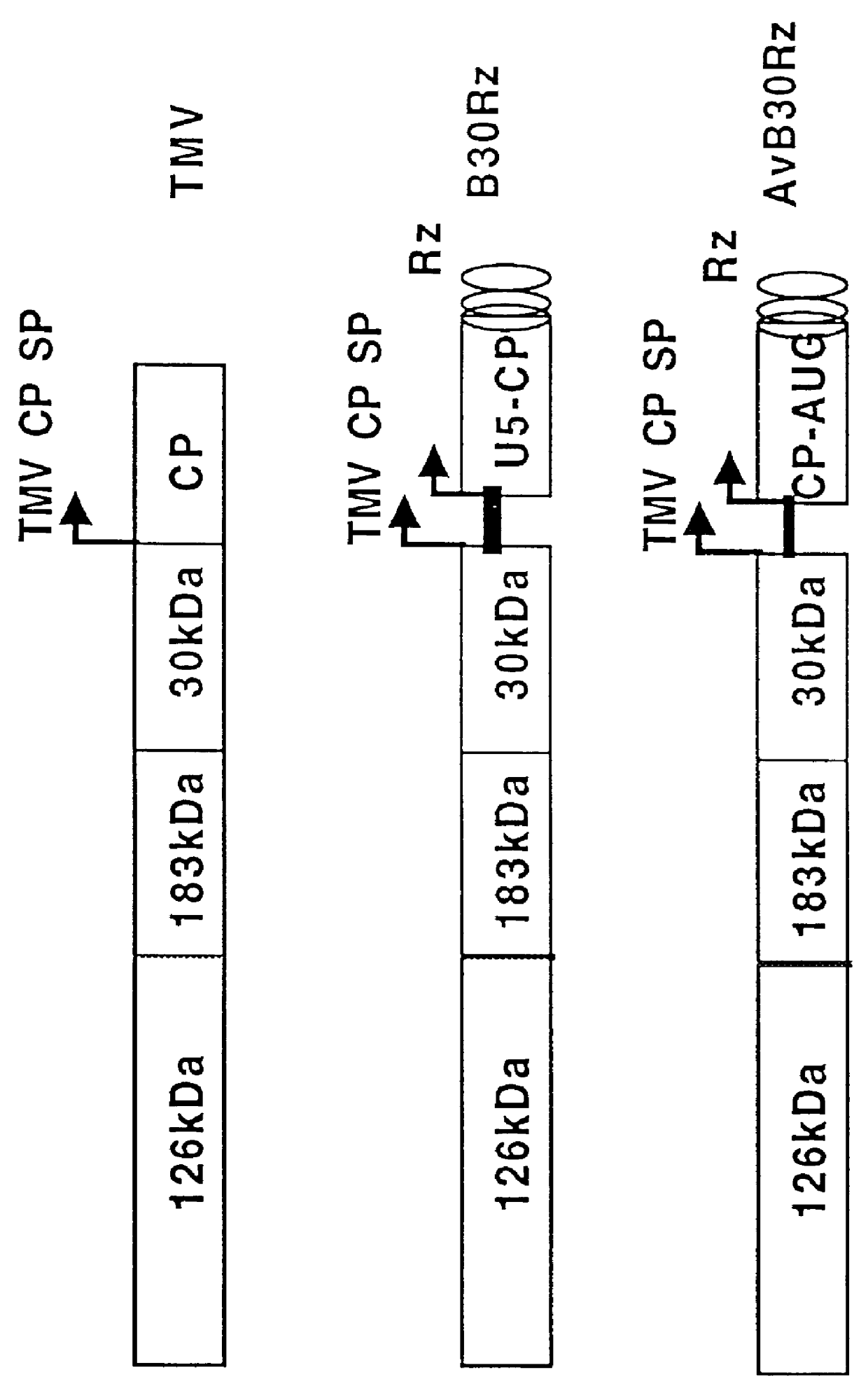
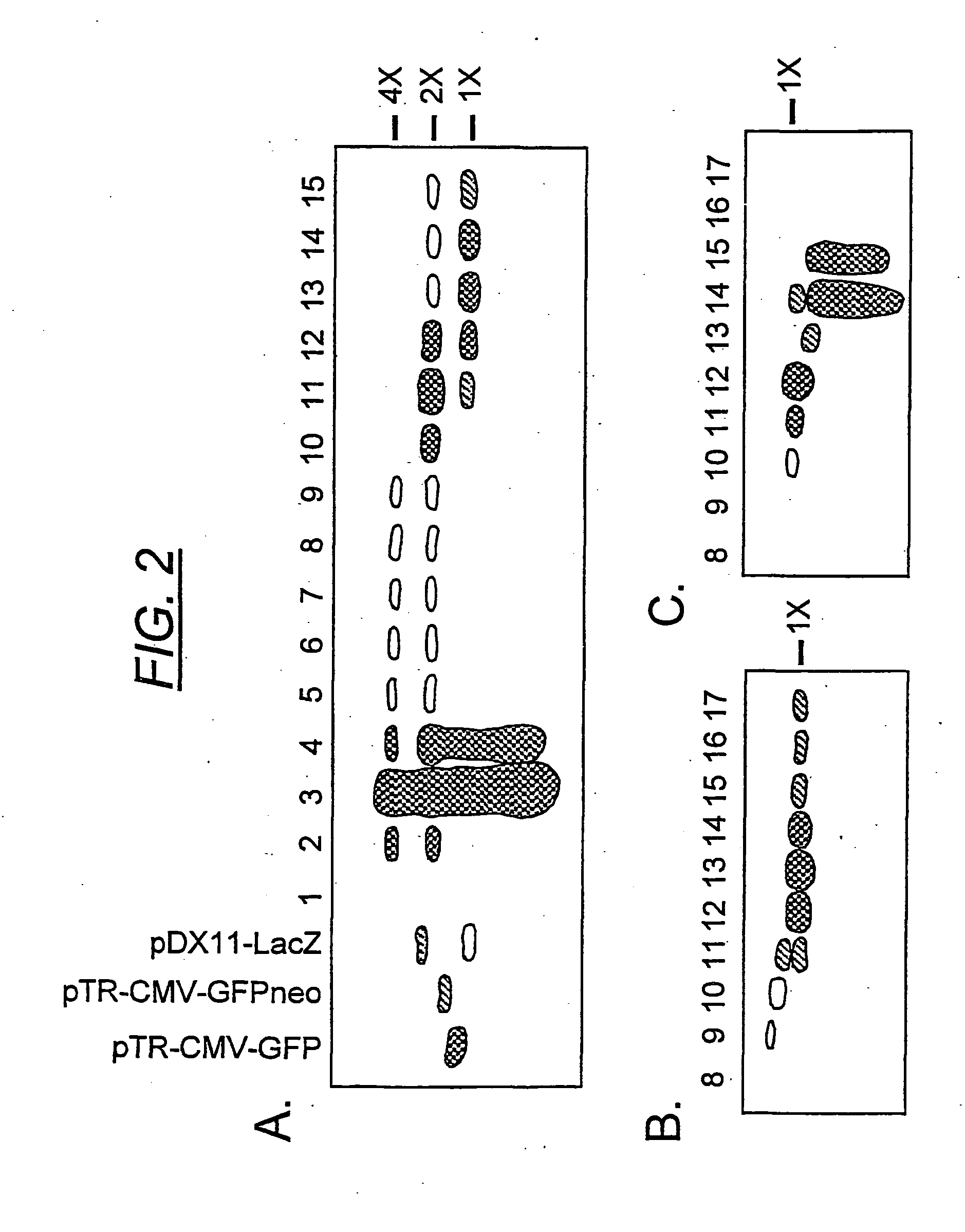
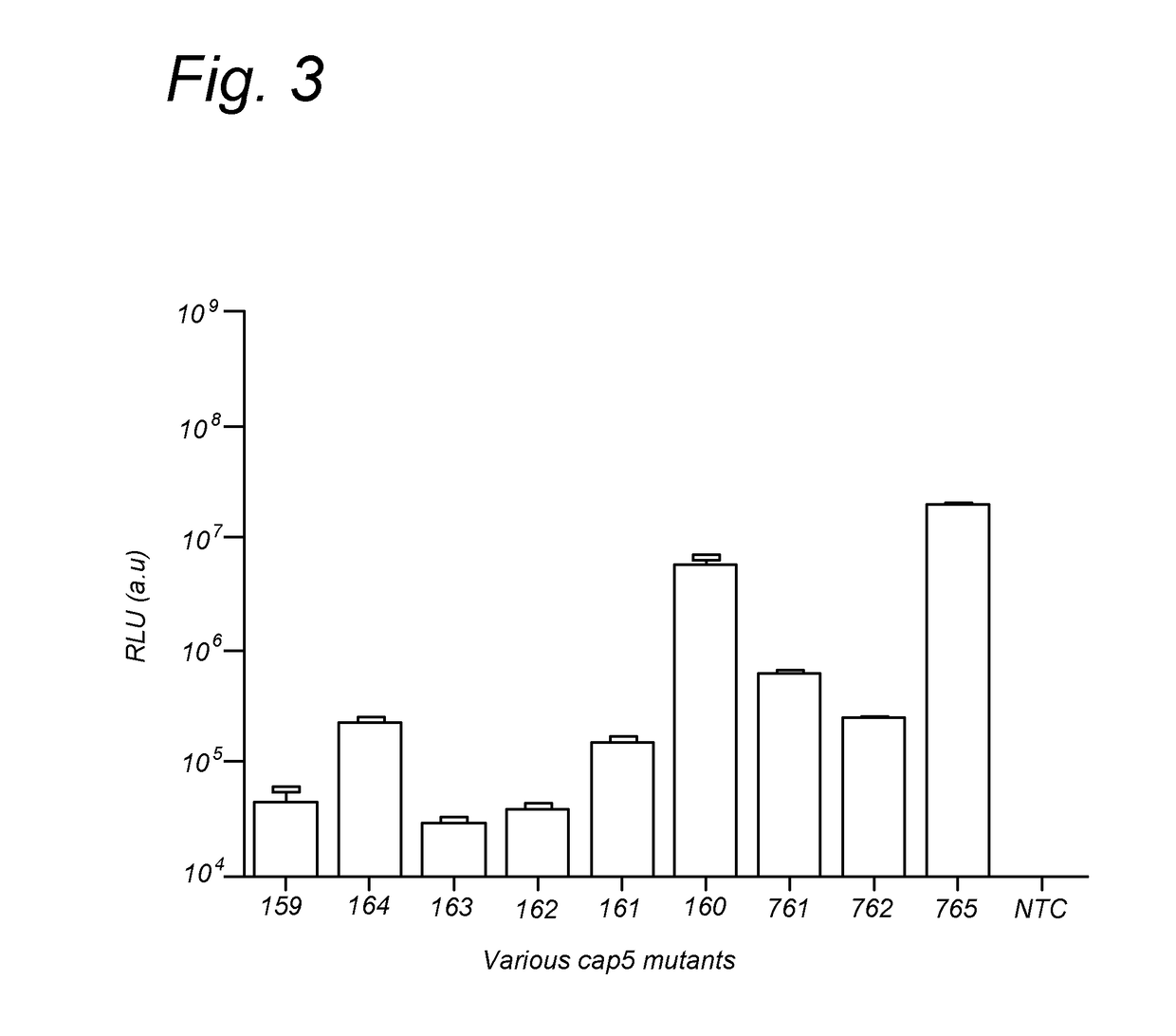
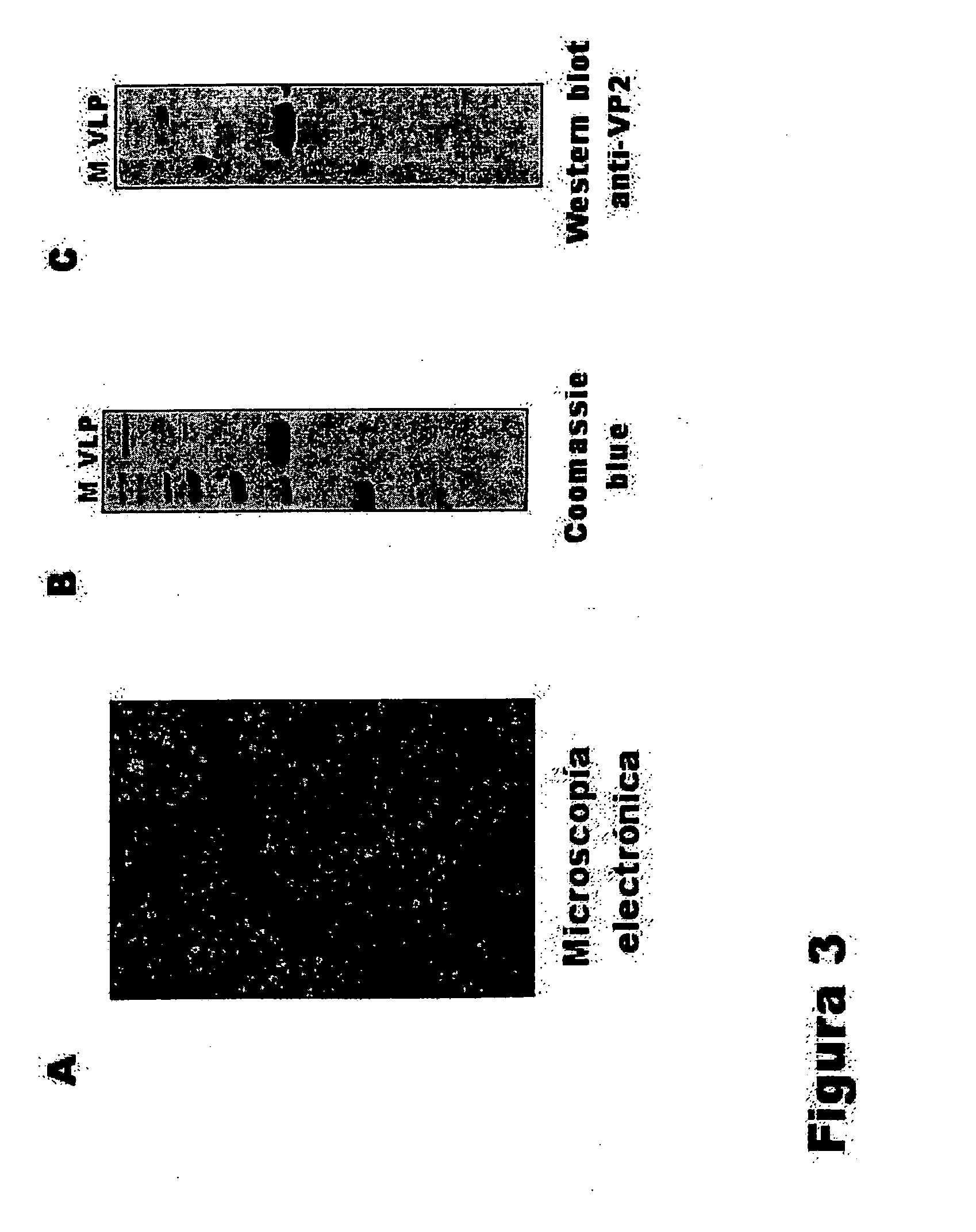
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
124 results about "Viral procapsid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A stable empty viral capsid produced during the assembly of viruses. [ISBN:0072370319, ISBN:1555811272]
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Self-assembling nanoparticle drug delivery system
InactiveUS20090226525A1High binding affinityHigh affinityPowder deliveryMicroencapsulation basedLipid formationMedicine
A self-assembling nanoparticle drug delivery system for the delivery of various bioactive agents including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the bioactive agent, a lipid layer or lipid / cholesterol layer coat and targeting or facilitating molecules anchored in the lipid layer. A method for construction of the self-assembling nanoparticle drug delivery system is also provided.
Owner:CHIMEROS
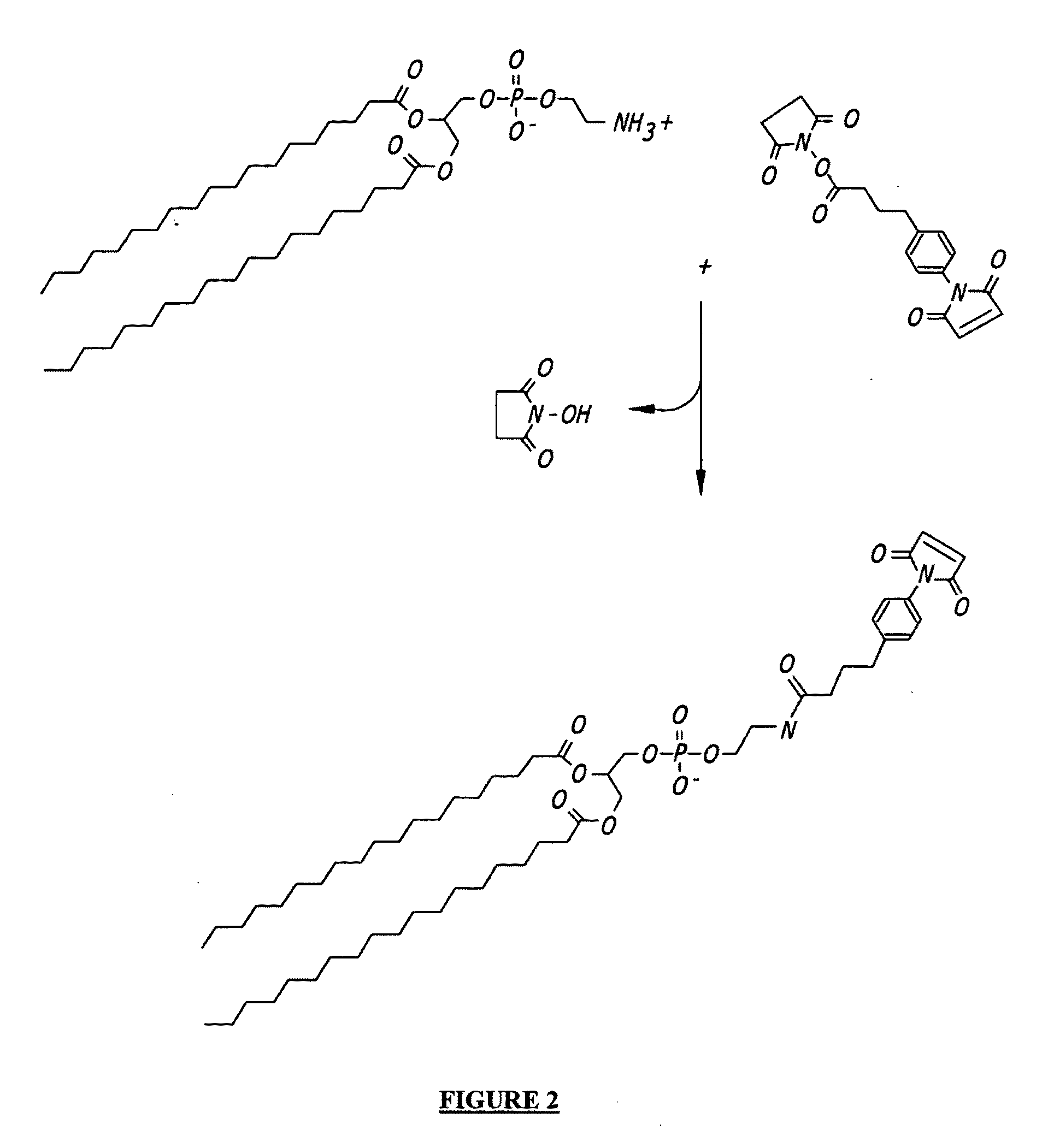
Duplexed parvovirus vectors
InactiveUS7465583B2High transduction efficiencyRapid onsetBiocidePeptide/protein ingredientsGeneticsViral vector
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Polypeptides fused with alfalfa mosaic virus or ilarvirus capsid proteins
InactiveUS6042832AEfficient presentation of antigenImprove protectionSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenIlarvirus
Owner:THOMAS JEFFERSON UNIV
Modified virus vectors and methods of making and using the same
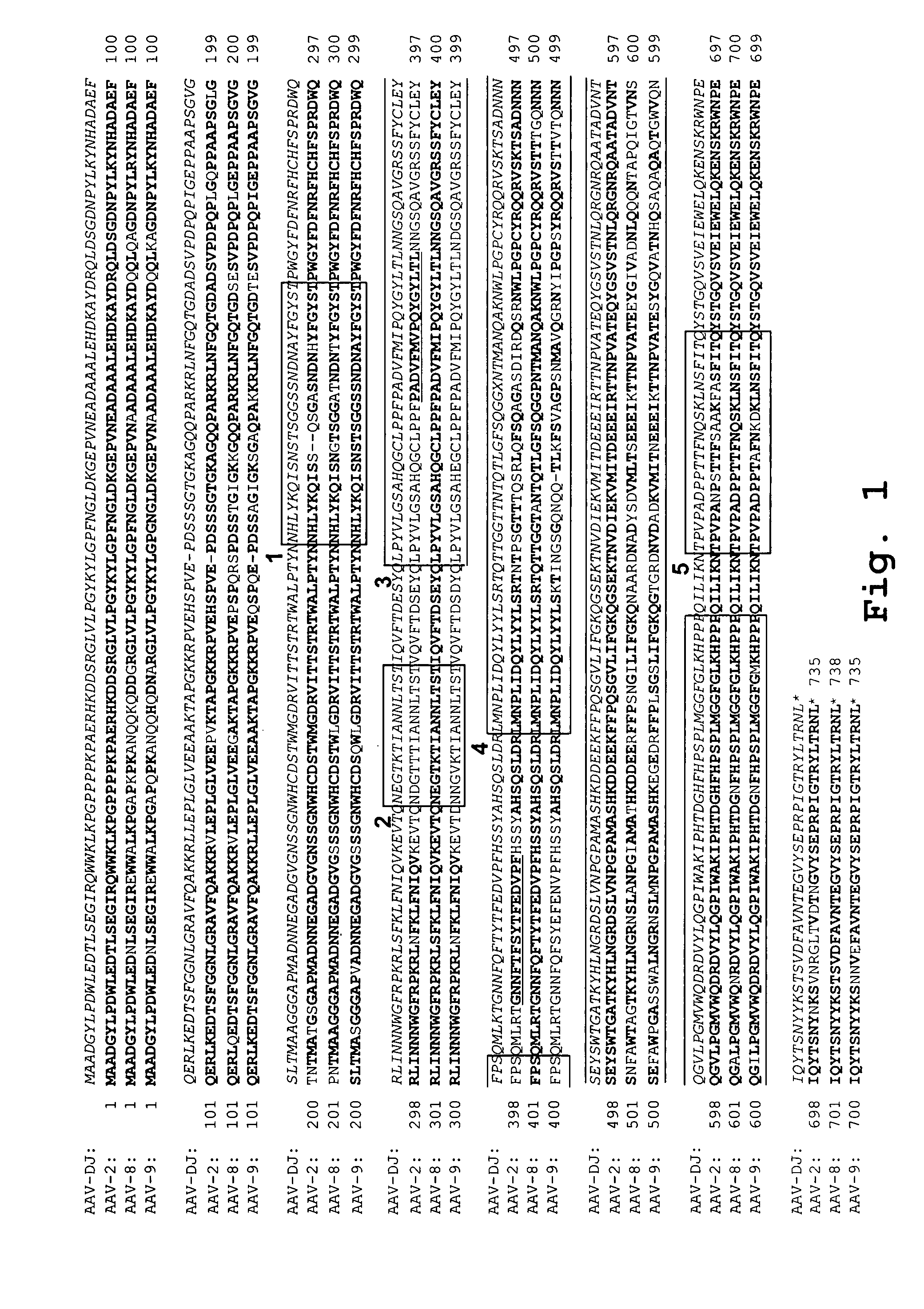
The present invention provides AAV capsid proteins (VP1, VP2 and / or VP3) comprising a modification in the amino acid sequence in the three-fold axis loop 4 and virus capsids and virus vectors comprising the modified AAV capsid protein. In particular embodiments, the modification comprises a substitution of one or more amino acids at amino acid positions 585 to 590 (inclusive) of the native AAV2 capsid protein sequence or the corresponding positions of other AAV capsid proteins. The invention also provides methods of administering the virus vectors and virus capsids of the invention to a cell or to a subject in vivo.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Capsid-modified recombinant adenovirus and methods of use
InactiveUS6955808B2Low yieldEfficiencyBiocideAntibody mimetics/scaffoldsSingle-Chain AntibodiesNoninvasive imaging
The present invention describes recombinant adenoviral vectors modified by incorporating targeting ligands or label into viral capsid or structural proteins. In one embodiment, single-chain antibody was introduced into the minor capsid proteins pIIIa or pIX so that the adenoviral vector can be targeted to a particular cell type. In another embodiment, there is provided a noninvasive imaging strategy useful for monitoring the replication and spread of conditionally replicative adenoviral vectors. Viral structural proteins such as pIX capsid protein, core proteins mu, V and VII were expressed as fusion protein with a fluorescent label. Once incorporated into the virions, detection of the structural fusion protein label would indicate the localization of the disseminated viral progeny. The detected fluorescent signals also closely correlate with the level of viral replication and progeny production.
Owner:UAB RES FOUND
Modified Virus Vectors and Methods of Making and Using the Same
The present invention provides AAV capsid proteins (VP1, VP2 and / or VP3) comprising a modification in the amino acid sequence in the three-fold axis loop 4 and virus capsids and virus vectors comprising the modified AAV capsid protein. In particular embodiments, the modification comprises a substitution of one or more amino acids at amino acid positions 585 to 590 (inclusive) of the native AAV2 capsid protein sequence or the corresponding positions of other AAV capsid proteins. The invention also provides methods of administering the virus vectors and virus capsids of the invention to a cell or to a subject in vivo.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Optimization of gene sequences of virus-like particles for expression in insect cells
InactiveUS20040121465A1Improve the level ofMinimize the numberAnimal cellsViral antigen ingredientsPolynucleotideTGE VACCINE
Codon optimized polynucleotides for optimal expression of recombinant proteins in eukaryotic cells are provided. The codon optimized polynucleotides encode a viral capsid protein that self assembles into a virus-like particle. The virus-like particle is expressed extracellularly and exhibits conformational antigenic epitopes capable of raising neutralizing antibodies. Pharmaceutical compositions, vaccines, and diagnostic test kits containing the gene products of the codon-optimized polynucleotides are also provided.
Owner:NOVAVAX
Vectors and methods for immunization against Norwalk virus using transgenic plants
InactiveUS20050155113A1SsRNA viruses positive-senseBacteriaNucleic acid moleculeNorwalk Virus Infections
The present invention relates to a synthetic plant-optimized nucleic acid molecule having a Norwalk virus capsid protein coding nucleotide sequence, and nucleic acid constructs, host cells, expression systems, and plants having the plant-optimized Norwalk virus nucleic acid molecule. The present invention also relates to a method of producing Norwalk virus capsid protein virus-like particles in a transgenic plant or transgenic plant seed transformed with a plant-optimized nucleic acid molecule encoding Norwalk virus capsid protein. The plant or a component thereof can be administered to a subject under conditions effective to immunize the subject against disease resulting from infection by a Norovirus, including Norwalk virus. An oral vaccine for immunization of a subject against Norwalk virus infection is also disclosed.
Owner:BOYCE THOMPSON INST FOR PLANT RES
Adeno-associated virus variant capsids and methods of use thereof
Provided herein are variant adeno-associated virus (AAV) capsid proteins having one or more modifications in amino acid sequence relative to a parental AAV capsid protein, which, when present in an AAV virion, confer increased infectivity of one or more types of retinal cells as compared to the infectivity of the retinal cells by an AA V virion comprising the unmodified parental capsid protein. Also provided are recombinant AAV virions and pharmaceutical compositions thereof comprising a variant AAV capsid protein as described herein, methods of making these rAAV capsid proteins and virions, and methods for using these rAAV capsid proteins and virions in research and in clinical practice, for example in, e.g., the delivery of nucleic acid sequences to one or more cells of the retina for the treatment of retinal disorders and diseases.
Owner:4D MOLECULAR THERAPEUTICS INC
Foot-and-mouth disease virus capsid protein tandem coexpressions and virus-like particle preparation method
ActiveCN104404074AHigh activityNatural binding activityBacteriaInactivation/attenuationEscherichia coliVirus-like particle
The invention relates to escherichia coli-derived single-plasmid-tandem soluble coexpression foot-and-mouth disease virus capsid proteins VP0 (which is a VP4 and VP2 fusion gene), VP1 and VP3, and a foot-and-mouth disease virus capsid protein virus-like particle preparation method. Foot-and-mouth disease virus capsid protein virus-like particles can be used for preparation of a foot-and-mouth disease vaccine. According to the method, a plurality of aspects of escherichia coli-derived soluble coexpression foot-and-mouth disease virus capsid protein are studied, by comprehensive use of tandem coexpression and SUMO(suggested upper merged ontology) technology with a tag for soluble coexpression of the foot-and-mouth disease virus capsid proteins VP0 (which is the VP4 and VP2 fusion gene), VP1 and VP3, the ultimate objective protein accounts for about 20% of total bacterial protein, and the foot-and-mouth disease virus capsid proteins obtained by purification can be successfully assembled into the virus like particles.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Novel Recombinant Adeno-Associated Virus Capsids with Enhanced Human Skeletal Muscle Tropism
ActiveUS20170159026A1Enhanced neutralization profileEnhanced transductionHydrolasesGenetic material ingredientsMuscle tissueSkeletal muscle
The present invention relates to variant AAV capsid polypeptides, wherein the variant AAV capsid polypeptides exhibit increased transduction and / or tropism in human muscle tissue or cells as compared non-variant parent capsid polypeptides.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Targeted heterologous antigen presentation on calicivirus virus-like particles
Owner:TAKEDA VACCINES INC
Duplexed parvovirus vectors
ActiveUS20070110724A1Increase efficiencyHigh levelBiocidePeptide/protein ingredientsParvovirusDouble strand
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Recombinant adeno-associated virus capsids with enhanced human skeletal muscle tropism
The present invention relates to variant AAV capsid polypeptides, wherein the variant AAV capsid polypeptides exhibit increased transduction and / or tropism in human muscle tissue or cells as compared non-variant parent capsid polypeptides.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Altered virus capsid protein and use thereof
InactiveUS20090298955A1Controlling specificity and efficiencyGood structural stabilityBiocidePeptide/protein ingredientsPeptidePapovavirus
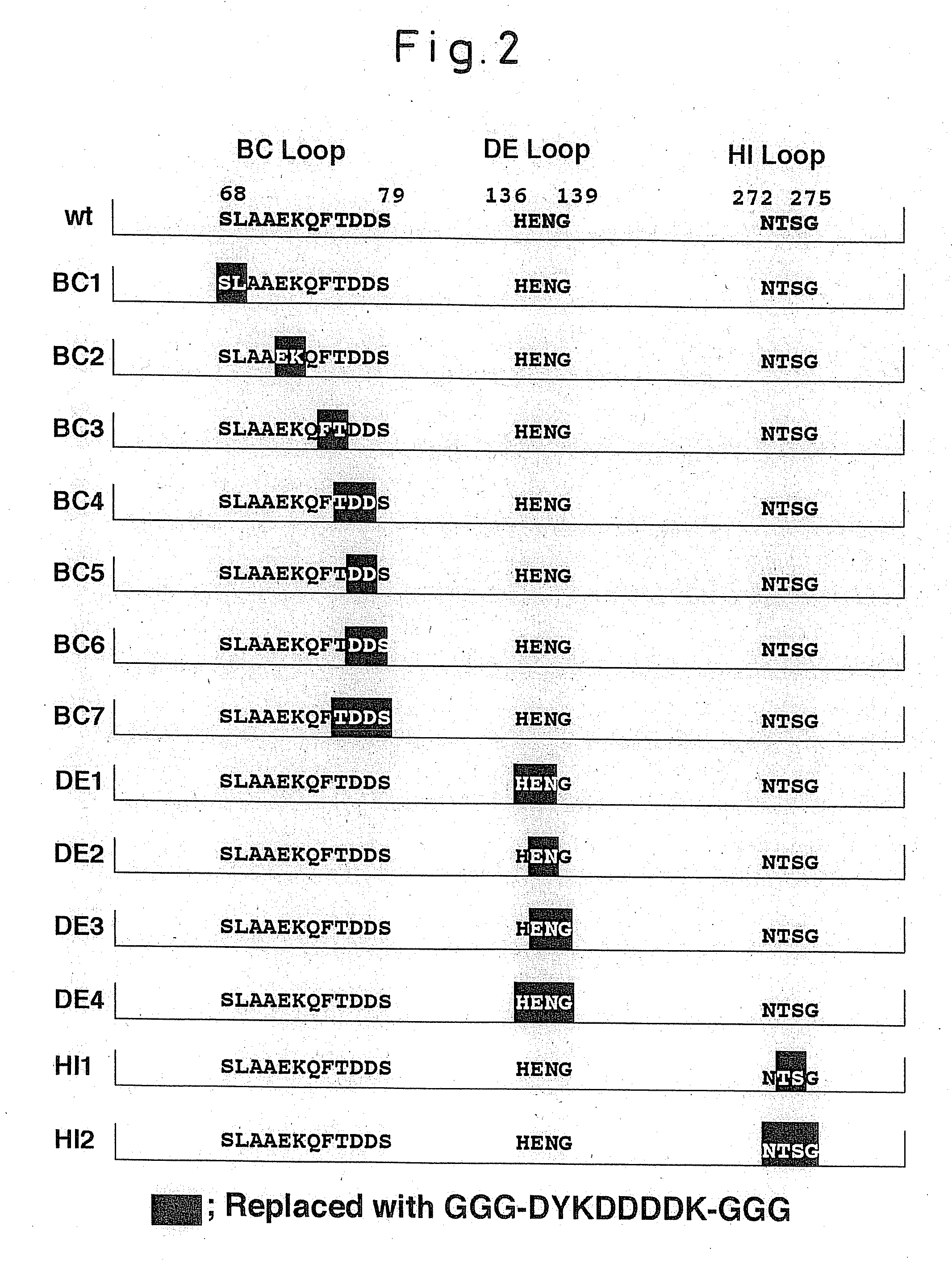
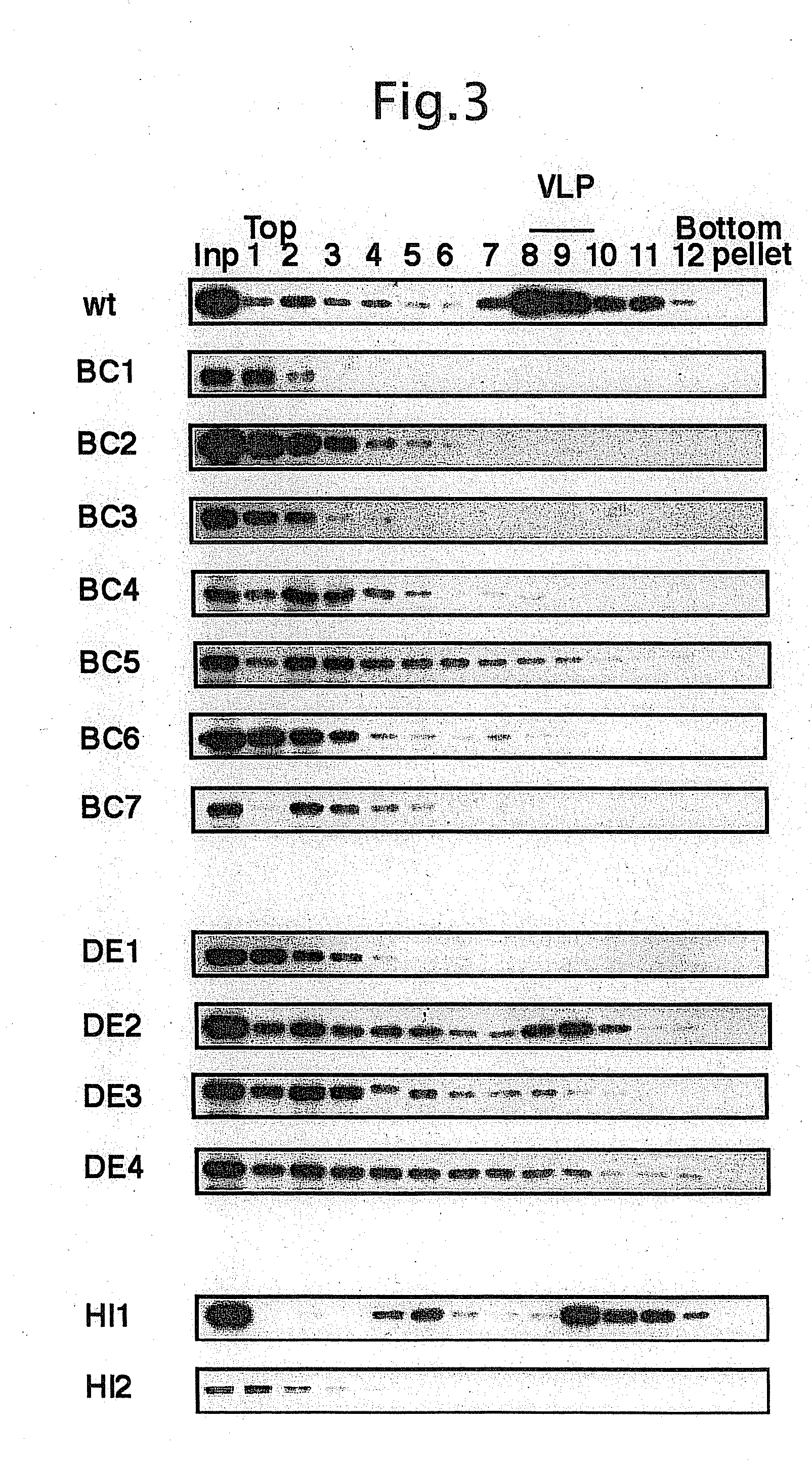
An altered capsid protein of a primate-infective papovavirus in which a foreign peptide sandwiched by 1 to several glycine residues at each end is inserted into at least one of the DE-loop or the HI-loop of the capsid protein of the primate-infective papovavirus, and a virus-like particle formed from the altered capsid protein.
Owner:KONICA MINOLTA INC +1
HIV capsid assembly-associated compositions and method
InactiveUS7348134B2Inhibiting HIV capsid formationInhibition formationSsRNA viruses positive-senseMicrobiological testing/measurementCell freeScreening method
A cell-free method for translation and assembly of retroviral, particularly HIV, capsid and capsid intermediates is disclosed. Also disclosed are novel HIV capsid assembly intermediates and novel host proteins which bind to such assembly intermediates. The invention also includes a screening method for compounds that alter retrovirus capsid assembly, and a method of treating HIV using compounds which inhibit the HIV capsid assembly pathway.
Owner:RGT UNIV OF CALIFORNIA
Synthetic liver-tropic adeno-associated virus capsids and uses thereof
The invention relates to synthetic adeno-associated virus capsids targeted to the liver and virus vectors comprising the same. The invention further relates to methods of using the vectors to target the liver and provide liver-specific expression, as well as transduce human primary hepatocytes and cell lines.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Further Improved AAV Vectors Produced in Insect Cells
ActiveUS20170356008A1Improve stabilityReduced expression levelVectorsVirus peptidesWild typeViral vector
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon and wherein the coding sequence for one or more amino acid residues have been inserted between the suboptimal translation initiation codon and the codon encoding the amino acid residue that corresponds to the amino acid residue at position 2 of the wild type capsid amino acid sequence of which the first amino acid residue is alanine, glycine valine, aspartic acid or glutamic acid. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins.
Owner:UNIQURE IP BV
Recombinant protein containing SARS virus RBD antigen and baculovirus displaying RBD protein
InactiveCN104292339AAchieve surface displayHigh clinical application valueGenetic material ingredientsAntiviralsShuttle vectorTransmembrane domain
The invention discloses a recombinant protein containing an SARS virus RBD antigen and a baculovirus displaying an RBD protein. The recombinant protein SP-RBD-TM is formed by connecting an N- end of the RBD protein of an SARS virus with a signal peptide SP of a baculovirus envelope protein GP64, and connecting a C- end with a transmembrane domain TM of the baculovirus envelope protein GP64. The recombinant baculovirus having the surface displaying the SARS antigen RBD protein is the recombinant baculovirus obtained by the steps of inserting a cording gene of the SP-RBD-TM into a donor plasmid, carrying out homologous recombination with a genome of a shuttle vector Bacmid through transposition to obtain a recombinant baculovirus genome, then transfecting a bombyx mori cell with the recombinant baculovirus genome, and packaging in the cell to obtain the recombinant baculovirus. The recombinant baculovirus allows the RBD protein of the SARS virus to be fused with the bombyx mori baculovirus envelope protein GP64, realizes display of the RBD protein on the surface of a viral capsid, and has good immunogenicity.
Owner:特菲(天津)生物医药科技有限公司
Aav vectors produced in insect cells
ActiveUS20120028357A1Improve stabilityReduced expression levelAnimal cellsGenetic material ingredientsNucleotideViral vector
The present invention relates to the production of adeno-associated viral vectors in insect cells. The insect cells therefore comprise a first nucleotide sequence encoding the adeno-associated virus (AAV) capsid proteins, whereby the initiation codon for translation of the AAV VP1 capsid protein is a non-ATG, suboptimal initiation codon. The insect cell further comprises a second nucleotide sequence comprising at least one AAV inverted terminal repeat (ITR) nucleotide sequence; a third nucleotide sequence comprising a Rep52 or a Rep40 coding sequence operably linked to expression control sequences for expression in an insect cell; and, a fourth nucleotide sequence comprising a Rep78 or a Rep68 coding sequence operably linked to expression control sequences for expression in an insect cell. The invention further relates to adeno-associated viral vectors with an altered ratio of the viral capsid proteins that provides improved infectivity of the viral particles.
Owner:UNIQURE IP BV
Adeno-associated virus variant capsids and use for inhibiting angiogenesis
Provided herein are variant adeno-associated virus (AAV) capsid proteins having one or more modifications in amino acid sequence relative to a parental AAV capsid protein, which, when present in an AAV virion, confer increased infectivity of one or more types of retinal cells as compared to the infectivity of the retinal cells by an AAV virion comprising the unmodified parental AAV capsid protein.Also provided are recombinant AAV virions and pharmaceutical compositions thereof comprising a variant AAV capsid protein as described herein, methods of making these rAAV capsid proteins and virions, and methods for using these rAAV capsid proteins and virions in research and in clinical practice, for example in, e.g., the delivery of nucleic acid sequences to one or more cells of the retina forthe treatment of retinal disorders and diseases.
Owner:4D MOLECULAR THERAPEUTICS INC
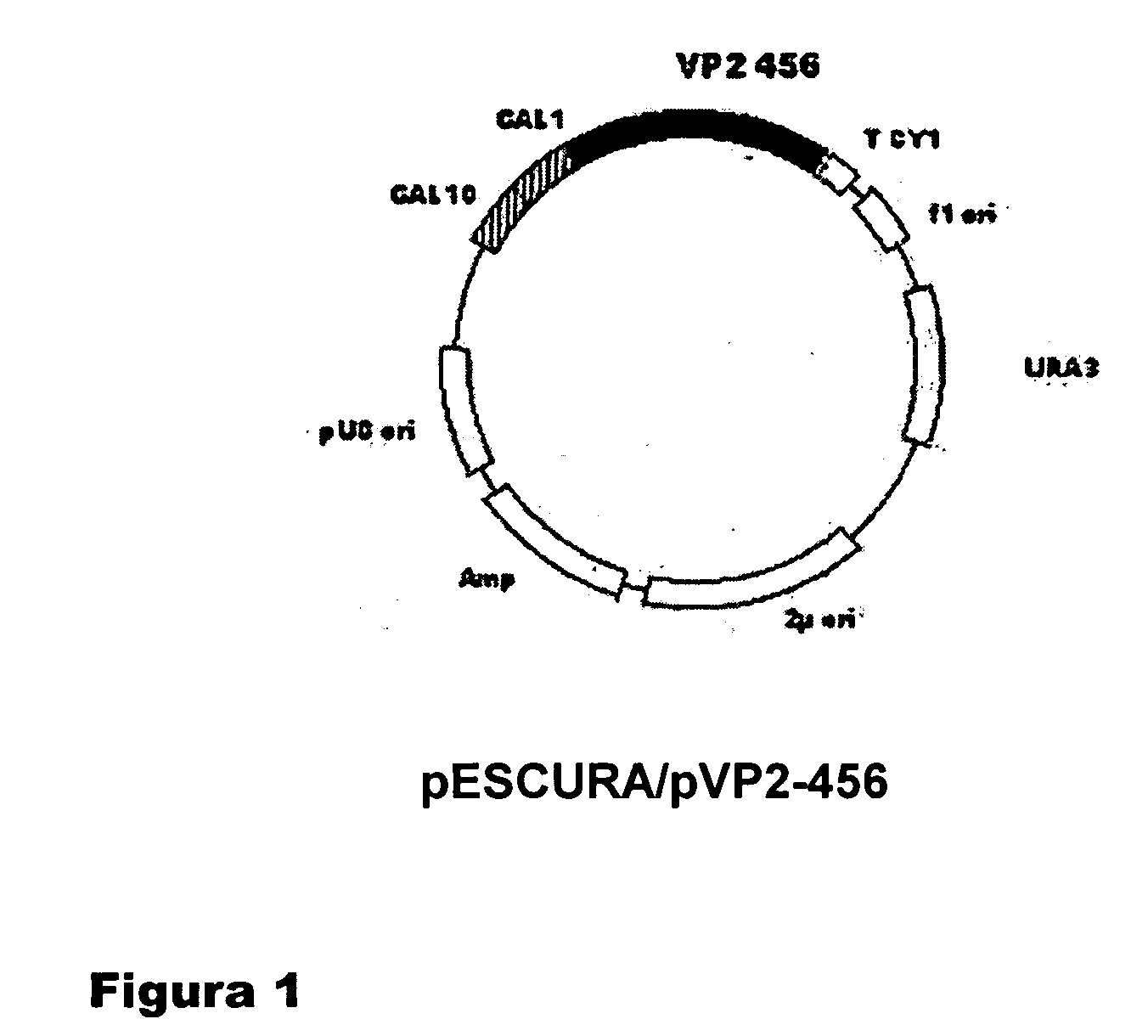
Process For Producing In Yeast Empty Viral Capsids Consisting Of Proteins Derived From Pvp2 Of The Infectious Bursal Disease Virus (Ibdv)
InactiveUS20070212375A1High yieldReduce economic costsFungiViral antigen ingredientsYeastProtein composition
The empty capsids of the infectious bursal disease virus (IBDV) are formed by the assembly of proteins derived from the pVP2 protein of IBDV, with a different size and with an application in producing vaccines and in preparing gene therapy vectors.
Owner:CHIMERA PHARMA S L U +1
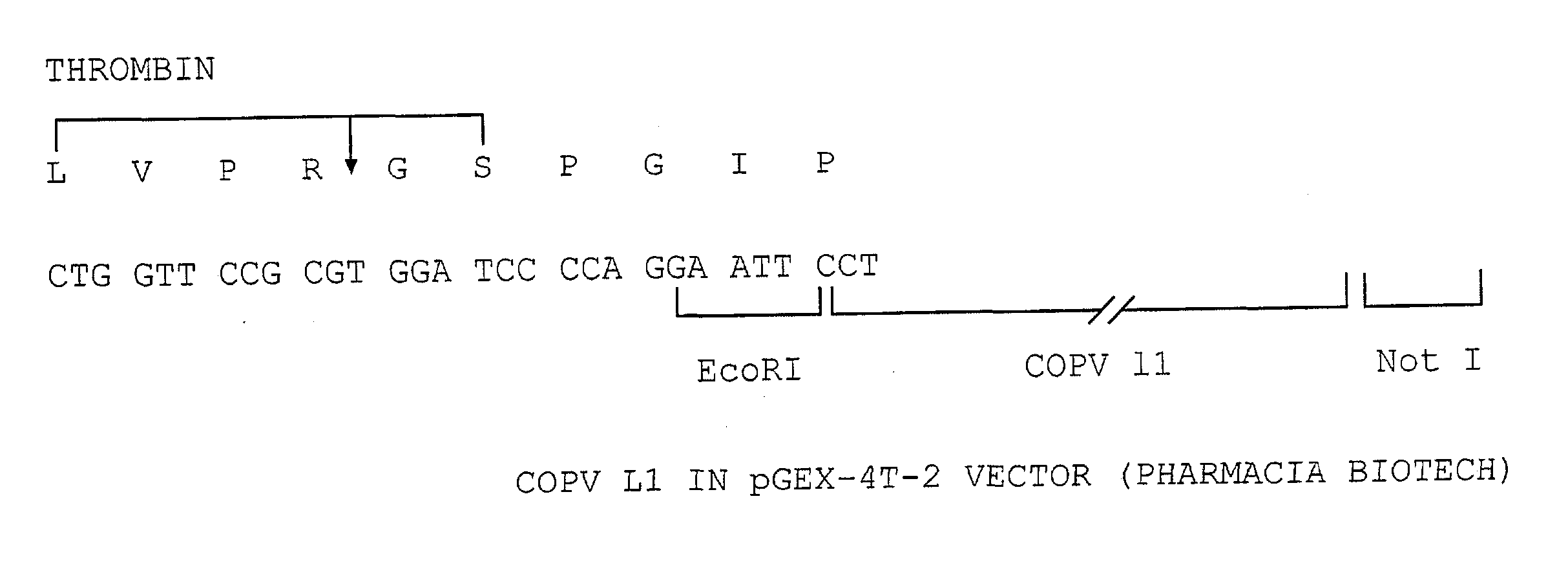
Stable (FIXED) forms of viral capsid proteins, and viral capsid protein fusions, preferably papillomavirus l1 proteins, and uses thereof
InactiveUS20070212374A1Easy to produceSuitable for useAntibody mimetics/scaffoldsViral antigen ingredientsAlcoholVirology
Owner:UNIV OF COLORADO THE REGENTS OF +1
Gene delivery vectors provided with a tissue tropism for smooth muscle cells, and/or endothelial cells
A gene delivery vehicle having been provided with at least a tissue tropism for cells selected from the group of smooth muscle cells, endothelial cells, and / or liver cells. The tissue tropism is generally provided by a virus capsid, such as one comprising protein fragments from at least two different viruses, such as two different adenoviruses, including adenovirus of subgroup C or subgroup B (for example, adenovirus 16). The protein fragments can comprises a tissue tropism determining fragment of a fiber protein derived from a subgroup B adenovirus. Also, cells for producing such gene delivery vehicles and pharmaceutical compositions containing said gene delivery vehicles. Further, a method of delivering nucleic acid to cells such as smooth muscle cells and / or endothelial cells which involves administering to the cells an adenovirus capsid having proteins from at least two different adenoviruses and wherein at least a tissue tropism determining fragment of a fiber protein is derived from a subgroup B adenovirus. Particular construct are also disclosed.
Owner:JANSSEN VACCINES & PREVENTION BV
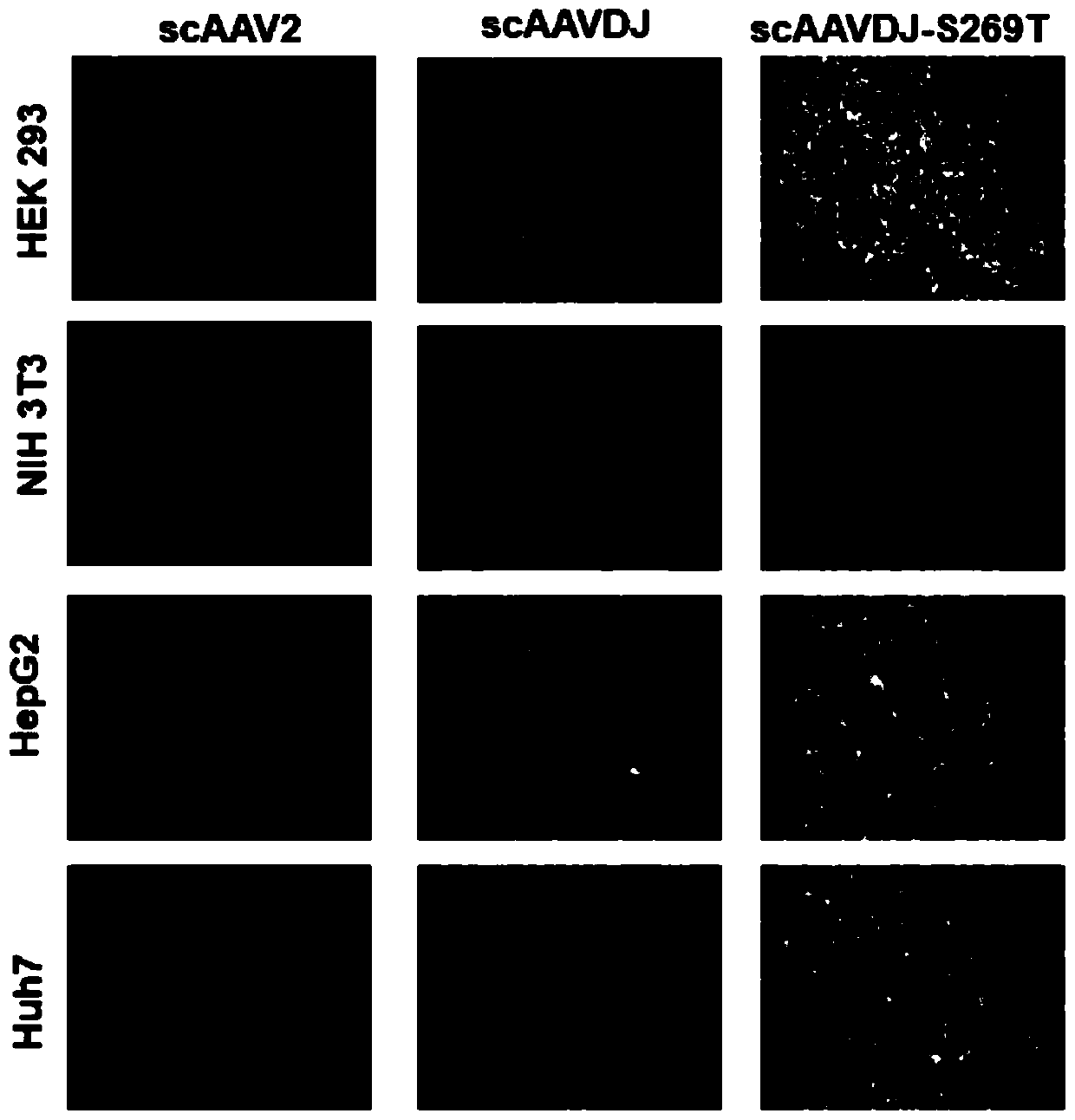
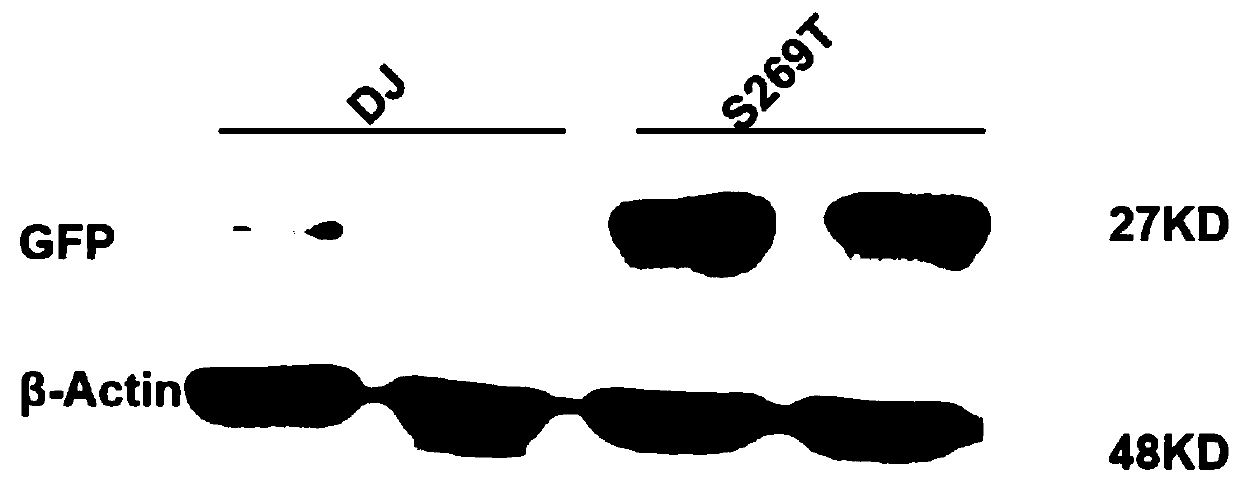
Capsid protein of adeno-associated virus (AAV), vector of AAV as well as construction method and application of vector of AAV
ActiveCN110950934AReduce dosageSimple production processGenetic material ingredientsVirus peptidesViral vectorImmunity response
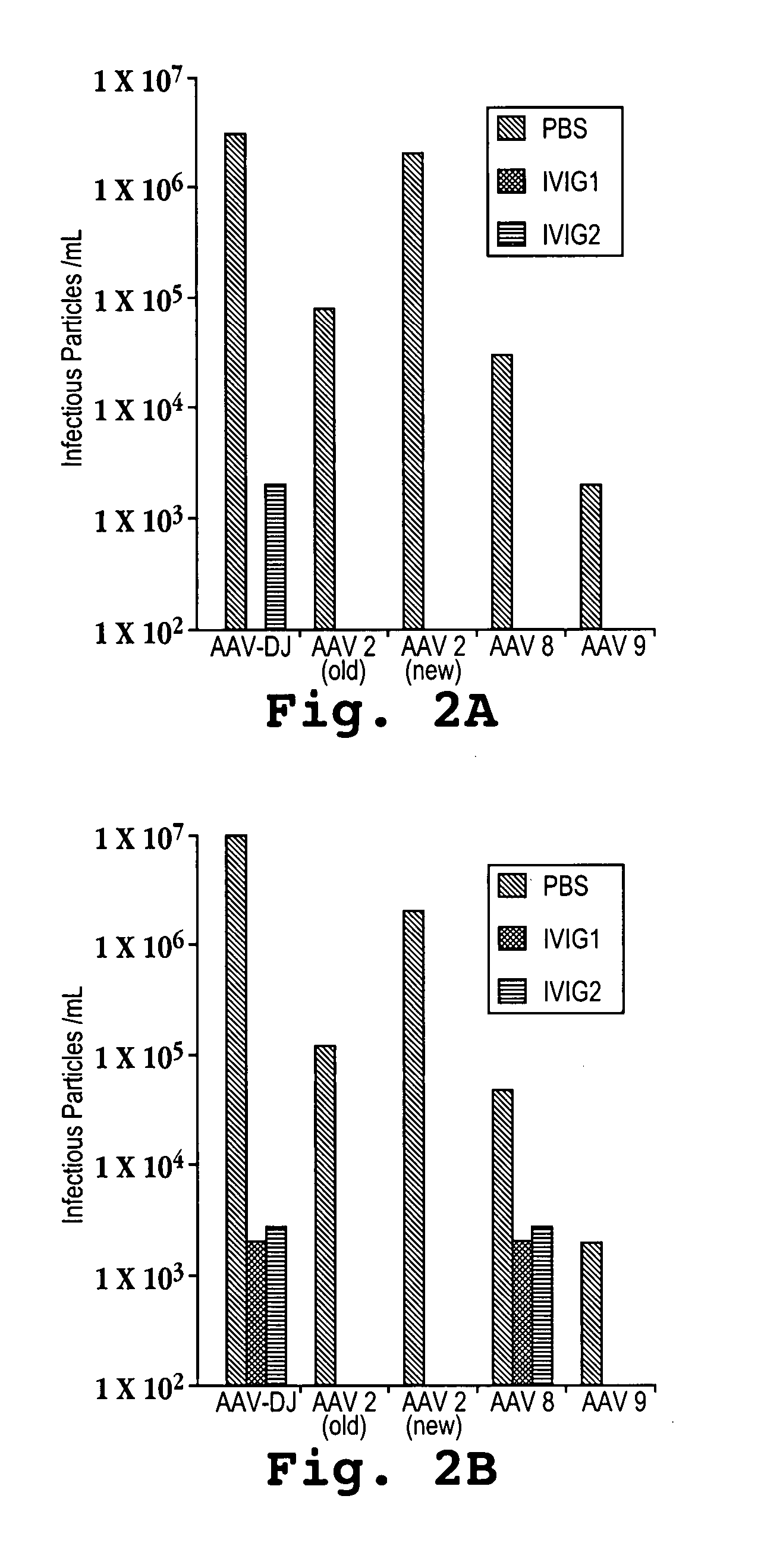
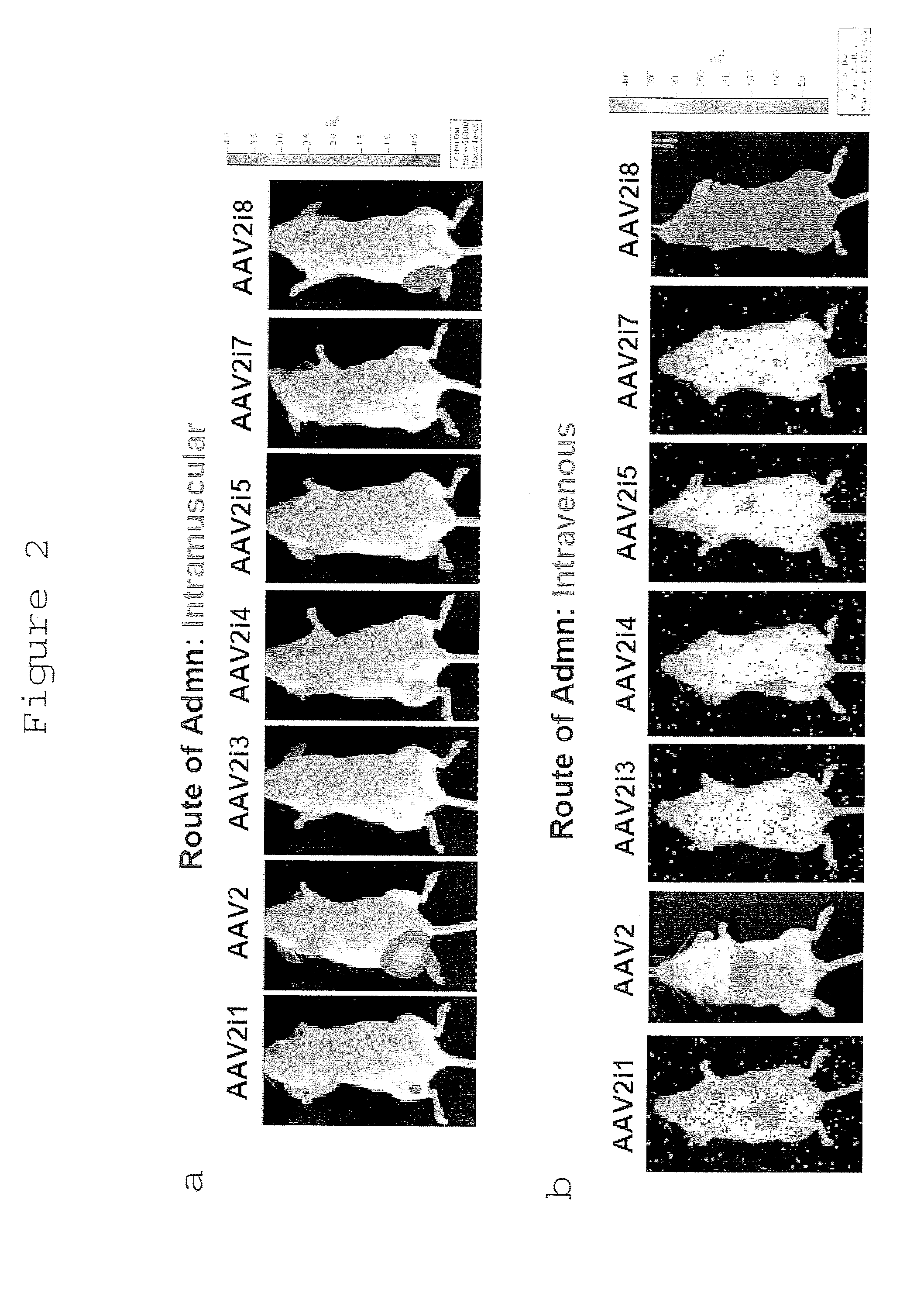
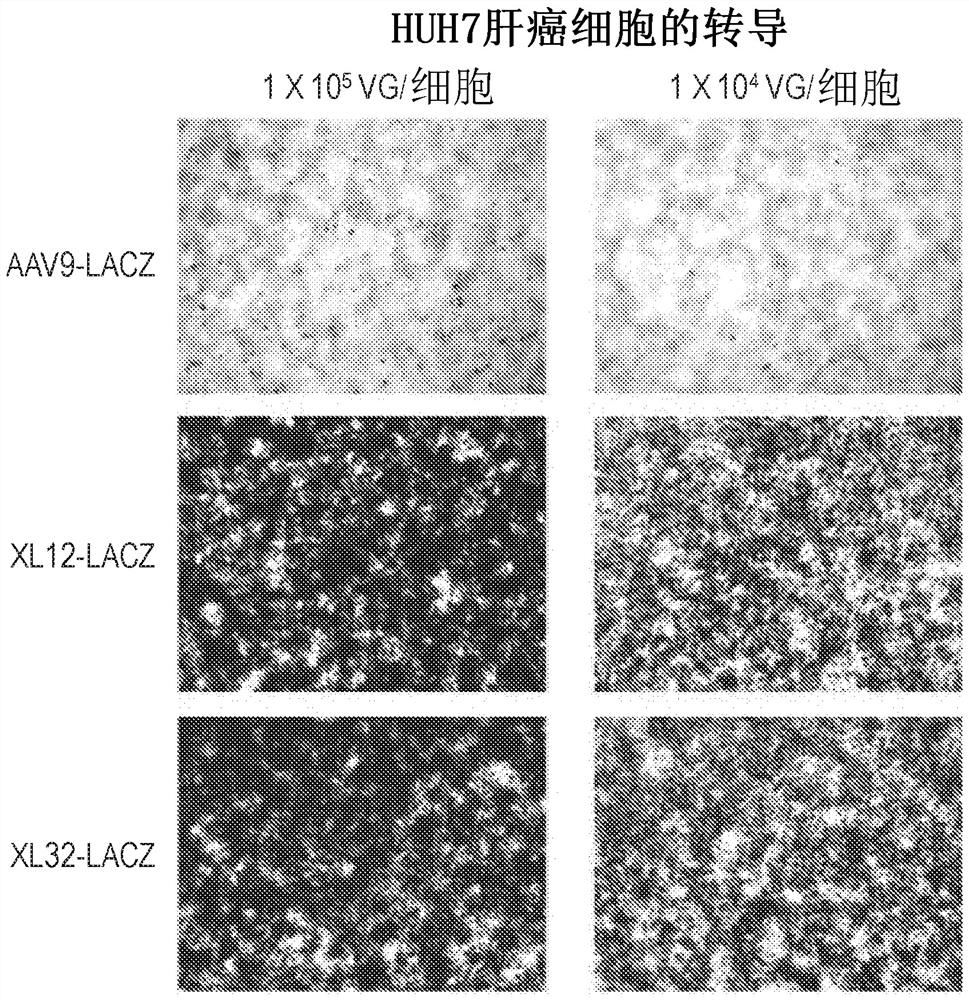
The invention discloses a capsid protein of an adeno-associated virus (AAV) which has an amino acid sequence shown as SEQ ID NO: 1. The invention also discloses an isolated nucleic acid molecule whichencodes the capsid protein of the AAV. The invention further discloses a vector of the AAV which encodes the capsid protein of the AAV. The invention still further discloses a construction method andapplication of the vector of the AAV. According to the capsid protein of the AAV, modification is performed on the capsid protein of the AAV-DJ , so that the AAV-DJ has significantly improved transduction efficiency in cell lines, including HEK293, various liver cancer cells, mouse fibroblasts and the like, and mouse livers compared with wild-type AAV, so that immune response in subjects caused by high-dose injection of virus vectors is avoided; and moreover, production process of the AAV is optimized so as to reduce production cost. Thus, clinical progress of AAV gene therapy products is promoted
Owner:FUDAN UNIV
EMA-ddPCR primer and probe for detecting infectious ASFV and application
ActiveCN113502352AGood repeatabilityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationVirus inactivationViral test
The invention belongs to the field of African swine fever virus detection, and provides an EMA-ddPCR primer and a probe for detecting infectious ASFV and application of the EMA-ddPCR primer and the probe for detecting the ASFV, and the ddPCR primer and the probe for detecting the ASFV can well distinguish nucleic acid positive and virus positive of the African swine fever virus and have the advantages of being high in sensitivity and good in repeatability. The primer probe and the detection method are applied to a ddPCR kit for detecting infectious ASFVs, and all experiments can be completed within 2-3 hours. Compared with a conventional infectious ASFV detection test, the method has the advantages that the test period of infectious ASFV detection is greatly shortened, and the requirements on scientific research conditions and equipment are reduced; nucleic acid and infectious virions of virus inactivation caused by virus capsid protein damage are effectively distinguished, the detection accuracy is improved, and the false positive phenomenon occurring in actual detection is reduced.
Owner:HUAZHONG AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com