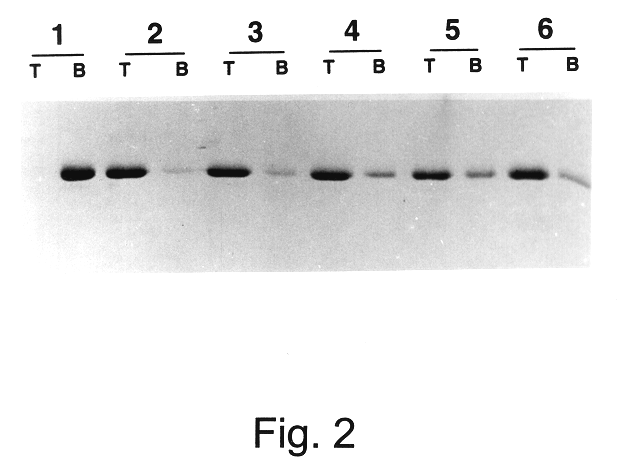
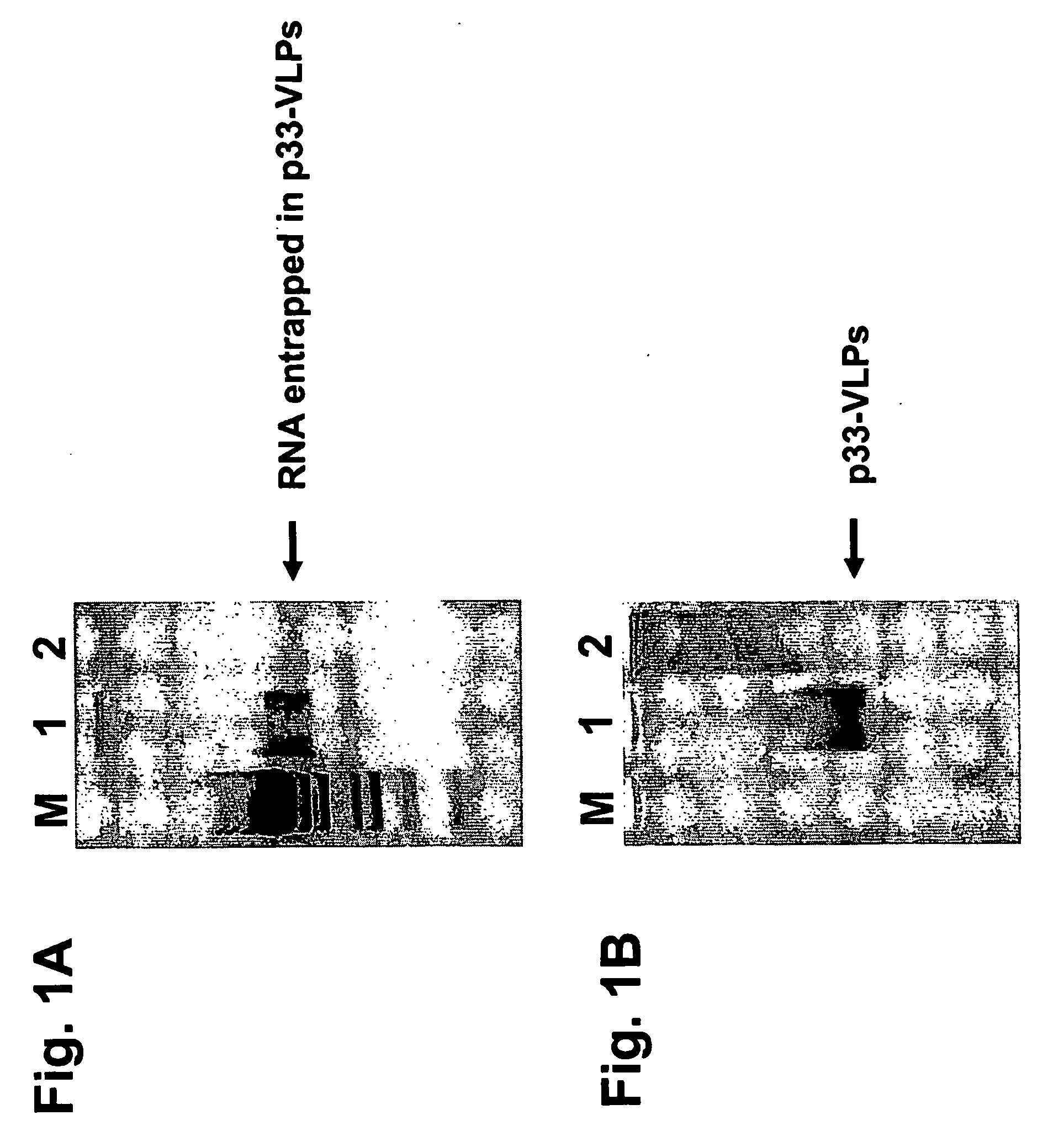
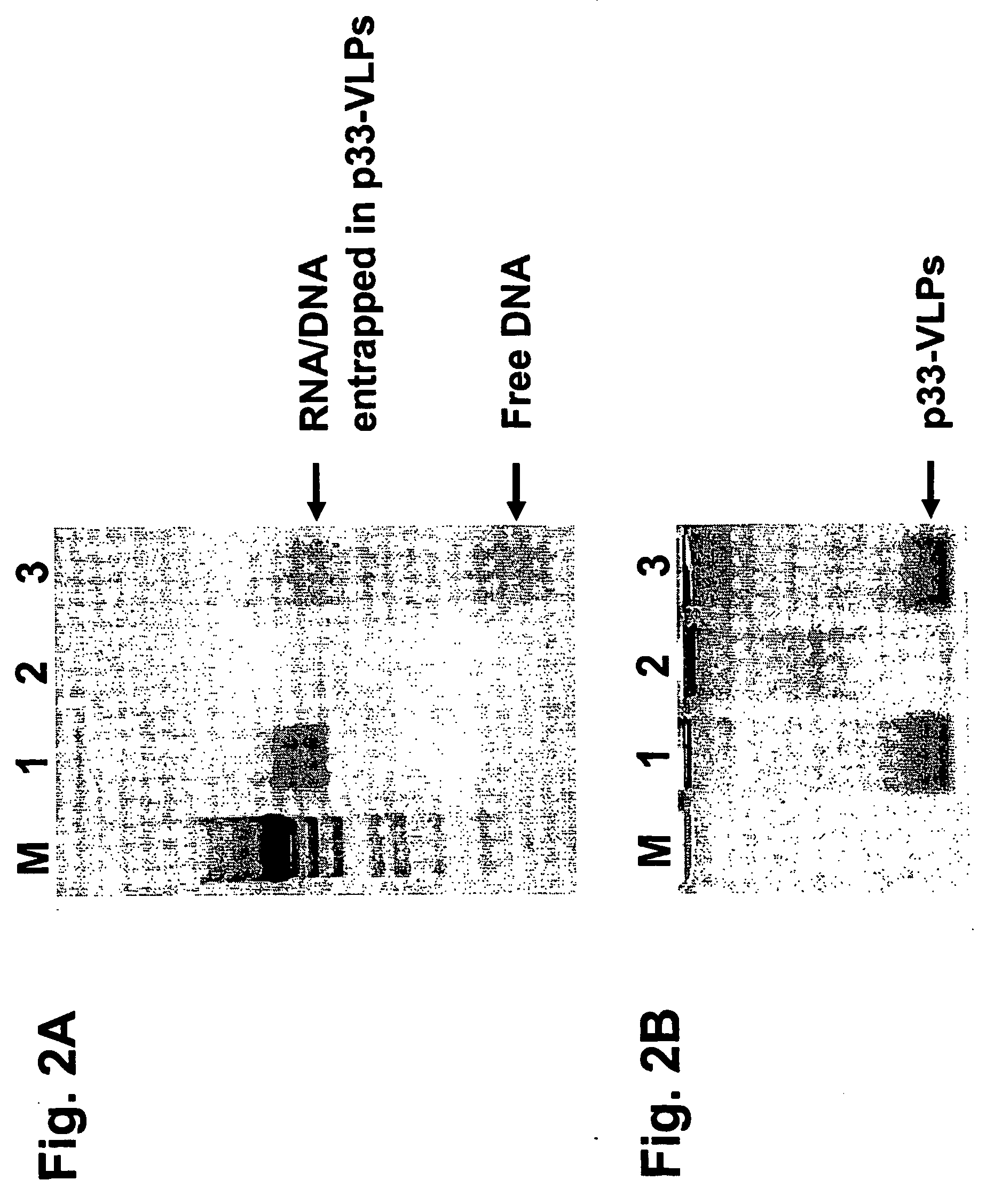
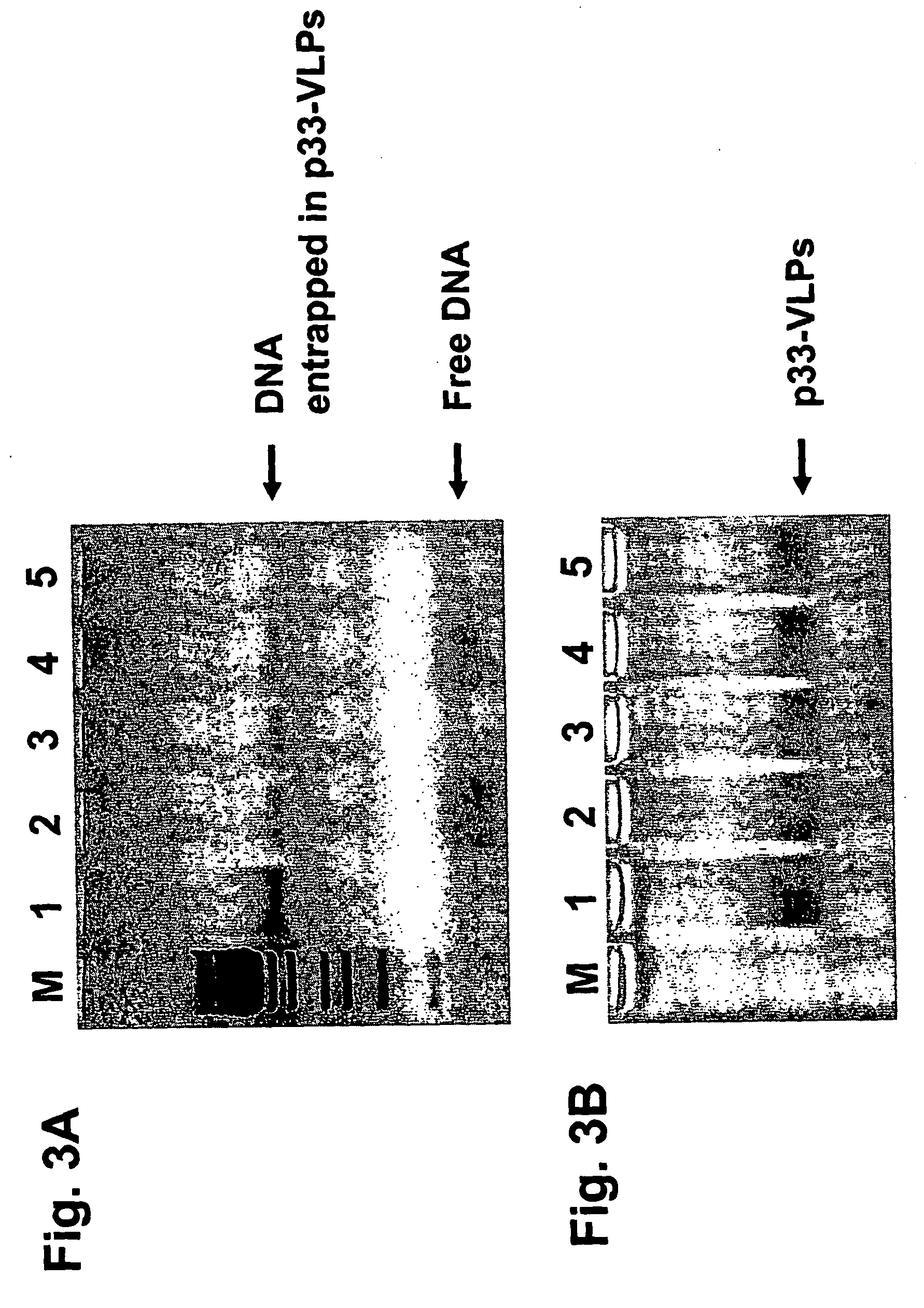
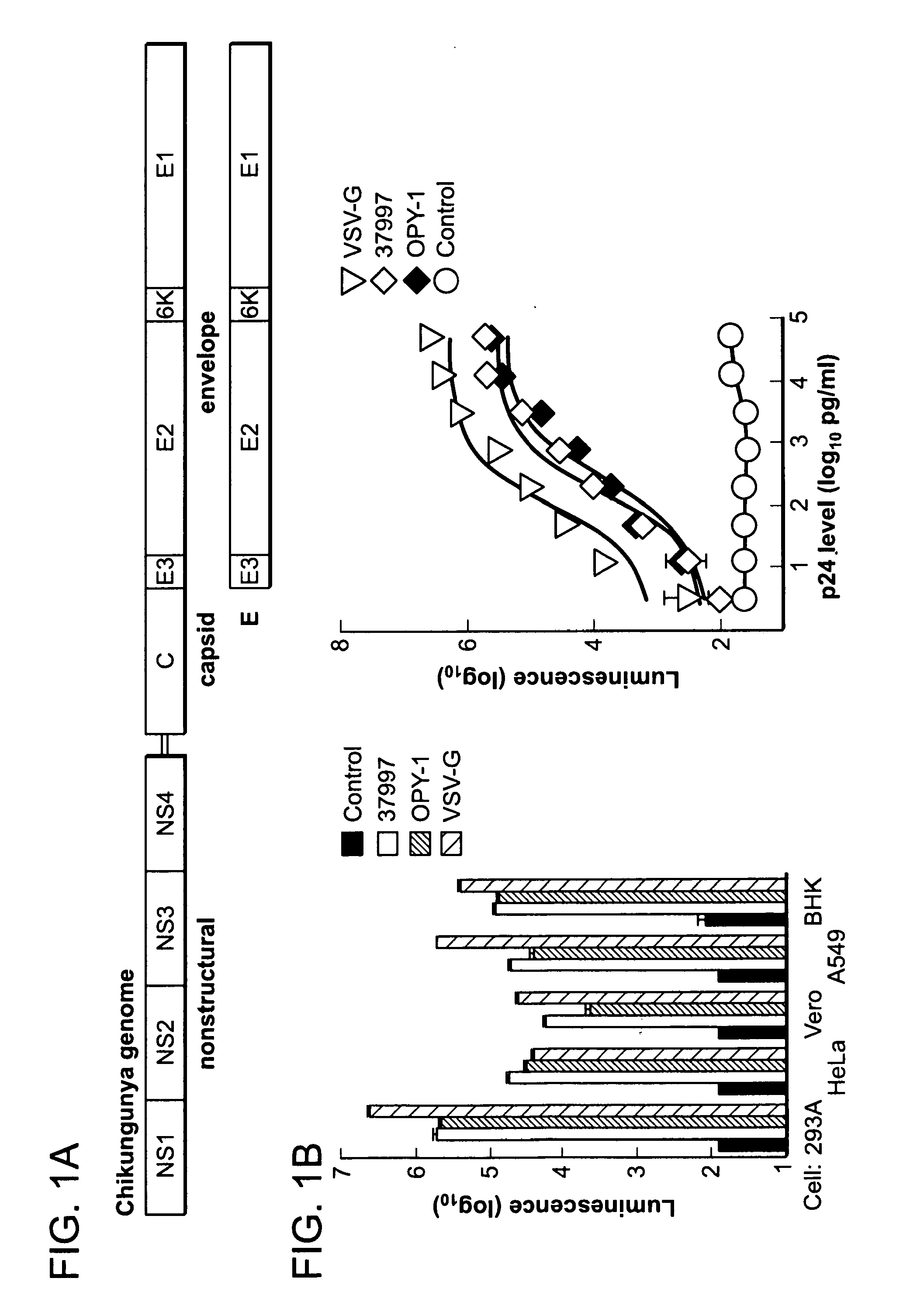
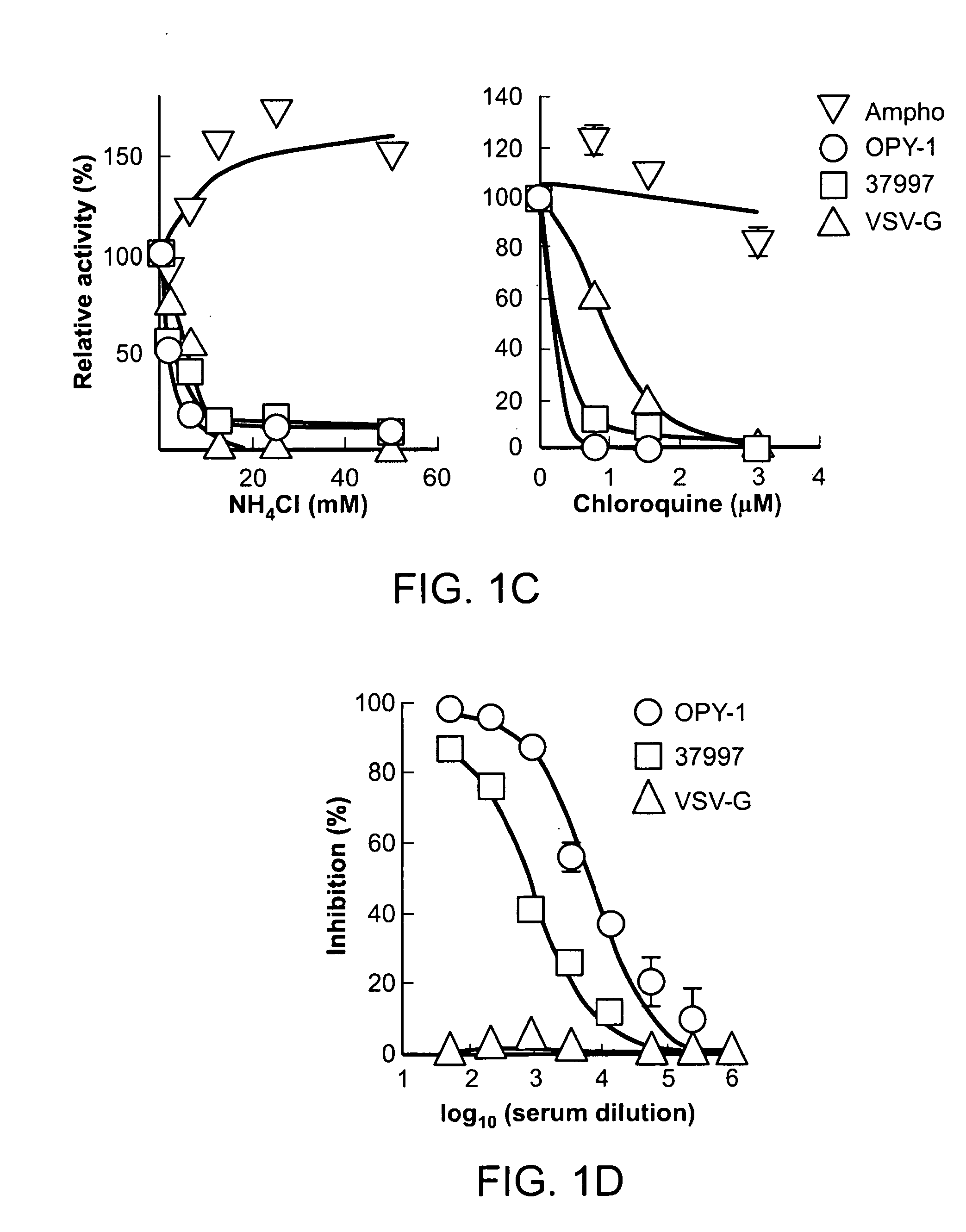
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1027 results about "Virus-like particle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs.VLPs derived from the Hepatitis B virus and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae (e.g. adeno-associated virus), Retroviridae (e.g. HIV), Flaviviridae (e.g. Hepatitis C virus) , Paramyxoviridae (e.g. Nipah) and bacteriophages (e.g. Qβ, AP205). VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.
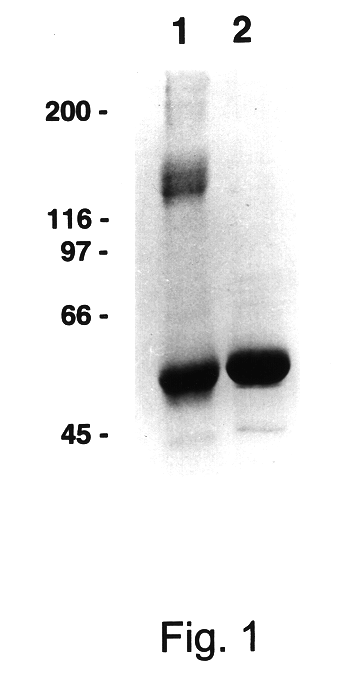
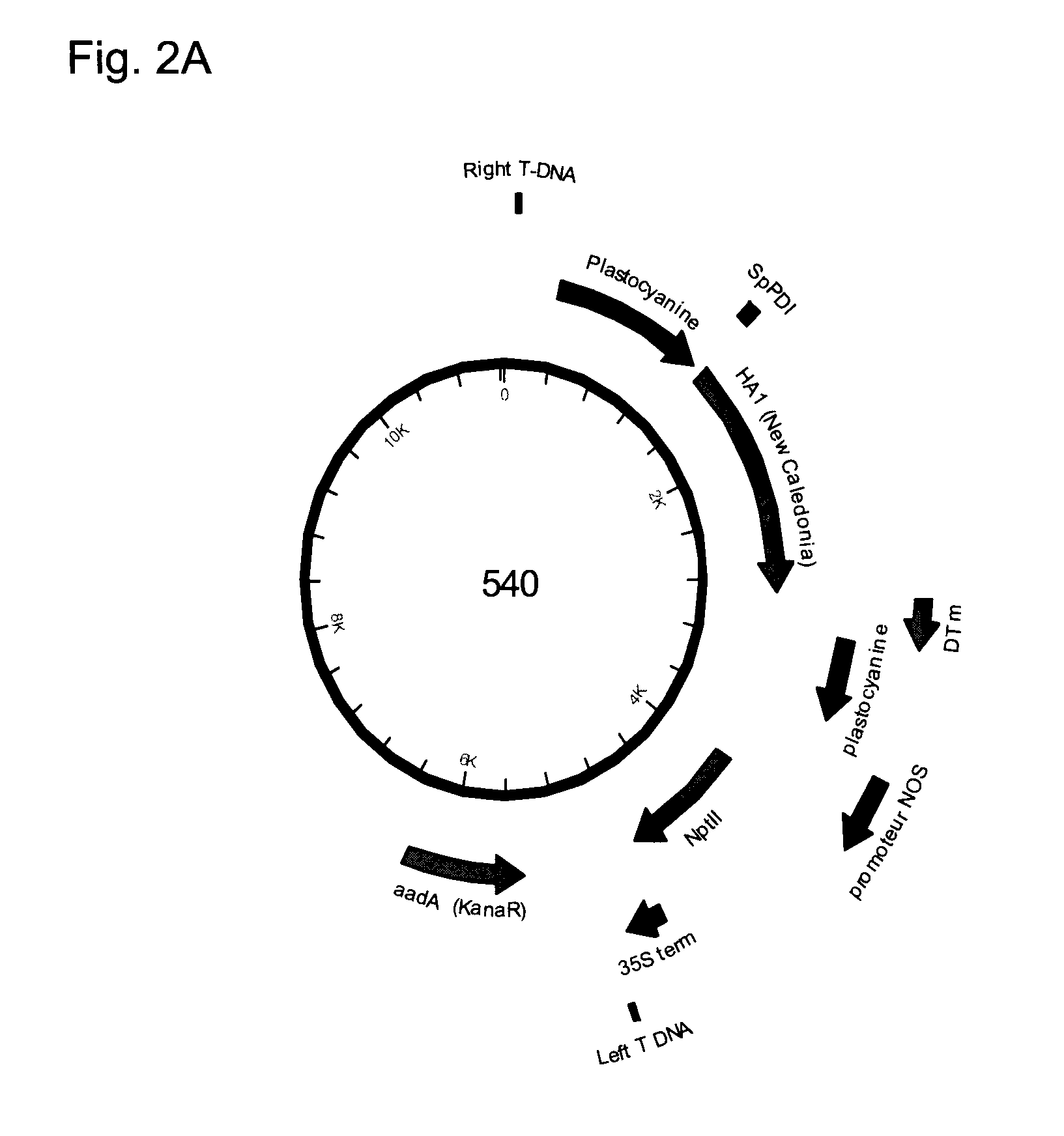
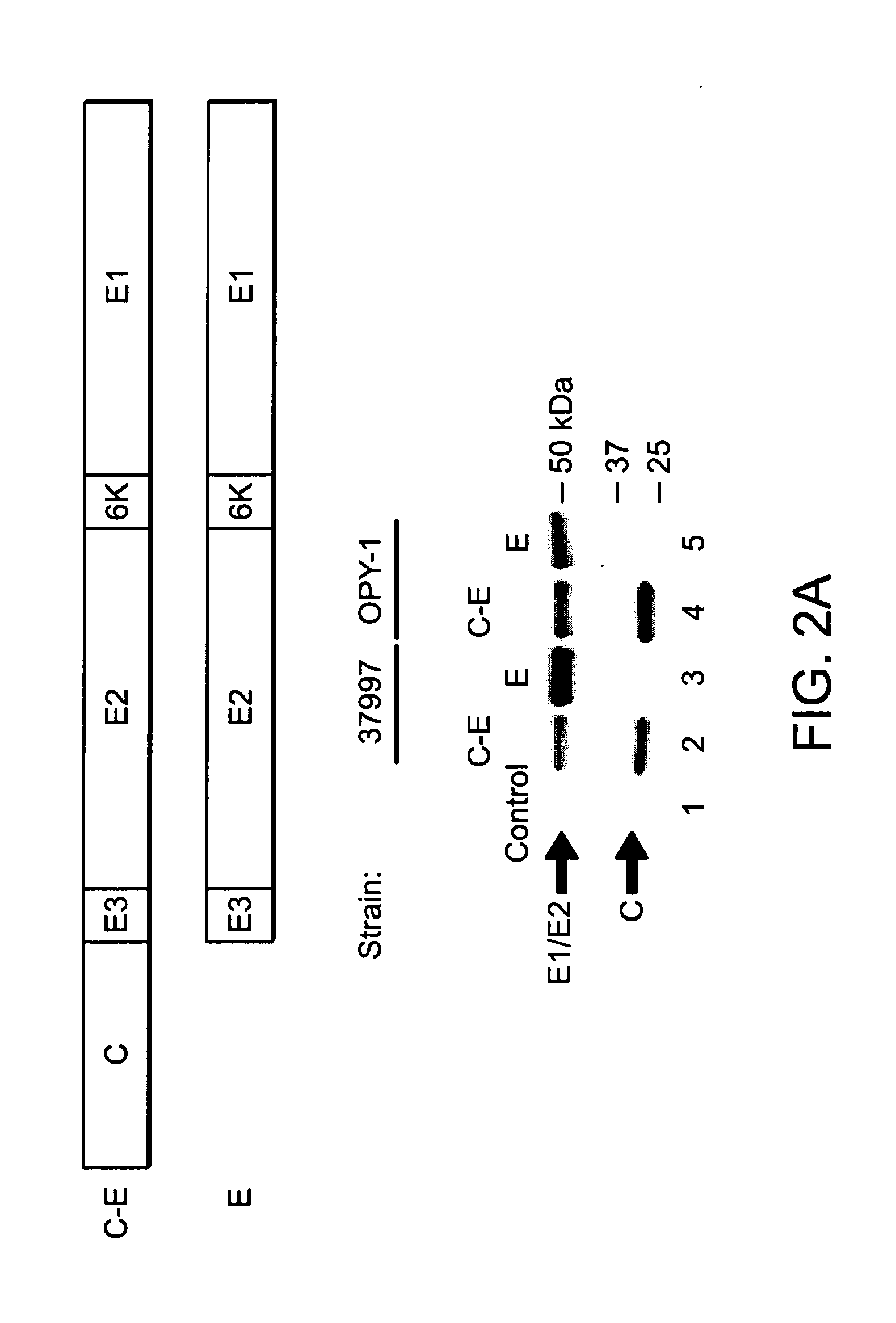
Assembly of wild-type and chimeric influenza virus-like particles (VLPs)
InactiveUS20050186621A1Minimal numberSsRNA viruses negative-senseFungiHeterologousVirus-like particle
Influenza virus-like particles (VLPs) comprising the structural proteins HA, NA, M1 and M2 are described. VLPs are also generated containing M1 alone, as are VLPs with M1 and any one or two of HA, NA and M2. VLPs with HA from one influenza subtype and NA from a different influenza subtype are also described, as are VLPs in which a portion or all of HA or NA is replaced by a heterologous moiety not produced by influenza virus, so as to comprise chimeric VLPs.
Owner:WYETH HOLDINGS LLC
Modified plant virus particles and uses therefor
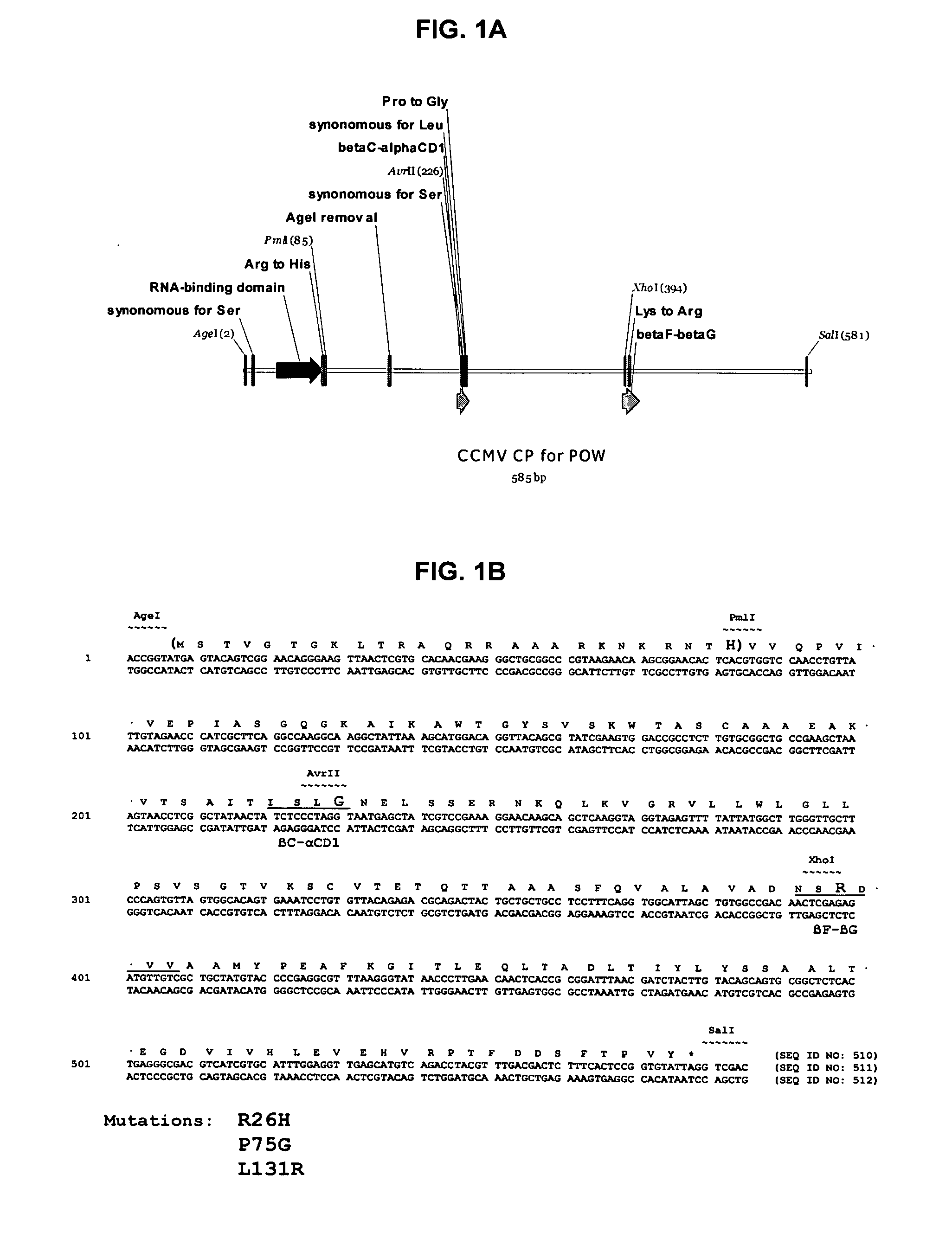
InactiveUS20120015899A1Broadening arrayEasy to assembleFusion with RNA-binding domainBiocideVirus-like particleCoat Proteins
Aspects of the invention provide modified virus-like particles that are designed for therapeutic applications. In particular, aspects of the invention provide CCMV coat proteins that are modified to generate virus-like particles, including mosaic virus-like particles, that can package and / or deliver one or more diagnostic and / or therapeutic agents. The invention also provides methods for treating subjects with one or more modified virus-like particles.
Owner:PLANT BIOSCI LTD +1
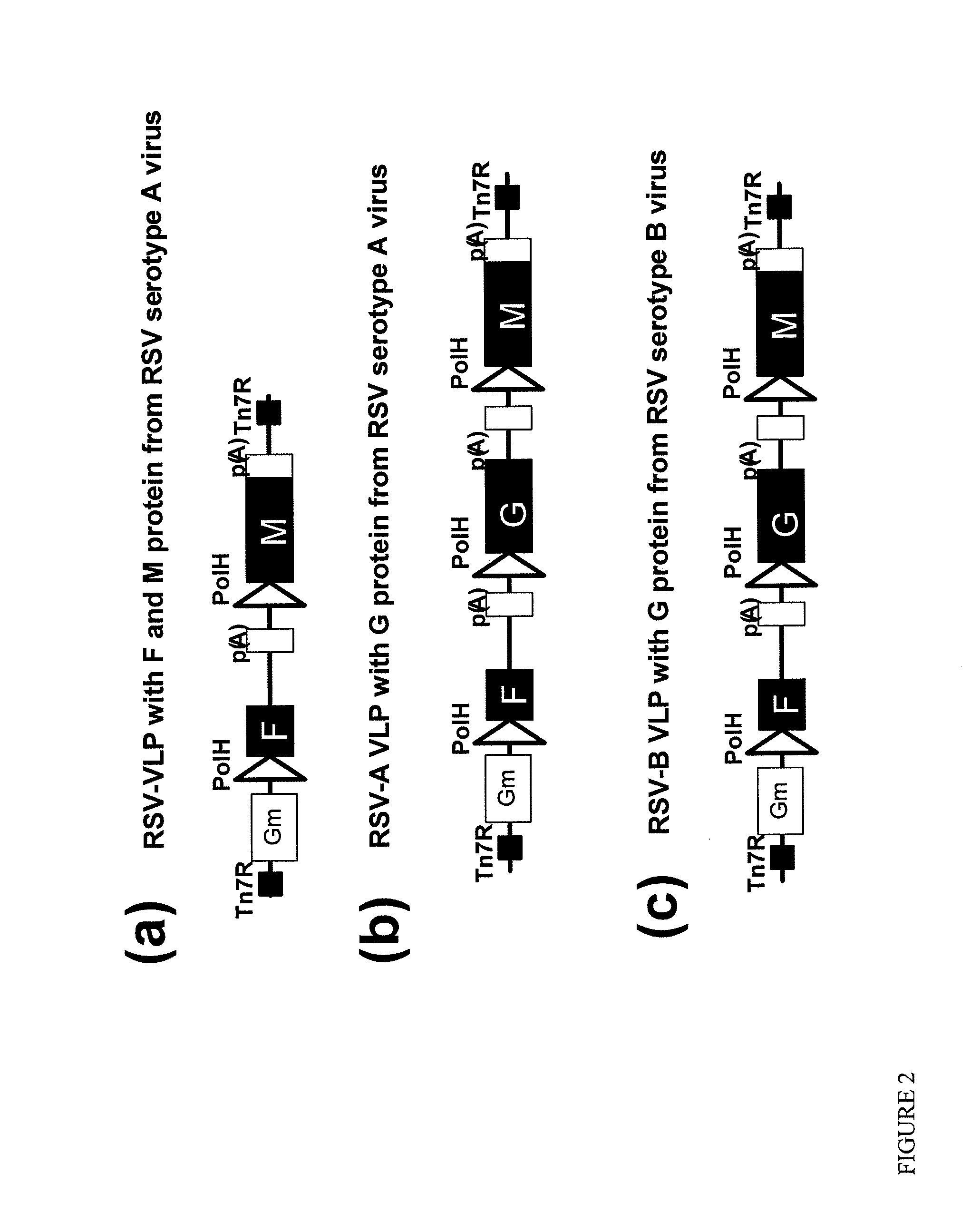
Respiratory syncytial virus-virus like particle (VLPS)
InactiveUS20080233150A1SsRNA viruses negative-senseViral antigen ingredientsVirus-like particleVertebrate
The present invention discloses and claims virus like particles (VLPs) that express and / or contains RSV proteins. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing immunity to infections, including RSV.
Owner:NOVAVAX
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPS)
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
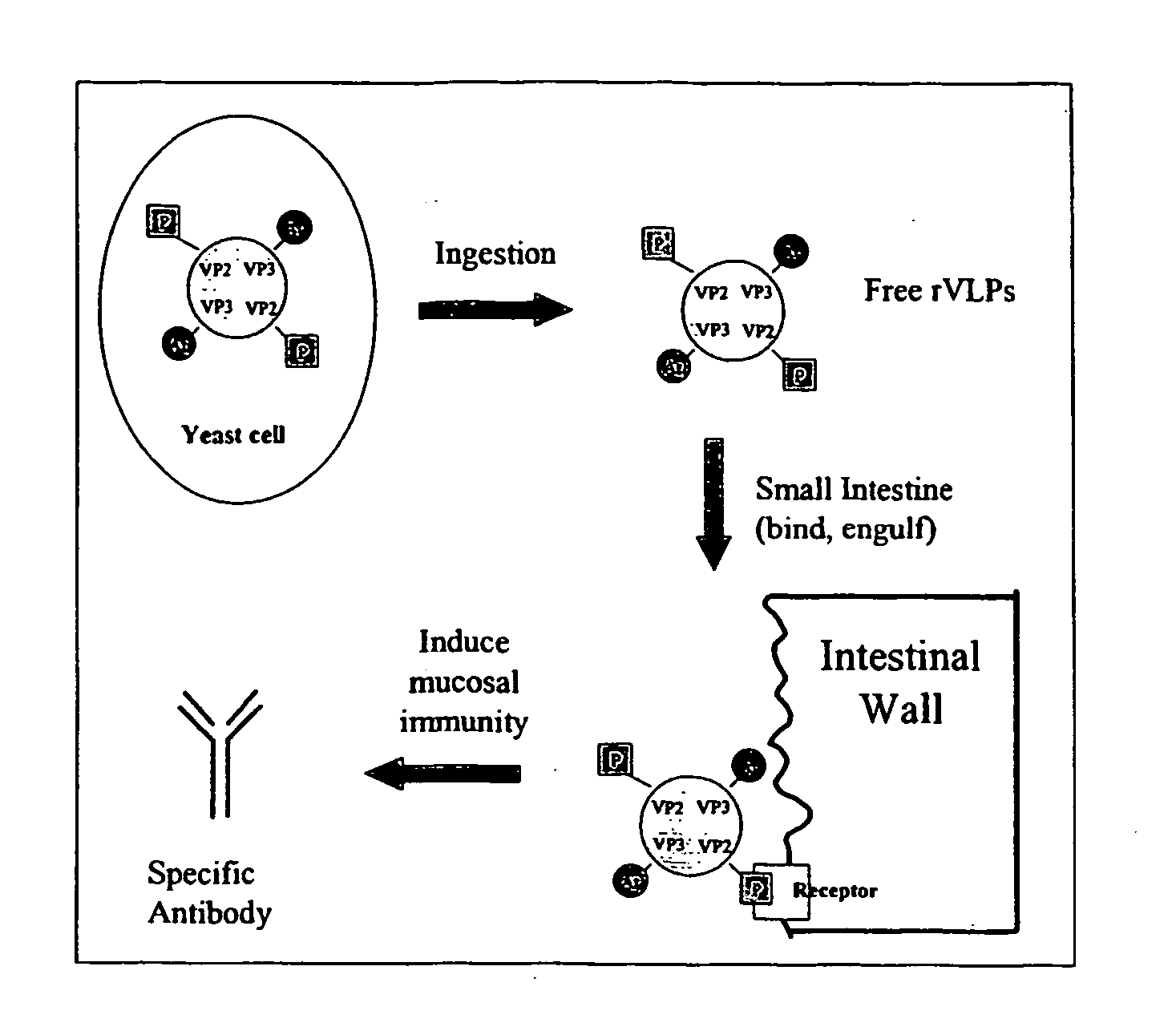
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
Hepatitis C virus asialoglycoproteins
InactiveUS6074852AFacilitate secretionFacilitated releaseNanotechFungiSialic acid aldolaseVirus-like particle
Two Hepatitis C Virus envelope proteins (E1 and E2) are expressed without sialylation. Recombinant expression of these proteins in lower eukaryotes, or in mammalian cells in which terminal glycosylation is blocked, results in recombinant proteins which are more similar to native HCV glycoproteins. When isolated by GNA lectin affinity, the E1 and E2 proteins aggregate into virus-like particles.
Owner:CHIRON CORP
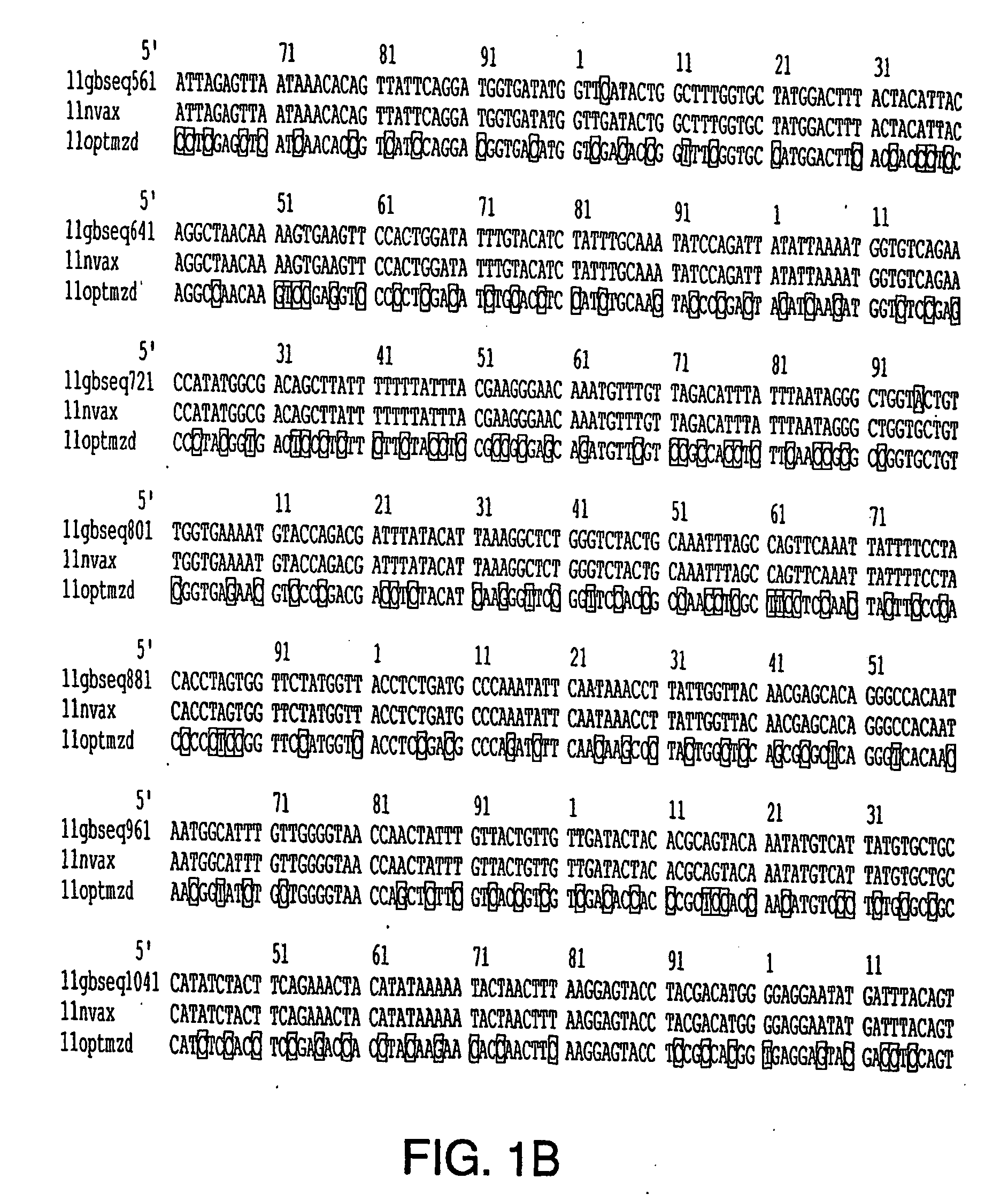
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Antigenically-marked non-infectious retrovirus-like particles
InactiveUS6291157B1Improve efficiencyLow backgroundSsRNA viruses negative-senseSsRNA viruses positive-sensePol genesIn vivo
Non-infectious, retrovirus-like particles comprise an assembly of an env gene product, a pol gene product and a gag gene product contain an antigenic marker which is non-retroviral or non-HIV retroviral. In one embodiment, the marker comprises an amino acid sequence containing an epitope inserted into the gag gene product at an antigenically-active insertion site. In another embodiment, the marker comprises an antigenic anchor sequence operatively connected to the env gene product replacing endogenous anchoring function. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in in vivo administration including to humans and in diagnosis. The presence of the antigenic marker enables recognition that antiserum containing anti-retroviral antibodies has been generated by exposure to the non-infectious retrovirus-like particles by testing for antibodies specific to the antigenic marker.
Owner:CONNAUGHT LAB
Influenza virus-like particles (VLPS) comprising hemagglutinin produced within a plant
InactiveUS20100239610A1Enhance immune responseEasy to captureSsRNA viruses negative-senseVirus peptidesHemagglutininLipid formation
A method for synthesizing influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of influenza HA in plants and the purification by size exclusion chromatography. The invention is also directed towards a VLP comprising influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Packaged virus-like particles
InactiveUS20060251623A1Improve responseEnhanced T cell responseSsRNA viruses negative-senseBiocideAbnormal tissue growthDisease
The present invention is related to the fields of vaccinology, immunology and medicine. The invention provides compositions and methods for enhancing immunological responses against antigens coupled or fused to virus-like particles (VLPs) packaged with immunostimulatory nucleic acids, preferably oligonucleotides containing at least one non-methylated CpG sequence and a toll-like receptor (TLR) ligand. The invention can be used to induce strong antibody and T cell responses particularly useful for the treatment of allergies, tumors and chronic viral diseases as well as other chronic diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Virus like particle compositions and methods of use
ActiveUS20120003266A1Improve purification effectAvoid infectionFungiSsRNA viruses positive-senseVirus-like particleChikungunya fever
The invention features compositions and methods for the prevention or treatment of one or more strains of Chikungunya virus, as well as other alphavirus-mediated diseases.
Owner:UNITED STATES OF AMERICA
Porcine circovirus II-type recombinant baculovirus as well as preparation method and application thereof
ActiveCN103122352AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliSpecific immunity
The invention discloses porcine circovirus II-type recombinant baculovirus as well as a preparation method and application thereof. ORF2 gene is artificially synthesized by referring to a PCV2b isolated strain ORF2 gene sequence; the synthesized ORF2 gene is connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHm 30RF2 is obtained. The baculovirus transfer vector pFBDPHm30RF2 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant rod granule rBac-PVR30RF2; the rod granule is transferred with a sf9 cell to obtain the recombinant baculovirus QP-Ac-30RF2. The recombinant baculovirus can be used for efficiently expressing the PCV20RF2 protein and forming virus-like particles. The VLP which is expressed and packaged by the recombinant baculovirus disclosed by the invention is used for preparing inactivated vaccine, and the organism is induced to generate specific immunity response after a 28-day-aged piglet is immunized, and the pig body can be completely protected from virulent attacks of the porcine circovirus.
Owner:HUAZHONG AGRI UNIV
Melan-A- carrier conjugates
InactiveUS7537767B2Improve responseMore immunogenicSsRNA viruses negative-senseBiocideDiseaseAllergy
Owner:CYTOS BIOTECHNOLOGY AG
Optimization of gene sequences of virus-like particles for expression in insect cells
InactiveUS20040121465A1Improve the level ofMinimize the numberAnimal cellsViral antigen ingredientsPolynucleotideTGE VACCINE
Codon optimized polynucleotides for optimal expression of recombinant proteins in eukaryotic cells are provided. The codon optimized polynucleotides encode a viral capsid protein that self assembles into a virus-like particle. The virus-like particle is expressed extracellularly and exhibits conformational antigenic epitopes capable of raising neutralizing antibodies. Pharmaceutical compositions, vaccines, and diagnostic test kits containing the gene products of the codon-optimized polynucleotides are also provided.
Owner:NOVAVAX
Assembly of wild-type and chimeric influenza virus-like particles (VLPs)
Owner:WYETH HOLDINGS LLC
Virus-like platform for rapid vaccine discovery
ActiveUS20090054246A1Promotes self-assemblyPeptide librariesLibrary screeningHeterologousVirus-like particle
The invention is directed to virus-like particles (VLPs) of an RNA bacteriophage that (a) comprises a coat polypeptide of said phage modified by insertion of a heterologous peptide that is displayed on said VLP and (b) encapsidates said bacteriophage mRNA as well as populations of these VLPs, and their uses. The invention is further directed to VLPs that encapsidate heterologous substances, as well as populations of these VLPs and their uses.
Owner:STC UNM
Kit for treating gastrointestinal tract
InactiveUS20040063188A1Viral antigen ingredientsGenetically modified cellsPurification methodsVirus-like particle
Methods for isolation and purification or recombinant gene products are disclosed. In particular, methods for isolation and purification of extracellular and intracellular viral gene products, including virus-like particles, are disclosed herein.
Owner:NOVAVAX
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles
ActiveUS20100166769A1Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Virus-like particles (VLPs) of mammalian-hosted viruses, such as SARS-CoV and influenza viruses, have been recombinantly produced from Vero cells. The VLPs closely emulate the exterior of authentic virus particles and are highly immunogenic. They can elicit not only humoral but also cellular immune responses in a mammal. Compositions and methods related to the VLPs are also described.
Owner:ACAD SINIC
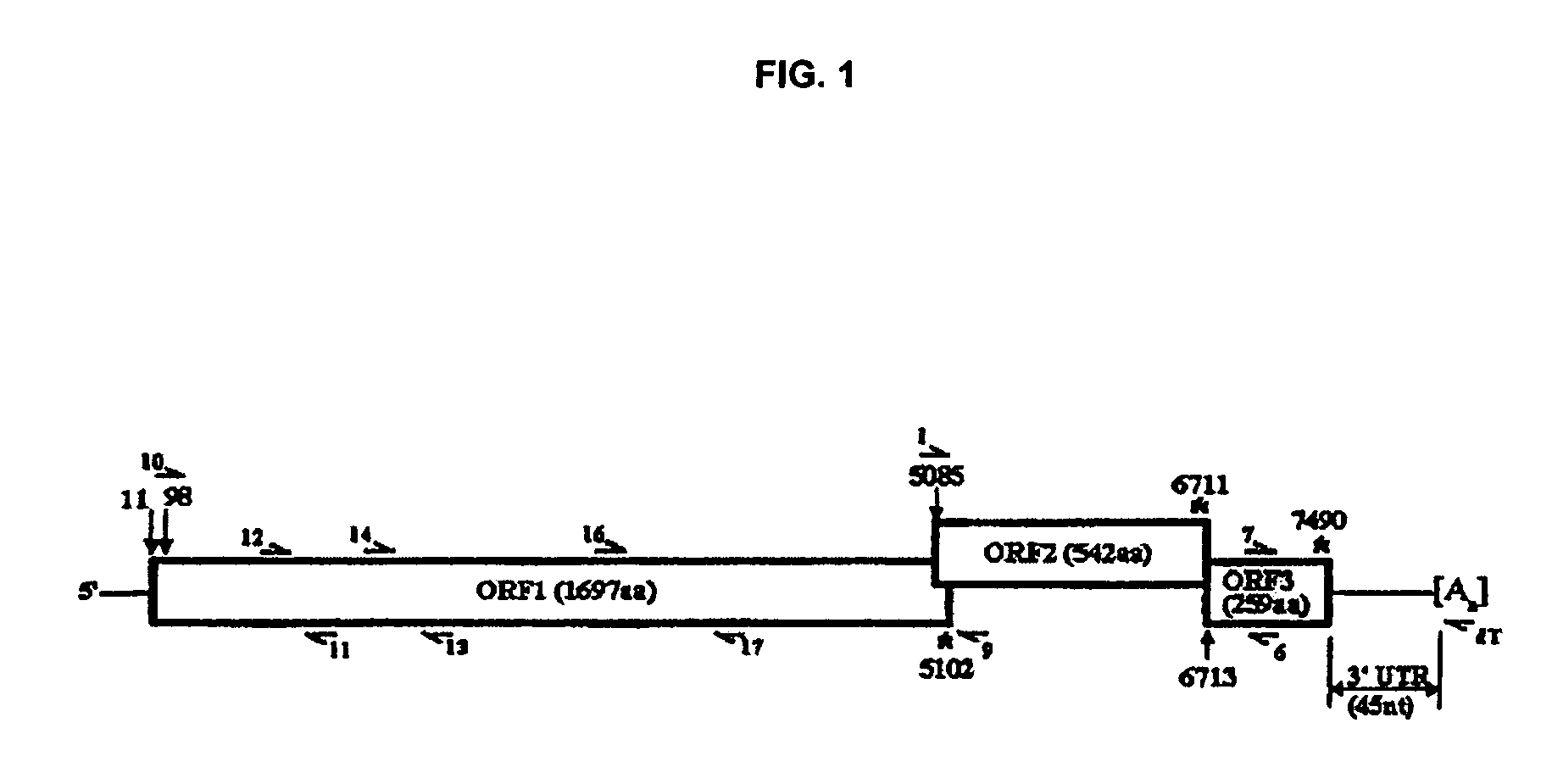
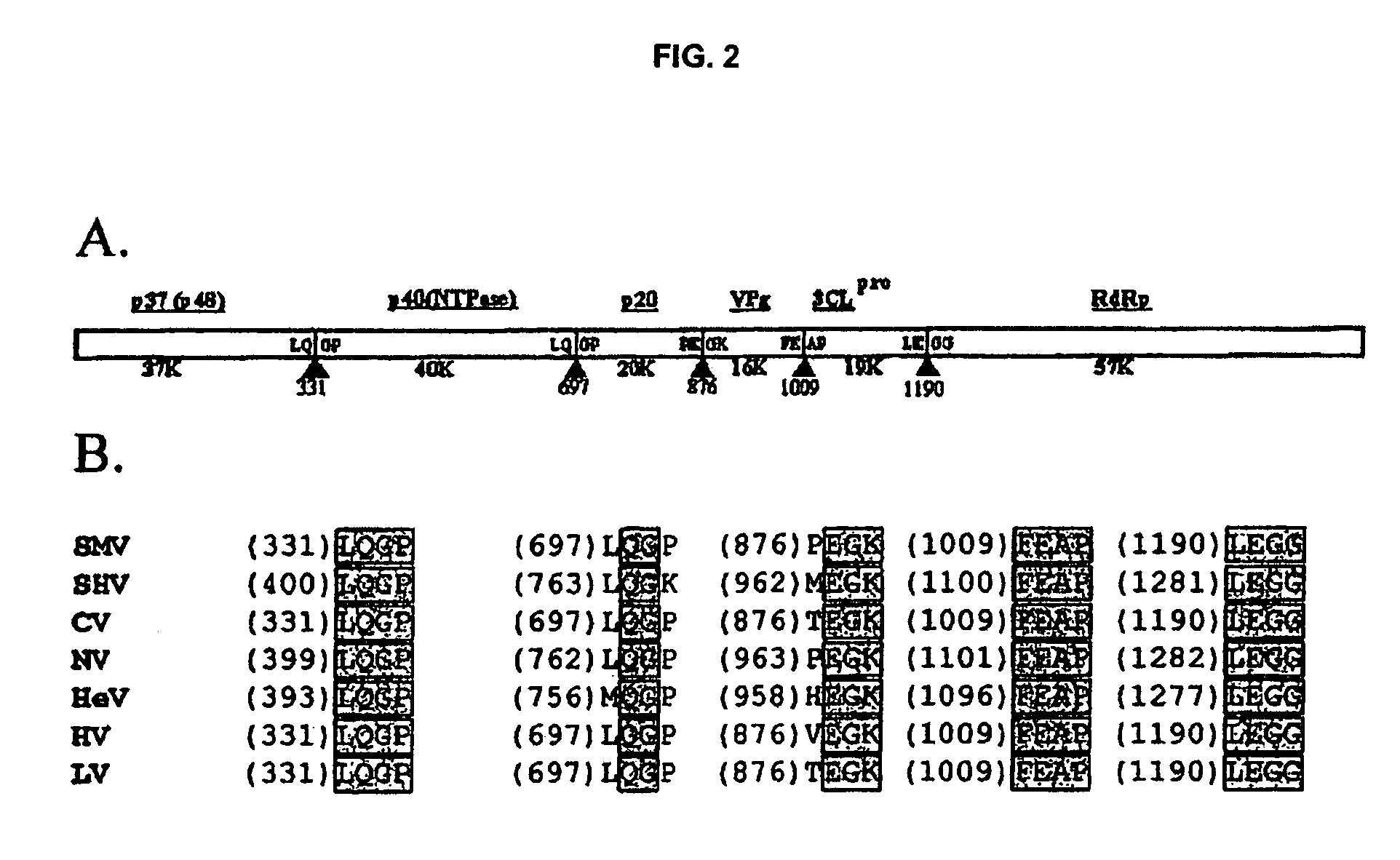
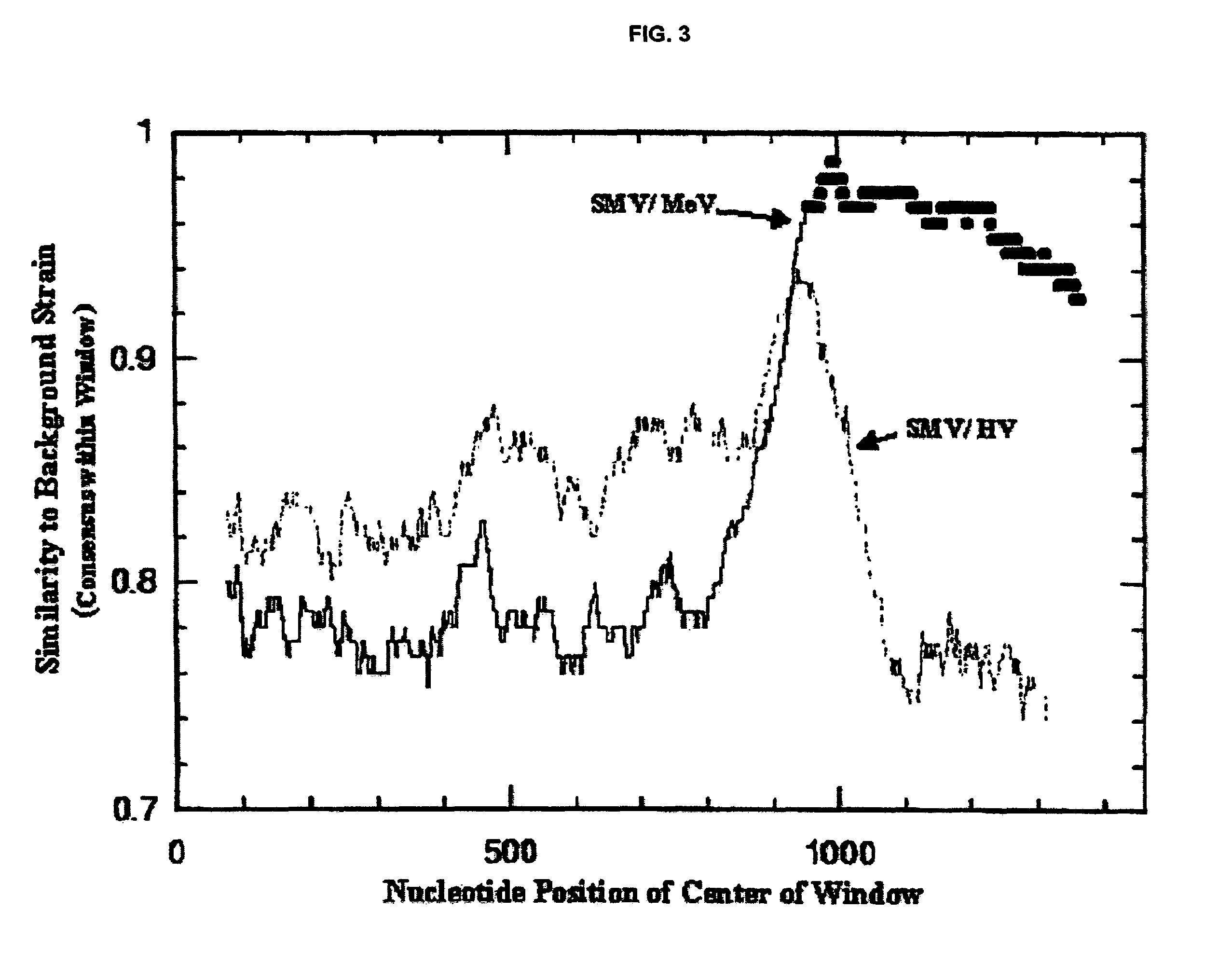
Snow mountain virus genome sequence, virus-like particles and methods of use
Snow Mountain Virus (SMV) belongs to the Norovirus genus of the Caliciviridae family. SMV is a genogroup II (GII) reference strain of human enteric caliciviruses associated with epidemic gastroenteritis. The positive sense RNA genome sequence of SMV was determined to be 7,537 nucleotides in length excluding the 3′ polyadenylated tract. The genome is organized into three open reading frames. Pairwise sequence alignments showed SMV ORF1 is highly conserved with other GII noroviruses, and most closely related to GII strains Melksham and Hawaii viruses. Comparative sequence analyses showed the SMV is a recombinant norovirus. VP1 / NP2 proteins assembled into virus-like particles (VLPs) when expressed in insect cells by a recombinant baculovirus. Characterization of one clone that expressed VP1 but failed to assemble into VLPs, identified histidine residue 91 as important for particle assembly.
Owner:MONTANA STATE UNIVERSITY
Functional influenza virus like particles (VLPs)
ActiveUS8080255B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
The present invention discloses and claims virus like particles (VLPs) that express and / or contains seasonal influenza virus proteins, avian influenza virus proteins and / or influenza virus proteins from viruses with pandemic potential. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing substantial immunity to either seasonal and avian influenza, or at least one symptom thereof.
Owner:NOVAVAX
Amyloid β1-6 antigen arrays
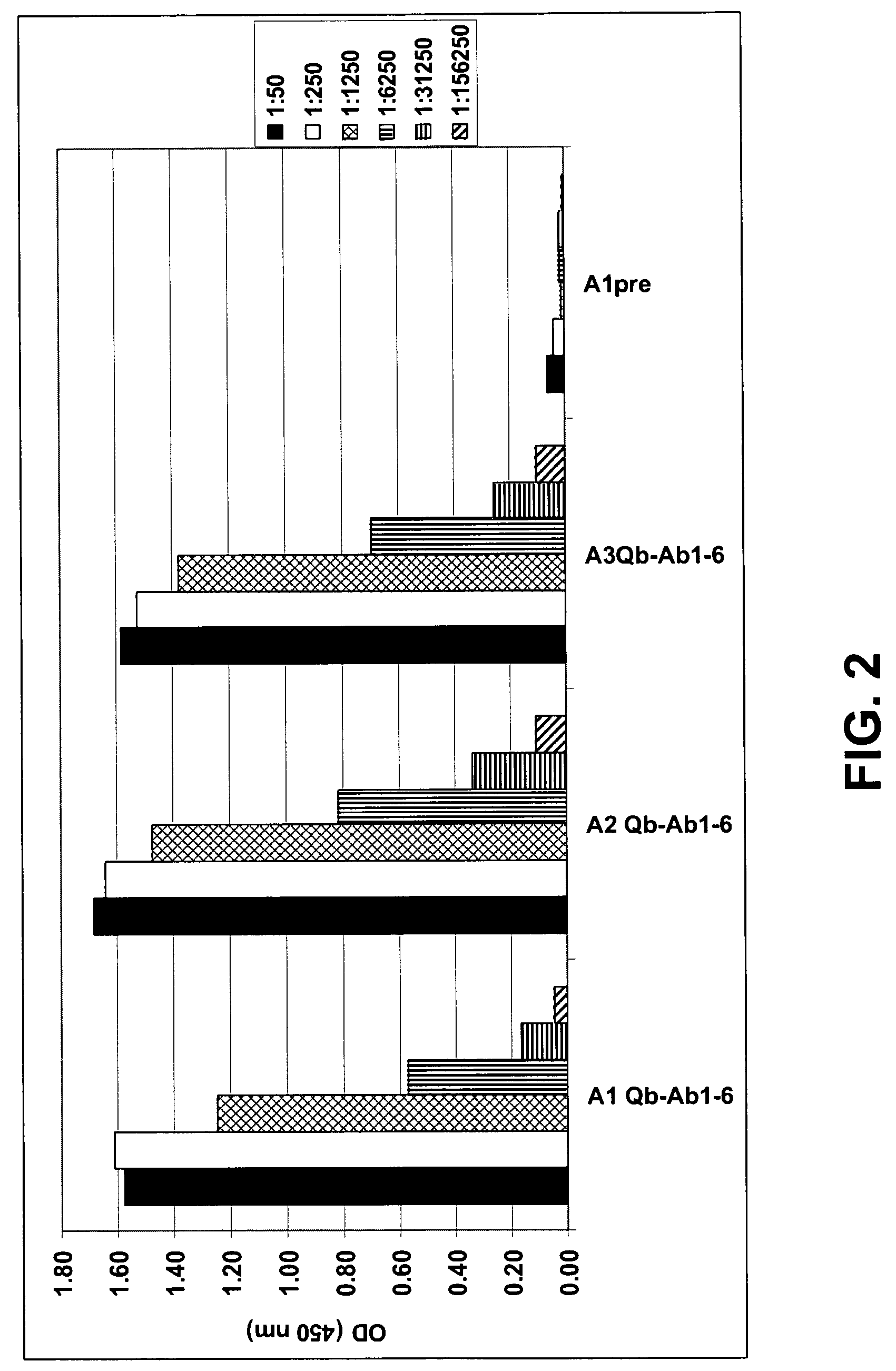
InactiveUS7279165B2High potencyEfficient inductionNervous disorderVirus peptidesVirus-like particleSpecific immunity
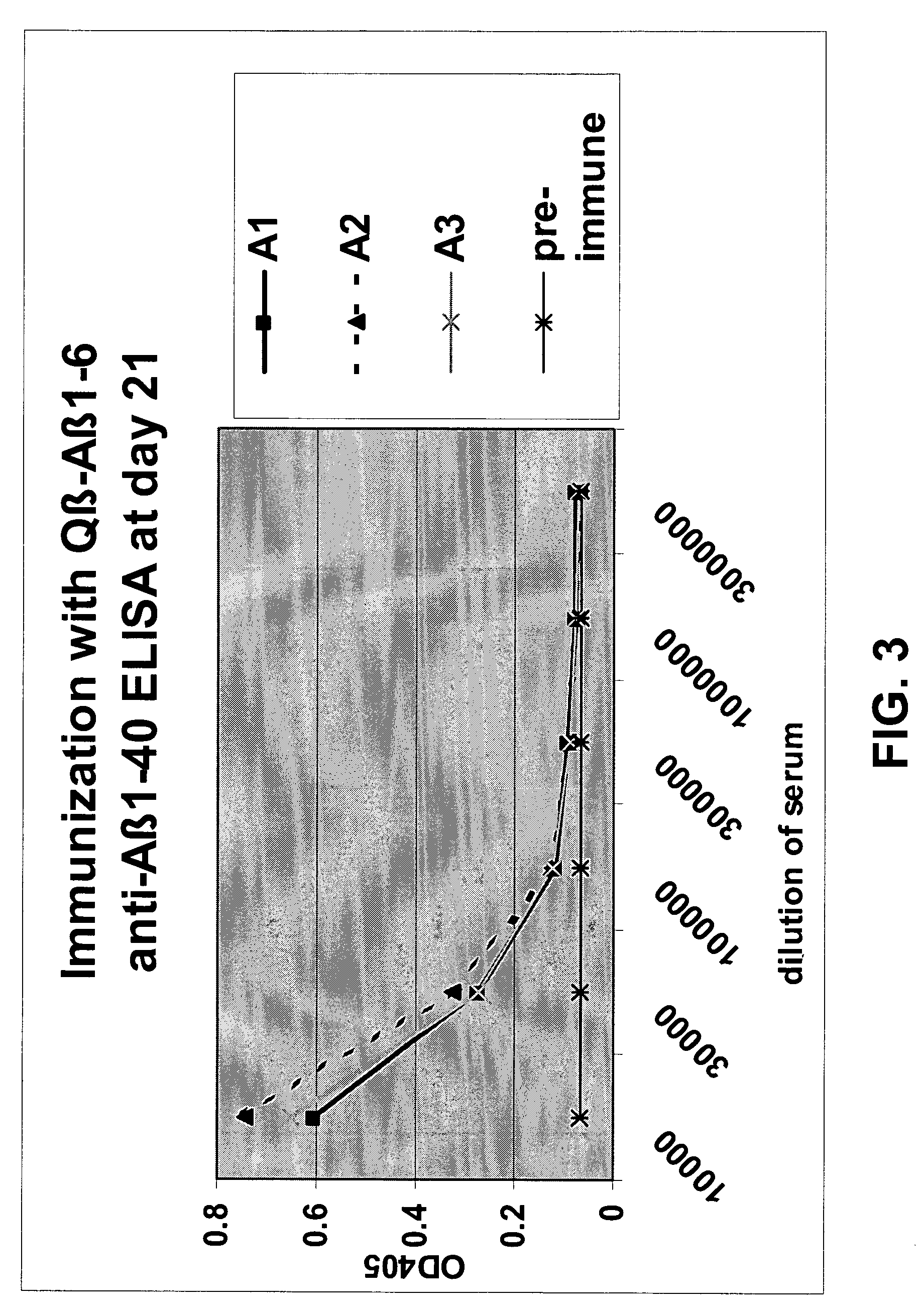
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising an ordered and repetitive antigen or antigenic determinant array, and in particular an Aβ1-6 peptide-VLP-composition. More specifically, the invention provides a composition comprising a virus-like particle and at least one Aβ1-6 peptide bound thereto. The invention also provides a process for producing the conjugates and the ordered and repetitive arrays, respectively. The compositions of the invention are useful in the production of vaccines for the treatment of Alzheimer's disease and as a pharmaccine to prevent or cure Alzheimer's disease and to efficiently induce immune responses, in particular antibody responses. Furthermore, the compositions of the invention are particularly useful to efficiently induce self-specific immune responses within the indicated context.
Owner:NOVARTIS AG
Functional influenza virus like particles (VLPs)
ActiveUS8506967B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus-like particle
Owner:NOVAVAX
In vitro method for disassmbly/reassembly of papillomavirus virus-like particles (VLPs). Homogeneous VLP and cavsomere compositions produced by said methods: use thereof as vehicle for improved purification, and delivery of active agents
InactiveUS7351533B2Increase ionic strengthStabilize VLPsMicroencapsulation basedMicrobiological testing/measurementEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavinis VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or marker” DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
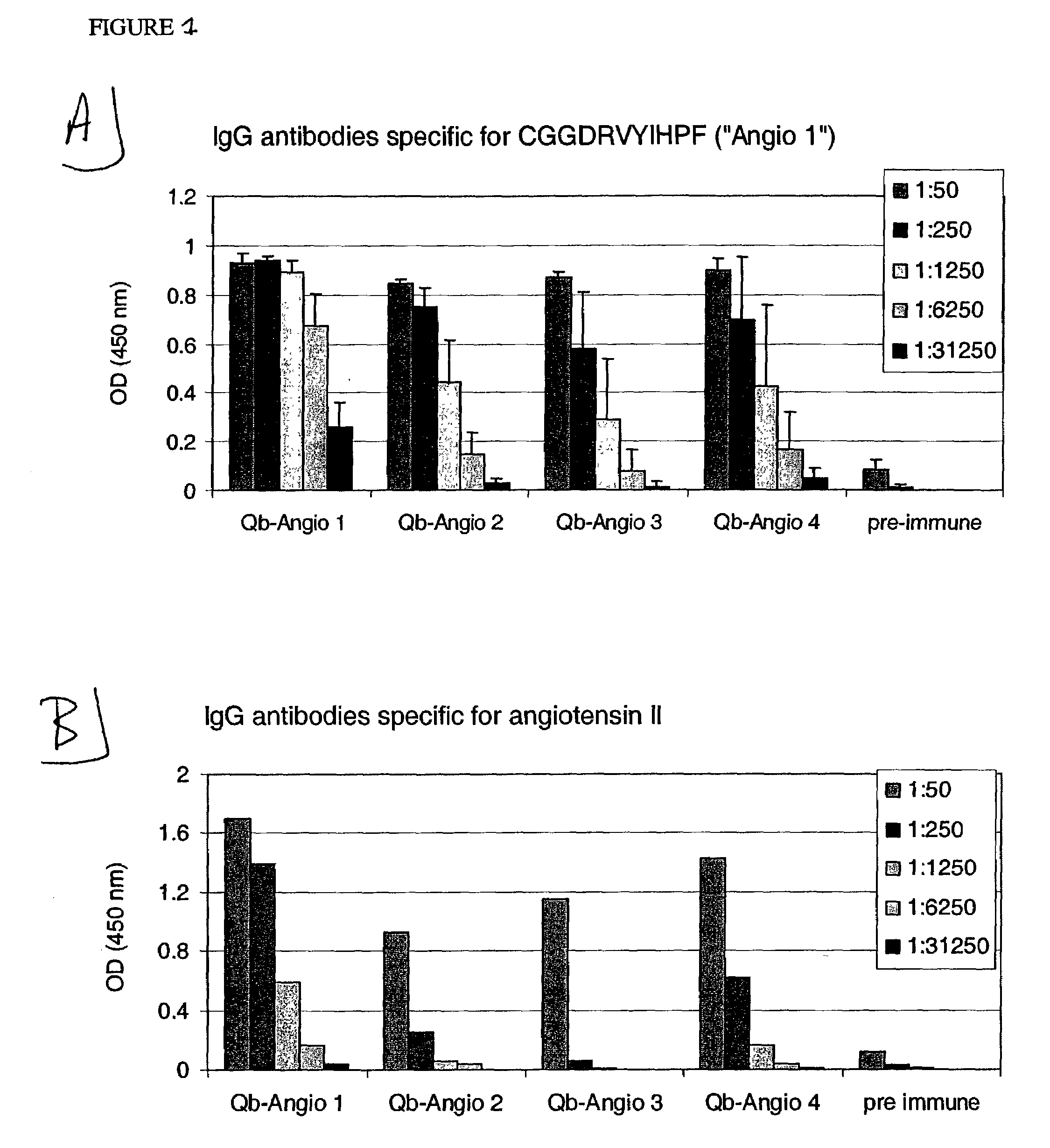
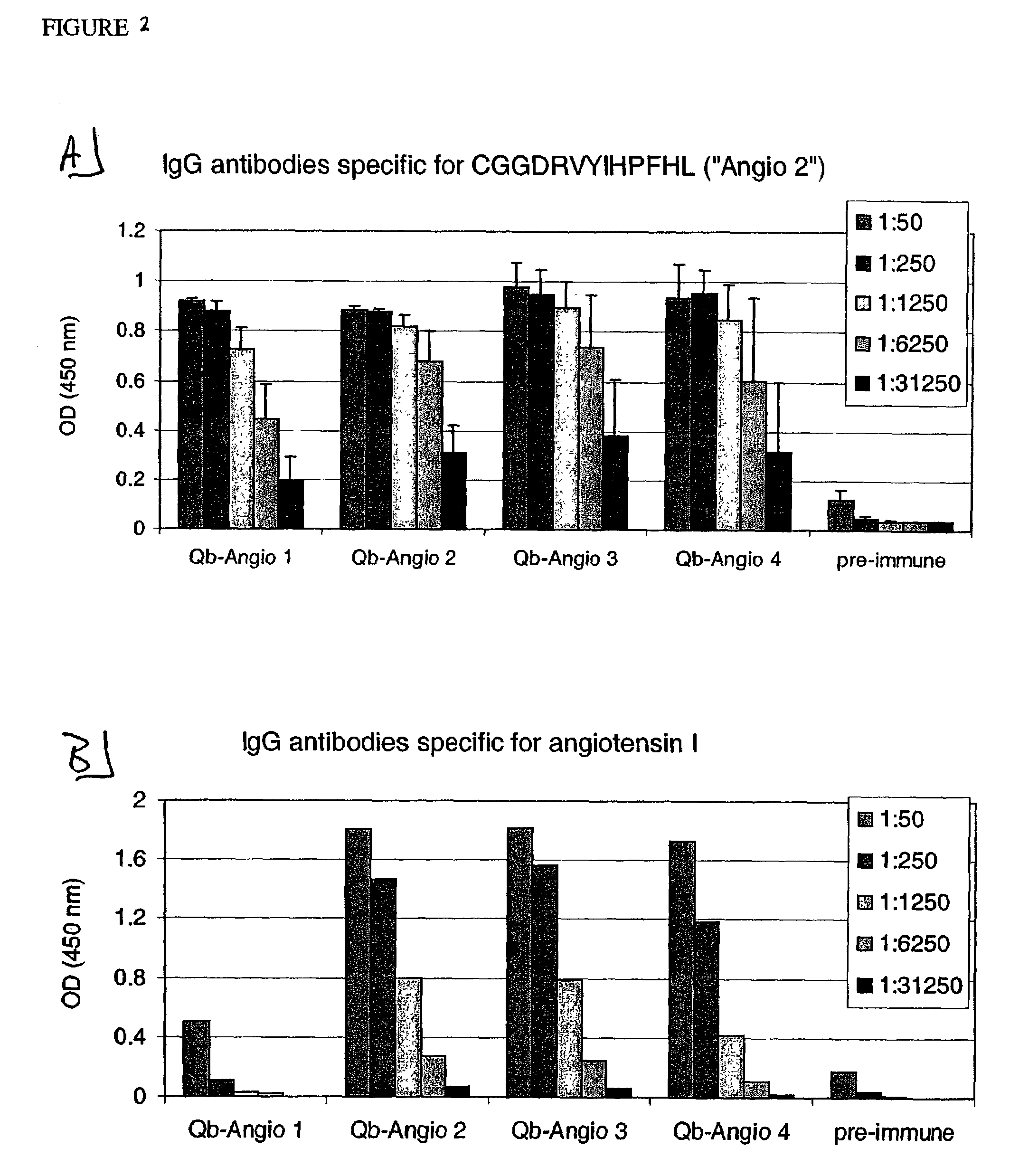
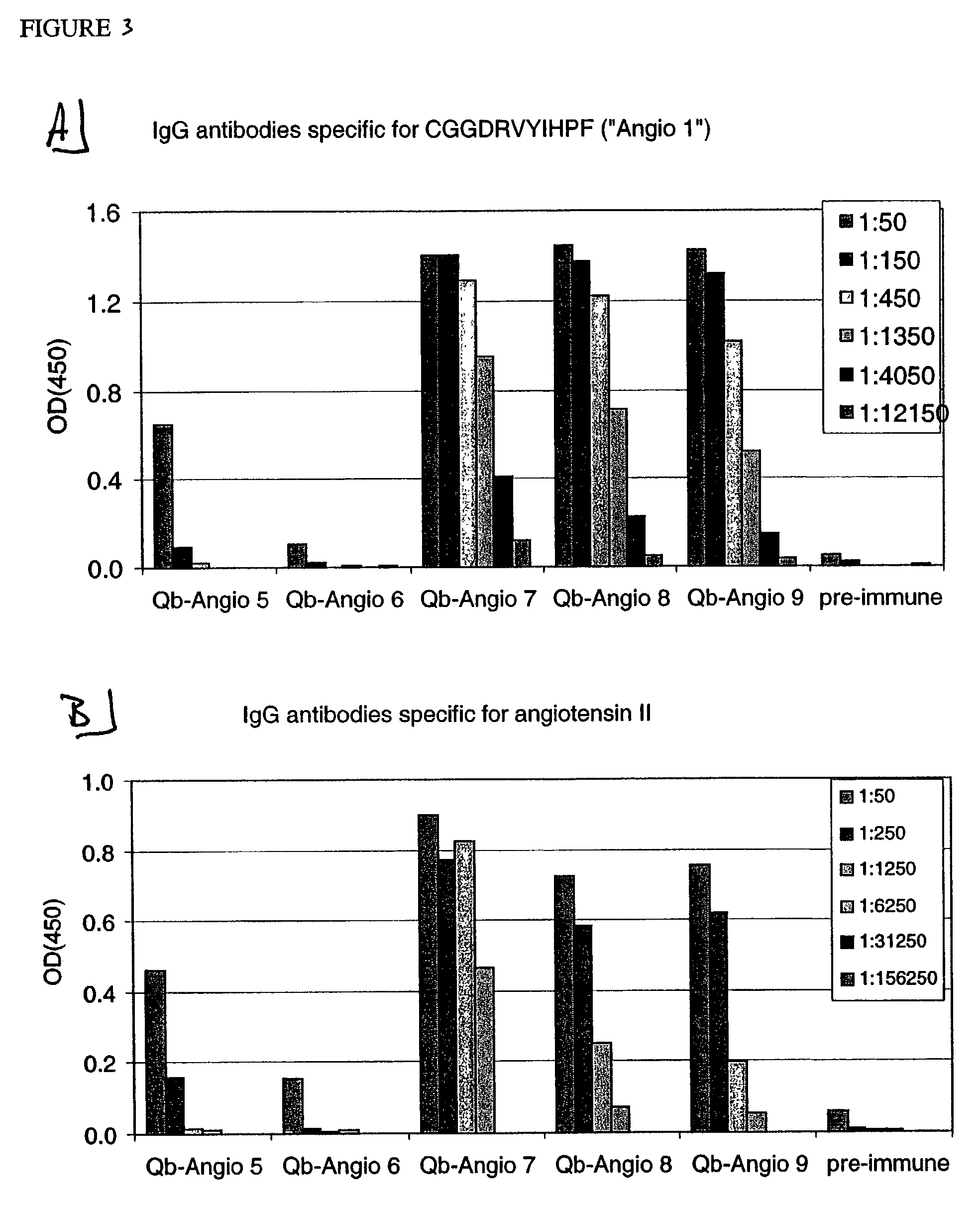
Angiotensin peptide-carrier conjugates and uses thereof
InactiveUS7115266B2Promote formationImprove complianceVirusesPeptide/protein ingredientsVirus-like particleAngiotensinogen mrna
The present invention provides conjugates of peptide derivatives of the mammalian peptide hormones angiotensinogen, angiotensin I and angiotensin II, presented in a repetitive scaffold by coupling the peptide derivatives to a carrier, particularly a virus-like particle (VLP). The invention also provides methods of producing such conjugates, and immunotherapeutic uses of the resulting immunogen conjugates for the therapy and prophylaxis of conditions associated with the renin-activated angiotensin system.
Owner:CYTOS BIOTECHNOLOGY AG
Method for preparing viral particles with cyclic dinucleotide and use of said particles for inducing immune response
ActiveUS20160074507A1Enhance immune responseInactivation/attenuationVertebrate cellsVirus-like particlePyrimidine Nucleotides
The present invention relates to methods for preparing virus-like particles comprising immunogenic cyclic dinucleotides.
Owner:INSTITUT CURIE +1
Foot-and-mouth disease virus capsid protein tandem coexpressions and virus-like particle preparation method
ActiveCN104404074AHigh activityNatural binding activityBacteriaInactivation/attenuationEscherichia coliVirus-like particle
The invention relates to escherichia coli-derived single-plasmid-tandem soluble coexpression foot-and-mouth disease virus capsid proteins VP0 (which is a VP4 and VP2 fusion gene), VP1 and VP3, and a foot-and-mouth disease virus capsid protein virus-like particle preparation method. Foot-and-mouth disease virus capsid protein virus-like particles can be used for preparation of a foot-and-mouth disease vaccine. According to the method, a plurality of aspects of escherichia coli-derived soluble coexpression foot-and-mouth disease virus capsid protein are studied, by comprehensive use of tandem coexpression and SUMO(suggested upper merged ontology) technology with a tag for soluble coexpression of the foot-and-mouth disease virus capsid proteins VP0 (which is the VP4 and VP2 fusion gene), VP1 and VP3, the ultimate objective protein accounts for about 20% of total bacterial protein, and the foot-and-mouth disease virus capsid proteins obtained by purification can be successfully assembled into the virus like particles.
Owner:SA BIOTECH (SUZHOU) PTE LTD
EV71virus-like particles as well as preparation method and application thereof
ActiveCN103255163AEfficient expressionGrow fastFungiSsRNA viruses positive-senseIon exchangeHigh pressure
Owner:BEIJING MINHAI BIOTECH +2
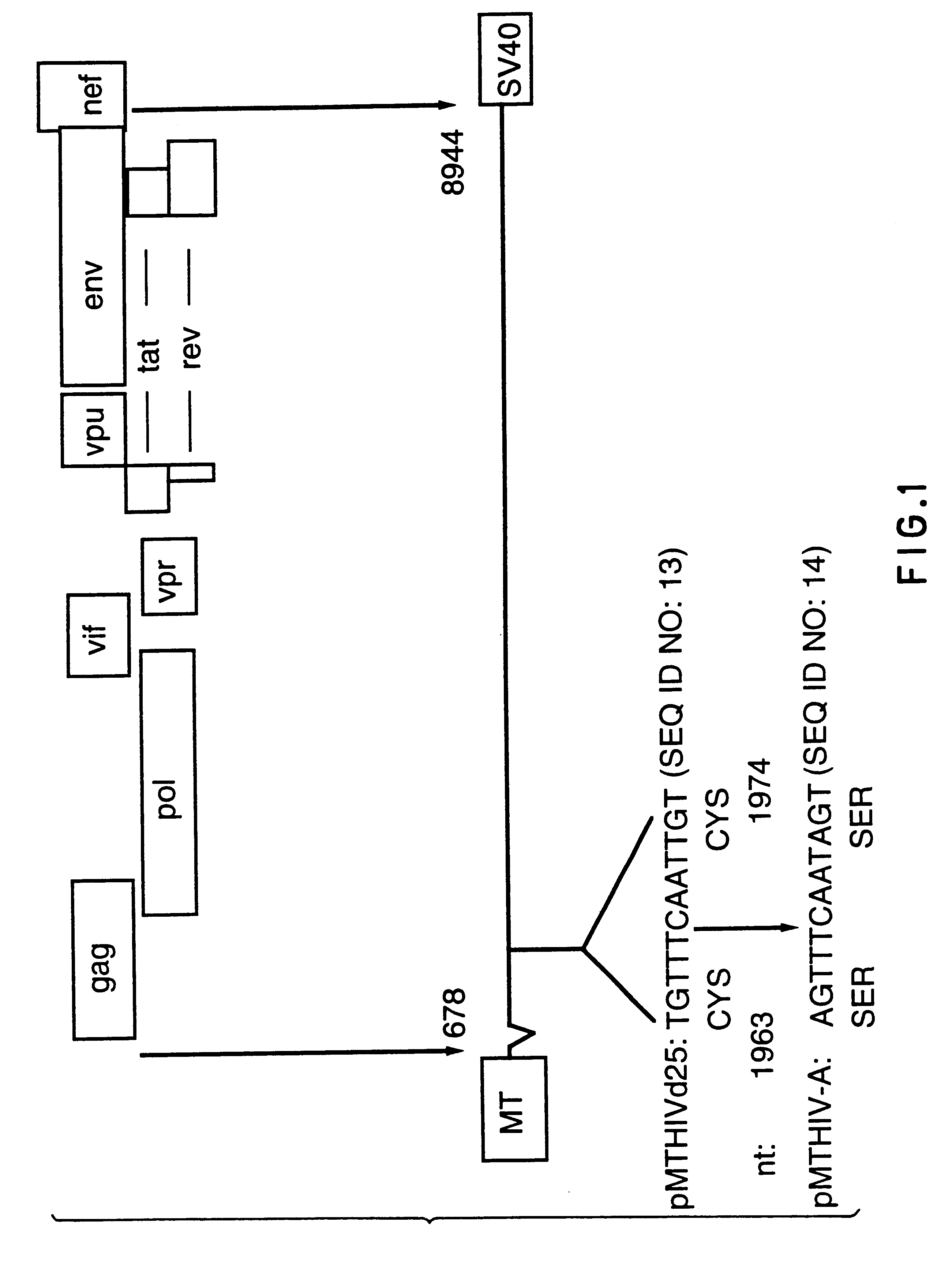
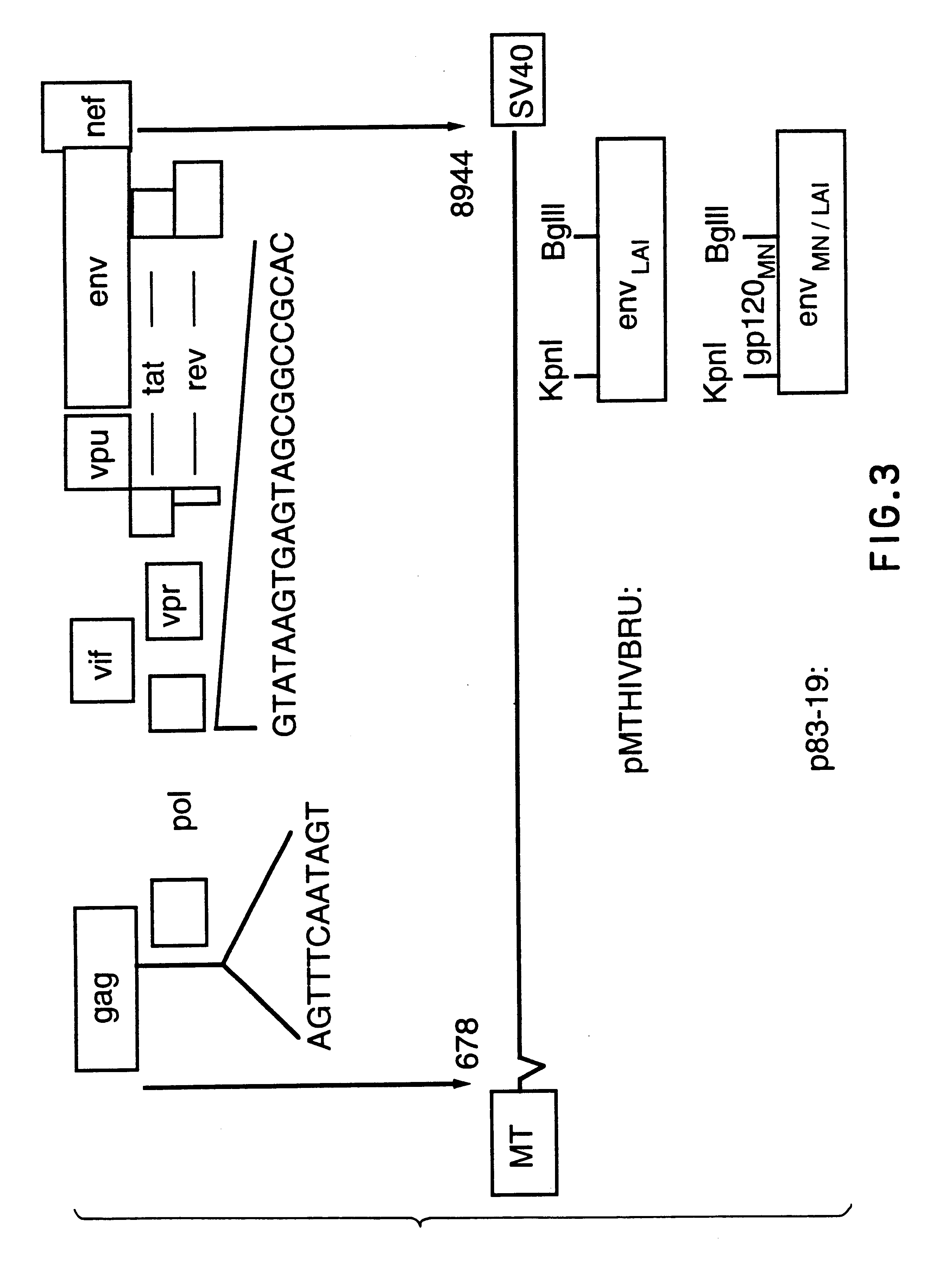
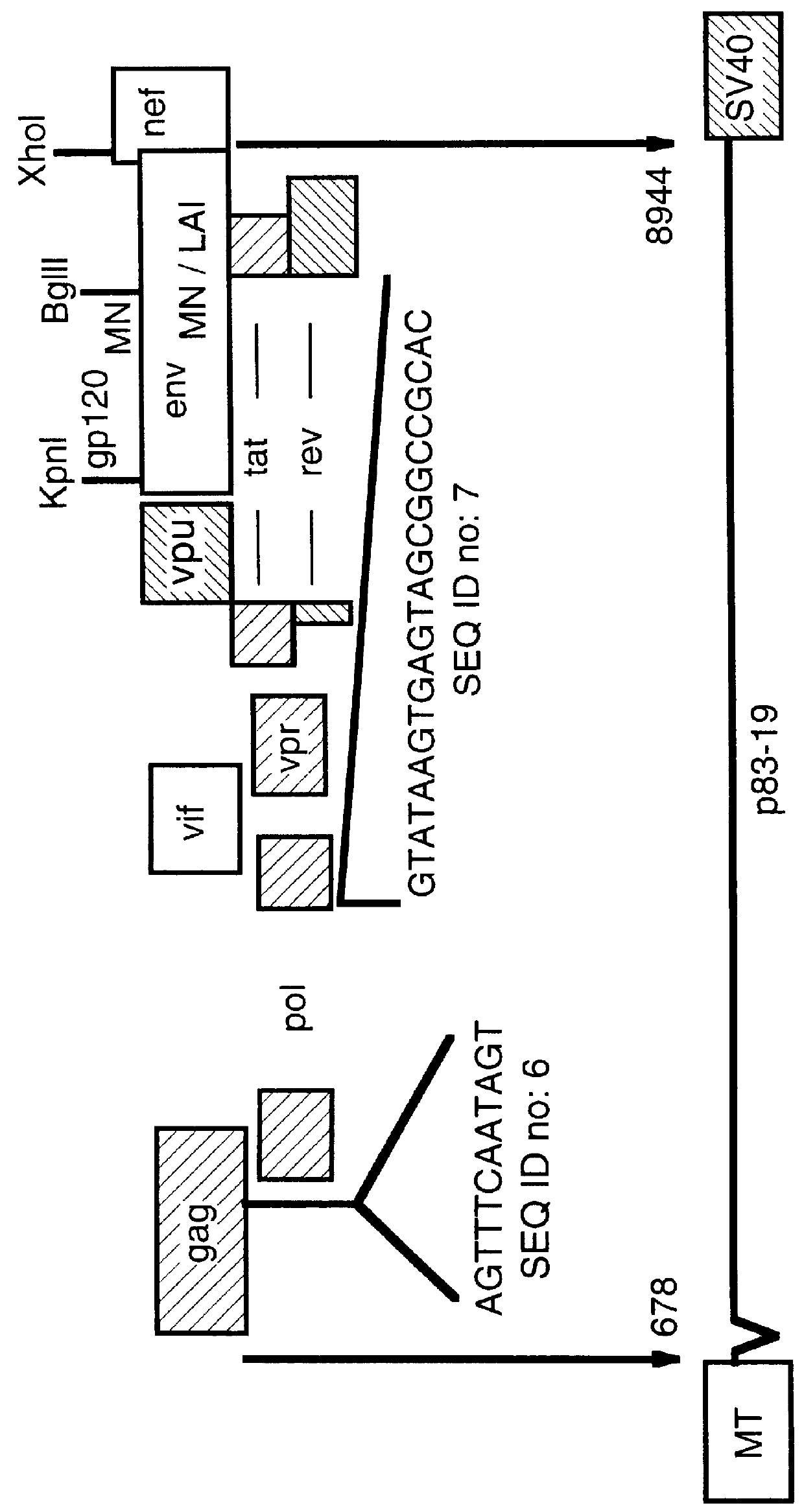
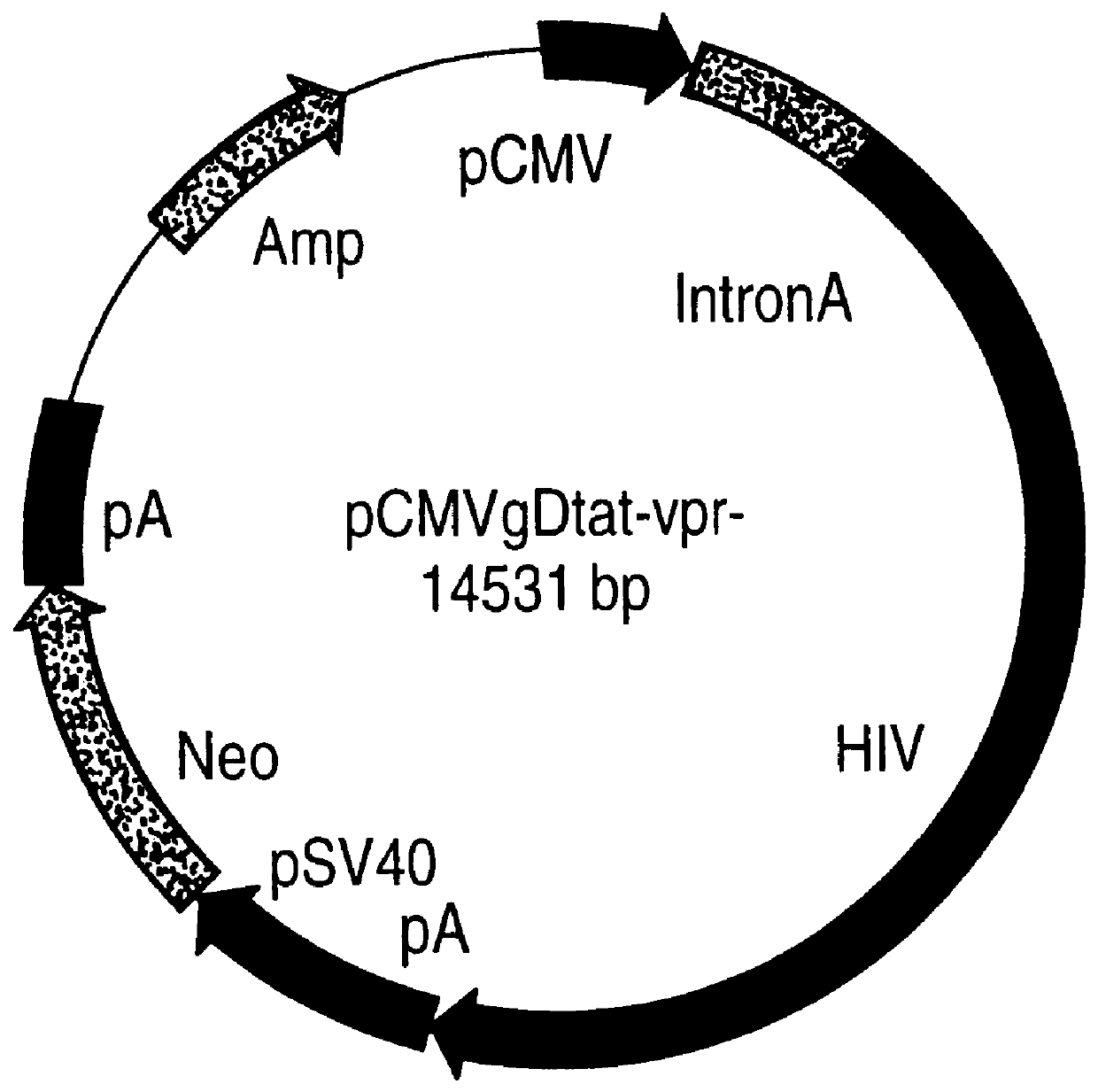
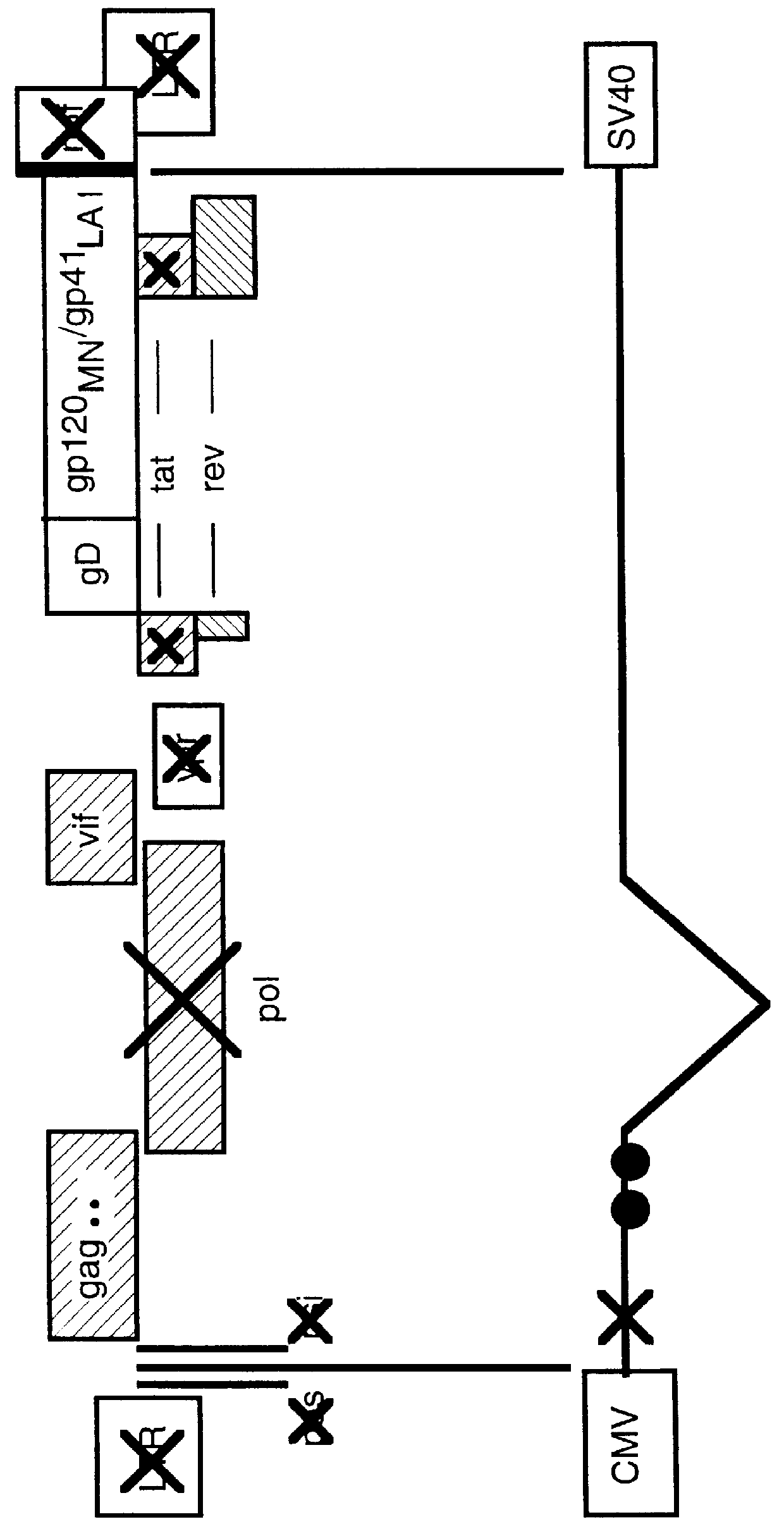
Constitutive expression of non-infectious HIV-like particles
Non-infectious, non-replicating immunogenic HIV-like particles are produced by stable longn-term constitutive expression in mammalian cells by eliminating elements toxic to the mammalian cells. An expression vector contains a nucleic acid molecule comprising a modified HIV genome devoid of long terminal repeats and wherein Tat and vpr sequences are functionally disabled and a constitutive promoter operatively connected to the modified HIV genome for constitutive expression of the modified genome to produce the HIV-like particles.
Owner:CONNAUGHT LAB
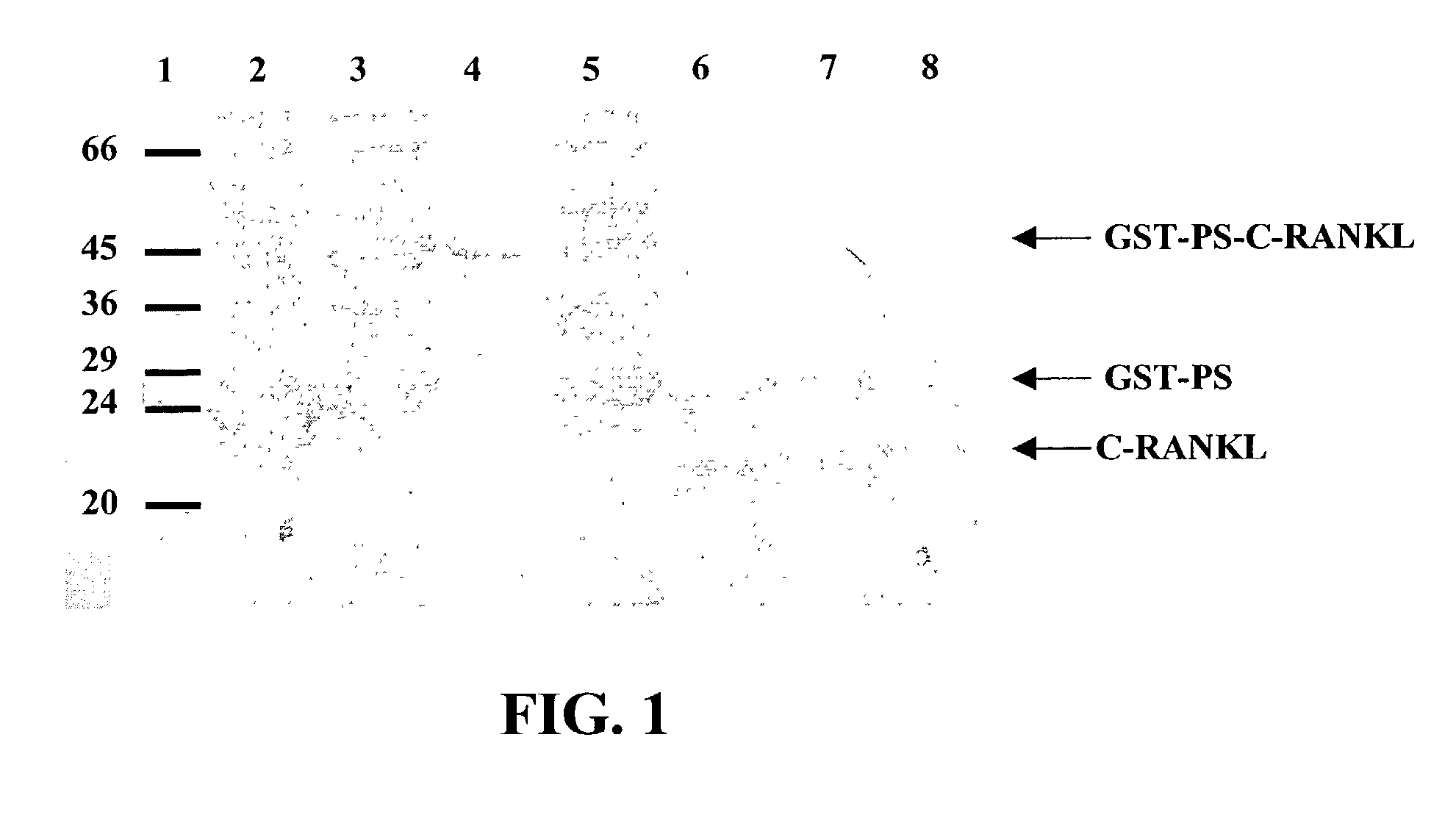
Antigen arrays for treatment of bone disease
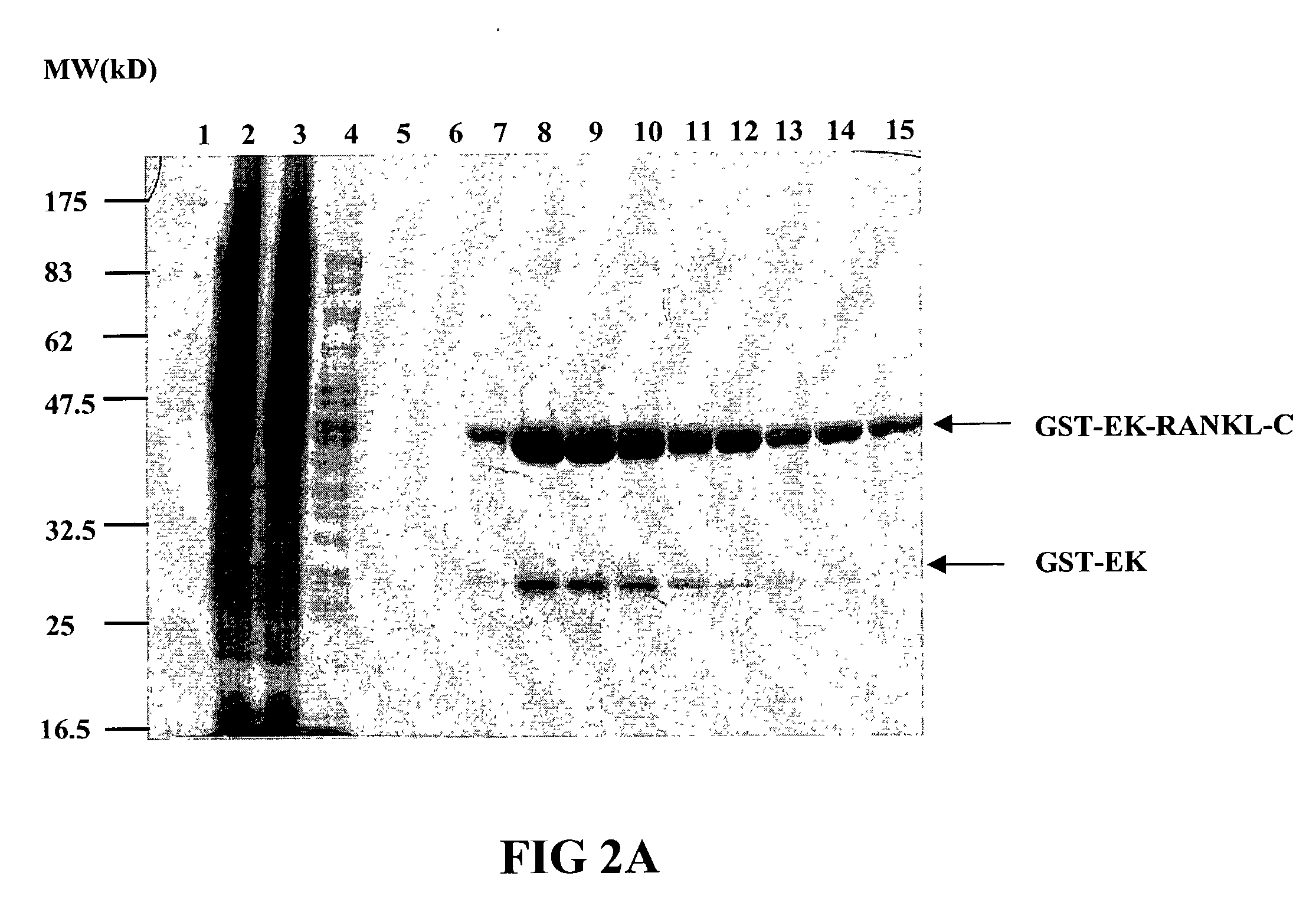
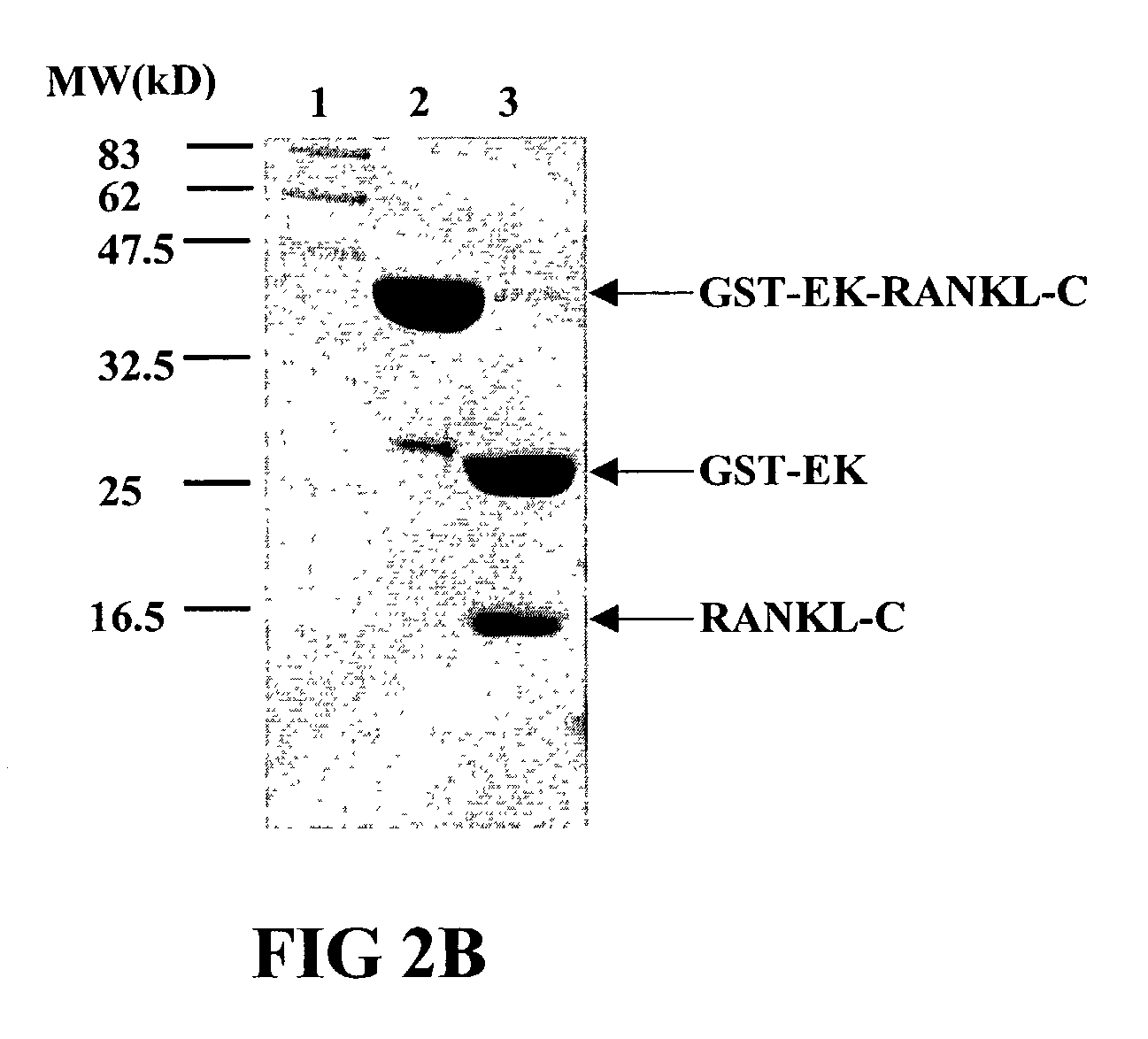
InactiveUS7128911B2Induce high titer of anti-RANKLEfficient inductionVirusesPeptide/protein ingredientsDiseaseRANKL Protein
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising an ordered and repetitive antigen or antigenic determinant array, and in particular a RANKL protein, RANKL fragment or RANKL peptide-VLP-array. More specifically, the invention provides a composition comprising a virus-like particle and at least one RANKL protein, RANKL fragment or RANKL peptide bound thereto. The invention also provides a process for producing the conjugates and the ordered and repetitive arrays, respectively. The compositions of the invention are useful in the production of vaccines for the treatment of bone diseases and as a pharmaccine to prevent or cure bone diseases and to efficiently induce immune responses, in particular antibody responses. Furthermore, the compositions of the invention are particularly useful to efficiently induce self-specific immune responses within the indicated context.
Owner:CYTOS BIOTECHNOLOGY AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com