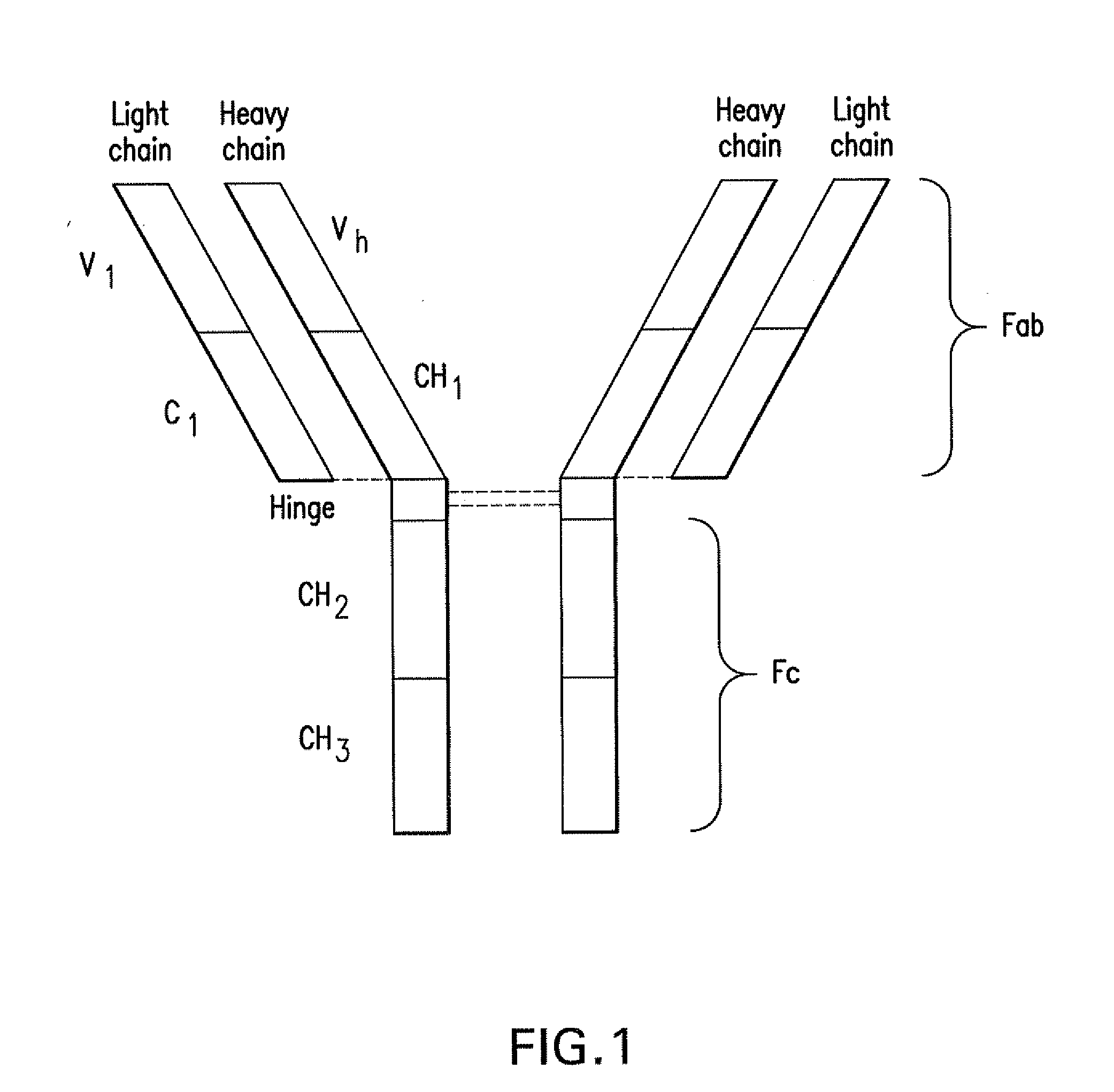
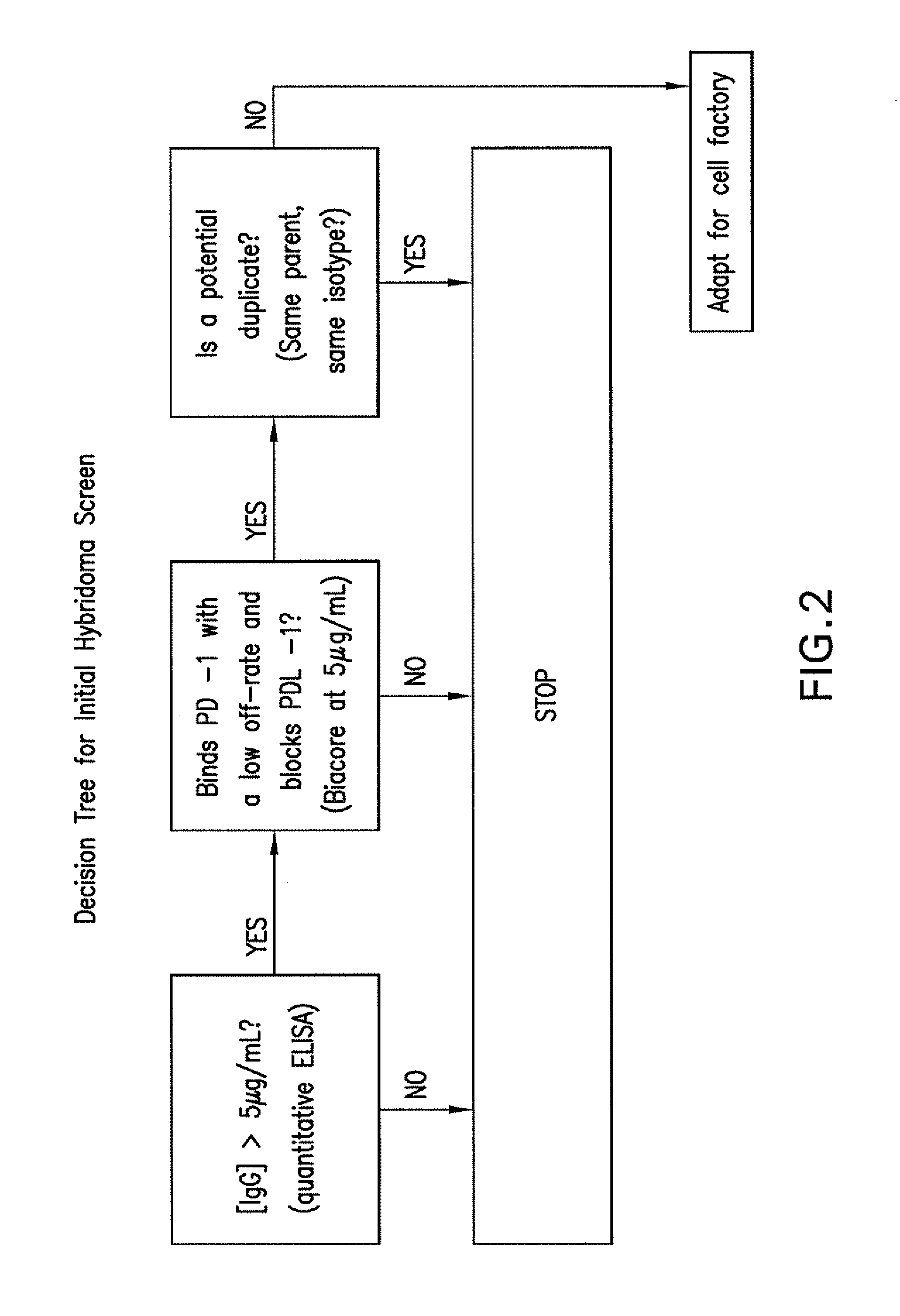
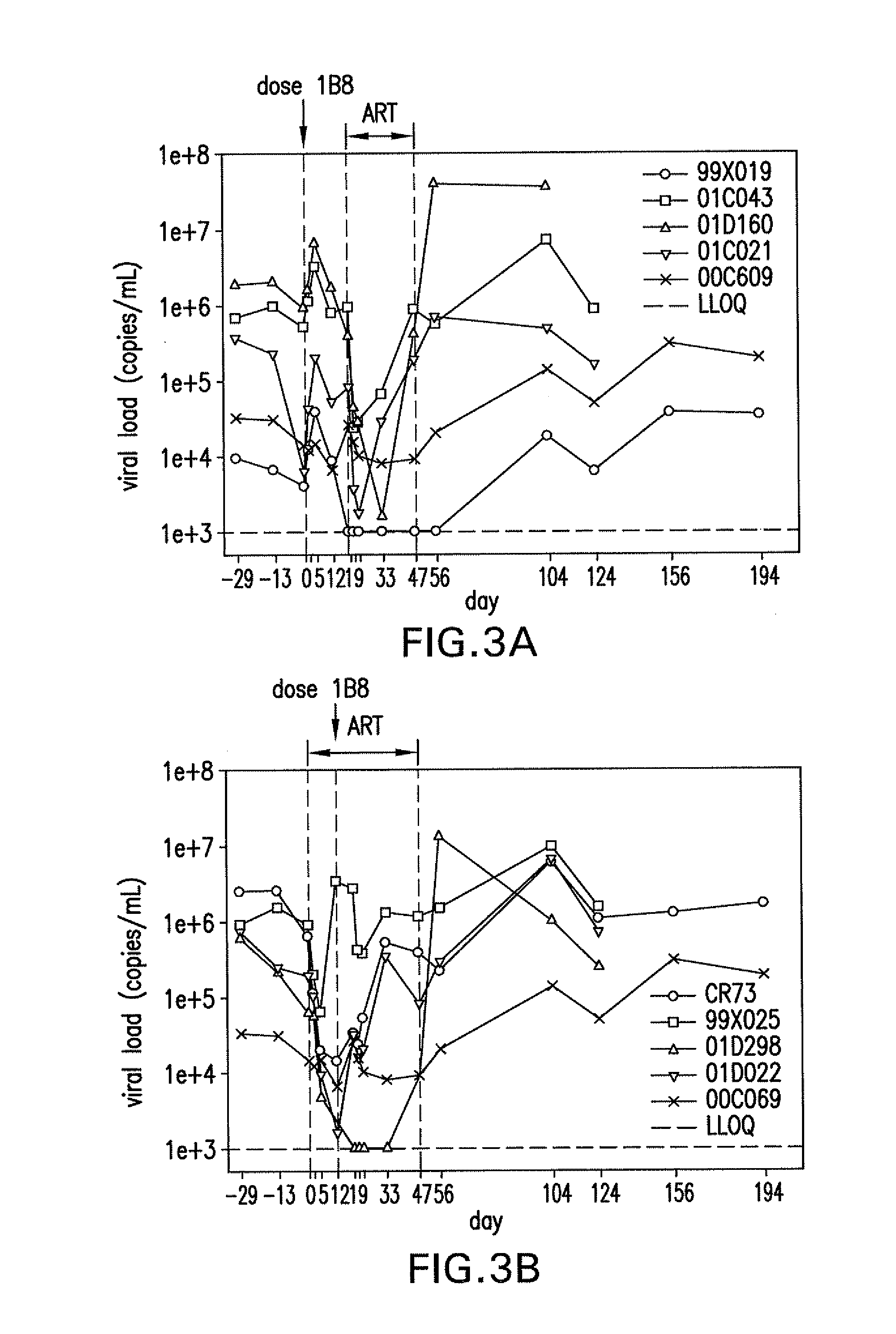
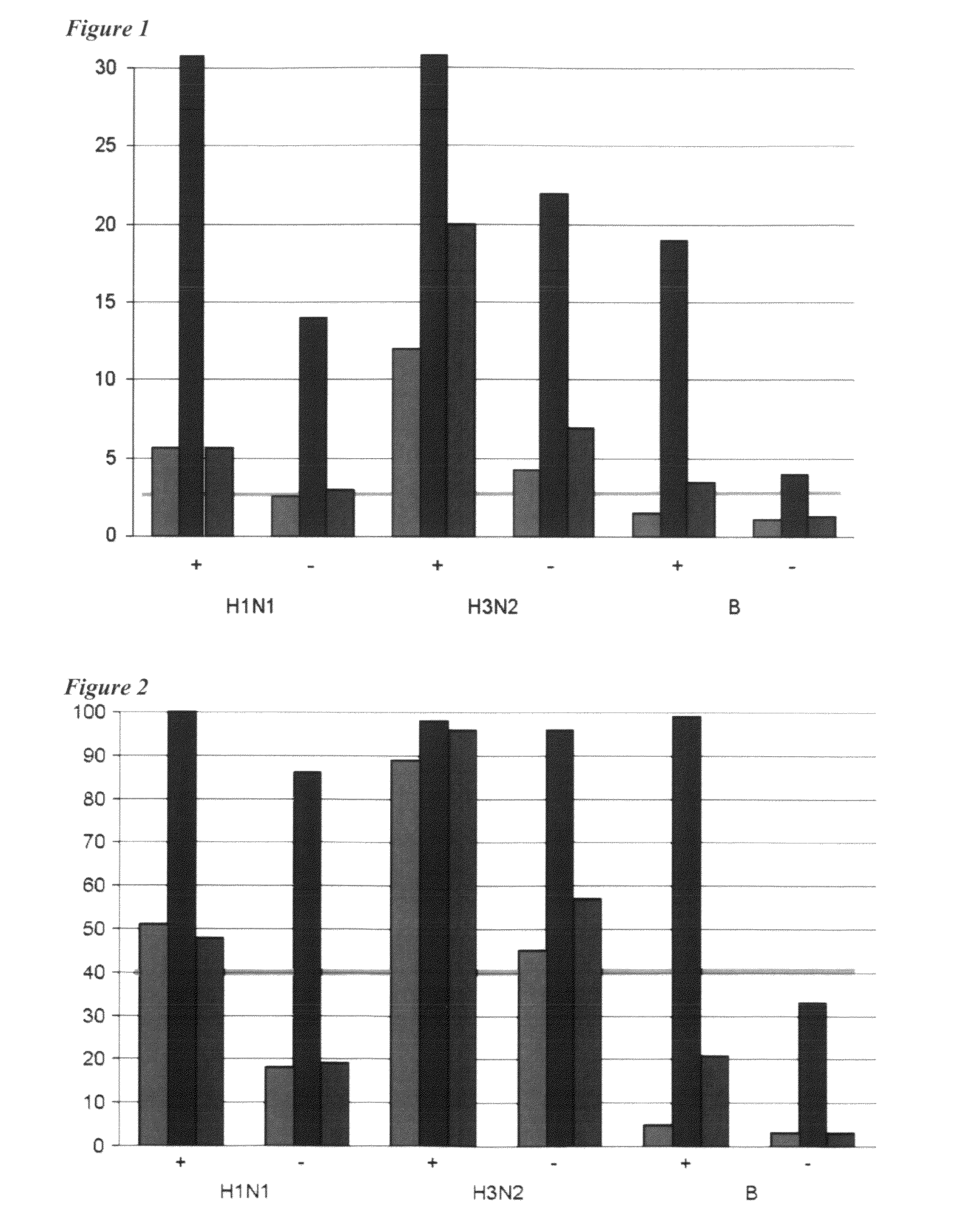
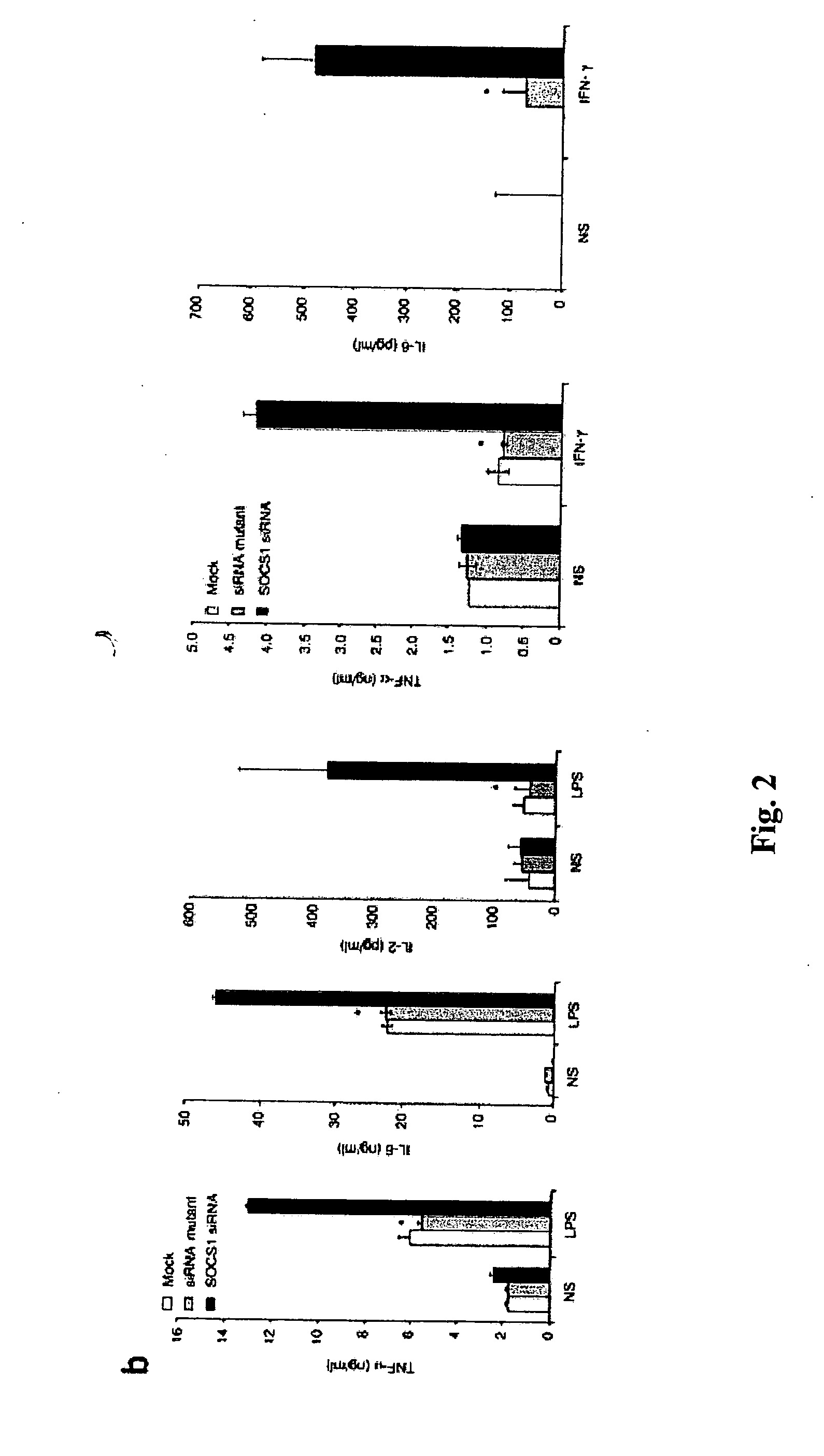
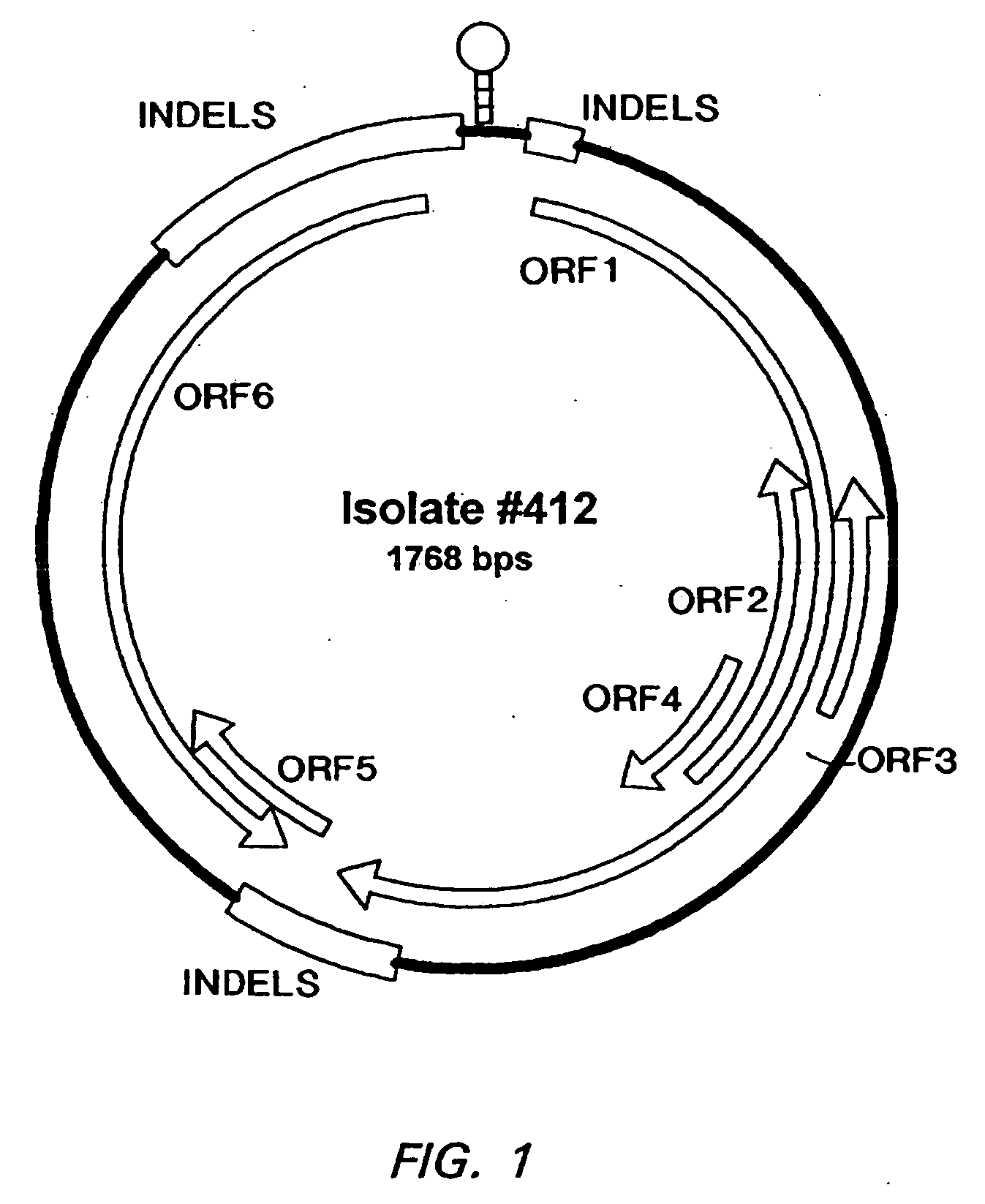
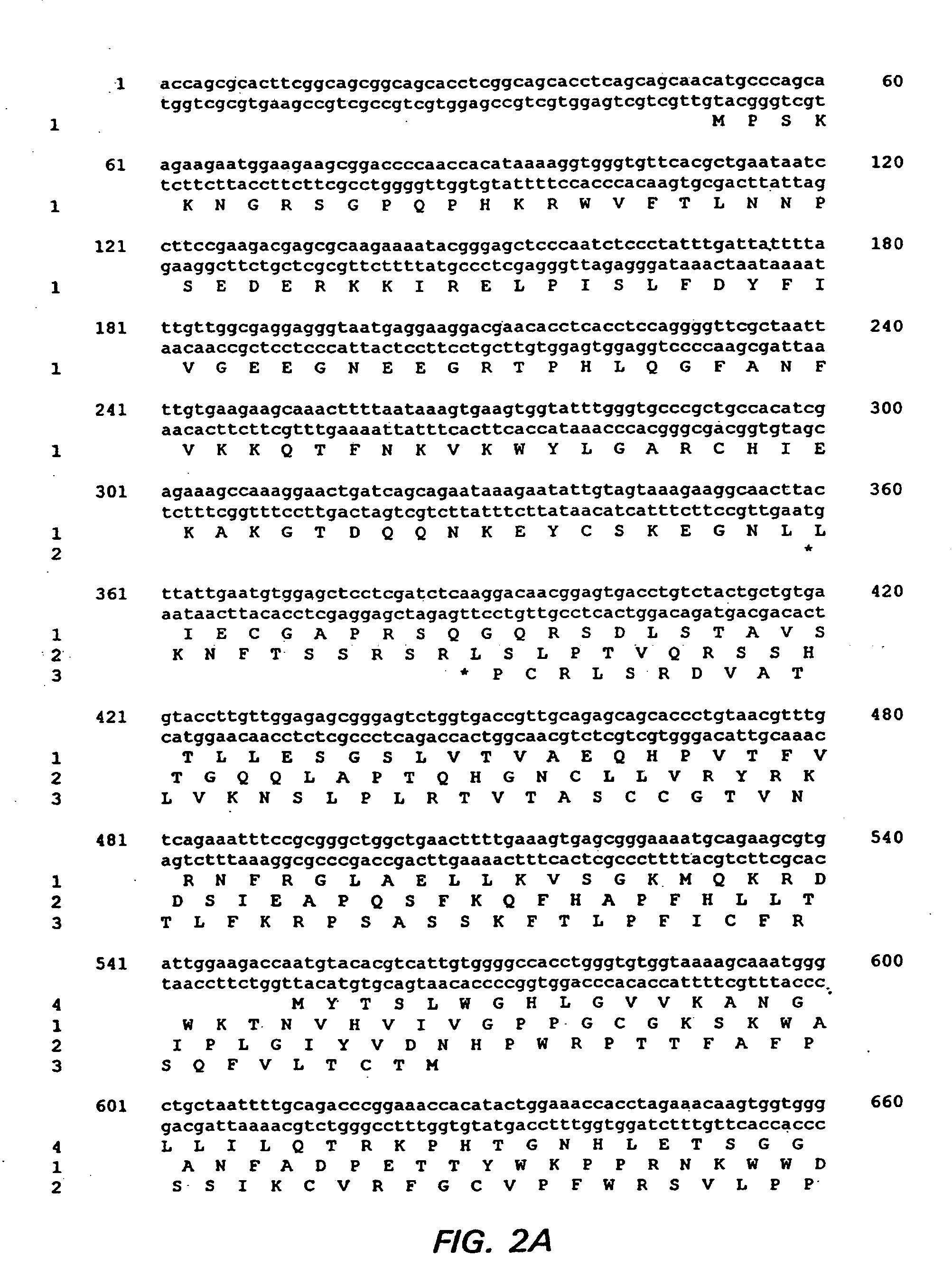
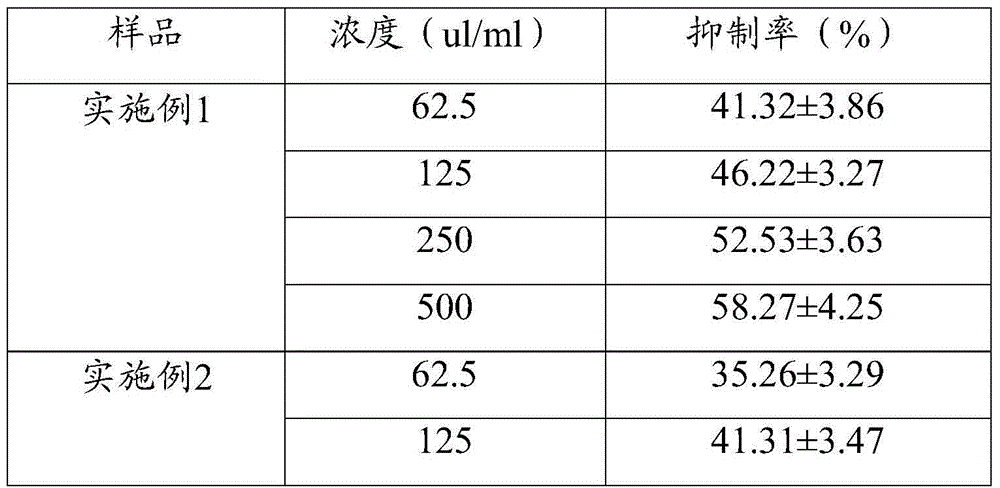
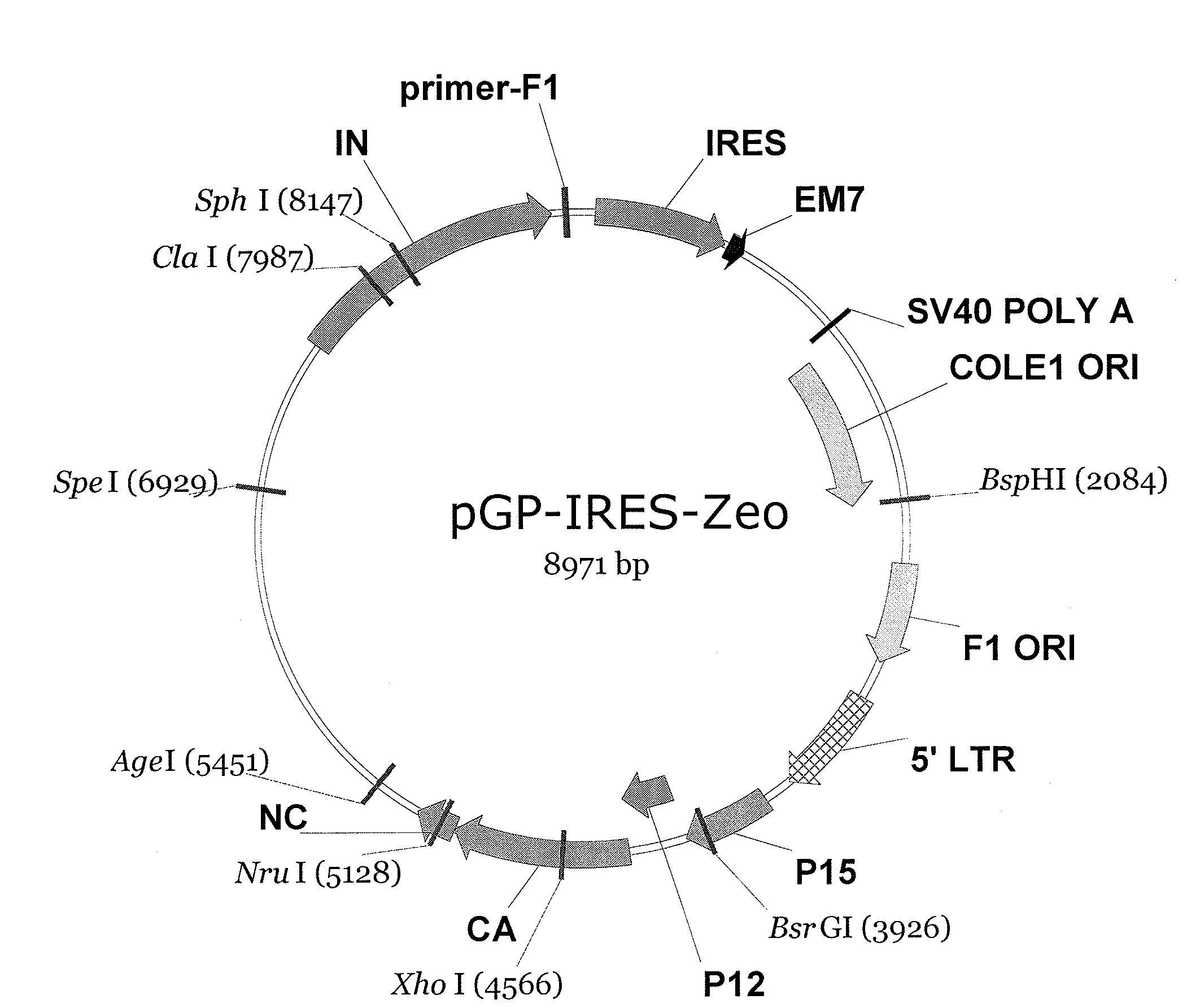
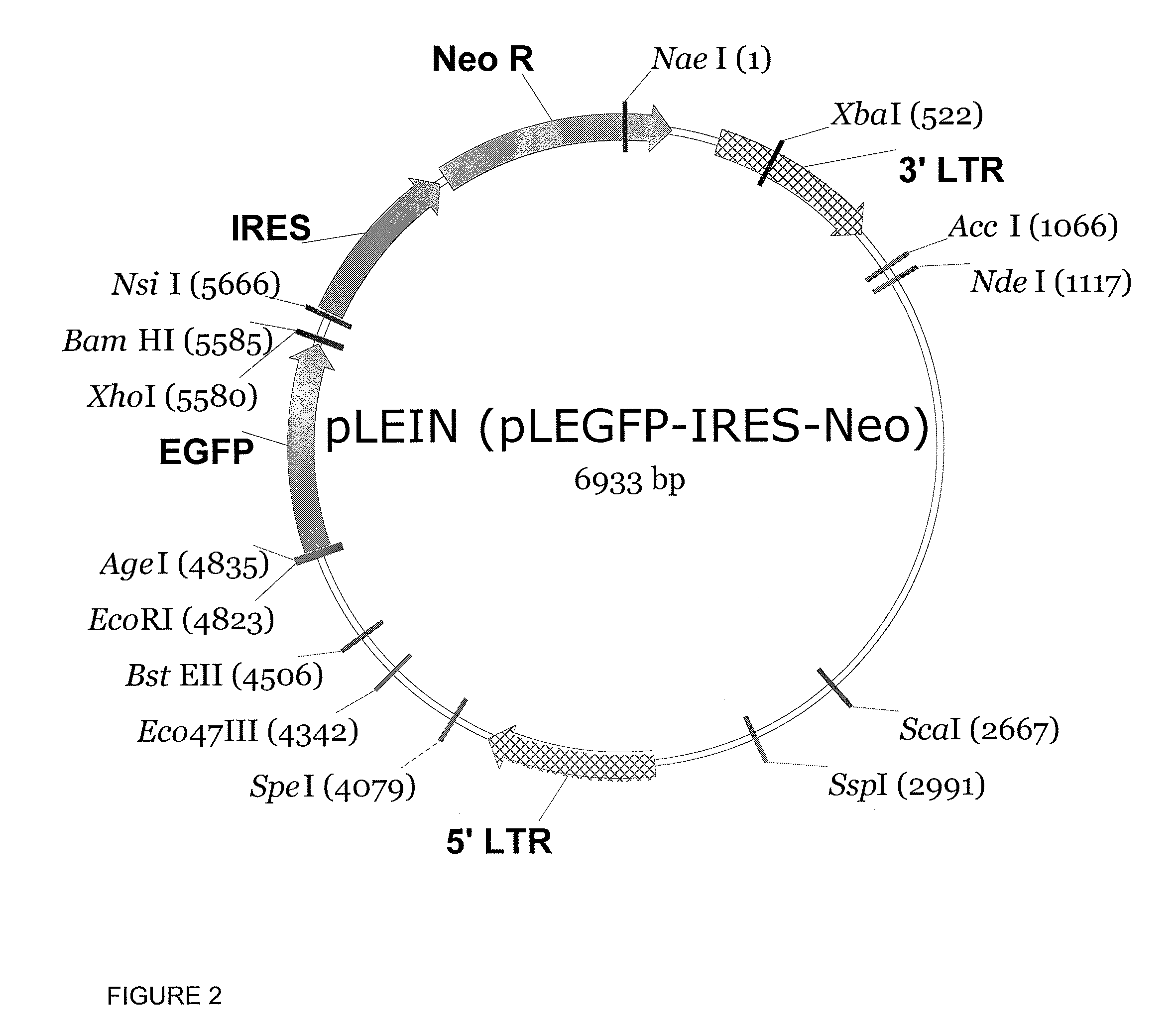
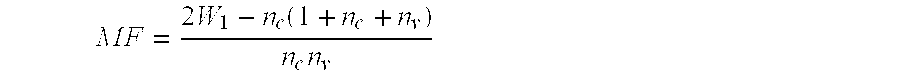Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
661 results about "Immunopotency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pd-1 binding proteins
ActiveUS20110008369A1Enhance host anti-microbial immunityImprove immunityAntibody mimetics/scaffoldsImmunoglobulins against animals/humansPD-L1Host immunity
The present invention features PD-1 binding proteins, a subset of which inhibits binding of PD-L1 to the PD-1 receptor. These binding proteins can be employed to modulate the immune system through the manipulation of the PD-1 signaling pathway, enhancing host immunity to treat infections and cancer.
Owner:MERCK SHARP & DOHME LLC
Adjuvanted influenza vaccines for pediatric use
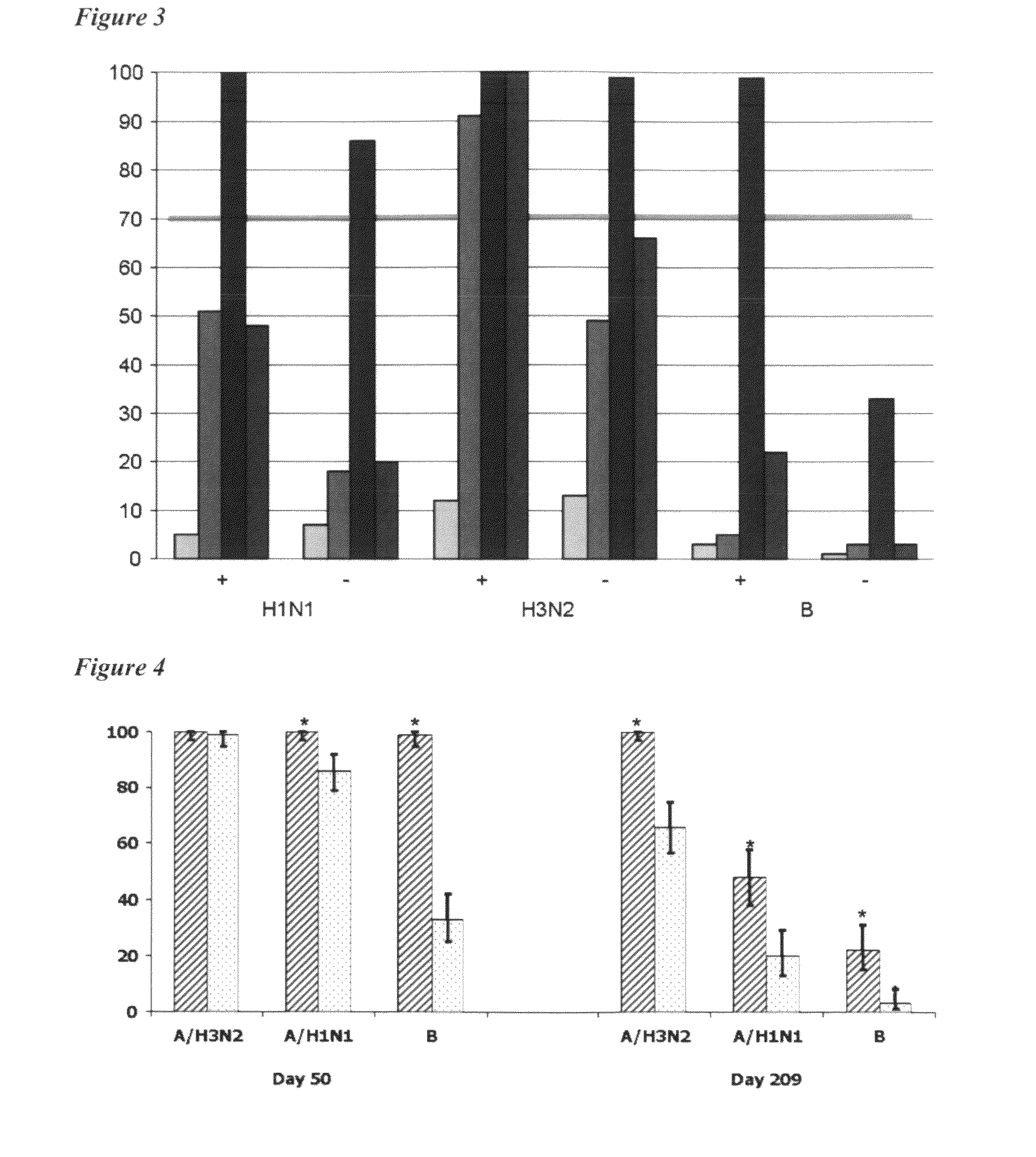
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Immunoconjugates Comprising Poxvirus-Derived Peptides and Antibodies Against Antigen-Presenting Cells for Subunit-Based Poxvirus Vaccines
InactiveUS20110064754A1Induces immunityHigh affinityViral antigen ingredientsImmunoglobulins against virusesTarget antigenPox virus
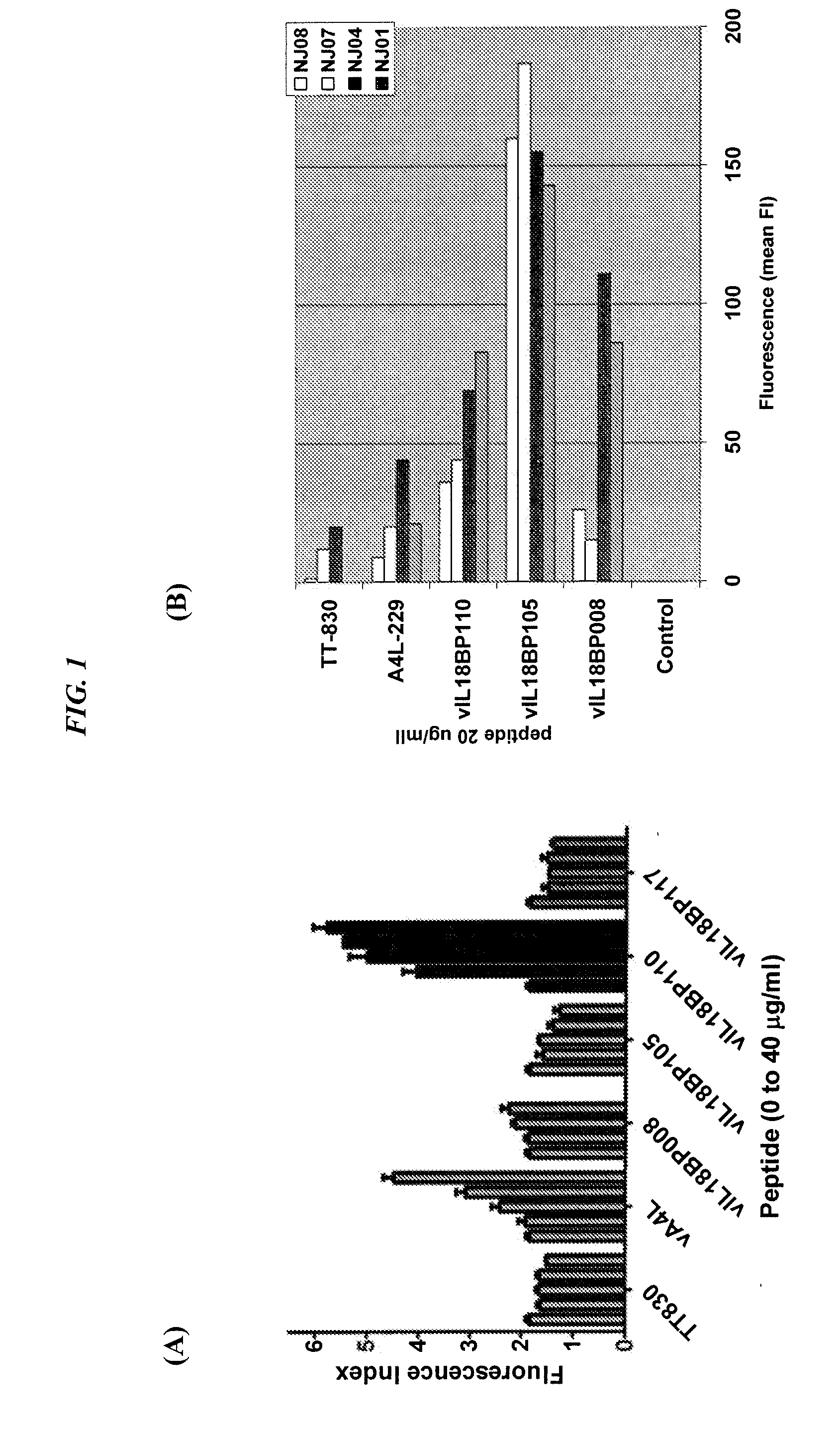
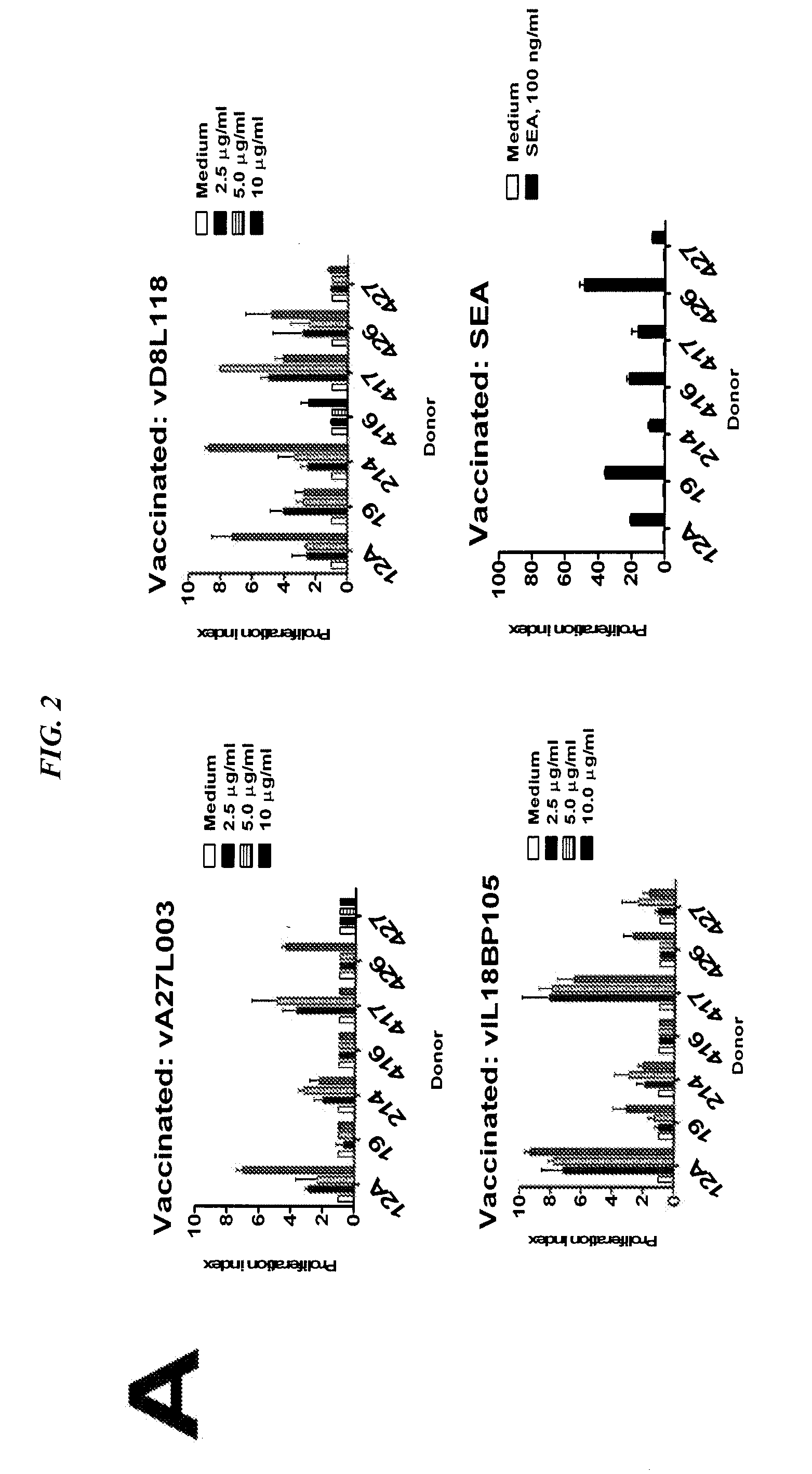
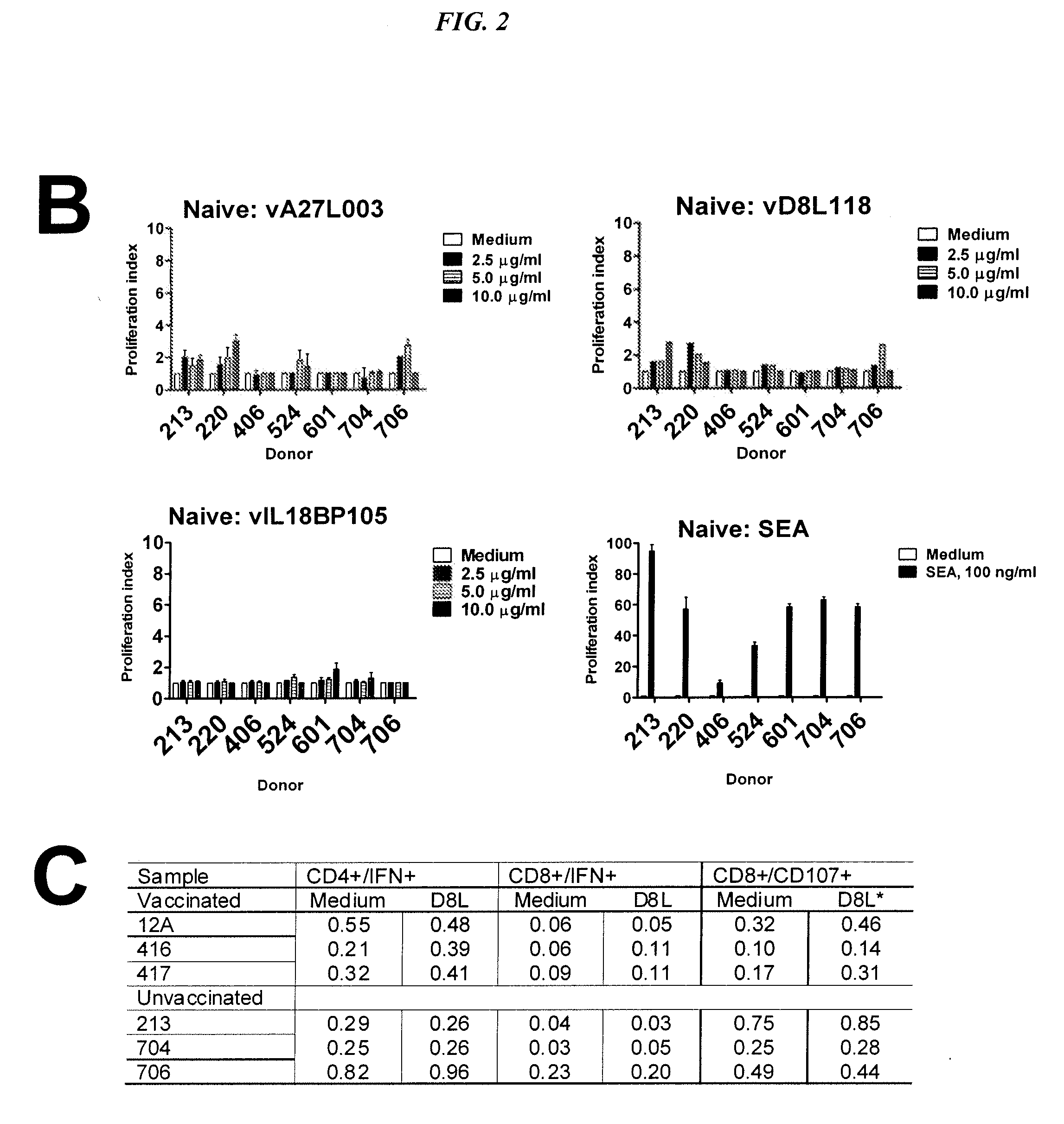
The present invention concerns methods and compositions for subunit-based vaccines for inducing immunity against poxvirus infections, such as smallpox. Preferred embodiments concern immunoconjugates comprising one or more subunit antigenic peptides attached to an antibody or fragment thereof that targets antigen-producing cells (APCs). More preferably, the antibody binds to HLA-DR and the antigenic peptide is from an immunomodulating factor, such as the viral IL-18 binding protein (vIL18BP). However, mixtures of antigenic peptides from different viral proteins may also be used. The vaccine is capable of inducing immunity against poxvirus without risk of disseminated infection in immunocompromised hosts or transmission to susceptible contacts.
Owner:CENT FOR MOLECULAR BIOLOGY & MEDICINE
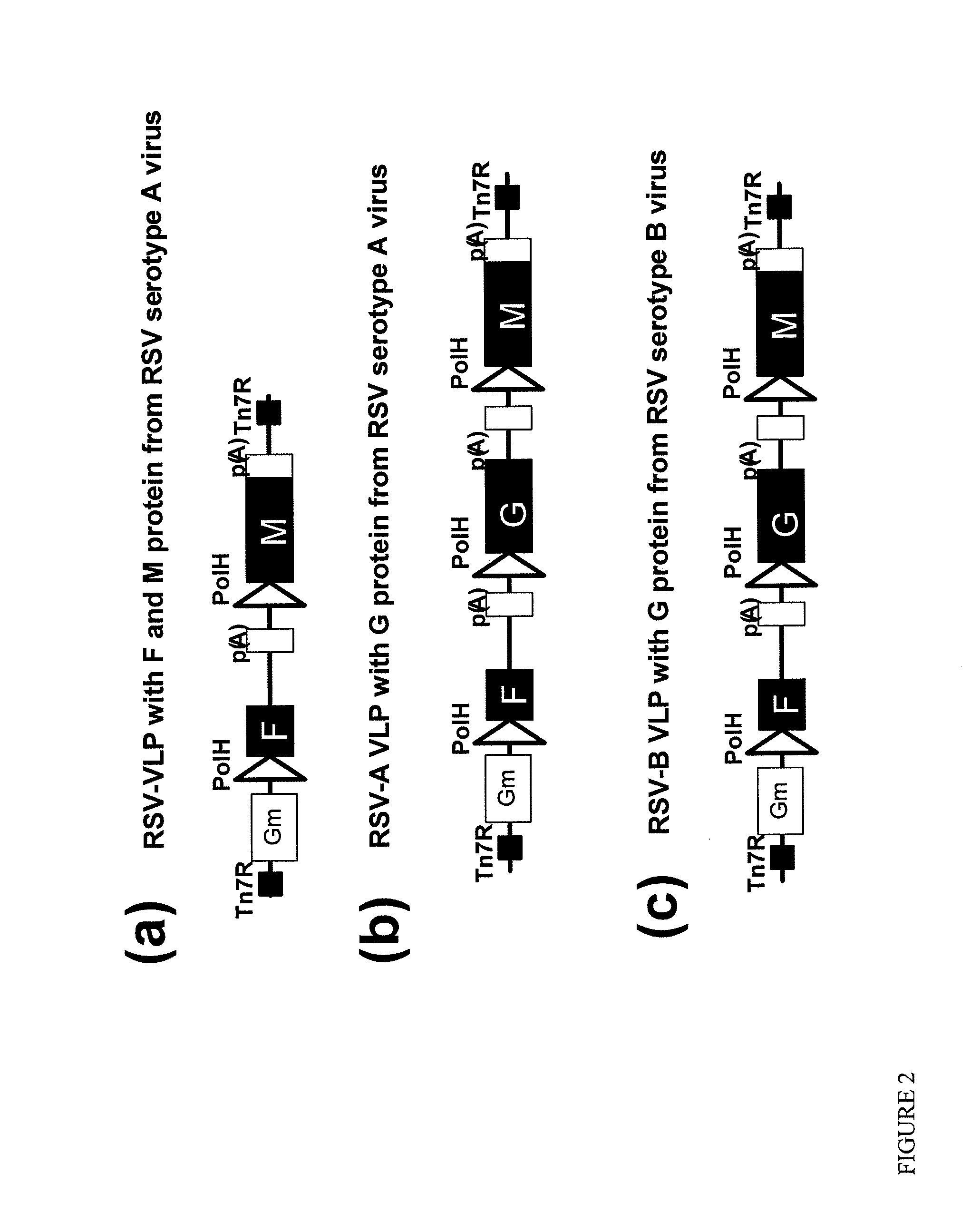
Respiratory syncytial virus-virus like particle (VLPS)
InactiveUS20080233150A1SsRNA viruses negative-senseViral antigen ingredientsVirus-like particleVertebrate
The present invention discloses and claims virus like particles (VLPs) that express and / or contains RSV proteins. The invention includes vector constructs comprising said proteins, cells comprising said constructs, formulations and vaccines comprising VLPs of the inventions. The invention also includes methods of making and administrating VLPs to vertebrates, including methods of inducing immunity to infections, including RSV.
Owner:NOVAVAX
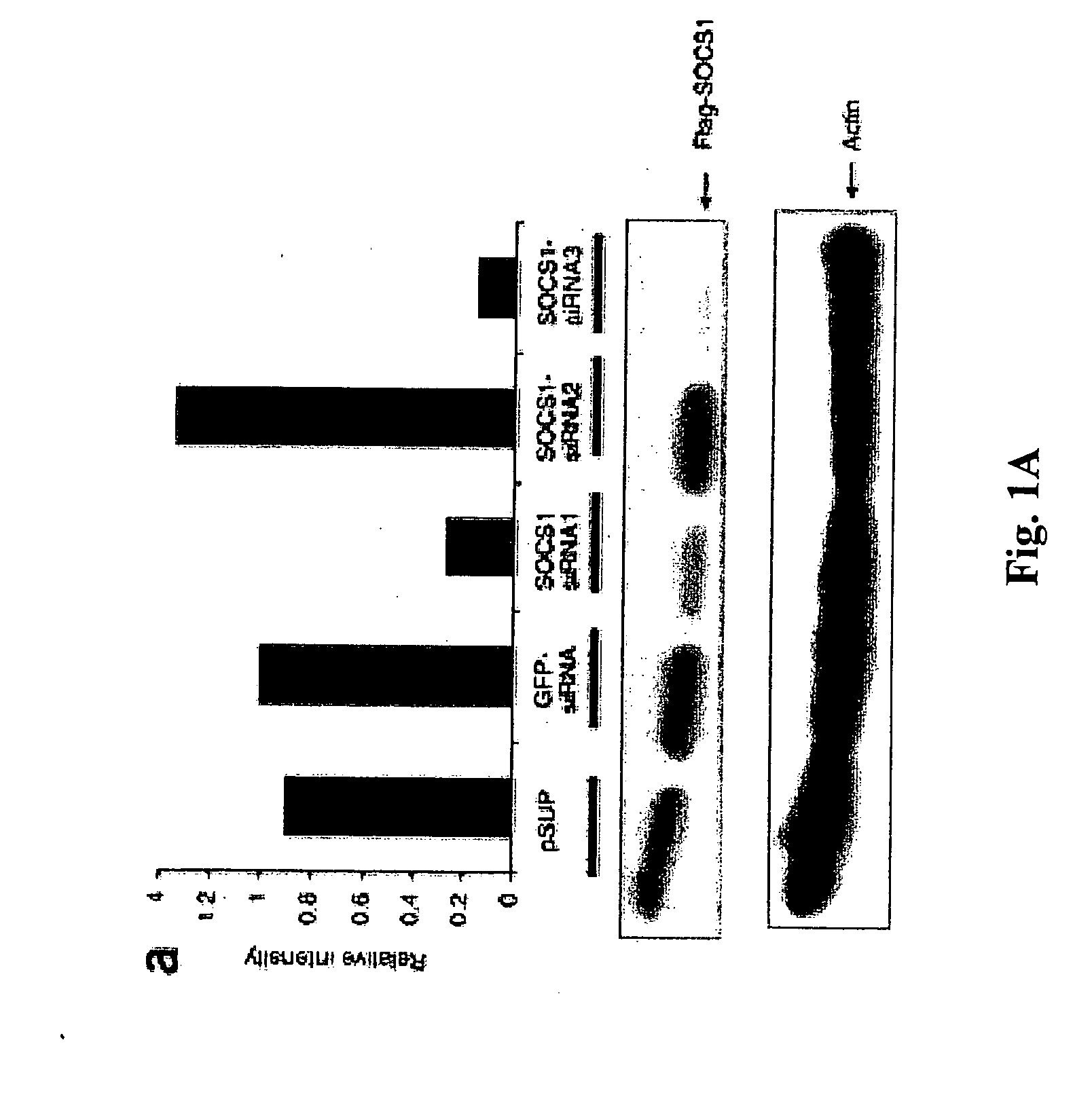
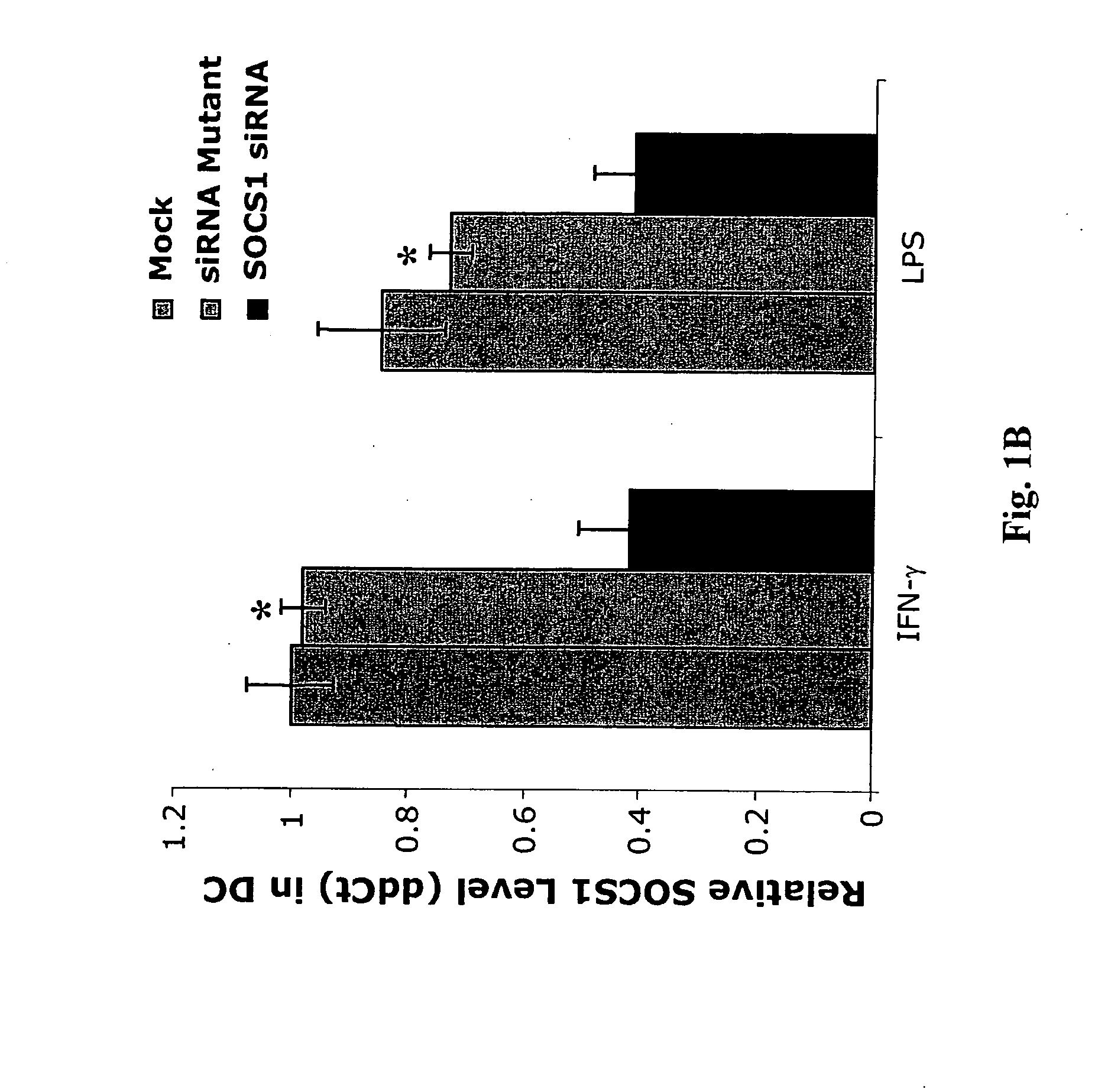
Modulation of negative immune regulators and applications for immunotherapy
ActiveUS20060292119A1Easy to integrateBiocideSsRNA viruses positive-senseVaccinationImmunocompetence
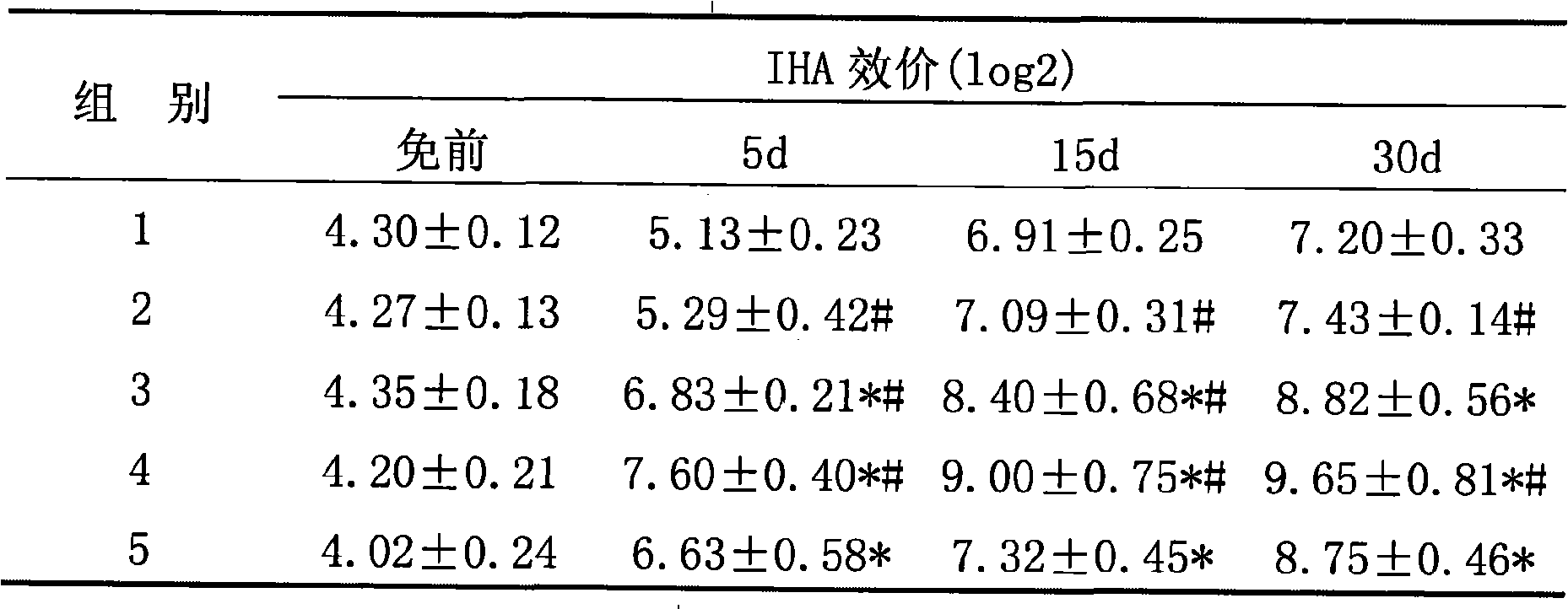
The invention includes compositions and methods for enhancing immunopotency of an immune cell by way of inhibiting a negative immune regulator in the cell. The present invention provides vaccines and therapies in which antigen presentation is enhanced through inhibition of negative immune regulators. The present invention also provides a mechanism to break self tolerance in tumor vaccination methods that rely on presentation of self tumor antigens.
Owner:BAYLOR COLLEGE OF MEDICINE
Porcine circovirus and Helicobacter combination vaccines and methods of use
InactiveUS20060029617A1Viral antigen ingredientsMicrobiological testing/measurementDiseasePorcine Circoviruses
The present invention is based on the discovery of novel species of the genus Helicobacter that are associated with gastro-esophageal ulceration in pigs. In particular, a novel species, H. cerdo, has been used as a source of antigenic material for the development of vaccine for the treatment of the gastro-esophageal disorders. Most advantageously, the novel Helicobacter and the porcine circoviruses associated with PMWS in pigs are useful for providing combination vaccines whereby immunogens derived from both types of pathogens may be codelivered to the target animal to stimulate the generation of protective antibodies and immunity. The invention, therefore, provides vaccines that are useful for the tratment of gastro-esophageal ulceration and PMWS in porcines. The present invention includes, therefore, multivalent immunogenic compositions and vaccines, multivaccine kits, and combined immunization or vaccination methods which make it possible to use such combined immunization or vaccination programmes.
Owner:MERIAL LTD
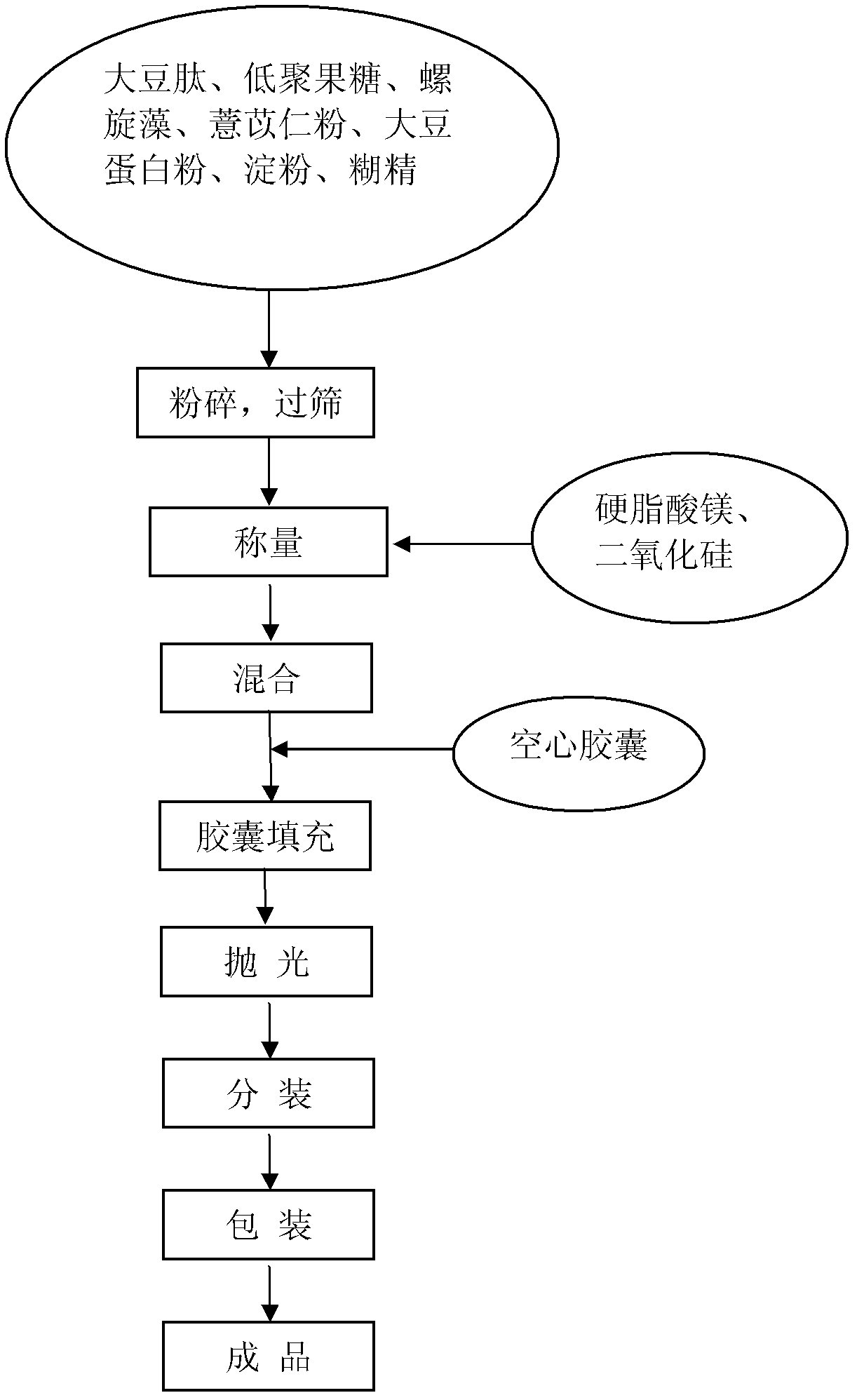
Healthcare food capable of strengthening immunity and preparing method thereof
The invention discloses a healthcare food capable of strengthening immunity. The healthcare food comprises, by weight percentage, 1% to 50% of soybean peptide, 0.5% to 30% of protein powder, 1% to 30% of fructo-oligosaccharide, 0.1% to 30% of coix seed powder, 0.1% to 10% of spirulina and 15% to 85% of auxiliary materials. According to the healthcare food, materials which are nutritious and effective are selected to be subjected to scientific formulating prescription, nutrition, safety, palatability and efficacy are taken as principles, and effects of strengthening cell immune function and humoral immune function are achieved, thereby immunity strengthening is achieved.
Owner:PERFECT CHINA
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
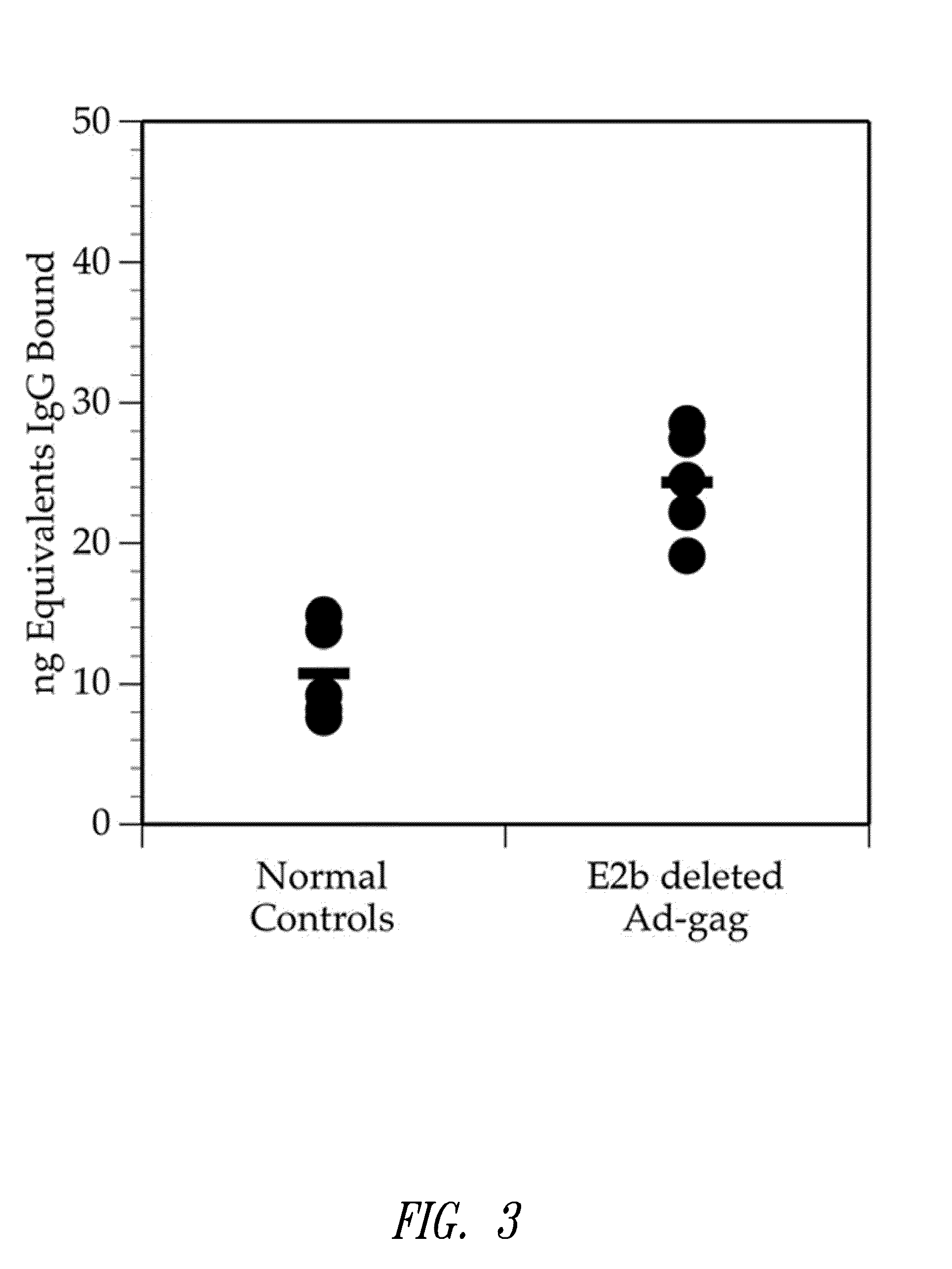
Sequential administration of a replication defective adenovirus vector in vaccination protocols
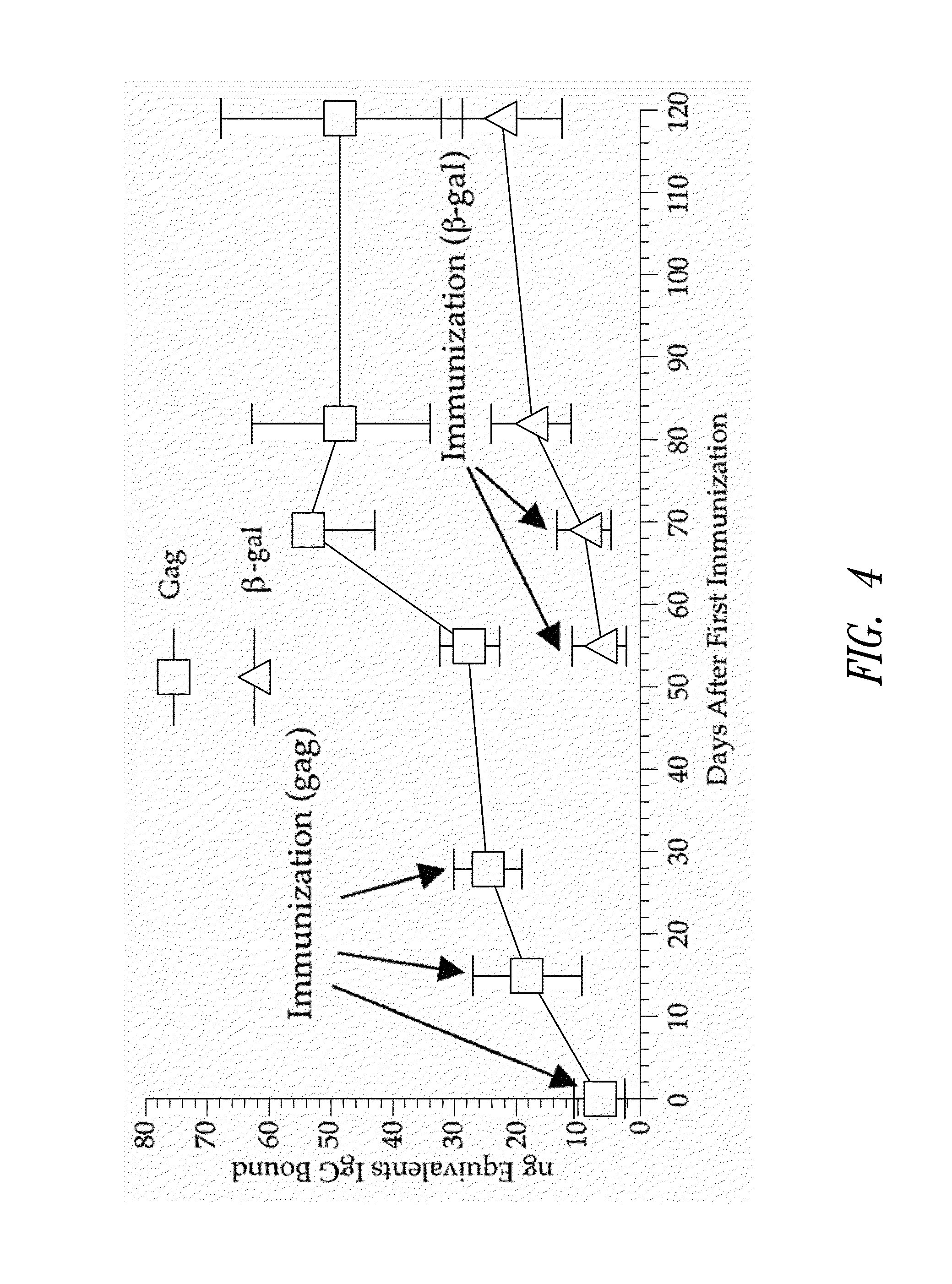
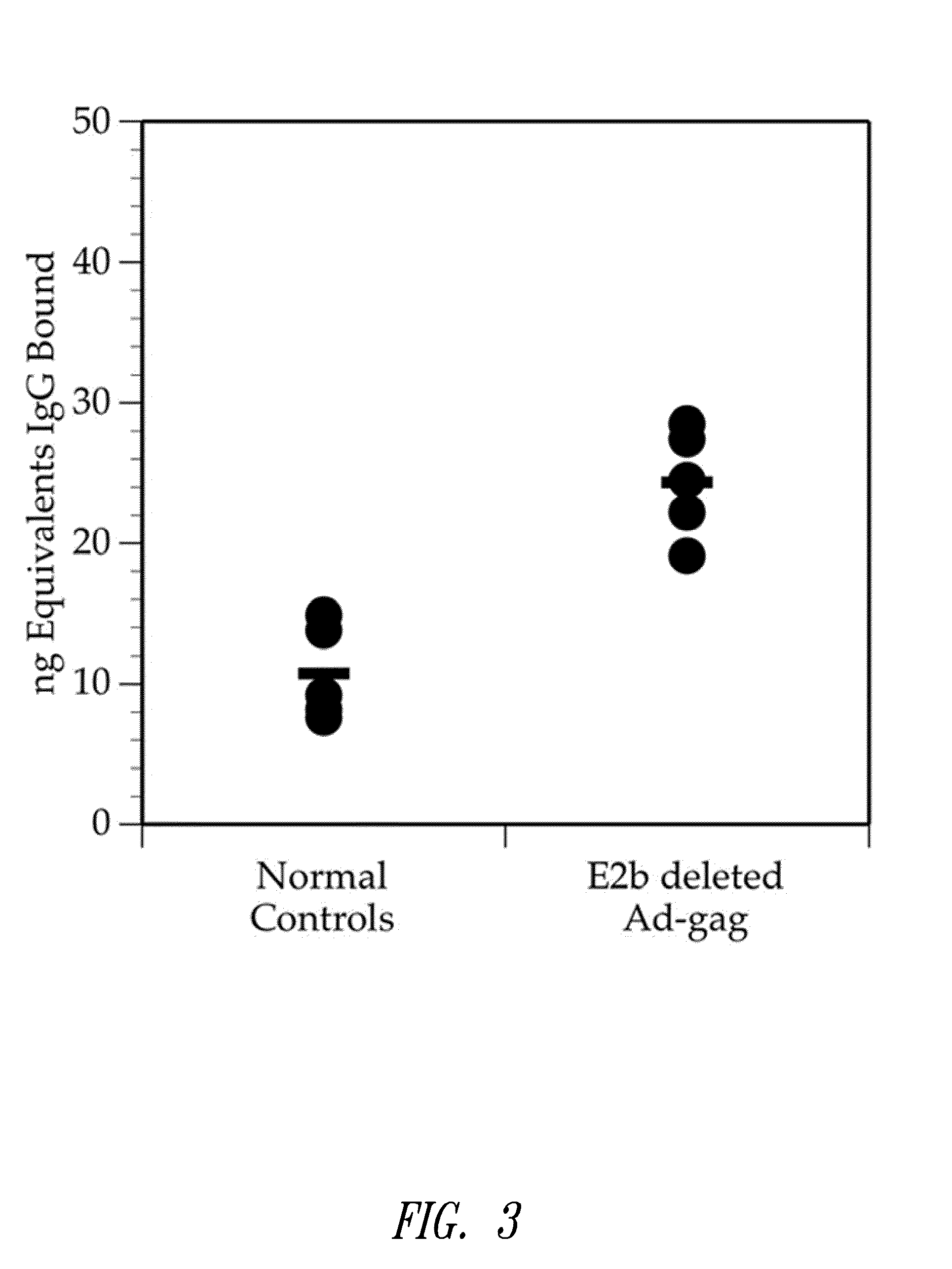
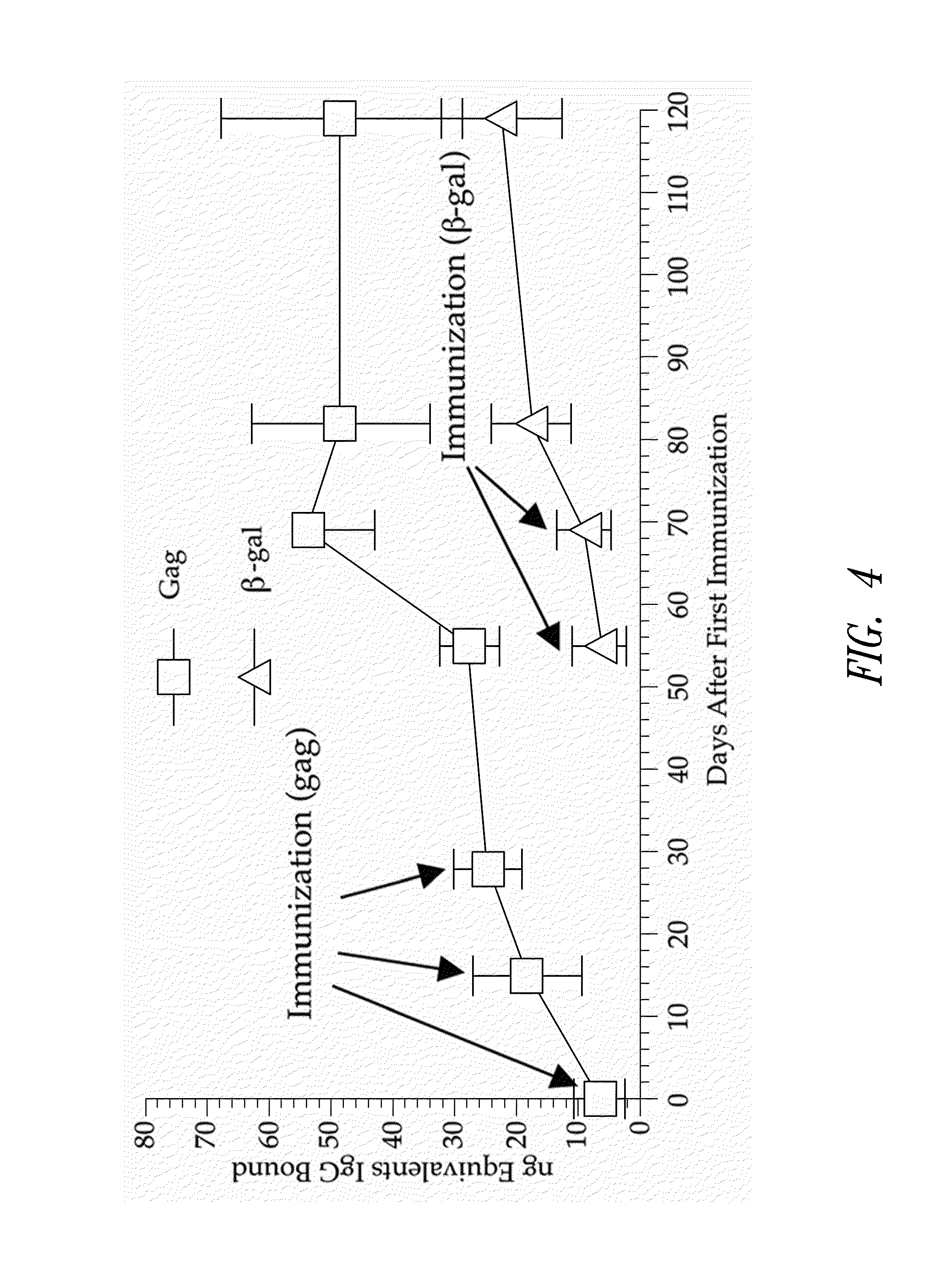
ActiveUS8298549B2Enhance immune responsePeptide/protein ingredientsVirus peptidesImmunopotencyVaccination
Methods for generating immune responses using adenovirus vectors that allow multiple vaccinations with the same adenovirus vector and vaccinations in individuals with preexisting immunity to adenovirus are provided.
Owner:ETUBICS CORP
Mycoplasma Hyopneumoniae Avirulent Adjuvanted Live Vaccine
ActiveUS20090117152A1Preventing and minimize severityElicit immune responseAntibacterial agentsBacterial antigen ingredientsViral antigensImmunogenicity
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS SERVICE LLC
Alga immunopotentiation compound feed for litopenaeus vannamei
ActiveCN103355491AImprove palatabilityFast food intakeAnimal feeding stuffBiotechnologyAntimicrobial action
The invention belongs to the field of aquaculture or feed, relates to a aquaculture feed, and especially relates to an alga immunopotentiation compound feed for litopenaeus vannamei. The invention at first provides an immunity strengthening agent for litopenaeus vannamei feed, and the agent is obtained through enzymatic hydrolysis and fermentation of an alga composite. The alga composite is composed of following components in parts by dried weight: 40 to 50 parts of asparagus, 15 to 25 parts of enteromorpha, 10 to 20 parts of ulva lactuca, 5 to 15 parts of laver and 5 to 15 parts of undaria pinnatifida. The invention further prepares a compound feed for litopenaeus vannamei by utilizing the immunity strengthening agent. The alga immunity strengthening agent contains algae polysaccharide, which has the effect of strengthening animal immunity, acrylic acid, terpenes, brominated phenolic and some sulfocompounds, which have the antiseptic effect, and sulphated polysaccharide, which has the antivirus effect. The litopenaeus vannameis fed on the alga immunopotentiation compound feed have a strong anti-stress performance, the body's immunity ability is improved, survival rate is high and culture benefit is remarkable.
Owner:SUN YAT SEN UNIV
Biological fermentation composition with anti-cancer effect and application of biological fermentation composition
ActiveCN104138527AImprove immunityPromote absorptionAntineoplastic agentsPlant ingredientsDiseaseAcute hyperglycaemia
The invention relates to a biological fermentation composition with an anti-cancer effect. The biological fermentation composition is prepared by using a method comprising the following steps: a, preparing a culture medium mixed solution; b, sterilizing; c, cooling; d, inoculating to obtain a fermentation solution; and e, standing to obtain the biological fermentation composition with the anti-cancer effect. The composition disclosed by the invention can be used for improving the in-vivo micro-ecological environment, balancing and regulating effective microbial communities in intestinal canals, repairing damaged cells, replenishing various in-vivo active bio-enzymes, amino acids, nucleic acids, trace elements and the like, improving the immunity of a human body, preventing diseases, effectively inhibiting cancer cells and stopping the dispersion and development of the cancer cells. Meanwhile, the biological fermentation composition can be used for improving an acidic physique to be a weak alkaline physique, is particularly suitable for the cancer crowd, the crowd with hypertension, hyperlipidemia and hyperglycemia and the fat crowd, can be prepared into various dosage forms such as pills, paste, aqueous solutions and the like, and has favorable supportive therapeutic effects on cancer, diabetes, digestive system diseases and the like.
Owner:贵州酵德生物科技有限公司
Momlv-based pseudovirion packaging cell line
The present invention discloses Moloney murine leukemia virus (MoMLV)-based viral packaging cell line for the production of anti-viral vaccines. The invention also includes methods of making, administering and formulating pseudovirions and replicon deficient viral particles of the invention and methods of inducing immunity.
Owner:BIOPROTECTION SYST
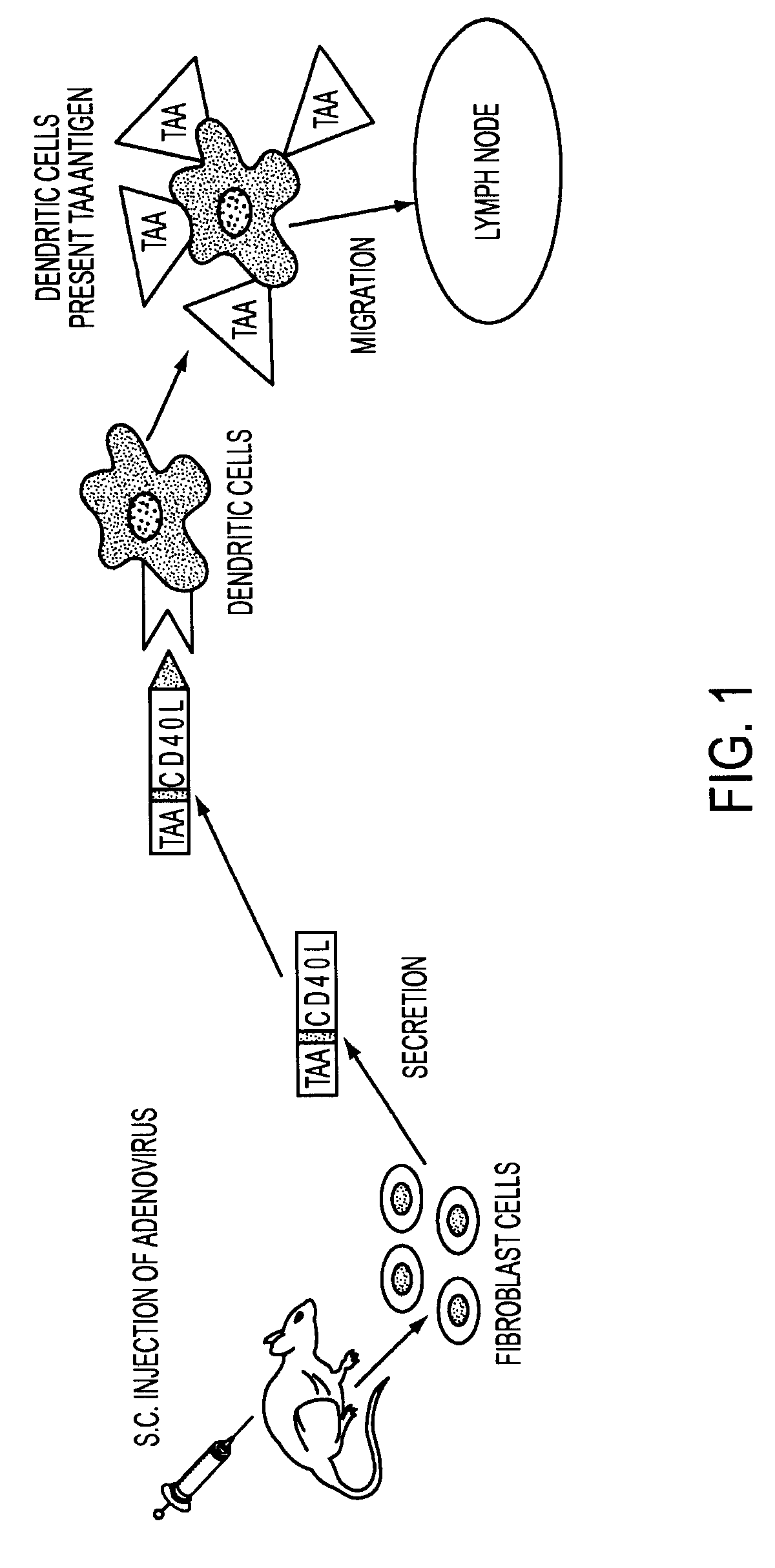
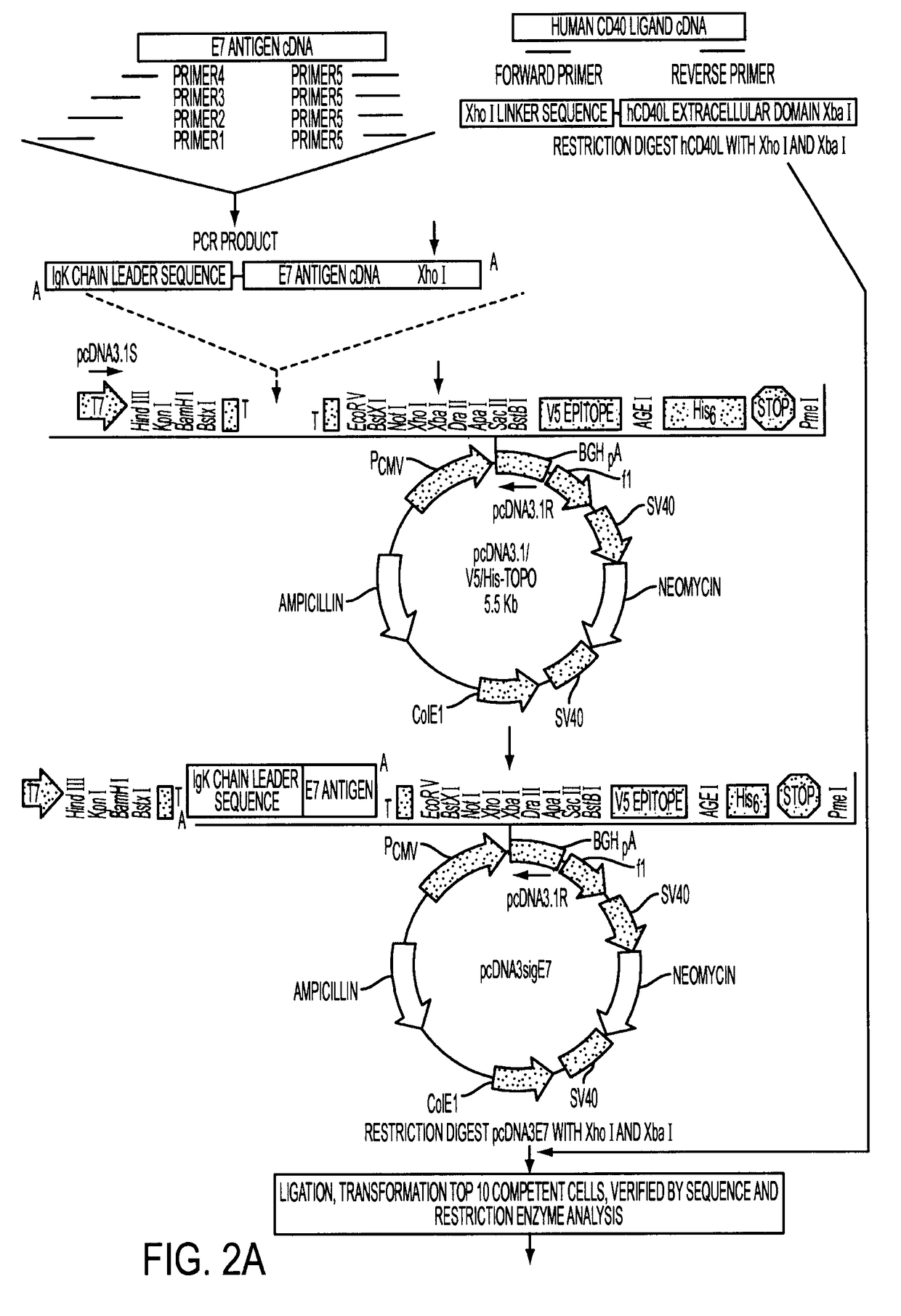
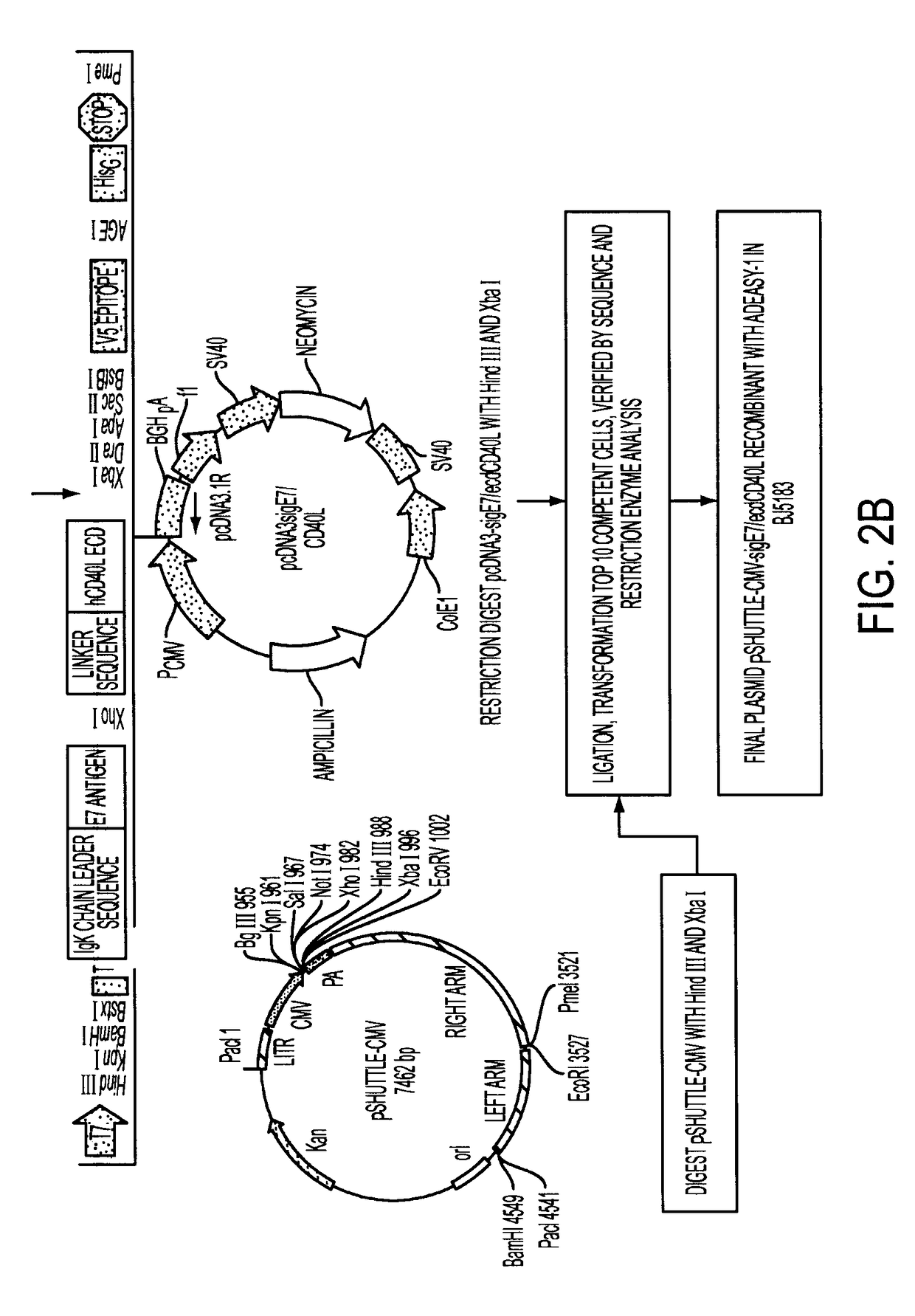
Adenoviral expression vector comprising a CD40L fusion protein adapted to elicit cellular immunity
ActiveUS8119117B2Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsTransmembrane domainTumor antigen
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
Health food for improving immunity and preparation method thereof
ActiveCN102415568AEnhance immune functionEnhance humoral immune functionFood preparationBiotechnologyDisease
The invention discloses a health food for improving immunity and a preparation method thereof. The health food is prepared from the following raw materials in part by weight: 1 to 3 parts of dendrobium officinale, 2 to 6 parts of American ginseng and 6 to 18 parts of Chinese wolfberry. Pharmacological experiments prove that: the health food has an obvious effect of promoting an immune system, canobviously improve a phagocytic index of macrophage of immunosuppressed mice and increase coefficients of immune organs in nonspecifc immune experiments, can improve the conversion rate of lymphocytes, improve the level of immunoglobulin IgG, IgA and IgM in serum of the immunosuppressed mice and assist in conditioning immunodeficiency disease, and diseases such as weakened immune system, low resistance and the like.
Owner:常熟雷允上制药有限公司
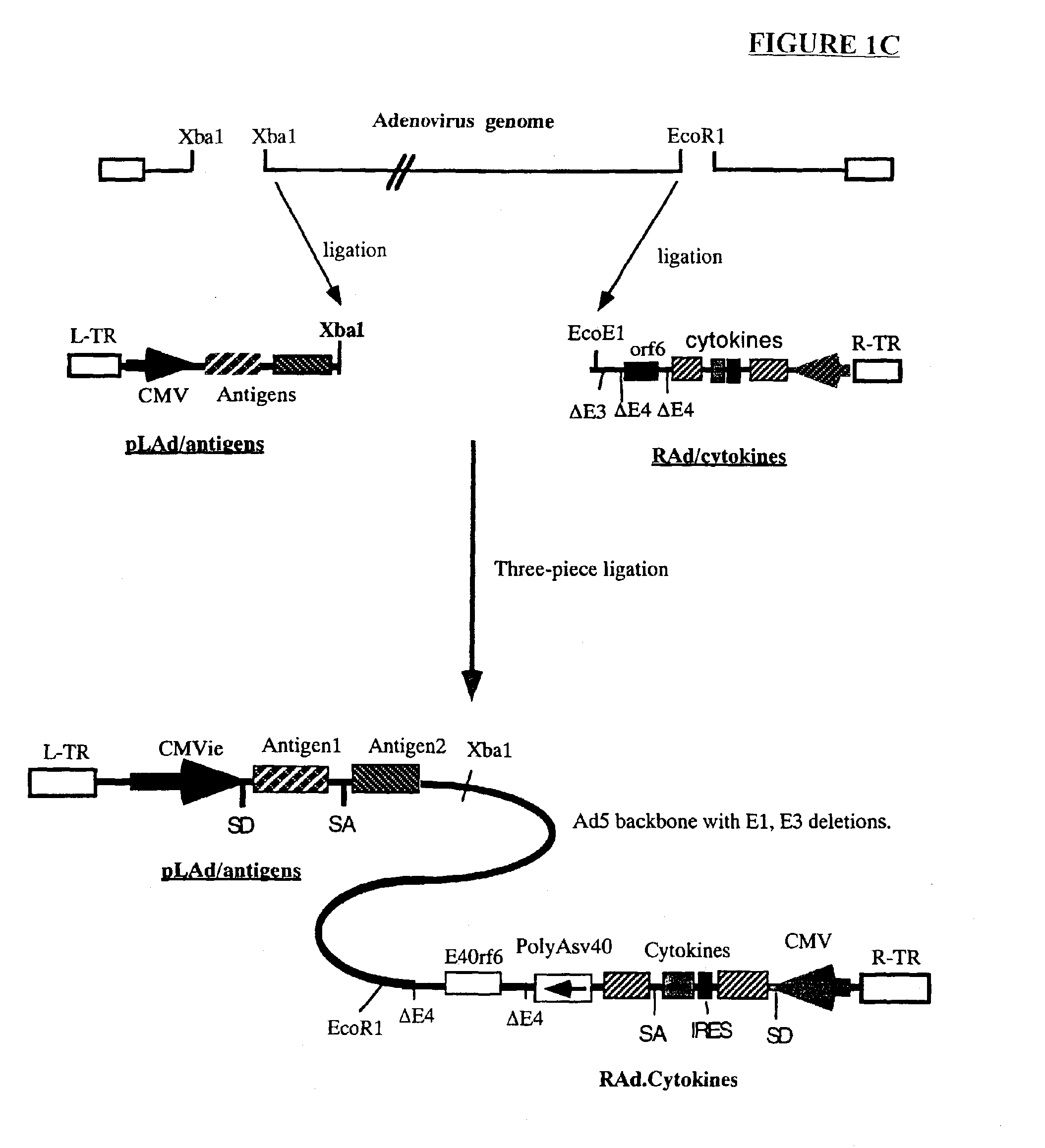
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
Safe compound feed of juvenile prawn of penaeus vannamei boone
InactiveCN101816380APromote growthImprove immunityAnimal feeding stuffAccessory food factorsBacillus licheniformisAdditive ingredient
The invention discloses a safe compound feed of juvenile prawn of penaeus vannamei boone, which consists of bean pulp, pea albumen powder, fish powder, blood cell albumen powder, cuttlefish powder, shrimp shell powder, double-low rapeseed meal, cotton pulp, monocalcium phosphate, No. 1 flour, gluten powder, 50% choline chloride, complex vitamin for shrimp, microelement additive for shrimp, bacillus licheniformis, decorticating element, lower polyxylose, fermented peptide for aquatic product, gene recombinant protein growth promoter, nutrition immunology aquatic product food attractant, phospholipids oil and fish oil. With the gene recombinant protein growth promoter, the nutrition immunology aquatic product food attractant, and the high antibacterial peptide-containing fermented peptide, the safe compound feed can effectively restrain various enteropathogenic bacteria, improves the digestibility of the nutrition constituent of the feed, promotes the growth of the penaeus vannamei boone, can improve the organism immunity of the penaeus vannamei boone, and can obviously improve the survival rate of the penaeus vannamei boone.
Owner:缪淑华
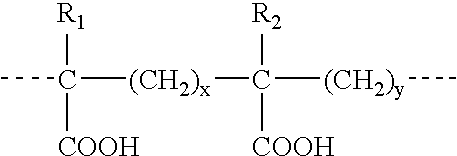
Propylene glycol derivatives, preparation method thereof, pharmaceutical composition and use thereof
InactiveCN102260177AOrganic chemistryImmunological disordersCurative effectOrgan transplant rejection
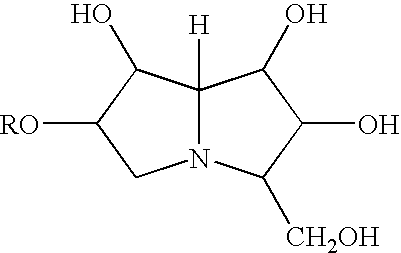
The invention discloses a new class of propylene glycol derivatives represented by the general formula (I), a preparation method thereof, a pharmaceutical composition containing them, and their use as medicines, especially immunomodulatory medicines. This kind of compound with excellent curative effect and low toxicity can be used for immune disorder and immunosuppression; and can be used for treatment and / or low immunity, rejection after organ transplantation and autoimmune disease. General formula (I)
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Mid-life vaccine and methods for boosting anti-mycobacterial immunity
InactiveUS7288261B2Boost immune responseIncrease resistanceAntibacterial agentsBacteriaImmunogenicityMycobacterium
Vaccine compositions for boosting immunity to mycobacteria when administered in mid-life in a subject who has been vaccinated neonatally or in early childhood with BCG and in whom protective immunity has waned comprise one or more purified immunogenic proteins from Mycobacterium tuberculosis from a group of 30 proteins that stimulate T cell immunity and interferon-γ secretion. A preferred protein is Ag85A, the secreted product (SEQ ID NO:31) of the Rv3084c gene. Also disclosed are methods for boosting immunity in such BCG-vaccinated subjects comprising administering an effective amount of the above vaccine composition.
Owner:COLORADO STATE UNIVERSITY
Veterinary compound acanthopanax granules with immunopotentiation and preparation method thereof
ActiveCN101559095AImprove immunityImprove disease resistanceGranular deliveryImmunological disordersDiseaseAnti stress
The invention provides veterinary compound acanthopanax granules with immunopotentiation, which is prepared from the following Chinese medicament raw materials by weight percentage: 70 to 60 percent of acanthopanax and the balance of astragalus root, wherein 100 grams of granules contain 100 grams of crud drugs. The compound acanthopanax granules produced by the method have good stability and safety, are mainly applied to strengthening the immunological effect of a vaccine and strengthening the immunity of organisms and the anti-stress efficacy clinically, and is applied to treating various immunosuppressive diseases of chickens and other animals and birds, strengthening the immunological effect, and treating symptoms of hypoimmunity, inappetence and the like caused by transportations, groups changes, abrupt climate changes and the like.
Owner:HENAN XINZHENGHAO BIO ENG
Antigen-presenting cell populations and their use as reagents for enhancing or reducing immune tolerance
InactiveUS20060292618A1Low levelImprove toleranceMicrobiological testing/measurementBlood/immune system cellsImmunologic disordersDisease
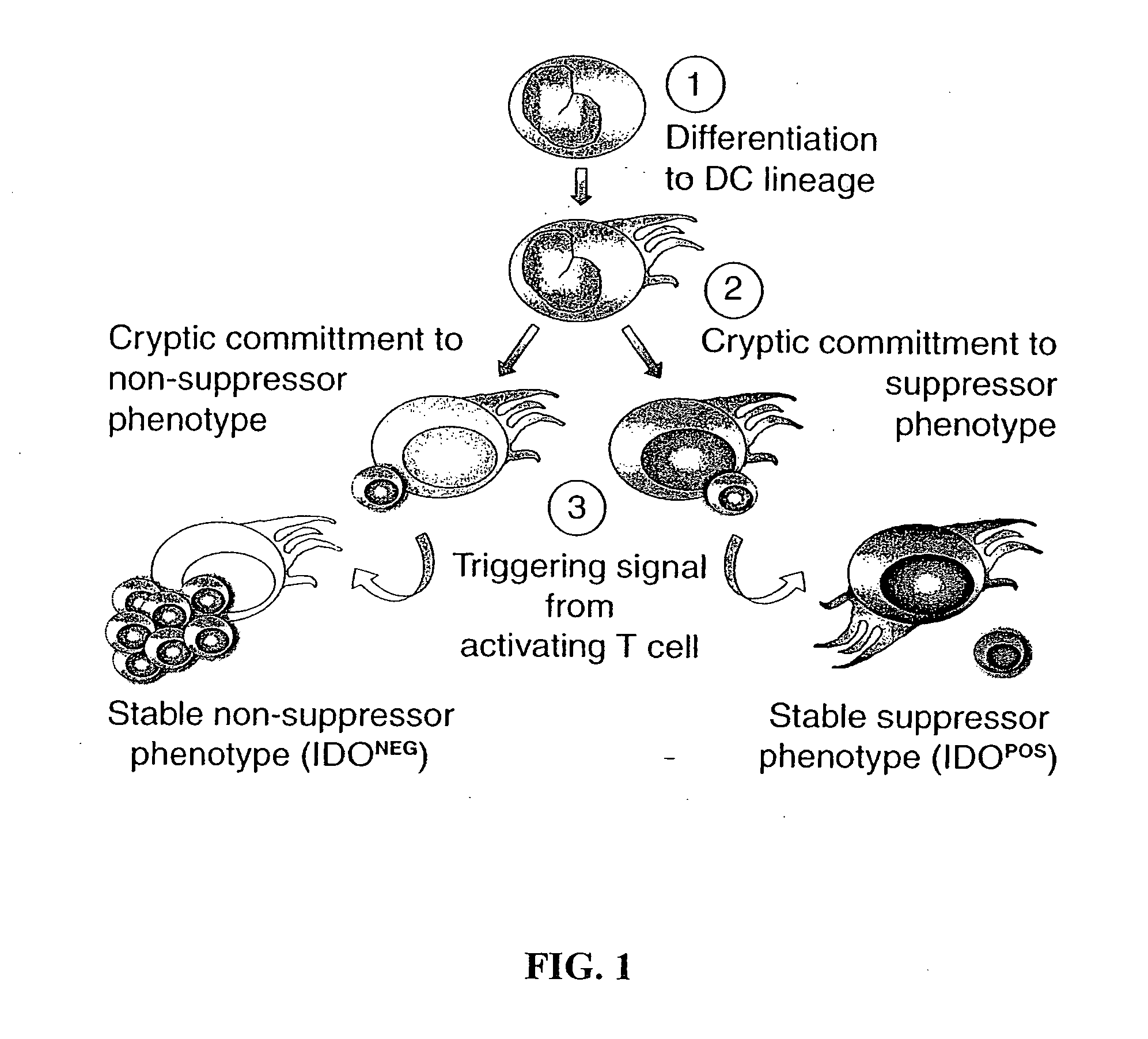
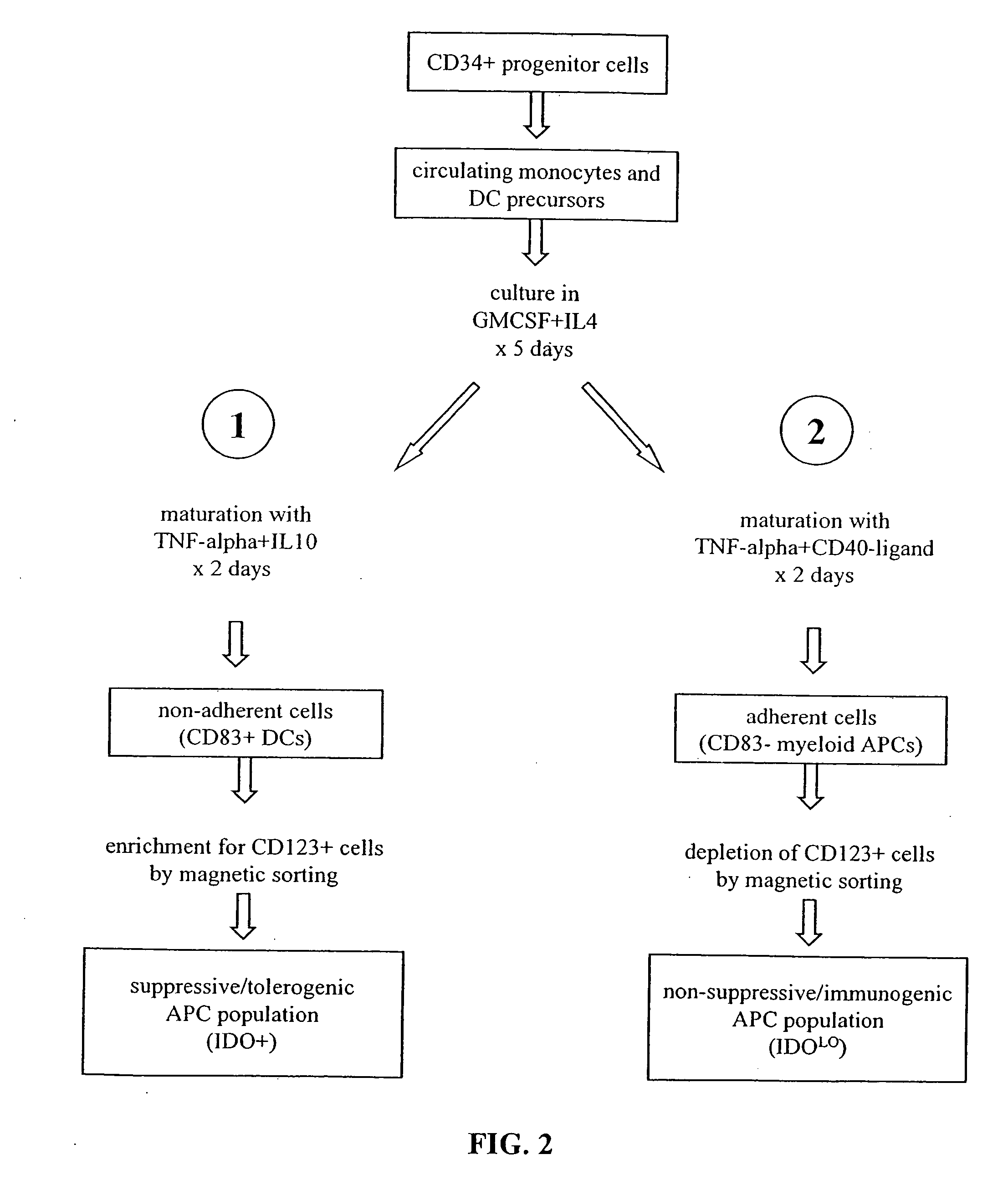
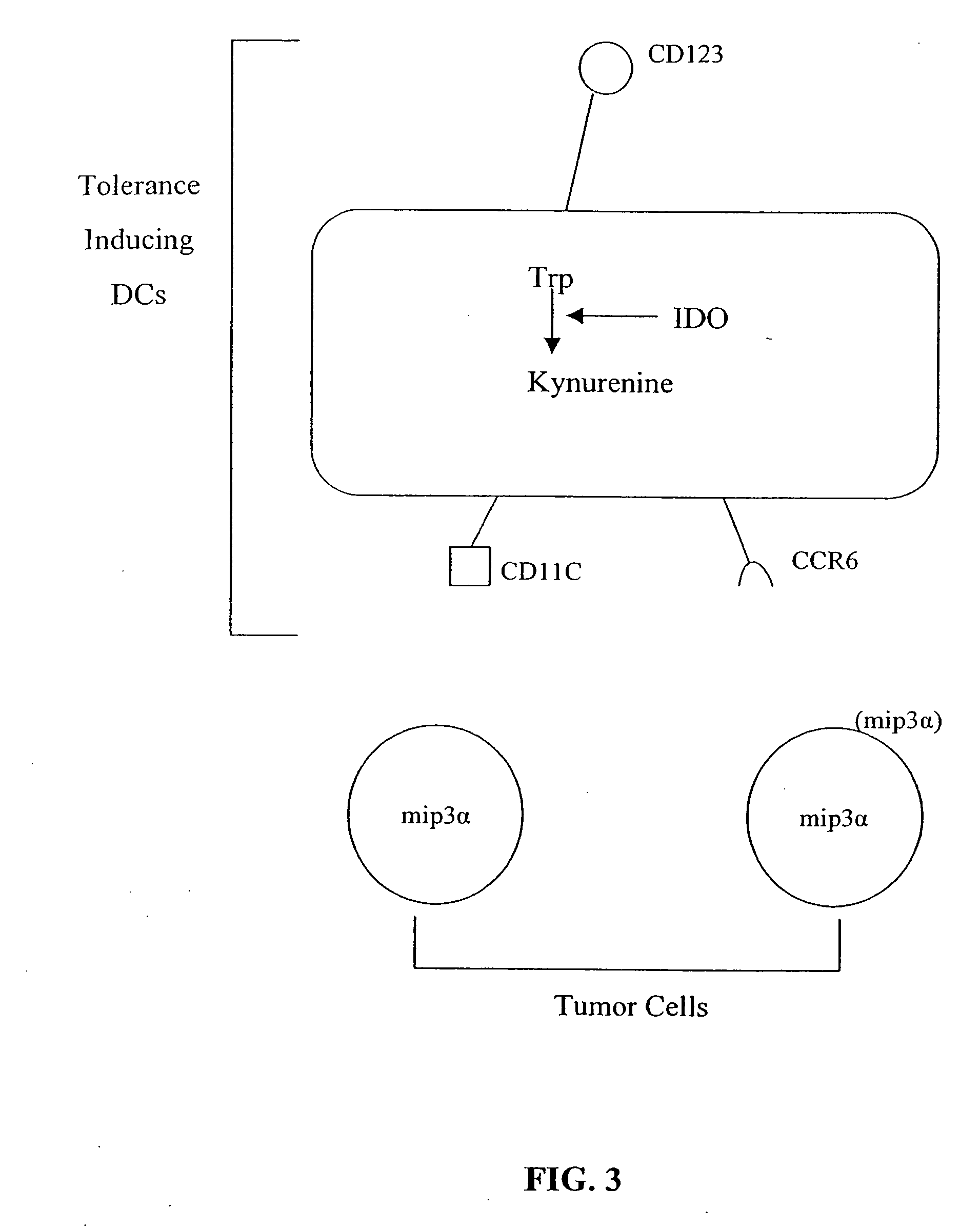
The present invention is based on the discovery antigen-presenting cells (APCs) may be generated to have predetermined levels of expression of the intracellular enzyme, indoleamine 2,3-dioxygenase (IDO). Because expression of high levels of IDO is correlated with a reduced ability to stimulate T cell responses and an enhanced ability to induce immunologic tolerance, APCs having high levels of IDO may be used to increase tolerance in the immune system, as for example in transplant therapy or treatment of autoimmune disorders. For example, APCs having high levels of IDO, and expressing or loaded with at least one antigen from a donor tissue may be used to increase tolerance of the recipient to the donor's tissue. Alternatively, APCs having reduced levels of IDO expression and expressing or loaded with at least one antigen from a cancer or infectious pathogen may be used as vaccines to promote T cell responses and increase immunity.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Synbiotics composition having immunity enhancing function and preparation and application thereof
InactiveCN108576822AInhibition of reproductionRestore pHFood ingredient functionsImmunocompetenceHealth food
The invention discloses a synbiotics composition having an immunity enhancing function. The synbiotics composition comprises prebiotics and probiotics with a weight ratio being 1-10:1-10. The invention also discloses a preparation of the synbiotics composition having the immunity enhancing function, and powder, tablets, a particulate agent and capsules prepared by pharmaceutically acceptable accessories are added in the synbiotics composition. The invention also discloses an application of the synbiotics composition in preparation of a drug or a health food for preventing / treating hypoimmunity. The synbiotics composition combines a plurality of probiotics and prebiotics according to a ratio, and is helpful for inhibiting harmful pathogen reproduction and recovering the pH value of intestinal tract, by simulating an immunization function in the intestinal tract, immunocompetence can be regulated to a normal state, ecological balance of the human body can be kept, so that function healthof human body can be effectively promoted, human body immunity can be enhanced, and pathogenic bacteria invasion can be prevented.
Owner:云南中京国建投资有限公司
Methods and compositions for producing an adenovirus vector for use with multiple vaccinations
ActiveUS20100183673A1Enhance immune responsePeptide/protein ingredientsVirus peptidesImmunopotencyVaccination
Methods for generating immune responses using adenovirus vectors that allow multiple vaccinations with the same adenovirus vector and vaccinations in individuals with preexisting immunity to adenovirus are provided.
Owner:ETUBICS CORP
Carcinoembryonic antigen fusions and uses thereof
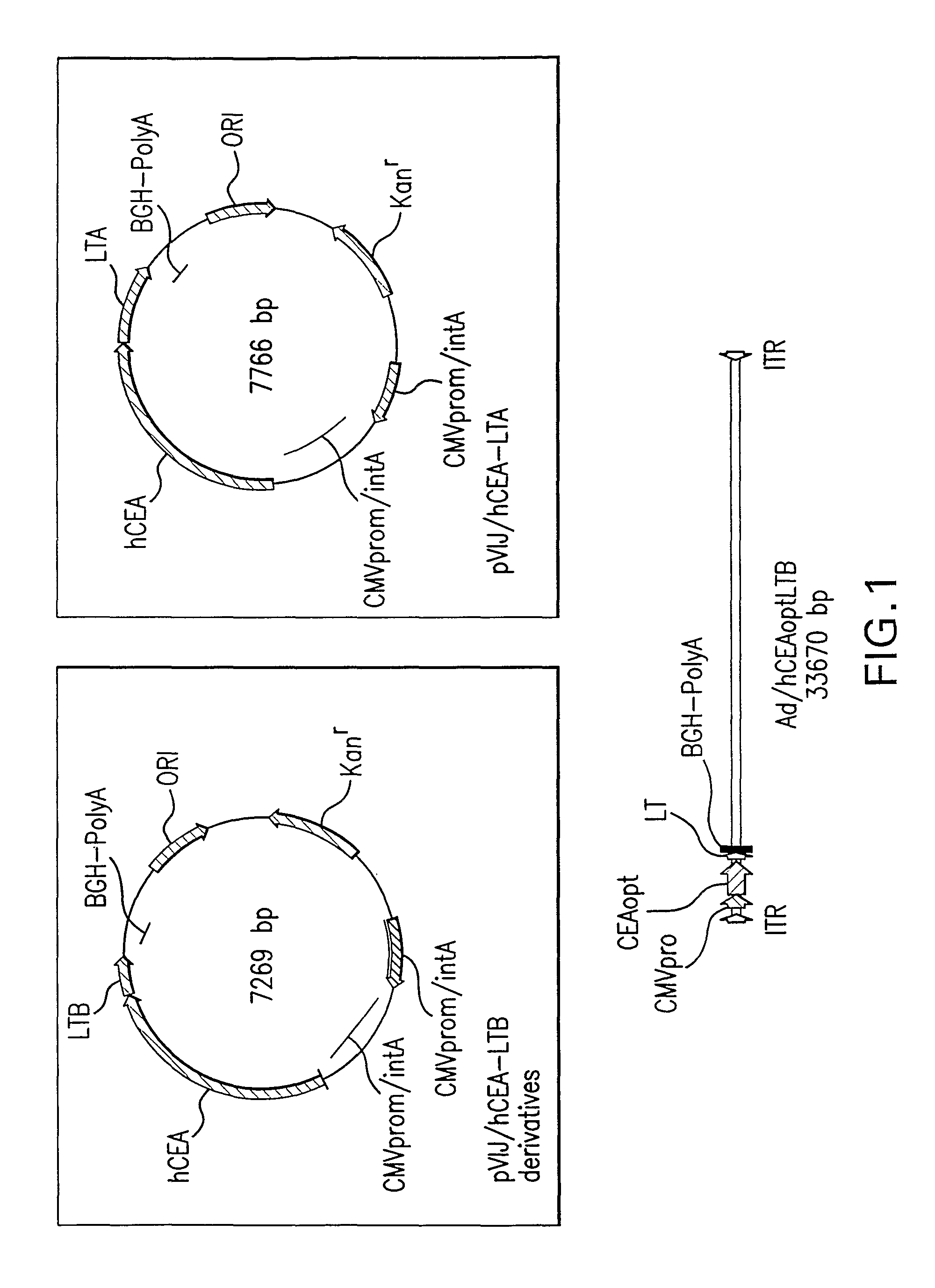
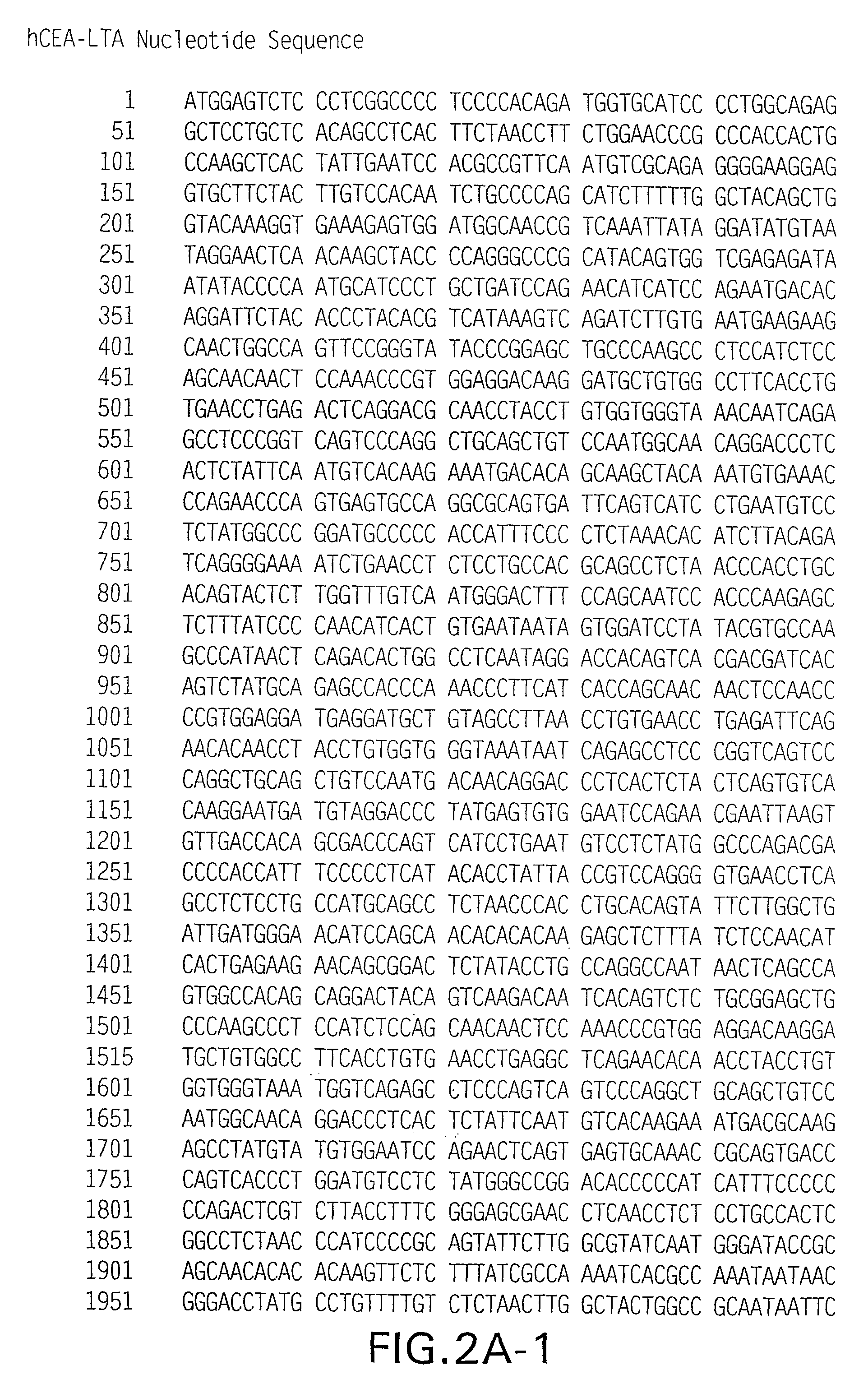
ActiveUS8188244B2Prevent and inhibit developmentEnhance immune responseAntibody mimetics/scaffoldsWhole-cell/virus/DNA/RNA ingredientsPolynucleotideTGE VACCINE
Polynucleotides encoding carcinoembryonic antigen (CEA) fusion proteins are provided, the CEA fusion proteins comprising a CEA protein, or functional variant thereof, fused to a substantial portion of an immunoenhancing element. The polynucleotides of the present invention can elicit an immune response in a mammal, which, in preferred embodiments, is stronger than the immune response elicited by a wild-type CEA. The gene encoding CEA is commonly associated with the development of human carcinomas. The present invention provides compositions and methods to elicit or enhance immunity to the protein product expressed by the CEA tumor-associated antigen, wherein aberrant CEA expression is associated with a carcinoma or its development. This invention specifically provides adenoviral vector and plasmid constructs carrying polynucleotides encoding CEA fusion proteins and discloses their use in vaccines and pharmaceutical compositions for preventing and treating cancer.
Owner:MSD ITAL
Haemophilus parasuis LC strain and application thereof
ActiveCN102399724AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacteriaHeterologousDisease
The invention relates to the field of haemophilus parasuis vaccines in veterinary biological products, in particular to a haemophilus parasuis LC strain. The collection number of the strain is CGMCC (China General Microbiological Culture Collection Center) No.5257. The invention also relates to application of the haemophilus parasuis LC strain to preparation of haemophilus parasuis inactivated vaccines. The haemophilus parasuis LC strain has stronger pathogenicity to pigs and has better immunogenicity; an inactivated alumina gel vaccine prepared by the strain is safe and reliable; not only a homologous attacking protection is provided, but also a better cross protection to blood serums type 4, type 5, type 10, type 12, type 14 and type 15 HPS (Hantavirus Pulmonary Syndrome) heterologous attacking can be provided; after the pigs are immunized, a stronger immunity can be generated and the morbidity and the mortality of the inoculated pigs are obviously reduced; the immune effect achieves or is better than the traditional commercialized vaccines in the market; the vaccine has the advantages to compete with like products at home and abroad and is capable of effectively preventing the epidemic and the transmission of a haemophilus parasuis disease and reducing the economic losses caused by the disease, so that the application range is wide.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Bioactive polypeptide QEPVL, and preparation and application thereof
ActiveCN102964427AImproves antioxidant activityBoosts immune activityPeptide/protein ingredientsMicroorganism based processesLymphocyteDrug biological activity
The invention relates to the field of a protein and particularly relates to a milk-derived bioactive polypeptide QEPVL with in-vitro antioxidant activity and body immunity promoting activity, wherein an amino acid sequence of the bioactive polypeptide QEPVL is Gln-Glu-Pro-Val-Leu. Through an in-vitro antioxidant test and an in-vitro immunity promoting test, the polypeptide QEPVL is proved to have relatively good antioxidant biological activity and immunity improving function; on one hand, free radicals in the body can be removed to reduce the harm on the human body caused by the free radicals; on the other hand, according to the bioactive polypeptide QEPVL, the immunity of the body can be further enhanced and the multiplication capacity of lymphocytes in vitro can be promoted, thereby improving the ability of the body to resist infections of external pathogens and reducing the incidence rate of the body without causing immunological rejections. The bioactive polypeptide QEPVL has great significance for the development of milk products, healthcare products and medicines with the antioxidant function and the immunity enhancement.
Owner:ZHEJIANG HUITAI LIFE HEALTH TECH CO LTD
Fodder additive having immunologic enhancement in growing and preparation method thereof
InactiveCN101032281AImprove immunityPromote growthBacteriaFood processingTotal nitrogenFeed additive
The present invention is one kind of feed additive for strengthening immunity and promoting growth. The feed additive is prepared with soybean residue, wheat bran, corn protein powder, corn starch, alcohol dreg, tropina powder, waste molasses and / or starch, and through inoculating probiotics seed and solid fermentation. It has total immune polysaccharide content not less of 0.3-1.0 %, immune energy matter content 0.3-1.0 %, lysine content not less than 4.0 %, amino nitrogen content 0.5-1.0 %, total nitrogen content not less than 10.0 %, and water not more than 12.0 %. The feed additive can raise the immunity of animal obviously, raise the speed of synthesizing protein, promote the growth of animal, appetize, promote the absorption of animal body on mineral elements, regulate physiological activity and improve nutritious condition.
Owner:上海邦成生物工程有限公司
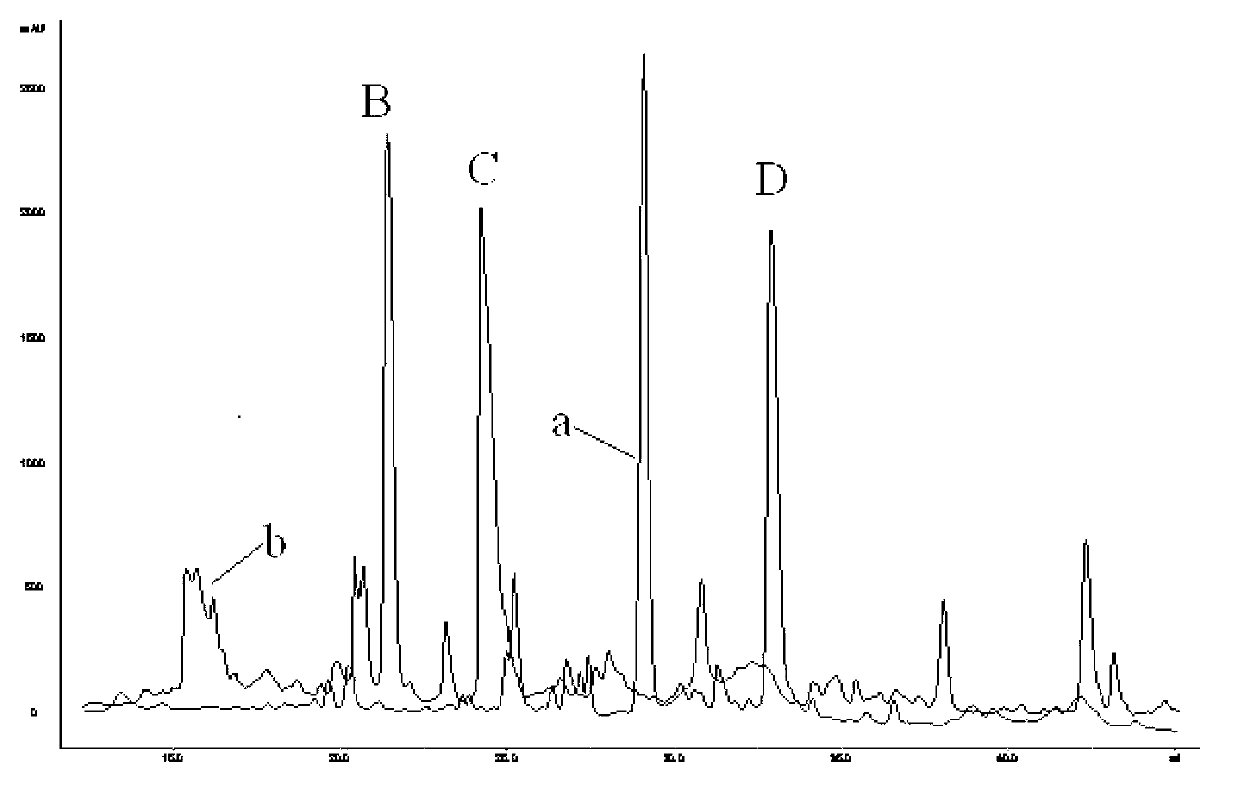
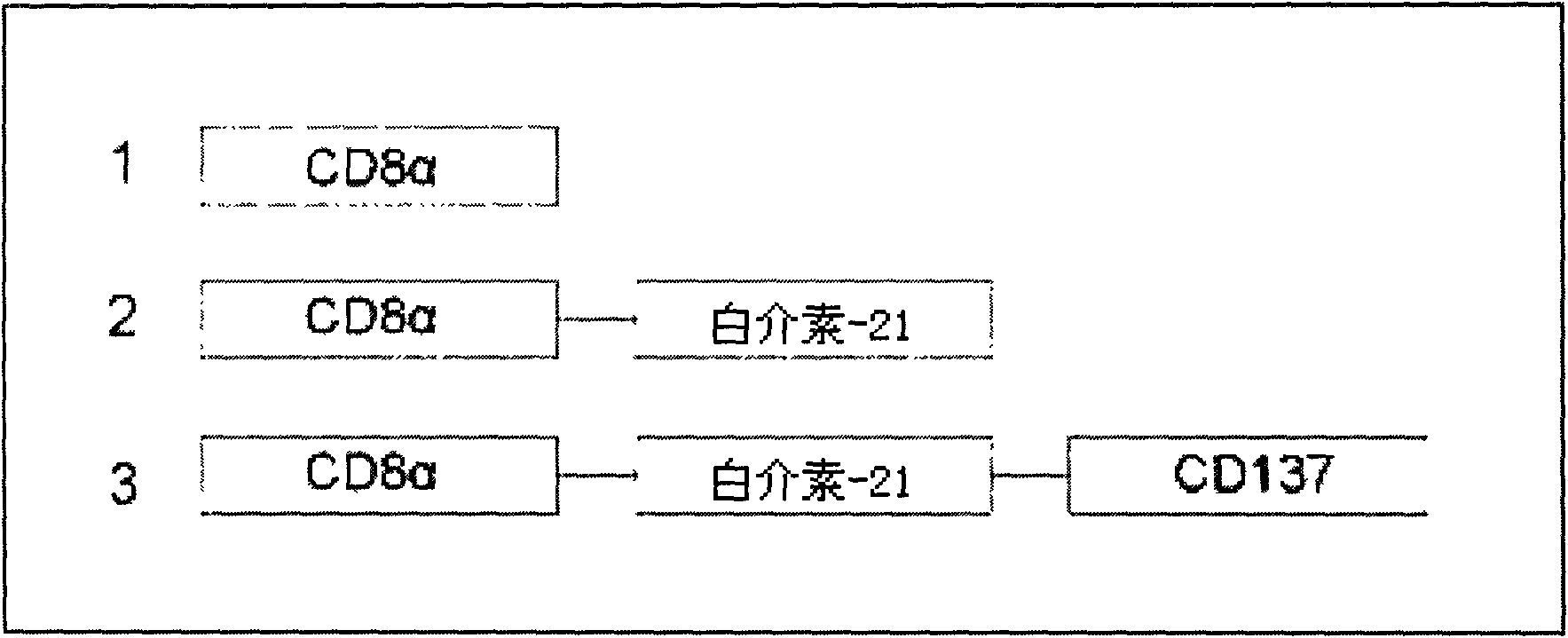
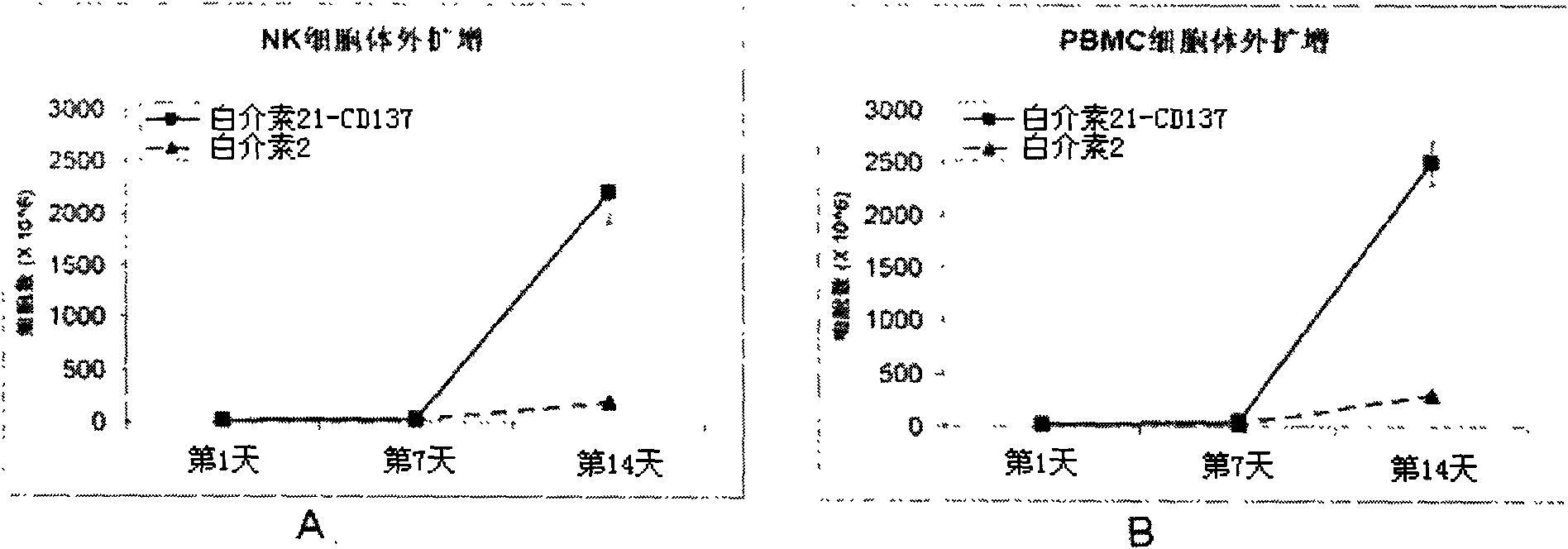
Method for amplifying and activating lymphocyte by using CD8 alpha-interleukin 21-CD137 compound
The invention relates to the field of immunology, in particular to a method for amplifying and activating a natural killer (NK) cell into a lymphokine-activated killer (LAK) cell by using a CD8 alpha-interleukin 21-CD137 compound. The method disclosed by the invention comprises the following steps of: forming the CD8 alpha-interleukin 21-CD137 compound by using CD8 alpha, interleukin 21 and a CD137 functional polypeptide, making an exogenous expression vector enter a host K562 cell, then activating a promoter and culturing a cell to obtain a cell for expressing a transmembrane interleukin 21-CD137 compound; and purifying the compound in the conventional way, and amplifying and activating a lymphocyte by using the purified compound to generate the LAK cell. The method has the advantage that: the LAK cell cultured and amplified by using the transmembrane interleukin 21-CD137 compound and a small dose of interleukin 2 is used for enhancing the immunity of a patient to help the patient resist tumors, viruses and bacteria. The method has a wide clinical using prospect.
Owner:杭州中赢生物医疗科技有限公司
Small molecule bioactive peptide, preparation method, composition and application thereof
InactiveCN1920049AEnhance immune functionPromote wound healingHydrolysed protein ingredientsSolution deliveryWound healingBioactive peptide
The invention discloses the little molecule biological activity peptide, which is from the mixture of soy protein, lactalbumin, egg and milk. The molecular weight of 90% of the little molecule biological activity peptide is less 5KDa. The invention also provides the preparing method. The invention also provides the composition of biological activity peptide, which comprises the said little molecule biological activity peptide and carriers of pharmacy and foodstuff. The little molecule biological activity peptide can improves the immunity, promote wound healing and enhance health.
Owner:SHANGHAI MIYANG BIOTECH
Lactobacillus rhamnosus and application thereof
ActiveCN110122877AGood intestinal adhesionBroad-spectrum antibacterialAntibacterial agentsConfectioneryInflammatory factorsLymphocyte proliferation
The invention provides an application of lactobacillus rhamnosus in regulating immunity. The lactobacillus rhamnosus is preserved in China General Microbiological Culture Collection Center on August 10, 2017, has the preservation number of CGMCC No.14511, and is classified and named as lactobacillus rhamnosus. The lactobacillus rhamnosus has good immunoregulatory effect on a body, has the inhibiting effect on the expression of inflammatory factors, the promotion effect on the anti-inflammatory factors and the promotion effect on lymphocyte proliferation significantly better than those of conventional commercial lactobacillus rhamnosus strains, and has immunoregulatory function on the body significantly better than that of the conventional lactobacillus rhamnosus strains.
Owner:深圳华大基因农业控股有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com