Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
113 results about "Toxoid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A toxoid is an inactivated toxin (usually an exotoxin) whose toxicity has been suppressed either by chemical (formalin) or heat treatment, while other properties, typically immunogenicity, are maintained. Toxins are secreted by bacteria, whereas toxoids are altered form of toxins; toxoids are not secreted by bacteria. Thus, when used during vaccination, an immune response is mounted and immunological memory is formed against the molecular markers of the toxoid without resulting in toxin-induced illness. Such a preparation is also known as an anatoxin. There are toxoids for prevention of diphtheria, tetanus and botulism.
DIG-3 insecticidal Cry toxins
DIG-3 Cry toxins, polynucleotides encoding such toxins, and transgenic plants that produce such toxins are useful to control insect pests.
Owner:CORTEVA AGRISCIENCE LLC
DIG-11 insecticidal cry toxins
Owner:CORTEVA AGRISCIENCE LLC
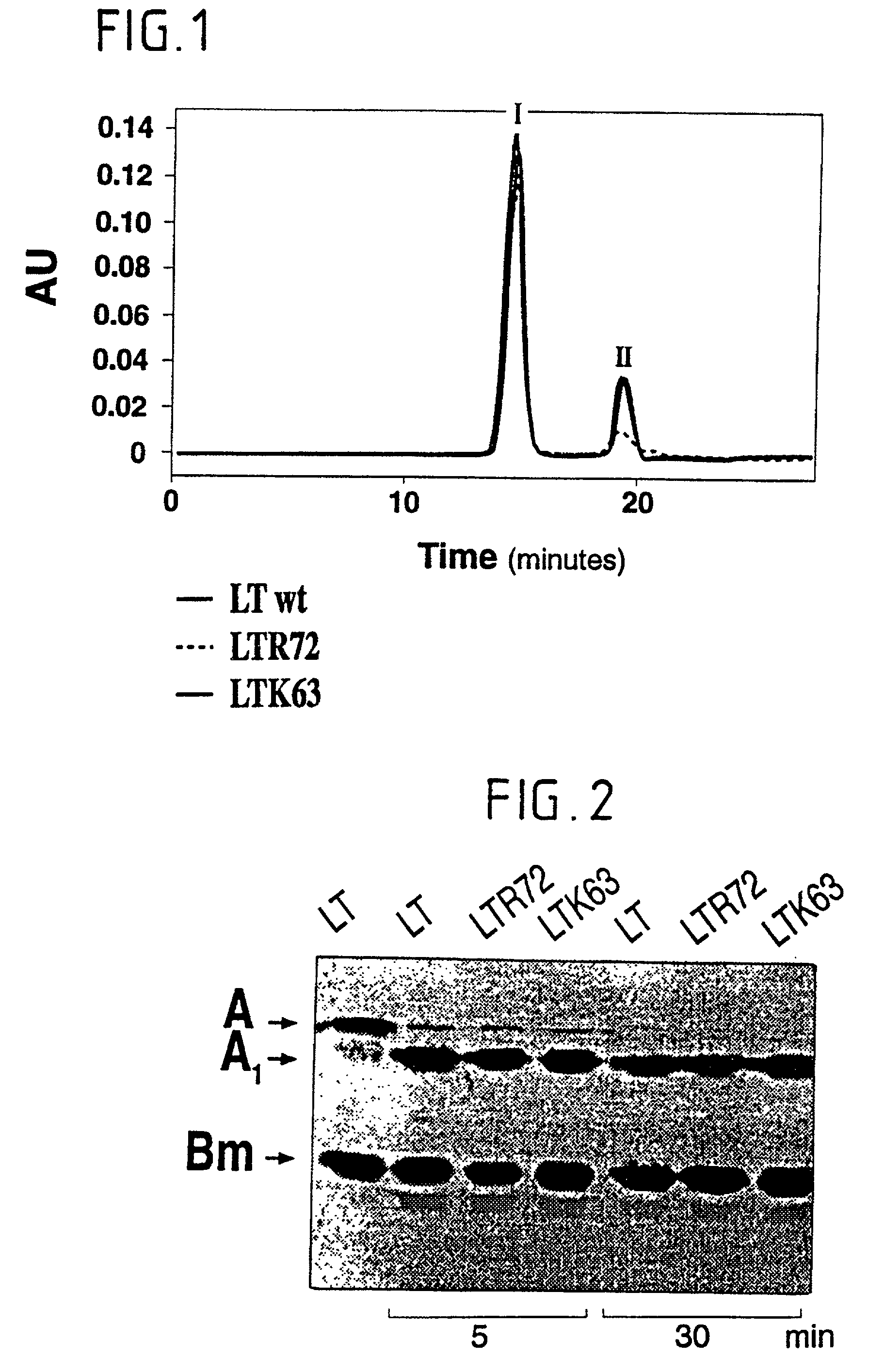
Immunogenic detoxified mutant E. coli LT-A-toxin
InactiveUS7115730B1Maximise adjuvanticityMaximise immunogenicityBacteriaSugar derivativesEscherichia coliAdjuvant
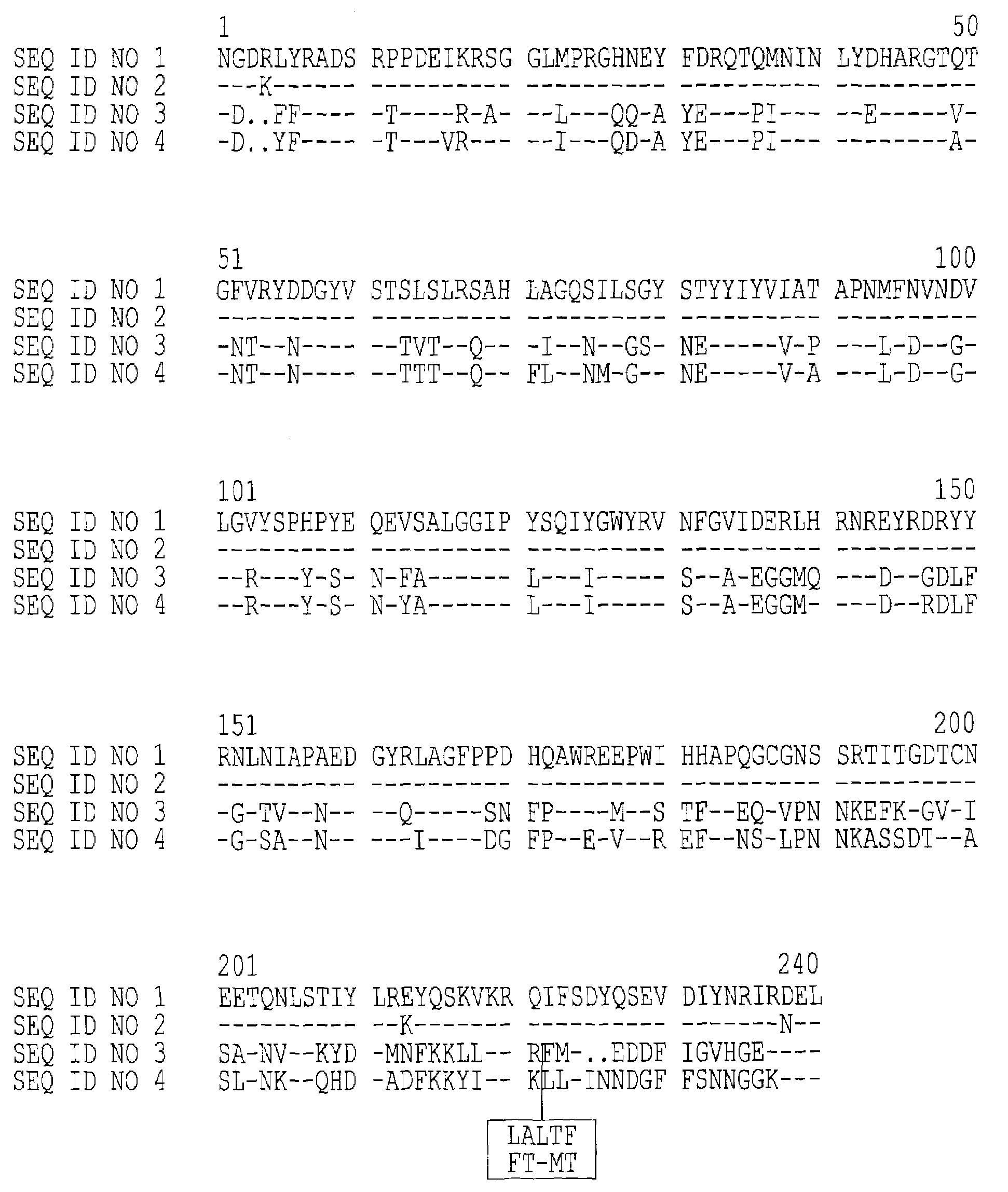
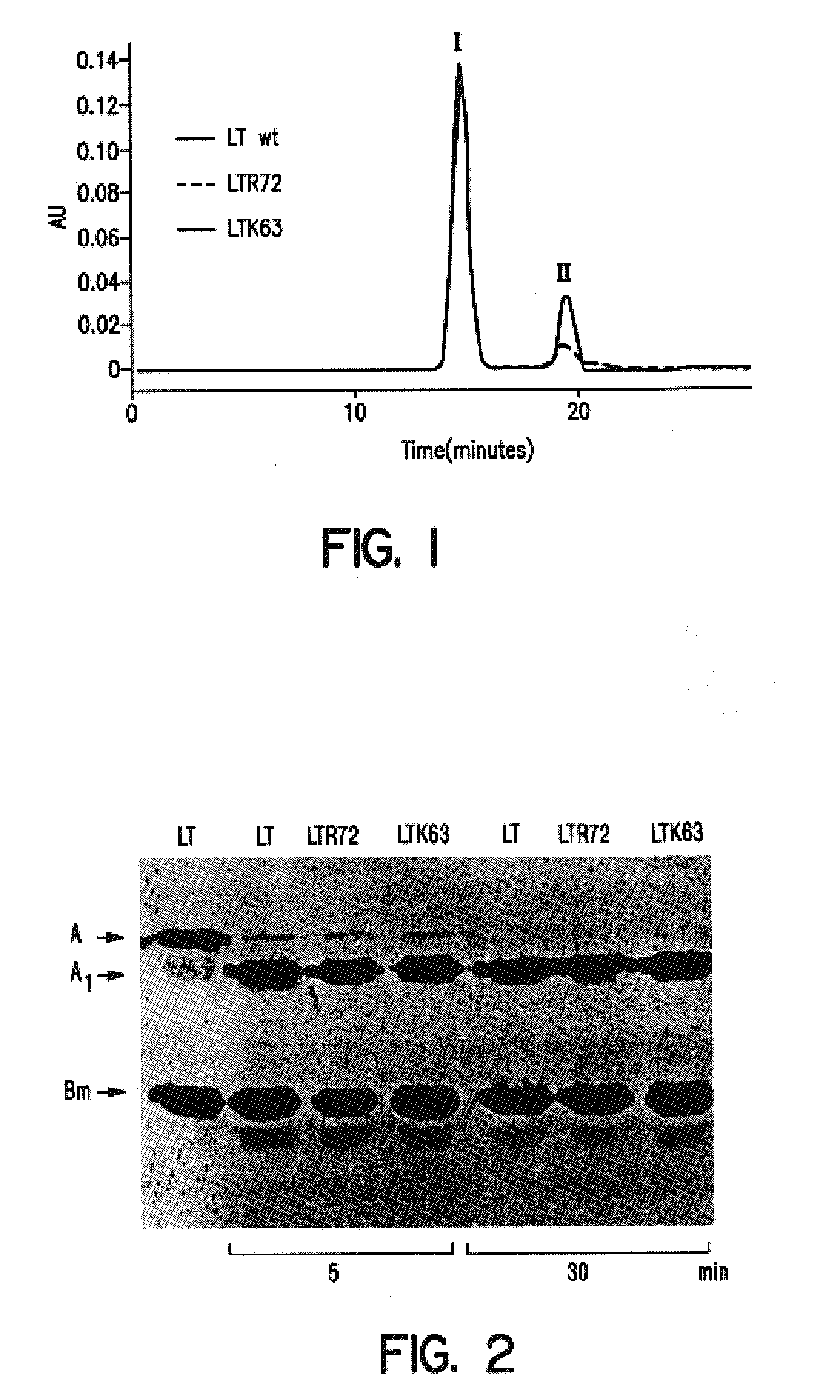
An immunogenic detoxified protein is provided which comprises the amino acid sequence of subunit A of an E. coli heat labile toxin (LT-A) or a fragment thereof in which at least amino acid Ala-72, numbered relative to SEQ ID NO:1, of the A subunits mutated, preferably by substitution with Arg. The toxoid is useful as vaccine against an enterotoxigenic strain of E. coli and is produced by recombinant DNA means by site-directed mutagenesis. It is also an effective adjuvant.
Owner:CHIRON CORP
Active immunization against clostridium difficile disease
InactiveUS6969520B2Prevent relapseImmune responseAntibacterial agentsBacterial antigen ingredientsDiseasePassive Immunizations
The invention provides active and passive immunization methods for preventing and treating Clostridium difficile infection, which involve percutaneous administration of C. difficile toxin-neutralizing polyclonal immune globulin, C. difficile toxoids, or combinations thereof. Also provided by the invention are C. difficile toxoids, C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying subjects that produce C. difficile toxin-neutralizing polyclonal immune globulin.
Owner:SANOFI PASTEUR BIOLOGICS CO
Dig-10 insecticidal cry toxins
Owner:CORTEVA AGRISCIENCE LLC
Immunization against clostridium difficile disease
InactiveUS20060029608A1Prevent relapseQuick treatmentAntibacterial agentsBacterial antigen ingredientsPassive ImmunizationsClostridium difficile infections
The invention provides active and passive immunization methods for preventing and treating Clostridium difficile infection, which involve percutaneous administration of C. difficile toxin-neutralizing polyclonal immune globulin, C. difficile toxoids, or combinations thereof. Also provided by the invention are C. difficile toxoids, C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying subjects that produce C. difficile toxin-neutralizing polyclonal immune globulin.
Owner:ACAMBIS INC
Vaccine Comprising Streptococcus Pneumoniae Capsular Polysaccharide Conjugates
InactiveUS20090017059A1Antibacterial agentsSenses disorderStreptococcus pneumoniae capsular polysaccharideStreptococcus mitis
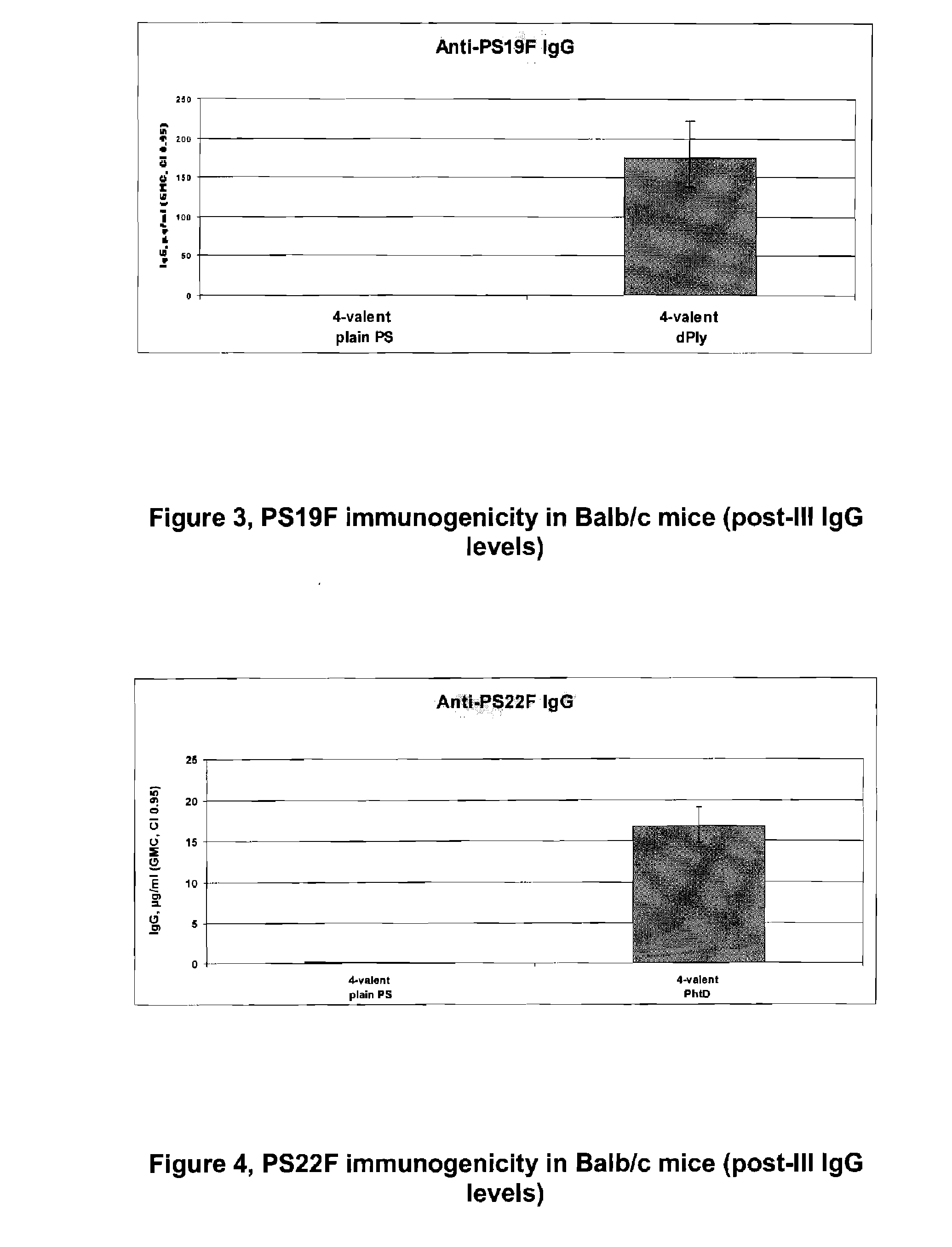
The present invention discloses an immunogenic composition comprising S. pneumoniae capsular saccharide conjugates from serotypes 19A and 19F wherein 19A is conjugated to a first bacterial toxoid and 19F is conjugated to a second bacterial toxoid. Vaccines, methods of making vaccines and uses of the vaccines are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6399076B2Antibacterial agentsBacterial antigen ingredientsToxinAcellular pertussis vaccines
Acellular pertussis vaccines comprise purified toxin or toxoid thereof, filamentous haemagglutinin, pertactin and fimbrial agglutinogens formulated to confer protection to at least 70% of members of an at-risk population. The fimbrial agglutinogens may be prepared from a Bordetella strain, particularly a B. pertussis strain, by a multiple step procedure involving extraction of the fimbrial agglutinogens from cell paste and concentrating and purifying the extracted material.
Owner:AVENTIS PASTEUR LTD
Gene chip for high-flux detection of pathogens and application thereof
InactiveCN102534013AStrong specificityDetermine the typeMicrobiological testing/measurementAgainst vector-borne diseasesYersinia pestisBrucella
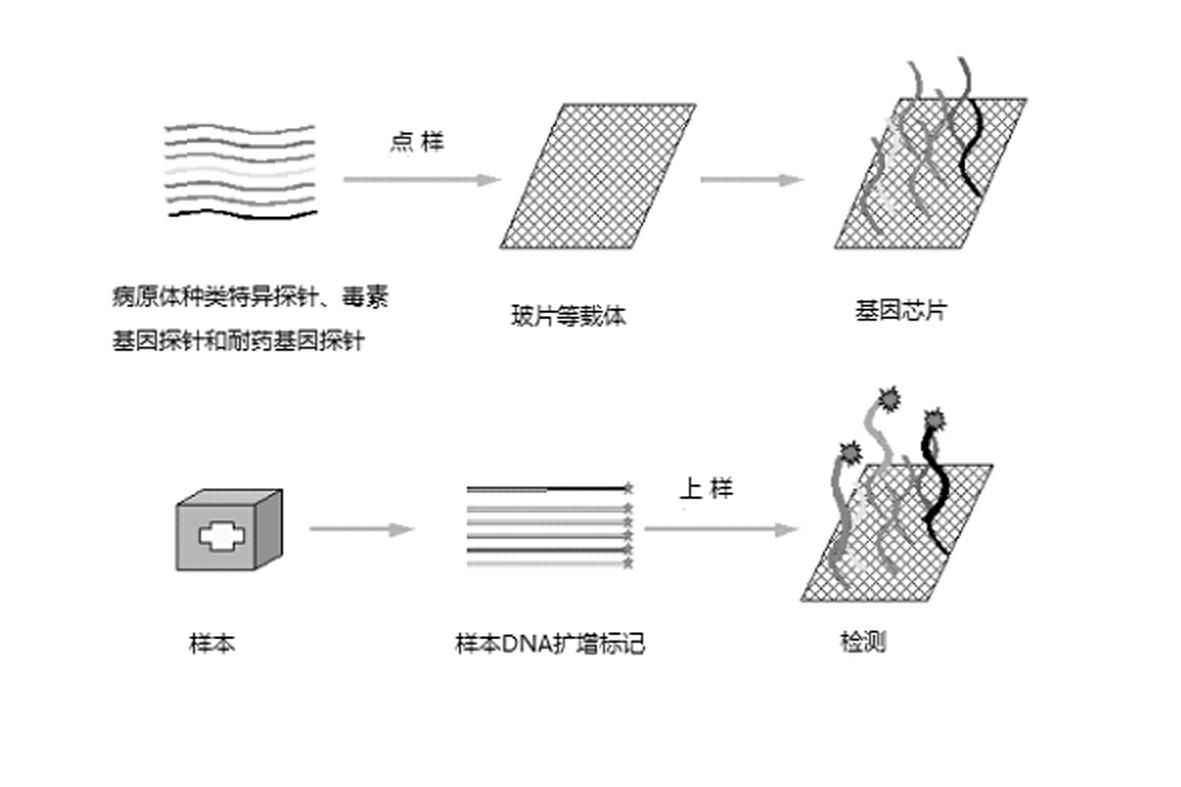
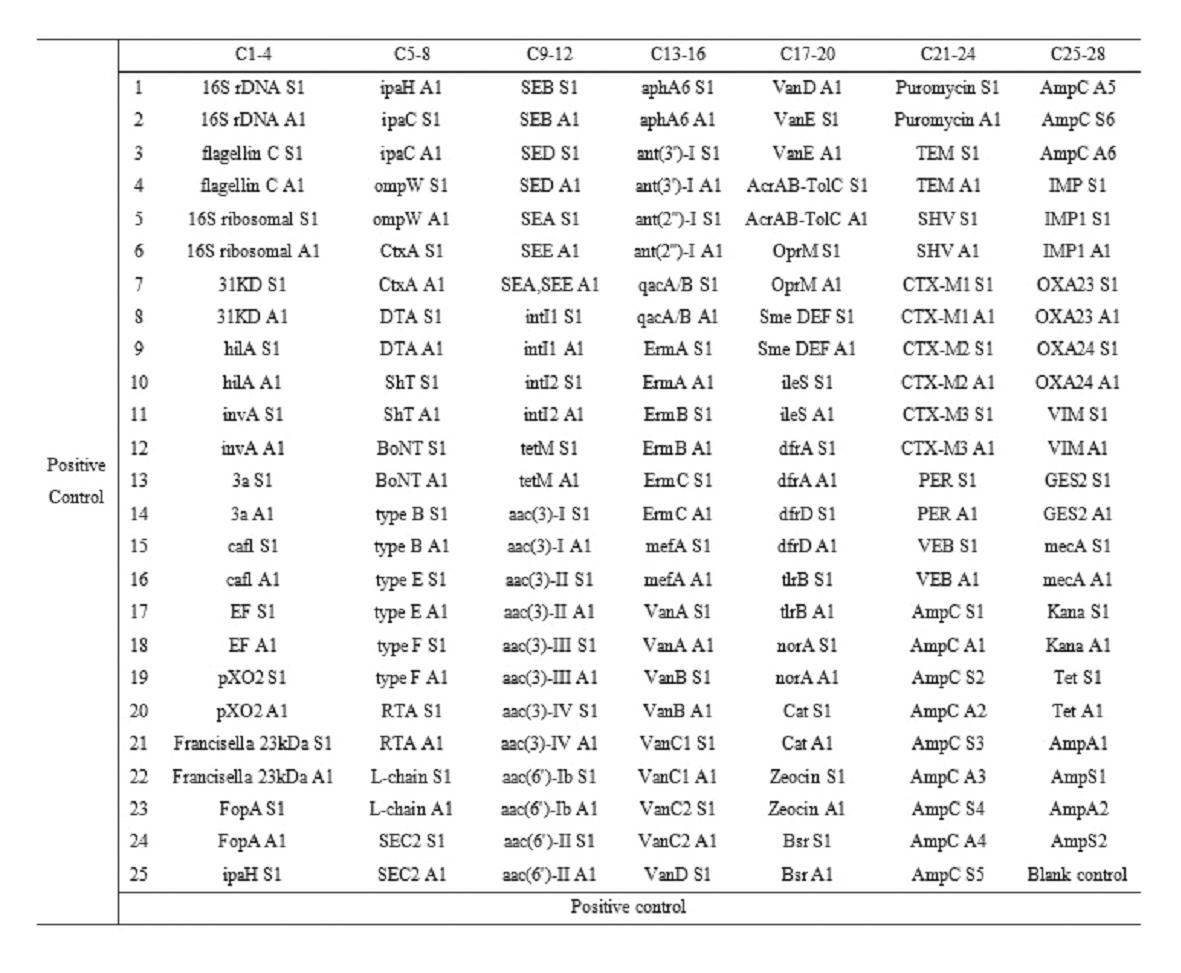
The invention relates to a gene chip for high-flux detection of pathogens and application thereof. The gene comprises (1) a combination of 174 oligonucleotide probes of pathogen variety specific genes, toxin genes and drug-resistant genes; and (2) a probe array, which is formed by curing the oligonucleotide probes on a carrier material by arm molecules. The gene chip comprises 174 gene probes, namely 32 pathogen variety specific gene probes of the following 8 pathogens of Burkholderia mallei, Burkholderia pseudomallei, Brucella, salmonella, Yersinia pestis, Bacillus anthracis, comma bacillus and the like, 25 toxin gene probe of the following 7 toxins of diphtheria toxin, Shiga toxin, staphylococcus enterotoxin, choleratoxin and the like, and 117 drug-resistant gene probes of 17 drug-resistant genes of extended-spectrum beta-lactamase, cephalosporinase, carbapenemase, integrase gene, common gene engineering carrier drug-resistant gene and the like. The gene chip can be used to detect multiple pathogen variety specific genes, toxin genes and drug-resistant genes.
Owner:李越希
Carboxymethylated retroviral regulatory proteins and interferon-α
InactiveUS7022326B1Simple and efficientPeptide/protein ingredientsAntibody mimetics/scaffoldsRetroviral infectionMammal
This invention relates to retroviral regulatory proteins or fragments thereof, or interferon alpha protein or fragments thereof, which are carboxymethylated. This chemical modification leads to new proteins or fragments which are biologically inactive but preserve their immunogenicity (toxoids). These proteins or fragments thereof, or interferon alpha or fragments thereof, can be utilized in the treatment and prevention of retroviral infections. The invention also relates to a pharmaceutical composition comprising at least one carboxymethylated protein or fragment of the invention, together with a pharmaceutically acceptable carrier. The invention also relates to a vaccine comprising at least one of the carboxymethylated proteins or fragments of the invention, together with an immunologically acceptable carrier. The invention also relates to a process for obtaining an immunogenic yet not toxic retroviral regulatory protein or fragment, or interferon alpha or fragment. The invention also relates to a method of inducing an immune response in a mammal, comprising administering the vaccine of the invention to a mammal in an immunologically effective amount.
Owner:BIOVACS +1
Mycoplasma hyopneumoniae avirulent adjuvanted live vaccine
InactiveCN101883581AAntibacterial agentsBacterial antigen ingredientsDiseaseVirulent characteristics
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS W LLC
Enhanced nasal composition of active peptide
InactiveUS20090035260A1Prevent oxidationImprove stabilityPeptide/protein ingredientsMetabolism disorderNasal cavityGoserelin
A pharmaceutical composition has a therapeutically effective amount of at least one of: a pharmaceutically active nasal peptide, its pharmaceutically acceptable salt and its peptidic fragment. The composition also contains an absorbefacient effective amount of THAM in a pharmaceutically acceptable, aqueous liquid diluent or carrier. The composition is provided in a convenient form for nasal administration. In one embodiment, the peptidic fragment may be selected physiologically active lymphokines and monokines, peptidic enzymes, proteic vaccines, peptidic toxoids and personalized proteins derived from genoma. In another embodiment, the peptidic fragment may be selected from the peptide hormones and hormone antagonists buserelin, desmopressin, vasopressin, angiotensin, felypressin, octreotide, somatropin, thyrotropin (TSH), somatostatin, gosereline, thryptorelin and insulin selected from the group consisting of cow and pig, synthetic and recombinant.
Owner:THERAPICON SRL
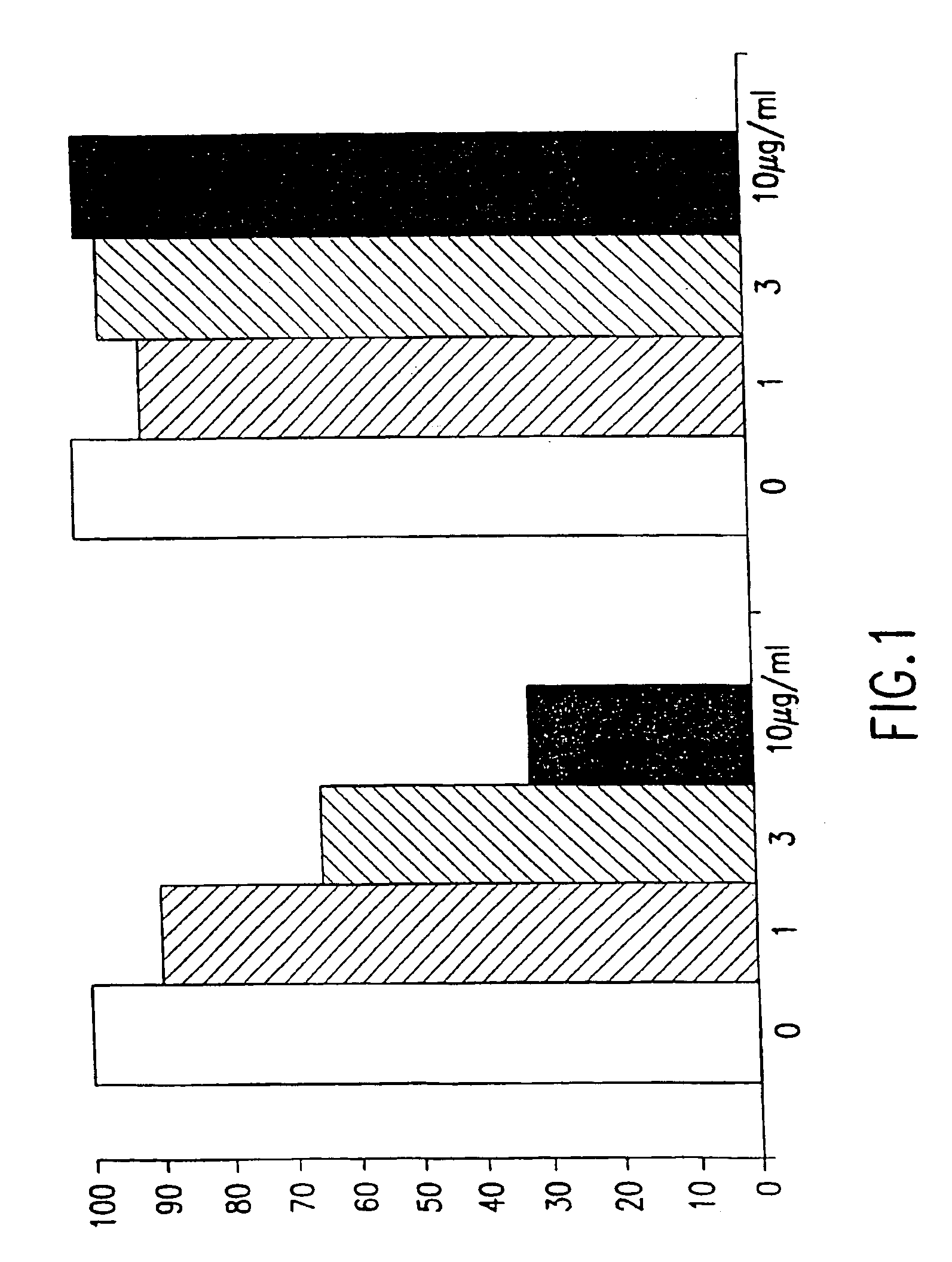
Botulinum antitoxin compositions and methods
InactiveUS20050042775A1Easy to understandAntibacterial agentsBacterial antigen ingredientsPepsin inhibitorSerotype
This invention provides botulinum antitoxin compositions and methods of production, and methods of treating animals and humans prophylactically and also those suspected of having contacted botulism toxin. The botulinum antitoxin is prepared by inoculating an animal with a monovalent botulinum toxoid and toxin. The animal's plasma is collected and purified at a high pH by affinity chromatography. The resulting monovalent immunoglobulins are de-speciated by digestion with pepsin. Monovalent antitoxins for all seven botulinum serotypes are then combined to produce a high titered heptavalent botulinum antitoxin composition.
Owner:INTRACEL RESOURCES
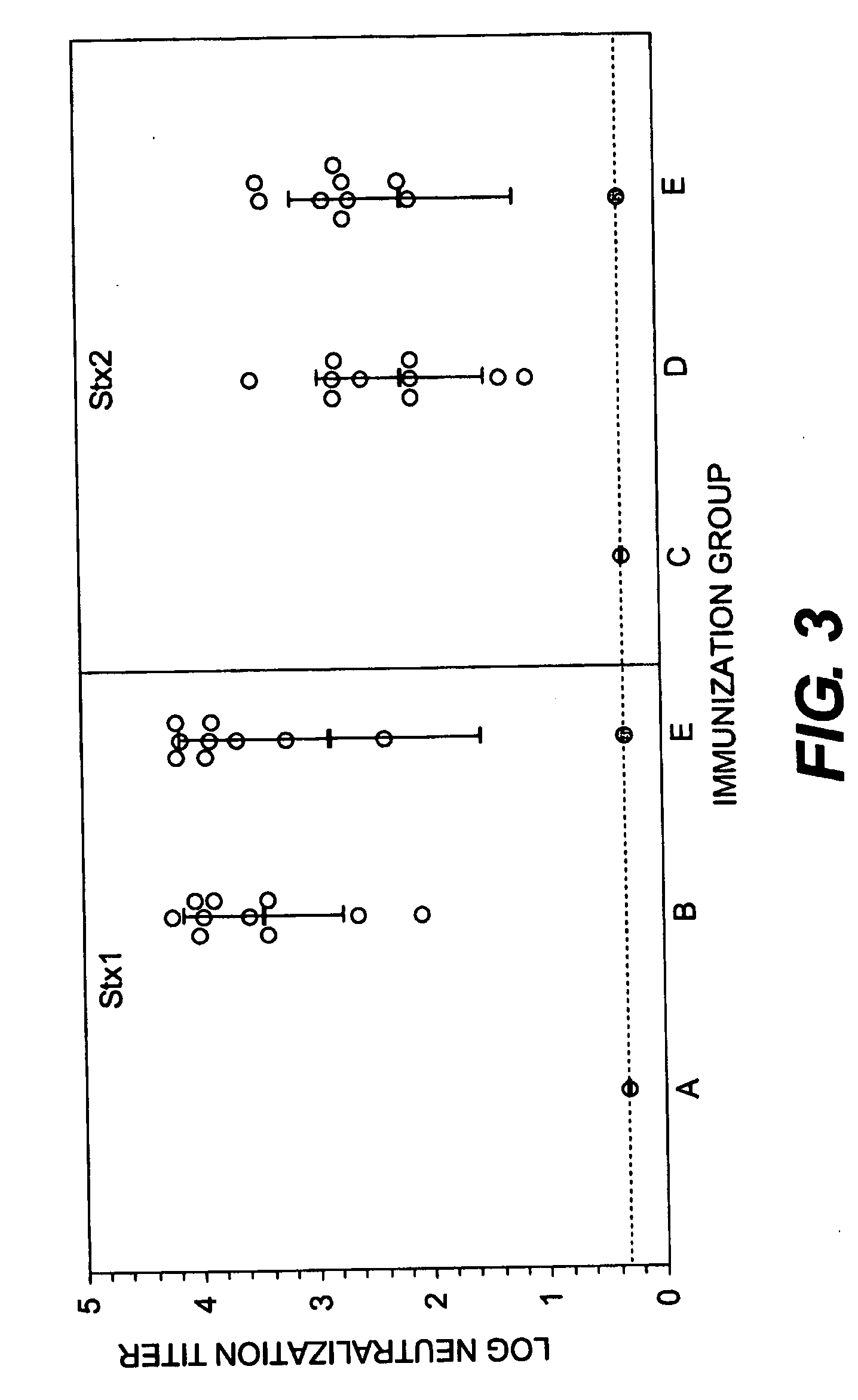
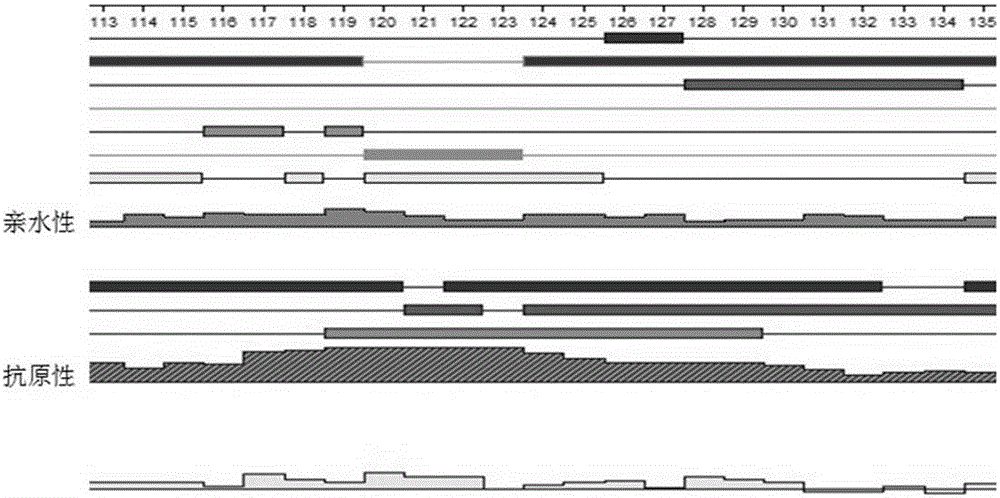
Shiga Toxoid Chimeric Proteins
InactiveUS20090226469A1Reducing and eliminating enzymatic activityReduced enzymatic activityImmunoglobulinsFermentationImmunogenicityShiga bacillus Dysentery
A chimeric Shiga toxoid according to the invention contains an enzymatically-inactivated StxA subunit and a native StxB subunit. This hybrid Shiga toxoid induces the production of broadly cross-reactive species of antibodies against Shiga toxin following immunization. The StxA subunit is modified so that it is enzymatically inactive. The invention thus encompasses the Shiga toxoid or fragments thereof and the nucleic acid sequence of the Shiga toxoid or fragments thereof. The invention further encompasses the production of a Shiga toxoid, the production of antibodies using the Shiga toxoid and methods of productions, and an immunogenic composition containing the Shiga toxoid.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Immunogenic detoxified mutant e. coli lt-a toxin
InactiveUS20030113338A1Maximise adjuvanticityMaximise immunogenicityAntibacterial agentsBiocideEscherichia coliAdjuvant
An immunogenic detoxified protein is provided which comprises the amino acid sequence of subunit A of an E. coli heat labile toxin (LT-A) or a fragment thereof in which at least amino acid Ala-72 of the A subunit is mutated, preferably by substitution with Arg. The toxoid is useful as vaccine against an enterotoxigenic strain of E. coli and is produced by recombinant DNA means by site-directed mutagenesis. It is also an effective adjuvant.
Owner:CHIRON CORP
Antibody Protective Agent And Methods Of Using Same
ActiveUS20110262626A1Improve accuracyImprove stabilityImmunoglobulins against bacteriaBiological material analysisAntibodyEnvironmentally friendly
The invention provides an effective and environmentally friendly antibody protective agent and the methods of using it in immunological detection. The antibody protective agent helps antibody to maintain relatively high immunological activity at room temperature. Working electrodes coated with antibodies and the antibody protective agent are installed in immunological detection devices to enhance stability and accuracy of immunological detection. The antibody protective agent is effectively used in the detection of a variety of toxins, for example, aflatoxin, staphylococcal enterotoxin, algae toxin, and vomitoxin.
Owner:JIANGNAN UNIV +1
Antigen-Drug Vehicle Enabling Switch From Selective Production of IgA Antibody to Production of Both of IgA and IgG Antibodies and Transnasal/Mucosal Vaccine Using the Same
ActiveUS20090130131A1Easy to solveReinforces and promotes prophylactic/therapeutic effectSsRNA viruses negative-senseViral antigen ingredientsMucosal vaccineBody fluid
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid; allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Toxoid preparation and uses thereof
The present invention relates to toxoid preparations comprising a non-disrupted and / or a non-denatured toxin associated with a particulate vector that minimizes or precludes said toxin from inflicting damage at an action site of said toxin. The present invention also relates to immunogenic compositions or vaccines comprising the toxoid preparations, and the methods of using the toxoid preparations, immunogenic compositions or vaccines.
Owner:ARYTHA BIOSCIENCES LLC
Antibody for broad-spectrum detection of Bt Cry1 toxoids as well as preparation method and application thereof
The invention discloses an antibody for broad-spectrum detection of Bt Cry1 toxoids as well as a preparation method and application thereof. The antibody is generated by secretion of hybridoma cells with the collection number of CCTCC NO:C2015145, obtained after coupling antigen polypeptide SEQ ID NO.2 and carrier protein KLH and then immunizing Balb / c mice. The ratio of the antibody to OD450 of six kinds of Bt Cry1 toxoids to negative holes (PBS solution) is far larger than 2.1, and the antibody has relatively high titer, has a broad-spectrum detection effect on the Bt Cry1 toxoids and makes up the blank of the broad-spectrum detection field of the Bt Cry1 toxoids.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Dig-10 insecticidal cry toxins
Owner:CORTEVA AGRISCIENCE LLC
Botulinum antitoxin compositions and methods
Owner:INTRACEL RESOURCES
Modified immunogenic pneumolysin compositions as vaccines
This invention relates to modified pneumolysin polypeptides that retain the immunogenic nature of pneumolysin but have reduced or undetectable hemolytic activity compared to native pneumolysin. The invention also provides a method for generating novel pneumolysin variants with these desired characteristic properties. The invention also provides immunogenic compositions useful as pharmaceutical compositions including vaccines in which non-toxic, modified pneumolysin is used to stimulate protective immunity against Streptococcus pneumoniae. The vaccines may be compositions in which the modified pneumolysin is conjugated to bacterial polysaccharides or may be carried on an attenuated viral vector. In addition, the invention also provides a method of using the non-toxic, modified pneumolysin toxoid in order to stimulate antibodies against Streptococcus pneumoniae in a treated individual which are then isolated and transferred to a second individual, thereby conferring protection against Streptococcus pneumoniae in the second individual.
Owner:MINETTI CONCEICAO +5
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS20010009666A1Antibacterial agentsBacterial antigen ingredientsToxoidAcellular pertussis vaccines
Acellular pertussis vaccines comprise purified toxin or toxoid thereof, filamentous haemagglutinin, pertactin and fimbrial agglutinogens formulated to confer protection to at least 70% of members of an at-risk population. The fimbrial agglutinogens may be prepared from a Bordetella strain, particularly a B. pertussis strain, by a multiple step procedure involving extraction of the fimbrial agglutinogens from cell paste and concentrating and purifying the extracted material.
Owner:AVENTIS PASTUER LTD
Mucosal vaccine enabling switching from production of IgA antibody to production of both of IgA and IgG antibodies
ActiveUS8211442B2Easy to solveReinforces and promotes effectSsRNA viruses negative-senseViral antigen ingredientsBody fluidMucosal vaccine
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid, allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
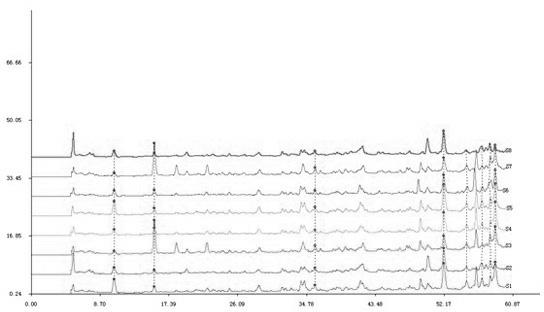
Method for detecting quality of south isatis root granules
The invention discloses a method for detecting the quality of south isatis root granules and belongs to the technical field of quality control of medicines. The method comprises the following steps of: preparing a comparison product solution; preparing a test article solution; performing gradient elution; making a standard fingerprint spectrum by using quinazoline dione as a reference peak; and controlling the quality of the fingerprint spectrum. In the method, quinazoline alkaloid is selected as an index for controlling the quality of south isatis root; the quinazoline alkaloid in south isatis root crude drugs has anti-toxoid and bacteriostatic actions; and the quinazoline alkaloid is relatively stable and small in content change in the south isatis root crude drugs, a preparation process and a preparation finished product. The standard fingerprint spectrum is established by using a high-efficiency liquid phase, the characteristics of active constituents of the south isatis root are taken as the principle things, and quantization parameters are obtained by using the fingerprint spectrum. The invention has the advantages that: the method is higher in accuracy and good in stabilityand repeatability; and the quality of a preparation can be more comprehensively and effectively controlled.
Owner:JIANGXI POZIN PHARMA
Combination vaccines with serogroup B meningococcus and D/T/P
InactiveUS9526776B2Low amountEnhance immune responseAntibacterial agentsImmunological disordersAdjuvantImmunity response
Serogroup B meningococcus antigens can successfully be combined with diphtheria, tetanus and pertussis toxoids (“DTP”) to provide effective combination vaccines for protecting against multiple pathogens. These combinations are effective with a range of different adjuvants, and with both pediatric-type and booster-type DTP ratios. The adjuvant can improve the immune response which the composition elicits; alternatively, by including an adjuvant it is possible for the compositions to have a relatively lower amount of antigen while nevertheless having immunogenicity which is comparable to unadjuvanted combination vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
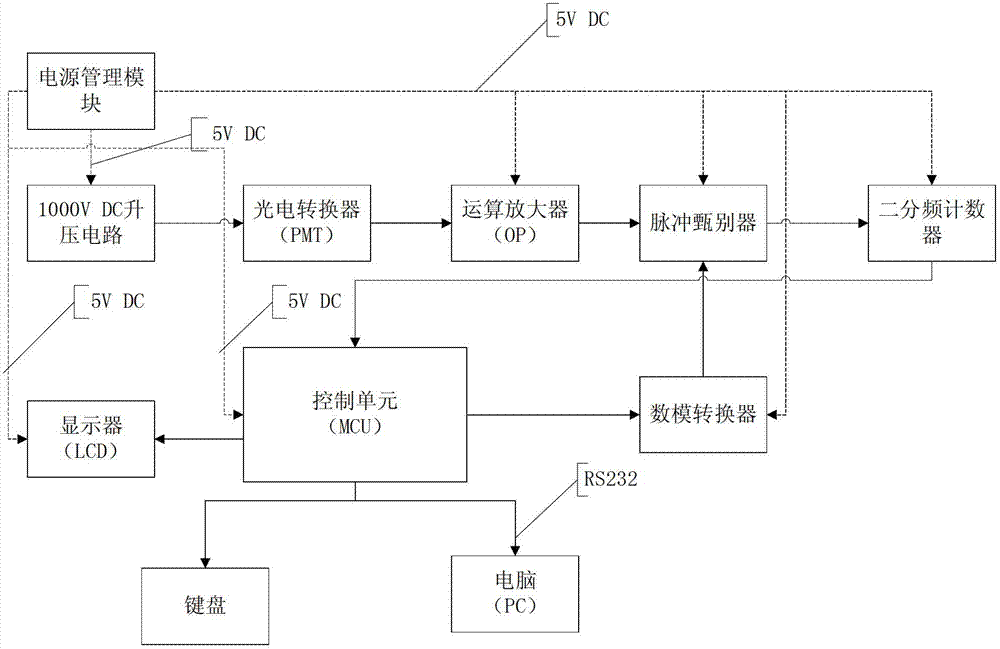
Device and method for acquiring dioxin toxoid signals
InactiveCN102967563AAchieve improvementHigh measurement accuracyColor/spectral properties measurementsFluorescence/phosphorescenceDiscriminatorVoltage amplitude
The invention discloses a device and a method for acquiring dioxin toxoid signals. The device comprises a photoelectric converter, a calculation amplifier, a pulse discriminator, a two divided-frequency counter, a control device and a digital-to-analog converter, wherein weak light emitted from living beings is converted into weak electric signals through the photoelectric converter, and subsequently amplified through the calculation amplifier and transmitted to the pulse discriminator; the pulse discriminator compares the signal voltage amplitude; when the pulse voltage amplitude exceeds a set threshold, a high level signal is output and is transmitted to the control unit through the two divided-frequency counter; the control unit transmits the set pulse voltage amplitude threshold to the pulse discriminator through the digital-to-analog converter; and the control unit is connected with an upper computer. The components are all supplied with power by a power supply management module. A working voltage of the photoelectric converter is supplied by a boost circuit. The invention further discloses an acquisition method based on the device. By utilizing the device and the method, the microsignal amplification is rapidly realized, background noise is effectively eliminated, and the measurement precision is improved.
Owner:GUANGZHOU BLT INSTR & METER
Feed active egg powder with nutrition, healthcare and growth promotion functions and production method thereof
The invention discloses feed active egg powder with nutrition, healthcare and growth promotion functions and a production method thereof, wherein the feed active egg powder with nutrition, healthcare and growth promotion functions is obtained by fully mixing weighed enterotoxin-resistant Escherichia coli tetravalent yolk antibody, C-type clostridium perfringens and beta toxoid-resistant divalent yolk antibody, rotavirus-resistant yolk antibody, transmissible gastroenteritis and epidemic diarrhea-resistant bigeminal yolk antibody, cholecystokinin / somatostatin-resistant double-fusion white and yolk antibody, lysozyme meringue powder, epidermal growth factor-containing meringue powder, common meringue powder and silica (antitackiness agent) for 60 to 100 seconds. The feed active egg powder with nutrition, healthcare and growth promotion functions provided by the invention has the beneficial effects of containing high-quality protein, fat and amino acid required for the growth and development of piglets, containing lysozyme, yolk antibody and epidermal growth factor for preventing disease, protecting health and promoting growth, and having four efficacies of enhancing the feed intake,accelerating the growth, boosting the immunity and controlling the diarrhea of the piglets before and after weaning.
Owner:赛法特(长沙)生物技术有限公司
Fermentation process
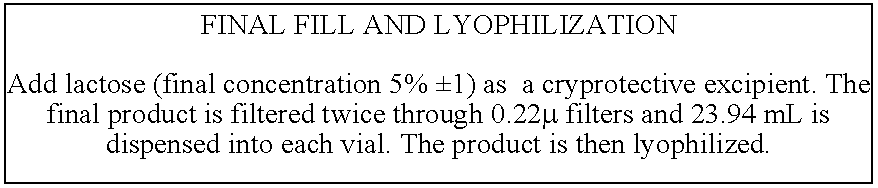
The present invention provides a process for periplasmic expression of a bacterial toxoid comprising the steps of:a) growing a culture of a gram negative host cell in a fermentation medium, wherein the host cell is transformed with a polynucleotide, and wherein the polynucleotide encodes the bacterial toxoid and a periplasmic signal sequence; or providing a gram negative host cell wherein the host cell is transformed with a polynucleotide, the polynucleotide encodes the bacterial toxoid and a periplasmic signal sequence and wherein the gram negative host cell comprises the bacterial toxoid expressed in the periplasm;a(i)) inducing expression of the bacterial toxoid;b) maturing the host cell, wherein the maturing step comprises:I) subjecting the host cell to a pH shock;II) incubating the host cell with no feed addition; and / orIII) subjecting the host cell to a temperature below −20° C.; andc) extracting the bacterial toxoid from the host cell wherein the extraction process comprises osmotic shock.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Shiga toxoid chimeric proteins
InactiveUS8846058B2Reducing and eliminating enzymatic activityBacterial antigen ingredientsDigestive systemAntiendomysial antibodiesImmunogenicity
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com