Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Mucosal vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
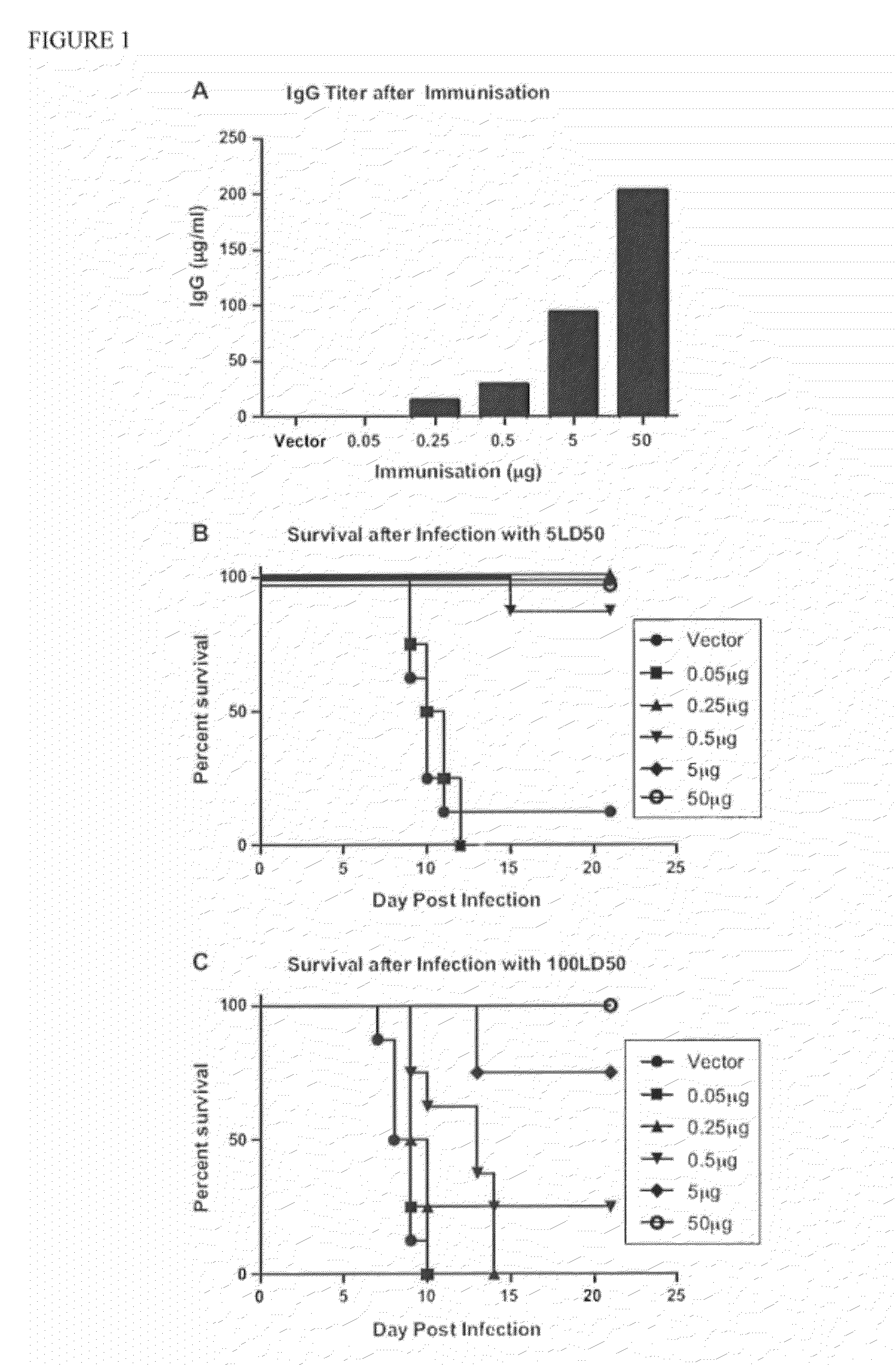
Mucosal vaccination involves the administration of vaccines at one or more mucosal sites leading to induction of immune responses at the mucosal site of administration, other mucosal sites, and/or systemically.
Novel, non-antigenic, mucosal adjuvant formulation which modulates the effects of substances, including vaccine antigens, in contact with mucosal body surfaces
InactiveUS20030104010A1Antibacterial agentsSsRNA viruses negative-senseMucosal adjuvantVaccine antigen
Adjuvant for mucosal vaccines which modulates the effects of substances, including vaccine antigens in contact with mucosal body surfaces.
Owner:BIOTEC PHARMACON
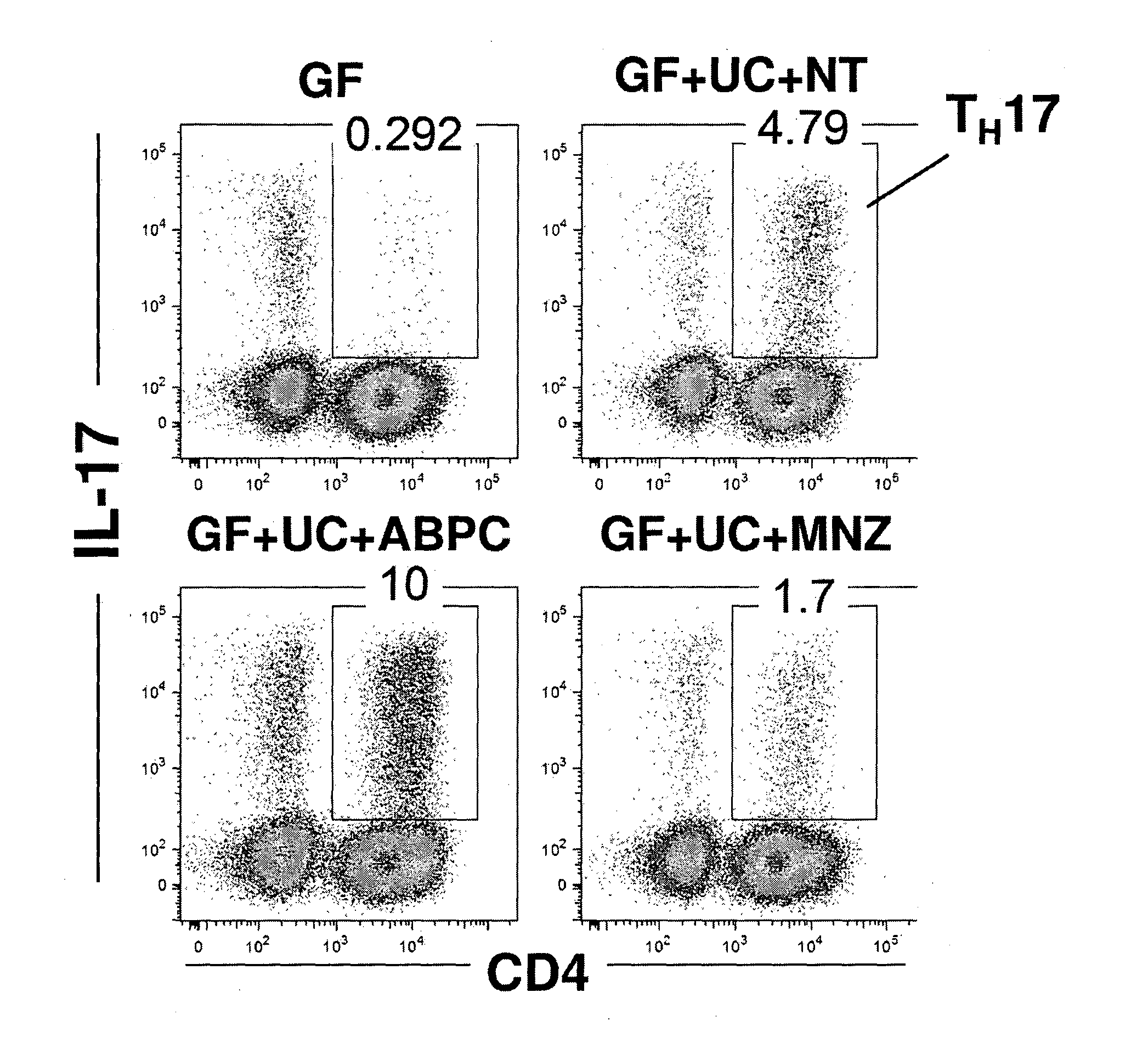
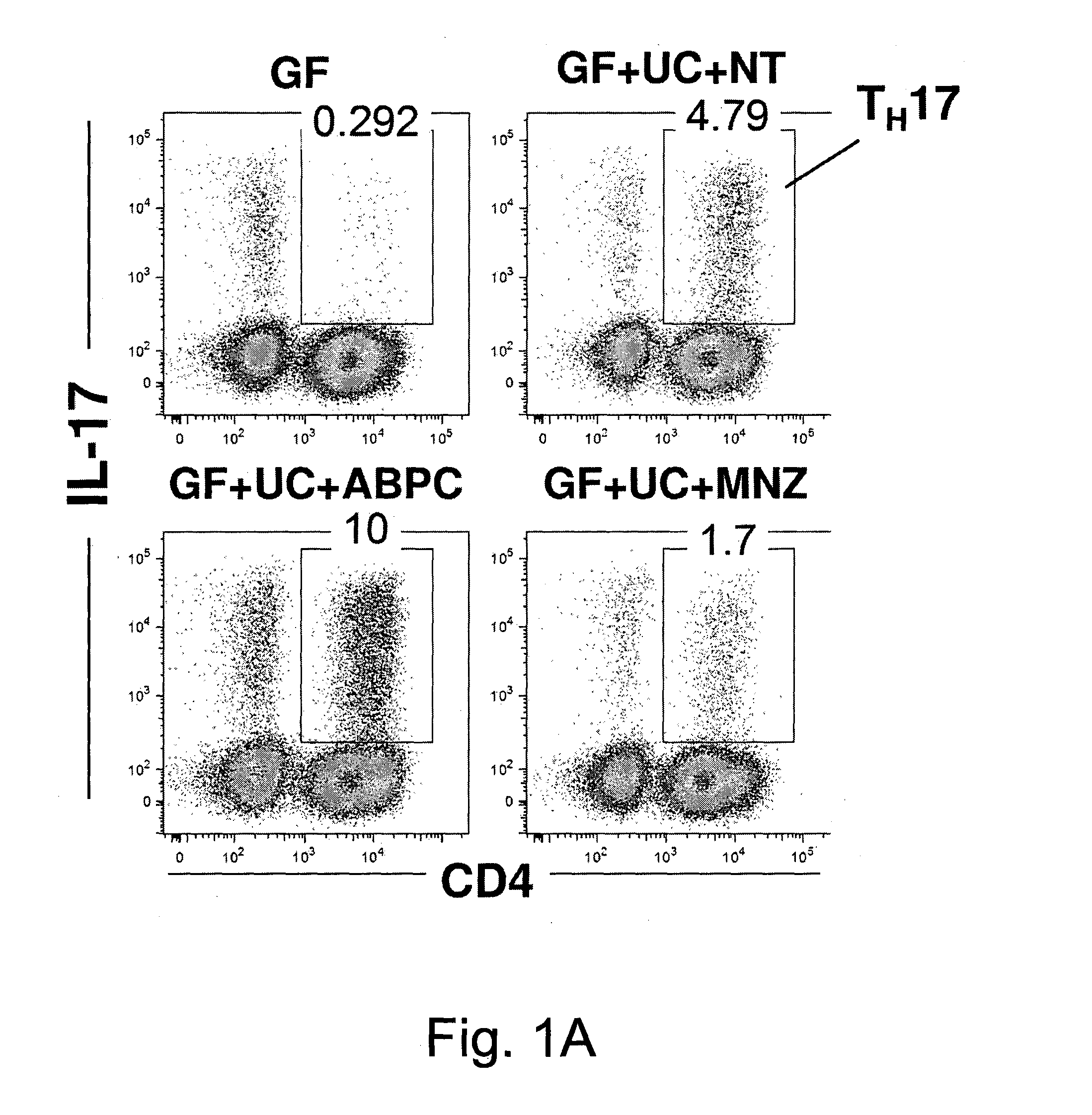
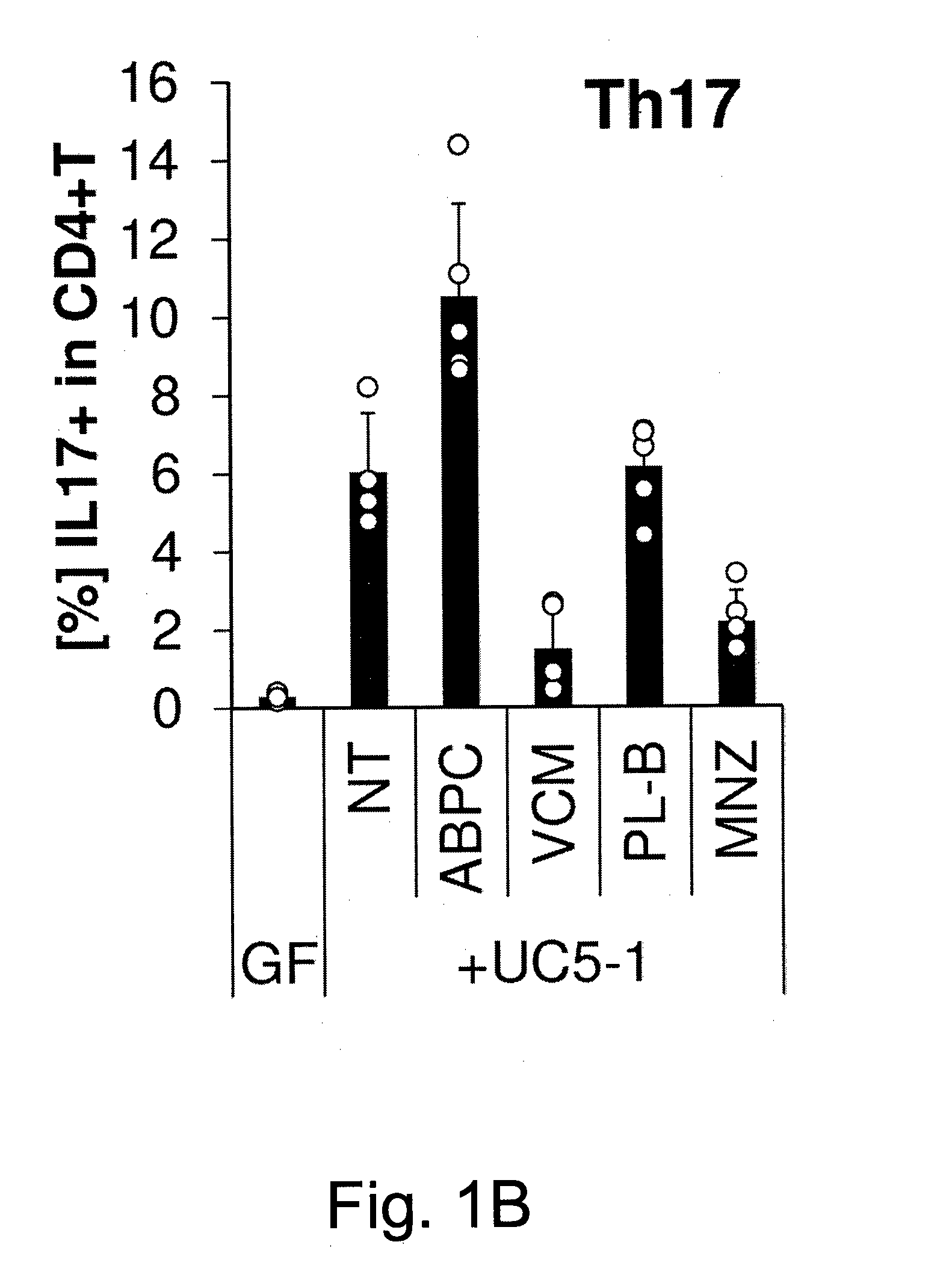
Compositions and methods for induction of th17 cells
ActiveUS20170028061A1Induced proliferationInduce accumulationBacteria material medical ingredientsSaccharide peptide ingredientsBacteroidesIntestinal structure
Owner:THE UNIV OF TOKYO +2
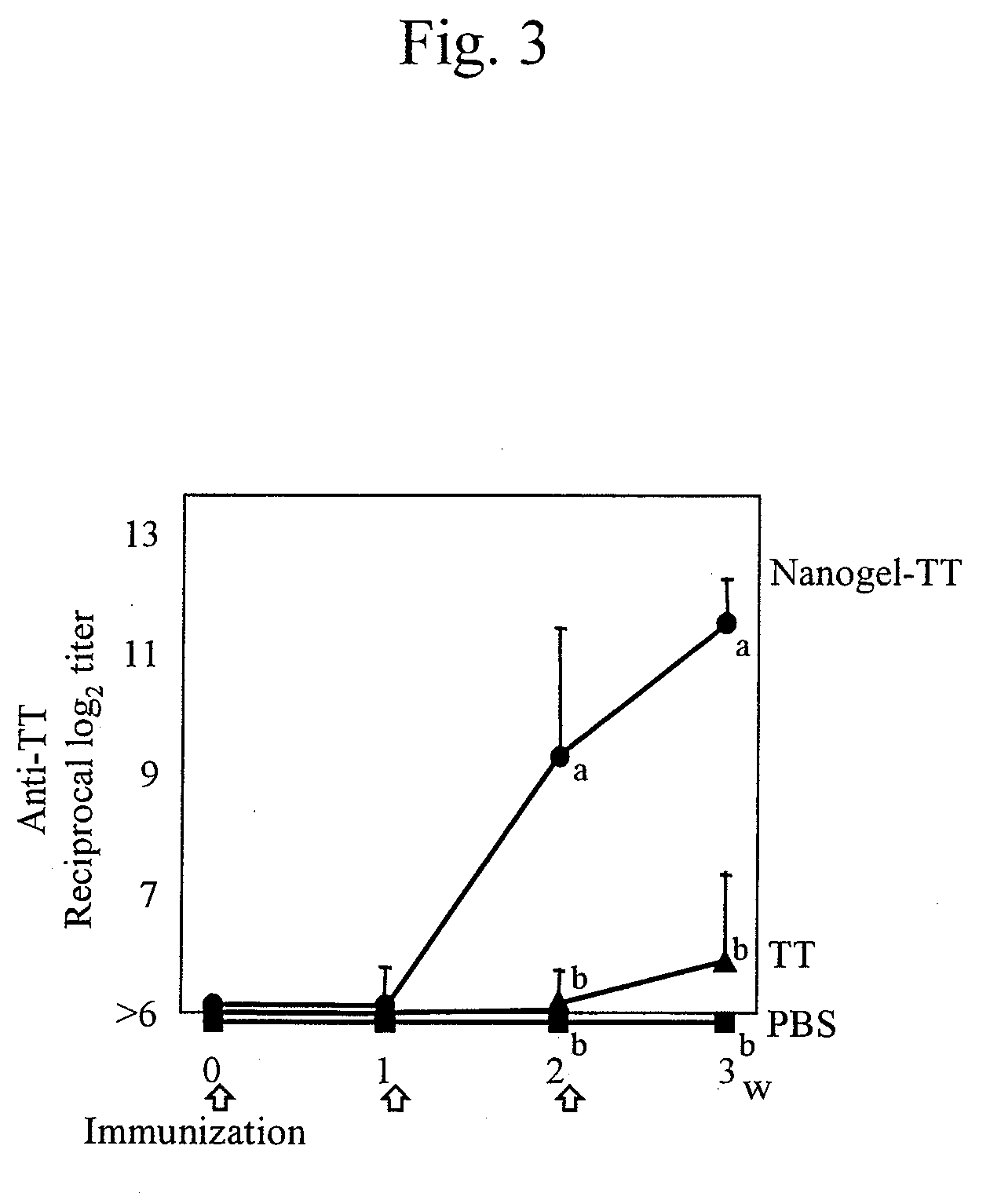
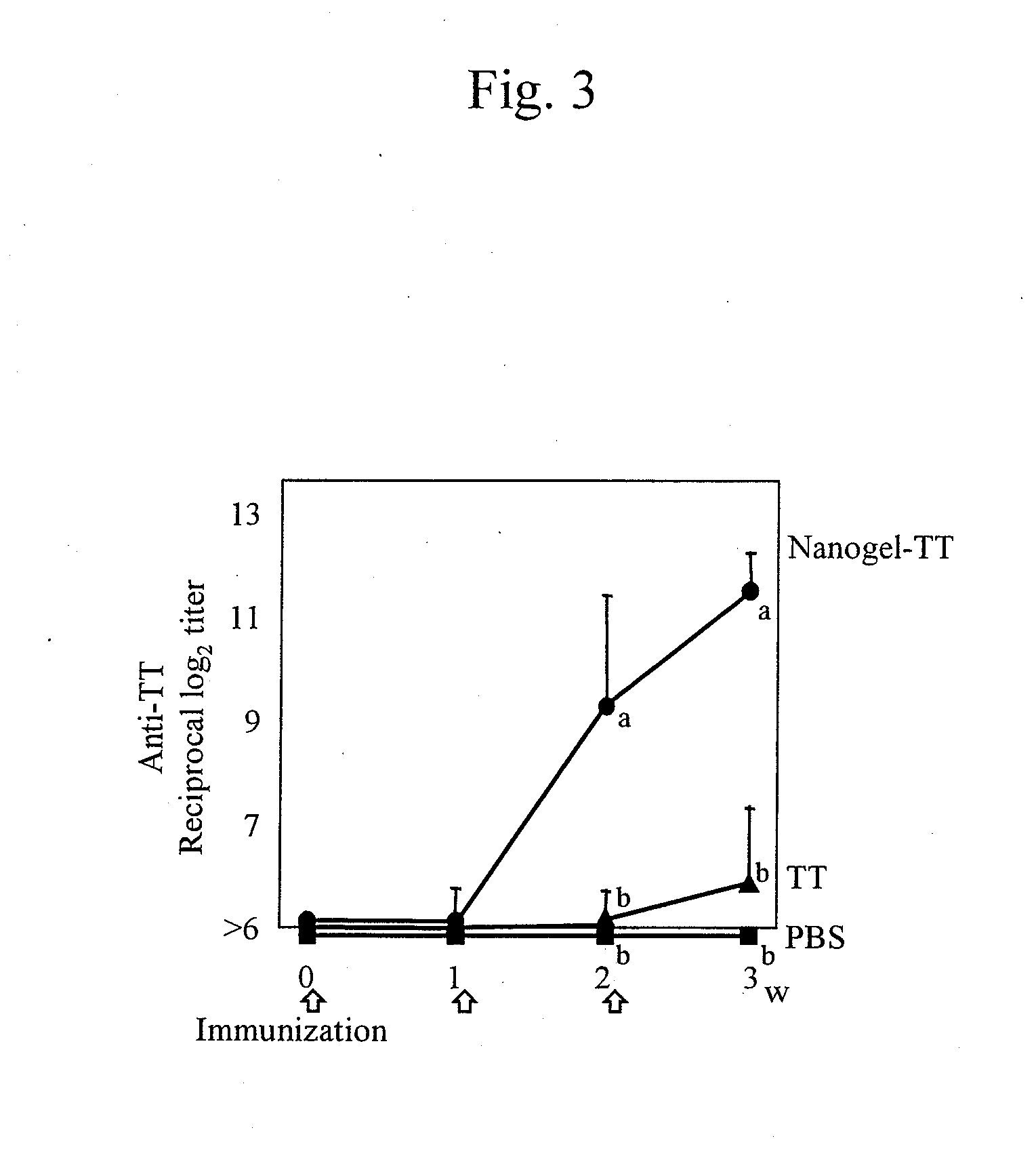
Mucosal vaccine using cationic nanogel
InactiveUS20110206729A1Effectively induces systemic and mucosal immune responseEfficient deliveryAntibacterial agentsBacterial antigen ingredientsMedicineCholesterol
A mucosal vaccine for the prevention or treatment of microbial infections is described that is capable of inducing vaccine antigen-specific immune responses in an organism without the addition of a mucosal adjuvant. The mucosal vaccine comprises a composite of a nanogel comprising a hydrophilic polysaccharide having a cationic functional group and a hydrophobic cholesterol added thereto as a side chain and a vaccine antigen. The vaccine is administered via a mucosal route.
Owner:NAT UNIV CORP TOKYO MEDICAL & DENTAL UNIV
Mucosal vaccine adjuvants containing bacterial flegellins as an active component
ActiveCN1909924ABacterial antigen ingredientsAntiinfectivesListerella paradoxaListeria monocytogenes
The present invention relates to mucosal vaccine adjuvants containing flagellins, the structural component of flagella, originated from Vibrio vulnificus, Salmonella typhimurium, and Listeria monocytogenes as an active component.
Owner:李浚行 +1
Mucosal vaccines with chitosan adjuvant and meningococcal antigens
InactiveUS20060051378A1Improve securityReduced dosAntibacterial agentsOrganic active ingredientsSalmonella serotype typhiCarrier protein
The invention provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The invention also provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred.
Owner:NOVARTIS AG +1
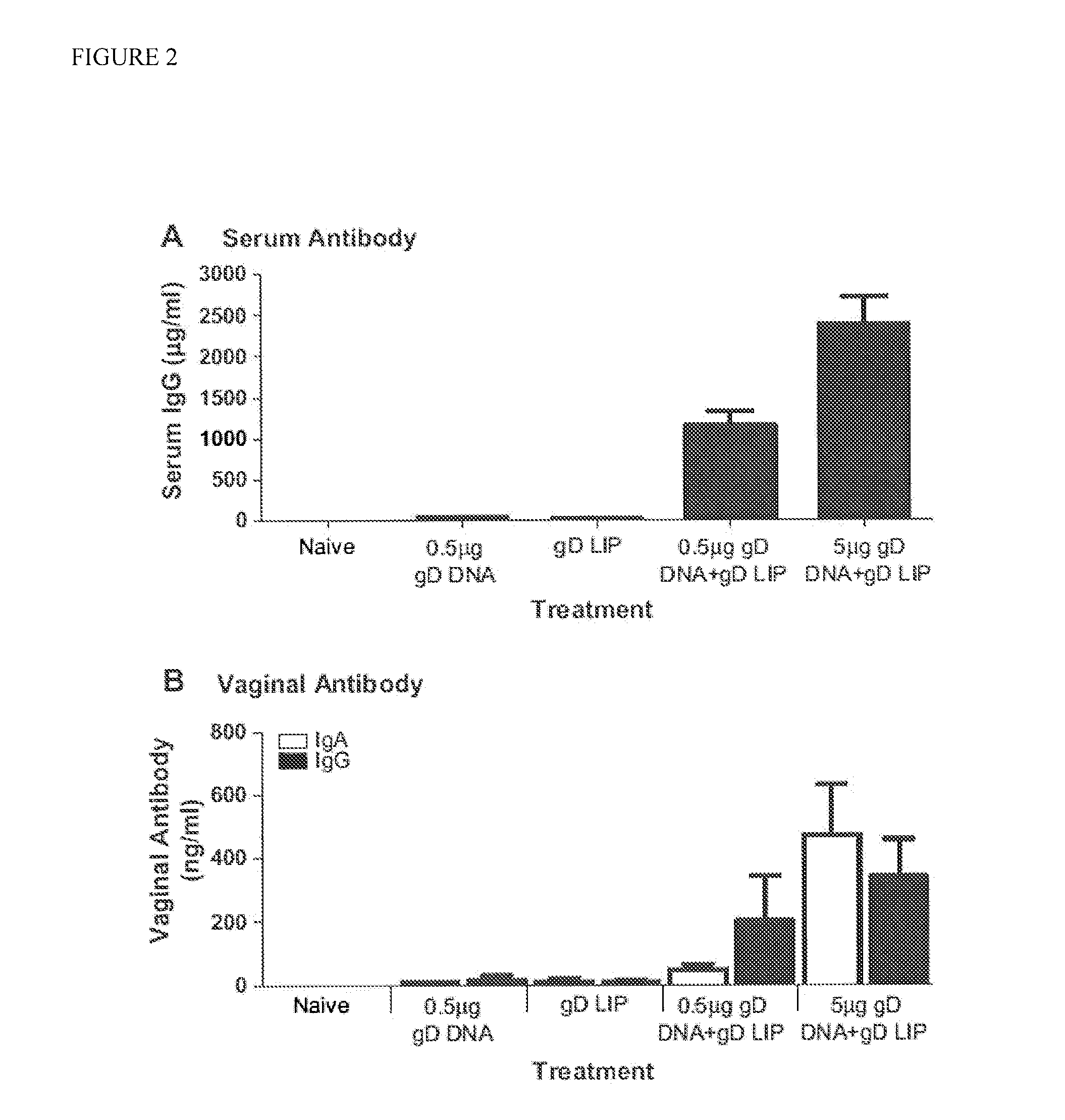
Novel mucosal vaccination approach for herpes simplex virus type-2
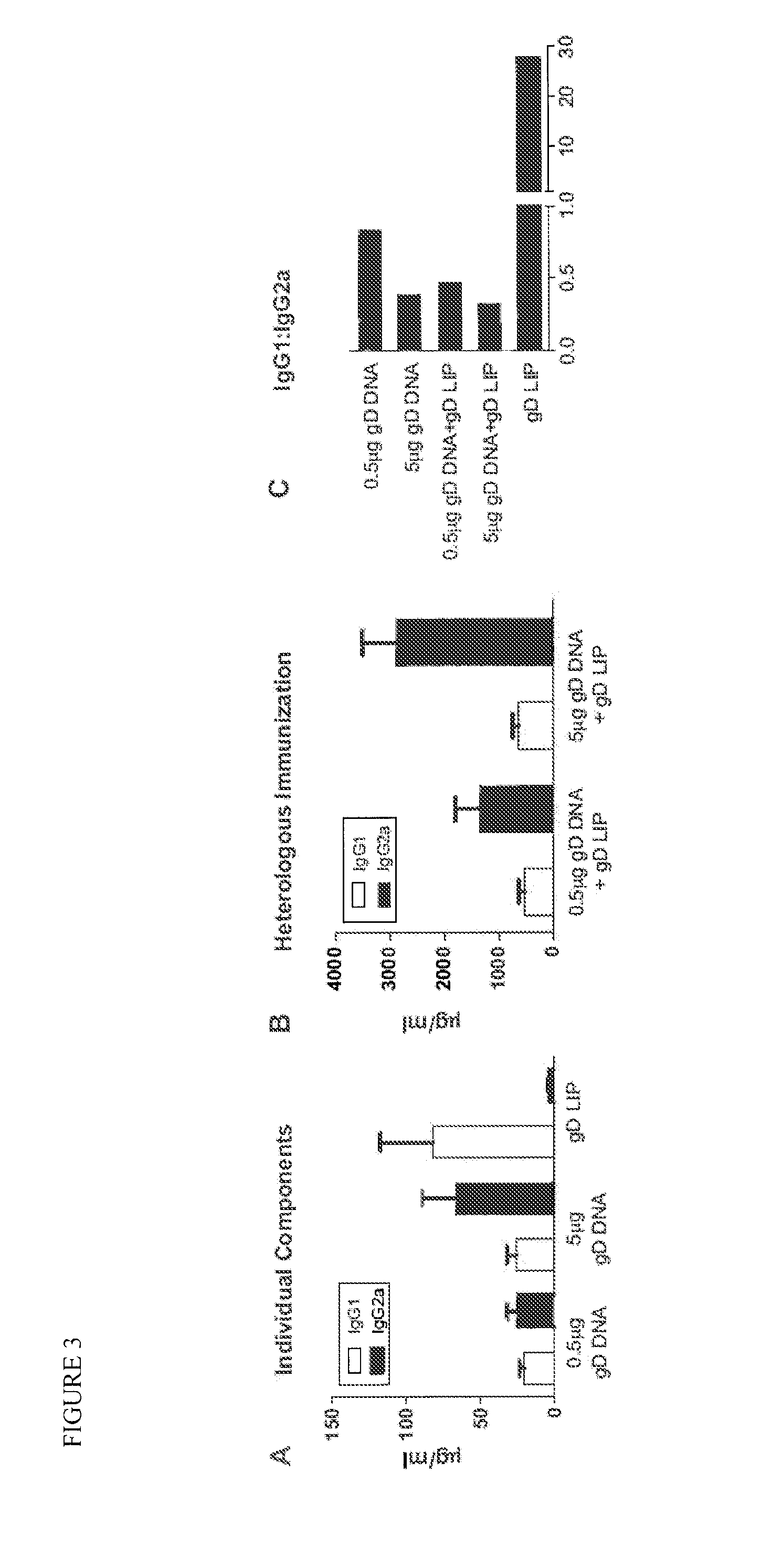
InactiveUS20120027841A1High potencyPrevent relapseOrganic active ingredientsViral antigen ingredientsHeterologousLiposome
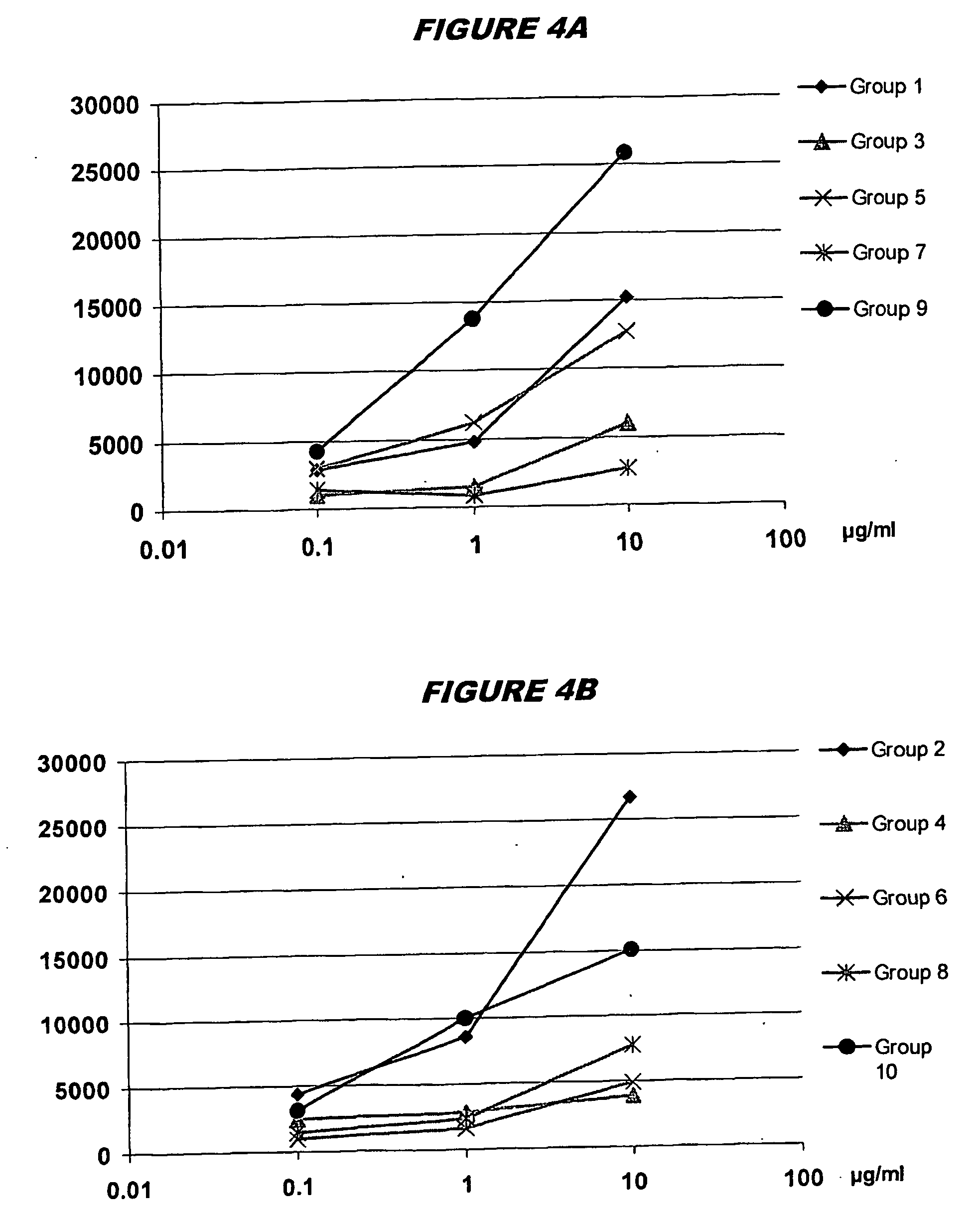
The invention provides methods and kits for immunizing animals (e.g. mammals) against viral antigens, including herpes-simplex virus type 2. The protective immune response elicited by the methods and kits of the invention is characterized by robust humoral, cellular, and mucosal immunity. In particular, the invention provides a heterologous immunization method comprising a priming DNA vaccine encoding an antigen and a boosting protein vaccine, in which the protein form of the antigen is encapsulated in liposomes. Methods of preventing primary acute, latent and recurrent viral infections, such as that caused by HSV-2 virus, and methods of providing passive protective immunity against a viral pathogen such as HSV-2 virus to a mammal are also disclosed.
Owner:MUCOSAL VACCINE TECH LLC
Antigen-Drug Vehicle Enabling Switch From Selective Production of IgA Antibody to Production of Both of IgA and IgG Antibodies and Transnasal/Mucosal Vaccine Using the Same
ActiveUS20090130131A1Easy to solveReinforces and promotes prophylactic/therapeutic effectSsRNA viruses negative-senseViral antigen ingredientsMucosal vaccineBody fluid
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid; allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Composite superimmunogen for bi-functional vaccine use for the treatment of illnesses associated with a stromal tissue disorder
The invention is relative to novel means of systemic or mucosal vaccinial therapy against some cancers, viral infections and allergy which are provided by the invention under the form of a family of composite superimmunogenic compounds for bifunctional vaccinial use able to induce an immune response raised towards two distinct targets, respectively, the causal pathogenic antigenic structure, on the one hand, and locally produced factors responsible for a subsequent immunotoxic or neoangiogenic stroma disorder, on the other hand.
Owner:NEOVACS SA
Novel, non-antigenic, mucosal adjuvant formulation which enhances the effects of substances, including vaccine antigens, in contact with mucosal body surfaces
InactiveUS20020009463A1Antibacterial agentsSsRNA viruses negative-senseMucosal adjuvantVaccine antigen
Adjuvant for mucosal vaccines which modulates the effects of substances, including vaccine antigens in contact with mucosal body surfaces.
Owner:BIOTEC PHARMACON
Novel prime-boost combinations of attenuated mycobacterium
InactiveUS20090304750A1Effective protectionOrganic active ingredientsBacterial antigen ingredientsPrime boostMucosal vaccine
The present invention provides vaccine compositions for effective induction of both mucosal and systemic immunity to pathogenic Mycobacterium species. Vaccination protocols are provided in which both parenteral and mucosal vaccine formulations are administered to a host. The parenteral and mucosal formulations comprise live, attenuated Mycobacteria.
Owner:HONE DAVID +1
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Mucosal vaccine enabling switching from production of IgA antibody to production of both of IgA and IgG antibodies
ActiveUS8211442B2Easy to solveReinforces and promotes effectSsRNA viruses negative-senseViral antigen ingredientsBody fluidMucosal vaccine
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid, allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Mucosal vaccine composition
InactiveUS20160213773A1Safe and effectiveSsRNA viruses negative-senseBacterial antigen ingredientsMucosal Immune ResponsesXanthomonas
The present invention aims at providing a vaccine composition capable of being administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is safe, useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and capable of effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, the mucosal vaccine composition containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof, wherein a mass ratio between the adjuvant and the antigen (total mass of the adjuvant / total mass of the antigen) is 0.002 to 500.
Owner:NITTO DENKO CORP
Curdlan sulfate/6-O-quaternized chitosan nanoparticles and application thereof in mucosal vaccines
InactiveCN107625961APromote proliferationPromote activationAntiinfectivesRespiratory disorderEpitheliumMucosal adjuvant
Nanoparticles are prepared from curdlan sulfate and 6-O-quaternized chitosan by an ion gelation method for the first time; the method is simple and convenient, green, safe and pollution-free, and through controlling experimental steps and parameters, the prepared curdlan sulfate / 6-O-quaternized chitosan nanoparticles have uniform size and good stability. Evaluation of the membrane penetrating ability, the immune regulation ability and the mucosal adjuvant effect of the curdlan sulfate / 6-O-quaternized chitosan nanoparticles shows that the positively charged characteristic of the nanoparticles make the nanoparticles more easily act on effective parts through epithelium mucosae, so as to play a role of immune enhancers and help an antigen reach the effective parts to play relatively good immune adjuvant effect; the nanoparticles have great industrial application value.
Owner:SHANDONG UNIV
Antigen-drug vehicle enabling transmucosal and transdermal administration, and method of inducing mucosal immunity and mucosal vaccine and DDS using the same
ActiveUS8268321B2Excellent infection defense effectReinforces and promotes preventive and therapeutic effect of drugPeptide/protein ingredientsViral antigen ingredientsSecretory IgA antibodyLocal immunity
Owner:UNIVERSITY OF TOKUSHIMA
Delivery system and application of chitosan mucosa compromising mucosal adjuvant
InactiveCN101204364AConvenient inductionOrganic active ingredientsViral antigen ingredientsMucosal adjuvantViral Myocarditis
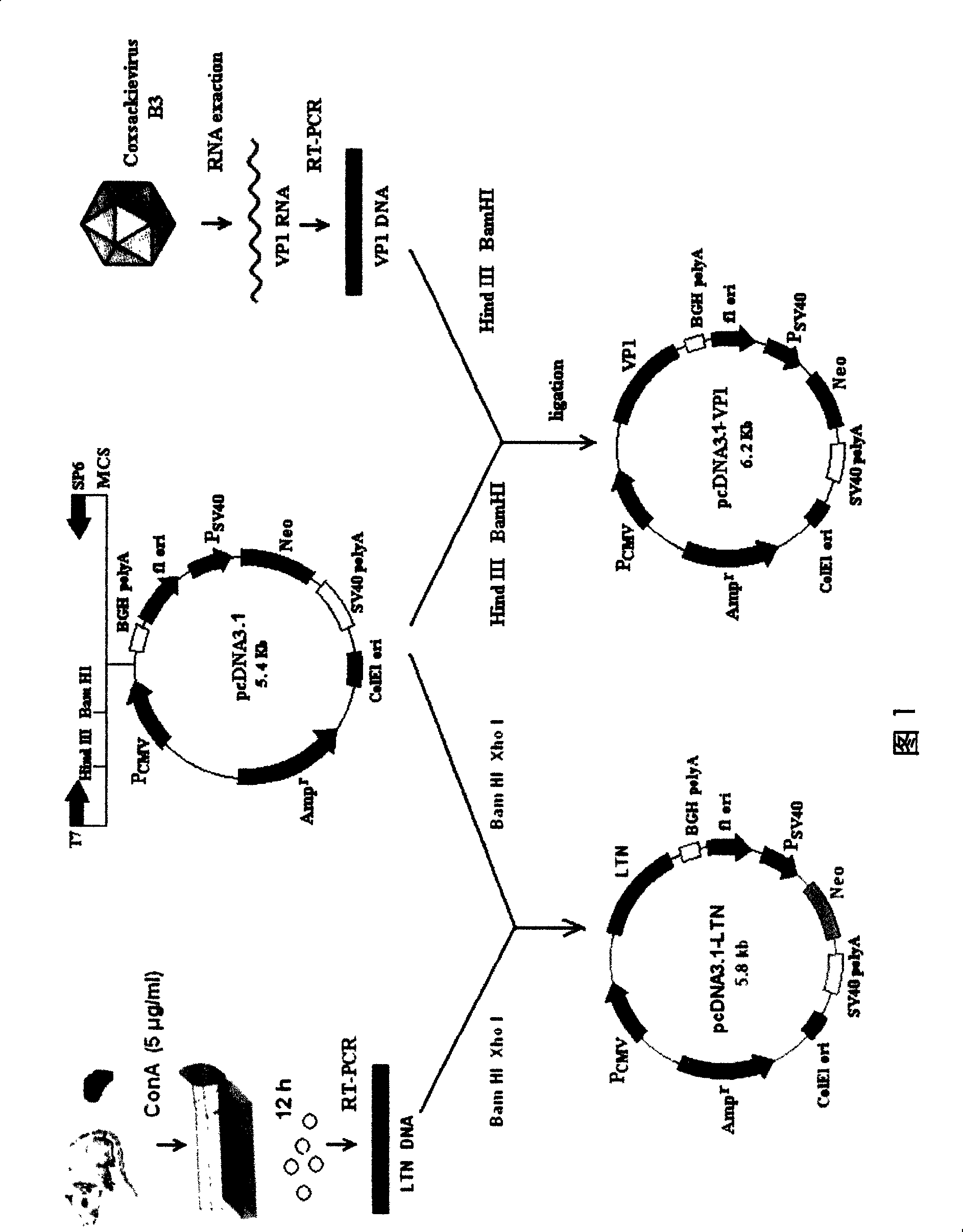
The invention relates to a chitosan mucosal delivery system, and the invention comprises two components which are the target antigen nanoparticles and the mucosal adjuvant nanoparticles. The target antigen nanoparticle comprises chitosan and target antigen coding plasmid DNA; the mucosal adjuvant nanoparticle comprises chitosan and mucosal adjuvant coding plasmid DNA. The mucosal vaccine which is produced according to the mucosal delivery system comprises two components, a component is the chitosan-VP1 nanoparticle which is formed by the chitosan and the plasmid of the coding CVB3 structural protein VP1 and the other component is the chitosan-VP1 nanoparticle which is formed by the chitosan and the plasmid of the coding lymphocyte chemotactic factor. By using the mucosal delivery system to carry out the mucosal immunity, the secreting of CVB3 specific generalization IgG and intestinal tract SIgA is induced effectively, and the occurrence of the Coxsackie vital myocarditis is effectively prevented.
Owner:FUDAN UNIV
Mucosal vaccine adjuvants containing bacterial flagellins as an active component
ActiveUS7914802B2Increasing mucosal IgAEasy to produceBacterial antigen ingredientsDepsipeptidesVibrio vulnificusActive component
The present invention relates to mucosal vaccine adjuvants containing flagellins, the structural component of flagella, originated from Vibrio vulnificus, Salmonella typhimurium, and Listeria monocytogenes as an active component.
Owner:RHEE JOON HAENG +1
Novel prime-boost combinations of attenuated mycobacterium
The present invention provides vaccine compositions for effective induction of both mucosal and systemic immunity to pathogenic Mycobacterium species. Vaccination protocols are provided in which both parenteral and mucosal vaccine formulations are administered to a host. The parenteral and mucosal formulations comprise live, attenuated Mycobacteria.
Owner:AERAS GLOBAL TB VACCINE FOUND
Antigen-and-drug vehicle comprising synthetic peptide, and mucosal vaccine using the same
ActiveUS8287887B2Higher phylactic effectGood for healthSsRNA viruses negative-sensePeptide-nucleic acidsSecretory IgA antibodyMucosal vaccine
The present invention is an antigen-and-drug (AD) vehicle and a mucosal vaccine utilizing a novel synthetic peptide. The antigen-and-drug (AD) vehicle is capable of inducing the production of secretory IgA antibodies, and is a complex of a synthetic peptide having the following amino acid sequence: PVHLKRLm (e.g., peptide of SEQ ID NO 1, 6, or 7) or KnLm (e.g., peptide of SEQ ID NO 2, 3, or 8), and a lipid(s). The mucosal vaccine is obtainable by allowing a mucosal-immunity-IgA-inducing amount of an antigen to coexist with, contact, be captured by, or be adsorbed onto the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Adjuvant for mucosal vaccine
InactiveUS20150306214A1High efficacyImprove securitySsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininProtein-protein complex
An object of the present invention is to provide an adjuvant for a mucosal vaccine with high safety that induces a sufficient immune response on the mucosa. According to the present invention, an adjuvant for a mucosal vaccine comprising a protein complex composed of hemagglutinin (HA) subcomponents HA1, HA2, and HA3 of botulinum toxin is provided.
Owner:DAIICHI SANKYO CO LTD +1
Mucosal Vaccine Adjuvants Containing Bacterial Flagellins as an Active Component
ActiveUS20080069844A1Easy to produceIncreasing mucosal IgABacterial antigen ingredientsDepsipeptidesVibrio vulnificusActive component
The present invention relates to mucosal vaccine adjuvants containing flagellins, the structural component of flagella, originated from Vibrio vulnificus, Salmonella typhimurium, and Listeria monocytogenes as an active component.
Owner:RHEE JOON HAENG +1
Mucosal vaccines with chitosan adjuvant and meningococcal antigens
InactiveUS8926992B2Protective efficacyImproving immunogenicityAntibacterial agentsBiocideSalmonella serotype typhiCarrier protein
The invention provides immunogenic compositions comprising (a) a capsular saccharide antigen from serogroup C of N. meningitidis, and (b) a chitosan adjuvant. The composition preferably comprises (c) one or more further antigens and / or (d) one or more further adjuvants. The compositions are particularly suitable for mucosal delivery, including intranasal delivery. The invention also provides immunogenic compositions for mucosal delivery comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of N. meningitidis. It is preferred that the capsular saccharides in the compositions of the invention are conjugated to carrier protein(s) and / or are oligosaccharides. Conjugated oligosaccharide antigens are particularly preferred.
Owner:NOVARTIS AG +1
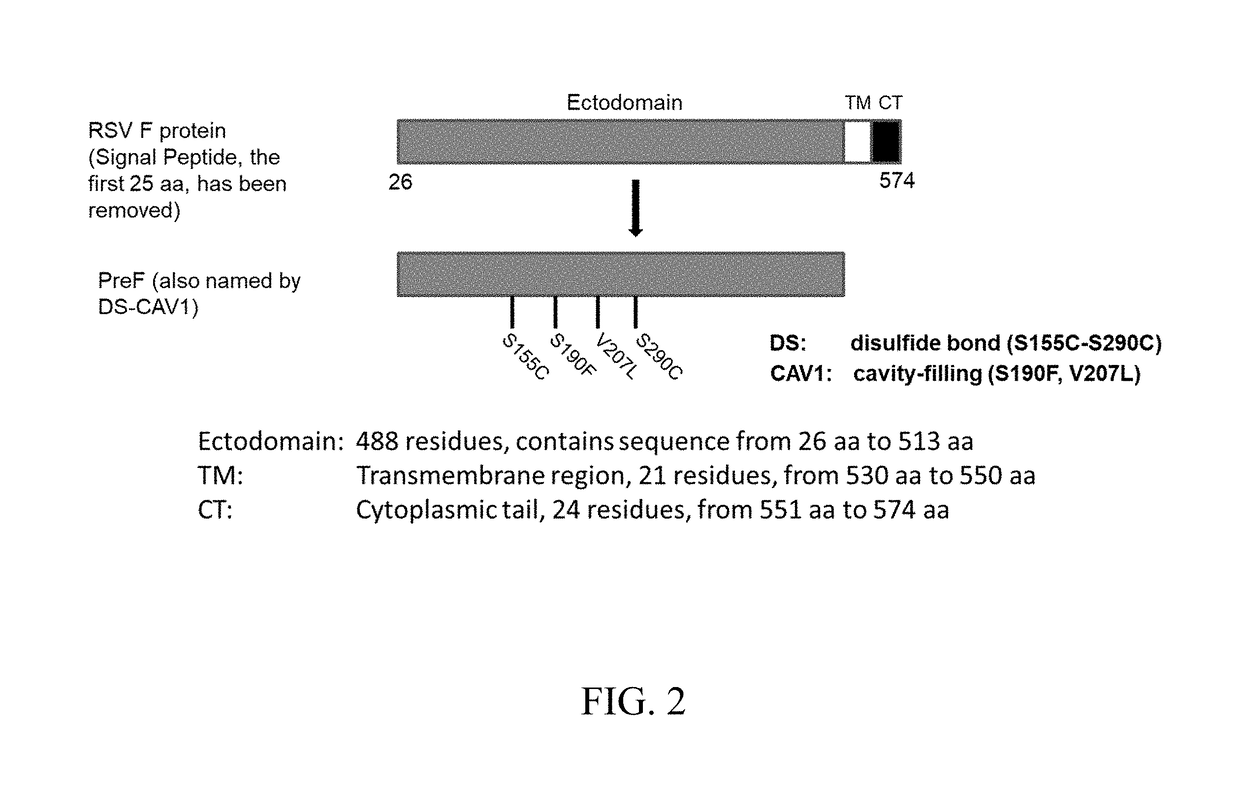
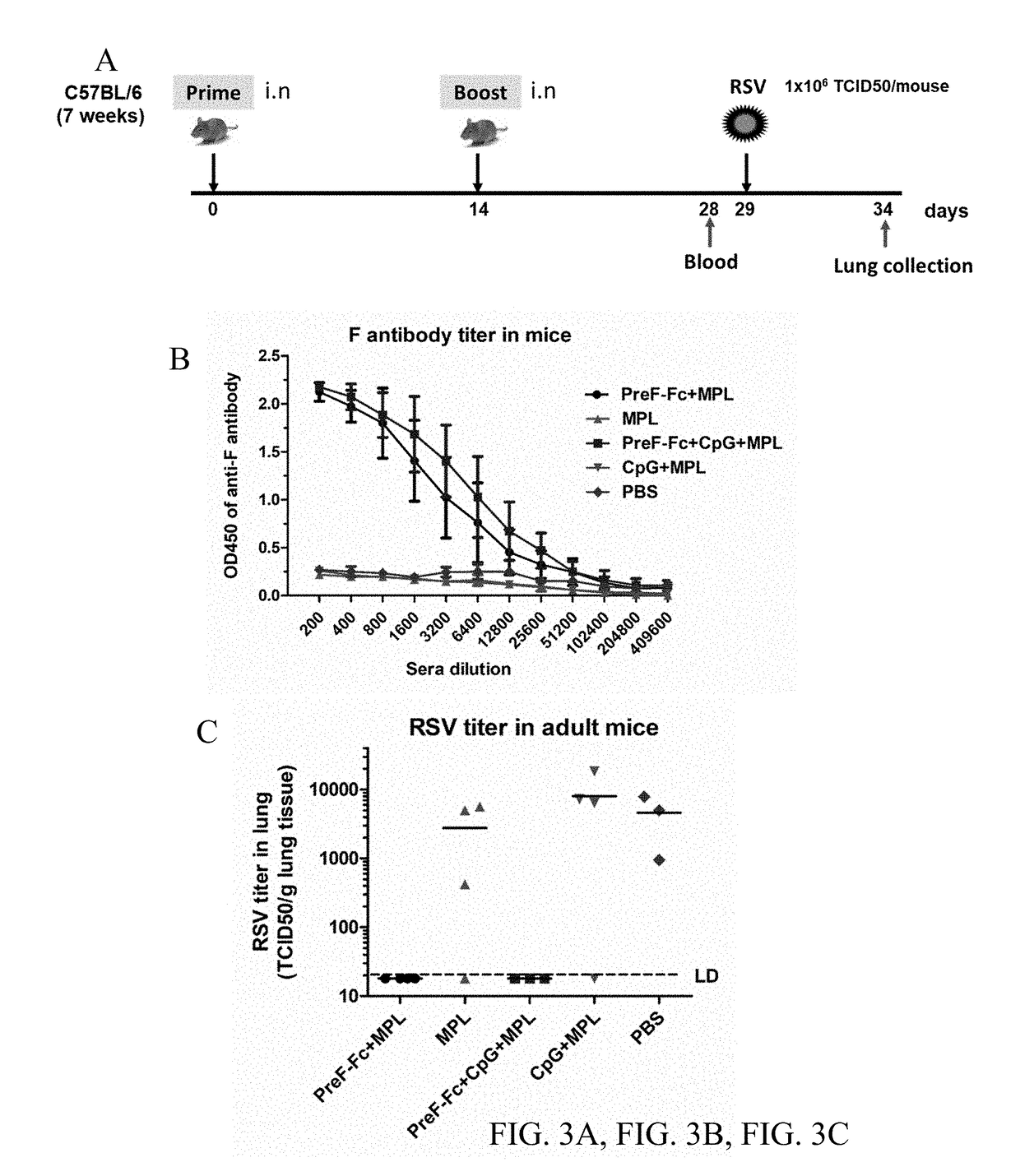
FCRn-Targeted Mucosal Vaccination Against RSV
ActiveUS20190060440A1Protective immune responseSsRNA viruses negative-senseAntibody mimetics/scaffoldsImmunglobulin eF protein
Disclosed are peptides comprising a monomeric Fc fragment of an immunoglobulin recognized by a neonatal receptor (FcRn); a modified pre-fusion respiratory syncytia virus (RSV) F protein; and a trimerization domain. Disclosed are nucleic acid sequences capable of encoding peptides comprising a monomeric Fc fragment of an immunoglobulin recognized by a neonatal receptor (FcRn); a modified pre-fusion respiratory syncytia virus (RSV) F protein; and a trimerization domain. Also disclosed are methods for eliciting a protective immune response against RSV comprising administering to a subject an effective amount of a composition comprising a monomeric Fc fragment of an immunoglobulin recognized by FcRn; a modified pre-fusion RSV F protein; and a trimerization domain, wherein the administering is to a mucosal epithelium.
Owner:UNIV OF MARYLAND
Adjuvant for mucosal vaccine
ActiveUS20160324960A1High efficacyImprove securitySsRNA viruses negative-senseBacterial antigen ingredientsHemagglutininProtein-protein complex
An object of the present invention is to provide an adjuvant for a mucosal vaccine with high safety that induces a sufficient immune response on the mucosa. According to the present invention, an adjuvant for a mucosal vaccine comprising a protein complex composed of hemagglutinin (HA) subcomponents HA1, HA2, and HA3 of botulinum toxin is provided.
Owner:KANAZAWA UNIV
Mucosal vaccine composition
ActiveUS20160193327A1Safe and effectiveSsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesXanthomonas
The present invention aims at providing a mucosal vaccine composition that can be administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and is capable of safely and effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Novel mucosal vaccination approach for herpes simplex virus type-2
InactiveUS20130195961A1High potencyPrevent relapseOrganic active ingredientsViral antigen ingredientsHeterologousLiposome
Methods and kits for immunizing animals (e.g. mammals) against viral antigens, including herpes-simplex virus type 2 are provided. The protective immune response elicited by the methods and kits is characterized by robust humoral, cellular, and mucosal immunity. In particular, a heterologous immunization method comprising a priming DNA vaccine encoding an antigen and a boosting protein vaccine, in which the protein form of the antigen is encapsulated in liposomes is provided. Methods of preventing primary acute, latent and recurrent viral infections, such as that caused by HSV-2 virus, and methods of providing passive protective immunity against a viral pathogen such as HSV-2 virus to a mammal are also disclosed.
Owner:MUCOSAL VACCINE TECH LLC
Composition and manufacturing of powders containing nanoadjuvants for mucosal vaccination
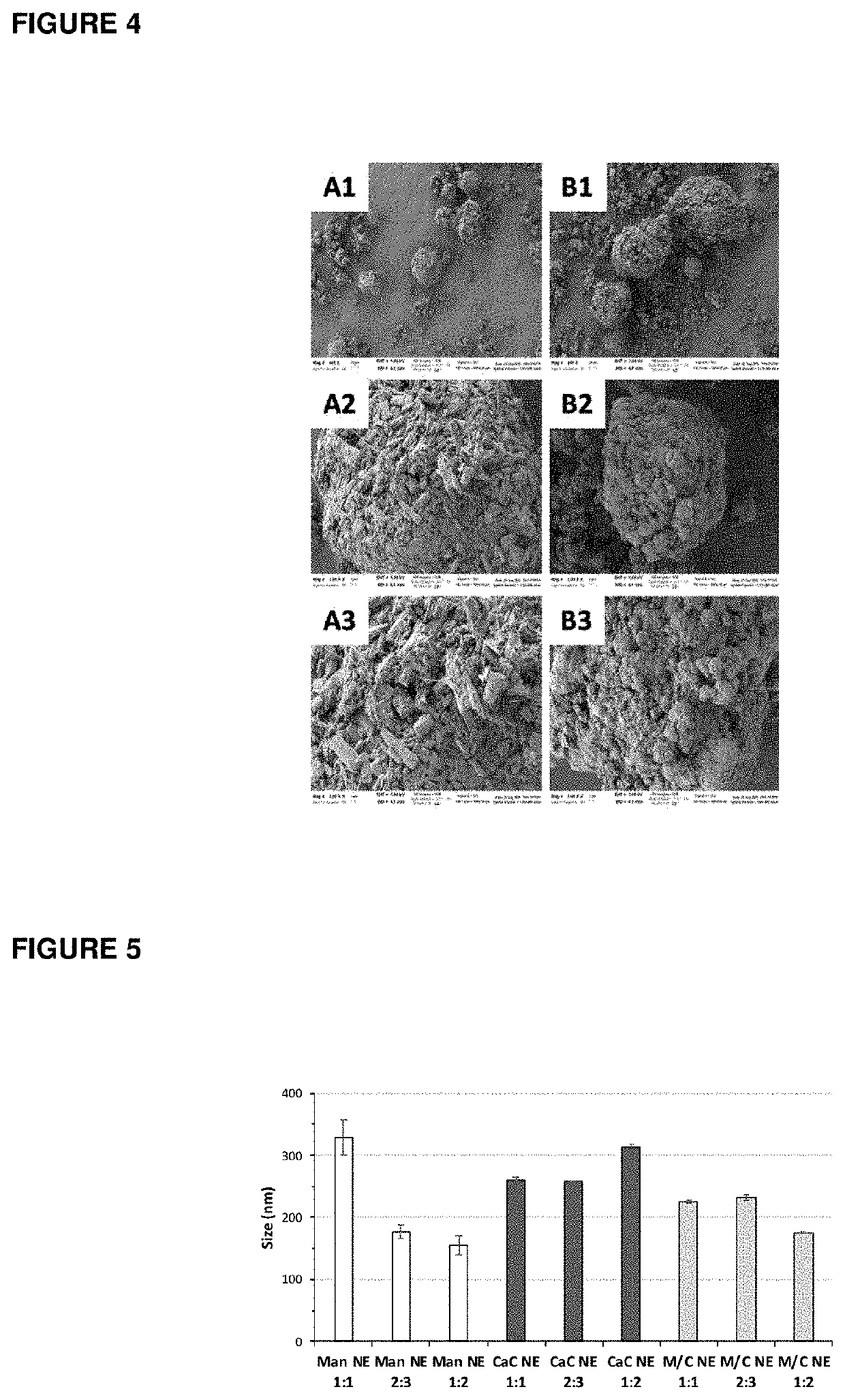
InactiveUS20200268880A1Reduce antigen activityEnhanced mixing processPowder deliveryInorganic non-active ingredientsMucosal vaccineTGE VACCINE
New preparative approach of dry powder vaccines for mucosal (e.g. nasal) administration for the purpose of human or animal immunization; it requires spraying a vaccine liquid dispersion, previously mixed with a sub-micron particulate adjuvant, onto a solid carrier while blending the mixture, followed by drying in mild conditions; the sub-micron particulate adjuvant is an O / W nanoemulsion stabilized with a polysaccharide. Improved dry powder vaccines are obtained in form of aggregated antigen-carrier particles, whereby the antigen is finely and firmly dispersed within the carrier; once in contact with the mucosal surface, the product quickly dissociates and releases the antigen component.
Owner:UNIV DEGLI STUDI DI PARMA
Mucosal Vaccine Using Cationic Nanogel
A mucosal vaccine for the prevention or treatment of microbial infections is described that is capable of inducing vaccine antigen-specific immune responses in an organism without the addition of a mucosal adjuvant. The mucosal vaccine comprises a composite of a nanogel comprising a hydrophilic polysaccharide having a cationic functional group and a hydrophobic cholesterol added thereto as a side chain and a vaccine antigen. The vaccine is administered via a mucosal route.
Owner:INTPROP STRATEGY NETWORK INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com