Mucosal vaccine using cationic nanogel
a technology of cationic nanogels and mucosal vaccines, applied in the field of mucosal vaccines, can solve problems such as the safety of mucosal vaccines on organisms, and achieve the effects of indubiting systemic and mucosal immune responses, facilitating immune response, and facilitating immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Mucosal Vaccine
[0056]Cationic nanogels (cationic CHP) in which the degree of cholesterol substitution was 1.4 and the degree of ethylenediamine substitution was 18 per 100 monosaccharides were used (CHPNH2 nanogels). A CHP derivative or cationic Pullulan was dissolved in a 1 mg / ml phosphate buffer solution (PBS). The CHPNH2 nanogels were subjected to sonication for 15 minutes and then filtered through a 0.22-mm filter.
[0057]The C-terminal avirulent region of a heavy chain of botulinus toxin (Hc; molecular weight: 45,000), tetanus toxoid (TT; molecular weight: 150,000), or the AIDS virus membrane antigen molecule (gag p24; molecular weight: 24,000) expressed in E. coli and purified was mixed with the equimolar amount of cationic nanogels prepared in the manner described above, and the resulting mixture was subjected to reaction at 45° C. for 5 hours to prepare a composite. The obtained antigen / cationic nanogel composite was used as a mucosal vaccine using cationic nano...
example 2
Transnasal Immunization
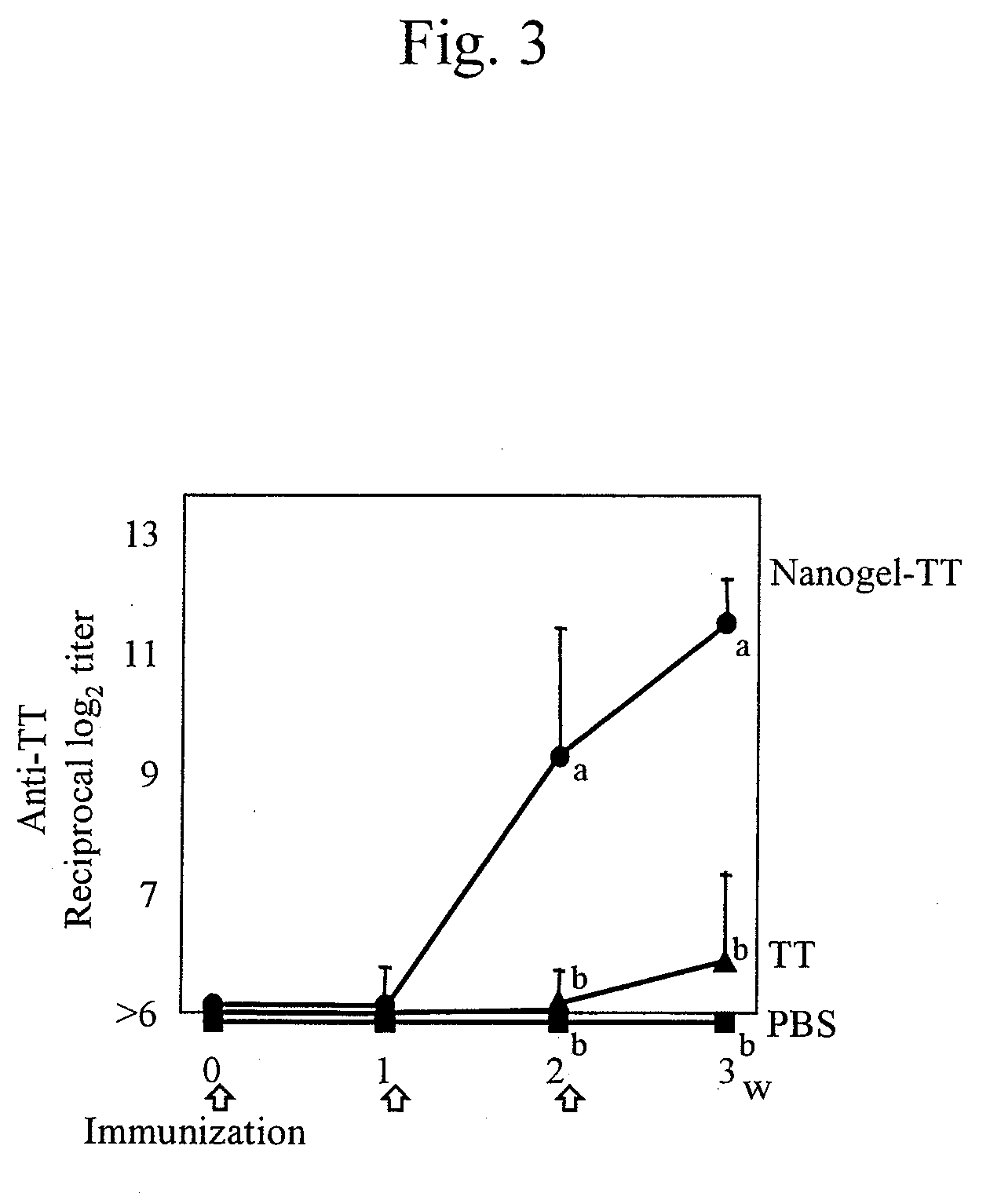
[0058]The mucosal vaccine using cationic nanogels prepared in Example 1 or the antigen alone was administered to 6- to 8-week-old Balb / c mice (female) through the nasal cavity in an amount of 10 μg of Hc (88.9 μg of nanogel), 30 μg of TT (80.0 μg of nanogel), or 10 μg of gag p24 (166.7 μg of nanogel) per mouse once a week (3 times in total) to immunize mice transnasally. The amount of antigens administered (i.e., the amount of the solution) was adjusted to 15 μl in every experimental group, and 7.5 μl of the solution was administered to each nostril. PBS was administered as a control.
[0059]The blood was sampled before immunization and a week after immunization, and IgG antibody titers to botulinus toxin, TT, or gag p24 in the blood serum were measured to evaluate the systemic immune responses. The nasal cavity was washed with 200 μl of PBS a week after the final immunization, and the IgA antibody titer in the nasal wash solution was measured to evaluate immune...
example 3
Neutralization Effects after Transnasal Immunization using Mucosal Vaccine using Nanogels
[0065]The vaccine using cationic nanogels using a C-terminal avirulent region of the heavy chain of botulinus toxin (Hc; molecular weight: 45,000) as the antigen prepared in Example 1 or Hc alone was administered transnasally to 5 mice for immunization in the same manner as in Example 2. PBS was administered as a negative control. After the mice were subjected to immunization 3 times, botulinus toxin (obtained from Professor Shunji Kozaki, Division of Veterinary Science, School of Life and Environmental Sciences, Osaka Prefecture University) was administered intraperitoneally in an amount 25,000 times greater than the lethal dose thereof via intraperitoneal administration (i.g., 500 ng) to analyze the survival effects. For the purpose of analyzing the neutralization effects of Hc-specific IgA induced in the nasal tissue, 10 μg of botulinum progenitor toxins (obtained from Wako Pure Chemical Indu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com