Mucosal vaccine adjuvants containing bacterial flagellins as an active component
a technology of flagellin and mucosal vaccine, which is applied in the direction of antibody medical ingredients, drug compositions, instruments, etc., can solve the problems of reducing immunogenicity, raising a significant social problem, and insufficient human use, so as to stimulate the production of interleukin-8 and increase the mucosal iga
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
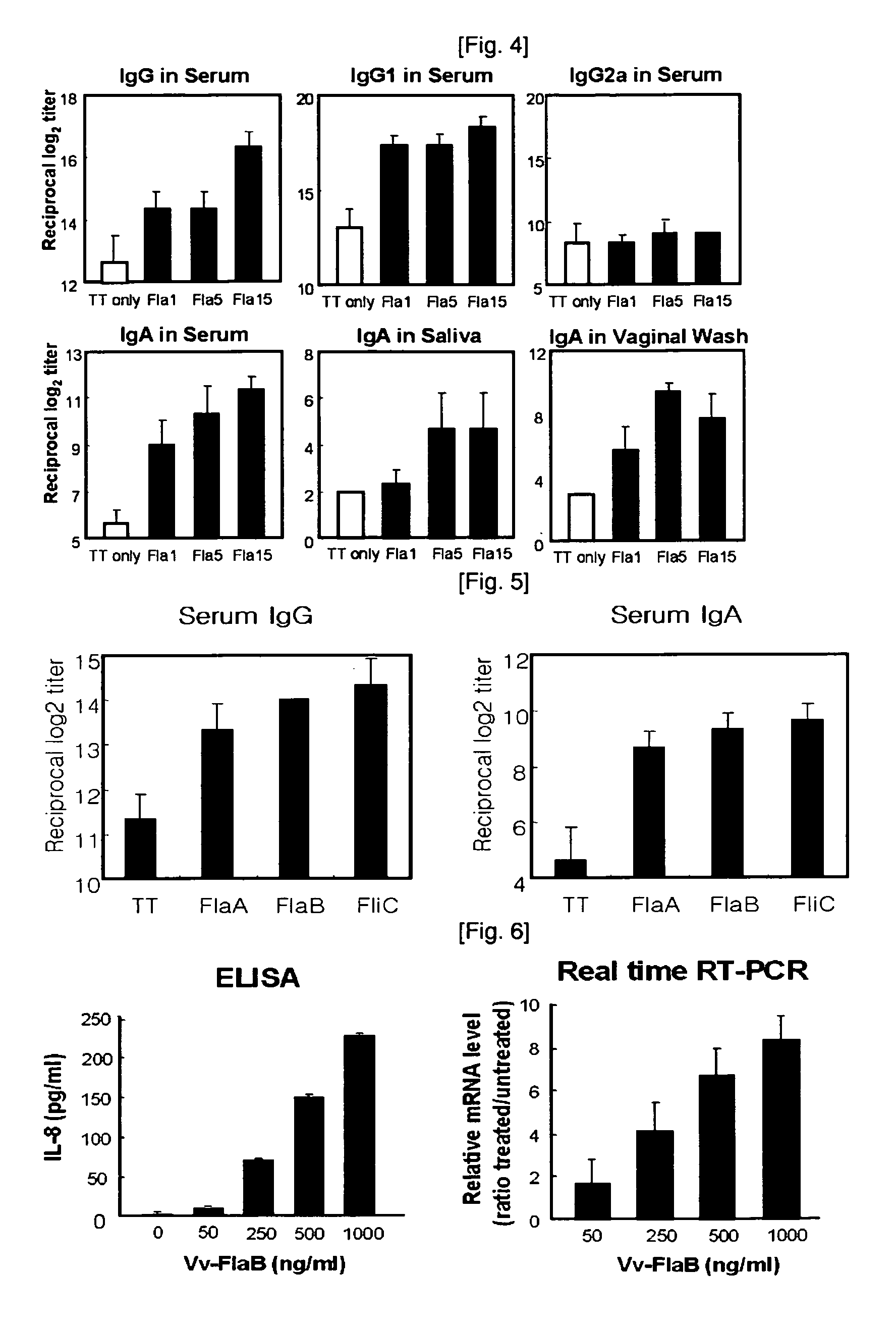
Image
Examples
example 1
Construction of Transposon Libraries
[0046]V. vulnificus MO6-24 / O type strains (obtained from J. Glenn Morris, Division of Hospital Epidemiology, University of Maryland School of Medicine, USA) and mini-Tn5 lacZ1 containing E. coli SM10λpir strains (obtained from Kenneth N. Timmis, GBF National Research Center for Biotechnology, Braunschweig, Germany) were cultured overnight at 37° C., 210 rpm in a stirring incubator, each were inoculated with single colony at 10 ml of 2.5 HI(2.5% NaCl heart infusion) broth media and 20 ml of LB (containing 100 μg / ml of Ampicillin and 100 μg / ml of Kanamicin) broth media
[0047]The following day these were centrifuged, and washed with antibiotic-free LB broth media and centrifuged two times, then suspended at 100 μl of new LB broth media. Each bacterial suspension of E. coli and V. vulnificus were mixted together and dropped on LB agar plate. After culturing it overnight at 37° C., 800 μl of new 2.5 HI broth media was added to the grown colonies on LB a...
example 2
Screening of Transposon Mutant Clones that Lose otility.
[0049]Each clone, prepared in Example 1, of the V. vulnificus MO6-24 / O transposon libraries was cultured overnight at 37° C., then inoculated to 0.3% agar containing semi-solid state HI (heart infusion) agar plates using sterilized toothpicks and cultured at 37° C. for 6 hours. Degrees of the motility of the bacteria were then determined by measuring the range of movement after growing the bacteria
[0050]3 transposon mutant clones that nearly completely lost motility were selected by screening procedures, and the experiment that would identify the mutant genes which insert transposons was progressed.
example 3
Identification of Flagellin Operon Genes
[0051]The cloning of genes nearby the transposon inserted region was carried out by screening the cosmid gene libraries, using DNA fragment as primer for amplification by arbitrary PCR methods. The amplification of DNA fragments nearby the transposon inserted site was used a two-step PCR amplification method. In the first PCR, the arbitrary primer 1 (5-GGCCACGCGTCGACTAGTCANNNNNNNNNNACGCCC-3) of sequence number 13, and the mini-Tn5 lacZ1 specific primer 1 (5-TTCTTCACGAGGCAGACCTCAGCGC-3) of sequence number 14, were used. The first PCR was set as follows; denaturing them for 30 seconds (sec) at 94° C., annealing for 30 sec at 30° C., and elongating for 1 minute (min) 30 sec at 72° C., with 5 cycles; afterwords a further 30 cycle PCR reaction was performed by denaturing for 30 seconds (sec) at 94° C., and annealing for 30 sec at 45° C., and elongating for 2 min at 72° C., with 30 cycles. The second PCR reaction was peformed using the products of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| adhesiveness | aaaaa | aaaaa |
| polar | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com