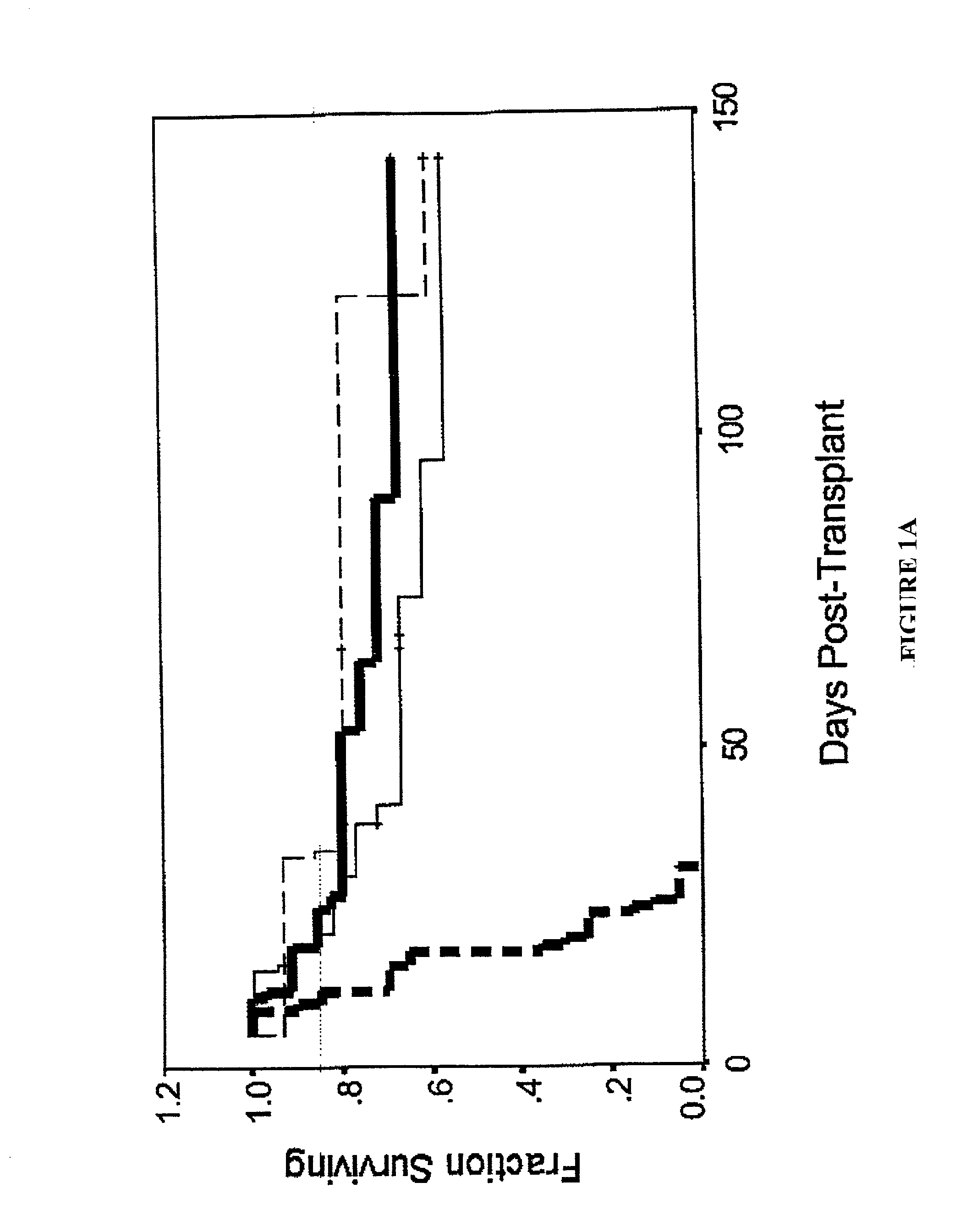
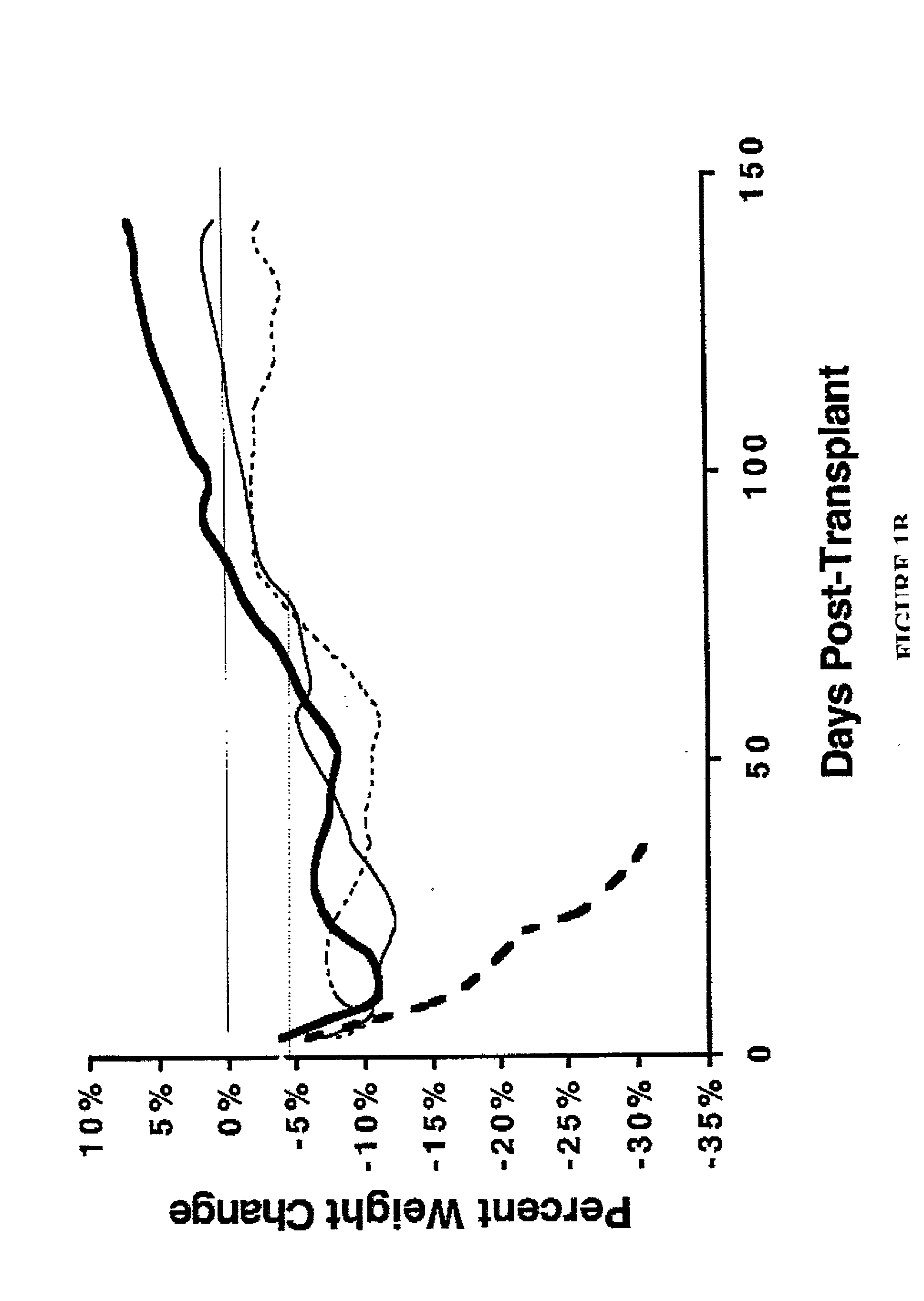
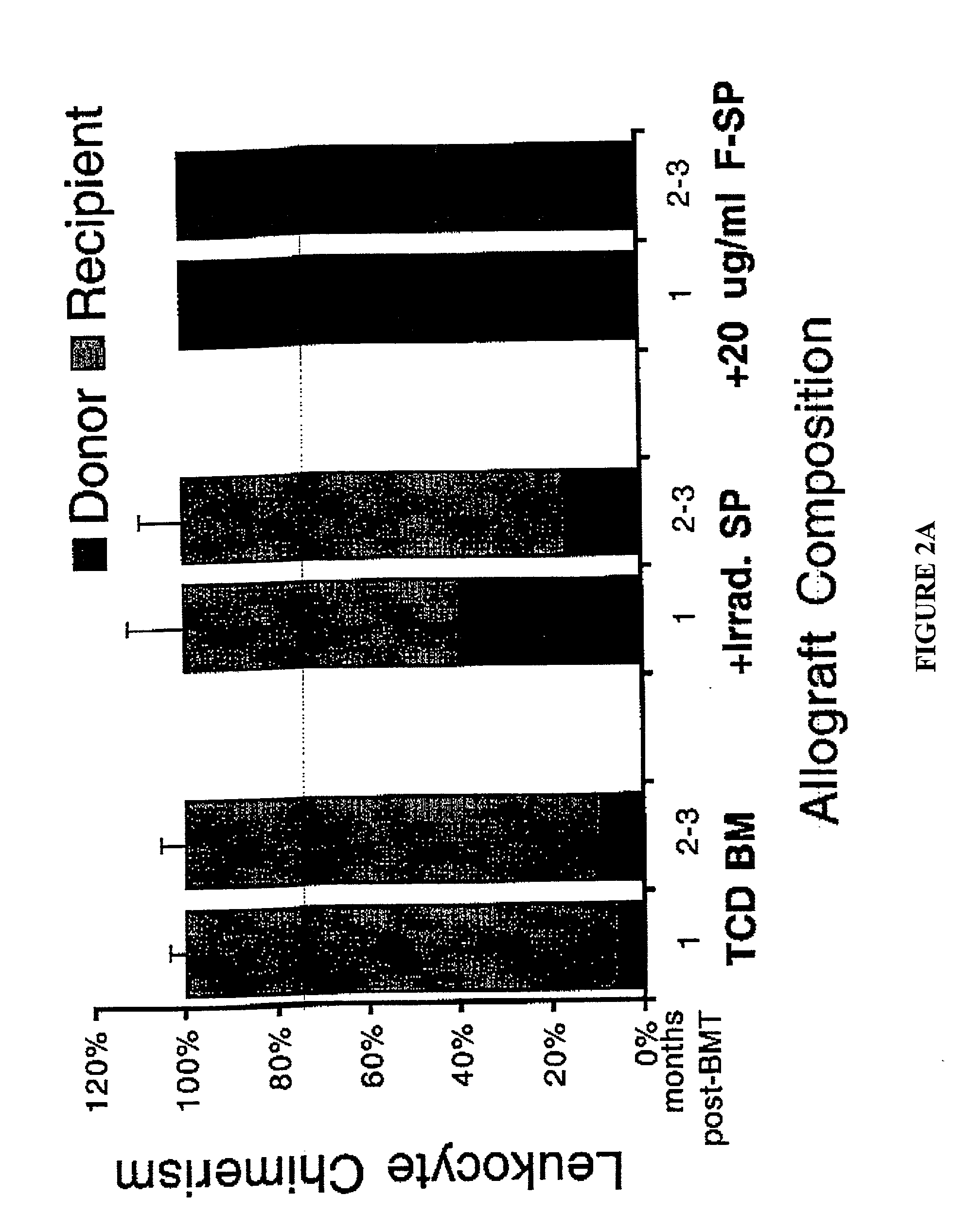
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1460 results about "Monocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monocytes are a type of leukocyte, or white blood cell. They are the largest type of leukocyte and can differentiate into macrophages and myeloid lineage dendritic cells. As a part of the vertebrate innate immune system monocytes also influence the process of adaptive immunity. There are at least three subclasses of monocytes in human blood based on their phenotypic receptors.
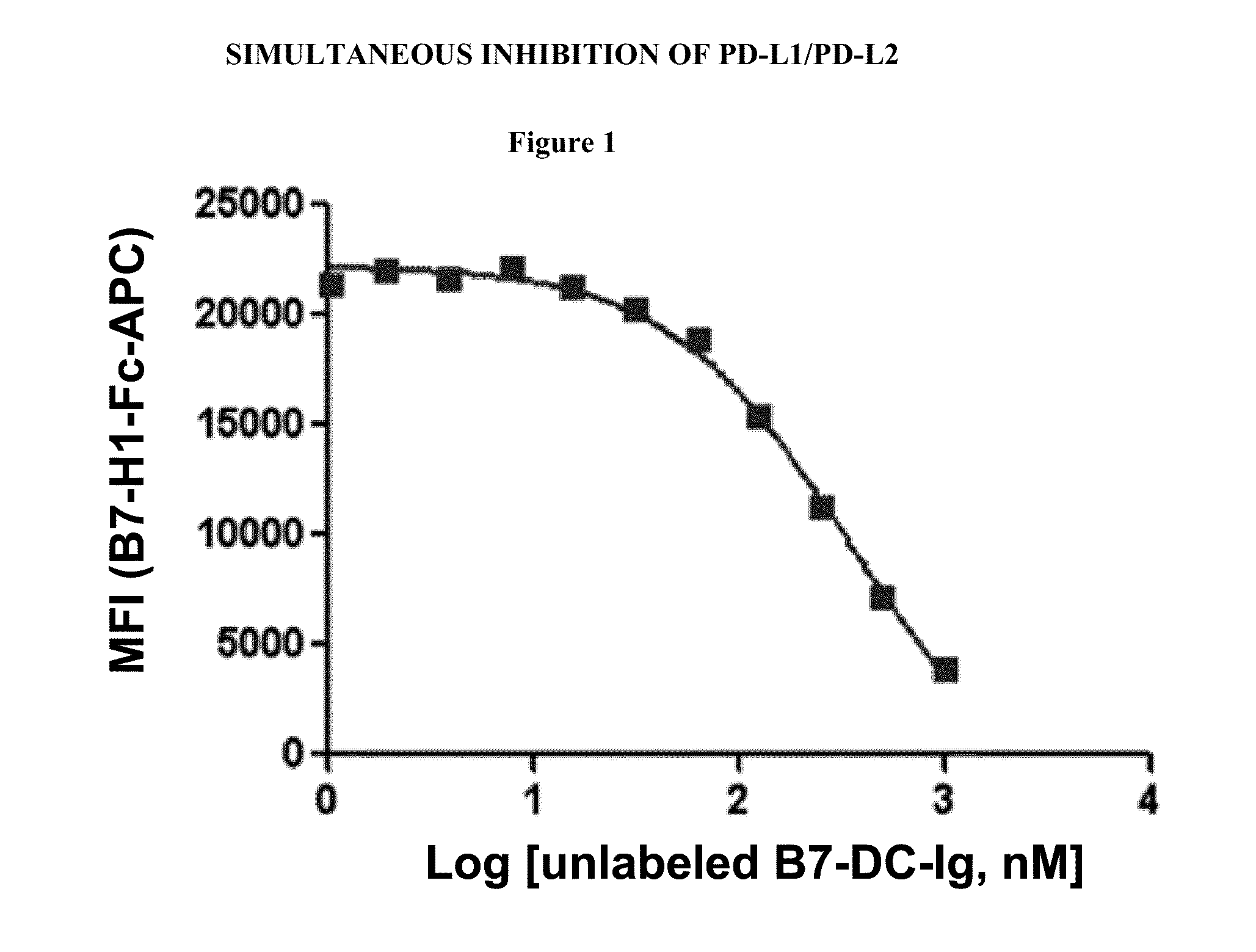
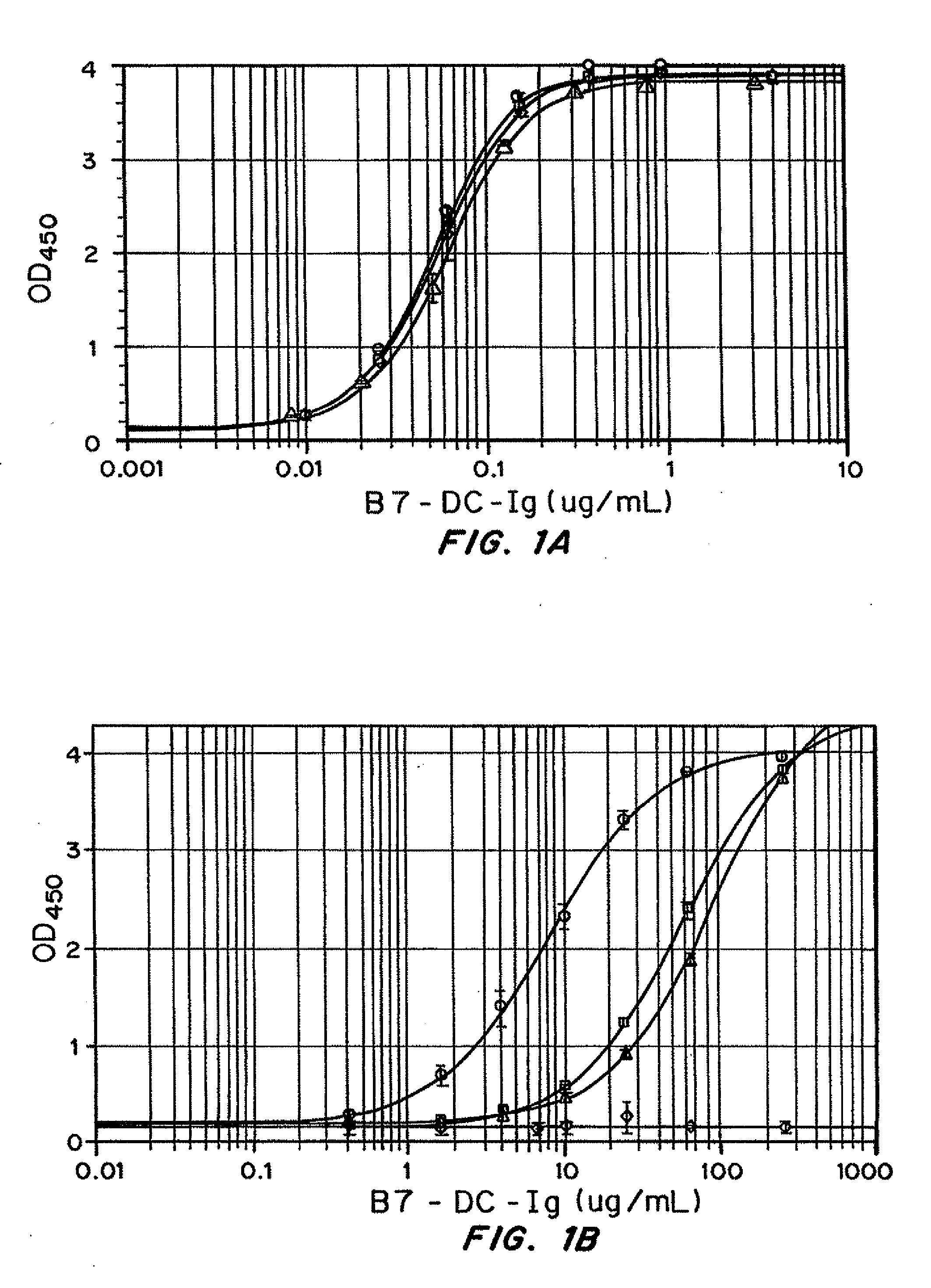
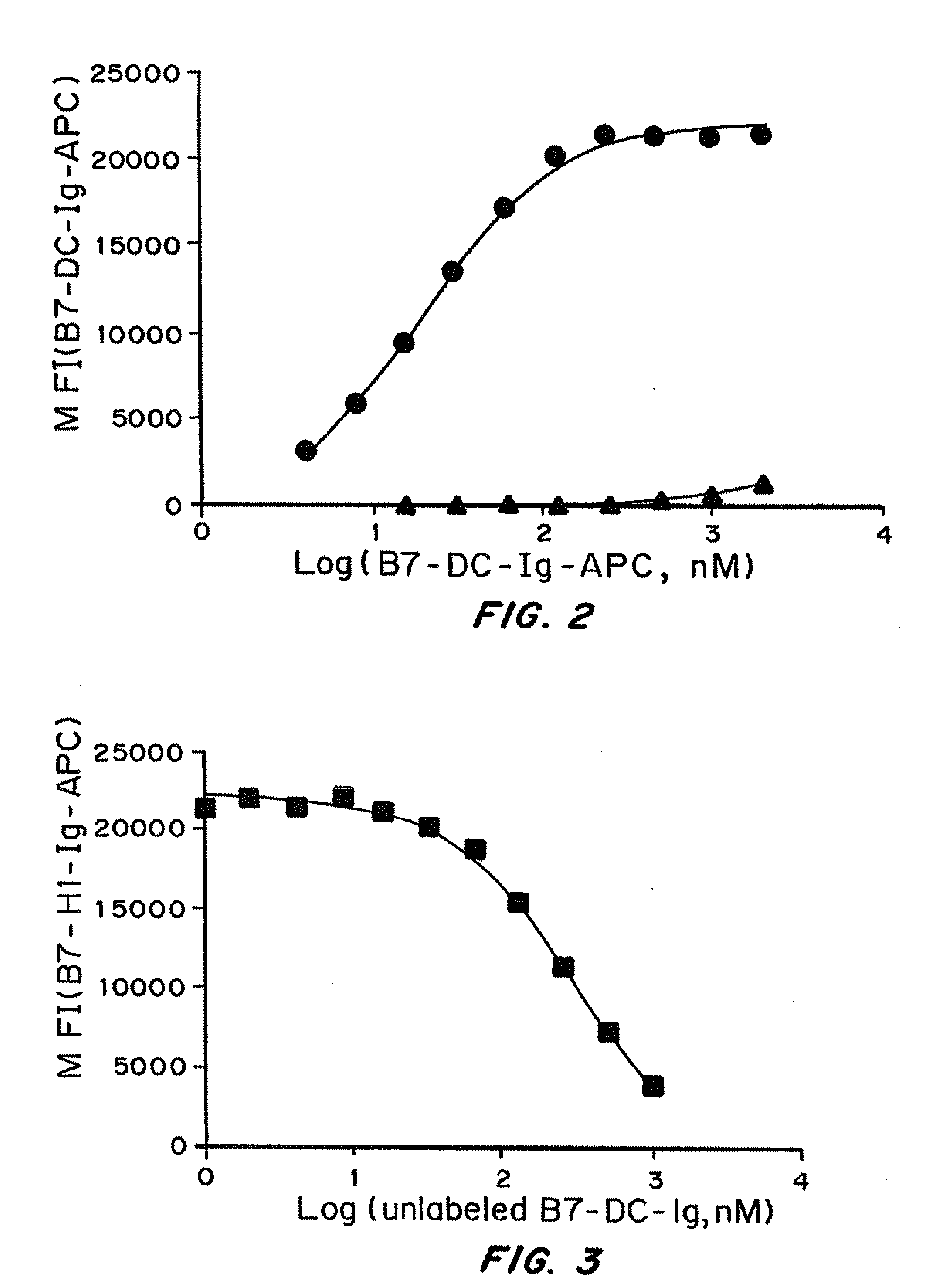
Simultaneous inhibition of pd-l1/pd-l2
InactiveUS20130017199A1Increase frequencyIncrease percentageAntibacterial agentsAntimycoticsDiseaseDendritic cell
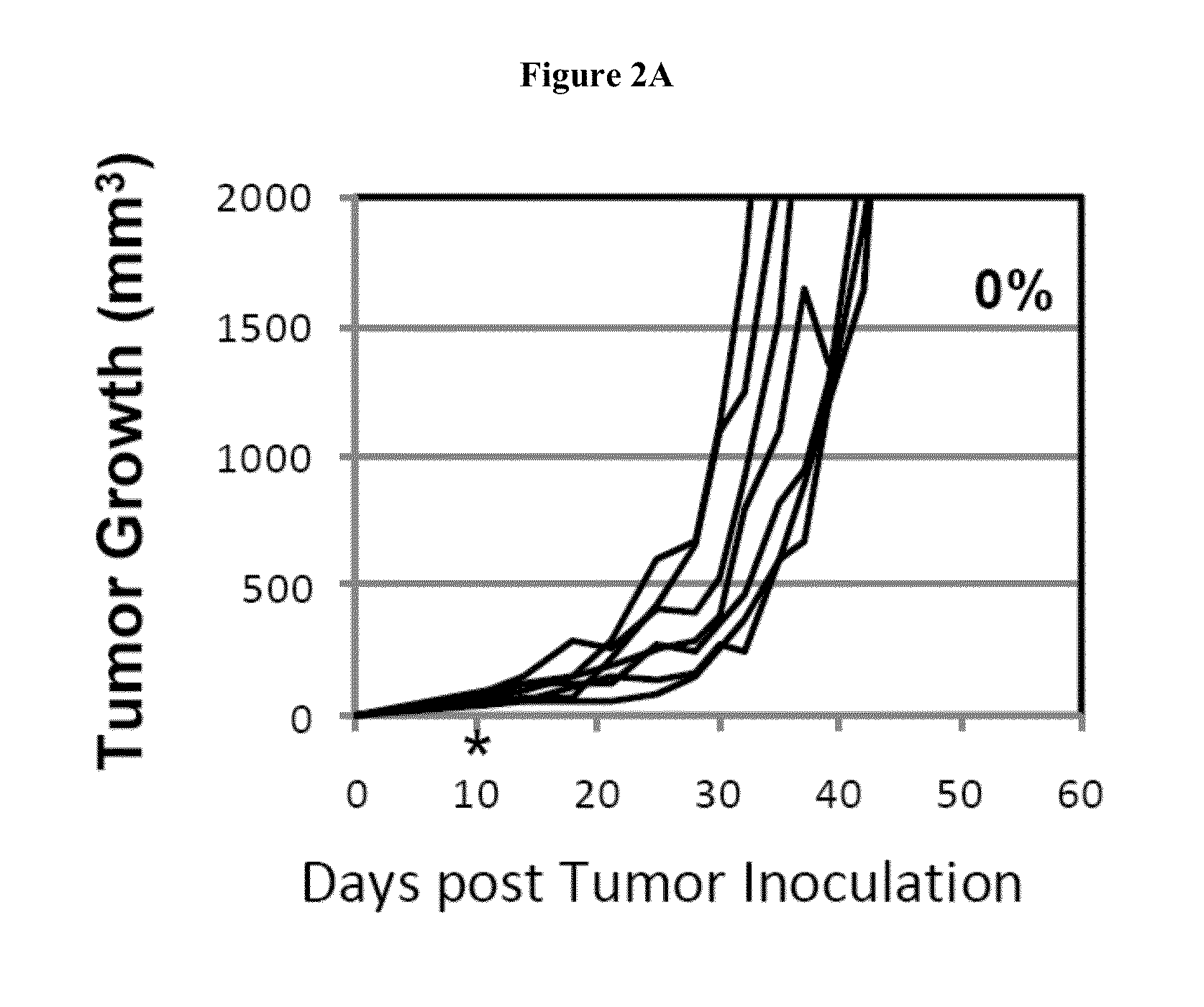
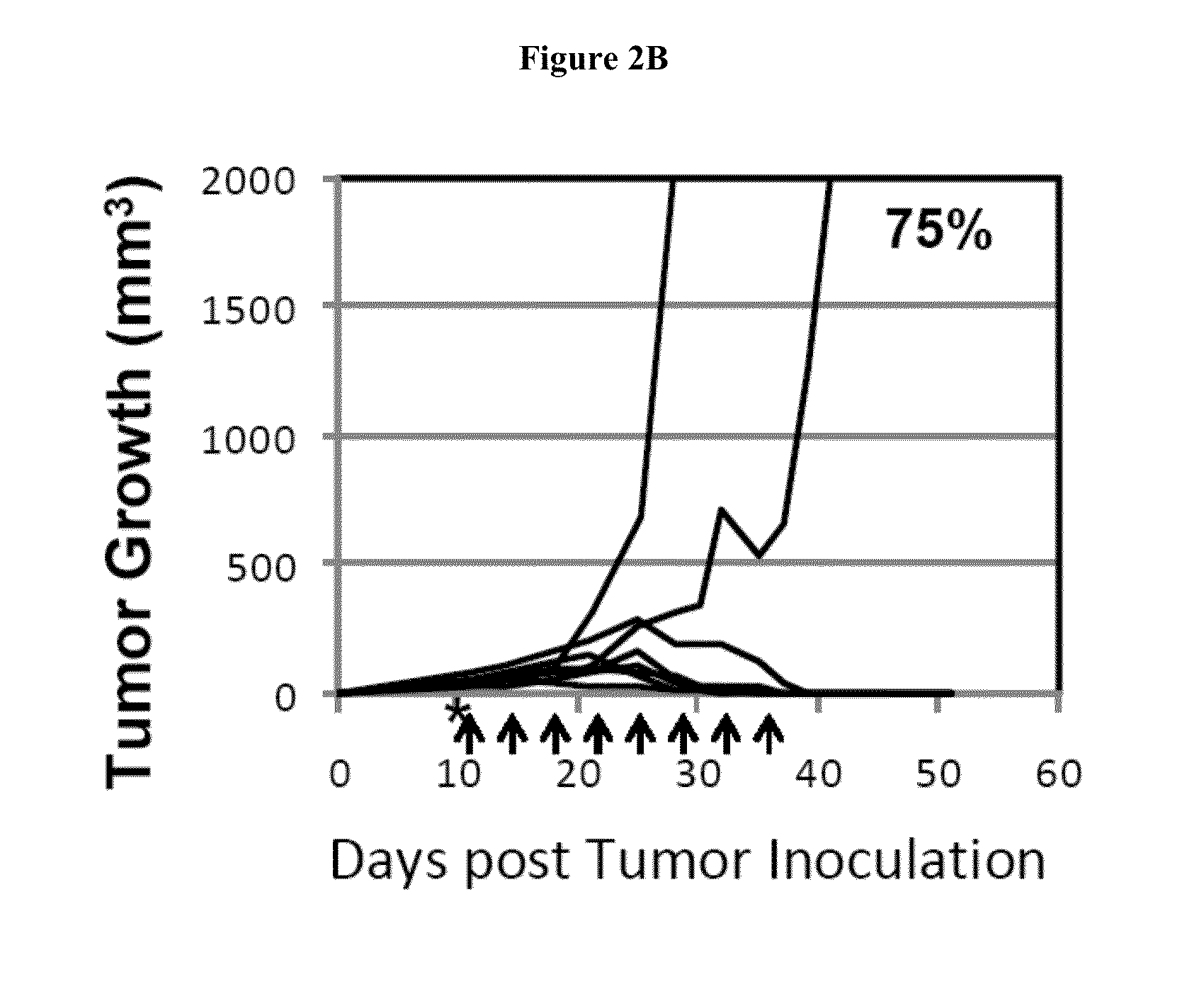
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by simultaneously inhibiting the PD-1 ligands, PD-L1 and PD-L2. The immune response can be modulated by providing antagonists which bind with different affinity, by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again. In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
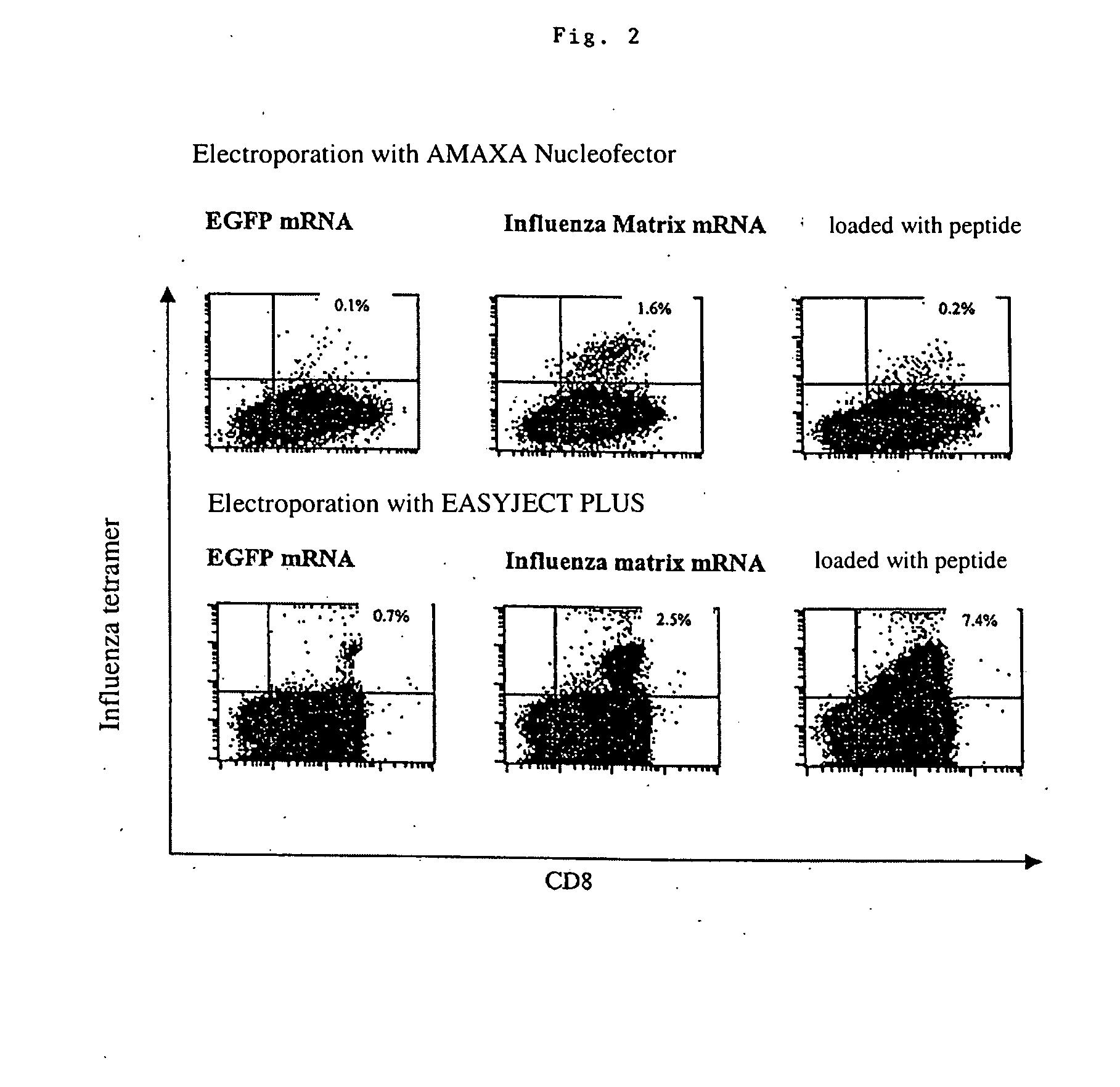
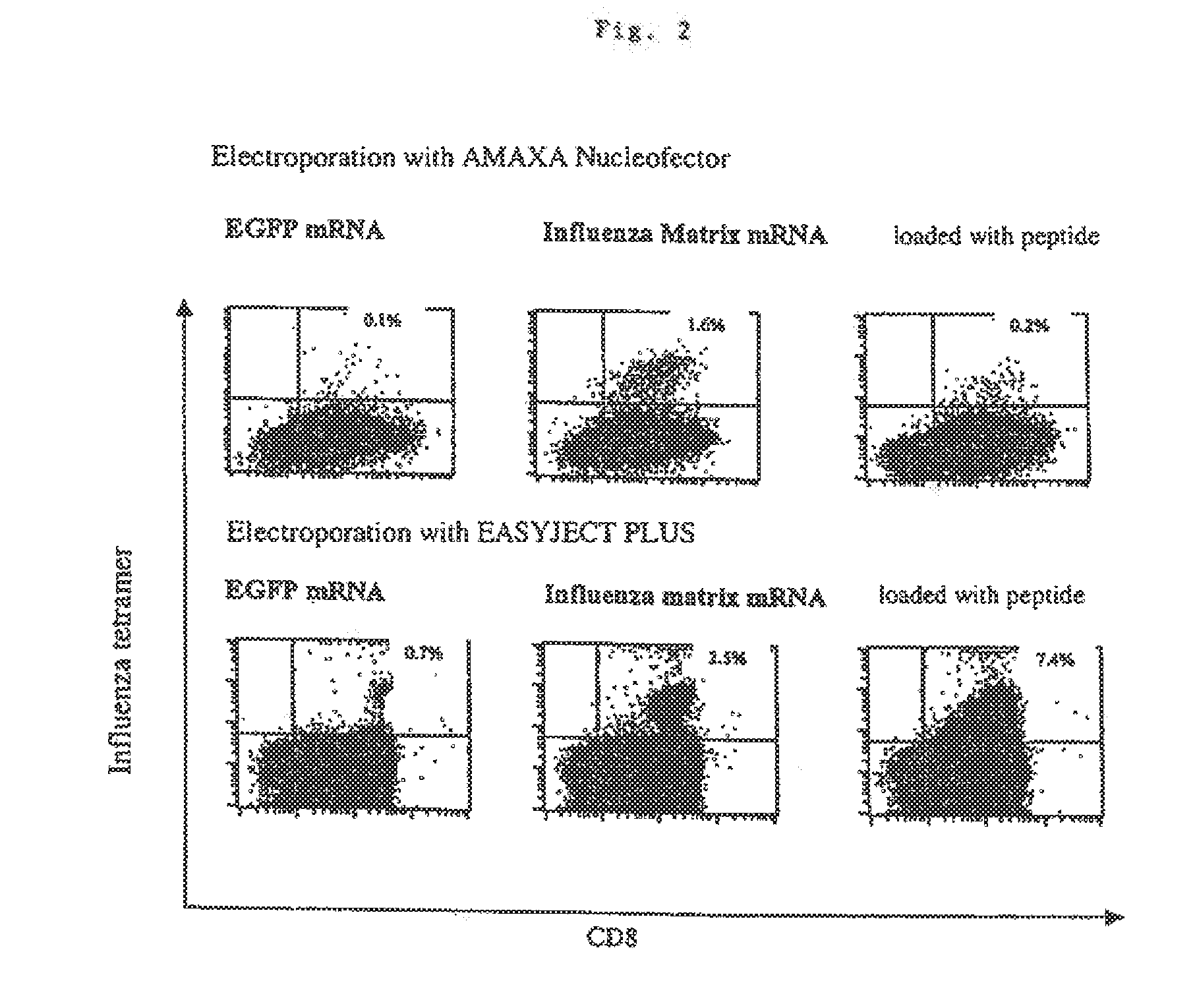
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
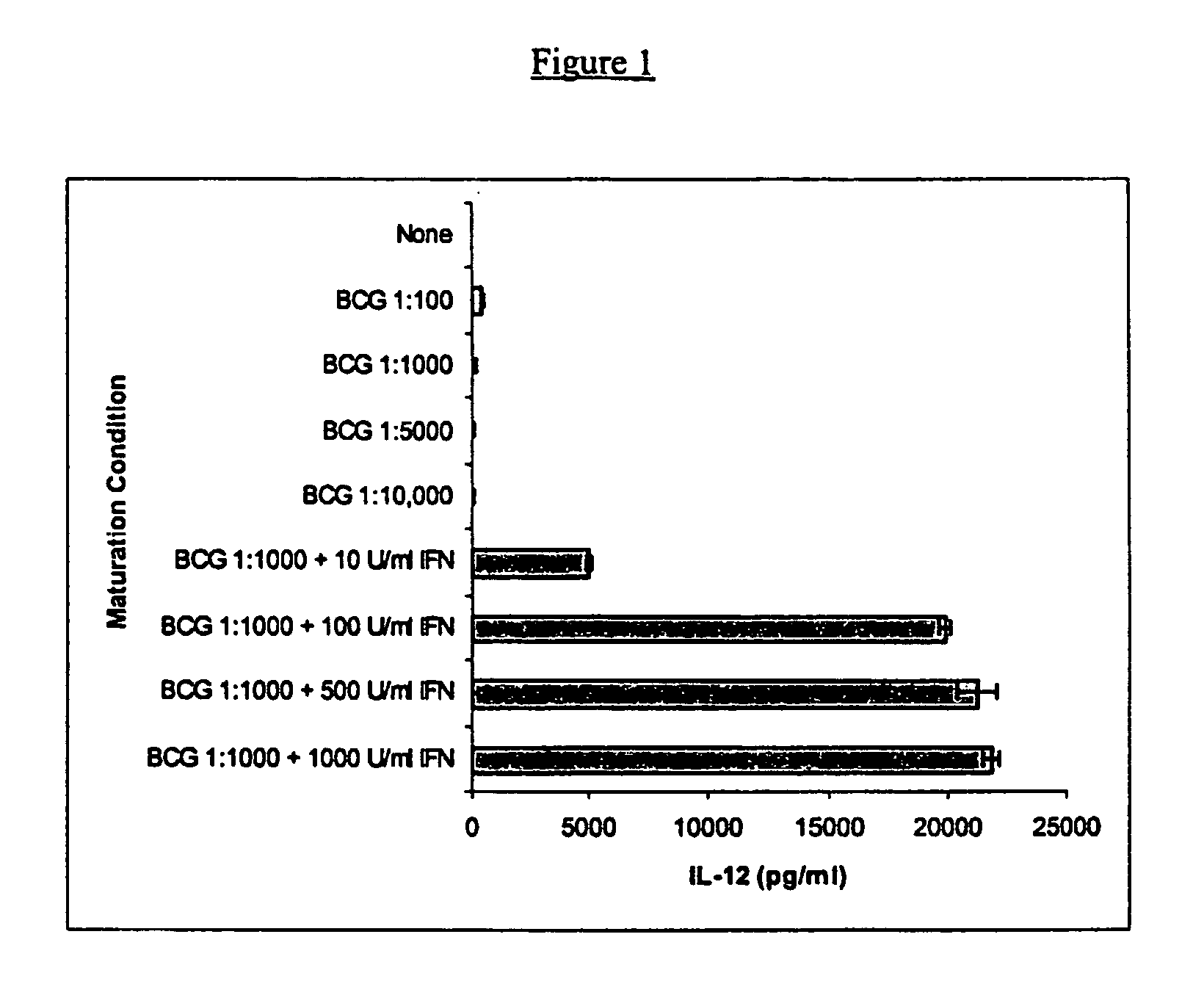
Compositions and methods for priming monocytic dendritic cells and t cells for th-1response
InactiveUS20050059151A1Mammal material medical ingredientsBlood/immune system cellsDendritic cellMonocyte
The present invention provides compositions and methods for inducing maturation of immature dendritic cells (DC) and for priming those cells for inducing a type 1 immune response. The present invention also provides dendritic cell populations useful for activating and for preparing T cells polarized towards production of type 1 cytokines and / or a type 1 response. Similarly, activated, polarized T cell populations, and methods of making the same are provided.
Owner:NORTHWEST BIOTHERAPEUTICS INC
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20120009221A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseViral antigen ingredientsAntigenPharmaceutical medicine
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
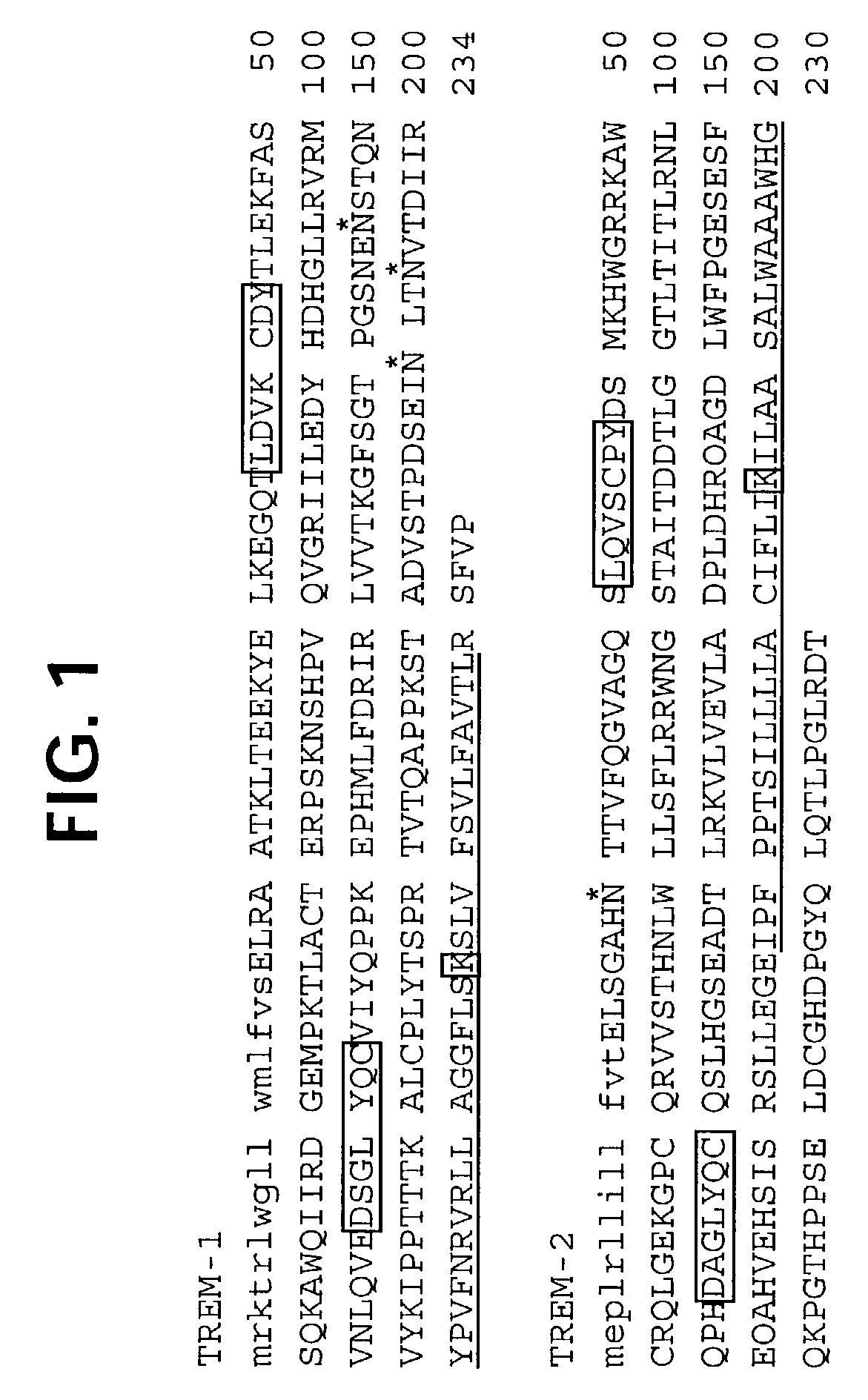
Novel receptor TREM (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20030165875A1Strong upregulationAntibacterial agentsAntimycoticsReceptor for activated C kinase 1Monocyte
Novel activating receptors of the Ig super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-beta. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:BIOXELL
Hematopoietic stem cells and methods of treatment of neovascular eye diseases therewith
InactiveUS20050063961A1Stably incorporated into neovasculature of the eyePromote repairBiocideSenses disorderDiseaseProgenitor
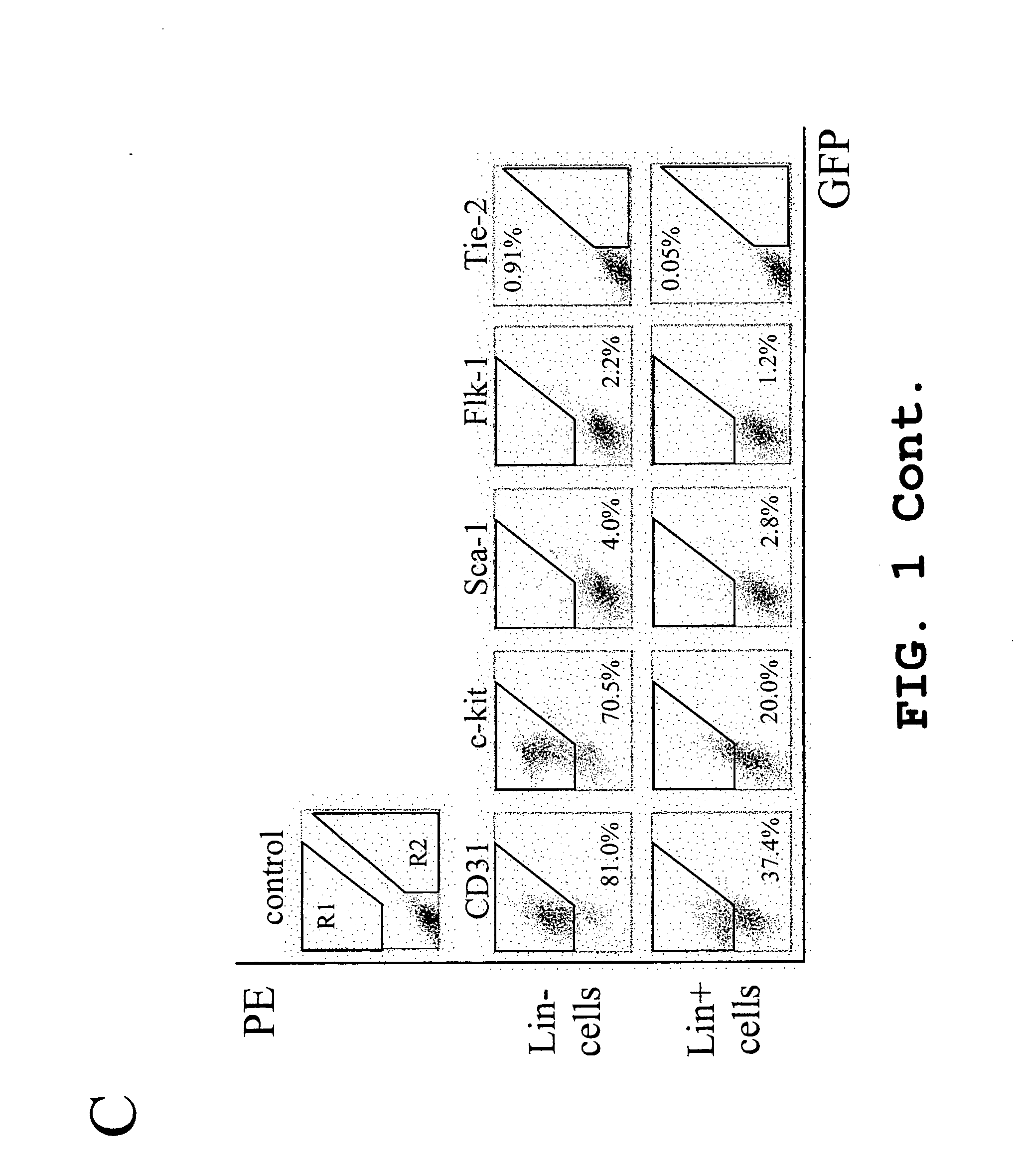
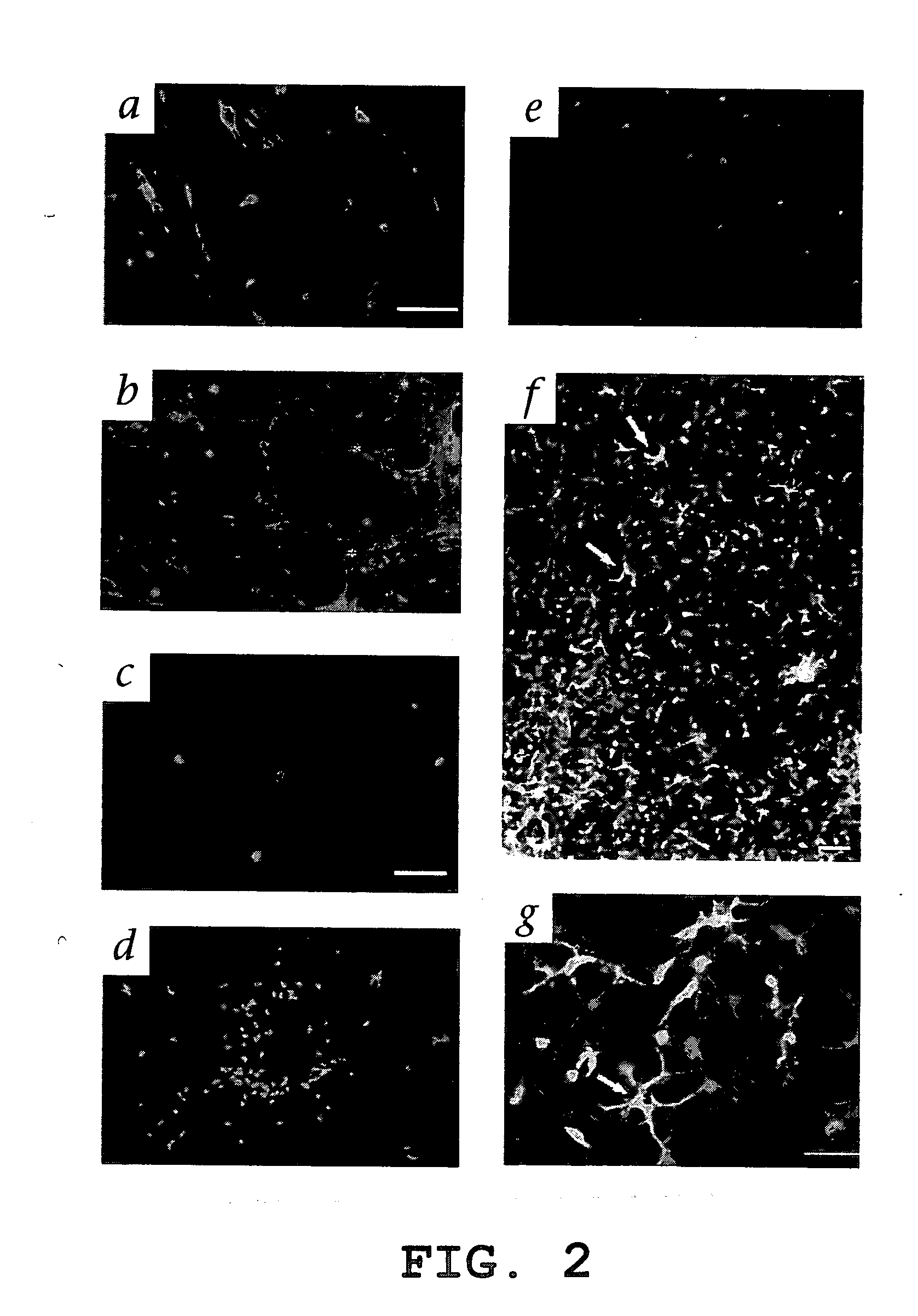
Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations (Lin− HSCs) contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. Preferably at least about 20% of the cells in the isolated Lin− HSCs express the cell surface antigen CD31. The isolated Lin− HSC populations are useful for treatment of ocular vascular diseases. In a preferred embodiment, the Lin− HSCs are isolated by extracting bone marrow from an adult mammal; separating a plurality of monocytes from the bone marrow; labeling the monocytes with biotin-conjugated lineage panel antibodies to one or more lineage surface antigens; removing of monocytes that are positive for the lineage surface antigens from the plurality of monocytes, and recovering a Lin− HSC population containing EPCs. Isolated Lin− HSCs that have been transfected with therapeutically useful genes are also provided, and are useful for delivering genes to the eye for cell-based gene therapy. Methods of preparing isolated stem cell populations of the invention, and methods of treating ocular diseases and injury are also described.
Owner:THE SCRIPPS RES INST
Generation of fully mature and stable dendritic cells from leukaphereses products for clinical applications
InactiveUS20040072347A1Bioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellPeripheral blood monocyte
The present invention provides a method for producing mature and stable dendritic cells or immature dendritic cells which comprises cultivating hematopoietic progenitor cells in a sterile cultivating apparatus, an apparatus suitable for said method and a method for preparing peripheral blood mononuclear cells, which are suitable for cultivation of dendritic cells.
Owner:MERIX BIOSCI
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20060140936A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Methods and compositions for immunotherapy of inflammatory and immune-dysregulatory diseases, using multispecific antagonists that target at least two different markers are disclosed. The different targets include (i) proinflammatory effectors of the innate immune system, (ii) coagulation factors, and (iii) targets specifically associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, wherein the targets included in group (iii) are neither a proinflammatory effector of the immune system nor a coagulation factor. When the multispecific antagonist reacts specifically with a target associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, it also binds specifically with at least one proinflammatory effector of the immune system or at least one coagulation factor. Thus, the multispecific antagonist contains at least one binding specificity related to the diseased cell or condition being treated and at least one specificity to a component of the immune system, such as a receptor or antigen of B cells, T cells, neutrophils, monocytes and macrophages, and dendritic cells, a modulator of coagulation, or a proinflammatory cytokine. The multispecific antagonists are used in the treatment of various diseases that are generated or exacerbated by, or otherwise involve, proinflammatory effectors of the innate immune system or coagulation factors. Such diseases more particularly include acute and chronic inflammatory disorders, autoimmune diseases, giant cell arteritis, septicemia and septic shock, coagulopathies (including diffuse intravascular coagulation), neuropathies, graft versus host disease, infectious diseases, acute respiratory distress syndrome, granulomatous diseases, transplant rejection, asthma, cachexia, myocardial ischemia, and atherosclerosis. Other diseases also responsive to these therapies include cancers and conditions with pathological angiogenesis.
Owner:IMMUNOMEDICS INC
Stem cell mediated treg activation/expansion for therapeutic immune modulation
ActiveUS20080159998A1High activityInduce upregulationBiocideGenetic material ingredientsAntigenT cell
Disclosed are cells, methods of modulating cells, and therapeutic uses of the cells for the immune modulation of mammals in need thereof. Immune modulation including alteration of cytokine profile, cytotoxic activity, antibody production and inflammatory states is achieved through the administration of various cell types that have been unmanipulated or manipulated in order to endow specific biological activity. Cellular subsets and administration of the subsets in combination with various agents are also provided. One embodiment teaches the previously unknown finding that adipose tissue derived mononuclear cells contain T cells with immune regulatory properties that alone or synergistically with various stem cells induce immune modulation upon administration. Another embodiment is the finding that stimulation of stem cell activation results in stem cell secondary activation of immune modulatory cells, one type which is T regulatory cells (Tregs). One specific embodiment involves extraction of a heterogenous stem cell pool, which contains T regulatory cells, treatment in culture of the population with agents known to stimulate stem cell activation, then subsequent extraction and administration of the purified Tregs. Other embodiments include expansion of Tregs in the presence of antigen in order to generate anti-specific Tregs.
Owner:XON CELLS
Composition for in vivo transplantation for treatment of human cervical cancer comprising mononuclear cells derived from umbilical cord blood
InactiveUS20100068194A1Good regenerative potentialReduced responseBiocideUnknown materialsSide effectMonocyte
Provided is a composition for in vivo transplantation for the treatment of human cervical cancer, comprising mononuclear cells derived from umbilical cord blood and a pharmaceutically acceptable carrier. When the umbilical cord blood-derived mononuclear cells are transplanted in vivo, cervical cancer can be effectively treated. In particular, the mononuclear cells derived from the umbilical cord blood retain high differentiation and proliferation abilities and exhibit very low graft-versus-host (GVH) reactions which are side effects caused by transplantation, and thus, can be transplanted to many patients.
Owner:KIM DONG KU
Method of treating acute coronary syndromes
InactiveUS20040266734A1Inhibitory activityInhibition amountBiocidePhosphorous compound active ingredientsPhagocytic CellManagement of acute coronary syndrome
The present invention relates to methods and compositions designed for the treatment or management of acute coronary syndromes, particularly, unstable angina and acute myocardial infarction. The methods of the invention comprise the administration of an effective amount of a formulation containing one or more therapeutic agents which specifically decreases or inhibits the activity of phagocytic cells and / or eliminates or diminishes the amount of phagocytic cells including, but not limited to, macrophages and monocytes. The formulations are specifically targeted to phagocytic cells. The invention also provides pharmaceutical compositions of formulations containing one or more therapeutic agents of the invention for administration to subjects currently suffering from or having recently suffered an acute coronary syndrome such as unstable angina and acute myocardial infarction.
Owner:ZULI HLDG LTD
Pd-1 antagonists and methods for treating infectious disease
InactiveUS20110159023A1Rapid induction of protectionRobust effector responseAntibacterial agentsOrganic active ingredientsDiseaseDendritic cell
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by binding to and blocking PD-1 to prevent or reduce inhibitory signal transduction, or by binding to ligands of PD-1 such as PD-L1, thereby preventing (in whole or in part) the ligand from binding to PD-1 to deliver an inhibitory signal. The immune response can be modulated by providing antagonists which bind with different affinity (i.e., more or less as required), by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again (similar to what occurs with antigen elicitation using priming and boosting). In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
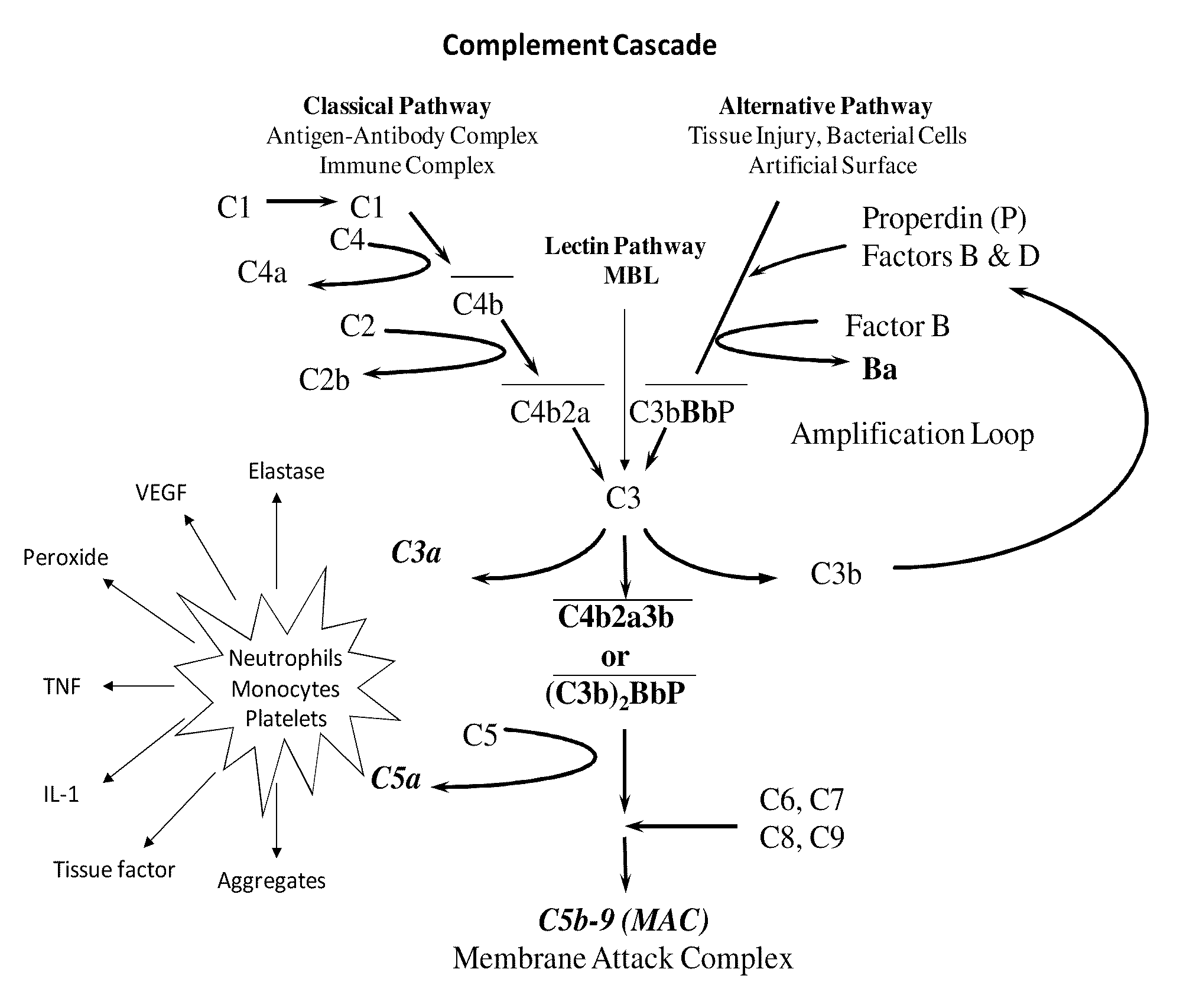
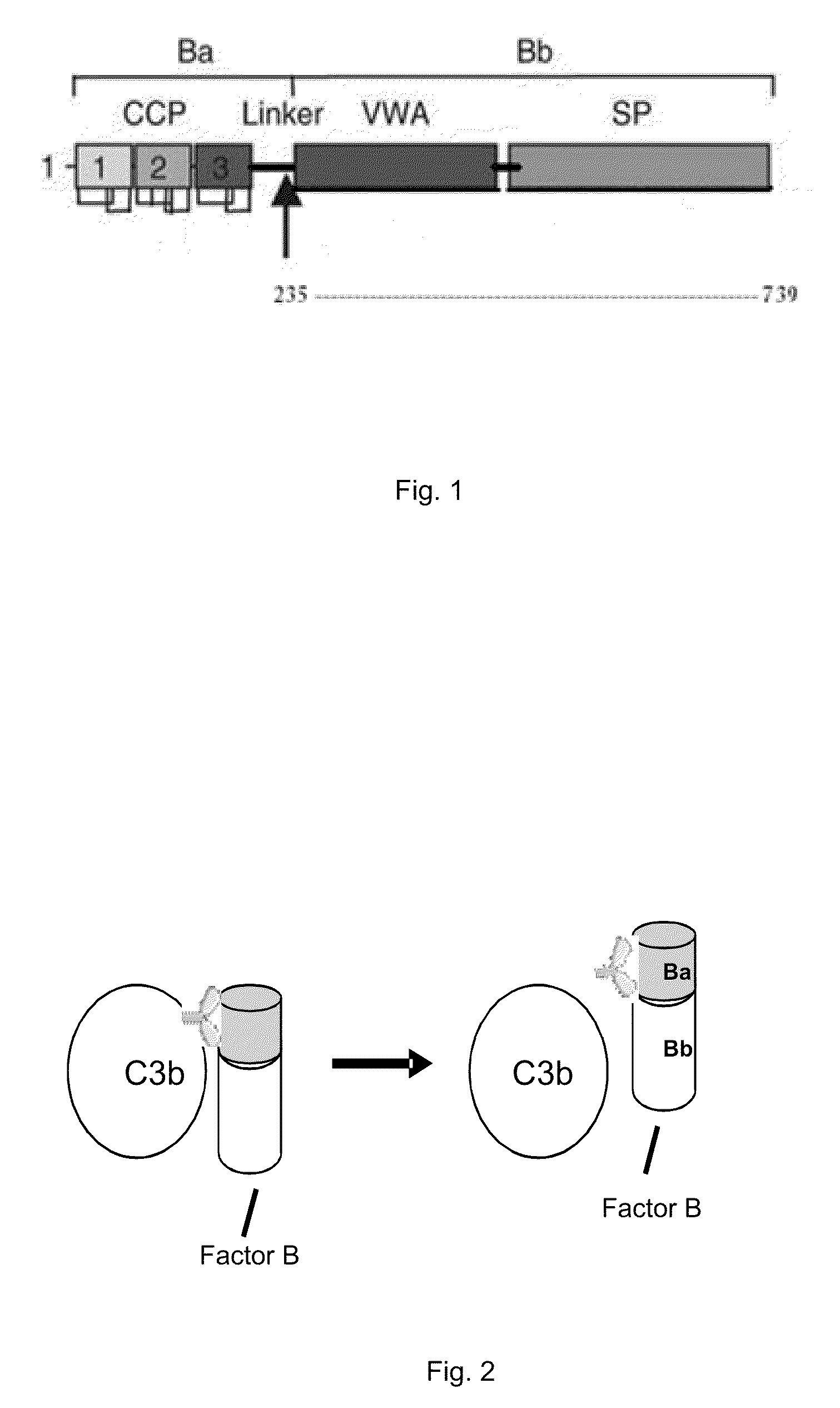
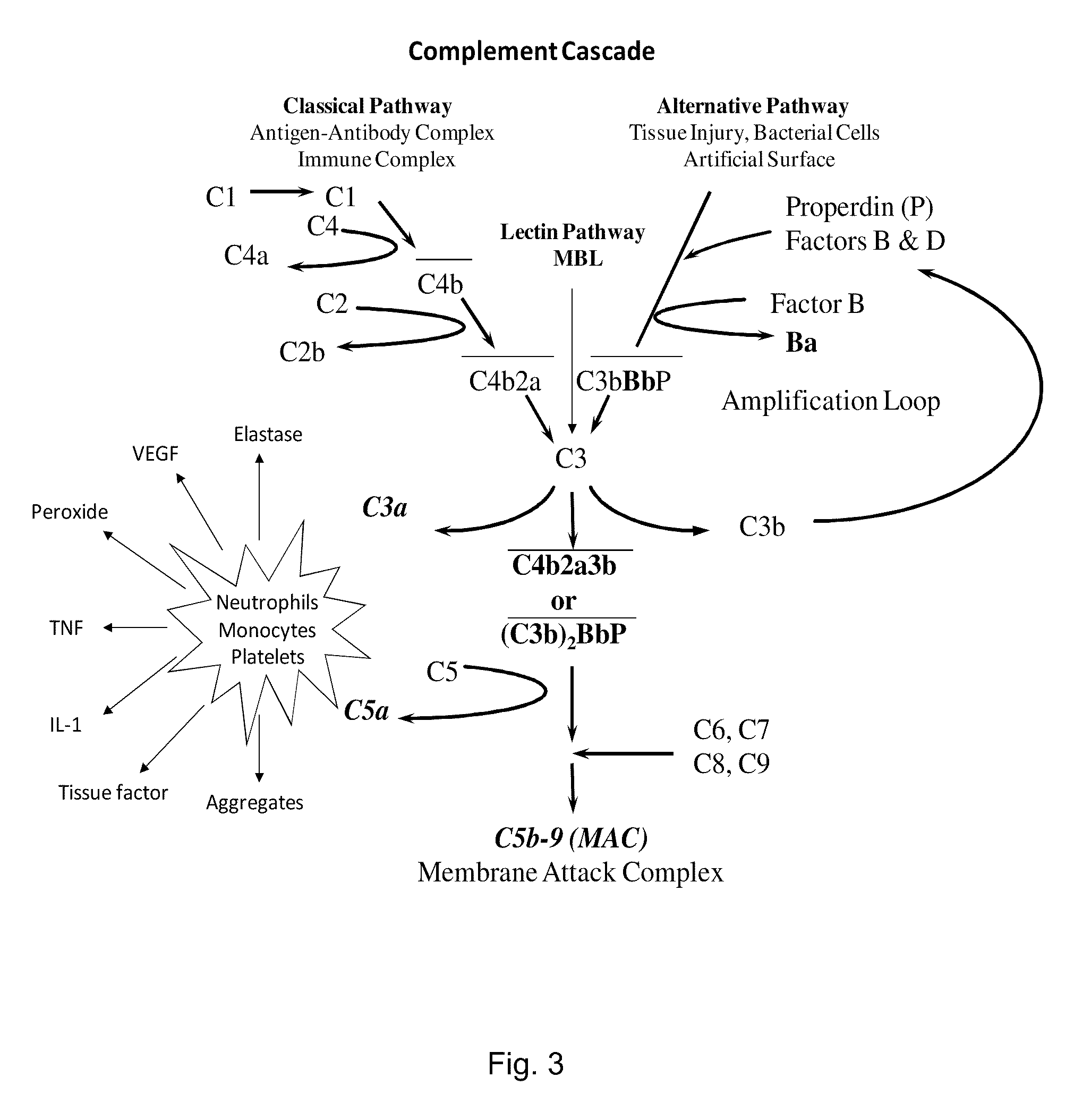
METHOD OF INHIBITING COMPLEMENT ACTIVATION WITH FACTOR Ba SPECIFIC ANTIBODIES AND USE THEREOF
InactiveUS20100015139A1Inhibiting complement activationEliminate side effectsAntibody ingredientsImmunoglobulins against enzymesFactor iiNeutrophil granulocyte
A method of inhibiting the adverse effects of alternative complement pathway activation products in a subject comprising administering to the subject an amount of anti-factor Ba antibody effective to selectively inhibit formation of an alternative complement pathway activation products C3a, C5a, and C5b-9, and activation of neutrophils, monocytes, and platelets.
Owner:NOVELMED THERAPEUTICS
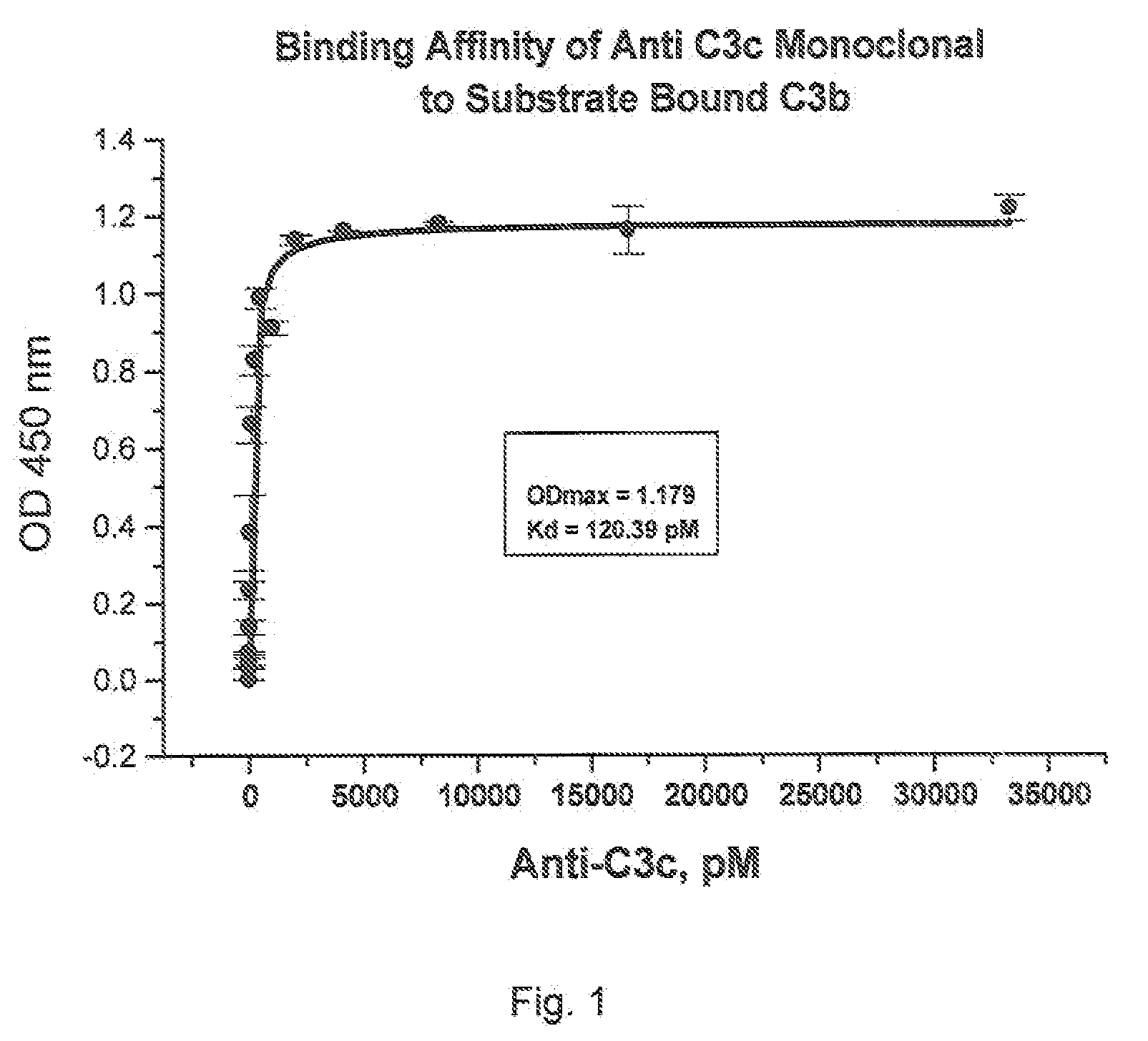
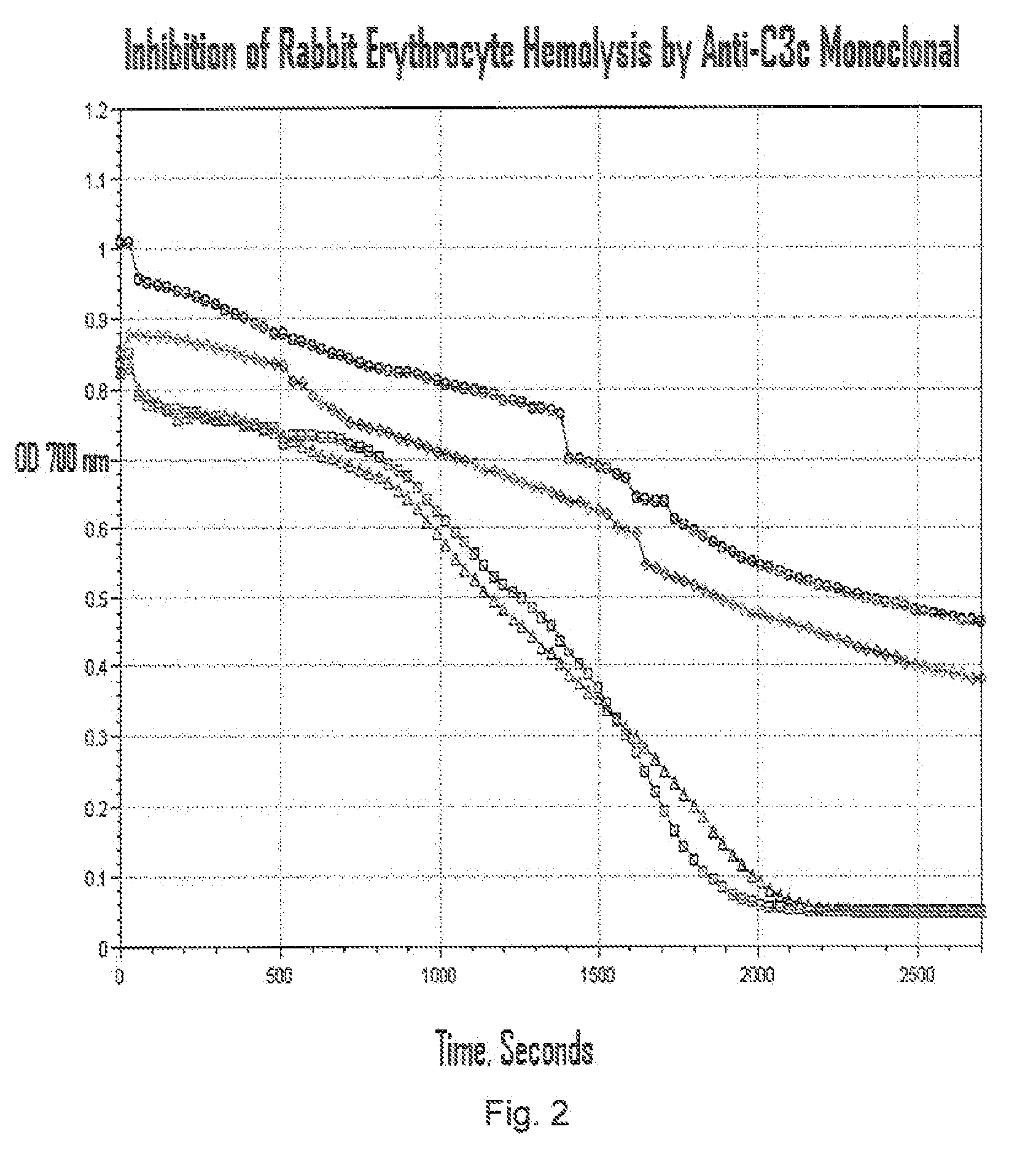
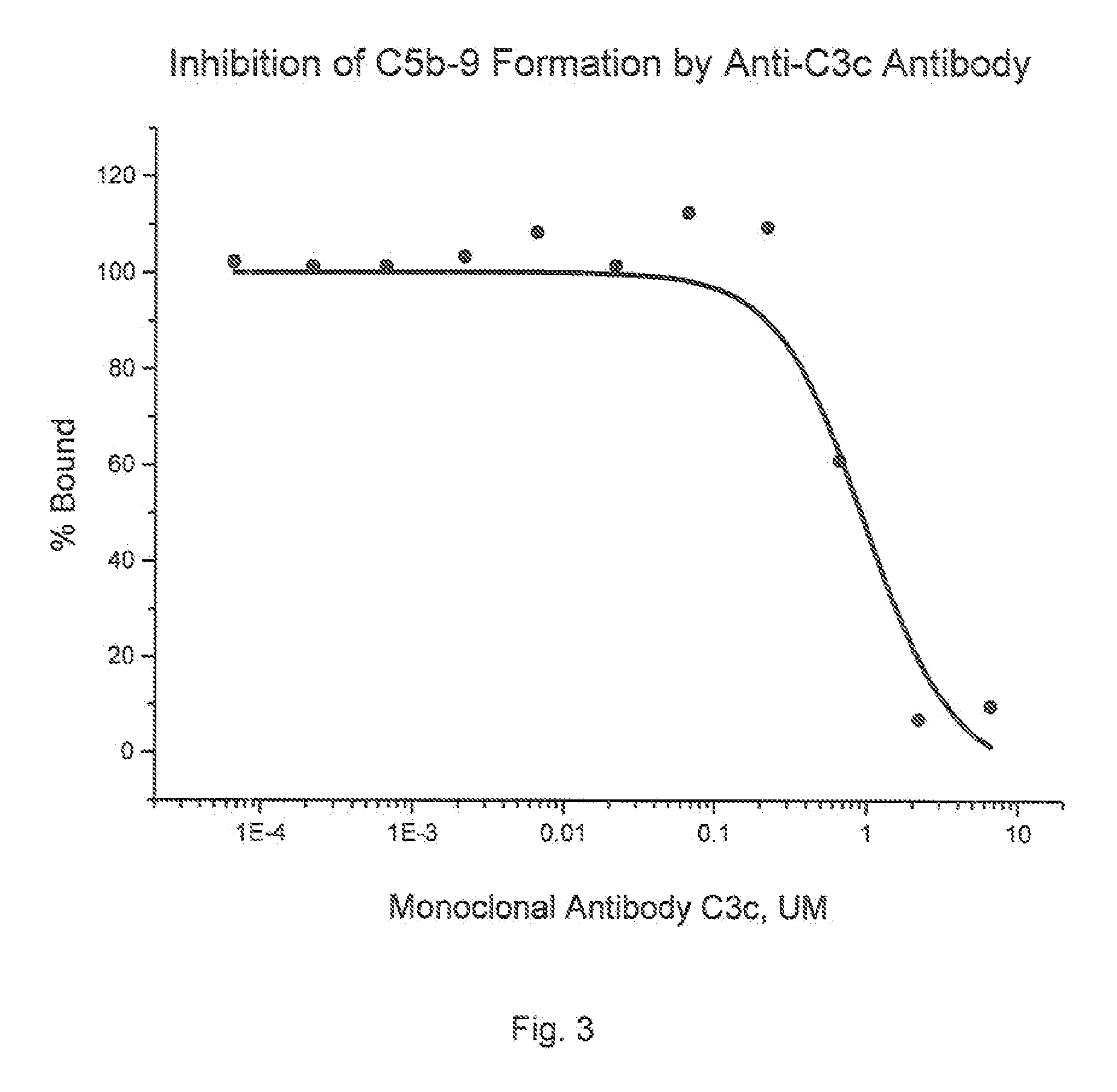
Method of inhibiting complement activation with human Anti-factor c3 antibodies and use thereof
ActiveUS20080233113A1Eliminate side effectsInhibiting complement activationSenses disorderNervous disorderProperdinNeutrophil granulocyte
A method of inhibiting complement activation mediated by C3b inhibitors in a subject includes administering a C3B inhibitor to the subject to inhibit at least one of C3b binding to factors B and properdin, inhibit C3 cleavage, inhibit the activation of neutrophils, monocytes, platelets, and endothelium; or inhibit the formation of C3a, C5a, and MAC.
Owner:NOVELMED THERAPEUTICS
Antibacterial composition and method of production
An electrolysis method is described for generating an aqueous solution of copper citrate that has bacteriocidal activity against methicillin resistant Staphylococcus aureus (MRSA) bacteria. Gram positive bacteria are known to be relatively more sensitive to the bacteriocidal activities of copper ions than are Gram negative bacteria. Situations exist in which a disinfectant that is relatively more toxic for Gram positive bacteria will be advantageous over a more broadly active disinfectant, such as that provided by most other disinfectants. In particular, a disinfectant that is relatively selective for Gram positive bacteria could help preserve various non-pathogenic Gram negative microbial populations. The residual Gram negative bacteria can potentially compete with, and thereby lessen the chances of the reintroduction of pathogenic Gram positive bacteria, such as MRSA, Streptococcus, Clostridium difficile and Listeria monocytogenes.
Owner:MI HOPE
Method of transplantation using chemotherapy-treated allogeneic cells that enhance immune responses without graft versus host disease
InactiveUS20020127208A1Effective in treating and preventing infectionReduce capacityBiocideGenetic material ingredientsAllogeneic cellHematopoietic cell
The present invention provides a method of transplanting hematopoietic cells between genetically unrelated individuals, comprising administering to the recipient, in combination with the administration of the hematopoietic cells, an amount of mononuclear cells which are treated so as to substantially reduce their ability to cause graft versus host disease while they retain their ability to proliferate in the recipient. The treated mononuclear cells can facilitate engraftment of hematopoietic cells when transplanted in combination with hematopoietic cells, treat or prevent infections, and treat cancer.
Owner:EMORY UNIVERSITY
Antibodies against csf-1r
ActiveUS20110243947A1Prevent dimerizationInhibit tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDocetaxel-PNPDocetaxel
The invention provides a human antibody that binds human CSF-1R with high affinity. Antibodies of the present invention have significant advantages over the antibodies known in the art by being multifunctional: inhibiting signaling of CSF-1R, internalizing and inducing CSF-1R degradation and stimulating ADCC in cell including tumors, macrophages and monocytes. They are also shown to be effective in treating leukemia, breast, endometrial and prostate cancer alone or in combination with docetaxel, paclitaxel, Herceptin® or doxorubicin.
Owner:IMCLONE SYSTEMS
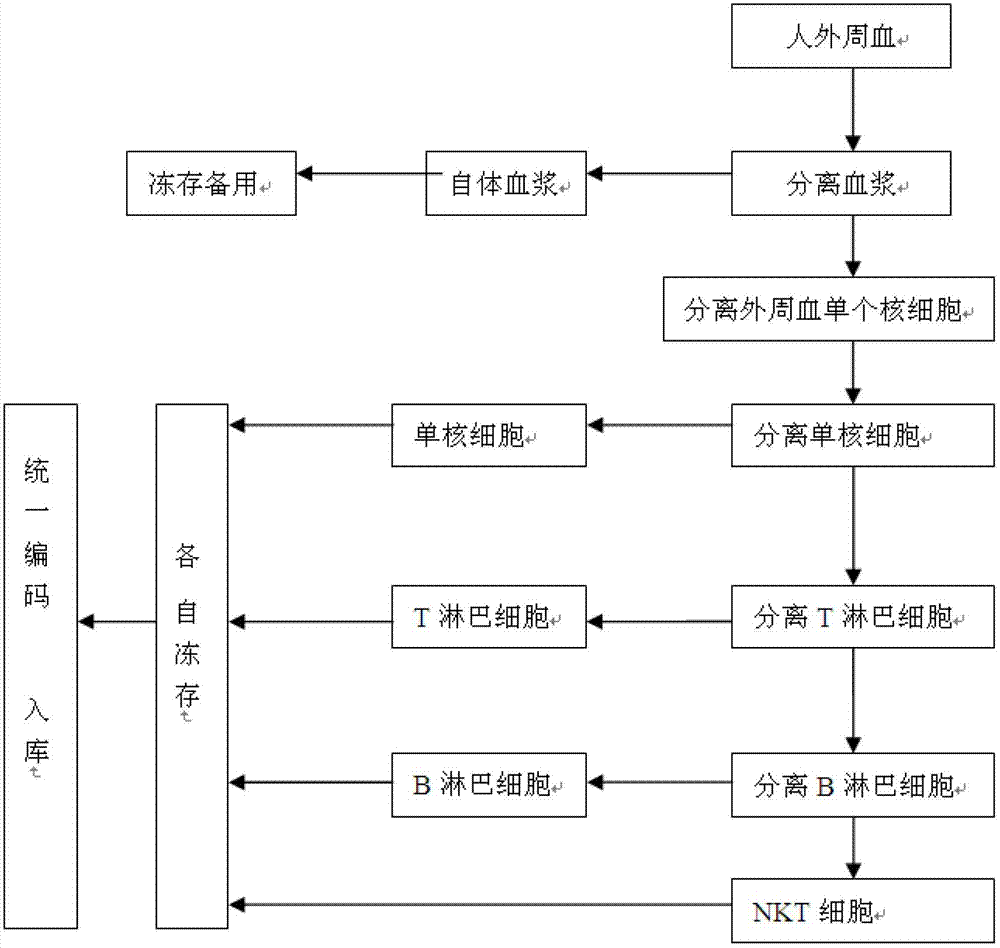
Method for constructing human peripheral blood immune cell bank
ActiveCN102758259AHigh activityHigh purityMicroorganism librariesBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for constructing a human peripheral blood immune cell bank. The method comprises the following steps of: collecting human peripheral blood, separating autologous plasma, separating a peripheral blood mononuclear cell, separating a mononuclear cell by the peripheral blood mononuclear cell and freezing, separating a T lymphocyte by the peripheral blood mononuclear cell and freezing, separating a B lymphocyte by the peripheral blood mononuclear cell and freezing, separating an NK cell by the peripheral blood mononuclear cell and freezing, and encoding and puttingin storage. According to the invention, immune cells of health or young people are separated and are respectively independently frozen, and relative numbers are stored and put into storage, so that the stored human immune cells have the characteristics of high activity, high purity and convenience for use, and fetal calf serum can be replaced by the human autologous plasma, so that introduction of a foreign protein can be avoided. Meanwhile, the method, provided by the invention, has the advantages of low cost, low requirements on laboratory conditions, and wide application.
Owner:济南赛尔生物科技股份有限公司
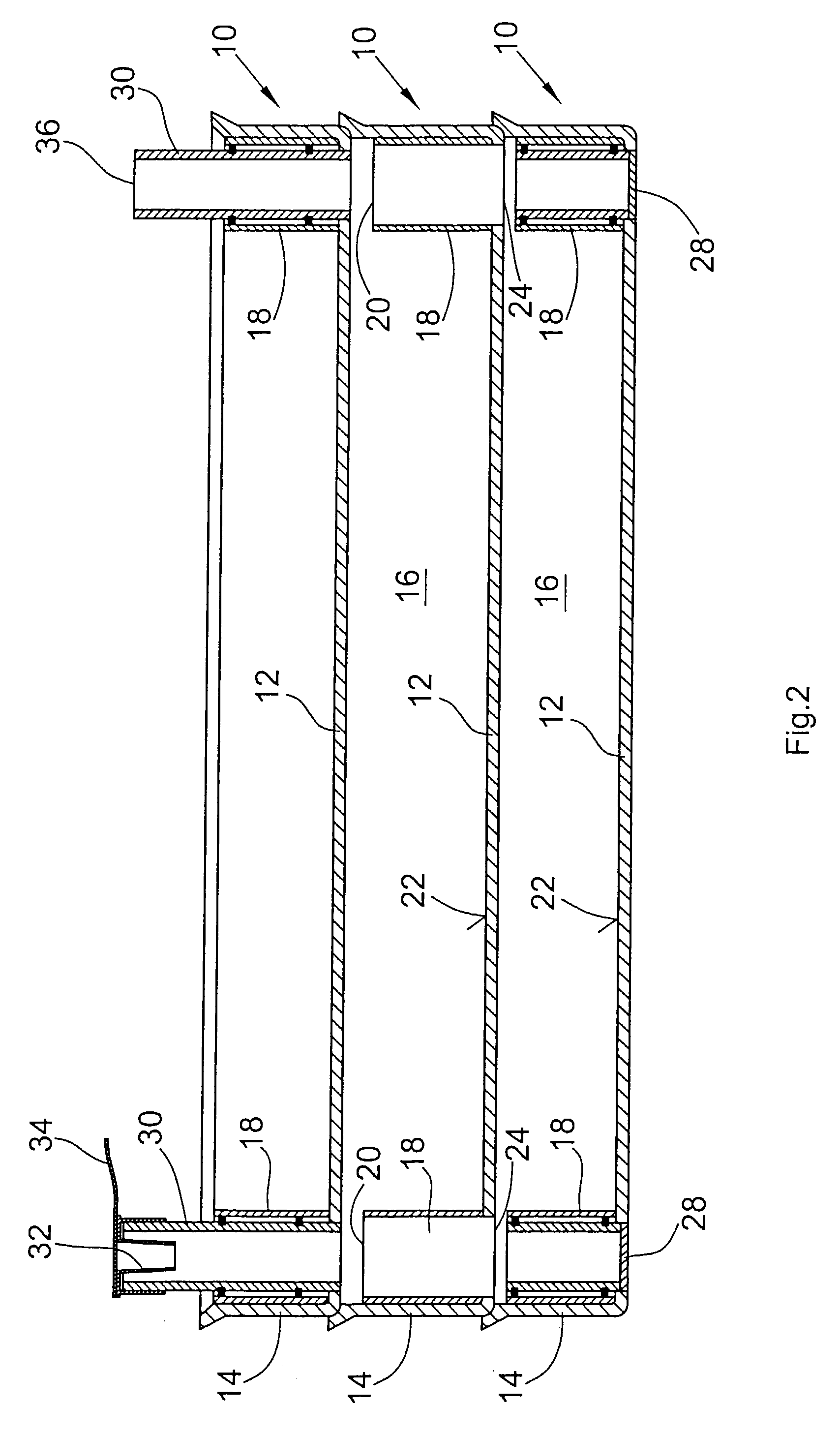
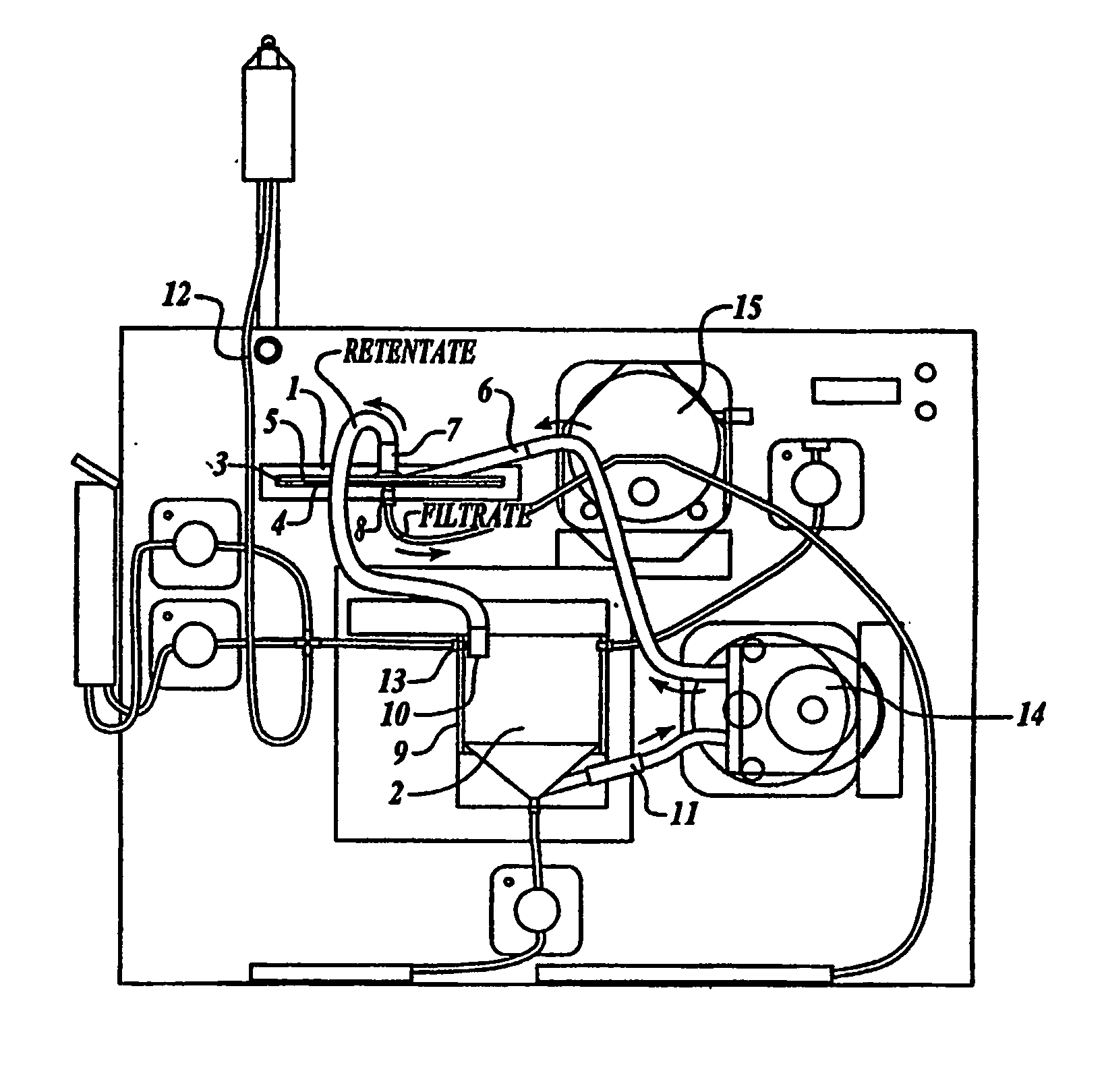
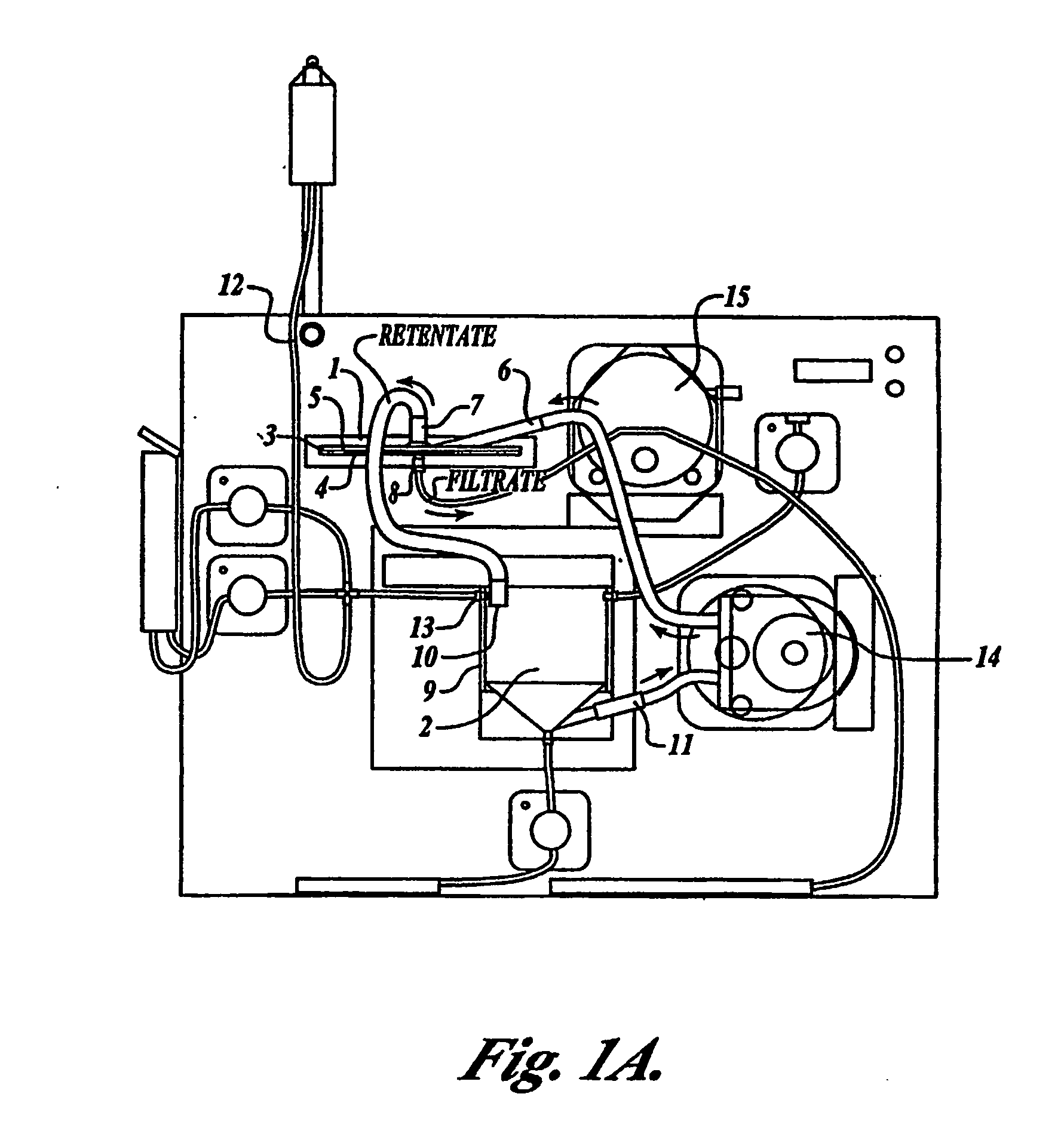
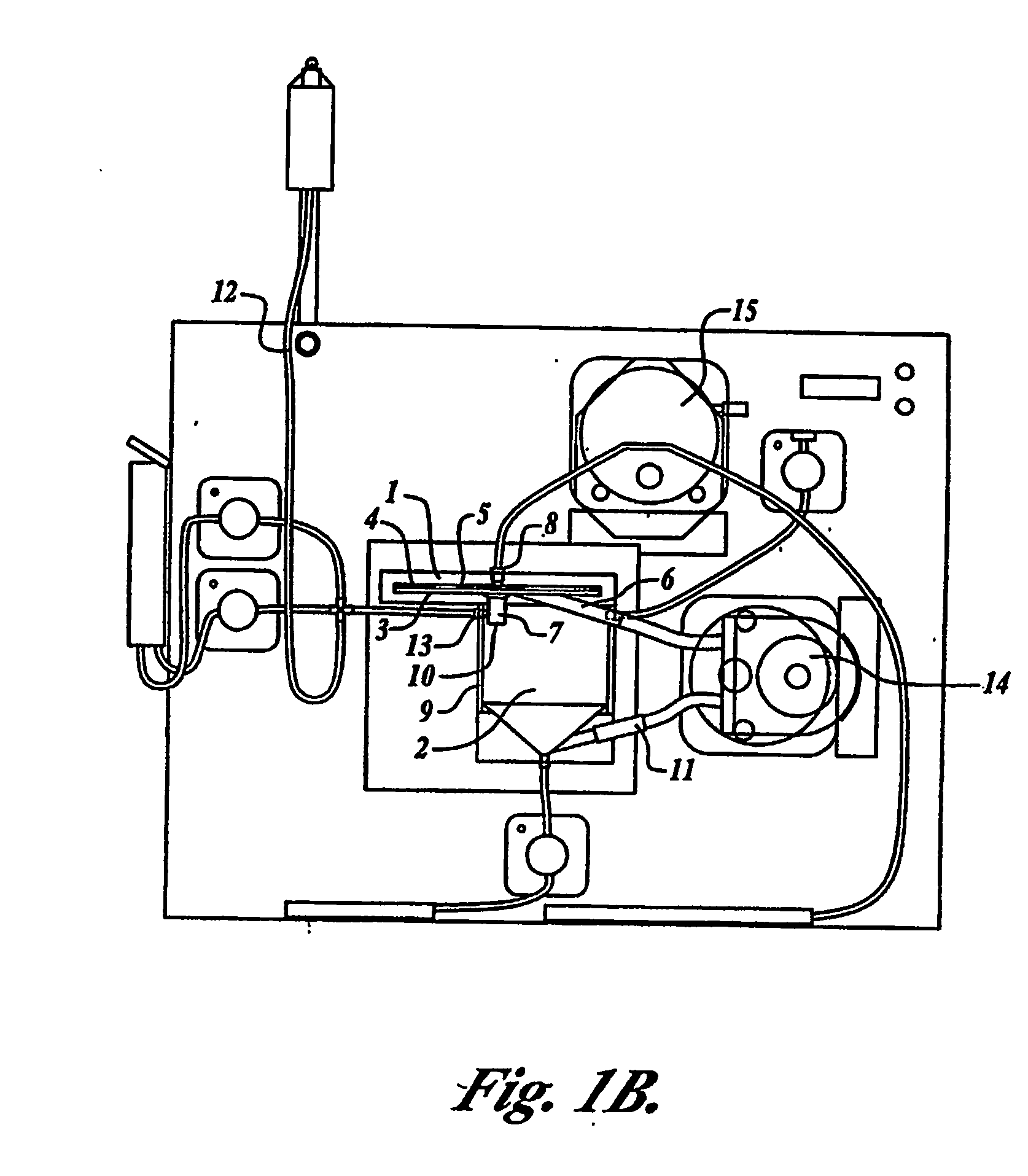
Tangential flow filtration devices and methods for leukocyte enrichment
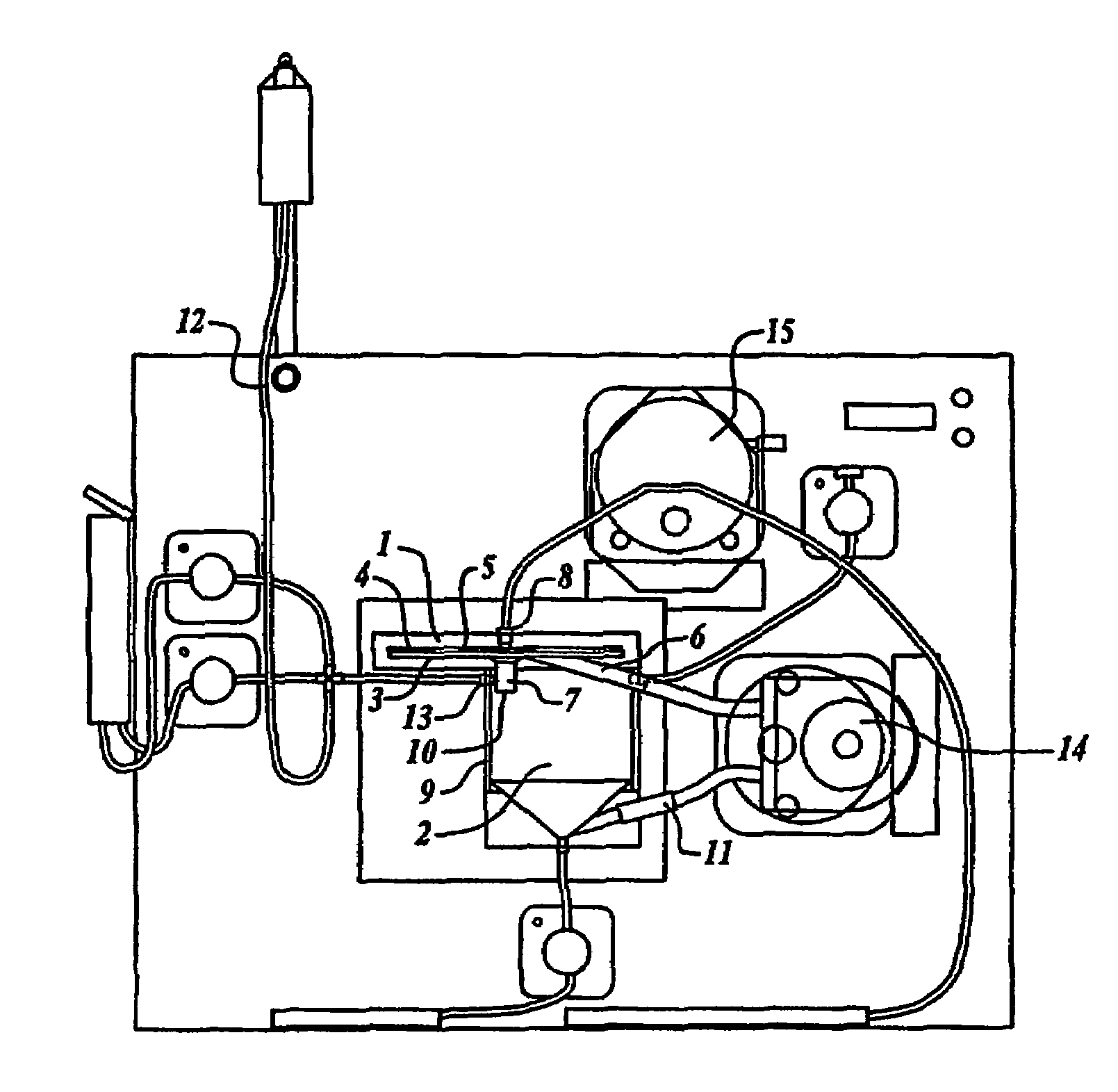
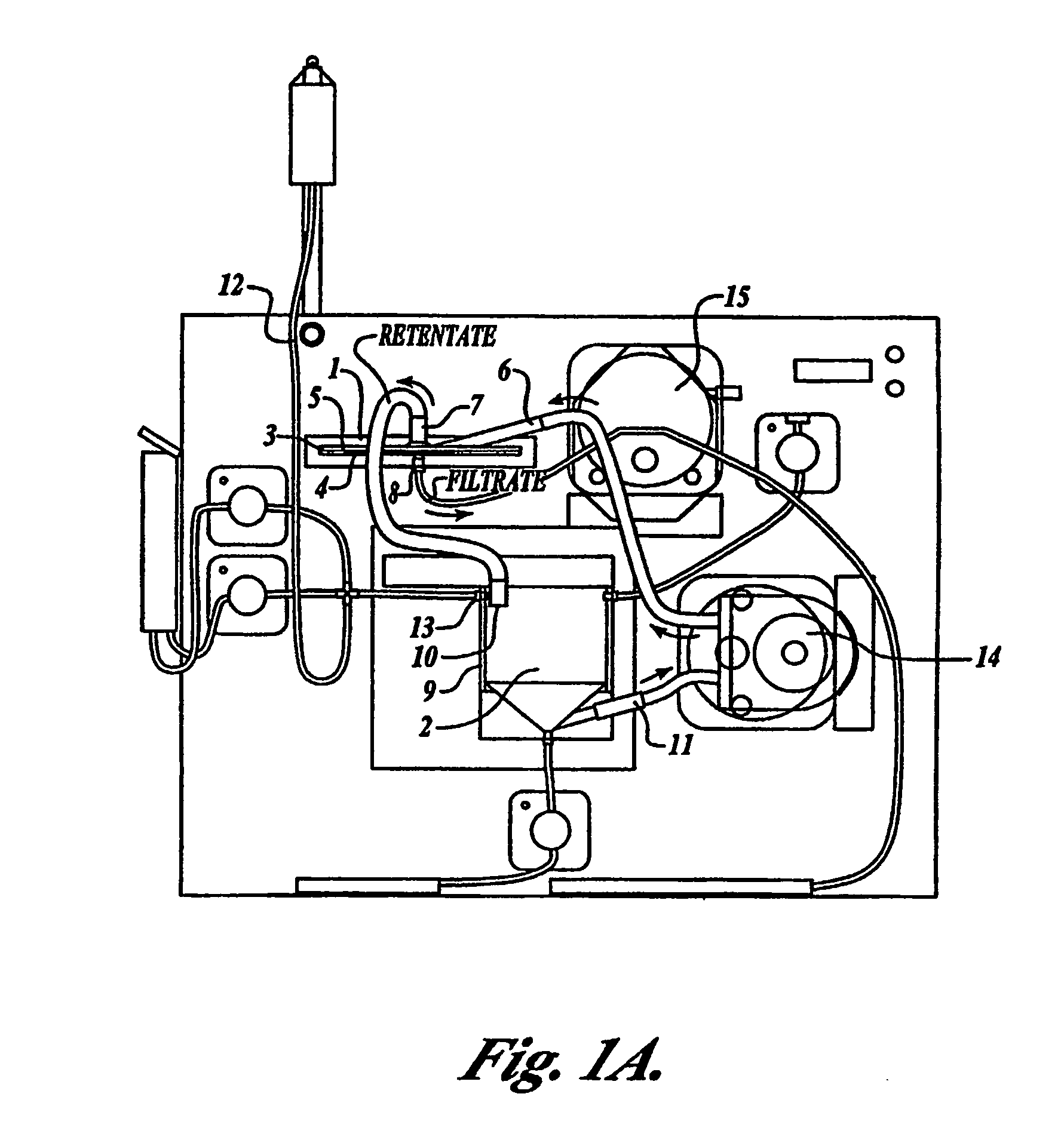
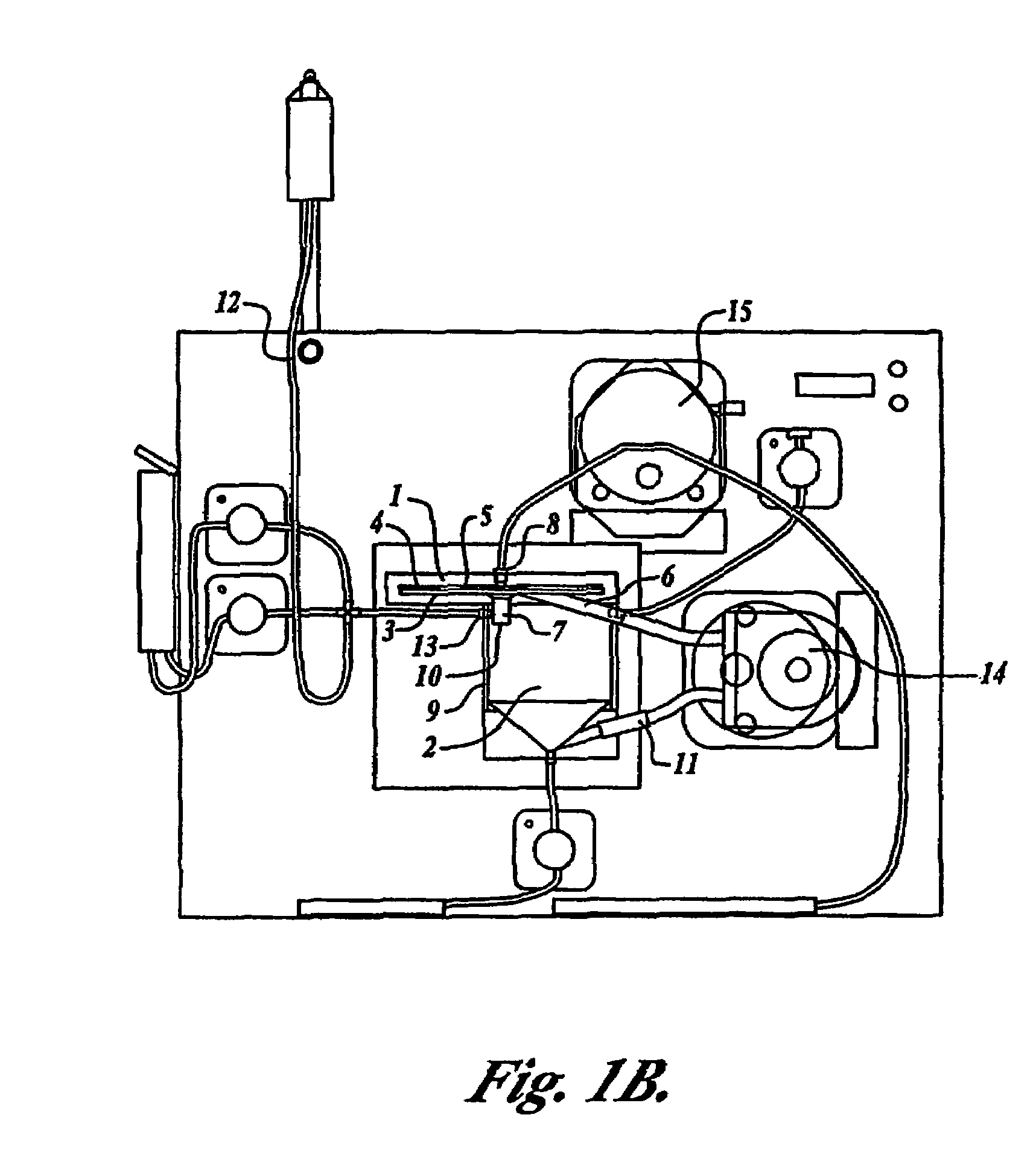
ActiveUS7695627B2Reduce rateLiquid separation auxillary apparatusOther blood circulation devicesMicropore FilterBlood component
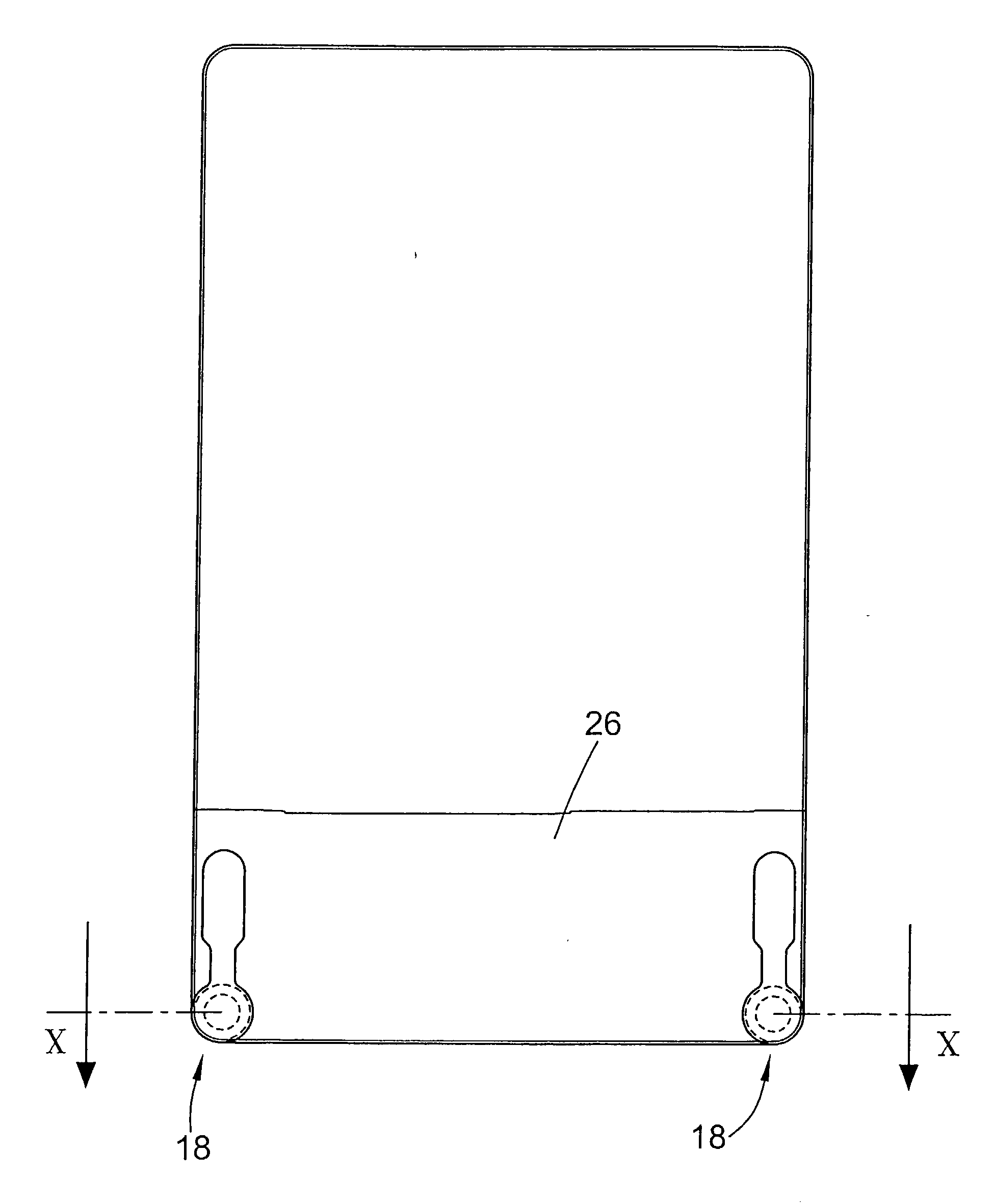
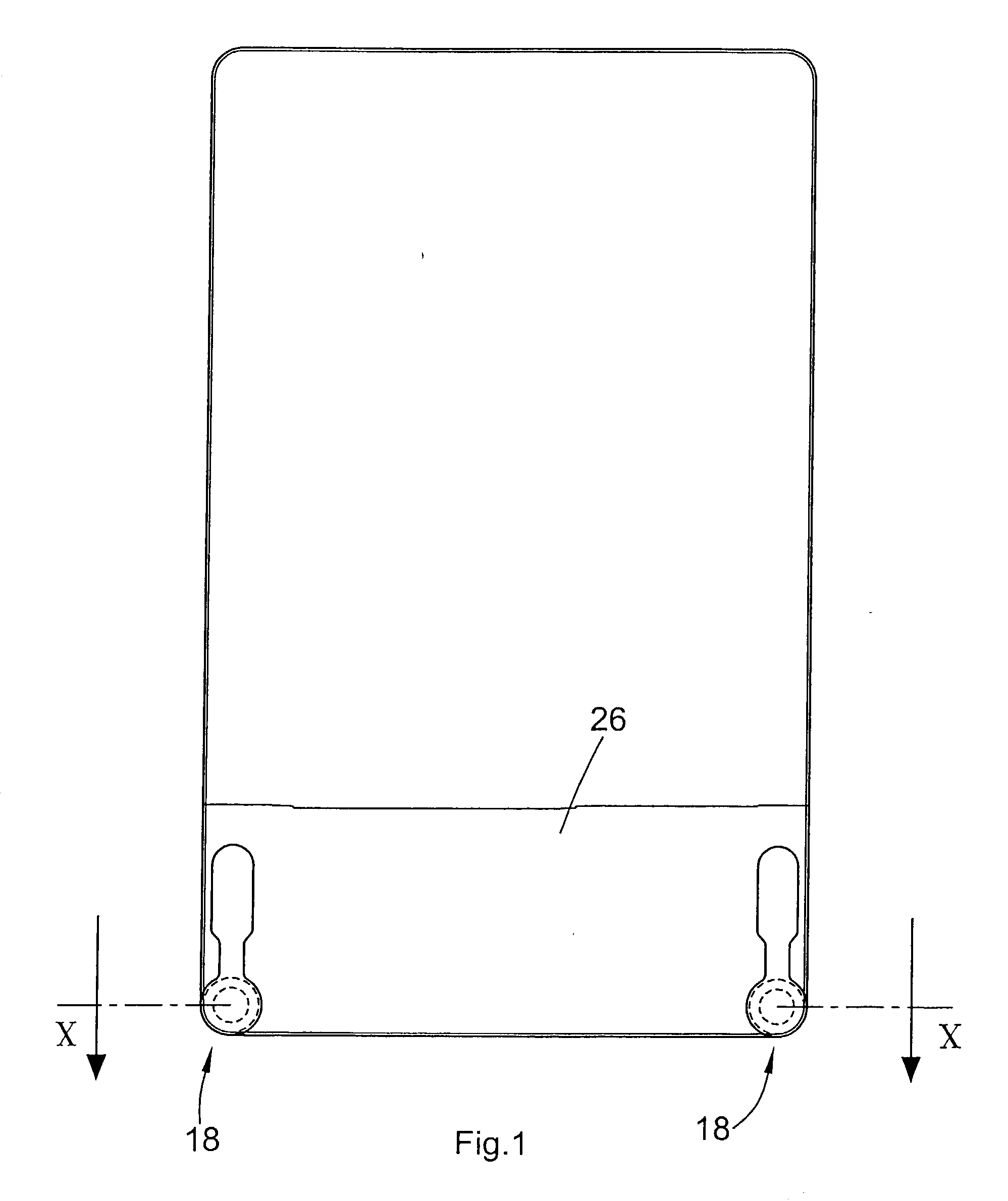
The present invention provides tangential flow filtration devices and methods for enriching a heterogeneous mixture of blood constituents for leukocytes by removal of non-leukocyte blood constituents. In one particular embodiment the device can provide a composition enriched in monocytes. One embodiment includes a remover unit (1) having a crossflow chamber (3) separated by a microporous filter (5) from a filtrate chamber (4), the remover unit (1) also having a tangential flow inlet (6), a fluid outlet (7) for a fluid enriched in leukocytes and a filtrate outlet (8).
Owner:NORTHWEST BIOTHERAPEUTICS INC
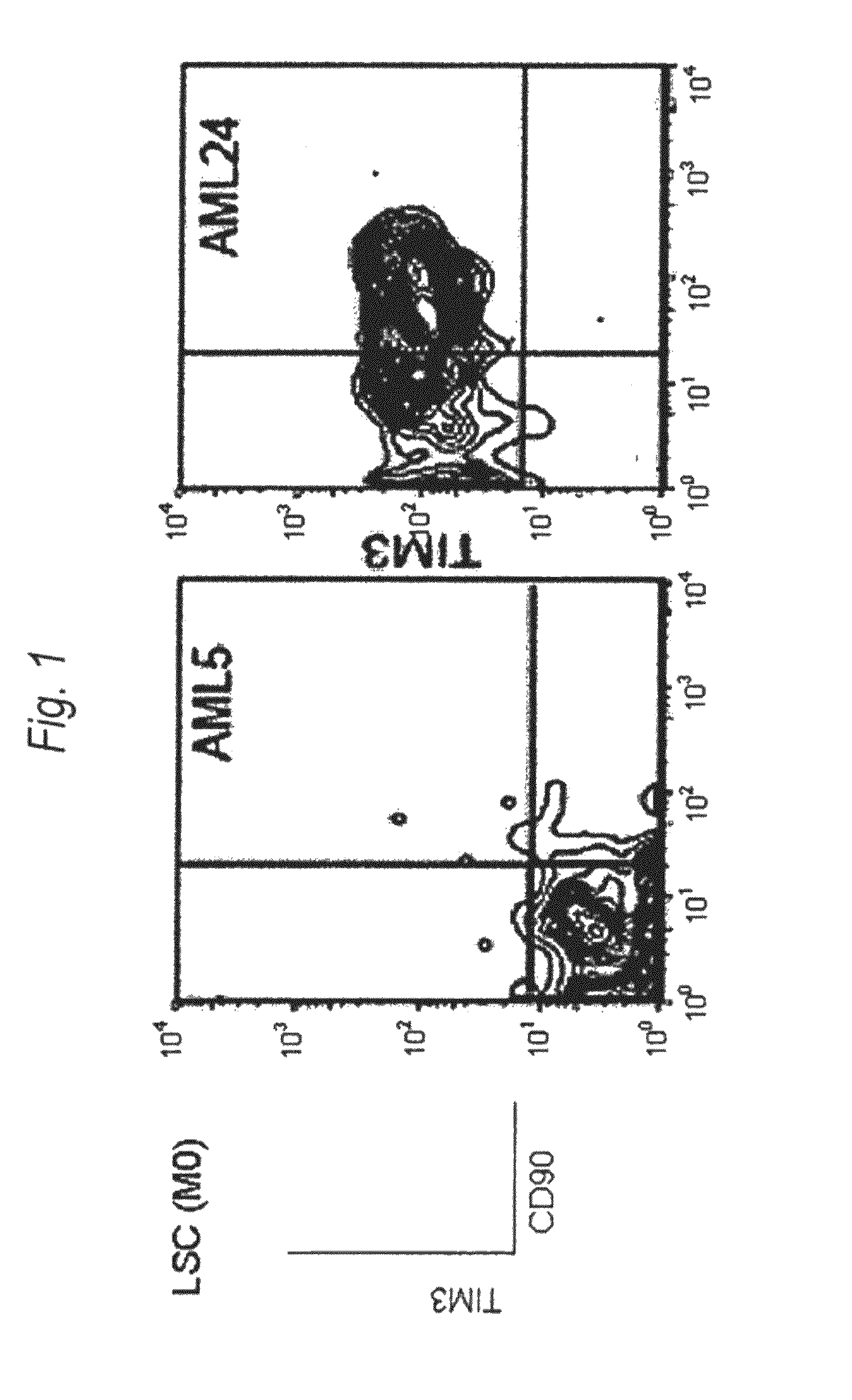
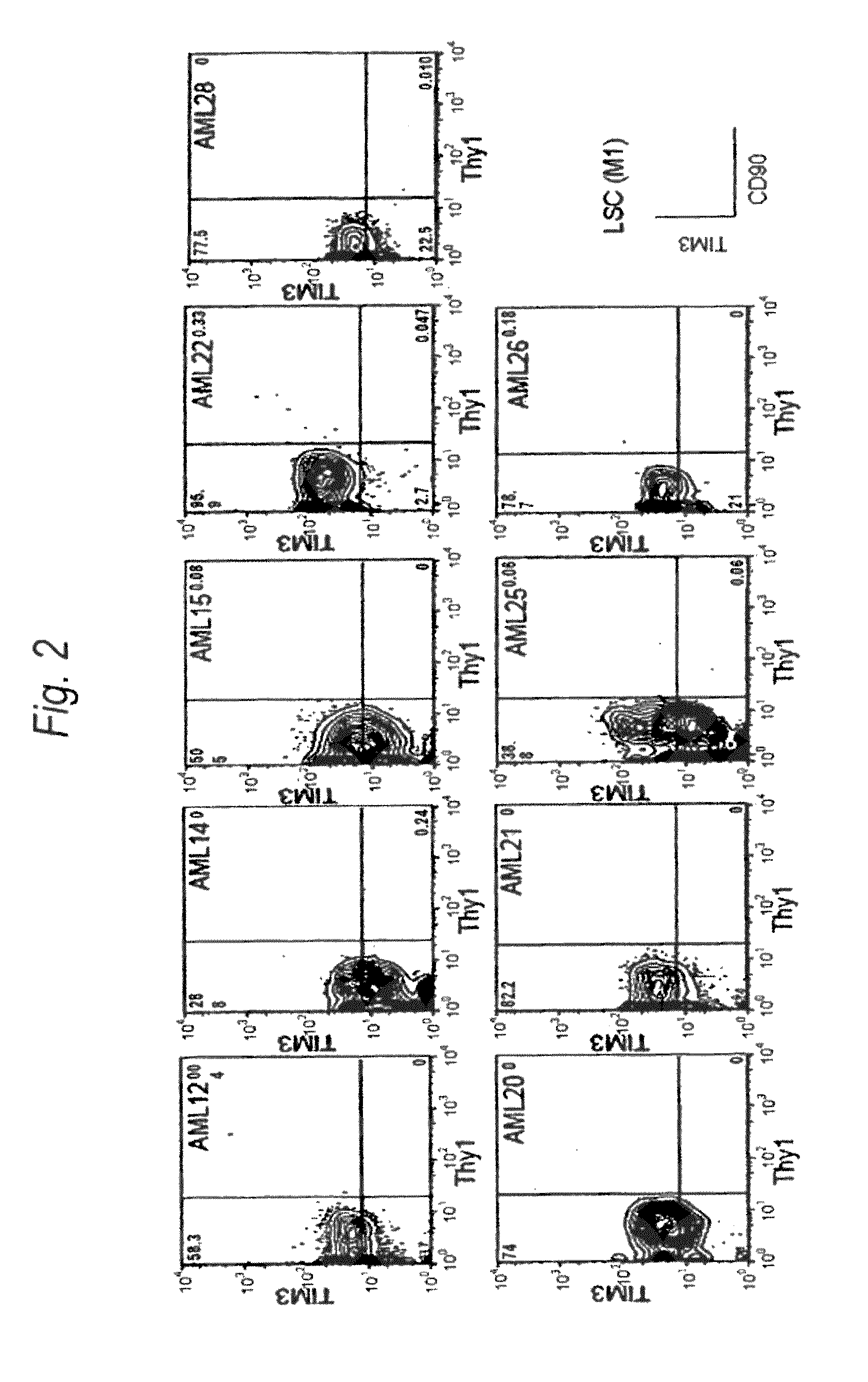
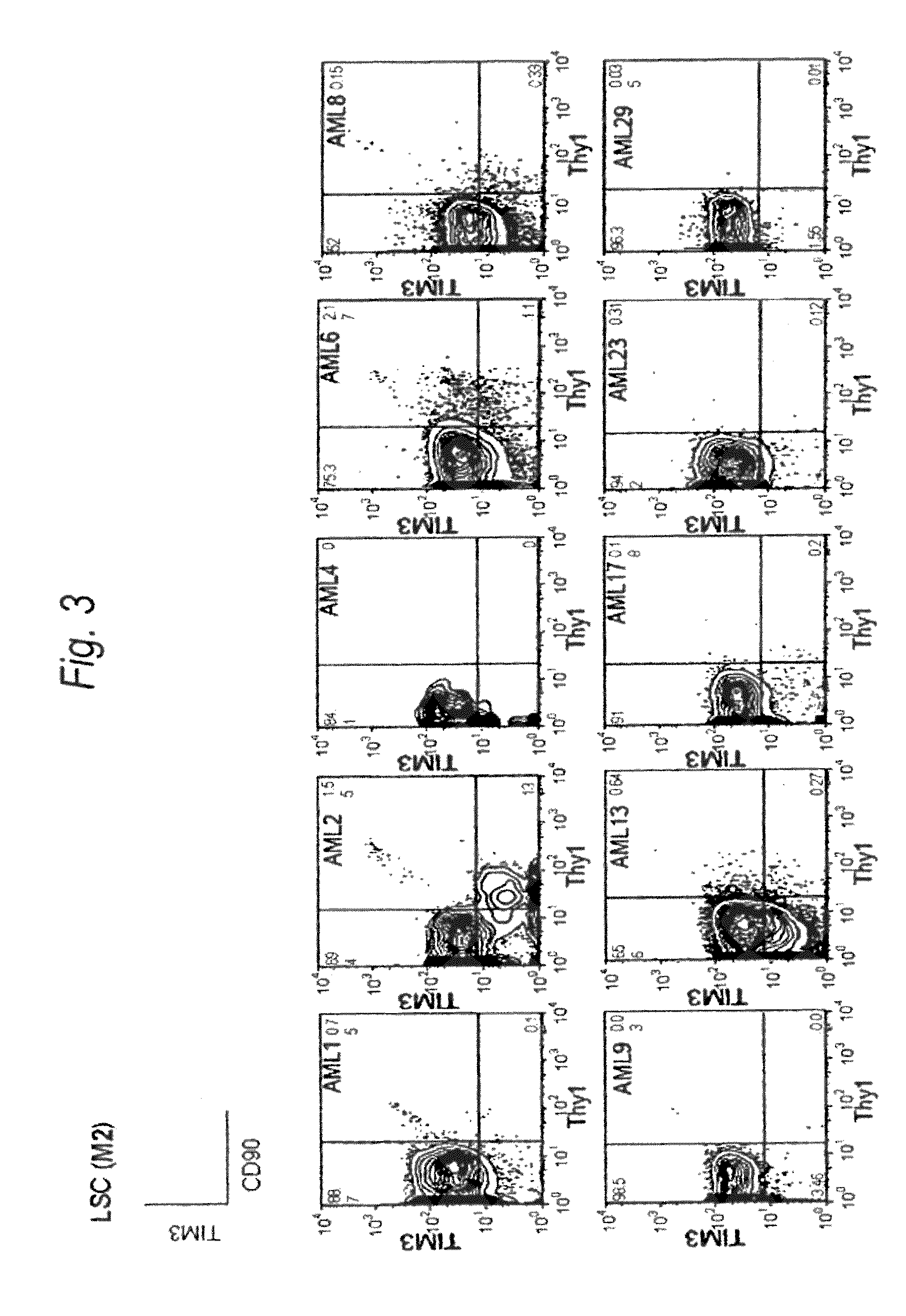
Method for treatment of blood tumor using anti-TIM-3 antibody
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveUS20070092967A1Increase success rateArtificial cell constructsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an αMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the αMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:HAN HOON
Tangential flow filtration devices and methods for leukocyte enrichment
ActiveUS20050173315A1Increase percentageReduce rateOther blood circulation devicesHaemofiltrationMedicineFiltration
The present invention provides tangential flow filtration devices and methods for enriching a heterogeneous mixture of blood constituents for leukocytes by removal of non-leukocyte blood constituents. In one particular embodiment the device can provide a composition enriched in monocytes. One embodiment includes a remover unit (1) having a crossflow chamber (3) separated by a microporous filter (5) from a filtrate chamber (4), the remover unit (1) also having a tangential flow inlet (6), a fluid outlet (7) for a fluid enriched in leukocytes and a filtrate outlet (8).
Owner:NORTHWEST BIOTHERAPEUTICS INC
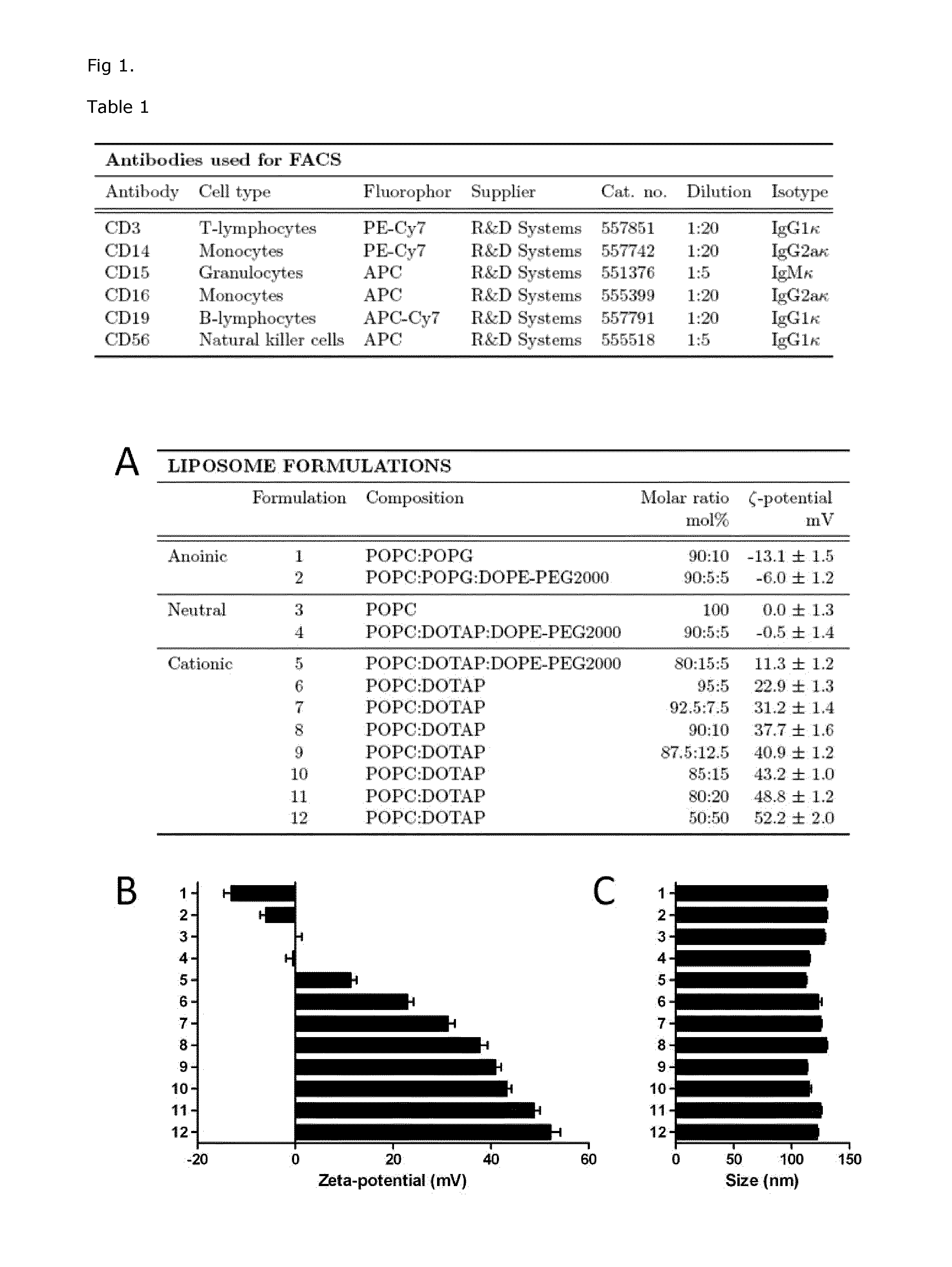
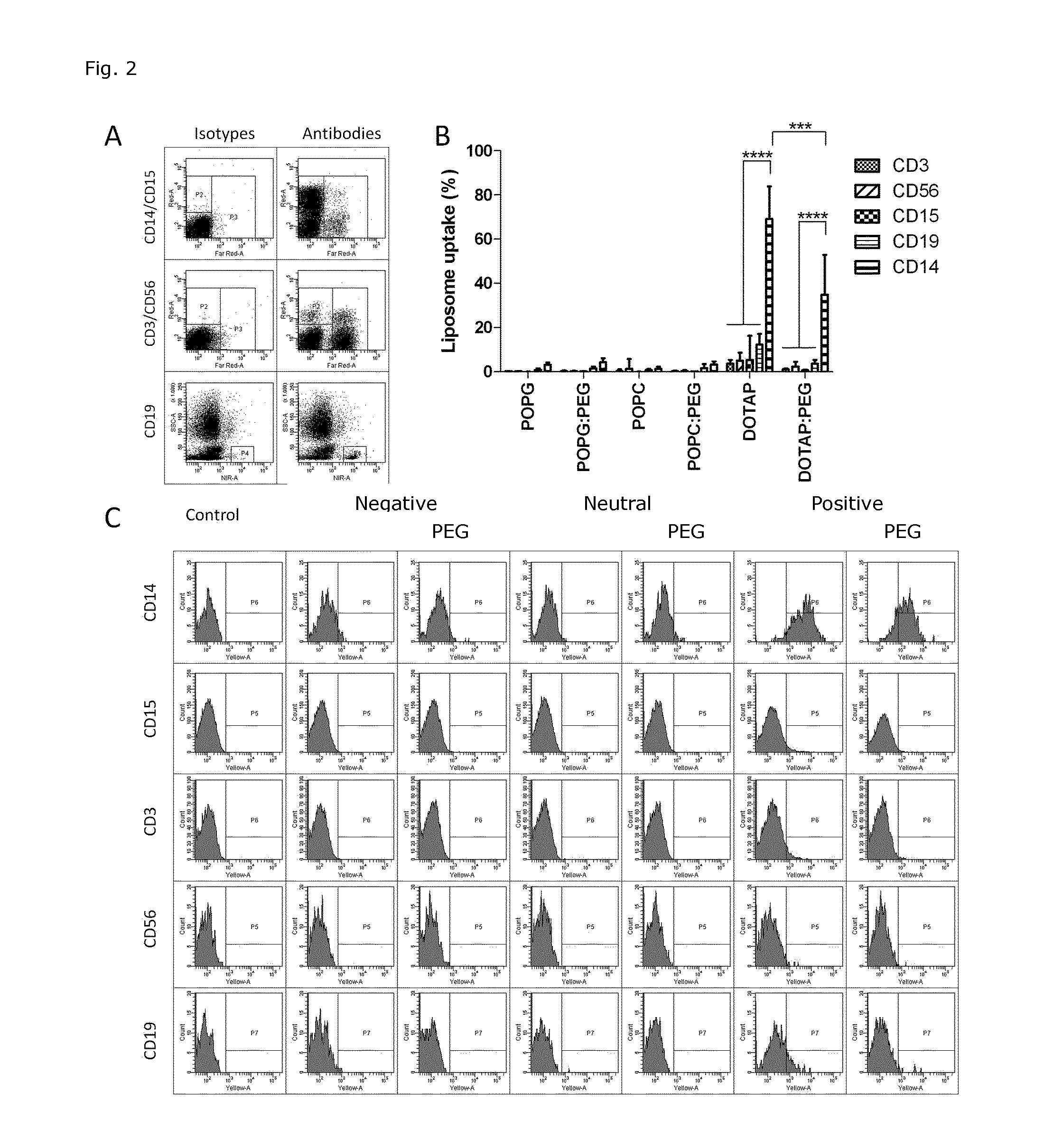
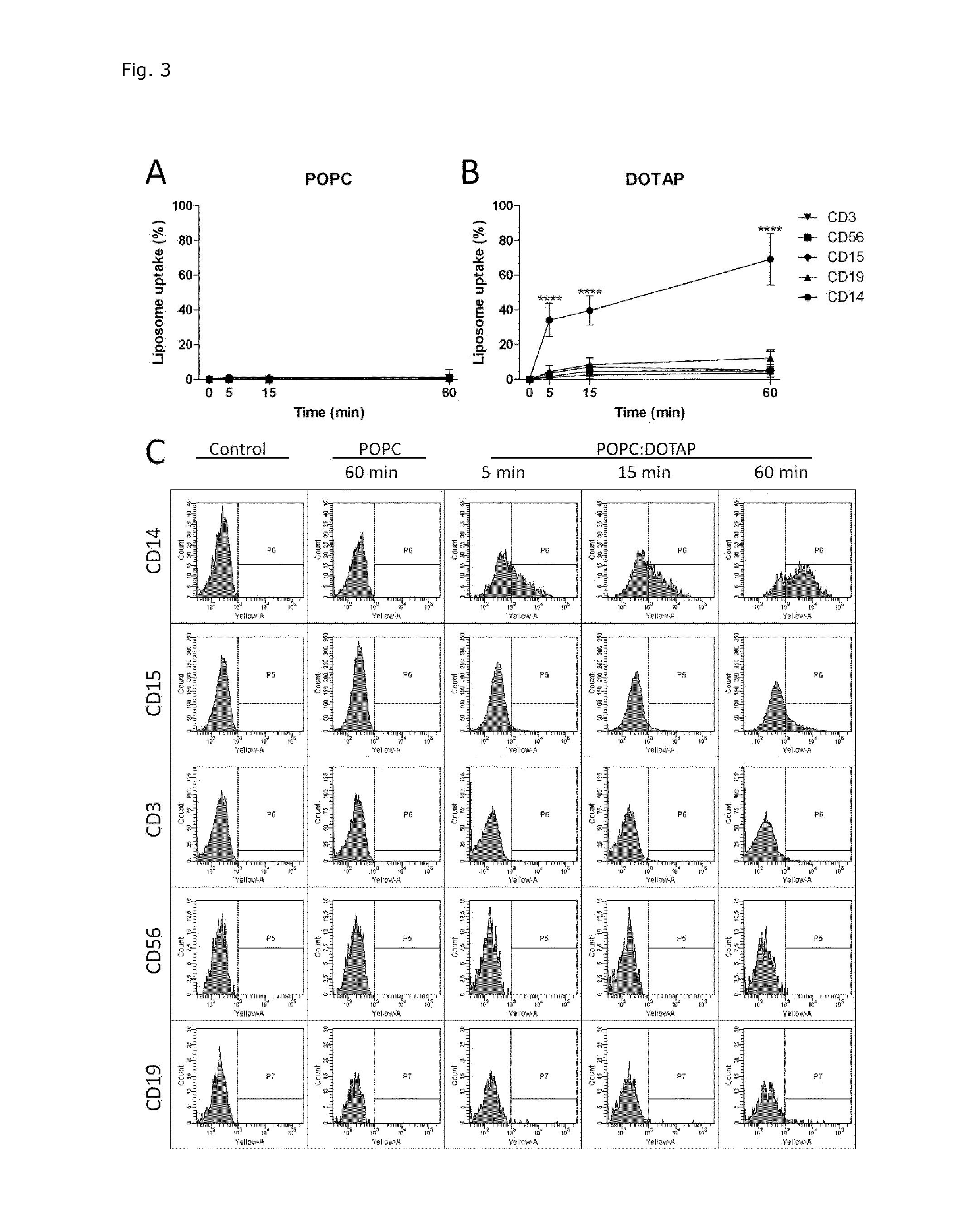
Cationic Liposomal Drug Delivery System for Specific Targeting of Human CD14+ Monocytes in Whole Blood
This invention concerns a liposome comprising lipids and at least one active ingredient, wherein at least one of the lipids is a cationic lipid; said liposome exhibiting a net positive charge at physiological conditions at which said liposome preferentially adheres to monocytes in freshly drawn blood when compared to adherence to granulocytes, T-lymphocytes, B-lymphocytes and / or NK cells in freshly drawn blood, to a lipid-based pharmaceutical composition comprising said liposomes and their use in monocytic associated prophylaxis, treatment or amelioration of a condition such as cancer, an infectious disease, an inflammatory disease, an autoimmune disease or allergy.
Owner:BIONEER +1
Materials and methods for prevention and treatment of RNA viral diseases
InactiveUS20040009152A1Preventing and decreasing severity of symptomBiocidePeptide/protein ingredientsDiseaseMononuclear cell infiltration
The subject invention concerns a method of inhibiting an RNA virus infection within a patient by increasing the amount of 2-5 oligoadenylate synthetase (2-5 AS) activity within the patient. Preferably, the preventative and therapeutic methods of the present invention involve administering a nucleotide encoding 2-5 AS, or at least one catalytically active fragment thereof, such as the p40, p69, p100 subunits, to a patient in need thereof. The present inventors have determined that overexpression of 2-5AS causes a reduction in epithelial cell damage, reduction in infiltration of mononuclear cells in the peribronchiolar and perivascular regions, and reduction in thickening of the septa in the lungs. Levels of chemokines, such as MIP1-alpha, are also reduced upon overexpression of 2-5AS. The subject invention also pertains to pharmaceutical compositions containing a nucleotide sequence encoding 2-5 AS and a pharmaceutically acceptable carrier, as well as vectors for delivery of the 2-5 AS nucleotide sequence.
Owner:IB SECURITYHOLDERS +1
Immunotherapy for immune suppressed patients
InactiveUS20060194242A1Organic active ingredientsPeptide/protein ingredientsDendritic cellCellular immunodeficiency
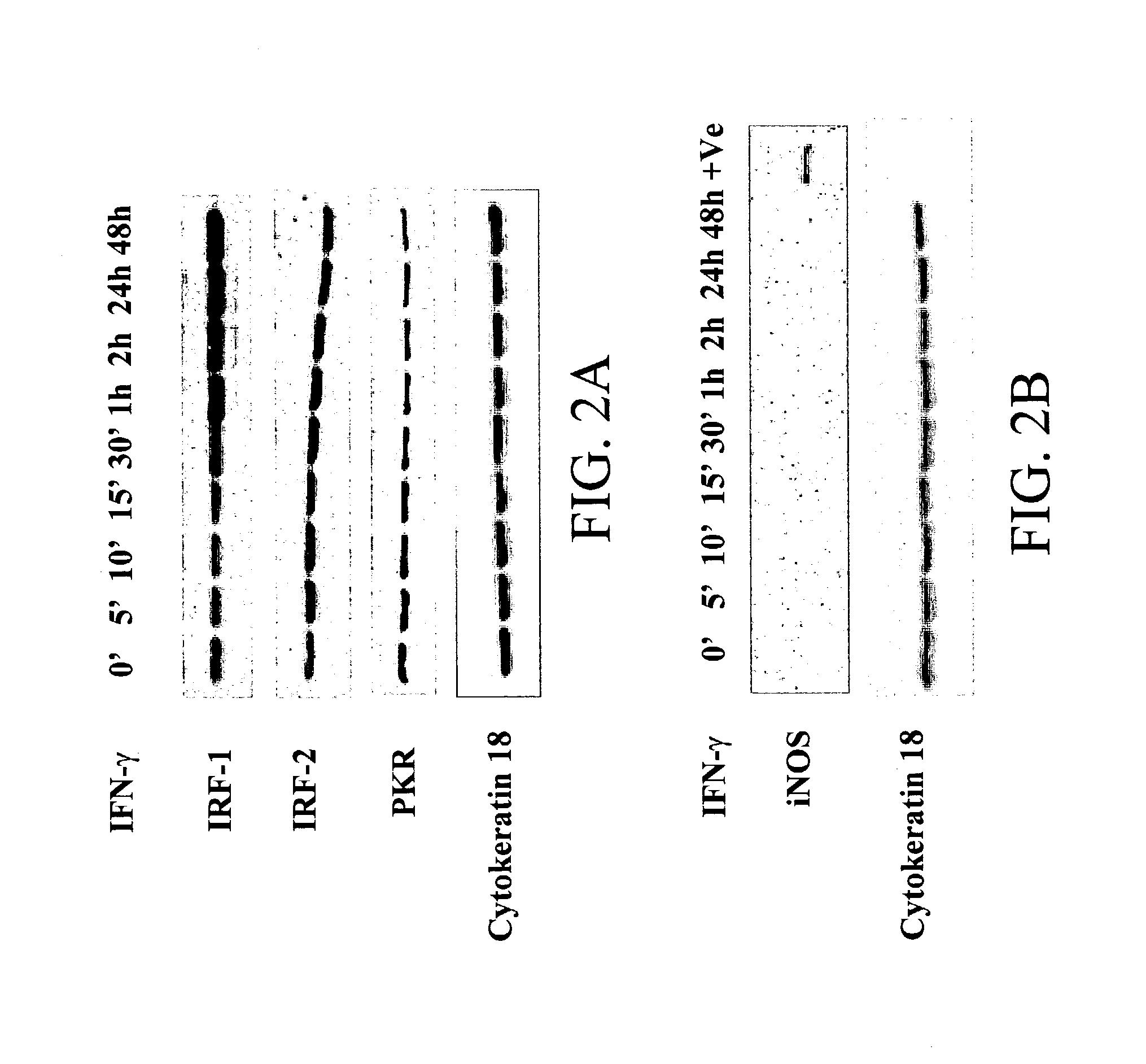
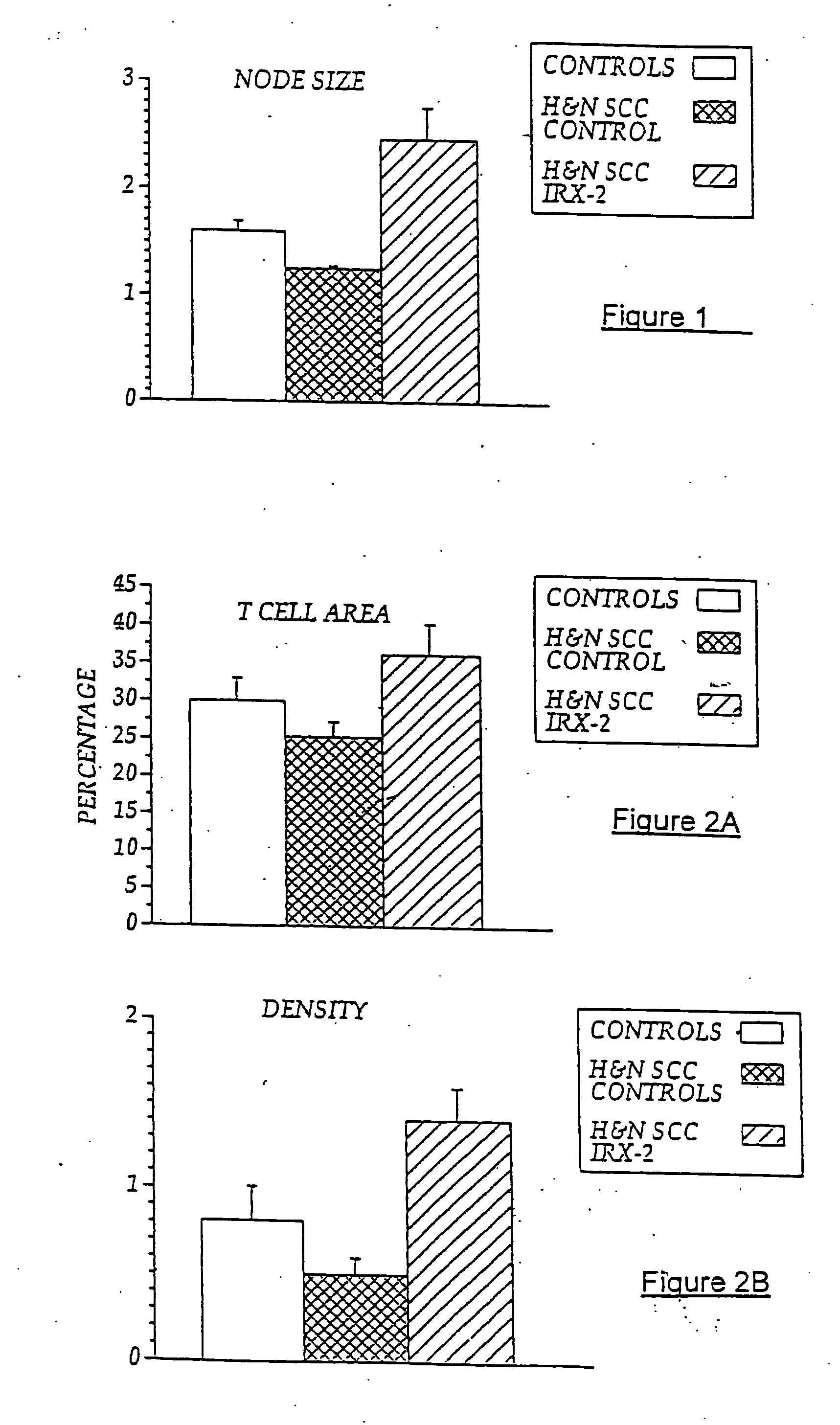
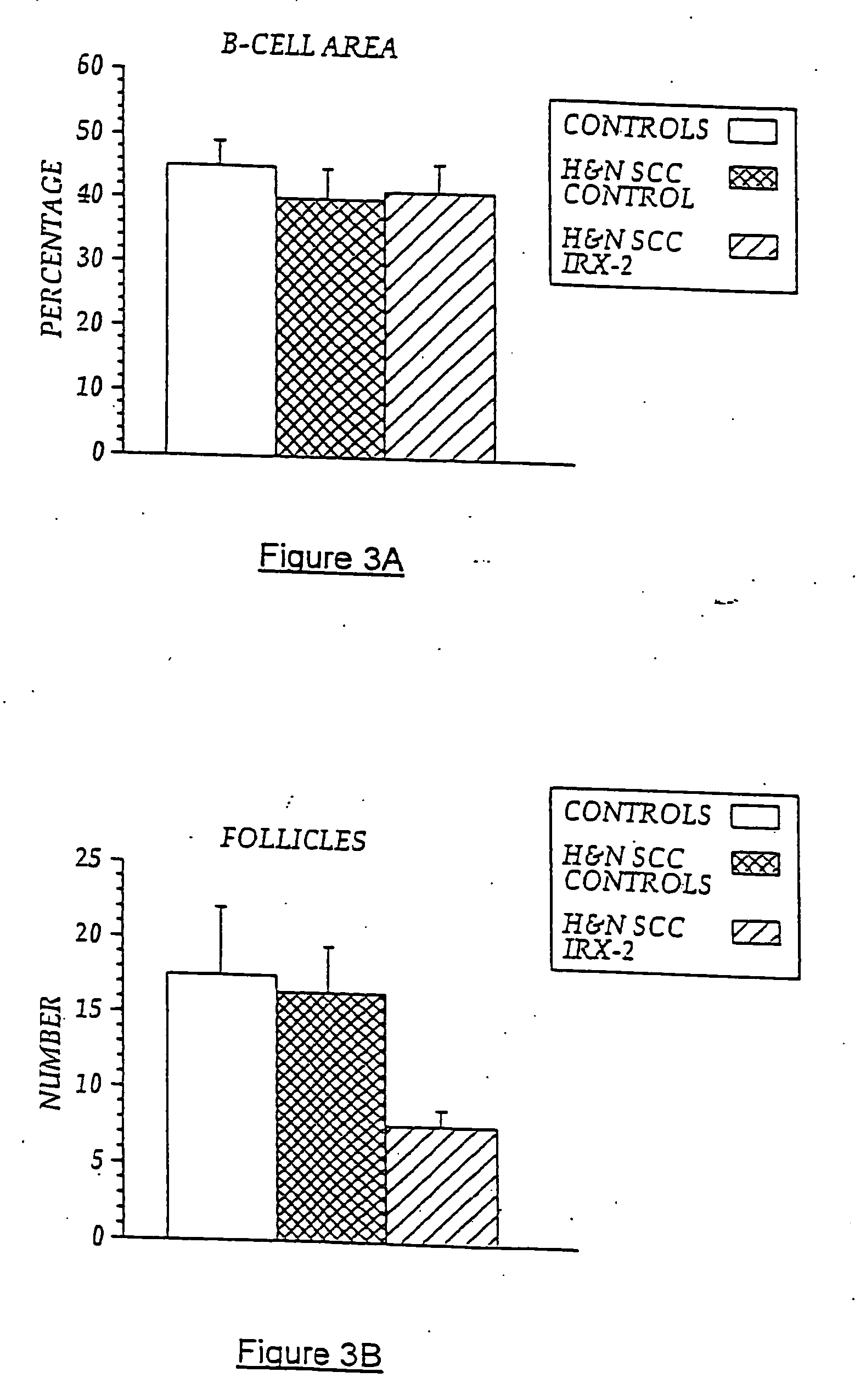
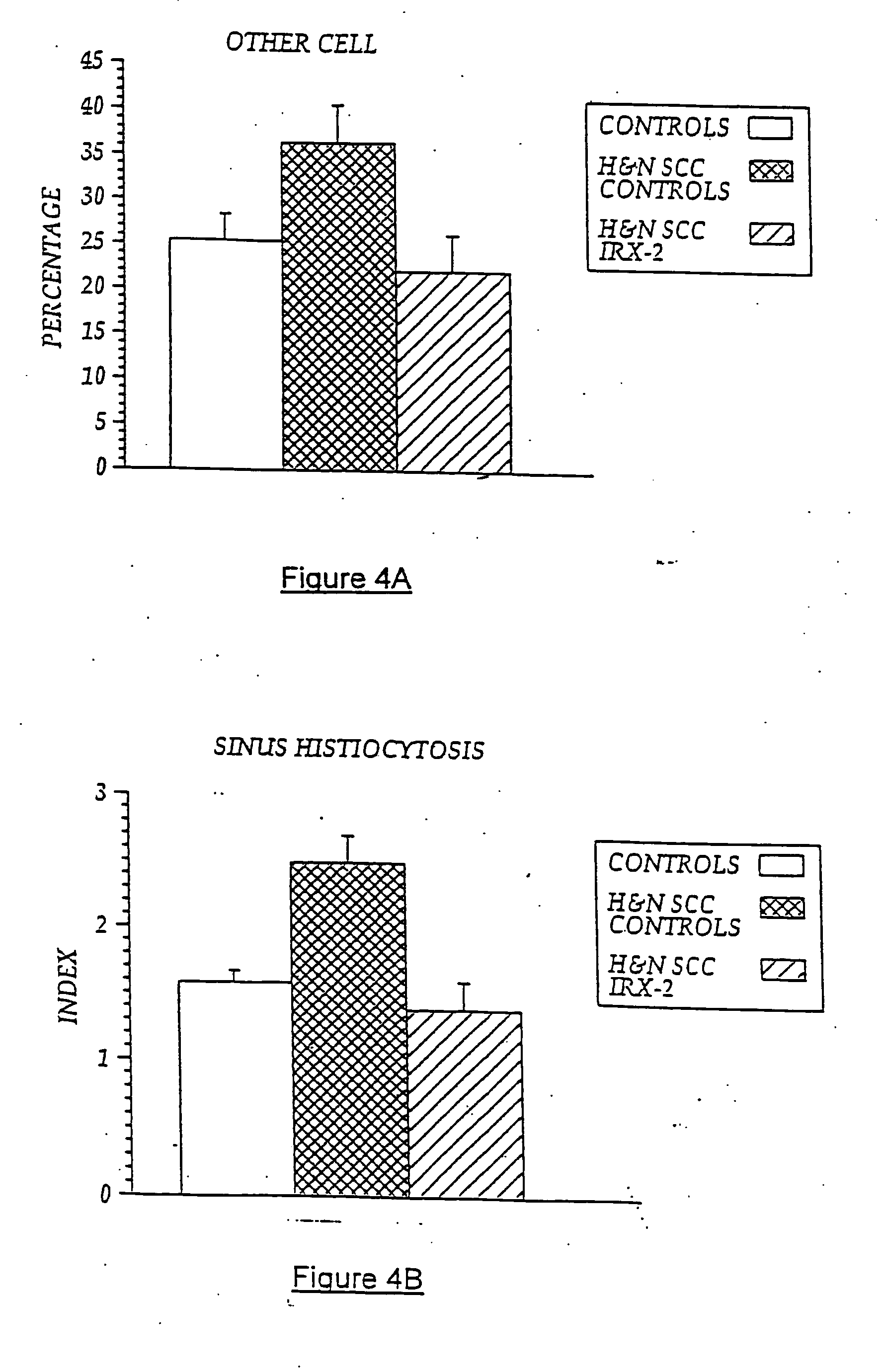
The present invention provides compositions of a natural cytokine mixture (NCM) for treating a cellular immunodeficiency characterized by T lymphocytopenia, one or more dendritic cell functional defects such as those associated with lymph node sinus histiocytosis, and / or one or more monocyte functional defects such as those associated with a negative skin test to NCM. The invention includes methods of treating these cellular immunodeficiences using the NCM of the invention. The compositions and methods are useful in the treatment of diseases associated with cellular immunodeficiencies such as cancer. Also provided are compositions and methods for reversing tumor-induced immune suppression comprising a chemical inhibitor and a non-steroidal anti-inflammatory drug (NSAID). The invention also provides a diagnostic skin test comprising NCM for predicting treatment outcome in cancer patients.
Owner:IRX THERAPEUTICS
Mononuclear phagocytes and their use to promote axonal regeneration
InactiveUS6267955B1Increase capacityEnhance phagocytic activityBiocideNervous disorderPhagocyteDisease
Methods and compositions are disclosed for the use of allogeneic mononuclear phagocytes to promote axonal regeneration in the central nervous system of a mammal. In one embodiment, allogeneic mononuclear phagocytes are cultured together with stimulatory tissue, such as skin, dermis or at least one nerve segment, and are subsequently administered into the central nervous system of a mammal at or near a site of injury or disease. In an alternative embodiment, autologous monocytes, preferably stimulated autologous monocytes, are administered into the central nervous system of a mammal at or near a site of injury or disease. CNS administration of mononuclear phagocytes may optionally be combined with administration of an adjuvant factor (e.g. aFGF) to the CNS, anti-inflammatory therapy of the mammal, or both. Methods for screening stimulatory tissue and cells and methods and compositions for cryopreserved allogeneic mononuclear phagocytes are also disclosed.
Owner:YEDA RES & DEV CO LTD
Nucleic acid encoding novel type-1 cytokine receptor GLM-R
Owner:GENENTECH INC
Cancer-associated antigen analogue peptides and uses thereof
InactiveUS20110229481A1Improve long-term prognosisPrevent recurrenceFungiBacteriaEpitopeActivation cells
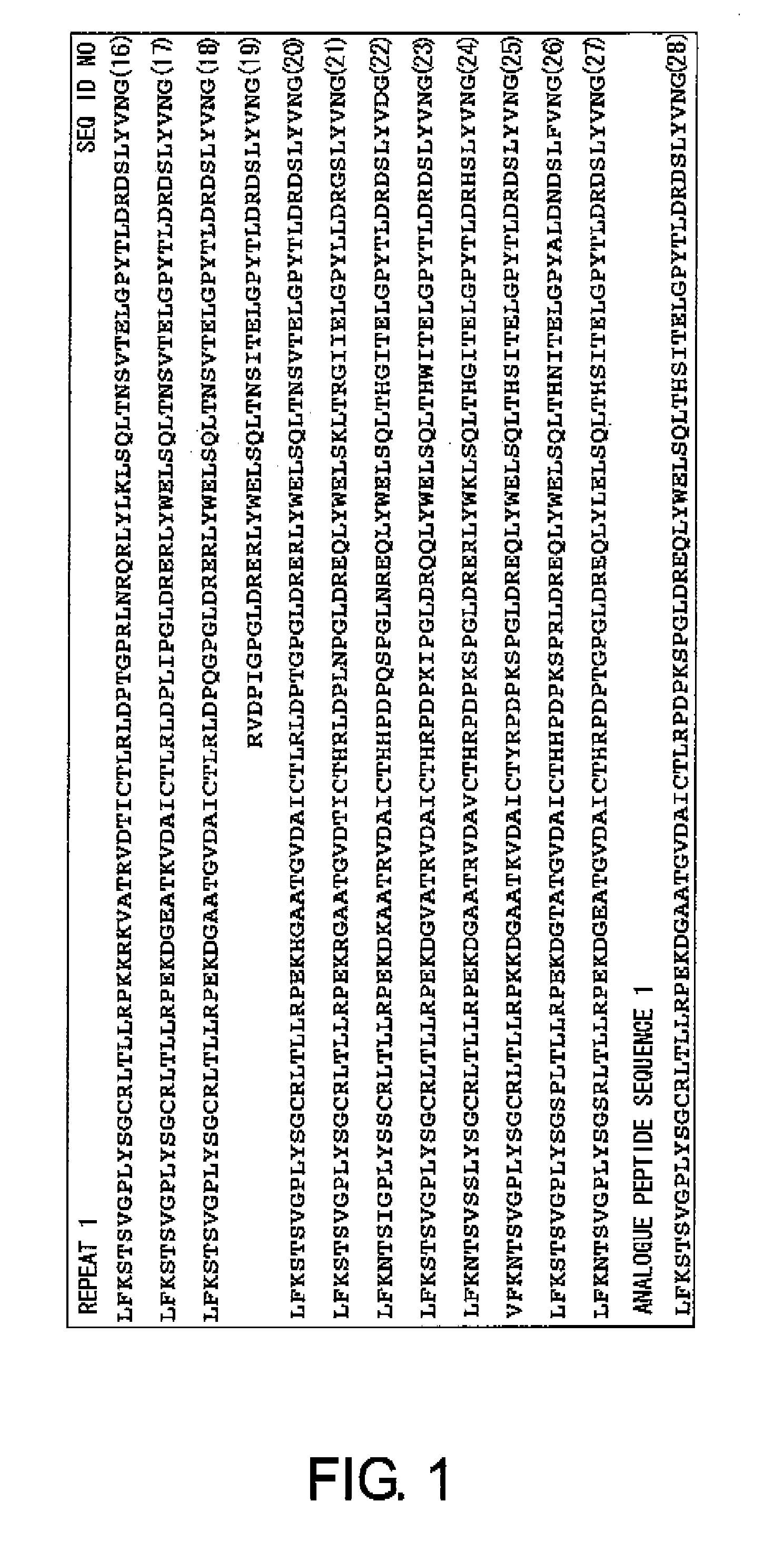
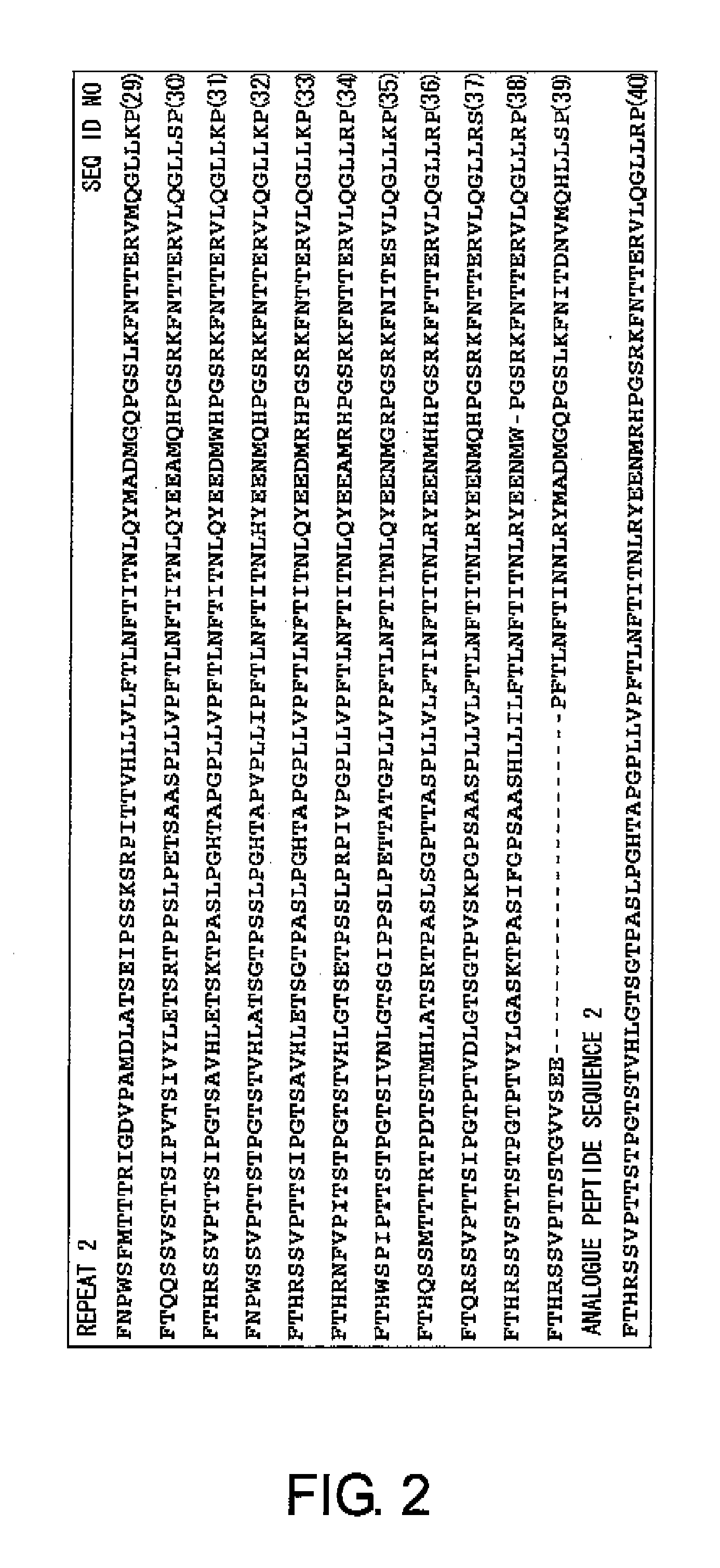
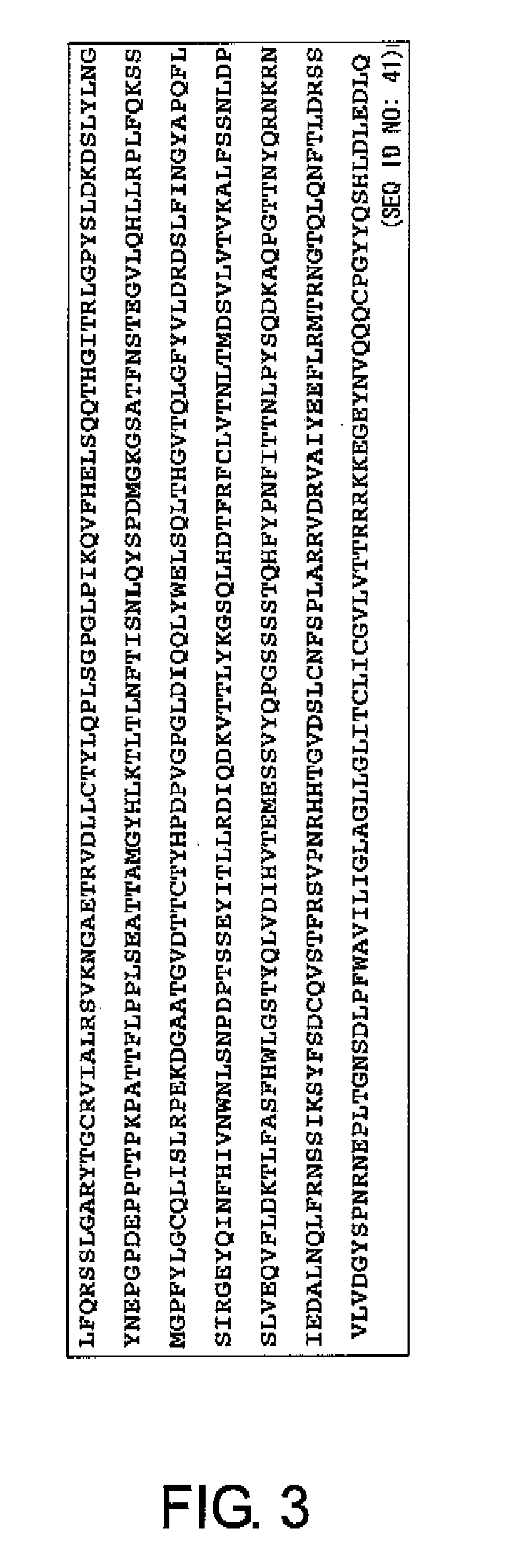
First, to solve the problems of the present invention, the present inventors confirmed the presence of ovarian cancer antigen-specific T cells in the peripheral blood of ovarian cancer patients by using an experimental system that detects combinations of CD4 and IL-4 or IFNγ. Next, using analogue peptides of the core protein MUC16 of the ovarian cancer-associated antibody C125, the present inventors revealed that antigen-specific CD4-positive T cells are present at an average frequency of about 4% in the peripheral mononuclear cells of patients and healthy subjects. Then, the present inventors analyzed the epitope for T cells in the core protein MUC16 of the ovarian cancer-associated antigen CA125, and determined the amino acid sequence FTLNFTITN (SEQ ID NO: 1) to be a shorter epitope. The present inventors further discovered that the analogue peptide OVCA11: GHTAPOPLLVPFTLNFTITN (SEQ ID NO: 11) is suitable for T cell activation.
Owner:CHUGAI PHARMA CO LTD
Growth of neural precursor cells using umbilical cord blood serum and a process for the preparation thereof for therapeutic purposes
InactiveUS20040203142A1Promote growthArtificial cell constructsBlood/immune system cellsCord blood stem cellCell culture media
This invention is concerned with stem cells derived from umbilical cord blood serum and a method for growing human embryonic stem cells and adult cells comprising sera separated from clotted umbilical cord blood, including growing and differentiating cord blood stem cells into neural precursors, comprising transdifferentiating CD34+ stem cells from mononuclear cells derived from umbilical cord blood to neural precursors. The stem cells obtained from the umbilical cord include pluripotent stem and progenitor cell population of mononuclear cells, and separating pluripotent stem and progenitor cell population of mononuclear cells obtained from the umbilical cord blood. A magnetic cell separator is used to separate out cells which contain a CD marker and then expanding the cells in a growth medium containing retinoic acid and one or more growth factors BDNF, GDNF, NGF and FGF as a differentiating agent. The invention is also concerned with the transplantation and repair of nerve damage, stokes, spinal injury, Parkinson's and Alzheimer's, prepared in accordance with the aforesaid method and a media for culturing umbilical cord blood stem calls consisting essentially of cord blood stem cells derived from umbilical cord blood serum.
Owner:RELIANCE LIFE SCI PYT
Absorbent and Column for Extracorporeal Circulation
InactiveUS20090275874A1Prolong lifeImprove the quality of lifeSemi-permeable membranesHaemofiltrationExtracorporeal circulationZeta potential
The present invention provides an absorbent which can remove cells present in blood including activated leukocytes such as granulocytes and monocytes, and cancer cells as well as can remove cytokines which facilitate the activation of the remaining cells, and further has no concern for pressure loss and has high configuration stability. That is, the present invention provides an absorbent which absorbs the granulocytes and the monocytes in blood, an absorbent for cancer therapy which absorbs an immunosuppressive protein and an absorbent having a bilayer structure of a net and a nonwoven fabric, having a zeta potential of −20 mV or more, as well as a blood circulation column containing any of the absorbents filled therein.
Owner:TORAY IND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com