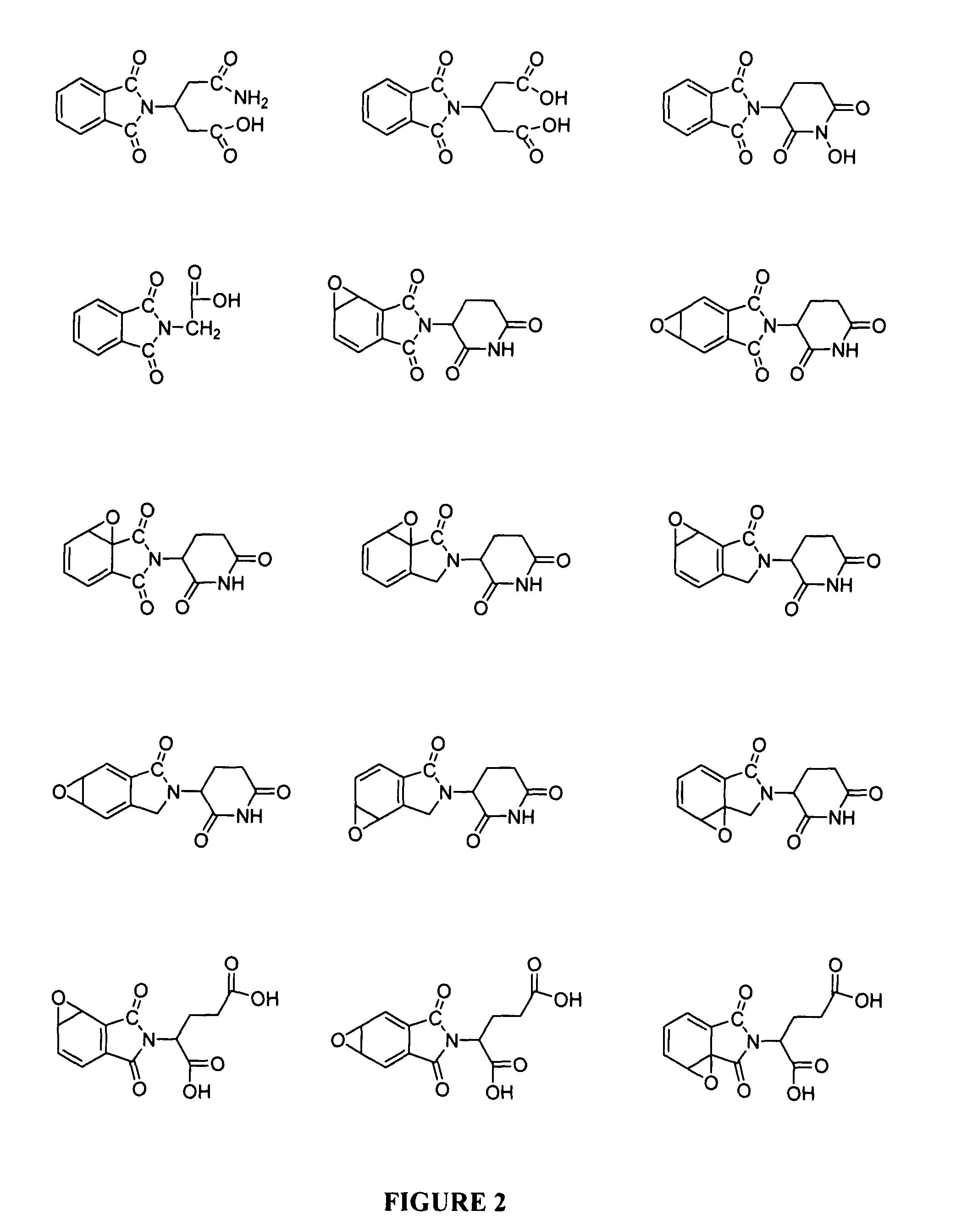
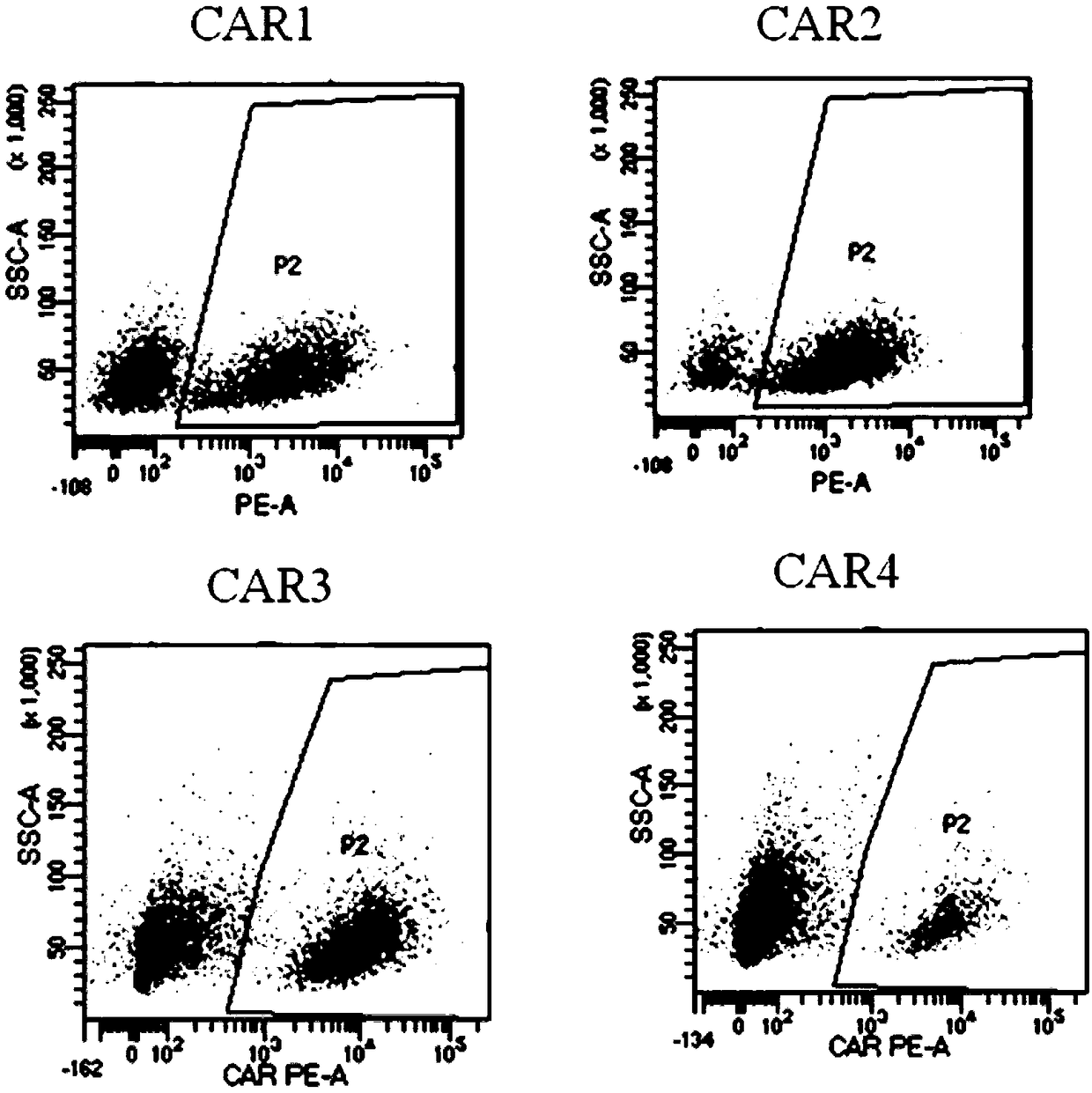
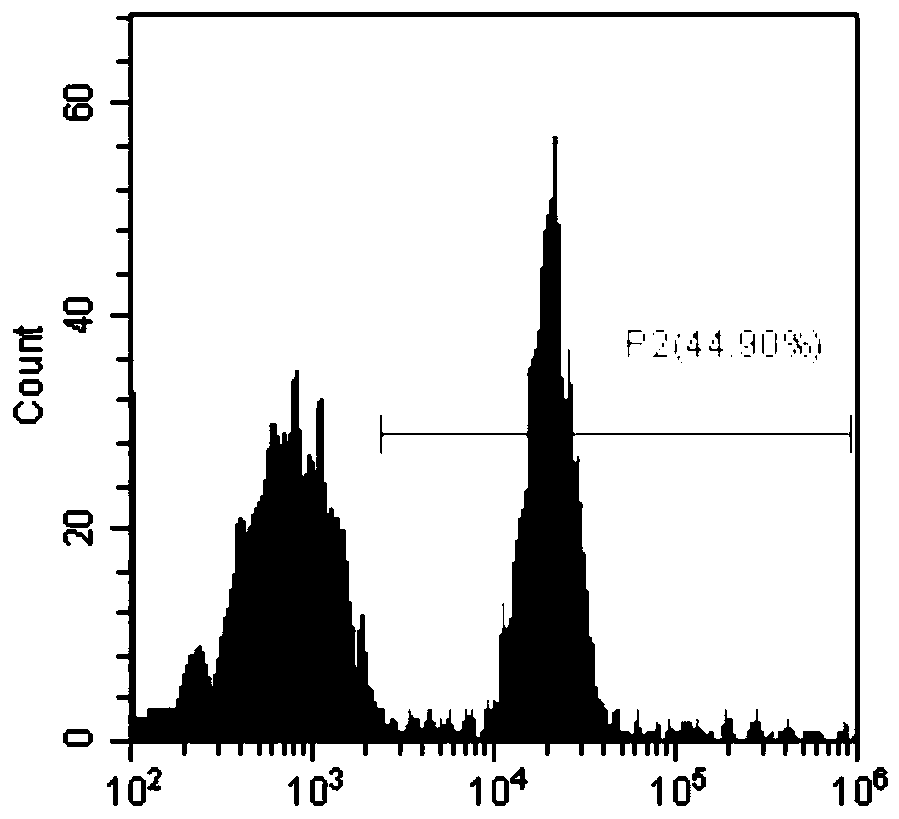
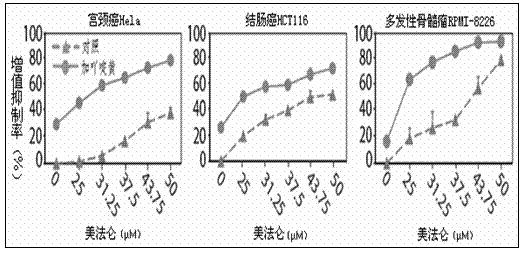
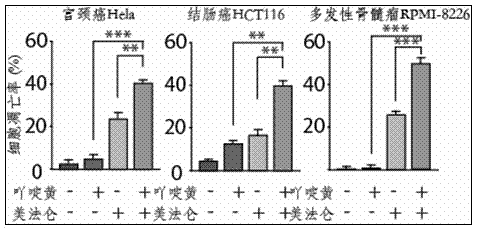
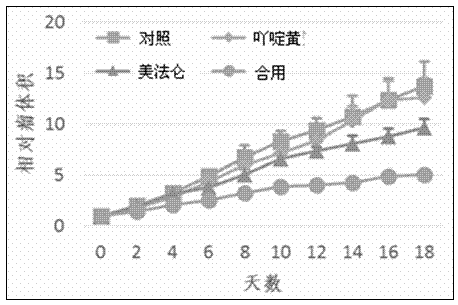
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
129 results about "Blood tumor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cancers, Tumors & Blood. Acute Lymphoblastic Leukemia (ALL) Acute lymphoblastic leukemia (ALL), also called acute lymphocytic leukemia, is a cancer of the white blood cells. In ALL, the bone marrow (the soft, spongy center of bones) produces too many lymphocytes, a type of white blood cells that do not mature as they should.
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS6896885B2Avoiding and decreasing and resistanceIncrease ratingsOrganic active ingredientsIn-vivo radioactive preparationsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies.The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN® (rituximab).
Owner:BIOGEN INC
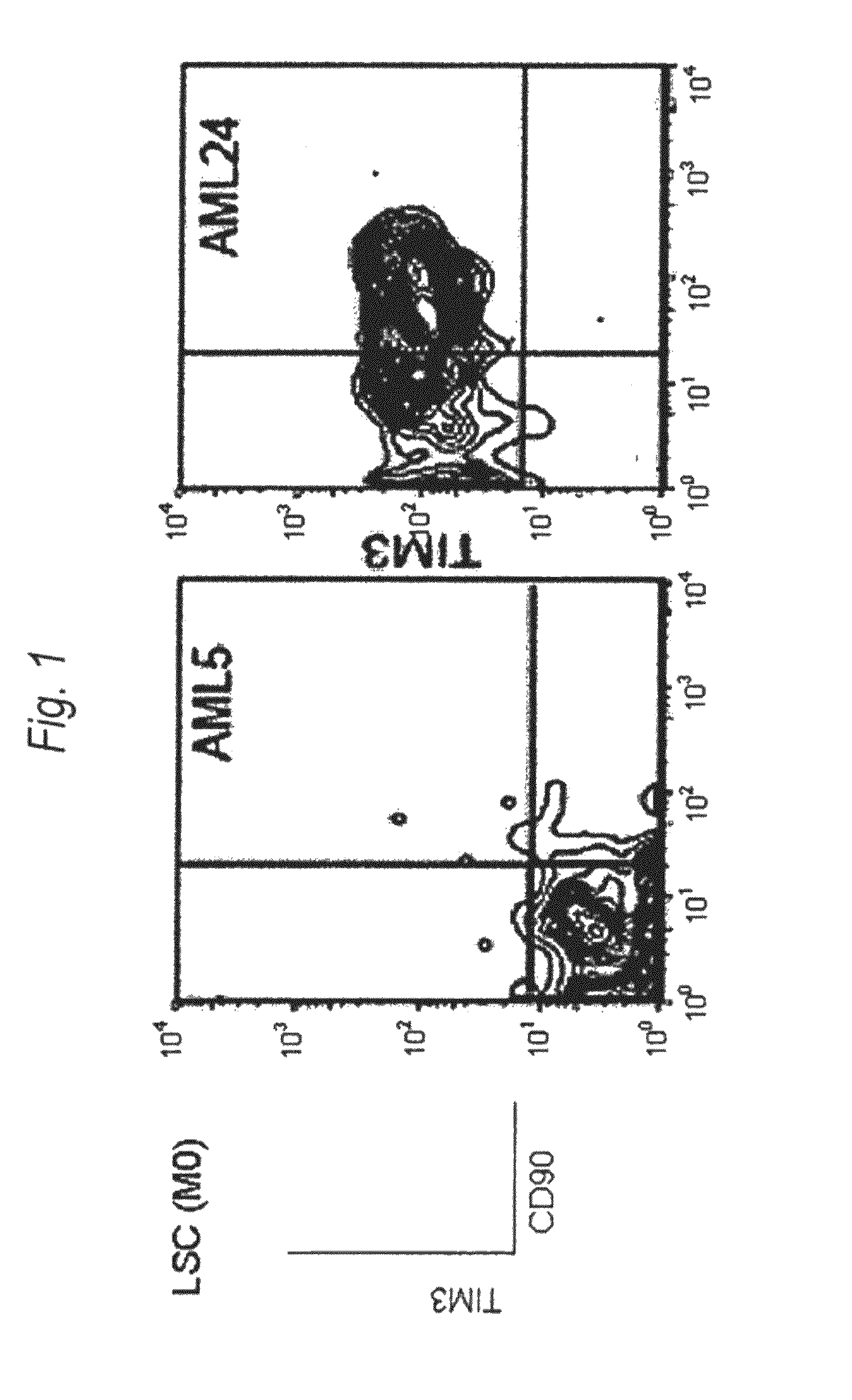
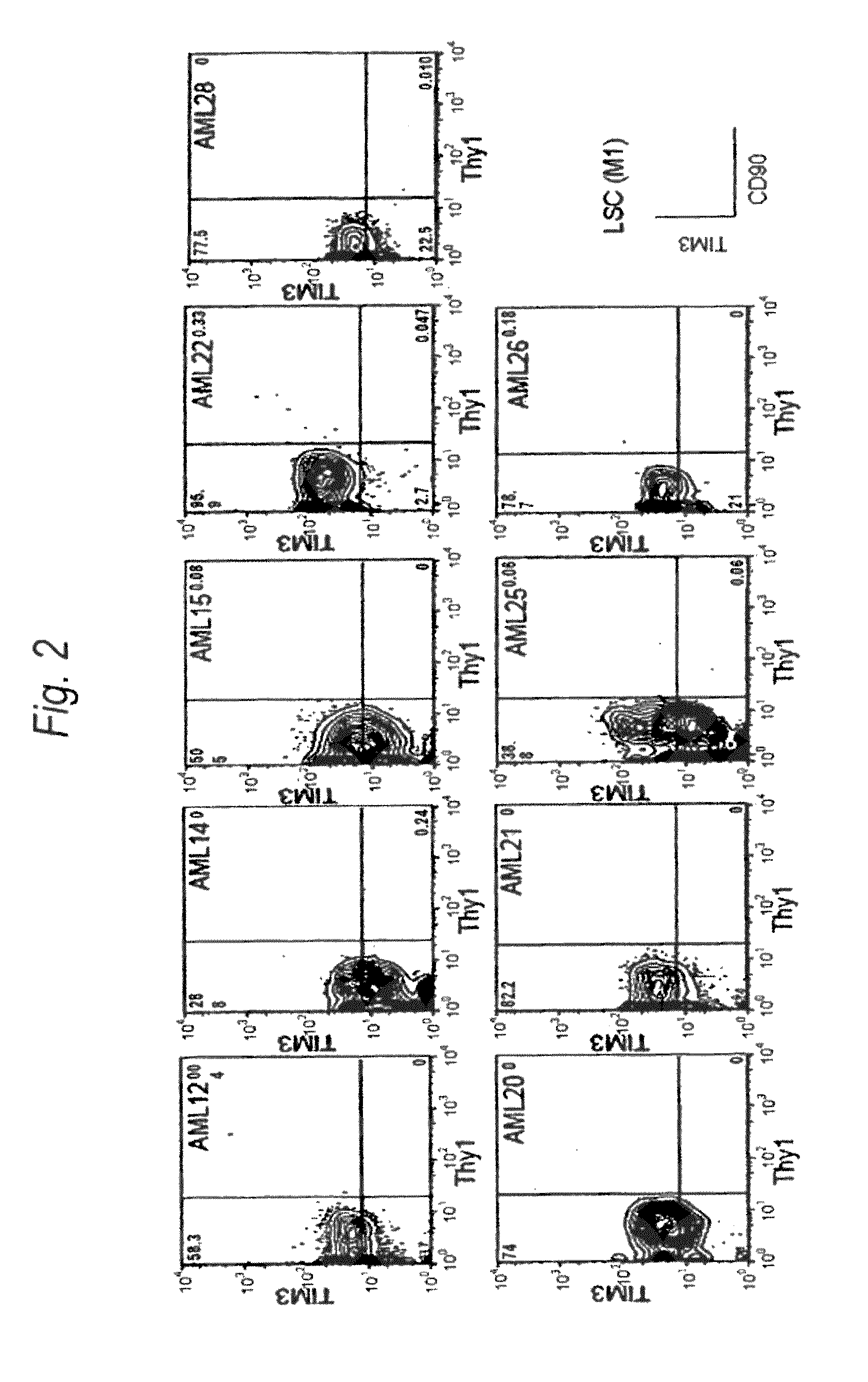
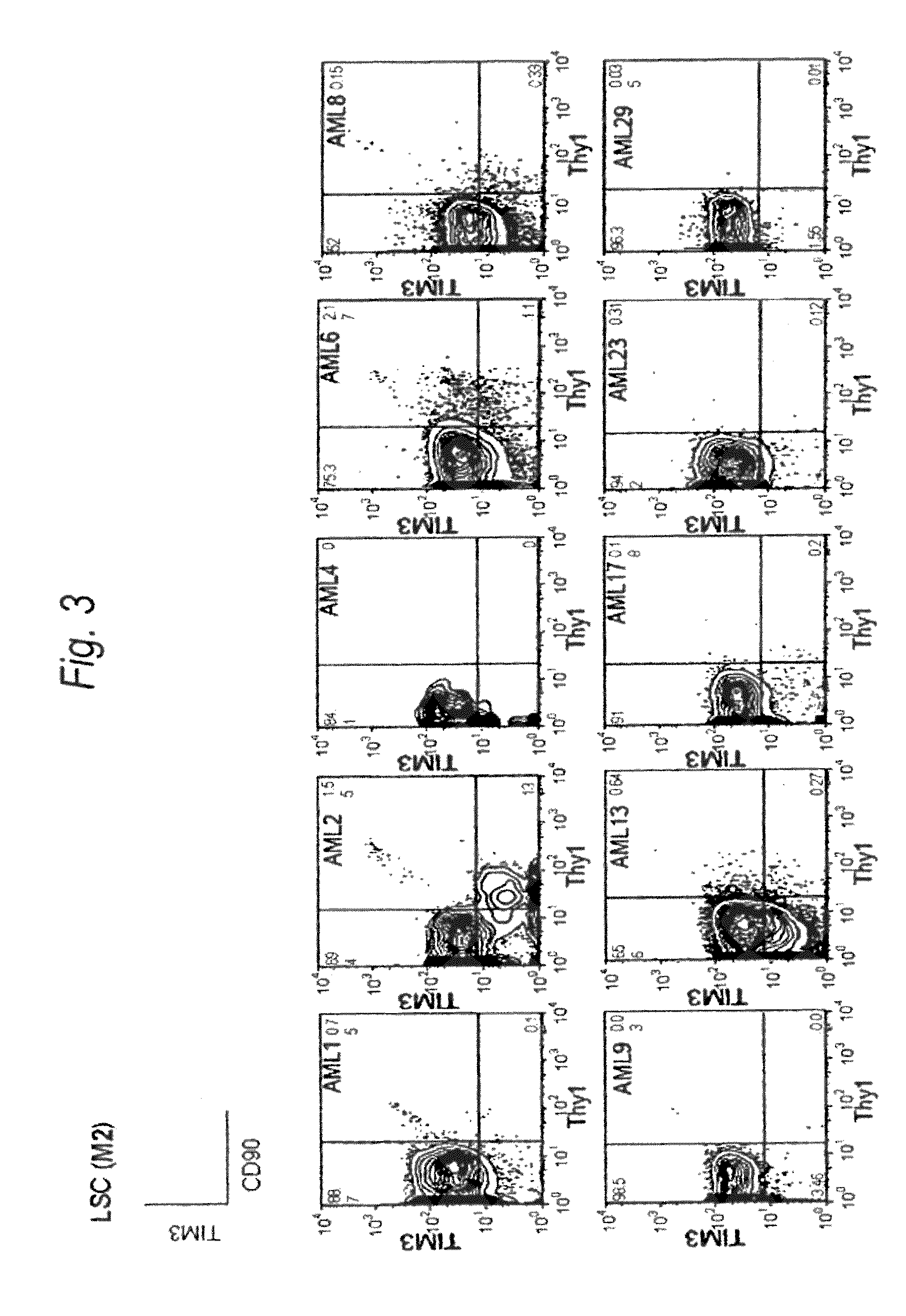
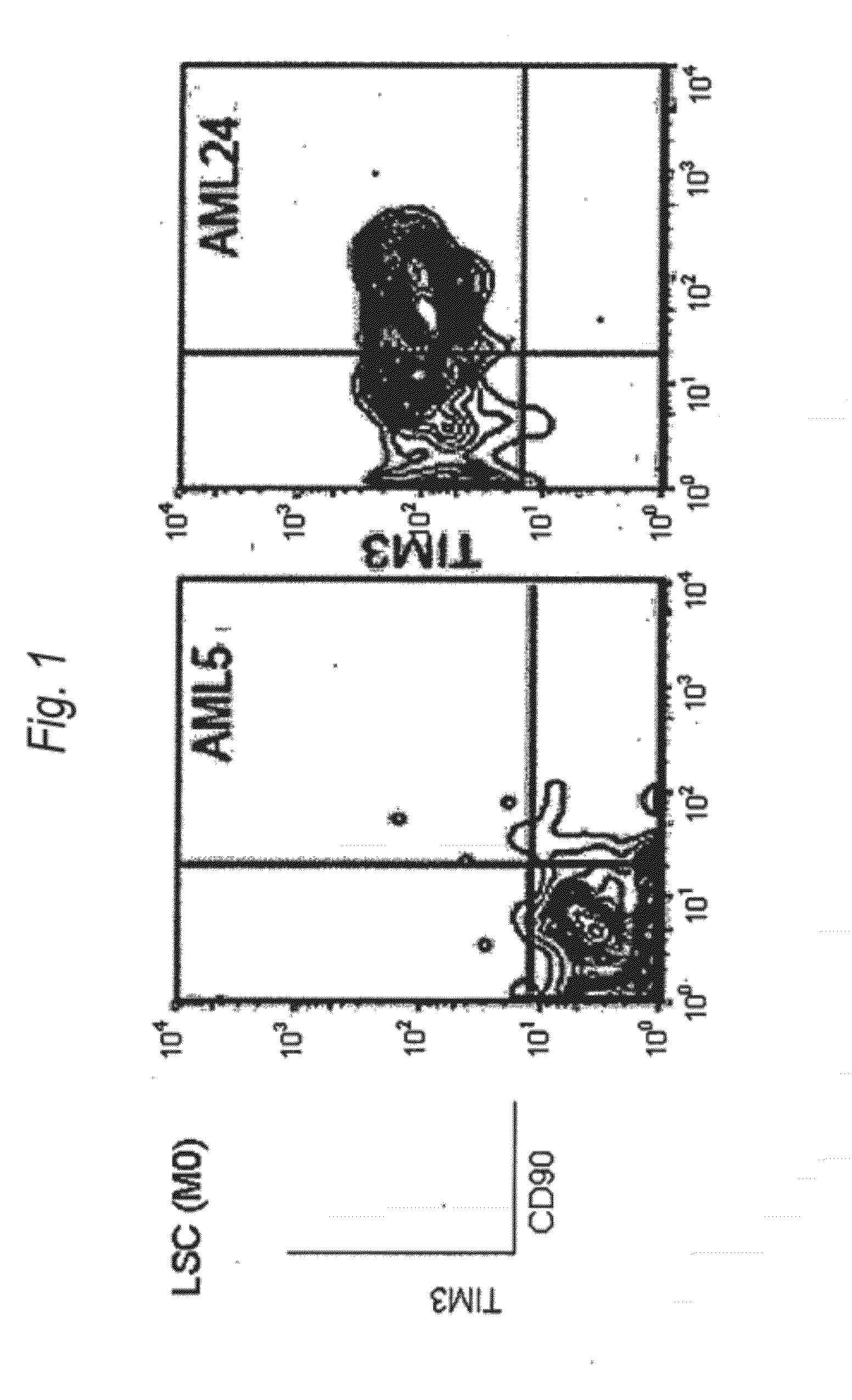
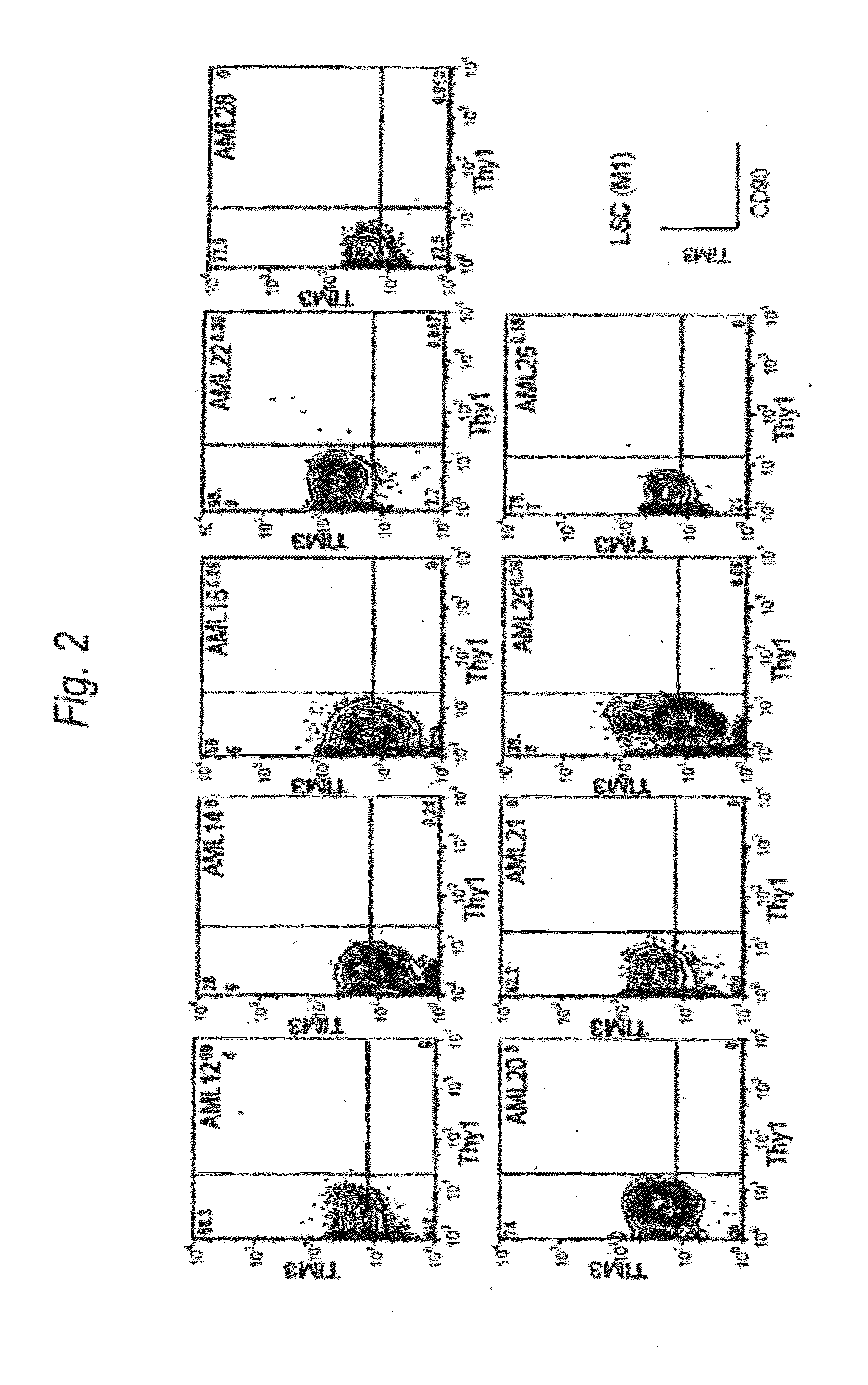
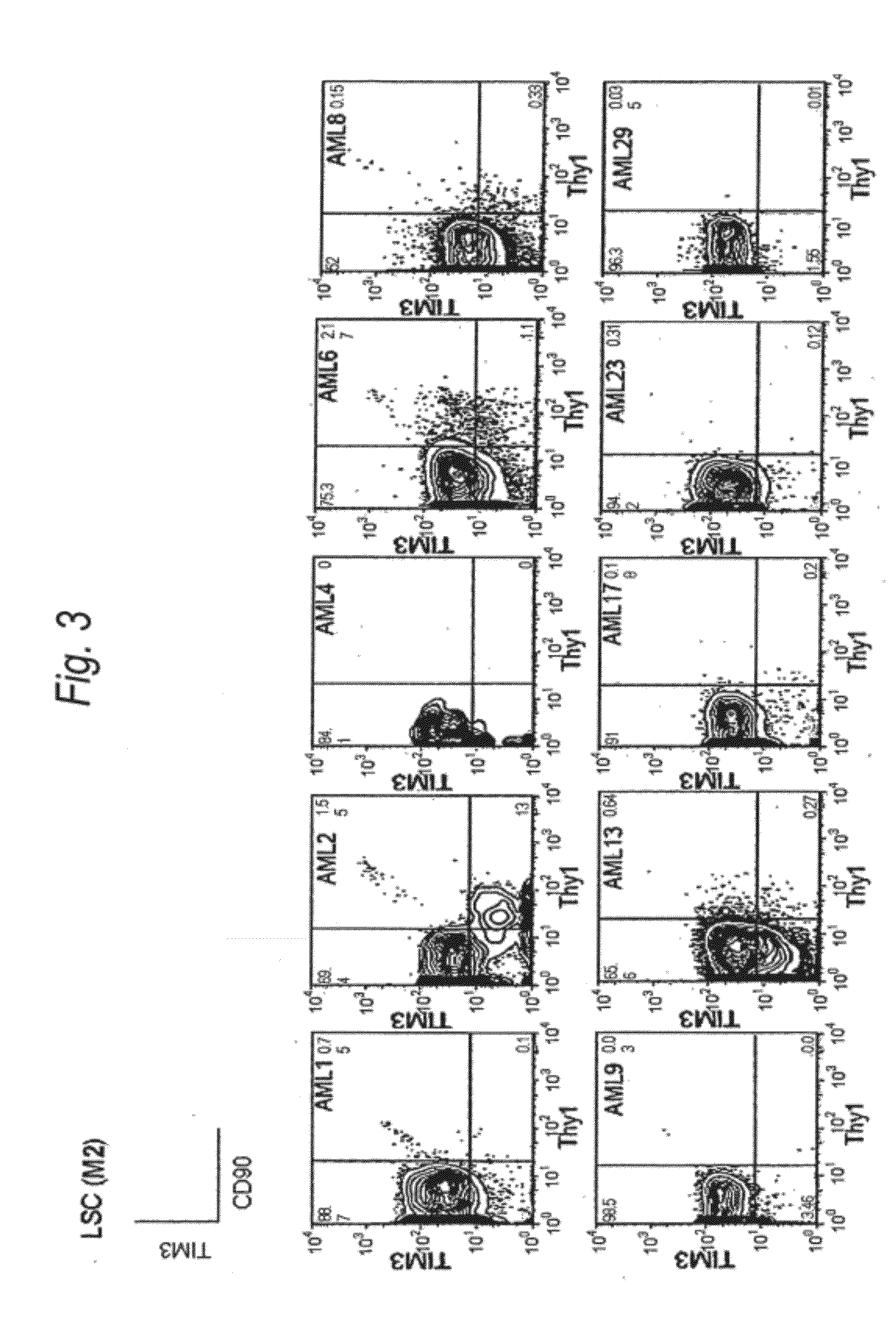
Method for treatment of blood tumor using anti-TIM-3 antibody
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20050180975A1Avoiding and decreasing and resistanceIncrease ratingsBiocidePeptide/protein ingredientsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN®.
Owner:BIOGEN INC
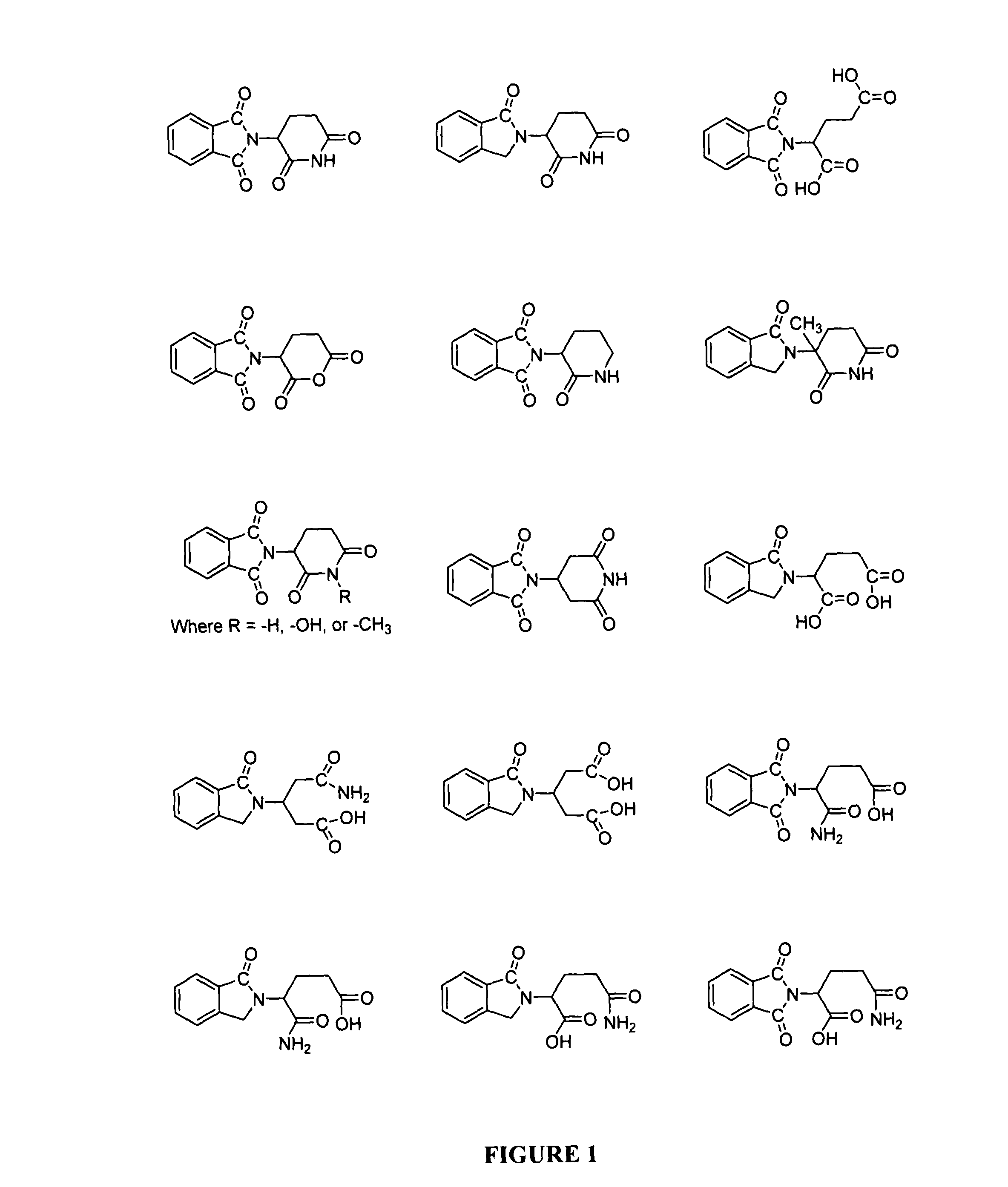
Methods for treating blood-born tumors with thalidomide
The present invention comprises a group of compounds that effectively inhibit angiogenesis. More specifically, thalidomide and various related compounds such as thalidomide precursors, analogs, metabolites and hydrolysis products have been shown to inhibit angiogenesis and to treat disease states resulting from angiogenesis. Importantly, these compounds can be administered orally.
Owner:CHILDRENS MEDICAL CENT CORP
Method for treatment of blood tumor using Anti-tim-3 antibody
ActiveUS20120100131A1Use to establishBiological material analysisAntibody ingredientsDiseaseCell Fraction
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
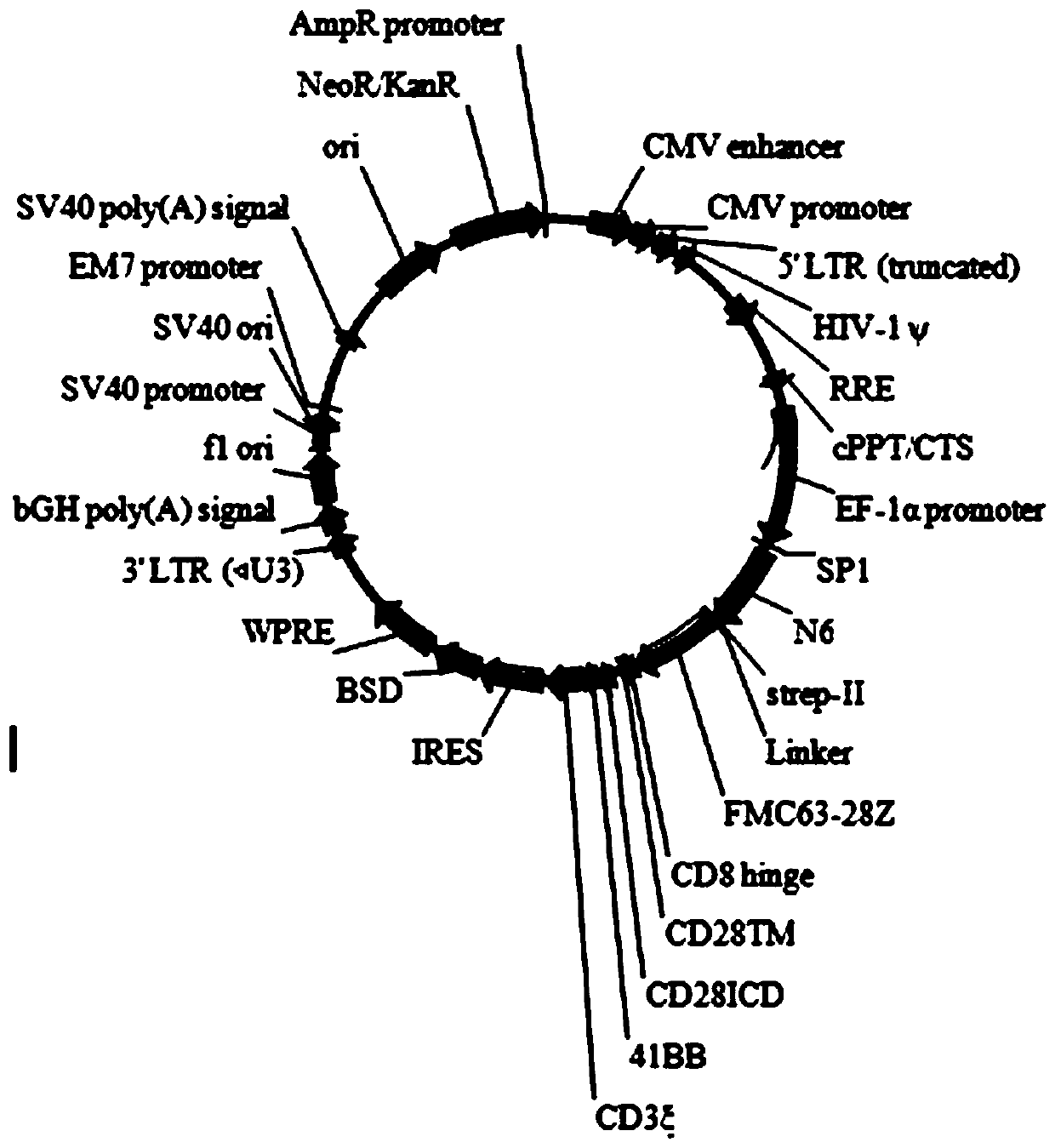
Chimeric antigen acceptor of target BCMA (B cell maturation antigen) and application of chimeric antigen acceptor
ActiveCN110041433ASmall molecular weightHighly effective specific therapyMammal material medical ingredientsImmunoglobulinsBCMA ProteinB-Cell Maturation Antigen
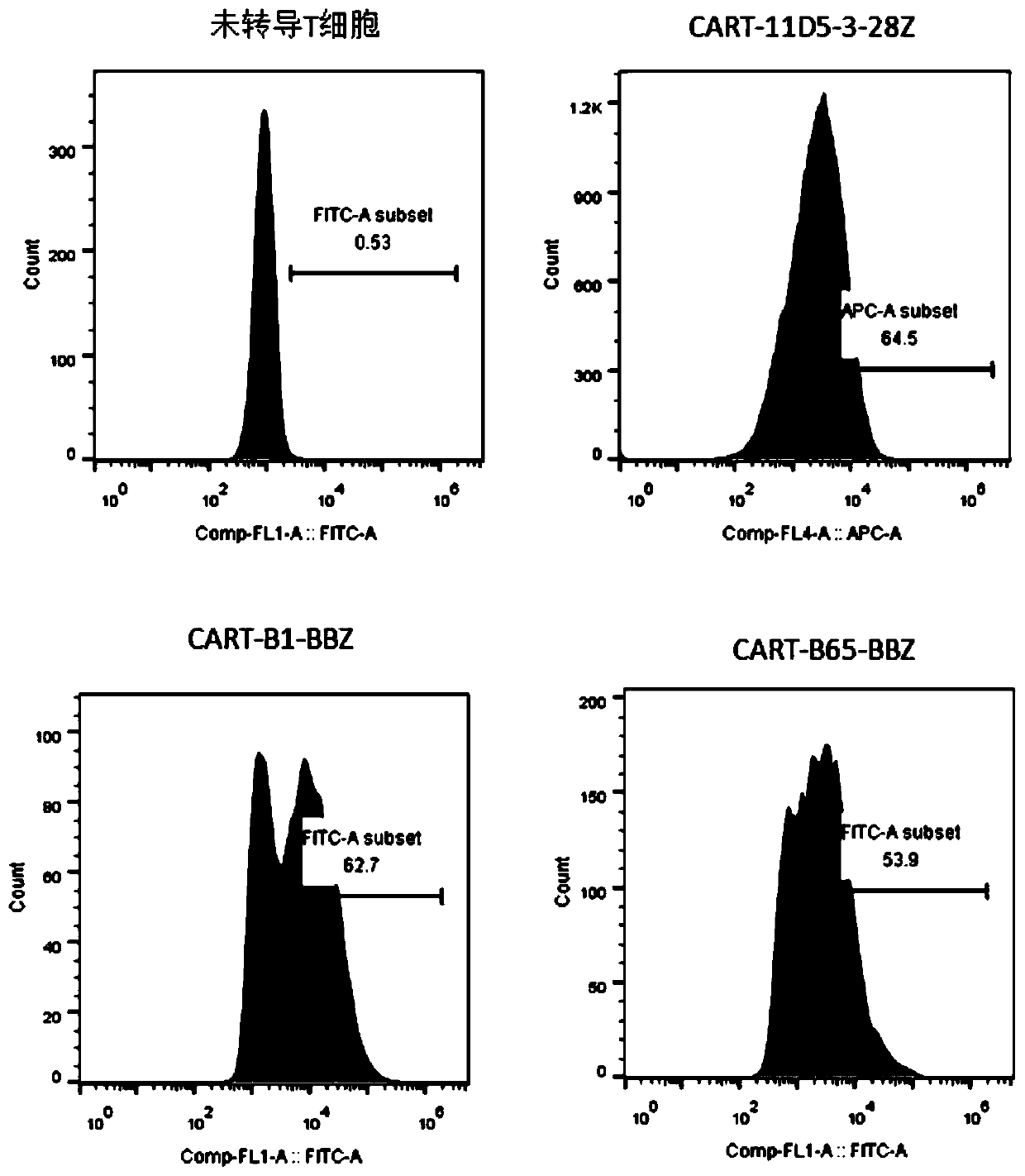
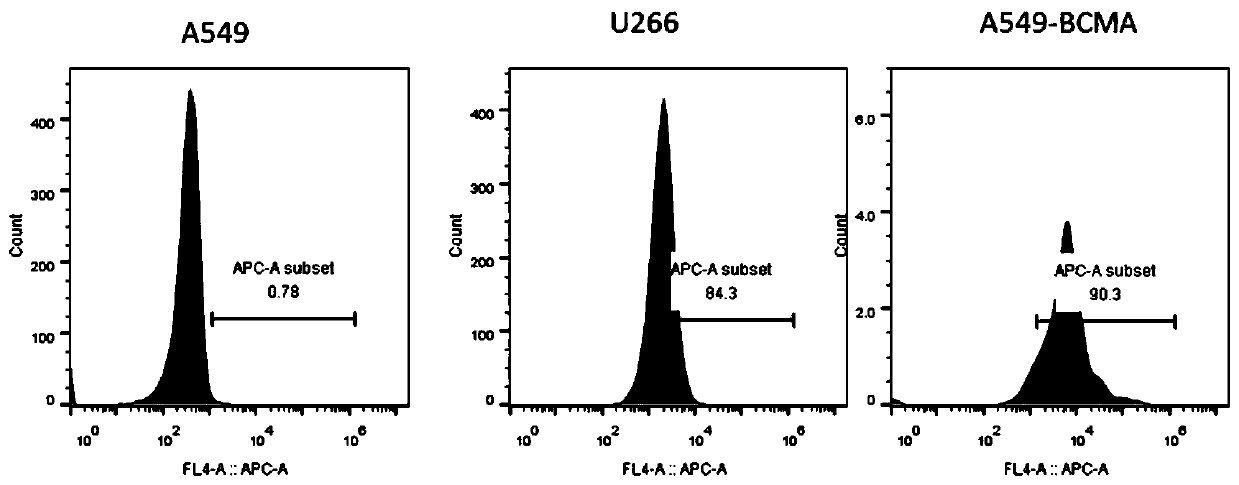
The invention relates to a chimeric antigen acceptor of a target BCMA (B cell maturation antigen). The chimeric antigen acceptor comprises an extracellular identification region, a hinge region, a transmembrane region and an intracellular signal region, wherein the extracellular identification region has a BCMA resisting nanometer antibody sequence, and the BCMA resisting nanometer antibody sequence is a heavy-chain variable region sequence combining with BCMA and from alpacas. More specifically, the BCMA resisting nanometer antibody sequence is BCMA monoclonal antibody B1 or B65, the amino acid sequence of the B1 is as shown in SEQID NO.1, and the amino acid sequence of the B65 is as shown in SEQID NO.2. According to the chimeric antigen acceptor disclosed by the invention, compared witha traditional mouse-derived SCFV or humanized SCFV, the nanometer antibody from the alpacas is used, and has small molecule quantity and immunogenicity, the possibility that CAR-T cells prepared on the base of the nanometer antibody produce HAMA effects in vivo is smaller, the remaining time of the CAR-T cells in vivo can be longer, the CAR-T cells pass through BCMA protein on the surfaces of target tumor cells and activate signal channels at the downstream part of T cells, the capacity for killing tumor cells having BCMA target points is given to the T cells, and BCMA positive blood tumor canbe efficiently and specifically treated.
Owner:上海科棋药业科技有限公司
ROR1 specific chimeric antigen receptor and application thereof
ActiveCN105924533AConfirmed specific killing effectDoes not affect the functionAntibody mimetics/scaffoldsMammal material medical ingredientsAntigen receptorHinge region
The invention discloses an ROR1 specific chimeric antigen receptor and an application thereof. The ROR1 specific chimeric antigen receptor is a protein formed through serially connecting scFv of anti-human ROR1, the hinge region and the transmembrane region of CD8alpha, the transmembrane region and the intracellular region of CD28 and the intracellular region of CD3zeta from the amino end to the carboxyl end. T cells modified with the chimeric antigen receptor have a specific killing effect on tumor cells, and are expected to be in widely used in treatment of all kinds of solid tumor and blood tumors.
Owner:BEIJING BIOHEALTHCARE BIOTECH
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
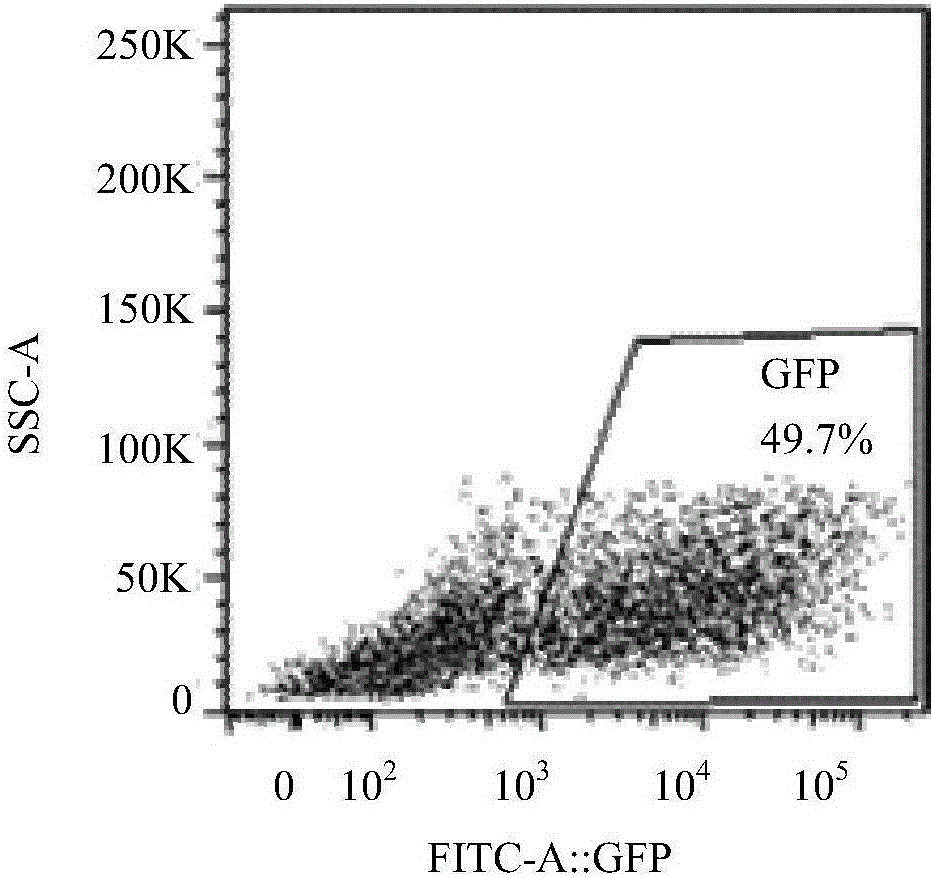
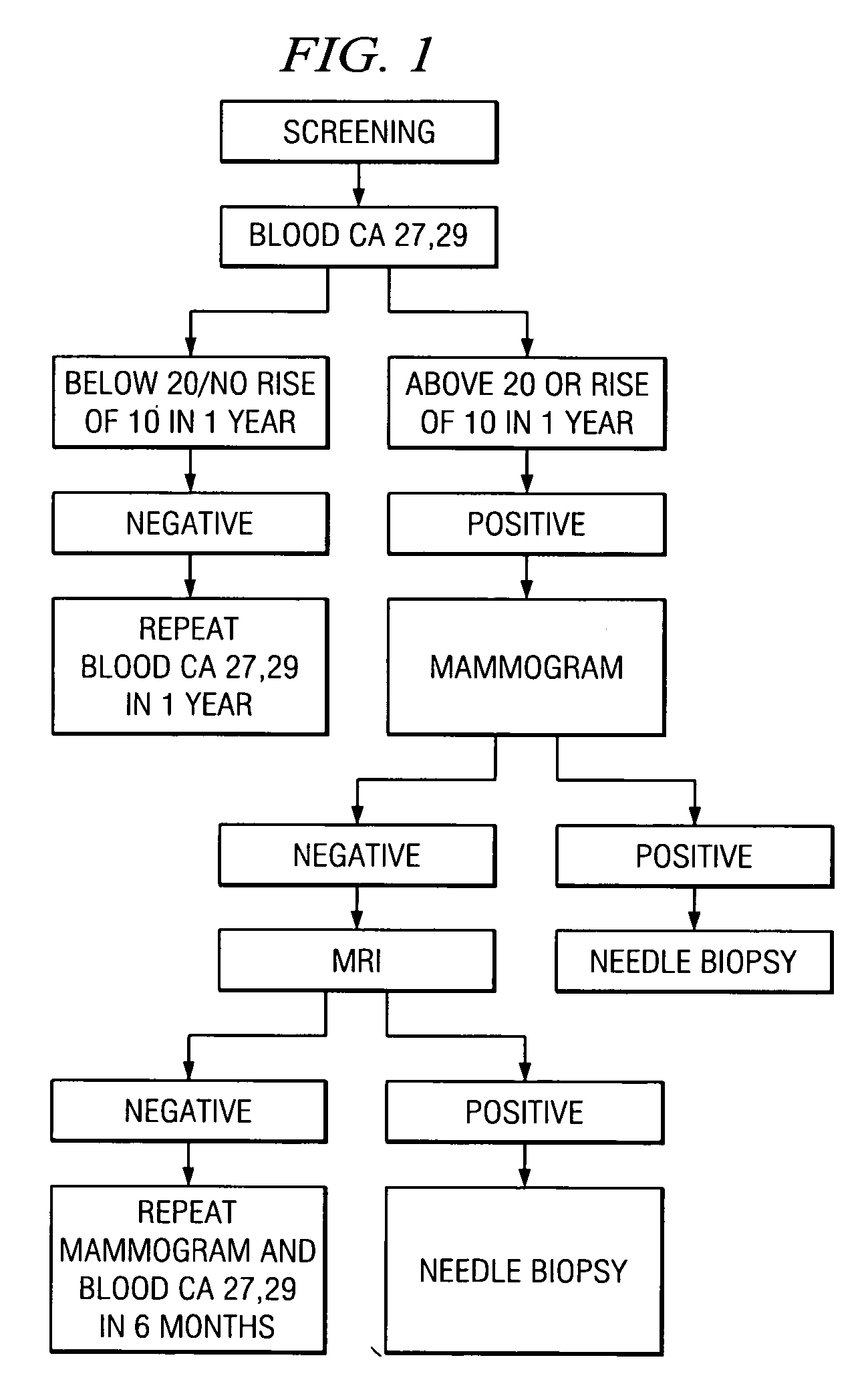
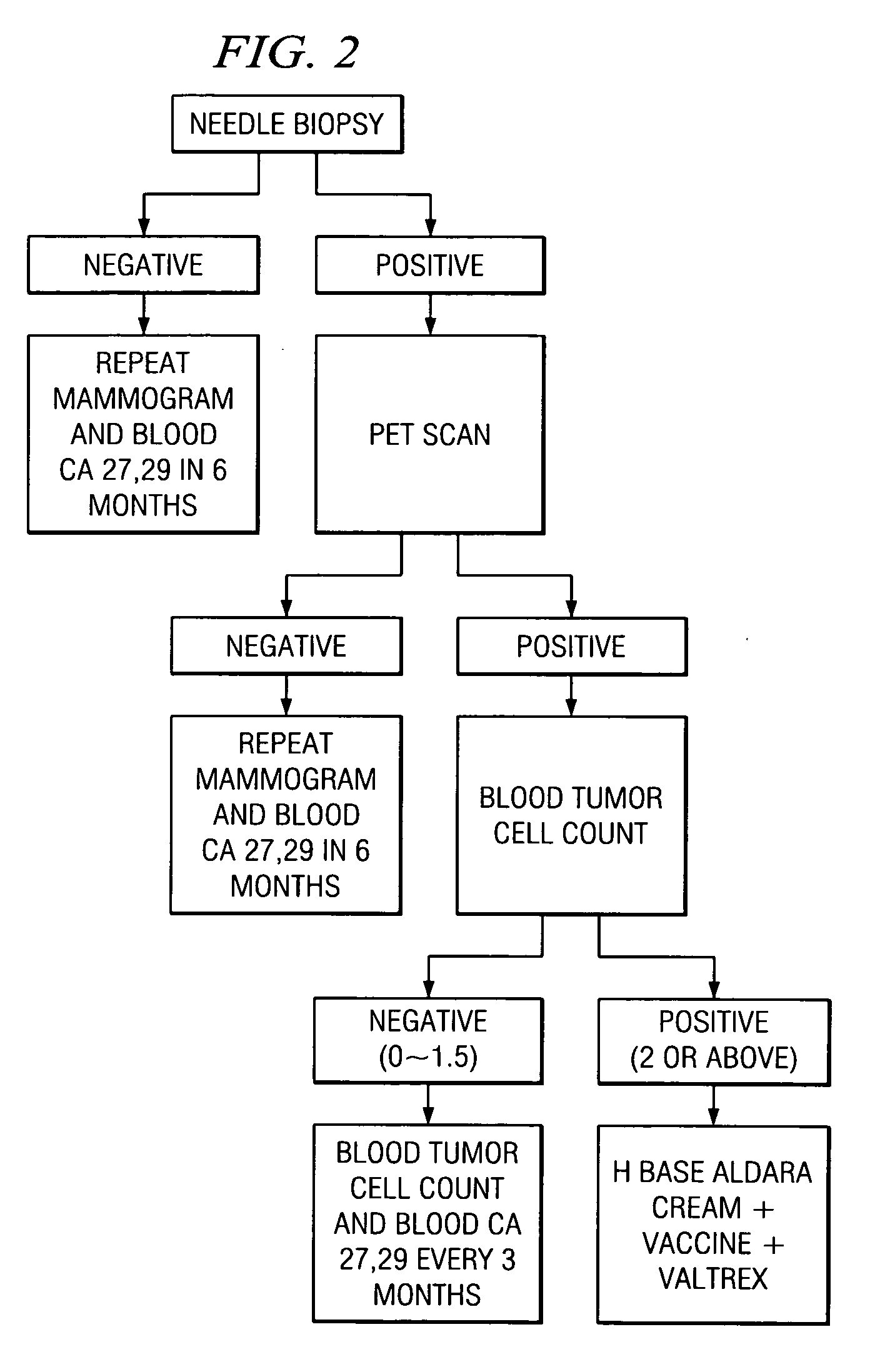
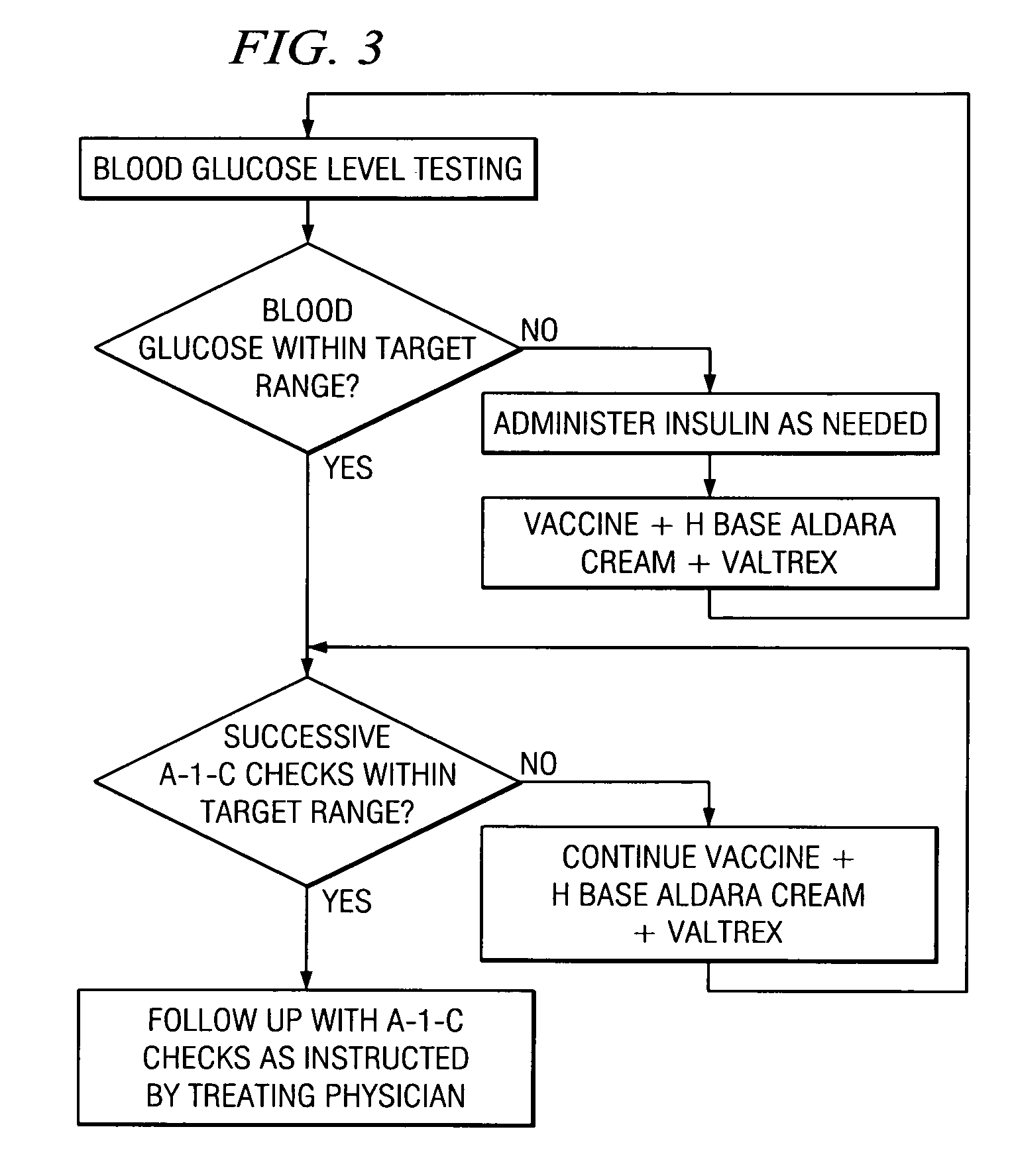
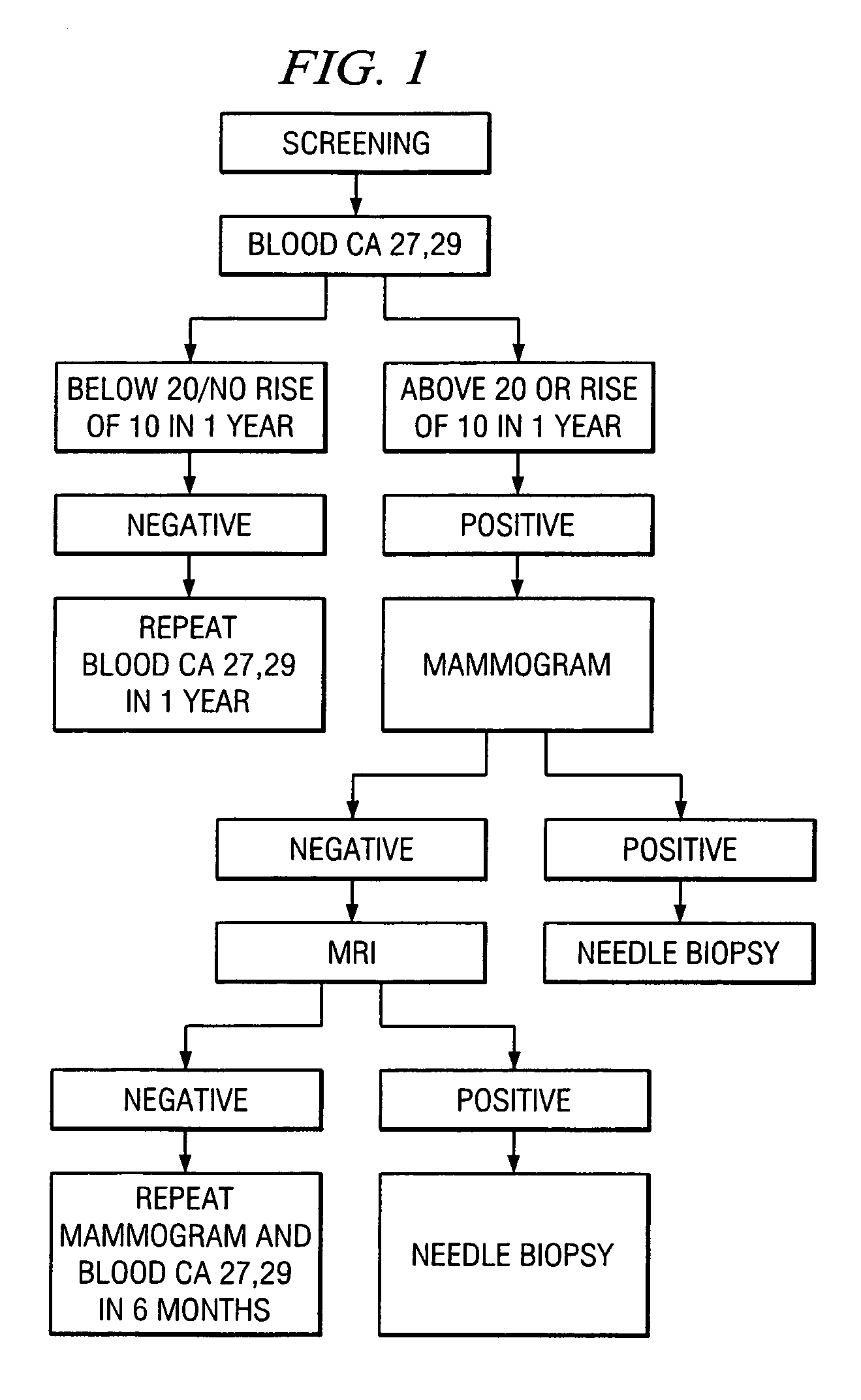
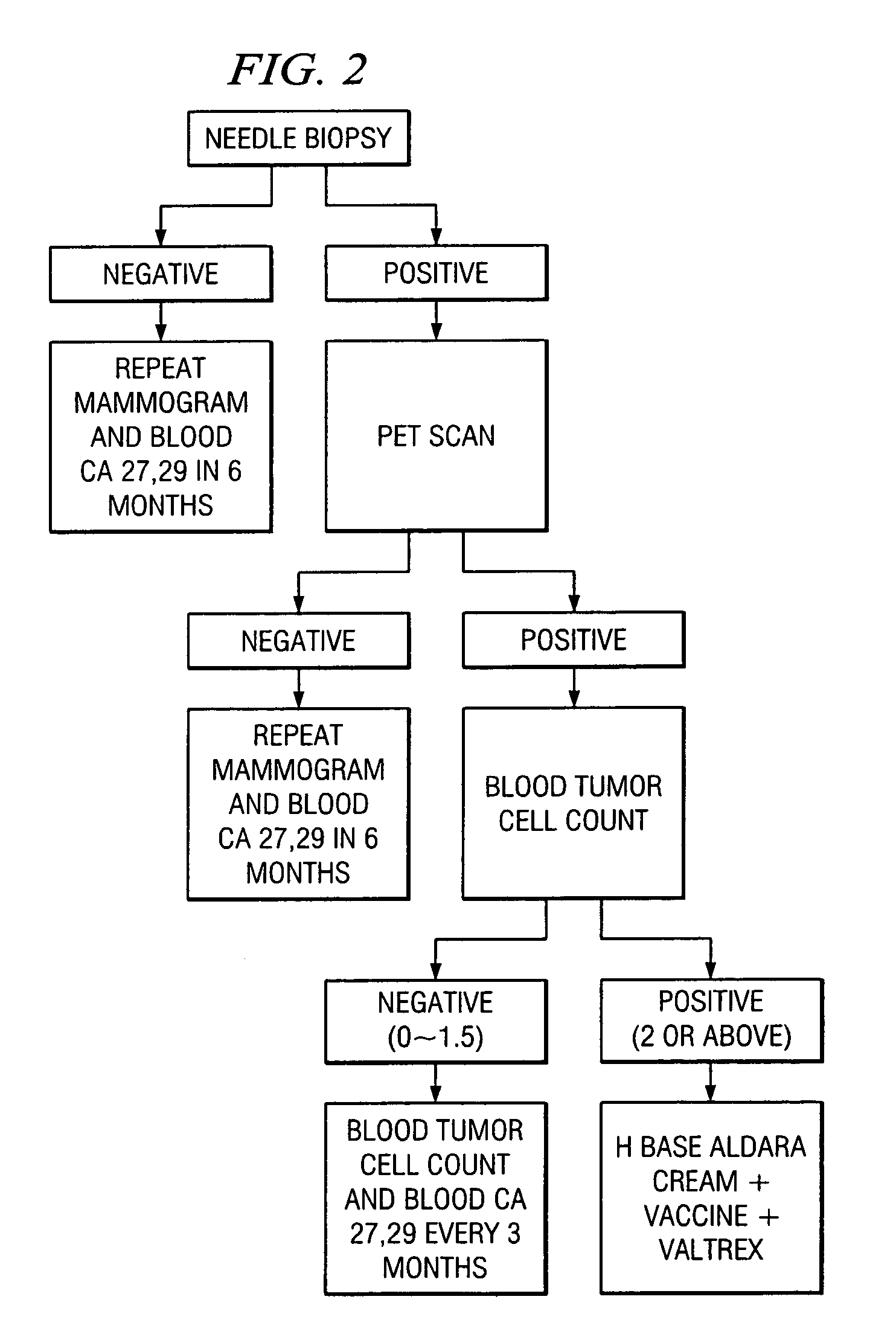
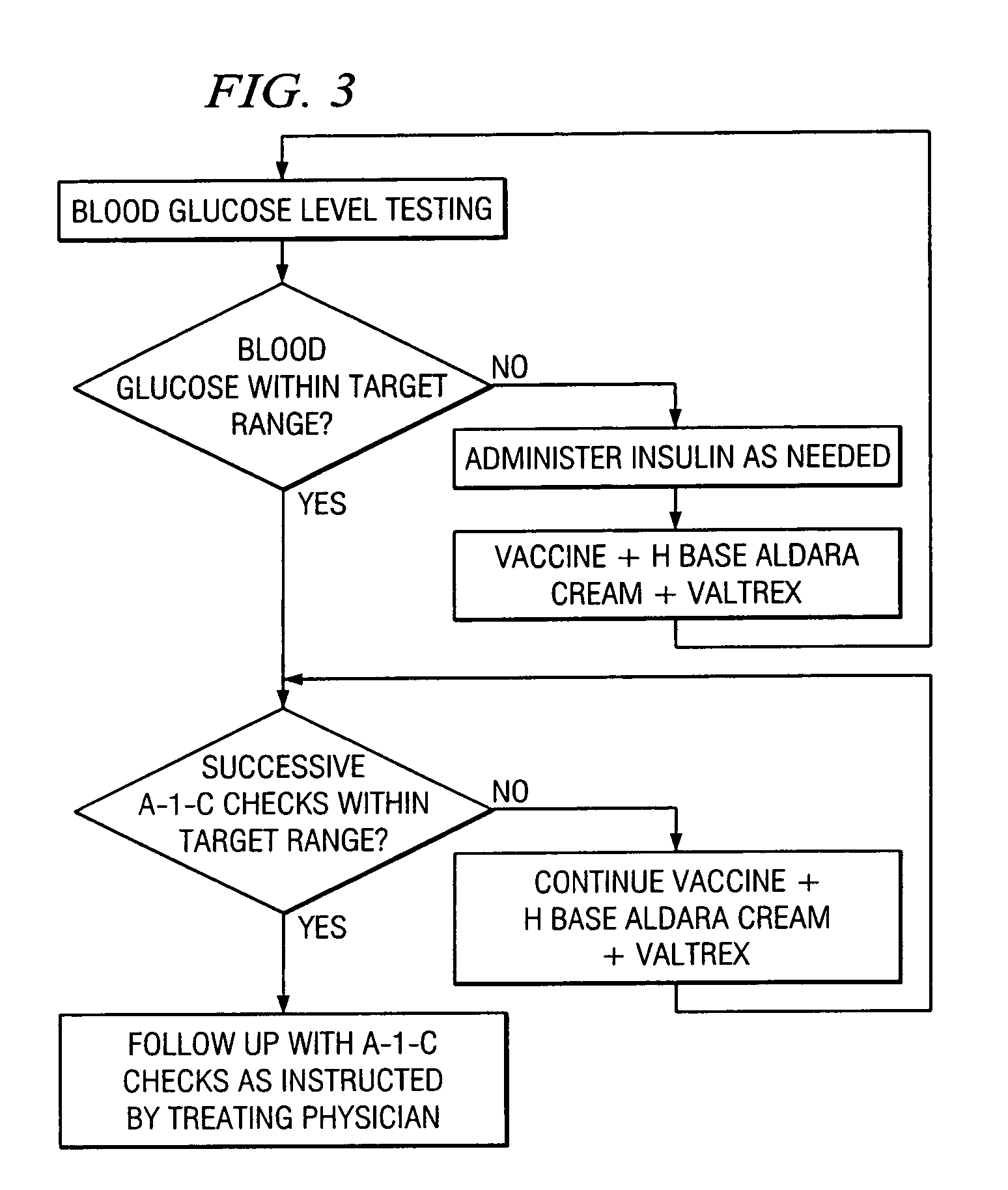
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering an needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA® (imiquimod) 5% cream with an equal amount of H base cream; administering a vaccine containing tumor necrosis factor, preferably the BCG vaccine; and orally administering VALTREX® (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:WOODWARD FAMILY LTD A PARTNERSHIP OF THE STATE OF TEXAS JOHN R WOODWARD GENERAL PARTNER +1
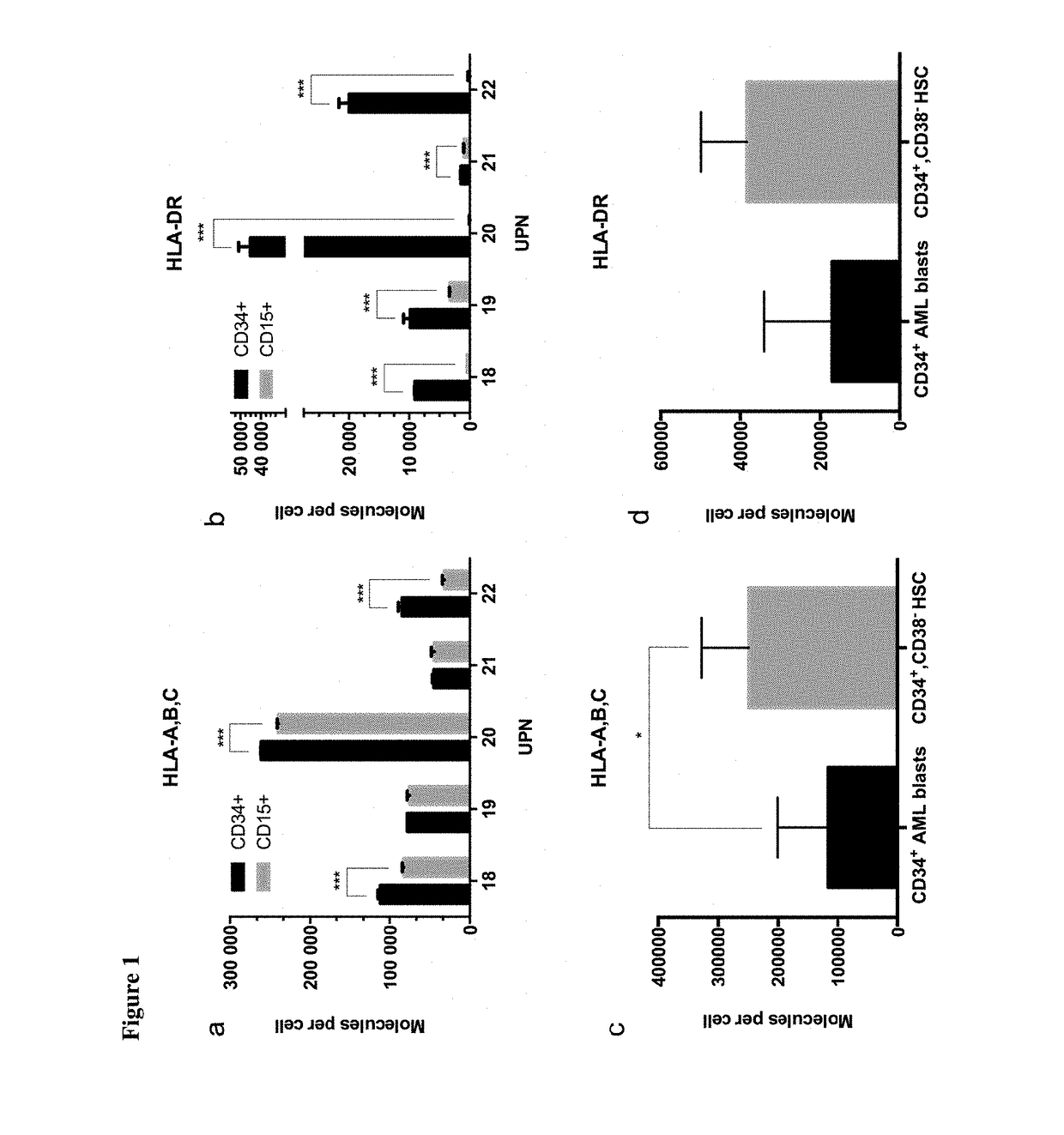
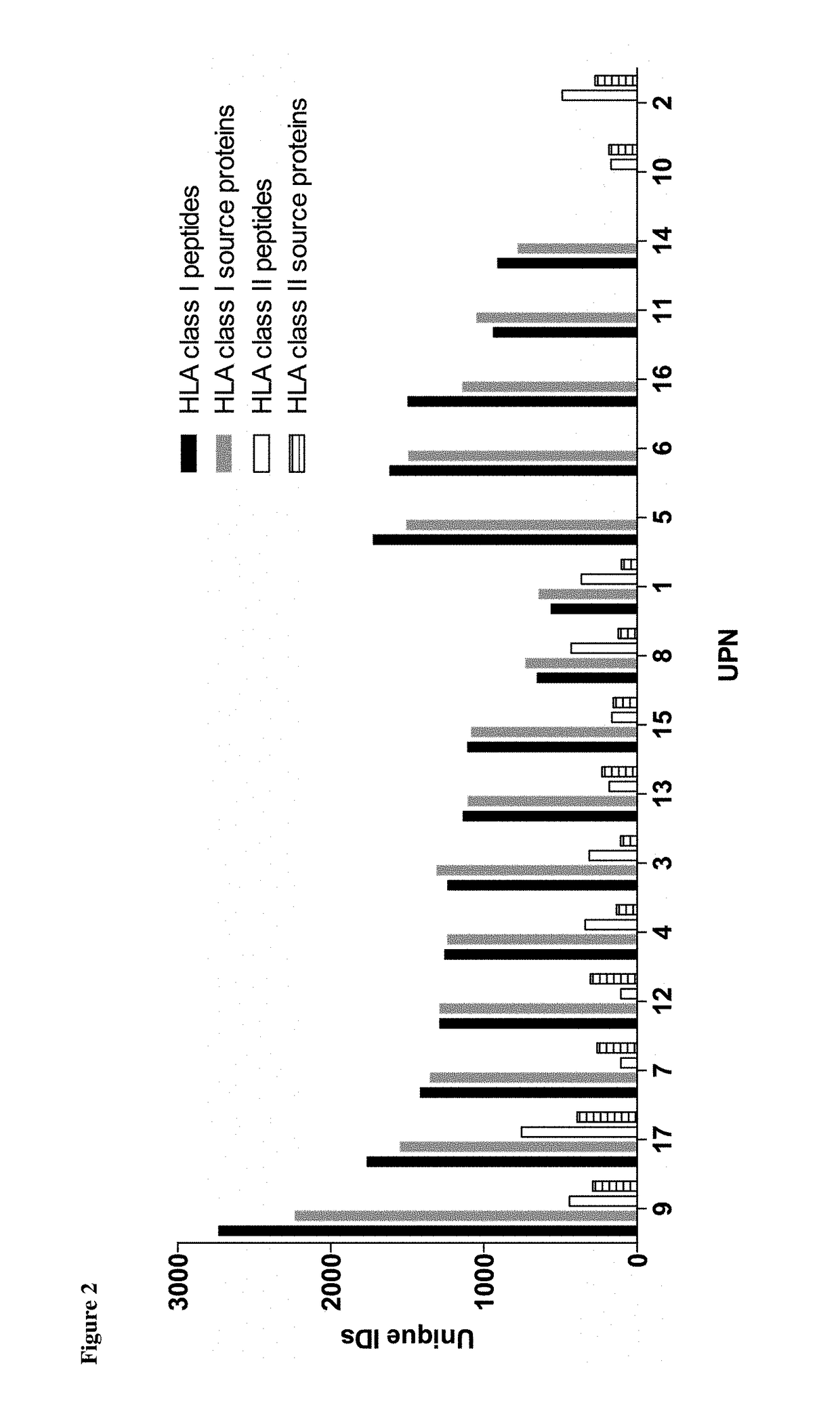
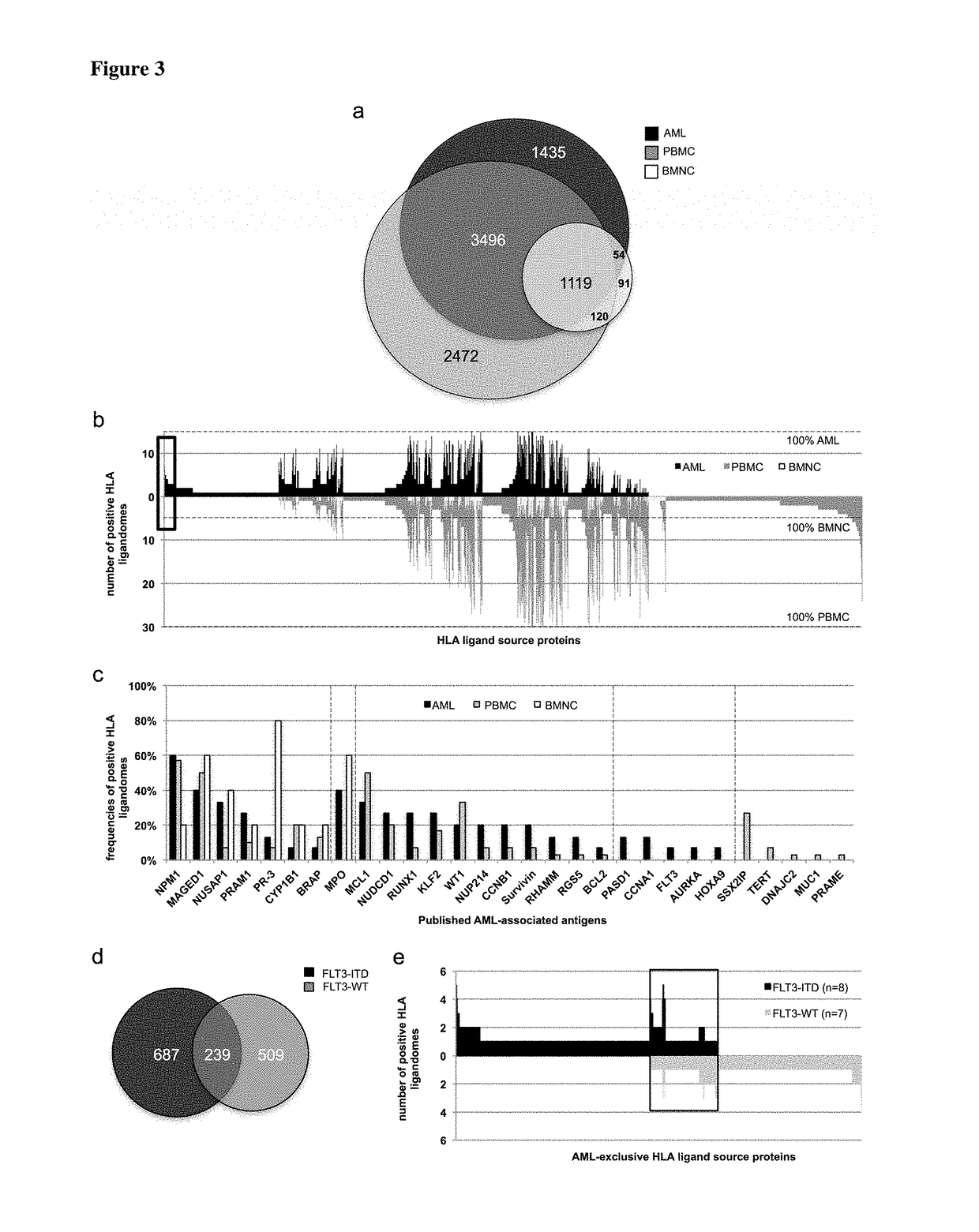
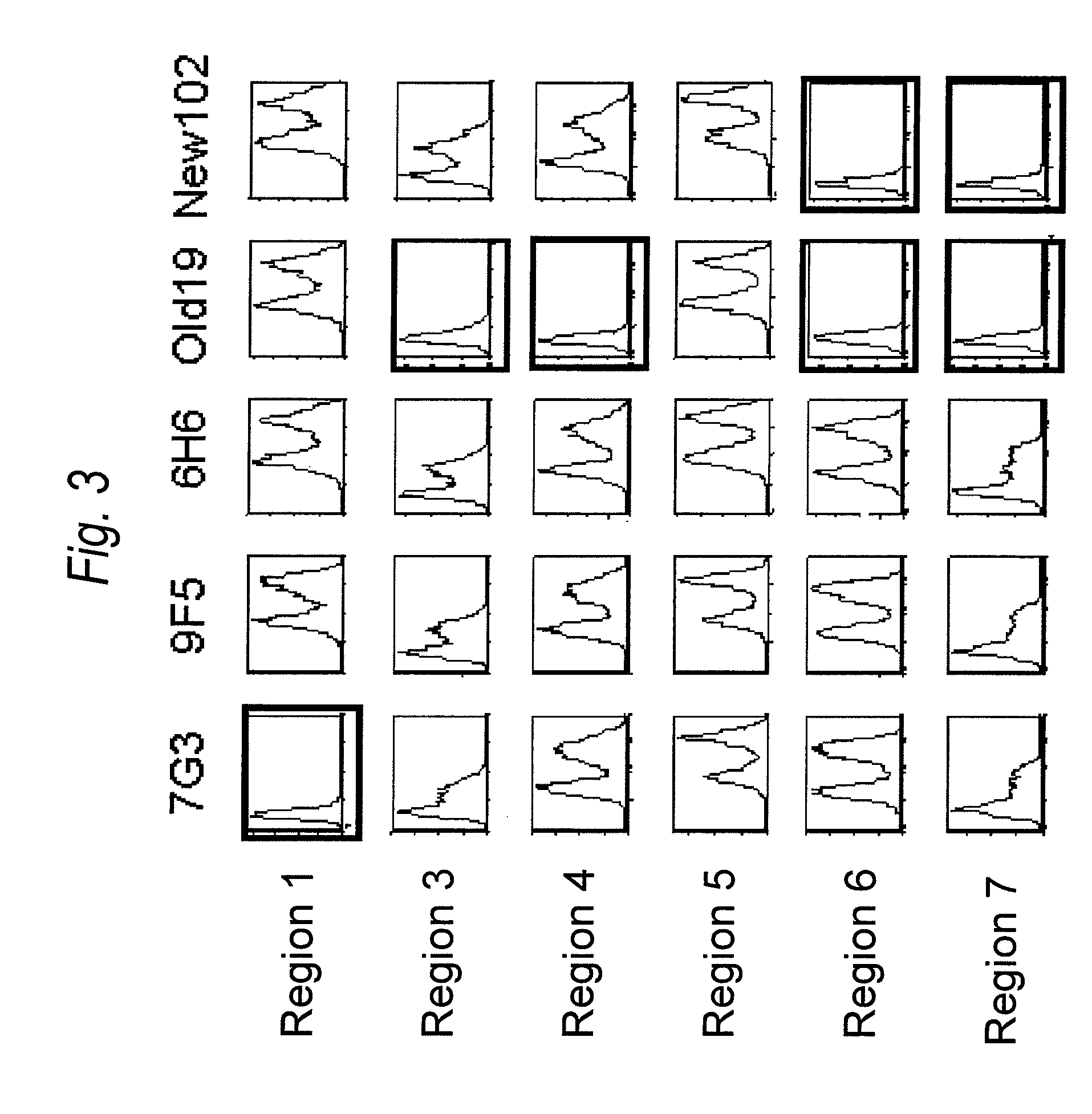
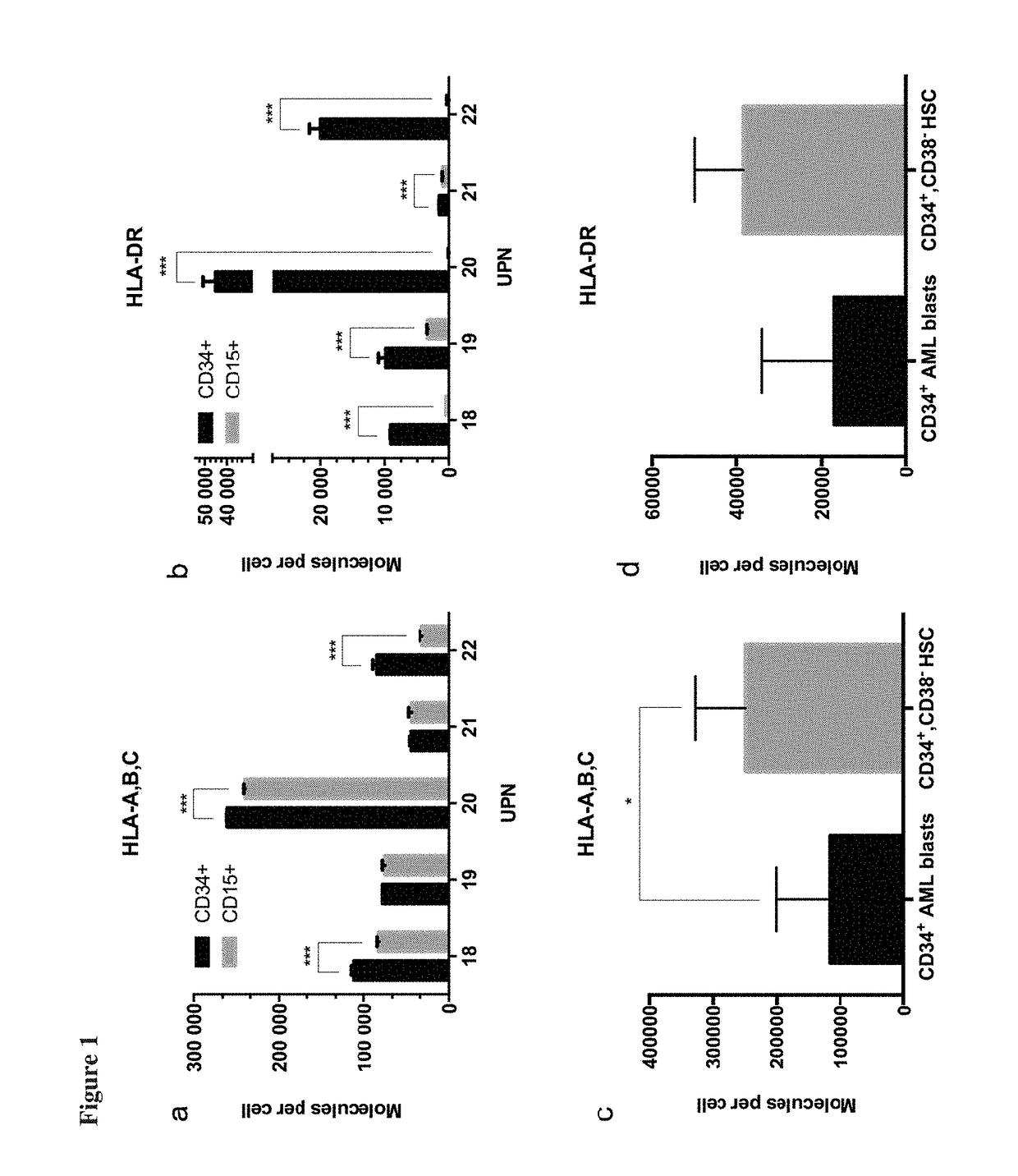
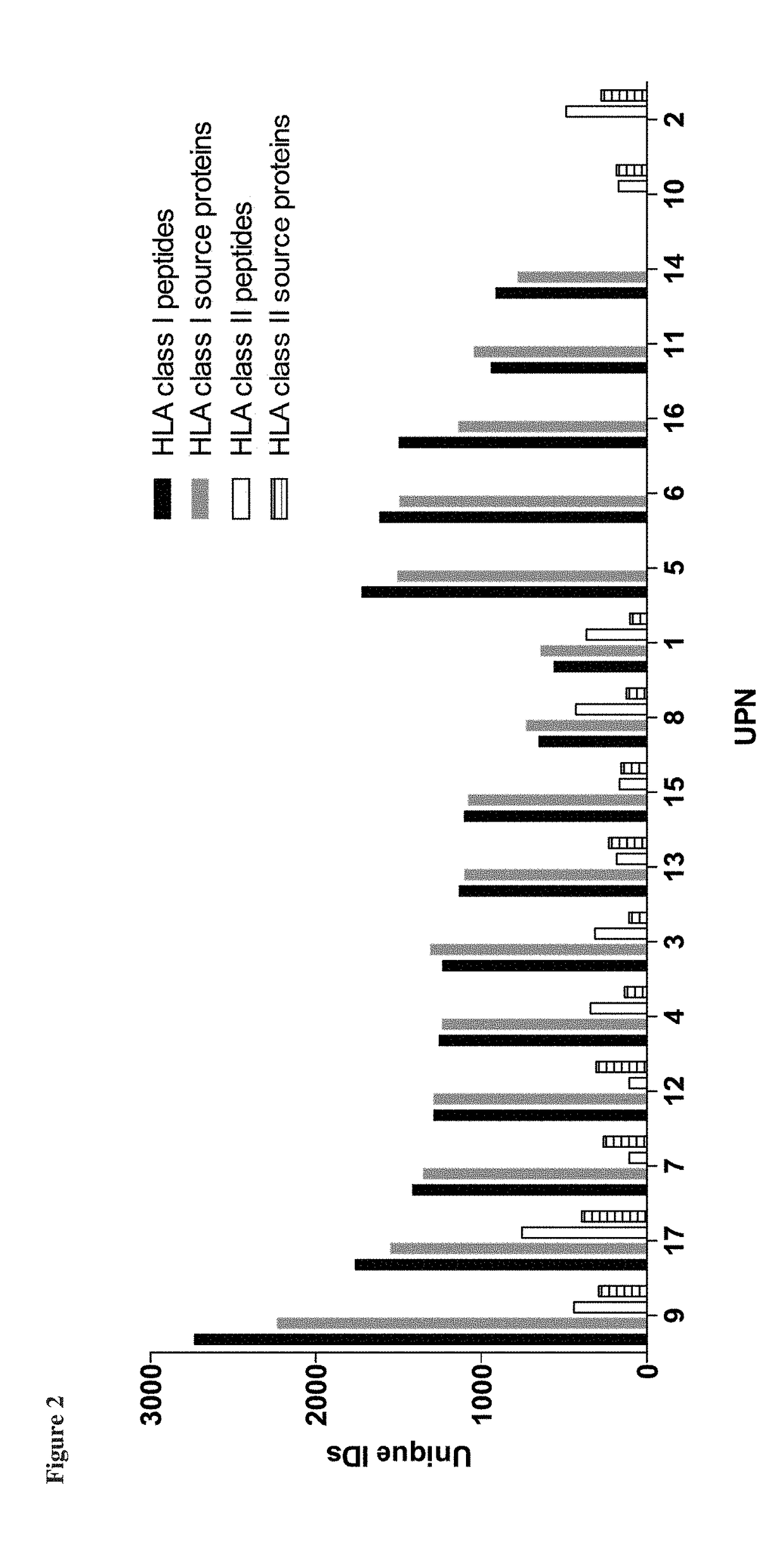
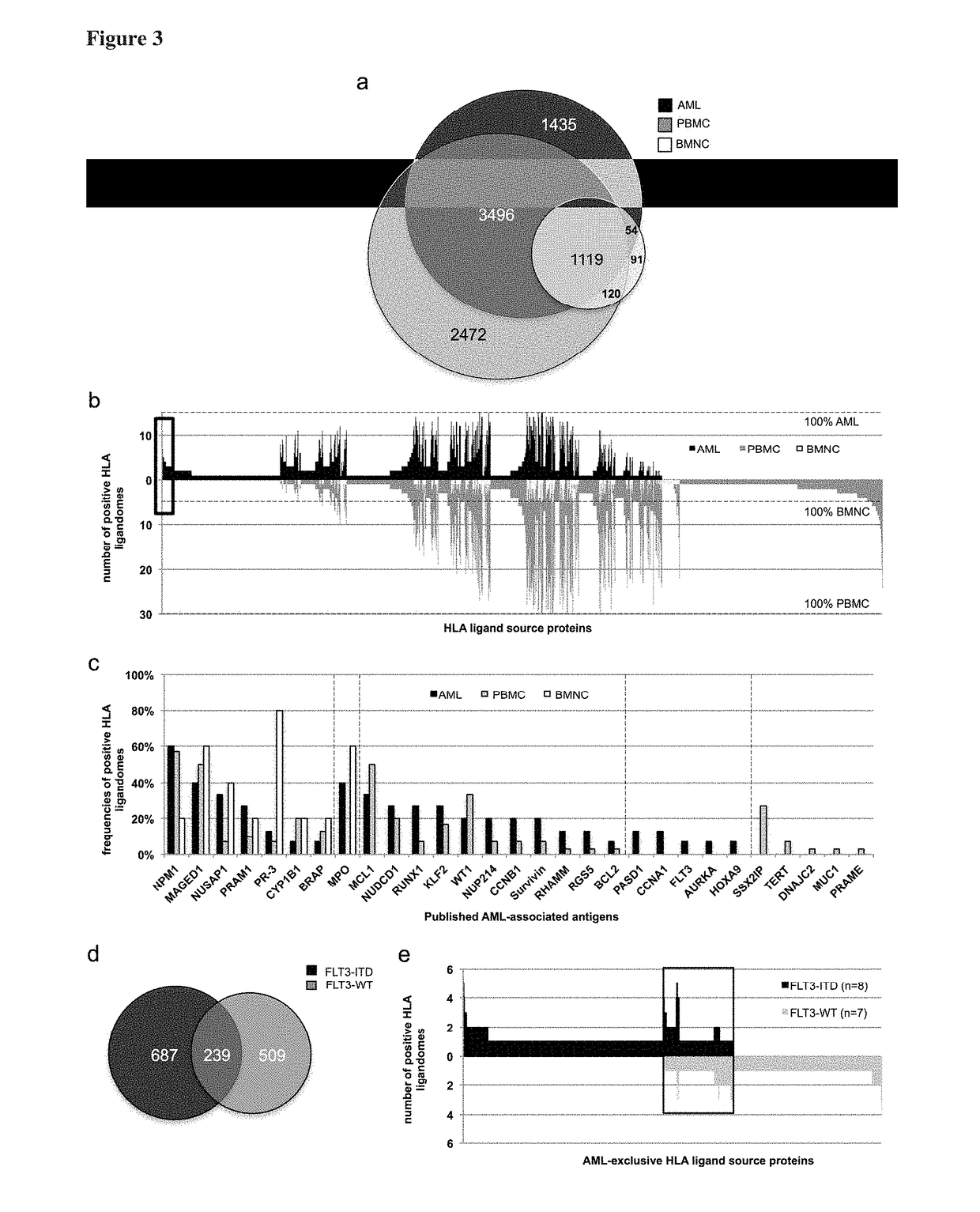
Immunotherapy against several tumors of the blood, such as acute myeloid leukemia (AML)
ActiveUS10064924B2Strong upregulationPromotes formationTumor rejection antigen precursorsHydrolasesHla class iiHuman tumor
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated cytotoxic T cell (CTL) peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. The present invention relates to several novel peptide sequences and their variants derived from HLA class I and HLA class II molecules of human tumor cells that can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
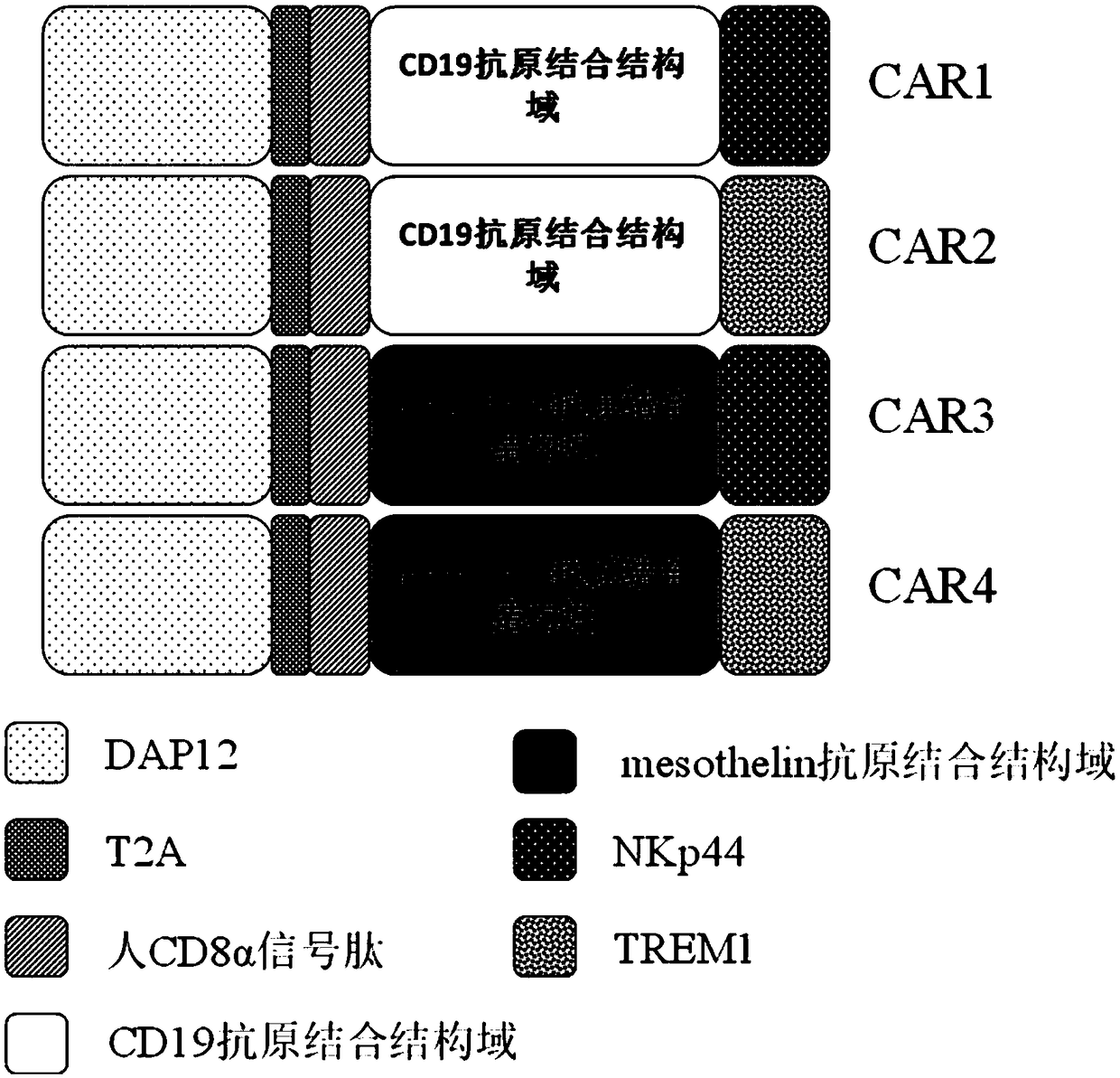
Application of novel CAR (Chimeric Antigen Receptor) modified T cells for treating cancer
ActiveCN109400713AMild release responseSignificant effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyExtracellular signalAbnormal tissue growth
The invention discloses a novel CAR (Chimeric Antigen Receptor) and application of novel CAR modified T cells for treating cancer. The novel CAR is formed by an extracellular signal peptide, an antigen binding structural domain, an intracellular first conduction structural domain and an intracellular second conduction structural domain, and contains an intracellular first conduction structural domain NKp44 or an intracellular first conduction structural domain TREM1. According to the novel CAR disclosed by the invention, multiple CAR nucleotide sequences are separated and purified, and the CARand CAR-T cells which specifically aims at CD19 malignant hematologic tumor and mesothelin malignant solid tumor antigens. In a hematologic tumor and solid tumor killing test, the killing capacity ofthe CAR-T cells to tumor cells is obviously enhanced, and good safety and good anti-tumor activity in clinic application are expressed.
Owner:NANJING CART MEDICAL TECH LTD
Method of cancer screening; method of cancer treatment; and method of diabetes treatment
A method of cancer screening comprising the steps of administering the Blood CA 27,29 testing procedure; if the result is positive administering a mammogram; if the result is positive administering a needle biopsy; if the result is positive administering a PET scan; if the result is positive administering a blood tumor cell count. If all of the foregoing steps are positive, the cancer is treated by applying imiquimod transdermally to rotating sites, preferably by mixing ALDARA (TM) (imiquimod) 5% cream with an equal amount of H base cream (TM); administering a vaccine that induces production of tumor necrosis factor, preferably the BCG vaccine; and orally administering Valtrex (TM) (valacyclovir) twice daily. The foregoing treatment method is also effective in treating Type I diabetes, MS, and other epidermal cancers.
Owner:LES MEDECINS
Method for establishing PDX (Patient Derived Xenograft) model of human blood tumor
ActiveCN109481666AHigh xenograft survival rateHigh tumor formation rateMammal material medical ingredientsAntibody ingredientsHuman leukemiaT lymphocyte
The invention discloses a method for establishing a PDX (Patient Derived Xenograft) model of human blood tumor. The method comprises the steps of extracting a blood tumor cell of a patient, adding rabbit anti-human thymocyte immunoglobulin ATG (Anti-Thymocyte Globulin) and patient autologous serum, mixing, incubating and after completing the incubation, re-suspending the obtained cell and inoculating in a mouse; and feeding the inoculated mouse with CsA (Cyclosporine A) and lasting for 5-10 days starting from 2 days before the inoculation of the blood tumor cell. The method disclosed by the invention has the beneficial effects that a novel highly immunodeficient NCG mouse independently developed in China is firstly adopted to establish a human leukemia PDX model; by orally taking human thymocyte immunoglobulin pretreatment sample combined with the cyclosporine for a long term, donor-derived T cells are removed and inhibited; and by combining the toxic effect of the ATG on lymphocytes with the functional blocking effect of the CsA on T lymphocytes, the immune function of the T lymphocytes can be persistently inhibited and the success rate of PDX modeling of the blood tumor is significantly improved.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Human interleukin 2-polyethylene glycol conjugate as well as preparation method and application thereof
ActiveCN112279906AReduced binding activityReduced stabilityPeptide/protein ingredientsDepsipeptidesWhite blood cellPolyethylene glycol
The invention discloses a human interleukin 2-polyethylene glycol conjugate as well as a preparation method and an application thereof. The human interleukin 2-polyethylene glycol conjugate comprisesrecombinant human interleukin 2 of at least one non-natural amino acid and PEG coupled to the at least one non-natural amino acid, the position of the at least one non-natural amino acid is selected from one or more sites of L36, M39, L40, M46, P47, L63, L66, E67, L70 and A73 corresponding to SEQ ID NO: 2, wherein the recombinant human interleukin 2 is a protein as shown in SEQ ID NO: 3 or a functional active fragment thereof. The human interleukin 2-polyethylene glycol conjugate provided by the invention can be used as a single drug or combined with an anti-tumor drug, and is used for treating solid tumors or blood tumors.
Owner:NOVOCODEX BIOPHARMACEUTICALS CO LTD
Application of engineered T cell with immune receptor for treatment of cancer
InactiveCN109734814AMild release responseEnhance killing activityMammal material medical ingredientsGenetic engineeringAntigenExtracellular signal
The invention discloses application of an engineered T cell with an immune receptor for treatment of cancer. A chimeric antigen receptor consists of an extracellular signal peptide, an antigen bindingstructural domain, a first intracellular conduction structural domain and a second intracellular conduction structural domain and comprises the first intracellular conduction structural domain in theform of the immune receptor. The invention provides a plurality of amino acid sequences of the chimeric antigen receptor and provides the chimeric antigen receptor specific for CD19 malignant hematological tumors and CAR-T cells. In a blood tumor killing test, the ability of the CAR-T cells to kill tumor cells is significantly improved, and high safety and high antitumor activity are shown in clinical application.
Owner:NANJING CART MEDICAL TECH LTD
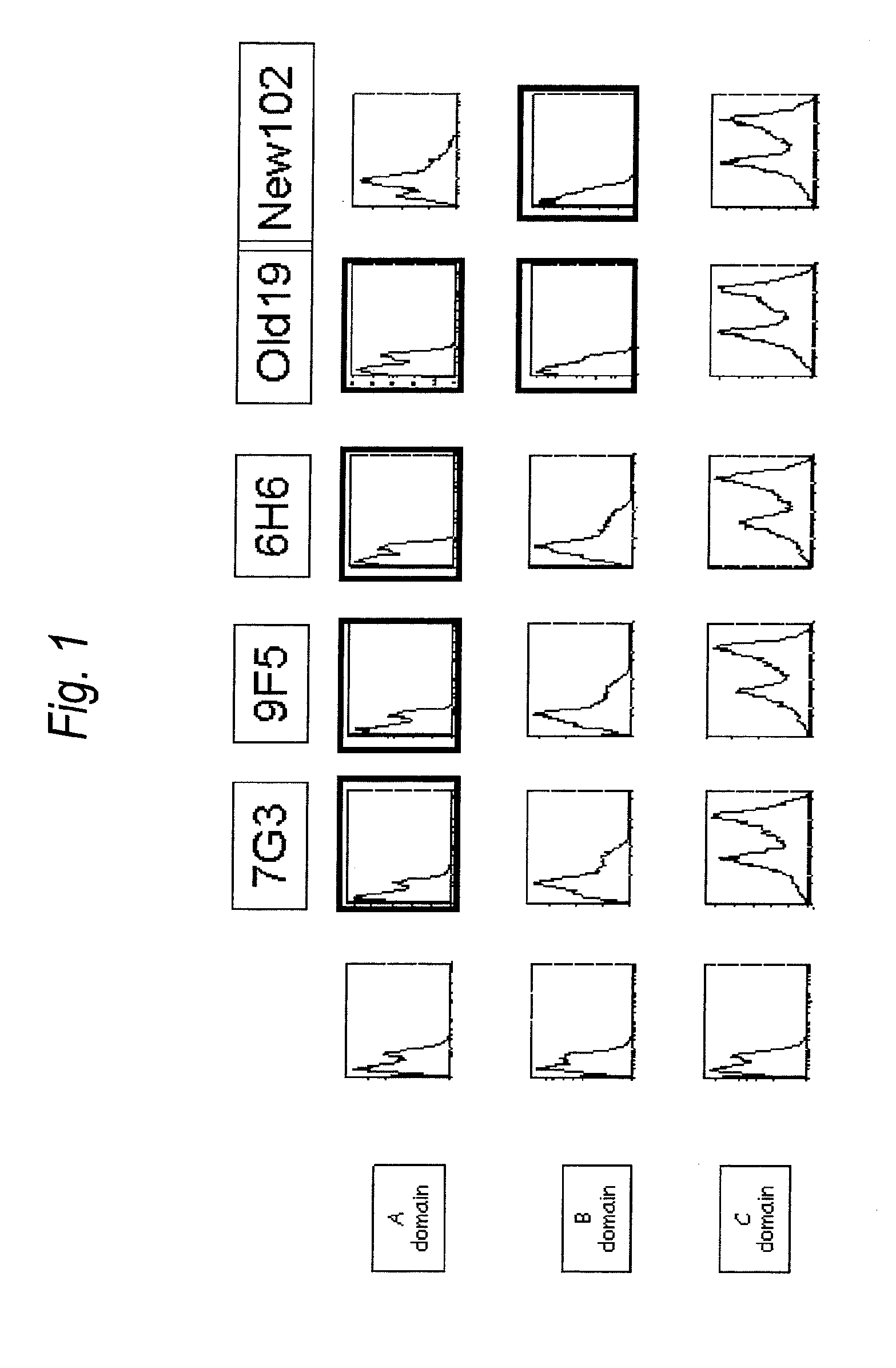
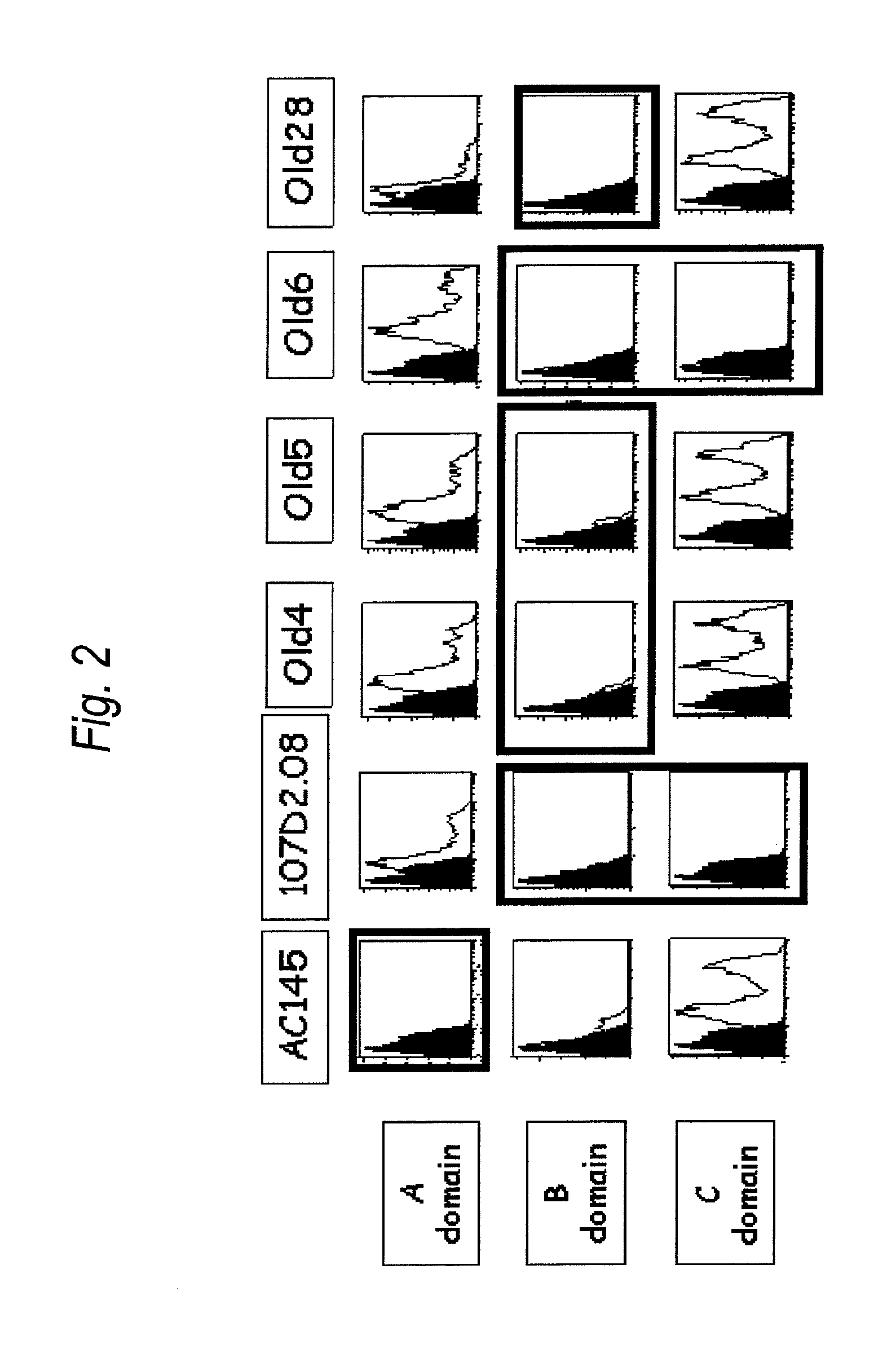
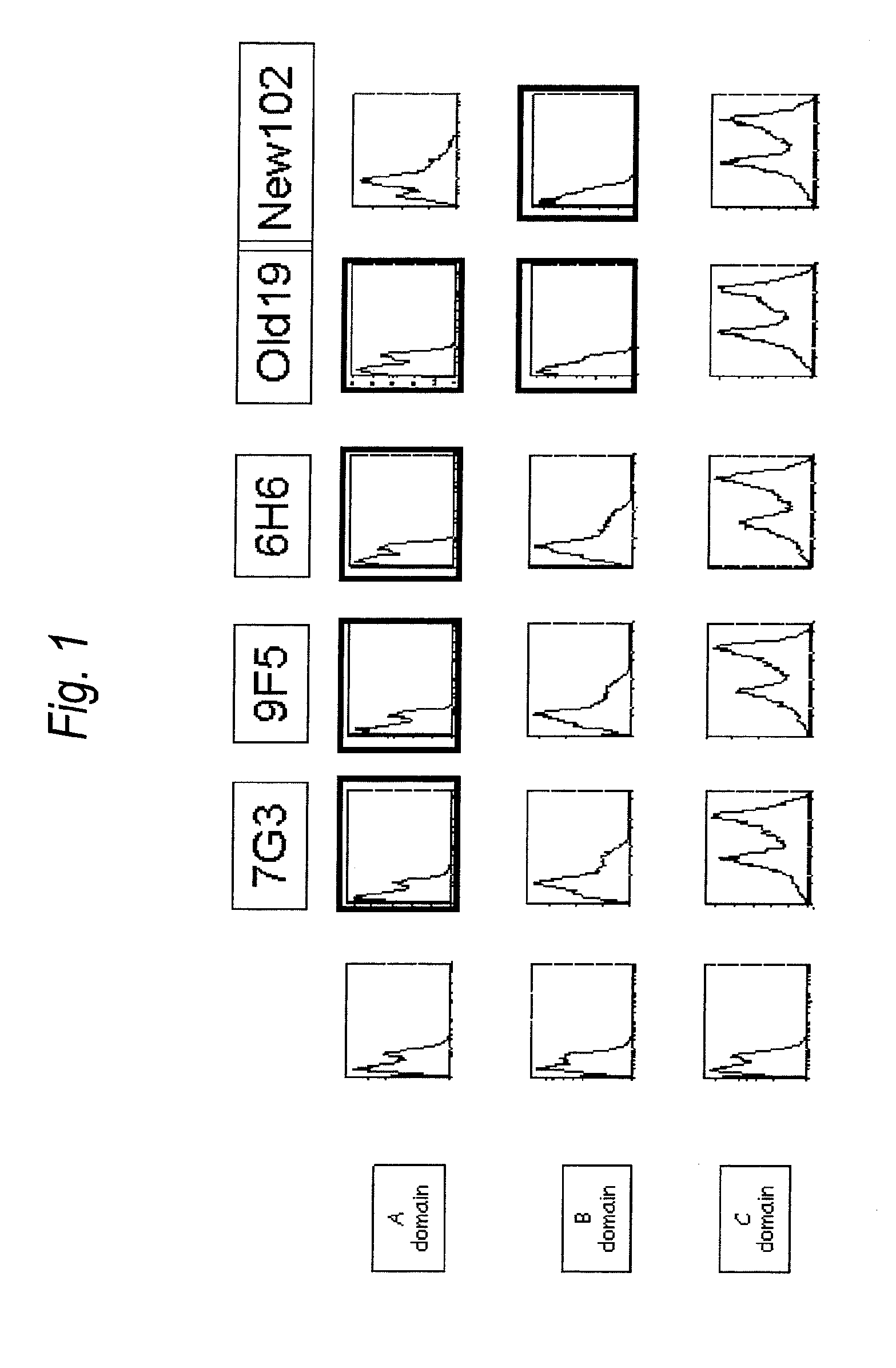
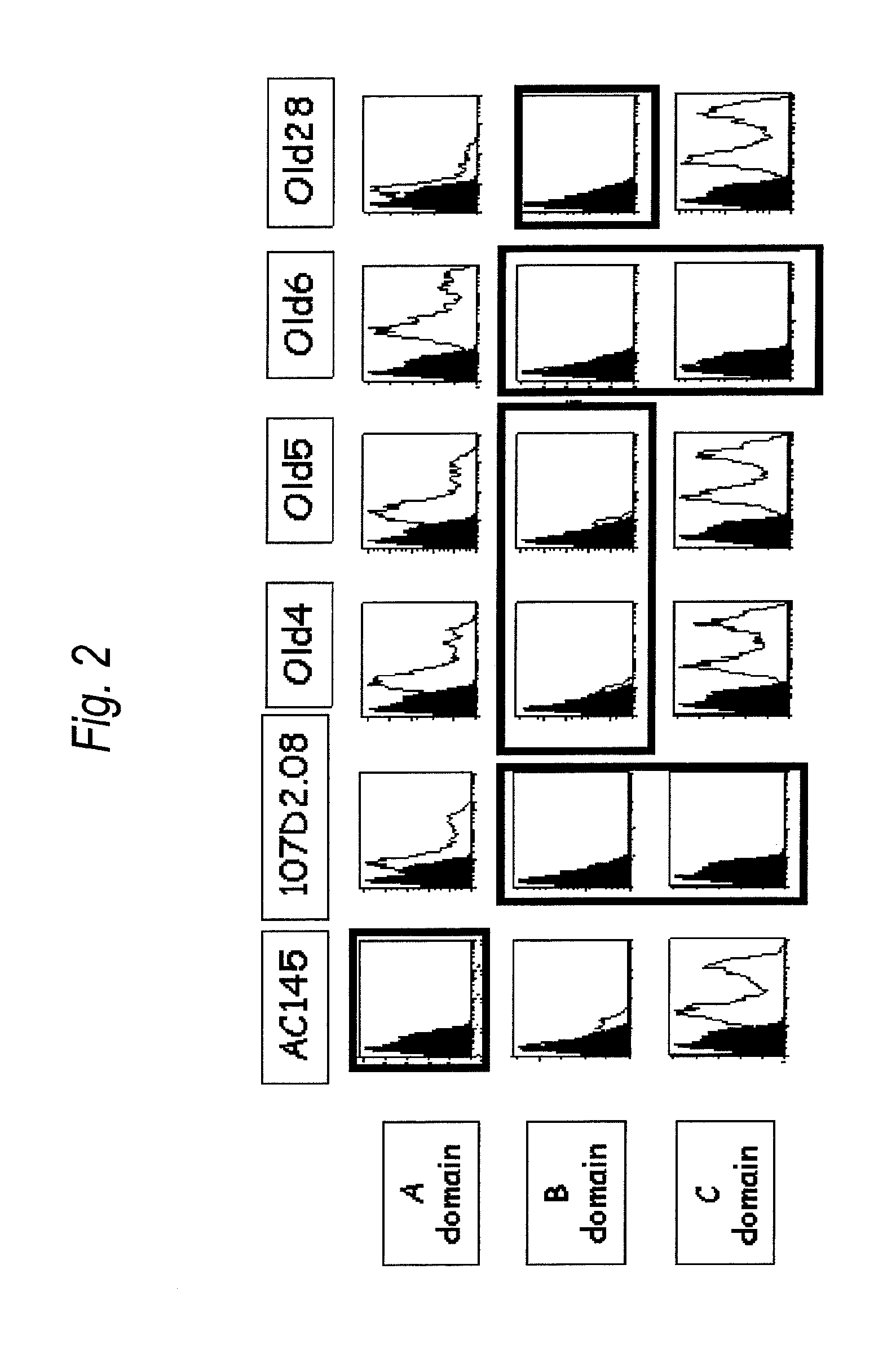
Anti-il-3ra antibody for use in treatment of blood tumor
ActiveUS20120070448A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsBULK ACTIVE INGREDIENTActive ingredient
The present invention provides an antibody to human IL-3Rα chain, which does not inhibit IL-3 signaling and binds to B domain of the human IL-3Rα chain but does not bind to C domain of the human IL-3Rα chain; a composition for preventing or treating a blood tumor in which a cell expressing IL-3Rα is found in bone marrow or peripheral blood of a subject, which comprises the antibody to human IL-3Rα as an active ingredient; and a method for treating a blood tumor in which a cell expressing IL-3Rα is found in bone marrow or peripheral blood, which comprises administering, to a subject, a composition comprising the IL-3Rα antibody as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Antibody to human IL-3 receptor alpha chain
ActiveUS8492119B2Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsBULK ACTIVE INGREDIENTActive ingredient
Owner:KYOWA HAKKO KIRIN CO LTD
Novel immunotherapy against several tumors of the blood, such as acute myeloid leukemia (AML)
ActiveUS20180311330A1Enhance stability and solubilityFlexibility in detectionTumor rejection antigen precursorsHydrolasesHla class iiHuman tumor
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated cytotoxic T cell (CTL) peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. The present invention relates to several novel peptide sequences and their variants derived from HLA class I and HLA class II molecules of human tumor cells that can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
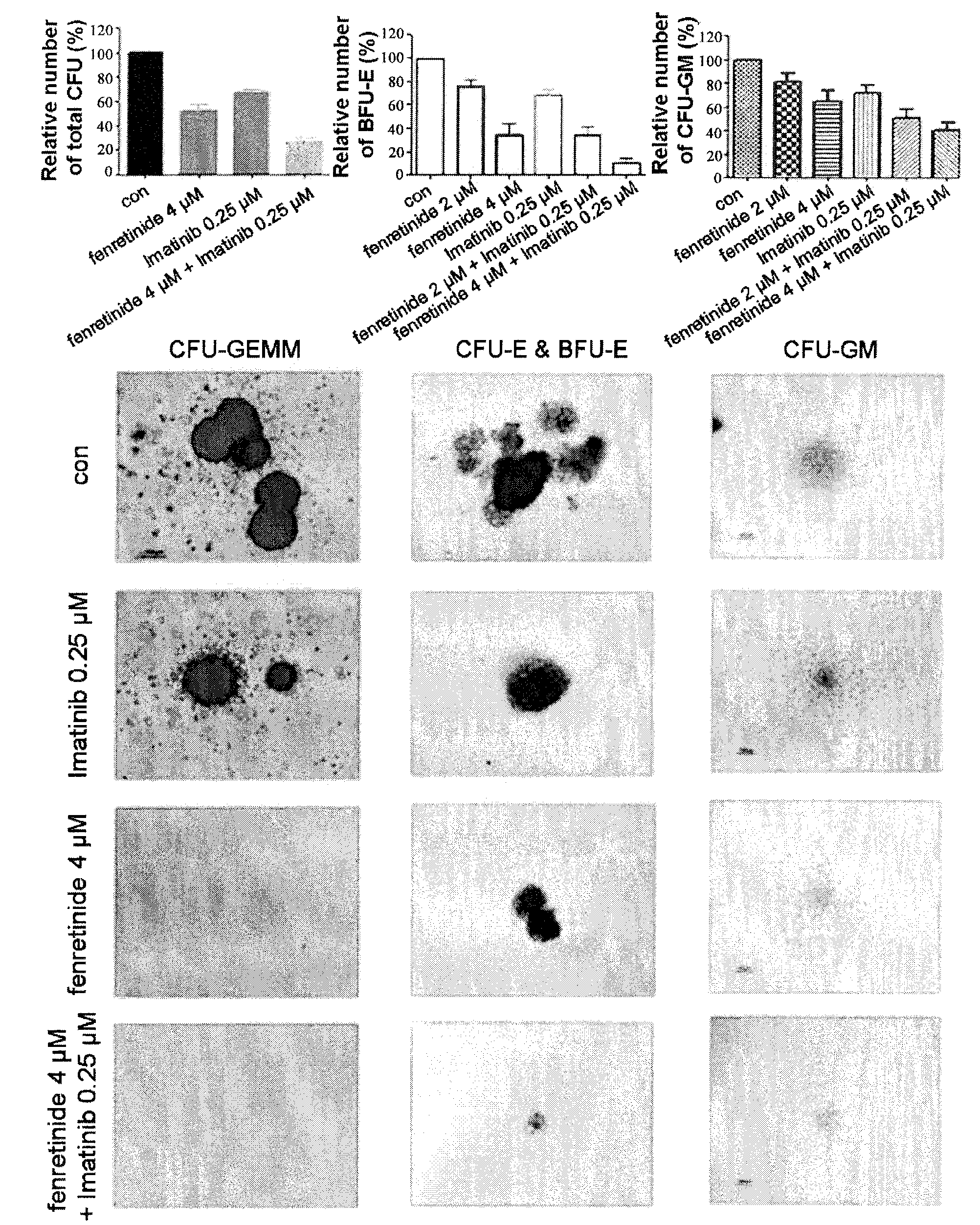
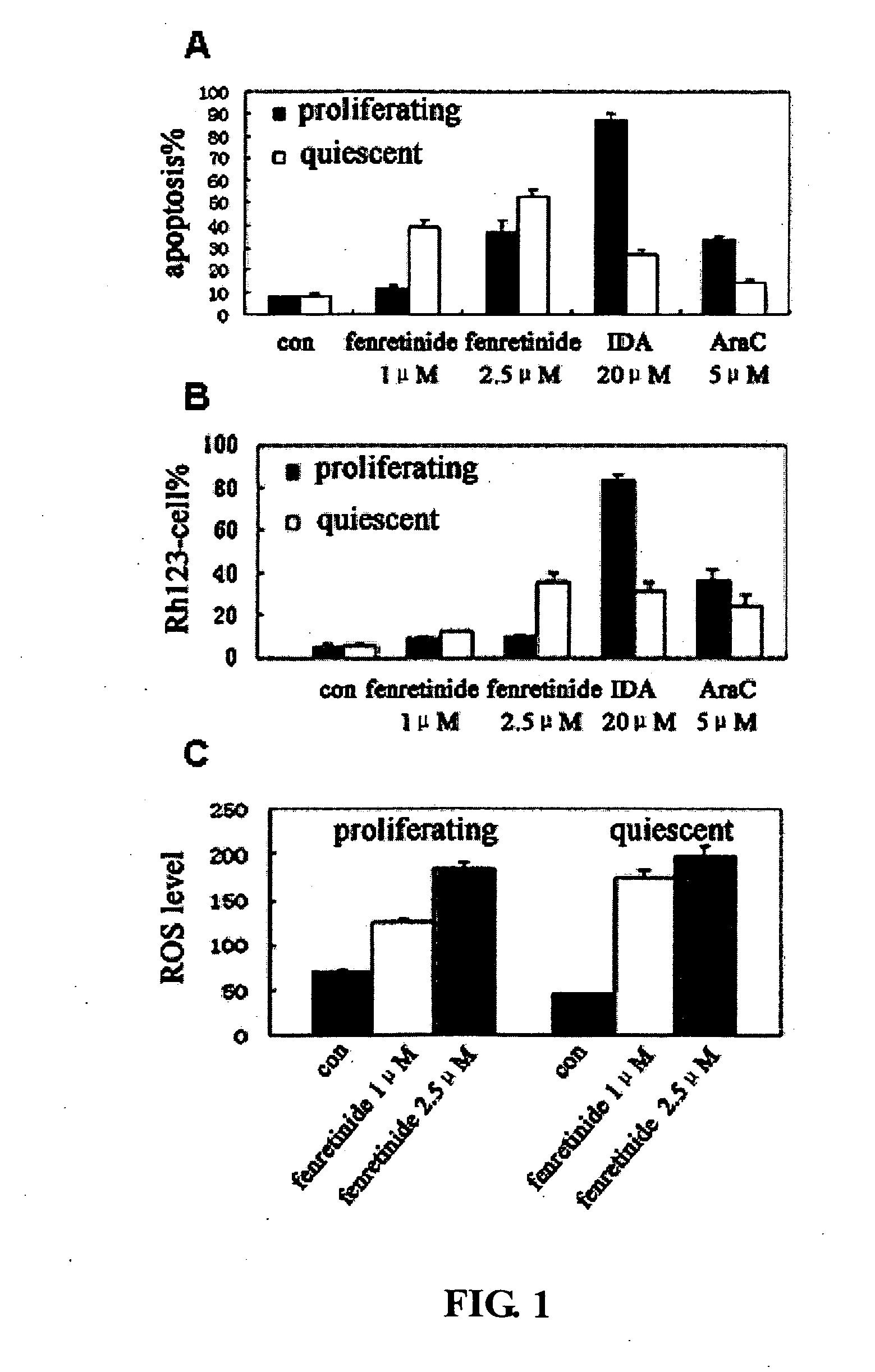
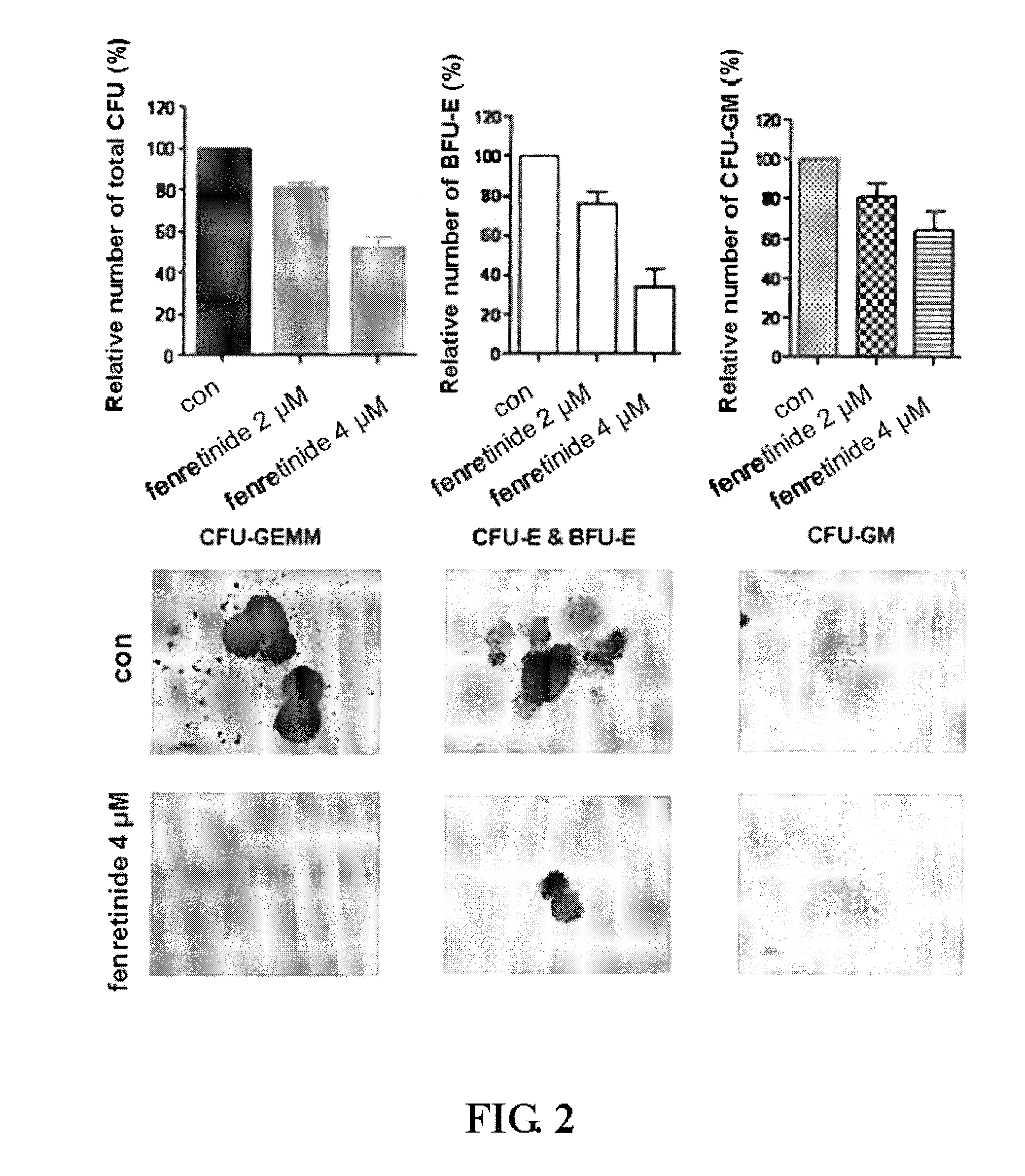
Use of Fenretinide or Bioactive Derivatives Thereof and Pharmaceutical Compositions Comprising the Same
ActiveUS20100008896A1Altering redox balanceIncreased oxygen levelsBiocideHeavy metal active ingredientsDiseaseMedicine
The present invention relates to a new medical use of fenretinide or bioactive derivatives thereof, particularly to the use of fenretinide or bioactive derivatives thereof in the preparation of a medicament for eliminating or killing tumor stem cells in a subject or for treating and / or preventing a tumor disease originating from tumor stem cells in a subject. The invention further relates to a new use of fenretinide or bioactive derivatives thereof in combination with other anti-tumor agents, a pharmaceutical composition comprising said fenretinide or bioactive derivatives thereof and at least one additional anti-tumor agent, a method of screening said other anti-tumor agent, a method of eliminating or killing tumor stem cells or particularly hematologic tumor stem cells in a subject by administrating said fenretinide or bioactive derivatives thereof, as well as a method of eliminating or killing tumor stem cells and tumor cells derived from tumor stem cells, particularly hematologic tumor stem cells and hematologic tumor cells derived from hematologic tumor stem cells in a subject by administrating said fenretinide or bioactive derivatives thereof in combination with other anti-tumor agent(s).
Owner:ZHANG JI +1
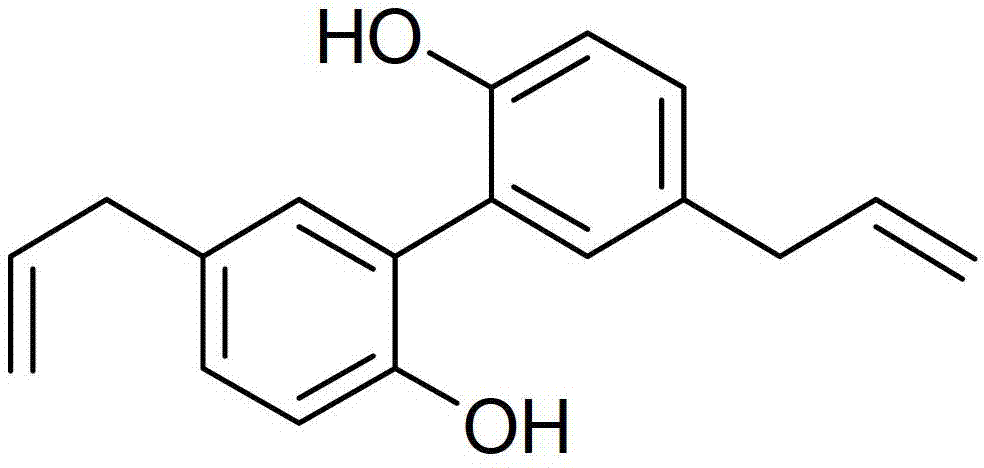
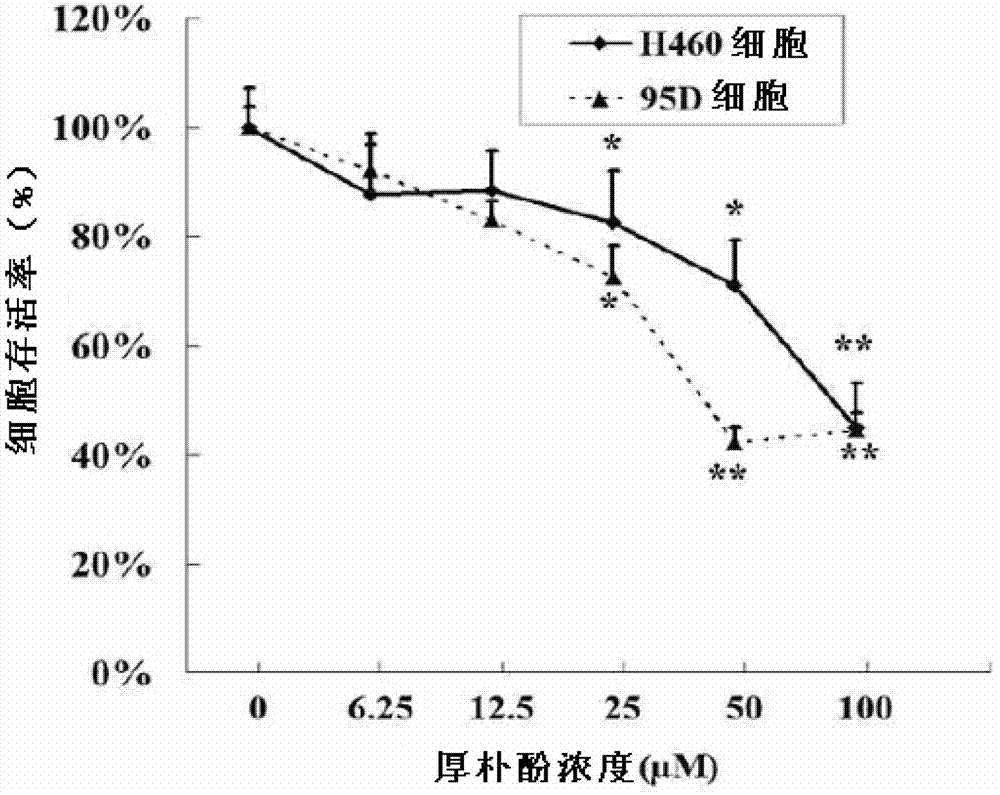
Application of biphenol and/or derivatives thereof in preparation of drugs for inhibiting cancer cell metastasis
InactiveCN103315981ALow toxicityAvoid stickingHydroxy compound active ingredientsAntineoplastic agentsCell adhesionCancer cell
The invention discloses application of biphenol and / or derivatives thereof in preparation of drugs for inhibiting metastasis of cancer cells, wherein the biphenol is magnolol and / or honokiol. The cancer cells are one or a plurality kinds of cells from cells of brain tumor, blood tumor, esophageal cancer, liver cancer, skin cancer, prostate cancer, bone cancer, breast cancer and lung cancer. One novel application of the magnolol, the honokiol and derivatives of both are provided. The magnolol and the like can effectively inhibit cell adhesion, migration, invasion and expression of MMP-2, so that the magnolol and the like can effectively inhibit the metastasis of the cancer cells. The magnolol, the honokiol and the derivatives of both have low toxicity, almost no toxic and harmful effect on human normal cells, and good clinical anti-tumor application prospects.
Owner:SHANGHAI PULMONARY HOSPITAL
Combined sorting purification device and sorting detection method for circulating blood tumor cells
ActiveCN106754344ARealize sortingAchieve purificationCell dissociation methodsBioreactor/fermenter combinationsEngineeringGuide wires
The invention relates to a combined sorting purification device for circulating blood tumor cells. The device comprises a sorting structure, a purifying structure and a control structure, wherein guide wires of the control structure extend to connect the sorting structure and the purifying structure; the control structure controls the sorting structure and the purifying structure; the sorting structure comprises a micro-fluidic chip which comprises triangular micro-array columns on two sides and a wide channel between the micro-arrays; the purifying structure comprises a purifying chip, a permanent magnet, two stepping motors for supporting the permanent magnet to move and supporting pieces for supporting parts, the permanent magnet is arranged below the purifying chip, the two stepping motors are vertically arranged, and the permanent magnet is arranged on one of the stepping motors; the micro-fluidic chip is connected to the purifying chip through a tubular structure; the control structure comprises a power supply and a control unit; and the control structure controls the stepping motors to move. The device can sort and purify tumor cells effectively, and is simpler and more convenient.
Owner:北京方远智汇科技有限公司
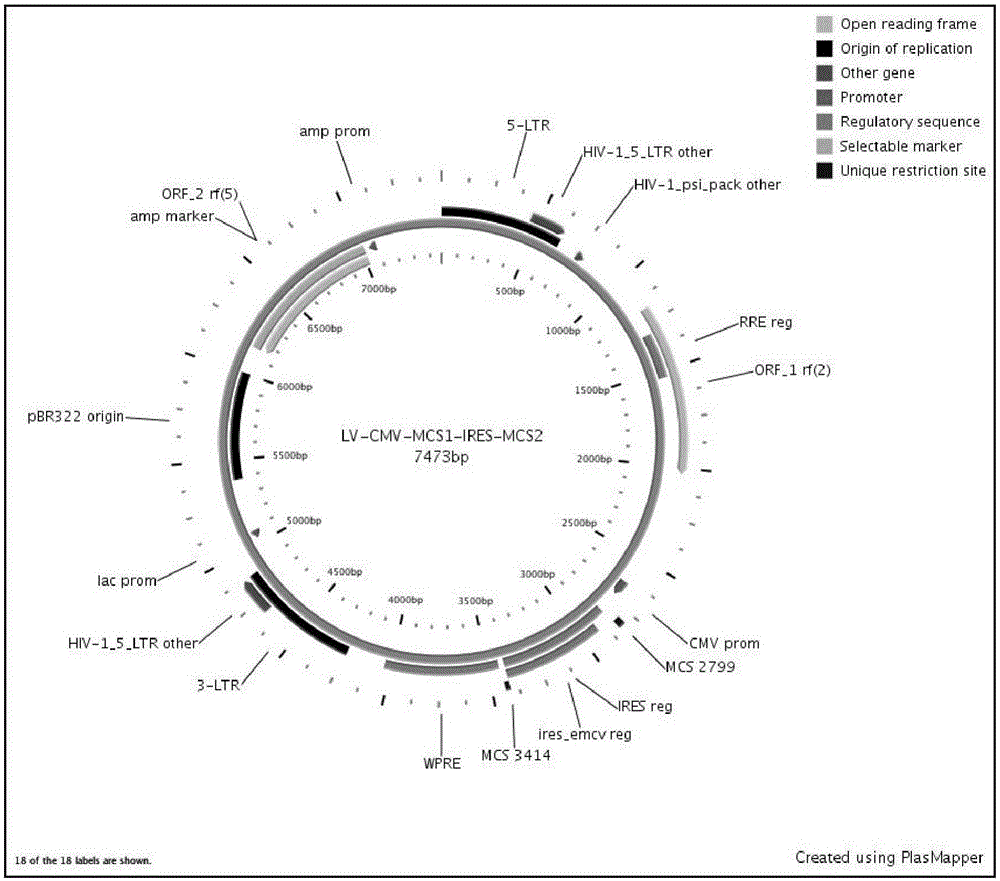
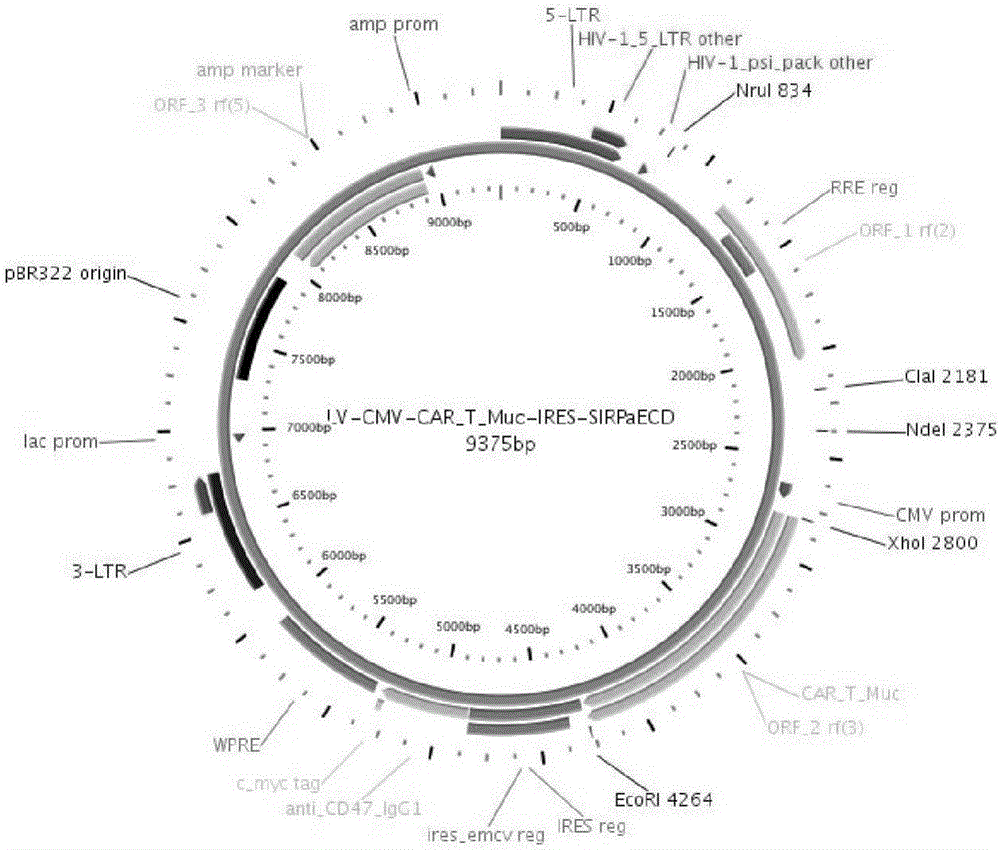
Construction and application of multiple-reading frame non-integrative lentiviral vector
The invention discloses construction and application of a multiple-reading frame non-integrative lentiviral vector, and relates to the technical field of gene / cell therapy. The lentiviral vector sequentially includes a 5'-LTR sequence, a promoter sequence, a multiple cloning site 1, one or more IRES, a multiple cloning site 2 and a 3'-LTR sequence from upstream to downstream; the vector also comprises a virus packaging signal sequence used for enhancing nuclear entry; and the multiple cloning site 1 and the multiple cloning site 2 are used for loading target exogenous expression gene. Experiments prove that vectors used for cellular immunity therapy of solid tumors, blood tumors, self immune diseases, stem cell therapy, viral diseases and genetic diseases can be effectively constructed on the basis of the lentiviral vector.
Owner:李因传
Bispecific chimeric antigen receptor for treating hematologic tumor complicated with HIV infection, gene thereof, and construction method and application of gene
ActiveCN111196858AAchieve the effect of treating two diseases at the same timeEasy to removeVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesAntiendomysial antibodies
The invention discloses a construction method and an application of a recombinant gene of a bispecific chimeric antigen receptor for treating HIV infection complicated with blood tumor. The chimeric antigen receptor is formed by sequentially connecting a signal peptide, an HIV gp120 antigen specific single-chain antibody and an anti-CD19 single-chain antibody and then sequentially connecting witha CD28 transmembrane region, a CD28 intracellular structural domain (ICD), a 4-1BB costimulatory structural domain and a CD3zeta intracellular signal transduction structural domain in series, or the chimeric antigen receptor comprises: first CAR composed of the signal peptide, the chimeric antigen receptor, the HIV gp120 antigen specific single-chain antibody, the CD28 transmembrane region, the CD28-ICD, the anti-CD19 single-chain antibody, the CD8 transmembrane region, the CD28-ICD, the 4-1BB costimulatory structural domain and the CD3zeta intracellular signal transduction domain; and secondCAR composed of and the signal peptide, the anti-CD19 single-chain antibody, the CD8 transmembrane region in parallel, connecting the CD28-ICD, the 4-1BB costimulatory structural domain and the CD3zeta intracellular signal transduction domain, wherein the first CAR and the second CAR are sequentially connected in parallel.
Owner:WUHAN UNIV OF SCI & TECH
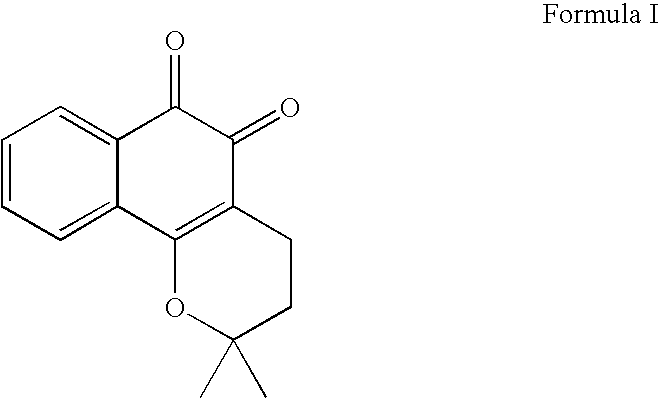
Treatment of hematologic tumors and cancers with beta-lapachone, a broad spectrum anti-cancer agent
The present invention provides for methods that utilize agents effective in the treatment of hematologic cancers and pre-cancerous hematologic cancer conditions. Moreover, the present invention provides agents capable of acting as an inhibitor of cell proliferation in hematologic cells.
Owner:ARQULE INC
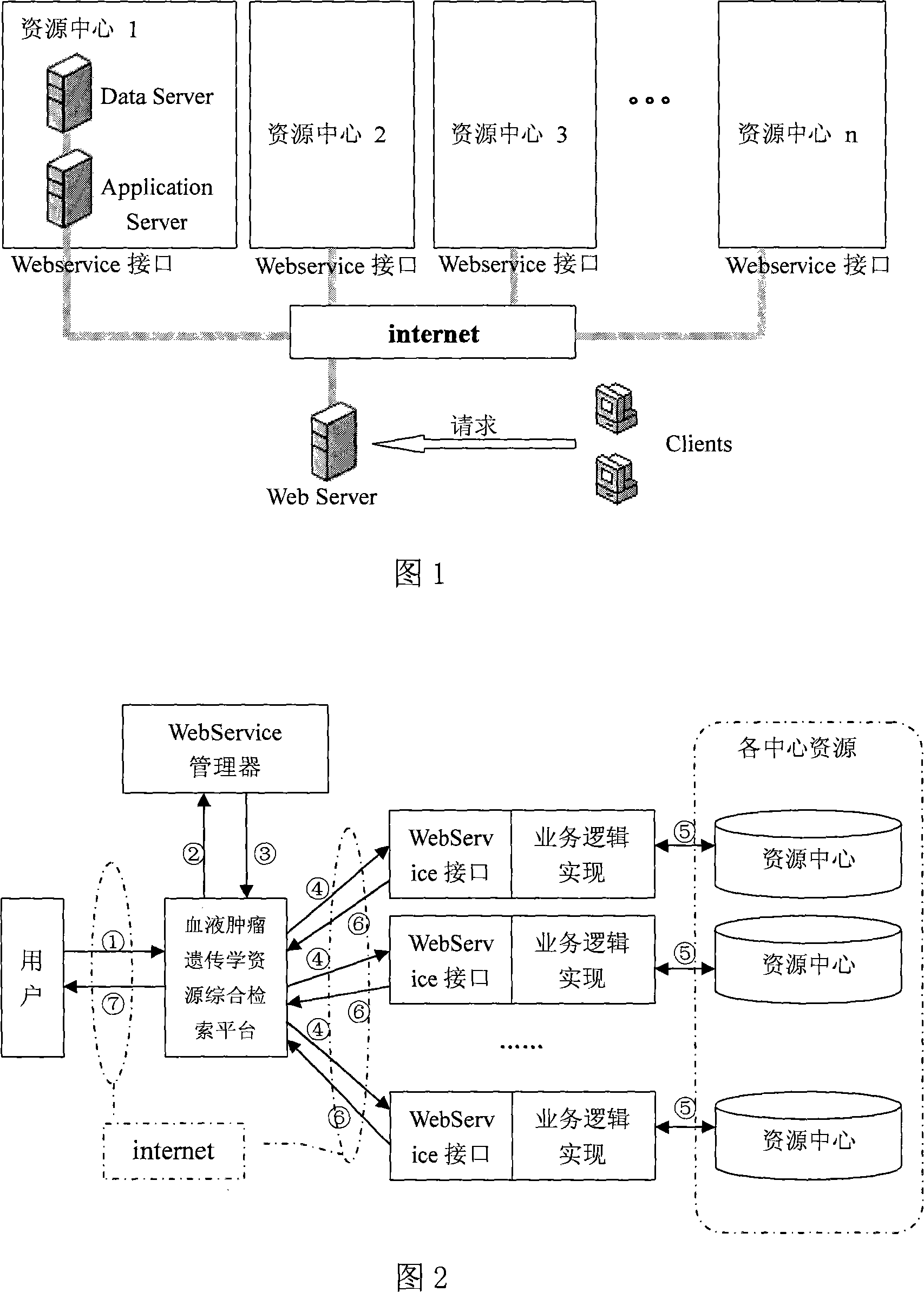
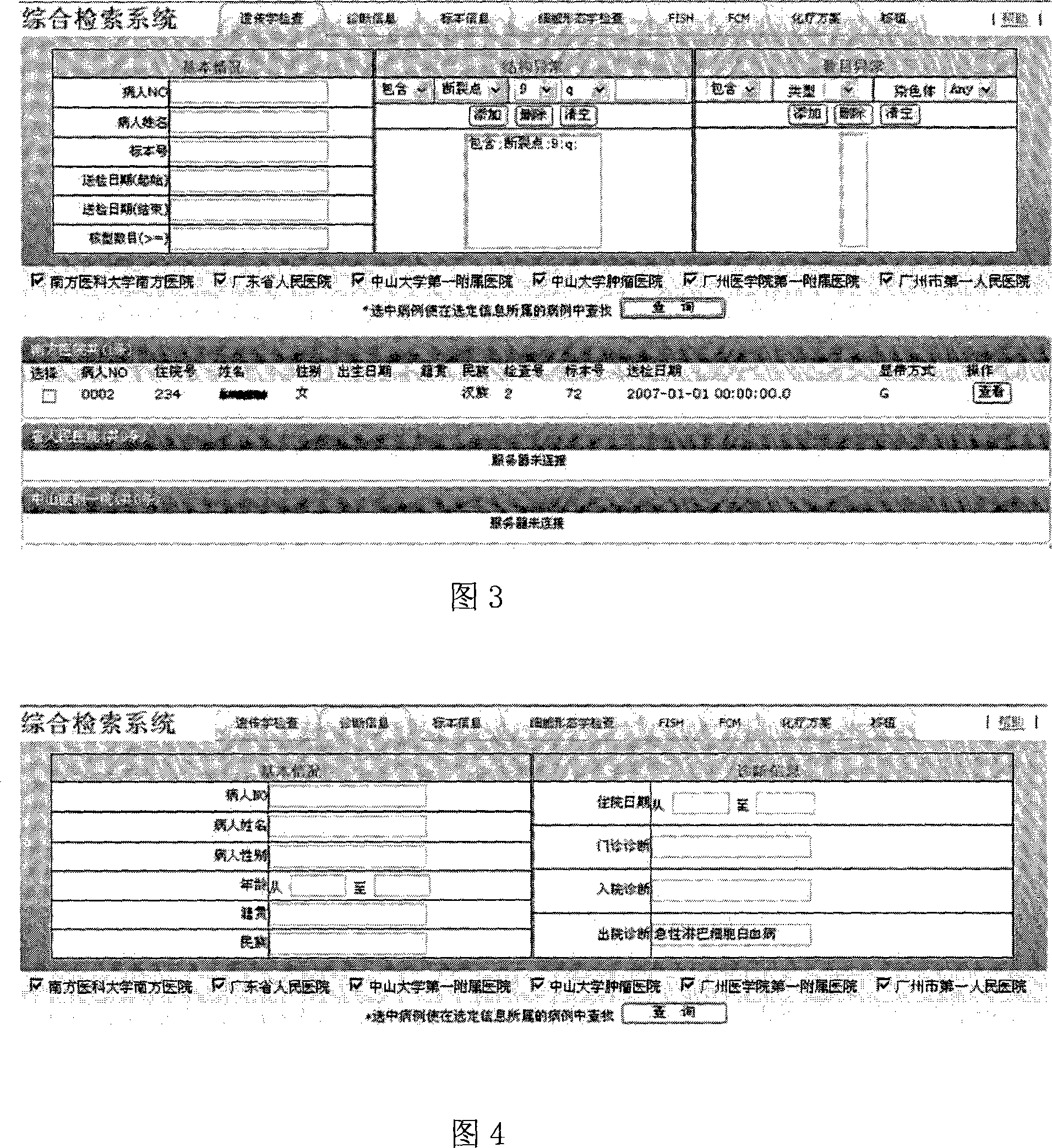
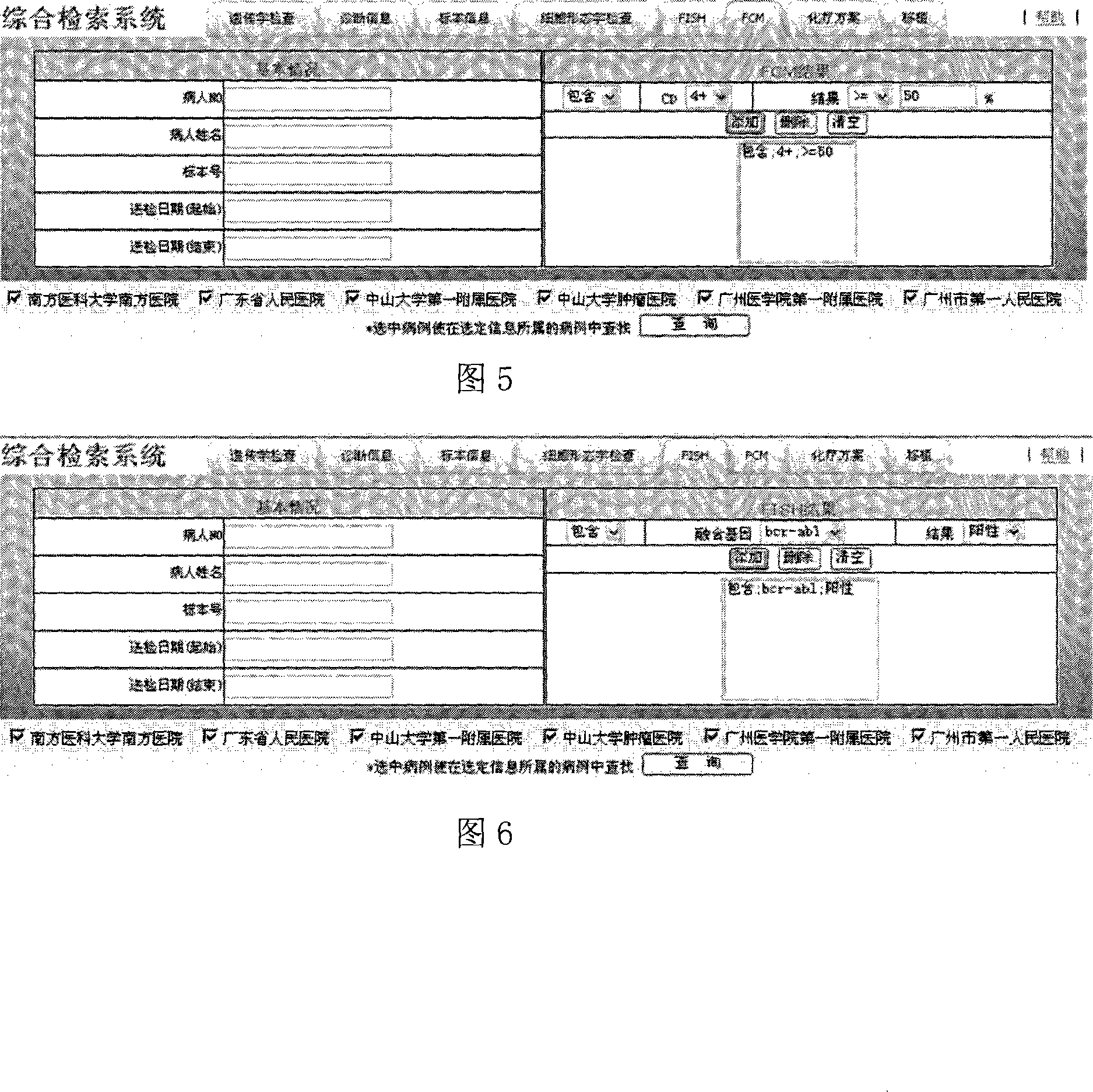
Combined retrieval system with multiple centers blood tumor genetics resource information
InactiveCN101136024ARealize the comprehensive search functionIncrease profitSpecial data processing applicationsData retrievalResource information
In the invention, installing webservice module at each blood disease center; the resource data of the blood disease center provides a standard data interface on the internet; in the data interface, the data service logic is defined and the access right is set up in order to provide relevant service information for different types of requests; the blood disease resource data retrieval platform can call the webservice interface of each blood disease center to get the data fitting the retrieval condition, and provides the retrieved data for the terminal user.
Owner:杜庆锋 +2
N-subsituted aromatic ring-2-amino pyrimidine compound and applications
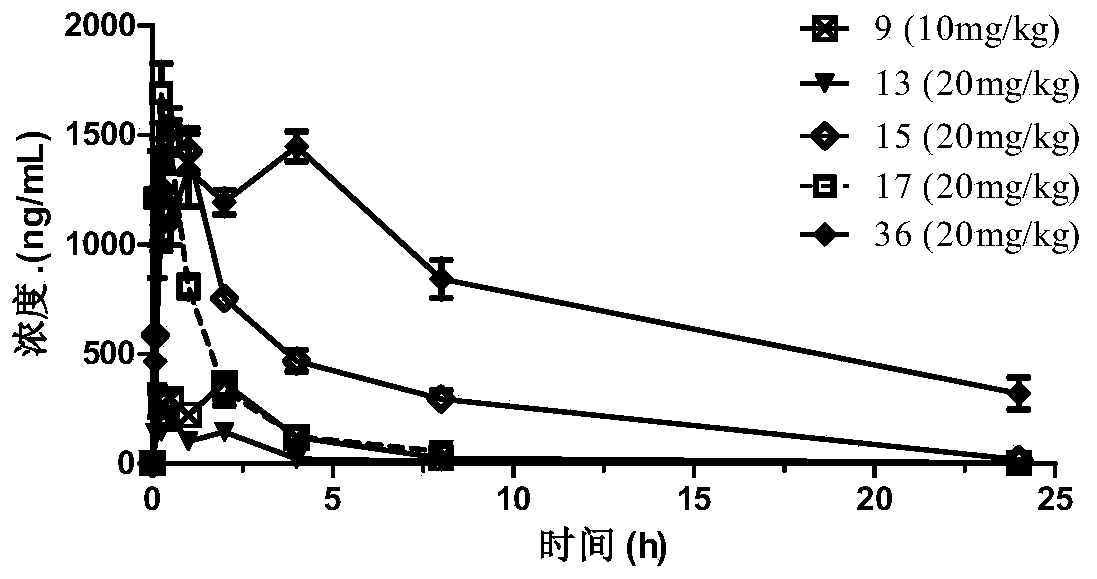
ActiveCN110872277AHas Chk1 inhibitory activityGood treatment effectOrganic chemistryAntineoplastic agentsEfficacyTherapeutic effect
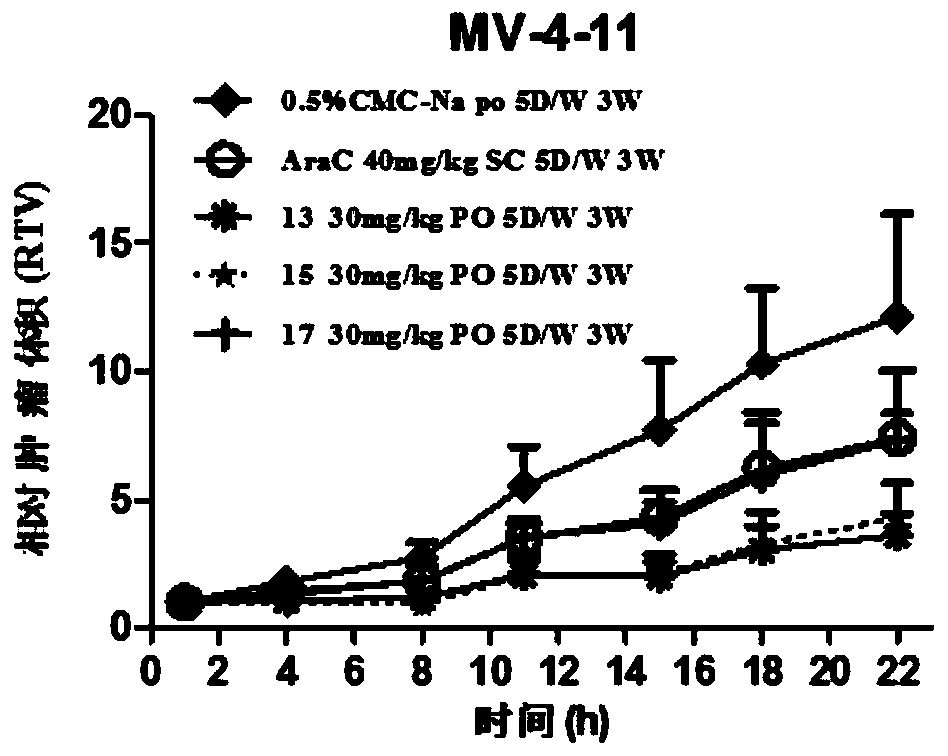
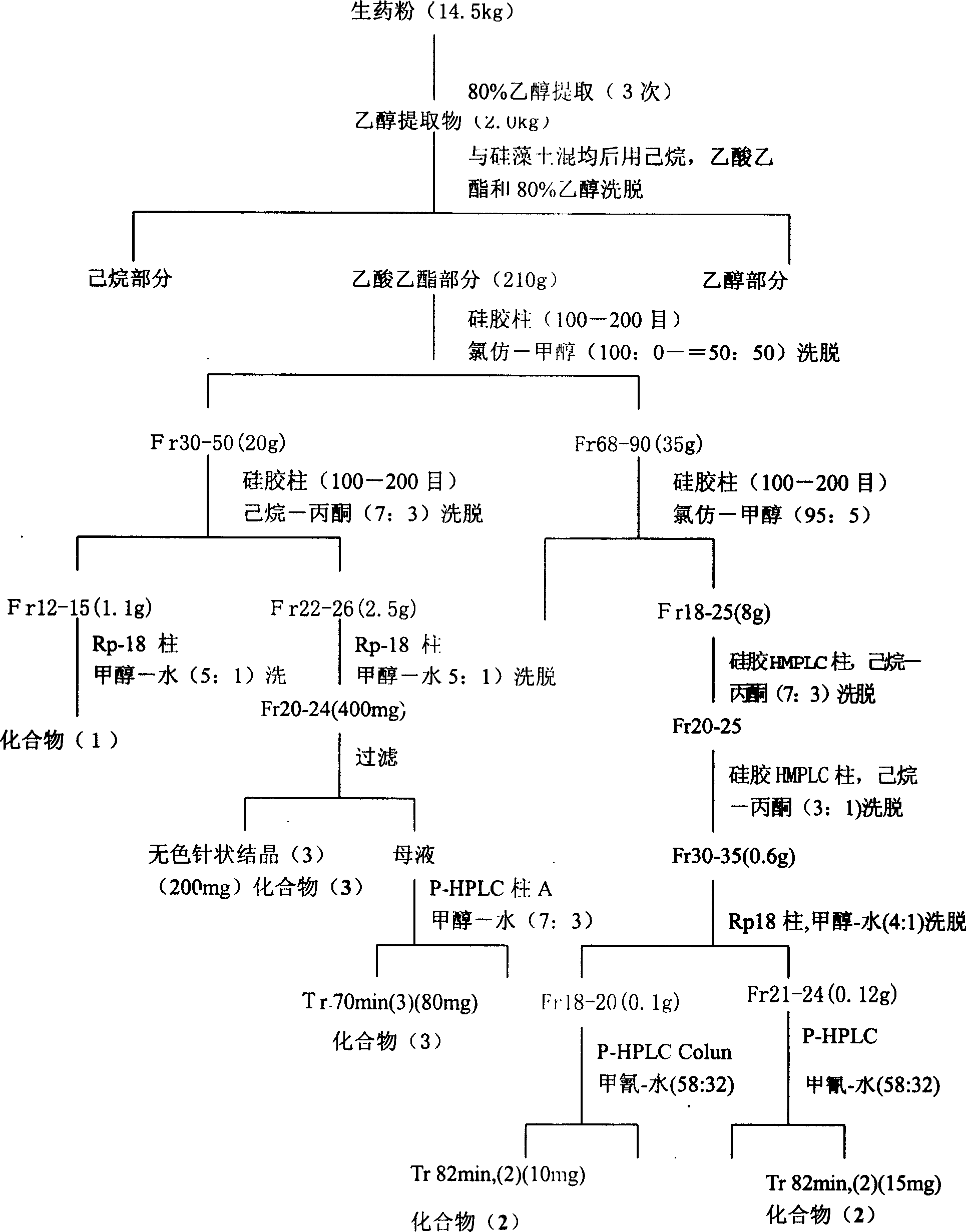
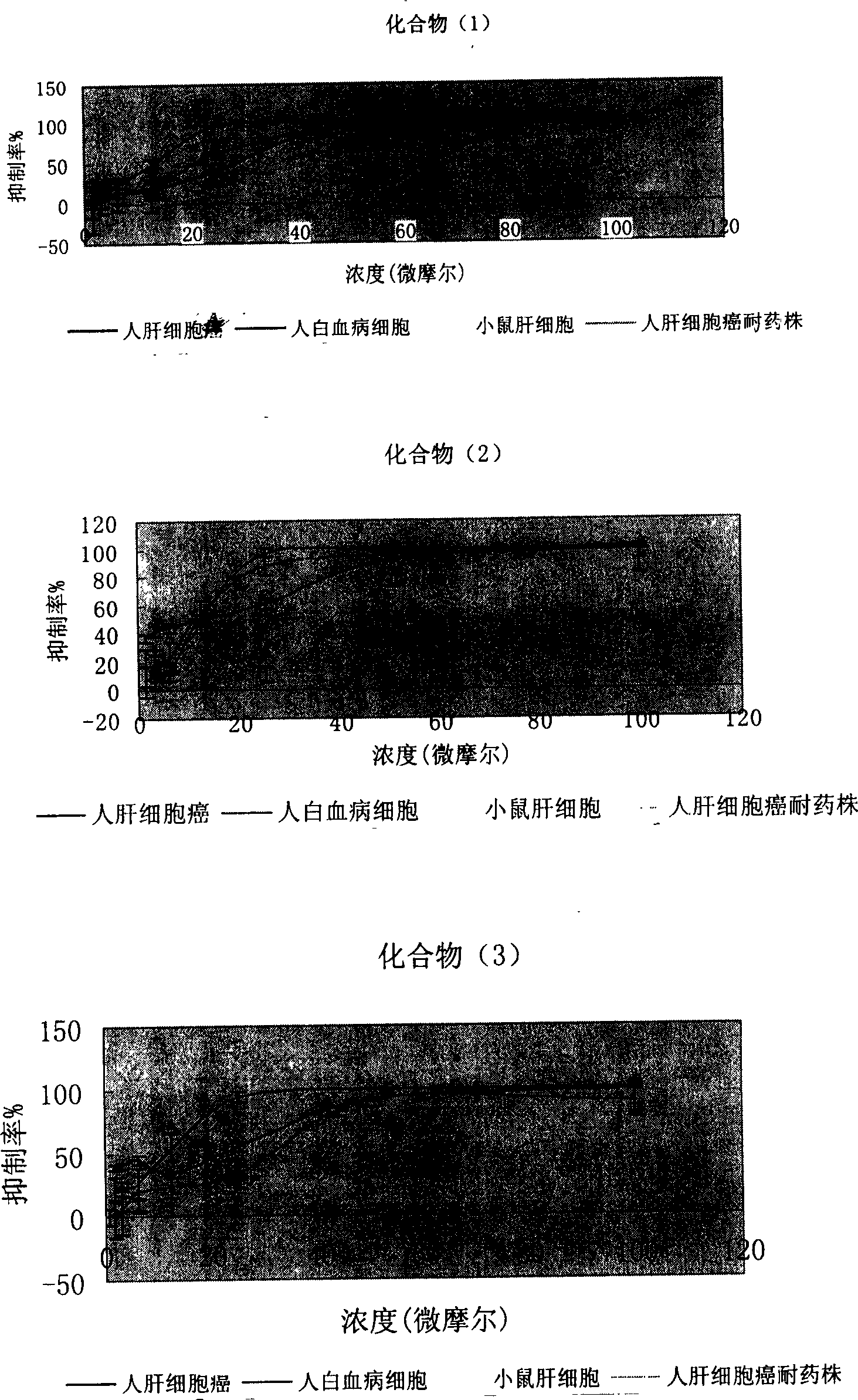
The invention provides an N-subsituted aromatic ring-2-amino pyrimidine compound and applications. The compound includes an optical isomer or a pharmaceutically acceptable salt. A test proves that theN-subsituted aromatic ring-2-amino pyrimidine having a novel framework has excellent CHK1 protein inhibition activity and has significant in-vitro proliferation inhibition effect on MV4-11, Z138 andthe like blood tumor cell strains; meanwhile, the compound also has excellent oral delivery effect. Further in-vivo efficacy test proves that the compound has great treatment effect on human acute myelogenous leukemia MV-4-11 Ba1b / c mouse transplantation tumor and has great treatment effect on tumor. The compound is reasonable in synthesis route design, is prepared from feasible raw materials andgentle reaction conditions, is high in yield in every step and is simple in preparation operation, and is suitable in industrial production. The N-subsituted aromatic ring-2-amino pyrimidine compoundhas a structural general formula as the specification.
Owner:ZHEJIANG UNIV +1
Anti-tumor effective part of traditional Chinese medicine lespedeza as well as preparation method and application thereof
The invention relates to an anti-tumor effective part of lespedeza extract and a preparation method thereof. The effective part is selected from one or the combination of a part A, an ethyl acetate part B, a normal butyl alcohol part C and a part D, wherein the part A is obtained by precipitating water solution of extracting solution extracted from lespedeza root under reflux of ethanol after ethanol recovery, the ethyl acetate part B is obtained by extracting the water solution with ethyl acetate, the normal butyl alcohol part C is obtained by extracting the water solution extracted by ethyl acetate with normal butyl alcohol, and the part D is finally obtained by concentrating and drying the treated water solution. Researches prove that the part can treat blood tumor, hysteromyoma, breast cancer and oophoroma. Therefore, the effective part can be used for preparing anti-tumor medicaments.
Owner:安徽省食品药品检验研究院 +1
Antineoplastic effect of a group of cycloart-one triterpene compound
InactiveCN1524532AStrong killing effectGood at scavenging free radicalsOrganic active ingredientsPharmaceutical delivery mechanismTriterpeneTriterpenoid
The invention relates to a the antineoplastic action of a group of looped pineapple confectionery terpenoid, which is three 9, 19 looped lanolinum alkyl triterpenoid (23-oxygen-acetyl cimicifugol - 3 -oxygen - beta - D - xyloside, 24 - oxygen - acetyl cimicifugol - 3 -oxygen-beta-D-xyloside and 25-oxygen-acetyl cimicifugol - 3 -oxygen - beta - D - xyloside) extracted from cimicifuga rhizome. Experimental investigation has shown that they have cell toxic action for blood tumor and entity tumor.
Owner:肖培根 +1
Chimeric antigen receptor capable of secreting bispecific antibody as well as expression vector and application thereof
ActiveCN110981972AEfficient killingAvoid immune escapePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Single-Chain Antibodies
The invention discloses a chimeric antigen receptor capable of secreting a bispecific antibody as well as an expression vector and an application thereof, which belong to the field of tumor immune drugs. The chimeric antigen receptor comprises a signal peptide, a CD19-targeting single-chain antibody, a lengthened CD8 alpha hinge region, a transmembrane region, a costimulatory factor, an intracellular signal peptide, a P2A connecting peptide, an IL2 signal peptide, a CD20-targeting single-chain antibody, a CD3-targeting single-chain antibody and a tag protein which are connected in sequence. The chimeric antigen receptor is the bispecific antibody capable of specifically targeting CD19 positive tumor cells and secreting targeting CD20 and targeting CD3 at the same time, the non-conductive Tcells can be targeted and activated, so that the tumor antigen CD20 is targeted, and the purpose that the CAR-T cells effectively kill malignant blood tumor cells of a B cell line in vivo is achieved. Meanwhile, the T cells of the chimeric antigen receptor can prevent CD19 positive tumor cells with low abundance expression from generating immune escape, so that the recurrence risk of malignant blood tumors of a B cell line is reduced.
Owner:华夏源细胞工程集团股份有限公司
Medicine composition for treating tumors and application thereof
ActiveCN103933044AImprove tumor treatment effectLow toxicityOrganic active ingredientsAntineoplastic agentsTumor therapyPharmaceutical medicine
The invention provides a medicine composition for treating tumors and an application thereof. The medicine composition is composed of acriflavine or pharmaceutically acceptable salts thereof, melphalan or pharmaceutically acceptable salts thereof, and a medicinal carrier, wherein the molar ratio of the acriflavine to the melphalan is 1:100 to 1:10, and the tumors comprise blood tumors and solid tumors. The forms of the medicine comprise single preparations prepared from acriflavine or melphalan respectively, or a compound preparation prepared from acriflavine and melphalan. The medicine composition provided by the invention has the effects of improving the curative effect on tumors, the toxicity of the medicine composition can be reduced, and the growth speeds of the tumors can be slowed down.
Owner:ZHEJIANG UNIV
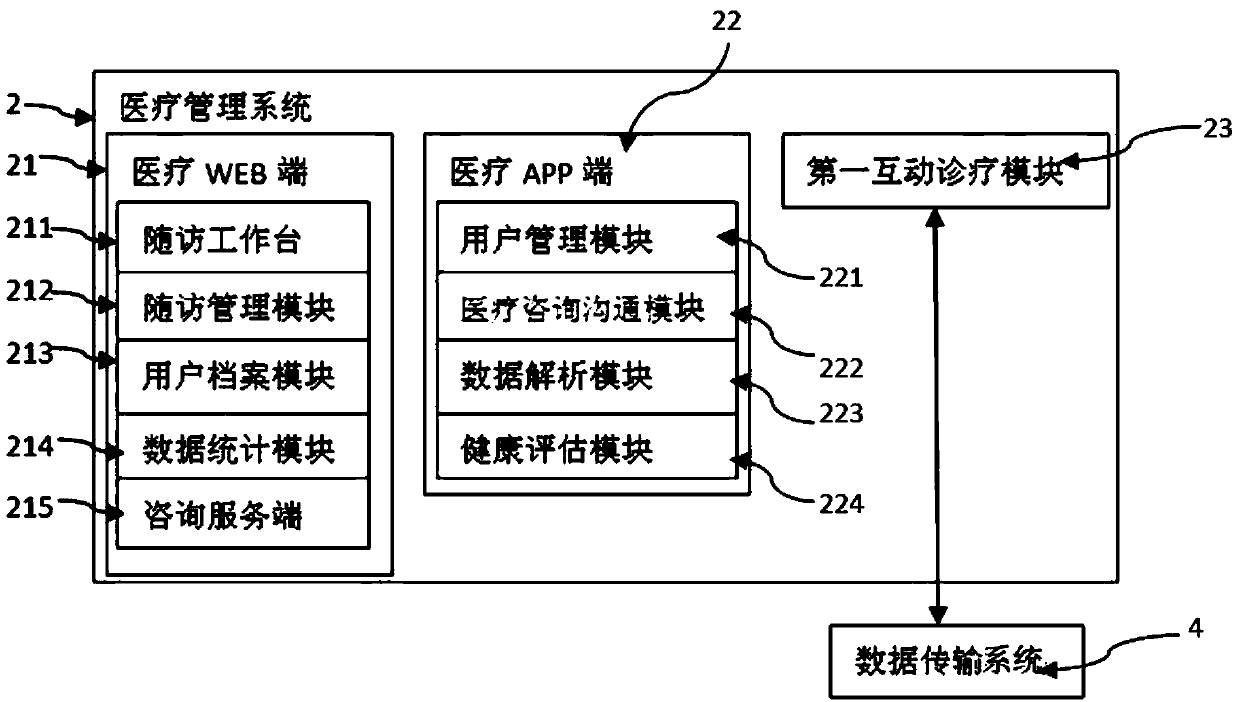
Blood tumor data acquisition and in-hospital and out-of-hospital integrated diagnosis and treatment support system and method
PendingCN110085304AImprove collection efficiencyHigh precisionMedical communicationEpidemiological alert systemsDiseaseModern medicine
The invention discloses a blood tumor data acquisition and in-hospital and out-of-hospital integrated diagnosis and treatment support system. The blood tumor data acquisition and in-hospital and out-of-hospital integrated diagnosis and treatment support system comprises a cloud follow-up visit platform, a medical management end system, a data transmission system and a user end system, wherein thecloud follow-up visit platform is connected with the medical management system and the client system through the data transmission system; and the medical management system is connected with the client system through the data transmission system. The blood tumor data acquisition and in-hospital and out-of-hospital integrated diagnosis and treatment support system has the advantages that the systemestablishes a data analysis system based on index data to form a rigorous diagnosis and treatment scheme; doctor-patient joint interaction is achieved, and the doctor-patient trust degree is improved; the intelligent reminding service effectively enhances the diagnosis and treatment executive force; professional follow-up visit management effectively improves data collection efficiency, accuracyand availability, promotes national blood disease prevention and management quality, and improves national health quality of China; and change rules of patients and diseases are comprehensively understood through data, and experience is summarized, and a treatment scheme is adjusted or improved, and the treatment effect and extrahospital life quality of the patients are improved, and development of modern medicine of the hematology department is promoted.
Owner:SHANGHAI BEISHENG MEDICAL EQUIP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com