Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
8076 results about "Guide wires" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
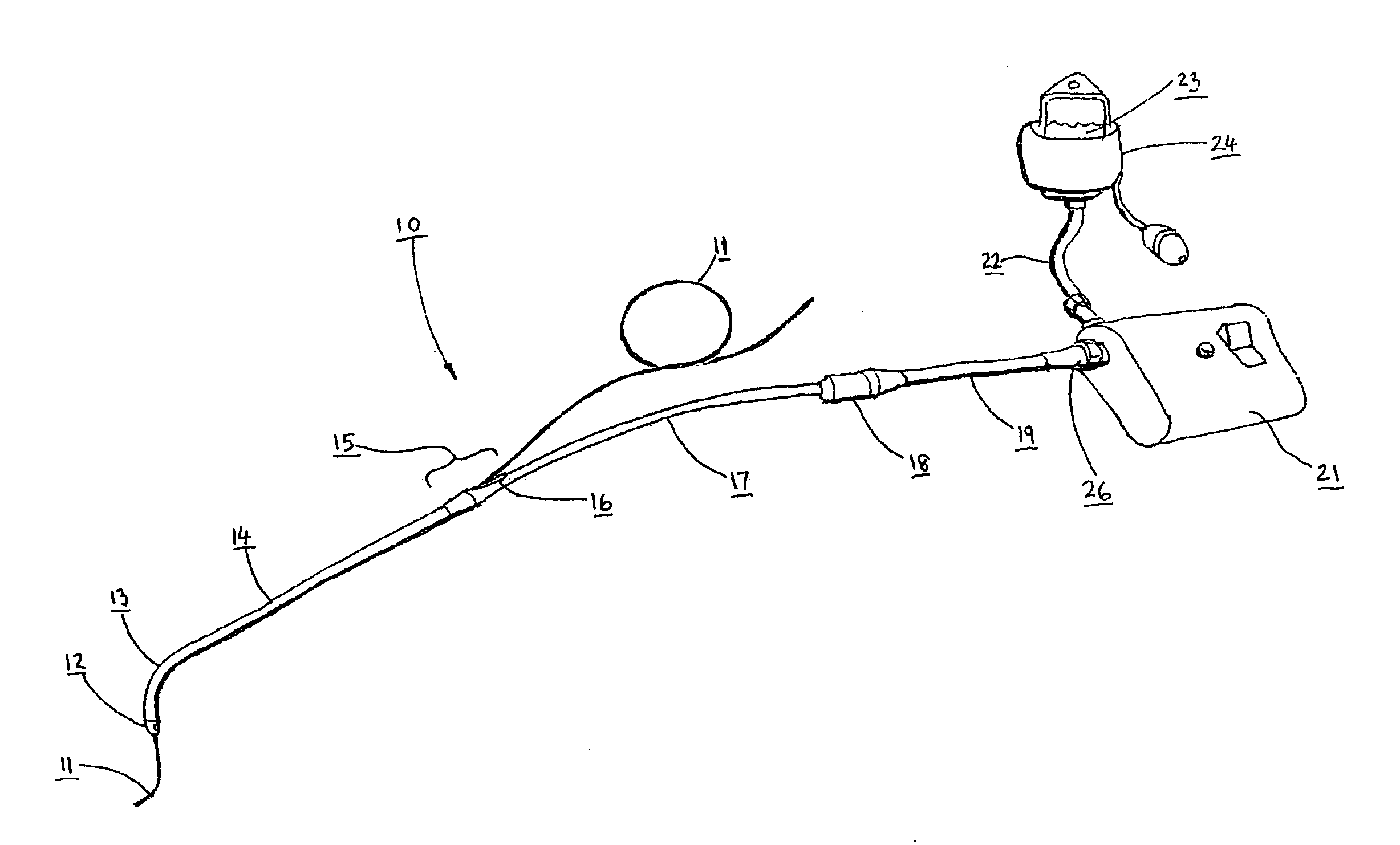
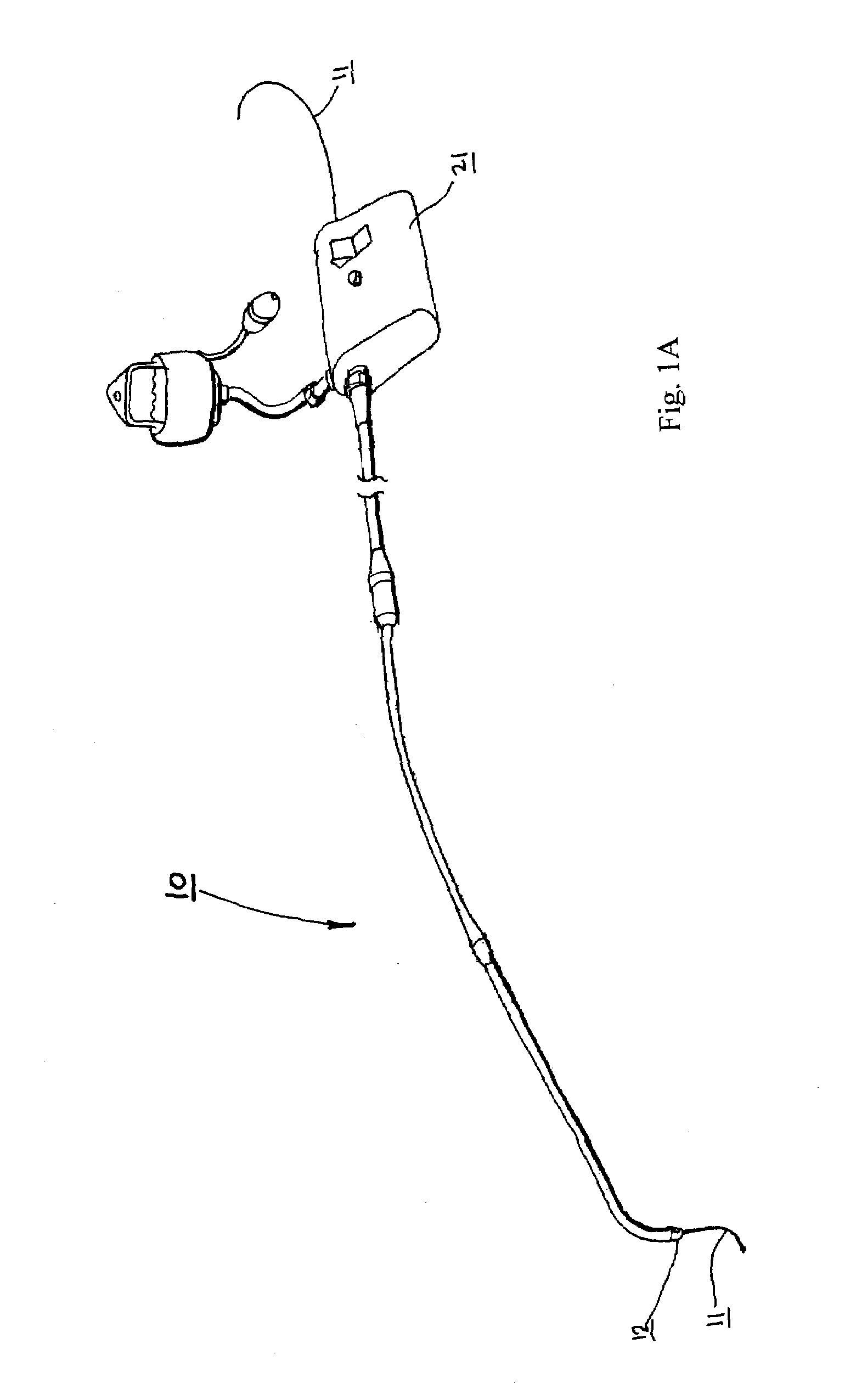
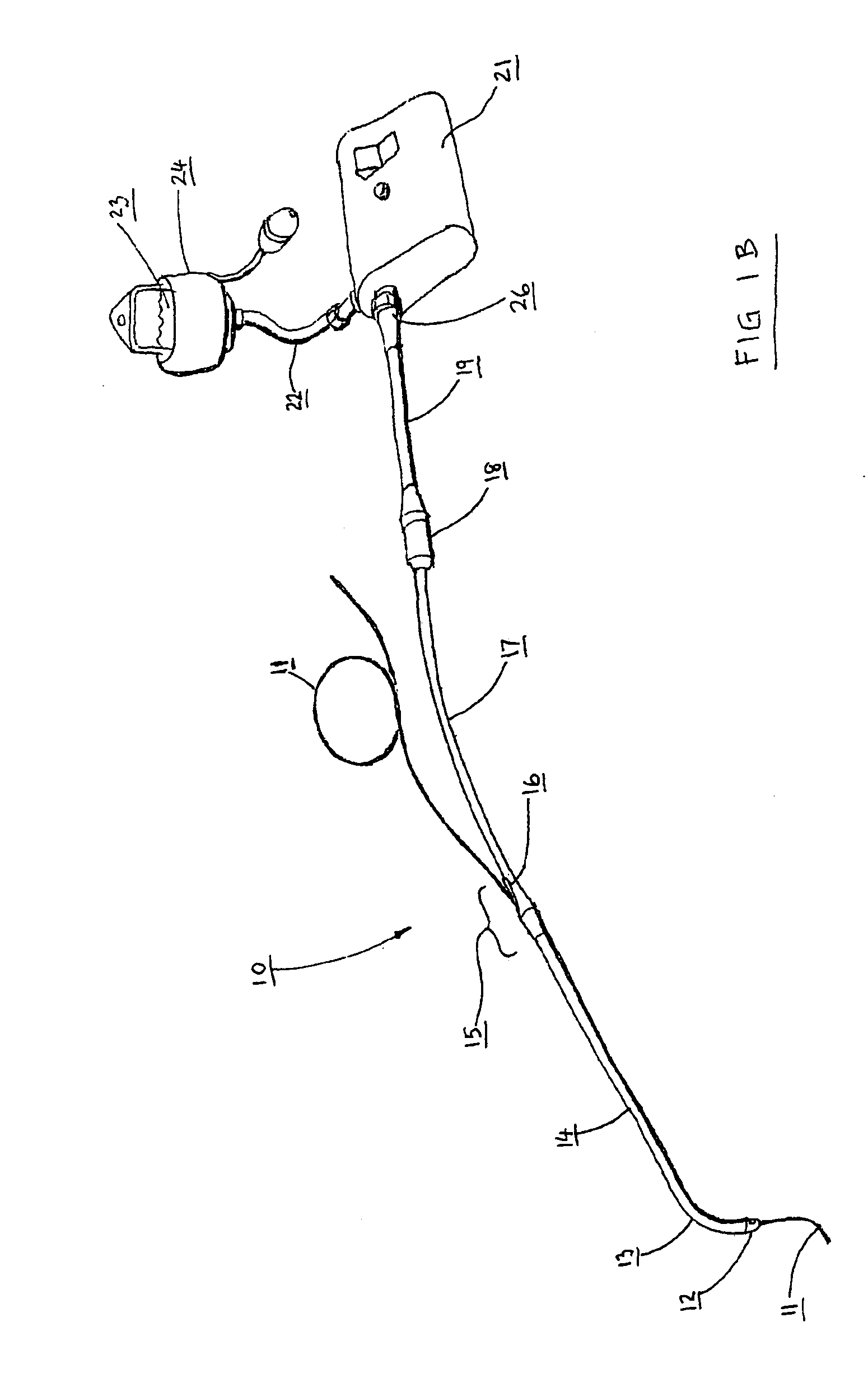
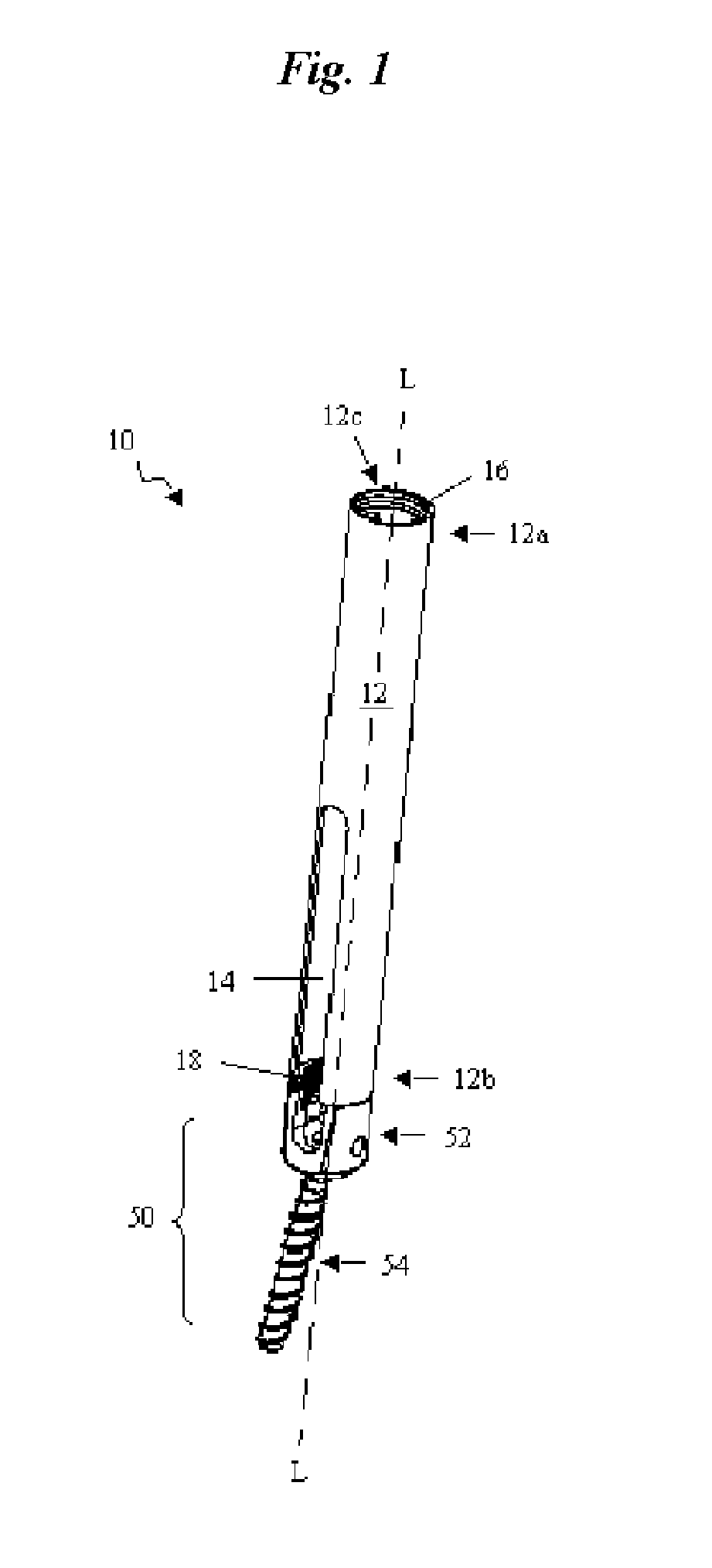
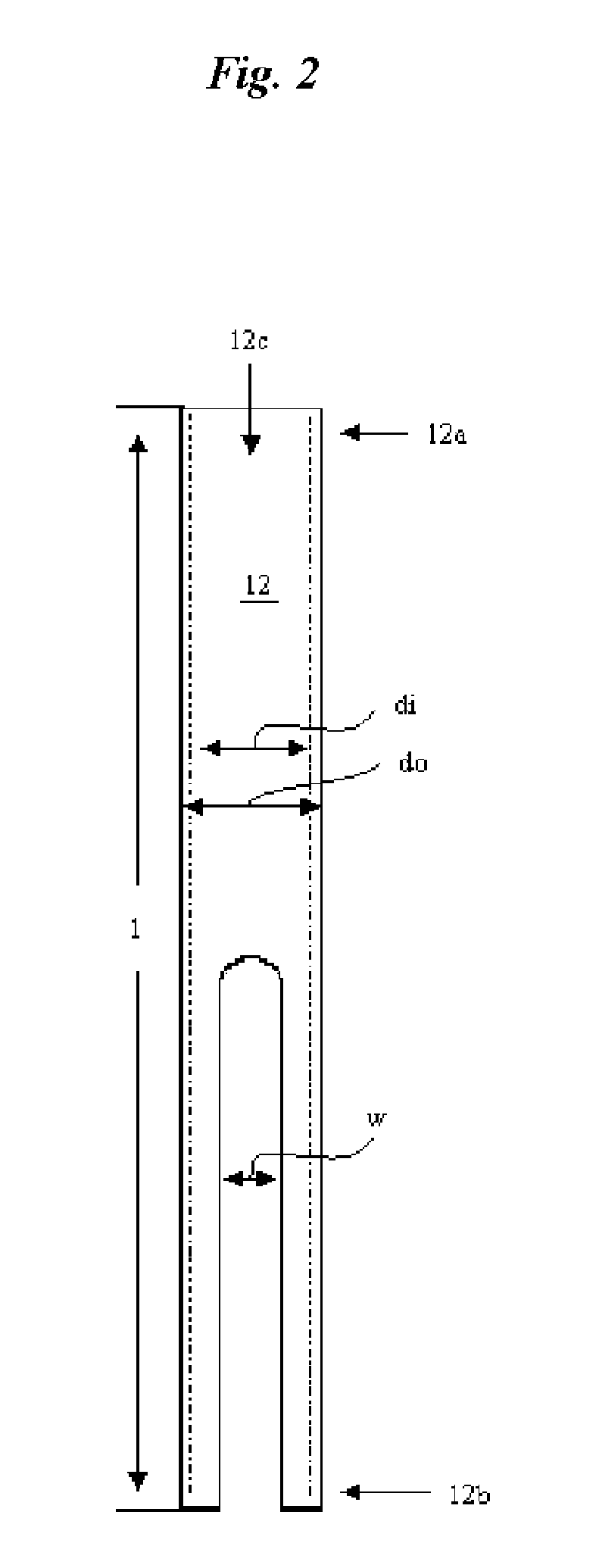
Catheter for conducting a procedure within a lumen, duct or organ of a living being
In an embodiment, the invention provides a catheter suitable for use in performing a procedure within a vessel, lumen or organ of a living having a distal end which is steerable, such as upon the application of compression. The catheter may be of the over the wire type, or alternatively may be a rapid exchange catheter. The catheter may provide for a rotating element which may be used to open a clogged vessel, or alternatively to provide information about adjacent tissues, such as may be generated by imaging or guiding arrangements using tissue detection systems known in the art, e.g., ultrasound, optical coherence reflectometry, etc. For rapid exchange catheters having a rotating element, there is provided an offset drive assembly to allow the rotary force to be directed from alongside the guidewire to a location coaxial to and over the guidewire.
Owner:KENSEY NASH CORP
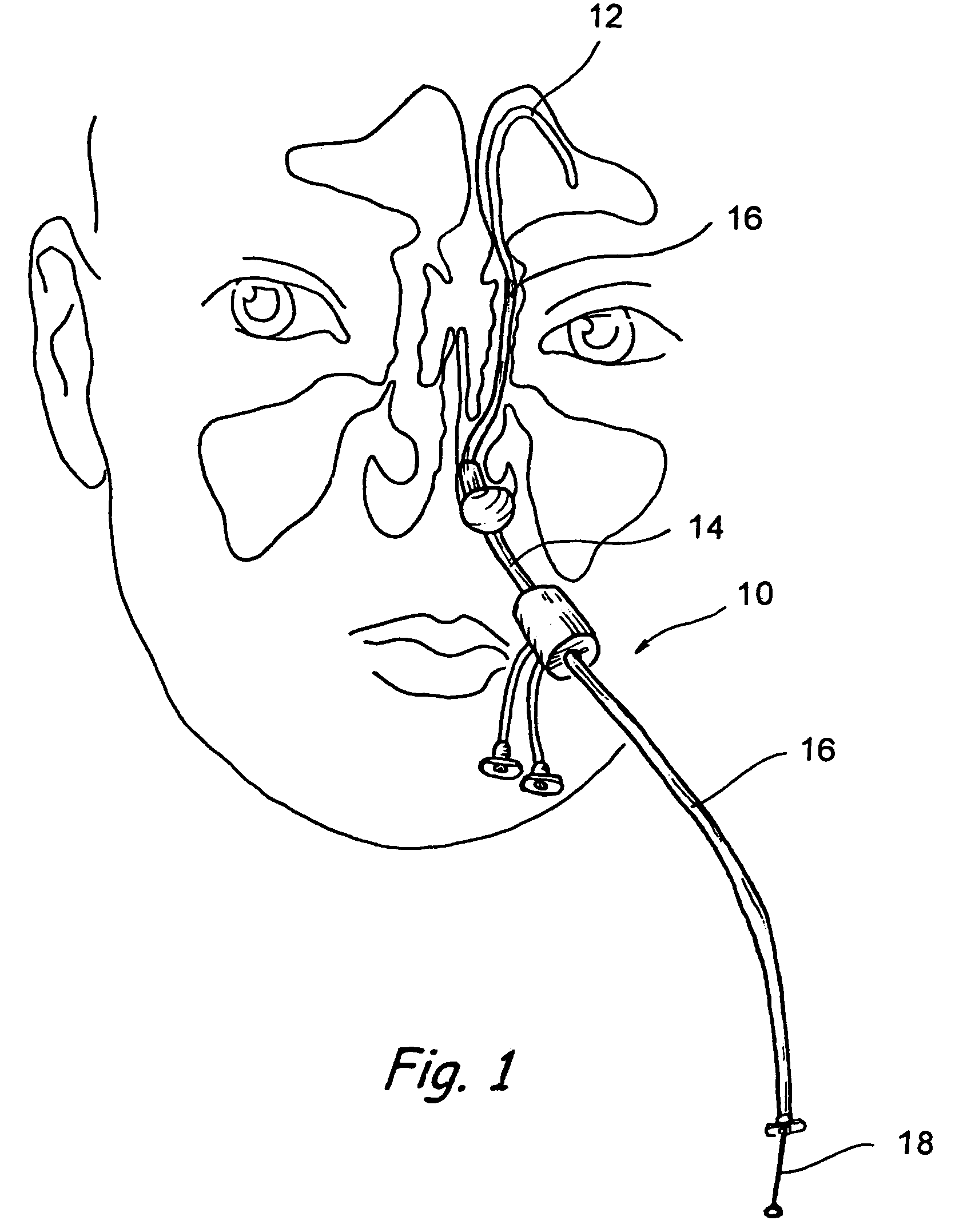
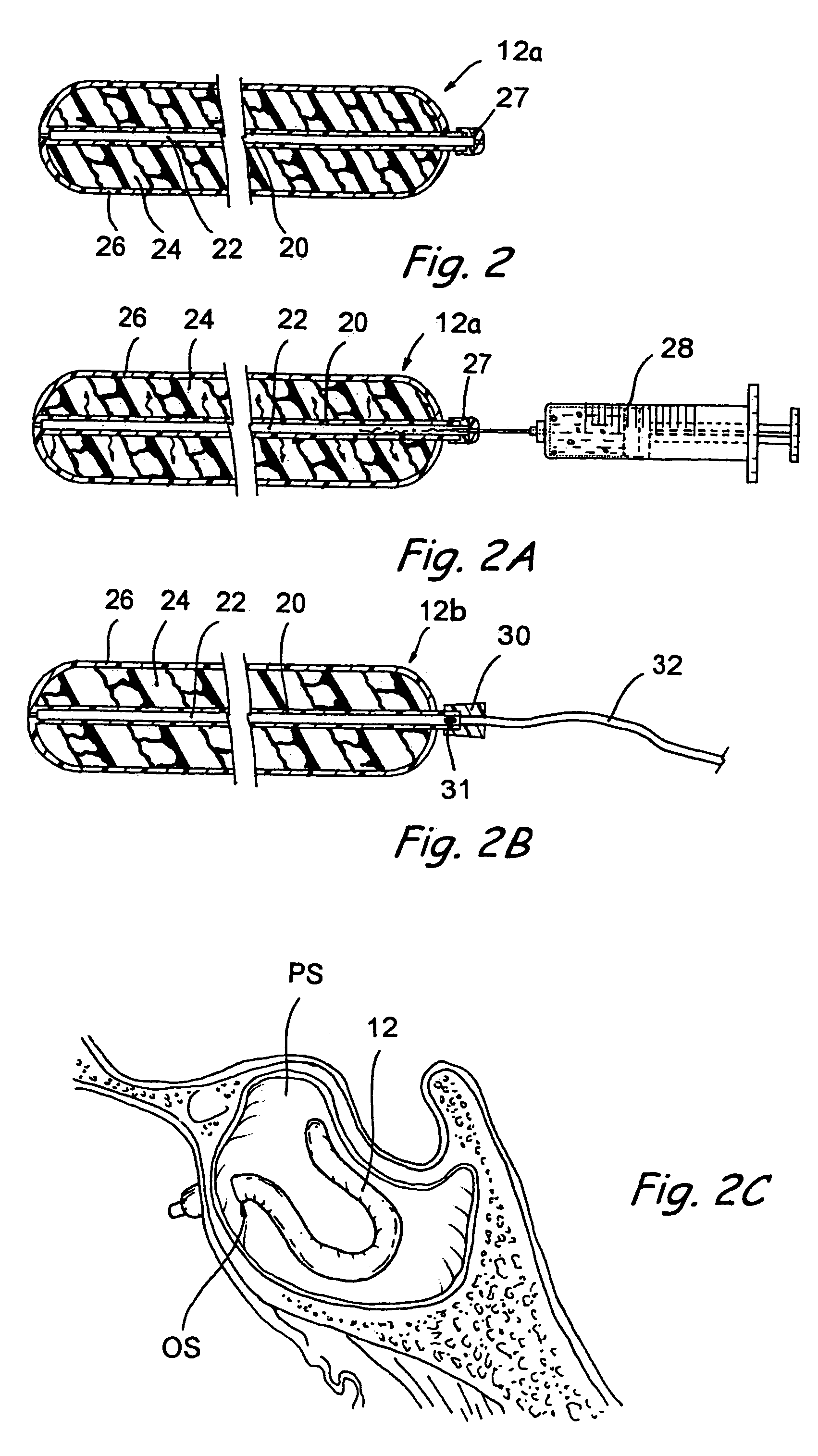
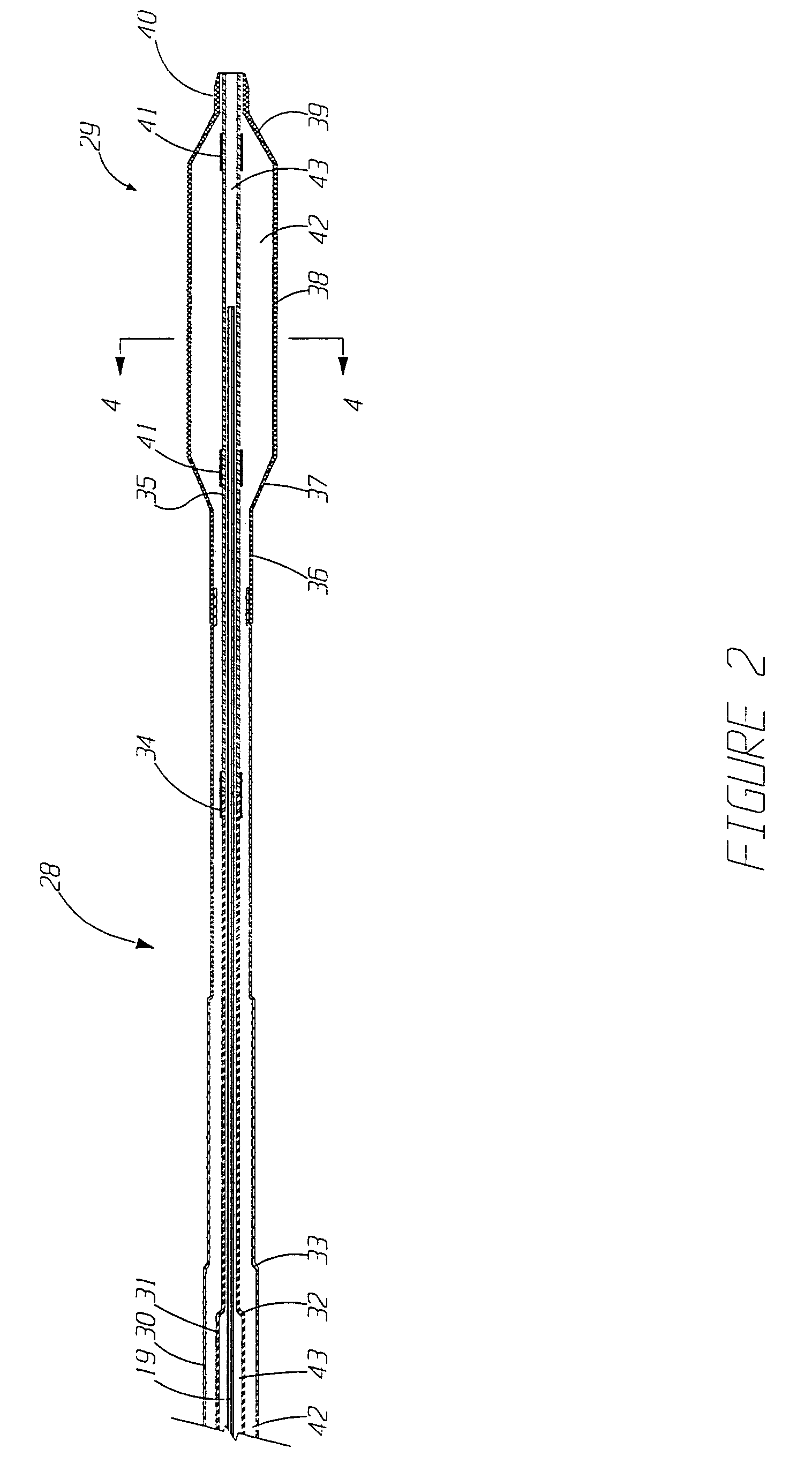
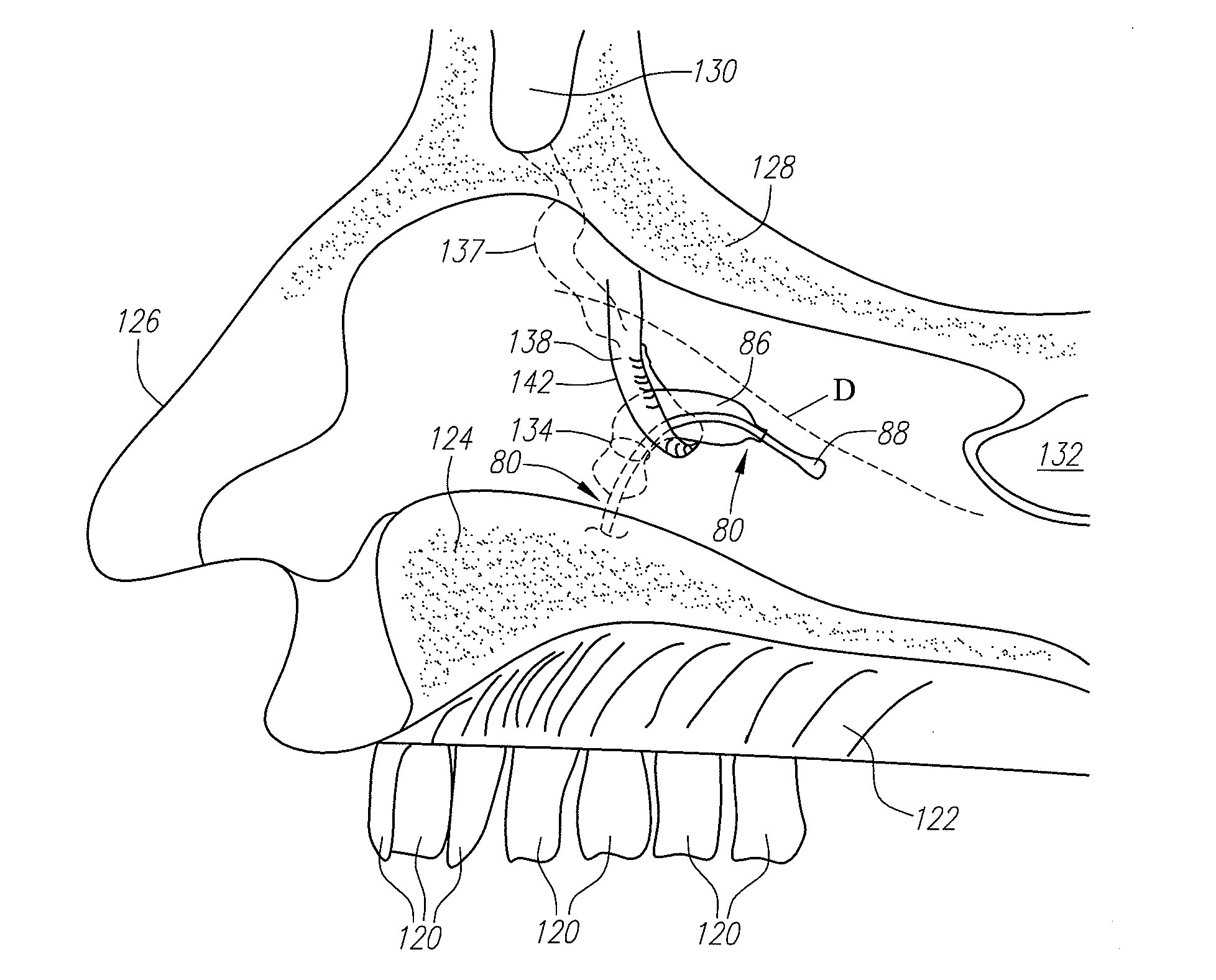
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
Stent-valves for valve replacement and associated methods and systems for surgery
InactiveUS20070213813A1Simple methodReduce riskStentsBalloon catheterLess invasive surgeryInsertion stent
Stent-valves (e.g., single-stent-valves and double-stent-valves), associated methods and systems for their delivery via minimally-invasive surgery, and guide-wire compatible closure devices for sealing access orifices are provided.
Owner:SYMETIS
Battery powered surgical tool with guide wire
ActiveUS8974932B2Primary cell to battery groupingInternal osteosythesisElectrical batteryEngineering
A battery pack for a use with a powered surgical tool. The battery pack may include a housing with an outer wall and opposing first and second ends. The housing may include an elongated shape that extends between the first and second ends. A first member may extend across the first end of the housing and include a first aperture, and a second end member may extend across the second end of the housing and may include a second aperture. A passage may extend through the housing with a first end that aligns with the first aperture and a second end that aligns with the second aperture. The housing may be sized for a plurality of storage locations positioned between the first and second members and around the passage, and each of the storage locations may be configured to store a power cell.
Owner:WARSAW ORTHOPEDIC INC
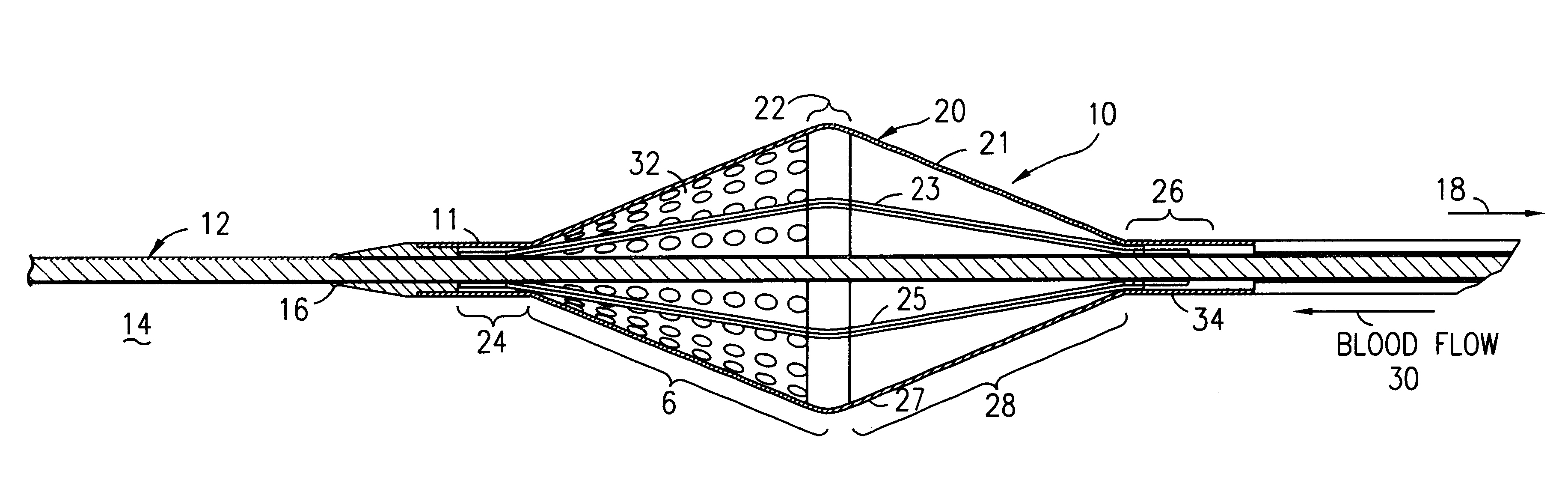
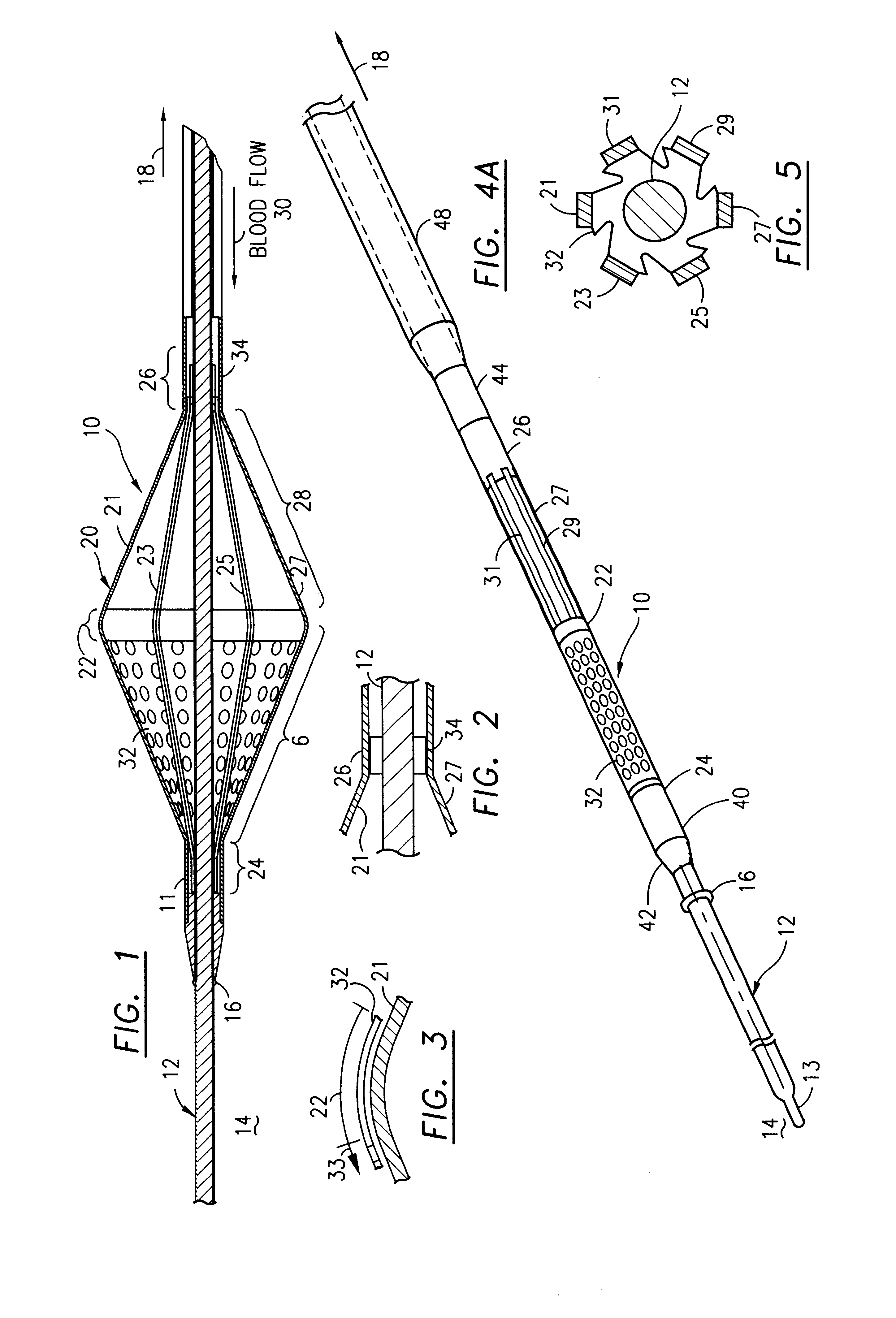
Filter for embolic material mounted on expandable frame
The filter device captures embolic material in a blood vessel and is placed in the blood vessel via a guide wire. The guide wire has a proximal end, a distal end and a stop near its distal end. The filter device includes an expandable frame of frame struts having a closed, radially compact form and an open, radially expanded form. The frame, in the radially expanded form, has frame struts forming a pair of facing frustoconical frame structures. Filter material is attached to one of the pair of frustoconical frame structures. In one embodiment, the filter material is a perforated membrane. The guide wire extends through the expandable frame and the expandable frame is freely movable over the guide wire (likewise, the guide wire is freely movable within the frame), both rotatably and longitudinally, except distally beyond the stop near the distal end of the guide wire. This mobility of the guide wire with respect to the expandable frame enables to guide wire to be guided by the operator through the blood vessel.
Owner:SCION CARDIO VASCULAR
Valve implanting device
ActiveUS6951571B1Easy to replaceIncreased durabilityVenous valvesBlood vesselsImplanted deviceGuide wires
Disclosed is a valve implanting device comprising a collapsible frame, inner and outer guide wires removably connected to the collapsible frame, and a plurality of valve flaps attached to the collapsible frame. The collapsible frame is inserted into a patient's femoral vein or artery, guided to a deployment position using the guide wires, expanded using the guide wires, and stabilized using the guide wires to manipulate fixating hubs on the collapsible frame. The collapsible frame includes a central hub, a plurality of spokes, fixating hubs, gripping members, and a plurality of valve flaps.
Owner:SRIVASTAVA ROHIT
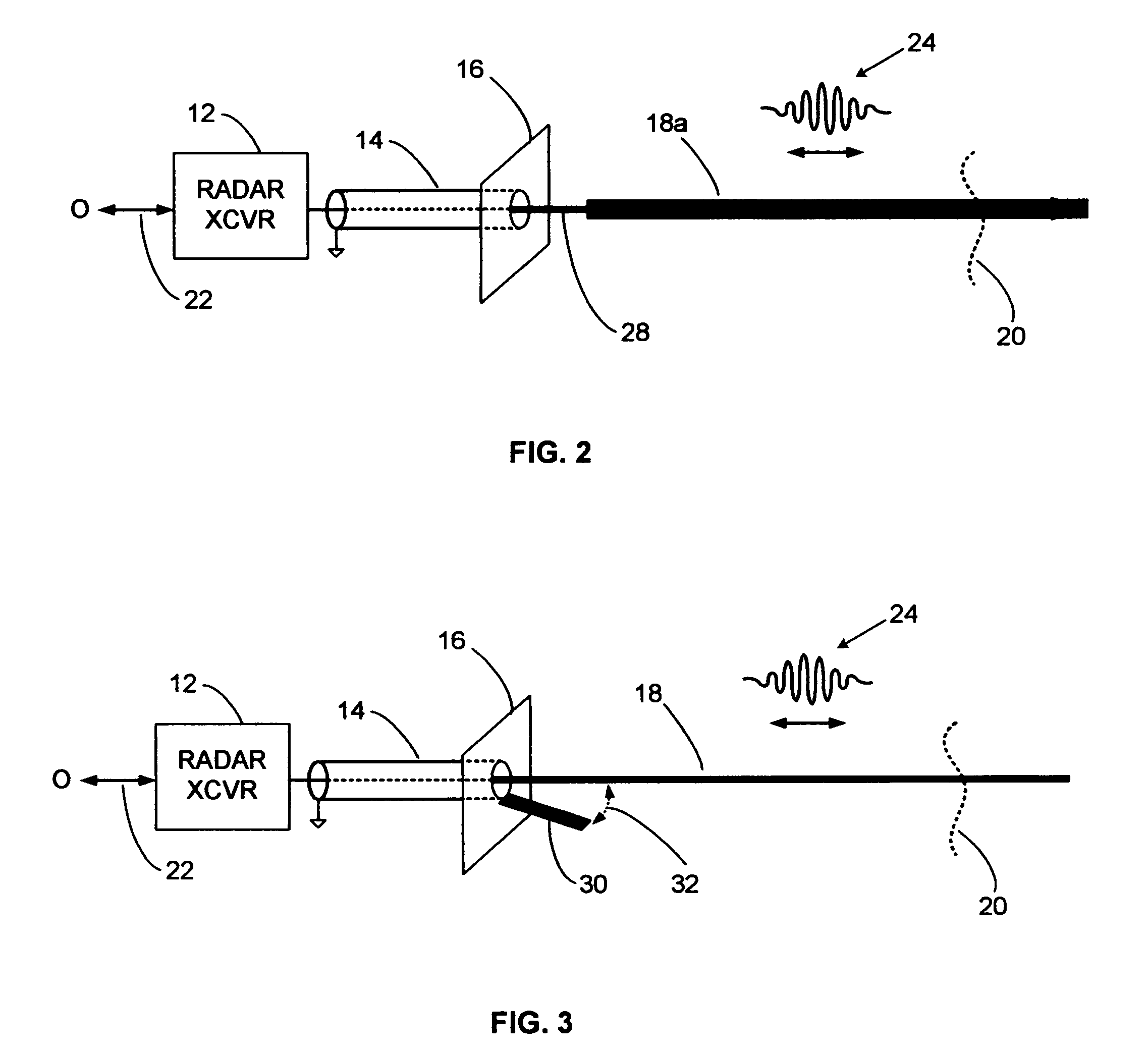
Reflection free launcher for electromagnetic guide wire
InactiveUS7345623B2Easy to measureSmall sizeMachines/enginesLevel indicatorsEngineeringImpedance matching
Guided wave radar (GWR) pulses are launched onto an electromagnetic guide wire using a compact launcher that includes an impedance matching element. The impedance matching element produces reflections that cancel launcher reflections. Short range echoes can be accurately detected after impedance matching. GWR operation in small tanks and in tanks containing low dielectric constant materials, such as propane, can be enhanced with the compact impedance-matched launcher.
Owner:MCEWAN TECH
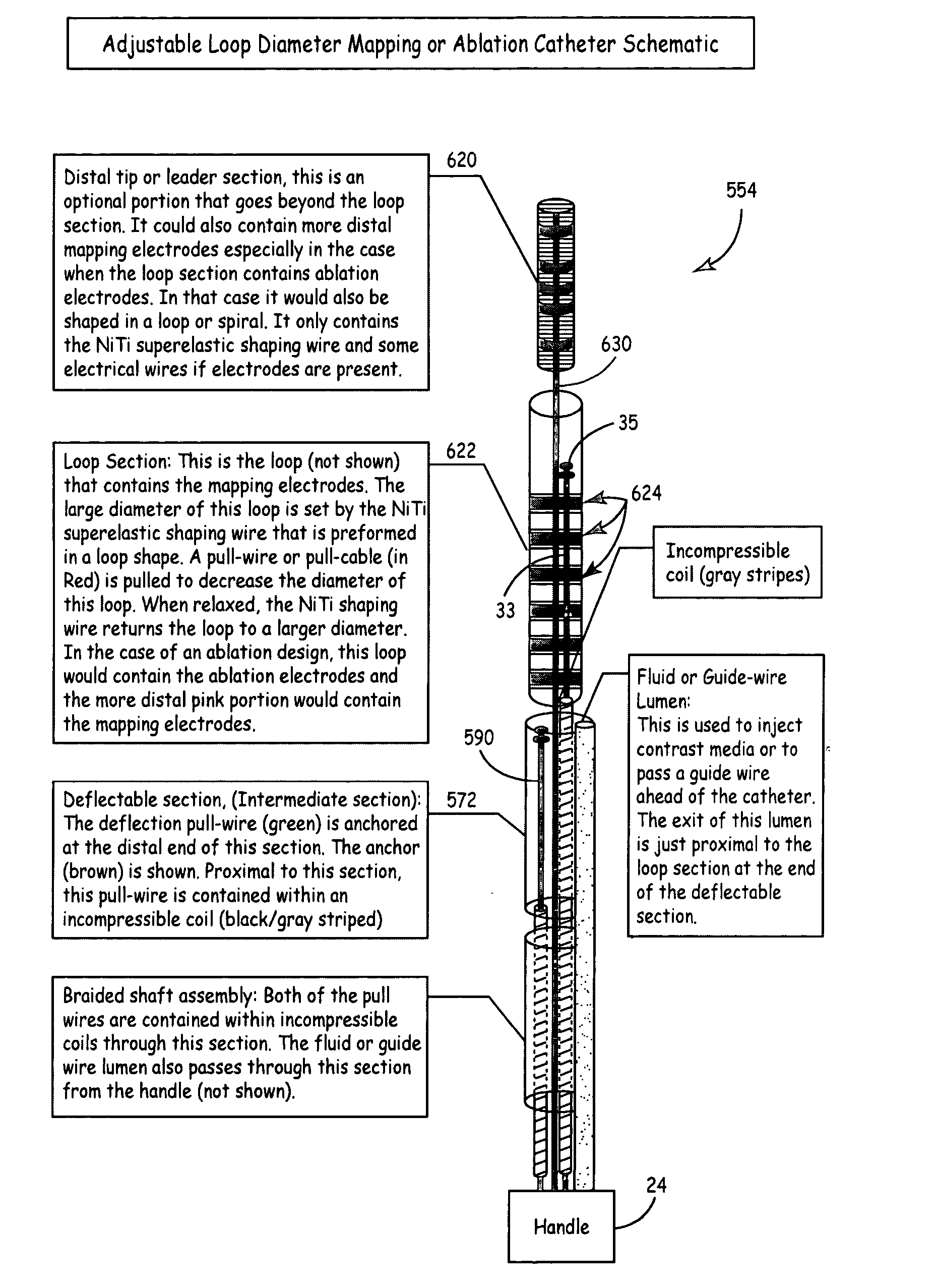
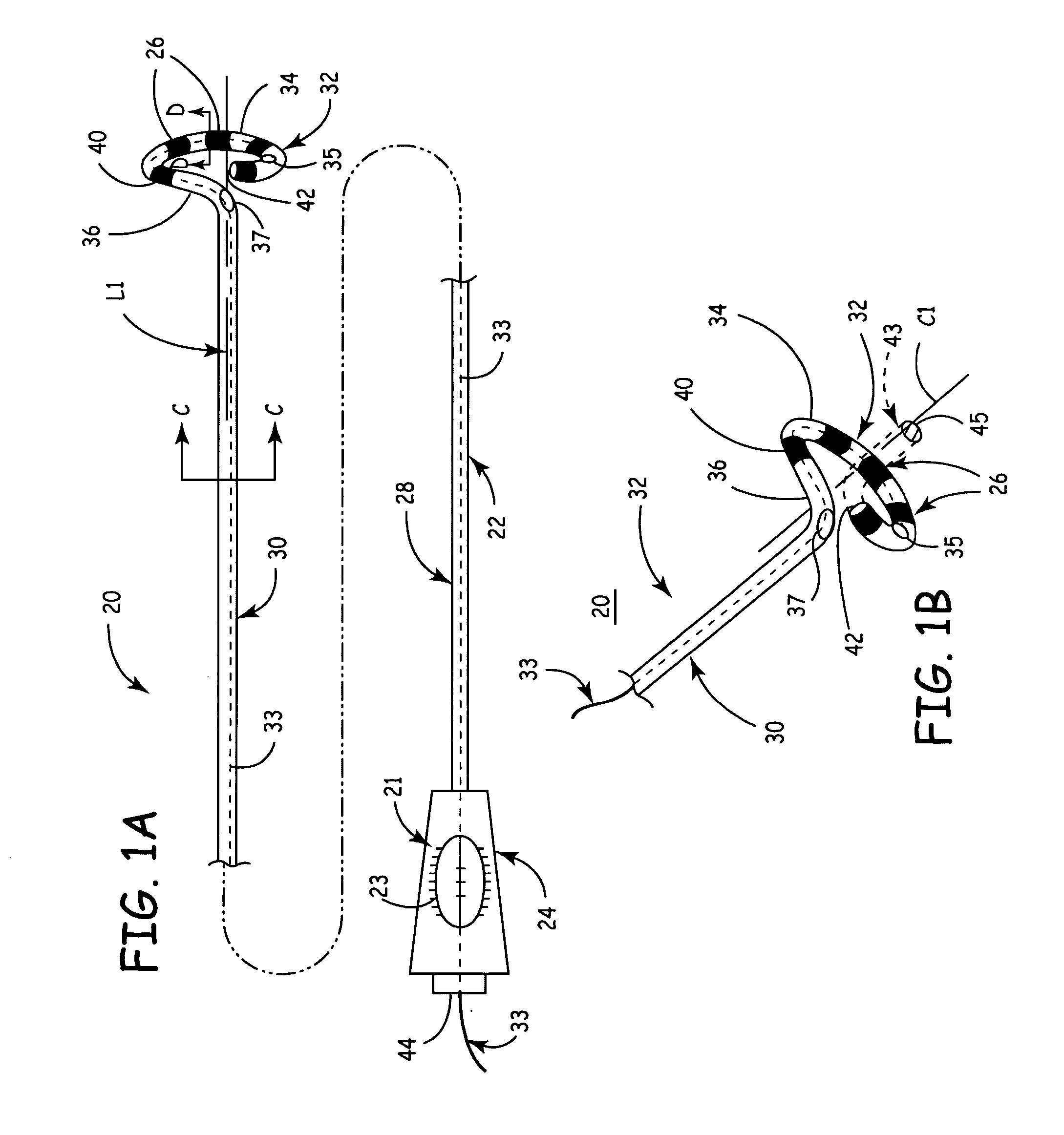
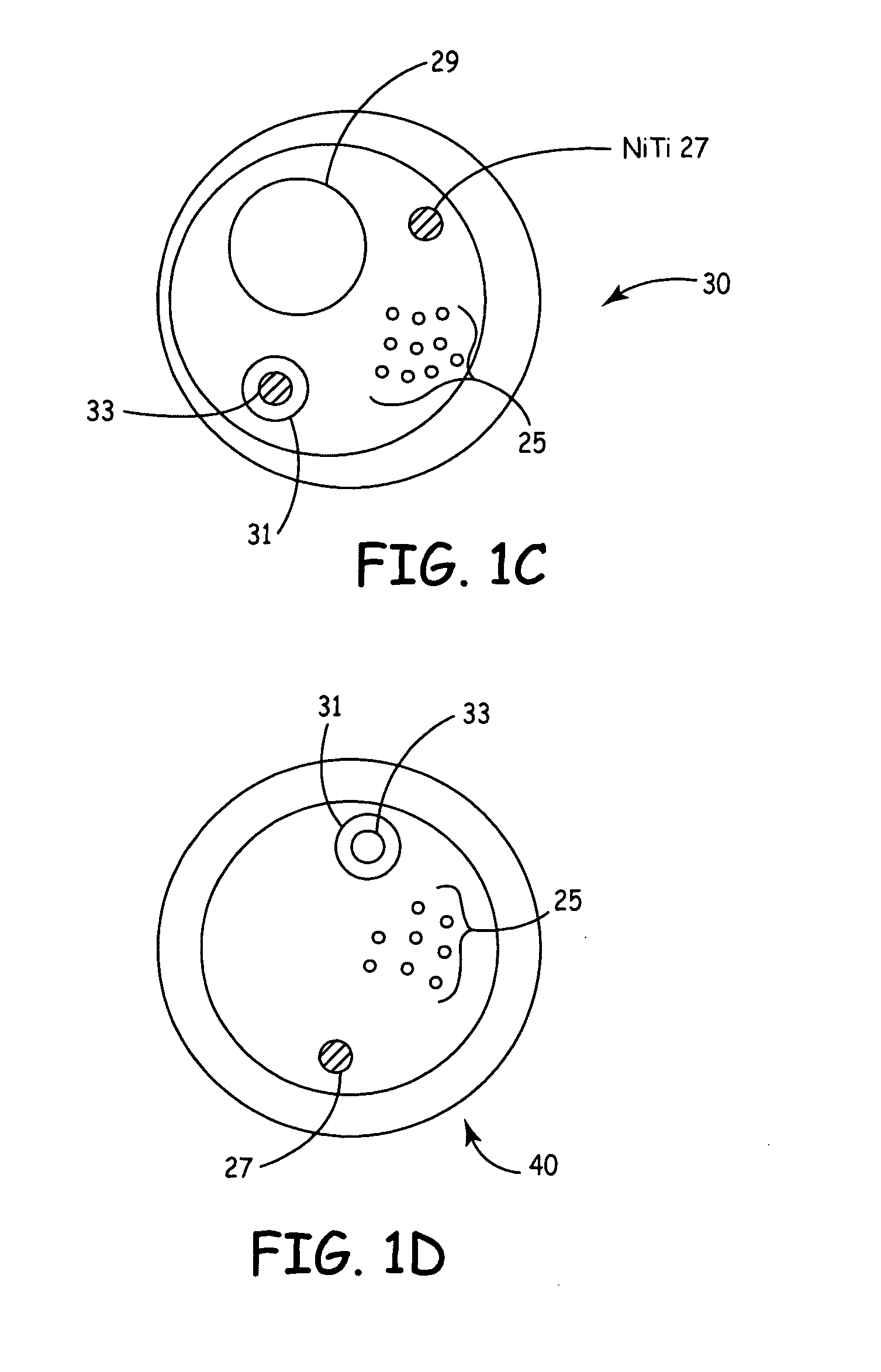
Multi-purpose catheter apparatus and method of use
InactiveUS20050010095A1Easy to adaptEfficiently and accurately deliverElectrocardiographySurgical needlesDistal portionSaline solutions
According to the present invention, a catheter having at least one multi-purpose lumen formed through the catheter terminates proximal a relatively complex-shaped distal portion thereof. In one form of this embodiment, the relatively complex-shaped distal portion comprises a looped portion having diagnostic- and / or ablation-type electrodes coupled thereto and an elongated diameter-adjusting member coupled proximal the distal end of the looped portion. The multi-purpose lumen may be used to alternately accommodate a variety of dedicated materials; such as, (i) a guide wire for initial deployment or later repositioning of the catheter, (ii) a volume or flow of a contrast media and the like, (iii) a deployable hollow needle or tube and the like used to biopsy adjacent tissue or dispense a therapeutic agent into a volume of tissue, and (iv) a cooling fluid, such as saline solution and the like dispensed at least during therapeutic tissue ablation procedures.
Owner:MEDTRONIC INC
Minimally invasive apparatus for implanting a sacral stimulation lead
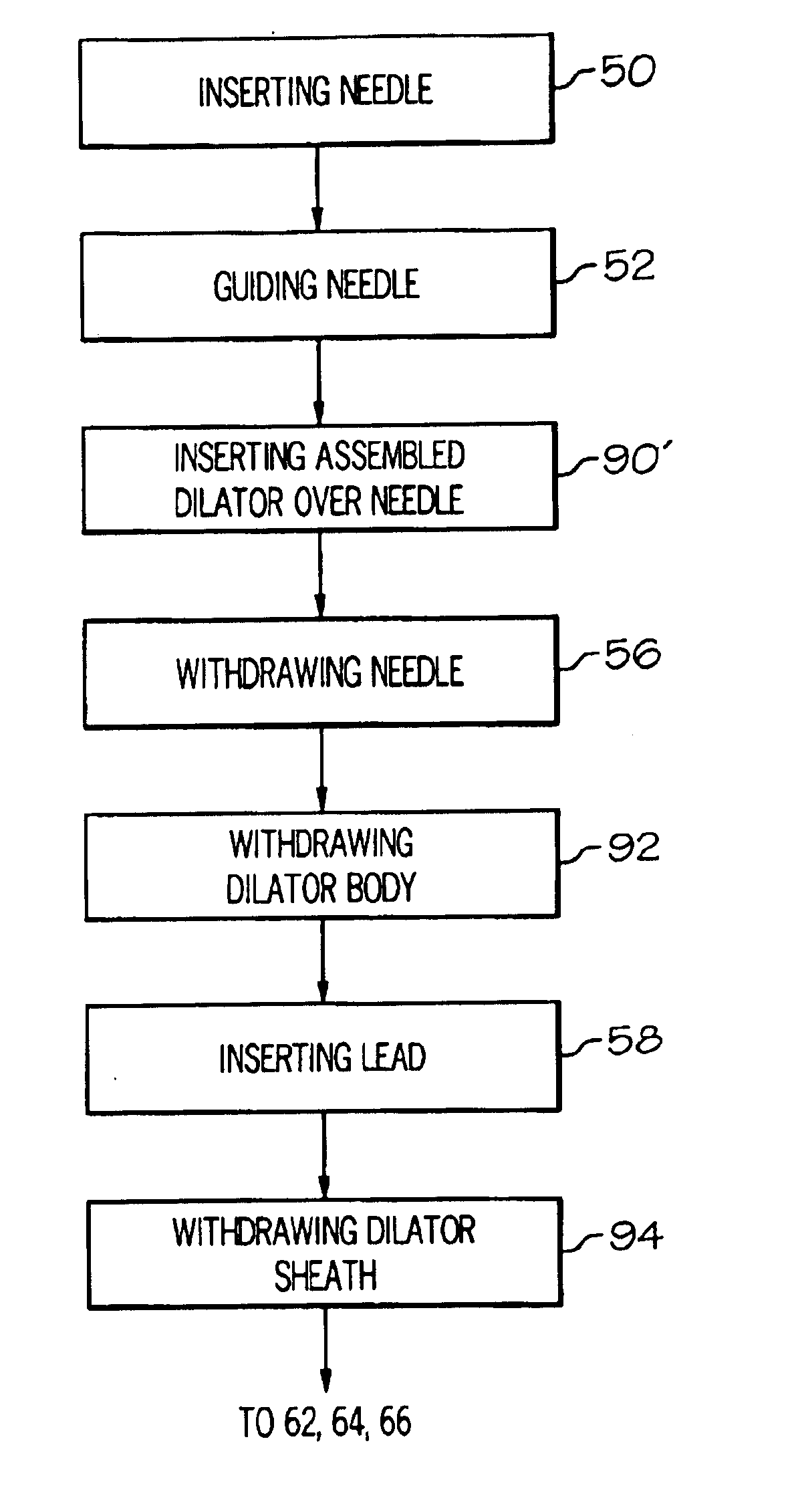
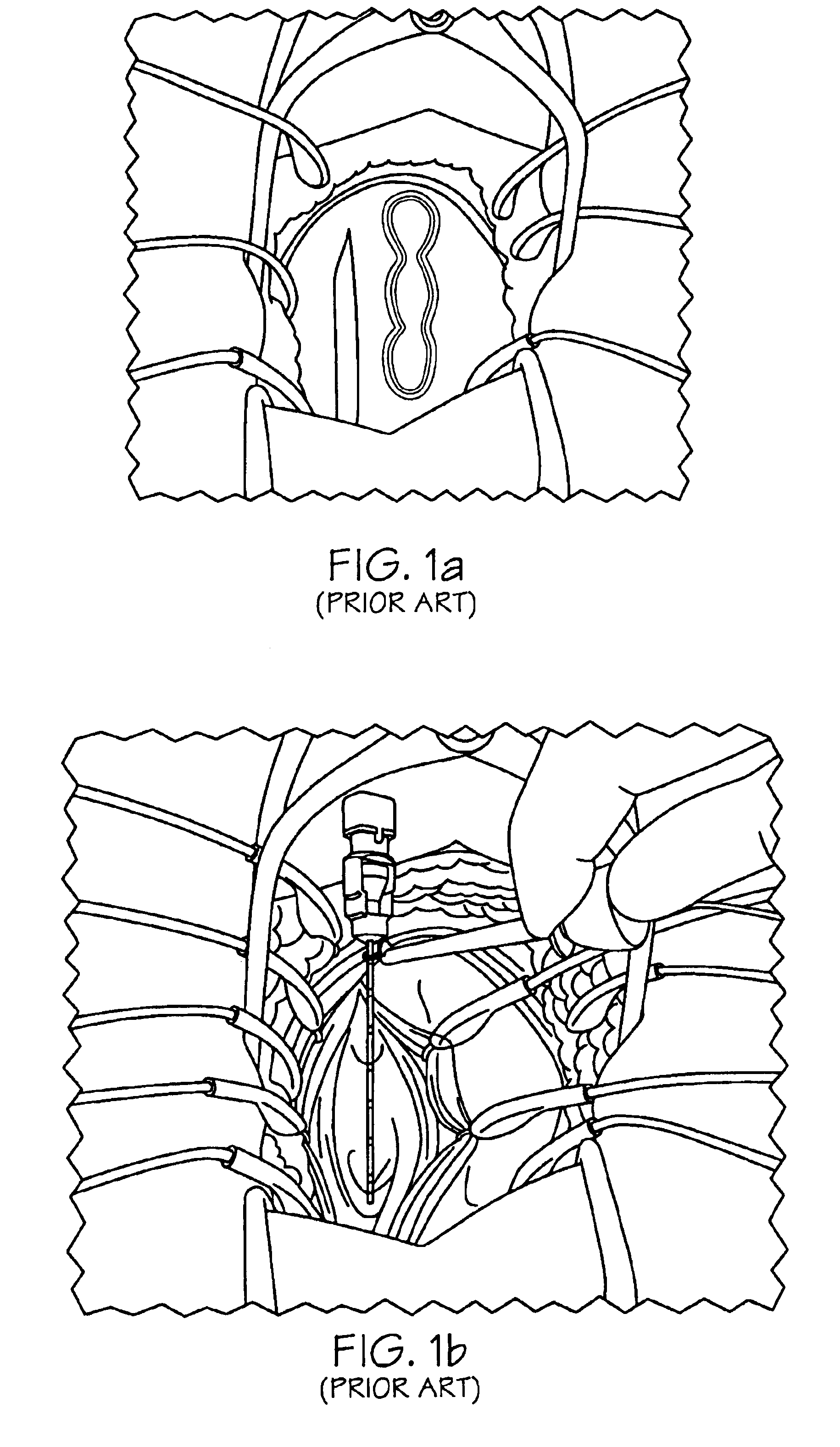
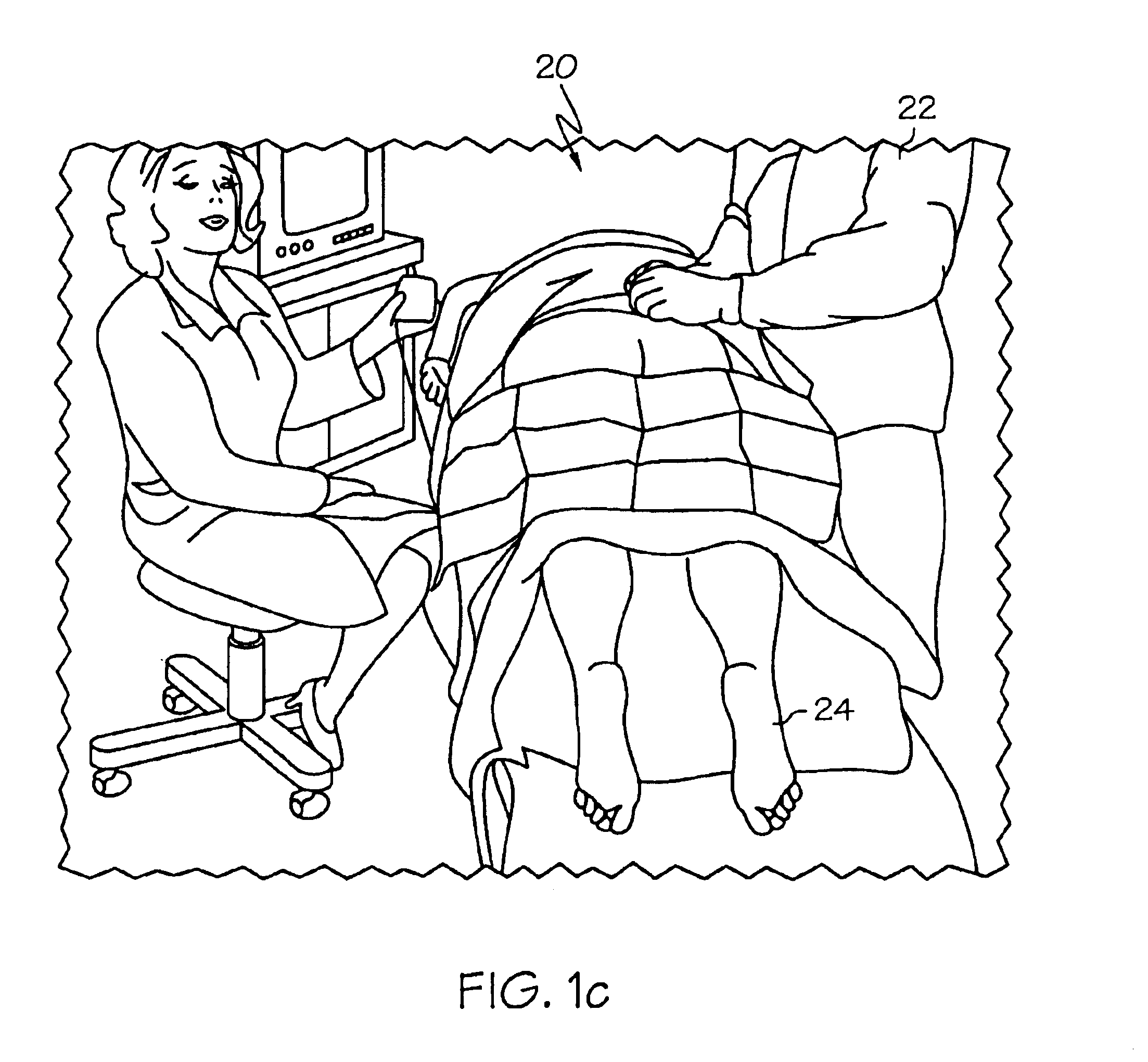
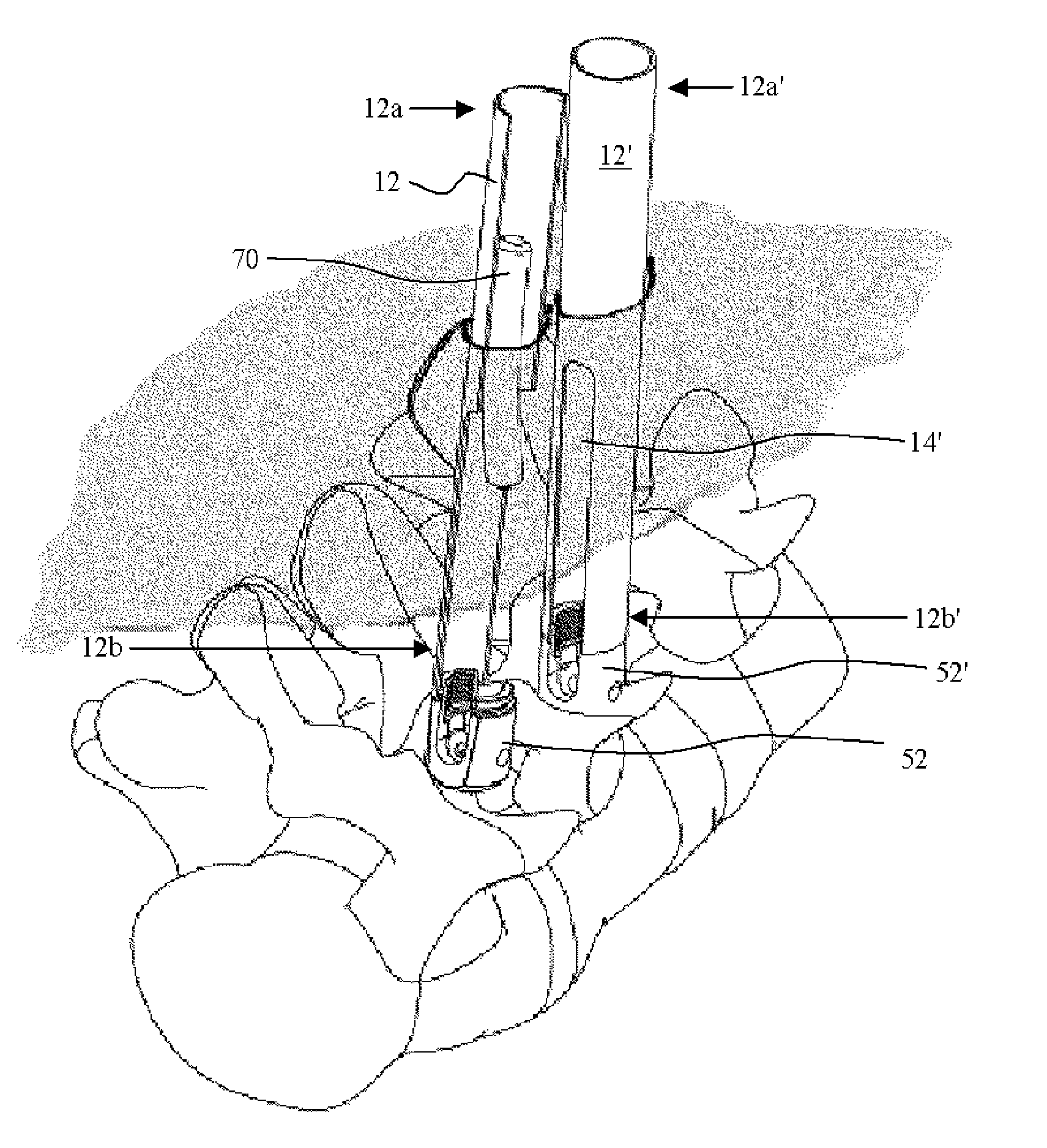
Methods and apparatus for implanting a stimulation lead in a patient's sacrum to deliver neurostimulation therapy that can reduce patient surgical complications, reduce patient recovery time, and reduce healthcare costs. A surgical instrumentation kit for minimally invasive implantation of a sacral stimulation lead through a foramen of the sacrum in a patient to electrically stimulate a sacral nerve comprises a needle and a dilator and optionally includes a guide wire. The needle is adapted to be inserted posterior to the sacrum through an entry point and guided into a foramen along an insertion path to a desired location. In one variation, a guide wire is inserted through a needle lumen, and the needle is withdrawn. The insertion path is dilated with a dilator inserted over the needle or over the guide wire to a diameter sufficient for inserting a stimulation lead, and the needle or guide wire is removed from the insertion path. The dilator optionally includes a dilator body and a dilator sheath fitted over the dilator body. The stimulation lead is inserted to the desired location through the dilator body lumen or the dilator sheath lumen after removal of the dilator body, and the dilator sheath or body is removed from the insertion path. If the clinician desires to separately anchor the stimulation lead, an incision is created through the entry point from an epidermis to a fascia layer, and the stimulation lead is anchored to the fascia layer. The stimulation lead can be connected to the neurostimulator to delivery therapies to treat pelvic floor disorders such as urinary control disorders, fecal control disorders, sexual dysfunction, and pelvic pain.
Owner:MEDTRONIC INC +1
Methods and devices for minimally invasive spinal fixation element placement
Owner:DEPUY SPINE INC (US)
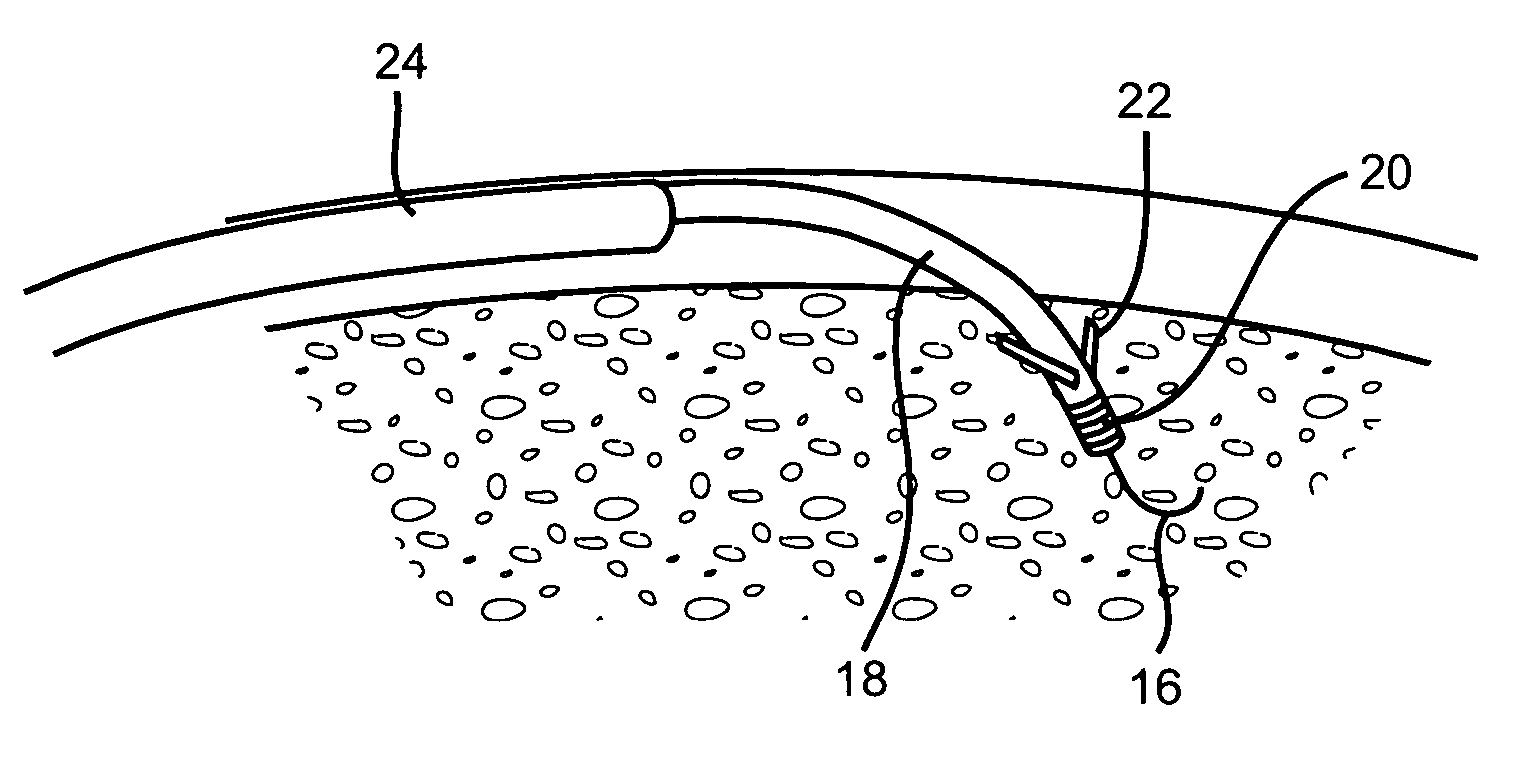
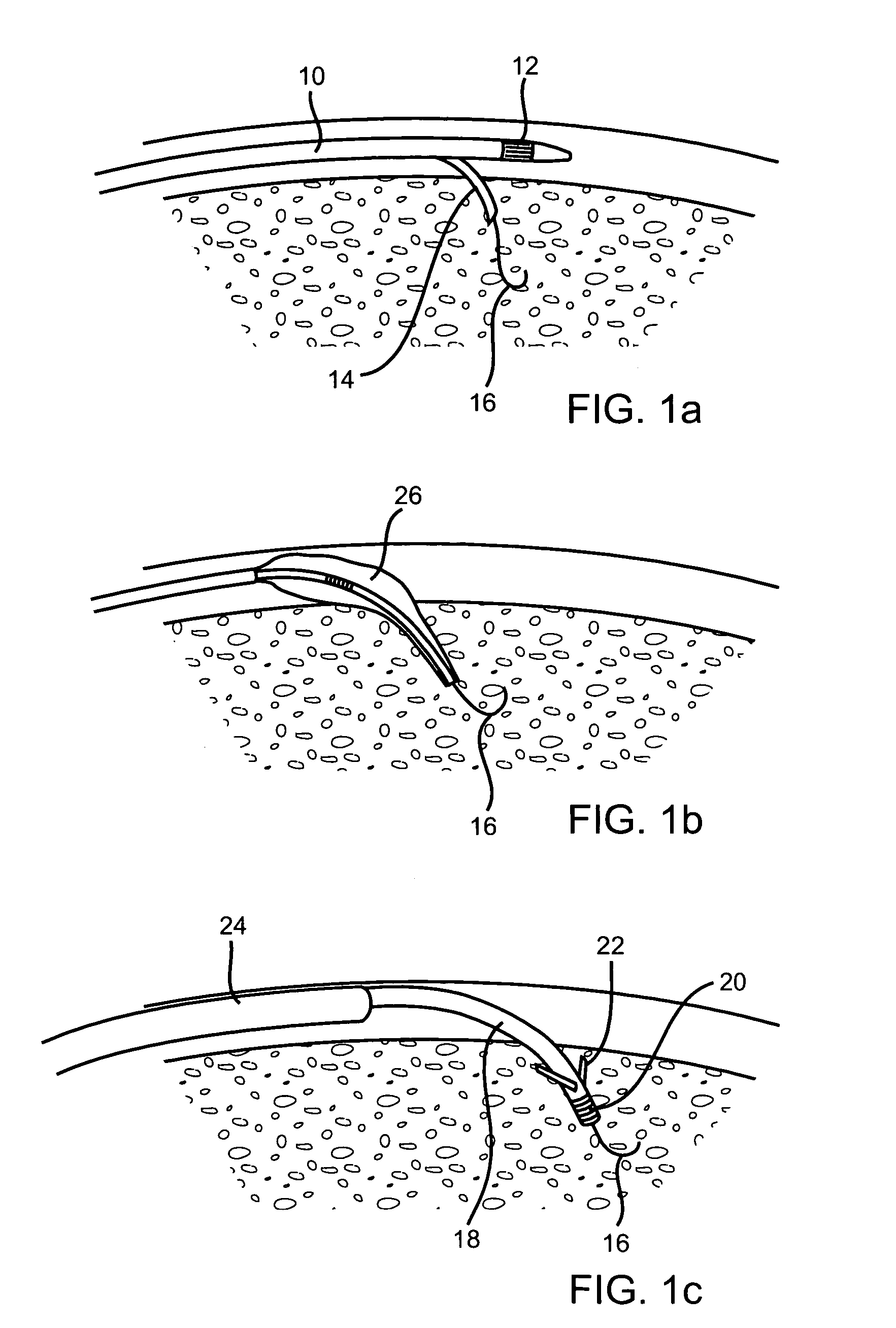
Devices and methods for transluminal or transthoracic interstitial electrode placement
ActiveUS7191015B2Epicardial electrodesTransvascular endocardial electrodesElectrode placementDevice implant
Methods and devices for implanting pacing electrodes or other apparatus, or for delivering substances, to the heart of other tissues within the body. A guided tissue penetrating catheter is inserted into a body lumen (e.g., blood vessel) or into a body cavity or space (e.g., the pericardial space) and a penetrator is advanced from the catheter to a target location. In some embodiments, a substance or an apparatus (such as an electrode) may be delivered through a lumen in the penetrator. In other embodiments, a guidewire may be advanced through the penetrator, the penetrating catheter may then be removed and an apparatus (e.g., electrode) may then be advanced over that guidewire. Also disclosed are various implantable electrodes and electrode anchoring apparatus.
Owner:MEDTRONIC VASCULAR INC
Method and device for locating guidewire and treating chronic total occlusions
InactiveUS20010031981A1Inhibit excessive separationPrevent slidingDiagnosticsDilatorsAtherectomyTotal occlusion
Devices, methods, kits, and methods remove obstructive material from the vasculature and other body lumens. Expansible baskets may be used as cooperating radially expansible shearing members. Helically oriented struts of each basket may wind in a uniform circumferential direction. The struts can be independently flexible, allowing the shearing members to flex axially together. The inner basket may be rotatably driven and may use an axial pump extending proximally from the shearing members and / or a distal penetrator for advancing into an occlusion which inhibits guidewire access. The struts may slide substantially continuously across each other, and may be sufficiently aggressive for highly effective thrombectomy. A rotationally static and axially flexible outer basket may provide a safe, limited atherectomy treatment.
Owner:TYCO HEALTHCARE GRP LP
Sigmoid valve and method for its percutaneous implantation
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is incorporated in self-expandable or inflatable endovascular stents or stents to form a combination which is introduced on a catheter with a guide wire into the circulatory system of the mammal to replace the diseased native valve. Once the combination is at the desired location the stent is caused to expand and affix itself to the patient's vessel wall. The prosthetic valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible circular support is affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible circular support is affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The circular supports maintain the shape of the valve during the surgical implantation procedure and thereafter.
Owner:THE INT HEART INST OF MONTANA FOUND
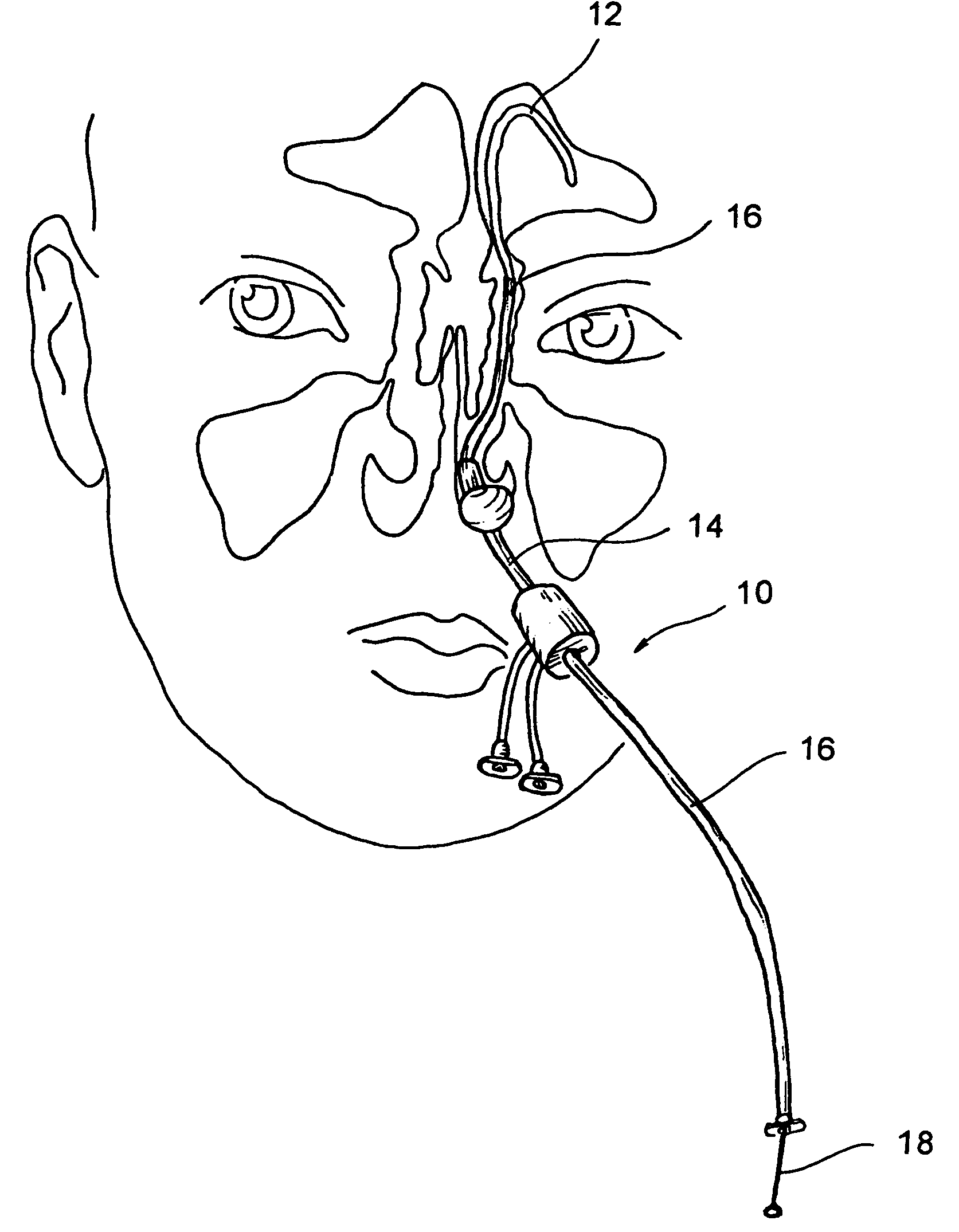
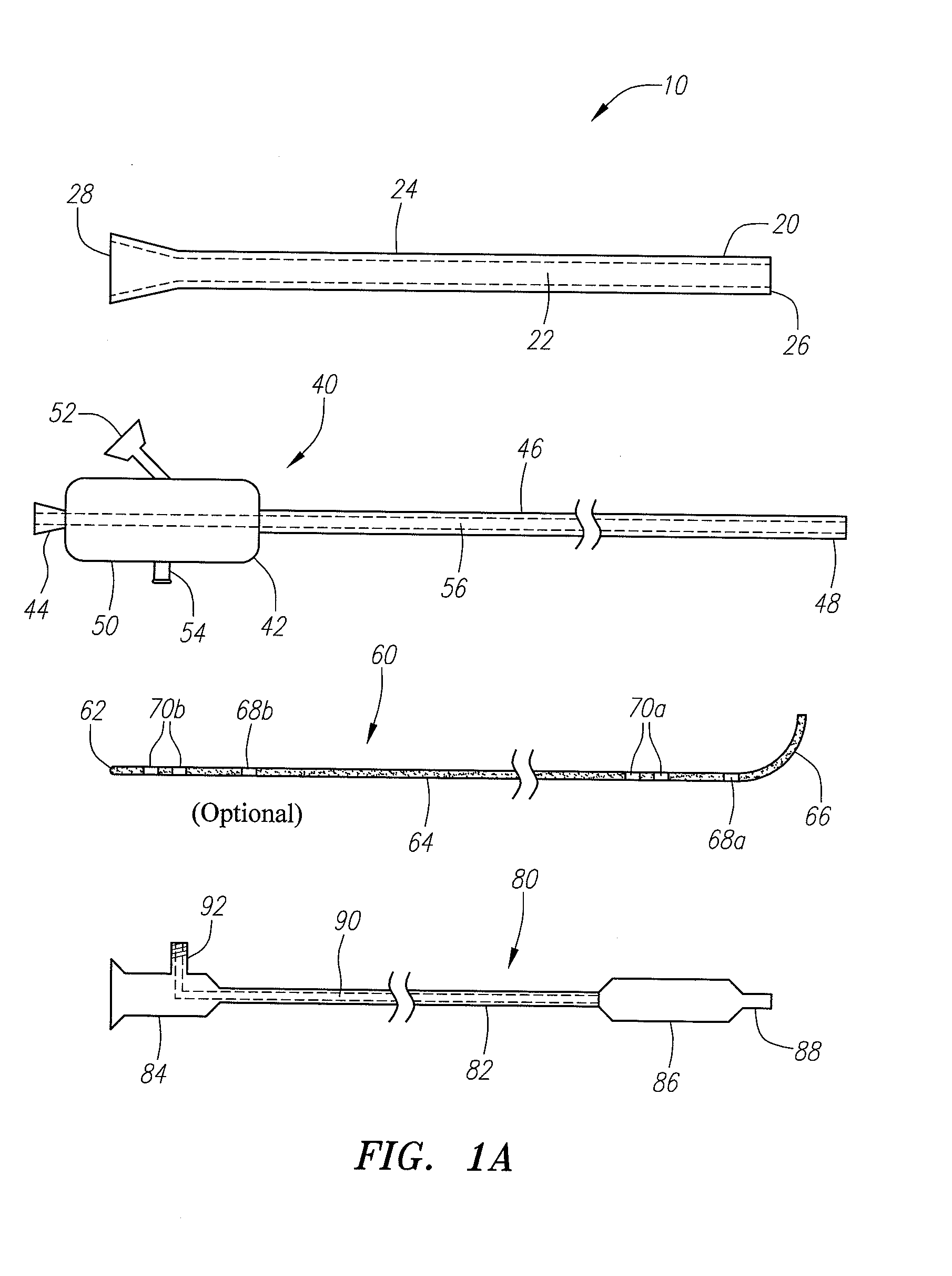
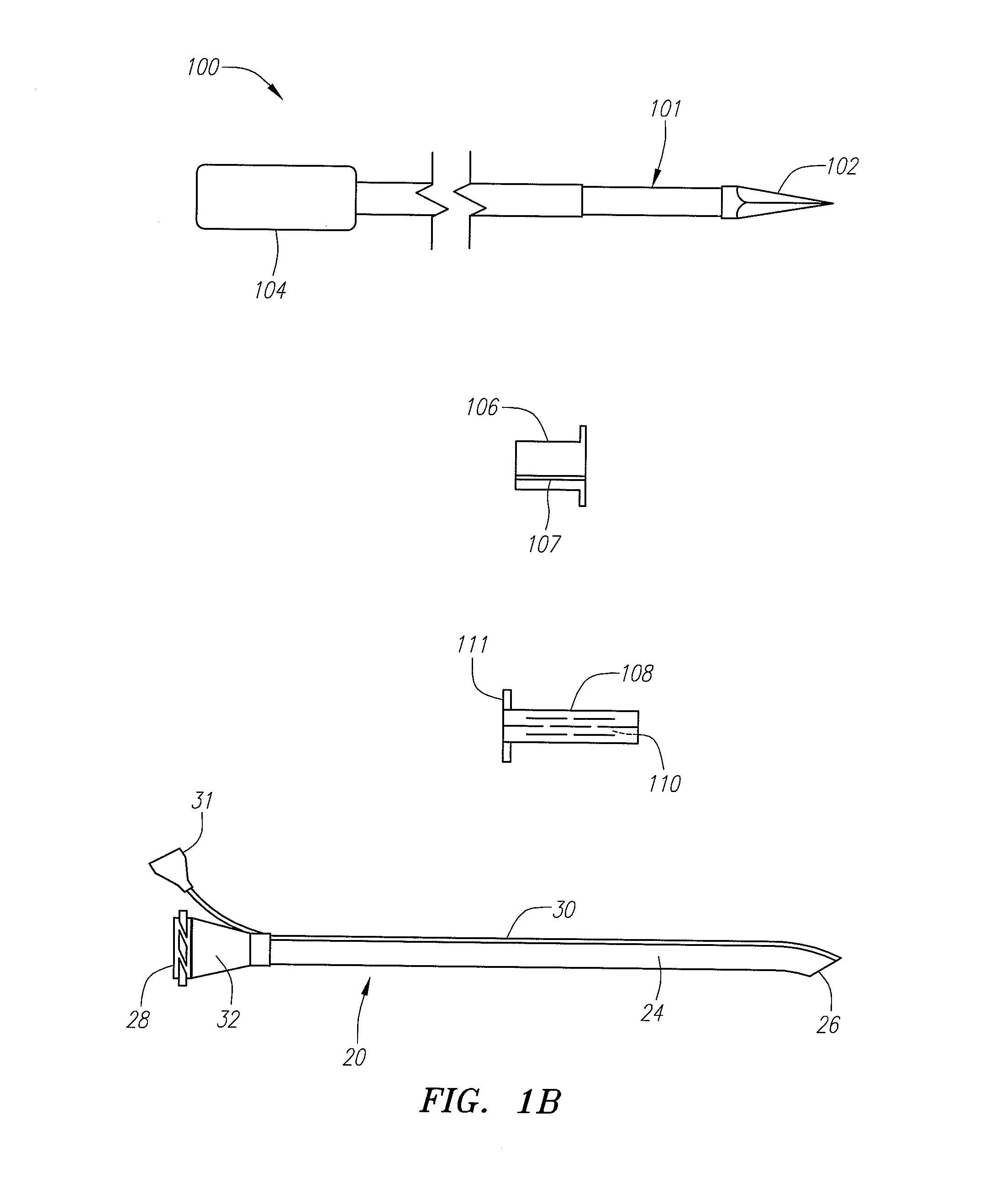
Devices, systems and methods useable for treating sinusitis
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Stent-valves for valve replacement and associated methods and systems for surgery
InactiveUS20090171447A1Simple methodReduce riskStentsBalloon catheterLess invasive surgeryGuide wires
Stent-valves (e.g., single-stent-valves and double-stent-valves), associated methods and systems for their delivery via minimally-invasive surgery, and guide-wire compatible closure devices for sealing access orifices are provided.
Owner:JENAVALVE TECH INC
Guidewire having deployable sheathless protective filter
InactiveUS20050021075A1Reduce the potential for damageAvoid damageGuide wiresSurgeryFiberEngineering
A protective system or apparatus for use in vascular procedures includes a tubular guidewire, a control cable slidable within the tubular guidewire, and a sheathless filter. The control cable is attached to a distal end of the sheathless filter and the tubular guidewire is attached to a proximal end of the sheathless filter. Selective displacement of the control cable radially expands the sheathless filter to create a proximal exterior convex primary filter surface that is positionable downstream from a site of a vascular procedure. The sheathless filter also presents a distal interior concave secondary filter surface. Preferably, the sheathless filter is constructed of a braided wire framework in the form of a tube over which woven polymer fibers or strands are applied to create a filter mesh having a softer filter surface.
Owner:MEDRAD INC.
Non cannulated dilators
A non-cannulated dilator that is designed with a rigid elongated solid body and with a judiciously configured tip is utilized as the first dilator of a series of dilators which are inserted into the body of a patient for minimally invasive spinal surgery and is made from a solid elongated body, that could be round, ovoid or other cross sectional configuration whose diameter is greater than one and a half (1½) millimeter, and that includes a tool engaging end portion at the proximal end is sufficiently rigid so as not to bend and has a pointed shaped insertion end portion at the distal end with the point of the pointed end being discreetly blunted and utilized in a surgical procedure as a replacement of the typical guide wire and is characterized as providing a “fee” to the surgeon as it penetrates through the tissue and muscle of the patient as it proceeds toward the target.
Owner:DEPUY SPINE INC (US) +1
Devices and methods for minimally invasive treatment of degenerated spinal discs
InactiveUS20050222681A1Accurate spacingBone debris is eliminatedBone implantJoint implantsExpandable cageRadio frequency
Spinal stabilization devices and their methods of insertion and use to treat degenerated lumbar, thoracic or cervical spinal discs in minimally invasive, outpatient procedures are described. In one embodiment, the spinal stabilization device is an expandable cage made of a coil or perforated cylindrical tube with a bulbous or bullet-shaped distal end and a flat or rounded proximal end. In a preferred embodiment, the spinal stabilization device is mechanically expanded to a larger diameter or is made of a superelastic nickel-titanium alloy which is thermally programmed to expand to a relatively larger diameter when a pre-determined transition temperature below body temperature is reached. To treat a degenerated disc, a guide wire is inserted into the disc and an endoscope is inserted through a posterolateral puncture in the back and advanced up to the facet of the spine. Mechanical tools or laser energy, under endoscopic visualization, are used to remove or vaporize a portion of the facet bone, creating an opening into the foraminal space in the spine for insertion of an endoscope, which enables the disc, vertebra and nerves to be seen. The passageway is expanded, mechanical tools or laser of RF energy are used to make a tunnel into the disc, and a delivery cannula is inserted up to the opening of the tunnel. An insertion tool is used to insert one or more spinal stabilization devices into the tunnel in the disc, preserving the mobility of the spine, while maintaining the proper space between the vertebra. Laser or radio frequency (RF) energy is used to coagulate bleeding, vaporize or remove debris and shrink the annulus of the disc to close, at least partially, the tunnel made in the disc.
Owner:TRIMEDYNE
Methods of using an intravascular balloon catheter in combination with an angioscope
A method of performing a medical procedure is disclosed which involves the use of a balloon catheter in combination with an angioscope to monitor an intravascular stent before, during or after deploying the stent. Specifically, the method may include the steps of (1) delivering an intravascular stent into the vasculature of a patient, (2) positioning a balloon catheter in the vasculature such that the inflatable balloon is adjacent the stent, (3) inserting an angioscope into the balloon catheter such that the distal end of the angioscope is adjacent an optically-transparent tube traversing the interior of the balloon, and (4) visually monitoring the stent before, during or after deploying the intravascular stent. The optically-transparent tube may define a guide wire lumen for use with a guide wire and the angioscope may be inserted into the guide wire lumen. The intravascular stent may be a balloon-expandable stent, a self-expanding stent, or a stent made of a photo-responsive polymer.
Owner:BOSTON SCI SCIMED INC
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
Suturing apparatus and method
InactiveUS6059800AEasy to optimizeImprove relationshipSuture equipmentsSurgical needlesEngineeringActuator
A suturing apparatus is adapted for joining two objects which define a near side and a far side. The apparatus includes an elongate support structure having a working channel and an axis extending between a proximal end and a distal end. The structural support is adapted to be positioned over a guidewire extending between the two objects, in an operative position wherein the proximal end is disposed on the near side and the distal end as disposed on the far side. Removal of the guidewire permits use of a stylet for positioning a suture to extend within the working channel from the proximal end to the distal end. A pair of hooks are movable from a proximal position at the proximal end of the support structure through the objects and a pair of slots in the support structure, to a distal position wherein the hooks engage the suture within the working channel at the distal end of the support structure. The actuator is then operable to move the hooks and the engaged suture from the distal position to the proximal position in order to facilitate tying of the suture and joining of the two objects. Alternatively, suture loops can be carried through the objects by unidirectional hooks and into the working channel. The hooks can then be withdrawn leaving the suture loops in the working channel where they can be engaged by a snare and withdrawn proximally between the two objects. Suturing pledgets can be added to the suturing apparatus prior to operative disposition, and a backing member can be provided to support at least the pledgets when the hook passes through the object.
Owner:APPL MEDICAL RESOURCES CORP
Plug with collet and apparatus and method for delivering such plugs
InactiveUS6896692B2Low profilePromote progressVaccination/ovulation diagnosticsTubular organ implantsEngineeringGuide wires
An apparatus for sealing a passage through tissue includes a plug including external threads, a lumen extending between its proximal and distal ends, and an annular collet slidably disposed within the lumen. The plug is carried on a distal end of a handle device that includes an inner member with a distal end that is slidable axially within the lumen of the body for moving the collet into a reduced cross-sectional region of the lumen, thereby compressing the collet to seal the lumen, and for deploying the plug from the handle device. During use, the plug is threaded into a passage through tissue over a guide wire. Once a desired location is reached, the plug is deployed from the handle device, thereby compressing the collet and sealing the lumen. The guide wire and handle device are removed, leaving the plug to seal the passage.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Implant delivery system with interlocked RX port orientation
A medical implant delivery system maintains an orientation between a guidewire lumen of an inner member of the system and a rapid-exchange port in an outer member. The medical device is disposed intermediate the inner and outer members and in friction or pressure-fit contact with the outer member. Once the guidewire lumen of the inner member and the rapid exchange port of the outer member are oriented, the friction or pressure-fit operates to maintain the orientation until deployment of the medical implant. Orientation is further maintained by a telescoping coupling of the guide wire lumen with the rapid exchange port.
Owner:TYCO HEALTHCARE GRP LP
Visualization apparatus for transseptal access
ActiveUS20070293724A1Easy to identify visuallyDifficult to identifyUltrasonic/sonic/infrasonic diagnosticsSurgerySeptal wallVia device
Visualization apparatus and methods for transseptal access are described herein where intravascular access across a septal wall is facilitated via devices which provide for direct visual viewing of tissue area. Such a system may include a deployment catheter and an attached imaging hood deployable into an expanded configuration. In use, the imaging hood is placed against or adjacent to the tissue to be imaged in a body lumen that is normally filled with an opaque bodily fluid such as blood. A translucent or transparent fluid can be pumped into the imaging hood until the fluid displaces any blood leaving a clear region of tissue to be imaged via an imaging element in the deployment catheter. Any number of therapeutic tools or a guidewire can be passed through the catheter and into the imaging hood for crossing the septal wall and passing the guidewire or instruments therethrough.
Owner:INTUITIVE SURGICAL OPERATIONS INC
Methods and apparatus for localized and semi-localized drug delivery
InactiveUS20050059931A1Turn easilyEasy to navigateStentsBalloon catheterVariable stiffnessThree vessels
A catheter system for localized or semi-localized administration of agents through the wall of a blood vessel is provided. Various catheter system constructions which use at least one expandable occluding device to create an isolated region are provided. Constructions using one catheter and one occlusion device are provided, along with constructions using two catheters and multiple occlusion devices. The catheter system may include a catheter with a variable stiffness along its length. The catheter system may also include a guide wire integrated with an inner catheter. The catheter can infuse the agent into the blood vessel in a pressure regulated manner. Methods for delivery and infusion of the agent within a blood vessel are also provided.
Owner:VENOMATRIX
Ratcheting mechanical driver for cannulated surgical systems
InactiveUS20090254094A1Control lengthPermit useProsthesisOsteosynthesis devicesCouplingMechanical advantage
A device for rotatably driving a cannulated tool along a guide wire. In one aspect, the device includes a coupling assembly for receiving the tool and guide wire as well as a ratchet mechanism permitting selective rotation between the device and the tool. The device further includes an elongate gripping portion extending transversely to the coupling assembly to provide a levered mechanical advantage while positioning the gripping portion out of the path of the guide wire. In another aspect, the device includes a handle having a through bore shorter than a predetermined length of a connection assembly through bore so that only a short portion of the guide wire is covered by the device. A method of driving a cannulated tool along a guide wire is also provided that includes rotating a gripping portion of a handle assembly about the tool in an arcuate path spaced from the tool.
Owner:PIONEER SURGICAL TECH INC
Temporary blood circulation assist device
InactiveUS6981942B2Avoid lacerationsPrevent perforationBlood pumpsIntravenous devicesImpellerEngineering
An inflatable circulation assist device is disclosed consisting of an inflatable stator housing an impeller with inflatable blades of varying shapes and sizes. The invention is introduced into the patient percutaneously. The circulation assist device is a small pump packaged into a compact form that is attached to a long flexible driveshaft. The pump is inserted along a guidewire to a target location, and then the pump is inflated. The circulation assist device's exterior is designed to expand only so much as to closely fit whatever cardiovascular system element in which it is placed for operation. The vascular assist device can be expanded either by inflation with a fluid. The driveshaft, which connects to the circulation assist device's impeller and extends outside the patient's body, is rotated by an external motor. After the circulation assist device is no longer needed, it is collapsed into a compact form and removed from the patient percutaneously.
Owner:RUTGERS THE STATE UNIV
Plug with detachable guidewire element and methods for use
InactiveUS6890343B2Low profilePromote progressVaccination/ovulation diagnosticsSurgical veterinaryDistal portionGuide wires
An apparatus for sealing a passage through tissue includes a bioabsorbable, threaded plug carried by a delivery device. A guide wire is receivable through lumens in the plug and delivery device that includes wings on a bioabsorbable distal portion. With the wings collapsed, the guide wire is advanced through the passage into a blood vessel, the wings expanding once located within the vessel, and the guide wire is withdrawn until the wings contact the vessel wall. The plug is threaded into the passage over the guide wire until the plug is disposed adjacent the wings. The distal portion of the guide wire is secured to the plug, e.g., by compressing a collet within the plug lumen that seals the lumen, and is severed from a proximal portion thereof. Thus, the plug and distal portion are deployed with the plug sealing the passage.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Guide wire control catheters for crossing occlusions and related methods of use
InactiveUS20040102719A1Simplify the viewing processStentsBalloon catheterPercutaneous angioplastyThree vessels
A wire control catheter for aligning and guiding a guide wire through a lesion in a vessel is provided. The wire control catheter includes a shaft having a guide wire lumen and a control wire lumen. A control wire passes through the control wire lumen and is used in combination with an articulation structure to deflect or curve a distal tip portion of the catheter. The distal catheter shaft may include a centering device for centering the catheter within the vessel. The distal catheter shaft also may include a pre-dilation balloon for dilating the lesion prior to performing angioplasty or other treatment on the lesion. Additionally, a sliding sheath catheter may be used to provide additional support to the guide wire. The sliding sheath catheter is sized to fit within the guide wire lumen of the control catheter and to allow the guide wire to pass through it. A method of treatment of a blood vessel includes inserting a guide wire into the blood vessel and advancing a control catheter over the guide wire until the distal tip of catheter is near the occlusion in the blood vessel. The tip of the catheter then is deflected via a control wire and an articulation structure. The guide wire is then advanced across the occlusion. The control catheter also may be advanced across the occlusion simultaneously with the guide wire or subsequent to the guide wire crossing. Prior to crossing the occlusion, the wire control catheter may be centered using a centering device. Subsequent to crossing the occlusion, the occlusion may be pre-dilated with a pre-dilation balloon of the wire control catheter.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Method and anchor for medical implant placement, and method of anchor manufacture
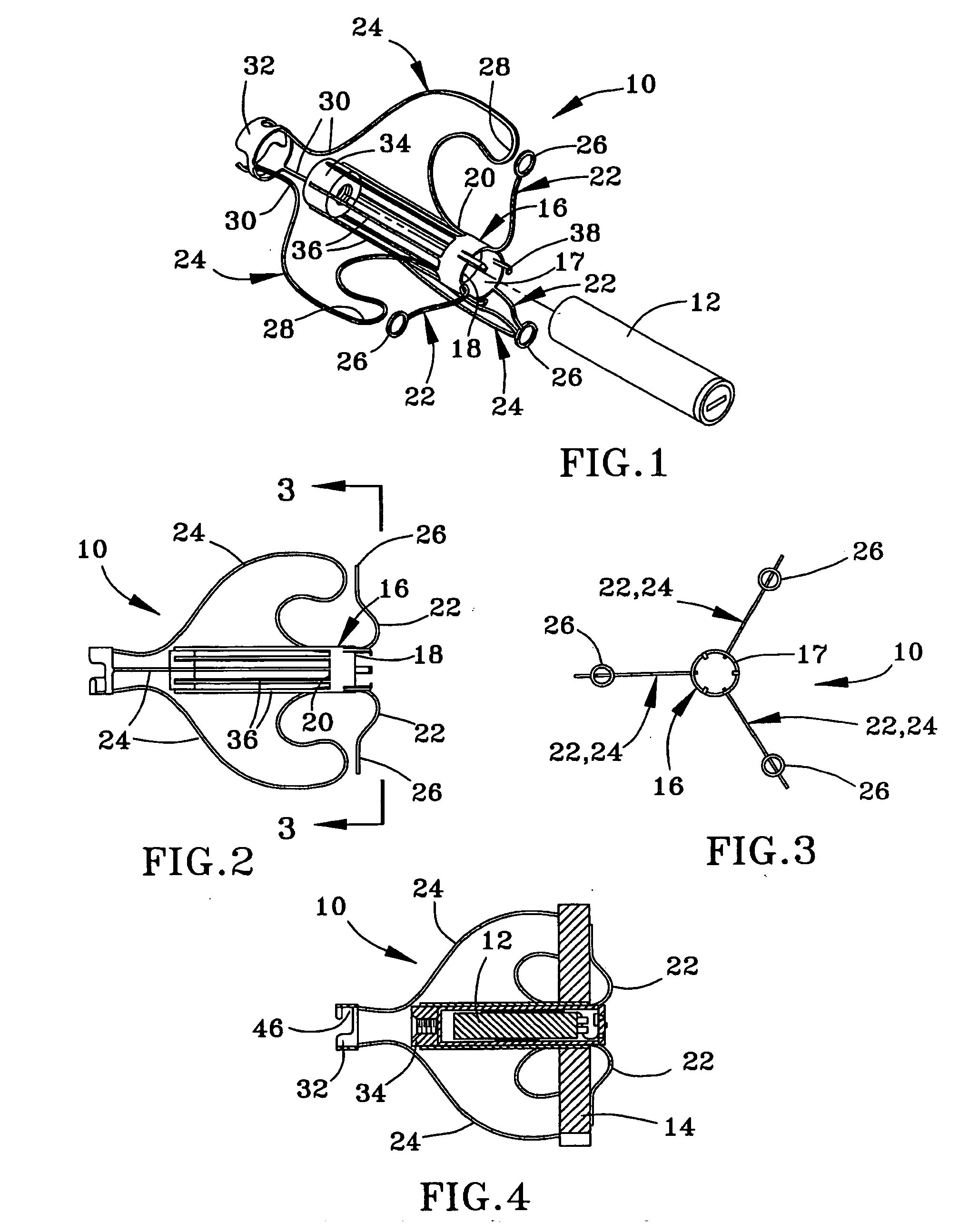
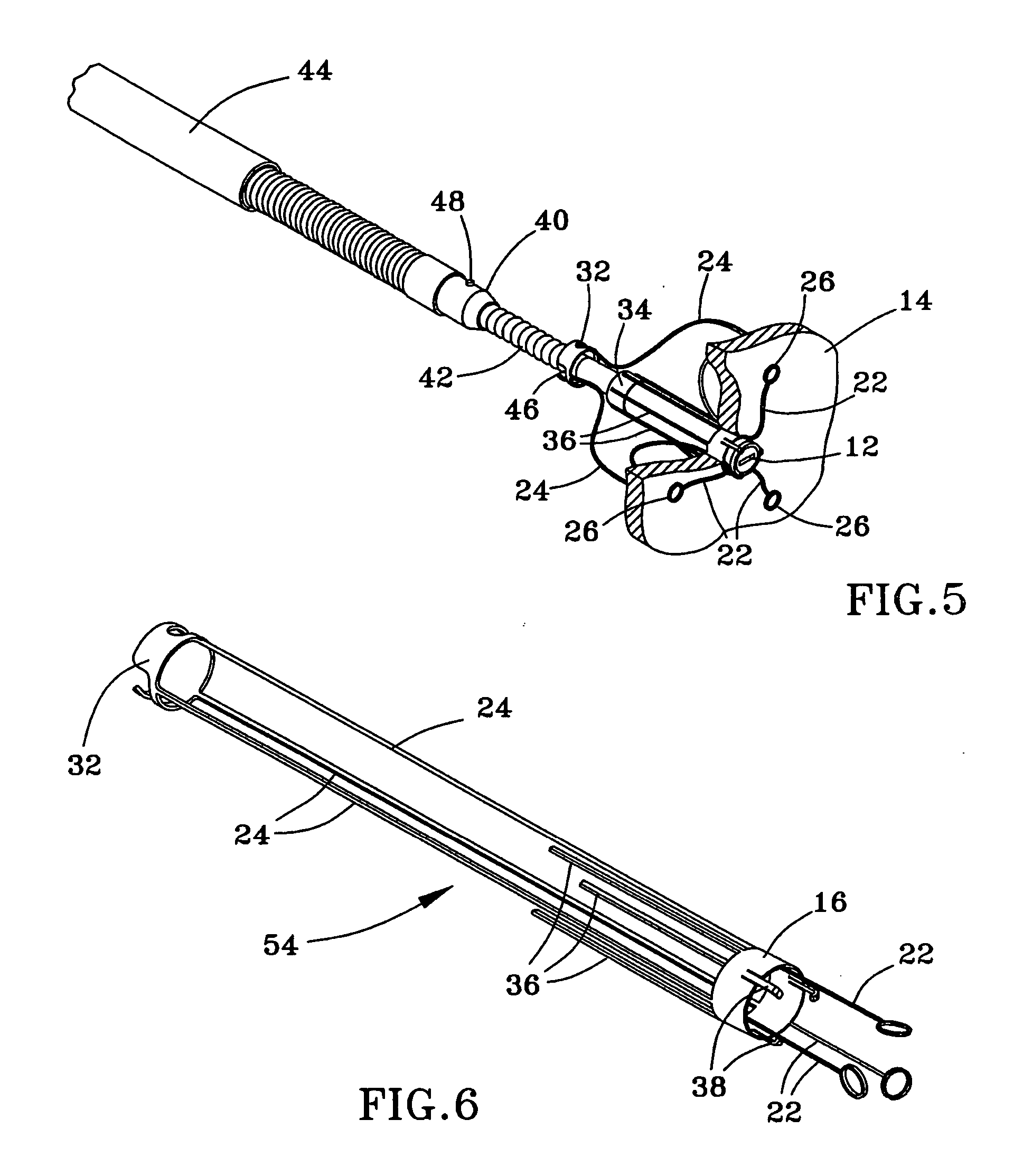
ActiveUS20050065589A1Transvascular endocardial electrodesCatheterDistal portionMonitoring physiological parameters
An anchor and procedure for placing a medical implant, such as for monitoring physiological parameters. The anchor includes a central body in which a medical implant can be received. arms and members extend radially from first and second ends, respectively, of the central body. Each member defines a leg extending toward distal portions of the arms to provide a clamping action. The anchor and its implant are placed by coupling first and second guidewires to first and second portions of the anchor, placing an end of a delivery catheter in a wall where implantation is desired, inserting the anchor in the catheter with the guidewires to locate the anchor within the wall, deploying the arms of the anchor at one side of the wall followed by deployment of the members at the opposite side of the wall, and thereafter decoupling the guidewires from the anchor.
Owner:UIM PRESSURE IMPLANT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com