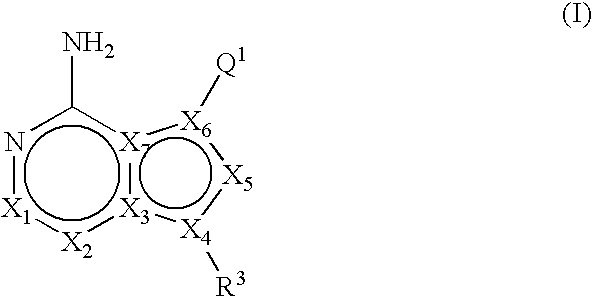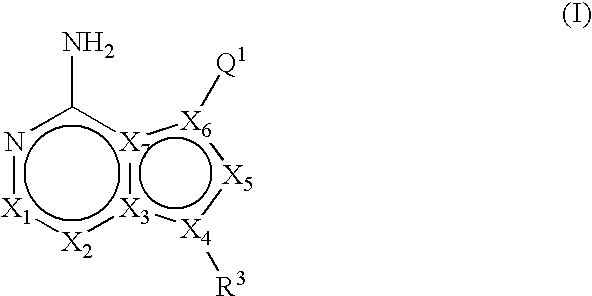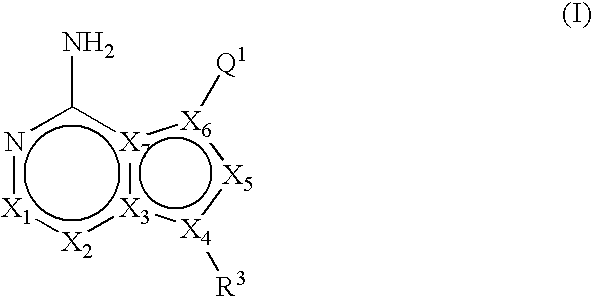Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
16628 results about "Pharmaceutical medicine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical medicine is a medical discipline concerned with the discovery, evaluation, registration, monitoring and clinical aspects of pharmaceutical development. All medical specialties overlap to some extent, and likewise the boundaries of pharmaceutical medicine are elastic. But, at its centre is the clinical testing of medicines, translation of pharmaceutical drug research into new medicines, safety and well-being of patients and research participants in clinical trials, and understanding the safety profile of medicines and their benefit-risk balance. Pharmaceutical physicians work in the pharmaceutical industry, universities / medical schools, drug regulatory authorities and contract research organisations, but have a close affinity with their medical colleagues elsewhere.
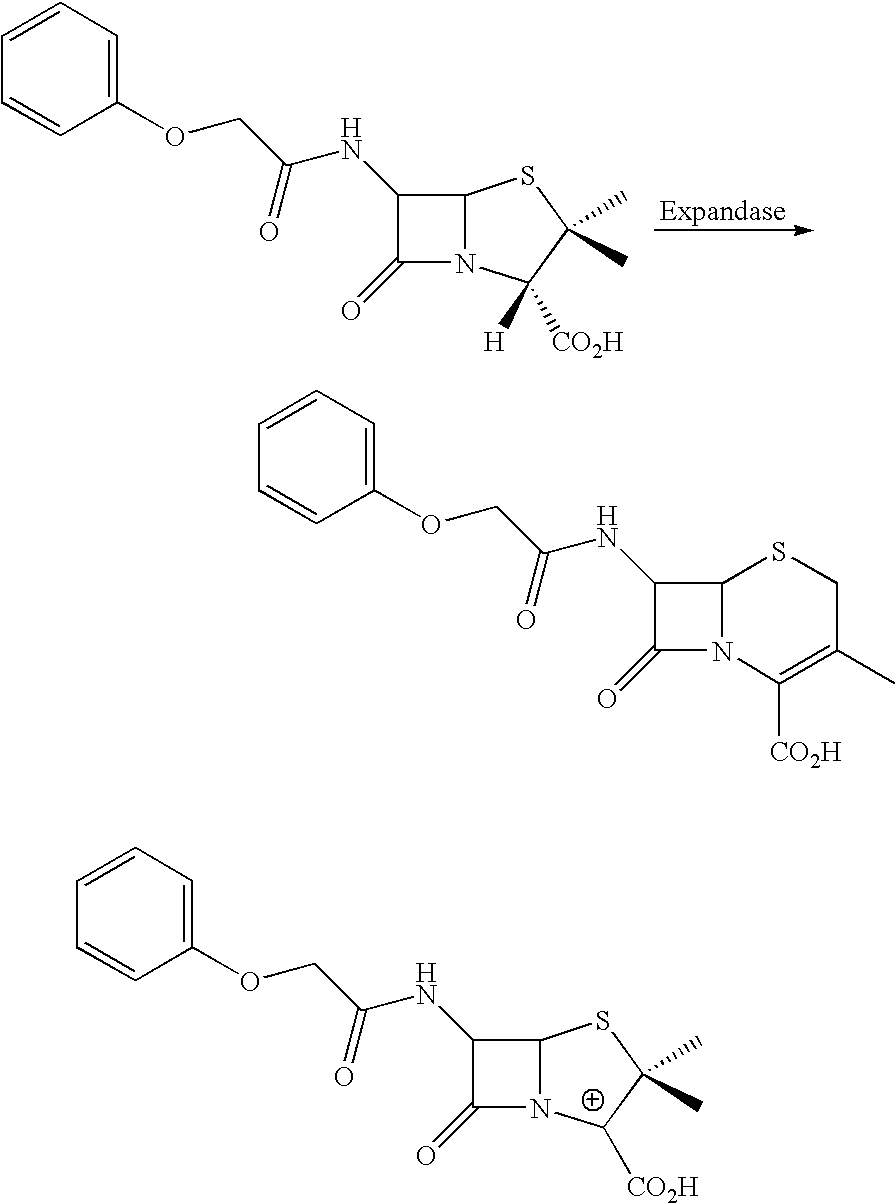
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
Heterocyclic compounds
InactiveUS6329381B1Excellent interferon biosynthesis inducing activityInhibition thicknessAntibacterial agentsBiocideBULK ACTIVE INGREDIENTInterferon inducer
The present invention relates to a heterocyclic compound of the following general formula (I):wherein X is sulfur atom, oxygen atom or -NR3- (R3 may form a heterocyclic ring or a substituted heterocyclic ring with R1 via the nitrogen atom),R1 is alkyl group, substituted alkyl group, aryl group, substituted aryl group, heterocyclic group or substituted heterocyclic group, andR2 is hydrogen atom, halogen atom etc.;or its pharmaceutically acceptable salt and interferon inducers, antiviral agents, anticancer agents and therapeutic agents for immunologic diseases comprising the compound (I) or its pharmaceutically acceptable salt as active ingredients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
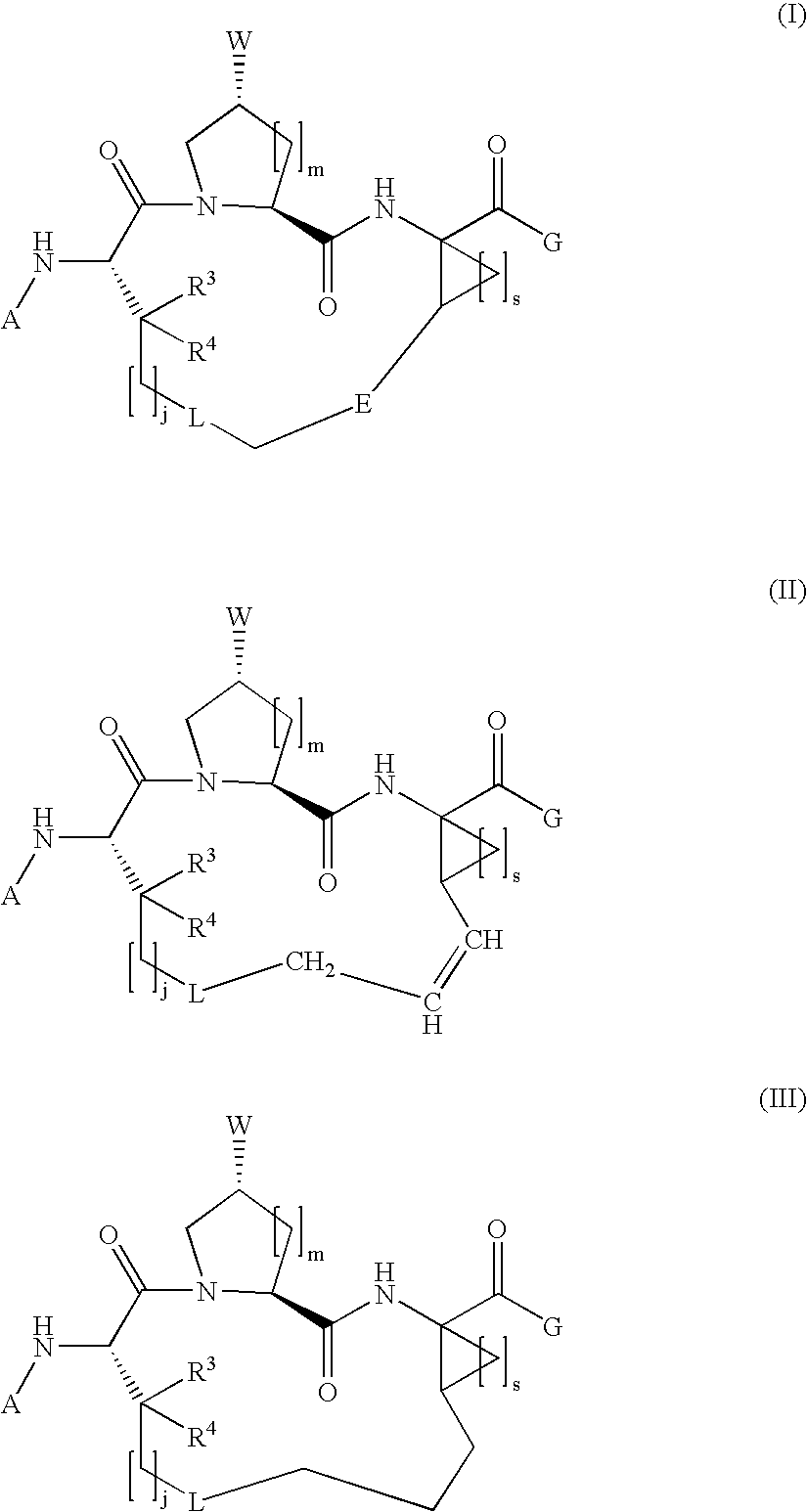
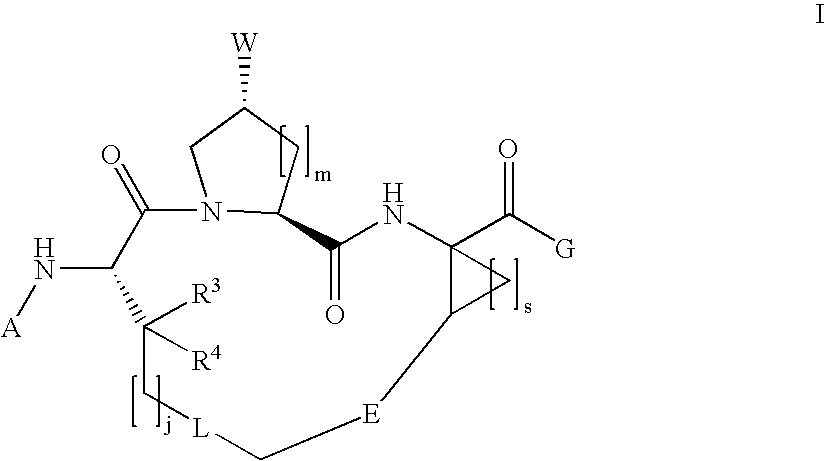
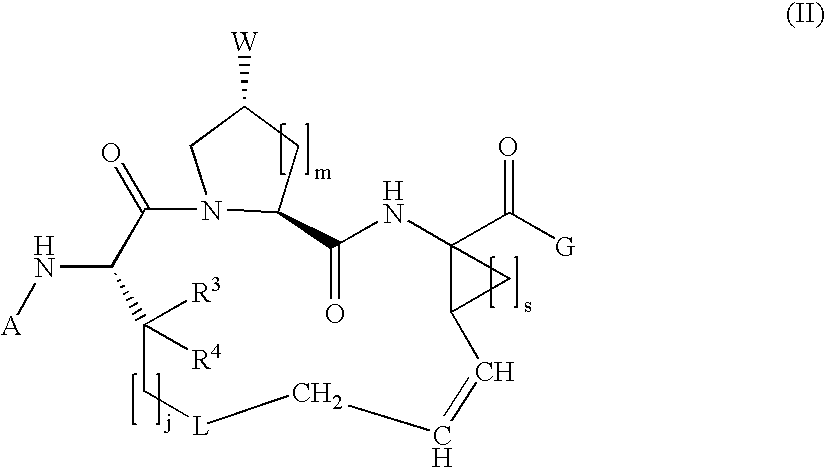
Macrocyclic hepatitis C serine protease inhibitors
The present invention relates to compounds of Formula I, II or Ill, or a pharmaceutically acceptable salt, ester, or prodrug, thereof: wherein W is a substituted or unsubstituted heterocyclic ring system. The compounds inhibit serine protease activity, particularly the activity of hepatitis c virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis c virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Methods and compositions for polypeptide engineering
Owner:CODEXIS MAYFLOWER HLDG LLC
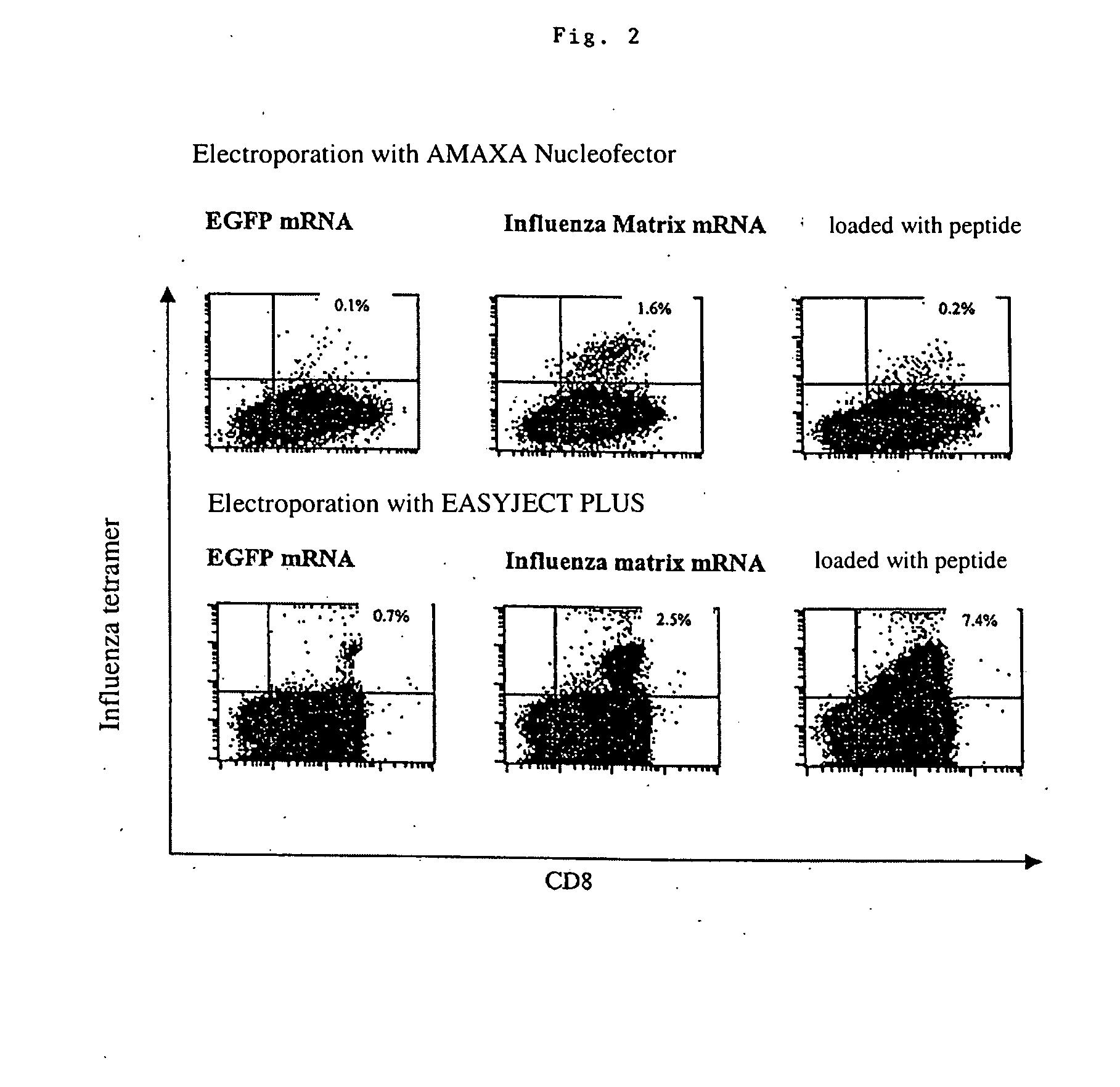
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
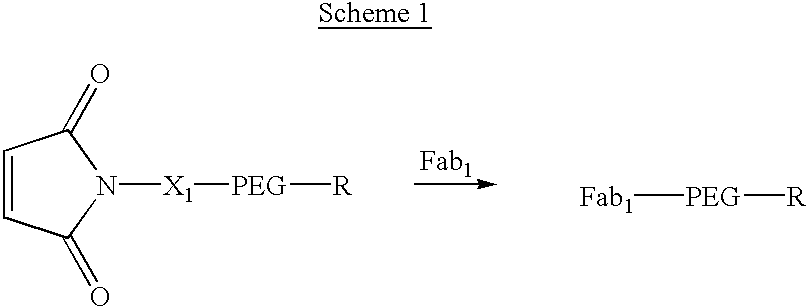
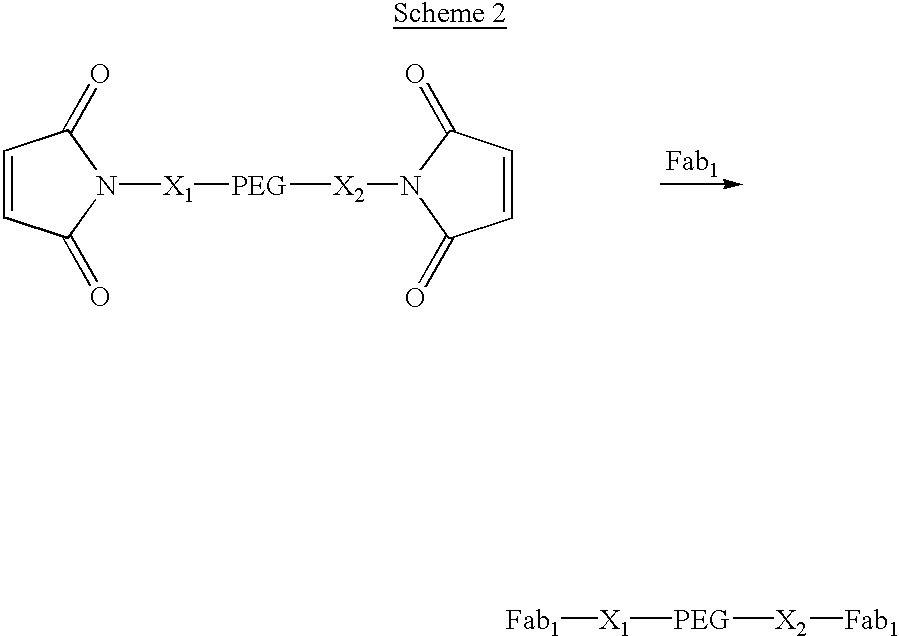
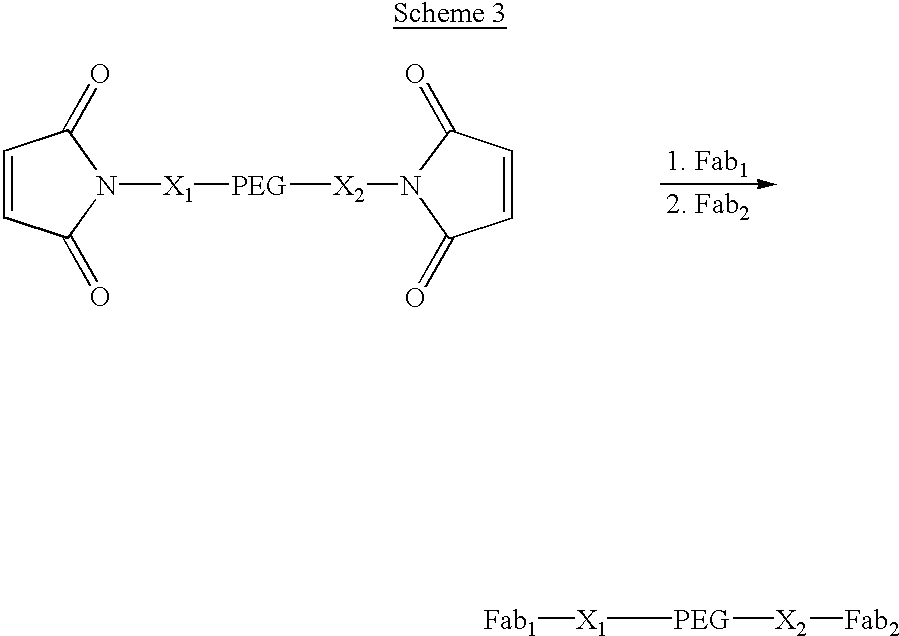
Pseudo-antibody constructs
InactiveUS20030211078A1Reduce productionInhibit synthesisOrganic active ingredientsBiocideHalf-lifeIn vivo
This invention relates to novel pharmaceutically useful compositions that bind to a biological molecule, having improved circulatory half-life, increased avidity, increased affinity, or multifunctionality, and methods of use thereof. The present invention provides a pseudo-antibody comprising an organic moiety covalenty coupled to at least two target-binding moieties, wherein the target-binding moieties are selected from the group consisting of a protein, a peptide, a peptidomimetic, and a non-peptide molecule that binds to a specific targeted biological molecule. The pseudo-antibody of the present invention may affect a specific ligand in vitro, in situ and / or in vivo. The pseudo-antibodies of the present invention can be used to measure or effect in an cell, tissue, organ or animal (including humans), to diagnose, monitor, modulate, treat, alleviate, help prevent the incidence of, or reduce the symptoms of, at least one condition.
Owner:CENTOCOR
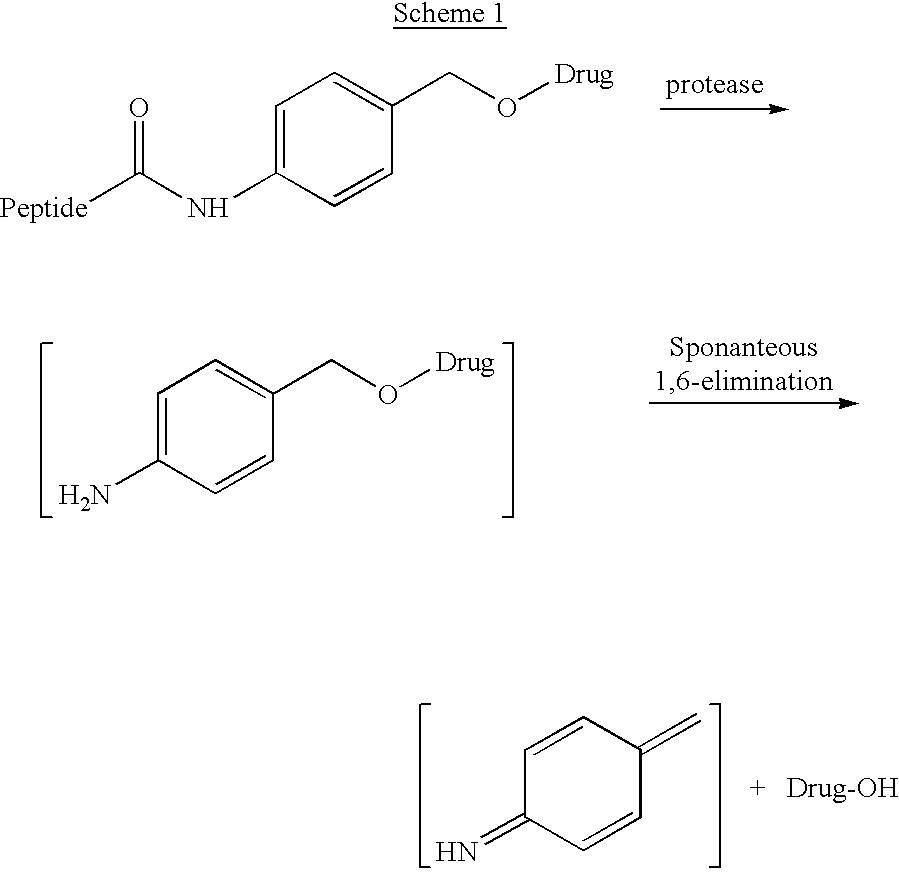
p-Amidobenzylethers in drug delivery agents
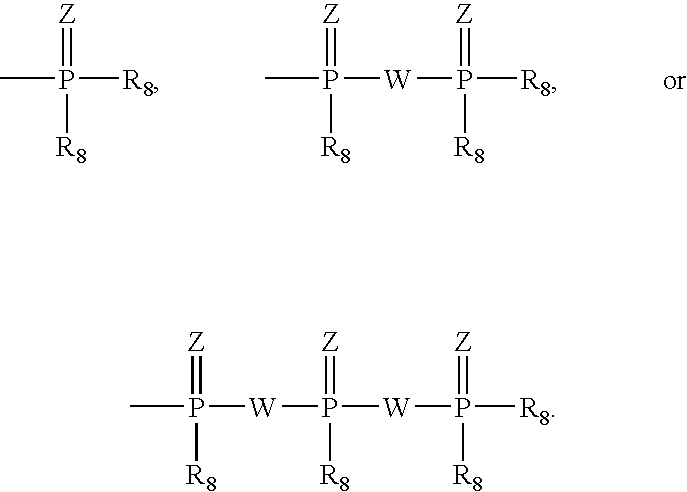
Compounds of the formulaeLAn-Z-X—WwD and BZ-X—WwDwherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide residue; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
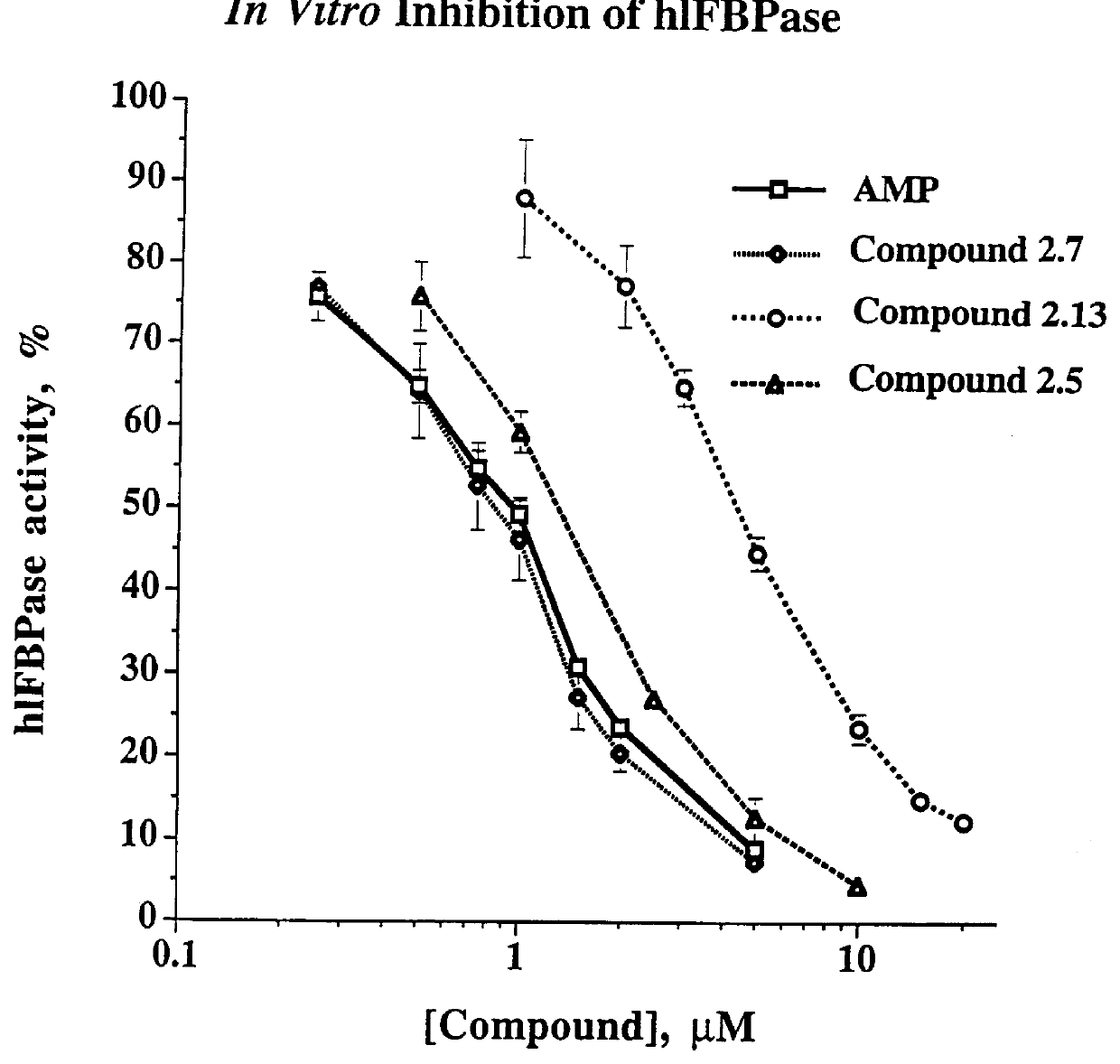
Indole and azaindole inhibitors of fructose-1,6-bisphosphatase
Novel indole and azaindole compounds of the following structure and their use as fructose-1,6-bisphosphatase inhibitors is described: and pharmaceutically acceptable prodrugs and salts thereof.
Owner:METABASIS THERAPEUTICS INC
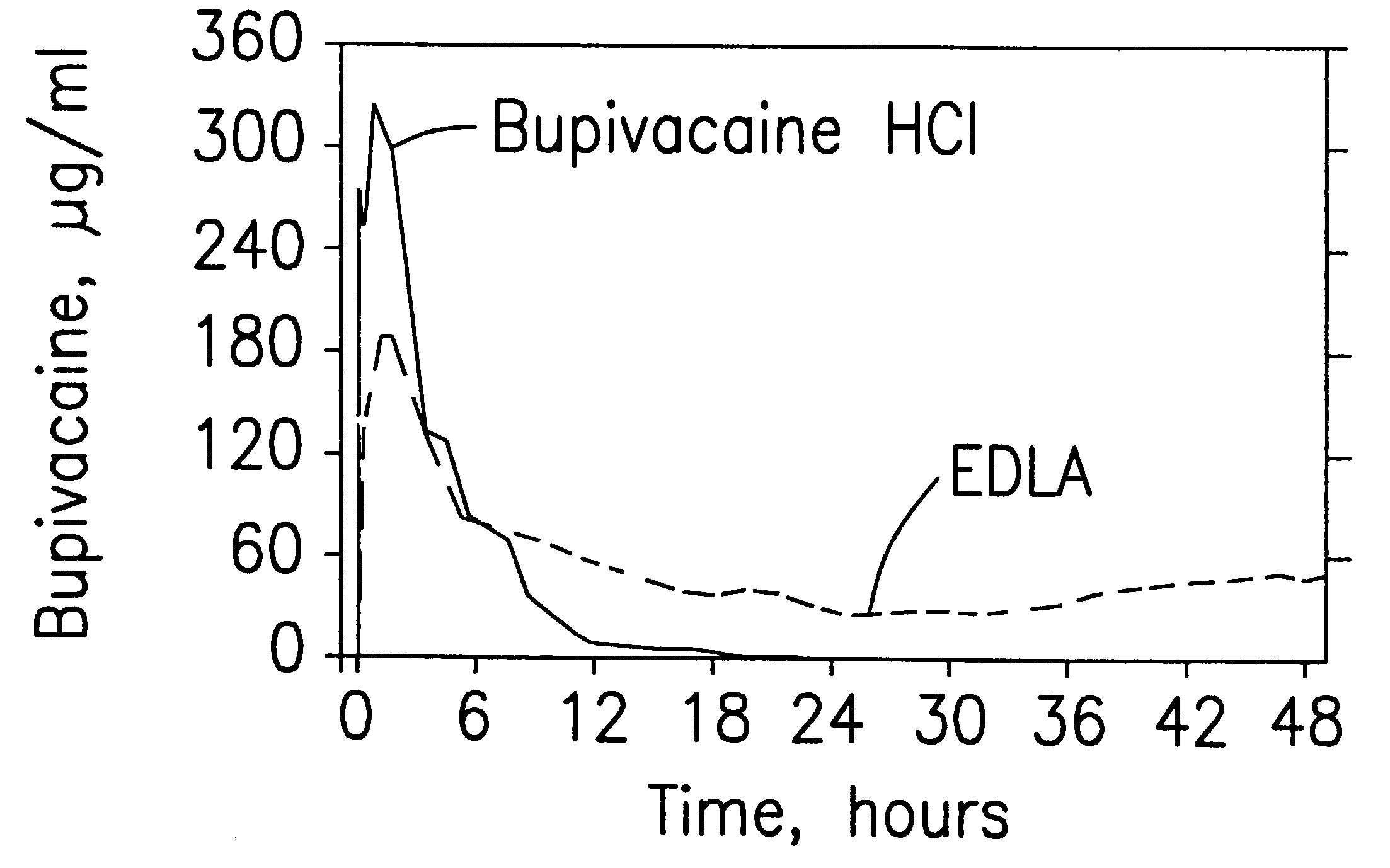
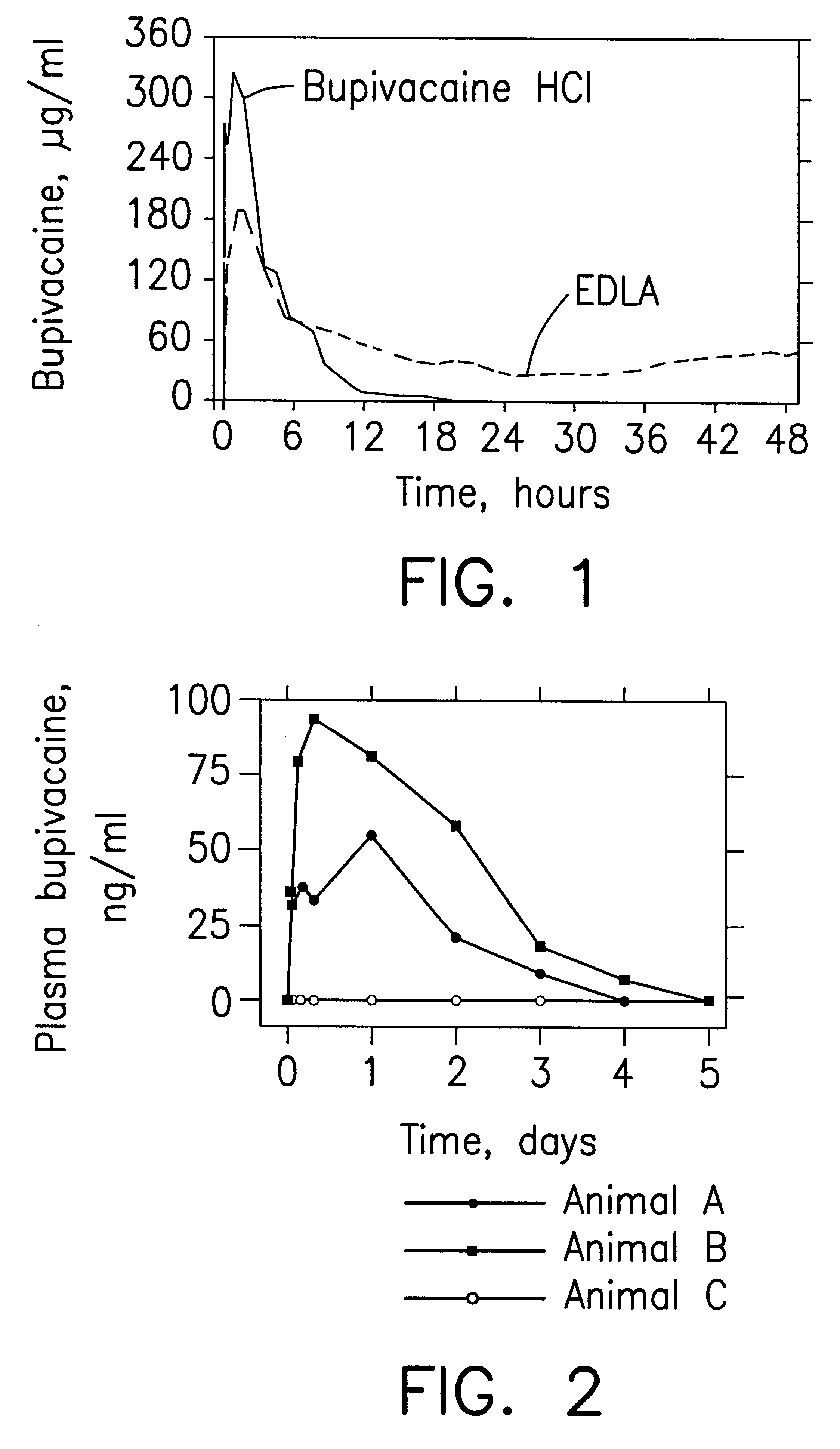
Prolonged anesthesia in joints and body spaces
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
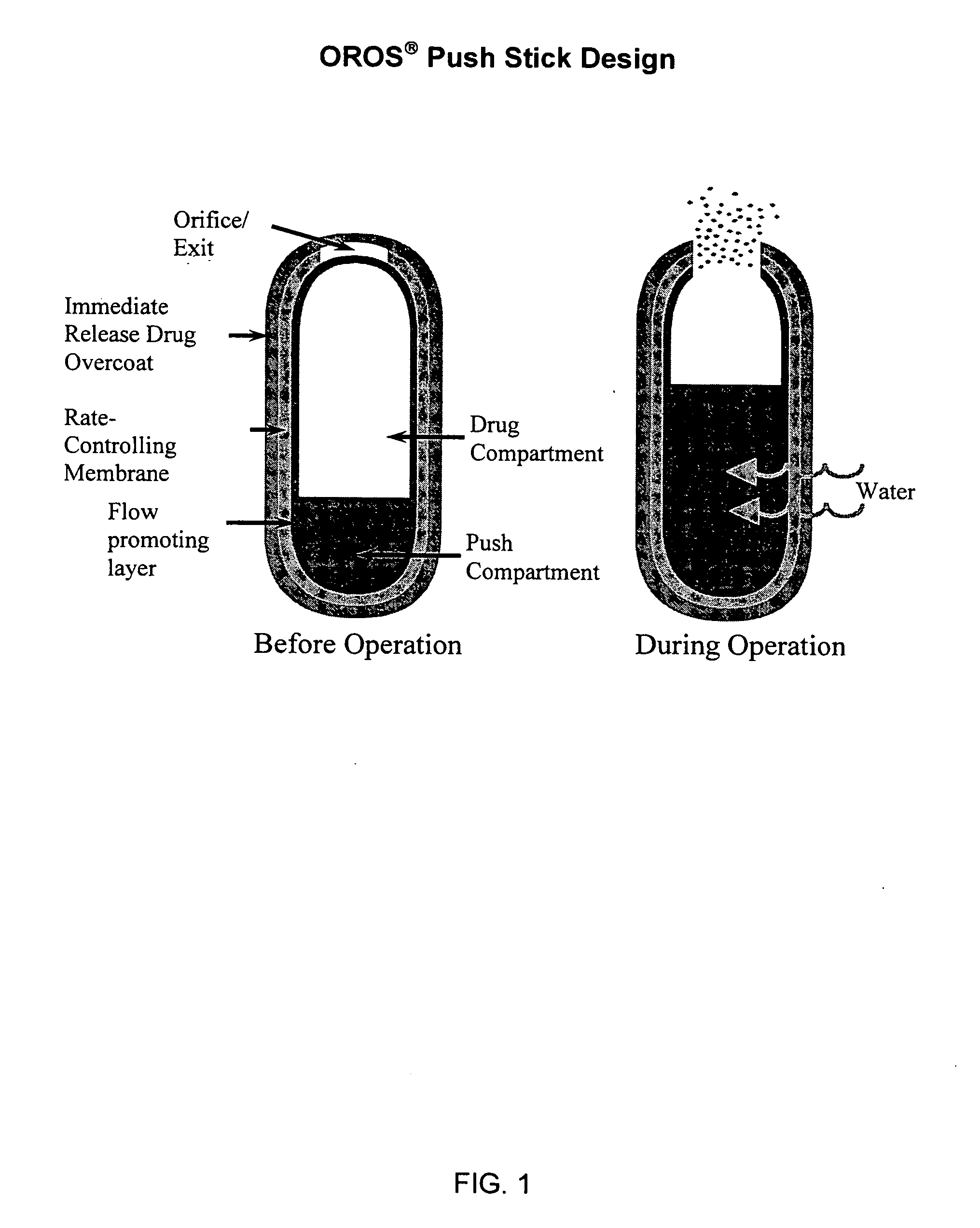
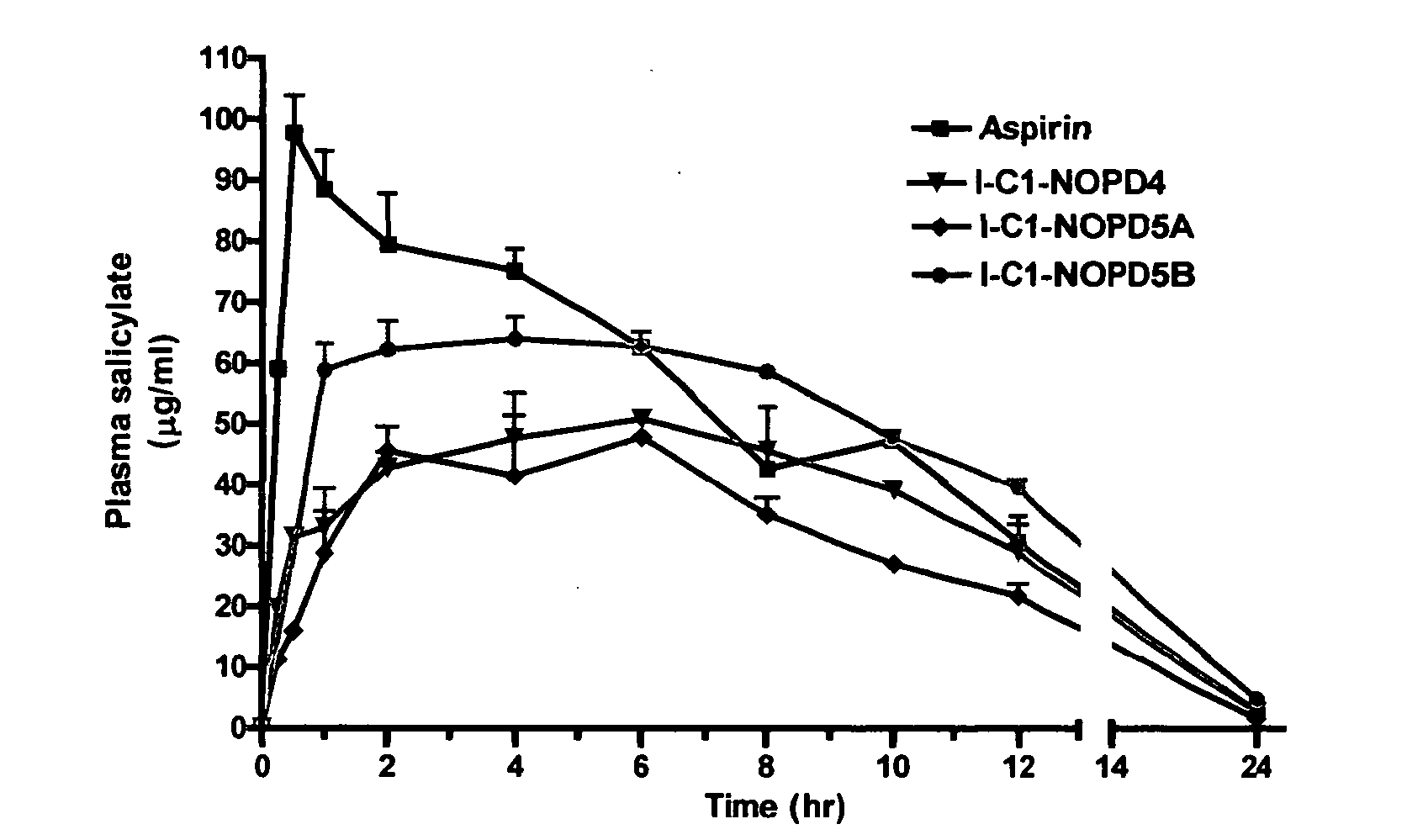
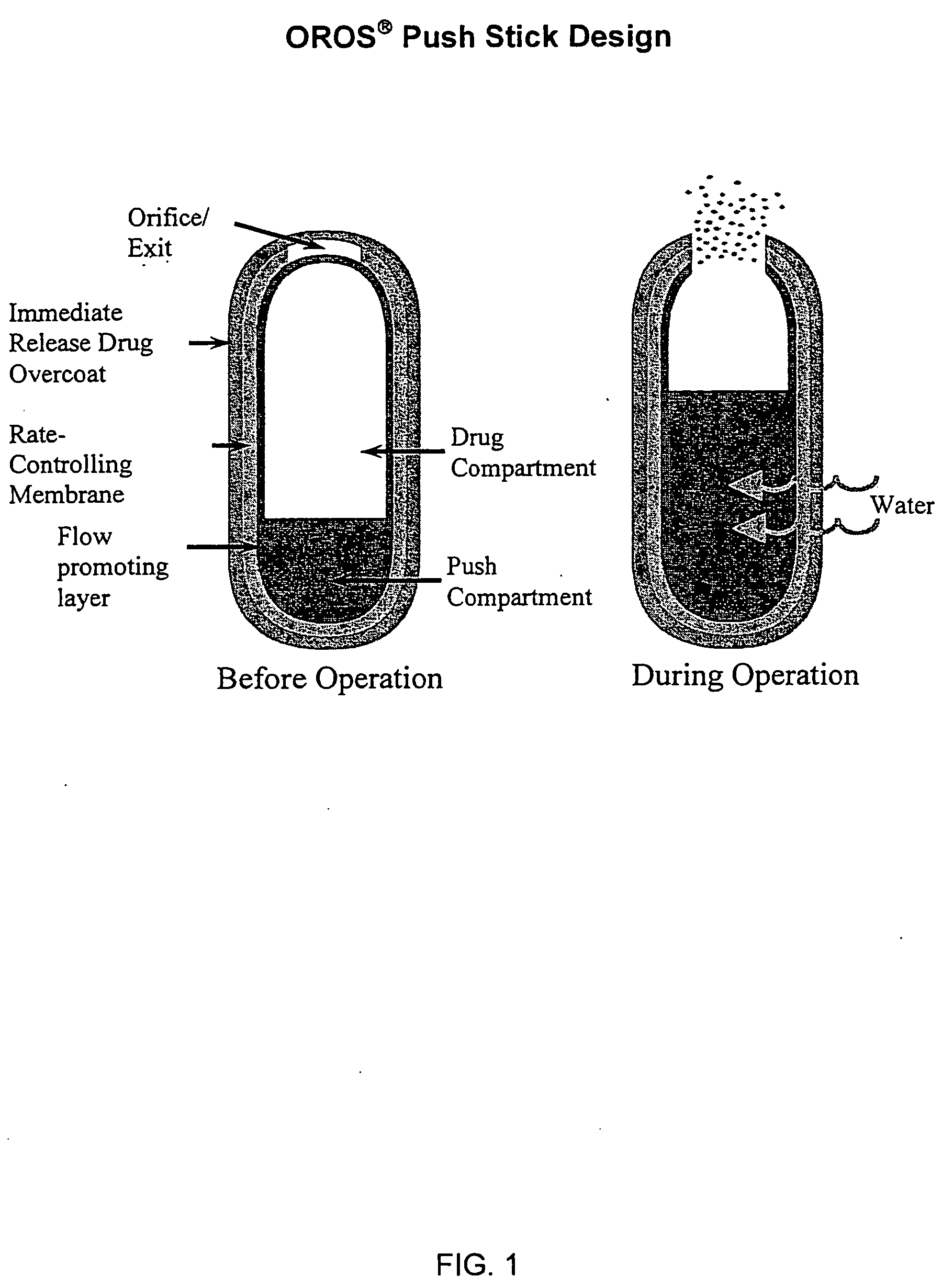
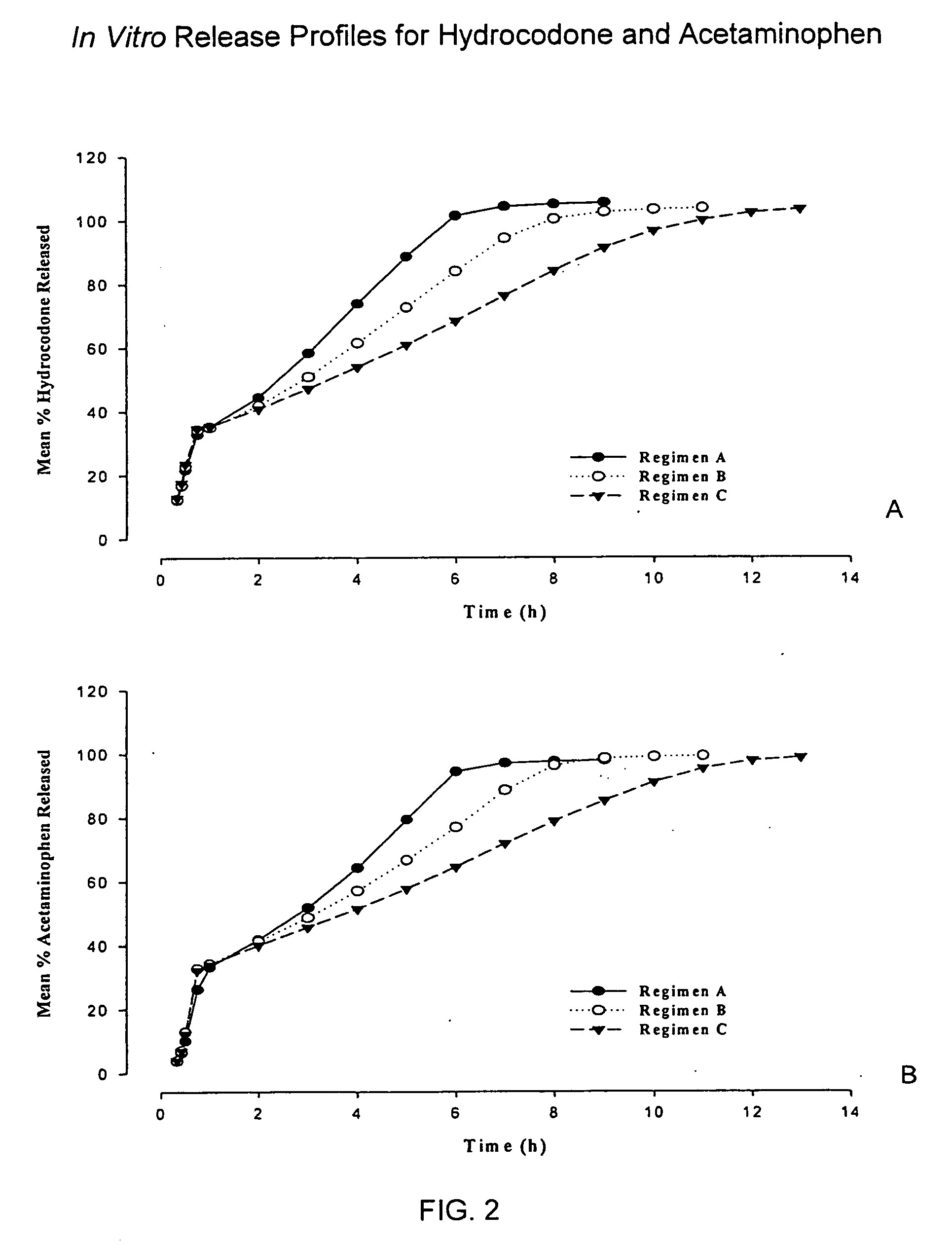
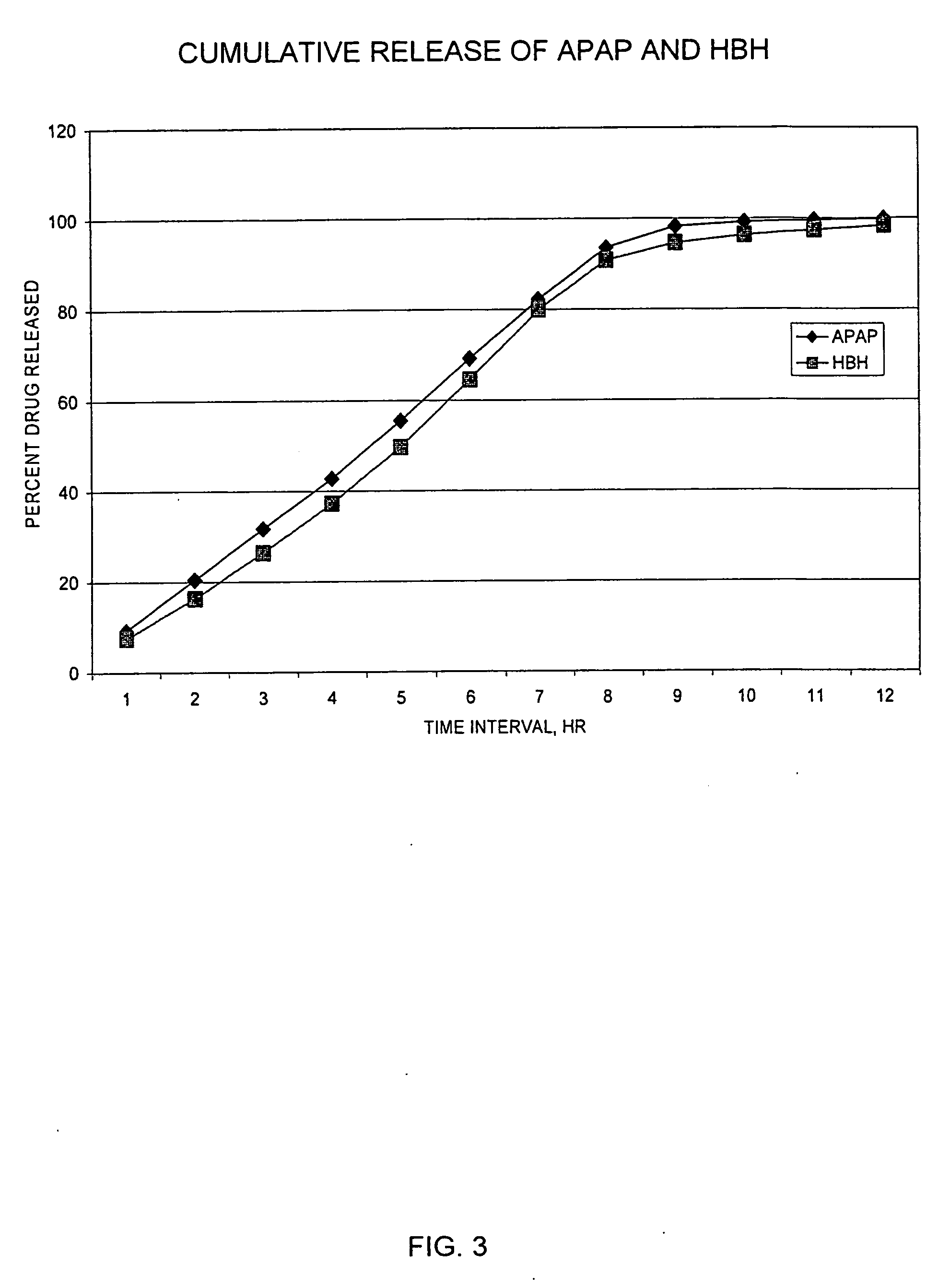
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
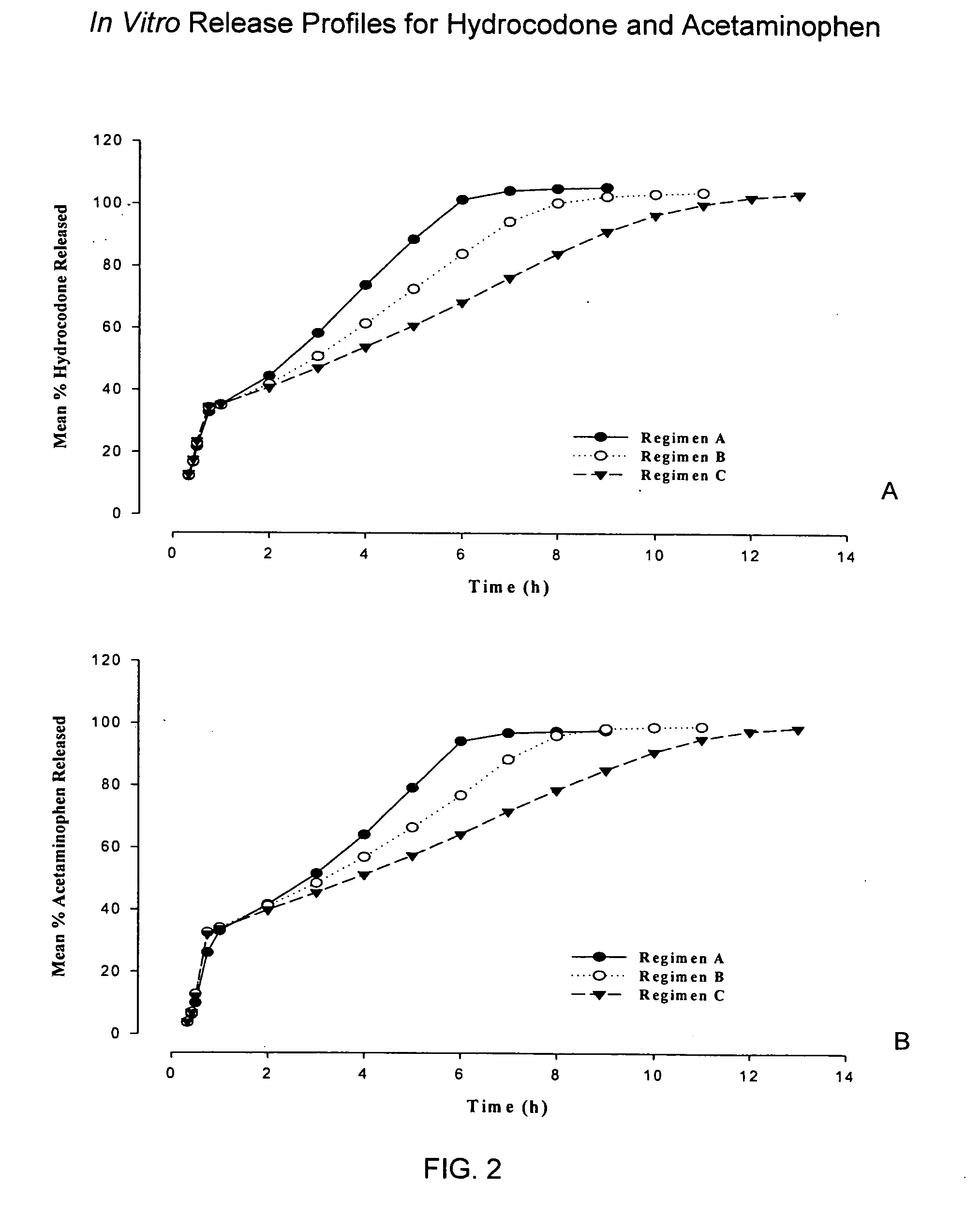
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Novel compounds
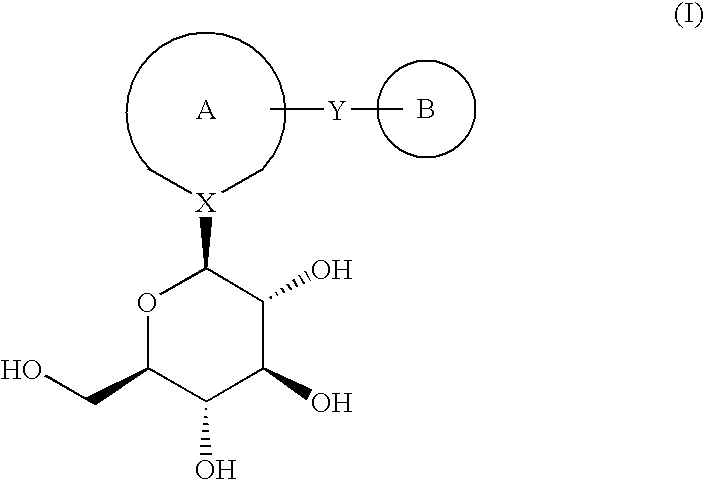
ActiveUS20050233988A1Delayed wound healingUseful in treatmentBiocideSenses disorderNitrogen atomCompound (substance)
A compound of the formula: wherein Ring A and Ring B are: (1) Ring A is an optionally substituted unsaturated monocyclic heterocyclic ring, and Ring B is an optionally substituted unsaturated monocyclic heterocyclic ring, an optionally substituted unsaturated fused heterobicyclic ring, or an optionally substituted benzene ring, (2) Ring A is an optionally substituted benzene ring, and Ring B is an optionally substituted unsaturated monocyclic heterocyclic ring or an optionally substituted unsaturated fused heterobicyclic ring, or (3) Ring A is an optionally substituted unsaturated fused heterobicyclic ring, and Ring B are independently an optionally substituted unsaturated monocyclic heterocyclic ring, an optionally substituted unsaturated fused heterobicyclic ring, or an optionally substituted benzene ring; X is a carbon atom or a nitrogen atom; Y is —(CH2)n— (n is 1 or 2); a pharmaceutically acceptable salt thereof, or a prodrug thereof.
Owner:MITSUBISHI TANABE PHARMA CORP
Compositions and methods for inhibiting expression of Eg5 gene
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of the Eg5 gene (Eg5 gene), comprising an antisense strand having a nucleotide sequence which is less that 30 nucleotides in length, generally 19-25 nucleotides in length, and which is substantially complementary to at least a part of the Eg5 gene. The invention also relates to a pharmaceutical composition comprising the dsRNA together with a pharmaceutically acceptable carrier; methods for treating diseases caused by Eg5 expression and the expression of the Eg5 gene using the pharmaceutical composition; and methods for inhibiting the expression of the Eg5 gene in a cell.
Owner:ALNYLAM PHARMA INC
Vitamin-targeted imaging agents
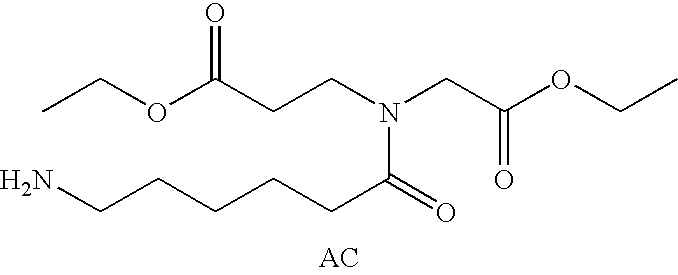
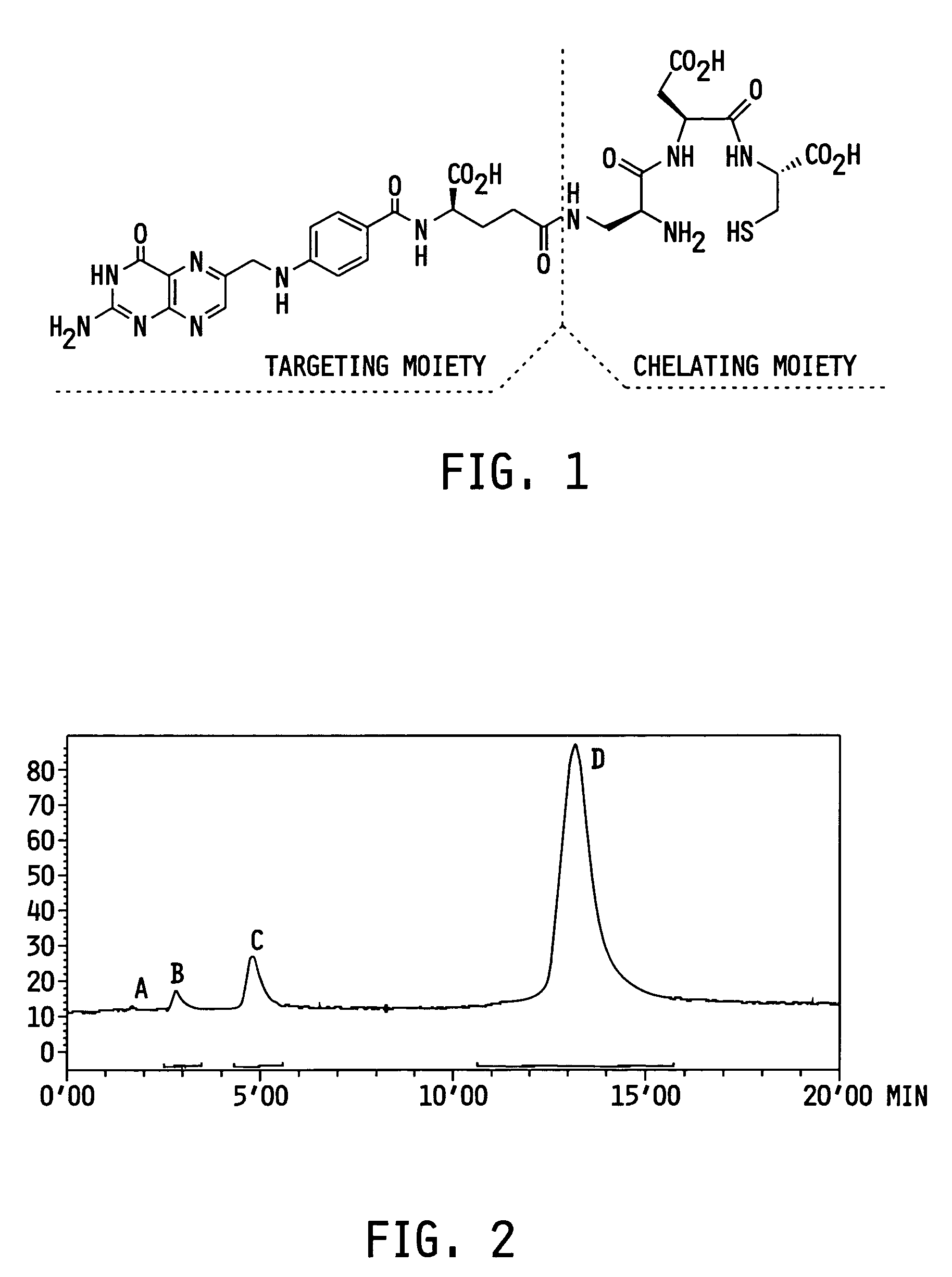
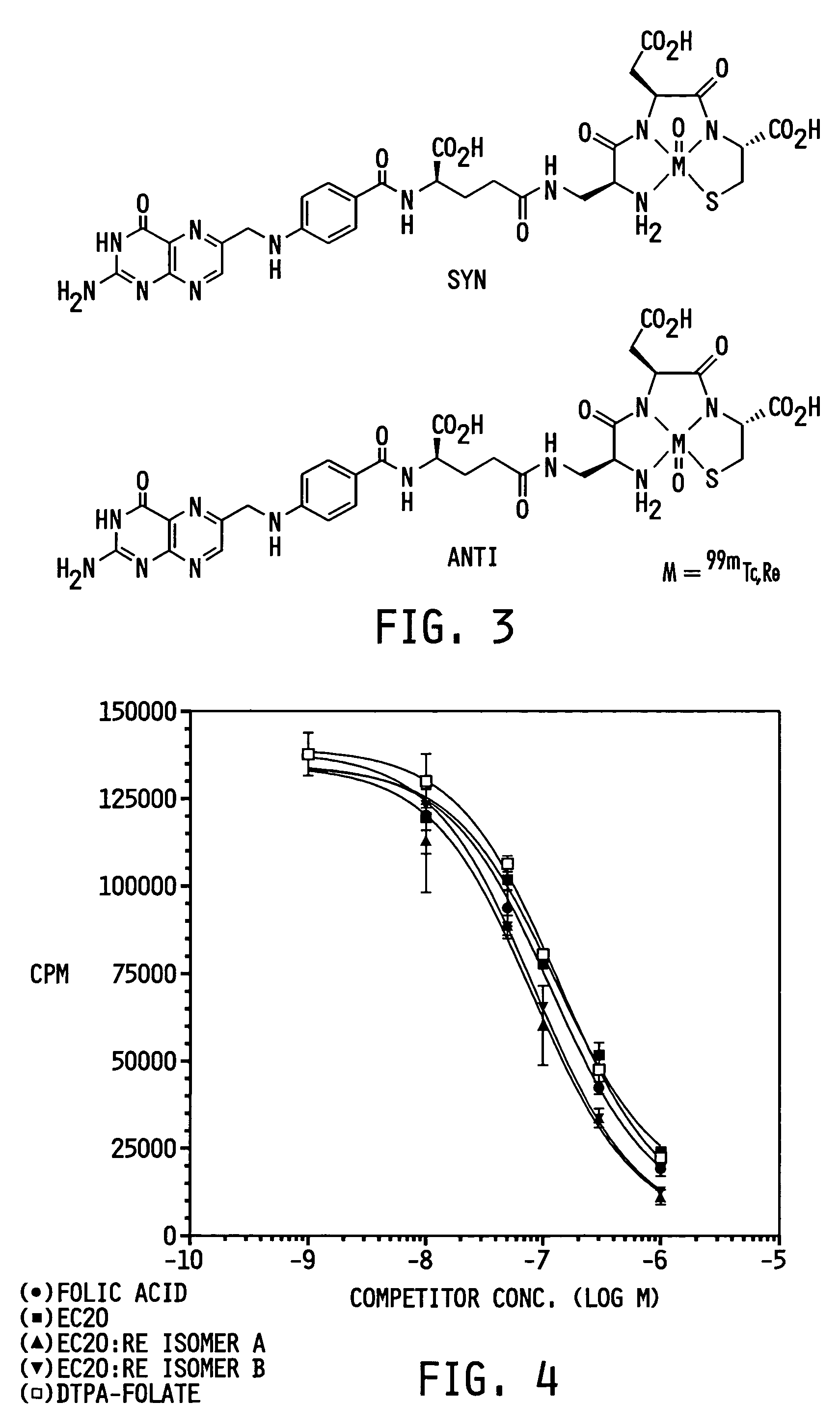
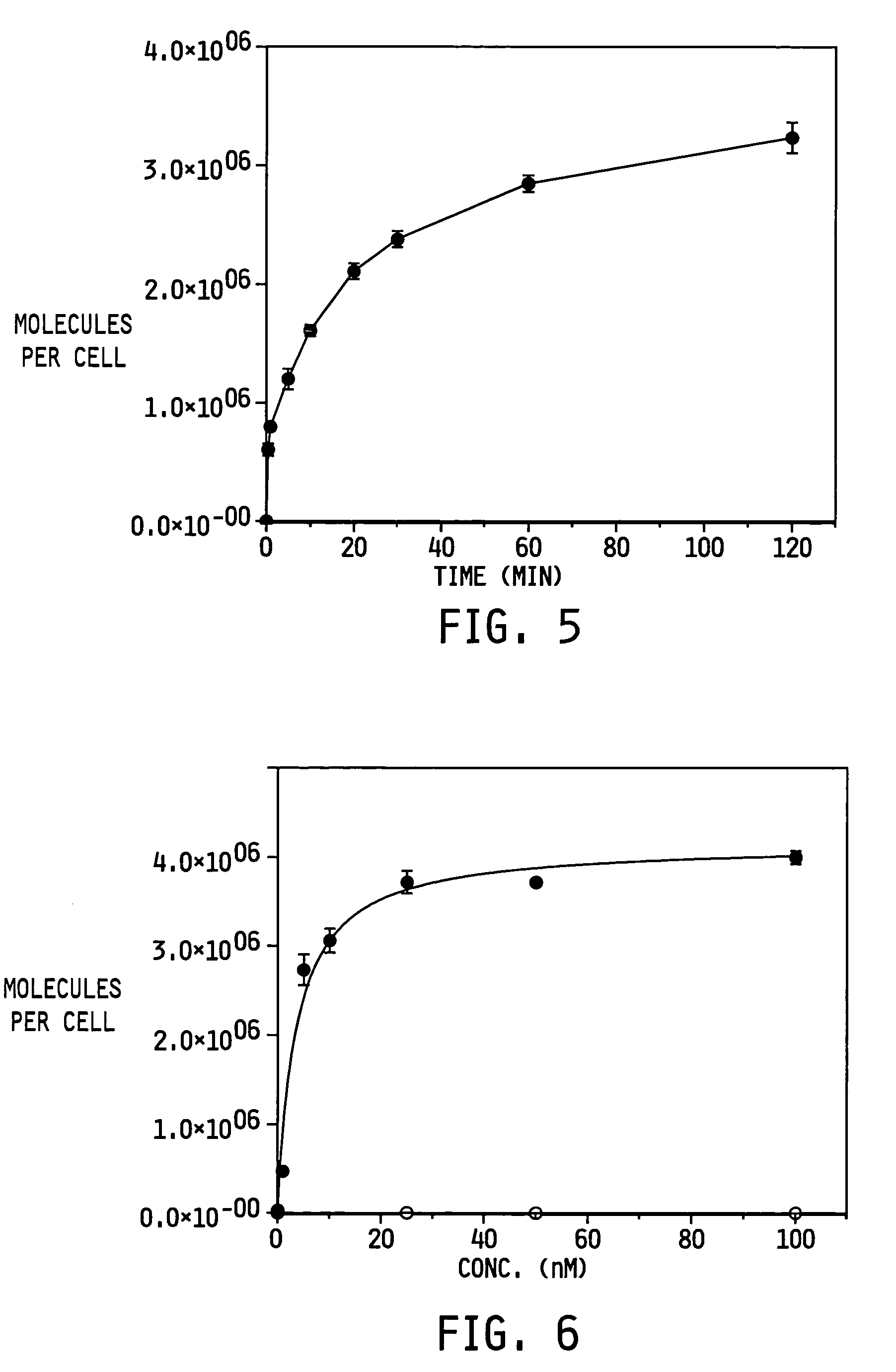
ActiveUS7128893B2Easy to getProducing cost is highPeptide/protein ingredientsRadioactive preparation carriersVitamin receptorSide chain
The invention relates to compounds and methods for targeting radionuclide-based imaging agents to cells having receptors for a vitamin, or vitamin receptor binding derivative or analog thereof, by using such a vitamin as the targeting ligand for the imaging agent. The invention provides a compound of the formulafor use in such methods. In the compound, V is a vitamin that is a substrate for receptor-mediated transmembrane transport in vivo, or a vitamin receptor binding derivative or analog thereof, L is a divalent linker, R is a side chain of an amino acid, M is a cation of a radionuclide, n is 1 or 0, K is 1 or 0, and the compound can be in a pharmaceutically acceptable carrier therefor. The vitamin-based compounds can be used to target radionuclides to cells, such as a variety of tumor cell types, for use in diagnostic imaging of the targeted cells.
Owner:ENDOCTYE INC
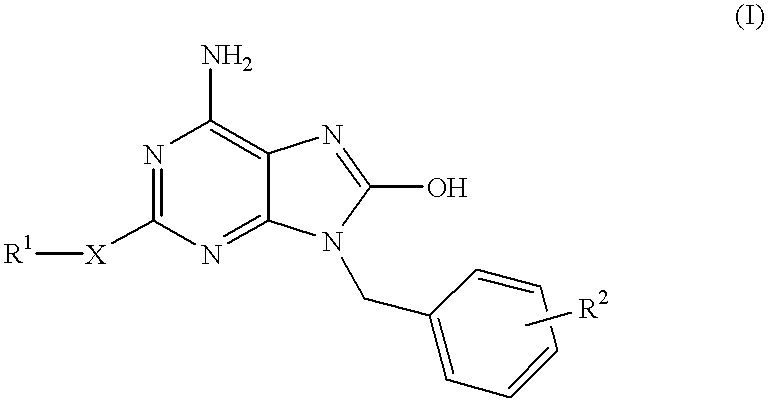
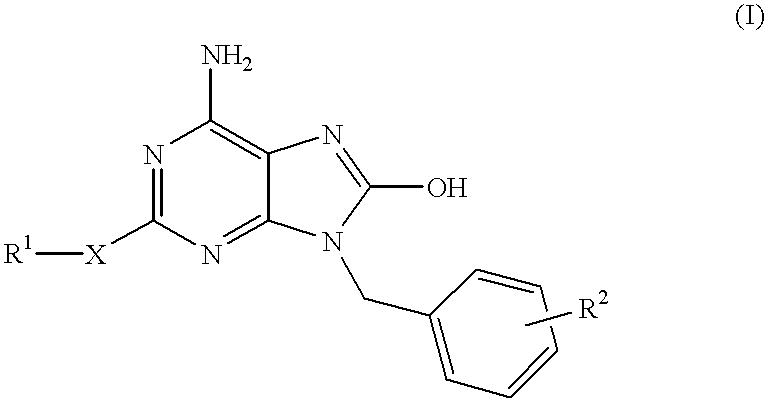
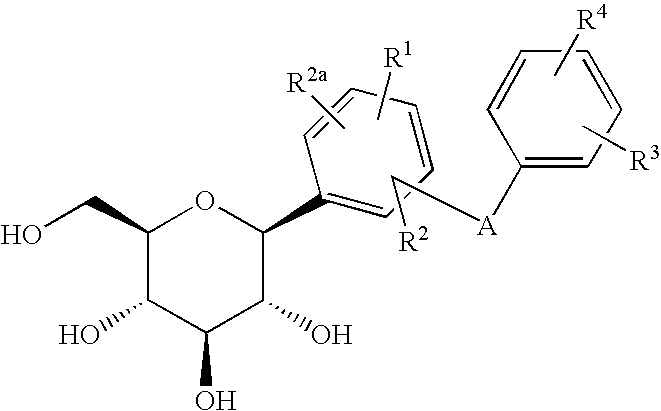
6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators
Compounds having the structure of formula I are provided. In formula I, R1 is H, OH, substituted or unsubstituted C1 to C3 alkyl, C1 to C3 perfluoroalkyl, or COR6; R6 is H, substituted or unsubstituted C1 to C4 alkyl, aryl, substituted or unsubstituted C1 to C4 alkoxy, substituted or unsubstituted C1 to C3 aminoalkyl; R2 and R3 are H, substituted or unsubstituted C1 to C6 alkyl, C1 to C6 perfluoroalkyl, substituted or unsubstituted C2 to C6 alkenyl, substituted or unsubstituted C2 to C6 alkynyl, substituted or unsubstituted C3 to C6 cycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heterocyclic; or R2 and R3 are fused to form spirocyclic rings; R4 is NHR7, OR7, NHSO2R7, or OSO2R7; Q is O, S, NR8, or CR9R10; or a pharmaceutically acceptable salt, ester, or prodrug thereof. Such compounds are useful as progesterone receptor modulators and for treating progesterone receptor related conditions.
Owner:WYETH LLC
SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR
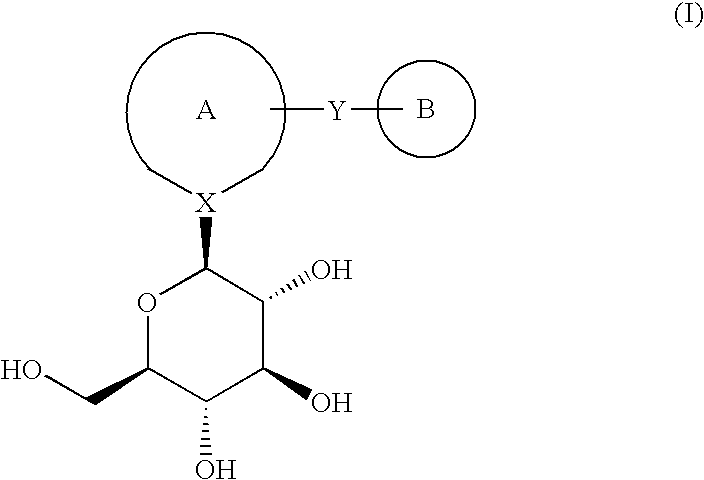
InactiveUS20140221380A1Strong inhibitory activityLess side effectsBiocideOrganic chemistryPyrazinePharmaceutical drug
[Summary][Problem] Provided is a substituted spiropyrido[1,2-a]pyrazine derivative or a pharmaceutically acceptable salt thereof, which is useful as an anti-HIV agent.[Solving Means] The present invention relates to a compound represented by the following formula [I] or [II] or a pharmaceutically acceptable salt thereof:wherein each symbol is as defined in the specification.
Owner:JAPAN TOBACCO INC
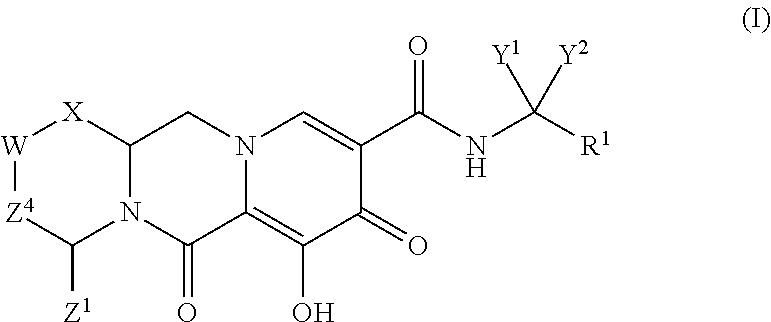
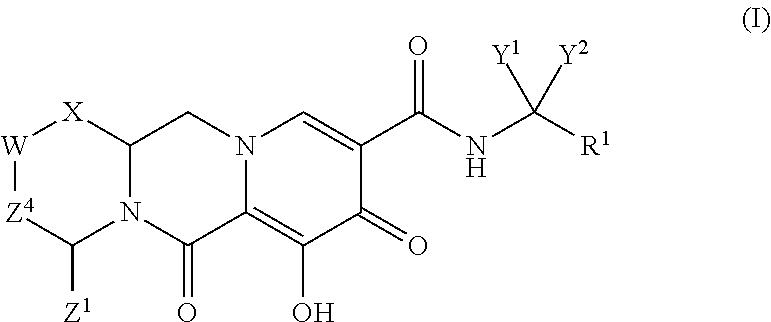
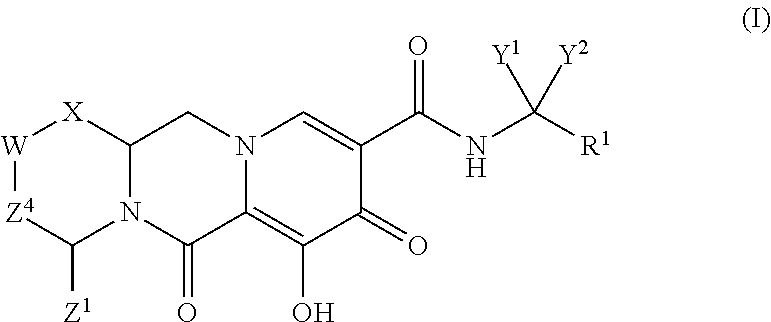
Polycyclic-carbamoylpyridone compounds and their pharmaceutical use
ActiveUS20140221356A1Inhibitory activityReduce HIV replicationBiocideOrganic chemistryImmunodeficiency virusAcyl group
Compounds for use in the treatment of human immunodeficiency virus (HIV) infection are disclosed. The compounds have the following Formula (I):including stereoisomers and pharmaceutically acceptable salts thereof, wherein R1, X, W, Y1, Y2, Z1, and Z4 are as defined herein. Methods associated with preparation and use of such compounds, as well as pharmaceutical compositions comprising such compounds, are also disclosed.
Owner:GILEAD SCI INC
Prodrugs containing novel bio-cleavable linkers
Owner:PIRAMAL ENTERPRISES LTD
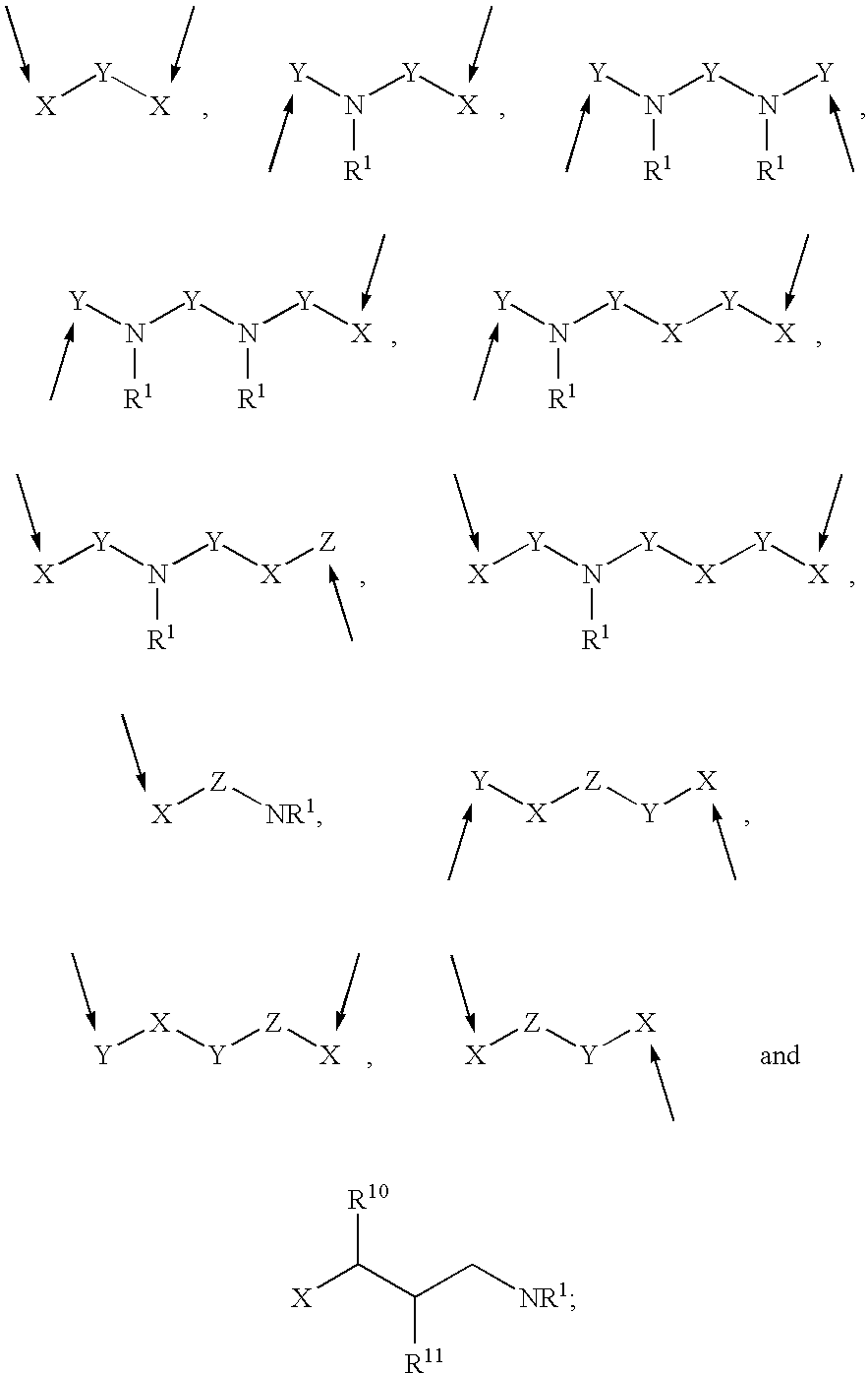
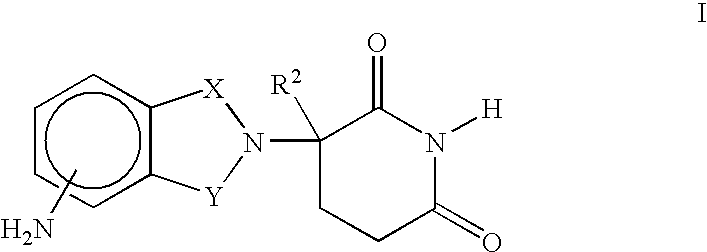
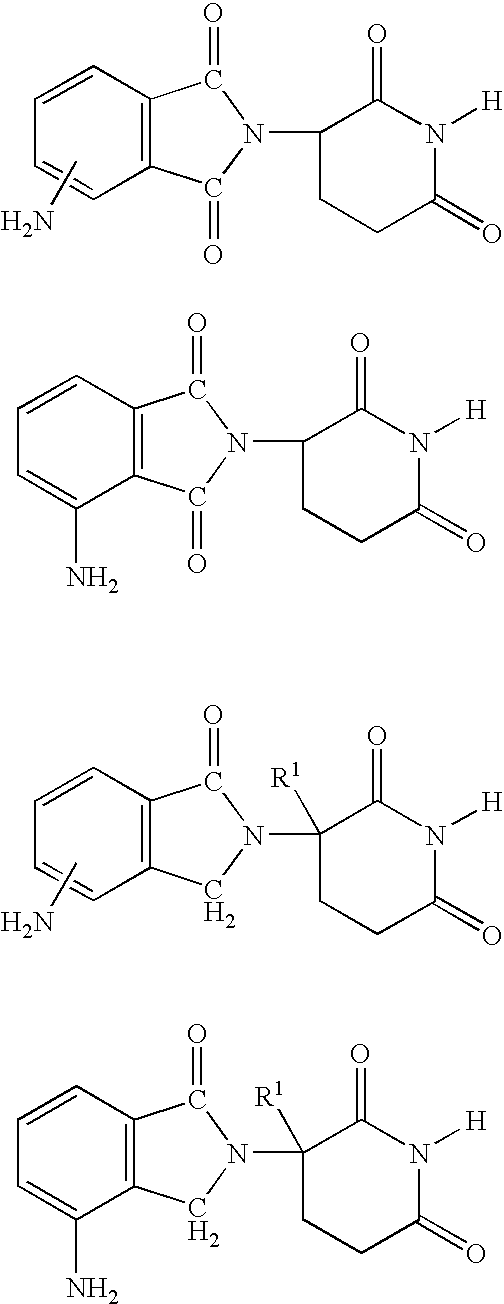
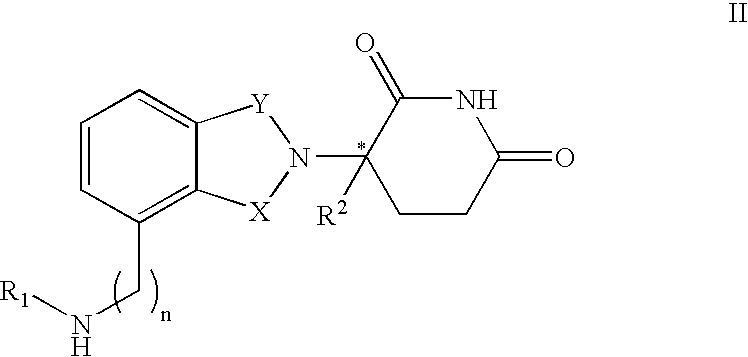
Methods of using 3-(4-amino-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for the treatment and management of myelodysplastic syndromes
Methods of treating, preventing and / or managing myclodysplastic syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient, and / or the transplantation of blood or cells. Specific second active ingredients are capable of affecting or blood cell production. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Therapeutic treatment and prevention of infections with a bioactive material(s) encapuslated within a biodegradable-bio-compatable polymeric matrix
InactiveUS6902743B1Induce productionSustained release of active agent over timePowder deliveryPeptide/protein ingredientsTherapeutic treatmentActive agent
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer having a molar composition of lactide / glycolide from 90 / 10 to 40 / 60, which may contain a pharmaceutically-acceptable adjuvant, as a blend of uncapped free carboxyl end group and end-capped forms ranging to ratios from 100 / 0 to 1 / 99.
Owner:ARMY UNITED STATES GOVERNMENT AS REPRESENTED BY THE SEC OF THE
6,6-Bicyclic ring substituted heterobicyclic protein kinase inhibitors
ActiveUS20060235031A1Treatment and/or prevention of hyperproliferative diseasesBiocideSenses disorderDiseasePTK Inhibitors
Compounds of the formula and pharmaceutically acceptable salts thereof, wherein X1, X2, X3, X4, X5, X6, X7, R1, and Q1 are defined herein, inhibit the IGF-1R enzyme and are useful for the treatment and / or prevention of hyperproliferative diseases such as cancer, inflammation, psoriasis, allergy / asthma, disease and conditions of the immune system, disease and conditions of the central nervous system.
Owner:ACERTA PHARMA BV
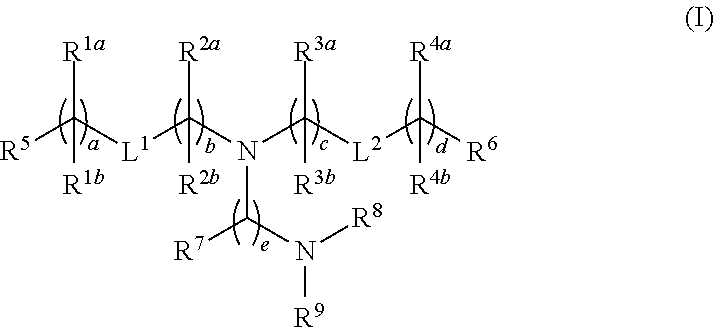
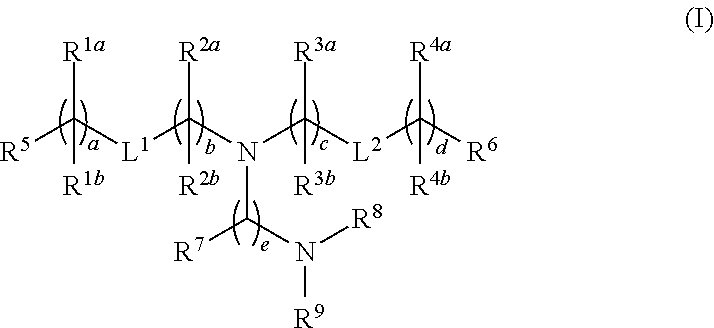
Novel lipids and lipid nanoparticle formulations for delivery of nucleic acids
Compounds are provided having the following structure:or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof, wherein R1a, R1b, R2a, R2b, R3a, R3b, R4a, R4b, R5, R6, R7, R8, R9, L1, L2, a, b, c, d and e are as defined herein. Use of the compounds as a component of lipid nanoparticle formulations for delivery of a therapeutic agent, compositions comprising the compounds and methods for their use and preparation are also provided.
Owner:ACUITAS THERAPEUTICS INC
Controlled release formulations of opioid and nonopioid analgesics
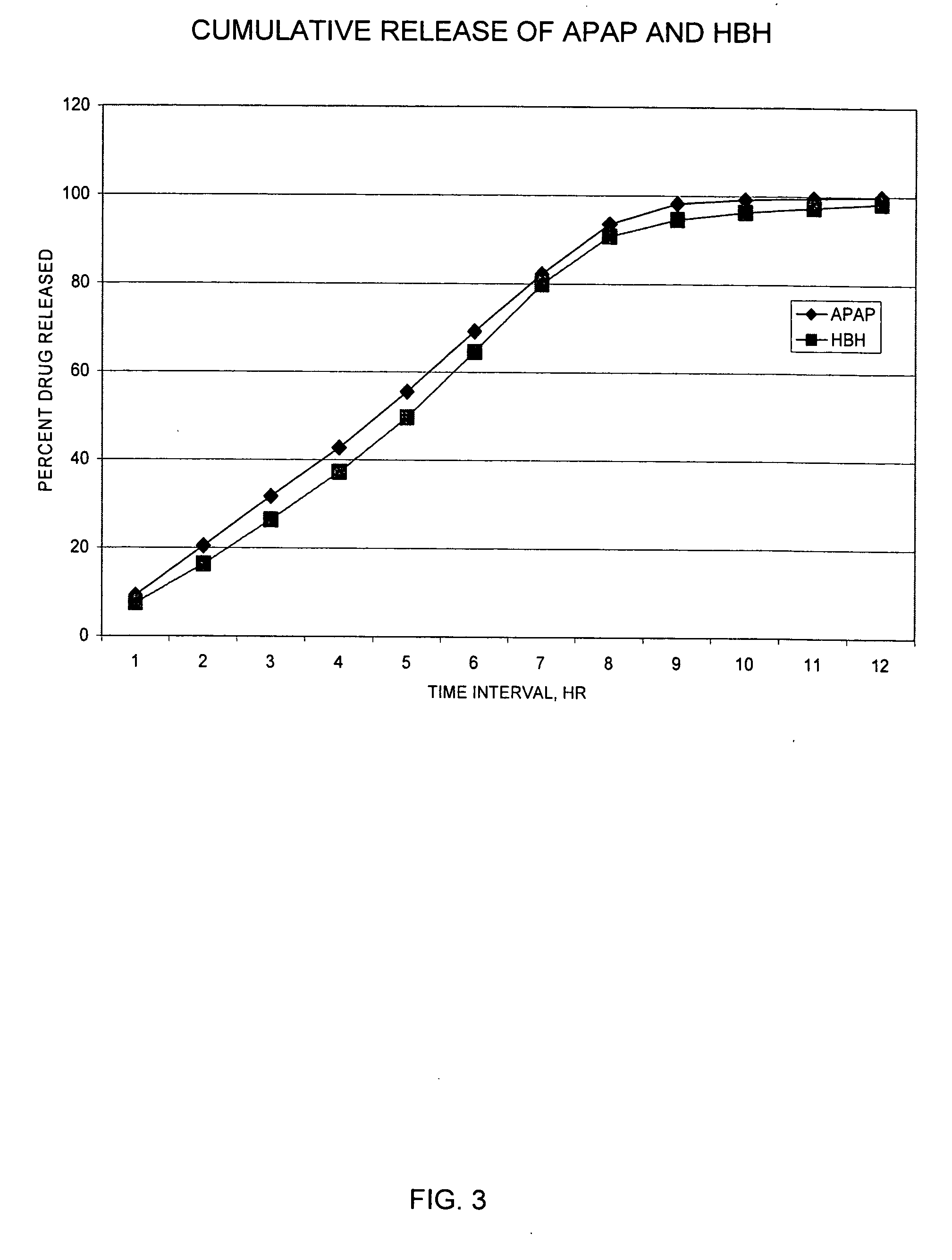
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
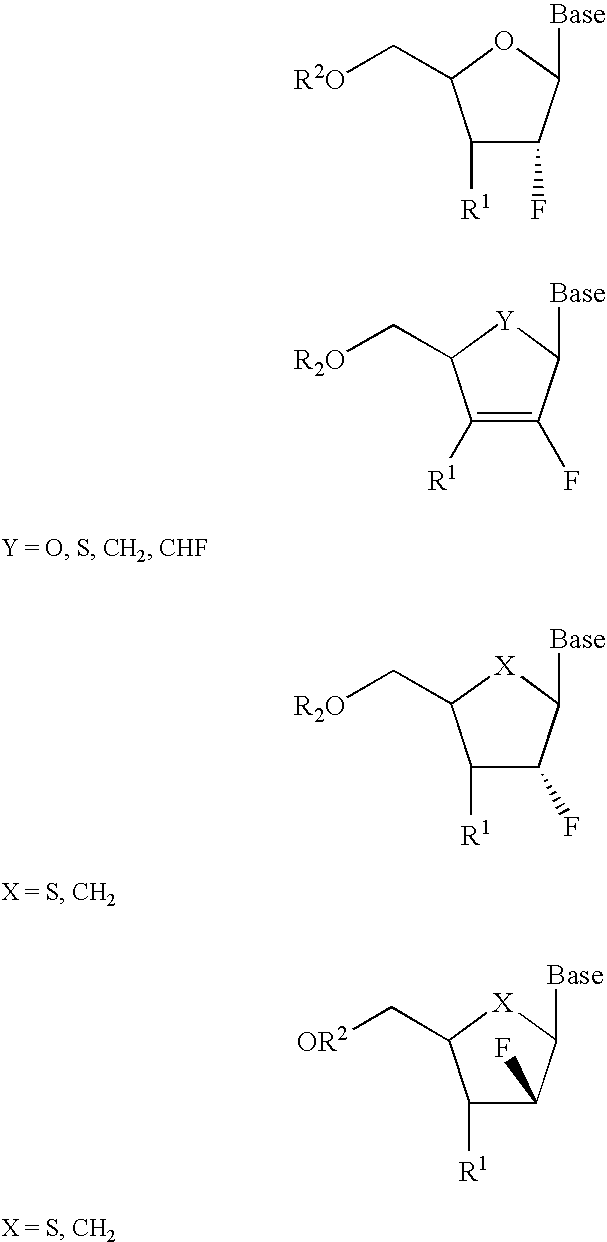
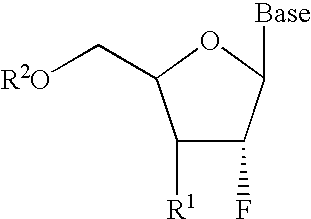
2′-fluoronucleosides
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
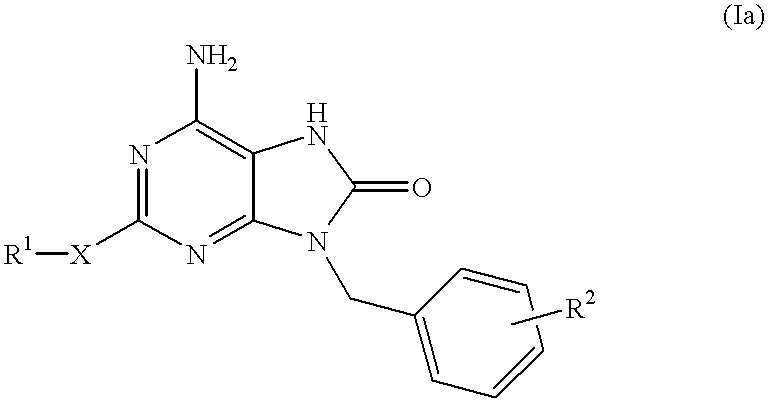
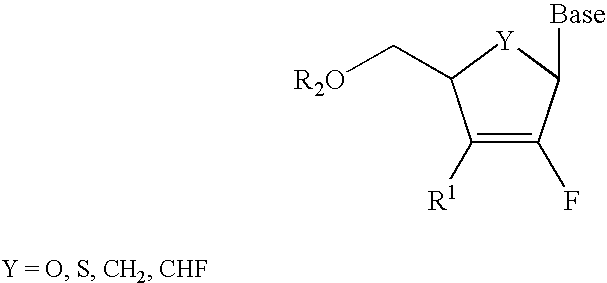
Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds
Compounds of Formula I-a and all pharmaceutically-acceptable forms thereof, are described herein. The variables R1, R2, R3, Z1, Q, and A shown in Formula I-a are defined herein. Pharmaceutical compositions containing one or more compounds of Formula I-a, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein. Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I-a effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases. Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity. Methods of treatment include administering a compound of Formula I-a as a single active agent or administering a compound of Formula I-a in combination with one or more other therapeutic agent. A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I-a under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
Substituted nucleosides, preparation thereof and use as inhibitors of RNA viral polymerases
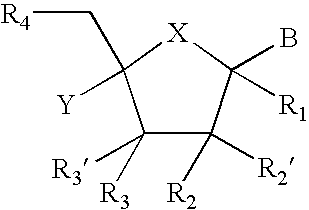
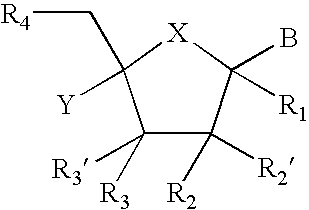
Provided are compounds represented by: X is O, S or NR6, R1 is H or (CH2)mR5, R2, R2', R3 and R3' are independently NO2, N3 or (CH2)mR5, OH R4 is H, OR6, SR6, NR6R6a, CN, C(O)OR6, C(O)NR6R6a, R6, OR7 or (CH2)nR7, R5 is H, halo, OR6, SR6, NR6R6a, CN, C(O)OR6, C(O)NR6R6a, R6, OR7 or (CH2)mR7, R6 and R6a are individually H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl or substituted aryl, R7 is: R8 is H, F, SR9 or OR9, R9 is H, alkyl, alkenyl, alkynyl, aryl or hydroxyprotecting group, Y is H, CH3 or (CH2)mR5, Z is O or S W is CH2, CF2, CHF or O, m is 0-4, B is adenine, guanine, cytosine, uracil, thymine, modified purines and pyrimidines substituted pyridines, five membered heterocycles substituted by at least one of amines, substituted amines, amides, substituted amides, esters, halogens, alkyls, ethers; and pharmaceutically acceptable salts thereof and prodrugs thereof. These ring systems may be substituted.
Owner:BIOCRYST PHARM INC
Fused bicyclic mTOR inhibitors
ActiveUS20070112005A1Useful in treatmentBiocideNervous disorderDiscovery and development of mTOR inhibitorsChemistry
Compounds represented by Formula (I) or a pharmaceutically acceptable salt thereof, are inhibitors of mTOR and useful in the treatment of cancer.
Owner:OSI PHARMA INC
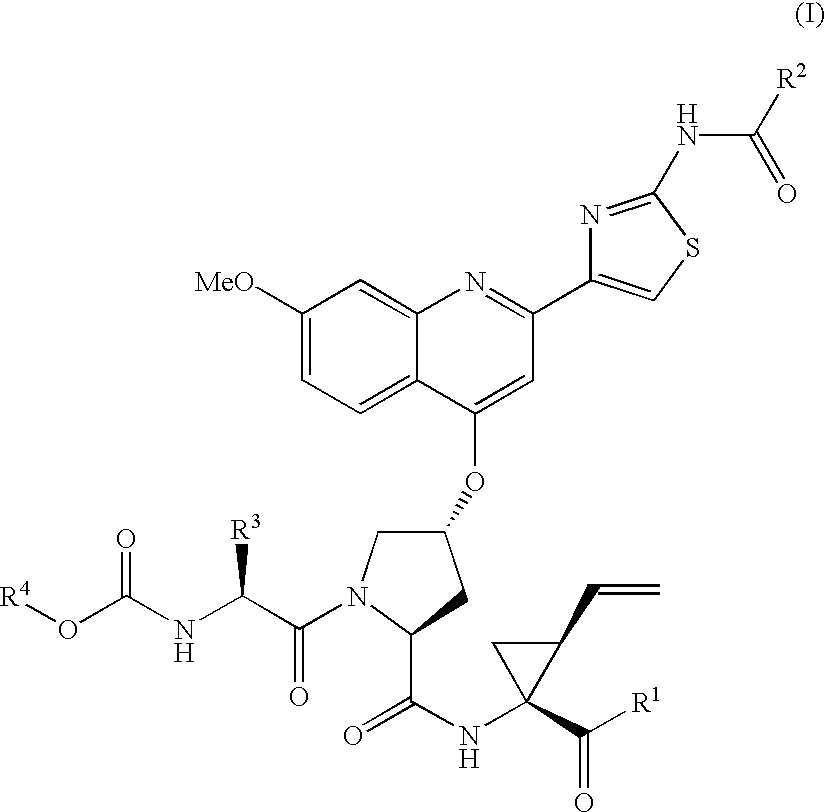
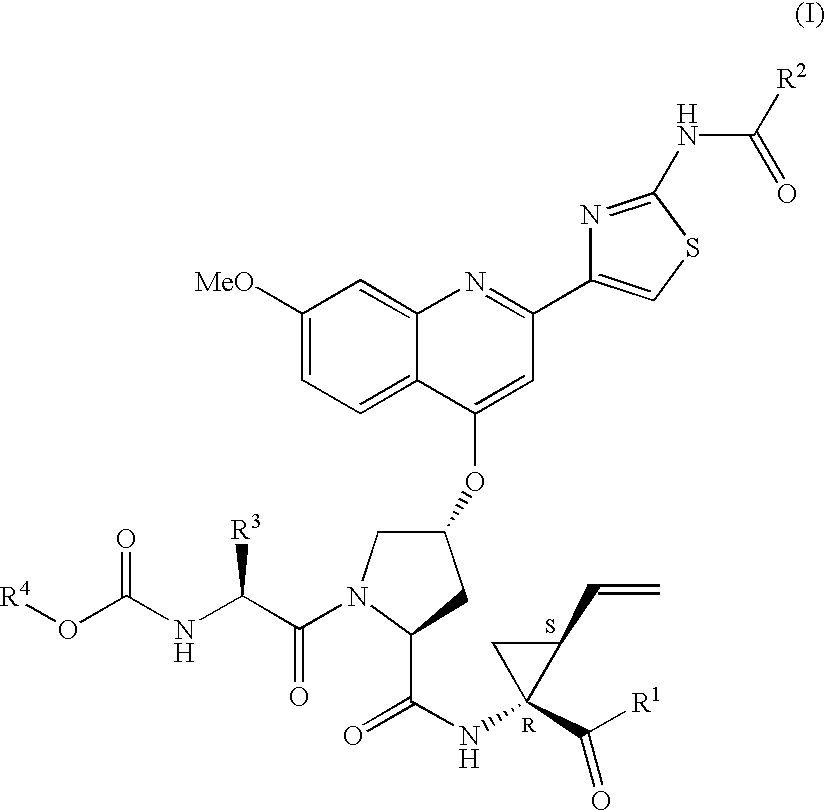
Hepatitis C inhibitor tri-peptides
ActiveUS7091184B2Better pharmacokinetic profileNot significant inhibitory activityBiocideDipeptide ingredientsHcv ns3 proteaseHepatitis C
Compounds of formula (I):wherein R1 is hydroxyl or sulfonamide derivative; R2 is t-butyl or —CH2—C(CH3)3 or —CH2-cyclopentyl; R3 is t-butyl or cyclohexyl and R4 is cyclobutyl, cyclopentyl or cyclohexyl; or a pharmaceutically acceptable salt thereof, are described as useful as inhibitor of the HCV NS3 protease.
Owner:BOEHRINGER INGELHEIM INT GMBH
Adjuvant in the form of a lipid-modified nucleic acid
The present invention relates to an immune-stimulating adjuvant in the form of a lipid-modified nucleic acid, optionally in combination with further adjuvants. The invention relates further to a pharmaceutical composition and to a vaccine, each containing an immune-stimulating adjuvant according to the invention, at least one active ingredient and optionally a pharmaceutically acceptable carrier and / or further auxiliary substances and additives and / or further adjuvants. The present invention relates likewise to the use of the pharmaceutical composition according to the invention and of the vaccine according to the invention for the treatment of infectious diseases or cancer diseases. Likewise, the present invention includes the use of the immune-stimulating adjuvant according to the invention in the preparation of a pharmaceutical composition for the treatment of cancer diseases or infectious diseases.
Owner:CUREVAC GMBH
Methods and related compositions for reduction of fat and skin tightening
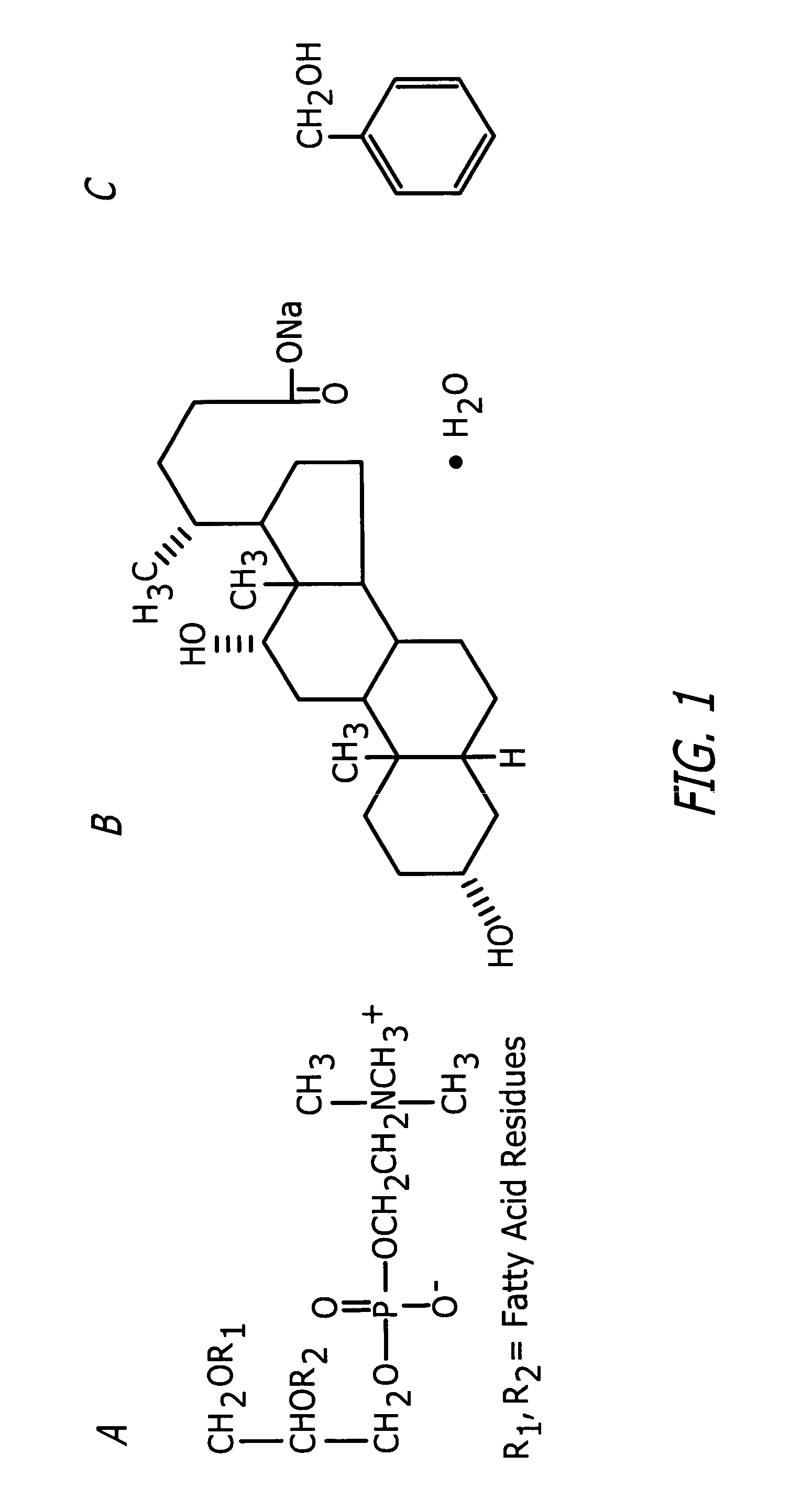
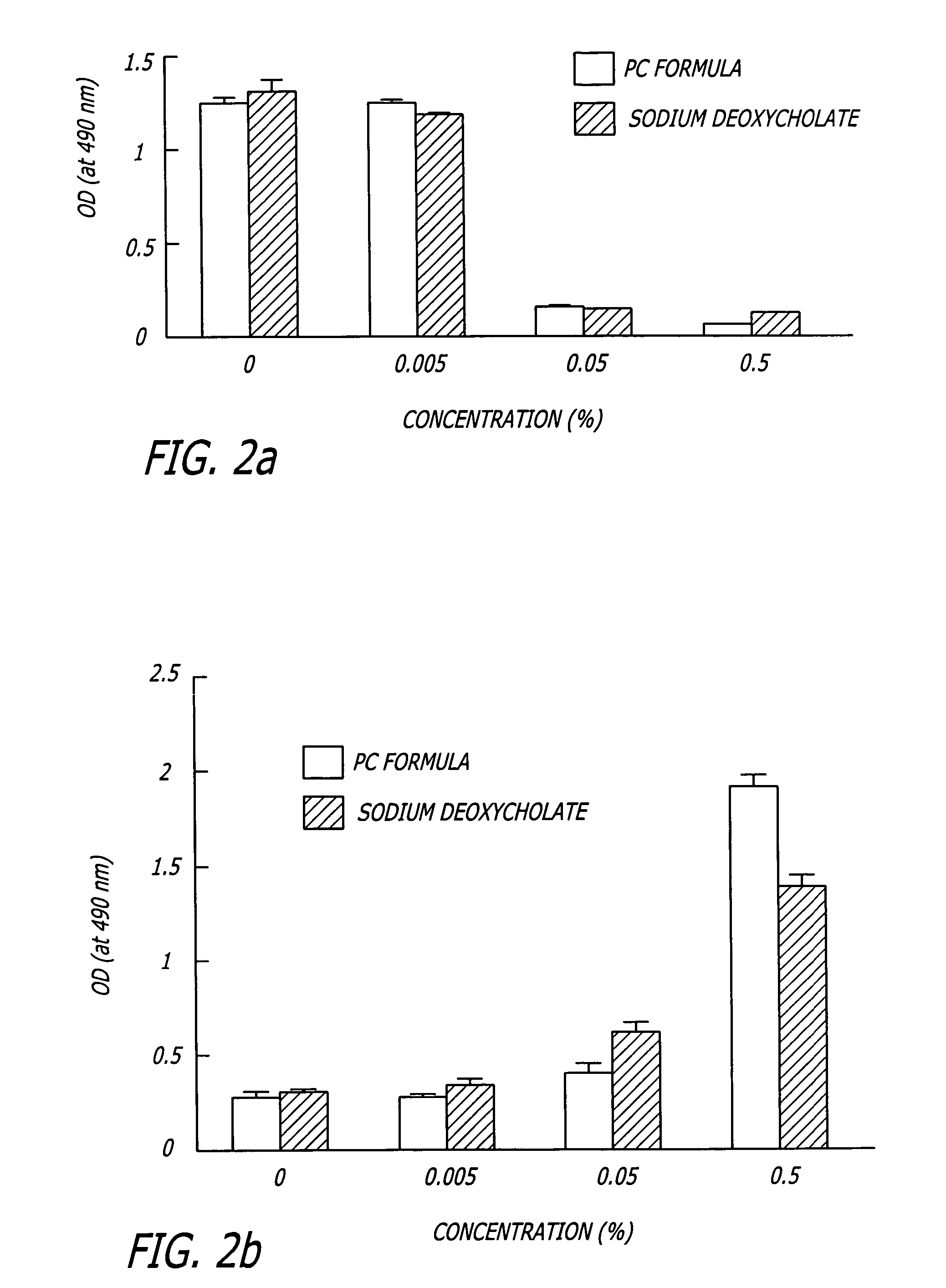
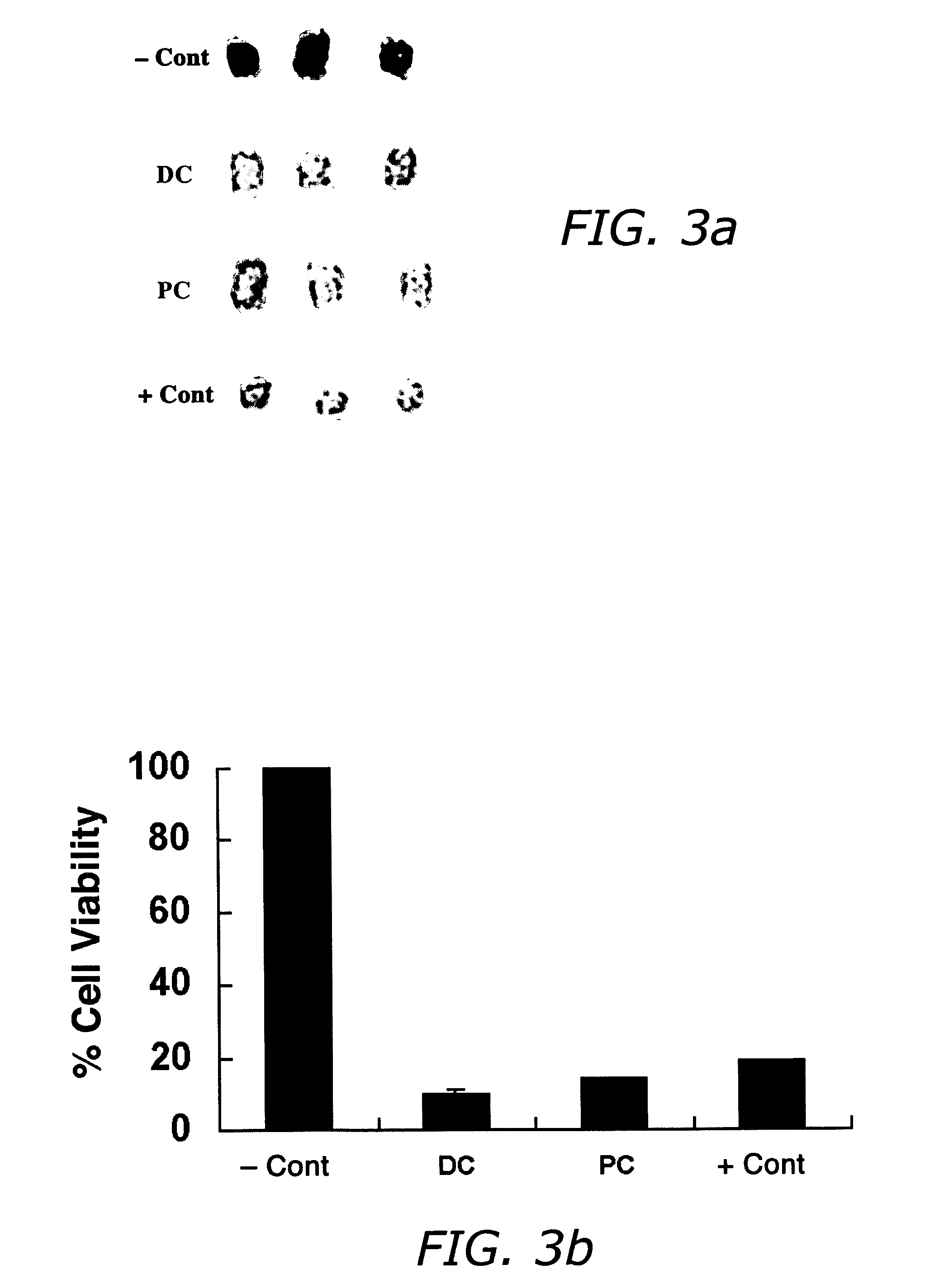
InactiveUS20060127468A1Efficient tighteningTighten regionBiocideCosmetic preparationsCelluliteExcipient
Compositions and methods useful in the reduction of localized fat deposits and tightening of loose skin in subjects in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion or anti-dispersion agents and pharmaceutically acceptable excipients. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including, for example, lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
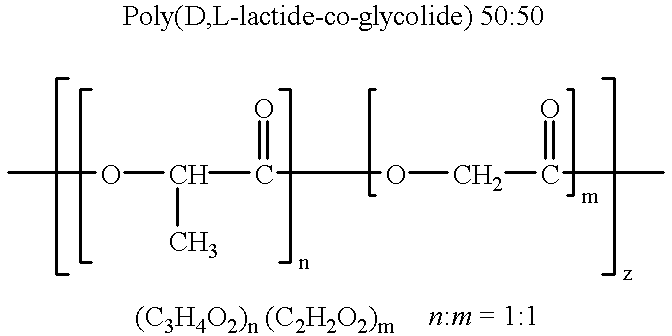
![6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators 6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f527f94c-1c91-4445-8bcc-7ae4c7ef053d/US07247625-20070724-C00001.png)
![6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators 6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f527f94c-1c91-4445-8bcc-7ae4c7ef053d/US07247625-20070724-C00002.png)
![6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators 6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f527f94c-1c91-4445-8bcc-7ae4c7ef053d/US07247625-20070724-C00003.png)
![SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaca0958-e088-450b-a622-0be890c729fb/US20140221380A1-20140807-C00001.png)
![SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaca0958-e088-450b-a622-0be890c729fb/US20140221380A1-20140807-C00002.png)
![SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaca0958-e088-450b-a622-0be890c729fb/US20140221380A1-20140807-C00003.png)
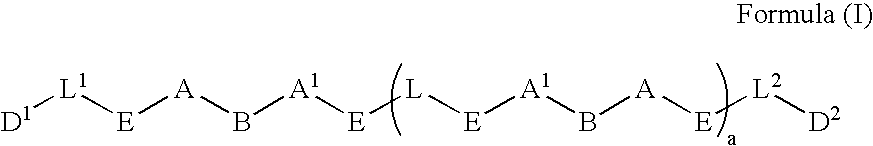
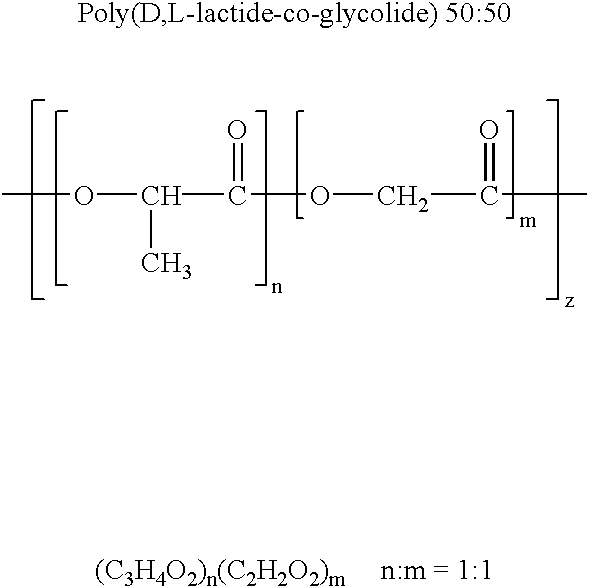



![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00001.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00002.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00003.png)