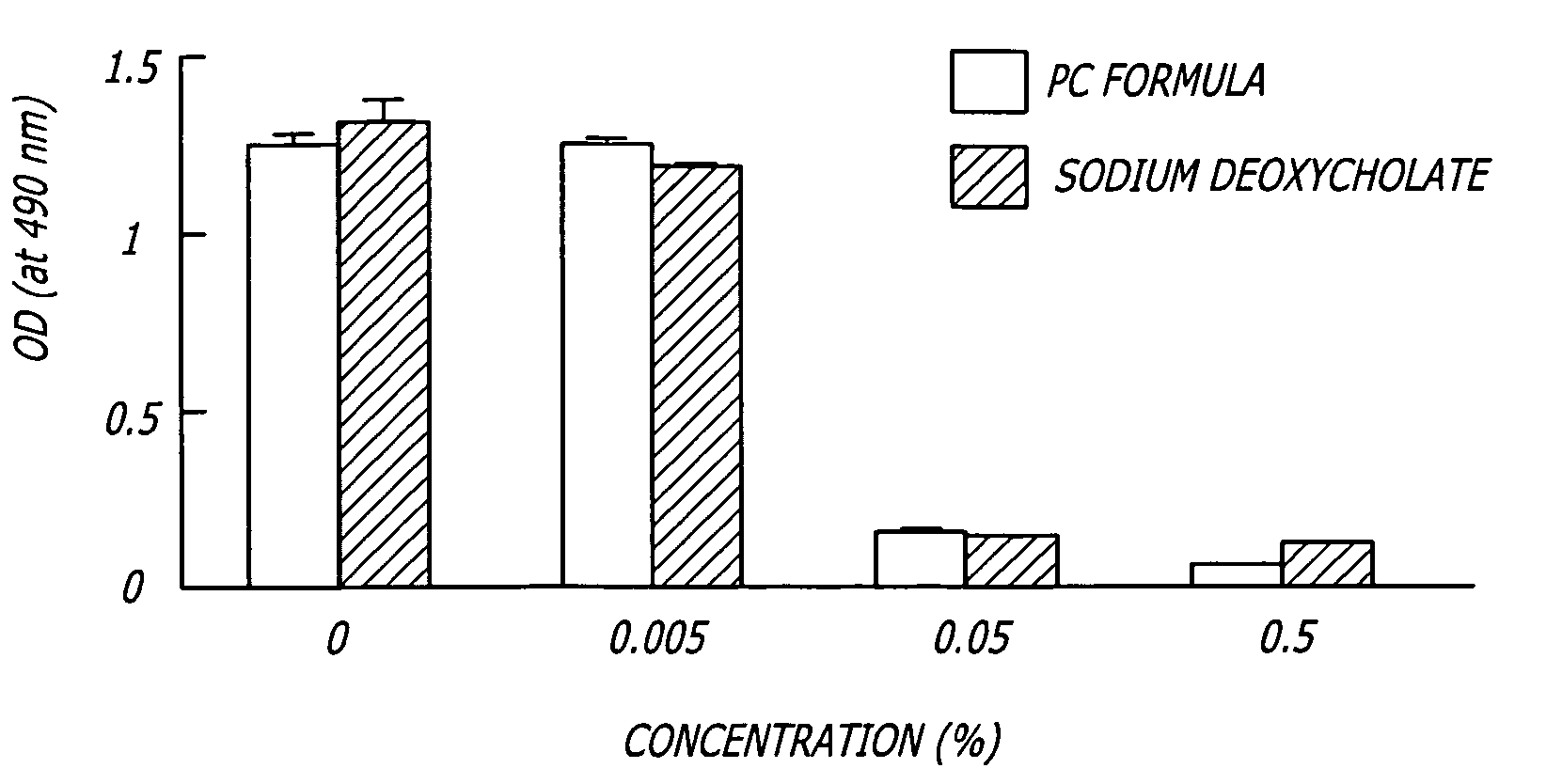
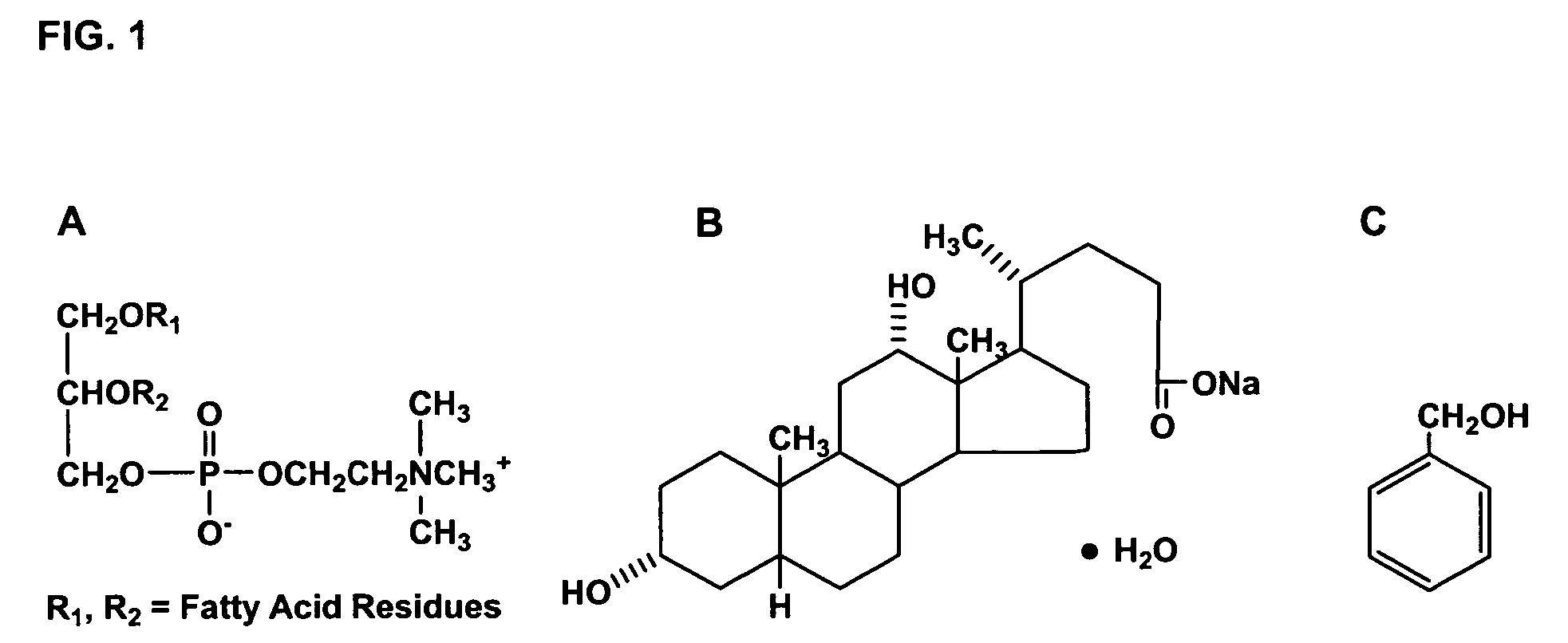
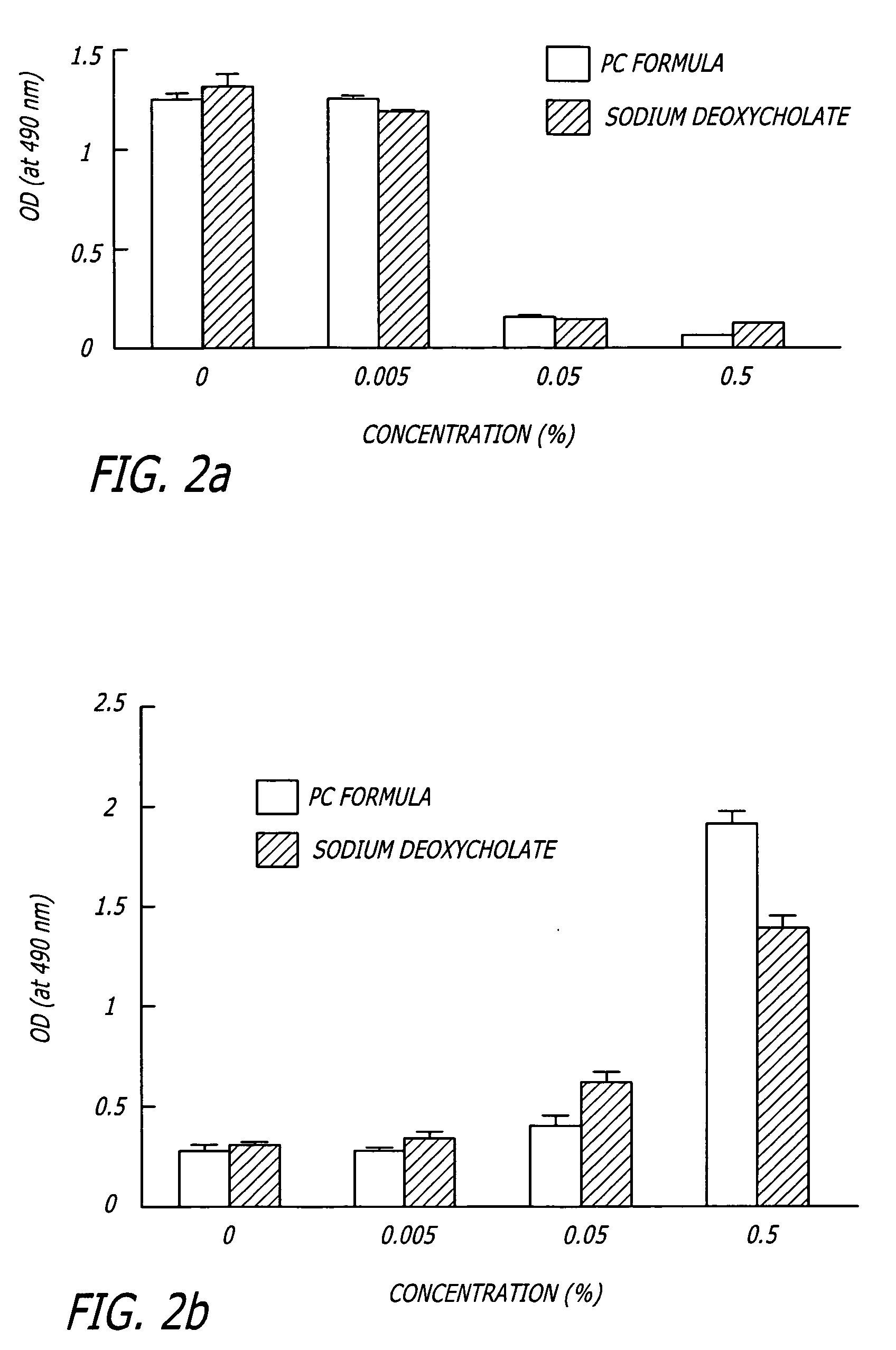
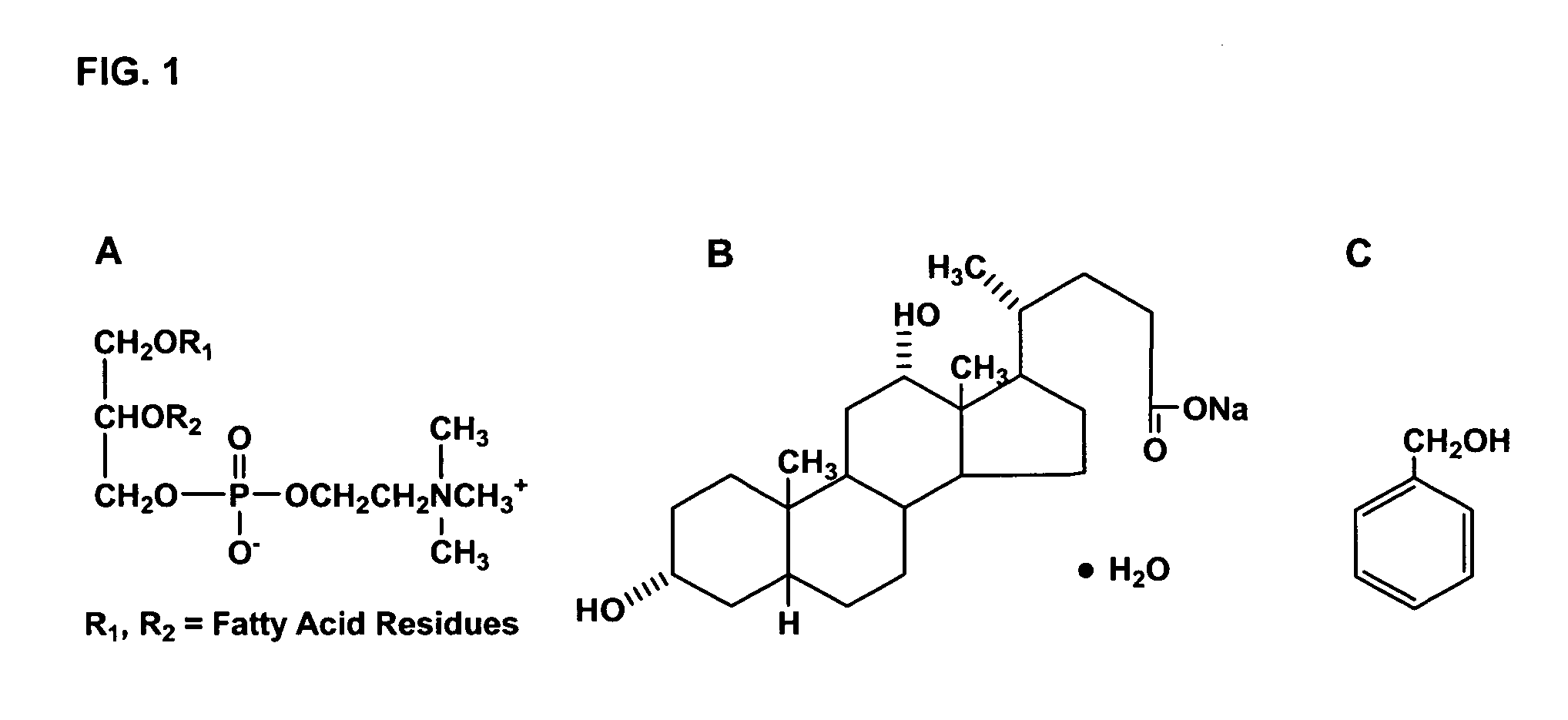
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
843 results about "Analgesic agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Other psychotropic analgesic agents include ketamine (an NMDA receptor antagonist), clonidine and other α2-adrenoreceptor agonists, and mexiletine and other local anaesthetic analogues.
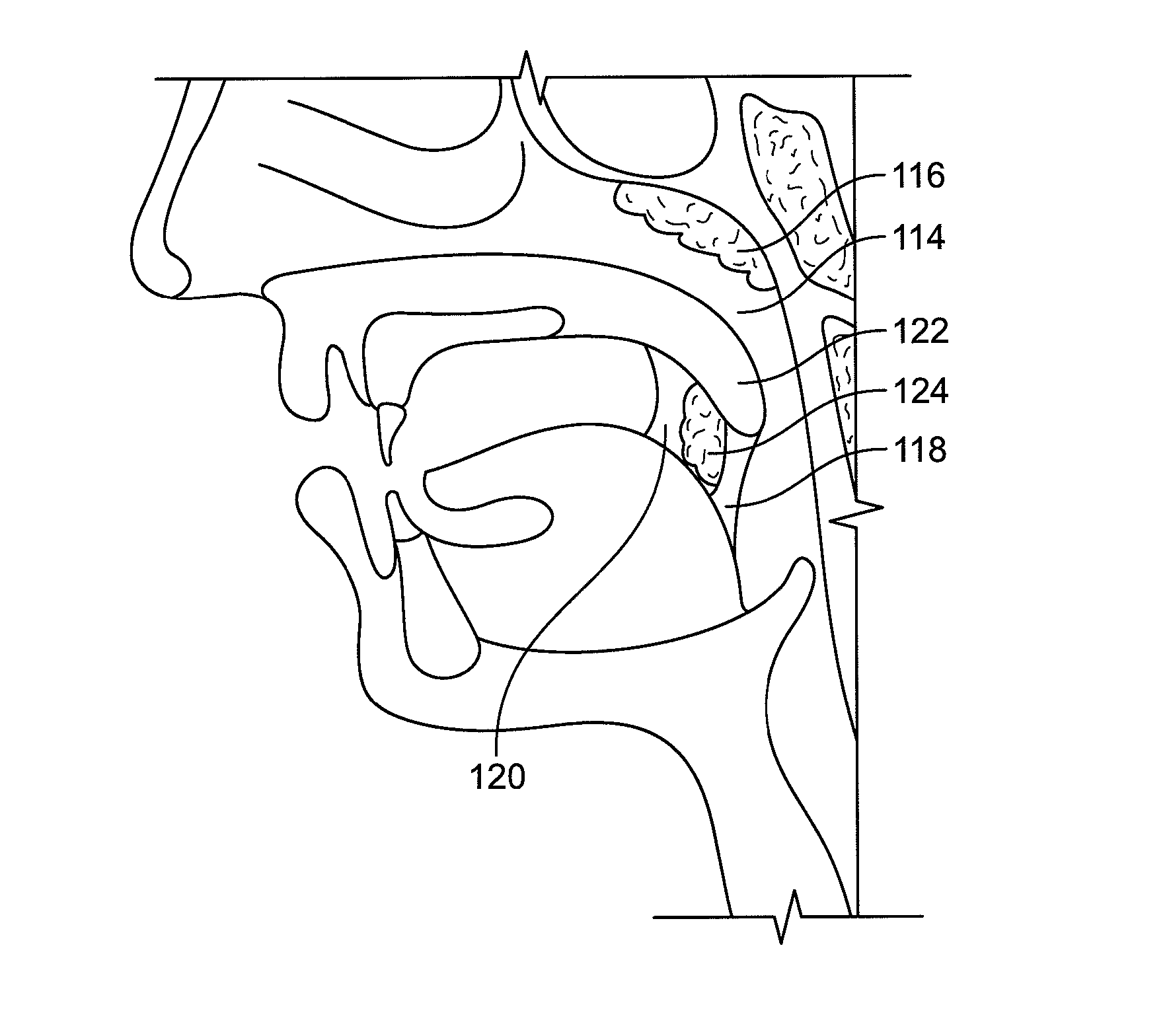
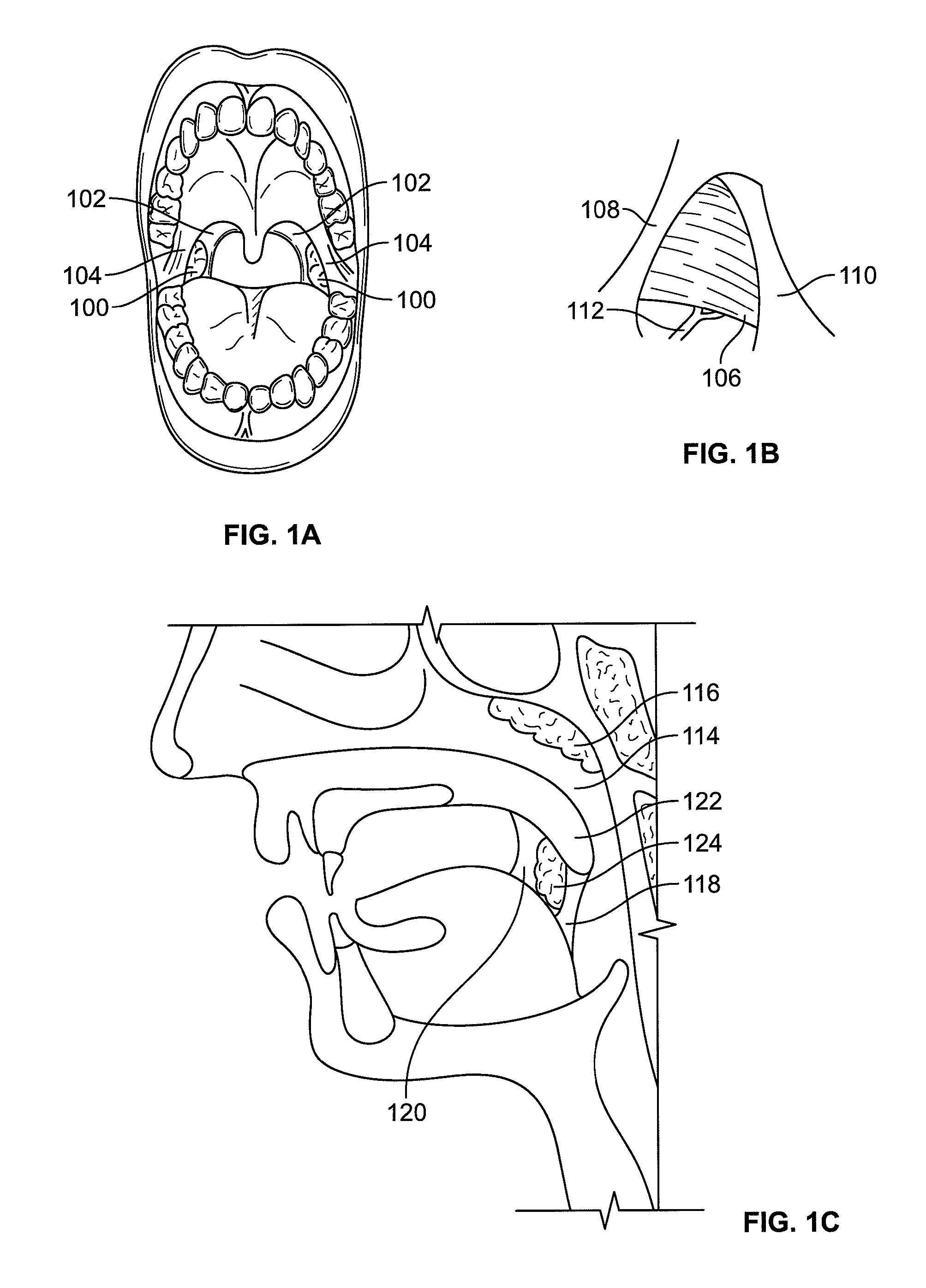
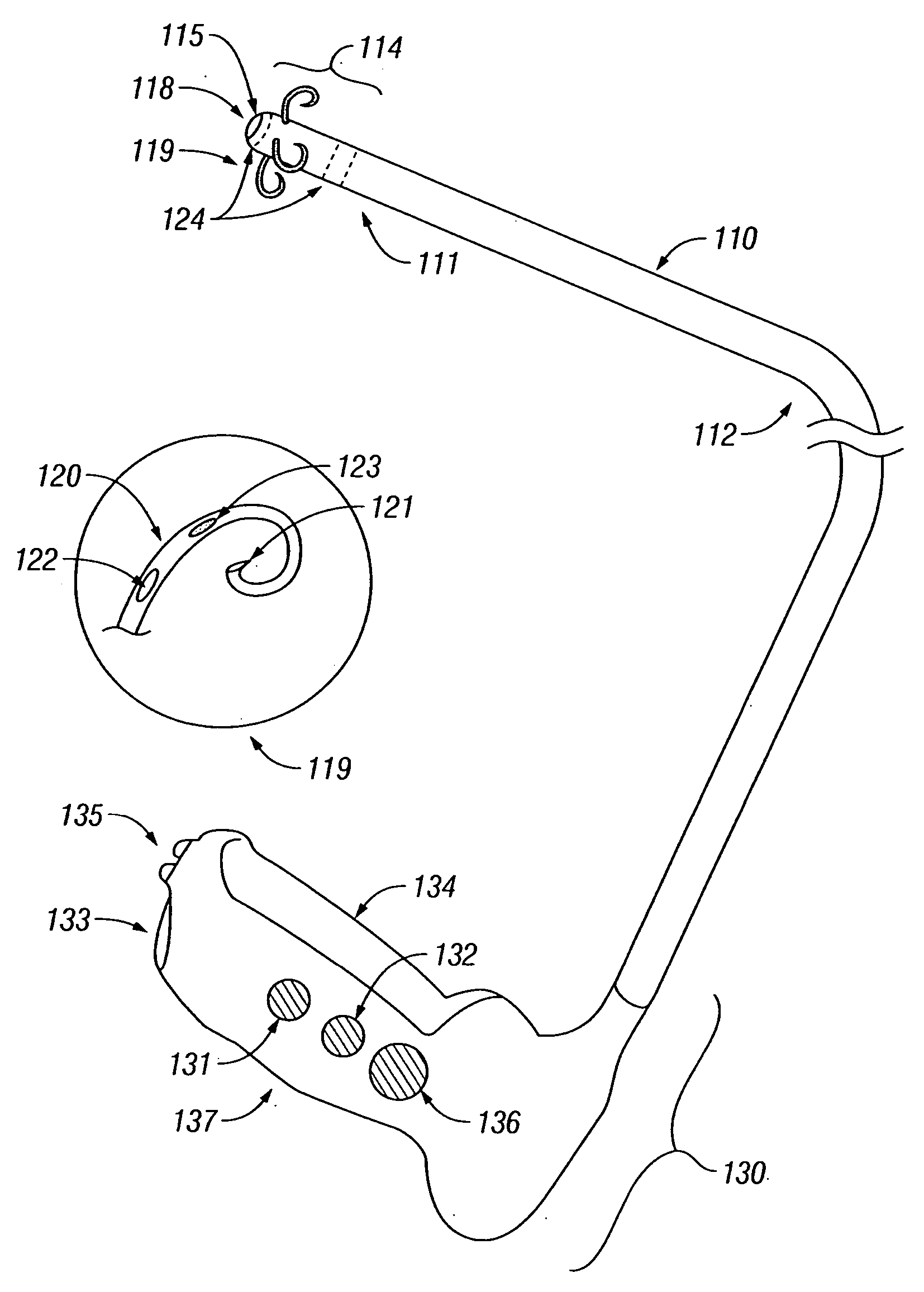
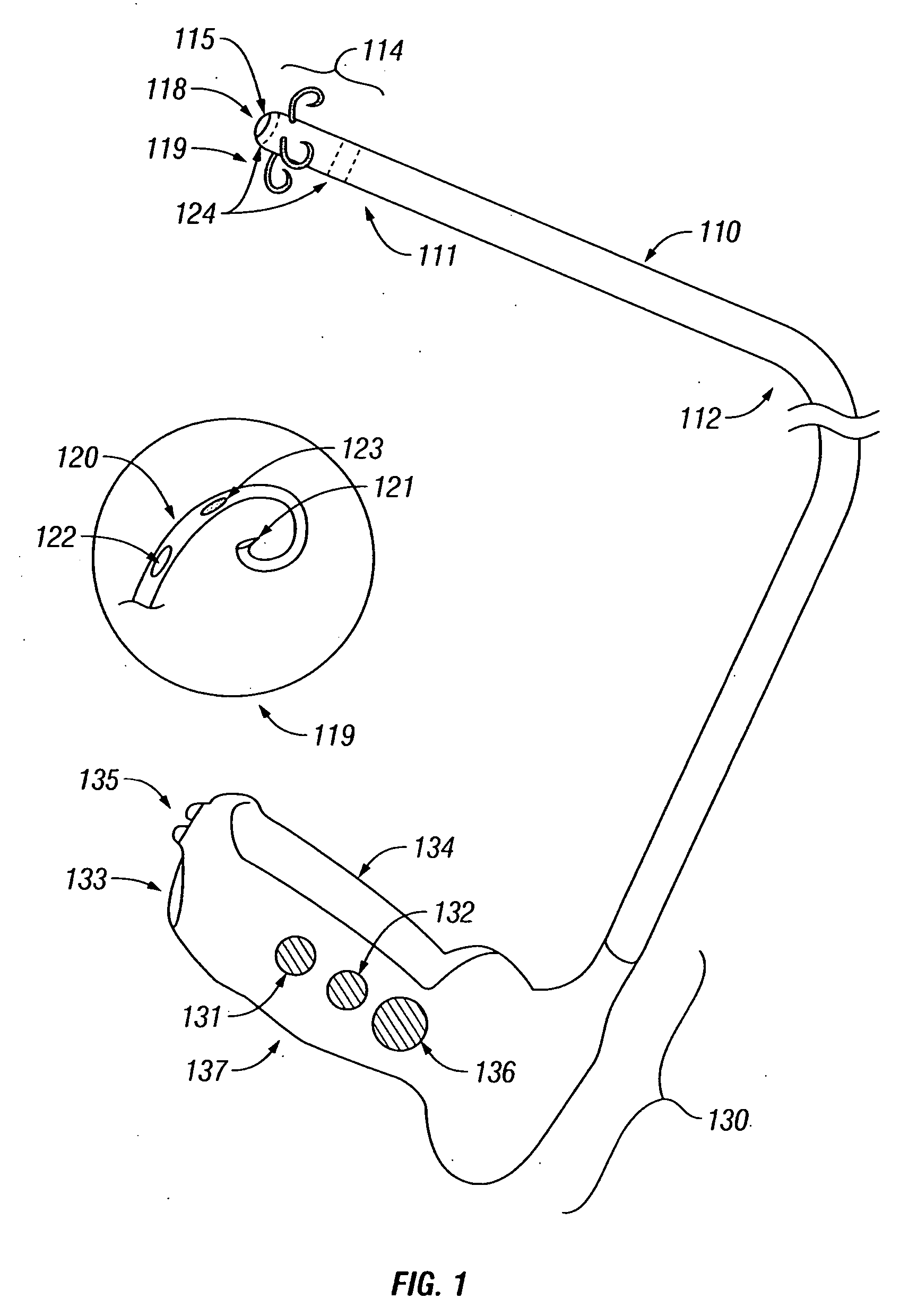
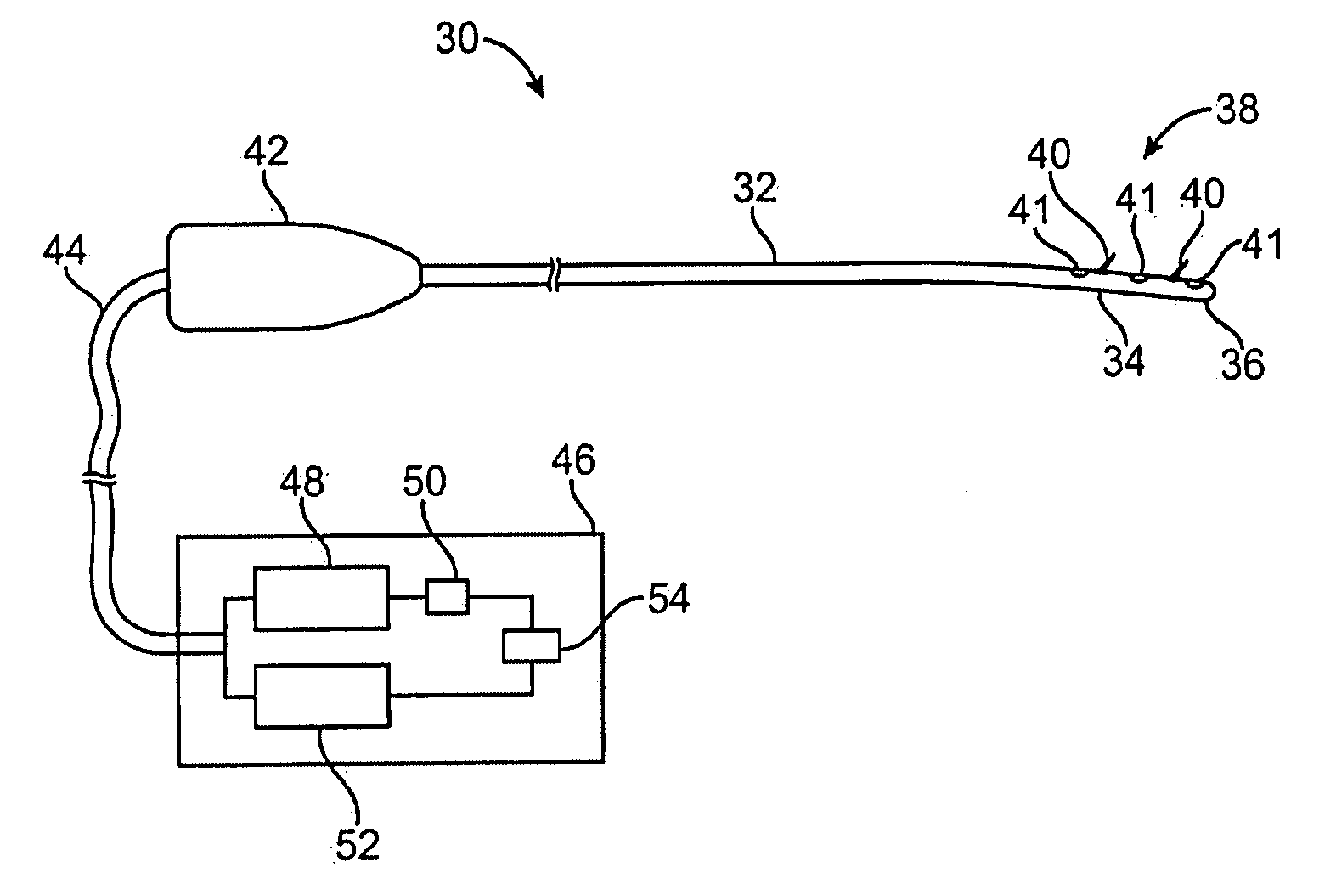
Devices and methods for treating pain associated with tonsillectomies
Described here are devices and methods for treating one or more conditions or symptoms associated with a tonsil procedure. In some variations, a drug-releasing device may be at least partially delivered to one or more tonsillar tissues before, during, or after a tonsil procedure. In some variations, the drug-releasing device may be configured to be biodegradable. In other variations, the drug-releasing device may comprise one or more hemostatic materials or one or more adhesives. The drug-releasing device may be configured to release one or more drugs or agents, such as, for example, one or more analgesics, local anesthetics, vasoconstrictors, antibiotics, combinations thereof and the like.
Owner:INTERSECT ENT INC
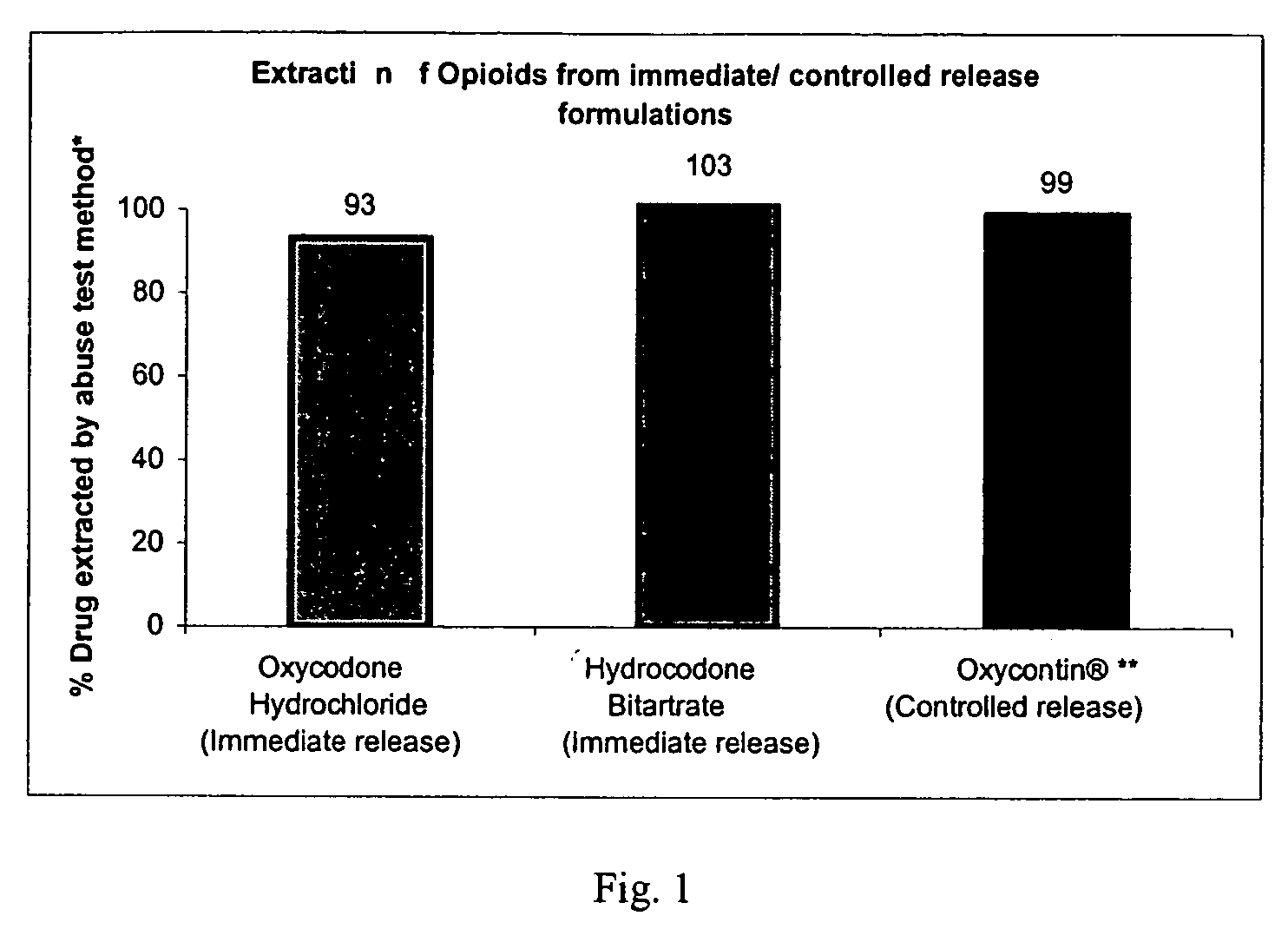
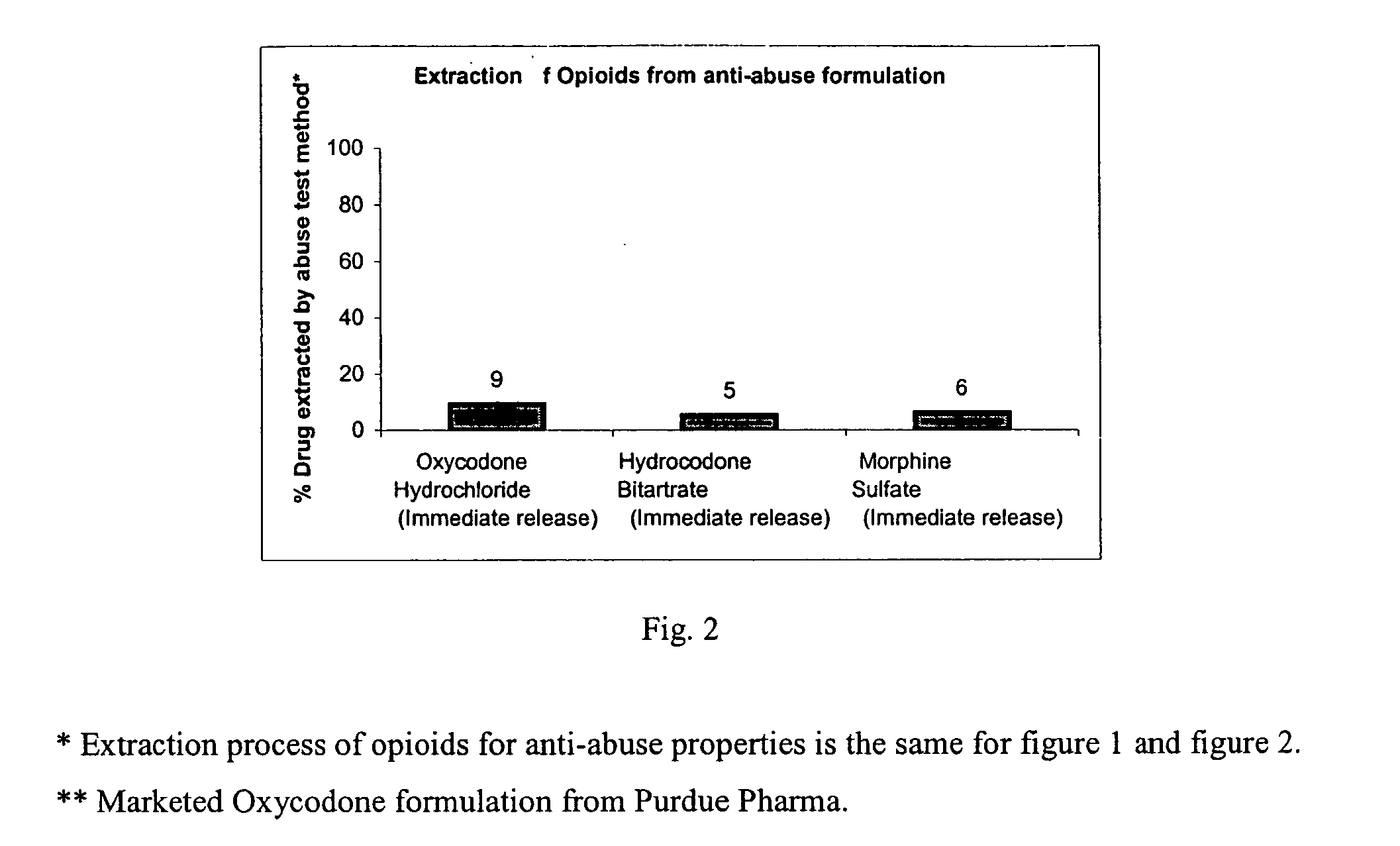
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
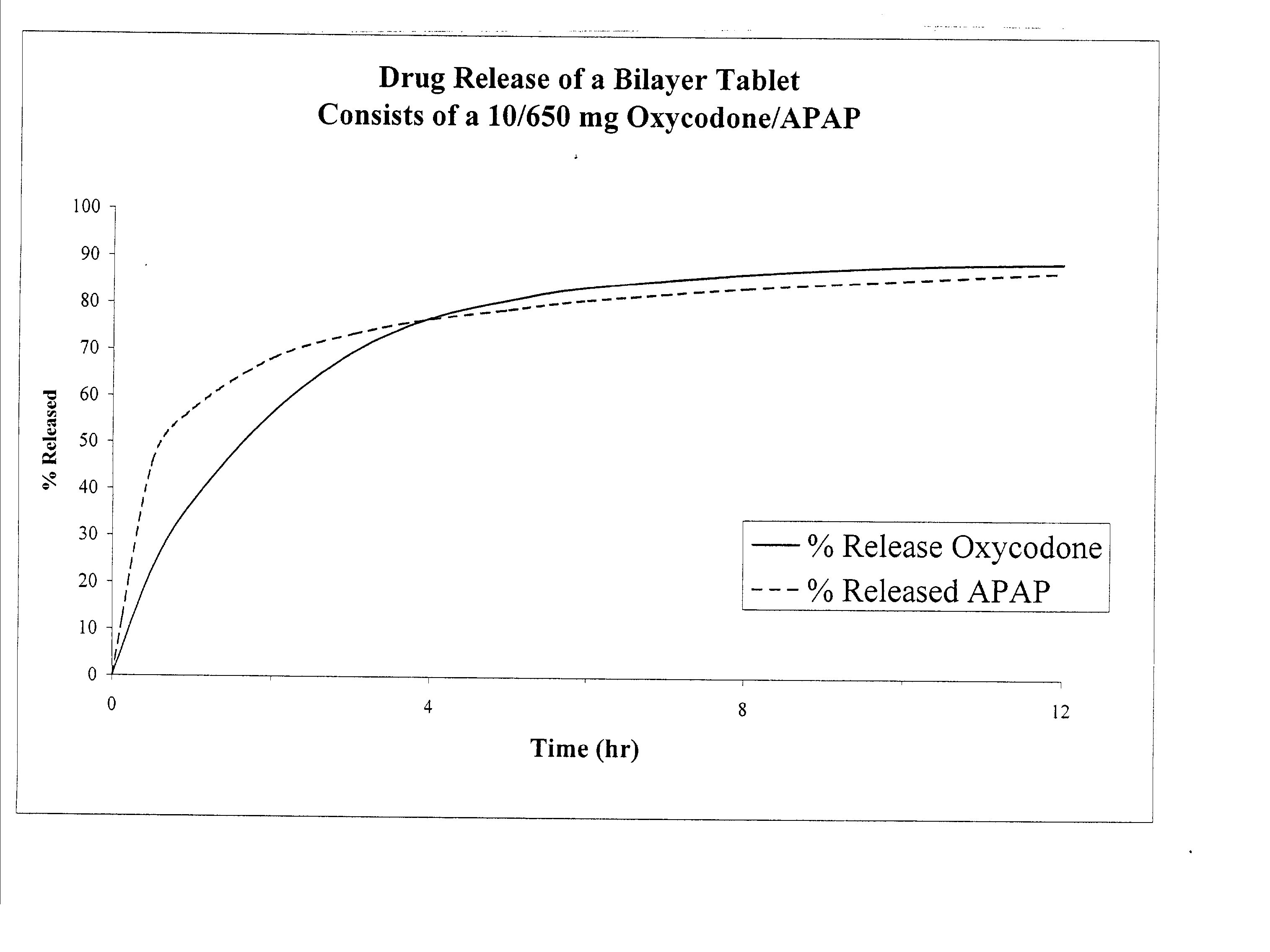
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
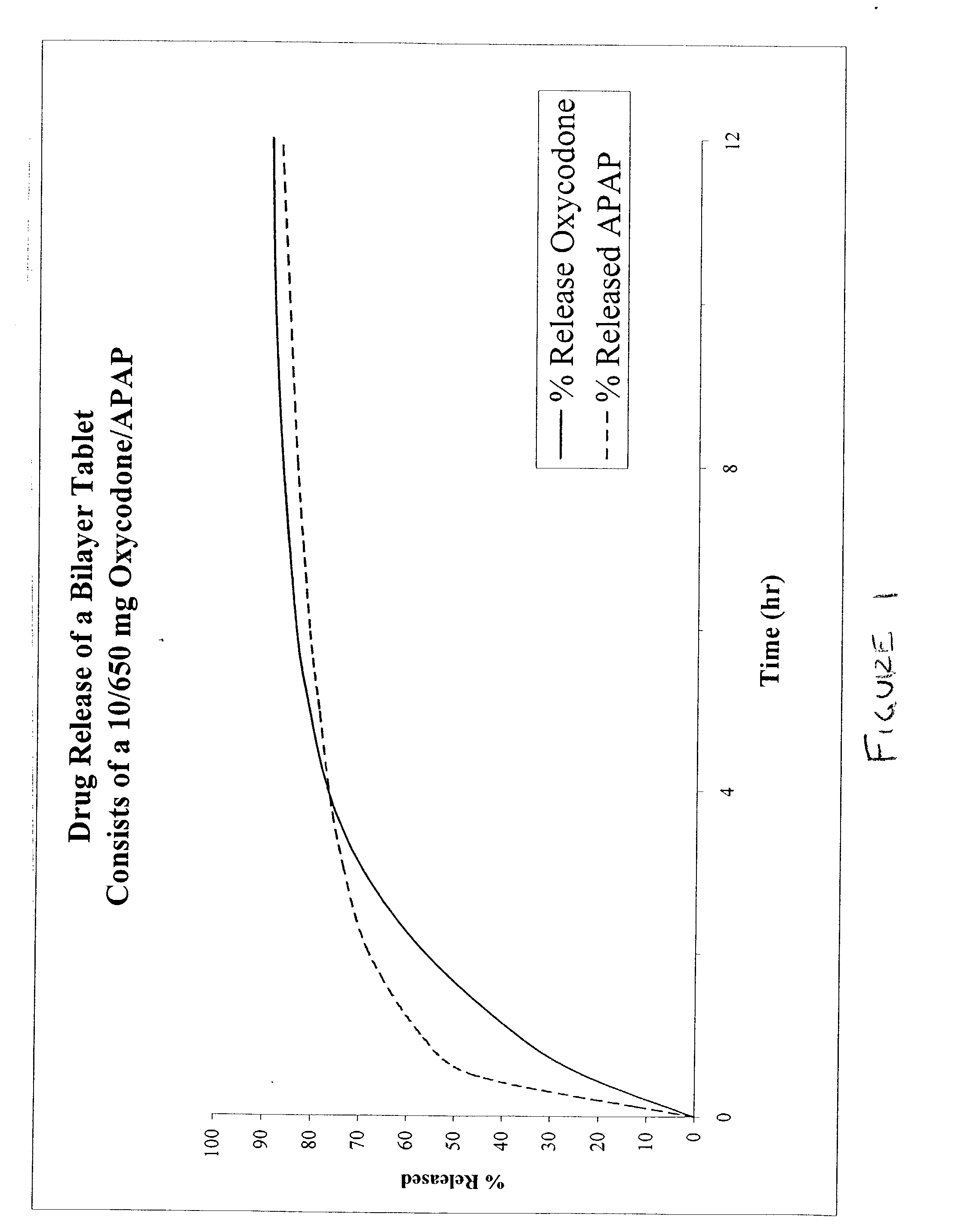
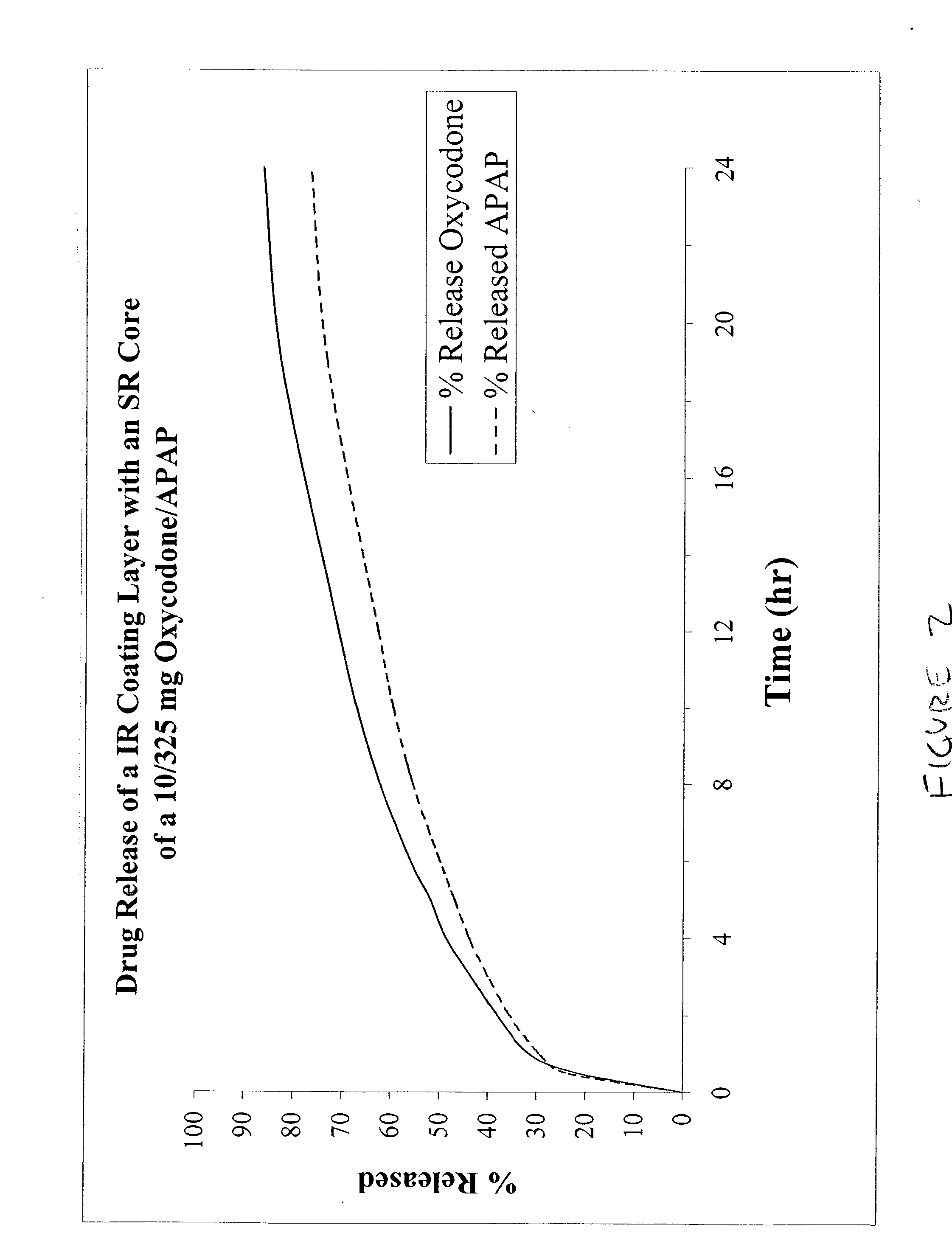
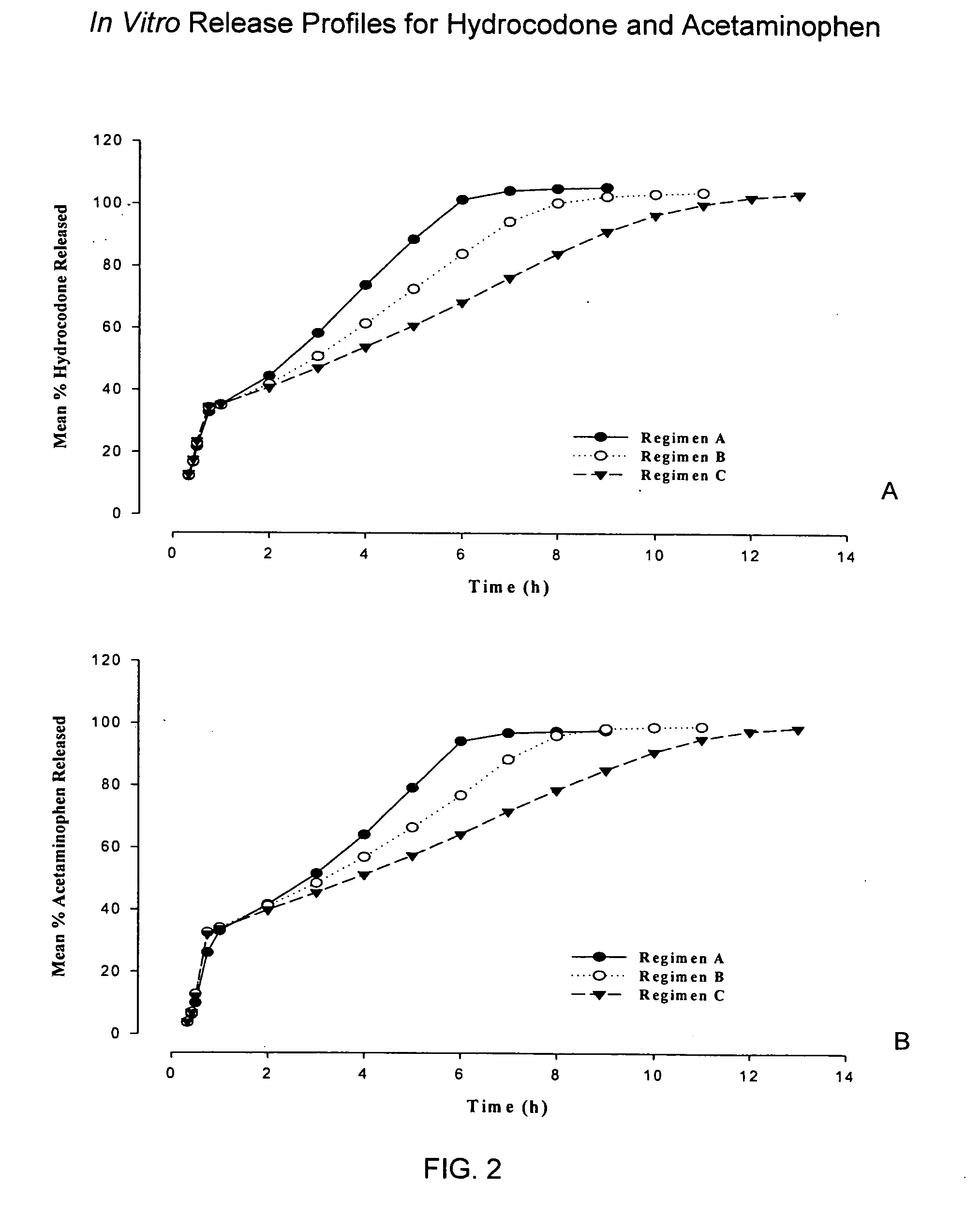
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
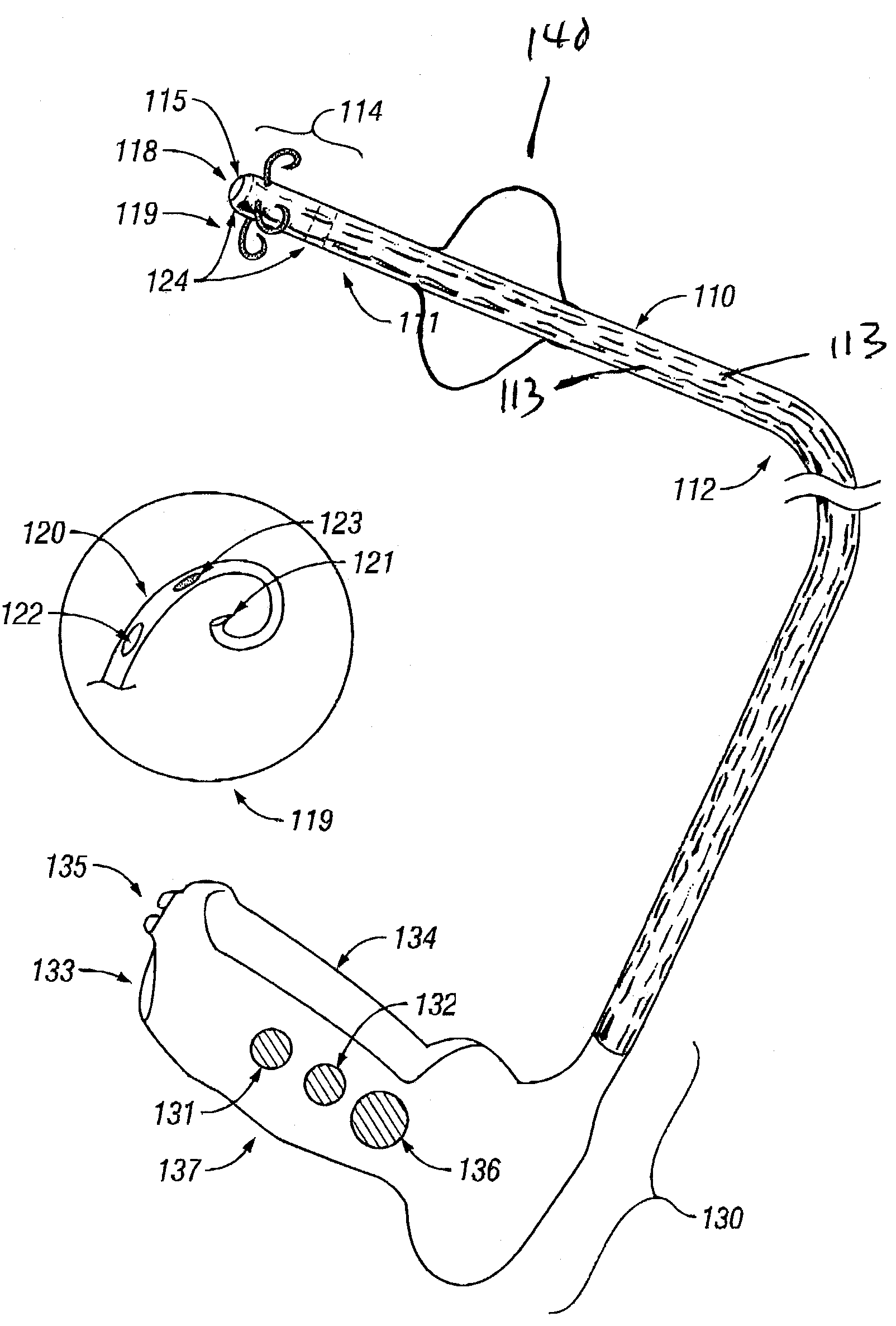
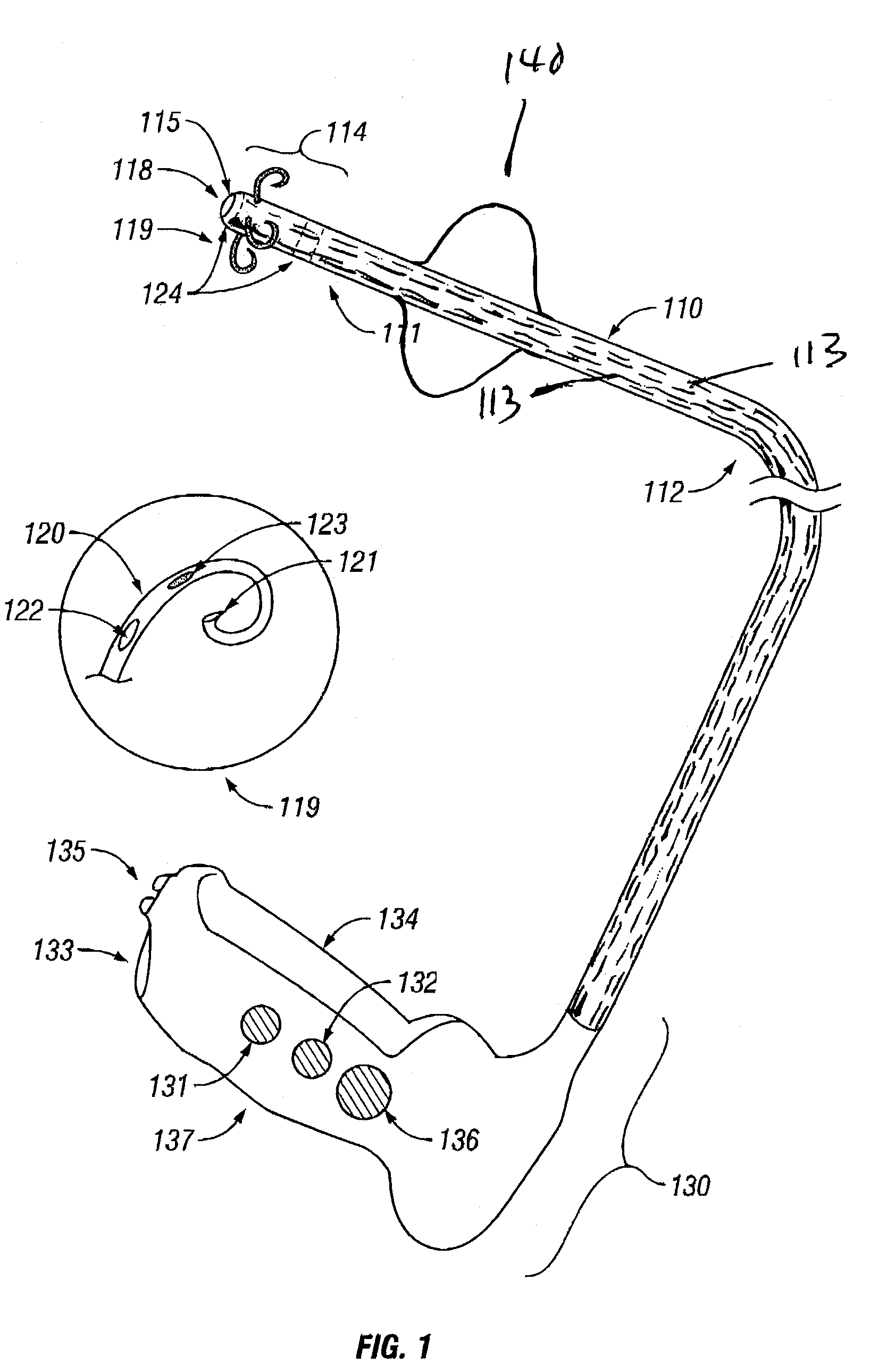
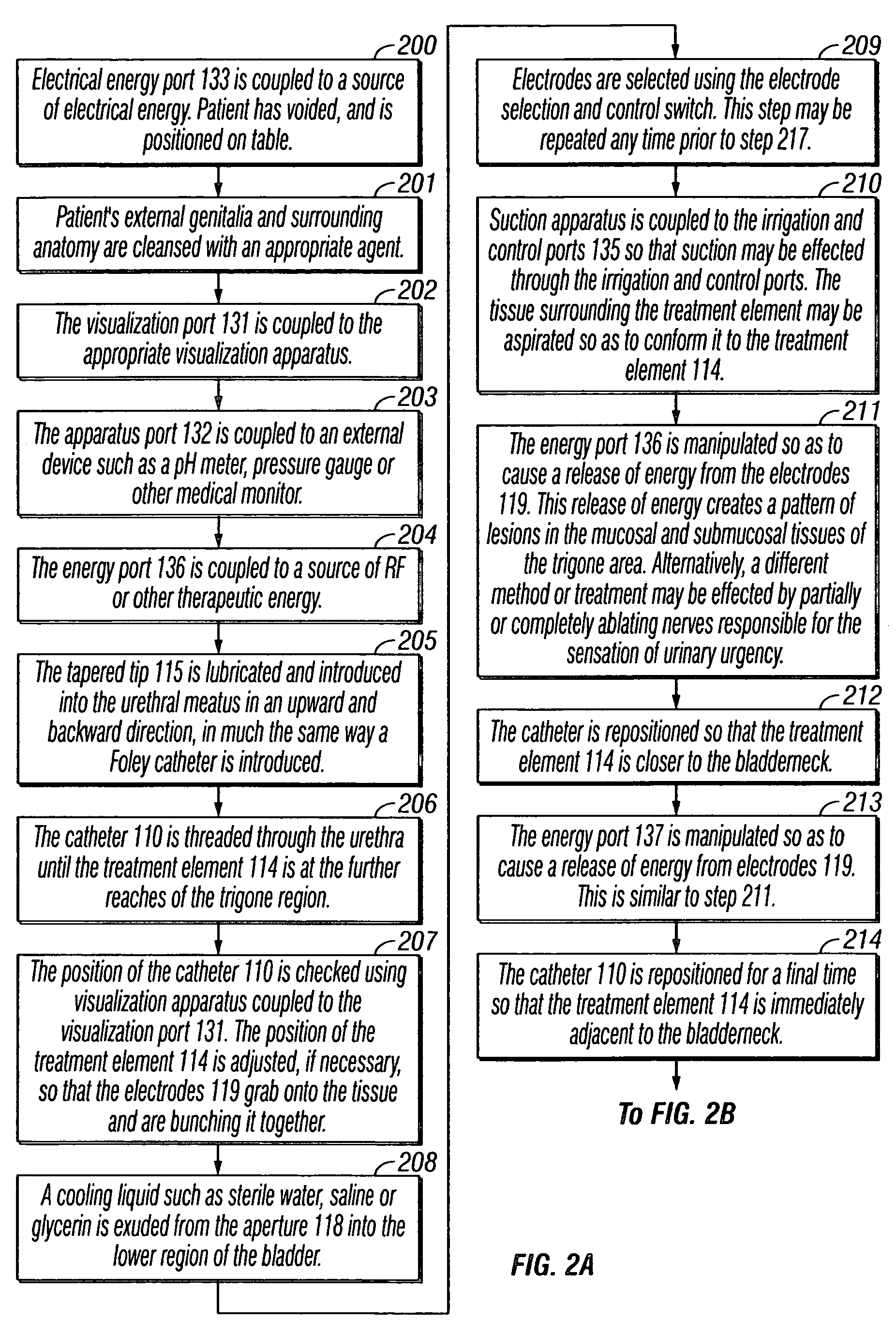
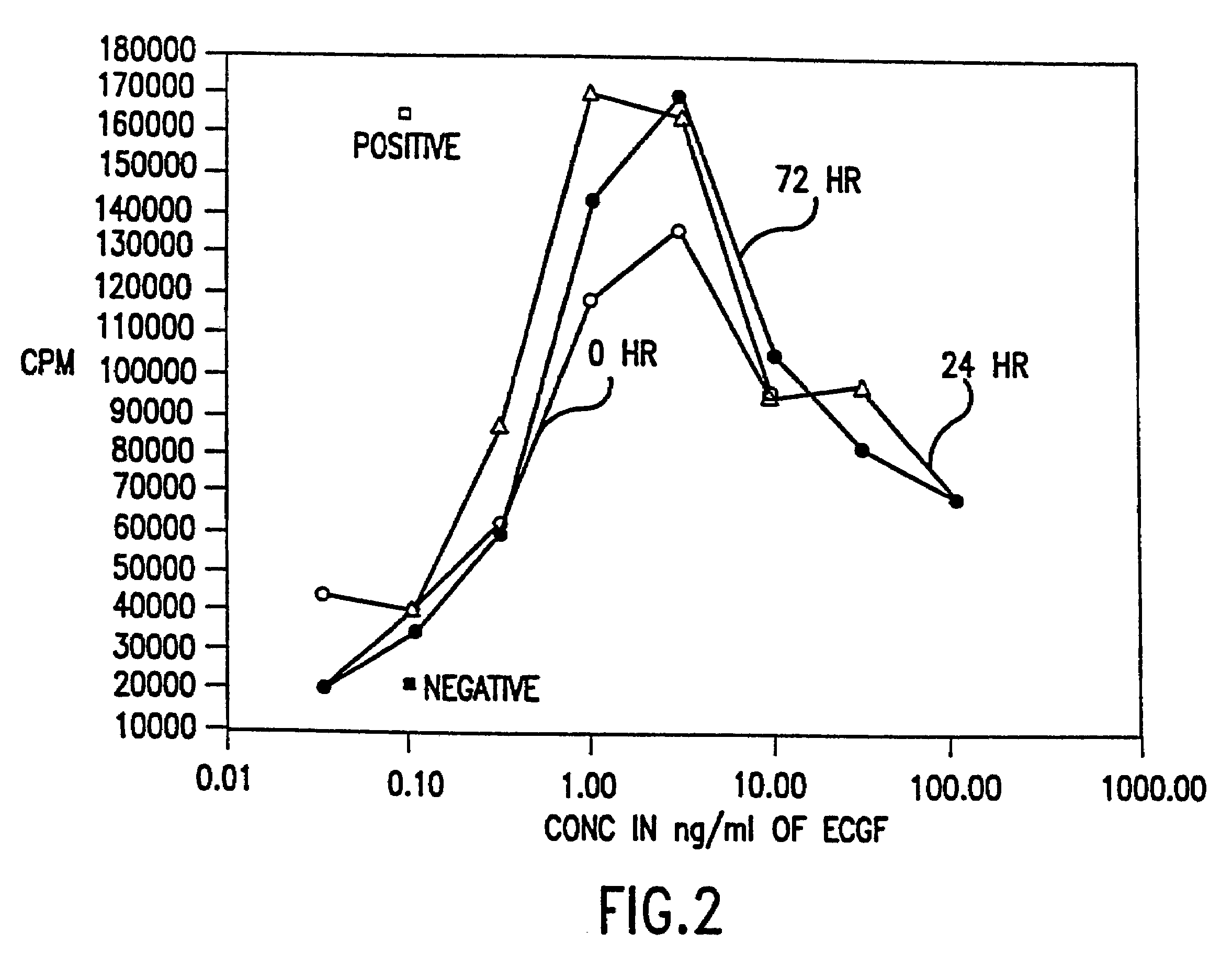
Treatment of urinary incontinence and other disorders by application of energy and drugs
The invention provides a method and system for treating disorders in parts of the body. A particular treatment can include on or more of, or some combination of: ablation, nerve modulation, three-dimensional tissue shaping, drug delivery, mapping stimulating, shrinking and reducing strain on structures by altering the geometry thereof and providing bulk to particularly defined regions. The particular body structures or tissues can include one or more of or some combination of region, including: the bladder, esophagus, vagina, penis, larynx, pharynx, aortic arch, abdominal aorta, thoracic, aorta, large intestine, sinus, auditory canal, uterus, vas deferens, trachea, and all associated sphincters. Types of energy that can be applied include radiofrequency, laser, microwave, infrared waves, ultrasound, or some combination thereof. Types of substances that can be applied include pharmaceutical agents such as analgesics, antibiotics, and anti-inflammatory drugs, bulking agents such as biologically non-reactive particles, cooling fluids, or dessicants such as liquid nitrogen for use in cryo-based treatments.
Owner:VERATHON
Methods and compositions for deterring abuse of opioid containing dosage forms
This invention relates to an abuse deterrent dosage form of opioid analgesics, wherein an analgesically effective amount of opioid analgesic is combined with a polymer to form a matrix.
Owner:HALSEY DRUG
Medical and dental implant devices for controlled drug delivery
Implantable devices and methods for use in the treatment of osteonecrosisare provided. The device includes at least one implant device body adapted for insertion into one or more channels or voids in bone tissue; a plurality of discrete reservoirs, which may preferably be microreservoirs, located in the surface of the at least one implant device body; and at least one release system disposed in one or more of the plurality of reservoirs, wherein the release system includes at least one drug selected from the group consisting of bone growth promoters, angiogenesis promoters, analgesics, anesthetics, antibiotics, and combinations thereof. The device body may be formed of a bone graft material, a polymer, a metal, a ceramic, or a combination thereof. The device body may be a monolithic structure, such as one having a cylindrical shape, or it may be in the form of multiple units, such as a plurality of beads.
Owner:MICROCHIPS INC
Sustained release opioid formulations and method of use
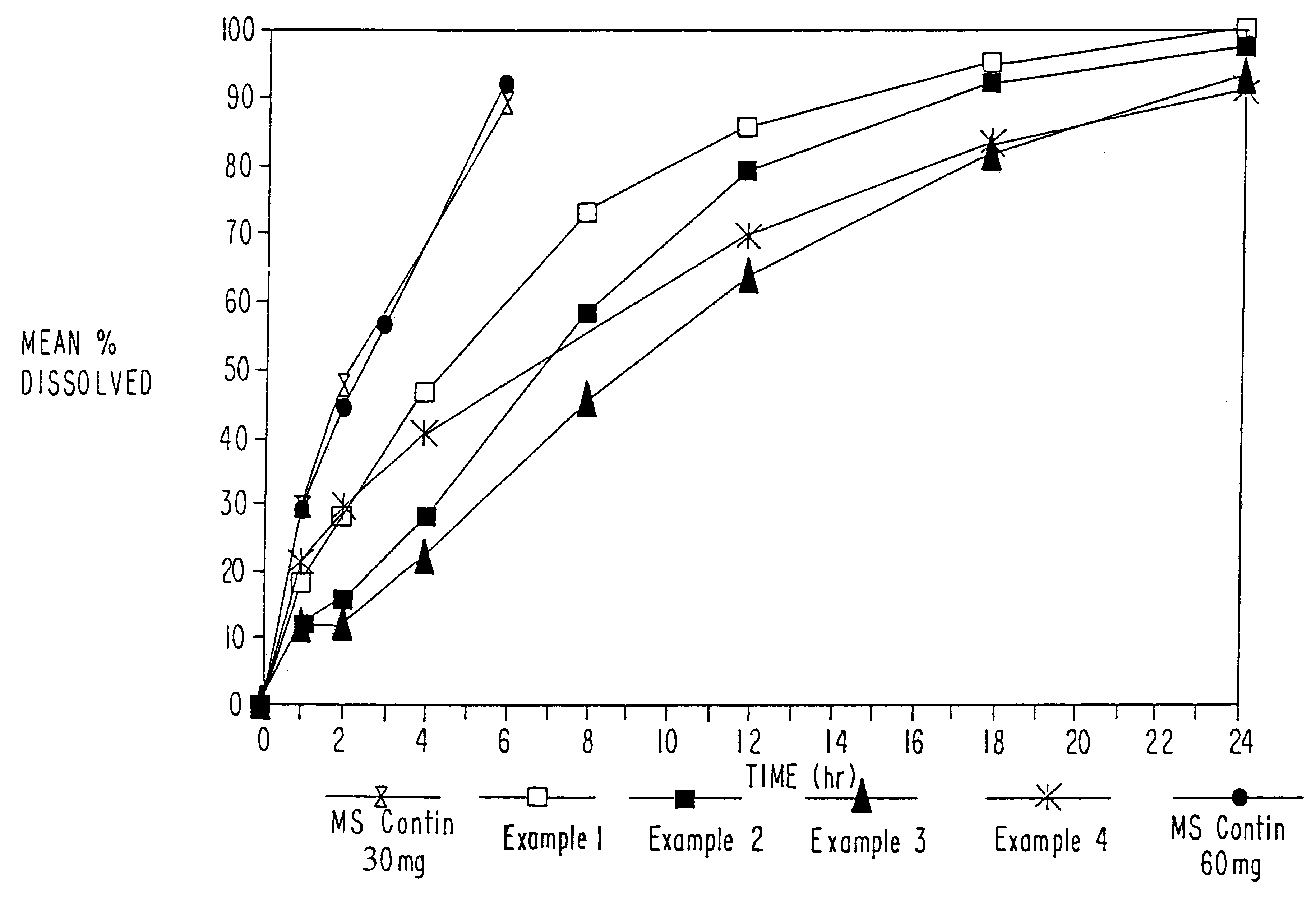
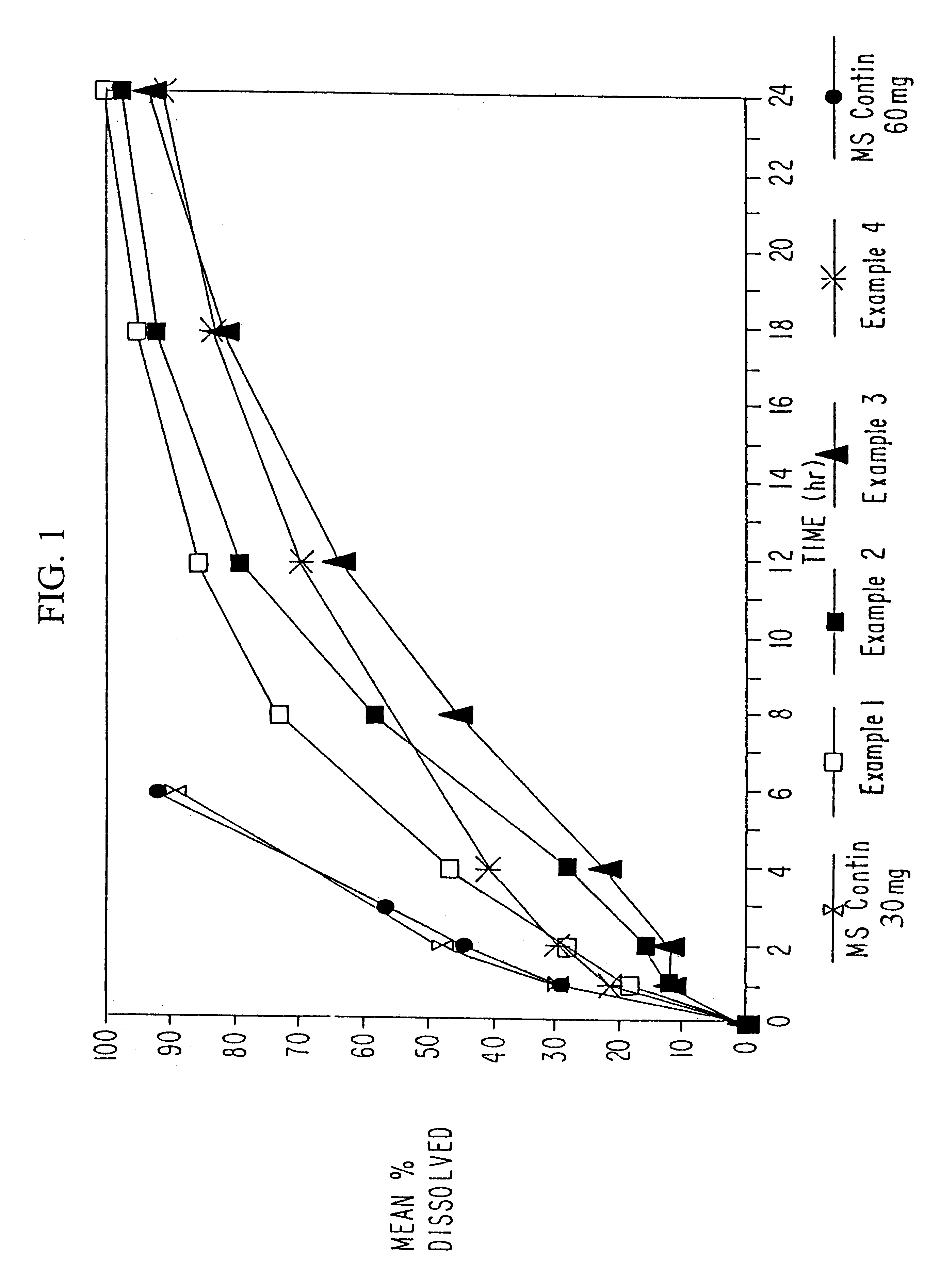
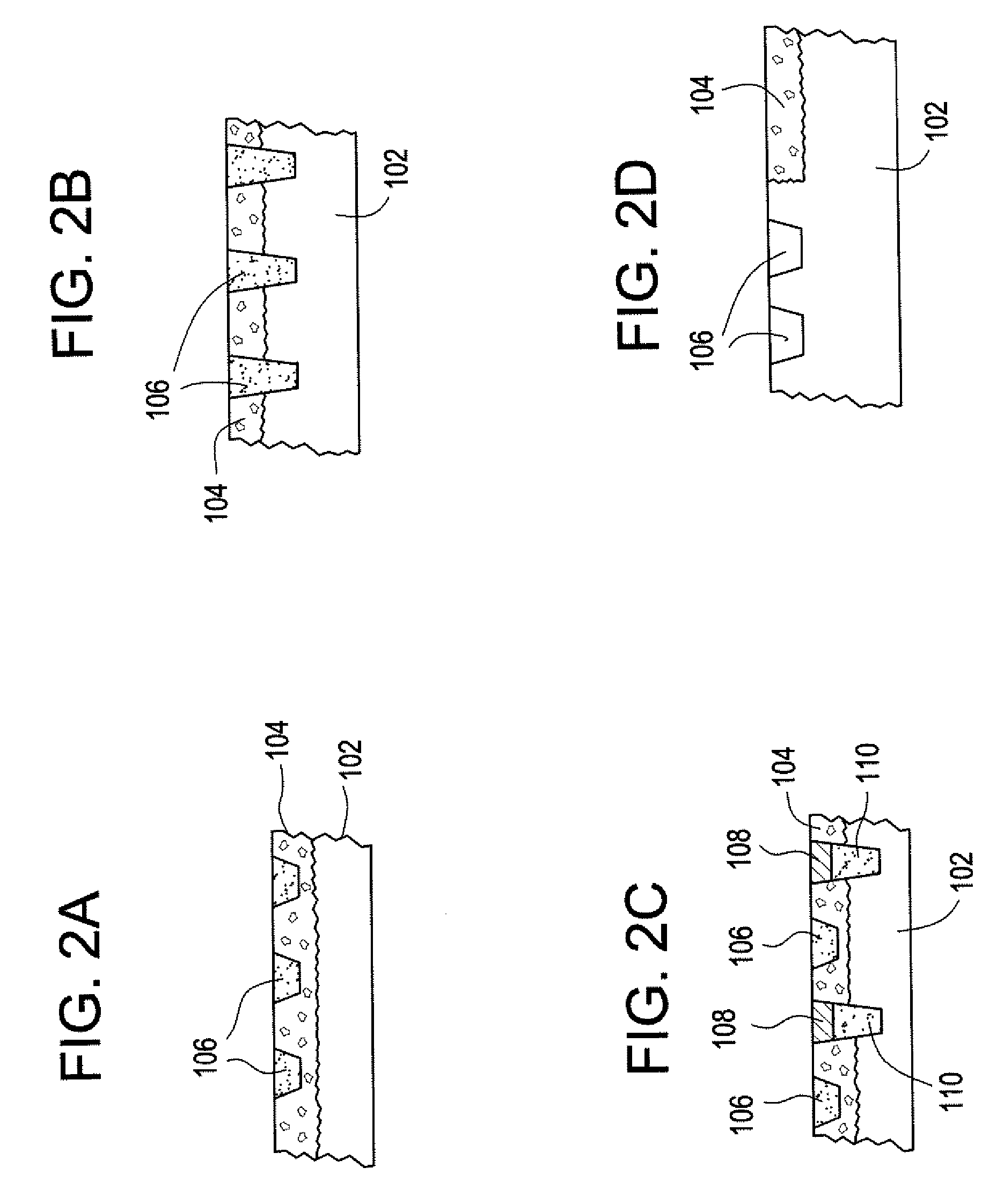
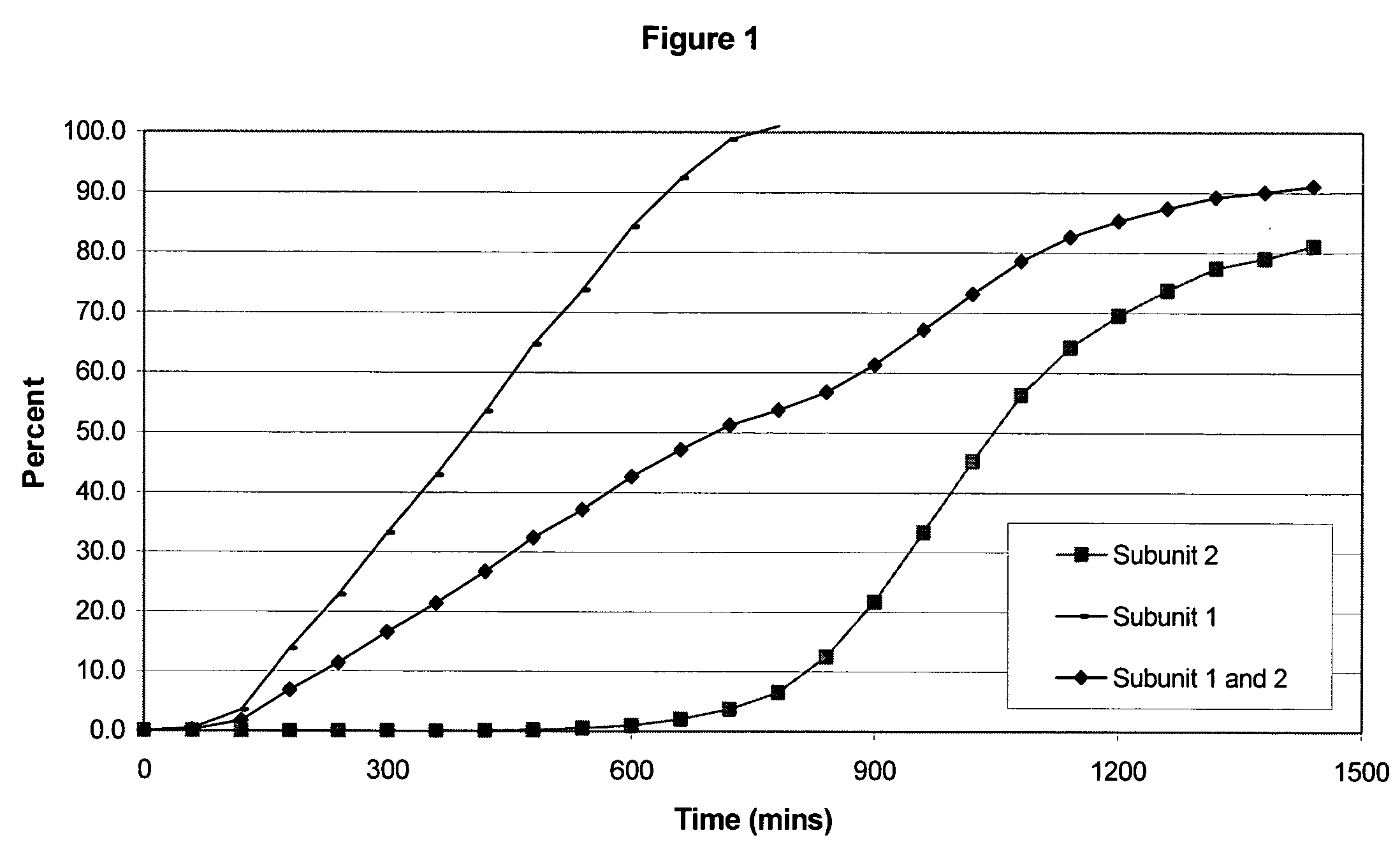
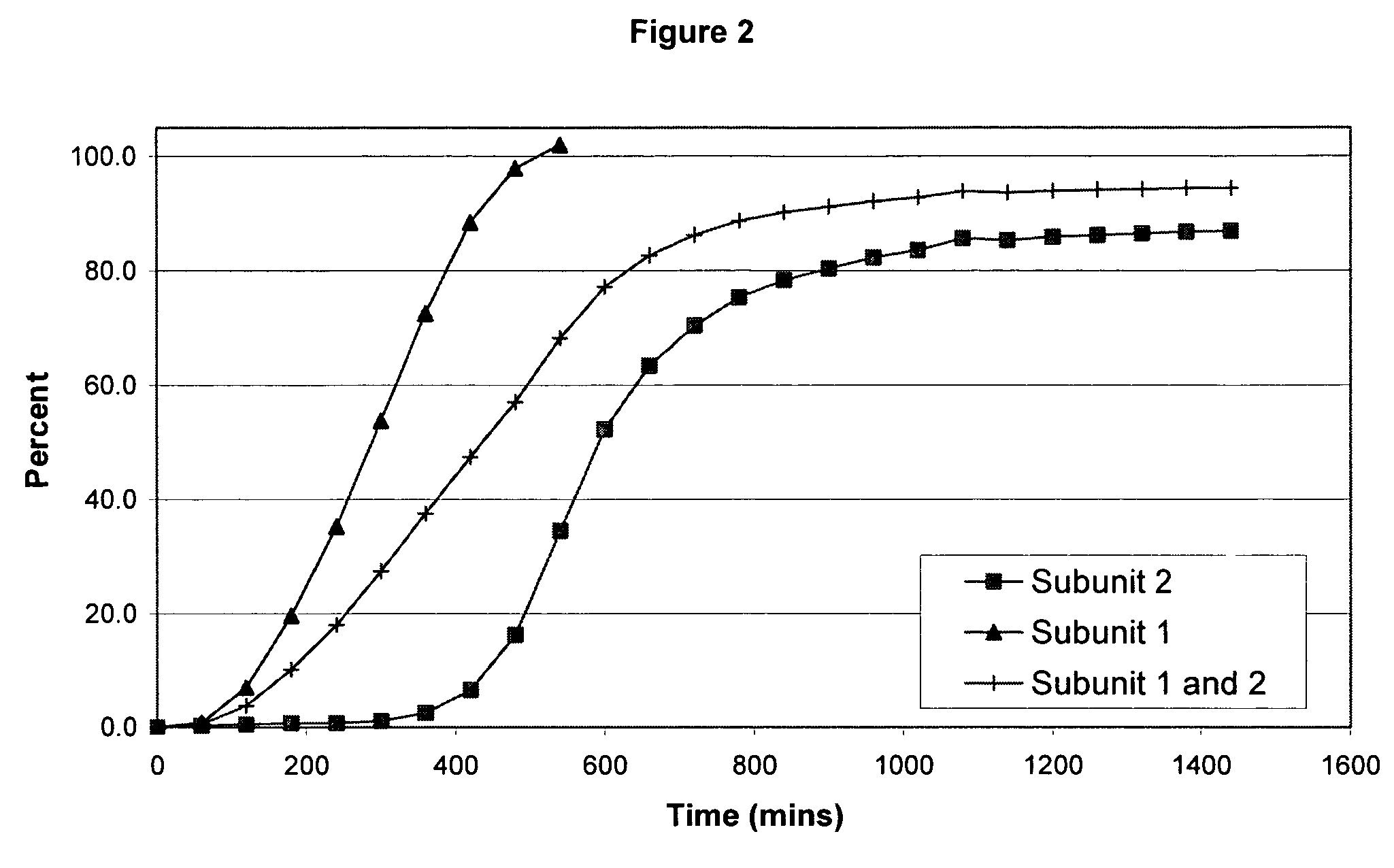
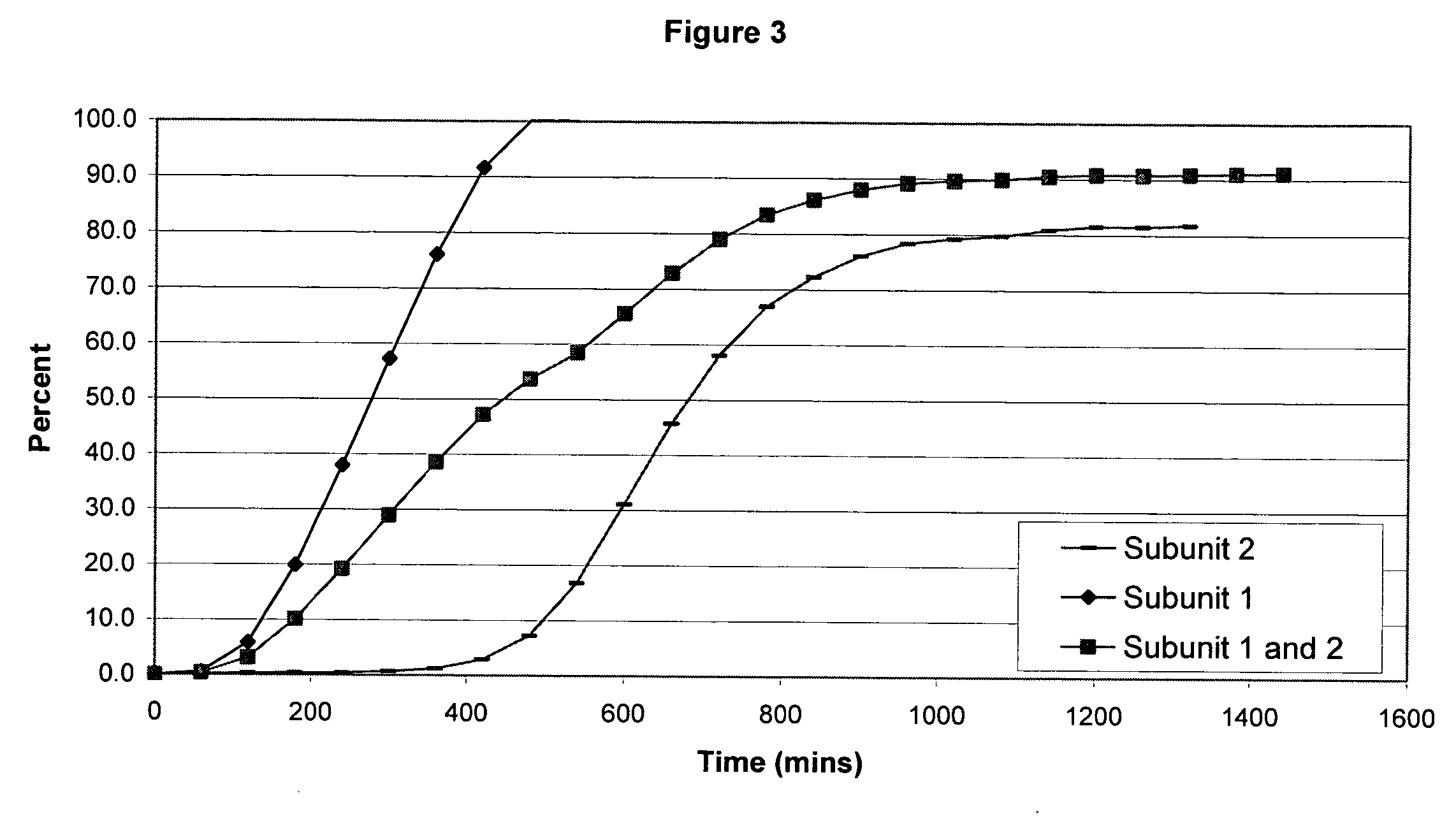
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
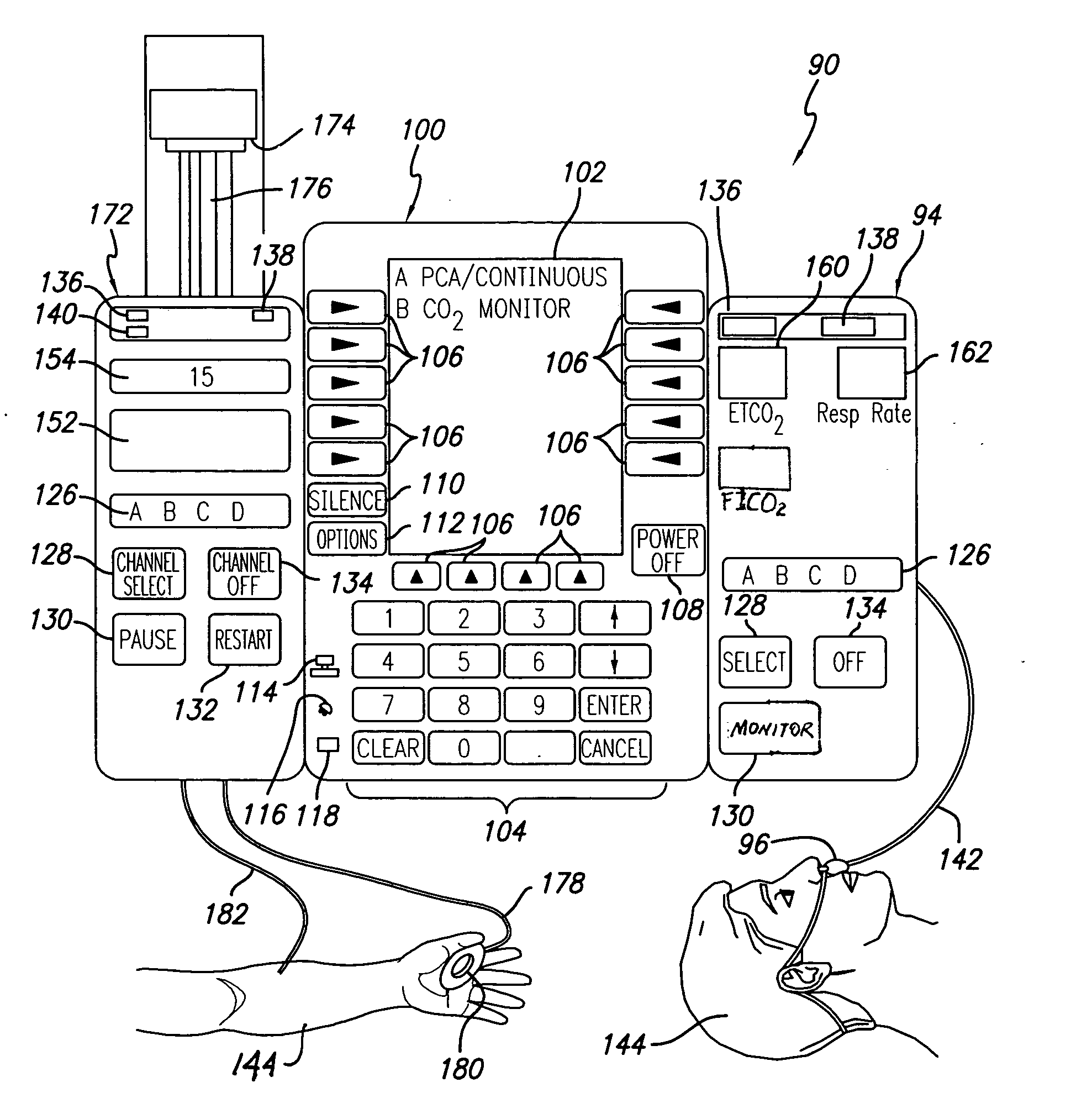
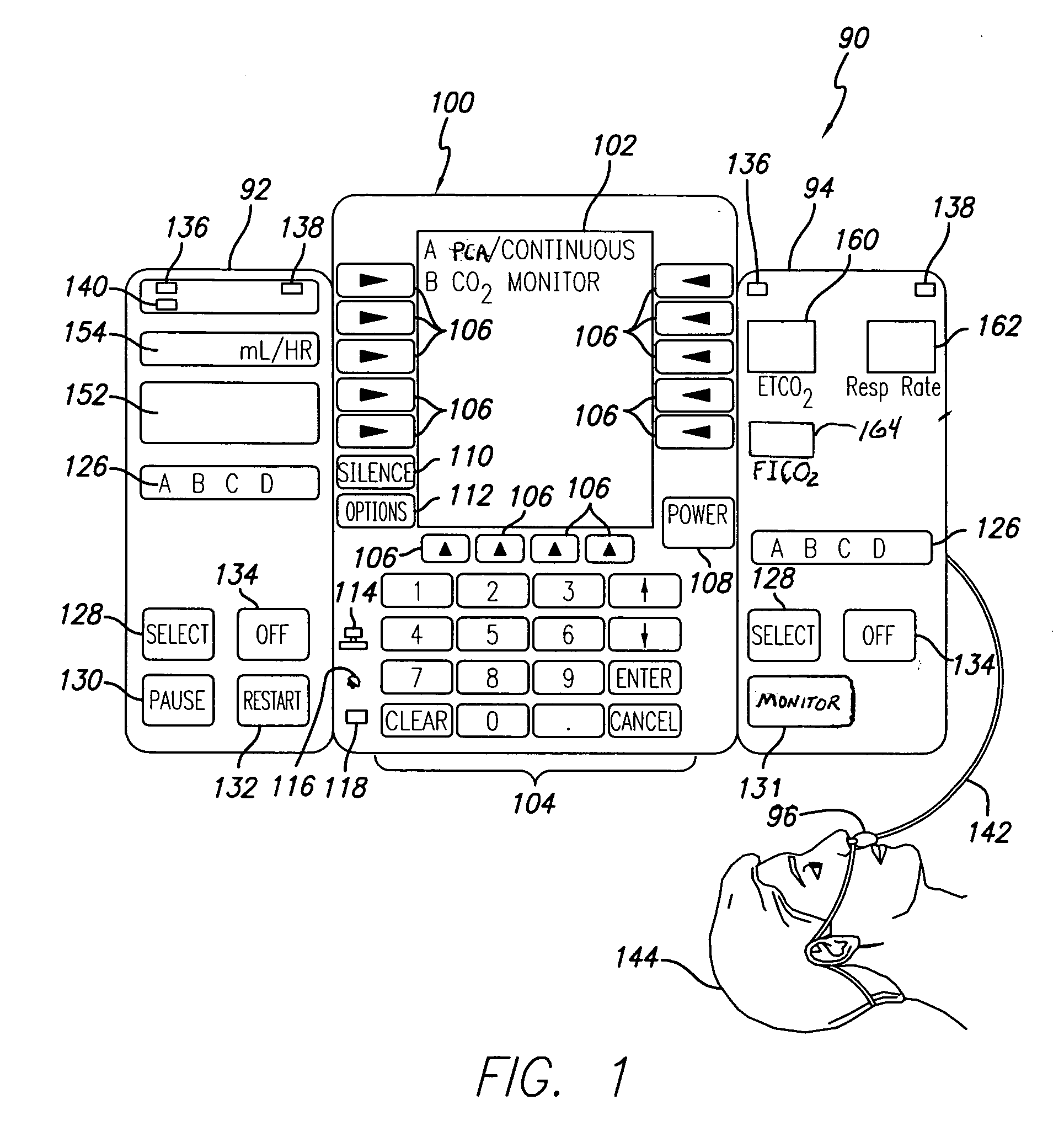
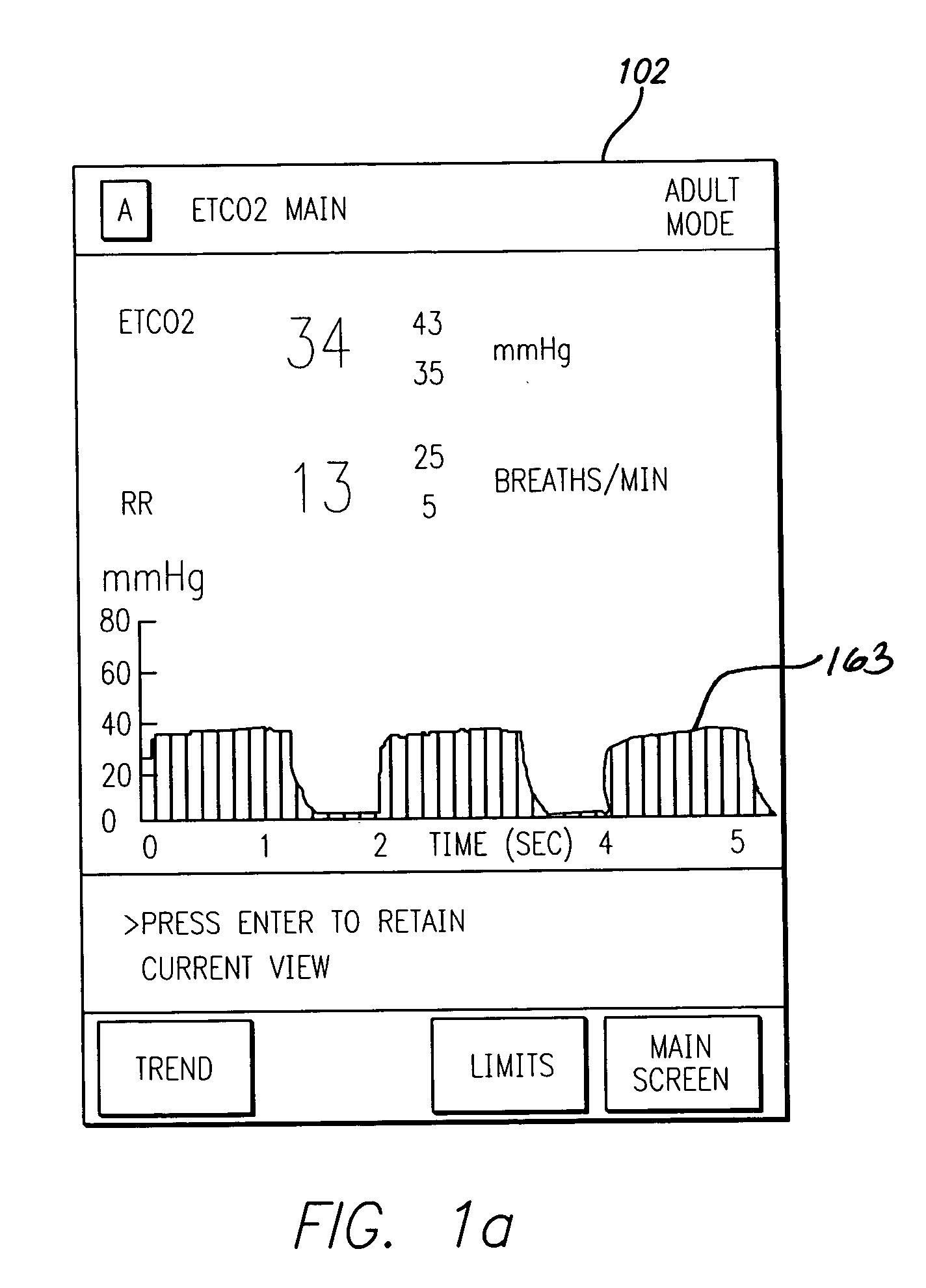
Patient-controlled analgesia with patient monitoring system and method
A patient care system in which a physiological parameter of a patient is monitored while the patient self-administers analgesic. A display presents a trend of the patient's physiological parameter along with the time of self-administration of the analgesic (“PCA”—patient controlled analgesic) such that the effect of the analgesic on the physiological parameter can be seen over selectable time periods. The physiological parameter may be ETCO2 or SpO2 or other. Also included is a drug library having acceptable pumping parameters as well as other PCA specific data. Should the operator program a pumping parameter that is outside an acceptable range, or should the patient attempt to self-administer more analgesic than the acceptable range permits, or should a patient's physiological parameter change during infusion such that a pumping parameter becomes outside an acceptable range, an indication of such will be given and action, such as stopping the pump, will be taken.
Owner:CAREFUSION 303 INC
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
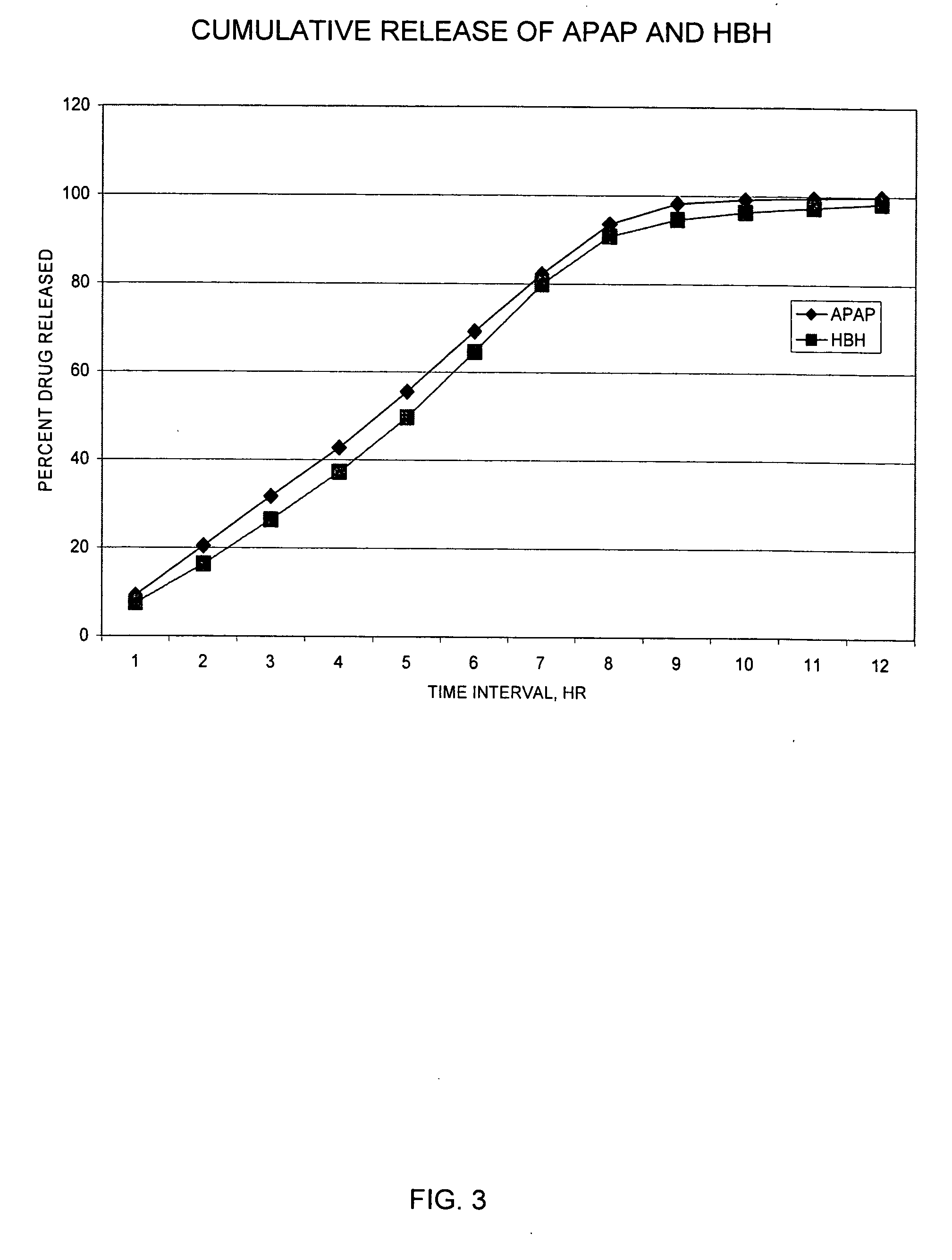
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
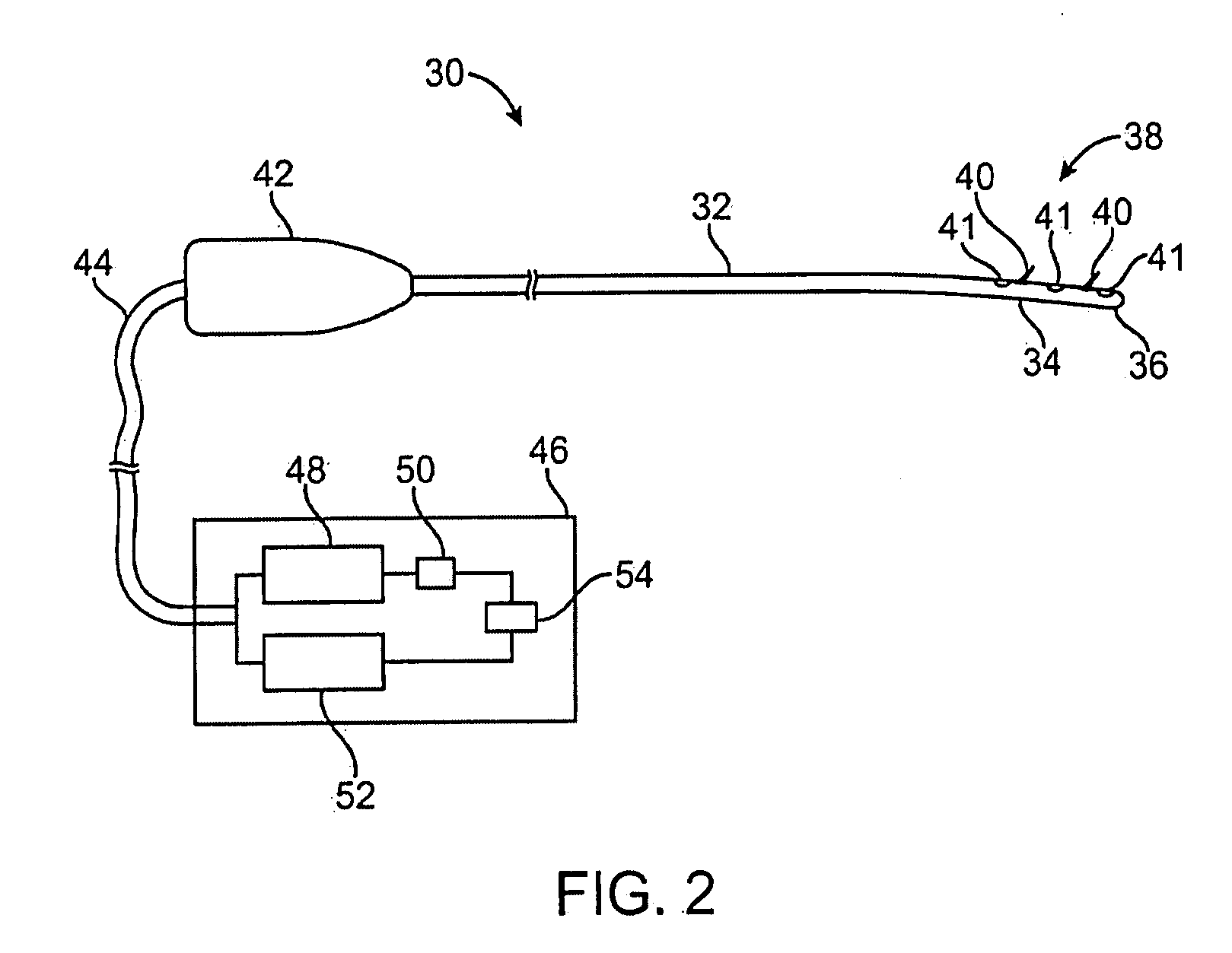
Programmable multi-dose intranasal drug delivery device
InactiveUS6948492B2Avoid diversionAvoid abuseRespiratorsLiquid surface applicatorsNasal sprayBiological activation
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
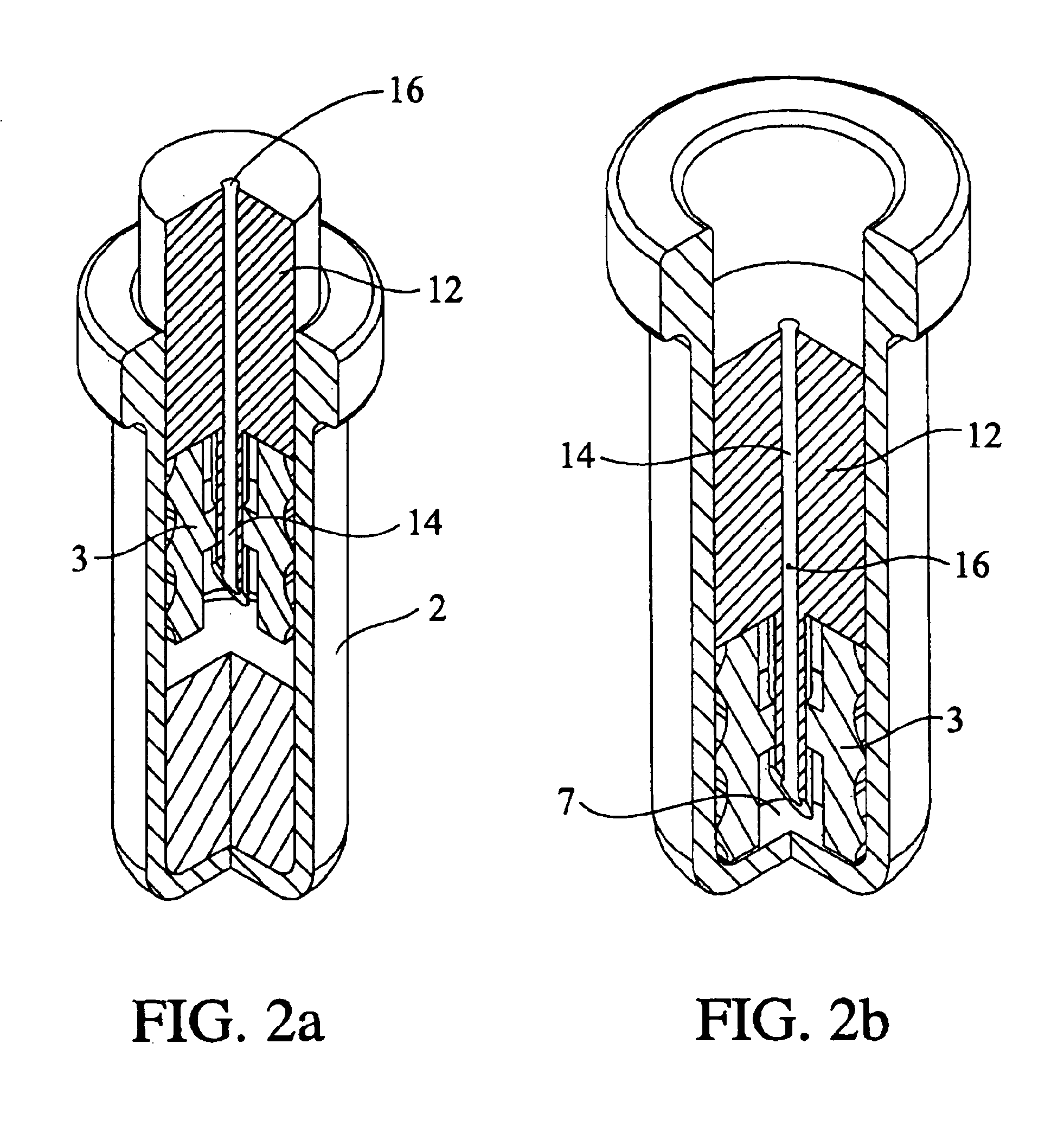
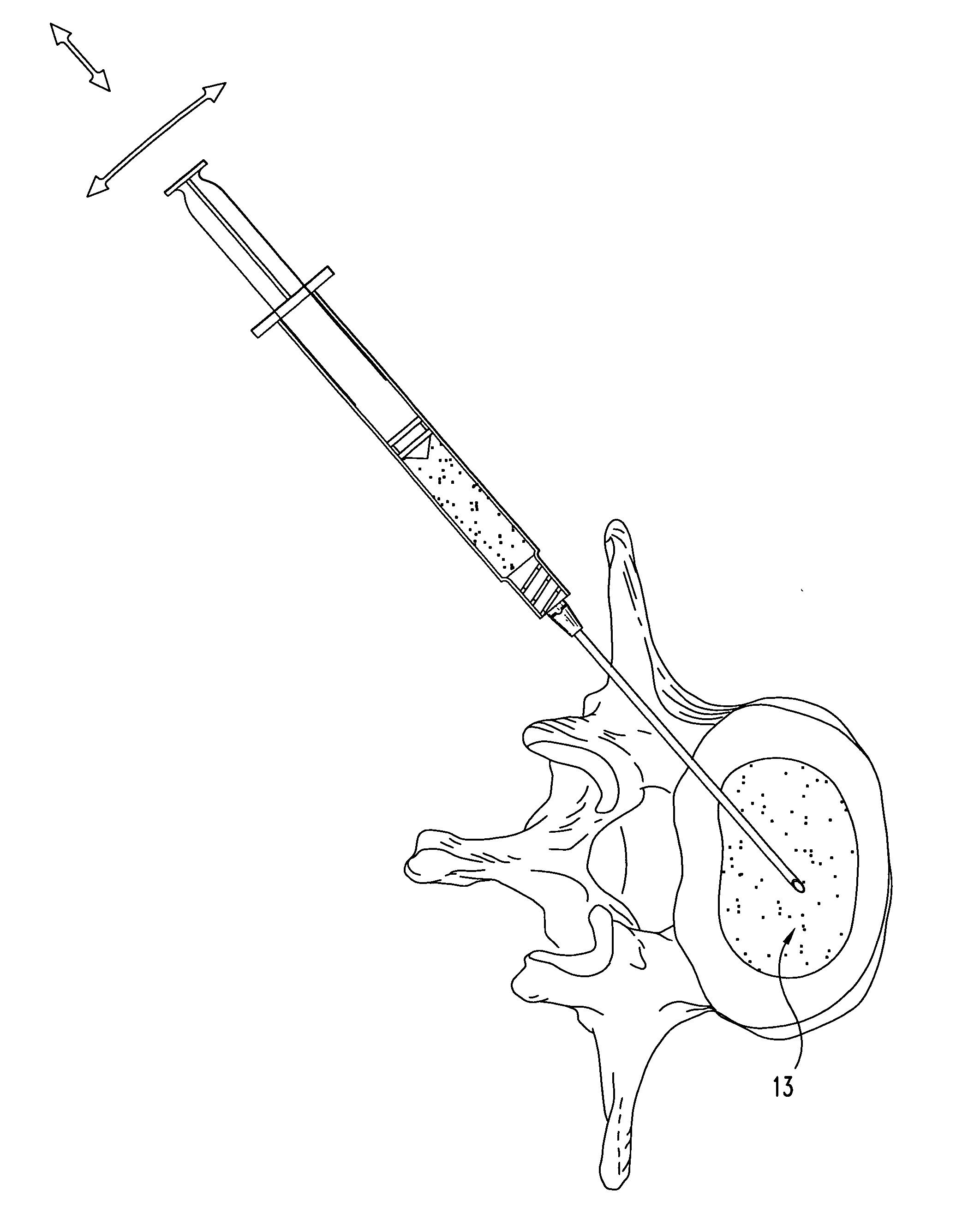
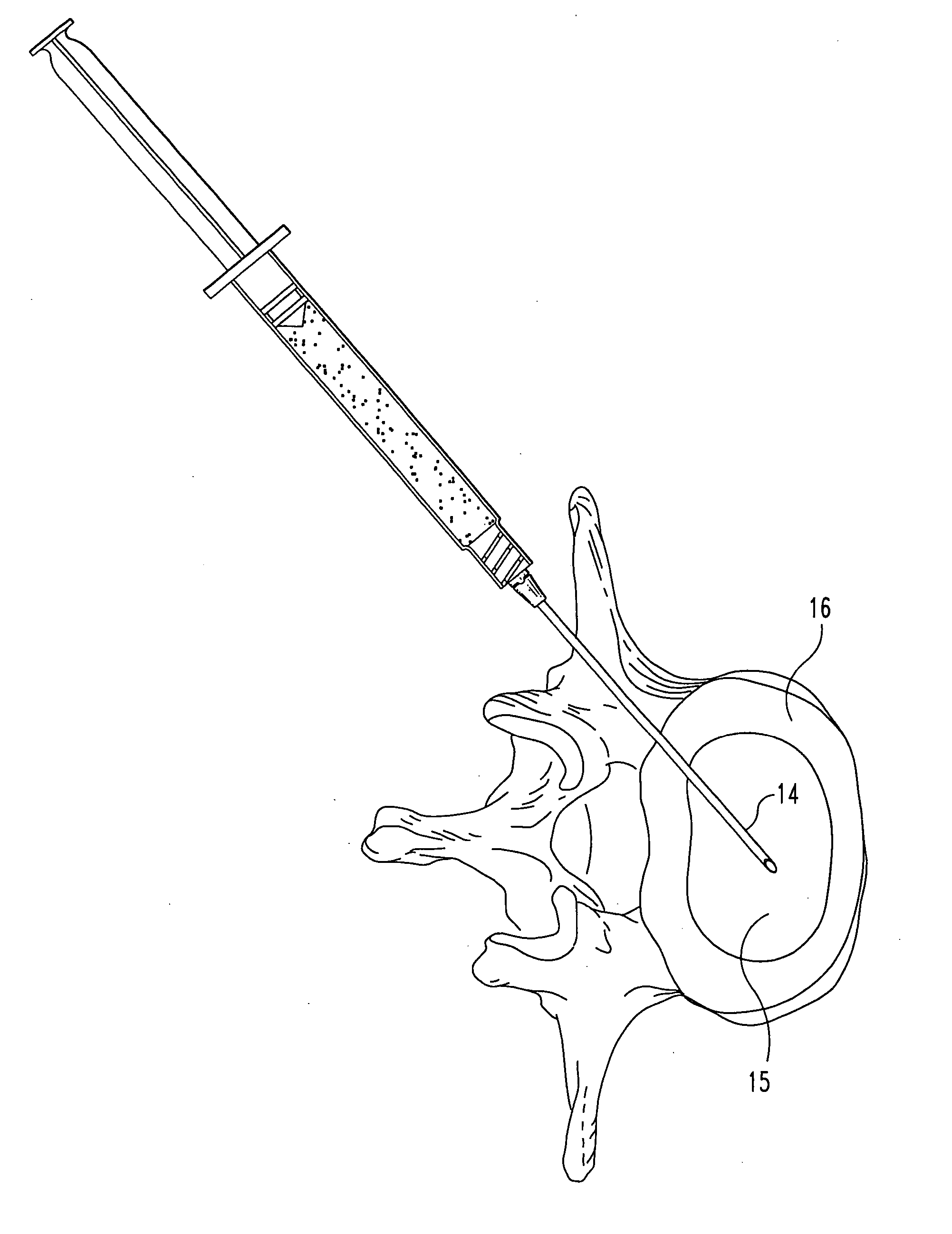
Compositions and methods for treating intervertebral discs with collagen-based materials
ActiveUS20050119754A1Enhance the imagePromote healingInternal osteosythesisLigamentsIntervertebral discInjected material
A method of augmenting an intervertebral disc nucleus by injecting or otherwise adding to a disc nucleus a plurality of particles of natural, collagen-rich tissue. The mean particle size of the pieces of natural, collagen-rich tissue may be between 0.25 mm and 1.0 mm. The particles may be dehydrated before implantation, and rehydrated after implantation, or they may be implanted in a “wet” state—such as a slurry or gel. Radiocontrast materials may be included to enhance imaging of the injected material. Other additives may include analgesics, antibiotics, proteoglycans, growth factors, stem cells, and / or other cells effective to promote healing and / or proper disc function.
Owner:WARSAW ORTHOPEDIC INC
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Implantable or insertable medical devices for controlled drug delivery
Implantable or insertable medical devices are provided, which comprises: (a) a biocompatible polymer; and (b) at least one therapeutic agent selected from an anti-inflammatory agent, an analgesic agent, an anesthetic agent, and an antispasmodic agent. The medical devices are adapted for implantation or insertion at a site associated with pain or discomfort upon implantation or insertion. In many embodiments, the therapeutic will be selected from at least one of (i) ketorolac and pharmaceutically acceptable salts thereof (e.g., ketorolac tromethamine) and (ii) 4-diethylamino-2-butynylphenylcyclohexyl glycolate and pharmaceutically acceptable salts thereof (e.g., oxybutynin chloride). Also provided are uses for the implantable or insertable medical devices, which uses comprise reducing pain or discomfort accompanying the implantation or insertion of such devices. Further uses may comprise reducing microbial buildup along the device. Methods for manufacturing implantable or insertable medical devices are also provided.
Owner:BOSTON SCI SCIMED INC
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20090317355A1Reduced and limited dose-dumping effectReduce interactionBiocideNervous disorderVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Methods and related compositions for reduction of fat and skin tightening
InactiveUS20060127468A1Efficient tighteningTighten regionBiocideCosmetic preparationsCelluliteExcipient
Compositions and methods useful in the reduction of localized fat deposits and tightening of loose skin in subjects in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion or anti-dispersion agents and pharmaceutically acceptable excipients. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including, for example, lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Topical dermal anaesthetic
A liquid composition applied transdermally for relief of pain comprising alcohol in an amount by weight of about 57 to about 91 percent; glycerin in an amount by weight of about 1 to about 12 percent; an analgesic agent in an amount by weight of about 2 to about 28 percent, the analgesic agent comprising a derivative of salicylic acid; methylsulfonylmethane in an amount by weight of about 0.02 to 5 percent; and emu oil in an amount by weight of about 0.01 to 3 percent, the liquid composition permeating skin to relieve pain. The composition further comprising, as an additional feature, aloe vera in an amount by weight of at least about 0.05 percent and having an amount by weight of about 0.05 to 4 percent. The composition features transdermal pain relief such that a patient can apply the analgesic agent directly to an area of pain without such side effects as stomach irritation which is normally associated with aspirin. The composition may be sprayed or rolled directly onto the painful area. Because of the unique formula, the composition is safe to vital internal organs, requires no mixing before use, and is shelf stable for marketing purposes.
Owner:VELTRAN LP
Methods and related compositions for reduction of fat
ActiveUS20050267080A1Reduce fat depositionReduce decreaseAntibacterial agentsBiocideCelluliteExcipient
Compositions and methods useful in the reduction of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion or anti-dispersion agents and pharmaceutically acceptable excipients. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including, for example, lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT +1
Methods and compositions for the non-surgical removal of fat
ActiveUS20050261258A1Easy to disassembleReduces blood lossCosmetic preparationsOrganic active ingredientsSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
Treatment of urinary incontinence and other disorders by application of energy and drugs
The invention provides a method and system for treating disorders in parts of the body. A particular treatment can include on or more of, or some combination of: ablation, nerve modulation, three-dimensional tissue shaping, drug delivery, mapping stimulating, shrinking and reducing strain on structures by altering the geometry thereof and providing bulk to particularly defined regions. The particular body structures or tissues can include one or more of or some combination of region, including: the bladder, esophagus, vagina, penis, larynx, pharynx, aortic arch, abdominal aorta, thoracic, aorta, large intestine, sinus, auditory canal, uterus, vas deferens, trachea, and all associated sphincters. Types of energy that can be applied include radiofrequency, laser, microwave, infrared waves, ultrasound, or some combination thereof. Types of substances that can be applied include pharmaceutical agents such as analgesics, antibiotics, and anti-inflammatory drugs, bulking agents such as biologically non-reactive particles, cooling fluids, or dessicants such as liquid nitrogen for use in cryo-based treatments.
Owner:VERATHON
Conjugates of galactose-binding lectins and clostridial neurotoxins as analgesics
InactiveUS7052702B1Pain reliefReduce and preferably prevent transmissionNervous disorderBacteriaGalactose binding lectinProtein translocation
A class of novel agents that are able to modify nociceptive afferent function is provided. The agents may inhibit the release of neurotransmitters from discrete populations of neurones and thereby reduce or preferably prevent the transmission of afferent pain signals from peripheral to central pain fibers. They comprise a galactose-binding lectin linked to a derivative of a clostridial neurotoxin. The derivative of the clostridial neurotoxin comprises the L-chain, or a fragment thereof, which includes the active proteolytic enzyme domain of the light (L) chain, linked to a molecule or domain with membrane translocating activity. The agents may be used in or as pharmaceuticals for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
Methods and related compositions for the non-surgical removal of fat
InactiveUS20060154906A1Easy to disassembleReduces blood lossCosmetic preparationsBiocideSurgical removalCellulite
Compositions and methods useful in the non-surgical removal of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion agents and pharmaceutically acceptable excipients but do not contain phosphotidylcholine. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
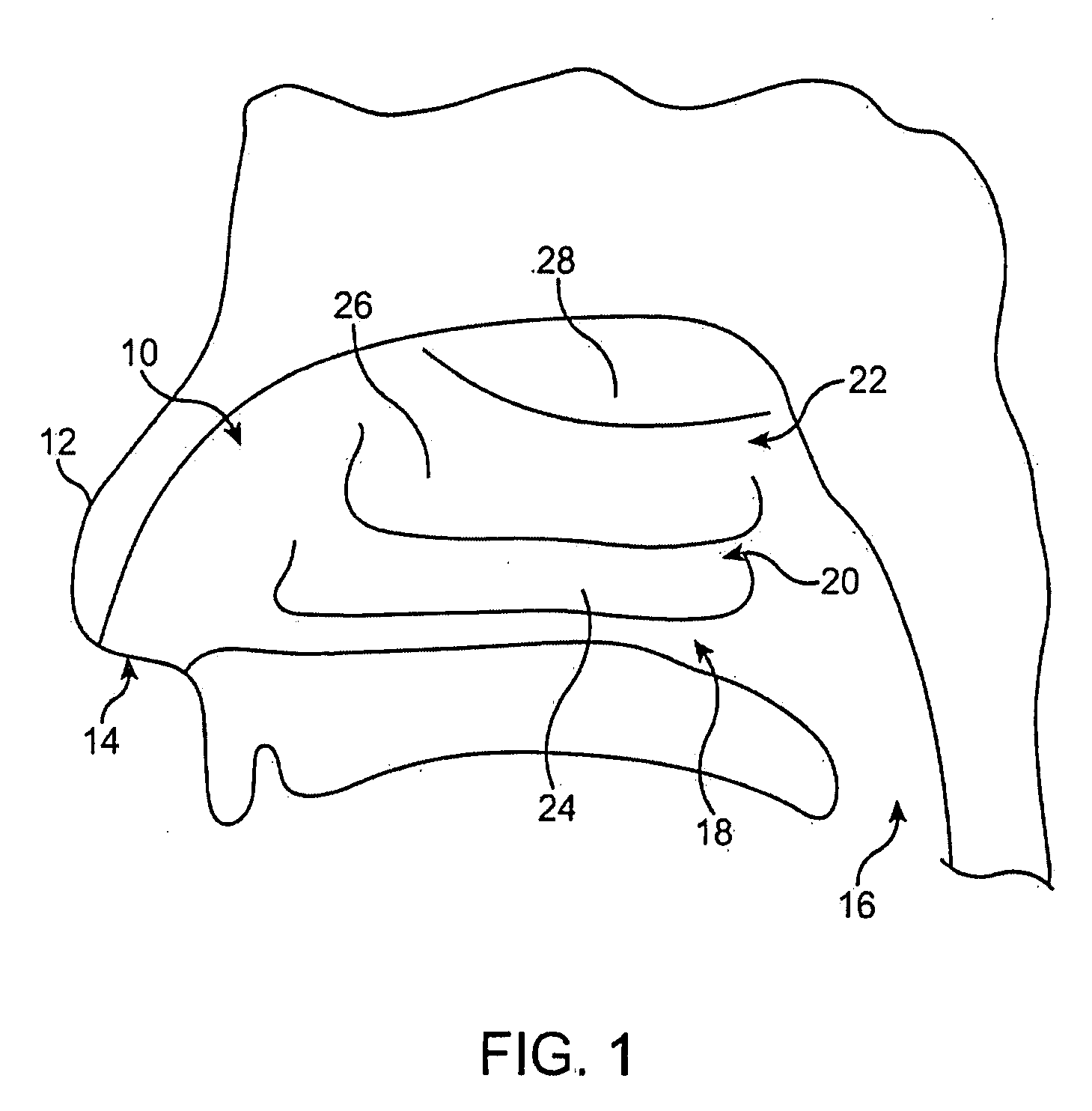
Systems for treatment of nasal tissue
InactiveUS20080027423A1Small sizeIncrease airflowUltrasound therapySurgical instrument detailsPain managementAnalgesic agents
Systems for the treatment of nasal tissue, particularly the nasal turbinates, are described. One method for reducing the size of the inferior nasal turbinate is to apply ultrasound energy to the tissue regions beneath the surface of the turbinate tissue. One instrument may be used to deliver ultrasound energy and provide an infusion or injection of a fluid directly into the turbinate being treated, e.g., to bulk up the size of the turbinate to ensure that the ultrasound energy is properly delivered directly into the intended turbinate tissue. Fluids containing anesthetics, fluids infused with analgesics, etc. may be used for pain management while other medications, such as non-steroidal drugs, steroidal drugs, anti-inflammatory drugs, anti-histamines, anti-bacterial drugs, etc., can also be used. Such assemblies can also be utilized with other instruments as a system. For example, such a probe can be used with nasal speculums or imaging instrument in treating tissue.
Owner:CHOI GEORGE Y
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant agent
InactiveUS20070014732A1Reducing abuse potential of dosage formLower potentialOrganic active ingredientsNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Collagen-based materials and methods for augmenting intervertebral discs
ActiveUS20050197707A1Enhance the imagePromote collagen crosslinkingInternal osteosythesisPeptide/protein ingredientsIntervertebral discInjected material
A method of augmenting an intervertebral disc by injecting particles of collagen-based material into the disc. The particles may be dehydrated before implantation, and rehydrated after implantation, or they may be implanted in a “wet” state—such as a slurry or gel. Radiocontrast materials may be included to enhance imaging of the injected material. Other additives may include analgesics, antibiotics, proteoglycans, growth factors, and / or other cells effective to promote healing and / or proper disc function.
Owner:WARSAW ORTHOPEDIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com