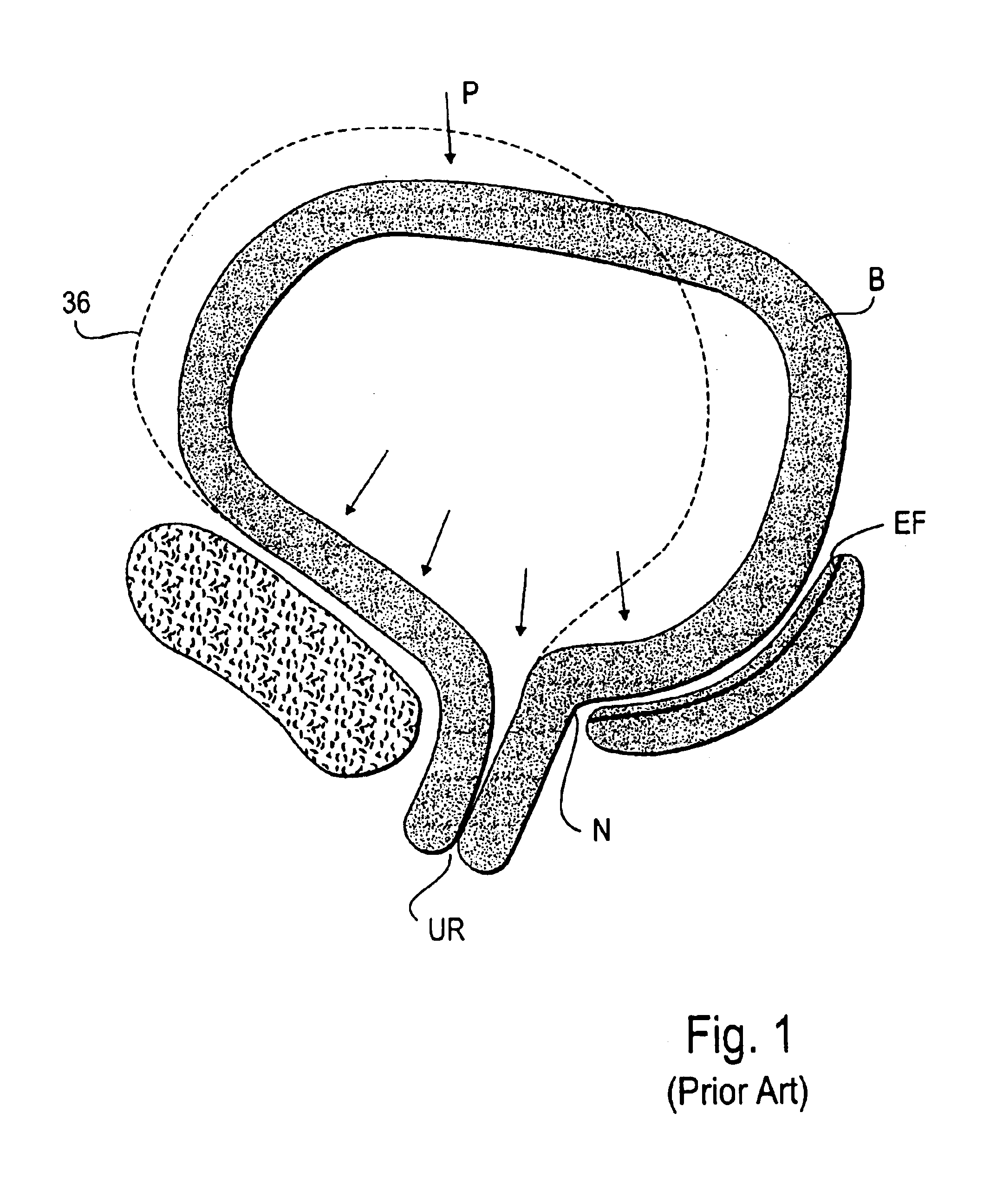
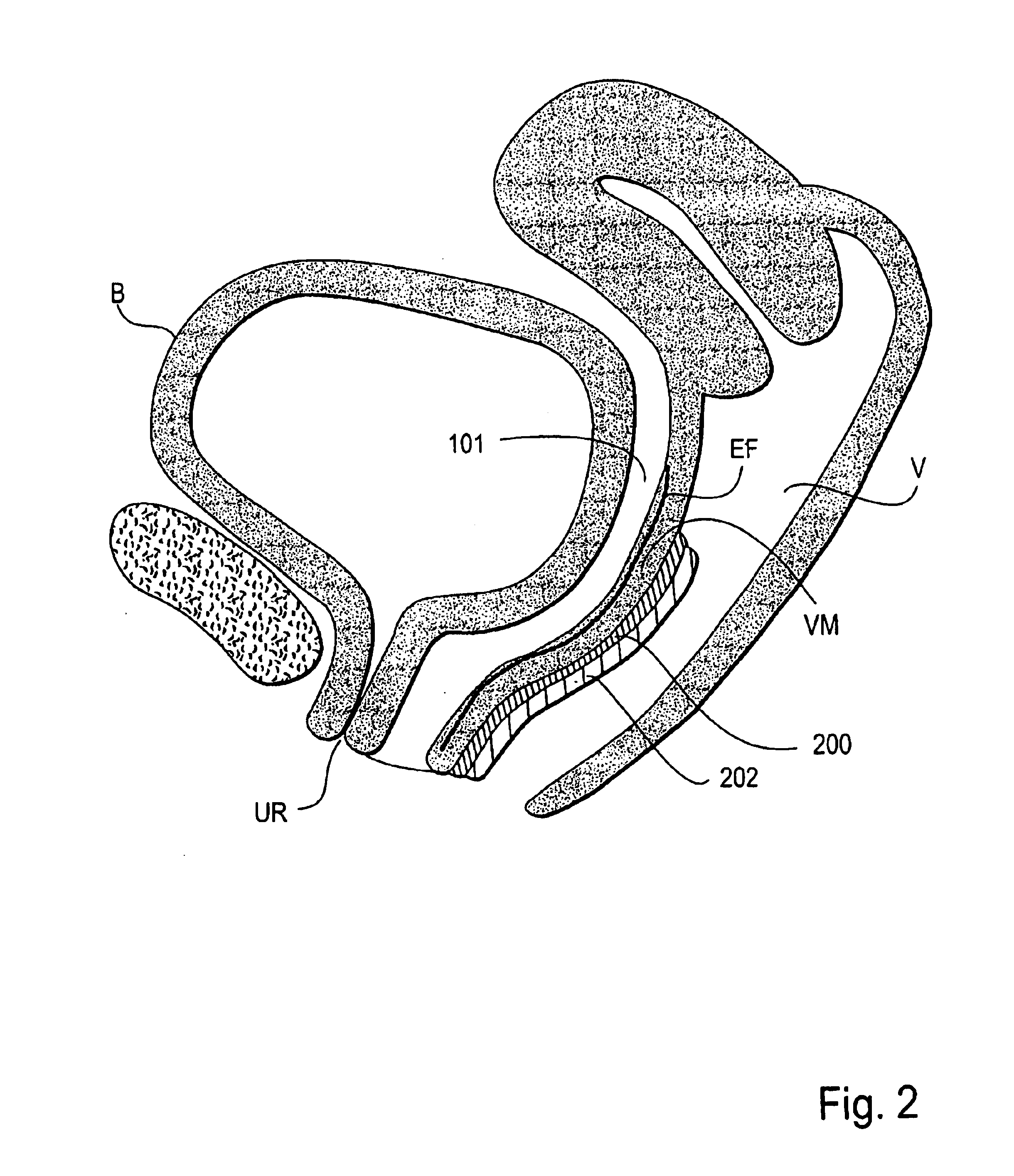
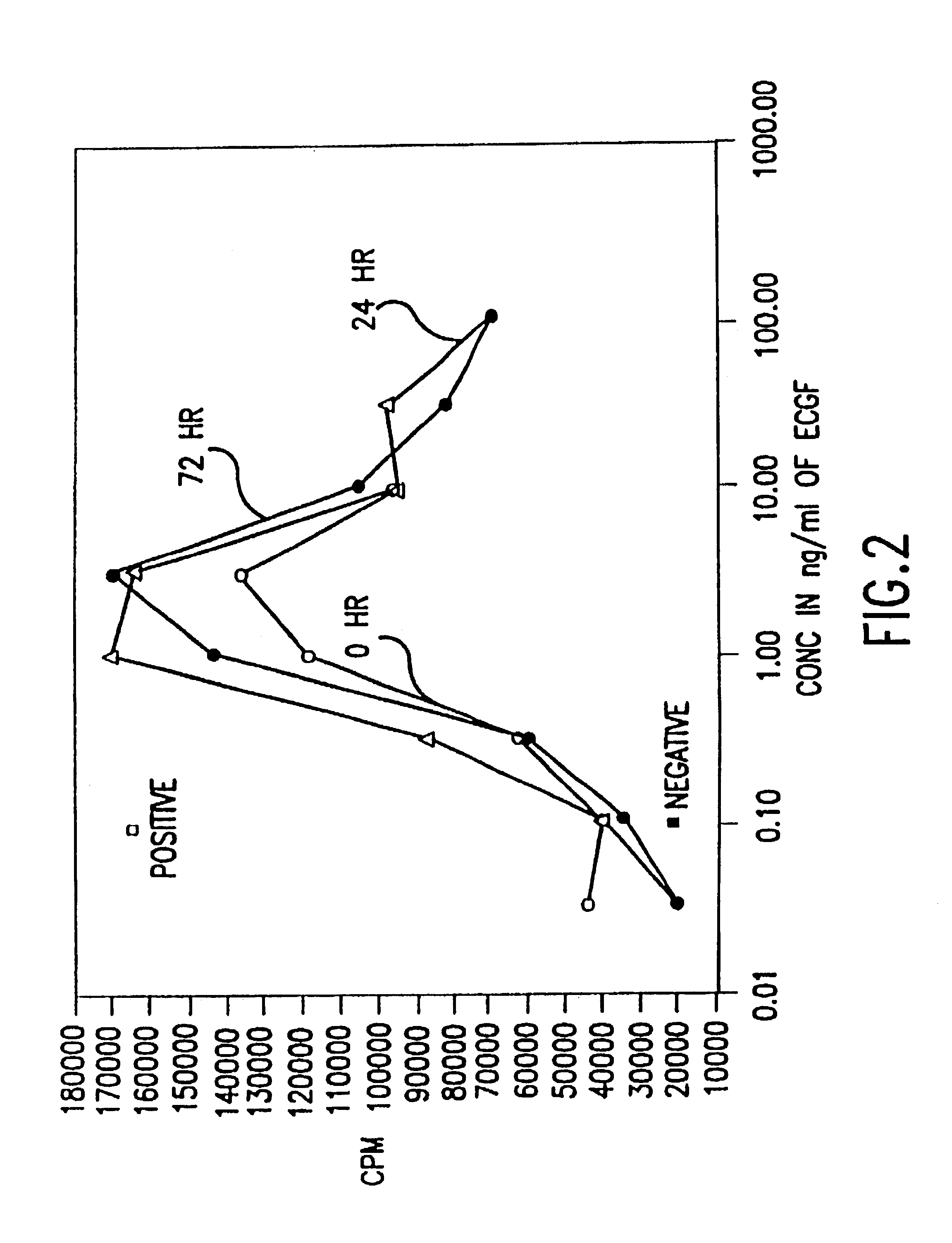
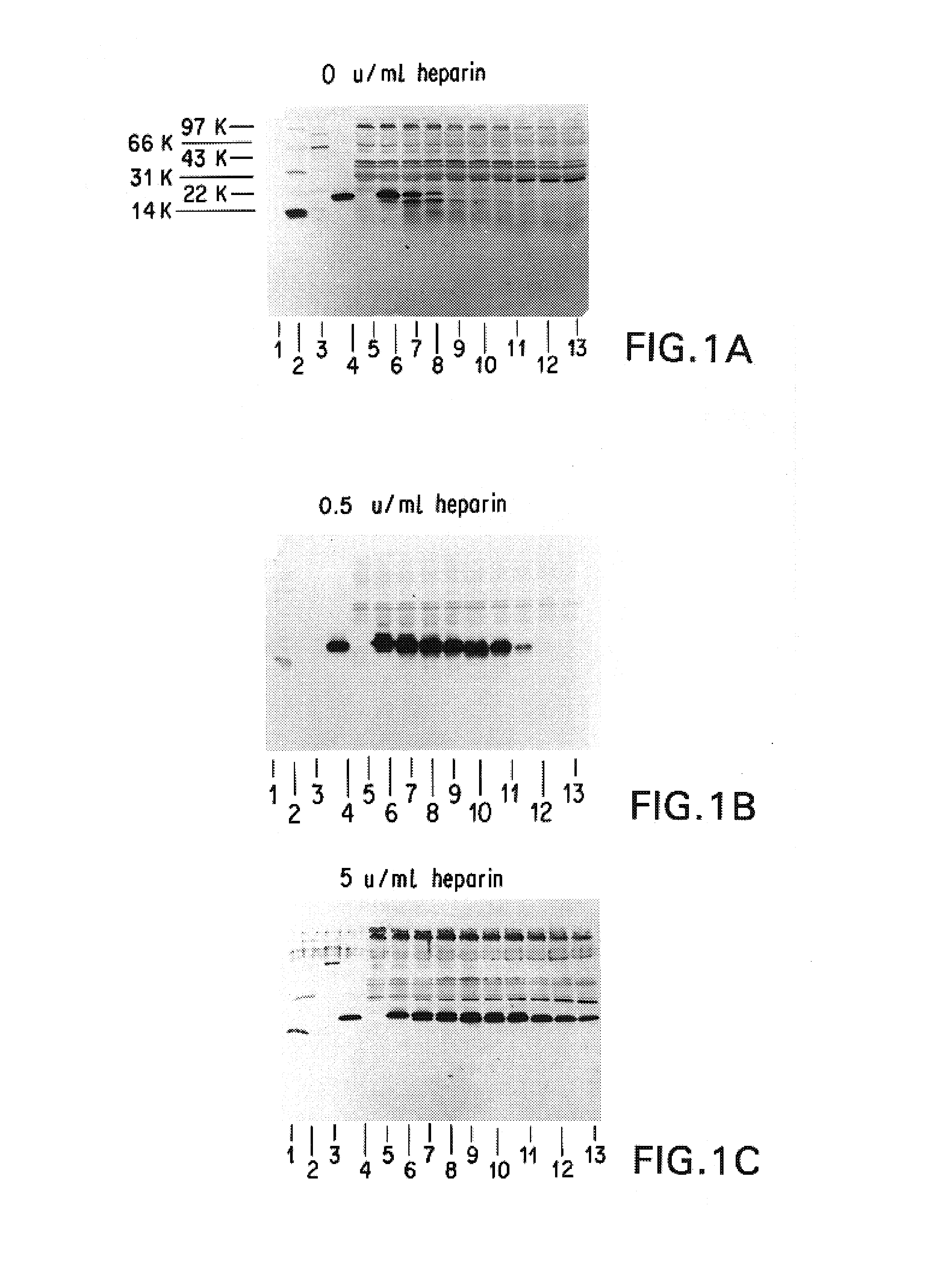
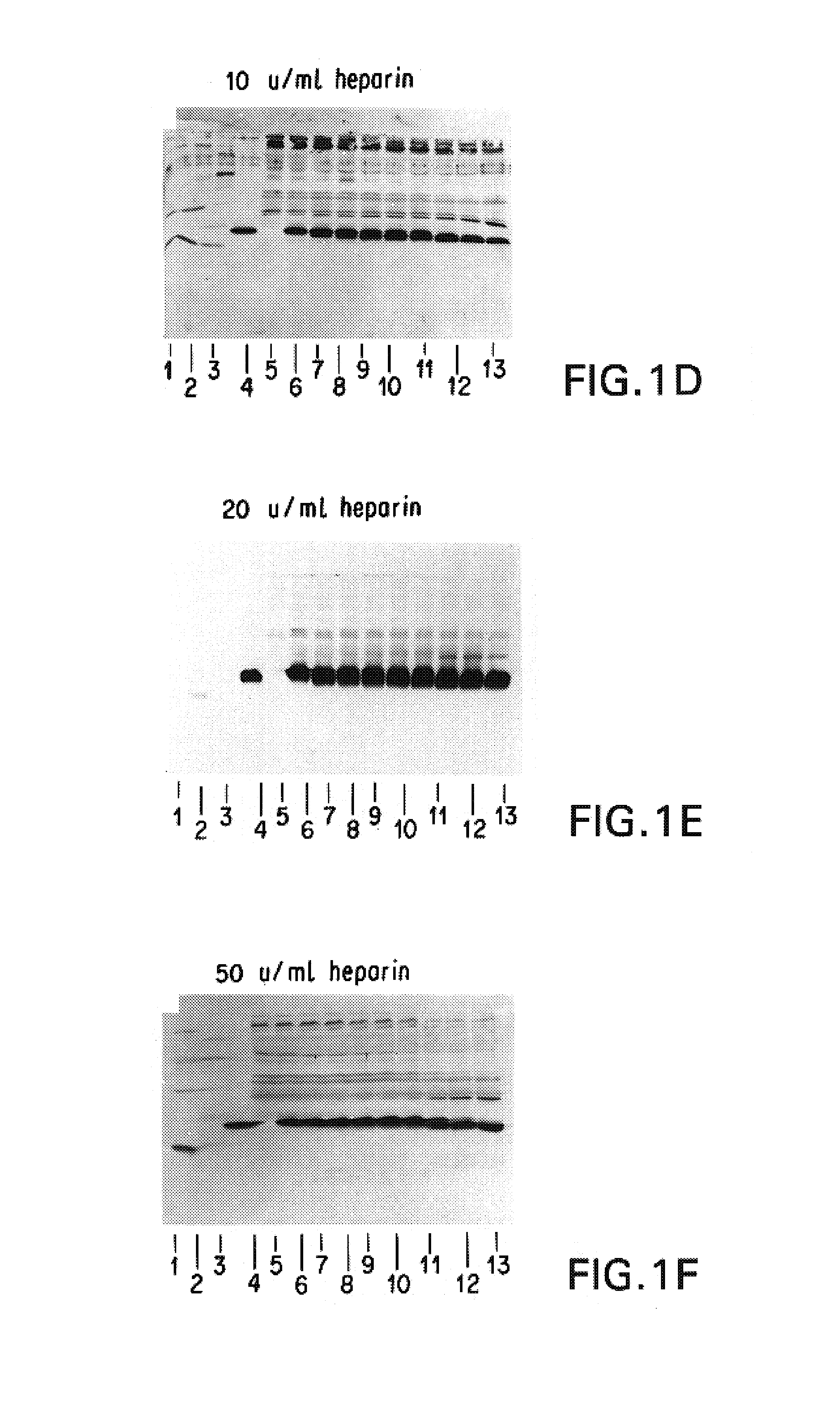
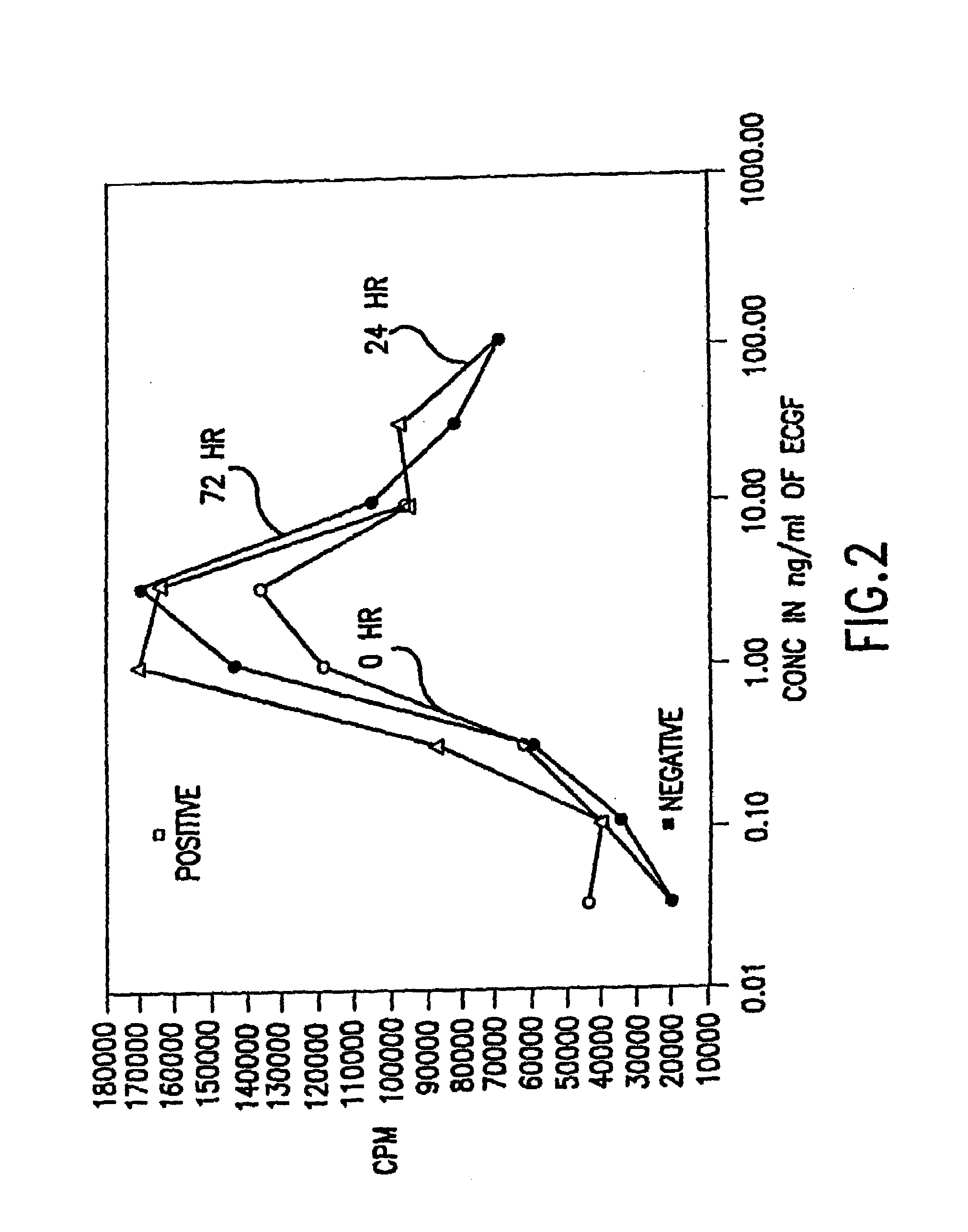
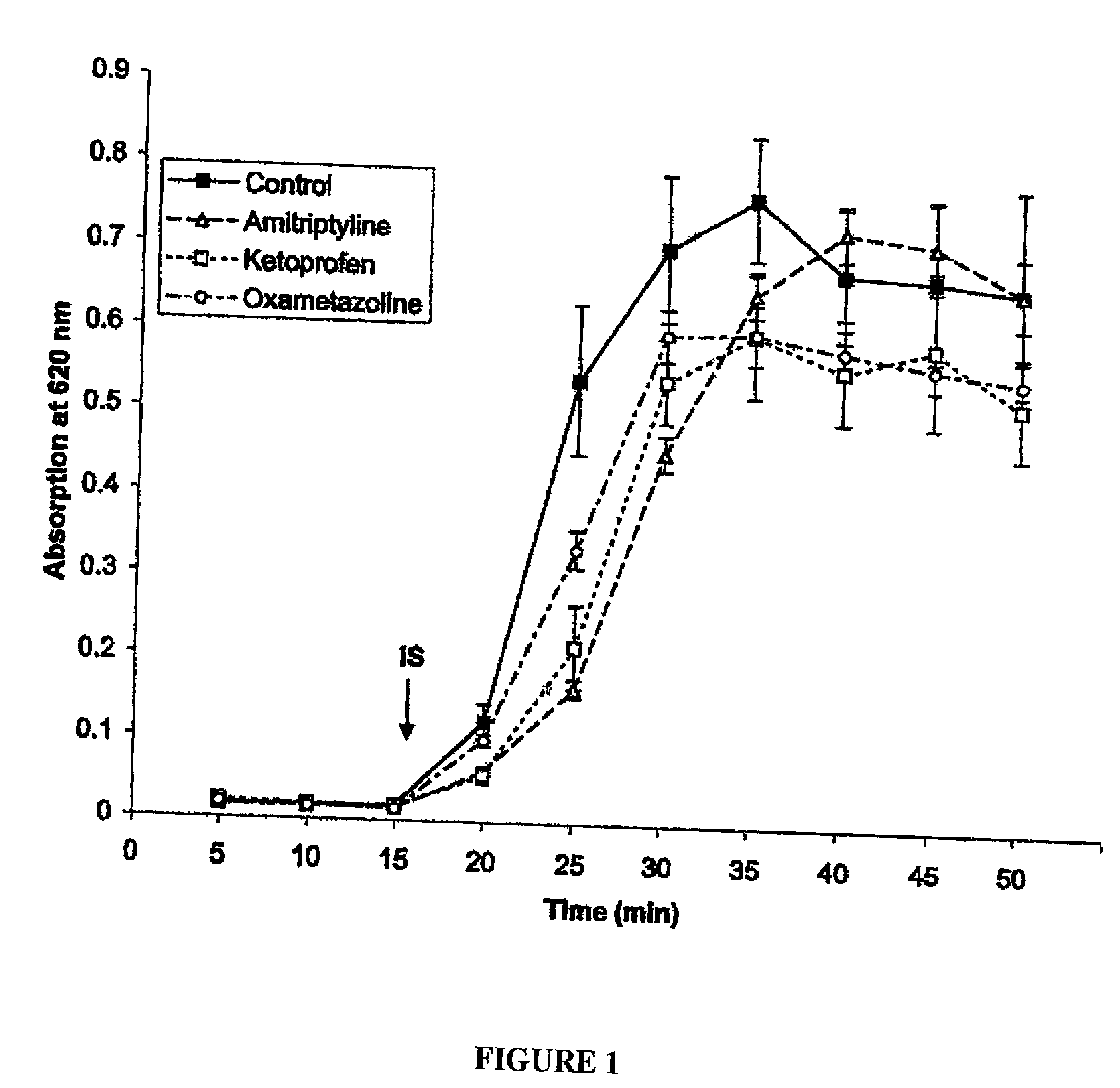
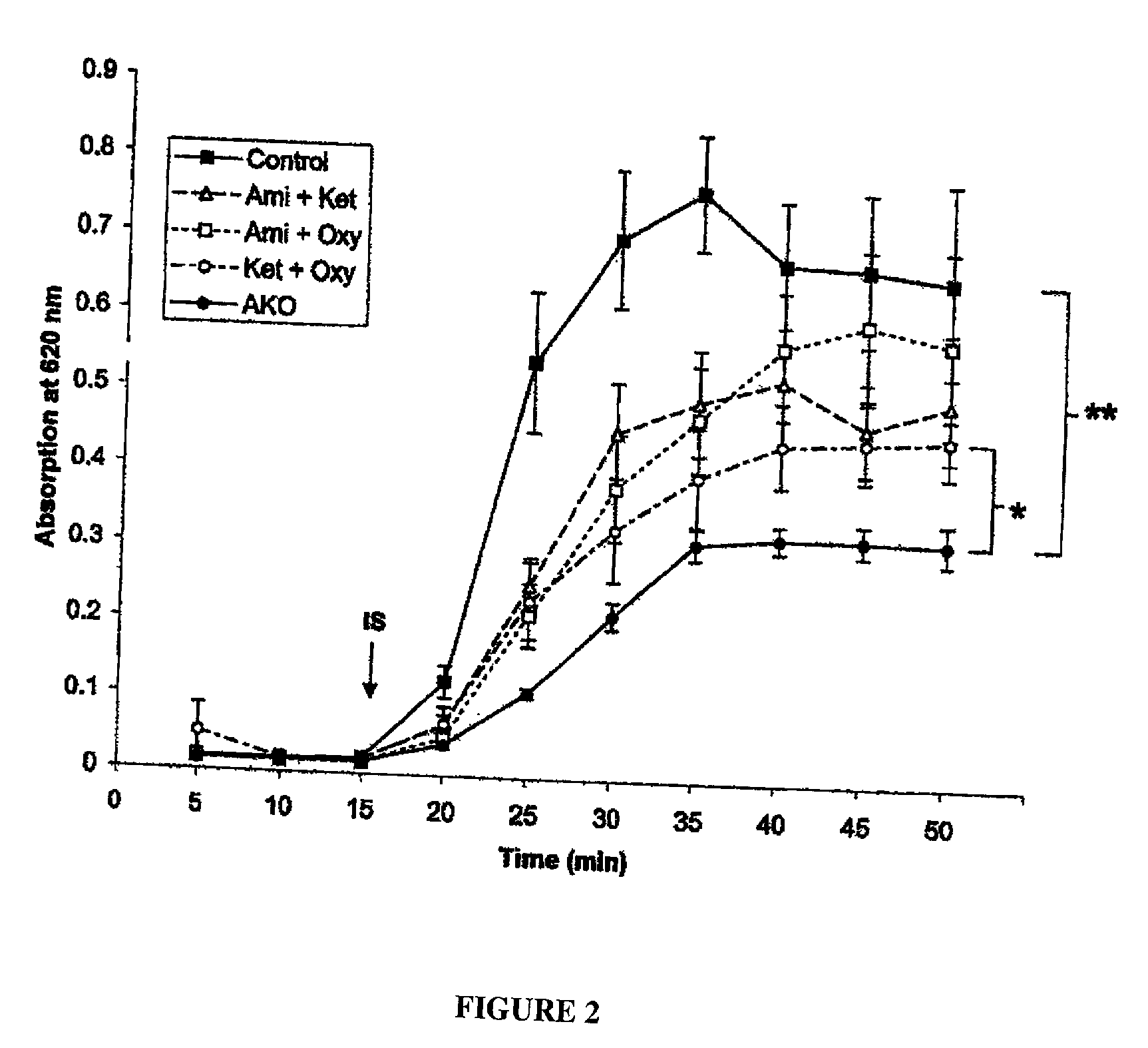
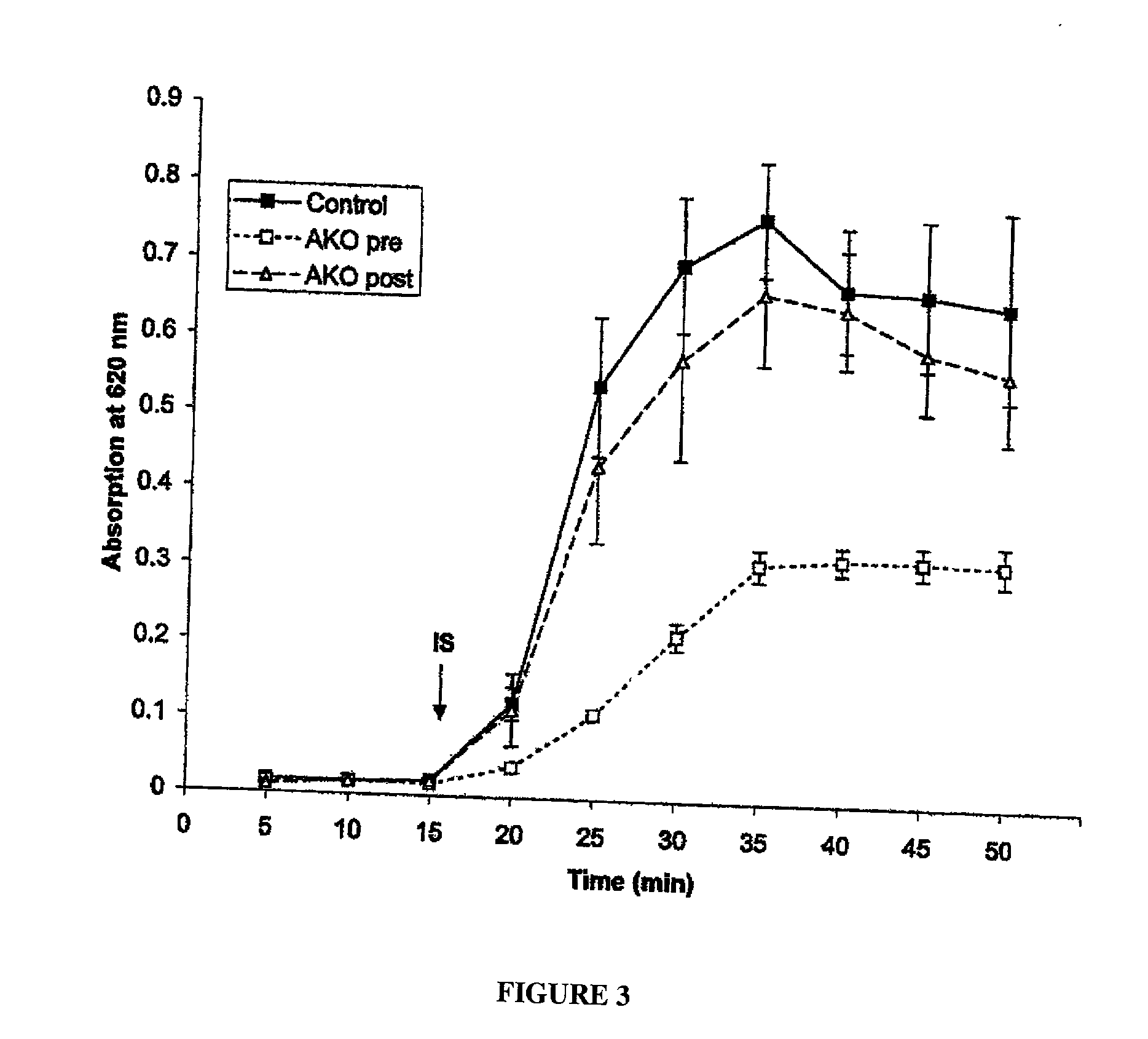
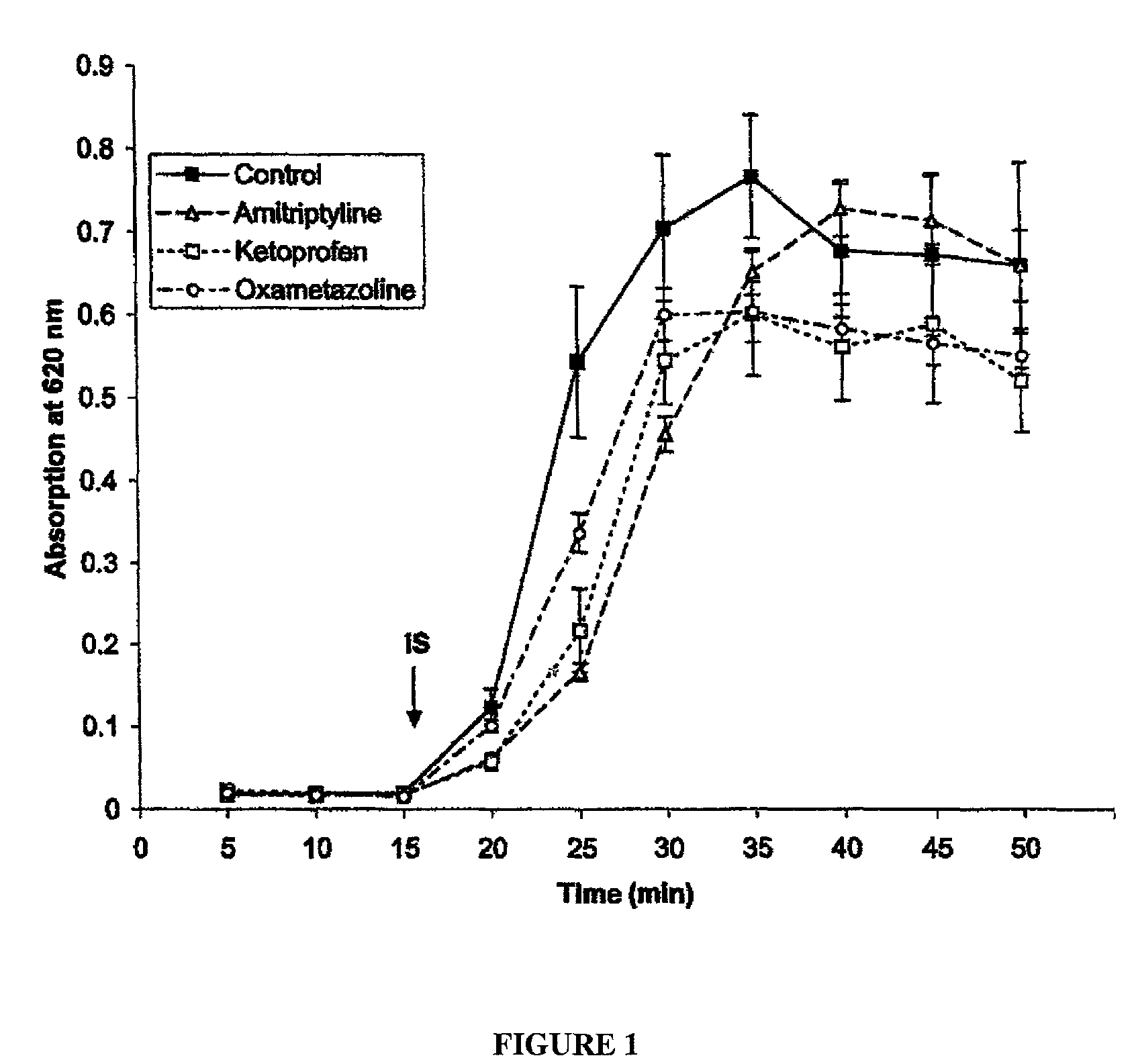
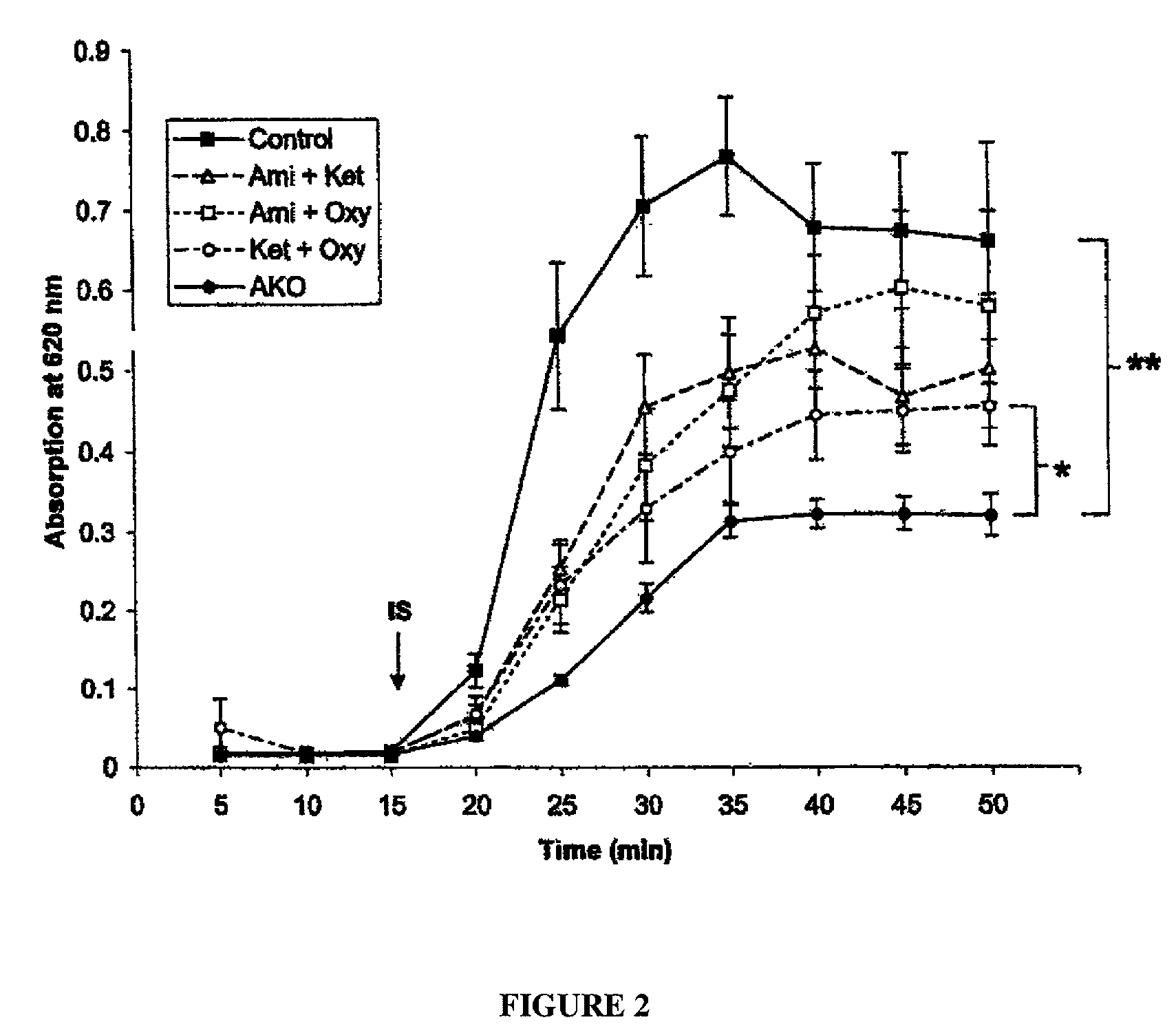
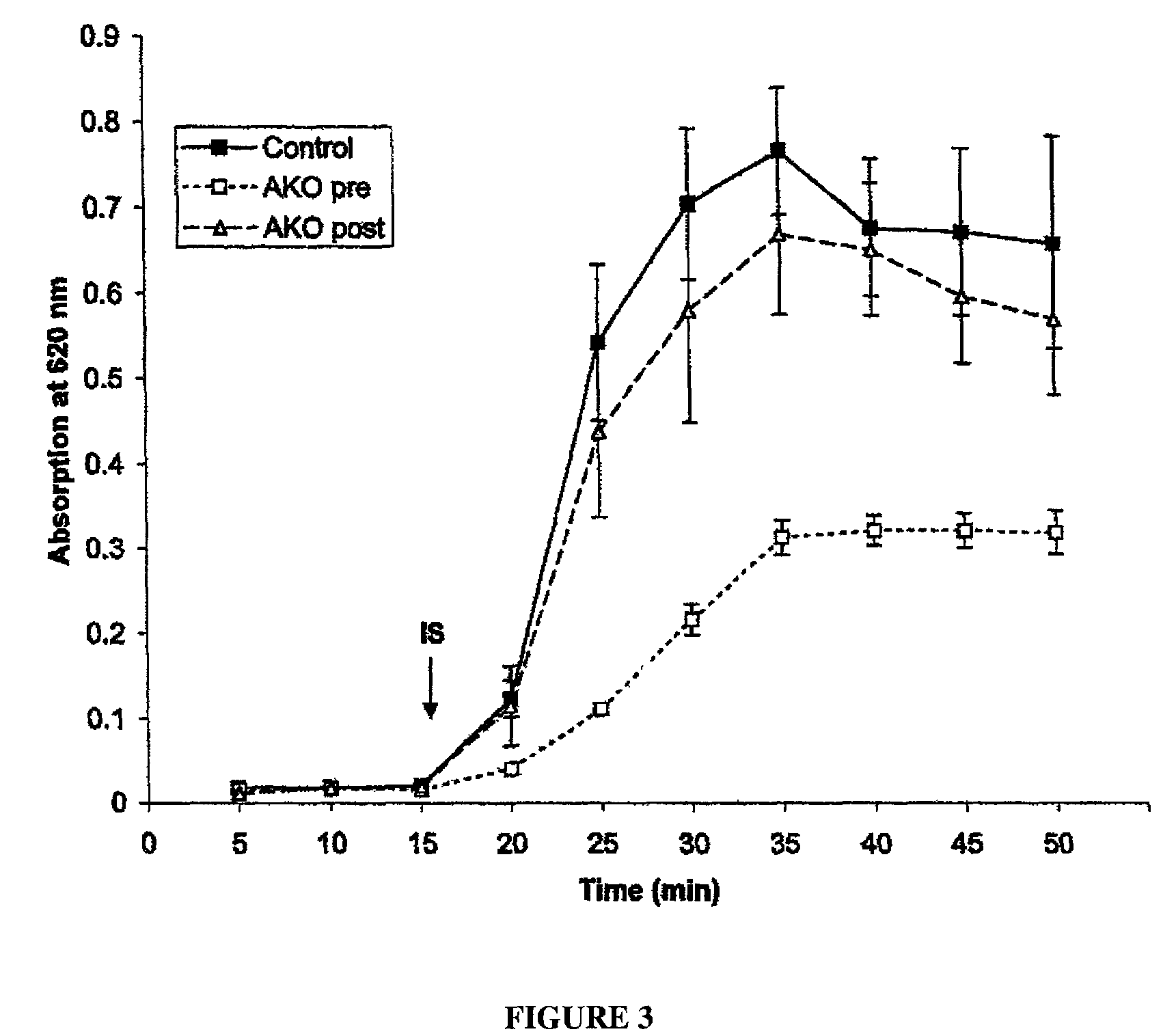
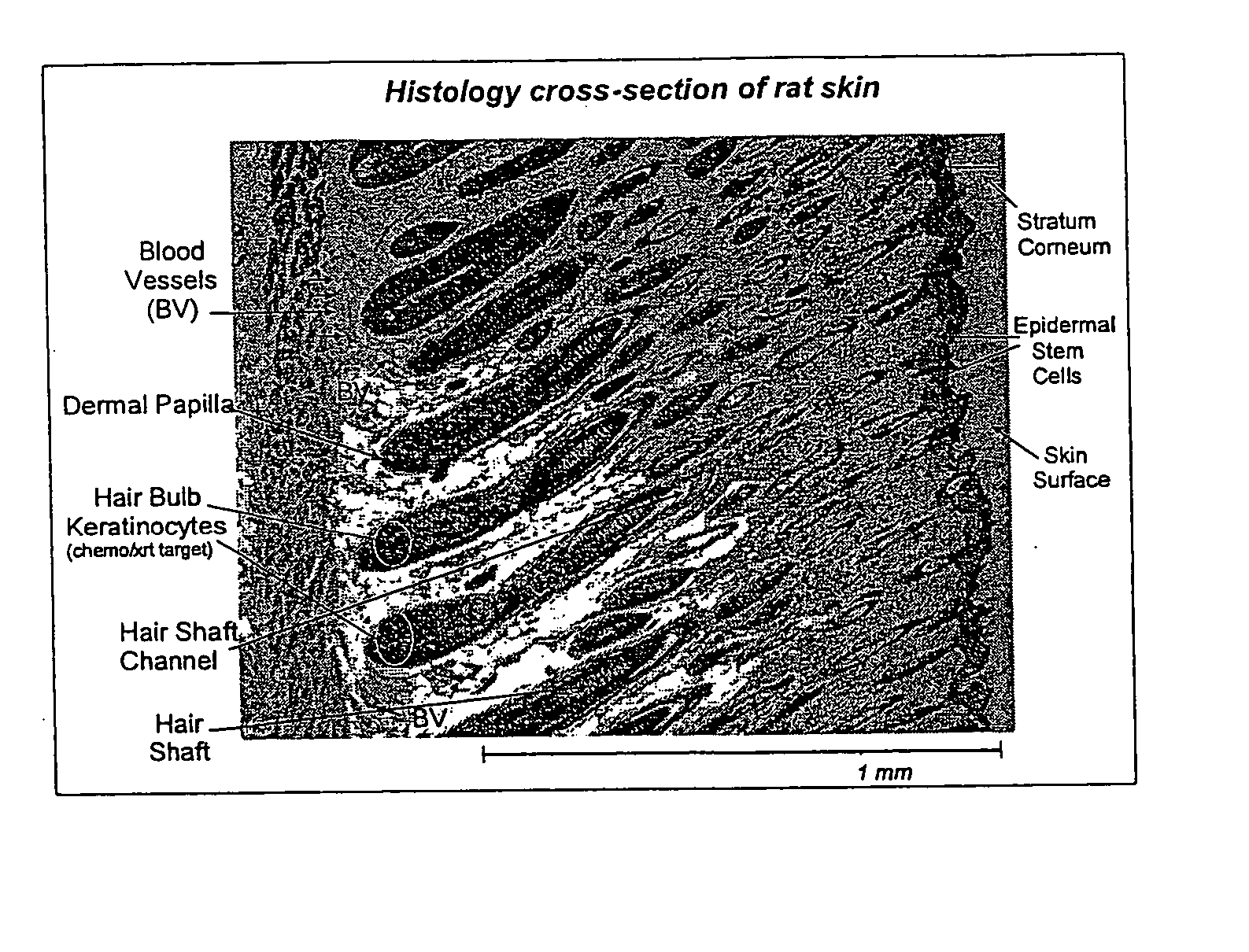
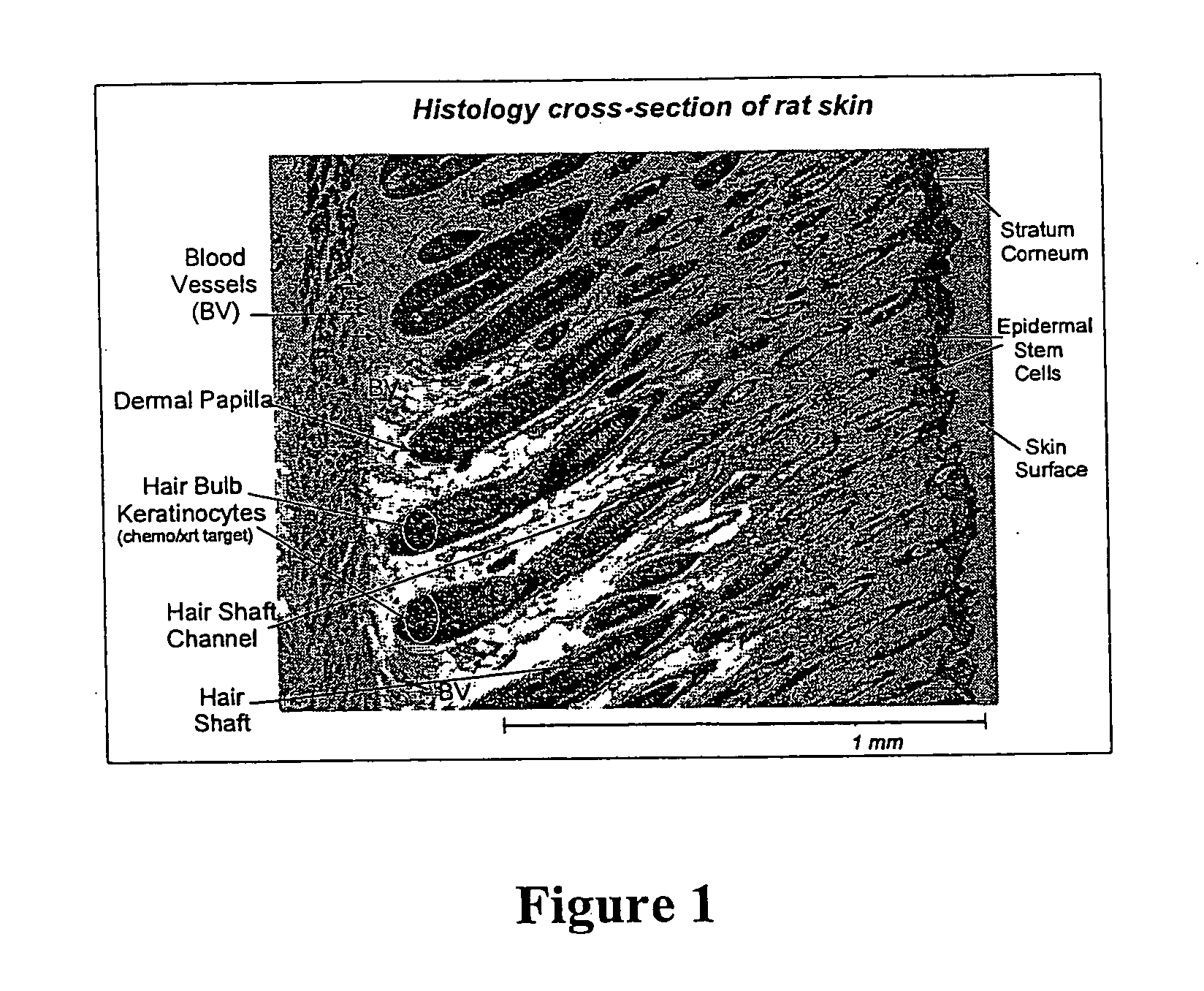
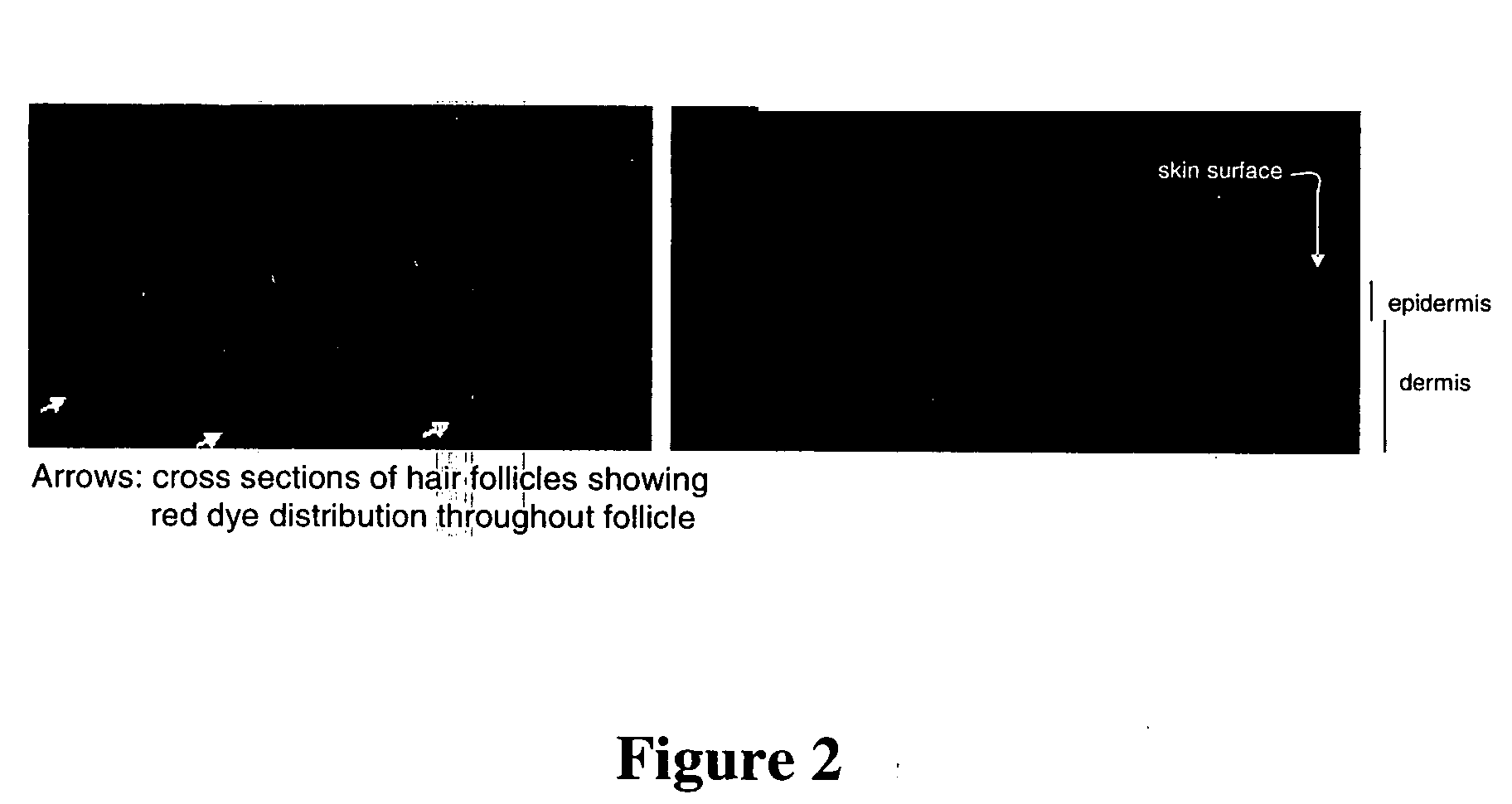
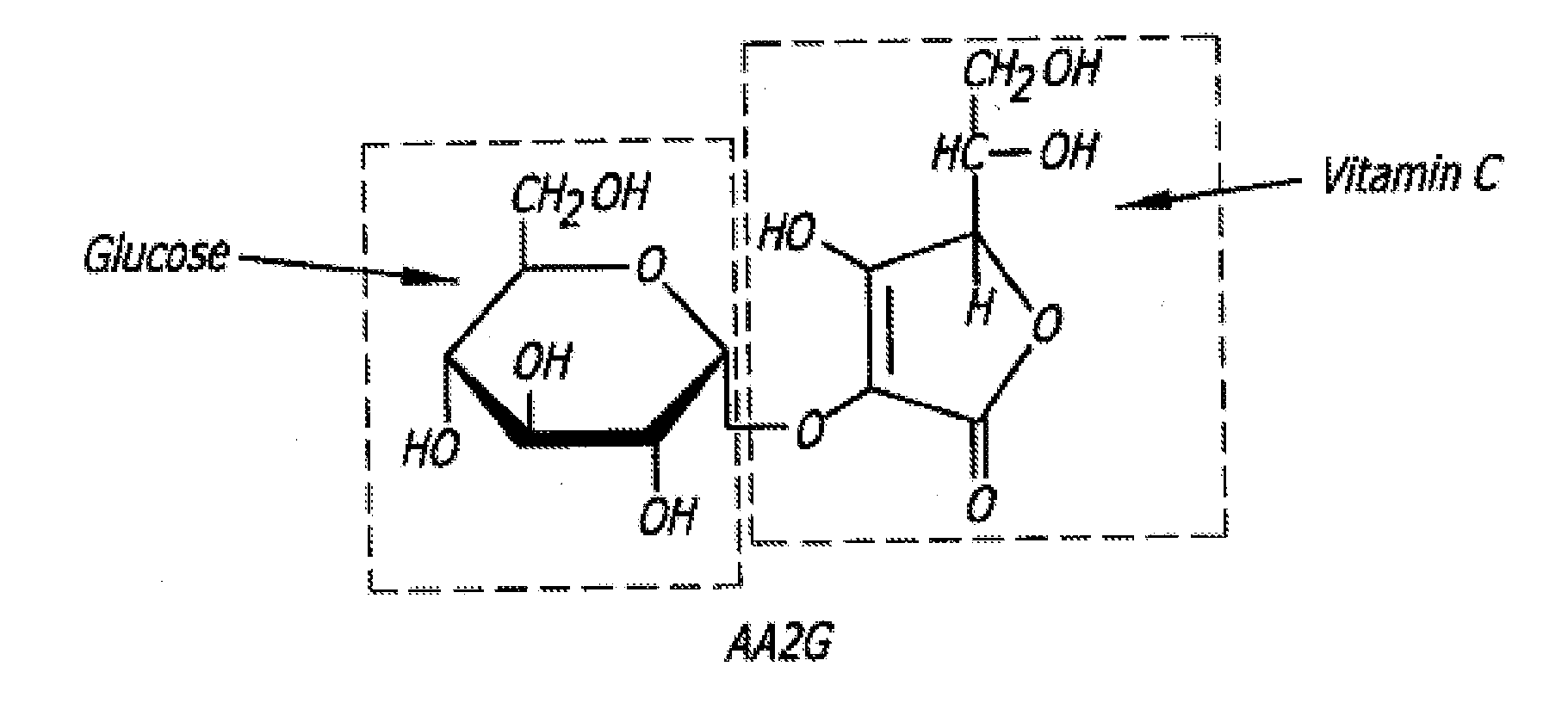
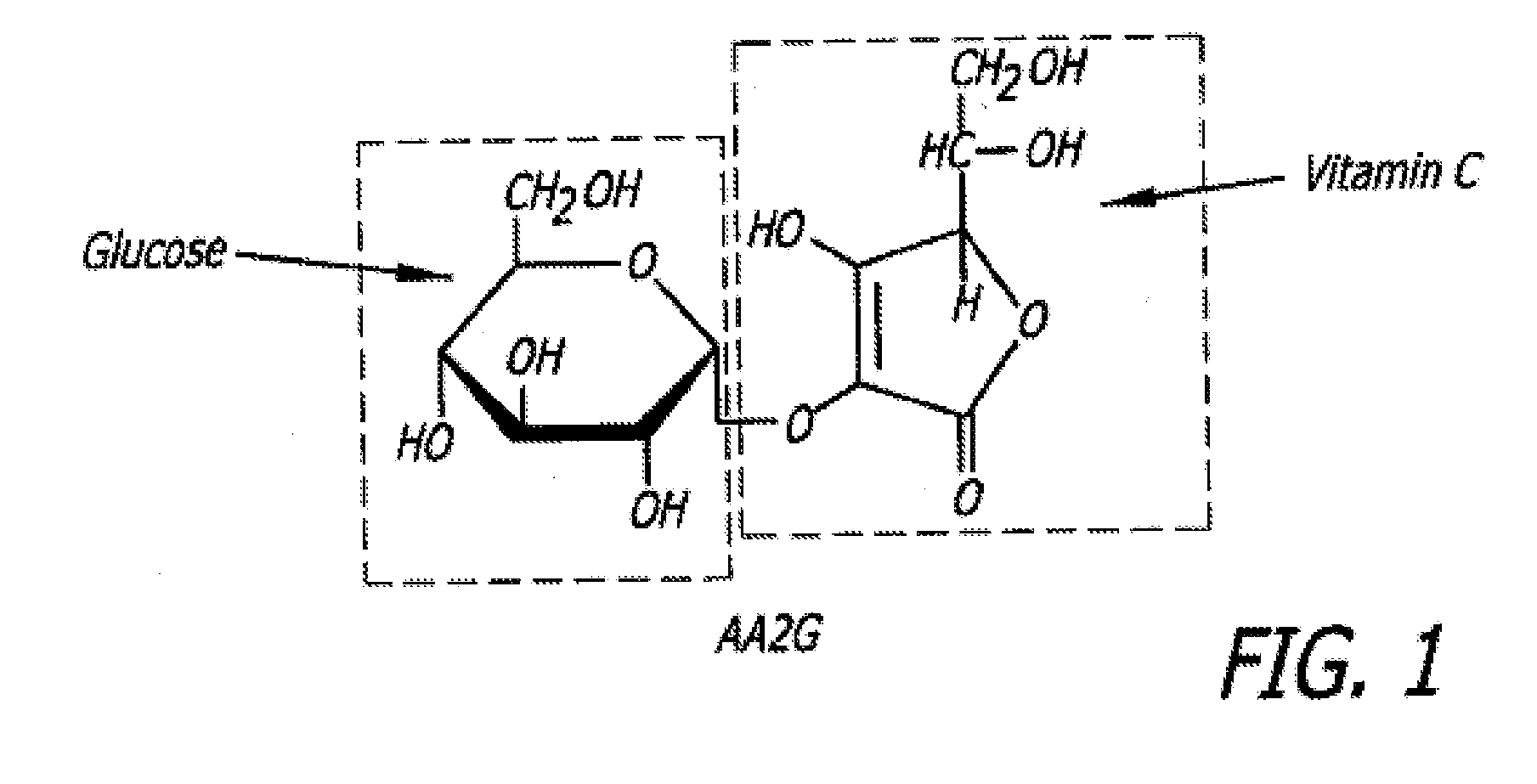
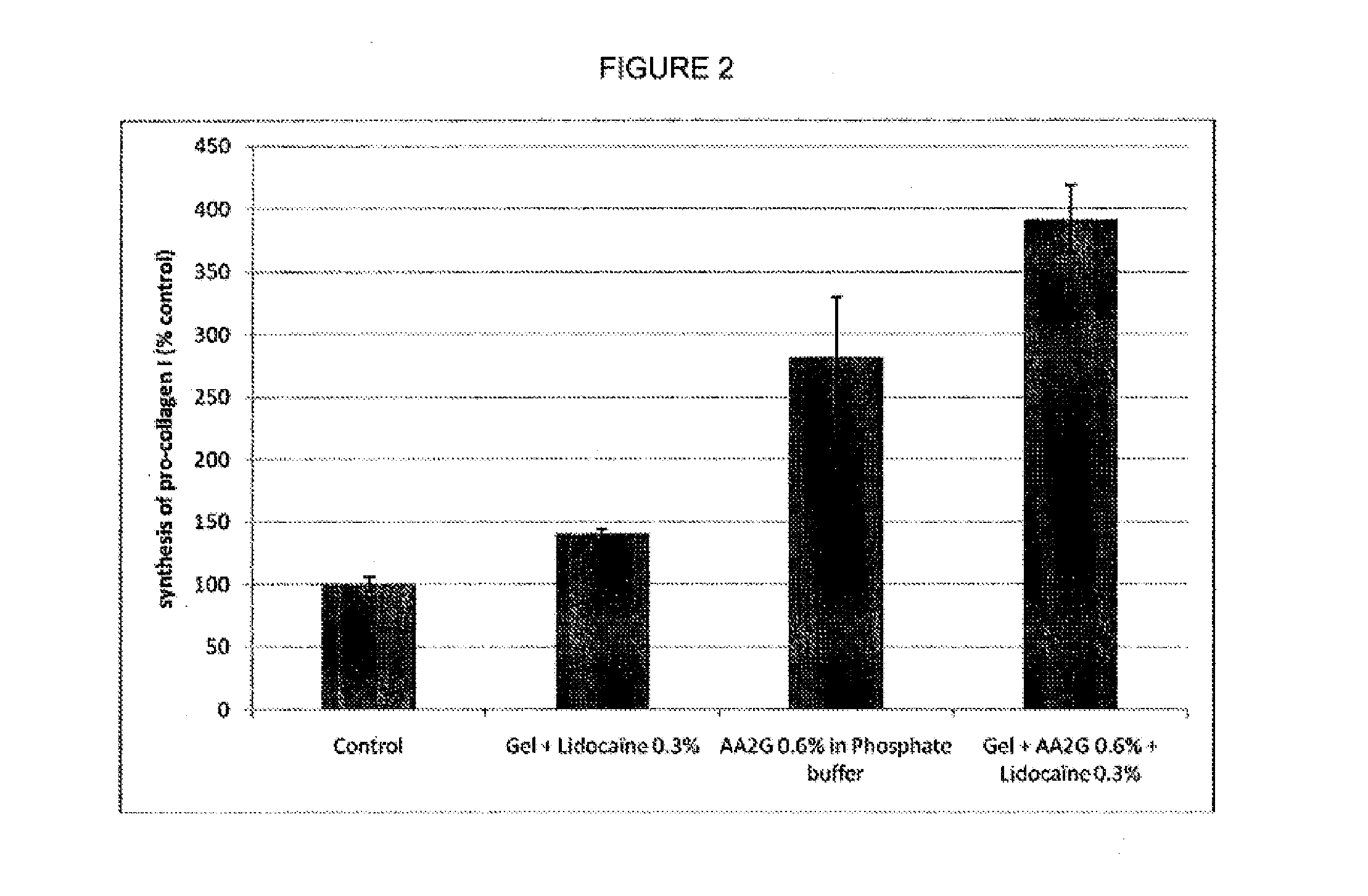
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109 results about "Vasoconstrictor Agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drugs used to cause constriction of the blood vessels.
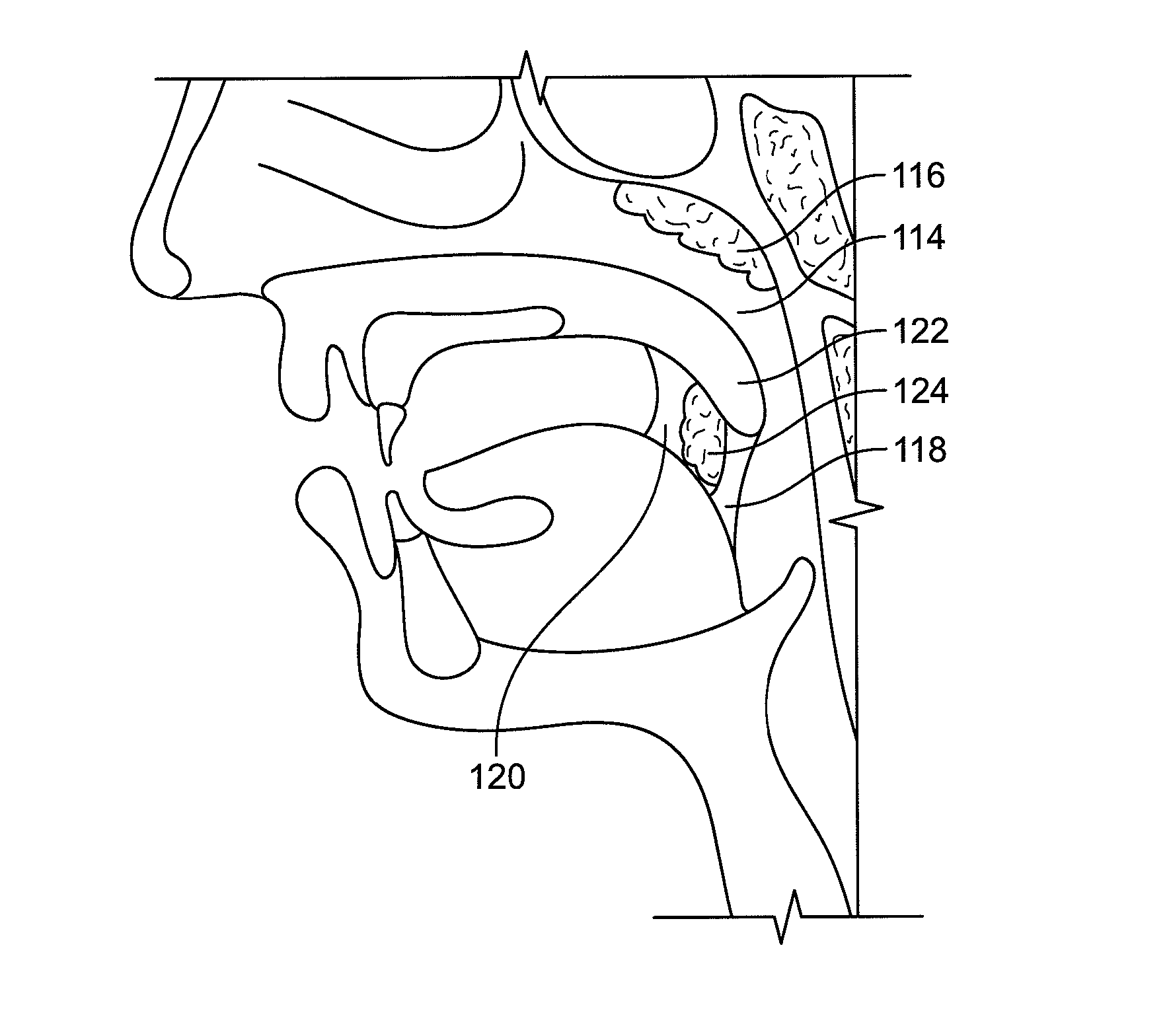
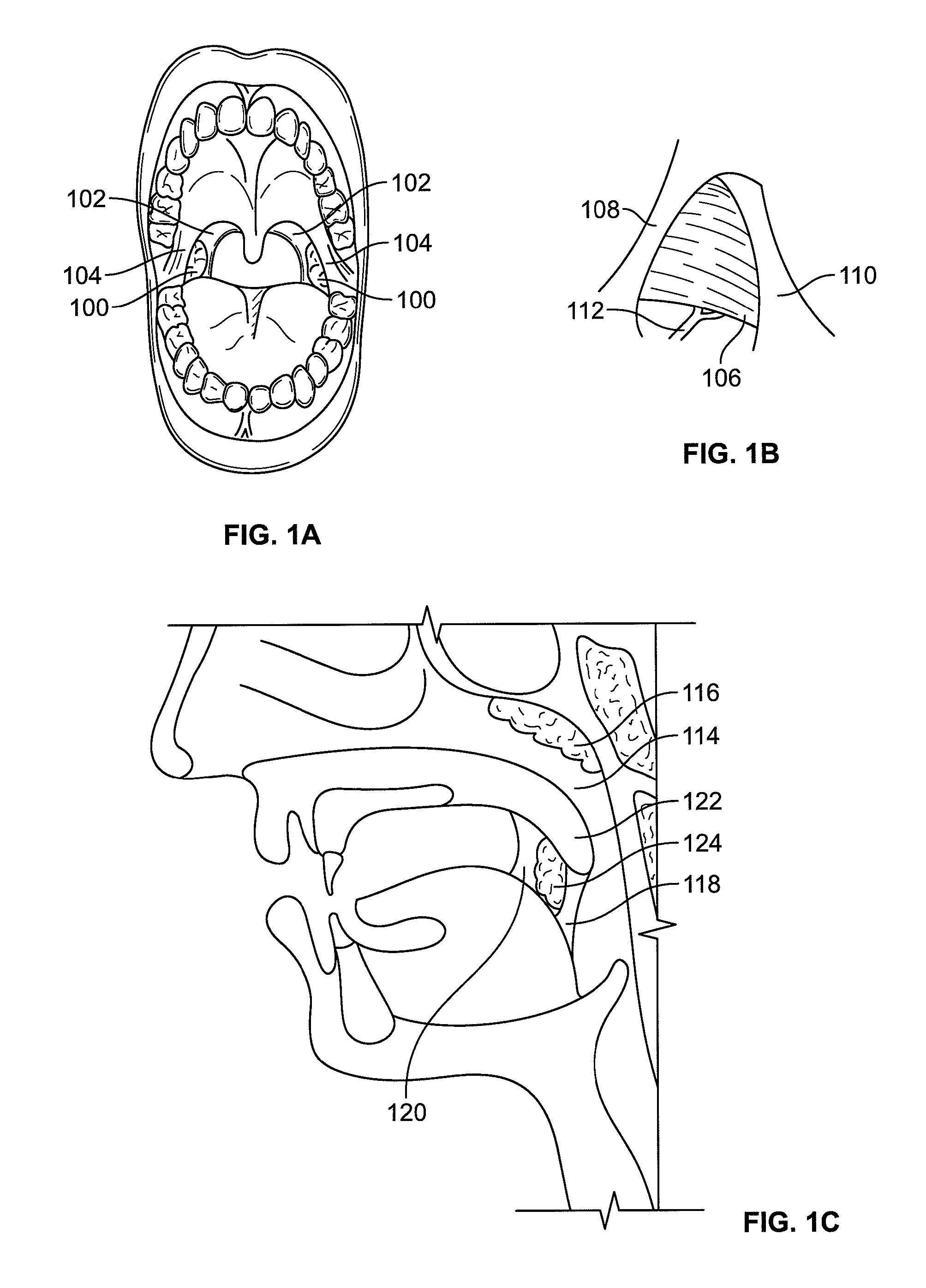
Devices and methods for treating pain associated with tonsillectomies
Described here are devices and methods for treating one or more conditions or symptoms associated with a tonsil procedure. In some variations, a drug-releasing device may be at least partially delivered to one or more tonsillar tissues before, during, or after a tonsil procedure. In some variations, the drug-releasing device may be configured to be biodegradable. In other variations, the drug-releasing device may comprise one or more hemostatic materials or one or more adhesives. The drug-releasing device may be configured to release one or more drugs or agents, such as, for example, one or more analgesics, local anesthetics, vasoconstrictors, antibiotics, combinations thereof and the like.
Owner:INTERSECT ENT INC
Skin resurfacing and treatment using biocompatible materials
InactiveUS20050059940A1Eliminate the problemAvoid infectionSurgeryMedical devicesHuman bodyCarrier fluid
Biocompatible materials are propelled at the skin with sufficient velocity to cause desired resurfacing of skin layers to the desired penetration depth. The materials, such as dry ice or water ice, are harmonious with the human body and thus eliminate foreign body reactions. Various materials may be used in combination, including local anesthetics and vasoconstrictors in solid or liquid form. The biocompatible solid or liquid particles are suspended in a cold carrier fluid and propelled through an insulated delivery system to the surface of the skin. The treatment of diseased skin lesions may be accomplished using the present invention as a drug delivery system.
Owner:PEARL TECHNOLOGY HOLDINGS LLC
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
Methods and kits for maxillary dental anesthesia by means of a nasal deliverable anesthetic
Methods and systems for anesthetizing a portion or all of a patient's maxillary dental arch using a nasal delivered anesthetizing composition. The process generates anesthesia sufficient for facilitation of operative dentistry, endodontics, periodontics or oral surgery for teeth of the maxillary arch. The dental nasal spray process consists of inserting one or more dispensing devices through the patient's nostril and delivering metered dosages of anesthetic solution or gel into the nasal cavity. The process may utilize a single solution which is a mixture of anesthetic agents, vasoconstricting agents and other physiological inert agents or two separate solutions, wherein one solution contains the vasoconstricting agents and the other solution contains the anesthetic agents. Anesthetic diffusion through the thin walls of the nasal cavity allows for the blocking of nerve impulses originating from the maxillary dentition and surrounding tissues. Anesthesia of specific oral regions such as right versus left sides of the dental arch, anterior versus posterior teeth, and soft tissue anesthesia may be controlled through modification of the dosage volume and the selection of right or left nostril insertion and agent delivery.
Owner:ST RENATUS +1
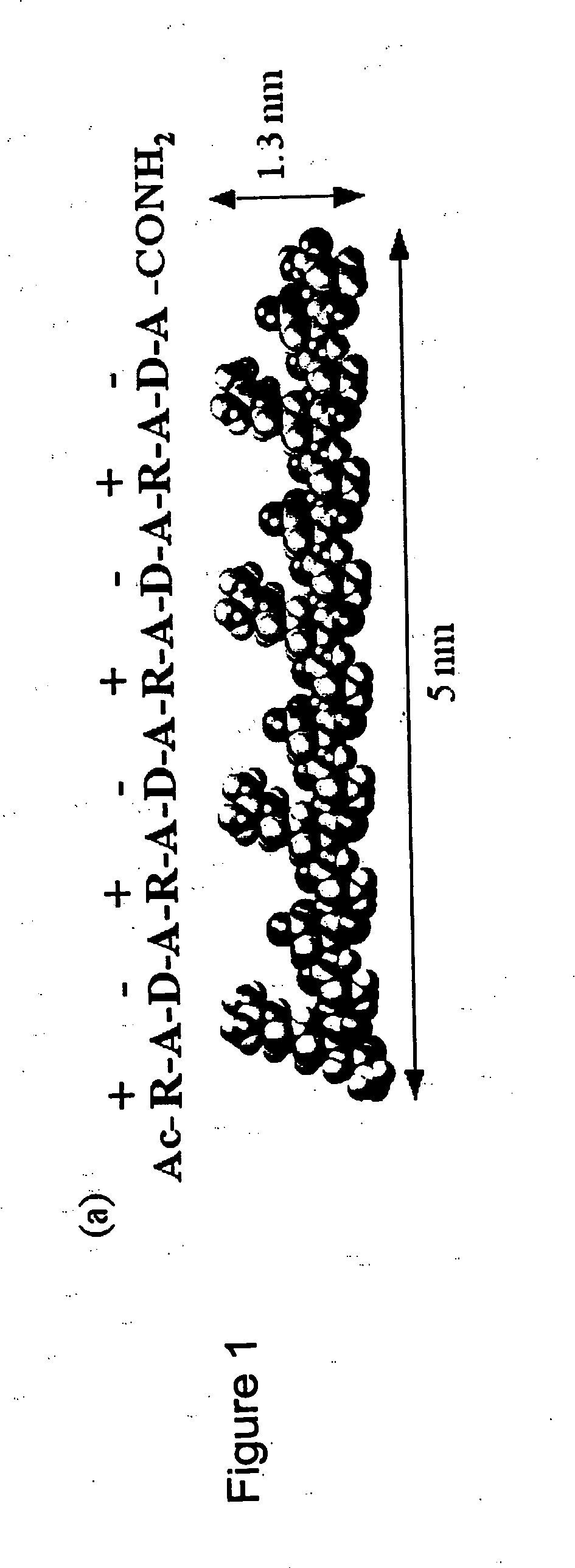
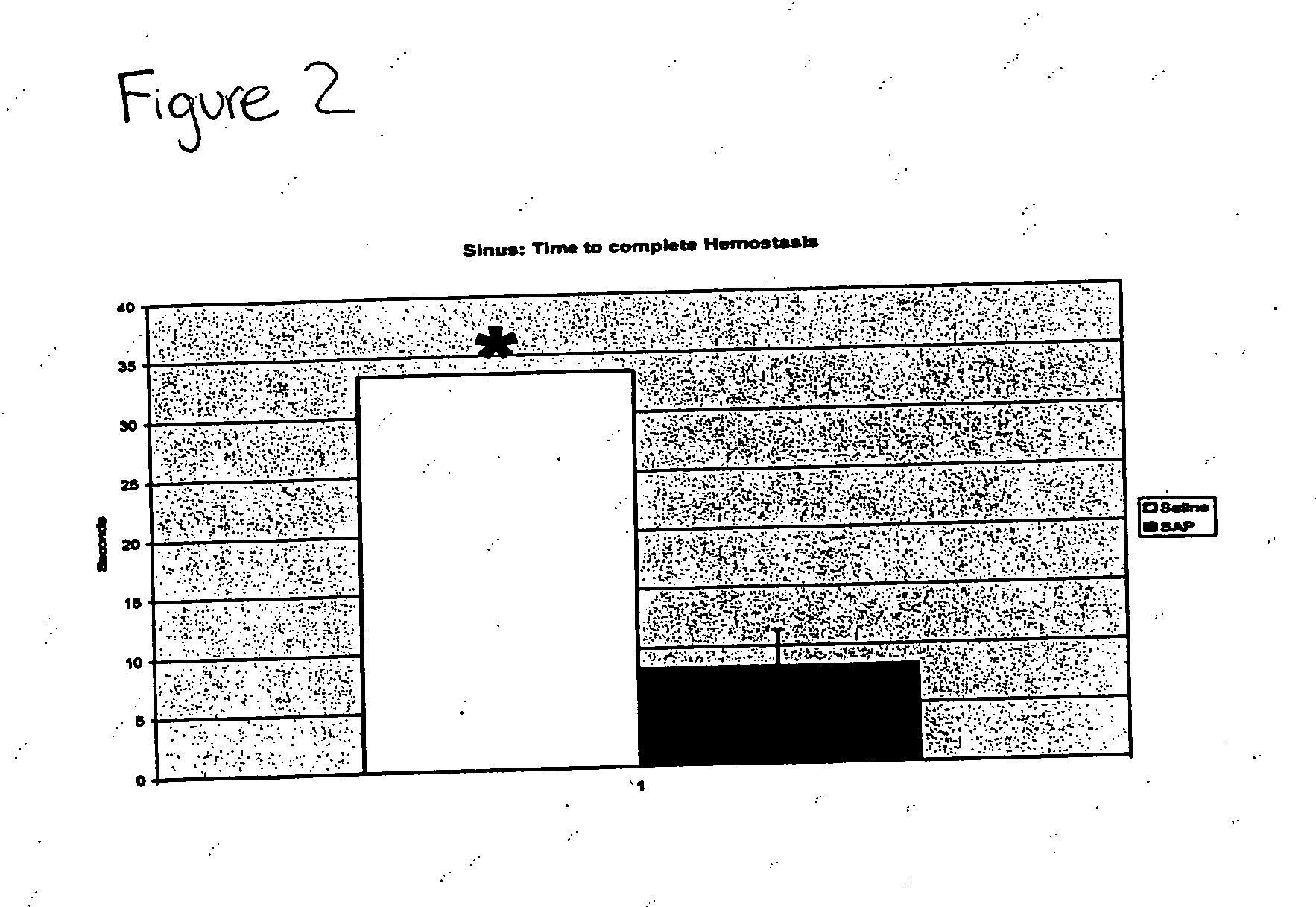
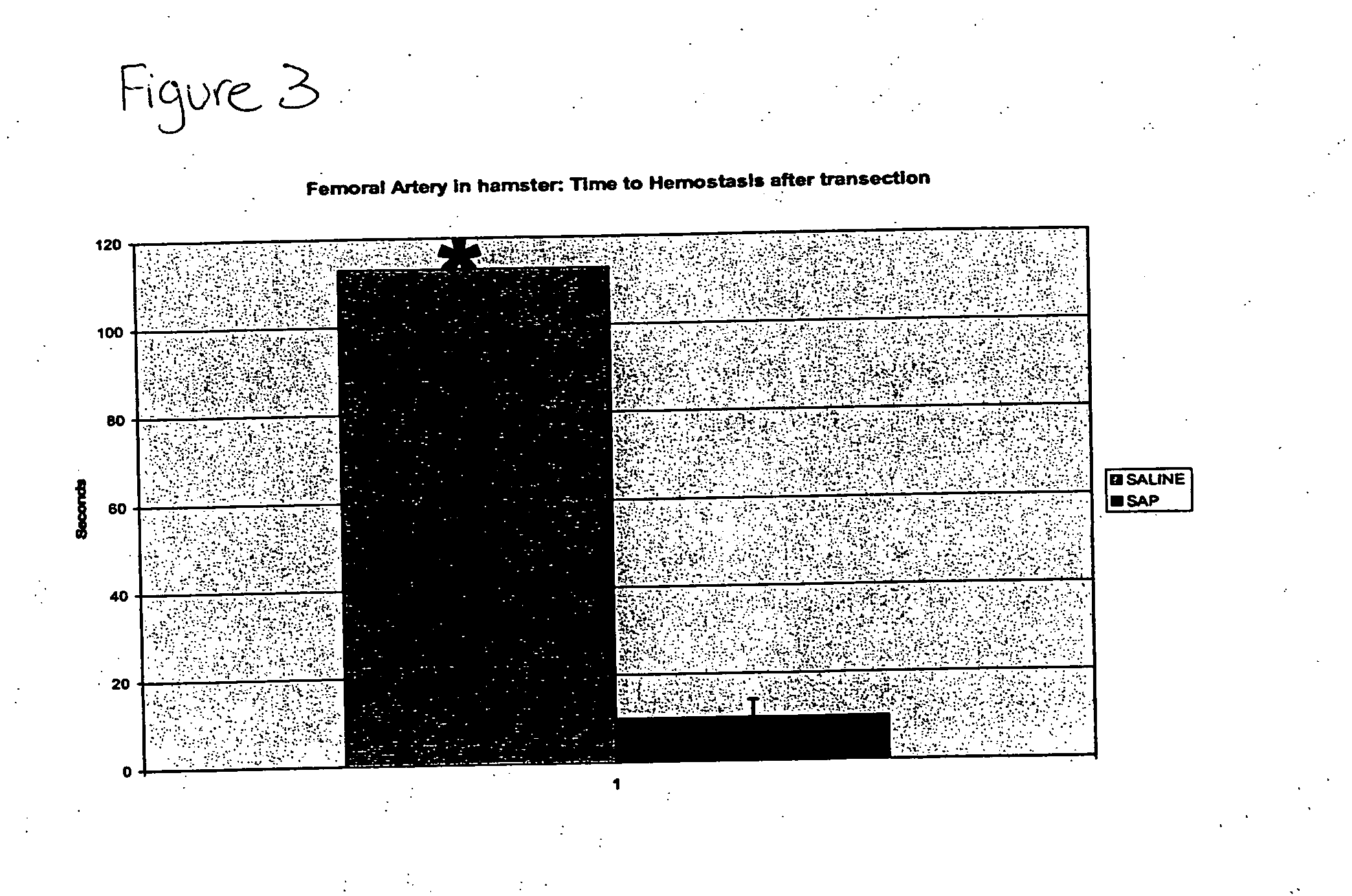
Compositions and methods for promoting hemostasis and other physiological activities
ActiveUS20070203062A1Good hemostasisControl bleedingBiocideTripeptide ingredientsWound dressingVasoconstrictor Agents
Compositions that include nanoscale structured materials or precursors thereof (e.g., self-assembling peptides) are described. The compositions can include other substances (e.g., a vasoconstrictor). Also described are methods for using the compositions to promote hemostasis, to protect the skin or wounds from contamination, to decontaminate a site upon removal of previously applied compositions that provided a protective coating, and to inhibit the movement of bodily substances other than blood. The compositions are also useful in isolating tissue, removing tissue, preserving tissue (for, e.g., subsequent transplantation or reattachment), and as bulking, stabilizing or hydrating agents. Medical devices that include the compositions (e.g., a stent or catheter), bandages or other wound dressings, sutures, and kits that include the compositions are also described.
Owner:VERSITECH LTD +1
Systems and methods using vasoconstriction for improved thermal treatment of tissues
InactiveUS6840954B2Improve the effectiveness of treatmentEnhance such thermal treatmentAnti-incontinence devicesSurgical instruments for heatingArteriolar VasoconstrictionTreatment effect
The present invention enhances the effectiveness of treatment of support tissue structures. Generally, such tissue structures support organs and hold the organs in their proper position for appropriate functioning. When such tissue structures become weak, hyper-elastic, and / or excessively lengthy, the organs of are no longer supported in their proper position. This often leads to physical manifestations such as incontinence, hernias, and the like. Remedies often involve thermal treatment of the support tissue structures, such as thermally inducted controlled shrinkage, contraction, or stiffening of the support tissue structure. To enhance such thermal treatment and diminish the possibility of undesirable heating and damage to nearby tissue surfaces, vasoconstrictive agents are used.
Owner:ASTORA WOMENS HEALTH
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Methods for treating wound tissue and forming a supplemented fibrin matrix
InactiveUS7196054B1Low antigenicityDecreasing thrombogenicityOrganic active ingredientsSurgical adhesivesTissue sealantVascular dilatation
Owner:AMERICAN NAT RED CROSS
Arthroscopic irrigation solution and method for peripheral vasoconstriction and inhibition of pain and inflammation
InactiveUS20030087962A1Control inflammationPain controlBiocideKallidin/bradykinin ingredientsArteriolar VasoconstrictionInflammation Process
A method and solution for perioperatively inhibiting a variety of pain and inflammation processes during arthroscopic procedures. The solution preferably includes a vasoconstrictor that demonstrates substantial agonist activity at alpha adrenergic receptors and that is selected for peripheral (local) vasoconstriction and one or more additional pain and inflammation inhibitory agents at dilute concentration in a physiologic carrier, such as saline or lactated Ringer's solution. The solution is applied by continuous irrigation of a wound during a surgical procedure for peripheral vasoconstriction and inhibition of pain and / or inflammation while avoiding undesirable side effects associated with systemic application of larger doses of the agents.
Owner:OMEROS CORP
Arthroscopic irrigation solution and method for peripheral vasoconstriction and inhibition of pain and inflammation
InactiveUS7973068B2Control inflammationPain controlKallidin/bradykinin ingredientsBiocideInflammation ProcessSide effect
A method and solution for perioperatively inhibiting a variety of pain and inflammation processes during arthroscopic procedures. The solution preferably includes a vasoconstrictor that exhibits alpha-adrenergic activity and one or more additional pain and inflammation inhibitory agents at dilute concentration in a physiologic carrier, such as saline or lactated Ringer's solution. The solution is applied by continuous irrigation of a wound during a surgical procedure for peripheral vasoconstriction and inhibition of pain and / or inflammation while avoiding undesirable side effects associated with systemic application of larger doses of the agents.
Owner:OMEROS CORP
Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy
ActiveUS20070077219A1Reduce and preferably prevent oral mucositisReduces and completely prevents oral mucositisBiocideCosmetic preparationsVasoconstrictor AgentsDermatology
Vasoconstrictors are administered topically to provide protection against the adverse effects, e.g., alopecia, mucositis or dermatitis, induced by chemotherapy or radiotherapy. Appropriate dosages and formulations of topical vasoconstrictors are provided. Methods for the use of such compositions are also provided.
Owner:WISCONSIN ALUMNI RES FOUND
Vascular treatment device and method
InactiveUS7163533B2Substantial timeSubstantial cost savingCatheterSurgical instrument detailsAnesthetic AgentVascular disease
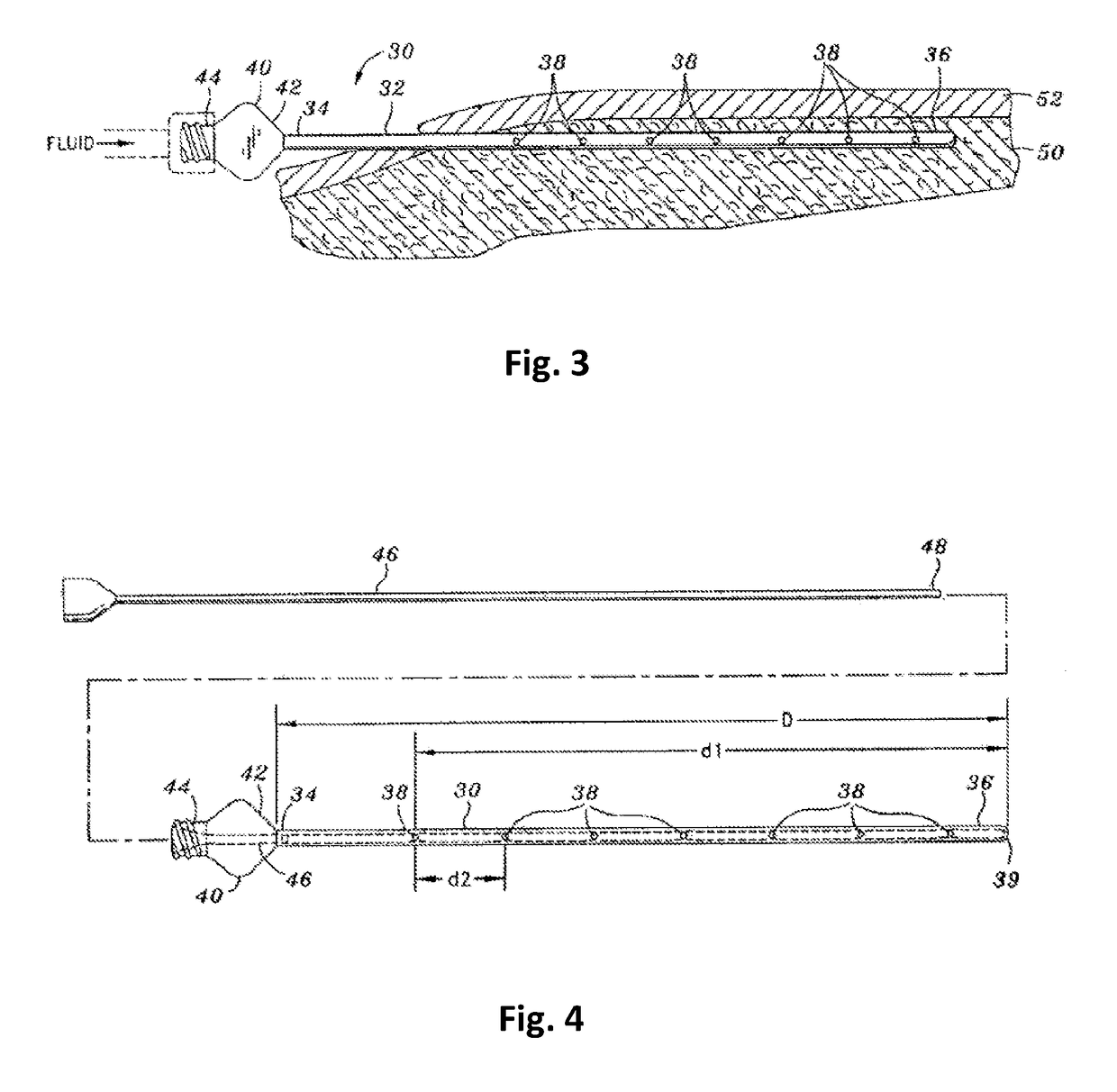
A catheter device for treating a vascular disease is provided. The catheter device includes an energy delivery device such as an optical fiber for delivering laser energy and a catheter. The catheter lumen receives the optical fiber and a fluid such as an anesthetic agent or vasoconstricting agent. According to the invention, a plurality of exits are formed in the sidewall of the catheter. The exits are in communication with the catheter lumen and administer the fluid into the blood vessel. By administering the fluid from within the catheter lumen, the present catheter device eliminates the need to make numerous external punctures to deliver fluid injections.
Owner:ANGIODYNAMICS INC
Hydrogel compositions comprising vasoconstricting and Anti-hemorrhagic agents for dermatological use
InactiveUS20110171310A1Improve stabilityProlonging dermal filler durationBiocideOrganic active ingredientsAnti-Hemorrhagic AgentVasoconstrictor Agents
The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions.
Owner:ALLERGAN IND
Compositions and methods for treatment of skin discoloration
ActiveUS20060057081A1Good lookingWhitening skinCosmetic preparationsToilet preparationsHuman skinAdditive ingredient
A cosmetically acceptable product for application to human skin is disclosed. The novel compositions are particularly suited for skin lightening and for diminishing the appearance of “dark circles” under the eyes. The compositions include any of several vasoconstrictors in a carrier with optionally added skin compatible ingredients.
Owner:EVERA LAB
Compositions and methods for promoting hemostasis and other physiological activities
ActiveUS20090111734A1Equally distributedPeptide/protein ingredientsDigestive systemWound dressingVasoconstrictor Agents
Compositions that include nanoscale structured materials or precursors thereof (e.g., self-assembling peptides) are described. The compositions can include other substances (e.g., a vasoconstrictor). Also described are methods for using the compositions to promote hemostasis, to protect the skin or wounds from contamination, to decontaiminate a site upon removal of previously applied compositions that provided a protective coating, and to inhibit the movement of bodily substances other than blood. The compositions are also useful in isolating tissue, removing tissue, preserving tissue (for, e.g., subsequent transplantation or reattachment), and as bulking, stabilizing or hydrating agents. Medical devices that include the compositions (e.g., a stent or catheter), bandages or other wound dressings, sutures, and kits that include the compositions are also described.
Owner:VERSITECH LTD +1
Devices and methods for selectively lysing cells
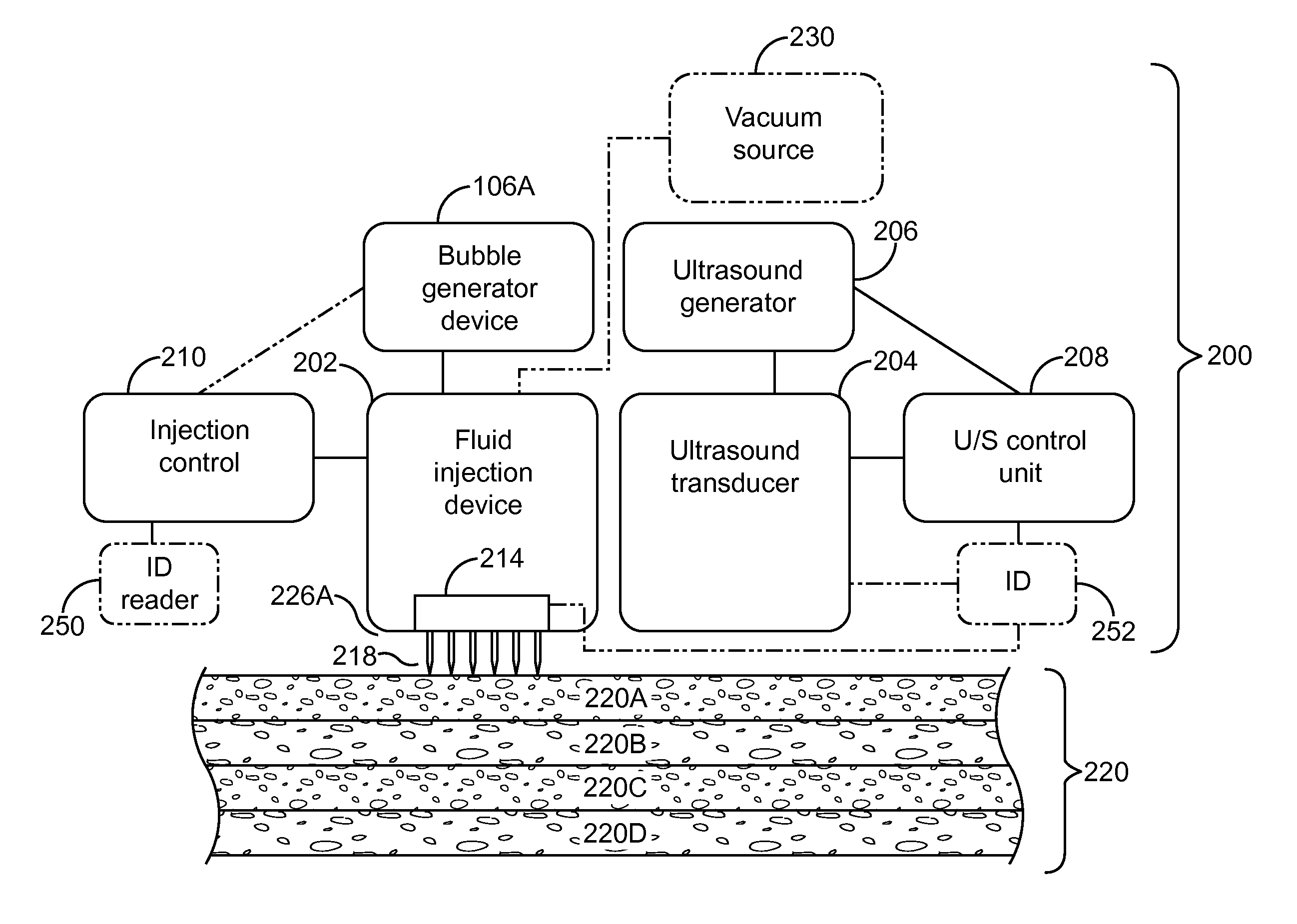
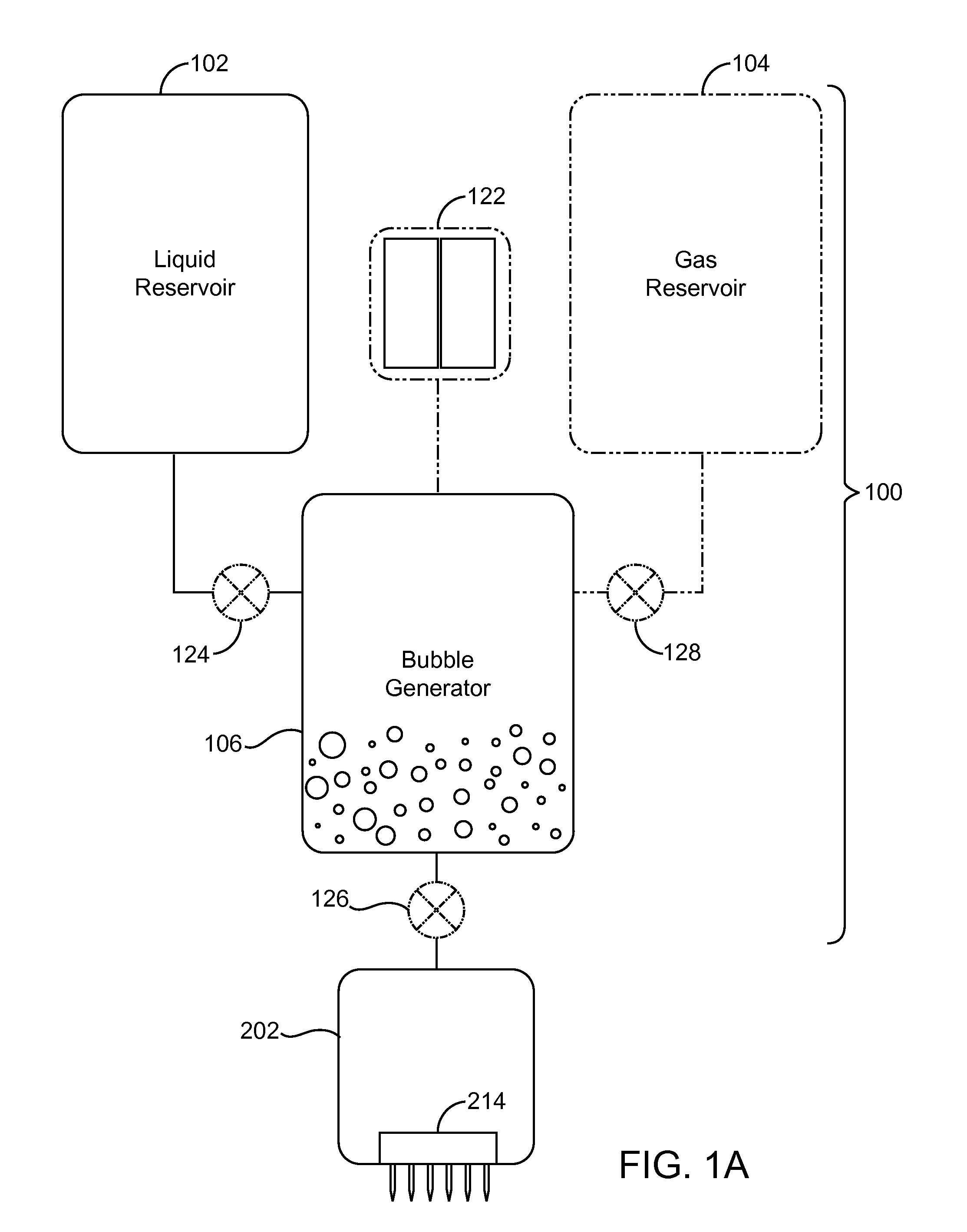
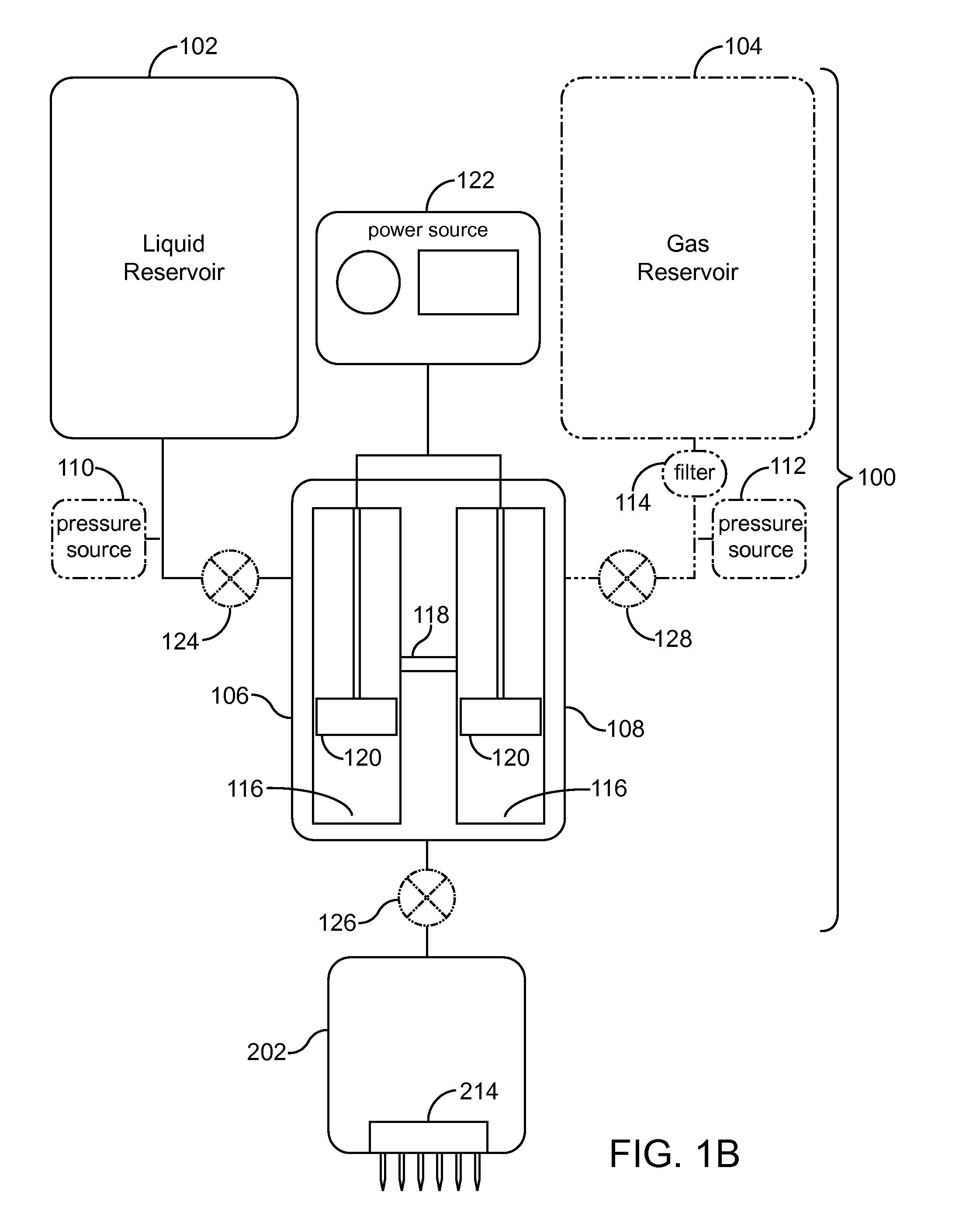
InactiveUS20080200863A1Promote absorptionFacilitates popping of bubblesUltrasound therapyElectrotherapyAnesthetic AgentVasoconstrictor Agents
A system comprising: a container containing a measured amount of a solution including at least one of a vasoconstrictor, a surfactant, and an anesthetic, the solution comprising a liquid and at least one of a gas and a fluid; a needle array in fluid connection with the container, the needle array including at least one needle.
Owner:ULTHERA INC
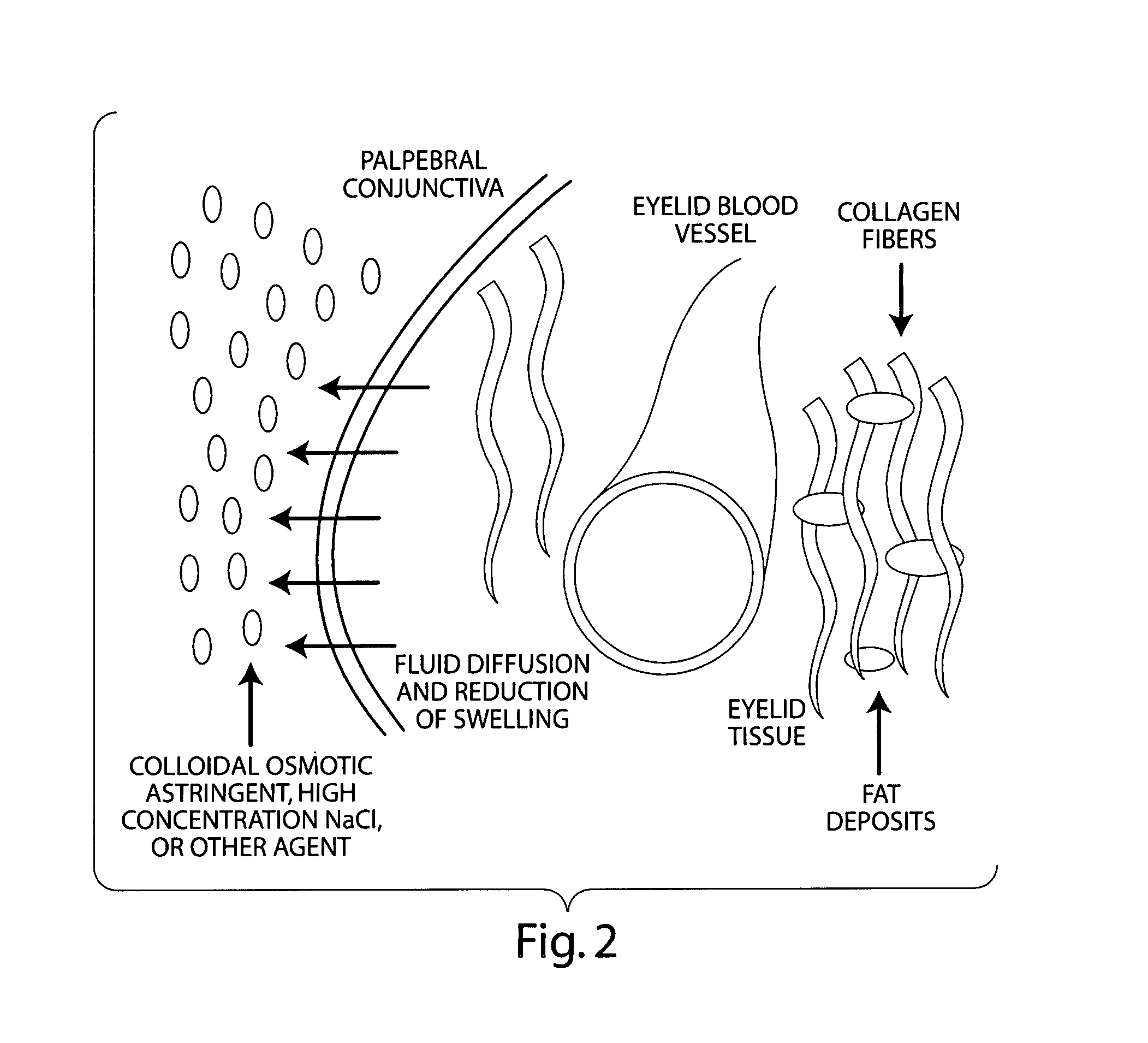
Compositions for the treatment and prevention of eyelid swelling
The invention features topical formulations comprising an osmotically active agent and / or a vasoconstrictor and / or an astringent agent for the treatment and prevention of eyelid swelling, and methods of use thereof.
Owner:NICOX OPHTHALMICS
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39192E1Increased longevityImprove stabilityAntibacterial agentsPowder deliveryTissue sealantVascular dilatation
This invention provides supplemented tissue sealants, methods for their production and use thereof. Disclosed are tissue sealants supplemented with at least one cytotoxin or cell proliferation inhibiting composition. The composition may be further supplemented with, for example, one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, cytokines, drugs, growth factors, interferons, hormones, lipids, deminearlized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like.
Owner:AMERICAN NAT RED CROSS
Devices and methods for treating pain associated with tonsillectomies
Described here are devices and methods for treating one or more conditions or symptoms associated with a tonsil procedure. In some variations, a drug-releasing device may be at least partially delivered to one or more tonsillar tissues before, during, or after a tonsil procedure. In some variations, the drug-releasing device may be configured to be biodegradable. In other variations, the drug-releasing device may comprise one or more hemostatic materials or one or more adhesives. The drug-releasing device may be configured to release one or more drugs or agents, such as, for example, one or more analgesics, local anesthetics, vasoconstrictors, antibiotics, combinations thereof and the like.
Owner:INTERSECT ENT INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39298E1Decreasing thrombogenicityLow antigenicityOrganic active ingredientsPowder deliveryTissue sealantVascular dilatation
This invention provides methods for the localized delivery of supplemented tissue sealants, wherein the supplemented tissue sealants comprise at least one composition which is selected from one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, antiproliferatives, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Further provided are methods of using the site-specific supplemented tissue sealants, including preparation of a biomaterial.
Owner:AMERICAN NAT RED CROSS
Tumescent infiltration drug delivery of high subcutaneous drug concentrations with prolonged local and systemic effects and minimal local or systemic toxicity
ActiveUS20170100331A1Increase local drug concentrationReduce blood viscosityInorganic non-active ingredientsPharmaceutical delivery mechanismTherapeutic effectVasoconstrictor Agents
Disclosed are methods of subcutaneous delivery of a drug or a therapeutic agent to a subject comprising administering to said subject a tumescent composition comprising: (a) the drug or the therapeutic agent, wherein a tumescent concentration of the drug is simultaneously: 1) below a threshold for local, subcutaneous tissue toxicity, 2) above a threshold for positive local therapeutic effect, and 3) above a concentration achievable by intravenous (IV), intramuscular (IM) or oral (PO) delivery; (b) a vasoconstrictor; and (c) a pharmaceutically acceptable carrier. Some embodiments relate to a method of treating or preventing sepsis or Systemic Inflammatory Response Syndrome (SIRS) in a subject. Some embodiments relate to a tumescent solution for treating a localized viral infection, e.g., varicella-zoster (shingles), the tumescent solution comprising an antiviral agent.
Owner:HK PHARMA INC
Topical preparation and method for transdermal delivery and localization of therapeutic agents
Disclosed herein is a preparation for topically delivering and localizing therapeutic agents, comprising: a vasoconstrictor for retarding vascular dispersion of a therapeutic agent; and a penetration enhancer for facilitating penetration of the vasoconstrictor and the therapeutic agent through a patient's skin. Further disclosed is an associated method of topically delivering and localizing therapeutic agents, comprising the steps of: using a vasoconstrictor for retarding vascular dispersion of a therapeutic agent; in combination with using a penetration enhancer for facilitating penetration of the vasoconstrictor and the therapeutic agent through a patient's skin. Also disclosed are various courses of treatment which comprise applying the various disclosed combinations of agents to the patient's skin.
Owner:RICHLIN DOHERTY
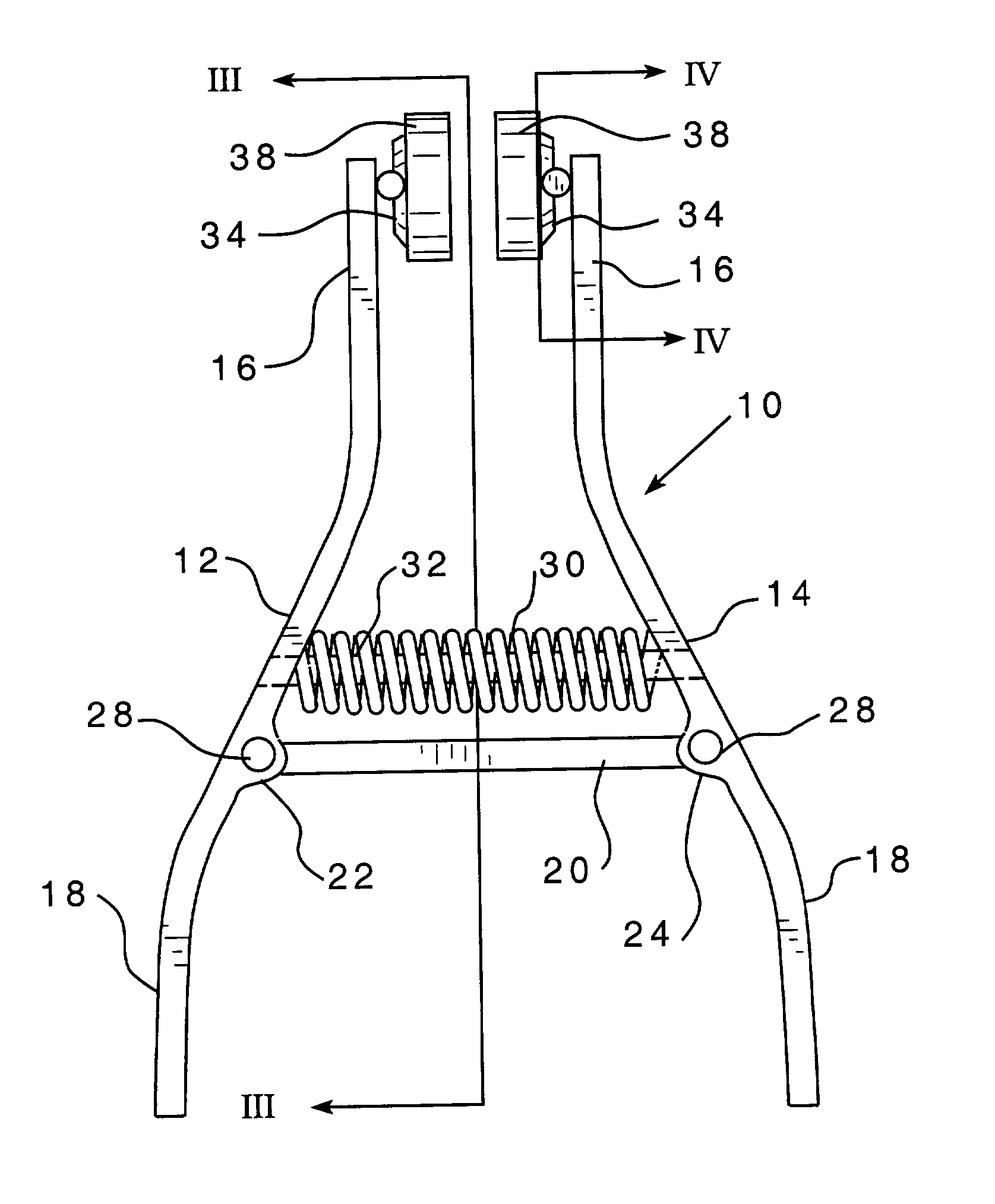
Apparatus fot treatment of a nose bleed
An apparatus for treatment of a nose bleed, in its preferred form having a pair of laterally spaced elongated arms pivotably connected to be selectively moved toward and away from each other like scissors or the like. A compression spring engages the arms to urge them into a desired position with respect to each other. Removable absorbent sponges are disposed on the upper ends of the arms and are arranged to engage both the septum of a nostril where typical nose bleeds would occur and the non-bleeding septum. The arms are squeezed to separate them and to insert the sponges into the nostrils and released to apply pressure on the bleeding and non-bleeding septums. The sponge engaging the bleeding area may be saturated with a vasoconstrictive agent to control the bleeding. That sponge may be removed and replaced with a sponge saturated with a local antiseptic and applied to formerly bleeding area in anticipation of cauterizing the same area using a sponge saturated with a cauterizing agent.
Owner:BUZARD RICHARD A
Compositions for treatment of common cold
InactiveUS7652030B2Efficient dosingReducing the runny noseBiocideAerosol deliveryCommon coldAnticholinergic Drugs
New stable compositions comprising the combination of a topically active vasoconstrictor and a topically active anticholinergic drug are disclosed. Preferably, the composition comprises ipratropium or a salt thereof in combination with xylometazoline hydrochloride and a salt thereof. Upon topically administering such compositions to a nasal mucosa in individuals suffering from the common cold the symptoms of rhinorrhea are significantly reduced.
Owner:TAKEDA PHARMA AS
Topical compositions for enhancing sexual responsiveness
A topical composition which enhances sexual responsiveness of a mammal is disclosed. An effective dosage of a peripheral vasodilator, an absorption enhancer and, optionally, a vasoconstrictor and an alpha receptor blocker are combined with a pharmaceutically-acceptable topical vehicle to produce the composition. The compositions are applied topically to the penis or labia majora and minora pudenda to enhance erection or vasocongestion.
Owner:BARMENSEN LABS
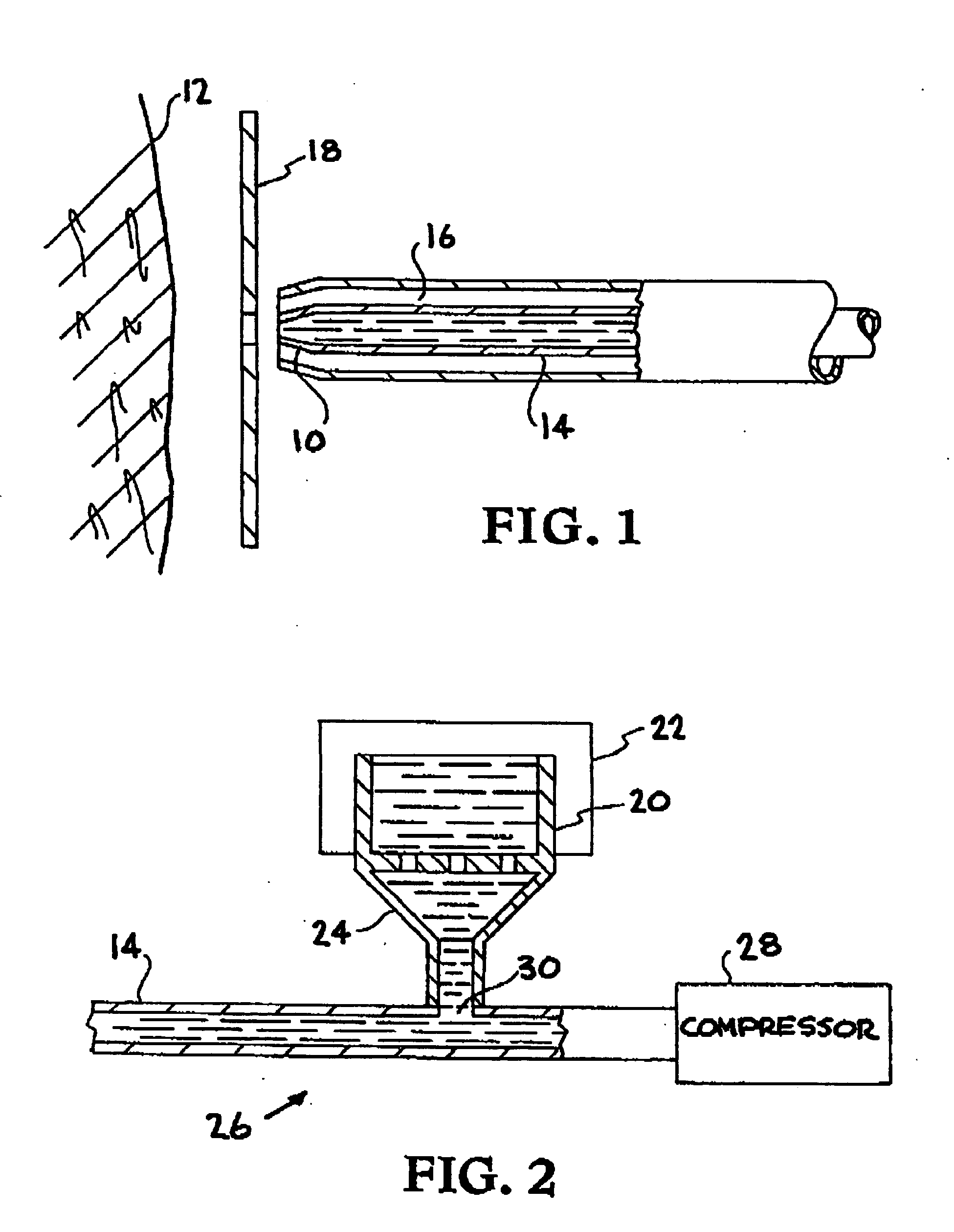
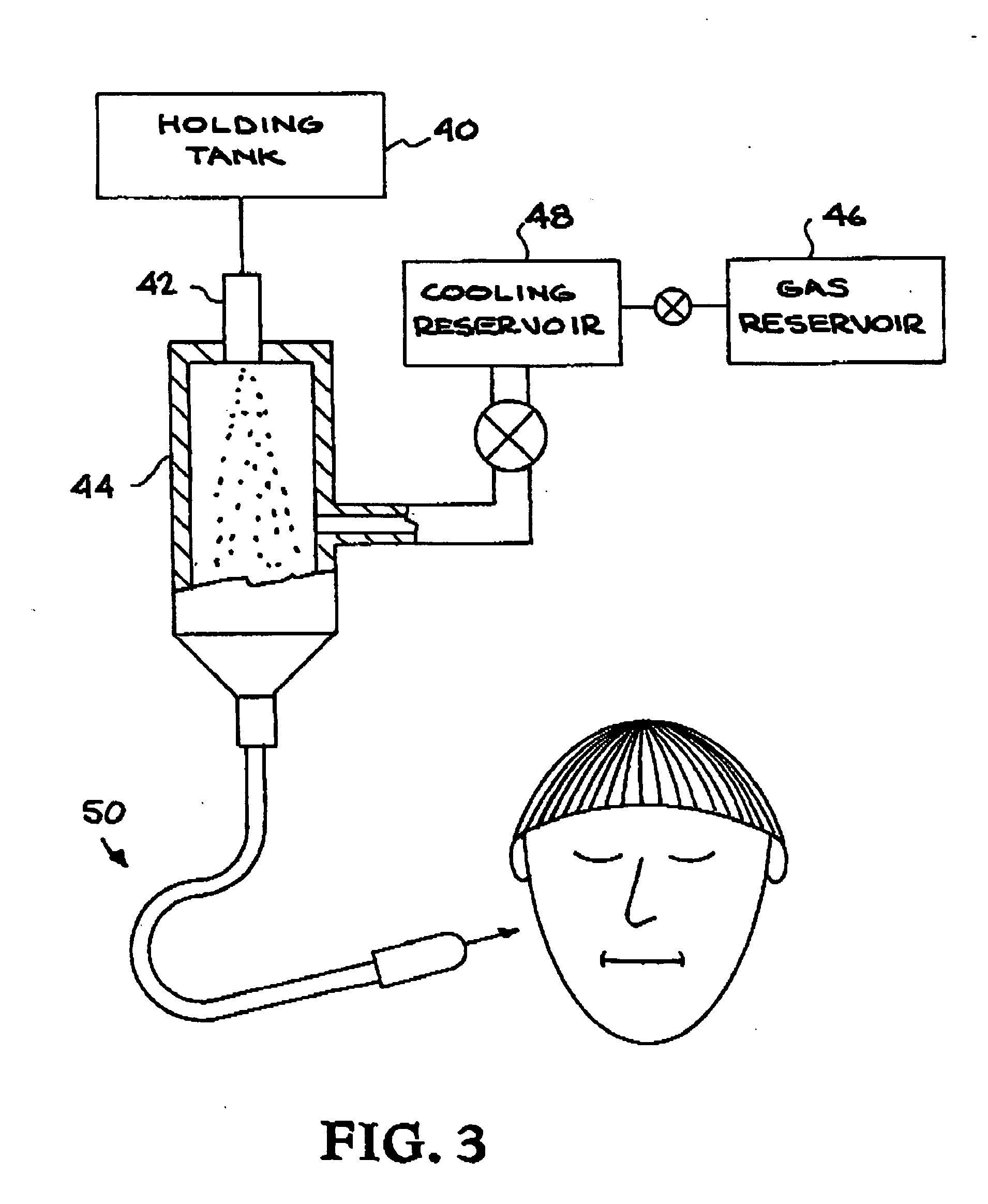
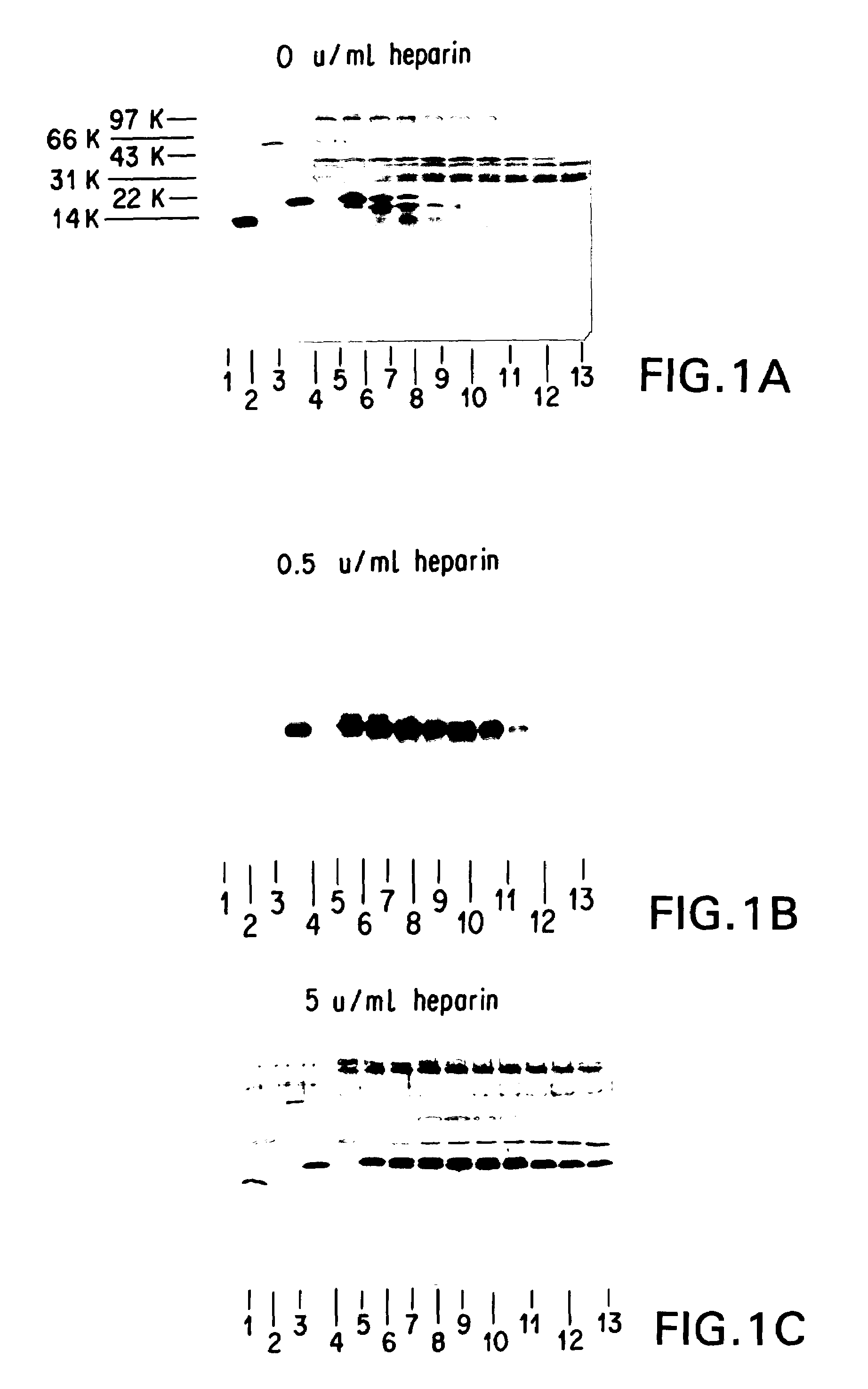
Methods and apparatus for the enhanced delivery of physiologic agents to tissue surfaces
InactiveUS20060172017A1Quick effectInterrupt and delay progressionBiocideInorganic active ingredientsNitric oxideNitrous oxide
Apparatus and methods deliver vasoconstrictive agents simultaneously with capnic gases. The capnic gases can enhance the effectiveness of the vasoconstrictive agent, lower the dosage of drug or concentration of agent necessary to achieve a therapeutic result, or both. Exemplary capnic gases include carbon dioxide, nitric oxide, nitrous oxide, and dilute acid gases.
Owner:CAPNIA INC
Topical Preparation and Method for Transdermal Delivery and Localization of Therapeutic Agents
InactiveUS20080293703A1Good dispersionImprove permeabilityAntipyreticAnalgesicsCombined useTopical preparation
Disclosed herein is a preparation for topically delivering and localizing therapeutic agents, comprising: a vasoconstrictor for retarding vascular dispersion of a therapeutic agent; and a penetration enhancer for facilitating penetration of the vasoconstrictor and the therapeutic agent through a patient's skin. Further disclosed is an associated method of topically delivering and localizing therapeutic agents, comprising the steps of: using a vasoconstrictor for retarding vascular dispersion of a therapeutic agent; in combination with using a penetration enhancer for facilitating penetration of the vasoconstrictor and the therapeutic agent through a patient's skin. Also disclosed are various courses of treatment, which comprise applying the various disclosed combinations of agents to the patient's skin.
Owner:RICHLIN DAVID M +1
Sclerotherapy for varicose veins
This invention is directed to pharmaceutical compositions and methods of treating varicose veins, including telangiectasias and reticular veins, and related symptoms and diseases. Embodiments comprise injection into a varicose vein of an amphiphilic block copolymer which has properties of conversion from liquid to gel state, surfactant properties, or combinations thereof. Other embodiments consist of the combination of an amphiphilic block copolymer with a co-solvent, also allowing the injected combination to form a gel within the vein. In other embodiments, an amphiphilic block copolymer is combined with a co-solvent, a sclerosant, a vasoconstrictor, water, or combinations thereof. Other embodiments of the invention include a combination of a sclerosant such as sodium tetradecyl sulfate or polidocanol with a vasoconstrictor, preferably a long-acting vasoconstrictor such as oxymetazoline. Other embodiments are directed to compositions and methods of treating venous and arteriovenous malformations and cancer.
Owner:VENAFAIR
Tumescent antibiotic solution
Disclosed herein are solutions and kits for tumescent antibiotic delivery. Embodiments of the solution comprise an antibiotic component, an anesthetic component and a vasoconstrictor component. In addition, a method of use is disclosed comprising subcutaneous delivery of the solution. The disclosed solution, kit and method of subcutaneous delivery can be used for a variety of surgical procedures including liposuction, mastectomy and others. The tumescent antibiotic solution can be administered to a patient in situations where establishing IV access is difficult, impossible, or undesirable. A variety of delivery methods appropriate for surgical settings, as well as for use by first responders are likewise disclosed.
Owner:HK PHARMA INC
Iontophoresis Drug Delivery Formulation Providing Acceptable Sensation and Dermal Anesthesia
InactiveUS20070078372A1Great confidenceFewer returnsElectrotherapyPharmaceutical delivery mechanismVasoconstrictor AgentsTransdermal medication
A shelf-stable electrically assisted transdermal drug delivery system for highly effective electrotransport of an anesthetic and a vasoconstrictor producing clinically acceptable dermal anesthesia and sensation is provided. In certain embodiments the anesthetic includes lidocaine and the vasoconstrictor includes epinephrine. Medicament delivery is affected to provide dermal anesthesia with little or no sensation during delivery, as measured by a variety of indicator tests. Methods of producing dermal anesthesia in patients are also provided.
Owner:VYTERIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com