Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
371 results about "Nasal spray" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nasal sprays, are used to deliver medications locally in the nasal cavities or systemically. They are used locally for conditions such as nasal congestion and allergic rhinitis. In some situations, the nasal delivery route is preferred for systemic therapy because it provides an agreeable alternative to injection or pills. Substances can be assimilated extremely quickly and directly through the nose. Many pharmaceutical drugs exist as nasal sprays for systemic administration (e.g. treatments for pain, migraine, osteoporosis and nausea). Other applications include hormone replacement therapy, treatment of Alzheimer's disease and Parkinson's disease. Nasal sprays are seen as a more efficient way of transporting drugs with potential use in crossing the blood–brain barrier.
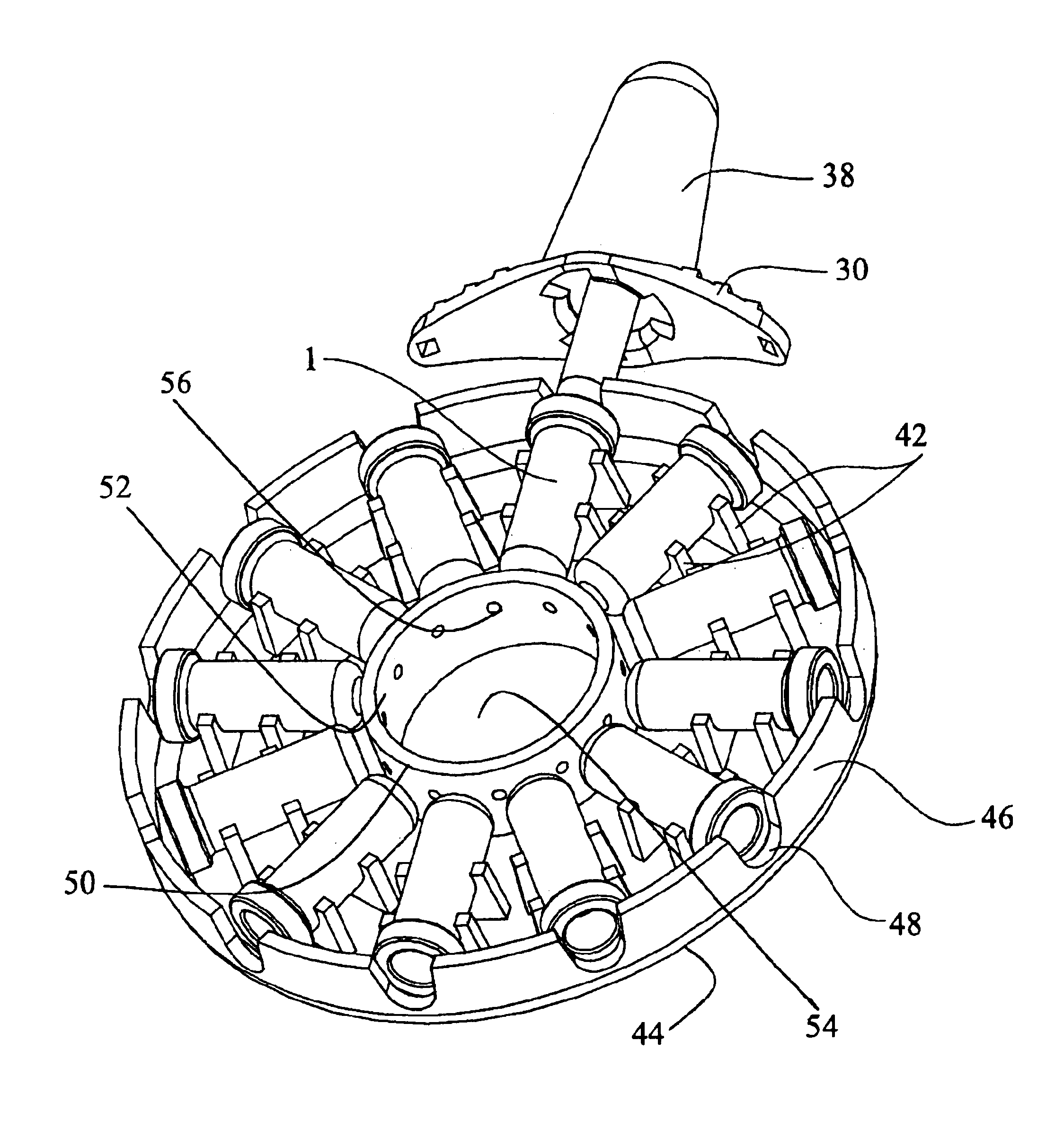
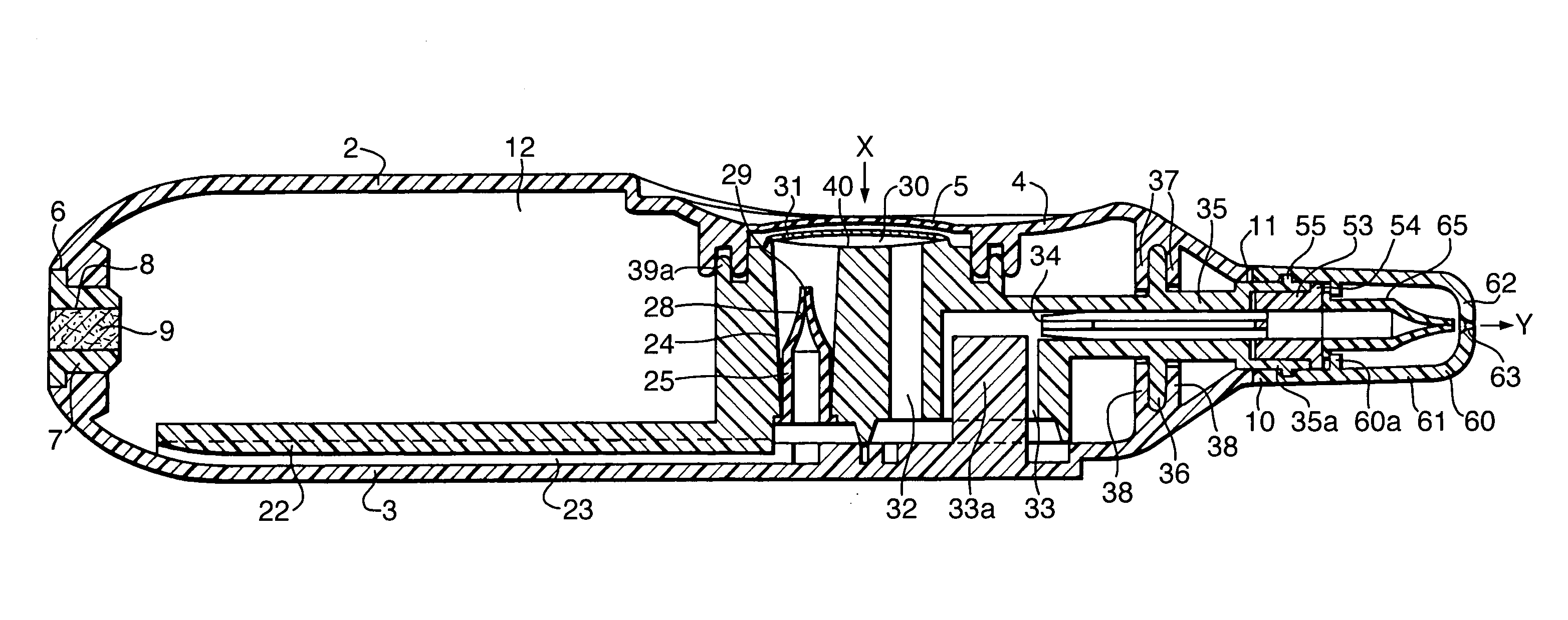
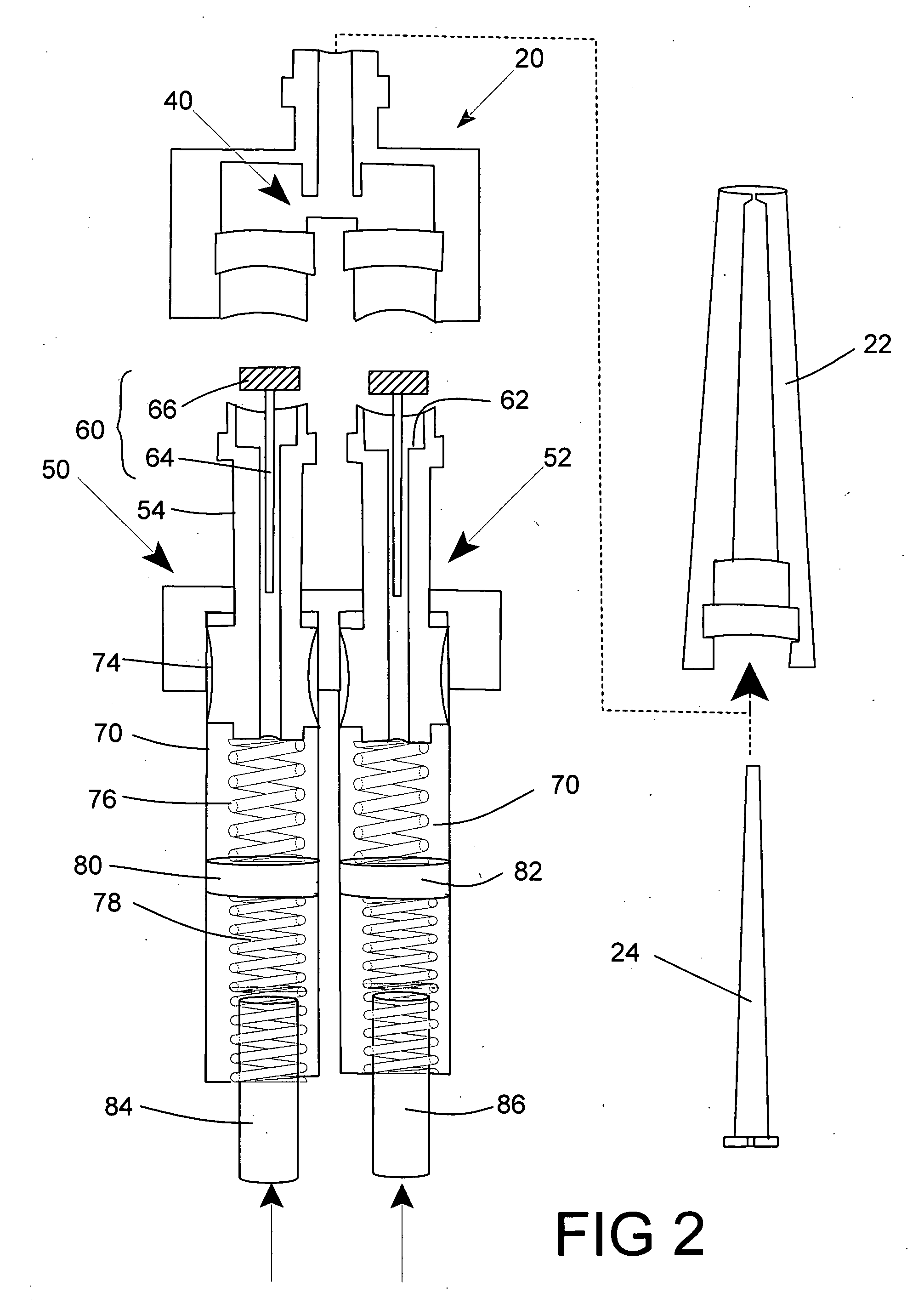
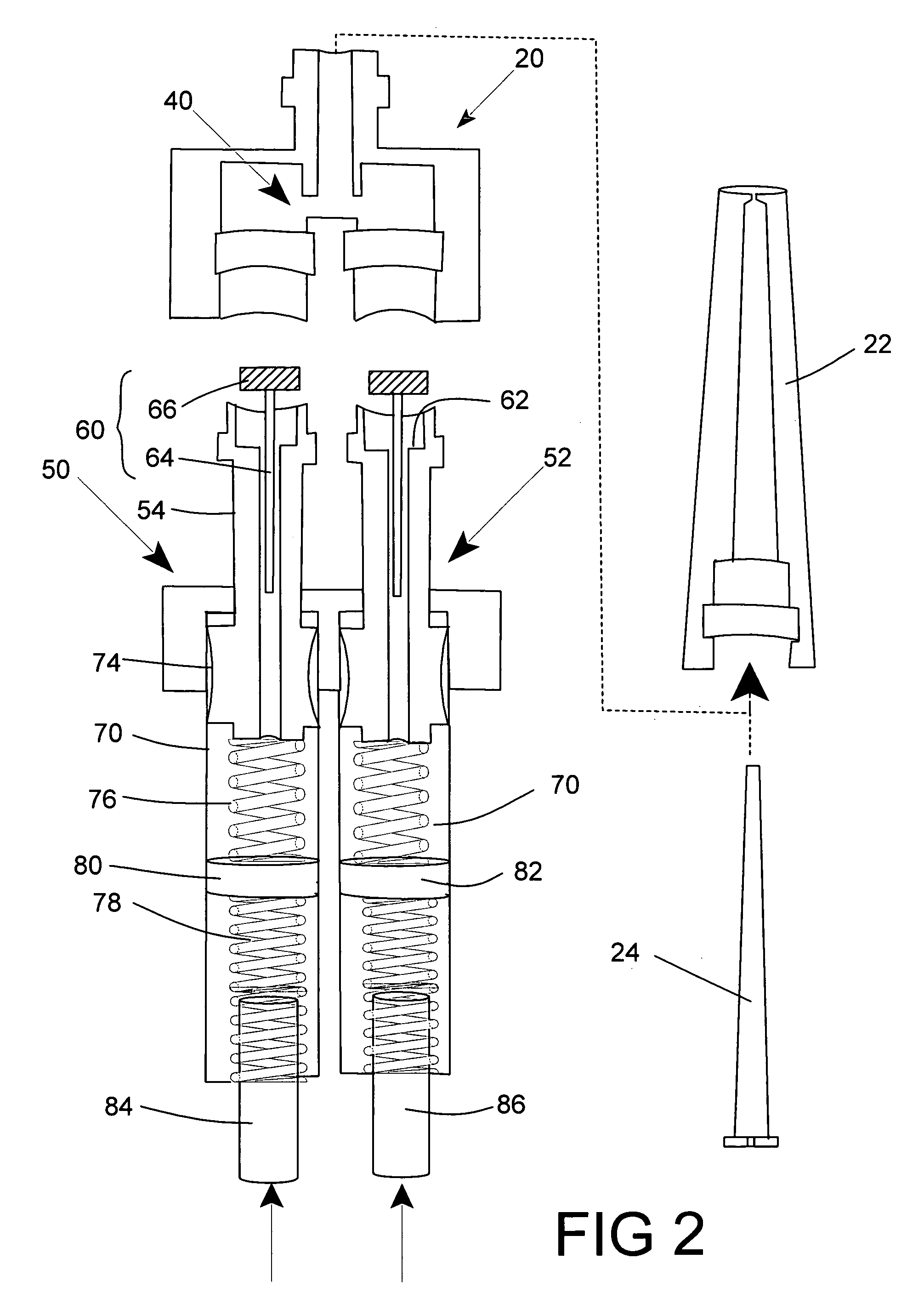
Programmable multi-dose intranasal drug delivery device
InactiveUS6948492B2Avoid diversionAvoid abuseRespiratorsLiquid surface applicatorsNasal sprayBiological activation
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
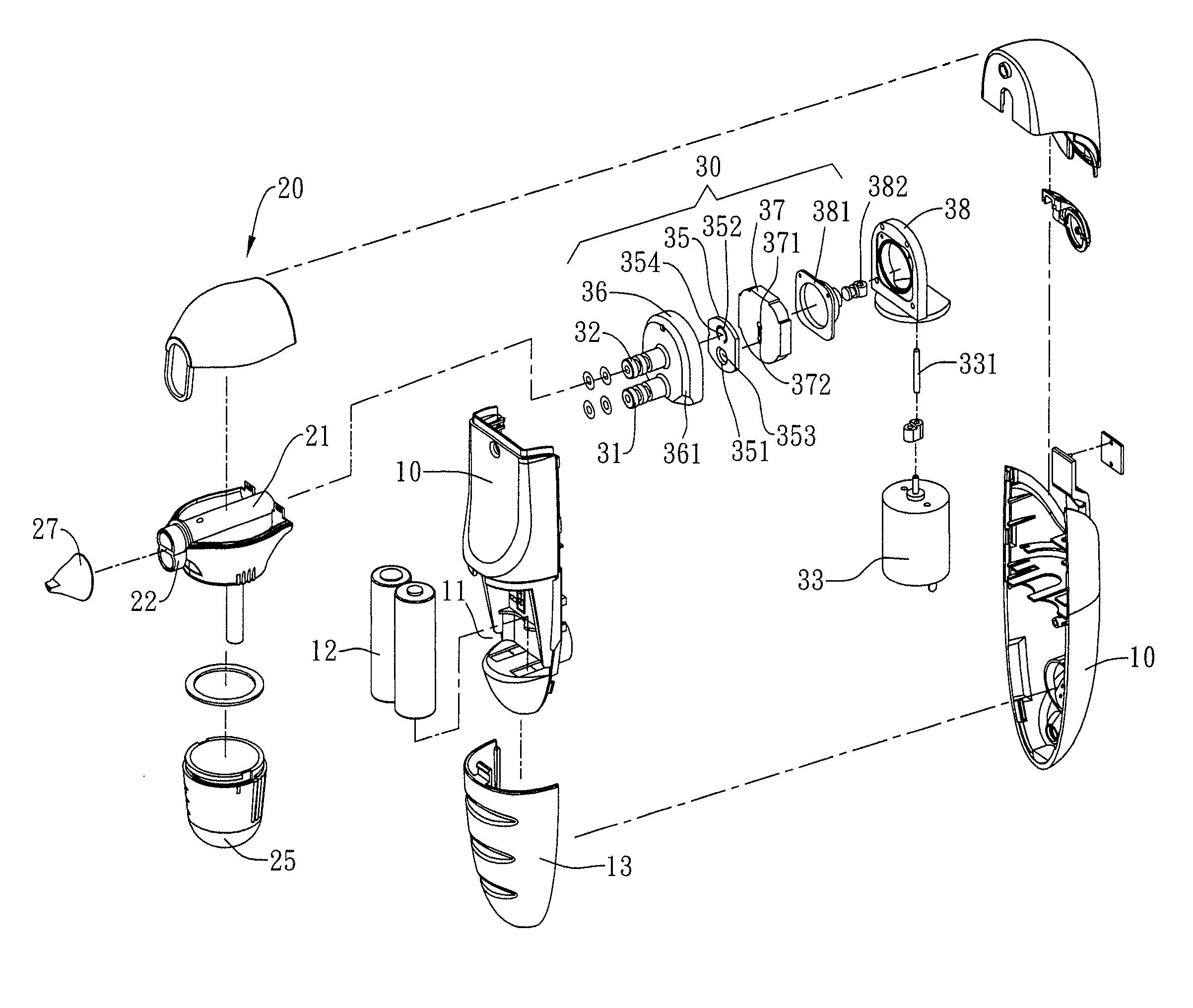
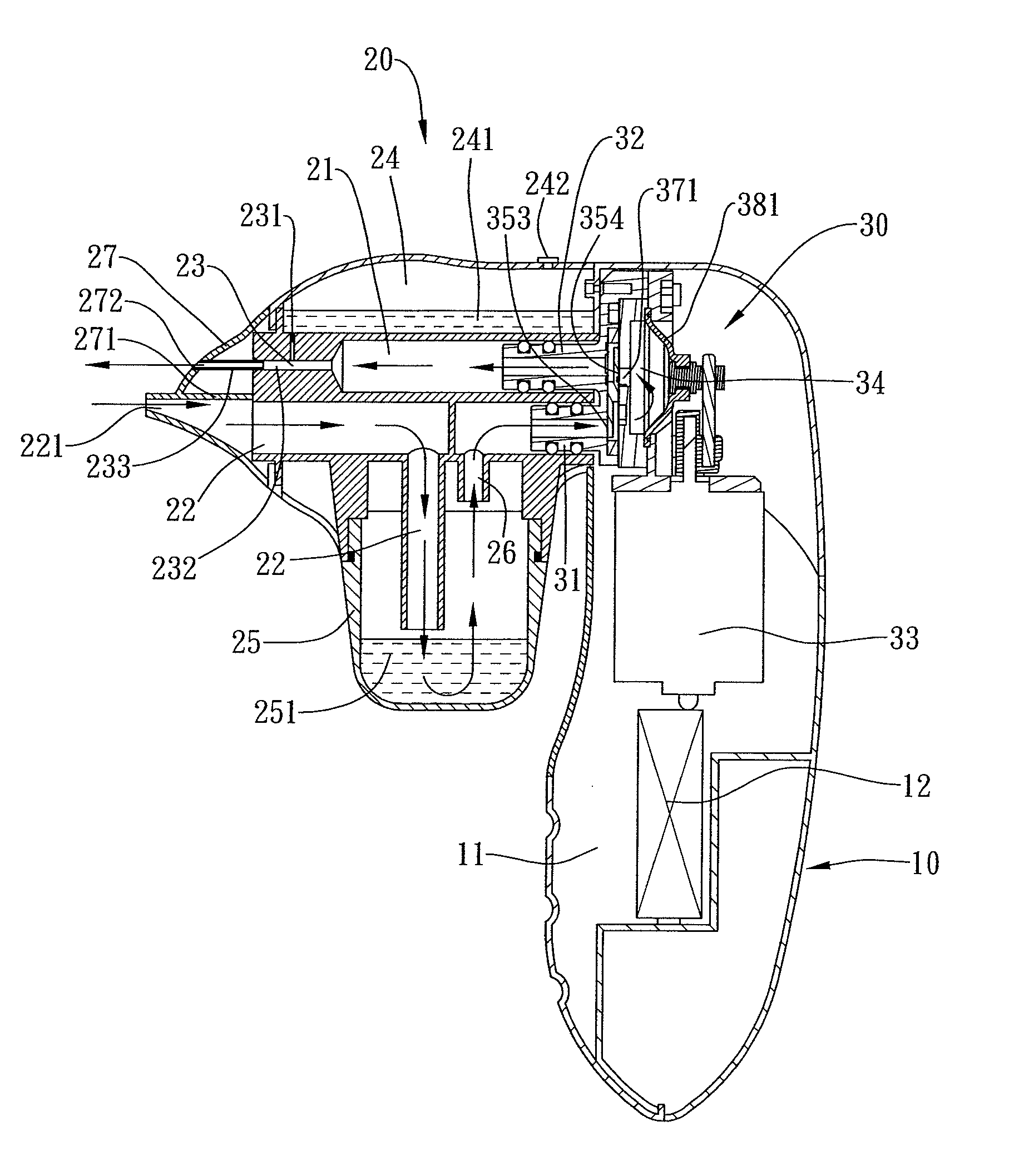
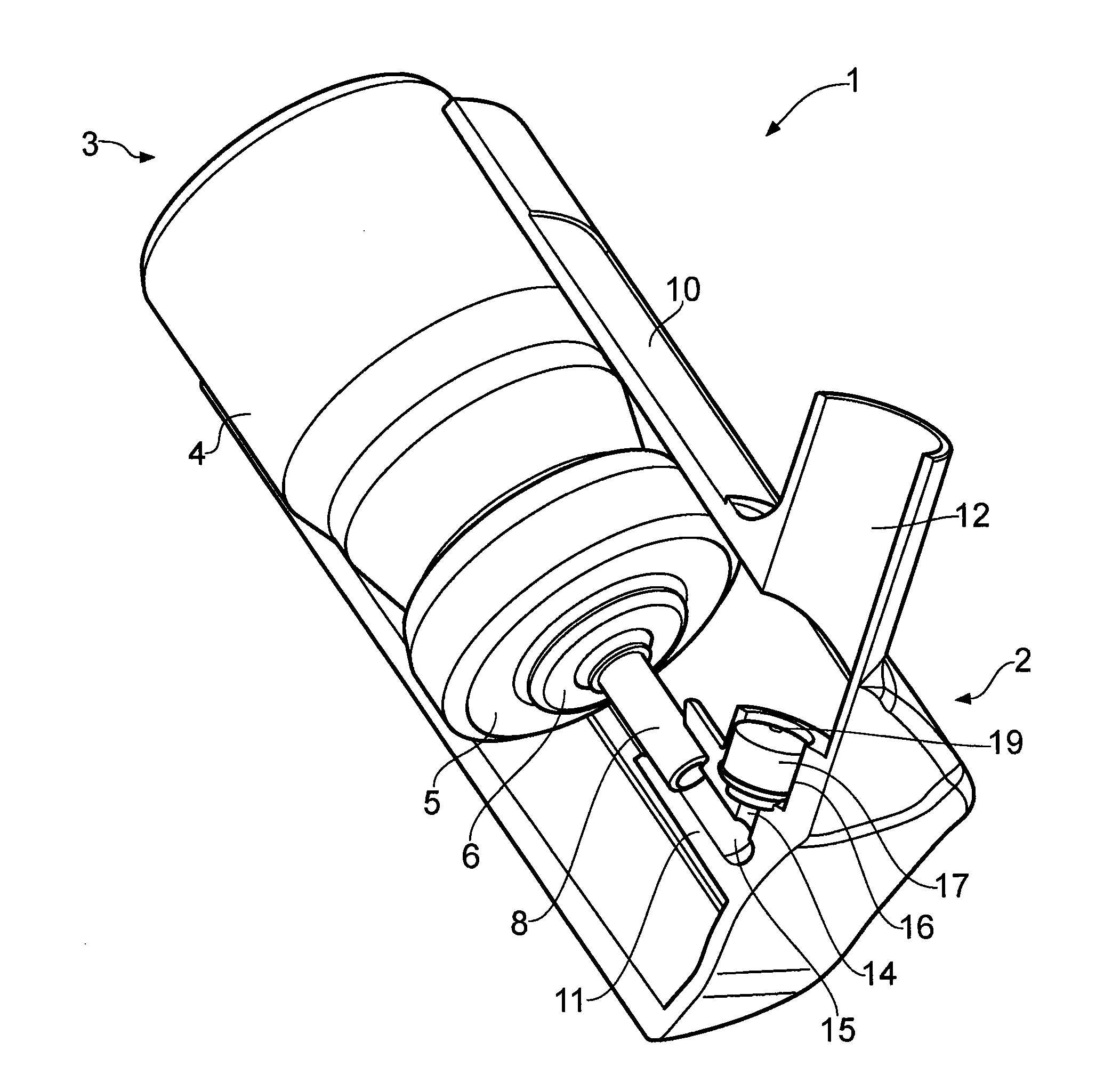
Combined nasal spray and aspirator device
ActiveUS7862536B2Present invention is physically more favorable for usersGood for healthEar treatmentCannulasNasal cavityNebulizer
Owner:AVITA CORP
Methods and kits for maxillary dental anesthesia by means of a nasal deliverable anesthetic
Methods and systems for anesthetizing a portion or all of a patient's maxillary dental arch using a nasal delivered anesthetizing composition. The process generates anesthesia sufficient for facilitation of operative dentistry, endodontics, periodontics or oral surgery for teeth of the maxillary arch. The dental nasal spray process consists of inserting one or more dispensing devices through the patient's nostril and delivering metered dosages of anesthetic solution or gel into the nasal cavity. The process may utilize a single solution which is a mixture of anesthetic agents, vasoconstricting agents and other physiological inert agents or two separate solutions, wherein one solution contains the vasoconstricting agents and the other solution contains the anesthetic agents. Anesthetic diffusion through the thin walls of the nasal cavity allows for the blocking of nerve impulses originating from the maxillary dentition and surrounding tissues. Anesthesia of specific oral regions such as right versus left sides of the dental arch, anterior versus posterior teeth, and soft tissue anesthesia may be controlled through modification of the dosage volume and the selection of right or left nostril insertion and agent delivery.
Owner:ST RENATUS +1
Programmable multi-dose intranasal drug delivery service
InactiveUS20060021614A1InterestingEasily interfaceRespiratorsLiquid surface applicatorsMedicineNasal spray
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
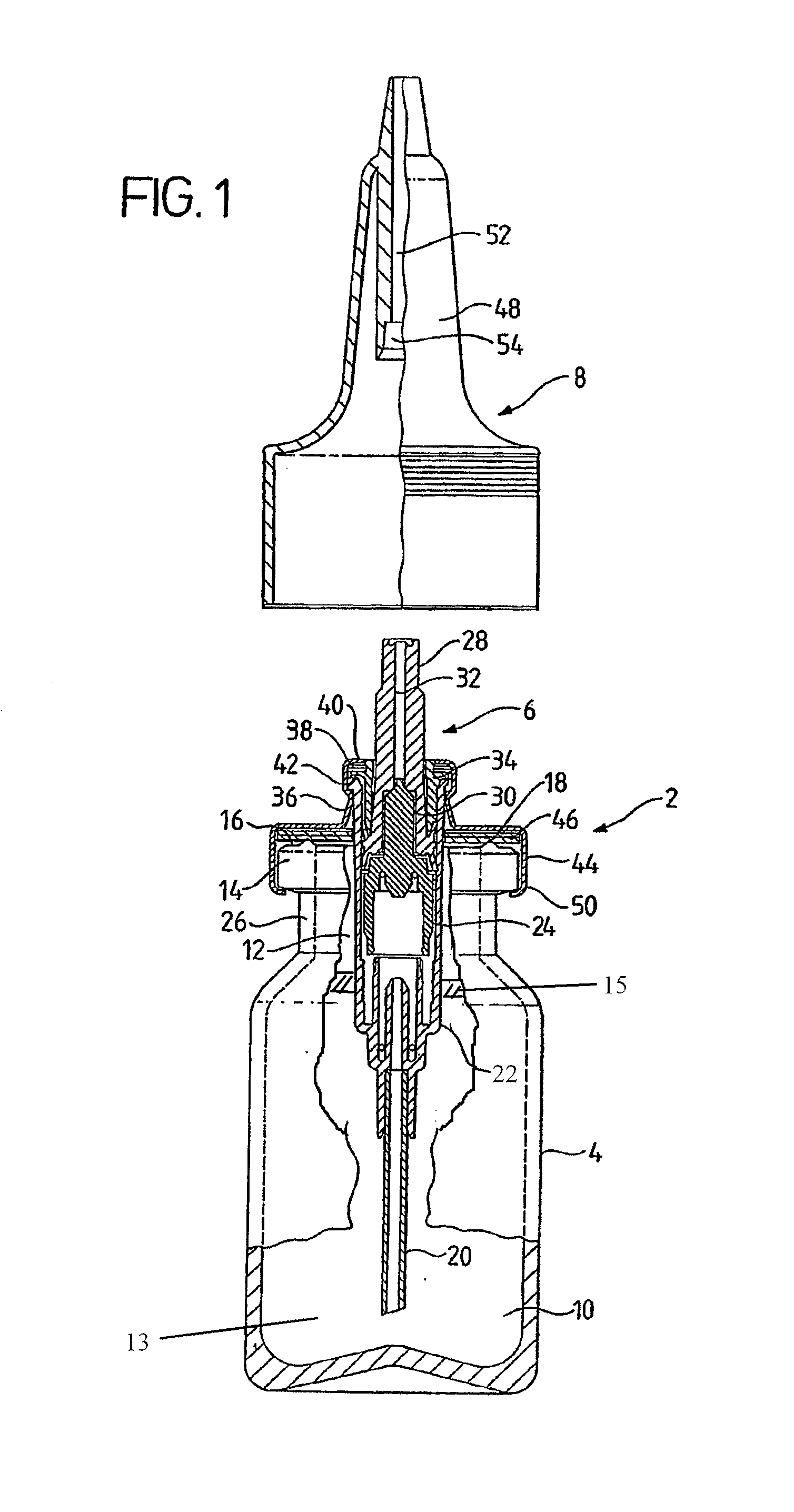
Dispenser for a liquid
A dispenser for a liquid is provided which is particularly, though not exclusively, useful for dispensing liquid medical compositions such as nasal sprays. The dispenser employs at least one, though preferably two, semi-rigid casing halves, defining a liquid reservoir, within which is mounted a bridge member which performs the functions of dip tube, dose metering pump, and outlet orifice. An air inlet may be provided into the reservoir, to allow air to enter as liquid leaves, in which case it may be provided with a liquid-impermeable, and preferably bacteria-impermeable, cover. Other constructions are described in which no such cover need be provided, or in which the need for an air inlet can be avoided altogether.
Owner:THE PROCTER & GAMBLE COMPANY
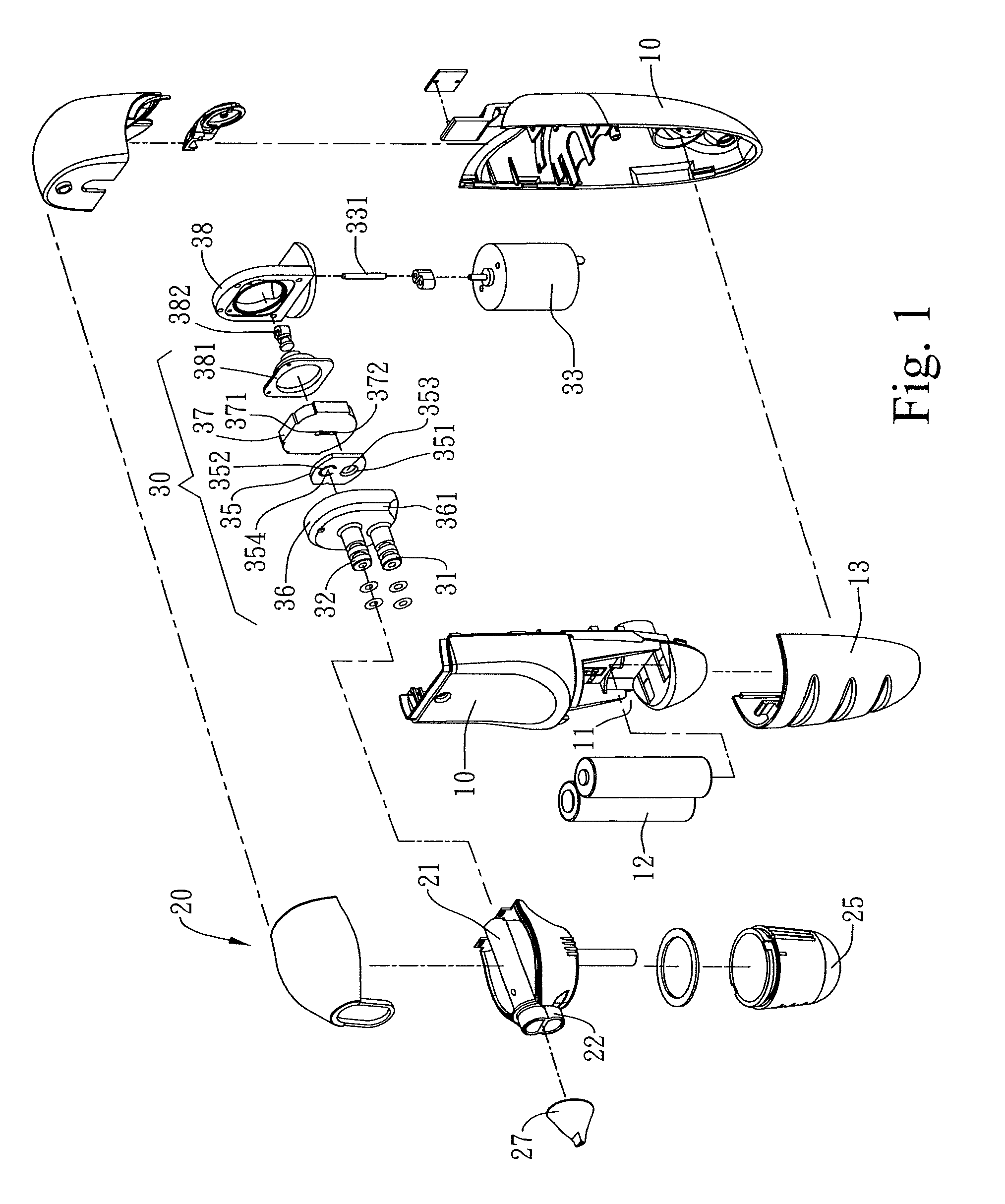
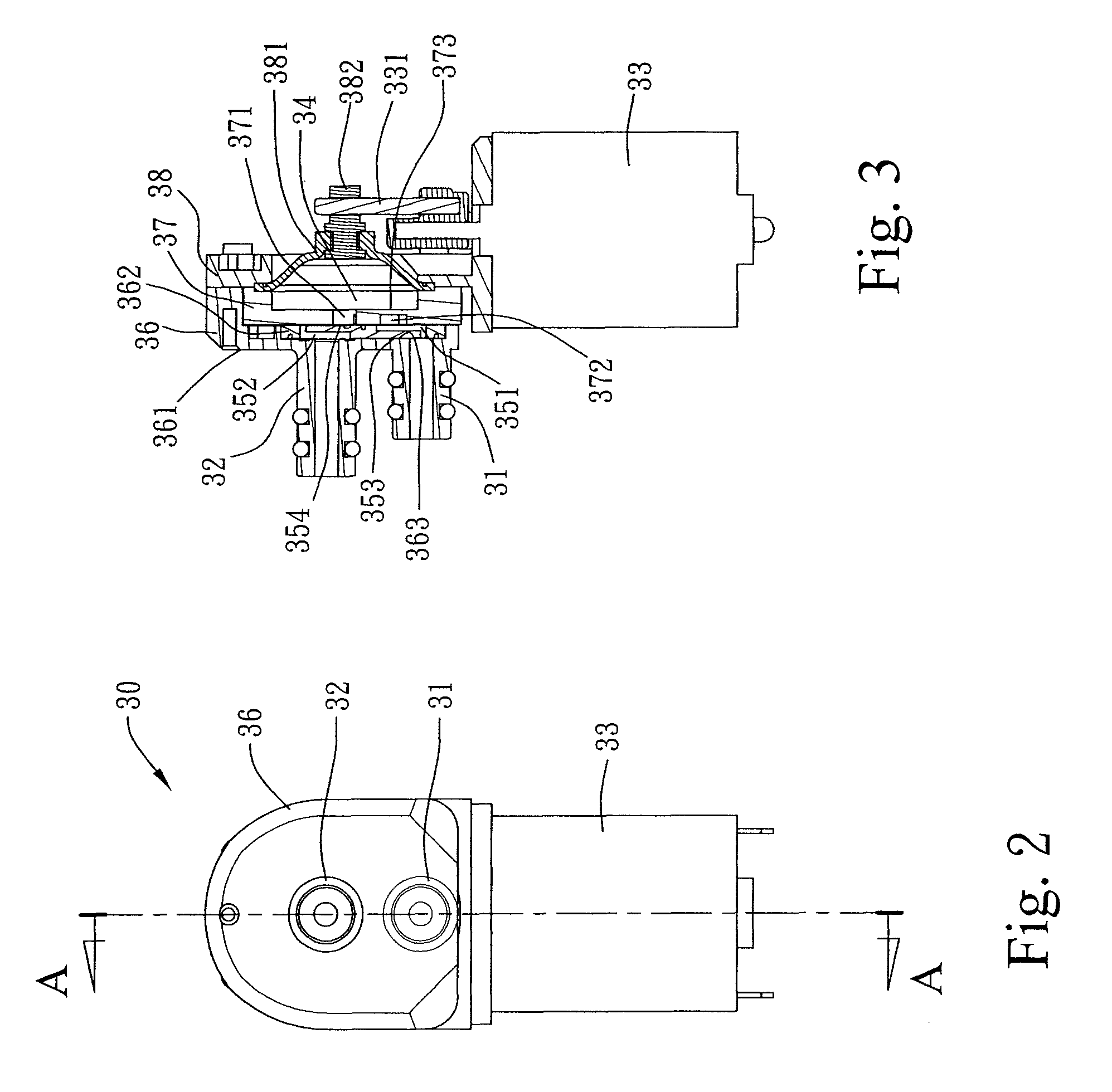
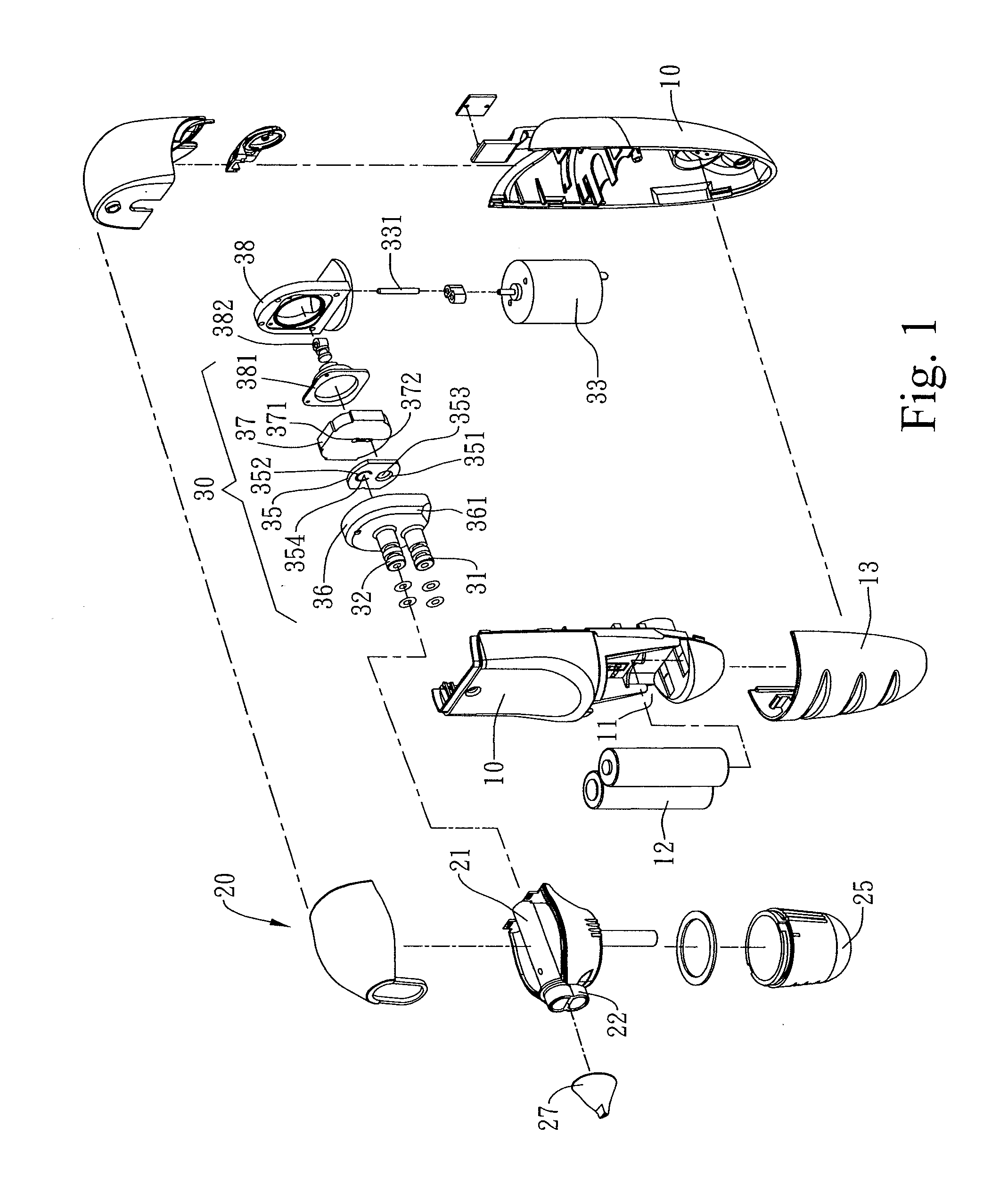
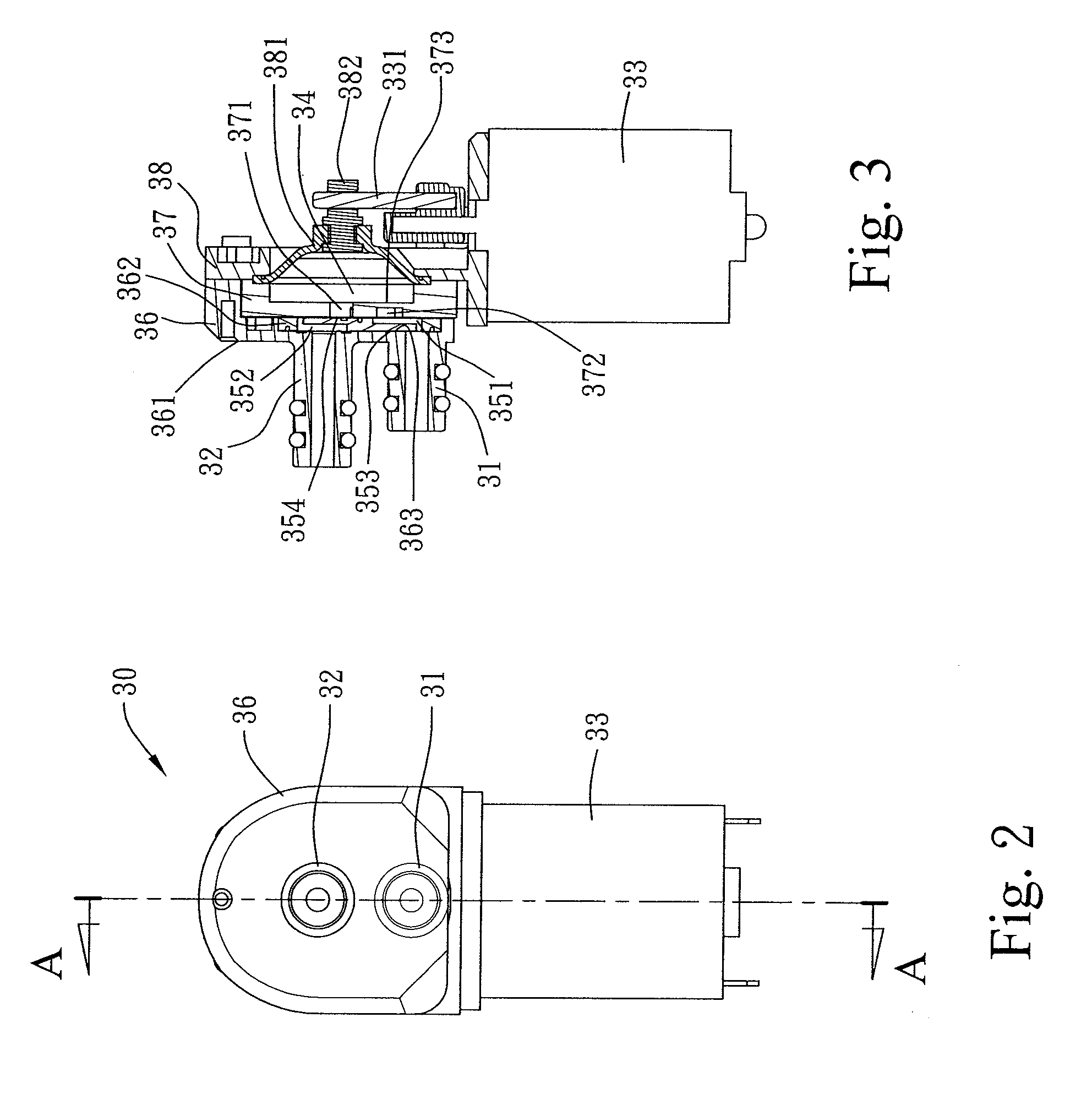
Combined nasal spray and aspirator device
ActiveUS20080312674A1Good for healthPresent invention is physically more favorable for usersEar treatmentCannulasNasal cavityNebulizer
A combined nasal spray and aspirator device includes an air pump disposed within a housing and comprises a spray and aspirator assembly. The air pump, at which an air suction connector and an air discharge connector are disposed, is driven by a driving component to perform air suction and discharge actions. The spray and aspirator assembly, mounted to the air suction connector and the air discharge connector, comprises a spray conduit and a first aspiration conduit; the spray conduit connects to an atomizer which is provided with liquids from a liquid storage container, and the first aspiration conduit connects to a mucus collector so that when some air carrying mucus enters the mucus collector, the mucus is left behind and the air enters the air pump through a second aspiration conduit. The device of the present invention integrates the operations of a nasal spray and a nasal aspirator, sucking the mucus away right after it sprays and liquefies the mucus, with said actions occurring almost simultaneously. The present invention is physically more favorable for users.
Owner:AVITA
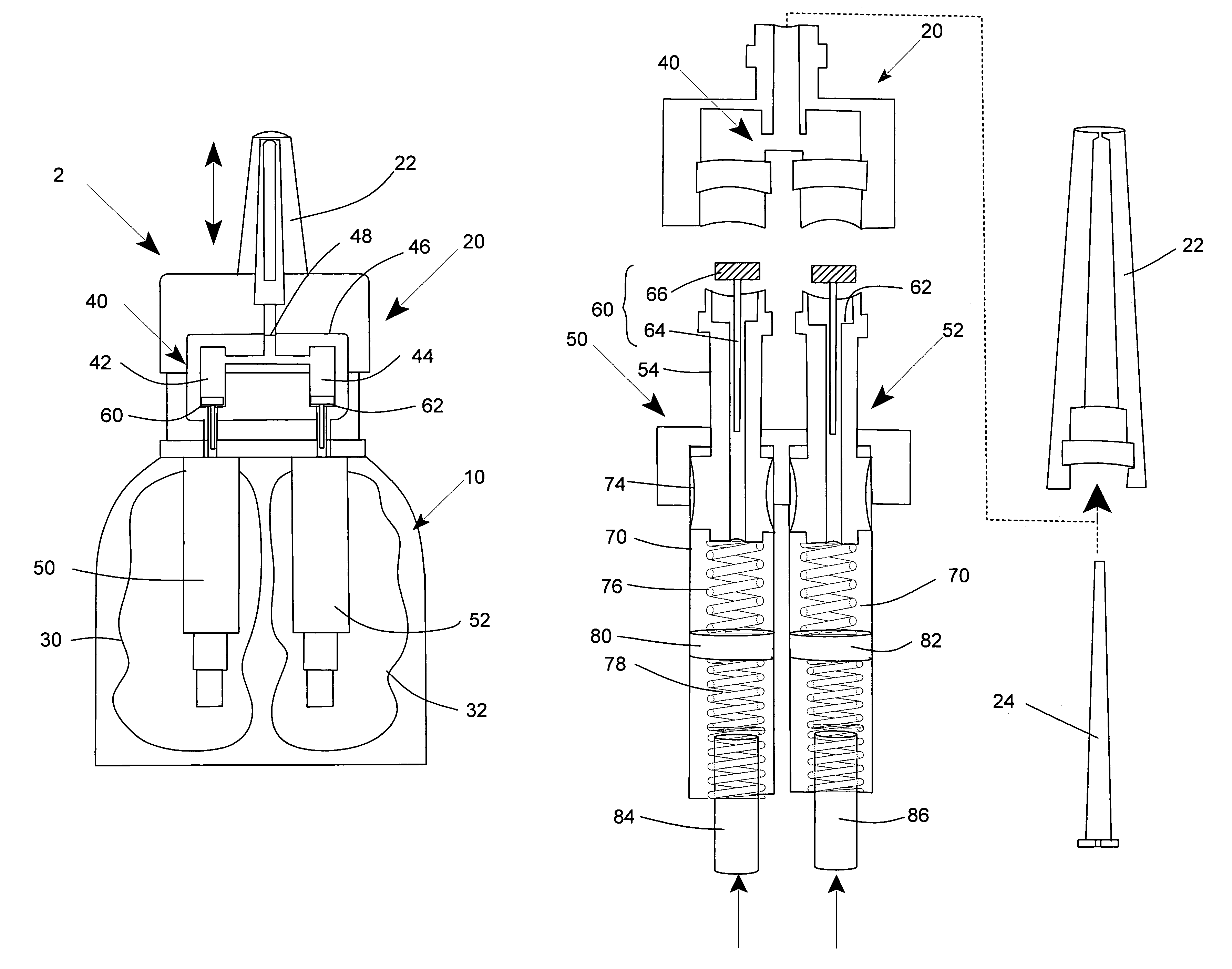
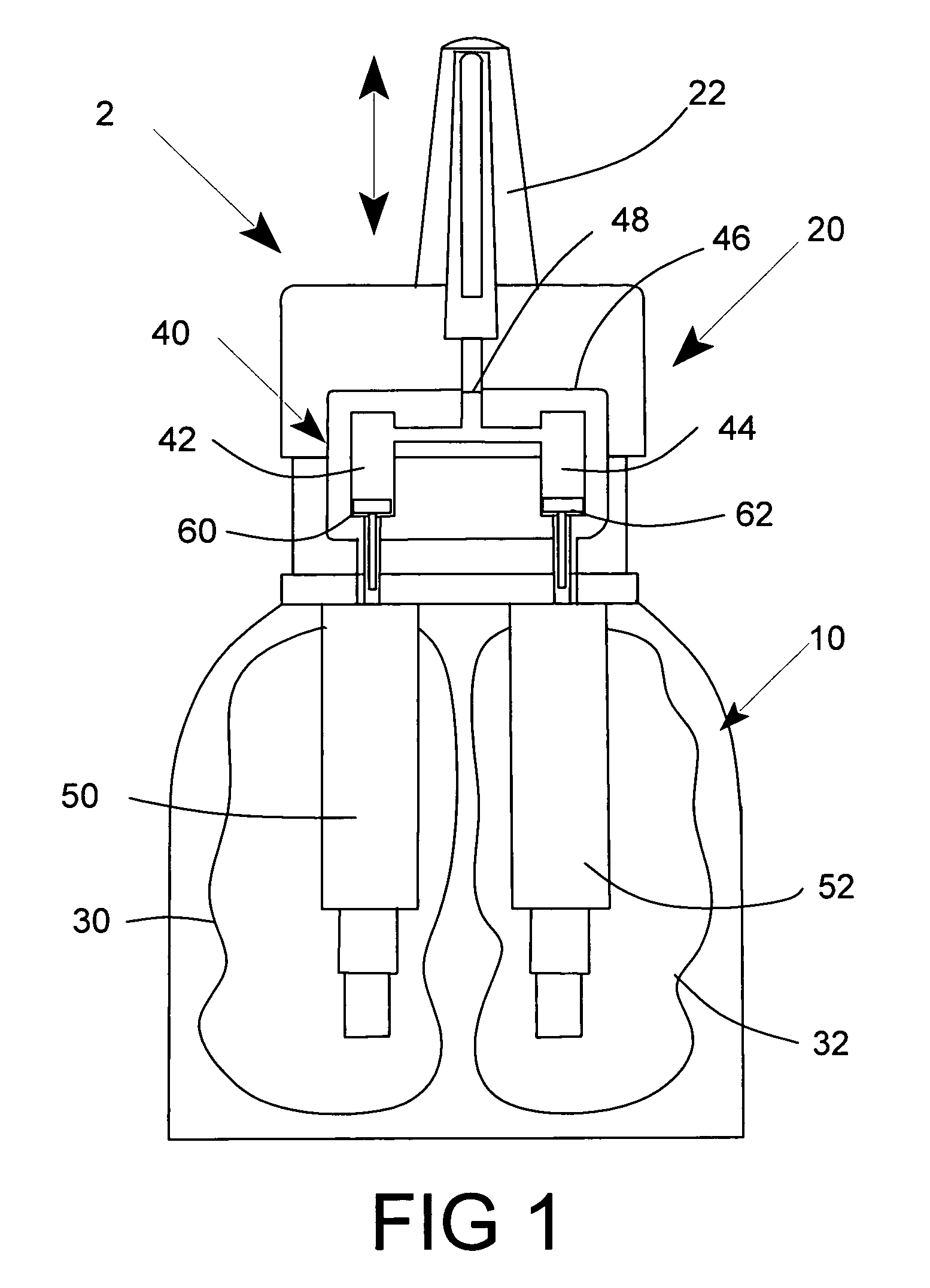
Multi medication nasal spray device and method
A nasal spray apparatus for simultaneously administrating metered amounts of multiple medicaments includes chambers for separately storing incompatible medicaments, such as an antihistamine and a steroid. Reciprocal piston pumps allow the medications to be sprayed into the user's nasal cavity. Two pumps can be used to separately transfer the medicaments to a receptacle where they can be initially mixed just prior to administration. A small volume receptacle is used to reduce the amount of mixture remaining after each stroke of the nasal spray apparatus. A check valve can be associated with each pump to further reduce medicament mixtures from cross contamination within storage chamber preparations. Collapsible components, including collapsible storage chambers or balloon capacitors can be employed to compensate for vacuums and back pressures as the medicaments are pumped to a spray nozzle.
Owner:MINOTTI AMERICO MICHAEL
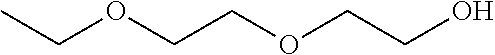
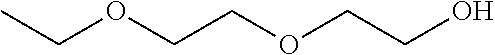
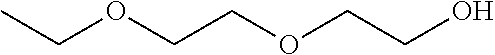
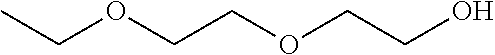
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS20140296191A1Less viscousLess denseBiocideOrganic chemistryDiethylene glycol monoethyl etherNasal spray
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Spray composition with reduced dripping
InactiveUS6565832B1Minimal tendency to drip and runMinimum tendencyAerosol deliveryAnaestheticsNasal sprayViscosity
An aqueous-based sprayable composition comprises a therapeutic or palliative agent, water and a mixture of microcrystalline cellulose and alkali metal carboxyalkylcellulose. In one embodiment, the composition is a non-Newtonian nasal spray exhibiting a very rapid viscosity recovery upon removal of shear forces.
Owner:BAYER HEALTHCARE LLC +1
Multi medication nasal spray device and method
A nasal spray apparatus for simultaneously administrating metered amounts of multiple medicaments includes chambers for separately storing incompatible medicaments, such as an antihistamine and a steroid. Reciprocal piston pumps allow the medications to be sprayed into the user's nasal cavity. Two pumps can be used to separately transfer the medicaments to a receptacle where they can be initially mixed just prior to administration. A small volume receptacle is used to reduce the amount of mixture remaining after each stroke of the nasal spray apparatus. A check valve can be associated with each pump to further reduce medicament mixtures from cross contamination within storage chamber preparations. Collapsible components, including collapsible storage chambers or balloon capacitors can be employed to compensate for vacuums and back pressures as the medicaments are pumped to a spray nozzle.
Owner:MINOTTI AMERICO MICHAEL
Naloxone hydrochloride nasal spray
The present invention is nasal cavity spray of Naloxine hydrochloride and its preparation process. The preparation of the present invention may be applied in single dosage form or multiple dosage form. The spray of the present invention includes Naloxine hydrochloride, osmotic pressure regulator, preservative, osmotic promoter and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Therapeutic nasal spray administered composition containing feverfew
A composition and delivery system are disclosed that will allow feverfew to be administered to a person in form in which an active ingredient of feverfew, particularly parthenolide can be readily and quickly assimilated by the person's body, particularly the central nervous system, and the therapeutic effects of the feverfew be rapidly imparted to the person. Feverfew is administered in the form of aqueous nasal spray composition, to provide therapeutic moisturization of nasal mucous membranes, relief of migraine headaches and antispasmodic effect, such as to relieve menstrual cramping or aid digestion. The effect is enhanced when the composition also contains nanoclustered resonant water. Vitamins, vitamin derivatives, surfactants, wetting agents, preservatives and emulsifiers may also be present.
Owner:LA JOLLA DIAGNOSTICS
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
Cucurbitacin lipsome preparation method and formulation
InactiveCN1504191AHigh encapsulation efficiencyOrganic active ingredientsDigestive systemCucurbitacin BMedicine
The invention relates to a cucurbitacin liposome composition and its preparation, which has rather high encapsulation efficiency, and can be administered through vein, muscle, oral and nasal. The constituent percentage by weight of the composition are, cucurbitacin BE or cucurbitacin B 0.001-0.1%, phospholipids 0.1-10%, cholesterin 0-5%, the phospholipids can be lecithin, di-stearoyl phosphatidyl choline, di- palmityl phosphatidyl choline, di-oleoyl phosphatidyl choline, di- palmityl phosphatidyl ethanolamine, di-stearoyl phosphatidylglycerol. The preparation according to the invention can be prepared in the form of injection, oral liquid, syrup, drop and nasal spray
Owner:SHENYANG PHARMA UNIVERSITY
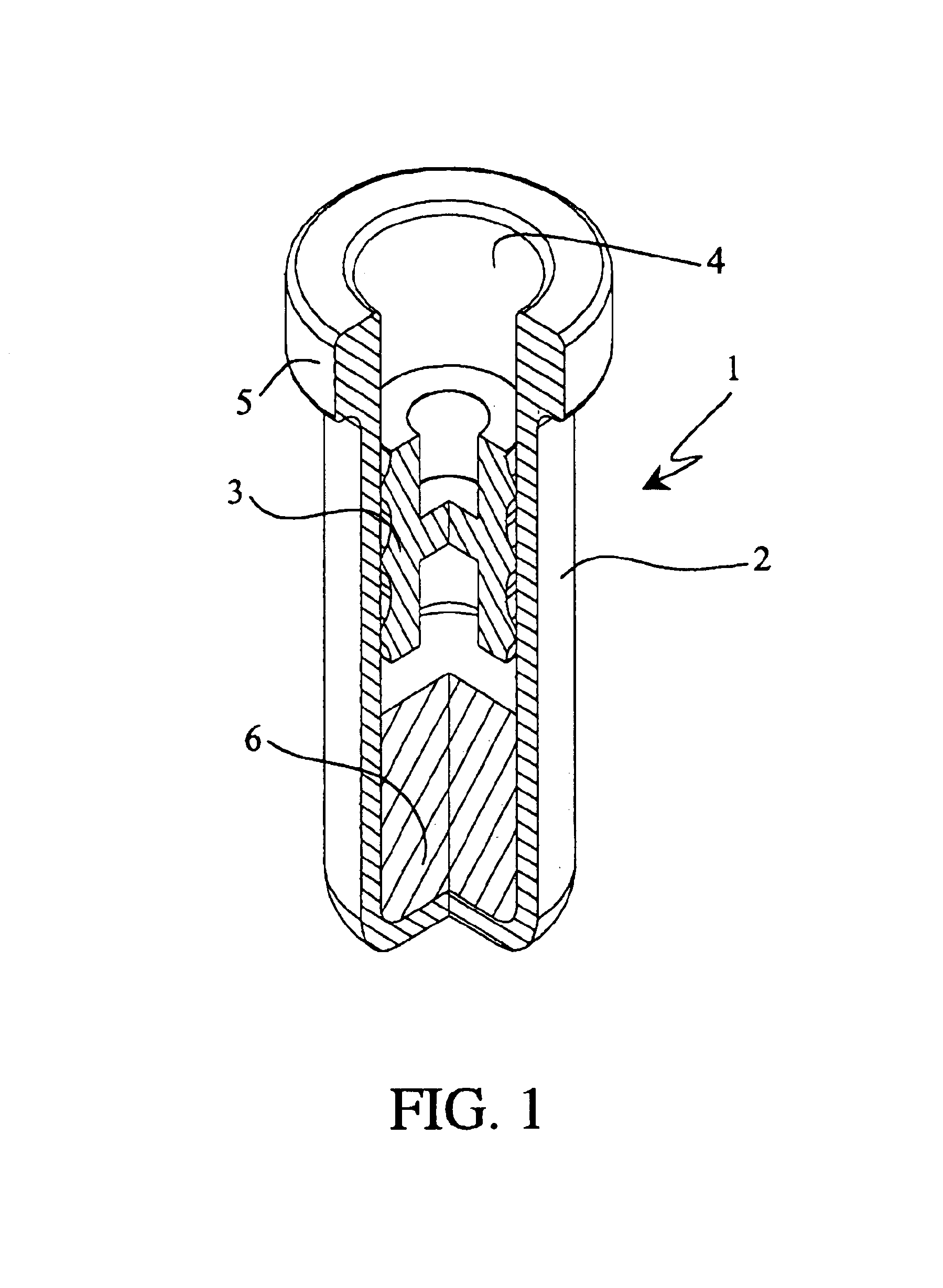
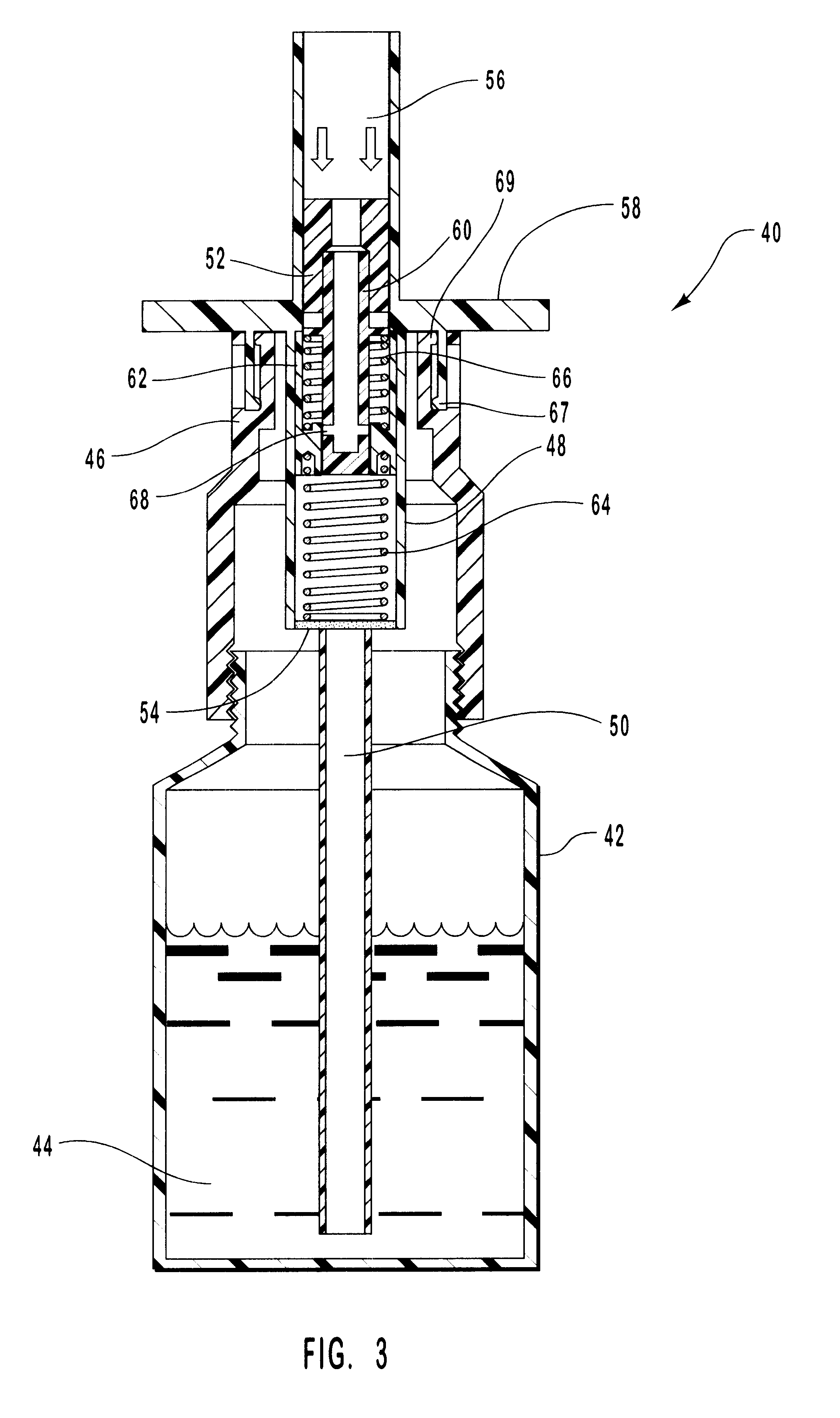
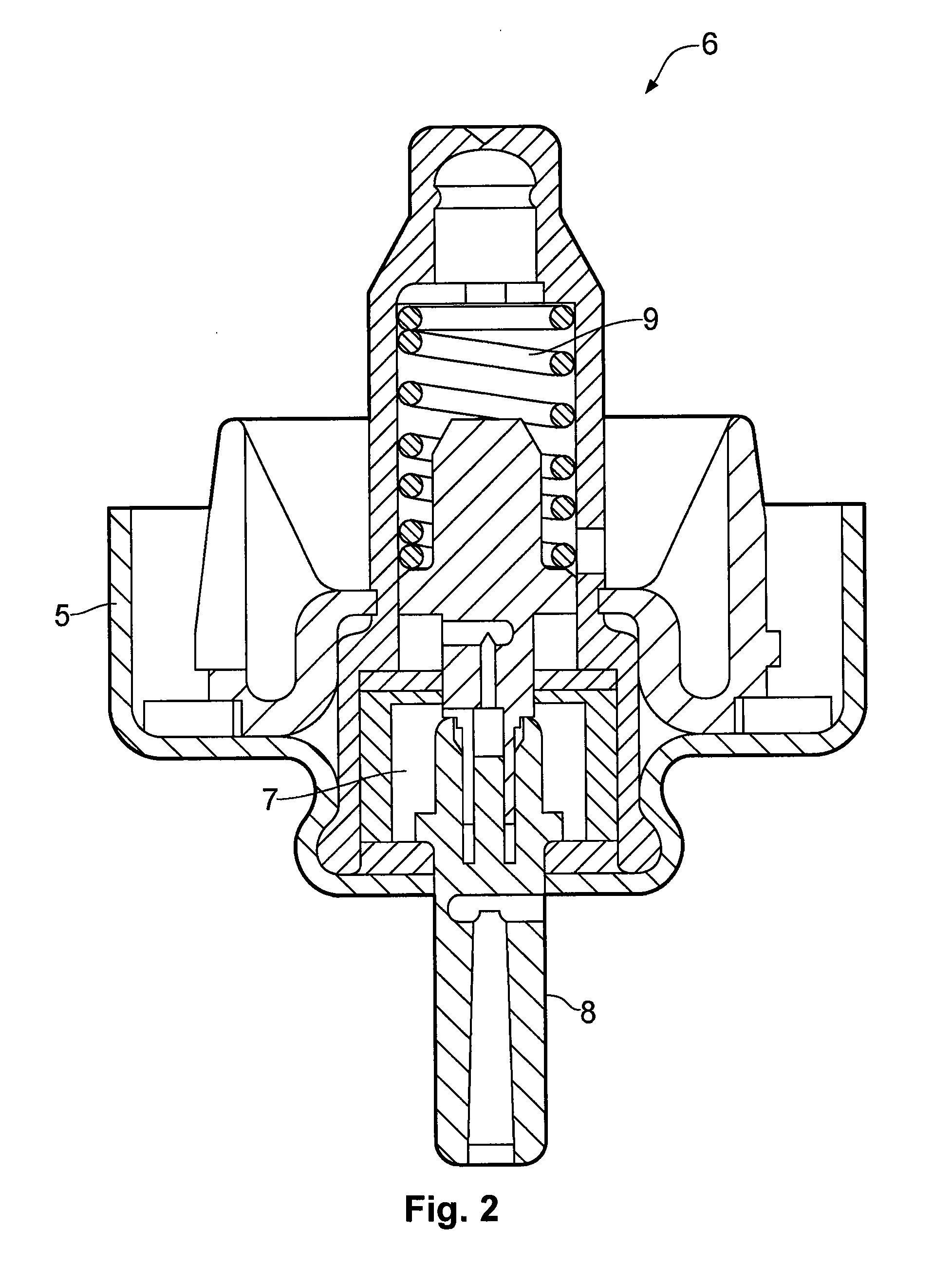
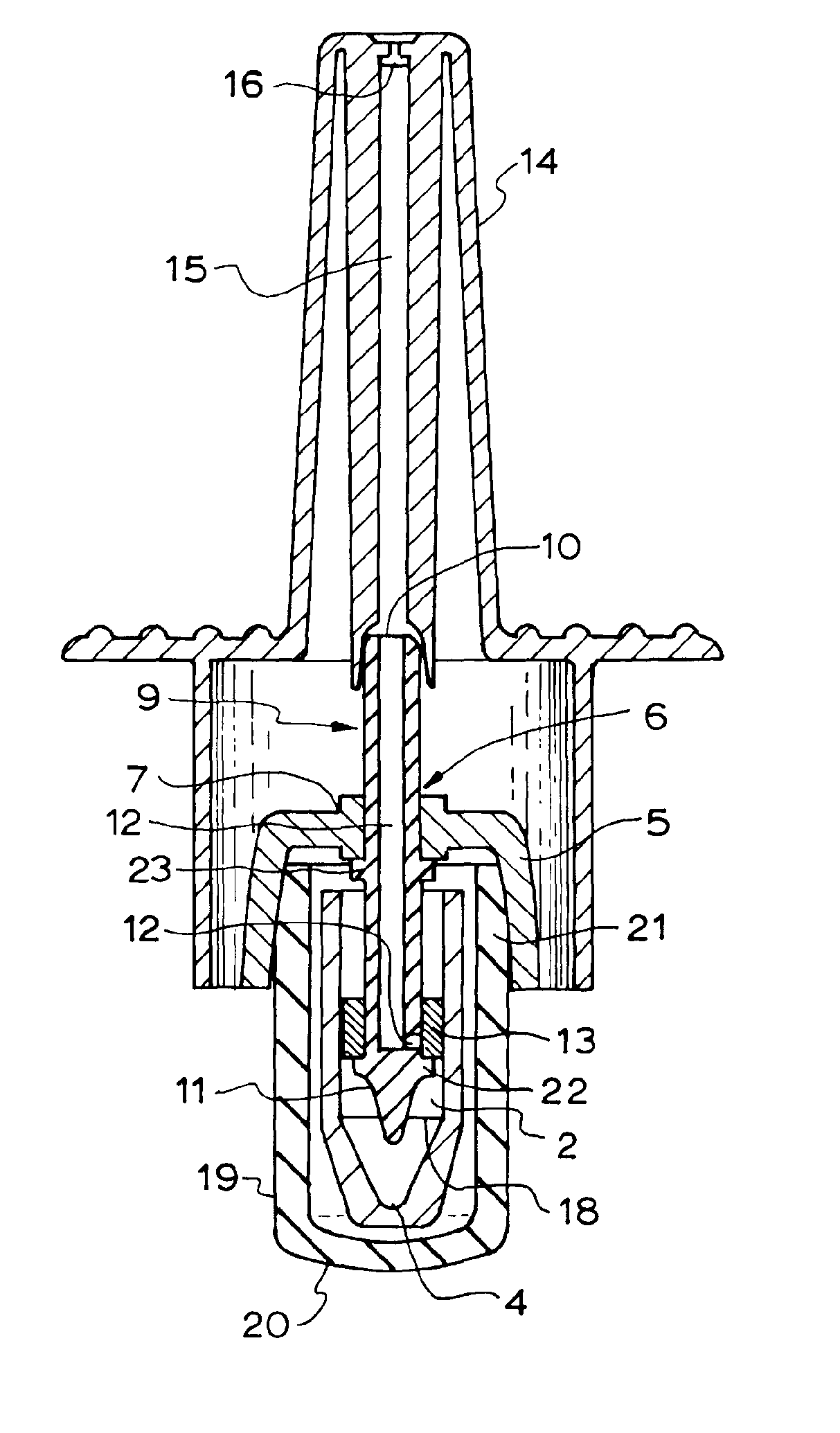
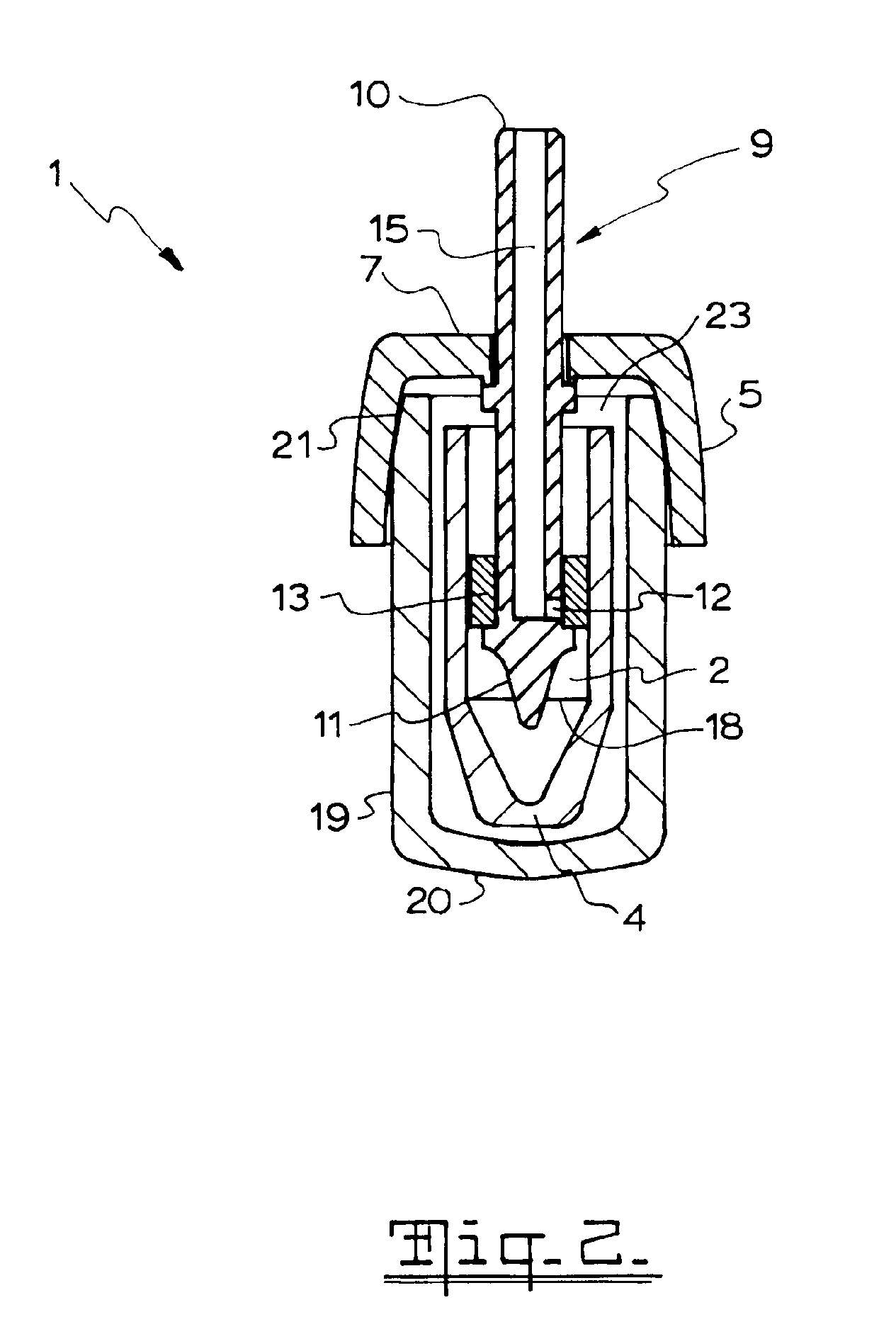
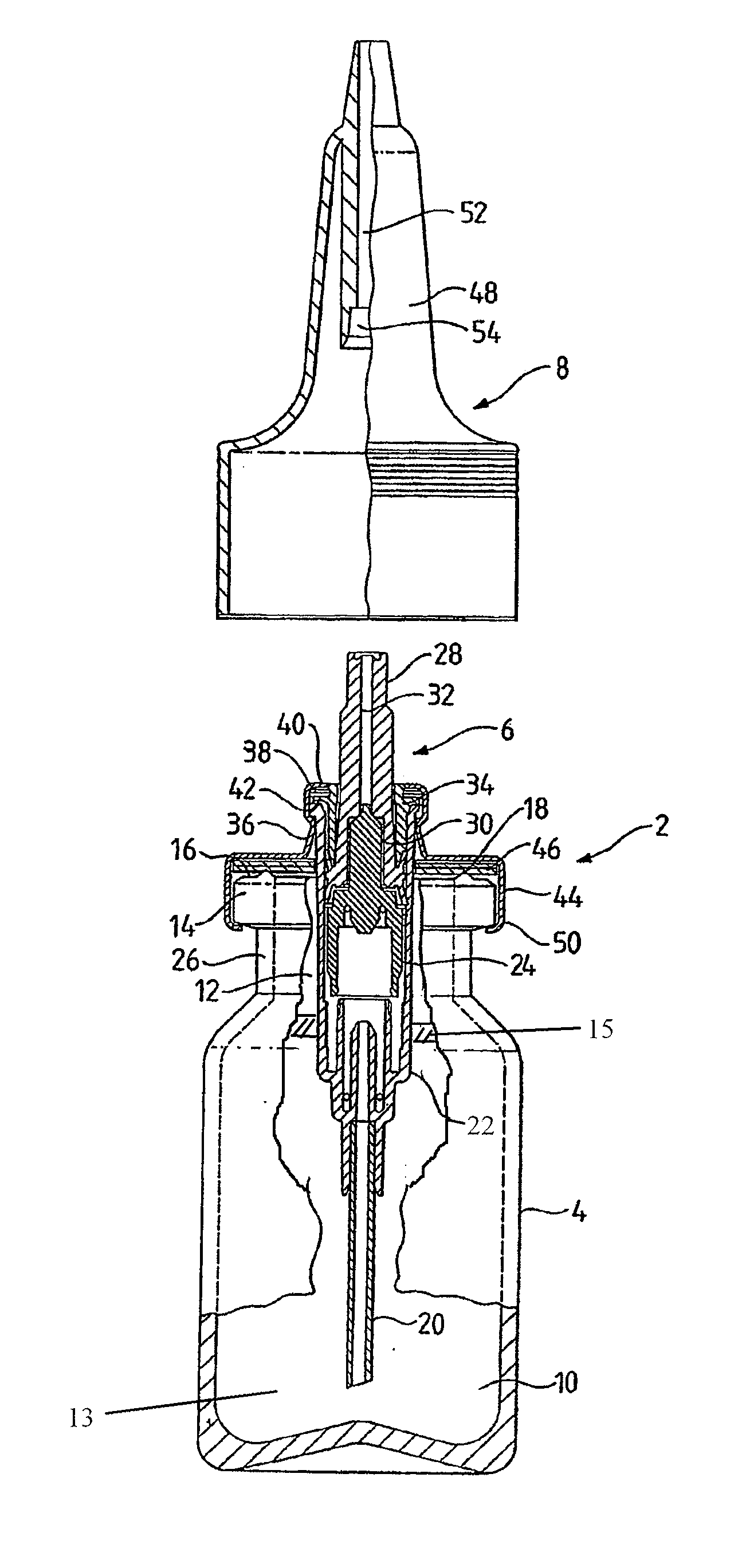
Nasal spray device
A nasal spray device for the delivery of a pharmaceutical formulation to the nasal cavity in metered doses. The device includes: a pressurised aerosol canister including a vial containing a pharmaceutical formulation including an active ingredient, a propellant and, optionally, a co-solvent, the aerosol canister further including a metering valve having a valve stem; and an actuator for the aerosol canister, the actuator including a stem block having a receptacle into which the valve stem of metering valve of the aerosol canister is received and axially located and being displaceable relative to the vial of the aerosol canister to actuate the metering valve of the aerosol canister, a sump extending below the receptacle, the stem block further defining a discharge orifice for the pharmaceutical formulation and a transfer channel through which a dispensed dose of the pharmaceutical formulation is able to pass from the sump to the discharge orifice.
Owner:TEVA BRANDED PHARMA PROD R & D +1
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
ActiveUS20180071390A1Less viscousLess denseOrganic active ingredientsAerosol deliveryUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Compstatin analogs for treatment of rhinosinusitis and nasal polyposis
In some aspects, the present invention provides methods treating a subject in need of treatment for chronic rhinosinusitis or nasal polyposis, the methods comprising administering a complement inhibitor such as a compstatin analog to the subject. In some embodiments, the complement inhibitor is administered intranasally, e.g., in a nasal spray.
Owner:APELLIS PHARMA
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
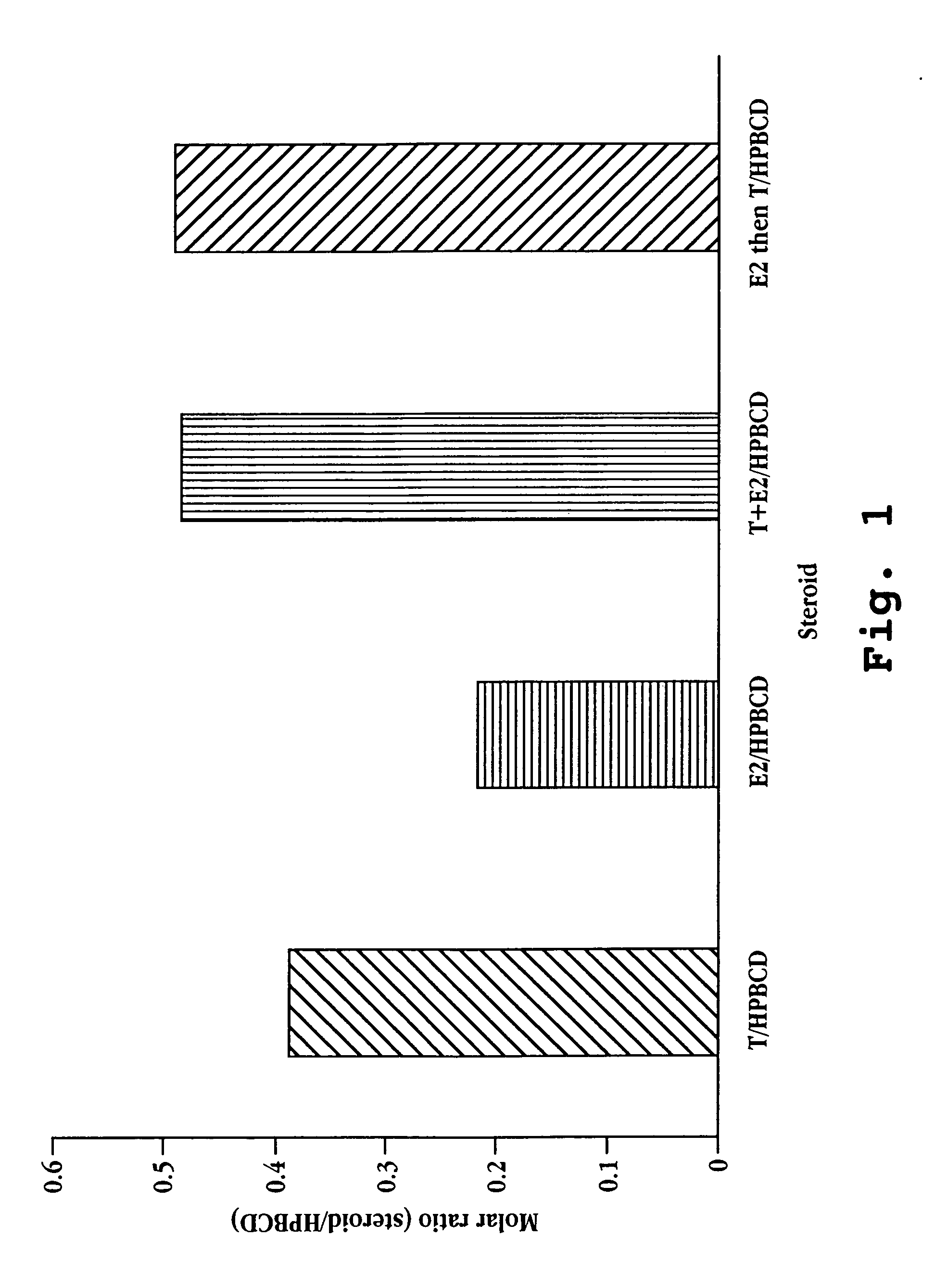
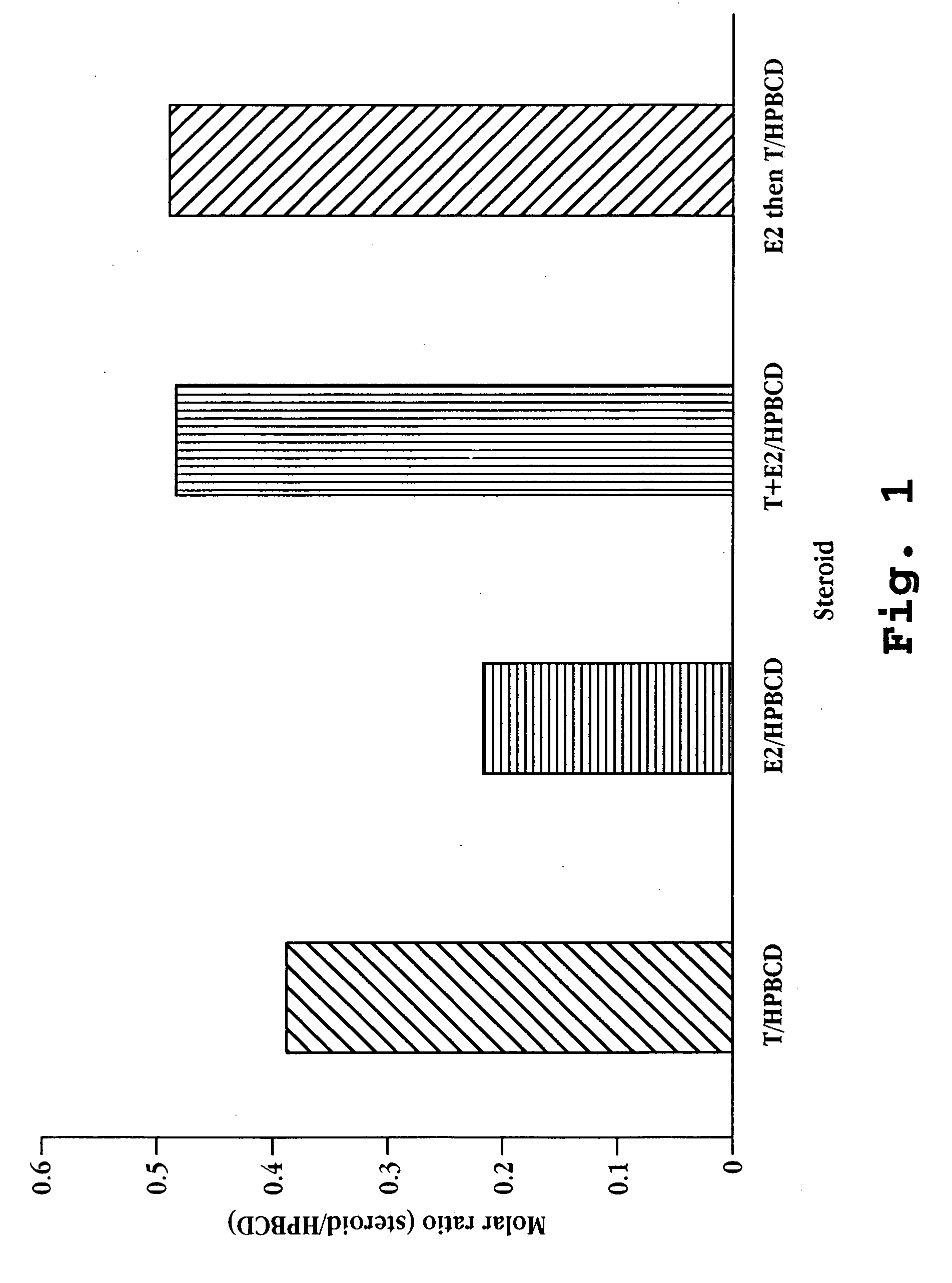
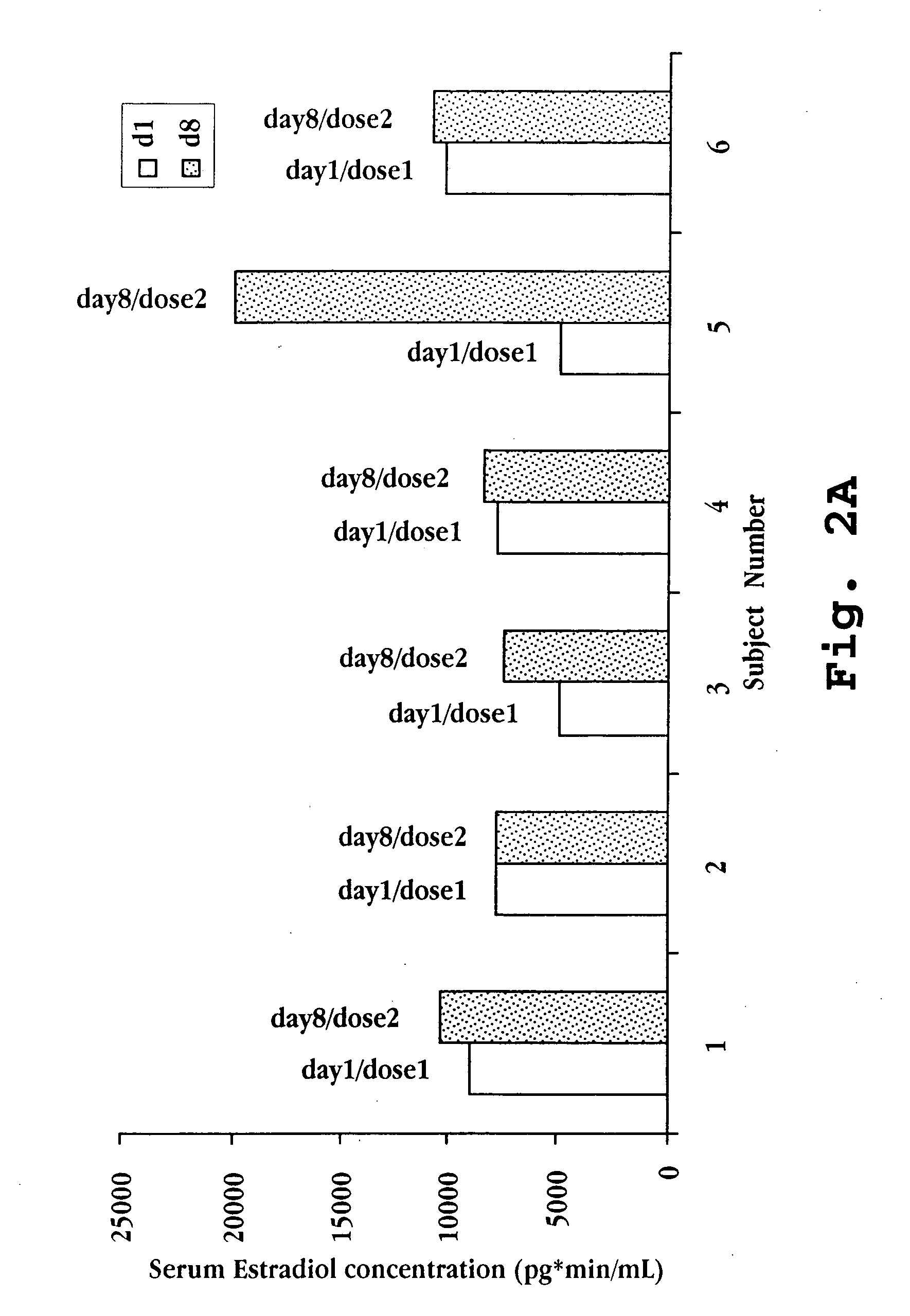
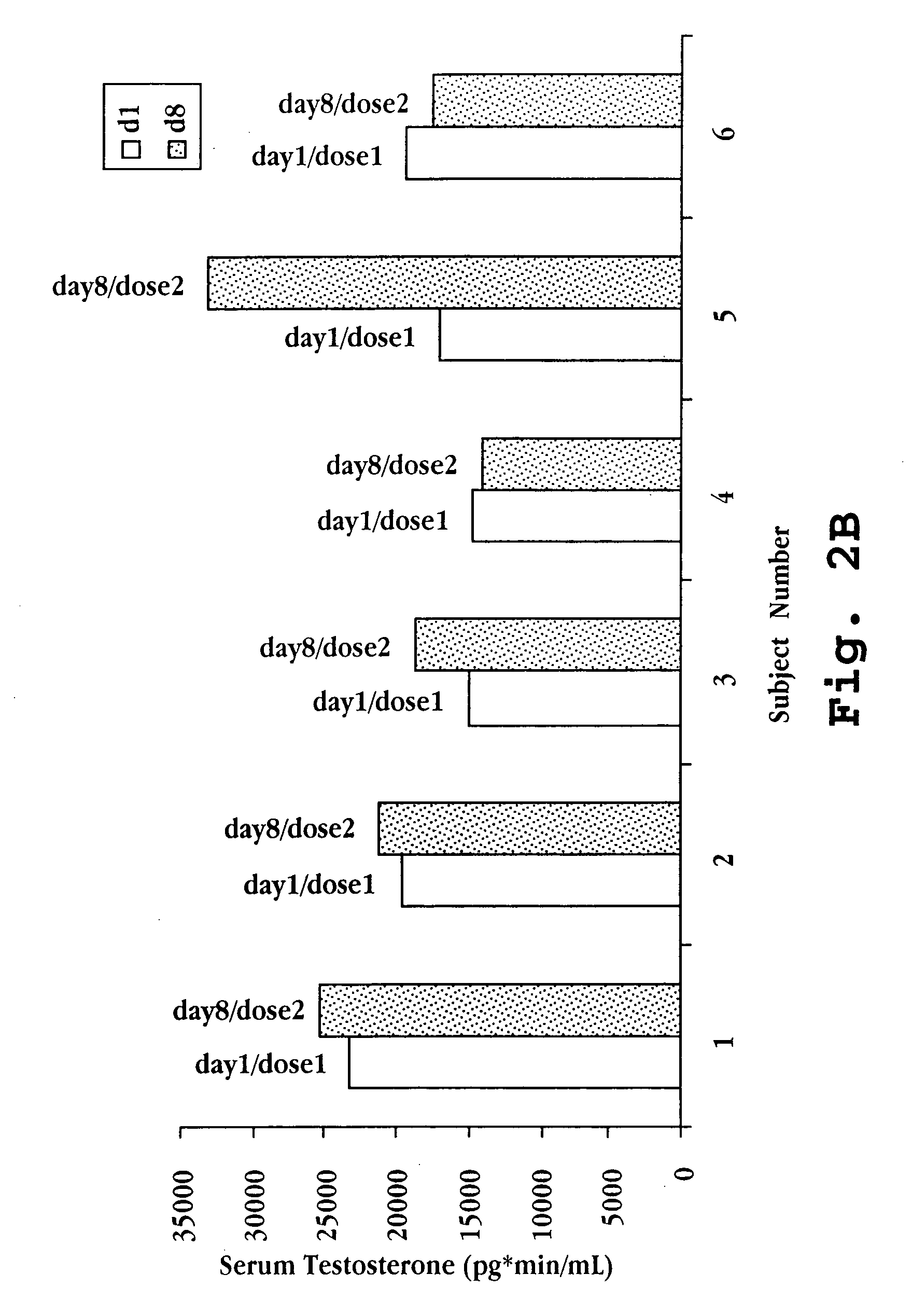
Nasal spray steroid formulation and method
The present invention relates to an improvement in a method of contraception, in treatment of benign gynecological disorders, and in hormone replacement. The improved method includes administering intranasally an estrogenic compound and an androgenic compound, and in some embodiments an optional progestin compound, in a once-daily bolus formulation comprised of the two or three steroids complexed with a cyclodextrin. An intranasal delivery system for administration of the formulation is also described.
Owner:BALANCE PHARMA
Oxymetazoline HCI and/or chlorpheniramine maleate nasal spray compositions
InactiveUS6316483B1Extend nasal muco-cilia clearance timeBiocidePharmaceutical delivery mechanismNasal sprayWater soluble
Aqueous nasal spray compositions comprising a medicament and an aqueous carrier comprising water soluble polymers selected from the group consisting of polyvinylpyrrolidone and mixtures thereof.
Owner:MSD CONSUMER CARE INC
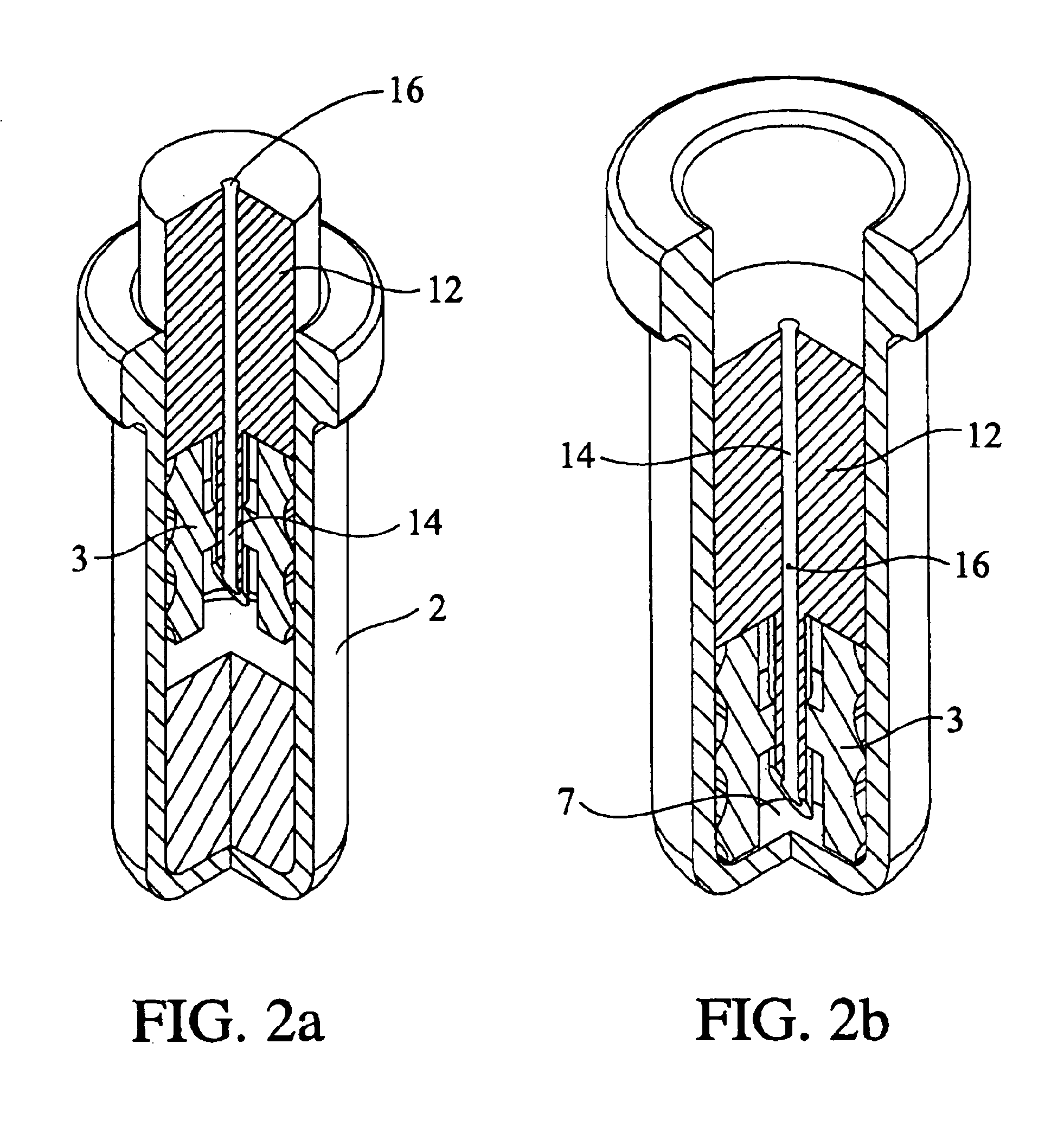
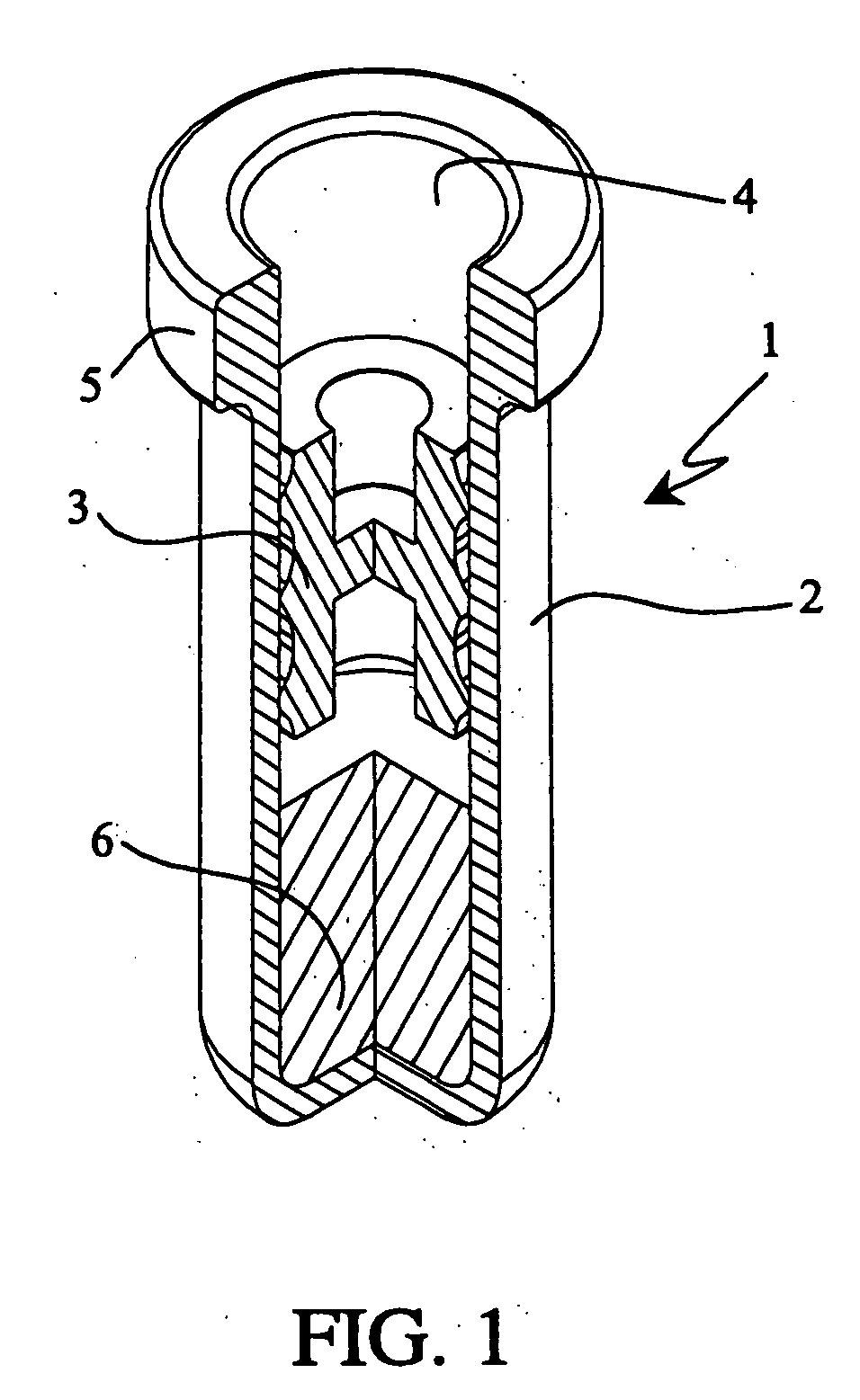
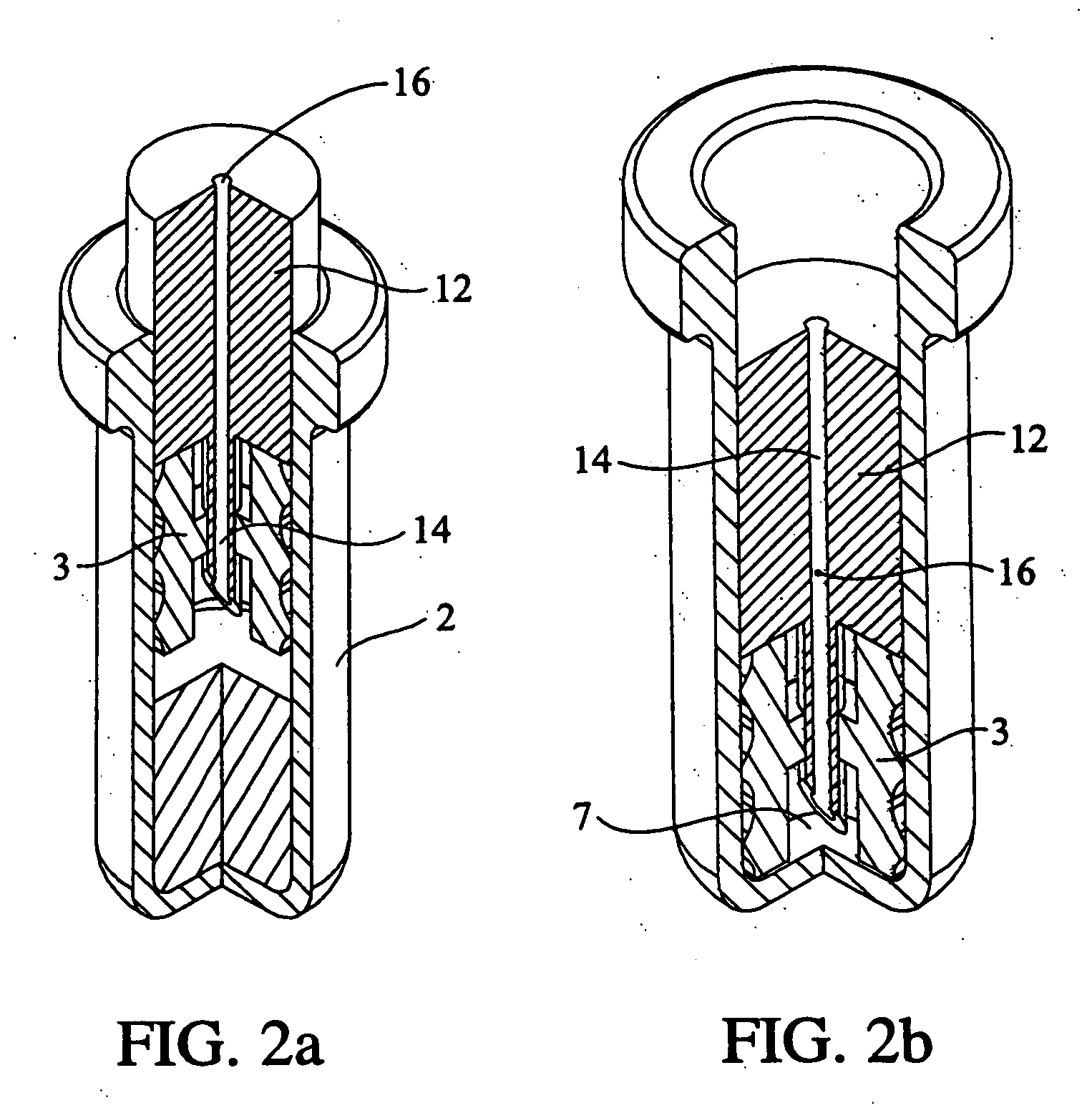
Single dose nasal spray pump
In one or more embodiments a single dose nasal spray pump includes a reservoir configured for holding a single dose of material to be dispensed, the reservoir extending between a closed end and an open end; a cap to be configured to be disposed over the open end of the reservoir, the cap including an aperture; a stem extending between an outlet end and a lower end, the stem having a conduit extending from the outlet end of the stem to a conduit inlet at or adjacent the lower end of the stem; and a piston disposed about the stem and configured for movement between a first position wherein the piston seals or substantially seals the conduit inlet and a second position wherein the piston is remote from the conduit inlet; wherein the stem is configured for movement from a first stem position wherein the piston is disposed in the reservoir in the first piston position and a second stem position wherein the stem is moved toward the closed end of the reservoir such that the piston is moved into the second piston position.
Owner:MK INT
Use of methylcobalamin nasal spray to treat disorders
A method of treating a disorder by nasally administering methylcobalamin, with or without folinic acid. The disorders addressed are: a) attention deficit hyperactivity disorder (ADHD); b) anxiety, depression, stress and chronic stress; c) socialization problems, mood problems, behavior problems, memory problems; d) dislexia, depth perception problems, color viewing problems, visual and auditory processing problems, light modulation problems, night vision problems; e) speech problems such as finding words, apraxia, and articulation problems, sleep regulation problems, eye or muscle movement problems; and f) chronic fatigue problems, digestion problems, sensitivity to chemicals, viral infection, inflammatory conditions such as rheumatoid arthritis, sciatica, and fibromyalgia, asthma, irritable bowel, colitis, tinnitus, migraines, nail biting, autoimmune problems. In some embodiments, the disorders that are particularly addressed are ADHD, anxiety, stress and chronic stress, and irritable bowel.
Owner:KURTZ STAN
Spray composition
InactiveUS20030185763A1Minimal tendency to drip and runMinimized in variation of characteristicPowder deliveryOrganic active ingredientsNasal sprayViscosity
An aqueous-based sprayable composition comprises a therapeutic or palliative agent, water and a mixture of microcrystalline cellulose and alkali metal carboxyalkylcellulose. In one embodiment, the composition is a non-Newtonian nasal spray exhibiting a very rapid viscosity recovery upon removal of shear forces.
Owner:BAYER HEALTHCARE LLC
Carbetocin pharmaceutical composition and preparation method thereof
The invention relates to a stable pharmaceutical composition containing carbetocin, and a preparation method thereof. The stable pharmaceutical composition containing carbetocin contains carbetocin, a pH regulator, an isoosmotic regulator and pharmaceutically acceptable auxiliary materials. The composition is used for preventing uterine atony and postpartum hemorrhage after selective epidural or spinal anesthesia cesarean section. The pharmaceutical composition mainly exists in a liquid form and is used through parenteral administration forms, mainly injection and nasal spray.
Owner:HYBIO PHARMA
Nasal spray formulation and method
InactiveUS20060008420A1Prevent signAvoid symptomsOrganic active ingredientsPeptide/protein ingredientsCyclodextrinNasal spray
A nasal spray formulation for use in female contraception or in the treatment of benign gynecological disorders is described. The nasal preparation is comprised of a GnRH compound and an estrogenic compound in the form of a water-soluble complex with a water-soluble cyclodextrin. The preparation effectively suppresses ovarian estrogen and progesterone production, and prevents signs and symptoms of estrogen deficiency, without a significant increase in the risk of endometrial hyperplasia.
Owner:BALANCE PHARMA
Sodium pyruvate nasal spray and preparation method thereof
ActiveCN102657611AGood antibacterial effectLess irritatingAerosol deliveryPharmaceutical non-active ingredientsNasal passageNasal passages
The present invention relates to a nasal spray containing sodium pyruvate and a preparation method thereof, belonging to the field of medical technology, being characterized in that: the nasal spray containing sodium pyruvate comprises the following components: sodium pyruvate, pyruvic acid, an isoosmotic adjusting agent, a preservative and water. Because the sodium pyruvate is capable of alleviating nasal obstruction and inflammation caused by rhinitis, a medical fluid provided in the invention can be made into a nasal spray medical fluid which is capable of entering nasal cavities by adopting a portable spraying device. The nasal spray medical fluid is particularly suitable for cleaning and removing harmful pollutants in nasal passages, paranasal sinuses and mucosal cilia and is capable of being used in nursing after a nasal cavity operation, relieving rhinostegnosis and reducing respiratory tract irritation and risks of infection. By adding the pyruvic acid to form a buffer solution, tingling caused by using the flushing fluid to wash the cavities can be reduced, and inflammation is alleviated. Allergic symptoms can be prevented or gradually alleviated for the people of special professional who often use the product provided in the invention to wash and care nasal cavities.
Owner:JIANG SU PHARMAMAXCORP +1
Nasal spray apparatus
A nasal spray comprises a container holding a nasal preparation containing sesame oil and a spray pump dispenser mounted to close an opening of the container. A dip tube from the dispenser extends down into the nasal preparation. The pump body is provided with a mounting collar operable to create an airtight seal with the top of the container. The nasal spray further comprises an inert substance covering a surface of the nasal preparation disposed within the container that is not contiguous with a wall of the container.
Owner:PHARMACURE HEALTH CARE
Compositions of pharmaceutical actives containing diethylene glycol monoethyl ether or other alkyl derivatives
InactiveUS9827315B2Less viscousLess denseOrganic active ingredientsBiocideUse medicationDiethylene glycol monoethyl ether
The present invention relates to pharmaceutical compositions of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle and / or to pharmaceutical compositions utilizing Diethylene glycol monoethyl ether or other alkyl derivatives thereof as a primary vehicle or as a solvent system in preparation of such pharmaceutical compositions. The pharmaceutical compositions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formulations containing such pharmaceutical actives and are suitable for use as injectables for intravenous and intramuscular administration, as well as for use as a preformed solution / liquid for filling in and preparation of capsules, tablets, nasal sprays, gargles, dermal applications, gels, topicals, liquid oral dosage forms and other dosage forms.
Owner:THEMIS MEDICARE LTD
Nasal spray agent
The present invention is nasal spray of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron, osmotic pressure regulator and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
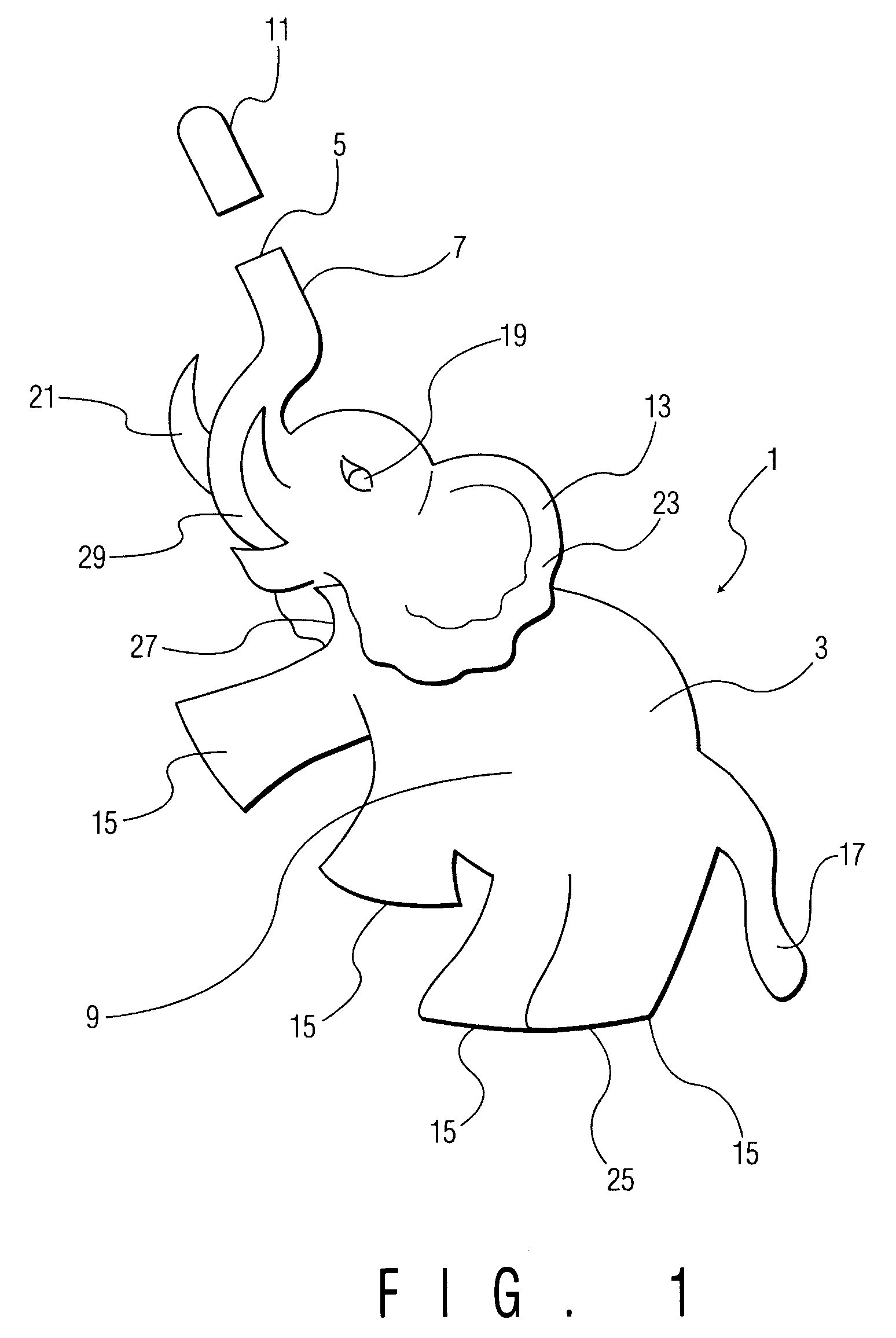
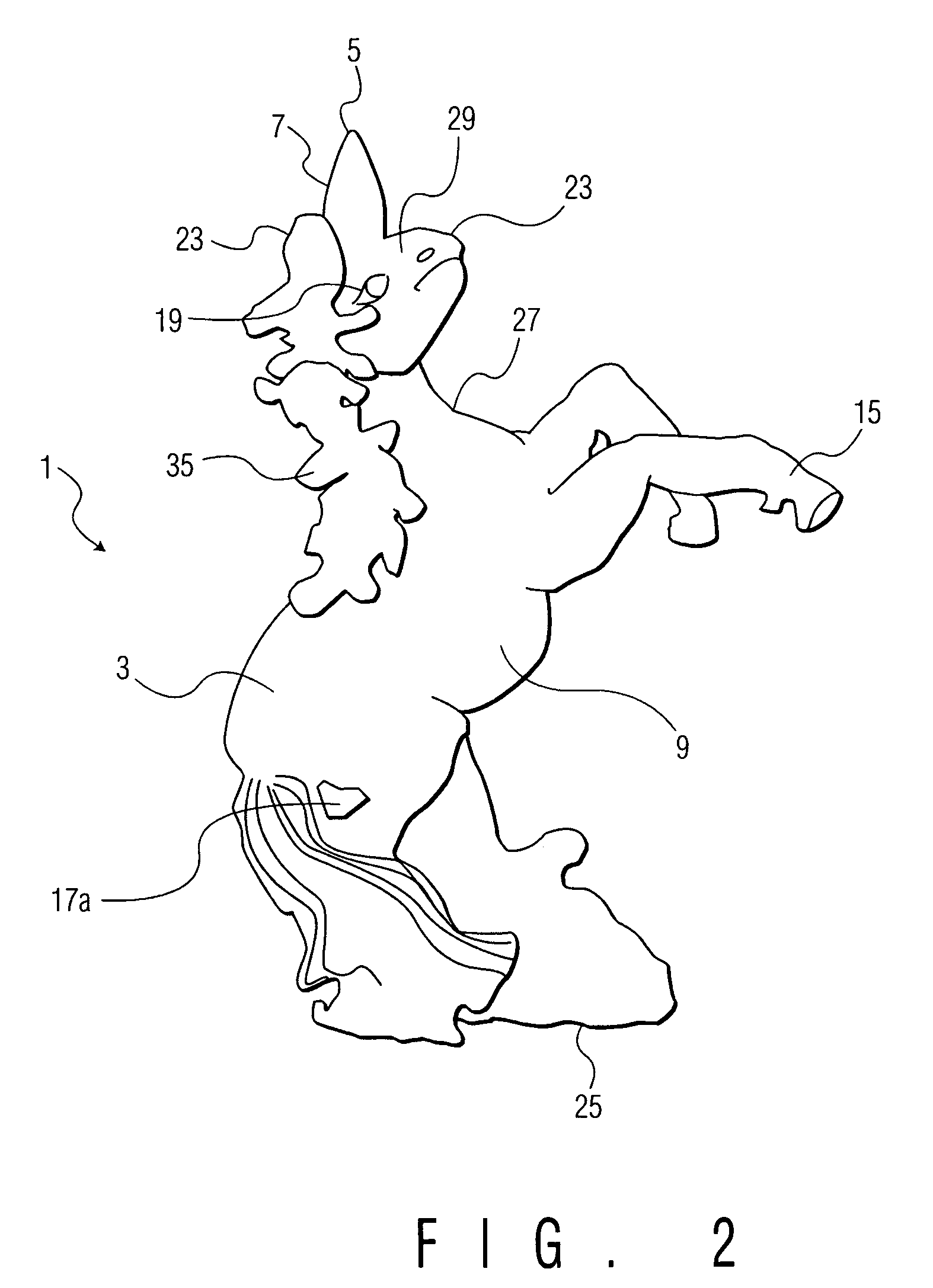
Children's nasal spray containers and inhalers
InactiveUS20050194005A1Easy to managePrecise managementMedical devicesMedical atomisersNasal cavityNasal spray
A container for dispensing nasal spray, ear drops, or an inhalant mist, formed in the three-dimensional shape of an entertaining figurine, such as an elephant, or a toy, having appendages which serve as handles for grasping and manipulating the container to administer the spray or mist to a child.
Owner:BERUBE PATRICIA J +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com