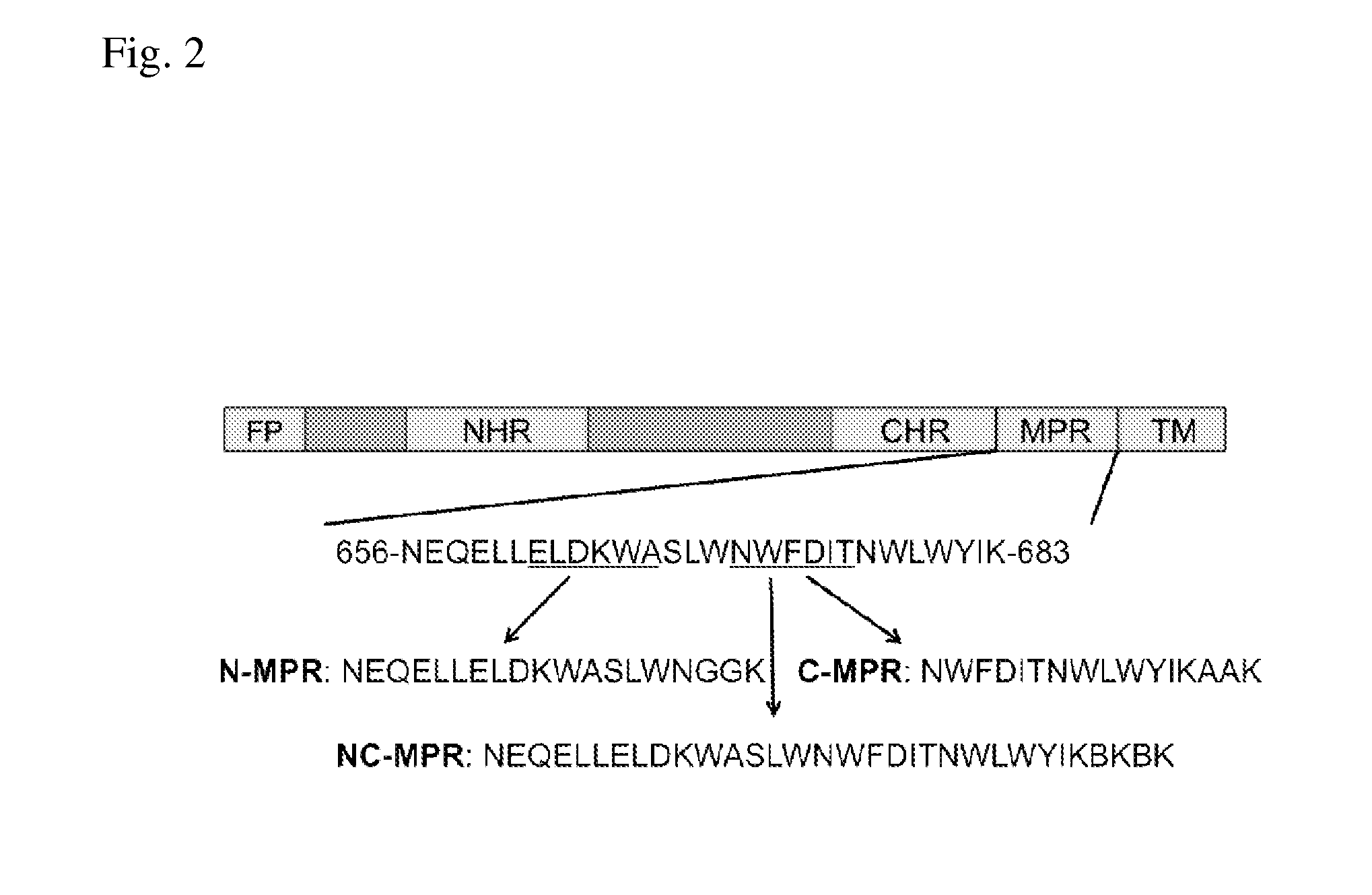
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "Phosphatidylglycerol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant. The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbone ester-bonded to either saturated or unsaturated fatty acids on carbons 1 and 2. The head group substituent glycerol is bonded through a phosphomonoester. It is the precursor of surfactant and its presence (>0.3) in the amniotic fluid of the newborn indicates fetal lung maturity.
Methods and compositions for liposomal formulation of antigens and uses thereof
The present invention relates to liposomal vaccine compositions, methods for the manufacture thereof, and methods for the use thereof to stimulate an immune response in an animal. These compositions comprise dimyristoylphosphatidylcholine (“DMPC”); either dimyristoylphosphatidylglycerol (“DMPG”) or dimyristoyltrimethylammonium propane (“DMTAP”) or both DMPC and DMTAP; and at least one sterol derivative providing a covalent anchor for one or more immunogenic polypeptide(s) or carbohydrate(s).
Owner:RGT UNIV OF CALIFORNIA +1
Formulation for spray-drying large porous particles
InactiveUS7279182B2Reduce and eliminate needEasy to preparePowder deliveryBiocidePrillVolumetric Mass Density
Particles having a tap density less than about 0.4 g / cm3 are formed by spray drying from a colloidal solution including a carboxylic acid or salt thereof, a phospholipid, a divalent salt and a solvent such as an aqueous-organic solvent. The colloidal solution can also include a therapeutic, prophylactic or diagnostic agent. Preferred carboxylic acids include at least two carboxyl groups. Preferred phospholipids include phosphatidylcholines, phosphatidylethanolamines, phosphatidylglycerols, phophstidylserines, phosphatidylinositols and combinations thereof. The particles are suitable for pulmonary delivery.
Owner:CIVITAS THERAPEUTICS
Pulmonary surfactant formulations
InactiveUS20060286038A1Increase ratingsImprove drug stabilityBiocideOrganic active ingredientsDiseaseDipalmitoyl Phosphatidylcholine
Synthetic pulmonary surfactant compositions comprising dipalmitoyl phosphatidylcholine, phosphatidylglycerol, and essentially neutral lipid, and having essentially no 1-palmitoyl 2-oleoyl phosphatidylglycerol and essentially no palmitic acid are provided. Methods for treating respiratory disease are also provided comprising administering a therapeutically effective amount of a synthetic pulmonary surfactant comprising dipalmitoyl phosphatidylcholine, phosphatidylglycerol, and essentially neutral lipid, and having essentially no 1-palmitoyl 2-oleoyl phosphatidylglycerol and essentially no palmitic acid.
Owner:DISCOVERY LABORATORIES INC
Cucurbitacin lipsome preparation method and formulation
InactiveCN1504191AHigh encapsulation efficiencyOrganic active ingredientsDigestive systemCucurbitacin BMedicine
The invention relates to a cucurbitacin liposome composition and its preparation, which has rather high encapsulation efficiency, and can be administered through vein, muscle, oral and nasal. The constituent percentage by weight of the composition are, cucurbitacin BE or cucurbitacin B 0.001-0.1%, phospholipids 0.1-10%, cholesterin 0-5%, the phospholipids can be lecithin, di-stearoyl phosphatidyl choline, di- palmityl phosphatidyl choline, di-oleoyl phosphatidyl choline, di- palmityl phosphatidyl ethanolamine, di-stearoyl phosphatidylglycerol. The preparation according to the invention can be prepared in the form of injection, oral liquid, syrup, drop and nasal spray
Owner:SHENYANG PHARMA UNIVERSITY
Method and reagent kit for measuring activated partial thromboplastin time
InactiveCN104076156APrevent precipitationLowering assayMicrobiological testing/measurementBiological material analysisBlood plasmaEnzyme
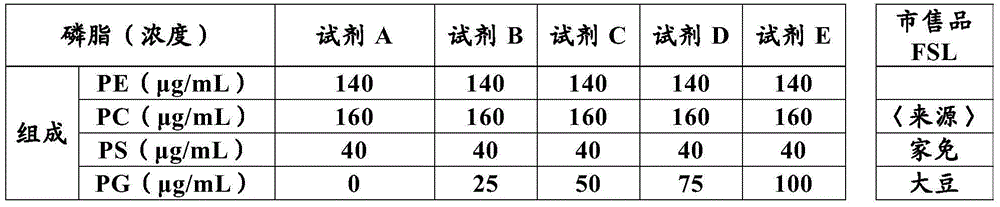
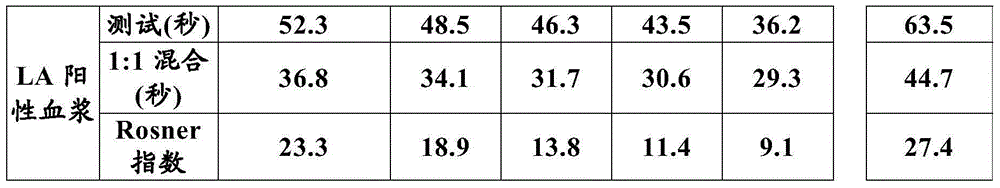
The present invention provides a method for measuring activated partial thromboplastin time. The method comprises: a first mixing step of mixing a blood plasma with a first reagent, wherein the first reagent comprises an activator and phosphatidylglycerol at a concentration equal to or greater than 25 µg / mL; a second mixing step of mixing a sample obtained in the first mixing step with a second reagent comprising a calcium salt; and a step of measuring coagulation time of the sample obtained in the second mixing step.
Owner:SYSMEX CORP
Oxaliplatin liposome, preparation method and application thereof
InactiveCN101897668AHigh encapsulation efficiencyHigh drug loadingAntineoplastic agentsLiposomal deliveryCholesterolLiposomal Oxaliplatin
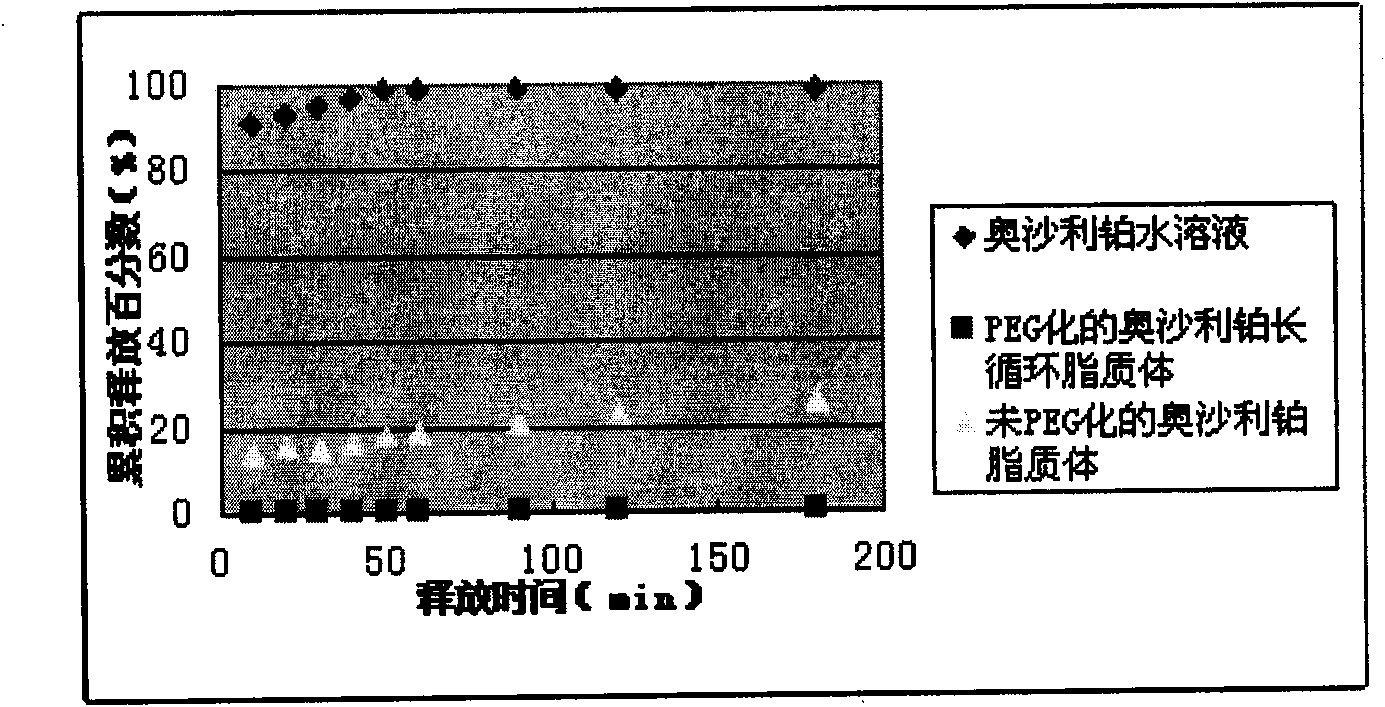
The invention discloses an oxaliplatin liposome, a preparation method and application thereof. The oxaliplatin liposome contains oxaliplatin, phospholipid and cholesterol, wherein, based on the total weight of the phospholipid, the phospholipid comprises the following components in percentage by weight: 75 to 90 percent of phosphatidylcholine (PC), and 10 to 25 percent of phosphatidyl glycerol (PG) and phosphatidic acid (PA); the weight ratio of the oxaliplatin to the phospholipid is 1:3-20, and the weight ratio of the cholesterol to the phospholipid is 1:2-10.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Lipids containing omega-3 and omega-6 fatty acids
InactiveUS20080085319A1Enhance memoryReduce stressBiocideEdible oils/fats ingredientsLipid formationAcyl group
A lipid preparation including a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidyl-inositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The preparation possesses an improved bioactivity, and is useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes.
Owner:ENZYMOTEC
Lipoidosis of Chinese herbal medicine alkaloid and its preparation
InactiveCN1446534AHigh encapsulation efficiencyReduce leakageAmine active ingredientsLiposomal deliveryCholesterolMedicine
A Chinese-medicinal alkaloid lipoid in the form of injection, orally applied medicine, syrup, drops, inhalation, or exteriorlly applied ointment for suppressing tumor contains Chinese-medicinal alkaloid (0.1-2 wt.%), phosphatide (lecithin, distearyl phosphatidylglycerol, etc). (0.5-10 wt.%) and cholesterol (0-5 wt.%). Its advantages are high active concentration in blood, and high curative effect.
Owner:SHENYANG PHARMA UNIVERSITY
Prasugrel liposome solid preparation
InactiveCN101816640AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsPill deliveryYolkSide effect
The invention provides a Prasugrel liposome solid preparation, which is characterized by being prepared by the following main components in parts by weight: 1 part of Prasugrel, 2-18 parts of yolk phosphatidylglycerol, 0.6-9 parts of cholesterol, 1.2-12 parts of sodium glycyl-cholate and 4-80 parts of other pharmaceutically accepted excipients. The Prasugrel liposome prepared by the invention has high entrapment rate and little side effect, improves stability of aqueous solubility thereof greatly and is favor of dissolving medicinal preparation out.
Owner:HAINAN LINGKANG PHARMA CO LTD
Lipids containing omega-3 and omega-6 fatty acids
InactiveUS20080085320A1Enhance memoryReduce stressOrganic active ingredientsBiocideLipid formationAcyl group
Owner:ENZYMOTEC
Method and reagent kit for measuring activated partial thromboplastin time
InactiveUS20140295470A1Less LA-induced impactOccurrence of preventedMicrobiological testing/measurementBiological material analysisBlood plasmaReagent
The present invention provides a method for measuring activated partial thromboplastin time. The method comprises: a first mixing step of mixing a blood plasma with a first reagent, wherein the first reagent comprises an activator and phosphatidylglycerol at a concentration equal to or greater than 25 μg / mL; a second mixing step of mixing a sample obtained in the first mixing step with a second reagent comprising a calcium salt; and a step of measuring coagulation time of the sample obtained in the second mixing step.
Owner:SYSMEX CORP
Protective effect of DMPC, DMPG, DMPC/DMPG, LysoPG and LysoPC against drugs that cause channelopathies
ActiveUS10238602B2Sufficient solubilityPreventing one or more cardiac channelopathiesKetone active ingredientsDisease diagnosisOral medicationActive agent
Owner:SIGNPATH PHARMA INC
Breviscapinum long-circulating nanoliposome and its preparation method
InactiveCN1843368ASimple preparation processImprove in vivo and in vitro stabilityOrganic active ingredientsLiposomal deliveryYolkFreeze-drying
The invention discloses a breviscapinum long-circulating nanoliposome and its preparation method, wherein the constituents include breviscapine, phosphatide, polyethylene glycol derived phosphatide (PEG2000-DSPE) and cholestrin by a molar ratio of 55:25-55:3-7:2.2-18.3, the dose type of the medicinal preparation includes injections, freeze-dried powder injections and sprays. The phospholipids can be soya bean lecithin, yolk phosphatidy icholine, hydrogenated soya bean lecithin, hydrogenated yolk lecithin, di-palmityl phosphatidyl choline or DPOG.
Owner:TSINGHUA UNIV
Injection alprostadil fat emulsion and preparing method thereof
ActiveCN105012248AReduce security risksEasy to operateOrganic active ingredientsPowder deliveryFat emulsionsAlprostadil Injection
An alprostadil lipid emulsion for injection and a preparation method therefor. The lipid emulsion contains alprostadil, phosphatidylcholine, phosphatidylglycerol, oil for injection and a lyophilized protective agent. Based on 1 part by weight of the alprostadil, the content of the phosphatidylcholine is 1200 to 4000 parts by weight, the content of the phosphatidylglycerol is 12 to 120 parts by weight, the content of the oil for injection is 2000 to 20000 parts by weight, and the content of the lyophilized protective agent is 16000 to 60000 parts by weight.
Owner:内蒙古多肽科技有限公司
Liposomal Vaccine Adjuvants and Methods of Making and Using Same
ActiveUS20140341974A1Stable temperatureBacterial antigen ingredientsSnake antigen ingredientsLipid formationCholesterol
A vaccine adjuvant composition comprising: a lipid selected from the group consisting of: dipalmitoyl phosphatidicholine (DPPC), dipalmitoyl phosphatidylglycerol (DPPG), dioleoyl phosphatidylcholine (DOPC), and cholesterol and containing a positively or negatively charged lipid with associated / entrapped protein antigen.
Owner:ENGIMATA
Macromolecular phosphatidylglycerol nanoliposome, and preparation methods and applications thereof
InactiveCN103565744AGood dispersionSuperparamagneticGenetic material ingredientsMacromolecular non-active ingredientsLipid formationEvaporation
The invention discloses a macromolecular phosphatidylglycerol nanoliposome, and preparation methods and applications thereof, and belongs to liposome preparation and application technologies. The phosphatidylglycerol nanoliposome is vesicles having a bilayer lipid membrane structure prepared by using phosphatidylglycerol chitosan to substitute phosphatide as a primary membrane material. The phosphatidylglycerol chitosan has a weight average molecular weight of above 2000, can be dissolved in organic solvents comprising chloroform, ethanol and the like, and contains a carboxyl or amino group. The preparation methods of the nanoliposome adopt common liposome preparation methods comprising a membrane dispersion method and an inverted evaporation method. The surface of like liposomes can be connected with a plurality of targeting preparations, and can simultaneously and respectively entrap water-soluble, oil-soluble or amphiphilic substances comprising medicines, proteins, genes, nutrients, vitamins, magnetic particles, quantum dots and the like. The methods have the advantages of high entrapment rate, simple operation, strong applicability and low cost, and the dressed macromolecular liposome has the advantages of uniform particle size distribution, adjustability in a range of 10-5000nm, stable system and strong slow release function.
Owner:SHANGHAI SENNA IND CO LTD
Protective effect of dmpc, dmpg, dmpc/dmpg, lysopg and lysopc against drugs that cause channelopathies
ActiveUS20180055770A1Sufficient solubilityPreventing one or more cardiac channelopathiesKetone active ingredientsDisease diagnosisOral medicationActive agent
The present invention includes compositions and methods for preventing one or more cardiac channelopathies or conditions resulting from irregularities or alterations in cardiac patterns caused by an active agent or a drug in a human or animal subject comprising: an amount of a phosphatidylglycerol adapted for oral administration effective to reduce or prevent one or more cardiac channelopathies or conditions resulting from irregularities or alterations in cardiac patterns caused by the active agent or drug, and one or more organoleptic, thixotropic, or both organoleptic and thixotropic agents.
Owner:SIGNPATH PHARMA INC
Propofol-containing fat emulsions
InactiveUS20060134145A1High emulsion stabilityAvoid painBiocideNervous disorderLocal anaestheticFat emulsion
The present invention provides a propofol-containing fat emulsion comprising propofol, an oily component, and an emulsifier, and further comprising a predetermined amount of a stabilizer, such as phosphatidylglycerol in which a specific fatty acid is a constituent fatty acid component. The present invention also provides a pain-relieving propofol-containing fat emulsion, which is obtained by mixing a local anaesthetic in advance with the above-described propofol-containing fat emulsion of the invention.
Owner:OTSUKA PHARM FAB INC
Glycerophospholipids containing omega-3 and omega-6 fatty acids
InactiveCN1897955AGuaranteed functionImprove and treat ADHDOrganic active ingredientsSenses disorderGlycerolOmega-6 fatty acid
Disclosed is a lipid preparation comprising a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidyl-inositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The disclosed preparations possess an improved bioactivity, and are useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes, for example ADHD.
Owner:ENZYMOTEC
Multiple sclerosis treatment
InactiveUS20060105032A1Reduce inflammationUseful in prophylaxis against developmentOrganic active ingredientsNervous disorderMedicineGlycerol
Symptoms, including biochemical correlates, of multiple sclerosis (MS) in a mammal are beneficially affected by administering to the mammal small doses of bodies, such as liposomes, of a size resembling that of mammalian cells, the bodies having phosphate glycerol head groups presented exteriorly on their surfaces. Preferred are liposomes comprised of 50-100% phosphatidylglycerol, with the phospho glycerol headgroups thereof exteriorly presented.
Owner:VASOGEN IRELAND LTD
Phospholipid detection method and application
PendingCN110007032AQuality improvementEasy to separateComponent separationRetention timeAcetonitrile
The invention discloses a phospholipid detection method and application. A phospholipid detection method is eluted with acetonitrile and ammonium acetate, and a phospholipid detection eluent comprisesacetonitrile and ammonium acetate, wherein by volume percentage, the acetonitrile accounts for 50% to 95%, and the ammonium acetate accounts for 5% to 50%. An application of the phospholipid detection method is used for detection of phosphatidylcholine, phosphatidylglycerol, phosphatidylserine, phosphatidyl ethanolamine, glycerophosphatidic acid, sphingomyelin and lysophosphatidylcholine. The analysis method provided by the invention has the advantages of simple operation, large number, good peak shape and short retention time of phospholipids analyzed, convenience and speed, reliability andpracticality.
Owner:JIANGNAN UNIV
Disodium clodronate liposome injection
InactiveCN103040863AInorganic phosphorous active ingredientsOil/fats/waxes non-active ingredientsSide effectCholesterol
The invention discloses disodium clodronate liposome injection and a preparation method thereof. The disodium clodronate liposome injection with excellent quality is made of disodium clodronate, distearoyl phosphatidyl glycerol, cholesterol, polyoxyethylene (40) hydrogenated castor oil according to specific weight ratio. Compared with the existing preparation, the injection has the advantages that the stability and the bioavailability of the injection are greatly improved, drug release is stable, the quality of injection products is improved, the toxic and side effect is reduced, and the curative effect is more obvious.
Owner:海南路易丹尼生物科技有限公司
Method for quickly extracting and separating lipid components in peony seeds
ActiveCN105296142AQuick how-toEasy to operateFatty-oils/fats refiningFatty-oils/fats productionSolid phase extractionSilica gel
The invention discloses a method for quickly extracting and separating lipid components in peony seeds. The method is characterized by loading a total lipid sample to a solid-phase extraction silica gel column through extracting total lipids in the peony seeds; then respectively carrying out elution by using chloroform, acetone and methanol as elution solutions to obtain neutral lipids, glycolipids and phospholipids; then respectively and further expanding the neutral lipids, the glycolipids and the phospholipids, separating triacylglycerol from the neutral lipids, separating MGDG (Monogalactosyl Diacylglycerol), DGDG (Digalactosyl Diglyceride) and SQDG (Sulfoquinovosyl Diacylglycerol) from the glycolipids, and separating phosphatidylethanolamine, phosphatidylcholine, phosphatidic acid, phosphatidylserine and phosphatidylglycerol from the phospholipids. According to the method disclosed by the invention, nine kinds of lipid components in grease of the peony seeds can be effectively separated, the variety of the obtained lipid components is rich, and fatty acid can be obtained after carrying out methyl esterification on the total lipids, the neutral lipids, the glycolipids and the phospholipids; the method is quick and simple, the cost is low, and a feasible analysis method is provided for fundamental researches.
Owner:SHANGHAI CHENSHAN BOTANICAL GARDEN +1
Ornithine aspartate liposome injection
InactiveCN101961315ASafety proofImprove stabilityOrganic active ingredientsNervous disorderSide effectMedicine
The invention discloses an ornithine aspartate liposome injection. The injection mainly comprises the following active ingredients: ornithine aspartate, dipalmitoyl phosphatidyl glycerol, cholesterol and antioxidant in part by weight of 1:3-20:1.8-12:0.05-0.2. The stability of the ornithine aspartate is improved; the valid period is prolonged; and the quality of the product is guaranteed. Because the liposome carriers, namely the dipalmitoyl phosphatidyl glycerol and the cholesterol, are degradable in bodies, the bioavailability is improved and the toxic and side effects are reduced. The injection has the advantages of simple production process, easy operation, low cost, high yield and suitability for mass production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Sialic acid derivative modified ibrutinib (IBR) nanocomposite and preparation method thereof
ActiveCN111973570ASolve the problem of no injectable formGood water solubilityOrganic active ingredientsAntipyreticTumor therapyEfficacy
The invention relate to a high-density sialic acid modified ibrutinib (IBR) nanocomposite and a preparation method thereof. The sialic acid modified IBR nanocomposites comprises IBR, phospholipid anda sialic acid derivative, wherein the molar ratio of IBR to phospholipid is 1:0.5 to 1:5, and the molar ratio of the sialic acid derivative to phospholipid is 1:19 to 1:1. The phospholipid is one or more of phosphatidylglycerol, phosphatidic acid, phosphatidylserine, phosphatidylinositol and cardiolipin. The nanocomposite can obviously improve the drug dissolution performance, provides an injectable product for reducing the daily dose of drugs, and can be used for clinical critical patients to solve the problem that the patients cannot take drugs orally. Based on the theory of ''immunopharmaceuticals'', the sialic acid is used to modify the composite, accumulation of the preparation in tumor site and the inhibitory effect on tumor microenvironment stromal cells are increased, so that the tumor therapeutic spectrum of IBR is expanded, and the drug efficacy is improved.
Owner:SHENYANG PHARMA UNIVERSITY
Biomarkers for epicardial adipose tissue
ActiveUS20160305968A1Fast quantificationFast reliable identificationOmicsDisease diagnosisLipid formationEpicardial adipose tissue
A method for predicting the level of epicardial adipose tissue (EAT) in a subject comprising: (a) determining a level of one or more lipid biomarkers in a sample from the subject, wherein the biomarkers are selected from the following: (i) phosphatidylcholine (PC) [16:1 / 16:1] (ii) diacylglycerol (DAG) [42:8] (iii) phosphatidylethanolamine-ether (PE-O) [32:5] (iv) phosphatidylcholine (PC) [18:0 / 22:5] (v) phosphatidylinositol (PI) [18:0 / 16:1] (vi) phosphatidylglycerol (PG) [20:3 / 20:3] (vii) phosphatidylglycerol (PG) [22:5 / 18:1] (b) comparing the levels of the biomarkers in the sample to reference values; wherein the levels of the biomarkers in the sample compared to the reference values are indicative of levels of EAT fat in the subject.
Owner:SOC DES PROD NESTLE SA
Liposomal vaccine adjuvants and methods of making and using same
ActiveUS9603799B2Stable temperatureBacterial antigen ingredientsVertebrate antigen ingredientsMedicineCholesterol
Owner:ENGIMATA
Molecular delivery vehicle
An isolated caveolin containing vesicle comprising a caveolin protein and at least one lipid, wherein at least about 30% of the at least one lipid is selected from phosphatidylethanolamine and phosphatidylglycerol is disclosed. Also disclosed is a method of making an isolated caveolin containing vesicle, an isolated caveolin containing vesicle comprising a recombinant caveolin protein, an isolated caveolin containing delivery vesicle, a method of making an isolated caveolin containing delivery vesicle and a method of treatment of a disease or condition by delivery of a molecule using the isolated caveolin containing delivery vesicle.
Owner:THE UNIV OF QUEENSLAND
Treatment of inflammation and vascular abnormalities of the eye
InactiveUS20070071805A1InflammationReduce inflammationOrganic active ingredientsBiocidePhosphateGlycerol
Inflammation and vascular abnormalities of the eye, including those related to ischemia, its prophylaxis and its alleviation, are treated by administration to the mammal of small amounts of phosphate-glycerol group presenting bodies such as phosphatidylglycerol liposomes.
Owner:VASOGEN IRELAND LTD
Clevidipine butyrate liquid liposome preparation
ActiveCN102335134AAvoid time costReduce economic costsOrganic active ingredientsPharmaceutical non-active ingredientsSterolClevidipine
The invention discloses a clevidipine butyrate liposome preparation and a preparation method thereof. The preparation includes 0.05wt%(wt%)-0.1%(wt%) of clevidipine butyrate, 40%(wt%) -70%(wt%) of phosphatidylcholine, 10%(wt%)-40%(wt%) of phosphatidyl glycerol, 10%(wt%)-30%(wt%) of sterol, and 0.55%(wt%)-3.3%(wt%) of stabilizer. The stabilizer contains a component A and a component B, wherein the component A is selected from one or more of oleic acid, sodium oleate, linoleic acid and sodium linoleate; and the component B is selected from one or more of vitamin E, coenzyme Q10, propyl gallate and ascorbyl palmitate. The liposome prepared by the method is in the form of liquid, and can be stored for a long time, therefore not only the potential safety hazard due to use of middle-chain and long-chain triglyceride in the traditional clevidipine butyrate emulsion is thoroughly solved, but also the freeze-drying operation in the traditional liposome preparation is omitted and the production cost is reduced; moreover, the liposome preparation is convenient for clinical application, overcomes the defect of uneven quality of redissolved freeze-drying liposome preparation and is beneficial to increase of the compliance and the medication safety of a patient.
Owner:BEIJING TIDE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com