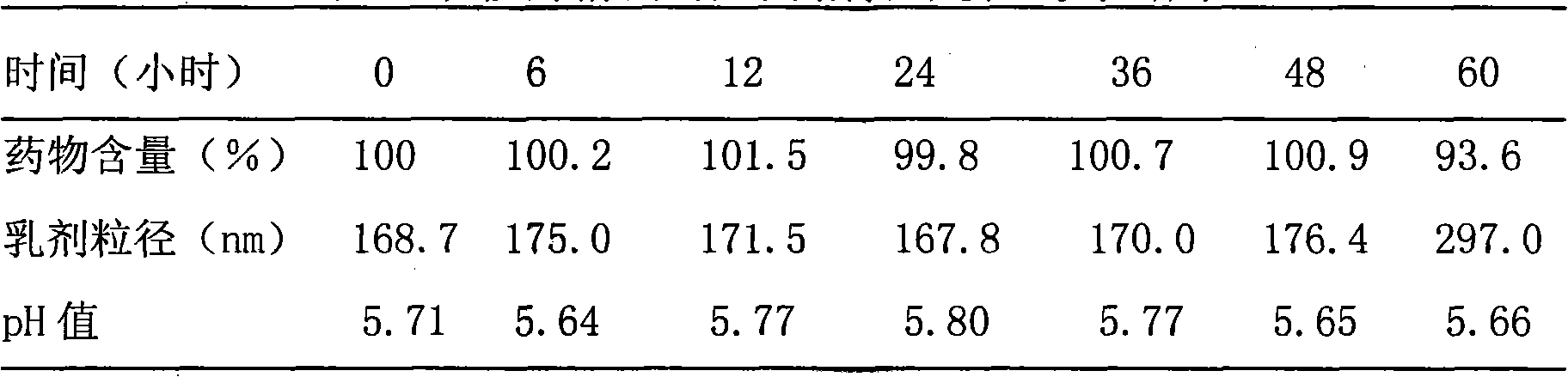
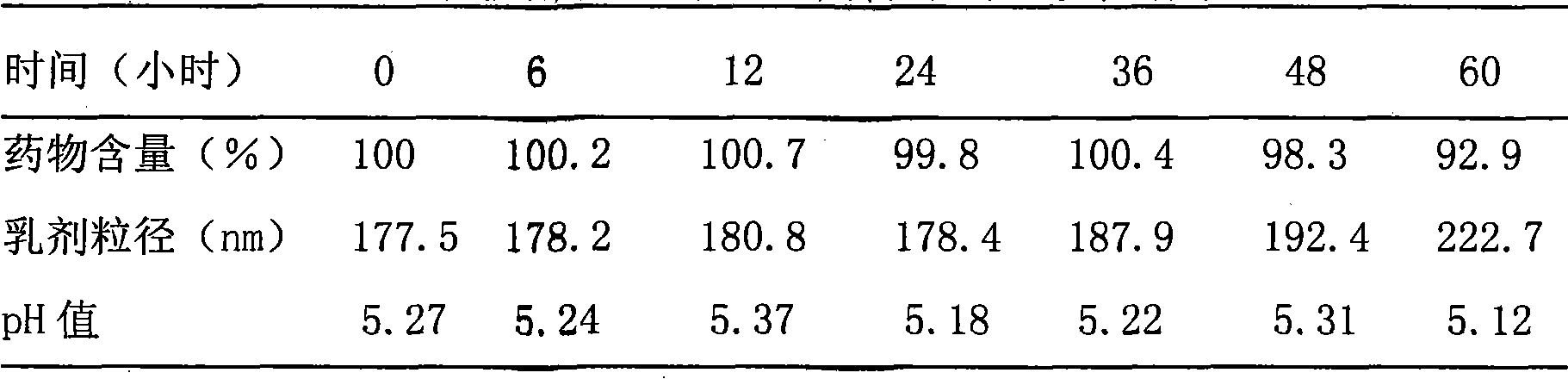
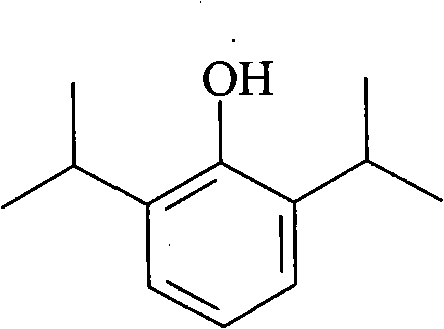
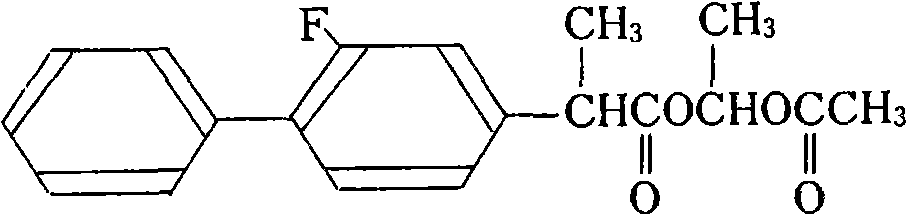
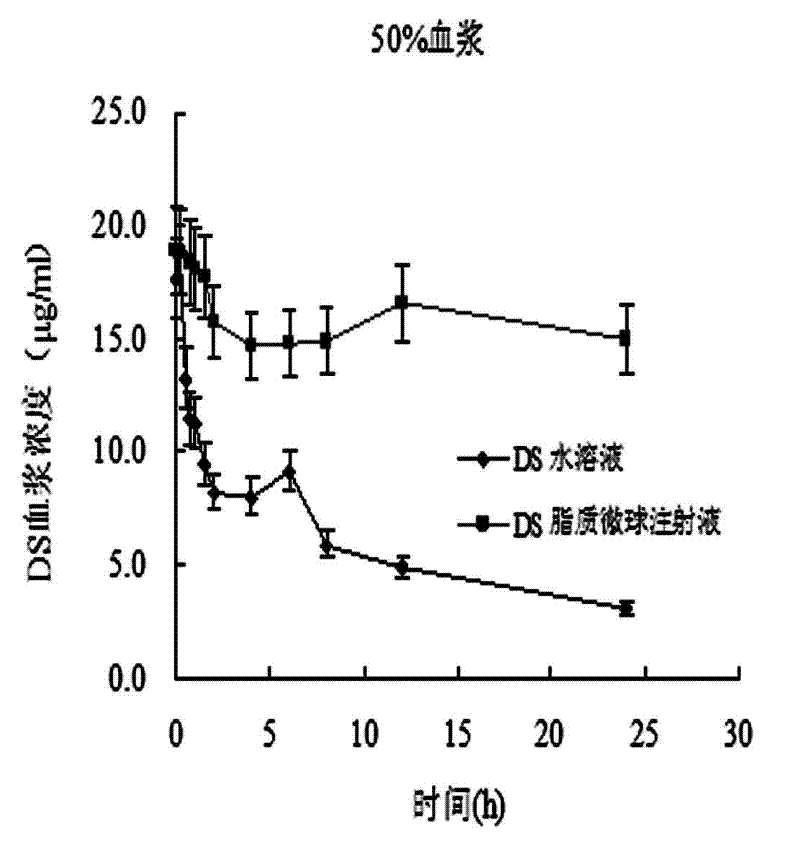
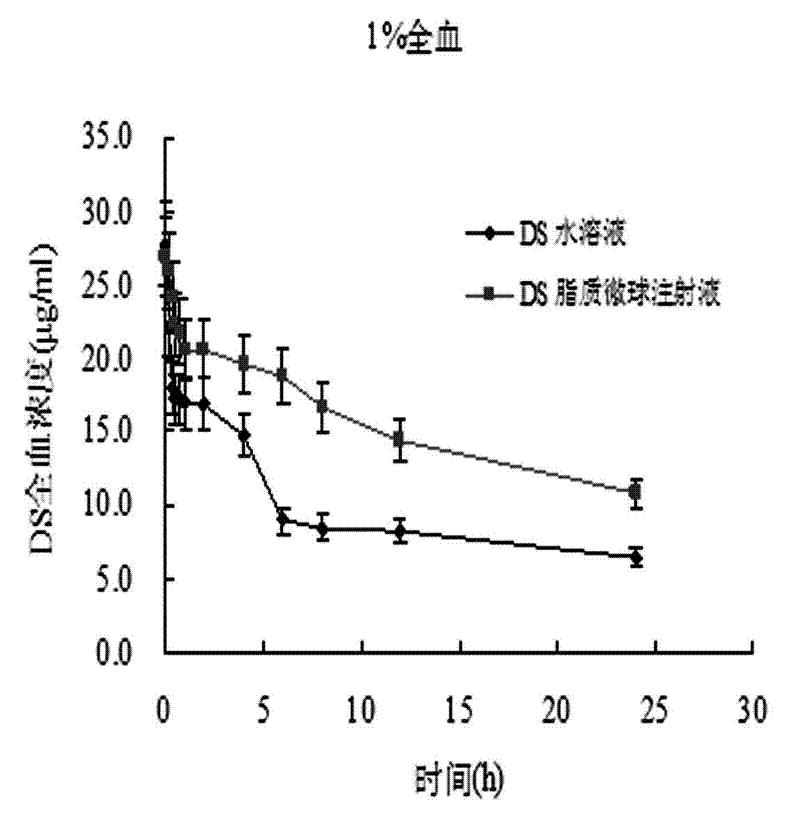
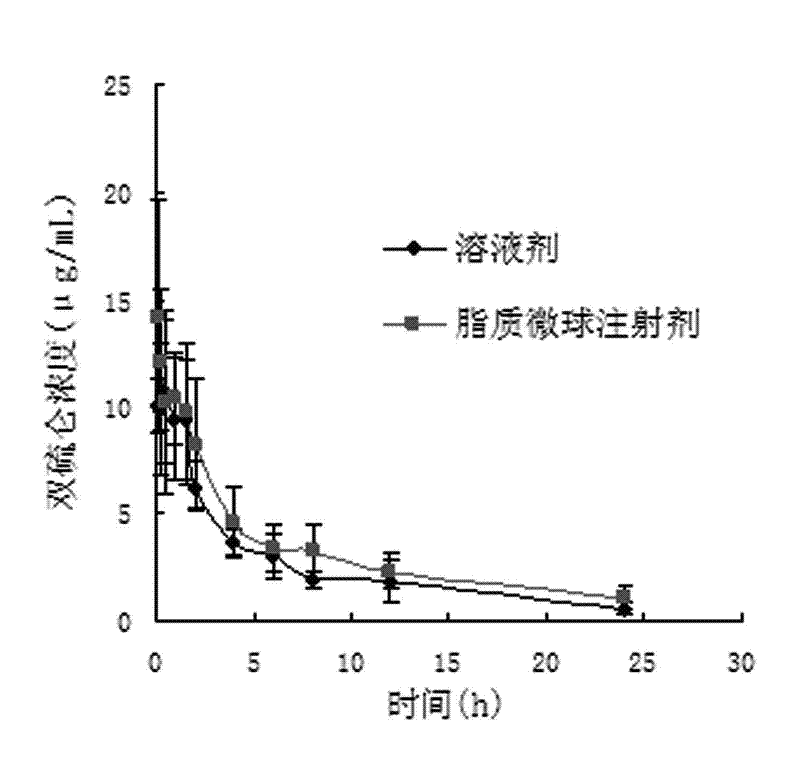
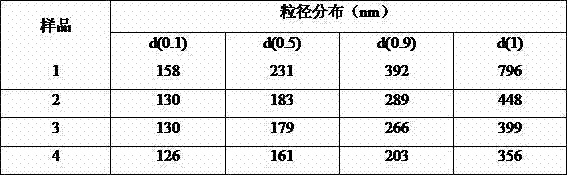
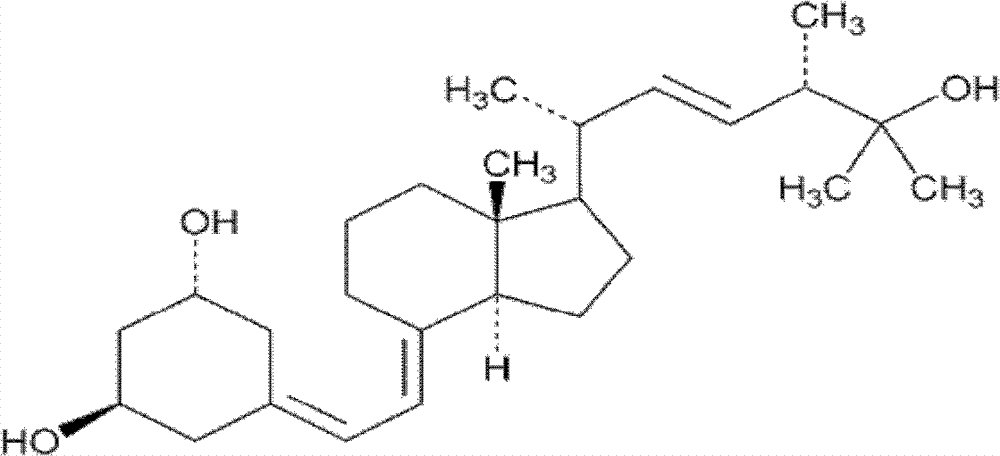
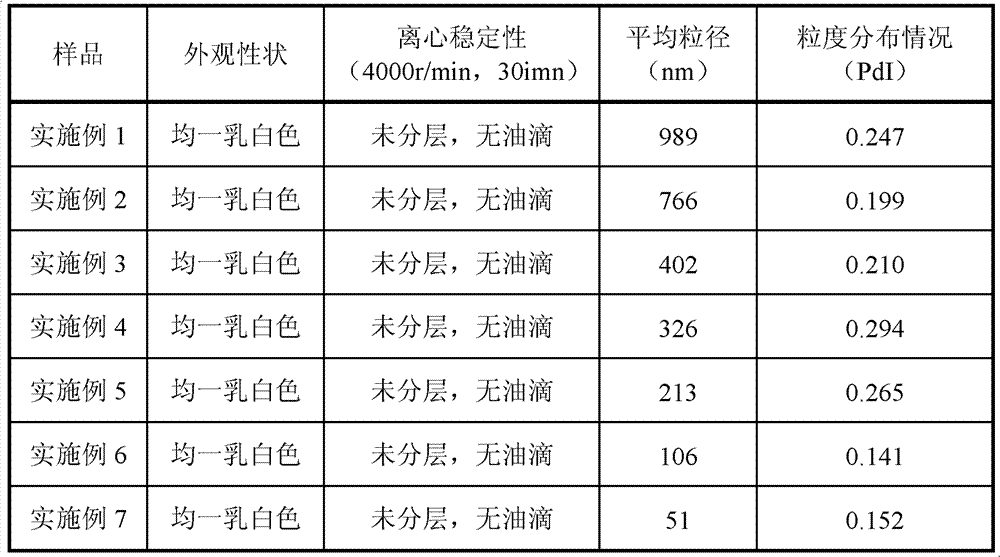
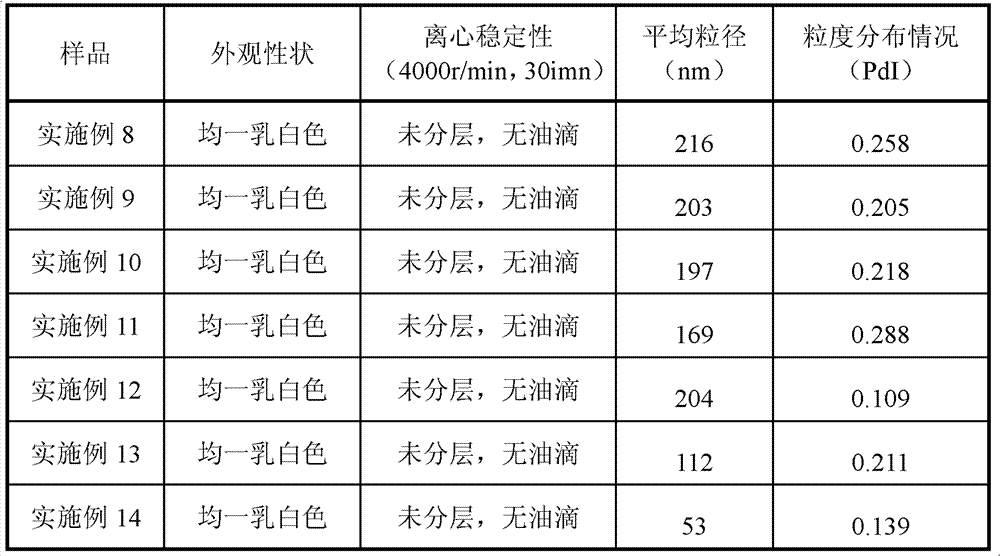
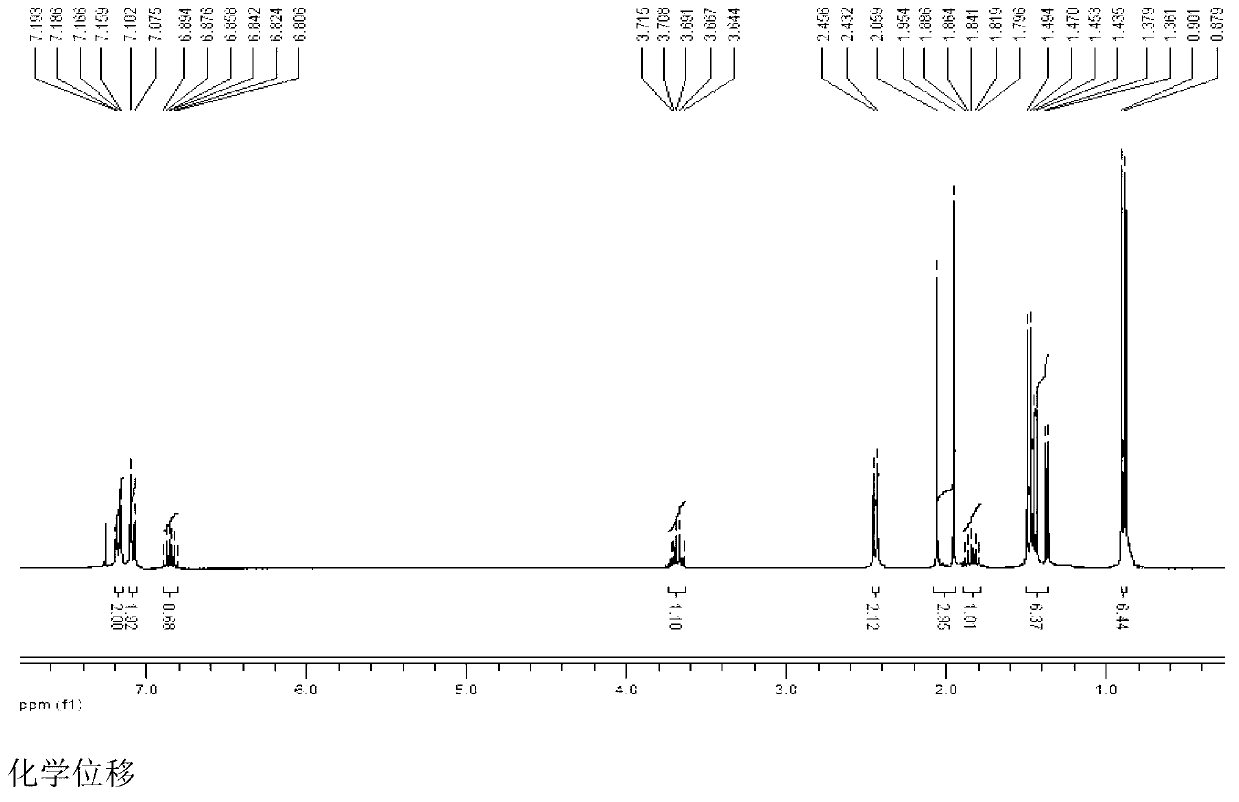
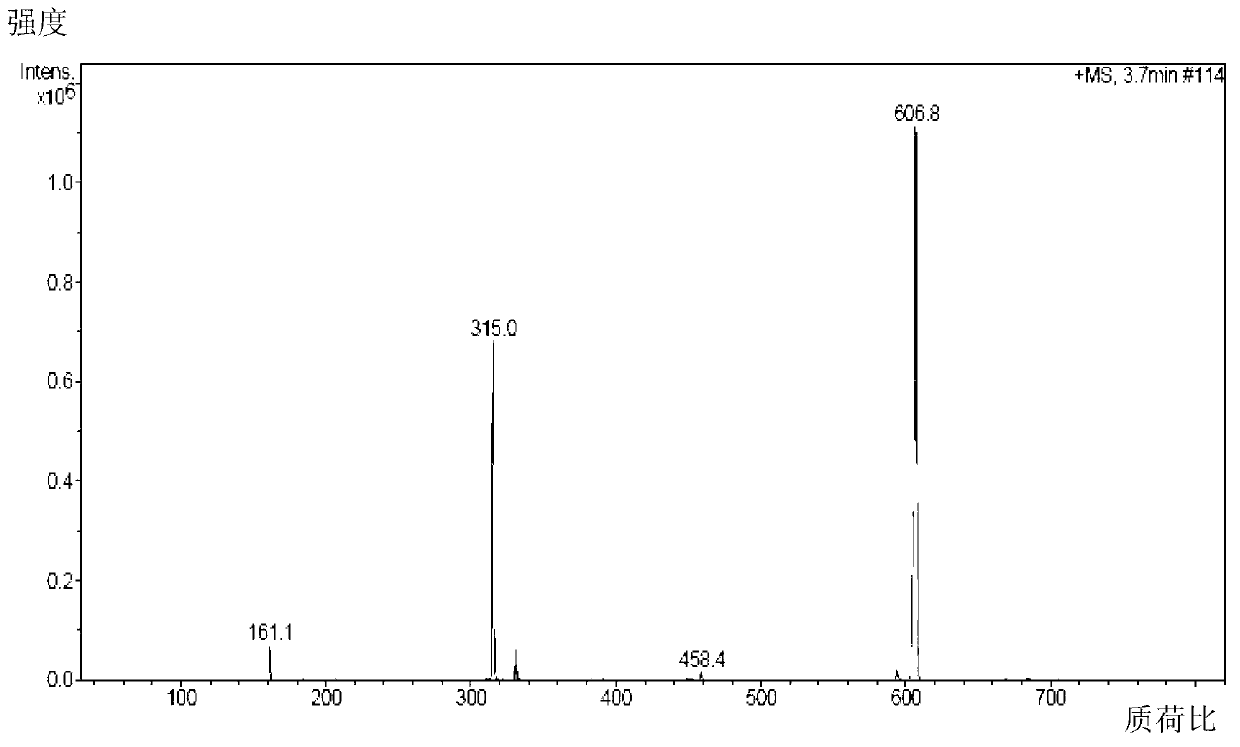
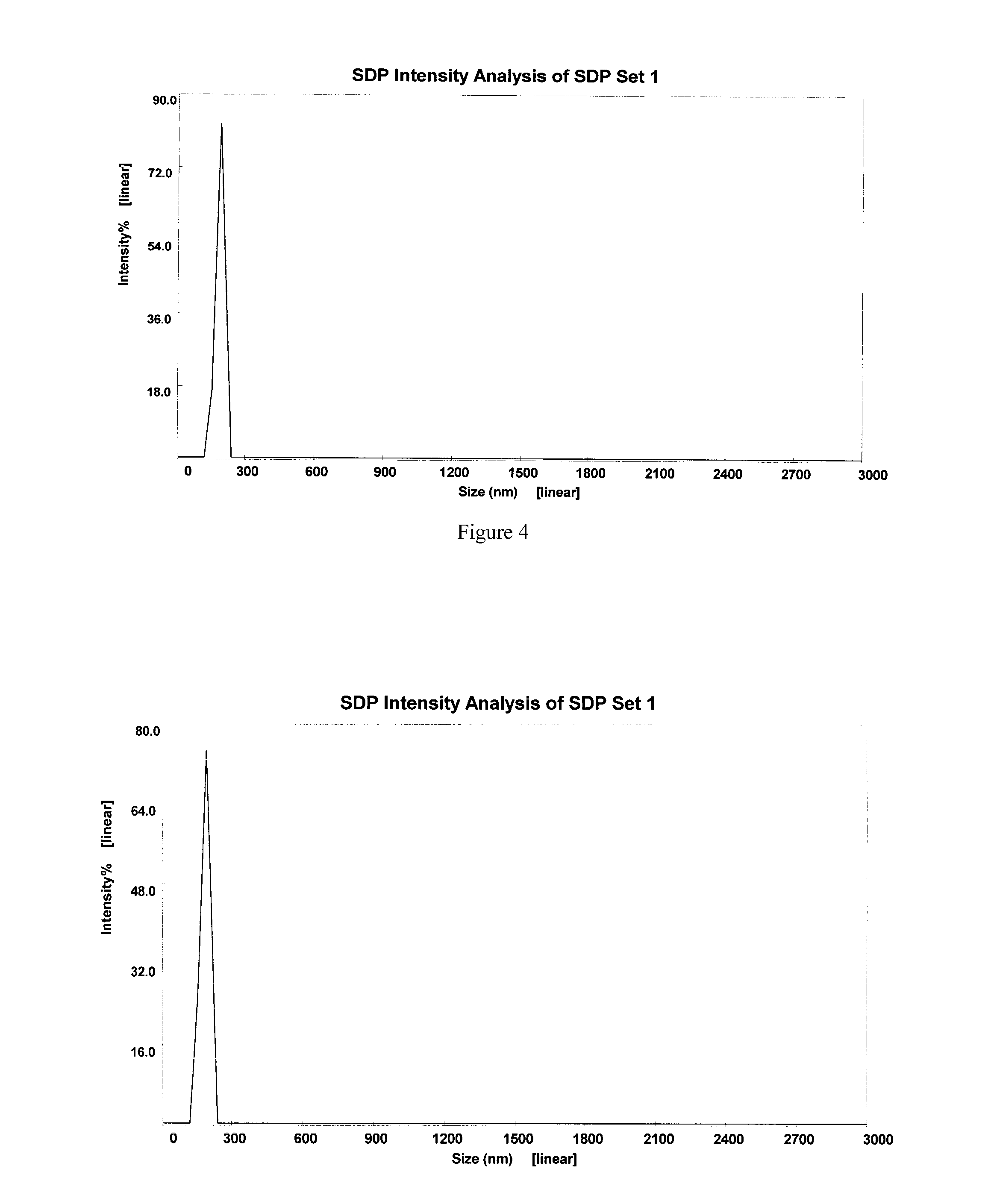
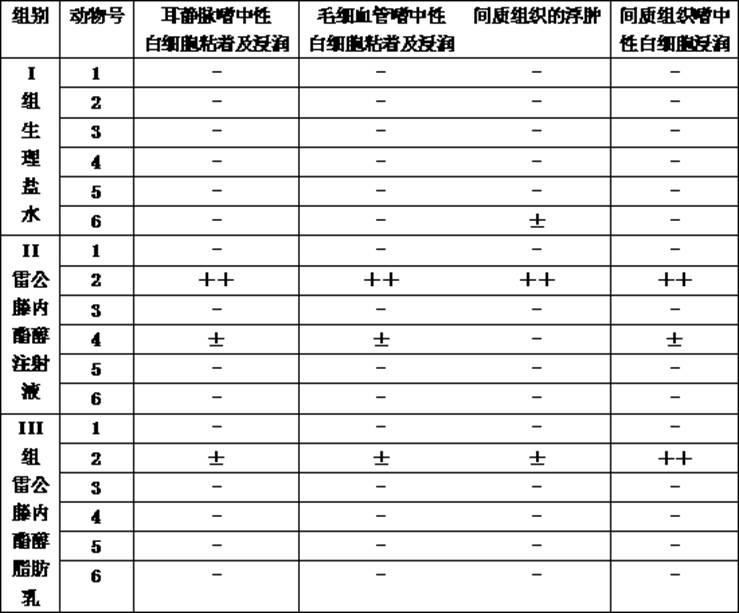
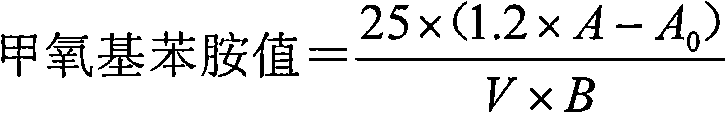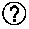Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
245 results about "Fat emulsions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
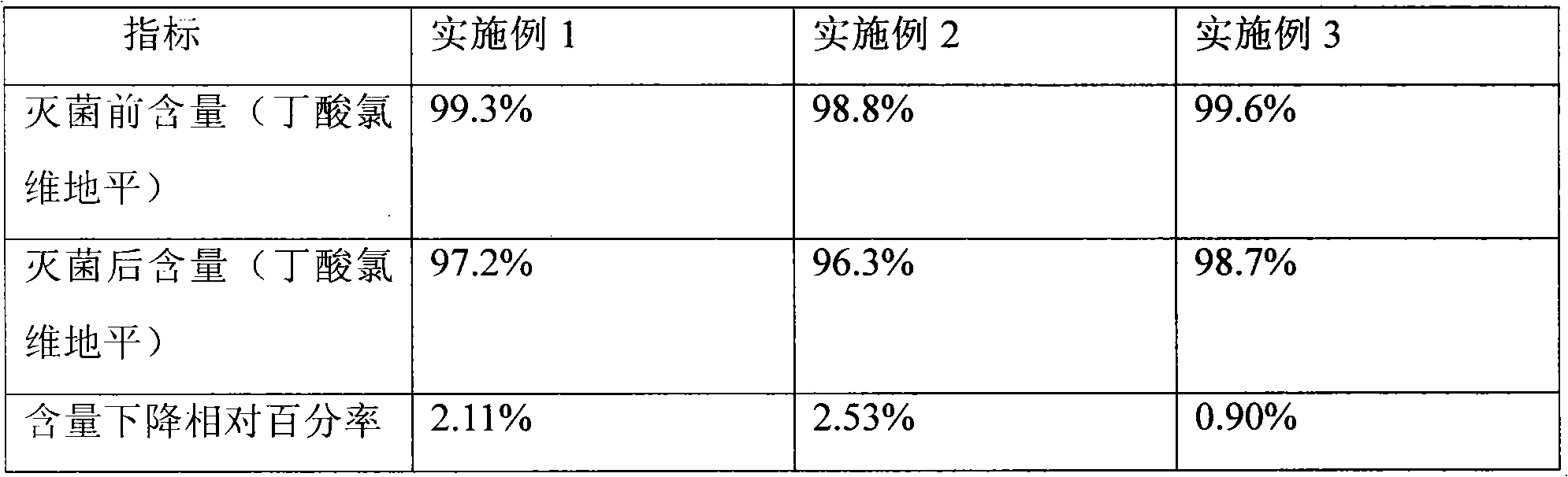
Fat emulsion injection of 'Xingnaojing' and its preparing process
InactiveCN1628779AHigh purityFully extractedMammal material medical ingredientsEmulsion deliveryHas active ingredientEmulsion
Disclosed is a fat emulsion injection of 'Xingnaojing' and its preparing process, which comprises using supercritical extraction technology and macroscopic adsorption resin technology for isolating and purifying the water soluble and fat soluble parts in the medicinal materials including musk, cape jasmine, and curcuma root, finally utilizing emulsion technology to obtain the end product.
Owner:JIANGSU QINGJIANG PHARMA
Fat emulsions
InactiveUS6277430B1Edible oils/fats ingredientsEdible oils/fats with reduced calorie/fat contentAlcoholPhospholipid
Novel fat-continous emulsion, containing a blend with emulsifying properties is obtained by the blending of: 0-90% of a partial glyceride (=A); 0-80% of a phospholipid (=B); 0.01-99.98% of a long chain alcohol having >20 C-atoms in the alcohol chain, while the amount of (A)+(B)>=0.02% and incorporating this blend in a fat-continuous emulsion.
Owner:UNILEVER PATENT HLDG BV
Fat emulsion injection liquid containing soybean oil, medium chain triglyceride, olive oil and fish oil and method for preparing the same
InactiveCN1965806AAvoid exhaustionImprove the outcome of standard clinical treatmentsOrganic active ingredientsMetabolism disorderYolkFish oil
The invention relates to an intralipid injection which contains soya oil, middle chain triglyceride, olive oil, and fish oil, wherein it comprises 48-72g.L soya oil, 48-72g / L middle chain triglyceride, 40-60g / L olive oil, and 24-36g / L fish oil, 108-162 mg / L dl-alpha-tocofecol, 9.6-14.4g / L lipovitellin, 22.5-27.5g / L pure glycerin, 240-360mg / L sodium oleate, 18-22mg / L caustic soda, and 1L injection water. The ratio between w-6 and w-3 aliphatic acid is 3.0-2.2:1. The invention optimizes and balances the aliphatic acid mode.
Owner:费森尤斯卡比华瑞制药有限公司
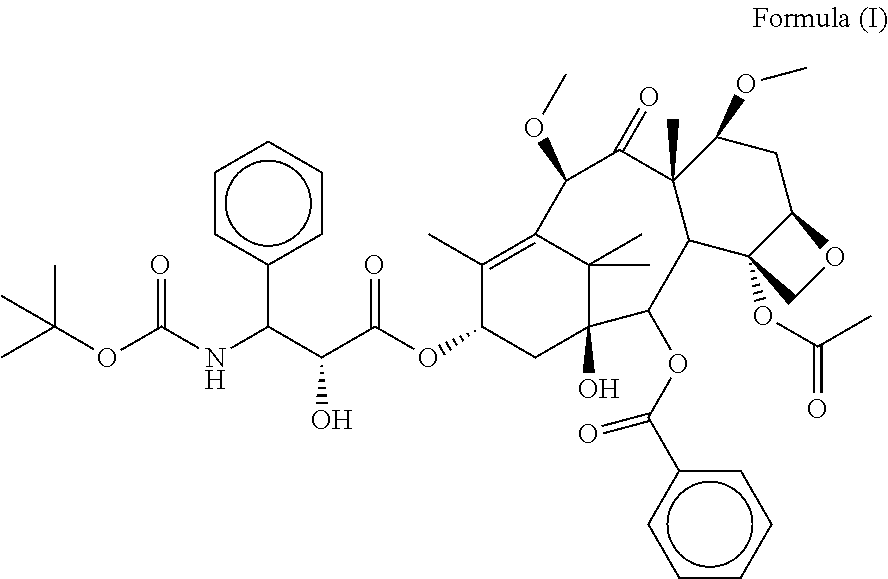
Taxanes medicine preparation for intravenous injection and preparation method thereof
ActiveCN101288642AGood biocompatibilityHigh tolerance in vivoOrganic active ingredientsSolution deliveryDrugs solutionDocetaxel
The invention relates to the technical field of medicine, which is a preparation of a taxane drug for intravenous drug delivery, consisting of two parts of drug solution and an emulsion. The drug solution is composed of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein, the solvent for injection is an organic solvent; the emulsion comprises a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When in use, the drug solution can be added and evenly mixed in the emulsion for direct intravenous drip according to the clinical drug dosage and can also be firstly added in the emulsion with the volume that is not less than 5 times of the volume of the drug solution according to the clinical drug dosage and then added with a certain amount of physiological saline or glucose injection for intravenous drip. The preparation of the invention does not contain solubilizer and has the advantages of little toxicity, safety, effectivity, stability and economy. The fat emulsion can also be taken as a nutrition replenisher for a patient, thus achieving better treatment effects. The physiological saline or the glucose injection can also replace a certain amount of the emulsion, so the storage and the transportation are convenient, and the preparation is more economical.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Propofol fat emulsion injection and preparation method thereof
ActiveCN102805728AImprove physical stabilityImprove stabilityNervous disorderHydroxy compound active ingredientsO-Phosphoric AcidMass-Volume Percentage
The invention discloses propofol fat emulsion injection and a preparation method thereof. The propofol fat emulsion injection comprises, by weight percent, 1% of propofol, 6-8% of soybean oil, 1.5-2.0% of soybean lecithin, 3-5% of glycerol, 0.1-0.8% of tween-80, 0.05-0.1% of phosphoric acid, appropriate amount of pH regulator and the balance of water for injection, wherein the amount of the pH regulator is enough to regulate pH of the water for injection to 7.5-8.0. The propofol fat emulsion injection improves drug delivery, decreases particle size, and improves uniformity of particle size, and accordingly stability of the injection is improved, pain during injection is lowered, and drug dependency of patients is increased.
Owner:NANJING CHIA TAI TIANQING PHARMA
Formula and preparation method of novel propofol fat emulsion preparation causing no pain and low injection stimulation
ActiveCN102085185ADissolve effectively and fullyHydroxy compound active ingredientsAnaestheticsGlycerolSugar
The invention discloses a formula and preparation method of a novel propofol fat emulsion preparation which causes no pain or can be used for obviously lowering injection stimulation and pain. The novel preparation comprises the main constituents of propofol, an emulsifier, refined soybean oil or other refined oil as an oil-soluble diluent, oleic acid or oleate with the effect of assisting emulsification, vitamin E or derivatives thereof with an antioxidation effect, an ionic complexing agent, and glycerol or micro molecular sugar as an isoosmotic adjusting agent.
Owner:XIAN LIBANG PHARMA
Flurbiprofen axetil medium-chain and long-chain fat emulsion and preparation method thereof
InactiveCN101940549AEasy to shapeSolve the real problemOrganic active ingredientsAntipyreticFat emulsionsFlurbiprofen axetil
The invention relates to a flurbiprofen axetil medium-chain and long-chain fat emulsion and a preparation method thereof. The fat emulsion comprises flurbiprofen axetil, long-chain oil for injection, medium-chain oil for injection, emulsifier, isotonic agent, pH regulator and water for injection, wherein the weight-volume percentage of the flurbiprofen axetil is 0.5-5%(w / v), the weight-volume percentage of the long-chain oil for injection is 5-10%(w / v), the weight-volume percentage of the medium-chain oil for injection is 5-10%(w / v), and the weight-volume percentage of the isotonic agent is 1-5%(w / v).
Owner:北京中海康医药科技发展有限公司
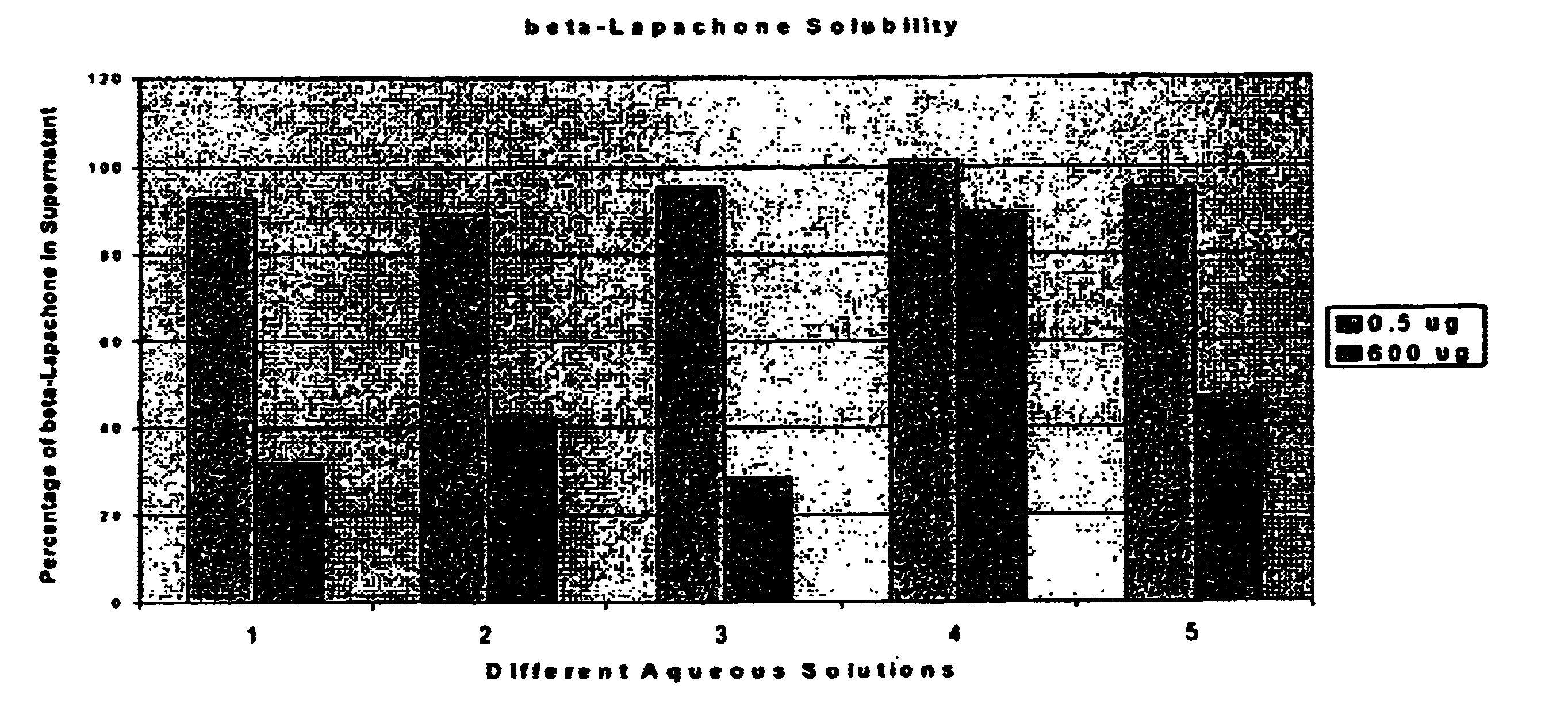
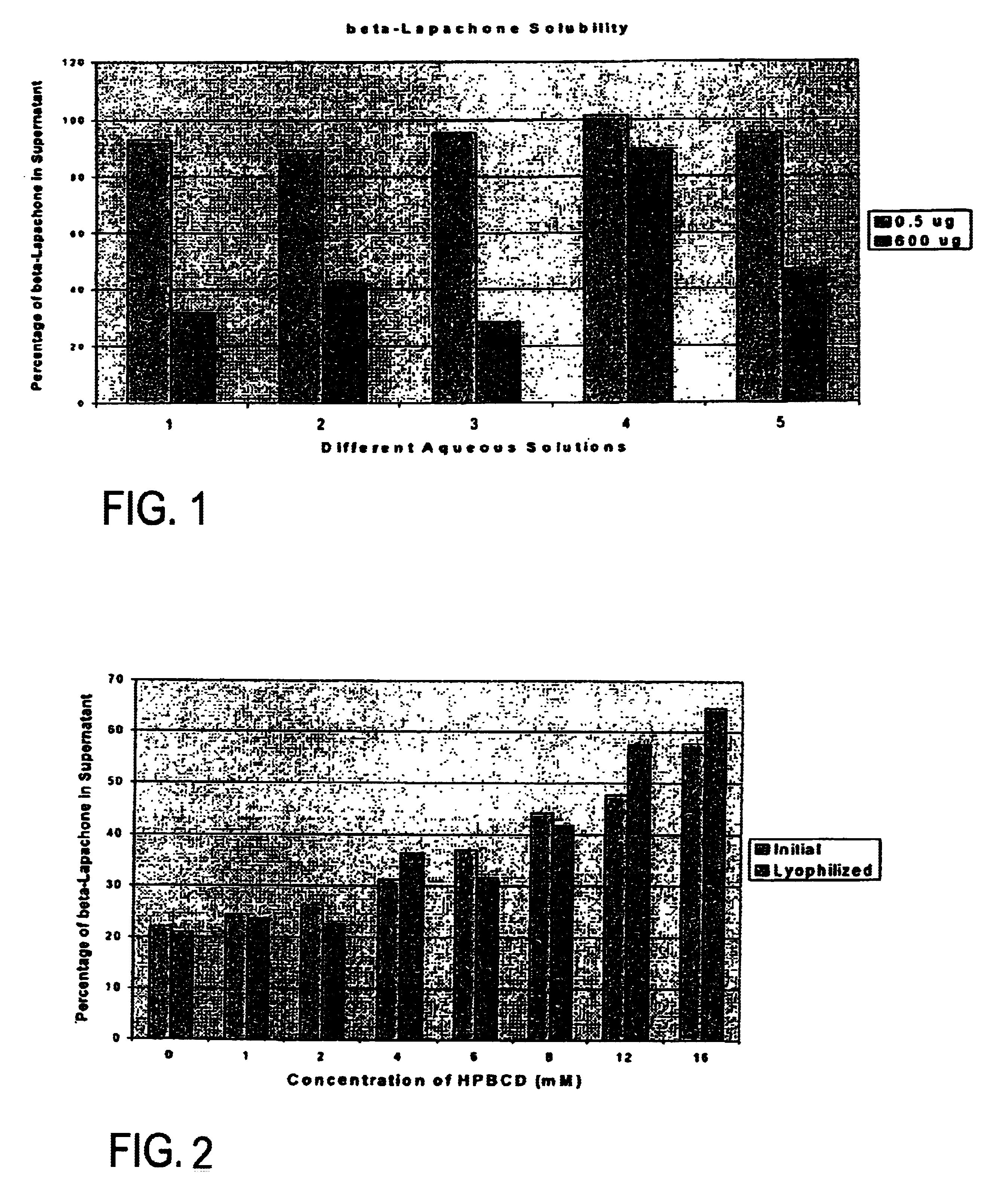
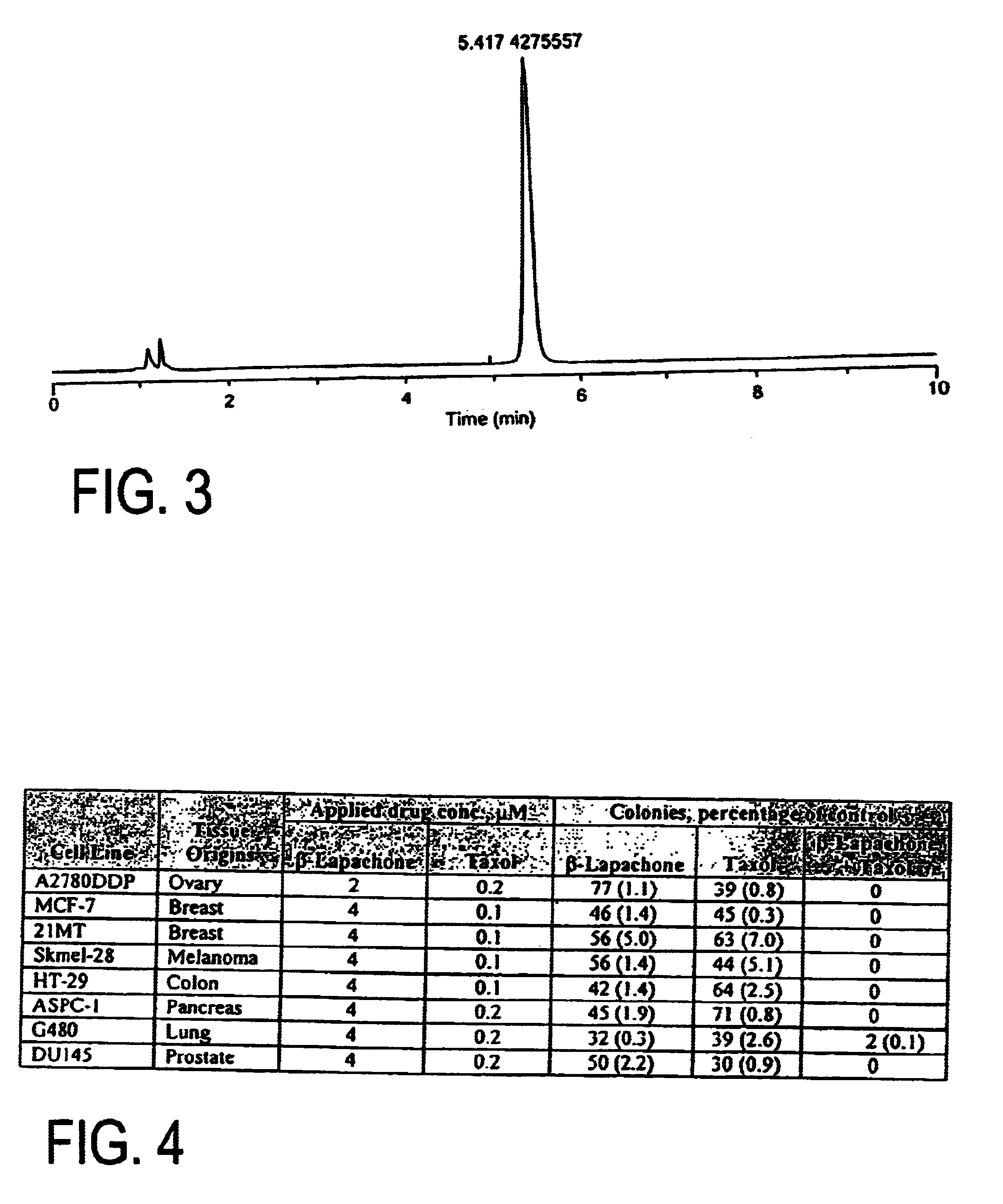
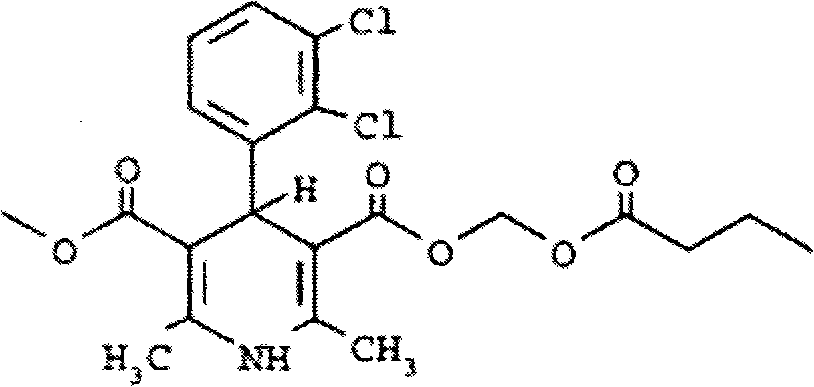
Pharmaceutical compositions containing beta-lapachone, or derivatives or analogs thereof, and methods of using same
Beta-lapachone, which is poorly soluble in most pharmaceutically acceptable solvents, has demonstrated significant antineoplastic activity against human cancer lines. The present invention overcomes this significant limitation by teaching novel pharmaceutical compositions comprising a therapeutically effective amount of Beta-lapachone, or a derivative or analog thereof, and a pharmaceutically acceptable solubilizing carrier molecule, which may be at water-solubilizing carrier molecule such as hydroxypropyl-β-cyclodextrin, or an oil-based solubilizing carrier molecule, for enhancing the solubility of Beta-lapachone in aqueous solution. The therapeutically effective amount of Beta-lapachone, or a derivative or analog thereof, may be complexed with the pharmaceutically acceptable solubilizing carrier molecule in aqueous solution. The novel pharmaceutical compositions may be administered with a second anticancer agent or in combination with radiation therapy. A formulation of Beta-lapachone or a derivative or analog thereof, complexed with a pharmaceutically acceptable solubilizing carrier molecule, wherein the complex can be freeze-dried and when subsequently reconstituted in aqueous solution is substantially soluble is also disclosed. Emulsions of Beta-Lapachone in a pharmaceutically acceptable fat emulsion vehicle are also provided. Also disclosed are methods for treating cancer by administering to a patient the novel pharmaceutical compositions and formulations. Pharmaceutical kits are also provided.
Owner:COPHARMA DEV +1
Clevidipine butyrate medium-length chain fat emulsion and preparation method thereof
InactiveCN102000027AGood quality indexSolve the real problemOrganic active ingredientsEmulsion deliveryClevidipineFat emulsions
The invention relates to a clevidipine butyrate medium-length chain fat emulsion comprising the following components: 0.05-0.1% (w / v) of clevidipine butyrate, 0.5-1.0% (w / v) of long chain oil for injection, 0.5-1.0% (w / v) of medium-chain oil for the injection, 1.0-2.0% (w / v) of emulsifying agents, 1.0-5.0% (w / v) of isotonic agents, pH regulating agents and water for the injection.
Owner:辽宁中海康生物制药股份有限公司
Non-PVC material for medical infusion products and preparation method thereof
The invention relates to a non-PVC material for medical infusion products and a preparation method thereof. The non-PVC material is characterized by comprising the following components by weight percent: 25-30% of SEBS, 45-49% of filling oil, 17-20% of PP, 6-7% of filler and 0.4-0.6% of antioxidant 1076. A disposable bag infusion set or a medical infusion product produced by the invention has the advantages of less precipitate, low drug adsorption, high transparency, light weight, more convenient treatment after use, environmental protection and the like. The non-PVC material does not react with paclitaxel and other fat emulsion anti-cancer drugs.
Owner:CHENGDU XINJIN SHIFENG MEDICAL APP ANDINSTR CO LTD
Medicinal composition fat emulsion injection containing eucalyptol, limonene and alpha-pinene and preparation method
ActiveCN101590033AActive ingredients are clearImprove bioavailabilityMetabolism disorderRespiratory disorderYolkGlycerol
The invention aims to develop medicinal composition fat emulsion injection containing eucalyptol, limonene and alpha-pinene and a preparation method. The preparation comprises the following components in percentage by weight: 0.0128 to 0.0320 percent of eucalyptol, 0.0084 to 0.0210 percent of limonene, 0.0028 to 0.0070 percent of alpha-pinene, 10 to 30 percent of soybean oil for injection, 1.0 to 1.5 percent of yolk lecithin for injection or 0.8 to 1.5 percent of soybean phospholipids for injection, 2.0 to 2.5 percent of glycerol for injection, and water for injection added to 100 milliliters. The preparation method has the characteristics of strong controllability of production quality, and definite treatment effect of a product. The invention fills up the blank of the medicinal composition fat emulsion injection preparation containing the eucalyptol, the limonene and the alpha-pinene.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
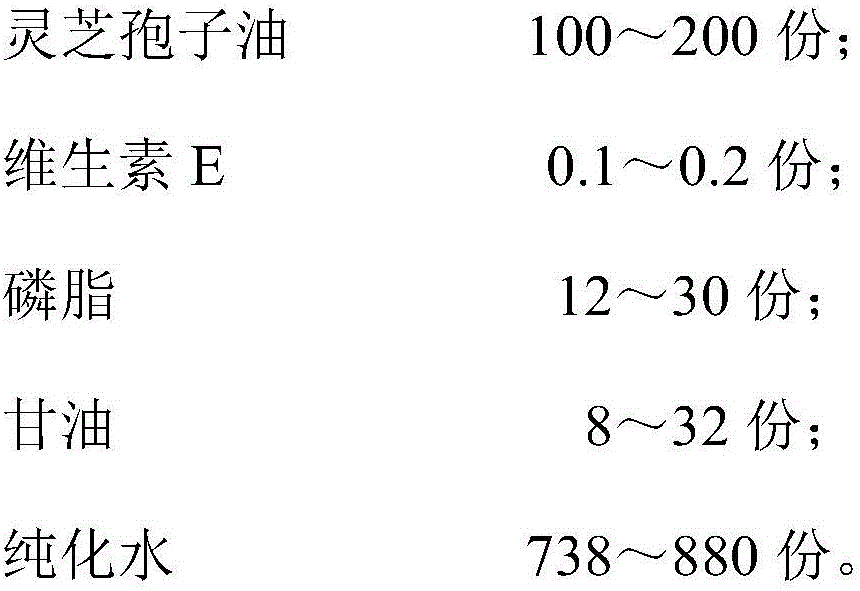
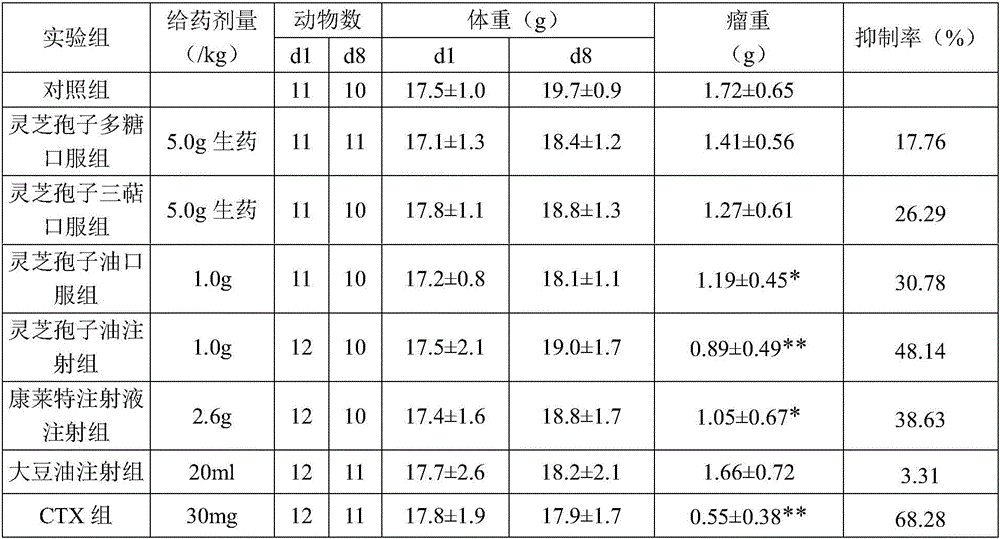
Preparation and application of ganoderma lucidum spore oil, ganoderma lucidum spore oil fat emulsion
InactiveCN106692213APrevent rancidityGuaranteed freshnessPharmaceutical non-active ingredientsEmulsion deliveryBULK ACTIVE INGREDIENTCooking oil
The invention discloses a preparation and application of ganoderma lucidum spore oil, ganoderma lucidum spore oil fat emulsion. The ganoderma lucidum spore oil is obtained by being directly prepared by two stage separation after wall-breaking, granulation, drying and supercritical CO2 extraction of the ganoderma lucidum spore. Conventional refining processes including deacidification and deodorization are not needed. The ganoderma lucidum spore oil is rich in monounsaturated fatty acid mainly comprising oleic acid, keeps the functional components of triterpene acid, ergosterol and fat-soluble vitamin peculiar to ganoderma lucidum and has good antitumor activity. Hygienic indexes of product acid value, peroxide value and the like meet cooking oil hygienic standards. The fat emulsion prepared from ganoderma lucidum spore oil by homogenization technique is convenient to take and has good effects of antitumor and effect-enhancing and toxicity-reducing, and active ingredients are more beneficial for human body absorption, and the bioavailability is high.
Owner:GUANGZHOU HANFANG PHARMA
Medium and long chain fat emulsion injection and preparation method thereof
ActiveCN103006751AImprove stabilityMetabolism disorderEmulsion deliveryGlycerolMedium-chain triglyceride
The invention aims to provide a medium and long chain fat emulsion injection and a preparation method thereof. Specifically, every 1,000 ml of the medium and long chain fat emulsion injection contains 50 to 100 grams of soybean oil for injection, 50 to 100 grams of medium chain triglycerides for injection, 12 grams of lecithin for injection, 25 grams of glycerin for injection and an appropriate quantity of water. By adopting a large quantity of process screening operations and combining pilot scale production, process conditions for a special prescription are determined to fully guarantee the effectiveness, controllability and stability of a product.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Hippophae rhamnoides seed oil fatty milk injection and technique of preparing the same
The invention discloses a medicament injection, which particularly relates to an injection which contains sea-buckthorn seed oil fat emulsion and a process for preparation. The sea-buckthorn seed oil fat emulsion of the invention is prepared according to the following components and proposition, 5 to 30g sea-buckthorn seed oil for injecting in each 100ml injection, 0.5 to 7.5g emulsifier, 1.5 to 3.6g isotonic regulator, 0.001 to 0.2g anti-oxidant, hydrochloric acid solution or sodium hydrate solution whose PH value is adjusted from 6.5 to 9.5, and the other are water for injecting. The proportions of the sea-buckthorn seed oil fat emulsion injection w-6 and w-3 are proper, and the stability is good, and the invention can not only provide heat energy for human bodies to replenish essential fatty acid for human bodies, but also the invention has the functions of improving body immunity, prompting tissue repair, anti-inflammatory anti-radiation and anti-mutation, and inhibiting cancer cell to diffuse. The invention particularly has a promoting effect to physical rapid restoration of postoperative patients, patients with large areas of burn, and cancer patients who have radiotheraphy and chemotherapy.
Owner:SHENYANG PHARMA UNIVERSITY
Cabazitaxel fat emulsion, and preparation method and use thereof
InactiveUS20180153848A1Excellent long-term storage stabilityExcellent resolubilityOrganic active ingredientsPharmaceutical non-active ingredientsCabazitaxelMedicine
Provided in the present invention is a cabazitaxel fat emulsion injection, wherein the cabazitaxel fat emulsion injection contains cabazitaxel, a medium chain triglyceride for injection, and lecithin. Also provided in the present invention are the method for preparing the cabazitaxel fat emulsion injection and the use thereof in preparing a drug for treating prostate cancer.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Walnut oil fat emulsion and manufacturing method thereof
InactiveCN101167793APrevent embolismGuaranteed damageMetabolism disorderAntinoxious agentsDiseasePN - Parenteral nutrition
The invention relates to walnut oil intralipid and process for preparation, belonging to the technical field of food and drug. The formulation comprises pure walnut oil, refining lecithin, pure glycerin, caustic soda and pyrogen-free pure water. The basic preparing procedures are as follows: pure walnut oil, refining lecithin, glycerin and water are vigorously stirred into primary milk and the pH value of the primary milk is adjusted to 7.5-8.5 through little caustic soda solution, and then high-pressured and homogenized, filtered by a filter, bottled, sealed and sterilized. The walnut oil intralipid is an artificial chyle product which is prepared by simulating natural chyle granules existing in blood and can be intravenously injected as 'parenteral nutrient solution' to rescue lives of some severe patients, (such as severely burned patients, traffic accident injured patients, patients with great operations, patients with advanced tumor and cachexia, etc.) and furthermore can be used for complementarily curing some dystrophic diseases and also can be orally taken as nutrition supplementary and auxiliary treatment. The invention can increase essential energy for body metabolism, provide biological film for body and essential 'polyunsaturated fatty acid' for biologically active substance metabolism, and prevent and correct deficiency of essential fatty acid for body. The invention has a plurality of therapeutic functions of blood sugar reduction, anti-oxidative damage, anti-anaphylaxis, anti-cell senescence, and anti-cardiovascular hardness and the like, and provides a new direct and convenient way through which edible and medical walnut effective component can enter human body.
Owner:陈朝银
Disulfiram lipid microsphere preparation for injection for treating tumor and preparation method thereof
InactiveCN102335140AInhibitory activityPrevent invasionOrganic active ingredientsPowder deliveryVegetable oilMicrosphere
The invention relates to a disulfiram lipid microsphere preparation for injection for treat tumor and a preparation method thereof, belonging to the field of pharmaceutical preparations. The disulfiram lipid microsphere preparation is prepared from the following components in mass percent: 0.1-4% of disulfiram or derivatives thereof, 5-30% of vegetable oil, 0.6-3% of lecithin, 0-4% of polyethylene glycol phospholipid or derivatives thereof, 0-4% of amino acid block copolymer, 0-0.6% of oleic acid or oleate, 0-1% of antioxidant, 0-0.4% of complexing agent, 1-3% of glycerol and balance of water for injection. According to the invention, due to the introduction of the polyethylene glycol phospholipid or derivatives thereof and the amino acid block copolymer, a fat emulsion can be kept in the blood for a longer time, an additional passive target function on tumor is provided for the medicament, the stability of the fat emulsion preparation is improved, and the effect of disulfiram on treating tumor is enhanced.
Owner:SHENYANG PHARMA UNIVERSITY
Etomidate fat emulsion concentrated solution, preparation method and application thereof
InactiveCN104706587ANo delaminationImprove liquidityOrganic active ingredientsAnaestheticsEngineeringSaline
An etomidate fat emulsion concentrated solution, a preparation method and an application thereof belong to the field of pharmacology and pharmaceutics. According to the invention, the defect that a traditional fat emulsion preparation technology is complex and stability is poor is overcome. The preparation technology provided by the invention is simple; the general physical stirring process is only needed; and no homogenization technology is required. A product prepared in the invention can be sterilized through a 0.22-micron microfiltration membrane; in clinical use, the product can be spontaneously emulsified after diluted by the use of an aqueous solution such as normal saline or a glucose solution, etc. and slightly oscillated; and under optimized conditions, average particle size is about 0.2 micron, and injection fat emulsion characteristics are fully embodied. The product has good fluidity, will not be retained on the wall, is single-phase, transparent and clear in appearance, can undergo clarification detection, will not cause preparation stratification after multigelation and is used in clinical uses such as induction of general anesthesia, short-time operative anesthesia and the like.
Owner:天津迈迪瑞康生物医药科技有限公司
Fat emulsion of oil of zedoary turmeric and preparation method
InactiveCN1795875ALess irritatingImprove complianceSolution deliveryEmulsion deliveryZedoary oilFat emulsion
An oil-in-water zedoary oil-fat emulsion for treating viral disease and cancer by injecting or oral taking is proportionally prepared from zedoary oil, soybean oil, lecithin, glycerin, oleic acid and the water for injection through high-speed stirring or ultrasonic oscillating, and high-pressure homogenizing. Its advantages are low by-effect and high curative effect.
Owner:董英杰
Fat emulsion of Paricalcitol, its preparation and preparation methods thereof
ActiveCN102772364ALess irritatingSafe and stable intravenous injectionPowder deliveryOrganic active ingredientsOrganic solventInjection emulsion
The invention relates to a fat emulsion of Paricalcitol. The fat emulsion comprises oil for injection, an emulsifier, a stabilizer, an isoosmotic adjusting agent, and a pH adjusting agent. Emulsion droplets have an average diameter of 50nm-1000nm. The fat emulsion can be prepared into injection emulsions, freeze-dried emulsions or capsules. Specifically, organic solvents cannot be employed as injections so as to reduce stimulation on blood vessels during injection and avoid injection package material impurity leaching caused by organic solvents, thus ensuring product quality and safety, and reducing the difficulty of product quality control.
Owner:CHONGQING HUAPONT PHARMA
High oxidation resistance long chain fat emulsion injection and preparation method thereof
InactiveCN101797225AReduce the degree of oxidationReduce oxidationMetabolism disorderPharmaceutical non-active ingredientsFiltrationOil phase
The invention provides high oxidation resistance long chain fat emulsion injection and a preparation method thereof. The fat injection consists of soybean oil for active component injection, emulsifier, isoosmotic adjusting agent, metal ion chelation agent and water for injection. The preparation method comprises the following steps: under inert gas environment, adding the emulsifier and coemulsifier into the heated soybean oil for injection, and uniformly stirring the mixture serving as oil phase at high speed; uniformly mixing the isoosmotic adjusting agent and the metal ion chelation agent with proper water for injection, and filtering the solution serving as water phase; in high speed stirring, combining the oil phase and the water phase, continuously stirring the mixture to foremilk at high speed, adjusting the pH of the foremilk to be between 9.0 and 10.0, adding the water for injection to the volume of 1,000ml; homogenizing the foremilk after the foremilk is uniformly mixed by a high pressure homogenizer until the milk particles are qualified; and preparing the fat emulsion injection through filling, nitrogen introducing, plugging, capping, sterilizing and cooling after filtration.
Owner:SHANGHAI INST OF PHARMA IND
Injection alprostadil fat emulsion and preparing method thereof
ActiveCN105012248AReduce security risksEasy to operateOrganic active ingredientsPowder deliveryFat emulsionsAlprostadil Injection
An alprostadil lipid emulsion for injection and a preparation method therefor. The lipid emulsion contains alprostadil, phosphatidylcholine, phosphatidylglycerol, oil for injection and a lyophilized protective agent. Based on 1 part by weight of the alprostadil, the content of the phosphatidylcholine is 1200 to 4000 parts by weight, the content of the phosphatidylglycerol is 12 to 120 parts by weight, the content of the oil for injection is 2000 to 20000 parts by weight, and the content of the lyophilized protective agent is 16000 to 60000 parts by weight.
Owner:内蒙古多肽科技有限公司
Sesquiterpene ketone injection, preparing method and use thereof
The present invention relates to the new types of plant extract containing germacrone, curcumone and / or arylcurcumone, that is, nano liposome type, precursor liposome type long-cycle liposome type, long-cycle nano liposome type, nano microemulsion type, venous emulsion type, nano liposome sphere type and fat emulsion type.
Owner:谢恬
Fat emulsion of biobalide B and preparation method
InactiveCN1759828AExcellent pharmaceutical characteristicsSignificant effectOrganic active ingredientsEmulsion deliveryEmulsionFat emulsion
A fatty emulsion of ginkalide B and its preparing process are disclosed. It has the strong antagonism to platelet activating factor.
Owner:刘晓东 +1
Compounds based on ibuprofen, preparation methods, uses and pharmaceutical preparation thereof
ActiveCN103003228AIncrease fat solubilityEffective aggregationOrganic active ingredientsOrganic compound preparationOrganic acidPropanoic acid
Disclosed are compounds based on ibuprofen, their preparation methods, uses and pharmaceutical preparation. The compounds have structures shown as formula(1), wherein m, n are integers and fulfill the requirement of 0.n.6, 0.m.6, respectively. The preparation methods for the compounds based on ibuprofen are as follows: contacting and reacting 2-(4-isobutyl phenyl)-propionic acid and an ester of an organic acid solution in the presence of a catalyst and under substitution reaction conditions. The present compounds can be used to prepare nonsteroidal anti-inflammatory drugs. The preparation can be preparation of fat emulsion, liposome, and dry emulsion and so on.
Owner:山东华鲁制药有限公司
Flurbiprofen axetil fat emulsion concentrate and preparation method and use thereof
InactiveCN104706575ANo delaminationImprove liquidityOrganic active ingredientsAntipyreticPharmacyFat emulsions
The invention relates to a flurbiprofen axetil fat emulsion concentrate and a preparation method and use thereof, and belongs to the field of medicine and pharmacy. The flurbiprofen axetil fat emulsion concentrate overcomes the defects of complex traditional fat milk preparation process and poor product stability. The flurbiprofen axetil fat emulsion concentrate is simple in preparation process only needing simple physical mixing without homogenization process. The product can be sterilized through a 0.22 mum millipore filter film, can spontaneously emulsify during clinical use by dilution with normal saline or glucose solution and other water solutions and slight oscillation, under the optimal conditions, emulsified average particle size is about 0.2 mum, and fully shows injection fat milk properties. The flurbiprofen axetil fat emulsion concentrate product has good liquidity, and may not be hung on the wall, the appearance is single-phase, transparent and clear, clarity detection is acceptable, and after repeated freezing and thawing, preparation stratification phenomenon does not occur. The flurbiprofen axetil fat emulsion concentrate product is used for postoperative and cancer analgesia and other clinical application.
Owner:天津迈迪瑞康生物医药科技有限公司
Ibuprofen-based compound, preparation method, use, and formulation of the same
InactiveUS20140112978A1Increase fat solubilityLong action timeOrganic active ingredientsBiocideOrganic acidPropanoic acid
Disclosed are compounds based on ibuprofen, their preparation methods, uses and pharmaceutical preparation. The compounds have structures shown as formula (1), wherein, m, n are integers and fulfill the requirements of 0≦n≦6, 0≦m≦6, respectively. The preparation methods for the compounds based on ibuprofen are as follows: contacting and reacting 2-(4-isobutyl-phenyl) propionic acid to have contact reaction with a solution of an organic acid ester in the presence of a catalyst under substitution reaction conditions The present compounds can be used to prepare nonsteroidal anti-inflammatory drugs. The preparation can be preparation of fat emulsion, liposome, and dried emulsion and so on.
Owner:HEILONGJIANG BAOQINGLONG BIOTECH
Preparations of Taxanes for Intravenous Administration and the Preparation Method Thereof
The present invention relates to the field of medical technology. More specifically, the present invention relates to a preparation of taxanes for intravenous administration, which consists of two parts: a drug solution and an emulsion. Said drug solution consists of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein said solvent for injection is an organic solvent. Said emulsion includes a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When used, the drug solution at the clinical dosage can be added and mixed homogeneously in the emulsion to perform intravenous drip directly; or the drug solution at the clinical dosage can also be firstly added into the emulsion with no less than 5 times volume of the drug solution and then a predetermined amount of normal saline or glucose solution for injection is added to perform intravenous drip. The preparation of the present invention does not contain solubilizer and has advantages of little toxicity, safety, effectiveness, stability and economy. The fat emulsion is also used as a nutritional replenishment, thus achieving a better therapeutic effect. In addition, the normal saline or glucose solution for injection can be used to replace a considerable amount of the emulsion, which makes the preparation, therefore, not only cost-efficient, but also convenient for transportation and storage in practice.
Owner:TASLY HLDG GRP CO LTD
Triptolide fat emulsion injection and preparation method thereof
InactiveCN102552137ASimple manufacturing processSuitable for industrialized mass productionOrganic active ingredientsEmulsion deliveryOil phaseNitrogen gas
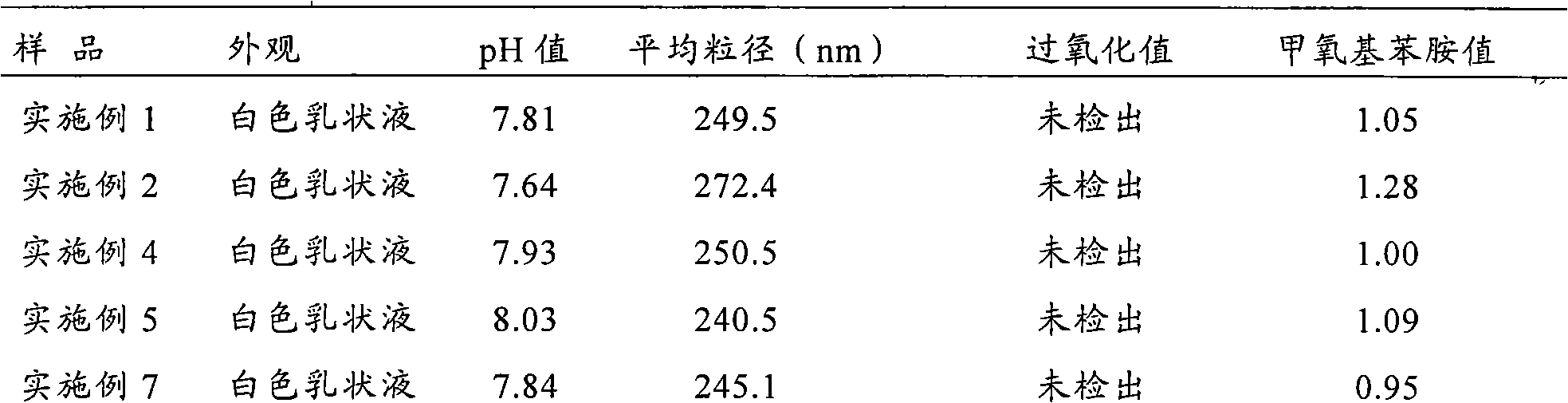
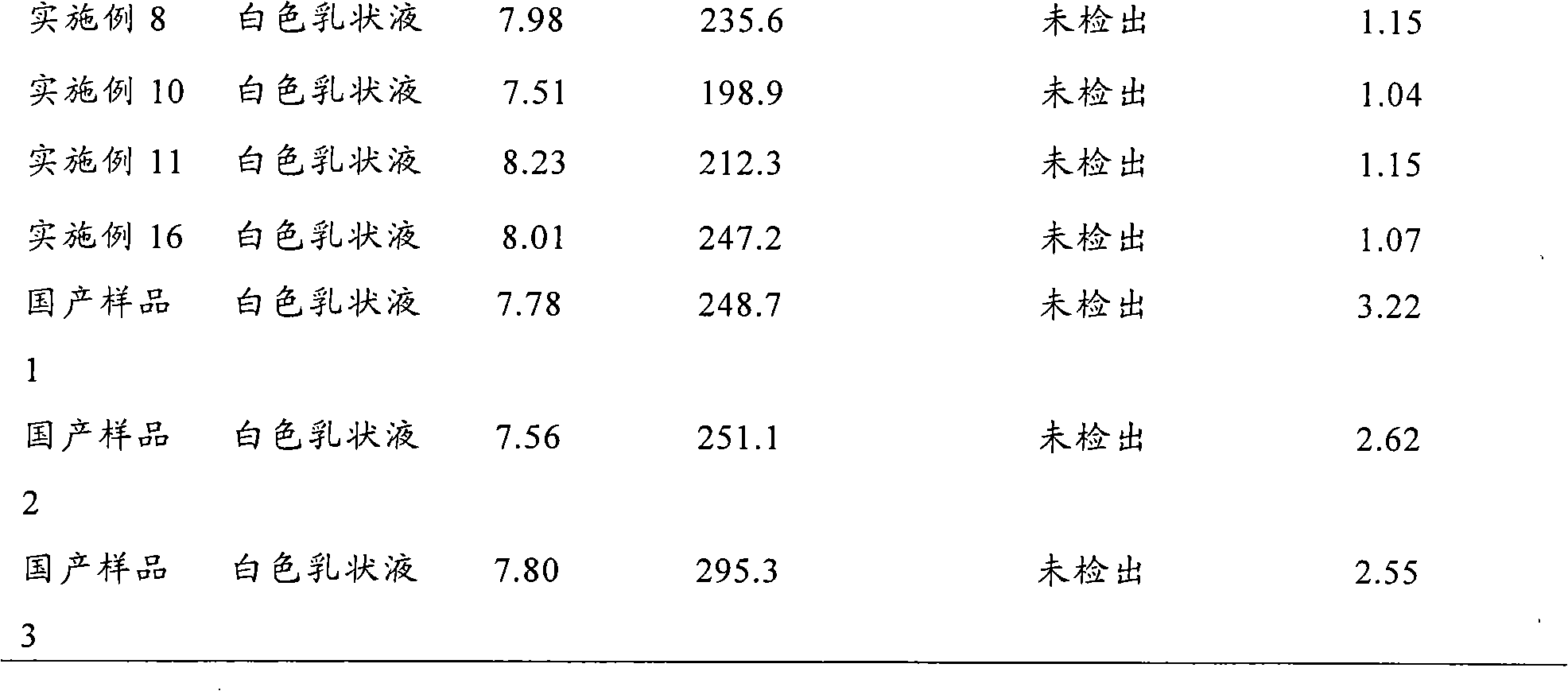
The invention relates to a fat emulsion of triptolide and a preparation method thereof. The fat emulsion injection comprises an antitumor effective dose of triptolide and pharmaceutically-acceptable auxiliary materials, wherein an oil phase is a mixture prepared from long-chain fat acid triglyceride and middle-chain fat acid triglyceride in the mass ratio of 4:1-1:1. The preparation method comprises the following steps of: mixing triptolide, an emulsifier, fat oil, an isotonic regulator, an anti-oxidizing agent and a stabilizer, and heating to obtain an oil phase; weighing water for injection, adding the isotonic regulator, and heating to obtain a water phase; heating and mixing the oil phase and the water phase under the protection of nitrogen gas, and stirring to obtain a primary emulsion; homogenizing the primary emulsion under a high pressure to form a uniform emulsion; and adjusting the pH value to 6-7, sterilizing rotationally to obtain a product, i.e., the triptolide fat emulsion injection, and sterilizing. The average particle diameter of the fat emulsion is 250.9+ / -60.0 nanometers, the polydispersion coefficient is 0.143, dispersion is uniform, a system is stable, the targeting property is high, and vessel irritation is low.
Owner:FUJIAN MEDICAL UNIV
Method for controlling quality of alprostadil injection
InactiveCN101581702AComponent separationPreparing sample for investigationIsoprostaglandin E1Silica gel
The invention discloses a method for measuring prostaglandin E1 and / or prostaglandin A1 in alprostadil fat emulsion injection, which comprises the following steps: (1) ultrasonically processing the alprostadil fat emulsion injection, obtaining an alprostadil solution of which the fat phase is removed; and (2) performing high performance liquid chromatography measurement to the alprostadil solution of which the fat phase is removed, obtaining the content of the prostaglandin E1 and / or prostaglandin A1, wherein the conditions of the high performance liquid chromatography measurement are as follows: stationary phase: octadecyl ether-bonded monolithic silica is filling agent, mobile phase: the ratio of phosphate buffer to acetonitrile is equal to 1-6:1, and the detection wavelength is 278 nm. The method can accurately measure the alprostadil and the degradation products thereof in the alprostadil fat emulsion injection.
Owner:上海万特医药科技(集团)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com