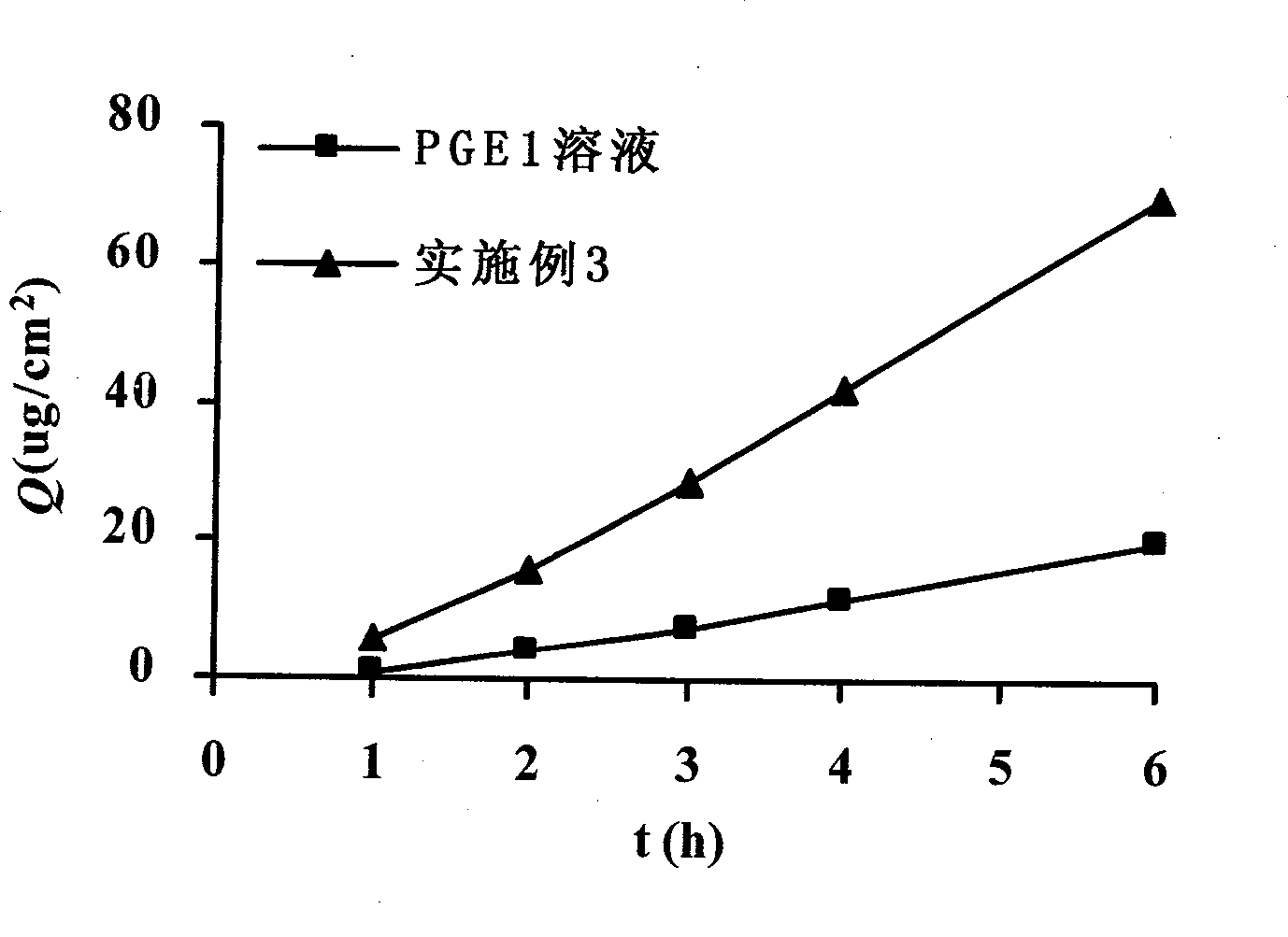
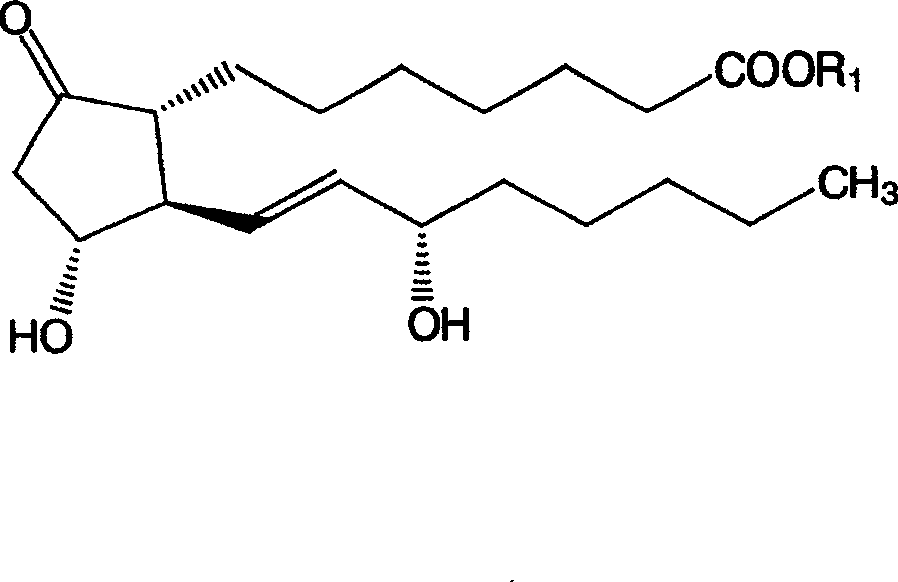
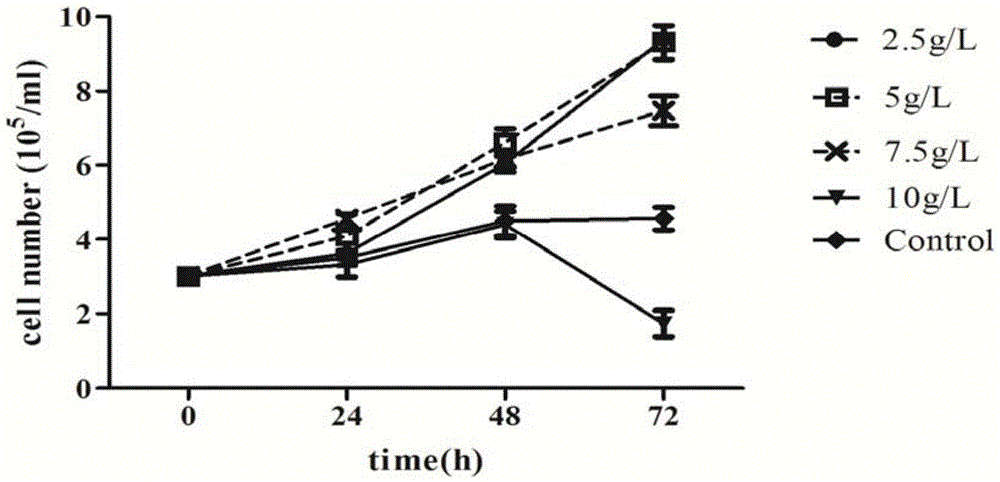
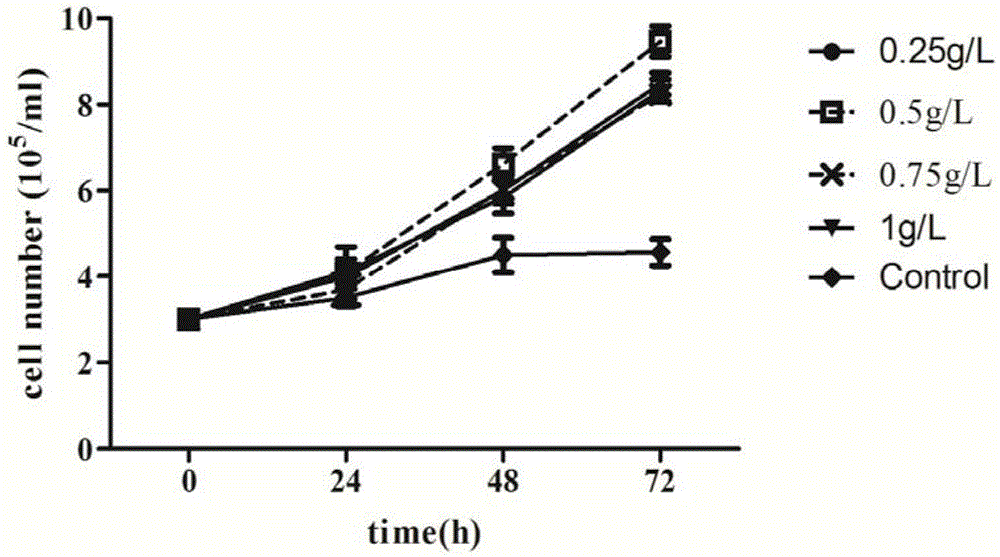
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Isoprostaglandin E1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nano emulsion injection of alprostadil and preparation method
InactiveCN1872072AImprove solubilityImprove stabilityOrganic active ingredientsEmulsion deliveryIsoprostaglandin E1Curative effect
A nano-emulsion injection of prostaglandin E1 contains the nano-emulsion particles of rostaglandin E1, the oil for injection, hydrophilic emulsifier, lipophilic emulsifier, isotonic agent, and stabilizer. Its preparing process and its quality control method are also disclosed.
Owner:广州中大创新药物研究与开发中心有限公司
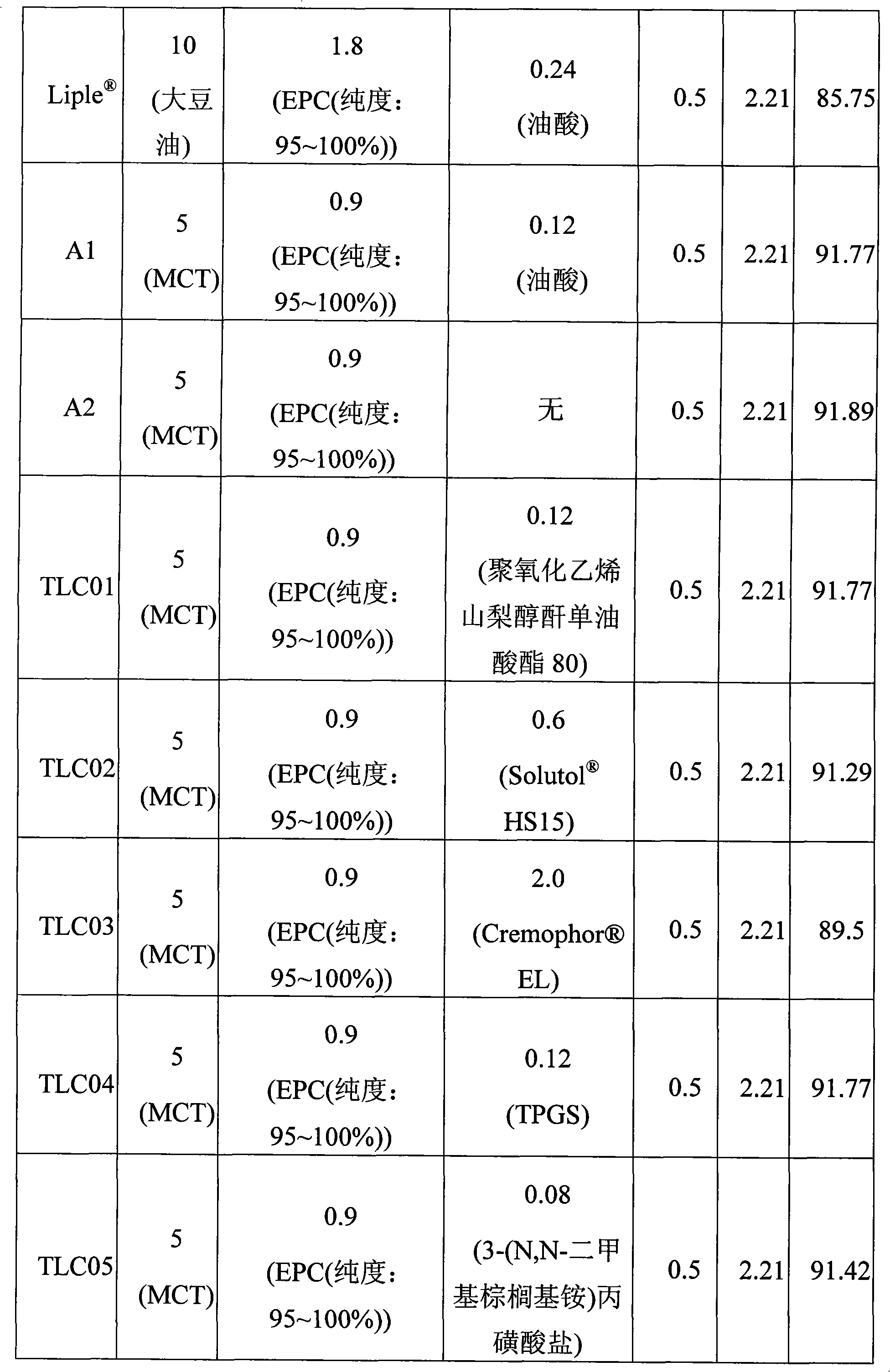
Emulsion composition comprising prostaglandin e1
An emulsion composition includes prostaglandin E1 (PGE1), a phospholipid with a high purity and a non-proton-providing surfactant that improves stability of PGE1. Embodiments of the emulsion composition include an effective amount of PGE1, about 1% to about 30% (w / w) of a pharmaceutically acceptable oil as an oil base based on the weight of the emulsion composition, about 1% to about 30% (w / w) of a phospholipid with a high purity based on the weight of the oil base, about 1.6% to about 40% (w / w) of a non-proton-providing surfactant based on the weight of the oil base, and the balance of the emulsion composition being water.
Owner:TAIWAN LIPOSOME CO LTD +1
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787AClinical application safetyEnsure safetyOrganic active ingredientsDigestive systemChemical structureLipid formation
The invention relates to a method for preparing a prostaglandin E1 lipid microsphere injection of a charging non-homogeneous phase (comprising a water phase, an oil / water interfacial film phase and an oil phase) dispersion system, of which the surface of the lipid microsphere can be charged with positive electricity or negative electricity. The prostaglandin E1 is alprostadil, of which the chemical structure comprises a basic skeleton of 20-carbon fatty acid with a 5-carbon ring and two side chains, wherein one side chain is provided with a hydrophilic carboxylic acid group, so that the prostaglandin E1 has the characteristic of light surface activity action. By utilizing the characteristic, and according to the formula and the preparation process provided in the invention, the prostaglandin E1 has an unique drug-carrying mode in a solution of lipid microsphere with the non-homogeneous phase dispersion system, and the prepared lipid microsphere injection is fundamentally different from an alprostadil injection(Kaishi, and is prepared by adopting the technology of the Japanese business corporation LTT Bio-Pharma Co., Ltd. already sold in markets, and the difference lies in that the drug-carrying mode is completely different, the content of degradation products in the preparation such as impurities is more than 50 percent lower than that of in the Kaishi, so that the prostaglandin E1 lipid microsphere injection and the alprostadil injection are fundamentally different. The invention relates to a method for preparing the prostaglandin E1 lipid microsphere injection and the drug-carrying characteristics thereof in a three-phase system; in the formula, 0.0001 to 0.1 weight portion of prostaglandin E1 is used as a drug, the prostaglandin E1 is added with auxiliary materials for medical purpose to prepare the prostaglandin E1 lipid microsphere injection, and the auxiliary materials for medical purpose comprises the following materials in portion by weight: 5 to 20.
Owner:李淑斌
Controlled platelet activation to monitor therapy of ADP antagonists
ActiveUS7790362B2Elcosanoid active ingredientsMicrobiological testing/measurementMedicineIsoprostaglandin E1
A method is provided of determining whether an individual has reduced ability to form platelet thrombi. An ADP platelet activator and one or platelet inhibitors are provided. At least one of the platelet inhibitors is Prostaglandin E1 (PGE1). An alternate signal transduction pathway is produced. A final concentration of ADP is 2 to 35 μM and a final concentration of PGE1 is 2 to 30 nM, preferably 20 to 25 nM.
Owner:INSTR LAB
Prostaglandin microemulsion gel rubber preparation and method of producing the same
InactiveCN101427993AOrganic active ingredientsPharmaceutical delivery mechanismDiseaseGel preparation
The invention relates to prostaglandin microemulsion-gel preparation and a preparation method thereof. The preparation comprises prostaglandin E1 or the derivative thereof, oil, emulsifier, assistant for emulsifying agent, gel substrate and water, and also possibly comprises a permeation accelerator, a preparation stabilizer, an antioxidant and antiseptics. The preparation has the advantages of good percutaneous permeability, no stimulation to skin and mucous membrane, simple preparation technique and stable quality, the preparation can be used for treating diseases such as ulcer of limbs caused by erectile dysfunction and chronic arterial occlusion and rest pain of limbs caused by microcirculatory disturbance.
Owner:李淑斌
Serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells
The invention provides a serum-free culture medium used for full-suspension culture of MDCK (Madin Darby Canine Kidney) cells. The culture medium is prepared from a DMEM / F12 culture medium containing 5g / L of NaCl and the following components: sodium stannite, recombinant human epidermal growth factors, hydrocortisone, recombinant full-chain insulin, prostaglandin E1, human transferrin, thyroxine (T3), vitamin E, cholesterol, ethanol amine, beta-mercaptoethanol, Tween-80, Hypep1510, recombinant human serum albumin ACF and mannitol. With the adoption of the culture medium provided by the invention, the full-suspension culture of the MDCK cells can be carried out very well under the condition that serum is not added; after the MDCK cells are continuously cultured for 20 generations, the average proliferation concentration of the MDCK cells is 2.308*10<6> / mL, the average cell viability is 97.4 percent and the average doubling time is 34.48 hours, so that the serum-free culture medium provides technical supports for developing influenza vaccines of cell matrixes of mammals in China.
Owner:马忠仁 +2
Prostaglandin E1 liposome frozen dry powder injection and production technology thereof
InactiveCN1449759AImprove stabilityLess side effectsOrganic active ingredientsLiposomal deliveryCholesterolIsoprostaglandin E1
The present invention provides a prostaglandin E1 liposome fronen dry powder injection and its production process. It is mainly characterized by that it uses soya lectithin and cholesterol to cover high-purity prostaglandin E1 so as to make them into freeze-dried powder injection. Said process can greatly raise stability of the finished product in production, can prolong expiry date, and can reduce side reaction.
Owner:CHEM PHARMA FACTORY NANYANG PUKANG GROUP
Compound recipe formula containing kurarinone prostaglandin E1 and aspirin, its preparation method and application
InactiveCN1415301ANew formulaExact therapeutic effectSalicyclic acid active ingredientsDigestive systemIsoprostaglandin E1Freeze-drying
A compound medicine containing kurarinol, prostaglandic E1 and aspirin is prepared through including the kurarinol and prostaglandin E1 by 6-0-malto-beta-cyclodextrin, mixing, adding others, and preparing the freeze dried powder injection. It can be used for treating cancers, cardiovascular and cerebrovascular diseases and hepatitis. Its advantages are sure curative effect, and no toxic by-effect.
Owner:蔡海德
Method for treating abdominal discomfort
A method for treating irritable bowel syndrome in a mammalian subject includes administering an effective amount of 13,14-dihydro-15-keto-16,16-difluoro-18-methyl-prostaglandin E1 or 13,14-dihydro-15-keto-16,16-difluoro-prostaglandin E1, or a salt, ether, ester or amide thereof, to the subject. A method for treating abdominal discomfort associated with irritable bowel syndrome in a mammalian subject includes administering an effective amount of 13,14-dihydro-15-keto-16,16-difluoro-18-methyl-prostaglandin E1 or 13,14-dihydro-15-keto-16,16-difluoro-prostaglandin E1, or a salt, ether, ester or amide thereof, to the subject.
Owner:SUCAMPO
Method for controlling quality of alprostadil injection
InactiveCN101581702AComponent separationPreparing sample for investigationIsoprostaglandin E1Silica gel
The invention discloses a method for measuring prostaglandin E1 and / or prostaglandin A1 in alprostadil fat emulsion injection, which comprises the following steps: (1) ultrasonically processing the alprostadil fat emulsion injection, obtaining an alprostadil solution of which the fat phase is removed; and (2) performing high performance liquid chromatography measurement to the alprostadil solution of which the fat phase is removed, obtaining the content of the prostaglandin E1 and / or prostaglandin A1, wherein the conditions of the high performance liquid chromatography measurement are as follows: stationary phase: octadecyl ether-bonded monolithic silica is filling agent, mobile phase: the ratio of phosphate buffer to acetonitrile is equal to 1-6:1, and the detection wavelength is 278 nm. The method can accurately measure the alprostadil and the degradation products thereof in the alprostadil fat emulsion injection.
Owner:上海万特医药科技(集团)有限公司
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787BGood physiological compatibilitySolve the problem of high doseOrganic active ingredientsDigestive systemLipid formationChemical structure
Owner:李淑斌
Stable fat emulsion containing prostaglandin e1
InactiveCN101743010AImprove stabilityActive ingredients will not decreaseOrganic active ingredientsSolution deliveryFat emulsionIsoprostaglandin E1
Disclosed is a fat emulsion containing a compound having prostaglandin E1 activity, which fat emulsion is hermetically sealed in a glass container and then heat sterilized. The residual amount of prostaglandin E1 activity after 16-month storage at 5 DEG C is not less than 65% but not more than 100% of the prostaglandin E1 activity at the beginning of storage.
Owner:MITSUBISHI TANABE PHARMA CORP +1
Compound prepn containing prostaglandin E1, prostaglandin A1 and prostaglandin B1 and its prepn process and use
InactiveCN1416815ANew formulaExact therapeutic effectOrganic active ingredientsNervous disorderSide effectIsoprostaglandin E1
The present invention discloses a compound injection in absolut ethyl alcohol or compound powdered injection preparation of prostaglandin E1, prostaglandin E1 potassium salt, prostaglandin E1 sodium salt or prostaglandin A1 and prostaglandin B1. The compound preparation is used in preventing and treating AIDS and abstaining from drugs. It has unique recipe, complementary components, determined curative effect and less toxic side effect.
Owner:蔡海德
Adhesion prevention and an endoscopic insufflation system therefor
InactiveUS7073512B2Effective preventionAvoid stickingOrganic active ingredientsInorganic active ingredientsWhite blood cellIsoprostaglandin E1
A method of treating or preventing adhesion formation during or following a surgical procedure comprising administering to a patient in need thereof at least one medicament selected from the group consisting of potassium channels; modulators of macrophage activation and leucocyte attraction through cytokines, or their inhibitors, antibodies or inhibitors blocking the effect of VEGF expression; prostaglandin E1; free radical scavengers, lipid peroxysomes; pregnatrienes; calcium antagonists; hypoxia; acidosis; MP; dopamine; and ATP-MgCl2, wherein the method prevents adhesion formation by preventing anoxemia.
Owner:SATURNUS
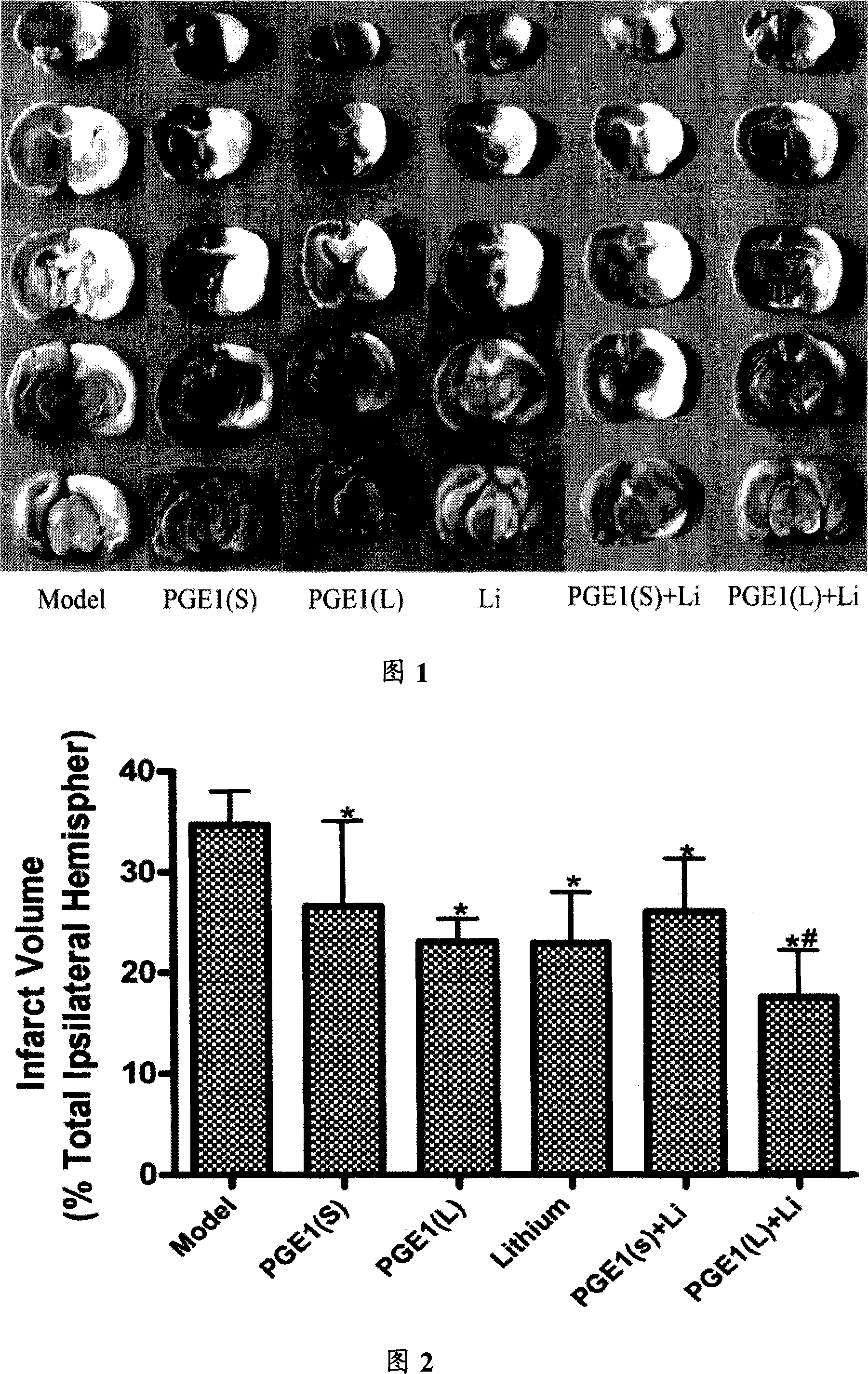
Composite preparations for curing schemic brain damage
InactiveCN101176735AReduce adverse reactionsGood curative effectOrganic active ingredientsNervous disorderIsoprostaglandin E1Clinical therapy
The invention relates to a compound preparation for treating the ischemic brain damage; wherein, the active component comprises prostaglandin E1 the lithium salt; the prostaglandin E1 and the lithium salt can be mixed into the intravenous injection or infusion mixture, and the prostaglandin E1 also can be made into the intravenous injection or infusion agent, meanwhile the lithium salt can be made into the subcutaneous injection. The invention has the advantages of enabling to be used on the people needing treatment or the experiment animal, inducing the formation of the heat shock protein, protects the neuron, increasingg the resistance of the cell hypoxia, and resisting the ischemic brain damage function through the synergistic interaction of the prostaglandin E1 and the lithium salt compound preparation. The invention also has an advantage that: the application dosage of two medicines in the prostaglandin E1 and the lithium salt compound preparation are both lower than the convention dosage of the two medicine in the clinic, so as to reduce the adverse reaction and improve the curative effect. The invention provides a new compound preparation for the ischemic cerebral apoplexy.
Owner:SUZHOU UNIV
Glycosides derivative of prostaglandin E1 and preparation method thereof
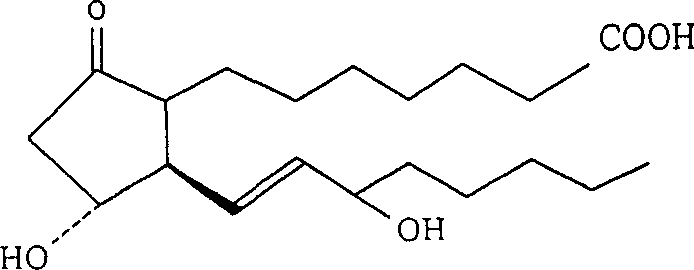
ActiveCN101671377AAdapt to treatment needsSugar derivativesSugar derivatives preparationHydrogenIsoprostaglandin E1
The invention discloses a derivative of prostaglandin, represented by the formula (I), the pharmaceutically acceptable salt and a preparation method thereof. In the formula, R1 is beta-D-glucosylsorbitol, beta-D-pyrane galactosyl, beta-D-pyrane xylosyl, alpha-L-pyrane rhamnoside, alpha-L-pyrane arabinose, alpha-D-pyrane mannose, alpha-D-fructofurano, beta-D-fructo ribosyl, beta-L-pyrane fucosyl, beta-D-glucosyl, beta-D-lactosyl, beta-D-cellobiose diglycosyl, and R2 is hydrogen or methyl. The invention provides a stable and long-acting derivative of prostaglandin E1 so as to adapt to treating requirement.
Owner:北京中海康医药科技发展有限公司
Method for preparing prostaglandin derivative
The invention relates to a method for preparing a compound with a structural formula (I). The compound with the formula (I) is a derivative of prostaglandin E1, and the chemical stability of the derivative is superior to that of the prostaglandin E1. The synthesis process disclosed by the invention is a complete synthesis method of the compound with the formula (I), and the method is simple and feasible.
Owner:沈阳万爱普利德医药科技有限公司
Methods for measuring platelet reactivity of individuals treated with drug eluting stents
ActiveUS20100184084A1Elcosanoid active ingredientsMicrobiological testing/measurementIsoprostaglandin E1Anticoagulant
A method is provided for measuring inhibition of platelet reactivity in an individual treated with a drug-eluting stent (DES). First, a blood sample is obtained from an individual treated with a DES and a P2Y12 antagonist. The blood sample is then mixed with particles comprising an attached GPIIb / IIIa receptor ligand, adenosine diphosphate (ADP) and prostaglandin E1 (PGE1). The mixture is incubated under conditions suitable for agglutinating particles, and platelet-mediated agglutination is assessed in the mixture. The absence or reduction of agglutination indicates that the individual treated with a DES has reduced platelet reactivity. Also provided is a kit for measuring inhibition of platelet aggregation by a P2Y12 receptor antagonist that includes a GPIIb / IIIa receptor ligand immobilized on a particle, adenosine diphosphate (ADP), prostaglandin E1 (PGE1), an anticoagulant, and a buffer to maintain the anticoagulated blood in a condition suitable for platelet aggregation.
Owner:INSTR LAB
Emulsion composition comprising prostaglandin E1
An emulsion composition includes prostaglandin E 1 (PGE 1 ), a phospholipid with a high purity and a non-proton-providing surfactant that improves stability of PGE 1 . Embodiments of the emulsion composition include an effective amount of PGE 1 , about 1 % to about 30% (w / w) of a pharmaceutically acceptable oil as an oil base based on the weight of the emulsion composition, about 1% to about 30% (w / w) of a phospholipid with a high purity based on the weight of the oil base, about 1.6% to about 40% (w / w) of a non-proton-providing surfactant based on the weight of the oil base, and the balance of the emulsion composition being water.
Owner:TAIWAN LIPOSOME CO LTD +1
Application of prostaglandin E1 in preparing medicines for treating cerebral hemorrhage
PendingCN110507659AReduce releaseImprove athletic recovery and neurological functionOrganic active ingredientsBlood disorderIsoprostaglandin E1Apoptosis
The invention provides an application of prostaglandin E1 in preparing medicines for treating cerebral hemorrhage, and belongs to the technical field of biological medicines. Compared with the prior art, the application has the advantages that when prostaglandin E1 (PGE1) is used for treating cerebral hemorrhage, compared with the effect of a NaCl blank control treatment group, the prognostic function of cerebral hemorrhage patients is obviously improved; recovery of exercise ability and improvement of nerve functions of cerebral hemorrhage mice are obviously improved; the number of apoptoticnerve cells in tissues around cerebral hematoma of the cerebral hemorrhage mice is obviously reduced; the number of survival nerve cells is obviously increased; and proliferation of astrocytes, activation of microglia and oxidative stress reactions are obviously inhibited. Meanwhile, PGE1 can reduce decrease of cell activity, release of lactic dehydrogenase (LDH) and apoptosis of neurons in in-vitro experiments.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Oral preparation of prostaglandin E1
InactiveCN1726921AEasy to administerEasy to storeOrganic active ingredientsMetabolism disorderIsoprostaglandin E1Pharmacology
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
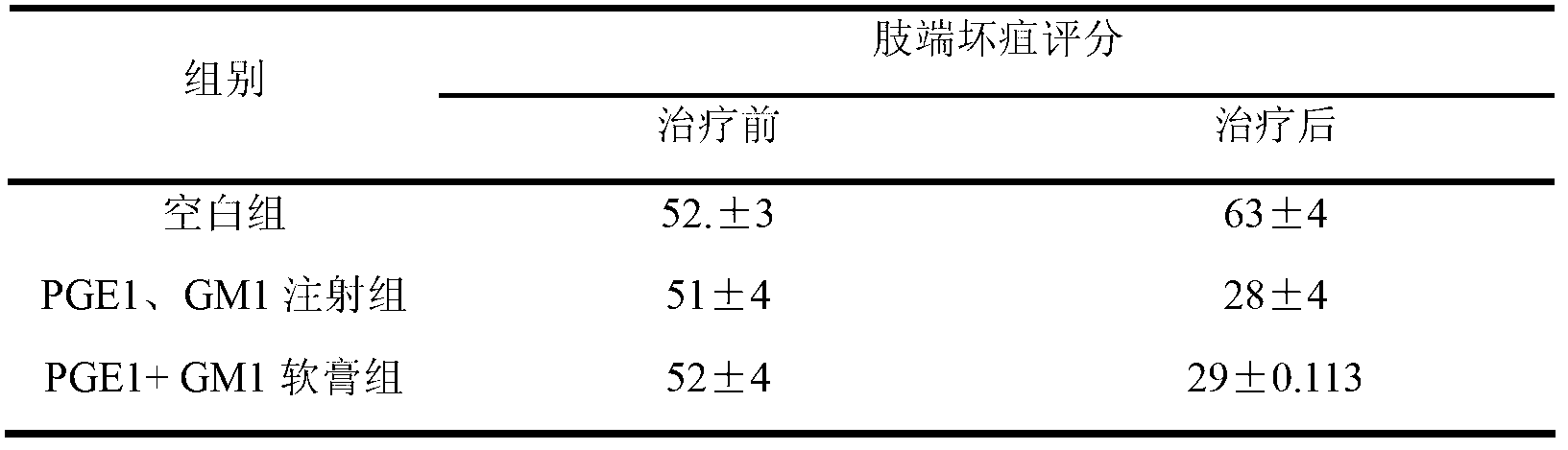
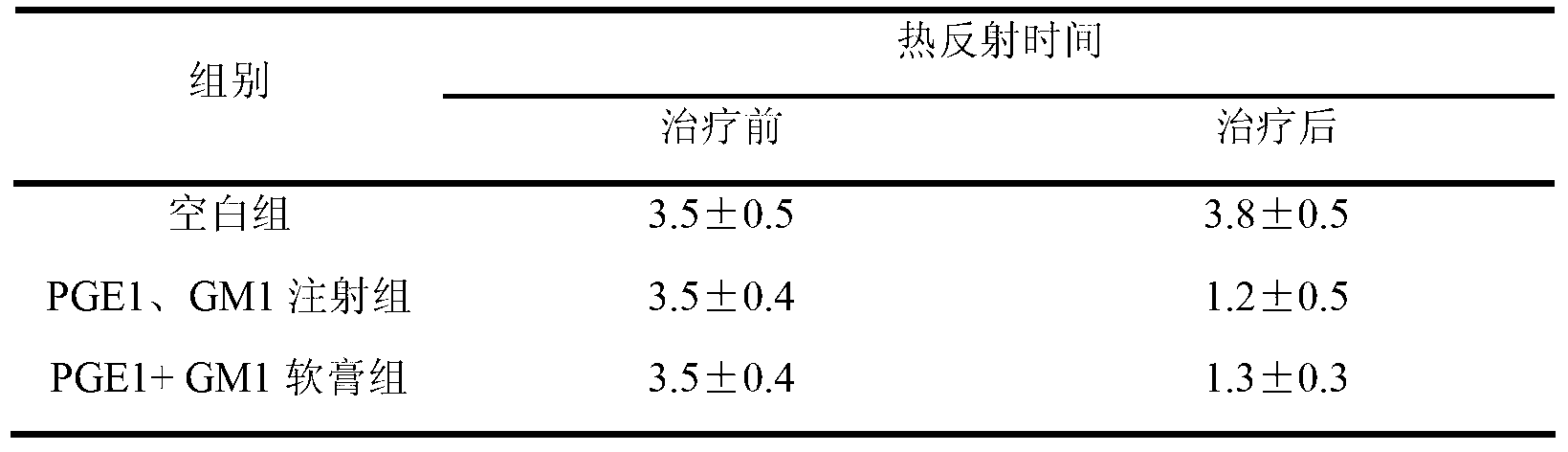
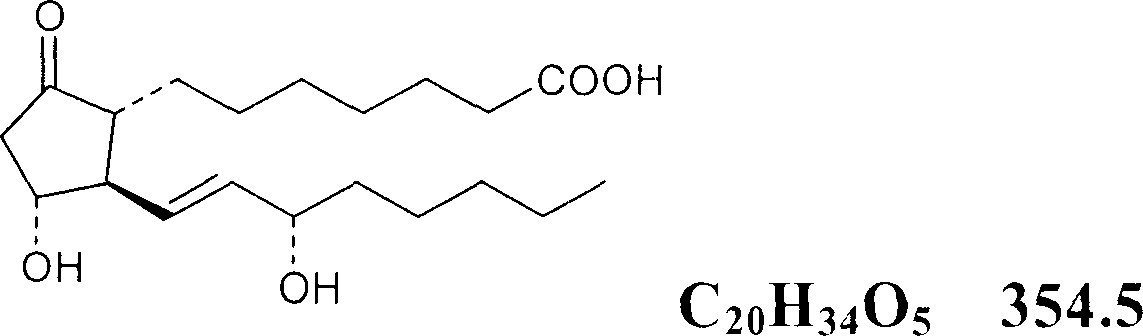
Pharmaceutical composition for treating diabetic feet caused by diabetes and acro-skin lesion and preparation method of pharmaceutical composition
ActiveCN103271930AHas the following beneficial effects: monosialotetrahexosyl ganglioside (GM1) can promote neurotrophicHas the following beneficial effects: Monosialotetrahexosylganglioside (GM1) promotes protectiveOrganic active ingredientsNervous disorderIsoprostaglandin E1Curative effect
The invention relates to pharmaceutical composition for treating diabetic feet caused by diabetes and acro-skin lesion and a preparation method of the pharmaceutical composition. The pharmaceutical composition is an ointment which is prepared by mixing prostaglandin E1 (PGE1) and monosialoteterahexosyl ganglioside (GM1) according to a proper proportion and adding an appropriate proportion of auxiliary materials. Experiments on animals show that the pharmaceutical composition has an ideal treatment effect on the diabetic feet caused by the diabetes and the acro-skin lesion, and is prepared into the ointment for external use and convenient to apply; an affected part is coated with the ointment directly; and the pharmaceutical composition can realize transdermal absorption, takes effect quickly, and is good in safety, clear in treatment effect, low in cost and good in adaptability of a patient.
Owner:JILIN YINGLIAN BIOPHARML
Nose taking powder of prostaglandin E
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method and composition for enhancement of male erectile function
InactiveUS20110263498A1Improve erectile functionEffective treatmentBiocideElcosanoid active ingredientsInsulin-like growth factorPhosphodiesterase 5 inhibitor
A pharmaceutical composition for enhancing male erectile function comprising an erectile function-enhancing amount of an insulin-like growth factor selected from the group consisting of IGF-1 (Somatmedin-C) and analogue LR3 IGF1 in admixture with a pharmaceutically-acceptable diluents or carrier. Such compositions optionally further comprise compounds selected from an androgen, particularly, testosterone and dihydrotestosterone, a vasodilator, PDE5 inhibitor and prostaglandin E1.
Owner:DR KENNETH ADAMS MEDICINE PROFESSIONAL CORPORATIO
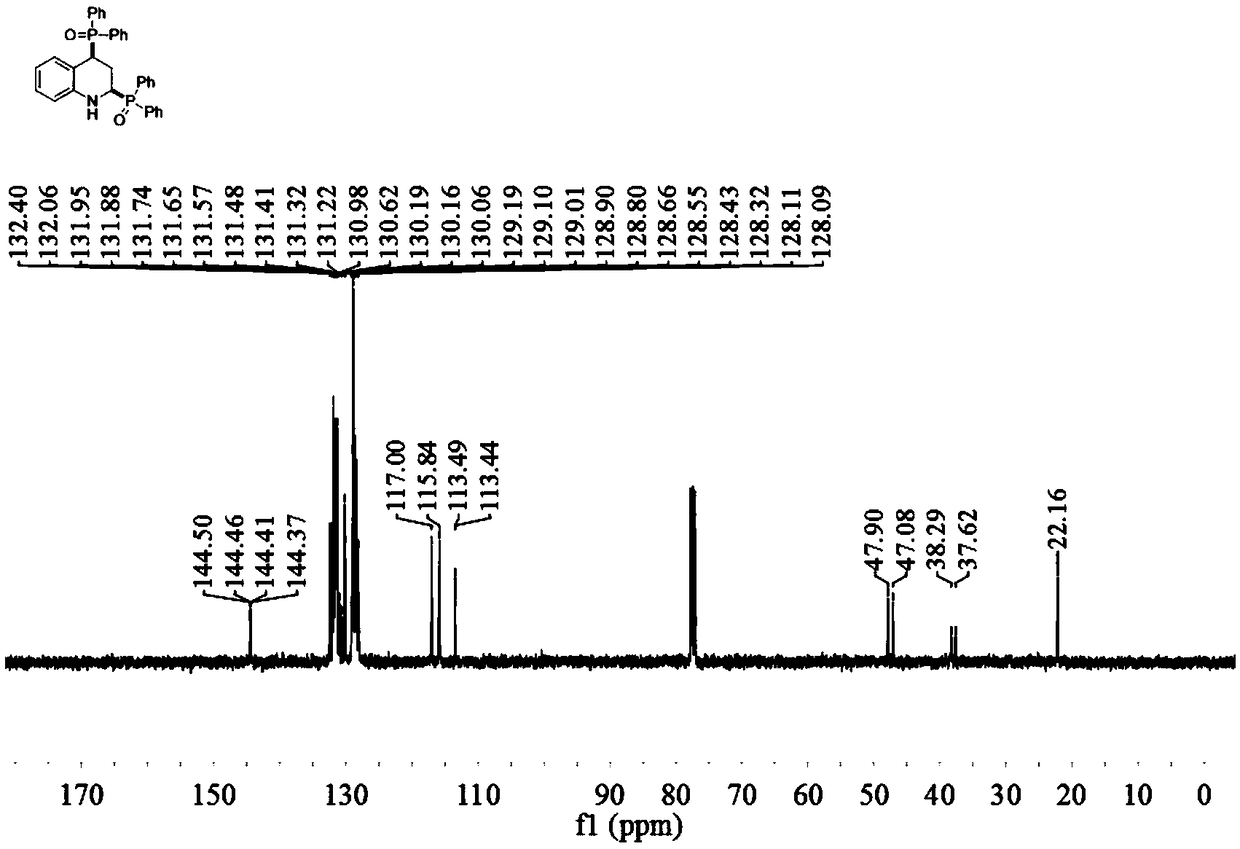
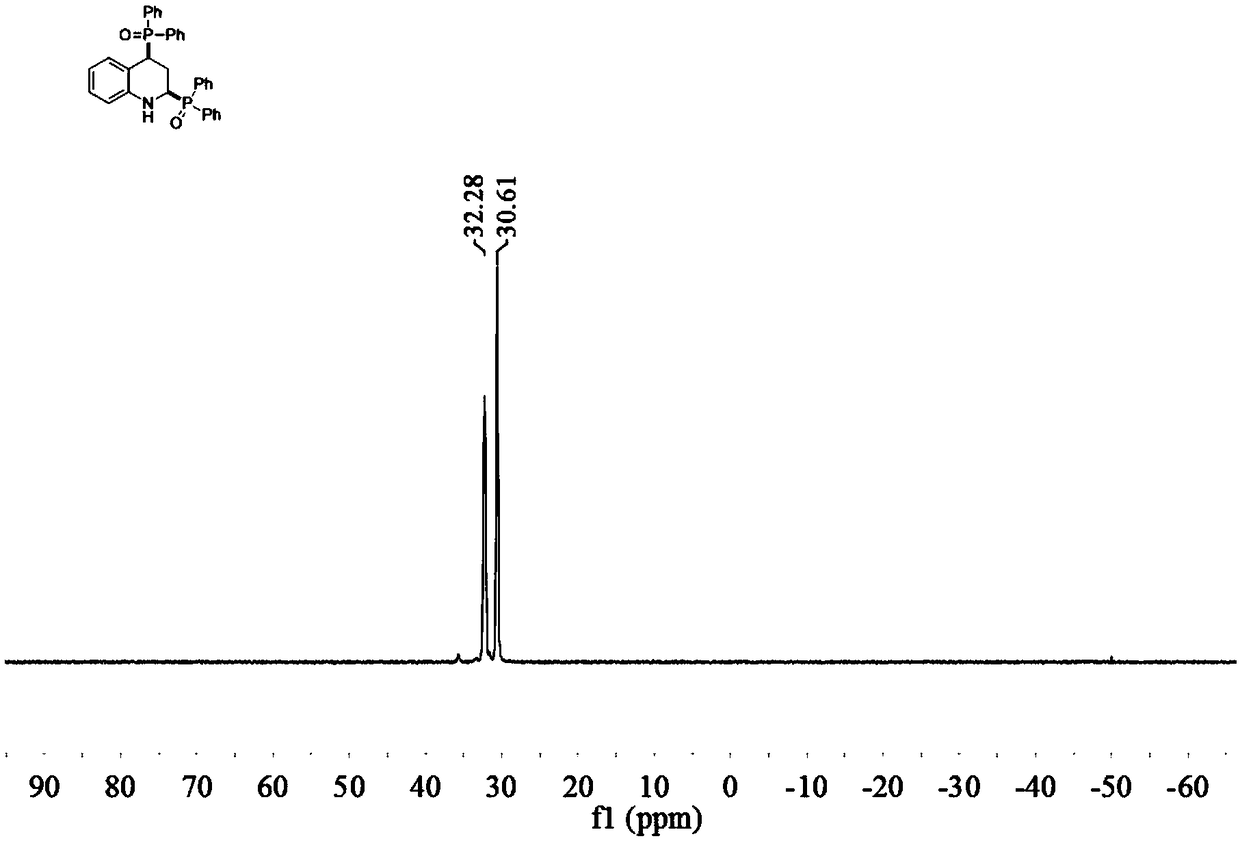
2,4-bis(diphenylphosphine oxide) tetrahydroquinoline compound and preparation method and application thereof
ActiveCN109134540AImprove compatibilityThe synthesis steps are simpleAntibacterial agentsNervous disorderIsoprostaglandin E1Evaporation
The invention discloses a 2,4-bis(diphenylphosphine oxide) tetrahydroquinoline compound and a preparation method and application thereof. The general molecular formula of the compound is shown in formula I as shown in specification, wherein R1 in formula I is H, Cl or Br. The preparation method comprises the following steps: S1, adding quinoline or a derivative of the quinoline and diphenylphosphine oxide in a reaction container under the condition that the molar ratio of the quinoline or the derivative of the quinoline to the diphenylphosphine oxide is 1: 2-3, adding a metal catalyst which accounts for 1-5% of that of the quinoline or the derivative of the quinoline, adding alcohol as a hydrogen donor, adding a solvent, and carrying out stirring reaction for 5-24 hours under the conditionof 80-160 DEG C; and S2, after reaction is finished, cooling to a room temperature, filtering, carrying out rotary evaporation under reduced pressure to remove a solvent so as to obtain a coarse product, and purifying the coarse product by column chromatography to obtain the final product. The compound has potential drug activity in the fields of prostaglandin E1 receptor antagonist, bacteria resistance, tyrosine kinase inhibition effect, tumor resistance and the like.
Owner:WUYI UNIV
P2Y12 receptor platelet aggregation detection reagent card and production method thereof
InactiveCN107607728AEfficient detectionEffective assessmentBiological testingIsoprostaglandin E1Thrombus
The invention provides a P2Y12 receptor platelet aggregation detection reagent card and a production method thereof. The P2Y12 receptor platelet aggregation detection reagent card comprises four detection channels, the first channel contains a lyophilized reagent coated with BSA and fibrinogen and contains no activators, the second channel contains a lyophilized reagent coated with the BSA and fibrinogen and also contains a platelet maximum activator iso-TRAP (thrombin receptor activating peptide), the third channel contains no lyophilized reagents, and the fourth channel contains the lyophilized reagent coated with the BSA and fibrinogen, and also contains a platelet P2Y12 receptor activator ADP (adenosine diphosphate) and a P2Y1 receptor antagonist PGE1 (prostaglandin E1). The patient'sP2Y12 receptor pathway-correlated platelet aggregation function is rapidly and effectively detected through optical nephelometry by using the detection reagent card. The card has the advantages of high sensitivity, strong specificity, easiness in operation, and suitableness for bedside fast detection, and can well predict the future thrombus or bleeding risk in patients treated with the P2Y12 receptor antagonists.
Owner:北京乐普诊断科技股份有限公司
Therapeutic agent for ameliorating prognosis after lower limb amputation surgery
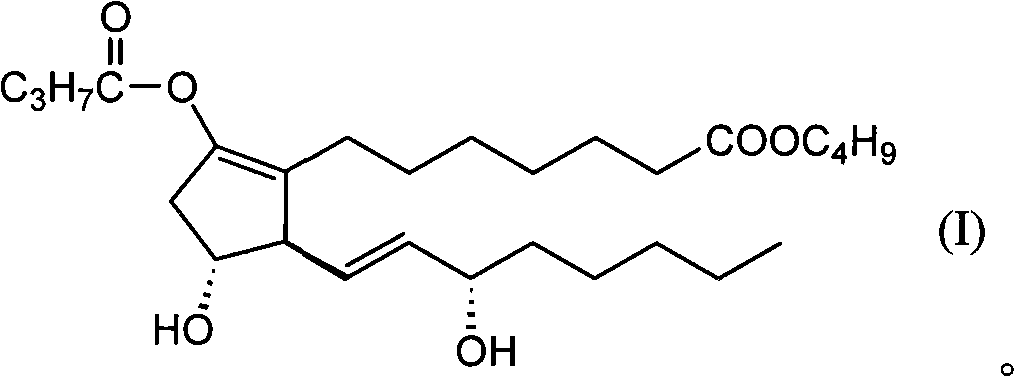
ActiveCN101622003AReduce deathImprove postoperative statusOrganic active ingredientsCardiovascular disorderVeinLipid particle
Disclosed is a therapeutic agent for ameliorating the prognosis after lower limb amputation surgery which is induced by severe peripheral arterial disease. The therapeutic agent comprises a prostaglandin E1 (PGE1) derivative shown by the formula (I) (AS-013) as an active ingredient. Preferably, the therapeutic agent takes a dosage form of a preparation for intravenous or local administration. More preferably, the preparation for injection takes a dosage form of a liposome preparation having the active ingredient AS-013 embedded in a lipid particle.
Owner:BEIJING TIDE PHARMA
Topical compositions for prostaglandin E1 delivery
InactiveCN1284862AAllergenicity reductionAvoid damageElcosanoid active ingredientsAerosol deliveryAlcoholChemical composition
A topical composition of a semi-solid consistency suitable is provided for transdermal application of prostaglandin E1. The composition comprises prostaglandin E1, a penetration enhancer, a polysaccharide gum, a lipophilic compound, and an acidic buffer system. The penetration enhancer is an alkyl-2-(N,N-disubstituted amino)-alkanoate ester, an (N,N-disubstituted amino)-alkanol alkanoate, or a mixture of these. The lipophilic compound may be an aliphatic C1 to C8 alcohol, an aliphatic C8 to C30 ester, or a mixture of these. The composition includes a buffer system capable of providing a buffered pH value for said composition in the range of about 3 to about 7.4.
Owner:FERRING INT CENT SA
Prostaglandin compositions and method of treatment for male erectile dysfunction
InactiveCN1394140AHigh impedanceEasy to presentElcosanoid active ingredientsPharmaceutical delivery mechanismFunctional disturbanceIsoprostaglandin E1
The invention provides methods of treating erectile dysfunction comprising the step of placing within the fossa navicularis of the patient an effective erection-inducing amount of a prostaglandin E1 composition of a semi-solid consistency. The composition comprises prostaglandin E1, a penetration enhancer, a polysaccharide gum, a lipophilic compound, and an acidic buffer system. The penetration enhancer is an alkyl-2-(N,N-disubstituted amino)-alkanoate ester, an (N,N-disubstituted amino)-alkanol alkanoate, or a mixture of these. The lipophilic compound may be an aliphatic C1 to C8 alcohol, an aliphatic C8 to C30 ester, or a mixture of these. The composition includes a buffer system capable of providing a buffered pH value for said composition in the range of about 3 to about 7.4.
Owner:NEXMED HLDG INC
Lavage preservation solution for pulmonary transplantation
A lavage preservation solution for lung transplantation relates to an organ transplantation technique. According to the scheme provided by the present invention, the lavage fluid contains potassium 3.0-6 mmol / liter, sodium 130-168 mmol / liter, magnesium 1.0-2.5 mmol / liter, chlorine 100-110 mmol / liter, Phosphate 30-40 mmol / L, sulfate 3.5-5.5 mmol / L, prostaglandin E1 108-150 mg / L, raffinose 30-50 mmol / L, low molecular dextran 40 20-38 g / L Lift. The lavage solution can prolong the preservation time of transplanted organs, improve the survival rate of transplanted organs, and preserve the functions of transplanted organs to the greatest extent possible.
Owner:陈静瑜
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com