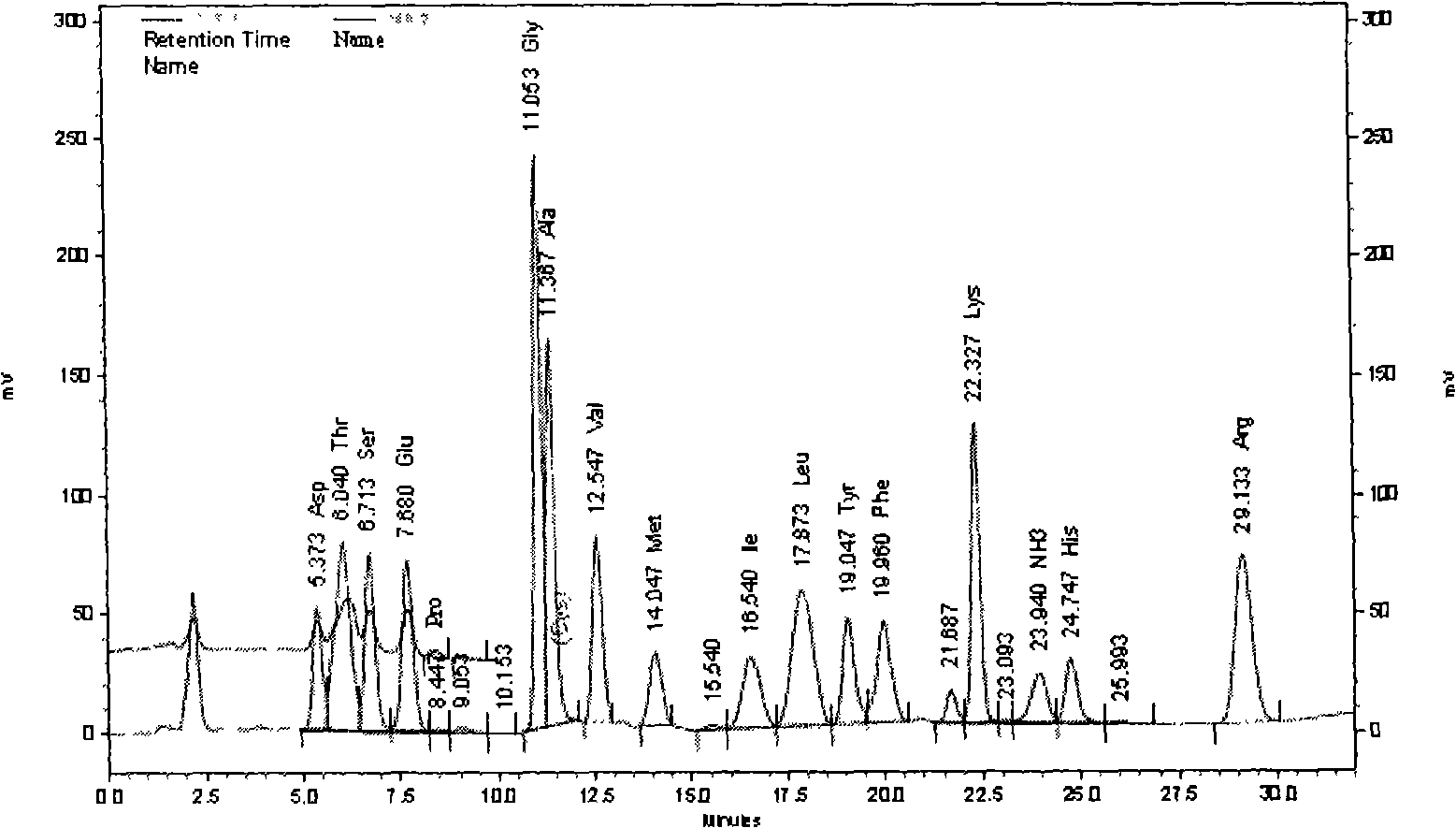
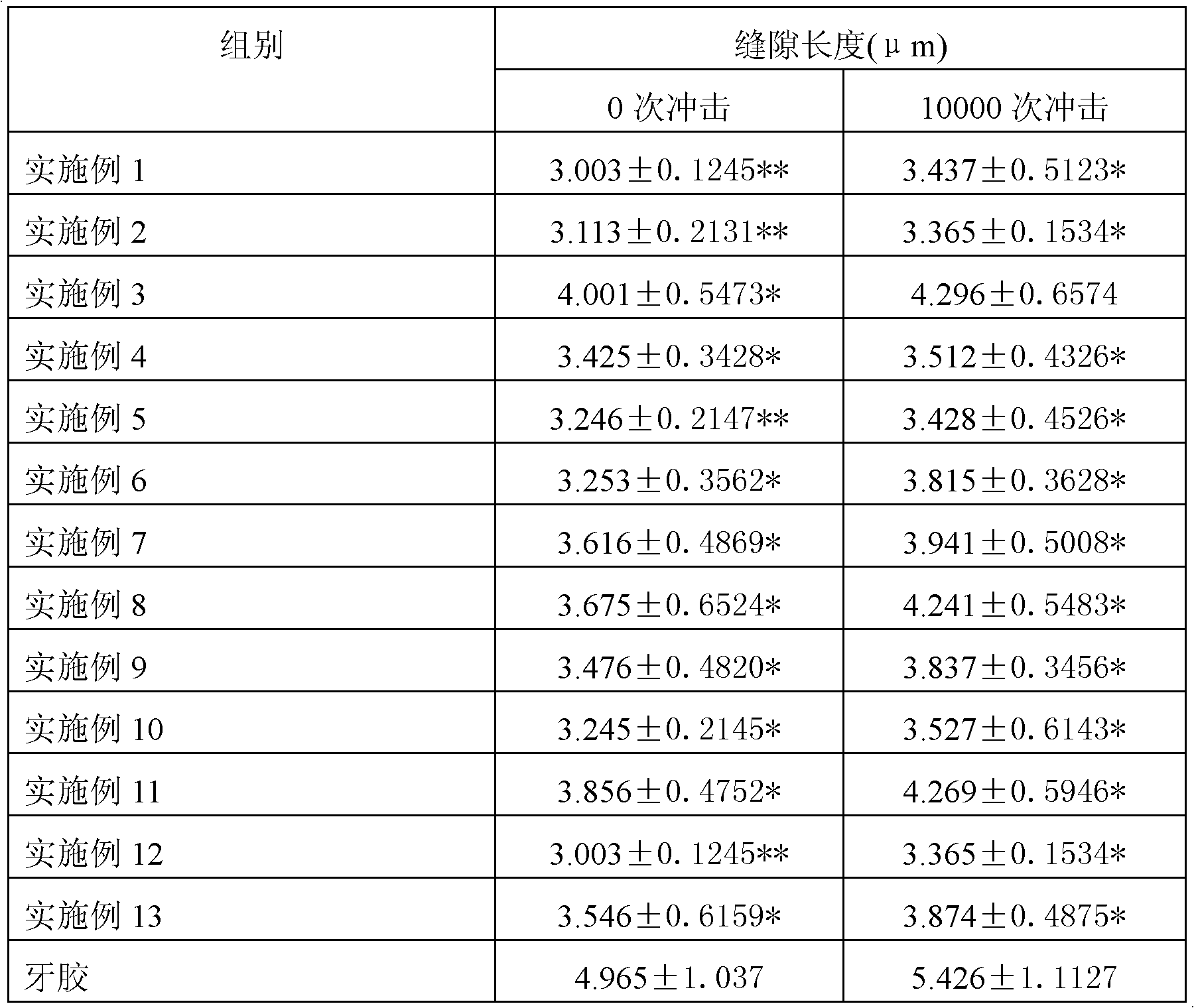
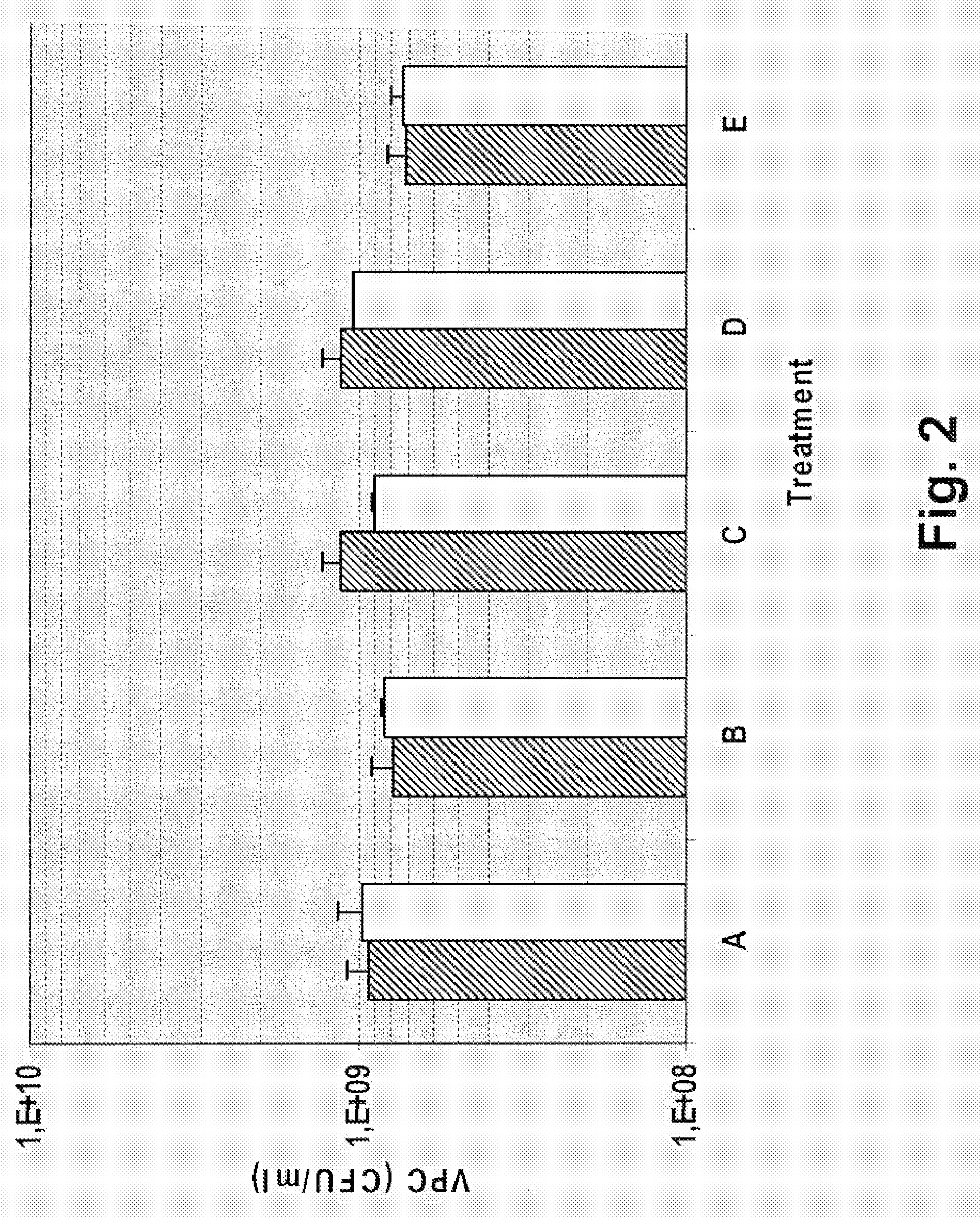
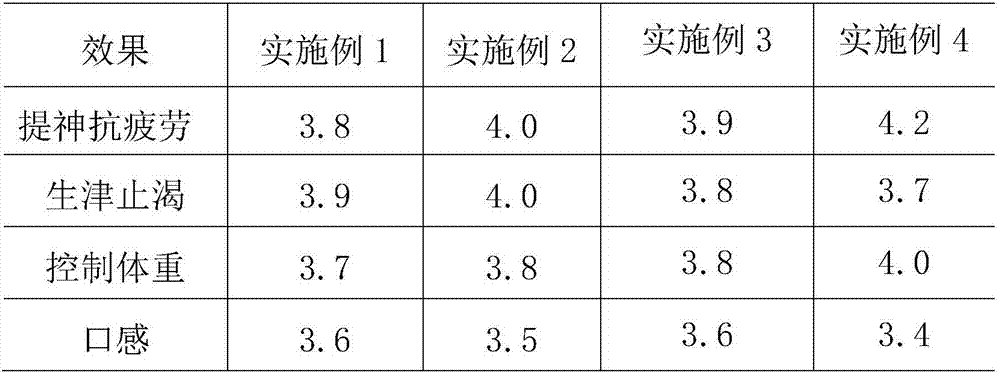
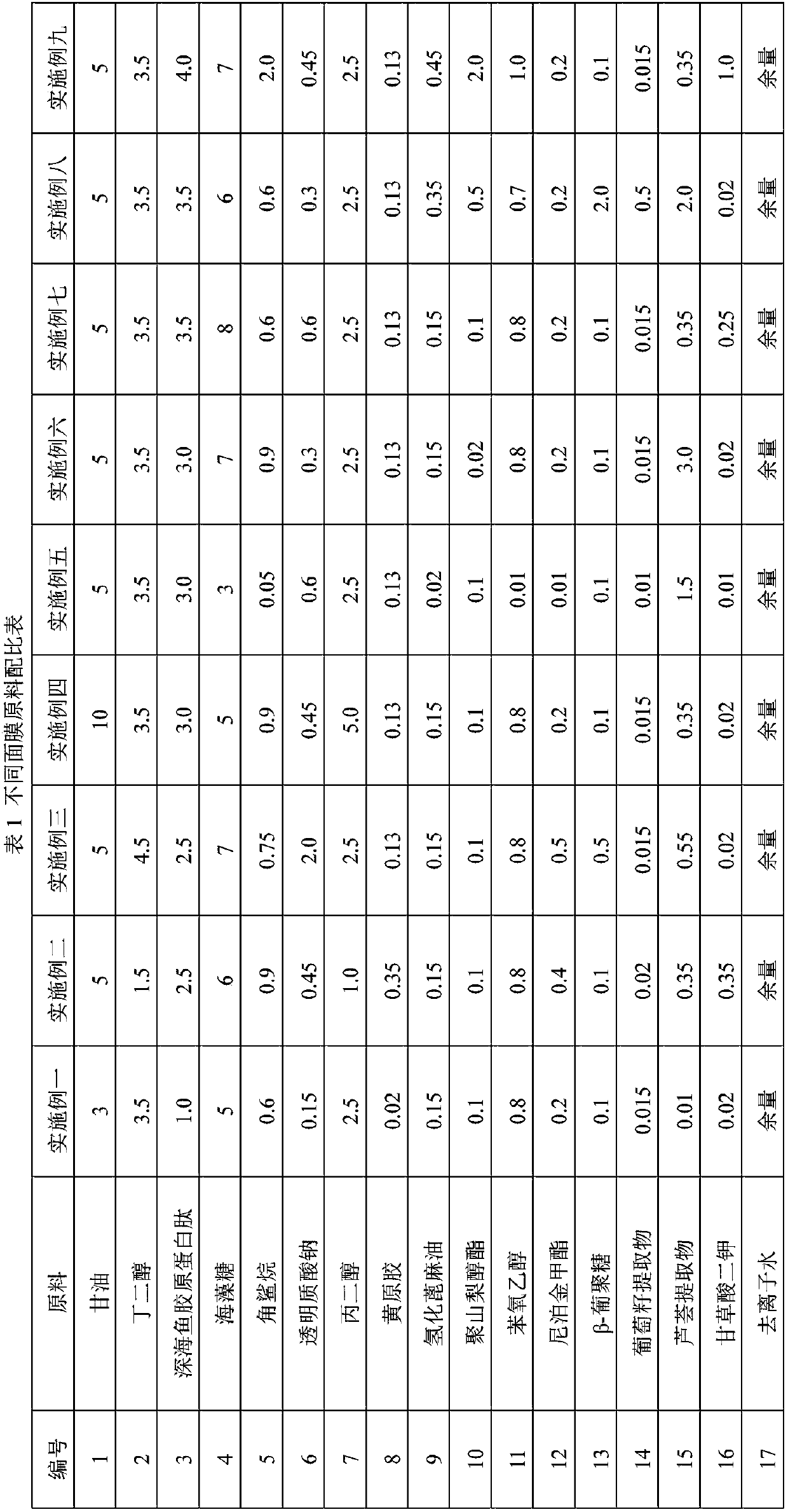
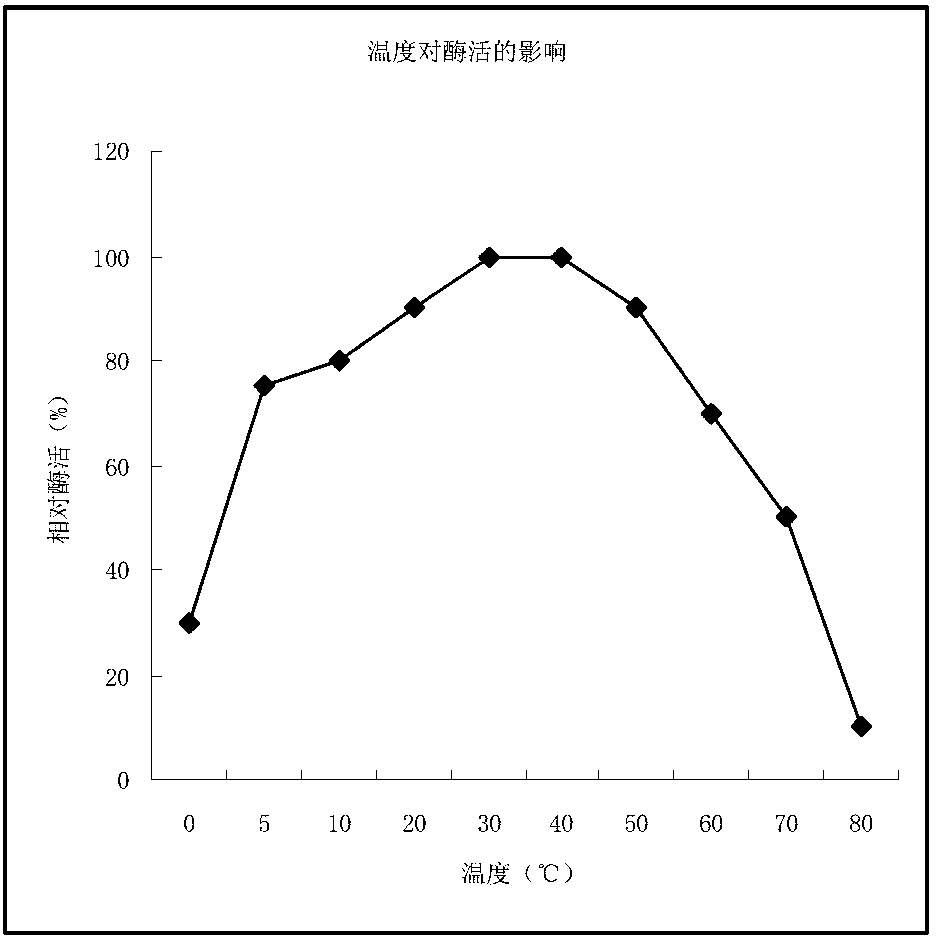
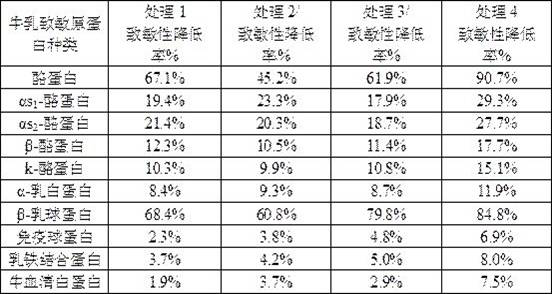
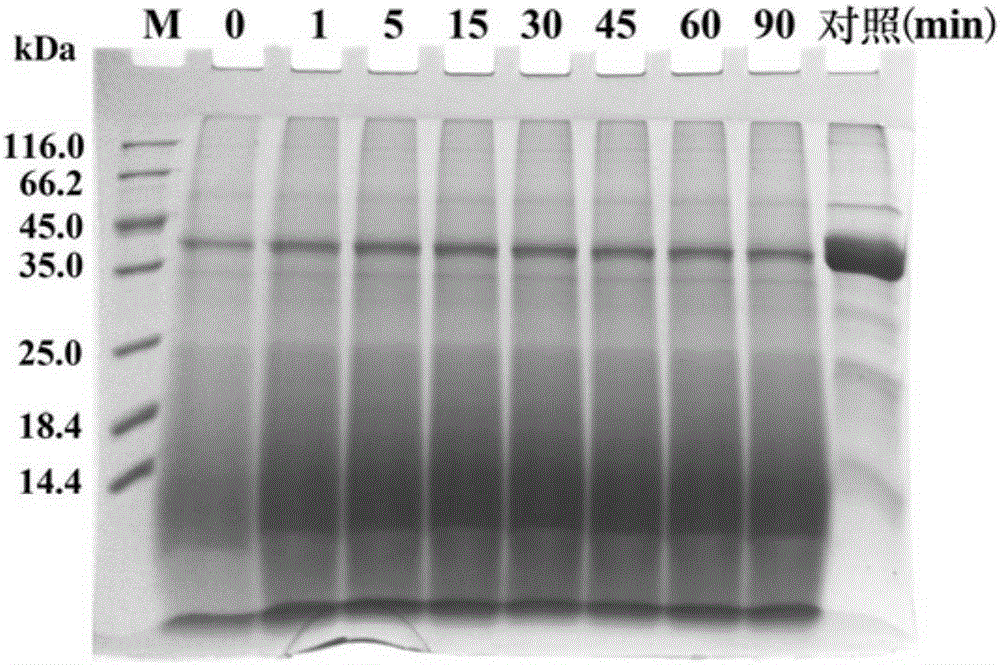
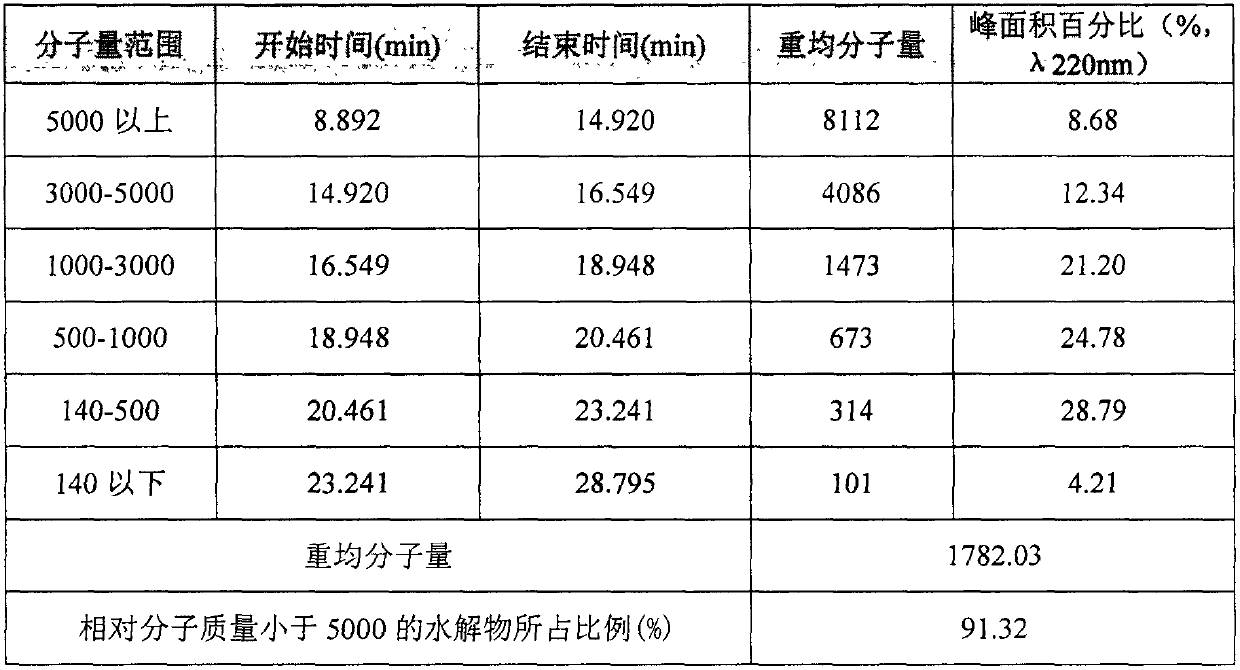
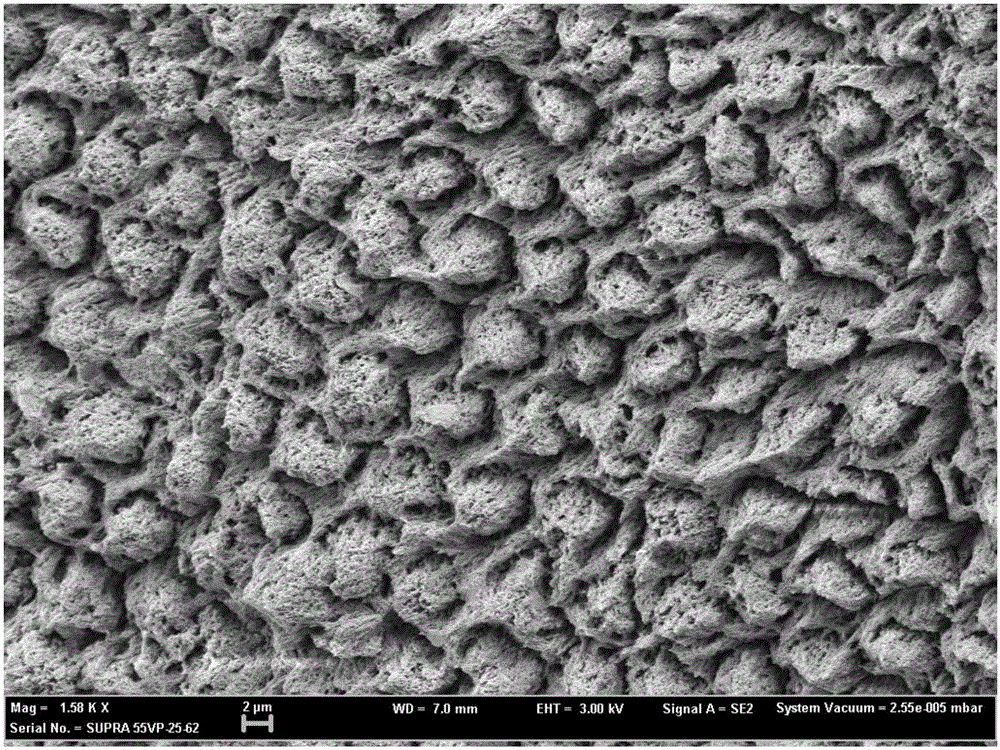
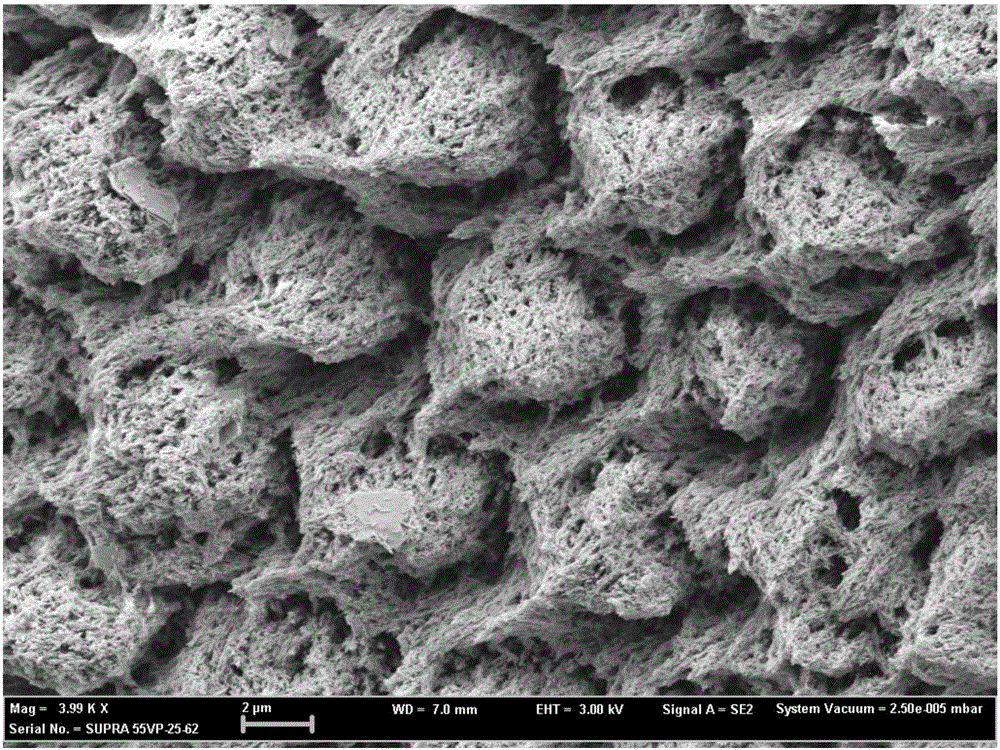
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
341results about How to "Allergenicity reduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-protein foaming compositions and methods of making the same
ActiveUS20060040034A1High densityHigh gas contentMilk preparationFrozen sweetsProtein freeProduct gas
A protein-free soluble foaming composition is provided which contains carbohydrate particles having a plurality of voids containing entrapped pressurized gas. The composition may include a surfactant and may be contained in a food product such as a beverage mix or an instant food. In addition, a method is provided for manufacturing the foaming composition in which the protein-free soluble foaming particles are heated and an external pressure exceeding atmospheric pressure is applied to the protein-free soluble foaming particles. The soluble foaming particles are cooled and the external gas pressure is released resulting in pressurized gas remaining in internal voids of the foaming composition.
Owner:INTERCONTINENTAL GREAT BRANDS +1
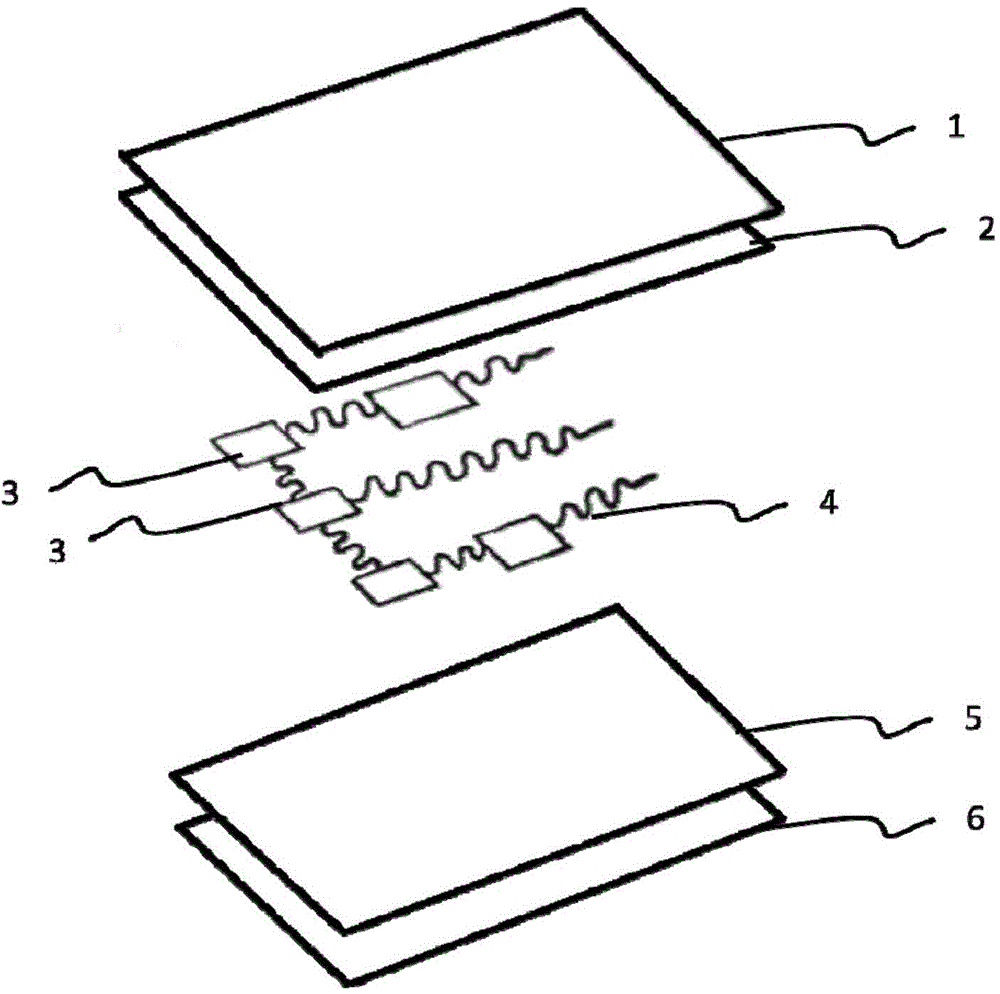
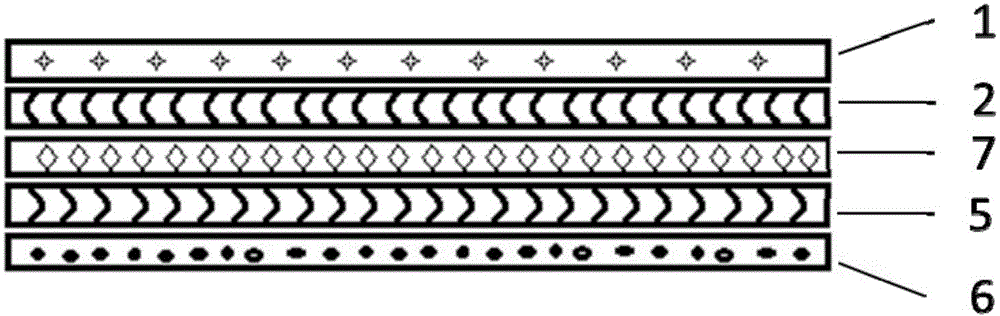
Flexible and extendable electronic device based on biocompatible films and manufacturing method
ActiveCN104523227AAvoid rednessAvoid reactionPorous dielectricsCircuit bendability/stretchabilityExtensibilityBiocompatibility Testing
The invention relates to a flexible and extendable electronic device based on biocompatible films and a manufacturing method, and belongs to the technical field of flexible and extendable electronic devices. The flexible and extendable electronic device is technically characterized in that the biocompatible films are used as an encapsulation layer and a substrate layer of the flexible and extendable electronic device, a bonding layer used for enhancing the boundary strength between the encapsulation layer and a functional layer can be further arranged between the encapsulation layer and the functional layer, and an adhesive layer used for enhancing the adhesive force of the device with the surface of an object to be tested is arranged underneath the substrate layer; the functional layer is of a flexible and extendable structure. In the manufacturing process of the flexible and extendable electronic device, a transfer printing technology based on a solution is mainly adopted to achieve integration of the functional layer and a flexible substrate. The flexible and extendable electronic device structurally keeps and even improves flexibility and extensibility. Meanwhile, compatibility features such as waterproofness, breathability and low allergenicity of the flexible and extendable electronic device allow the flexible and extendable electronic device to normally work on the surface of a human body for more than 24 hours without causing a foreign body feeling and discomfort, and skin soaking or rubefaction or other anaphylactic reactions caused by poor biocompatibility can be avoided.
Owner:浙江智柔科技有限公司
Fish protein powder and preparation method thereof
InactiveCN101595939AMeet the need for protein supplementationPromote digestionProtein composition from fishFood preparationWater solubleDigestion
The invention provides a fish protein powder which is water soluble with the color between light-yellow and pale-brown meal, wherein, the dry basis protein content of the fish protein powder is more than 80%; and a preparation method is provided for the fish protein powder. The method comprises the following steps: (1) preparing mincedfish seriflux; (2) preparing enzymatic hydrolyzate by the mincedfish seriflux; (3) preparing the fish protein powder by the enzymatic hydrolyzate. The fish protein powder prepared in the invention has the advantages of health, easy digestion, easy absorption, and the like; and can be widely used on protein-enhanced foods; the production process is simple, the production efficiency is high, and the raw materials have wide sources and can be obtained easily, thereby helping save cost.
Owner:SOUTH CHINA UNIV OF TECH
Method for producing sea-crab seasoner products material
The invention discloses a method for producing the raw materials of a seasoning product of sea crab; the method comprises the following steps: wastes of the sea crab are smashed; the sea crab is used for processing and cooking the concentrated solution of soup ingredient (namely, sea crab juice), heating is carried out to inactivate the enzyme, the process of mixed acid is used for carrying out deordorization, and filtering is carried out so as to remove the residue, etc. As the invention uses the hydrolyzation of papain to lead the protein in the waste of the sea crab to be decomposed furthest and the allergen to be removed, the fresh substances are released completely, while flavourzyme is adopted to carry out debittering process and inhibit the reproduction of microorganism in the process of enzymolysis; besides, as the invention uses weak acid process of citric acid and malic acid to carry out deodorization to the hydrolyzate or further uses low-temperature concentration and spray drying, the original nutrient ingredients and unique flavor of the product materials are maintained to the utmost degree and the property of the allergen is reduced.
Owner:JIMEI UNIV
Use of gel material in therapeutic process of dental disease
ActiveCN103006444AShorten the timeReduce the number of referralsImpression capsDentistry preparationsDiseaseMedicine
The invention discloses a use of a gel material in a therapeutic process (a dental filling process) of a dental disease. The use is characterized in that the gel material is applied to cavity preparation in the therapeutic process of the dental disease and then used for temporarily sealing in the therapeutic process of the dental disease, wherein the gel material is selected from temperature-sensitive type hydrogel or solvent-sensitive type gel; the phase transition temperature of the temperature-sensitive type hydrogel is 25 DEG C to 36 DEG C, i.e., when the temperature is lower than the phase transition temperature, the gel material is in a liquid state, and when the temperature is higher than the phase transition temperature, the gel material is in a solid state; the solvent-sensitive gel is insoluble in water, and freely soluble in other solvents; and the solvent-sensitive gel is in the liquid state in a process of dissolving in the solvents, the solvent-sensitive gel is in the solid state after the solvents are reduced.
Owner:韩冰
Pet food for reducing food allergy reactions
InactiveUS20070031534A1Reduce generationEasy to useFood processingAnimal feeding stuffBiotechnologyHypersensitive response
As a pet food produced to contain no proteins that can become allergens, a pet food that contains 1 or more types of amino acids or salts thereof instead of protein raw materials that can become allergens or a pet food produced to contain, in addition to the above amino acid(s), 1 or more types of protein raw materials with low allergenicity selected from any of potato, sweet potato, rice, foxtail millet, barnyard millet, kaoliang, corn, pea, brewer's yeast, and baker's yeast is provided.
Owner:NOSAN CORP +1
Non-protein foaming compositions and methods of making the same
ActiveUS7534461B2Improve the immunityAllergenicity reductionMilk preparationFrozen sweetsProduct gasAtmospheric pressure
A protein-free soluble foaming composition is provided which contains carbohydrate particles having a plurality of voids containing entrapped pressurized gas. The composition may include a surfactant and may be contained in a food product such as a beverage mix or an instant food. In addition, a method is provided for manufacturing the foaming composition in which the protein-free soluble foaming particles are heated and an external pressure exceeding atmospheric pressure is applied to the protein-free soluble foaming particles. The soluble foaming particles are cooled and the external gas pressure is released resulting in pressurized gas remaining in internal voids of the foaming composition.
Owner:INTERCONTINENTAL GREAT BRANDS LLC +1
Swelling-type hollow silk fibroin micro-needle drug delivery system and preparation method thereof
ActiveCN104888284AHigh drug loading rateAllergenicity reductionMicroneedlesSurgeryDermal sensitizationProcessing cost
The invention discloses a swelling-type hollow silk fibroin micro-needle drug delivery system and a preparation method thereof. According to the technical scheme of the preparation method, a swelling modified silk fibroin solution is casted in a PDMS mold to be vacuumized, dried and molded as a hollow needle-type housing. After that, a silk fibroin micro-needle array provided with cavities in the hollow needle-type housing is filled with powder-like or solution-like drugs, vacuumizing and drying are carried out to form a drug part. Finally, a layer of modified silk fibroin solution is cast, dried and molded as a cladded coating. The cladded coating is removed to obtain the swelling-type hollow silk fibroin micro-needle drug delivery system. The drugs are wrapped inside the cavities of the silk fibroin micro-needle arrays, so that the system is high in drug loading ratio and low in skin allergenicity and stimulation. The system facilitates the sustained release and the controlled release of micro-needle drugs. Meanwhile, according to the preparation method, since the swelling modified silk fibroin solution is cast, dried and molded in a mild processing condition, the system is low in processing cost and suitable for large-scale production. Drugs are directly wrapped in the cavities of the swelling hollow micro-needles, so that controlled-release drugs are swelled in the micro-needle base material. In this way, the biological activities of the controlled-release drugs are maintained, while the structure of the hollow silk fibroin micro-needle drug delivery system is greatly simplified. The system is better in practicality.
Owner:PHARSUN MEDICAL BIOTECHNICS (SHANGHAI) CO LTD
Light fasting full nutrition formula brewing instant bar and preparation method thereof
InactiveCN109645320AReasonable configurationEnsure balanceNatural extract food ingredientsFood ingredient functionsDietary fiberPreservative
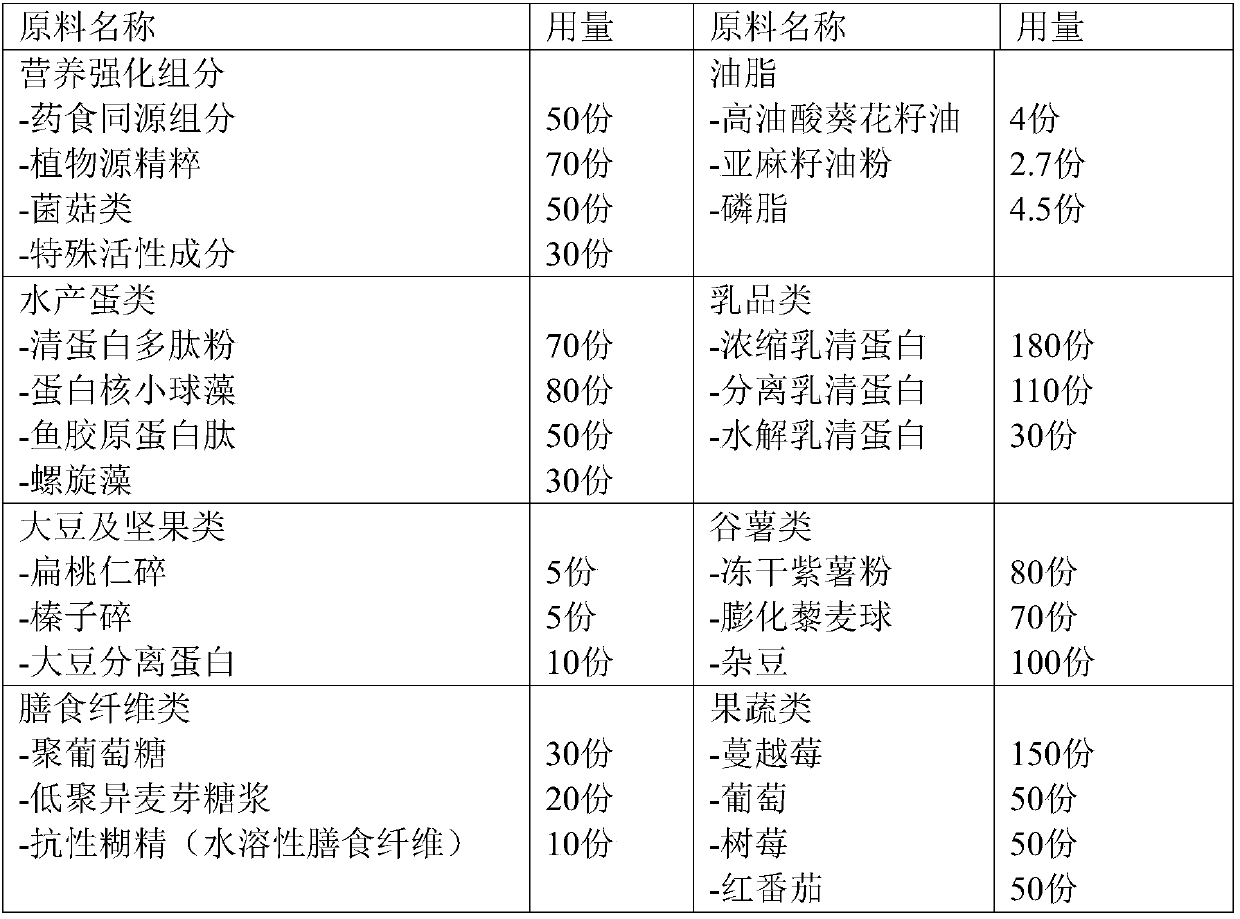
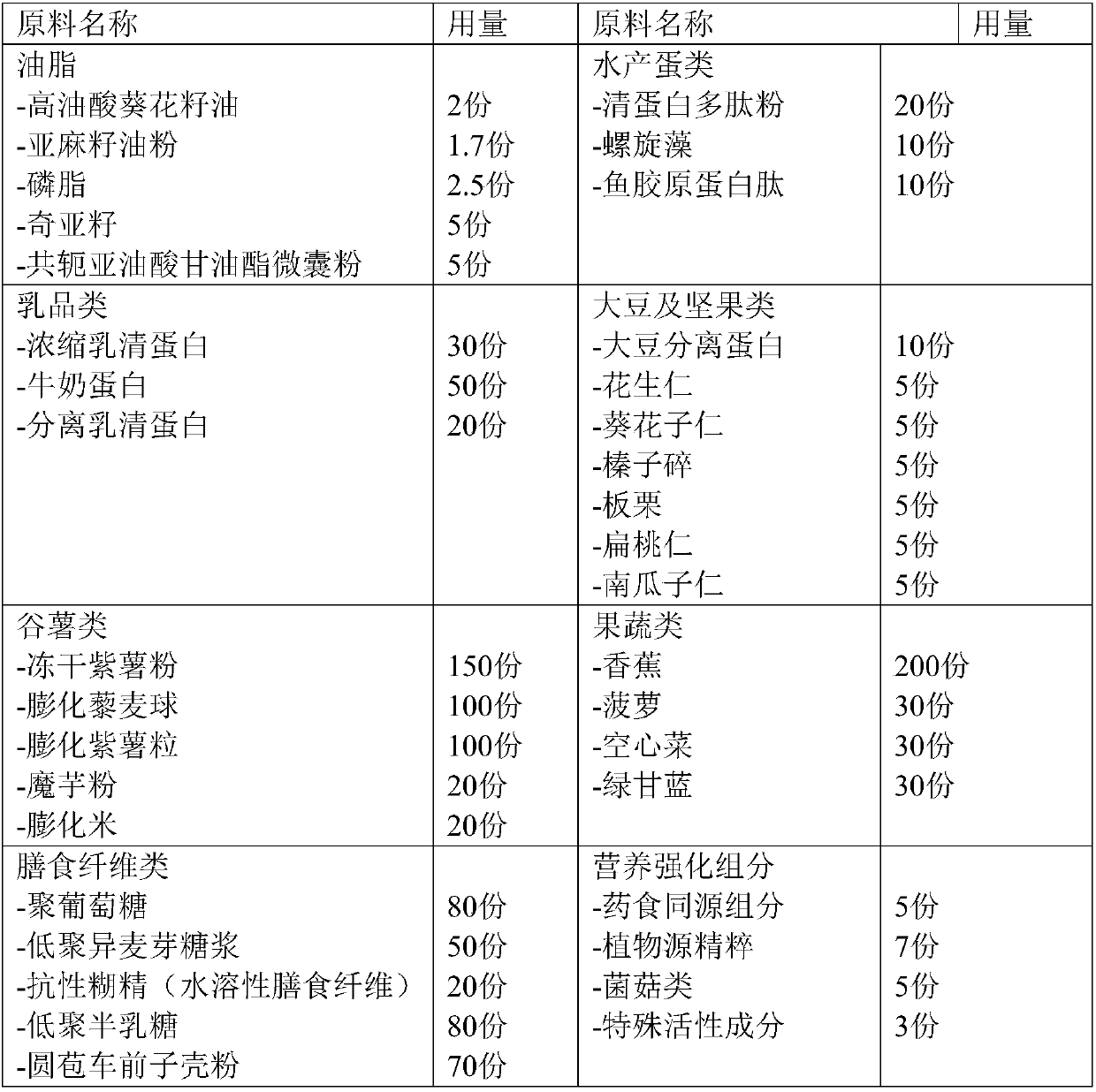
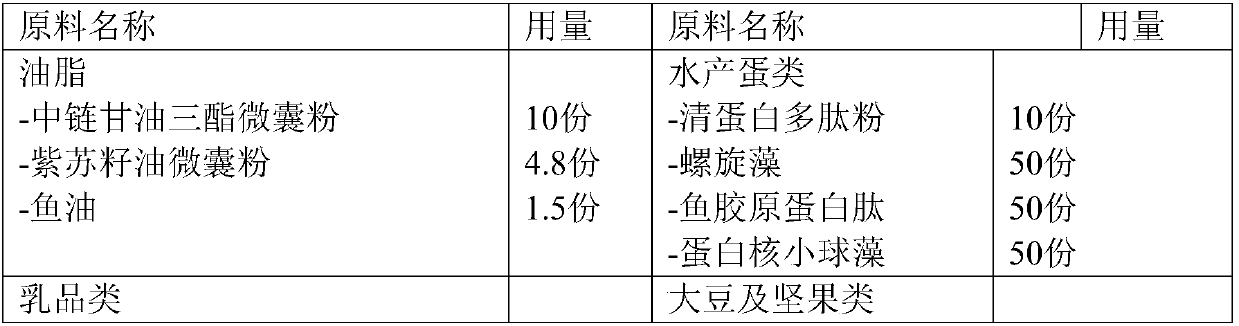
The invention provides a light fasting full nutrition formula brewing instant bar and a preparation method thereof. The formula can brew various components of instant nutrient nutrients according to the suggested configuration of the 'Chinese Dietary Guidelines', foods are diverse, and no preservatives, no sweeteners and no artificial pigments are added; the bar is prepared from, by weight, oil, aquatic eggs, dairy products, soybeans, nuts, cereal potatoes, dietary fibers, fruits and vegetables, nutritional strengthening components and the like, is convenient to eat, and is especially suitablefor meeting the full nutritional needs of a body during light fasting and fat reduction.
Owner:北京万莱康营养与健康食品科学技术研究院有限公司
Method of modifying lactoalbumin by enzymatic method and its application
ActiveCN1596676AImprove the degree of enzymatic hydrolysisHigh selectivityMilk preparationEnzyme methodProtein formation
A process for modifying the lactoprotein by enzyme method features that the immobilized composite enzyme, the ionically regulatory protein structure and heat treating technique are used as selectively hydrolyze the alpha S-casein and beta-lactoglobulin in lactoprotein, resulting in easy digestion and low sensitization. It can be used for composite milk for baby, old man and patient.
Owner:YINGTAN HUABAO FLAVORS & FRAGRANCES
Preparation method of improved di-lysine-aspirin
ActiveCN101633624AIncreased crystal fluidityShort reaction timeOrganic active ingredientsOrganic chemistryChemistryO-acetylsalicylic acid
The invention discloses a preparation method of improved di-lysine-aspirin, comprising the following steps: adding a DL-lysine aqueous solution with the mass percentage concentration being 25-30 percent to an alcobolic solution of aspirin at the temperature of 15-25 DEG C; enabling the mass ratio of DL-lysine to aspirin to be 1:(1.3-1):1.5; mixing and crystallizing for 5-15 minutes; then adding precipitation alcohol with the mass being 3-4 times of aspirin; reducing the temperature within 10 DEG C, and mixing and culturing crystal for 0.5-1.5 hours; filtering and then obtaining crystal; and washing the crystal by an amount of absolute ethyl alcohol for 1-2 times. The invention enables the acid value of a product to be reduced below 6.0 by changing the mixture ratio of reactants, enables the crystal fluidity of the product to be enhanced by changing the flow acceleration of materials and enables the appearance and free oxybenzoic acid of the product to be improved by reducing the temperature of the whole system, thereby reducing the acrimony and the sensitization of medicines and improving the quality of aspirin.
Owner:BENGBU BBCA MEDICINE SCI DEV
Acidophilus goat milk and method for making same
InactiveCN101081045AInhibition of reproductionRegulate stomachMilk preparationSoured milkMilk products
The present invention discloses one kind of sour ewe milk and its production process, and belongs to the field of sour milk producing technology. Fresh ewe milk or remade ewe milk as the material is produced into curd type sour ewe milk, stirred sour ewe milk or flavored sour ewe milk through purifying, standardizing, compounding, thickening, sterilizing, fermenting, cooling and other steps. These sour ewe milk products are healthful, and the production process is simple and suitable for industrial application.
Owner:张保钢
Hypoallergenic milk protein powder and preparation method thereof
ActiveCN104719610AEasy to prepareEase of industrial productionAnimal proteins working-upFlavorUltrafiltration
The invention relates to hypoallergenic milk protein powder and a preparation method thereof and belongs to the field of processing of milk products. The preparation method provided by the invention comprises the following steps: by taking milk protein powder as a raw material, fully dissolving in water, then adding compound proteinase, obtaining a milk protein enzymolysis solution by using a biological enzymolysis technology, performing ultrafiltration on the milk protein enzymolysis solution through a ceramic membrane to obtain a protein solution with molecular weight of less than 10000KD, concentrating and drying to obtain the hypoallergenic milk protein powder provided by the invention. The hypoallergenic milk protein powder is whole milk protein, so that the sensitization of whey protein and casein protein is reduced, and the local flavor is good; and the hypoallergenic milk protein powder is safe to eat, has no additives and can provide the complete milk protein for people sensitive to the whey protein. The method for preparing the milk protein powder is simple and easier to realize industrial production and has huge application prospects and market values.
Owner:CHINA AGRI UNIV
Formula for supplementing nutrients of bone and improving functions of bone and preparation method
InactiveCN110074250ACan adjust demandIntake can be adjustedVitamin food ingredientsFood ingredient functionsCalcium in biologyTreatment effect
The invention discloses a formula for supplementing nutrients of bone and improving functions of bone and a preparation method. The formula comprises the following components in percentage by mass: 30to 70% of bone collagen protein peptide powder, 1 to 10% of L-aspartic acid calcium, 1 to 10% of hydrolyzed II-type collagen, 1 to 10% of casein phosphopeptide powder, 1 to 10% of milk minerals, and0.0002 to 0.005% of vitamin D. The formula has the advantages that according to the cause of bone diseases, several kinds of pure animals and plants are used as the raw materials and are reasonably compounded and scientifically proportioned, so that while the content of calcium in a human body is supplemented, the bone collagen protein is supplemented, and the full absorbing of calcium is accelerated; after the formula is taken for a long time, the demand and intake amount of calcium by the human body can be adjusted, and the formula is especially suitable for patients with osteoporosis or arthritis; any side or toxic effect is avoided, the auxiliary treatment effect is realized on treatment of various bone diseases, the nutrients of the bone can be supplemented, the functions of the bonecan be improved, and the functions of enhancing the immunity of the human body, resisting the aging and resisting the fatigue are realized.
Owner:广东澳思瑞雅健康美容生物科技有限公司
Composition comprising enzymatically digested yeast cells and method of preparing same
The present invention relates to the field of fermentation media. More specifically, the invention provides a method for preparing a composition useful for culturing microbial cells wherein whole and / or autolysed yeast cells are enzymatically treated to obtain the composition. The microbial cultures obtained have increased stability and are useful in the manufacturing of food, feed and as a pharmaceutical product.
Owner:CHR HANSEN AS
Refreshing anti-fatigue solid beverage
InactiveCN106889412AVarious ingredientsFull of nutritionNatural extract food ingredientsFood ingredient functionsNutrientPeptide
The invention discloses a refreshing anti-fatigue solid beverage. The solid beverage is prepared from the following raw materials: plant extract, a sweetening agent, an acidity regulator, a nutrient supplement, maltodextrin, plant protein peptide powder and mixed-fruit flavored essence. The preparation process of the solid beverage comprises the steps of inspecting raw materials, preparing materials, mixing materials, sterilizing, quantitatively packaging, inspecting, filling into cartons and storing. The refreshing anti-fatigue solid beverage has rich nutrition and good refreshing and anti-fatigue effect by adding multiple plant extracts, is easy to prepare and convenient to carry, is especially suitable for people who operate computers for a long time, drivers and students. The solid beverage further contains electrolyte, can be taken by sporting groups to keep homeostasis and regulate muscle contraction, and cannot cause nausea, headache and other situations.
Owner:安徽元麦凡康食品有限公司
Hydrating and skin rejuvenating mask and preparation method thereof
ActiveCN108042388AAllergenicity reductionNo stimulationCosmetic preparationsToilet preparationsAbsorption of waterMoisture
The invention discloses a hydrating and skin rejuvenating mask and a preparation method thereof, and belongs to the technical field of masks. The hydrating and skin rejuvenating mask comprises the following raw materials in percentage by weight: 3 to 10 percent of glycerinum, 1.5 to 4.5 percent of butanediol, 1 to 4 percent of deep-sea fish collagen peptide, 3 to 8 percent of mycose, 0.05 to 2 percent of squalane, 0.15 to 2 percent of sodium hyaluronate, 1 to 5 percent of propylene glycol, 0.02 to 0.35 percent of xanthan gum, 0.02 to 0.45 percent of hydrogenated castor oil, 0.02 to 2 percent of polysorbate, 0.01 to 1 percent of phenoxyethanol, 0.01 to 0.5 percent of methylparaben, 0.1 to 2 percent of beta-glucan, 0.01 to 0.5 percent of a grape seed extract, 0.01 to 3 percent of an aloe extract, 0.01 to 1 percent of dipotassium glycyrrhizinate and the balance of deionized water. The mask provided by the invention promotes the absorption of water by the skin, is favorable for the skin topreserve moisture for a long time, is non-irritant and has a good effect of moisturizing skin.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD
Composition Comprising Carbohydrates and Peptides which Comprise Tryptophan
InactiveUS20090270337A1High tryptophan/LNAA ratioGood uptakeNervous disorderDipeptide ingredientsPeptideTryptophan
An edible composition comprising peptides rich in tryptophan, which edible composition further comprises a rapidly available glucose composition and a slowly available glucose composition.
Owner:CONOPCO INC D B A UNILEVER
Gelatin composition, and preparation method and application thereof
ActiveCN103263688AGood hemostatic abilityControl flexibilityAbsorbent padsMacromolecular non-active ingredientsGlutaminaseGelatin
The invention provides a gelatin composition. The gelatin composition comprises hydroxylated gelatin, microbial glutamine transaminase and protein glutaminase, wherein the hydroxylated gelatin is 20-60%proline-hydroxylated gelatin. The gelatin composition has an extremely-good bleeding stopping effect at a temperature in a range of 4-10DEG C. The invention also discloses a preparation method of the gelatin composition and an application of the gelatin composition.
Owner:李肯
Lactic acid bacteria goat milk beverage and preparing process thereof
InactiveCN101081046APrevents bloatingPrevent diarrheaMilk preparationLactic acid bacteriumSheep milk
The present invention discloses one kind of ewe milk beverage with lactic acid bacteria, and belongs to the field of beverage producing technology. Sour ewe milk as the main material is produced into the ewe milk beverage with lactic acid bacteria through mixing with water, sugar or sweetening agent, fruit juice, stabilizer and other components; filtering; sterilizing; homogenizing and other steps. The ewe milk beverage with lactic acid bacteria is healthful, and the production process is simple and suitable for industrial application.
Owner:DALIAN JIUYANG DAIRY
Liquid milk preparation method based on reduction of cow milk sensitization
PendingCN111631261AAllergenicity reductionHigh nutritional valueMilk preparationBiotechnologyPhysiology
The invention relates to a liquid milk preparation method based on reduction of milk sensitization. The preparation method comprises the following steps: (1) degreasing; (2) preheating; (3) enzymolysis: after the preheating, taking skimmed milk, sequentially adding three protease solutions into the skimmed milk in a dropwise adding manner, and carrying out enzymolysis, wherein the enzymolysis timeis 60-120 min, the temperature is 50-60 DEG C, and the pH value is adjusted in different time periods so as to obtain an enzymatic hydrolysate; and (4) enzyme deactivation. Small peptides with a molecular weight of less than 5000 Daltons in the hypoallergenic liquid milk account for about 99%. The liquid milk preparation method for reducing the milk sensitization is used for reducing the sensitization of a plurality of allergen proteins in the cow milk, and the allergen proteins in the cow milk comprise one or more of casein, alpha s1-casein, alpha s2-casein, beta-casein, k-casein, alpha-lactalbumin, beta-lactoglobulin, immunoglobulin and lactoferrin.
Owner:SHENYANG AGRI UNIV
Respiratory syncytial virus-like particles and preparation method and application thereof
ActiveCN103642761AHigh expressionIncrease productionInactivation/attenuationImmunoglobulins against virusesSucroseShuttle vector
The invention relates to respiratory syncytial virus-like particles, and establishes a novel method of preparing respiratory syncytial virus-like particles for animal immunization. The preparation method comprises the following steps: according to codon bias of mammalian cells, carrying out codon optimization for M, F and G genes which are respectively cloned to a shuttle vector to obtain recombinant adenovirus plasmids, and transfecting to obtain the recombinant adenovirus FGAd-Msyn, FGAd-Fsyn and FGAd-Gsyn; infecting the recombinant adenovirus by use of Vero cells to obtain the virus-like particles; purifying the virus-like particles by adopting a sucrose density gradient centrifugation method; applying the particles to animal immunization. According to the invention, the virus-like particles are prepared by adopting the adenovirus vector, and a novel virus-like particle preparation method is established. The protein expression quantity is improved and the yield of the virus-like particles is improved through codon optimization, so that a better immune effect is obtained, and the respiratory syncytial virus-like particles are relatively safe.
Owner:BEIJING JIAOTONG UNIV
Surfactant-free oil-in-water type emulsion, process for preparation thereof and its uses
InactiveUS20130224133A1Reduced and no irritabilitySuitable sensationCosmetic preparationsTransportation and packagingOil phaseCrosslinked polymers
There is described an oil-in-water emulsion free of surfactant agents comprising: A) At least a cross-polymer acrylate / alkyl-acrylate or derivatives thereof, or mixtures thereof in the aqueous phase of the emulsion; b) At least a polyacrylate or derivatives thereof, or mixtures thereof in the oily phase of the emulsion, and c) At least a neutralizing agent. Further, the present invention refers to a process of obtaining a surfactant-free emulsion and its use in cosmetics and pharmaceuticals compositions. The emulsion in question can be applied to all kinds of skin, being also indicated for people with sensitive skin.
Owner:NATURA COSMETICOS SA
Processing method of dried crab meat floss with low allergy
The invention discloses a processing method of dried crab meat floss with low allergy, and relates to the field of crab meat processing method. The processing method comprises the following steps: washing crabs to remove sand, mud, and dirt on the surface of crabs, removing crab shell, crab gill, and umbilical part of crab, washing the crab bodies, picking out crab meat; soaking the obtained crab meat in a flavoring liquid, then cooking the flavoring liquid, heating the flavoring liquid by slow fire to keep the flavoring liquid in a boiling state until the flavoring liquid is completely evaporated; baking the crab meat until the water content reaches 50%, tearing the crab meat into filaments, putting the crab filaments into a frying pan containing plant oil, frying the crab meat by slow fire until the crab meat become loose and uniform; baking the obtained crab meat in a preheated baking oven to dry the crab meat floss; sterilizing the dried crab meat floss, packing dried crab meat floss into bags, and finishing to obtain finished products. Polyphenol oxidase and caffeic acid are used to soak crab meat for a short time; at the same time, processing technologies such as boiling, baking, and the like are adopted so as to maximally preserve the unique flavor and nutrients of crab meat; moreover, the allergen of crab meat carries out enzymatic crosslinking reactions, and thus the sensitization is reduced. The processing method has the characteristics of simple operation, low cost, and suitability for massive production.
Owner:JIMEI UNIV
Composition comprising carbohydrates and peptides which comprise tryptophan
ActiveUS20110166085A1Raise the ratioPromote absorptionNervous disorderDipeptide ingredientsCompound (substance)D-Glucose
An edible composition comprising peptides rich in tryptophan, which edible composition further comprises a rapidly available glucose composition and a slowly available glucose composition.
Owner:DSM IP ASSETS BV
Method for preparation of egg white oligopeptide having high F value
ActiveCN101012472AHas the effect of protecting the liverReduce odorFermentationPre treatmentOligopeptide
The invention discloses a making method of high-F value egg oligopeptide, which comprises the following steps: a. predisposing raw material; b. hydrolyzing protein for two steps; c. adsorbing through active carbon; d. desalting; obtaining the product with molecular weight at 300-1000 and F value more than 20.
Owner:JILIN JINYI FOOD CO LTD
Nutrient energy type yak milk protein carbohydrate and preparation method thereof
The invention discloses nutrient energy type yak milk protein carbohydrate and a preparation method thereof. The protein carbohydrate is prepared by adding yak milk proteins, protein peptides and other auxiliary nutrient elements including taurine, inositol and the like. The protein carbohydrate is mainly used for crowds overfatiguing because of staying up late and crowds requiring to replenish energy after strenuous exercise; various carbohydrate sources are added to meet the rapid demands of organisms on energy; meanwhile, the nutrients including the yak milk proteins, the protein peptides, fat and the like are added, so that the continuous energy supply is realized; functional factors such as inositol, taurine, vitamins, and mineral substances are matched, so that the energy deficiency required by the organisms is replenished, the fatigue is relieved, the immunities of the organisms are enhanced, and the normal operations of the organisms are ensured.
Owner:GANNAN BEIYITE BIOLOGICAL SCI & TECH
Industrial manufacturing method and use of allergen-eliminated partly appropriately-hydrolyzed casein peptide
InactiveCN103966292AAllergenicity reductionMilk preparationHydrolysed protein ingredientsBiotechnologyNutrition
The invention discloses an industrial manufacturing method and a use of an allergen-eliminated partly appropriately-hydrolyzed casein peptide, and belongs to the technical field of food nutrition and biotechnology. The industrial manufacturing method utilizes food-grade casein as a raw material and realizes preparation of the appropriately-hydrolyzed casein peptide powder by a composite enzymatic hydrolysis-ultrafiltration combined technology. The allergen-eliminated partly appropriately-hydrolyzed casein peptide has average molecular weight of 1500Da-3000Da, protein antigenicity reduced by more than 85%, and has main allergen alpha s1-casein content reduced by more than 95% than casein content. Compared with other appropriately-hydrolyzed casein products on the market, the allergen-eliminated partly appropriately-hydrolyzed casein peptide has obviously reduced sensitization activity and has a small bitter taste and good processing performances. The allergen-eliminated partly appropriately-hydrolyzed casein peptide prepared by the industrial manufacturing method can be used as a novel functional nutrient in common foods, sports functional foods, infant formula milk powder and foods, adult milk powder and foods, milk powder and foods for middle and old age, health foods and medicines and has a wide market prospect.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Remineralizing material for use on demineralized enamel surface and preparation method thereof
ActiveCN106074184AReasonable structural designEasy to prepareImpression capsDentistry preparationsAtherion elymusHydrogen phosphate
The present invention provides a remineralizing material for use on a demineralized enamel surface and a preparation method thereof; the remineralizing material is prepared through the following steps: using carboxymethyl chitosan of certain concentration to stabilize hydrogen phosphate ions in supersaturated ionic solution with hydrogen phosphate and calcium ions in supersaturated calcium-containing ionic liquid, so as to form nano amorphous calcium phosphate particles, and adding sodium hypochlorite solution of certain concentration and glycine into sodium hypochlorite solution of certain concentration to obtain the remineralizing material. The demineralized enamel oriented ordered mineralizing material described herein has reasonable structural design, is simple to prepare and can transform quickly into crystal and specifically binds and firmly binds with the demineralized enamel surface; arranging newly-formed hydroxyapatite in a certain direction in order; the material is nontoxic and nonirritating, has good biocompatibility and low allergicity and has ideal result.
Owner:STOMATOLOGICAL HOSPITAL TIANJIN MEDICAL UNIV
Instant crab leg processing method
InactiveCN105767953AAllergenicity reductionSimple and efficient operationFood ingredient functionsReducing sugarSensitization
The present invention discloses an instant crab leg processing method and relates to the crab processing. The processing method includes the following steps: 1) crab leg pre-treating: crab legs are washed and the washed crab legs are placed into salt and sugar water to be soaked; 2) pre-cooking: the soaked crab legs are put into a steaming and cooking machine to be pre-cooked, and the pre-cooked crab legs are cooled to a room temperature; 3) low-temperature pickling: the pre-cooked crab legs are immersed in the salt and sugar water again to conduct a low-temperature pickling; and 4) packaging: the low-temperature pickled crab legs are loaded into a compound packaging bag according to specifications, the loaded pickled crab legs are vacuum packaged, and the vacuum packaged crab legs are subjected to a high-temperature and high-pressure steam sterilization to thereby obtain a flexibly packaged crab leg finished product. In the production processing process of the instant crab legs, the salt and sugar water is used to conduct a short-time soaking, so that the crab meat proteins and the reducing sugar produce a Maillard reaction in the pre-cooking and the high-temperature and high-pressure sterilization process. The processing method significantly reduces the sensitization of the crab leg products, solves the cost problem that the crab leg meat obtaining needs a large amount of manual costs, and is simple in operation, low in cost, easy to achieve a large-scale production, etc.
Owner:JIMEI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com